- 1School of Agriculture and Bioengineering, Longdong University, Qingyang, China
- 2Collaborative Innovation Center for Longdong Dryland Crop Germplasm Improvement and Industrialization, Longdong University, Qingyang, China
- 3Gansu Dryland Research Center of Winter Wheat Germplasm Innovation and Application Engineering, Longdong University, Qingyang, China
- 4Gansu Collaborative Innovation of Academicians and Experts on Dryland Agriculture in the Loess Plateau, Longdong University, Qingyang, China
- 5Agronomy College, Gansu Agriculture University, Lanzhou, China
The threshold range of reactive oxygen species (ROS) concentration remains a critical challenge and focal point in future research concerning its influence on the growth and development of both beneficial and harmful plants. This study demonstrates that as the concentration of hydrogen peroxide (H2O2) increases from 0.0% to 0.6%, the seed germination rate gradually rises. At 0.6% H2O2, the germination rate peaks at 94.67%, accompanied by the maximum activities of superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT). However, with further increases in H2O2 concentration (0.7% – 1.3%), the seed germination rate and antioxidant enzyme activity gradually decline, while the levels of superoxide anion (O2-) and H2O2 accumulate progressively. This suggests that higher H2O2 concentrations impair the ROS scavenging capacity in cabbage-type rapeseed, leading to increased ROS production and subsequent inhibition of growth and development. At half-lethal H2O2 concentrations (1.4%–1.5%), the seed germination rate before rehydration is significantly reduced to 10.97% and 9.03%, respectively, but can recover to approximately 50% after rehydration. H2O2 concentrations exceeding 2.2% are lethal, resulting in a 0% seed germination rate both before and after rehydration; notably, the post-rehydration germination rate remains below 10%. At these concentrations, the levels of O2-, SOD, POD, and CAT decrease to their minimum values, indicating that high exogenous H2O2 concentrations induce cell death, which in turn suppresses ROS production and inactivates ROS-scavenging enzyme activity. Consequently, cellular osmotic potential increases, leading to the accumulation of high concentrations of exogenous H2O2 within cells.
1 Introduction
Seed germination represents the initial and pivotal step in a plant’s life cycle (Chen et al., 2000). Meanwhile, seed germination rate - serving as a core indicator of a seed’s germination capacity - acts as a key determinant of successful crop growth and directly influences final crop yields (Tania et al., 2022). Numerous research reports have demonstrated that reactive oxygen species (ROS) can induce and promote seed germination (Bailly, 2019) playing diverse roles throughout the plant growth process (Chu et al., 2022). Kranner et al. (2010) conducted experiments using pea seeds and observed high levels of hydrogen peroxide (H2O2) and low concentrations of superoxide radicals (O2-) at the early stage of imbibition. Furthermore, the elongation of the radicle following germination was found to be significantly correlated with an increase in O2- levels. This suggests that ROS production is an early and consistent event in the imbibition and germination processes across different seed types (Liu et al., 2017). Studies have found that exogenous application of H2O2 can break the dormancy of potato tubers (Liu et al., 2017), Arabidopsis (Oracz et al., 2007), Hedysarum scoparium (Su et al., 2016) and sugarbeet (Chu et al., 2022)seeds. According to the “oxidative window” hypothesis proposed by Bailly (2019), only a critical range of ROS concentrations alleviates dormancy while levels below or above the critical range impair germination. Recent studies have further confirmed that the effects of exogenous H2O2 on promoting or inhibiting Arabidopsis seed germination and seedling establishment are dose-dependent (Wang et al., 2025). Specifically, low concentrations of H2O2 facilitate Arabidopsis seed germination, whereas higher concentrations lead to a significant suppression of germination rate (Zhou et al., 2018; Wang et al., 2025), and this process is accompanied by changes in the activities of peroxidase (POD) and superoxide dismutase (SOD) (Jiang et al., 2012; Zheng et al., 2018). In summary, these findings collectively demonstrate the significant regulatory role of exogenous H2O2 in both seed germination and early seedling growth. Accordingly, growth regulators containing H2O2 can be applied through various methods, including seed soaking prior to sowing, incorporation into the growth medium, or foliar spraying on young plants, which can enhance both agricultural productivity and economic returns. It is widely accepted that only H2O2 within an appropriate range can promote seed germination. Both excessively low and high H2O2 concentrations are detrimental to germination, with high concentrations inhibiting plant growth and triggering programmed cell death. A key question then arises: what is the threshold range within which H2O2 act as signaling molecules to participate in plant growth and development? This remains a prominent and challenging issue in ROS research.
Currently, research on the ROS threshold in cruciferous rapeseed varieties remains relatively limited. Accordingly, this study employs 16NTS309, a cold-resistant rapeseed variety bred by our research group, as the research subject. B. napus of 16NTS309 having a strong cold tolerance, which could over winter in the 36°73′N area at an altitude of 1,517 m. These are the essential B. napus germplasm resources having strong cold tolerance levels used for breeding in northern China. The present study examined the effects of different concentrations of H2O2 (a type of ROS) on rapeseed, including its germination rate and the contents of ROS (O2- and H2O2) as well as antioxidant enzymes (SOD, POD, and CAT). The objectives were to clarify the threshold range at which ROS promote B.napus growth and to verify the impact of high H2O2 concentrations on rapeseed growth and development. This study furnishes valuable theoretical guidance for the application of H2O2 in agricultural production, thereby facilitating yield improvement in rapeseed varieties. Moreover, it yields practical reference value for relevant agricultural practices and production management.
2 Experimental materials and treatment methods
2.1 Experimental materials
Mature and plump seeds of B.napus winter rapeseed cultivar 16NTS309 were selected, thoroughly washed, air-dried, and vernalized at a low temperature of 4 °C for 12 hours to break seed dormancy.
2.2 Experimental treatments
2.2.1 Exploration of ROS threshold range
In this experiment, 31 different concentrations of H2O2 treatments were established to investigate the effects of varying H2O2 levels on the germination rate, plant growth length, and intracellular ROS, including O2- and H2O2, in B.napus seeds. The objective was to determine the threshold range within which ROS either promotes or inhibits plant growth.
Initially, a 30% H2O2 solution was used as the stock solution, and various dilutions were prepared according to the formula C1V1 = C2V2 (30% * X = C2 * 100). The 31 gradient concentrations included: 0.0% (ddH2O control), 0.1%, 0.2%, 0.3%, 0.4%, 0.5%, 0.6%, 0.7%, 0.8%, 0.9%, 1%, 1.1%, 1.2%, 1.3%, 1.4%, 1.5%, 1.6%, 1.7%, 1.8%, 1.9%, 2%, 2.1%, 2.2%, 2.3%, 2.4%, 2.5%, 2.6%, 2.7%, 2.8%, 2.9%, and 3%. Each treatment involved 1000 seeds, evenly distributed into 100 culture bottles with 10 seeds per bottle, and the experiment was conducted in triplicate.
Subsequently, the seeds were rinsed three times with distilled water and vernalized at 4 °C for 24 hours. Culture bottles were sterilized, dried, and lined with a single layer of sterile filter paper. Each bottle was then treated with the corresponding concentration of H2O2. Seeds were evenly placed in the bottles and cultivated in a controlled growth chamber maintained at 25°C with a light intensity of 3000 Lx under a 16-hour photoperiod. Due to the volatility of H2O2, which may affect the accuracy of the results, we have strictly controlled the experimental conditions through the following operations: 1)Every 24 hours, leave the culture bottles open for 3 hours, and use filter paper to absorb the excess H2O2 liquid at the bottom of the bottles, then add a small amount of freshly prepared H2O2 of each concentration gradient; 2) Immediately cover the bottles after each H2O2 replacement to ensure the airtightness of the culture environment. All these operations are to ensure the reliability of the experimental data.
This procedure was repeated daily for 4d, during which germination rates and growth trends were recorded. On the 4d, intracellular levels of H2O2, O2-, SOD, POD, and CAT were measured.
2.2.2 Validation experiment of ROS lethal concentration and semi-lethal concentration
2.2.2.1 Seed rehydration experiment
For each H2O2 treatment gradient with a germination rate below 50%, a rehydration experiment was conducted to assess the recovery of seed germination following rehydration and to determine the threshold concentrations representing the lethal and semi-lethal levels of H2O2. Initially, 21 H2O2 gradients ranging from 1% to 3% were repeated. Each treatment involved 1000 seeds, which were evenly distributed into 100 culture bottles (10 seeds per bottle). Following 4d of cultivation, the germination rates for each H2O2 concentration were recorded. Subsequently, seeds treated with 1%, 1.1%, 1.2%, 1.3%, 1.4%, 1.5%, 1.6%, 1.7%, 1.8%, 1.9%, 2%, 2.1%, 2.2%, 2.3%, 2.4%, 2.5%, 2.6%, 2.7%, 2.8%, 2.9%, and 3% H2O2 were carefully rinsed five times with distilled water. The culture bottles were also cleaned and dried. Subsequently, the seeds from each treatment were evenly placed back into the respective culture bottles, and an equal volume of ddH2O was added. Cultivation was continued under controlled conditions at 25 °C with a light intensity of 3000 Lx and a photoperiod of 16 hours per day for an additional 4d. Daily observations of germination rates were recorded throughout this period.
