Abstract
P2-type Ca2+ ATPases are responsible for cellular Ca2+ transport, which plays an important role in plant development and tolerance to biotic and abiotic stresses. However, the role of P2-type Ca2+ ATPases in stress response and stomatal regulation is still elusive in soybean. In this study, a total of 12 P2-type Ca2+ ATPases genes (GmACAs and GmECAs) were identified from the genome of Glycine max. We analyzed the evolutionary relationship, conserved motif, functional domain, gene structure and location, and promoter elements of the family. Chlorophyll fluorescence imaging analysis showed that vegetable soybean leaves are damaged to different extents under salt, drought, cold, and shade stresses. Real-time quantitative PCR (RT-qPCR) analysis demonstrated that most of the GmACAs and GmECAs are up-regulated after drought, cold, and NaCl treatment, but are down-regulated after shading stress. Microscopic observation showed that different stresses caused significant stomatal closure. Spatial location and temporal expression analysis suggested that GmACA8, GmACA9, GmACA10, GmACA12, GmACA13, and GmACA11 might promote stomatal closure under drought, cold, and salt stress. GmECA1 might regulate stomatal closure in shading stress. GmACA1 and GmECA3 might have a negative function on cold stress. The results laid an important foundation for further study on the function of P2-type Ca2+ ATPase genes GmACAs and GmECAs for breeding abiotic stress-tolerant vegetable soybean.
Introduction
Vegetable soybean (Glycine max L., also named “Maodou” in China and “edamame” in Japan) serves as a fresh vegetable and has higher nutritional content than grain- and oil-type soybean (Dong et al., 2014; Liu et al., 2017a). In recent years, the production of vegetable soybean has increased with the shift in consumer preference. Abiotic stresses such as low temperature, drought, and salinity have become important factors influencing the yield, taste, and nutritional quality of vegetable soybean (Xu et al., 2016). However, there is limited research work on the abiotic stress tolerance of vegetable soybean.
Cytosolic calcium concentration ([Ca2+]cyt) is a key cellular second messenger that plays a crucial role in plant growth, development, and stress response (Chen et al., 2010; Kudla et al., 2010; Seybold et al., 2014). In plants, guard cells integrate environmental and endogenous signals to regulate the aperture of stomatal pores and [Ca2+]cyt oscillations are essential components of stomatal closure. [Ca2+]cyt oscillation can regulate the closing of stomata through two mechanisms. Short-term calcium [Ca2+]cyt oscillation can quickly close the stomata, while long-term [Ca2+]cyt oscillation is controlled by a stable oscillation frequency, transient number, duration and amplitude that regulate stomatal closure (Allen et al., 2001; Fuglsang and Palmgren, 2021). In addition, calcium signaling can also participate in hormonal pathways to regulate stomatal movement under biotic and abiotic stresses (Tagliani, 2020; Thor et al., 2020; Hsu et al., 2021; Ou et al., 2022). Ca2+ has minimal physical mobility and is rarely transported between cells (Marschner, 2012). Therefore, it is necessary for plants to regulate intracellular Ca2+ concentration depending on the coordinated activities of pumps, channels, and co-transporters on the plasma membrane, tonoplast and membranes of different organelles (Bush, 1995; Sze et al., 2000; Tuteja and Mahajan, 2007). When external environmental stimuli induce the opening of Ca2+ channels, a rapid increase of [Ca2+]cyt is perceived and decoded to give an appropriate response. Elevation of Ca2+ causes changes in the Ca2+ regulatory proteins and their targets, leading to the activation of downstream signaling events in different plant cell types (Clapham, 2007; Kudla et al., 2010; Bredow and Monaghan, 2018; Zhang et al., 2019).
Ca2+ transport in plant cells is mainly mediated by Ca2+ channels, Ca2+ antiporters, and Ca2+ pumps (White, 2000; Spalding and Harper, 2011; Wang et al., 2016; Demidchik et al., 2018). Ca2+ pumps belong to a large family of phosphorylated (P)-type ATPases located at the plasma membrane (PM), tonoplast, endoplasmic reticulum (ER), and Golgi (Table 1; Urbina et al., 2006; Hilleary et al., 2020). Generally, Ca2+ pumps are divided into ER-type Ca2+ ATPases (ECAs, P2A ATPases) and autoinhibited Ca2+ ATPases (ACAs, P2B ATPases) in plants, which have a higher affinity for Ca2+ transportation than Ca2+ channels and Ca2+ antiporters (Carafoli, 1991; Huda et al., 2013b; García Bossi et al., 2019). P2A ATPases serve housekeeping functions to load intracellular compartments with Ca2+ and/or Mn2+ whereas P2B ATPases are tightly regulated Ca2+-pumps that regulate cytoplasmic Ca2+ concentrations (subject to regulation by calmodulin and phosphorylation) that have the main role in signal transduction (Fuglsang and Palmgren, 2021). P2B-type pumps in plants contained an auto-inhibitory region that partly overlaps the calmodulin-binding domain at the N-terminal (Geisler et al., 2000b). Thus, calcium transport and ATP hydrolysis are inhibited in the absence of calmodulin, but this inhibition was eliminated by the binding of Ca2+ to the calmodulin-binding domain (Hwang et al., 2000; Lone et al., 2006; Lee et al., 2007).
Table 1
| Gene Name | Locus name | Gene Location | Protein length (aa) | MW (Da) | pI | GRAVY | Arabidopsis orthologs | Arabidopsis orthologs subcellular localization |
|---|---|---|---|---|---|---|---|---|
| GmACA1 | GLYMA_04G045400 | Chr4: 3622850..3633108: + | 1,019 | 111255.82 | 5.92 | 0.181 | ACA1 | Endoplasmic reticuluma |
| GmACA2 | GLYMA_01G193600 | Chr1: 52814383..52820169: − | 1,014 | 110604.22 | 5.72 | 0.173 | ACA2 | Endoplasmic reticulumb |
| GmACA8 | GLYMA_15G1675001 | Chr15: 14792771..14837236: + | 1,082 | 117916.60 | 8.06 | 0.078 | ACA8 | Plasma membranec |
| GmACA9 | GLYMA_08G222200 | Chr8: 038317..18058442: + | 1,092 | 119274.47 | 6.39 | 0.030 | ACA9 | Plasma membraned |
| GmACA10 | GLYMA_17G057800 | Chr17: 4370750..4397516: − | 1,105 | 120782.81 | 7.81 | 0.091 | ACA10 | n.r. |
| GmACA11 | GLYMA_19G136400 | Chr19: 39763468..39774889: − | 1,035 | 113482.71 | 5.75 | 0.166 | ACA11 | Vacuole membranee |
| GmACA12 | GLYMA_19G159900 | Chr19: 42082444..42086717: − | 1,069 | 118014.41 | 7.07 | 0.052 | ACA12 | Plasma membranef |
| GmAC13 | GLYMA_19G038600 | Chr19: 5397711..5401490: − | 1,029 | 113059.19 | 7.06 | 0.075 | ACA13 | n.r. |
| GmECA1 | GLYMA_03G175200 | Chr3: 38873627..38880295: + | 1,060 | 116399.97 | 5.30 | 0.039 | ECA1 | Endoplasmic reticulumg |
| GmECA2 | GLYMA_07G053100 | Chr7: 4650513..4656886: − | 1,073 | 118776.88 | 5.52 | 0.084 | ECA2 | n.r. |
| GmECA3 | GLYMA_04G046700 | Chr4: 3723235..3748189: + | 1,001 | 109910.84 | 5.67 | 0.245 | ECA3 | Golgih |
| GmECA4 | GLYMA_19G175900 | Chr19: 43587386..43593918: + | 1,060 | 116530.04 | 5.22 | 0.031 | ECA4 | n.r. |
Summary of Ca2+-ATPase genes in Glycine max and the identity of Arabidopsis homologs.
PI, isoelectric point; MW, molecular weight; and GRAVY, Grand Average of Hydropathy; n.r.: Not reported.
Ca2+ pumps are essential in many aspects of plant growth and development, including pollen tube growth, programmed cell death, and polarized tip growth in roots (Allen et al., 2001; Rajiv and Robinson, 2004; Kurusu et al., 2005; Young et al., 2006; Pandey et al., 2007; Monshausen et al., 2008; Hocking et al., 2017), as well as responses to abiotic stresses (e.g., shading, heat, cold, salt, drought, and osmotic stress), biotic stresses and symbiosis (e.g., fungi and bacteria; Lillo, 1994; Sanders et al., 1999; Rahman et al., 2016; Liu et al., 2017c; Yuan et al., 2018; Li et al., 2019). In Arabidopsis, pollen of loss-of-function mutant aca9 displayed a reduced growth potential and a high frequency of aborted fertilization, resulting reduction in seed set (Schiøtt et al., 2004). Ca2+ ATPases are also available to be involved in the activation of salicylic acid (SA)-dependent programmed cell death regulated by AtACA4 and AtACA11 in Arabidopsis (Clapham, 2007; Yann et al., 2010). Ca2+-stimulated root growth and the detoxification of high Mn2+ are modulated by AtECA3 (Li et al., 2008). AtACA8 functions in the response to cold stress (Schiøtt and Palmgren, 2005), and AtACA8 and AtACA11 are involved in hypoxia stress (Wang et al., 2016). Overexpression of Arabidopsis AtACA4 and AtACA2 in yeast improved salt tolerance (Geisler et al., 2000a; Anil et al., 2007). In other plant species, overexpression of rice OsACA6 in tobacco can effectively regulate ROS mechanism and proline synthesis and improve resistance to salt and drought stress (Huda et al., 2013a). The expression level of soybean Ca2+-ATPase 1 (SCA1) was highly and rapidly induced by salt stress and a fungal elicitor (Chung et al., 2000). P2B-type Ca2+ ATPase loss-of-function mutants from the moss Physcomitrella patens exhibit susceptibility to salt stress (Enas et al., 2009).
Stomatal movement facilitates transpiration and photosynthesis, and actively regulates responses to biotic and abiotic stresses in plants (Melotto et al., 2008; Chen et al., 2017; Liu et al., 2017b; Wang and Chen, 2020). Stomatal closure stimuli such as abscisic acid (ABA), hydrogen peroxide, drought, salinity, cold, elevated external Ca2+ and elevated atmospheric CO2 (Gilroy et al., 1990; McAinsh et al., 1990, 2000; Allen et al., 2001; Sanders et al., 2002; Young et al., 2006; Chen et al., 2017). There is emerging evidence linking the Ca2+ ATPases with stomatal regulation. For instance, BONZAI1 (AtBON1) positively regulated the activities of AtACA8 and AtACA10. The bon1, aca10, and aca8 knock-out mutants have defects in stomatal closure in response to environmental stimuli (Yang et al., 2017). However, the specific roles of other P2-type Ca2+ ATPases in stomatal function under external environmental stimulation have not yet been elucidated.
