- 1State Key Laboratory of Dao-di Herbs, National Resource Center for Chinese Materia Medica, China Academy of Chinese Medical Sciences, Beijing, China
- 2Institute of Healthcare China Academy of Chinese Medical Sciences, Nanchang, China
Commercial cultivation of the medicinal plant Atractylodes lancea is significantly restricted by low survival rates and reduced yields. Intercropping can reasonably coordinate interspecific interactions, effectively utilize environmental resources, and increase survival and yield. We conducted a field experiment from 2014 to 2016 to analyze the advantages and effects of intercropping on A. lancea survival, growth traits, individual volatile oil content, and total volatile oil content. In addition to A. lancea monoculture (AL), five intercropping combinations were planted: Zea mays L. (ZM) + A. lancea, Tagetes erecta L. (TE) + A. lancea, Calendula officinalis L. (CO) + A. lancea, Glycine max (Linn.) Merr. (GM) + A. lancea, and Polygonum hydropiper L. (PH) + A. lancea. The survival and average rhizome weight of A. lancea was higher in the ZM, CO, and TE treatments than in the monoculture treatment, and the average plant height was higher in all intercropping treatments than in the monoculture. The volatile oil content of A. lancea from the ZM and CO treatments was significantly improved relative to that of monoculture plants. The volatile oil harvest was higher in the ZM, CO, and TE treatments than in the monoculture. We conclude that intercropping is an effective way to increase the survival and yield of A. lancea. Furthermore, intercropping with ZM, CO, and TE increases the harvest of four volatile oils from A. lancea.
Introduction
The rhizomes of Atractylodes lancea (Thunb.) DC. (Chinese: Cangzhu) are commonly used in traditional Chinese medicine as a remedy for rheumatic diseases, digestive disorders, night blindness, and influenza (Oseko et al., 2005). In the past few decades, the demand for A. lancea has been increasing, as the use of its active compounds in the pharmaceutical industry has grown substantially. During the 2020 novel coronavirus pneumonia outbreak, A. lancea was one of the main traditional Chinese medicinal materials used for the prevention of COVID-19 infection (Yang et al., 2020; Zhao et al., 2020). A. lancea is a perennial plant that is typically harvested from the field after 2 – 3 years of cultivation. Because wild A. lancea resources are increasingly endangered, the market depends heavily on artificial cultivation. However, industrial A. lancea monoculture systems face great hazards associated with continuous cropping, including the suppression of soil fertility, reduced productivity, and increased pest and disease damage. The disease incidence rate on A. lancea, especially that of root rot disease, can reach up to 80%, causing serious reductions in growth and productivity (Wang et al., 2016). Guo et al. (2005) have previously reported that autotoxicity may be another negative effect of the continuous cropping of single cultivars.
Compared with the planting of single cultivars, intercropping has significant agro-ecological advantages (Power, 1989; Khan et al., 1997; Brooker et al., 2015). The disease problems associated with continuously cropping patchouli can be ameliorated by intercropping with turmeric and ginger (Zeng et al., 2020). Maize/soybean intercropping suppressed the occurrence of soybean red crown rot (Gao et al., 2014), and maize/pepper intercropping can reduce disease levels of soil-borne Phytophthora on pepper (Yang et al., 2015). Intercropping Chinese chive cultivars with banana can reduce the incidence of Panama disease (Li Z. et al., 2020). Traditional intercropping usually aims at the improvement of crop yields (Li C. et al., 2020), which consist mainly of primary metabolites. By contrast, the aim of medicinal plant cultivation is usually the production of more secondary metabolites. Previous research has reported that intercropping may lead to changes in plant accumulation of secondary metabolites (Maffei and Mucciarelli, 2003; Ngwene et al., 2017; Zeng et al., 2020).
In this study, we carried out two years of field experimentation to determine the effects of intercropping on A. lancea. The objectives of the study were: (i) to compare important growth indicators in different intercropping systems; (ii) to compare plant yield and the accumulation of secondary metabolites in different intercropping systems; and (iii) to investigate the different accumulation patterns of major active components under five intercropping systems.
Materials and Methods
Experimental Site
Field experiments were conducted on newly developed terraces in Huadun village, Laibang Town, Yuexi County, Anhui Province (30°56′7.15′′N, 116°1′40.43′E, altitude 620 m) in 2015 and 2016. This site is located in the north subtropical humid monsoon climate area, and its frost-free period is 220 days. The mean annual temperature is 17°C, the mean annual ground temperature is 17°C, the average annual precipitation is 2434.6 mm, and the average sunshine duration is 2070.5 h. Meteorological data from 2015 and 2016 were collected by automatic weather stations near the test site.
Experiment Design and Field Management
In this study, intercropping partners were selected on the basis of their functions. Selected species are all common native and agricultural species. The gramineous roots of Zea mays L. (ZM) can activate soil microbial flora (Abbott and Robson, 1991), and its aboveground parts are tall and dense, providing a degree of shade. Both marigold and calendula are from the Asteraceae family, and their above- and belowground parts contain volatile oils that provide resistance to pests and diseases (Mansoor and Mashkoor, 1988; Natarajan et al., 2006). It has been shown that Calendula officinalis L. (CO) can be used for pest control, and the calendula oil contained therein can be used as a repellent to prevent egg laying by flies (Pudasaini et al., 2008; Riaz et al., 2009). Extracts of Tagetes erecta L. (TE), leaves, and roots are toxic to the nematode that is closely associated with root rot (Wang et al., 2003; Hooks et al., 2010). The Glycine max (Linn.) Merr. (GM) root system harbors nitrogen-fixing rhizobia and has the effect of enhancing soil fertility. Finally, Polygonum hydropiper L. (PH) is the natural companion species of A. lancea.
Seedlings of A. lancea were derived from A. lancea rhizomes growing in Huoshan, Anhui Province, and seedlings of similar size were used for the experiment. Seeds of maize, soybean, marigold, calendula, and P. hydropiper were those of commercial cultivars.
Five intercropping treatments and an A. lancea monoculture treatment were used in the experiment: A. lancea alone, A. lancea + Zea mays L. (ZM), A. lancea + Glycine max (Linn.) Merr. (GM), A. lancea + Tagetes erecta L. (TE), A. lancea + Calendula officinalis L. (CO), and A. lancea + Polygonum hydropiper L. (PH). The row spacing between A. lancea and A. lancea is 20 × 30 cm, and the row spacing between A. lancea and partner plants is 20 × 30 cm, and the spacing between partner plants and partner plants is also 20 × 30 cm. The experiment used a randomized complete block design with four replications, and each experimental plot was 10 m2 (2 m × 5 m). Therefore, there are 187 A. lancea in AL treatment and 99 A. lancea in intercropping treatment.
All plants were planted by hand. A. lancea was only planted in December 2014 and has been grown in the field for two years. And the partner plants were planted for the first time in April 2015 and the second planted in April 2016. A. lancea was planted by rhizome propagation after sterilization (soaking for 30 min at room temperature in 50% carbendazim diluted 800–1000 times), and rhizomes were buried 1–2 cm underground. Intercropping plants were grown from seed sown 1–2 cm deep. The plantings were weeded in March, June, and November of each year, and no pesticides or fertilizers were used throughout the experimental period.
Measurement Parameters and Methods
Plant Biomass and Yield
At the end of November 2015 and 2016, ten A. lancea were selected from each experimental plot for biomass and yield analysis, including both their above- and belowground parts. Measurements included plant height, number of branches, number of apical and lateral buds on the rhizome, and rhizome fresh weight.
