- 1Research Unit for Plant-produced Pharmaceuticals, Chulalongkorn University, Bangkok, Thailand
- 2Department of Pharmacognosy and Pharmaceutical Botany, Faculty of Pharmaceutical Sciences, Chulalongkorn University, Bangkok, Thailand
- 3Department of Microbiology, Faculty of Science, Mahidol University, Bangkok, Thailand
The ongoing coronavirus disease 2019 (COVID-19) outbreak caused by novel zoonotic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was initially reported in Wuhan city, Hubei Province of China, in late December 2019. The rapid global spread of the virus calls for the urgent development of vaccines or therapeutics for human applications to combat the coronavirus infection. Monoclonal antibodies (mAbs) have been utilized as effective therapeutics for treating various infectious diseases. In the present study, we evaluated the feasibility of plant expression system for the rapid production of recently identified therapeutically suitable human anti-SARS-CoV-2 mAbs B38 and H4. Transient co-expression of heavy-chain and light-chain sequences of both the antibodies by using plant expression geminiviral vector resulted in rapid accumulation of assembled mAbs in Nicotiana benthamiana leaves within 4 days post-infiltration. Furthermore, both the mAbs were purified from the plant crude extracts with single-step protein A affinity column chromatography. The expression level of mAb B38 and H4 was estimated to be 4 and 35 μg/g leaf fresh weight, respectively. Both plant-produced mAbs demonstrated specific binding to receptor binding domain (RBD) of SARS-CoV-2 and exhibited efficient virus neutralization activity in vitro. To the best of our knowledge, this is the first report of functional anti-SARS-CoV-2 mAbs produced in plants, which demonstrates the ability of using a plant expression system as a suitable platform for the production of effective, safe, and affordable SARS-CoV-2 mAbs to fight against the spread of this highly infectious pathogen.
Introduction
Coronavirus (CoV) outbreaks in the last two decades cause devastating impact on the human population and global economy. The zoonotic outbreaks of severe acute respiratory syndrome coronavirus (SARS-CoV) in China and Middle East respiratory syndrome coronavirus (MERS-CoV) in Saudi Arabia on 2002–2003 and 2012, respectively are the highly pathogenic betacoronaviruses reported in the 21st century. SARS-CoV-2 has recently been added to this list, which is the causative agent for the ongoing pandemic coronavirus disease 2019 (COVID-19). SARS-CoV-2, a member of the betacoronavirus genus in the family Coronaviridae, has emerged in China in late December 2019 and causes severe fever, respiratory illness, and pneumonia (Wang et al., 2020a; Zhu et al., 2020). The virus spreads rapidly, with cases of >40 million confirmed in several countries, and has become a global public health crisis. Because of its high virulence, the virus has taken down the lives of >1 million people worldwide as of October 2020 (World Health Organization [WHO], 2020). Although many vaccine or drug candidates are in clinical trials, there are no clinically approved vaccines or targeted antiviral therapeutics available to treat coronavirus infections until now. Several efforts are in progress to develop an effective vaccine or therapeutic to prevent and treat this infection (Malla et al., 2020; Thanh Le et al., 2020).
In recent decades, monoclonal antibodies (mAbs) have been studied for its application in immunotherapies and treatments for several infectious diseases. Specific mAbs against SARS-CoV-2 are highly essential for early diagnosis and disease treatment. The potential of antibodies as efficacious anti-SARS-CoV-2 therapeutics was reported in recent studies (Cao et al., 2020; Wang et al., 2020b). mAbs specific to antigenic sites on viral surface proteins have shown therapeutic efficacy and are considered as a potential therapeutic intervention against several viral infections. Similar to SARS-CoV, the receptor binding domain (RBD) located on the surface spike (S) protein of SARS-CoV-2 binds with the functional cell receptor angiotensin-converting enzyme 2 (ACE2) in humans and mediates the viral fusion and entry into the host cells (Li et al., 2003; Shang et al., 2020). The neutralizing antibodies directed against SARS-CoV-2 mainly target the RBD of S protein, which plays an important role in virus pathogenesis and antigenicity (Poh et al., 2020; Prompetchara et al., 2020; Shanmugaraj et al., 2020c). Several research groups have characterized SARS-CoV-2 neutralizing mAbs, and more recently, two human mAbs B38 and H4 were identified by a group of researchers from China in a convalescent COVID-19 patient (Wu et al., 2020) that recognize and bind to the different RBD epitopes located on the SARS-CoV-2 virus glycoprotein, thereby preventing virus ability to bind to the host cell ACE2 receptor. These mAbs exhibited great potency and are considered as a potential prophylactic and therapeutic candidates, since they efficiently neutralize SARS-CoV-2 (Jahanshahlu and Rezaei, 2020). While these mAbs represent important candidate molecules for use as diagnostic reagents and for passive immunotherapy, effective expression platforms are needed to ensure the affordability, safety, and rapid scalability of these mAbs or any other similar candidates for clinical development.
The development of recombinant DNA technology has a major impact on biotechnology that enables the production of a wide range of biopharmaceuticals in different expression hosts. The therapeutic proteins including mAbs and their derivatives have been produced in conventional expression systems based on yeast, insect, and mammalian cells. mAbs represent a successful and fastest-growing class of biopharmaceutical products that have a greatest impact on modern medicine. Unfortunately, the mAbs produced in mammalian expression systems are prohibitively expensive, which cannot be affordable to many, especially to those who are in middle- and low-income countries (Waheed et al., 2016; Kesik-Brodacka, 2018).
In recent decades, plants are considered as a safe, cost-effective, and scalable platform for the production of recombinant proteins including vaccine antigens and mAbs (Twyman et al., 2003; Fischer and Buyel, 2020). The plant expression systems offer unique advantages compared to conventional systems in terms of low production costs, scalability, low risk of pathogen contamination, flexibility, and robustness of the system, which make them a promising platform to produce commercially valuable biopharmaceuticals (Ma et al., 2013; Tschofen et al., 2016). The importance and the role of plant-made biopharmaceuticals in the fight against SARS-CoV-2 have been quite extensively commented in recent reviews (Capell et al., 2020; Rosales-Mendoza et al., 2020; Shanmugaraj et al., 2020b). The rapid nature of plant transient expression can expedite the protein production to mitigate potential pandemics. The plant-produced recombinant mAbs have been reported as potential candidates against various viral infections including chikungunya (Iyappan et al., 2018; Hurtado et al., 2020), dengue (Dent et al., 2016), Ebola (Davey et al., 2016), HIV (Teh et al., 2014), rabies (van Dolleweerd et al., 2014), West Nile virus (He et al., 2014; Sun et al., 2017), and Zika (Diamos et al., 2019). Plant-derived recombinant proteins can be produced either by stable transformation (nucleus/chloroplast) of target gene or through transient expression via agroinfiltration. Every plant transformation technology has its own advantages, which can be chosen based on the nature of target protein or final product. However, the latest advancement of transient plant expression systems using viral vectors and agroinfiltration demonstrates the rapid production of recombinant proteins in large-scale biomanufacturing facilities within a few days, which make them a convenient platform for the production of rapid-response vaccines or therapeutics to tackle epidemic or pandemic situations (Gelvin, 2010; Holtz et al., 2015; Buyel et al., 2017; Buyel, 2018).
Here, in the present study, we explore the potential of a plant expression system for producing therapeutically suitable human anti-SARS-CoV-2 mAbs B38 and H4 in order to use as a diagnostic reagent or therapeutics. In this context, both the mAbs were efficiently expressed by using geminiviral vector and assembled in Nicotiana benthamiana leaves. The expression of recombinant mAbs was determined by Western blotting and quantified by ELISA. Further, the plant-produced mAbs was purified by single-step affinity chromatography, and antigen binding specificity was assessed. Interestingly, both the plant-produced mAbs showed neutralization efficacy but with different potency against SARS-CoV-2 in vitro. This study provides a proof of principle for the rapid production of SARS-CoV-2 therapeutic candidates in plants to tackle emergency situations, and the successful production of functional anti-SARS-CoV-2 mAbs in plants could address the safety, cost, and other economic issues related to mAb production in other production platforms.
Materials and Methods
Plant Expression Vector Construction
The institutional review board of Chulalongkorn University approved the present study protocol, and all methods were performed in accordance with the relevant guidelines and regulations. The geminiviral vector (Chen et al., 2011) used in this study was kindly provided by Hugh S. Mason, Arizona State University, United States. The amino acid sequences of the human heavy-chain (HC) and light-chain (LC) variable regions (VH and VL) of anti-SARS-CoV-2 mAb B38 and H4 were retrieved from the previous report (Wu et al., 2020). The amino acid sequence of both the antibodies was presented in the Supplementary File. Both the antibody sequences were codon-optimized to facilitate expression in N. benthamiana, and the gene sequences were synthesized (Genewiz, Suzhou, China). The synthesized VH and VL gene fragments were fused to the corresponding sequences of codon-optimized human immunoglobulin G (IgG) constant HC (CH) and constant LC (CL) regions, respectively. The synthesized VH gene was digested with XbaI and BmtI, and the VL gene was digested with XbaI and AflII and gel eluted. Similarly, the backbone of the geminiviral plant expression vector pBYR2eK2Md (pBYK-2e) containing either CH or CL gene fragments was obtained by digesting with respective enzymes. The gel purified products were then ligated with T4 DNA ligase (New England Biolabs, United Kingdom) following the manufacturer’s instructions. The murine leader sequence (MGWSCIILFLVATATGVHS) was included in the amino-terminal (N-terminal) and the retention signal peptide SEKDEL was added in the carboxyl-terminal (C-terminal) of the HC and LC sequences. The resulting plasmids were transformed into Escherichia coli strain DH10B competent cells by heat shock method and eventually moved into Agrobacterium tumefaciens (GV3101) via electroporation. Agrobacterium clones were screened by PCR using gene-specific forward primer and the vector-specific reverse primer (2e-R). The list of primers used in the study was provided in Table 1. The PCR cycling conditions were as follows: initial denaturation at 94°C for 5 min followed by 30 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 60–90 s and a final extension at 72°C for 10 min. PCR amplification was performed by using Taq DNA polymerase (Vivantis Technologies, Malaysia). The PCR products were observed on a 1% agarose gel. The confirmed Agrobacterium cells containing the recombinant plasmids were used for plant transformation.
