- 1Dept of Biosciences, School of Liberal Arts and Sciences, Mody University of Science and Technology, Lakshmangarh, Sikar, Rajasthan, India
- 2Department of Agriculture, Medi-Caps University, Pigdamber Road, Rau, Indore, Madhya Pradesh, India
- 3Department of Physics, Faculty of Sciences, University 20 Août 1955, Skikda, Algeria
- 4Department of Life Sciences, Hemchandracharya North Gujarat University, Patan, Gujarat, India
- 5Department of Biotechnology, Noida International University, Noida, U.P., India
- 6Radiological Sciences Department, College of Applied Medical Sciences, King Khalid University, Abha, Saudi Arabia
- 7BioImaging Unit, Space Research Centre, University of Leicester, Leicester, United Kingdom
- 8Department of Biotechnology, Deen Dayal Upadhyaya (D.D.U.) Gorakhpur University, Gorakhpur, Uttar Pradesh, India
- 9Department of Medical Laboratory Sciences, College of Applied Medical Sciences, Prince Sattam Bin AbdulAziz University- Al-Kharj, Riyadh, Saudi Arabia
- 10Department of Pathology, College of Medicine, Qassim University, Buraidah, Saudi Arabia
- 11Department of Earth Resources and Environmental Engineering, Hanyang University, Seoul, Republic of Korea
The most significant issues that humans face today include a growing population, an altering climate, an growing reliance on pesticides, the appearance of novel infectious agents, and an accumulation of industrial waste. The production of agricultural goods has also been subject to a great number of significant shifts, often known as agricultural revolutions, which have been influenced by the progression of civilization, technology, and general human advancement. Sustainable measures that can be applied in agriculture, the environment, medicine, and industry are needed to lessen the harmful effects of the aforementioned problems. Endophytes, which might be bacterial or fungal, could be a successful solution. They protect plants and promote growth by producing phytohormones and by providing biotic and abiotic stress tolerance. Endophytes produce the diverse type of bioactive compounds such as alkaloids, saponins, flavonoids, tannins, terpenoids, quinones, chinones, phenolic acids etc. and are known for various therapeutic advantages such as anticancer, antitumor, antidiabetic, antifungal, antiviral, antimicrobial, antimalarial, antioxidant activity. Proteases, pectinases, amylases, cellulases, xylanases, laccases, lipases, and other types of enzymes that are vital for many different industries can also be produced by endophytes. Due to the presence of all these bioactive compounds in endophytes, they have preferred sources for the green synthesis of nanoparticles. This review aims to comprehend the contributions and uses of endophytes in agriculture, medicinal, industrial sectors and bio-nanotechnology with their mechanism of action.
1 Introduction
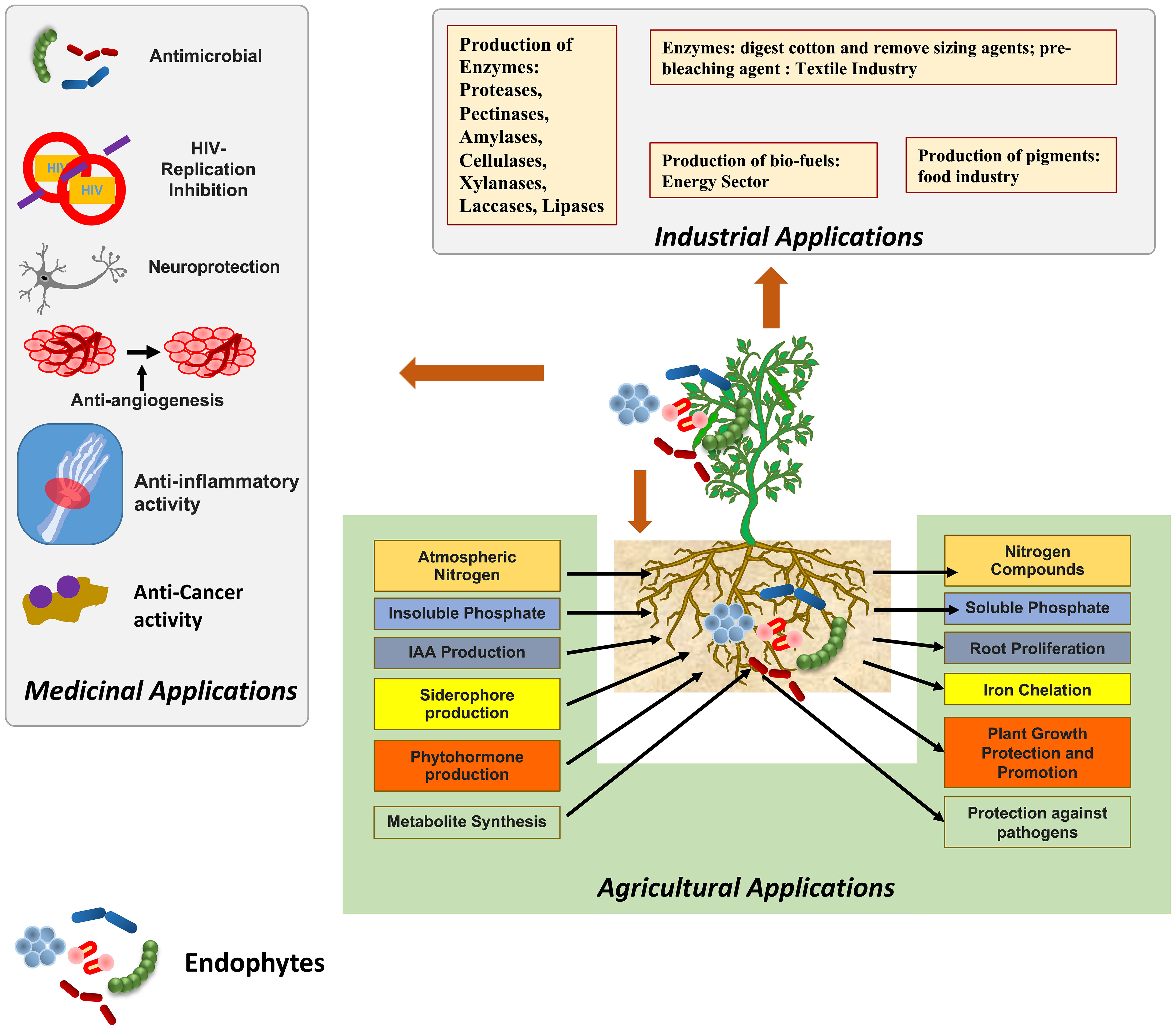
According to the report published by the IPCC (2018), the probability of limiting the effects of global warming to 1.5°C is majorly determined by the cumulative emission of carbon dioxide (CO2) and future non-CO2 radiative forcing. The devastating impact of climate change can be observed in sustainable agriculture systems and overall agriculture productivity (Verma et al., 2022). Agricultural activities in the 21st century are largely depending on the extensive use of fertilizers, pesticides, fungicides etc. and also sometimes involve the over-irrigation and use of high-yielding crop varieties (Hegazy et al., 2017). Such practices have a negative impact on the environment and lead to low fertility of the soil by decreasing the symbiotic association of fungal and bacterial communities in the soil. Such practices also lead to groundwater pollution due to the leaching out of nitrogen and phosphorus from the soil into the groundwater (Rafi et al., 2019). In a similar vein, many biotic factors, such as bacteria, fungi, viruses, weeds, insects, and nematodes, are major constraints of stress that tend to increase the reactive oxygen species that affect the physiological and molecular functioning of plants and also lead to the decrease in crop productivity (Chaudhary et al., 2022). In addition, the increased temperatures, atmospheric CO2 levels, and precipitation patterns also affect agricultural production and insect infestations (Skendžić et al., 2021). In order to reduce the negative impact of such practices and to maintain the fertility of the soil, various eco-friendly farming techniques are employed such as the inculcation of microorganisms as fertilizers encompassing nutrient mobilizing capacity (Gamez et al., 2019). The symbiotic relationship between plants and microorganisms exerts various benefits on plants such as an increase in height and weight, the high nutritional value of plants etc. It results in increasing crop yield, nutrient cycling and fertility of the soil (Card et al., 2021). A single plant is colonised by a large number of microbes, these microbes can be termed epiphytes and endophytes. Endophytes, symbiotic bacterial and fungal communities, are present in intercellular and intracellular spaces of plant parts, such as stems, roots, leaves, etc. (Oukala et al., 2021). Endophytes are also accommodated by weeds inflorescences, petioles, buds, and dead and hollow hyaline cells of plants, fruits and seeds (Lonkar and Bodade, 2021). Either the full or some part of the life cycle of endophyte microbes occur inside the host plant, without causing any negative impact on the plant (Joo et al., 2021). Endophytic associations are reported in various types of plants such as soybeans, chickpeas, cowpeas, sunflower, pearl millet, rice, maize, mustard, sugarcane, cotton, tomatoes, etc (Fadiji and Babalola, 2020). Endophytes are classified on the basis of their biological nature, mode of transmission, and diversity into two classes: transient endophytes and true endophytes (Sharma et al., 2021). In addition, on the basis of their association with host plants, endophytes are classified as obligate and facultative endophytes. Endophytes that spread among plants vertically and completely rely on plant metabolism for survival are referred to as “obligate endophytes,” whereas endophytes that enter plants from neighbouring soil or environment and only partially rely on the host plant, completing only some part of the host plant’s lifecycle, are referred as “facultative endophytes” (Khare et al., 2018). The endophytic world has gained popularity among researchers due to its significant contributions as it produces various types of bioactive compounds that play important roles in various industries such as agricultural, pharmaceutical, medical, and biotechnological industries (Figure 1) (Latz et al., 2018; Tiwari et al., 2023).
Endophytes have a significant impact on the health of their hosts, including their ability to absorb nutrients, produce phytohormones, and reduce the damage caused by pathogens through antibiosis, the generation of lytic enzymes, the activation of secondary metabolites, and the activation of hormones (Chaudhary et al., 2022). However, the complete elucidation of total metabolites produced by endophytes, their functions, protein-protein interactions, and the factors which influence the interaction between fungal, and bacterial endophytes with different plants is still in inferencing.
Nowadays, both fungal and bacterial endophytes are important in the industrial, pharmacological, and biotechnological sectors. This is because they produce different types of metabolites that are used as antitumor, antiviral, and antimicrobial agents, plant growth promoters, bio-control agents, stress tolerance of plants, and immunosuppressants. They also produce different types of compounds that make them good antibiotic, anti-diabetic, and antioxidant agents (Gouda et al., 2016). Several researchers have demonstrated the potential of these endophytes in the synthesis of novel nanomaterials and the role of microbial endophytes in agriculture (Yadav et al., 2020), (Dhara et al., 2023).
In the present review, the authors have emphasized the importance of both fungal and bacterial endophytes in industries, pharmaceuticals and biotechnology. This study addressed recent research on endophytes in order to address a gap in the field and give a detailed application of the metabolites or bioactive components that have been isolated from endophytes and their potential application to bio-nanotechnology. In addition, this review has emphasised on the importance of endophytes-mediated synthesis of nanoparticles as well as their applicability in a variety of sectors.
2 Various approaches for screening bioactive compounds in endophytic culture
From the various pieces of literature, it has been proven that there are various approaches for the screening of bioactive compounds from the culture of endophytes like axenic culture, OSMAC approach, and elicitors (Gakuubi et al., 2021). All these three approaches play an important role in the activation of cryptic gene clusters in the endophyte’s genome. In addition to this, there are several instrument-based approaches like solid-phase microextraction-gas chromatography-mass spectroscopy (SPME GCMS), high-performance liquid chromatography high-resolution mass spectroscopy (HPLC-HRMS) and matrix-associated laser desorption ionization-HRMS (MALDI-HRMS). SPGE-GC-MS helps in the screening of volatile compounds especially signalling compounds secreted by the endophytes. HPLC-HRMS helps in the screening for bioactive compounds mainly antimicrobial and anti-cancerous. MALDI-HRMS, the technique could help in the screening of the distribution and release of target compounds in host plants’ apoplast (Mishra et al., 2022). Figure 2 is showing a schematic diagram for various approaches for screening bioactive compounds from endophytic culture.
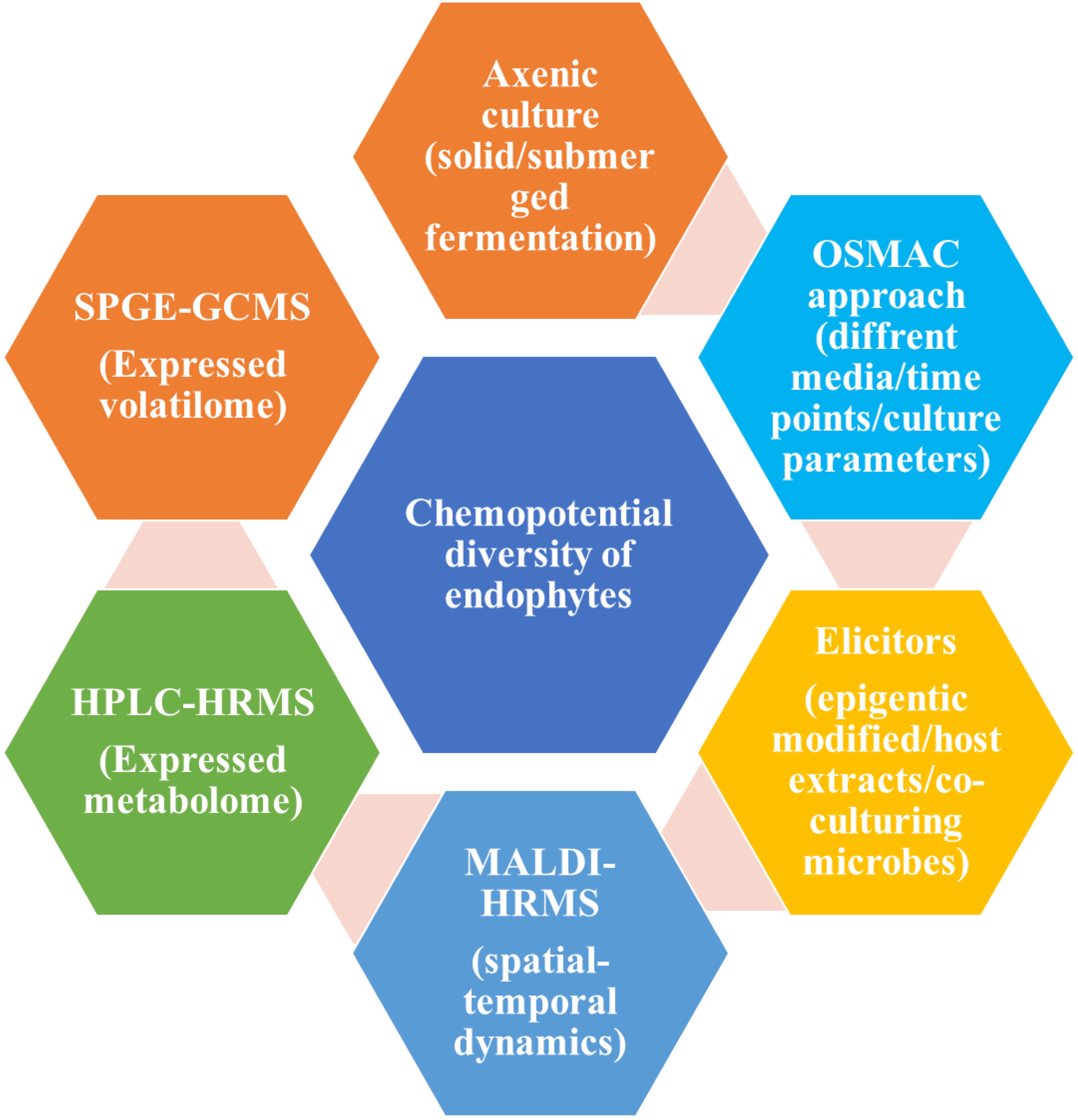
Figure 2 Schematic diagram for screening of various bioactive compounds from endophytic culture adopted from (Mishra et al., 2022).
3 The mechanism employed by endophytes in plant growth promotion and protection
Endophytes protect plants by employing two types of mechanisms: indirect and direct. In direct mechanism, endophytes directly promote plant cell elongation and proliferation by producing phytohormones, indole-3-acetic acid (IAA), siderophores, 1-aminocyclopropane-1-carboxylic acid, phosphate and potassium solubilization antibiosis, and by suppressing the pathogens, etc.