The objective of this study was to characterize P2-type Ca2+ ATPase genes in soybean by bioinformatics, physiological and molecular approaches. We hypothesized that GmACAs and GmECAs are key components of abiotic stress tolerance via the regulation of Ca2+ signaling in soybean. We analyzed potential family members of Ca2+ ATPases that may be involved in the regulation of abiotic stress and stomatal movement. We provide important information for further understanding of the biological functions of P2-type Ca2+ ATPases and utilization of P2-type Ca2+ ATPase genes for improving stress resistance and crop yield in soybean.
Materials and Methods
Plant Materials, Growth Conditions, and Abiotic Stress Treatments
For expression pattern analysis of P2-type Ca2+ ATPase genes under different abiotic stresses, the seeds of vegetable soybean cultivar “Zhexian 9” were placed in two rows (each row contained eight seeds) on double-layer filter papers (20 × 30 cm) with 3 cm away from the upper and lower edges. Then a layer of wet filter paper was covered on the seeds. The three-layer filter paper with seeds was rolled up from left to right and placed in a covered bucket (20 × 20 cm). Sterile water was added to the bucket every day. The bucket was placed in a light incubator under the conditions of 25°C/25°C (day/night), 12 h/12 h photoperiod (day/night) and 70% relative humidity for germination. Seedings with consistent germination were then transplanted into a pot filled with peat/vermiculite (2:1). Three seedlings in each pot were cultured in a light incubator in the same condition for germination and irrigated with water.
Two-week-old soybean seedlings (V4 stage, with three fully expanded leaves) were used to study the expression patterns of GmACAs and GmECAs after salt, drought, cold and shading stresses. Soybean seedlings under normal growth conditions were used as a control. In salt treatment, soybean seedlings were transferred to 250 mM NaCl solution for root soaking treatment (Yong et al., 2008). For drought treatment, seedlings were placed on dry filter papers in a light incubator (Chen et al., 2007; Chen et al., 2014). Potted seedlings were cultured in a light incubator at 4°C for cold treatment (Du et al., 2009). Moreover, potted seedlings were transferred to a light incubator with a photoperiod of 24 h (night) for shading treatment (Guimarães-Dias et al., 2012). The first fully unfolded true leaves of soybean were collected as samples at 0, 1, 6, 12, 24, and 48 h after treatment and immediately frozen in liquid nitrogen and stored at −80°C for RNA extraction. The control and each treatment had three biological repeats.
Identification of the Soybean P2-Type Ca2+ ATPase Genes and Sequence Analysis
The genome sequence and gene annotation of G. max, Ostreococcus tauri, Chlamydomonas reinhardtii, P. patens, Selaginella moellendorffii, Oryza sativa, Zea mays, Capsicum annuum, and Arabidopsis thaliana were downloaded from the National Center for Biotechnology Information (NCBI) database.1 Hidden Markov Model (HMM) was used to predict soybean P2-type Ca2+ ATPase genes. The gene family sequence feature file E1-E2 ATPase (PF00122.20) was obtained from Pfam.2 PF00122.20 feature sequence file was alignment to soybean proteome database through Hmmer search online version 2.39.03 with the E value at 10−10. The obtained protein sequences were searched for protein domains through the alignment Pfam-A database of the Hmmscan program. Sequences that did not contain family characteristic domains were deleted. BLASTp program4 was used for protein sequence alignment to search the genome sequence of A. thaliana and O. sativa for orthologs. Computation of the theoretical isoelectric point (pI) and molecular weight (Mw) of soybean P2-type Ca2+ ATPase proteins was performed using the compute pI/Mw online tool.5 Grand average of hydropathicity (GRAVY) was calculated using Protparam6 and ProtComp 9.0 was used to predict the sub-cellular localization.7
Phylogenetic Analysis
Multiple amino acid sequences of identified P2-type Ca2+ ATPase genes were aligned using ClustalX2.1.0.0 software (Larkin et al., 2007). The phylogenetic trees comparing soybean and multiple species (O. tauri, C. reinhardtii, P. patens, S. moellendorffii, O. sativa, Z. mays, C. annuum, A. thaliana, and G. max) were utilized the neighbor-joining method and a graphical representation was produced with MEGA-X software and bootstrap analysis. The robustness of each node in the tree was determined using 1,000 bootstrap replicates with the pairwise deletion option. The phylogenetic trees comparing soybean and A. thaliana were also constructed with Mega-X by the parameters above. The MEME 5.0.5 online program (Bailey et al., 2015)8 was used for the identification of motifs in the P2-type Ca2+ATPase proteins sequences. Evolutionary bioinformatics was conducted as described in (Zhao et al., 2019). Briefly, candidate protein sequences were selected using the 1,000 Plant Transcriptome (1KP) database (Leebens-Mack et al., 2019).9 The amino acid sequences were employed as the query sequences to access the transcriptome data with the criterion of E-value <10−10 and coverage >50% by using BLASTP. MAFFT10 was applied to align the protein sequences and the phylogenies constructed with the online toolkit RAxML of CIPRES (Miller et al., 2010). The Interactive Tree of Life resource11 was used to annotate gene trees.
Bioinformatic Analysis
The location of the functional domain was to align the identified soybean P2-type Ca2+ ATPase protein sequence one by one using PHMMER online website.12 In addition, the online Gene Structure Display Service (GSDS2.0)13 was used to predict the intron structure by comparing the cDNA of the soybean P2-type Ca2+ ATPase genes with the corresponding genomic DNA sequences.
All the soybean P2-type Ca2+ ATPase genes were mapped to the chromosomes from the physical location information obtained from the soybean genomic database using Circos (Krzywinski et al., 2009). Multiple collinear scanning toolkit (MCScanX) was used to analyze gene duplication events with the default parameters (Wang et al., 2012). The syntenic analysis maps were constructed using the Dual Synteny Plotter software.14
The upstream 2 kb regions from the transcription start site of each soybean P2-type Ca2+ ATPases genes were extracted from the soybean genome sequence, and used to identify cis-elements by the PlantCARE server.15
Chlorophyll Fluorescence Imaging and Stomatal Aperture Analysis
Chlorophyll fluorescence imaging (Chaerle et al., 2007) was performed using V4 stage soybean seedlings at the control and abiotic stress treatment for 12 h. Whole seedlings were photographed with Manual Plant Explorer™ (PhenoVation, Holland). Each measurement was performed on three replicates for each treatment, with 15 seedlings from each replicate. Stomatal aperture images were taken using Digital Microscope VHX-7000 Series (KEYENCE, Japan) according to (O’Carrigan et al., 2014). Each measurement was performed on three replicates for each treatment, with 50 stomata from each replicate.
Real-Time Quantitative PCR Analysis of Gene Responses to Abiotic Stresses
Total RNA was isolated from leaves using the EZNA Plant RNA Kit (Omega Bio-Tec, United States) following the manufacturer’s instructions. RNA integrity was verified with 1% agar gel electrophoresis and the RNA concentration was measured using BioDrop uLite (BioDrop, United Kingdom). The first cDNA strand was synthesized from 1 μg of total RNA using Hifair® III 1st Strand cDNA Synthesis Kit with dsDNase (Yeasen, China) according to the manufacturer’s instructions. Real-time quantitative PCR (qPCR) was conducted in Bio-rad CFX96™ (Bio-rad, United States) using Hieff® qPCR SYBR Green Master Mix (None ROX; Yeasen, China). The 15 μl reaction mixture contained 2 μl of a diluted template (10 μl of the generated first-strand cDNA diluted by 90 μl ddH2O), 7.5 μl of Hieff® qPCR SYBR Green Master Mix, and 0.4 μl of each of the two gene specific primers (10 μM), 4.7 μl ddH2O. The reactions were performed as follows: 95°C for 30 s, 40 cycles at 95°C for 10 s, 60°C for 15 s, and 72°C for 20 s. A melting curve analysis was conducted following each assay to confirm the specificity of the amplicon for each primer pair. Gene-specific primers were designed using Primer 3 (Untergasser et al., 2012). Relative gene expression values were calculated using the 2−ΔΔCT method with the soybean GmEF1b as the reference gene (Hu et al., 2009). The gene-specific primers are listed in Supplementary Table S1.
Data Analysis
Student’s t-tests were used to determine significance levels for control and treatment phenotypic data (Fv/Fm and stomatal aperture). Significance levels: 0.01 < *p ≤ 0.05, 0.001 < **p ≤ 0.01, ***p ≤ 0.001. The relative transcript expression levels of each vegetable soybean P2-type Ca2+ ATPase genes were transformed to log2. The data clustering analysis and the quantitative color scheme were performed by Amazing Heatmap.14
Results
Identification of the P2-Type Ca2+ ATPase Genes in Glycine max
In this study, we first obtained the P2-type Ca2+ ATPase gene sequences from the G. max genome by HmmerWeb version 2.39.0. Twelve P2-type calcium ATPase genes from G. max were identified by further BLASTp methods of NCBI. We renamed the Glycine max P2B-type Ca2+ ATPase genes (GmACA1, GmACA2, GmACA8, GmACA9, GmACA10, GmACA11, GmACA12, and GmACA13) and P2A-type Ca2+ ATPase genes (GmECA1, GmECA2, GmECA3, and GmECA4) based on close homology to the corresponding Arabidopsis genes (Table 1). The results showed that GmACAs and GmECAs were distributed on eight soybean chromosomes (Table 1). The lengths of the 12 P2-type Ca2+ ATPases varied from 1,001 (GmECA3) to 1,105 (GmACA10) residues with an average of 1,053 amino acids. Molecular weights of GmACAs and GmECAs ranged from 109.9 to 120.8 kDa and their theoretical isoelectric points (pIs) ranged from 5.22 (GmECA4) to 8.06 (GmACA8).
Evolutionary Analysis of the P2-Type Ca2+ ATPases
To deduce the evolutionary relationship of the P2-type Ca2+ ATPase genes, a phylogenetic analysis was performed for nine green plant species (Figure 1). Phylogenetic analysis showed that the P2-type Ca2+ ATPase genes are divided into seven groups. The P2B-type Ca2+ ATPase genes were assigned to the I–V Groups. Groups I and II contain the most CAs of land plants while Groups III–IV belong to algae (Figure 1). The P2A-type Ca2+ ATPase proteins were assigned to VI and VII Groups. The GmACAs proteins were most closely related to ACAs in Arabidopsis (Figure 1B). All of the five members (CrCA1, CrCA2, CrCA3, CrCA4, and CrCA6) of C. reinhardtii in the order of Chlamydomonadales in Group III were CrCAs. OtCA1 of O. tauri in the order of Mamiellales was the only member in Group IV (Figure 1A). The full-length amino acid sequences of GmACA8 and GmECA3 were used to further analyze the evolutionary origin of this family of genes using the 1KP database (Figure 2). We found that the homologs of ECA3s and CA8s were highly conserved in plants and algae with 510 and 820 out of the 1,300 OneKP species. As is presented in Figure 2, the homologs of ECA3s and CA8s have existed in all tested clades of the land plant lineage with high similarity (Bryophyta, Lycophyta, Ferns, Gymnosperm, and angiosperm), as well as the algae (Glaucophyta, Rhodophyta, Chlorophyta, and Streptophytina). This is consisting with the previous study that the P2A ATPases and P2B ATPases are common in both prokaryotes and eukaryotes (Palmgren et al., 2020; Figure 2).
