Abstract
Leptochloa chinensis (L.) Nees, a noxious weed species commonly found in rice fields, has become a significant challenge in Jiangsu Province, China, as it has developed resistance to multiple herbicides due to extensive and continuous herbicide use in recent years. Therefore, this study was conducted to elucidate sensitivity differences and the mechanisms underlying the resistance of L. chinensis (L.) Nees populations to commonly used herbicides across different regions of Jiangsu Province, China. A whole-plant bioassay was used to assess the sensitivity of 46 L. chinensis populations collected from various areas within Jiangsu to several herbicides frequently applied in paddy fields, including: cyhalofop-butyl, fenoxaprop-P-ethyl, pyraclonil, benzobicyclon, anilofos, and oxaziclomefone. After treatment with cyhalofop-butyl, 38 out of 46 populations showed relative resistance-index values that were over four times that of the controls, indicating significant resistance to cyhalofop-butyl. All 41 cyhalofop-butyl-resistant populations showed cross-resistance to fenoxaprop-P-ethyl but remained susceptible to pyraclonil, benzobicyclon, anilofos, and oxaziclomefone. The proportion of populations resistant to acetyl-CoA carboxylase (ACCase)-inhibiting herbicides increased progressively from the south to the north of Jiangsu. Cross-resistance was evident between cyhalofop-butyl and fenoxaprop-P-ethyl; however, all resistant populations were susceptible to pyraclonil, benzobicyclon, anilofos, and oxaziclomefone. Furthermore, mutations in the ACCase gene were identified as a crucial mechanism for cyhalofop-butyl resistance. Specifically, we found ACCase mutations I1781L, W1999C, W2027C/L/S, I2041N, and D2078G in cyhalofop-butyl-resistant L. chinensis populations, among which, W1999C and W2027C accounted for a relatively high proportion, while I1781L, W2027L/S, I2041N, and D2078G were found in one population each. ACCase gene mutations are seemingly a key mechanism for the development of resistance to cyhalofop-butyl, thus, our study provides useful information for developing effective weed-management strategies for controlling this noxious weed species, while ensuring sustainable agricultural practices.
1 Introduction
Leptochloa chinensis (L.) Nees is a tetraploid (2n=4x=40) belonging to the Chloridoideae subfamily of the Poaceae(grass) family, with high drought and waterlogging resistance, moisture preference, and strong reproductive and tillering abilities. Furthermore, this grass species is globally recognized as a noxious agricultural weed (Chauhan and Johnson, 2008; Wen et al., 2017; Zhu et al., 2018). The spread and harmful effect of L. chinensis in the middle and lower reaches of the Yangtze River Basin in China have shown a clear increasing trend over time, which has been exacerbated by the widespread adoption of direct-seeded rice cultivation practices and prolonged, exclusive use of herbicides, posing a severe threat to rice production security (Wu et al., 2015; Yuan et al., 2022).
Cyhalofop-butyl is an acetyl-CoA carboxylase (ACCase, EC.6.4.12) inhibitor herbicide traditionally used in paddy fields for the management of Poaceae weeds, including L. chinensis and Echinochloa crus-galli (Ruiz-Santaella et al., 2006). However, the efficacy of cyhalofop-butyl against L. chinensis has been declining owing to its long-term, exclusive use. This has led to the development of resistance among weeds, as indicated by an increasing number of reports from farmers stating that recommended doses are ineffective in controlling the weed. Exclusive use of ACCase inhibitor herbicides over extended periods can induce resistance in weeds. To date, 49 species of weeds have reportedly developed resistance to this class of herbicides globally (Heap, 2024; Heap and Knight, 1986; Maneechote et al., 2005). Thus, a population of L. chinensis resistant to ACCase inhibitors, including cyhalofop-butyl, was identified in Bangkok, Thailand in 2002 (Heap, 2024). Similarly, L. chinensis populations resistant to cyhalofop-butyl were detected in the paddy fields of Hubei Province, China in 2011; L. chinensis populations showing cross-resistance to cyhalofop-butyl and fenoxaprop-P-ethyl were reported from South Korea in 2012 (Sahid et al., 2011; Wei et al., 2015), and L. chinensis populations resistant to cyhalofop-butyl were identified in Malaysia in 2014 (Rahman et al., 2014). Currently, L. chinensis populations resistant to cyhalofop-butyl have been identified in Jiangsu (Deng et al., 2019), Shanghai (Yuan et al., 2022), Anhui (Jiang et al., 2022), and Hunan (Peng et al., 2020). Several mutations, such as Ile-1781-Leu, Leu-1818-Phe, Trp-1999-Cys, Trp-2027-Cys, Trp-2027-Gly, Trp-2027-Leu, Trp-2027-Ser, Ile-2041-Asn, Cys-2088-Arg, and Gly-2096-Ala, in ACCase have been identified as the primary cause of L. chinensis resistance (Kurniadie et al., 2022; Zhang et al., 2022; Zhao et al., 2022; Liao et al., 2024; Deng et al., 2023; Jiang et al., 2024).
In turn, Deng et al. (2019) detected cyhalofop-butyl-resistant populations of L. chinensis in the paddy fields of Jiangsu Province in 2019. However, variation in sensitivity to cyhalofop-butyl and other commonly used herbicides among different geographic populations of L. chinensis in Jiangsu, along with their distribution and the underlying mechanisms responsible for resistance, remain unclear. Thus, in this study, we systematically collected seed samples of L. chinensis from paddy fields across the southern, central, and northern regions of Jiangsu Province to test their sensitivity to the commonly used herbicides, the aryloxyphenoxypropionate(APP) family of ACCase inhibitor cyhalofop-butyl(HRAC 1, inhibition of Acetyl CoA Carboxulase) and fenoxaprop-P-ethyl(HRAC 1, inhibition of Acetyl CoA Carboxulase), the protoporphyrinogen oxidase(PPO) inhibitor pyraclonil(HRAC 14, inhibition of Protoporphyrinogen Oxidase), the 4-hydroxyphenylpyruvate dioxygenase inhibitor benzobicyclon(HRAC 27, inhibition of Hydroxyphenyl Pyruvate Dioxygenose), the organophosphorus herbicide anilofos(HRAC 15, inhibition of Very Long-Chain Fatty Acid Synthesis), and the organoheterocyclic herbicide oxaziclomefone(HRAC 30, inhibition of Fatty Acid Thioesterase), with the aim to analyze the distribution of resistant populations and the key molecular mechanisms responsible for resistance. Our study aimed to provide technical guidance for the effective management and control of L. chinensis in paddy fields.
2 Materials and methods
2.1 Seeds
Seeds were randomly collected from various locations across southern and northern Jiangsu Province during seed maturation period, from September to November 2020. The LENJ-001 population is a known susceptible population. Specific collection sites are listed in Table 1.
