- 1State Key Laboratory of Environmental Criteria and Risk Assessment, Chinese Research Academy of Environmental Sciences, Beijing, China
- 2National Center for Science & Technology Evaluation, Beijing, China
- 3College of Environmental Science and Engineering, Tongji University, Shanghai, China
In situ plant–fungal combined remediation technology for arsenic (As)-contaminated soil has emerged as a dominant technology in soil pollution remediation both domestically and internationally. However, the lack of systematic studies on in situ plants and rhizosphere fungal diversity in As-contaminated soils, particularly in heavily polluted area, limits the application of the plant–fungal combined remediation technology. In this study, we surveyed and identified the distribution of dominant native plant in highly arsenic-contaminated area, and then we used 18S rDNA technology to analyze the diversity of rhizosphere fungi and related factors from the area. The results revealed that Pteris vittata (L.) of Pteridaceae and Imperata cylindrica (L.) Beauv. of Poaceae are the dominant native plants in highly arsenic-contaminated area. The concentrations of As in the rhizosphere soils of the dominant plants in the area exceeded the As soil limits set by the European Union and the World Health Organization. A large quantity of As resulted in the dominance of fungi from the phyla Glomeromycota, Ascomycota, and Basidiomycota in the contaminated area soils, while relative abundance of fungi is varied in different sites. Additionally, soil acidity and alkalinity (pH), available phosphorus (AP), and As had the most notable effects on fungal diversity in Shihuangsi village and Linkuang village, whereas the low soil organic carbon (SOC) content in Heshan village was the primary limiting environmental factor for fungal diversity. The results of this study provide a theoretical foundation and technical guidance for the development of novel plant–fungal combined remediation technologies aimed to the control of As pollution in plant and soil.
1 Introduction
Arsenic (As) is a highly toxic metalloid element, which can enter the human body through diet, skin contact, and breathing, leading to cardiovascular disease, neurotoxicity, and skin cancer (Fayiga and Saha, 2016; Al-Makishah et al., 2020). Therefore, As is classified as a Group 1 carcinogen by the International Agency for Research on Cancer (IARC) and the World Health Organization (WHO) (Fayiga and Saha, 2016). Arsenic is widely distributed in the natural environment, with soil being the primary medium for its presence as a result of ore mining, smelting, and the use of As-containing products such as pesticides and animal feed additives (Kayode et al., 2021; Feng et al., 2024). For example, As content in the agricultural soils of the Badhshir Plain in southeastern Iran was as high as 50.26 mg/kg (Khajehpour et al., 2022), and that in the agricultural topsoil in China was as high as 3.71 × 106 t (Gong et al., 2020). Soil As contamination is even more severe in mining areas. The As content in the soil of the abandoned Shimen realgar mine in China was 86.32–43,997.76 mg/kg (Chen et al., 2020). Moreover, As present in the soil can enter the food chain through plant uptake and ultimately pose a threat to human health. Therefore, addressing soil As contamination in soil has always been a major focus. Currently, traditional physical and chemical remediation techniques are being increasingly substituted with in situ phytoremediation, primarily for their cost-effectiveness and reduced risk of secondary pollution. For example, native dominant hyperaccumulator plants such as Pteris vittata, Pityrogramma calomelanos, and Vetiveria zizanoides are used to accumulate or immobilise As in their roots (Anh et al., 2018; Eze and Harvey, 2018), thus achieving ecological remediation of As-contaminated soils. However, for highly As-contaminated soils, the high concentration of As limits the growth of dominant plants, reducing phytoremediation efficiency (Wu et al., 2024; Yan et al., 2024). Therefore, it is imperative to prioritise the development of innovative and efficient technologies for the remediation of As-contaminated soils, particularly those with elevated levels of the contaminant.
In recent years, several studies have indicated that certain microorganisms, especially fungi such as arbuscular mycorrhizal fungi (AMF), can interact with plants to enhance their survival in heavy metals-contaminated soils, thereby improving the ecological remediation efficiency of soils with high concentrations of heavy metals (Gupta et al., 2021; Salari et al., 2024). For example, Cr(III) can be precipitated by the negatively charged phosphate groups (or analogues) present on the surface of AMF fungi, others can combined with histidine analogues or carboxylic acid ligands and be adsorbed onto the fungal cell wall (Wu et al., 2016). Rhizophagus irregularis reduced the uptake and translocation of Bi in the plant organs by enhancing the exudation of polyphenols and organic acids into the rhizospheric soil (Mohammed et al., 2023). The symbiotic network by AMF and root can effectively immobilize and capture As through chitin on the cell wall, thereby reducing the absorption of As by plants and improving the tolerance of plants to As (Khalid et al., 2021). Claroideoglomus etunicatum promotes the transformation of As from crystalline forms to an iron - aluminium oxides that is readily available for plant utilization in the rhizosphere soil of Pteris vittata (L.) by regulating plant rhizosphere exudates such as organic acids, thus activating As in the rhizosphere soil and enhancing its uptake by plants. This also changes the valence state of As in the soil, improving plant survival and increasing plant efficiency in remediating As contamination (Pan et al., 2024). As-resistant fungi such as Fimetariella rabenhorstii and Hormonema viticola can enhance plant resistance to As by upregulating genes associated with metallothioneins, superoxide dismutases, ascorbate peroxidases, and phytochelatins, thereby promoting plant growth in As-contaminated areas (Soto et al., 2019). The As-resistant Trichoderma sp. promotes the conversion of As from inorganic to organic forms in plants, reducing As toxicity and increasing plant tolerance to As stress (Tripathi et al., 2017). AMF participates in the process of reducing As(III) to As(V). As(III) can forms complexes with thiol compounds, ultimately being stored in plant vacuoles or excreted into the environment, thereby reducing arsenic toxicity to the plants (Zhang et al., 2015). Cantamessa et al. (2020) carried out research in a large outdoor area (an abandoned As mining site in northern Italy) to investigate the impact of AMF on the growth of P. vittata, and found that AMF can increase the biomass of plant. In conclusion, the formation of the aforementioned microbial-plant co-remediation system primarily involves AMF enhancing plant tolerance to heavy metals through both direct and indirect effects. Regarding direct effects, AMF forms symbiotic associations (hyphae) with plants, increasing the specific surface area of root. This promotes the uptake of mineral elements such as nitrogen (N) and phosphorus (P), thereby benefiting plant growth (Kuang et al., 2025). Simultaneously, thiol groups on their extraradical hyphae and polyphosphate within the hyphae can directly immobilize and chelate heavy metals, reducing their toxicity to plants (Chen et al., 2018; García-Sánchez et al., 2021). Regarding indirect effects, AMF can regulate rhizospheric soil environment by altering plant root exudates, such as pH, redox potential and organic matter composition, thereby affecting speciation and bioavailability of heavy metal in rhizosphere soil (Dhalaria et al., 2020). In addition, AMF can also enhance plant resistance to heavy metals by regulating changes in root morphology, increasing antioxidant enzyme activity, and modulating the expression of genes encoding heavy metal transporters (Ferrol et al., 2016; Riaz et al., 2021).
Therefore, the combination of dominant fungi and plants in soil presents a promising approach for remediating high levels of As contamination. Thus, most research on the combined use of microbes (such as AMF) and plants to remediate As-contaminated soil have been conducted under controlled greenhouse conditions (Akhtar et al., 2024; Asif et al., 2025). The application of locally advantageous As-accumulating plants and in situ microorganisms in practical environments has become a key constraint on the efficient remediation of As-contaminated soil. However, there is limited research on the dominant microorganisms in As-contaminated soils, and the diversity of rhizosphere fungi in these areas remain unclear. This lack of information presents challenges in identifying dominant microorganisms and hinders the development and application of fungal-plant cooperative remediation techniques for highly As-contaminated areas. Therefore, identifying the structure and composition of native dominant As-accumulating plants and in-situ microbial communities in typical As-contaminated soil is a necessary prerequisite for the application of the above-mentioned combined remediation methods in actual soil environments.
Based on the above background and current situation, we conducted a study on the fungal diversity in a highly As-contaminated area—the Shimenshan Realgar mining area, which is located in Shimen County, Hunan Province, and is the largest realgar mining area in Asia. The area has a mining heritage that spans over 1500 years, but mining activities ceased in 2011 (Yang et al., 2013). Due to the previous intensive mining and smelting activities, the soil in this area was severely contaminated, with the soil As content ranging from 86.32 to 43997.76 mg/kg (Chen et al., 2020). The soil As content in the surrounding Heshan Village also exhibited elevated levels, reaching as high as 5240.8 mg/kg (Tang et al., 2016). In the present study, a plant survey was conducted in the beneficiation, smelting, and tailings areas of the realgar mining area. The dominant plants were identified, and rhizosphere soil samples were collected. The fungal diversity in the rhizosphere soil was investigated using 18S rDNA high-throughput sequencing technology. Further, (1) the dominant phyla, classes, and orders of rhizosphere soil fungi in different functional zones; (2) the similarities and differences in the composition and distribution of fungal communities in rhizosphere soil across different functional zones; and (3) the relationship between fungal diversity in the rhizosphere soil and soil environmental factors were determined. The findings of this study are anticipated to enhance the understanding of the fungal community diversity in As-contaminated areas, particular focusing on fungi in symbiosis with plants, and establish a theoretical foundation for the future advancement of plant–fungal combined remediation technologies in As contaminated areas.
