- 1Subtropical Horticulture Research Station, United States Department of Agriculture, Agricultural Research Service, Miami, FL, United States
- 2Department of Pharmaceutical Botany, Faculty of Gulhane Pharmacy, University of Health Sciences, Ankara, Türkiye
- 3Department of Pharmacognosy, Faculty of Pharmacy, Anadolu University, Eskisehir, Türkiye
Introduction: Fruit flies, belonging to the family Tephritidae, such as the Mediterranean fruit fly (Ceratitis capitata Wiedemann) and the Caribbean fruit fly (Anastrepha suspensa Loew), are recognized as major agricultural pests worldwide. Their larval stages infest a wide array of fruits and vegetables, causing significant economic losses through direct damage to crops and restrictions on international trade. Conventional pest management, heavily reliant on synthetic pesticides, has led to health concerns and the emergence of pesticide resistance. In response, semiochemicals, particularly essential oils and their constituents, are emerging as promising alternatives.
Methods: In this study, authenticated Artemisia absinthium L. (wormwood, Asteraceae) (Asd) essential oil (EO) was compared with five lab-distilled commercial A. absinthium EOs (A1 to A5) using gas chromatography with a flame ionization detector (GC–FID) and gas chromatography–mass spectrometry (GC–MS), and essential oils were tested in behavioral assays on sterile male C. capitata. Key components were evaluated for their potential attraction of male C. capitata, and the toxicities of compounds to female A. suspensa were determined.
Results: Cluster analysis revealed three major groups of compounds: β-thujone and (Z)-β-ocimene epoxide, β-thujone and camphor, and only camphor-rich. In short-range attraction bioassays, Asd and A1 samples captured the most male C. capitata at 30 minutes. These findings were linked to a higher α,β-thujone content in samples Asd (41.04%) and A1 (29.6%). A set of bioassays were conducted to compare the response of C. capitata to α-thujone, α,β-thujone, and tea tree oil, a strong medfly attractant. Medflies were similarly attracted to both α-thujone and α,β-thujone from 30 to 90 minutes. In a subsequent bioassay, α-thujone and α,β-thujone demonstrated strong toxicity to adult female A. suspensa, with the LD50 values being 0.21 and 0.14 μg/μL, respectively.
Discussion: These findings demonstrate that thujones have both attractant properties for male C. capitata and significant toxicity to A. suspensa, making them promising candidate compounds for integration into comprehensive integrated pest management strategies.
1 Introduction
Tephritid fruit flies are economically important insect pests of fruit commodities that threaten tropical, subtropical, and Mediterranean regions. They are considered highly polyphagous and potential exotic invaders (Fimiani, 1989; Vera et al., 2002; Puche et al., 2005; Suckling et al., 2008; Vargas et al., 2010; Navarro-Campos et al., 2011; Aluja et al., 2014; Epsky et al., 2014; Papadopoulos, 2014; Qin et al., 2015; Suckling et al., 2016; Weldon et al., 2018; Dumont and Oliva, 2024; Zhao et al., 2024). Among the invaders, Mediterranean fruit fly (medfly), Ceratitis capitata Wiedemann, Mexican fruit fly (mexfly), Anastrepha ludens Loew, and Oriental fruit fly, Bactrocera dorsalis Hendel, are of major quarantine importance to the United States (Froerer et al., 2010; Mangan et al., 2011; Steck et al., 2019; Duyck et al., 2022; Salas et al., 2023; Davydova et al., 2023; Shelly et al., 2023; Roh et al., 2023). Florida faces a high risk from invasive insect pests. This is due to several factors—its unique geology, favorable ecological conditions, numerous international ports of entry, and increasing tourism—all of which make the state particularly vulnerable and alter the ecosystem (Frank and McCoy, 1995; Brown et al., 2006; Wagner and Van Driesche, 2010; Pinkerton et al., 2019; Inserra et al., 2023; Raparelli et al., 2025).