Table 1
| Order | Population code | Site of collection | Geographical location |
|---|---|---|---|
| 1 | LESZ-001 | Tongli Town, Wujiang District, Suzhou City | 31°6′N, 120°43′E |
| 2 | LESZ-003 | Guli Town, Changshu County, Suzhou City | 31°40′N, 120°49′E |
| 3 | LESZ-005 | Daxin Town, Zhangjiagang City, Suzhou City | 31°57′N, 120°31′E |
| 4 | LEJY-001 | Songjiawei Village, Jiangyin City, Wuxi City | 31°52′N, 120°7′E |
| 5 | LEHS-003 | Yuqi Street, Huishan District, Wuxi City | 31°42′N, 120°10′E |
| 6 | LEYX-003 | Dingshu Town, Yixing City, Wuxi City | 31°16′N, 119°50′E |
| 7 | LELY-003 | Nandu Town, Liyang City, Changzhou City | 31°26′N, 119°19′E |
| 8 | LEJT-001 | Zhiqian Town, Jintan District, Changzhou City | 31°39′N, 119°28′E |
| 9 | LELS-001 | Baima Town, Lishui District, Nanjing City | 31°34′N, 119°10′E |
| 10 | LEJR-001 | Shishi Town, Jurong City, Zhenjiang City | 31°56′N, 119°9′E |
| 11 | LEJR-002 | Guozhuang Town, Jurong City, Zhenjiang City | 31°48′N, 119°1′E |
| 12 | LELH-003 | Chengqiao Town, Liuhe District, Nanjing City | 32°22′N, 118°43′E |
| 13 | LEYZ-004 | Qianjin Village, Yizheng City, Yangzhou City | 32°18′N, 119°4′E |
| 14 | LEYL-001 | Yiling Town, Jiangdu District, Yangzhou City | 32°28′N, 119°40′E |
| 15 | LEJD-001 | Daqiao Town, Jiangdu District, Yangzhou City | 32°21′N, 119°42′E |
| 16 | LEJD-003 | Dinggou Town, Jiangdu District, Yangzhou City | 32°33′N, 119°39′E |
| 17 | LEGY-001 | Ganduo Town, Gaoyou City, Yangzhou City | 32°49′N, 119°46′E |
| 18 | LEGY-002 | Ganduo Town, Gaoyou City, Yangzhou City | 32°50′N, 119°44′E |
| 19 | LERG-001 | Changjiang Town, Rugao City, Nantong City | 32°6′N, 120°36′E |
| 20 | LERG-002 | Wuyao Town, Rugao City, Nantong City | 32°12′N, 120°32′E |
| 21 | LERG-003 | Wuyao Town, Rugao City, Nantong City | 32°12′N, 120°32′E |
| 22 | LEJJ-001 | Dongxing Town, Jingjiang City, Taizhou City | 31°58′N, 120°9′E |
| 23 | LETX-002 | Huangqiao Town, Taixing City, Taizhou City | 32°14′N, 120°14′E |
| 24 | LEXH-002 | Xingdong Town, Xinghua City, Taizhou City | 32°58′N, 119°54′E |
| 25 | LEXH-003 | Diaoyu Town, Xinghua City, Taizhou City | 33°4′N, 119°58′E |
| 26 | LEXH-006 | Diduo Town, Xinghua City, Taizhou City | 32°50′N, 120°5′E |
| 27 | LEHQ-001 | Hongqi Farm, Jiangyan District, Taizhou City | 32°33′N, 120°1′E |
| 28 | LEDF-007 | Fangqiang Farm, Dafeng District, Yancheng City | 33°29′N, 120°28′E |
| 29 | LESY-005 | Haitong Town, Sheyang County, Yancheng City | 33°48′N, 120°20′E |
| 30 | LEFN-001 | Chenliang Town, Funing County, Yancheng City | 33°41′N, 119°56′E |
| 31 | LEHX-001 | Huanghai Farm, Xiangshui County, Yancheng City | 34°17′N, 120°1′E |
| 32 | LEHX-002 | Huanghai Farm, Xiangshui County, Yancheng City | 34°17′N, 120°1′E |
| 33 | LEBH-001 | Binhuai Farm, Binhai County, Yancheng City | 34°15′N, 120°6′E |
| 34 | LEHH-001 | Huaihai Farm, Sheyang County, Yancheng City | 34°9′N, 120°14′E |
| 35 | LESQ-001 | Wangji Town, Siyang County, Suqian City | 33°52′N, 118°44′E |
| 36 | LESQ-002 | Chuancheng Town, Siyang County, Suqian City | 33°36′N, 118°37′E |
| 37 | LESQ-003 | Lailong Town, Suyu District, Suqian City | 34°2′N, 118°29′E |
| 38 | LESN-001 | Daodao, Weiji Town, Suining County, Xuzhou City | 34°1′N, 117°57′E |
| 39 | LESN-002 | Weiji Town, Suining County, Xuzhou City | 34°1′N, 117°57′E |
| 40 | LELSH-001 | Gaozhuang, Lianshui County, Huai’an City | 33°58′N, 119°5′E |
| 41 | LEBYH-001 | Huai’an Baoying Lake Farm | 33°36′N, 119°1′E |
| 42 | LEGY-001 | Songzhuang Town, Ganyu District, Lianyungang City | 34°46′N, 119°8′E |
| 43 | LEGYD-001 | Dongxin Farm, Guanyun County, Lianyungang City | 34°35′N, 119°22′E |
| 44 | LEYT-002 | Yuntai Farm, Haizhou District, Lianyungang City | 34°39′N, 119°23′E |
| 45 | LEDX-003 | Dongxin Farm, Lianyun District, Lianyungang City | 34°32′N, 119°22′E |
| 46 | LENJ-001 | Xuanwu District, Nanjing City | 30°2’N, 118°52’E |
Leptochloa chinensis (L.) Nees seed collection sites.
2.2 Herbicide treatments
Test chemicals and the manufacturers are shown in Table 2. The technical materials of cyhalofop -butyl, fenoxaprop-P-ethyl, pyraclonil, benzobicyclon, anilofos, and oxaziclomefone were individually dissolved in 1mL acetone and then diluted to a series of solutions with water containing 0.1% (v/v) Tween-80 aqueous solution.
Table 2
| Herbicide | Test doses (g a.i. ha-1) |
Manufacturer |
|---|---|---|
| 98% Cyhalofop-butyl TC | 105 | Jiangsu Agrochem Laboratory Co., Ltd., Jiangsu, China |
| 96% Fenoxaprop-P-ethyl TC | 37.5 | Jiangsu Agrochem Laboratory Co., Ltd., Jiangsu, China |
| 97% Pyraclonil TC | 210 | Hubei Xianghe Precision Chemistry Co., Ltd., Hubei, China |
| 98% Benzobicyclon TC | 225 | SDS Biotech K.K., Tokyo, Japan |
| 95% Anilofos TC | 315 | Shandong Binnong Technology Co., Ltd., Shandong, China |
| 97% Oxaziclomefone TC | 45 | Jiangsu Agrochem Laboratory Co., Ltd., Jiangsu, China |
Herbicides and manufacturers.
TC, Technical Material.
2.3 Test methods
2.3.1 Cultivation of the experimental materials
Whole-plant bioassay was used in this experiment. White plastic pots (7 cm in length and width and 9 cm in height), with holes in the bottom, were filled to approximately three-quarters of their capacity with air-dried fine previously sieved soil and placed inside a plastic turnover box. Water was added to the bottom to ensure the soil was saturated with moisture. The soil used for the tests was a loam (pH 6.7) with 1.6% organic matter content. The seeds were sown separately, covered with a thin layer of soil of approximately 0.2–0.3-cm thick and cultivated in a solar greenhouse (temperature: daytime, 25 ± 5°C, nighttime, 25 ± 5°C; light regime: 12-:12-h light/dark). Once the weeds had emerged uniformly, seedlings were thinned to 12 per pot.