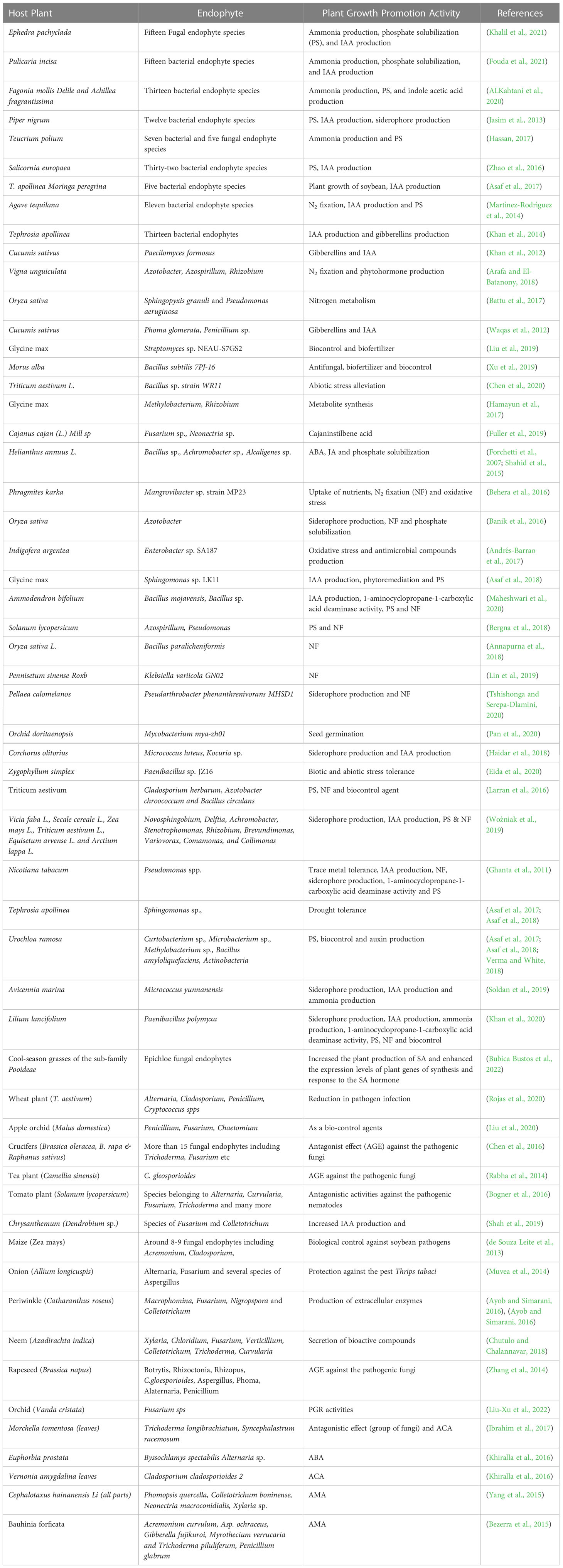
In addition, endophytes promote the capacity to convert atmospheric nitrogen into ammonia, which is required for the synthesis of proteins and nucleic acids and provides tolerance against salt and drought by synthesizing sugar molecules (Muthu Narayanan et al., 2022). Several studies have highlighted the role of endophytic fungi in mitigating biotic and abiotic stresses, making them an essential component of climate-smart and sustainable agriculture (Verma et al., 2022), (Tyagi et al., 2022). The endophytes are known to produce various types of siderophores such as carboxylate, catecholate, phenolates, and hydroxamates. These siderophores perform a variety of functions, including the biocontrol of phytopathogens by limiting the pathogens’ ability to absorb iron, the reduction of heavy metal toxicity, and the induction of induced systemic resistance (ISR) (Chaudhary et al., 2022), (Gómez-Godínez et al., 2023). Several microbial species such as Rhodococcus spp, Bacillus spp, Enterobacter spp, Methylobacterium spp, Pseudomonas fluorescens, Pseudomonas putida, Pantoea ananatis and Pantoea agglomerans, etc. are positively shown to produce siderophores (Karuppiah et al., 2022; Singh et al., 2022). Endophytes emit different organic acids such as malic, gluconic, and citric acids that convert insoluble soil phosphate (apatite, fluorapatite and hydroxyapatite) into soluble orthophosphates by chelating cations attached to the phosphate (Fadiji and Babalola, 2020), (Yadav et al., 2018). Various bacterial and fungal endophytes have been reported to date for phosphate solubilisation activity, phosphate solubilisation, phytohormone production and nitrogen fixation activity (Supplementary Table 1). Endophytic associative nitrogen-fixing microbes are superior to rhizosphere microorganisms in terms of their ability to enable plant life to flourish in nitrogen-deficient soil and to support the overall health and growth of plants (Afzal et al., 2019). Curvularia geniculata, a dark septate root endophytic fungus isolated from the roots of Parthenium hysterophorus, is known to stimulate the growth of plants by solubilising phosphorus (P) and producing phytohormones (Mehta et al., 2019). Colonisation by the endophytic fungus Serendipita indicia enhances nutrient uptake and helps maintain ionic homeostasis by limiting the passage of sodium (Na+) and potassium (K+) ions in plants and enhancing gene transcription, both of which play important roles in Na+ and K+ homeostasis (Tyagi et al., 2022). Endophytes like Colletotrichum, Pseudomonas, Bacillus. Herbaspirillum, Alcaligenes, Streptomyces, Piriformospora indica, Sebacina vermifera, and Penicillium have gained particular interest amongst others because of the propensity to produce phytohormones such as auxins, gibberellins (GA), cytokinins and ethylene that favour improved plant development under harsh conditions (Burragoni and Jeon, 2021), (Omomowo et al., 2023). It has been found that the pestalotiopsis microspore produces pestalotin analogue, a metabolite with gibberellin activity that promotes faster germination (Li et al., 2018). Likewise, Cladosporium sphaerospermum, an endophyte of Glycine max, is responsible for the production of gibberellic acid, which is known to encourage the growth of rice and soybean plants (Omomowo et al., 2023).
The indirect mechanism adopted by endophytes promotes the plant growth by enhancing the plant defence system using various mechanisms like plant resistance induction, environmental stress tolerance, predation and hyperparasite, stimulation of secondary metabolites in plants etc (Tian et al., 2008). When a plant is under attack from a biotrophic pathogen, signalling molecules like salicylic acid (SA) and associated pathogenesis-related (PR) proteins act to induce “systemic acquired resistance” (SAR), which in turn triggers “local resistance” by producing a hypersensitivity reaction (HR) in the infected and surrounding areas of the plant (Muthu Narayanan et al., 2022). For instance, pre-treatment of Pisum sativum seeds with Pseudomonas fluorescens (OKC) and Trichoderma asperellum (T42) prevents powdery mildew disease by stimulating the defence response by upregulation of phytohormone, SA, and PR-1 protein (Patel and Saraf, 2017).
Induced system resistance (ISR) is the second defence mechanism plants use to fend against infections (Qin et al., 2021). By triggering the release and transport of signalling molecules like JA and associated PR proteins to the affected areas, it helps plants defend themselves against necrotrophic diseases. Neither the pathogenic virus nor its replication is directly hindered by the ISR approach. On the contrary, it reinforces the plants’ inherent physical or chemical defences (Muthu Narayanan et al., 2022). For example, the modulation of signalling pathways by JA and its product JA-isoleucine (JA-Ile) hormone is often achieved by the utilisation of abscisic acid (ABA) or ethylene (necrotrophic pathogens defender) (Muthu Narayanan et al., 2022). ISR is induced by Bacillus subtilis PTA-271 and Pseudomonas fluorescens PTACT2 to prevent canker and grey mould disease caused by Pseudomonas syringae Pst DC3000 and Botrytis cinerea, respectively, in the Arabidopsis plants. Infected plant leaves provide evidence of their antagonistic impact through an increase in JA and ABA (Nguyen and Nguyen, 2020).
In antibiosis, various secondary metabolites such as lipopeptides antibiotics, amino acid-rich peptides (neomycin), and cyclic cationic lipopeptides are produced by different endophytes that serve as biocontrol agents as they exhibit antifungal, antibacterial, and nematocidal activities against phytopathogens (Muthu Narayanan et al., 2022). For instance, to protect the leaves of the W. somnifera plant from infection by A. alternata, endophytic bacteria like B. amyloliquefaciens and P. fluorescens enhance callose deposition in guard cells (Mishra et al., 2018). Epichloe is a monophyletic genus of filamentous fungi that develop everlasting symbioses with cool-season grasses (Card et al., 2021). Epichloe fungal endophytes not only boost plant immunity against chewing insects by creating protective alkaloids, but they also promote plant immunity by increasing endogenous defence responses mediated by the jasmonic acid (JA) pathway (Card et al., 2021). The foliar endophytic fungus Colletotrichum tropicale isolated from T. cocoa protects the cocoa tree from black pod disease caused by Phytophthora spp. by upregulating genes related to cellulose and lignin deposition and host cell wall hardening (Sadoral and Cumagun, 2021). Bacillus atrophaeus and Bacillus mojavensis, isolated from the Glycyrrhiza uralensis (Licorice) plant, have antifungal activity due to the presence of various compounds such as 1,2-bezenedicarboxyl acid, methyl ester, decanodioic acid, and bis (2-ethylhexyl) ester (Mohamad et al., 2018). The antibacterial activity of Aspergillus sp., endophyte, isolated from the Bauhinia guianensis plant has been reported due to the presence of fumigaclavine C and pseurtotin C (Wen et al., 2022). In another study, the protein bacillomycin D was produced by B. amyloliquefaciens, which was shown to have antagonistic action against the fungus F. graminearum (Gu et al., 2017).
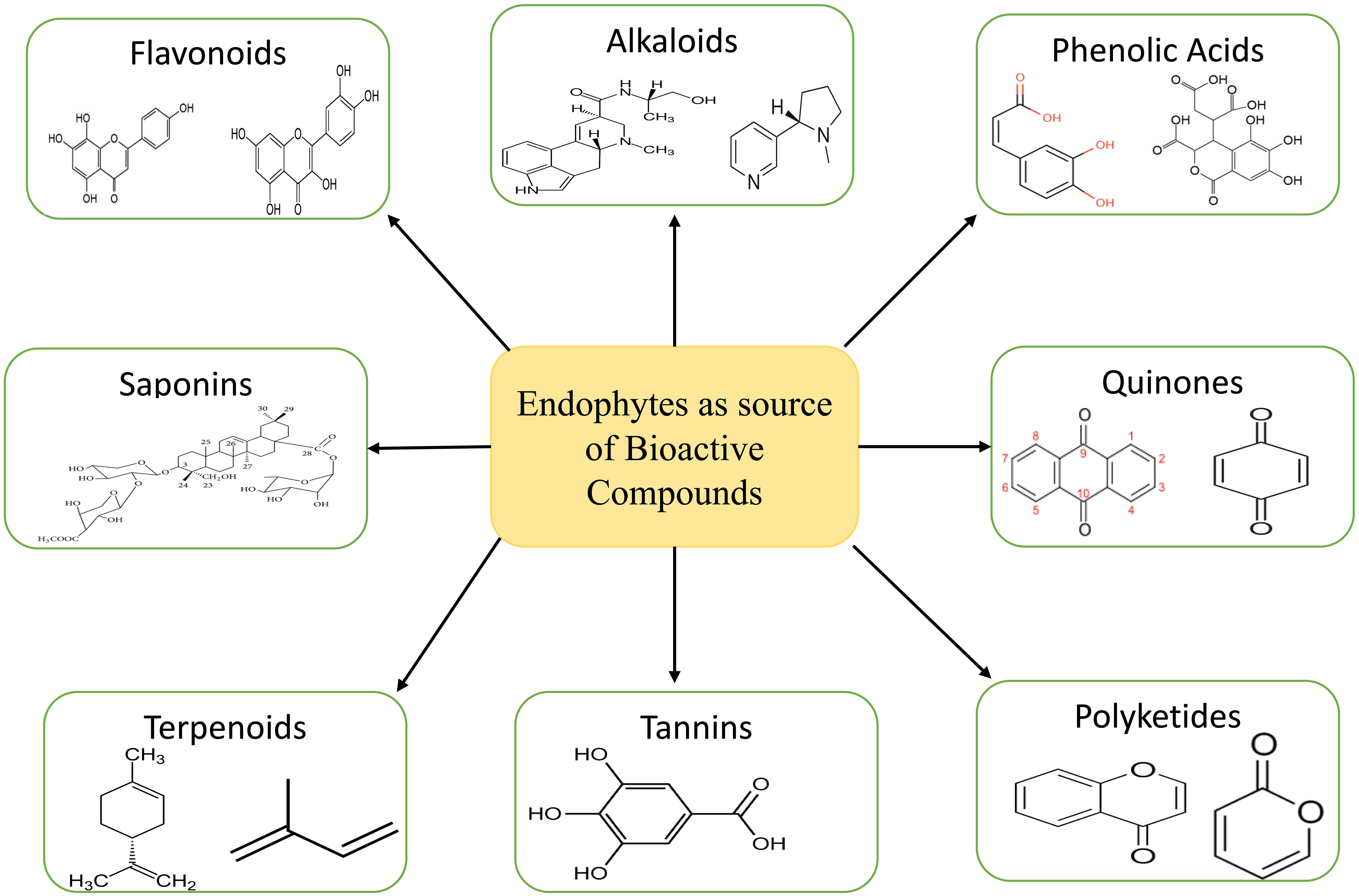
4 Endophytic bioactive components of medicinal significance
The ecosystem is a treasure trove of medicinal plants that contain chemicals that have the potential to serve as a substitute for medications produced synthetically (Michel et al., 2020). The major problem with collecting these compounds from medicinal plants is that there aren’t very many of them, while the demand is high. This leads to overexploitation, which in turn reduces plant population (Palanichamy et al., 2018). In order to fulfil the large-scale production of these compounds, alternative approaches like tissue culture, semi-synthesis, exploitation of endophytes for these compound syntheses, heterologous production, etc. are adopted (Sandargo et al., 2019). Endophytes present in plants are considered a treasure house of various bioactive compounds such as tannins, alkaloids, terpenoids, benzopyranones, quinones, polyketides, chinones, saponins, flavonoids, phenolic acids, steroids, xanthones etc. (Figure 3) (Kumari et al., 2020; Vaid et al., 2020). These bioactive compounds are known to have various uses in the medical sector.