Note: If the germination rate increased significantly after rehydration and exceeded 60%, it indicated that the high H2O2 concentration had temporarily inhibited germination. If the germination rate remained around 50% after rehydration, the corresponding H2O2 concentration was considered the semi-lethal concentration for B.napus growth and development. If the germination rate remained below 10% after rehydration, the H2O2 concentration was classified as the lethal concentration for B.napus.
2.2.2.2 Verification experiment
To further validate the reliability of the experimental data, normal B.napus seedlings were sprayed with H2O2 solutions at concentrations ranging from 1% to 3% to observe their growth responses. Initially, healthy seedlings were cultivated in culture bottles. When the plants reached a height of approximately 7 cm, they were treated by spraying with 1%, 1.2%, 1.3%, 1.4%, 1.5%, 1.6%, 1.7%, 1.8%, 1.9%, 2%, 2.1%, 2.2%, 2.3%, 2.4%, 2.5%, 2.6%, 2.7%, 2.8%, 2.9%, and 3% H2O2 solutions, respectively. Each treatment consisted of 30 culture bottles, with 10 seedlings per bottle. The treatment was continued for 4d, and the growth status of the rapeseed seedlings was observed and recorded on the fourth day.
2.3 Index measurement
2.3.1 Seed germination
Daily observations were conducted to record germination parameters, including germination rate and seedling length. The germination rate was calculated using the following formula:
2.3.2 Qualitative detection of H2O2 and O2-
Seedlings were placed in the test tubes and immersed in 3, 3'-Diaminobenzidine (DAB) and Nitrotetrazolium blue chloride (NBT) staining solution to detect H2O2 and O2− as described by (Wang et al., 2025). Plants were immersed for 8 h with DAB and NBT staining solution that solution should be placed away from light. After infiltration, the stained plants were bleached in an acetic acid:glycerol: ethanol (1:1:3, v/v/v) solution at 100 °C for 10 - 20min, then stored in 95% (v/v) ethanol until scanned. The experiment was repeated six times.
2.3.3 Quantitative detection of SOD, POD, CAT, H2O2, and O2-
Following four days of treatment, the levels of H2O2 and O2-, as well as the enzymatic activities of SOD, POD, and CAT, were quantitatively measured in B.napus seedlings exposed to each H2O2 concentration gradient. POD, SOD and CAT activities were measured as described by (Qi et al., 2020).
2.4 Data analysis
The data were analyzed using SPSS 19.0 software, with one-way analysis of variance (ANOVA) applied to identify significant differences between the two treatment groups (P< 0.05). Graphical illustrations were prepared using Adobe Photoshop CC 2018 (Adobe Inc., San Jose, CA, USA).
3 Results
3.1 Effects of exogenous H2O2 on rapeseed germination rate exogenous
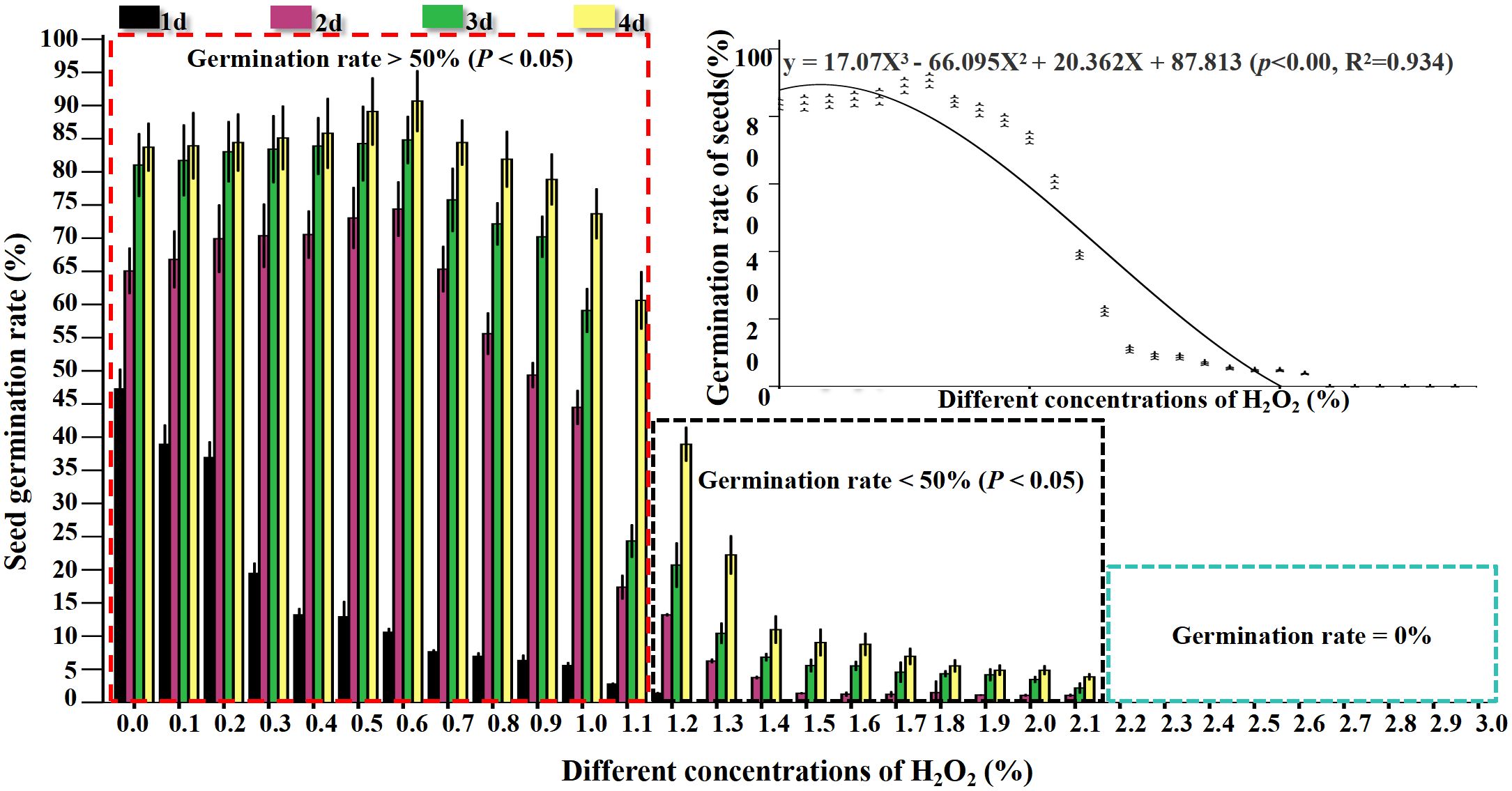
H2O2 at different concentrations exerted distinct effects on the viability of rapeseed seeds, with variations in germination rate observed across different treatment durations, as detailed below: After 1 day of treatment, the germination rate of seeds subjected to 0.0% (CK) to 3% H2O2 treatments exhibited a downward trend (Figure 1; Appendix Table 1). Among these, seeds treated with 0.0% (CK) to 1.2% H2O2 sequentially underwent swelling, embryo breakthrough of the seed coat, and accumulation of substantial O2- at the radicle tip (Figure 2), with relatively low H2O2 accumulation (Figure 3). Specifically, the germination rate of the 0.0% (CK) treatment reached 47.22%, which was significantly higher (P< 0.5) than those of the 0.1% to 3% H2O2 treatments. The germination rates of the 0.1% and 0.2% H2O2 treatments followed, at 38.89% and 36.72%, respectively. For the 0.3% to 1.2% H2O2 treatments, germination rates ranged from 19.44% to 1.39%, while no germination was observed in the 1.3% to 3% H2O2 treatments.