Collection and Analysis of Volatiles
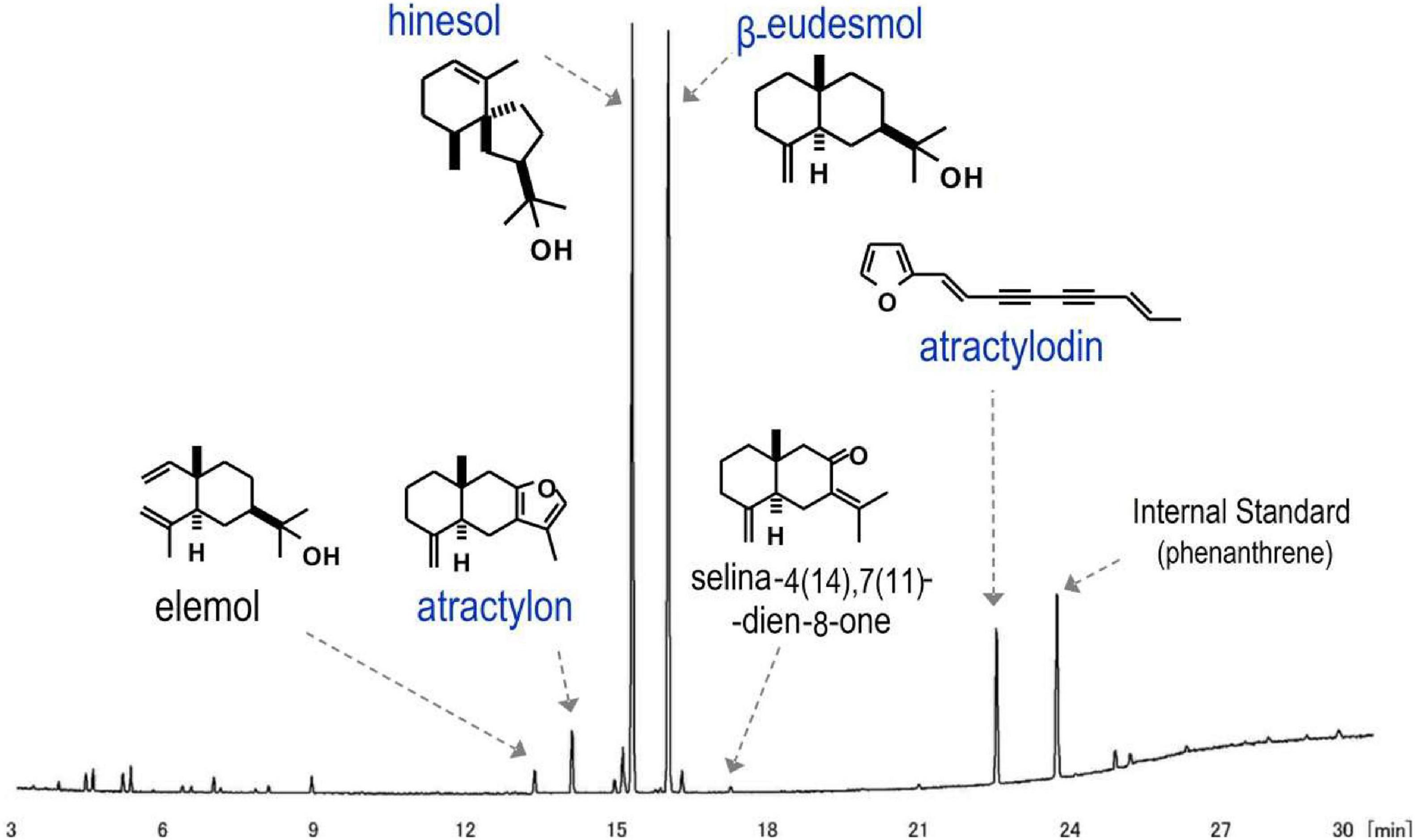
Atractylodes lancea rhizomes were collected and dried in a 40°C oven for one week to constant weight, then crushed to <0.3 mm. A 500-mg sample of the resulting powder was placed into a 50-ml centrifuge tube, 25 ml of n-hexane was added, and the mixture was first shaken (250 min–1, 15 min) and then centrifuged at 3000 rpm for 10 min. The supernatant was removed for subsequent use, 20 ml of n-hexane was added, and the above process was repeated. Both supernatants were combined in a volumetric flask, 1.0 ml of the internal standard solution was added, the sample was diluted to 50 ml, and a 1-ml sample was injected into the gas chromatograph/mass spectrometer (GC/MS) (Trace 1310 gas chromatograph and TSQ 8000 mass spectrometer).
The contents of four volatile active ingredients, atractylon, hinesol, β-camphor (β-eudesmol), and atractylodin, were measured using the method of Li (2018) with an Agilent DB-5ms series column (0.25 mm id × 30 m, 0.25 μm). The carrier gas was helium (flow rate: 1 ml/min), the injection mode was split (proportion 50:1), the injection volume was 1 ml, and the inlet temperature was 240°C. The column temperature was 120°C for the first 2 min; then the temperature was programmed to rise to 240°C at 5°C/min and was maintained at 240°C for 5 min. The MSD ionization mode had the following parameters: ionization voltage (EI) 70 V, ion source 230°C, and quadrupole 150°C. The MSD data acquisition mode was scanning (40–500 AMU). As shown in Figure 1, the method is accurate, fast, and reproducible.
Data Analysis
Tukey’s HSD test was used to test for differences among intercropping treatments. Pearson’s correlation coefficient was used to analyze the correlations among A. lancea growth and biochemical indices under different intercropping treatments. Statistics and correlation analysis were performed using SPSS v16.0 (SPSS Inc., Chicago, United States) and Microsoft Excel 2003.
Results
Effects of Different Intercropping Treatments on the Survival of A. lancea
The survival percentage of A. lancea from different treatments was investigated two years after planting (Figure 2). Plant survival was significantly higher in the TE treatment (69 ± 4.2%) than in the monoculture (52 ± 8.3%), followed by the CO (60 ± 7.2%) and ZM (59 ± 1.6%) treatments. By contrast, the survival of plants in the PH (49 ± 5.7%) and GM (37 ± 1.8%) treatments was significantly lower than that of monoculture plants.
