- 1State Key Laboratory of Black Soils Conservation and Utilization, Northeast Institute of Geography and Agroecology, Chinese Academy of Sciences, Changchun, China
- 2University of Chinese Academy of Sciences, Beijing, China
Aims: Plants have developed sophisticated mechanisms to adapt to changing environments. The strategies for biomass allocation is critical for plant to ensure fitness. Increasing soil salinity has a dramatic impact on plant growth and reproduction from the individual to community level. However, our understanding of how plants adapt their biomass allocation strategies to changes in soil salinity and community structure is incomplete.
Methods: We investigated 122 individuals of perennial herb Allium ramosum from 70 plots along a soil gradient in Songnen grassland. Field investigations determined the physicochemical properties of the soil, aboveground biomass, richness and individual and each organ (root, stem, leaf, flower, and bulb) biomass of A. ramosum.
Results: The results showed that plant community aboveground biomass and community-weighted height decreased as soil salinity increased. A. ramosum individual size decreased, the allometric exponents between reproductive organs (flowers) and storage organs (bulbs) also decreased, with more biomass allocated to flowers. However, this trend was indirectly influenced by salinization through a reduction in community weighted height (reducing light competition) and community aboveground biomass (altering competitive pressure for resources), rather than a direct response to soil salinity.
Conclusions: This study highlights the complex interplay between community structure and individual plant adaptation strategies in response to environmental gradients, emphasizing the role of community-scale processes in regulating individual resource allocation.
Introduction
Plants maintain population survival and development by adjusting life history strategies, including nutrient acquisition and reproductive strategies (Bonser, 2013; Zemunik et al., 2015; Wong et al., 2024). Changes in such strategies vary depending on the environment and life history of different plant populations (Planas-Sitja et al., 2023; Van de Walle et al., 2023; Stott et al., 2024). Biomass allocation patterns reflect the trade-offs plants make between growth, reproduction, and survival, as total resources for these functions are limited (Poorter et al., 2012; White et al., 2022). When resources are limited, plants may prioritize their growth and reproduction strategies to ensure population survival. For example, in low-light conditions, plants may grow taller with increased stem biomass to capture more light, while in nutrient-poor soils, they may allocate more biomass to roots to enhance nutrient uptake (Xiao et al., 2021; Bartuskova et al., 2022). Thus, changes in soil characteristics may have a dramatic impact on plant life history strategies. Soil salinity degradation is a common type of soil degradation, which is exacerbated by climate change and anthropogenic impacts (Cao et al., 2021; Corwin, 2021; Garcia-Franco et al., 2021). Soil salinization results in elevated soil pH, while soil nutrient content decreases. Further restrict plant transpiration by decreasing soil water potential or have a toxic effect on plant roots due to high osmotic pressure (Li et al., 2022; Liu et al., 2024).
Consequently, empirical evidence indicates that fluctuations in soil salinity induce structure and functional modifications within plant communities (Cao et al., 2021; Heng et al., 2022; Xiao et al., 2025). Given that species belonging to diverse families and functional groups exhibit a spectrum of phenotypic plasticity in response to environmental gradients, this response often leads to shifts in population dynamics and subsequent alterations in ecosystem processes (Zhou et al., 2021; Van de Walle et al., 2023; Beccari and Carmona, 2024; Wu et al., 2024). It is especially critical to understand how plants adapt to soil salinization and community changes by adjusting biomass allocation to construct new leaves, stems, and roots in order to maintain growth and reproduction (Chen et al., 2021; Bartuskova et al., 2022; Dolezal et al., 2024). However, our understanding of how plants adapt to biotic and abiotic factors by adjusting their life history strategies to maintain populations in different communities remains limited. The trade-off between growth, nutrient acquisition and reproduction derives from the fact that when limited resources are allocated to one function (e.g., reproduction), they are not available for others (Weiner et al., 2009; Skarpaas et al., 2016; Umana et al., 2021). Some species may allocate more resources to growth and nutrient acquisition to maintain their survival, while others may prefer to allocate resources to increase the number of offspring, reflecting a coordinated allocation of biomass among organs due to variation in life history strategies (Bonser, 2013; Fujita et al., 2014; Bartuskova et al., 2022). For example, in nutrient-rich environments, plants may have easier access to nutrients and thus more energy for reproduction, whereas in situations where resources are scarce, plants may prioritize securing growth but not reproduction (Hulshof et al., 2012; Hu et al., 2021; Fang et al., 2023; Dolezal et al., 2024). The duration of plant life history and reproductive patterns can also influence the trade-off between nutrient acquisition and reproduction (Weiner et al., 2009; Stott et al., 2024). Typically, annual species usually concentrate their growth and reproduction in a short period, whereas perennial plants can acquire nutrients and produce seeds over multiple growing seasons (Hu et al., 2021; Fang et al., 2023).
However, the West, Brown, Enquist (WBE) model suggests that the approximate fractal structure of vascular plants allows the parts of individuals to grow in a specific proportion, which is not isometric but rather allometric (West et al., 1999; Zhang et al., 2021b). For instance, there is an allometric exponent of 3/4 observed between stem-leaf and root-leaf biomass (Enquist and Niklas, 2002). Similarly, an allometric relationship exists between reproduction and aboveground biomass (Weiner et al., 2009). Studies on biomass allocation patterns in various plants revealed that the same allometric relationships were present (Tang et al., 2022). In other words, plants differing in communities and functions exhibited uniform allometric relationships (Enquist and Niklas, 2002). Recent studies have found that changes in environmental conditions can alter the allometric exponent among plant organs, indicating that the pattern of plant biomass partitioning is modified (Zhang et al., 2021b; Tang et al., 2022; Zhang et al., 2022). Soil nutrient gradients were significantly correlated with stem and leaf allocation of biomass at the community level, with nutrient-enriched environments promoting high allocation of stems to compete for light, whereas in infertile habitats communities tended to allocate more biomass to leaves than stems (Yan et al., 2016). Similar results also reported in nutrient-rich forests, where species tend to increase stems (Kim et al., 2020). In addition, plants adapted to phosphorus-limited habitats were found to tend to reduce resource allocation for sexual reproduction (Fujita et al., 2014). The existing studies have primarily concentrated on the direct effects of soil nutrient content on plant life history strategies, while neglecting to consider the impact of soil-dependent plant communities on these life history strategies. Furthermore, numerous studies have examined interspecific variation in plant strategies, yet these have scarcely addressed how the life history strategies of a particular species vary with soil and community characteristics (Fujita et al., 2014; Yan et al., 2016; Dolezal et al., 2021; Fang et al., 2023; Tsogtsaikhan et al., 2025).
The Allium ramosum L. is a perennial herb belong to the Liliaceae. It is found in grasslands of northern China, where it frequently occurs as a component of communities with other species (Ge et al., 2020). Allium L. species are characterized by the presence of a bulb, which functions as an underground storage organ (Chope et al., 2012; Hsiao et al., 2019). The physiological and ecological functions of bulbs are of significance to the life cycle of plants (Ashagrie et al., 2021). They are the primary nutrient storage organ, storing substantial amounts of starch, sugar, and other nutrients (Howard and Cellinese, 2020). These stored nutrients provide energy and nutrients during plant growth and under unfavorable conditions, thereby facilitating survival and growth (Hsiao et al., 2019). Thus, utilizing Allium ramosum as a model species enables us to more directly investigate the trade-offs between growth, storage, and reproduction in plants.
