- Department of Plant Science and Landscape Architecture, University of Maryland, College Park, MD, United States
Fruit drop, cracking, and advanced ripening prior to fruit harvest can promote significant losses in important apple cultivars such as ‘Ambrosia’ and ‘Fuji’ grown in the mid-Atlantic. These losses result from environmental factors, delays in harvest due to the lack of red skin color development, and cultivar-specific characteristics, among others. Aminoethoxyvinylglycine (AVG) and 1-methylcyclopropene (1-MCP) are ethylene-inhibiting plant growth regulators (PGRs) that can alter preharvest fruit drop, cracking, maturity, and quality by impeding ethylene biosynthesis and perception, respectively. However, there is a knowledge gap on understanding the impacts of specific timings and dosages of such PGR treatments on ‘Ambrosia’ and ‘Fuji’ under mid-Atlantic environmental conditions. The objective of this work was to characterize and compare the impacts of specific rates of preharvest ethylene-inhibiting PGRs on fruit drop and cracking, ethylene production, physicochemical properties, skin color, and associated gene expression in ‘Ambrosia’ and ‘Fuji’ apples during on-the-tree ripening. Multivariate statistical methods for identifying meaningful correlations among the measured variables were applied. Our results show that both full-rate AVG (130 mg a.i. L−1) and 1-MCP (150 mg a.i. L−1) significantly reduced preharvest fruit cracking compared with control fruits in ‘Ambrosia’, but not in ‘Fuji’. Furthermore, among all treatments, full-rate AVG (130 mg a.i. L−1) treatment displayed the lowest ethylene production and gene expression of ethylene- related genes. 1-MCP (150 mg a.i. L−1) and half-rate AVG (65 mg a.i. L−1) treatments presented a reduction in the ethylene production and gene expression of ethylene-related genes when compared to control fruits. Regarding color, apple skin blush and anthocyanin-related gene expression were the lowest in full-rate AVG (130 mg a.i. L−1)-treated fruits, explaining why these fruits met the 50% red blush coverage 1 week later than all other treatments in both cultivars. Correlations amongst the assessed features were also identified. These results suggest a cultivar-specific effectiveness under mid-Atlantic conditions and specifies a framework for the use of ethylene-inhibiting PGRs under mid-Atlantic environmental conditions.
1 Introduction
Preharvest fruit drop and cracking due to abscission from the tree and fruit fissures prior to the optimal maturity increases yield losses (Arseneault and Cline, 2016; Li et al., 2021). The strategy of harvesting apple fruit before the optimal maturity to reduce fruit drop and cracking negatively impacts red skin color development while reducing fruit quality and marketability (Kvikliene et al., 2011; Anbesse Girma et al., 2022). Therefore, finding new horticultural practices that allow harvest at optimal maturity while enhancing red skin coloration, but without promoting fruit drop or cracking, is a critical challenge for commercially important cultivars such as ‘Ambrosia’ and ‘Fuji’ grown under mid-Atlantic conditions.
‘Ambrosia’ is an emerging mid-season cultivar in the USA, primarily restricted to Canada until 2017 (Warner, 2025). This cultivar presents a challenge to the industry, as it possesses a narrow harvest period that is hard to navigate when managing cultivars with overlapping harvest windows and seems to present susceptibility to fruit drop, yet predisposition to cracking is generally unknown (Mennell, 2002; Crassweller et al., 2007; Toivonen, 2015). ‘Fuji’ is a late-season cultivar, favored by growers and consumers and maintaining popularity in the top five U.S. cultivars (Peng and Fu, 2023). Previous work has reported ‘Fuji’ to display a high sensitivity to preharvest fruit cracking, contributing to yield losses, although susceptibility to fruit drop has been inconsistent under different environmental conditions where the fruits are grown (Kasai et al., 2008; Li et al., 2010). To our knowledge, understanding of the predisposition of ‘Ambrosia’ and ‘Fuji’ cultivars to fruit drop and cracking under mid-Atlantic conditions is limited.
Preharvest fruit drop, cracking, and fruit quality are known to be highly regulated by ethylene, a key plant hormone responsible for fruit ripening along with a host of other physiological processes (Bleecker and Schaller, 1996; Santos et al., 2023). Apples are climacteric fruits and thus exhibit a surge in ethylene production concurrent with an increase in respiration throughout ripening (Giovannoni, 2004; Chen et al., 2018). As a positive regulator of abscission, ethylene induces intracellular tissue and cell wall degradation in the abscission zone of the fruit pedicel and therefore increases preharvest fruit drop as the ripening proceeds throughout the season (Estornell et al., 2013; Arseneault and Cline, 2016; Miah and Farcuh, 2024a). Further, ethylene seems to be implicated in the regulation of fruit cracking through indirect mechanisms, which increases with advanced fruit maturity and ripening and is additionally associated with cell wall regulation-related genes that are ethylene-responsive (Khadivi-Khub, 2015; La Spada et al., 2024). The ethylene pathway has been widely studied, and its components are well documented (Klee, 2004; Binder, 2020; Liu et al., 2020; Cocetta and Natalini, 2022; Kader, 2022). The ethylene pathway is composed of ethylene biosynthesis, involving the enzymatic catalysis of S-adenosyl-l-methionine (SAM) to 1-aminocyclopropane-1-carboxylate (ACC) by the enzyme ACS and to ethylene by the enzyme 1-aminocyclopropane-1-carboxylate oxidase (ACO) (Adams and Yang, 1979; Bleecker and Schaller, 1996; Farcuh et al., 2018; Wang and Faust, 2019); ethylene perception, comprising copper-binding membrane-associated receptors [ethylene response sensor (ERS) and ethylene receptor type (ETR)] and a negative regulator of ethylene response [constitutive triple response 1 (MdCTR1), a Ref-like serine/threonine-protein kinase] (Lee et al., 1998; Dal Cin et al., 2005, 2006; Wiersma et al., 2007; Yuan and Li, 2008; Busatto et al., 2017; Dolgikh et al., 2019; Bleecker, 1999); and lastly, ethylene signaling, activated by several transcription factors, including the ethylene response factors (ERFs) (Li et al., 2016).
Ethylene-inhibiting plant growth regulators (PGRs) that alter the ethylene pathway are commonly utilized to control preharvest fruit drop and cracking in apples. Two such PGRs are aminoethoxyvinylglycine (AVG), which impedes ACS activity in ethylene biosynthesis (Brasil and Siddiqui, 2018; Liu et al., 2022; Miah and Farcuh, 2024c), and 1-methylcyclopropene (1-MCP), which binds to and saturates receptor sites involved in ethylene perception (Doerflinger et al., 2019). The rate and timing of AVG and 1-MCP have been reported to impact their effectiveness on apple fruit drop and cracking, while location, yearly variation, and the cultivar type also contribute to the efficacy of these ethylene-inhibiting PGRs (Argenta et al., 2018a). Although previous research has shown that AVG and 1-MCP both exhibit a reduction in preharvest fruit drop in ‘Delicious’ (Yuan and Li, 2008; Malladi et al., 2023), ‘Honeycrisp’ (Arseneault and Cline, 2017; Johnson and Farcuh, 2024; Miah and Farcuh, 2024a), ‘Gala’ (Layne et al., 2002; Argenta et al., 2018a; Liu et al., 2022), ‘McIntosh’ (Schupp and Greene, 2004; Greene, 2005), and ‘Golden Supreme’ (Yuan and Carbaugh, 2007) apples, while additionally demonstrating reduced cracking in ‘Gala’ (Lee et al., 2016; Liu et al., 2022), different dosages and timings of application were used in each study, depending on cultivar as well as growing environment, hence indicating that generalizations on recommendations cannot be made and that specific studies are necessary for different cultivars grown under different environmental conditions to define the most efficient application dosages and timing. To date, we are not aware of any previous work that has investigated the impacts of specific dosages and timings of these ethylene-inhibiting PGRs on ‘Ambrosia’ and ‘Fuji’ grown under mid-Atlantic conditions. This work is of critical need in the region, directly boosting fruit quality, production, and profitability.
Ethylene plays a pivotal role in modulating fruit ripening and influencing key quality attributes, including texture, flavor, and color, which are crucial for consumer preference (Giovannoni, 2004; Kumar et al., 2014; Miah et al., 2023). Fruits treated with ethylene-inhibiting PGRs, particularly AVG and 1-MCP, have been shown to maintain apple flesh firmness and decrease starch hydrolysis to sugars in ‘Gala’ (Argenta et al., 2018b), ‘Cripps Pink’ (do Amarante et al., 2022), ‘McIntosh’ (Doerflinger et al., 2019), and ‘Red Delicious’ apples (Boyacı, 2022). However, changes in fruit titratable acidity and soluble solids content have exhibited incongruities, with no significant changes after ethylene-inhibiting PGR treatments (McArtney et al., 2008; do Amarante et al., 2022) in some cultivars and higher acidity and lower soluble solids in others (Liu et al., 2022; Malladi et al., 2023; Doerflinger et al., 2024; Miah and Farcuh, 2024a). Moreover, different ethylene-inhibiting PGR treatments have also shown discrepancies in the development of apple red skin coloration, proving problematic for the stringent requirements for U.S. standards of marketability (USDA, 2019). Preharvest AVG-treated fruits have previously exhibited delays to red coloration in ‘Gala’ (Liu et al., 2022) and ‘Cripps Pink’ (Whale et al., 2008), yet did not display significant changes to coloring in ‘Delicious’ (Yuan and Li, 2008), while preharvest 1-MCP-treated apples showed red skin color development delays in ‘Anna’ (Farag et al., 2015) and ‘Delicious’ (Yuan and Li, 2008) and no differences in red skin coloration in ‘Scarletspur Delicious’ (Elfving et al., 2007). These inconsistencies have been shown to result from the alteration in the biosynthesis of anthocyanins, the primary pigment responsible for apple red skin coloration, which can directly impact apple fruit marketability (Liu et al., 2021; Fanyuk et al., 2022). Anthocyanin biosynthesis occurs via the phenylpropanoid metabolic pathway, beginning with the precursor phenylalanine, derived from the shikimate pathway and enzymatically converted to anthocyanin in the flavonoid pathway (Whale et al., 2008; Fanyuk et al., 2022). The enzymes included in the conversion are phenylalanine ammonia-lyase (PAL), chalcone synthase (CHS), chalcone isomerase (CHI), flavanone 3-hydroxylase (F3H), dihydroflavonol 4-reductase (DFR), leucoanthocyanidin dioxygenase (LDOX), and UDP-glucose-flavonoid 3-O-glucosyltransferase (UFGT), as well as MdMYB10, a regulatory transcription factor exhibiting a rise in parallel with anthocyanin content (Whale et al., 2008; Feng et al., 2013; Ma et al., 2021; Li et al., 2022; Shi et al., 2022; Sunil and Shetty, 2022; Wang et al., 2022). Although ethylene has been shown to play a major role in upregulating anthocyanin biosynthesis-related genes (Elfving et al., 2007; Onik et al., 2018; Zhang et al., 2018; Farcuh et al., 2022), notably, as described above, different ethylene-inhibiting PGR treatments have displayed inconsistent results in red skin coloration when applied to different apple cultivars. This may indicate that other factors, in addition to ethylene, such as cultivar, environmental conditions, specific ethylene PGR applied, and timings and dosages of each PGR, may also play key roles, which needs further investigation. Particularly for ‘Ambrosia’ and ‘Fuji’ apples grown in the mid-Atlantic, information is lacking on the impact of different ethylene-inhibiting PGRs on fruit red skin coloration.
Based on the available literature, studies examining the influence of ethylene-inhibiting PGR treatments on preharvest fruit drop, cracking, fruit maturity, and quality characteristics, as well as associated ethylene and anthocyanin transcript accumulation, are lacking, particularly in important cultivars for the mid-Atlantic region, such as ‘Ambrosia’ and ‘Fuji’. Accordingly, the objective of this study was to depict and contrast the effects of specific rates of preharvest ethylene-inhibiting PGRs (AVG and 1-MCP) on fruit drop and cracking, ethylene production, physicochemical characteristics, skin color, and expression of genes involved in ethylene biosynthesis and its perception, as well as genes responsible for anthocyanin biosynthesis in ‘Ambrosia’ and ‘Fuji’ apples during preharvest ripening. Multivariate statistical methods were applied to identify meaningful correlations among the measured variables.
2 Materials and methods
2.1 Plant materials and treatments
2.1.1 Experiment 1: ‘Ambrosia’
A field trial was conducted over two consecutive years using a 6-year-old ‘Ambrosia’ apple orchard (Malus domestica ‘Ambrosia’/M9) located in Aspers, PA. The trees were trained to a modified central leader system on a trellis and planted at a spacing of 1 × 4 m. A randomized complete block design was implemented, consisting of four treatments, each replicated four times with 40 trees per replicate. Four treatments were applied: AVG at 130 and 65 mg a.i. L−1 (ReTain, Valent BioSciences Corporation, Libertyville, IL, USA), 1-MCP at 150 mg a.i. L−1 MCP (Harvista 1.3 SC, AgroFresh, Philadelphia, PA, USA), and an untreated control. The full-rate (130 mg a.i. L−1) of AVG was applied 4 weeks prior to the expected commercial harvest date, and the half-rate (65 mg a.i. L−1) was applied 1 week prior to the expected commercial harvest date. Rates and application timings were based on manufacturer recommendations. ‘Ambrosia’ is suggested to be a sensitive cultivar that, in addition to the full-rate, may respond to lower application rates closer to the anticipated harvest (Valent BioSciences, 2022). 1-MCP was applied when the starch pattern index reached 3, based on manufacturer recommendations, using an AgroFresh formulation tank, injection pump, and calibration tube attached to a sprayer. AVG and 1-MCP treatments were combined with 1.0 mL L−1 Silwet-77 organosilicone surfactant and applied with a pressurized orchard sprayer.
Maturity indices were assessed throughout the season [i.e., surface and background color, skin blush, flesh firmness, starch pattern index (SPI), soluble solids content (SSC), and titratable acidity (TA)] to determine the anticipated commercial harvest date following prior methodology (Miah and Farcuh, 2024c) and using control fruits as the reference. Three evaluation timepoints during on-the-tree ripening were selected: 1 week before commercial harvest (1WBCH), commercial harvest (CH), and 1 week after commercial harvest (CH + 1W). At each timepoint, eight fruits per replicate were harvested for each treatment and promptly brought to the laboratory. Per replication, four fruits were used for measuring ethylene production rate and rinsed, the skin was removed (skin tissue), and the remaining flesh was cut (flesh tissue). The remaining fruits were evaluated for physicochemical parameters.
2.1.2 Experiment 2: Fuji
A field trial was conducted over two consecutive years using a 15-year-old ‘Fuji’ apple orchard (M. domestica ‘Fuji’/M9) located in Aspers, PA. The trees were trained to a tall spindle and planted at a spacing of 1 × 4 m. A randomized complete block design was implemented, consisting of three treatments, each replicated four times with 40 trees per replicate. The three treatments included full-rate AVG at 130 mg a.i. L−1, 1-MCP at 150 mg a.i. L−1 MCP, and an untreated control, applied as described above for Experiment 1.
Fruits were harvested at three different timepoints during the tree ripening—1WBCH, CH, and CH + 1W—following what was described above for Experiment 1.
2.2 Determination of preharvest fruit drop and cracking
For both experiments, independently, preharvest fruit drop was assessed as previously described (Johnson and Farcuh, 2024; Miah and Farcuh, 2024b) for three treatments: full-rate AVG at 130 mg a.i. L−1, 1-MCP at 150 mg a.i. L−1 MCP, and the control. For each replication, preharvest fruit drop was calculated from five pre-selected limbs labeled 2 weeks before CH, containing 20 fruits each on alternate sides of different trees throughout the block. Fruit drop was assessed on each harvest timepoint, i.e., starting at 1WBCH and ending at CH + 1W. The number of fruits on each limb was counted at each evaluation timepoint, and fruit drop was calculated as a percentage of the original fruit count on that limb. The percentage of cracked fruits was additionally determined as a count of cracked fruits per limb at each timepoint (Byers, 1997).
2.3 Ethylene production assessment
For Experiment 1 (‘Ambrosia’), ethylene production rate (μL C2H4 kg−1 h−1) was measured via a static system as previously described (Tong et al., 2003; Kim et al., 2015a; Farcuh and Hopfer, 2023). ‘Ambrosia’ fruits were incubated in a hermetic 1-L jar equipped with rubber stoppers for 1 hour at 20°C. One milliliter of headspace gas was drawn from each jar with a syringe. For Experiment 2 (‘Fuji’), internal ethylene concentration (IEC) was determined from 1-mL samples of internal gas pulled from the core cavity, as portrayed in previous research (Miah et al., 2023; Miah and Farcuh, 2024a). The obtained gas samples were injected into a gas chromatograph (GC-2014C, Shimadzu Co., Kyoto, Japan) equipped with an activated alumina column and a Flame ionization detector (FID) as previously described (Johnson and Farcuh, 2024).
2.4 Fruit skin color and physicochemical analyses
Evaluation of blush percentage, skin color, index of absorbance difference (IAD), flesh firmness, SPI, SSC, and TA was conducted as reported earlier (Miah et al., 2023; Miah and Farcuh, 2024c). Fruit blush percentage was visually estimated to the nearest 5% on each apple. Surface and background color were evaluated using a colorimeter (Konica Minolta CR400 Chroma Meter, Konica Minolta Sensing, Inc., Osaka, Japan), and hue angle (hue °) was estimated as previously explained (Infante et al., 2008). The IAD was measured utilizing a Delta Absorbance (DA) Meter (TR Turoni, Forli, Italy) with measurements taken from three distinct fruit surface points and averaged (Ziosi et al., 2008). Flesh firmness was assessed on the two opposing fruit sides, with approximately 2-mm thickness peeled from each side using a TA.XT Plus Connect texture analyzer (Texture Technologies Corp., Scarsdale, NY, USA) equipped with an 11.1-mm-diameter probe, a 50-kg load cell, and the Exponent TE32 software (v6.0, Texture Technologies Corp., Scarsdale, NY, USA) operated at 8 mm with a speed of 8 mm s−1. For SPI measurements, each fruit was halved transversally and rated between 1 (100% stained starch) and 8 (0% stained starch) using the Cornell generic chart (Blanpied and Silsby, 1992). SSC and malic acid were measured using a hand-held digital refractometer (Atago, Tokyo, Japan) and an automatic titrator (855 Robotic Titrosampler; Metrohm, Riverview, FL, USA), respectively (Farcuh et al., 2020, 2018; Miah and Farcuh, 2024b).
2.5 Total RNA extraction and real-time quantitative polymerase chain reaction
The isolation of RNA took place from skin and flesh tissue from each replication at each evaluation timepoint using a modified cetyltrimethylammonium bromide (CTAB)/NaCl method (Chang et al., 1993) as explained earlier (Kim et al., 2015b; Farcuh et al., 2018; Miah and Farcuh, 2024a). The synthesis of first-strand complementary DNA, primer design, and quantitative real-time polymerase chain reaction occurred as reported earlier (Kim et al., 2015b), with primer sequences shown in Supplementary Table 1. Relative gene expression examination was performed employing the Comparative Cycle Threshold Method (Livak and Schmittgen, 2001) with the reference gene actin (MdACT).
2.6 Statistical analyses
A factorial experiment under a randomized complete block design was analyzed in RStudio using linear mixed models with treatment and evaluation timepoints as fixed factors and replications as the random factor (R ver 2024.04.2 + 764). Normality was confirmed from the residuals, and Tukey’s honestly significant difference (HSD) at 5% significance level was used to compare the treatments when the model was statistically significant.
Pearson’s correlation coefficients were calculated for each pairwise combination of factors using mean-centered data in the software package JMP (ver 15.2, SAS Institute, Cary, NC, USA). A “biplot” graph was used to visualize the principal component analysis (PCA) for associations among the assessed treatments and evaluation timepoints, as well as analyzed features (fruit drop, fruit cracking, ethylene production, skin color, physicochemical parameters, and gene expression values), with the number of principal components determined using the scree test.