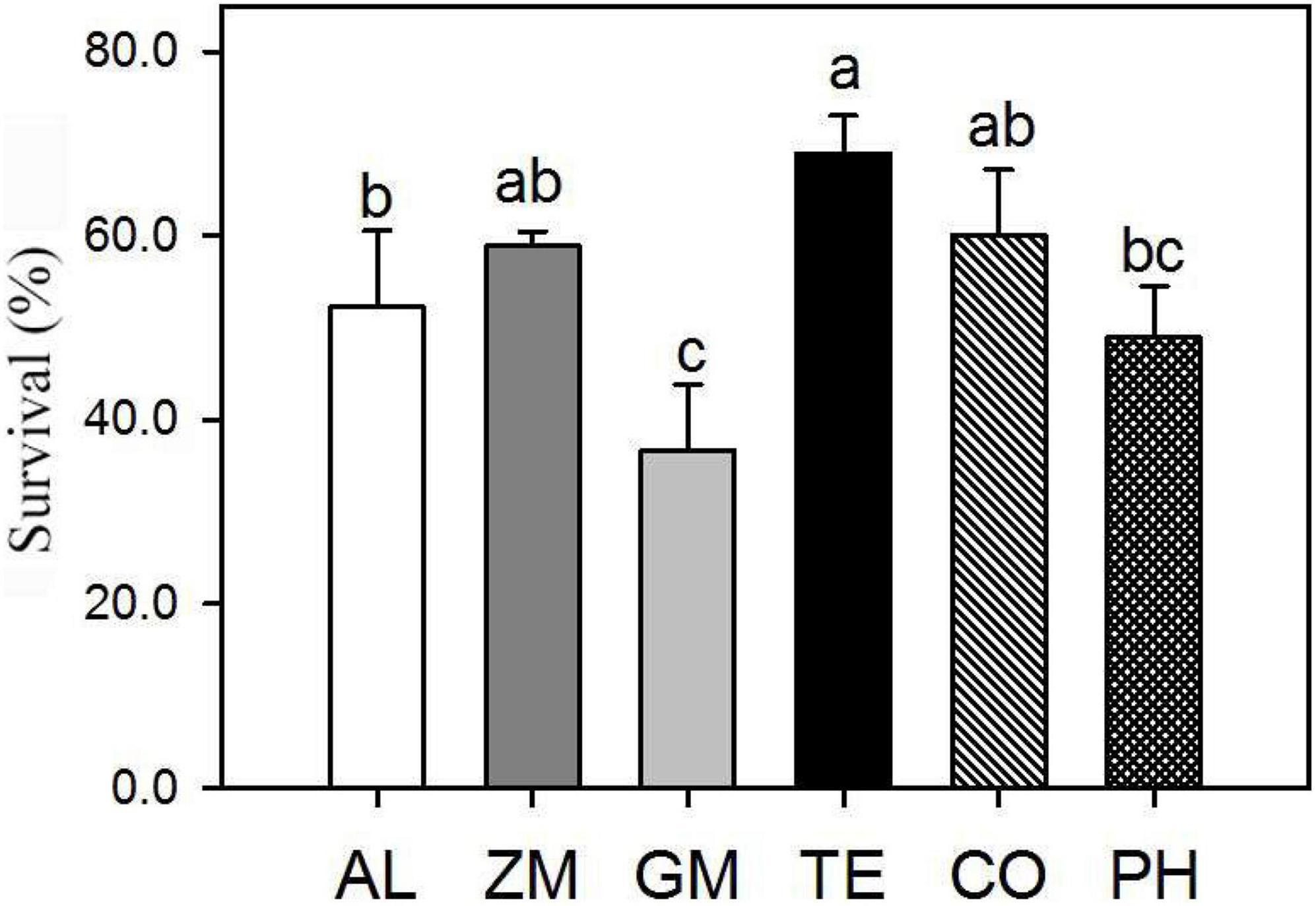
Figure 2. Effect of different intercropping treatments on the percentage survival of A. lancea in 2016. Different lowercase letters indicate significant differences at the 5% significance level.
Effects of Different Intercropping Treatments on Growth and Yield of A. lancea
In 2015, the average plant height was significantly greater in the ZM and TE treatments (both ∼34 cm) than in the monoculture (26 ± 1.6 cm) (Figure 3). Likewise, the average rhizome weight was significantly greater in the ZM and TE treatments (109 ± 8.8 g and 96 ± 11.3 g, respectively) than in the monoculture (72 ± 7.5 g) (Figure 3). In 2016, the average plant height was significantly greater in all the intercropping treatments than in the monoculture (Figure 3). Similarly, the average rhizome weights in the ZM, TE, and CO treatments were 141 ± 13.0 g, 150 ± 10.9 g, and 161 ± 19.2 g, all significantly greater than that in the monoculture (90 ± 8.7 g) (Figure 3).
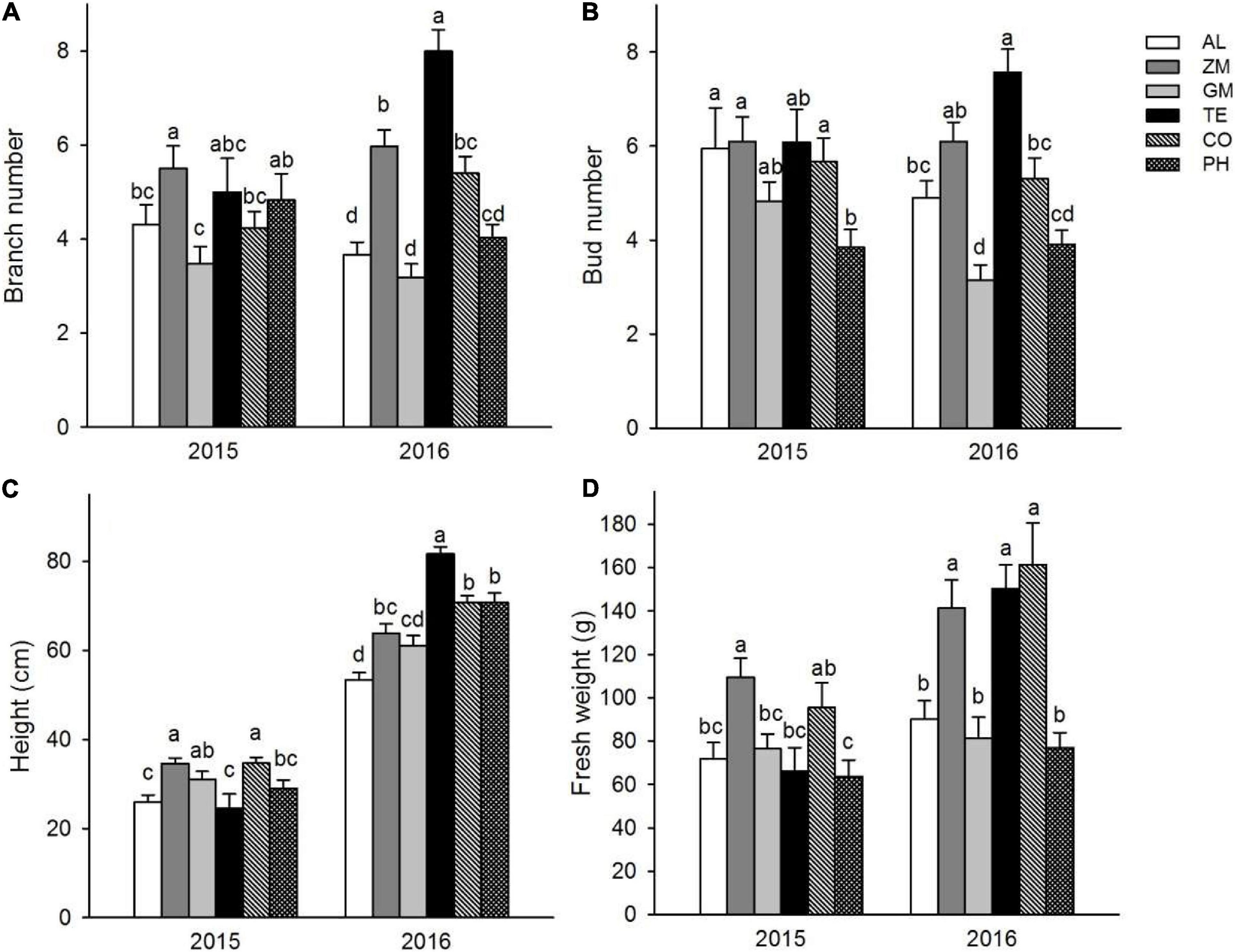
Figure 3. Effect of different intercropping treatments on branch number (A), bud number (B), height (C), and fresh weight (D) of A. lancea in 2015 and 2016. Different lowercase letters indicate significant differences at the 5% significance level.
