- 1Faculty of Chemistry, University of Belgrade, Belgrade, Serbia
- 2Medical Faculty of the Military Medical Academy, University of Defence, Belgrade, Serbia
- 3Department of Chemistry, Faculty of Science, University of Hradec Kralove, Hradec Králové, Czechia
- 4Department for Experimental Toxicology and Pharmacology, National Poison Control Centre, Military Medical Academy, Belgrade, Serbia
In plants, there is a complex and multilevel network of the antioxidative system (AOS) operating to counteract harmful reactive species (RS), the foremost important of which are reactive oxygen species (ROS), and maintain homeostasis within the cell. Specific AOSs for plant cells are, first and foremost, enzymes of the glutathione-ascorbate cycle (Asc-GSH), followed by phenolic compounds and lipophilic antioxidants like carotenoids and tocopherols. Evidence that plant cells have excellent antioxidative defense systems is their ability to survive at H2O2 concentrations incompatible with animal cell life. For the survival of stressed plants, it is of particular importance that AOS cooperate and participate in redox reactions, therefore, providing better protection and regeneration of the active reduced forms. Considering that plants abound in antioxidant compounds, and humans are not predisposed to synthesize the majority of them, new fields of research have emerged. Antioxidant potential of plant compounds has been exploited for anti-aging formulations preparation, food fortification and preservation but also in designing new therapies for diseases with oxidative stress implicated in etiology.
Introduction
Plants are multicellular organisms which, thanks to their inability to makeover, have very well-developed adaptation systems and mechanisms of protection to varying environmental conditions. External factors like drought, high and low temperatures; also as high levels of radiation have an adverse effect on plants. A standard characteristic of varied stressors is their potential to promote the generation of reactive oxygen species (ROS) in plant tissue, the build-up of which within the cell causes oxidative stress. This term was initially introduced by Sies and Cadenas (1985). Namely, oxidative stress implies an interruption of the redox equilibrium as a consequence of the increased level of ROS within the cell itself; however, the most recent version of definition may be “imbalance between oxidants and antioxidants in favor of the oxidants, leading to a disruption of redox signaling and control and/or molecular damage” (Sies, 2018). Also, recently, oxidative stress is classified in subforms: oxidative stress present in physiological conditions (eustress), and oxidative stress expressing deleterious effects on macromolecules (distress; Lushchak, 2014).
Paradoxically, oxygen as a molecule which sustains aerobic life, against being essential for energy metabolism and respiration, is involved within the mechanism of the onset of various diseases and degenerative conditions (Sies et al., 2017). With the evolution of photosynthesis, initially by cyanobacteria and afterwards by plants, over 2 billion years ago, the quantity of oxygen on Earth has increased significantly. Molecular oxygen is made as a by-product during this process by operation of the oxygen-evolving complex (OEC), which is a component of the photosystem (PS) II (Yachandra et al., 1996). The massive quantities of present oxygen enabled the production of more ATP via aerobic respiration but also increased the danger of ROS formation. Aerobic organisms are ready to survive by virtue of the event of antioxidant protection mechanisms, exhibiting a defensive role against a vast number of ROS (Dumont and Rivoal, 2019).
Molecular oxygen can act as an oxidant, but despite its high thermodynamic reactivity, its reactions are kinetically slow thanks to the prevailing spin restriction (Krieger-Liszkay, 2005). In its ground state, oxygen appears as a triplet (3O2), with two unpaired electrons (biradical) of parallel spins in two separate orbitals, which makes it paramagnetic and thus shows no affinity for organic molecules unless activated. Oxygen activation is often achieved by two mechanisms (Apel and Hirt, 2004):
1. Absorbing excess energy sufficient to rotate the spin of one unpaired electron to make a single state (1O2), during which two electrons are of opposite spin.
2. A multi-step monovalent reduction to the formation of superoxide radical (O2•−), hydrogen peroxide (H2O2), hydroxyl radical (•OH), and eventually water.
By activation, the spin restriction has been surpassed and 1O2 can interact in two-electron transfer reactions, while its oxidizing capacity is greatly increased. The gradual reduction of triplet oxygen, exposing to high energy or electron transfer reactions, results in the formation of ROS (Sharma et al., 2012). Numerous defense mechanisms are implicated within the battle against these highly reactive molecules, the foremost important being the antioxidative system (AOS). The goal of such a system is to guard cells against ROS and oxidative stress that happens if the influence of ROS prevails (Huang et al., 2019). When determining if some molecule would behave as anti‐ or prooxidant of particular importance are micro-conditions (pH, presence of trace metals, etc.) restricted for specific cell compartment.
In this short review paper, we have selected the ROS, briefly summarized their main characteristics, described their prooxidant activities, and outlined the most prominent antioxidants in plants.
Reactive Oxygen Species
Reactive species (RS) are a broad term and include ROS, nitrogen [reactive nitrogen species (RNS)], sulfur [reactive sulfur species (RSS)], and other species, several of which are free radicals, and each has the potential to cause oxidative stress as a result of their accumulation within the cell to a level that exceeds the capacity to remove them (Mittler, 2017). ROS are the most vital group of RS and include, additionally to free radicals, non-radical forms which do not have unpaired electrons but also are highly reactive, e.g., H2O2, 1O2, hypochlorous acid (HClO), and ozone (O3). Free radicals are known since the twentieth century within the world of chemistry and were originally described as intermediate compounds in organic and inorganic chemistry (Kohen and Nyska, 2002). These molecules that have one or more unpaired electrons, leading to high reactivity, are formed when an atom or molecule “loses” or “gains” one electron, or during homolytic cleavage of a covalent bond. Conversely, when two free radicals share their unpaired electrons, non-radical species are formed (Arora et al., 2002). The term “Reactive” may be a relative term, while •OH indiscriminately reacts with all biological molecules in its vicinity, O2•− and H2O2 are highly selective. From the pathophysiological, as well as the physiological, point of view, the foremost significant ROS are •OH, O2•−, organic alkoxy (RO•), and organic peroxyl radicals (ROO•) as well as non-radical species: 1O2, H2O2, and O3 (Dumont and Rivoal, 2019).
Toxicity is not necessarily associated with reactivity. In many cases, the longer half-life of ROS provides an extended time for diffusion and consequently the power to succeed in sensitive sites within a cell where it can react with biomolecules far away from the location of its generation. For instance, the relatively long-lived O2•− (with a half-life of 1–4 μs and migration distance of 30 nm) generated on the mitochondrial membrane, diffuses toward the mitochondrial genome and reduces the transition metals within the genome itself. Singlet oxygen has approximately equivalent properties as O2•− with the best affinity to Trp, His, Tyr, and Cys residues of proteins. Hydrogen peroxide could live quite 1 ms, and its migration distance is in range of 1 μm, enabling to react with DNA and Cys and Met residues of protein far away from its origin (Mittler, 2017). On the contrary, extremely reactive •OH features a half-life of roughly 1 ns and migration distance of 1 nm, therefore, reacting with all neighboring biomolecules like DNA, RNA, lipids, and proteins (Figure 1).
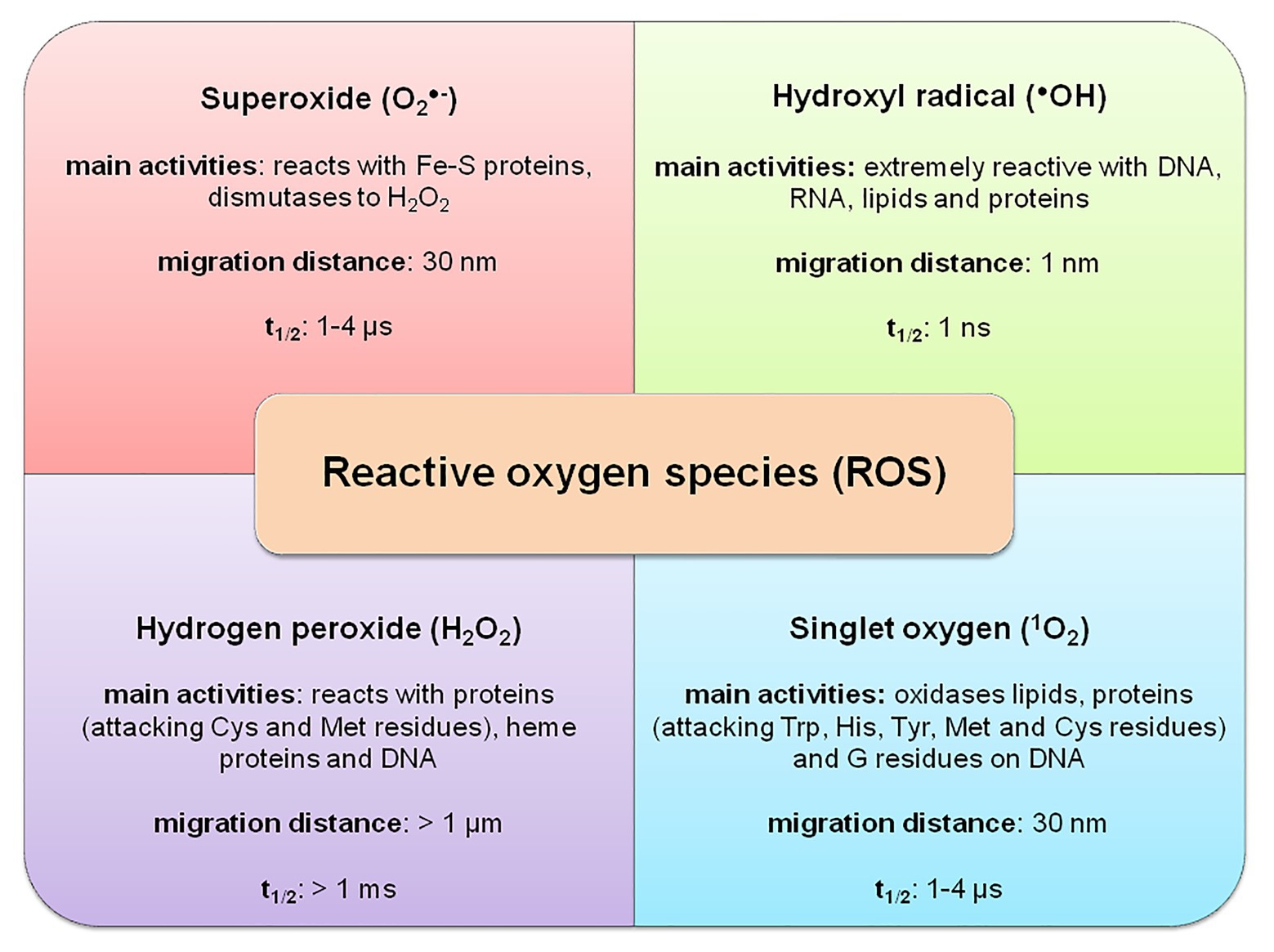
Figure 1. Basic properties of reactive oxygen species (ROS) in a plant cell – adapted from: Mittler (2017; https://creativecommons.org/licenses/by-nc-nd/4.0/).
