Abstract
Flowering time is an important trait for all major market classes of hemp (Cannabis sativa), affecting yields and quality of grain, fiber, and cannabinoids. C. sativa is usually considered a short-day plant, flowering once night length reaches a critical threshold. Variations in flowering time within and across cultivars in outdoor grown populations have been previously identified, likely corresponding to genetic differences in this critical night length. Further, some C. sativa are photoperiod insensitive, colloquially referred to as “autoflowering.” This trait has anecdotally been described as a simple recessive trait with major impacts on phenology and yield. In this work, the locus responsible for the “autoflower” trait (Autoflower1), as well as a major-effect flowering time locus, Early1, were mapped using bulked segregant analysis. Breeder-friendly high-throughput molecular marker assays were subsequently developed for both loci. Also detailed are the flowering responses of diverse cultivars grown in continuous light and the result of crossing two photoperiod insensitive cultivars of differing pedigree.
Introduction
Hemp (Cannabis sativa) is a multi-use crop that is widely considered a photoperiod sensitive, short-day plant. Flowering time is important for all major market classes, and uniform flowering dates within a cultivar are essential for ease of harvest. Fiber hemp benefits from a long growing season, as harvest usually occurs around the flowering date, and early flowering results in a shorter vegetative growth phase to accumulate biomass (Salentijn et al., 2019). Grain hemp must flower early enough such that grain can be harvested before frost if growing in temperate latitudes, but precocious flowering can lead to severe yield penalties due to a lack of time to accumulate biomass that provides photosynthate for grain filling. This is especially challenging for subtropical and tropical latitudes where most days of the year have nights longer than the critical night threshold (Zhang et al., 2021). For cannabinoid production, as with grain production, precocious flowering may result in reduced floral biomass yield, while plants that do not flower by the end of the season fail to accumulate high concentrations of cannabinoids (Stack et al., 2021). Additionally, cannabinoid profiles change throughout the maturation of the inflorescence, making initiation of flowering an important factor in timing regulatory compliance testing and harvest (Toth et al., 2021; Zhang et al., 2021).
Previous work has outlined differences in photoperiod threshold across different cultivars, in controlled environments as well as field conditions (Stack et al., 2021; Zhang et al., 2021). It has also been well established that some plants are photoperiod insensitive (day neutral), a trait proposed to have been introgressed from high-latitude populations, which have been classified by some as a putative species, Cannabis ruderalis (McPartland, 2018). Photoperiod insensitivity is sometimes referred to colloquially as “autoflower” (Gloss, 2015). This trait has been suggested to be inherited in a simple, recessive, Mendelian fashion, but there are limited data on this in the peer-reviewed literature (Green, 2005). A patent covering molecular markers and biotechnological manipulation of genes responsible for “autoflower” is held by Phylos Biosciences (Phylos Bioscience, International Patent WO 2021/097496 A2). In addition to commercial high-cannabinoid “autoflower” cultivars, several grain cultivars, such as ‘FINOLA,’ have been referred to as “autoflowering” in the literature (Van Bakel et al., 2011), but have a distinct phenotype compared to photoperiod-insensitive high-cannabinoid cultivars in that the height of mature ‘FINOLA’ depends greatly on latitude (being shorter at lower latitudes), while “autoflower” high-cannabinoid cultivars do not appear to exhibit this phenomenon (Callaway, 2002; Yang et al., 2020; Stack et al., 2021). However, it is not clear if the genetic mechanism for photoperiod insensitivity is the same in all cultivars (Zhang et al., 2021). Diagnostic molecular markers and complementation assays could help resolve this question.
It is a well-established phenomenon that there is significant population structure in C. sativa, associated at least in part with recent breeding history and geography (Carlson et al., 2021). While there is ongoing debate on the specifics of the nature of this population structure (hindered in part because of the ease of intercrossing between subgroups of C. sativa), there is strong support for at least two subpopulations, which have been described as subspecies (McPartland, 2018). The two subspecies that have been described differ in end use and likely origin, with C. sativa ssp. sativa grown for grain and fiber originally in northern European latitudes and C. sativa ssp. indica grown for cannabinoid production originally in Southeast Asia, including India. Various other subpopulations have been described, including a distinct clade of C. sativa with geographic origins in China (Carlson et al., 2021; Ren et al., 2021). Different taxonomic classifications have also been proposed, including the putative species C. ruderalis, which has been considered the source of the “autoflower” trait in all C. sativa populations (Green, 2005).
Genetic pathways for the induction of flowering are largely conserved across dicot plants, with major photosensory, thermosensory, and age-related pathways converging on major floral integrator genes (Mouradov et al., 2002; Jung and Müller, 2009). These floral integrator genes, including CO, FT, SOC1, and FLC, result in the expression of floral meristem identity genes such as AP1 and LFY (Mouradov et al., 2002). The expression of these floral meristem identity genes result in a switch from a vegetative phase to a reproductive phase. Upstream of the major floral integrator genes are a host of other well-established genes including PRR37, casein kinase I, AP2 group genes, and others, which have been associated with flowering time in various plants including rice (Hori et al., 2013), sorghum (Murphy et al., 2011), and pepper (Yuan et al., 2021).
There has been extensive research into genes involved in photoperiod response and earliness per se in a variety of crops, aided by extensive work in Arabidopsis (Blümel et al., 2015). However, there has been relatively little research in this area in C. sativa. A recent genome-wide association study (GWAS) in fiber hemp implicated genes in the major photosensory, thermosensory, and age-related pathways as well as a host of transcription factors in time to flower (Petit et al., 2020). While the genes implicated in this study serve as a potential starting point, more study on the genetics of flowering time control in C. sativa is required for predictive breeding efforts. The conserved nature of flowering induction may ease the discovery of relevant genes in extant germplasm.
Our group previously identified several populations marketed as F1 hybrids (‘Umpqua’ and ‘Deschutes’) that have individuals with two distinct flowering times, approximately a month apart (Stack et al., 2021). This segregation ratio would be expected if one parent was heterozygous for a major effect flowering time locus while the other parent was homozygous at that locus. If this were the case, such a significant locus would be well suited for development of high-throughput molecular markers such as PACE (PCR Allele Competitive Extension, 3CR). As differences in flowering are not obvious when the plant is in an early vegetative state, early screening with molecular markers for this trait or the autoflower trait could be very useful (Toth et al., 2018).
For essentially qualitative traits controlled by major effect flowering time loci as described here, bulk segregant analysis (BSA) has been successfully used to map genes and generate molecular markers and related assays (Song et al., 2017). Bulk segregant analysis is a technique that utilizes the sequencing of pooled DNA samples from individuals with the same phenotype in contrasting groups in a segregating population, and has been used effectively in a range of crops including C. sativa (Ban and Xu, 2020; Welling et al., 2020). Bulk segregant analysis usually involves short-read sequencing and subsequent alignment to a reference genome, but BSA involving long reads and reference-free techniques have been developed (Nordström et al., 2013; Segawa et al., 2021). The number of individuals in the pools must be sufficiently large to randomize the association of all regions of the genome except the region or regions associated with the trait of interest. Compared with other methods of mapping, this technique has the advantage of obtaining whole genome sequences of the region of interest, alleviating the issue of ascertainment bias present in other methods of sequencing mapping populations such as single nucleotide polymorphism (SNP) chips or genotyping-by-sequencing (GBS). It is also cheaper and results in higher read depth than individually sequencing genomes, but multiple sequencing efforts would be required for mapping more than one trait. Bulk segregant analysis can also be conducted using pre-defined molecular markers instead of direct sequencing, but the decreasing cost of sequencing has made these approaches less common (Zhang et al., 2009; Becker et al., 2011).
