- 1ICAR-National Institute of Biotic Stress Management, Raipur, India
- 2ICAR-National Center for Integrated Pest Management, New Delhi, India
- 3ICAR-Indian Agricultural Research Institute, New Delhi, India
- 4ICAR-Natioal Bureau of Agricultural Insects Resources, Bengaluru, India
- 5ICAR-National Rice Research Institute, Cuttack, India
- 6Department of Entomology, DKS, College of Agriculture and Technology, Bhatapara, Chhattisgarh, India
Climate change factors, including elevated carbon dioxide (eCO2), elevated ozone (e03), and the combined effect of elevated temperature and CO2 (eT+eCO2), significantly influence the population dynamics, development, and feeding behavior of the Brown Planthopper (Nilaparvata lugens, BPH) and its impact on rice yield. A two-year field study (2019–2020) showed that BPH populations were highest under eCO2 (61.6 ± 13.5 and 50.6 ± 12.3 N. lugens/hill) and moderate under eT+eCO2 (44.5 ± 9.4 and 47.5 ± 12.1 N. lugens/hill), while e03 drastically reduced populations (17.7 ± 3.1 and 25.1 ± 7.0 N. lugens/hill). Fecundity followed a similar trend, with the highest egg production under eCO2 (219.7 ± 3.3 and 234.3 ± 9.7 eggs/female), moderate under eT+eCO2 (194.2 ± 6.3 and 223.5 ± 9.2 eggs/female), and lowest under e03 (108.4 ± 6.0 and 135.6 ± 3.7 eggs/female). Developmental duration was shortest under eT+eCO2 (14.9 ± 0.3 and 15.9 ± 0.4 days) and longest under e03 (18.2 ± 0.40 and 21.7 ± 0.40 days). Feeding intensity, indicated by honeydew excretion, was highest under eCO2 (124.8 ± 5.3 and 131.3 ± 4.2 mm²), reduced under eT+eCO2 (105.7 ± 4.9 and 107.6 ± 3.4 mm²), and lowest under e03 (44.2 ± 2.5 and 48.9 ± 2.6 mm²). Results indicated that eCO2 promoted overall plant growth, with the highest plant height (65.4 ± 0.8 cm) and reproductive tillers (22.2 ± 0.6). However, under BPH infestation, eCO2 also resulted in the highest yield reduction (15.9%) despite producing the highest grain yield under uninfested conditions (40.1 ± 0.3 g/hill). The eT+eCO2 treatment exhibited moderate reductions in plant height (62.4 ± 0.6 cm) and grain yield (38.1 ± 0.4 g/hill), with a yield loss of 11.5% under infestation. The e03 treatment negatively impacted plant growth, significantly reducing plant height (54.8 ± 1.0 cm), total tillers (17.7 ± 0.9), and grain yield (27.5 ± 0.2 g/hill) in uninfested conditions, with a lower yield reduction (8.72%) under infestation. The findings of this study indicate that pests and host plants benefited under eCO2 and eT+eCO2 conditions; however, increasing BPH populations caused yield losses. Nevertheless, e03 had a detrimental effect on pests as well as host plants. The results pertaining to the collective impact of climate change factors on both the host plant and pests have the potential to contribute to the advancement of insect pest management strategies in response to shifting climates.
1 Introduction
Over half of the world’s population receives nutrition from rice (Oryza sativa L.), making it the most significant staple food in the world (Khush, 2004). In the fiscal year 2023, India is expected to have produced more than 135 million metric tons of rice. This indicated that rice production has been rising steadily since 2017. In India, rice is a staple food that is consumed on a large scale. The area planted to scented rice varieties, particularly Basmati, is increasing year on year due to both domestic and international demand. However, because of a number of biotic and abiotic factors, rice productivity is declining in India (Behura et al., 2011). Despite this, the country’s population boom will cause a continued increase in the demand for grain in the ensuing decades. The brown plant hopper (BPH), Nilaparvata lugens (Stal.) outbreaks have happened throughout rice cultivation history, but with the advent of green revolution, improved rice varieties and input intensive farm practices, the outbreaks became more frequent and intense. N. lugens causes direct damage to the rice plant by sucking the phloem sap, causing them to wilt and completely dry out- a phenomenon known as “hopper burn”, inflicting a yield loss of 70% (Krishnaiah et al., 2008) and transmits viral diseases such as grassy stunt and ragged stunt virus.
The incidence of herbivorous insect pests and crop yield are both affected by major climate change factors, such as increased atmospheric CO2 concentration, rising temperatures, and elevated tropospheric ozone levels (Raderschall et al., 2021; IPCC, 2022). By 2050, atmospheric CO2 levels are anticipated to attain 550 ppm as a result of the increase in anthropogenic greenhouse gas emissions. This acceleration is anticipated to result in a global temperature increase of 1.8 to 4°C by the century’s end (IPCC, 2022). Similarly the concentration of ground level ozone have enhanced significantly since the industrial revolution (Morgan et al., 2006), and have risen from 10 ppb in late 1800s to average levels of 40 ppb currently (Brauer et al., 2016).
The effects of elevated CO2 and temperature on plant physiology and phytochemistry are well-documented (Ainsworth et al., 2007). Elevated CO2 increases leaf mass per area and the C:N ratio by promoting carbohydrate accumulation while diluting nitrogen content (Coley et al., 2002). In contrast, elevated temperatures enhance leaf biomass and nitrogen content (Way and Oren, 2010). In context of insect, elevated CO2 indirectly affects insects by driving herbivores to feed more nutrient-deficient plants to meet nitrogen demands resulting in population expansion (Srinivasa Rao et al., 2009). A similar outcome was observed in the wheat aphid Sitobion avenae, which showed a significant increase under elevated CO2 conditions compared to ambient levels (Chen et al., 2004). Elevated temperatures are likely to directly affect insect development by raising metabolic rates, which shortens development (Bale et al., 2002). Numerous investigations have indicated that the duration of various aphid instars decreased with rising temperatures (Wang and Tsai, 2001). Elevated ozone level affects insect fitness by modifying growth rate, developmental duration, survival, feeding behavior, and oviposition (Ward and Masters, 2007; Brownlie and Johnson, 2009). The impacts may be positive, negative, or neutral (Couture and Lindroth, 2012; Capone et al., 2013). It is typically not directly associated with the nutritional attributes of plants. Elevated CO2, temperature, and ozone levels may have a direct impact on agricultural production as well as an indirect impact due to insect pests (Coakley et al., 1999). Previous research suggests that rice agriculture faces considerable threats from anticipated environmental changes (Long, 2012).
Most research has examined the individual effects of elevated CO2, temperature, and ozone on crop yields and crop growth in controlled environments. However, studies on their interactive effects CO2 & temperature, and ozone remain scarce. In the Indian context, research on climate change’s impact on crop-pest interactions is even more limited. Given this gap, it is crucial to assess how rising CO2, temperature, and ozone influence rice crops and their major sucking pest, the brown planthopper (BPH).
2 Materials and methods
2.1 Collection and maintenance of N. lugens
The BPH population (nymphs and adults) was collected from unsprayed rice fields of ICAR-Indian Agricultural Research Institute (IARI), Research Farm (28°64 N, 77°17 E and 228.61 m). Thereafter, the BPH population was transferred to a glass house at the Division of Entomology, IARI, New Delhi, under controlled conditions of temperature (28 ± 2°C), relative humidity (70 ± 5%), and photoperiod (14L:10D) on a susceptible rice variety, Taichung Native 1, in order to facilitate mass multiplication. To obtain a homogeneous BPH population, ten pairs of adults (males and females) were placed onto uninfested rice pots (40 days old), allowed 24 hours to oviposit, and then employed for further study (Babu et al., 2022).