To prevent the interaction between radicals and biological molecules, antioxidants should be in close vicinity to the radical’s place of formation, being in competition with the free radical for the biological substrate (Arora et al., 2002). If an antioxidant is not present in sufficient quantity to neutralize ROS, oxidation of biomolecules, like lipid peroxidation, protein damage (carbonylation of aminoalkanoic acid residues), oxidation of single DNA and RNA nucleotides, enzyme inhibition, and activation of apoptosis, will occur (Gill and Tuteja, 2010).
One of the foremost studied ROS is hydrogen peroxide. It exhibits a dual role: in low concentrations, it participates in signal transduction, while in high concentrations, it exerts a toxic effect on the cell. Under physiological conditions, the extent of H2O2 in leaves ranges approximately 1 μmol per gram of fresh tissue weight, i.e., 10 μmol/L H2O2 in peroxisomes (Cheeseman, 2006; Foyer and Noctor, 2016). Its presence in apoplast is essential for normal cell development. Acting as a substrate for class III peroxidase, H2O2 participates in phenolic compounds’ oxidation and, consequently, polymerization and cell wall lignin formation (Lewis and Yamamoto, 1990; Almagro et al., 2008; Moural et al., 2017; Pandey et al., 2017). On the other side, H2O2 expresses toxicity for several cells within the concentration range of 10–100 μmol/L, leading to aging or apoptosis. The mechanism of its toxicity is direct inactivation of enzymes by oxidation of cysteine (–SH) or methionine (–SCH3) residues necessary for catalysis. An indirect effect of H2O2 is expressed by crossing the cellular membranes via peroxiporins and by reacting with the Fe2+ or Cu+ ions, resulting in the formation of more potent toxic species such as •OH, and exactly •OH is liable for the bulk, or maybe all of the damage caused to DNA molecules in cells treated with H2O2. Sometimes exposure of cells to H2O2 could increase O2•− production due to NOX (EC 1.6.3.1) activation. Furthermore, H2O2 has long been recognized as a potent inhibitor of photosynthesis since, even at low H2O2 concentrations (10 μmol/L) could inhibit CO2 fixation by 50% by oxidizing enzymes included in Calvin cycle (Foyer and Shigeoka, 2011).
Plants are especially exposed to oxidative stress caused by 1O2 since they are rich in chlorophyll (Chl) which acts as a photosensitizer, and 1O2 is consistently generated in leaves. Chlorophyll is an efficient pigment which absorbs light within the so-called light-harvesting complexes (LHCs), intrinsic antennas, and PS II reaction centers, with the extra advantage that its excited state is long-lived enough to supply excitatory energy conversion to electrochemical potential via the method of charge separation during photosynthesis (Krieger-Liszkay, 2005; Xiulan et al., 2019). However, the excited (triplet) state of chlorophyll (3Chl) could supply nearby molecular oxygen with sufficient energy, leading to 1O2 formation if the energy is not efficiently used, or effective scavenger is lacking. Singlet oxygen is taken into account to be the foremost important ROS liable for the light-induced loss of PS II activity via degradation of the D1 protein, also as for the so-called “bleaching” of pigment (Krieger-Liszkay, 2005). Plants use two strategies to guard the photosynthetic apparatus from photoinhibition. The primary is non-photochemical quenching, i.e., dissipation of excessive excitation energy of 3Chl in antennas of PS II within the sort of heat. Second, carotenoid-dependent mechanism, or “quenching,” is predicated on the power of PS II to transfer electrons to varied acceptors in its proximity (carotenoids, α-tocopherol), which liberate excess energy within the sort of heat, returning to their ground state (Trebst, 2003; Apel and Hirt, 2004). Although this chlorophyll-carotenoid transfer is extremely effective, still 5% of 3Chl remains and this incomplete quenching is the evidence that antenna pigments are considered as a possible source of 1O2 in chloroplasts with a tendency for damaging D1 protein (Triantaphylidès and Havaux, 2009).
Superoxide anion radical (O2•−) is the primary cell-generated ROS which triggers a cascade of reactions and, therefore, the formation of secondary ROS, either directly or via enzymatic and metal-catalyzed processes, counting on the cell compartment. Generated via single-electron reduction of molecular oxygen within the cell, it is rapidly converted to H2O2 by superoxide dismutase (SOD, EC 1.15.1.1) activity, preventing the build-up of O2•−, and thereupon damage and inactivation of proteins containing Fe-S clusters (Schieber and Chandel, 2014). The foremost important reaction of O2•− is dismutation, non-enzymatic, or by SOD, where it reacts with another molecule of O2•− resulting in oxidation of its one molecule to oxygen and reduction of the second to H2O2. The reaction is the most effective in acidic pH, and on the contrary, slower in the basic environment (Kohen and Nyska, 2002; Birben et al., 2012; Noctor et al., 2018).
Hydroxyl radical (•OH) is taken into account as the most potent oxidant which, owing to its short half-life, very positive redox potential (close to +2 V) and high affinity for biomolecules, non-selectively oxidize DNA, proteins, lipids, amino acids, sugars, and metals, leading to damage or genetic instability. Generally, •OH is formed from H2O2 within the presence of iron or copper ions within the reaction described as well-known Fenton reaction (Fenton, 1984). For this reason, cells have various mechanisms for maintaining iron homeostasis, since no enzymes are found within the cell to eliminate •OH (Schieber and Chandel, 2014). As a consequence of metal absorption from the soil, plants are more suspectable to oxidative stress, and special care is taken in preventing reaction between transition metals and H2O2 by their sequestration. For this reason, plants are heeled with ferritins and metallothioneins capable of storing iron, copper, and zinc (Halliwell and Gutteridge, 2015). Prooxidants, such as O2•− and Asc, could re-reduce Fe3+ (Cu2+) to Fe2+ (Cu1+) and restore these ions in the game (Salehi et al., 2020).
Sources of ROS in Plant Cells
Free radicals and other oxygen derivatives are inevitable by-products of biological redox reactions, as well as a consequence of aerobic metabolism in plants (Figure 2).
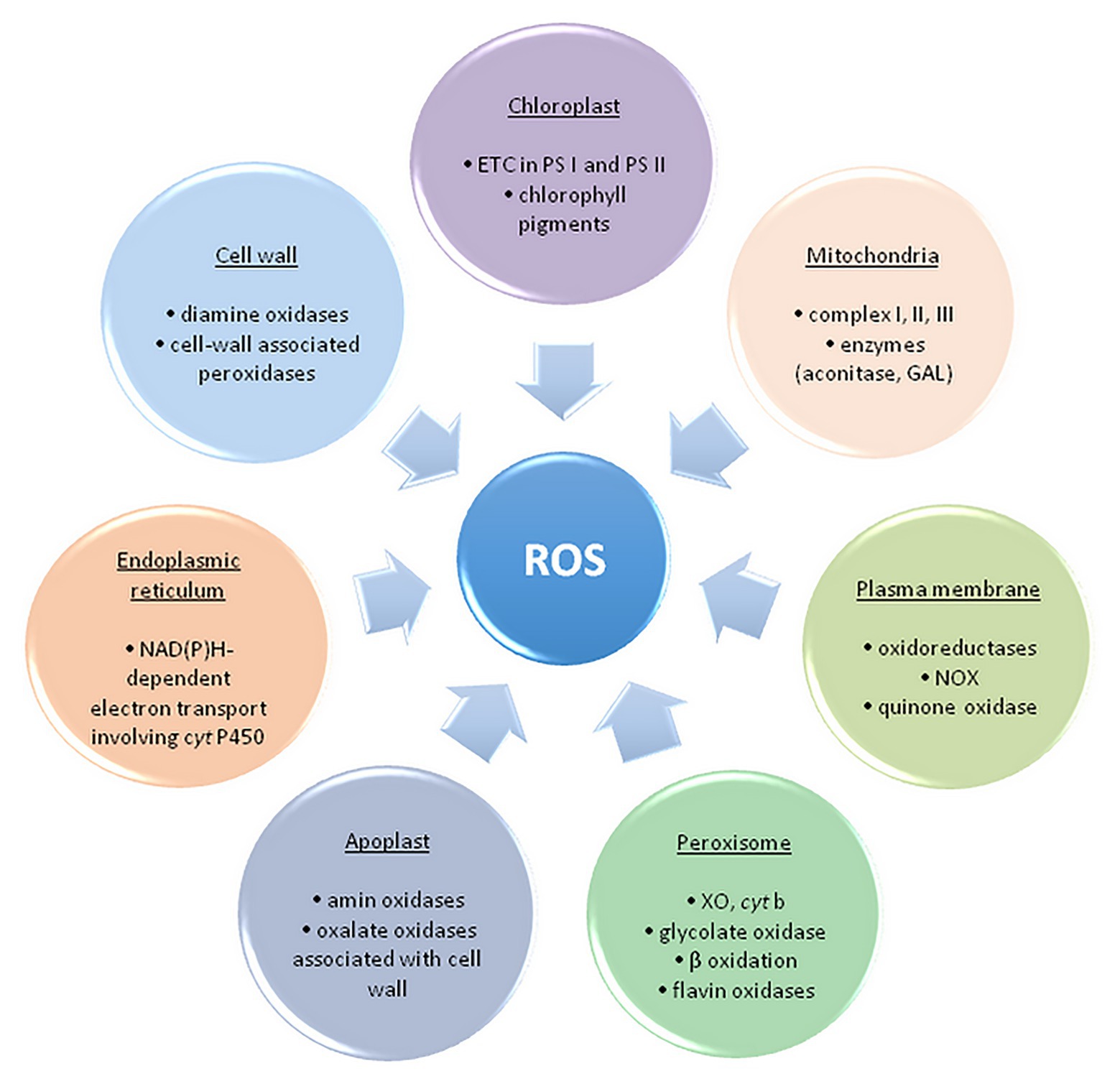
Figure 2. The main sites of ROS formation in a plant cell. ETC, electron transport chain; PS, photosystem; NOX, NAD(P)H oxidase; GAL, galactono-γ lactone dehydrogenase; XO, xanthine oxidase – adapted from: Sharma et al. (2012; https://creativecommons.org/licenses/by/3.0/).
