- Tropical Agriculture Research Front, Japan International Research Center for Agricultural Sciences, Ishigaki, Okinawa, Japan
Introduction: Sugarcane (Saccharum spp.) is often grown under unstable rainfall and drought conditions, highlighting the need for improved drought tolerance. Erianthus arundinaceus, a closely related species, shows high intrinsic water use efficiency (iWUE) and robust root formation capacity. However, research on improving sugarcane leaf traits using Erianthus is limited. This study aimed to evaluate the water use efficiency and associated leaf traits of sugarcane × Erianthus intergeneric F1 hybrids and their parental genotypes under both wet and dry pot conditions in a greenhouse to assess the potential for improving drought tolerance through intergeneric hybridization.
Methods: The sugarcane cultivars (drought-susceptible NiF8 and drought-tolerant Ni9), Erianthus accessions (JIRCAS1 and JW630), and their intergeneric F1 hybrids (NiF8 × JIRCAS1 and NiF8 × JW630) were evaluated for gas exchange and leaf morphology.
Results: Erianthus accessions had superior stomatal responses, lower stomatal conductance, and higher iWUE than NiF8, with JW630 showing higher iWUE than Ni9. However, Erianthus accessions had lower gravimetric water use efficiencies (gWUE) than the sugarcane cultivars, likely due to the higher leaf area ratio (LAR). The hybrids displayed higher iWUE, with dry matter partitioning characteristics resembling those of sugarcane (low LAR, high shoot/root ratio, and high partitioning to the stem), suggesting potential for higher gWUE under field canopy conditions. The high iWUEs of Erianthus and F1 hybrids were suggested to be attributed to the fewer stomata on the abaxial surface.
Conclusion: This study highlights Erianthus’s potential in improving leaf characteristics to enhance sugarcane drought tolerance because the hybrids demonstrated “best of both worlds” scenario, where they inherited high iWUE from Erianthus with favorable biomass partitioning characteristics from sugarcane.
1 Introduction
Water deficits or droughts are primary climatic factors that constraint global sugarcane (Saccharum spp.) production, regardless of whether the final product is sugar or biomass. Drought stress impairs key physiological functions, including photosynthesis and associated enzymatic activities (Du et al., 1996; Dinh et al., 2017; Marchiori et al., 2017; Zhao et al., 2017), leading to reduced biomass production and lower final yield in sugarcane (Robertson et al., 1999; Basnayake et al., 2012, 2015). Therefore, enhancing water use efficiency (WUE)—crop productivity per amount of water resources applied to the field or used by the plant—is crucial for optimizing the yield and profitability of sugarcane production under both rainfed and irrigated conditions (Basnayake et al., 2012; Natarajan et al., 2020), which also could save water resource. Yield stability under variable water conditions can be achieved through improved breeding strategies and effective crop management (Ferreira et al., 2017; Singels et al., 2019; Dlamini, 2021; Watanabe et al., 2021). However, despite advanced crop management techniques, variety selection remains essential, as varieties are typically classified as either drought-tolerant or drought-susceptible (Inman-Bamber and Stead, 1990). While the development of new drought-tolerant varieties through breeding is considered the most effective strategy for offering growers viable options, the limited availability of such varieties in drought-prone regions and cropping seasons suggests that breeding and selection efforts are currently insufficient in terms of efficiency (Acreche, 2017). This is likely due to the difficulty in evaluating the impact of environmental factors on WUE, as the sugarcane growth period is long and the effects of these factors are substantial and complex (Basnayake et al., 2012; Liu et al., 2016; Natarajan et al., 2020). Therefore, scaling down WUE along both the time/phenology axis and the size axis is considered effective to help understand the physiological mechanisms of WUE. Intrinsic WUE (iWUE), also known as transpiration efficiency, which is the ratio of individual leaf photosynthetic rate (A) to its transpiration indicator, namely stomatal conductance (gs), is recognized as the minimum unit of WUE and has been proposed as an important target for crop breeding (Condon et al., 2004). Besides a ratio of A and gs, stomatal responsiveness, indicated by A vs. gs regression, is also important to consider determinants for WUE (Battle et al., 2024).
The narrow genetic base of previous sugarcane cultivars, with several specific genotypes in their pedigrees, is a major limitation for enhancing yield and stress tolerance (Wei and & Jackson, 2016; Jackson, 2019). Erianthus, a genus within the closely related Saccharum complex, is considered a promising genetic resource for sugarcane improvement, having played a key role in the establishment of sugarcane species (Jackson and Henry, 2011). Among species in Saccharum complex such as S. spontaneum and Miscanthus, Erianthus has been reported to exhibit exceptional tolerance to wider range of biotic and abiotic stresses, including nematodes (Bhuiyan et al., 2014, 2016), drought (Matsuo et al., 2002; Augustine et al., 2015; Manoj et al., 2019), soil acidity (Matsuo et al., 2002; Takaragawa et al., 2023), and salt (Manoj et al., 2019). Although limited agronomic studies have focused on its vigorous growth and stress tolerance (Jackson and Henry, 2011), the morphological and physiological traits contributing to its resilience are becoming increasingly understood. The robust growth of Erianthus is often linked to its high root-forming capacity (Matsuo et al., 2002; Takaragawa et al., 2022; Terajima et al., 2023). However, both above-ground and below-ground characteristics contribute to its drought tolerance. For instance, Erianthus exhibits higher iWUE, as indicated by the gas exchange properties of individual leaves under well-watered conditions, compared to commercial sugarcane cultivars, even in a limited root zone under pot culture (Jackson et al., 2016; Li et al., 2017). Furthermore, Erianthus showed higher iWUE than sugarcane when grown in pots under both wet and dry soil conditions, attributed to factors such as lower stomatal density on the abaxial surface of the leaves and the accumulation of specific leaf metabolites, including betaine and GABA (Takaragawa and Wakayama, 2024). Erianthus has been used for intergeneric hybridization to improve sugarcane productivity traits, with reported gains in biomass productivity (Nair et al., 2017; Pachakkil et al., 2019; Meena et al., 2020) and root system characteristics (Fukuhara et al., 2013; Bhuiyan et al., 2016; Takaragawa et al., 2022; Terajima et al., 2023). However, reports on the improvement of sugarcane leaf traits using Erianthus are limited.
Closely related genetic resources other than Erianthus have been used to improve gas exchange characteristics and stomatal morphology in sugarcane. Hybridization with S. officinarum and S. spontaneum improved leaf morphology, including stomatal distribution and leaf width, and photosynthetic characteristics of the interspecific hybrid F1 (Rao, 1951; Irvine, 1975). Additionally, interspecific hybridization between commercial sugarcane cultivars and S. spontaneum has led to improvement in leaf anatomical characteristics such as leaf thickness and cellular arrangement (Jumkudling et al., 2022). Furthermore, intergeneric hybrids between sugarcane cultivars and Miscanthus germplasm have demonstrated improved photosynthetic capacity at low temperatures (Glowacka et al., 2016; Kar et al., 2019, 2020). Investigation of leaf traits related to drought tolerance in Erianthus using back cross (BC)1F1 lines of S. officinarum and E. arundinaceus suggested potential improvements in metabolites such as proline and several enzymes through intergeneric hybridization (Deng et al., 2019). However, the benefits of intergeneric hybridizations could not be demonstrated due to the low composition of Erianthus-derived genes owing to the extensive backcrossing to sugarcane. Moreover, the authors compared the hybrid lines with major sugarcane cultivars rather than with the parental genotypes of sugarcane and Erianthus. The gas exchange characteristics of sugarcane cultivars and interspecific/intergeneric hybrids have been compared, but without using parental genotypes as reference controls (Jackson et al., 2016; Li et al., 2017). Therefore, a more comprehensive evaluation of the intergeneric hybrid F1, including both parental genotypes as comparators, is necessary to assess the potential for introducing the superior leaf traits of Erianthus into sugarcane. Additionally, no studies have examined the response of leaf characteristics such as physiological and anatomical traits under soil drying conditions in intergeneric hybrids with Erianthus.
Therefore, in this study, we aimed to investigate the water use efficiency and associated leaf characteristics of sugarcane × Erianthus intergeneric F1 hybrids and their parental genotypes under both wet and dry pot conditions in a greenhouse to assess the potential for improving drought tolerance through intergeneric hybridization.
2 Materials and methods
2.1 Plant materials and treatments
The sugarcane cultivars NiF8 (drought-susceptible) and Ni9 (drought-tolerant); Erianthus accessions JIRCAS1 (unknown origin) and JW630 (collected in Shizuoka, Japan); and their intergeneric hybrids F1 J08-12 (NiF8 × JIRCAS1) and J16-77 (NiF8 × JW630) were included in the study. The hybrids J08–12 and J16–77 were confirmed true intergeneric hybrids using PCR-based simple sequence repeat (SSR) markers (D'Hont et al., 1995) and nuclear DNA content by flow cytometry assays (Pachakkil et al., 2019) (Supplementary Figure S1). Two Erianthus accessions are known to belong to genetically distinct groups (Tsuruta et al., 2012, 2017) and both show robust root system in the field (Terajima et al., 2023). The plants were grown in a temperature- and humidity-controlled glasshouse at the Tropical Agriculture Research Front, Japan International Research Center for Agricultural Sciences (24°22'43" N, 124°11'4" E). The day temperature was maintained at 31°C from 7:00 am to 7:00 pm, while the night temperature was set at 27°C; the relative humidity was maintained at 60% (Supplementary Figure S2). The daily cumulative solar radiation in the greenhouse during the growing season averaged 12.4 ± 5.1 mol m-2 day-1.
The pot experiment was performed with a two-factorial design examining sugarcane genotypes and soil moisture conditions (6 genotypes × 2 water regimes). Seedlings of single-bud setts were raised in containers filled with potting mix (Minori, JA Okinawa, Okinawa, Japan) from May 14 (for Erianthus) and June 2 (for sugarcane and intergeneric hybrids) 2021. Due to the slower germination and initial growth of Erianthus, their seedlings were germinated approximately two weeks earlier to synchronize with the growth stage of the other plants. On July 19, 2021, 12 plants of each genotype were transplanted into 1/2000a Wagner pots filled with 10 kg of FW potting mix. Fertilization was performed at transplantation using a solid slow-release fertilizer with a nutrient ratio of N:P:K = 2.4:0.7:1.0 g pot-1. To reduce evaporation, cobble gravel was spread at a depth of 2 cm on the soil surface, as described in Jackson et al. (2016). Irrigation was initially provided three times daily using an automatic drip system until irrigation control began. On August 18, 2021, drainage was stopped using rubber plugs, and manual irrigation control was implemented. Irrigation was controlled according to Dinh et al. (2017) by reading the volumetric water content (VWC) at 8:00 am using a soil moisture sensor (EC-5, Meter) placed at a soil depth of 13 cm (center of the pot) and estimating the water consumption per pot from the previously obtained soil bulk density. Beginning August 25, 2021, the soil pF value was estimated from the VWC (Supplementary Figure S4) using the moisture characteristic curve of the test soil obtained earlier (Supplementary Figure S3). Two treatments were applied: a wet treatment where the soil was irrigated to a well-watered condition (0.445 m3 m-3; pF 1.4), and a dry treatment, where irrigation was gradually reduced by approximately 1% until reaching the permanent wilt point (0.131 m3 m-3; pF 4.2). The pots were randomly placed with four replicates per treatment.
