- 1Division of Genetics, ICAR-Indian Agricultural Research Institute, New Delhi, India
- 2Division of Physiology, Biochemistry and PHT, ICAR-Central Plantation, Kasaragod, India
- 3Germplasm Evaluation Division, ICAR-National Bureau of Plant Genetic Resources, New Delhi, India
- 4Division of Biochemistry, ICAR-Indian Agricultural Research Institute, New Delhi, India
- 5Division of Plant Pathology, ICAR-Indian Agricultural Research Institute, New Delhi, India
- 6Division of Seed Science and Technology, ICAR-Indian Agricultural Research Institute, New Delhi, India
- 7World Vegetable Center, South Asia, ICRISAT Campus, Patancheru, Hyderabad, India
Globally, yellow mosaic disease (YMD) remains a major constraint of mungbean production, and management of this deadly disease is still the biggest challenge. Thus, finding ways to manage YMD including development of varieties possessing resistance against mungbean yellow mosaic virus (MYMV) and mungbean yellow mosaic India virus (MYMIV) is a research priority for mungbean crop. Characterization of YMD resistance using various advanced molecular and biochemical approaches during plant–virus interactions has unfolded a comprehensive network of pathogen survival, disease severity, and the response of plants to pathogen attack, including mechanisms of YMD resistance in mungbean. The biggest challenge in YMD management is the effective utilization of an array of information gained so far, in an integrated manner for the development of genotypes having durable resistance against yellow mosaic virus (YMV) infection. In this backdrop, this review summarizes the role of various begomoviruses, its genomic components, and vector whiteflies, including cryptic species in the YMD expression. Also, information about the genetics of YMD in both mungbean and blackgram crops is comprehensively presented, as both the species are crossable, and same viral strains are also found affecting these crops. Also, implications of various management strategies including the use of resistance sources, the primary source of inoculums and vector management, wide-hybridization, mutation breeding, marker-assisted selection (MAS), and pathogen-derived resistance (PDR) are thoroughly discussed. Finally, the prospects of employing various powerful emerging tools like translational genomics, and gene editing using CRISPR/Cas9 are also highlighted to complete the YMD management perspective in mungbean.
Introduction
Mungbean (Vigna radiata (L.) Wilczek) is indigenous to India or Indo-Burma region and is the third most important self-pollinated, short-duration grain legume crop after chickpea and pigeonpea. The central Asian region is believed to be the primary center of genetic diversity for mungbean (Kumar and Kumar, 2014). The genome size of mungbean is relatively small (579 Mb) and the 2n number of chromosomes is 22 (Parida et al., 1990; Kang et al., 2014). It is also known as greengram, greenbean, mashbean, goldengram, and greensoy (Markam et al., 2018). Mungbean is an important and cheap source of food protein across Asia, especially for the poor, thus plays an imperative role in the alleviation of protein malnutrition especially in the developing countries (Selvi et al., 2006). It contains a relatively high proportion of easily digestible good quality protein (24%) with low flatulence and is also rich in iron contents (40–70 ppm), making it an ultimate choice for balanced diets (Selvi et al., 2006; Vairam et al., 2016).
Besides seeds, its sprouts, which contain high vitamin C and folate are also very much relished in Asian cuisine (Nair et al., 2013); while its foliage can also be used as fodder, feed, and hay. Rhizobium and Bradyrhizobium bacteria which are present in the root-nodules of mungbean, fix the atmospheric nitrogen and thus improve the soil fertility, and benefit the succeeding crops. Mungbean is being cultivated across a wide range of latitudes (40 N or S) covering tropical and sub-tropical regions of the world and is suitably adapted to a range of cropping systems (http://avrdc.org/intl-mungbean-network/). Globally, mungbean is being grown in over 7.0 million ha area, yielding 3.5 million tons of grains mainly from Asia but spreading to other parts of the world (Nair et al., 2019). The major mungbean growing countries include India, China, Pakistan, Bangladesh, Sri Lanka, Thailand, Myanmar, Vietnam, Indonesia, Australia, and the Philippines (Alam et al., 2014b). Worldwide, India is the largest mungbean producer, yielding 2.17 million tons of grains from about 4.32 m ha area. However, the average productivity of mungbean in India is quite low (~502 kg/ha), even lower than most of the other pulse crops (Project Coordinators Report-2018).
In mungbean, yellow mosaic disease (YMD) caused by yellow mosaic viruses (YMVs) is of key importance especially in South and Southeast Asia. Besides mungbean, YMD also affect various leguminous crops including blackgram (Vigna mungo), mothbean (Vigna aconitifolia), Lima bean (P. lunatus), pigeonpea (Cajanus cajan), French bean (Phaseolus vulgaris), cowpea (Vigna unguiculata), Dolichos (Lablab purpureus), horsegram (Macrotyloma uniflorum), and soybean (Glycine max) (Ramesh et al., 2017b; Dikshit et al., 2020). The overall crop yield loss may range between 10 and 100%, depending on the mungbean genotype and stage of crop infection (Singh, 1980a; Marimuthu et al., 1981; Bashir et al., 2006).
YMD spread to the mungbean crop through whitefly (Bemisia tabaci Gennadius)—an insect vector for YMVs (Selvi et al., 2006). Although, YMD has been reported throughout the world (except Australia); but its heavy incidence is mainly reported from countries like India, Bangladesh, and Pakistan (Pathak and Jhamaria, 2004; Biswas et al., 2008; Salam et al., 2011). The virus enters the phloem cells of the host through the whitefly proboscis and the viral aggregates appear in the host cell nuclei roughly two days before the symptom appearance (Thongmeearkom et al., 1981). The visible symptoms appear as scattered yellow-color spots on the young leaves which later turns into a yellow mosaic pattern and ultimately results in complete yellowing, drying and withering of leaves (Figure 1). The pods on the infected mungbean plant become smaller in size, yellowing of the leaves decreases the photosynthetic efficiency which ultimately manifested as severe yield penalty (Malathi and John, 2009).

Figure 1 Field view of YMD susceptible (yellowing of the plants) and resistant expression (normal green plants) in various mungbean genotypes.
In India, MYMV was first reported from the mungbean fields of Indian Agricultural Research Institute (IARI), New Delhi during 1950s (Nariani, 1960). In general, MYMV is the major isolate infecting mungbean crop in western and southern India, Thailand, and Indonesia; whereas, MYMIV isolate in central, eastern and northern India, Pakistan, Bangladesh, Nepal, and Vietnam (Malathi and John, 2009). With this background, this review systematically deals with the scientific developments about YMVs infecting mungbean, its vector and also various YMD management challenges including the prospective use of recent tools like—omics approaches and translational genomics, across the world.
Begomovirus and YMD in Vigna
The family Geminiviridae comprised of nine genera, viz., Becurtovirus, Begomovirus, Capulovirus, Curtovirus, Eragrovirus, Grablovirus, Mastrevirus, Topucovirus, and Turncurtovirus, and the viruses are attributed to respective genus depending on its host, vector and genome arrangements (Varsani et al., 2017; Zerbini et al., 2017). The genus name Begomovirus was derived from the type member, Bean Golden MOsaic virus (BGMV), causing golden mosaic disease in beans. Begomovirus is the largest genus of a family Geminiviridae having twinned quasi-icosahedral particles (20 × 30 nm) encapsidating circular ss-DNA. These are mostly bipartite, with vector specificity and have specific amino acid sequences in its coat protein (Briddon and Markham, 2000). It comprises of nearly 322 species and more than 500 isolates, infecting various economically important dicot crops (Fauquet et al., 2008; Varsani et al., 2014; Varsani et al., 2017).
In pulses, depending on the viral nucleotide sequence identity, yellow mosaic disease (YMD) is caused by four distinct begomoviruses namely, (i) MYMV, (ii) MYMIV, (iii) dolichos yellow mosaic virus (DoYMV) and (iv) horsegram yellow mosaic virus (HgYMV); which are collectively known as yellow mosaic viruses (YMVs) (Qazi et al., 2007; Malathi and John, 2009; Naimuddin et al., 2016). The term ‘Legumoviruses’ has been used to refer the legume infecting bipartite begomoviruses (Briddon et al., 2010).
MYMV particles were first observed and purified in the leaf cells of mungbean by Thongmeearkom et al. (1981) and Honda et al. (1983), respectively. The genome of Thailand isolates of MYMV (Morinaga et al., 1993) and the isolate from North India (Mandal et al., 1997) was found sharing <89% similarity (Fauquet et al., 2008), hence considered as a distinct species, and later was named as MYMIV. The detailed historical perspectives of YMD in mungbean are presented in chronological order in Figure 2.
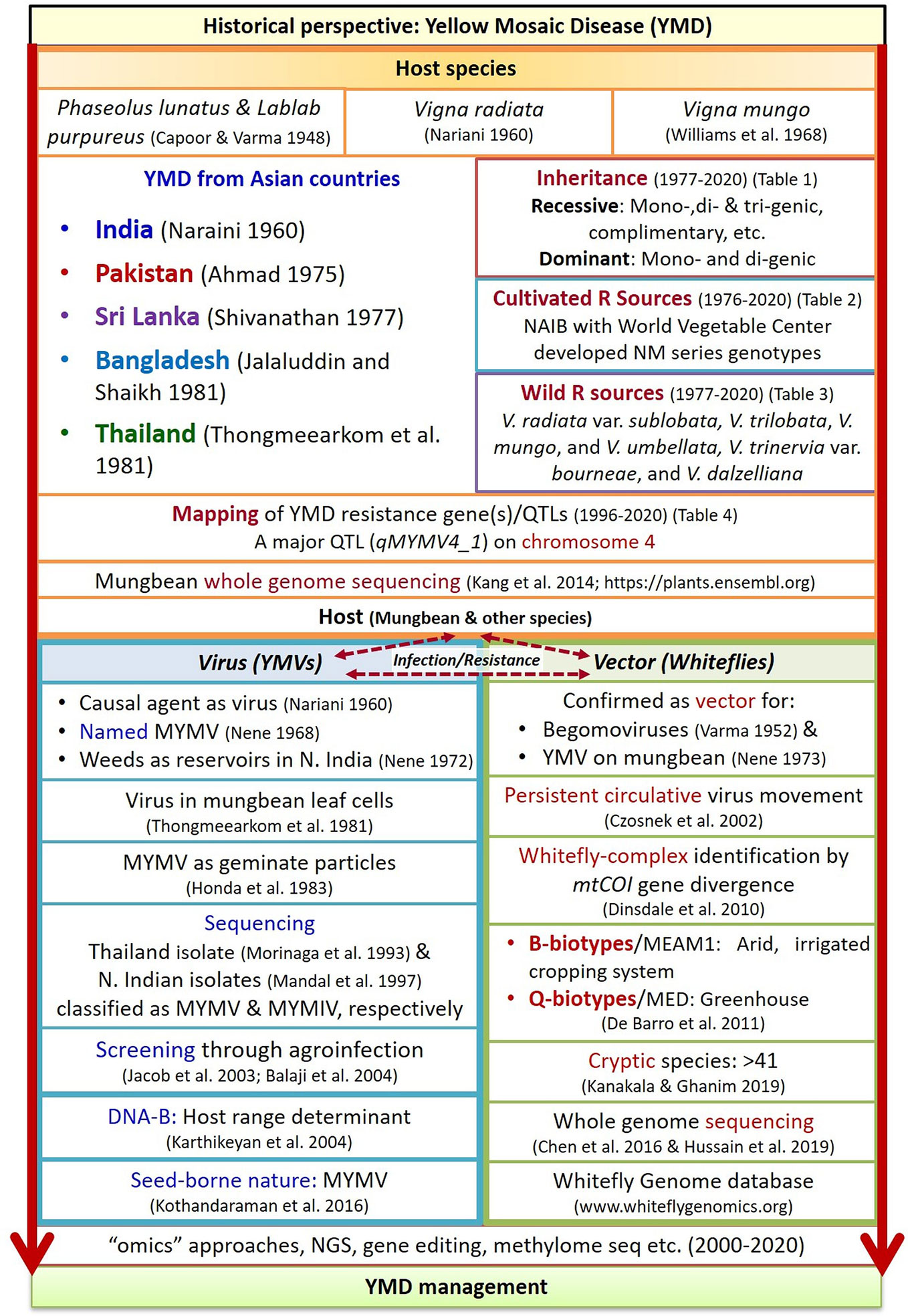
Figure 2 Historical sketch of YMD in mungbean crop (Derived from Capoor and Varma, 1948; Capoor and Varma, 1950; Varma, 1952; Nariani, 1960; Nene, 1968; Williams et al., 1968; Nene, 1972; Nene, 1973; Ahmad, 1975; Shivanathan, 1977; Jalaluddin and Shaikh, 1981; Thongmeearkom et al., 1981; Honda et al., 1983; Morinaga et al., 1993; Mandal et al., 1997; Czosnek et al., 2002; Jacob et al., 2003; Balaji et al., 2004; Karthikeyan et al., 2004; Dinsdale et al., 2010; De Barro et al., 2011; Kang et al., 2014; Chen et al., 2016; Kothandaraman et al., 2016; Hussain et al., 2019; Kanakala and Ghanim, 2019). Where, R, resistance; YMVs, yellow mosaic viruses; NGS, next generation sequencing.
Role of various DNA components in the YMD Expression and Molecular Characterization of Begomoviruses
Initially, all the begomoviruses were considered to be monopartite and DNA-B was believed to be generated as a satellite, which later got established as an integral part of the genome. The DNA-A and DNA-B of bipartite begomoviruses were supposed to be unique and diversification in these is due to the component exchange during evolution (Briddon et al., 2010). The viral sense strand of DNA-A encodes the coat protein (CP, ~29.7 kDa) and movement or pre-coat protein (~12.8 kDa) from AV1 and AV2 genes, respectively. The MYMIV-AV2 protein was also reported modulating the functions of Rep protein by affecting the ratio between open circular and supercoiled DNA forms (Rouhibakhsh et al., 2011).
The viral complementary sense strand encodes four proteins namely, replication-associated protein (Rep, ~40.2 kDa; ORF AC1), replication enhancer protein (REn, ~15.6 kDa; ORF AC3) and transcription activator protein (TrAP, ~19.6 kDa; ORF AC2). The AC4 (~12.0 kDa) is believed to regulate symptom expression; whereas, AC5 which is located downstream of AC3 (in antisense orientation of DNA-A) codes for a pathogenicity determinant in MYMIV, suppressing only sense RNA-induced gene silencing (Li et al., 2015) (Figure 3).
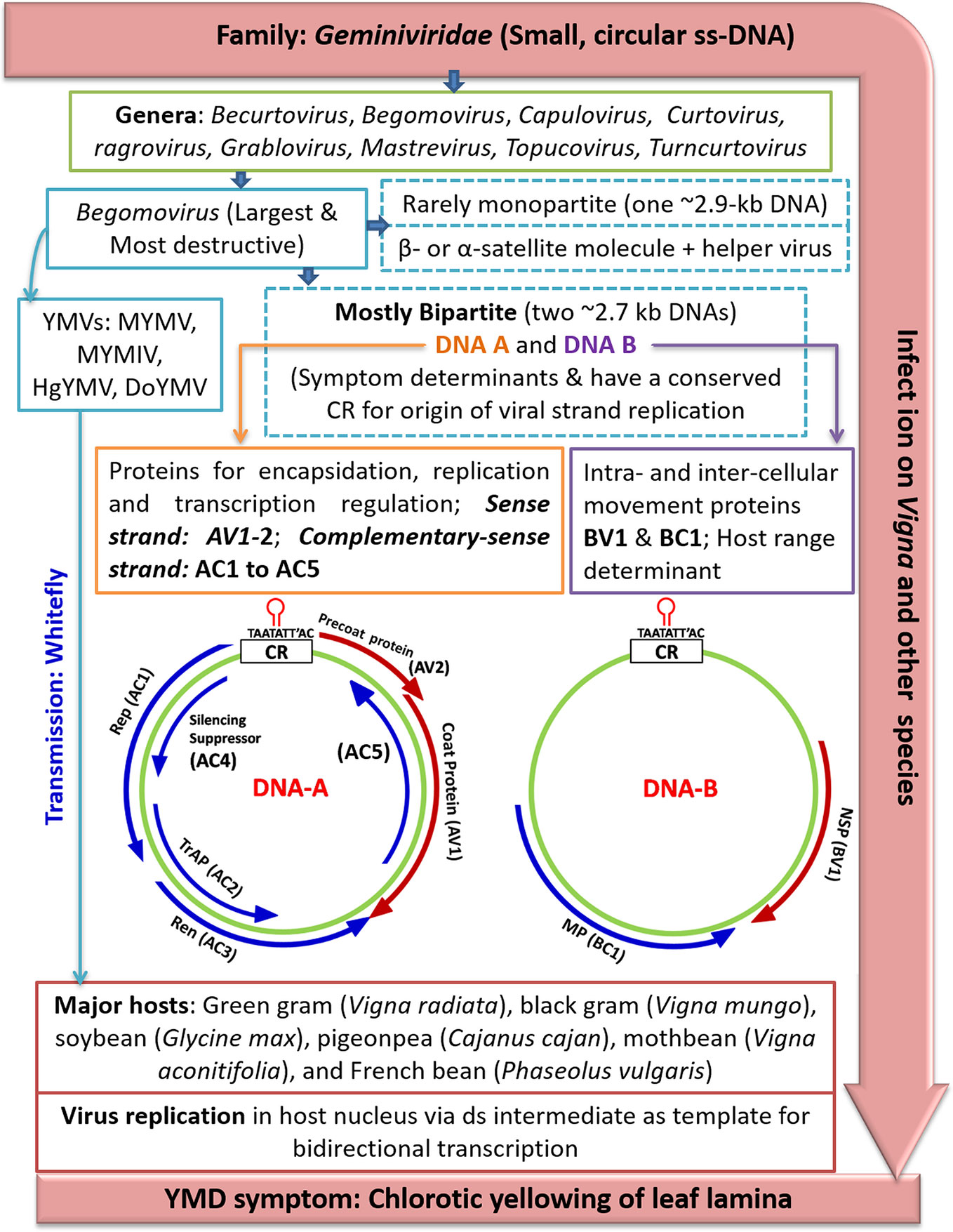
Figure 3 An outline of YMD development in mungbean. Where, YMD (yellow mosaic disease), MYMV (mungbean yellow mosaic virus), MYMIV (mungbean yellow mosaic India virus), HgYMV (horsegram yellow mosaic virus), DoYMV (dolichos yellow mosaic virus), AV2 (precoat protein), CP/AV1 (coat protein), Rep/AC1 (replication protein), TrAP/AC2 (transcriptional activator protein), REn/AC3 (rep enhancer protein), AC4 (silencing suppressor), CR (common region). The maps of YMV genomic DNA-A and DNA-B components are derived from Kumar et al. (2017b) and Shivaprasad et al. (2005) in which the ORF (open reading frames) are presented as bar arrows with the head representing 3′-terminus.