Florida has sporadically faced numerous incursions of C. capitata; however, due to strict control strategies and successful eradication programs implemented by regulatory agencies, the medfly has not become an established pest in Florida (Simanton, 1958; Cunningham, 1989; Pinero et al., 2014; Tabanca et al., 2019; Skelley et al., 2025). However, the Caribbean fruit fly (caribfly), Anastrepha suspensa Loew, is established in Florida, where it is a production pest of guava and a quarantine pest of citrus. To ensure that citrus crops remain free of this pest, they must be grown in areas certified under the “Fly-Free Zone Certification Protocol” (Gould, 1998). These zones must be clear of host plants, such as loquat, Eriobotrya japonica Lindl., rose apple, Syzygium jambos (L.) Alst., Cattley guava, Psidium cattleianum Sabine, common guava, Psidium guajava L., and Surinam cherry, Eugenia uniflora L (Nguyen et al., 1992; Simpson, 1993; Gould, 1998; Weems et al., 2001; Heve et al., 2017). Fruit fly management relies on monitoring through a network of lure-baited traps and insecticide applications; however, continuous use of insecticides raises concerns of resistance development, environmental contamination, and negative impacts on non-target organisms (Troetschler, 1983; Wang and Messing, 2006; Shelly et al., 2012; Hsu et al., 2011; Shikano et al., 2022; Antonio et al., 2023; Hoskins et al., 2023). Therefore, there is an urgent need to develop biorational alternatives for managing tephritid fruit flies. One such biorational management tool is the sterile insect technique (SIT) for controlling and eradicating C. capitata (Juan-Blasco et al., 2013; Manrakhan and Addison, 2014; Pimentel et al., 2017; Mason, 2024). In theory, sterile males are released and mate with wild females, thereby preventing reproduction and leading to population suppression. The success of SIT requires sterile males to compete with wild males (Suckling et al., 2011; Diouf et al., 2024; Li et al., 2024).
Semiochemicals, defined as chemical signals involved in inter- or intra-specific insect communication, are constantly investigated and utilized as biorational tools for pest management. Their primary applications involve either attracting male and female fruit flies into traps for monitoring or mass capture or promoting repellency to protect exposed fruits from infestation. Furthermore, semiochemicals play a role in male lek formation (grouping to attract females), calling males within these aggregations, and releasing pheromones to draw in females by improving the competitiveness of sterile males (Vanickova et al., 2012; Shelly, 2018; Tejada et al., 2020; Dumont and Oliva, 2024). Male-specific semiochemicals are also used in traps for monitoring C. capitata. For instance, the synthetic male-specific attractant trimedlure [tert-butyl 4 (and 5)-chloro-2-methylcyclohexane-1-carboxylate] is a key component in traps targeting male C. capitata (Cunningham, 1989; Shelly et al., 1996; Tan et al., 2014; Shelly and Cloonan, 2023). Despite its effectiveness, trimedlure is produced by only one manufacturer globally. This limited supply highlights the need to develop alternative, plant-based male-specific attractants for C. capitata. The success of SIT programs is directly linked to the quality and competitiveness of the released sterile males. The use of volatile attractants is crucial to ensure that they exhibit mating behavior similar to their wild counterparts and effectively compete for mates.
Essential oils (EOs), rich in diverse bioactive compounds, are considered a prime resource of male-specific kairomones for C. capitata. For example, a sesquiterpene hydrocarbon, (+)-α-copaene, found in Angelica archangelica L. EO, was identified as highly attractive to medfly males, and the oil was used in monitoring traps during early eradication programs in Florida before the identification of trimedlure (Steiner et al., 1957; Simanton, 1958; Segura et al., 2018). While research efforts testing the attractiveness of (+)-α-copaene have been successful and found that it is three times more attractive to male C. capitata than trimedlure, it has not been widely used in the field. This is because isolating and synthesizing it is expensive, and its availability from natural sources is limited (Flath et al., 1994a, b; Shelly et al., 2023; Lull et al., 2023; Koening et al., 1994). Additional studies have shown that α-copaene analogs, such as α-ylangene and β-copaene, demonstrated short-range attractiveness to C. capitata males but were not strong attractants in the field (Flath et al., 1994a, b). Accordingly, research efforts were devoted to developing alternative natural sources of medfly attractants. Ginger root oil, Zingiber officinale Roscoe, another non-host plant, also increased the mating competitiveness of males, and the effect was long-lasting (Shelly and Mclnnis, 2001). Searching for new natural sources of α-copaene, volatile chemicals from avocado (Persea americana Mill.), litchi (Litchi chinensis Sonn.), and ficus (Ficus benjamina L.) wood were investigated. Behavior and electroantennogram responses to male C. capitata showed that litchi elicited the highest responses and ficus the lowest, and the attraction to avocado genotypes varied. This study did not directly correlate medfly attraction with α-copaene levels, suggesting that other sesquiterpenes may influence male responses (Niogret et al., 2011). Following the attraction of medfly males to ginger root EO, short-range bioassays demonstrated that linalool was primarily responsible for the attraction to ginger root oil (Niogret et al., 2017). In studies of the short-range attraction of sterile C. capitata, we found that Melaleuca alternifolia (Maiden & Betche) Cheel, Tetradenia riparia (Hochst.) Codd, and Juniperus foetidissima Willd EOs were highly attractive (Shelly and Epsky, 2015; Tabanca et al., 2020; Blythe et al, 2020; Kurtca et al., 2021).