Taxol (Paclitaxel) is a diterpene alkaloid which is produced in Taxus sp. by different endophytes such as- Taxomyces andreanae, Fusarium solani, Metarhizium anisopliae and many others which exhibit anticancer (antitumor) activity (Table 1). Taxol acts as a mitotic inhibitor and causing microtubules to break down at the time of cell division. Whereas other bioactive compounds such as- Brefeldin-A, Phomopsin-A/B/C, Cytosporone-B/C, Terpene, and cryptocandin produced in different plants Quercus variabilis Blume, Excoecaria agallocha L., Allamanda cathartica L. and Tvipterigeum wilfordii Hook. f. by different endophytes such as Cladosporium sp., Phomopsis sp., and Cryptosporiopsis quercina are known to have antimicrobial, antifungal, antibacterial and antimycotic activities respectively. Similarly, Lovastatin is produced in the Solanum xanthocarpum plant by Phomopsis vexans endophyte known to have blood cholesterol-lowering properties (Parthasarathy and Sathiyabama, 2015). Lovastatin inhibits the HMG-CoA reductase enzyme (hydroxyl methylglutaryl coenzyme A reductase), which plays an important role in the regulation of the rate-limiting step of cholesterol biosynthesis as this enzyme catalyses the conversion of HMG-CoA to mevalonate, in a competitive manner (Bhargavi et al., 2018). Ligustrazine which is also known as TMP (Tetra methylpyrazine) can stimulate neuronal differentiation by controlling Topoisomerase IIβ epigenetic activity (Lin et al., 2022). It safeguards against the oxygen-glucose deprivation-induced degeneration of neurons, encourages the migration of brain progenitor/precursor cells and also inhibits H2O2-induced apoptosis in bone marrow-derived mesenchymal stem cells by controlling the PI3K/Akt and ERK1/2 signalling pathways. 3-Nitropropionic acid and tenuazonic acid exhibit strong antitubercular effects on M. tuberculosis H37Ra by disrupting the isocitrate lyase enzyme pathways required for the metabolism and virulence of the pathogen (Adeleke and Babalola, 2021). HupA (huperzine A) acts as a cholinesterase inhibitor (ChEI) which function to decrease the breakdown of acetylcholine and is used in dementia and Alzheimer’s treatment (Dou et al., 2018). Likewise; DPT (Deoxypodophyllotoxin) cyclolignan compound, isolated from various plants accompanied by endophytes (Table 1). The anti-cancer effect of DPT on colorectal cancer cells through induction of apoptosis, by destabilization of microtubules, activation of mitochondrial apoptotic pathway via regulation of B-cell lymphoma 2 (Bcl-2) family proteins, (decreasing Bcl-xL and increasing Bcl-2 associated X (BAX)) and suppression of tumorigenesis have been reported recently (Gamage et al., 2019). In addition, bilobalide, sesquiterpene tri-lactone, obtained from Ginkgo biloba, could be a potential therapeutic agent for brain ischemia and neurodegeneration due to its upregulation of mitochondrial DNA-encoded COX III subunit of cytochrome c oxidase and the ND1 subunit of NADH dehydrogenase genes. Both genes are involved in neuroprotection through the preservation of mitochondrial functions and hindrance in apoptosis. Nowadays the prodrug approach is often used to combat pharmacokinetic, pharmaceutical, and thermodynamic barriers that limit the inculcation of new drugs. The influenza virus is a deadly virus causing severe damage to human beings. Neuraminidase (NA) inhibitor drugs nowadays are used to treat influenza infections. But due to its antigenic drift and antigenic shift, the influenza virus is continuously evolving and may become resistant to previous drugs. Recently, cyclosporine A (CsA) and its analogues have been reported for antiviral activity against influenza A and B strains (Ma et al., 2016). Cyclosporine is a natural product and can be produced by endophytes (Supplementary Table 1). Likewise, Human cytomegalovirus (hCMV) encodes a 256 amino acid serine protease which is responsible for capsid assembly, an essential process for herpes virus production. Cytonic acids A and B, protease inhibitors, obtained from endophytic fungi Cytonaema sp., prevent the development of infectious herpes viruses by blocking the assembly (Tiwari et al., 2023).
5 Endophytes of agricultural significance
Endophytes exhibits numerous plant growth-promoting activities such as phosphate solubilization, siderophore production, IAA production, nitrogen fixation, ammonia production, etc. (Hashim et al., 2020; Zamin et al., 2020) Piriformospora indica, an endophytic basidiomycete fungus that colonises many plant roots, is employed most often to promote the growth of plants (Burragoni and Jeon, 2021). Biopesticides and biofertilizers are becoming formulated with Trichoderma species like T. hamatum, T. harzianum, T. polysporum, and T. virideare because of their ability to colonise root tissues and interact with the host plant via molecular crosstalk, thereby improving nutrient and water uptake, inducing disease resistance, degrading toxic compounds, and ultimately promoting plant growth (Topolovec-Pintarić, 2019). Endophytes defend plants from environmental stresses such as salinity, drought, and others through a variety of mechanisms. One of these mechanisms is the increased production of abscisic acid (ABA), which in turn produces proteins that assist plants in reducing the amount of water lost through transpiration and oxidative stress (Sah et al., 2016). In addition, increased tryptophan production leads to the production of IAA, which is the plant growth hormone auxin and promotes plant growth and rooting (Khan et al., 2019). In a similar fashion, 1-Aminocyclopropane-1-carboxylase (ACC) deaminase hydrolyses the ACC and reduces the production of ethylene, which is responsible for the senescence of the plant, while promoting the production of ammonia and alpha ketobutyrate, which are potential plant growth promoters (Nascimento et al., 2018). Endophytes confer drought tolerance on their hosts by increasing tissue solute accumulation, decreasing water conduction through the leaf, lowering transpiration rates, or thickening the cuticle of the leaf and through osmoregulation for instance, endophytic Neotyphodium spp. improves grass plant drought tolerance (Chhipa and Deshmukh, 2019). Secretion of antioxidant metabolites such as ascorbate and glutathione by endophytes reduces host tissue reactive oxygen species and promotes salt stress tolerance (He et al., 2017). These mechanisms work together to improve plant growth under abiotic and biotic stress conditions by increasing root length and density, increasing nutrient supply to plants, suppressing phytopathogens (Supplementary Table 1), and improving relative water content, osmotic adjustment, and antioxidant property (Khan et al., 2019). Investigators have shown that fungal endophytes have an important role in the host plant, especially in Phyto-stimulation, phytoremediation, phyto-immobilization, phytotransformation and biological control. In addition to this such fungal endophytes produce secondary metabolites which play a role in the reduction of heavy metal toxicity (Radziemska et al., 2021), (Anamika et al., 2018). These boost the plant’s antioxidative mechanism, leading to detoxification and allowing it to grow in polluted soil, thereby increasing the plant’s resistance to heavy metals (Radziemska et al., 2021). Besides this, fungal endophytes have also several beneficial effect on the host plant which is shown below in Figure 4. Hydrophobic, organic molecules with a low molecular weight (300 Da) and a high vapour pressure (0.01 kPa at 20°C) are known as volatile organic compounds (VOCs). Most of these chemicals are derivatives of amino acids, benzenoid compounds, fatty acids, phenylpropanoids, or terpenoids (Kaddes et al., 2019). Endophytic bacteria and fungi produce volatile organic compounds (VOCs) that effectively prevent plant diseases caused by phytopathogens (Kaddes et al., 2019), (Etminani and Harighi, 2018; Etminani et al., 2022).
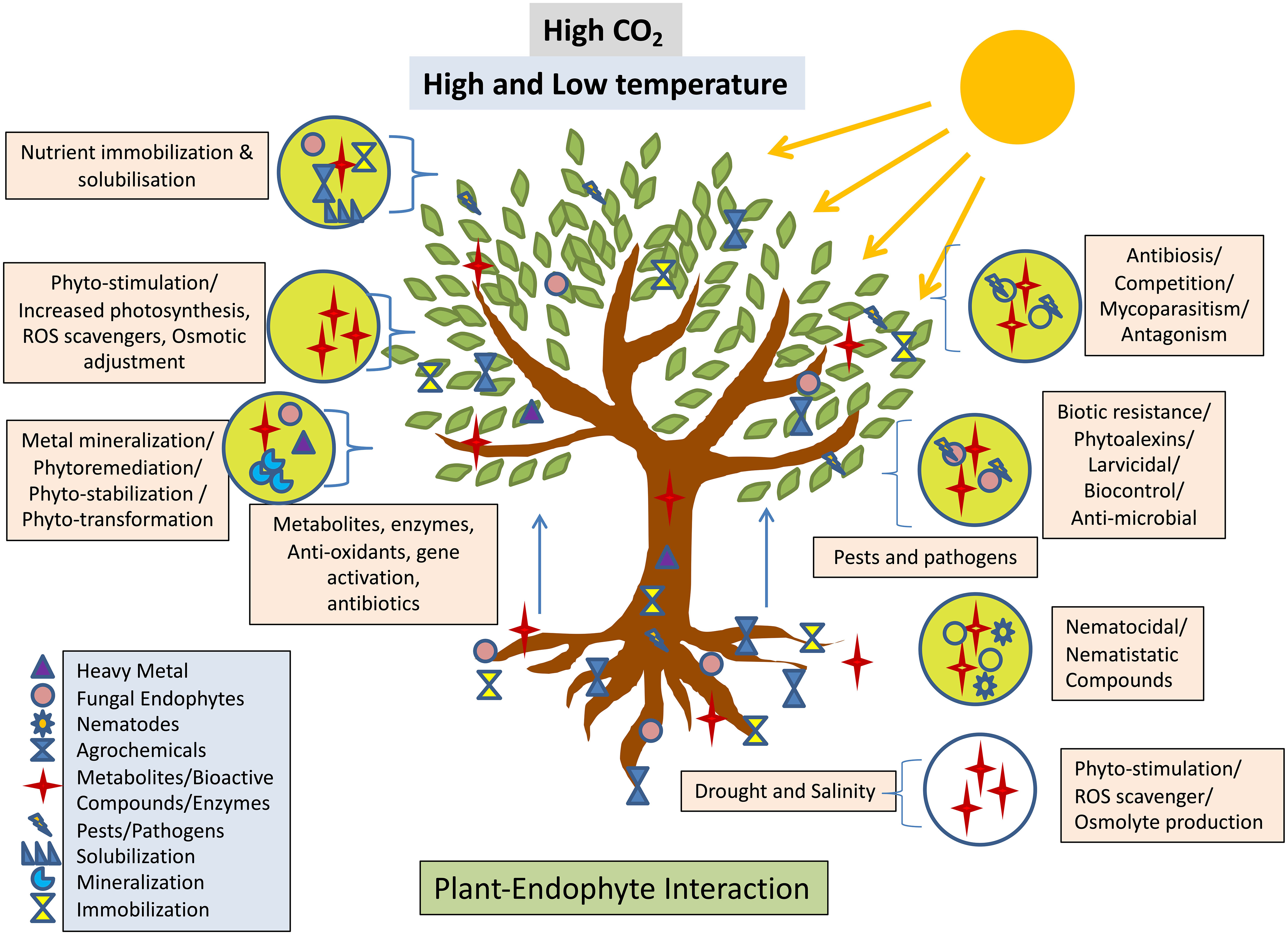
Figure 4 Interaction between fungal endophyte and plant expediates Phyto-stimulation conferring the stress response adopted from (Verma et al., 2022).
The endophytic fungus Trichoderma harzianum, isolated from the tomato plant Solanum lycopersicum, produces the volatile organic compound diterpene. This compound inhibits the growth of the phytopathogen Botrytis cinerea by inducing the expression of tomato defence genes related to salicylic acid (SA) (Faucon et al., 2017). Grapevine endophytic bacteria such as Pantoea sp. Sa14, Pseudomonas sp. Sn48, Pseudomonas sp. Ou22, Pseudomonas sp. Ba35, Serratia sp. Ba10, and Enterobacter sp. Ou80 all produce volatile organic compounds (VOCs) that impede the growth of Agrobacterium tumefaciens in a number of ways, such as inhibiting the chemotaxis, motility, biofilm growth, and root attachment (Etminani and Harighi, 2018; Etminani et al., 2022) Antiherbivore defences in grasses are bolstered by the presence of the endophytic fungus Epichloe, both through alkaloid-dependent and-independent pathways (Bastias et al., 2017). Epichloe endophytes not only defend host plants against herbivores but also from several pathogens. For instance, the presence of an Epichloe endophyte within plants reduced the symptoms of plant diseases caused by the biotrophic fungal infections Blumeria graminis, Claviceps purpurea, Ustilago bullata, and Laetisaria fuciformis (Kou et al., 2021).
6 Endophytes of industrial significance
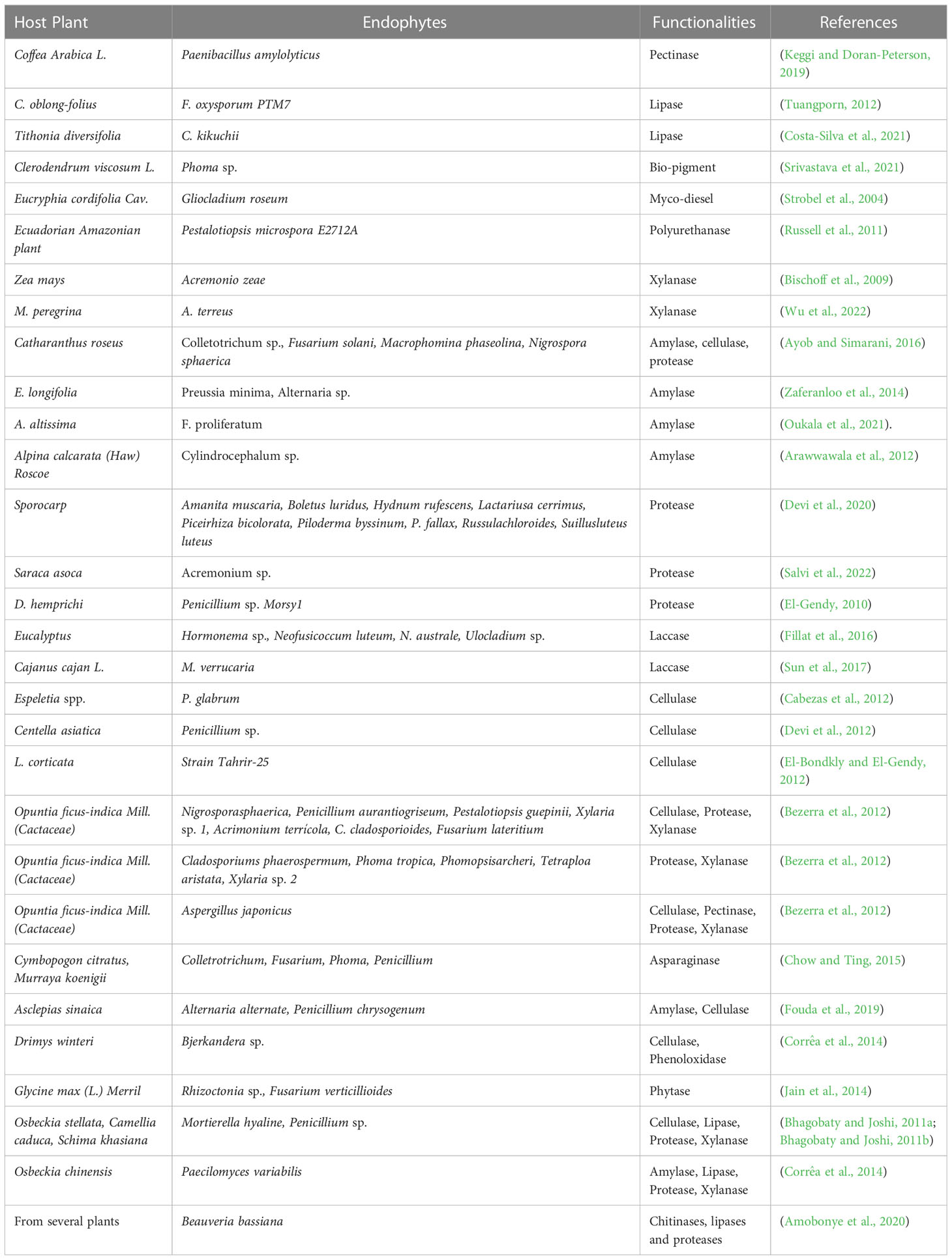
Bacterial and fungal endophytes are the greatest sources of enzyme production which can be used in several industries (Patel et al., 2017), (Hirata et al., 2018). Endophyte species produce several enzymes, including proteases, pectinases, amylases, cellulases, xylanases, laccases, lipases, and others, which are significant in many industrial industries (Table 2) (Zaferanloo et al., 2014). Additionally, some enzymes generated by endophytic species play crucial roles in a wide range of industries, including the production of biofuels in the energy sector, which is used as an alternative source of conventional fuel; the development of pigments for the food industry; the manufacturing of enzymes to degrade polyurethane; and many more (Mengistu, 2020; Singh et al., 2023). Endophytes Phomopsis, Cephalosporium, Microsphaeropsis, and Nigrospora were isolated from plants Taxus chinensis var. mairei Mast, Cupressus torulosa D. Don, Keteleeria davidiana varchienpeii, Sabina chinensis cv. Kaizuca and Keteleeria evelyniana Mast, are known to synthesize enzymes which aid in the extraction of biofuels (Tiwari et al., 2023). In place of toxic chemicals, the textile industry uses a combination of pectinase with amylase, lipase, cellulase, and hemicellulase to digest cotton and remove sizing agents. Pectinase has been investigated extensively in oil extraction from several sources, such as flaxseed, dates, and olives (Haile and Ayele, 2022). Currently, immobilised lipases are used in a wide range of commercial processes, including the manufacture of biosensors, biodiesel and cleansers as well as the organic synthesis of various substances, including cosmetics, meals, medications, fragrances, and tastes (Ismail and Baek, 2020). Xylanase is used as a pre-bleaching agent in the paper and pulp industries. It also has biotechnological applications in the biofuel, food, textile, and feed industries (Singh et al., 2019). Chemical, beverage, textile, food, biofuel, and paper sectors are just a few of the many that rely on the starch-digesting enzyme amylase. It is widely used in the pharmaceutical industry to hydrolyse starch to create various sugars including glucose and maltose, which have a variety of applications. The starch industry uses amylases most frequently to hydrolyze starch during the starch liquefaction process, which turns starch into fructose and glucose syrups (Mehta and Satyanarayana, 2016). Proteases are essential industrial enzymes with numerous uses in chemical and biological reactions. Proteases are also utilised in many other industries, including the production of detergents, the food industry, the tanning of leather, the manufacturing of paper, the recovery of silver from photographic films, the manufacturing of paper, bioremediation procedures and employed therapeutically to cure inflammation and dangerous lesions (Abdel Wahab and Ahmed, 2018; Othman et al., 2018). Cellulases are in great demand across many different industries, including the food and beverage industry, the paper and pulp businesses the manufacturing of textiles, the pharmaceutical field, the cleaning products industry, and the biofuels sector (Raghav et al., 2022). Cellulases are crucial in the selective processing of lignocellulosic biological materials (Payne et al., 2015; Jayasekara and Ratnayake, 2019). Lipopeptides are an important class of secondary metabolites produced by bacterial endophytes and consist of cyclic or linear peptides that are connected to lipophilic molecules. Antibiotic efficacy against numerous diseases places these lipopeptides among the most potent available compounds (Narayanan and Glick, 2022), (al Ayed et al., 2022). Endophytes producing lipopeptides were reported in the medicinal plant Cordia dichotoma L., which is native to the Jammu region. These endophytes belonged to the genera Acidomonas, Alcaligenes, Bacillus, Pseudomonas, Peaenibacillus, Ralstonia, Streptococcus, Micrococcus, and Staphylococcus. Many of the lipopeptide-producing endophytes demonstrated antibacterial activity against a wide variety of bacteria, including Salmonella typhi, Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis, Staphylococcus aureus, Klebsiella pneumoniae (Sharma and Mallubhotla, 2022). Aspergillus sp. A9, Aspergillus sp. A36, Penicillium sp. P5, and Penicillium sp. P15, an endophytic fungus isolated from M. guianensis, was found to be an excellent producer of hydrolase enzymes. The lipase and protease produced by Penicillium P15 and Penicillium sp. P5 were able to break down the S. aureus biofilm (Matias et al., 2021). Hydrolytic enzymes like peptidase, amylase, xylanase, and carboxylase are produced by endophytes, which lyse the rigid peptidoglycan or murein that protects bacterial cell walls (Muthu Narayanan et al., 2022). Endophytic hydrolytic enzymes have the ability to degrade the chitin cell walls that are present in pathogenic fungi, which in turn protects plants from becoming infected (Loc et al., 2020).