The DNA-B harbors two genes viz., BV1 (in viral sense strand) and BC1 (in complementary sense strand) encoding nuclear shuttle protein (NSP; ~33.1 kDa) and movement protein (MP; ~29.6 kDa), respectively. The MP regulates the cell to cell movement of viruses via plasmodesmata, while NSP helps in the movement of viral DNA between the host cell nucleus and cytoplasm and also their long-distance movement through host vascular system (Hanley-Bowdoin et al., 1999). Since the BV1 and BC1 are absent in monopartite begomoviruses, the function of NSP is found played by CP (AV1) gene (Polston et al., 1997). Also, the mungbean plant proteins are reported influenced by the plant–virus interaction and are simultaneously used by the viruses for its growth, multiplication, and cell-to-cell movement (Cayalvizhi et al., 2015).
A 200 bp region common to both DNA-A and DNA-B of bipartite begomoviruses is known as the common region (CR). The intergenic region in begomoviruses possesses an origin of replication (ori), a highly conserved stem-loop or hairpin structure having a nonanucleotide motif (TAATATT↓AC) and ‘iterons’ or direct repeat motifs of 5–7 nucleotide length (Hanley-Bowdoin et al., 1999; Pant et al., 2001).
Iterons function by recognizing the Rep proteins which nick the nonanucleotide motif and start the rolling circle DNA replication (Argüello-Astorga et al., 1994). Both DNA-A and DNA-B contains very similar iteron sequences which ensure that the DNA-A encoded Rep can initiate replication of both components (Shafiq et al., 2010). Highly specific Rep-Iteron interaction prevents the interaction between distinct begomovirus species (Chatterji et al., 2000) and thereby maintains the bipartite genome integrity. The iteron sequence (GGTGT) of MYMV, MYMIV, and DoYMV are similar, whereas HgYMV has a different sequence (GGTAT), thus are unable to readily exchange its components with other legume yellow mosaic viruses (LYMVs). Moreover, due to recombination, there is a replacement between ori of DNA-B with that of DNA-A, resulting in component capture between distinct species as reported for the HgYMV-like DNA-B sequence containing iteron motifs GGTGT (Girish and Usha, 2005).
Molecular Characterization of YMVs
YMVs are mostly characterized either by complete sequencing or by sequencing of various DNA-A and DNA-B components. Molecular characterization of YMVs infecting mungbean in Bangladesh and Pakistan revealed 97 and 94% sequence similarity for the CP and NSP-genes of MYMIV, respectively (Hussain et al., 2004; Islam et al., 2012). Similarly, sequence-based phylogenetic analysis of legume-infecting begomoviruses from Indonesia and Vietnam has identified Indonesian isolates as MYMIV strain-A, while Vietnam isolates as MYMV strain-B (Tsai et al., 2013). Furthermore, sequencing of 44 components (23 DNA-A, 19 DNA-B, and 2 betasatellites) of various LYMVs occurring across Pakistan revealed the presence of showed MYMIV with two distinct types (Ilyas et al., 2010). Molecular analysis of a begomovirus infecting V. mungo var. Silvestris, revealed it to be a strain of MYMIV and is designated as MYMIV-VSKN (Naimuddin et al., 2011b). CP gene characterization revealed considerable genetic variability in the MYMV-Tamil Nadu isolates of blackgram, cowpea and mungbean samples (Maheshwari et al., 2014). Molecular studies identified MYMIV isolates causing YMD in blackgram collected from Andhra Pradesh (India); whereas, MYMV isolate was found in the neighboring state of Tamil Nadu (Reddy et al., 2015). Recently, a new isolate (Mg-mungbean-1) of MYMIV having a recombinant DNA-B component was identified from Meghalaya (India). The DNA-A based phylogenetic tree also confirmed this novel isolate as a MYMIV (Banerjee et al., 2018).
Role of DNA Components in Infection by YMVs
Less durability of resistance of a legume genotype against begomoviruses may be due to recombination and component exchanges occurring in the viruses. However, no comprehensive evidence indicating the interaction of virus infecting various legume species exist, which means legume infecting begomoviruses are evolving independently of those infecting other plant families (Qazi et al., 2007). The DNA-B of an HgYMV isolate showed very high sequence similarity (96%) with that of soybean MYMV isolate, while it was only 70–73% with MYMV and MYMIV DNA-B which is speculated to be due to the component exchange and appears as host range expansion adaptation mechanism (Qazi et al., 2007).
Kumar et al. (2017a) through agroinoculation of dimeric infectious clones (having both DNA-A and DNA-B of MYMIV) have confirmed the pathogenicity of cowpea strain of MYMIV in cowpea and mungbean. Further sequence analysis has confirmed it as MYMIV isolate harboring a distinct DNA-B component playing a key role in symptom expression. Interestingly, viral clones were infectious to various crops (viz. cowpea, mungbean, blackgram, and French bean), but wild-type isolates are transmissible via whiteflies to only cowpea and not to blackgram or mungbean, suggesting the role of insect vector determining the natural host range (John et al., 2008). Ilyas et al. (2010) using sequence information of LYMVs revealed that either recombination with nonlegume viruses or interactions with betasatellites of begomoviruses is the reason for the emergence of more virulent variants affecting various legumes.
The comparison of blackgram isolate of MYMV (IMYMV-Bg) showed sequence divergence for the common region (CR) between DNA-A and DNA-B, while overexpression of IMYMV-Bg Rep protein in E. coli showed its specific binding to the CR-sequences. In addition, ATP-upregulated cleavage and ATP-mediated conformational change of Rep was also recorded (Pant et al., 2001). The agroinoculation of partial dimers of KA27 and KA22 DNA-Bs with DNA-A in blackgram and mungbean, established DNA-B of MYMV-Vig as a vital host-range determinant (Balaji et al., 2004). The swapping of the KA27 DNA-B component with the KA22 DNA-B nuclear shuttle protein (NSP) gene in MYMV-Vig has resulted in mild-yellow symptoms, suggesting NSP as major symptom determinant (Mahajan et al., 2011). The cloned DNA-A and five different DNA-Bs of MYMV-Vig when agroinoculated with mixed cultures of Agrobacterium showed co-infection ability of all DNA-B components to V. mungo (Karthikeyan et al., 2004). Thus, the co-existence of multiple DNA-B components of MYMV-Vig appears helping its host range expansion, while additional DNA-B components may help in infecting V. radiata and V. aconitifolia (Karthikeyan et al., 2004). Hence, it looks obligatory to find a more precise role being played by different DNA components of various YMVs affecting diverse Vigna species. A comprehensive list of primers amplifying different MYMV components as reported by different researchers is presented in Table S1.
Genetics of YMD Resistance IN Vigna
Most commonly exploited measure for YMD management in mungbean is the development and use of resistant varieties. However, the nature of the gene(s) controlling the YMD resistance in Vigna is reported varying in different genetic backgrounds and gene actions differ from a single dominant gene (Markam et al., 2018) to the recessive gene inheritance (Khattak et al., 2000; Dhole and Reddy, 2012) (Table 1). YMD tolerance and resistance were found regulated by one and two recessive genes, respectively in different cross combinations, while pairs of genes having dominant and recessive epistasis were also found governing resistance in interspecific crosses (Singh and Sharma, 1983).
Differential mungbean−YMV interaction appears the most probable reason for the identification of various types of resistance reactions to the YMD. The dominant MYMV resistance gene action indicates gain-of-function, while recessive inheritance signifies loss of host genes function which appears essential for virus infection, replication, and cell-to-cell movement (Diaz-Pendon et al., 2007). Weather parameters regulating whitefly activity is another very crucial factor for the viral disease expression under open field conditions (Sudha et al., 2013b). Since YMD resistance in mungbean was mostly reported controlled by digenic dominant interaction with some modifier genes, therefore the use of recombination breeding and delayed selection method should be more effective for the incorporation of YMD resistance (Mahalingam et al., 2018; Dikshit et al., 2020). The recessive nature of YMD resistance also emphasizes the significance of marker-assisted selection (MAS) for quick and precise YMD resistance breeding programs in mungbean (Chen et al., 2013).
Insight About Whiteflies as a Vector and YMD Development
Whitefly (Bemisia tabaci Gennadius) (Hemiptera, Aleyrodidae), a polyphagous pest of Indian origin, causes severe damage to over 1,000 plant species, not only by sucking the plant sap but also as a vector of several viral diseases (Fishpool and Burban, 1994). It can transmit nearly 300 virus species of multiple virus genera including Begomovirus (~90%), Carlavirus, Crinivirus, Closterovirus, and Ipomovirus (4%) (Simon et al., 2003; Castillo et al., 2011; www.whiteflygenomics.org). The mouthparts of the whiteflies are designed to retain the virus through their stylet, while feeding on the phloem sap from the plant. After entering the vector, the virus moves in a persistent circulative manner (Czosnek et al., 2002) and during its next feeding on a healthy plant the virus is injected with salivary secretion. The virus circulates (do not replicate in the whitefly) from the foregut, midgut, hindgut, hemolymph, and finally to the salivary glands of the whitefly before their release into the plants (Fiallo-Olivé et al., 2020).
For acquisition and inoculation of virus through phloem sap, the vector requires at least 15 to 60 min and 15 to 30 min, respectively. However, 8 h of minimum latent period is a must between acquisition and inoculation, for successful transmission of viruses (Ghanim et al., 2001; Czosnek, 2008). Whitefly transmission ability is directly proportional to its acquisition access period (AAP) while gender and age of the vector also influences the virus transmission efficiency (Czosnek et al., 2002). The persistent mode depends on the minimum AAP and maximum duration of retention (generally 3 days for male and 10 days for female whiteflies) of the virions in the whiteflies.
Although, whitefly nymphs can get the virus from infected leaves, however, the virus cannot traverse to the eggs. Moreover, infectivity cannot be retained for the lifetime by either male or female whitefly (Karthikeyan et al., 2014). The interaction between the highly conserved virus CP and the receptors in the gut and salivary glands of the whitefly imparts Begomovirus-whitefly specificity, and any alteration in the virus CP also alters their vector preferences. Various proteins encoded by the whitefly like molecular chaperone proteins, HSP70 to assist the efficient circulative transmission of viruses (Brown and Czosnek, 2002; Varun and Saxena, 2017).
Murugan and Nadarajan (2012) reported no correlation between the presence of leaf trichomes in blackgram and whitefly activities and thus resistance to YMV, however, no such report is available for mungbean. Begomoviruses can negatively influence the longevity and fecundity of whiteflies to enhance their transmission; while whitefly behavior and feeding habits also influences the genetic composition and evolution of viruses (Varun and Saxena, 2017).
The globally accepted identification method of B. tabaci complex is the identification of divergence threshold of mitochondrial cytochrome oxidase subunit I (mtCOI) gene which was earlier considered at 3.5% (Dinsdale et al., 2010) and later changed to 4.0% (Lee et al., 2013). Sequence analysis of mtCOI has partitioned them into more than 41 morphologically indistinguishable groups or cryptic species (Dinsdale et al., 2010; De Barro et al., 2011; Mugerwa et al., 2018; Hu et al., 2018; Kanakala and Ghanim, 2019). However, these cryptic species do possess considerable variations for traits like host-range, insecticide resistance, and dispersing capability (Simon et al., 2003; Nair et al., 2017). In general, maximum whitefly diversity is reported from Asia. Of 11 genetic groups reported from India, Asia-I and Asia II-1 are found predominant with a significantly higher transmission efficiency of Asia-I (Anokhe et al., 2018).
The B-biotypes or Middle East-Asia Minor 1 (MEAM) 1 are found in arid, irrigated cropping system while Q-biotypes or Mediterranean MED species can adapt to greenhouse environments (Dinsdale et al., 2010; De Barro et al., 2011; Horowitz and Ishaaya, 2014). Recent whitefly whole genome sequencing has revealed that the Asia II-1 and Middle East Asia Minor 1 (MEAM1) species differ for the genes involved in virus transmission and insecticide resistance (Hussain et al., 2019). This indicates the need to generate more sequence information for different whiteflies biotypes across the world for holistic management of disease. Detailed studies on the whitefly-Begomovirus co-evolution in terms of their transmission, YMV-whitefly interactions and proteins associated in virus movement inside the whitefly can assist in the formulation of novel and more effective ways of YMV management (Varun and Saxena, 2017).
Screening Methods and Varying YMD Resistance Expression in Vigna
Since mechanical transmission of YMV is not possible, therefore screening of mungbean for YMD resistance is mostly performed at the YMV hot spots. However, screening using viruliferous whiteflies and agroinoculation techniques which are more precise are on the rise. The details are discussed in this section.
Screening of Genotypes at YMV Hot-Spots
The evaluation of mungbean against YMD under hot-spot conditions are carried out using infector-row technique in certain standard statistical experimental design. Generally, one row of a most susceptible spreader genotype of that region is sown after every two (Habib et al., 2007), three (Nair et al., 2017) or 10 rows of the test-genotypes (Sai et al., 2017) and also two rows of spreader may be planted all around the experimental area for having sufficient YMV load (Habib et al., 2007). Recommended cultural practices with no insecticide spray should be followed so as to encourage the whitefly population for sufficient infection and spread of YMD. Since whitefly starts infecting the plants soon after germination and YMD symptom is first visible during 2nd week after planting (continues till 6th week), therefore crop should be constantly watched for the presence of whitefly and YMD development. The disease can be scored as per the scale of Khan et al. (2007) when 90% of the infector rows express the YMD incidence and the genotypes can be categorized in various groups from resistant till susceptible (Selvi et al., 2006; Habib et al., 2007).
The major limitation under hot-spot screening is that the causative viruses and also the whitefly biotypes remain unknown (Shivaprasad et al., 2006). In addition, there is always chance of non-uniform disease development due to the varying whitefly population which simultaneously depends on the planting locations and season (Laosatit et al., 2020). Under field conditions, more whitefly built-up were reported at a higher temperature; whereas, high-rainfall and high-humidity results in a negative impact on the whitefly population (Rahman et al., 2006; Islam et al., 2008). Besides, a negative correlation between high-altitude regions with low-humidity and YMD incidence highlights the significance of various environmental factors influencing the YMD severity (Alam et al., 2014c).
Screening Using Viruliferous Whiteflies
The screening of genotypes for YMD resistance under the net-houses using viruliferous whiteflies appears a better option (Shivaprasad et al., 2006). The whiteflies were first made viruliferous by force-feeding on YMV agroinfected mungbean plants for nearly 24 h, also known as acquisition access period (AAP) and these were then used for the inoculation of healthy plants for approximately 24–48 h, also known as inoculation access period (IAP). Whitefly is an extremely efficient vector as even a single viruliferous adult can transmit the YMV within 24 h of AAP and IAP (Malathi and John, 2009). Govindan et al. (2014) reported 10 viruliferous adult whiteflies after 24 h each of AAP and IAP causing YMD with 70.50% virus transmission efficiency, while 20 viruliferous whitefly adults after 48 h of AAP and 24 h of IAP has resulted in 85% virus transmission efficiency. Since YMD can be very effectively spread by very low densities of adult whiteflies; therefore, no correlation could be established between the number of whiteflies and YMD severity (Akhtar et al., 2011).
Screening Through Agroinfection
As the YMV can only be transmitted by the whitefly vector, thus agroinoculation based genotypic screening is considered better option for the identification of YMD resistance sources (Sudha et al., 2013c). Agroinoculation in mungbean are performed on surface sterilized 2 d sprouted seeds (Jacob et al., 2003) grown in Hoagland’s solution by removing the seed coat and then pricking near the hypocotyl region and then immersing the pricked seeds in A. tumefaciens culture (Sudha et al., 2013b; Sudha et al., 2013c; Sai et al., 2017). After overnight incubation, seeds are washed using distilled water and sown in pots. Afterwards, the inoculated plants should be grown in a growth chamber with 16/18 h photoperiod, 25°C temperature and 60–70% of relative humidity (Karthikeyan et al., 2011). The appearance of YMD symptoms in the leaves can be noted from 7th to 12th day of inoculation, while infectivity (%) can be calculated based on the number of infected plants to the total number of inoculated plants (Sudha et al., 2015). Plants should be transferred to the greenhouse after 15 days after symptom appearance (Balaji et al., 2004). The biggest advantage with agroinoculation is that it creates uniform disease conditions than those of natural conditions and thus the symptoms are also easier to compare (Sudha et al., 2015).
A field-based screening of 78 mungbean genotypes for MYMV has identified 28 genotypes as resistant while on agroinoculation of same genotypes only 03 (ML1108, KMG189 and SP84) and 01 (ML818) genotypes were found resistant to the VA221 (KA30 DNA-A and KA22 DNA-B) and VA239 (KA30 DNA-A and KA27 DNA-B) strains, respectively (Sudha et al., 2013c). Thus, it was assumed that the resistance expressed at the field could be because of certain mechanisms preventing the viral entry into the plant through insect vectors.
Generally, for bipartite geminiviruses, the agroinfection is performed by mixing two Agrobacterium strains harboring partial tandem repeats of DNA-A and DNA-B components, independently. However, Jacob et al. (2003) reported a ‘single strain agroinfection method’ of a bipartite begomovirus, which employs a combination of binary vectors, pGA1.9A and pPZP1.9B having MYMV-Vi DNA-A and DNA-B partial tandem repeats, respectively in the same Agrobacterium strain. This method consistently gave 100% agroinfection in blackgram (Figure 4). Moreover, when viral load is minimal and also for the asymptomatic plants; real-time PCR assay should be opted over conventional PCR assays (Sudha et al., 2013c). Thus, not only understanding the YMV resistance mechanism, but also quantification of viral load in the virus challenged plants appears essential while evaluating the YMV resistance.