2.3.2 Determination of the sensitivity of L. chinensis populations to cyhalofop-butyl
When plants reached the 2–3-leaf stage, stem and leaf spray treatments were applied. Spraying was performed with a 3WPSH-500D bioassay spray tower developed by the Nanjing Institute of Agricultural Mechanization of the Ministry of Agriculture, with a disk diameter of 50 cm, a spindle rotation speed of 6 rpm, nozzle aperture of 0.3 mm, spray pressure of 0.3 MPa, droplet diameter of 100 μm, and a nozzle flow rate of 90 mL/min. The volume of water used was 450 L ha-1. There were six experimental doses: 1/4, 1/2, 1, 2, 4, and 8 times the upper limit of the recommended field application rate of cyhalofop-butyl, which is 105 g a.i.ha-1, resulting in doses of 26.25, 52.5, 105, 210, 420, and 840 g a.i. ha-1, respectively. The herbicide doses were 6.56, 13.12, 26.24, 52.48, 104.96, and 209.92 g a.i. ha-1 for the LENJ-001 population. For each L. chinensis population, an untreated blank control was set up, and four replications per treatment were included. At 20 d after treatment, the fresh weight of the aerial parts of the L. chinensis plants was measured. Each treatment had four replicates, and the experiment was repeated twice. Excel Software was used to analyze the inhibition of biomass accumulation in the areal plant body parts. The statistical analysis software SigmaPlot v.14.0 (Systat Software, San Jose, CA, USA) was used to perform a four parameter double logistic nonlinear regression analysis on the data using the formula y = C+(D-C)/[1+(x/GR50)b] and calculate the herbicide dosage required to inhibit 50% plant growth (GR50). In the formula, x is the specific herbicide dosage, y is the relative fresh weight of weeds under x treatment, C is the lower response limit; D is the upper response limit, and b is the slope of the curve at GR50. Relative resistance index (RI) was calculated as the GR50 of the resistant population divided by the GR50 of the susceptible population. RI ≤ 2 indicates a susceptible population (S), 2 < RI ≤ 5 indicates low resistance (LR), 5 < RI ≤ 10 indicates moderate resistance (MR), and RI > 10 indicates high resistance (HR).
2.3.3 Analysis of ACCase mutations in L. chinensis populations
Based on the results of the cyhalofop-butyl-resistance test above, all populations were selected, with at least 10 plants per population. Fresh leaf tissue was sampled for genomic DNA extraction. Primers were designed according to the method described by Jiang et al. (2022) (F: 5′-GTGGTGAGGTTATTTGGATTATTGAC-3′; R: 5′-CACCTTGGAGTTTTGCTTTCAG-3′), covering seven amino acid variation sites known to contribute to ACCase inhibitors resistance (I1781, W1999, W2027, I2041, D2078, C2088, and G2096). The target fragment with 1128 bp was amplified using TaKaRa LA Taq polymerase for PCR under the following conditions: initial denaturation at 98°C for 3 min; 35 cycles at 98°C for 30 s, 56°C for 30 s, and 72°C for 30 s; followed by a final extension at 72°C for 10 min. Subsequently, the polymerase chain reaction (PCR) products were separated using electrophoresis, recovered and purified using agar gel with TIAN gelMidi Purification Kit (Tiangen, Beijing, China). The purified products were cloned into the pMD20-T vector (TaKaRa Biotechnology). The recombinant plasmids containing the gene were extracted and sequenced. In order to distinguish the genes encoding plasticity ACCase in L. chinensis, at least 20 clones were randomly selected from each biological replicate for sequencing. The resulting sequences were aligned and compared using DNAMAN v 6.0 software (Lynnon, Quebec, Canada).
2.3.4 Determination of the level of sensitivity of L. chinensis to other herbicides
When plants reached the 2–3-leaf stage, the stems and leaves were sprayed using a 3WPSH-500D bioassay spray tower described above. Information on the herbicides used in the whole-plant doseresponse experiments are showed in Table 2. An untreated blank control was prepared for each L. chinensis population; each treatment included four replicates and the experiment was conducted in duplicate. At 20 d after treatment, fresh weight of the aerial parts of L. chinensis was measured, and the rate of inhibition of biomass accumulation was analyzed using Microsoft Excel.
3 Results
3.1 Differences in the sensitivity of L. chinensis populations to cyhalofop-butyl
A whole-plant bioassay was used to determine the sensitivity of 46 L. chinensis populations collected from various areas of Jiangsu Province to cyhalofop-butyl, a commonly used aryloxyphenoxypropionate herbicide in paddy fields. The results (Table 3) indicated that 41 out of the 46 L. chinensis populations exhibited 2.07- to 14.58 times resistance to cyhalofop-butyl. Although the GR50 values of LESE-001, LESZ-005, LEJY-001, LELY-001 populations were lower than 50% of the field dose, they were still classified as LR. Several researchers think that if for 50% growth inhibition of the population less than ½ of the recommended herbicide dose is sufficient, then it is not a resistant population. Based this rule, the LESE-001, LESZ-005, LEJY-001, LELY-001 populations should be classified as S. The area south of the Yangtze River in Jiangsu Province was defined as the southern Jiangsu region, the area from the Yangtze River to the northern Jiangsu main irrigation canal was defined as the Central Jiangsu region, and the area north of the northern Jiangsu main irrigation canal was defined as the northern Jiangsu region (Figure 1). Analysis of the distribution of herbicide resistance across different geographic populations in Jiangsu Province revealed that 7 out of 11 populations in the southern Jiangsu region had 2.07- to 4.96 times resistance, with 63.64% of the populations showing resistance. Meanwhile, in the Central Jiangsu region, all 19 populations showed 4.96- to 11.09 times resistance to cyhalofop-butyl, with 100% of the populations being resistant. In turn, in the Northern Jiangsu region, all 15 populations showed 5.57- to 14.58 times resistance, i.e., again, 100% of the populations were resistant.
