- 1International Maize and Wheat Improvement Center (CIMMYT), Nairobi, Kenya
- 2Independent Consultant, Ames, IA, United States
- 3National Livestock Resources Research Institute, National Agricultural Research Organization, Kampala, Uganda
Grain yield (GY) in maize (Zea mays L.) is influenced by multiple component traits, with ear- and tassel-related traits playing a significant role. Despite their importance, these traits receive less emphasis in tropical maize breeding. This study aimed to: (i) assess the inheritance and heterosis of ear and tassel traits, and (ii) investigate their genetic correlation with GY. Thirty tropical maize inbred lines were used to develop 150 hybrids, which were evaluated under artificial Striga hermonthica infestation, managed drought stress, and rainfed conditions over two years. General (GCA) and specific (SCA) combining ability mean squares were significant (P< 0.05) for most traits, indicating the contribution of both additive and nonadditive gene action. GCA sums of squares predominated over SCA, suggesting that additive gene action controlled the inheritance of ear, husk, and tassel traits. Broad-sense heritability was high for husk (H2 = 0.59–0.89), ear (H2 = 0.71–0.93), and tassel (H2 = 0.78–0.95) traits. Fifteen inbred lines exhibited significant positive GCA effects for ear traits, suggesting the presence of favorable alleles associated with increased ear length (ERL) and circumference (ERC). Additionally, 23 inbred lines exhibited favorable GCA effects for reduced tassel size. Mid-parent heterosis for ERL (41%) and ERC (22%) was greater under managed drought stress. Both ERL and ERC were strongly correlated with GY (rg = 0.58–0.96), suggesting their suitability for inclusion in a selection index. Path analysis identified ERL and ERC as having significant positive direct effects on GY, while ear aspect had a negative direct effect on GY across all conditions. Selection for ear and tassel traits in tropical maize is recommended to develop more efficient inbred lines for higher grain yield.
Introduction
Maize (Zea mays L.) contributes significantly to caloric intake and livelihoods in sub-Saharan Africa (SSA). Projections indicate that maize demand will grow at an annual rate of approximately 1.3%, largely driven by its versatility in food, feed, and industrial applications (Ranum et al., 2014). To meet current and future demands, maize productivity per unit area for maize must increase (Mansfield and Mumm, 2014). In SSA, maize yield averages approximately 2.0 t ha-1, while the global average is about 5.8 t ha-1 (Erenstein et al., 2022). More recent estimates indicate an average maize yield of 2.2 t ha-1 in 2023 in SSA (FAOSTAT, 2024). The low maize yields in SSA are attributed to various factors: biotic and abiotic stresses, limited use of inputs, poor agronomy, limited availability of certified hybrid seed, and the prevalence of old maize varieties (Bänziger et al., 2006; Mahuku et al., 2015; Abate et al., 2017; Cairns et al., 2021). Achieving significant maize yield gains, particularly through the development and dissemination of multiple stress-tolerant varieties, is essential for improving maize productivity in SSA. Furthermore, the development of stress tolerant varieties must consider maize genotypes with optimal plant architecture that efficiently utilize assimilates.
The development of efficient plant types using yield components and improved morphological and other physiological traits has been suggested as a key component in crop improvement strategies (Donald, 1968). In many regions, maize yield gains have been attributed to breeding, the use of hybrids, improved crop management practices, and increased plant density (Duvick, 2005; Fasoula and Fasoula, 2002; Lee and Tollenaar, 2007). The relationship between grain yield and morphological characteristics, specifically the process of assimilate production (source), and dry matter accumulation (sink) is critical and a fundamental determinant of maize grain yield (Lee and Tollenaar, 2007; Egli, 2015). Key source traits linked to increased maize productivity include smaller tassels, erect leaves, increased stay-green characteristics during kernel filling, better radiation use efficiency, slower leaf senescence, longer kernel filling periods, reduced lodging, and improved disease and insect resistance (Cavalieri and Smith, 1985; Duvick et al., 2004; Duvick, 2005; Tollenaar and Aguilera, 1992). Sink traits such as ear size (ear length and diameter), ears per plant, kernel number, kernel size, kernel rows per ear have also been associated with higher yield (for a review, see Egli, 2015).
Breeding for reduced tassel size in maize has the potential to enhance photosynthetic efficiency and improve grain yield. Smaller tassels require fewer resources for development, allowing for greater allocation of photosynthetic assimilates and energy towards grain filling (Grogan, 1956; Duncan et al., 1967). Sangoi (2001) reported a 20% reduction in allocation of assimilates to the tassel in improved hybrids. Large tassels reduce light interception in the upper maize canopy (Grogan, 1956), and studies have shown negative correlations between tassel branch numbers and grain yield (Geraldi et al., 1985). Lambert and Johnson (1978) noted that increased grain yield in case of reduced tassel branches results from reduced shading effects in the maize canopy. Lauer et al. (2012) reported a downward trend in tassel branch numbers in temperate maize inbred lines from the 1930s to the 2000s.
Maize ear length and circumference are key morphological traits determining maize grain yield (Huo et al., 2016), with the ear serving as a significant storage reservoir for photosynthetic assimilates. Environmental stress can affect the expression of ear length, leading to variability across different growing conditions (Nielsen, 2003). Maize ear husks are important photosynthetic organs of maize, with high carbon assimilation efficiency and a significant contribution to kernel dry matter per unit area (Fujita et al., 1995). Husk traits such as husk width, husk length, and husk number are important for mechanical harvesting of maize (Zhou et al., 2020), with fewer husks being more conducive for mechanical harvesting (Troyer and Ambrose, 1971). Studies on temperate and other maize germplasm have highlighted the presence of genetic correlations among husk, ear, and tassel traits tropical germplasm (Zhou et al., 2020).
Understanding of the variability and inheritance mechanisms of important source-sink traits in maize is crucial for developing improved high-yield, efficient maize varieties. Combining ability studies have reported the importance of additive gene action over non-additive gene action for ear traits (Mock and Schuetz, 1974; Han and Hallauer, 1989; Fan et al., 2008), husk traits (Brewbaker and Kim, 1979), and tassel branch number (Betrán and Hallauer, 1996; Brewbaker, 2015) in temperate maize. However, in tropical maize, ear length, diameter, and circumference were under the influence of both additive and nonadditive effects (Dhillon and Singh, 1977; Kamara et al., 2020). While evidence of genetic improvements in key agronomic traits associated with increased grain yield, such as ear and tassel characteristics, is available for temperate maize (Duvick et al., 2004, Duvick, 2005; Lauer et al., 2012), comparable data for tropical maize is limited.
Few studies have investigated the genetics of ear or tassel traits in tropical maize, with research limited to one to two traits under optimal management conditions (Kamara et al., 2014; Nardino et al., 2016; Kamara et al., 2020; Onejeme et al., 2020). None of these studies have investigated ear, husk, and tassel traits in a large set of African-adapted tropical maize inbred lines under managed stress environments in a single study. The objectives of this study were to: (i) investigate the inheritance of ear, and tassel traits among tropical maize inbred lines and estimate the heterosis, and (ii) examine genetic correlations between grain yield and other yield component traits under contrasting management conditions.
Materials and methods
Genetic materials
This study used 30 inbred lines of diverse origins and breeding history, including 25 lines from the International Maize and Wheat Improvement Center (CIMMYT) and five Striga-resistant lines from the International Institute of Tropical Agriculture (IITA) (Supplementary Table S1). The CIMMYT lines included 11 doubled haploid (DH) lines developed from F2 populations and 10 lines developed through pedigree breeding. Additionally, four elite CIMMYT lines (CML312, CML543, CML610A, and CKL12128), which express varying levels of drought tolerance were included in the study. The 30 inbred lines were grouped into six sets, with each set having five inbred lines. Lines from one set (females) were crossed with lines from another set (males) in a North Carolina Design II (NC II) mating scheme (Comstock and Robinson, 1948) resulting in 150 experimental single-cross hybrids. Each inbred line was used once as either a female or male in different sets. We used the NC II mating design with sets to reduce the number of hybrids generated when crossing many inbred lines. More details about the NCII with sets design and its use can be found in Hallauer and Miranda Filho (1988, p. 67–72).
Experimental design, test locations
Hybrid trials
The 150 single-cross hybrids, along with two internal genetic gain and four commercial checks were grown in nine trials planted at five locations in Kenya in 2020 and 2021 (Supplementary Table S2). The experimental design was a 4 × 39 alpha-lattice design (Patterson and Williams, 1976), with two replications. Each experimental unit consisted of one row 4 m long, spaced 0.75 m between rows and 0.20 m between plants, to give a final plant population density of approximately 66,666 plants ha-1 at all locations. The 156 hybrids were evaluated in five field trials under artificial Striga infestation at the Kenya Agricultural and Livestock Research Organization (KALRO) research stations at Kibos and Alupe, and at Siaya ATC. Two hybrid trials were planted under rainfed conditions at KALRO Kakamega. Two managed drought stress trials were planted at KALRO Kiboko Research Center. All hybrids were tested in every environment. The location characteristics and soil types at the five locations have been described previously by Makumbi et al. (2015; 2018) and Kimutai et al. (2024). Standard agronomic and cultural practices were followed as recommended for each location.
Line trials
A line evaluation trial, comprising of the 30 parental lines of the 150 experimental hybrids, was formed and laid out as a 3 × 10 alpha-lattice design with two replications. Each experimental unit consisted of two 5 m long rows, spaced 0.75 m between rows and 0.25 m between plants. The inbred line trials were evaluated at the same locations and conditions as the hybrid trials, with the line trials planted side by side with the hybrid trial at all sites. In total, nine inbred line trials were conducted, including five under artificial Striga infestation, two under rainfed conditions, and two under managed drought stress. Standard agronomic and cultural practices, as recommended for each location, were followed.
Artificial Striga infestation and managed drought stress
Artificial Striga hermonthica infestation (hereafter referred to as Striga) was used to ensure uniform exposure to Striga for all genotypes at three locations: Alupe, Kibos, and Siaya ATC. The fields at Kibos and Alupe research had been previously used for imazapyr herbicide studies (Makumbi et al., 2015; Kanampiu et al., 2018). Due to the residual toxicity of imazapyr (Alister and Kogan, 2005), any Striga seeds present in the soil from prior experiments is killed. Striga inoculum was prepared and applied following protocols detailed by Makumbi et al. (2015) and Kanampiu et al. (2018). Di-ammonium phosphate (DAP, 18:46:0) fertilizer was applied at half the recommended rate (30 kg ha-1) at planting to promote plant establishment without suppressing Striga germination. A half dose (30 kg ha-1) of calcium ammonium nitrate (CAN, 26%) fertilizer was applied for topdressing at 4 weeks after planting. Hand weeding was conducted to remove all weeds except Striga plants. The managed drought stress trials were carried out under irrigated conditions during the rain-free period (June–October) at Kiboko. Irrigation water was applied using sprinklers and drip lines at planting to establish a good plant stand, with regular watering during vegetative growth to prevent water stress. During the study period, the average minimum and maximum temperature were 13.3°C and 32.7°C in 2020, and 13.6°C and 31.5°C in 2021. Irrigation water in the drought trial was withdrawn 45 days (V15 stage) after planting. Detailed drought stress management procedures are described in the manual by Bänziger et al. (2000). The anthesis-silking interval (ASI) was regularly calculated to determine any need for additional irrigation water during or after flowering. The average ASI for the hybrids and parental inbred lines was 0.9 and 2.4 days, respectively – both within the acceptable range for good drought stress management, as outlined by Bänziger et al. (2000). As a result, no further irrigation was necessary in the trials. Standard agronomic and cultural practices were followed as recommended for drought stress trials.
Data collection
Data were recorded on agronomic, ear traits, and tassel on plot basis. The agronomic traits recorded included plant aspect, ear rot, and ear weight. Plant aspect was recorded on a scale of 1 to 5, where 1 indicated excellent and 5 indicated poor plant type. Bad husk cover was measured as percentage of plants with ears that are not completely covered by the husks. Ear aspect was recorded on a scale of 1 to 5, based on proper grain filling, ear uniformity, and ear rot infection, with 1 = uniform, large well-filled and clean ears, and 5 = ears with undesirable characteristics. Ear rot was recorded as percentage of harvested ears that are affected due to a combined effect of various fungal diseases. Ear weight was used to calculate grain yield (GY) expressed in t ha-1, adjusted to 80% shelling percentage and 12.5% grain moisture content. Husk traits (husk length, HSL; husk width, HSW, and husk number, HSN), and ear traits (ear length, ERL, and ear circumference, ERC) were measured. Husk traits were phenotyped at harvest following the method outlined by Cui et al. (2016). In brief, husk number was recorded from the outermost layer to the innermost layer, while husk length and width were measured on the 3rd husk from the outside. Husk width was measured at the midpoint of the 3rd husk. Ear length was measured from the base to the tip on eight cobs of well‐bordered plants, and ear circumference was measured on the same cobs. Data on ear traits was recorded on eight plants in a plot. Tassel branch number (TBN) and tassel branch length (TBL), were recorded by counting the number of tassel branches and measuring the branch length on 10 plants in a plot.
Statistical analyses
Analysis of variance
All data were tested for normality using the Shapiro-Wilk test (Shapiro and Wilk, 1965) before analysis of variance. Analyses of variance were performed using PROC MIXED of SAS (SAS Institute, 2016). Entries were considered fixed effects while locations were considered random effects. The linear model below was used for combined analysis across each environment:
where Yijrk is the mean of the ith genotype, in the rth replicate within the kth subblock of the jth environment; μ is the grand mean; αi is the effect of the ith genotype; βj is the effect of the jth environment; ρr is the effect of the rth replicate; ρr(βj) is the effect of the replicates within environments; λk[ρr(βj)] is the effect of the incomplete blocks within replicates and environments; αβij is the effect of genotype × environment interaction; and ϵijrk is the residual error.
In the across-environment analysis of variance, the significance of the genotype effects was tested using the corresponding genotype × environment interaction as the error term, while the genotype × environment interaction was tested using the pooled error. Each location-year combination was considered a separate environment. All factors were considered random effects to estimate variance components. The best linear unbiased estimates (BLUEs) and the best linear unbiased predictions (BLUPs) were computed using META-R (Alvarado et al., 2020).
Broad-sense heritability was estimated for combined environments according to Hallauer et al. (2010) as:
where is the genotypic variance, is the variance of the interaction between the genotype and environment, is the number of environments, is the number of replicates, and the is the residual variance.
Genotypic correlations were estimated using META-R for pairs of traits following Holland (2006) as:
where is the estimated genotypic covariance between traits i and j, and are the estimated genotypic standard deviations for traits i and j, respectively.
Mid-parent (MPH) and high-parent (HPH) heterosis of all traits were calculated using the BLUEs of the hybrids and inbred lines. Mid-parent heterosis was calculated as where, F1 is the hybrid mean performance, and MP = (P1 + P2)/2 where P1 and P2 are the means of the two parents. High-parent heterosis was calculated as where HP is mean of the best parent.
Design II analysis
To estimate combining ability of the lines and hybrids, an analysis of variance was conducted for the 150 experimental hybrids using the PROC GLM of SAS (SAS, 2016) following the North Carolina Design II model (Comstock and Robinson, 1948). The following general linear model was used for the analysis across environments.
where Yijkl is the observed trait value, µ is the grand mean, mi is the effect of the ith male, fj is the effect of the jth female, (m × f)ij is the effect of interaction between ith male and jth female, (m × e)ik is the effect of the ith male in the kth environment, (f × e)jk is the effect of the jth female in the kth environment, (m × f × e)ijk is the interaction effect between ith male and jth female in the kth environment, ek is the effect of the kth environment, rl(ek) is the effect of lth replication in the kth environment, and ϵijkl is the residual error.
In an NC II sets design, the variance components of variance for hybrids within sets are partitioned into those attributable to male (sets), female (sets), and the female × male (sets) interaction (Hallauer et al., 2010). The proportion of GCA-male, GCA-female, and SCA for each trait was computed as a percentage of the sum of squares for the hybrids in each environment. Estimates of GCA effects for agronomic, tassel, and ear traits for the inbred lines and SCA effects for each hybrid, were computed from BLUEs across environments using PROC MEANS of SAS (SAS, 2016).
Path analysis
To examine cause and effect relationships among grain yield, tassel, ear, and agronomic traits, BLUPs for these traits were subjected to sequential path analysis to mitigate against multicollinearity which occurs when two or more independent variables in a regression model are highly correlated (Samonte et al., 1998; Sserumaga et al., 2020). In sequential path analysis, traits were classified into first, second, or third order (or higher) based on their impact on the total variation in grain yield, using stepwise regression. Path coefficient analysis was performed separately for artificial Striga infestation and rainfed conditions, and a combination of both using SPSS version 20 (IBM, 2011).