2 Materials and methods
2.1 Overview of the study area
The study area is located in a highly As-contaminated area, the realgar mining area, northwest of Shimen County, Changde City, Hunan Province (Supplementary Figure S1). It has a subtropical monsoon climate, with an average annual precipitation of 1567.3 mm and an average annual temperature of 18.4°C.
2.2 Investigation and analysis of plants and fungi in the study area
Based on detailed investigation of realgar mining area and literature on heavy arsenic contamination (Zeng et al., 2016), samples were collected from three areas: Shihuangsi village (W-B) nearby beneficiation area, Heshan village (W-S) nearby smelting area, and Linkuang village (W-T) nearby tailings area (Supplementary Table S1). In each area, we randomly set up 10 shrub quadrats, each measuring 10 m × 10 m. Within each of these quadrats, we further set up 1 small quadrats, each measuring 1 m × 1 m, for the investigation of herbaceous plants. These plots were set up in locations with similar slope positions and aspects with dimensions. Subsequently, we carried out a survey of the plant distribution to determine the dominant native plants in As-contaminated areas. The proportion of a certain plant in the study area is shown in Equation (1).
Where Pi (m2) is the distribution area of a certain plant in the study area, Si (m2) is the area of each quadrat, n is the numbers of sampling point.
After the dominant native plants were identified, the rhizospheric soil of the dominant plants was randomly collected at 3 points in the W-B, W-S and W-T areas. A total of 2 kg of rhizosphere soil (0–20 cm) of the dominant plants was collected, mixed, and divided into two portions. One portion was stored in a 4°C mini fridge (SAST, China) and transported to the laboratory for the analysis of physicochemical properties. The other portion of soil sample was placed in a liquid nitrogen tank and immediately transported to the laboratory for microbial diversity analysis.
2.3 Soil property analysis
The soil samples were air-dried, ground, and sieved through a 2 mm sieve, and soil property analysis was conducted. In a beaker containing 10 g of soil, 25 mL of deionised water was added, magnetically stirred for 10 min, and allowed to stand for 30 min. The pH of the supernatant was measured using a pH meter (PHBJ-260, Leici, China). The air-dried soil was sieved through a 100-mesh sieve and divided into two portions. One portion was treated with hydrochloric acid (HCl) to eliminate inorganic carbon, followed by air-drying, grinding, and analysis for total organic carbon (TOC) using an elemental analyser (Elementar variomacro EL, Germany), and the other portion was analysed for nitrogen (N) and sulphur (S) contents by elemental analyser. Available potassium (AK) was quantified using flame photometry (AA-6880, Shimadzu, Shanghai, China). Soil-available phosphorus (AP) was determined using the sodium bicarbonate (NaHCO3) extraction method (Duboc et al., 2022).
The measurement of total arsenic (TAs) and total antimony (TSb) in soil samples was performed as follows: A solution of 8 mL acid mixture (nitric acid, hydrochloric acid, and hydrofluoric acid in a 5:2:1 ratio) was added to a PTFE tube containing 50 mg of soil and allowed to stand overnight, followed by digestion for 2 h using a microwave system (CEM, MARs5, USA). After cooling the digestion tube to 20°C, the sample was transferred to a 90°C water bath until the solution became clear. The solution from the tube was then transferred to a 50 mL centrifuge tube and filtered through a 0.45 μm polyethersulfone membrane. The volume was adjusted to 50 mL by adding 5% hydrochloric acid. The concentrations of As and Sb were measured using an inductively coupled plasma optical emission spectrometer (ICP-OES, Agilent 5900, USA). Quality control was maintained throughout the analysis as follows. The recovery rate of As in soil was 70.7%–110.0%, and that of Sb was 80.0%–120.0%. Standard solutions of As and Sb were calibrated by standard curves, and the correlation coefficient (r) was greater than 0.995. The relative standard deviation (rsd) of repeated samples in soil was less than 10%.
2.4 Determination of soil microbial diversity using 18S rDNA technology
Total DNA extraction for different microbial colonies was performed using DNA extraction kits (TianGen Biotech, Beijing, China). The primers for fungal 18S rDNA gene amplification were 817F: 5′-GCCTCCCTCGCGCCATCAG (10 bp MID) CAGCCGCGGTAATTCCAGCT-3′ and 1196R: 5′-GCCTTGCCAGCCCGCTCAG GTTTCCCGTAAGGCGCCGAA-3′. Samples were amplified using PCR (ABI GenAmp®9700, USA) with barcode-specific primers and analysed by million agarose gel electrophoresis (E-Gel Power Snap, Thermo Fisher Scientific, America). Quantitative detection of PCR products was performed using a fluorescent blue quantitative system (QuantiFlourTM-ST, USA), and the products were mixed in different proportions according to sequencing needs. The PCR products were amplified to construct a MiSeq library template, and single-stranded DNA was obtained via alkaline denaturation. DNA fragments complementary to the primers were fixed onto the chip, and complementary DNA fragments from other primers were randomly generated, creating relevant bridges. Amplification bridges were formed to produce DNA clusters. Linear biased DNA amplicons became single-stranded. Four types of fluorescent-labelled dNTPs and modified DNA polymerase were added to the PCR, synthesising a single base in each loop. The nucleotide sequence of each template was read from the template sequence of each reaction. The “terminator” and “fluorophore” were chemically cleavaged to restore 3′ terminal activity, enabling the synthesis of the next nucleotide. Based on the fluorescence signal result, the DNA template sequence was obtained and identified. The data presented in the study are deposited in the NCBI repository, accession number PRJNA1334568.
2.5 Statistical analysis
Data analysis was performed using the SPSS®19.0 software package. Analysis of variance (ANOVA) was used to assess the differences between the mean values of soil characteristics, and the t-test was applied for comparison at the 0.05 probability level (p=0.05). Observed richness indices such as Chao, Shannon, and Good’s coverage were calculated using Mothur (version 1.80.2). Non-metric multidimensional scaling (NMDS) and redundancy (RDA) analyses were performed using Canoco 5 software. The figures were processed using Origin®2019b.
3 Results and discussion
3.1 Identification of dominant native plant in arsenic mining area
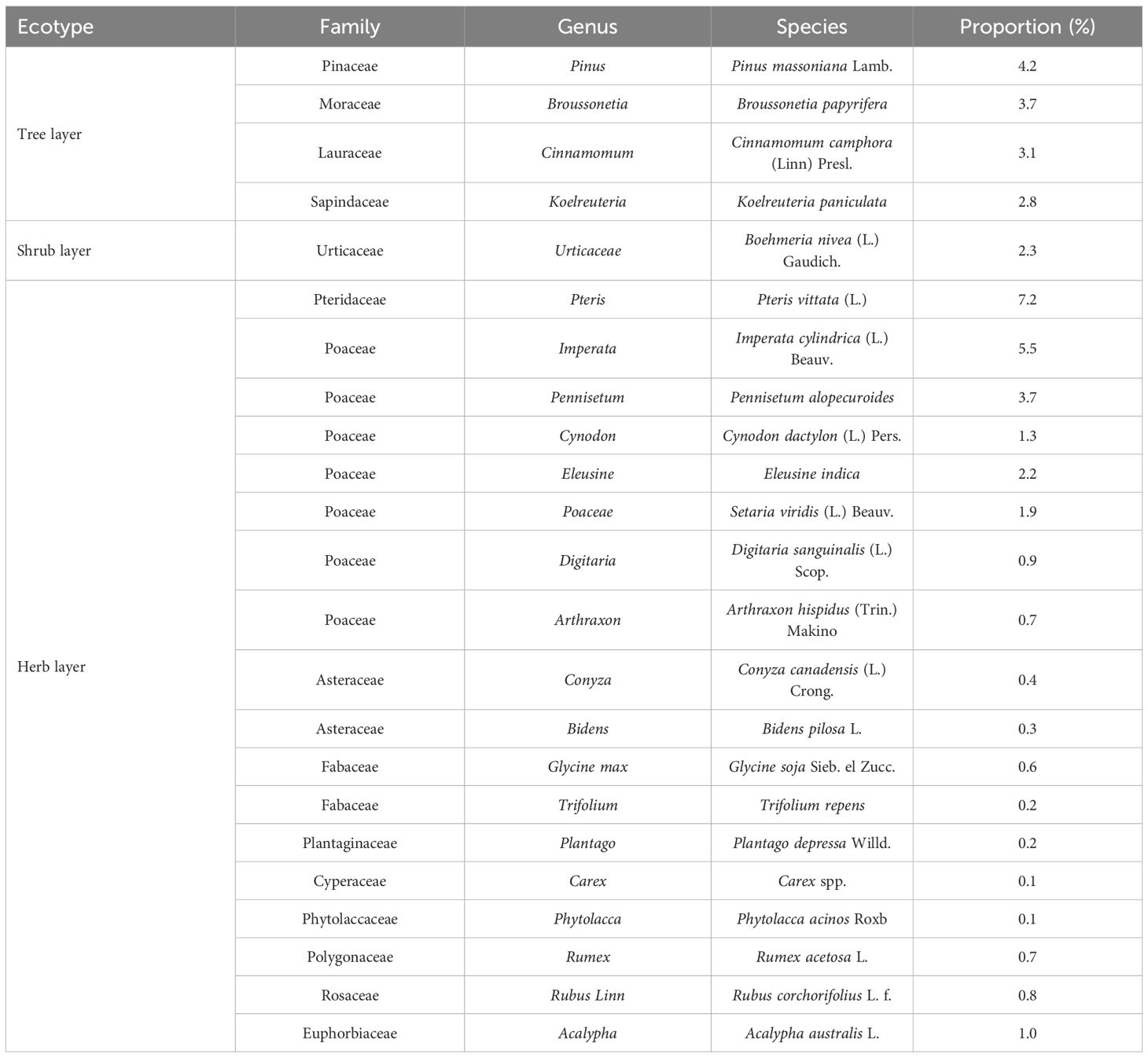
Plant community composition is shown in Table 1, there are 23 species in As-contaminated areas, belonging to 13 families and 23 generas. Among them, there are 4 species of trees, 1 species of shrubs and 18 species of herbs. In herbaceous plants, characteristics of the most diverse and widely distributed plant species in Poaceae. Among them, the distribution area of Poaceae accounted for 16.2% of the total quadrat area. Based on the comprehensive analysis of plant community composition and distribution, Poaceae are identified as the dominant native plant category in As-contaminated soil. Further to analysis distribution of Poaceae revealed that the distribution area of Imperata cylindrica (L.) Beauv in As-contaminated soil accounts for 5.5% of the total sample quadrat area. Concurrently, extensive researches indicate that Imperata cylindrica (L.) Beauv also serves as a primary pioneer plant in other high-concentration abandoned heavy metal mining area. It can widely exist in mining areas and simultaneously accumulate large amounts of heavy metals such as chromium and copper (Fu et al., 2016; Zheng et al., 2019; Mao et al., 2024). Considering the significant proportion of Imperata cylindrica (L.) Beauv among herbaceous plants, it can be considered a potentially dominant native species for ecological remediation in As-contaminated soil. In addition, Pteris vittata (L.) accounts for 7.2% of the total sample quadrat area. The Pteridaceae to which Pteris vittata (L.) belongs, is not dominant native family in As-contaminated soil, a large number of articles have demonstrated that Pteris vittata (L.) is a hyperaccumulator of As (Nguyen et al., 2011; Bai et al., 2023). Therefore, Pteris vittata (L.) is regarded as an excellent candidate for phytoremediation of highly arsenic-contaminated soils. In summary, considering the distribution of plants in the community and special ecological functions, the potential dominant native species for ecological remediation in high soil As concentration areas are Pteris vittata (L.) and Imperata cylindrica (L.) Beauv.