Table 3
| Entry | Population | Logistic equation | Correlation coefficient | GR50 g a.i. ha-1 |
Susceptibility | Mutation | Frequency of plants with mutation(s) % | RI* |
|---|---|---|---|---|---|---|---|---|
| 1 | LESZ-001 | y=-9.1964 + 109.4264/ (1+(x/37.0971) -2.2368) |
1.0000 | 37.0971 | LR | WT | – | 2.53 |
| 2 | LESZ-003 | y=-53.6186 + 154.2167/(1+(x/23.7359) -1.7999) | 0.9999 | 23.7359 | S | – | – | 1.62 |
| 3 | LESZ-005 | y=-17.2467 + 117.5376/(1+(x/30.3213)-1.9665) | 0.9999 | 30.3213 | LR | WT | – | 2.07 |
| 4 | LEJY-001 | y=6.8889 + 93.1705/ (1+(x/39.2875) -2.6445) |
1.0000 | 39.2875 | LR | WT | – | 2.68 |
| 5 | LEHS-003 | y=25.2840 + 74.8558/ (1+(x/63.3494)-4.3050) |
1.0000 | 63.3494 | LR | WT | – | 4.32 |
| 6 | LEYX-003 | y=-28.2482 + 128.5793/(1+(x/26.9161)-1.7531) | 0.9993 | 26.9161 | S | – | – | 1.83 |
| 7 | LELY-003 | y=-10.4796 + 110.8085/(1+(x/31.4017)-2.0106) | 1.0000 | 31.4017 | LR | WT | – | 2.14 |
| 8 | LEJT-001 | y=-94.2302 + 194.3745/(1+(x/18.5684)-1.7307) | 0.9990 | 18.5648 | S | – | – | 1.27 |
| 9 | LELS-001 | y=16.7391 + 78.8823/ (1+(x/79.5397)-3.4533) |
0.9902 | 79.5397 | MR | W2027C | 90 | 5.42 |
| 10 | LEJR-001 | y=11.9962 + 82.8761/ (1+(x/72.7335)-2.5779) |
0.9833 | 72.7335 | LR | WT | – | 4.96 |
| 11 | LEJR-002 | y=-129.5258 + 229.5656/ (1+(x/16.1023)-1.6053) |
0.9965 | 16.1023 | S | – | – | 1.10 |
| 12 | LELH-003 | y=14.6115 + 80.9054/ (1+(x/73.0230)-3.4343) |
0.9921 | 73.0230 | LR | WT | – | 4.98 |
| 13 | LEYZ-004 | y=18.1072 + 76.2527/ (1+(x/87.7839)-5.4614) |
0.9861 | 87.7839 | MR | W2027C | 100 | 5.98 |
| 14 | LEYL-001 | y=8.4036 + 79.0351/ (1+(x/82.1600)-3.5896) |
0.9651 | 82.1600 | MR | W2027C | 100 | 5.60 |
| 15 | LEJD-001 | y=9.4320 + 88.7853/ (1+(x/72.8151)^-2.2299) |
0.9966 | 72.8151 | LR | WT | – | 4.96 |
| 16 | LEJD-003 | y=-5.4165 + 101.7864/ (1+(x/104.7670)-1.2963) |
0.9928 | 104.7670 | MR | W2027C | 100 | 7.14 |
| 17 | LEGY-001 | y=7.7252 + 84.6088/ (1+(x/75.8842)-2.6572) |
0.987 | 75.8842 | MR | W2027L | 80 | 5.17 |
| 18 | LEGY-002 | y=8.3153 + 87.4225/ (1+(x/77.1450)-2.5675) |
0.9898 | 77.1450 | MR | I2041N | 70 | 5.26 |
| 19 | LERG-001 | y=9.0839 + 85.7091/ (1+(x/85.0290)-2.4508) |
0.9788 | 85.0290 | MR | W1999C | 70 | 5.79 |
| 20 | LERG-002 | y=6.5286 + 92.8018/ (1+(x/82.4145)-1.8677) |
0.9875 | 82.4145 | MR | WT | – | 5.62 |
| 21 | LERG-003 | y=-0.7142 + 93.4364/ (1+(x/102.3075)-1.6654) |
0.9918 | 102.3075 | MR | W2027C | 100 | 6.97 |
| 22 | LEJJ-001 | y=-12.4920 + 104.4448/(1+(x/82.3830)-1.3574) | 0.9800 | 82.3830 | MR | I1781L | 90 | 5.61 |
| 23 | LETX-002 | y=11.9585 + 81.4586/ (1+(x/76.1895)-3.6082) |
0.9846 | 76.1895 | MR | W2027C | 90 | 5.19 |
| 24 | LEXH-002 | y=4.7908 + 84.3971/ (1+(x/82.0110)-2.8081) |
0.9862 | 82.0110 | MR | W1999C | 90 | 5.59 |
| 25 | LEXH-003 | y=6.6055 + 84.8186/ (1+(x/102.8400)-2.3092) |
0.9964 | 102.8400 | MR | W2027C | 100 | 7.01 |
| 26 | LEXH-006 | y=6.1199 + 95.4984/ (1+(x/74.3895)-1.6790) |
0.9986 | 74.3895 | MR | W1999C | 90 | 5.07 |
| 27 | LEHQ-001 | y=7.6368 + 82.5764/ (1+(x/83.4090)-2.7359) |
0.9876 | 83.4090 | MR | W2027C | 100 | 5.68 |
| 28 | LEDF-007 | y=4.5508 + 93.8466/ (1+(x/162.8010)-1.7952) |
0.9995 | 162.8010 | HR | W2027S | 100 | 11.09 |
| 29 | LESY-005 | y=7.2280 + 88.2909/ (1+(x/81.0360)-3.1872) |
0.9928 | 81.0360 | MR | W2027C | 90 | 5.52 |
| 30 | LEFN-001 | y=0.9214 + 93.8226/ (1+(x/81.9435)-1.8817) |
0.9978 | 81.9435 | MR | W2027C | 100 | 5.58 |
| 31 | LEHX-001 | y=3.6921 + 84.0791/ (1+(x/167.4600)-1.9868) |
0.9995 | 167.4600 | HR | W2027C | 100 | 11.41 |
| 32 | LEHX-002 | y=-1.0350 + 105.9160/ (1+(x/156.9210)-1.2119) |
0.9995 | 156.9210 | HR | W1999C | 100 | 10.69 |
| 33 | LEBH-001 | y=7.8959 + 84.2202/ (1+(x/207.4485)-2.0099) |
0.9979 | 207.4485 | HR | W2027C | 100 | 14.14 |
| 34 | LEHH-001 | y=1.9036 + 95.0790/ (1+(x/150.8805)-1.6903) |
0.9976 | 150.8805 | HR | W2027C | 90 | 10.28 |
| 35 | LESQ-001 | y=7.2445 + 87.2385/ (1+(x/149.8895)-1.7711) |
0.9976 | 149.8995 | HR | W2027C | 100 | 10.21 |
| 36 | LESQ-002 | y=-2.6873 + 121.1010/ (1+(x/213.9915)-0.9235) |
0.907 | 213.9915 | HR | W2027C | 100 | 14.58 |
| 37 | LESQ-003 | y=6.3374 + 88.2822/ (1+(x/121.3575)-1.6015) |
0.9878 | 121.3575 | MR | W1999C | 100 | 8.27 |
| 38 | LESN-001 | y=4.5054 + 93.4969/ (1+(x/106.6470)-1.5236) |
0.998 | 106.6470 | MR | WT | – | 7.27 |
| 39 | LESN-002 | y=-26.3543 + 138.2501/(1+(x/97.4535)-0.8147) | 0.9994 | 97.4535 | MR | W1999C | 100 | 6.64 |
| 40 | LELSH-001 | y=-16.0025 + 116.9730/(1+(x/81.7770)-1.1393) | 0.9951 | 81.7770 | MR | WT | – | 5.57 |
| 41 | LEBYH-001 | y=6.5731 + 85.5943/ (1+(x/202.1970)-1.7603) |
0.9985 | 202.1970 | HR | W1999C | 100 | 13.78 |
| 42 | LEGY-001 | y=1.4817 + 94.8060/ (1+(x/147.0945)-1.3893) |
0.9973 | 147.0945 | HR | W2027C | 100 | 10.02 |
| 43 | LEGYD-001 | y=4.1636 + 95.5794/ (1+(x/203.1885)-1.5127) |
0.9963 | 203.1885 | HR | W1999C | 100 | 13.85 |
| 44 | LEYT-002 | y=-6.0716 + 101.6062/ (1+(x/121.9425)-1.3588) |
0.9993 | 121.9425 | HR | D2078G | 100 | 8.31 |
| 45 | LEDX-003 | y=-7.9602 + 104.9971/ (1+(x/126.5910)-1.1405) |
0.9998 | 126.5910 | HR | W2027 | 100 | 8.63 |
| 46 | LENJ-001 | y=-167.1343 + 267.6653/(1+(x/14.6750)-1.7750) | 0.9999 | 14.6750 | S |
Sensitivity of different geographical populations of Leptochloa chinensis (L.) Nees, to cyhalofop-butyl.
*Resistance index value.