3 Results
3.1 Experiment 1: effects of AVG and 1-MCP on ‘Ambrosia’ fruit drop, cracking, ethylene production, physicochemical parameters, skin color, and gene expression during on-the-tree ripening
3.1.1 Fruit drop and cracking in ‘Ambrosia’
For preharvest fruit drop and cracking in ‘Ambrosia’ fruits, only three out of the four treatments were evaluated throughout on-the-tree ripening at the three harvest timepoints (full-rate AVG, 1-MCP, and control) (Table 1). In the case of fruit drop, a trend of increased fruit drop was observed for most treatments throughout on-the-tree ripening over both years. Although differences were not statistically significant between treatments in preharvest fruit drop, at CH + 1W, AVG treatments exhibited a 50% reduction in fruit drop compared to the control in 2023, while in 2024, both AVG and 1-MCP treatments displayed 1.5-fold lower fruit drop percentages than control fruits.
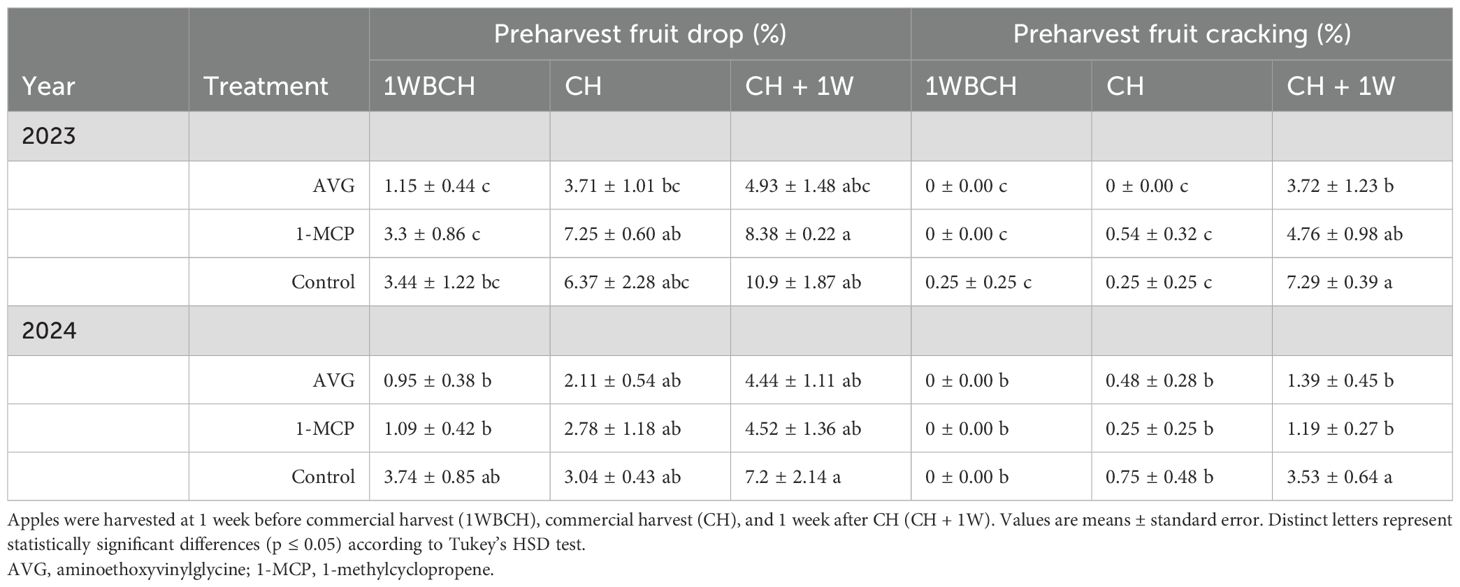
Table 1. Effect of AVG and 1-MCP on ‘Ambrosia’ preharvest fruit drop and cracking evaluated during on-the-tree ripening in Aspers, PA, during 2023 and 2024 production seasons.
Regarding fruit cracking, values increased remarkably across ripening timepoints from 1WBCH to CH + 1W for all treatments in 2023 and control fruits in 2024. In 2023, AVG-treated fruits exhibited a significant twofold reduction in fruit cracking compared to the control, while in 2024, both AVG- and 1-MCP-treated fruits displayed a significant 2.5-fold decrease in values compared to the control.
3.1.2 Ethylene production and physicochemical parameters in ‘Ambrosia’
Ethylene production rate displayed a significant increase from 1WBCH to CH + 1W for full-rate and half-rate AVG during the tree ripening in 2023 and for every treatment in 2024, presenting the highest values at CH + 1W for each treatment when compared across the assessed timepoints (Figures 1A, B). During both assayed years, control fruits showed the statistically highest ethylene production rates as compared to all other treatments during most timepoints. In 2023, half-rate AVG-treated fruits showed the least ethylene production rates at all assessed timepoints, followed by full-rate AVG-treated fruits, then 1-MCP-treated fruits, and lastly by control fruits with the highest values (Figure 1A). In 2024, full-rate AVG-treated fruits displayed the least ethylene production rate at 1WBCH as well as CH + 1W, and at the latter, it was followed by 1-MCP-treated fruits, then by half-rate AVG-treated fruits, and finally by control fruits, exhibiting the highest values (Figure 1B).
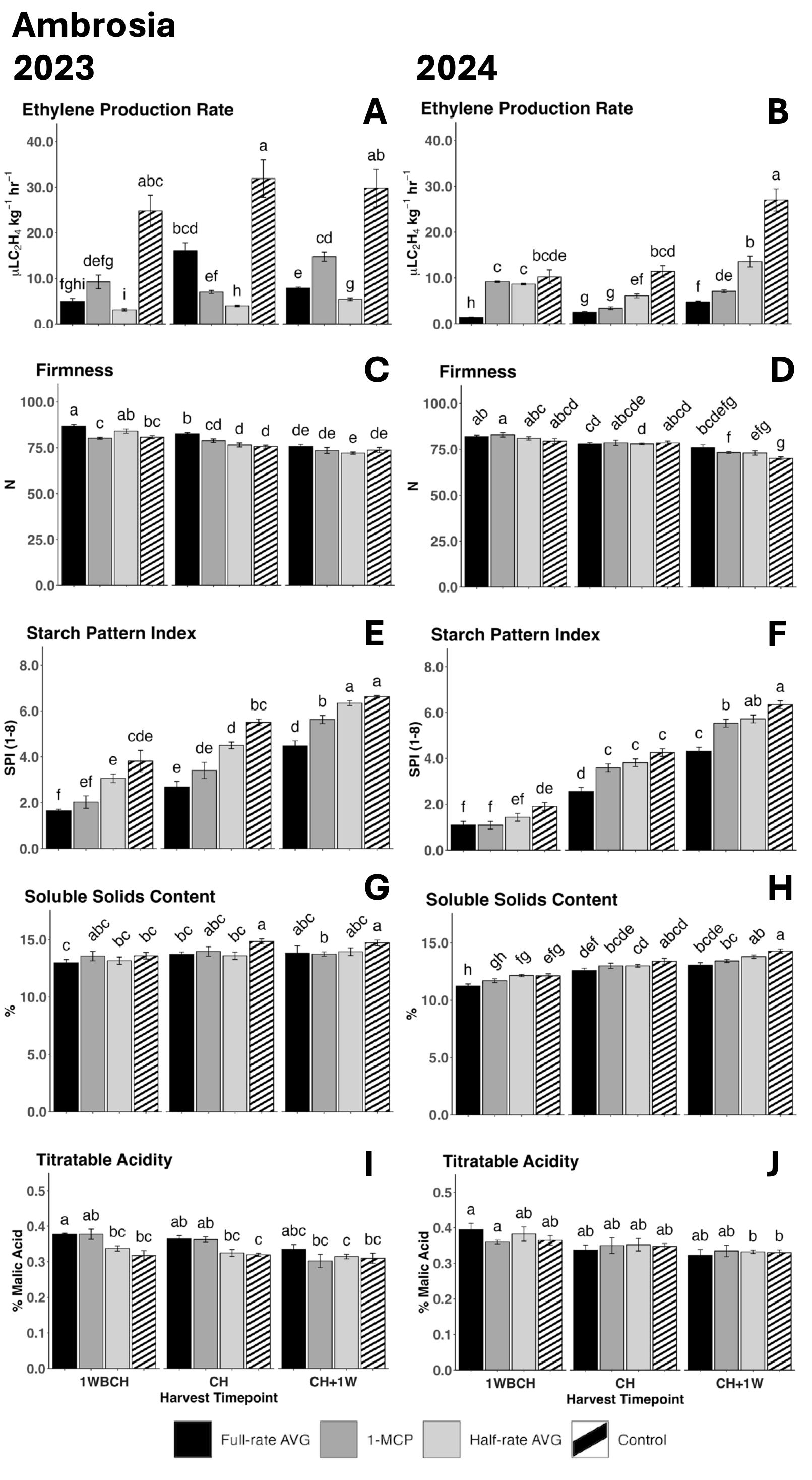
Figure 1. Effect of full-rate AVG, half-rate AVG, and 1-MCP on ‘Ambrosia’ ethylene production rate and physicochemical properties during on-the-tree ripening in Aspers, PA, in (A, C, F, H, J) 2023 and (B, E, G, I, K) 2024. (A, B) Ethylene production rate, (C, D) flesh firmness, (E, F) starch content (starch pattern index), (G, H) soluble solids content, and (I, J) titratable acidity (malic acid). Apple evaluations were conducted at 1 week before commercial harvest (1WBCH), commercial harvest (CH), and 1 week after commercial harvest (CH + 1W). Values are means ± standard error. Distinct letters represent statistically significant differences (p ≤ 0.05) according to Tukey’s HSD test. AVG, aminoethoxyvinylglycine; 1-MCP, 1-methylcyclopropene.
Flesh firmness was significantly reduced from 1WBCH to CH + 1W for all treatments throughout the three assayed timepoints, consistently in both years (Figures 1C, D). In 2023, full-rate AVG-treated fruits exhibited significantly higher firmness values than 1-MCP-treated and control fruits at 1WBCH than all other treatments at CH (Figure 1C). In 2024, 1-MCP-treated fruits exhibited statistically higher firmness values than control fruits at CH + 1W (Figure 1D).
Starch pattern index values steadily increased in both years for all treatments from 1WBCH to CH + 1W, indicative of increased starch disappearance throughout ripening (Figures 1E, F). In 2023, SPI values were the lowest for full-rate AVG-treated fruits at 1WBCH and CH, followed by 1-MCP-treated fruits, half-rate AVG-treated fruits, and lastly the control fruits, with the highest exhibited values (Figure 1E). In 2024, full-rate AVG- and 1-MCP-treated fruits presented SPI values that were significantly lower than those of control fruits at 1WBCH, while full-rate AVG-treated fruits displayed the statistically lowest SPI values at CH (Figure 1F). Finally, at CH + 1W, full-rate AVG-treated fruits presented the most reduced SPI values in both years among all treatments; in 2024, 1-MCP- and half-rate AVG-treated fruits exhibited intermediate SPI values compared with full-rate AVG-treated and control fruits (Figures 1E, F).
SSC significantly increased from 1WBCH to CH+ 1W throughout the three assessed on-the-tree ripening timepoints in all treatments during 2024 (Figure 1H), yet not in 2023 (Figure 1G). In this same year, full-rate AVG-treated fruits displayed lower SSC values than half-rate AVG-treated and control fruits at 1WBCH, while at CH + 1W, both full-rate AVG- and 1-MCP-treated fruits exhibited statistically lower SSC values than control fruits.
TA values displayed a decreasing trend as on-the-tree ripening progressed from 1WBCH to CH + 1W for each treatment in 2023 and 2024 (Figures 1I, J). In 2023, full-rate AVG- and 1-MCP-treated fruits displayed statistically significantly higher TA values than control fruits at CH (Figure 1I).
3.1.3 Skin coloration in ‘Ambrosia’
A significant decrease in surface skin hue values from 1WBCH to CH + 1W was observed in both years, revealing an increased red skin coloration (Figures 2A, B). In 2023, at 1WBCH, full-rate AVG- and 1-MCP-treated fruits showed the highest surface skin values, followed by half-rate AVG-treated fruits, and finally by control fruits, while at CH, full-rate AVG-treated fruits significantly differentiated with the highest values from all other treatments (Figure 2A). Control fruits exhibited the statistically lowest values at CH + 1W (Figure 2A). In 2024, full-rate AVG-treated fruits displayed the highest surface skin hue values at 1WBCH and CH (Figure 2B). Correspondingly, skin blush percentage increased significantly, for all treatments, as fruits ripened on the tree (Figures 2C, D). Skin blush percentage was statistically the lowest for full-rate AVG- and 1-MCP-treated fruits, followed by half-rate AVG-treated fruits, and finally by control fruits, which exhibited the highest blush values at 1WBCH in 2023 (Figure 2C). At CH in 2023 and 1WBCH and CH in 2024, full-rate AVG treatment displayed the least blush values compared with all other treatments. Notably, 1-MCP-treated, half-rate AVG-treated, and control fruits reached a blush percentage >50% at CH, while full-rate AVG-treated fruits achieved this 1 week later at CH + 1W in both years (Figures 2C, D).
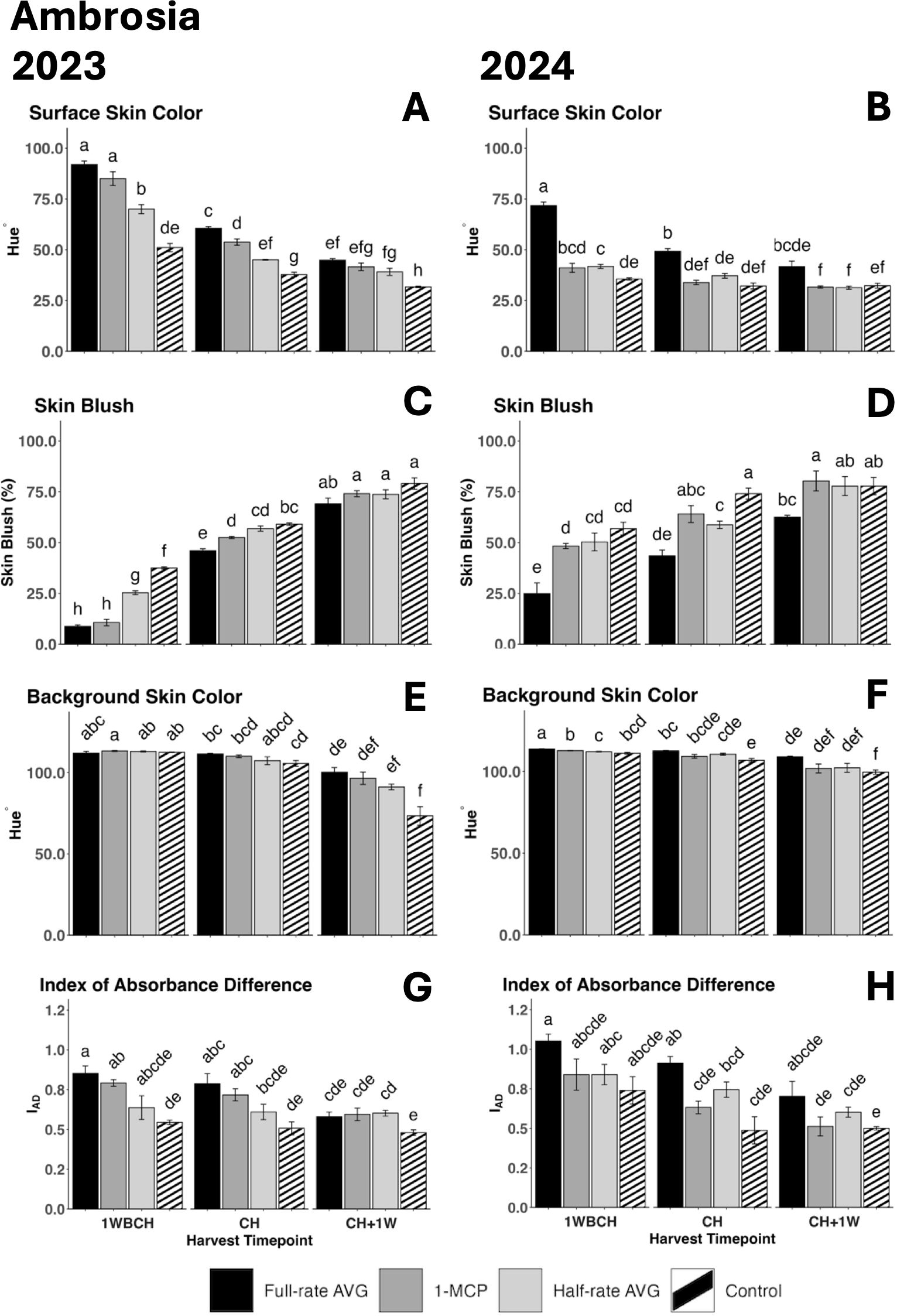
Figure 2. Effect of full-rate AVG, half-rate AVG, and 1-MCP on ‘Ambrosia’ fruit color during on-the-tree ripening in Aspers, PA, in (A, C, E, G) 2023 and (B, D, F, H) 2024. (A, B) Surface skin color, (C, D) skin blush, (E, F) background skin color, and (G, H) index of absorbance difference. Apple evaluations were conducted at 1 week before commercial harvest (1WBCH), commercial harvest (CH), and 1 week after commercial harvest (CH + 1W). Values are means ± standard error. Distinct letters represent statistically significant differences (p ≤ 0.05) according to Tukey’s HSD test. AVG, aminoethoxyvinylglycine; 1-MCP, 1-methylcyclopropene.
A significant reduction in background skin hue values was observed in both years for every treatment from 1WBCH to CH + 1W, revealing a green to yellow color conversion (Figures 2E, F). Furthermore, at CH + 1W, control fruits displayed the lowest background skin hue angle values, statistically differing from full-rate AVG-treated fruits in both years (Figures 2E, F). Similarly, IAD values were significantly lower in control fruits than in full-rate AVG-treated fruits at 1WBCH and CH in 2023 and at CH in 2024, while the rest of the treatments displayed intermediate values (Figures 2G, H).
3.1.4 Expression of ethylene biosynthesis and perception genes in ‘Ambrosia’
Ethylene biosynthesis genes, MdACS1 and MdACO1, displayed a significant upregulation during on-the-tree ripening from 1WBCH to CH + 1W for all evaluated treatments over both years (Figures 3A–D). In 2023, both genes displayed the highest transcript accumulation for control fruits at all stages, followed by 1-MCP-treated fruits, and lastly by half-rate- and full-rate AVG-treated fruits, which exhibited the lowest values (Figures 3A, C). The exception to this was at 1WBCH and CH, where full-rate AVG- and half-rate AVG-treated fruits did not differ. The control maintained the highest gene expression levels for both MdACS1 and MdACO1, significantly greater than those of full-rate AVG- and 1-MCP-treated fruits at CH and CH + 1W in 2023 and 2024. 1-MCP-treated fruits followed, displaying intermediate gene expression between both rates of AVG-treated fruits and the control at CH and CH + 1W in 2023. In contrast, in 2024, the expression levels of MdACS1 in half-rate AVG-treated fruits were greater than those in 1-MCP- and full-rate AVG-treated fruits at CH + 1W (Figure 3B).