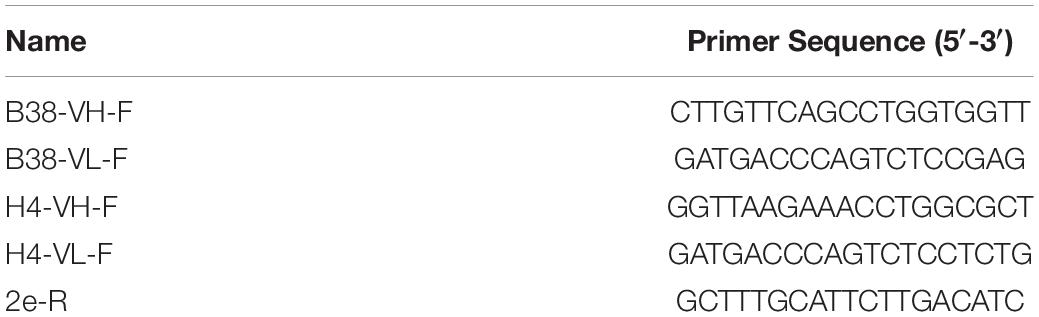
Table 1. Sequences of the oligonucleotides used for the E. coli/Agrobacterium clone confirmation by PCR.
Transient Expression of Monoclonal Antibodies B38 and H4 in N. benthamiana Leaves
Wild-type N. benthamiana plants grown under controlled conditions in the greenhouse (8-h dark/16-h light cycle) were agroinfiltrated with recombinant A. tumefaciens strain that contained either HC or LC. Briefly, A. tumefaciens harboring the plant expression vector containing either HC or LC was grown in Luria–Bertani (LB) broth supplemented with 50 mg/L kanamycin, 50 mg/L gentamycin, 50 mg/L of rifampin at 28°C overnight with continuous shaking at 200 rpm. Then, the overnight-grown culture was centrifuged at 5,000 g for 10 min at 28°C in a tabletop centrifuge, and the pellet was resuspended in the sterile infiltration buffer [10 mM MgSO4, 10 mM 2-(N-morpholino)ethanesulfonic acid (MES) pH 5.5] to get the final optical density (OD)600 of 0.2. The cell suspension containing HC and LC construct was equally mixed in a 1:1 ratio and co-infiltrated in the leaves of 6–8-week-old N. benthamiana plants by vacuum infiltration. The infiltrated plants were incubated in a controlled environment with high humidity at 28°C for 4 days. The agroinfiltrated leaves were harvested and processed for further analysis.
Protein Extraction and Purification
To purify anti-SARS-CoV-2 mAb (B38 and H4) from plants, the infiltrated plant leaves were harvested 4 days post infiltration (dpi) and purified by protein A column chromatography as described previously (Rattanapisit et al., 2019a). Briefly, the harvested leaves were homogenized with extraction buffer 1 × phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.47 mM KH2PO4) at a ratio of 1 g leaf tissue to 2 ml buffer, and the plant crude extracts were centrifuged at 26,000 g for 40 min at 4°C. After centrifugation, supernatant was collected and filtered through 0.45-μm membrane filter. Amintra protein A resin (Expedeon, United Kingdom) was packed in the column and equilibrated with 1 × PBS (pH 7.4). After equilibration, the recombinant mAbs in the filtered protein extract was loaded into a protein A bead column. The column was further washed with 15 column volumes of 1 × PBS, and the bound protein was eluted using elution buffer (0.1 M glycine, pH 2.7) and neutralized with 1.5 M Tris-HCl (pH 8.8). Following the purification, the eluted fractions were analyzed on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel stained with Coomassie brilliant blue (AppliChem, Germany) and Western blotting.
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis and Western Blotting
The plant-produced mAbs were mixed with non-reducing sample buffer [125 mM Tris-HCl pH 6.8, 12% (w/v) SDS, 10% (v/v) glycerol, 0.001% (w/v) bromophenol blue] and separated on 6%–15% polyacrylamide gel as described previously (Rattanapisit et al., 2019b). For reducing conditions, 22% (v/v) β-mercaptoethanol was added in the sample buffer and separated on 6–15% polyacrylamide gel. The protein samples were boiled at 95°C for 5 min and separated in the gel for 90 min at 100 V. All blue prestained protein standards (Bio-Rad, United States) were included in SDS-PAGE and Western blots as protein molecular weight markers. After electrophoresis, gels were stained with Coomassie brilliant blue, and the protein bands were visualized. For Western blotting, the protein in the gel was electro-transferred on to 0.45-μm nitrocellulose membrane (Bio-Rad, United States) for 90 min at 100 V. The membrane was blocked with 5% skim milk in 1 × PBS for 1 h at room temperature, washed with PBST (PBS with 0.05% Tween-20) thrice for 5 min each, and probed with either anti-human gamma antibody (The Binding Site, United Kingdom) or anti-human kappa antibody conjugated with horseradish peroxidase (HRP) (Southern Biotech, United States) diluted 1:5,000 in 3% skim milk in 1 × PBS. After incubation for 2 h, the plates were washed with PBST thrice, and the bound antibody was detected by chemiluminescence using ECL plus detection reagent (GE Healthcare, United Kingdom) in accordance with the manufacturer’s procedures.
Monoclonal Antibody Expression Level Quantification by ELISA
The expression level of plant-produced mAbs was determined by ELISA using commercially available human IgG1 kappa isotype antibody (Abcam, United Kingdom) as standard. Technical triplicates were assayed for all the samples and standard. Briefly, the 96-well plates were coated with anti-human IgG-Fc fragment specific (Abcam, United Kingdom) and incubated overnight at 4°C. Then, the plates were washed with PBST for three times and blocked with 5% skim milk powder in 1 × PBS for 2 h at 37°C. After being washed with PBST, the dilutions of plant crude extracts and standard were added to the plate. After 2-h incubation, the plate was washed and HRP-conjugated anti-human kappa antibody (Southern Biotech, United States) with a dilution of 1:1,000 in 1 × PBS was added and incubated for 1 h at 37°C. The plate was then washed three times with PBST, developed with 3,3′,5,5′-tetramethylbenzidine (TMB) substrate (Promega, United States). The reaction was stopped by adding 1 M H2SO4, and absorbance was measured at 450 nm.
Binding Efficiency of Plant-Produced Monoclonal Antibody to SARS-CoV-2 Receptor Binding Domain Protein
ELISA to examine the binding of mAb B38 and H4 to SARS-CoV-2 RBD protein was performed. Three biological replicates were performed, and each sample was subjected to three technical replicates to assess the mAb binding efficiency. Briefly, RBD of SARS-CoV-2 protein (His tagged RBD produced in Sf9 insect cells, Z03479; Genscript Biotech, United States) was bound to 96-well microplates (Greiner Bio-One GmbH, Frickenhausen, Germany). After overnight incubation, the plates were blocked with 5% skim milk (BD, Franklin Lakes, NJ, United States) in 1 × PBS for 2 h, washed three times with PBST, and incubated with plant-produced mAbs B38 and H4. After 2-h incubation at 37°C, sheep anti-human kappa LC conjugated with HRP (Southern Biotech, United States) at a dilution of 1:1,000 in 1 × PBS was added, and samples were incubated for 1 h at 37°C. The plate was then washed three times with PBST, developed using TMB substrate (Promega, United States), and the absorbance was read at 450 nm. The commercially available human IgG1 (Abcam, United Kingdom) and plant-produced human anti-PD1 antibody (Rattanapisit et al., 2019b) was used as negative controls.
Severe Acute Respiratory Syndrome Coronavirus 2 Neutralization
All the experiments with live SARS-CoV-2 virus were performed at a certified biosafety level 3 facility, Department of Microbiology, Faculty of Science, Mahidol University, Thailand. The experimental protocol was approved by Mahidol University, and all methods were performed by following standard protocols approved by the institutional review committee. In this study, we used SARS-CoV-2 virus (SARS-CoV-2/01/human/Jan2020/Thailand) isolated from a confirmed COVID-19 patient at Bamrasnaradura Infectious Diseases Institute, Nonthaburi, Thailand, for in vitro experiments. Positive convalescent serum collected from a different COVID-19 patient was approved to be used as a clinical specimen by the Faculty of Medicine, Ramathibodi Hospital. The informed consent was waived by the institutional review boards that approved the present study.