Effects of Different Intercropping Treatments on the Concentrations of Volatile Oils in A. lancea
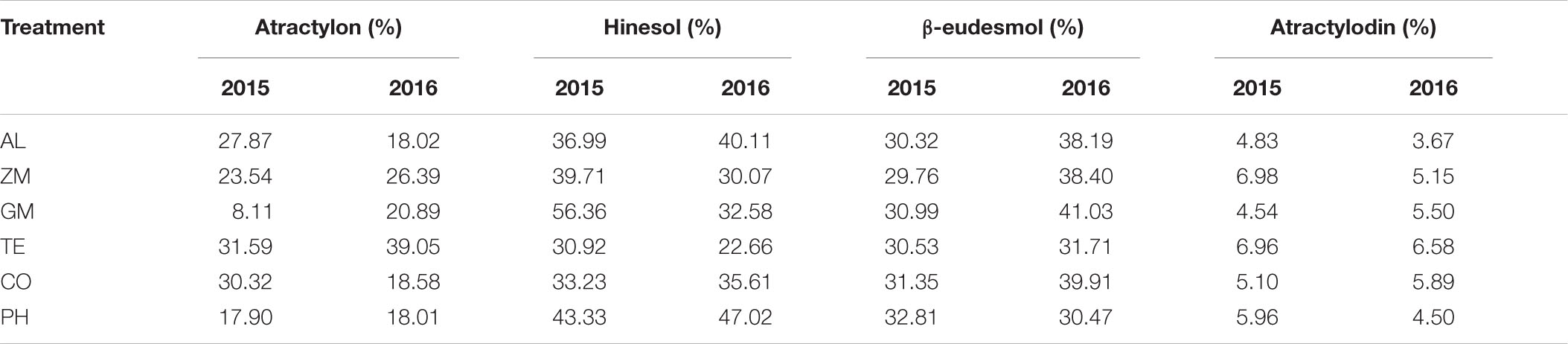
The concentrations of the four main volatile oils in A. lancea were analyzed in 2015 and 2016 (Figure 4). The concentration is the ratio of the mass of volatile oil to the mass of A. lancea rhizome. In 2015, the atractylon concentration of A. lancea was lowest in the GM treatment, the hinesol concentration of A. lancea was lowest in the TE treatment, and the atractylodin concentration of A. lancea was lowest in the CO treatment and the monoculture. In 2016, the hinesol and β-eudesmol concentrations of A. lancea were significantly lower in the TE treatment than in any other treatment. There were no other significant differences in concentrations of the four volatile oils among the treatments in 2015 and 2016.
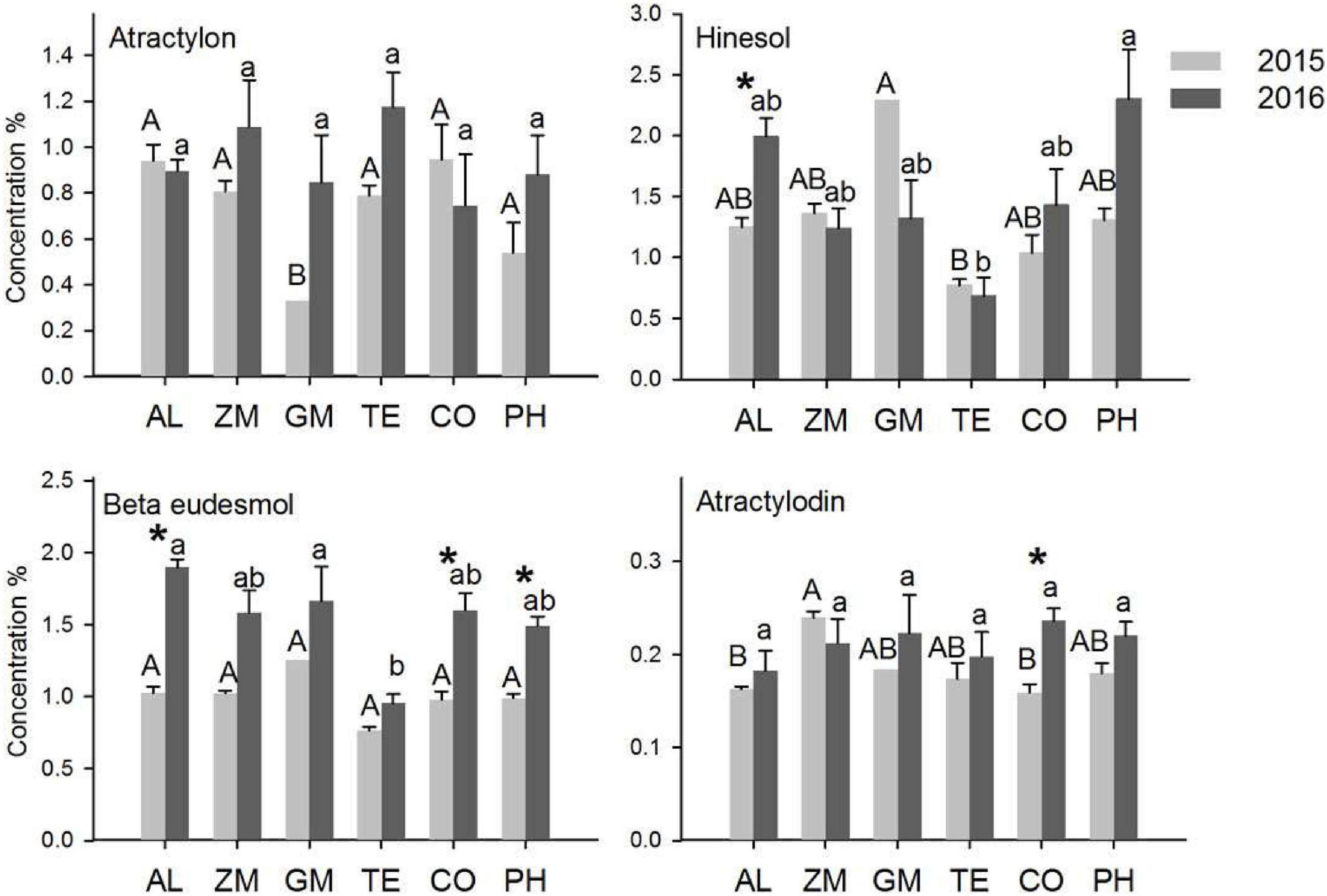
Figure 4. The effect of different intercropping treatments on the concentrations of four volatile oils from A. lancea in 2015 and 2016. Different capital (2015) and lowercase (2016) letters indicate significant differences at the 5% significance level, and asterisks indicate significant differences between 2015 and 2016 within individual treatments.
The concentrations of the four volatile oils increased significantly from 2015 to 2016 in a few treatments. The hinesol concentration of A. lancea in the monoculture, the β-eudesmol concentration of A. lancea in the CO and PH treatments, and the atractylodin concentration of A. lancea in the CO treatment all increased significantly from 2015 to 2016. However, the hinesol concentration of A. lancea decreased from 2015 to 2016 in the GM treatment.
Effects of Different Intercropping Treatments on Volatile Oil Content of A. lancea
The content indicates the quality of volatile oil from A. lancea. Based on the analysis of the average rhizome weight and the concentrations of the four main volatile oils in A. lancea, the content of the four volatile oils was calculated for individual plants. In 2015, the atractylon content of A. lancea was significantly higher in the CO treatment than in the GM, TE, and PH treatments. The hinesol content of A. lancea was significantly higher in the ZM and GM treatments than in other treatments. The β-eudesmol content was significantly higher in the ZM treatment than in the TE and PH treatments, and the atractylodin content was significantly higher in the ZM treatment than in the other treatments. In 2016, the atractylon content was significantly higher in the TE, ZM, and CO treatments than in the other treatments. The hinesol content was significantly higher in the CO treatment than in the monoculture, and the hinesol content was significantly lower in the GM and TE treatments than in the monoculture. The β-eudesmol content was significantly higher in the ZM and CO treatments than in the monoculture, and the β-eudesmol content was significantly lower in the GM and PH treatments than in the monoculture. The atractylodin content was significantly higher in the CO, ZM, and TE treatments than in the monoculture.