In our ongoing search for new monitoring and control tools of C. capitata and other tephritid fruit flies, we explored the EOs of Artemisia absinthium L. (Asteraceae) as a potential source of kairomones. A. absinthium, commonly known as wormwood, is a strong aromatic plant native mainly to Europe, but represented in the Mediterranean region and Asia (Mihajilov-Krstev et al., 2014; Nemeth, 2016). Both EO and wormwood extracts have been widely used in many gastric herbal preparations, dietary supplements, and alcoholic beverages, and for agricultural purposes, such as acaricidal, antifungal, and insecticidal properties and allelopathic effects (Lachenmeier and Uebelacker, 2010; Abad et al., 2012; Umpierrez et al., 2012; Llorens-Molina and Vacas, 2015). Its EO is connected with its potential natural insecticide primarily due to the presence of thujone isomers (Abad et al., 2012; Mihajilov-Krstev et al., 2014; Nguyen and Nemeth, 2016). Thujones are found in various EOs, including A. absinthium, with their ratio varying depending on the plant source and developmental stage (Nemeth, 2016).
The current study aimed to i) analyze the chemical composition of authenticated A. absinthium EO and compare it with lab-distilled A. absinthium EOs purchased as plant tissues from various local herbal supplies in Turkey using gas chromatography–flame ionization detection (GC–FID) and gas chromatography–mass spectrometry (GC–MS), ii) evaluate A. absinthium EOs in behavioral assays with sterile male C. capitata, and iii) evaluate the key compounds in the behavioral assays for C. capitata and determine their toxicity against female Caribbean fruit flies, A. suspensa.
2 Materials and methods
2.1 Materials
A. absinthium used as an authenticated/standard (Asd) was obtained from the natural sources of Istanbul, Zeytinburnu Medicinal Plants Garden in Turkey. Commercial samples A1 to A5 were purchased from Turkish herbal shops as loose plant parts per sample. The A. absinthium aerial parts were subjected to hydrodistillation for 3 hours using a Clevenger-type apparatus to obtain essential oils. The percentage yields were calculated on a dry weight basis as given in Supplementary Table S1 (Supplementary Material). The oils were dried over anhydrous sodium sulfate to remove moisture and stored at +4°C until analysis and tested further. α-Thujone (CAS No. 546-80-5) and α,β-thujone mixture (Product No. PHL82669) were purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA). A schematic representation of the methodology is given in Figure 1.
Figure 1. Schematic representation of the methodology used to analyze Artemisia absinthium EOs by GC–MS system and test the key compounds in the behavioral and toxicity assays for Ceratitis capitata and Anastrepha suspensa. EOs, essential oils; GC–MS, gas chromatography–mass spectrometry.
2.2 Analysis of essential oils
2.2.1 Gas chromatography–mass spectrometry analysis
The GC–MS analysis was carried out with an Agilent 5975 GC-MSD system (Santa Clara, CA, USA). Innowax FSC column (60 m × 0.25 mm, 0.25 µm film thickness) was used with helium as carrier gas (0.8 mL/min). GC oven temperature was kept at 60°C for 10 minutes, programmed to 220°C at a rate of 4°C/min, kept constant at 220°C for 10 minutes, and then programmed to 240°C at a rate of 1°C/min. The split ratio was adjusted to 40:1. The injector temperature was set to 250°C. Mass spectra were recorded at 70 eV. Mass range was from m/z 35 to 450.
2.2.2 Gas chromatography with flame ionization detector analysis
The GC analysis was carried out using an Agilent 6890N GC system. The FID detector temperature was 300°C. To obtain the same elution order using GC–MS, simultaneous auto-injection was conducted on a duplicate of the same column, applying the same operational conditions. Relative percentage amounts of the separated compounds were calculated from FID chromatograms. The analysis results are given in Table 1.