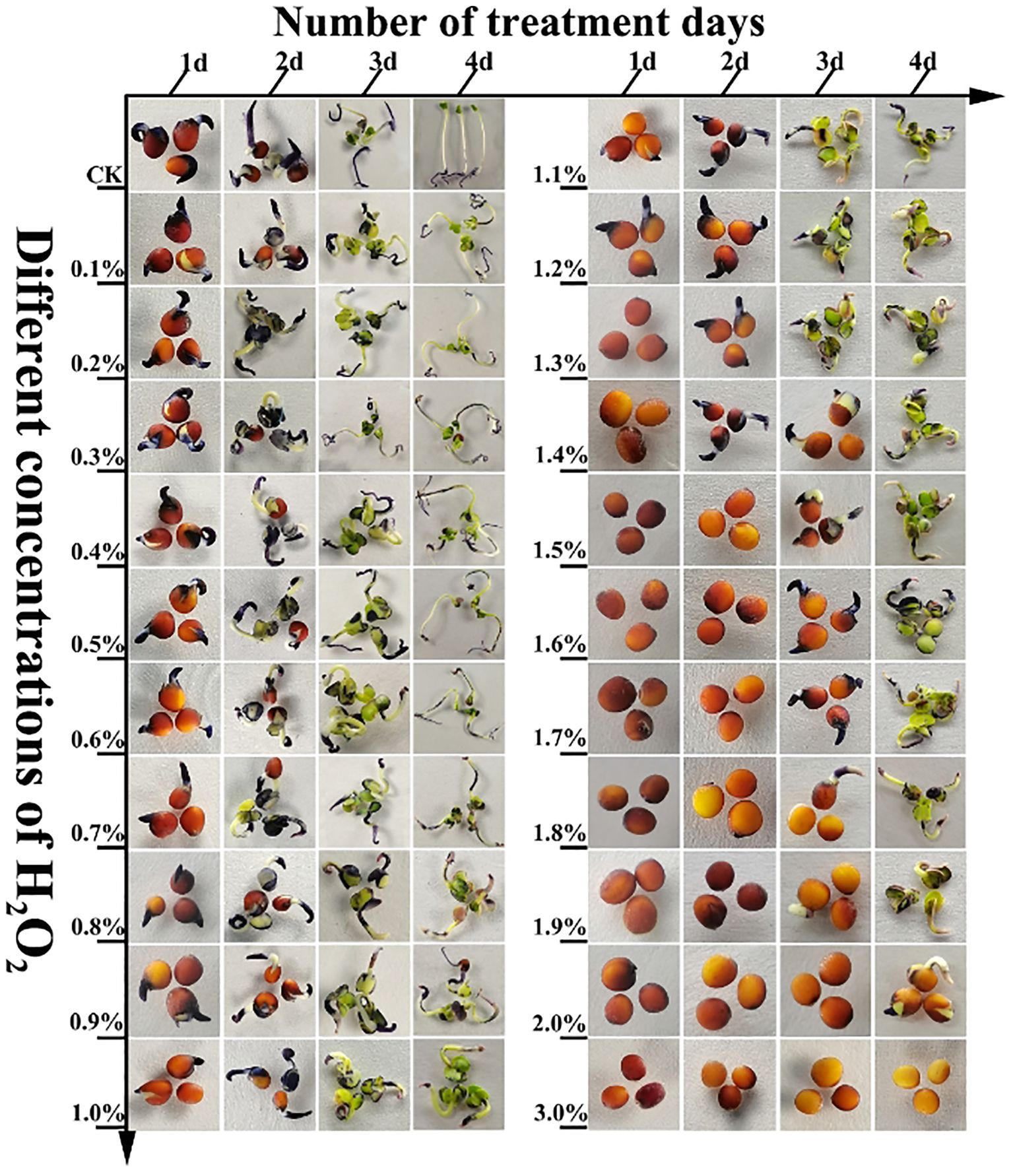
Figure 2. The seeds of B.napus 16NTS309 were treated with H2O2 and then stained with NBT to observe the distribution of O2- in the hypocotyl, cotyledon and embryo.

Figure 3. The seeds of B.napus 16NTS309 were treated with H2O2 and then stained with DAB to observe the distribution of H2O2 in the hypocotyl, cotyledon and embryo.
After 2 days of treatment, the germination rate of seeds across the 0.0% (CK) to 3% H2O2 treatments displayed a trend of first increasing and then decreasing (Figure 1; Appendix Table 1). Specifically, germination rates rose in the 0.0% (CK) to 0.6% H2O2 range, peaking at 74.31% in the 0.6% H2O2 treatment (Appendix Table 1), indicating that an appropriate concentration of H2O2 promotes rapeseed germination. In contrast, germination rates declined in the 0.7% to 2.1% H2O2 treatments, ranging from 65.28% to 1.08%, and no germination occurred at H2O2 concentrations ≥ 2.2%, suggesting that higher H2O2 concentrations inhibit seed germination. NBT (Figure 2) and DAB(Figure 3) staining results revealed increased accumulation of O2- in the radicle tips and cotyledon regions, with relatively low H2O2 accumulation (Figure 3).
After 3 days of treatment, compared with the 2nd day, the germination rate in each treatment gradient showed the most significant increase; however, seeds treated with 2.2% to 3% H2O2 still failed to germinate. Germination rates in the 0.0% (CK) to 0.9% H2O2 treatments ranged from 70.41% to 84.72%, with the highest rate (84.72%) observed at 0.6% H2O2. Seeds treated with 1% to 2.1% H2O2 exhibited varying degrees of swelling and radicle breakthrough of the seed coat, with germination rates ranging from 2.17% to 59.03% (Figure 1; Appendix Table 1).
On the 4th day, the germination rate across the 0.0% (CK) to 3% H2O2 treatments maintained the trend of first increasing and then decreasing, reaching a maximum of 94.67% at 0.6% H2O2. However, the rate of increase in germination slowed compared to the 3rd day, and no germination was still observed in the 2.2% to 3% H2O2 treatments (Figure 1; Appendix Table 1). These results confirm that an appropriate concentration of exogenous H2O2 facilitates seed germination, while both lower and higher concentrations exert inhibitory effects. Under the 0.5% and 0.6% H2O2 treatments, the germination rate of rapeseed ranged from 89.01% to 94.67%, indicating that this concentration was the most favorable for the germination of rapeseed (Figure 1; Appendix Table 1). At 1% and 1.1% H2O2, germination rates were 73.61% and 60.56%, respectively, significantly lower than that of the 0.0% (CK) treatment, confirming that higher concentrations hinder germination. At 1.2% H2O2, the germination rate was 38.89%, which is speculated to be the semi-lethal concentration for cabbage-type rapeseed seeds. At H2O2 concentrations > 1.5%, germination rates dropped below 10%, potentially representing the lethal concentration for these seeds (Figure 1; Appendix Table 1).
The curve fitting yielded the following cubic polynomial equation: y = 17.07X³ - 66.095X² + 20.362X + 87.813 (p< 0.001, R² = 0.934). Statistical validation of this model demonstrates two key findings: The p-value is less than 0.001, which confirms the extremely high statistical significance of the overall regression relationship, indicating that the association between the independent variable X and dependent variable y is not by chance. The coefficient of determination R² = 0.934 means the model accounts for approximately 93.4% of the total variation in the dependent variable y, reflecting excellent goodness of fit.
3.2 Effects of exogenous H2O2 on H2O2 and O2- contents in B.napus seeds
In this study, B.napus seeds of 16NTS309 were continuously treated with 31 gradient concentrations of exogenous H2O2 (0.0%–3%) for 4 days. After treatment, NBT staining (for O2-, Figure 2) and DAB staining (for H2O2, Figure 3) were performed on the seeds or seedlings to investigate the variation patterns of H2O2 and O2- contents. Staining results showed that germinating seeds accumulated abundant blue precipitates (indicating O2-) at the radicle tip, with relatively few brown precipitates (indicating H2O2). This suggests that a large amount of O2- accumulates at the germinating tip (Figure 3), which is beneficial for cell division in this region. During the later stages of seed growth and development, O2- and H2O2 were also detected in cotyledons and hypocotyls, further confirming that H2O2 and O2- play crucial roles in seed growth and development.
However, with increasing concentrations of exogenous H2O2 (1.7%–2.1%), the accumulation of blue precipitates (O2-) decreased (Figure 2), while brown precipitates (H2O2) darkened progressively (Figure 3). This indicates that high concentrations of exogenous H2O2 inhibit the reactive oxygen species (ROS) generation mechanism, leading to reduced O2- accumulation and increased H2O2 levels. The elevated H2O2 content may be attributed to enhanced membrane permeability caused by high exogenous H2O2 concentrations, which allows external H2O2 to penetrate into cells—likely the primary reason for the dark brown staining observed in DAB assays.