The contents of individual volatile oils increased significantly from 2015 to 2016 in most of the intercropping systems (Figure 5). The atractylon content was significantly higher in 2016 than in 2015 in the TE, ZM, CO, and PH treatments. Likewise, the contents of hinesol (except in the monoculture), β-eudesmol, and atractylodin (except in the ZM treatment) were significantly higher in all treatments in 2016 than in 2015.
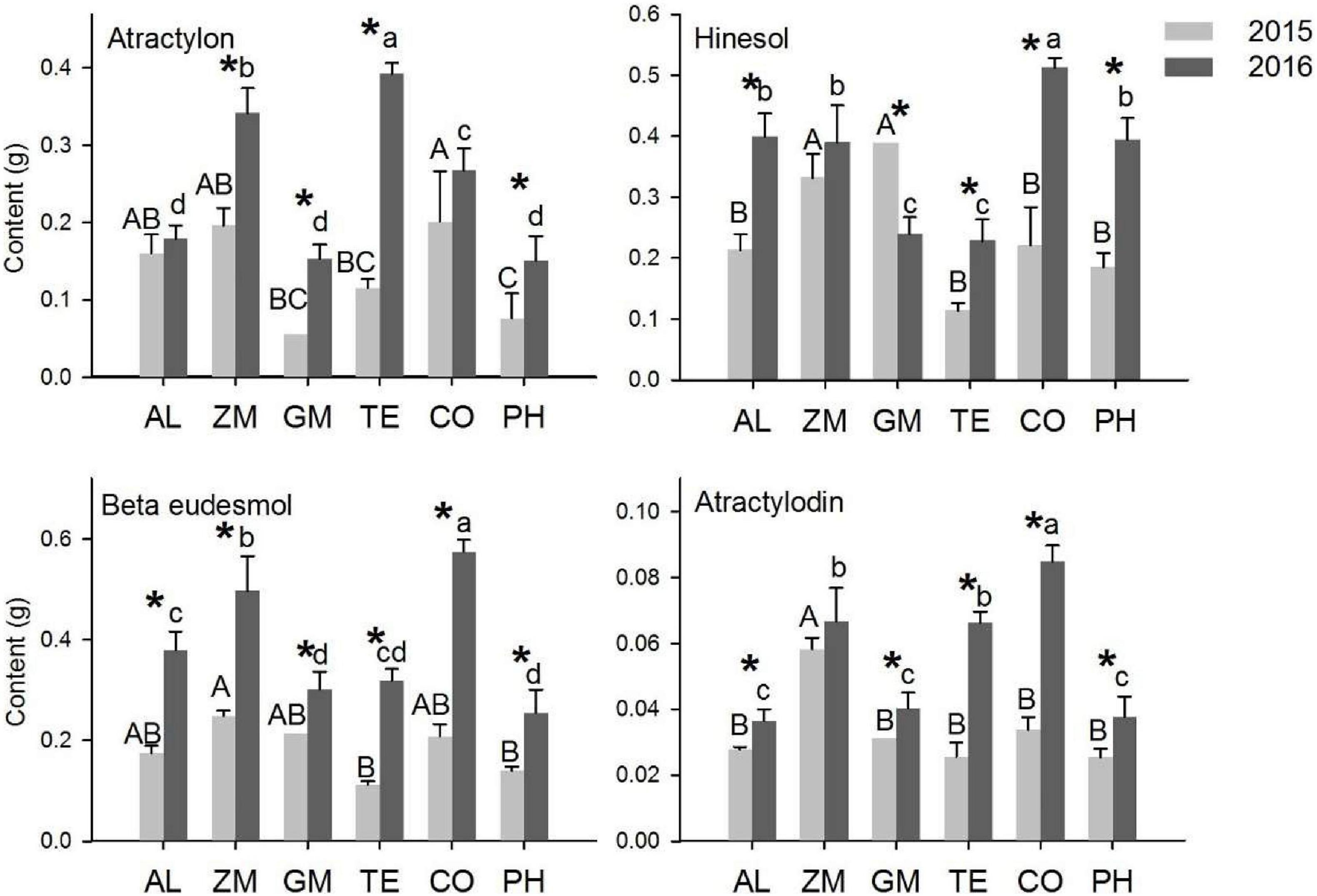
Figure 5. The effect of different intercropping treatments on the contents of four volatile oils in 2015 and 2016. Different capital (2015) and lowercase (2016) letters indicate a significant difference at the 5% significance level, and asterisks indicate significant differences between 2015 and 2016 within individual treatments.
Effects of Different Intercropping Treatments on the Accumulation of Volatile Oils in A. lancea
The proportion of different volatile oils is an important characteristic of Daodi herbs (Guo et al., 2005). The proportion of individual volatile oil concentrations to the total concentration of all four volatile oils changed markedly from 2015 to 2016 in all treatments (Figure 6, Appendix A.). The total volatile oil concentration increased significantly from 2015 to 2016 in only the AL and PH treatments. The relative concentration of atractylon was higher in 2015 than in 2016 in the AL and CO treatments; the opposite pattern was found in other treatments, particularly for GM, in which the atractylon concentration was significantly higher in 2016. The relative concentration of hinesol was significantly higher in 2015 than in 2016 for the ZM, GM, and TE treatments, and this difference was significant for GM. With the exception of the PH treatment, the relative concentration of β-eudesmol was higher in 2016 than in 2015 for all treatments. The relative concentration of atractylodin did not change from 2015 to 2016 in the GM and TE treatments, whereas its relative concentration was lower in 2016 than in 2015 for the other treatments.