7 Role of endophytes in bio-nanotechnology
Nanotechnology and nanoparticles (NPs) have gained huge attention in the last decade due to their unique and remarkable properties like high surface area to volume ratio and high surface energies (Yadav et al., 2020). Due to these features, NPs are widely used in medicine, research, drug delivery, electronics and environmental clean-up (Modi et al., 2022). When it comes to drug delivery and medicine biocompatible and non-toxic nanomaterials are the first preference. So, biocompatible NPs could be easily synthesized by using fungal and bacterial endophytes. From the various pieces of literature, it has been revealed that numerous investigators have used both prokaryotic and eukaryotic endophytes for the synthesis of both metal NPs and metal oxide NPs (Ahmad and Kalra, 2020; Modi et al., 2022). All these methods mainly involve bottom-up approaches which involve exposure of metallic ions to the desired endophytes under desired conditions. Investigators have proven that the positively charged metal ions come closer to the negatively charged endophytic surfaces by electrostatic attraction. Further, these ions then get transported to the internal structure of the endophytes via ions channels where the metal ions get reduced to their zero-valent atomic states., which further then get aggregated to form NPs (Yadav et al., 2020), (Dhara et al., 2023). Figure 5 is showing basic steps involved in the green synthesis of NPs by using endophytic microorganisms.
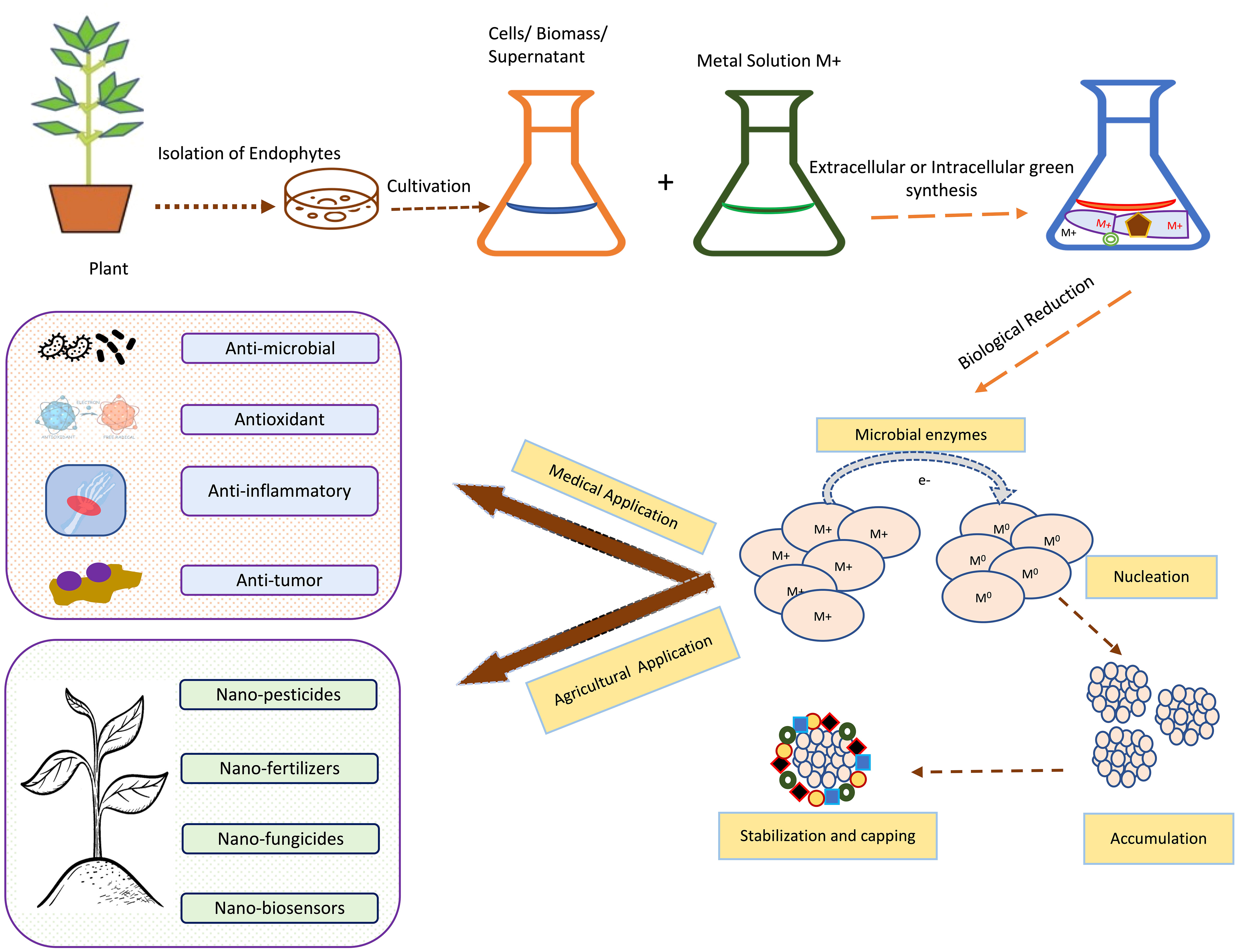
Figure 5 Schematic representation of the general steps for green synthesis of metal-based NPs using endophytic microorganisms isolated from tropical plant adopted from Bogas et al., (Bogas et al., 2022).
Till date investigators have synthesized gold, silver, and copper metal NPs from the endophytes, which have been used in all the domains of science. In addition to this, investigators have reported the synthesis of zinc sulfide, copper oxide, cobalt oxide, nickel oxide, etc from endophytes isolated from terrestrial and marine regions. Fadiji and their colleagues showed the role of various NPs synthesized from the bacterial and fungal endophytes in sustainable agriculture by enhancing plant growth and improving disease resistance (Fadiji et al., 2022). Bogas and their team have shown that these endophytes could act as biofactories for nanoparticles. NPs synthesized from such endophytes have immense potential in healthcare applications (Bogas et al., 2022). In addition, Rathore and his colleagues have placed an emphasis on bacterial endophytes, discussing the recent biomedical scope of these organisms, as well as their synthesis, associated challenges, and importance in bio-nanotechnology (Rathore et al., 2022). Mishra and their groups have also emphasized the green synthesis of NPs by using fungal endophytes which have easy scale-up, downstream processing and eco-friendly nature (Misra et al., 2021). The majority of these NPs synthesized from the endophytes have a role as an antimicrobial agent or as an anti-cancer agent. The antimicrobial activity of endophyte-mediated synthesis NPs is shown in Figure 6 while Figure 7 is showing anticancer activity of the NPs synthesized from endophytes. Table 3 is showing a summarized form of various nanoparticle syntheses from endophytes, along with their applications.
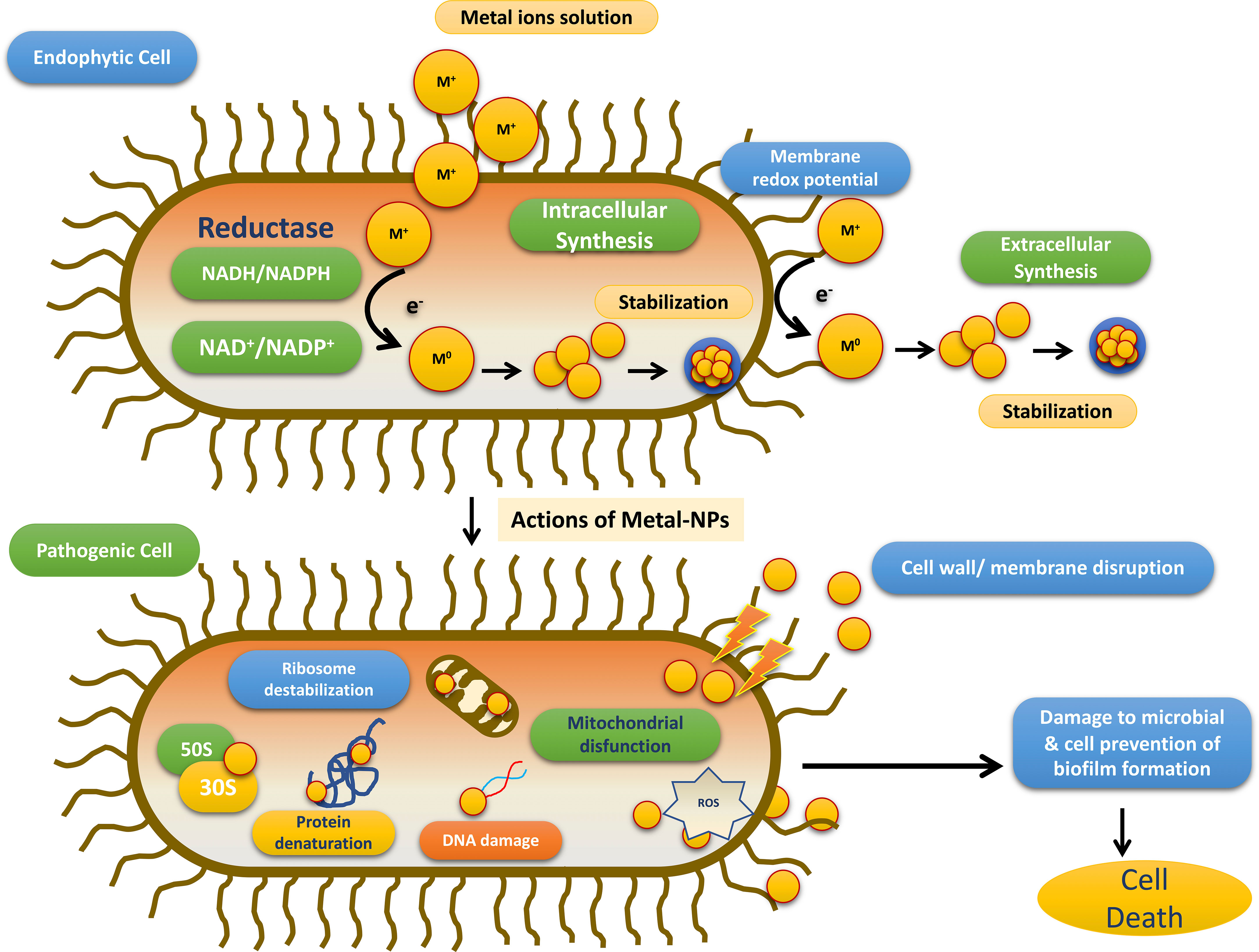
Figure 6 Schematic diagram for the steps involved in the antimicrobial activity of NPs synthesized by endophytes adopted from Bogas et al., (Bogas et al., 2022).
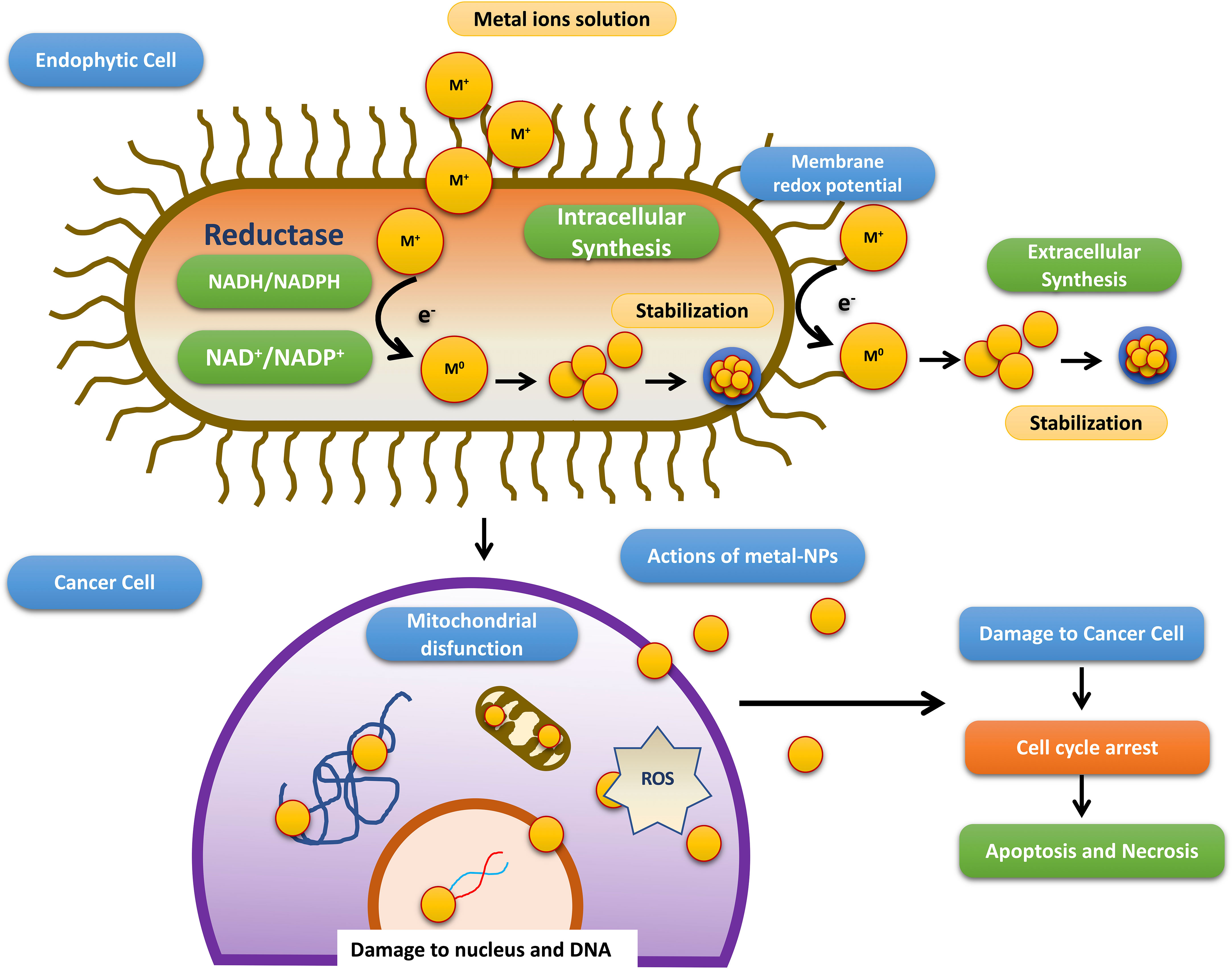
Figure 7 Schematic diagram representing steps involved in the anti-cancer activity of NPs synthesized by endophytes adopted from Bogas et al., (Bogas et al., 2022).