3.3 Quantitative analysis of H2O2 and O2- contents
To further validate the reliability of the qualitative test results for H2O2 and O2-, this study quantitatively analyzed the contents of H2O2 and O2- in 31 samples under each H2O2 concentration (0.0% – 3.0%) after 4 days of treatment. The results indicated that as the H2O2 concentration increased, the O2- (Figure 4A) content exhibited a fluctuating trend, initially increasing, then decreasing, followed by a second increase and a final decrease (Figure 4; Appendix Table 2). In contrast, H2O2 (Figure 4B) content displayed a trend of initial increase, subsequent decrease, and a second increase. Significant differences were observed in both O2- (Figure 4A) and H2O2 (Figure 4B) contents across different treatments (P< 0.05), indicating that varying H2O2 concentrations exerted significant effects on plant growth and germination.
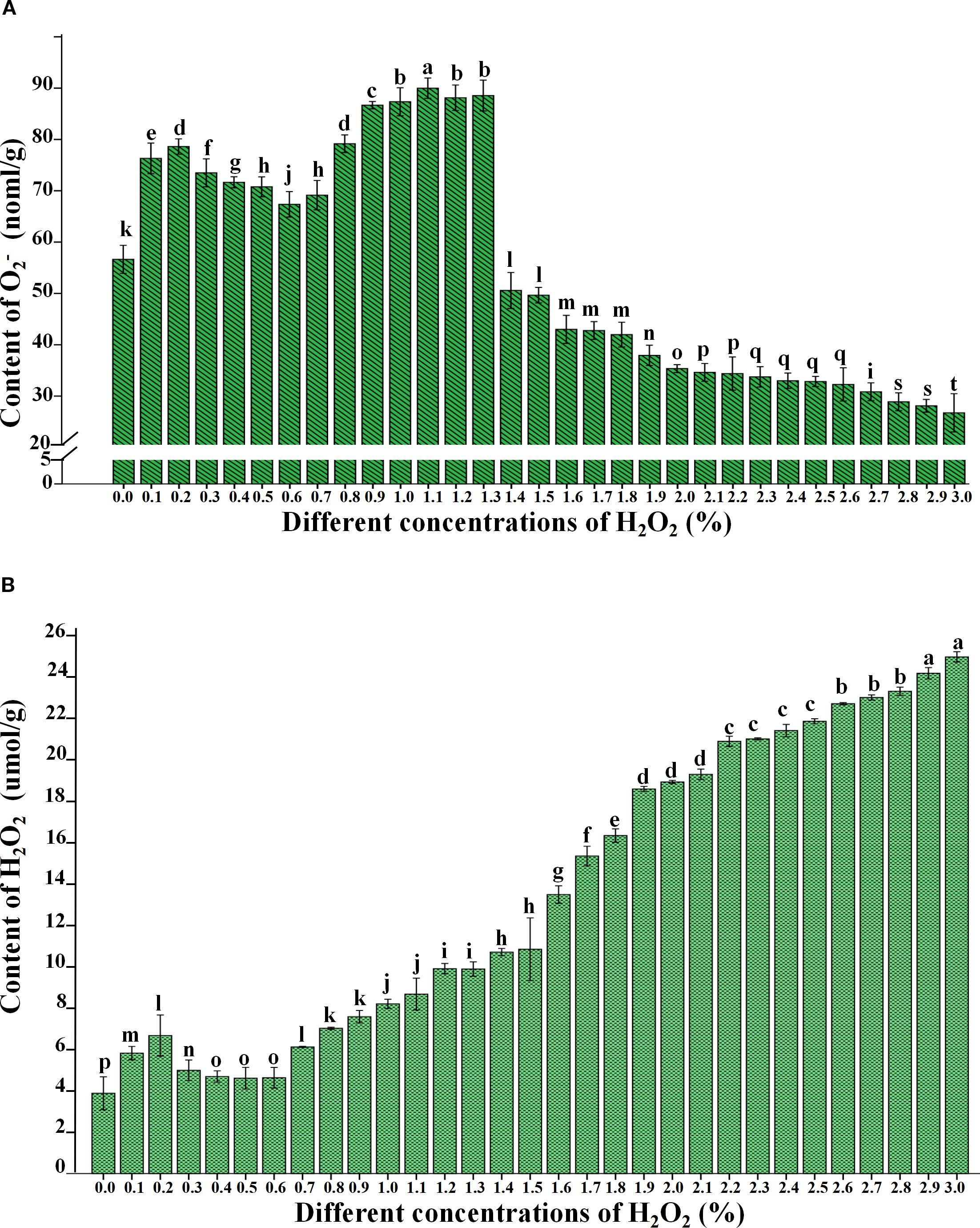
Figure 4. Change of O2- (A) and H2O2 (B) content in B.napus seed with treated by different concentrations of H2O2. Lowercase letters (i.e., a, b, c, etc.) are used to denote the results of differential analysis, where distinct letters indicate statistically significant differences at the P < 0.05 level.
Compared with the control treatment (0.0% H2O2), the O2− and H2O2 (Figure 4; Appendix Table 2) contents in seeds treated with 0.1% and 0.2% H2O2 showed an increasing trend and were significantly higher than those in the control group. Specifically, the O2− contents were 76.32 nmol/g and 78.62 nmol/g, while the H2O2 contents were 5.82 μmol/g and 6.67 μmol/g, respectively. These findings suggest that low concentrations of exogenous H2O2 can stimulate the intracellular accumulation of ROS, including O2− and H2O2. However, higher concentrations of ROS did not show a significant promoting effect on rapeseed germination. Moreover, the O2− and H2O2 contents in seeds treated with 0.3%–0.6% H2O2 were significantly higher than in the control group but markedly lower than those in seeds treated with lower (0.1%–0.2%) and higher (0.8%–1.3%) H2O2 concentrations. This suggests that both low and high concentrations of H2O2 can induce greater ROS production within seeds, which may partially inhibit germination.
Upon treatment with 1.4% H2O2, the O2− content in seeds exhibited a sharp decline (Figure 4A; Appendix Table 2), whereas the H2O2 content showed a rapid increase. This observation was consistent with the NBT (Figure 2) and DAB (Figure 3) staining results. A plausible explanation is that high H2O2 concentrations may lead to seed mortality, thereby severely impairing the cellular capacity to produce and scavenge ROS. Despite this, the ongoing processes of absorption and imbibition within the non-viable seeds allowed for the accumulation of exogenous H2O2, resulting in a sharp rise in H2O2 levels between 1.9% and 3.0%. This result aligns with the DAB staining data.
3.4 Effects of exogenous H2O2 on endogenous SOD, POD, and CAT activities in B.napus seeds
This study determined the activities of SOD, POD, and CAT in 31 samples after 4 days of treatment with exogenous H2O2 at concentrations ranging from 0.0% to 3%. The results showed that as the H2O2 concentration increased, the activities of SOD (Figure 5A), POD (Figure 5B), and CAT (Figure 5C) exhibited a consistent trend of initial increase followed by decrease. Statistical analysis revealed significant differences in enzyme activities among all treatments (P<0.05), indicating that varying H2O2 concentrations significantly affect the dynamics of SOD, POD, and CAT activities during seed germination (Figure 5; Appendix Table 2). Notably, SOD, POD, and CAT activities were significantly higher in seeds treated with 0.3% – 0.6% H2O2 compared to other treatments. This finding aligns with the earlier observation that 0.3%–0.6% H2O2 is optimal for seed germination, suggesting that these enzymes play a critical role in maintaining ROS (H2O2 and O2-) at physiologically appropriate levels-likely explaining the lower intracellular ROS content observed under these concentrations. However, compared with the normal treatment (0.0%), the activities of SOD (Figure 5A), POD (Figure 5B), and CAT (Figure 5C) enzymes were significantly enhanced by low concentrations of H2O2 (0.1% and 0.2%). Nevertheless, these enzyme activities were markedly reduced at higher H2O2 concentrations (0.3%–0.6%). When the H2O2 concentration exceeded 0.7%, increased H2O2 levels progressively inhibited seed germination as well as the activities of SOD (Figure 5A), POD (Figure 5B), and CAT (Figure 5C) enzymes. This suggests that elevated H2O2 concentrations are detrimental to seed germination, as they suppress antioxidant enzyme activities, thereby impairing the capacity to scavenge ROS and ultimately leading to increased seed mortality.