In the present study we focused on plant biomass allocation patterns and investigated whether they were affected not only by soil features but also by plant community structure along an soil gradient in the natural grassland. We hypothesized that 1) along the soil gradient the biomass allocation strategy of Allium ramosum is plastic, e.g. wild leeks allocate more biomass to reproductive organs (flowers) at sites with heavier soil salinization and more biomass to growth and nutrient storage organs (leaves, bulbs) at sites with lighter soil salinization; 2) The altered biomass allocation strategy of Allium ramosum was not influenced by soil characteristics alone (N and P content), but by a combination of soil and plant community characteristics.
Materials and methods
Study site and plant sampling
The study was conducted at the Changling Grassland Research Station of the Chinese Academy of Sciences, located in Jilin Province, on the southwestern part of the Songnen Plain. Geographically, the site is positioned at 121°30′-123°44′-E, 44°44′-48°40′N, situated in the eastern part of the Inner Mongolian Plateau. The altitude of the area ranges from 138 to 145 meters above sea level. Since 2001, the site has been fenced to prevent grazing, thereby minimizing its impact on the vegetation (Huang et al., 2016). The Songnen grassland, situated near the Mongolia Plateau, experiences a typical continental semi-arid monsoon climate. The study area receives an average annual rainfall of approximately 470 mm, which varies significantly from year to year. In contrast, the annual evaporation rate exceeds 1,500 mm, more than three times the average annual precipitation. The mean annual temperature hovers around 4.9 °C, and the frost-free period lasts between 120 and 150 days. Characterized as a typical meadow grassland, the plant community is predominantly composed of Stipa baicalensis in areas with lower soil salinization. However, the vegetation is complex and diverse due to the influence of soil salinity. In more saline areas, the dominant species are mainly salt-tolerant graminoids such as Leymus chinensis and Puccinellia tenuiflora, and Suaeda glauca, which appear in the area of heavier salinity (Huang et al., 2019).
Plant traits and soil feature measurements
We selected 7 community types along the soil gradient (Table 1), and ten 1 m×1 m plots were selected for each community. Within each plot, 1–2 A. ramosum plants were randomly selected. Thus,10–20 individuals were taken from each community. A total of 122 individuals of Allium ramosum from 70 plots were sampled.
For each Allium ramosum individuals from different communities, we measured the height and separated the organs into roots, stems, leaves, flowers and bulbs. They were weighed after drying at 65 °C for 48 hours. At the same time, we took three soil samples at 0–15 cm depth in each plot and pooled them as a composite sample to measure soil nutrients. Some of the sieved samples were used to quantify available nutrients. Soil pH and electrical conductivity (EC) were measured using pH meter and conductivity meter in a soil:water suspension (1:5) after 5 min of shaking, respectively. We used potassium dichromate oxidation external heating method to determined soil organic carbon (SOC) content. The total nitrogen content (TN) and total phosphorus (TP) was measured by spectrophotometry. Soil available P was extracted by NaHCO3 and determined by Molybdate colorimetric method (Murphy and Riley, 1962). Available N was determined by the alkali diffusion method.
Data analysis
The assessment of soil fertility based on individual soil nutrients may not be comprehensive, as the co-limitation of multiple nutrients is a common occurrence. Consequently, we employed principal component analysis (PCA) to condense the complexity of soil nutrients (soil available nitrogen, phosphorus, organic carbon, total nitrogen, total phosphorus, and the N/P ratio) into fewer dimensions using the ‘psych’ package. The use of multiple unrelated nutrients in PCA may reduce the explanatory power of the first axis, but the principal components along the axes better capture nutrient variation along a soil gradient. To select an appropriate axis for the soil nutrient gradient, we also analyzed the correlations between the first four principal axis and each soil indicator.
Community-weighted means were used to describe the height of the plant community.
For each species in the community, we randomly selected five plants to measure height, and subsequently calculated plant community-weighted heights based on the ratio of species biomass in the community.
Coefficients of variation were used to describe the stability of plant communities and species as soil salinity increased. Standard principal axis regression analyses were used to obtain coefficients of biomass partitioning among Allium ramosum organs by ‘smatr’ package, and linear regression models were used to analyze the relationships between soil principal components and community and Allium ramosum traits. Finally structural equation modelling was used for analyzing the direct and indirect effects of soil salinity on plant biomass allocation by ‘lavaan’ package. All analyses were carried out in R 4.2.0.
Results
Soil gradients in the natural grassland
Soil salinity, as indicated by pH and electrical conductivity (EC), demonstrated an inverse relationship with nutrient content. Specifically, an increase in soil salinity corresponded to a decrease in soil organic carbon (SOC) content and overall nutrient availability (Figure 1). The first principal axis explained 77% of the variation in soil salinity and nutrient indicators (Figure 1). The pH and EC exhibited a negative correlation with the first principal axis, while SOC, total nitrogen (TN) and other nutrient indicators exhibited a positive correlation with the first principal axis (Supplementary Table S1). Consequently, the first principal axis can be interpreted as a representation of the soil salinity gradient, ranging from high salinity and low nutrients to low salinity and high nutrients. A significant and positive correlation was identified between plant community above-ground biomass and soilPC1, suggesting that as soil salinity decreased and nutrients increased, plant community biomass and stability increased (Figures 2A, B). The trend of species richness and community above-ground biomass exhibited consistency (Figures 2C, D). Soil salinity did not alter the relationship between plant community biomass and diversity; however, species evenness demonstrated a significant decrease as plant community aboveground biomass increased (Supplementary Figure S1). The Allium ramosum population biomass and stability remained relatively unresponsive to variations in soil gradient, although the size of individual Allium ramosum plants exhibited an increasing trend with increasing soil gradient (Figure 2E, F). However, the variability in individual size did not demonstrate a significant linear relationship with soil gradient (Figure 2H). Concurrently, the height of individual Allium ramosum plants and the biomass of each organ increased with soil gradient, particularly the aboveground biomass. Notably, the flower biomass exhibited no significant change with soil gradient (Supplementary Figure S2).

Figure 1. Principal component analysis (PCA) of soil nutrients. SOC, soil organic carbon; TN, total nitrogen; TP, total phosphorus; AN, available nitrogen; AP, available phosphorus; NP, nitrogen-phosphorus ratio.