Results
Analysis of variance
The combined ANOVA for the hybrids revealed significant (P< 0.01) mean squares for environment (E) and genotype (G) across most ear, tassel, and plant aspect traits under artificial Striga infestation, rainfed, and managed drought stress conditions (Tables 1, 2). However, the genotype effect was not significant for ear rot (EROT) under artificial Striga infestation, and the environment effect was not significant for bad husk cover (BHC) under managed drought stress conditions. Partition of the genotype source of variation showed significant (P< 0.05) mean squares for GCAm/sets, GCAf/sets and SCA/sets for all traits under artificial Striga infestation except SCA/sets for EROT (Table 1). Under rainfed conditions, GCAm/sets, GCAf/sets and SCA/sets mean squares were highly significant (P< 0.001) for all traits except GCAf/sets for BHC, and SCA/sets for HSL, HSW, BHC, and tassel branch length (TBL) (Table 2). Under managed drought stress, GCAm/sets, GCAf/sets and SCA/sets mean squares were significant (P< 0.05) for all traits except GCAm/sets for BHC, and SCA/sets for HSW.
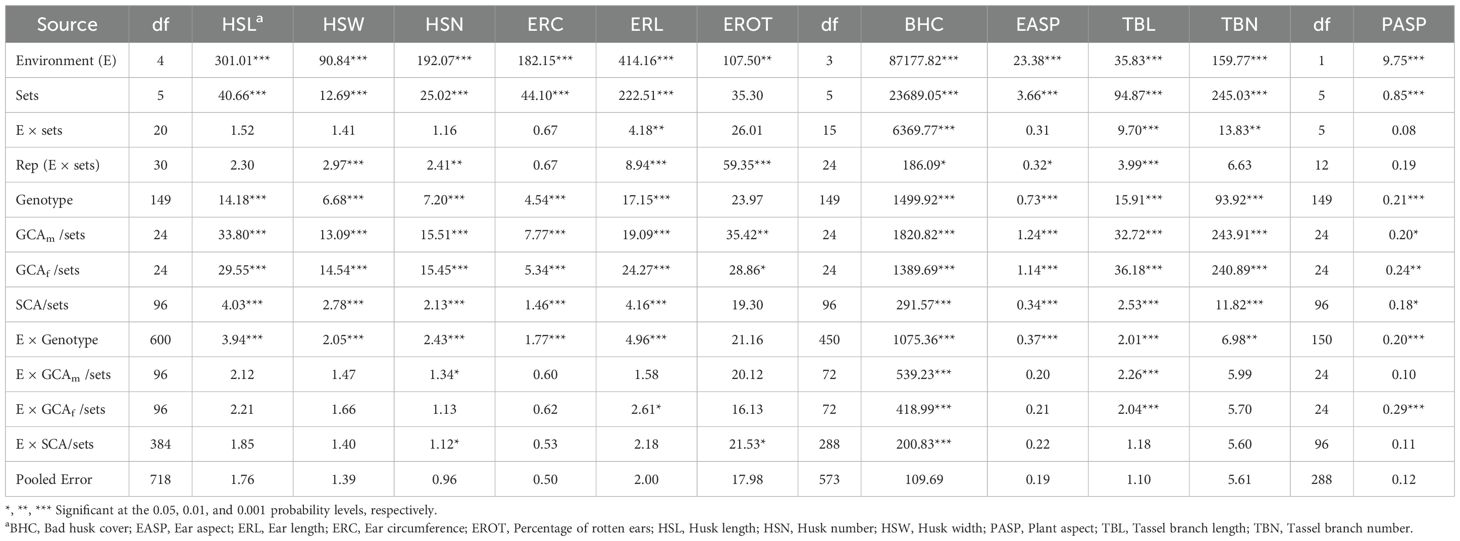
Table 1. Mean squares from combined ANOVA for husk, ear, tassel, and plant aspect traits of 150 NCII hybrids evaluated under artificial Striga infestation at three locations in 2020 and 2021.
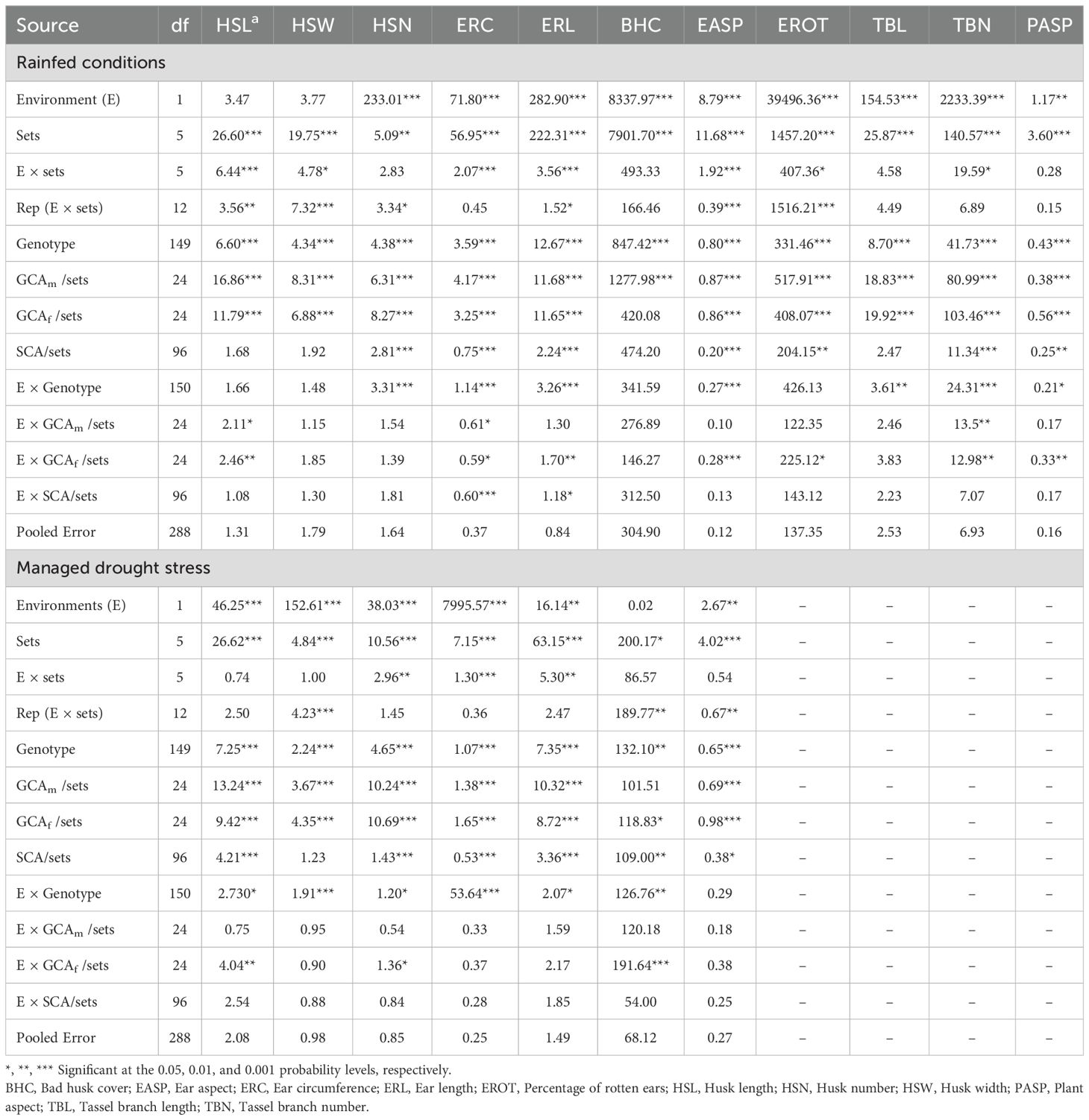
Table 2. Mean squares from combined ANOVA for husk, ear, tassel, and plant aspect traits of 150 NCII hybrids evaluated under rainfed conditions at Kakamega and managed drought stress at Kiboko in 2020 and 2021.
The G × E interaction was significant (P< 0.01) for all traits under artificial Striga infestation except for EROT (Table 1). However, the G × E interaction was not significant for husk traits (HSL, HSW and BHC) and EROT under rainfed conditions, and ear aspect under managed drought stress conditions (Table 2). The GCAm/sets × E interaction was significant (P< 0.05) for HSN, BHC, and TBL under artificial Striga infestation, while GCAf/sets × E interaction was significant (P< 0.05) for ear length (ERL), BHC, TBL, and plant aspect (PASP). In contrast, both GCAm/sets × E and GCAf/sets × E interactions were significant for HSL, ear circumference (ERC), and tassel branch number (TBN) under rainfed conditions.
The partitioning of the genotype sums of squares into GCA (GCAf + GCAm) and SCA revealed that GCA accounted for 71.3 to 79.7% of the total variation for husk traits (HSL, HSW, HSN) and 69.2 to 72.2% for ear traits (ERC, ERL) among hybrids under artificial Striga infestation (Figure 1). Similarly, GCA accounted for a larger proportion of the variation among hybrids for tassel traits TBN and TBL (87.2 to 91.1%) and BHC (73.4%). Under rainfed conditions, GCA accounted for a larger proportion to the total variation among hybrids, contributing 56.4% to 81.1% for husk traits, 71.3% to 72.3% for ear traits, and 79.7% to 82.2% for tassel traits, while SCA sums of squares explained 52.8% of the variation among hybrids. Under managed drought stress conditions, GCA sums of squares were of greater magnitude than SCA sum of squares for all measured traits (Supplementary Table S3). A comparison of the contributions of GCAm and GCAf showed that GCAf was greater than GCAm for HSL, ERC, and BHC, while both effects were equal in magnitude for HSN and TBN under artificial Striga infestation. Under rainfed conditions GCAf was greater than GCAm for HSL, HSW, ERC, BHC, and EROT, but both effects were equal in magnitude for ERL and ear aspect (EASP).
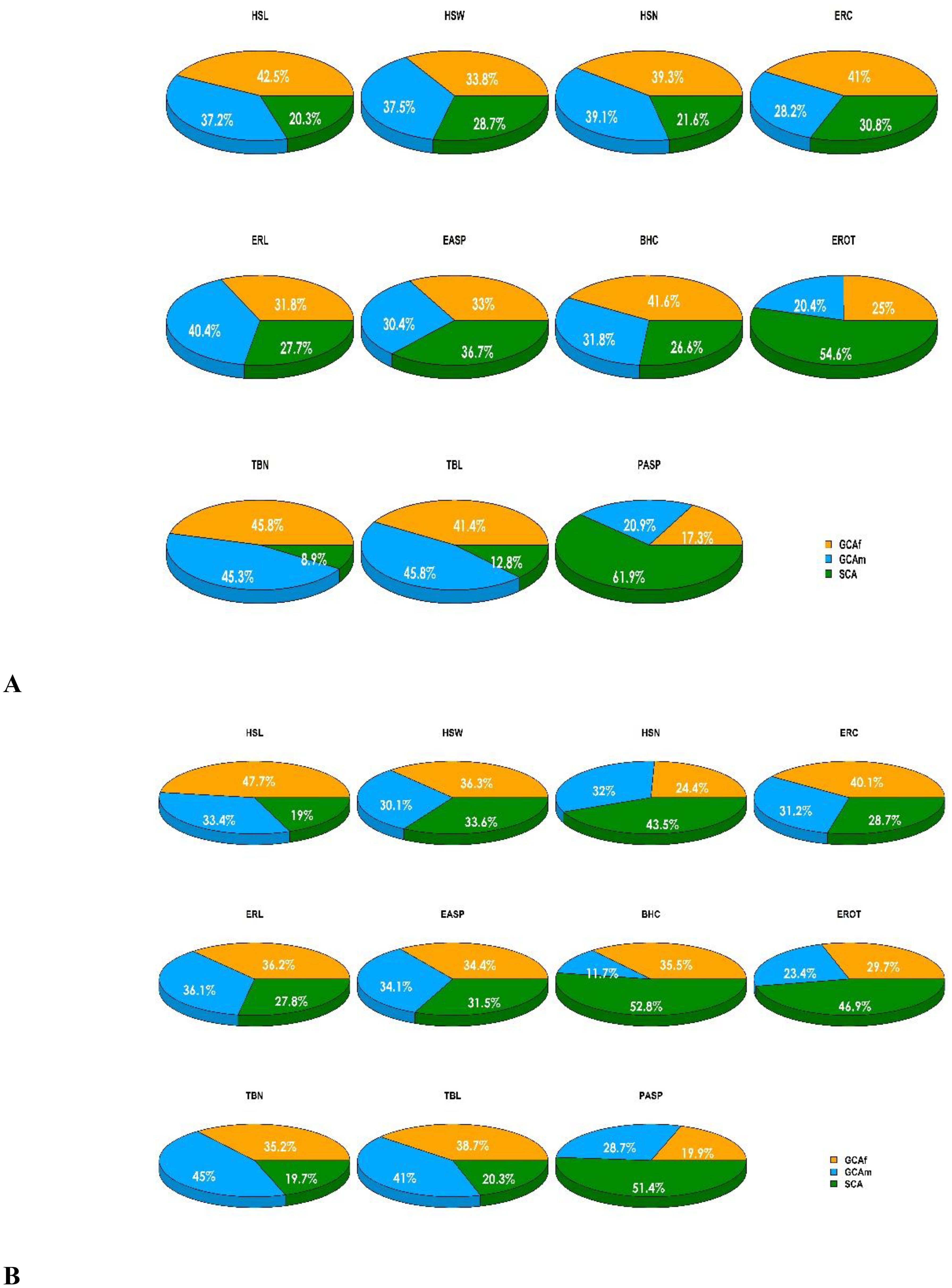
Figure 1. Graphical representation of the sums of squares contribution for husk, ear, tassel, and plant aspect traits under two conditions: (A) artificial Striga infestation, and (B) rainfed conditions. BHC, Bad husk cover; EASP, Ear aspect; ERL, Ear length; ERC, Ear circumference; EROT, Percentage of rotten ears; HSL, Husk length; HSN, Husk number; HSW, Husk width; PASP, Plant aspect; TBL, Tassel branch length; TBN, Tassel branch number.
Means, variance components, and broad-sense heritability
The summary statistics for traits measured under contrasting management conditions are shown in Table 3; Figure 2. The mean HSL showed slight variation across the three management conditions, with values of 20.8 cm under artificial Striga infestation, 22.7 cm under rainfed conditions, and 22.1 cm under managed drought stress conditions. The means for HSN were similar for rainfed conditions and managed drought stress, but slightly lower for artificial Striga infestation. The ear traits ERC and ERL showed smaller values under stressed conditions, with means of 13.8 cm and 15.7 cm under artificial Striga infestation, and 8.5 cm and 14.3 cm under managed drought stress. In contrast, under rainfed conditions, the means were higher: 15.4 cm for ERC and 17.8 cm for ERL. The means for tassel traits TBL and TBN were largely identical under artificial Striga infestation and rainfed conditions.
