- 1Austrian Centre of Industrial Biotechnology (ACIB), Graz, Austria
- 2Institute of Environmental Biotechnology, Graz University of Technology, Graz, Austria
- 3SAN Agrow Holding GmbH, Herzogenburg, Austria
- 4Microbiome Biotechnology, Leibniz Institute for Agricultural Engeneering and Bioeconomy (ATB), Potsdam, Germany
- 5Institute for Biochemistry and Biology, University of Potsdam, Potsdam, Germany
Aureobasidium pullulans is a globally distributed fungus commonly found in plant-associated and anthropogenic environments. Known for its antagonistic activity against plant pathogens, it is widely used as a biocontrol agent in sustainable agriculture. Despite its prevalence in edible plant tissues and frequent environmental exposure, its broader role within microbiomes and potential relevance for human health remain underexplored. In this perspective article, we highlight the global distribution of A. pullulans based on publicly available sequencing data and examine its ecological function from a microbiome-based viewpoint. Our synthesis supports the view of A. pullulans as a safe, plant-beneficial symbiont with high value for sustainable crop protection and potential relevance for the One Health framework. Future microbiome research should further explore its functional roles within plant and human-associated microbiomes to better harness its benefits while ensuring biosafety across ecosystems.
Introduction
The fungus Aureobasidium pullulans (De Bary) Arnaud, commonly known as the ‘black yeast’, was first described 150 years ago (Cooke, 1959). At that time, like the majority of microorganisms, A. pullulans was subjected to an anthropocentric perspective on the microbial world, i.e., the mere presence of a microorganism implies disease (Heidenreich et al., 1997). Though ahead of the times, Cooke discussed the ecological life history of A. pullulans in 1959 and highlighted that more intense studies may demonstrate its independence from saprobic and pathogenic strains (Cooke, 1959). The discovery of the microbiome, a term first defined by Whipps et al. (1988) and newly conceptualized by Berg et al. (2020) has provided an alternative to the anthropocentric perspective on microbial life. Microorganisms are ubiquitous providers of key ecosystem services and are, thus, intrinsically associated with the health of eukaryotic hosts. This has led to the definition of the holobiont, which refers to a host organism together with all of its associated microorganisms, including bacteria, archaea, fungi, viruses, and protists, forming a complex ecological unit (Vandenkoornhuyse et al., 2015). This concept emphasizes that the biology, evolution, and health of the host cannot be fully understood without considering its microbial interactions and co-evolutionary dynamics. Furthermore, the microbiome interconnects holobionts; for example, plant-associated bacteria in food can withstand human digestion (Wicaksono et al., 2022) and may inhabit the human gut, representing an underexplored but important component of the exposome (Wicaksono et al., 2023a), which is defined as the sum of exposures to which an individual is subjected during their lifespan.
A. pullulans is a frequent member of the environmental microbiome. Due to its targeted antagonistic activity, the fungus can protect crops against various plant pathogens, such as Monilinia laxa, Botrytis cinerea, Alternaria alternata, and Fusarium spp (Zajc et al., 2020; Iqbal et al., 2021; Wachowska et al., 2021; Di Francesco et al., 2023). Initially, A. pullulans was categorized into four subspecies: A. pullulans var. pullulans, var. melanogenum, var. subglaciale, and var. namibiae (Zalar et al., 2008). However, significant genomic differences among these groups warranted their reclassification as four distinct species: A. pullulans, A. subglaciale, A. namibiae, and A. melanogenum (Gostinčar et al., 2014). This revised taxonomy is particularly important for biotechnological applications in agriculture, as it clearly distinguishes A. melanogenum – a species with strains that may possess pathogenic potential for humans – from the agriculturally relevant species A. pullulans (Gostinčar et al., 2014; Černoša et al., 2025). A. pullulans has been considered safe for various agricultural applications (European Food Safety Authority, 2013; Prasongsuk et al., 2018), and its unparalleled global distribution and use for a sustainable economy (Rensink et al., 2024) calls for studying it in relation to the One Health concept. The importance of A. pullulans as an effective biocontrol agent, as well as its applicability in diverse sectors of the sustainable food industry, has been comprehensively reviewed by Di Francesco and colleagues (Di Francesco et al., 2023). However, a microbiome-based perspective on the global and host-associated role of A. pullulans is still missing. In this perspective paper, we review the literature on A. pullulans occurrence from a microbiome-based perspective to gain new insights into its global prevalence in different biomes and the potential for human exposure by representing a common member of the edible plant microbiome.
A. pullulans is prevalent in anthropogenic environments
A. pullulans is known for its host- and non-host-associated lifestyles. The fungus has been detected in numerous ecosystems, ranging from soils (Ignatova et al., 2015; Ademakinwa and Agboola, 2016; Bennamoun et al., 2016), freshwater and marine environments (Gunde-Cimerman et al., 2000; Wang et al., 2009), deserts and drylands (Coleine et al., 2021), glaciers’ ice and permafrost (Branda et al., 2010; Sannino et al., 2020), as well as in the air and atmosphere (Shelton et al., 2002; Griffin, 2007). A. pullulans survives in acidic and alkaline surroundings (Cooke, 1959), and saline soils (Bennamoun et al., 2016). Due to its prevalence in extreme habitats, the fungus was described as a polyextremotolerant microorganism (Gostinčar et al., 2023) because it can survive cold as well as hot temperatures up to 50°C (Zajc et al., 2020). However, A. pullulans does not grow well at 37°C (Zajc et al., 2020). Interestingly, the fungus does not show substantial specialization in any of these habitats at the genomic level (Gostinčar et al., 2019). Frequent recombination between A. pullulans strains could diminish the structuring of the global A. pullulans population (Gostinčar et al., 2019).