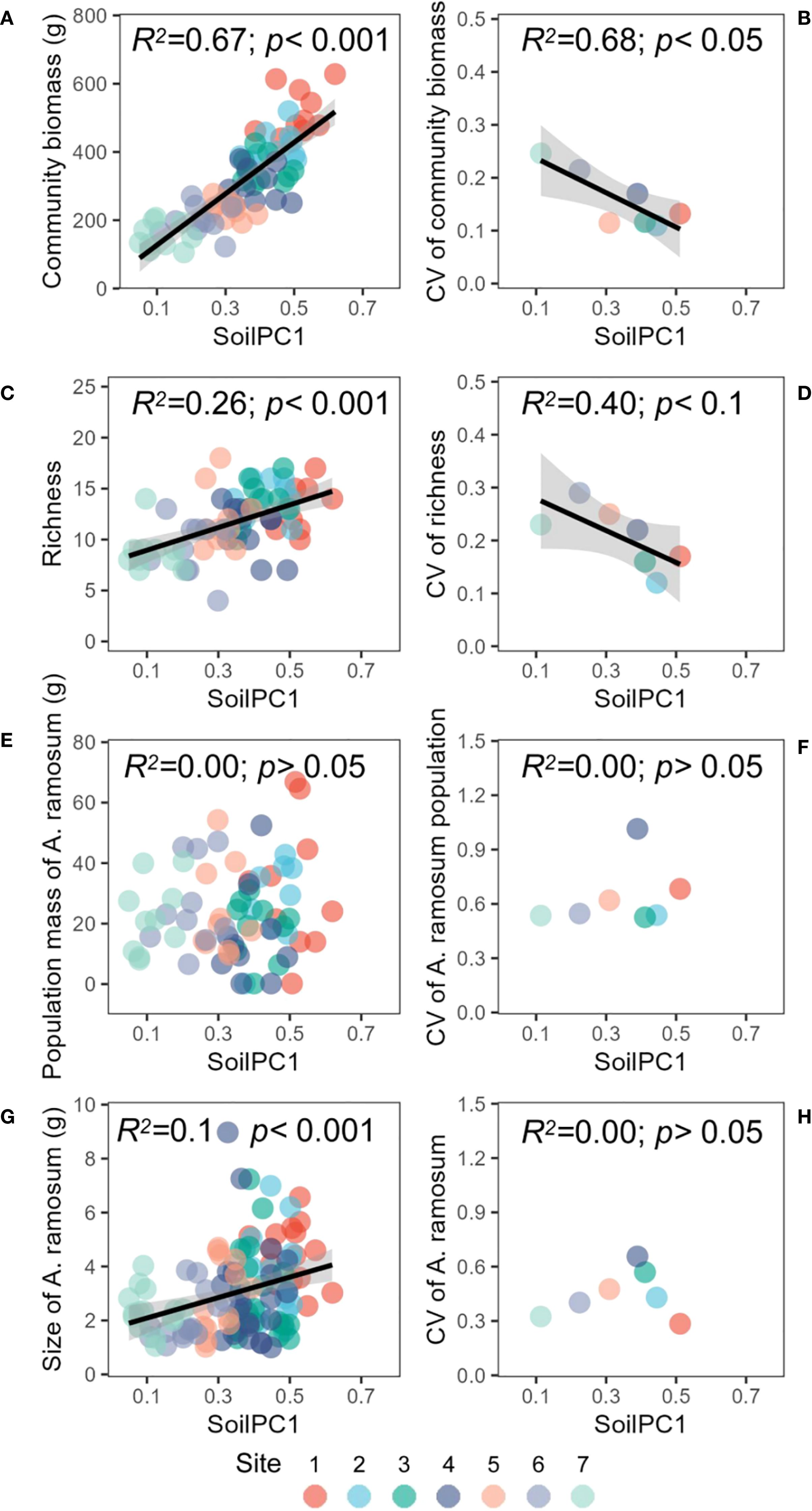
Figure 2. Relationships between the soil gradient (PC1) and (A) community above ground biomass, (B) CV of community above ground biomass, (C) richness, (D) CV of richness, (E) population mass of A. ramosum, (F) CV of A. ramosum population mass, (G) size of A. ramosum, (H) CV of A. ramosum size. The colors of the points represent different sites (dominant species of the plant community).
Changes in biomass allocation patterns across soil gradients
With soil gradient, the allometric exponent between leaf and flower biomass and between bulb and flower biomass decreased (i.e. plants tended to allocate more biomass to flowers relative to plant growth and storage organs (leaves, bulbs)) (Figures 3C, G). Similarly, the allometric exponent between leaves and stems decreased with soilPC1 (more biomass was allocated to stems) (Figure 3A). Significant allometric partitioning among other organs of Allium ramosum existed, but did not vary significantly with soil gradients or plant community aboveground biomass (Supplementary Table S3, Supplementary Figure S3).
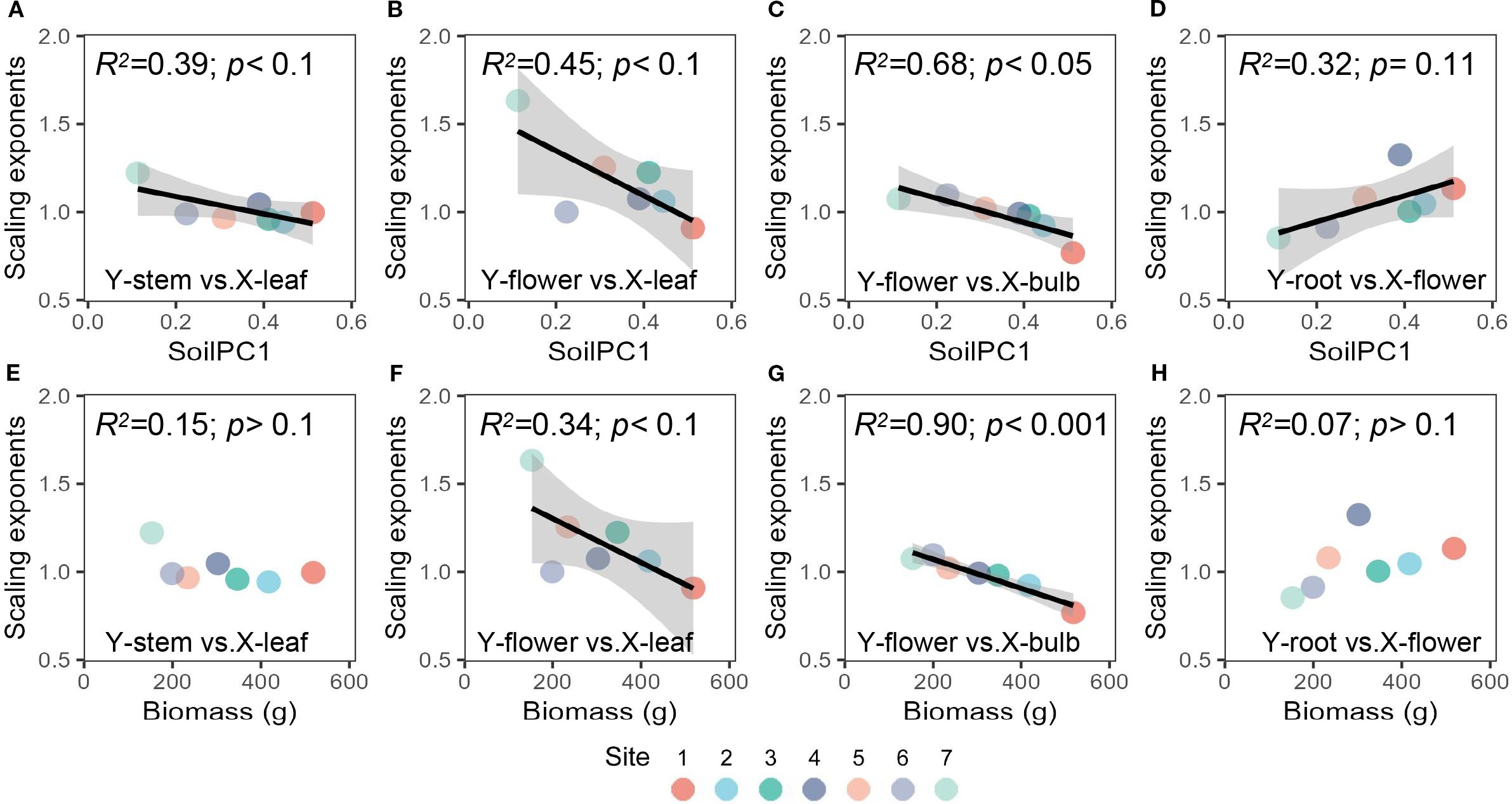
Figure 3. Relationships between the soil gradient (PC1) and scaling exponents of (A) leaf vs stem, (B) leaf vs flower, (C) bulb vs flower (D) flower vs root, and relationships between community above-ground biomass and (E) leaf vs stem, (F) leaf vs flower, (G) bulb vs flower (H) flower vs root. The colors of the points represent different sites (dominant species of the plant community).