Briefly, the plant-produced mAbs B38 or H4 or positive control or negative control was twofold serially diluted in 96-well plates. Then, 100 × TCID50 (50% tissue culture infectious dose) of the SARS-CoV-2 virus was added to the mAb dilutions and incubated for 1 h at 37°C. The Vero-E6 cells pre-seeded in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 2% fetal bovine serum (FBS), 100 U/ml of penicillin, and 0.1 mg/ml of streptomycin (1 × 104 cells/well, duplicates) were then infected with the mAb–virus mixture for 2 days. The cells were then fixed and permeablized with ice-cold 1:1 methanol/acetone fixative for 20 min at 4°C. The fixed plates were washed three times with 1 × PBS containing 0.05% Tween 20 (wash buffer) and blocked with blocking buffer containing 2% bovine serum albumin (BSA) and 0.1% tween 20 in 1 × PBS for 1 h. After washing the plates thrice with wash buffer, the SARS-CoV/SARS-CoV-2 nucleocapsid mAb (40143-R001; Sino Biological, United States) with a dilution of 1:5,000 in 1 × PBS containing 0.5% BSA and 0.1% Tween 20 was added to each well and incubated for 2 h at 37°C. The plates were then washed three times with wash buffer, and HRP-conjugated goat anti-rabbit polyclonal antibody (P0448; Dako, Denmark) was used as secondary antibody at a dilution 1:2,000. After incubation for 1 h at 37°C, the plates were washed thrice and detected with SureBlue TMB 1 component microwell peroxidase substrate (SeraCare Life Sciences, United States). The reaction was stopped with 1N HCl. The absorbance was measured at 450 and 620 nm (reference wavelength) with an ELISA plate reader. Both virus control (no antibody) and cell control (no virus, no antibody) were included in the plates. Convalescent serum collected from the COVID-19 patient (heat inactivated at 56°C, 30 min) was used as a positive control; anti-human PD1 antibody (Rattanapisit et al., 2019b) and negative serum were used as negative controls. The experiment was performed with three technical replicates for each sample. The average ODs at 450 and 620 nm were determined for virus control and cell control wells, and the neutralizing endpoint was determined by 50% specific signal calculation. The neutralizing endpoint titer was expressed as the reciprocal of the highest serum dilution with an OD value less than X (Rowe et al., 1999; World Health Organization [WHO], 2011), which was calculated as follows:
Sera which tested negative at 1:10 dilution were assigned a titer of <10. Sera were considered positive if neutralization titer is ≥20.
Results
Expression of Monoclonal Antibodies Using Geminiviral Plant Expression Vector
The expression of mAbs B38 and H4 in N. benthamiana was accomplished by cloning codon-optimized HC and LC sequences into the geminiviral vector pBYK-2e to create the expression cassettes pBYK-2e-B38 HC, pBYK-2e-B38 LC, pBYK-2e-H4 HC, and pBYK-2e-H4 LC. The coding sequences of the antibody genes were incorporated into the vector under the control of the CaMV 35S promoter with duplicated enhancer to drive maximal transcription, and the RNA silencing suppressor P19 was also present in the vector for enhanced protein production (Diamos and Mason, 2019). The generalized schematic diagram of the T-DNA regions of the plant expression vectors used in the present study was shown in Figure 1. The LC and HC expression cassette was co-infiltrated into the leaves of wild-type N. benthamiana by vacuum agroinfiltration. The protein extracts were collected from the agroinfiltrated leaves 4 dpi, and the recombinant mAbs were purified. The assembled mAbs produced from N. benthamiana were quantified by ELISA. The expression levels of mAbs B38 and H4 were estimated to be 4 and 35 μg/g fresh weight (FW), respectively (Figure 2).
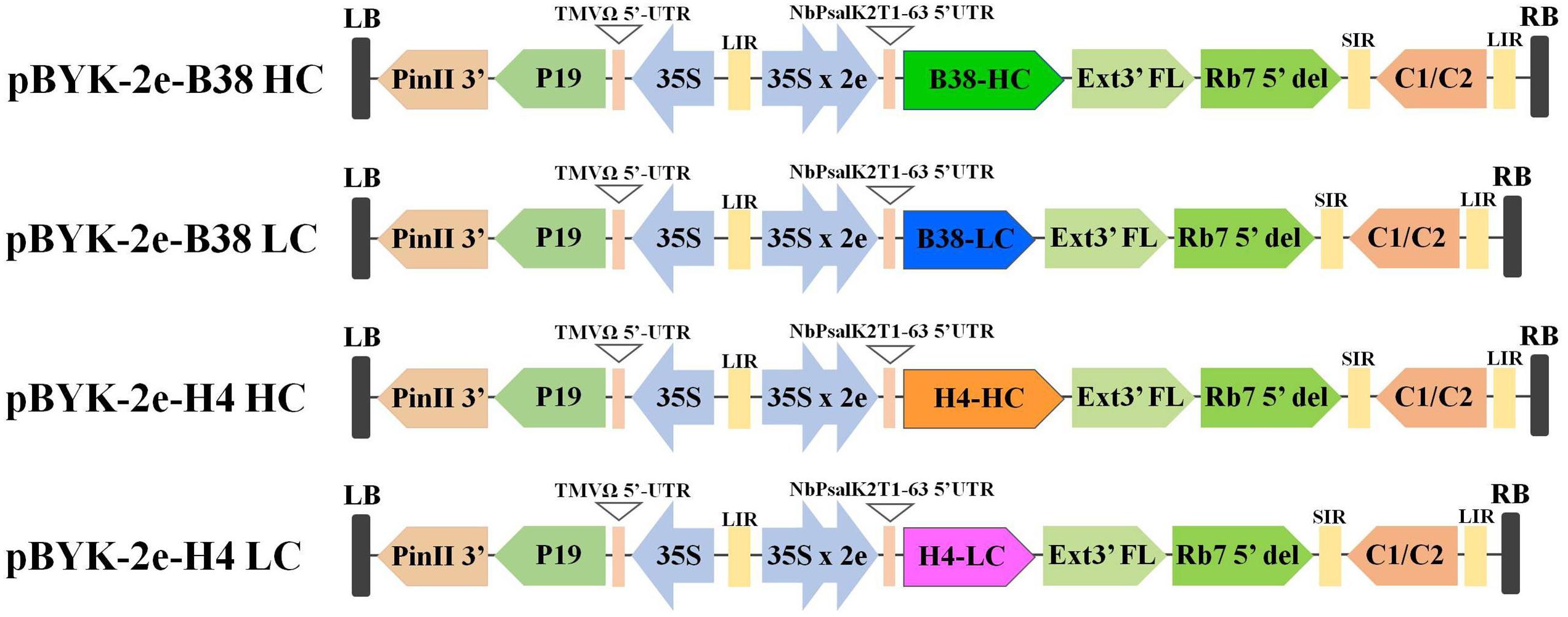
Figure 1. Generalized schematic representation of the monoclonal antibodies (mAbs) B38 and H4 heavy chain (HC) and light chain (LC) in plant expression constructs used in the present study. RB and LB, the right and left borders of the T-DNA region transferred by Agrobacterium into plant cells; 35S, Cauliflower Mosaic Virus (CaMV) 35S promoter; 35S x 2e, CaMV 35S promoter with duplicated enhancer; NbPsalK2T1-63 5′UTR, 5′ untranslated region; B38-HC, HC of B38 antibody; B38-LC, LC of B38 antibody; H4-HC, HC of H4 antibody; H4-LC, LC of H4 antibody; Ext3′FL, 3′ region of tobacco extension gene; Rb7 5’ del, tobacco RB7 promoter; SIR, short intergenic region of BeYDV; LIR, long intergenic region of BeYDV; C2/C1, Bean Yellow Dwarf Virus (BeYDV) ORFs C1 and C2 encoding for replication initiation protein (Rep) and RepA; TMVΩ 5′-UTR, 5′ untranslated region of tobacco mosaic virus Ω; P19, the RNA silencing suppressor from tomato bushy stunt virus; PinII 3′, the terminator from potato proteinase inhibitor II gene.