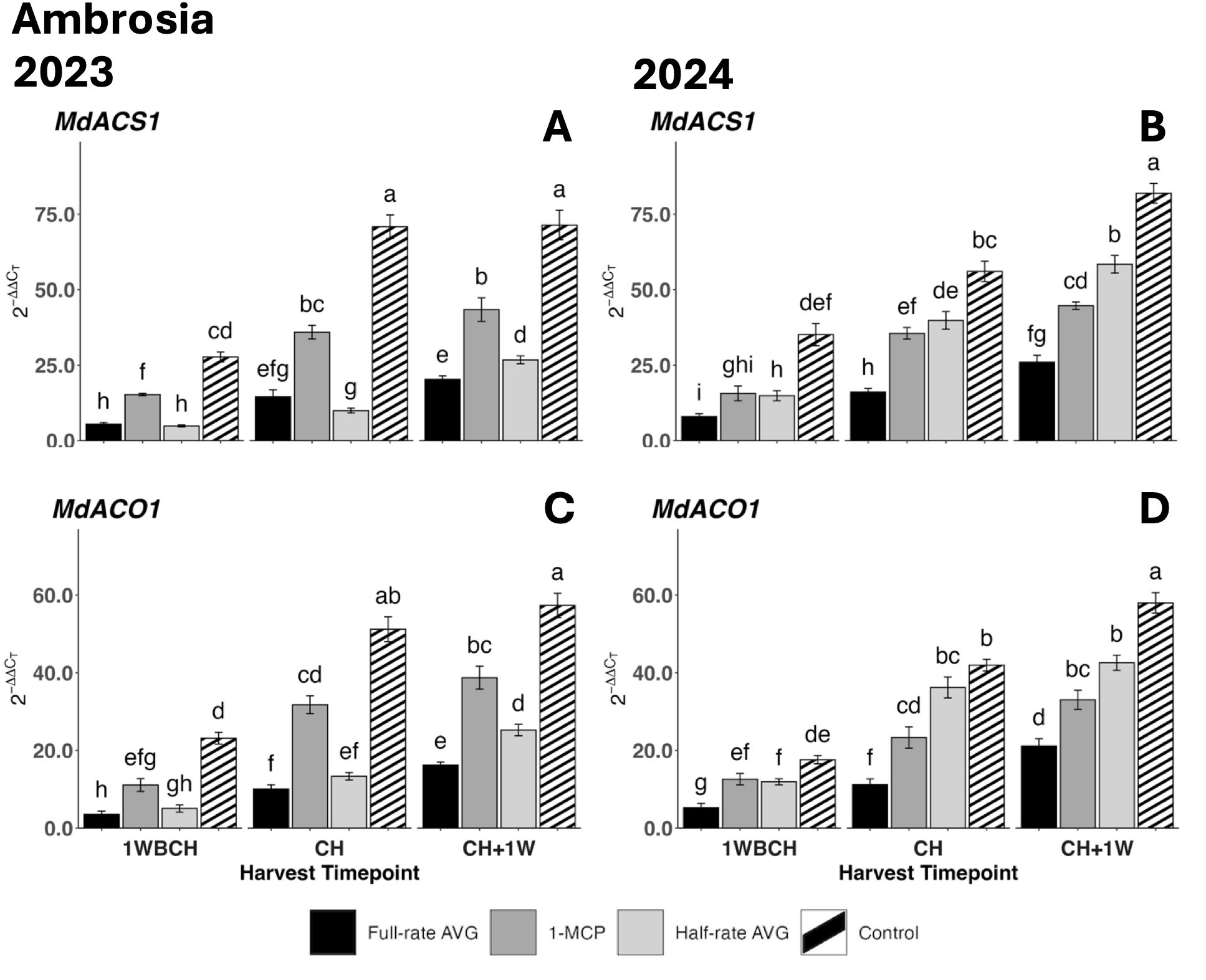
Figure 3. Effect of full-rate AVG, half-rate AVG, and 1-MCP on relative expression of ethylene biosynthesis genes in ‘Ambrosia’ during on-the-tree ripening in Aspers, PA, in (A, C) 2023 and (B, D) 2024. (A, B) MdACS1 and (C, D) MdACO1. Apple evaluations were conducted at 1 week before commercial harvest (1WBCH), commercial harvest (CH), and 1 week after commercial harvest (CH + 1W). Values are means ± standard error. Distinct letters represent statistically significant differences (p ≤ 0.05) according to Tukey’s HSD test. ACS, 1-aminocyclopropane-carboxylase synthase; ACO, 1-aminocyclopropane-1-carboxylate oxidase; AVG, aminoethoxyvinylglycine; 1-MCP, 1-methylcyclopropene.
Likewise, the expression levels of ethylene perception-related genes rose significantly throughout ripening from 1WBCH to CH + 1W for each treatment (Figure 4). The exception to this was MdETR1, MdETR2, and MdETR5, which displayed a significant increase in most but not all treatments from 1WBCH to CH + 1W in 2023 (Figures 4G, I). In both years, control fruits displayed significantly higher transcript accumulation than all regulator treatments at CH and CH + 1W for MdERS1, MdETR1, and MdETR5, as well as for MdCTR1 at CH (Figures 4A, B, E, F, I–L). For these same genes at CH + 1W in 2024, the highest expression was observed in half-rate AVG-treated fruits, then 1-MCP-treated fruits, and lastly full-rate AVG-treated fruits. Contrarily, 1-MCP fruits showed significantly higher expression than both AVG treatments at CH + 1W for MdETR1 and MdETR2 in 2023 (Figures 4E, G). MdERS2 did not exhibit significant differences among treatments across the harvest timepoints in either year (Figures 4C, D).
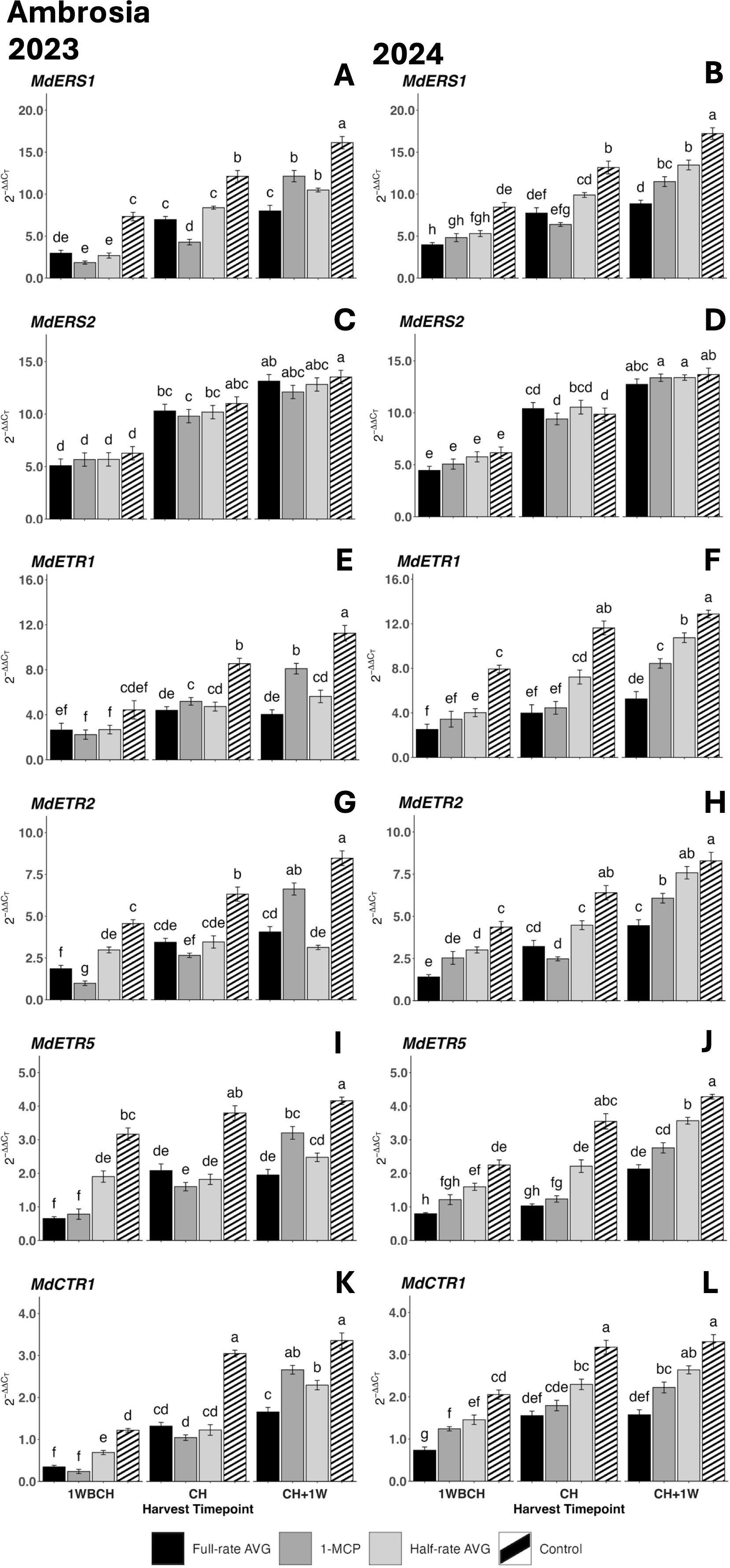
Figure 4. Effect of full-rate AVG, half-rate AVG, and 1-MCP on the relative expression of ethylene perception genes in ‘Ambrosia’ apples during on-the-tree ripening in Aspers, PA, in (A, C, E, G, I, K) 2023 and (B, D, F, H, J, L) 2024. (A, B) MdERS1, (C, D) MdERS2, (E, F) MdETR1, (G, H) MdETR2, (I, J) MdETR5, and (K, L) MdCTR1. Apple evaluations were conducted at 1 week before commercial harvest (1WBCH), commercial harvest (CH), and 1 week after commercial harvest (CH + 1W). Values are means ± standard error. Distinct letters represent statistically significant differences (p ≤ 0.05) according to Tukey’s HSD test. ERS, ethylene response sensor; ETR, ethylene receptor type; CTR, constitutive triple response; AVG, aminoethoxyvinylglycine; 1-MCP, 1-methylcyclopropene.
3.1.5 Expression of anthocyanin biosynthesis genes in ‘Ambrosia’
Transcript accumulation for all anthocyanin biosynthesis genes increased significantly from 1WBCH to CH + 1W in both years (Figure 5). The exception to this was control fruits, which had no significant rise in the expression of MdPAL in 2023 or MdCHS in 2024, as well as full-rate AVG-treated fruits, which had no significant rise in expression of MdUFGT in 2023 (Figures 5A, D, M). In both years, AVG-treated fruits displayed the lowest gene expression levels, followed by 1-MCP- and half-rate AVG-treated fruits, and finally by control fruits. The latter presented statistically higher transcript accumulation at CH across all treatments for each gene as compared to full-rate AVG-treated fruits. 1-MCP- and half-rate AVG-treated fruits did not differ at any timepoint for most genes, and 1-MCP-treated, half-rate AVG-treated, and control fruits did not differ at CH + 1W for MdPAL, MdCHI, MdF3H, and MdLDOX in both years (Figures 5A, B, E–H, K, L). No difference in expression across all treatments was exhibited at CH + 1W for MdDFR1 in either year (Figures 5I, J).
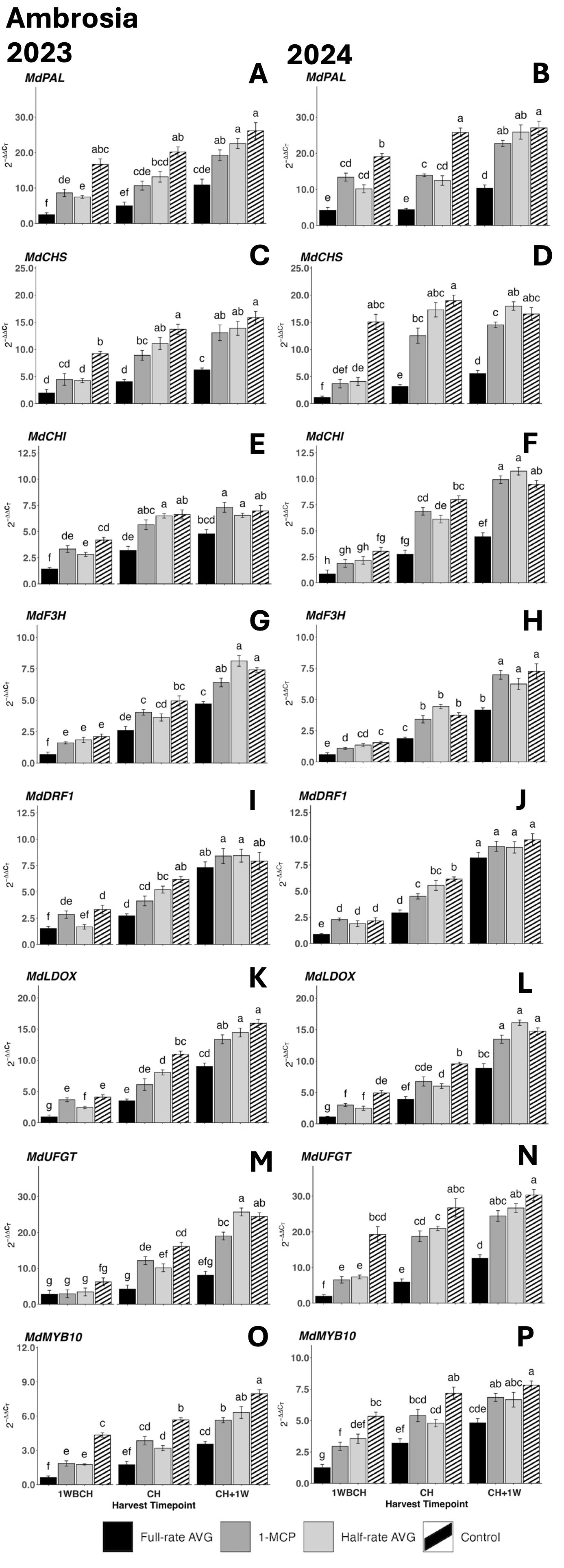
Figure 5. Effect of full-rate AVG, half-rate AVG, and 1-MCP on relative gene expression of anthocyanin biosynthesis-related genes of ‘Ambrosia’ during on-the-tree ripening in Aspers, PA, in (A, C, E, G, I, K, M, O) 2023 and (B, D, F, H, J, L, N,P) 2024. (A, B) MdPAL, (C, D) MdCHS, (E, F) MdCHI, (G, H) MdF3H, (I, J) MdDFR1, (K, L) MdLOX, (M, N) MdUFGT, and (O, P) MdMYB10. Apple evaluations were conducted at 1 week before commercial harvest (1WBCH), commercial harvest (CH), and 1 week after commercial harvest (CH + 1W). Values are means ± standard error. Distinct letters represent statistically significant differences (p ≤ 0.05) according to Tukey’s HSD test. PAL, phenylalanine ammonia-lyase; CHS, chalcone synthase; CHI, chalcone isomerase; F3H, flavanone 3-hydroxylase; DFR, dihydroflavonol 4-reductase; LDOX, leucoanthocyanidin dioxygenase; UFGT, UDP-glucose-flavonoid 3-O-glucosyltransferase; MYB, MYB transcription factor; AVG, aminoethoxyvinylglycine; 1-MCP, 1-methylcyclopropene.
3.1.6 Relationships among fruit drop, cracking, ethylene production, physicochemical parameters, skin color, and gene expression during on-the-tree ripening in ‘Ambrosia’
Pearson’s correlation coefficients were obtained (Supplementary Table 2), and a principal component analysis (Figure 6) was performed for ‘Ambrosia’ fruits in 2023 and 2024, including all evaluated parameters in the above results. Preharvest fruit drop and cracking displayed positive correlations with ethylene production rate (r = 0.67 and 0.54, respectively), blush percentage (r = 0.92 and 0.75), SPI (r = 0.95 and 0.85), SSC (r = 0.90 and 0.70), expression of all anthocyanin biosynthesis genes (r ≥ 0.80 and 0.51), and gene expression of ethylene biosynthesis (r ≥ 0.87 and 0.67) and perception (r ≥ 0.83 and 0.68). Drop and cracking were negatively correlated with surface skin hue angle (r = −0.85 and −0.59), background skin hue angle (r = −0.93 and −0.97), IAD (r = −0.86 and −0.64), firmness (r = −0.95 and −0.88), and TA (r = −0.84 and −0.76).
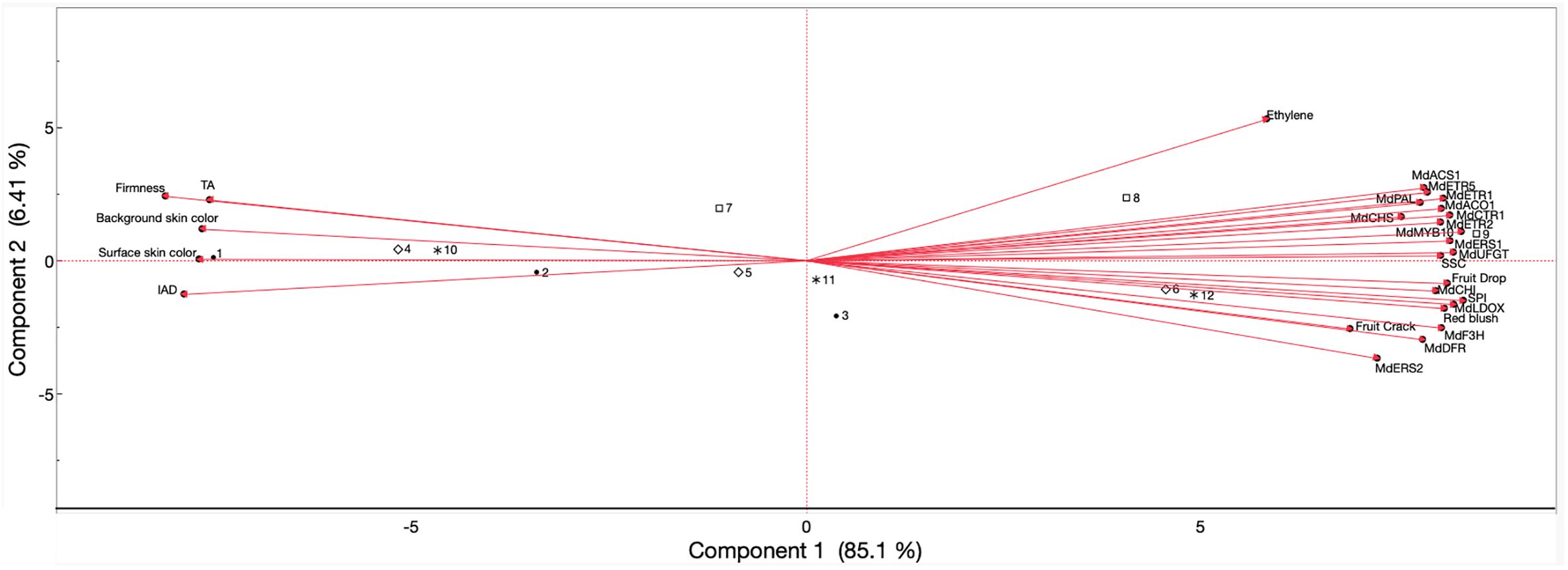
Figure 6. Principal component analysis of values obtained from preharvest fruit drop and cracking, ethylene production, physicochemical properties, skin color, expression of ethylene biosynthesis-related and perception-related genes, and anthocyanin biosynthesis-related genes of ‘Ambrosia’ apples subjected to AVG and 1-MCP treatments and evaluated during on-the-tree ripening. Numbers correspond to the different treatments and evaluation timepoints that were examined. •1, full-rate AVG_1WBCH; •2, full rate-AVG_CH; •3, full-AVG_CH + 1W; ⋄4, 1-MCP_1WBCH; ⋄5, 1-MCP_CH; ⋄6, 1-MCP_CH + 1W; □7, control_1WBCH; □8, control_CH; □9, control_CH + 1W; *10, half-rate AVG_1WBCH; *11, half-rate AVG_CH; *12, half-rate AVG_CH + 1W. SPI, starch pattern index; SSC, soluble solids content; TA, titratable acidity; IAD, index of absorbance difference; AVG, aminoethoxyvinylglycine; 1-MCP, 1-methylcyclopropene. Gene coding is defined in Figures 3–5.
‘Ambrosia’ ethylene production rate displayed positive associations with skin blush percentage (r = 0.49), SPI (r = 0.54), SSC (r = 0.67), expression of all anthocyanin biosynthesis genes (r ≥ 0.42), and gene expression of ethylene biosynthesis (r ≥ 0.78) and perception (r ≥ 0.34). Conversely, ethylene rate exhibited negative correlations with surface skin hue (r = −0.56), background skin hue (r = −0.61), IAD (r = −0.68), firmness (r = −0.49), and TA (r = −0.45).