We conducted a taxonomy-based search for A. pullulans in the GlobalFungi database (Větrovský et al., 2020), which contains high-throughput sequencing metabarcoding studies, to illustrate its global distribution (Figure 1). We used two search terms to query the database: (i) an empty prompt to retrieve all samples and (ii) “Aureobasidium pullulans” to obtain samples where A. pullulans was detected. These two tables were merged, and a total of 57’184 samples were obtained (as of 02.11.2023). We included only samples from 515 studies that were not flagged as “manipulated”, used non-nested primers (covering ITS1, ITS2, or full-length ITS), and contained at least 500 samples per study. This resulted in a total of (i) 50’084 total samples and (ii) 10’191 samples in which A. pullulans was detected. Due to the compositional nature of the sequencing data and the high variability among reads between the different primers (Supplementary Figures 1, 2), we used presence/absence to delineate the global distribution of A. pullulans. Nonetheless, with an increasing number of reads, the detection of A. pullulans was more likely, and this was reflected in an increased prevalence (Supplementary Figure 3). Hence, we used prevalence for the purpose of delineating the occurrence probability of A. pullulans globally.
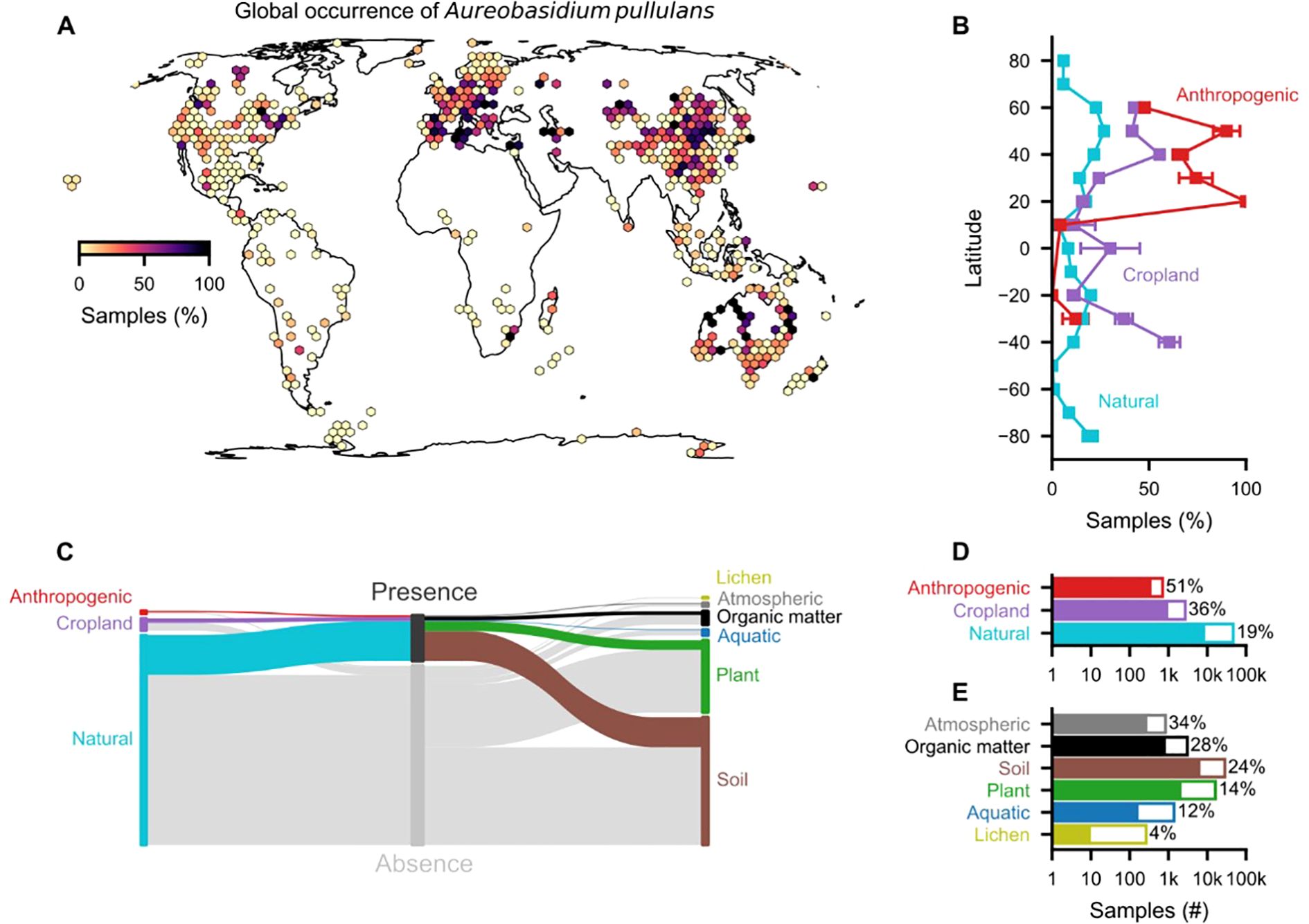
Figure 1. Global occurrence of A. pullulans. (A) Percentage of samples with A. pullulans out of 515 studies based on ITS sequences (ITS1, ITS2, full-length ITS), showing a total of 57’184 samples. (B) Latitudinal occurrence patterns in different environments (mean ± SE). (C) Distribution of samples with the presence/absence of A. pullulans in different environments and sample types. (D) Total number of samples in different environments and (E) sample types; percentages are represented by the colored part of the bars and indicate the fraction of samples with A. pullulans. The 50’084 samples were obtained from the GlobalFungi database (02.11.2023; Větrovský et al., 2020).