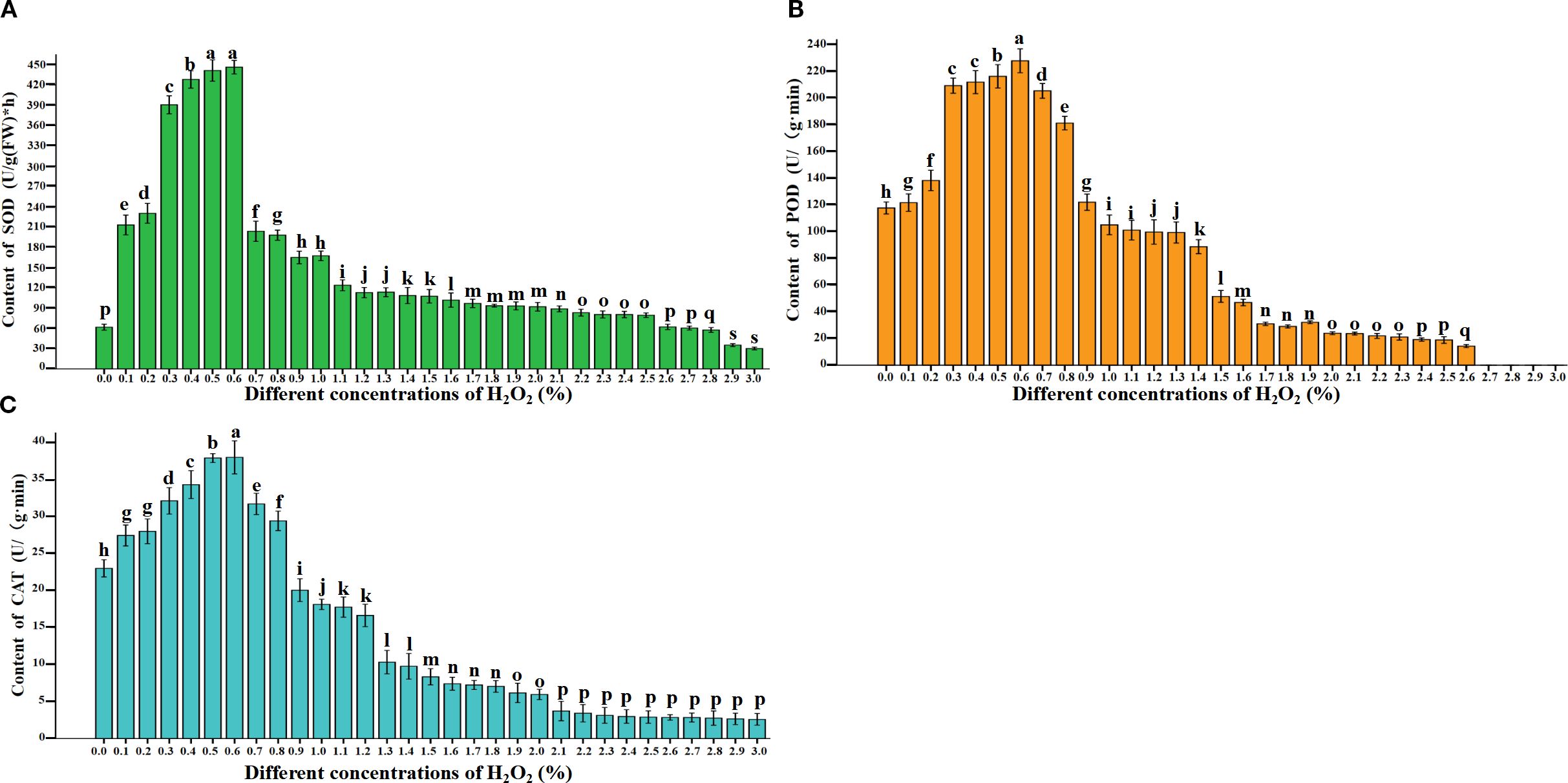
Figure 5. Effects of exogenous H2O2 concentration treatments on SOD (A), peroxidase POD, (B), and catalase CAT, (C) in rapeseed seedlings. Lowercase letters (i.e., a, b, c, etc.) are used to denote the results of differential analysis, where distinct letters indicate statistically significant differences at the P < 0.05 level.
3.5 Verification experiment for ROS threshold
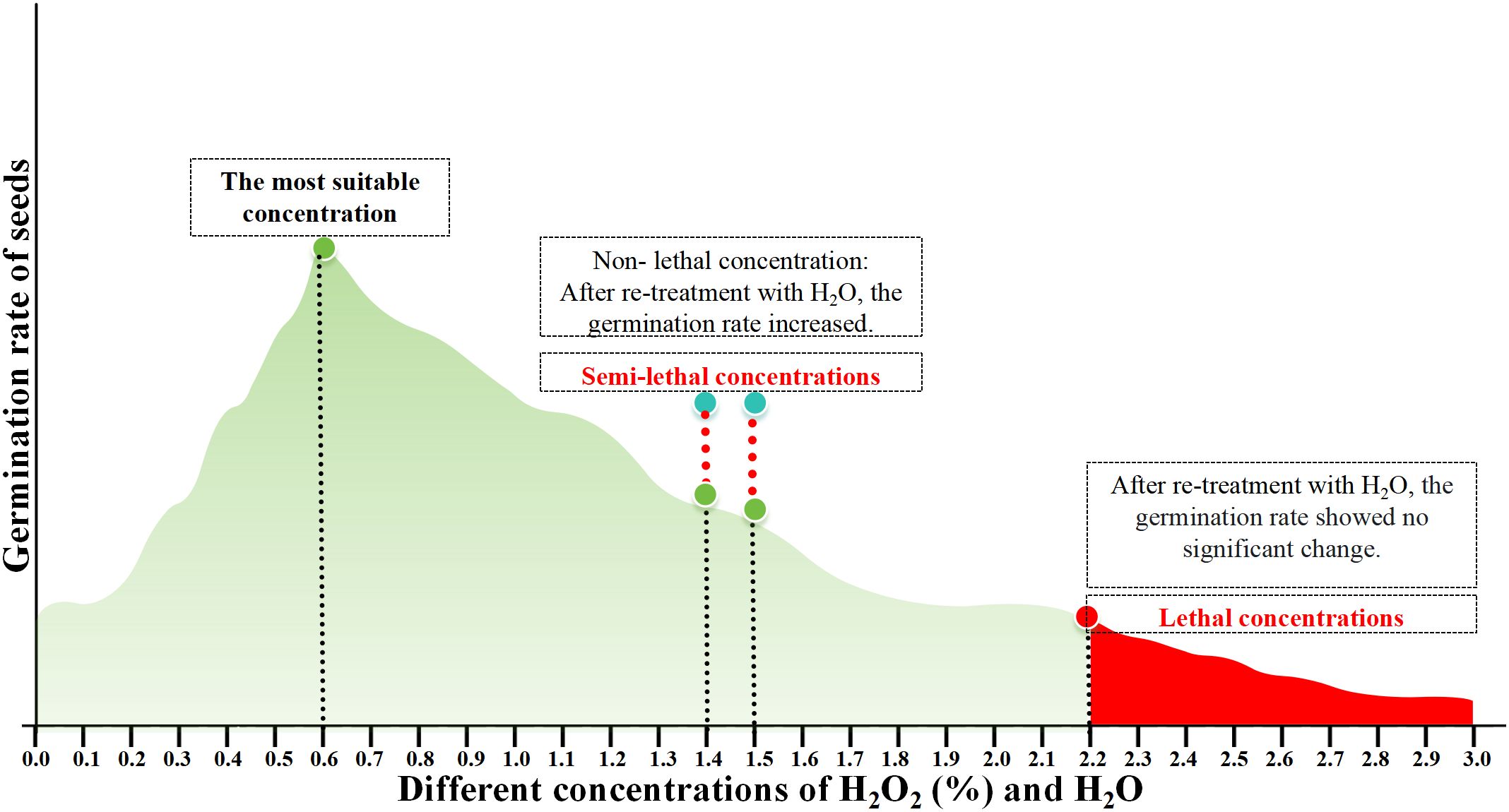
Previous results indicated that as exogenous H2O2 concentration increased (1% – 3%), seed growth and development were significantly inhibited. After treatment with 1.2% H2O2, the seed germination rate was 35.6%, from which it can be inferred that this concentration represents the half-lethal threshold. In contrast, following treatment with 1.5% H2O2, the seed germination rate dropped to 9.5%, thereby indicating that this concentration is the lethal concentration of H2O2. To verify this experimental result, in this study, the seeds that had been treated with H2O2 for 4 days were subjected to H2O treatment again.
The results revealed significant changes in germination rates across all H2O2 treatments after rehydration, compared to pre-rehydration levels (Figure 6; Appendix Table 3). Specifically: Seeds treated with 1%–1.3% H2O2 exhibited germination rates of 93.3%, 83.3%, 82.2%, and 80.0% after rehydration, with particularly notable increases in the 1.2% and 1.3% groups (Figure 6; Appendix Table 3). This suggests that while high H2O2 concentrations initially inhibit germination, rehydration can alleviate this inhibition, thereby increasing germination rates. Consequently, 1.2% H2O2 is not within the semi-lethal concentration range.
