- 1Bioinformatics and Software Development Team, Genetwister Technologies B.V., Wageningen, Netherlands
- 2Laboratory of Phytopathology, Wageningen University, Wageningen, Netherlands
- 3Molecular Plant Pathology, Swammerdam Institute for Life Sciences, University of Amsterdam, Amsterdam, Netherlands
The fungus Fusarium oxysporum is infamous for its devastating effects on economically important crops worldwide. F. oxysporum isolates are grouped into formae speciales based on their ability to cause disease on different hosts. Assigning F. oxysporum strains to formae speciales using non-experimental procedures has proven to be challenging due to their genetic heterogeneity and polyphyletic nature. However, genetically diverse isolates of the same forma specialis encode similar repertoires of effectors, proteins that are secreted by the fungus and contribute to the establishment of compatibility with the host. Based on this observation, we previously designed the F. oxysporum Effector Clustering (FoEC) pipeline which is able to classify F. oxysporum strains by forma specialis based on hierarchical clustering of the presence of predicted putative effector sequences, solely using genome assemblies as input. Here we present the updated FoEC2 pipeline which is more user friendly, customizable and, due to multithreading, has improved scalability. It is designed as a Snakemake pipeline and incorporates a new interactive visualization app. We showcase FoEC2 by clustering 537 publicly available F. oxysporum genomes and further analysis of putative effector families as multiple sequence alignments. We confirm classification of isolates into formae speciales and are able to further identify their subtypes. The pipeline is available on github: https://github.com/pvdam3/FoEC2.
1 Introduction
The cosmopolitan Fusarium oxysporum species complex (FOSC) includes well known plant pathogens that cause diseases in a broad range of hosts such as tomato, onion and banana (Edel-Hermann and Lecomte, 2019). Pathogenic F. oxysporum strains are a significant threat to crop production and cause devastating decreases in yield and economic losses (Cook et al., 2015; Panno et al., 2021).
F. oxysporum strains are grouped into a forma specialis (f. sp.) depending on the hosts they are capable of infecting. More than a hundred formae speciales (ff. spp.) have been documented to date, varying in their host range from a single species to multiple genera (Edel-Hermann and Lecomte, 2019). Examples include f. sp. cubense on banana, f. sp. melonis on melon, f. sp. cepae on onion and f. sp. radicis-cucumerinum affecting multiple cucurbit crops. However, strains grouped into the same forma specialis are not necessarily phylogenetically closely related (Baayen et al., 2000). The polyphyletic nature of host range in FOSC has hampered the determination of forma specialis of uncharacterized F. oxysporum strains using conserved gene sequences. Experimental methods can be used and remain the gold standard for host determination, but are less favorable due to their time- and labor-intensive nature.
Effector genes encode small secreted proteins that enable host colonization, for instance by suppressing host immunity. The genome of F. oxysporum f. sp. lycopersici encodes 14 such effectors named Secreted In Xylem (SIX1 - SIX14) which have been identified in xylem sap of infected tomato plants (Rep et al., 2004; Houterman et al., 2007; Ma et al., 2010; Schmidt et al., 2013). Genomic analyses uncovered that the promoter regions of SIX genes frequently contain miniature impala (mimp) transposable elements (TEs) (Schmidt et al., 2013). While mimps do not seem to be directly involved in the transcriptional regulation of SIX genes (Schmidt et al., 2013), their presence is correlated to putative horizontal gene transfer events involving effector genes (van Dam and Rep 2017b). Horizontal chromosome transfer (HCT) of accessory chromosomes or chromosome fragments is believed to be one of the driving factors facilitating the spread of host specificity between isolates (Ma et al., 2010; Li et al., 2020).
The F. oxysporum Effector Clustering (FoEC) pipeline (van Dam et al., 2016) is a computational method to predict candidate effector genes and cluster these based on their presence/absence patterns. Traditional gene prediction methods have trouble detecting effector genes due to their short sequence, fast evolutionary rate and localization in complex genomic regions (Gibriel et al., 2016). To circumvent these, the FoEC pipeline exploits the presence of (partial) mimps in the promoter regions of F. oxysporum effector genes, reducing the search space significantly. Mimps are easily identified by their terminal inverted repeats (TIRs), which can be found by their consensus sequence (Bergemann et al., 2008). When using this pipeline, F. oxysporum strains that belong to the same forma specialis are typically grouped together, solidifying the hypothesis that the effector repertoire of a F. oxysporum genome plays a role in determining the host range of a strain. FoEC has been used to classify uncharacterized F. oxysporum strains into potential formae speciales based on presence/absence patterns of putative effector genes (Urbaniak et al., 2019; Constantin et al., 2021; Sabahi et al., 2021), considerably narrowing down the number of hosts to test in experimental procedures.
Here we present the updated FoEC2 pipeline that improves upon both usability and functionality. FoEC2 is implemented in Python3 and based in Snakemake (Mölder et al., 2021) to help manage external tools and internal scripts, as well as improve scalability with multithreading, i. e. performing multiple tasks simultaneously on multiple processors where possible. It uses hidden Markov models (HMM) profile search instead of BLAST to increase the sensitivity of searches, and R Shiny to visualize presence/absence patterns of putative effectors and their multiple sequence alignments (MSAs). We benchmark and demonstrate FoEC2 on a dataset of 537 currently available FOSC genomes and demonstrate that previously uncharacterized isolates can be assigned to their most probable formae speciales.
2 Materials and methods
2.1 Implementation
FoEC2 is available on github: https://github.com/pvdam3/FoEC2. It is based on Python3 and Snakemake (v. 6.15.1) and uses conda environments for installation of dependencies. A minimal command to run FoEC2 requires a directory containing F. oxysporum genomes as input. Optional input files include genome annotations to supplement the predictions made by FoEC2 and known effector sequences to skip the prediction part of the pipeline.
Several configuration files are used in the Snakemake pipeline. The genome configuration file contains the paths to all the input genomes along with their labels (by default, the file name), as well as the paths to their annotation files if provided. A bash script is used to facilitate writing this file, taking the provided input files and producing the genome configuration file. Filtering thresholds such as ORF length, number of cysteines, three- and six-frame translation and size of search region can also be modified in the main configuration file.
2.1.1 Identification of putative effectors
Mimp TIRs are identified by a regular expression with their consensus sequence (“TT[TA]TTGCNNCCCACTGNN”) (Bergemann et al., 2008; van Dam and Rep 2017b). Open reading frames (ORFs) are located in either three- or six-frame translation mode using a custom Python3 script. In three-frame mode, which is used throughout this manuscript, ORFs must be downstream of a TIR and point away from the TIR. Six-frame mode is included to locate putative effectors anywhere surrounding the TE, a feature which may be valuable when searching for other types of genomic elements associated with effectors.