Our analysis demonstrates that A. pullulans can occur in various environments. Although A. pullulans occurred on all continents, it was more often detected in anthropogenic environments (51% of n = 707 samples) and croplands (36%, n = 2’713) compared to natural environments (19%, n = 46’664). Interestingly, A. pullulans was most prevalent in atmospheric samples, including air and dust. The high prevalence in soil (24%, n = 28’433), organic matter (28%, n = 3043), and atmospheric samples (34%, n = 838) suggests that it is often present in our surroundings. Hence, we expect frequent human exposure to A. pullulans across different environments with a low risk for hazard incidents (Prasongsuk et al., 2018).
A. pullulans is an effective biological agent in agriculture
The prevalence of A. pullulans in croplands is likely attributable to its broad use as an effective biocontrol agent against bacterial and fungal phytopathogens. Several organic disease control products based on A. pullulans are already on the market (e.g., Boni Protect, Blossom Protect, Botector) and are highly promising alternatives to problematic chemicals in viticulture and horticulture, both pre- and postharvest. This is of high importance considering, for instance, the European Green Deal proposing a reduction of the use and risk of pesticides by 50% by 2030.
Over the past decades, A. pullulans strains have been applied as single organisms, and in combination with other organisms and chemical peptides (Zajc et al., 2020). Nevertheless, most studies focus on fruit crops, whereas the potential impact of A. pullulans on cereals and legumes is less explored. Pre-harvest applications have shown effectiveness against pathogens such as Erwinia amylovora in apples (Slack et al., 2019; Zeng et al., 2023), Diplodia seriata in grapevines (Pinto et al., 2018), and Verticillium dahliae in olive trees (López-Moral et al., 2021, 2022). Post-harvest studies have focused mainly on fruits, demonstrating biocontrol of Botrytis cinerea in strawberries, apples, and grapes (Schena et al., 2003; Mari et al., 2012; Iqbal et al., 2022), Monilinia laxa in stone fruits (Zhang et al., 2010; Di Francesco et al., 2018), or diverse Penicillium species in tropical and non-tropical fruits (Ippolito et al., 2000; Janisiewicz et al., 2000; Zhang et al., 2010, p. 20; Mari et al., 2012; Parafati et al., 2017), just to name some examples. In some cases, it has been used successfully in microbial consortia with Bacillus subtilis (Bellamy et al., 2022). In a recent study, A. pullulans was transmitted via bees to strawberry flowers, resulting in decreased strawberry post-harvest infections with B. cinerea (Iqbal et al., 2022). The potential impact of A. pullulans on insects and their microbiome has not been assessed so far (Davis and Landolt, 2013; Hung et al., 2015). Overall, A. pullulans is mainly used as a direct antagonist towards phytopathogens, and the main mode of action of A. pullulans is referred to as a competition for space and nutrients. However, A. pullulans might also have the ability, due to its wide genetic equipment, to induce systemic resistance in plants, which was shown lately by Zeng et al. (2023), demonstrating increased gene expression of pathogenesis-related genes. Further evidence for A. pullulans’ broad spectrum application potential is given by its postulated function in abiotic stress management of coniferous trees under drought stress (Mannaa et al., 2023), underlining the global potential of A. pullulans.
Another important consideration for the use of biocontrol agents is their interaction with the native microbiome in plants and soil, although this aspect has been less explored to date. A recent study showed that the dominance of A. pullulans on fruit surfaces resulted in a decreased abundance of naturally occurring phytopathogenic fungi and an increased proportion of bacteria with plant growth-promoting properties (Shi et al., 2022). Since the application and occurrence of A. pullulans seems to potentially result in benefits for the plant, we suggest that A. pullulans could be considered a fungal soterobiont. This term has recently been postulated to describe microorganisms – artificially applied or native to the plant – that can extend the host plant´s immune system by providing active protection against pathogens, which results in resistant phenotypes (Cernava and Berg, 2022). However, both genetic and functional aspects of A. pullulans, the host plant, and other members of the plant microbiota must be considered to build a comprehensive understanding of the dynamics within the holobiont.
A. pullulans is a native member of the plant and the edible microbiome
The native plant microbiome assists the host plant in acquiring nutrients, suppressing pathogens, enhancing stress tolerance, and regulating plant hormones (Sánchez-Cañizares et al., 2017; Berg et al., 2022; Gross, 2022). Thus, plants rely on their associated microbiota and are unlikely to survive without them under natural conditions (Paasch et al., 2023). A. pullulans displays a plethora of properties and was observed to natively colonize various plants, including wheat (Wachowska et al., 2020), apple (Abdelfattah et al., 2022), grapevine (Wassermann et al., 2021), olive trees (López-Moral et al., 2021), Ficus (Singh and Saini, 2008), wildflowers (Choudhury et al., 2011), and seeds of native alpine plants (Wassermann et al., 2019a). In addition, A. pullulans was frequently documented to occur in the edible parts of fresh produce, such as apples (Wassermann et al., 2019b; Abdelfattah et al., 2021; Zhimo et al., 2022), cherries (Schena et al., 2003; Molnárová et al., 2014), peaches (Zhang et al., 2010; Molnárová et al., 2014), citrus (Ferraz et al., 2016), and strawberries (Adikaram et al., 2002), and a large body of literature observed A. pullulans in berries of grapevine (Fleet, 2003; Martini et al., 2009; Verginer et al., 2010; Grube et al., 2011; Barata et al., 2012; Pinto et al., 2014). However, these observations are mainly based on PCR-based marker gene profiling or microbial cultivation methods, which do not provide direct information regarding actual microbial loads.