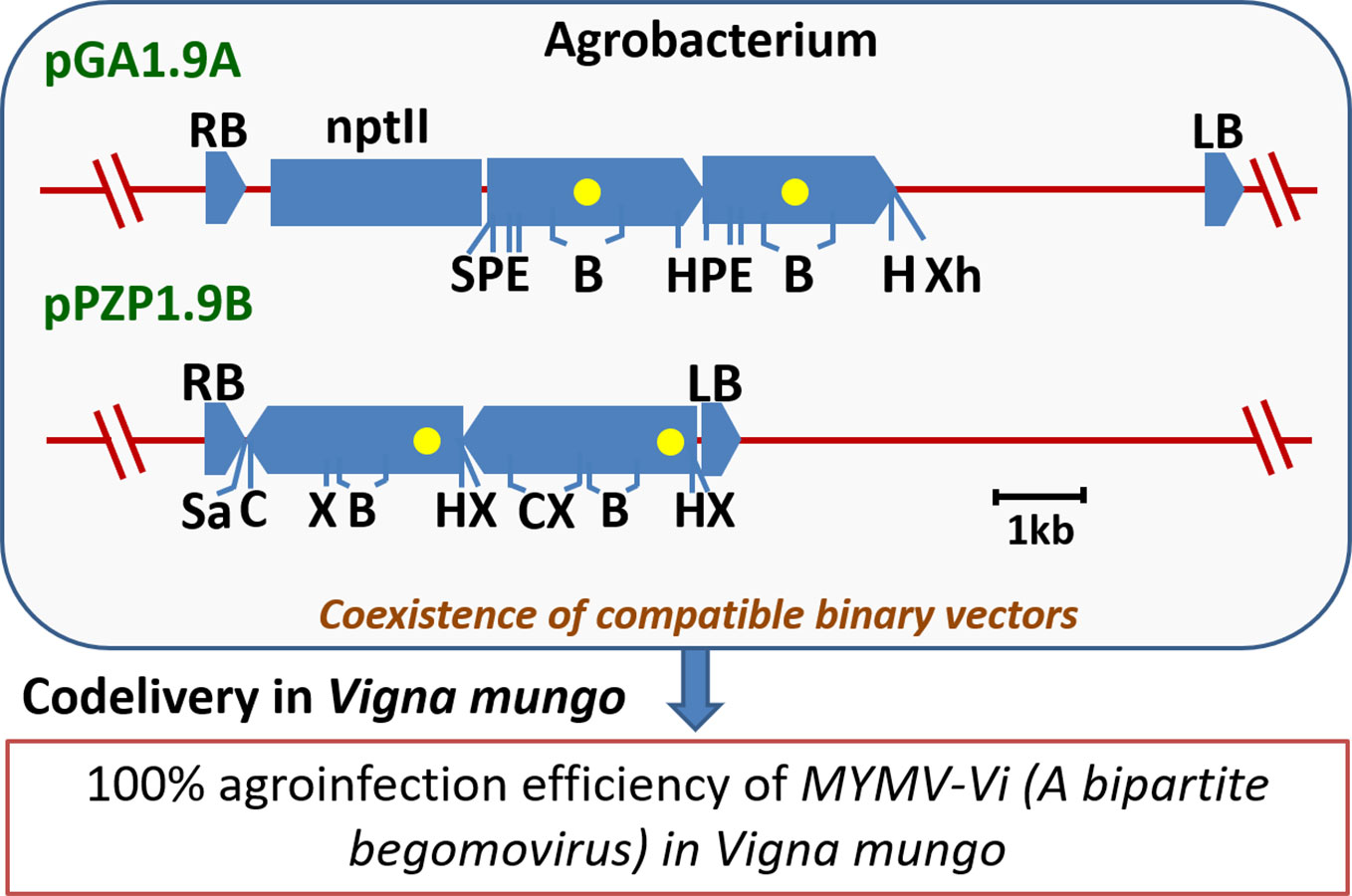
Figure 4 An outline of a simple and efficient, ‘single-strain agroinfection method’ of a bipartite begomovirus MYMV-Vi in Vigna. The linear maps of binary vectors represent MYMV-Vi partial tandem repeat regions of DNA-A (pGA1.9A) and DNA-B (pPZP1.9B) having full-length 1-mer portion and the 0.3-mer or 0.9-mer repeat portions of the virus as boxed arrows; Yellow dots: common region; RB and LB: right and left T-DNA borders, respectively; nptII: neomycin phospho-transferase II; B, BamHI; C, ClaI; E, EcoRI; H, HindIII; P, PstI; S, SacI; Sa, SalI; X, XbaI; and Xh, XhoI (Derived from Jacob et al., 2003).
Seed Borne Nature of YMVs
Begomoviruses are mostly confined to the phloem parenchyma and cambium, and rarely to mesophyll parenchymatous tissue, thus they can reach seed parts only till seed coat hilum (Rojas et al., 2005; Kothandaraman et al., 2016). However, the early symptom appearance as yellowing of the very first trifoliate leaf of the blackgram seedling in the field indicated the possibility of seed-borne nature of YMV. PCR amplicons sequencing, DAS-ELISA, immunosorbent electron microscopy, and confocal microscopy confirmed the presence of MYMV in the seed coat, cotyledon, and embryonic axes. However, the seeding growth tests revealed no YMD symptoms, though both DNA-A and B components of MYMV could be detected in 32% of the seedlings (Kothandaraman et al., 2016). It was speculated that the vigorous metabolic environment of seedling could be inhibiting the efficient build-up and translocation of the virus leading to no symptom. However, whitefly transmission of the virus was not demonstrated from the PCR confirmed symptomless seedlings.
On contrary, when seeds (with yellow patches on the seed coat) from MYMIV infected mungbean plants when used for PCR amplification do showed the presence of virus, but it could not be detected in the seedlings of PCR positive seeds, and the seed-borne nature of YMD in mungbean was ruled out (Naimuddin et al., 2016). Except for the report of Kothandaraman et al. (2016), there was no other report confirming or validating the seed-borne nature of YMVs in any other Vigna species. Thus, detailed analysis is still needed to confirm the exact mechanism of seed-borne nature of YMVs in different Vigna species.
Biochemical Changes During Mungbean–YMV Interactions
Upon YMV infection, the compatible reaction results in systemic infection leading to symptom expression (Yang et al., 2007). During YMV-host incompatible reaction, the resistance gene expression gets activated upon interaction with viral avirulence (avr) proteins which then triggers a cascade of defence genes including pathogenesis-related (PR) proteins which are also associated with systemic acquired resistance (SAR) (Sels et al., 2008). All these ultimately results in the ceased replication and arrested movement of the YMVs (Figure 5). Various ROS-scavenging enzymes viz. ascorbate peroxidases, superoxide dismutases, and catalases are reportedly maintaining the ROS homeostasis in the plant cells which eventually inactivates the virus (Torres, 2010; Oliveira et al., 2012).
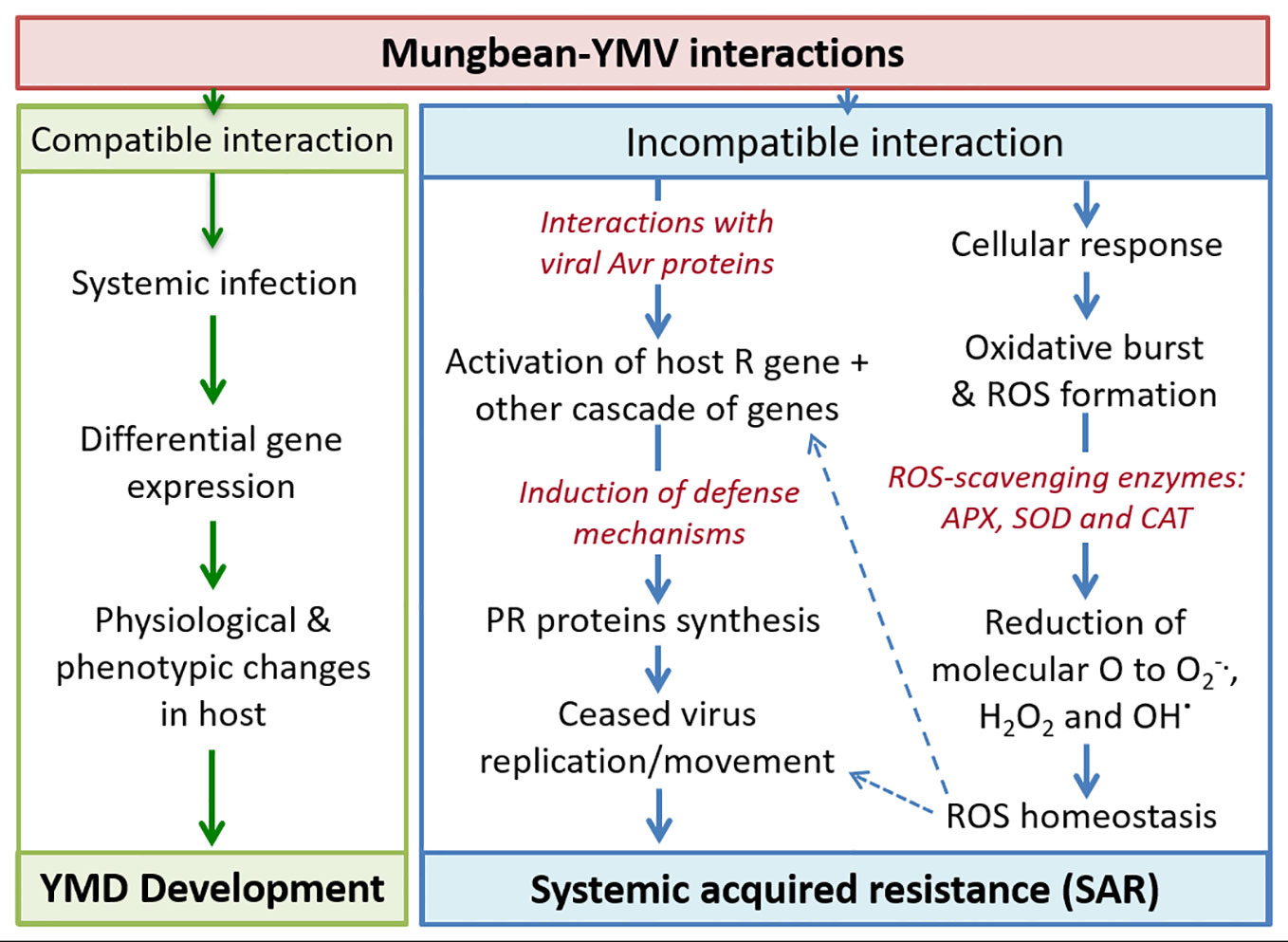
Figure 5 Schematic presentation of plant-virus interaction leading to disease development and resistance expression in mungbean. Where, Avr, Avirulence; R, Resistance; PR, Pathogenesis-Related; ROS, Reactive Oxygen Species; APX, Ascorbate peroxidases; SOD, Superoxide dismutases; CAT, Catalase.
The relative expressions of defense and signal transduction associated proteins are important for the induction of YMD resistance. Photosynthesis pathway proteins, especially PS-II electron transport pathway are mainly affected in susceptible genotypes under YMV-stress. In addition, significantly elevated levels are recorded for phenolics, H2O2, and carbohydrates in YMV incompatible interaction over compatible reaction. The pathways associated with the induction of defense response carries various core proteins, of which ascorbate peroxidase, rubisco activase, and serine/glycine hydroxyl-methyltransferase are the nodal hub which results in defense response. Also, YMV resistance in blackgram was reported channelizing the carbohydrate flux towards the pentose phosphate pathway (Kundu et al., 2013). Thus, the role of various biochemicals (involved in the ROS homeostasis) in imparting YMD resistance in mungbean should be established in a gene-network derived pathway mode using high throughput transcriptomic studies, during both compatible and incompatible reactions.
YMD Management Challenges and Opportunities
The YMD management using insecticides (to control whiteflies) has been considered effective, but due to the development of insecticide resistance in vectors, the disease is on the rise. In addition, excessive use of chemicals resulted in detrimental impacts on both environment and human health (Mishra et al., 2017). Several YMD management strategies for the sustainable management of YMD are thoroughly discussed in this section.
The Management of Primary Hosts of YMV and Its Vector
Eradication of primary hosts of YMV such as perennial weeds and summer whiteflies will facilitate YMD management (Malathi and John, 2009; Karthikeyan et al., 2014). The significant YMV hosts include, V. radiata, V. mungo, V. aconitifolia, V. unguiculata, Cajanus cajan, Glycine max and Phaseolus vulgaris (Varma et al., 1992; Karthikeyan et al., 2004; Qazi et al., 2007; Karthikeyan et al., 2014). Alternatively, other leguminous hosts namely, V. hainiana and V. trilobata, have also been confirmed as natural hosts (Naimuddin et al., 2011a; Ramesh et al., 2017b). Besides, ‘infected tolerant plants’ or ‘symptomless carriers’ may also act as a virus-host.
Managing whiteflies is quite complex, as they attack in hundreds and even one attack is enough for the severe weakening of a plant. In the Northern and Southern Indian conditions, two indigenous cryptic species viz. Asia II-1 and Asia II-8, respectively are reported predominant (Nair et al., 2017). Since whitefly species differ significantly in its sensitivity to various insecticides, therefore inclusive information about the abundance of whitefly species of any region is essential for the rational use of insecticides (Horowitz and Ishaaya, 2014). The application of systemic insecticide combinations at the early growth stage proved effective for whitefly management, as it kills the vector and simultaneously protects the plant against further attack (Wang et al., 2009; Dubey and Singh, 2013). Also, field-sanitation, plucking of infested leaves, water-sprays, avoiding an excess of nitrogen fertilizer are also recommended to curb the whitefly population (Karthikeyan et al., 2014). In addition, 8 h of seed hydro-priming was reported effective for lowering the incidence and severity of YMV infection in mungbean (Rashid et al., 2004).
Resistance Sources for YMD in Mungbean and Blackgram
The YMD resistance is generally assessed by the appearance of symptoms using a commonly accepted disease scoring scale (Khattak et al., 2008; Iqbal et al., 2011; Panduranga et al., 2011). However, while selecting any genotype as resistant, utmost care should be taken and any symptomless carrier should never be used in the YMV resistance breeding program as a resistance source. Therefore, the resistance sources screening under open field conditions should also include the simultaneous identification of viral strains (Karthikeyan et al., 2014).
There are abundance of reports stating absolute YMD resistance among certain mungbean lines, but most of them were poor yielder (Pathak and Jhamaria, 2004; Salam et al., 2009). Generally, the mungbean germplasm having good yield potential is reported susceptible to the YMD (Khattak et al., 2008; Akhtar et al., 2011). Success has been achieved via shuttle breeding program between the World Vegetable Center (AVRDC) and NIAB (Nuclear Institute for Agriculture and Biology-Pakistan), which has resulted in the development of several mungbean varieties having YMV resistant (AVRDC, 1995; Khattak et al., 2008).
Most of the reports about the identification of YMD resistance sources in mungbean across the world are based on field screening (Table 2). A few Indian mungbean genotypes like IPM-02-03, PDM-139, Pusa0672, and HUM16 are reported resistant by different workers under different field conditions in different years (Asthana, 1998; Datta et al., 2012; Paul et al., 2013; Mohan et al., 2014; Subedi et al., 2016).

Table 2 A timeline of mungbean and blackgram genotypes, reported as YMD resistant and susceptible by different researchers.
Notably, the genotype found resistant in one location may not be resistant under other locations, as the resistance is viral strain specific. Thus, while selecting the resistant parent for YMD resistance breeding, it is advised to first screen all the genotypes at any given location, and depending on the results, crossing programme should be designed. Confirmation of YMD resistance using agro-inoculation of age no type appears as the best option, as it results in significantly uniform disease expression (Sudha et al., 2013c).
Wild Relatives and Wide-Hybridization for YMD Resistance
Some wild relatives of mungbean, like a few accessions of V. radiata var. sublobata (Singh and Ahuja, 1977), V. trilobata, V. mungo, and V. umbellata (Pandiyan et al., 2008), have been reported as YMD resistant. Seven Vigna accessions viz., Vigna synthetic allotetraploid, V. umbellata, V. mungo var. mungo, V. trilobata, V. trinervia var. bourneae, V. radiata var. sublobata and V. dalzelliana were reported free from YMD (Gautam et al., 2014). Also, certain accessions of V. umbellata were found resistant to a few isolates of MYMV, which can be used for the transfer of MYMV resistance into V. radiata and V. mungo via inter-specific cross (Sudha et al., 2015).
On contrary, MYMIV was recorded as the predominant virus causing YMD in 40 accessions of different wild species of Vigna. Likewise, V. hainiana (IC331450) was found infected with MYMV (Gautam et al., 2014). Naimuddin et al. (2011a) also reported natural infections of MYMIV in two wild Vigna species, viz. V. hainiana (IC-331615) and V. trilobata (IC-331436) under Indian conditions. Thus, care must be taken while selecting the wild Vigna species for the transfer of YMD resistance in mungbean through wide-hybridization.
At present, the World Vegetable Center (AVRDC) holds nearly 12,153 Vigna accessions which is the largest collection, representing a vital resource for inter-specific hybridization (Kim et al., 2015). The cross-compatibility among Vigna species is not very well defined and for widening the genetic base of V. radiata, the crossing using secondary gene pool including blackgram (Vigna mungo (L.) Hepper), rice bean [V. umbellata (Thunb.)], V. radiata var. sublobata and V. trilobata have been attempted with some success (Table 3). Unfortunately, wide hybridization in mungbean recorded severe cross-barriers like development of a few, small and mostly non-viable hybrid seeds, embryo death or hybrid sterility, incompatibility in chromosomal pairing and chromosome elimination (Pandiyan et al., 2012; Sudha et al., 2013a; Pratap et al., 2018). DNA marker analysis has also shown severe segregation distortion and chromosome elimination in an F2 population derived from a cross between mungbean and rice bean (Sudha et al., 2013a).
Measures such as the use of mentor pollination, embryo rescue, and hormonal manipulations are reported to increase the success of interspecific crosses. To overcome the cross-compatibility problem of mungbean with rice bean, use of either 100 ppm E-Amino Caproic Acid (EACA), or V. radiata var. sublobata as a bridge species was reported successful (Kumar and Kumar, 2014). The hybrids between the cross of V. mungo × V. radiata were obtained through sequential embryo rescue (Gosal and Bajaj, 1983; Verma and Singh, 1986). In India, till now only three mungbean varieties namely, HUM1, Pant Moong4 and IPM99-125 having a high level of YMD resistance could be released using mungbean × blackgram crosses. Thus, more concerted efforts are required to not only overcome the cross-compatibility barrier but also to prevent chromosome elimination while attempting for the wide hybrids.