3.2 Characteristics of arsenic and associated metal content in different regions
As in soil can negatively impact plant physiology and growth, thereby reducing the efficiency of phytoremediation for As-contaminated soil. Therefore, we measure the concentrations of As in rhizospheric soil of Pteris vittata (L.) and Imperata cylindrica (L.) Beauv. The heavy metal content in soils from different sample points of As-contaminated areas is shown in Figure 1A. The average concentrations of As in the soils of W-B, W-S, and W-T areas were 964.69, 1574.04, and 2268.30 mg/kg, respectively, which far exceed the TAs concentration limit set by the European union soil guidelines (10 mg/kg) (Anawar et al., 2006). This indicated that soils in all sample points were severely contaminated with As. There were significant differences in the soil As content across different sample points, with W-S area exhibiting the highest average As concentration, followed by W-S area. Both areas were1.63 and 2.35 times higher than the As concentration (p < 0.05) in W-B area, respectively. As and Sb usually exist together (Mi et al., 2023). Hence, we further investigated the Sb content in soils from the different sample points. The results (Figure 1B) indicated that the soil Sb concentrations from W-B, W-S, and W-T areas were 509.73, 602.56, and 515.62 mg/kg, respectively. These concentrations exceeded the soil safety limit established by the WHO (36 mg/kg) by 14.16, 16.74, and 14.32 times, respectively (Jin and Peng, 2024). Sb concentrations in soil of W-S and W-T areas were higher than in W-B area (p < 0.05). The above results showed that the heavy metal content in soils of Heshan village and Linkuang village was significantly higher than that in W-B Shihuangsi village. This could be attributed to the fact that W-B area near by the beneficiation area involves only physical processing of the ore, resulting in a reduction in ore volume. Consequently, a large number of heavy metals still exist in mineral forms, such as AsS, As2S3, or FeAsS, without being released into the soil (Kim et al., 2014; Liu et al., 2022). In contrast, W-S near by the smelting and W-T nearby tailings areas, respectively. The ore in the W-S and W-T area undergo further physical or chemical processing, which facilitates the release of heavy metals and their accumulation in the soils (Timofeev et al., 2018).
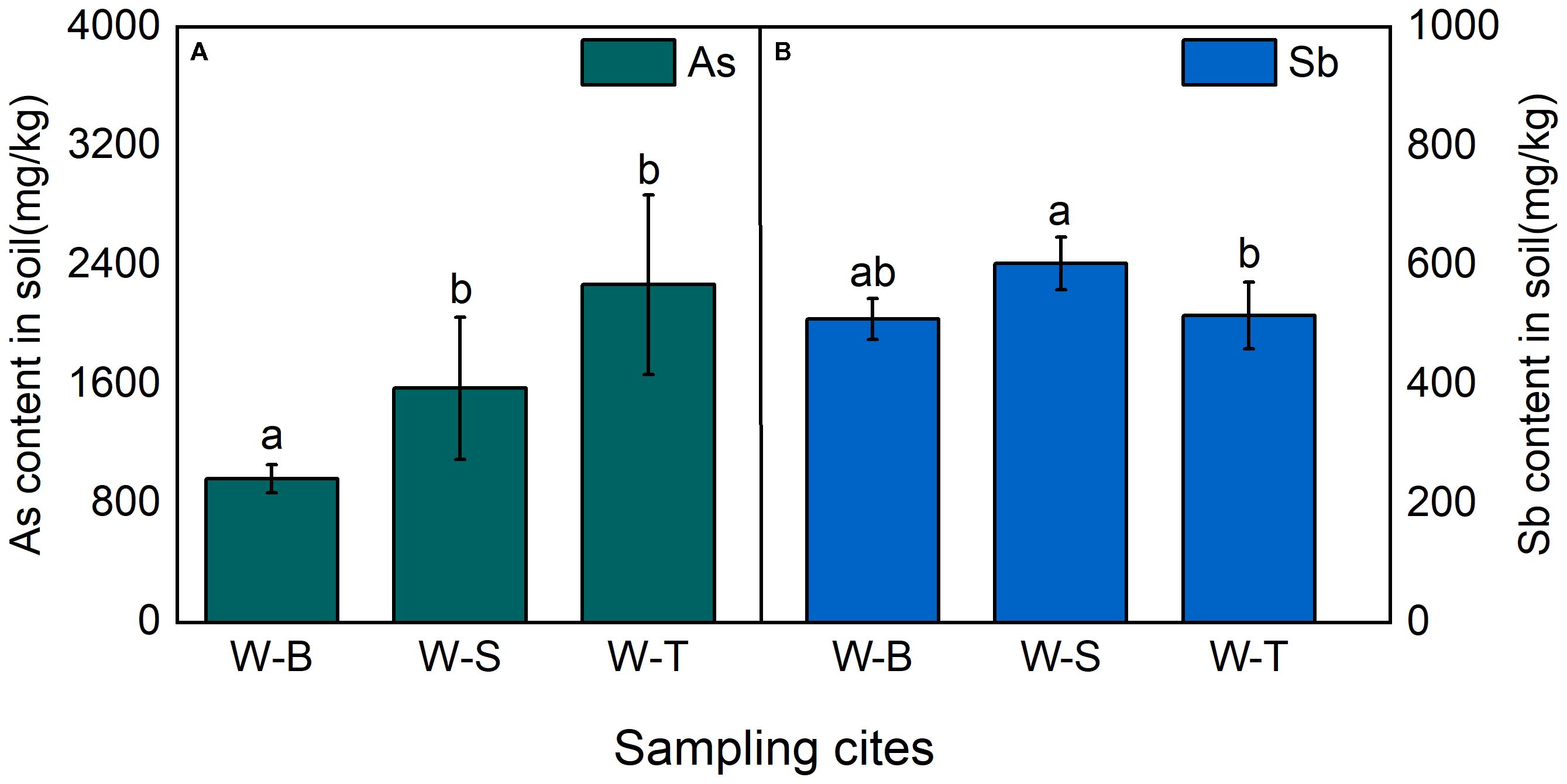
Figure 1. As and Sb contents of soil in As-contaminated areas, (A) As content in soil; (B) Sb content in soil. Shihuangsi village (W-B); Heshan village (W-S); Linkuang village (W-T).
3.3 Sequencing data and fungal taxonomic richness
The forms and content of As and associated metal in the soil can significantly affect the composition and structure of the dominant plant rhizosphere fungal communities (Chodak et al., 2013; Tang et al., 2022). Lin et al. (2020) analysed the primary factors influencing soil microbial diversity by collecting soil samples from 23 regions, revealing a significant negative correlation between fungal community abundance and evenness in soil and cadmium (Cd) content. We performed operational taxonomic unit (OTU) classification for all sequences at a 97% similarity level, and the results indicated that the average number of OTUs in W-B, W-S, and W-T area were 3401, 2758, and 3571, respectively (Supplementary Figure S2). Therefore, we hypothesised that the fungal diversity in W-B and W-T areas may be higher than in W-S area.