The results of the structural equation model showed that the direct effect of soilPC1 on the leaf-flower scaling exponents was not significant, but there was a significant positive effect on the plant community-weighted height, i.e. the plant community-weighted height increased as soil salinity decreased, but the community-weighted height had a significant negative effect on the leaf-flower scaling exponents, and thus there was an indirect negative effect of soilPC1 on the leaf-flower scaling exponents. SoilPC1 and plant community-weighted height together explained 81% of the variation in leaf-flower scaling exponents (Figure 4A). Similarly, soilPC1 indirectly influenced the scaling exponents between bulbs and flowers by influencing the aboveground biomass of the community, explaining 94% of the variance (Figure 4B).
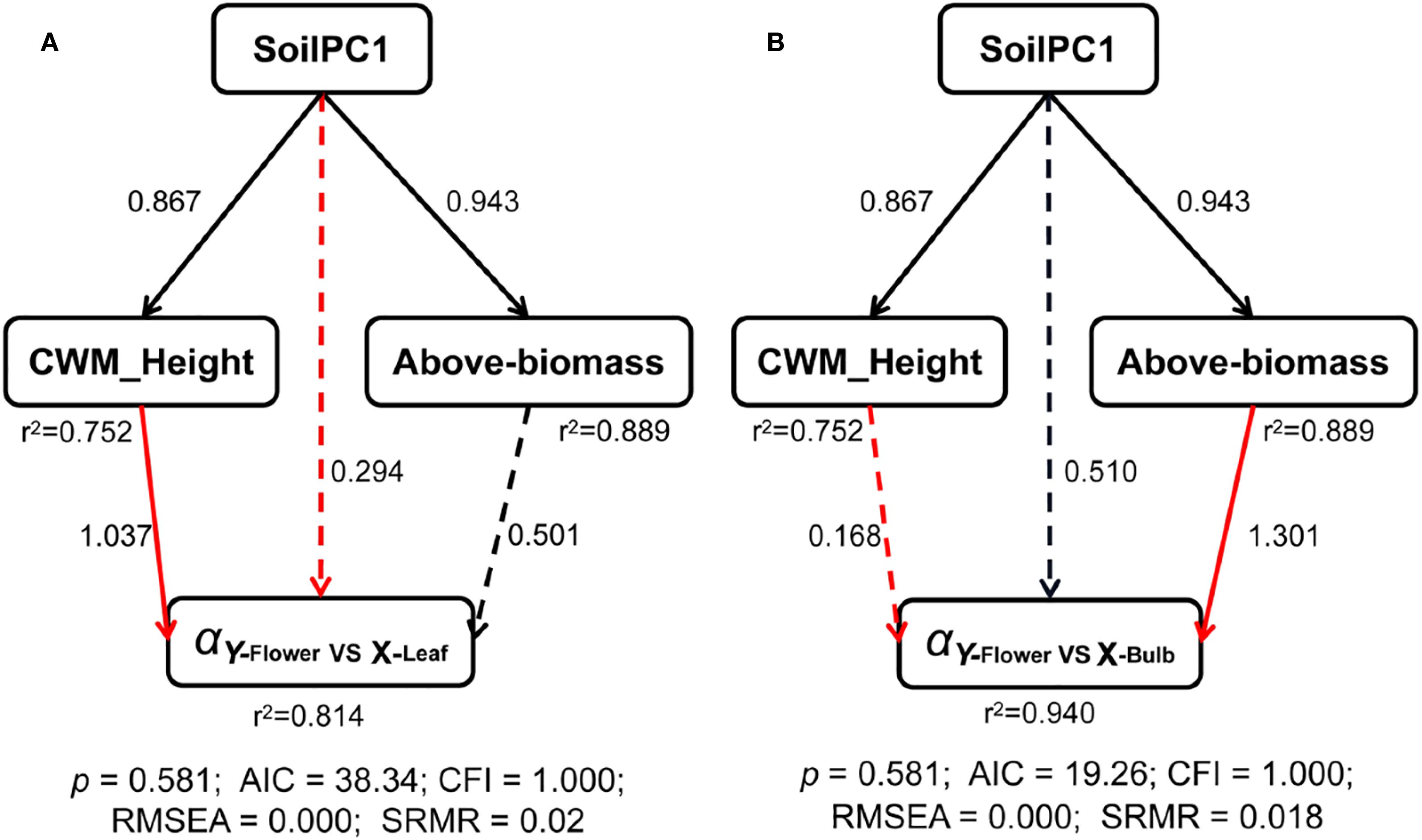
Figure 4. Structural equation models of driving mechanisms of multiple factors on scaling exponents of (A) leaf vs flower and (B) bulb vs flower. Black and red solid arrows represent significant (P < 0.05) positive and negative paths, respectively. Dashed black and red arrows represent non-significant (P > 0.05) positive and negative paths, respectively. Numbers represent the standardized path coefficients. The r2 represents the proportion of explained variance for exponents.
Discussion
Changes in soil salinity gradients and plant communities
Soil salinization is a synergistic process of salt accumulation and nutrient loss. Studies have shown that salinity indicators (e.g. pH) are significantly negatively correlated with soil nutrients (organic carbon, nitrogen and phosphorus) in several ecosystems (Corwin, 2021; Garcia-Franco et al., 2021; Huang et al., 2024). Principal component analysis (PCA) further indicated that pH and EC could be the core indicators of soil degradation on the salinization gradient, while organic carbon and nitrogen and phosphorus became sensitive parameters for nutrient loss. The PCA showed that the salinity indicators (pH and EC) showed significant negative correlation with the nutrient indicators (organic carbon, nitrogen and phosphorus) (Figure 1), which revealed the close coupling between soil degradation and nutrient loss in the salinization process. This result is consistent with the general pattern of saline grassland degradation in the global scale (Pan et al., 2013; Heng et al., 2022; Li et al., 2022). For example, in saline soils in western Jilin Province, organic matter and quick potassium content decreased significantly with increasing sodium ion (Na+) content and EC, while total phosphorus content remained at a low level for a long time (Cao et al., 2021). This negative correlation can be explained by the following mechanism: ion competition and nutrient fixation: under high salinity conditions, Na+ displaces nutrients such as calcium (Ca2+) and magnesium (Mg2+) in the soil colloid by cation exchange, leading to their leaching or fixation (Zhou et al., 2023). Salt reduces organic matter mineralization and nutrient release by increasing soil osmotic pressure and toxicity and inhibiting microbial decomposition functions (Zhang et al., 2015; Chen et al., 2024). Experiments on the improvement of saline soils showed that the organic matter content of unimproved soils was only 50% of that of improved soils, verifying the negative effect of salts on soil carbon and nitrogen cycling (Xiao et al., 2025). Salinization leads to soil sloughing and reduced porosity, further hindering nutrient uptake by the root system (Garcia-Franco et al., 2021). The process forms a positive feedback of ‘salt accumulation-nutrient loss-soil degradation’, which ultimately leads to a systematic decline in soil fertility.
Natural environmental gradients shape patterns of plant diversity and are important factors influencing plant community dynamics. For example, along large-scale gradients (latitude, elevation), biodiversity tends to increase with energy, temperature and environmental stability, while within regional scales, such as degradation gradients and soil nutrient gradients, also significantly influence plant community structure and function (Zhou et al., 2021; Heng et al., 2022; Wu et al., 2024; Yang et al., 2025). We showed that plant community biomass and diversity decreased significantly with increasing salinization, while community variability increased (Figure 2). Salinization not only directly stresses plant physiology, but also affects community biomass and diversity by changing community structure and resource competition (Zhang et al., 2015). This result is consistent with studies in saline grassland, where salinization led to a large reduction in microbial community diversity (Heng et al., 2022). This change may be related to the fact that salinity inhibits plant photosynthesis and water uptake, leading to a decrease in overall community productivity (Corwin, 2021). At the same time, nutrient deprivation increases competition between species, and only a few salt-tolerant species can maintain population stability through phenotypic plasticity (e.g., reduced individual size, increased reproductive investment) (Tang et al., 2022). On the salinity gradient, local differences in soil physicochemical properties (e.g. pH and salinity patchy distribution) led to increased community variability (Zhang et al., 2015).