2.2 Measurement of gas exchange parameters
The gas exchange parameters—photosynthetic rate (A), stomatal conductance (gs), transpiration rate (E), and intercellular CO2 concentration (Ci)—of the uppermost fully expanded leaves were measured using a portable gas exchange measurement device (LI-6400, LI-COR BioSciences, Lincoln, Nebraska, USA) on August 24 (prior to the start of the irrigation treatment), and on September 6, 16, 23, and October 1 during the treatment period in 2021. A 6-cm2 (2 cm × 3 cm) LED chamber (LI-6400B, LI-COR) was used, with two light intensity levels: unsaturated (500 µmol m-2 s-1) and saturated (2000 µmol m-2 s-1) photosynthetic photon flux density (PPFD). Light curves previously measured for NiF8 and JW630 confirmed no difference between the two species regarding light saturation and unsaturation (Takaragawa and Matsuda, 2023). The flow rate and reference CO2 concentration were set to 400 µmol s-1 and 400 µmol mol-1, respectively. Leaf temperature was maintained at 30.9 ± 1.0°C via a block temperature set at 30°C. Leaf vapor pressure deficit (VPD) was manually controlled at 1.9 ± 0.2 kPa using a desiccant bulb filled with Drierite® (W. A. Hammond Drierite Co., Xenia, OH, USA). iWUE was calculated from the obtained A and gs, using the equation:
The choice of gs to calculate gas exchange water use efficiency is based on its role as a transpiration index that accounts for VPD. This approach is easier and equitable, facilitating comparison across studies. In contrast to using transpiration rate or photosynthetic water use efficiency (A/E), gs provides a more consistent and fairer metric for comparison with other literatures (Jackson et al., 2016; Li et al., 2017; Nakabaru et al., 2020).
2.3 Survey of leaf anatomical features
A thin layer of nail polish was applied to both sides of the leaf used for gas exchange measurements, and stomatal samples were collected using double-sided tape (Wu and Zhao, 2017; Takaragawa and Wakayama, 2024). Cross-sections of the tested leaves were prepared manually and fixed onto glass slides to measure the interveinal distance—defined as the distance between vascular bundles. Observations were made using an optical microscope system (Eclipse E800, Nikon, Tokyo, Japan) equipped with image analysis software (NIS-elements, Nikon).
2.4 Evaluation of plant growth
At the beginning of the treatment, four plants per genotype were harvested, with all remaining plants harvested 49 days after treatment (on October 13, 2021). The culm length of the main stem, total leaf area, and dry matter weight of each organ were recorded. Leaf area was measured using a leaf area meter (LI-3100, LI-COR). The rate of main-stem elongation during the treatment period was calculated based on the culm length before and after treatment. Underground parts were washed to remove soil and separated into roots and underground stems (stubbles). The underground stem weight was included in the aboveground weight. The leaf area ratio (LAR) during the treatment period was calculated using the leaf area (L1, L2) and total dry matter weight (W1, W2) measurements taken before and after the treatment, according to the following equation:
The water use efficiency of biomass production, defined as gravimetric WUE (gWUE), was calculated by dividing the increment in dry matter (ΔDW) by the water consumed (ΔWU) during the treatment period, using the following equation (Dinh et al., 2017):
Total nitrogen content of each plant part was analyzed using an NC analyzer (NC22F; Sumika Chemical Analysis Service, Ltd., Osaka, Japan) to calculate the nitrogen uptake (ΔNU) during the treatment period, and the nitrogen use efficiency (NUE) was calculated using the following equation:
2.5 Statistical analysis
Data analysis was conducted using the Bell Curve for Excel statistical analysis software (Social Survey Research Information Co., Ltd., Tokyo, Japan). A two-way factorial analysis of variance (ANOVA) was performed to assess the effects of genotype (six genotypes), water regime (two water regimes), and their interactions on leaf anatomical and dry matter parameters. A four-way factorial ANOVA was also conducted to evaluate the effects of genotype, water regime, PPFD for measurement (two levels), measurement date (five dates), and their interactions with gas exchange parameters. Results of ANOVA were shown with percentage of each factorial variance to total variance. Differences among mean values of the examined parameters for each genotype were determined using Tukey’s test, with statistical significance assumed at P < 0.05 (n = 4). Measured A and gs values were plotted for each genotype under each PPFD condition, and a correlation analysis was conducted to derive the A vs. gs slope. Differences in the A vs. gs slope values between NiF8 and each genotype were assessed using a t-test, with statistical significance assumed at P < 0.05, 0.01, and 0.001.
3 Results
3.1 Comparison of stomatal responses to drought among genotypes
The soil water conditions during the water treatment are shown in Figure 1. Based on changes in VWC under dry conditions, leaf gas exchange measurements were performed on August 24, September 6, September 16, September 23, and October 1, with mean VWC values of 0.43, 0.32, 0.25, 0.16, and 0.14 m3 m-3, respectively (Table 1).
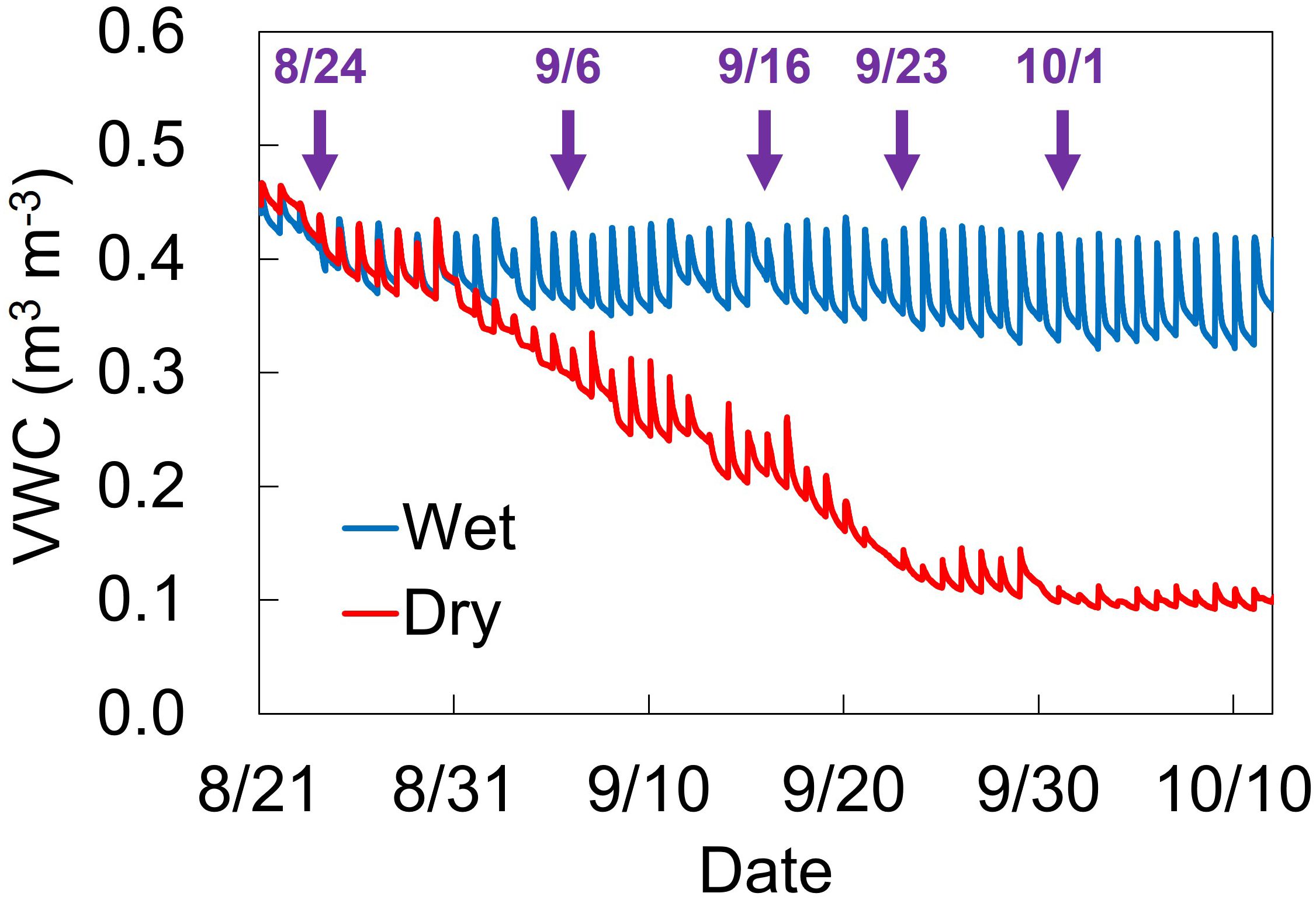
Figure 1. Changes of soil volume water content (VWC) at 15-cm depth under wet and dry conditions. Blue and red lines indicate VWC values under wet and dry conditions, respectively. Manual irrigation control and stress treatment were started from 8/20 and 8/30, respectively. Arrows indicate five dates for gas exchange measurement.
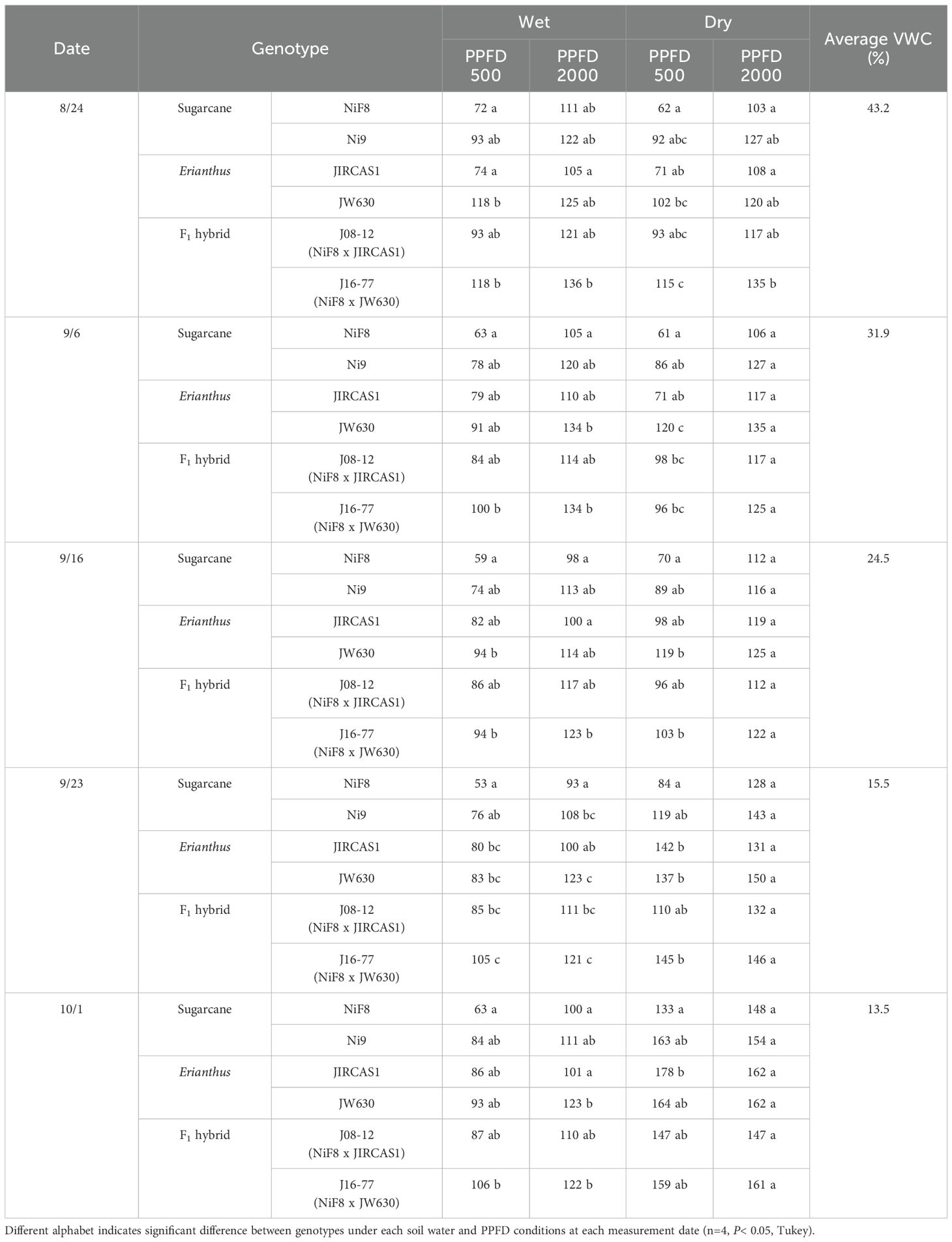
Table 1. Intrinsic water use efficiency (iWUE) of sugarcane, Erianthus, and intergeneric F1 hybrid under wet and dry conditions.