2.2 Experimental setup
The experiments were carried out at the Free Air Temperature Enrichment (FATE) facility of the Division of Environmental Science, ICAR-IARI, New Delhi, during the rainy season from July to October in 2019 and 2020. Each FATE ring had a circular area of 28 m2 and the elevated CO2 level was maintained only during the day time. CO2 concentration was measured by Infra-Red Gas Analyzer (IRGA, PP system, SBA-5) placed at the center of each ring. The temperature inside the FATE rings was elevated using infra-red heaters. O3 was produced by an ozone generator (Eltech Engineers, Mumbai) by converting atmospheric oxygen into O3 using UV lamps and its concentration inside the rings was monitored by an ozone analyzer (2B Technologies). Four FATE rings were used to conduct the experiments. Ring one had an elevated carbon dioxide (eCO2) condition of 600 ± 25 ppm. Ring two was equipped with elevated temperature (Ambient+3°C) and carbon dioxide (600 ± 25 ppm) (eT+eCO2) conditions. Ring three was equipped with only elevated ozone (65 ± 5 ppb) (e03) conditions. Ring number four was used as a control with ambient temperature, CO2 and O3 conditions (AM). Hereafter, all four exposure conditions will be referred to as eT+eCO2, eCO2, e03, and AM throughout the manuscript. Rice plants (Pusa Basmati 1121) were grown under these conditions and then insects were released to assess the effect of eT+eCO2; eCO2; e03 and AM on N. lugens development and survival along with yield parameters.
2.3 Study of demographic structure of N. lugens
To study the population structure of N. lugens, 25-day-old potted rice plants were transferred to all four FATE rings (eT+eCO2, eCO2, e03, and AM) and enclosed in Mylar cages with two windows for aeration. After ten days of crop exposure to each condition, five pairs of gravid brachypterous females and males were released into 10 replications in each treatment (Pandi et al., 2018a). The number of nymphs, males, and females per hill was recorded weekly in 2019 and 2020 for BPH demographic study.
2.4 Study on biological parameters of N. lugens
In this experiment we studied development duration, adult longevity and fecundity. First instars of N. lugens nymphs were released in each exposure treatment in order to analyze the nymphal period. The nymphal duration for each instar and the total nymphal period were calculated based on the moulting data of N. lugens (Pandi et al., 2018a). Adult lifespan was assessed by transferring newly emerged males and females to each treatment conditions and recording their longevity till their death. To assess fecundity, a freshly emerged pair, consisting of a brachypterous female and a winged male, was introduced into each exposure treatment for mating and egg-laying (Cheng et al., 2001). After seven days, the leaf sheath of rice plant was carefully removed using fine forceps and a scalpel. The exposed inner tissue was examined under a stereomicroscope (40× magnification) to visualize the embedded eggs. The total number of eggs per plant was counted manually using a fine needle to mark the counted eggs (Horgan et al., 2021). Experiment consisted of 10 replications and was carried out throughout two rainy seasons during 2019 and 2020.
2.5 Feeding potential of N. lugens by honeydew test
Feeding potential of N. lugens was assessed by measuring the honeydew excreted by newly emerged females on Whatman No. 1 filter paper treated with a 0.5% bromocresol solution. After oven-drying at 100°C for 5 minutes, the honeydew stains developed a violet or purple color due to their amino acid content. The excreted spots were traced onto tracing paper, and their areas were quantified using millimeter-scale graph paper by counting the enclosed squares (Paguia et al., 1980). Each treatment was replicated ten times across two consecutive seasons to ensure the reliability of the results.
2.6 Plant parameters
To study plant parameters viz., plant height, number of tillers, reproductive tillers, panicle length, grain per panicle, test weight, and yield per hill were recorded for each treatment.
2.7 Statistical analysis
The data of N. lugens population dynamics were normalized using the square root transformation and subjected to two-way analysis of variance (ANOVA). F-tests were used to determine the significance of differences between treatments and weeks tested by F- tests, while least significant differences (LSD) were used to compare treatment means at P=0.05. Data on biological parameters of N. lugens and plant parameters were subjected to analysis of variance (ANOVA), and the significance of differences between the treatments was tested by F-tests, while the treatment means were compared by the least significant differences (LSD) at P=0.05 using the statistical software SAS version 9.2.
3 Results
3.1 Population dynamics of N. lugens in FATE
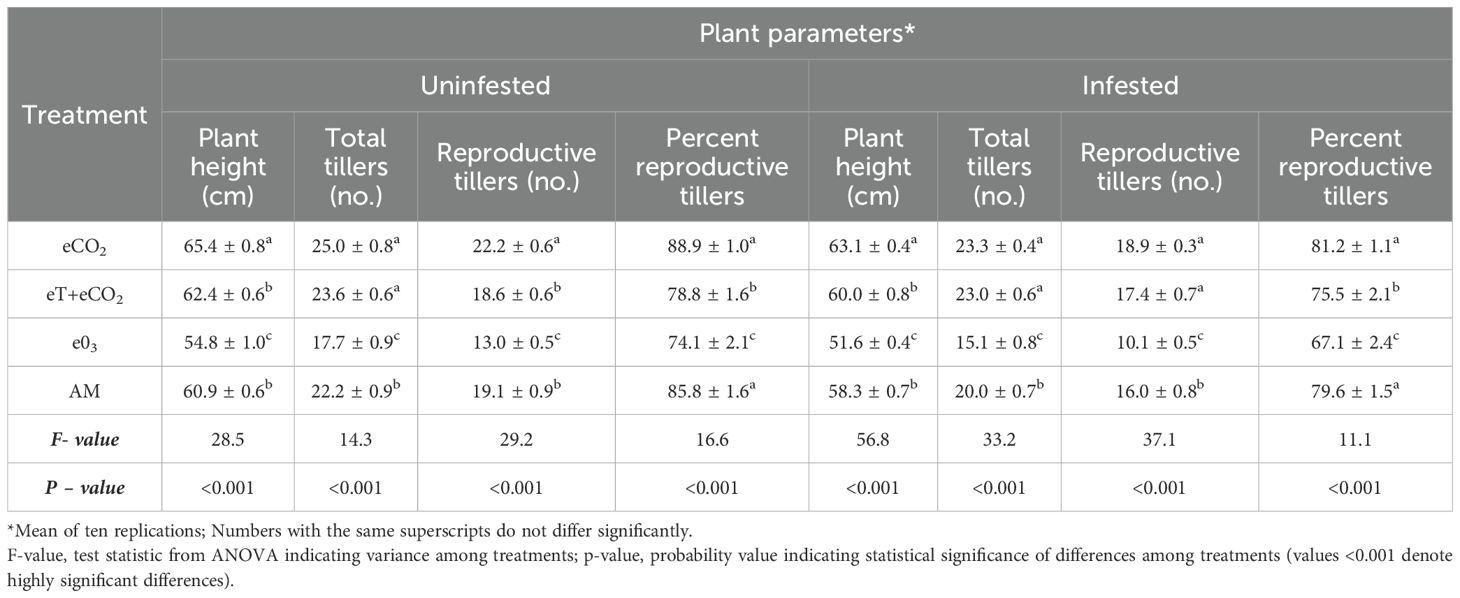
N. lugens populations significantly differed across treatments (F=373.5, P<0.001 for 2019; F=113.19, P<0.001 for 2020), weeks (F=313.6, P<0.001 for 2019; F=450.7, P<0.001 for 2020), and treatment-week interactions (F=14.8, P<0.001 for 2019; F=5.6, P<0.001 for 2020), as shown in Supplementary Tables 1, 5. In both 2019 and 2020, the population of N. lugens was higher in eCO2, followed by eT+eCO2, and lowest in e03 when compared to the ambient population. In 2019, two peaks were recorded in eCO2: first in the 5th weeks after release (WAR) (114.6 ± 6.2 N. lugens/hill) and second in the 8th WAR (100.7 ± 5.6 N. lugens/hill) whereas in 2020, first in the 3rd WAR (72.2 ± 3.4 N. lugens/hill) and second in the 4th WAR (84.3 ± 5.0 N. lugens/hill). In 2019 and 2020, eCO2 had the highest mean population density (61.6 ± 13.5 and 50.6 ± 12.3 N. lugens/hill) compared to the other treatments (Figures 1a, b). Conversely, the e03 had the lowest average population density of N. lugens per hill, with observed values of 17.7 ± 3.1 and 25.1 ± 7.0 for the years 2019 and 2020, respectively. During 2019 and 2020, under eT+eCO2, average population density of N. lugens (44.5 ± 9.4 and 47.5 ± 12.1 N. lugens/hill) found to be significantly higher than ambient control but was significantly lower than eCO2 (Figures 1a, b). Furthermore, there were significant variations in the populations of nymphs and adults (male and female) among the treatments during both seasons (Figures 1a, b; Supplementary Tables 2, 3, 6, 7). In both seasons, N. lugens nymphs and adults are most abundant in the eCO2, followed by the eT+eCO2, AM, and e03 (Figures 1a, b). On the other hand, e03 reduced the population of N. lugens and impeded rice crop development.