For physicochemical parameters, firmness presented positive associations with surface skin hue (r = 0.86), background skin hue (r = 0.90), IAD (r = 0.84), and TA (r = 0.93) while showing negative associations with blush percentage (r = −0.96), SPI (r = −0.98), SSC (r = −0.89), expression of all anthocyanin biosynthesis genes (r ≤ −0.80), and gene expression of ethylene biosynthesis (r ≤ −0.79) and perception (r ≤ −0.80). SPI exhibited positive correlations with blush percentage (r = 0.97), SSC (r = 0.92), expression of all anthocyanin biosynthesis genes (r ≥ 0.85), and gene expression of ethylene biosynthesis (r ≥ 0.84) and perception (r ≥ 0.86). Nevertheless, negative correlations were observed between SPI and surface skin hue (r = −0.88), background skin hue (r = −0.90), IAD (r = −0.88), and TA (r = −0.91). The parameter SSC presented positive associations with blush percentage (r = 0.92), expression of all anthocyanin biosynthesis genes (r ≥ 0.82), and gene expression of ethylene biosynthesis (r ≥ 0.91) and perception (r ≥ 0.96) while contrarily displaying significantly negative correlations with surface skin hue (r = −0.89), background skin hue (r = −0.82), IAD (r = −0.88), and TA (r = −0.80). There was a positive association between TA and surface skin hue (r = 0.90), background skin hue (r = 0.77), and IAD (r = 0.84) while correlating negatively with blush percentage (r = −0.94), expression of all anthocyanin biosynthesis genes (r ≤ −0.75), and gene expression of ethylene biosynthesis (r ≤ −0.67) and perception (r ≤ −0.76).
For color-related parameters, surface and background skin hue angle and IAD were positively associated (r ≥ 0.67), yet all exhibited negative associations with blush percentage (r ≤ −0.81), anthocyanin biosynthesis gene expression (r ≤ −0.67), and gene expression of ethylene biosynthesis (r ≤ −0.79) and perception (r ≤ −0.70). Blush percentage displayed juxtaposed positive associations with gene expression of anthocyanin biosynthesis (r ≥ 0.81), ethylene biosynthesis (r ≥ 0.80), and ethylene perception (r ≥ 0.82).
The expression of all anthocyanin biosynthesis-related genes was positively associated with each other (r ≥ 0.73), as was the expression of all ethylene biosynthesis (r ≥ 0.99) and ethylene perception assessed genes (r ≥ 0.66). Additionally, correlations were positive among anthocyanin and ethylene gene expression (r ≥ 0.60) and gene expression of ethylene biosynthesis and perception (r ≥ 0.66).
The PCA showed the allocation of the four treatments across the evaluated harvest timepoints with a total variation of 91.51% explained by the first (85.1%) and second (6.41%) principal components (Figure 6). The parameters of TA, firmness, background and surface skin hue angles, and IAD comprised the negative side of the first component axis (associated with full-rate AVG and 1-MCP at 1WBCH and CH, and the control and half-rate AVG at 1WBCH only), while ethylene production, expression of all analyzed anthocyanin and ethylene genes, fruit drop and cracking, blush percentage, SPI, and SSC defined the positive side of the axis (associated with 1-MCP and half-rate AVG at CH + 1W, and the control at CH and CH + 1W). Half-rate AVG at CH and full-rate AVG at CH + 1W were associated with very low positive values of the first component axis.
3.2 Experiment 2: effects of AVG and 1-MCP on ‘Fuji’ fruit drop, cracking, ethylene production, physicochemical parameters, skin color, and gene expression during on-the-tree ripening
3.2.1 Fruit drop and cracking in ‘Fuji’
For preharvest fruit drop and cracking, the three treatments (full-rate AVG, 1-MCP, and control) were evaluated throughout on-the-tree ripening at three harvest timepoints (Table 2). A rise was observed for preharvest fruit drop from 1WBCH to CH + 1W in 2022, with over an eightfold increase for full-rate AVG and 1-MCP treatments at CH + 1W and over a 20-fold increase for control fruits at CH + 1W, although insignificant.
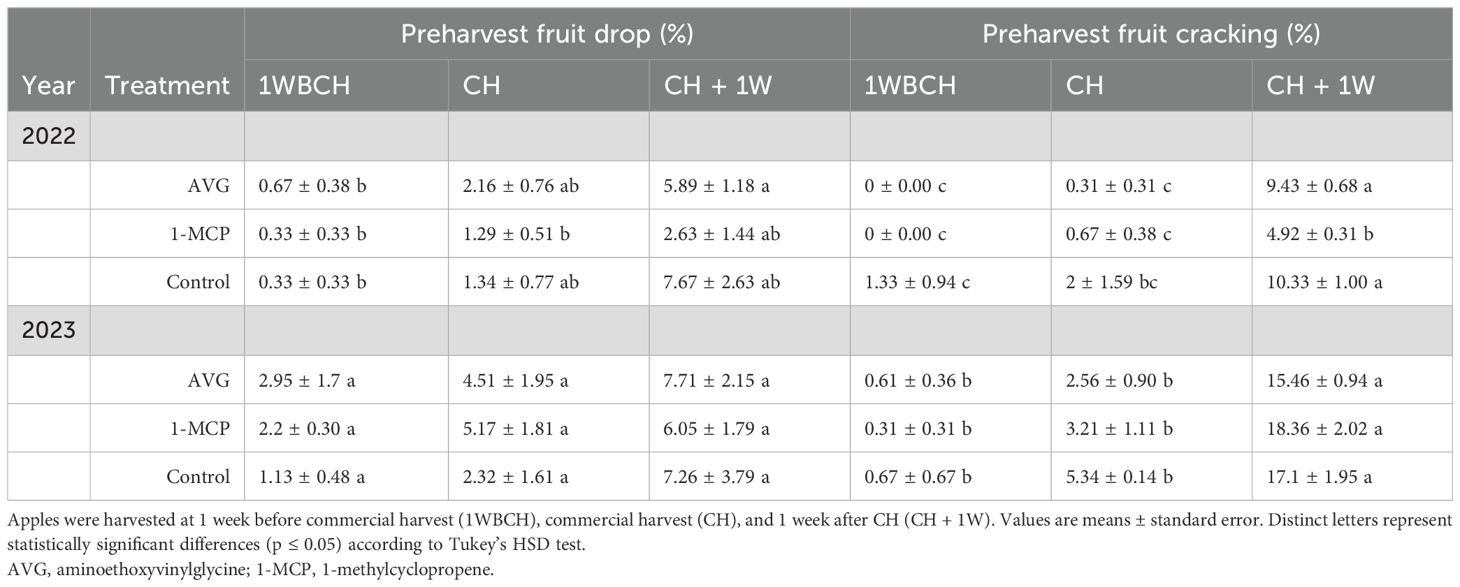
Table 2. Effect of AVG and 1-MCP on ‘Fuji’ preharvest fruit drop and cracking evaluated during on-the-tree ripening in Aspers, PA, during 2022 and 2023 production seasons.
Regarding fruit cracking, statistically higher values were displayed at CH + 1W compared with 1WBCH for each treatment in 2022 and 2023 (Table 2). In 2022, 1-MCP displayed twofold reduction in fruit cracking at CH + 1W compared to control and full-rate AVG-treated fruits.
3.2.2 Ethylene production and physicochemical parameters in ‘Fuji’
IEC increased significantly for control fruits throughout ripening from 1WBCH to CH + 1W in 2022 (Figure 7A) and for 1-MCP-treated fruits in 2023 (Figure 7B). At CH, in both years, control fruits only statistically differentiated from full-rate AVG-treated fruits (Figures 7A, B), while in 2023, IEC for full-rate AVG-treated fruits was statistically lower than that for 1-MCP-treated fruits (Figure 7B). At CH + 1W, in both years, control fruits exhibited significantly higher IEC than the two assessed ethylene-inhibiting regulator treatments (Figures 7A, B).
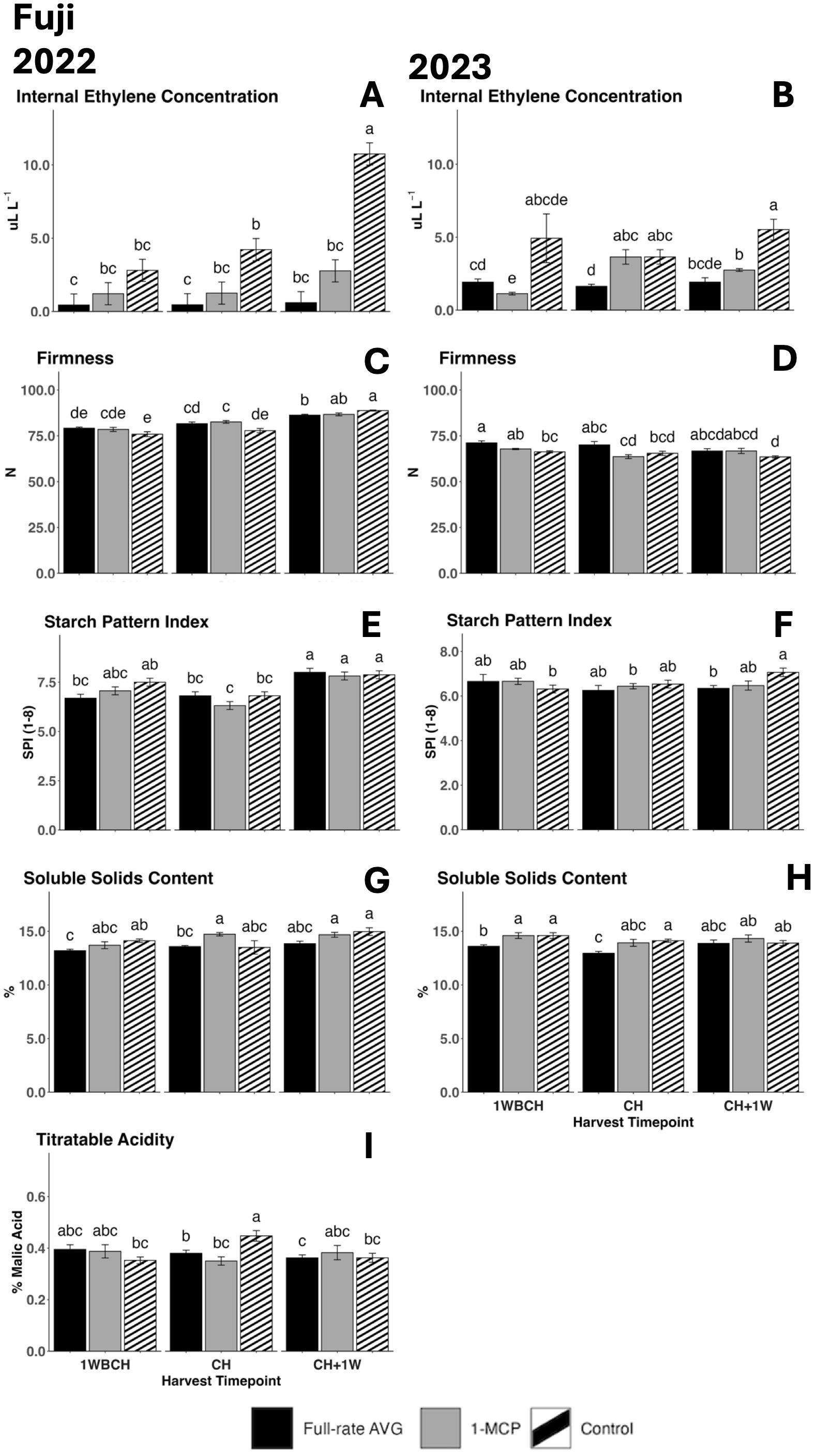
Figure 7. Effect of full-rate AVG and 1-MCP on ‘Fuji’ internal ethylene concentration and physicochemical properties during on-the-tree ripening in Aspers, PA, in (A, C, E, G, I) 2022 and (B, D, F, H) 2023. (A, B) Internal ethylene concentration, (C, D) flesh firmness, (E, F) starch content (starch pattern index), (G, H) soluble solids content, and (I) titratable acidity (malic acid). Apple evaluations were conducted at 1 week before commercial harvest (1WBCH), commercial harvest (CH), and 1 week after commercial harvest (CH + 1W). Values are means ± standard error. Distinct letters represent statistically significant differences (p ≤ 0.05) according to Tukey’s HSD test. AVG, aminoethoxyvinylglycine; 1-MCP, 1-methylcyclopropene.
Flesh firmness decreased significantly from 1WBCH to CH + 1W in 2023 in control fruits only (Figure 7D). An increased firmness was exhibited in full-rate AVG-treated fruits at 1WBCH as compared to the control in 2023, yet it did not differentiate from 1-MCP-treated fruits or control fruits at any other timepoint. In 2022, 1-MCP-treated fruits displayed significantly higher firmness values compared to the control at CH, while the control was significantly higher than full-rate AVG-treated fruits in firmness at CH + 1W (Figure 7C).
SPI values steadily increased during on-the-tree ripening from 1WBCH to CH + 1W for full-rate AVG-treated fruits in 2022, while SPI values for 1-MCP-treated and control fruits remained generally constant (Figure 7E). In 2023, only control fruits displayed a significant increase in SPI, as full-rate AVG-treated fruits displayed lower values at CH + 1W compared to control fruits, indicative of increased starch disappearance in the latter (Figure 7F).
Regarding SSC, values were constant throughout the three assessed timepoints in all treatments for both years (Figures 7G, H). Full-rate AVG-treated fruits exhibited significantly lower SSC as compared to the control at 1WBCH and as compared to 1-MCP-treated fruits at CH in 2022 (Figure 7G). In 2023, full-rate AVG-treated fruits exhibited the lowest SSC as compared to all treatments at 1WBCH and presented statistically reduced values than control fruits at CH (Figure 7H).
TA generally remained constant across treatments throughout the assessed ripening timepoints in 2022, although control fruits presented significantly higher TA values at CH compared with 1WBCH (Figure 7I). Full-rate AVG- and 1-MCP-treated fruits displayed significantly reduced TA values than the control at CH in that same year. TA was not able to be measured during 2023 due to an unexpected technical problem.
3.2.3 Skin coloration in ‘Fuji’
Surface skin hue showed a decreasing trend from 1WBCH to CH + 1W throughout ripening in all treatments in both years (Figures 8A, B). In 2022, full-rate AVG-treated fruits showed the highest surface skin values at each timepoint, remaining statistically higher than those of 1-MCP-treated fruits and control fruits at 1WBCH and CH and higher than those of control fruits at CH + 1W (Figure 8A). 1-MCP-treated fruits presented statistically increased surface skin values over control fruits at CH (Figure 8A). In 2023, full-rate AVG-treated fruits exhibited higher surface skin values than 1-MCP-treated fruits and control fruits at CH and than 1-MCP-treated fruits at CH + 1W (Figure 8B). Correspondingly, skin blush percentage presented a tendency to increase as ripening progressed on the tree from 1WBCH to CH + 1W in both years (Figures 8C, D). In 2022, full-rate AVG-treated fruits showed significantly decreased blush percentage values than 1-MCP-treated fruits and the control at 1WBCH and than the control at CH (Figure 8C). In 2023, full-rate AVG-treated fruits exhibited significantly lower blush than control fruits at CH and 1-MCP-treated fruits at CH + 1W (Figure 8D). 1-MCP treatment only significantly reduced blush compared with the control at CH in 2022 (Figure 8C). Notably, full-rate AVG was the singular treatment that failed to meet the 50% blush percentage requirement at the first timepoint (1WBCH), only reaching 50% 1 week later at CH in both years.
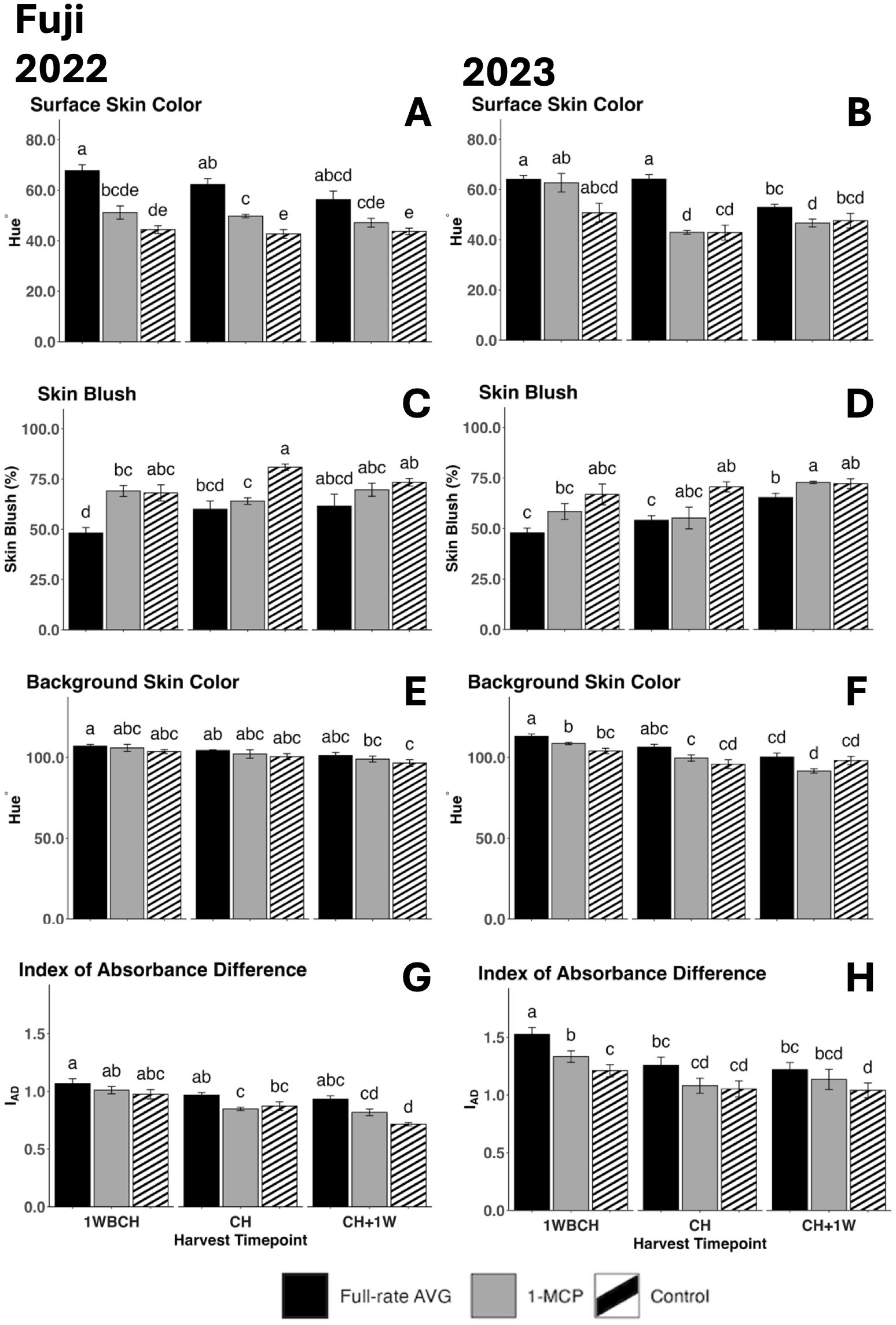
Figure 8. Effect of full-rate AVG and 1-MCP on ‘Fuji’ fruit color during on-the-tree ripening in Aspers, PA, in (A, C, E, G) 2022 and (B, D, F, H) 2023. (A, B) Surface skin color, (C, D) skin blush, (E, F) background skin color, and (G, H) index of absorbance difference. Apple evaluations were conducted at 1 week before commercial harvest (1WBCH), commercial harvest (CH), and 1 week after commercial harvest (CH + 1W). Values are means ± standard error. Distinct letters represent statistically significant differences (p ≤ 0.05) according to Tukey’s HSD test. AVG, aminoethoxyvinylglycine; 1-MCP, 1-methylcyclopropene.