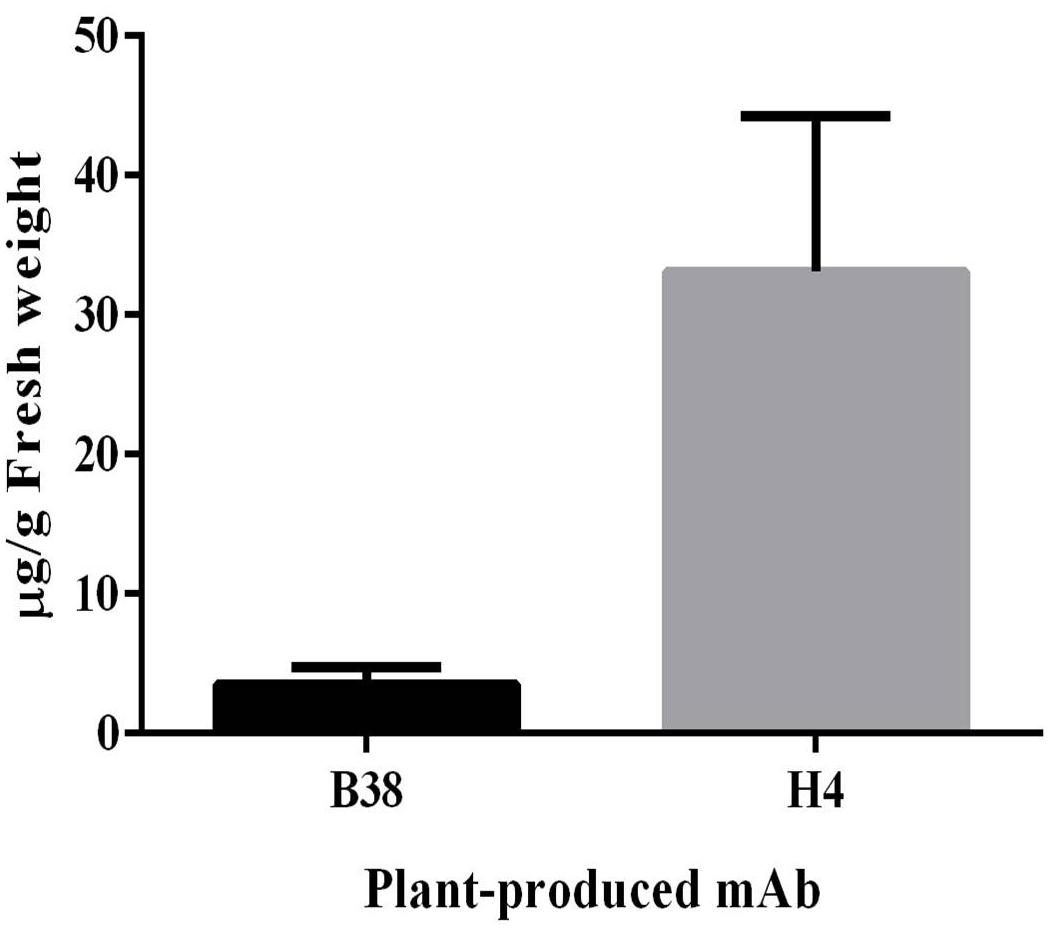
Figure 2. Expression levels of plant-produced monoclonal antibodies (mAbs) B38 and H4 were measured by ELISA. N. benthamiana leaves agroinfiltrated with heavy-chain (HC) and light-chain (LC) constructs were harvested at 4 days post infiltration (dpi), and the expression was analyzed using human immunoglobulin G (IgG) as standard. The result was presented on the leaf fresh weight (FW) basis. The data are expressed as mean ± standard deviation of three independent replicates.
Purification of Recombinant Monoclonal Antibodies From Plant Crude Extract
The anti-SARS-CoV-2 mAbs (B38 and H4) from the leaf crude extract of N. benthamiana plants were purified by using protein A affinity column chromatography. The identity of the purified mAbs was confirmed by SDS-PAGE and Western blotting. The separated bands in the SDS-PAGE gel was stained with Coomassie brilliant blue stain, and the bands were visualized. For Western blotting, the separated proteins were transferred to the nitrocellulose membrane and detected with HRP-conjugated anti-human gamma chain antibody or anti-human kappa chain antibody. As shown in Figure 3, HC and LC bands of both the mAbs were observed with the expected molecular size of approximately 150 kDa under non-reducing conditions (Figures 3A–C, Lanes 2 and 4), which is possibly the heterotetramer of the fully assembled antibody (mAb B38 and H4). Some additional molecular weight bands are also observed, probably indicating the assembly intermediates or proteolytic fragments (Hehle et al., 2011; van Dolleweerd et al., 2014). Under reducing conditions, predominant ∼50 kDa band was typically visible in Coomassie-stained SDS-PAGE gel (Figure 3A, Lanes 1 and 3) and Western blot probed with HRP-conjugated anti-human gamma chain antibody corresponding to the expected size for HC (Figure 3B, Lanes 1 and 3) of the antibody, whereas ∼25 kDa band was visible in Coomassie-stained SDS-PAGE gel (Figure 3A, Lanes 1 and 3) and Western blot probed with HRP-conjugated anti-human kappa chain antibody (Figure 3C, Lanes 1 and 3), which corresponds to LC of both the plant-produced antibodies. This result confirms the co-expression of HC and LC resulting in the expression of fully assembled anti-SARS-CoV-2 mAbs B38 and H4 in N. benthamiana.
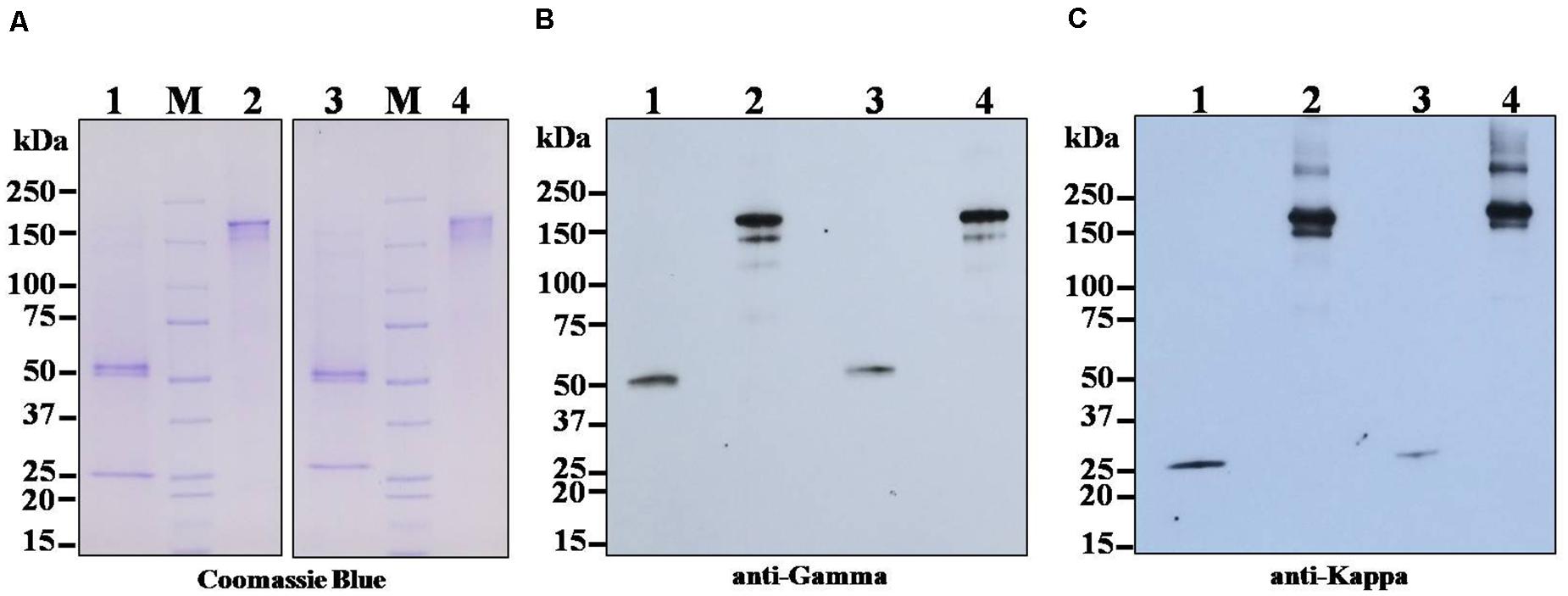
Figure 3. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis of transiently expressed monoclonal antibodies (mAbs) B38 and H4 in N. benthamiana leaves. Agrobacterium containing the plant expression geminiviral cassette with heavy chain (HC) or light chain (LC) of the antibody was co-infiltrated into the N. benthamiana leaves. The leaf samples were harvested 4 days post infiltration (dpi), and protein extracts were separated by SDS-PAGE and stained with Coomassie blue stain (A). For Western blot, the protein was electroblot transferred onto a nitrocellulose membrane, and the membrane was probed with either HRP-conjugated anti-human gamma chain antibody (B) or anti-human kappa chain antibody (C). Lane 1: Plant-produced mAb B38 under reducing condition; Lane 2: Plant-produced mAb B38 under non-reducing condition; Lane 3: Plant-produced mAb H4 under reducing condition; Lane 4: Plant-produced mAb H4 under non-reducing condition.
Recognition of Plant-Produced Monoclonal Antibody to SARS-CoV-2 Receptor Binding Domain Protein
Since both the human mAbs H4 and B38 were reported to recognize different epitopes on RBD domain of S protein on SARS-CoV-2, it was indispensable to show that the plant-produced recombinant mAb versions have the ability to recognize and bind the RBD domain of SARS-CoV-2. ELISA was performed in which SARS-CoV-2 RBD was coated on 96-well microplates followed by adding serial dilutions of purified recombinant mAbs B38 and H4. As shown in Figure 4, the plant-produced antibody recognized the RBD protein, whereas negligible reactivity was observed in the negative controls. Together, these data suggested that the plant-produced recombinant antibodies have the ability to bind the RBD protein of SARS-CoV-2.