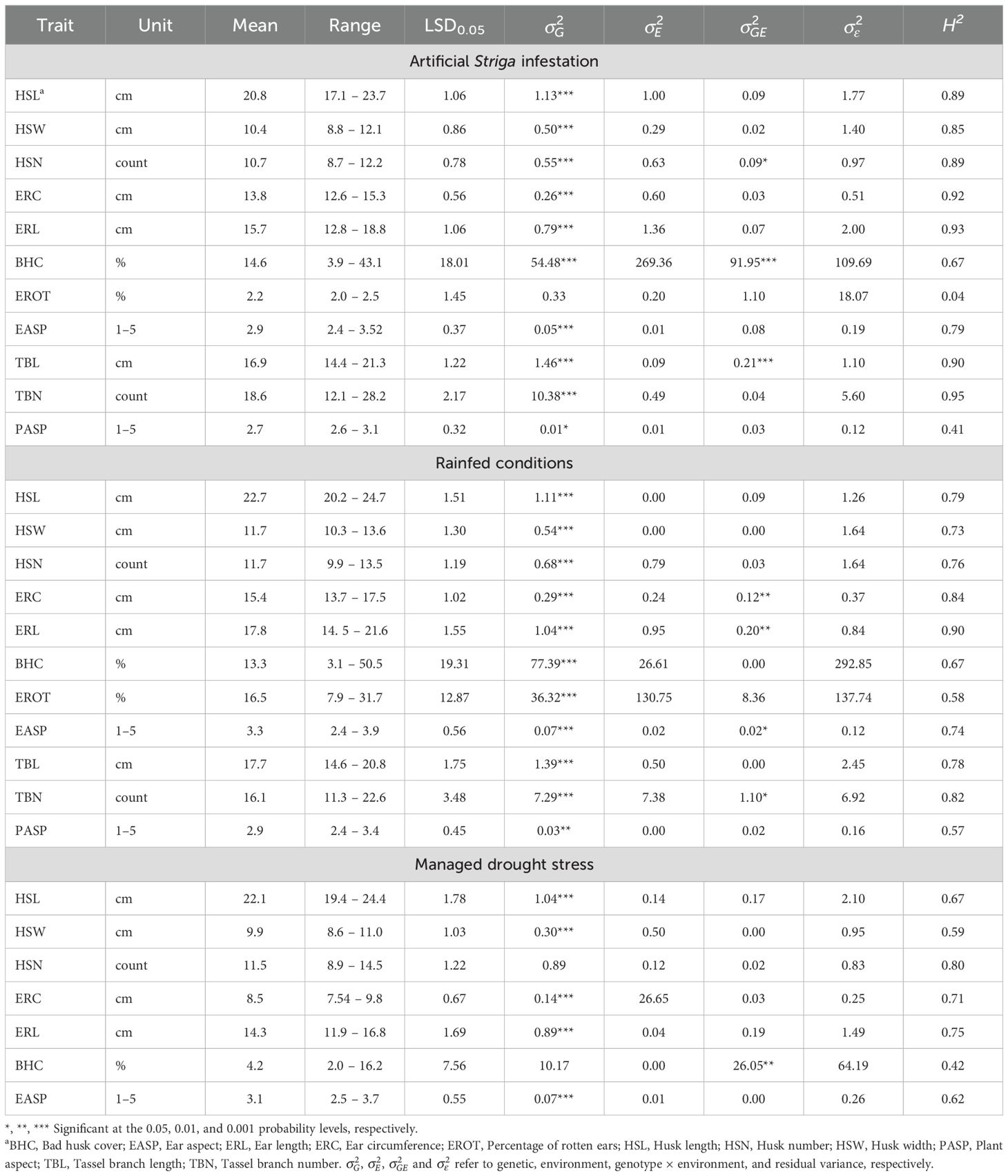
Table 3. Summary statistics, variance component and broad-sense heritability (H2) estimates for husk, ear, and tassel traits of 150 maize hybrids under artificial Striga infestation, rainfed, and managed drought stress conditions.
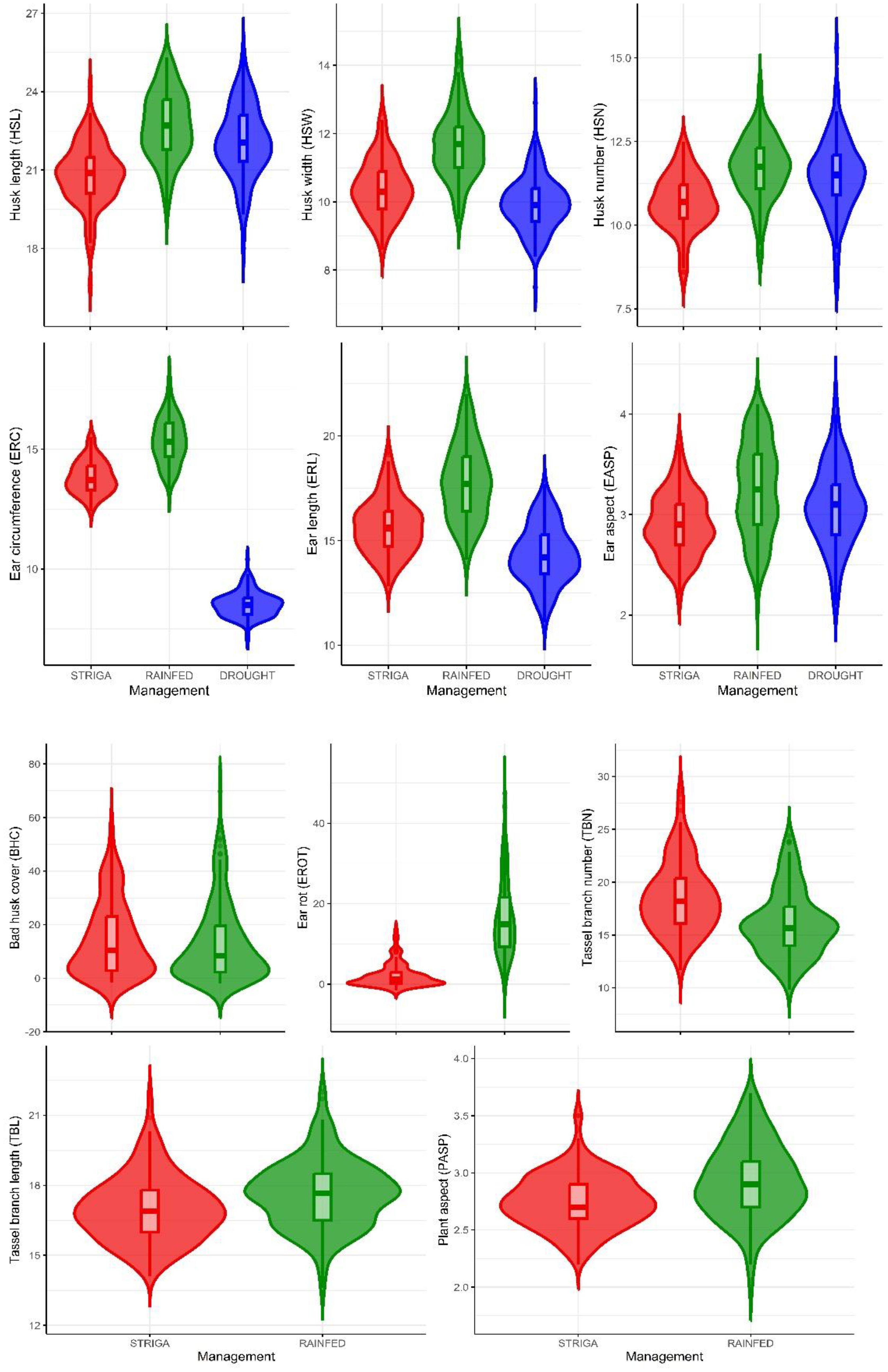
Figure 2. Means of husk, ear, tassel, and plant aspect traits of 150 maize hybrids. Red, green, and blue violin plots represent artificial Striga infestation, rainfed, and managed drought stress conditions, respectively.
Genetic variance estimates were significantly different from zero for all traits under all management conditions, except for EROT under artificial Striga infestation and HSN and BHC under managed drought stress. Under artificial Striga infestation, genetic variance exceeded environmental variance for two husk traits (HSL and HSW), EASP, and tassel traits (TBL and TBN). Broad-sense heritability estimates were generally high across all management conditions, ranging from 0.67 to 0.95 (Table 3). Under artificial Striga infestation, husk traits (0.85 to 0.89), ear traits (0.92 to 0.93), and tassel traits (0.90 to 0.95) exhibited particularly high heritability estimates. Exceptions included moderate heritability for PASP, EROT, HSW, and BHC (0.41 to 0.59) and low heritability for EROT under artificial Striga infestation.
Combining ability effects
The GCA effects for husk, ear, and tassel traits under artificial Striga infestation are shown in Table 4. Nine inbred lines including TZSTR189, CKDHL171092, CKDHL171119, CKDHL171527, CKL17604, CKL17517, CKL17531, CKL17719, and CML610A exhibited significant (P< 0.05) positive GCAf and GCAm effects for HSL. For HSW, significant positive GCAf and GCAm effects were observed in eight inbred lines, with two of these lines displaying significant positive GCAf and GCAm effects for both HSL and HSW. For HSN, one line (CKDHL171162) had significant negative GCAf and GCAm effects, while five lines showed significant negative GCAf effects, and 11 lines showed significant negative GCAm effects. Conversely, three lines (CKL17513, CML543 and CML312) showed significant positive GCAf effects and significant negative GCAm effects for HSN. The GCA effects for ERC and ERL varied among the lines. Seven inbred lines exhibited significant (P< 0.05) positive GCAf and GCAm effects for ERC, while eight lines showed significant positive GCAf and GCAm effects for ERL. Notably, CKL17571 showed significant positive GCAf and GCAm effects for both ERC and ERL. For tassel traits, 11 inbred lines exhibited significant negative GCAf and GCAm effects for TBL, while 13 inbred lines showed significant negative GCAf and GCAm effects for TBN. Four lines (TEISTR1159, CKL17961, CKDHL171527 and CKL17611) showed significant negative GCAf and GCAm effects for both TBL and TBN.
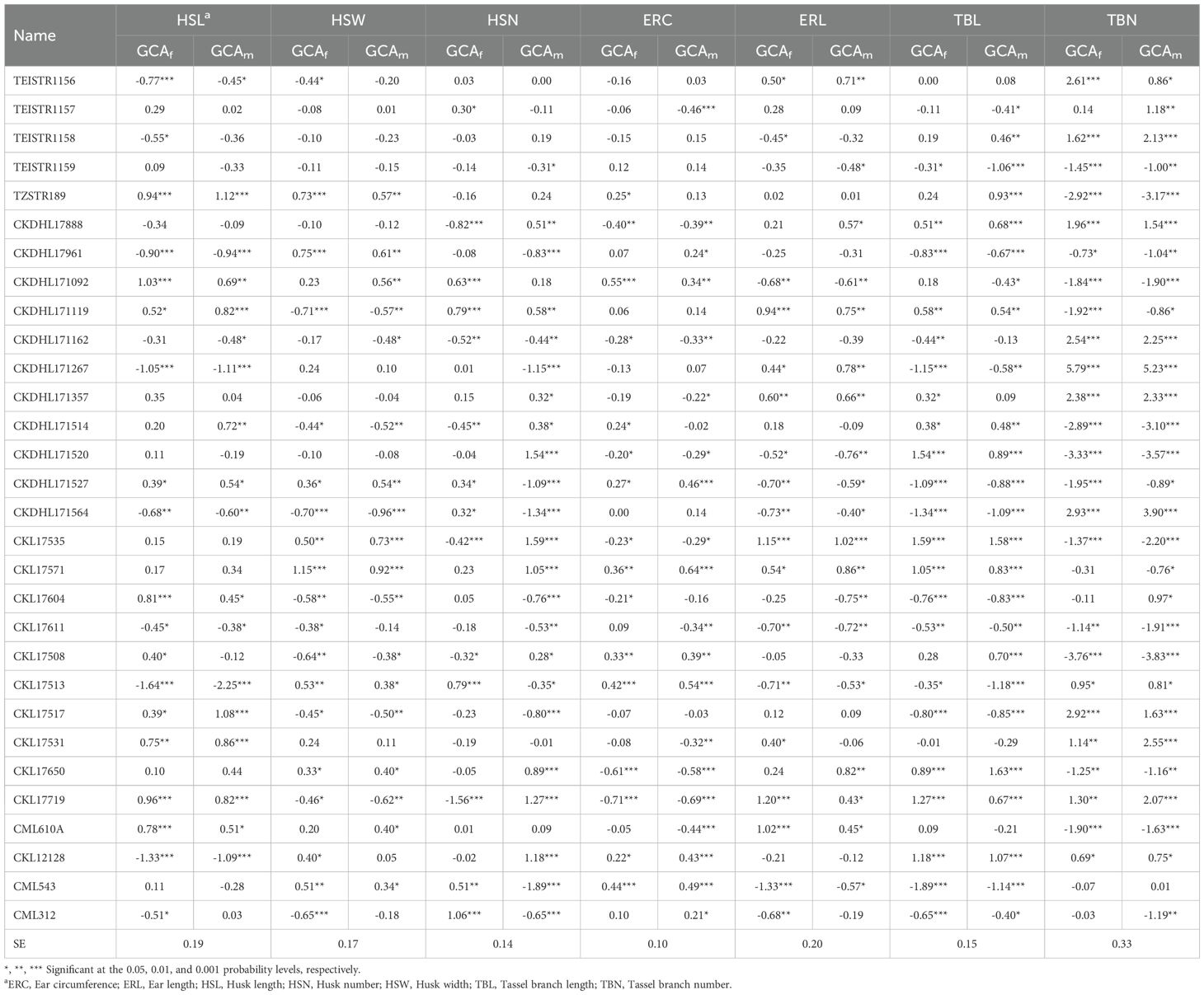
Table 4. Estimates of general combining ability effects of females (GCAf) and males (GCAm) for husk, ear, and tassel traits of 30 tropical maize inbred lines evaluated under artificial Striga infestation across five environments in 2020 and 2021.
Five inbred lines exhibited significant (P< 0.05) desirable (negative) GCAf and GCAm effects for BHC, while four additional lines showed significant desirable GCAf effects (Supplementary Table S4). Furthermore, two lines (CKDHL171092 and CKL17508) showed significant desirable GCAm effects for BHC. Inbred lines CKDHL171514 and CKL17650 showed significant desirable (negative) GCAm effects for EROT. Favorable (negative) significant GCAf and GCAm effects for EASP were observed on five lines, while two lines showed favorable GCAm effects.
The GCA effects for husk, ear, and tassel traits under rainfed conditions are presented in Table 5. Seven inbred lines showed significant (P< 0.05) positive GCAf and GCAm effects for HSL. Additionally, two lines (CKL17650 and CML543) had significant positive GCAm effects. Only two lines exhibited significant positive GCAf and GCAm effects for HSW. However, six additional lines showed either significant positive GCAf or GCAm effects. For HSN, three lines exhibited significant negative GCAf and GCAm effects for HSN, with three more lines showing either significant negative GCAf or GCAm effects. Significant positive GCAf and GCAm effects for ERC were observed in five lines (TZSTR189, CKDHL171092, CKL17571, CKL17513, and CML543), while six lines (CKDHL171119, CKDHL171267, CKL17535, CKL17571, CKL17531, and CML610A) showed significant positive GCAf and GCAm effects for ERL. An additional five lines showed significant positive GCAm effects. Four lines (CKDHL171527, CKL17513, CKL17517, and CML543) showed significant GCAf and GCAm effects for reduced TBL, and seven more lines had either significant GCAf or GCAm effects. In contrast, seven lines showed significant GCAf and GCAm effects for reduced TBN. Additionally, three lines exhibited significant GCAf effects, and two lines showed significant GCAm effects for reduced TBN.
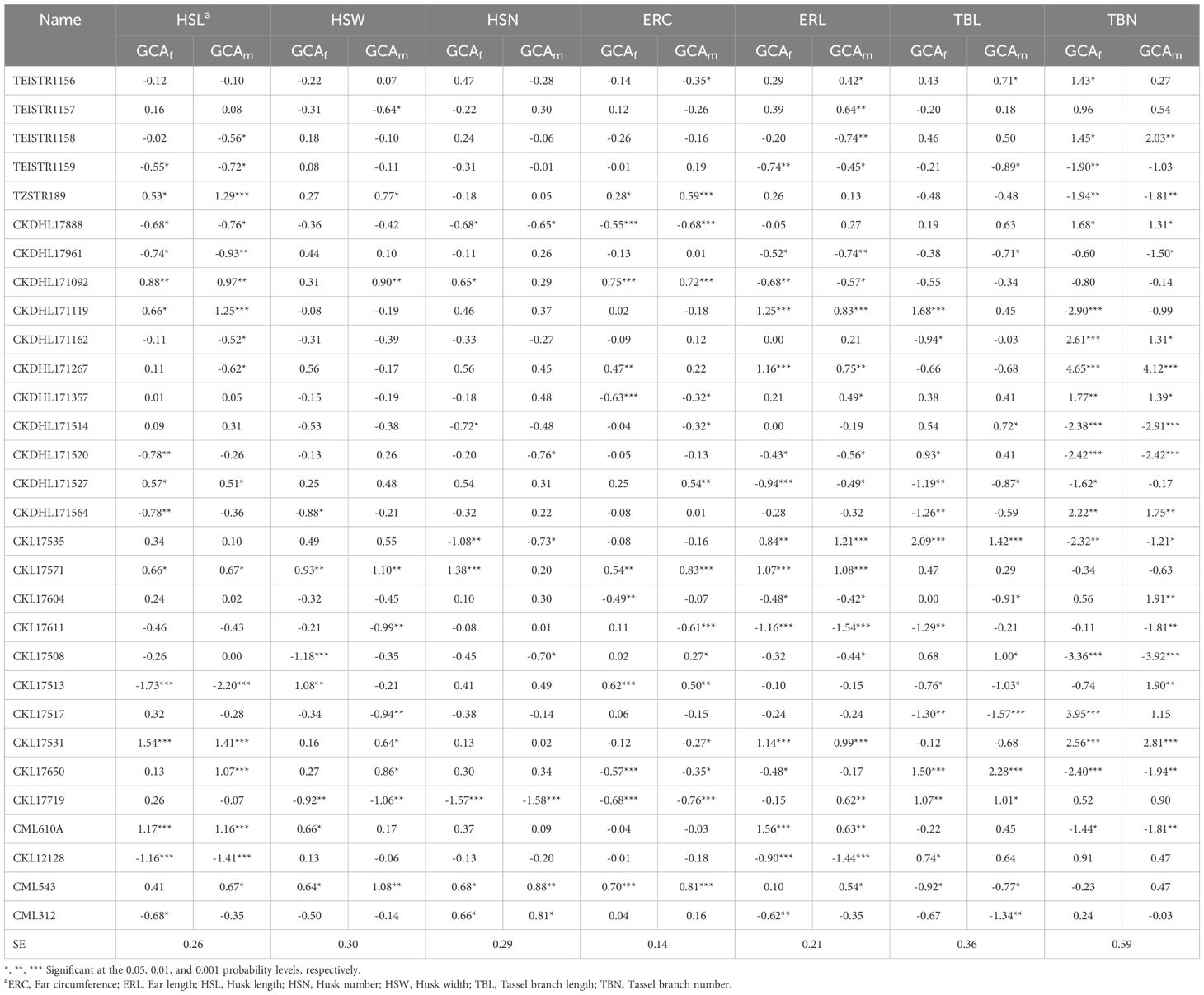
Table 5. Estimates of general combining ability effects of females (GCAf) and males (GCAm) for husk, ear, and tassel traits of 30 tropical maize inbred lines evaluated under rainfed conditions in 2020 and 2021.