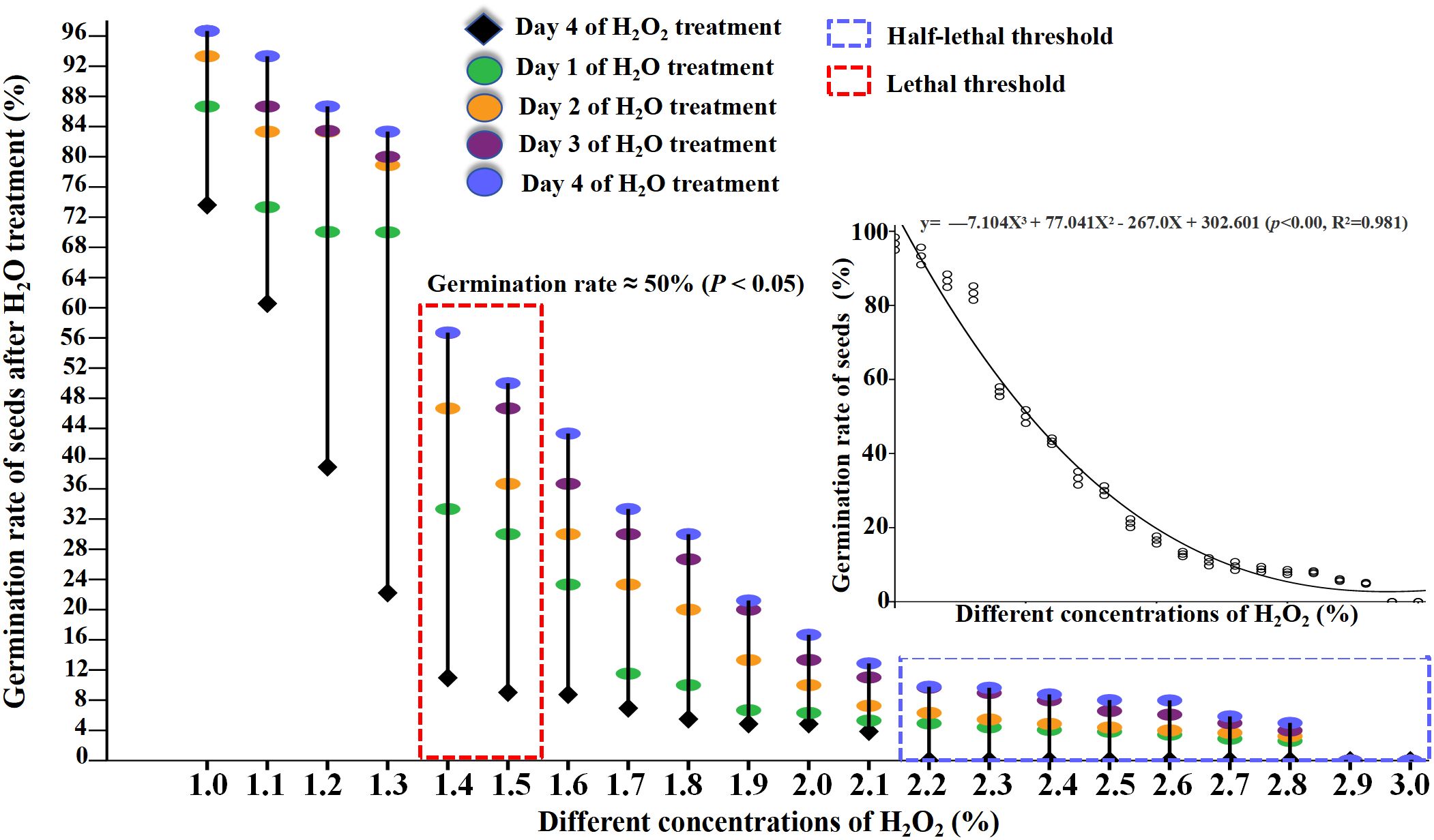
Figure 6. After the seeds were pretreatment with H2O2 (1%-3%) for 4d and then re-watering (4d), effect of H2O2 and re-watering on seed germination rate of B.napus were counted.
After 4 days of supplementary H2O treatment, the germination rates of seeds previously treated with 1.4% and 1.5% H2O2 increased to 56.72% and 50%, respectively (Figure 6; Appendix Table 3). This indicates that 1.4% and 1.5% H2O2 may instead represent the semi-lethal concentrations for the germination of cabbage-type rapeseed. While those treated with 2.2%–3% H2O2 maintained germination rates below 10% (approaching 0 with increasing concentration). These results indicate that H2O2 concentrations exceeding 2.2% are likely lethal for cabbage-type rapeseed germination (Figure 7).
To further confirm these findings, an additional experiment was conducted: normal-growing cabbage-type rapeseed plants were sprayed with high-concentration H2O2 (1.0%–3.0%). Observations showed that leaf yellowing intensified with increasing H2O2 concentration, and extensive leaf necrosis occurred at concentrations >2.2% (Figure 8). These results, coupled with the inverse correlation between chlorophyll content and H2O2 concentration, further validate that high levels of ROS (specifically H2O2) exert inhibitory or lethal effects on the growth and development of cabbage-type rapeseed.

Figure 8. The normal growth of B. napus 16NTS309 seedlings were treated by spraying high concentration H2O2 (1.0%-3.0%) to verify the effect of high concentration H2O2 on the growth and development of 16NTS309 seedlings.
The cubic polynomial equation derived from curve fitting is: y = -7.104X³ + 77.041X² - 267.0X + 302.601 (p< 0.001, R² = 0.981). Statistical indicators confirm the model’s excellent fitting performance, as elaborated below: Statistical significance (p< 0.001):This indicates the overall regression relationship of the cubic polynomial is statistically highly significant. It effectively rules out the possibility that the association between the independent variable (X) and dependent variable (y) is driven by random errors. Goodness of fit (R² = 0.981):The coefficient of determination (R²) reaches 0.981, meaning the model accounts for approximately 98.1% of the total variation in the dependent variable (y). This reflects the model’s strong explanatory power for the data, high fitting accuracy, and its ability to well capture the nonlinear relationship between X and y.
4 Discussion
4.1 Appropriate H2O2 concentration promotes the germination of B.napus seeds
Studies have indicated that the germination of seeds is closely associated with the production of ROS (Zheng et al., 2009). A growing body of research by scholars has confirmed that ROS can act as signaling molecules to facilitate seed germination, including soybeans (Li et al., 2023), beans (Pereira et al., 2017), cotton (Wang et al., 2022), peas (Barba-Espín et al., 2012b), wheat (Momeni et al., 2023), and cucumbers (Ahmad et al., 2021). Nevertheless, exogenous H2O2 at different concentrations exerts distinct effects on crop growth and development: low concentrations promote seed germination, while high concentrations inhibit it (Wang et al., 2025). Chuesaard et al (2023) demonstrated that a low concentration of H2O2 (25 mM) can accelerate the germination of Oryza sativa seeds. Similarly, when wheat seeds were treated with H2O2 at different concentrations (0.5%, 1%, and 3%), the low concentration 0.5%H2O2 significantly increased the germination percentage. Li et al. (2014) treated Pinus bungeana seeds with gradient concentrations of H2O2 and recorded the highest germination rate (76.0%) at a 0.6%H2O2 concentration. In the present study, 31 H2O2 concentration gradients (ranging from 0.0% to 3.0%) were tested to assess their impact on B.napus seed germination. The results revealed that: with H2O2 concentration increased, the germination rate first rose and then declined. Lower concentrations of H2O2 (0.3%-0.6%)promote seed germination, resulting in a germination rate exceeding 85.303%, which is significantly higher than that of the control group (0.0%). notably, the maximum germination rate (94.67%) was achieved at 0.6% H2O2. Our research findings are consistent with Li et al (2014); Wang et al (2025) and Chuesaard et al (2023) studies. These findings indicate that a 0.6% H2O2 concentration is conducive to B.napus seed germination.