SignalP (Almagro Armenteros et al., 2019) is used to detect signal peptides in translated ORFs (‘-format short -gff3 -batch 10000’). FoEC2 supports SignalP versions 4.1 and 5; version 5.0b was used for the analysis described below. SignalP is under an academic software license and is therefore the only tool which requires manual installation. AUGUSTUS (Stanke et al., 2006; v3.4.0; parameters ‘–species=fusarium –genemodel=complete –noInFrameStop=true –predictionStart=X –predictionEnd=X –strand=X’) is used to update the ORFs with signal peptides. In case of overlap, the gene model by AUGUSTUS is retained. The resulting ORFs are filtered on length and presence of cysteines (defaults: 20aa ≤ size ≤ 600aa; 0 ≤ cysteines) and are considered putative effectors.
2.1.2 Identification of effector clusters
Clustering on all putative effector protein sequences is performed using Diamond v2.0.13 (Buchfink et al., 2015) with Diamond BLASTP. Next, MCL v14.137 (Enright et al., 2002; Van Dongen and Abreu-Goodger, 2012; i = 1.2) creates clusters of putative effectors.
2.1.3 Presence/absence variation
An MSA of each gene cluster was generated with MAFFT v7.490 (Katoh and Standley, 2013; default parameters), followed by constructing a hidden Markov model (HMM) profile with HMMER v3.3.2 (Eddy, 2011). The putative effector HMM profiles queries for the input genomes using HMMER’s nhmmer function (Wheeler and Eddy, 2013). A hit is valid if the E-value (default = 10E-10) and query coverage (default = 80%) meet the provided thresholds.
2.1.4 Clustering and visualization
R Shiny was used to visualize results with R v4.0.4 (R Core Team, 2020). The distance and clustering methods for both genomes (rows) and putative effectors (columns) can be adjusted by the user and are set at ‘binary’ distance and ‘average’ clustering by default. The following packages were used: shiny v1.7.1, shinythemes v1.2.0, dendextend v1.15.2, RColorBrewer v1.1-2, pals v1.7, pheatmap v1.0.12, phylogram v2.1.0, DT v0.20, rhandsontable v0.3.8, msaR v0.6.0 (for the BioJS MSA viewer (Yachdav et al., 2016).
2.2 Datasets
All publicly available FOSC genomes (as of January 18, 2022) were obtained from NCBI (Supplementary Table S1). Duplicates of the F. oxysporum strain 4287 (GCA_003315725.1, GCA_001703185.1, GCA_001703175.2, GCF_000149955.1) and f. sp. melonis NRRL_26406 (GCA_002318975.1) were removed, as were the samples VCG0125, VCG0120 and VCG01220 (GCA_016802195.1, GCA_016802205.1, GCA_016802225.1) as they were in silico Minimum Information about a Single Amplified Genome (MISAG) hybrids where sequencing data from f. sp. cubense was imputed onto the reference genome of Fol4287. Due to low quality of some genomes, the completeness of each accession was determined using BUSCO (Manni et al., 2021; v. 5.30; using lineage ‘hypocreales_odb10’, 4494 genes). Only genomes with a BUSCO completeness score of 70% or higher were used for further analysis, leaving a total of 537 F. oxysporum samples. The F. verticillioides genome (GCF_000149555) was added as an outgroup. An overview of all removed samples can be seen in Supplementary Table S2. To benchmark the FoEC2 pipeline, the dataset with 59 isolates described by (van Dam et al. 2016) (Supplementary Table S3) was used, as well as the FoEC pipeline available at https://github.com/pvdam3/FoEC (default settings).
The sequences of SIX genes were obtained from (van Dam et al. 2016). SIX genes (SIX1-SIX14) were identified among the final putative effectors with blastn (v. 2.12.0+; task = blastn-short; E-value threshold 1E-5).
2.3 Phylogeny construction
A phylogeny was constructed using the set of 537 publicly available F. oxysporum genomes (Supplementary Table S1), as well the F. verticillioides 7600 genome (GCF_000149555). This phylogeny was based on single copy BUSCO genes found in each genome using the ‘hypocreales_odb10’ lineage. BUSCO genes found in at least 98% of all genomes were used as input for MAFFT v7.490 to generate MSAs based on translated amino acid sequences. The resulting MSAs were trimmed with trimAl v1.4 (Capella-Gutiérrez et al., 2009; parameter ‘gappyout’) and concatenated into a single FASTA file, with a total length of 1,444,221 aligned amino acid positions. The concatenated FASTA file was then used as input for IQ-TREE v2.2.0 (Nguyen et al., 2015; parameters ‘-m JTT+F+R2 -bb 1000’) to create a phylogeny.
2.4 Comparison to effectorP
FoEC2 was compared to using SignalP (Almagro Armenteros et al., 2019) along with EffectorP (Sperschneider et al., 2016), a frequently used method to predict effector genes. All 33 publicly available F. oxysporum genomes which had gene annotations were used for this comparison (Supplementary Table S4).
Proteomes were generated with the gene annotations and were used as input for SignalP v5.0b (‘-format short -gff3 -batch 10000’). The proteins with predicted signal peptides were then fed into EffectorP v3.0 (‘-E’), which produced a FASTA file with putative effectors per genome. A modified version of FoEC2, which skipped the effector predictions and started at the identification of effector clusters, was run on all the predicted effectors. This resulted in a putative effector family presence/absence plot for each method. To compare the completeness of the predicted effectors, SIX genes (SIX1-SIX14) were identified among the final putative effectors with blastn (v. 2.12.0+; task = blastn-short; E-value threshold 1E-5).