Biomass allocation strategy for Allium ramosum in response to soil gradient
There was no significant trend observed in the biomass or stability of Allium ramosum populations along the soil gradient (Figure 2e). This phenomenon may be attributed to the physiological plasticity exhibited by Allium ramosum. Individuals within the species demonstrate a capacity to adapt to stressful environments through a reduction in body size, which leads to a decrease in resource requirements, and an increase in phenotypic variation, which can be considered a non-linear response (Huang et al., 2019; Ren et al., 2020; Matesanz et al., 2021). The impact of soil features on Allium ramosum populations may also be influenced by species interactions within the community. Our results demonstrated that individual size, plant height and the size of organs of Allium ramosum increased significantly with soil gradient (Figure 2, Supplementary Figure S2). These correlations indicate that natural selection may have prevented independent evolution of traits, suggesting a need for coordination among traits contributing to the same function (He et al., 2020; Zhou et al., 2022; Bin et al., 2024). The observed correlations among these traits can be regarded as a facet of the broader coordination between traits and organs in plants (Matesanz et al., 2021; Sanchez-Bermejo et al., 2023). Furthermore, in accordance with other studies, environmental stress did not modify the allometric distribution of biomass among organs, yet the allometric indices and constants exhibited a significant trend with soil salinity (Eziz et al., 2017; Peng et al., 2022). Growth and reproduction represent two of the most fundamental processes in plants, with the biomass of leaves, stems and roots determining the ability to capture light and access soil resources to provide photosynthetic products and nutrients for reproduction (Poorter et al., 2012; Yan et al., 2016; White et al., 2022). Allium ramosum exhibit an adaptive strategy for biomass allocation priority across salinity gradients. As soil salinity increase, the allocation of biomass to sexual reproductive organs (e.g. flowers) exceeds that allocated to nutritive organs (leaves, bulbs) (Figure 3).
Based on a field study conducted in the Sugan Lake wetland on the Qinghai-Tibet Plateau. Soil salinity conditions across three distinct habitats: inland salt marsh, oasis wetland, and seasonal river wetland significantly influenced Saussurea salsa biomass allocation and morphology. Under high salinity, plants developed large, thick leaves with low specific leaf area (SLA) and formed roots with moderate diameter and length, allocating minimal biomass to roots to mitigate water stress and ion toxicity by enhancing water storage in leaves and reducing root exposure (Li et al., 2021). This strategic allocation of biomass ensures the survival of offspring by increasing reproductive output under stressful conditions (Zhang et al., 2021a; Van de Walle et al., 2023; Stott et al., 2024). This variation in the strategy for biomass allocation is also subject to change in response to climate change and across large climatic and soil gradients, where increased resource stress (e.g. drought, low soil nutrient content) is experienced. Artemisia spp. exhibit increased biomass for reproduction allocation and decreased biomass allocation to leaves (Tsogtsaikhan et al., 2025). The decrease in leaves may be due to individual size limitation and reduced water consumption from transpiration, since more saline soils have poorer water-holding capacity (Zhou et al., 2023). However, it is important to note that the results of structural equation modelling suggest that this allocation pattern does not constitute a direct response to soil features, but is mediated through changes in community above-ground biomass and structure (Figure 4). The effect of soil salinization on the pattern of biological allocation between leaves and flowers of Allium ramosum was found to be indirectly influenced by affecting the community-weighted height of the plant. The decrease in community weighted height was attributed to salinization, resulting in the replacement of dominant species with low salt-tolerant species (Janousek and Folger, 2014; Token et al., 2022). At higher community heights, light competition is increased and plants allocate more biomass to growth, favoring plant extension for more light (Xiao et al., 2021). One reason why plants allocate fewer resources to reproduction may be mechanical limitations of mechanical support when plants are taller (West et al., 1999). According to Corner’s rules, larger inflorescences require thicker stems to support them (Fajardo et al., 2020). Hence, for Allium ramosum, taller stems is required so that the flowers can reach a sufficient height to favor pollination and fruit set. If plants devote more biomass to producing taller and thicker stems, this can lead to a less competitive plant, with stems being considered luxury organs (Xiao et al., 2021), especially in Allium ramosum plants, which only serve to support the inflorescence. Thus, as the community weighted height of the plant increases, the plant tends to allocate more biomass to leaves rather than flowers (Figure 4a).
It is also noteworthy that Allium ramosum is a perennial plant and that there will be a trade-off between storage and reproduction (Zhang et al., 2022). Our results also indicated that as soil salinity increased, plants similarly allocated more biomass to reproduction than to storage (Figure 4b). This strategy may reduce competition for light resources due to declining biomass in the plant community, prompting Allium ramosum to divert resources to reproduction in order to extend their dispersal advantage (Bonser, 2013). Conversely, in communities with less saline soils, increased aboveground biomass caused plants to allocate resources inside the storage organ - bulbs, to allow plants to have larger plants in the next growing season, as bulb size was significantly correlated with individual plant size (Howard and Cellinese, 2020; Ashagrie et al., 2021).
Notably, phosphorus has been found to be an essential element for plants, influencing the entire reproductive process, from bud differentiation to seed maturity (Fortier and Wright, 2021; Velez-Mora et al., 2024). At the intraspecific level, P-limited conditions resulted in later flowering onset, shorter individual flowering duration, and reduced flower/inflorescence production per plant. Interspecifically, species adapted to P-limited environments exhibited earlier flowering onset, longer seed stalks and panicles, but also shorter flowering periods and fewer flowers per plant (Wang et al., 2022). Critically, P limitation consistently constrained investment in sexual reproduction (e.g., reduced flower production, shorter flowering periods), potentially impairing dispersal capacity. Significant confounding effects of soil pH and moisture were also revealed which covaried with nutrient regimes—on reproductive traits, complicating the interpretation of N:P effects (Wang et al., 2022).
Based on the analysis of 599 Eurasian herbaceous sites, phosphorus (P) limitation (indicated by high N:P ratios in plant biomass) strongly influences plant reproductive strategies, with significant implications for endangered species. Plants in P-limited communities exhibit markedly reduced investment in sexual reproduction compared to nitrogen (N)-limited communities, manifested through lower seed production, diminished seed mass, shorter flowering periods, delayed flowering onset, and greater reliance on vegetative propagation and perennial lifespans (Fujita et al., 2014).
The experimental study investigated how absolute and relative nitrogen (N) and phosphorus (P) supply affect sexual reproduction traits in a common grass (Holcus lanatus) and an endangered forb (Parnassia palustris) demonstrated that the effect of N:P supply ratio on sexual reproduction investment is critically dependent on the absolute nutrient supply level: at low absolute nutrient supply, N:P ratio had minimal impact on reproduction traits, whereas at high absolute nutrient supply, a high N:P ratio (indicating low relative P availability) significantly reduced investment in sexual reproduction for H. lanatus (Wang et al., 2019). Low relative P-supply (high N:P ratio) restricted the positive response of sexual reproduction traits to increased absolute nutrient supply, essentially limiting the potential benefits of higher nutrient availability. While data for the endangered P. palustris were limited due to high mortality, its survival patterns mirrored the reproduction response of H. lanatus, suggesting similar constraints under low relative P-supply at high nutrient levels (Wang et al., 2019). This is consistent with our results that, as a perennial nondominant species, reproduction of Allium ramosum may be more strongly limited by phosphorus along the soil gradient (Figure 1, Supplementary Table S1).