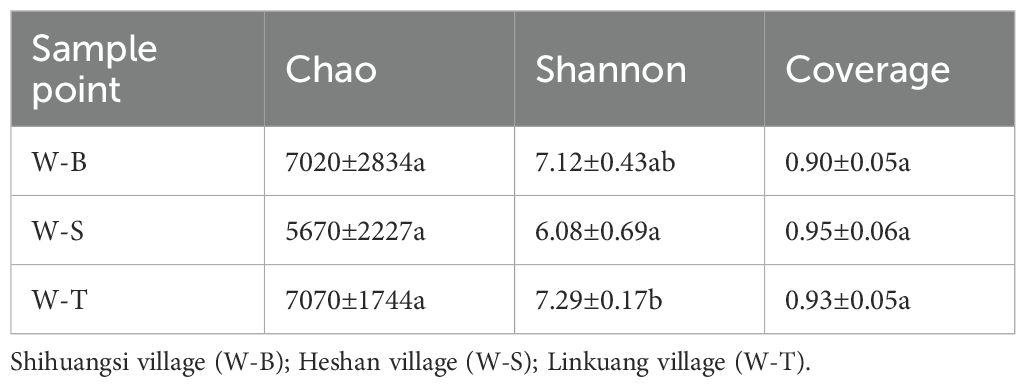
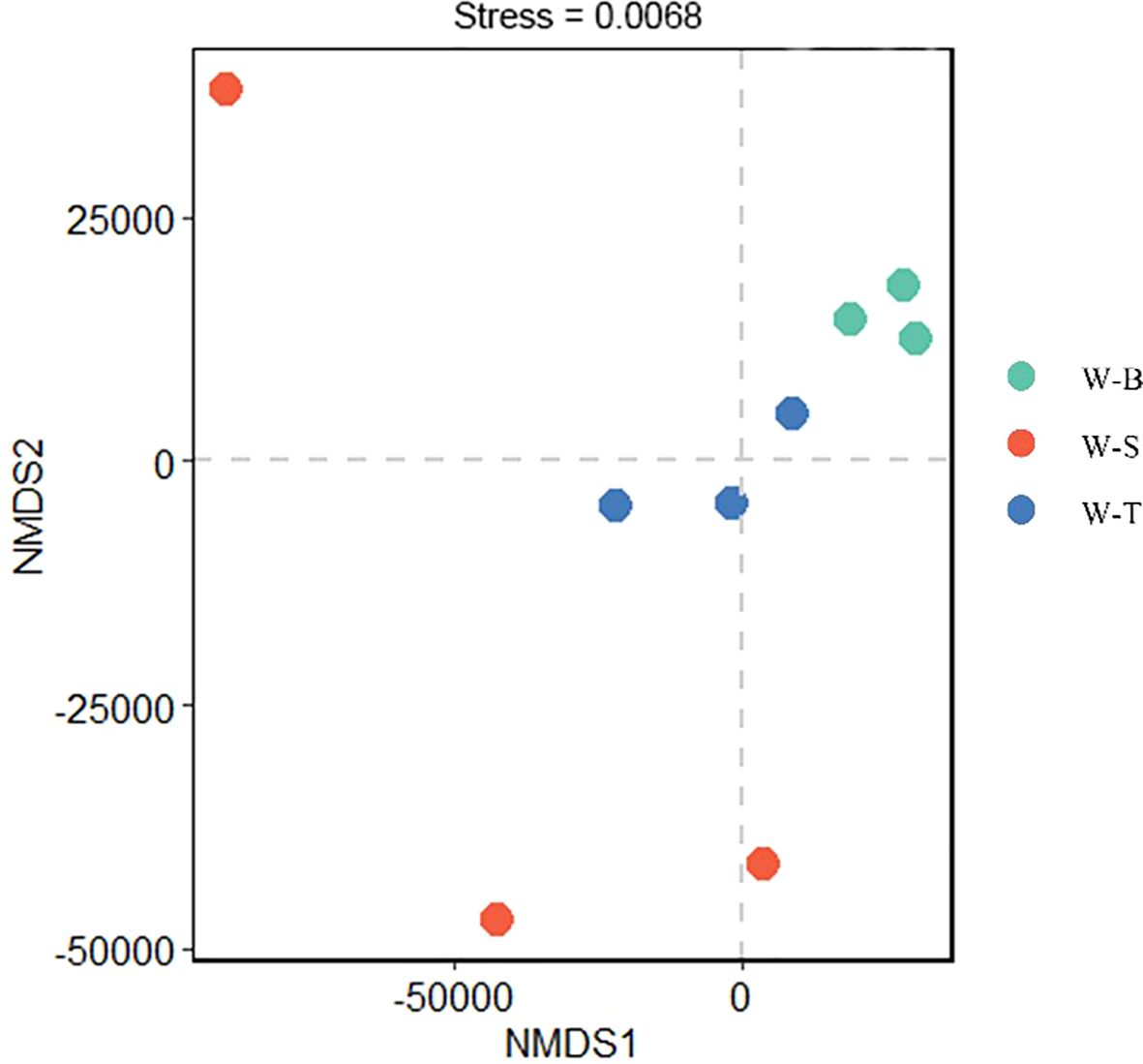
To further validate this hypothesis, we examined the α-diversity of fungi in the different sample points. The sample coverage results are shown in Table 2, indicating no significant differences in coverage between the three sample points. The coverage rate reached 90%, indicating that the sequencing results effectively portray the fungal community diversity characteristics of the three sample points. We further analysed the Chao index to estimate the number of OTUs in the samples, which can evaluate the total species number. The results (Table 2) indicated that the Chao index decreased in the following order: W-T area > W-B area > W-S area, with the Chao index for W-S area being only 80% of that in W-B and W-T areas. This suggested that the total number of fungal species W-S area is lower than in the other two sample points; however, there was no significant difference in the total fungal species between the three sample points (p < 0.05). Chao index in the uncontaminated area reported by Xin (2025) exceeded 10000, significantly higher than the Chao indices at the three sampling sites in our study, which indicates diversity index of fungi in uncontaminated area is significant greater than that observed at the three sampling sites. We also analysed the Shannon index to evaluate the relative abundance (evenness) of microbes in the samples. The results (Table 2) showed that the relative abundance of fungi is highest in W-T area, followed by W-B area, with W-S area having the lowest fungal relative abundance. The Shannon index of W-T area was significantly higher than that of W-S area (p < 0.05); however, there was no significant difference in fungal relative abundance between W-T and W-B areas. Similarly, the Shannon index for fungi in the uncontaminated area exceeded 7.5, surpassing the corresponding indices at the three sampling sites. This indicates a higher relative abundance (evenness) of fungal species in the uncontaminated sites. This may be attributable to the selective pressure exerted by high heavy metal concentrations on fungal communities, leading to a more concentrated distribution of species within communities exhibiting greater tolerance to heavy metals, thereby reducing relative abundance (evenness). In summary, the α-diversity was highest in W-T area, followed by W-B area, and lowest in W-S area, which was consistent with the OTU classification results of the three sample points (Supplementary Figure S2). In addition to the α-diversity of fungal communities, we used Non-metric multidimensional scaling (NMDS) based on Bray–Curtis distances to determine the β-diversity of fungal communities in different sample points. NMDS is an ordination technique used to analyse species composition data and is often used for interpreting the microbial community distributions (Li et al., 2022; Sajjad et al., 2024). The NMDS results are shown in Figure 2, with a stress value of 0.0068 (<0.05), indicating that the results are highly representative. W-B area was in close proximity to W-T area and farther from W-S area, indicating that the fungal diversity and composition of W-B area closely resemble those of W-T area.
3.4 Fungal community composition and distribution in the different sample points
To further show the microbial composition in the different sample points, we analysed the fungal composition at the phylum, class, and order levels in the soil samples from the three sample points. As shown in Figure 3A, eight common fungal phyla were detected in the experimental soils of the three sample points. Among them, the phyla Glomeromycota, Ascomycota, and Basidiomycota exhibited higher relative abundances in W-B, W-S, and W-T areas. This indicates that these three phyla comprise the dominant microorganisms in As-contaminated areas, with the relative abundance of Glomeromycota exceeding 80% in all three sample points. Glomeromycota constitutes the primary microbial phylum to which AMF belong. It suggests that AMF communities may dominate fungal community in soil. At the phylum level, the relative abundance of non-AMF-associated Ascomycota and Basidiomycota was highest in fungal communities from uncontaminated site, followed by the Glomeromycota (10%). Notably, the relative abundances of Ascomycota and Basidiomycota were significantly higher than that of Glomeromycota, which may be due to the selective pressure exerted by heavy metals in contaminated areas upon microbial communities. In the high concentration of arsenic, Glomeromycota exhibits greater adaptability compared to the phyla Ascomycota and Basidiomycota. Furthermore, variations in fungal community diversity were observed among the different sample points. For example, the relative abundance of Glomeromycota in the W-S and W-T areas were lower than in W-B area, possibly due to the stronger inhibitory effect of high concentrations of As on the growth of specific Glomeromycota fungi (Yang et al., 2023).