A significant reduction in background skin hue values throughout the evaluated harvest timepoints from 1WBCH to CH + 1W, revealing a green to yellow color conversion, was observed in both years for most treatments, with significant differences between 1WBCH and CH + 1W for full-rate AVG- and 1-MCP-treated fruits in 2023 (Figures 8E, F). Further, full-rate AVG-treated fruits displayed significantly higher background skin values than 1-MCP-treated and control fruits at 1WBCH in 2023 (Figure 8F). Correspondingly, IAD values decreased throughout ripening in both years (Figures 8G, H). In 2023, at 1WBCH, full-rate AVG treatment produced statistically higher IAD values, followed by 1-MCP, and lastly the control (Figure 8H). Full-rate AVG-treated fruits additionally exhibited statistically higher IAD values than control fruits at CH + 1W in both years (Figures 8G, H).
3.2.4 Expression of ethylene biosynthesis and perception genes in ‘Fuji’
Transcript accumulation for both assessed ethylene biosynthesis genes, MdACS1 and MdACO1, increased significantly as on-the-tree ripening progressed from 1WBCH to CH + 1W in each treatment in both years (Figure 9). In 2022, full-rate AVG-treated fruits displayed the statistically lowest gene expression, followed by 1-MCP-treated fruits, and lastly the control at each timepoint (Figures 9A, C). The same trend was observed in 2023 in CH and CH + 1W, while at 1WBCH, there were no differences between full-rate AVG- and 1-MCP-treated fruits (Figures 9B, D). Control fruits exhibited significantly higher gene expression values of MdACS1 and MdACO1 than either ethylene regulator treatment at all timepoints in both years.
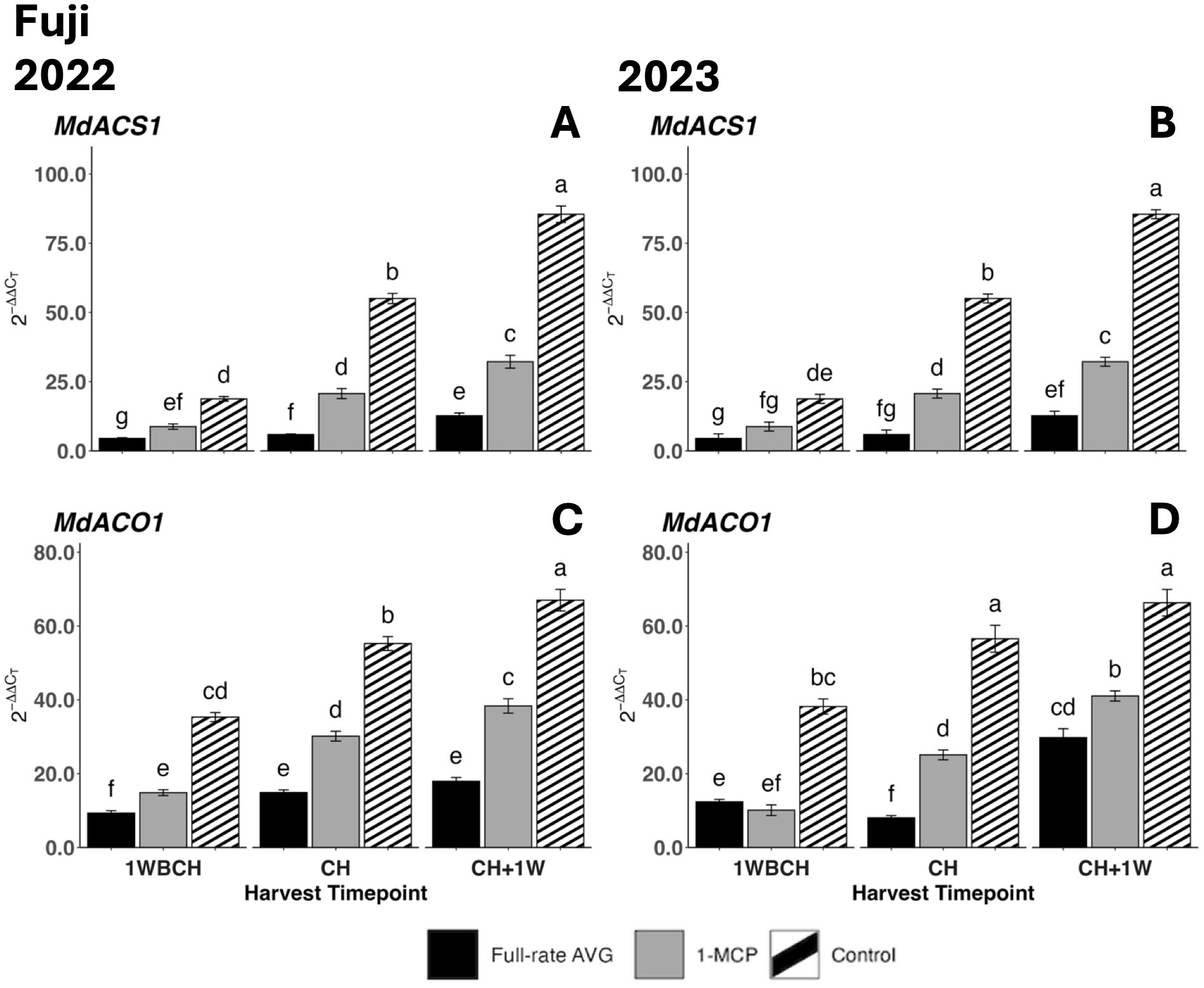
Figure 9. Effect of full-rate AVG and 1-MCP on relative expression of ethylene biosynthesis genes in ‘Fuji’ during on-the-tree ripening in Aspers, PA, in (A, C) 2022 and (B, D) 2023. (A, B) MdACS1 and (C, D) MdACO1. Apple evaluations were conducted at 1 week before commercial harvest (1WBCH), commercial harvest (CH), and 1 week after commercial harvest (CH + 1W). Values are means ± standard error. Distinct letters represent statistically significant differences (p ≤ 0.05) according to Tukey’s HSD test. ACS, 1-aminocyclopropane-carboxylase synthase; ACO, 1-aminocyclopropane-1-carboxylate oxidas; AVG, aminoethoxyvinylglycine; 1-MCP, 1-methylcyclopropene.
Equally, the transcript accumulation of all ethylene perception genes increased significantly from 1WBCH to CH + 1W in all treatments in both years, except for full-rate AVG treatment for MdETR2 and MdCTR1 in 2023 (Figures 10H, L). Control fruits displayed significantly higher gene expression values than full-rate AVG- and 1-MCP-treated fruits at 1WBCH for all assessed genes in both years (Figures 10A, B, E–L) and at CH in 2022. In 2023, at CH, full-rate AVG-treated fruits exhibited the lowest transcript accumulation values for all assessed genes, while there were no differences between 1-MCP-treated and control fruits for MdERS1, MdETR1, and MdCTR1 (Figures 10B, F, L). At CH + 1W, for both years, all evaluated genes presented the lowest transcript accumulation for full-rate AVG-treated fruits, followed by 1-MCP-treated fruits, and the statistically highest values were for control fruits, with only MdETR5 showing no differences between 1-MCP-treated and control fruits. MdERS2 gene expression levels were an exception to all the above, as treatments exhibiting a lack of significant differences at any timepoint were observed in either production season (Figures 10C, D).
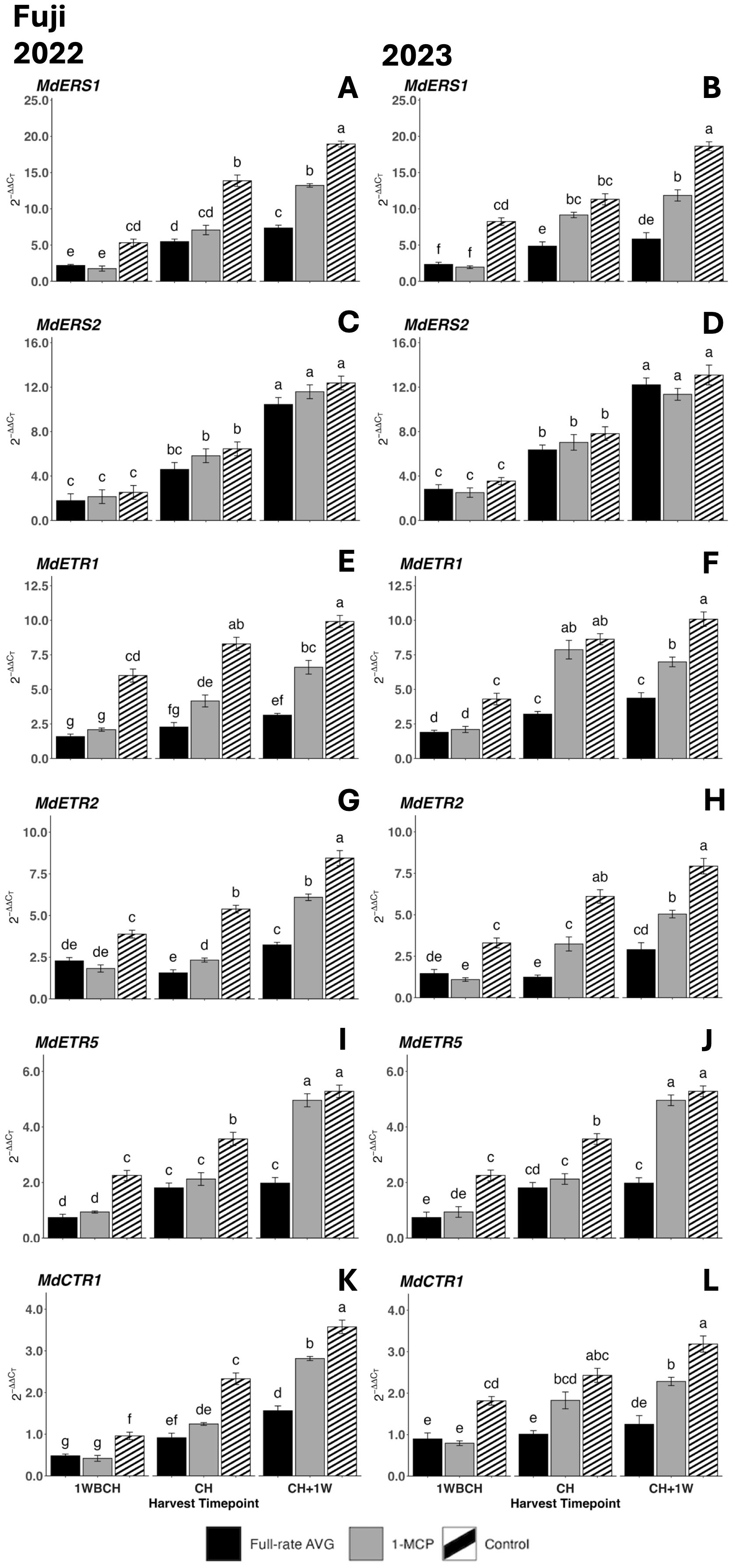
Figure 10. Effect of full-rate AVG and 1-MCP on the relative expression of ethylene perception genes in ‘Fuji’ apples during on-the-tree ripening in Aspers, PA, in (A, C, E, G, I, K) 2022 and (B, D, F, H, J, L) 2023. (A, B) MdERS1, (C, D) MdERS2, (E, F) MdETR1, (G, H) MdETR2, (I, J) MdETR5, and (K,L) MdCTR1. Apple evaluations were conducted at 1 week before commercial harvest (1WBCH), commercial harvest (CH), and 1 week after commercial harvest (CH + 1W). Values are means ± standard error. Distinct letters represent statistically significant differences (p ≤ 0.05) according to Tukey’s HSD test. ERS, ethylene response sensor; ETR, ethylene receptor type; CTR, constitutive triple response; AVG, aminoethoxyvinylglycine; 1-MCP, 1-methylcyclopropene.
3.2.5 Expression of anthocyanin biosynthesis genes in ‘Fuji’
There was a significant rise in gene expression level from the first to last evaluated harvest timepoints for all assessed anthocyanin biosynthesis genes in both years (Figure 11). Full-rate AVG-treated fruits presented the statistically lowest transcript accumulation at each timepoint for MdPAL, MdCHI, and MdLDOX in 2022 and for MdCHS and MdDFR1 in 2023, while control fruits displayed the highest transcript accumulation (Figures 11A, E, K, D, J). 1-MCP-treated and control fruits presented no significant differences at the final timepoint (CH + 1W) in either year for any gene.
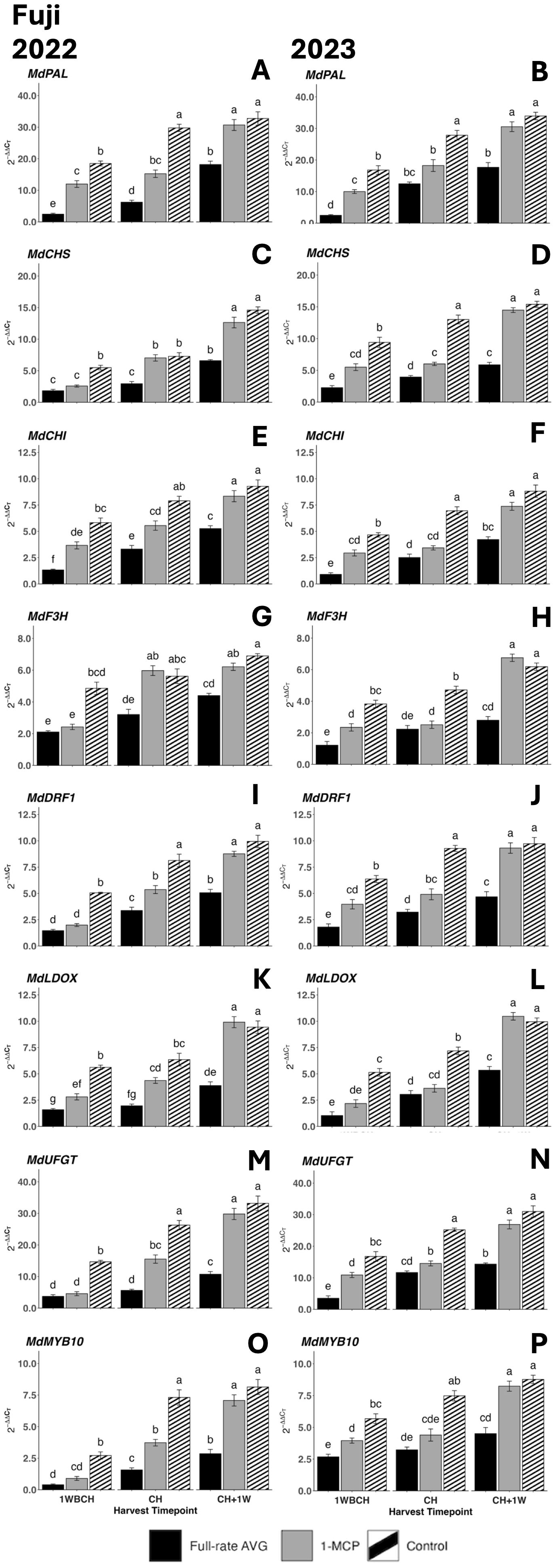
Figure 11. Effect of 1-MCP and full-rate AVG on relative gene expression of anthocyanin biosynthesis-related genes of ‘Fuji’ during on-the-tree ripening in Aspers, PA, in (A, C, E, G, I, K, M, O) 2022 and (B, D, F, H, J, L, N, P) 2023. (A, B) MdPAL, (C, D) MdCHS, (E, F) MdCHI, (G, H) MdF3H, (I, J) MdDFR1, (K, L) MdLOX, (M, N) MdUFGT, and (O, P) MdMYB10. Apple evaluations were conducted at 1 week before commercial harvest (1WBCH), commercial harvest (CH), and 1 week after commercial harvest (CH + 1W). Values are means ± standard error. Distinct letters represent statistically significant differences (p ≤ 0.05) according to Tukey’s HSD test. PAL, phenylalanine ammonia-lyase; CHS, chalcone synthase; CHI, chalcone isomerase; F3H, flavanone 3-hydroxylase; DFR, dihydroflavonol 4-reductase; LDOX, leucoanthocyanidin dioxygenase; UFGT, UDP-glucose-flavonoid 3-O-glucosyltransferase; MYB, MYB transcription factor; AVG, aminoethoxyvinylglycine; 1-MCP, 1-methylcyclopropene.
3.2.6 Relationships among fruit drop, cracking, ethylene production, physicochemical parameters, skin color, and gene expression during on-the-tree ripening in ‘Fuji’
Pearson’s correlation coefficients were obtained (Supplementary Table 3), and a principal component analysis (Figure 12) was performed for ‘Fuji’ fruits in 2022 and 2023, including all evaluated parameters in the results above. Preharvest fruit drop and cracking were positively correlated with each another (r = 0.90), and fruit drop correlated less strongly with other parameters over cracking: IEC (r = 0.41 and 0.51, respectively), SPI (r = 0.66 and 0.83), anthocyanin biosynthesis gene expression (r ≥ 0.45 and 0.68), and gene expression of ethylene biosynthesis (r ≥ 0.42 and 0.57) and perception (r ≥ 0.39 and 0.57). Background skin hue angle (r = −0.56 and −0.76), IAD (r = −0.53 and −0.61), and firmness (r = −0.66 and −0.76) all negatively correlated with fruit drop and cracking. Only fruit cracking positively correlated with blush percentage (r = 0.52).
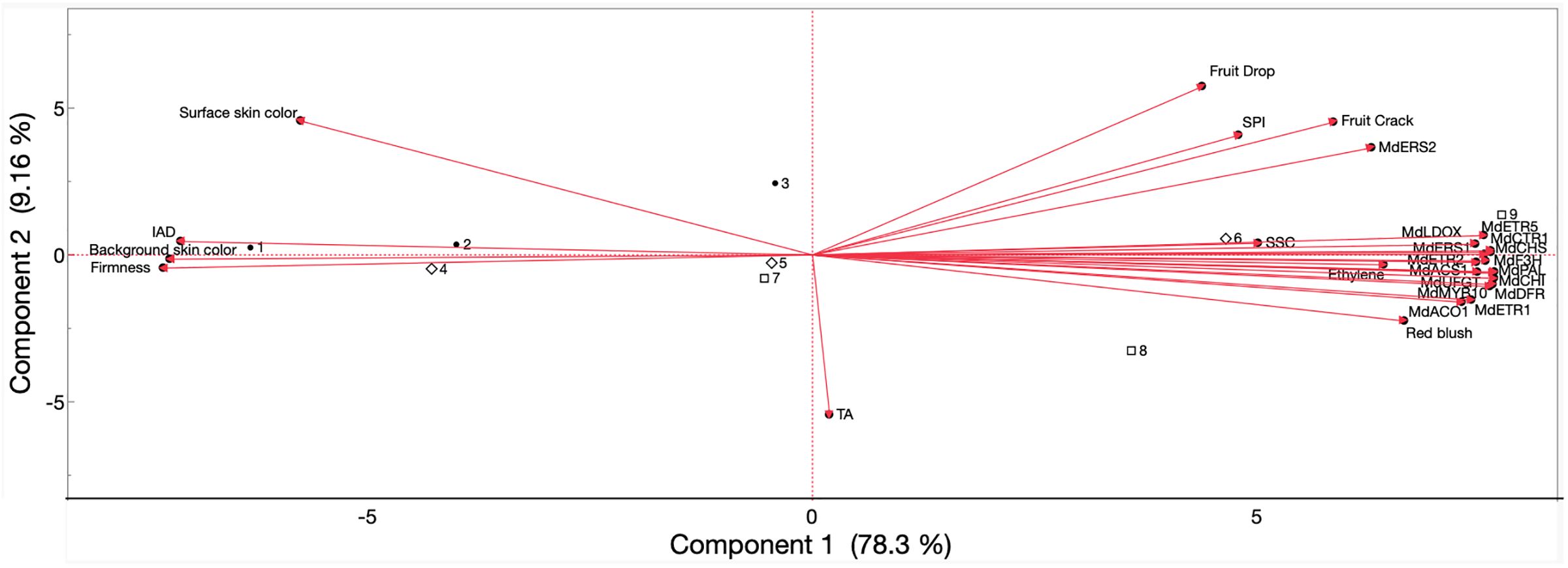
Figure 12. Principal component analysis of values obtained from preharvest fruit drop and cracking, IEC, physicochemical properties, skin color, expression of ethylene biosynthesis-related and perception-related genes, and anthocyanin biosynthesis-related genes of ‘Fuji’ apples subjected to AVG and 1-MCP treatments and evaluated during on-the-tree ripening. Numbers correspond to the different treatments and evaluation timepoints that were examined. •1, full-rate AVG_1WBCH; •2, full-rate AVG_CH; •3, full-rate AVG_CH + 1W; ⋄4, 1-MCP_1WBCH; ⋄5, 1-MCP_CH; ⋄6, 1-MCP_CH + 1W; □7, control_1WBCH; □8, control_CH; □9, control_CH + 1W. SPI, starch pattern index; SSC, soluble solids content; TA, titratable acidity (for 2022 only); IAD, index of absorbance difference. Gene coding is defined in Figures 9–11. IEC; internal ethylene concentration; AVG, aminoethoxyvinylglycine; 1-MCP, 1-methylcyclopropene.