The relationship between A and gs was plotted for all measurements for both dry and wet treatments across each genotype (Figure 2). The correlation between A and gs was statistically significant under both unsaturated and saturated light conditions, with a steeper slope observed under saturated light than under unsaturated light. Among the genotypes, NiF8 exhibited a consistent tendency for higher gs (>0.3 mol m-2 s-1), regardless of light conditions. The slope under saturated light was significantly higher for Ni9, J08-12, and J16–77 than for NiF8, while JIRCAS1 and JW630 showed higher but not statistically significant trends. Under unsaturated light conditions, the slope was significantly higher for J08–12 and J16–77 compared with NiF8, whereas Ni9 and JW630 exhibited higher but non-significant trends.
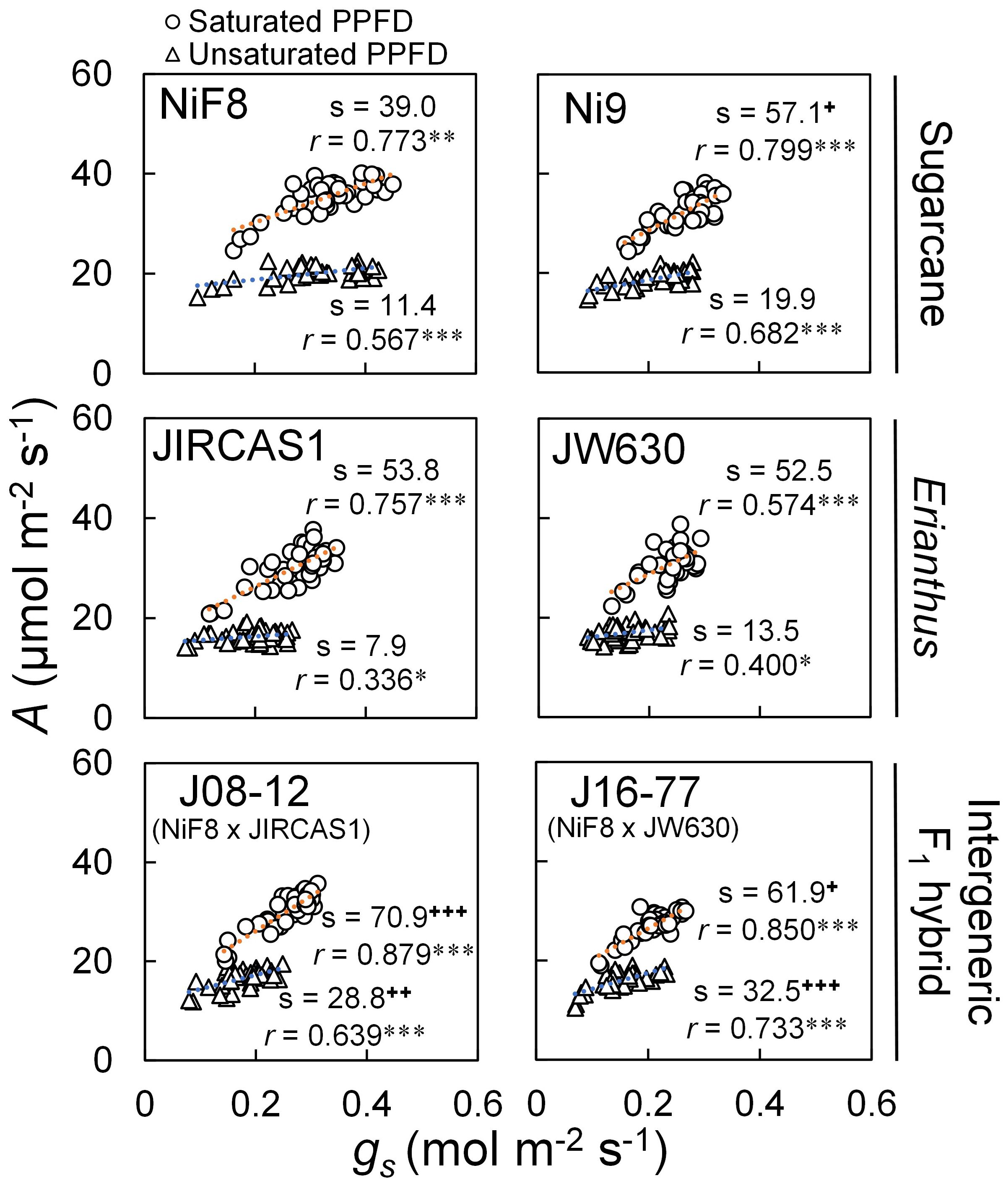
Figure 2. Phenotypic correlation between photosynthetic rate (A) and stomatal conductance (gs) under unsaturated and unsaturated light conditions. Gas exchange measurements were conducted at 2000 (○) and 500 (△) μmol m-2 s-1 of PPFD using LED for saturated and unsaturated light conditions, respectively. Orange and blue dotted line indicates linear regression line for measurements under saturated and unsaturated light conditions, respectively (n=40). “s” and “r” indicate regression slope and correlation coefficient, respectively. “*”, “**” and “***” indicate significant regression at P < 0.05, 0.01 and 0.001, respectively. “+”, “++” and “+++” indicate significantly different slope from one of NiF8 under each PPFD condition at P < 0.05, 0.01 and 0.001, respectively (t-test). Whereas intrinsic water use efficiency (iWUE) is expressed as the ratio of photosynthetic rate to stomatal conductance, the A-gs relation is a visual representation of the stomatal response of photosynthesis, whose regression equation slope indicates the ability of stomatal opening/closing to respond to soil moisture, allowing a linear interpretation of iWUE.
The relation of A, gs, and iWUE to soil moisture was plotted to observe genotype-specific differences relative to NiF8 (Supplementary Figures S5, S6, Figure 3). Under saturated light conditions, NiF8 exhibited a higher A and more pronounced inter-genotype differences (Supplementary Figure S5). NiF8 also showed higher gs, with higher inter-genotype variation observed under unsaturated light than under saturated light conditions (Supplementary Figure S6). Both A and gs exhibited minimal inter-genotype differences under extremely dry conditions (VWC< 0.2 m3 m-3) (Supplementary Figures S5, S6). The differences in iWUE between genotypes were smaller under saturated light than under unsaturated light as well as under conditions of extreme dryness (VWC< 0.2 m3 m-3) compared to wetter conditions (Figure 3, Table 1). The iWUE of Ni9 consistently remained higher than that of NiF8 under both unsaturated and saturated light conditions, regardless of soil moisture levels (Figures 3A, B). Although the difference in iWUE between Erianthus JIRCAS1 and NiF8 was minimal under saturated light, the iWUE of JIRCAS1 tended to remain higher than that of NiF8 under unsaturated light conditions (Figures 3C, D). The iWUE of Erianthus JW630 was higher than that of NiF8 under both unsaturated and saturated light conditions (Figures 3E, F). The iWUE of the intergeneric hybrids J08–12 and J16–77 was comparable to or higher than that of their Erianthus parents JIRCAS1 and JW630, respectively (Figures 3C–F).
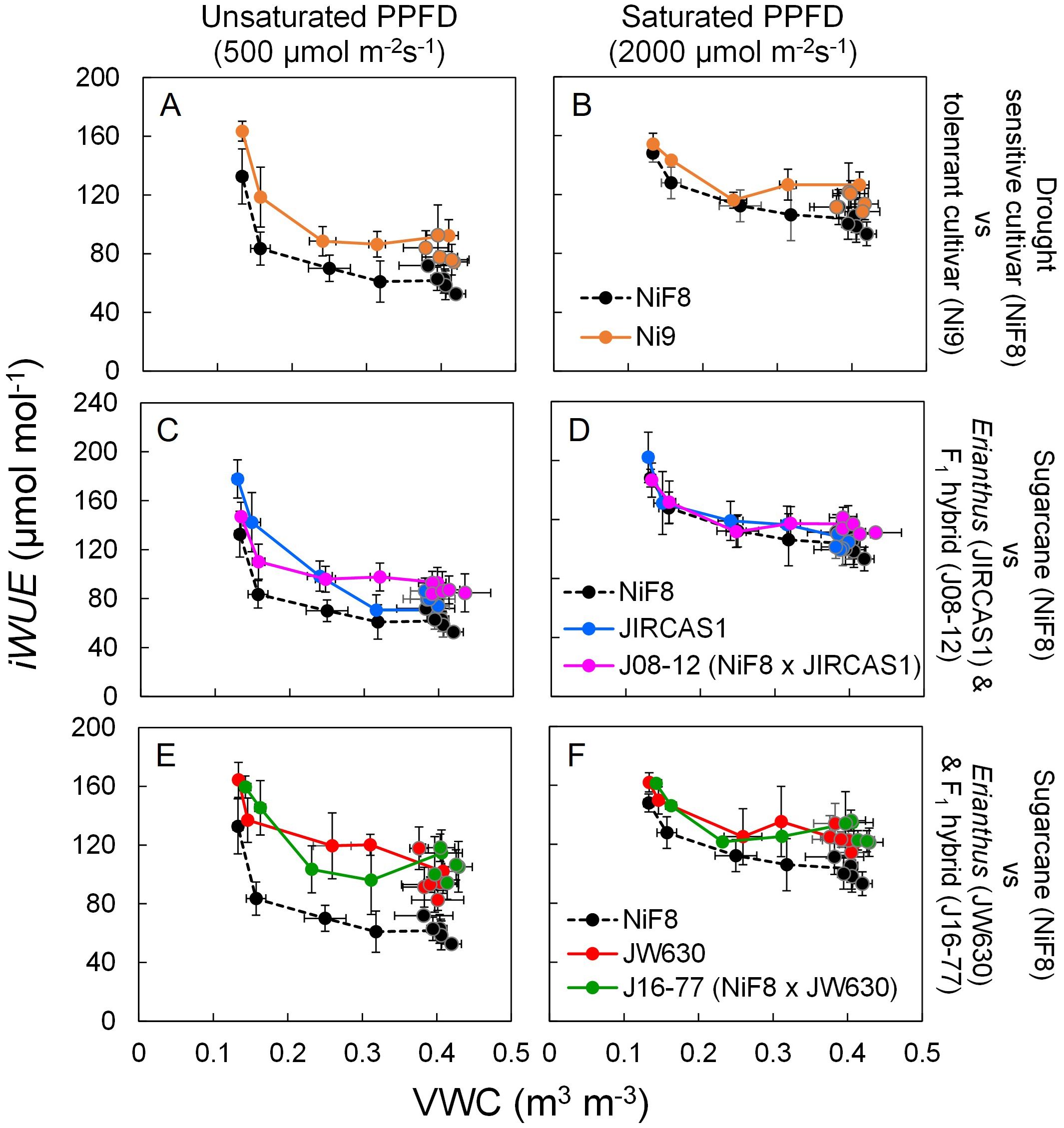
Figure 3. Responses of intrinsic water use efficiency (iWUE) to soil water changes under unsaturated and saturated light conditions. Average data under each soil water condition (wet or dry) at each measurement date were plotted with error bars for standard deviations (n=4 per genotype). Closed circle with and without line indicate the value under soil dry and wet conditions, respectively. Significant genotypic differences for iWUE were shown in the Table 1 which shows the differences of values obtained at each date. (A, B), (C, D), (E, F) labels show the relations of NiF8 with Ni9, JIRCAS1, and JW630, respectively. (A, C, E) show data under unsaturated PPFD conditions while (B, D, F) show data under saturated PPFD condition.