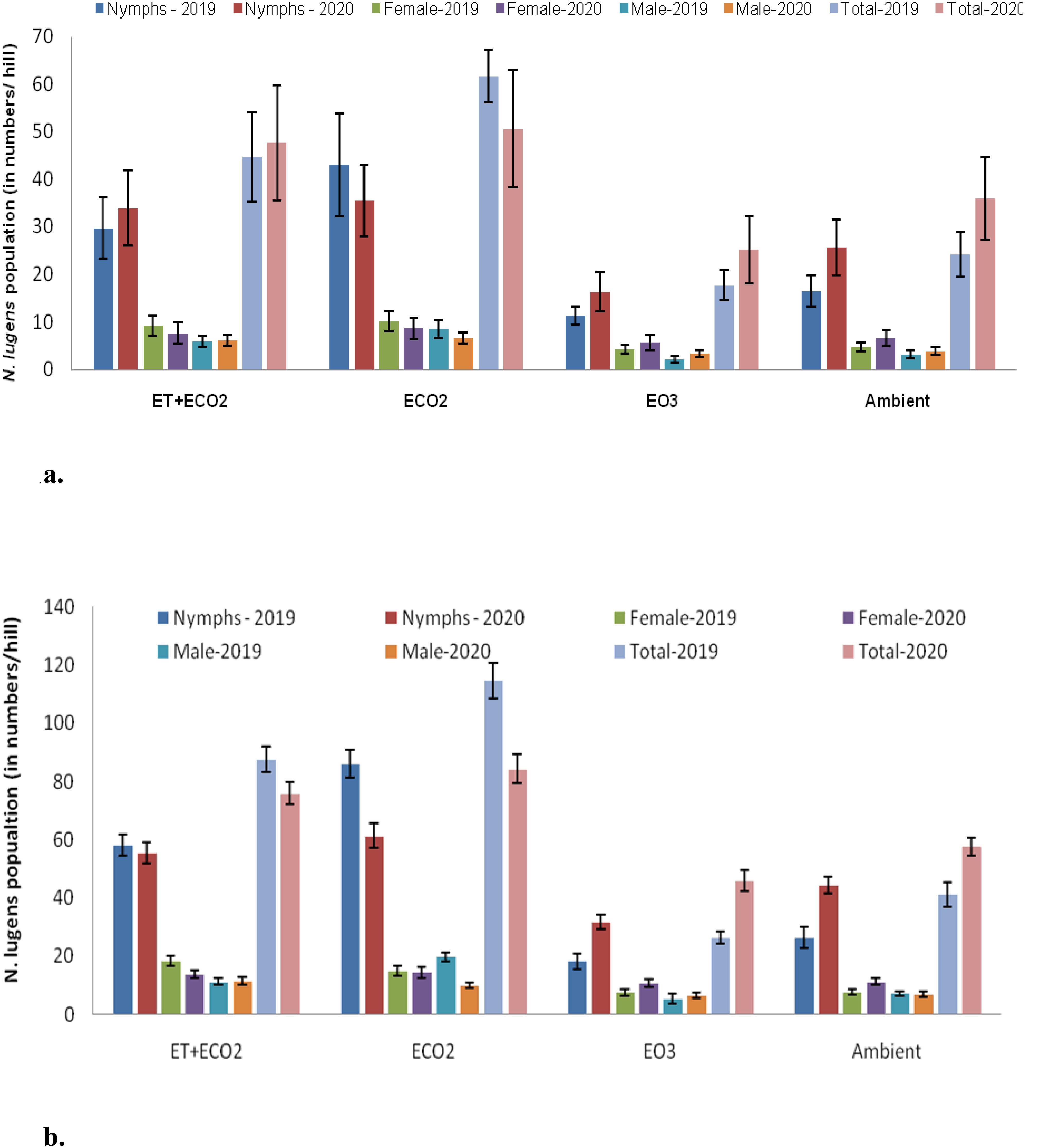
Figure 1. (a) Mean population of different stages of brown planthopper N. lugens recorded during the rainy season of 2019 and 2020. (b) Peak emergence of different stages of brown planthopper N. lugens recorded during the rainy season of 2019 and 2020.
3.2 Biological parameters of N. lugens in FATE
3.2.1 Fecundity and related parameters of N. lugens
The fecundity of N. lugens on rice plants showed significant difference among treatments in both years (F=90.1, P<0.001 and F=42.5, P<0.001). Females deposited the most eggs on rice plants exposed to eCO2 (219.7 ± 3.3 & 234.3 ± 9.7) followed by eT+eCO2 (194.2 ± 6.3 & 223.5 ± 9.2) and AM (128.6 & 163.1) in both years 2019 and 2020 (Table 1). In contrast to other treatments and the control, N. lugens fertility dramatically dropped on e03-treated plants (108.4 ± 6.0 and 135.6 ± 3.7 eggs/female) in both seasons. In 2019 and 2020, rice plants that were exposed under eCO2 exhibited the highest number of eggs per female (16.9 ± 0.2 & 14.5 ± 0.4), while plants that were exposed under e03 exhibited the lowest number of eggs per female (10.4 ± 0.3 & 10.0 ± 0.3). Egg masses under eT+eCO2 (13.5 ± 0.4 &12.6 ± 0.4) were considerably greater than ambient (11.1 ± 0.3 & 11.6), but lower than eCO2 in both the seasons.
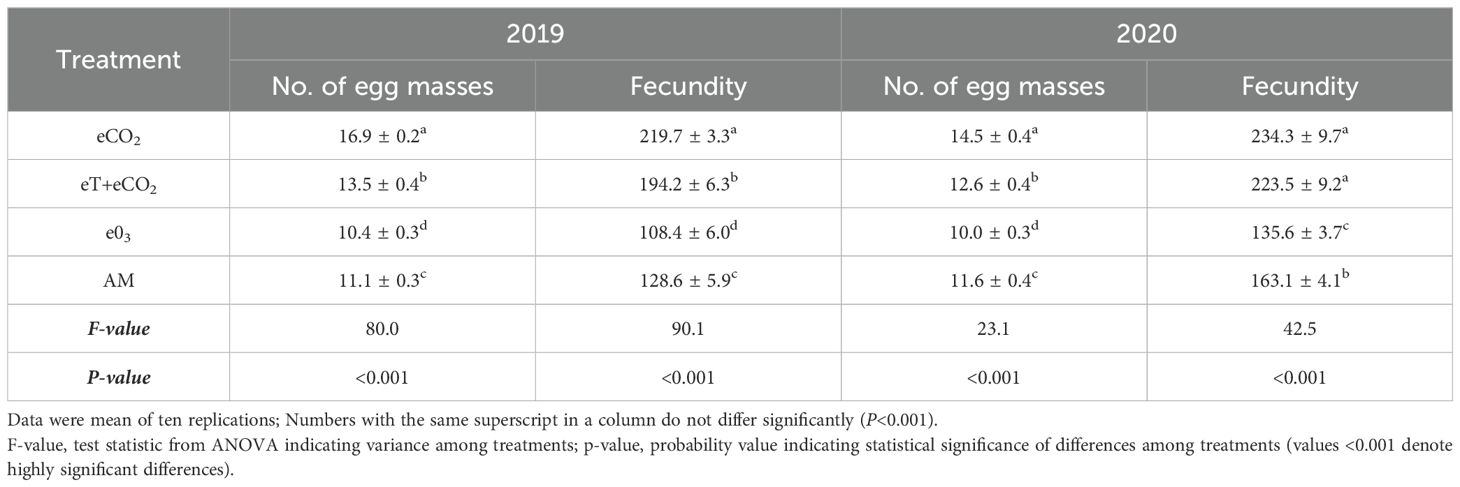
Table 1. The combined effect of elevated temperature+CO2 and ozone on N. lugens fecundity and egg masses during the rainy season of 2019 and 2020.