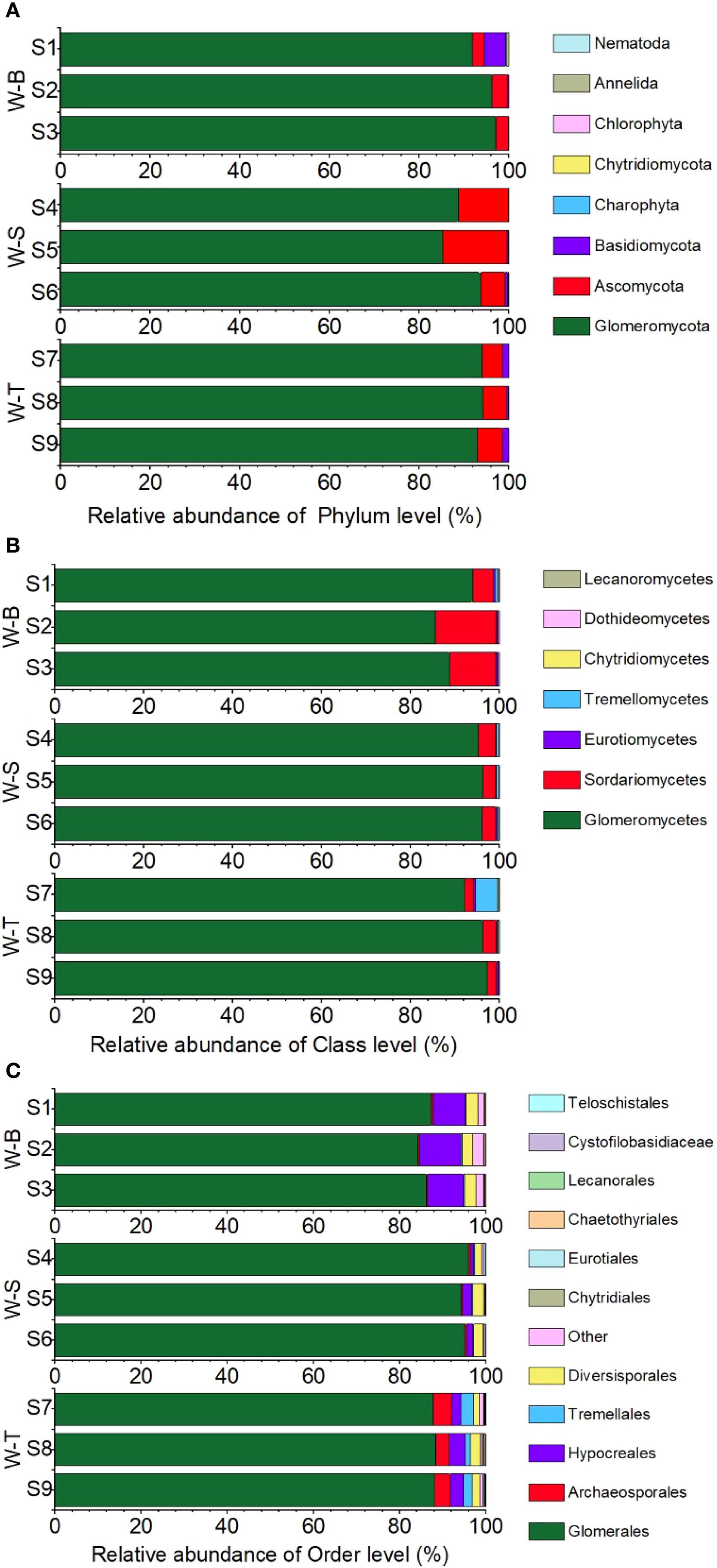
Figure 3. Fungi composition and abundance for each pollution level at phylum (A), class (B) and order levels (C). Shihuangsi village (W-B); Heshan village (W-S); Linkuang village (W-T).
At the class level, Glomeromycetes belonging to the phylum Glomeromycota exhibited the highest relative abundance at the class level in all three sample points, with relative abundances of 96.24%, 89.41%, and 94.81% in W-B, W-S, and W-T areas, respectively (Figure 3B). Sordariomycetes exhibited the second highest relative abundance in all three sample points. Glomeromycetes and Sordariomycetes accounted for 95% of the microbial classes in the three sample points, suggesting that, at the class level, Glomeromycetes and Sordariomycetes are the dominant microorganisms in As-contaminated areas. Similarly, variations were observed in the fungal diversity at the class level between the different sample points. The relative abundance of Glomeromycetes in the W-S and W-T areas were higher than in W-B area. Interestingly, the class Glomeromycetes, which had the highest relative abundance at the class level, belongs to Glomeromycota and is also a class of arbuscular mycorrhizal fungi (AMF). AMF exhibit wide distribution and forms symbiotic relationships with plants. It substantially improves plant survival under stress by enhancing the plant’s nutrient absorption, improves soil structure, and regulates the expression of proteins related to plant stress resistance (Allsup et al., 2023; Martin and van der Heijden, 2024). Even in environments with severe heavy metal contamination, wastewater irrigation, and salt marshes, AMF exhibit widespread distribution and plays an essential ecological role (Arriagada et al., 2009; Sonjak et al., 2009; Marro et al., 2022). Furthermore, Sordariomycetes, belonging to the phylum Ascomycota, plays a crucial role in the decomposition of organic matter and in soil nutrient cycling (Zhang et al., 2006). These results indicate that functional microorganisms that play a key role in As-contaminated areas exhibit a high level of tolerance to heavy metal pollution.
At the order level, a total of 32 orders were identified in the soil samples, among which Glomerales, Archaeosporales, Hypocreales, and Diversisporales were common to all three sample points (Figure 3C). In the W-B and W-T areas, Glomerales, Hypocreales, and Diversisporales were most abundant, with relative abundances of 98.66% and 96.99%, respectively. In the W-S area, the three orders with the highest relative abundance were Glomerales, Archaeosporales, and Hypocreales. Glomerales was the most abundant order in all three sample points and is an important order in the AMF classification, further supporting the idea that AMF shows a high level of tolerance to As contamination. Additionally, the study indicated a marked decrease in the relative abundance of Glomerales in the W-S and W-T areas compared to W-B area (Figure 3C). This divergence could be attributed to the increased susceptibility of Glomerales to elevated levels of heavy metal contamination (Dai et al., 2012).
3.5 Influence of soil physicochemical properties on microbial diversity
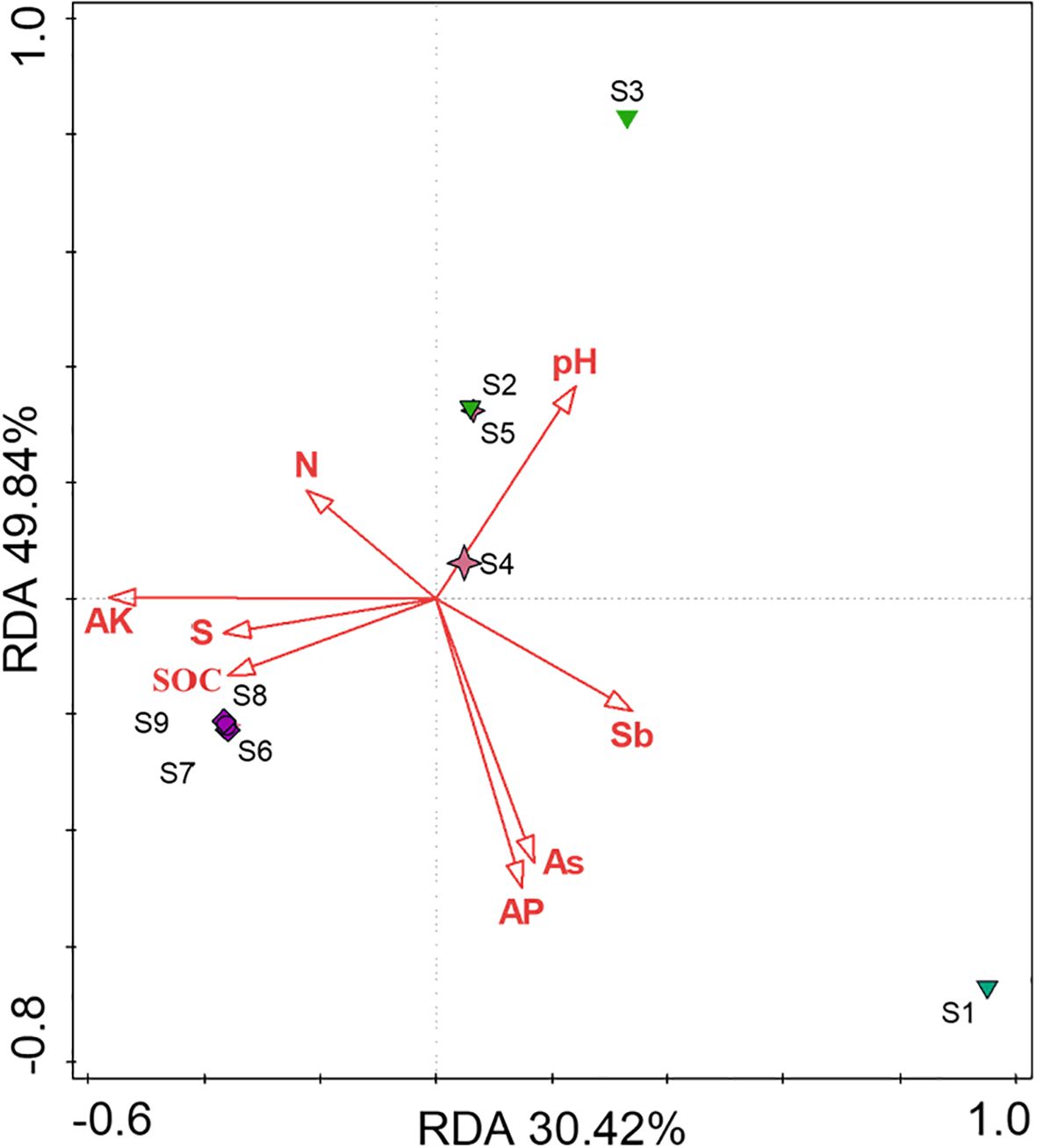
To further explore the main environmental factors influencing fungal diversity in the different sample points, this study used redundancy analysis (RDA) to validate the correlation between eight environmental factors and soil fungal diversity. As a constrained linear by gradient sorting method, RDA can reflect the influence of environmental factors on species composition and also express the explanatory value of each environmental factor. The degree of correlation between the environmental factor arrows and microbial arrows is represented by the cosine of the angle between them. The results of the RDA are shown in Figure 4. Overall, the soil AP, AK, and As contents had the most notable effects on fungal diversity in each functional area. Additionally, in the W-S and W-T areas, soil pH, AP, and As had the most notable effects on fungal diversity. In W-B area, SOC had the greatest impact on fungal diversity. These results indicate variations in the environmental factors influencing microbial diversity across the different sample points. In both W-S and W-T areas, fungal diversity was positively correlated with soil pH and negatively correlated with the concentrations of AP and As. This relationship may be associated with the dominance of AMF in the fungal communities of the W-S and W-T areas. AMF is an oligotrophic symbiotic microorganism that provides nutrients for plant growth by absorbing more phosphorus from the soil and exchanging it for plant carbon sources (Duan et al., 2024). At elevated soil AP concentrations, the phosphorus uptake by plants through their roots is sufficient to fulfil their growth requirements. This results in reduced carbon supply from plants to AMF, thereby leading to a decrease in the abundance and diversity of AMF (Olsson et al., 2010; Basyal and Emery, 2021). Furthermore, heavy metals are another important factor influencing AMF diversity. Excessive concentrations of heavy metals can have toxic effects on AMF, thereby affecting the composition and structure of AMF communities (Betancur-Agudel et al., Chen et al., 2023). This could potentially elucidate the negative relationships among AP, As, and AMF. Soil pH influences the form of As and phosphorus, indirectly affecting the composition and structure of the AMF community. When soil pH decreases, insoluble phosphates are released, increasing the AP content. Meanwhile, lowered pH can lead to the release of As bound to carbonates in the soil, thereby increasing the concentration of bioavailable As and increasing the toxic effects of heavy metals on AMF, ultimately reducing its diversity. Therefore, there is a positive correlation between soil pH and microbial community structure. In W-B area, soil SOC had the most notable effect on fungal diversity, which is consistent with the findings of Bi et al. (2020). This is likely because the germination of AMF spores and hyphae necessitates elevated levels of SOC. The soil in W-B area is primarily composed of As2S3 minerals and has an extremely low organic carbon content. This deficiency in SOC inhibits the growth of spores and hyphae, rendering SOC the primary factor influencing the composition of the AMF community in the rhizosphere soil or roots (Stürmer et al., 2018; Luo et al., 2019).
4 Conclusions