ROS are primarily formed in chloroplasts, mitochondria, plasma membranes, peroxisomes, apoplast, and endoplasmic reticulum (Elstner, 1982; Sharma et al., 2012). The maximum amount as 1% of the oxygen employed by plants is diverted to ROS synthesis in several cell compartments (Bhattacharjee, 2005). The main source of ROS in plants is photosynthesis, precisely, electron transport chain (ETC) and photorespiration in peroxisomes (Foyer and Shigeoka, 2011). Photosystems within ETC, PS II and PS I, respectively, generate 1O2 and O2•− within the so-called Mehler reaction (Mehler and Brown, 1956; Apel and Hirt, 2004).
Additionally, production of ROS (primarily O2•− and H2O2) occurs when molecular oxygen is reduced by mainly electron leakage in mitochondrial complex I and III (about 1–5% of oxygen is converted to H2O2; Rhoads et al., 2006; Černý et al., 2018). In peroxisomes, the process of photorespiration (glycolate pathway) takes place in C3 plants, during which glycolate formed within the chloroplast stroma is oxidized. Hydrogen peroxide is made as a by-product, and peroxisomes are considered the main site of its intracellular production. Homologues of NAD(P)H oxidase (NOX, EC 1.6.3.1) are found in numerous plants and are named the respiratory burst oxidase homolog (RBOH). RBOHs catalyze the transfer of electrons from NAD(P)H within the cytoplasm to the molecular oxygen and create O2•− during the defense of plants against pathogens (Bhattacharjee, 2005). Additionally, to the cell wall, RBOHs are also expressed in vacuoles, endoplasmic reticulum, nucleus, and mitochondria (Mittler, 2017). Oxidative burst is taken into account one among the plant’s main responses to (a)biotic stress (high intensity of UV and photosynthetically active radiation (PAR) drought, high and low temperatures, inappropriately high concentration of Zn2+, Cu2+ and Cd2+, air pollution, herbicides, mechanical damage, pathogen attack, etc.), however, it is also essential for normal cell growth and development (Jia et al., 2013). Also, in response to varied adverse environmental conditions, class III peroxidases from apoplasts might be a source of ROS, contributing to oxidative burst, alongside with NOX. These peroxidases could form •OH within the presence of NADH, also as H2O2 (Swanson and Gilroy, 2010). ROS appearing within the apoplast may originate from other enzymes of the cell wall, for instance, oxalate oxidase also referred to as Germin, which releases H2O2 and CO2 from oxalic acid (Sharma et al., 2012).
Role of ROS in Signal Transduction
Reactive oxygen species do not have an exclusively detrimental effect on the cell and its components. Namely, increasing attention is focused on the benefits of ROS for plants since ROS support cell proliferation, physiological processes, and viability and maintaining the basal level of ROS within the cell is specifically important. ROS, created by various enzymes in plants, perform fine-tuning of signal transduction process associated with plant growth and defense against biotic and abiotic stressors. Regulated production of low concentrations of ROS features a signal role. RBOH-dependent ROS are related to plant’s defense response to pathogens but also with plant growth. Namely, a temporary ROS increase within the apoplast is essential for leaf and root growth and differentiation (Suzuki et al., 2011; Morales and Munné-Bosch, 2016). Furthermore, peroxidases within the apoplast are involved in ROS signaling and accumulation in various cellular compartments, including chloroplasts, mitochondria, peroxisomes, and nucleus (Mittler, 2017).
Therefore, ROS act as activators of signaling pathways for biological processes initiation. Signal translation mediated by redox reactions occurs primarily by oxidation and reduction of cysteine residues. Hence, for instance, H2O2 mediated oxidation of cysteine residues occur within the presence of nanomolar concentrations of H2O2. In contrast, H2O2 present in higher levels may irreversibly oxidize thiolate anions to sulfuric (SO2−) or sulfonic (SO3−) species, consequently promoting oxidative damage of biomolecules (Schieber and Chandel, 2014). For this reason, cells have enzymes which prevent the formation of intracellular H2O2, for instance, peroxiredoxins (PRX), glutathione peroxidase (GPx, EC 1.11.1.9), and ascorbate peroxidase (APX, EC 1.11.1.11; Exposito-Rodriguez et al., 2017; Mittler, 2017). Hence, a dominant concept in redox transmission is the balance between prooxidants on the one hand and antioxidants on the opposite. Counting on the oxidation degree, triggered programmed cell death and/or acclimatization of the plant and increased stress tolerance may occur (Jia et al., 2013; Noctor et al., 2018). Numerous authors emphasize the importance of maintaining the basal level of ROS above cytostatic and below cytotoxic, which allows redox reactions and essential processes regulation to happen (Truong and Carroll, 2013; Schieber and Chandel, 2014; Reczek and Chandel, 2015; Diebold and Chandel, 2016; Mittler, 2017). Too high or too low level of ROS impairs plant growth and development while sustaining an optimal level improves its progress, and therefore, responses to ROS are considered as dose-dependent. ROS and hormonal signaling are tightly intertwined where ROS acts as intrinsic growth and development signals activating many essential developmental processes, such as root hair growth, root elongation and gravitropism (thru auxin), stomatal closure (thru abscisic acid, ABA), lignin synthesis (thru jasmonic acid), leaf shape, trichome development, seed germination, etc. Beside phytohormones are involved in those adaptive responses of plants to environmental conditions, gibberellic acid (GA) is involved in process of apoptosis (Mhamdi et al., 2010; Gupta and Chakrabarty, 2013; Han et al., 2013; Talaat et al., 2019). Namely, GA-induced degradation of nuclear growth-repressing regulators, called DELLA proteins, is the key component of this mechanism. Additionally, it has been shown that triggering of programmed cell death by GA is tightly-related with ROS, predominantly H2O2, in aleurone cells of barley (Ishibashi et al., 2012). GA decreases the activity of catalase (CAT), ascorbate peroxidase (APX), and superoxide dismutase (SOD), leading to reduced scavenging ability for ROS, consequently resulting in peroxidative damage of membranes followed by release of hydrolytic enzymes (Jones and Smirnoff, 2005; Figure 3).
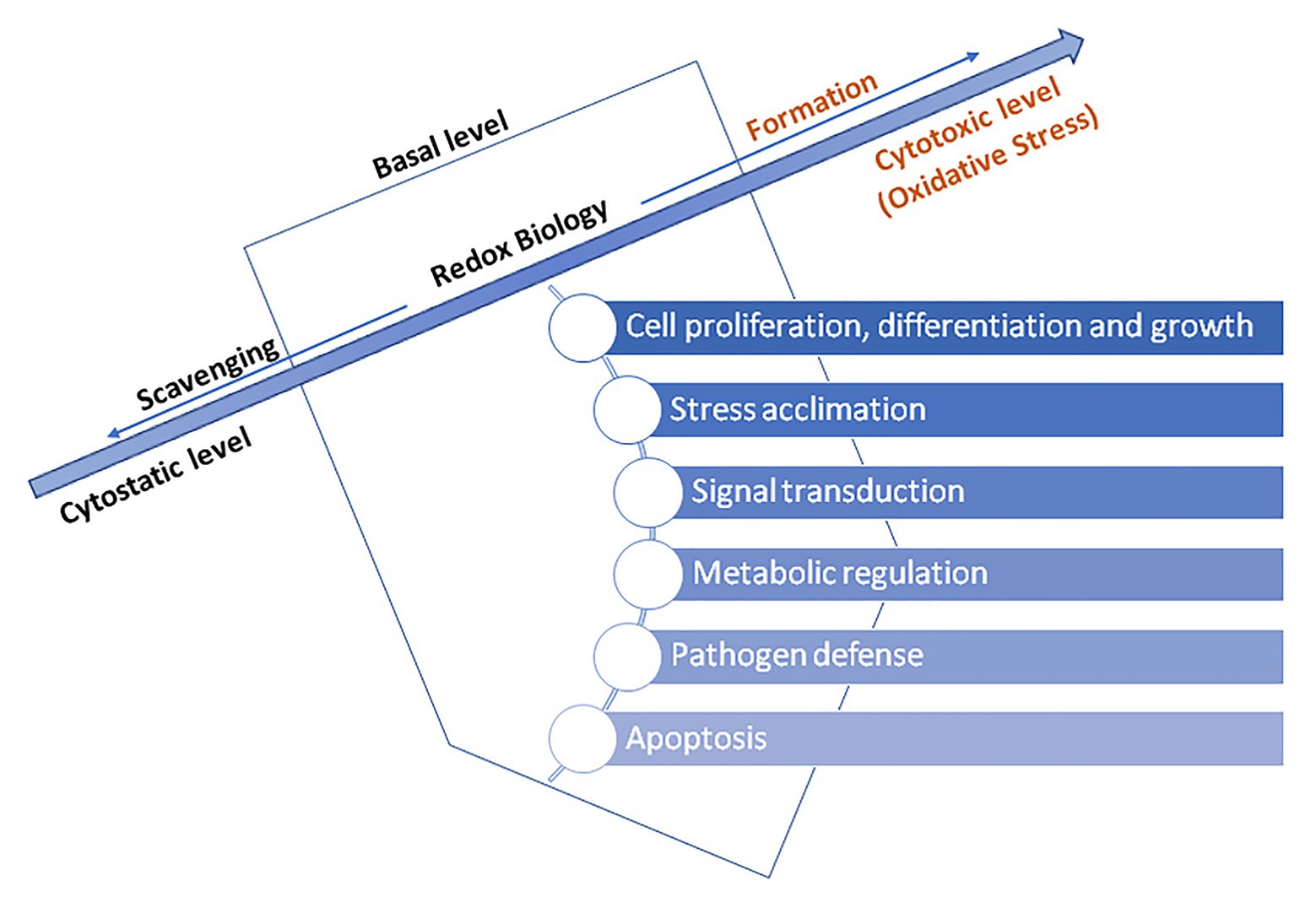
Figure 3. The basal level ROS-induced regulation of essential processes in a plant cells – adapted from: Mittler (2017; https://creativecommons.org/licenses/by-nc-nd/4.0/).
Plant Antioxidant Defense System
Excessive oxidation and reduction of cell components are equally detrimental, so maintaining redox homeostasis is crucial (Foyer and Shigeoka, 2011). For this reason, plants are extremely rich in compounds with antioxidative activity. Although antioxidative protection is different from species to species, its presence is ubiquitous (Wachter et al., 2005; Pulido et al., 2009; Diaz Vivancos et al., 2010a,b).
By definition, antioxidants represent molecules capable of inhibiting or quenching free radical reactions and delaying or preventing cell damage, and, in lower concentration than potential substrate which might be oxidized, significantly delay or hinder its oxidation (Nimse and Pal, 2015; Dumont and Rivoal, 2019). The foremost prominent low relative molecular mass antioxidants in plants are water-soluble ascorbate (Asc), glutathione, and phenols, and liposoluble tocopherols, tocotrienols, and carotenoids (Figure 4).