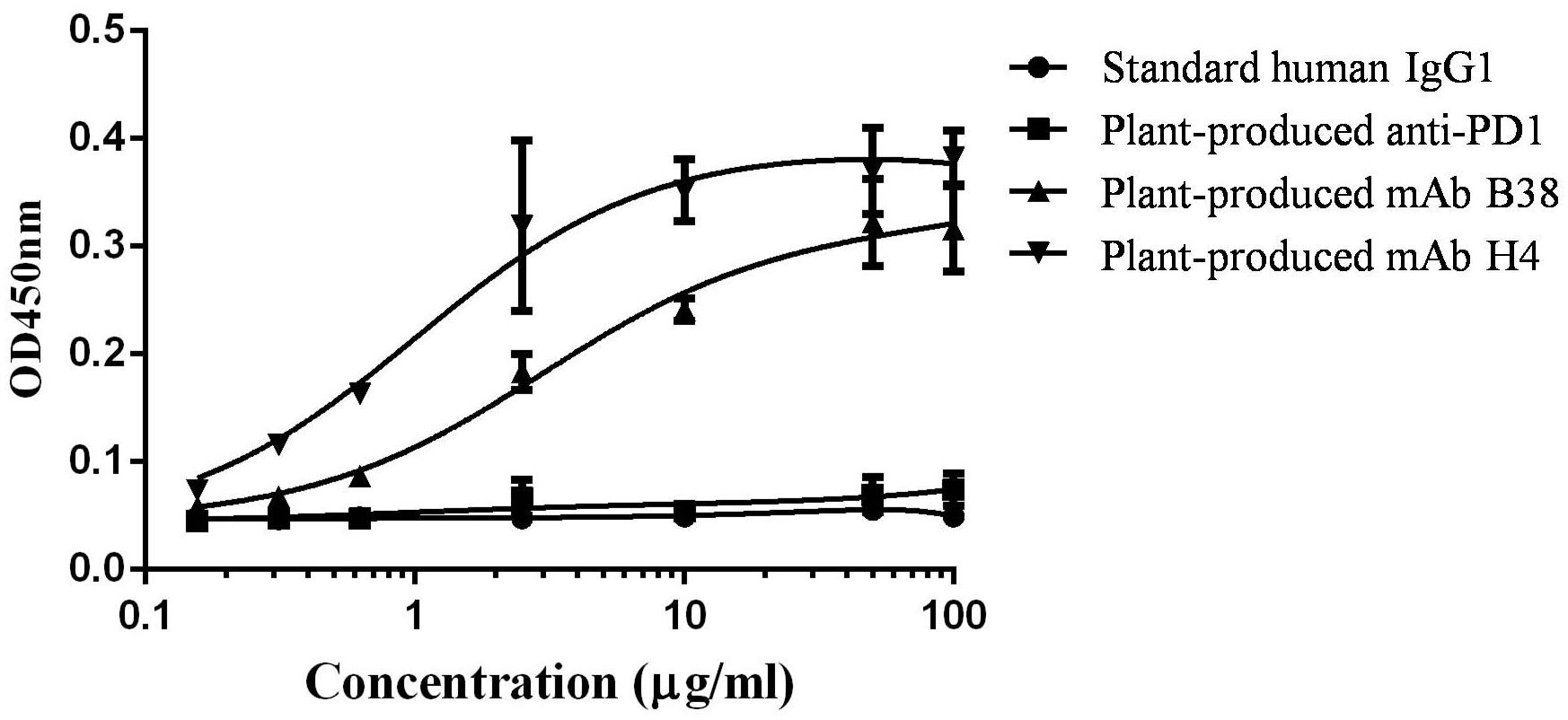
Figure 4. Binding of plant-produced monoclonal antibodies (mAbs) B38 and H4 to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) receptor binding domain (RBD) protein. The ability of the plant-produced mAbs to recognize RBD protein of SARS-CoV-2 was assessed by ELISA. The plant-produced mAbs B38 and H4, standard human immunoglobulin G (IgG)1, and plant-produced anti- PD1 antibody (as negative control) (Rattanapisit et al., 2019b) were incubated on plates coated with commercial SARS-CoV-2 RBD. The bound antibody was detected with a horseradish peroxidase (HRP)-conjugated anti-human kappa antibody. The data are the mean values of technical triplicates per concentration.
Neutralization Activity of Monoclonal Antibody Against SARS-CoV-2
The neutralization potential of plant-produced mAbs B38 and H4 against SARS-CoV-2 was evaluated. The detection of nucleocapsid protein indicates virus infection and lack of neutralizing antibodies. The results (Table 2) show that the plant-produced antibodies B38 exhibit a neutralization titer of 40 at the concentration of 5.45 μg/ml, and H4 showed a neutralization titer of 640 at the concentration of 0.492 μg/ml against SARS-CoV-2 in vitro, while the negative controls, i.e., anti-human PD1 antibody and negative serum (<10) did not neutralize SARS-CoV-2. The neutralizing titer of plant-derived mAb H4 was superior to that of plant-derived mAb B38. These results proved that the anti-SARS-CoV-2 mAbs produced in N. benthamiana are functional and exhibited neutralizing activity against highly infectious SARS-CoV-2.
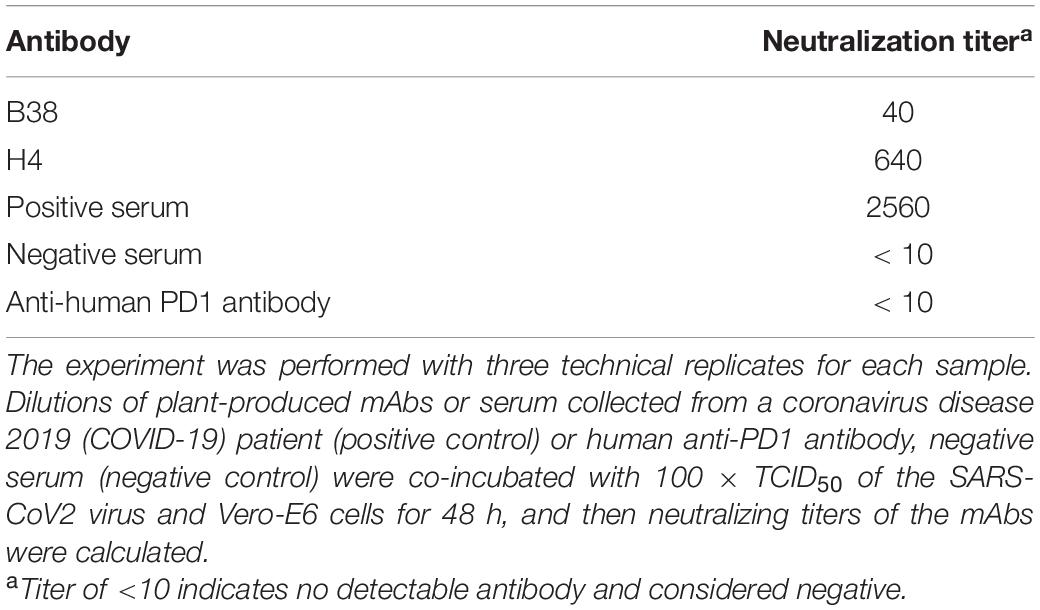
Table 2. The neutralizing activity of plant-produced B38 and H4 monoclonal antibodies (mAbs) against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Discussion
The unprecedented speed spread of the SARS-CoV-2 is responsible for the current pandemic, and the explosion of the number of COVID-19 cases associated with increasing mortality rates demands for effective diagnosis and treatment. Although many groups are working toward developing a COVID-19 vaccine, there is an equally vital competition going on parallelly to identify and produce potential targeted mAbs that could provide an immediate boost to the immune system to fight against the virus. Indeed, human mAbs targeting against S glycoprotein of SARS-CoV-2 have been shown to be protective against SARS-CoV-2 infection (Pinto et al., 2020; Wang et al., 2020b; Wec et al., 2020). The recently identified human neutralizing antibodies B38 and H4 shown to reduce virus titers in animal models are considered potential candidates to use either alone or in combination with other mAbs to treat COVID-19 and also possibly other future coronavirus infections (Wu et al., 2020). These findings suggest the utility of the neutralizing antibodies as a promising prophylactic or therapeutic candidate against SARS-CoV-2. However, the high production cost of therapeutic mAbs in conventional expression systems might limit their use in developing and underdeveloped countries (Kelley, 2009). The global pandemic highlights the need for expression platforms that can bring the therapeutic mAbs to the market with rapid production speed, mass production, and affordability.
The production of biopharmaceutical proteins in plants has received much attention in recent years, and several proteins with commercial, industrial, or pharmaceutical applications have been produced by plant cells, tissues, or whole plants (Tschofen et al., 2016; Donini and Marusic, 2019). Remarkably, the US Food and Drug Administration (FDA) approval of the first plant-made pharmaceutical ElelysoTM (taliglucerase alfa) for the treatment of Gaucher’s disease (Fox, 2012) and first in-human clinical trial of the transgenic tobacco-derived anti-HIV antibody 2G12 (Ma et al., 2015) made a significant breakthrough in the field of plant molecular farming that has established the regulatory framework and opens the way for large pharmaceutical companies to manufacture plant-made pharmaceuticals. Further, the rapid nature of the transient expression system was well demonstrated during the Ebola outbreak by Mapp Biopharmaceutical Inc. (Davey et al., 2016). In addition, extensive work has been done to optimize and express various vaccine antigens and therapeutic antibodies in plant expression systems for the diagnosis, prevention, and treatment of several infectious diseases (Shahid and Daniell, 2016; Zhang et al., 2017; Massa et al., 2018; Schillberg et al., 2019; Rybicki, 2020; Shanmugaraj et al., 2020a).