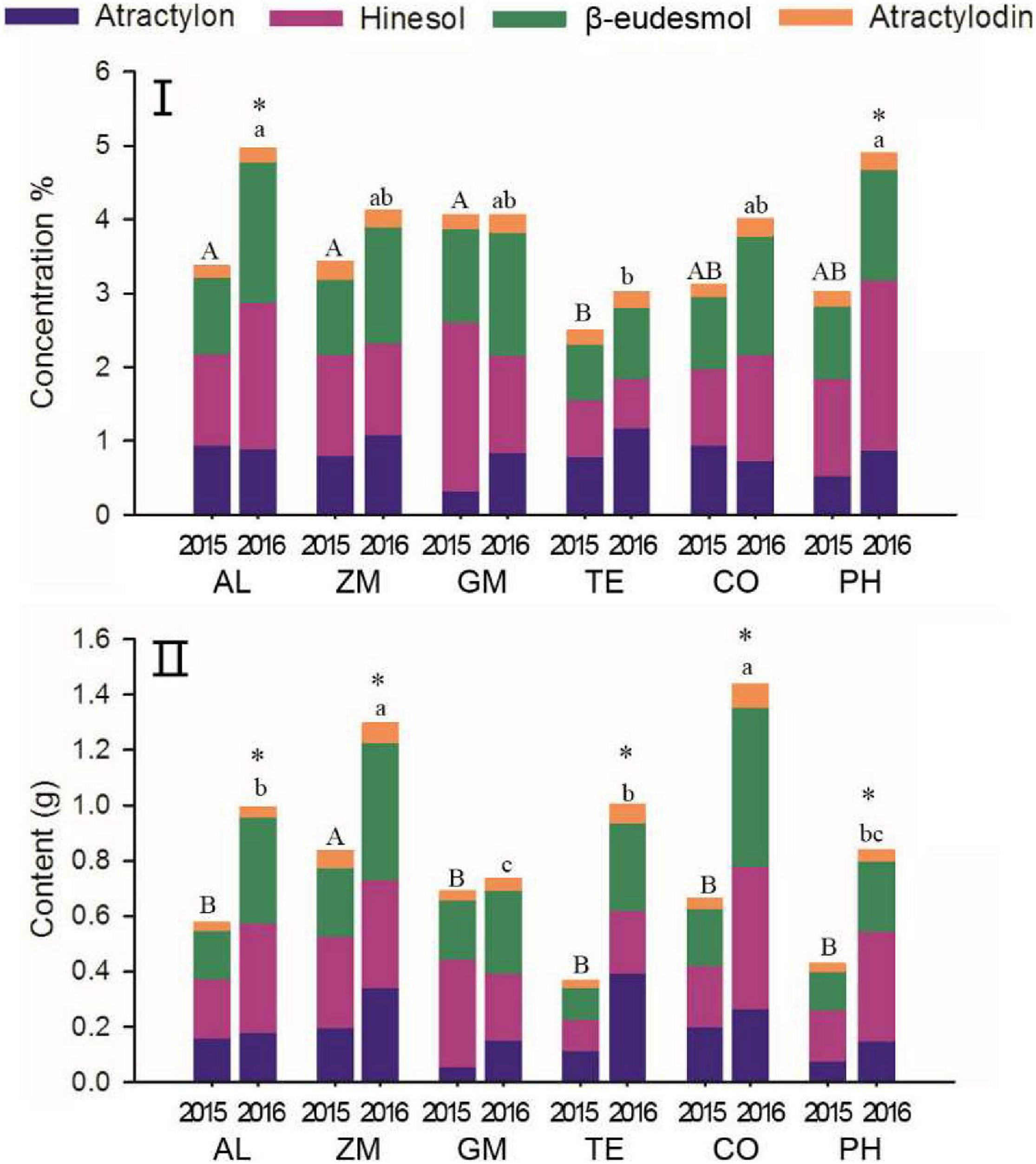
Figure 6. The effect of different intercropping treatments on concentration (I) and content (II) of total volatile oils in 2015 and 2016. Different capital (2015) and lowercase (2016) letters indicate significant differences at the 5% significance level, and asterisks indicate significant differences between 2015 and 2016 within individual treatments.
As biomass increased through time, the total volatile oil content increased in all treatments (Figure 6(II)) and increased significantly in the AL, ZM, TE, CO, and PH treatments. Total volatile oil content was highest in the CO treatment, followed by the ZM treatment; it was lowest in the GM treatment. Although the increase in content of individual volatile oils was slight in some treatments, the contents of all four individual volatile oils increased with time in all treatments (with the exception of hinesol in the GM treatment). Contents of individual volatile oils also differed among the treatments. For example, the AL and CO treatments showed clear increases in contents of all four volatile oils, whereas the ZM treatment showed increases primarily in atractylon and β-eudesmol content. The TE treatment showed increases mainly in atractylon and β-eudesmol content, and the PH treatment showed increases mainly in atractylon, β-eudesmol and hinesol content.
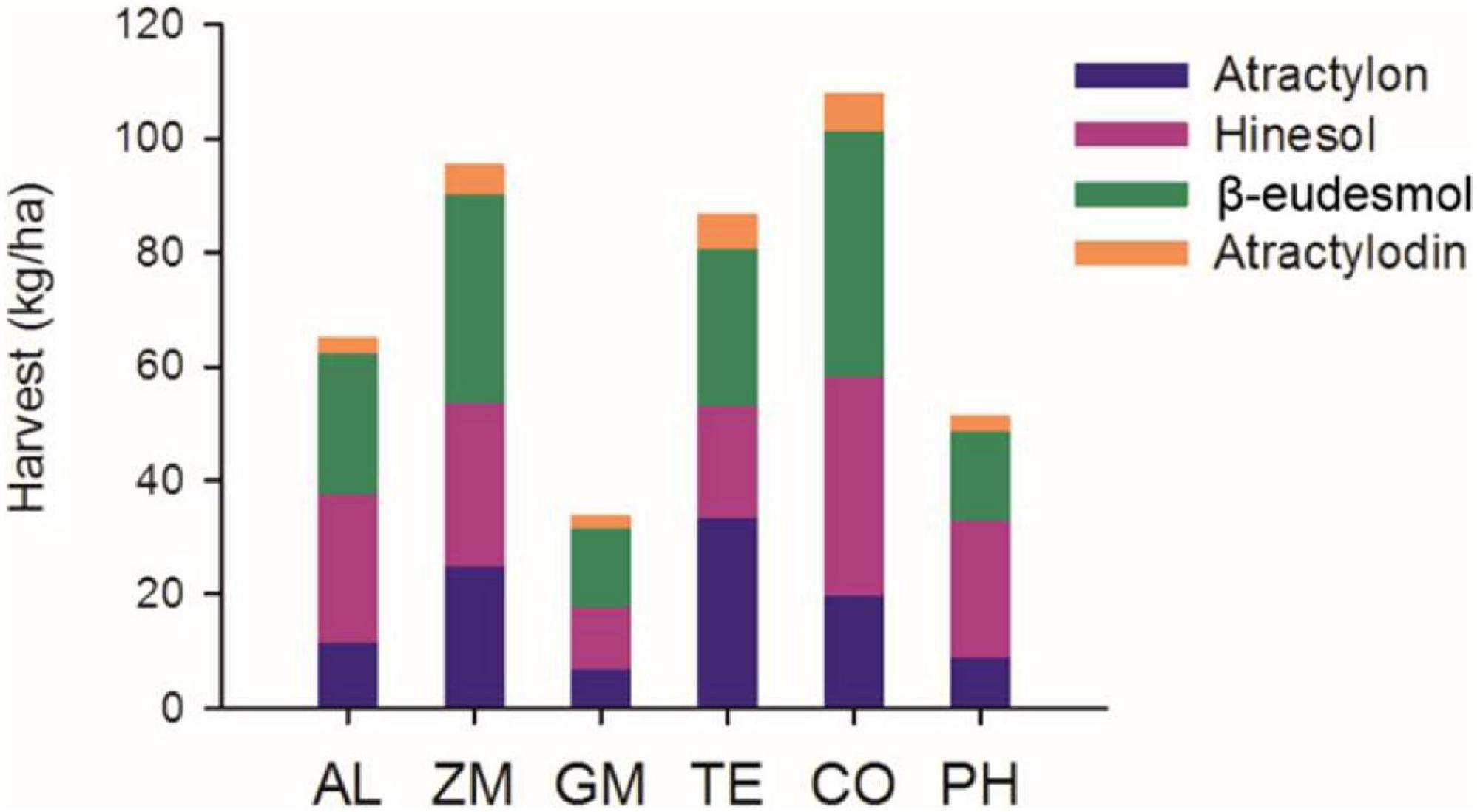
Based on plant survival, rhizome weight, and the number of plants per hectare, we calculated the total harvest of the four volatile oils in 2016 (Figure 7). The total volatile oil harvest was higher in the CO, ZM, and TE treatments than in the AL monoculture, whereas that of other treatments was lower than in the AL monoculture.
Discussion
Improved Biomass of A. lancea Under Intercropping
Complementary patterns of root distribution and plant phenology are important mechanisms by which intercropping improves yield (Keating and Carberry, 1993; Martin-Guay et al., 2018). Intercropping a deeply rooted plant with a more shallowly rooted plant efficiently utilizes belowground space and reduces root competition (Li et al., 2001; Chapagain et al., 2018; Zhang et al., 2020). Phenological complementation of different species can also reduce nutrient competition and increase resource use efficiency. In this study, ZM, TE, and CO treatments markedly improved biomass and the accumulation of four volatile oils in A. lancea. In the ZM treatment, A. lancea has a shallow root system, whereas ZM has a deep root system. In addition, the phenological phases of ZM, TE, and CO differ from those of A. lancea, thereby potentially providing suitable environmental conditions compared with the monoculture.