Figure 1
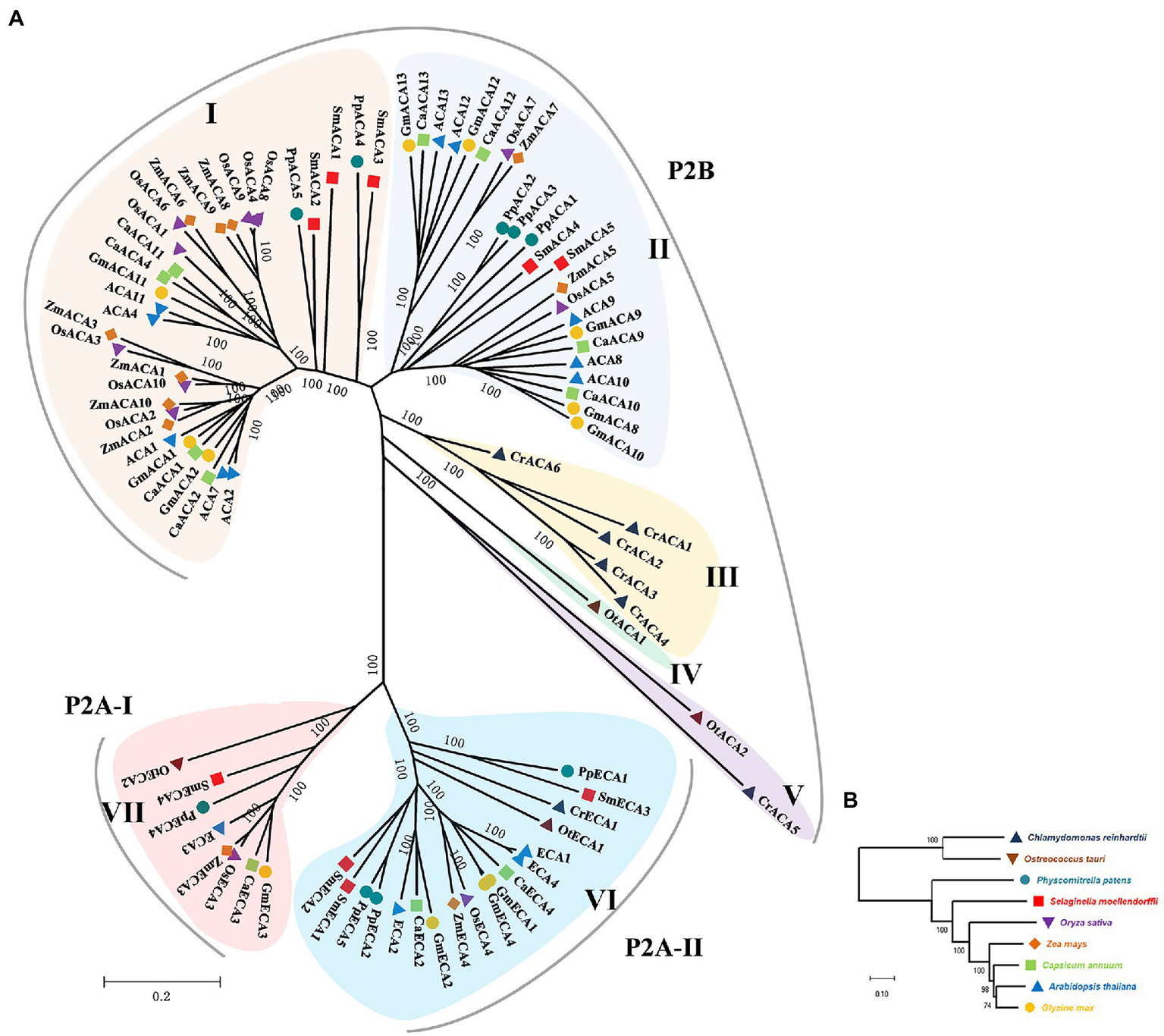
The phylogenetic tree for the P2- type ATPases. (A) The tree was constructed from a complete alignment of Chlamydomonas reinhardtii, Ostreococcus tauri, Physcomitrella patens, Selaginella moellendorffii, Oryza sativa, Zea mays, Capsicum annuum, Arabidopsis thaliana, and Glycine max P2-type ATPases and generated with the MEGA-X program using the Neighbor-Joining method. The resulting groups are shown in different shades of colors. (B) Evolutionary relationship of P2-type ATPases between green species used to construct phylogenetic trees.
Figure 2

Phylogenetic analysis of GmACA8 and GmECA3. Genes sampled from Chromia algae were used as the outgroup (in the shade of dark brown) and the root of the tree, and the Interactive Tree of Life resource was used to annotate gene trees. The location of GmACA8 and GmECA3 were shown on the phylogenetic trees. The blue bar chart outside the phylogenetic tree represents the percentage of identical matches, which is range from 0 to 100.
Ten conserved motifs were detected in the P2-type Ca2+ ATPase protein sequences of all species (Supplementary Figure S1, Supplementary Table S2). Except for CrCA6, 10 motifs were detected in all members of Group I, II, III, and IV. OtCA2 in Group V did not contain motif 5. CrCA5 only contained six motifs without motifs 5, 6, 8, and 9. Motif 7 was missing from all members in Groups VI and VII. Motif 5 was not found in SmECA4 and OtECA2. Overall, P2-type Ca2+ ATPases in the same evolutionary lineage of green plants had similar motif compositions and arrangements (Supplementary Figure S1).
Functional Domains in Glycine max P2-Type Ca2+ ATPases
In order to understand the evolutionary trend and potential function of GmACAs and GmECAs, we carried out phylogenetic analysis based on the full-length protein sequences and conserved functional domain analysis of P2-type Ca2+ ATPases between G. max and Arabidopsis (Supplementary Figures S2, S3). P2-type Ca2+ ATPases from Glycine max and Arabidopsis were divided into three evolutionary groups and GmECAs were distributed into two groups. We identified seven conserved domains, containing typical Cation transporter/ATPase, N-terminus, E1-E2 ATPase, Cation transporting ATPase, C-terminus and 8–10 transmembrane, and signal domains. Except for ACA12 and ACA13, all members of Groups I and II contained the N terminal auto-inhibitory domain (Supplementary Figure S2).
Gene Structure Localization and Synteny Analysis of P2-Type Ca2+ ATPase Genes
The diversity of genetic structure is one of the mechanisms that promote the evolution of multiple gene families (Garris et al., 2005; Schad et al., 2013). We compared the number and location of exons and introns in the P2-type Ca2+ ATPase gene sequences of Arabidopsis and G. max (Supplementary Figure S4). The results showed that the homologous genes in the same evolutionary groups exhibited similar exon-intron composition. The number of exons in GmACAs and GmECAs varied greatly from 1 to 42. Members in Group II of P2-type Ca2+ ATPase genes contained the largest and least number of exons, GmACA10 contained the most 42 exons, GmACA8 contained 38 exons, while GmACA12 and GmACA13 only contained 1 exon. In Group VII and VI, GmECA3 contained 35 exons, GmECA2 contained nine exons, GmECA1 and GmECA4 contained eight exons (Supplementary Figure S4).
We then analyzed the gene duplication of GmACAs and GmECAs in the G. max genome. A total of 12 P2-type Ca2+ ATPase genes were unevenly distributed in eight chromosomes. Chromosome (Chr) 19 had the most genes, including GmACA11, GmACA12, GmACA13, and GmECA4. There was no tandem duplication in the GmACAs and GmECAs, but there were nine pairs of segmental duplicates. For instance, GmACA2 has three homologous genes on chromosomes 5, 11, and 17, respectively (Figure 3; Supplementary Figure S5).
Figure 3

Chromosome localization and collinearity analysis of Glycine max P2-type ATPases family proteins. Colored lines indicate P2-type ATPases syntenic blocks in the soybean genome. Gray lines indicate all syntenic blocks except P2-type ATPases. The shades of color in the inner chromosome chromosomes represent the number of genes at the location of the chromosome, and yellow to red indicates the number of genes from low to high.
Cis–Elements Analysis of the Promoters of Glycine max P2-Type Ca2+ ATPase Genes
Different cis-elements in gene promoters determine gene spatial and temporal-specific expression (Wang et al., 2013). Thus, we analyzed the cis-elements in the promoter region of GmACAs and GmECAs (Figure 4). The results showed that the cis-elements in the promoters of GmACAs and GmECAs are mainly divided into two types. Type I was relevant to phytohormones, including MeJA, gibberellin, salicylic acid, abscisic acid, and auxin responsiveness cis-elements. Type II was related to abiotic stresses, including light, anaerobic, low-temperature, and drought responsiveness cis-elements (Figure 4).
Figure 4
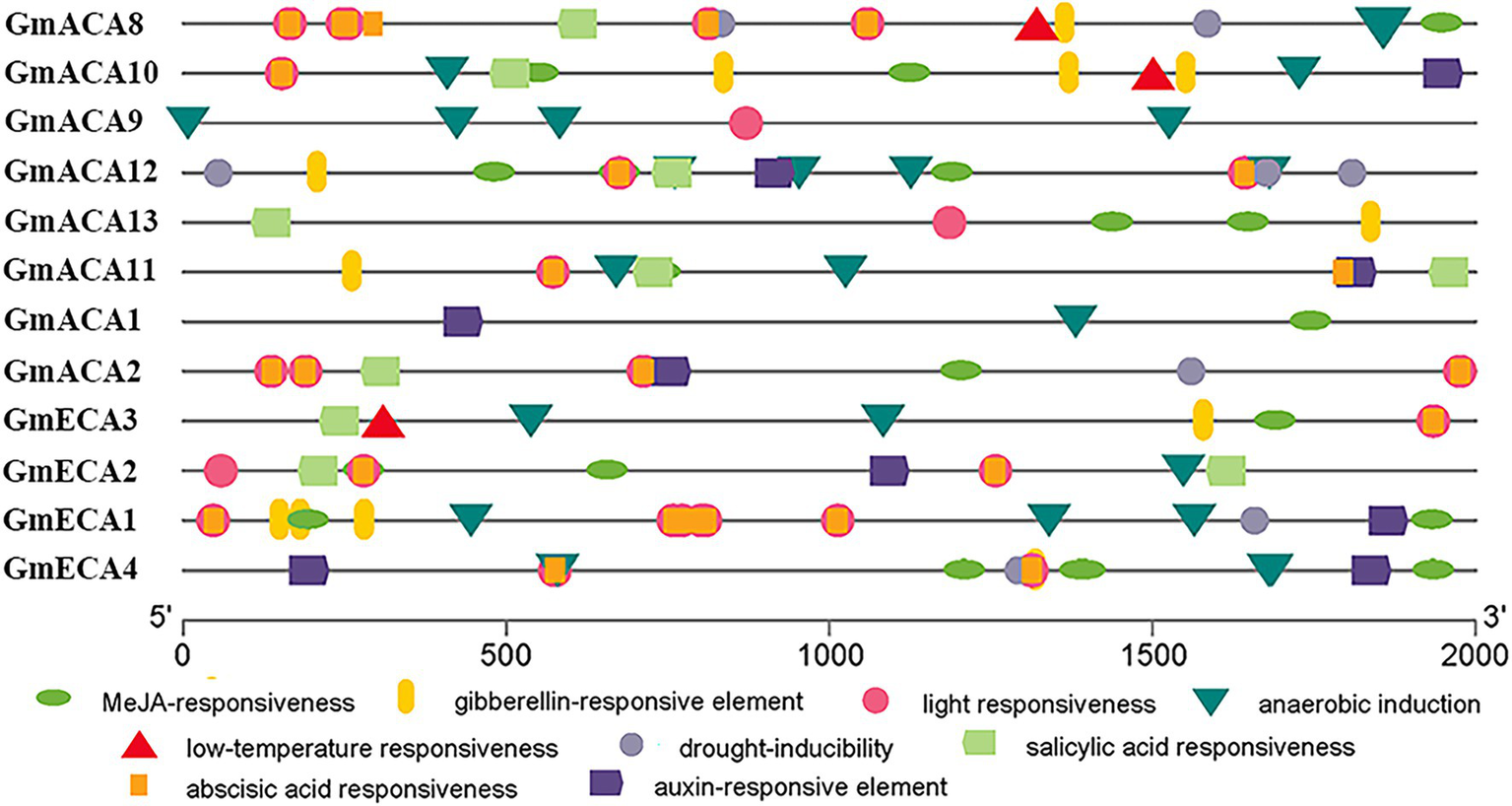
Analysis of the cis-elements of the 2,000 bp promoter sequence upstream of the initial codon of Glycine max P2-type ATPases genes from the evolutionary level. Different color graphics represent different cis-elements and positions on the promoter.