3 Results
3.1 The FoEC2 pipeline
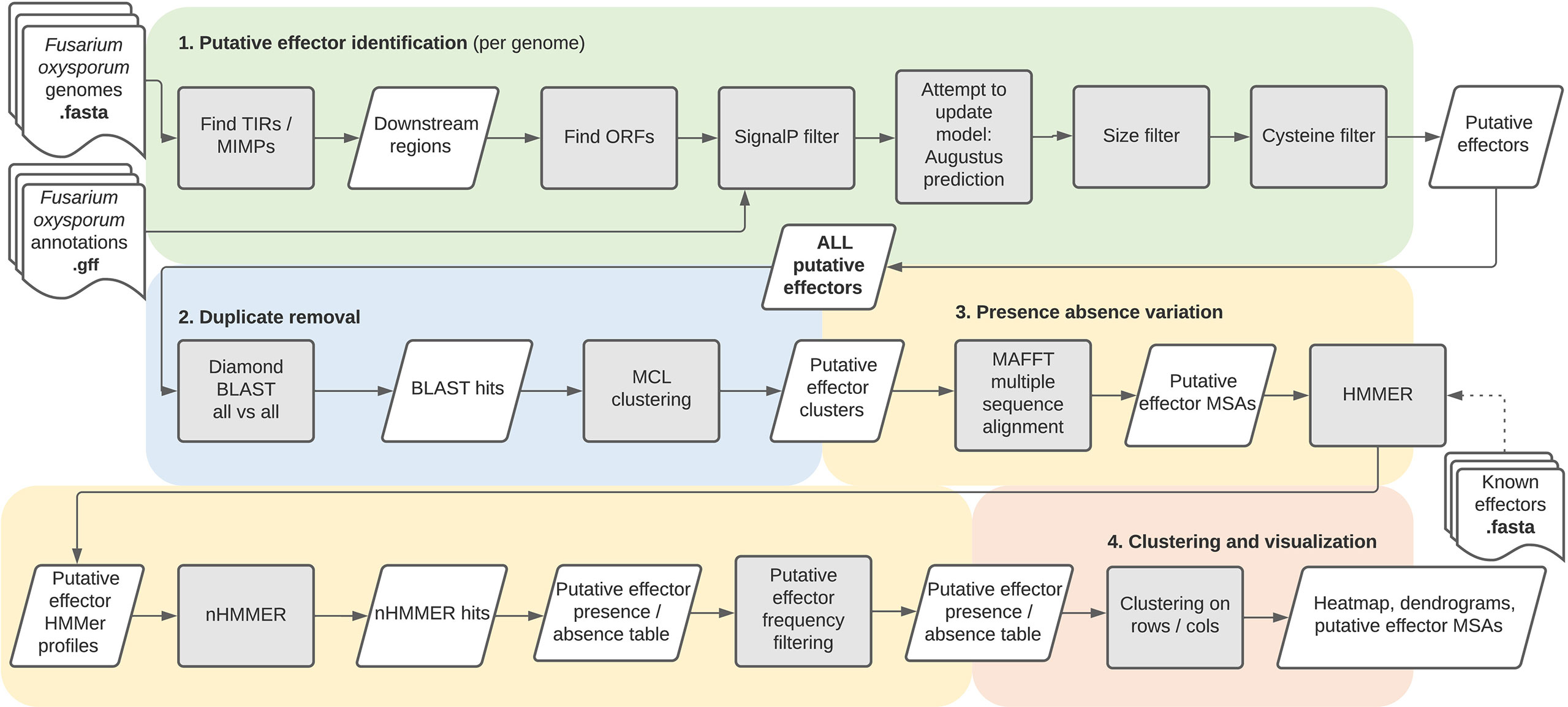
The FoEC2 pipeline, like its predecessor, is divided into four main parts (Figure 1): (i) identification of putative effectors, (ii) identification of effector families, (iii) establishment of presence/absence variation, and (iv) clustering and visualization. The first three parts are executed by a Snakemake pipeline, which produces the output needed for the fourth part which performs clustering and visualization of presence/absence patterns.
First, mimp TIRs are searched for throughout each genome using their consensus sequence. The detection of mimp TIRs can lead to the identification of complete mimps, in which two mimp TIRs in opposite direction are found within 400 bp of one another, and incomplete mimps (solo-mimp TIRs).
The next step searches for ORFs in the vicinity (<2500 bp) of the previously identified (in)complete mimps. Translation of the input genome assembly is performed in three-frame mode, where ORFs must be downstream of a TIR and point away from that TIR. ORFs identified encode a methionine followed by sequence of at least 20 amino acids and a stop codon. Next, genes from the (optional) user-provided annotations are merged with the ORFs detected by the pipeline if they are located in the ORF search regions. In the case of overlapping genes/ORFs from both sources, the user-provided gene takes precedence over the pipeline ORF prediction. Because effector proteins are secreted, SignalP (Almagro Armenteros et al., 2019) is then used select ORFs with a signal peptide. These genomic regions, containing ORFs with a predicted signal peptide, are used as input sequences for AUGUSTUS (Stanke et al., 2006) to predict a gene model. If no gene model is predicted by AUGUSTUS, the original ORF (without introns) is taken along as is. These ORFs and gene models are then optionally filtered by size and cysteine content to decrease the number of false positives. The sequences remaining after these filtering steps are considered to be putative effectors and are available as both nucleotide and protein FASTA files.
This first part of the pipeline predicts putative effectors for each genome. Multiple isolates can share the same or very similar effectors and one isolate can have multiple copies of an effector. The pipeline removes this redundancy by clustering homologous effectors into effector families based on sequence similarity. To determine the presence of putative effector families in the initial set of genomes, each cluster is represented by an HMM profile. Searching with an HMM profile instead of a single representative sequence is one of the major differences with the original FoEC pipeline. It potentially increases sensitivity as it accounts for sequence diversity. The putative effector hits are then tallied per genome, resulting in a presence/absence variation table for each putative effector family and each genome. The final filtering step removes putative effectors which have over 20 hits in any given genome, or which have 10 or more hits on average across all genomes, as these are not likely to be effector genes but more probably (part of) transposable elements.
Results are visualized in an R Shiny app (Supplementary Figure S1), showing a customizable heatmap depicting clusters, dendrograms for genomes and putative effectors, and MSAs of putative effector clusters. Metadata for both genomes and putative effectors may also be uploaded, edited and again downloaded. Several aesthetic modifications can be made and data can be downloaded.
3.2 Comparison to FoEC
First, the results of FoEC2 were compared to the FoEC pipeline using the 59 isolates analyzed by (van Dam et al. 2016). These include isolates of the ff. spp. niveum, cucumerinum, radicis-cucumerinum, melonis, lycopersici, conglutinans, radicis-lycopersici, cubense, pisi and vasinfectum. This analysis was completed after 2.2 hours on 50 CPUs. FoEC finished more rapidly on this small number of genomes (1.5 hours on similar infrastructure).
The FoEC2 pipeline resulted in 227 putative effector clusters (Supplementary Figure S2), compared to the 215 clusters found by FoEC (van Dam et al., 2016). Next, the presence/absence profiles of effector families were used to cluster the isolates. On the original dataset of 59 genomes, putative effector patterns resulted in clusters that consistently grouped samples from the same forma specialis together. Formae speciales were mostly contained in single clusters, the exception being F. oxysporum f. sp. cucumerinum, which was split into two clusters, as was observed in FoEC (van Dam et al., 2016).
3.3 Effector clustering patterns: Filling in the host specificity gaps
To test the capability of FoEC2, the pipeline was run on all 537 publicly available F. oxysporum genomes meeting the quality thresholds described in the Materials and Methods (Figure 2; Supplementary Table S1). The genome of F. verticillioides was added as an outgroup. In total, 222 out of 537 genomes contained a forma specialis annotation in their description. This analysis was completed after 29.7 hours on 50 CPUs.