Based on the global data set, we found a wide range of sequence reads of A. pullulans across the different samples, reaching high percentages in certain samples (Supplementary Figure 2). However, microbiome-based quantitative data on the fungal plant microbiota remain limited. To estimate the load of A. pullulans in plant tissues, we analyzed a previously published dataset on the apple microbiome (Abdelfattah et al., 2022). The data includes marker gene sequences (ITS) and quantitative real-time PCR (ITS gene copy numbers (GCN) per centimeter of shoot length) measurements for the endophytic microbiota of 61 apple accessions from 11 Malus species. The total fungal load ranged from 106 GCN cm-1 to 109 GCN cm-1 in domesticated apples (Abdelfattah et al., 2022), and A. pullulans sequences accounted for 36% to 51% of all fungal GCN in these samples, indicating that the fungus is a native and significant member of the apple microbiome. This example supports A. pullulans’ environmental prevalence and shows its potential for host interaction, as microbial abundance is a key determinant of ecological relevance and functional impact on the host (Lloréns-Rico et al., 2021).
In general, while all niche-specific microbiota play a functional role for the plant (Trivedi et al., 2020), and for humans as consumers, microbes associated with the edible parts of a plant can pose health benefits and risks (Berg et al., 2015; Kim et al., 2020). The risks are deeply studied, yet human pathogens causing food-borne outbreaks, as well as opportunistic pathogens that cause healthcare-associated infections (Mehta et al., 2017), are still of global concern, accelerated by the drivers of the Anthropocene (Flandroy et al., 2018). However, edible plants are colonized by a huge diversity of microorganisms, and only a very small fraction may have adverse impacts on healthy humans (Berg et al., 2015). Those microbes represent the edible plant microbiome and are an important component of the exposome (Berg et al., 2015; Wicaksono et al., 2023a). Studies suggest that the edible microbiome may positively impact the gut microbiome and human health. Fruit and vegetable-derived bacteria are, despite their low abundance, consistently present in the human gut, enriching the functional diversity of the gut microbiota due to the presence of genes associated with benefits for human health (Wicaksono et al., 2023b). Besides bacteria, eukaryotic organisms are important components of the gut microbiome (Zhang et al., 2022); yet, to our knowledge, no comparable studies on fruit- and vegetable-transmitted fungi in the human gut have been conducted so far (Laforest-Lapointe and Arrieta, 2018). Nonetheless, A. pullulans has been detected in stool samples of healthy humans (Maas et al., 2023). In addition, it is known that fungal β-glucans play an important role in the human immune system (Zhang et al., 2022) and also the compounds produced by A. pullulans may have potential health benefits for humans (Ikewaki et al., 2023; Raghavan et al., 2023). Analyzing the diversity of fungi and other eukaryotes in the human gut and whether they are delivered via plant consumption will help uncover those microorganisms’ roles for host health.
Conclusion
A. pullulans is a globally distributed fungus commonly found in anthropogenic environments and croplands. Research indicates that A. pullulans pose minimal health risks to humans (Gostinčar et al., 2014). Yet, given its widespread occurrence in food and the environment, animal and human exposure is probable, necessitating comprehensive risk assessments to ensure safety. The fungus’s beneficial properties for plants, including pathogen suppression and crop protection, along with its native abundance in wild plants and crops, underscore its potential relevance for future agricultural practices aligned with the One Health framework. Future research should focus on its interaction with the native plant and the environmental microbiome. Further, its role within the human exposome and gut microbiome needs to be explored to better understand its interactions and any potential health implications.
Author contributions
NB: Writing – original draft, Writing – review & editing. BW: Writing – original draft, Writing – review & editing. SB: Writing – original draft, Writing – review & editing. RO: Writing – review & editing. AM: Writing – review & editing. GB: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The COMET center: acib: Next Generation Bioproduction is funded by BMIMI, BMWET, SFG, Standortagentur Tirol, Government of Lower Austria aund Vienna Business Agency in the framework of COMET -Competence Centers for Excellent Technologies. The COMET-Funding Program is managed by the Austrian Research Promotion Agency FFG. This work was supported by TU Graz Open Access Publishing Fund.
Conflict of interest
Author RO and AM were employed by the company SAN Agrow Holding GmbH.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1652366/full#supplementary-material
References