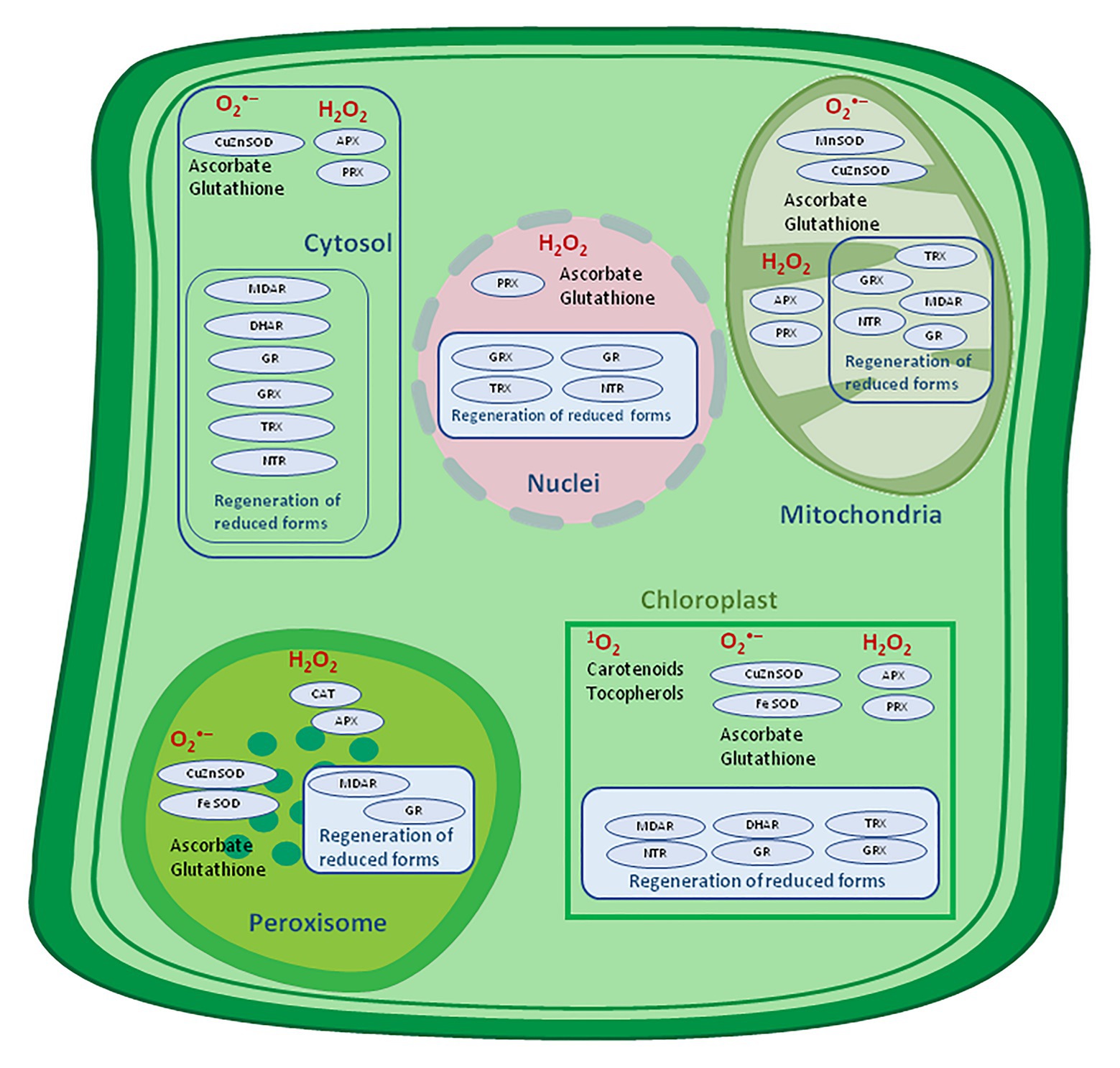
Figure 4. Antioxidative system location in a plant cell. APX, ascorbate peroxidase; CAT, catalase, DHAR, dehydroascorbate reductase; MDAR, monodehydroascorbate reductase; GR, glutathione reductase; GRX, glutaredoxin; SOD, superoxide dismutase; NTR, NADPH-thioredoxin reductase; PRX, peroxiredoxin; TRX, thioredoxin – adapted from: Noctor et al. (2018; http://creativecommons.org/licenses/by-nc-sa/4.0).
These molecules could self-react with ROS, but the removal efficiency is higher in enzyme-mediated reactions, like those catalyzed by APX (EC 1.11.1.11), ascorbate oxidase (AscO, EC 1.10.3.3), SOD, catalase (CAT, EC 1.11.1.6), and GPx (EC 1.11.1.9; Allen, 2009; Mhamdi et al., 2012; Noctor and Foyer, 2016; Noctor et al., 2018). Low relative molecular mass antioxidants remove ROS both indirectly and directly. Specifically, the indirect mechanism is chelation of transition metals, which prevents participation within the Haber-Weiss (Kehrer, 2000) or Fenton reaction, while the direct mechanism involves donating or receiving of electrons, scavenging radicals, and consequently preventing their reaction with biological molecules.
The antioxidant, which donates or receives electrons, is stabilized by π-electrons delocalization and resonance, and this is the case with Asc, phenolic compounds, and tocopherols. However, the advantage of scavengers over enzymatic antioxidants is their small size, which allows them to diffuse through cell membranes and localize near biological molecules which are potential targets of ROS (Kohen and Nyska, 2002). Additionally to those primary antioxidants, biomolecules, such as amino acids, sugars, pigments, also as secondary metabolites like flavonoids and terpenes, own antioxidant activity. Furthermore, secondary antioxidants are capable of regenerating oxidized primary antioxidant, as exemplified by Asc capable of regenerating oxidized α-tocopherol and α-tocopherol further regenerate β-carotene. Also, both created liposoluble radicals are often reduced by Asc, thereby exhibiting their antioxidant action within the membrane protection against lipid peroxidation. This is often an example of the synergistic action of AOS in preserving membrane integrity (Yachandra et al., 1996).
The most significant antioxidant in plant tissue, present at millimolar concentrations in chloroplasts, is Asc, followed by glutathione (GSH), which is present at 1,000 times lower concentration than Asc but is additionally vital. Specific enzyme systems (peroxidases) create the chance to rapidly react with H2O2, and their oxidized forms are regenerated by specific high-capacity reductases. In most cases, the entire amount of those antioxidants is greatly reduced (over 95%) within the cytosol, chloroplast, and mitochondria, with oxidized forms accumulating only in compartments with less efficient redox recycling mechanisms, like vacuoles and apoplasts (Noctor et al., 2018). Although these antioxidants scavenge ROS separately, they have long been thought to co-operate within the so-called water-water (Asada, 1999) and Asc-GSH cycle (Foyer and Halliwell, 1976) to metabolize H2O2 and keep sufficient excitatory energy under control within the chloroplasts (Foyer and Shigeoka, 2011). On the contrary, catalases play a serious role in H2O2 metabolism in peroxisomes (Mhamdi et al., 2012).
Water-Water and Asc-GSH Cycle
The water-water cycle begins by reducing the ground state of molecular oxygen to O2•− on the acceptor side of PS I in Mehler’s reaction. Under physiological conditions, O2•− is rapidly reduced to water by superoxide dismutase (SOD; Asada, 1999). However, O2•− could even be non-enzymatically disproportionate to H2O2 and O2. The resulting H2O2 is detoxified by APX which in turns oxidizes two Asc molecules, its specific electron donor. Simultaneously, the formation of short-lived radical, monodehydroascorbate (MDA•), happen, which may be spontaneously converted to Asc and dehydroascorbate (DHA), and/or might be rapidly reduced to Asc by NAD(P)H-dependent monodehydroascorbate reductase (MDAR, EC 1.6.5.4; Foyer and Shigeoka, 2011). MDAR is found within the cytosol, chloroplasts, peroxisomes, mitochondria, and the plasma membrane (Noctor and Foyer, 1998). Unlike Asc, DHA lacks antioxidative capability and is converted back to Asc by the addition of two electrons from GSH by DHA reductases (DHAR, EC 1.8.5.1; Nimse and Pal, 2015). GSH-dependent DHAR activity is expressed in chloroplasts, mitochondria, and peroxisomes (Jimenez et al., 1997; Scheibe et al., 2005; Malik et al., 2011; Queval et al., 2011; Noctor and Foyer, 2016). GSH is regenerated from its oxidized state, glutathione disulphide (GSSG), by the action of glutathione reductase (GR, EC 1.8.1.7) using electrons from NAD(P)H, thus closing the regeneration cycle of Asc and GSH (Figure 5).
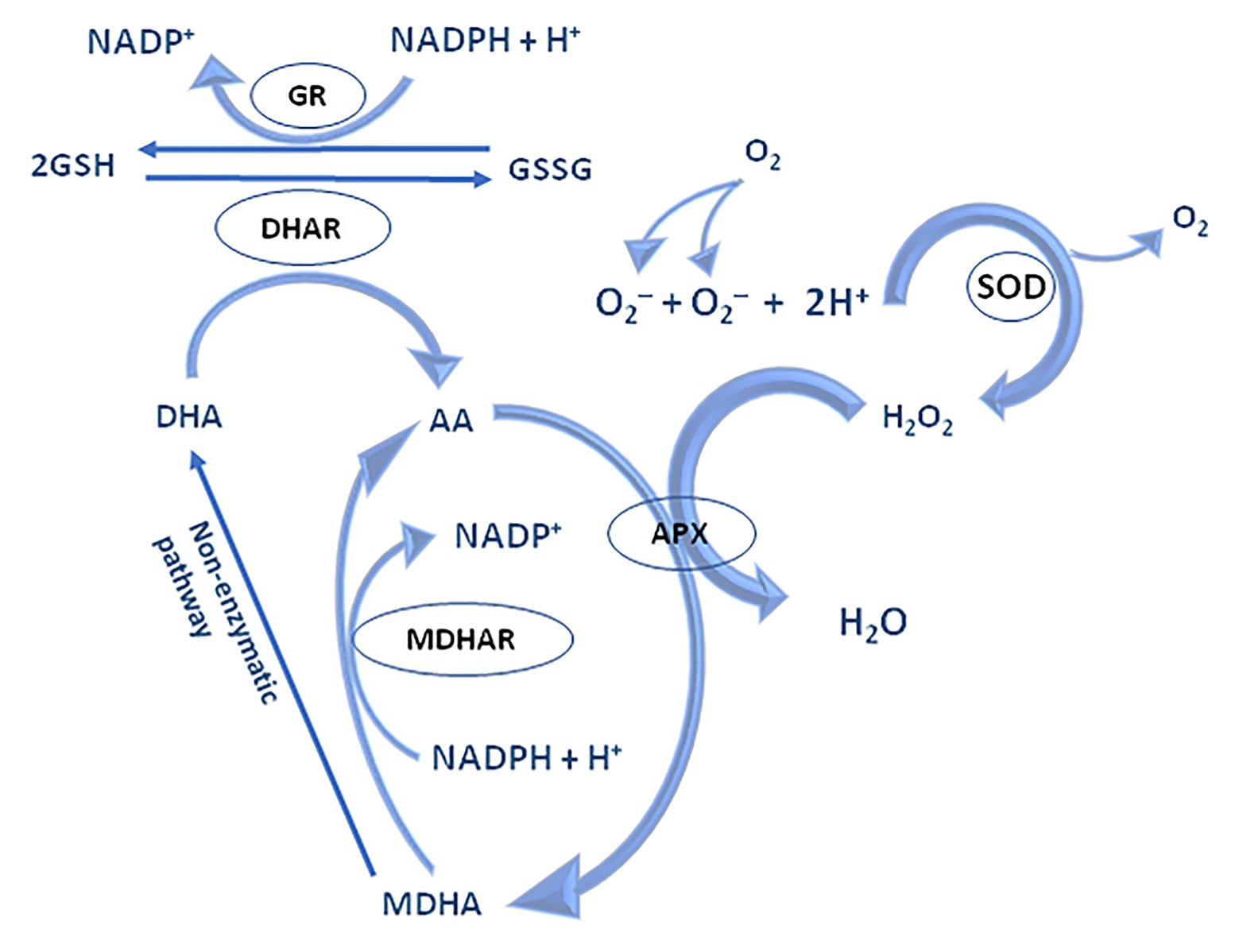
Figure 5. The mechanism of water-water and Asc-GSH cycle – adapted from: Noctor and Foyer (1998; https://creativecommons.org/publicdomain/mark/1.0/).