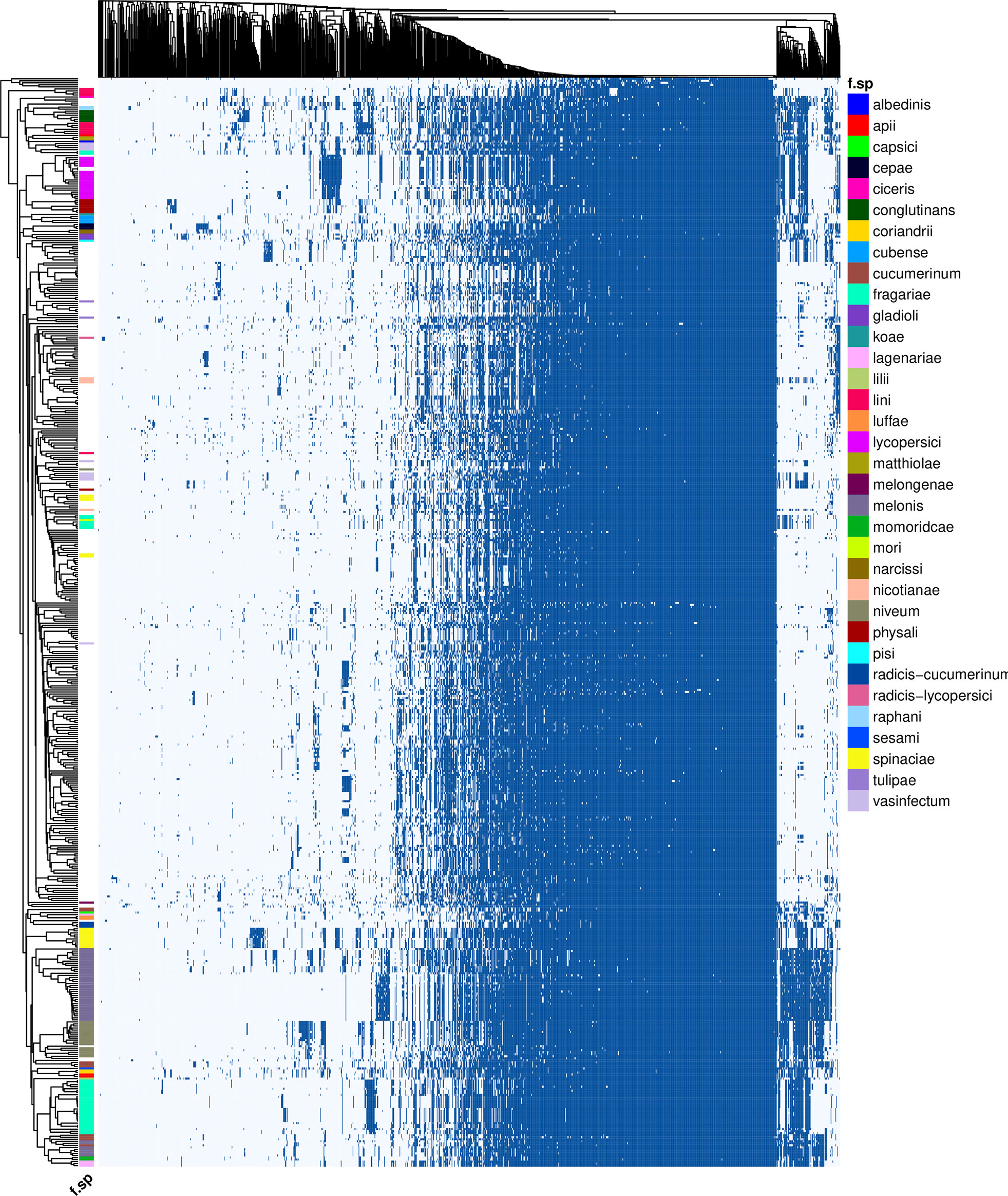
Figure 2 Presence (dark blue)/absence (light blue) variation of 1,283 putative effectors (columns) across 538 Fusarium genomes (rows). Genomes are annotated by forma specialis when possible. Presence is determined by nhmmer, given the thresholds E-value ≤ 10E-10, query coverage ≥ 80%. Clusters were generated with a binary distance matrix and average clustering.
In total, 1,251 putative effector families were identified. Hierarchical clustering of the presence/absence patterns of these families in the 537 F. oxysporum genomes (Figure 2) shows that in many cases (e.g., ff. spp. lycopersici, niveum, conglutinans, raphani, cubense, physalis, and others), these groups encompass all representatives of a single forma specialis. This approach thus allows for inference of the pathogenic potential of a F. oxysporum genome for which no forma specialis is described. For example, assemblies RBG7064 (GCA_009298205.1) and RBG7070 (GCA_009298435.1) are not assigned to a forma specialis (Figure 3A). However, they are found in a cluster of genomes annotated as f. sp. niveum and their putative effector presence pattern matches that of various niveum isolates. The metadata available for these two assemblies show that the samples were collected from Citrullus lanatus (watermelon). This means that these assemblies are likely F. oxysporum f. sp. niveum. Another example is assemblies VPRI32264 (GCA_009298805.1), VPRI16234 (GCA_009298505.1) and VPRI11681 (GCA_009298875.1) that do not have a forma specialis annotation, but group inside the F. oxysporum f. sp. lycopersici cluster (Figure 3B). While all three samples originated from Solanum samples, only VPRI32264 and VPRI11681 were collected from Solanum lycopersicum (tomato), whereas VPRI16234 was collected from Solanum tuberosum (potato). This could be an indication of an opportunistic infection of potato by a F. oxysporum f. sp. lycopersici isolate. A similar situation is shown in Figure 3C; nine isolates collected from Pisum sativum plants and one collected from soil (RBG5783, GCA_009297405.1) cluster closely together with the F. oxysporum f. sp. pisi HDV247 isolate originally sequenced by the Broad institute, indicative of being the same forma specialis. Indeed, they all possess a similar set of ‘pisi’ putative effectors (arrow, Figure 3C). The isolates mentioned for these three example cases were all sequenced and assembled in a study on phylogenetic relationships between Australian F. oxysporum isolates (Achari et al., 2020).