The GCA effects for agronomic traits under rainfed conditions are shown in Supplementary Table S4. One inbred line exhibited significant (P< 0.05) desirable (negative) GCAf and GCAm effects for BHC, while three additional lines showed significant desirable GCAm effects. Inbred lines TEISTR1157 and CKDHL171527 showed significant desirable (negative) GCAf and GCAm effects for EROT, with five other lines exhibiting significant desirable GCAf or GCAm effects. Five inbred lines showed desirable significant (P< 0.05) GCAf and GCAm effects for EASP. Four lines showed significant negative GCAf effects for PASP, while two lines exhibited significant negative GCAm effects.
Under managed drought stress conditions, six inbred lines showed significant (P< 0.05) positive GCAf effects for HSL, while five lines exhibited significant positive GCAm effects (Table 6). Only one line (CML610A) showed significant positive GCAf and GCAm effects for HSL. Similarly, six inbred lines showed significant positive GCAf effects for HSW, with two lines (CKL17535 and CML610A) showing significant positive GCAf and GCAm effects. Seven lines exhibited significant negative GCAf and GCAm effects for HSN, while five lines showed significant positive GCAf and GCAm effects for the same trait. Significant and positive GCAf and GCAm effects for ERC were exhibited by five lines (TEISTR1159, CKL17571, CKL17513, CKL12128, and CML543), while four lines (CKDHL171267, CKDHL171357, CKL17535, and CKL17531) exhibited significant positive GCAf and GCAm effects for ERL. Six inbred lines showed significant negative GCAf effects for TBL, and three lines exhibited significant negative GCAm effects. Two lines showed significant negative GCAf and GCAm effects, with an additional five lines exhibiting significant negative GCAf effects.
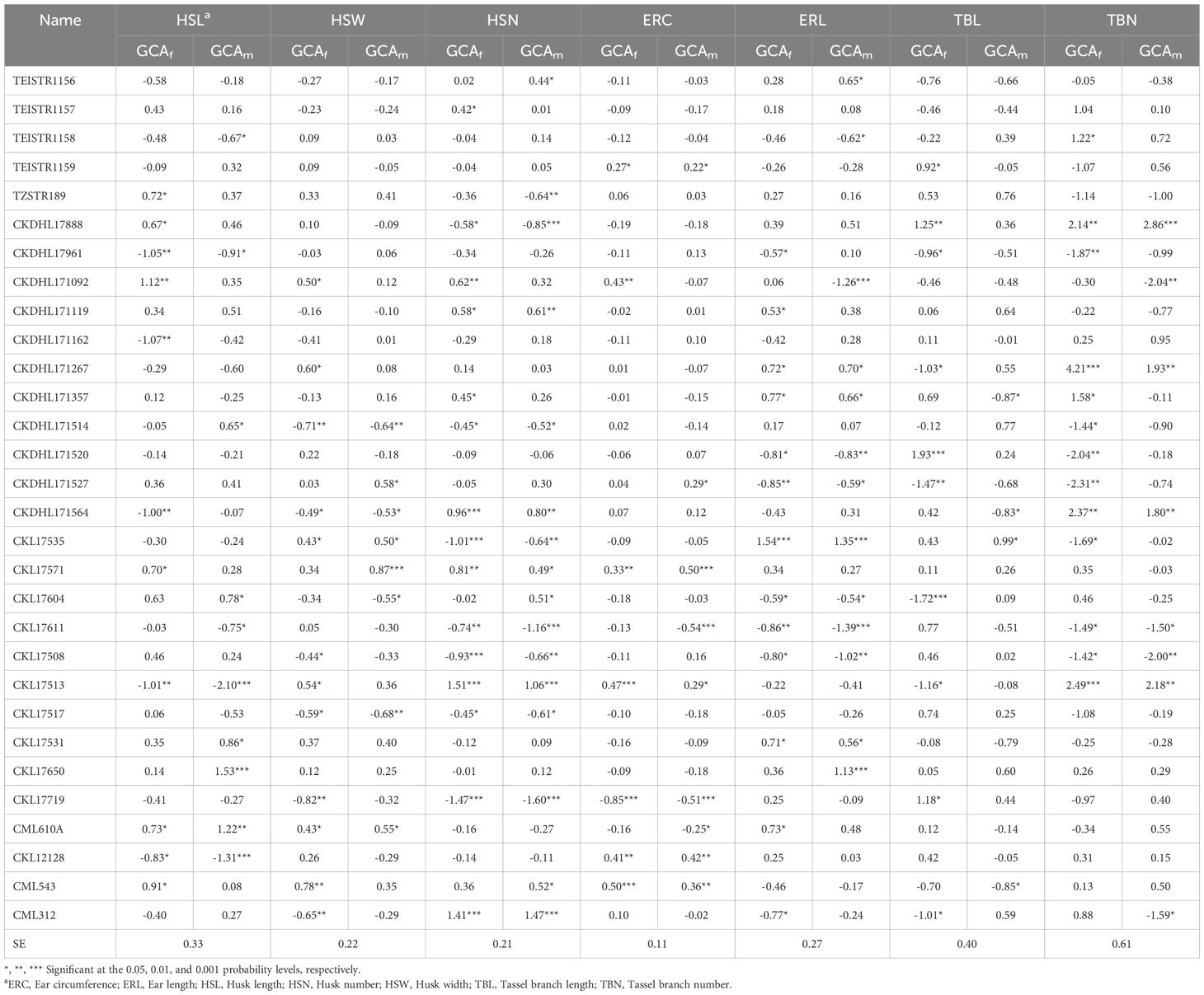
Table 6. Estimates of general combining ability effects of females (GCAf) and males (GCAm) for husk, ear, and tassel traits of 30 lines evaluated under managed drought stress conditions at Kiboko in 2020 and 2021.
ANOVA for inbred lines and per se performance
The combined ANOVA for inbred lines revealed significant environment (E), genotype (G), and G × E interaction mean squares for all traits, except for G × E interaction for HSN and BHC under artificial Striga infestation (Supplementary Table S5). Significant variation attributable to both E and G was observed for the measured traits under rainfed and managed drought stress conditions. Additionally, the G × E interaction was significant for PASP under rainfed conditions, and for BHC and ERC under managed drought stress conditions. Under artificial Striga infestation, the mean ERC was 11.69 cm, ranging from 10.82 to 13.03 cm, while ERL ranged from 11.15 to 14.90 cm, with a mean of 12.85 cm. The TBL values ranged from 10.7 to 19.2, and TBN varied from 8 to 24. Under managed drought stress conditions, the average values for ERC and ERL were lower than those recorded under artificial Striga infestation. Broad-sense heritability estimates were moderate for BHC under both artificial Striga infestation (0.56) and managed drought stress conditions (0.46), as well as for EROT under artificial Striga infestation (0.50). For all other traits, except for PASP, broad-sense heritability estimates were high, ranging from 0.67 to 0.95 (Supplementary Table S5).
Genotypic correlations among traits
Genotypic correlations among the measured traits under artificial Striga infestation and rainfed conditions are presented in Figure 3. Of the 66 trait pairs under artificial Striga infestation, 19 exhibited significant positive genotypic correlations, while 31 pairs showed significant negative correlations. Ear-related traits (HSL, HSW, HSN, ERC, ERL) and BHC were negatively correlated with PASP (rg = –0.29 to –0.61, P< 0.001). HSL and HSW had significant (P< 0.01) correlations with EASP and TBN. HSW and HSN were positively correlated with ERC (P< 0.001). Both ERC and ERL were positively and significantly (P< 0.001) correlated with BHC, and ERL was positively correlated with TBL (P< 0.001). TBN was negatively correlated with TBL (–0.38, P< 0.001). Grain yield (GY) had significant (P< 0.001) positive correlations with BHC, HSW, ERC, ERL and TBL. Significant (P< 0.05) negative correlations were observed between GY and EROT, PASP, TBN and EASP. Under rainfed conditions, a similar pattern of negative genotypic correlations between PASP and ear related traits (HSL, HSW, HSN, ERC, ERL) and BHC was observed. The husk traits (HSL, HSW, HSN) were positively correlated with ERC and ERL (rg = 0.18 to 0.69, P< 0.05). ERL was also positively correlated with both TBL and TBN (rg = 0.21 to 0.24, P< 0.05). Significant positive correlations (P< 0.01) were observed between GY and BHC, husk traits, ERC, ERL, and TBN. Under managed drought stress conditions, genotypic correlations among traits revealed that BHC exhibited a significant negative correlation with all recorded traits (rg = –0.19 to –0.71, P< 0.01) (Figure 3). HSL was negatively correlated with both HSW and HSN, while ERC and ERL were positively correlated with both HSW and HSN. Significant (P< 0.0) positive correlations between GY and HSL, HSW, HSN, ERC, and ERL was observed.
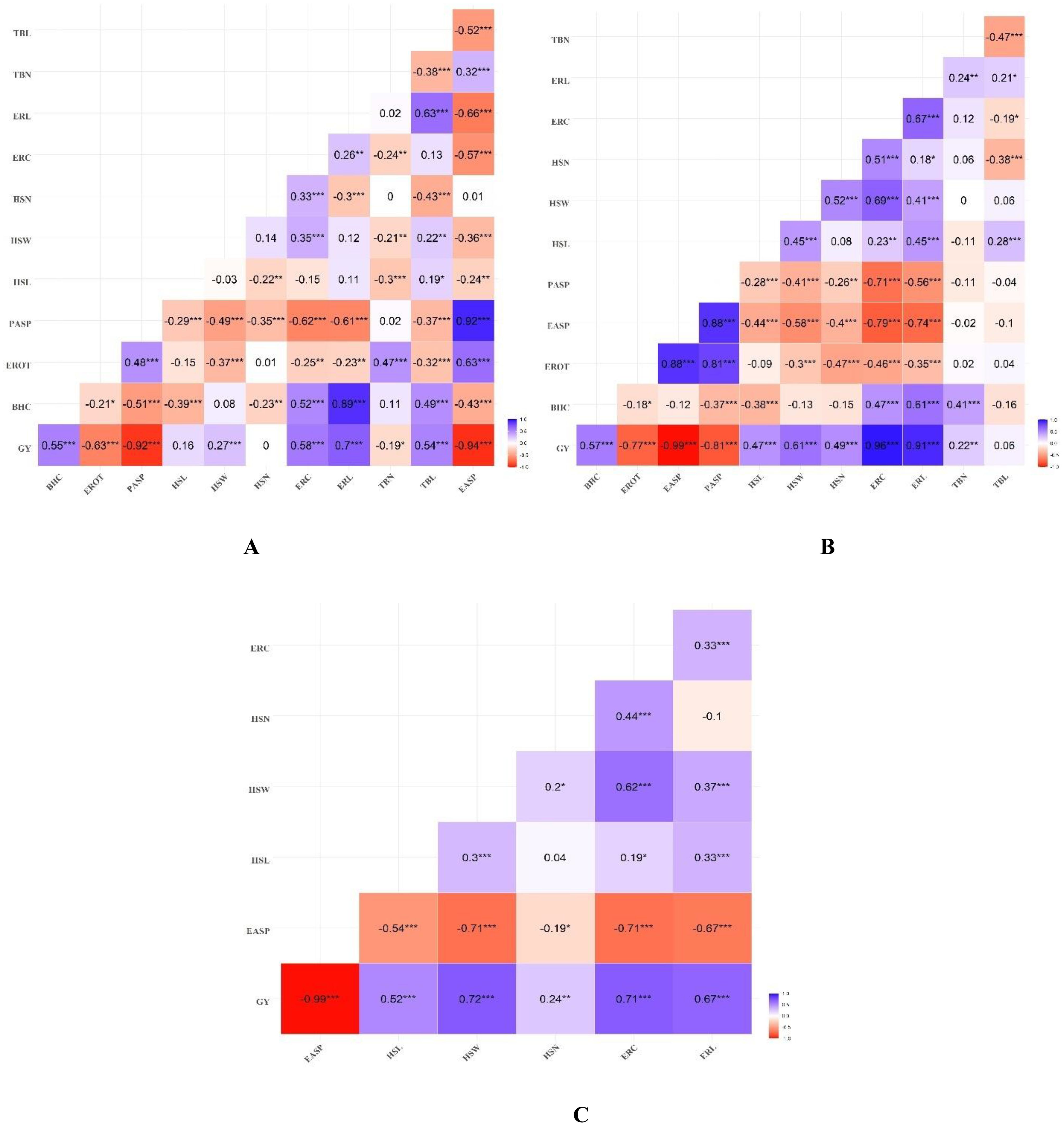
Figure 3. Heatmap of genotypic correlations between grain yield, husk, ear, and tassel related traits under (A) artificial Striga infestation, (B) rainfed, and (C) managed drought stress conditions. *, **, *** Significant at 0.05, 0.01, and 0.001 probability levels, respectively.
Heterosis estimates for ear and tassel traits
Mid-parent (MPH) and high-parent heterosis (HPH) estimates varied across the three management conditions (Figure 4). Under artificial Striga infestation, the average MPH for ERL was 22.8%, ranging from -1.57% to 39.90%, with a similar range observed for HPH. Tassel branch number (TBN) exhibited greater heterosis compared to TBL, with MPH of 53.2% and 24%, respectively. Under managed drought stress, MPH and HPH for ERC and ERL were of higher magnitude than those under artificial Striga infestation, with a two-fold increase in MPH for ERL. The MPH ranged from 2.27% to 45.50% for TBL and from 1.96% to 102.70% for TBN under rainfed conditions. The range of heterosis for TBL and TBN was larger under rainfed conditions compared to artificial Striga infestation.