This study investigated the distribution of plant community composition in highly As contaminated area, the realgar mining area, in Shimen county, Hunan province. The results showed that Pteris vittata (L.) and Imperata cylindrica (L.) Beauv. are the dominant native species in this area. In addition, this study investigated the diversity of plant-symbiotic fungi in this area, among which the relative abundances of three fungal phyla, Glomeromycota, Ascomycota, and Basidiomycota, were highest in the W-S, W-T, and W-B areas. Glomerales (the order to which AMF belongs) was identified as the dominant order in highly As-contaminated areas. Furthermore, As and AP were negatively correlated in the W-S and W-T areas and showed a significant positive correlation with soil pH. This could indicate that bioavailable As contamination disrupted the ecological composition of the fungi. In W-B area, organic carbon had the most notable effect on fungal diversity, which could be attributed to the low organic carbon content in W-B area, and is insufficient to meet the carbon source requirements for the growth of Glomerales. These findings offer valuable insights for the development of future plant–fungal combined ecological remediation technologies for As-contaminated soil, especially the soil with high As concentration. This study provides a potential strategy to utilize plant-symbiotic fungi (AMF) to reduce As toxicity to plants. Furthermore, some studies indicate that during practical soil remediation for heavy metals, the efficacy of AMF is influenced by factors such as ambient temperature, soil particle size, and other composite pollutants (e.g., plastics, chemical fertilisers, and pesticides). Previous studies have reported that combined pollution from heavy metals and microplastics can affect the diversity of AMF communities. Based on the above contents, when applying AMF to practical arsenic (As)-contaminated soil remediation in the future, it will be necessary to comprehensively consider actual environmental conditions and scientifically and rationally utilise locally dominant AMF. This approach will effectively enhance the application efficacy of AMF in soil remediation.
Data availability statement
The data presented in the study are deposited in the NCBI repository, accession number PRJNA1334568.
Author contributions
ZM: Methodology, Data curation, Investigation, Writing – original draft. SF: Writing – review & editing, Methodology, Data curation. LX: Writing – review & editing, Methodology, Formal analysis. HS: Formal analysis, Writing – review & editing, Methodology. WY: Investigation, Writing – review & editing. WF: Validation, Supervision, Writing – review & editing, Funding acquisition.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was financially supported by the special fund of the Chinese central government for basic scientific research operations in commonweal research institutes (2024YSKY-27) and the National Key Research and Development Program of China (2021YFC3201001 and 2023YFC3708004), Huanghuai Lab Sci-Tech Innovation Project (240700005).
Acknowledgments
We gratefully acknowledge the financial support provided by these foundations.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1608933/full#supplementary-material
References
Akhtar, O., Pandey, D., Zoomi, I., Singh, U., Chaudhary, K. L., Mishra, R., et al. (2024). Role of arbuscular mycorrhizal fungi in heavy metals homoeostasis in plants. J. Plant Growth Regul. 43, 3971–3985. doi: 10.1007/s00344-024-11393-w
Allsup, C. M., George, I., and Lankau, R. A. (2023). Shifting microbial communities can enhance tree tolerance to changing climates. Sci. 380, 835–840. doi: 10.1126/science.adf2027, PMID: 37228219
Al-Makishah, N. H., Taleb, M. A., and Barakat, M. A. (2020). Arsenic bioaccumulation in arsenic-contaminated soil: a review. Chem. Pap. 74, 2743–2757. doi: 10.1007/s11696-020-01122-4
Anawar, H. M., Garcia-Sanchez, A., Murciego, A., and Buyolo, T. (2006). Exposure and bioavailability of arsenic in contaminated soils from the La Parrilla mine, Spain. Environ. Geol. 50, 170–179. doi: 10.1007/s00254-006-0196-2
Anh, B. T. K., Minh, N. N., Ha, N. T. H., Kim, D. D., Kien, N. T., Trung, N. Q., et al. (2018). Field survey and comparative study of Pteris Vittata and Pityrogramma Calomelanos grown on arsenic contaminated lands with different soil pH. B. Environ. Contam. Tox. 100, 720–726. doi: 10.1007/s00128-018-2325-5, PMID: 29557492
Arriagada, C., Sampedro, I., Garcia-Romera, I., and Ocampo, J. (2009). Improvement of growth of Eucalyptus globulus and soil biological parameters by amendment with sewage sludge and inoculation with arbuscular mycorrhizal and saprobe fungi. Sci. Total Environ. 407, 4799–4806. doi: 10.1016/j.scitotenv.2009.05.021, PMID: 19515400
Asif, A., Koner, S., Hsu, P. C., He, B. J., Paul, S., and Hussain, B. (2025). Synergistic interactions between AMF and MHB communities in the rhizospheric microenvironment facilitated endemic hyperaccumulator plants growth thrive under heavy metal stress in ultramafic soil. J. Hazard. Mater. 492, 138233. doi: 10.1016/j.jhazmat.2025.138233, PMID: 40228454
Bai, Y., Wan, X. M., Lei, M., Wang, L. Q., and Chen, T. B. (2023). Research advances in mechanisms of arsenic hyperaccumulation of Pteris vittata: Perspectives from plant physiology, molecular biology, and phylogeny. J. Hazard. Mater. 460, 132463. doi: 10.1016/j.jhazmat.2023.132463, PMID: 37690196
Basyal, B. and Emery, S. M. (2021). An arbuscular mycorrhizal fungus alters switchgrass growth, root architecture, and cell wall chemistry across a soil moisture gradient. Mycorrhiza 31, 251–258. doi: 10.1007/s00572-020-00992-6, PMID: 33105490
Bi, Y. L., Xiao, L., Guo, C., and Christie, P. (2020). Revegetation type drives rhizosphere arbuscular mycorrhizal fungi and soil organic carbon fractions in the mining subsidence area of northwest China. Catena 195, 104791. doi: 10.1016/j.catena.2020.104791
Cantamessa, S., Massa, N., Gamalero, E., and Berta, G. (2020). Phytoremediation of a highly arsenic polluted site, using Pteris vittata L. and arbuscular mycorrhizal fungi. Plant-Basel 9, 1211. doi: 10.3390/plants9091211, PMID: 32947777
Chen, B. D., Nayuki, K., Kuga, Y., Zhang, X., Wu, S. L., and Ohtomo, R. (2018). Uptake and intraradical immobilization of cadmium by arbuscular mycorrhizal fungi as revealed by a stable isotope tracer and synchrotron radiation μX-ray fluorescence analysis. Microbes Environ. 33, 257–263. doi: 10.1264/jsme2.ME18010, PMID: 30122692
Chen, X. M., Zeng, X. C., Kawa, Y. K., Wu, W. W., Zhu, X. B., Ullah, Z., et al. (2020). Microbial reactions and environmental factors affecting the dissolution and release of arsenic in the severely contaminated soils under anaerobic or aerobic conditions. Ecotox. Environ. Saf. 189, 109946. doi: 10.1016/j.ecoenv.2019.109946, PMID: 31759742
Chen, Y., Zhou, X. Y., Wang, Z., Su, X., Liu, F. Q., and Tian, X. Y. (2023). Cd contamination determined assembly processes and network stability of AM fungal communities in an urban green space ecosystem. Sci. Total Environ. 899, 166372. doi: 10.1016/j.scitotenv.2023.166372, PMID: 37598964