3.2.2 Developmental parameters of N. lugens
Total nymphal duration, adult longevity and developmental period differed significantly across the treatments and seasons (Tables 2A–C). In both 2019 and 2020, the total nymphal duration was considerably shorter on plants grown under eT+eCO2 (14.9 ± 0.3 & 15.9 ± 0.4 days) compared to other treatments such as eCO2 (15.5 ± 0.17 & 17.3 ± 0.42 days), e03 (18.2 ± 0.40 & 21.7 ± 0.40 days), and ambient (16.7 ± 0.15 & 18.8 ± 0.84 days). The total developmental period was significantly shorter under eT+eCO2 and eCO2 than ambient conditions, but no difference was observed between e03 and ambient conditions in both season (Tables 2A, B). In comparison to ambient condition, stress condition drastically shortens the longevity of both sexes (Table 2C). In 2019, female and male longevity was lowest in eT+eCO2 (9.7 ± 0.3&8.7 ± 0.1days). In contrast, during 2020, e03 had the lowest female and male longevity (11.1 ± 0.2 & 9.5 ± 0.5 days).
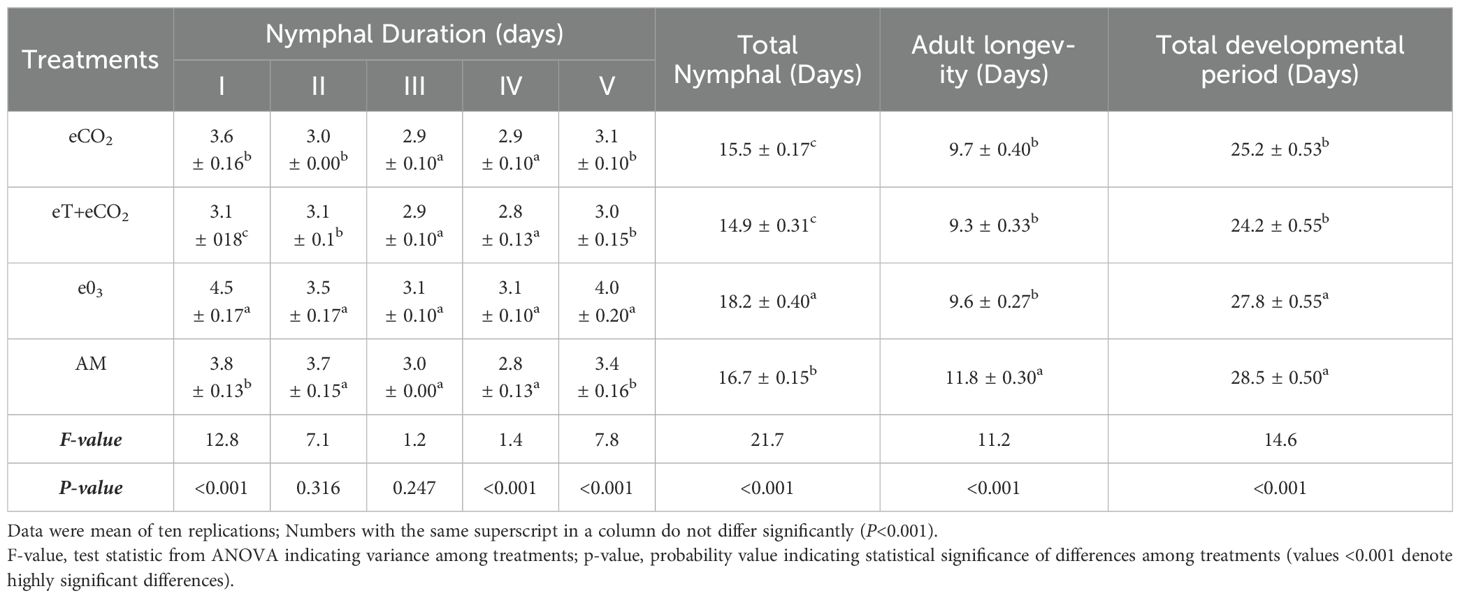
Table 2a. The combined effect of elevated temperature+CO2 and ozone on developmental stages of N. lugens in Free Air Temperature Enrichment (FATE) during the rainy season 2019.
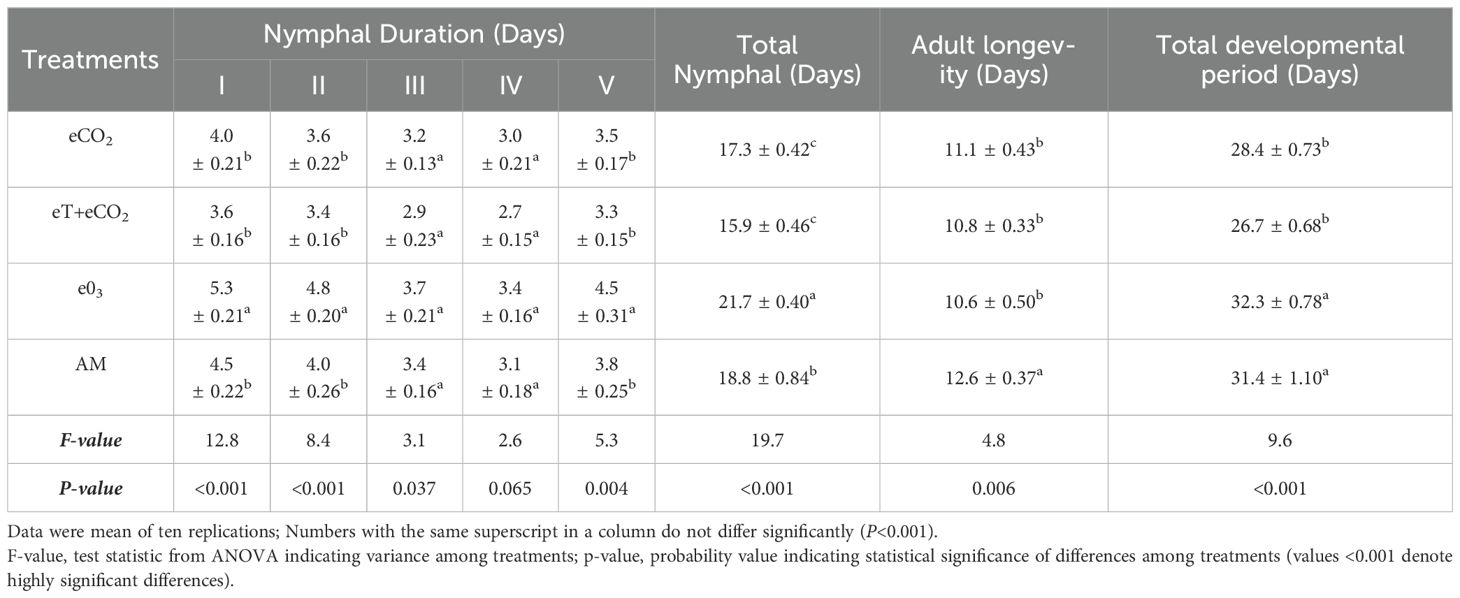
Table 2b. The combined effect of elevated temperature+CO2 and ozone on developmental stages of N. lugens in Free Air Temperature Enrichment (FATE) during the rainy season 2020.
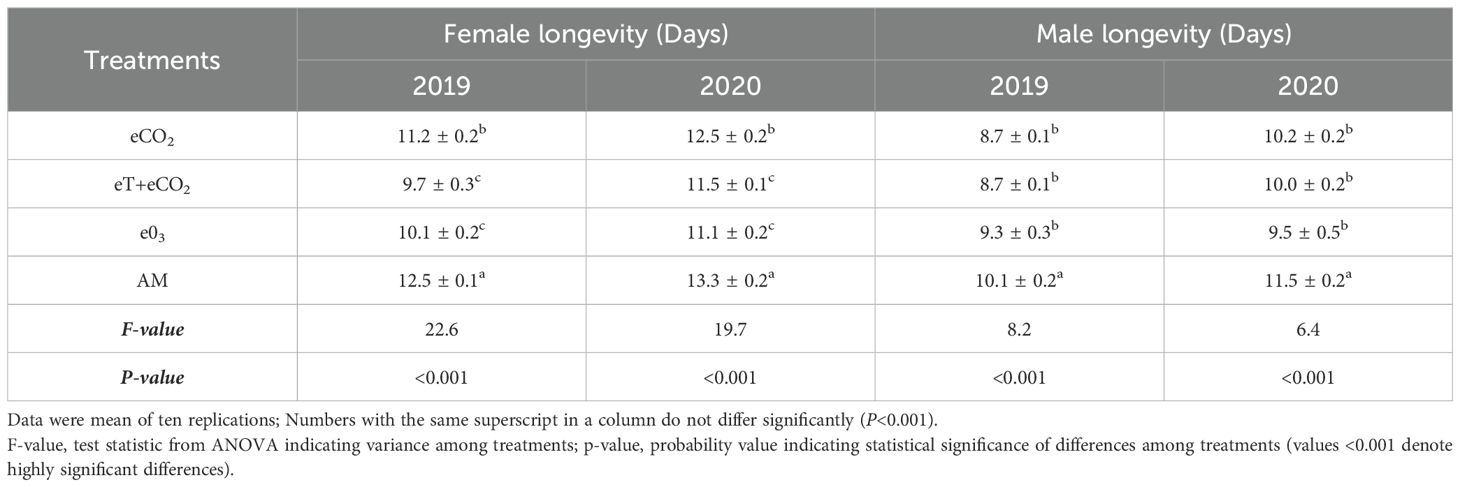
Table 2c. The combined effect of elevated temperature+CO2 and ozone on N. lugens longevity during the rainy season of 2019 and 2020.