ANOVA results based on the mean of each genotype across all measurement conditions and dates indicated that gas exchange parameters were significantly influenced by soil moisture conditions, light conditions, and genotype (Supplementary Table S1). Among these parameters, A, iWUE, Ci, E, and A/E (photosynthetic water use efficiency) were most strongly affected by PPFD, whereas gs was primarily influenced by genotype. Significant differences in iWUE between genotypes were observed on each measurement date, with JW630 and J16–77 typically exhibiting significantly higher iWUE than NiF8, except under saturated light conditions during the dry treatment (Table 1).
3.2 Comparison of leaf anatomical features among genotypes
The stomatal distribution of the test genotypes, including those of Erianthus and intergeneric hybrids, was amphistomatous, with a higher density of stomata on the abaxial surface than on the adaxial surface, consistent with other Poaceae species (Supplementary Figure S7, Table 2). ANOVA results revealed that genotype had a significant effect on all anatomical traits, whereas the effects of water regime and genotype–water interaction were relatively small (Table 2). Regardless of soil moisture conditions, NiF8 exhibited significantly higher stomatal density on the abaxial surface than JW630 and J16-77. The stomatal density on the adaxial surface was generally lower in NiF8 than in other genotypes, regardless of soil moisture conditions, with significant differences observed only in J08–12 under wet conditions and in JIRCAS1 and J16–77 under dry conditions. Overall, JW630 showed a lower stomatal density than the other genotypes. The ratio of adaxial to abaxial stomatal density under wet conditions was highest for JW630 and significantly higher for all other genotypes compared to NiF8. No significant differences in stomatal density ratios were observed between sugarcane cultivars or Erianthus accessions under dry conditions; however, Erianthus and intergeneric hybrids showed higher values compared to the two sugarcane cultivars. The interveinal distance did not differ among the sugarcane cultivars, whereas significant differences were found among Erianthus accessions (Table 2, Supplementary Figure S8). Specifically, Erianthus JW630 and the intergeneric hybrid J16–77 showed significantly longer interveinal distances than NiF8, whereas the intergeneric hybrid J08–12 showed a tendency for longer interveinal distance, although not significantly.
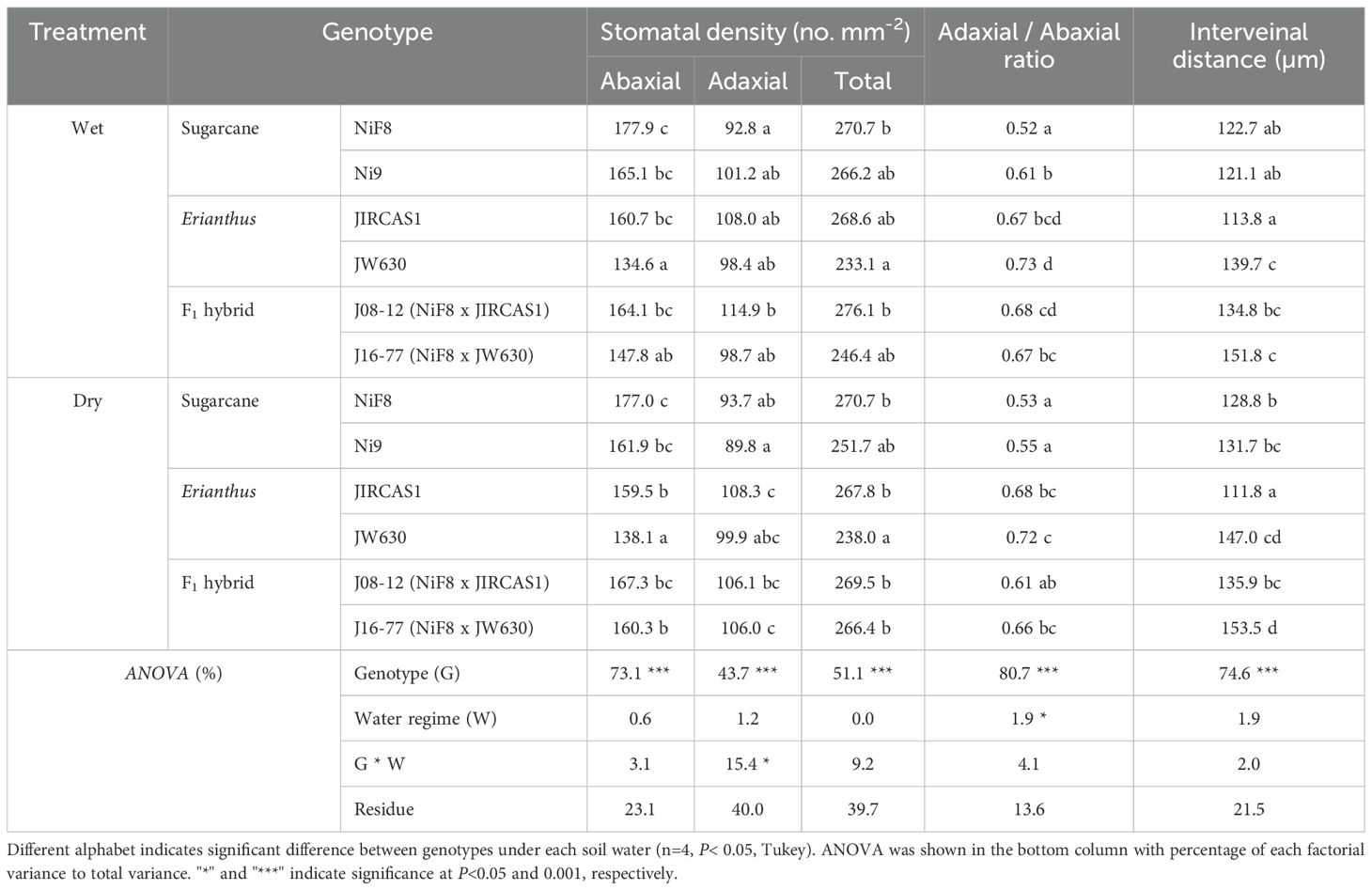
Table 2. Stomatal density and leaf interveinal distance of sugarcane, Erianthus, and intergeneric F1, hybrid under wet and dry conditions.
3.3 Comparison of biomass production response to drought among genotypes
ANOVA results indicated that both genotype and water regime significantly affected all parameters related to dry matter production (Table 3), except for NUE (Supplementary Table S2). Furthermore, the interaction between genotype and water regime was significant for all parameters, except for LAR and NUE. Genotypic differences in gWUE displayed varying trends across treatments. Under wet conditions, the genotypic differences in the gWUE of shoot dry mass were not significant, though the gWUE tended to be higher for the two sugarcane cultivars and the intergeneric hybrid J08–12 than for the two Erianthus accessions and the intergeneric hybrid J16-77. However, under dry conditions, genotypic differences in the gWUE of shoot dry mass were significant, with the two Erianthus accessions showing the lowest values, followed by the two sugarcane cultivars and the two intergeneric hybrids. Comparing the values between two treatments, the gWUE ratios of dry to wet conditions for average shoot dry mass tended to be higher for sugarcane and the intergeneric hybrids than for Erianthus, with similar trends observed for the gWUE of total dry mass. LAR was minimally affected by soil moisture conditions, with clear genotypic differences. The two Erianthus accessions exhibited significantly higher LAR values than the other genotypes. The shoot mass/root mass (S/R) ratios showed similar trends under both dry and wet conditions, being lower for Erianthus and higher for both sugarcane and intergeneric hybrids. The S/R ratio was the lowest for Erianthus JW630, and intermediate to higher for intergeneric hybrids compared to those for the parental genotypes. Under wet conditions, the stem elongation rate was significantly higher for the other genotypes than for Erianthus JW630, whereas under dry conditions, it was significantly higher or tended to be higher for the other genotypes than for the two Erianthus accessions. NUE variation among replicates was large, and genotypic differences were unclear (Supplementary Table S2).
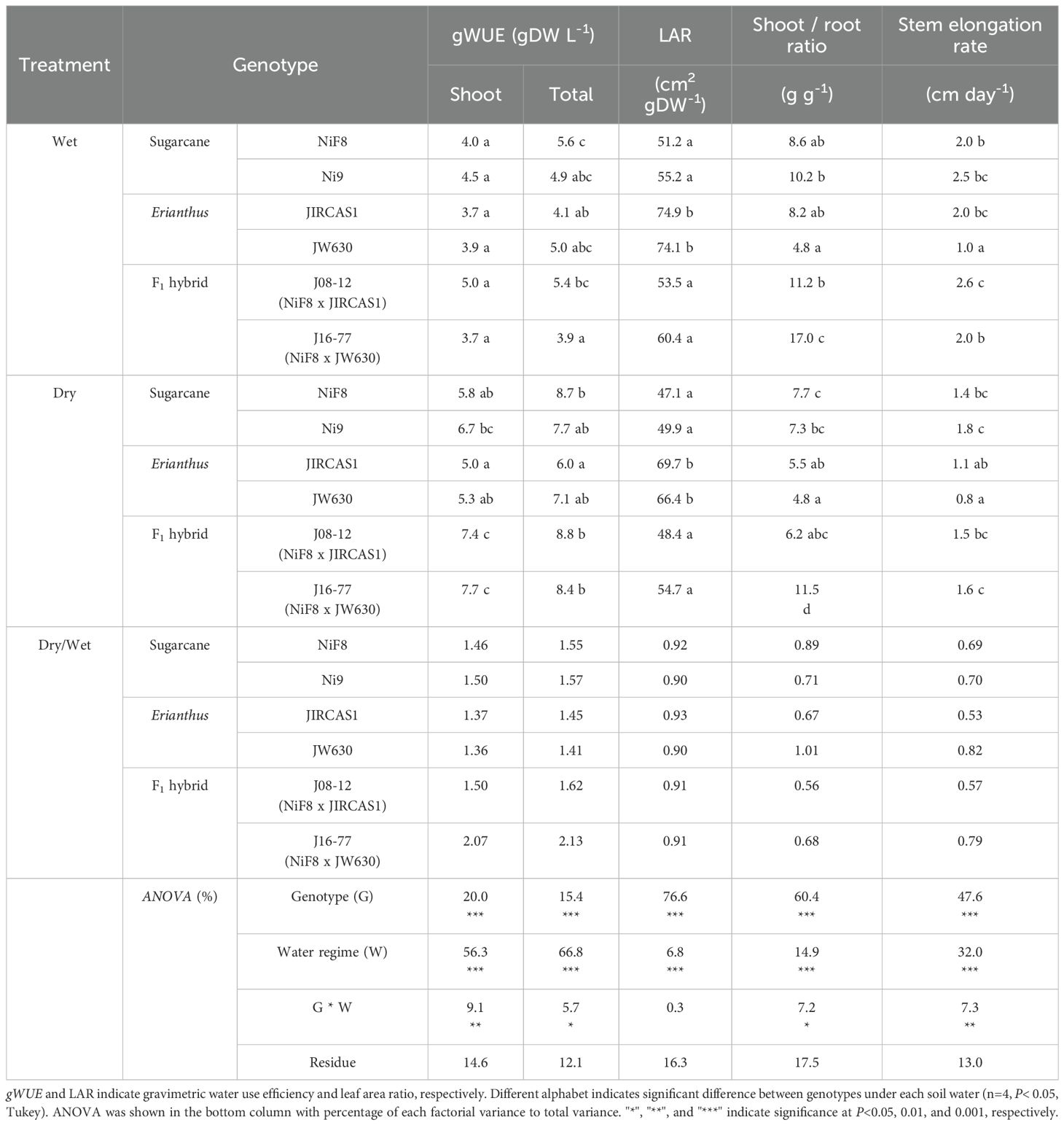
Table 3. Shoot growth parameters of sugarcane, Erianthus, and intergeneric F1 hybrid under wet and dry conditions.