Chlorophyll Fluorescence Imaging Assay of Soybean Under Abiotic Stresses
We then conducted experiments to validate these predictions for GmACAs and GmECAs in soybean. False color images of chlorophyll fluorescence yield (Fv/Fm) from leaves indicated a significant difference in damage to soybean plants under drought, cold, salt, and shading stresses (Figure 5). The average Fv/Fm of leaves was significantly reduced by drought, salt, and cold stresses, implicating a serious impact on the overall photosynthetic capacity of leaves. However, less damage was observed in leaves as indicated by Fv/Fm values after shading treatment.
Figure 5
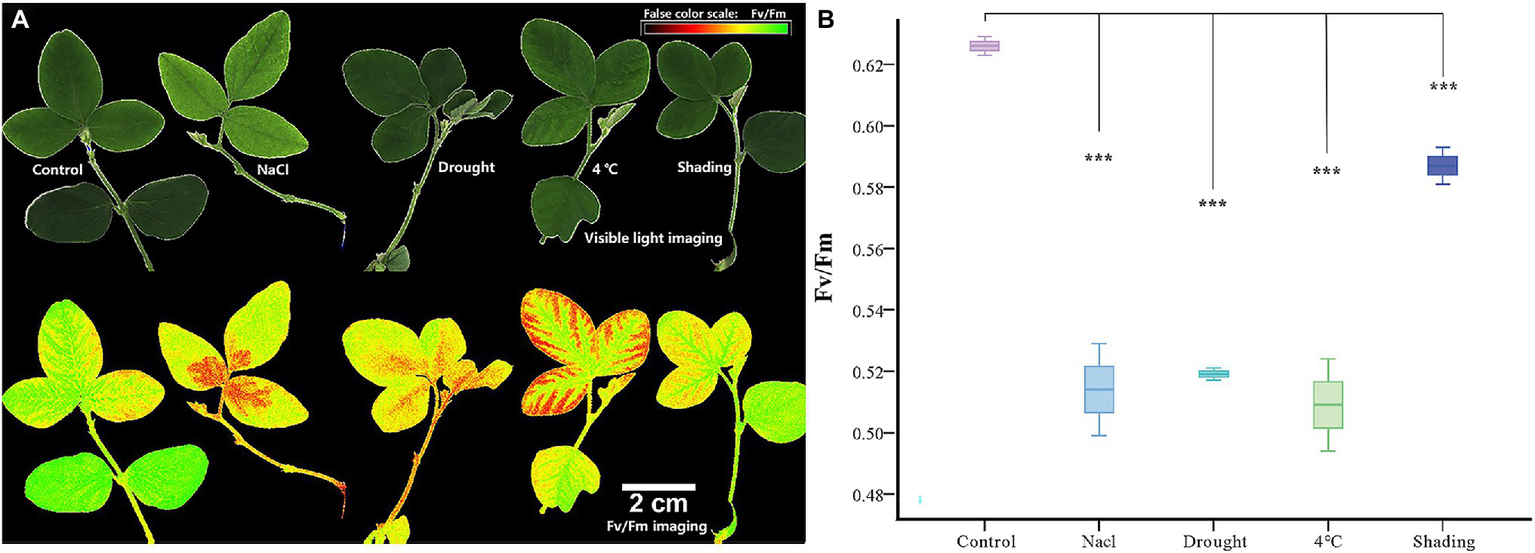
Chlorophyll fluorescence imaging and chlorophyll fluorescence yield (Fv/Fm) index in vegetable soybean under control and abiotic stress. (A) Abiotic stress damage in soybean leaves is visualized by chlorophyll fluorescence imaging. The panels show false-color images of the chlorophyll fluorescence yield (Fv/Fm) of leaves after 12 h stress. (B) Quantitative comparison of abiotic stress damage to soybean leaves determined by chlorophyll fluorescence imaging (Fv/Fm). ***p ≤ 0.001.
Expression Patterns of GmACAs and GmECAs in Response to Abiotic Stress
The expression of all 12 P2-type Ca2+ ATPase genes was analyzed under different abiotic stresses to further validate their potential functions (Figure 6). Overall, drought, salt, and cold stress caused more serious damage to vegetable soybean, where the expression of GmACAs and GmECAs were largely up-regulated. Meanwhile, less damage was found under shading stress and P2-type Ca2+ ATPase genes were mainly down-regulated. Most of GmACAs and GmECAs were up-regulated during the five time points under drought stress, except for GmACA2 (down-regulated in 12, 24, and 48 h), as well as GmACA8, GmACA9, and GmACA11 (down-regulated in 12 h; Figure 6A). Similar results were found under the cold treatment (Figure 6B). Only the expression of GmACA1, GmECA3, and GmACA11 decreased compared with the control. Interestingly, GmACA2 showed a significantly high expression from 6 to 48 h of cold stress as compared to other GmACAs and GmECAs. Under salt stress, most of GmACAs and GmECAs showed an increase throughout the treatment. There was a sudden drop in the expression of GmACA8, GmACA9, GmACA10, GmACA11, GmACA13, and GmECA2 at 6 h of salt treatment. The expression level of GmECA3 reached peaks at 12 h followed by a decrease at 24 and 48 h (Figure 6C). Moreover, the expression pattern of GmACAs and GmECAs under shading stress was dramatically different in contrast to drought, salinity, and cold treatments. There was a short-term up-regulation in the expression level of all 12 GmACAs and GmECAs under 1-h shading stress (Figure 6D). Then, almost all of the genes showed down-regulation except for GmECA1, which kept higher expression compared to the control.
Figure 6

Heat map expression profiles of P2-type ATPases genes in leaf under four different abiotic stresses. (A) drought stress; (B) cold (4°C); (C) salinity stress (250 mM); (D) shading stress. The color bar in each panel represents log2 expression values, blue indicates lower and red higher transcript abundance compared to the relevant control. The annotated color quantitation scale is seen as a vertical column on the right side of the heat map. Abiotic stress stages used for expression profiling are mentioned at the bottom of each column.
Stomatal Aperture Assay of Soybean Under Abiotic Stresses
Taking into consideration that the function of GmACAs and GmECAs in regulating [Ca2+]cyt are closely related to the stomatal aperture. We performed an imaging analysis of the stomatal state under different stresses. The stomatal opening degree was quantified using the ImageJ program (Figure 7A). The results showed that the stomatal opening of soybean was significantly reduced by the drought, cold and salt stresses to almost complete closure (Figure 7B). However, the stomata displayed less degree of closure in response to the shading treatment. We further conducted a correlation analysis between the expression levels of Ca2+ ATPase genes and the stomatal aperture under four abiotic stresses, and found that the expression of Ca2+ ATPase genes was negatively correlated with the stomatal aperture (Supplementary Figure S6).
Figure 7
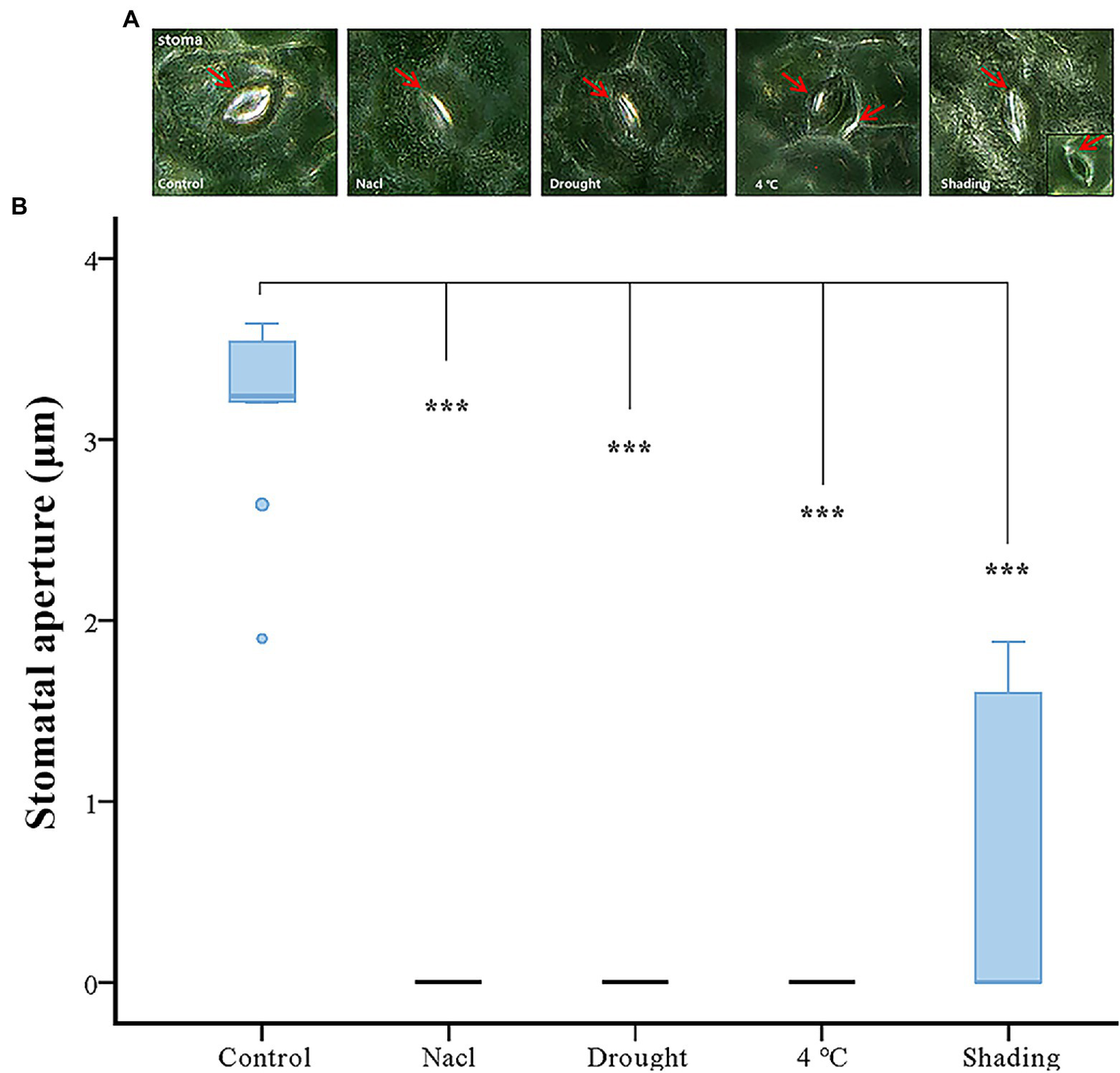
Effect of abiotic stress on stomatal aperture. (A) The microscope of stomatal movement of vegetable soybean leaves under control and abiotic stress. The red arrow indicates the position of the stoma. (B) Inhibition of stomatal aperture by abiotic stress. ***p ≤ 0.001.