In this study, we demonstrated the production and efficacy of two candidate SARS-CoV-2 mAbs B38 and H4 in N. benthamiana in order to develop affordable antibody-based therapeutics against SARS-CoV-2. The nucleotide sequences of both the mAbs were codon-optimized to boost the recombinant protein expression in plants. The plant expression geminiviral vector was used for the transient expression of both the mAbs in N. benthamiana. Earlier reports have shown that the transient Agrobacterium-mediated transformation with geminiviral replicon vector derived from bean yellow dwarf virus produces mAbs in tobacco (Chen et al., 2011; Diamos and Mason, 2019). Our results showed that both mAbs B38 and H4 accumulated efficiently in N. benthamiana leaves within 4 dpi at a level of 4 and 35 μg/g FW, respectively. Transient expression offers several advantages and is the method of choice to manufacture high levels of proteins to meet the unforeseen demand of recombinant viral proteins or therapeutic antibodies during epidemic situations. The striking feature of the plant transient expression system is that it requires a few weeks to produce recombinant proteins after constructing the plant expression vector. Hence, it is considered as a promising alternative to the generation of stable transformants by transgenic approach, which usually takes 3–6 months (Peyret and Lomonossoff, 2015; Yao et al., 2015; Rybicki, 2017).
Further, the antigen binding ability of purified mAbs B38 and H4 to SARS-CoV-2 RBD was determined. ELISA results showed that plant-produced anti-SARS-CoV-2 mAbs can bind to the RBD protein of SARS-CoV-2, whereas no binding was observed in the negative control. Hence, it was confirmed that the mAbs were correctly assembled in the plant system and retained its ability to bind to RBD protein of SARS-CoV-2. The neutralizing activity of the plant-produced mAbs was also investigated in vitro, and the results demonstrated that both the plant-produced antibodies have a neutralizing activity against SARS-CoV-2, highlighting the potency of plant-produced anti-SARS-CoV-2 mAbs. Wu et al. (2020) reported that HEK-293T-produced antibodies B38 and H4 exhibited a neutralizing activity in Vero E6 cells and protective efficacy in a mouse model. In either case, B38 was shown to be effective compared to H4, whereas our results showed that plant-produced H4 was found to be better than B38 in RBD binding and neutralizing SARS-CoV-2 in vitro. Although the cause of this disparity is unclear at this moment, we speculate that both B38 and H4 produced in wild-type N. benthamiana carried plant-specific glycans that might have an impact on the antigen binding and biological function of plant-produced mAbs (Ko et al., 2003; Miranda et al., 2007; Scallon et al., 2007; Strasser et al., 2009). One way of circumventing the difference in the complex glycans between mammals and plants is to use gycoengineered plants for the production of structurally similar mAbs as those produced in mammalian platforms, which could improve the therapeutic utility of mAbs (Schahs et al., 2007; Strasser et al., 2008; Montero-Morales and Steinkellner, 2018; Sukenik et al., 2018). In this way, anti-SARS-CoV-2 mAbs can be produced with more homogeneous human-like glycans, and its efficacy can be compared with mammalian counterparts. Available evidence suggests that some of the plant-produced recombinant proteins exhibit equivalent or better activity compared to their mammalian-produced counterparts in vitro and in vivo (Zeitlin et al., 2011; Olinger et al., 2012; Lai et al., 2014).
Our group has previously reported that the anti-SARS-CoV neutralizing antibody CR3022 (ter Meulen et al., 2006) can be efficiently produced in plants, which could be used as a diagnostic reagent for SARS-CoV-2 detection. In contrary to the present results, the plant-produced CR3022 can effectively bind with RBD of SARS-CoV-2 but did not neutralize SARS-CoV-2 in vitro, which could be due to the fact that epitope of CR3022 does not overlap with the ACE2 binding site (Rattanapisit et al., 2020; Tian et al., 2020). Unlike CR3022, mAbs B38 and H4 exhibit competition with ACE2 for RBD binding and recognize different epitopes on the RBD of SARS-CoV-2, allowing them to effectively neutralize SARS-CoV-2 by preventing the virus binding with the cellular receptor ACE2 (Wu et al., 2020). Altogether, the results from antigen binding specificity and neutralization ability demonstrate that the plant-produced mAbs B38 and H4 were properly folded, fully functional, and retained their biological activity after purification.
In order to effectively control the spread of the infection, a therapeutic approach based on mAbs should provide sufficient breadth of protection against different strains of SARS-CoV-2. In such cases, a single mAb might not be sufficient to prevent viral escape and extend the breadth of protection. Hence, the application of two or more mAb cocktails for effective passive immunoprophylaxis is needed, which requires a cost-effective platform to manufacture such mAbs, so that it can be accessible outside the developed world. The successful demonstration of rapid production of mAbs B38 and H4 in plants might help to address the safety and cost-related issues of immunotherapeutics, since earlier studies have shown that the plant recombinant proteins can be produced with significant reduction in manufacturing costs and capital expenditures. Further plant-based production of biologics can reduce the upstream production cost to $1.00–$2.00 per kg of protein, which can ensure equitable access to affordable therapeutics. However, a high level of recombinant protein expression in plant expression systems and product recovery warrants the future success of the plant-derived biopharmaceuticals (Chen, 2008; Tuse et al., 2014; Chen and Davis, 2016; Nandi et al., 2016; McNulty et al., 2020). Indeed, a detailed techno-economic assessment of the economic significance and rapid nature of plant system is critical to estimate the production cost of anti-SARS-CoV-2 mAbs in N. benthamiana at the commercial scale. In summary, we demonstrated the rapid production, purification, and protective efficacy of two therapeutic candidate mAbs B38 and H4 in N. benthamiana. To our knowledge, this is the first report of the plant-produced mAb that neutralizes SARS-CoV-2 in vitro. However, safety, in vivo breadth, and potency studies in animal models warrant the efficacy of these plant-produced mAbs toward clinical development. Taken together, our results may lead as a proof of principle study that can readily be applied to generate safer antibody-based therapeutics against SARS-CoV-2 or other emerging infectious disease threats.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Human Research Ethics Committee, Faculty of Medicine Ramathibodi Hospital, Mahidol University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
WP and AT conceived and designed the experiments. BS and KR constructed the plant expression vectors and performed the protein expression, protein purification, and antigen binding by ELISA. SM and AT performed the in vitro neutralization experiment. BS, WP, and AT analyzed the data and wrote the manuscript. All the authors read and approved the final version of the manuscript.
Funding
This study was funded by Baiya Phytopharm Co., Ltd., and the Thailand Research Fund grant No. RSA628006. This study was also financially supported by The Second Century Fund (C2F), Chulalongkorn University (BS), and Graduate School, Ratchadapisek Somphot Fund, Chulalongkorn University (KR).
Conflict of Interest
WP from Chulalongkorn University is a founder/shareholder of Baiya Phytopharm Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the Department of Disease Control, Ministry of Public Health, Thailand, for providing clinical specimens for the viral isolate and sera from a COVID-19 survivor. We would like to thank Professor Hugh Mason (Arizona State University) for providing the geminiviral vector. We would also like to thank the Second Century Fund (C2F), Chulalongkorn University (BS), and Graduate School, Ratchadapisek Somphot Fund, Chulalongkorn University (KR), for providing the financial support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2020.589995/full#supplementary-material
References
Buyel, J. F. (2018). Plant molecular farming - Integration and exploitation of side streams to achieve sustainable biomanufacturing. Front. Plant Sci. 9:1893. doi: 10.3389/fpls.2018.01893
Buyel, J. F., Twyman, R. M., and Fischer, R. (2017). Very-large-scale production of antibodies in plants: the biologization of manufacturing. Biotechnol. Adv. 35, 458–465. doi: 10.1016/j.biotechadv.2017.03.011