Chodak, M., Golebiewski, M., Morawska-Ploskonka, J., Kuduk, K., and Niklinska, M. (2013). Diversity of microorganisms from forest soils differently polluted with heavy metals. Appl. Soil Ecol. 64, 7–14. doi: 10.1016/j.apsoil.2012.11.004
Dai, M. L., Hamel, C., Arnaud, M. S., He, Y., Grant, C., and Lupwayi, N. (2012). Arbuscular mycorrhizal fungi assemblages in Chernozem great groups revealed by massively parallel pyrosequencing. Can. J. Microbiol. 58, 81–92. doi: 10.1139/W11-111, PMID: 22220554
Dhalaria, R., Kumar, D., Kumar, H., Nepovimova, E., Kuca, K., Islam, M. T., et al. (2020). Arbuscular mycorrhizal fungi as potential agents in ameliorating heavy metal stress in plants. Agronomy-Basel 10, 815. doi: 10.3390/agronomy10060815
Duan, S. L., Feng, G., Limpens, E., Bonfante, P., Xie, X. N., and Zhang, L. (2024). Cross-kingdom nutrient exchange in the plant-arbuscular mycorrhizal fungus-bacterium continuum. Nat. Rev. Microbiol. 22, 773–790. doi: 10.1038/s41579-024-01073-7, PMID: 39014094
Duboc, O., Hernandez-Mora, A., Wenzel, W. W., and Santner, J. (2022). Improving the prediction of fertilizer phosphorus availability to plants with simple, but non-standardized extraction techniques. Sci. Total Environ. 806, 150486. doi: 10.1016/j.scitotenv.2021.150486, PMID: 34601180
Eze, V. C. and Harvey, A. P. (2018). Extractive recovery and valorisation of arsenic from contaminated soil through phytoremediation using Pteris cretica. Chemosphere 208, 484–492. doi: 10.1016/j.chemosphere.2018.06.027, PMID: 29886337
Fayiga, A. O. and Saha, U. K. (2016). Arsenic hyperaccumulating fern: Implications for remediation of arsenic contaminated soils. Geoderma 284, 132–143. doi: 10.1016/j.geoderma.2016.09.003
Feng, Y., Xu, S. D., Xu, J. H., Li, X. F., Jiang, J. P., Wu, C. F., et al. (2024). Arsenic behavior in soil-plant system under the manure application with the combination of antibiotic and roxarsone. Sci. Total Environ. 946, 174274. doi: 10.1016/j.scitotenv.2024.174274, PMID: 38942320
Ferrol, N., Tamayo, E., and Vargas, P. (2016). The heavy metal paradox in arbuscular mycorrhizas: from mechanisms to biotechnological applications. J. Exp. Bot. 67, 6253–6265. doi: 10.1093/jxb/erw403, PMID: 27799283
Fu, S., Wei, C. Y., and Li, L. H.. (2016). Characterizing the accumulation of various heavy metals in native plants growing around an old antimony mine. Hum. Ecol. Risk Assess. 22, 882–898. doi: 10.1080/10807039.2015.1118676
García-Sánchez, M., Silva-Castro, G. A., Sanchez, A., Arriagada, C., and García-Romera, I. (2021). Effect of arbuscular mycorrhizal fungi and mycoremediated dry olive residue in lead uptake in wheat plants. Appl. Soil Ecol. 159, 103838. doi: 10.1016/j.apsoil.2020.103838
Gong, Y. W., Qu, Y. J., Yang, S. H., Tao, S. Y., Shi, T. R., Liu, Q. Y., et al. (2020). Status of arsenic accumulation in agricultural soils across China, (1985-2016). Environ. Res. 186, 109525. doi: 10.1016/j.envres.2020.109525, PMID: 32330770
Gupta, S., Thokchom, S. D., and Kapoor, R. (2021). Arbuscular mycorrhiza improves photosynthesis and restores alteration in sugar metabolism in Triticum aestivum L. grown in arsenic contaminated soil. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.640379, PMID: 33777073
Jin, C. and Peng, L. Z. (2024). Antimony’s environmental impact in China. Sci. 386, 280–281. doi: 10.1126/science.adr3933, PMID: 39418371
Kayode, O., Aizebeokhai, A. P., and Odukoya, M. (2021). Arsenic in agricultural soils and implications for sustainable agriculture. Earth Environ. Sci. 655, 12081. doi: 10.1088/17551315/655/1/012081
Khajehpour, S., Karbassi, A., Honarmand, M., and Shariat, M. (2022). Exposure risk assessment, pollution level, and source identification of arsenic in soil: A case study of the Bardsir Plain (southeastern Iran). Int. J. Environ. Heal. R. 32, 1123–1136. doi: 10.1080/09603123.2020.1836134, PMID: 33153289
Khalid, M., Ur-Rahman, S., Hassani, D., Hayat, K., Zhou, P., and Hui, N. (2021). Advances in fungal-assisted phytoremediation of heavy metals: A review. Pedosphere 31, 475–495. doi: 10.1016/S1002-0160(20)60091-1
Kim, E. J., Yoo, J. C., and Baek, K. (2014). Arsenic speciation and bioaccessibility in arsenic-contaminated soils: Sequential extraction and mineralogical investigation. Environ. pollut. 186, 29–35. doi: 10.1016/j.envpol.2013.11.032, PMID: 24361561
Kuang, Q. Q., Wu, Y. J., Gao, Y. M., An, T. T., Liu, S., Liang, L.Y., et al. (2025). Arbuscular mycorrhizal fungi mitigate cadmium stress in maize. Ecotoxicol. Environ. Saf. 289, 117600. doi: 10.1016/j.ecoenv.2024.117600, PMID: 39752916
Li, Q., He, G. X., Wen, T., Zhang, D. G., and Liu, X. N. (2022). Distribution pattern of soil fungi community diversity in alpine meadow in Qilian Mountains of eastern Qinghai-Tibetan Plateau. Ecol. Indic. 141, 109054. doi: 10.1016/j.ecolind.2022.109054
Lin, L. T., Chen, Y., Qu, L. Y., Zhang, Y. X., and Ma, K. M. (2020). Cd heavy metal and plants, rather than soil nutrient conditions, affect soil arbuscular mycorrhizal fungal diversity in green spaces during urbanization. Sci. Total Environ. 726, 138594. doi: 10.1016/j.scitotenv.2020.138594, PMID: 32320884
Liu, S. J., Yuan, R. X., Wang, X. D., and Yan, Z. G. (2022). Soil tungsten contamination and health risk assessment of an abandoned tungsten mine site. Sci. Total Environ. 852, 158461. doi: 10.1016/j.scitotenv.2022.158461, PMID: 36063943
Luo, X., He, X. H., Luo, X. M., Liu, Y. N., Wang, J. Q., and Dong, J. Y. (2019). Soil organic carbon shapes AMF communities in soils and roots of Cynodon dactylon under anti-seasonal drying-wetting cycles. Diversity-Basel 11, 197. doi: 10.3390/d11100197
Mao, W. Q., Wu, Y., Li, Q. H., Xiang, Y. Y., Tang, W. T., Hu, H. Y., et al. (2024). Seed endophytes and rhizosphere microbiome of Imperata cylindrica, a pioneer plant of abandoned mine lands. Front. Microbiol. 15, 1415329. doi: 10.3389/fmicb.2024.1415329, PMID: 39113844
Marro, N., Grilli, G., Soteras, F., Caccia, M., Longo, S., and Cofré, N. (2022). The effects of arbuscular mycorrhizal fungal species and taxonomic groups on stressed and unstressed plants: a global meta-analysis. New Phytol. 235, 320–332. doi: 10.1111/nph.18102, PMID: 35302658
Martin, F. M. and van der Heijden, M. G. A. (2024). The mycorrhizal symbiosis: Research frontiers in genomics, ecology, and agricultural application. New Phytol. 242, 1486–1506. doi: 10.1111/nph.19541, PMID: 38297461
Mi, Y. D., Xu, C., Li, X. R., Zhou, M., Cao, K., and Dong, C. M. (2023). Arbuscular mycorrhizal fungi community analysis revealed the significant impact of arsenic in antimony- and arsenic-contaminated soil in three Guizhou regions. Front. Microbiol. 14. doi: 10.3389/fmicb.2023.1189400, PMID: 37275177
Mohammed, A. E., Pawelzik, E., Nour, M. M., Alotaibi, M. O., Abdelgawad, H., and Saleh, A. M. (2023). Mycorrhized wheat and bean plants tolerate bismuth contaminated soil via improved metal detoxification and antioxidant defense systems. Plant Physiol. Biochem. 205, 108148. doi: 10.1016/j.plaphy.2023.108148, PMID: 37977026