Once sequencing data are obtained from contrasting pools, a comparison of regions that differ in allelic frequency can be performed (Magwene et al., 2011). In the case of a simple recessive trait in an F2 population, one pool should be homozygous for a region containing the causative gene, while the other pool should have an alternative allelic frequency of ∼33% in that region. In the case of a major gene in a backcross, one pool should be homozygous in a region and the other pool should be heterozygous. The difference between allele frequencies can be represented in a number of ways, including comparing the number of significantly different SNPs in a region determined through Fisher’s Exact Test, a G-Test statistic, or through the delta-allele frequency method (also called the delta-SNP method) which involves determining the difference in allele frequency directly (Zhang and Panthee, 2020).
Materials and methods
Field and greenhouse trials of populations segregating for flowering time
A population segregating for photoperiod insensitivity was developed by first crossing a female autoflower plant from a feminized (all-female) seed lot numbered KG9202 (generously provided by Kayagene, Hollister, CA, United States) with a late flowering, photoperiod sensitive ‘Otto II’ plant (generously provided by Edgar Winters, WinterFox Farms, Klamath Falls, OR) determined to be male and cannabinoid chemotype III using molecular markers (Toth et al., 2020) to produce F1 family GVA-H-19-1148. These parental cultivar populations were previously trialed in the 2019 Cornell high-cannabinoid hemp field trial (Stack et al., 2021). One selected photoperiod-sensitive female F1 plant (GVA-H-19-1148-002) was multiplied by rooting stem cuttings, then one ramet was treated with silver thiosulfate to induce male flowers that pollinated multiple genetically identical female plants to generate F2 seed labeled GVA-H-20-1080 (Carlson et al., 2021). This technique results in an entirely female, or feminized, population (Lubell and Brand, 2018).
A second population segregating for photoperiod insensitivity was ‘TJ’s CBG’ (generously provided by Stem Holdings Agri, Eugene, OR), which was evaluated in the 2020 Cornell CBG hemp field trials and displayed a CBG-dominant chemotype (chemotype IV).
For initial assessment of photoperiod insensitivity in the segregating populations, seeds of each were sown in potting mix in 50-cell SureRoot trays on December 16, 2019 and grown in a greenhouse with a 16L:8D light schedule. Eighty-eight healthy plants in each population were transplanted to one-gallon pots on February 3, 2020. While flowering was evident on some plants at this point, rating for terminal flowering as previously defined (Stack et al., 2021) was completed on March 23, 2020.
The high-CBD cultivar ‘Umpqua’ (generously provided originally by Industrial Seed Innovations) was evaluated in the Cornell high-cannabinoid hemp field trials in 2019 and 2020 where flowering time was carefully assessed. Details about the 2019 trial are available in Stack et al. (2021). The 2020 trial was executed using similar protocols, but with a different seed lot of ‘Umpqua’ generously provided by Arcadia Biosciences (Davis, CA). An additional 100 plants taken equally from both seed lots were transplanted outdoors on July 22, 2021 in a trial to evaluate flowering time in Geneva, NY using similar protocols. The 2021 flowering time field trial also included 96 individuals from the KG9202 × ‘Otto II’ F2 population GVA-H-20-1080 and 26 plants of ‘Hempress’ (generously provided by Point3 Farma, Center, CO). Height and wet biomass was recorded for each plant in population GVA-H-20-1080 in this trial. Additional populations segregating for photoperiod insensitivity were identified in the 2020 Cornell CBG hemp field trial.1
To evaluate photoperiod insensitivity across diverse germplasm, 50 seeds of each population were sown in potting mix in 50-cell SureRoot trays on April 20, 2021 unless otherwise noted and grown in a greenhouse under continuous supplemental lighting from high pressure sodium lamps. Flowering was assessed weekly. Male flowering was considered to have started when the length of internodes at the apex of the plant shortened and male buds were clearly visible at the growing tip.
A complementation cross was completed between two photoperiod-insensitive plants: pollen parent ‘Picolo’ (generously provided by Hemp Genetics International, Saskatoon, SK), and a homozygous Autoflower1/Autoflower1 seed parent from the ‘Le Crème’ cultivar population (generously provided by Ventura Seed Company, Camarillo, CA, segregating 1:3 for photoperiod insensitivity). The F1 plants from this cross were grown under 16L:8D with a 1 h night break in 50-cell SureRoot trays alongside known photoperiod sensitive and insensitive cultivars. Ten plants from each population were established on January 5, 2022. ‘Auto CBD’ and ‘Le Crème’ were feminized populations with no males. ‘RN16’ was a dioecious photoperiod-sensitive high CBD hemp cultivar (Stack et al., 2021).
Bulk segregant analysis sequencing
DNA was extracted using a Qiagen DNeasy 96-well kit from young leaf tissue collected from plants in population GVA-H-20-1080 and dried on silica gel. Two pools were created by combining equal amounts of DNA from 28 flowering, photoperiod-insensitive plants and 25 non-flowering, photoperiod-sensitive plants. Illumina TruSeq libraries with an insert size of ∼500 bp were constructed for each pool by the Cornell Institute of Biotechnology then paired end 151 bp sequencing was performed on the Illumina NextSeq 2000 platform with ∼35× coverage.
DNA was extracted from dried, milled floral biomass samples of ‘Umpqua’ as previously described (Toth et al., 2020) for 15 early flowering and 15 late-flowering plants from the 2019 and 2020 trials and from 15 early flowering and 15 late-flowering samples from the 2021 flowering time trial. Illumina TruSeq libraries were constructed for each phenological pool and sequenced on the Illumina NextSeq 2000 platform, as described above.
Reads were aligned to the CBDRx-cs10 (GCF_900626175.2) genome assembly (Grassa et al., 2021) using Geneious Prime software (Biomatters, Inc., San Diego, CA, United States) using the Geneious mapper algorithm at the fastest speed with three iterations. Variants were also called in the Geneious Prime environment (coverage > 3, minimum variant frequency > 0.05). Variant calls were exported and modified using a custom Python script to be compatible with PyBSASeq (Zhang and Panthee, 2020). PyBSASeq was run using the “BulksOnly” protocol, assuming an F2 population structure for GVA-H-20-1080 and a backcross population structure for ‘Umpqua’ bulks. Significant SNPs and regions were calculated using Fisher’s Exact Test, a G-Test statistic, and the delta-allele frequency method.
PCR allele competitive extension (PACE) genotyping assays
PCR allele competitive extension assays were designed manually in the Geneious Prime environment. PACE reactions were run according to the product manual (3CR Bioscience Ltd., Essex, United Kingdom). Polymorphic SNP in the Autoflower1 region identified as perfectly associated with photoperiod phenotype in GVA-H-20-1080 pools were converted to PACE markers (Table 1 and Supplementary Table 1). These were assayed across multiple populations including the individual plants that formed the pool, a field grown population of GVA-H-20-1080, cultivars segregating for flowering time when grown under field conditions, and diverse photoperiod-sensitive and photoperiod insensitive cultivars. Unless otherwise noted, 8 plants from each cultivar or population were tested. For wide-germplasm testing, the primer sets AUTO-2 and EAR<jrn>LY-1</jrn> were used.