Figure 1
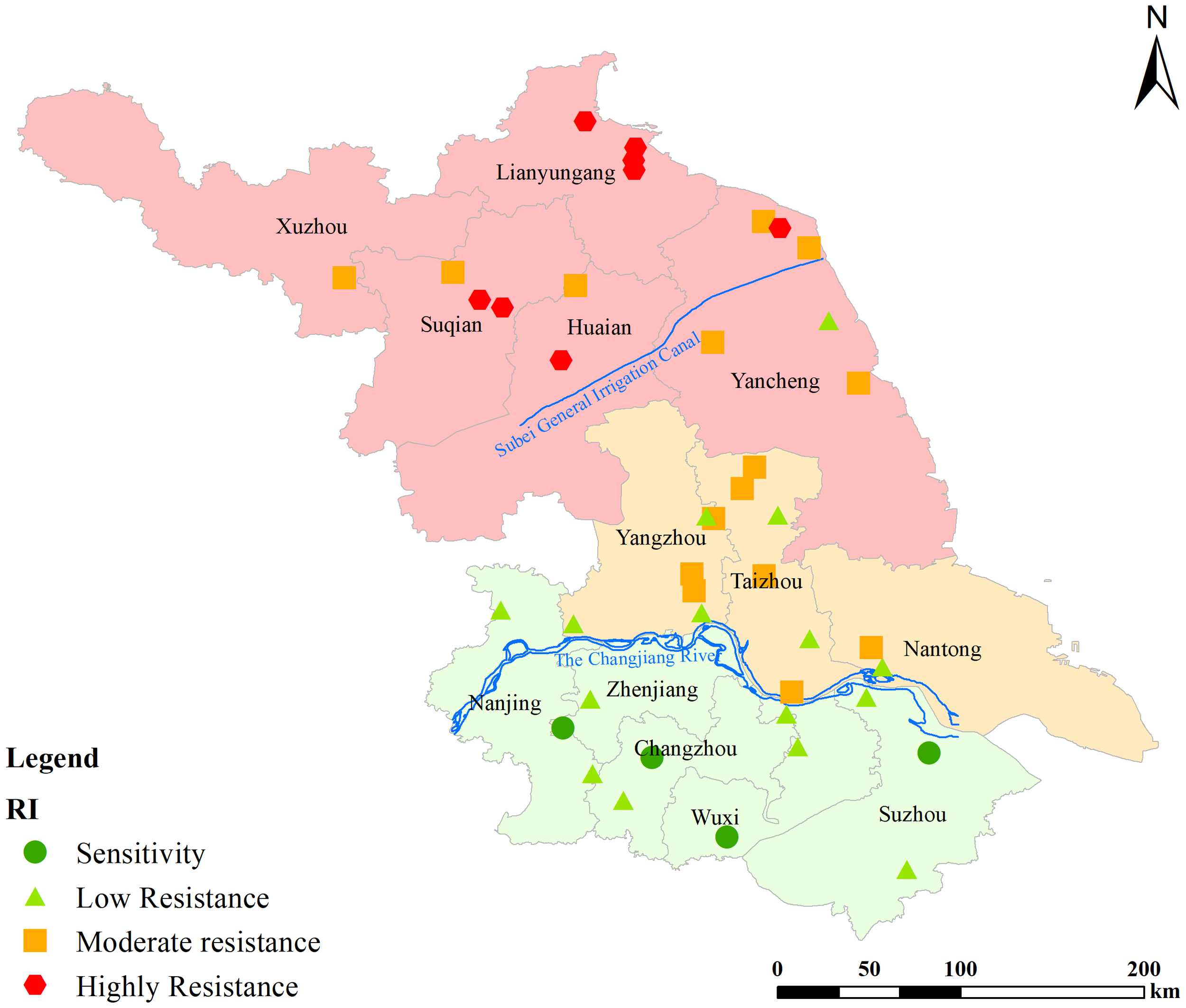
Distribution of different geographical populations of Leptochloa chinensis (L.) Nees in Jiangsu Province, China.
3.2 Analysis of ACCase mutations in resistant population of L. chinesis
An amplified sequence fragment of 1,128 bp including seven known mutation sites showed >96% homology with the corresponding genes of Alopecurus myosuroides, a species closely related to L. chinensis. A comparison of the translated amino acid sequences with those of A. myosuroides revealed mutations I1781L, W1999C, W2027C/L/S, I2041N, and D2078G in ACCase of cyhalofop-butyl-resistant L. chinensis populations in the paddy fields of Jiangsu Province (Table 3); however, no mutations were detected at the C2088 or G2096 sites. Mutations W1999C and W2027C were relatively prevalent, whereas mutations I1781L, W2027L/S, I2041N, and D2078G were each found in a different population.
No resistance mutations were observed in LR populations with an RI of 4.98 or below. Similarly, no resistance mutations were detected in the LELSH-001 population with an RI of 5.57, or the LESN-001 population, with an RI of 7.27. The LELSH-001 and LESN-001 populations ranked as MR to cyhalofop-butyl without any mutations in the ACCase amino acid sequence, suggesting that resistance may be attributed to non-target site mechanisms or enhanced ACCase expression. The exact causes of this resistance warrant further investigation (Table 3).
3.3 Cross resistance and multiple resistance in L. chinensis populations
After treatment with fenoxaprop-P-ethyl, another member of the aryloxyphenoxypropionate family of ACCase inhibitor herbicides, at 37.5 g a.i. ha-1, the inhibition rates of biomass accumulation in the aerial parts of the LEJY-001, LELY-003, and LEJR-002 populations were all >80%, whereas those of the remaining 43 populations were all <80% (Table 4). Among the 30 populations with a biomass inhibition rate of <50% following treatment with fenoxaprop-P-ethyl at 37.5 g a.i. ha-1, 15 were from the northern Jiangsu region and 15 from the Central Jiangsu region, accounting for 100% of the total number of populations in northern and 75% in Central Jiangsu, respectively.