Mutation Breeding
Mutation breeding is an instant way of accelerating the genetic variation for various traits including YMD resistance in crop plants. In mungbean, 10–30 KR dose was found quite effective for getting the desirable mutants for traits like earliness, synchronous maturity, and YMD resistance (Singh and Chaturvedi, 1981). While performing the mutation breeding, the breeders generally select one or a few target traits for the improvement purpose. Single plant selections were performed under disease pressure conditions during M2 and onwards generation to find the plant(s) with YMD resistance and high yield through the selection of various other traits like fertile branches per plant, pods per plant and seed yield per plant, etc. These mutant lines may be released as such as a variety or the traits may be incorporated in other varieties through backcross breeding (Pratap et al., 2020).
At Nuclear Institute for Agriculture and Biology (NIAB, Pakistan) the mungbean improvement was initiated in 1970s with major focus to create variations through induced mutations (gamma irradiation) and hybridization, to develop high yielding and YMV resistant varieties (Haq, 2009). NAIB in collaboration with World Vegetable Center started the crossing program using a mutant YMV resistant line with KPS1, which resulted in the development of to two advanced YMD-resistant lines namely NM-92 (NIAB Mungbean-1992) and NM94 (Ali et al., 1997) which were introduced to various countries in South Asia (Shanmugasundaram et al., 2009). Two very popular summer mungbean cultivar of India, Pusa Vishal and SML-668 was also derived from NM-92 and NM-94 respectively, through selection for YMD resistance and synchronous maturity. NM-92 also became very popular in other countries like Bangladesh and Myanmar (Brar et al., 2006).
Several mutant varieties in mungbean have been developed across the world which are both high yielding and also resistant to many biotic stresses including YMD (Table 4). Based on field scoring, Vairam et al. (2016) have identified 05 mutants (viz., M5, M18, M26, M70, and M71) which in M3 generation showed YMD resistance. Thus, the mutation breeding approach looks promising not only for the creation of YMD resistance but also for the yield improvement without severely altering the existing genetic architecture (Vairam et al., 2016).
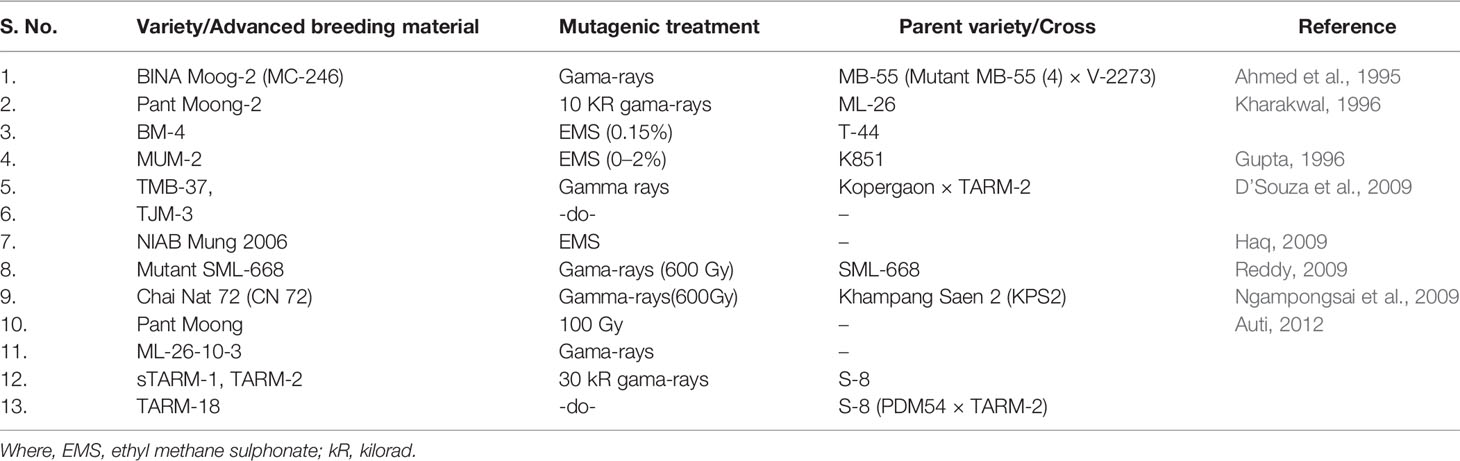
Table 4 List of YMD resistant mungbean varieties/advanced breeding lines developed through the mutagenesis approach.
Marker Assisted Selection (MAS)
The identification of tightly linked molecular marker(s) with the YMV resistance gene and screening of genotypes through MAS can augment the selection precision for the YMD resistance (Laosatit et al., 2020). Additionally, the recessive expression of YMV resistance also highlights the importance of MAS for mungbean breeding programs (Chen et al., 2013). Although, a large number of DNA markers reported linked with YMD resistance in both mungbean and blackgram (Table 5), but not yet very successfully used in the breeding programme, possibly due to the poor linkage or parental polymorphism (Selvi et al., 2006; Souframanien and Gopalakrishna, 2006).
Markers Linked With the YMD Resistance
Linkage map comparisons have revealed same linkage group (could be the same locus) for three major QTLs imparting YMD resistance viz., MYMIVE9_25, qMYMIV4/qMYMIV1, and qMYMIV2.1 from the genotypes NM92, NM10-12-1 and BARImung6, respectively (Kitsanachandee et al., 2013; Alam et al., 2014b). Interestingly, the resistance in these genotypes was derived from a common genotype 6601 (Laosatit et al., 2020). Thus, fine-mapping and cloning of the region should be attempted on priority for these QTLs for finding the functional details of this region.
Although ricebean is nearly immune to YMD (Sudha et al., 2015), yet due to low cross-compatibility this is occasionally used for the transfer of YMD resistance to mungbean (Sudha et al., 2013a; Bhanu et al., 2017b). Recently, QTL mapping of YMD resistance gene(s) using a RIL population (Mungbean-VRM (Gg)1 × Ricebean-TNAU RED) through GBS has revealed 05 QTLs having PVE from 10.11 to 20.04%. One major QTL qMYMV4_1 was found located in a 1.2Mb (14,504,302–15,788,321) region on mungbean chromosome 4 having 83 annotated genes of which 18 are considered as candidate genes (Figure 6) imparting resistance (Mathivathana et al., 2019). Since this is a big region, therefore adding more markers to this region will help in reducing the number of candidate genes for YMD resistance. However, the number of QTLs identified for the YMD resistance in TNAU RED is contrary to the fact that the resistance in ricebean is under the control of a single recessive gene (Sudha et al., 2013b).
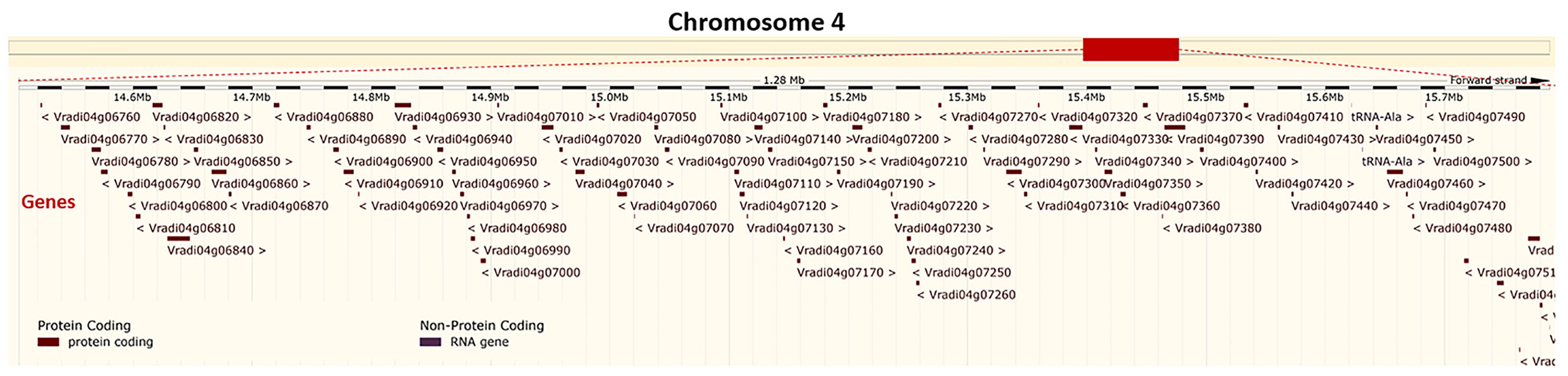
Figure 6 Physical location of a major QTL (qMYMV4_1) on the mungbean chromosome 4: 14,504,302-15,788,321. This region possesses 18 candidate genes imparting YMD resistance (Derived from: Mathivathana et al., 2019; https://plants.ensembl.org/Vigna_radiata/Info/Index).
However, Sai et al. (2017) have identified the MYMV resistance genes on chromosome 3 (using SCAR marker CM9); whereas, Yen et al. (2016) have reported several MYMV linked SNPs on the mungbean chromosomes 2, 5, 7, 9, and 10 (using CEL-I nuclease-based genotyping) (Yen et al., 2016). Till now, the chromosomal location of other markers linked with MYMV/MYMIV resistance genes or QTLs in mungbean are not yet worked out using integration studies. The details of markers linked with the YMD resistance genes/QTLs in mungbean and blackgram are presented in Table 5.
The detail gene mapping for the YMD resistance revealed that the genes imparting resistance to MYMIV (at least 02 loci) and MYMV in mungbean are different (Laosatit et al., 2020). Similarly, Alam et al. (2014b) also reported a SCAR marker (MYMVR-583) linked to a recessive gene imparting MYMV resistance in the genotype TM-99-37; but this marker was found not associated with the QTLs for the MYMIV resistance.
Candidate Gene for YMD Resistance
In both V. mungo and V. radiata, based on the role of ‘R genes’ in imparting plant virus resistance, the RGA markers (YR4 and CYR1) are reported completely linked with the resistance to MYMIV, suggesting that CYR1 could be a part of the candidate disease resistance gene (Pal et al., 2007; Maiti et al., 2011). Interestingly, CYR1 is also found associated, but not completely linked with MYMIV resistance in mungbean, indicating that the gene(s) for the resistance is not same and more than one locus is involved in imparting the resistance. Full-length sequence analysis of blackgram R gene CYR1 revealed it as 1,176 amino acids protein of non-TIR-NBS-LRR subfamily which by interacting with MYMIV-CP may act as a signaling molecule for recognizing the effector molecule of the pathosystem imparting resistance (Maiti et al., 2012).
Recently, BLASTN analysis of the CYR1 gene and linked SSR marker sequences for MYMIV resistance in NM10-12-1, NM92, and BARImung6 on the reference genomes of mungbean and azuki bean (V. angularis) showed that the CYR1 gene and other QTLs are present on different chromosomes (Laosatit et al., 2020). This has again reconfirmed that the resistance to MYMIV in mungbean and blackgram is different. The presence of different YMD resistant genes between mungbean, blackgram, and ricebean allows developing more-durable resistant genotypes via gene pyramiding.
Validation of Markers Linked With YMD Resistance
Of four markers (viz. VMYR1, YR4, CYR1, and SCARISSR811) reported linked with the YMD resistance when tested in a set of 14 blackgram genotypes revealed validation of three markers (YR4, CYR1, and SCARISSR811); while the marker VMYR1 produced monomorphic expression (Sowmini and Jayamani, 2014). Further, Binyamin et al. (2015) showed validation of two SCAR markers in 15 mungbean genotypes, which were reported linked with the MYMV resistance gene in both mungbean (Dhole and Reddy, 2013) and blackgram (Souframanien and Gopalakrishna, 2006). There are quite a good number of DNA markers known linked with the YMD resistance in mungbean and blackgram (Table 5), which still needs validation in a diverse set of genotypes. Such marker validation studies will not only help in speeding up the introgression of YMD resistance in different backgrounds, but also quick development of YMD-resistant genotypes without the need for artificial screening.
Pathogen-Derived Resistance (PDR) Based Strategy
PDR refers to the ectopic expression of viral genomic sequences as RNA or protein, to impart resistance against the homologous (sequence wise related) or heterologous (unrelated) viruses, which can be deployed for expressing varied functional or dysfunctional YMV genes like coat protein (CP), protease, membrane protein (MP), replicase, etc. in mungbean, or gene silencing technology may also be used (Karthikeyan et al., 2014). In geminiviruses, CP, and Rep gene expression are mostly used for PDR (Kunik et al., 1994), but use of this technology in blackgram or mungbean is not yet successful due to their recalcitrant nature to Agrobacterium-mediated transformation.
Shivaprasad et al. (2006) in tobacco leaf disc assay showed MYMV genes-based PDR using CP, Rep-sense, Rep-antisense, T-Rep, NSP, and MP genes. Similarly, the effect of AC4-sense and AC4 hpRNA genes on MYMV DNA accumulation in tobacco leaf-disc assay has also revealed the potential of the AC4 hpRNA gene in imparting YMD resistance (Sunitha et al., 2013). However, the blackgram did not express any YMD resistance, when an MYMV derived DNA-A bidirectional promoter was used to activate PTGS against YMD (Pooggin et al., 2003). In another study, when mungbean plants were inoculated with infectious MYMIV clones containing the complementary-sense gene (ACI) encoding Rep, showed 64% infection (Haq et al., 2010). However, when co-inoculation was performed with the Anti-Rep construct, both symptom severity and infection percentage become negligible. The deletion in the CP amino-acids at N0 (75 and 150) of MYMIV has found affecting both systemic spread and pathogenicity (Haq et al., 2011), while agro-inoculation of the CP hairpin construct (Cphp) reported preventing the viral pathogenesis in mungbean (Kumari and Malathi, 2012). Kumar et al. (2017b) demonstrated RNAi-derived resistance to MYMIV in cowpea, where agro-infection of transgenic lines expressing AC2-hp and AC2+AC4-hp RNA showed nearly absolute resistance. These lines also reported accumulating transgene-specific siRNAs and very low level of viral DNA titers. In the era of rapid biotechnological advancements, very soon PDR will become a reality for YMD management in Vigna.
Management of Single and Multiple Viral Infections
Since single and multiple viral infections are quite common under open field conditions. Mixed infections with MYMIV, GBNV, and ULCD were reported in blackgram which varied in different cultivars and seasons of the different year (Biswas et al., 2009). Thus, understanding the pattern of mixed viral infection in Vigna crops in different seasons will help in the identification of various factors leading to the multiple viral infections and ultimately help in the planning of better management strategies (Biswas et al., 2009).
Scope of CRISPR-Cas9 Technology for the Imposition of YMD Resistance in Vigna
CRISPR (clustered regularly interspaced short palindromic repeat)–CRISPR associated 9 (CAS9) or CRISPR/Cas9 technology has been deployed to engineer the plants and confer resistance against begomovirus infection by using sgRNAs designed to target viral genomic DNAs (Khatodia et al., 2016; Zaidi et al., 2016). The CRISPR–Cas9 system using viral intergenic region (IR), CP, and Rep genes have been successfully used to impart resistance to BSCTV (Beet Severe Curly Top Virus) in transgenic Nicotiana benthamiana and Arabidopsis thaliana (Ji et al., 2015). However, Ali et al. (2015) showed the imposition of resistance to multiple geminiviruses viz. Tomato yellow leaf curl virus (TYLCV), Beet curly top virus (BCTV), and Merremia mosaic virus (MeMV) through CRISPR/Cas9 system in N. benthamiana by deploying a sgRNA aimed to recognize a conserved sequence (TAATATTAC) of IR (a characteristic of betasatellites).
Recently, CRISPR/Cas9-mediated genome editing tool was successfully used in cowpea (V. unguiculata) for the disruption of symbiotic nitrogen fixation gene by targeting symbiosis receptor-like kinase gene, which showed ~67% mutagenic efficiency as the complete blockage of nodule formation (Ji et al., 2019). Thus, the success of CRISPR/Cas9 in a Vigna system is expected to quickly promote functional genomics analyses for various other traits including YMD resistance in other Vigna species too. Like Cas9, Cpf1 is another type of CRISPR nuclease which is more efficient and result in lower off-target effect (Ji et al., 2019), and appears better alternative for the editing of various Vigna genomes, including mungbean for YMD resistance. Thus, CRISPR based genome editing approaches should be aimed to impose multiple virus resistance. Additionally, the CRISPR/Cas9 system may also be used for the identification of host factors controlling plant resistance through targeted mutagenesis (Zaidi et al., 2016).
Conclusion and Future Thrust
Both mungbean crop diversity and MYMV affected area have gradually increased since the mid-nineties, which can be attributed to intensive mungbean farming. Any single YMD management strategy may not be a viable option as the resistance is governed by a range of factors like plant genotype, stains of YMVs, whitefly biotypes, ambient weather conditions, and presence of alternate hosts. Other YMD management challenges include (i) lack of precise infection mechanism of various YMV strains at the molecular level; (ii) development of multiple viral strain-specific resistant lines; (iii) reduction of vector population below threshold under field conditions. An inclusive outline of YMD development and management strategies are outlined in Figure 7.