TABLE 1
| Primer | Group/location | Primer sequence |
| AUTO-1 | 18464905 | |
| FAM | WT | GAAGGTGACCAAGTTCATGCTATCCAGGGTCTGGCTTTAAAAA |
| HEX | AUTO | GAAGGTCGGAGTCAACGGATTATCCAGGGTCTGGCTTTAAAAT |
| REV | CCATAAAATGATAAGTACACTCTAC | |
| AUTO-2 | 19701425 | |
| FAM | WT | GAAGGTGACCAAGTTCATGCTTTGGACTTCACCAAATGAGCCC |
| HEX | AUTO | GAAGGTCGGAGTCAACGGATTTTGGACTTCACCAAATGAGCCT |
| REV | CTTCTAACCCTTTGCATGAATG | |
| AUTO-3 | 19731625 | |
| FAM | AUTO | GAAGGTGACCAAGTTCATGCTCACAAGAATAATGCCCAAGAT |
| HEX | WT | GAAGGTCGGAGTCAACGGATTCACAAGAATAATGCCCAAGAC |
| REV | CCTAGGTTGACATAGCCACCA | |
| AUTO-4 | 19991224 | |
| FAM | AUTO | GAAGGTGACCAAGTTCATGCTTCTCACTTTCTGTCTTTTTCCCT |
| HEX | WT | GAAGGTCGGAGTCAACGGATTTCTCACTTTCTGTCTTTTTCCCC |
| REV | TCACAGTCTCAACAGGAGTGG | |
| AUTO-5 | 21536161 | |
| FAM | AUTO | GAAGGTGACCAAGTTCATGCTTTTTCATTTTCGGTGGGGTTTC |
| HEX | WT | GAAGGTCGGAGTCAACGGATTTTTTCATTTTCGGTGGGGTTTT |
| REV | GGTTGGATGTTTCAGCTGAAG | |
| EARLY-1 | 41445929 | |
| FAM | EARLY | GAAGGTGACCAAGTTCATGCTGGATACTAGCCACTAGAAAGGTTT |
| HEX | WT | GAAGGTCGGAGTCAACGGATTGGATACTAGCCACTAGAAAGGTTG |
| REV | CGAAGGAGATAAAGACTGTGAG | |
| EARLY-2 | 46288769 | |
| FAM | EARLY | GAAGGTGACCAAGTTCATGCTATGTGTGTGTGCCTGTAGAACC |
| HEX | WT | GAAGGTCGGAGTCAACGGATTATGTGTGTGTGCCTGTAGAACT |
| REV | GTCCTAACCTTCAGAAACTCCTAG |
PACE primers designed for the Autoflower1 (AUTO) and Early1 (EARLY) loci.
Additional PACE primers segregating in GVA-H-20-1080 associated with Autoflower1 are listed in Supplementary Table 1.
Results
Autoflower1 photoperiod insensitivity is a recessive Mendelian trait
Two populations segregating for photoperiod insensitivity (GVA-H-20-1080 and ‘TJ’s CBG’) were planted under non-inductive, long day conditions (16L:8D dark). In the GVA-H-20-1080 population, 28/88 plants flowered (31.8%), and in the ‘TJ’s CBG’ population, 24/88 plants flowered (27.3%). These data are not significantly different from 25% of the plants flowering (χ2 P > 0.05), consistent with a recessive allele at a single locus we are designating Autoflower1 that was homozygous in KG9202, heterozygous in the photoperiod sensitive F1 progeny of KG9202 × ‘Otto II,’ and segregating 1:2:1 in the GVA-H-20-1080 F2 and ‘TJ’s CBG’ populations. The parents of the GVA-H-20-1080 population were also grown under these conditions, with KG9202 flowering alongside the Autoflower1/Autoflower1 homozygotes and ‘Otto II’ not being induced to flower.
Mapping of the Autoflower1 locus
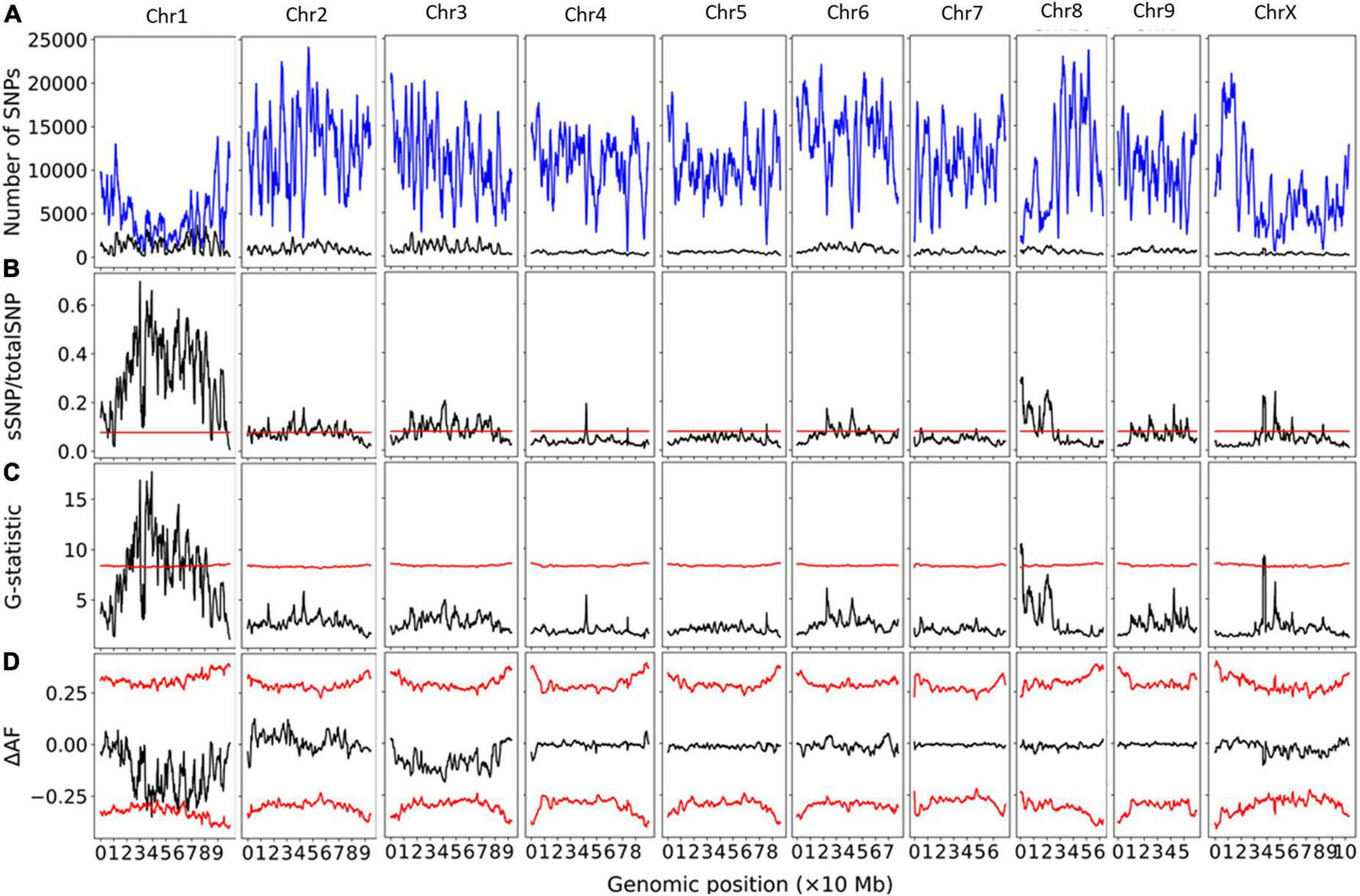
Bulk segregant analysis of Illumina sequence pools of photoperiod-sensitive and -insensitive individuals showed clear statistical significance for the G-Test statistic in a region of Chromosome 1 (NC_044371.1) for population GVA-H-20-1080 (Figure 1). 230420 SNPs were included on Chromosome 1 in this analysis. No other chromosome reached significance by this metric (Figure 1). The significant region associated with Autoflower1 spanned 17.74–22.94 Mb on Chromosome 1, with highly significant genomic windows (G-Test statistic > 11.5) at 18.59–19.70 Mb.
FIGURE 1

Bulk segregant analysis examining pools of photoperiod-insensitive and photoperiod-sensitive plants from GVA-H-20-1080. A physical genomic window size of 2 Mb and step size of 10 kb is shown. (A) Chromosomal distribution of SNPs. Total SNP number is in blue and significant SNP number (Fisher Exact Test P < 0.01) is in black. (B) Ratio of significant SNPs:Total SNPs across chromosomes. (C) G-Test statistic values across chromosomes. (D) Delta-allele frequency (ΔAF) values across chromosomes. Red lines represents significance thresholds determined by simulation of 10000 SNPs given allele depths at the 99.5th percentile for significant SNPs:Total SNPs and G-statistic, and the 99% confidence interval for ΔAF values.
Using a delta-allele frequency approach, none of the genome reached significance, but the region identified on Chromosome 1 neared significance, and there were non-significant peaks on Chromosomes 8 and X.