Effect of Root Exudates on Survival of A. lancea
Root exudates play an important role in plant health (Bais et al., 2006; Sasse et al., 2018; Olanrewaju et al., 2019). In the second year of intercropping, plant survival was higher in the ZM, TE, and CO treatments than in the other treatments (Figure 2), perhaps related to the growth-promoting effect of their root exudates. For example, maize root exudates can enrich plant growth-promoting rhizobacteria and enhance the metabolic capacity of soil bacteria (Baudoin et al., 2002; Benizri et al., 2002; Mendes et al., 2013; Vejan et al., 2016). Moreover, studies have confirmed that the root exudates of CO can inhibit the occurrence of pests and diseases, and it is widely used for this purpose in the field (Ploeg, 2000). Tagetes erecta L. has a similar inhibitory effect on root-knot nematodes (Steiner, 1941) and can effectively reduce the density of harmful nematodes (Akhtar and Mashkoor Alam, 1992; Reynolds et al., 2000).
Effects of Shading on the Accumulation of Four Volatile Oils in A. lancea
The accumulation of secondary metabolites is an important index for the evaluation of medicinal materials (Zofou et al., 2013; Song et al., 2014). Secondary metabolites are small molecular organic substances that can assist plants in adapting to the external environment (Kong et al., 2016). In our previous experiments, we found that shading increased the biomass and the content of four volatile oils in A. lancea in the short term (Li, 2018). We speculate that the shading effect of the maize plant is responsible for the increased volatile oil content of A. lancea. Maize was the tallest plant in this study, and the first year’s results showed that the atractylodin content was significantly higher in the ZM treatment than in the monoculture. Likewise, in 2016, the total volatile oil harvest of A. lancea was significantly higher in the ZM treatment than in the monoculture and was the second highest among all the treatments.
Conclusion
Five plant species were chosen as intercropping partners for A. lancea, and the growth traits, survival, and volatile oil production of A. lancea were analyzed to evaluate each intercropping combination. Compared with the monoculture, intercropping with ZM, CO, and TE significantly increased the survival and rhizome weight of A. lancea. Two years after planting, A. lancea intercropped with ZM, TE, and CO showed a great advantage in total volatile oil harvest. The underlying mechanisms of plant interaction in these systems remain to be explored in the future.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author Contributions
ZP: writing – original draft and data curation. YZ: investigation, methodology, project administration, and funding acquisition. BY, YX, XG, ZZ, XlC, XpC, and XW: data curation. TW, SW, and CK: data curation and methodology. LH: conceptualization and supervision. LG: investigation, conceptualization, supervision, project administration, and funding acquisition. KS: sample collection, conceptualization, methodology, data curation, and writing – review and editing. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the following projects: the National Natural Science Foundation of China (81874337, 81503194, and 81891014), the National Key Research and Development Program (2017YFC1700701 and 2018YFD0201100), and the Fundamental Research Funds for the Central Public Welfare Research Institutes (ZZ13-YQ-094).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abbott, L. K., and Robson, A. D. (1991). Factors influencing the occurrence of vesicular-arbuscular mycorrhizas. Agric. Ecosyst. Environ. 35, 121–150. doi: 10.1016/0167-8809(91)90048-3
Akhtar, M., and Mashkoor Alam, M. (1992). Effect of crop residues amendments to soil for the control of plant-parasitic nematodes. Bioresour. Technol. 41, 81–83. doi: 10.1016/0960-8524(92)90102-4
Bais, H. P., Weir, T. L., Perry, L. G., Gilroy, S., and Vivanco, J. M. (2006). The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 57, 233–266. doi: 10.1146/annurev.arplant.57.032905.105159
Baudoin, E., Benizri, E., and Guckert, A. (2002). Impact of growth stage on the bacterial community structure along maize roots, as determined by metabolic and genetic fingerprinting. Appl. Soil Ecol. 19, 135–145. doi: 10.1016/S0929-1393(01)00185-8
Benizri, E., Dedourge, O., Dibattista-Leboeuf, C., Piutti, S., Nguyen, C., and Guckert, A. (2002). Effect of maize rhizodeposits on soil microbial community structure. Appl. Soil Ecol. 21, 261–265. doi: 10.1016/S0929-1393(02)00094-X
Brooker, R. W., Bennett, A. E., Cong, W. F., Daniell, T. J., George, T. S., Hallett, P. D., et al. (2015). Improving intercropping: a synthesis of research in agronomy, plant physiology and ecology. New Phytol. 206, 107–117. doi: 10.1111/nph.13132
Chapagain, T., Pudasaini, R., Ghimire, B., Gurung, K., Choi, K., Rai, L., et al. (2018). Intercropping of maize, millet, mustard, wheat and ginger increased land productivity and potential economic returns for smallholder terrace farmers in Nepal. F. Crop. Res. 227, 91–101. doi: 10.1016/j.fcr.2018.07.016
Gao, X., Wu, M., Xu, R., Wang, X., Pan, R., Kim, H. J., et al. (2014). Root interactions in a maize/soybean intercropping system control soybean soil-borne disease, red crown rot. PLoS One 9:31. doi: 10.1371/journal.pone.0095031
Guo, L. P., Huang, L. Q., and Jiang, Y. X. (2005). Study on Ecological Factors Affecting the Quality of Atractylodes Lancea (Thunb.) DC. Beijing: China Academy of Chinese Medicine Sciences.
Hooks, C. R. R., Wang, K.-H., Ploeg, A., and McSorley, R. (2010). Using marigold (Tagetes spp.) as a cover crop to protect crops from plant-parasitic nematodes. Appl. Soil Ecol. 46, 307–320. doi: 10.1016/j.apsoil.2010.09.005
Keating, B. A., and Carberry, P. S. (1993). Resource capture and use in intercropping: solar radiation. F. Crop. Res. 34, 273–301. doi: 10.1016/0378-4290(93)90118-7
Khan, Z., Ampongnyarko, K., Chiliswa, P., Hassanali, A., Kimani, S., Lwande, W., et al. (1997). Intercropping increases parasitism of pests. Nature 388:631. doi: 10.1038/41677
Li, C., Hoffland, E., Kuyper, T. W., Yu, Y., Zhang, C., Li, H., et al. (2020). Syndromes of production in intercropping impact yield gains. Nat. Plants 6, 653–660. doi: 10.1038/s41477-020-0680-9
Li, L., Sun, J., Zhang, F., Li, X., Yang, S., and Rengel, Z. (2001). Wheat/maize or wheat/soybean strip intercropping I. Yield advantage and interspecific interactions on nutrients. F. Crop. Res. 71, 123–137. doi: 10.1016/S0378-4290(01)00156-3
Li, Q. (2018). Mechanism of Biosynthesis of Sesquiterpenes Volatile Oil in Atractylodes lancea Under Different Light. Guangdong Pharmaceutical University.
Li, Z., Wang, T., He, C., Cheng, K., Zeng, R., and Song, Y. (2020). Control of Panama disease of banana by intercropping with Chinese chive (Allium tuberosum Rottler): cultivar differences. BMC Plant Biol. 20:432. doi: 10.1186/s12870-020-02640-9
Maffei, M., and Mucciarelli, M. (2003). Essential oil yield in peppermint/soybean strip intercropping. F. Crop. Res. 84, 229–240. doi: 10.1016/S0378-4290(03)00092-3
Mansoor, A. S., and Mashkoor, A. (1988). Studies on the nemato-toxicity of root exudates of certain species of tagetes. Indian J. Nematol. 18, 335–337.