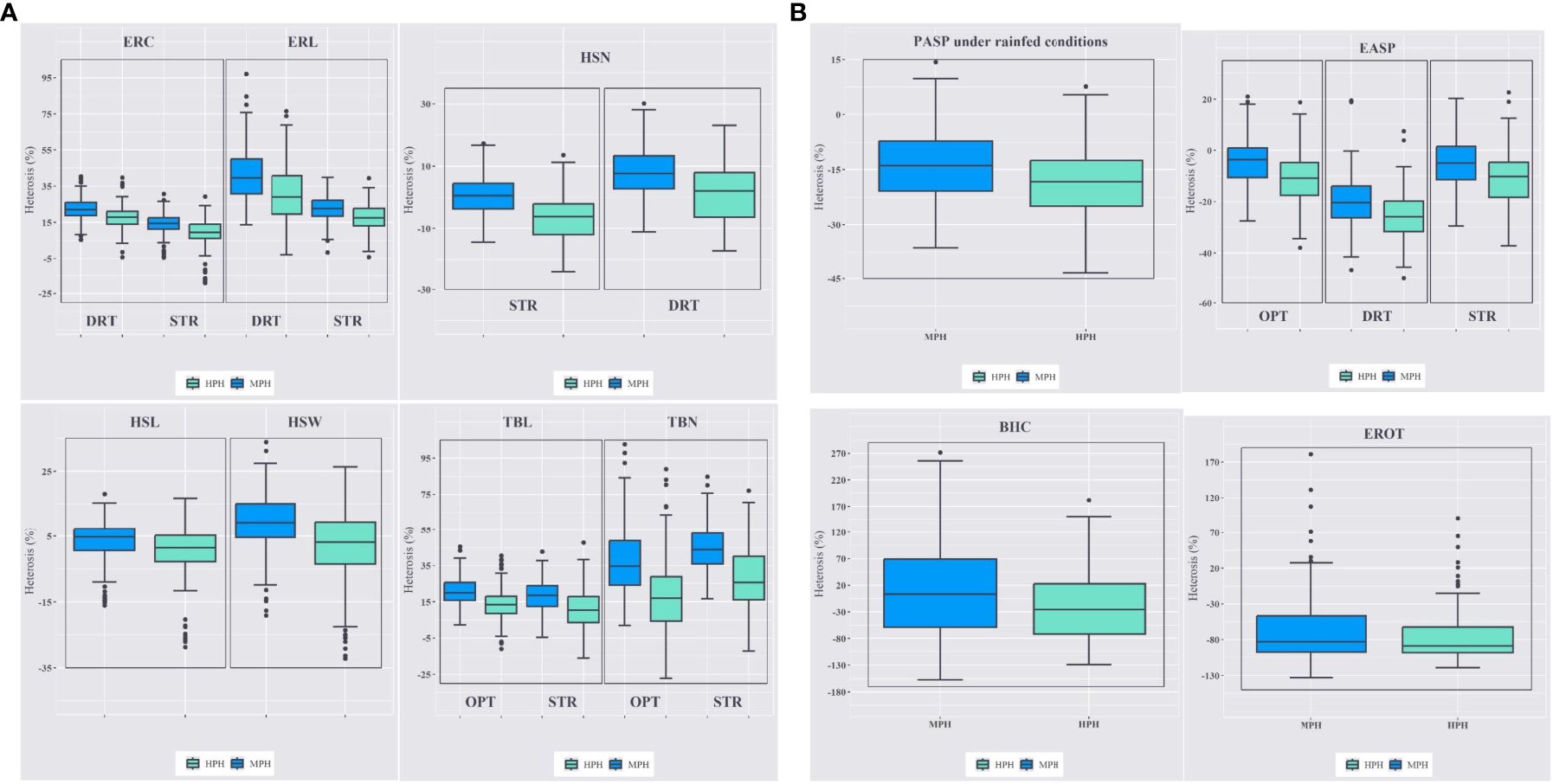
Figure 4. (A) Mid-parent and high-parent heterosis estimates for ear, husk and tassel traits under managed drought stress (DRT), artificial Striga infestation (STR), and rainfed (OPT) conditions. ERC, ear circumference; ERL, ear length; HSN, husk number; HSL, husk length; HSW, husk width; TBL, tassel branch length; TBN, tassel branch number. (B) Mid-parent and high-parent heterosis estimates for plant aspect (PASP), ear aspect (EASP); bad husk cover (BHC), and percentage of rotten ears (EROT) under rainfed, artificial Striga infestation, and managed drought stress conditions.
Path analysis
Path analysis using stepwise regression identified EASP, ERL, and ERC as first-order traits, which together explained 77% of the variation in GY under artificial Striga infestation (Figure 5A). Ear length and ERC had positive path coefficients (0.22 and 0.15, respectively), while EASP had a negative direct path coefficient of –0.64 with GY. Among the second-order traits, TBL had the largest indirect effect (0.60) on GY. Second-order traits PASP, TBL, and TBN influenced GY indirectly via ERL, while HSW, HSN, and PASP affected GY through ERC.
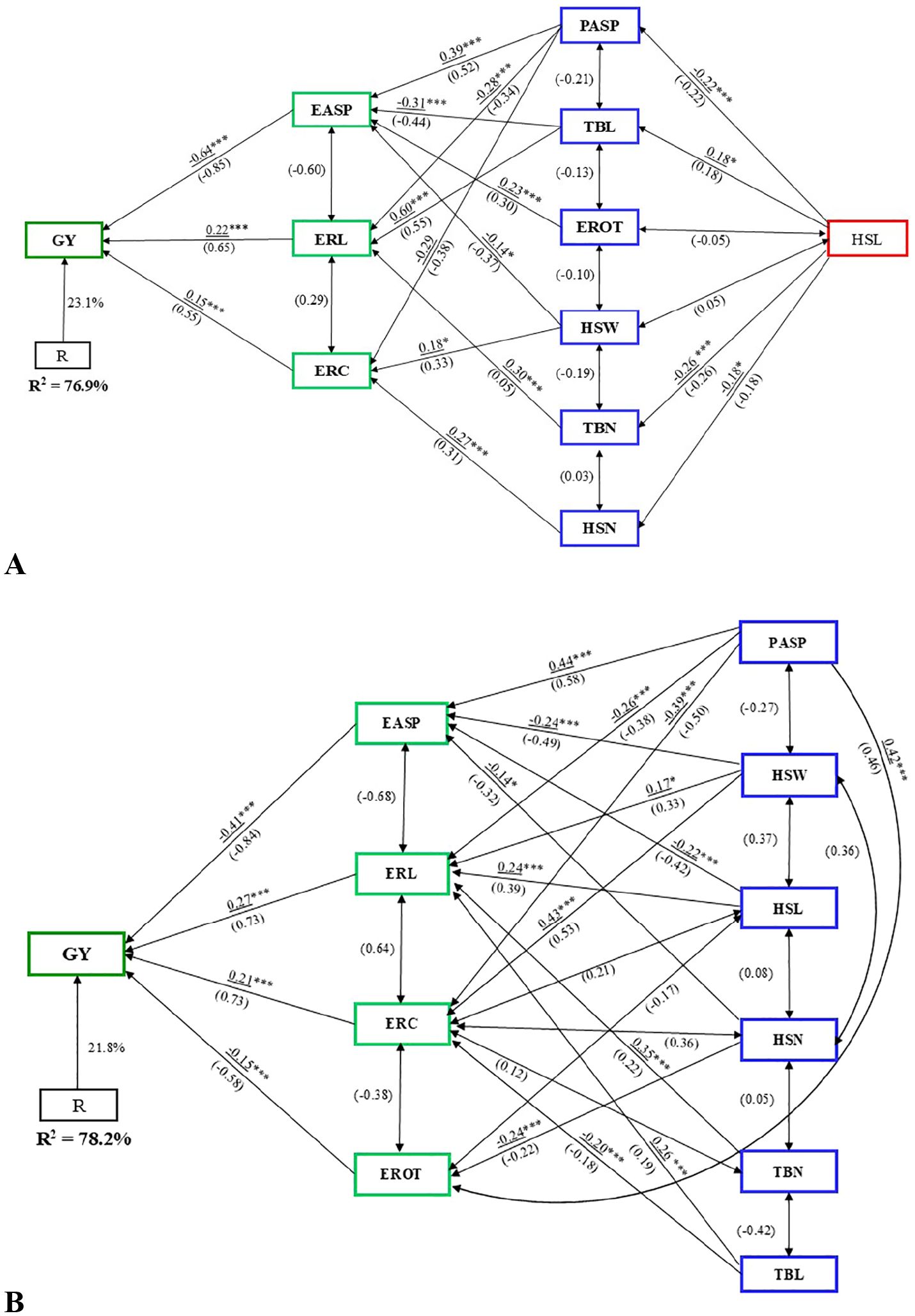
Figure 5. Path analysis model illustrating the causal relationships between grain yield (GY) and husk, ear, tassel, and agronomic traits under (A) artificial Striga infestation, and (B) rainfed conditions. Underlined values represent direct path coefficients, while values in parenthesis indicate correlation coefficients. Single-arrow lines represent path coefficients, while double-arrow lines denote correlation coefficients. EASP, ear aspect; ERC, ear circumference; ERL, ear length; EROT, Percentage of rotten ears; HSL, husk length; HSN, husk number; HSW, husk width; PASP, plant aspect; TBL, tassel branch length; TBN, tassel branch number. *, **, *** Significant at the 0.05 and 0.001 probability levels, respectively.
Under rainfed conditions, path analysis revealed EASP, ERL, ERC, and EROT as first-order traits, accounting for 78% of the variation in GY (Figure 5B). ERL and ERC had positive path coefficients of 0.27 and 0.21, respectively, while EASP and EROT had negative direct path coefficients with GY. The rest of the traits were categorized as second-order traits. The second-order traits, except HSN, influenced GY indirectly through ERL. Additionally, PASP, HSW, and TBL had indirect effects on GY via ERC, while HSN and PASP impacted GY through EROT. Among the second-order traits, PASP had the largest indirect effect (0.44), followed by HSW (0.43).
In the combined analysis across artificial Striga infestation and rainfed conditions, the first-order traits – EASP, ERL, ERC, and PASP – explained 84% of the variability in GY (Figure 6). ERL and ERC had positive direct path coefficients (0.21 and 0.13, respectively), while EASP and PASP showed negative direct path coefficients (–0.59 and –0.13, respectively) with GY. Similar to the analysis under rainfed conditions, only first- and second-order traits were identified. Second-order traits EROT, HSW, TBL, and TBN influenced GY indirectly through ERL. TBL and EROT had the largest indirect effects (0.56 and 0.53, respectively).
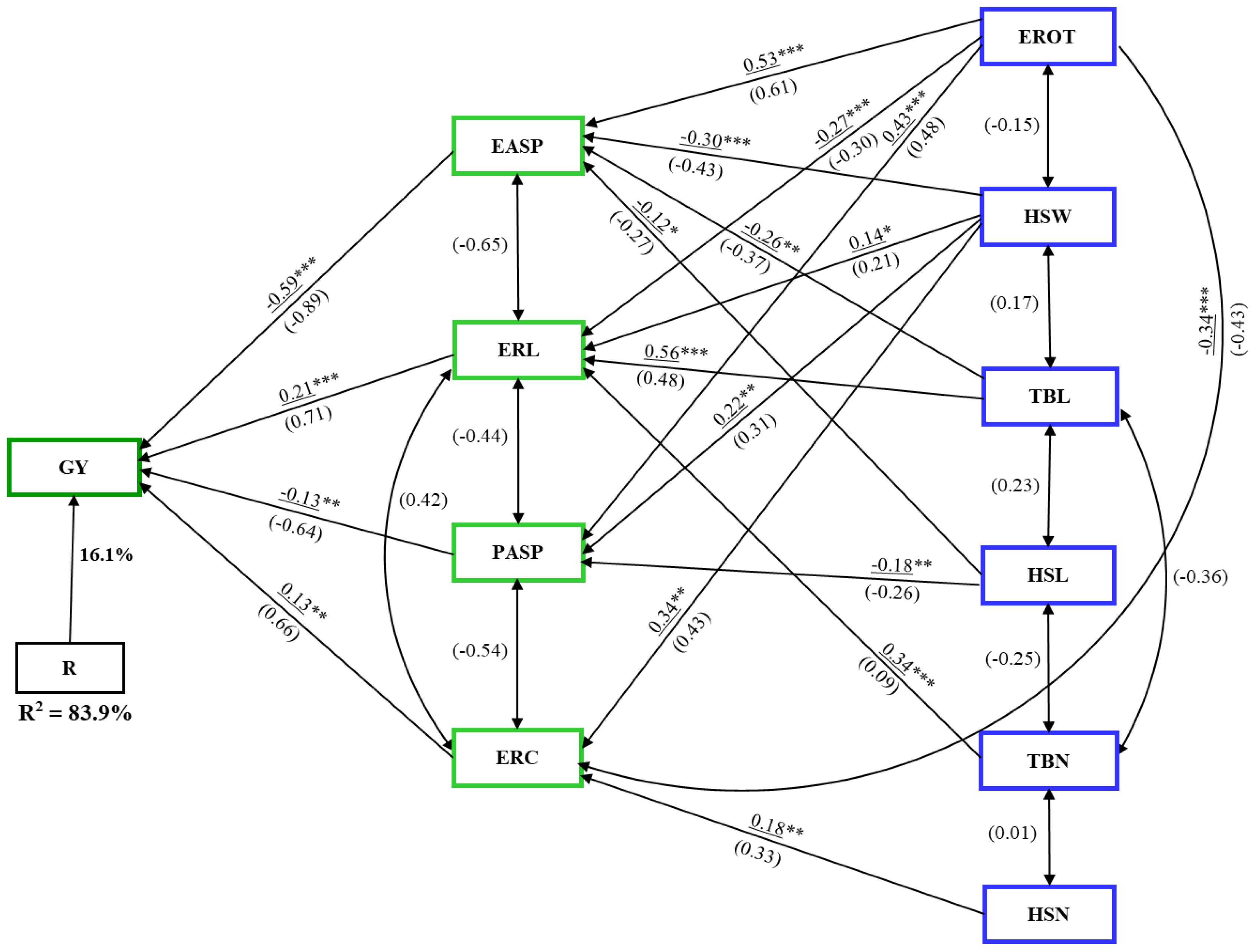
Figure 6. Path analysis model illustrating the causal relationships between grain yield (GY), husk, ear, tassel, and agronomic traits under combined artificial Striga infestation and rainfed conditions. Underlined values represent direct path coefficients, while values in parenthesis indicate correlation coefficients. Single-arrow lines represent path coefficients, while double-arrow lines denote correlation coefficients. EASP, ear aspect; ERC, ear circumference; ERL, ear length; EROT, Percentage of rotten ears; HSL, husk length; HSN, husk number; HSW, husk width; PASP, plant aspect; TBL, tassel branch length; TBN, tassel branch number. *, **, *** Significant at the 0.05, 0.01, and 0.001 probability levels, respectively.
Discussion
Maize grain yield is influenced by the partitioning of assimilates between vegetative and reproductive tissues, particularly during critical growth phases such as flowering and grain-filling. Improved yields in temperate maize have been attributed to changes in plant and ear morphology, an extended grain-filling period, and tolerance to both biotic and abiotic stresses (Duvick, 2005; Fischer and Edmeades, 2010; Lauer et al., 2012; Egli, 2015). Among the traits associated with increased yields are those related to the ear and tassel. A limited number of studies have explored the variability in some of the plant and ear morphological traits that are crucial for grain yield in tropical maize (Guei and Wassom, 1996; Sofi, 2007; Sorsa et al., 2023). This study examined the genetic variation and inheritance of husk, ear, and tassel traits in mid-altitude adapted tropical maize lines under different management conditions.
Genetic variability and heritability
Breeding programs rely on genetic variability to make selections leading to genetic improvements in traits of interest. The significant genotypic differences observed among the parental lines and hybrids for most of the traits indicate sufficient genetic variability in the germplasm used in this study to enable progress through selection for these traits. This result is in agreement with findings from other studies on maize under varying stress and non-stress conditions (Betrán et al., 2003; Makumbi et al., 2018; Badu-Apraku et al., 2020). Furthermore, genetic variances were significant and generally exceeded the magnitude of G × E interaction variances for most traits, consistent with findings from studies on Striga-resistant germplasm with diverse genetic backgrounds (Menkir and Kling, 2007; Kimutai et al., 2024). The significant G × E interactions for most traits suggest variability in genotype responses, likely influenced by differing climatic conditions across the seasons of evaluation. Similar results have been reported for various traits under Striga infestation across seasons (Menkir et al., 2012; Makumbi et al., 2015; Kanampiu et al., 2018; Kimutai et al., 2024), as well as under rainfed and managed stress conditions (Bolaños, 1995; Makumbi et al., 2011, 2018; Mageto et al., 2017; Worku et al., 2016), underscoring the importance of multi-environment or multi-year evaluations for identifying stable genotypes. Conversely, the non-significant G × E interaction for some traits (HSL and HSW) under rainfed conditions suggests these traits were stable across environments, and that genotype evaluation for these traits can rely primarily on genetic differences, with minimal environmental influence, potentially reducing the need for extensive multi-environment testing.
Broad-sense heritability estimates for husk, ear, and tassel traits in this study were predominantly high for both hybrids and lines across all management conditions, except for moderate estimates for ERC, HSL, and HSW under managed drought stress. These results suggested that much of the observed variability is due to genetic factors, indicating strong potential for genetic gains through selection. Consequently, narrow-sense heritability estimates for these traits is also expected to be high (Falconer and Mackay, 1996). Brewbaker (2015) reported high narrow-sense heritability for TBN in some biparental populations of temperate maize, whereas Guei and Wassom (1996) found low narrow-sense heritability for TBN in tropical maize. Sorsa et al. (2023), reported low to moderate broad-sense heritability estimates for ERL and ear diameter in tropical maize. Multiple studies have reported moderate to high heritability estimates for ear traits such as ERL and ear diameter (Han and Hallauer, 1989; Yu et al., 2020; Yang et al., 2021; Wang J. et al., 2023; Chang et al., 2024), tassel traits like TBN and TBL (Mock and Schuetz, 1974; Schuetz and Mock, 1978; Geraldi et al., 1985; Upadyayula et al., 2006; Yi et al., 2019; Zeng et al., 2022), and husk traits including HSL, HSN, and HSW (Zhou et al., 2020; Zhang et al., 2021, 2022) in temperate maize.