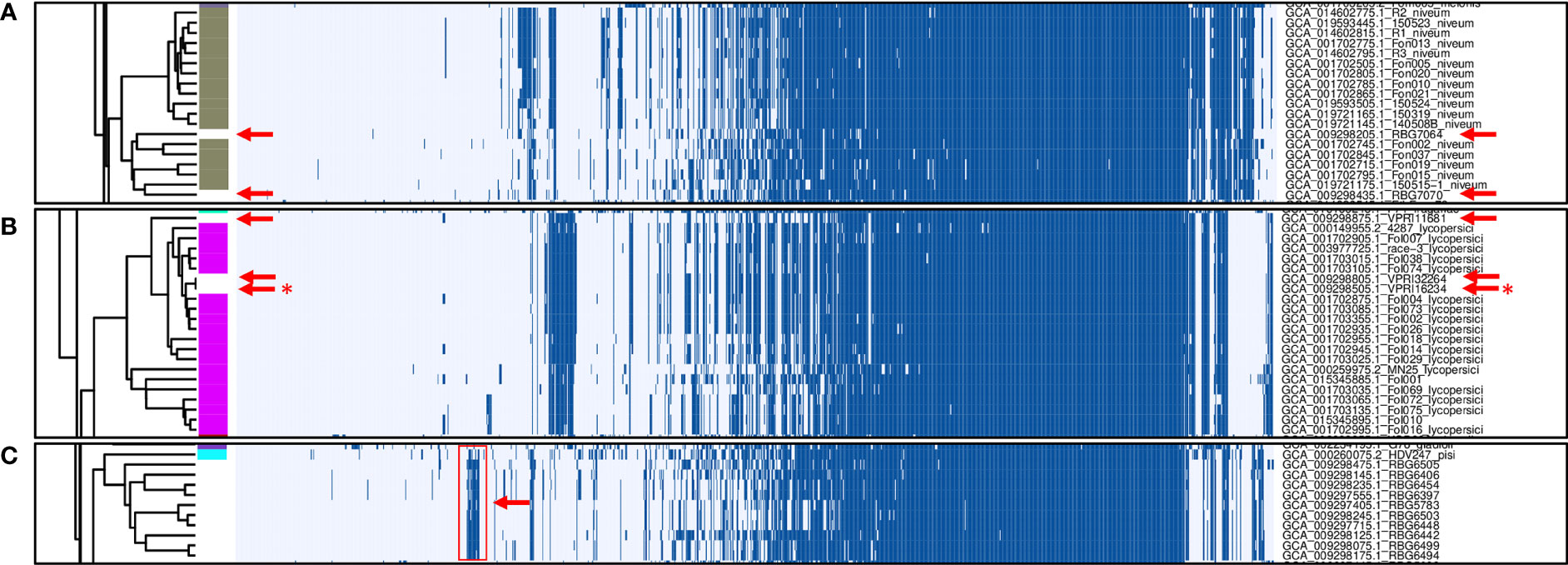
Figure 3 Zoomed-in examples of presence/absence variation in F. oxysporum genomes without a forma specialis (f. sp.) annotation. (A) Gaps in annotated f. sp. niveum strains (gray) correspond to genomes originating from Citrullus lanatus samples (red arrows). (B) Gaps in annotated f. sp. lycopersici strains (magenta) are filled by genomes resulting from Solanum lycopersicum samples (red arrows) and one genome associated with a Solanum tuberosum sample (red asterisk). (C) non-annotated samples clustering together with the f. sp. pisi HDV247 sample.
While in the examples above all members of a forma specialis reside in a single cluster, other formae speciales are distributed in multiple clusters. In some cases, this reflects differences in disease symptoms. For example, in strawberry-infecting isolates (f. sp. fragariae) distinct disease phenotypes have been described. These two phenotypes, ‘yellowing’ and ‘wilting’, likely require different sets of genetic tools to successfully cause disease in the host and have traversed independent evolutionary paths to pathogenicity towards cultivated strawberry (Henry et al., 2021). In line with these previous observations, three main strawberry/berry affecting groups reside in completely different clusters. The largest group (Figure 2, bottom) is the strawberry-yellows causing clade. In contrast to the wilt-type fragariae isolates, the yellows isolates possess a pathogenicity chromosomal fragment “chrY”. Indeed, a yellows-specific group of putative effectors is clearly visible in these isolates. Previously, strawberry-wilting isolates have been classified into three groups based on a phylogeny of conserved core genes, where W1 and W2 consist of Australian isolates that cluster phylogenetically with strawberries-yellow isolates, but here form a separate cluster with f. sp. mori, affecting blackberry, and W3 consists of Spanish isolates, that are in a distinct cluster with isolates that are assigned to f. sp. vasinfectum. Similarly, F. oxysporum f. sp. radicis-cucumerinum, which affects multiple cucurbit crops and causes root and shoot rot rather than the wilt inducing f. sp. cucumerinum isolates, forms a separate, distinct cluster from other cucurbit-infecting isolates, together with two luffae isolates and one lagenariae isolate. Finally, in line with the observations by (Batson et al. 2021), the two pathogenicity types that can be distinguished in f. sp. spinaciae also form two groups, albeit within one cluster.
Several formae speciales are dispersed in distinct clusters that do not correspond to known different pathogenicity types or symptoms. For example, four clusters of cucurbit-infecting strains were observed. The first is comprised of a large number of melonis isolates. The second contains the ‘C1’ and ‘C2’ subclades off. sp. melonis identified previously (Sabahi et al., 2021), as well as isolates of the ff. spp. momordicae, lagenariae and cucumerinum. The third clade groups together representatives of f. sp. niveum and cucumerinum isolates Foc 018, 021 and 030 (van Dam et al., 2016). Like melonis, f. sp. cucumerinum is also represented in both of the main cucurbit clades in the plot. This was also observed in (van Dam et al. 2016), where the abovementioned three cucumerinum isolates grouped away from the rest of the cucumerinum isolates. Similarly, f. sp. vasinfectum has four individual effector pattern groups. Finally, formae speciales that infect closely related hosts do not necessarily contain similar sets of effectors. For example, the placement of f. sp. nicotianae far away from other Solanaceae infecting isolates (F. oxysporum f. sp. lycopersici, physali) indicates different genes underlying pathogenicity of F. oxysporum towards these related plant species. For reference, the cluster plot including sample names, accession numbers and formae speciales is included in Supplementary Figure S3.
3.4 Core phylogeny highlights the polyphyletic nature of many formae speciales
To compare the clustering of effectors with the phylogenetic association of the analyzed isolates, a phylogenetic tree was generated based on core genes (Supplementary Figure S4). This tree was built based on a concatenated multiple sequence alignment of 3,037 conserved BUSCO genes, with a total length of 1,444,221 amino acid positions. Three major taxonomic clades are recognized within the Fusarium oxysporum species complex: clades 1, 2 and 3 (O’Donnell et al., 1998; Laurence et al., 2014). The majority (381) of the 537 F. oxysporum isolates fall inside main clade 2; 127 isolates belong to clade 3 and only 27 to clade 1. Two isolates do not clearly fall within one of the three clades in this tree; Fo65 (GCA_014324465.1) falls outside clade 1 and Fo24 (GCA_014337855.1) is positioned between clades 1 and 2/3, in line with the findings by (Constantin et al. 2021). Many formae speciales clustering together in Figure 2 are polyphyletic, i.e., they occur in distinct clades within the core phylogeny. Two very pronounced examples of this include F. oxysporum f. sp. lycopersici and F. oxysporum f. sp. niveum (Supplementary Figure S4, red and blue text highlights). These two formae speciales are each grouped in a single cluster in Figure 2, while in the core phylogeny they are located in four and five distinct clades, respectively. Various other examples of this can be found and are supportive of HCT as the mechanism of host-specific effector gene transfer.