Martin-Guay, M. O., Paquette, A., Dupras, J., and Rivest, D. (2018). The new Green Revolution: sustainable intensification of agriculture by intercropping. Sci. Total Environ. 615, 767–772. doi: 10.1016/j.scitotenv.2017.10.024
Mendes, R., Garbeva, P., and Raaijmakers, J. M. (2013). The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 37, 634–663. doi: 10.1111/1574-6976.12028
Natarajan, N., Cork, A., Boomathi, N., Pandi, R., Velavan, S., and Dhakshnamoorthy, G. (2006). Cold aqueous extracts of African marigold, Tagetes erecta for control tomato root knot nematode, Meloidogyne incognita. Crop Prot. 25, 1210–1213. doi: 10.1016/j.cropro.2006.03.008
Ngwene, B., Neugart, S., Baldermann, S., Ravi, B., and Schreiner, M. (2017). Intercropping induces changes in specific secondary metabolite concentration in ethiopian kale (Brassica carinata) and African nightshade (Solanum scabrum) under controlled conditions. Front. Plant Sci. 8:1700. doi: 10.3389/fpls.2017.01700
Olanrewaju, O. S., Ayangbenro, A. S., Glick, B. R., and Babalola, O. O. (2019). Plant health: feedback effect of root exudates-rhizobiome interactions. Appl. Microbiol. Biotechnol. 103, 1155–1166. doi: 10.1007/s00253-018-9556-6
Oseko, J., Onyango, M. A., and Onyango, J. (2005). “Performance of african kale (Brassica carinata) to intercropping with other indigenous vegetables,” in Proceedings of the Fifth Workshop on Sustainable Horticultural Production in the Tropics.
Ploeg, A. (2000). Effects of amending soil with Tagetes patula cv. Single Gold on Meloidogyne incognita infestation of tomato. Nematology 2, 489–493. doi: 10.1163/156854100509394
Power, A. G. (1989). The ecology of intercropping. Trends Ecol. Evol. 4, 324–325. doi: 10.1016/0169-5347(89)90048-7
Pudasaini, M., Viaene, N., and Moens, M. (2008). Hatching of the root-lesion nematode, Pratylenchus penetrans, under the influence of temperature and host. Nematology 10, 47–54. doi: 10.1163/156854108783360078
Reynolds, L. B., Potter, J. W., and Ball-Coelho, B. R. (2000). Crop rotation with tagetes sp. is an alternative to chemical fumigation for control of root-lesion nematodes. Agron. J. 92, 957–966. doi: 10.2134/agronj2000.925957x
Riaz, T., Khan, S. N., and Javaid, A. (2009). Effect of co-cultivation and crop rotation on corm rot disease of Gladiolus. Sci. Hortic. (Amsterdam). 121, 218–222. doi: 10.1016/j.scienta.2009.01.041
Sasse, J., Martinoia, E., and Northen, T. (2018). Feed your friends: do plant exudates shape the root microbiome? Trends Plant Sci. 23, 25–41. doi: 10.1016/j.tplants.2017.09.003
Song, M. C., Kim, E. J., Kim, E., Rathwell, K., Nam, S.-J., and Yoon, Y. J. (2014). Microbial biosynthesis of medicinally important plant secondary metabolites. Nat. Prod. Rep. 31, 1497–1509. doi: 10.1039/c4np00057a
Steiner, G. (1941). Nematodes parasitic on and associated with roots of marigolds (Tagetes hybrids). Proc. Biol. Soc. Washingt. 54, 31–34.
Vejan, P., Abdullah, R., Khadiran, T., and Ismail, S. (2016). Role of plant growth promoting rhizobacteria in agricultural sustainability—a review. Molecules 21, 1–17. doi: 10.3390/molecules21050573
Wang, K.-H., Sipes, B. S., and Schmitt, D. P. (2003). Intercropping cover crops with pineapple for the management of Rotylenchulus reniformis. J. Nematol. 35, 39–47.
Wang, T. L., Guo, L. P., Zhang, Y., Chen, M. L., and Guan, W. (2016). Pathogen identification, regularity of development and control measures of diseases on Atractylodes lancea. Zhongguo Zhongyao Zazhi 41, 2411–2415. doi: 10.4268/cjcmm20161307
Yang, M., Zhang, Y., Qi, L., Mei, X., Liao, J., Ding, X., et al. (2015). Plant-plant-microbe mechanisms involved in soil-borne disease suppression on a maize and pepper intercropping system. PLoS One 9:e115052. doi: 10.1371/journal.pone.0115052
Yang, Y., Islam, M. S., Wang, J., Li, Y., and Chen, X. (2020). Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int. J. Biol. Sci. 16, 1708–1717. doi: 10.7150/ijbs.45538
Zeng, J., Liu, J., Lu, C., Ou, X., Luo, K., Li, C., et al. (2020). Intercropping with turmeric or ginger reduce the continuous cropping obstacles that affect pogostemon cablin (Patchouli). Front. Microbiol. 11:579719. doi: 10.3389/fmicb.2020.579719
Zhang, D., Sun, Z., Feng, L., Bai, W., Yang, N., Zhang, Z., et al. (2020). Maize plant density affects yield, growth and source-sink relationship of crops in maize/peanut intercropping. F. Crop. Res. 257:107926. doi: 10.1016/j.fcr.2020.107926
Zhao, Z., Li, Y., Zhou, L., Zhou, X., Xie, B., Zhang, W., et al. (2020). Prevention and treatment of COVID-19 using traditional Chinese medicine: a review. Phytomedicine 85:153308. doi: 10.1016/j.phymed.2020.153308
Zofou, D., Ntie-Kang, F., Sippl, W., and Efange, S. M. N. (2013). Bioactive natural products derived from the Central African flora against neglected tropical diseases and HIV. Nat. Prod. Rep. 30, 1098–1120. doi: 10.1039/c3np70030e
Appendix A
The relative proportion of individual volatile oils to all four volatile oils in A. lancea in 2015 and 2016.
Keywords: Atractylodes lancea (Thunb.) DC., intercropping, survival, production, volatile oil
Citation: Peng Z, Zhang Y, Yan B, Zhan Z, Chi X, Xu Y, Guo X, Cui X, Wang T, Wang S, Kang C, Wan X, Sun K, Huang L and Guo L (2021) Diverse Intercropping Patterns Enhance the Productivity and Volatile Oil Yield of Atractylodes lancea (Thunb.) DC.. Front. Plant Sci. 12:663730. doi: 10.3389/fpls.2021.663730
Received: 10 February 2021; Accepted: 17 June 2021;
Published: 20 July 2021.
Edited by:
Martin Weih, Swedish University of Agricultural Sciences, SwedenReviewed by:
Yong Suk Chung, Jeju National University, South KoreaChristel Baum, University of Rostock, Germany
Copyright © 2021 Peng, Zhang, Yan, Zhan, Chi, Xu, Guo, Cui, Wang, Wang, Kang, Wan, Sun, Huang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kai Sun, c3VuLWthaUBwa3UuZWR1LmNu; Luqi Huang, aHVhbmdsdXFpMDFAMTI2LmNvbQ==; Lanping Guo, Z2xwMDFAMTI2LmNvbQ==
†These authors share first authorship
 Zheng Peng
Zheng Peng Yan Zhang1†
Yan Zhang1† Xiuzhi Guo
Xiuzhi Guo Tielin Wang
Tielin Wang Sheng Wang
Sheng Wang Chuanzhi Kang
Chuanzhi Kang Luqi Huang
Luqi Huang Lanping Guo
Lanping Guo