Abdelfattah, A., Freilich, S., Bartuv, R., Zhimo, V. Y., Kumar, A., Biasi, A., et al. (2021). Global analysis of the apple fruit microbiome: are all apples the same? Environ. Microbiol. 23, 6038–6055. doi: 10.1111/1462-2920.15469
Abdelfattah, A., Tack, A. J. M., Wasserman, B., Liu, J., Berg, G., Norelli, J., et al. (2022). Evidence for host–microbiome co-evolution in apple. New Phytol. 234, 2088–2100. doi: 10.1111/nph.17820
Ademakinwa, A. N. and Agboola, F. K. (2016). Biochemical characterization and kinetic studies on a purified yellow laccase from newly isolated Aureobasidium pullulans NAC8 obtained from soil containing decayed plant matter. J. Genet. Eng. Biotechnol. 14, 143–151. doi: 10.1016/j.jgeb.2016.05.004
Adikaram, N. K. B., Joyce, D. C., and Terryc, L. A. (2002). Biocontrol activity and induced resistance as a possible mode of action for Aureobasidium pullulans against grey mould of strawberry fruit. Australas. Plant Pathol. 31, 223–229. doi: 10.1071/AP02017
Barata, A., Malfeito-Ferreira, M., and Loureiro, V. (2012). The microbial ecology of wine grape berries. Int. J. Food Microbiol. 153, 243–259. doi: 10.1016/j.ijfoodmicro.2011.11.025
Bellamy, S., Shaw, M., and Xu, X. (2022). Field application of Bacillus subtilis and Aureobasidium pullulans to reduce Monilinia laxa post-harvest rot on cherry. Eur. J. Plant Pathol. 163, 761–766. doi: 10.1007/s10658-022-02508-8
Bennamoun, L., Hiligsmann, S., Dakhmouche, S., Ait-Kaki, A., Labbani, F.-Z., Nouadri, T., et al. (2016). Production and Properties of a Thermostable, pH—Stable Exo-Polygalacturonase Using Aureobasidium pullulans Isolated from Saharan Soil of Algeria Grown on Tomato Pomace. Foods 5, 72. doi: 10.3390/foods5040072
Berg, G., Erlacher, A., and Grube, M. (2015). “The edible plant microbiome: importance and health issues,” in Principles of Plant-Microbe Interactions (Springer International Publishing, Cham), 419–426. doi: 10.1007/978-3-319-08575-3_44
Berg, G., Rybakova, D., Fischer, D., Cernava, T., Vergès, M.-C. C., Charles, T., et al. (2020). Microbiome definition re-visited: old concepts and new challenges. Microbiome 8, 103. doi: 10.1186/s40168-020-00875-0
Berg, G., Kusstatscher, P., Wassermann, B., Cernava, T., and Abdelfattah, A.. (2022). “Beneficial microbes for agriculture,” in Good Microbes in Medicine, Food Production, Biotechnology, Bioremediation, and Agriculture, eds. De Bruijn, F. J., Smidt, H., Cocolin, L. S., Sauer, M., Dowling, D., and Thomashow, L. (Wiley), 427–443. doi: 10.1002/9781119762621.ch34
Branda, E., Turchetti, B., Diolaiuti, G., Pecci, M., Smiraglia, C., and Buzzini, P. (2010). Yeast and yeast-like diversity in the southernmost glacier of Europe (Calderone Glacier, Apennines, Italy). FEMS Microbiol. Ecol. 72, 354–369. doi: 10.1111/j.1574-6941.2010.00864.x
Cernava, T. and Berg, G. (2022). The emergence of disease-preventing bacteria within the plant microbiota. Environ. Microbiol. 24, 3259–3263. doi: 10.1111/1462-2920.15896
Černoša, A., Gostinčar, C., Holcar, M., Kostanjšek, R., Lenassi, M., and Gunde-Cimerman, N. (2025). The impact of Aureobasidium melanogenum cells and extracellular vesicles on human cell lines. Sci. Rep. 15, 1413. doi: 10.1038/s41598-024-84189-3
Choudhury, A. R., Saluja, P., and Prasad, G. S. (2011). Pullulan production by an osmotolerant Aureobasidium pullulans RBF-4A3 isolated from flowers of Caesulia axillaris. Carbohydr. Polymers 83, 1547–1552. doi: 10.1016/j.carbpol.2010.10.003
Coleine, C., Stajich, J. E., de los Ríos, A., and Selbmann, L. (2021). Beyond the extremes: Rocks as ultimate refuge for fungi in drylands. Mycologia 113, 108–133. doi: 10.1080/00275514.2020.1816761
Cooke, W. (1959). An ecological life history of Aureobasidium pullulans (de Bary) Arnaud. Mycopathologia Mycologia Applicata 12, 1–45. doi: 10.1007/BF02118435
Davis, T. S. and Landolt, P. J. (2013). A survey of insect assemblages responding to volatiles from a ubiquitous fungus in an agricultural landscape. J. Chem. Ecol. 39, 860–868. doi: 10.1007/s10886-013-0278-z
Di Francesco, A., Mari, M., Ugolini, L., and Baraldi, E. (2018). Effect of Aureobasidium pullulans strains against Botrytis cinerea on kiwifruit during storage and on fruit nutritional composition. Food Microbiol. 72, 67–72. doi: 10.1016/j.fm.2017.11.010
Di Francesco, A., Zajc, J., and Stenberg, J. A. (2023). Aureobasidium spp.: diversity, versatility, and agricultural utility. Horticulturae 9, 59. doi: 10.3390/horticulturae9010059
European Food Safety Authority (2013). Conclusion on the peer review of the pesticide risk assessment of the active substance Aureobasidium pullulans (strains DSM 14940 and DSM 14941). EFSA J. 11. doi: 10.2903/j.efsa.2013.3183
Ferraz, L. P., da Cunha, T., da Silva, A. C., and Kupper, K. C. (2016). Biocontrol ability and putative mode of action of yeasts against Geotrichum citri-aurantii in citrus fruit. Microbiological Res., 188–189, 72–79. doi: 10.1016/j.micres.2016.04.012
Flandroy, L., Poutahidis, T., Berg, G., Clarke, G., Dao, M.-C., Decaestecker, E., et al. (2018). The impact of human activities and lifestyles on the interlinked microbiota and health of humans and of ecosystems. Sci. Total Environ. 627, 1018–1038. doi: 10.1016/j.scitotenv.2018.01.288