2.2.3 Identification of essential oil constituents
The identification of the EO components was carried out by co-injection with available standard compounds, which were purchased from commercial sources or obtained from natural sources. In addition, computer matching against commercial, Wiley GC/MS Library, MassFinder software 4.0 (McLafferty and Stauffer, 1989; Hochmuth, 2008), and in-house “Başer Library of Essential Oil Constituents” built up by genuine compounds and components of known oils, as well as MS literature data, was used for the identification. A C9–C40 n-alkane standard solution (Fluka, Buchs, Switzerland) was used to spike the samples for the determination of chromatographic retention indices (RIs).
2.3 Laboratory bioassays
2.3.1 Short-range attraction bioassays with C. capitata
Bioassays were conducted using sterile male C. capitata, consistent with the insect source, rearing procedures, and short-range attraction methods detailed by Tabanca et al. (2020). All experiments took place at room temperature within screened cages (20.3 cm3, BioQuip Products, Rancho Dominguez, CA, USA). Fifty flies, 7–15 days old, were introduced into each cage 1 hour before starting an experiment. Each assay began by placing two Petri dishes (53-mm diameter × 12-mm height) containing a Whatman #1 filter paper disk (3.5-cm diameter) soaked with 10 μL of a 10% dilution of the test compound in acetone on two opposing sides of the cages spaced 25 cm apart. All flies were discarded and not reused after assay termination. Bioassay development was divided into three parts.
Experiment 1: In the first set of preliminary bioassays, single, separate cages were used to evaluate the C. capitata response to six different combinations: 1) tea tree oil (TTO) versus acetone, 2) Asd versus acetone, 3) A1 versus acetone, 4) A2 versus acetone, 5) A3 versus acetone, 6) A4 versus acetone, and 7) A5 versus acetone.
Experiment 2: In the second set of bioassays, cages were used to evaluate the C. capitata response to 1) TTO versus acetone, 2) α-thujone versus acetone, and 3) α,β-thujone versus acetone
Experiment 3: In the third set of bioassays, cages were used to evaluate the C. capitata response to 1) TTO versus α-thujone, 2) TTO versus α,β-thujone, and 3) α-thujone versus α,β-thujone.
Fly responses were measured by counting the number of flies within the Petri dish at 5, 10, 15, 30, 45, 60, 75, and 90 minutes. The first set of preliminary bioassays tested one cage of each combination. Each test in the second and third sets of bioassays was replicated 12 times, with cage positions randomized between runs. TTO [M. alternifolia (Maiden & Betche) Cheel.] was included as a positive control due to its powerful short-range attraction to male C. capitata (Shelly and Epsky, 2015; Tabanca et al., 2020).
2.3.2 Toxicity of thujones against female adult A. suspensa
The colony of Caribbean fruit fly, A. suspensa, was maintained in rearing cages (30 × 30 × 30 cm) in an insectary in the toxicology laboratory in the Horticultural Research Station in the USDA-ARS at Miami, Florida, since August 2020. A. suspensa originated from field-collected guava fruits that were infested by A. suspensa in 2020, and since then, it has been maintained on an artificial diet in the insectary under 26.0°C ± 0.5°C, 70% ± 5% relative humidity, and 12:12 (light:dark), without exposure to any insecticides.
Topical bioassays using thoracic application to adult female A. suspensa were conducted to determine the toxicities of α-thujone and α,β-thujone under laboratory conditions similar to those mentioned above in the toxicology laboratory. Stock solutions of α-thujone and α,β-thujone were first prepared by dissolving 100 μg of each compound in 1 μL of acetone to establish a 100 μg/μL solution. Stock solution of each compound was then diluted serially to establish 0.05, 0.1, 0.2, 0.25, 0.5, and 0.75 μg/μL solutions, and each dilution was used in topical bioassays.