3.2.3 Feeding potential of N. lugens by honeydew test
The amount of honeydew excreted by N. lugens females differed significantly across treatments in both seasons (F=79.7, P<0.001in 2019 and F=145.1, P<0.001in 2020) (Table 3). During both seasons, brachypterous females fed on eCO2-exposed rice plants had a higher percent increase in honey dew excretion above ambient (224 and 229%), but e03-exposed rice plants had a decrease percentage of honey dew excretion (-14 to 20%). In both years of study, females fed on ET+ EC exposed rice plants excreted much more honeydew than AM and e03, but significantly less than eCO2 (Table 3).
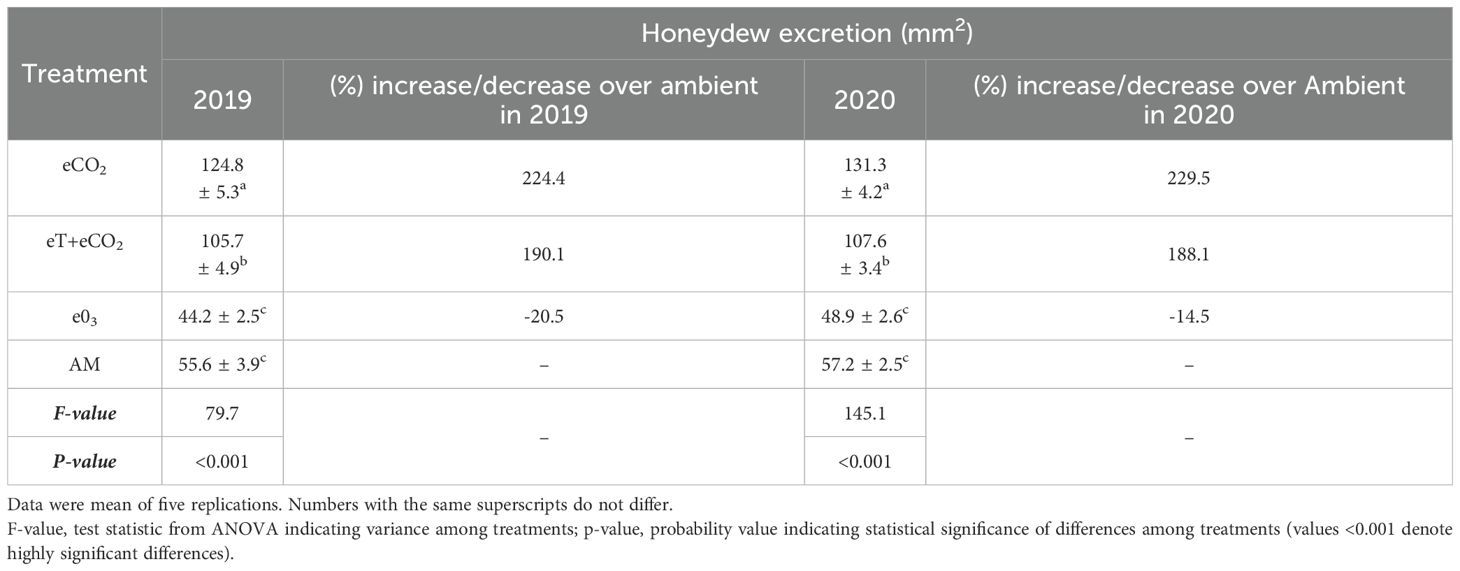
Table 3. The combined effect of elevated temperature + CO2 and ozone on honeydew excretion during the rainy season of 2019 and 2020.
3.3 Impact of climate change parameters on plant growth and yield of rice in FATE
The present study demonstrated that the N. lugens infestation had a deleterious effect on plant parameters in all treatments, including ambient. As a result, all plant parameters are much better under BPH-uninfested than infested conditions. In both infested and uninfested conditions, eCO2 improved plant parameters whereas e03 significantly reduced them in contrast to eT+eCO2 and ambient (Table 4). Therefore, under uninfested condition the plant parameters such as plant height (65.4 ± 0.8cm), total tillers (25.0 ± 0.8), reproductive tillers (22.2 ± 0.6) and percent reproductive tillers (88.9 ± 1.0%) were recorded maximum in eCO2 condition. On the other hand, plant height (54.8 ± 1.0 cm), total tillers (17.7 ± 0.9), reproductive tillers (13.0 ± 0.5) and percent reproductive tillers (74.12 ± 2.1%) were recorded as the lowest in e03. Under eT+eCO2, plant height (62.4 ± 0.6 cm) and total tillers (23.6 ± 0.6) were enhanced but reproductive tillers (18.6 ± 0.6) and percent reproductive tillers (78.8 ± 1.6%) were negatively affected as compared to ambient control (Table 4). Under N. lugens infestation, the plant parameters were recorded lower in all the treatments including ambient conditions. The plant height (63.1 ± 0.4 cm), total tillers (23.3 ± 0.4), reproductive tillers (18.9 ± 0.3) and percent reproductive tillers (81.2 ± 1.1%) were recorded maximum in eCO2 condition. On the other hand, plant height (51.6 ± 0.4 cm), total tillers (15.1 ± 0.8), reproductive tillers (10.1 ± 0.5) and percent reproductive tillers (67.1 ± 2.4%) were recorded as the lowest in e03 infested with N. lugens among all the treatments (Table 4). Similarly, grain and yield traits significantly differed among the treatments in both uninfested, and infested conditions. The eCO2 has a positive effect on the length of the panicle (27.5 ± 0.3 cm), grain per panicle (99.7 ± 1.9), grain yield (40.1 ± 0.3 gm/hill), 1000 seed weight (40.1 ± 0.3 gm) as well as decrease the unfilled grain percentage (5.2 ± 0.5%) (Table 5). However, grains per panicle drastically decreased in N. lugens infestation (86.0 ± 2.8) under eCO2. The e03 negatively hampered grain parameters like grains per panicle (74.7 ± 1.5), grain yield (27.5 ± 0.2 gm/hill) and 1000 seed weight (18.3 ± 0.4 gm). Also, the percentage of unfilled grain (12.2 ± 0.8%) under e03 was the highest among all treatments. The length of the panicle under eT+eCO2 (26.8 ± 0.2 cm) increased significantly in uninfested conditions but it was at par with ambient control in infested conditions (Table 5).