Fleet, G. (2003). Yeast interactions and wine flavour. Int. J. Food Microbiol. 86, 11–22. doi: 10.1016/S0168-1605(03)00245-9
Gostinčar, C., Ohm, R. A., Kogej, T., Sonjak, S., Turk, M., Zajc, J., et al. (2014). Genome sequencing of four Aureobasidium pullulans varieties: biotechnological potential, stress tolerance, and description of new species. BMC Genomics 15, 549. doi: 10.1186/1471-2164-15-549
Gostinčar, C., Turk, M., Zajc, J., and Gunde‐Cimerman, N. (2019). Fifty Aureobasidium pullulans genomes reveal a recombining polyextremotolerant generalist. Environ. Microbiol. 21, 3638–3652. doi: 10.1111/1462-2920.14693
Gostinčar, C., Stajich, J. E., and Gunde-Cimerman, N. (2023). Extremophilic and extremotolerant fungi. Curr. Biol. 33, R752–R756. doi: 10.1016/j.cub.2023.06.011
Griffin, D. W. (2007). Atmospheric movement of microorganisms in clouds of desert dust and implications for human health. Clin. Microbiol. Rev. 20, 459–477. doi: 10.1128/CMR.00039-06
Gross, M. (2022). How plants grow their microbiome. Curr. Biol. 32, R97–R100. doi: 10.1016/j.cub.2022.01.044
Grube, M., Schmid, F., and Berg, G. (2011). Black fungi and associated bacterial communities in the phyllosphere of grapevine. Fungal Biol. 115, 978–986. doi: 10.1016/j.funbio.2011.04.004
Gunde-Cimerman, N., Zalar, P., Hoog, S., and PlemenitaÅš, A. (2000). Hypersaline waters in salterns â&128;&147; natural ecological niches for halophilic black yeasts’. FEMS Microbiol. Ecol. 32, 235–240. doi: 10.1111/j.1574-6941.2000.tb00716.x
Heidenreich, M. C. M., Corral-Garcia, M. R., Momol, E. A., and Burr, T. J. (1997). Russet of Apple Fruit Caused by Aureobasidium pullulans and Rhodotorula glutinis. Plant Dis. 81, 337–342. doi: 10.1094/PDIS.1997.81.4.337
Hung, K. Y., Michailides, T. J., Millar, J. G., Wayadande, A., and Gerry, A. C. (2015). House fly (Musca domestica L.) attraction to insect honeydew. PloS One 10, e0124746. doi: 10.1371/journal.pone.0124746
Ignatova, L. V., Brazhnikova, Y. V., Berzhanova, R. Z., and Mukasheva, T. D. (2015). Plant growth-promoting and antifungal activity of yeasts from dark chestnut soil. Microbiological Res. 175, 78–83. doi: 10.1016/j.micres.2015.03.008
Ikewaki, N., Sonoda, T., Kurosawa, G., Iwasaki, M., Devaprasad Dedeepiya, V., Senthilkumar, R., et al. (2023). Beta 1,3-1,6 glucans produced by two novel strains of aureobasidium pullulans exert immune and metabolic beneficial effects in healthy middle-aged Japanese men: results of an exploratory randomized control study. J. Aging Res. Lifestyle 12, 61–71. doi: 10.14283/jarlife.2023.11
Ippolito, A., El Ghaouth, A., Wilson, C. L., and Wisniewski, M. (2000). Control of postharvest decay of apple fruit by Aureobasidium pullulans and induction of defense responses. Postharvest Biol. Technol. 19, 265–272. doi: 10.1016/S0925-5214(00)00104-6
Iqbal, M., Jamshaid, M., Zahid, M. A., Andreasson, E., Vetukuri, R. R., and Stenberg, J. A. (2021). Biological control of strawberry crown rot, root rot and grey mould by the beneficial fungus Aureobasidium pullulans. BioControl 66, 535–545. doi: 10.1007/s10526-021-10083-w
Iqbal, M., Jützeler, M., França, S. C., Wäckers, F., Andreasson, E., and Stenberg, J. A. (2022). Bee-vectored Aureobasidium pullulans for biological control of Gray Mold in strawberry. Phytopathology® 112, 232–237. doi: 10.1094/PHYTO-05-21-0205-R
Janisiewicz, W. J., Tworkoski, T. J., and Sharer, C. (2000). Characterizing the mechanism of biological control of postharvest diseases on fruits with a simple method to study competition for nutrients. Phytopathology® 90, 1196–1200. doi: 10.1094/PHYTO.2000.90.11.1196
Kim, J., Yoon, S.-J., Park, Y.-J., Kim, S.-Y., and Ryu, C.-M. (2020). Crossing the kingdom border: Human diseases caused by plant pathogens. Environ. Microbiol. 22, 2485–2495. doi: 10.1111/1462-2920.15028
Laforest-Lapointe, I. and Arrieta, M.-C. (2018). Microbial eukaryotes: a missing link in gut microbiome studies. mSystems 3, e00201–e00217. doi: 10.1128/mSystems.00201-17
Lloréns-Rico, V., Vieira-Silva, S., Gonçalves, P. J., Falony, G., and Raes, J. (2021). Benchmarking microbiome transformations favors experimental quantitative approaches to address compositionality and sampling depth biases. Nat. Commun. 12. doi: 10.1038/s41467-021-23821-6
López-Moral, A., Agustí-Brisach, C., and Trapero, A. (2021). Plant biostimulants: new insights into the biological control of verticillium wilt of olive. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.662178
López-Moral, A., Llorens, E., Scalschi, L., García-Agustín, P., Trapero, A., and Agustí-Brisach, C. (2022). Resistance induction in olive tree (Olea europaea) against verticillium wilt by two beneficial microorganisms and a copper phosphite fertilizer. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.831794
Maas, E., Penders, J., and Venema, K. (2023). Fungal-bacterial interactions in the human gut of healthy individuals. J. Fungi 9, 139. doi: 10.3390/jof9020139