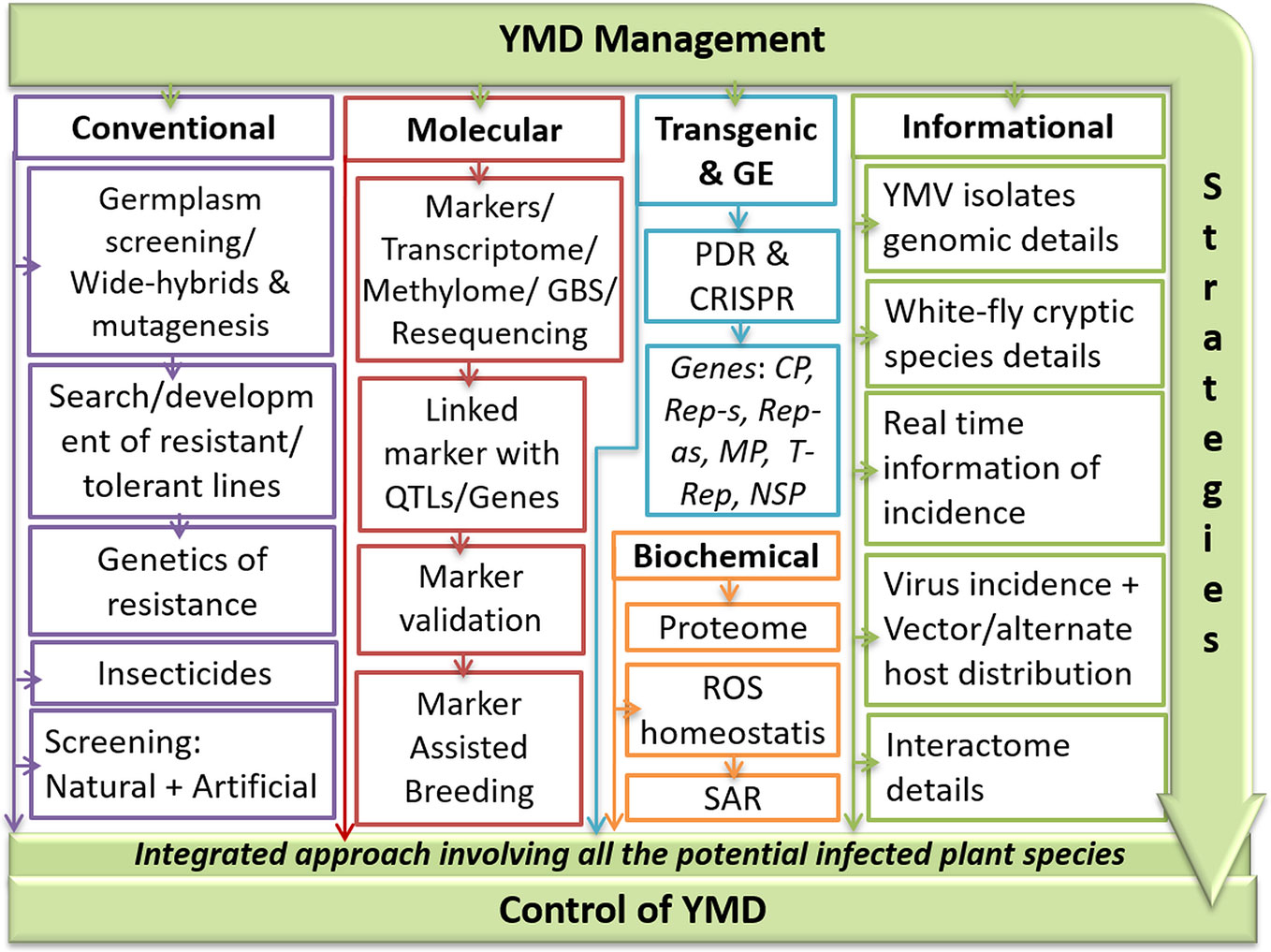
Figure 7 An outline of integrated YMD management strategies in mungbean. Where, CP, coat protein; CRISPR, clustered regularly interspaced short palindromic repeat; GBS, genotype by sequencing; GE, genome editing; MP, movement protein; NSP, nuclear shuttle protein; PDR, pathogen-derived resistance; QTL, quantitative trait loci; Rep, replication protein; ROS, reactive oxygen species; SAR, systemic acquired resistance; YMD, yellow mosaic disease; YMV, yellow mosaic virus.
The main reasons for not obtaining any durable resistance even after four-decades of YMD resistance breeding in mungbean could be due to the field-based germplasm screening without considering the natural existence of various begomoviruses along with the presence of whitefly cryptic species (Nair et al., 2017). Thus, any efficient YMD management strategy in Vigna should take into account the strains of YMVs, whitefly biotypes and their distribution in the target area (Nair et al., 2017) along with artificially screening through forced feeding and agroinoculation (Basak et al., 2005; Mohan et al., 2014).
The presence of various non-leguminous begomoviruses in legumes, suggests recombination in the virus, resulting in the appearance of more severe races, causing widespread crop loss (Ilyas et al., 2010). This again reiterates the pressing need for generating an exhaustive genomic database about the viral isolates affecting various crops across the world. The database should possess detailed phylogenetic information about MYMV and other isolates infecting different grain legumes. This will eventually facilitate in identifying the best strategy for the deployment of resistance sources having a mismatch of resistance gene(s) (Karthikeyan et al., 2014; Prema and Rangaswamy, 2018).
Comprehensive real-time information at the global level should be constantly generated in a network mode for the intensity of virus incidence and spatial distribution of vectors and alternate hosts for monitoring and giving early warnings about the possible occurrence of YMD (Meti et al., 2017). Such a system will also assist in making an appropriate judgment about the preventive and control measures, spray schedules, and other required practices for minimizing the YMD incidence.
The small genome size of the mungbean looks beneficial to the breeders for attempting genomic assisted breeding on a fast track for the development of YMD resistant varieties. The rapid advent of relatively low-cost RNA-seq technologies is also expected to assist in the mapping of the gene(s) or QTLs and MAS for YMD resistance. Although, various markers are reported closely linked with the YMD resistance gene, yet these are specific to some population and not yet validated across different sets of mungbean genotypes. Thus, a large number of linked SNP markers with the YMD resistance gene(s) should be identified with the aim of map-based cloning of the gene(s) (Maiti et al., 2011). Resequencing of different YMD resistant wild Vigna species from different geographical regions is expected to capture the allelic variations for the YMD resistance, whereas the use of advanced backcross-QTL (AB-QTL) may assist in the identification and transfer of valuable QTLs governing YMD resistance (Tanksley and Nelson, 1996). Detailed studies involving leaf proteome of different Vigna species may provide a deep insight into the YMD resistance response at the biochemical level. The flavin-containing monooxygenase identified through the association of proteomics data should be taken forward for overexpression analysis (Sai et al., 2017). Thus, information about the YMV infected host cell transcriptome, proteome, interactome, and degradome may give greater insight about the changes in the host cells and ultimately leading to the establishment of viral infection (Ramesh et al., 2017a). These -omics studies will also help in precise identification of various functional components which shows significant differential changes during both compatible and incompatible interactions.
The detailed information about the origin of dsRNA or the activation of plants RNA silencing machinery, when exposed to the YMV infection for imparting antiviral immunity in mungbean is still lacking. The Ty-1 and Ty-3 are the only host resistance genes identified for geminivirus infection in tomato, showing homology to host RNA-dependent RNA polymerases (Verlaan et al., 2013). This gave the clue about the role of secondary siRNAs as an effector in imparting RNA silencing-based antiviral resistance, but it warrants further evidentiary confirmation.
Due to its multiple host range, small genome size and larger carrying capacity, a geminivirus offers great prospects for its use in various novel applications including VIGS (virus-induced gene silencing) and genome modification involving ZFN (zinc-finger nucleases) (Kim et al., 1996), TALENs (transcription activator-like effector nucleases) (Miller et al., 2011), and CRISPR/Cas system (Zaidi et al., 2016). Thus, for sustainable YMD management, various novel and advanced biotechnological approaches especially gene editing, whole genome methylome studies, QTL-Seq, RNA-Seq and genome-wide association studies (GWAS) should be deployed for the inclusive understanding and management of YMD in mungbean.
Author Contributions
GM, HD, RS, RN, SP: Conceptualization, writing and editing of manuscript. AK, AS, AR, KT, MA, NK, Priti, RK, UD: Collection and compilation of information.
Funding
Financial support received from Indian Council of Agricultural Research (ICAR), New Delhi, and SERB, New Delhi (CRG/2019/002024) is gratefully acknowledged.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to Mr. Dilip Kumar for his technical support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2020.00918/full#supplementary-material
References
Ahmad, S., Sajjad, M., Nawaz, R., Hussain, M. A., Aslam, M. N. (2017). Identification of resistance sources to mungbean yellow mosaic virus among mungbean germplasm. Asian J. Agri. Biol. 5 (1), 26–31.
Ahmad, M. (1975). Screening of mungbean (Vigna radiata) and urdbean (V. mungo) for germplasms resistance to yellow mosaic virus. J. Agric. Res. 13, 349–354.
Ahmed, Z. U., Shaikh, M. A. Q., Begum, S. (1995). “Development of the high yielding and disease resistant mungbean variety, BINA Moong-2 using nuclear and cross breeding techniques,” in Induced Mutations and Molecular Techniques for Crop Improvement. Proceedings of an International Symposium on the Use of Induced Mutations and Molecular Techniques for Crop Improvement Jointly Organized by the International Atomic Energy Agency and the Food and Agriculture Organization of the United Nations (June 19-23, 1995). (Cambridge University Press), 638–640. doi: 10.1017/S002185969621398X
Akhtar, K. P., Sarwar, G., Abbas, G., Asghar, M. J., Sarwar, N., Shah, T. M. (2011). Screening of mungbean germplasm against Mungbean Yellow Mosaic India Virus and its vector Bemisia tabaci. Crop Prot. 30, 1202–1209. doi: 10.1016/j.cropro.2011.05.012
Alam, A. K. M. M., Somta, P., Srinives, P. (2014a). Generation mean and path analyses of reaction to mungbean yellow mosaic virus (MYMV) and yield-related traits in mungbean (Vigna radiata (L.) Wilczek). SABRAO J. Breed Genet. 46 (1), 150–159.
Alam, A. K. M. M., Somta, P., Srinives, P. (2014b). Identification and confirmation of quantitative trait loci controlling resistance to mungbean yellow mosaic disease in mungbean [Vigna radiata (L.) Wilczekl]. Mol. Breed. 34, 1497–1506. doi: 10.1007/s11032-014-0133-0
Alam, A. K. M. M., Somta, P., Jompuk, C., Chatwachirawong, P., Srinives, P. (2014c). Evaluation of mungbean genotypes based on yield stability and reaction to mungbean yellow mosaic virus disease. Plant Pathol. J. 30 (3), 261–268. doi: 10.5423/PPJ.OA.03.2014.0023
Ali, M., Malik, I. A., Sabir, H. M., Ahmad, B. (1997). The mungbean green revolution in Pakistan. Technical Bulletin No. 24. AVRDC. (Shanhua, Taiwan: © Asian Vegetable Research and Development Center), 66.
Ali, Z., Abulfaraj, A., Idris, A., Ali, S., Tashkandi, M., Mahfouz, M. M. (2015). CRISPR/Cas9-mediated viral interference in plants. Genome Biol. 16, 238. doi: 10.1186/s13059-015-0799-6
Ammavasai, S., Phogat, D. S., Solanki, I. S. (2004). Inheritance of resistance to Mungbean Yellow Mosaic Virus (MYMV) in greengram (Vigna radiata L. Wilczek). Indian J. Genet. 64 (2), 146.
Anokhe, A., Mandal, B., Subramanian, S. (2018). Characterization of mung bean yellow mosaic virus transmission by Asia-I and Asia II-1 genetic group of Bemisia tabaci Gennadius. J. Entomol. Zool Stud. 6 (1), 487–491.
Argüello-Astorga, G. R., Guevara-González, L. R., Herrera-Estrella, L. R., Rivera-Bustamante, R. F. (1994). Geminivirus replication origins have a group-specific organization of iterative elements: a model for replication. Virology 203, 90–100. doi: 10.1006/viro.1994.1458
Auti, S. G. (2012). Induced morphological and quantitative mutations in mungbean. Biorem. Biodivers. Bioavailability 6 (1), 27–39.
Babu, R. R., Nandan, M., Sudipta, P., Chandra, S. N. (2019). Screening of some blackgram (Vigna mungo (L.) Hepper) genotypes for resistance to yellow mosaic virus during summer season. Electron J. Plant Breed. 10 (3), 1329–1332. doi: 10.5958/0975-928X.2019.00170.4
Balaji, V., Vanitharani, R., Karthikeyan, A. S., Anbalagan, S., Veluthambi, K. (2004). Infectivity analysis of two variable DNA B components of Mungbean yellow mosaic virus-Vigna in Vigna mungo and Vigna radiata. J. Biosci. 29, 297–308. doi: 10.1007/BF02702612
Banerjee, A., Umbrey, Y., Yadav, R. M., Roy, S. (2018). Molecular evidence of an isolate of Mungbean Yellow Mosaic India Virus with a recombinant DNA B component occurring on mungbean from mid-hills of Meghalaya, India. Virus Dis. 29 (1), 68–74. doi: 10.1007/s13337-018-0429-5
Basak, J., Kundagramy, S., Ghose, T. K., Pal, A. (2005). Development of yellow mosaic virus (YMV) resistance linked DNA-marker in Vigna mungo from population segregating for YMV reaction. Mol. Breed. 14, 375–383. doi: 10.1007/s11032-005-0238-6
Bashir, M., Ahmad, Z., Mansoor, S. (2006). Occurrence and distribution of viral diseases of mungbean and mashbean in Punjab, Pakistan. Pak J. Bot. 38, 1341–1351.
Bhanu, A. N., Kumar, P., Singh, M. N., Srivastava, K., Hemantaranjan, A. (2017a). Assessment of genetic purity of inter-specific F1 hybrids involving Vigna radiata and Vigna umbellata. J. Exp. Biol. Agric. Sci. 5 (5), 636–643. doi: 10.18006/2017.5(5).636.643
Bhanu, A. N., Singh, M. N., Srivastava, K. (2017b). Screening mungbean [Vigna radiata (L.) Wilczek] genotypes for mungbean yellow mosaic virus resistance under natural condition. Adv. Plants Agric. Res. 7 (6), 00276. doi: 10.15406/apar.2017.07.00276
Bhanu, A. N., Singh, M. N., Srivastava, K. (2019). Genetic analysis of gene-specific resistance to mungbean yellow mosaic virus in mungbean. (Vigna radiata). Plant Breed. 138 (2), 202–206. doi: 10.1111/pbr.12675
Bhaskar, A. V. (2017). Genotypes against major diseases in green gram and black gram under natural field conditions. Int. J. Curr. Microbiol. App Sci. 6 (6), 832–843. doi: 10.20546/ijcmas.2017.606.098
Binyamin, R., Khan, M. A., Khan, N. A., Khan, A. I. (2015). Application of SCAR markers linked with mungbean yellow mosaic virus disease-resistance gene in Pakistan mungbean germplasm. Genet. Mol. Res. 14 (1), 2825–2830. doi: 10.4238/2015.March.31.13
Biswas, K. K., Malath, V. G., Varma, A. (2008). Diagnosis of symptomless Yellow mosaic begomovirus infection in pigeonpea by using cloned mungbean yellow mosaic India virus as probe. J. Plant Biochem. Biotechnol. 17 (1), 9–14. doi: 10.1007/BF03263253
Biswas, K. K., Tarafdar, A., Kumar, A., Dikshit, H. K., Malathi, V. G. (2009). Multiple infection in urdbean (Vigna mungo) in natural condition by begomovirus, tospovirus and urdbean leaf crinkle virus complex. Indian Phytopathol. 62 (1), 75–82.
Brar, J. S., Bains, T. S., Shanmugasundaram, S., Singh, S. (2006). “Developing short duration mungbean genotypes suitable for rice-wheat cropping system,” in Proceedings of Final workshop and planning meeting on mungbean. AVRDC–DFID Mungbean Project 2002-2004. Ed. Shanmugasundaram, S., (Ludhiana, India: Punjab Agricultural University) 61–81. https://assets.publishing.service.gov.uk/media/57a08c4ae5274a27b20010df/mungbean_03india.pdf
Briddon, R. W., Markham, P. G. (2000). Cotton leaf curl virus disease. Virus Res. 71 (1/2), 151–159. doi: 10.1016/S0168-1702(00)00195-7
Briddon, R. W., Patil, B. L., Basavaraj, B., Nawaz-ul-Rehman, M. S., Fauquet, C. M. (2010). Distinct evolutionary histories of the DNA-A and DNA-B components of bipartite begomoviruses. BMC Evol. Biol. 10, 97. doi: 10.1186/1471-2148-10-97
Brown, J. K., Czosnek, H. (2002). Whitefly transmission of plant viruses. Adv. Bot. Res. 36, 65–76. doi: 10.1016/S0065-2296(02)36059-2
Castillo, N. J., Fiallo-Olive, E., Sanchez-Campos, S. (2011). Emerging virus diseases transmitted by whiteflies. Annu. Rev. Phytopathol. 49, 219–248. doi: 10.1146/annurev-phyto-072910-095235
Cayalvizhi, B., Nagarajan, P., Raveendran, M., Rabindran, R., Selvam, N. J., Kannan Bapu, J. R., et al. (2015). Unraveling the responses of mungbean (Vigna radiata) to mungbean yellow mosaic virus through 2D-protein expression. Physiol. Mol. Plant Pathol. 90, 65–77. doi: 10.1016/j.pmpp.2015.03.001
Chatterji, A., Chatterji, U., Beachy, R. N., Fauquet, C. M. (2000). Sequence parameters that determine specificity of binding of the replication associated protein to its cognate site in two strains of Tomato leaf curl virus-New Delhi. Virology 273, 341–350. doi: 10.1006/viro.2000.0434
Cheema, H. K., Pratap, A., Sujayanand, G. K., Arora, R., Sandhu, S. (2017). “Breeding for insect resistance in mungbean and urdbean breeding insect resistant crops for sustainable agriculture,” in Breeding insect resistant crops for sustainable agriculture. Ed. Arora, R, Sandhu, S. (Singapore: Springer Singapore), 353–385. doi: 10.1007/978-981-10-6056-4
Chen, H. M., Ku, H. M., Schafleitner, R., Bains, T. S., Kuo, C. G., Liu, C. A., et al. (2013). The major quantitative trait locus for mungbean yellow mosaic Indian virus resistance is tightly linked in repulsion phase to the major bruchid resistance locus in a cross between mungbean [Vigna radiata (L.) Wilczek] and its wild relative Vigna radiata ssp. sublobata. Euphytica 192, 205–216. doi: 10.1007/s10681-012-0831-9
Chen, W., Hasegawa, D. K., Kaur, N., Kliot, A., Pinheiro, P. V., Luan, J., et al. (2016). The draft genome of whitefly Bemisia tabaci MEAM1, a global crop pest, provides novel insights into virus transmission, host adaptation, and insecticide resistance. BMC Biol. 14, 110. doi: 10.1186/s12915-016-0321-y
Chitra, S., Kalaimagal, T., Thirumurugan, T. (2018). Development of MYMV resistant pre-breeding lines in mungbean (Vigna radiata) through interspecific hybridization. Int. J. Curr. Microbiol. App Sci. 7 (1), 2674–2677. doi: 10.20546/ijcmas.2018.701.320
Czosnek, H., Ghanim, M., Ghanim, M. (2002). Circulative pathway of begomoviruses in the whitefly vector Bemisia tabaci– insights from studies with tomato yellow leaf curl virus. Ann. Appl. Biol. 140, 215–231. doi: 10.1111/j.1744-7348.2002.tb00175.x
Czosnek, H. (2008). “Acquisition, circulation and transmission of begomoviruses by their whitefly vectors,” in Viruses in the environment 37/661(2) (Trivandrum: Research Signpost). ISBN: 978-81-308-0235-0.