4 Discussion
4.1 Impact of climate change parameters on population dynamics of N. lugens
To effectively manage N. lugens in changing climatic scenarios, it is necessary to comprehend the incidence and dynamics of pest populations. The present research found that the population of N. lugens, which includes nymphs and brachypterous females, was maximum under eCO2 condition. The population of N. lugens reached its highest peak in early season, and a second peak was seen later in the season. This is in line with Pandi et al., 2018a; Daravath et al., 2018; and Tenguri et al., 2023, who suggested that eCO2 concentration increased photosynthesis, plant canopy size, and tillering, creating an ideal environment for N. lugens and faster population growth. The eT+eCO2 had a higher N. lugens population than ambient but lower than eCO2 conditions due to the simultaneous increase in atmospheric CO2 and temperature, which may affect insect-plant interaction directly and indirectly (Shi et al., 2014). The combined effect of eT+eCO2 boosts the population of N. lugens, but not to the same extent as eCO2 alone, because, in addition to the effect of CO2, increased temperature causes decrease in stem water levels, affecting the population of BPH that feeds on phloem Shi et al. (2014). On the other hand, e03 impeded the population expansion of N. lugens. The population’s growth may have been inhibited by a decrease in feeding rate, lower fertility, and a reduction in the number of females as a result of e03. Our findings match with the results of previous studies (Walling, 2000; Yan et al., 2018) who suggested the elevated e03 conditions altered the plant nutritional profile, synthesis and accumulate more secondary metabolite. Menendez et al. (2010) found a similar finding for the green peach aphid M. persicae. The impact of elevated ozone on insect development appears to be variable across different species. Some studies have reported that increased ozone levels enhance insect growth, while others have found a negative or negligible effect (Heliovaara and Vaisanen, 1993; Guo et al., 2020). For instance, in Bemisia tabaci (Q biotype), Hong et al. (2016) observed that elevated ozone led to increased egg production, shorter development time, and higher survivorship. Conversely, Mina et al. (2012) reported that elevated ozone had a detrimental effect on the development of Chilo partellus. In eCO2 and eT+eCO2, fecundity and egg masses per female were increased. Despite this, fecundity was greater in eCO2 conditions than in eT+eCO2. Elevated CO2 levels led to a greater population of N. lugens, indicating enhanced fecundity and a higher proportion of brachypterous females. This trend might be due to favorable microclimatic circumstances caused by dense plant growth and enhanced tillering (Prasannakumar et al., 2012). Elevated CO2 levels increased the number of brachypterous females, possibly increasing to the BPH population. These females produced eggs at a higher rate. Similarly, Krishnaiah et al. (2008) discovered that brachypterous females produce more eggs and contribute more to population growth than macropterous females. The eT+eCO2 increased fertility in rice plant hoppers, N. lugens (Pandi et al., 2018a), and maize leaf aphids, R. maidis (Xie et al., 2014), indicating a positive effect on multiplication. Plant nutritional quality, favorable microclimatic conditions, improved feeding, and a greater sucking rate by females may have contributed to higher fecundity. In contrast BPH strongly avoided rice plants that had been subjected to elevated ozone while choosing sites to lay eggs. This is most likely due to the changes in the chemical signals given by the plant, which are commonly employed to decide whether a host plant is accepted or not for feeding and egg laying (Hilker and Meiners, 2011). Our findings are consistent with prior study done by Cui et al., 2019 and Inoue et al., 2016. The studies also found that higher ozone levels affected fertility in the whitefly B. tabaci and fewer eggs were deposited by the leaf beetle A. Coerulea.
In eCO2 and eT+eCO2, the pest development period, including nymphal length and adult lifespan significantly decreased. The shortened developmental time may be attributed to the increased C:N ratio, higher sugar, and reduced nitrogen levels in the rice plants (SChadler et al., 2007). As a result, insects expended additional energy on feeding to make up the dietary deficiency of their food which ultimately shortened developmental period. Some recent researches have indicated that N. lugens on rice under CO2 enriched settings had shorter developmental period, shortened nymphal period, and short female and male lifespan (Pandi et al., 2018b; Daravath et al., 2018). In a study conducted by Auad et al. (2012), it was shown that the nymphal longevity of the yellow sugarcane aphid, S. flava, was dramatically reduced under elevated CO2+tempreture. Similarly, Shi et al. (2014) and Xie et al. (2014) found that female N. lugens had a shorter lifespan and had substantially shorter developmental durations at each life stage when exposed to elevated CO2 and temperature. However, elevated ozone extended nymphal and total developmental period of N. lugens. In both seasons, elevated ozone levels reduced adult lifetime, as well as female and male longevity. The elevated ozone level stimulated the plants to produce and store secondary metabolites. This might impact several facets of insect behavior and performance, such as feeding patterns, ability to lay eggs, longevity, and reproductive potential. These modifications have the capacity to modify the abundance and structure of herbivorous insects (Lindroth, 2010; Couture and Lindroth, 2012; Cui et al., 2012, 2014).
Honeydew production by insects is directly proportional to the sap sucking (Tenguri et al., 2023). The N. lugens reared under eCO2, and eT+eCO2 produced considerably more honeydew than AM. It shows that N. lugens females are sucking more to compensate for their inferior nutritional condition and the plant’s greater C: N ratio. Prior research has indicated that N. lugens exhibited an increased rate of sap-sucking under increasing CO2, as earlier reported by Shi et al. (2014); Pandi et al. (2018b), and Daravath et al. (2018). In contrast, significant lower honeydew production was reported by females fed on plants raised under elevated ozone conditions. Walling (2000) and Yan et al. (2018) observed that when plants are grown under elevated ozone concentrations, they produce more secondary metabolites and anti-nutritional chemicals, which reduce the female sap sucking rate.
4.2 Impact of climate change parameters on plant growth and yield attributes of rice
Agricultural crops are heavily influenced by variations in climatic circumstances such as CO2 levels, temperature, and ozone. Elevated CO2 has a nutritional and fertilization effect on plant growth and reproduction fertilization effect on plant growth and reproduction. This, in the end, results in higher biomass and productivity specifically in C3 plants (Hasegawa et al., 2007; Ainsworth et al., 2007; Reddy et al., 2010). Increasing CO2 concentrations will enhance the degree of damage caused by insect pests, in addition to promoting the growth of plants (Gregory et al., 2009). In this study, both eCO2 and the combination of eT+eCO2 had a positive effect on rice growth indices, such as plant height and the number of tillers. Similar impact is observed on grain characteristics such as panicle length, grain per panicle, test weight, and yield in uninfested rice crops. N. lugens infestation in rice plants causes more severe damage, notwithstanding the advantageous impact, when plants are subjected to elevated CO2 levels and the combined effect of elevated temperature and CO2. The increased level of damage was attributed to their higher fecundity, an increased number of wingless females, and intensified sap-sucking behavior (Prasannakumar et al., 2012). Shi et al. (2014) found that the combination of eT+eCO2 had a favorable effect on various biological parameters of both the rice plant and the plant hopper. Thus, N. lugens population significantly increases under eCO2 alone as well as its combination eT+eCO2, thereby increasing yield loss. In contrast, ozone impedes several reproductive processes, including germination of pollen, fertilization, and the abortion of flowers, pods, and individual ovules or seeds (Black et al., 2000), and reduces grain yield, straw yield and harvest index (Bhatia et al., 2012). Elevated ozone had a detrimental impact on plant development and yield characteristics, as plant height, total tillers and reproductive tillers, panicle length, and seeds per panicle were all considerably lower than under ambient condition. Negative responses of rice to elevated ozone have been attributed to impaired growth, photosynthetic performance, reproduction and quality of the grain ultimately showing a reduction in grain yield (Bhatia et al., 2021; Oksanen et al., 2013).
Overall, the findings demonstrated that when rice plants are exposed to elevated CO2, their nutritional, biochemical, and developmental properties improve. As a result, N. lugens grows and develops more effectively. The interactive effect of elevated CO2+temperature had a favorable impact on the growth and development of N. lugens, while the higher temperature may have counteracted and diminished the amplified favorable effect of elevated CO2. On the other hand, elevated ozone levels decreased plant nutrition by interfering with certain plant growth factors. Hence, the number of N. lugens decreased and its peak was delayed than expected.