The procedure of the topical bioassay was similar to our previous study (Tok et al., 2023). To collect the synchronized female adult A. suspensa for the topical bioassay, pupae of A. suspensa were collected from rearing cages in the insectary and placed in a tray inside a screen cage (30 cm × 30 cm × 30 cm) under laboratory conditions. After adult emergence, female adults (<3 days old) were then collected using an aspirator into a plastic vial (3 cm in diameter × 8 cm in height). To begin the bioassay, vials containing female adults were placed in a refrigerator first at 10°C for 5 minutes to calm the flies, and calmed flies were then removed from the refrigerator to a Petri dish for the topical application. On each fly, a repeating dispenser equipped with a gastight and microliter syringe (50 μL) (PB600, Hamilton Company, Reno, NV, USA) was used to apply a 1-μL dilution at each concentration of each compound on the dorsal thorax of the calmed adult flies. After topical application, the adult flies were immediately transferred into a plastic cup (6 cm in diameter × 7.4 cm in height) and covered with a mesh screen for post-treatment observation. A block of sugar and yeast hydrolysate mixture (4:1 per weight) (1 cm3) and a block of water agar (1 cm3) were placed on top of the mesh screen to supply the food and water for the tested flies. To remove the chilling effect from the treatment, only adult flies recovered from chill were used for the experiment after a topical bioassay. After 24 hours, the numbers of live and dead flies were documented, and the mortality of A. suspensa in each treatment was calculated. Untreated female adults and those treated with acetone alone were used as controls. For each dilution of α-thujone and α,β-thujone, 10–15 female adult flies were treated, and for the control treatment, 10 female adult flies were treated with acetone. Each treatment was replicated three times. In total, 260 female flies were used in the evaluation of thujones.
2.4 Statistical analysis
Principal component analysis (PCA) and hierarchical cluster analysis (HCA) were carried out using the JMP Pro version 18.2.1 (SAS Institute, Cary, NC, USA). The covariance data matrix for the volatile compounds was an X × X matrix (28 individual compounds × 6 samples = 168 data). The PCA was performed using the JMP default standard method. The HCA was performed using Ward’s method. Short-range attraction data in the second set of bioassays were analyzed using JMP Pro 16 (SAS Institute). All short-range attraction data were found to meet assumptions of normality.
A mixed model was utilized for the separate analysis of the effect of factors on fly response to individual compounds within each of the six combinations tested. Fly age and time (minutes) were treated as fixed effects, while replicate was treated as a random effect. Time was treated as a repeated structure, with replicate as the subject. A compound symmetry structure was chosen, and Tukey’s pairwise comparisons of fly responses were performed between time points for each compound. Multiple comparison tests were used to test for differences in male responses at each time point to compounds within the six comparison tests.
The mortality data of A. suspensa in toxicity bioassays were used to calculate the lethal dose (LD50 and LD99) for α-thujone and α,β-thujone. Mortality data for each treatment were corrected by mortalities in the untreated control using Abbott’s formula (Abbott, 1925) before the analysis. A probit analysis was then used to calculate the lethal dose corresponding to a 50% and 99% reduction (LD50 and LD99) in A. suspensa’s survival based on the regression curve. The statistical analysis was performed using SAS (version 9.4, SAS Institute Inc., Cary, NC, USA).
3 Results
3.1 Chemical composition of A. absinthium essential oils
Essential oil yield is a key factor for economically important EOs such as A. absinthium EO. The conventional hydrodistillation method was used for yielding. Pale yellow EOs were obtained with yields of 1.0 to 3.5 mL/kg (0.1% to 0.35%) for A1 to A5 samples. According to the European Pharmacopoeia, the EO yield for A. absinthium should not be less than 2 mL/kg (0.2%), and the wormwood herb consists of basal leaves, slightly leafy, flowering tops, or a mixture of these dried organs (European Pharmacopeia, 2008). The highest EO yield was obtained when plant material was collected at full bloom and distilled shortly after collection [e.g., Asd sample, 3.8 mL/kg (0.38%), Supplementary Table S1]. EO production is highly influenced by resources, storage conditions (packed or unpacked), age of plants, and plant population density (flowers, leaves, stems, and branches).