Similarly, IEC rate in ‘Fuji’ fruits displayed positive associations with blush percentage (r = 0.66), SPI (r = 0.57), SSC (r = 0.55), anthocyanin gene expression (r ≥ 0.74), and gene expression of ethylene biosynthesis (r ≥ 0.91) and perception (r ≥ 0.50). Negative associations were exhibited with firmness (r = −0.71), surface skin hue (r = −0.57), background color (r = −0.58), and IAD (r = −0.72).
For the physicochemical parameters, firmness showed positive associations with surface skin hue (r = 0.74), background skin hue (r = 0.95), and IAD (r = 0.96) while presenting negative associations with blush percentage (r = −0.77), SSC (r = −0.57), expression of all anthocyanin genes (r ≤ −0.84), ethylene biosynthesis gene expression (r ≤ −0.86), and ethylene perception gene expression (r ≤ −0.86). SPI, likewise, displayed positive correlations with blush percentage (r = 0.52), SSC (r = 0.48), expression of all anthocyanin genes (r = 0.55), and gene expression of ethylene biosynthesis (r ≥ 0.52) and perception (r ≥ 0.55), except for MdETR1. SSC again followed the same positive correlation with blush percentage (r = 0.63), anthocyanin genes (r ≥ 0.60), ethylene biosynthesis (r ≥ 0.56), and perception genes (r ≥ 0.52), with the exception of MdERS2, and negative correlations with background skin hue (r = −0.60) and IAD (r = −0.62).
For skin color parameters, blush percentage positively correlated with expression of all anthocyanin genes (r ≥ 0.83), ethylene biosynthesis genes (r ≥ 0.84), and ethylene perception genes (r ≥ 0.75) and negatively correlated with surface skin hue angle (r = −0.85), background skin hue angle (r = −0.81), and IAD (r = −0.79). Conversely, surface skin hue, background skin hue, and IAD were each negatively associated with anthocyanin biosynthesis gene expression (r ≤ −0.65), ethylene biosynthesis gene expression (r ≤ −0.74), and ethylene perception gene expression (r ≤ −0.62), with the exception of MdERS2.
Expression of all anthocyanin biosynthesis-related genes showed positive associations with each other (r ≥ 0.96), as did all ethylene biosynthesis (r ≥ 0.97) and ethylene perception assessed genes (r ≥ 0.67). Additionally, correlations were positive among anthocyanin and ethylene gene expression (r ≥ 0.75) and gene expression of ethylene biosynthesis and perception (r ≥ 0.62).
The PCA explained 87.5% of the variation among the three harvest timepoints and the three treatments for all evaluated parameters in the first (78.3%) and second (9.16%) components (Figure 12). The negative axis of the first component comprised surface skin color, background skin color, firmness, and IAD and included full-rate AVG at all assessed timepoints (1WBCH, CH, and CH + 1W), 1-MCP at 1WBCH and CH, and the control at 1WBCH. The positive axis comprised parameters preharvest fruit drop and cracking, SPI, SSC, internal ethylene concentration, blush percentage, and all analyzed ethylene- and anthocyanin-related genes and was associated with 1-MCP at CH + 1W and the control at CH and CH + 1W.
4 Discussion
Apple fruit yield, marketability, and profitability are directly dependent on preharvest fruit drop, cracking, and physicochemical and color parameters, which can all be altered by the application of ethylene-inhibiting PGRs (Argenta et al., 2022; do Amarante et al., 2022; Liu et al., 2022; Malladi et al., 2023; Johnson and Farcuh, 2024). Ethylene has exhibited a strong influence on preharvest fruit drop and cracking (Klee, 2004; Byers et al., 2005), which is confirmed by the positive associations found between these three parameters in this study. Although not statistically significant potentially due to high data variability, the ~50% reduction in preharvest fruit drop observed in ‘Ambrosia’ fruits with the use of ethylene-inhibiting PGRs compared with control in this work is of critical importance for mid-Atlantic growers, and it also agrees with previous research showing that AVG decreases preharvest fruit drop due to the inhibition of ethylene production in cultivars such as ‘Golden Delicious’ (Sakaldas and Gundogdu, 2016; Byers et al., 2019) and ‘Honeycrisp’ (Arseneault and Cline, 2017; Johnson and Farcuh, 2024), as does 1-MCP in ‘Golden Delicious’ (Sakaldas and Gundogdu, 2016). These trends also align with the reduction in the transcript accumulation of analyzed ethylene biosynthesis and perception genes for both ethylene-inhibiting PGR treatments. Contrarily, the lack of significant reduction in fruit drop with the application of ethylene-inhibiting PGRs in ‘Fuji’ fruits in this study is supported by previous research indicating that this cultivar’s susceptibility to fruit drop has been inconsistent under different environmental conditions where the fruits are grown (Kasai et al., 2008; Li et al., 2010). This study shows that under mid-Atlantic conditions, ‘Fuji’ fruits do not have a high predisposition to fruit drop, which explains the lack of differences among the assessed treatments in this parameter. In fact, AVG drop mitigation has been observed to be most effective when natural drop is heavier (Amarante et al., 2002; Unrath et al., 2023), suggesting that decreased ethylene concentration is not the only factor reducing fruit drop, and weather conditions during the growing season, as well as apple cultivar, play a critical role. Additionally, future studies could consider extending the analyzed time period used in this work to study fruit drop, as it may reveal higher fruit drop percentages and thus significant differences among treatments.
The increase in fruit cracking during on-the-tree ripening for all treatments is consistent with prior findings (Byers, 1997). For ‘Ambrosia’, both ethylene-inhibiting PGR treatments exhibited a reduction in cracking, supported by work in ‘Gala’ (Liu et al., 2022), ‘Arlet’ (Byers et al., 2005), ‘Rome’, and ‘Delicious’ (Byers, 1997). In sweet cherry and litchi fruits, it has been shown that ethylene biosynthesis and fruit cracking share the key genes 1-aminocyclopropane-1-carboxylate synthase (ACS) and ACO (Wang et al., 2019; Michailidis et al., 2021; Santos et al., 2023). The latter could support the alteration of fruit cracking observed in this study after ethylene-inhibiting PGR treatments. Furthermore, higher firmness and delayed fruit ripening may aid in the prevention of fruit cracking (Opara et al., 1997), supporting the impact of ethylene-inhibiting PGRs on decreasing ‘Ambrosia’ fruit cracking in this work. In contrast, the lack of consistent results in ‘Fuji’ for ethylene-inhibiting PGRs reducing fruit cracking differs from earlier studies showing decreased fruit cracking with AVG treatments ranging from 60 to 130 mg a.i. L−1 (Petri et al., 2011). These differences could be explained by the major influence on fruit cracking development that location and climate can have, as cracking in ‘Gala’ apples varied considerably across a single orchard (Opara et al., 2000). ‘Fuji’ has been categorized as a cracking-sensitive cultivar, and vulnerability to cracking is additionally advanced by factors like environmental conditions, orchard management, and a lower ability of the fruit’s skin to resist surface tensions due to fruit expansion (Ginzberg and Stern, 2016). In conjunction with the lack of response to ethylene-inhibiting PGR treatment on fruit drop in this study, ‘Fuji’ may require further research into rates and timings of ethylene-inhibiting PGRs to assess the impact on fruit cracking in the mid-Atlantic.
Increased ethylene production throughout ripening in apples is a result of their climacteric nature (Chen et al., 2018; Miah et al., 2023). ACS activity is known to act as the key limiting factor in ethylene biosynthesis during the ripening process, followed by ACO (Brasil and Siddiqui, 2018). The significantly lower ethylene production observed in this work for both cultivars treated with ethylene-inhibiting PGRs, as compared to control fruits, could be a result of ACS enzyme inactivity, which consequently limits ACO. The significantly decreased transcript accumulation of MdACS1 and MdACO1 in PGR-treated fruits could be an indirect consequence of this enzyme inactivity and is consistent with prior work in apples (Miah and Farcuh, 2024c) and pears (He et al., 2023). Among ethylene-inhibiting PGR treatments, the most significant impact of full-rate AVG on reducing MdACS1 and MdACO1 gene expression levels, compared with 1-MCP, could be explained by its different mode of action. While AVG directly impedes the action of ACS and thus of ethylene biosynthesis, 1-MCP acts as an antagonist to the receptor binding sites of ethylene, binding to and consequently preventing downstream signal transduction (Tatsuki et al., 2007; Bulens et al., 2012), and only then impacting ethylene autocatalysis and reducing the transcript accumulation of ethylene biosynthesis-related genes (Yang and Hoffman, 1984). Research has suggested that 1-MCP also upregulates members of the ERF gene family, which correspond to transcription factors in the last step along the ethylene signaling cascade. ERF members have been shown to act as negative regulators of fruit ripening, ultimately suppressing MdACS1 transcription (Liu et al., 2015; Li et al., 2016). Furthermore, the lower gene expression levels of MdACS1 and MdACO1 in half-rate AVG-treated fruits as compared to full-rate AVG-treated fruits could be a result of a respective reduced degree of ethylene biosynthesis. The latter is consistent with findings in ‘Red Delicious’ exhibiting decreasing ethylene production with increasing AVG rates (Malladi et al., 2023). Moreover, AVG is not an ethylene-specific inhibitor and is known to block other pyridoxal phosphate (PLP)-dependent enzymes in the cell, such as tryptophan aminotransferases (TAAs), which are crucial for auxin biosynthesis (Song et al., 2016). As auxin production appears to trigger ethylene production in apples (Arseneault and Cline, 2016), the application of AVG can potentially suppress the biosynthesis of both hormones, although this needs further study.
Ethylene perception occurs via receptors reported to be negative regulators of downstream ethylene responses (Kevany et al., 2007). As receptor protein degradation is triggered by the binding of ethylene, ethylene binding maintains receptors in the “off” state, and therefore, decreased receptor presence due to degradation results in an enhanced sensitivity to ethylene (Klee and Giovannoni, 2011; Cherian et al., 2014). With the mode of action for 1-MCP directly inhibiting ethylene perception by binding to and preventing the degradation of the receptors, we expected lower expression of perception genes for this treatment with reduced sensitivity to ethylene (Sisler and Serek, 1997). In agreement, in our study, MdETR1, MdETR2, MdETR5, and MdERS1, all reported as negative regulators of ethylene in apples and other fruits, such as pear (Xie et al., 2015), displayed significantly lower transcript accumulation for 1-MCP-treated fruits as compared to the control in ‘Fuji’ and ‘Ambrosia’. Full-rate AVG treatment similarly decreased perception-related gene expression, which could be due to the downstream effects of decreased biosynthesis delaying the degradation of the negative ethylene receptors (Kevany et al., 2007; Dal Cin et al., 2008). In fact, AVG treatment at the full rate was generally most effective at reducing the expression of ethylene perception-related genes compared to the other treatments, likely because ethylene biosynthesis was directly impaired by AVG application before perception could occur (Johnson and Farcuh, 2024). Conversely, MdERS2 presented no significant differences across treatments. This trend in MdERS2 parallels previous research suggesting that this receptor is ethylene-independent and is instead developmentally dependent, complementary to the rise of expression that we observed throughout the ripening period in both evaluated cultivars (Li and Yuan, 2008; Johnson and Farcuh, 2024). MdCTR1, which plays a crucial role as a negative regulator of ethylene, prevents relaying of ethylene signaling and subsequent responses (Kieber et al., 1993; Solano et al., 1998; Alonso et al., 1999; Yang et al., 2013). All ethylene-inhibiting PGR treatments significantly reduced the expression of MdCTR1 transcripts in this work compared with the control, aligning with prior findings in 1-MCP in ‘Golden Delicious’ (Yang et al., 2013) and both AVG and 1-MCP in ‘Honeycrisp’ (Johnson and Farcuh, 2024). Furthermore, the upregulation of MdCTR1 expression observed in this work throughout on-the-tree ripening for both cultivars is consistent with previous CTR1 gene expression studies in different fruits such as plum (Dal Cin et al., 2006; El-Sharkawy et al., 2007; Farcuh et al., 2019), pear (El-Sharkawy et al., 2003), and tomato (Leclercq et al., 2002), suggesting that MdCTR1, in its role of negative expression regulator of ethylene responses, may act as a controlling mechanism in response to increased ethylene concentrations, ultimately aiding in slowing the ripening process (Klee, 2002; Yang et al., 2013).
Delayed ethylene production has also been observed to result in delayed fruit ripening on the tree due to the primary role of ethylene in driving apple maturity (Liu et al., 2022). ‘Ambrosia’ fruits treated with ethylene-inhibiting PGRs displayed higher flesh firmness and decreased SPI values (and therefore lower starch disappearance) than control fruits, indicative of delayed maturity. Higher fruit firmness may be a result of the reduced activity of cell wall-degrading enzymes, which have been widely reported to be regulated by ethylene (Johnston et al., 2009; Arseneault and Cline, 2016; Wang et al., 2021; Wu et al., 2023), as observed in other fruits such as melons (Hung et al., 2010) and peaches (Bregoli et al., 2002). Lower starch disappearance parallels prior results in ‘Honeycrisp’ (Johnson and Farcuh, 2024) and ‘Red Delicious’ (Malladi et al., 2023), suggesting that starch degradation may be ethylene-dependent in ‘Ambrosia’. This notion is additionally supported by ethylene production displaying negative associations with firmness and positive associations with SPI in this work. The variable results exhibited by ‘Fuji’ in firmness and SPI with ethylene-inhibiting PGR treatment align with inconsistent values in previous research (Escalada and Archbold, 2019). The decreased SSC values and the higher TA values observed in fruits from both cultivars treated with ethylene-inhibiting PGRs compared with those in the control coincide with prior reports (Argenta et al., 2018a; Tomala et al., 2020a, 2020b; Liu et al., 2022; Doerflinger et al., 2024). The involvement of ethylene in fruit ripening is further supported by ethylene production negatively associating with TA values and positively associating with SSC values in this work, although some studies have attributed a lack of effect on SSC and TA with 1-MCP (McArtney et al., 2008; do Amarante et al., 2022) and AVG (Yuan and Carbaugh, 2007) to seasonal differences, regional location, and application timing (Doerflinger et al., 2019). In general, among ethylene-inhibiting PGR treatments, full-rate AVG was the most efficient in delaying fruit maturity, followed by 1-MCP, and lastly, half-rate AVG. This is likely related to the significantly lower ethylene production in full-rate AVG-treated fruits than in 1-MCP- and half-rate AVG-treated apple fruit. Higher rates of AVG have been documented with greater efficacy than lower rates in delaying apple fruit ripening in prior findings in accordance with higher ethylene biosynthesis inhibition (Greene and Schupp, 2004; Ozturk et al., 2015). The results of the current study are further supported by previous reports indicating that AVG treatment exhibits greater influence on apple fruit maturity than 1-MCP (Scolaro et al., 2015; Argenta et al., 2018b; Johnson and Farcuh, 2024), reinforcing the differential impact of ethylene-inhibiting PGRs on fruit quality.
Red coloration is additionally important for commercial marketability and is the result of anthocyanin accumulation (Whale and Singh, 2007). In our work, ethylene production was positively correlated with the assessed anthocyanin biosynthesis-related genes, suggesting the participation of endogenous ethylene in the regulation of anthocyanin accumulation throughout fruit ripening, in agreement with previous work (Faragher and Brohier, 1984; Blankenship and Richard Unrath, 1988; Wang and Dilley, 2001; Shafiq et al., 2014; Farcuh et al., 2022; Shi et al., 2022). Ethylene production and the assessed anthocyanin biosynthesis genes were moreover positively correlated with blush percentage and negatively correlated with surface skin color, background skin color, and IAD. Full-rate AVG in particular has been shown to decrease red coloration in several cultivars, including ‘Gala’ (Argenta et al., 2018b), ‘Cripps Pink’ (Whale et al., 2008), and ‘Honeycrisp’ (Miah and Farcuh, 2024a). Peaches (Belding and Lokaj, 2002) and pears (Clayton et al., 2000) have exhibited similar coloration inhibition. In this study, full-rate AVG-treated fruits reached >50% red blush coverage 1 week later than any other treatment for both assessed cultivars. The latter can be explained by the patterns observed in the analyzed anthocyanin biosynthesis-related genes, where the lowest transcript accumulation was observed in full-rate AVG treatment. Half-rate AVG- and 1-MCP-treated fruits displayed intermediate reductions in coloration and color-related transcript accumulation compared with control and full-rate AVG-treated fruits, which could be due to the different modes of action of the ethylene-inhibiting PGRs and the different rates of AVG. Transcription factor MdMYB10 plays a key role in regulating anthocyanin biosynthesis and has been shown to positively interact with ethylene biosynthesis genes ACS and ACO (Wang and Dilley, 2001; Espley et al., 2007; Jia et al., 2020; Li et al., 2022; Shi et al., 2022). The differences in red skin coloration among ethylene-inhibiting PGR treatments could also be explained by the fact that AVG directly inhibits ACS and therefore may alter MdMYB10 transcript accumulation and thus red color development beyond 1-MCP treatment, as 1-MCP represses ethylene perception (Byers, 1997; Yuan and Li, 2008).
The differences discussed above were summarized in the PCA for both ‘Fuji’ and ‘Ambrosia’, where the positioning of full-rate AVG-treated fruits can be explained by these fruits presenting the lowest ethylene production, ethylene biosynthesis and perception, and anthocyanin biosynthesis-related gene expression, and thus the most delayed fruit maturity. A midway maturity relative to full-rate AVG-treated and control fruits for all assessed features was associated with 1-MCP- and half-rate AVG-treated fruits, while the most advanced maturity was exhibited by control fruits. Regarding color, the lowest values for apple skin blush and anthocyanin-related gene expression in full-rate AVG-treated fruits contribute to their placement in the PCA and explain why these fruits only met the minimum requirement of 50% red blush coverage 1 week later than all other treatments in both assessed cultivars. Current work is ongoing in assessing the impact of 1-MCP and AVG on these economically important apple cultivars during postharvest storage.
Our study on the impact of different ethylene-inhibiting plant growth regulators on emerging and prevalent cultivars such as ‘Ambrosia’ and ‘Fuji’ throughout on-the-tree ripening in the mid-Atlantic showed that ethylene production was strongly correlated with fruit drop and that full-rate AVG treatment most efficiently decreased ethylene production as compared to other treatments. Moreover, this work revealed a reduction in fruit cracking compared with control fruits throughout ripening with full-rate AVG and 1-MCP treatments. Full-rate AVG further downregulated the transcript accumulation of ethylene biosynthesis- and perception-related genes, ultimately resulting in the greatest delay to fruit ripening relative to the other treatments in this study. Nevertheless, full-rate AVG treatment presented a challenge for red skin color development, reaching the 50% blush only 1 week after all other treatments trialed in this study in both assessed cultivars. Furthermore, this work demonstrated that although 1-MCP- and half-rate AVG-treated fruits did not delay red skin color development to the extent of the full-rate AVG treatment, they exhibited a midway maturity between the latter and control fruits, revealed by their intermediate ethylene production rate and related gene expression values. This study provides a promising framework for mid-Atlantic growers to select the best ethylene-inhibiting plant growth regulator to aid in harvest and fruit quality management in these economically important apple cultivars.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
EJ: Conceptualization, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. MF: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by the State Horticultural Association of Pennsylvania and by start-up funds from the College of Agriculture and Natural Resources and the Department of Plant Science and Landscape Architecture (UMD) to MF.