5 Conclusion
In conclusion, the study revealed that eCO2 alone had a positive effect on rice plant growth and yield parameters but simultaneously also stimulated the N. lugens population development. The surge in N. lugens population might be due to the formation of a favorable micro-climate by denser plant growth which resulted in a higher number of brachypterous females and nymph population, and also higher fecundity by females. This increased brown plant hopper development coupled with higher sap sucking rate under enriched CO2 resulted in greater yield losses compared to ambient conditions. Similarly, the interactive effect of elevated temperature and CO2 also had certain positive effects on rice plant growth, reproductive and grain parameters. However, it favored the pest multiplication and perpetuation causing higher grain yield losses than ambient conditions. Also, the elevated ozone concentration above threshold level had a significant negative effect on rice plant growth and yield, this negative effect of Ozone accompanied with N. lugens infestation aggravates more yield losses than ambient conditions. The N. lugens populations are expected to aggravate in future climate change conditions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
YY: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. SC: Conceptualization, Investigation, Methodology, Resources, Supervision, Validation, Writing – review & editing. SS: Conceptualization, Investigation, Methodology, Resources, Supervision, Writing – original draft, Writing – review & editing. PT: Investigation, Methodology, Writing – review & editing. AS: Data curation, Formal Analysis, Writing – review & editing. GG: Data curation, Formal Analysis, Software, Writing – original draft, Writing – review & editing. AB: Methodology, Resources, Supervision, Writing – original draft, Writing – review & editing. CP: Formal Analysis, Software, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
Authors are grateful to the Division of Entomology, Indian Agricultural Research Institute, New Delhi for providing all necessary facilities and support for the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1518361/full#supplementary-material
Abbreviations
BPH, Brown Plant Hopper; C:N, Carbon to nitrogen ratio; e03, Elevated Ozone; eCO2, Elevated CO2; eT+eCO2, Elevated temperature and Elevated CO2; FATE, Free Air Temperature Enrichment; IARI, Indian Agricultural Research Institute, New Delhi; IRGA, Infra-Red Gas Analyzer; WAR, Weeks After Release.
References
Ainsworth, E. A., Rogers, A., Leakey, A. D., Heady, L. E., Gibon, Y., Stitt, M., et al. (2007). Does elevated atmospheric [CO2] alter diurnal C uptake and the balance of C and N metabolites in growing and fully expanded soybean leaves? J. Exp. Botany. 58, 579–591. doi: 10.1093/jxb/erl233
Auad, A. M., Fonseca, M. G., Resende, T. T., and Maddalena, I. S. C. P. (2012). Effect of climate change on longevity and reproduction of Sipha flava (Hemiptera: Aphididae). Florida Entomologist. 95, 433–444. doi: 10.2307/23268565
Babu, S. B., Parameswaran, C., Anant, A. K., Padhi, J., Bansal, R., Priyadarsini, S., et al. (2022). Genomic analysis and finding of candidate genes for Nilaparvata lugens (stål) resistance in Indian pigmented and other indigenous rice genotypes. Crop Protection. 156, 105959. doi: 10.1016/j.cropro.2022.105959
Bale, J. S., Masters, G. J., Hodkinson, I. D., Awmack, C., Bezemer, T. M., Brown, V. K, et al. (2002). Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Glob Chang Biol. 8, 1–16. doi: 10.1046/j.1365-2486.2002.00451.x
Behura, N., Sen, P., and Kar, M. K. (2011). Introgression of yellow stem borer (Scirphophaga oryzae) resistance gene, into cultivated rice (Oryza sp.) from wild spp. Indian J. Agric. Sci. 81, 359–362.
Bhatia, A., Mina, U., Kumar, V., Tomer, R., Kumar, A., Chakrabarti, B., et al. (2021). Effect of elevated ozone and carbon dioxide interaction on growth, yield, nutrient content and wilt disease severity in chickpea grown in Northern India. Heliyon. 7, e06049. doi: 10.1016/j.heliyon.2021.e06049
Bhatia, A., Tomer, R., Kumar, V., Singh, S. D., and Pathak, H. (2012). Impact of tropospheric ozone on crop growth and productivity–a review. J. scient. Ind. Res. 71, 97–112.
Black, V. J., Black, C. R., Roberts, J. A., and Stewart, C. A. (2000). Impact of ozone on the reproductive development of plants. New Phytologist. 147, 421–447. doi: 10.1046/j.1469-8137.2000.00721.x
Brauer, M., Freedman, G., Frostad, J., Van Donkelaar, A., Martin, R. V., Dentener, F., et al. (2016). Ambient air pollution exposure estimation for the global burden of disease 2013. Environ. Sci. Technol. 50, 79–88. doi: 10.1021/acs.est.5b03709
Brownlie, J. C. and Johnson, K. N. (2009). Symbiont-mediated protection in insect hosts. Trends Microbiol. 17, 348–354. doi: 10.1016/j.tim.2009.05.005
Capone, A., Ricci, I., Damiani, C., Mosca, M., Rossi, P., Scuppa, P., et al. (2013). Interactions between Asaia, Plasmodium and Anopheles: new insights into mosquito symbiosis and implications in malaria symbiotic control. Parasit Vectors. 6, 182. doi: 10.1186/1756-3305-6-182
Chen, F. J., Wu, G., and Ge, F. (2004). Impacts of elevated CO2 on the population abundance and reproductive activity of aphid Sitobion avenae Fabricius feeding on spring wheat. J. Appl. Entomol. 128, 723–730. doi: 10.1111/j.1439-0418.2004.00921.x
Cheng, J., Zhao, W., Lou, Y., and Zhu, Z. (2001). Intra- and inter-specific effects of the brown planthopper and white backed planthopper on their population performance. J. Asia-Pac. Entomol. 4, 85–92. doi: 10.1016/S1226-8615(08)60108-9
Coakley, S. M., Scherm, H., and Chakraborty, S. (1999). Climate change and disease management. Annu. Rev. Phytopathol. 37, 399–426. doi: 10.1146/annurev.phyto.37.1.399
Coley, P., Massa, M., Lovelock, C., and Winter, K. (2002). Effects of elevated CO2 on foliar chemistry of saplings of nine species of tropical tree. Oecologia. 133, 62–69. doi: 10.1007/s00442-002-1005-6
Couture, J. and Lindroth, R. L. (2012). Atmospheric change alters performance of an invasive forest insect. Glob Chang Biol. 18, 3543–3557. doi: 10.1111/gcb.12014
Cui, H., Sun, Y., Sun, J., Ren, Q., Li, C., and Ge, F. (2012). Elevated O3 reduces the fitness of Bemisia tabaci via enhancement of the SA-dependent defense of the tomato plant. Arthropod-Plant Interact. 6, 425–437. doi: 10.1007/s11829-012-9189-0
Cui, H., Sun, J., Wei, Y., Hu, F., and Ge, F. (2014). Elevated O3 enhances the attraction of whitefly infested tomato plants to Encarsia formosa. Sci. Rep. 4, 1–6. doi: 10.1038/srep05350
Cui, H., Sun, Y., Zhao, Z., and Zhang, Y. (2019). The combined effect of elevated O3 levels and TYLCV infection increases the fitness of Bemisia tabaci Mediterranean on tomato plants. Environ. Entomol. 48, 1425–1433. doi: 10.1093/ee/nvz113
Daravath, V., Chander, S., and Mandla, R. (2018). Impact of elevated CO2 on Nilaparvata lugens (stal), rice crop and feeding of Pardosa pseudoannulata. Indian J. Entomol. 80, 662–667. doi: 10.5958/0974-8172.2018.00220.1
Gregory, P. J., Johnson, S. N., Newton, A. C., and Ingram, J. S. I. (2009). Integrating pests and pathogens into the climate change/food security debate. J. Exp. Bot. 60, 2827–2838. doi: 10.1093/jxb/erp080
Guo, H., Sun, Y., Yan, H., Li, C., and Ge, F. (2020). O3-induced priming défense associated with the abscisic acid signaling pathway enhances plant resistance to bemisia tabaci. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.00093
Hasegawa, T., Shimono, H., Yang, L. X., Kim, H. Y., Kobayashi, T., Sakai, H., et al. (2007). “Response of rice to increasing CO2 and temperature: recent findings from large-scale free-air CO2 enrichment (FACE) experiments,” in Proceedings of the 26th international rice conference, 9–12 Octobe. Eds. Aggarwal, P., Ladha, J., Singh, R., Devakumar, C., and Hardy, B. (New Delhi, India: International Rice Research Institute, Indian Council of Agricultural Research and National Academy of Agricultural Sciences), 439–447.
Heliovaara, K. and Vaisanen, R. (1993). “Pollution in terrestrial ecosystems,” in Insects and Pollution (CRC Press, Boca Raton, Florida), 55–160.