4.2 The influence of higher concentrations of H2O2 on the growth and development of B.napus seeds
The dual role of H2O2 exerts a marked dual effect: at low concentrations, it influences seed germination and seedling growth, whereas at higher concentrations, it induces seed death (Jeevan et al., 2015), with such impacts exhibiting a dosage-dependent pattern. In light of this characteristic, Wang et al (2025) conducted an analysis on the influence of varying concentrations of H2O2 on Arabidopsis seed germination, the results revealed that 2 mM H2O2 serves as a critical threshold: when this concentration is exceeded, both Arabidopsis seed germination and seedling growth exhibit a gradual inhibition. Chuesaard et al. (2023) demonstrated that higher concentrations H2O2(100–200 mM) exert a delaying effect on the process. Similar concentration-dependent effects have been observed across various plant species. For instance, in soybean germination experiments, exogenous application of H2O2 at 10 mM or 20 mM was found to inhibit germination (Barba-Espín et al., 2012b). Arooj et al (2024) found that higher concentrations H2O2 (1% and 3%) significantly impaired germination. After treating B.napus seeds with 31 distinct concentrations of H2O2, we observed comparable results: 0.6% H2O2 emerged as a critical threshold. As the concentration increased to the range of 0.7%–3.0%, the seed germination rate exhibited a declining trend, with germination completely ceasing (germination rate = 0) once the concentration reached 2.2%. A similar effect was observed in our study: higher concentrations of H2O2 either inhibited seed germination or led to seed death. This raises an important question: are seeds treated with higher concentrations of H2O2 irreversibly dead, or is their germination merely temporarily suppressed?
4.3 Does an elevated concentration of H2O2 induce cell death in B.napus?
Li et al. (2014) investigated the effects of varying H2O2 concentrations on Pinus bungeana seed germination and found that concentrations exceeding 1.2% significantly reduced germination rates compared to the control group, with higher concentrations resulting in progressively lower germination performance. In this study, treatment with 1.2% and 1.3% H2O2 resulted in germination rates of only 38.89% and 22.22%, respectively. These findings are consistent with those reported by Li et al. (2014). To verify this experimental result, in this study, the seeds that had been treated with H2O2 for 4 days were subjected to H2O treatment again. Notably, following rehydration, the germination rates of seeds treated with 1.2% and 1.3% H2O2 increased by approximately 30- and 40-fold, respectively. Similarly, the germination rates of seeds treated with 1.4% and 1.5% H2O2 improved by nearly 50-fold after rehydration, reaching up to 50%. Collectively, these results suggest that high concentrations of H2O2 inhibit the germination of B.napus seeds; however, the inhibitory effect is partially alleviated through rehydration. Therefore, 1.2% and 1.3% H2O2 cannot be classified as semi-lethal concentrations for B.napus seed germination, whereas 1.4% and 1.5% H2O2 may represent the semi-lethal thresholds for ROS during the growth and development of this species.
Higher concentrations of H2O2 reduce cell survival rates. For instance, when cells were cultured with hydrogen peroxide alone, their survival rates were 41.61% at a concentration of 100 mM and 7.95% at 200 mM (Mok et al., 2014). Lapshina et al (2016) found that tobacco leaf treatment with 100 mM H2O2 increased their content of endogenous H2O2 and changes in the cell structure. Sun et al. (2020) treated strains with H2O2 at concentrations of 0.25%, 0.50%, 0.75%, 1.00%, 1.50%, 2.00%, 2.50%, and 3.00%. Their findings revealed that when H2O2 concentrations exceeded 2%, strain biomass approached zero, with 2.5% identified as the lethal concentration for the tested strains. Our study revealed that exposure to 2.2%–3% H2O2 resulted in germination rates below 10%, with a trend toward near-zero germination as the concentration increased. These results suggest that H2O2 concentrations exceeding 2.2% are likely lethal to the germination of cabbage-type rapeseed. To further verify these findings, higher concentrations of H2O2 were applied to healthy plants via spraying. Observations indicated that as H2O2 concentrations increased, leaves gradually yellowed, with concentrations exceeding 2.2%H2O2 leading to leaf death. Yang et al (2007) reported that elevated concentrations of H2O2 resulted in reduced contents of chlorophyll a, chlorophyll b, and total chlorophyll in garlic leaves. These findings, together with the aforementioned observations, indicate that chlorophyll content exhibits a negative correlation with H2O2 concentration. Furthermore, high levels of ROS—specifically, H2O2 concentrations exceeding 2.1%—can induce cell death in B.napus. Collectively, these results confirming that high levels of H2O2 concentrations exceeding 2.2% — induce cell death in nasturtium-type rapeseed.
4.4 Analysis of changes in the contents of H2O2, O2-, SOD, POD and CAT after treatment with different concentrations of H2O2
Research reported that the levels of ROS generated in cells are tightly controlled by ROS-scavenging enzymes, including SOD, CAT, and POD. These enzymes help maintain ROS at critical concentrations—referred to as the “oxidative window”—enabling them to function as cellular messengers (Bailly et al., 2008). Specifically, O2- are rapidly converted to H2O2 by cellular SOD. Subsequently, the activities of POD and CAT further regulate H2O2 within the “oxidative window” (Anand et al., 2019). When the concentration of H2O2 exceeds the critical threshold, it induces the inactivation of relevant antioxidant enzymes. This disruption perturbs the dynamic equilibrium of intracellular ROS (H2O2 and O2-), resulting in their substantial accumulation and subsequent triggering of cellular programmed death. After treating wheat (Li et al., 2011), cucumber (Ahmad et al., 2021)seedlings with exogenous H2O2, it was found that an appropriate concentration of H2O2 could significantly increase the enzyme activities of SOD, CAT, POD, etc. in the seedlings, reduce the production of H2O2 and O2- in the seedlings. Li et al (2011) reported that pretreatment with 0.05 μM H2O2 (equivalent to<0.1% H2O2) enhanced the activities of CAT, SOD, and POD in wheat seedlings. Studies have shown that treating Tartary buckwheat seeds with an appropriate concentration of hydrogen peroxide (5–10 mM) can effectively enhance the activities of SOD, CAT, and POD; in contrast, a high concentration of hydrogen peroxide (100 mM) results in reduced activities of these enzymes (Yao et al., 2021). This study found that when the H2O2 concentration within the appropriate range (0.3% - 0.6%) was applied to B.napus seeds, the germination rate, SOD, POD and CAT enzyme activities reached the highest values and were significantly higher than the normal (0.0%) treatment. The higher enzyme activity further reduced the accumulation of H2O2 and O2- in the B.napus seed, this result is consistent with the results of NBT and DAB staining. This study further confirmed the previous research viewpoint: lower concentrations of H2O2 promote the antioxidant enzyme activity of ROS.
However, high concentrations of H2O2 reduce the antioxidant enzyme activity and have a significant inhibitory effect on plant growth and development (Li et al., 2011; Yao et al., 2021). This study found that as the H2O2 (0.7%-3.0%)concentration increased, the antioxidant enzyme activity gradually dropped to the lowest level. After 2.2% H2O2 treatment, the germination rate was 0%, and the enzyme activities of SOD, POD and CAT dropped to the lowest values. There was no significant difference in the enzyme activities among the treatments. This further proved that the H2O2 concentration (>2.2%) is the lethal concentration threshold for the growth and development of B.napus. This result can also reasonably explain that under high concentration H2O2 treatment, the generation and clearance ability of ROS drop to the lowest level, the seeds die, and the cell permeability of the seeds increases, more exogenous H2O2 penetrates into the seeds, so the content of H2O2 in the range of 2.2% - 3% shows a sharp increase trend. This further proves the previous researchers’ viewpoints, high concentrations of H2O2 reduce the antioxidant enzyme activity and have a significant inhibitory effect on plant growth and development.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Author contributions
WQ: Writing – original draft, Writing – review & editing. WS: Resources, Software, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was financially supported by the National Nature Science Foundation regional fund project (32360455); Qingyang City Joint Research Fund Project - Major Project (QY-STK-2024A-046); Doctoral foundation of Longdong University (XYBYZK2107). University Teachers Innovation Fund Project of Gansu Province (2025A-198).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1673768/full#supplementary-material
References
Ahmad, D., Jain, S. K., Joshi, M. A., Anand, A., Tomar, B. S., Kumar, S., et al. (2021). H2O2 as a better index of seed quality and mechanism of cucumber (Cucumis sativus) seed deterioration. Indian J. Agric. Sci. 91, 1500–1504. doi: 10.56093/ijas.v91i10.117515
Anand, A., Kumari, A., Thakur, M., and Koul, A. (2019). Hydrogen peroxide signaling integrates with phytohormones during the germination of magnetoprimed tomato seeds. Sci. Rep. 9 (1), 8814. doi: 10.1038/s41598-019-45102-5
Arooj, I., Gohar, I., Mustansir, R., Bibi, R., Khan, N. U., and Raza, M. (2024). Early growth response of wheat genotypes to varying concentrations of hydrogen peroxide (H2O2). Int. J. Agric. Innov. Cutting-Edge Res. (HEC Recognised) 2, 8–13. doi: zenodo.org/records/14589434
Bailly, C. (2019). The signalling role of ROS in the regulation of seed germination and dormancy. Biochem. J. 476, 3019–3032. doi: 10.1042/BCJ20190159
Bailly, C., El-Maarouf-Bouteau, H., and Corbineau, F. (2008). From intracellular signaling networks to cell death: the dual role of reactive oxygen species in seed physiology. C. R. Biol. 331, 806–814. doi: 10.1016/j.crvi.2008.07.022
Barba-Espín, G., Hernández, J. A., and Diaz-Vivancos, P. (2012b). Role of H2O2 in pea seed germination. Plant Signaling Behav. 7, 193–1195.