8 Conclusion
Endophytes are bacterial and fungal species beneficial to plants by fulfilling their requirements for growth and protection. Recent applications of endophytes in the agriculture sector not only accelerate plant growth by providing tolerance against various stresses but also reduces the use of numerous agrochemicals like chemical fertilizers and pesticides and this would make agriculture more sustainable and productive. In addition, the exploration of the insecticidal, antimicrobial, and pest-control activities of endophytes will make them good friends of farmers. Endophytes produce several bioactive compounds with huge industrial and medicinal applications as well as can be involved in the bio-transformation of hazardous chemicals like toxins, pollutants and heavy metals. Although the bio-transforming activities of endophytes is still in their infancy. Thus, future efforts should focus on the industrial and medicinal applications of the bio-transforming endophytes and strengthen their eco-friendly and cost-effective approaches in food safety and in the pharma sector. Endophytes exert various therapeutic activities such as anti-cancer, anti-diabetic, anti-inflammatory etc activities by their bioactive compounds. To date, there is no report on commercially available antibiotics derived from endophytes. Intensive research is required which emphasizes the development of new drugs or antibiotics from endophytes and their mechanism of action. The use of endophytic microbes is a relatively new area of study for the environmentally friendly synthesis of nanoparticles, especially when compared to saprophytic microorganisms. Endophyte-derived NPs have potential applications in medicine, including the elimination of multidrug-resistant bacteria, the transport of genetic elements in genetic engineering, and the detection of disease. At the interface of biology and nanotechnology, this area of study has the potential to usher in a plethora of novel nanomaterials. Incorporating metagenomics, metabolomics, and metabolic profiling approaches for elucidating the biosynthetic pathways adopted by endophytes and plants, as well as creating protein-protein interaction maps, and exploring endophytic nanoparticles, will greatly illuminate future applications of endophytes in agriculture, environment, medicine, and industry.
Author contributions
NC, ND, AG, RV and B-H J contributed in conceptualisation, supervision, review editing and writing, and VY, MC, UB, RG and RC reviewed the manuscript, involved in formal analysis, validation and review editing. MA, LE, WA and NC prepared the final draft. MA, LE, WA, RV and B-HJ contributed in visualisation, supervision, review editing and funding acquisition and RV, RG, B-HJ finalized and submitted the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University (KKU) for funding this research through the Research Group Program Under the Grant Number: (R.G.P.2/513/44). This work was supported by the Mid-Career Researcher Program (grant no. 2020R1A2C3004237) through the National Research Foundation of the Republic of Korea. The authors are also thankful to the Department of Biosciences; Mody University of Science and Technology, Lakshmangarh Sikar, Rajasthan (SM/2020-21/008 and SM/2022-23/008) for providing financial support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2023.1193573/full#supplementary-material
Abbreviations
JA, Jasmonic Acid; OSMAC, approach; SPME GCMS, solid-phase microextraction-gas chromatography-mass spectroscopy; HPLCHRMS, highperformance liquid chromatography high-resolution mass spectroscopy; MALDIHRMS, matrix-associated laser desorption ionization-HRMS; IAA, indole-3-acetic acid; SAR, systemic acquired resistance; SA, salicylic acid; PR, Pathogenesis Related; HR, hypersensitivity reaction; ISR, induced system resistance; HMG-CoA reductase enzyme, hydroxyl methylglutaryl coenzyme A reductase; TMP, Tetramethylpyrazine; HupA, huperzine A; ChEI, cholinesterase inhibitor; TMP, Tetramethylpyrazine; DPT, Deoxypodophyllotoxin; ABA, Abscisic acid; ACC, 1-aminocyclopropane-1-carboxylic acid; VOCs, volatile organic compounds; PS, phosphate solubilization; NF, Nitrogen fixation; CE, Cyclopeptides echinocandins.
References
Abdel Wahab, W. A., Ahmed, S. A. (2018). Response surface methodology for production, characterization and application of solvent, salt and alkali-tolerant alkaline protease from isolated fungal strain aspergillus niger WA 2017. Int. J. Biol. Macromol 115, 447–458. doi: 10.1016/j.ijbiomac.2018.04.041
Adeleke, B. S., Babalola, O. O. (2021). Pharmacological potential of fungal endophytes associated with medicinal plants: a review. J. Fungi 7, 1–16. doi: 10.3390/jof7020147
Afzal, I., Shinwari, Z. K., Sikandar, S., Shahzad, S. (2019). Plant beneficial endophytic bacteria: mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 221, 36–49. doi: 10.1016/j.micres.2019.02.001
Ahmad, W., Kalra, D. (2020). Green synthesis, characterization and anti microbial activities of ZnO nanoparticles using euphorbia hirta leaf extract. J. King Saud Univ Sci. 32, 2358–2364. doi: 10.1016/j.jksus.2020.03.014
al Ayed, K., Ballantine, R. D., Hoekstra, M., Bann, S. J., Wesseling, C. M. J., Bakker, A. T., et al. (2022). Synthetic studies with the brevicidine and laterocidine lipopeptide antibiotics including analogues with enhanced properties and in vivo efficacy. Chem. Sci. 13, 3563–3570. doi: 10.1039/D2SC00143H
ALKahtani, M. D. F., Fouda, A., Attia, K. A., Al-Otaibi, F., Eid, A. M., El-Din Ewais, E., et al. (2020). Isolation and characterization of plant growth promoting endophytic bacteria from desert plants and their application as bioinoculants for sustainable agriculture. Agronomy 10. doi: 10.3390/agronomy10091325
Amobonye, A., Bhagwat, P., Pandey, A., Singh, S., Pillai, S. (2020). Biotechnological potential of beauveria bassiana as a source of novel biocatalysts and metabolites. Crit. Rev. Biotechnol. 40, 1019–1034. doi: 10.1080/07388551.2020.1805403
Andrés-Barrao, C., Lafi, F. F., Alam, I., de Zélicourt, A., Eida, A. A., Bokhari, A., et al. (2017). Complete genome sequence analysis of enterobacter sp. SA187, a plant multi-stress tolerance promoting endophytic bacterium. Front. Microbiol. 8. doi: 10.3389/fmicb.2017.02023
Annapurna, K., Govindasamy, V., Sharma, M., Ghosh, A., Chikara, S. K. (2018). Whole genome shotgun sequence of bacillus paralicheniformis strain KMS 80, a rhizobacterial endophyte isolated from rice (Oryza sativa l.). 3 Biotech. 8, 223. doi: 10.1007/s13205-018-1242-y
Arafa, M. M., El-Batanony, N. H. (2018). Growth, yield and chemical composition response of some legume crops to inoculation with non-rhizobial endophytic bacteria from melilotus indicus (L.). All. Nodules. 9, 353–358. doi: 10.21608/jpp.2018.35727
Arawwawala, L. D. A. M., Arambewela, L. S. R., Ratnasooriya, W. D. (2012). Alpinia calcarata Roscoe: a potent anti inflammatory agent. J. Ethnopharmacol 139, 889–892. doi: 10.1016/j.jep.2011.12.036
Arya, S., Sonawane, H., Math, S., Tambade, P., Chaskar, M., Shinde, D. (2021). Biogenic titanium nanoparticles (TiO2NPs) from tricoderma citrinoviride extract: synthesis, characterization and antibacterial activity against extremely drug-resistant pseudomonas aeruginosa. Int. Nano Lett. 11, 35–42. doi: 10.1007/s40089-020-00320-y
Asaf, S., Khan, A. L., Khan, M. A., Al-Harrasi, A., Lee, I.-J. (2018). Complete genome sequencing and analysis of endophytic sphingomonas sp. LK11 and its potential in plant growth. 3 Biotech. 8, 389. doi: 10.1007/s13205-018-1403-z
Asaf, S., Khan, M. A., Khan, A. L., Waqas, M., Shahzad, R., Kim, A.-Y., et al. (2017). Bacterial endophytes from arid land plants regulate endogenous hormone content and promote growth in crop plants: an example of sphingomonas sp. and serratia marcescens. J. Plant Interact. 12, 31–38. doi: 10.1080/17429145.2016.1274060
Ayob, F. W., Simarani, K. (2016). Endophytic filamentous fungi from a catharanthus roseus: identification and its hydrolytic enzymes. Saudi Pharm. J. 24, 273–278. doi: 10.1016/j.jsps.2016.04.019
Bagur, H., Medidi, R. S., Somu, P., Choudhury, P. W. J., Karua, C. S., Guttula, P. K., et al. (2022). Endophyte fungal isolate mediated biogenic synthesis and evaluation of biomedical applications of silver nanoparticles. Materials Technol. 37, 167–178. doi: 10.1080/10667857.2020.1819089
Bagur, H., Poojari, C. C., Melappa, G., Rangappa, R., Chandrasekhar, N., Somu, P. (2020). Biogenically synthesized silver nanoparticles using endophyte fungal extract of ocimum tenuiflorum and evaluation of biomedical properties. J. Clust Sci. 31, 1241–1255. doi: 10.1007/s10876-019-01731-4
Balakumaran, M. D., Ramachandran, R., Balashanmugam, P., Mukeshkumar, D. J., Kalaichelvan, P. T. (2016). Mycosynthesis of silver and gold nanoparticles: optimization, characterization and antimicrobial activity against human pathogens. Microbiol. Res. 182, 8–20. doi: 10.1016/j.micres.2015.09.009
Balakumaran, M. D., Ramachandran, R., Kalaichelvan, P. T. (2015). Exploitation of endophytic fungus, guignardia mangiferae for extracellular synthesis of silver nanoparticles and their in vitro biological activities. Microbiol. Res. 178, 9–17. doi: 10.1016/j.micres.2015.05.009
Banik, A., Mukhopadhaya, S. K., Dangar, T. K. (2016). Characterization of N2-fixing plant growth promoting endophytic and epiphytic bacterial community of Indian cultivated and wild rice (Oryza spp.) genotypes. Planta 243, 799–812. doi: 10.1007/s00425-015-2444-8
Bastias, D. A., Martínez-Ghersa, M. A., Ballaré, C. L., Gundel, P. E. (2017). Epichloe fungal endophytes and plant defenses: not just alkaloids. Trends Plant Sci. 22, 939–948. doi: 10.1016/j.tplants.2017.08.005
Battu, L., Reddy, M. M., Goud, B. S., Ulaganathan, K., Kandasamy, U. (2017). Genome inside genome: NGS based identification and assembly of endophytic sphingopyxis granuli and pseudomonas aeruginosa genomes from rice genomic reads. Genomics 109, 141–146. doi: 10.1016/j.ygeno.2017.02.002
Behera, P., Vaishampayan, P., Singh, N. K., Mishra, S. R., Raina, V., Suar, M., et al. (2016). The draft genome sequence of mangrovibacter sp. strain MP23, an endophyte isolated from the roots of phragmites karka. Genom Data 9, 128–129. doi: 10.1016/j.gdata.2016.07.007
Bergna, A., Cernava, T., Rändler, M., Grosch, R., Zachow, C., Berg, G. (2018). Tomato seeds preferably transmit plant beneficial endophytes. Phytobiomes J. 2, 183–193. doi: 10.1094/PBIOMES-06-18-0029-R
Bezerra, J. D. P., Nascimento, C. C. F., Barbosa, R., do, N., da Silva, D. C. V., Svedese, V. M., et al. (2015). Endophytic fungi from medicinal plant bauhinia forficata: diversity and biotechnological potential. Braz. J. Microbiol. 46, 49–57. doi: 10.1590/S1517-838246120130657
Bezerra, J. D. P., Santos, M. G. S., Svedese, V. M., Lima, D. M. M., Fernandes, M. J. S., Paiva, L. M., et al. (2012). Richness of endophytic fungi isolated from opuntia ficus-indica mill. (Cactaceae) and preliminary screening for enzyme production. World J. Microbiol. Biotechnol. 28, 1989–1995. doi: 10.1007/s11274-011-1001-2
Bhagobaty, R. K., Joshi, S. R. (2011a). Fungal endophytes of five medicinal plants prevalent in the traditionally preserved ‘Sacred forests’ of meghalaya, India. For. Sci. Technol. 7, 151–154. doi: 10.1080/21580103.2011.621381
Bhagobaty, R. K., Joshi, S. R. (2011b). Multi-loci molecular characterization of endophytic fungi isolated from five medicinal plants of meghalaya, India. Mycobiology 39, 71–78. doi: 10.4489/MYCO.2011.39.2.071
Bhargavi, S. D., Praveen, V. K., Anil Kumar, M., Savitha, J. (2018). Comparative study on whole genome sequences of aspergillus terreus (Soil fungus) and diaporthe ampelina (Endophytic fungus) with reference to lovastatin production. Curr. Microbiol. 75, 84–91. doi: 10.1007/s00284-017-1353-4
Bischoff, K. M., Wicklow, D. T., Jordan, D. B., de Rezende, S. T., Liu, S., Hughes, S. R., et al. (2009). Extracellular hemicellulolytic enzymes from the maize endophyte acremonium zeae. Curr. Microbiol. 58, 499–503. doi: 10.1007/s00284-008-9353-z
Bogas, A. C., Henrique Rodrigues, S., Gonçalves, M. O., de Assis, M., Longo, E., Paiva De Sousa, C. (2022). Endophytic microorganisms from the tropics as biofactories for the synthesis of metal-based nanoparticles: healthcare applications. Front. Nanotechnology 4. doi: 10.3389/fnano.2022.823236
Bogner, C. W., Kariuki, G. M., Elashry, A., Sichtermann, G., Buch, A.-K., Mishra, B., et al. (2016). Fungal root endophytes of tomato from Kenya and their nematode biocontrol potential. Mycol Prog. 15, 30. doi: 10.1007/s11557-016-1169-9
Bubica Bustos, L. M., Ueno, A. C., Biganzoli, F., Card, S. D., Mace, W. J., Martínez-Ghersa, M. A., et al. (2022). Can aphid herbivory induce intergenerational effects of endophyte-conferred resistance in grasses? J. Chem. Ecol. 48, 867–881. doi: 10.1007/s10886-022-01390-2
Burragoni, S. G., Jeon, J. (2021). Applications of endophytic microbes in agriculture, biotechnology, medicine, and beyond. Microbiol. Res. 245, 126691. doi: 10.1016/j.micres.2020.126691
Cabezas, L., Calderon, C., Medina, L. M., Bahamon, I., Cardenas, M., Bernal, A. J., et al. (2012). Characterization of cellulases of fungal endophytes isolated from espeletia spp. J. Microbiol. 50, 1009–1013. doi: 10.1007/s12275-012-2130-5
Card, S. D., Bastías, D. A., Caradus, J. R. (2021). Antagonism to plant pathogens by epichloë fungal endophytes–a review. Plants 10. doi: 10.3390/plants10101997
Chaudhary, P., Singh, S., Chaudhary, A., Sharma, A., Kumar, G. (2022). Overview of biofertilizers in crop production and stress management for sustainable agriculture. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.930340