Autoflower1 candidate gene analysis
Within the G-statistic significant region of Autoflower1 on Chromosome 1 defined by the BSA of GVA-H-20-1080, 237 annotated genes were identified using the NCBI Genome Data Viewer. Of these, 75 were uncharacterized. Candidate genes potentially involved in controlling flowering time include: DOF zinc finger nucleases (LOC115704700, LOC115704742), nuclear transcription factor Y subunit B-1 (NFYB1, LOC115706176), floral homeotic protein APETALA 2 (AP2, LOC115708151), regulator of nonsense transcripts UPF2 (LOC115706264), zinc finger CCCH domain-containing protein 11 (LOC115706080), two-component response regulator-like PRR37 (PRR37, LOC115705128), protein FAR1-RELATED SEQUENCE 5-like (LOC115703878, LOC115703890), and protein LONG AFTER FAR RED 3 (LOC115705698). The 237 genes within the significant region are detailed in Supplementary Table 2.
Germplasm screening with Autoflower1 molecular assays
Within the region significantly associated with Autoflower1, some SNP alleles that were homozygous in the photoperiod-insensitive bulk and had an allele frequency of ∼33% in the photoperiod-sensitive bulk were converted to PACE assays and screened on diverse germplasm (Table 2). As photoperiod-sensitive plants may be heterozygous for Autoflower1, the Autoflower1 marker assay was considered to be perfect if the homozygous allelic group associated with photoperiod-insensitive plants from the bulk was associated with photoperiod-insensitive plants only, while photoperiod-sensitive plants were either heterozygous or in the alternate homozygous allelic group.
TABLE 2
| Cultivar or population | Source | Autoflower11 | n | AUTO-12 (18.464 Mb) | AUTO-2 (19.701 Mb) | AUTO-3 (19.731 Mb) | AUTO-4 (19.991 Mb) | AUTO-5 (21.536 Mb) |
| CASPL4D1 | NFYB1 | AP2 | PRR37 | LOC11570 3889 | ||||
| ‘Anka’ | UniSeeds Inc. | WT/WT | 4 | A/A | C/C | C/C | T/T | T/T |
| Bish Feral | Bish Enterprises | Unknown | 8 | A/A | C/C | C/C | C/C | T/T |
| C16 | Arcadia | WT/WT | 2 | A/A | C/C | C/C | T/T | T/T |
| ‘CFX-2’ | Hemp Genetics Intl. | Unknown | 8 | A/A | C/C | C/C | C/C | T/T |
| ‘Henola’ | Intl. Hemp | Unknown | 8 | A/A | C/C | C/C | T/T | T/T |
| ‘Picolo’ | Hemp Genetics Intl. | Unknown | 16 | A/A | C/C | C/C | Seg* | T/T |
| ‘Puma’ | CN Kenaf and Hemp | WT/WT | 4 | A/A | C/C | C/C | Seg* | T/T |
| RN13A | Paul Smith Denver Co. | WT/WT | 4 | A/A | C/C | C/C | T/T | T/T |
| RN17 | Paul Smith Denver Co. | WT/WT | 8 | A/A | C/C | C/C | Seg* | T/T |
| ‘Si-1’ | CN Kenaf and Hemp | WT/WT | 19 | A/A | C/C | C/C | Seg* | T/T |
| ‘Canda’ | Parkland Ind. | Unknown | 8 | A/A | C/C | C/C | C/C | T/T |
| Missouri Feral | John Fike (40.2, −94.6) | Unknown | 8 | A/A | C/C | C/C | C/C | T/T |
| ‘Nebraska’ | Winter Fox Farms | Unknown | 8 | A/A | C/C | C/C | Seg* | T/T |
| ‘NWG-Elite’ | New West Genetics | Unknown | 8 | A/A | C/C | C/C | Seg* | T/T |
| ‘T2’ | Boring Hemp Co. | WT/WT | 8 | A/A | C/C | C/C | Seg* | T/T |
| ‘USO-31’ | UniSeeds Inc. | Unknown | 8 | A/A | C/C | C/C | C/C | T/T |
| ‘CBG Delight’ | Flura | Segregating | 32 | Seg† | Seg† | Seg† | Seg† | Seg* |
| ‘H5’ | American Hemp Co. | Segregating | 32 | Seg* | Seg† | Seg† | Seg* | Seg* |
| ‘Hempress’ | Point3 Farma | Segregating | 24 | Seg† | Seg† | Seg† | Seg* | Seg† |
| ‘Le Crème’ | Ventura Seed Co. | Segregating | 44 | ND | Seg† | Seg† | ND | ND |
| GVA-H-20-1080 | Cornell Hemp | Segregating | 184 | Seg* | Seg† | Seg† | Seg† | Seg* |
| ‘TJ’s CBG’ | Stem Holdings Agri | Segregating | 88 | Seg† | Seg† | Seg† | Seg† | Seg* |
| ‘Suver Haze’ | Oregon CBD | WT/Autoflower1 | 8 | T/C | T/C | T/C | C/C | C/T |
| ‘Umpqua’ | Ind. Seed Innovations | WT/ Autoflower1 | 4 | T/C | T/C | T/C | T/T | T/C |
| AD1010 | Phylos Bioscience | Autoflower1/ Autoflower1 | 4 | T/T | T/T | T/T | T/T | Seg* |
| ‘Alpha Explorer’ | Phylos Bioscience | Autoflower1/ Autoflower1 | 4 | T/T | T/T | T/T | T/T | Seg*’ |
| ‘Alpha Nebula’ | Phylos Bioscience | Autoflower1/ Autoflower1 | 4 | T/T | T/T | T/T | T/T | Seg* |
| ‘Auto CBD’ | Phylos Bioscience | Autoflower1/ Autoflower1 | 4 | T/T | T/T | T/T | T/T | C/C |
| ‘Auto CBG’ | Oregon CBD | Autoflower1/ Autoflower1 | 4 | T/T | T/T | T/T | T/T | Seg* |
| DNCBD | Arcadia Bioscience | Autoflower1/ Autoflower1 | 4 | T/T | T/T | T/T | T/T | C/C |
| ‘Dr. Chunk’ | Kayagene | Autoflower1/ Autoflower1 | 4 | T/T | T/T | T/T | T/T | C/C |
| ‘Maverick’ | Kayagene | Autoflower1/ Autoflower1 | 4 | T/T | T/T | T/T | T/T | C/C |
| ‘Purple Star’ | Atlas Seeds | Autoflower1/ Autoflower1 | 4 | T/T | T/T | T/T | T/T | C/C |
| ‘Rincon’ | Kayagene | Autoflower1/ Autoflower1 | 4 | T/T | T/T | T/T | T/T | Seg* |
| ‘Sour Citron’ | Kayagene | Autoflower1/ Autoflower1 | 4 | T/T | T/T | T/T | T/T | C/C |
| ‘Sour RNA Seedless’ (triploid) | Oregon CBD | Autoflower1/ Autoflower1/ Autoflower1 | 4 | T/T(/T) | T/T(/T) | T/T(/T) | T/T(/T) | C/C(/C) |
Genotype group calls for the Autoflower1 locus by cultivar or population.
1Expected Autoflower1 locus status based on phenotype and breeding history. Segregating populations all segregating 3:1 photoperiod sensitive: photoperiod insensitive.
2Seg†, segregating perfectly; Seg*, segregating imperfectly; ND, not determined.
Effect of Autoflower1 genotype on agronomic performance
Ninety-six individuals of GVA-H-20-1080 grown in the 2021 flowering time field trial were genotyped at Autoflower1 using the AUTO-2 marker to determine the additive effect of this locus when grown under field conditions. There was a significant effect of the allelic group on flowering date, height, and biomass, with heterozygotes being intermediate with respect to flowering date, height, and wet biomass (Figure 2).