Acknowledgments
We thank Joy Cline and Bear Mountain Orchards for their assistance with this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1629445/full#supplementary-material
References
Adams, D. O. and Yang, S. F. (1979). Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc. Natl. Acad. Sci. 76, 170–174. doi: 10.1073/pnas.76.1.170
Alonso, J. M., Hirayama, T., Roman, G., Nourizadeh, S., and Ecker, J. R. (1999). EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Sci. (1979) 284, 2148–2152. doi: 10.1126/science.284.5423.2148
Amarante, C. V. T., Simioni, A., Megguer, C. A., and Blum, L. E. B. (2002). Effect of aminoethoxyvinilglycine (AVG) on preharvest fruit drop and maturity of apples. Rev. Bras. Frutic 24, 661–664. doi: 10.1590/s0100-29452002000300022
Anbesse Girma, S., Daniel Alemu, G., and Mikiyas Kebede, A. (2022). Review on postharvest quality and handling of apple. Int. J. Agric. Sci. Food Technol. 8, 28–32. doi: 10.17352/2455-815x.000141
Argenta, L. C., Scolaro, A. M. T., Do Amarante, C. V. T., and Vieira, M. J. (2018a). Preharvest treatment of ‘Gala’ apples with 1-MCP and AVG – II: Effects on fruit quality after storage in. Acta Horticulturae 1194, 127–134. doi: 10.17660/ActaHortic.2018.1194.20
Argenta, L. C., Scolaro, A. M. T., Do Amarante, C. V. T., Vieira, M. J., and Werner, S. S. (2018b). Preharvest treatment of ‘Gala’ apples with 1-MCP and AVG – I: Effects on fruit maturation on the tree., in. Acta Horticulturae 1194, 113–120. doi: 10.17660/ActaHortic.2018.1194.18
Argenta, L. C., Wood, R. M., De Angelis Monteiro Terra, F., and Neuwald, D. A. (2022). Effect of preharvest ethylene inhibitor application on ‘Fuji’ apple on-tree maturation and quality after storage. Acta Horticulturae 1344, 219–226. doi: 10.17660/ActaHortic.2022.1344.32
Arseneault, M. H. and Cline, J. A. (2016). A review of apple preharvest fruit drop and practices for horticultural management. Sci. Hortic. 211, 40–52. doi: 10.1016/j.scienta.2016.08.002
Arseneault, M. H. and Cline, J. A. (2017). AVG, NAA, boron, and magnesium influence preharvest fruit drop and fruit quality of ‘Honeycrisp’ apples. Can. J. Plant Sci. 98, 741–752. doi: 10.1139/cjps-2017-0170
Belding, R. D. and Lokaj, G. R. W. (2002). Aminoethoxyvinylglycine treatment of peach fruit reduces ethylene production and softening. HortScience 37, 1065–1068. doi: 10.21273/hortsci.37.7.1065
Binder, B. M. (2020). Ethylene signaling in plants. J. Biol. Chem. 295, 7710–7725. doi: 10.1074/jbc.REV120.010854
Blankenship, S. M. and Richard Unrath, C. (1988). Pal and ethylene content during maturation of red and golden delicious apples. Phytochemistry 27, 1001–1003. doi: 10.1016/0031-9422(88)80260-7
Blanpied, G. D. and Silsby, K. (1992). Predicting harvest date windows for apples. A cornell cooperative extension 221, 1–18. Available online at: https://ecommons.cornell.edu/server/api/core/bitstreams/242b2cd3-40b2-4843-a2dc-bde02d4c2f0d/content.
Bleecker, A. B. (1999). Ethylene perception and signaling: An evolutionary perspective. Trends Plant Sci. 4, 269–274. doi: 10.1016/S1360-1385(99)01427-2
Bleecker, A. and Schaller, E. (1996). The mechanism of ethylene perception. Plant Physiol. 111, 653–660. doi: 10.1104/pp.111.3.653
Boyacı, S. (2022). Effect of aminoethoxyvinylglycine (AVG) applications on pre-harvest drop and fruit quality of ‘Red delicious, red chief’ Apple cultivar. Erwerbs-Obstbau 64, 395–400. doi: 10.1007/s10341-022-00662-1
Brasil, I. M. and Siddiqui, M. W. (2018). Postharvest quality of fruits and vegetables: an overview. Preharvest Modulation Postharvest Fruit Vegetable Quality 1–40. doi: 10.1016/B978-0-12-809807-3.00001-9
Bregoli, A. M., Scaramagli, S., Costa, G., Sabatini, E., Ziosi, V., Biondi, S., et al. (2002). Peach (Prunus persica) fruit ripening: Aminoethoxyvinylglycine (AVG) and exogenous polyamines affect ethylene emission and flesh firmness. Physiol. Plant 114, 472–481. doi: 10.1034/j.1399-3054.2002.1140317.x
Bulens, I., Van de Poel, B., Hertog, M. L. A. T. M., De Proft, M. P., Geeraerd, A. H., and Nicolai, B. M. (2012). Influence of harvest time and 1-MCP application on postharvest ripening and ethylene biosynthesis of “Jonagold” apple. Postharvest Biol. Technol. 72, 11–19. doi: 10.1016/j.postharvbio.2012.05.002
Busatto, N., Tadiello, A., Trainotti, L., and Costa, F. (2017). Climacteric ripening of apple fruit is regulated by transcriptional circuits stimulated by cross-talks between ethylene and auxin. Plant Signal Behav. 12, e1268312. doi: 10.1080/15592324.2016.1268312
Byers, R. E. (1997). Effects of aminoethoxyvinylglycine (AVG) on preharvest fruit drop, maturity, and cracking of several apple cultivars. J. Tree Fruit Production 2, 77–97. doi: 10.1300/J072v02n01_06
Byers, R. E., Carbaugh, D. H., and Combs, L. D. (2005). Ethylene inhibitors delay fruit drop, maturity, and increase fruit size of “Arlet” apples. HortScience 40, 2061–2065. doi: 10.21273/hortsci.40.7.2061
Byers, R. E., Carbaugh, D. H., and Combs, L. D. (2019). 116 preharvest fruit drop studies, harvest quality, and cold storage of `Golden delicious’ and `Rome’ Apples. HortScience 35, 409A–409. doi: 10.21273/hortsci.35.3.409a
Chang, S., Puryear, J., and Cairney, J.. (1993). A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Report 11. doi: 10.1007/BF02670468
Chen, Y., Grimplet, J., David, K., Castellarin, S. D., Terol, J., Wong, D. C. J., et al. (2018). Ethylene receptors and related proteins in climacteric and non-climacteric fruits. Plant Sci. 276, 63–72. doi: 10.1016/j.plantsci.2018.07.012
Cherian, S., Figueroa, C. R., and Nair, H. (2014). Movers and shakers” in the regulation of fruit ripening: A cross-dissection of climacteric versus non-climacteric fruit. J. Exp. Bot. 65, 4705–4722. doi: 10.1093/jxb/eru280
Clayton, M., Biasi, W. V., Southwick, S. M., and Mitcham, E. J. (2000). ReTain™ affects maturity and ripening of `Bartlett’ pear. HortScience 35, 1294–1299. doi: 10.21273/hortsci.35.7.1294
Cocetta, G. and Natalini, A. (2022). Ethylene: Management and breeding for postharvest quality in vegetable crops. A review. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.968315
Crassweller, R., McNew, R., Greene, D., Miller, S., Cline, J., Azarenko, A., et al. (2007). Performance of apple cultivars in the 1999 NE-183 regional project planting. I. Growth and yield characteristics. J. Am. Pomological Soc. 61, 6967–6979. Available online at: https://www.pubhort.org/aps/61/v61_n2_a13.htm.
Dal Cin, V., Danesin, M., Boschetti, A., Dorigoni, A., and Ramina, A. (2005). Ethylene biosynthesis and perception in apple fruitlet abscission (Malus domestica L. Borck). J. Exp. Bot. 56, 2995–3005. doi: 10.1093/jxb/eri296
Dal Cin, V., Danesin, M., Botton, A., Boschetti, A., Dorigoni, A., and Ramina, A. (2008). Ethylene and preharvest drop: The effect of AVG and NAA on fruit abscission in apple (Malus domestica L. Borkh). Plant Growth Regul. 56, 317–325. doi: 10.1007/s10725-008-9312-5
Dal Cin, V., Rizzini, F. M., Botton, A., and Tonutti, P. (2006). The ethylene biosynthetic and signal transduction pathways are differently affected by 1-MCP in apple and peach fruit. Postharvest Biol. Technol. 42, 125–133. doi: 10.1016/j.postharvbio.2006.06.008
do Amarante, C. V. T., Argenta, L. C., de Freitas, S. T., and Steffens, C. A. (2022). Efficiency of pre-harvest application of 1-MCP (Harvista™ 1.3 SC) to delay maturation of ‘Cripps Pink’ apple fruit. Sci. Hortic. 293, 110715. doi: 10.1016/j.scienta.2021.110715
Doerflinger, F. C., Al Shoffe, Y., Sutanto, G., Nock, J. F., and Watkins, C. B. (2024). Preharvest 1-methylcyclopropene (1-MCP) treatment effects on quality of spot and strip picked ‘Gala’ apples at harvest and after storage as affected by postharvest 1-MCP and temperature conditioning treatments. Sci. Hortic. 325, 112682. doi: 10.1016/j.scienta.2023.112682
Doerflinger, F. C., Nock, J. F., Miller, W. B., and Watkins, C. B. (2019). Preharvest aminoethoxyvinylglycine (AVG) and 1-methylcyclopropene (1-MCP) effects on ethylene and starch concentrations of ‘Empire’ and ‘McIntosh’ apples. Sci. Hortic. 244, 134–140. doi: 10.1016/j.scienta.2018.09.031
Dolgikh, V. A., Pukhovaya, E. M., and Zemlyanskaya, E. V. (2019). Shaping ethylene response: the role of EIN3/EIL1 transcription factors. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.01030
Elfving, D. C., Drake, S. R., Reed, A. N., and Visser, D. B. (2007). Preharvest applications of sprayable 1-methylcyclopropene in the orchard for management of apple harvest and postharvest condition. HortScience 42, 1192–1199. doi: 10.21273/hortsci.42.5.1192
El-Sharkawy, I., Jones, B., Li, Z. G., Lelièvre, J. M., Pech, J. C., and Latché, A. (2003). Isolation and characterization of four ethylene perception elements and their expression during ripening in pears (Pyrus communis L.) with/without cold requirement. J. Exp. Bot. 54, 1615–1625. doi: 10.1093/jxb/erg158
El-Sharkawy, I., Kim, W. S., El-Kereamy, A., Jayasankar, S., Svircev, A. M., and Brown, D. C. W. (2007). Isolation and characterization of four ethylene signal transduction elements in plums (Prunus salicina L.). J. Exp. Bot. 58, 3631–3643. doi: 10.1093/jxb/erm213
Escalada, V. S. and Archbold, D. D. (2019). Cultivar variation in response to AVG and heat treatments for sustaining apple fruit quality in cold storage. HortScience 39, 781D–782. doi: 10.21273/hortsci.39.4.781d
Espley, R. V., Hellens, R. P., Putterill, J., Stevenson, D. E., Kutty-Amma, S., and Allan, A. C. (2007). Red colouration in apple fruit is due to the activity of the MYB transcription factor, MdMYB10. Plant J. 49, 414–427. doi: 10.1111/j.1365-313X.2006.02964.x
Estornell, L. H., Agustí, J., Merelo, P., Talón, M., and Tadeo, F. R. (2013). Elucidating mechanisms underlying organ abscission. Plant Sci. 199–200, 48–60. doi: 10.1016/j.plantsci.2012.10.008
Fanyuk, M., Patel, M. K., Ovadia, R., Maurer, D., Feygenberg, O., Oren-Shamir, M., et al. (2022). Preharvest application of phenylalanine induces red color in mango and apple fruit’s skin. Antioxidants 11, 491. doi: 10.3390/antiox11030491
Farag, K. M., Nagy, N. M., Haikal, A. M., and Derhab, S. (2015). Mitigation of ethephon and protone influence while improving “anna” apples coloration, fruit quality and storability by preharvest application of sprayable 1-MCP. J. Agric. Environ. Sci. 14, 1–23. Available online at: https://d1wqtxts1xzle7.cloudfront.net/40324551/apple1-libre.pdf?1448360296=&response-content-disposition=inline%3B+filename%3DMITIGATION_OF_ETHEPHON_AND_PROTONE_INFLU.pdf&Expires=1755097942&Signature=dB7GJ6-3EAYO6-V8bhwbFvvTNYest~FnYZIYpEahOIILA68rr-O-qlHnx3RSRDHBHQOnYb5t6QW7~jRSOTeiOuWKMlzqMMDlgM6wCbN~gccNcy996qUA-VZ6YC5zt5uBq~2AYYa9aTWK5Z0TNeIJW5QZbGIT7aDlczMQf4EUvarksJdGuxmIq2UYPIsNqBiwYkiI8qTGt~VOXMaeftJzYd5W80yGnxLihHT9nSysiVJ~E3YtTxVn2oi6U2FPw3gGDvCp6UTZGj9YCArIAl1BtwLY4FmXe4zYbbEYFS8QbvGMdq0D-FmIw5HBZQBhjaSa0Ncm-nqWW3ICIOCywFwtqg__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA.
Faragher, J. D. and Brohier, R. L. (1984). Anthocyanin accumulation in apple skin during ripening: Regulation by ethylene and phenylalanine ammonia-lyase. Sci. Hortic. 22, 89–96. doi: 10.1016/0304-4238(84)90087-6
Farcuh, M., Copes, B., Le-Navenec, G., Marroquin, J., Cantu, D., Bradford, K. J., et al. (2020). Sensory, physicochemical and volatile compound analysis of short and long shelf-life melon (Cucumis melo L.) genotypes at harvest and after postharvest storage. Food Chem. X 8, 100107. doi: 10.1016/j.fochx.2020.100107
Farcuh, M. and Hopfer, H. (2023). Aroma volatiles as predictors of chilling injury development during peach (Prunus persica (L) Batsch) cold storage and subsequent shelf-life. Postharvest Biol. Technol. 195, 112137. doi: 10.1016/j.postharvbio.2022.112137
Farcuh, M., Rivero, R. M., Sadka, A., and Blumwald, E. (2018). Ethylene regulation of sugar metabolism in climacteric and non-climacteric plums. Postharvest Biol. Technol. 139, 20–30. doi: 10.1016/j.postharvbio.2018.01.012
Farcuh, M., Tajima, H., Lerno, L. A., and Blumwald, E. (2022). Changes in ethylene and sugar metabolism regulate flavonoid composition in climacteric and non-climacteric plums during postharvest storage. Food Chemistry: Mol. Sci. 4, 100075. doi: 10.1016/j.fochms.2022.100075
Farcuh, M., Toubiana, D., Sade, N., Rivero, R. M., Doron-Faigenboim, A., Nambara, E., et al. (2019). Hormone balance in a climacteric plum fruit and its non-climacteric bud mutant during ripening. Plant Sci. 280, 51–65. doi: 10.1016/j.plantsci.2018.11.001
Feng, F., Li, M., Ma, F., and Cheng, L. (2013). Phenylpropanoid metabolites and expression of key genes involved inanthocyanin biosynthesis in the shaded peel of apple fruit in response to sun exposure. Plant Physiol. Biochem. 69, 54–61. doi: 10.1016/j.plaphy.2013.04.020
Ginzberg, I. and Stern, R. A. (2016). Strengthening fruit-skin resistance to growth strain by application of plant growth regulators. Sci. Hortic. 198, 150–153. doi: 10.1016/j.scienta.2015.11.016
Giovannoni, J. J. (2004). Genetic regulation of fruit development and ripening. Plant Cell 16, S170–180. doi: 10.1105/tpc.019158
Greene, D. W. (2005). Time of aminoethoxyvinylglycine application influences preharvest drop and fruit quality of ‘McIntosh’ apples. HortScience 40, 2056–2060. doi: 10.21273/hortsci.40.7.2056
Greene, D. W. and Schupp, J. R. (2004). Effect of aminothoxyyinylglycine (AVG) on preharvest drop, fruit quality, and maturation of “McIntosh” apples. II. Effect of timing and concentration relationships and spray volume. HortScience 39, 1036–1041. doi: 10.21273/hortsci.39.5.1036
He, J., Feng, Y., Cheng, Y., and Guan, J. (2023). Effects of preharvest aminoethoxyvinylglycine (AVG) treatment on fruit ripening, core browning and related gene expression in ‘Huangguan’ Pear (Pyrus bretschneideri rehd.). Horticulturae 9, 179. doi: 10.3390/horticulturae9020179
Hung, D., McConchie, R., and Morris, S. (2010). Pre-harvest treatment with aminoethoxyvinylglycine influences fruit ripening and post-storage quality of benzothiadiazole-treated rockmelon (Cucumis melo L.). Hortic. Environ. Biotechnol. 51, 288–294. Available online at: https://www.cabidigitallibrary.org/doi/pdf/10.5555/20103309725.
Infante, R., Farcuh, M., and Meneses, C. (2008). Monitoring the sensorial quality and aroma through an electronic nose in peaches during cold storage. J. Sci. Food Agric. 88, 2073–2078. doi: 10.1002/jsfa.3316
Jia, D., Li, Z., Dang, Q., Shang, L., Shen, J., Leng, X., et al. (2020). Anthocyanin biosynthesis and methylation of the mdMYB10 promoter are associated with the red blushed-skin mutant in the red striped-skin “Changfu 2” apple. J. Agric. Food Chem. 68, 4292–4304. doi: 10.1021/acs.jafc.9b07098
Johnson, E. and Farcuh, M. (2024). Aminoethoxyvinylglicine and 1-methylcyclopropene: effects on preharvest drop, fruit maturity, quality, and associated gene expression of “Honeycrisp” Apples in the US mid-atlantic. Plants (Basel) 13, 2524. doi: 10.3390/plants13172524
Johnston, J. W., Gunaseelan, K., Pidakala, P., Wang, M., and Schaffer, R. J. (2009). Co-ordination of early and late ripening events in apples is regulated through differential sensitivities to ethylene. J. Exp. Bot. 60, 2689–2699. doi: 10.1093/jxb/erp122
Kader, A. A. (2022). Ethylene-induced senescence and physiological disorders in harvested horticultural crops. HortScience 20, 54–57. doi: 10.21273/hortsci.20.1.54
Kasai, S., Hayama, H., Kashimura, Y., Kudo, S., and Osanai, Y. (2008). Relationship between fruit cracking and expression of the expansin gene MdEXPA3 in “Fuji” apples (Malus domestica Borkh.). Sci. Hortic. 116, 194–198. doi: 10.1016/j.scienta.2007.12.002
Kevany, B. M., Tieman, D. M., Taylor, M. G., Cin, V. D., and Klee, H. J. (2007). Ethylene receptor degradation controls the timing of ripening in tomato fruit. Plant J. 51, 458–467. doi: 10.1111/j.1365-313X.2007.03170.x
Khadivi-Khub, A. (2015). Physiological and genetic factors influencing fruit cracking. Acta Physiol. Plant 37, 1718. doi: 10.1007/s11738-014-1718-2
Kieber, J. J., Rothenberg, M., Roman, G., Feldmann, K. A., and Ecker, J. R. (1993). CTR1, a negative regulator of the ethylene response pathway in arabidopsis, encodes a member of the Raf family of protein kinases. Cell 72, 427–441. doi: 10.1016/0092-8674(93)90119-B
Kim, H. Y., Farcuh, M., Cohen, Y., Crisosto, C., Sadka, A., and Blumwald, E. (2015a). Non-climacteric ripening and sorbitol homeostasis in plum fruits. Plant Sci. 231, 30–39. doi: 10.1016/j.plantsci.2014.11.002
Kim, H. Y., Saha, P., Farcuh, M., Li, B., Sadka, A., and Blumwald, E. (2015b). RNA-seq analysis of spatiotemporal gene expression patterns during fruit development revealed reference genes for transcript normalization in plums. Plant Mol. Biol. Rep. 33, 1634–1649. doi: 10.1007/s11105-015-0860-3
Klee, H. J. (2002). Control of ethylene-mediated processes in tomato at the level of receptors. J. Exp. Bot. 53, 2057–2063. doi: 10.1093/jxb/erf062
Klee, H. J. (2004). Ethylene signal transduction. Moving beyond arabidopsis. Plant Physiol. 135, 660–667. doi: 10.1104/pp.104.040998
Klee, H. J. and Giovannoni, J. J. (2011). Genetics and control of tomato fruit ripening and quality attributes. Annu. Rev. Genet. 45, 41–59. doi: 10.1146/annurev-genet-110410-132507
Kumar, R., Khurana, A., and Sharma, A. K. (2014). Role of plant hormones and their interplay in development and ripening of fleshy fruits. J. Exp. Bot. 65, 4561–4575. doi: 10.1093/jxb/eru277
Kvikliene, N., Kviklys, D., Valiuškaite, A., Viskelis, P., Uselis, N., Lanauskas, J., et al. (2011). Effect of harvest date on fruit maturity, quality and storability of “Lodel. apples. J. Food Agric. Environ. 9, 210–213.