Discussion
The Evolution of P2-Type Ca2+ ATPases Is Linked to Stress Tolerance in Land Plants
P2-type Ca2+ ATPases are involved in maintaining the accurate concentration of Ca2+, Mn2+, and Zn2+ in the cytosol located in different membranes (Feng et al., 2002; Li et al., 2008). With the completion of many plant genome assemblies, it is convenient to compare the characteristics of gene families to understand the evolutionary relationships among different species. Previous studies showed that there were 14 P2-type Ca2+ ATPase genes in A. thaliana (Rahmati Ishka, 2015), 29 in Glycine soja (Sun et al., 2016), 11 in rice (Li et al., 2015), 13 in wheat (Aslam et al., 2017), 4 in O. tauri, 7 in C. reinhardtii, 11 in P. patens, and 9 in S. moellendorffii (Pedersen et al., 2012). In this study, 12 P2-type ATPase genes were firstly identified from the G. max genome and the evolutionary origin, the expression level of GmACAs and GmECAs and their potential function on the stomatal movement of vegetable soybean leaves under abiotic stress were characterized.
P2-type Ca2+ ATPases system is responsible for the extrusion of cytosol Ca2+. Its high affinity and low turnover efflux ability could make the cytosol Ca2+ concentration back to a few micromolar after signal mediated influx (Kudla et al., 2010). In the process of plant evolution, different members of the P2-type Ca2+ ATPase family seemed to have their unique spatial positioning and functions. In Arabidopsis, It was reported that AtACA1, AtACA2, and AtECA1 are localized at the endoplasmic reticulum; AtACA8, AtACA9, AtACA10, and AtACA12 are localized at the plasma membrane; AtACA11 is localized at the vacuolar membrane; AtECA3 was localized at the Golgi (Table 1; Liang and Sze, 1998; Bonza et al., 2000; Hwang et al., 2000; Schiøtt et al., 2004; Lee et al., 2007; George et al., 2008; Li et al., 2008; Limonta et al., 2014; Rahmati Ishka, 2015). Ostreococcus Tauri is a unicellular alga, which does not have the spatial ability to contain bigger organizational structures (Raven, 2002), indicating less requirement for Ca2+ transport. Therefore, the regulation of calcium signals in Ostreococcus Tauri cells is relatively simple compared with the vascular plants such as Arabidopsis and soybean. Phylogenetic tree analysis found that Ostreococcus Tauri contained only 4 P2-type Ca2+ ATPase members. From the evolutionary origin of GmACA8 and GmECA3 (Figure 2), it was found that the genetic relationship of P2-type Ca2+ ATPases was farther from that of algae, and the number of species distributed by GmECA3 (103 species) in algae is less than the number of species distributed by GmACA8 (129 species). The results suggested that there are differences between P2-type Ca2+ ATPase genes in algae and vascular plants, which might be a sign of functional differentiation in calcium signal regulation and stress tolerance.
GmACAs and GmECAs Are Key Genes in Abiotic Stress Response in Soybean
Calcium signals were reported to be involved in abiotic stress response and induced stomatal closure. Drought stress, abscisic acid (ABA), hydrogen peroxide, cold, the elevation of external Ca2+, and atmospheric CO2 all induce stomatal closure. [Ca2+]cyt signals could be generated by influx and efflux of the ion from the extracellular space, or by storage and release from intracellular compartments, such as the endoplasmic reticulum (ER), Golgi, plastid, and vacuole. By recruiting different stores, distinct spatial patterns of [Ca2+]cyt could be generated (Radin, 1984; Allen et al., 2001; Lim et al., 2015; Tombesi et al., 2015). Ca2+ pumps (CAs and ECAs) are responsible for the release of [Ca2+]cyt to the extracellular space and the influx to the intracellular Ca2+ stores. In rice, OsCAs family genes were regulated by four different osmotic-related abiotic stresses (PEG, NaCl, drain, and ABA; Li et al., 2015). In wheat, ECAs induced up-regulation with the increase of Ca2+ concentration. We analyzed, for the first time, the expression patterns of P2-type Ca2+ ATPase family genes in soybean under four abiotic stresses (drought, cold, salt, and shading; Figure 6). The results showed that the expression levels of GmACAs and GmECAs were regulated by abiotic stress (Figure 6). Correlation analysis between the stomatal aperture and the expression levels of Ca2+ ATPase genes under four abiotic stresses showed that soybean Ca2+ ATPase genes were negatively correlated with the stomatal aperture, indicating that the up-regulated expression of Ca2+ ATPase genes may regulate stomatal closure (Supplementary Figure S6). Further research found that all drought, cold, salt, and shading stress leads to stomatal closure (Figure 7), which is consistent with previous studies (Gibbons and Smillie, 1980; Pieruschka et al., 2006; Wang et al., 2015). We speculated that different subcellular localization of P2-type Ca2+ ATPases on intracellular membranes might be involved in regulating Ca2+ signal under different abiotic stresses. Therefore, it is of great significance to study the expression pattern of P2-type Ca2+ ATPase genes under abiotic stress for its potential function in regulating stomatal movement (Figures 6, 7).
Based on our research and previous studies, we summarized the transport regulation system of the Ca2+ pump in vegetable soybean cells (Figure 8). The 12 P2-type Ca2+ATPase family genes were divided into four types according to their subcellular localization. The four types of GmACAs and GmECAs are located in the plasma membrane (GmACA8, GmACA9, GmACA10, GmACA12, and GmACA13), endoplasmic reticulum (GmACA1, GmACA2, GmECA1, GmECA2, and GmECA4), tonoplast (GmACA11), and Golgi (GmECA3) intracellular membrane systems, respectively. According to the temporal and spatial expression of GmACAs and GmECAs under different stresses, all the genes located on the plasma membrane (GmACA8, GmACA9, GmACA10, GmACA12, and GmACA13) and the tonoplast (GmACA11) were up-regulated by drought, cold, and salt stress, and down-regulated by shading stress. Among the genes located at the endoplasmic reticulum, only GmECA2 and GmECA4 showed similar expression profiles. The results revealed that P2-type Ca2+ ATPase genes located at the endoplasmic reticulum (GmACA1, GmACA2, GmECA1, GmECA2, and GmECA4) might produce functional differentiation in response to different abiotic stresses. It has also shown that when plants are severely stressed, Ca2+ pumps located on the plasma membrane and tonoplast are preferentially mobilized in a unified manner, which can rapidly generate Ca2+ oscillation to regulate plant responses to external stresses, such as stomata closure. Moreover, GmACA1 might be involved in the negative regulation of cold and shading stress. GmECA1 was up-regulated under four abiotic stresses, especially under shading stress, which might positively regulate stomatal closure under shading stress. SCA1, the homologous gene of GmACA2 in this study, was up-regulated after salt stress (Aslam et al., 2017). Expression patterns of the most homologous genes GmACA2 under salt stress are consistent with that of AtACA2 and AtACA4 (Geisler et al., 2000a; Anil et al., 2007). It showed that GmACA2 could positively regulate cold and salt stress. The expression level of AtACA8 was found to be up-regulated under cold stress which was consistent with the results of a previous study (Schiøtt and Palmgren, 2005). GmECA3 may play a negative regulatory role under cold, salt, and shading stress. OsACA6 transcript levels were enhanced in response to salt, drought, abscisic acid and heat, and overexpression of OsACA6 in tobacco can improve salt, drought, and cold tolerance of transgenic lines (Huda et al., 2013a, 2014). It is suggested that the OsCA6 homologous gene GmACA11 may regulate plant stress resistance by regulating stomatal closure.
Figure 8
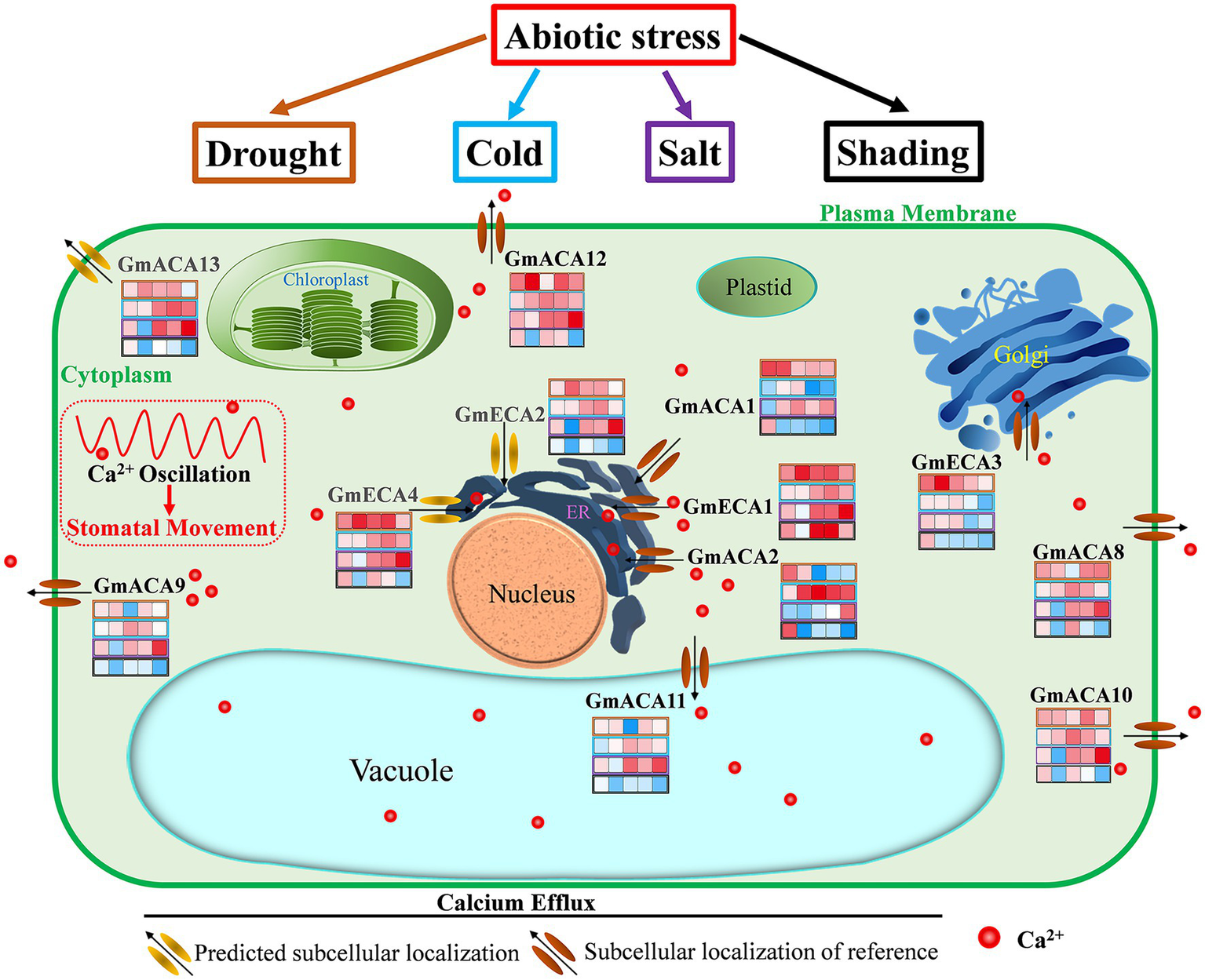
Overview of the regulation of calcium efflux by P2-type Ca2+ pump (GmACAs and GmECAs) in vegetable soybean cells. By referring to the identified transport system of the Ca2+ pump in Arabidopsis, the schematic representation of the P2-type Ca2+ pump transport system in soybean was summarized. Grey colors of gene names represent predicted subcellular localizations. The heat map represents the expression level of each gene at 1, 6, 12, 24, and 48 h after drought, cold, salt, and shading stress (refer to Figure 6). See the text and Table 1 for discussion and references.