FIGURE 2
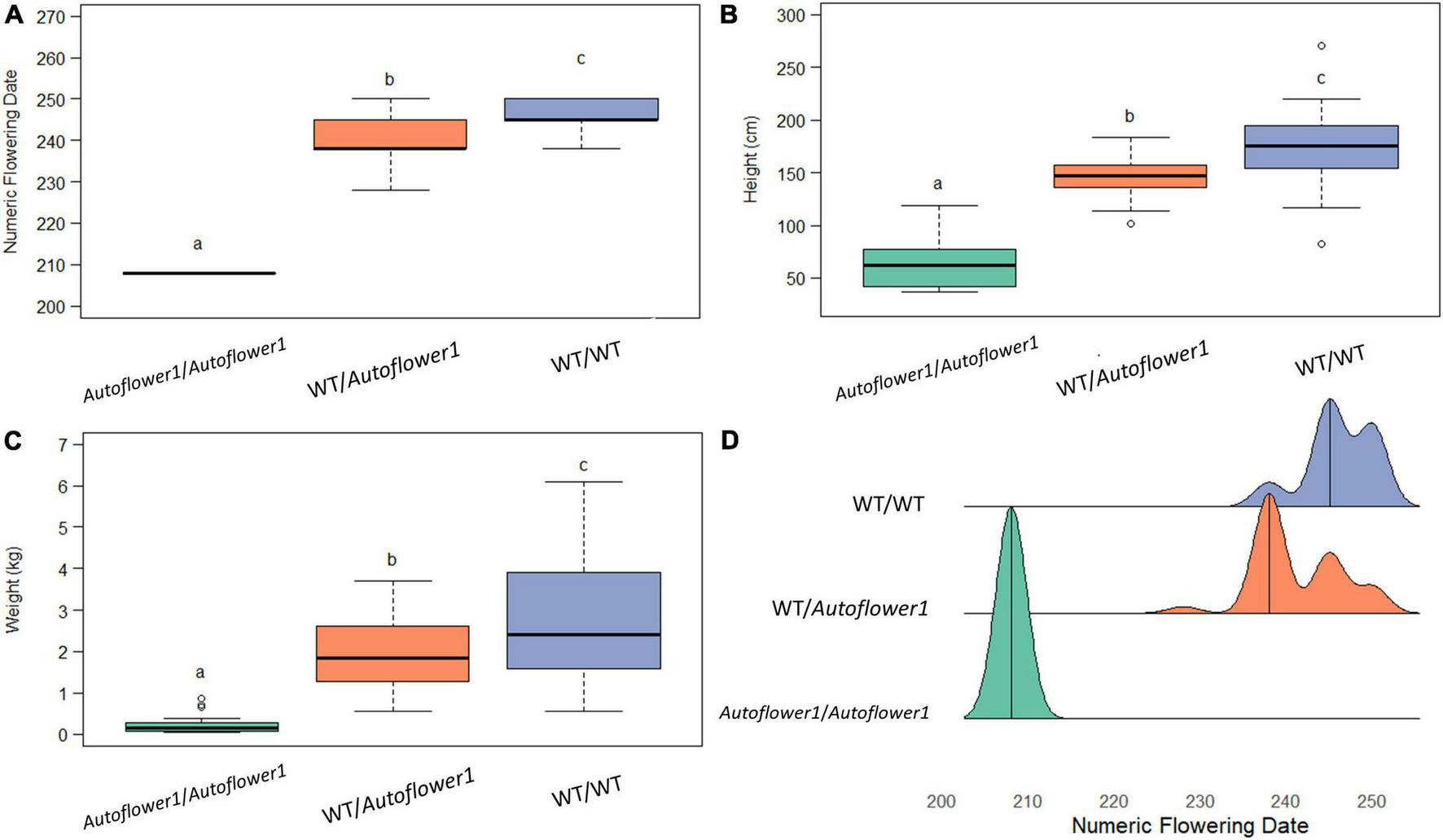
Effect of genotype at Autoflower1 on agronomic traits. (A) Numeric (ordinal) flowering day. (B) Height, measured from base to tip at end of season. (C) Wet biomass. (D) Density ridge plot of flowering times within groups. Letters depict Tukey post-hoc test groupings (α = 0.05).
Flowering of diverse germplasm under continuous light
Several populations were grown under continuous light to determine if non-Autoflower1/Autoflower1 cultivars could be induced to flower. There were distinct patterns of flowering time behavior among and within populations (Table 3). Most high-cannabinoid cultivars did not flower under continuous light, except for those homozygous for Autoflower1. Notably, plants heterozygous at the Autoflower1 locus did not flower under continuous light. In general, cultivated fiber and Chinese cultivars did not terminally flower, while male and female plants in closely related feral populations did produce terminal and axillary (solitary) flowers, respectively. Also flowering under continuous light were the Canadian grain cultivars, ‘Picolo’ and ‘CFX-2’ (Hemp Genetics International, Saskatoon, SK).
TABLE 3
| Cultivar or population | Males present1 | Taxonomic group2 | AUTO -FLOWER11 | EARLY11 | Source | Flowering date1,3 | ||||
| May 10 | May 17 | May 24 | May 31 | June 28 | ||||||
| ‘Carmagnola’ | + | Fiber/Feral | –/– | –/– | Schiavi Seed | – | – | – | – | – |
| ‘Puma’ | + | Chinese | –/– | –/– | CN Kenaf and Hemp | – | – | – | – | – |
| GVA-H-19-1052 | – | West Coast | –/– | +/+, ±, –/– | Cornell Hemp | – | – | – | – | – |
| RN16 | + | T1/R4 | –/– | –/– | Paul Smith Denver Co. | – | – | – | – | – |
| ‘Umpqua’ | – | West Coast | ± | ±, –/– | Arcadia Bioscience | – | – | – | – | – |
| NS52 | – | Not tested | ±, –/– | ±, –/– | Phytonyx | – | – | – | – | – |
| ‘Fedora 17’ | – | Grain/Dual | –/– | +/+, ±, –/– | UniSeeds, Inc. | – | – | – | – | Axillary |
| ‘A2R4’ | + | Fiber/Feral | –/– | –/– | WinterFox Farms | – | – | – | – | Axillary |
| ‘BaOx’ | + | BaOx/Otto II | –/– | –/– | Ryes Creek | – | – | – | – | M, axillary F |
| ‘Nebraska’ | + | Fiber/Feral | –/– | –/– | WinterFox Farms | – | – | – | – | M, axillary F |
| Missouri Feral | + | Fiber/Feral | –/– | –/– | J. Fike | – | – | – | M only | M, axillary F |
| GVA-H-20-1080 | – | Intercross | +/+, ±, –/– | –/– | Cornell Hemp | – | 1/4 | 1/4 | 1/4 | 1/4 |
| ‘Auto CBD’ | – | Not tested | +/+ | –/– | Phylos Bioscience | – | + | + | + | + |
| ‘Auto CBG’ | – | Not tested | +/+ | –/– | Oregon CBD | – | + | + | + | + |
| ‘Socati Auto’ | – | Not tested | +/+ | –/– | Boring Hemp Co. | – | + | + | + | + |
| KG9202 | – | West Coast | +/+ | –/– | Kayagene | – | + | + | + | + |
| ‘Anka’ | + | Grain/Dual | –/– | –/– | UniSeeds Inc. | – | M only | M only | M, axillary F | M, axillary F |
| ‘Henola’ | – | Grain/Dual | –/– | + /+ | Bija Hemp | – | Some | + | + | + |
| ‘CFX-2’ | + | Grain/Dual | –/– | –/– | HGI | M only | + | + | + | + |
| ‘Picolo’ | + | Grain/Dual | –/– | –/– | HGI | M only | + | + | + | + |
Time to flower of various C. sativa cultivars and populations under continuous light.
1XY plants present in population; yes (+), no (–); Alleles; non-WT (+), WT (–); Flowers present on date, yes (+), no (–).
2Taxonomical group data described in Carlson et al. (2021).