Mannaa, M., Han, G., Jung, H., Park, J., Kim, J.-C., Park, A. R., et al. (2023). Aureobasidium pullulans Treatment Mitigates Drought Stress in Abies koreana via Rhizosphere Microbiome Modulation. Plants 12, 3653. doi: 10.3390/plants12203653
Mari, M., Martini, C., Spadoni, A., Rouissi, W., and Bertolini, P. (2012). Biocontrol of apple postharvest decay by Aureobasidium pullulans. Postharvest Biol. Technol. 73, 56–62. doi: 10.1016/j.postharvbio.2012.05.014
Martini, M., Musetti, R., Grisan, S., Polizzotto, R., Borselli, S., Pavan, F., et al. (2009). DNA-Dependent Detection of the Grapevine Fungal Endophytes Aureobasidium pullulans and Epicoccum nigrum. Plant Dis. 93, 993–998. doi: 10.1094/PDIS-93-10-0993
Mehta, S. R., Johns, S., Stark, P., and Fierer, J. (2017). Successful treatment of Aureobasidium pullulans central catheter-related fungemia and septic pulmonary emboli. IDCases 10, 65–67. doi: 10.1016/j.idcr.2017.08.017
Molnárová, J., Vadkertiová, R., and Stratilová, E. (2014). Extracellular enzymatic activities and physiological profiles of yeasts colonizing fruit trees. J. Basic Microbiol. 54, S74–S84. doi: 10.1002/jobm.201300072
Paasch, B. C., Sohrabi, R., Kremer, J. M., Nomura, K., Cheng, Y. T., Martz, J., et al. (2023). A critical role of a eubiotic microbiota in gating proper immunocompetence in Arabidopsis. Nat. Plants 9, 1468–1480. doi: 10.1038/s41477-023-01501-1
Parafati, L., Vitale, A., Restuccia, C., and Cirvilleri, G. (2017). Performance evaluation of volatile organic compounds by antagonistic yeasts immobilized on hydrogel spheres against gray, green and blue postharvest decays. Food Microbiol. 63, 191–198. doi: 10.1016/j.fm.2016.11.021
Pinto, C., Pinho, D., Sousa, S., Pinheiro, M., Egas, C., and Gomes, A. C. (2014). Unravelling the diversity of grapevine microbiome. PloS One 9, e85622. doi: 10.1371/journal.pone.0085622
Pinto, C., Custódio, V., Nunes, M., Songy, A., Rabenoelina, F., Courteaux, B., et al. (2018). Understand the potential role of Aureobasidium pullulans, a resident microorganism from grapevine, to prevent the infection caused by Diplodia seriata. Front. Microbiol. 9, 3047. doi: 10.3389/fmicb.2018.03047
Prasongsuk, S., Lotrakul, P., Ali, I., Bankeeree, W., and Punnapayak, H. (2018). The current status of Aureobasidium pullulans in biotechnology. Folia Microbiologica 63, 129–140. doi: 10.1007/s12223-017-0561-4
Raghavan, K., Dedeepiya, V. D., Yamamoto, N., Ikewaki, N., Sonoda, T., Iwasaki, M., et al. (2023). Benefits of gut microbiota reconstitution by beta 1,3–1,6 glucans in subjects with autism spectrum disorder and other neurodegenerative diseases. J. Alzheimer’s Dis. 94, S241–S252. doi: 10.3233/jad-220388
Rensink, S., Van Nieuwenhuijzen, E. J., Sailer, M. F., Struck, C., and Wösten, H. A. B. (2024). Use of Aureobasidium in a sustainable economy. Appl. Microbiol. Biotechnol. 108, 202. doi: 10.1007/s00253-024-13025-5
Sánchez-Cañizares, C., Jorrín, B., Poole, P. S., and Tkacz, A. (2017). Understanding the holobiont: the interdependence of plants and their microbiome. Curr. Opin. Microbiol. 38, 188–196. doi: 10.1016/j.mib.2017.07.001
Sannino, C., Borruso, L., Mezzasoma, A., Battistel, D., Zucconi, L., Selbmann, L., et al. (2020). Intra- and inter-cores fungal diversity suggests interconnection of different habitats in an Antarctic frozen lake (Boulder Clay, Northern Victoria Land). Environ. Microbiol. 22, 3463–3477. doi: 10.1111/1462-2920.15117
Schena, L., Nigro, F., Pentimone, I., Ligorio, A., and Ippolito, A. (2003). Control of postharvest rots of sweet cherries and table grapes with endophytic isolates of Aureobasidium pullulans. Postharvest Biol. Technol. 30, 209–220. doi: 10.1016/S0925-5214(03)00111-X
Shelton, B. G., Kirkland, K. H., Flanders, W. D., and Morris, G. K. (2002). Profiles of airborne fungi in buildings and outdoor environments in the United States. Appl. Environ. Microbiol. 68, 1743–1753. doi: 10.1128/AEM.68.4.1743-1753.2002
Shi, Y., Yang, Q., Zhao, Q., Dhanasekaran, S., Ahima, J., Zhang, X., et al. (2022). Aureobasidium pullulans S-2 reduced the disease incidence of tomato by influencing the postharvest microbiome during storage. Postharvest Biol. Technol. 185, 111809. doi: 10.1016/j.postharvbio.2021.111809
Singh, R. S. and Saini, G. K. (2008). Pullulan-hyperproducing color variant strain of Aureobasidium pullulans FB-1 newly isolated from phylloplane of Ficus sp. Bioresource Technol. 99, 3896–3899. doi: 10.1016/j.biortech.2007.08.003
Slack, S. M., Outwater, C. A., Grieshop, M. J., and Sundin, G. W. (2019). Evaluation of a contact sterilant as a niche-clearing method to enhance the colonization of apple flowers and efficacy of Aureobasidium pullulans in the biological control of fire blight. Biol. Control 139, 104073. doi: 10.1016/j.biocontrol.2019.104073
Trivedi, P., Leach, J. E., Tringe, S. G., Sa, T., and Singh, B. K. (2020). Plant–microbiome interactions: from community assembly to plant health. Nat. Rev. Microbiol. 18, 607–621. doi: 10.1038/s41579-020-0412-1
Vandenkoornhuyse, P., Quaiser, A., Duhamel, M., Le Van, A., and Dufresne, A. (2015). The importance of the microbiome of the plant holobiont. New Phytol. 206, 1196–1206. doi: 10.1111/nph.13312