The Asc-GSH cycle is operating in various cellular compartments including the cytosol, mitochondria, chloroplast, and peroxisomes. Different isoforms of APX and SOD are localized within the stroma and thylakoid membrane, whereas chloroplast GR and DHAR are located within the stroma (Sharma et al., 2012). Despite the Asc-GSH cycle significance, newer evidence suggests overlapping of the role of peroxiredoxins (PRX) in maintaining a relevant level of H2O2 in chloroplasts.
Peroxiredoxins, which belong to the family of peroxidases, are important in ROS detoxification since they reduce H2O2 and organic peroxides and add cooperation with thioredoxin (TRX) and TRX-like proteins in chloroplasts (Dietz, 2011). PRXs utilize thiols to scale back H2O2. The Asc-GSH cycle has greater specificity for H2O2, and chloroplast APX has greater activity than PRX, however, PRXs are specific for lipid peroxides and/or RNS (Foyer and Noctor, 2016).
Enzymatic Antioxidants
Superoxide Dismutase
Superoxide dismutase plays a serious role in oxidative stress by catalyzing the rapid dismutation of O2•− and thus reducing the danger of •OH formation via metal-catalyzed reactions. SOD-catalyzed dismutation is 10,000 times faster than spontaneous reactions. The enzyme is present in all aerobic cells and subcellular compartments sensitive to oxidative stress (Bowler et al., 1992). There are three types of SOD metalloenzymes in plants counting on metal cofactor present within the active center. The foremost abundant isoenzyme is Cu/Zn-SOD found in chloroplast stroma, cytosol, peroxisomes, and apoplast. Mn-SOD is expressed in mitochondria and peroxisomes but has also been detected in both apoplast and cell wall, while Fe-SOD is present to a lesser extent. However, this isoenzyme is restricted for the chloroplast stroma of certain plant species (Mittler et al., 2004).
These isoenzymes are differentiating in their sensitivity to H2O2 and KCN (Bannister et al., 1987). Genes coding SOD are sensitive to environmental stressors, and increased activity is usually correlated with increased plant tolerance for environmental stress (Sharma et al., 2012). SOD acts as the first level of protection against ROS, transforming O2•− into H2O2, and APX, GPx, and CAT further detoxify the resulting H2O2 (Apel and Hirt, 2004). The knock-down mutants of chloroplast Cu/Zn-SOD show suppressed growth, reduced size of chloroplasts, and impaired photosynthetic activity (Rizhsky et al., 2003).
Catalase and Peroxidase
The intracellular level of H2O2 is regulated by several enzymes, the foremost important of which are catalases (CATs) and peroxidases participating within the fine regulation of ROS concentration through the cell (Baby and Jini, 2010). Considering cellular compartmentalization, CATs might be found in large quantities in peroxisomes, whereas this enzyme has not been found in chloroplasts. It captures the H2O2 created in peroxisomes during the process of photorespiration and β-oxidation of fatty acids. The catalases contain four heme subunits with Fe2+ ions undergoing oxidation and catalyze the dissociation of two H2O2 molecules into water and oxygen (Arora et al., 2002).
Catalases are very efficient in H2O2 removal with a unique ability to convert two H2O2 molecules into water and molecular oxygen with no need for reduction equivalent. Precisely, this happens via oxidation of Fe2+ ion in heme, after which Fe2+ is reduced by reaction with H2O2. The Km value for CAT is within the millimolar range, which may be a far higher concentration of H2O2 within the cell than physiological, implying its role predominantly under stress conditions (Černý et al., 2018). On the other hand, CATs express low activity against organic peroxides. Comparing with peroxidases, they have higher Km value for H2O2 hence peroxidases could remove H2O2 albeit present in low concentrations (Sharma et al., 2012). On the other hand, peroxidases-driven reactions are using low relative molecular mass antioxidants, GSH and Asc as electron donors, so removal of H2O2 using this pathway may be a very energy-consuming reaction for cell since it utilizes important molecules from the cell environment: two GSH molecules are consumed for removal of one H2O2 molecule (Kohen and Nyska, 2002). Regarding the aminoalkanoic acid sequence, peroxidases are divided into three classes (Welinder, 1992). The APX is assessed as a first-class and differs from the class III peroxidase-like well-known horseradish peroxidase (HRP). The importance of APX within a plant cell is indicated by the very fact that APX isoenzymes are distributed in as many as five cell compartments: stroma (sAPX) and thylakoids (tAPX) in chloroplasts, microbodies (including glyoxysomes and peroxisomes; mAPX), cytosol (cAPX), and mitochondria (mitAPX, as a membrane-bound form; Chen and Asada, 1987; Jimenez et al., 1997; De Leonardis et al., 2000). Mutants with tAPX deficiency are considered lethal while plants with overexpressed tAPX are tolerant of methyl viologen (paraquat) induced stress (Yabuta et al., 2002). Also, overexpression of cytosolic APX in chloroplasts led to the rise of plant’s tolerance to drought and high salinity (Badawi et al., 2004), whereas knock-out mutants have compromised chloroplast scavenging system and increased H2O2 accumulation in leaves (Davletova et al., 2005). However, APX isoenzymes aren’t so effective in reducing lipid hydroperoxides. A salient feature of APX, especially chloroplast, is its sensitivity to oxidative inactivation within the absence of Asc. At low concentrations of Asc (less than 20 mmol/L), chloroplast APX activity is rapidly decreased in the presence of H2O2, with an inactivation half-life of the 30 s. In contrast, cytosolic and peroxisomal isoforms lose their activity after quite 1 h (Miyake and Asada, 1996). Depletion of chloroplast Asc and inactivation of chloroplast APX are considered for limitations of photosynthetic efficiency under stress conditions and thus potential targets for improvement (Ishikawa and Shigeoka, 2008). The sole H2O2-scavenging enzyme within the apoplastic and vacuolar space of all plant organs is class III peroxidase. They catalyze the oxidation of different substrates with H2O2 acting as an electron acceptor, consequently generating radicals (mainly phenolic), and such reaction is described as Peroxidase/Phenolics/Ascorbate (PPA) system (Takahama, 2004). Induced in response to various environmental stresses, this family of isoenzymes has an imperative role in cross-talk between primary and secondary antioxidants (Veljović-Jovanović et al., 2018).
Glutathione peroxidases, which even have strong activity against H2O2, could use both GSH and TRX as reducing substrates and will eliminate lipid peroxides additionally to H2O2 (Herbette et al., 2002). Besides their role in H2O2 neutralization, CAT and GPx together with SOD enzyme show a synergistic effect in O2•− elimination.
Non-enzymatic Antioxidants
Ascorbic Acid
Ascorbate is taken into account a potent antioxidant thanks to its ability to donate electrons in an exceedingly wide selection of enzymatic and non-enzymatic reactions. It is especially present within the leaves and in higher concentration compared to GSH (Das and Roychoudhury, 2014). Under physiological conditions, within the sort of monoanionic, Asc mainly exists in its reduced form (90%) in chloroplasts, with the remainder within the sort of DHA which lacks antioxidative activity (Yachandra et al., 1996; Noctor and Foyer, 1998). Ascorbate could donate two electrons, whereby donation of one is followed by the assembly of semidehydroascorbate or ascorbate, and donation of the second electron is related to DHA production. It might be regenerated by DHAR utilizing two GSH molecules.
Ascorbate occurs in all subcellular compartments including the cell wall except for vacuoles where is present in low concentrations (Das and Roychoudhury, 2014). Nevertheless, the bulk of Asc is found within the cytoplasm, but unlike other soluble antioxidants, a substantial portion is exported to the apoplast where it is present at millimolar concentrations and is taken into account to be the primary line of defense against potentially harmful external prooxidants. Chloroplast’s Asc is also a cofactor for violaxanthin de-epoxidase where it participates within the production of xanthophylls which are directly involved in quenching of excessive excitatory energy on PS II (Davey et al., 2000).
Ascorbate, as quantitatively dominant antioxidant in plant cells, is found altogether subcellular compartments including the apoplast with a mean concentration of 2–25 mmol/L or more within the chloroplast stroma (Foyer and Lelandais, 1996). Intracellular concentrations are up to millimolar range (i.e., 20 mmol/L within the cytosol and 20–300 mmol/L in chloroplast stroma; Larson, 1988). Asc can directly capture •OH, O2•−, and 1O2 and also to reduce H2O2 to water via an ascorbate peroxidase reaction (Noctor and Foyer, 1998). However, Asc could acts as prooxidant reducing Fe, Cu, and Mn ions and consequently providing a chance to re-engage in one among the redox reactions.
Glutathione
The tripeptide, γ-glutamyl-cysteinyl glycine, the foremost abundant low relative molecular mass thiol within the cell, has been found in large quantities in every cell compartment: cytosol, chloroplast, endoplasmic reticulum, vacuoles, and mitochondria. It is not only specific to plant cells but also plays a really important role as a redox buffer amid Asc (Liu and Li, 2019).