Relative importance of additive and nonadditive effects, and combining ability
The significant GCA and SCA suggested that both additive and nonadditive genetic effects influenced the inheritance of husk, ear, and tassel traits across all contrasting environments. This result suggested that simple selection methods could be effective for improving traits such as ERC and TBN. Previous inheritance studies in temperate and tropical maize have reported variation in the relative importance of additive and nonadditive genetic effects for these traits. For TBN, our results are consistent with the findings in several studies that highlighted the importance of additive genetic effects in temperate and tropical maize (Mock and Schuetz, 1974; Schuetz and Mock, 1978; Guei and Wassom, 1996; Nardino et al., 2016; Onejeme et al., 2020; Kamara et al., 2020). Husk number is primarily under the influence of additive genetic effects (Brewbaker and Kim, 1979). Similarly, both additive and nonadditive effects were reported to influence ear length, diameter, and circumference in maize of different adaptations (Dhillon and Singh, 1977; Onejeme et al., 2020; Kamara et al., 2020). Kamara et al. (2014) reported the importance of non-additive gene action over additive gene action for ear length and diameter.
In this study, the GCA sums of squares predominated the SCA sums of squares for husk, ear, and tassel traits across all management conditions. These findings indicate that additive gene action was predominant in the inheritance of ear, husk, and tassel traits across all management conditions. This is consistent with previous studies that reported significant contribution of additive gene action to traits such as ERL and ear diameter (Han and Hallauer, 1989; Fan et al., 2008; Kamara et al., 2020), husk number (Brewbaker and Kim, 1979), and TBN (Betrán and Hallauer, 1996; Brewbaker, 2015). Wolf and Hallauer (1997) reported that epistatic effects were important for ERL in temperate maize. The study revealed significant GCAm/sets × E, GCAf/sets × E, and SCA/sets × E interactions for most traits under artificial Striga infestation, rainfed, and managed drought stress conditions, indicating that the GCA effects and hybrid performance (SCA) varied across environments. Similar results have been reported for various agronomic traits under Striga infestation, low N, and managed drought stress conditions (Makumbi et al., 2011; Badu-Apraku et al., 2016; Njeri et al., 2017).
The GCA effects for husk, ear, and tassel traits varied across contrasting environments. The consistently significant positive GCA (GCAf and GCAm) effects for HSL expressed by six inbred lines (CML610A, CKDHL171092, CKDHL171119, CKDHL171527, CKL17531 and TZSTR189) under both artificial Striga infestation and rainfed conditions, indicated that these inbreds possess favorable alleles for longer husks. Notably, CML610A also exhibited significant GCA effects for HSL under managed drought stress, indicating consistency of its GCA effects across environments. Nine inbred lines showed significant positive GCAf and GCAm effects for HSW, suggesting that these lines carry favorable alleles for increased HSW. Interestingly, CML610A, which had positive GCA effects for HSL, also showed positive GCA effects for HSW, indicating that this line carries good favorable allele combinations that can be exploited in breeding programs. The results under artificial Striga infestation showed that 16 lines carried favorable alleles for increased HSN (significant positive GCAf or GCAm effects), while others exhibited significant negative GCAf or GCAm effects for HSN. Similar patterns were observed under rainfed and managed drought stress conditions. These findings suggest that both maternal and paternal effects influence inheritance of HSN, implying that the choice of a line as either the female or male parent could impact the expression of husk number in a hybrid.
Husks in maize serve various functions, from contributing to photosynthesis to protecting developing kernels from birds, insect pests, and diseases. The GCA effects observed in this study suggest that this germplasm offers favorable alleles for breeding maize suited to different needs. For instance, increased husk numbers have been linked to reduced damage from fall armyworm (FAW) and corn earworm (CEW) (Brewbaker and Kim, 1979), making lines with favorable GCA effects for increased HSN valuable for breeding programs. However, shorter husk leaves may increase the vulnerability of kernels to insect and bird damage. Inbred lines with favorable GCA effects for longer husks can be used to develop hybrids with better husk coverage to protect kernels and avoid open tips. An interesting area for future research would be to evaluate the impact of husk traits such as number, tightness, and length on FAW ear damage in sub-Saharan Africa (SSA). Additionally, husk tightness has been identified as a barrier to Fusarium growth (Warfield and Davis, 1996; Cao et al., 2014) and aflatoxin contamination in maize (Barry et al., 1986). While increased husk number may be beneficial in some environments, fewer husks are preferred in temperate regions for faster drying (Troyer and Ambrose, 1971), and suitability for mechanical harvesting.
In this study, some inbred lines exhibited significant positive GCAf and GCAm effects for ERC and ERL artificial Striga infestation, suggesting that these inbred lines possess favorable alleles for larger and longer ears. The six lines with significant positive GCAf and GCAm effects for ERL (TEISTR1156, CKDHL171119, CKDHL171267, CKDHL171357, CKL17535, and CKL17571) have a breeding history of Striga resistance, with one line among these (TEISTR1156) showing promising results when tested in hybrid combination under artificial Striga infestation (Menkir et al., 2012). The putative Striga resistance in these lines may have contributed to their favorable ear characteristics under stress conditions caused by Striga infestation. Additionally, four lines expressed consistent significant positive GCAf and GCAm effects for ERL under both rainfed and artificial Striga infestation conditions, indicating that these lines possess favorable alleles for these traits under both stressed and non-stressed conditions. Three inbred lines (CKDHL171267, CKDHL171357, and CKL17535) exhibited consistently significant positive GCAf and GCAm effects for ERL under both artificial Striga infestation and managed drought conditions, indicating their genetic value in maintaining ear length under biotic and abiotic stress conditions. Ear length and ear diameter are some of the critical grain yield components in maize, and traits like these can be used as indirect selection criteria for improved grain yield. Inbred lines with desirable GCA effects for increased ear length identified in this study are potential candidates for use in a selection program aimed at improving ear length in mid-altitude tropical maize germplasm.
Grain yield results from both dry matter accumulation and its partitioning to the grain at physiological maturity, with the latter being a function of kernels per plant and weight per kernel (Tollenaar and Lee, 2006; Lee and Tollenaar, 2007). Grain yield limitations in maize other than those induced by biotic and abiotic stresses can be examined by considering the availability of assimilates to the developing grain (source), and the capacity of the grains to store the assimilates (sink) (Tollenaar, 1977). Sink size can be a limiting factor in maize, but it can be overcome by increasing ear size (Egli, 2015). The two ear traits (ERL and ERC) described herein are important in determination of kernels per plant and therefore contribute to the size of the sink, and ultimately grain yield in maize. Therefore, breeding efforts to develop genotypes with longer or larger ears can lead to yield increase. In-depth studies using a targeted number of hybrids developed from inbred lines with contrasting GCA effects for ear traits identified in this study could enhance our understanding of source-sink relationships in this germplasm. This approach would be especially valuable under Striga infestation, where the balance between assimilate production and utilization is affected. Previous research involving a limited number of hybrids has successfully explored such dynamics (e.g. Rajcan and Tollenaar, 1999; Tollenaar and Daynard, 1978, 1982; Uhart and Andrade, 1991), providing a foundation for applying similar methodologies to this unique set of germplasm. Furthermore, genomic approaches could be employed to investigate source-sink relationships under artificial Striga infestation, offering deeper insights into the underlying mechanisms (e.g. Welcker et al., 2007; Kumar et al., 2023).
In this study, 10 inbred lines exhibited significant negative GCAf effects for TBL, and 13 lines showed significant negative GCAm effects for TBN under artificial Striga infestation, indicating favorable alleles for reduced tassel branch length and number. Seven inbred lines showed GCA effects for reduced TBN under both rainfed and artificial Striga infestation, reflecting their consistency in expression of favorable alleles for a lower TBN across a range of environments. Studies have shown that reduced TBN is associated with increased grain yield primarily due to better light interception and reduced competition for assimilates between the tassel and the ear (Hunter et al., 1969; Mock and Pearce, 1975; Sangoi, 2001). Our results revealed that there are several inbred lines with potential for reduced TBN, which could lead to higher grain yield when used in hybrid combinations. These lines would be valuable for breeding programs, especially if they also combine low TBN with multiple stress tolerance, a key requirement for maize breeding in SSA. A study by Lauer et al. (2012) reported that TBN in US inbred lines has steadily decreased from an average of 12 in the 1930s to an average of 6 in the 2000s, a trend attributed to systematic selection for smaller tassel size.
Genetic correlations, heterosis, and path analysis
Genetic correlations between traits provide insights into breeding strategies. The results of this study revealed significant positive genetic correlations between ear traits (ERL and ERC) and grain yield, indicating that selecting for increased ear length and circumference can enhance grain yield. The high heritability estimates for these two traits combined with their strong genetic correlation with grain yield, suggest that they could serve as effective indirect selection criteria for grain yield improvement. Conversely, grain yield showed a negative correlation with TBN under Striga infestation, but a positive correlation with TBN under rainfed conditions. In a study with tropical × temperate hybrids, Ndou et al. (2021) did not find a significant correlation between GY and TBN. This suggests that the genes controlling the two traits are differentially affected by different physiological mechanisms depending on the environment (Falconer and Mackay, 1996). While genetic correlations are typically population specific, a previous study also reported a negative correlation between TBN and grain yield (Geraldi et al., 1985). Interestingly, no strong correlation was found between TBN and husk traits under rainfed conditions, which contrasts with a study by Brewbaker (2015) which reported a strong correlation between TBN and HSN in tropical maize.
Our results revealed that heterosis for ERL and ERC was more expressed under managed drought stress compared to Striga infestation. This aligns with previous studies (Betrán et al., 2003; Makumbi et al., 2011; 2018) in which greater magnitude of heterosis under managed drought stress than other stress conditions was reported. The heterosis estimate for ERL under Striga infestation in this study was comparable to that reported by Yu et al. (2020). Since heterosis is generally more pronounced in ERL (Troyer and Ambrose, 1971), breeding programs should use lines with desirable GCA effects for ERL such as those identified in this study to develop hybrids that can express significant heterosis for ERL. Under managed drought stress, inbred line performance is more severely impacted than that of hybrids, resulting in larger heterosis estimates.
Path coefficient analysis revealed that EASP had the largest negative direct path coefficient, while ERL and ERC consistently showed positive direct path coefficient with GY across all conditions. This suggests that EASP, along with ear traits ERL and ERC, are critical when evaluating GY potential of genotypes under both artificial Striga infestation and rainfed conditions. These traits should be integrated into a selection index to facilitate hybrid advancement (e.g., Badu-Apraku et al., 2014; Makumbi et al., 2018; Crispim-Filho et al., 2020). In particular, a base index that incorporates trait-specific heritability estimates would be most effective (e.g. Smith et al., 1981; Makumbi et al., 2018). Additionally, these traits can serve as indirect selection criteria to improve GY. A high and significant genetic correlation between GY and these traits further supports this conclusion. Similarly, Silveira et al. (2021) proposed tassel traits as indirect selection criteria. While measuring ERL and ERC can be time-consuming, the adoption of new high-throughput ear phenotyping techniques (Miller et al., 2017; Makanza et al., 2018; Oury et al., 2022; Liu et al., 2023; Wang J. et al., 2023) is expected to significantly enhance the speed and efficiency of capturing these traits in breeding programs.
Genetic improvement of ear, husk, and tassel traits in tropical maize
The genetic improvement of tropical maize in sub-Saharan Africa and Latin America has primarily focused on biotic and abiotic stress tolerance, as well as improved grain yield, with notable success (Bänziger et al., 2006; Badu-Apraku et al., 2013; Prasanna et al., 2020; 2021). This work targeted selection for traits related to stress tolerance, such as ears per plant, reduced anthesis-silking interval, resistance to stalk and root lodging, and resistance to foliar and virus diseases, alongside improving final grain yield in the hybrids and synthetics. However, there was limited focus on selection for individual traits that enhance efficiency in assimilate utilization and ultimately increase grain yield. Such traits include but are not limited to reduced tassel size and branch number, ear length and diameter, kernel rows per ear, husk tightness and extension length, and leaf architecture. In contrast, temperate maize breeding programs have consistently selected for these traits, resulting in inbred lines with better plant architecture and higher grain yield per se and in hybrid combinations (Russell, 1991; Duvick, 2005; Wang et al., 2011; Lauer et al., 2012; Liu et al., 2021). Earlier efforts to systematically improve traits like reduced TBN in tropical maize were reported (Fischer et al., 1987; Chapman and Edmeades, 1999). We believe more attention should be given to systematically improving these traits in tropical maize, particularly in SSA, to develop new inbred lines that efficiently convert nutrients into higher grain yields. Selection for these traits has been successful in temperate regions (Fakorede and Mock, 1978; Schuetz and Mock, 1978; Cortez-Mendoza and Hallauer, 1979; Lopez-Reynoso and Hallauer, 1998). The improvement of these traits in tropical maize could be achieved through systematic introgression of ex-PVP temperate germplasm that carries favorable alleles for some of these traits (e.g., Abadassi and Hervé, 2000; Cupertino-Rodrigues et al., 2020; Dao et al., 2020; Musundire et al., 2021; Ndou et al., 2021). A reduction in TBN in tropical × temperate maize crosses has been reported (Ndou et al., 2021). A promising breeding strategy could involve developing biparental populations by crossing lines with reduced TBN and increased ERL and ERC identified in this study, with ex-PVP lines for DH induction. However, this approach must carefully consider the alignment of heterotic patterns between tropical and temperate maize. Faster and more efficient identification of inbred lines with favorable allele combinations for traits such TBN, ERL, ERC, and HSN can be achieved through marker-assisted selection (MAS), following fine mapping of quantitative trait loci (QTL) reported in several studies (Huo et al., 2016; Khatun et al., 2022; Ruidong et al., 2023; Wang X. et al., 2023; Zeng et al., 2022; Xiao et al., 2016; Zhou et al., 2020; Zhu et al., 2018).
Conclusions
The study revealed that additive gene action predominated in the inheritance of ear, husk, and tassel traits across all management conditions. Inbred lines with consistently favorable GCA effects for increased ERL and ERC, along with favorable GCA effects for reduced TBN and TBL, were identified, indicating their suitability for hybrid development, and the formation of biparental breeding populations. Heterosis estimates were higher for ear and tassel traits under stress conditions. Genetic correlations between grain yield and ERL and ERC were strong and positive. Path analysis revealed ERL, ERC, and EASP were the first-order traits most strongly correlated with grain yield, highlighting their value for inclusion in a selection index. The broad-sense heritability estimates for husk, ear, and tassel traits were mostly high, indicating the potential for significant genetic gains from selection for these traits. The inbred lines evaluated in this study exhibited higher average TBN compared to US inbred lines. Tropical maize inbred lines could be improved for the key grain yield component traits through the introgression of temperate germplasm to develop more efficient and higher yielding inbred lines.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
TK: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. DM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Writing – original draft, Writing – review & editing. EM: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. HK: Data curation, Investigation, Visualization, Writing – original draft, Writing – review & editing. GO: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. CA: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. JSK: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. JJK: Formal analysis, Visualization, Writing – original draft, Writing – review & editing. RK: Formal analysis, Visualization, Writing – original draft, Writing – review & editing. JS: Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was supported by grants from the Bill and Melinda Gates Foundation (BMGF) through the Stress Tolerant Maize for Africa (STMA, OPP1134248) project and through the Accelerating Genetic Gains in Maize and Wheat for Improved Livelihoods project (INV–003439) funded by the Bill and Melinda Gates Foundation (BMGF), Foundation for Food and Agriculture Research (FFAR) and the United States Agency for International Development (USAID).
Acknowledgments
We thank technical assistants Patricia Onyango at KALRO-Alupe Research Station, and Gabriel Ambani and Christine Litali at KALRO-Kakamega for assistance with different aspects of this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1618054/full#supplementary-material
Supplementary Table 1 | List, origin, and set information of 30 inbred lines used in a North Carolina Design II mating scheme to generate 150 hybrids.
Supplementary Table 2 | Test location characteristics (coordinates, elevation, rainfall, and temperature) in 2020 and 2021.