Conclusion
This study was the first genome-wide analysis of the P2-type Ca2+ ATPase family in G. max responsive to different abiotic stresses (drought, cold, salt and shading stress). The effect of the Ca2+ pump on stomatal opening under different stress was speculated through the spatial location and temporal expression of the P2-type Ca2+ ATPase family genes in soybean. GmACA8, GmACA9, GmACA10, GmACA12, GmACA13, and GmACA11 might promote stomatal closure under drought, cold and salt stress; GmECA1 might regulate stomatal closure in shading stress. GmACA1 and GmECA3 might have a negative function on cold stress. The roles of GmACAs in regulating tolerance to different stress should be further evaluated through genetic transformation and functional analysis in soybean and other model organisms such as Arabidopsis, yeast, and Xenopus oocytes.
Significance Statement
The Ca2+ pump belongs to the P2-type calcium ATPases gene family, which is responsible for cellular Ca2+ transport and plays an important role in plant development and response to biotic and abiotic stresses. The results laid an important foundation for further study on the function of P2-type calcium ATPases genes GmACAs and GmECAs for soybean abiotic-resistant breeding.
Funding
The research was supported by grants from the Key R&D Program of Zhejiang Province (2021C02009) and National Key R&D Program of China (2018YFD0100901).
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
SX, JW, XF, and GC conceived and designed the experiments. JW, XF, SZ, SL, TS, and YZ performed the experiments. JW, GC, Z-HC, and SX analyzed the data and wrote the paper. JW, YZ, and FX contributed to reagents/materials/analysis tools. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.898256/full#supplementary-material
Supplementary Figure S1Motif compositions of the P2-type ATPases from nine different plant species. Inner layer: An unrooted phylogenetic tree constructed using MEGA-X with the neighbor-joining method. The resulting groups are shown in different shades of colors. P2-type ATPases in soybeans are shown with a highlighted background. Outer layer: Distribution of the conserved motifs in P2-type ATPases proteins. The differently colored boxes represent different motifs and their positions in each P2-type ATPases protein sequence.
Supplementary Figure S2Functional domains analysis of P2-type ATPases proteins in Arabidopsis and Glycine max from an evolutionary level. The differently colored boxes represent different conserved domains and their positions in each P2-type ATPases protein sequence.
Supplementary Figure S3Alignment of multiple soybean P2-type ATPases proteins and conserved domains amino acid sequences.
Supplementary Figure S4Gene structures composition of the P2-type ATPases genes from Arabidopsis and Glycine max. Exon-intron structures of P2-type ATPases genes. Solid green boxes indicate untranslated 5′- and 3′-regions; solid yellow boxes indicate exons; and black lines indicate introns. The number indicates the phases of the corresponding introns. The protein length can be estimated using the different scales at the bottom.
Supplementary Figure S5Schematic representations of the chromosomal distribution of the soybean P2-type ATPases genes. The chromosome number is indicated to the left of each chromosome.
Supplementary Figure S6Correlation analysis of expression levels and the stomatal aperture of P2-type ATPases genes under four abiotic stresses.
Footnotes
1.^https://www.ncbi.nlm.nih.gov
3.^https://www.ebi.ac.uk/Tools/hmmer/search/hmmsearch
4.^https://blast.ncbi.nlm.nih.gov
5.^http://web.expasy.org/compute_pi/
6.^http://web.expasy.org/protparam/
7.^http://linux1.softberry.com
8.^http://meme-suite.org/tools/meme
9.^https://sites.google.com/a/ualberta.ca/onekp/
10.^https://mafft.cbrc.jp/alignment/software/
12.^https://www.ebi.ac.uk/Tools/hmmer/search/phmmer
13.^http://gsds.cbi.pku.edu.cn
14.^https://github.com/CJ-Chen/TBtools
15.^http://bioinformatics.psb.ugent.be/webtools/plantcare/html/
References
1
AllenG. J.ChuS. P.HarringtonC. L.SchumacherK.HoffmannT.TangY. Y.et al. (2001). A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature411, 1053–1057. doi: 10.1038/35082575
2
AnilV. S.PremrajR.KumarP.MathewM. (2007). A plant Ca-pump, ACA2, relieves salt hypersensitivity in yeast: modulation of cytosolic calcium signature and activation of adaptive Na+ homeostasis. J. Biol. Chem.283, 3497–3506. doi: 10.1074/jbc.M700766200
3
AslamR.WilliamsL. E.BhattiM. F.VirkN. (2017). Genome-wide analysis of wheat calcium ATPases and potential role of selected ACA s and ECA s in calcium stress. BMC Plant Biol.17:174. doi: 10.1186/s12870-017-1112-5
4
BaileyT. L.JohnsonJ.GrantC. E.NobleW. S. (2015). The MEME suite. Nucleic Acids Res.43, W39–W49. doi: 10.1093/nar/gkv416
5
BonzaM. C.MorandiniP.LuoniL.GeislerM.PalmgrenM. G.MichelisM. I. D. (2000). At-ACA8 encodes a plasma membrane-localized calcium-ATPase of Arabidopsis with a calmodulin-binding domain at the N terminus. Plant Physiol.123, 1495–1506. doi: 10.1104/pp.123.4.1495
6
BredowM.MonaghanJ. (2018). Regulation of plant immune signaling by calcium-dependent protein kinases. Mol. Plant. Microbe Interact.32, 6–19. doi: 10.1094/MPMI-09-18-0267-FI
7
BushD. S. (1995). Calcium regulation in plant cells and its role in signaling. Annu. Rev. Plant Physiol. Plant Mol. Biol.46, 95–122. doi: 10.1146/annurev.pp.46.060195.000523
8
CarafoliE. (1991). Calcium pump of the plasma membrane. Physiol. Rev.71, 129–153. doi: 10.1152/physrev.1991.71.1.129
9
ChaerleL.LeinonenI.JonesH.Van Der StraetenD. (2007). Monitoring and screening plant populations with combined thermal and chlorophyll fluorescence imaging. J. Exp. Bot.58, 773–784. doi: 10.1093/jxb/erl257
10
ChenZ. H.ChenG.DaiF.WangY.HillsA.RuanY. L.et al. (2017). Molecular evolution of grass stomata. Trends Plant Sci.22, 124–139. doi: 10.1016/j.tplants.2016.09.005
11
ChenX.ChenZ.ZhaoH.ZhaoY.ChengB.XiangY. (2014). Genome-wide analysis of soybean HD-zip gene family and expression profiling under salinity and drought treatments. PLoS One9:e87156. doi: 10.1371/journal.pone.0087156
12
ChenZ. H.HillsA.LimC. K.BlattM. R. (2010). Dynamic regulation of guard cell anion channels by cytosolic free Ca2+ concentration and protein phosphorylation. Plant J.61, 816–825. doi: 10.1111/j.1365-313X.2009.04108.x
13
ChenM.WangQ. Y.ChengX. G.XuZ. S.LiL. C.YeX. G.et al. (2007). GmDREB2, a soybean DRE-binding transcription factor, conferred drought and high-salt tolerance in transgenic plants. Biochem. Biophys. Res. Commun.353, 299–305. doi: 10.1016/j.bbrc.2006.12.027
14
ChungW. S.LeeS. H.KimJ. C.HeoW. D.KimM. C.ParkC. Y.et al. (2000). Identification of a calmodulin-regulated soybean Ca(2+)-ATPase (SCA1) that is located in the plasma membrane. Plant Cell12, 1393–1407. doi: 10.1105/tpc.12.8.1393
15
ClaphamD. E. (2007). Calcium signaling. Cell131, 1047–1058. doi: 10.1016/j.cell.2007.11.028
16
DemidchikV.ShabalaS.IsayenkovS.CuinT. A.PottosinI. (2018). Calcium transport across plant membranes: mechanisms and functions. New Phytol.220, 49–69. doi: 10.1111/nph.15266
17
DongD. K.FuX. J.YuanF. J.ChenP. Y.ZhuS. L.LiB. Q.et al. (2014). Genetic diversity and population structure of vegetable soybean (Glycine max (L.) Merr.) in China as revealed by SSR markers. Genet. Resour. Crop Evol.61, 173–183. doi: 10.1007/s10722-013-0024-y
18
DuQ. L.CuiW. Z.ChenJ.YuD. Y. (2009). GmRFP1 encodes a previously unknown RING-type E3 ubiquitin ligase in soybean (Glycine max). Mol. Biol. Rep.37, 685–693. doi: 10.1007/s11033-009-9535-1
19
EnasQ.FaltuszA. M. C.GlenW.DanielL.HaukeH.ColinB.et al. (2009). A PIIB-type Ca2+-ATPase is essential for stress adaptation in Physcomitrella patens. Proc. Natl. Acad. Sci. U. S. A.105, 19555–19560. doi: 10.1073/pnas.0800864105
20
FengL.YoungJ. C.SussmanM. R.HarperJ. F.SzeH. (2002). An endoplasmic reticulum-bound Ca2+/Mn2+ pump, ECA1, supports plant growth and confers tolerance to Mn2+ stress. Plant Physiol.130, 128–137. doi: 10.1104/pp.004440
21
FuglsangA.PalmgrenM. (2021). Proton and calcium pumping P-type ATPases and their regulation of plant responses to the environment. Plant Physiol.187, 1856–1875. doi: 10.1093/plphys/kiab330