La Spada, P., Dominguez, E., Continella, A., Heredia, A., and Gentile, A. (2024). Factors influencing fruit cracking: an environmental and agronomic perspective. Front. Plant Sci. 15. doi: 10.3389/fpls.2024.1343452
Layne, D. R., Jiang, Z., and Rushing, J. W. (2002). The influence of reflective film and ReTain on red skin coloration and maturity of “Gala” apples. Horttechnology 12, 640–645. doi: 10.21273/horttech.12.4.640
Leclercq, J., Adams-Phillips, L. C., Zegzouti, H., Jones, B., Latché, A., Giovannoni, J. J., et al. (2002). LeCTR1, a tomato CTR1-like gene, demonstrates ethylene signaling ability in arabidopsis and novel expression patterns in tomato. Plant Physiol. 130, 1132–1142. doi: 10.1104/pp.009415
Lee, J., Mattheis, J. P., and Rudell, D. R. (2016). Storage temperature and 1-methylcyclopropene treatment affect storage disorders and physiological attributes of ‘Royal Gala’ apples. HortScience 51, 84–93. doi: 10.21273/hortsci.51.1.84
Lee, S., Ross, G., and Gardener, R. (1998). An apple homolog of the ethylene receptor gene ETR1 (Accession no. AF 032448). Plant Physiol. 117, 1126.
Li, T., Jiang, Z., Zhang, L., Tan, D., Wei, Y., Yuan, H., et al. (2016). Apple (Malus domestica) MdERF2 negatively affects ethylene biosynthesis during fruit ripening by suppressing MdACS1 transcription. Plant J. 88, 735–748. doi: 10.1111/tpj.13289
Li, H., Liu, G., Tian, H., and Fu, D. (2021). Fruit cracking: a review. Shengwu Gongcheng Xuebao/Chinese J. Biotechnol. 37, 2737–2752. doi: 10.13345/j.cjb.200553
Li, C., Yu, W., Xu, J., Lu, X., and Liu, Y. (2022). Anthocyanin biosynthesis induced by MYB transcription factors in plants. Int. J. Mol. Sci. 23, 11701. doi: 10.3390/ijms231911701
Li, J. and Yuan, R. (2008). NAA and ethylene regulate expression of genes related to ethylene biosynthesis, perception, and cell wall degradation during fruit abscission and ripening in “delicious” apples. J. Plant Growth Regul. 27, 283–295. doi: 10.1007/s00344-008-9055-6
Li, J., Zhu, H., and Yuan, R. (2010). Profiling the expression of genes related to ethylene biosynthesis, ethylene perception, and cell wall degradation during fruit abscission and fruit ripening in apple. J. Am. Soc. Hortic. Sci. 135, 391–401. doi: 10.21273/jashs.135.5.391
Liu, J., Islam, M. T., and Sherif, S. M. (2022). Effects of aminoethoxyvinylglycine (AVG) and 1-methylcyclopropene (1-MCP) on the pre-harvest drop rate, fruit quality, and stem-end splitting in ‘Gala’ Apples. Horticulturae 8, 1100. doi: 10.3390/horticulturae8121100
Liu, H., Liu, Z., Wu, Y., Zheng, L., and Zhang, G. (2021). Regulatory mechanisms of anthocyanin biosynthesis in apple and pear. Int. J. Mol. Sci. 22, 8441. doi: 10.3390/ijms22168441
Liu, M., Pirrello, J., CHERVIN, C., Roustan, J.-P., and Bouzayen, M. (2015). Ethylene control of fruit ripening: revisiting the complex network of transcriptional regulation. Plant Physiol. pp, 2380–2390. doi: 10.1104/pp.15.01361
Liu, Y., Tang, M., Liu, M., Su, D., Chen, J., Gao, Y., et al. (2020). The molecular regulation of ethylene in fruit ripening. Small Methods 4, 1900485. doi: 10.1002/smtd.201900485
Livak, K. J. and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25. doi: 10.1006/meth.2001.1262
Ma, Y., Ma, X., Gao, X., Wu, W., and Zhou, B. (2021). Light induced regulation pathway of anthocyanin biosynthesis in plants. Int. J. Mol. Sci. 22, 11116. doi: 10.3390/ijms222011116
Malladi, A., Tonapi, K. V., and Kon, T. M. (2023). Aminoethoxyvinylglycine reduces preharvest fruit drop and fruit ethylene evolution in ‘Red delicious’ Apple but affects fruit size and quality inconsistently. HortScience 58, 1410–1417. doi: 10.21273/HORTSCI17287-23
McArtney, S. J., Obermiller, J. D., Schupp, J. R., Parker, M. L., and Edgington, T. B. (2008). Preharvest 1-methylcyclopropene delays fruit maturity and reduces softening and superficial scald of apples during long-term storage. HortScience 43, 366–371. doi: 10.21273/hortsci.43.2.366
Miah, M. S. and Farcuh, M. (2024a). Combining the use of reflective groundcovers and aminoethoxyvinylglycine to assess effects on skin color, preharvest drop, and quality of ‘Honeycrisp’ Apples in the mid-atlantic US. Horticulturae 10, 179. doi: 10.3390/horticulturae10020179
Miah, M. S. and Farcuh, M. (2024b). Reflective groundcovers promote anthocyanin content and advance fruit maturity of ‘Evercrisp’ apples grown in the Mid-Atlantic US. Front. Plant Sci. 15. doi: 10.3389/fpls.2024.1478498
Miah, M. S. and Farcuh, M. (2024c). The expression of key ethylene and anthocyanin biosynthetic genes of ‘Honeycrisp’ Apples subjected to the combined use of reflective groundcovers and aminoethoxyvinylglycine in the mid-atlantic US. Plants 13, 1141. doi: 10.3390/plants13081141
Miah, M. S., Hinson, C., and Farcuh, M. (2023). Assessing fruit maturity and quality of ‘Buckeye gala’ Grown on a diverse panel of apple (Malus domestica borkh.) rootstocks in western maryland. Agronomy 13, 2528. doi: 10.3390/agronomy13102528
Michailidis, M., Karagiannis, E., Bazakos, C., Tanou, G., Ganopoulos, I., and Molassiotis, A. (2021). Genotype- and tissue-specific metabolic networks and hub genes involved in water-induced distinct sweet cherry fruit cracking phenotypes. Comput. Struct. Biotechnol. J. 19, 5406–5420. doi: 10.1016/j.csbj.2021.09.030
Onik, J. C., Hu, X., Lin, Q., and Wang, Z. (2018). Comparative transcriptomic profiling to understand pre- and post-ripening hormonal regulations and anthocyanin biosynthesis in early ripening apple fruit. Molecules 23, 1908. doi: 10.3390/molecules23081908
Opara, L. U., Hodson, A. J., and Studman, C. J. (2000). Stem-end splitting and internal ring-cracking of “Gala” apples as influenced by orchard management practices. J. Hortic. Sci. Biotechnol. 75, 465–469. doi: 10.1080/14620316.2000.11511270
Opara, L. U., Studman, C. J., and Banks, N. H. (1997). Sunlight affects the incidence of internal ring cracking and other physical attributes of ‘Gala’ apples. J. Tree Fruit Production 2, 45–52. doi: 10.1300/J072v02n01_04
Ozturk, B., Yildiz, K., Ozkan, Y., and Kilic, K. (2015). Effects of aminoethoxyvinylglycine treatments on pre-harvest fruit drop and fruit quality of Braeburn apples. Bangladesh J. Bot. 44, 299–307. doi: 10.3329/bjb.v44i2.38520
Peng, Z. and Fu, D. (2023). Effects of 1-methylcyclopropene treatment on the quality of red “Fuji” apples fruit during short-term storage. Food Qual. Saf. 7, fyac074. doi: 10.1093/fqsafe/fyac074
Petri, J. L., Hawerroth, F. J., Leite, G. B., and Couto, M. (2011). Concentração e época de aplicação de aminoetoxivinilglicine (AVG) na maturação de macieiras “Fuji Suprema. Rev. Bras. Frutic 33, 335–344. doi: 10.1590/S0100-29452011005000070
Sakaldas, M. and Gundogdu, M. A. (2016). The effects of preharvest 1-methylcyclopropene (Harvista) treatments on harvest maturity of “Golden Delicious” apple cultivar., in. Acta Horticulturae 1139, 601–608. doi: 10.17660/ActaHortic.2016.1139.104
Santos, M., Egea-Cortines, M., Gonçalves, B., and Matos, M. (2023). Molecular mechanisms involved in fruit cracking: A review. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1130857
Schupp, J. R. and Greene, D. W. (2004). Effect of aminoethoxyvinylglycine (AVG) on preharvest drop, fruit quality, and maturation of “McIntosh” apples. I. Concentration and timing of dilute applications of AVG. HortScience 39, 1030–1035. doi: 10.21273/hortsci.39.5.1030
Scolaro, A. M. T., Argenta, L. C., Do Amarante, C. V. T., Petri, J. L., and Hawerroth, F. J. (2015). Preharvest control of ‘Royal Gala’ apple fruit maturation by the inhibition of ethylene action or synthesis. Rev. Bras. Frutic 37, 38–47. doi: 10.1590/0100-2945-010/14
Shafiq, M., Singh, Z., and Khan, A. S. (2014). Pre-harvest ethephon application and training systems affect colour development, accumulation of flavonoids and fruit quality of “Cripps Pink” apple. Aust. J. Crop Sci. 8, 093006.
Shi, C., Liu, L., Wei, Z., Liu, J., Li, M., Yan, Z., et al. (2022). Anthocyanin accumulation and molecular analysis of correlated genes by metabolomics and transcriptomics in sister line apple cultivars. Life 12, 1246. doi: 10.3390/life12081246
Sisler, E. C. and Serek, M. (1997). Inhibitors of ethylene responses in plants at the receptor level: Recent developments. Physiol. Plant 100, 577–582. doi: 10.1034/j.1399-3054.1997.1000320.x
Solano, R., Stepanova, A., Chao, Q., and Ecker, J. R. (1998). Nuclear events in ethylene signaling: A transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev. 12, 3703–3714. doi: 10.1101/gad.12.23.3703
Song, C., Zhang, D., Zhang, J., Zheng, L., Zhao, C., Ma, J., et al. (2016). Expression analysis of key auxin synthesis, transport, and metabolism genes in different young dwarfing apple trees. Acta Physiol. Plant 38, 43. doi: 10.1007/s11738-016-2065-2
Sunil, L. and Shetty, N. P. (2022). Biosynthesis and regulation of anthocyanin pathway genes. Appl. Microbiol. Biotechnol. 106, 1783–1798. doi: 10.1007/s00253-022-11835-z
Tatsuki, M., Endo, A., and Ohkawa, H. (2007). Influence of time from harvest to 1-MCP treatment on apple fruit quality and expression of genes for ethylene biosynthesis enzymes and ethylene receptors. Postharvest Biol. Technol. 43, 28–35. doi: 10.1016/j.postharvbio.2006.08.010
Toivonen, P. M. A. (2015). Comparison of IAD and starch-iodine indices at harvestand how they relate to post-storage firmness retentionin ambrosiaTM apples over three growing seasons. Can. J. Plant Sci. 95, 1177–1180. doi: 10.4141/CJPS-2015-185
Tomala, K., Grzęda, M., Guzek, D., Głąbska, D., and Gutkowska, K. (2020b). The effects of preharvest 1-methylcyclopropene (1-MCP) treatment on the fruit quality parameters of cold-stored ‘szampion’ cultivar apples. Agric. (Switzerland) 10, 80. doi: 10.3390/agriculture10030080
Tomala, K., Grzeda, M., Guzek, D., Głabska, D., and Gutkowska, K. (2020a). Analysis of possibility to apply preharvest 1-methylcyclopropene (1-MCP) treatment to delay harvesting of red Jonaprince apples. Sustainability (Switzerland) 12, 4575. doi: 10.3390/su12114575
Tong, C. B. S., Bedford, D. S., Luby, J. J., Propsom, F. M., Beaudry, R. M., Mattheis, J. P., et al. (2003). Location and temperature effects on soft scald in ‘Honeycrisp’ apples. HortScience 38, 1153–1155. doi: 10.21273/hortsci.38.6.1153
Unrath, C. R., Obermiller, J. D., Green, A., and McArtney, S. J. (2023). The effects of aminoethoxyvinylglycine and naphthaleneacetic acid treatments on abscission and firmness of ‘Scarletspur delicious’ Apples at normal and delayed harvests. Horttechnology 19, 620–625. doi: 10.21273/horttech.19.3.620
USDA (2019). United states standards for grades of apples (Washington, D.C., USA: USDA). Available online at: https://www.ams.usda.gov/sites/default/files/media/Apple_Standards.pdf.
Wang, Z. and Dilley, D. R. (2001). Aminoethoxyvinylglycine, combined with ethephon, can enhance red color development without over-ripening apples. HortScience 36, 328–331. doi: 10.21273/hortsci.36.2.328
Wang, S. Y. and Faust, M. (2019). Ethylene biosynthesis and polyamine accumulation in apples with watercore. J. Am. Soc. Hortic. Sci. 117, 133–138. doi: 10.21273/jashs.117.1.133
Wang, J. G., Gao, X. M., Ma, Z. L., Chen, J., Liu, Y. N., and Shi, W. Q. (2019). Metabolomic and transcriptomic profiling of three types of litchi pericarps reveals that changes in the hormone balance constitute the molecular basis of the fruit cracking susceptibility of Litchi chinensis cv. Baitangying. Mol. Biol. Rep. 46, 154–162. doi: 10.1007/s11033-019-04986-2
Wang, M. M., Li, T. X., Wu, Y., Song, S. W., Bai, T. H., Jiao, J., et al. (2021). Genome-wide identification of microRNAs involved in the regulation of fruit ripening in apple (Malus domestica). Sci. Hortic. 289, 110416. doi: 10.1016/j.scienta.2021.110416
Wang, S., Li, L. X., Zhang, Z., Fang, Y., Li, D., Chen, X., et al. (2022). Ethylene precisely regulates anthocyanin synthesis in apple via a module comprising MdEIL1, MdMYB1, and MdMYB17. Hortic. Res. 9, uhac034. doi: 10.1093/hr/uhac034
Warner, G. (2025). Ambrosia restrictions to end (Yakima, Washington, USA: Good Fruit Grower). Available online at: https://www.goodfruit.com/ambrosia-restrictions-to-end/.
Whale, S. K. and Singh, Z. (2007). Endogenous ethylene and color development in the skin of “Pink Lady” apple. J. Am. Soc. Hortic. Sci. 132, 20–28. doi: 10.21273/jashs.132.1.20
Whale, S. K., Singh, Z., Behboudian, M. H., Janes, J., and Dhaliwal, S. S. (2008). Fruit quality in “Cripp’s Pink” apple, especially colour, as affected by preharvest sprays of aminoethoxyvinylglycine and ethephon. Sci. Hortic. 115, 342–351. doi: 10.1016/j.scienta.2007.10.015
Wiersma, P. A., Zhang, H., Lu, C., Quail, A., and Toivonen, P. M. A. (2007). Survey of the expression of genes for ethylene synthesis and perception during maturation and ripening of “Sunrise” and “Golden Delicious” apple fruit. Postharvest Biol. Technol. 44, 204–211. doi: 10.1016/j.postharvbio.2006.12.016
Wu, M., Luo, Z., and Cao, S. (2023). Promoter variation of the key apple fruit texture related gene mdPG1 and the upstream regulation analysis. Plants 12, 1452. doi: 10.3390/plants12071452
Xie, X., Einhorn, T., and Wang, Y. (2015). Inhibition of ethylene biosynthesis and associated gene expression by aminoethoxyvinylglycine and 1-methylcyclopropene and their consequences on eating quality and internal browning of ‘Starkrimson’ pears. J. Am. Soc. Hortic. Sci. 140, 587–596. doi: 10.21273/jashs.140.6.587
Yang, S. F. and Hoffman, N. E. (1984). Ethylene biosynthesis and its regulation in higher plants. Annu. Rev. Plant Physiol. 35, 155–189. doi: 10.1146/annurev.pp.35.060184.001103
Yang, X., Song, J., Campbell-Palmer, L., Fillmore, S., and Zhang, Z. (2013). Effect of ethylene and 1-MCP on expression of genes involved in ethylene biosynthesis and perception during ripening of apple fruit. Postharvest Biol. Technol. 78, 55–66. doi: 10.1016/j.postharvbio.2012.11.012
Yuan, R. and Carbaugh, D. H. (2007). Effects of NAA, AVG, and 1-MCP on ethylene biosynthesis, preharvest fruit drop, fruit maturity, and quality of “Golden Supreme” and “Golden Delicious” apples. HortScience 42, 101–105. doi: 10.21273/hortsci.42.1.101
Yuan, R. and Li, J. (2008). Effect of sprayable 1-MCP, AVG, and NAA on ethylene biosynthesis, preharvest fruit drop, fruit maturity, and quality of “delicious” apples. HortScience 43, 1454–1460. doi: 10.21273/hortsci.43.5.1454
Zhang, J., Xu, H., Wang, N., Jiang, S., Fang, H., Zhang, Z., et al. (2018). The ethylene response factor MdERF1B regulates anthocyanin and proanthocyanidin biosynthesis in apple. Plant Mol. Biol. 98, 205–218. doi: 10.1007/s11103-018-0770-5
Keywords: plant growth regulators, fruit drop, cracking, ethylene, apples, gene expression
Citation: Johnson E and Farcuh M (2025) Assessing the impacts of aminoethoxyvinylglycine and 1-methylcyclopropene on fruit drop, cracking, quality, and related transcript accumulation in ‘Ambrosia’ and ‘Fuji’ apples during on-the-tree ripening. Front. Plant Sci. 16:1629445. doi: 10.3389/fpls.2025.1629445
Received: 16 May 2025; Accepted: 29 July 2025;
Published: 22 August 2025.
Edited by:
Agata Kućko, Warsaw University of Life Sciences, PolandReviewed by:
Anish Malladi, University of Georgia, United StatesThabiso Kenneth Satekge, University of Limpopo, South Africa
Copyright © 2025 Johnson and Farcuh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Macarena Farcuh, bWZhcmN1aEB1bWQuZWR1
 Emily Johnson
Emily Johnson Macarena Farcuh
Macarena Farcuh