Table 4
| Population | Inhibition (%) of biomass accumulation | Population | Inhibition (%) of biomass accumulation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fenoxaprop-P-ethyl | Pyraclonil | Benzobicyclon | Anilofos | Oxaziclomefone | Fenoxaprop-P-ethyl | Pyraclonil | Benzobicyclon | Anilofos | Oxaziclomefone | ||
| LESZ-001 | 71.25 | 100 | 100 | 100 | 100 | LEXH-002 | 25.45 | 100 | 100 | 100 | 100 |
| LESZ-003 | 68.65 | 100 | 100 | 100 | 100 | LEXH-003 | 36.45 | 100 | 100 | 100 | 98.75 |
| LESZ-005 | 75.62 | 100 | 100 | 100 | 100 | LEXH-006 | 48.25 | 100 | 100 | 100 | 100 |
| LEJY-001 | 80.36 | 100 | 100 | 100 | 100 | LEHQ-001 | 22.35 | 100 | 100 | 100 | 97.75 |
| LEHS-003 | 76.95 | 100 | 100 | 100 | 100 | LEDF-007 | 19.85 | 100 | 100 | 100 | 100 |
| LEYX-003 | 66.35 | 100 | 100 | 100 | 100 | LESY-005 | 35.68 | 100 | 100 | 100 | 99.85 |
| LELY-003 | 80.25 | 100 | 100 | 100 | 100 | LEFN-001 | 42.35 | 100 | 100 | 100 | 100 |
| LEJT-001 | 100 | 100 | 100 | 100 | 100 | LEHX-001 | 12.35 | 100 | 100 | 100 | 100 |
| LELS-001 | 60.55 | 100 | 100 | 100 | 100 | LEHX-002 | 15.47 | 100 | 100 | 100 | 92.35 |
| LEJR-001 | 77.50 | 100 | 100 | 100 | 100 | LEBH-001 | 24.78 | 100 | 100 | 100 | 100 |
| LEJR-002 | 100 | 100 | 100 | 100 | 100 | LEHH-001 | 25.65 | 100 | 100 | 100 | 90.05 |
| LELH-003 | 54.45 | 100 | 100 | 100 | 95.45 | LESQ-001 | 28.35 | 100 | 100 | 100 | 100 |
| LEYZ-004 | 45.35 | 100 | 100 | 100 | 97.20 | LESQ-002 | 15.65 | 100 | 100 | 100 | 100 |
| LEYL-001 | 50.35 | 100 | 100 | 100 | 92.35 | LESQ-003 | 12.85 | 100 | 100 | 100 | 94.35 |
| LEJD-001 | 60.21 | 100 | 100 | 100 | 98.85 | LESN-001 | 34.62 | 100 | 100 | 100 | 100 |
| LEJD-003 | 45.85 | 100 | 100 | 100 | 100 | LESN-002 | 29.63 | 100 | 100 | 100 | 100 |
| LEGY-001 | 52.36 | 100 | 100 | 100 | 95.35 | LELSH-001 | 21.35 | 100 | 100 | 100 | 100 |
| LEGY-002 | 44.38 | 100 | 100 | 100 | 100 | LEBYH-001 | 18.75 | 100 | 100 | 100 | 97.55 |
| LERG-001 | 35.86 | 100 | 100 | 100 | 95.8 | LEGY-001 | 34.15 | 100 | 100 | 100 | 100 |
| LERG-002 | 41.25 | 100 | 100 | 100 | 100 | LEGYD-001 | 40.35 | 100 | 100 | 100 | 100 |
| LERG-003 | 33.68 | 100 | 100 | 100 | 96.85 | LEYT-002 | 25.68 | 100 | 100 | 100 | 95.8 |
| LEJJ-001 | 36.85 | 100 | 100 | 100 | 90.35 | LEDX-003 | 19.68 | 100 | 100 | 100 | 100 |
| LETX-002 | 42.85 | 100 | 100 | 100 | 100 | LENJ-001 | 100 | 100 | 100 | 100 | 100 |
Biomass reduction of different geographical populations of Leptochloa chinensis (L.) Nees by herbicide treatment.
Treatment with protoporphyrinogen oxidase-inhibitor herbicide pyraclonil at 210 g a.i. ha-1, novel bicyclooctane herbicide benzobicyclon at 225 g a.i. ha-1, and organophosphorus herbicide anilofos, at 315 g a.i. ha-1 resulted in 100% inhibition of biomass accumulation across all 46 L. chinensis populations in Jiangsu Province.
Treatment of L. chinensis with 45 g a.i. ha-1 of the organoheterocyclic herbicide oxaziclomefone at the two-leaf stage led to <97% inhibition of biomass accumulation in 10 out of the 46 L. chinensis populations collected from Jiangsu Province, including six populations located in Central and four in northern Jiangsu, whereas the other 36 populations showed 100% inhibition of biomass accumulation.
4 Discussion
Cyhalofop-butyl and fenoxaprop-P-ethyl are ACCase inhibitor herbicides (Wei et al., 2015) that have been used continuously for many years in Jiangsu Province as common herbicidal options for controlling Poaceae weeds in paddy fields. In this study, we found that 41 out of the 46 geographical populations of L. chinensis present in Jiangsu Province have developed significant resistance to cyhalofop-butyl, and 43 showed significant cross-resistance to fenoxaprop-P-ethyl, with resistance rates reaching 91%–93%. This explains the failure of conventional recommended dosages of cyhalofop-butyl and fenoxaprop-P-ethyl in effectively controlling L. chinensis infestations in many areas of Jiangsu Province. The results of resistance distribution of different geographical populations in Jiangsu Province showed that 63.64% of the resistance populations were in southern Jiangsu Province. In the middle of Jiangsu Province, the resistant population accounted for 100%. In northern Jiangsu, the resistant population of L. chinensis accounted for 100%. Furthermore, we observed a gradual increase trend in the proportion of L. chinensis populations resistant to ACCase inhibitor herbicides from the southern to the northern region of Jiangsu, possibly owing to the higher frequency of use of these herbicides in the latter (Zhu et al., 2024).
Research evidence indicates that target-site resistance mechanisms, such as mutations or increased expression of ACCase, along with non-target-site resistance mediated by enhanced metabolism, are the two primary mechanisms underlying weed resistance to ACCase inhibitor herbicides (Han et al., 2016; Wang et al., 2013). Often, target-site mutations within ACCase are the principal cause of herbicide resistance (Beckie and Tardif, 2012). Thus, to date, 14 types of amino acid mutations at seven amino acid sites within the ACCase carboxyltransferase (CT) domain have been identified in weeds that are resistant to ACCase inhibitor herbicides, namely, I1781L, I1781V, I1781T, W1999C/S/L, W2027C, I2041N, I2041N, D2078G, C2088R, G2096A, and G2096S (Beckie and Tardif, 2012; Délye et al., 2005; Guo et al., 2017; Powles and Yu, 2010). In this study, we identified only previously reported resistance mutations within the ACCase CT domain of resistant L. chinensis populations and did not detect any new type of mutation. Previous research has shown that mutations at multiple sites can coexist within the same resistant weed species. For instance, Zhang and Powles (2006) reported six different amino acid mutations in the ACCase CT domain of resistant Italian ryegrass, while Yu et al. (2013) found three distinct ACCase mutations on a single plant of wild oats. Furthermore, Phongphitak et al. (2014) conducted a cross-breeding experiment with fenoxaprop-P-ethyl-resistant L. chinensis in Kedah, Malaysia, and found that resistance in this case might be controlled by a single gene. Similarly, Yu et al. (2017) discovered a mutation from tryptophan to cysteine at site 2027 in L. chinensis populations highly resistant to cyhalofop-butyl, marking the first case of detection of a target-gene mutation in this weed species, with the mutation rate exceeding 50%. Additionally, Wu et al. (2019) identified a replacement of tryptophan with serine at site 1999 in the ACCase CT domain of cyhalofop-butyl-resistant L. chinensis populations in paddy fields in East China, possibly one of the important factors leading to the observed resistance. So far, Ile-1781-Leu, Leu-1818-Phe, Trp-1999-Cys, Trp-2027-Cys, Trp-2027-Gly, Trp-2027-Leu, Trp-2027-Ser, Ile-2041-Asn, Cys-2088-Arg, and Gly-2096-Ala, in ACCase have been identified as the primary cause of L. chinensis resistance to Cyhalofop-butyl (Kurniadie et al., 2022; Zhang et al., 2022; Zhao et al., 2022; Liao et al., 2024; Deng et al., 2023; Jiang et al., 2024). In this study, resistant L. chinensis populations in Jiangsu Province showed mutations I1781L, W1999C, W2027C/L/S, I2041N, and D2078G in ACCase, with no mutations found at the C2088 or G2096 sites. In particular, the W1999C and W2027C mutations were relatively prevalent, whereas I1781L, W2027L/S, I2041N, and D2078G each occurred in a different population. Gene mutations of the target enzyme ACCase are a crucial mechanism underlying the resistance of L. chinensis populations to cyhalofop-butyl and fenoxaprop-P-ethyl in the paddy fields of Jiangsu province. Nonetheless, the LELSH-001 and LEHX-002 populations, which showed no ACCase amino acid mutations, were categorized as MR to cyhalofop-butyl, suggesting that their resistance may result from non-target mechanisms or enhanced ACCase expression. It had been found that the GSTF1, GSTU1, GSTU6, CYP707A5, CYP71C4, and CYP 71C1 genes expressed highly in the R population may be responsible for cyhalofop-butyl resistance in L. chinensis. (Zhang et al., 2022; Cao et al., 2023). The causes of resistance to cyhalofop-butyl in the LELSH-001 and LEHX-002 populations need to further investigation. All 41 cyhalofop-butyl-resistant L. chinensis populations collected in Jiangsu Province were susceptible to oxaziclomefone, with the recommended doses achieving over 90% control efficacy. Similarly, the regular recommended doses of pyraclonil, benzobicyclon, and anilofos showed 100% efficacy in inhibiting biomass accumulation in the 46 geographic populations of L. chinensis in Jiangsu Province studied herein, effectively controlling the weed.