Complementation test of photoperiod-insensitive cultivars
The Canadian grain hemp cultivar ‘Picolo’ and a subset of the individuals in the ‘Le Crème’ population both flowered under continuous light, but had contrasting PACE marker calls at the Autoflower1 locus. A complementation test was performed to determine if there were distinct genes underlying their respective photoperiod insensitivity. All F1 plants from this cross were induced to flower, although the time to flower was distinct from the parents (Table 4). Homozygous Autoflower1/Autoflower1 female plants flowered 3 weeks earlier than female ‘Picolo’ and female F1 plants, and male ‘Picolo’ plants flowered 2 weeks earlier than the male F1 plants. Female ‘Picolo’ and F1 plants were morphologically similar, while ‘Le Crème’ Autoflower1 plants were distinct (Figure 3). All plants of a given sex and genotype flowered on the same day.
TABLE 4
| Cultivar or pedigree | Males (weeks) | Females (weeks) |
| ‘AutoCBD’, ‘Le Crème’ (Autoflower1/Autoflower1) | n/a | 4 (AutoCBD n = 10, Le Crème (Autoflower1/ Autoflower1) n = 3) |
| ‘Picolo’ | 4 (n = 7) | 7 (n = 3) |
| ‘Le Crème’ (Autoflower1/Autoflower1) × ‘Picolo’ (F1) | 6 (n = 4) | 7 (n = 6) |
| RN16 | None | None |
Time to flower under long day (16L:8D) lighting.
FIGURE 3

Photoperiod-insensitive C. sativa grown under long days (16L:8D) and photographed 85 days after planting. Representative female plants of panel (A) ‘Picolo,’ (B) ‘Le Crème’ (Autoflower1/Autoflower1), and (C) ‘Le Crème’ (Autoflower1/Autoflower1) × ‘Picolo’ F1. Receptive white pistils were present at the apex of plants in panels (A,C) at 85 days after planting, but only brown desiccated pistils were present on plants in panel (B).
Segregation for flowering time in ‘Umpqua’
The cultivar ‘Umpqua’ has been grown in Cornell field trials in 2019, 2020, and 2021. In each year, two distinct flowering times were noted (Figure 4). Over the course of 3 years, clear grouping was apparent, with 78 plants total in the early flowering group and 97 plants total in the later flowering group. These data are consistent with a 1:1 segregation of early and late phenotypes (χ2 = 2.063, P = 0.15), characteristic of a backcross involving a major effect gene (designated here as Early1) that is heterozygous in one parent and homozygous in the other. Neither phenotype was induced to flower under continuous light (Table 3).
FIGURE 4

Density ridge plot of ‘Umpqua’ flowering time in the field over 3 years in Geneva, NY.
Mapping of Early1 in ‘Umpqua’
Bulk segregant analysis showed clear statistical significance for the Early1 locus on Chromosome 1 (NC_044371.1), reaching significance using the delta-allele frequency approach (Figure 5). 247814 SNPs on Chromosome 1 were used in this analysis. Examination of the significant SNP data showed that the early flowering ‘Umpqua’ group was heterozygous at Early1 while late flowering ‘Umpqua’ group was not.
FIGURE 5
Bulk segregant analysis for pools of early- and late-flowering plants from ‘Umpqua.’ Physical genomic window size is 2 Mb and step size is 10 kb. (A) Chromosomal distribution of SNPs. Total SNP number is in blue and significant SNP number (Fisher Exact Test P < 0.01) is in black. (B) Ratio of significant SNPs:Total SNPs across chromosomes. (C) G-statistic values across chromosomes. (D) Delta-allele frequency (ΔAF) values across chromosomes. Red lines represents significance thresholds determined by simulation of 10000 SNPs given allele depths at the 99.5th percentile for significant SNPs:Total SNPs and G-statistic, and the 99% confidence interval for ΔAF values.
Using a G-statistic threshold, most of Chromosome 1, as well as small peaks on Chromosomes 8 and X, were deemed significant.
Early1 candidate gene analysis
For the two ‘Umpqua’ pools, there were several small peaks that exceeded significance levels for the delta-allele frequency metric on Chromosome 1, spanning the intervals 35.26–36.23, 38.67–39.36, and 59.8–59.9 Mb. A total of 45 genes are annotated within these regions, of which the strongest candidate gene for Early1 based on molecular function is LOC115705415 (annotated to encode Casein kinase 1-like protein 1), located on Chromosome 1 at 39.265 Mb (Supplementary Table 3). Polymorphic SNPs that were heterozygous for Early1 in the early flowering pool were developed into high-throughput PACE marker assays (Table 1). Genotype assays correlated perfectly with the early- and late-flowering phenotypes of ‘Umpqua’ across all tested plants (N = 175).
Discussion
Autoflower1
Autoflower1 confers photoperiod insensitivity in diverse C. sativa germplasm, and segregates in a simple, recessive (Mendelian) manner. Using BSA, we mapped the Autoflower1 locus derived from KG9202 controlling the photoperiod insensitive phenotype in the GVA-H-20-1080 population to a small region on C. sativa Chromosome 1. Polymorphic markers from the Illumina data were used to develop Autoflower1 molecular assays that accurately reported cultivars marketed as “Autoflowers,” and did not report any photoperiod sensitive plant as photoperiod insensitive. The markers were not effective at predicting photoperiod insensitivity across all germplasm, but this may be due to multiple causes of photoperiod insensitivity.
While Autoflower1 is recessive with respect to photoperiod insensitivity, plants that were heterozygous for Autoflower1 flowered approximately 2 weeks earlier than plants that were homozygous for Autoflower1 under field conditions. This earlier flowering resulted in smaller plants with less total biomass, but may be useful for higher latitudes, as cultivars that are heterozygous for Autoflower1 can produce very high yields in a shorter growing season (Stack et al., 2021). Many available cultivars are heterozygous for Autoflower1, which may be used as an effective breeding strategy for intellectual property protection. The prevalence of segregating populations marketed as cultivars suggests that some (perhaps unscrupulous or novice) breeders used parents that were heterozygous at Autoflower1 leading to photoperiod-insensitive plants in the seed population. Populations segregating with ∼1/4 photoperiod-insensitive individuals, such as ‘TJ’s CBG,’ suggest production by a cross between two parents heterozygous for Autoflower1, possibly the self-pollination of a plant heterozygous for Autoflower1. As detailed in Table 2, several commercially marketed cultivar populations from multiple sources were segregating for Autoflower1, which may have resulted in poor cultivar performance for growers due to variation in photoperiod sensitivity.
Further work to identify the taxonomic origin of Autoflower1 is pertinent. Autoflower1 is often ascribed in the gray literature as derived from C. ruderalis, but the most recent and in-depth genomic studies do not support the existence of this group (Green, 2005; Carlson et al., 2021; Ren et al., 2021). Autoflower1 would be expected to have evolved either at very high or very low latitudes, where photoperiod insensitivity is evolutionarily advantageous because seasonal variation in daylength is minimal. Further plant collecting expeditions and population genomics analyses would help resolve the evolutionary origin of Autoflower1 as well as the extent of the genetic and phenotypic diversity of photoperiod sensitivity in C. sativa.
Future work to determine the causative gene at Autoflower1 will allow biotechnological manipulation of the photoperiod sensitivity phenotype and more facile conversion of elite cultivars to and from photoperiod insensitivity. There were strong candidate genes for Autoflower1 based on annotated predicted molecular function in the significant QTL interval identified for the GVA-H-20-1080 pools. Notably, SNPs near the genes for nuclear transcription factor Y subunit B-1 (NFYB1, LOC115706176) and floral homeotic gene APETALA 2 (AP2, LOC115708151) were in linkage disequilibrium and were perfectly associated with predicted trait phenotype across all individuals tested. These genes have the potential to be causative for the trait, as a Nuclear factor Y gene (DTH8) plays an important repressive role related to photoperiod in rice (Wei et al., 2010) while AP2 homologs are also important flowering time repressors in pepper (Yuan et al., 2021) and Arabidopsis (Yant et al., 2010). Future gene silencing or knockouts of these and other potential candidate genes may lead to identification of the true gene or set of genes responsible for this trait, although a patent is already held covering biotechnological manipulation of genes within this genetic interval (Phylos Bioscience, International Patent WO 2021/097496 A2).