Supplementary Table 3 | Percentage of total genotypic sum of squares contribution for ear traits, plant aspect and tassel traits of 150 hybrids attributable to GCA-males (GCAm), GCA-females (GCAf), and SCA across managed drought stress conditions, 2020-2021.
Supplementary Table 4 | Estimates of general combining ability effects of females (GCAf) and males (GCAm) of 30 tropical maize lines for plant and ear traits evaluated under artificial Striga infestation, rainfed, and managed drought stress conditions. aBHC, Bad husk cover; EASP, Ear aspect; EROT, Percentage of rotten ears; PASP, Plant aspect.
Supplementary Table 5 | Summary of mean squares from combined ANOVA, descriptive statistics, and broad-sense heritability estimates for ear and tassel traits of inbred lines under different management conditions in Kenya (2020–2021). aBHC, Bad husk cover; EASP, Ear aspect; ERL, Ear length; ERC, Ear circumference; EROT, Percentage of rotten ears; HSL, Husk length; HSN, Husk number; HSW, Husk width; PASP, Plant aspect; TBL, Tassel branch length; TBN, Tassel branch number.
References
Abadassi, J. and Hervé, Y. (2000). Introgression of temperate germplasm to improve an elite tropical maize population. Euphytica 113, 125–133. doi: 10.1023/A:1003916928181
Abate, T., Fisher, M., Abdoulaye, T., Kassie, G. T., Lunduka, R., Marenya, P., et al. (2017). Characteristics of maize cultivars in Africa: How modern are they and how many do smallholder farmers grow? Agric. Food Secur 6, 30. doi: 10.1186/s40066-017-0108-6
Alister, C. and Kogan, M. (2005). Efficacy of imidazolinone herbicides applied to imidazolinone resistant maize and their carryover effect on rotational crops. Crop Prot. 24, 375–379. doi: 10.1016/j.cropro.2004.09.011
Alvarado, G., Rodríguez, F. M., Pacheco, A., Burgueño, J., Crossa, J., Vargas, M., et al. (2020). META-R: A software to analyze data from multi-environment plant breeding trials. Crop J. 8, 745–756. doi: 10.1016/j.cj.2020.03.010
Badu-Apraku, B., Akinwale, R. O., and Oyekunle, M. (2014). Efficiency of secondary traits in selecting for improved grain yield in extra-early maize under Striga-infested and Striga-free environments. Plant Breed. 133, 373–380. doi: 10.1111/pbr.12163
Badu-Apraku, B., Fakorede, M. A. B., Oyekunle, M., Akinwale, R. O., and Aderounmu, M. (2020). Combining ability, heterotic patterns, and genetic diversity of extra-early maturing yellow maize inbreds under contrasting environments. Field Crops Res. 252, 107793. doi: 10.1016/j.fcr.2020.107793
Badu-Apraku, B., Fakorede, M. A. B., Talabi, A. O., Oyekunle, M., Akaogu, I. C., Akinwale, R. O., et al. (2016). Gene action and heterotic groups of early white quality protein maize inbreds under multiple stress environments. Crop Sci. 56, 183–199. doi: 10.2135/cropsci2015.05.0276
Badu-Apraku, B., Yallou, C. G., and Oyekunle, M. (2013). Genetic gains from selection for high grain yield and Striga resistance in early maturing maize cultivars of three breeding periods under Striga-infested and Striga-free environments. Field Crops Res. 147, 54–67. doi: 10.1016/j.fcr.2013.03.022
Bänziger, M., Edmeades, G. O., Beck, D., and Bellon, M. (2000). Breeding for drought and N stress tolerance in maize: From theory to practice (Mexico City: CIMMYT).
Bänziger, M., Setimela, P. S., Hodson, D., and Vivek, B. (2006). Breeding for improved drought tolerance in maize adapted to southern Africa. Agric. Water Manage. 80, 212–224. doi: 10.1016/j.agwat.2005.07.014
Barry, D., Lillehoj, E. B., Widstrom, N. W., McMillan, W. W., Zuber, M. S., Kwolek, W. F., et al. (1986). Effect of husk tightness and insect (Lepidoptera) infestation on aflatoxin contamination of preharvest maize. Environ. Entomol. 15, 1116–1118. doi: 10.1093/ee/15.6.1116
Betrán, F. J., Beck, D., Bänziger, M., and Edmeades, G. O. (2003). Secondary traits in parental inbreds and hybrids under stress and non-stress environments in tropical maize. Field Crops Res. 83, 51–65. doi: 10.1016/S0378-4290(03)00061-3
Betrán, F. J. and Hallauer, A. R. (1996). Characterization of interpopulation genetic variability in three hybrid maize populations. J. Heredity 87, 319–328. doi: 10.1093/oxfordjournals.jhered.a023006
Bolaños, J. (1995). Physiological bases for yield differences in selected maize cultivars from Central America. Field Crops Res. 42, 69–80. doi: 10.1016/0378-4290(95)00022-I
Brewbaker, J. L. (2015). Diversity and genetics of tassel branch numbers in maize. Crop Sci. 55, 65–78. doi: 10.2135/cropsci2014.03.0248
Brewbaker, J. L. and Kim, S. K. (1979). Inheritance of husk numbers and ear insect damage in maize. Crop Sci. 19, 32–36. doi: 10.2135/cropsci1979.0011183X001900010008x
Cairns, J. E., Chamberlin, J., Rutsaert, P., Voss, R. C., Ndhlela, T., and Magorokosho, C. (2021). Challenges for sustainable maize production in sub-Saharan Africa. J. Cereal Sci. 101, 103274. doi: 10.1016/j.jcs.2021.103274
Cao, A., Santiago, R., Ramos, A. J., Souto, X. C., Aguín, O., Malvar, R. A., et al. (2014). Critical environmental and genotypic factors for Fusarium verticillioides infection, fungal growth and fumonisin contamination in maize grown in northwestern Spain. Int. J. Food Microbiol. 177, 63–71. doi: 10.1016/j.ijfoodmicro.2014.02.004
Cavalieri, A. J. and Smith, O. S. (1985). Grain filling and field drying of a set of maize hybrids released from 1930 to 1982. Crop Sci. 25, 856–860. doi: 10.2135/cropsci1985.0011183X002500050031x
Chang, L., He, K., and Liu, J. (2024). Decoding the genetic basis of ear-related traits in maize (Zea mays L.) using linkage mapping, association mapping and genomic prediction. Plant Breed. 143, 840–856. doi: 10.1111/pbr.13212
Chapman, S. C. and Edmeades, G. O. (1999). Selection improves drought tolerance in tropical maize populations. II. Direct and correlated responses among secondary traits. Crop Sci. 39, 1315–1324. doi: 10.2135/cropsci1999.3951315x
Comstock, R. E. and Robinson, H. F. (1948). The components of genetic variance in populations of biparental progenies and their use in estimating the average degree of dominance. Biometrics 4, 254–266. doi: 10.2307/3001412
Cortez-Mendoza, H. and Hallauer, A. R. (1979). Divergent mass selection for ear length in maize. Crop Sci. 19, 175–178. doi: 10.2135/cropsci1979.0011183X001900020001x
Crispim-Filho, A. J., dos Santos, F. P., Pinto, J. F. N., Melo, P. G. S., dos Reis, E. F., and Mendes-Resende, M. P. (2020). Dealing with multiple traits in maize: A new approach for selecting progenies. Crop Sci. 60, 3151–3165. doi: 10.1002/csc2.20292
Cui, Z., Luo, J., Qi, C., Ruan, Y., Li, J., Zhang, A., et al. (2016). Genome-wide association study (GWAS) reveals the genetic architecture of four husk traits in maize. BMC Genomics 17, 946. doi: 10.1186/s12864-016–3229-6
Cupertino-Rodrigues, M., Dhliwayo, T., Trachsel, S., Guo, R., and San Vicente, F. (2020). Evaluation of U.S. inbred lines with expired plant variety protection for mid-altitude tropical maize breeding. Euphytica 216, 44. doi: 10.1007/s10681-020-02584-z
Dao, A., Sanou, R., Sanon, R. D., Zeba, I., Coulibaly, S., and Lübberstedt, T. (2020). Exploring the potential usefulness of U.S. maize expired Plant Variety Protection Act lines for maize breeding in sub-Saharan Africa. Crop Sci. 60, 2251–2265. doi: 10.1002/csc2.20189
Dhillon, B. S. and Singh, J. (1977). Combining ability and heterosis in diallel crosses of maize. Theor. Appl. Genet. 49, 117–122. doi: 10.1007/BF00281709
Donald, C. M. (1968). The breeding of crop ideotypes. Euphytica 17, 385–403. doi: 10.1007/BF00056241
Duncan, W. G., Williams, W. A., and Loomis, R. S. (1967). Tassels and the productivity of maize. Crop Sci. 7, 37–39. doi: 10.2135/cropsci1967.0011183X000700010013x
Duvick, D. N. (2005). The contribution of breeding to yield advances in maize. Adv. Agron. 86, 83–145. doi: 10.1016/S0065-2113(05)86002-X
Duvick, D. N., Smith, J. S. C., and Cooper, M. (2004). Long-term selection in a commercial hybrid maize breeding program. Plant Breed. Rev. 24, 109–151. doi: 10.1002/9780470650288.ch4
Egli, D. B. (2015). Is there a role for sink size in understanding maize population–yield relationships? Crop Sci. 55, 2453–2462. doi: 10.2135/cropsci2015.04.0227
Erenstein, O., Jaleta, M., Sonder, K., Mottaleb, K., and Prasanna, B. M. (2022). Global maize production, consumption and trade: trends and R and D implications. Food Secur. 14, 1295–1319. doi: 10.1007/s12571-022-01288-7
Fakorede, M. A. B. and Mock, J. J. (1978). Changes in morphological and physiological traits associated with recurrent selection for grain yield in maize. Euphytica 27, 397–409. doi: 10.1007/BF00043165
Falconer, D. S. and Mackay, T. F. C. (1996). Introduction to quantitative genetics. 4th ed (Essex, UK: Longman Group).
Fan, X. M., Chen, H. M., Tan, J., Xu, C. X., Zhang, Y. D., Luo, L. M., et al. (2008). Combining abilities for yield and yield components in maize. Maydica 53, 39–46.
FAOSTAT (2024). Food and Agriculture Organization of the United Nations (FAO). Available online at: https://www.fao.org/faostat/en/data/QCL/metadata (Accessed April 8, 2025).
Fasoula, V. A. and Fasoula, D. A. (2002). Principles underlying genetic improvement for high and stable crop yield potential. Field Crops Res. 75, 191–209. doi: 10.1016/S0378-4290(02)00026-6
Fischer, R. A. and Edmeades, G. O. (2010). Breeding and cereal yield progress. Crop Sci. 50, S85–S98. doi: 10.2135/cropsci2009.10.0564
Fischer, K. S., Edmeades, G. O., and Johnson, E. C. (1987). Recurrent selection for reduced tassel branch number and reduced leaf area density above the ear in tropical maize populations. Crop Sci. 27, 1150–1156. doi: 10.2135/cropsci1987.0011183X002700060013x
Fujita, K., Sato, H., Sawada, O., and Sendo, S. (1995). Husk leaves contribution to dry matter and grain production as well as distribution in flint corn (Zea mays L.) genotypes differing in husk leaf area. Soil Sci. Plant Nutr. 41, 587–596. doi: 10.1080/00380768.1995.10419620
Geraldi, I. O., Miranda-Filho, J. B., and Vencovsky, R. (1985). Estimates of genetic parameters for tassel characters in maize (Zea mays L.) and breeding perspectives. Maydica 30, 1–14.
Grogan, C. O. (1956). Detasseling responses in corn. Agron. J. 48, 247–249. doi: 10.2134/agronj1956.00021962004800060001x
Guei, R. G. and Wassom, C. E. (1996). Genetic analysis of tassel size and leaf senescence and their relationships with yield in two tropical lowland maize populations. Afr. Crop Sci. J. 4, 275–281.
Hallauer, A. R., Carena, M., and Miranda Filho, J. B. (2010). Quantitative genetics in maize breeding (New York: Springer).
Hallauer, A. R. and Miranda Filho, J. B. (1988). Quantitative genetics in maize breeding (Ames: Iowa State University Press).
Han, G.-C. and Hallauer, A. R. (1989). Estimates of genetic variability in F2 maize populations. Jour Iowa Acad. Sci. 96, 14–19.
Holland, J. B. (2006). Estimating genotypic correlations and their standard errors using multivariate restricted maximum likelihood estimation with SAS Proc MIXED. Crop Sci. 46, 642–654. doi: 10.2135/cropsci2005.0191
Hunter, R. B., Daynard, T. B., Hume, D. J., Tanner, J. W., Curtis, J. D., and Kannenberg, L. W. (1969). Effect of tassel removal on grain yield of corn (Zea mays L.). Crop Sci. 9, 405–406. doi: 10.2135/cropsci1969.0011183X000900040003x
Huo, D., Ning, Q., Shen, X., Liu, L., and Zhang, Z. (2016). QTL mapping of kernel number related traits and validation of one major QTL for ear length in maize. PloS One 11, e0155506. doi: 10.1371/journal.pone.0155506
Kamara, M. K., El-Degwy, I. S., and Koyama, H. (2014). Estimation combining ability of some maize inbred lines using line × tester mating design under two nitrogen levels. Aust. J. Crop Sci. 8, 1336–1342.