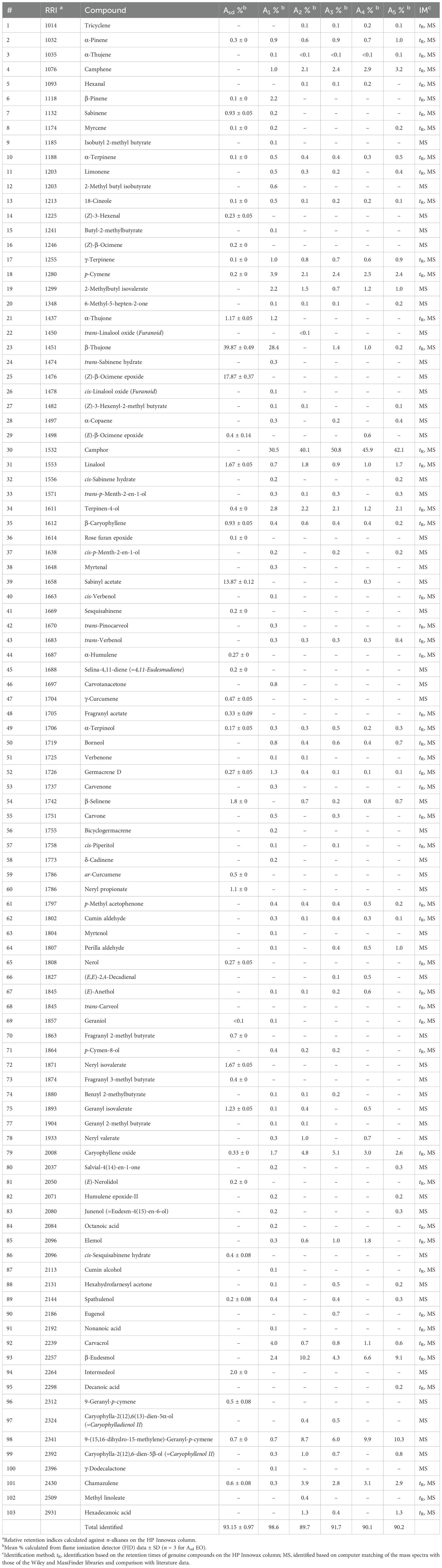
GC–FID and GC–MS techniques were used to analyze the chemical profile of A. absinthium EOs. A total of 103 compounds constituting 89.7% to 98.6% of the total oil were detected in the six A. absinthium samples. The identified compounds, their relative percentages, and retention indices are listed in Table 1 in order of their elution time on the polar column. The EOs are composed of monoterpene hydrocarbons (2.03%–10.5%), oxygenated monoterpenes (46.5%–76.22%), sesquiterpene hydrocarbons (0.9%–4.64%), oxygenated sesquiterpenes (6.03%–18.5%), oxygenated diterpenes (0.7%–10.3%), fatty acids+esters (0%–1.7%), and other non-terpenoids (3.03%–6.2%) (Figure 2). In examined oils, β-thujone (39.87%), (Z)-β-ocimene epoxide (17.87%), and sabinyl acetate (13.87%) were the major constituents of Asd oil, followed by intermedeol (2.0%), β-selinene (1.8%), neryl isovalerate (1.67%), linalool (1.67%), geranyl isovalerate (1.23%), α-thujene (1.17%), and neryl propionate (1.1%); in contrast, A1–A5 EOs were dominated by camphor (30.5%–50.8%), followed by β-thujone (0%–28.4%), β-eudesmol (2.4%–10.2%), and diterpene 9-(15,16-dihydro-15-methylene)-geranyl-p-cymene (0.7%–10.3%). The remaining most common compounds that occurred were caryophyllene oxide (1.7%–5.1%), carvacrol (0.7%–4%), chamazulene (0.3%–3.9%), p-cymene (2.1%–3.9%), camphene (1.0%–3.2%), terpinen-4-ol (1.2%–2.8%), and 2-methylbutyl isovalerate (0.7%–2.2%). From all the identified compounds, β-thujone was determined as a major compound in Asd EO, whereas camphor was the main compound in five samples, A1 to A5 EOs. β-Thujone and α-thujone were assessed only as Asd and A1 EOs, and camphor was absent from the Asd EO. In addition, (Z)-β-ocimene epoxide, sabinyl acetate, β-eudesmol, and pleasant floral compounds, neryl propionate, neryl isovalerate, nerol, and (E)-nerolidol, were detected only in Asd EO. The situation was similar with A1 to A5 samples: compounds camphene, 2-methylbutyl isovalerate, α-terpineol, borneol, and carvacrol were present in 1% and over only in A1 to A5 samples, while γ-terpinene, p-cymene, terpinen-4-ol, caryophyllene oxide, 9-(15,16-dihydro-15-methylene)-geranyl-p-cymene, and chamazulene were found in all six samples (Asd and A1 to A5).