The strategy for biomass allocation at the plant community level also produces adaptive variation in response to environmental change (Fant and Ghedini, 2024; Wu et al., 2024). A strategy for biomass allocation prioritizing survival is favored in arid environments, where more biomass is allocated to the root system for water, whereas investment in the above-ground fraction is prioritized in wetter areas with better soil nutrient (Wu et al., 2024). Global-scale studies indicate that the species composition and diversity of plant communities can buffer the effects of environmental gradients on biomass allocation (Skarpaas et al., 2016; Dolezal et al., 2021). For example, species turnover reduces the degree of variability in the overall allocation strategy of the community through functional trait complementarities (Zhou et al., 2021; Fang et al., 2023; Krak et al., 2025).
Recent studies of biomass allocation patterns have demonstrated considerable variation in this ratio across diverse environmental contexts (Chen et al., 2021; Beccari and Carmona, 2024; Dolezal et al., 2024). However, the majority of these studies have been conducted on individual plants, focusing exclusively on the abiotic environment and species traits, without considering the interactive dynamics among species within communities (Zhang et al., 2021a; Zhou et al., 2022; Krak et al., 2025; Tsogtsaikhan et al., 2025). Consequently, our understanding of how plants respond to the combined effects of soil salinity stress and species competition remains limited. The findings of this study contradict traditional, simplified models of direct soil-plant response and underscore the critical role of community-scale processes in individual adaptation strategies. At the community level, interspecific roles and environmental stresses may interact, complicating the individual to community level scaling transitions (Zhou et al., 2021; Fant and Ghedini, 2024). Consequently, we would like to propose that future studies include more characteristics of plant communities and populations when analyzing strategies for biomass allocation in response to environmental gradients or environmental changes.
Conclusion
In this study, we investigated the biomass allocation patterns of various plant communities and Allium ramosum within these communities across a soil gradient. The results demonstrated that plant community diversity, community weighted height and aboveground biomass increased with soil gradient. Concurrently, the allometric exponent between leaf-flower and bulb-flower biomass exhibited a significant decreasing trend, with a greater allocation of biomass to sexual reproductive organs (flowers) than to vegetative organs (leaves and bulbs). The biomass allocation strategy of Allium ramosum was influenced by a decrease in community weighted height driven by soil salinization (reduced light competition) and changes in community aboveground biomass (competitive pressure for resources). These results highlight the influence of soil conditions and plant communities on plant life history strategies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
CF: Writing – original draft, Writing – review & editing. JF: Writing – original draft. GF: Writing – original draft. HW: Investigation, Writing – original draft. CW: Funding acquisition, Writing – review & editing. WQ: Writing – review & editing. DY: Investigation, Methodology, Writing – review & editing. YH: Funding acquisition, Writing – review & editing.
Ethics statement
Because the sampling site is located at Songnen Grassland Research Station (part of the Northeast Institute of Geography and Agroecology, Chinese Academy of Sciences), we were able to sample plants directly without permission.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by Strategic Priority Research Program of the Chinese Academy of Sciences (XDA28110201) and National Natural Science Foundation of China (42471071) of Yingxin Huang. And was supported by Jilin Provincial Natural Science Foundation (YDZJ202401482ZYTS) of Congwen Wang.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1627304/full#supplementary-material
References
Ashagrie, T., Belew, D., and Nebiyu, A. (2021). Influence of planting date and bulb size on yield and quality of onion (Allium cepa L.) seed production. Cogent Food Agric. 7, 1908656. doi: 10.1080/23311932.2021.1908656
Bartuskova, A., Lubbe, F. C., Qian, J., Herben, T., and Klimesova, J. (2022). The effect of moisture, nutrients and disturbance on storage organ size and persistence in temperate herbs. Funct. Ecol. 36, 314–325. doi: 10.1111/1365-2435.13997
Beccari, E. and Carmona, C. P. (2024). Aboveground and belowground sizes are aligned in the unified spectrum of plant form and function. Nat. Commun. 15, 9199. doi: 10.1038/s41467-024-53180-x
Bin, Y., Russo, S. E., Zhang, J., Li, Y., Cao, H., Ye, W., et al. (2024). Functional traits are more strongly correlated with biomass than diameter growth. J. Ecol. 112, 1225–1239. doi: 10.1111/1365-2745.14281
Bonser, S. P. (2013). High reproductive efficiency as an adaptive strategy in competitive environments. Funct. Ecol. 27, 876–885. doi: 10.1111/1365-2435.12064
Cao, C., Tao, S., Cui, Z., and Zhang, Y. (2021). Response of soil properties and microbial communities to increasing salinization in the meadow grassland of northeast China. Microbial Ecol. 82, 722–735. doi: 10.1007/s00248-021-01695-x
Chen, R., Ran, J., Hu, W., Dong, L., Ji, M., Jia, X., et al. (2021). Effects of biotic and abiotic factors on forest biomass fractions. Natl. Sci. Rev. 8. doi: 10.1093/nsr/nwab025
Chen, S., Hou, H., Zhang, X., Gao, Z., Wang, H., Yuan, Y., et al (2024). Relationship between nutrient accumulation in broomcorn millet (Panicum miliaceum L.) and microbial community under different salinity soils. Plant Soil. 511, 1285–1302. doi: 10.1007/s11104-024-07046-2
Chope, G. A., Cools, K., Hammond, J. P., Thompson, A. J., and Terry, L. A. (2012). Physiological, biochemical and transcriptional analysis of onion bulbs during storage. Ann. Bot. 109, 819–831. doi: 10.1093/aob/mcr318
Corwin, D. L. (2021). Climate change impacts on soil salinity in agricultural areas. Eur. J. Soil Sci. 72, 842–862. doi: 10.1111/ejss.13010
Dolezal, J., Chondol, T., Chlumska, Z., Altman, J., Capkova, K., Dvorsky, M., et al. (2024). Contrasting biomass allocations explain adaptations to cold and drought in the world’s highest-growing angiosperms. Ann. Bot. 134, 401–414.