D’Souza, S. F., Reddy, K. S., Badigannavar, A. M., Manjaya, J. G., Jambhulkar, S. J. (2009). “Mutation breeding in oilseeds and grain legumes in India: accomplishments and socio-economic impact,” in Induced plant mutations in the genomic era food and agricultural organization of the United Nations. Ed. Shu, Q. Y., 55. (Food and Agricultural Organization of the United Nations, Rome: © FAO & IAEA)
Datta, S., Gangwar, S., Kumar, S., Gupta, S., Rai, R., Kaashyap, M., et al. (2012). Genetic diversity in selected Indian mungbean [Vigna radiata (L.) Wilczek] cultivars using RAPD markers. Am. J. Plant Sci. 3, 1085–1091. doi: 10.4236/ajps.2012.38130
De Barro, P. J., Liu, S. S., Boykin, L. M., Dinsdale, A. B. (2011). Bemisia tabaci: a statement of species status. Ann. Rev. Entomol. 56, 1–19. doi: 10.1146/annurev-ento-112408-085504
Dharajiya, D. T., Ravindrababu, Y. (2019). Identifcation of molecular marker associated with mungbean yellow mosaic virus resistance in mungbean [Vigna radiata (L.) Wilczek]. Vegetos 32, 532–539. doi: 10.1007/s42535-019-00063-y
Dhole, V. J., Reddy, K. S. (2012). Genetic analysis of resistance to mungbean yellow mosaic virus in mungbean (Vigna radiata). Plant Breed. 131, 414–417. doi: 10.1111/j.1439-0523.2012.01964.x
Dhole, V. J., Reddy, K. S. (2013). Development of a SCAR marker linked with a MYMV resistance gene in mungbean (Vigna radiata L. Wilcek). Plant Breed. 132, 127–132. doi: 10.1111/pbr.12006
Diaz-Pendon, J. A., Li, F., Li, W. X., Ding, S. W. (2007). Suppression of antiviral silencing by Cucumber mosaic virus 2b protein in Arabidopsis is associated with drastically reduced accumulation of three classes of viral small interfering RNAs. Plant Cell 19, 2053–2063. doi: 10.1105/tpc.106.047449
Dikshit, H. K., Mishra, G. P., Somta, P., Shwe, T., Alam, A. K. M. M., Bains, T. S., et al. (2020). “Classical genetics and traditional breeding in mungbean,” in The Mungbean Genome, Compendium of Plant Genomes. Ed. Nair, R. M. (Switzerland AG: © Springer Nature), 43–54. doi: 10.1007/978-3-030-20008-4_4
Dinsdale, A., Cook, I., Riginos, C., Buckley, Y. M., De Barro, P. (2010). Refined global analysis of Bemisia tabaci (Hemiptera: Sternorrhyncha: Aleyrodoidea: Aleyrodidae) mitochondrial cytochrome oxidase 1 to identify species level genetic boundaries. Ann. Entomol. Soc. Am. 103, 196–208. doi: 10.1603/AN09061
Dubey, S. C., Singh, B. (2013). Integrated management of major diseases of mungbean by seed treatment and foliar application of insecticide, fungicides and bioagent. Crop Prot. 47, 55–60. doi: 10.1016/j.cropro.2012.12.025
Durga Prasad, A. V. S., Murugan, E., Vanniarajan, C. (2015). Inheritance of resistance of mungbean yellow mosaic virus in Urdbean (Vigna mungo (L.) Hepper). Curr. Biotica. 8 (4), 413–417.
Dwivedi, S., Singh, D. P. (1985). Inheritance of resistance to yellow mosaic virus in a wide cross of blackgram (Vigna mungo L). Z Pflanzenzucht. 95, 281–284.
Fauquet, C. M., Briddon, R. W., Brown, J. K., Moriones, E., Stanley, J., Zerbini, M., et al. (2008). Geminivirus strain demarcation and nomenclature. Arch. Virol. 153, 783–821. doi: 10.1007/s00705-008-0037-6
Fiallo-Olivé, E., Pan, L. L., Liu, S. S., Navas-Castillo, J. (2020). Transmission of begomoviruses and other whitefly-borne viruses: dependence on the vector species. Phytopathol. 10 (1), 10–17. doi: 10.1094/PHYTO-07-19-0273-FI
Fishpool, L. D. C., Burban, C. (1994). Bemisia tabaci: the whitefly vector of African cassava mosaic geminivirus. Trop. Sci. 34 (1), 55–72.
Gautam, N. K., Akram, M., Akhtar, J., Khan, J., Dwivedi, N. K., Latha, M., et al. (2014). Responses of wild Vigna species/ sub-species to yellow mosaic disease viruses, detected by a PCR-based method. Phytopathol. Mediterr. 53 (3), 428–437. doi: 10.14601/Phytopathol_Mediterr-13600
Ghanim, M., Morin, S., Czosnek, H. (2001). Rate of tomato yellow leaf curl virus (TYLCV) translocation in the circulative transmission pathway of its vector, the whitefly Bemisia tabaci. Phytopathol. 91, 188–196. doi: 10.1094/PHYTO.2001.91.2.188
Girish, K. R., Usha, R. (2005). Molecular characterization of two soybean-infecting begomoviruses from India and evidence for recombination among legume-infecting begomoviruses from South-East Asia. Virus Res. 108, 167–176. doi: 10.1016/j.virusres.2004.09.006
Gopi, P., Satyanarayana, A., Krishna, R. A., Sambasiva Rao, K. R. S. (2016). Evaluation of blackgram germplasm for resistance against YMV. J. Plant Pathol. Microbiol. 7, 7. doi: 10.4172/2157-7471.1000368
Gosal, S. S., Bajaj, Y. P. S. (1983). Interspecific hybridization between Vigna mungo and Vigna radiata through embryo culture. Euphytica 32, 129–137. doi: 10.1007/BF00036873
Govindan, K., Nagarajan, P., Angappan, K. (2014). Molecular studies on transmission of mung bean yellow mosaic virus (MYMV) by Bemisia tabaci Genn. in mungbean. Afr J. Agril. Res. 9 (38), 2874–2879. doi: 10.5897/AJAR2013.7237
Gupta, S., Kumar, S., Singh, R. A., Chandra, S. (2005). Identification of a single dominant gene for resistance to mungbean yellow mosaic virus in blackgram. SABRAO J. Breed Genet. 37, 85–89.
Gupta, S., Gupta, D. S., Anjum, T. K., Pratap, A., Kumar, J. (2013). Inheritance and molecular tagging of MYMIV resistance gene in blackgram (Vigna mungo L. Hepper). Euphytica ,193, 27–37. doi: 10.1007/s10681-013-0884-4
Gupta, P. K. (1996). “Mutation breeding in mungbean,” in Recent advances in mungbean research. Eds. Asthana, A. N., Kim, D. H. (India: Indian Society of Pulses Research, IIPR, Kanpur-208024), 124–136.
Habib, S., Shad, N., Javaid, A., Iqbal, U. (2007). Screening of mungbean germplasm for resistance/tolerance against yellow mosaic disease. Mycopath 5 (2), 89–94.
Hanley-Bowdoin, L., Settlage, S. B., Orozco, B. M., Nagar, S., Robertson, D. (1999). Geminiviruses: models for plant DNA replication, transcription, and cell cycle regulation. Crit. Rev. Plant Sci. 18, 71–106. doi: 10.1016/S0735-2689(99)00383-4
Haq, Q. M. I., Arif, A., Malathi, V. G. (2010). Engineering resistance against mungbean yellow mosaic India virus using antisense RNA. Indian J. Virol. 21, 82–85. doi: 10.1007/s13337-010-0003-2
Haq, Q. M. I., Jyothsna, P., Arif, A., Malathi, V. G. (2011). Coat protein deletion mutation of mungbean yellow mosaic India virus (MYMIV). J. Plant Biochem. Biotechnol. 20 (2), 182–189. doi: 10.1007/s13562-011-0044-7
Haq, M. A. (2009). “Development of mutant varieties of crop plants at NIAB and the impact on agricultural production in Pakistan,” in Induced plant mutations in the genomic era. Food and Agricultural Organization of the United Nations, vol. p . Ed. Shu, Q. Y., 61.(Food and Agricultural Organization of the United Nations, Rome: © FAO & IAEA)
Holeyachi, P., Savithramma, D. L. (2013). Identification of RAPD markers linked to MYMV resistance in mungbean (Vigna radiata (L). Wilczek). Bioscan. 8 (4), 1409–1411.
Honda, Y., Iwaki, M., Saito, Y. (1983). Mechanical transmission, purification and some properties of whitefly-borne mungbean yellow mosaic virus in Thailand. Plant Dis. 67, 801–804. doi: 10.1094/PD-67-801
Horowitz, A. R., Ishaaya, I. (2014). Dynamics of biotypes B and Q of the whitefly Bemisia tabaci and its impact on insecticide resistance. Pest Manag. Sci. 70, 1568–1572. doi: 10.1002/ps.3752
Hu, J., Zhang, X., Jiang, Z., Zhang, F., Liu, Y., Li, Z., et al. (2018). New putative cryptic species detection and genetic network analysis of Bemisia tabaci (Hempitera: Aleyrodidae) in China based on mitochondrial COI sequences. Mitochondrial DNA Part A. 29, 474–484. doi: 10.1080/24701394.2017.1307974
Hussain, M., Qazi, J., Mansoor, S., Iram, S., Bashir, M., Zafar, Y. (2004). First report of Mungbean Yellow Mosaic India Virus on mungbean in Pakistan. Plant Pathol. 53, 518. doi: 10.1111/j.1365-3059.2004.01037.x
Hussain, S., Farooq, M., Malik, H. J., Amin, I., Scheffler, B. E., Scheffler, J. A., et al. (2019). Whole genome sequencing of Asia II 1 species of whitefly reveals that genes involved in virus transmission and insecticide resistance have genetic variances between Asia II 1 and MEAM1 species. BMC Genomics 20, 507. doi: 10.1186/s12864-019-5877-9
Ilyas, M., Qazi, J., Mansoor, S., Briddon, R. W. (2010). Genetic diversity and phylogeography of begomoviruses infecting legumes in Pakistan. J. Gen. Virol. 91, 2091–2101. doi: 10.1099/vir.0.020404-0
Iqbal, U., Iqbal, M. S., Afzal, R., Jamal, A., Farooq, M. A., Zahid, A. (2011). Screening of mungbean germplasm against mungbean yellow mosaic virus (MYMV) under field conditions. Pak J. Phytopathol. 23 (1), 48–51.
Islam, M. S., Latif, M. A., Ali, M., Hossain, M. S. (2008). Population dynamics of white fly on some recommended mungbean varieties in Bangladesh and its impact on incidence of mungbean yellow mosaic virus disease and yield. Int. J. Agri. Sci. Tech. 2, 41–47.
Islam, M. N., Sony, S. K., Borna, R. S. (2012). Molecular characterization of mungbean yellow mosaic disease and coat protein gene in mungbean varieties of Bangladesh. Plant Tiss Cult Biotech. 22 (1), 73–81. doi: 10.3329/ptcb.v22i1.11263
Jacob, S. S., Vanitharani, R., Karthikeyan, A. S., Chinchore, Y., Thillaichidambaram, P., Veluthambi, K. (2003). Mungbean yellow mosaic virus-Vi agroinfection by codelivery of DNA A and DNA B from one Agrobacterium strain. Plant Dis. 87, 247–251. doi: 10.1094/PDIS.2003.87.3.247
Jalaluddin, M., Shaikh, M. A. Q. (1981). Evaluation of mungbean (Vigana radiata L.) germplasm for resistance to mungbean yellow mosaic virus. SABRAO J. 13, 61–68.
Ji, X., Zhang, H., Zhang, Y., Wang, Y., Gao, C. (2015). Establishing a CRISPR–Cas-like immune system conferring DNA virus resistance in plants. Nat. Plants 1, 15144. doi: 10.1038/nplants.2015.144
Ji, J., Zhang, C., Sun, Z., Wang, L., Duanmu, D., Fan, Q. (2019). Genome editing in cowpea Vigna unguiculata using CRISPR-Cas9. Int. J. Mol. Sci. 20, 2471. doi: 10.3390/ijms20102471
John, P., Sivalingam, P. N., Haq, Q. M. I., Kumar, N., Mishra, A., Briddon, R. W., et al. (2008). Cowpea golden mosaic disease in Gujarat is caused by a mungbean yellow mosaic India virus isolate with a DNA B variant. Arch. Virol. 153 (7), 1359–1365. doi: 10.1007/s00705-008-0116-8
Kanakala, S., Ghanim, M. (2019). Global genetic diversity and geographical distribution of Bemisia tabaci and its bacterial endosymbionts. PloS One 14 (3), e0213946. doi: 10.1371/journal.pone.0213946
Kang, Y. J., Kim, S. K., Kim, M. Y., Lestari, P., Kim, K. H., Ha, B. K., et al. (2014). Genome sequence of mungbean and insights into evolution within Vigna species. Nat. Commun. 5, 5443. doi: 10.1038/ncomms6443
Karthikeyan, A. S., Vanitharani, R., Balaji, V., Anuradha, S., Thillaichidambaram, P., Shivaprasad, P. V., et al. (2004). Analysis of an isolate of Mungbean Yellow Mosaic Virus (MYMV) with a highly variable DNA-B component. Arch. Virol. 149, 643–1652. doi: 10.1007/s00705-004-0313-z
Karthikeyan, A., Sudha, M., Pandiyan, M., Senthil, N., Shobana, V. G., Nagarajan, P. (2011). Screening of MYMV resistant mungbean (Vigna radiata L. Wilczek) progenies through agroinoculation. Int. J. Plant Pathol. 2, 115–125. doi: 10.3923/ijpp.2011.115.125
Karthikeyan, A., Sudha, M., Senthil, N., Pandiyan, M., Raveendran, M., Nagarajan, P. (2012). Screening and identification of RAPD markers linked to MYMV resistance in mungbean (Vigna radiata (L) Wilczek). Arch. Phytopathol. Plant Prot. 45 (6), 712–716. doi: 10.1080/03235408.2011.592016
Karthikeyan, A., Shobhana, V. G., Sudha, M., Raveendran, M., Senthil, N., Pandiyan, M., et al. (2014). Mungbean yellow mosaic virus (MYMV): a threat to green gram (Vigna radiata) production in Asia. Int. J. Pest Manage. 60 (4), 314–324. doi: 10.1080/09670874.2014.982230
Kaushal, R. P., Singh, B. M. (1988). Inheritance of disease resistance in blackgram (Vigna mungo (L.) Hepper) to mungbean yellow mosaic virus. Indian J. Agric. Sci. 58, 123–124.
Khan, M. G., Ahmad, W., Khattak, G. S. S., Siraj-ud-Din Ahmad, H. (2007). Mode of inheritance of resistance to mungbean yellow mosaic virus (MYMV) in mungbean (Vigna radiata (l.) Wilczek). Sarhad J. Agric. 23 (4), 1071–1074.
Kharakwal, M. C. (1996). “Accomplishment of mutation breeding in crop improvement in India,” in Isotopes and Radiations in Agriculture and Environment Research. Eds. Sachdev, M. S., Sachdev, P., Deb, D. L. (New Delhi, India: Indian Society of Nuclear Techniques in Agriculture and Biology), 196–218.
Khatodia, S., Bhatotia, K., Passricha, N., Khurana, S. M. P., Tuteja, N. (2016). The CRISPR/Cas Genome-Editing Tool: Application in Improvement of Crops. Front. Plant Sci. 7, 506. doi: 10.3389/fpls.2016.00506
Khattak, G. S. S., Haq, M. A., Ashraf, M., Elahi, T. (2000). Genetics of Mungbean Yellow Mosaic Virus (MYMV) in mungbean (Vigna radiata L.) Wilczek. J. Genet. Breed. 54 (4), 237–243.
Khattak, M. K., Shafqat, A., Chisti, J. I. (2004). Varietal resistance of mungbean (Vigna radiata L.) against whitefly (Bemisia tabaci Genn.), jassid (Amrasca devastans Dist.), and thrips (Thrips tabaci Lind.). Pak Entomol. 26, 9–12.
Khattak, G. S. S., Iqbal, S., Shah, S. A. (2008). Breeding high yielding and disease resistant mungbean (Vigna radiata (L.) Wilczek) genotypes. Pak J. Bot. 40, 1411–1417.
Kim, Y. G., Cha, J., Chandrasegaran, S. (1996). Hybrid restriction enzymes: Zinc finger fusions to Fok I cleavage domain. P Natl. Acad. Sci. USA. 93, 1156–1160. doi: 10.1073/pnas.93.3.1156
Kim, S. K., Nair, R. M., Lee, J., Lee, S.-H. (2015). Genomic resources in mungbean for future breeding programs. Front. Plant Sci. 6, 626. doi: 10.3389/fpls.2015.00626
Kitsanachandee, R., Somta, P., Chatchawankanphanich, O., Akhtar, K. P., Shah, M., Nair, R. M., et al. (2013). Detection of quantitative trait loci for Mungbean Yellow Mosaic India Virus (MYMIV) resistance in mungbean (Vigna radiata (L.) Wilczek) in India and Pakistan. Breed Sci. 63, 367–373. doi: 10.1270/jsbbs.63.367
Kothandaraman, S. V., Devadason, A., Ganesan, M. V. (2016). Seed-borne nature of a begomovirus, Mung bean yellow mosaic virus in black gram. Appl. Microbiol. Biotechnol. 100, 1925–1933. doi: 10.1007/s00253-015-7188-7
Kumar, S., Kumar, R. (2014). Genetic improvement of mungbean for yield, nutrition and resistance to stresses-A review. Int. J. Trop. Agril. 32 (3-4), 683–687.