Dry matter partitioning for each organ is shown in Figure 4. In sugarcane, a higher proportion of dry matter was allocated to the stem, with reduced allocation to the leaves due to drought, leading to an increase in dead tissue. In contrast, the Erianthus accessions exhibited higher partitioning to leaves and roots. Although intergeneric hybrids tended to increase root partitioning under drought, their overall dry matter allocation was similar to that of sugarcane, with more dry matter directed to the stems.
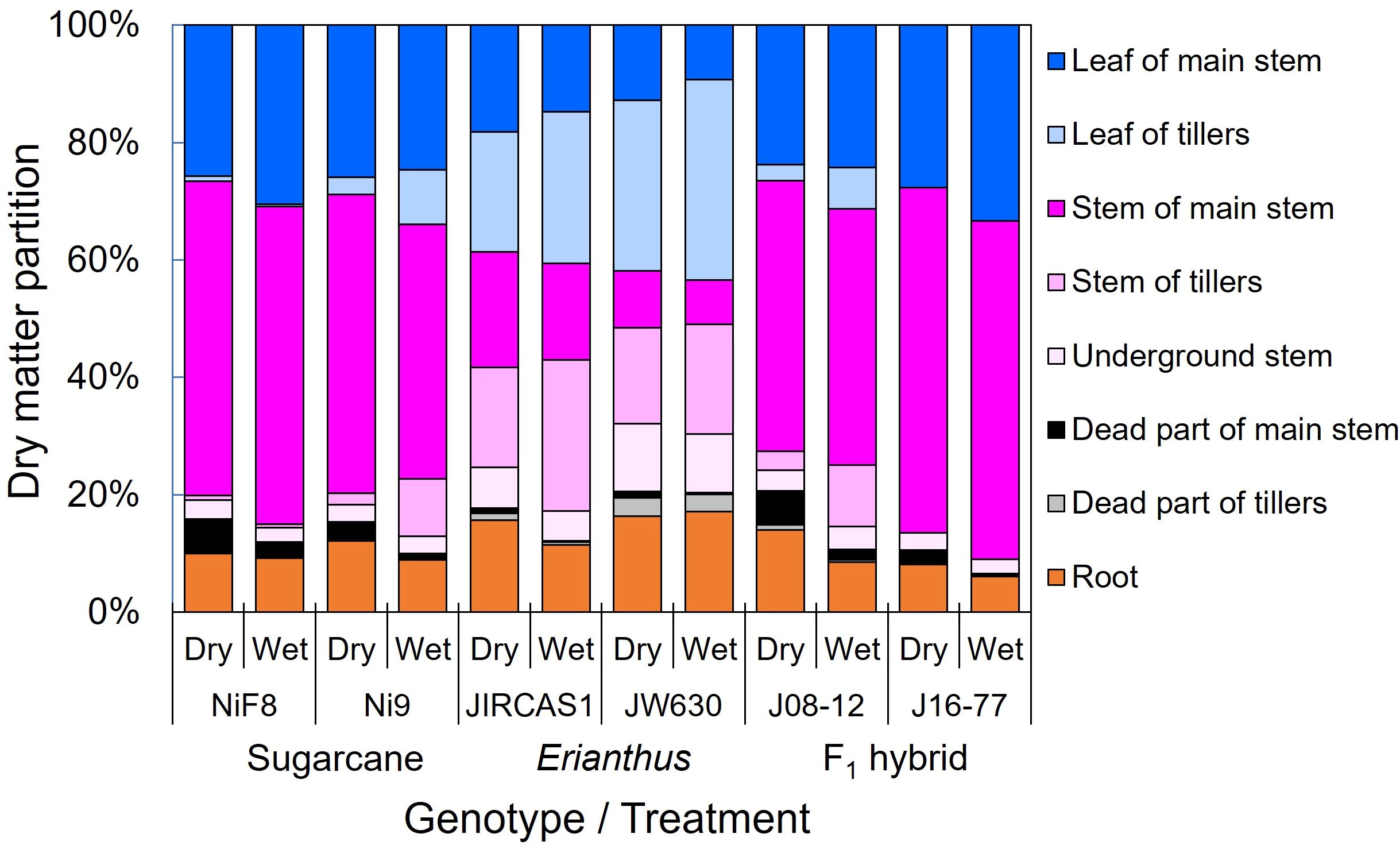
Figure 4. Dry matter partitions of sugarcane, Erianthus, and intergeneric F1 hybrid under wet and dry conditions. Blue (deep: main stem, light: tillers), purple (deep: main stem, semi-light: tillers, light: underground (stubble)), black (deep: main stem, light: tillers), and orange colors indicate leaf parts, stem parts, dead parts, and root parts, respectively. This figure visually shows the differences and similarities in dry matter distribution and supplements the dry matter distribution parameters expressed in terms of LAR and shoot/root ratio (Table 3). Here, we can visually see that sugarcane and intergeneric hybrids show similar dry matter partitioning.
4 Discussion
4.1 Comparison of stomatal responses to soil water conditions in sugarcane and Erianthus
The relationship between A and gs (Figure 2) indicated that Erianthus exhibited a more sensitive stomatal response, with lower gs and lesser transpiration than the drought-susceptible cultivar NiF8, regardless of light conditions. This trend was particularly evident under unsaturated light conditions. In contrast, compared to that of the drought-tolerant cultivar Ni9, the A vs gs slope for Erianthus was not high, indicating that stomatal responsiveness in Erianthus was not necessarily higher than that in sugarcane (Figure 2). A key feature of gas exchange in Erianthus, as compared to sugarcane, is the high stomatal responsiveness while maintaining gs at consistent low levels, which indicates the presence of an underlying anatomical mechanism (Lawson and Blatt, 2014; Bertolino et al., 2019). Typically, longer interveinal distances and fewer stomata result in lower gs (Kawamitsu et al., 2002; Xu and Zhou, 2008; Pitaloka et al., 2022). When factors affecting stomatal responses, such as water status (soil moisture, VPD, etc.) and solar radiation, are variable, the stomatal reactivity—the ability to adjust stomatal opening and closing in response to these factors—plays a critical role in maintaining high iWUE (Kawamitsu et al., 1993; Tominaga et al., 2014; Matthews et al., 2017; Dinh et al., 2019; Lawson and Vialet-Chabrand, 2019; Ozeki et al., 2022). A better stomatal response has been reported in leaves that are more amphistomatous, with a higher distribution of stomata on the adaxial surface relative to the abaxial surface (Haworth et al., 2018; Drake et al., 2019; Xiong and Flexas, 2020). Amphistomatous leaves contribute to maintaining optimal leaf water status in response to transpiration demand. When stomata are open, the temperature gradient between the atmosphere, stomatal cavity, and leaf chloroplast is reduced (Drake et al., 2019), suggesting that stomatal responses to VPD—the driving force for transpiration—can be effectively regulated (Kawamitsu et al., 1993, 2002). Furthermore, in C4 grasses, more stomata on the adaxial surface can increase the surface area of mesophyll cells in contact with intracellular air space, enhancing iWUE and mesophyll conductance (Pathare et al., 2020). In the present study, the Erianthus species, particularly JW630, exhibited fewer stomata on the abaxial surface (resulting in a longer interveinal distance) and a more amphistomatous stomatal distribution (Table 2), which may explain its heightened stomatal responsiveness.
Furthermore, Erianthus JIRCAS1 exhibited a trend toward higher iWUE than the susceptible cultivar NiF8 under both wet and dry conditions, although its iWUE was not consistently higher than that of the drought-tolerant cultivar Ni9 (Figure 3, Table 1). In contrast, Erianthus JW630 consistently showed significantly higher iWUE than both NiF8 and Ni9. The high iWUE of Erianthus JW630 was likely attributed to leaf anatomy, including low stomatal density, (Table 2), leading to low gs, and may have a different physiological mechanism compared to that of drought-tolerant Ni9. The ability of Erianthus to maintain high A despite a low gs (that is, a high iWUE) may be linked to ultrastructural features such as mesophyll cell wall thickness and surface area in contact with the stomatal cavity, both of which are involved in bundle sheath leakiness (von Caemmerer and Furbank, 2003). Further investigation of gas exchange characteristics, such as A-Ci curves, and anatomical features of this species will provide deeper insights into these mechanisms.
Erianthus exhibits genetically distinct lineages (Tsuruta et al., 2012, 2017, 2022) which influence variations in its morphological, ecological (Tagane et al., 2012), and agronomic (Terajima et al., 2022) traits. In the current study, variation in iWUE was observed between two Erianthus accessions (Table 1) which are classified into different genetic groups (Tsuruta et al., 2012, 2017). The accession JW630 was collected from Shizuoka Prefecture, a temperate region in Japan, while the origin of JIRCAS1 remains unknown. Previous studies have primarily focused on tropical accessions, such as the IJ series, which also exhibit high iWUE and related variations (Jackson et al., 2016; Li et al., 2017). These findings highlight the need for further investigation into the variation in leaf characteristics across different genotypic groups in Erianthus. Additionally, selecting Erianthus genotypes for improving drought tolerance in sugarcane will require considering both root system and aboveground traits.
4.2 Potential for enhancing sugarcane gWUE via iWUE improvement through intergeneric hybridization with Erianthus
Jackson et al. (2016) reported a strong correlation between iWUE and gWUE in sugarcane germplasm, with several Erianthus accessions showing higher values for both parameters compared to sugarcane. However, in the current study, high iWUE did not necessarily lead to high gWUE in Erianthus. This discrepancy may be attributed to differences in growing conditions: Jackson et al. (2016) conducted their trial in larger pots under outdoor conditions, while our study, was performed in smaller pots in a glasshouse. These differences may have limited branching in sugarcane varieties and caused greater root restriction in Erianthus. It is recommended that gWUE evaluation and screening in pot trials should take into account pot size and that evaluation of leaf traits at very early growth stages, rather than gWUE, would be more appropriate to validly evaluate genotypic differences under pot experiments. Despite this discrepancy with previous studies, low iWUE in sugarcane may result in low gWUE under field conditions, particularly under field canopy conditions, due to the larger leaf area and high LAR (or high LAI) of tillers. Additionally, because many leaves under a shaded canopy perform photosynthesis under low-light conditions (Almeida et al., 2022), where iWUE, which exhibits higher genotypic variation under low-light conditions, may have a more pronounced impact on gWUE. Erianthus, recognized for its drought-tolerance, may achieve high gWUE even at high LAR (caused by presence of many tillers), owing to its robust root system in the field (Terajima et al., 2023), in addition to its high iWUE (Figure 3, Table 1). The intergeneric F1 hybrids, which exhibited high gWUE under pot conditions—unlike the parental Erianthus accessions—showed high iWUE (Table 1) and demonstrated sugarcane-like dry matter partitioning characteristics (low LAR, high S/R ratio, and higher stem partitioning) (Table 3, Figure 4). Consequently, these hybrids may potentially maintain high gWUE even under field canopy conditions with high LAR. Although the intergeneric hybrids exhibited limited dry matter partitioning to roots in this study, which focused on relatively early growth under pot conditions, field studies have shown that the hybrid J08–12 forms roots with intermediate potential between parental species, exhibiting higher root mass and depth than sugarcane (Takaragawa et al., 2022; Terajima et al., 2023). These findings in the present study highlighted the "best of both worlds" scenario demonstrated by the hybrids, where they inherited high iWUE from Erianthus with favorable biomass partitioning characteristics from sugarcane. This fact could represent the ideal outcome for breeding drought-tolerant varieties via intergeneric hybridization with Erianthus. Further field trials will assess the relationships among iWUE, canopy coverage, root system formation, and gWUE using a hybrid population derived from several sets of parental genotypes.