Verginer, M., Leitner, E., and Berg, G. (2010). Production of volatile metabolites by grape-associated microorganisms. J. Agric. Food Chem. 58, 8344–8350. doi: 10.1021/jf100393w
Větrovský, T., Morais, D., Kohout, P., Lepinay, C., Algora, C., Awokunle Hollá, S., et al. (2020). GlobalFungi, a global database of fungal occurrences from high-throughput-sequencing metabarcoding studies. Sci. Data 7, 228. doi: 10.1038/s41597-020-0567-7
Wachowska, U., Kwiatkowska, E., and Pluskota, W. (2021). Alternaria alternata as a Seed-Transmitted Pathogen of Sida hermaphrodita (Malvaceae) and Its Suppression by Aureobasidium pullulans. Agriculture 11, 1264. doi: 10.3390/agriculture11121264
Wachowska, U., Stuper-Szablewska, K., and Perkowski, J. (2020). Yeasts isolated from wheat grain can suppress fusarium head blight and decrease trichothecene concentrations in bread wheat and durum wheat grain. Polish J. Environ. Stud. 29, 4345–4360. doi: 10.15244/pjoes/118427
Wang, W. L., Chi, Z. M., Chi, Z., Li, J., and Wang, X. H. (2009). Siderophore production by the marine-derived Aureobasidium pullulans and its antimicrobial activity. Bioresource Technol. 100, 2639–2641. doi: 10.1016/j.biortech.2008.12.010
Wassermann, B., Cernava, T., Müller, H., Berg, C., and Berg, G. (2019a). Seeds of native alpine plants host unique microbial communities embedded in cross-kingdom networks. Microbiome 7, 108. doi: 10.1186/s40168-019-0723-5
Wassermann, B., Korsten, L., and Berg, G. (2021). Plant health and sound vibration: analyzing implications of the microbiome in grape wine leaves. Pathogens 10, 63. doi: 10.3390/pathogens10010063
Wassermann, B., Kusstatscher, P., and Berg, G. (2019b). Microbiome response to hot water treatment and potential synergy with biological control on stored apples. Front. Microbiol. 10. doi: 10.3389/fmicb.2019.02502
Whipps, J., Lewis, K., and Cooke, R. (1988). “Mycoparasitism and plant disease control,” in Fungi in biological control systems (Manchester University Press), 161–187.
Wicaksono, W. A., Buko, A., Kusstatscher, P., Cernava, T., Sinkkonen, A., Laitinen, O. H., et al. (2023a). Impact of cultivation and origin on the fruit microbiome of apples and blueberries and implications for the exposome. Microbial Ecol. 86, 973–984. doi: 10.1007/s00248-022-02157-8
Wicaksono, W. A., Buko, A., Kusstatscher, P., Sinkkonen, A., Laitinen, O. H., Virtanen, S. M., et al. (2022). Modulation of the food microbiome by apple fruit processing. Food Microbiol. 108, 104103. doi: 10.1016/j.fm.2022.104103
Wicaksono, W. A., Cernava, T., Wassermann, B., Abdelfattah, A., Soto-Giron, M. J., Toledo, G. V., et al. (2023b). The edible plant microbiome: evidence for the occurrence of fruit and vegetable bacteria in the human gut. Gut Microbes 15, 2258565. doi: 10.1080/19490976.2023.2258565
Zajc, J., Černoša, A., Di Francesco, A., Castoria, R., Curtis, F. D., Lima, G., et al. (2020). Characterization of aureobasidium pullulans isolates selected as biocontrol agents against fruit decay pathogens. Fungal Genom. Biol. 10, 163. doi: 10.35248/2165-8056.20.10.163
Zalar, P., Gostinčar, C., De Hoog, G. S., Uršič, V., Sudhadham, M., and Gunde-Cimerman, N. (2008). Redefinition of Aureobasidium pullulans and its varieties. Stud. Mycology 61, 21–38. doi: 10.3114/sim.2008.61.02
Zeng, Q., Johnson, K. B., Mukhtar, S., Nason, S., Huntley, R., Millet, F., et al. (2023). Aureobasidium pullulans from the fire blight biocontrol product, blossom protect, induces host resistance in apple flowers. Phytopathology® 113, 1192–1201. doi: 10.1094/PHYTO-12-22-0452-R
Zhang, D., Spadaro, D., Garibaldi, A., and Gullino, M. L. (2010). Efficacy of the antagonist Aureobasidium pullulans PL5 against postharvest pathogens of peach, apple and plum and its modes of action. Biol. Control 54, 172–180. doi: 10.1016/j.biocontrol.2010.05.003
Zhang, F., Aschenbrenner, D., Yoo, J. Y., and Zuo, T. (2022). The gut mycobiome in health, disease, and clinical applications in association with the gut bacterial microbiome assembly. Lancet Microbe 3, e969–e983. doi: 10.1016/s2666-5247(22)00203-8
Keywords: one health, crop protection, global occurrence, Aureobasidium pullulans, edible microbiome
Citation: Bziuk N, Wassermann B, Bickel S, Omidvar R, Manica A and Berg G (2025) Aureobasidium pullulans: a microbiome-based perspective from global biomes to edible plant tissues. Front. Plant Sci. 16:1652366. doi: 10.3389/fpls.2025.1652366
Received: 23 June 2025; Accepted: 12 August 2025;
Published: 24 September 2025.
Edited by:
Zhen Wang, Yunnan Agricultural University, ChinaReviewed by:
Adil Zahoor, University of Arkansas, United StatesYohannes Ebabuye Andargie, Kyungpook National University, Republic of Korea
Copyright © 2025 Bziuk, Wassermann, Bickel, Omidvar, Manica and Berg. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Birgit Wassermann, QmlyZ2l0Lndhc3Nlcm1hbm5AdHVncmF6LmF0
†These authors have contributed equally to this work
 Nina Bziuk
Nina Bziuk Birgit Wassermann
Birgit Wassermann Samuel Bickel
Samuel Bickel Reza Omidvar3
Reza Omidvar3 Gabriele Berg
Gabriele Berg