Nguyen, T. H. H., Sakakibara, M., Sano, S., and Mai, T. N. (2011). Uptake of metals and metalloids by plants growing in a lead-zinc mine area, Northern Vietnam. J. Hazard. Mater. 186, 1384–1391. doi: 10.1016/j.jhazmat.2010.12.020, PMID: 21227580
Olsson, P. A., Rahm, J., and Aliasgharzad, N. (2010). Carbon dynamics in mycorrhizal symbioses is linked to carbon costs and phosphorus benefits. FEMS Microbiol. Ecol. 72, 123–131. doi: 10.1111/j.1574-6941.2009.00833.x, PMID: 20459516
Pan, G. F., Li, W. Z., Huang, L. K., Mo, G. Z., and Wang, X. L. (2024). Arbuscular mycorrhizal fungi promote arsenic accumulation in Pteris vittata L. through arsenic solubilization in rhizosphere soil and arsenic uptake by hyphae. J. Hazard. Mater. 466, 133579. doi: 10.1016/j.jhazmat.2024.133579, PMID: 38290333
Riaz, M., Kamran, M., Fang, Y. Z., Wang, Q. Q., Cao, H. Y., Yang, G. L., et al. (2021). Arbuscular mycorrhizal fungi-induced mitigation of heavy metal phytotoxicity in metal contaminated soils: A critical review. J. Hazard. Mater. 402, 123919. doi: 10.1016/j.jhazmat.2020.123919, PMID: 33254825
Sajjad, W., Ilahi, N., Kang, S. C., Bahadur, A., Banerjee, A., and Zada, S. (2024). Microbial diversity and community structure dynamics in acid mine drainage: Acidic fire with dissolved heavy metals. Sci. Total Environ. 909, 168635. doi: 10.1016/j.scitotenv.2023.168635, PMID: 37981161
Salari, H., Amooaghaie, R., Mozafari, H., Ghorbanpour, M., and Sedaghati, E. (2024). Impact of two arbuscular mycorrhizal fungi species on arsenic tolerance and accumulation in safflower (Carthamus tinctorius L.). BMC Plant Biol. 24, 1174. doi: 10.1186/s12870-024-05906-8, PMID: 39654066
Sonjak, S., Udovic, M., Wraber, T., Likar, M., and Regvar, M. (2009). Diversity of halophytes and identification of arbuscular mycorrhizal fungi colonising their roots in an abandoned and sustained part of Secovlje salterns. Soil Biol. Biochem. 41, 1847–1856. doi: 10.1016/j.soilbio.2009.06.006
Soto, J., Ortiz, J., Herrera, H., Fuentes, A., Almonacid, L., Charles, T. C., et al. (2019). Enhanced arsenic tolerance in Triticum aestivum inoculated with arsenic-resistant and plant growth promoter microorganisms from a heavy metal-polluted soil. Microorganisms 7, 348. doi: 10.3390/microorganisms7090348, PMID: 31547348
Stürmer, S. L., Kemmelmeier, K., Moreira, B. C., Kasuya, M. C. M., Pereira, G. M. D., and da Silva, K. (2018). Arbuscular mycorrhizal fungi (Glomeromycota) communities in tropical savannas of Roraima, Brazil. Mycol. Prog. 17, 1149–1159. doi: 10.1007/s11557-018-1430-5
Tang, J. X., Liao, Y. P., Yang, Z. H., Chai, L. Y., and Yang, W. C. (2016). Characterization of arsenic serious-contaminated soils from Shimen realgar mine area, the Asian largest realgar deposit in China. J. Soil Sediment 16, 1519–1528. doi: 10.1007/s11368-015-1345-6
Tang, B., Xu, H. P., Song, F. M., Ge, H. G., and Yue, S. Y. (2022). Effects of heavy metals on microorganisms and enzymes in soils of lead-zinc tailing ponds. Environ. Res. 207, 112174. doi: 10.1016/j.envres.2021.112174, PMID: 34637758
Timofeev, I., Kosheleva, N., and Kasimov, N. (2018). Contamination of soils by potentially toxic elements in the impact zone of tungstenmolybdenum ore mine in the Baikal region: A survey and risk assessment. Sci. Total Environ. 642, 63–76. doi: 10.1016/j.scitotenv.2018.06.042, PMID: 29894883
Tripathi, P., Singh, P. C., Mishra, A., Srivastava, S., Chauhan, R., and Awasthi, S. (2017). Arsenic tolerant Trichoderma sp reduces arsenic induced stress in chickpea (Cicer arietinum). Environ. pollut. 223, 137–145. doi: 10.1016/j.envpol.2016.12.073, PMID: 28153415
Wu, Z. H., Li, F. L., Wang, F. F., Jin, R. Z., Li, Y. Y., Li, S. L., et al. (2024). A synthetic bacterial consortium improved the phytoremediation efficiency of ryegrass on polymetallic contaminated soil. Ecotox. Environ. Saf. 282, 116691. doi: 10.1016/j.ecoenv.2024.116691, PMID: 38981391
Wu, S. L., Zhang, X., Chen, B. D., Wu, Z. X., Li, T., Hu, Y. J., et al. (2016). Chromium immobilization by extraradical mycelium of arbuscular mycorrhiza contributes to plant chromium tolerance. Environ. Exp. Bot. 122, 10–18. doi: 10.1016/j.envexpbot.2015.08.006
Xin, Y.. (2025). Rhizosphere fungal diversity of Rhus chinensis and its effects on seedling growth under manganese stress. Changsha. Central South University of Forest and Technology. doi: 10.27662/d.cnki.gznlc.2025.000931
Yan, S., Wang, J., Zhang, J. Q., Ning, J. L., Chen, S. L., and Xie, S. G. (2024). Bacterial community composition and function vary with farmland type and soil depth around a mining area. Environ. pollut. 360, 124510. doi: 10.1016/j.envpol.2024.124510, PMID: 39002750
Yang, R. Q., Ma, G. G., Liu, C. L., Wang, C., Kang, X. Y., and Wu, M. H. (2023). Effects of different heavy metal pollution levels on microbial community structure and risk assessment in Zn-Pb mining soils. Environ. Sci. pollut. Res. 30, 52749–52761. doi: 10.1007/s11356-023-26074-6, PMID: 36843164
Yang, Y., Yang, L., and Xiang, X. M. (2013). Bacterial diversity and community structure analysis for two samples of mine drainage from Shimen Realgar Mine, China. Adv. Mater. Res. 641, 246–252. doi: 10.4028/www.scientific.net/AMR.641-642.246
Zeng, X. C., Guoji, E., Wang, J. N., Chen, X. M., Mu, Y., Li, H., et al. (2016). Functions and unique diversity of genes and microorganisms involved in arsenite oxidation from tha realgar mine. Appli. Environ. Microbiol. 82, 7019–7029. doi: 10.1128/AEM.02190-16, PMID: 27663031
Zhang, N., Castlebury, L. A., Miller, A. N., Huhndorf, S. M., Schoch, C. L., and Seifert, K. A. (2006). An overview of the systematics of the Sordariomycetes based on a four-gene phylogeny. Mycologia 98, 1076–1087. doi: 10.3852/mycologia.98.6.1076, PMID: 17486982
Zhang, X., Ren, B. H., Wu, S. L., Sun, Y. Q., Lin, G., and Chen, B. D. (2015). Arbuscular mycorrhizal symbiosis influences arsenic accumulation and speciation in Medicago truncatula L. in arsenic-contaminated soil. Chemosphere 119, 224–230. doi: 10.1016/j.chemosphere.2014.06.042, PMID: 25016555
Keywords: soil remediation, dominant native plant, dominant microbes, arsenic, 18S rDNA
Citation: Zhou M, Shi F, Li X, Su H, Wei Y and Wang F (2025) Revealing the critical role of in-situ plant and microbe community structure in remediation of typical high-arsenic soil through molecular analysis. Front. Plant Sci. 16:1608933. doi: 10.3389/fpls.2025.1608933
Received: 09 April 2025; Accepted: 22 September 2025;
Published: 10 October 2025.
Edited by:
Hongcheng Wang, Guizhou Normal University, ChinaReviewed by:
Manivannan Paramasivan, Bharathidasan University, IndiaMengran Yang, University of Missouri, United States
Copyright © 2025 Zhou, Shi, Li, Su, Wei and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fanfan Wang, d3dmZmFhbm5hemFAMTI2LmNvbQ==
 Min Zhou
Min Zhou Feng Shi2
Feng Shi2 Hailei Su
Hailei Su