The reduced form of glutathione, GSH, may be a major sulfur depo form and plays important roles in various biological processes, including cellular growth, development, regulation of sulfur transport, signal transduction, protein and nucleic acid synthesis, phytochelatin synthesis for metal chelation, xenobiotic detoxification, and expression of genes liable for stress (Bartoli et al., 2017). Besides, GSH is synthesized both in chloroplasts and within the cytosol. It can chelate Cu2+ ions and cease them from participating in the Haber-Weiss reaction (Zlobin et al., 2017).
Together with its oxidized form, GSSG, reduced glutathione maintains redox balance within the cell. The cysteine residue in the molecule center is liable for the high reduction potential of GSH. As low relative molecular mass antioxidants, GSH could scavenge H2O2, or react non-enzymatically with 1O2, O2•−, and •OH (Krasnovsky, 1998). However, the main role of GSH as an antioxidant is its ability to regenerate another potent hydrophilic antioxidant, ascorbic acid, precisely through the Asc-GSH cycle. GSH helps to recycle oxidized Asc to the reduced state employing DHAR. GSH also can reduce DHA non-enzymatically at pH > 7 and GSH concentrations >1 mmol/l (Sharma et al., 2012).
Carotenoids
Carotenoids, such as lycopene, β-carotene, xanthophyll, lutein, and zeaxanthin, are lipophilic antioxidants capable of detoxifying various ROS and most effectively capture the lipid peroxyl radical (LOO•), thus providing membrane protection. Carotenoids react with LOO• and form lipid hydroperoxide (LOOH) and a carotenoid radical which will be regenerated by tocopherol, and both tocopherol and carotenoid radicals might be reduced by Asc subsequently (Yachandra et al., 1996).
Present in plants, they may capture 3Chl, 1O2, also as excited chlorophyll (Chl∗) to guard the photosynthetic apparatus. Hence, β-carotene captures 1O2 with greater efficiency compared to α-tocopherol (Kehrer, 2000; Sharma et al., 2012). The conjugated double bond system owned by carotenoids provides easy absorption of energy from the excited molecule and dissipation of excess within the sort of heat. For instance, zeaxanthin is involved within the non-photochemical quenching of excess excitatory energy at PS II (Asada, 1999).
Tocopherols and Tocotrienols
Tocopherols and tocotrienols are essential components of the cell membrane where they express both antioxidant and non-antioxidant functions. There are four tocopherol and tocotrienol isomers (α, β, γ, and δ). Tocopherols are a gaggle of lipophilic antioxidants and are synthesized by photosynthetic organisms and present in green, photosynthetically active parts of the plant only. The antioxidant activity of tocopherol is predicated on the electron donor properties of the chromanol ring.
These antioxidants protect lipids and other membrane components by physically trapping and chemically reacting with 1O2 in chloroplasts, preserving the structure and performance of PS II. The method of 1O2 capture is extremely efficient and it has been estimated that 1 α-tocopherol molecule can neutralize up to 220 molecules of 1O2 in vitro before its degradation (Sharma et al., 2012). However, their rate is two times lower compared with β-carotene and they are not effective in capturing •OH and alkoxy radicals (RO•) in vivo (Kohen and Nyska, 2002; Blokhina et al., 2003). Regeneration of oxidized tocopherol might be achieved via Asc, GSH, or ubiquinone.
The relative antioxidant activity of the isomers in vivo corresponds to the subsequent order α > β > γ > δ due to the methylation pattern and the number of methyl groups added to the polar head of the phenolic ring. Also, α-tocopherol, with its three methyl substituents, has the very best antioxidant activity. However, α-tocotrienol has been shown to possess better antioxidant activity than α-tocopherol within the membrane environment. The chloroplast membrane contains predominantly α isomer of tocopherol; therefore, they are well protected from photooxidative damage (Smirnoff, 2000). Furthermore, α-tocopherol is the main form within the leaves, while γ-tocopherol is within the seed.
Vitamin E (collective term for tocopherols and tocotrienols) has the potential for regenerating lipid peroxyl, alkyl, and alkoxy radicals formed during the polyunsaturated fatty acids oxidation whereby directly prevent a sequence propagation during auto-oxidation of the lipid layer. By donating hydrogen atoms to the radical, vitamin E becomes tocopherol radical which is resonantly stabilized and not sufficiently reactive for independent initiation of membrane peroxidation, which is additionally a basic criterion for good antioxidants (Kohen and Nyska, 2002). Also, tocopherol present in high concentrations acts as prooxidant alongside transition metal ions and lipid peroxides.
Phenolic Compounds
Phenols are a multifarious group of secondary metabolites (flavonoids, tannins, hydroxycinnamate esters, lignin, etc.) present in plant tissue (Sakakibara et al., 2003). The antioxidant capacity of phenols is related to their structure (aromatic ring with –OH or –OCH3 substituents) very suitable for trapping free radicals. They have a robust capacity to donate an electron or hydrogen atom also because the ability to rapidly stabilize formed phenol radical and have shown greater activity compared to Asc and α-tocopherol in in vitro system. Phenols containing o-dihydroxy groups within their structure can complex metal ions and prevent the formation of ROS in the Haber-Weiss reaction (Fenton, 1984; Rice-Evans et al., 1996). Furthermore, they will directly capture 1O2 and inhibit lipid peroxidation by trapping lipid alkoxy radicals (Sharma et al., 2012). Another mechanism associated with antioxidant properties of phenols is the ability to modify the kinetics of peroxidation by altering the lipid package and reducing membrane fluidity. These changes can limit the diffusion of free radicals and reduce the peroxidation reaction. It has also been shown that phenolic compounds could be involved within the H2O2 capture cascade (Takahama and Oniki, 1992). Increased accumulation of phenolic compounds under biotic also as abiotic stress has been demonstrated and certain anthocyanins and flavanols have up to fourfold higher antioxidative activity than Asc (Rice-Evans et al., 1996).
The cooperation between Asc and phenols has been shown within the hydrogen-peroxide-peroxidase system which takes place in vacuole where H2O2 diffuses and may be reduced by peroxidases, and phenols are used because of the primary electron donors. Both Asc and MDA• radicals can reduce the phenoxy radical. If Asc regeneration takes place within the cytosol and Asc is delivered back to the vacuole, the peroxidase/phenols/Asc system can operate within the vacuole and capture H2O2. This mechanism is restricted to plant tissue and enhances plant tolerance during oxidative stress (Krasnovsky, 1998). The MDAR enzyme has also been shown to be capable of reducing the phenoxy radical, like quercetin radical, to phenol (Sakihama et al., 2000).
Conclusion and Outlooks
Accordingly, available data indicate that ROS detoxification pathways are not present to the extent they might remove all ROS from the cellular environment, but that there is a level of coordination between the processes which generate ROS and those which remove them, therefore, maintaining the optimal amount of ROS within the cellular environment.
A really popular trend for testing AOSs is the use of genetically transformed plants, with overexpressed or removed a selected component of AOS, as well as the application of artificial environmental conditions to cause oxidative stress. Extensive literature indicates that enhancing the expression of certain enzymes likes SOD, GR, and DHAR, utilizing gene-splicing, can improve plant tolerance to abiotic stress. Certainly, the enhancement of chloroplast antioxidative protection has been proven to be one among the foremost effective pathways for shielding plant cells from abiotic stress.
In addition to the fact that antioxidants are essential for plant’s subsistence, they may benefit humans and well. This claim has been supported by plenty of antioxidant formulations offered and available to us in markets. The main constituents of those formulations are principally plant’s extracts containing biologically active compounds well-known for some favorable effect. For instance, it has been investigated that natural compounds could help in the prevention of neurodegenerative diseases for instance. Also, the fact that many of those antioxidants cannot be synthesized within human cells due to lack of enzymes in the first place, qualify them as essential nutrients for our population.
Extensive research is being conducted to investigate natural compounds which may curb or alleviate oxidative stress and thereupon empower the immune system and nowadays, we have a growing number of plant-based nutrition supporters. The last decade is supported by investigations of potentially beneficial mild prooxidant effects. Namely, moderate-dose exposure to noxious agents or factors induces an adaptive response of cells termed as hormesis. Overall, although six decades-long, this multiplex field of research is still dynamic and subject to evolve due to acquiring deeper insights and new knowledge of this intricate network of molecules and their reactions. Although particular antioxidant compounds express extraordinary antioxidant capacity in vitro, more challengeable and complex in vivo studies, which will perfectly simulate an intracellular environment with the presence of an orchestrated network of pro‐ and antioxidants should be conducted.
Author Contributions
JD and VJ: conceptualization, investigation, resources, and writing – original draft preparation and visualization. VJ, EN, MN, and KK: validation. MN and VJ: formal analysis, writing – review, and editing. KK: supervision and funding acquisition. EN: project administration. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the financial support of the University of Hradec Kralove (Faculty of Science, VT2019-2021) and Excellence project Prf-2206, the University of Hradec Kralove, Hradec Kralove, Czechia. This work is also supported by the Medical Faculty of the Military Medical Academy, University of Defense, Belgrade, Republic of Serbia (MFVMA/04/20-22).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Allen, J. F. (2009). Why chloroplasts and mitochondria retain their own genomes and genetic systems: collocation for redox regulation of gene expression. Proc. Natl. Acad. Sci. U. S. A. 112, 10231–10238. doi: 10.1073/pnas.1500012112
Almagro, L., Gómez Ros, L. V., Belchi-Navarro, S., Bru, R., Ros Barceló, A., and Pedreño, M. A. (2008). Class III peroxidases in plant defence reactions. J. Exp. Bot. 60, 377–390. doi: 10.1093/jxb/ern277
Apel, K., and Hirt, H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373–399. doi: 10.1146/annurev.arplant.55.031903.141701
Arora, A., Sairam, R. K., and Srivastava, G. C. (2002). Oxidative stress and antioxidative system in plants. Curr. Sci. 82, 1227–1238.
Asada, K. (1999). The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Plant Biol. Ann. Rev. 50, 601–639. doi: 10.1146/annurev.arplant.50.1.601
Baby, J., and Jini, D. (2010). Insight into the role of antioxidant enzymes for salt tolerance in plants. Int. J. Bot. 6, 456–464. doi: 10.3923/ijb.2010.456.464
Badawi, G. H., Kawano, N., Yamauchi, Y., Shimada, E., Sasaki, R., Kubo, A., et al. (2004). Over-expression of ascorbate peroxidase in tobacco chloroplasts enhances tolerance to salt stress and water deficit. Physiol. Plant. 121, 231–238. doi: 10.1111/j.0031-9317.2004.00308.x
Bannister, J. V., Bannister, W. H., and Rotilio, G. (1987). Aspects of the structure, function, and applications of superoxide dismutase. Crit. Rev. Biochem. 22, 111–180. doi: 10.3109/10409238709083738
Bartoli, C. G., Buet, A., Grozeff, G. G., Galatro, A., and Simontacchi, M. (2017). “Ascorbate-glutathione cycle and abiotic stress tolerance in plants” in Ascorbic acid in plant growth, development and stress tolerance. eds. M. A. Hossain, S. Munné-Bosch, D. J. Burritt, P. Diaz-Vivancos, M. Fujita, and A. Lorence (Switzerland: Springer), 177–200.