Chen, J.-L., Sun, S.-Z., Miao, C.-P., Wu, K., Chen, Y.-W., Xu, L.-H., et al. (2016). Endophytic trichoderma gamsii YIM PH30019: a promising biocontrol agent with hyperosmolar, mycoparasitism, and antagonistic activities of induced volatile organic compounds on root-rot pathogenic fungi of panax notoginseng. J. Ginseng Res. 40, 315–324. doi: 10.1016/j.jgr.2015.09.006
Chen, C., Yue, Z., Chu, C., Ma, K., Li, L., Sun, Z. (2020). Complete genome sequence of bacillus sp. strain WR11, an endophyte isolated from wheat root providing genomic insights into its plant growth-promoting effects. Mol. Plant-Microbe Interactions® 33, 876–879. doi: 10.1094/MPMI-02-20-0030-A
Chhipa, H., Deshmukh, S. K. (2019). “Fungal endophytes: rising tools in sustainable agriculture production,” in Endophytes and secondary metabolites. Ed. Jha, S. (Cham: Springer International Publishing), 631–655. doi: 10.1007/978-3-319-90484-9_26
Chow, Y., Ting, A. S. Y. (2015). Endophytic l-asparaginase-producing fungi from plants associated with anticancer properties. J. Adv. Res. 6, 869–876. doi: 10.1016/j.jare.2014.07.005
Chowdhury, D. R., Chatterjee, S. K., Kanti Roy, S. (2016). Studies on endophytic fungi of calotropis procera (L.) R.Br. with a view to their antimicrobial and antioxidant actvities mediated by extracellular synthesised silver nanoparticles. IOSR J. Pharm. Biol. Sci. 11, 113–121. doi: 10.9790/3008-110502113121
Chutulo, E. C., Chalannavar, R. K. (2018). Endophytic mycoflora and their bioactive compounds from azadirachta indica: a comprehensive review. J. Fungi 4. doi: 10.3390/jof4020042
Corrêa, R. C. G., Rhoden, S. A., Mota, T. R., Azevedo, J. L., Pamphile, J. A., de Souza, C. G. M., et al. (2014). Endophytic fungi: expanding the arsenal of industrial enzyme producers. J. Ind. Microbiol. Biotechnol. 41, 1467–1478. doi: 10.1007/s10295-014-1496-2
Costa-Silva, T. A., Carvalho, A. K. F., Souza, C. R. F., de Castro, H. F., Bachmann, L., Said, S., et al. (2021). Enhancement lipase activity via immobilization onto chitosan beads used as seed particles during fluidized bed drying: application in butyl butyrate production. Appl. Catal A Gen. 622, 118217. doi: 10.1016/j.apcata.2021.118217
de Souza Leite, T., Cnossen-Fassoni, A., Pereira, O. L., Mizubuti, E. S. G., de Araújo, E. F., de Queiroz, M. V. (2013). Novel and highly diverse fungal endophytes in soybean revealed by the consortium of two different techniques. J. Microbiol. 51, 56–69. doi: 10.1007/s12275-013-2356-x
Devi, R., Kaur, T., Guleria, G., Rana, K. L., Kour, D., Yadav, N., et al. (2020). “Chapter 9 - fungal secondary metabolites and their biotechnological applications for human health,” in New and future developments in microbial biotechnology and bioengineering. Eds. Rastegari, A. A., Yadav, A. N., Yadav, N. (Elsevier), 147–161. doi: 10.1016/B978-0-12-820528-0.00010-7
Devi, N. N., Prabakaran, J. J., Wahab, F. (2012). Phytochemical analysis and enzyme analysis of endophytic fungi from centella asiatica. Asian Pac J. Trop. BioMed. 2, S1280–S1284. doi: 10.1016/S2221-1691(12)60400-6
Dhara, M., Mahakud, D., Naik, U. C. (2023). “17 - molecular mechanism for production of nanoparticles by endophytes,” in Endophytic association: what, why and how. Eds. Shah, M., Deka, D. (Academic Press), 353–367. doi: 10.1016/B978-0-323-91245-7.00018-3
Dong, Z.-Y., Narsing Rao, M. P., Xiao, M., Wang, H.-F., Hozzein, W. N., Chen, W., et al. (2017). Antibacterial activity of silver nanoparticles against staphylococcus warneri synthesized using endophytic bacteria by photo-irradiation. Front. Microbiol. 8. doi: 10.3389/fmicb.2017.01090
Dou, K.-X., Tan, M.-S., Tan, C.-C., Cao, X.-P., Hou, X.-H., Guo, Q.-H., et al. (2018). Comparative safety and effectiveness of cholinesterase inhibitors and memantine for alzheimer’s disease: a network meta-analysis of 41 randomized controlled trials. Alzheimers Res. Ther. 10, 126. doi: 10.1186/s13195-018-0457-9
Eid, A. M., Fouda, A., Niedbała, G., Hassan, S. E. D., Salem, S. S., Abdo, A. M., et al. (2020). Endophytic streptomyces laurentii mediated green synthesis of Ag-NPs with antibacterial and anticancer properties for developing functional textile fabric properties. Antibiotics 9, 1–18. doi: 10.3390/antibiotics9100641
Eida, A. A., Bougouffa, S., Alam, I., Hirt, H., Saad, M. M. (2020). Complete genome sequence of paenibacillus sp. JZ16, a plant growth promoting root endophytic bacterium of the desert halophyte zygophyllum simplex. Curr. Microbiol. 77, 1097–1103. doi: 10.1007/s00284-020-01908-5
El-Bondkly, A. M. A., El-Gendy, M. M. A. (2012). Cellulase production from agricultural residues by recombinant fusant strain of a fungal endophyte of the marine sponge latrunculia corticata for production of ethanol. Antonie Van Leeuwenhoek 101, 331–346. doi: 10.1007/s10482-011-9639-1
El-Gendy, M. M. A. (2010). Keratinase production by endophytic penicillium spp. Morsy1 under solid-state fermentation using rice straw. Appl. Biochem. Biotechnol. 162, 780–794. doi: 10.1007/s12010-009-8802-x
Etminani, F., Harighi, B. (2018). Isolation and identification of endophytic bacteria with plant growth promoting activity and biocontrol potential from wild pistachio trees. Plant Pathol. J. 34, 208–217. doi: 10.5423/PPJ.OA.07.2017.0158
Etminani, F., Harighi, B., Mozafari, A. A. (2022). Effect of volatile compounds produced by endophytic bacteria on virulence traits of grapevine crown gall pathogen, agrobacterium tumefaciens. Sci. Rep. 12. doi: 10.1038/s41598-022-14864-w
Fadiji, A. E., Babalola, O. O. (2020). Elucidating mechanisms of endophytes used in plant protection and other bioactivities with multifunctional prospects. Front. Bioeng Biotechnol. 8. doi: 10.3389/fbioe.2020.00467
Fadiji, A. E., Mortimer, P. E., Xu, J., Ebenso, E. E., Babalola, O. O. (2022). Biosynthesis of nanoparticles using endophytes: a novel approach for enhancing plant growth and sustainable agriculture. Sustainability (Switzerland) 14. doi: 10.3390/su141710839
Faucon, M.-P., Houben, D., Lambers, H. (2017). Plant functional traits: soil and ecosystem services. Trends Plant Sci. 22, 385–394. doi: 10.1016/j.tplants.2017.01.005
Fillat, Ú., Martín-Sampedro, R., Macaya-Sanz, D., Martín, J. A., Ibarra, D., Martínez, M. J., et al. (2016). Screening of eucalyptus wood endophytes for laccase activity. Process Biochem. 51, 589–598. doi: 10.1016/j.procbio.2016.02.006
Forchetti, G., Masciarelli, O., Alemano, S., Alvarez, D., Abdala, G. (2007). Endophytic bacteria in sunflower (Helianthus annuus l.): isolation, characterization, and production of jasmonates and abscisic acid in culture medium. Appl. Microbiol. Biotechnol. 76, 1145–1152. doi: 10.1007/s00253-007-1077-7
Fouda, A., Eid, A. M., Elsaied, A., El-Belely, E. F., Barghoth, M. G., Azab, E., et al. (2021). Plant growth-promoting endophytic bacterial community inhabiting the leaves of pulicaria incisa (LAM.) DC inherent to arid regions. Plants 10, 1–22. doi: 10.3390/plants10010076
Fouda, A., Hassan, S. E. D., Eid, A. M., El-Din Ewais, E. (2019). “The interaction between plants and bacterial endophytes under salinity stress,” in Endophytes and secondary metabolites. Ed. Jha, S. (Cham: Springer International Publishing), 591–607. doi: 10.1007/978-3-319-90484-9_15
Fuller, D. Q., Murphy, C., Kingwell-Banham, E., Castillo, C. C., Naik, S. (2019). Cajanus cajan (L.) millsp. origins and domestication: the south and southeast Asian archaeobotanical evidence. Genet. Resour Crop Evol. 66, 1175–1188. doi: 10.1007/s10722-019-00774-w
Gakuubi, M. M., Munusamy, M., Liang, Z. X. (2021). And ng, s Fungal endophytes: a promising frontier for discovery of novel bioactive compounds. B. J. Fungi 7. doi: 10.3390/jof7100786
Gamage, C. D. B., Park, S. Y., Yang, Y., Zhou, R., Taş, İ., Bae, W. K., et al. (2019). Deoxypodophyllotoxin exerts anti-cancer effects on colorectal cancer cells through induction of apoptosis and suppression of tumorigenesis. Int. J. Mol. Sci. 20. doi: 10.3390/ijms20112612
Gamez, R., Cardinale, M., Montes, M., Ramirez, S., Schnell, S., Rodriguez, F. (2019). Screening, plant growth promotion and root colonization pattern of two rhizobacteria (Pseudomonas fluorescens Ps006 and bacillus amyloliquefaciens Bs006) on banana cv. williams (Musa acuminata colla). Microbiol. Res. 220, 12–20. doi: 10.1016/j.micres.2018.11.006
Ghanta, S., Bhattacharyya, D., Sinha, R., Banerjee, A., Chattopadhyay, S. (2011). Nicotiana tabacum overexpressing γ-ECS exhibits biotic stress tolerance likely through NPR1-dependent salicylic acid-mediated pathway. Planta 233, 895–910. doi: 10.1007/s00425-011-1349-4
Gómez-Godínez, L. J., Aguirre-Noyola, J. L., Martínez-Romero, E., Arteaga-Garibay, R. I., Ireta-Moreno, J., Ruvalcaba-Gómez, J. M. (2023). A look at plant-Growth-Promoting bacteria. Plants 12, 1668. doi: 10.3390/plants12081668
Gouda, S., Das, G., Sen, S. K., Shin, H. S., Patra, J. K. (2016). Endophytes: a treasure house of bioactive compounds of medicinal importance. Front. Microbiol. 7. doi: 10.3389/fmicb.2016.01538
Gu, Q., Yang, Y., Yuan, Q., Shi, G., Wu, L., Lou, Z., et al. (2017). Bacillomycin d produced by bacillus amyloliquefaciens is involved in the antagonistic interaction with the plantpathogenic fungus fusarium graminearum. Appl. Environ. Microbiol. 83. doi: 10.1128/AEM.01075-17
Haidar, B., Ferdous, M., Fatema, B., Ferdous, A. S., Islam, M. R., Khan, H. (2018). Population diversity of bacterial endophytes from jute (Corchorus olitorius) and evaluation of their potential role as bioinoculants. Microbiol. Res. 208, 43–53. doi: 10.1016/j.micres.2018.01.008
Haile, S., Ayele, A. (2022). Pectinase from microorganisms and its industrial applications. Sci. World J. 2022. doi: 10.1155/2022/1881305
Hamayun, M., Hussain, A., Khan, S. A., Kim, H. Y., Khan, A. L., Waqas, M., et al. (2017). Gibberellins producing endophytic fungus porostereum spadiceum AGH786 rescues growth of salt affected soybean. Front. Microbiol. 8. doi: 10.3389/fmicb.2017.00686
Hashim, A. M., Alharbi, B. M., Abdulmajeed, A. M., Elkelish, A., Hassan, H. M., Hozzein, W. N. (2020). Oxidative stress responses of some endemic plants to high altitudes by intensifying antioxidants and secondary metabolites content. Plants 9, 1–23. doi: 10.3390/plants9070869
Hassan, S. E.-D. (2017). Plant growth-promoting activities for bacterial and fungal endophytes isolated from medicinal plant of teucrium polium l. J. Adv. Res. 8, 687–695. doi: 10.1016/j.jare.2017.09.001
Hassan, S. E.-D., Salem, S. S., Fouda, A., Awad, M. A., El-Gamal, M. S., Abdo, A. M. (2018). New approach for antimicrobial activity and bio-control of various pathogens by biosynthesized copper nanoparticles using endophytic actinomycetes. J. Radiat. Res. Appl. Sci. 11, 262–270. doi: 10.1016/j.jrras.2018.05.003
Hatamzadeh, S., Rahnama, K., Nasrollahnejad, S., Fotouhifar, K. B., Hemmati, K., White, J. F., et al. (2020). Isolation and identification of l-asparaginase-producing endophytic fungi from the asteraceae family plant species of Iran. PeerJ 2020. doi: 10.7717/peerj.8309
He, L., He, T., Farrar, S., Ji, L., Liu, T., Ma, X. (2017). Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem. 44, 532–553. doi: 10.1159/000485089
Hegazy, A. K., Emam, M. H., Lovett-Doust, L., Azab, E., El-Khatib, A. A. (2017). Response of duckweed to lead exposure: phytomining, bioindicators and bioremediation. Desalination Water Treat 70, 227–234. doi: 10.5004/dwt.2017.20545
Hemashekhar, B., Chandrappa, C. P., Govindappa, M., Chandrashekar, N. (2019). Endophytic fungus alternaria spp isolated from rauvolfia tetraphylla root arbitrate synthesis of gold nanoparticles and evaluation of their antibacterial, antioxidant and antimitotic activities. Adv. Natural Sciences: Nanoscience Nanotechnology 10, 035010. doi: 10.1088/2043-6254/ab38b0
Hirata, Y., Yamada, C., Ito, Y., Yamamoto, S., Nagase, H., Oh-hashi, K., et al. (2018). Novel oxindole derivatives prevent oxidative stress-induced cell death in mouse hippocampal HT22 cells. Neuropharmacology 135, 242–252. doi: 10.1016/j.neuropharm.2018.03.015
Ibrahim, M., Kaushik, N., Sowemimo, A., Chhipa, H., Koekemoer, T., van de Venter, M., et al. (2017). Antifungal and antiproliferative activities of endophytic fungi isolated from the leaves of markhamia tomentosa. Pharm. Biol. 55, 590–595. doi: 10.1080/13880209.2016.1263671
Ismail, A. R., Baek, K.-H. (2020). Lipase immobilization with support materials, preparation techniques, and applications: present and future aspects. Int. J. Biol. Macromol 163, 1624–1639. doi: 10.1016/j.ijbiomac.2020.09.021
Jain, S., Vaishnav, A., Kasotia, A., Kumari, S., Gaur, R. K., Choudhary, D. K. (2014). Rhizobacterium-mediated growth promotion and expression of stress enzymes in glycine max l. Merrill against fusarium wilt upon challenge inoculation. World J. Microbiol. Biotechnol. 30, 399–406. doi: 10.1007/s11274-013-1455-5
Jasim, B., John Jimtha, C., Jyothis, M., Radhakrishnan, E. K. (2013). Plant growth promoting potential of endophytic bacteria isolated from piper nigrum. Plant Growth Regul. 71, 1–11. doi: 10.1007/s10725-013-9802-y
Jayasekara, S., Ratnayake, R. (2019). “Microbial cellulases: an overview and applications,” in Cellulose. Eds. Pascual, A. R., Martín, M. E. E. (Rijeka: IntechOpen), 5. doi: 10.5772/intechopen.84531
Joo, H.-S., Deyrup, S. T., Shim, S. H. (2021). Endophyte-produced antimicrobials: a review of potential lead compounds with a focus on quorum-sensing disruptors. Phytochem. Rev. 20, 543–568. doi: 10.1007/s11101-020-09711-7
Kaddes, A., Fauconnier, M. L., Sassi, K., Nasraoui, B., Jijakli, M. H. (2019). Endophytic fungal volatile compounds as solution for sustainable agriculture. Molecules 24. doi: 10.3390/molecules24061065
Karuppiah, V., Natarajan, S., Gangatharan, M., Aldayel, M. F., Alsowayeh, N., Thangavel, K. (2022). Development of siderophore-based rhizobacterial consortium for the mitigation of biotic and abiotic environmental stresses in tomatoes: an in vitro and in planta approach. J. Appl. Microbiol. n/a. 133, 3276–3287. doi: 10.1111/jam.15625