The PPFD for gas exchange measurements had the greatest influence on iWUE (Supplementary Table S1). Genotypic differences in iWUE were particularly pronounced under unsaturated light (500 µmol m-2 s-1) conditions than under saturated light (2000 µmol m-2 s-1) conditions (Figure 3, Table 1). Additionally, the slope of the A vs gs curve was smaller, and the stomatal response was notably lower under unsaturated light conditions than under saturated light conditions (Figure 2). Therefore, iWUE screening under low-light conditions may prove effective and provide practical implications for developing high-throughput phenotyping protocols for drought tolerance screening. In contrast, such differences in stomatal responses due to varying light conditions suggest that obtaining stable results when measuring the response at multiple sites under field conditions may be challenging, especially in regions such as Okinawa (which comprise small islands and represent our study site), where the weather frequently shifts between cloudy and sunny within short time frames. Genotypic differences in iWUE vary depending on the measurement date; therefore, measuring within a moderate gs range (0.2–0.3 mol m-2 s-1, Jackson et al., 2016; 0.1–0.4 mol m-2 s-1, Natarajan et al., 2021) or averaging multiple measurements, is recommended (Jackson et al., 2016; Li et al., 2017). Considering this climate instability, investigating genotypic differences in response to fluctuating light conditions (Eyland et al., 2021; Tanaka et al., 2019) and exploring non-destructive methods for measuring daily variations in gas exchange, such as sap flow for transpiration (Contreras and Ozawa, 2005), are essential.
Gas exchange measurements are strongly influenced by environmental variations during data collection, which can compromise the stability and efficiency of the measurements. In recent years, the throughput of photosynthesis measurements has been enhanced by reducing measurement time through the use of closed-type equipment (Honda et al., 2021; Tanaka et al., 2021; Takaragawa and Matsuda, 2023). There have been no previous reports on high-throughput estimating and screening iWUE using UAVs and hyperspectral images, while component parameters for iWUE can be estimated by aerial image analysis: transpiration indices from leaf or canopy temperatures obtained from thermal images (Basnayake et al., 2016; Iseki and Olaleye, 2020; Natarajan et al., 2019) and photosynthetic activity using hyperspectral images (Kohzuma et al., 2021). However, despite recent advancements and attempts (Takaragawa et al., 2025), improvements in the measurement throughput of iWUE, which requires simultaneous measurement of photosynthesis and gs, remain incomplete.
Gas exchange is governed by complex biochemical processes influenced by metabolites, enzymes, and morphology (Sage et al., 2013). Among these, leaf morphological and anatomical traits, particularly stomatal characteristics, play a critical role in supporting gas exchange and mechanical function (Franks and Farquhar, 2007; Haworth et al., 2021). Although anatomical traits, such as stomatal density, are not sufficiently robust or universal enough to be used for species classification (Davis, 1987), they exhibit a smaller environmental variation compared to gas exchange characteristics and can show stable genotypic variation (Moreno-Sotomayor et al., 2002). The current study also demonstrated that environmental variation in leaf anatomical traits was relatively small as indicated by ANOVA results (Table 2, Supplementary Table S1). The leaf anatomical characteristics of the intergeneric F1 hybrids were intermediate between the parental genotypes, with intergeneric hybridization with Erianthus resulting in a progeny having longer interveinal distances, fewer stomata on the abaxial surface, and a higher stomatal distribution ratio (Table 2). These findings suggest that the improvement in iWUE through intergeneric hybridization was facilitated by changes in leaf anatomy. When examining hybrid populations, the throughput of morphological and anatomical observations may need to be enhanced through rapid image acquisition or other methods (Strock et al., 2022).
Although intergeneric hybridization offers potential for improving leaf traits of sugarcane, F1 hybrids typically exhibit lower sugar content with higher fiber content than sugarcane parents (Pachakkil et al., 2019), which discourages their direct utilization in breeding programs for sugar industry. Therefore, backcrossing using sugarcane variety must be performed to improve sugar content of hybrids, requiring a further investigation of leaf traits in the backcross populations.
5 Conclusions
We attempted to assess the potential for introducing the superior leaf traits of Erianthus into sugarcane by comparing the response of leaf traits to drought among sugarcane × Erianthus intergeneric F1 hybrids and their parental genotypes. In conclusion, the use of Erianthus germplasm, not drought-tolerant sugarcane cultivars, for improving drought tolerance in sugarcane remains a subject of debate. However, our study shows that incorporating Erianthus species into breeding programs could enhance the overall drought tolerance of sugarcane because intergeneric F1 hybrid exhibited favorable trait combinations inherited from both sugarcane and Erianthus parents (Figure 5). Erianthus has the potential to significantly improve not only leaf physiological and morphological characteristics, as demonstrated in the current study, but also the root system formation ability (Fukuhara et al., 2013; Takaragawa et al., 2022; Terajima et al., 2023). Future research will focus on comparing several F1 and BC hybrid populations, incorporating both drought-tolerant cultivars and Erianthus, to further assess their potential for improving drought resilience in sugarcane under field conditions.
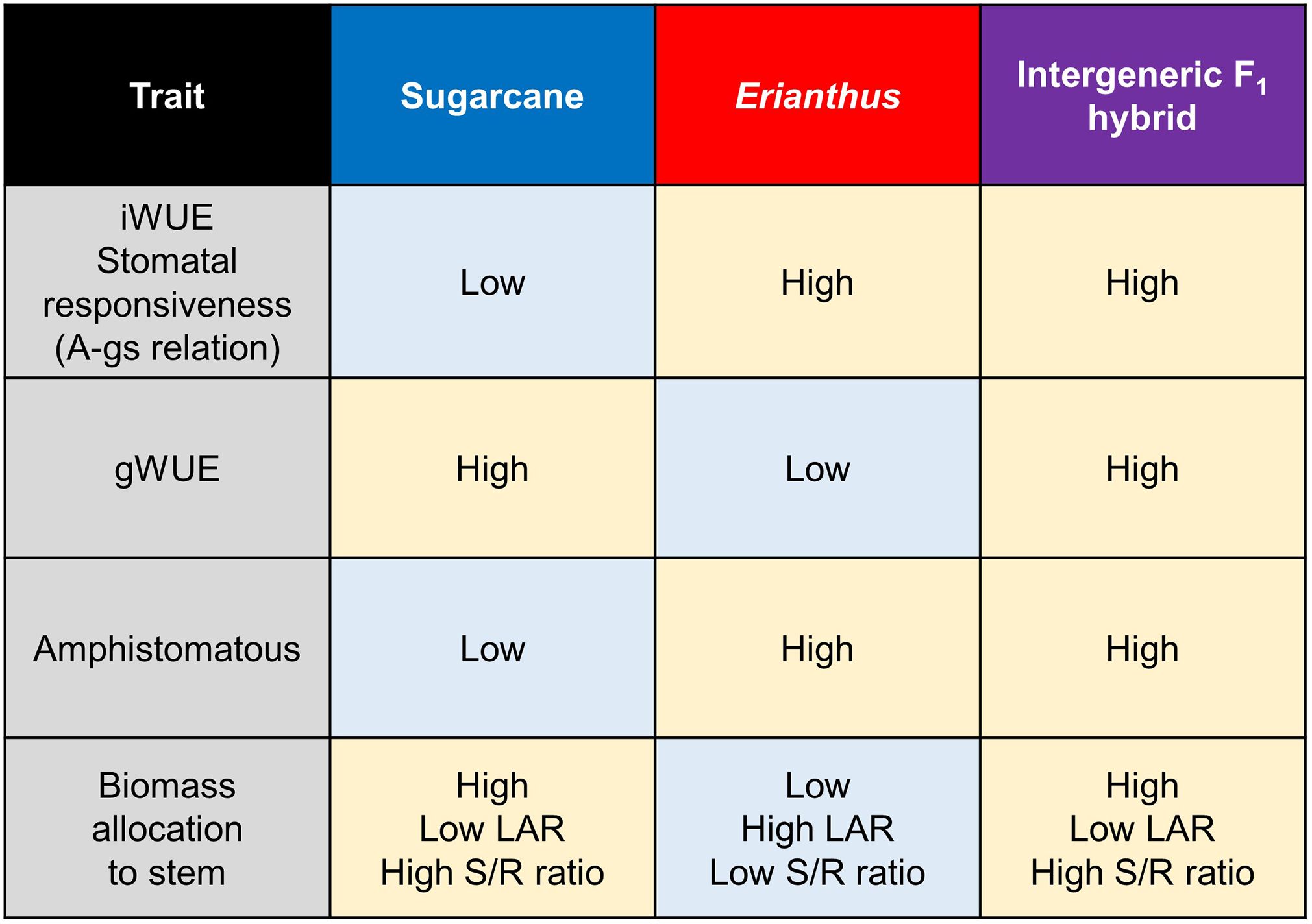
Figure 5. A schematic summary diagram for key differences between sugarcane, Erianthus, and intergeneric F1 hybrids. iWUE, gWUE, LAR, and S/R ratio indicate intrinsic water use efficiency, gravimetric water use efficiency, leaf area ratio, and shoot biomass/root baiomass ratio, respectively. Amphistomatous indicates high stomatal density ratio of adaxial to abaxial leaf surface. The diagram highlighted the "best of both worlds" scenario demonstrated by the hybrids.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
HT: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. YT: Funding acquisition, Resources, Writing – review & editing. KO: Data curation, Investigation, Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was conducted as part of a project entitled “Tropical crop genetic resources: Advancement of tropical crop genetic resources utilization through the development of database, technologies and research networking (2022-2027),” funded by our affiliate JIRCAS.
Acknowledgments
We express our sincere gratitude to the JIRCAS-TARF staff for their assistance with experimental management and measurements. We also thank Dr. Masakazu Nakayama, Dr. Kosuke Hamada, and Dr. Hiroshi Matsuda for their support with leaf area measurements, calibration of EC-5 sensors, and statistical analysis of genotypic differences in A vs. gs slopes, respectively.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1649112/full#supplementary-material
Abbreviations
A, photosynthetic rate; Ci, intercellular carbon dioxide; E, transpiration rate; gs, stomatal conductance; gWUE, gravimetric water use efficiency; iWUE, intrinsic water use efficiency; LAR, leaf area ratio; NUE, nitrogen use efficiency; pF, soil matric potential; PPFD, photosynthetic photon flux density; VPD, vapor pressure deficit; VWC, volume water content.
References
Acreche, M. M. (2017). Nitrogen-, water- and radiation-use efficiencies affected by sugarcane breeding in Argentina. Plant Breed. 136, 174–181. doi: 10.1111/pbr.12440
Almeida, R. L., Silveira, N. M., Miranda, M. T., Pacheco, V. S., Cruz, L. P., Xavier, M. A., et al. (2022). Evidence of photosynthetic acclimation to self-shading in sugarcane canopies. Photosynthetica 60, 521–528. doi: 10.32615/ps.2022.045
Augustine, S. M., Syamaladevi, D. P., Premachandran, M. N., Ravichandran, V., and Subramonian, N. (2015). Physiological and molecular insights to drought responsiveness in Erianthus spp. Sugar Tech 17, 121–129. doi: 10.1007/s12355-014-0312-7
Basnayake, J., Jackson, P. A., Inman-Bamber, N. G., and Lakshmanan, P. (2012). Sugarcane for water-limited environments: Genetic variation in cane yield and sugar content in response to water stress. J. Exp. Bot. 63, 6023–6033. doi: 10.1093/jxb/ers251
Basnayake, J., Jackson, P. A., Inman-Bamber, N. G., and Lakshmanan, P. (2015). Sugarcane for water-limited environments. Variation in stomatal conductance and its genetic correlation with crop productivity. J. Exp. Bot. 66, 3945–3958. doi: 10.1093/jxb/erv194
Basnayake, J., Lakshmanan, P., Jackson, P., Chapman, S., and Natarajan, S. (2016). Canopy temperature: a predictor of sugarcane yield for irrigated and rainfed conditions. Proceeding Int. Soc. Sugar Cane Technol. 29, 50–59.