Bhattacharjee, S. (2005). Reactive oxygen species and oxidative burst: roles in stress, senescence and signal transduction in plants. Curr. Sci. 89, 1113–1121.
Birben, E., Sahiner, U. M., Sackesen, C., Erzurum, S., and Kalayci, O. (2012). Oxidative stress and antioxidant defence. World Allergy Organ. J. 5, 9–19. doi: 10.1097/WOX.0b013e3182439613
Blokhina, O., Virolainen, E., and Fagerstedt, K. V. (2003). Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann. Bot. 91, 179–194. doi: 10.1093/aob/mcf118
Bowler, C., Montagu, M. V., and Inzé, D. (1992). Superoxide dismutase and stress tolerance. Plant Biol. Ann. Rev. 43, 83–116. doi: 10.1146/annurev.pp.43.060192.000503
Černý, M., Habánová, H., Berka, M., Luklová, M., and Brzobohatý, B. (2018). Hydrogen peroxide: its role in plant biology and crosstalk with signalling networks. Int. J. Mol. Sci. 19:E2812. doi: 10.3390/ijms19092812
Cheeseman, J. M. (2006). Hydrogen peroxide concentrations and leaves under natural conditions. J. Exp. Bot. 57, 2435–2444. doi: 10.1093/jxb/erl004
Chen, G. X., and Asada, K. (1987). Ascorbate peroxidase in tea leaves: occurrence of two isozymes and differences in their enzymatic and molecular properties. Plant Cell Physiol. 30, 987–998. doi: 10.1093/oxfordjournals.pcp.a077844
Das, K., and Roychoudhury, A. (2014). Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2:53. doi: 10.3389/fenvs.2014.00053
Davey, M. W., Montagu, M. V., Inze, D., Sanmartin, M., Kanellis, A., Smirnoff, N., et al. (2000). Plant L-ascorbic acid: chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 80, 825–860. doi: 10.1002/(SICI)1097-0010(20000515)80:7<825::AID-JSFA598>3.0.CO;2-6
Davletova, S., Rizhsky, L., Liang, H., Shengqiang, Z., Oliver, D. J., Coutu, J., et al. (2005). Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 17, 268–281. doi: 10.1105/tpc.104.026971
De Leonardis, S., Dipierro, N., and Dipierro, S. (2000). Purification and characterization of an ascorbate peroxidase from potato tuber mitochondria. Plant Physiol. Biochem. 38, 773–779. doi: 10.1016/S0981-9428(00)01188-8
Diaz Vivancos, P., Dong, Y., Ziegler, K., Markovic, J., Pallardó, F. V., Pellny, T. K., et al. (2010a). Recruitment of glutathione into the nucleus during cell proliferation adjusts whole-cell redox homeostasis in Arabidopsis thaliana and lowers the oxidative defence shield: recruitment of GSH into the nucleus. Plant J. 64, 825–838. doi: 10.1111/j.1365-313X.2010.04371.x
Diaz Vivancos, P., Wolff, T., Markovic, J., Pallardó, F. V., and Foyer, C. H. (2010b). A nuclear glutathione cycle within the cell cycle. Biochem. J. 431, 169–178. doi: 10.1042/BJ20100409
Diebold, L., and Chandel, N. S. (2016). Mitochondrial ROS regulation of proliferating cells. Free Radic. Biol. Med. 100, 86–93. doi: 10.1016/j.freeradbiomed.2016.04.198
Dietz, K. J. (2011). Peroxiredoxins in plants and cyanobacteria. Antioxid. Redox Signal. 15, 1129–1159. doi: 10.1089/ars.2010.3657
Dumont, S., and Rivoal, J. (2019). Consequences of oxidative stress on plant glycolytic and respiratory metabolism. Front. Plant Sci. 10:166. doi: 10.3389/fpls.2019.00166
Elstner, E. F. (1982). Oxygen activation and oxygen toxicity. Annu. Rev. Plant Biol. 33, 73–96. doi: 10.1146/annurev.pp.33.060182.000445
Exposito-Rodriguez, M., Laissue, P. P., Yvon-Durocher, G., Smirnoff, N., and Mullineaux, P. M. (2017). Photosynthesis-dependent H2O2 transfer from chloroplasts to nuclei provides a high-light signalling mechanism. Nat. Commun. 8:49. doi: 10.1038/s41467-017-00074-w
Fenton, H. J. H. (1984). Oxidation of tartaric acid in the presence of iron. J. Chem. Soc. Trans. 65, 899–910. doi: 10.1039/ct8946500899
Foyer, C. H., and Halliwell, B. (1976). The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133, 21–25. doi: 10.1007/BF00386001
Foyer, C. H., and Lelandais, M. (1996). A comparison of the relative rates of ascorbate and glucose transport across the thylakoid, chloroplast, and plasmalemma membranes of pea leaf mesophyll cells. J. Plant Physiol. 148, 391–398. doi: 10.1016/S0176-1617(96)80271-9
Foyer, C. H., and Noctor, G. (2016). Stress-triggered redox signalling: what’s in pROSpect? Plant Cell Environ. 39, 951–964. doi: 10.1111/pce.12621
Foyer, C. H., and Shigeoka, S. (2011). Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 155, 93–100. doi: 10.1104/pp.110.166181
Gill, S. S., and Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48, 909–930. doi: 10.1016/j.plaphy.2010.08.016
Gupta, R., and Chakrabarty, S. K. (2013). Gibberellic acid in plant: still a mystery unresolved. Plant Signal. Behav. 8:e25504. doi: 10.4161/psb.25504
Halliwell, B., and Gutteridge, J. M. (2015). Free radicals in biology and medicine. New York: Oxford University Press.
Han, Y., Mhamdi, A., Chaouch, S., and Noctor, G. (2013). Regulation of basal and oxidative stress-triggered jasmonic acid-related gene expression by glutathione. Plant Cell Environ. 36, 1135–1146. doi: 10.1111/pce.12048
Herbette, S., Lenne, C., Leblanc, N., Julien, J. L., Drevet, J. R., and Roeckel-Drevet, P. (2002). Two GPX-like proteins from Lycopersicon esculentum and Helianthus annuus are antioxidant enzymes with phospholipid hydroperoxide glutathione peroxidase and thioredoxin peroxidase activities. Eur. J. Biochem. 269, 2414–2420. doi: 10.1046/j.1432-1033.2002.02905.x
Huang, H., Ullah, F., Zhou, D. -X., Yi, M., and Zhao, Y. (2019). Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 10:800. doi: 10.3389/fpls.2019.00800
Ishibashi, Y., Tawaratsumida, T., Kondo, K., Kasa, S., Sakamoto, M., Aoki, N., et al. (2012). Reactive oxygen species are involved in gibberellin/abscisic acid signaling in barley aleurone cells. Plant Physiol. 158, 1705–1714. doi: 10.1104/pp.111.192740
Ishikawa, T., and Shigeoka, S. (2008). Recent advances in ascorbate biosynthesis and the physiological significance of ascorbate peroxidase in photosynthesizing organisms. Biosci. Biotechnol. Biochem. 72, 1143–1154. doi: 10.1271/bbb.80062
Jia, L., Xu, W., Li, W., Ye, N., Liu, R., Shi, L., et al. (2013). Class III peroxidases are activated in proanthocyanidin-deficient Arabidopsis thaliana seeds. Ann. Bot. 111, 839–847. doi: 10.1093/aob/mct045
Jimenez, A., Hernandez, J. A., del Río, L. A., and Sevilla, F. (1997). Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol. 114, 275–284. doi: 10.1104/pp.114.1.275
Jones, M., and Smirnoff, N. (2005). “Reactive oxygen species in plant development and pathogen defence” in Antioxidants and reactive oxygen species in plants. ed. N. Smirnoff (United Kingdom: Wiley Bleckwell), 197–214.
Kehrer, J. P. (2000). The Haber–Weiss reaction and mechanisms of toxicity. Toxicology 149, 43–50. doi: 10.1016/s0300-483x(00)00231-6
Kohen, R., and Nyska, A. (2002). Invited review: oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 30, 620–650. doi: 10.1080/01926230290166724
Krasnovsky, J. A. (1998). Singlet molecular oxygen in photo biochemical systems: IR phosphorescence studies. Memb. Cell Biol. 12, 665–690.