Keggi, C., Doran-Peterson, J. (2019). Paenibacillus amylolyticus 27C64 has a diverse set of carbohydrate-active enzymes and complete pectin deconstruction system. J. Ind. Microbiol. Biotechnol. 46, 1–11. doi: 10.1007/s10295-018-2098-1
Khalil, A. M. A., Hassan, S. E. D., Alsharif, S. M., Eid, A. M., Ewais, E. E. D., Azab, E., et al. (2021). Isolation and characterization of fungal endophytes isolated from medicinal plant ephedra pachyclada as plant growth-promoting. Biomolecules 11, 1–18. doi: 10.3390/biom11020140
Khan, M. S., Gao, J., Chen, X., Zhang, M., Yang, F., Du, Y., et al. (2020). Isolation and characterization of plant growth-promoting endophytic bacteria paenibacillus polymyxa SK1 from lilium lancifolium. BioMed. Res. Int. 2020. doi: 10.1155/2020/8650957
Khan, A. L., Hamayun, M., Kang, S.-M., Kim, Y.-H., Jung, H.-Y., Lee, J.-H., et al. (2012). Endophytic fungal association via gibberellins and indole acetic acid can improve plant growth under abiotic stress: an example of paecilomyces formosus LHL10. BMC Microbiol. 12, 3. doi: 10.1186/1471-2180-12-3
Khan, I., Ullah, N., Zha, L., Bai, Y., Khan, A., Zhao, T., et al. (2019). Pathogens alteration of gut microbiota in inflammatory bowel disease (IBD): cause or consequence? IBD Treat Targeting Gut Microbiome. doi: 10.3390/pathogens8030126
Khan, A. L., Waqas, M., Kang, S.-M., Al-Harrasi, A., Hussain, J., Al-Rawahi, A., et al. (2014). Bacterial endophyte sphingomonas sp. LK11 produces gibberellins and IAA and promotes tomato plant growth. J. Microbiol. 52, 689–695. doi: 10.1007/s12275-014-4002-7
Khare, E., Mishra, J., Arora, N. K. (2018). Multifaceted interactions between endophytes and plant: developments and prospects. Front. Microbiol. 9. doi: 10.3389/fmicb.2018.02732
Khiralla, A., Mohamed, I. E., Tzanova, T., Schohn, H., Slezack-Deschaumes, S., Hehn, A., et al. (2016). Endophytic fungi associated with Sudanese medicinal plants show cytotoxic and antibiotic potential. FEMS Microbiol. Lett. 363, fnw089. doi: 10.1093/femsle/fnw089
Kou, M. Z., Bastías, D. A., Christensen, M. J., Zhong, R., Nan, Z. B., Zhang, X. X. (2021). The plant salicylic acid signalling pathway regulates the infection of a biotrophic pathogen in grasses associated with an epichloë endophyte. J. Fungi 7. doi: 10.3390/jof7080633
Kumari, P., Sharma, P., Sharma, S. (2020). Synergism of rhizobium and rhizobacteria on growth, symbiotic parameters, soil quality and grain yield in summer mungbean (Vigna radiata l. wilczek). Int. J. Curr. Microbiol. Appl. Sci. 9, 136–151. doi: 10.20546/ijcmas.2020.903.017
Larran, S., Simón, M. R., Moreno, M., Siurana, M. P. S., Perelló, A. (2016). Endophytes from wheat as biocontrol agents against tan spot disease. Biol. Control 92, 17–23. doi: 10.1016/j.biocontrol.2015.09.002
Latz, M. A. C., Jensen, B., Collinge, D. B., Jørgensen, H. J. L. (2018). Endophytic fungi as biocontrol agents: elucidating mechanisms in disease suppression. Plant Ecol. Divers. 11, 555–567. doi: 10.1080/17550874.2018.1534146
Li, S.-J., Zhang, X., Wang, X.-H., Zhao, C.-Q. (2018). Novel natural compounds from endophytic fungi with anticancer activity. Eur. J. Med. Chem. 156, 316–343. doi: 10.1016/j.ejmech.2018.07.015
Lin, B., Song, Z., Jia, Y., Zhang, Y., Wang, L., Fan, J., et al. (2019). Biological characteristics and genome-wide sequence analysis of endophytic nitrogen-fixing bacteria klebsiella variicola GN02. Biotechnol. Biotechnol. Equip. 33, 108–117. doi: 10.1080/13102818.2018.1555010
Lin, J., Wang, Q., Zhou, S., Xu, S., Yao, K. (2022). Tetramethylpyrazine: a review on its mechanisms and functions. Biomedicine Pharmacotherapy 150, 113005. doi: 10.1016/j.biopha.2022.113005
Liu, J., Ridgway, H. J., Jones, E. E. (2020). Apple endophyte community is shaped by tissue type, cultivar and site and has members with biocontrol potential against neonectria ditissima. J. Appl. Microbiol. 128, 1735–1753. doi: 10.1111/jam.14587
Liu, D., Yan, R., Fu, Y., Wang, X., Zhang, J., Xiang, W. (2019). Antifungal, plant growth-promoting, and genomic properties of an endophytic actinobacterium streptomyces sp. NEAU-S7GS2. Front. Microbiol. 10. doi: 10.3389/fmicb.2019.02077
Liu-Xu, L., Vicedo, B., García-Agustín, P., Llorens, E. (2022). Advances in endophytic fungi research: a data analysis of 25 years of achievements and challenges. J. Plant Interact. 17, 244–266. doi: 10.1080/17429145.2022.2032429
Loc, N. H., Huy, N. D., Quang, H. T., Lan, T. T., Thu Ha, T. T. (2020). Characterisation and antifungal activity of extracellular chitinase from a biocontrol fungus, trichoderma asperellum PQ34. Mycology 11, 38–48. doi: 10.1080/21501203.2019.1703839
Lonkar, K., Bodade, R. (2021). “Potential role of endophytes in weeds and herbicide tolerance in plants,” in Plant growth-promoting microbes for sustainable biotic and abiotic stress management. Eds. Mohamed, H. I., El-Beltagi, H. E.-D. S., Abd-Elsalam, K. A. (Cham: Springer International Publishing), 227–250. doi: 10.1007/978-3-030-66587-6_9
Ma, C., Li, F., Musharrafieh, R. G., Wang, J. (2016). Discovery of cyclosporine a and its analogs as broad-spectrum anti-influenza drugs with a high in vitro genetic barrier of drug resistance. Antiviral Res. 133, 62–72. doi: 10.1016/j.antiviral.2016.07.019
Maheshwari, R., Bhutani, N., Suneja, P. (2020). Isolation and characterization of ACC deaminase producing endophytic bacillus mojavensis PRN2 from pisum sativum. Iran J. Biotechnol. 18, 11–20. doi: 10.30498/IJB.2020.137279.2308
Mani, V. M., Kalaivani, S., Sabarathinam, S., Vasuki, M., Soundari, A. J. P. G., Ayyappa Das, M. P., et al. (2021). Copper oxide nanoparticles synthesized from an endophytic fungus aspergillus terreus: bioactivity and anti-cancer evaluations. Environ. Res. 201, 111502. doi: 10.1016/j.envres.2021.111502
Manjunath, M. H., Joshi, C. G., Danagoudar, A., Poyya, J., Kudva, A. K., BL, D. (2017). Biogenic synthesis of gold nanoparticles by marine endophytic fungus-cladosporium cladosporioides isolated from seaweed and evaluation of their antioxidant and antimicrobial properties. Process Biochem. 63, 137–144. doi: 10.1016/j.procbio.2017.09.008
Martínez-Rodríguez, J. D. C., de la Mora-Amutio, M., Plascencia-Correa, L. A., Audelo-Regalado, E., Guardado, F. R., Hernández-Sánchez, E., et al. (2014) Cultivable endophytic bacteria from leaf bases of agave tequilana and their role as plant growth promoters. Available at: www.sbmicrobiologia.org.br.
Matias, R. R., Sepúlveda, A. M. G., Batista, B. N., de Lucena, J. M. V. M., Albuquerque, P. M. (2021). Degradation of staphylococcus aureus biofilm using hydrolytic enzymes produced by Amazonian endophytic fungi. Appl. Biochem. Biotechnol. 193, 2145–2161. doi: 10.1007/s12010-021-03542-8
Mehta, D., Satyanarayana, T. (2016). Bacterial and archaeal α-amylases: diversity and amelioration of the desirable characteristics for industrial applications. Front. Microbiol. 7. doi: 10.3389/fmicb.2016.01129
Mehta, P., Sharma, R., Putatunda, C., Walia, A., Singh, B. P. (2019). “Endophytic fungi: role in phosphate solubilization,” in Advances in endophytic fungal research: present status and future challenges (Cham: Springer International Publishing), 183–209. doi: 10.1007/978-3-030-03589-1_9
Mengistu, A. A. (2020). Endophytes: colonization, behaviour, and their role in defense mechanism. Int. J. Microbiol. 2020. doi: 10.1155/2020/6927219
Michel, J., Abd Rani, N. Z., Husain, K. (2020). A review on the potential use of medicinal plants from asteraceae and lamiaceae plant family in cardiovascular diseases. Front. Pharmacol. 11. doi: 10.3389/fphar.2020.00852
Mishra, A., Pratap, S. S., Sahil, M., Pratap, S. S., Arpita, B., Nishtha, M., et al. (2018). Endophyte-mediated modulation of defense-related genes and systemic resistance in withania somnifera (L.) dunal under alternaria alternata stress. Appl. Environ. Microbiol. 84, e02845–e02817. doi: 10.1128/AEM.02845-17
Mishra, S., Priyanka Sharma, S. (2022). Metabolomic insights into endophyte-derived bioactive compounds. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.835931
Misra, M., Sachan, A., Sachan, S. G. (2021). “Chapter 15 - role of fungal endophytes in the green synthesis of nanoparticles and the mechanism,” in Fungi bio-prospects in sustainable agriculture, environment and nano-technology. Eds. Sharma, V. K., Shah, M. P., Parmar, S., Kumar, A. (Academic Press), 489–513. doi: 10.1016/B978-0-12-821734-4.00001-0
Modi, S., Inwati, G. K., Gacem, A., Saquib Abullais, S., Prajapati, R., Yadav, V. K., et al. (2022). Nanostructured antibiotics and their emerging medicinal applications: an overview of nanoantibiotics. Antibiotics 11, 708. doi: 10.3390/antibiotics11060708
Mohamad, O. A. A., Li, L., Ma, J. B., Hatab, S., Xu, L., Guo, J. W., et al. (2018). Evaluation of the antimicrobial activity of endophytic bacterial populations from Chinese traditional medicinal plant licorice and characterization of the bioactive secondary metabolites produced by bacillus atrophaeus against verticillium dahliae. Front. Microbiol. 9. doi: 10.3389/fmicb.2018.00924
Mousa, S. A., El-Sayed, E.-S. R., Mohamed, S. S., Abo El-Seoud, M. A., Elmehlawy, A. A., Abdou, D. A. M. (2021). Novel mycosynthesis of Co3O4, CuO, Fe3O4, NiO, and ZnO nanoparticles by the endophytic aspergillus terreus and evaluation of their antioxidant and antimicrobial activities. Appl. Microbiol. Biotechnol. 105, 741–753. doi: 10.1007/s00253-020-11046-4
Munawer, U., Raghavendra, V. B., Ningaraju, S., Krishna, K. L., Ghosh, A. R., Melappa, G., et al. (2020). Biofabrication of gold nanoparticles mediated by the endophytic cladosporium species: photodegradation, in vitro anticancer activity and in vivo antitumor studies. Int. J. Pharm. 588, 119729. doi: 10.1016/j.ijpharm.2020.119729
Muthu Narayanan, M., Ahmad, N., Shivanand, P., Metali, F. (2022). The role of endophytes in combating fungal- and bacterial-induced stress in plants. Molecules 27. doi: 10.3390/molecules27196549
Muvea, A. M., Meyhöfer, R., Subramanian, S., Poehling, H.-M., Ekesi, S., Maniania, N. K. (2014). Colonization of onions by endophytic fungi and their impacts on the biology of thrips tabaci. PloS One 9, e108242-. doi: 10.1371/journal.pone.0108242
Narayanan, Z., Glick, B. R. (2022). Secondary metabolites produced by plant growth-promoting bacterial endophytes. Microorganisms 10. doi: 10.3390/microorganisms10102008
Nascimento, F. X., Rossi, M. J., Glick, B. R. (2018). Ethylene and 1-aminocyclopropane-1-carboxylate (ACC) in plant–bacterial interactions. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.00114
Nguyen, N. T., Nguyen, V. A. (2020). Synthesis, characterization, and photocatalytic activity of ZnO nanomaterials prepared by a green, nonchemical route. J. Nanomater 2020. doi: 10.1155/2020/1768371
Omomowo, I. O., Amao, J. A., Abubakar, A., Ogundola, A. F., Ezediuno, L. O., Bamigboye, C. O. (2023). A review on the trends of endophytic fungi bioactivities. Sci. Afr 20, e01594. doi: 10.1016/j.sciaf.2023.e01594
Othman, A. M., Elsayed, M. A., Elshafei, A. M., Hassan, M. M. (2018). Purification and biochemical characterization of two isolated laccase isoforms from agaricus bisporus CU13 and their potency in dye decolorization. Int. J. Biol. Macromol 113, 1142–1148. doi: 10.1016/j.ijbiomac.2018.03.043
Oukala, N., Pastor, V., Aissat, K. (2021). Bacterial endophytes: the hidden actor in plant immune responses against biotic stress. Plants 10. doi: 10.3390/plants10051012
Palanichamy, P., Krishnamoorthy, G., Kannan, S., Marudhamuthu, M. (2018). Bioactive potential of secondary metabolites derived from medicinal plant endophytes. Egyptian J. Basic Appl. Sci. 5, 303–312. doi: 10.1016/j.ejbas.2018.07.002
Pan, L., Chen, J., Ren, S., Shen, H., Rong, B., Liu, W., et al. (2020). Complete genome sequence of mycobacterium mya-zh01, an endophytic bacterium, promotes plant growth and seed germination isolated from flower stalk of doritaenopsis. Arch. Microbiol. 202, 1965–1976. doi: 10.1007/s00203-020-01924-w
Parthasarathy, R., Sathiyabama, M. (2015). Lovastatin-producing endophytic fungus isolated from a medicinal plant solanum xanthocarpum. Nat. Prod Res. 29, 2282–2286. doi: 10.1080/14786419.2015.1016938
Patel, T., Saraf, M. (2017). Biosynthesis of phytohormones from novel rhizobacterial isolates and their in vitro plant growth-promoting efficacy. J. Plant Interact. 12, 480–487. doi: 10.1080/17429145.2017.1392625
Patel, A. K., Singhania, R. R., Pandey, A. (2017). “Chapter 2 - production, purification, and application of microbial enzymes,” in Biotechnology of microbial enzymes. Ed. Brahmachari, G. (Academic Press), 13–41. doi: 10.1016/B978-0-12-803725-6.00002-9
Payne, C. M., Knott, B. C., Mayes, H. B., Hansson, H., Himmel, M. E., Sandgren, M., et al. (2015). Fungal cellulases. Chem. Rev. 115, 1308–1448. doi: 10.1021/cr500351c
Qin, L., Tian, P., Cui, Q., Hu, S., Jian, W., Xie, C., et al. (2021). Bacillus circulans GN03 alters the microbiota, promotes cotton seedling growth and disease resistance, and increases the expression of phytohormone synthesis and disease resistance-related genes. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.644597
Rabha, A. J., Naglot, A., Sharma, G. D., Gogoi, H. K., Veer, V. (2014). In vitro evaluation of antagonism of endophytic colletotrichum gloeosporioides against potent fungal pathogens of camellia sinensis. Indian J. Microbiol. 54, 302–309. doi: 10.1007/s12088-014-0458-8
Radziemska, M., Gusiatin, Z. M., Cydzik-Kwiatkowska, A., Cerdà, A., Pecina, V., Bęś, A., et al. (2021). Insight into metal immobilization and microbial community structure in soil from a steel disposal dump phytostabilized with composted, pyrolyzed or gasified wastes. Chemosphere 272, 129576. doi: 10.1016/j.chemosphere.2021.129576