Kumar, S., Tanti, B., Mukherjee, S. K., Sahoo, L. (2017a). Molecular characterization and infectivity of Mungbean Yellow Mosaic India virus associated with yellow mosaic disease of cowpea and mungbean. Biocat. Agril. Biotechnol. 11 (1), 183–191. doi: 10.1016/j.bcab.2017.07.004
Kumar, S., Tanti, B., Patil, B. L., Mukherjee, S. K., Sahoo, L. (2017b). RNAi-derived transgenic resistance to Mungbean yellow mosaic India virus in cowpea. PloS One 12 (10), e0186786. doi: 10.1371/journal.pone.0186786
Kumari, A., Malathi, V. G. (2012).“RNAi-Mediated strategy to develop transgenic resistance in grain legumes targeting the mungbean yellow mosaic India virus coat protein gene,” in Proceedings of the International Conference on Plant Biotechnology for Food Security: New Frontiers. (New Delhi, India), 156
Kumari, S., Sahni, S., Prasad, B. D. (2020). Screening of urdbean germplasm for high seed yield coupled with resistance to Mungbean yellow mosaic virus (MYMV). Curr. J. Appl. Sci. Technol. 39 (1), 100–106. doi: 10.9734/CJAST/2020/v39i130485
Kundu, S., Chakraborty, D., Kundu, A., Pal, A. (2013). Proteomics approach combined with biochemical attributes to elucidate compatible and incompatible plant-virus interactions between Vigna mungo and Mungbean Yellow Mosaic India Virus. Proteome Sci. 11, 15. doi: 10.1186/1477-5956-11-15
Kunik, T., Salomon, R., Zamir, D., Navot, N., Zeidan, M., Michelson, I., et al. (1994). Transgenic tomato plants expressing the tomato yellow leaf curl virus capsid protein are resistant to the virus. Nat. Biotechnol. 12, 500–504. doi: 10.1038/nbt0594-500
Lambrides, C. J., Diatloff, C., Liu, J., Imrie, B. C. (1999).“Molecular marker studies in mungbean Vigna radiata,” in Proceeding of 11th Australasian Plant Breeding Conference, (Adelaide, Australia), 19–23.
Laosatit, K., Somta, P., Chen, X., Srinives, P. (2020). “Genomic Approaches to Biotic Stresses,” in The Mungbean Genome, Compendium of Plant Genomes. Ed. Nair, R. M. (Switzerland AG: © Springer Nature), 133–167. doi: 10.1007/978-3-030-20008-4_10
Lee, W., Park, J., Lee, G.-S., Lee, S., Akimoto, S. (2013). Taxonomic status of the Bemisia tabaci complex (Hemiptera: Aleyrodidae) and reassessment of the number of its constituent species. PloS One 8, e63817. doi: 10.1371/journal.pone.0063817
Lekhi, P., Gill, R. K., Kaur, S., Bains, T. S. (2018). Generation of interspecific hybrids for introgression of mungbean yellow mosaic virus resistance in Vigna radiata (L.) Wilczek. Legume Res. 41, 526–531. doi: 10.18805/LR-3808
Li, F., Xu, X., Huang, C., Gu, Z., Cao, L., Hu, T., et al. (2015). The AC5 protein encoded by Mungbean yellow mosaic India virus is a pathogenicity determinant that suppresses RNA silencing-based antiviral defences. New Phytol. 208, 555–569. doi: 10.1111/nph.13473
Mahajan, N., Parameswari, C., Veluthambi, K. (2011). Severe stunting in blackgram caused by the Mungbean yellow mosaic virus (MYMV) KA27 DNA B component is ameliorated by co-infection or post-infection with the KA22 DNA B: MYMV nuclear shuttle protein is the symptom determinant. Virus Res. 157, 25–34. doi: 10.1016/j.virusres.2011.01.013
Mahalingam, A., Satya, V. K., Manivannan, N., Narayanan, S. L., Sathya, P. (2018). Inheritance of mungbean yellow mosaic virus disease resistance in greengram [Vigna radiata (L.) Wilczek]. Int. J. Curr. Microbiol. Appl. Sci. 7 (01), 880–885. doi: 10.20546/ijcmas.2018.701.107
Maheshwari, R., Panigrahi, G., Angappan, K. (2014). Molecular characterization of distinct YMV (Yellow mosaic virus) isolates affecting pulses in India with the aid of coat protein gene as a marker for identification. Mol. Biol. Rep. 41, 2635–2644. doi: 10.1007/s11033-014-3122-9
Maiti, S., Basak, J., Kundagrami, S., Kundu, A., Pal, A. (2011). Molecular marker-assisted genotyping of Mungbean Yellow Mosaic India Virus resistant germplasms of mungbean and urdbean. Mol. Biotechnol. 47, 95–104. doi: 10.1007/s12033-010-9314-1
Maiti, S., Paul, S., Pal, A. (2012). Isolation characterization and structural analysis of non-TR-NBS-LRR encoding candidate gene from MYMIV-resistant Vigna mungo. Mol. Biotechnol. 52, 217–233. doi: 10.1007/s12033-011-9488-1
Malathi, V. C., John, P. (2009). “Mungbean yellow mosaic viruses,” in Desk encyclopedia of plant and fungal virology. Eds. Van Regenmortal, M., Mahy, B. (London: Academic press), 217–226.
Mandal, B., Varma, A., Malathi, V. G. (1997). Systemic infection of Vigna mungo using the cloned DNAs of the blackgram isolate of mungbean yellow mosaic geminivirus through agroinoculation and transmission of the progeny virus by whiteflies. J. Phytopathol. 145, 505–510. doi: 10.1111/j.1439-0434.1997.tb00358.x
Manivannan, N., Sethuraman, N. K., Natarajan, S. (2001). Screening of greengram [Vigna radiata (L.) Wilczek] germplasm for yellow mosaic resistance. Legume Res. 24, 268–271.
Marimuthu, T., Subramanian, C. L., Mohan, R. (1981). Assessment of yield losses due to yellow mosaic infection in mungbean. Pulse Crop Newsl. 1, 104.
Markam, N. K., Nair, S. K., Nanda, H. C., Lakpale, N. (2018). Studies on allelic relationship for resistance to mungbean yellow mosaic virus disease in mungbean genotypes. Int. J. Chem. Stud. 6 (2), 2401–2403.
Mathivathana, M. K., Murukarthick, J., Karthikeyan, A., Jang, W., Dhasarathan, M., Jagadeeshselvam, N., et al. (2019). Detection of QTLs associated with mungbean yellow mosaic virus (MYMV) resistance using the interspecific cross of Vigna radiata × Vigna umbellata. J. Appl. Genet. 60, 255–268. doi: 10.1007/s13353-019-00506-x
Meti, M., Kenganal, M., Sunkad, G., Aswathanarayana, D. S., Shanwad, U. K. (2017). Spatial variability of mungbean yellow mosaic virus (MYMV) in North Eastern Karnataka. Int. J. Plant Prot. 10 (2), 420–428. doi: 10.15740/HAS/IJPP/10.2/420-428
Miller, J. C., Siyuan, T., Guijuan, Q., Kyle, A. B., Wang, J., Xia, D. F., et al. (2011). A TALE nuclease architecture for efficient genome editing. Nat. Biotechnol. 29, 143–148. doi: 10.1038/nbt.1755
Mishra, S. P., Asthana, A. N. (1996). “Inheritance of yellow mosaic virus resistance in mungbean (Vigna radiata (L.) Wilczek),” in Recent Advances in Mungbean Research. Eds. Asthana, A. N., Kim, D. H. (India: Indian Society of Pulses Research, IIPR, Kanpur-208024), 214–219.
Mishra, G. P., Singh, B., Seth, T., Singh, A. K., Halder, J., Krishnan, N., et al. (2017). Biotechnological advancements and begomovirus management in okra (Abelmoschus esculentus L.): Status and perspectives. Front. Plant Sci. 8, 360. doi: 10.3389/fpls.2017.00360
Mohan, S., Sheeba, A., Murugan, E., Ibrahim, S. M. (2014). Screening of mungbean germplasm for resistance to mungbean yellow mosaic virus under natural condition. Indian J. Sci. Technol. 7 (7), 891–896.
Morinaga, T., Ikegami, M., Miura, K. (1993). The nucleotide sequence and genome structure of mungbean yellow mosaic geminivirus. Microbiol. Immunol. 37, 471–476. doi: 10.1111/j.1348-0421.1993.tb03238.x
Mugerwa, H., Seal, S., Wang, H. L., Patel, M. V., Kabaalu, R., Omongo, C. A., et al. (2018). African ancestry of New World, Bemisia tabaci-whitefly species. Sci. Rep. 8, 2734. doi: 10.1038/s41598-018-20956-3
Murugan, E., Nadarajan, N. (2012). Genetic studies on differential expression of mungbean yellow mosaic virus resistance related to trichome density in urd bean (Vigna mungo (L.) Hepper). Indian J. Plant Genet. Resour. 25 (2), 135–138.
Nagaraj, Basavaraj, S., Padmaja, A. S., Nagaraju, N., Ramesh, S. (2019). Identification of stable sources of resistance to mungbean yellow mosaic virus (MYMV) disease in mungbean [Vigna radiata (L.) Wilczek]. Plant Genet. Res.: Charact. Utilization 17 (4), 362–370. doi: 10.1017/s1479262119000121
Naimuddin, Akram, M., Aditya, P. (2011a). First report of natural infection of mungbean yellow mosaic India virus in two wild species of Vigna. New Dis. Rep. 23, 21–22. doi: 10.5197/j.2044-0588.2011.023.021
Naimuddin, K., Akram, M., Sanjeev, G. (2011b). Identification of mungbean yellow mosaic India virus infecting Vigna mungo var. silvestris L. Phytopathol. Mediterr. 50, 94–100. doi: 10.14601/Phytopathol_Mediterr-8740
Naimuddin, K., Akram, M., Singh, N. P. (2016). Yellow mosaic of mungbean and urdbean: current status and future strategies. J. Food Legumes 29 (2), 77–93.
Nair, R. M., Yang, R. Y., Easdown, W. J., Thavarajah, D., Thavarajah, P., Hughes, J. D., et al. (2013). Biofortification of mungbean (Vigna radiata) as a whole food to enhance human health. J. Sci. Food Agric. 93, 1805–1813. doi: 10.1002/jsfa.6110
Nair, R. M., Götz, M., Winter, S., Giri, R. R., Boddepalli, V. N., Sirari, A., et al. (2017). Identification of mungbean lines with tolerance or resistance to yellow mosaic in fields in India where different begomovirus species and different Bemisia tabaci cryptic species predominate. Eur. J. Plant Pathol. 149 (2), 349–365. doi: 10.1007/s10658-017-1187-8
Nair, R. M., Pandey, A. K., War, A. R., Hanumantharao, B., Shwe, T., Alam, A., et al. (2019). Biotic and abiotic constraints in mungbean production-progress in genetic improvement. Front. Plant Sci. 10, 1340. doi: 10.3389/fpls.2019.01340
Narasimhan, A., Patil, B. R., Datta, S., Kaashyap, M. (2010). Genetic diversity assessment across different genotypes of mungbean and urdbean using molecular markers. Electron J. Plant Breed. 1 (4), 379–383.
Nene, Y. L. (1968). “A survey of the viral disease of pulses crops in Uttar Pradesh, India,” in First Annual Report (Pantnagar: UP Agricultural University), 358.
Nene, Y. L. (1972). A survey of viral diseases of pulse crops in Uttar Pradesh. G. B. Pant Univer Agricul Technol. Pantnagar Res. Bull. 4, 191, 88.
Nene, Y. L. (1973). Control of Bemisia tabaci Genn., a vector of several plant viruses. Indian J. Agric. Sci. 43, 433–436.
Ngampongsai, S., Watanasit, A., Srisombun, S., Srinives, P., Masari, A. (2009). “Current Status of Mungbean and the Use of Mutation Breeding in Thailand,” in Induced plant mutations in the genomic era. Food and Agricultural Organization of the United Nations. Ed. Shu, Q. Y., (Food and Agricultural Organization of the United Nations, Rome: © FAO & IAEA) 355–357.
Oliveira, J. T. A., Andrade, N. C., Miranda, A. S. M., Soares, A. A., Gondim, D. M. F., Filho, J. H. A., et al. (2012). Differential expression of antioxidant enzymes and PR-proteins in compatible and incompatible interactions of cowpea (Vigna unguiculata) and the root-knot nematode Meloidogyne incognita. Plant Physiol. Biochem. 51, 145–152. doi: 10.1016/j.plaphy.2011.10.008
Pal, S. S., Dhaliwal, H. S., Bains, S. S. (1991). Inheritance of resistance to yellow mosaic virus in some Vigna species. Plant Breed. 106, 168–171. doi: 10.1111/j.1439-0523.1991.tb00496.x
Pal, S. S., Singh, J. J., Singh, I. (2000). Transfer of YMV resistance in cultivar SML32 of Vigna radiata from other related Vigna species. Plant Dis. Res. 15, 67–69.
Pal, A., Chakrabarti, A., Basak, J. (2007). New motifs within the NB-ARC domain of R proteins: Probable mechanisms of integration of geminiviral signatures within the host species of Fabaceae family and implications in conferring disease resistance. J. Theor. Biol. 246 (3), 564–573. doi: 10.1016/j.jtbi.2007.01.013
Pandiyan, M., Ramamoorthi, N., Ganesh, S. K., Jebaraj, S., Pagarajan, P., Balasubramanian, P. (2008). Broadening the genetic base and introgression of MYMV resistance and yield improvement through unexplored genes from wild relatives in mungbean. Plant Mutat. Rep. 2 (1), 33–38.
Pandiyan, M., Senthil, N., Suersh, R., Chakravarthy, N., Packiaraj, D., Jagadeesh, S. (2012). Interspecific hybridization of Vigna radiata x Vigna trilobata. Wudpecker J. Agril. Res. 1 (6), 233–234.
Panduranga, G. S., Vijayalakshmi, K., Reddy, K., Loka Rajashekara, H. (2011). Evaluation of mungbean germplasm for resistance against whitefly (Bemisia tabaci Genn.) and mungbean yellow mosaic virus (MYMV) disease. Indian J. Entomol. 73 (4), 338–342.
Pandya, B. P., Singh, D. P., Sharma, B. L. (1977). Screening of mungbean germplasm for field resistance to yellow mosaic virus. Trop. Grain Legume Bull. 7, 13–14.
Pant, V., Gupta, D., Choudhury, N. R., Malathi, V. G., Varma, A., Mukherjee, S. K. (2001). Molecular characterization of the Rep protein of the black gram isolate of Indian mungbean yellow mosaic virus. J. Gen. Virol. 82, 2559–2567. doi: 10.1099/0022-1317-82-10-2559
Parida, A., Raina, S. N., Narayan, R. K. J. (1990). Quantitative DNA variation between and within chromosome complements of Vigna species (Fabaceae). Genetica 82, 125–133. doi: 10.1007/BF00124642
Pathak, A. K., Jhamaria, S. L. (2004). Evaluation of mungbean (Vigna radiata L.) varieties to yellow mosaic virus. J. Mycol. Plant Pathol. 34 (1), 64–65.
Paul, P. C., Biswas, M. K., Mandal, D., Pal, P. (2013). Studies on host resistance of Mungbean against Mungbean Yellow Mosaic Virus in the agro-ecological condition of lateritic zone of West bengal. Bioscan. 8 (2), 583–587.
Polston, J. E., Bois, D., Ano, G., Poliakoff, F., Urbino, C. (1997). Occurrence of a strain of Potato yellow mosaic geminivirus infecting tomato in the Eastern Caribbean. Plant Dis. 82, 126. doi: 10.1094/PDIS.1998.82.1.126B
Pooggin, M., Shivaprasad, P. V., Veluthambi, K., Hohn, T. (2003). RNAi targeting of a DNA virus in plants. Nat. Biotechnol. 21, 131–132. doi: 10.1038/nbt0203-131b
Pratap, A., Prajapati, U., Singh, C. M., Gupta, S., Rathore, M., Malviya, N., et al. (2018). Potential, constraints and applications of in vitro methods in improving grain legumes. Plant Breed. 137, 235–249. doi: 10.1111/pbr.12590
Pratap, A., Douglas, C., Prajapati, U., Kumari, G., War, A. R., Tomar, R., et al. (2020). “Breeding Progress and Future Challenges: Biotic Stresses,” in The Mungbean Genome, Compendium of Plant Genomes. Ed. Nair, R. M. (Switzerland AG: ©Springer Nature), 80. doi: 10.1007/978-3-030-20008-4_5
Prema, G. U., Rangaswamy, K. T. (2018). Molecular detection and characterization of coat protein gene of Mungbean Yellow Mosaic Virus (MYMV) from Karnataka. Int. J. Agril. Sci. 10 (3), 5118–5122. doi: 10.9735/0975-3710.10.3.5118-5122
Project Coordinators Report-2018 (2018). All India Coordinated Research Project on MULLaRP (Mungbean, Urdbean, Lentil, Lathyrus, Rajmash, Fieldpea) (Kalyanpur, Kanpur: ICAR-Indian Institute of Pulses Research), 1–46.
Qazi, J., Ilyas, M., Mansoor, S., Briddon, R. (2007). Legume yellow mosaic viruses: genetically isolated begomoviruses. Mol. Plant Pathol. 8, 343–348. doi: 10.1111/j.1364-3703.2007.00402.x
Rahman, A. H. M., Akanda, A. M., Ashraful, A. K. M. (2006). Relationship of whitefly population build up with the spread of TYLCV on eight tomato varieties. J. Agric. Rural Dev. 4, 67–74. doi: 10.3329/jard.v4i1.770
Ramesh, S. V., Sahu, P. P., Prasad, M., Praveen, S., Pappu, H. R. (2017a). Geminiviruses and plant hosts: A closer examination of the molecular arms race. Viruses 9, 256. doi: 10.3390/v9090256
Ramesh, S. V., Chouhan, B. S., Ramteke, R. (2017b). Molecular detection of Begomovirus (family: Geminiviridae) infecting Glycine max (L.) Merr. and associated weed Vigna trilobata. J. Crop Weed 13 (2), 64–67.