Continuous light
Plants from diverse germplasm had different flowering responses to continuous light. Photoperiod-sensitive high-cannabinoid cultivars and modern European and Chinese fiber cultivars did not flower under continuous light. Fiber cultivars have been selected for their ability to continue to grow vegetatively until late in the season, which maximizes stem biomass yield. Some feral populations, which are closely related to European fiber cultivars (Carlson et al., 2021), displayed male flowering, but not terminal female flowering, perhaps indicating some selective advantage to photoperiod-insensitive male flowering outside of cultivation. This may also reflect the ancestral genetics of the progenitors of these feral populations, but it is difficult to know the original provenance of their progenitors.
Despite not being reported by the Autoflower1 markers, Canadian grain cultivars ‘Picolo’ and ‘CFX-2’ flowered readily under continuous light conditions. This could be due to the molecular markers not being polymorphic or effective in these populations, or due to an alternate genetic basis for photoperiod insensitivity. Different genetic mechanisms may be resolved with a complementation test. If the same gene was responsible for photoperiod sensitivity in ‘Picolo’ and Autoflower1 ‘Le Crème,’ F1 progeny from an intercross should be uniformly photoperiod-insensitive. Otherwise, other genes, dominance, or epistasis may be involved. The results (Table 4) were inconclusive, as all plants flowered under long days, but the timing and architecture of flowering (Figure 3) suggests more complex genetic regulation in photoperiod-insensitive plants across broad germplasm.
Early1
Beyond segregation for Autoflower1, several elite populations marketed as cultivars have been demonstrated as segregating 1:1 for a major-effect early flowering time phenotype (Stack et al., 2021). The Early1 locus, which confers an apparent effect size of 2–4 weeks earlier flowering in ‘Umpqua,’ was also mapped to Chromosome 1 using BSA, but to a different location than Autoflower1. The apparent different effect in 2019 compared to 2020 and 2021 may have been due to differences in flowering time rating or non-uniform planting dates. Molecular markers for Early1 were identified and high-throughput assays developed for this locus, which could further aid in development of cultivars with uniform flowering time.
In searching for candidate genes in the confidence interval for the Early1 locus identified in ‘Umpqua’ populations, only a small portion of Chromosome 1 was found to exceed significance thresholds by the delta-allele frequency method. One possible candidate gene for early flowering within this small interval encodes a Casein kinase 1-like protein 1 (LOC115705415). This gene is homologous to the major flowering time gene Early flowering 1/Heading date 16 in rice, another short day plant (Hori et al., 2013). Future validation work could involve genetic engineering or genome editing to accomplish gene knockout or gene knock-in to confirm loss or gain of function. The molecular markers and assays for Early1 presented in this work will be considerably valuable in breeding. Studies to further explore the interactions between these two flowering time loci, Autoflower1 and Early1, will likely lead to a better understanding of the genetics of flowering time and development of stable cultivars with unique flowering times. As early flowering ‘Umpqua’ plants were heterozygous for both traits, the progeny of an inbred population would be expected to form nine genotypic groups, whose phenotypes would reveal the role of epistasis between these loci.
There were some differences in the statistical outcomes of the BSA for Autoflower1 in comparison to Early1 in ‘Umpqua.’ The statistically significant region of Early1 in ‘Umpqua’ as determined by the G-Test statistic was much larger and broader than that of Autoflower1. This is not surprising if this segregation truly is the result of a simple backcross, as recombination occurs only in one parent, rather than in both parents. This reduces the number of recombination events and therefore increases the apparent QTL size. However, analysis using the delta-allele frequency method resulted in a small peak and reliable diagnostic molecular assays were developed for the Early1 locus.
Curiously, mapping both Autoflower1 and Early1 had apparent peaks on Chromosomes 8 and X, reaching statistical significance by the G-statistic threshold in the case of mapping Early1. It is unlikely that a 3-locus model explains the observed segregation in flowering time, so this is likely an experimental artifact. The unusual peaks may be due to errors in mapping or genome assembly, with segments on Chromosomes 8 and X being highly homologous to Chromosome 1 leading to inappropriate mapping, or errors in assembly with those significant regions actually residing on Chromosome 1. The CBDRx-cs10 genome used is known to be incomplete and may not be correctly physically ordered (Kovalchuk et al., 2020).
Many C. sativa cultivars produced during the rapid expansion of the cannabinoid industry were segregating for flowering time. There is a critical need in the industry to develop uniform and stable cultivars that represent a range of critical photoperiods. Cultivars with known critical photoperiods can be more effectively matched with the latitude of agricultural regions and environmental conditions in controlled environment cultivation. A better understanding of the genetic basis of flowering time in C. sativa, coupled with molecular tools to accelerate breeding and selection, will enable the development of new uniform cultivars to meet this need.
Statements
Data availability statement
The data presented in this study are deposited in the NCBI SRA repository, accession numbers: SRR20046529-SRR20046532.
Author contributions
JT: conceptualization, methodology, formal analysis, and writing—original draft. GS and CC: conceptualization, resources, and writing—review and editing. LS: conceptualization, supervision, writing—review and editing, and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This work was partially supported by the New York State Department of Agriculture and Markets through grants AC477 and AC483 from Empire State Development Corporation, a grant from the Foundation for Food and Agriculture Research Hemp Research Consortium (NexGen-Hemp-0000000008) in cooperation with Scotts Corporation, and a sponsored research agreement with Pyxus International. The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Acknowledgments
We are grateful to the technical staff of the Smart Lab, especially Rebecca Wilk, Teagan Zingg, Allison DeSario, Deanna Gentner, Lauren Carlson, Brian Nardone, Michael Quade, Alexander Wares, and McKenzie Schessl. We would like to thank the generous contribution of germplasm by the companies and individuals listed in Table 3.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.991680/full#supplementary-material
References
1
BanS.XuK. (2020). Identification of two QTLs associated with high fruit acidity in apple using pooled genome sequencing analysis.Hortic. Res.7:171. 10.1038/s41438-020-00393-y
2
BeckerA.ChaoD.-Y.ZhangX.SaltD. E.BaxterI. (2011). Bulk segregant analysis using single nucleotide polymorphism microarrays.PLoS One6:e15993. 10.1371/journal.pone.0015993
3
BlümelM.DallyN.JungC. (2015). Flowering time regulation in crops—what did we learn from Arabidopsis?Curr. Opin. Biotechnol.32121–129. 10.1016/j.copbio.2014.11.023
4
CallawayJ. (2002). Hemp as food at high latitudes.J. Ind. Hemp7105–117. 10.1300/J237v07n01_09
5
CarlsonC. H.StackG. M.JiangY.TaşkıranB.CalaA. R.TothJ. A.et al (2021). Morphometric relationships and their contribution to biomass and cannabinoid yield in hybrids of hemp (Cannabis sativa).J. Exp. Bot.727694–7709. 10.1093/jxb/erab346
6
GlossD. (2015). An overview of products and bias in research.Neurotherapeutics12731–734. 10.1007/s13311-015-0370-x
7
GrassaC. J.WeiblenG. D.WengerJ. P.DabneyC.PoplawskiS. G.Timothy MotleyS.et al (2021). A new Cannabis genome assembly associates elevated cannabidiol (CBD) with hemp introgressed into marijuana.New Phytol.2301665–1679. 10.1111/nph.17243
8
GreenG. (2005). The cannabis breeder’s bible: The definitive guide to marijuana genetics, cannabis botany and creating strains for the seed market.San Francisco, CA: Green Candy Press.