Interestingly, the core phylogeny (Supplementary Figure S4) shows that three of the nicotianae samples are phylogenetically close to a group of lycopersici and physali isolates, while their effector profiles are very different.
3.5 Most SIX genes are detected by FoEC2
In order to evaluate whether verified effectors are identified, SIX1-SIX14 were searched for in the putative effector families produced by FoEC and FoEC2. Similar to the original pipeline, not all SIX genes were detected by FoEC2. SIX5 and SIX12 were not detected by either version of the pipeline, whereas SIX10 was found by FoEC2 using 538 genomes, but not by FoEC or FoEC2 in the set of 59 genomes. The remaining SIX genes were all found at least once among the final putative effector clusters.
3.6 Comparing FoEC2 to effectorP
Lastly, FoEC2 was compared to another computational effector prediction tool, EffectorP, a machine learning based approach which was trained on a curated set of fungal and oomycete effectors (Sperschneider et al., 2016). For this comparison, we used all 33 publicly available F. oxysporum genomes with gene annotations.
The method involving EffectorP resulted in 1,372 putative effector families (Supplementary Figure S5), compared to the 394 putative effector families obtained when using FoEC2 (Supplementary Figure S6). The number of predicted effectors per genome is 50 to 100 times higher while using EffectorP. Moreover, the number of predicted effectors is consistently high, regardless of whether the isolate is pathogenic or not, whereas FoEC2 predicted fewer effectors for non-pathogenic isolates (Supplementary Table S4).
Clustering of host specificity worked well using the FoEC2 predicted effector families (Supplementary Figure S6), whereas the clustering of EffectorP showed more influence from the core phylogeny (Supplementary Figure S6). For example, the EffectorP method did not cluster the two f. sp. lycopersici or the multiple non-pathogenic isolates together. F. oxysporum f. sp. melonis isolate NRRL26406 (GCA_000260495.2) is placed next to F. oxysporum f. sp. lycopersici isolate 4287 (GCA_000149955.2). These isolates are very close to each other in the core phylogeny (Supplementary Figure S4). The FoEC2 clustering shows a clade of Brassicaceae infecting isolates (ff. spp. conglutinans, matthiolae, raphani), whereas these formae speciales are not together in the EffectorP clustering. Like in the larger set, FoEC2 was able to detect all SIX genes except for SIX5 and SIX12 within the final putative effector families. EffectorP was only able to detect half of the SIX genes (SIX1, SIX2, SIX6, SIX9, SIX10, SIX11 and SIX13).
Overall, this comparison shows that FoEC2, due to its use of the association between mimps and effectors, is best suited to predict candidate effectors in F. oxysporum.
4 Discussion
4.1 Clustering based on putative effector profiles gives an indication of possible host species and supports horizontal chromosome transfer
In this paper, we generated the largest set of putative effectors in F. oxysporum to date: 1,251 effector families in 537 F. oxysporum genomes. It is likely that a substantial part of this number represents false positives. The pipeline allows stricter filtering by altering certain thresholds (e.g., cysteine content, protein length, etc.) while identifying putative effectors, but in this paper, settings that resulted in as few false negatives as possible were used, assessed through the detection of SIX genes. The formae speciales clusters (Figure 2) largely align with earlier observations in other studies. The fact that various formae speciales are broken up into multiple clades supports the theory that pathogenicity towards a plant host sometimes evolved through multiple independent events. This seems to be the case for F. oxysporum f. sp. fragariae (three clades, Henry et al., 2021), melonis (three clades, Sabahi et al., 2021), cucumerinum (three clades) spinaciae (two clades, Batson et al., 2021). Isolates with a similar effector repertoire and position in the hierarchical clustering (Figure 2), but with distinct core phylogenies (Supplementary Figure S4) support the prevalence of HCT within the FOSC. HCT of (partial) pathogenicity chromosomes and thus effector genes has been experimentally shown for multiple formae speciales, including lycopersici and radicis-cucumerinum (Ma et al., 2010; Vlaardingerbroek et al., 2016; van Dam et al., 2017a; Li et al., 2020).
Traces of core phylogeny similarities (and thus influence of non-lineage specific candidate effector genes) can be seen in the effector clustering plot (Figure 2), but these seem to have a limited effect. For example, F. oxysporum f. sp. cucumerinum 011 and 013 (GCA_001703455.1 and GCA_001702495.1) are close to cubense TR4 isolates even though they do not share a related plant host. These are all core phylogeny clade 1 isolates. However, in between is a F. oxysporum f. sp. koae isolate, which is in core phylogeny clade 2.
The clustering of non-annotated isolates with isolates that do have a known forma specialis description (Figure 3) provides support for their hypothesized forma specialis. This should still be confirmed by performing virulence assays on the suspected plant host species, but this analysis provides an important clue on what host an uncharacterized isolate may cause disease on. Additionally, the results can serve to identify forma specialis or even subclade specific marker sequences (van Dam et al., 2018).
4.2 False negatives due to absence of a signal peptide prediction or large sequence diversity
Some SIX genes were not found among the final list of putative effectors returned by FoEC2. SIX5 and SIX12 have not been predicted by either version of the pipeline. In the case of SIX12, its product does not have a signal peptide (Schmidt et al., 2013) and is thus filtered out by the pipeline. The SIX5 product does seem to have a signal peptide but this was not predicted by SignalP. Obviously, effectors without a signal peptide recognized by SignalP are not identified with this pipeline.
While running FoEC2 on 59 genomes, SIX10 was found and deemed a putative effector after the first part of the pipeline, but the nhmmer hits never met the minimum query coverage of 80% due to the large sequence variation inside intronic regions. Sometimes, several of these hits were found on the same contig. To improve upon the detection of SIX10 and similar effectors, the pipeline could consider multiple hits as a single entity when calculating the query coverage if they could potentially represent a single gene. Another solution could be to simply reduce the query coverage threshold, but that would result in a larger number of false positives.
4.3 The use of nhmmer increases sensitivity
One of the significant changes in FoEC2 in comparison to FoEC is the use of HMM profiles to represent clusters of putative effectors instead of the longest sequence in a cluster. HMM profiles are capable of capturing more information, keeping track of position-specific nucleotide conservation and gap or insertion frequency. The longest sequence in a cluster does not necessarily reflect information which is true for all sequences, and sequence variation is not captured when searching for presence/absence. For instance, an insertion in the longest sequence could have a negative influence on the BLAST search results. Using nhmmer is more time-consuming than blastn, but nhmmer’s increased sensitivity can identify sequences with lower similarity.
4.4 A more efficient and user-friendly effector detection pipeline
Previously, a few preparation steps were sometimes needed to run FoEC. One of these steps included changing FASTA file extensions to ‘.fa’ or ‘.fasta’ if ‘.fna’ was used. This was a common occurrence, given that files downloaded from NCBI typically have the ‘.fna’ extension. FoEC2 accepts multiple FASTA file extensions.