Cao, Y., Su, B., Guo, X., Sun, W., Deng, Y., Bao, L., et al. (2020). Potent neutralizing antibodies against SARS-CoV-2 identified by high-throughput single-cell sequencing of convalescent patients’ B cells. Cell 182, 73.e16–84.e16. doi: 10.1016/j.cell.2020.05.025
Capell, T., Twyman, R. M., Armario-Najera, V., Ma, J. K. C., Schillberg, S., and Christou, P. (2020). Potential applications of plant biotechnology against SARS-CoV-2. Trends Plant Sci. 25, 635–643. doi: 10.1016/j.tplants.2020.04.009
Chen, Q. (2008). Expression and purification of pharmaceutical proteins in plants. Biol. Eng. 1, 291–321. doi: 10.13031/2013.26854
Chen, Q., and Davis, K. R. (2016). The potential of plants as a system for the development and production of human biologics. F1000Research 5:F1000 Faculty Rev-912. doi: 10.12688/f1000research.8010.1
Chen, Q., He, J., Phoolcharoen, W., and Mason, H. S. (2011). Geminiviral vectors based on bean yellow dwarf virus for production of vaccine antigens and monoclonal antibodies in plants. Hum. Vaccin. 7, 331–338. doi: 10.4161/hv.7.3.14262
Davey, R. T. Jr., Dodd, L., Proschan, M. A., Neaton, J., Neuhaus Nordwall, J., Koopmeiners, J. S., et al. (2016). A randomized, controlled trial of ZMapp for ebola virus infection. N. Engl. J. Med. 375, 1448–1456. doi: 10.1056/NEJMoa1604330
Dent, M., Hurtado, J., Paul, A. M., Sun, H., Lai, H., Yang, M., et al. (2016). Plant-produced anti-dengue virus monoclonal antibodies exhibit reduced antibody-dependent enhancement of infection activity. J. Gen. Virol. 97, 3280–3290. doi: 10.1099/jgv.0.000635
Diamos, A. G., Hunter, J. G. L., Pardhe, M. D., Rosenthal, S. H., Sun, H., Foster, B. C., et al. (2019). High level production of monoclonal antibodies using an optimized plant expression system. Front. Bioeng. Biotechnol. 7:472. doi: 10.3389/fbioe.2019.00472
Diamos, A. G., and Mason, H. S. (2019). Modifying the replication of geminiviral vectors reduces cell death and enhances expression of biopharmaceutical proteins in Nicotiana benthamiana leaves. Front. Plant Sci. 9:1974. doi: 10.3389/fpls.2018.01974
Donini, M., and Marusic, C. (2019). Current state-of-the-art in plant-based antibody production systems. Biotechnol. Lett. 41, 335–346. doi: 10.1007/s10529-019-02651-z
Fischer, R., and Buyel, J. F. (2020). Molecular farming - The slope of enlightenment. Biotechnol. Adv. 40:107519. doi: 10.1016/j.biotechadv.2020.107519
Fox, J. L. (2012). First plant-made biologic approved. Nat. Biotechnol. 30, 472–472. doi: 10.1038/nbt0612-472
Gelvin, S. B. (2010). Plant proteins involved in Agrobacterium-mediated genetic transformation. Annu. Rev. Phytopathol. 48, 45–68. doi: 10.1146/annurev-phyto-080508-081852
He, J., Lai, H., Engle, M., Gorlatov, S., Gruber, C., Steinkellner, H., et al. (2014). Generation and analysis of novel plant-derived antibody-based therapeutic molecules against West Nile virus. PLoS One 9:e93541. doi: 10.1371/journal.pone.0093541
Hehle, V. K., Paul, M. J., Drake, P. M., Ma, J. K. C., and van Dolleweerd, C. J. (2011). Antibody degradation in tobacco plants: a predominantly apoplastic process. BMC Biotechnol. 11:128. doi: 10.1186/1472-6750-11-128
Holtz, B. R., Berquist, B. R., Bennett, L. D., Kommineni, V. J. M., Munigunti, R. K., White, E. L., et al. (2015). Commercial-scale biotherapeutics manufacturing facility for plant-made pharmaceuticals. Plant Biotechnol. J. 13, 1180–1190. doi: 10.1111/pbi.12469
Hurtado, J., Acharya, D., Lai, H., Sun, H., Kallolimath, S., Steinkellner, H., et al. (2020). In vitro and in vivo efficacy of anti-chikungunya virus monoclonal antibodies produced in wild-type and glycoengineered Nicotiana benthamiana plants. Plant Biotechnol. J. 18, 266–273. doi: 10.1111/pbi.13194
Iyappan, G., Shanmugaraj, B. M., Inchakalody, V., Ma, J. K. C., and Ramalingam, S. (2018). Potential of plant biologics to tackle the epidemic like situations - case studies involving viral and bacterial candidates. Int. J. Infect. Dis. 73:363. doi: 10.1016/j.ijid.2018.04.4236
Jahanshahlu, L., and Rezaei, N. (2020). Monoclonal antibody as a potential anti-COVID-19. Biomed. Pharmacother. 129:110337. doi: 10.1016/j.biopha.2020.110337
Kelley, B. (2009). Industrialization of mAb production technology: the bioprocessing industry at a crossroads. mAbs 1, 443–452. doi: 10.4161/mabs.1.5.9448
Kesik-Brodacka, M. (2018). Progress in biopharmaceutical development. Biotechnol. Appl. Biochem. 65, 306–322. doi: 10.1002/bab.1617
Ko, K., Tekoah, Y., Rudd, P. M., Harvey, D. J., Dwek, R. A., Spitsin, S., et al. (2003). Function and glycosylation of plant-derived antiviral monoclonal antibody. Proc. Natl. Acad. Sci. U.S.A. 100, 8013–8018. doi: 10.1073/pnas.0832472100
Lai, H., He, J., Hurtado, J., Stahnke, J., Fuchs, A., Mehlhop, E., et al. (2014). Structural and functional characterization of an anti-West Nile virus monoclonal antibody and its single-chain variant produced in glycoengineered plants. Plant Biotechnol. J. 12, 1098–1107. doi: 10.1111/pbi.12217
Li, W., Moore, M. J., Vasilieva, N., Sui, J., Wong, S. K., Berne, M. A., et al. (2003). Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426, 450–454. doi: 10.1038/nature02145
Ma, J. K.-C., Christou, P., Chikwamba, R., Haydon, H., Paul, M., Ferrer, M. P., et al. (2013). Realising the value of plant molecular pharming to benefit the poor in developing countries and emerging economies. Plant Biotechnol. J. 11, 1029–1033. doi: 10.1111/pbi.12127
Ma, J. K.-C., Drossard, J., Lewis, D., Altmann, F., Boyle, J., Christou, P., et al. (2015). Regulatory approval and a first-in-human phase I clinical trial of a monoclonal antibody produced in transgenic tobacco plants. Plant Biotechnol. J. 13, 1106–1120. doi: 10.1111/pbi.12416
Malla, A., Shanmugaraj, B., and Ramalingam, S. (2020). Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): an emerging zoonotic respiratory pathogen in humans. J. Pure Appl. Microbiol. 14(Suppl. 1), 931–936. doi: 10.22207/JPAM.14.SPL1.30
Massa, S., Presenti, O., and Benvenuto, E. (2018). Engineering plants for the future: farming with value-added harvest. Prog. Bot. 80, 65–108. doi: 10.1007/124_2018_20
McNulty, M. J., Gleba, Y., Tusé, D., Hahn-Löbmann, S., Giritch, A., Nandi, S., et al. (2020). Techno-economic analysis of a plant-based platform for manufacturing antimicrobial proteins for food safety. Biotechnol. Prog. 36:e2896. doi: 10.1002/btpr.2896
Miranda, L. R., Duval, M., Doherty, H., Seaman, M. S., Posner, M. R., and Cavacini, L. A. (2007). The neutralization properties of a HIV-specific antibody are markedly altered by glycosylation events outside the antigen-binding domain. J. Immunol. 178, 7132–7138. doi: 10.4049/jimmunol.178.11.7132
Montero-Morales, L., and Steinkellner, H. (2018). Advanced plant-based glycan engineering. Front. Bioeng. Biotechnol. 6:81. doi: 10.3389/fbioe.2018.00081
Nandi, S., Kwong, A. T., Holtz, B. R., Erwin, R. L., Marcel, S., and McDonald, K. A. (2016). Techno-economic analysis of a transient plant-based platform for monoclonal antibody production. mAbs 8, 1456–1466. doi: 10.1080/19420862.2016.1227901
Olinger, G. G., Pettitt, J., Kim, D., Working, C., Bohorov, O., Bratcher, B., et al. (2012). Delayed treatment of Ebola virus infection with plant-derived monoclonal antibodies provides protection in rhesus macaques. Proc. Natl. Acad. Sci. U.S.A. 109, 18030–18035. doi: 10.1073/pnas.1213709109
Peyret, H., and Lomonossoff, G. P. (2015). When plant virology met Agrobacterium: the rise of the deconstructed clones. Plant Biotechnol. J. 13, 1121–1135. doi: 10.1111/pbi.12412
Pinto, D., Park, Y.-J., Beltramello, M., Walls, A. C., Tortorici, M. A., Bianchi, S., et al. (2020). Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature 583, 290–295. doi: 10.1038/s41586-020-2349-y
Poh, C. M., Carissimo, G., Wang, B., Amrun, S. N., Lee, C. Y.-P., Chee, R. S.-L., et al. (2020). Two linear epitopes on the SARS-CoV-2 spike protein that elicit neutralising antibodies in COVID-19 patients. Nat. Commun. 11:2806. doi: 10.1038/s41467-020-16638-2
Prompetchara, E., Ketloy, C., and Palaga, T. (2020). Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 38, 1–9. doi: 10.12932/ap-200220-0772
Rattanapisit, K., Chao, Z., Siriwattananon, K., Huang, Z., and Phoolcharoen, W. (2019a). Plant-produced anti-enterovirus 71 (EV71) monoclonal antibody efficiently protects mice against EV71 infection. Plants 8:560. doi: 10.3390/plants8120560
Rattanapisit, K., Phakham, T., Buranapraditkun, S., Siriwattananon, K., Boonkrai, C., Pisitkun, T., et al. (2019b). Structural and in vitro functional analyses of novel plant-produced anti-human PD1 antibody. Sci. Rep. 9:15205. doi: 10.1038/s41598-019-51656-1