Dolezal, J., Jandova, V., Macek, M., and Liancourt, P. (2021). Contrasting biomass allocation responses across ontogeny and stress gradients reveal plant adaptations to drought and cold. Funct. Ecol. 35, 32–42. doi: 10.1111/1365-2435.13687
Enquist, B. J. and Niklas, K. J. (2002). Global allocation rules for patterns of biomass partitioning in seed plants. Science 295, 1517–1520. doi: 10.1126/science.1066360
Eziz, A., Yan, Z., Tian, D., Han, W., Tang, Z., and Fang, J. (2017). Drought effect on plant biomass allocation: A meta-analysis. Ecol. Evol. 7, 11002–11010. doi: 10.1002/ece3.3630
Fajardo, A., Mora, J. P., and Robert, E. (2020). Corner’s rules pass the test of time: little effect of phenology on leaf-shoot and other scaling relationships. Ann. Bot. 126, 1129–1139. doi: 10.1093/aob/mcaa124
Fang, T., Rao, M., Chen, Q., Liu, S., Lai, J., Chen, T., et al. (2023). Different biomass allocation strategies of geophytes and non-geophytes along an altitude gradient. Ecol. Indic. 146. doi: 10.1016/j.ecolind.2022.109805
Fant, L. and Ghedini, G. (2024). Biomass competition connects individual and community scaling patterns. Nat. Commun. 15, 9916. doi: 10.1038/s41467-024-54307-w
Fortier, R. and Wright, S. (2021). Nutrient limitation of plant reproduction in a tropical moist forest. Ecology 102, e03469. doi: 10.1002/ecy.3469
Fujita, Y., Venterink, H. O., van Bodegom, P. M., Douma, J. C., Heil, G. W., Hoelzel, N., et al. (2014). Low investment in sexual reproduction threatens plants adapted to phosphorus limitation. Nature 505, 82–86. doi: 10.1038/nature12733
Garcia-Franco, N., Wiesmeier, M., Hurtarte, L. C. C., Fella, F., Martinez-Mena, M., Almagro, M., et al. (2021). Pruning residues incorporation and reduced tillage improve soil organic matter stabilization and structure of salt-affected soils in a semi-arid Citrus tree orchard. Soil Tillage Res. 213, 105129. doi: 10.1016/j.still.2021.105129
Ge, W., Bu, H., Wang, X., Martinez, S. A., and Du, G. (2020). Inter- and intra-specific difference in the effect of elevation and seed mass on germinability of eight Allium species. Global Ecol. Conserv. 22, e01016. doi: 10.1016/j.gecco.2020.e01016
He, N., Li, Y., Liu, C., Xu, L., Li, M., Zhang, J., et al. (2020). Plant trait networks: improved resolution of the dimensionality of adaptation. Trends Ecol. Evol. 35, 908–918. doi: 10.1016/j.tree.2020.06.003
Heng, T., Yang, L., Hermansen, C., de Jonge, L. W., Zhang, Z., Wu, B., et al. (2022). Linking microbial community compositions to cotton nitrogen utilization along soil salinity gradients. Field Crops Res. 288. doi: 10.1016/j.fcr.2022.108697
Howard, C. C. and Cellinese, N. (2020). Tunicate bulb size variation in monocots explained by temperature and phenology. Ecol. Evol. 10, 2299–2309. doi: 10.1002/ece3.5996
Hsiao, J., Yun, K., Moon, K. H., and Kim, S.-H. (2019). A process-based model for leaf development and growth in hardneck garlic (Allium sativum). Ann. Bot. 124, 1143–1160. doi: 10.1093/aob/mcz060
Hu, J., Yu, H., Li, Y., Wang, J., Lv, T., Liu, C., et al. (2021). Variation in resource allocation strategies and environmental driving factors for different life-forms of aquatic plants in cold temperate zones. J. Ecol. 109, 3046–3059. doi: 10.1111/1365-2745.13719
Huang, Y., Fan, G., Zhou, D., and Pang, J. (2019). Phenotypic plasticity of four Chenopodiaceae species with contrasting saline-sodic tolerance in response to increased salinity-sodicity. Ecol. Evol. 9, 1545–1553. doi: 10.1002/ece3.4515
Huang, K., Kuai, J., Jing, F., Liu, X., Wang, J., Lin, J, et al (2024). Effects of understory intercropping with salt-tolerant legumes on soil organic carbon pool in coastal saline-alkali land. J. Environ. Manage. 370. doi: 10.1016/j.jenvman.2024.122677
Huang, Y., Lechowicz, M. J., Price, C. A., Li, L., Wang, Y., and Zhou, D. (2016). The underlying basis for the trade-off between leaf size and leafing intensity. Funct. Ecol. 30, 199–205. doi: 10.1111/1365-2435.12491
Hulshof, C. M., Stegen, J. C., Swenson, N. G., Enquist, C. A. F., and Enquist, B. J. (2012). Interannual variability of growth and reproduction in Bursera simAruba: the role of allometry and resource variability. Ecology 93, 180–190. doi: 10.1890/11-0740.1
Janousek, C. N. and Folger, C. L. (2014). Variation in tidal wetland plant diversity and composition within and among coastal estuaries: assessing the relative importance of environmental gradients. J. Vegetation Sci. 25, 534–545. doi: 10.1111/jvs.12107
Kim, D., Medvigy, D., Maier, C. A., Johnsen, K., and Palmroth, S. (2020). Biomass increases attributed to both faster tree growth and altered allometric relationships under long-term carbon dioxide enrichment at a temperate forest. Global Change Biol. 26, 2519–2533. doi: 10.1111/gcb.14971
Krak, K., Balsankova, T., Semberova, K., Hadincova, V., Pechackova, S., Skalova, H., et al. (2025). Species-specific root-shoot ratios in a diverse grassland community. Funct. Ecol. 39, 51–63. doi: 10.1111/1365-2435.14716
Li, J., Liu, Y., Zhang, M., Xu, H., Ning, K., Wang, B., et al. (2022). Melatonin increases growth and salt tolerance of Limonium bicolor by improving photosynthetic and antioxidant capacity. BMC Plant Biol. 22. doi: 10.1186/s12870-021-03402-x
Li, Q., Zhao, C., Kang, M., and Li, X. (2021). The relationship of the main root-shoot morphological characteristics and biomass allocation of Saussurea salsa under different habitat conditions in Sugan lake wetland on the northern margin of the Qinghai-Tibet Plateau. Ecol. Indic. 128. doi: 10.1016/j.ecolind.2021.107836
Liu, M., Cao, J., Wang, C., Wang, B., and Xue, R. (2024). Vermicompost enhances the salt tolerance of maize by reshaping the rhizosphere microenvironment. Appl. Soil Ecol. 203. doi: 10.1016/j.apsoil.2024.105633
Matesanz, S., Blanco-Sanchez, M., Ramos-Munoz, M., de la Cruz, M., Benavides, R., and Escudero, A. (2021). Phenotypic integration does not constrain phenotypic plasticity: differential plasticity of traits is associated to their integration across environments. New Phytol. 231, 2359–2370. doi: 10.1111/nph.17536
Murphy, J. and Riley, J. (1962). A modified single solution method for thedetermination of phosphate in natural waters. Analytica Chimica Acta 27, 31–36. doi: 10.1016/S0003-2670(00)88444-5
Pan, C., Liu, C., Zhao, H., and Wang, Y. (2013). Changes of soil physico-chemical properties and enzyme activities in relation to grassland salinization. Eur. J. Soil Biol. 55, 13–19. doi: 10.1016/j.ejsobi.2012.09.009
Peng, Y., Fornara, D. A., Yue, K., Peng, X., Peng, C., Wu, Q., et al. (2022). Globally limited individual and combined effects of multiple global change factors on allometric biomass partitioning. Global Ecol. Biogeography 31, 454–469. doi: 10.1111/geb.13438
Planas-Sitja, I., Monnin, T., Loeuille, N., and Cronin, A. L. (2023). To disperse or compete? Coevolution of traits leads to a limited number of reproductive strategies. Oikos 2023. doi: 10.1111/oik.09972
Poorter, H., Niklas, K. J., Reich, P. B., Oleksyn, J., Poot, P., and Mommer, L. (2012). Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytol. 193, 30–50. doi: 10.1111/j.1469-8137.2011.03952.x
Ren, L., Guo, X., Liu, S., Yu, T., Guo, W., Wang, R., et al. (2020). Intraspecific variation in Phragmites australis: Clinal adaption of functional traits and phenotypic plasticity vary with latitude of origin. J. Ecol. 108, 2531–2543. doi: 10.1111/1365-2745.13401
Sanchez-Bermejo, P. C., Davrinche, A., Matesanz, S., Harpole, W. S., and Haider, S. (2023). Within-individual leaf trait variation increases with phenotypic integration in a subtropical tree diversity experiment. New Phytol. 240, 1390–1404.