Battle, M. W., Vialet-Chabrand, S., Kasznicki, P., Simkin, A. J., and Lawson, T. (2024). Fast stomatal kinetics in sorghum enable tight coordination with photosynthetic responses to dynamic light intensity and safeguard high water use efficiency. J. Exp. Bot. 75, 6796–6809. doi: 10.1093/jxb/erae389
Bertolino, L. T., Caine, R. S., and Gray, J. E. (2019). Impact of stomatal density and morphology on water-use efficiency in a changing world. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.00225
Bhuiyan, S. A., Croft, B. J., Stirling, G., Meagher, L. M., and Wong, E. (2014). Development of methods for screening sugarcane and Erianthus germplasm for resistance to plant-parasitic nematodes. Proceeding Aust. Soc. Sugar Cane Technol. 36, 166–176.
Bhuiyan, S. A., Croft, B. J., Stirling, G. R., Wong, E., Jackson, P., and Cox, M. (2016). Assessment of resistance to root-lesion and root-knot nematodes in Australian hybrid clones of sugarcane and its wild relatives. Australas. Plant Pathol. 45, 165–173. doi: 10.1007/s13313-016-0400-0
Condon, A. G., Richards, R. A., Rebetzke, G. J., and Farquhar, G. D. (2004). Breeding for high water-use efficiency. Journal of experimental botany, 55(407), 2447–2460.
Contreras, S. M. and Ozawa, K. (2005). Hardpan effect on sugarcane [Saccharum officinarum] transpiration, growth and yield. J. Agric. Meteorology 61, 23–28. doi: 10.2480/agrmet.61.23
D’Hont, A., Rao, P. S., Feldmann, P., Grivet, L., Islam-Faridi, N., Taylor, P., et al. (1995). Identification and characterisation of sugarcane intergeneric hybrids, Saccharum officinarum x Erianthus arundinaceus, with molecular markers and DNA in situ hybridisation. Theor. Appl. Genet. 91, 320–326. doi: 10.1007/BF00220894
Davis, J. I. (1987). Genetic and environmental determination of leaf epidermal anatomy in Puccinellia (Poaceae). Am. J. Bot. 74, 1744–1749. doi: 10.1002/j.1537-2197.1987.tb08776.x
Deng, Q., Dou, Z., Chen, J., Wang, K., and Shen, W. (2019). Drought tolerance evaluation of intergeneric hybrids of BC 3 F 1 lines of Saccharum officinarum× Erianthus arundinaceus. Euphytica 215, 1–13. doi: 10.1007/s10681-019-2513-3
Dinh, T. H., Takaragawa, H., Watanabe, K., Nakabaru, M., and Kawamitsu, Y. (2019). Leaf photosynthesis response to change of soil moisture content in sugarcane. Sugar Tech 21, 949–958. doi: 10.1007/s12355-019-00735-8
Dinh, T. H., Watanabe, K., Takaragawa, H., Nakabaru, M., and Kawamitsu, Y. (2017). Photosynthetic response and nitrogen use efficiency of sugarcane under drought stress conditions with different nitrogen application levels. Plant Production Sci. 20, 412–422. doi: 10.1080/1343943X.2017.1371570
Dlamini, P. J. (2021). Drought stress tolerance mechanisms and breeding effort in sugarcane: A review of progress and constraints in South Africa. Plant Stress 2, 100027. doi: 10.1016/j.stress.2021.100027
Drake, P. L., De Boer, H. J., Schymanski, S. J., and Veneklaas, E. J. (2019). Two sides to every leaf: water and CO2 transport in hypostomatous and amphistomatous leaves. New Phytol. 222, 1179–1187. doi: 10.1111/nph.15652
Du, Y. C., Kawamitsu, Y., Nose, A., Hiyane, S., Murayama, S., Wasano, K., et al. (1996). Effects of water stress on carbon exchange rate and activities of photosynthetic enzymes in leaves of sugarcane (Saccharum sp.). Funct. Plant Biol. 23, 719–726. doi: 10.1071/PP9960719
Eyland, D., van Wesemael, J., Lawson, T., and Carpentier, S. (2021). The impact of slow stomatal kinetics on photosynthesis and water use efficiency under fluctuating light. Plant Physiol. 186, 998–1012. doi: 10.1093/plphys/kiab114
Ferreira, T. H., Tsunada, M. S., Bassi, D., Araújo, P., Mattiello, L., Guidelli, G. V., et al. (2017). Sugarcane water stress tolerance mechanisms and its implications on developing biotechnology solutions. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.01077
Franks, P. J. and Farquhar, G. D. (2007). The mechanical diversity of stomata and its significance in gas-exchange control. Plant Physiol. 143, 78–87. doi: 10.1104/pp.106.089367
Fukuhara, S., Terajima, Y., Irei, S., Sakaigaichi, T., Ujihara, K., Sugimoto, A., et al. (2013). Identification and characterization of intergeneric hybrid of commercial sugarcane (Saccharum spp. hybrid) and Erianthus arundinaceus (Retz.) Jeswiet. Euphytica 189, 321–327. doi: 10.1007/s10681-012-0748-3
Glowacka, K., Ahmed, A., Sharma, S., Abbott, T., Comstock, J. C., Long, S. P., et al. (2016). Can chilling tolerance of C4 photosynthesis in Miscanthus be transferred to sugarcane? Gcb Bioenergy 8, 407–418. doi: 10.1111/gcbb.12283
Haworth, M., Marino, G., Loreto, F., and Centritto, M. (2021). Integrating stomatal physiology and morphology: evolution of stomatal control and development of future crops. Oecologia 197, 867–883. doi: 10.1007/s00442-021-04857-3
Haworth, M., Scutt, C. P., Douthe, C., Marino, G., Gomes, M. T. G., Loreto, F., et al. (2018). Allocation of the epidermis to stomata relates to stomatal physiological control: stomatal factors involved in the evolutionary diversification of the angiosperms and development of amphistomaty. Environ. Exp. Bot. 151, 55–63. doi: 10.1016/j.envexpbot.2018.04.010
Honda, S., Ohkubo, S., San, N. S., Nakkasame, A., Tomisawa, K., Katsura, K., et al. (2021). Maintaining higher leaf photosynthesis after heading stage could promote biomass accumulation in rice. Sci. Rep. 11, 7579. doi: 10.1038/s41598-021-86983-9
Inman-Bamber, N. G. and Stead, B. A. (1990). A method for choosing the most profitable commercial sugarcane variety. Proc. South Afr. Sugar Technologists’ Assoc. 64, 1–4.
Irvine, J. E. (1975). Relations of photosynthetic rates and leaf and Canopy Characters to sugarcane yield 1. Crop Sci. 15, 671–676. doi: 10.2135/cropsci1975.0011183X001500050017x
Iseki, K. and Olaleye, O. (2020). A new indicator of leaf stomatal conductance based on thermal imaging for field grown cowpea. Plant Production Sci. 23, 136–147. doi: 10.1080/1343943X.2019.1625273
Jackson, P. (2019). Why are yields of sugarcane not increasing as much as sugar beet (or other crops). Proc. Int. Soc. Sugar Cane Technol. 30, 128–137.
Jackson, P., Basnayake, J., Inman-Bamber, G., Lakshmanan, P., Natarajan, S., and Stokes, C. (2016). Genetic variation in transpiration efficiency and relationships between whole plant and leaf gas exchange measurements in Saccharum spp. and related germplasm. J. Exp. Bot. 67, 861–871. doi: 10.1093/jxb/erv505
Jackson, P. and Henry, R. J. (2011). “Erianthus,” in Wild crop relatives: Genomic and breeding resources: Industrial crops (Springer Berlin Heidelberg, Berlin, Heidelberg), 97–107. doi: 10.1007/978-3-642-21102-7_5
Jumkudling, S., Songsri, P., Taratima, W., and Jongrungklang, N. (2022). Diversity and distribution of anatomical characteristics involved with drought resistance of inter-specific (Saccharum spp. hybrid× S. spontaneum) sugarcane F1 hybrid population. Sugar Tech 24, 1342–1356. doi: 10.1007/s12355-021-01067-2
Kar, S., Weng, T. Y., Nakashima, T., Villanueva-Morales, A., Stewart, J. R., Sacks, E. J., et al. (2020). Field performance of Saccharum× Miscanthus intergeneric hybrids (Miscanes) under cool climatic conditions of northern Japan. Bioenergy Res. 13, 132–146. doi: 10.1007/s12155-019-10066-x
Kar, S., Zhang, N., Nakashima, T., Villanueva-Morales, A., Stewart, J. R., Sacks, E. J., et al. (2019). Saccharum× Miscanthus intergeneric hybrids (miscanes) exhibit greater chilling tolerance of C4 photosynthesis and postchilling recovery than sugarcane (Saccharum spp. hybrids). GCB Bioenergy 11, 1318–1333. doi: 10.1111/gcbb.12632
Kawamitsu, Y., Hiyane, S. I., Tamahiro, Y., and Hakoyama, S. (2002). Regulation of photosynthesis and water use efficiency in relation to stomatal frequency and interveinal distance in C3-and C4-grass species. Environ. Control Biol. 40, 365–374. doi: 10.2525/ecb1963.40.365
Kawamitsu, Y., Yoda, S., and Agata, W. (1993). Humidity pretreatment affects the responses of stomata and CO2 assimilation to vapor pressure difference in C3 and C4 plants. Plant Cell Physiol. 34, 113–119. doi: 10.1093/oxfordjournals.pcp.a078384
Kohzuma, K., Tamaki, M., and Hikosaka, K. (2021). Corrected photochemical reflectance index (PRI) is an effective tool for detecting environmental stresses in agricultural crops under light conditions. J. Plant Res. 134, 683–694. doi: 10.1007/s10265-021-01316-1
Lawson, T. and Blatt, M. R. (2014). Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 164, 1556–1570. doi: 10.1104/pp.114.237107
Lawson, T. and Vialet-Chabrand, S. (2019). Speedy stomata, photosynthesis and plant water use efficiency. New Phytol. 221, 93–98. doi: 10.1111/nph.15330
Li, C., Jackson, P., Lu, X., Xu, C., Cai, Q., Basnayake, J., et al. (2017). Genotypic variation in transpiration efficiency due to differences in photosynthetic capacity among sugarcane-related clones. J. Exp. Bot. 68, 2377–2385. doi: 10.1093/jxb/erx107
Liu, J., Basnayake, J., Jackson, P. A., Chen, X., Zhao, J., Zhao, P., and Fan, Y. (2016). Growth and yield of sugarcane genotypes are strongly correlated across irrigated and rainfed environments. Field Crops Research, 196, 418–425.
Manoj, V. M., Anunanthini, P., Swathik, P. C., Dharshini, S., Ashwin Narayan, J., Manickavasagam, M., et al. (2019). Comparative analysis of glyoxalase pathway genes in Erianthus arundinaceus and commercial sugarcane hybrid under salinity and drought conditions. BMC Genomics 19, 1–16. doi: 10.1186/s12864-018-5349-7
Marchiori, P. E., MaChado, E. C., Sales, C. R., Espinoza-Núñez, E., Magalhães Filho, J. R., Souza, G. M., et al. (2017). Physiological plasticity is important for maintaining sugarcane growth under water deficit. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.02148
Matsuo, K., Chuenpreecha, T., Matsuo, N., and Ponragdee, W. (2002). Eco-physiological characteristics of Erianthus spp. and yielding abilities of three forages under conditions of cattle feces application. JIRCAS working Rep. 30, 187–194.