Rashid, A., Harris, D., Hollington, P., Ali, S. (2004). On-farm seed priming reduces yield losses of mungbean (Vigna radiata) associated with Mungbean Yellow Mosaic Virus in the North West frontier province of Pakistan. Crop Prot. 23, 1119–1124. doi: 10.1016/j.cropro.2004.04.002
Reddy, B. V. B., Obaiah, S., Prasanthi, L., Sivaprasad, Y., Sujitha, A., Krishna, T. G. (2015). Mungbean yellow mosaic India virus is associated with yellow mosaic disease of blackgram (Vigna mungo L.) in Andhra Pradesh, India. Arch. Phytopathol. Plant Prot. 48 (4), 345–353. doi: 10.1080/03235408.2014.888874
Reddy, K. S. (2009). “A new mutant for yellow mosaic virus resistance in mungbean (Vigna radiata L. Wilczek) variety SML-668 by recurrent gamma-ray irradiation,” in Induced Plant Mutation in the Genomics Era. Ed. Shu, G. Y. (Rome: Food and Agriculture Organization of the United Nations), 361–362.
Rojas, M. R., Hagen, C., Lucas, W. J., Gilbertson, R. L. (2005). Exploiting chinks in the plant’s armor: evolution and emergence of geminiviruses. Annu. Rev. Phytopathol. 43, 361–394. doi: 10.1146/annurev.phyto.43.040204.135939
Rouhibakhsh, A., Haq, Q. M. I., Malathi, V. G. (2011). Mutagenesis in ORF AV2 affects viral replication in Mungbean Yellow Mosaic India Virus. J. Biosci. 36, 329–340. doi: 10.1007/s12038-011-9041-1
Sai, C. B., Nagarajan, P., Raveendran, M., Rabindran, R., Kannan Bapu, J. R., Senthil, N. (2017). Understanding the inheritance of mungbean yellow mosaic virus (MYMV) resistance in mungbean (Vigna radiata L. Wilczek). Mol. Breed. 37, 63. doi: 10.1007/s11032-017-0650-8
Salam, S. A., Patil, M. S., Salimath, P. M. (2009). Evaluation of mungbean cultures against MYMV in Karnataka under natural conditions. Legume Res. 32 (4), 286–289.
Salam, S. A., Patil, M. S., Byadgi, A. S. (2011). Status of mungbean yellow mosaic virus disease incidence on green gram. Karnataka J. Agric. Sci. 24 (2), 247–248.
Sandhu, T. S., Brar, J. S., Sandhu, S. S., Verma, M. M. (1985). Inheritance of resistance to mungbean yellow mosaic virus in greengram. J. Res. Punjab Agril. Univ. 22 (1), 607–611.
Sehrawat, N., Yadav, M., Bhat, K. V., Sairam, R. K., Jaiwal, P. K. (2016). Introgression of mungbean yellow mosaic virus resistance in Vigna mungo (L.) Hepper and purity testing of F1 hybrids using SSRs. Turk J. Agril. For. 40, 95–100. doi: 10.3906/tar-1407-169
Sels, J., Mathys, J., De Coninck, B. M. A., Cammue, B. P. A., De Bolle, M. F. C. (2008). Plant pathogenesis-related (PR) proteins: A focus on PR peptides. Plant Physiol. Biochem. 46, 941–950. doi: 10.1016/j.plaphy.2008.06.011
Selvi, R., Muthiah, A. R., Manivannan, N. (2006). Tagging of RAPD marker for MYMV resistance in mungbean (Vigna radiata (L.) Wilczek). Asian J. Plant Sci. 5, 277–280. doi: 10.3923/ajps.2006.277.280
Shafiq, M., Shaheen, A., Zafar, Y., Briddon, R. W., Mansoor, S. (2010). Pepper leaf curl Lahore virus requires the DNA B component of Tomato leaf curl New Delhi virus to cause leaf curl symptoms. Virol. J. 7, 367. doi: 10.1186/1743-422X-7-367
Shanmugasundaram, S., Keatinge, J. D. H., d’Arros, Hughes, J. (2009). “The mungbean transformation. Diversifying crops, defeating malnutrition,” in Millions fed: proven successes in agricultural development. Eds. Spielman, D. J., Pandya-Lorch, R. (Washington, DC: International Food Policy Research Institute), 103–108.
Shanmugasundaram (1996). Mungbean varietal improvements. Tainan: Asian Vegetable Research and Development Center. (© AVRDC-The World Vegetable Center), 53–54, http://203.64.245.61/fulltext_pdf/EAM/1991-2000/eam0117.pdf.
Shivanathan (1977). Tropical Agriculture Research Series No. 10 (Japan: Tropical Agriculture Research Center), 65–68.
Shivaprasad, P. V., Akbergenov, R., Trinks, D., Rajeswaran, R., Veluthambi, K., Hohn, T., et al. (2005). Promoters, transcripts, and regulatory proteins of mungbean Yellow Mosaic Geminivirus. J. Virol. 79 (13), 8149–8163. doi: 10.1128/JVI.79.13.8149–8163.2005
Shivaprasad, P. V., Thillaichidambaram, P., Balaji, V., Veluthambi, K. (2006). Expression of full-length and truncated Rep genes from Mungbean Yellow Mosaic Virus-Vigna inhibits viral replication in transgenic tobacco. Virus Genes 33, 365–374. doi: 10.1007/s11262-006-0077-5
Shukla, G. P., Pandya, B. P. (1985). Resistance to yellow mosaic in greengram. SABRAO J. 17 (3), 165–171.
Simon, B., Cenis, J. L., Demichelis, S., Rapisarda, C., Caciagli, P. (2003). Bosco, D.Survey of Bemisia tabaci (Hemiptera: Aleyrodidae) biotypes in Italy with the description of a new biotype (T) from. Euphorbia characias. Bull. Entomol. Res. 93, 259–264. doi: 10.1079/BER2003233
Singh, B. V., Ahuja, M. R. (1977). Phaseolus sublobatus Roxb. A source of resistance to yellow mosaic virus for cultivated mung. Indian J. Genet. Plant Breed. 37, 130–132.
Singh, V. P., Chaturvedi, S. N. (1981). Gamma-ray induced male sterile mutants in mungbean. Pulse Crops Newsl. 1, 41–42.
Singh, D. P., Sharma, B. L. (1983). Inheritance of resistance to yellow mosaic virus in Vigna radiata x Vigna radiata var. sublobata crosses. XV. Intl. Cong. Genet. New Delhi (Abs), Oxford and IBH Publishing Company, Part II, 755.
Singh, S. K., Singh, P. S. (2014). Screening of mungbean (Vigna radiata) genotypes against major insects. Curr. Adv. Agric. Sci. 6 (1), 85–87.
Singh, K. B., Sandhu, J. S., Malhotra, R. S., Brar, J. S. (1977). ML-5 A new moong. Indian Farming, 27 (5), 7–8.
Singh, A. K., Verma, V. S., Singh, P., Rizvi, S. E. H., Singh, B. (2008). Evaluation and screening of urdbean germplasm for yield and genetic resistance against whitefly and yellow mosaic virus under rainfed conditions of Jammu. Plant Dis. Res. 23 (2), 68–72.
Singh, G., Singh, S., Sheoran, O. P. (2013). Inheritance of Mungbean Yellow Mosaic Virus (MYMV) resistance in mungbean [Vigna radiata (l.) Wilczek]. Legume Res. 36 (2), 131–137.
Singh, N., Mallick, J., Sagolsem, D., Mandal, N., Bhattacharyya, S. (2018). Mapping of molecular markers linked with MYMIV and yield attributing traits in mungbean. Indian J. Genet. 78 (1), 118–126. doi: 10.5958/0975-6906.2018.00014.7
Singh, C. M., Pratap, A., Gupta, S., Biradar, R. S., Singh, N. P. (2020). Association mapping for mungbean yellow mosaic India virus resistance in mungbean (Vigna radiata L. Wilczek). 3Biotech 10 (2), 33. doi: 10.1007/s13205-019-2035-7
Singh, D. P. (1980a). Inheritance of resistance to yellow mosaic virus in blackgram (Vigna mungo L.). Theor. Appl. Genet. 52, 233–235. doi: 10.1007/BF00264676
Singh, J. P. (1980b). Effect of virus diseases on growth components and yield of mungbean and urdbean. Indian Phytopathol. 33, 405–408.
Souframanien, J., Gopalakrishna, T. (2006). ISSR and SCAR markers linked to the mungbean yellow mosaic virus (MYMV) resistance gene in blackgram [Vigna mungo (L.) Hepper]. Plant Breed. 125, 619–622. doi: 10.1111/j.1439-0523.2006.01260.x
Sowmini, K., Jayamani, P. (2014). Validation of molecular markers linked with yellow mosaic disease resistance in blackgram Vigna mungo (L.) Hepper. Legume Genomics Genet. 5 (4), 25–30. doi: 10.5376/lgg.2014.05.0004
Subedi, S., Neupane, S., Ghimire, T. N. (2016). Screening of mungbean and black gram genotypes as sources of genetic resistance against Mungbean Yellow Mosaic Disease. Nepal J. Agril. Sci. 14, 148–155.
Sudha, M., Anusuya, P., Ganesh, N. M., Karthikeyan, A., Nagarajan, P., Raveendran, M., et al. (2013a). Molecular studies on mungbean [Vigna radiata (L.) Wilczek] and ricebean [Vigna umbellate (Thunb.)] interspecific hybridization for Mungbean Yellow Mosaic Virus resistance and development of species specific SCAR marker for ricebean. Arch. Phytopathol. Plant Prot. 46 (5), 503–551. doi: 10.1080/03235408.2012.745055
Sudha, M., Karthikeyan, A., Anusuya, P., Ganesh, N. M., Pandiyan, M., Senthil, N., et al. (2013b). Inheritance of resistance to mungbean yellow mosaic virus (MYMV) in inter and intra specific crosses of mungbean (Vigna radiata). Am. J. Plant Sci. 4, 1924–1927. doi: 10.4236/ajps.2013.410236
Sudha, M., Karthikeyan, A., Nagarajan, P., Raveendran, M., Senthil, N., Pandiyan, M., et al. (2013c). Screening of mungbean (Vigna radiata) germplasm for resistance to mungbean yellow mosaic virus using agroinoculation. Can. J. Plant Pathol. 35 (3), 424–430. doi: 10.1080/07060661.2013.827134
Sudha, M., Karthikeyan, A., Shobhana, V. G., Nagarajan, P., Raveendran, M., Senthil, N., et al. (2015). Search for Vigna species conferring resistance to mungbean yellow mosaic virus in mungbean. Plant Genet. Resour-C. 13 (2), 162–167. doi: 10.1017/S1479262114000859
Sunitha, S., Shanmugapriya, G., Balamani, V., Veluthambi, K. (2013). Mungbean yellow mosaic virus (MYMV) AC4 suppresses post-transcriptional gene silencing and an AC4 hairpin RNA gene reduces MYMV DNA accumulation in transgenic tobacco. Virus Genes 46 (3), 496–504. doi: 10.1007/s11262-013-0889-z
Tamilzharasi, M., Vanniarajan, C., Karthikeyan, A., Souframanien, J., Pillai, M. A., Meenakshisundram, P. (2019). Evaluation of urdbean (Vigna mungo) genotypes for mungbean yellow mosaic virus resistance through phenotypic reaction and genotypic analysis. Legume Res. LR4035, 1–7. doi: 10.18805/LR-4035
Tanksley, S. D., Nelson, J. C. (1996). Advanced backcross QTL analysis: a method for the simultaneous discovery and transfer of valuable QTLs from unadapted germplasm into elite breeding lines. Theor. Appl. Genet. 92, 191–203. doi: 10.1007/BF00223376
Thamodhran, G., Geetha, S., Ramalingam, A. (2016). Genetic study in urd bean (Vigna mungo (L.) Hepper) for inheritance of Mungbean Yellow Mosaic Virus resistance. Int. J. Agril. Environ. Biotechnol. 9 (1), 33–37. doi: 10.5958/2230-732X.2016.00005.X
Thongmeearkom, P., Honda, Y., Saito, Y., Syamananda, R. (1981). Nuclear ultra structural changes and aggregates of virus like particles in mungbean cells affected by mungbean yellow mosaic disease. Phytopathol. 71, 41–44. doi: 10.1094/Phyto-71-41
Torres, M. A. (2010). ROS in biotic interactions. Physiol. Plant. 138, 414–429. doi: 10.1111/j.1399-3054.2009.01326.x
Tsai, W. S., Shih, S. L., Rauf, A., Safitri, R., Hidayati, N., Huyen, B. T. T., et al. (2013). Genetic diversity of legume yellow mosaic begomoviruses in Indonesia and Vietnam. Ann. Appl. Biol. ,163, 367–377. doi: 10.1111/aab.12063
Vairam, N., Lavanya, S. A., Muthamilan, M., Vanniarajan, C. (2016). Screening of M3 mutants for yellow vein mosaic virus resistance in greengram [Vigna radiata (L.) wilczek]. Int. J. Plant Sci. 11 (2), 265–269. doi: 10.15740/HAS/IJPS/11.2/265-269
Varma, A., Dhar, A. K., Mandal, B. (1992). “MYMV transmission and control in India,” in Mungbean Yellow Mosaic Disease. Eds. Green, S. K., Kim, D. (Taipei: Asian Vegetable Research and Development Centre), 8–27.
Varma, P. M. (1952). Studies on the relationship of the Bhendi yellow vein mosaic virus and its vector, the whitefly (Bemisia tabaci Gen.). Indian J. Agril. Sci. 22, 75–91.
Varsani, A., Navas-Castillo, J., Moriones, E., Hernández-Zepeda, C., Idris, A., Brown, J. K. (2014). Establishment of three new genera in the family Geminiviridae: Becurtovirus, Eragrovirus and Turncurtovirus. Arch. Virol. 159 (8), 2193–2203. doi: 10.1007/s00705-014-2050-2
Varsani, A., Roumagnac, P., Fuchs, M., Navas-Castillo, J., Moriones, E., Idris, A., et al. (2017). Capulavirus and Grablovirus: two new genera in the family Geminiviridae. Arch. Virol. 162 (6), 1819–1831. doi: 10.1007/s00705-017-3268-6
Varun, P., Saxena, S. (2017). “Transmission of Begomoviruses,” in Begomoviruses: Occurrence and Management in Asia and Africa. Eds. Saxena, S., Tiwari, A. K. (Singapore: © Springer Nature), 51–70. doi: 10.1007/978-981-10-5984-1
Verlaan, M. G., Hutton, S. F., Ibrahem, R. M., Kormelink, R., Visser, R. G. F., Scott, J. W., et al. (2013). The tomato yellow leaf curl virus resistance genes Ty-1 and Ty-3 are allelic and code for DFDGD-Class RNA-dependent RNA polymerases. PloS Genet. 9, e1003399. doi: 10.1371/journal.pgen.1003399
Verma, R. P. S., Singh, D. P. (1986). The allelic relationship of genes giving resistance to mungbean yellow mosaic virus in blackgram. Theor. Appl. Genet. 72, 737–738. doi: 10.1007/BF00266537
Virmani, S. S., Singh, K. B., Singh, K. (1976). Note on the screening of greengram germplasm against yellow mosaic virus disease in India. Indian J. Agric. Sci. 46, 243–245.
Vishalakshi, B., Umakanth, B., Shanbhag, A. P., Ghatak, A., Sathyanarayanan, N., Madhav, M. S., et al. (2017). RAPD assisted selection of black gram (Vigna mungo L. Hepper) towards the development of multiple disease resistant germplasm. 3Biotech 7 (1), 1. doi: 10.1007/s13205-016-0582-8
Wang, Z., Yao, M., Wu, Y. (2009). Cross-resistance, inheritance and biochemical mechanisms of imidacloprid resistance in B-biotype Bemisia tabaci. Pest Manag. Sci. 65, 1189–1194. doi: 10.1002/ps.1808
Williams, F. J., Grewal, J. S., Amin, K. S. (1968). Serious and new diseases of pulse crops in India in 1966. Plant Dis. Rep. 52, 300–304.
Yadav, G. S., Dahiya, B. (2000). Screening of some mungbean genotypes against major insect pests and yellow mosaic virus. Ann. Agric. Bio Res. 5, 71–73.
Yang, C., Guo, R., Jie, F., Nettleton, D., Peng, J., Carr, T., et al. (2007). Spatial analysis of Arabidopsis thaliana gene expression in response to Turnip mosaic virus infection. Mol. Plant Microbe Interact. 20, 359–370. doi: 10.1094/MPMI-20-4-0358
Yen, J. Y., Bui, T. G., Schafleitner, R., Lin, C. Y., Huang, S. M., Chen, L. F., et al. (2016)."Marker-assisted selection of disease and pest resistant mungbean linesusing Cel-I genotyping," in Tropentag 2016: Solidarity in a competing world-fair use of resources. Eds.Freyer, B., Tielkes, E (Göttingen: © CUVILLIER VERLAG), 82.
Zaidi, S. S. A., Mansoor, S., Ali, Z., Tashkandi, M., Mahfouz, M. M. (2016). Engineering Plants for Geminivirus Resistance with CRISPR/Cas9 System. Trends Plant Sci. 21 (4), 279–281. doi: 10.1016/j.tplants.2016.01.023
Keywords: begomovirus, gene editing, greengram, pathogen derived resistance, translational genomics, vector management
Citation: Mishra GP, Dikshit HK, S. V. R, Tripathi K, Kumar RR, Aski M, Singh A, Roy A, Priti, Kumari N, Dasgupta U, Kumar A, Praveen S and Nair RM (2020) Yellow Mosaic Disease (YMD) of Mungbean (Vigna radiata (L.) Wilczek): Current Status and Management Opportunities. Front. Plant Sci. 11:918. doi: 10.3389/fpls.2020.00918
Received: 19 March 2020; Accepted: 04 June 2020;
Published: 24 June 2020.
Edited by:
Ralf Georg Dietzgen, The University of Queensland, AustraliaReviewed by:
Senthil Natesan, Tamil Nadu Agricultural University, IndiaS. Kanakala, North Carolina State University, United States
Copyright © 2020 Mishra, Dikshit, S. V., Tripathi, Kumar, Aski, Singh, Roy, Priti, Kumari, Dasgupta, Kumar, Praveen and Nair. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gyan P. Mishra, Z3lhbi5nZW5lQGdtYWlsLmNvbQ==
 Gyan P. Mishra
Gyan P. Mishra Harsh K. Dikshit1
Harsh K. Dikshit1 Ramesh S. V.
Ramesh S. V. Kuldeep Tripathi
Kuldeep Tripathi Ramakrishnan M. Nair
Ramakrishnan M. Nair