22
García BossiJ.KumarK.BarberiniM.DomínguezG.RondonY.Marino-BusljeC.et al. (2019). Role of P-type IIA (ECA) and P-type IIB (ACA) Ca2+-ATPases in plant development and growth. J. Exp. Bot.71, 1239–1248. doi: 10.1093/jxb/erz521
23
GarrisA. J.TaiT. H.CoburnJ.KresovichS.McCouchS. (2005). Genetic structure and diversity in Oryza sativa L. Genetics169, 1631–1638. doi: 10.1534/genetics.104.035642
24
GeislerM.AxelsenK. B.HarperJ. F.PalmgrenM. G. (2000b). Molecular aspects of higher plant P-type Ca(2+)-ATPases. Biochim. Biophys. Acta Biomembr.1465, 52–78. doi: 10.1016/S0005-2736(00)00131-0
25
GeislerM.FrangneN.GomèsE.MartinoiaE.PalmgrenM. G. (2000a). The ACA4 gene of Arabidopsis encodes a vacuolar membrane calcium pump that improves salt tolerance in yeast. Plant Physiol.124, 1814–1827. doi: 10.1104/pp.124.4.1814
26
GeorgeL.RomanowskyS. M.HarperJ. F.SharrockR. A. (2008). The ACA10 Ca2+-ATPase regulates adult vegetative development and inflorescence architecture in Arabidopsis. Plant Physiol.146, 323–324. doi: 10.1104/pp.107.108118
27
GibbonsG. C.SmillieR. M. (1980). Chlorophyll fluorescence photography to detect mutants, chilling injury and heat stress. Carlsb. Res. Commun.45, 269–282. doi: 10.1007/BF02906179
28
GilroyS.ReadN. D.TrewavasA. J. (1990). Elevation of cytoplasmic calcium by caged calcium or caged inositol trisphosphate initiates stomatal closure. Nature346, 769–771. doi: 10.1038/346769a0
29
Guimarães-DiasF.Neves-BorgesA. C.VianaA. A. B.MesquitaR. O.RomanoE.Grossi-de-SáM. d. F.et al. (2012). Expression analysis in response to drought stress in soybean: shedding light on the regulation of metabolic pathway genes. Genet. Mol. Biol.35, 222–232. doi: 10.1590/S1415-47572012000200004
30
HillearyR.Paez-ValenciaJ.VensC.ToyotaM.PalmgrenM.GilroyS. (2020). Tonoplast-localized Ca2+ pumps regulate Ca2+ signals during pattern-triggered immunity in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U. S. A.117, 18849–18857. doi: 10.1073/pnas.2004183117
31
HockingB.ConnS. J.ManoharM.XuB.AthmanA.StancombeM. A.et al. (2017). Heterodimerization of Arabidopsis calcium/proton exchangers contributes to regulation of guard cell dynamics and plant defense responses. J. Exp. Bot.68, 4171–4183. doi: 10.1093/jxb/erx209
32
HsuP. K.DubeauxG.TakahashiY.SchroederJ. I. (2021). Signaling mechanisms in abscisic acid-mediated stomatal closure. Plant J.105, 307–321. doi: 10.1111/tpj.15067
33
HuR.FanC.LiH.ZhangQ.FuY. F. (2009). Evaluation of putative reference genes for gene expression normalization in soybean by quantitative real-time RT-PCR. BMC Mol. Biol.10:93. doi: 10.1186/1471-2199-10-93
34
HudaK. M.BanuM. S.GargB.TulaS.TutejaR.TutejaN. (2013a). OsACA6, a P-type IIB Ca2+ ATPase promotes salinity and drought stress tolerance in tobacco by ROS scavenging and enhancing the expression of stress-responsive genes. Plant J.76, 997–1015. doi: 10.1111/tpj.12352
35
HudaK. M. K.BanuM. S. A.YadavS.SahooR. K.TutejaR.TutejaN. (2014). Salinity and drought tolerant OsACA6 enhances cold tolerance in transgenic tobacco by interacting with stress-inducible proteins. Plant Physiol. Biochem.82, 229–238. doi: 10.1016/j.plaphy.2014.06.007
36
HudaK. M. K.YadavS.BanuM. S. A.TrivediD. K.TutejaN. (2013b). Genome-wide analysis of plant-type II Ca2+ ATPases gene family from rice and Arabidopsis: potential role in abiotic stresses. Plant Physiol. Biochem.65, 32–47. doi: 10.1016/j.plaphy.2013.01.002
37
HwangI.SzeH.HarperJ. F. (2000). A calcium-dependent protein kinase can inhibit a Calmodulin-stimulated Ca2+ pump (ACA2) located in the endoplasmic reticulum of Arabidopsis. Proc. Natl. Acad. Sci. U. S. A.97, 6224–6229. doi: 10.1073/pnas.97.11.6224
38
KrzywinskiM.ScheinJ.Birolİ.ConnorsJ.GascoyneR.HorsmanD.et al. (2009). Circos: an information aesthetic for comparative genomics. Genome Res.19, 1639–1645. doi: 10.1101/gr.092759.109
39
KudlaJ.BatističO.HashimotoK. (2010). Calcium signals: the Lead currency of plant information processing. Plant Cell22, 541–563. doi: 10.1105/tpc.109.072686
40
KurusuT.YagalaT.MiyaoA.HirochikaH.KuchitsuK. (2005). Identification of a putative voltage-gated Ca2+ channel as a key regulator of elicitor-induced hypersensitive cell death and mitogen-activated protein kinase activation in rice. Plant J.42, 798–809. doi: 10.1111/j.1365-313X.2005.02415.x
41
LarkinM. A.BlackshieldsG.BrownN. P.ChennaR.McgettiganP. A.McwilliamH.et al. (2007). Clustal W and Clustal X version 2.0. Bioinformatics23, 2947–2948. doi: 10.1093/bioinformatics/btm404
42
LeeS. M.KimH. S.HanH. J.MoonB. C.KimC. Y.HarperJ. F.et al. (2007). Identification of a calmodulin-regulated autoinhibited Ca2+-ATPase (ACA11) that is localized to vacuole membranes in Arabidopsis. FEBS Lett.581, 3943–3949. doi: 10.1016/j.febslet.2007.07.023
43
Leebens-MackJ.WickettN.DeyholosM.DeGironimoL.PokornyL.LiF. W.et al. (2019). One thousand plant transcriptomes and the phylogenomics of green plants. Nature574, 679–685. doi: 10.1038/s41586-019-1693-2
44
LiX.ChanrojS.WuZ.RomanowskyS.HarperJ. F.SzeH. (2008). A distinct endosomal Ca2+/Mn2+ pump affects root growth through the secretory process. Plant Physiol.147, 1675–1689. doi: 10.1104/pp.108.119909
45
LiZ. G.YeX. Y.QiuX. M. (2019). Glutamate signaling enhances the heat tolerance of maize seedlings by plant glutamate receptor-like channels-mediated calcium signaling. Protoplasma256, 1165–1169. doi: 10.1007/s00709-019-01351-9
46
LiY.YuanF.WenZ.LiY.WangF.ZhuT.et al. (2015). Genome-wide survey and expression analysis of the OSCA gene family in rice. BMC Plant Biol.15:261. doi: 10.1186/s12870-015-0653-8
47
LiangF.SzeH. (1998). A high-affinity Ca2+ pump, ECA1, from the endoplasmic reticulum is inhibited by cyclopiazonic acid but not by thapsigargin. Plant Physiol.118, 817–825. doi: 10.1104/pp.118.3.817
48
LilloC. (1994). Light/dark regulation of higher plant nitrate reductase related to hysteresis and calcium/magnesium inhibition. Physiol. Plant.91, 295–299. doi: 10.1111/j.1399-3054.1994.tb00434.x
49
LimC. W.BaekW.JungJ.KimJ. H.LeeS. C. (2015). Function of ABA in stomatal defense against biotic and drought stresses. Int. J. Mol. Sci.16, 15251–15270. doi: 10.3390/ijms160715251
50
LimontaM.RomanowskyS.OlivariC.BonzaM. C.LuoniL.RosenbergA.et al. (2014). ACA12 is a deregulated isoform of plasma membrane Ca2+-ATPase of Arabidopsis thaliana. Plant Mol. Biol.84, 387–397. doi: 10.1007/s11103-013-0138-9
51
LiuX.FanY.MakM.BablaM.HolfordP.WangF.et al. (2017b). QTLs for stomatal and photosynthetic traits related to salinity tolerance in barley. BMC Genomics18:9. doi: 10.1186/s12864-016-3380-0
52
LiuX.LiX.DaiC.ZhouJ.YanT.ZhangJ. (2017c). Improved short-term drought response of transgenic rice over-expressing maize C4 phosphoenolpyruvate carboxylase via calcium signal cascade. J. Plant Physiol.218, 206–221. doi: 10.1016/j.jplph.2017.08.005
53
LiuC. K.TuB. J.LiY. S.TianB. W.ZhangQ. Y.LiuX. B.et al. (2017a). Potassium application affects key enzyme activities of sucrose metabolism during seed filling in vegetable soybean. Crop Sci.57, 2707–2717. doi: 10.2135/cropsci2016.08.0648
54
LoneB.LauraL.Maria IdaD. M.PalmgrenM. G. (2006). The plant plasma membrane Ca2+ pump ACA8 contains overlapping as well as physically separated autoinhibitory and calmodulin-binding domains. J. Biol. Chem.281, 1058–1065. doi: 10.1074/jbc.M508299200
55
MarschnerH. (2012). Mineral nutrition of higher plants. J. Ecol.76:1250. doi: 10.1016/B978-012473542-2/50019-5
56
McAinshM. R.BrownleeC.HetheringtonA. M. (1990). Abscisic acid-induced elevation of guard cell cytosolic Ca2+ precedes stomatal closure. Nature343, 186–188. doi: 10.1038/343186a0
57
McAinshM. R.GrayJ. E.HetheringtonA. M.LeckieC. P.NgC. (2000). Ca2+ signalling in stomatal guard cells. Biochem. Soc. Trans.28, 476–481. doi: 10.1042/bst0280476
58
MelottoM.UnderwoodW.HeS. Y. (2008). Role of stomata in plant innate immunity and foliar bacterial diseases. Annu. Rev. Phytopathol.46, 101–122. doi: 10.1146/annurev.phyto.121107.104959
59
MillerM. A.PfeifferW.SchwartzT. (2010). “Creating the CIPRES science gateway for inference of large phylogenetic trees,” in Gateway Computing Environments Workshop (GCE), 1–8.