Another preparation step needed involved changing sequence headers in all genomes to a consistent format (e.g., ‘contig_1’) before running the pipeline. This naming scheme was used because FoEC stored information in the headers. FoEC2 instead uses GFF3 files and tables to store information. This eliminates the need to change the headers before a run and provides the advantage of having easily accessible information. Researchers can also visualize elements such as mimps, TIRs and putative effectors in a genome browser of their choice thanks to the new GFF3 output.
4.5 Effector clustering in F. oxysporum works best with FoEC2
FoEC2 significantly reduces the search space within a genome by focusing on regions in the vicinity of mimps. This explains why there are much fewer putative effector clusters when using FoEC2 rather than the commonly used approach of running EffectorP on a predicted secretome. The complete predicted effectorome produced by EffectorP also captures elements of the core phylogeny, meaning that it is not as well-suited for inferring formae speciales (Supplementary Figures S5, S6). However, it is clear that FoEC2 is not a viable alternative for EffectorP with other species because often such a relationship between effectors and genetic elements like mimps is not known.
5 Conclusion
The updated FoEC2 pipeline provides an easy way for researchers to determine an initial forma specialis classification for F. oxysporum genomes and search for putative effectors within them. This can directly influence the time and resources needed to characterize newly assembled F. oxysporum genomes by reducing the number of experiments required to confirm host specificity. The increased run time caused by using nhmmer searching is compensated by the use of multithreading facilitated by Snakemake, the chosen framework for FoEC2. The new RShiny app provides the visualization and allows users to customize plots to suit their needs. The added MSA visualization also provides an opportunity to dive into the determined putative effector clusters. Overall, the updated FoEC2 pipeline has multiple new features contained within a modularized pipeline, better accommodating the needs of users as well as making it possible to easily expand its range of functions in the future.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
All authors contributed to conceiving and designing the analysis. MG collected the data and performed the analysis. Testing of the code was performed by PD and LF. MG, LB and PD wrote the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
Authors MG, LB and PD were employed by Genetwister Technologies B.V.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.1012688/full#supplementary-material
References
Achari, S. R., Kaur, J., Dinh, Q., Mann, R., Sawbridge, T., Summerell, B. A., et al. (2020). Phylogenetic relationship between Australian Fusarium oxysporum isolates and resolving the species complex using the multispecies coalescent model. BMC Genomics 21 (1), 1–20. doi: 10.1186/s12864-020-6640-y
Almagro Armenteros, J. J., Tsirigos, K. D., Sønderby, C. K., Petersen, T. N., Winther, O., Brunak, S., et al. (2019). SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 37 (4), 420–423. doi: 10.1038/s41587-019-0036-z
Baayen, R. P., O'Donnell, K., Bonants, P. J., Cigelnik, E., Kroon, L. P., Roebroeck, E. J., et al. (2000). Gene genealogies and AFLP analyses in the Fusarium oxysporum complex identify monophyletic and nonmonophyletic formae speciales causing wilt and rot disease. Phytopathology 90 (8), 891–900. doi: 10.1094/PHYTO.2000.90.8.891
Batson, A. M., Fokkens, L., Rep, M., du Toit, L. J. (2021). Putative effector genes distinguish two pathogenicity groups of fusarium oxysporum f. sp. spinaciae. Mol. Plant-Microbe Interact. 34 (2), 141–156. doi: 10.1094/MPMI-06-20-0145-R
Bergemann, M., Lespinet, O., M’Barek, S. B., Daboussi, M. J., Dufresne, M. (2008). Genome-wide analysis of the Fusarium oxysporum mimp family of MITEs and mobilization of both native and de novo created mimps. J. Mol. Evol. 67 (6), 631–642. doi: 10.1007/s00239-008-9164-7
Buchfink, B., Xie, C., Huson, D. H. (2015). Fast and sensitive protein alignment using DIAMOND. Nat. Methods 12 (1), 59–60. doi: 10.1038/nmeth.3176
Capella-Gutiérrez, S., Silla-Martínez, J. M., Gabaldón, T. (2009). trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25 (15), 1972–1973. doi: 10.1093/bioinformatics/btp348
Constantin, M. E., Fokkens, L., De Sain, M., Takken, F. L., Rep, M. (2021). Number of candidate effector genes in accessory genomes differentiates pathogenic from endophytic Fusarium oxysporum strains. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.761740
Cook, D. C., Taylor, A. S., Meldrum, R. A., Drenth, A. (2015). Potential economic impact of Panama disease (tropical race 4) on the Australian banana industry. J. Plant Dis. Prot. 122 (5), 229–237. doi: 10.1007/BF03356557
Eddy, S. R. (2011). Accelerated profile HMM searches. PloS Comput. Biol. 7 (10), e1002195. doi: 10.1371/journal.pcbi.1002195
Edel-Hermann, V., Lecomte, C. (2019). Current status of Fusarium oxysporum formae speciales and races. Phytopathology 109 (4), 512–530. doi: 10.1094/PHYTO-08-18-0320-RVW
Enright, A. J., Van Dongen, S., Ouzounis, C. A. (2002). An efficient algorithm for large-scale detection of protein families. Nucleic Acids Res. 30 (7), 1575–1584. doi: 10.1093/nar/30.7.1575
Gibriel, H. A., Thomma, B. P., Seidl, M. F. (2016). The age of effectors: Genome-based discovery and applications. Phytopathology 106 (10), 1206–1212. doi: 10.1094/PHYTO-02-16-0110-FI
Henry, P. M., Pincot, D. D., Jenner, B. N., Borrero, C., Aviles, M., Nam, M. H., et al. (2021). Horizontal chromosome transfer and independent evolution drive diversification in Fusarium oxysporum f. sp. fragariae. New Phytol. 230 (1), 327–340s. doi: 10.1111/nph.17141
Houterman, P. M., Speijer, D., Dekker, H. L., de Koster, C. G., Cornelissen, B. J., Rep, M. (2007). The mixed xylem sap proteome of Fusarium oxysporum-infected tomato plants. Mol. Plant Pathol. 8 (2), 215–221. doi: 10.1111/j.1364-3703.2007.00384.x
Katoh, K., Standley, D. M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30 (4), 772–780. doi: 10.1093/molbev/mst010
Laurence, M. H., Summerell, B. A., Burgess, L. W., Liew, E. C. (2014). Genealogical concordance phylogenetic species recognition in the Fusarium oxysporum species complex. Fungal Biol. 118 (4), 374–384. doi: 10.1016/j.funbio.2014.02.002
Li, J., Fokkens, L., Conneely, L. J., Rep, M. (2020). Partial pathogenicity chromosomes in Fusarium oxysporum are sufficient to cause disease and can be horizontally transferred. Environ. Microbiol. 22 (12), 4985–5004. doi: 10.1111/1462-2920.15095
Manni, M., Berkeley, M. R., Seppey, M., Simão, F. A., Zdobnov, E. M. (2021). BUSCO update: novel and streamlined workflows along with broader and deeper phylogenetic coverage for scoring of eukaryotic, prokaryotic, and viral genomes. Mol. Biol. Evol. 38 (10), 4647–4654. doi: 10.1093/molbev/msab199
Ma, L. J., van der Does, H. C., Borkovich, K. A., Coleman, J. J., Daboussi, M. J., Di Pietro, A., et al. (2010). Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature 464 (7287), 367–373. doi: 10.1038/nature08850
Mölder, F., Jablonski, K. P., Letcher, B., Hall, M. B., Tomkins-Tinch, C. H., Sochat, V., et al. (2021). Sustainable data analysis with snakemake. F1000Research 10, 33. doi: 10.12688/f1000research.29032.2
Nguyen, L. T., Schmidt, H. A., Von Haeseler, A., Minh, B. Q. (2015). IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32 (1), 268–274. doi: 10.1093/molbev/msu300
O’Donnell, K., Kistler, H. C., Cigelnik, E., Ploetz, R. C. (1998). Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. 95 (5), 2044–2049. doi: 10.1073/pnas.95.5.2044
Panno, S., Davino, S., Caruso, A. G., Bertacca, S., Crnogorac, A., Mandić, A., et al. (2021). A review of the most common and economically important diseases that undermine the cultivation of tomato crop in the mediterranean basin. Agronomy 11 (11), 2188. doi: 10.3390/agronomy11112188
R Core Team (2020) R: A language and environment for statistical computing. Available at: https://www.R-project.org/ (Accessed May 15, 2022).