Hilker, M. and Meiners, T. (2011). Plants and insect eggs: how do they affect each other? Phytochemistry. 72, 1612–1623. doi: 10.1016/j.phytochem.2011.02.018
Hong, Y., Yi, T., Tan, X., Zhao, Z., and Ge, F. (2016). High ozone (O3) affects the fitness associated with the microbial composition and abundance of Q biotype. Bemisia tabaci. Front. Microbiol. 7. doi: 10.3389/fmicb.2016.01593
Horgan, F. G., Arida, A., Ardestani, G., and Almazan, M. L. P. (2007). Elevated temperatures diminish the effects of a highly resistant rice variety on the brown planthopper. Sci. Rep. 11(1), 262. doi: 10.1038/s41598-020-80704-4
Inoue, W. A., Vanderstock, T., Sakikawa, M., Nakamura, H., Saito, H., Shibuya, M., et al. (2016). The interaction between insects and deciduous broadleaved trees under different O3 concentrations and soil fertilities. Boreal For. Res. 64, 30.
IPCC (2022). “Summary for Policymakers,” in Climate Change 2022: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change (Cambridge University Press, Cambridge, UK and New York, NY, USA), 3–33. doi: 10.1017/9781009325844.001
Khush, G. S. (2004). Harnessing science and technology for sustainable rice-based production systems. Int. Rice Commission Newslett. 53, 17–23.
Krishnaiah, N. V., Lekshmi, V. J., Passlu, I. C., Kalti, G. R., and Padmavathi, C. (2008). Insecticide in rice-IPM, past, present and future, DRR, hyderabad, India 148.
Lindroth, R. L. (2010). Impacts of elevated atmospheric CO2 and O3 on forests: Phytochemistry, trophic interactions, and ecosystem dynamics. J. Chem. Ecol. 36, 2–21. doi: 10.1007/s10886-009-9731-4
Long, S. P. (2012). Virtual special issue on food security—greater than anticipated impacts of near-term global atmospheric change on rice and wheat. Glob Chang Biol. 18, 1489–1490. doi: 10.1111/j.1365-2486.2012.02676.x
Menendez, A. I., Romero, A. M., Folcia, A. M., and Martinez-Ghersaet, M. A. (2010). Aphid and episodic O3 injury in arugula plants (Eruca sativa Mill.) grown in open-top field chambers. Agric. Ecosyst. Environ. 135, 10–14. doi: 10.1016/j.agee.2009.08.005
Mina, U., Bhatia, A., Chakrabarti, B., Harit, R. C., and Kumar, U. (2012). Impact of ozone and carbon dioxide on growth and development of Chilo partellus swinhoe (maize stalk borer). J. Entomol. Res. 36, 363–366.
Morgan, P. B., Mies, T. A., Bollero, A., Nelson, R. L., and Long, S. P. (2006). Season-long elevation of ozone concentration to projected 2050 levels under fully open-air conditions substantially decreases the growth and production of soybean. New Phytol. 170, 333–343. doi: 10.1111/j.1469-8137.2006.01679.x
Oksanen, E., Pandey, V., Pandey, A. K., Keski-Saari, S., Kontunen-Soppela, S., and Sharma, S. (2013). Impacts of increasing ozone on Indian plants. Environ. Pollut. 177, 189–200. doi: 10.1016/j.envpol.2013.02.010
Paguia, P., Pathak, M. D., and Heinrichs, E. A. (1980). Honeydew excretion measurement techniques for determining differential feeding activity of biotype of Nilaparvata lugens on rice varieties. J. Econ. Entomol. 73, 35–40. doi: 10.1093/jee/73.1.35
Pandi, G. G. P., Chander, S., Pal, M., and Soumia, P. S. (2018b). Impact of elevated CO2 on Oryza sativa phenology and brown planthopper, Nilaparvata lugens (Hemiptera: Delphacidae) population. Curr. Sci. 114, 1767–1777. doi: 10.18520/cs/v114/i08/1767-1777
Pandi, G. G. P., Chander, S., Singh, M. P., and Pathak, H. (2018a). Impact of elevated CO2 and temperature on brown planthopper population in rice ecosystem. Proc. Natl. Acad. Sci. India Sect B Biol. Sci. 88, 57–64. doi: 10.1007/s40011-016-0727-x
Prasannakumar, N., Chander, S., and Pal, M. (2012). Assessment of impact of climate change with reference to elevated CO2 on rice brown planthopper, Nilaparvata lugens (Stal.) and crop yield. Curr. Sci. 103, 1201–1205.
Raderschall, C., Vico, G., Lundin, O., Taylor, A., and Bommarco, R. (2021). Water stress and insect herbivory interactively reduce crop yield while the insect pollination benefit is conserved. Glob Chang Biol. 27, 1–13. doi: 10.1111/gcb.15386
Reddy, A. R., Rasineni, G. K., and Raghavendra, A. S. (2010). The impact of global elevated CO2 concentration on photosynthesis and plant productivity. Curr. Sci. 99, 46–57.
SChadler, M., Roeder, M., Brandal, R., and Matthies, D. (2007). Interacting effects of elevated CO2, nutrient availability and plant species on a generalist invertebrate herbivore. Glob Chang Biol. 13, 1005–1015. doi: 10.1111/j.1365-2486.2007.01319.x
Shi, B. K., Huang, J. L., Hu, C. X., and Hou, M. L. (2014). Interactive effects of elevated CO2 and temperature on rice planthopper, Nilaparvata lugens. J. Integr. Agric. 13, 1520–1529. doi: 10.1016/S2095-3119(14)60804-2
Srinivasa Rao, M., Srinivas, K., Vanaja, M., Rao, G. S. N., Venkateswarlu, B., and Ramakrishna, Y. S. (2009). Host plant (Ricinus communis Linn.) mediated effects of elevated CO2 on growth performance of two insect folivores. Curr. Sci. 97, 1047–1054.
Tenguri, P., Chander, S., Ellur, R. K., Yele, Y., Sundaran, A. P., Nagaraju, M. T., et al. (2023). Effect of silicon application to the rice plants on feeding behavior of the brown planthopper, nilaparvata lugens (Stål) under elevated CO2. Silicon. 15, 5811–5820. doi: 10.1007/s12633-023-02480-w
Walling, L. L. (2000). The myriad plant responses to herbivores. J. Plant Growth Regul. 19, 195–216. doi: 10.1007/s003440000026
Wang, J. J. and Tsai, J. H. (2001). Development, survival and reproduction of black citrus aphid, Toxoptera aurantii (Hemiptera: Aphididae), as a function of temperature. Bull. Entomol. Res. 91, 477–487. doi: 10.1079/BER2001120
Ward, N. L. and Masters, G. L. (2007). Linking climate change and species invasion: an illustration using insect herbivores. Glob Chang Biol. 13, 1605–1615. doi: 10.1111/j.1365-2486.2007.01399.x
Way, D. A. and Oren, R. (2010). Differential responses to changes in growth temperature between trees from different functional groups and biomes: a review and synthesis of data. Tree Physiol. 30, 669–688. doi: 10.1093/treephys/tpq015
Xie, H., Zhao, L., Wang, W., Wang, Z., Ni, X., Cai, W., et al. (2014). Changes in life history parameters of Rhopalosiphum maidis (Homoptera: Aphididae) under four different elevated temperature and CO2 combinations. J. Econ. Entomol. 107, 1411–1418. doi: 10.1603/EC13302
Keywords: brown planthopper, climate change, elevated ozone, insects, pest management, rice
Citation: Yele Y, Chander S, Suroshe SS, Tenguri P, Sundaran AP, Pandi GGP, Bhatia A and Patel C (2025) “Physiological and demographic responses of Nilaparvata lugens to combined climate stressors: CO2, temperature, and ozone”. Front. Plant Sci. 16:1518361. doi: 10.3389/fpls.2025.1518361
Received: 28 October 2024; Accepted: 13 May 2025;
Published: 05 June 2025.
Edited by:
Rahul Kumar Tiwari, Indian Institute of Sugarcane Research (ICAR), IndiaReviewed by:
Jaba Jagdish, International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), IndiaPradeep Kumar Dalal, Acharya Narendra Deva University of Agriculture and Technology, India
Copyright © 2025 Yele, Chander, Suroshe, Tenguri, Sundaran, Pandi, Bhatia and Patel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yogesh Yele, eW9nZXNoeWVsZTEzQGdtYWlsLmNvbQ==
 Yogesh Yele
Yogesh Yele Subhash Chander2
Subhash Chander2 G. Guru Pirasanna Pandi
G. Guru Pirasanna Pandi Arti Bhatia
Arti Bhatia Chenesh Patel
Chenesh Patel