Matthews, J. S., Vialet-Chabrand, S. R., and Lawson, T. (2017). Diurnal variation in gas exchange: the balance between carbon fixation and water loss. Plant Physiol. 174, 614–623. doi: 10.1104/pp.17.00152
Meena, M. R., Kumar, R., Ramaiyan, K., Chhabra, M. L., Raja, A. K., Krishnasamy, M., et al. (2020). Biomass potential of novel interspecific and intergeneric hybrids of Saccharum grown in sub-tropical climates. Sci. Rep. 10, 21560. doi: 10.1038/s41598-020-78329-8
Moreno-Sotomayor, A., Weiss, A., Paparozzi, E. T., and Arkebauer, T. J. (2002). Stability of leaf anatomy and light response curves of field grown maize as a function of age and nitrogen status. J. Plant Physiol. 159, 819–826. doi: 10.1078/0176-1617-00809
Nair, N. V., Mohanraj, K., Sunadaravelpandian, K., Suganya, A., Selvi, A., and Appunu, C. (2017). Characterization of an intergeneric hybrid of Erianthus procerus × Saccharum officinarum and its backcross progenies. Euphytica 213, 1–11. doi: 10.1007/s10681-017-2053-7
Nakabaru, M., Hoang, D. T., Watanabe, K., Takaragawa, H., Yabuta, S., Ueno, M., et al. (2020). Responses of leaf gas exchange rate to acute soil drying in Jatropha curcas L. Plant Production Sci. 23, 333–342. doi: 10.1080/1343943X.2020.1730699
Natarajan, S., Basnayake, J., Lakshmanan, P., and Fukai, S. (2020). Limited contribution of water availability in genotype-by-environment interaction in sugarcane yield and yield components. J. Agron. Crop Sci. 206, 665–678. doi: 10.1111/jac.12407
Natarajan, S., Basnayake, J., Lakshmanan, P., and Fukai, S. (2021). Genotypic variation in intrinsic transpiration efficiency correlates with sugarcane yield under rainfed and irrigated field conditions. Physiologia Plantarum 172, 976–989. doi: 10.1111/ppl.13221
Natarajan, S., Basnayake, J., Wei, X., and Lakshmanan, P. (2019). High-throughput phenotyping of indirect traits for early-stage selection in sugarcane breeding. Remote Sens. 11, 2952. doi: 10.3390/rs11242952
Ozeki, K., Miyazawa, Y., and Sugiura, D. (2022). Rapid stomatal closure contributes to higher water use efficiency in major C4 compared to C3 Poaceae crops. Plant Physiol. 189, 188–203. doi: 10.1093/plphys/kiac040
Pachakkil, B., Terajima, Y., Ohmido, N., Ebina, M., Irei, S., Hayashi, H., et al. (2019). Cytogenetic and agronomic characterization of intergeneric hybrids between Saccharum spp. hybrid and Erianthus arundinaceus. Sci. Rep. 9, 1748. doi: 10.1038/s41598-018-38316-6
Pathare, V. S., Koteyeva, N., and Cousins, A. B. (2020). Increased adaxial stomatal density is associated with greater mesophyll surface area exposed to intercellular air spaces and mesophyll conductance in diverse C4 grasses. New Phytol. 225, 169–182. doi: 10.1111/nph.16106
Pitaloka, M. K., Caine, R. S., Hepworth, C., Harrison, E. L., Sloan, J., Chutteang, C., et al. (2022). Induced genetic variations in stomatal density and size of rice strongly affects water use efficiency and responses to drought stresses. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.801706
Rao, J. T. (1951). Xeromorphic adaptations in sugar cane for resistance to drought. Proc. Int. Soc. Sugar Cane Technol. 7, 82–89.
Robertson, M. J., Inman-Bamber, N. G., Muchow, R. C., and Wood, A. W. (1999). Physiology and productivity of sugarcane with early and mid-season water deficit. Field Crops Res. 64, 211–227. doi: 10.1016/S0378-4290(98)00167-1
Sage, R. F., Peixoto, M. M., and Sage, T. L. (2013). “Photosynthesis in sugarcane,” in Sugarcane: Physiology, biochemistry, and functional biology, 121–154. doi: 10.1002/9781118771280.ch6. NJ, USA: John Wiley & Sons, Inc.
Singels, A., Paraskevopoulos, A. L., and Mashabela, M. L. (2019). Farm level decision support for sugarcane irrigation management during drought. Agric. Water Manage. 222, 274–285. doi: 10.1016/j.agwat.2019.05.048
Strock, C. F., Schneider, H. M., and Lynch, J. P. (2022). Anatomics: High-throughput phenotyping of plant anatomy. Trends Plant Sci. 27, 520–523. doi: 10.1016/j.tplants.2022.02.009
Tagane, S., Ponragdee, W., Sansayawichai, T., Sugimoto, A., and Terajima, Y. (2012). Characterization and taxonomical note about Thai Erianthus germplasm collection: the morphology, flowering phenology and biogeography among E. procerus and three types of E. arundinaceus. Genet. Resour. Crop Evol. 59, 769–781. doi: 10.1007/s10722-011-9717-2
Takaragawa, H., Asahi, T., Mitsuoka, M., Taira, E., and Kawamitsu, Y. (2025). Establishment of a low-cost photosynthesis measurement system based on a single-board microcomputer and CO2 sensors. Photosynthesis Res. 163(5), 52. doi: 10.1007/s11120-025-01170-5
Takaragawa, H. and Matsuda, H. (2023). Rapid evaluation of leaf photosynthesis using a closed-chamber system in a C4 plant, sugarcane. Plant Production Sci. 26, 174–186. doi: 10.1080/1343943X.2023.2210766
Takaragawa, H., Matsuda, H., and Terajima, Y. (2023). Acidic soil tolerance of sugarcane and Erianthus root assessed by cell membrane stability. Plant Root 17, 26–35. doi: 10.3117/plantroot.17.26
Takaragawa, H., Okamoto, K., Terajima, Y., and Anzai, T. (2022). Evaluation of root distribution and nitrate leaching in sugarcane, Erianthus, and their intergeneric hybrid at new planting. Plant Production Sci. 25, 298–310. doi: 10.1080/1343943X.2022.2097098
Takaragawa, H. and Wakayama, M. (2024). Responses of leaf gas exchange and metabolites to drought stress in different organs of sugarcane and its closely related species Erianthus arundinaceus. Planta 260, 90. doi: 10.1007/s00425-024-04508-w
Tanaka, Y., Adachi, S., and Yamori, W. (2019). Natural genetic variation of the photosynthetic induction response to fluctuating light environment. Curr. Opin. Plant Biol. 49, 52–59. doi: 10.1016/j.pbi.2019.04.010
Tanaka, Y., Taniyoshi, K., Imamura, A., Mukai, R., Sukemura, S., Sakoda, K., et al. (2021). MIC-100, a new system for high-throughput phenotyping of instantaneous leaf photosynthetic rate in the field. Funct. Plant Biol. 49, 496–504. doi: 10.1071/FP21029
Terajima, Y., Ponragdee, W., Sansayawichai, T., Tippayawat, A., Chanachai, S., Ebina, M., et al. (2022). Genetic variation in agronomic traits of Erianthus germplasm under multiple-ratoon crops in Thailand. Crop Sci. 62, 1531–1549. doi: 10.1002/csc2.20697
Terajima, Y., Sugimoto, A., Tippayawat, A., Irei, S., and Hayashi, H. (2023). Root distribution and fibre composition of intergeneric F1 hybrid between sugarcane and E. arundinaceus. Field Crops Res. 297, 108920. doi: 10.1016/j.fcr.2023.108920
Tominaga, J., Inafuku, S., Coetzee, T., and Kawamitsu, Y. (2014). Diurnal regulation of photosynthesis in Jatropha curcas under drought during summer in a semi-arid region. Biomass Bioenergy 67, 279–287. doi: 10.1016/j.biombioe.2014.05.010
Tsuruta, S. I., Ebina, M., Kobayashi, M., Hattori, T., and Terauchi, T. (2012). Analysis of genetic diversity in the bioenergy plant Erianthus arundinaceus (Poaceae: Andropogoneae) using amplified fragment length polymorphism markers. Grassland Sci. 58, 174–177. doi: 10.1111/j.1744-697X.2012.00258.x
Tsuruta, S. I., Ebina, M., Terajima, Y., Kobayashi, M., and Takahashi, W. (2017). Genetic variability in Erianthus arundinaceus accessions native to Japan based on nuclear DNA content and simple sequence repeat markers. Acta Physiologiae Plantarum 39, 1–13. doi: 10.1007/s11738-017-2519-1
Tsuruta, S. I., Srithawong, S., Sakuanrungsirikul, S., Ebina, M., Kobayashi, M., Terajima, Y., et al. (2022). Erianthus germplasm collection in Thailand: genetic structure and phylogenetic aspects of tetraploid and hexaploid accessions. BMC Plant Biol. 22, 45. doi: 10.1186/s12870-021-03418-3
von Caemmerer, S. and Furbank, R. T. (2003). The C4 pathway: an efficient CO2 pump. Photosynthesis Res. 77, 191–207. doi: 10.1023/A:1025830019591
Watanabe, K., Takaragawa, H., Fukuzawa, Y., Ueno, M., and Kawamitsu, Y. (2021). Relationships between water balance and the growth and yield of ratoon sugarcane on Minamidaito island. Japanese J. Crop Sci. 90, 324–333. doi: 10.1626/jcs.90.324
Wei, X. and & Jackson, P. (2016). Addressing slow rates of long-term genetic gain in sugarcane. Proc. Int. Soc. Sugar Cane Technol. 29, 1923–1930.
Wu, S. and Zhao, B. (2017). Using clear nail polish to make Arabidopsis epidermal impressions for measuring the change of stomatal aperture size in immune response. In: Shan, L. and He, P. (eds) Plant pattern recognition receptors. Methods and protocols. Methods Mol. Biol. 1578, 243–248. doi: 10.1007/978-1-4939-6859-6_20
Xiong, D. and Flexas, J. (2020). From one side to two sides: the effects of stomatal distribution on photosynthesis. New Phytol. 228, 1754–1766. doi: 10.1111/nph.16801
Xu, Z. and Zhou, G. (2008). Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot. 59, 3317–3325. doi: 10.1093/jxb/ern185
Keywords: assimilation rate, A vs gs curve, drought tolerance, intergeneric F1 hybrid, photosynthetic rate, stomatal conductance, stomatal density, transpiration efficiency
Citation: Takaragawa H, Terajima Y and Okamoto K (2025) Improvement in the intrinsic water use efficiency of sugarcane by intergeneric hybridization with Erianthus arundinaceus. Front. Plant Sci. 16:1649112. doi: 10.3389/fpls.2025.1649112
Received: 18 June 2025; Accepted: 09 October 2025;
Published: 21 November 2025.
Edited by:
Takaki Yamauchi, Nagoya University, JapanReviewed by:
Congcong Guo, Hebei Agricultural University, ChinaNakorn Jongrungklang, Khon Kaen University, Thailand
Copyright © 2025 Takaragawa, Terajima and Okamoto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hiroo Takaragawa, dGFrYXJhZ2F3YWgwMzE4QGppcmNhcy5nby5qcA==
 Hiroo Takaragawa
Hiroo Takaragawa Yoshifumi Terajima
Yoshifumi Terajima Ken Okamoto
Ken Okamoto