Rep, M., van der Does, H. C., Meijer, M., Van Wijk, R., Houterman, P. M., Dekker, H. L., et al. (2004). A small, cysteine-rich protein secreted by Fusarium oxysporum during colonization of xylem vessels is required for I-3-mediated resistance in tomato. Mol. Microbiol. 53 (5), 373–1383. doi: 10.1111/j.1365-2958.2004.04177.x
Sabahi, F., Sain, M. D., Banihashemi, Z., Rep, M. (2021). Comparative genomics of Fusarium oxysporum f. sp. melonis strains reveals nine lineages and a new sequence type of AvrFom2. Environ. Microbiol. 23 (4), 2035–2053. doi: 10.1111/1462-2920.15339
Schmidt, S. M., Houterman, P. M., Schreiver, I., Ma, L., Amyotte, S., Chellappan, B., et al. (2013). MITEs in the promoters of effector genes allow prediction of novel virulence genes in Fusarium oxysporum. BMC genomics 14, 1, 1–21. doi: 10.1186/1471-2164-14-119
Sperschneider, J., Gardiner, D. M., Dodds, P. N., Tini, F., Covarelli, L., Singh, K. B., et al. (2016). EffectorP: predicting fungal effector proteins from secretomes using machine learning. New Phytol. 210 (2), 743–761. doi: 10.1111/nph.13794
Stanke, M., Keller, O., Gunduz, I., Hayes, A., Waack, S., Morgenstern, B. (2006). AUGUSTUS: ab initio prediction of alternative transcripts. Nucleic Acids Res. 34 (suppl_2), W435–W439. doi: 10.1093/nar/gkl200
Urbaniak, C., van Dam, P., Zaborin, A., Zaborina, O., Gilbert, J. A., Torok, T., et al. (2019). Genomic characterization and virulence potential of two Fusarium oxysporum isolates cultured from the international space station. Msystems 4 (2), .e00345-18. doi: 10.1128/mSystems.00345-18
van Dam, P., de Sain, M., Ter Horst, A., van der Gragt, M., Rep, M. (2018). Use of comparative genomics-based markers for discrimination of host specificity in Fusarium oxysporum. Appl. Environ. Microbiol. 84 (1), e01868-17. doi: 10.1128/AEM.01868-17
van Dam, P., Fokkens, L., Ayukawa, Y., van der Gragt, M., Ter Horst, A., Brankovics, B., et al. (2017a). A mobile pathogenicity chromosome in Fusarium oxysporum for infection of multiple cucurbit species. Sci. Rep. 7 (1), 1–15. doi: 10.1038/s41598-017-07995-y
van Dam, P., Fokkens, L., Schmidt, S. M., Linmans, J. H., Kistler, H. C., Ma, L. J., et al. (2016). Effector profiles distinguish formae speciales of Fusarium oxysporum. Environ. Microbiol. 18 (11), 4087–4102. doi: 10.1111/1462-2920.13445
van Dam, P., Rep, M. (2017b). The distribution of miniature impala elements and SIX genes in the Fusarium genus is suggestive of horizontal gene transfer. J. Mol. Evol. 85 (1), 14–25. doi: 10.1007/s00239-017-9801-0
Van Dongen, S., Abreu-Goodger, C. (2012). “Using MCL to extract clusters from networks,” in Bacterial molecular networks (New York, NY: Springer), (pp. 281–295).
Vlaardingerbroek, I., Beerens, B., Rose, L., Fokkens, L., Cornelissen, B. J., Rep, M. (2016). Exchange of core chromosomes and horizontal transfer of lineage-specific chromosomes in Fusarium oxysporum. Environ. Microbiol. 18 (11), 3702–3713. doi: 10.1111/1462-2920.13281
Wheeler, T. J., Eddy, S. R. (2013). Nhmmer: DNA homology search with profile HMMs. Bioinformatics 29 (19), 2487–2489. doi: 10.1093/bioinformatics/btt403
Keywords: effectors, mimp, effector prediction, host specificity, snakemake, SIX genes, Fusarium oxysporum, FoEC2
Citation: Brenes Guallar MA, Fokkens L, Rep M, Berke L and van Dam P (2022) Fusarium oxysporum effector clustering version 2: An updated pipeline to infer host range. Front. Plant Sci. 13:1012688. doi: 10.3389/fpls.2022.1012688
Received: 05 August 2022; Accepted: 26 September 2022;
Published: 19 October 2022.
Edited by:
Juan Emilio Palomares-Rius, Spanish National Research Council (CSIC), SpainReviewed by:
Salim Bourras, Swedish University of Agricultural Sciences, SwedenHelena Gil Azinheira, University of Lisbon, Portugal
Copyright © 2022 Brenes Guallar, Fokkens, Rep, Berke and van Dam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter van Dam, cC52YW5kYW1AZ2VuZXR3aXN0ZXIubmw=
 Megan A. Brenes Guallar
Megan A. Brenes Guallar Like Fokkens2,3
Like Fokkens2,3 Martijn Rep
Martijn Rep Lidija Berke
Lidija Berke Peter van Dam
Peter van Dam