Krieger-Liszkay, A. (2005). Singlet oxygen production in photosynthesis. J. Exp. Bot. 56, 337–346. doi: 10.1093/jxb/erh237
Lewis, N. G., and Yamamoto, E. (1990). Lignin: occurrence, biogenesis and biodegradation. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 41, 455–496. doi: 10.1146/annurev.pp.41.060190.002323
Liu, L., and Li, J. (2019). Communications between the endoplasmic reticulum and other organelles during abiotic stress response in plants. Front. Plant Sci. 10:749. doi: 10.3389/fpls.2019.00749
Lushchak, V. I. (2014). Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 224, 164–175. doi: 10.1016/j.cbi.2014.10.016
Malik, S. I., Hussain, A., Yun, B. W., Spoel, S. H., and Loake, G. J. (2011). GSNOR-mediated de-nitrosylation in the plant defence response. Plant Sci. 181, 540–544. doi: 10.1016/j.plantsci.2011.04.004
Mehler, H., and Brown, H. (1956). Studies on reactions of illuminated chloroplasts III. Simultaneous photoproduction and consumption of oxygen studied with oxygen isotopes. Arch. Biochem. Biophys. 38, 365–370. doi: 10.1016/0003-9861(52)90042-8
Mhamdi, A., Hager, J., Chaouch, S., Queval, G., Han, Y., Taconnat, Y., et al. (2010). Arabidopsis glutathione Reductase 1 is essential for the metabolism of intracellular H2O2 and to enable appropriate gene expression through both salicylic acid and jasmonic acid signalling pathways. Plant Physiol. 153, 1144–1160. doi: 10.1104/pp.110.153767
Mhamdi, A., Noctor, G., and Baker, A. (2012). Plant catalases: peroxisomal redox guardians. Arch. Biochem. Biophys. 525, 181–194. doi: 10.1016/j.abb.2012.04.015
Mittler, R., Vanderauwera, S., Gollery, M., and Van Breusegem, F. (2004). Reactive oxygen gene network of plants. Trends Plant Sci. 9, 490–498. doi: 10.1016/j.tplants.2004.08.009
Miyake, C., and Asada, K. (1996). Inactivation mechanism of ascorbate peroxidase at low concentrations of ascorbate; hydrogen peroxide decomposes compound I of ascorbate peroxidase. Plant Cell Physiol. 37, 423–430. doi: 10.1093/oxfordjournals.pcp.a028963
Morales, M., and Munné-Bosch, S. (2016). Oxidative stress: a master regulator of plant trade-offs? Trends Plant Sci. 21, 996–999. doi: 10.1016/j.tplants.2016.09.002
Moural, T. W., Lewis, K. M., Barnaba, C., Zhu, F., Palmer, N. A., Sarath, G., et al. (2017). Characterization of class III peroxidases from switchgrass. Plant Physiol. 173, 417–433. doi: 10.1104/pp.16.01426
Nimse, S. B., and Pal, D. (2015). Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 5, 27986–28006. doi: 10.1039/C4RA13315C
Noctor, G., and Foyer, C. H. (1998). Ascorbate and glutathione: keeping active oxygen under control. Plant Biol. Ann. Rev. 49, 249–279. doi: 10.1146/annurev.arplant.49.1.249
Noctor, G., and Foyer, C. H. (2016). Intracellular redox compartmentation and ROS-related communication in regulation and signalling. Plant Physiol. 171, 1581–1592. doi: 10.1104/pp.16.00346
Noctor, G., Reichheld, J. P., and Foyer, C. H. (2018). ROS-related redox regulation and signalling in plants. Semin. Cell Dev. Biol. 80, 3–12. doi: 10.1016/j.semcdb.2017.07.013
Pandey, V. P., Awasthi, M., Singh, S., Tiwari, S., and Dwivedi, U. N. (2017). A comprehensive review on the function and application of plant peroxidases. Biochem. Anal. Biochem. 6:308. doi: 10.4172/2161-1009.1000308
Pulido, P., Cazalis, R., and Cejudo, F. J. (2009). An antioxidant redox system in the nucleus of wheat seed cells suffering oxidative stress. Plant J. 57, 132–145. doi: 10.1111/j.1365-313X.2008.03675.x
Queval, G., Jaillard, D., Zechmann, B., and Noctor, G. (2011). Increased intracellular H2O2 availability preferentially drives glutathione accumulation in vacuoles and chloroplasts. Plant Cell Environ. 34, 21–32. doi: 10.1111/j.1365-3040.2010.02222.x
Reczek, C. R., and Chandel, N. S. (2015). ROS-dependent signal transduction. Curr. Opin. Cell Biol. 33, 8–13. doi: 10.1016/j.ceb.2014.09.010
Rhoads, D. M., Umbach, A. L., Subbaiah, C. C., and Siedow, J. N. (2006). Mitochondrial reactive oxygen species. Contribution to oxidative stress and interorganellar signaling. Plant Physiol. 141, 357–366. doi: 10.1104/pp.106.079129
Rice-Evans, C. A., Miller, N. J., and Paganga, G. (1996). Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 20, 933–956. doi: 10.1016/0891-5849(95)02227-9
Rizhsky, L., Liang, H., and Mittler, R. (2003). The water-water cycle is essential for chloroplast protection in the absence of stress. J. Biol. Chem. 278, 38921–38925. doi: 10.1074/jbc.M304987200
Sakakibara, H., Honda, Y., Nakagawa, S., Ashida, H., and Kanazawa, K. (2003). Simultaneous determination of all polyphenols in vegetables, fruits, and teas. J. Agric. Food Chem. 51, 571–581. doi: 10.1021/jf020926l
Sakihama, Y., Mano, J. I., Sano, S., Asada, K., and Yamasaki, H. (2000). Reduction of phenoxyl radicals mediated by monodehydroascorbate reductase. Biochem. Biophys. Res. Commun. 279, 949–954. doi: 10.1006/bbrc.2000.4053
Salehi, B., Azzini, E., Zucca, P., Maria Varoni, E. V., Anil Kumar, N., Dini, L., et al. (2020). Plant-derived bioactives and oxidative stress-related disorders: a key trend towards healthy aging and longevity promotion. Appl. Sci. 10:947. doi: 10.3390/app10030947
Scheibe, R., Backhausen, J. E., Emmerlich, V., and Holtgrefe, S. (2005). Strategies to maintain redox homeostasis during photosynthesis under changing conditions. J. Exp. Bot. 56, 1481–1489. doi: 10.1093/jxb/eri181
Schieber, M., and Chandel, N. S. (2014). ROS function in redox signalling and oxidative stress. Curr. Biol. 24, R453–R462. doi: 10.1016/j.cub.2014.03.034
Sharma, P., Jha, A. B., Dubey, R. S., and Pessarakli, M. (2012). Reactive oxygen species, oxidative damage, and antioxidant defence mechanism in plants under stressful conditions. J. Bot. 2012:217037. doi: 10.1155/2012/217037
Sies, H. (2018). On the history of oxidative stress: concept and some aspects of current development. Curr. Opin. Toxicol. 7, 122–126. doi: 10.1016/j.cotox.2018.01.002
Sies, H., Berndt, C., and Jones, D. P. (2017). Oxidative stress. Ann. Rev. Boichem. 86, 715–748. doi: 10.1146/annurev-biochem-061516-045037
Sies, H., and Cadenas, E. (1985). Oxidative stress: damage to intact cells and organs. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 311, 617–631. doi: 10.1098/rstb.1985.0168
Smirnoff, N. (2000). Ascorbic acid: metabolism and functions of a multi-facetted molecule. Curr. Opin. Plant Biol. 3, 229–235.
Suzuki, N., Miller, G., Morales, J., Shulaev, V., Torres, M. A., and Mittler, R. (2011). Respiratory burst oxidases: the engines of ROS signalling. Curr. Opin. Plant Biol. 14, 691–699. doi: 10.1016/j.pbi.2011.07.014
Swanson, S., and Gilroy, S. (2010). ROS and plant development. Physiol. Plant. 138, 384–392. doi: 10.1111/j.1399-3054.2009.01313.x
Takahama, U. (2004). Oxidation of vacuolar and apoplastic phenolic substrates by peroxidase: the physiological significance of the oxidation reactions. Phytochem. Rev. 3, 207–219. doi: 10.1023/B:PHYT.0000047805.08470.e3
Takahama, U., and Oniki, T. (1992). Regulation of peroxidase-dependent oxidation of phenolics in the apoplast of spinach leaves by ascorbate. Plant Cell Physiol. 33, 379–387. doi: 10.1093/oxfordjournals.pcp.a078265
Talaat, N. B., Hasanuzzaman, M., Fotopoulos, V., Nahar, K., and Fujita, M. (2019). “Role of reactive oxygen species signalling in plant growth and development” in Reactive oxygen, nitrogen and sulfur species in plants. eds. H. Hasanuzzaman, V. Fotopoulos, K. Nahar, and M. Fujita (United Kingdom: Wiley Blackwell), 225–266.
Trebst, A. (2003). Function of β-carotene and tocopherol in photosystem II. Z. Naturforsch C. J. Biosci. 58, 609–620. doi: 10.1515/znc-2003-9-1001
Triantaphylidès, C., and Havaux, M. (2009). Singlet oxygen in plants: production, detoxification and signaling. Trends Plant Sci. 14, 219–228. doi: 10.1016/j.tplants.2009.01.008
Truong, T. H., and Carroll, K. S. (2013). Redox regulation of protein kinases. Crit. Rev. Biochem. Mol. Biol. 48, 332–356. doi: 10.3109/10409238.2013.790873
Veljović-Jovanović, S., Kukavica, B., Vidović, M., Morina, F., and Menckhoff, L. J. (2018). “Class III peroxidases: functions, localization and redox regulation of isoenzymes” in Antioxidants and antioxidant enzymes in higher plants. eds. D. K. Gupta, J. M. Palma, and F. J. Corpas (Switzerland: Springer Nature), 269–300.
Wachter, A., Wolf, S., Steiniger, H., Bogs, J., and Rausch, T. (2005). Differential targeting of GSH1 and GSH2 are achieved by multiple transcription initiation: implications for the compartmentation of glutathione biosynthesis in the Brassicaceae. Plant J. 41, 15–30. doi: 10.1111/j.1365-313X.2004.02269.x
Welinder, K. G. (1992). Superfamily of plant, fungal and bacterial peroxidases. Curr. Opin. Struct. Biol. 2, 388–393. doi: 10.1016/0959-440X(92)90230-5
Xiulan, X., Zhouqing, H., Nifan, C., Zizhong, T., Qiang, W., and Yi, C. (2019). The roles of environmental factors in the regulation of oxidative stress in the plant. Biomed. Res. Int. 2019:9732325. doi: 10.1155/2019/9732325
Yabuta, Y., Motoki, T., Yoshimura, K., Takeda, T., Ishikawa, T., and Shigeoka, S. (2002). Thylakoid membrane-bound ascorbate peroxidase is a limiting factor of antioxidant systems under photo-oxidative stress. Plant J. 32, 915–925. doi: 10.1046/j.1365-313x.2002.01476.x
Yachandra, V., Sauer, K., and Klein, M. (1996). Manganese cluster in photosynthesis: where plants oxidize water to dioxygen. Chem. Rev. 96, 2927–2950. doi: 10.1021/cr950052k
Keywords: oxidative stress, reactive oxygen species, antioxidative defence system, cell, plants
Citation: Dumanović J, Nepovimova E, Natić M, Kuča K and Jaćević V (2021) The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 11:552969. doi: 10.3389/fpls.2020.552969
Edited by:
Julian Eaton-Rye, University of Otago, New ZealandReviewed by:
Christine Helen Foyer, University of Birmingham, United KingdomQiang-Sheng Wu, Yangtze University, China
Copyright © 2021 Dumanović, Nepovimova, Natić, Kuča and Jaćević. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kamil Kuča, a2FtaWwua3VjYUB1aGsuY3o=; a2FtaWwua3VjYUBmbmhrLmN6; Vesna Jaćević, dl9qYWNldmljQHlhaG9vLmNvbQ==
†ORCID: Jelena Dumanović, orcid.org/0000-0002-8078-9878
Eugenie Nepovimova, orcid.org/0000-0003-0281-246X
Maja Natić, orcid.org/0000-0002-6610-297X
Kamil Kuča, orcid.org/0000-0001-9664-1109
Vesna Jaćević, orcid.org/0000-0001-5137-2638
‡These authors have contributed equally to this work
 Jelena Dumanović
Jelena Dumanović Eugenie Nepovimova
Eugenie Nepovimova Maja Natić1†
Maja Natić1† Kamil Kuča
Kamil Kuča Vesna Jaćević
Vesna Jaćević