Rafi, M. M., Krishnaveni, M. S., Charyulu, P. B. B. N. (2019). “Chapter 17 - phosphate-solubilizing microorganisms and their emerging role in sustainable agriculture,” in Recent developments in applied microbiology and biochemistry. Ed. Buddolla, V. (Academic Press), 223–233. doi: 10.1016/B978-0-12-816328-3.00017-9
Raghav, D., Jyoti, A., Siddiqui, A. J., Saxena, J. (2022). Plant-associated endophytic fungi as potential bio-factories for extracellular enzymes: progress, challenges and strain improvement with precision approaches. J. Appl. Microbiol. 133, 287–310. doi: 10.1111/jam.15574
Ramos, M. M., dos S. Morais, E., da S. Sena, I., Lima, A. L., de Oliveira, F. R., de Freitas, C. M., et al. (2020). Silver nanoparticle from whole cells of the fungi trichoderma spp. isolated from Brazilian Amazon. Biotechnol. Lett. 42, 833–843. doi: 10.1007/s10529-020-02819-y
Rathore, S., Ujjainwal, M., Kaushik, A., Bala, J. (2022). “Bacterial endophytes and bio-nanotechnology,” in Bacterial endophytes for sustainable agriculture and environmental management. Eds. Singh, A. K., Tripathi, V., Shukla, A. K., Kumar, P. (Singapore: Springer Singapore), 201–212. doi: 10.1007/978-981-16-4497-9_10
Rodrigues, A. G., Ping, L. Y., Marcato, P. D., Alves, O. L., Silva, M. C. P., Ruiz, R. C., et al. (2013). Biogenic antimicrobial silver nanoparticles produced by fungi. Appl. Microbiol. Biotechnol. 97, 775–782. doi: 10.1007/s00253-012-4209-7
Rojas, E. C., Jensen, B., Jørgensen, H. J. L., Latz, M. A. C., Esteban, P., Ding, Y., et al. (2020). Selection of fungal endophytes with biocontrol potential against fusarium head blight in wheat. Biol. Control 144, 104222. doi: 10.1016/j.biocontrol.2020.104222
Russell, J. R., Jeffrey, H., Pria, A., Kaury, K., Sandoval., A. G., Dantzler., K. W., et al. (2011). Biodegradation of polyester polyurethane by endophytic fungi. Appl. Environ. Microbiol. 77, 6076–6084. doi: 10.1128/AEM.00521-11
Sadoral, J. P., Cumagun, C. J. R. (2021). Observations on the potential of an endophytic fungus associated with cacao leaves against phytophthora palmivora. Microbiol. Res. (Pavia) 12, 528–538. doi: 10.3390/microbiolres12030037
Sah, S. K., Reddy, K. R., Li, J. (2016). Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.00571
Salvi, S., Varghese, R., Digholkar, G., Deshpande, A., Malvankar, C., Pawar, A., et al. (2022). Saraca asoca: a scoping review on the phytoconstituents, bioactives and their therapeutic effects. German J. Pharm. Biomaterials 1, 03–13. doi: 10.5530/gjpb.2022.3.11
Sandargo, B., Chepkirui, C., Cheng, T., Chaverra-Muñoz, L., Thongbai, B., Stadler, M., et al. (2019). Biological and chemical diversity go hand in hand: basidiomycota as source of new pharmaceuticals and agrochemicals. Biotechnol. Adv. 37, 107344. doi: 10.1016/j.biotechadv.2019.01.011
Shah, S., Shrestha, R., Maharjan, S., Selosse, M. A., Pant, B. (2019). Isolation and characterization of plant growth-promoting endophytic fungi from the roots of dendrobium moniliforme. Plants 8. doi: 10.3390/plants8010005
Shahid, M., Hameed, S., Tariq, M., Zafar, M., Ali, A., Ahmad, N. (2015). Characterization of mineral phosphate-solubilizing bacteria for enhanced sunflower growth and yield-attributing traits. Ann. Microbiol. 65, 1525–1536. doi: 10.1007/s13213-014-0991-z
Sharma, M., Mallubhotla, S. (2022). Diversity, antimicrobial activity, and antibiotic susceptibility pattern of endophytic bacteria sourced from cordia dichotoma l. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.879386
Sharma, H., Rai, A. K., Dahiya, D., Chettri, R., Nigam, P. S. (2021). Exploring endophytes for in vitro synthesis of bioactive compounds similar to metabolites produced in vivo by host plants. AIMS Microbiol. 7, 175–199. doi: 10.3934/MICROBIOL.2021012
Singh, D., Rathod, V., Ninganagouda, S., Hiremath, J., Singh, A. K., Mathew, J. (2014). Optimization and characterization of silver nanoparticle by endophytic fungi Penicillium sp. isolated from Curcuma longa (Turmeric) and application studies against MDR E. coli and S. aureus. Bioinorg Chem. Appl. 2014, 408021. doi: 10.1155/2014/408021
Singh, S., Sidhu, G. K., Kumar, V., Dhanjal, D. S., Datta, S., Singh, J. (2019). “Fungal xylanases: sources, types, and biotechnological applications,” in Recent advancement in white biotechnology through fungi: volume 1: diversity and enzymes perspectives. Eds. Yadav, A. N., Mishra, S., Singh, S., Gupta, A. (Cham: Springer International Publishing), 405–428. doi: 10.1007/978-3-030-10480-1_12
Singh, D., Yadav, V. K., Ali, D., Soni, S., Kumar, G., Dawane, V., et al. (2022). Isolation and characterization of siderophores producing chemolithotrophic bacteria from the coal samples of the aluminum industry. Geomicrobiol J. 40(4), 307–314. doi: 10.1080/01490451.2022.2128114
Singh, A., Yadav, V. K., Chundawat, R. S., Soltane, R., Awwad, N. S., Ibrahium, H. A., et al. (2023). Enhancing plant growth promoting rhizobacterial activities through consortium exposure: a review. Front. Bioeng Biotechnol. 11. doi: 10.3389/fbioe.2023.1099999
Skendžić, S., Zovko, M., Živković, I. P., Lešić, V., Lemić, D. (2021). The impact of climate change on agricultural insect pests. Insects 12. doi: 10.3390/insects12050440
Soldan, R., Mapelli, F., Crotti, E., Schnell, S., Daffonchio, D., Marasco, R., et al. (2019). Bacterial endophytes of mangrove propagules elicit early establishment of the natural host and promote growth of cereal crops under salt stress. Microbiol. Res., 223–225. doi: 10.1016/j.micres.2019.03.008
Srivastava, M., Maurya, P., Jyotshna Shanker, K. (2021). Clerodendrum viscosum: a critical review on phytochemistry, pharmacology, quality assurance, and safety data. Medicinal Chem. Res. 30, 2145–2167. doi: 10.1007/s00044-021-02804-8
Strobel, G., Daisy, B., Castillo, U., Harper, J. (2004). Natural products from endophytic microorganisms. J. Nat. Prod 67, 257–268. doi: 10.1021/np030397v
Sun, J., Guo, N., Niu, L. L., Wang, Q. F., Zang, Y. P., Zu, Y. G., et al. (2017). Production of laccase by a new myrothecium verrucaria MD-R-16 isolated from pigeon pea [Cajanus cajan (L.) millsp.] and its application on dye decolorization. Molecules 22. doi: 10.3390/molecules22040673
Tian, P., Nan, Z., Li, C., Spangenberg, G. (2008). Effect of the endophyte neotyphodium lolii on susceptibility and host physiological response of perennial ryegrass to fungal pathogens. Eur. J. Plant Pathol. 122, 593–602. doi: 10.1007/s10658-008-9329-7
Tiwari, P., Kang, S., Bae, H. (2023). Plant-endophyte associations: rich yet under-explored sources of novel bioactive molecules and applications. Microbiol. Res. 266, 127241. doi: 10.1016/j.micres.2022.127241
Topolovec-Pintarić, S. (2019). “Trichoderm : Invisible Partner for Visible Impact on Agriculture,” in Trichoderma - The Most Widely Used Fungicide (London, UK: IntechOpen). doi: 10.5772/intechopen.83363
Tshishonga, K., Serepa-Dlamini, M. H. (2020). Draft genome sequence of pseudarthrobacter phenanthrenivorans strain MHSD1, a bacterial endophyte isolated from the medicinal plant pellaea calomelanos. Evolutionary Bioinf. 16, 1176934320913257. doi: 10.1177/1176934320913257
Tuangporn, P. (2012). An extracellular lipase from the endophytic fungi fusarium oxysporum isolated from the Thai medicinal plant, croton oblongifolius roxb. Afr J. Microbiol. Res. 6, 2622–2638. doi: 10.5897/ajmr11.965
Tyagi, J., Chaudhary, P., Jyotsana Bhagwati, U., Bhandari, G., Chaudhary, A. (2022). “Chapter 17 impact of endophytic fungi in biotic stress management,” in From chemicals to biologicals. Eds. Soni, R., Suyal, D. C., Goel, R., 447–462. doi: 10.1515/9783110771558-017
Uddandarao, P., Balakrishnan, R. M., Ashok, A., Swarup, S., Sinha, P. (2019). Bioinspired ZnS: gd nanoparticles synthesized from an endophytic fungi aspergillus flavus for fluorescence-based metal detection. Biomimetics 4. doi: 10.3390/biomimetics4010011
Uddandarao, P., Mohan, R. B. (2016). ZnS semiconductor quantum dots production by an endophytic fungus aspergillus flavus. Materials Sci. Engineering: B 207, 26–32. doi: 10.1016/j.mseb.2016.01.013
Vaid, S. K., Srivastava, P. C., Pachauri, S. P., Sharma, A., Rawat, D., Shankhadhar, S. C., et al. (2020). Effective zinc mobilization to rice grains using rhizobacterial consortium. Isr. J. Plant Sci. 67, 145–157. doi: 10.1163/22238980-20201085
Verma, V. C., Kharwar, R. N., Gange, A. C. (2009). Biosynthesis of antimicrobial silver nanoparticles by the endophytic fungus aspergillus clavatus. Nanomedicine 5, 33–40. doi: 10.2217/nnm.09.77
Verma, A., Shameem, N., Jatav, H. S., Sathyanarayana, E., Parray, J. A., Poczai, P., et al. (2022). Fungal endophytes to combat biotic and abiotic stresses for climate-smart and sustainable agriculture. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.953836
Verma, S. K., White, J. F. (2018). Indigenous endophytic seed bacteria promote seedling development and defend against fungal disease in browntop millet (Urochloa ramosa l.). J. Appl. Microbiol. 124, 764–778. doi: 10.1111/jam.13673
Vijayanandan, A. S., Balakrishnan, R. M. (2018). Biosynthesis of cobalt oxide nanoparticles using endophytic fungus aspergillus nidulans. J. Environ. Manage 218, 442–450. doi: 10.1016/j.jenvman.2018.04.032
Waqas, M., Khan, A. L., Kamran, M., Hamayun, M., Kang, S. M., Kim, Y. H., et al. (2012). Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules 17, 10754–10773. doi: 10.3390/molecules170910754
Wen, J., Okyere, S. K., Wang, S., Wang, J., Xie, L., Ran, Y., et al. (2022). Endophytic fungi: an effective alternative source of plant-derived bioactive compounds for pharmacological studies. J. Fungi 8. doi: 10.3390/jof8020205
Woźniak, M., Gałązka, A., Tyśkiewicz, R., Jaroszuk-ściseł, J. (2019). Endophytic bacteria potentially promote plant growth by synthesizing different metabolites and their phenotypic/physiological profiles in the biolog gen iii microplateTM test. Int. J. Mol. Sci. 20. doi: 10.3390/ijms20215283
Wu, Y., Shu, T., Li, P., Wang, Z., Wang, H., Pan, F., et al. (2022). A novel endo-β-1,4-xylanase xyl-1 from aspergillus terreus HG-52 for high-efficiency ramie degumming. J. Natural Fibers 19, 13890–13900. doi: 10.1080/15440478.2022.2111390
Xu, W., Ren, H., Ou, T., Lei, T., Wei, J., Huang, C., et al. (2019). Genomic and functional characterization of the endophytic bacillus subtilis 7PJ-16 strain, a potential biocontrol agent of mulberry fruit sclerotiniose. Microb. Ecol. 77, 651–663. doi: 10.1007/s00248-018-1247-4
Yadav, V. K., Khan, S. H., Malik, P., Thappa, A., Suriyaprabha, R., Ravi, R. K., et al. (2020). “Microbial synthesis of nanoparticles and their applications for wastewater treatment,” in Microbial biotechnology: basic research and applications (Springer), 147–187.
Yadav, A. N., Kumar, V., Dhaliwal, H. S., Prasad, R., Saxena, A. K. (2018). “Chapter 15 - microbiome in crops: diversity, distribution, and potential role in crop improvement,” in Crop improvement through microbial biotechnology. Eds. Prasad, R., Gill, S. S., Tuteja, N. (Elsevier), 305–332. doi: 10.1016/B978-0-444-63987-5.00015-3
Yang, H. R., Hu, X. P., Jiang, C. J., Qi, J., Wu, Y. C., Li, W., et al. (2015). Diversity and antimicrobial activity of endophytic fungi isolated from cephalotaxus hainanensis Li, a well-known medicinal plant in China. Lett. Appl. Microbiol. 61, 484–490. doi: 10.1111/lam.12483
Zaferanloo, B., Bhattacharjee, S., Ghorbani, M. M., Mahon, P. J., Palombo, E. A. (2014). Amylase production by preussia minima, a fungus of endophytic origin: optimization of fermentation conditions and analysis of fungal secretome by LC-MS. BMC Microbiol. 14, 55. doi: 10.1186/1471-2180-14-55
Zamin, M., Fahad, S., Khattak, A. M., Adnan, M., Wahid, F., Raza, A., et al. (2020). Developing the first halophytic turfgrasses for the urban landscape from native Arabian desert grass. Environ. Sci. pollut. Res. 27, 39702–39716. doi: 10.1007/s11356-019-06218-3
Zhang, Q., Zhang, J., Yang, L., Zhang, L., Jiang, D., Chen, W., et al. (2014). Diversity and biocontrol potential of endophytic fungi in brassica napus. Biol. Control 72, 98–108. doi: 10.1016/j.biocontrol.2014.02.018
Zhao, S., Zhou, N., Zhao, Z.-Y., Zhang, K., Wu, G.-H., Tian, C.-Y. (2016). Isolation of endophytic plant growth-promoting bacteria associated with the halophyte salicornia europaea and evaluation of their promoting activity under salt stress. Curr. Microbiol. 73, 574–581. doi: 10.1007/s00284-016-1096-7
Keywords: nano particle, plant growth promotion, larvicidal, bioactive component, endophytes
Citation: Choudhary N, Dhingra N, Gacem A, Yadav VK, Verma RK, Choudhary M, Bhardwaj U, Chundawat RS, Alqahtani MS, Gaur RK, Eltayeb LB, Al Abdulmonem W and Jeon B-H (2023) Towards further understanding the applications of endophytes: enriched source of bioactive compounds and bio factories for nanoparticles. Front. Plant Sci. 14:1193573. doi: 10.3389/fpls.2023.1193573
Received: 25 March 2023; Accepted: 31 May 2023;
Published: 10 July 2023.
Edited by:
Ricardo Aroca, Spanish National Research Council (CSIC), SpainReviewed by:
Parul Chaudhary, Graphic Era Hill University, IndiaShipra Pandey, Indian Institute of Technology Bombay, India
Copyright © 2023 Choudhary, Dhingra, Gacem, Yadav, Verma, Choudhary, Bhardwaj, Chundawat, Alqahtani, Gaur, Eltayeb, Al Abdulmonem and Jeon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rakesh Kumar Verma, cmt3YXQ0QHlhaG9vLmNvbQ==; Byong-Hun Jeon, YmhqZW9uQGhhbnlhbmcuYWMua3I=; Rajarshi Kumar Gaur, Z2F1cnJhamFyc2hpQGhvdG1haWwuY29t
 Nisha Choudhary
Nisha Choudhary Naveen Dhingra
Naveen Dhingra Amel Gacem
Amel Gacem Virendra Kumar Yadav
Virendra Kumar Yadav Rakesh Kumar Verma
Rakesh Kumar Verma Mahima Choudhary1
Mahima Choudhary1 Rajendra Singh Chundawat
Rajendra Singh Chundawat Rajarshi Kumar Gaur
Rajarshi Kumar Gaur Waleed Al Abdulmonem
Waleed Al Abdulmonem Byong-Hun Jeon
Byong-Hun Jeon