9
HoriK.Ogiso-TanakaE.MatsubaraK.YamanouchiU.EbanaK.YanoM. (2013). Hd16, a gene for casein kinase I, is involved in the control of rice flowering time by modulating the day-length response.Plant J.7636–46. 10.1111/tpj.12268
10
JungC.MüllerA. E. (2009). Flowering time control and applications in plant breeding.Trends Plant Sci.14563–573. 10.1016/j.tplants.2009.07.005
11
KovalchukI.PellinoM.RigaultP.Van VelzenR.EbersbachJ.AshnestJ. R.et al (2020). The genomics of Cannabis and its close relatives.Annu. Rev. Plant Biol.71713–739. 10.1146/annurev-arplant-081519-040203
12
LubellJ. D.BrandM. H. (2018). Foliar sprays of silver thiosulfate produce male flowers on female hemp plants.Horttechnology28743–747. 10.21273/HORTTECH04188-18
13
MagweneP. M.WillisJ. H.KellyJ. K. (2011). The statistics of bulk segregant analysis using next generation sequencing.PLoS Comput. Biol.7:e1002255. 10.1371/journal.pcbi.1002255
14
McPartlandJ. M. (2018). Cannabis systematics at the levels of family, genus, and species.Cannabis Cannabinoid Res.3203–212. 10.1089/can.2018.0039
15
MouradovA.CremerF.CouplandG. (2002). Control of flowering time : Interacting pathways as a basis for diversity.Plant Cell14S111–S130. 10.1105/tpc.001362
16
MurphyR. L.KleinR. R.MorishigeD. T.BradyJ. A.RooneyW. L.MillerF. R.et al (2011). Coincident light and clock regulation of pseudoresponse regulator protein 37 (PRR37) controls photoperiodic flowering in sorghum.Proc. Natl. Acad. Sci. U.S.A.10816469–16474. 10.1073/pnas.1106212108
17
NordströmK. J.AlbaniM. C.JamesG. V.GutjahrC.HartwigB.TurckF.et al (2013). Mutation identification by direct comparison of whole-genome sequencing data from mutant and wild-type individuals using k-mers.Nat. Biotechnol.31325–330. 10.1038/nbt.2515
18
PetitJ.SalentijnE. M.PauloM.-J.DenneboomC.TrindadeL. M. (2020). Genetic architecture of flowering time and sex determination in hemp (Cannabis sativa L.): A genome-wide association study.Front. Plant Sci.11:1704. 10.3389/fpls.2020.569958
19
RenG.ZhangX.LiY.RidoutK.Serrano-SerranoM. L.YangY.et al (2021). Large-scale whole-genome resequencing unravels the domestication history of Cannabis sativa.Sci. Adv.7:eabg2286. 10.1126/sciadv.abg2286
20
SalentijnE. M.PetitJ.TrindadeL. M. (2019). The complex interactions between flowering behavior and fiber quality in hemp.Front. Plant Sci.10:614. 10.3389/fpls.2019.00614
21
SegawaT.NishiyamaC.Tamiru-OliM.SugiharaY.AbeA.SoneH.et al (2021). Sat-BSA: An NGS-based method using local de novo assembly of long reads for rapid identification of genomic structural variations associated with agronomic traits.Breed. Sci.71:20148. 10.1270/jsbbs.20148
22
SongJ.LiZ.LiuZ.GuoY.QiuL.-J. (2017). Next-generation sequencing from bulked-segregant analysis accelerates the simultaneous identification of two qualitative genes in soybean.Front. Plant Sci.8:919. 10.3389/fpls.2017.00919
23
StackG. M.TothJ. A.CarlsonC. H.CalaA. R.Marrero-GonzálezM. I.WilkR. L.et al (2021). Season-long characterization of high-cannabinoid hemp (Cannabis sativa L.) reveals variation in cannabinoid accumulation, flowering time, and disease resistance.GCB Bioenergy13546–561. 10.1111/gcbb.12793
24
TothJ. A.SmartL. B.SmartC. D.StackG. M.CarlsonC. H.PhilippeG.et al (2021). Limited effect of environmental stress on cannabinoid profiles in high-cannabidiol hemp (Cannabis sativa L.).GCB Bioenergy131666–1674. 10.1111/gcbb.12880
25
TothJ. A.StackG. M.CalaA. R.CarlsonC. H.WilkR. L.CrawfordJ. L.et al (2020). Development and validation of genetic markers for sex and cannabinoid chemotype in Cannabis sativa L.GCB Bioenergy12213–222. 10.1111/gcbb.12667
26
TothJ.PanduranganS.BurtA.Mitchell FetchJ.KumarS. (2018). Marker-assisted breeding of hexaploid spring wheat in the Canadian prairies.Can. J. Plant Sci.99111–127. 10.1139/cjps-2018-0183
27
Van BakelH.StoutJ. M.CoteA. G.TallonC. M.SharpeA. G.HughesT. R.et al (2011). The draft genome and transcriptome of Cannabis sativa.Genome Biol.12:R102. 10.1186/gb-2011-12-10-r102
28
WeiX.XuJ.GuoH.JiangL.ChenS.YuC.et al (2010). DTH8 suppresses flowering in rice, influencing plant height and yield potential simultaneously.Plant Physiol.1531747–1758. 10.1104/pp.110.156943
29
WellingM. T.LiuL.KretzschmarT.MauleonR.AnsariO.KingG. J. (2020). An extreme-phenotype genome-wide association study identifies candidate cannabinoid pathway genes in Cannabis.Sci. Rep.101–14. 10.1038/s41598-020-75271-7
30
YangR.BertholdE.McCurdyC. R.da Silva BenevenuteS.BrymZ. T.FreemanJ. H. (2020). Development of cannabinoids in flowers of industrial hemp (Cannabis sativa L.)—a pilot study.J. Agric. Food Chem.686058–6064. 10.1021/acs.jafc.0c01211
31
YantL.MathieuJ.DinhT. T.OttF.LanzC.WollmannH.et al (2010). Orchestration of the floral transition and floral development in Arabidopsis by the bifunctional transcription factor APETALA2.Plant Cell222156–2170. 10.1105/tpc.110.075606
32
YuanX.FangR.ZhouK.HuangY.LeiG.WangX.et al (2021). The APETALA2 homolog CaFFN regulates flowering time in pepper.Hortic. Res.8:208. 10.1038/s41438-021-00643-7
33
ZhangG.-L.ChenL.-Y.XiaoG.-Y.XiaoY.-H.ChenX.-B.ZhangS.-T. (2009). Bulked segregant analysis to detect QTL related to heat tolerance in rice (Oryza sativa L.) using SSR markers.Agric. Sci. China8482–487. 10.1016/S1671-2927(08)60235-7
34
ZhangJ.PantheeD. R. (2020). PyBSASeq: A simple and effective algorithm for bulked segregant analysis with whole-genome sequencing data.BMC Bioinform.21:99. 10.1186/s12859-020-3435-8
35
ZhangM.AndersonS. L.BrymZ. T.PearsonB. J. (2021). Photoperiodic flowering response of essential oil, grain, and fiber hemp (Cannabis sativa L.) cultivars.Front. Plant Sci.12:694153. 10.3389/fpls.2021.694153
Summary
Keywords
hemp (Cannabis sativa L.), bulk segregant analysis (BSA), flowering time, autoflower, early flowering, continuous light
Citation
Toth JA, Stack GM, Carlson CH and Smart LB (2022) Identification and mapping of major-effect flowering time loci Autoflower1 and Early1 in Cannabis sativa L.. Front. Plant Sci. 13:991680. doi: 10.3389/fpls.2022.991680
Received
11 July 2022
Accepted
22 August 2022
Published
21 September 2022
Volume
13 - 2022
Edited by
Dilip R. Panthee, North Carolina State University, United States
Reviewed by
Abhinandan Surgonda Patil, International Rice Research Institute (IRRI), Philippines; Alicja Macko-Podgórni, University of Agriculture in Kraków, Poland
Updates
Copyright
© 2022 Toth, Stack, Carlson and Smart.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lawrence B. Smart, lbs33@cornell.edu
†Present Address: Craig H. Carlson, Cereal Crops Research Unit, Edward T. Schafer Agricultural Research Center, USDA, ARS, Fargo, ND, United States
This article was submitted to Plant Breeding, a section of the journal Frontiers in Plant Science
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.