Chen, W., Xing, D., and Tang, Y. (2020). Ultraweak biophoton emission of new and aged rice seeds during early imbibition. Biomedical Photonics and Optoelectronic Imaging. SPIE 4224, 214–217. doi: 10.1117/12.403976
Chu, C., Poore, R. C., Bolton, M. D., and Fugate, K. K. (2022). Mechanism of sugar beet seed germination enhanced by hydrogen peroxide. Front. Plant Sci. 13, 888519. doi: 10.3389/fpls.2022.888519
Chuesaard, T., Peankid, P., Thaworn, S., Jaradrattanapaiboon, A., Veerana, M., and Panngom, K. (2023). Different effects of reactive species generated from chemical donors on seed germination, growth, and chemical contents of Oryza sativa L. Plants 12, 765. doi: 10.3390/plants12040765
Jeevan, S. P., Rajendra Prasad, S., Banerjee, R., and Thammineni, ,. C. (2015). Seed birth to death: dual functions of reactive oxygen species in seed physiology. Ann. Bot. 116, 663–668. doi: 10.1093/aob/mcv098
Jiang, J. L., Su, M., Wang, L. Y., Jiao, C. J., Sun, Z. X., Cheng, W., et al. (2012). Exogenous hydrogen peroxide reversibly inhibits root gravitropism and induces horizontal curvature of primary root during grass pea germination. Plant Physiol. Biochem. 53, 84–93. doi: 10.1016/j.plaphy.2012.01.017
Kranner, I., Roach, T., Beckett, R. P., Whitaker, C., and Minibayeva, F. V. (2010). Extracellular production of reactive oxygen species during seed germination and early seedling growth in Pisum sativum. J. Plant Physiol. 167, 805–811. doi: 10.1016/j.jplph.2010.01.019
Lapshina, L. A., Reunov, A. V., and Nagorskaya, V. P. (2016). Effects of exogenous H2O2 on the content of endogenous H2O2, activities of catalase and hydrolases, and cell ultrastructure in tobacco leaves. Biol. Bull. 43, 419–425. doi: 10.1134/S1062359016050058
Li, X. H., Liu, Y. H., Luo, C. Y., Liu, C. Y., and He, Y. (2014). Influence of different dispositions on germination rate of Pinus Bungeana. J. Yu lin Univ. 24, 21–23.
Li, J. T., Qiu, Z. B., Zhang, X. W., and Wang, L. S. (2011). Exogenous hydro gen peroxide can enhance tolerance of wheat seedlings to salt stress. Acta Physiol. Plant 33, 835–842. doi: 10.1007/s11738-010-0608-5
Li, M., Zhang, P., Guo, Z., Cao, W., Gao, L., Li, Y., et al. (2023). Molybdenum nanofertilizer boosts biological nitrogen fixation and yield of soybean through delaying nodule senescence and nutrition enhancement. ACS nano 17, 14761–14774. doi: 10.1021/acsnano.3c02783
Liu, B. L., Zhao, S., Tan, F., Zhao, H., Wang, D. D., Si, H. J., et al. (2017). Changes in ROS production and antioxidant capacity during tuber sprouting in potato. Food Chem. 237, 205–213. doi: 10.1016/j.foodchem.2017.05.107
Mok, J. W., Chang, D. J., and Joo, C. K. (2014). Antiapoptotic effects of anthocyanin from the seed coat of black soybean against oxidative damage of human lens epithelial cell induced by H2O2. Curr. Eye Res. 39, 1090–1098. doi: 10.3109/02713683.2014.903497
Momeni, M. M., Kalantar, M., and Dehghani-Zahedani, M. (2023). H2O2 seed priming improves tolerance to salinity stress in durum wheat. Cereal Res. Commun. 51, 391–401. doi: 10.1007/s42976-022-00307-9
Oracz, K., Bouteau, H. E. M., Farrant, J. M., Cooper, K., Belghazi, M., Job, C., et al. (2007). ROS production and protein oxidation as a novel mechanism for seed dormancy alleviation. Plant J. 50, 452–465. doi: 10.1111/j.1365-313X.2007.03063.x
Pereira, A. E. S., Silva, P. M., Oliveira, J. L., Oliveira, H. C., and Fraceto, L. F. (2017). Chitosan nanoparticles as carrier systems for the plant growth hormone gibberellic acid. Colloids Surf B Biointerfaces 150, 141–152. doi: 10.1016/j.colsurfb.2016.11.027
Qi, W., Ma, L., Wang, F., Wang, P., Wu, J., Wang, J., et al. (2020). Physiological and biochemical mechanisms and cytology of cold tolerance in Brassica napus. Front. In Plant Sci. 11, 1241. doi: 10.3389/fpls.2020.01241
Su, L. Q., Lan, Q. Y., Pritchard, H. W., Xue, H., and Wang, X. H. (2016). Reactive oxy gen species induced by cold stratification promote germi nation of Hedysarum scoparium seeds. Plant Physiol. Biochem. 109, 406–415. doi: 10.1016/j.plaphy.2016.10.025
Sun, Z. Y., Zhang, Y., Pan, L. H., Yang, S. L., Wei, C. T., and Luo, J. P. (2020). Screening of high selenium-enriched yeast strains and analysis of their selenium-enriched characteristics. China Brewing 39, 116–120. doi: 10.11882/j.issn.0254-5071.2020.09.022
Tania, S. S., Rhaman, M. S., Rauf, F., Rahaman, M. M., Kabir, M. H., Hoque, M. A., et al. (2022). Alleviation of salt-inhibited germination and seedling growth of kidney bean by seed priming and exogenous application of salicylic acid (SA) and hydrogen peroxide (H2O2). Seeds 1, 87–98. doi: 10.3390/seeds1020008
Wang, Y. K., Liu, X. H., Sun, X. Y., Mao, X. N., Wang, Z. Y., Peng, J., et al. (2025). The promotive and repressive effects of exogenous H2O2 on Arabidopsis seed germination and seedling establishment depend on application dose. Physiologia Plantarum 177, e70098. doi: 10.1111/ppl.70098
Wang, W., Zhang, C., Zheng, W., Lv, H., Li, J., Liang, B., et al. (2022). Seed priming with protein hydrolysate promotes seed germination via reserve mobilization, osmolyte accumulation and antioxidant systems under PEG-induced drought stress. Plant Cell Rep. 41, 2173–2186. doi: 10.1007/s00299-022-02914-6
Yang, Y., Wu, Z., Li, C. H., Long, Y. J., and Zeng, X. R. (2007). Vitrification of the Garlic Plantlet under Exogenous H2O2 Stress in vitro. Acta Botanica Boreali-Occidentalia Sin. 08), 1637–1642.
Yao, X., Zhou, M., Ruan, J., Peng, Y., Yang, H., Tang, Y., et al. (2021). Pretreatment with H2O2 alleviates the negative impacts of naCl stress on seed germination of tartary buckwheat (Fagopyrum tataricum). Plants 10, 1784. doi: 10.3390/plants10091784
Zheng, C. F., Jiang, D., Liu, F. L., Dai, T. B., Liu, W. C., Jing, Q., et al. (2009). Exogenous nitric oxide improves seed germination in wheat against mitochondrial oxidative damage induced by high salinity. Environ. And Exp. Bot. 67, 222–227. doi: 10.1016/j.envexpbot.2009.05.002
Zheng, Y., Yin, X., and Ma, H. (2018). Effects of hydrogen peroxide on seed germination, seedling growth and physiological characteristics of Bombax ceiba after heat shock. Pak J. Bot. 50, 1327–1333.
Keywords: Brassica napus, O2-, H2O2, seed germination, ROS - reactive oxygen species
Citation: Qi W and Sun W (2025) Analysis of the threshold range of ROS concentration in winter rapeseed of the Brassica napus type. Front. Plant Sci. 16:1673768. doi: 10.3389/fpls.2025.1673768
Received: 26 July 2025; Accepted: 25 August 2025;
Published: 15 September 2025.
Edited by:
Guodong Wang, Shaanxi Normal University, ChinaCopyright © 2025 Qi and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiliang Qi, cXdsOTE5NkAxNjMuY29t
 Weiliang Qi
Weiliang Qi Wancang Sun5
Wancang Sun5