Skarpaas, O., Meineri, E., Bargmann, T., Potsch, C., Topper, J., and Vandvik, V. (2016). Biomass partitioning in grassland plants along independent gradients in temperature and precipitation. Perspect. Plant Ecol. Evol. Systematics 19, 1–11. doi: 10.1016/j.ppees.2016.01.006
Stott, I., Salguero-Gomez, R., Jones, O. R., Ezard, T. H. G., Gamelon, M., Lachish, S., et al. (2024). Life histories are not just fast or slow. Trends Ecol. Evol. 39, 830–840. doi: 10.1016/j.tree.2024.06.001
Tang, L., Zhou, Q. S., Gao, Y., and Li, P. (2022). Biomass allocation in response to salinity and competition in native and invasive species. Ecosphere 13, 14. doi: 10.1002/ecs2.3900
Token, S., Jiang, L., Zhang, L., and Ly, G. (2022). Effects of plant diversity on primary productivity and community stability along soil water and salinity gradients. Global Ecol. Conserv. 36. doi: 10.1016/j.gecco.2022.e02095
Tsogtsaikhan, T., Yang, X., Gao, R., Liu, J., Tang, W., Liu, G., et al. (2025). Biomass allocation between reproductive and vegetative organs of Artemisia along a large environmental gradient. BMC Plant Biol. 25, 1–13. doi: 10.1186/s12870-024-06030-3
Umana, M. N., Cao, M., Lin, L., Swenson, N. G., and Zhang, C. (2021). Trade-offs in above- and below-ground biomass allocation influencing seedling growth in a tropical forest. J. Ecol. 109, 1184–1193.
Van de Walle, J., Fay, R., Gaillard, J.-M., Pelletier, F., Hamel, S., Gamelon, M., et al. (2023). Individual life histories: neither slow nor fast, just diverse. Proc. R. Soc. B-Biological Sci. 290. doi: 10.1098/rspb.2023.0511
Velez-Mora, D., Trigueros-Alatorre, K., Duncan, D., and Quintana-Ascencio, P. (2024). Natural and anthropogenic factors influence flowering synchrony and reproduction of a dominant plant in an inter-Andean scrub. Am. J. Bot. 111. doi: 10.1002/ajb2.16416
Wang, S., van Dijk, J., and Wassen, M. (2019). Sexual reproduction traits of Holcus lanatus L. and Parnassia palustris L. @ in response to absolute and relative supply of nitrogen and phosphorus. Environ. Exp. Bot. 168. doi: 10.1016/j.envexpbot.2019.103813
Wang, S., van Dijk, J., and Wassen, M. (2022). Sexual reproduction trait expressions of grassland species along a gradient of nitrogen: phosphorus stoichiometry. Plant Soil 473, 215–234. doi: 10.1007/s11104-021-05230-2
Weiner, J., Campbell, L. G., Pino, J., and Echarte, L. (2009). The allometry of reproduction within plant populations. J. Ecol. 97, 1220–1233. doi: 10.1111/j.1365-2745.2009.01559.x
West, G. B., Brown, J. H., and Enquist, B. J. (1999). A general model for the structure and allometry of plant vascular systems. Nature 400, 664–667. doi: 10.1038/23251
White, C. R., Alton, L. A., Bywater, C. L., Lombardi, E. J., and Marshall, D. J. (2022). Metabolic scaling is the product of life-history optimization. Science 377, 834–83+. doi: 10.1126/science.abm7649
Wong, M. Y., Wurzburger, N., Hall, J. S., Wright, S. J., Tang, W., Hedin, L. O, et al (2024). Trees adjust nutrient acquisition strategies across tropical forest secondary succession. New Phytol. 243, 132–144.
Wu, W., Sun, R., Liu, X., Li, L., Qi, M., Zhang, F., et al. (2024). Driving mechanisms of community biomass allocation along environmental gradients in different grasslands in China. Ecol. Indic. 160. doi: 10.1016/j.ecolind.2024.111886
Xiao, M., Jiang, S., Li, J., Li, W., Fu, P., Liu, G., et al. (2025). Synergistic effects of bio-organic fertilizer and different soil amendments on salt reduction, soil fertility, and yield enhancement in salt-affected coastal soils. Soil Tillage Res. 248. doi: 10.1016/j.still.2024.106433
Xiao, Y., Liu, X., Zhang, L., Song, Z., and Zhou, S. (2021). The allometry of plant height explains species loss under nitrogen addition. Ecol. Lett. 24, 553–562. doi: 10.1111/ele.13673
Yan, B., Ji, Z., Fan, B., Wang, X., He, G., Shi, L., et al. (2016). Plants adapted to nutrient limitation allocate less biomass into stems in an arid-hot grassland. New Phytol. 211, 1232–1240. doi: 10.1111/nph.13970
Yang, C., Chen, Y., Sun, W., Zhang, Q., Diao, M., and Sun, J. (2025). Extreme soil salinity reduces N and P metabolism and related microbial network complexity and community immigration rate. Environ. Res. 264. doi: 10.1016/j.envres.2024.120361
Zemunik, G., Turner, B. L., Lambers, H., and Laliberte, E. (2015). Diversity of plant nutrient-acquisition strategies increases during long-term ecosystem development. Nat. Plants 1, 1–4. doi: 10.1038/nplants.2015.50
Zhang, Y., Cao, C., Guo, L., Wu, Q., and Cui, Z. (2015). Soil properties, bacterial community composition, and metabolic diversity responses to soil salinization of a semiarid grassland in northeast China. J. Soil Water Conserv. 70, 110–120. doi: 10.2489/jswc.70.2.110
Zhang, L., Khamphilavong, K., Zhu, H., Li, H., He, X., Shen, X., et al. (2021b). Allometric scaling relationships of Larix potaninii subsp. chinensis traits across topographical gradients. Ecol. Indic. 125. doi: 10.1016/j.ecolind.2021.107492
Zhang, J., Xie, H., Biswas, A., Shan, Y., Qi, X., and Cao, J. (2021a). Response of different organs’ stoichiometry of Phragmites australis to soil salinity in arid marshes, China. Global Ecol. Conserv. 31. doi: 10.1016/j.gecco.2021.e01843
Zhang, L., Yang, L., Zhou, H., Ren, L., Li, W., Bai, W., et al. (2022). Carbon allocation patterns in forbs and grasses differ in responses to mowing and nitrogen fertilization in a temperate grassland. Ecol. Indic. 135, 108588. doi: 10.1016/j.ecolind.2022.108588
Zhou, J., Cieraad, E., and van Bodegom, P. M. (2022). Global analysis of trait-trait relationships within and between species. New Phytol. 233, 1643–1656. doi: 10.1111/nph.17879
Zhou, L., Liu, W., Duan, H., Dong, H., Li, J., Zhang, S., et al. (2023). Improved effects of combined application of nitrogen-fixing bacteria Azotobacter beijerinckii and microalgae Chlorella pyrenoidosa on wheat growth and saline-alkali soil quality. Chemosphere 313. doi: 10.1016/j.chemosphere.2022.137409
Keywords: life history strategies, biomass allocation, allometric, soil salinity, perennial herbs
Citation: Fu C, Fan J, Fan G, Wang H, Wang C, Qiang W, Yu D and Huang Y (2025) Soil and plant communities co-regulating plant biomass allocation patterns along a saline-alkali gradient, case study of Allium ramosum in Songnen Grassland, Northeast China. Front. Plant Sci. 16:1627304. doi: 10.3389/fpls.2025.1627304
Received: 13 May 2025; Accepted: 25 August 2025;
Published: 24 September 2025.
Edited by:
Chaohe Huangfu, Anhui University, ChinaReviewed by:
Weizhou Xu, Yulin University, ChinaKhalil Kadaoui, Abdelmalek Essaâdi University, Morocco
Suwan Ji, Xinjiang University, China
Copyright © 2025 Fu, Fan, Fan, Wang, Wang, Qiang, Yu and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingxin Huang, aHVhbmd5eEBpZ2EuYWMuY24=
 Changxing Fu
Changxing Fu Jiayan Fan1,2
Jiayan Fan1,2 Heqi Wang
Heqi Wang Congwen Wang
Congwen Wang Dafu Yu
Dafu Yu Yingxin Huang
Yingxin Huang