Kamara, M. K., Rehan, M., Ibrahim, K. M., Alsohim, A. S., Elsharkawy, M. M., and Kheir, A.M.S. (2020). Genetic diversity and combining ability of white maize inbred lines under different plant densities. Plants 9, 1140. doi: 10.3390/plants9091140
Kanampiu, F., Makumbi, D., Mageto, E., Omanya, G., Waruingi, S., Musyoka, P., et al. (2018). Assessment of management options on Striga infestation and maize grain yield in Kenya. Weed Sci. 66, 516–524. doi: 10.1017/wsc.2018.4
Khatun, M., Monir, M. M., Lou, X., Zhu, J., and Xu, H. (2022). Genome-wide association studies revealed complex genetic architecture and breeding perspective of maize ear traits. BMC Plant Biol. 22, 537. doi: 10.1186/s12870-022-03913-1
Kimutai, J. J. C., Makumbi, D., Burgueño, J., Pérez-Rodríguez, P., Crossa, J., Gowda, M., et al. (2024). Genomic prediction of the performance of tropical doubled haploid maize lines under artificial Striga hermonthica (Del.) Benth. infestation. G3 Genes|Genomes|Genetics 14, jkae186. doi: 10.1093/g3journal/jkae186
Kumar, R., Brar, M. S., Kunduru, B., Ackerman, A. J., Yang, Y., Luo, F., et al. (2023). Genetic architecture of source–sink-regulated senescence in maize. Plant Physiol. 193, 2459–2479. doi: 10.1093/plphys/kiad460
Lambert, R. J. and Johnson, R. R. (1978). Leaf angle, tassel morphology, and the performance of maize hybrids. Crop Sci. 18, 499–502. doi: 10.2135/cropsci1978.0011183X001800030037x
Lauer, S., Hall, B. D., MuLaosmanovic, E., Anderson, S. R., Nelson, B., and Smith, S. (2012). Morphological changes in parental lines of Pioneer Brand maize hybrids in the U.S. Central Corn Belt. Crop Sci. 52, 1033–1043. doi: 10.2135/cropsci2011.05.0274
Lee, E. A. and Tollenaar, M. (2007). Physiological basis of successful breeding strategies for maize grain yield. Crop Sci. 47, S202–S215. doi: 10.2135/cropsci2007.04.0010IPBS
Liu, G., Yang, H., Xie, R., Yang, Y., Liu, W., Guo, X., et al. (2021). Genetic gains in maize yield and related traits for high-yielding cultivars released during 1980s to 2010s in China. Field Crops Res. 270, 108223. doi: 10.1016/j.fcr.2021.108223
Liu, M., Zhang, S., Li, W., Zhao, X., and Wang, X. (2023). Identifying yield-related genes in maize based on ear trait plasticity. Genome Biol. 24, 94. doi: 10.1186/s13059-023-02937-6
Lopez-Reynoso, J. J. and Hallauer, A. R. (1998). Twenty-seven cycles of divergent mass selection for ear length in maize. Crop Sci. 38, 1099–1107. doi: 10.2135/cropsci1998.0011183X003800040035x
Mageto, E. K., Makumbi, D., Njoroge, K., and Nyankanga, R. (2017). Genetic analysis of early-maturing maize (Zea mays L.) inbred lines under stress and nonstress conditions. J. Crop Improv 31, 560–588. doi: 10.1080/15427528.2017.1315625
Mahuku, G., Lockhart, B. E., Wanjala, B., Jones, M. W., Kimunye, J. N., Stewart, L. R., et al. (2015). Maize lethal necrosis (MLN), an emerging threat to maize-based food security in sub-Saharan Africa. Phytopathology 105, 956–965. doi: 10.1094/PHYTO-12-14-0367-FI
Makanza, R., Zaman−Allah, M., Cairns, J. E., Eyre, J., Burgueño, J., Pacheco, A., et al. (2018). High−throughput method for ear phenotyping and kernel weight estimation in maize using ear digital imaging. Plant Methods 14, 49. doi: 10.1186/s13007-018-0317-4
Makumbi, D., Assanga, S., Diallo, A., Magorokosho, C., Asea, G., Worku, M., et al. (2018). Genetic analysis of tropical midaltitude-adapted maize populations under stress and nonstress conditions. Crop Sci. 58, 1492–1507. doi: 10.2135/cropsci2017.09.0531
Makumbi, D., Betrán, F. J., Bänziger, M., and Ribaut, J. M. (2011). Combining ability, heterosis and genetic diversity in tropical maize (Zea mays L.) under stress and non-stress conditions. Euphytica 180, 143–162. doi: 10.1007/s10681-010-0334-5
Makumbi, D., Diallo, A., Kanampiu, F., Mugo, S., and Karaya, H. (2015). Agronomic performance and genotype × environment interaction of herbicide-resistant maize varieties in eastern Africa. Crop Sci. 55, 540–555. doi: 10.2135/cropsci2014.08.0593
Mansfield, B. D. and Mumm, R. H. (2014). Survey of plant density tolerance in US maize germplasm. Crop Sci. 54, 157–173. doi: 10.2135/cropsci2013.04.0252
Menkir, A. and Kling, J. (2007). Response to recurrent selection for resistance to Striga hermonthica (Del.) Benth. in a tropical maize population. Crop Sci. 47, 672–682. doi: 10.2135/cropsci2006.07.0494
Menkir, A., Makumbi, D., and Franco, J. (2012). Assessment of reaction patterns of hybrids to Striga hermonthica (Del.) Benth. under artificial infestation in Kenya and Nigeria. Crop Sci. 52, 2528–2537. doi: 10.2135/cropsci2012.05.0307
Miller, N. D., Haase, N. J., Lee, J., Kaeppler, S. M., de Leon, N., and Spalding, E. P. (2017). A robust, high-throughput method for computing maize ear, cob, and kernel attributes automatically from images. Plant J. 89, 169–178. doi: 10.1111/tpj.13320
Mock, J. J. and Pearce, R. B. (1975). An ideotype of maize. Euphytica 24, 613–623. doi: 10.1007/BF00132898
Mock, J. J. and Schuetz, S. H. (1974). Inheritance of tassel branch number in maize. Crop Sci. 14, 885–888. doi: 10.2135/cropsci1974.0011183X001400060033x
Musundire, L., Derera, J., Dari, S., and Tongoona, P. (2021). Assessment of genetic gains for grain yield and components from introgression of temperate donor inbred line into tropical elite maize inbred lines: II. Performance inter se. Euphytica 217, 18. doi: 10.1007/s10681-020-02749-w
Nardino, M., Queiróz de Souza, V., Baretta, D., Konflanz, V. A., Follmann, D. N., Carvalho, I. R., et al. (2016). Partial diallel analysis among maize lines for characteristics related to the tassel and the productivity. Afr. J. Agric. Res. 11, 974–982. doi: 10.5897/AJAR2014.10314
Ndou, V., Gasura, E., Chivenge, P., and Derera, J. (2021). Grain yield gains and associated traits in tropical 3 temperate maize germplasm under high and low plant density. Euphytica 217, 186. doi: 10.1007/s10681-021-02918-5
Nielsen, R. L. (2003). Ear initiation and size determination in corn (Indiana, USA, Corny News Network, Department of Agronomy, Purdue University). Available online at: http://www.kingcorn.org/news/articles.03/EarSize-0609.html (Accessed December 30, 2024).
Njeri, S. G., Makumbi, D., Warburton, M. L., Diallo, A., Jumbo, M. B., and Chemining’wa, G. (2017). Genetic analysis of tropical quality protein maize (Zea mays L.) germplasm. Euphytica 213, 261. doi: 10.1007/s10681-017-2048-4
Onejeme, F. C., Okporie, E. O., and Eze, C. E. (2020). Combining ability and heterosis in diallel analysis of maize (Zea mays L.) lines. Intern. Ann. Sci. 9, 188–200. doi: 10.21467/ias.9.1.188-200
Oury, V., Leroux, T., Turc, O., Chapuis, R., Palaffre, C., Tardieu, F., et al. (2022). Earbox, an open tool for high−throughput measurement of the spatial organization of maize ears and inference of novel traits. Plant Methods 18, 96. doi: 10.1186/s13007-022-00925-8
Patterson, H. D. and Williams, E. R. (1976). A new class of resolvable incomplete block designs. Biometrika 63, 83–92. doi: 10.1093/biomet/63.1.83
Prasanna, B. M., Cairns, J. E., Zaidi, P. H., Beyene, Y., Makumbi, D., Gowda, M., et al. (2021). Beat the stress: breeding for climate resilience in maize for the tropical rainfed environments. Theor. Appl. Genet. 134, 1729–1752. doi: 10.1007/s00122-021-03773-7
Prasanna, B. M., Suresh, L. M., Mwatuni, F., Beyene, Y., Makumbi, D., Gowda, M., et al. (2020). Maize lethal necrosis (MLN): Efforts toward containing the spread and impact of a devastating transboundary disease in sub-Saharan Africa. Virus Res. 282, 197943. doi: 10.1016/j.viruses.2020.197943
Rajcan, I. and Tollenaar, M. (1999). Source:sink ratio and leaf senescence in maize: I. Dry matter accumulation and partitioning during grain filling. Field Crops Res. 60, 245–253. doi: 10.1016/S0378-4290(98)00142-7
Ranum, P., Peña-Rosas, J. P., and Garcia-Casal, M. N. (2014). Global maize production, utilization, and consumption. Ann. N. Y Acad. Sci. 1312, 105–112. doi: 10.1111/nyas.12396
Ruidong, S., Shijin, H., Yuwei, Q., Yimeng, L., Xiaohang, Z., Ying, L., et al. (2023). Identification of QTLs and their candidate genes for the number of maize tassel branches in F2 from two higher generation sister lines using QTL mapping and RNA-seq analysis. Front. Plant Sci. 14, 1202755. doi: 10.3389/fpls.2023.1202755
Russell, W. A. (1991). Genetic improvement of maize yields. Adv. Agron. 46, 245–298. doi: 10.1016/S0065-2113(08)60582-9
Samonte, S. O. P. B., Wilson, L. T., and McClung, A. M. (1998). Path analyses of yield and yield-related traits of fifteen diverse rice genotypes. Crop Sci. 38, 1130–1136. doi: 10.2135/cropsci1998.0011183X003800050004x
Sangoi, L. (2001). Understanding plant density effects on maize growth and development: an important issue to maximize grain yield. Cienc. Rur 31, 159–168. doi: 10.1590/S0103-84782001000100027
Schuetz, S. H. and Mock, J. J. (1978). Genetics of tassel branch number in maize and its implications for a selection program for small tassel size. Theor. Appl. Genet. 53, 265–271. doi: 10.1007/BF00280990
Shapiro, S. S. and Wilk, M. B. (1965). An analysis of variance test for normality (complete samples). Biometrika 52, 591–611. doi: 10.1093/biomet/52.3-4.591
Silveira, D. L., Filho, A. C., Wartha, C. A., Carini, F., Bandeira, C. T., Pezzini, R. V., et al. (2021). Linear relationships between grain yield and tassel traits in maize. Aust. J. Crop Sci. 15, 1164–1171. doi: 10.21475/ajcs.21.15.08.p3228
Smith, O. S., Hallauer, A. R., and Russell, W. A. (1981). Use of index selection in recurrent selection programs in maize. Euphytica 30, 611–618. doi: 10.1007/BF00038788
Sofi, P. A. (2007). Genetic analysis of tassel and ear characters in maize (Zea mays L.) using triple test cross. Asian J. Plant Sci. 6, 881–883. doi: 10.3923/ajps.2007.881.883
Sorsa, Z., Mohammed, W., Wegary, D., and Tarkegne, A. (2023). Performances of three-way cross hybrids over their respective single crosses and related heterosis of maize (Zea mays L.) hybrids evaluated in Ethiopia. Heliyon 9, e15513. doi: 10.1016/j.heliyon.2023.e15513
Sserumaga, J. P., Makumbi, D., Assanga, S. O., Mageto, E. K., Njeri, S. G., Jumbo, B. M., et al. (2020). Identification and diversity of tropical maize inbred lines with resistance to common rust (Puccinia sorghi Schwein). Crop Sci. 60, 2971–2989. doi: 10.1002/csc2.20345
Tollenaar, M. (1977). Sink-source relationships during reproductive development in maize. A review. Maydica 22, 49–75.
Tollenaar, M. and Aguilera, A. (1992). Radiation use efficiency of old and modern hybrids. Agron. J. 84, 536–541. doi: 10.2134/agronj199.2.00021962008400030033x
Tollenaar, M. and Daynard, T. B. (1978). Relationship between assimilate source and reproductive sink in maize grown in short-season environment. Agron. J. 70, 219–223. doi: 10.2134/agronj1978.00021962007000020003x
Tollenaar, M. and Daynard, T. B. (1982). Effect of source-sink ratio on dry matter accumulation and leaf senescence of maize. Can. J. Plant Sci. 62, 855–860. doi: 10.4141/cjps82-128
Tollenaar, M. and Lee, E. A. (2006). Physiological dissection of grain yield in maize by examining genetic improvement and heterosis. Maydica 51, 399–408.
Troyer, A. F. and Ambrose, W. B. (1971). Plant characteristics affecting field drying rate of ear corn. Crop Sci. 11, 529–531. doi: 10.2135/cropsci1971.0011183X001100040019x
Uhart, S. A. and Andrade, F. H. (1991). Source-sink relationships in maize grown in a cool-temperate area. Agronomie 11, 863–875. doi: 10.1051/agro:19911004
Upadyayula, N., Wassom, J., Bohn, M. O., and Rocheford, T. R. (2006). Quantitative trait loci analysis of phenotypic traits and principal components of maize tassel inflorescence architecture. Theor. Appl. Genet. 113, 1395–1407. doi: 10.1007/s00122-006-0359-2
Wang, X., Li, W., Han, L., Liang, C., Li, J., Shang, X., et al. (2023). QTG-Miner aids rapid dissection of the genetic base of tassel branch number in maize. Nat. Commun. 14, 5232. doi: 10.1038/s41467-023-41022-1
Wang, T., Ma, X., Li, Y., Bai, D., Liu, C., Liu, Z., et al. (2011). Changes in yield and yield components of single-cross maize hybrids released in China between 1964 and 2001. Crop Sci. 51, 512–525. doi: 10.2135/cropsci2010.06.0383
Wang, J., Zhao, S., Zhang, Y., Lu, X., Du, J., Wang, C., et al. (2023). Investigating the genetic basis of maize ear characteristics: a comprehensive genome-wide study utilizing high-throughput phenotypic measurement method and system. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1248446
Warfield, C. Y. and Davis, R. M. (1996). Importance of the husk covering on the susceptibility of corn hybrids to Fusarium ear rot. Plant Dis. 80, 208–210. doi: 10.1094/PD-80-0208
Welcker, C., Boussuge, B., Bencivenni, C., Ribaut, J.-M., and Tardieu, F. (2007). Are source and sink strengths genetically linked in maize plants subjected to water deficit? A QTL study of the responses of leaf growth and of anthesis-silking interval to water deficit. J. Exp. Bot. 58, 339–349. doi: 10.1093/jxb/erl227
Wolf, D. P. and Hallauer, A. R. (1997). Triple testcross analysis to detect epistasis in maize. Crop Sci. 37, 763–770. doi: 10.2135/cropsci1997.0011183X003700030012x
Worku, M., Makumbi, D., Beyene, Y., Das, B., Mugo, S., Pixley, K., et al. (2016). Grain yield performance and flowering synchrony of CIMMYT’s tropical maize (Zea mays L.) parental inbred lines and single crosses. Euphytica 211, 395–409. doi: 10.1007/s10681-016-1758-3
Xiao, Y., Tong, H., Yang, X., Xu, S., Pan, Q., Qiao, F., et al. (2016). Genome-wide dissection of the maize ear genetic architecture using multiple populations. New Phytol. 210, 1095–1106. doi: 10.1111/nph.13814
Yang, L., Li, T., Liu, B., Li, R., Yu, R., Zhang, X., et al. (2021). Genetic analysis of ear-related traits under different pollination treatments in maize (Zea mays). Plant Breed. 140, 211–222. doi: 10.1111/pbr.12887
Yi, Q., Hou, X., Liu, Y., Zhang, X., Zhang, J., Liu, H., et al. (2019). QTL analysis for plant architecture-related traits in maize under two different plant density conditions. Euphytica 215, 148. doi: 10.1007/s10681-019-2446-x
Yu, K., Wang, H., Liu, X., Xu, C., Li, Z., Xu, X., et al. (2020). Large-scale analysis of combining ability and heterosis for development of hybrid maize breeding strategies using diverse germplasm resources. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.00660
Zeng, T., Meng, Z., Yue, R., Lu, S., Li, W., Li, W., et al. (2022). Genome wide association analysis for yield related traits in maize. BMC Plant Biol. 22, 449. doi: 10.1186/s12870-022-03812-5
Zhang, X., Lu, M., Xia, A., Xu, T., Cui, Z., Zhang, R., et al. (2021). Genetic analysis of three maize husk traits by QTL mapping in a maize-teosinte population. BMC Genomics 22, 386. doi: 10.1186/s12864-021-07723-x
Zhang, J., Zhang, F., Tian, L., Ding, Y., Qi, J., Zhang, H., et al. (2022). Molecular mapping of quantitative trait loci for 3 husk traits using genotyping by sequencing in maize (Zea mays L.). G3: Genes|Genomes|Genetics 12, jkac198. doi: 10.1093/g3journal/jkac198
Zhou, G., Mao, Y., Xue, L., Chen, G., Lu, H., Shi, M., et al. (2020). Genetic dissection of husk number and length across multiple environments and fine-mapping of a major-effect QTL for husk number in maize (Zea mays L.). Crop J. 8, 1071–1080. doi: 10.1016/j.cj.2020.03.009
Keywords: combining ability, ear length, ear circumference, heritability, heterosis, path analysis, tassel traits, Striga hermonthica
Citation: Kosgei T, Makumbi D, Mageto EK, Kavai HM, Ochieng GO, Adhiambo CA, Kasango JS, Kimutai JJC, Kamau RM and Sserumaga JP (2025) Genetic analysis of ear, husk, and tassel traits in tropical maize under diverse environments. Front. Plant Sci. 16:1618054. doi: 10.3389/fpls.2025.1618054
Received: 25 April 2025; Accepted: 07 July 2025;
Published: 11 August 2025.
Edited by:
Cándido López-Castañeda, Colegio de Postgraduados (COLPOS), MexicoReviewed by:
Lovepreet Singh, Michigan State University, United StatesEdmore Gasura, University of Zimbabwe, Zimbabwe
Ashok Singamsetti, Banaras Hindu University, India
Copyright © 2025 Kosgei, Makumbi, Mageto, Kavai, Ochieng, Adhiambo, Kasango, Kimutai, Kamau and Sserumaga. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dan Makumbi, ZC5tYWt1bWJpQGNnaWFyLm9yZw==
†ORCID: Titus Kosgei, orcid.org/0009-0002-6546-5151
Dan Makumbi, orcid.org/0000-0002-1801-5986
Edna K. Mageto, orcid.org/0000-0002-3946-3219
Hilda M. Kavai, orcid.org/0009-0001-6600-4528
George O. Ochieng, orcid.org/0009-0002-9791-3801
Carolyne A. Adhiambo, orcid.org/0009-0001-6663-7261
Joseph S. Kasango, orcid.org/0009-0006-5440-2648
Joan J. C. Kimutai, orcid.org/0000-0002-8266-9503
Rachael M. Kamau, orcid.org/0000-0002-5164-6536
Julius P. Sserumaga, orcid.org/0000-0001-5152-2835
 Titus Kosgei
Titus Kosgei Dan Makumbi
Dan Makumbi Edna K. Mageto1,2†
Edna K. Mageto1,2† Hilda M. Kavai
Hilda M. Kavai Julius P. Sserumaga
Julius P. Sserumaga