60
MonshausenG. B.MesserliM. A.SimonG. (2008). Imaging of the yellow cameleon 3.6 indicator reveals that elevations in cytosolic Ca2+ follow oscillating increases in growth in root hairs of Arabidopsis. Plant Physiol.147, 1690–1698. doi: 10.1104/pp.108.123638
61
O’CarriganA.HindeE.LuN.XuX. Q.DuanH.HuangG.et al. (2014). Effects of light irradiance on stomatal regulation and growth of tomato. Environ. Exp. Bot.98, 65–73. doi: 10.1016/j.envexpbot.2013.10.007
62
OuX.LiT.ZhaoY.ChangY.WuL.ChenG.et al. (2022). Calcium-dependent ABA signaling functions in stomatal immunity by regulating rapid SA responses in guard cells. J. Plant Physiol.268:153585. doi: 10.1016/j.jplph.2021.153585
63
PalmgrenM.SørensenD. M.HallströmB. M.SällT.BrobergK. (2020). Evolution of P2A and P5A ATPases: ancient gene duplications and the red algal connection to green plants revisited. Physiol. Plant.168, 630–647. doi: 10.1111/ppl.13008
64
PandeyS.ZhangW.AssmannS. M. (2007). Roles of ion channels and transporters in guard cell signal transduction. FEBS Lett.581, 2325–2336. doi: 10.1016/j.febslet.2007.04.008
65
PedersenC. N.AxelsenK. B.HarperJ. F.PalmgrenM. G. (2012). Evolution of plant p-type ATPases. Front. Plant Sci.3:31. doi: 10.3389/fpls.2012.00031
66
PieruschkaR.SchurrU.JensenM.WolffW. F.JahnkeS. (2006). Lateral diffusion of CO2 from shaded to illuminated leaf parts affects photosynthesis inside homobaric leaves. New Phytol.169, 779–788. doi: 10.1111/j.1469-8137.2005.01605.x
67
RadinW. J. (1984). Stomatal responses to water stress and to abscisic acid in phosphorus-deficient cotton plants. Plant Physiol.76, 392–394. doi: 10.1104/pp.76.2.392
68
RahmanA.NaharK.HasanuzzamanM.FujitaM. (2016). Calcium supplementation improves Na+/K+ ratio, antioxidant defense and glyoxalase systems in salt-stressed rice seedlings. Front. Plant Sci.7:609. doi: 10.3389/fpls.2016.00609
69
Rahmati IshkaM. (2015). Genetic analyses of Ca2+ circuits in Arabidopsis vegetative and reproductive development. dissertation/doctoral thesis. Reno, NV: University of Nevada.
70
RajivD.RobinsonK. R. (2004). Identification and characterization of stretch-activated ion channels in pollen protoplasts. Plant Physiol.135, 1398–1406. doi: 10.1104/pp.104.041483
71
RavenJ. A. (2002). Selection pressures on stomatal evolution. New Phytol.153, 371–386. doi: 10.1046/j.0028-646X.2001.00334.x
72
SandersD.BrownleeC.HarperJ. F. (1999). Communicating with calcium. Plant Cell11, 691–706. doi: 10.1105/tpc.11.4.691
73
SandersD.PellouxJ.BrownleeC.HarperJ. F. (2002). Editorial ICOM at the cross-roads?MMC12, 3–10. doi: 10.1016/0260-4779(93)90002-7
74
SchadE.KalmarL.TompaP. (2013). Exon-phase symmetry and intrinsic structural disorder promote modular evolution in the human genome. Nucleic Acids Res.41, 4409–4422. doi: 10.1093/nar/gkt110
75
SchiøttM.PalmgrenM. G. (2005). Two plant Ca2+ pumps expressed in stomatal guard cells show opposite expression patterns during cold stress. Physiol. Plant.124, 278–283. doi: 10.1111/j.1399-3054.2005.00512.x
76
SchiøttM.RomanowskyS. M.BaekgaardL.JakobsenM. K.PalmgrenM. G.HarperJ. F. (2004). A plant plasma membrane Ca2+ pump is required for normal pollen tube growth and fertilization. Proc. Natl. Acad. Sci. U. S. A.101, 9502–9507. doi: 10.1073/pnas.0401542101
77
SeyboldH.TrempelF.RanfS.ScheelD.RomeisT.LeeJ. (2014). Ca2+ signalling in plant immune response: from pattern recognition receptors to Ca2+ decoding mechanisms. New Phytol.204, 782–790. doi: 10.1111/nph.13031
78
SpaldingE. P.HarperJ. F. (2011). The ins and outs of cellular Ca2+ transport. Curr. Opin. Plant Biol.14, 715–720. doi: 10.1016/j.pbi.2011.08.001
79
SunM.JiaB.CuiN.WenY.DuanmuH.YuQ.et al. (2016). Functional characterization of a Glycine soja Ca2+ATPase in salt–alkaline stress responses. Plant Mol. Biol.90, 419–434. doi: 10.1007/s11103-015-0426-7
80
SzeH.LiangF.HwangI.CurranA. C.HarperJ. F. (2000). Diversity and regulation of plant Ca2+ pumps: insights from expression in yeast. Annu. Rev. Plant Physiol. Plant Mol. Biol.51, 433–462. doi: 10.1146/annurev.arplant.51.1.433
81
TaglianiA. (2020). Stomatal immunity: a calcium channel essential for pathogen-induced Stomatal closure. Mol. Plant13:1349. doi: 10.1016/j.molp.2020.09.012
82
ThorK.JiangS.MichardE.GeorgeJ.ScherzerS.HuangS.et al. (2020). The calcium-permeable channel OSCA1.3 regulates plant stomatal immunity. Nature585, 569–573. doi: 10.1038/s41586-020-2702-1
83
TombesiS.NardiniA.FrioniT.SoccoliniM.ZadraC.FarinelliD.et al. (2015). Stomatal closure is induced by hydraulic signals and maintained by ABA in drought-stressed grapevine. Sci. Rep.5:12449. doi: 10.1038/srep12449
84
TutejaN.MahajanS. (2007). Calcium signaling network in plants. Plant Signal. Behav.2, 79–85. doi: 10.4161/psb.2.2.4176
85
UntergasserA.CutcutacheI.KoressaarT.YeJ.FairclothB. C.RemmM.et al. (2012). Primer 3—new capabilities and interfaces. Nucleic Acids Res.40:e115. doi: 10.1093/nar/gks596
86
UrbinaD. C.SilvaH.MeiselL. A. (2006). The Ca2+ pump inhibitor, thapsigargin, inhibits root gravitropism in Arabidopsis thaliana. Biol. Res.39, 289–296. doi: 10.4067/s0716-97602006000200011
87
WangY.ChenZ. H. (2020). Does molecular and structural evolution shape the speedy grass stomata?Front. Plant Sci.11:333. doi: 10.3389/fpls.2020.00333
88
WangF.ChenZ. H.LiuX.ColmerT. D.ZhouM.ShabalaS. (2016). Tissue-specific root ion profiling reveals essential roles of the CAX and ACA calcium transport systems in response to hypoxia in Arabidopsis. J. Exp. Bot.67, 3747–3762. doi: 10.1093/jxb/erw034
89
WangH.LiH.LiangX.XuL. (2015). A new indicator in early drought diagnosis of cucumber with chlorophyll fluorescence imaging. Proc. SPIE Int. Soc. Opt. Eng.9530, 1–7. doi: 10.1117/12.2184618
90
WangY.TangH.DebarryJ. D.TanX.LiJ.WangX.et al. (2012). MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res.40:e49. doi: 10.1093/nar/gkr1293
91
WangK.ZhangX.ZhaoY.ChenF.XiaG. (2013). Structure, variation and expression analysis of glutenin gene promoters from Triticum aestivum cultivar Chinese spring shows the distal region of promoter 1Bx7 is key regulatory sequence. Gene527, 484–490. doi: 10.1016/j.gene.2013.06.068
92
WhiteP. J. (2000). Calcium channels in higher plants. Biochim. Biophys. Acta1465, 171–189. doi: 10.1016/s0005-2736(00)00137-1
93
XuS. C.LiuN.MaoW. H.HuQ. Z.WangG. F.GongY. M. (2016). Identification of chilling-responsive microRNAs and their targets in vegetable soybean (Glycine max L.). Sci. Rep.6:26619. doi: 10.1038/srep26619
94
YangD. L.ShiZ.BaoY.YanJ.YangZ.YuH.et al. (2017). Calcium pumps and interacting BON1 protein modulate calcium signature, stomatal closure, and plant immunity. Plant Physiol.175, 424–437. doi: 10.1104/pp.17.00495
95
YannB.MinL. S.ShawnR.RobertB.ChrisS.Woo SikC.et al. (2010). Disruption of the vacuolar calcium-ATPases in Arabidopsis results in the activation of a salicylic acid-dependent programmed cell death pathway. Plant Physiol.154, 1158–1171. doi: 10.1104/pp.110.159038
96
YongL.ZhangJ. S.ChenS. Y.ZhangW. K. (2008). Role of soybean GmbZIP132 under abscisic acid and salt stresses. J. Integr. Plant Biol.50, 221–230. doi: 10.1111/j.1744-7909.2007.00593.x
97
YoungJ. J.MehtaS.IsraelssonM.GodoskiJ.GrillE.SchroederJ. I. (2006). CO2 signaling in guard cells: calcium sensitivity response modulation, a Ca2+-independent phase, and CO2 insensitivity of the gca2 mutant. Proc. Natl. Acad. Sci. U. S. A.103, 7506–7511. doi: 10.1073/pnas.0602225103
98
YuanP.YangT.PoovaiahB. (2018). Calcium signaling-mediated plant response to cold stress. Int. J. Mol. Sci.19:3896. doi: 10.3390/ijms19123896
99
ZhangZ.HouC.TianW.LiL.ZhuH. (2019). Electrophysiological studies revealed CaM1-mediated regulation of the Arabidopsis calcium channel CNGC12. Front. Plant Sci.10:1090. doi: 10.3389/fpls.2019.01090
100
ZhaoC.WangY.ChanK. X.MarchantD. B.FranksP. J.RandallD.et al. (2019). Evolution of chloroplast retrograde signaling facilitates green plant adaptation to land. Proc. Natl. Acad. Sci. U. S. A.116, 5015–5020. doi: 10.1073/pnas.1812092116
Summary
Keywords
abiotic stresses, Ca2+ ATPases, Glycine max L., phylogenetic analysis, stomatal regulation
Citation
Wang J, Fu X, Zhang S, Chen G, Li S, Shangguan T, Zheng Y, Xu F, Chen Z-H and Xu S (2022) Evolutionary and Regulatory Pattern Analysis of Soybean Ca2+ ATPases for Abiotic Stress Tolerance. Front. Plant Sci. 13:898256. doi: 10.3389/fpls.2022.898256
Received
17 March 2022
Accepted
02 May 2022
Published
19 May 2022
Volume
13 - 2022
Edited by
Yizhou Wang, Zhejiang University, China
Reviewed by
Zhoufei Wang, South China Agricultural University, China; Ye Zihong, China Jiliang University, China
Updates
Copyright
© 2022 Wang, Fu, Zhang, Chen, Li, Shangguan, Zheng, Xu, Chen and Xu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shengchun Xu, shengchun2001@163.com
†These authors have contributed equally to this work
This article was submitted to Plant Membrane Traffic and Transport, a section of the journal Frontiers in Plant Science
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.