Scientific rotation of herbicide types with different mechanisms of action is an effective strategy for controlling noxious weeds and preventing herbicide-resistance development (Evans et al., 2016; Tranel and Wright, 2002). This study revealed that L. chinensis populations across different regions of Jiangsu province have developed significant resistance to two members of the aryloxyphenoxypropionate family of ACCase inhibiting herbicides, cyhalofop-butyl and fenoxaprop-P-ethyl. However, all populations were susceptible to the PPO-inhibitor herbicide pyraclonil, the novel bicyclooctane herbicide benzobicyclon, the organophosphorus herbicide anilofos, and the organoheterocyclic herbicide oxaziclomefone. These findings are consistent with those reported by Gu et al. (2021) and Tian et al. (2022), who noted that pyraclonil and benzobicyclon could be used for resistance management in L. chinensis. Therefore, using either cyhalofop-butyl or fenoxaprop-P-ethyl alone should be minimized in agricultural practice. Instead, adopting tank mixes and rotation strategies using pyraclonil, benzobicyclon, anilofos, and oxaziclomefone could help delay the development and spread of herbicide resistance in L. chinensis populations.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
PX: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. KW: Data curation, Writing – review & editing. YJ: Formal Analysis, Writing – review & editing. YF: Writing – review & editing. AZ: Investigation, Writing – review & editing. KC: Investigation, Writing – review & editing. HW: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Jiangsu Agricultural Science and Technology Innovation Fund (grant number CX(22)5003), and by the National Natural Science Foundation of China (grant number 31801755).
Acknowledgments
This is a short text to acknowledge the contributions of specific colleagues, institutions, or agencies that aided the efforts of the authors.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Beckie H. J. Tardif F. J. (2012). Herbicide cross resistance in weeds. Crop Prot.35, 15–28. doi: 10.1016/j.cropro.2011.12.018
2
Cao Z. Liu M. Cao W. Zhan Y. Bai L. Pan L. (2023). A glutathione S-transferase and a cytochrome P450 may confer cyhalofop-butyl resistance in Leptochloa chinensis (L.) Nees. Pestic. Biochem. Physiol.197, 105691. doi: 10.1016/j.pestbp.2023.105691
3
Chauhan B. S. Johnson D. E. (2008). Germination ecology of Chinese sprangletop (Leptochloa chinensis) in the Philippines. Weed Sci.56, 820–825. doi: 10.1614/WS-08-070.1
4
Délye C. Zhang X. Q. Michel S. Matéjicek A. M. B. Powles S. B. (2005). Molecular bases for sensitivity to acetyl-coenzyme A carboxylase inhibitors in black-grass. Plant Physiol.137, 794–806. doi: 10.1104/pp.104.046144
5
Deng W. Cai J. Zhang J. Chen Y. Y. Chen Y. R. Di Y. J. et al . (2019). Molecular basis of resistance to ACCase-inhibiting herbicide cyhalofop-butyl in Chinese sprangletop (Leptochloa chinensis (L.) Nees) from China. Pestic. Biochem. Physiol.158, 143–148. doi: 10.1016/j.pestbp.2019.05.004
6
Deng W. Li Y. Yao S. Wu J. Zhu A. Yang Q. et al . (2023). Current status of cyhalofop-butyl and metamifop resistance and diversity of the ACCase gene mutations in Chinese sprangletop (Leptochloa chinensis) from China. Pestic. Biochem. Physiol.200, 105826. doi: 10.1016/j.pestbp.2023.105648
7
Evans J. A. Tranel P. J. Hager A. G. Schutte B. Wu C. Chatham L. A. et al . (2016). Managing the evolution of herbicide resistance. Pest Manage. Sci.72, 74–80. doi: 10.1002/ps.4009
8
Gu H. P. Yuan G. H. Gao Y. Tian Z. H. (2021). Safety and herbicidal activity of 4% pyraclonil in direct-seeding rice field. Acta Agric. Shanghai.37, 68–73. doi: 10.15955/j.issn1000-3924.2021.04.13
9
Guo W. L. Zhang L. L. Wang H. Z. Li Q. Liu W. T. Wang J. X. (2017). A rare Ile-2041-Thr mutation in the ACCase gene confers resistance to ACCase-inhibiting herbicides in shortawn foxtail (Alopecurus aequalis). Weed Sci.65, 239–246. doi: 10.1017/wsc.2016.32
10
Han H. P. Yu Q. Owen M. J. Cawthray G. R. Powles S. B. (2016). Widespread occurrence of both metabolic and target-site herbicide resistance mechanisms in Lolium rigidum populations. Pest Manage. Sci.72, 255–263. doi: 10.1002/ps.3995
11
Heap I. (2024). The international survey of herbicide resistant weeds. Available online at: http://www.weedscience.org (Accessed 13 May 2024).
12
Heap I. Knight R. (1986). The occurrence of herbicide cross-resistance in a population of annual ryegrass, Lolium rigidum, resistant to diclofop-methyl. Aust. J. Agric. Res.37, 149–156. doi: 10.1071/AR9860149
13
Jiang M. Wang X. Hu W. Wang Z. Guan H. Zhao N. et al . (2024). A novel mutation Trp-2027-Gly in acetyl-CoA carboxylase confers resistance to cyhalofop-butyl in Chinese sprangletop (Leptochloa chinensis). Pest Manage. Sci.80, 6243–6250. doi: 10.1002/ps.8353
14
Jiang M. H. Wang Y. F. Li W. Li Q. Zhang J. X. Liao M. et al . (2022). Investigating resistance levels to cyhalofop-butyl and mechanisms involved in Chinese sprangletop (Leptochloa chinensis L.) from Anhui Province, China. Pestic. Biochem. Physiol.186, 105165. doi: 10.1016/j.pestbp.2022.105165
15
Kurniadie D. Widianto R. Aprilia A. N. Damayanti F. (2022). Confirmation of the mechanisms of resistance to ACCase-inhibiting herbicides in Chinese Sprangletop (Leptochloa chinensis (L.) Nees) from South Sulawesi, Indonesia. Agronomy12, 3152. doi: 10.3390/agronomy12123152
16
Liao M. Jiang M. Wang X. Hu W. Zhao N. Cao H. (2024). The Cys-2088-Arg mutation in the ACCase gene and enhanced metabolism confer cyhalofop-butyl resistance in Chinese sprangletop (Leptochloa chinensis). Pestic. Biochem. Physiol.200, 105826. doi: 10.1016/j.pestbp.2024.105826
17
Maneechote C. Samanwong S. Zhang X. Q. Powles S. B. (2005). Resistance to ACCase-inhibiting herbicides in sprangletop (Leptochloa chinensis). Weed Sci.53, 290–295. doi: 10.1614/WS-04-164R
18
Peng Y. J. Pan L. Liu D. C. Cheng X. M. Ma G. L. Li S. F. et al . (2020). Confirmation and characterization of cyhalofop-butyl-resistant Chinese sprangletop (Leptochloa chinensis) populations from China. Weed Sci.68, 253–259. doi: 10.1017/wsc.2020.15
19
Phongphitak E. Maneechote C. Rerkasem B. Jamjod S. (2014). Inheritance of resistance to fenoxaprop-p-ethyl in sprangletop (Leptochloa chinensis L. Nees). Weed Biol. Manage.14, 159–166. doi: 10.1111/wbm.12043
20
Powles S. B. Yu Q. (2010). Evolution in action: plants resistant to herbicides. Annu. Rev. Plant Biol.61, 317–347. doi: 10.1146/annurev-arplant-042809-112119
21
Rahman M. M. Islam M. A. Sofian-Azirun M. Amru N. B. Ismail S. (2014). Control measures of sprangletop (Leptochloa chinensis) resistant biotype using propanil, quinclorac and cyhalofop-butyl. Int. J. Agric. Biol.16, 801–806. Available at: http://www.fspublishers.org (Accessed June 01, 2014).