Rattanapisit, K., Shanmugaraj, B., Manopwisedjaroen, S., Purwono, P. B., Siriwattananon, K., Khorattanakulchai, N., et al. (2020). Rapid production of SARS-CoV-2 receptor binding domain (RBD) and spike specific monoclonal antibody CR3022 in Nicotiana benthamiana. Sci. Rep. 10:17698. doi: 10.1038/s41598-020-74904-1
Rosales-Mendoza, S., Marquez-Escobar, V. A., Gonzalez-Ortega, O., Nieto-Gomez, R., and Arevalo-Villalobos, J. I. (2020). What does plant-based vaccine technology offer to the fight against COVID-19? Vaccines 8:183. doi: 10.3390/vaccines8020183
Rowe, T., Abernathy, R. A., Hu-Primmer, J., Thompson, W. W., Lu, X., Lim, W., et al. (1999). Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J. Clin. Microbiol. 37, 937–943. doi: 10.1128/JCM.37.4.937-943.1999
Rybicki, E. P. (2017). Plant-made vaccines and reagents for the one health initiative. Hum. Vaccin. Immunother. 13, 2912–2917. doi: 10.1080/21645515.2017.1356497
Rybicki, E. P. (2020). Plant molecular farming of virus-like nanoparticles as vaccines and reagents. WIREs Nanomed. Nanobiotechnol. 12:e1587. doi: 10.1002/wnan.1587
Scallon, B. J., Tam, S. H., McCarthy, S. G., Cai, A. N., and Raju, T. S. (2007). Higher levels of sialylated Fc glycans in immunoglobulin G molecules can adversely impact functionality. Mol. Immunol. 44, 1524–1534. doi: 10.1016/j.molimm.2006.09.005
Schahs, M., Strasser, R., Stadlmann, J., Kunert, R., Rademacher, T., and Steinkellner, H. (2007). Production of a monoclonal antibody in plants with a humanized N-glycosylation pattern. Plant Biotechnol. J. 5, 657–663. doi: 10.1111/j.1467-7652.2007.00273.x
Schillberg, S., Raven, N., Spiegel, H., Rasche, S., and Buntru, M. (2019). Critical analysis of the commercial potential of plants for the production of recombinant proteins. Front. Plant Sci. 10:720. doi: 10.3389/fpls.2019.00720
Shahid, N., and Daniell, H. (2016). Plant-based oral vaccines against zoonotic and non-zoonotic diseases. Plant. Biotechnol. J. 14, 2079–2099. doi: 10.1111/pbi.12604
Shang, J., Ye, G., Shi, K., Wan, Y., Luo, C., Aihara, H., et al. (2020). Structural basis of receptor recognition by SARS-CoV-2. Nature 581, 221–224. doi: 10.1038/s41586-020-2179-y
Shanmugaraj, B., Bulaon, C. J., and Phoolcharoen, W. (2020a). Plant molecular farming: a viable platform for recombinant biopharmaceutical production. Plants 9:842. doi: 10.3390/plants9070842
Shanmugaraj, B., Malla, A., and Phoolcharoen, W. (2020b). Emergence of novel coronavirus 2019-nCoV: need for rapid vaccine and biologics development. Pathogens 9:148. doi: 10.3390/pathogens9020148
Shanmugaraj, B., Siriwattananon, K., Wangkanont, K., and Phoolcharoen, W. (2020c). Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19). Asian Pac. J. Allergy Immunol. 38, 10–18. doi: 10.12932/AP-200220-0773
Strasser, R., Castilho, A., Stadlmann, J., Kunert, R., Quendler, H., Gattinger, P., et al. (2009). Improved virus neutralization by plant-produced anti-HIV antibodies with a homogeneous beta1,4-galactosylated N-glycan profile. J. Biol. Chem. 284, 20479–20485. doi: 10.1074/jbc.M109.014126
Strasser, R., Stadlmann, J., Schahs, M., Stiegler, G., Quendler, H., Mach, L., et al. (2008). Generation of glyco-engineered Nicotiana benthamiana for the production of monoclonal antibodies with a homogeneous human-like N-glycan structure. Plant Biotechnol. J. 6, 392–402. doi: 10.1111/j.1467-7652.2008.00330.x
Sukenik, S. C., Karuppanan, K., Li, Q., Lebrilla, C. B., Nandi, S., and McDonald, K. A. (2018). Transient recombinant protein production in glycoengineered Nicotiana benthamiana cell suspension culture. Int. J. Mol. Sci. 19:1205. doi: 10.3390/ijms19041205
Sun, H., Chen, Q., and Lai, H. (2017). Development of antibody therapeutics against flaviviruses. Int. J. Mol. Sci. 19:54. doi: 10.3390/ijms19010054
Teh, A. Y., Maresch, D., Klein, K., and Ma, J. K. (2014). Characterization of VRC01, a potent and broadly neutralizing anti-HIV mAb, produced in transiently and stably transformed tobacco. Plant Biotechnol. J. 12, 300–311. doi: 10.1111/pbi.12137
ter Meulen, J., van den Brink, E. N., Poon, L. L., Marissen, W. E., Leung, C. S., Cox, F., et al. (2006). Human monoclonal antibody combination against SARS coronavirus: synergy and coverage of escape mutants. PLoS Med. 3:e0030237. doi: 10.1371/journal.pmed.0030237
Thanh Le, T., Andreadakis, Z., Kumar, A., Gomez Roman, R., Tollefsen, S., Saville, M., et al. (2020). The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 19, 305–306. doi: 10.1038/d41573-020-00073-5
Tian, X., Li, C., Huang, A., Xia, S., Lu, S., Shi, Z., et al. (2020). Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg. Microbes Infect. 9, 382–385. doi: 10.1080/22221751.2020.1729069
Tschofen, M., Knopp, D., Hood, E., and Stöger, E. (2016). Plant molecular farming: much more than medicines. Annu. Rev. Anal. Chem. 9, 271–294. doi: 10.1146/annurev-anchem-071015-041706
Tuse, D., Tu, T., and McDonald, K. A. (2014). Manufacturing economics of plant-made biologics: case studies in therapeutic and industrial enzymes. Biomed. Res. Int. 2014:256135. doi: 10.1155/2014/256135
Twyman, R. M., Stoger, E., Schillberg, S., Christou, P., and Fischer, R. (2003). Molecular farming in plants: host systems and expression technology. Trends Biotechnol. 21, 570–578. doi: 10.1016/j.tibtech.2003.10.002
van Dolleweerd, C. J., Teh, A. Y., Banyard, A. C., Both, L., Lotter-Stark, H. C., Tsekoa, T., et al. (2014). Engineering, expression in transgenic plants and characterisation of E559, a rabies virus-neutralising monoclonal antibody. J. Infect. Dis. 210, 200–208. doi: 10.1093/infdis/jiu085
Waheed, M. T., Sameeullah, M., Khan, F. A., Syed, T., Ilahi, M., Gottschamel, J., et al. (2016). Need of cost-effective vaccines in developing countries: what plant biotechnology can offer? Springerplus 5:65. doi: 10.1186/s40064-016-1713-8
Wang, C., Horby, P. W., Hayden, F. G., and Gao, G. F. (2020a). A novel coronavirus outbreak of global health concern. Lancet 395, 470–473. doi: 10.1016/S0140-6736(20)30185-9
Wang, C., Li, W., Drabek, D., Okba, N. M. A., van Haperen, R., Osterhaus, A. D. M. E., et al. (2020b). A human monoclonal antibody blocking SARS-CoV-2 infection. Nat. Commun. 11:2251. doi: 10.1038/s41467-020-16256-y
Wec, A. Z., Wrapp, D., Herbert, A. S., Maurer, D. P., Haslwanter, D., Sakharkar, M., et al. (2020). Broad neutralization of SARS-related viruses by human monoclonal antibodies. Science 369, 731–736. doi: 10.1126/science.abc7424
World Health Organization [WHO] (2011). WHO Global Influenza. Surveillance Network. WHO Global Influenza Surveillance Network: Manual for The Laboratory Diagnosis and Virological Surveillance of Influenza. Geneva: World Health Organization [WHO].
World Health Organization [WHO] (2020). WHO Coronavirus Disease (COVID-19) Dashboard. Geneva: World Health Organization [WHO].
Wu, Y., Wang, F., Shen, C., Peng, W., Li, D., Zhao, C., et al. (2020). A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science 368, 1274–1278. doi: 10.1126/science.abc2241
Yao, J., Weng, Y., Dickey, A., and Wang, K. Y. (2015). Plants as factories for human pharmaceuticals: applications and challenges. Int. J. Mol. Sci. 16, 28549–28565. doi: 10.3390/ijms161226122
Zeitlin, L., Pettitt, J., Scully, C., Bohorova, N., Kim, D., Pauly, M., et al. (2011). Enhanced potency of a fucose-free monoclonal antibody being developed as an Ebola virus immunoprotectant. Proc. Natl. Acad. Sci. U.S.A. 108, 20690–20694. doi: 10.1073/pnas.1108360108
Zhang, B., Shanmugaraj, B., and Daniell, H. (2017). Expression and functional evaluation of biopharmaceuticals made in plant chloroplasts. Curr. Opin. Chem. Biol. 38, 17–23. doi: 10.1016/j.cbpa.2017.02.007
Keywords: molecular farming, transient expression, Nicotiana benthamiana, plant recombinant proteins, plant-produced monoclonal antibody, SARS-CoV-2, COVID-19
Citation: Shanmugaraj B, Rattanapisit K, Manopwisedjaroen S, Thitithanyanont A and Phoolcharoen W (2020) Monoclonal Antibodies B38 and H4 Produced in Nicotiana benthamiana Neutralize SARS-CoV-2 in vitro. Front. Plant Sci. 11:589995. doi: 10.3389/fpls.2020.589995
Received: 31 July 2020; Accepted: 28 October 2020;
Published: 27 November 2020.
Edited by:
Nobuyuki Matoba, University of Louisville, United StatesReviewed by:
Sandiswa Mbewana, University of Cape Town, South AfricaKaren Ann McDonald, University of California, Davis, United States
Copyright © 2020 Shanmugaraj, Rattanapisit, Manopwisedjaroen, Thitithanyanont and Phoolcharoen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Waranyoo Phoolcharoen, V2FyYW55b28uUEBjaHVsYS5hYy50aA==
†These authors have contributed equally to this work
 Balamurugan Shanmugaraj
Balamurugan Shanmugaraj Kaewta Rattanapisit1,2†
Kaewta Rattanapisit1,2† Suwimon Manopwisedjaroen
Suwimon Manopwisedjaroen Waranyoo Phoolcharoen
Waranyoo Phoolcharoen