22
Ruiz-Santaella J. P. Heredia A. Prado R. D. (2006). Basis of selectivity of cyhalofop-butyl in Oryza sativa L. Planta223, 191–199. doi: 10.1007/s00425-005-0075-1
23
Sahid I. B. Karso J. Chuah T. S. (2011). Resistance mechanism of Leptochloa chinensis Nees to propanil. Weed Biol. Manage.11, 57–63. doi: 10.1111/j.1445-6664.2011.00405.x
24
Tian Z. H. Yuan G. H. Gao Y. Shen G. H. (2022). Application status, problems and suggestions of benzobicyclon in rice field in China. Plant Prot.48, 248–254. doi: 10.16688/j.zwbh.2022298
25
Tranel P. J. Wright T. R. (2002). Resistance of weeds to ALS-inhibiting herbicides: what have we learned? Weed Sci.50, 700–712. doi: 10.1614/0043-1745(2002)050[0700:RROWTA]2.0.CO;2
26
Wang H. Li J. Lv B. Lou Y. Dong L. (2013). The role of cytochrome P450 monooxygenase in the different responses to fenoxaprop-P-ethyl in annual bluegrass (Poa annua L.) and short awned foxtail (Alopecurus aequalis Sobol.). Pestic. Biochem. Physiol.107, 334–342. doi: 10.1016/j.pestbp.2013.10.001
27
Wei S. Li P. Ji M. Dong Q. Wang H. (2015). Target-site resistance to bensulfuron-methyl in Sagittaria trifolia L. populations. Pestic. Biochem. Physiol.124, 81–85. doi: 10.1016/j.pestbp.2015.05.001
28
Wen M. Q. Zhou X. M. Liu J. Zhou Y. Mo B. C. Zhu Z. Y. (2017). Resistance of Leptochloa chinensis (L.) Nees to cyhalofop-butyl in rice field of direct seeding and resistance biochemical mechanism research. J. South. Agric.48, 647–652. doi: 10.3969/j.issn.2095-1191.2017.04.013
29
Wu X. W. Wang F. G. Cao Q. (2019). Resistance of Leptochloa chinensis populations to cyhalofop-butyl in rice fields of eastern China. Chin. J. Pestic. Sci.21, 285–290. doi: 10.16801/j.issn.1008-7303.2019.0049
30
Wu S. Zhang J. L. Li B. T. Tang L. M. (2015). Influence of Leptochloa chinensis on the growth of paddy rice and its economic threshold. Sci. Agric. Sin.48, 469–478. doi: 10.3864/j.issn.0578-1752.2015.03.07
31
Yu Q. Ahmad-Hamdani M. S. Han H. Christoffers M. J. Powles S. B. (2013). Herbicide resistance-endowing ACCase gene mutations in hexaploid wild oat (Avena fatua): insights into resistance evolution in a hexaploid species. Heredity110, 220–231. doi: 10.1038/hdy.2012.69
32
Yu J. X. Gao H. T. Pan L. Yao Z. W. Dong L. Y. (2017). Mechanism of resistance to cyhalofop-butyl in Chinese sprangletop (Leptochloa chinensis (L.) Nees). Pestic. Biochem. Physiol.143, 306–311. doi: 10.1016/j.pestbp.2016.11.001
33
Yuan G. H. Tian Z. H. Gao Y. Shen G. H. (2022). Resistance status of Leptochloa chinensis to three acetyl-CoA carboxylase (ACCase) inhibitors in rice fields in Shanghai and involved ACCase gene mutations. Chin. J. Pestic. Sci.24, 492–500. doi: 10.16801/j.issn.1008-7303.2022.0012
34
Zhang Y. Chen L. Song W. Cang T. Xu M. Wu C. (2022). Diverse mechanisms associated with cyhalofop-butyl resistance in Chinese sprangletop (Leptochloa chinensis (L.) Nees): Characterization of target-site mutations and metabolic resistance-related genes in two resistant populations. Front. Plant Sci.13. doi: 10.3389/fpls.2022.990085
35
Zhang X. Q. Powles S. B. (2006). Six amino acid substitutions in the carboxyl-transferase domain of the plastidic acetyl-CoA carboxylase gene are linked with resistance to herbicides in a Lolium rigidum population. New Phytol.172, 636–645. doi: 10.1111/j.1469-8137.2006.01879.x
36
Zhao N. Jiang M. Li Q. Gao Q. Zhang J. Liao M. et al . (2022). Cyhalofop-butyl resistance conferred by a novel Trp-2027-Leu mutation of acetyl-CoA carboxylase and enhanced metabolism in Leptochloa chinensis. Pest Manage. Sci.78, 1176–1186. doi: 10.1002/ps.6734
37
Zhu A. Shen Q. Chen L. Jia Y. Zhou J. Yu J. et al . (2024). Occurrence characteristics and control countermeasures of weeds in rice fields of Jiangsu Province in 2022. China Plant Protec.44, 32–38. doi: 10.3969/j.issn.1672-6820.2024.07.005
38
Zhu W. D. Zhou P. G. He Y. H. Yang J. Lin R. H. Qi W. Q. et al . (2018). Influence of Leptochloa chinensis (L.) Nees on growth and yield properties of rice and its economic threshold of control. J. South. Agric.49, 863–869. doi: 10.3969/j.issn.2095-1191.2018.05.06
Summary
Keywords
Leptochloa chinensis (L.) Nees, geographical populations, herbicide, cyhalofop-butyl, sensitivity, target mutation
Citation
Xu P, Wang K, Ju Y, Fu Y, Zhu A, Cao K and Wang H (2025) Herbicide resistance in Leptochloa chinensis (L.) Nees populations from different regions of Jiangsu Province, China: sensitivity differences and underlying mechanisms. Front. Plant Sci. 16:1535877. doi: 10.3389/fpls.2025.1535877
Received
28 November 2024
Accepted
20 January 2025
Published
04 February 2025
Volume
16 - 2025
Edited by
Ye Liu, Nanjing Agricultural University, China
Reviewed by
Minghao Jiang, Anhui Agricultural University, China
Marta Stankiewicz-Kosyl, Warsaw University of Life Sciences, Poland
Updates
Copyright
© 2025 Xu, Wang, Ju, Fu, Zhu, Cao and Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongchun Wang, whc23@jaas.ac.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.