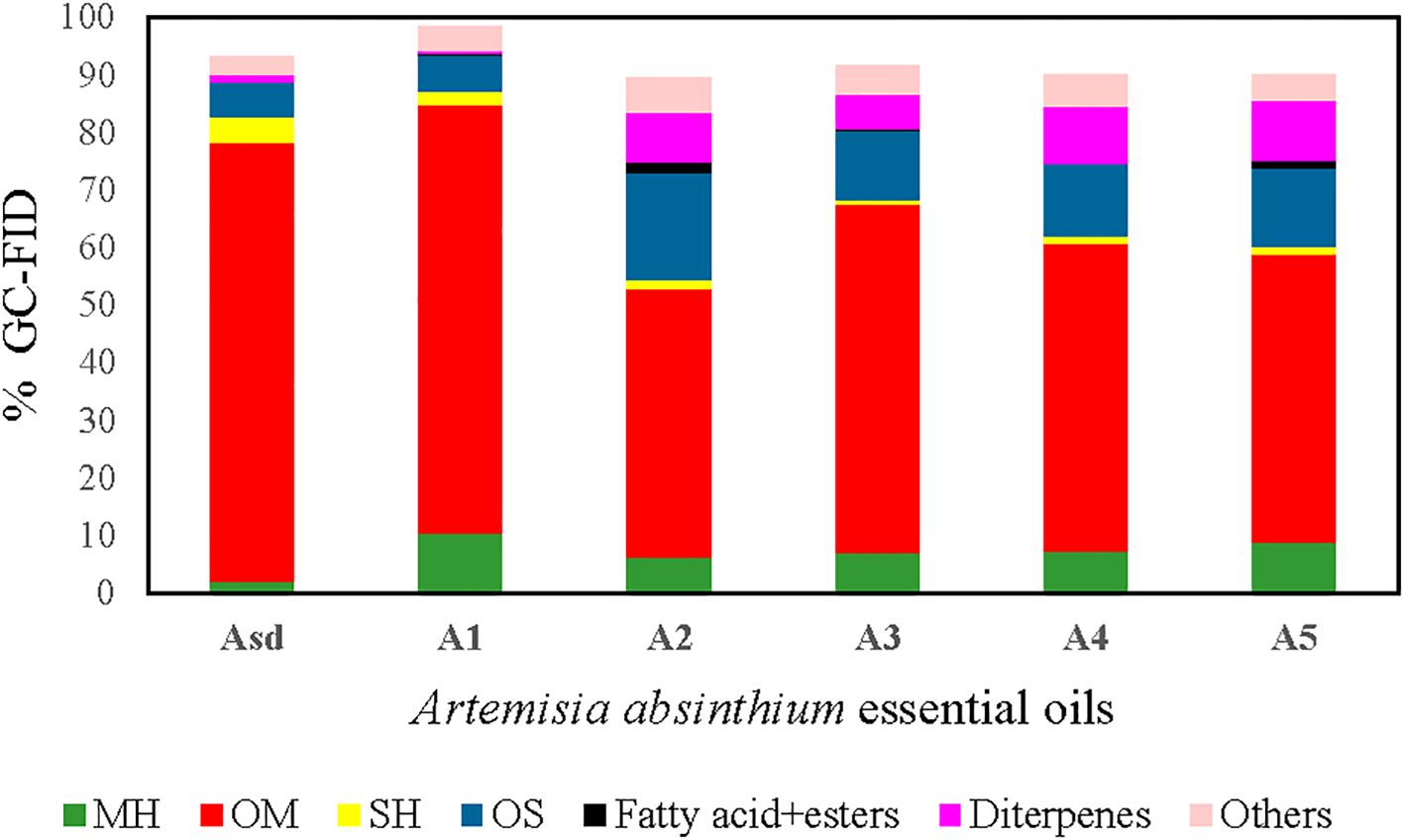
Figure 2. Percent composition of terpene groups in the Artemisia absinthium EOs: MH (monoterpene hydrocarbons), OM (oxygenated monoterpenes), SH (sesquiterpene hydrocarbons), OS (oxygenated sesquiterpenes), aliphatic fatty acid+esters, diterpenes, and others. EOs, essential oils.
3.2 Multivariate analysis of the A. absinthium essential oils
Multivariate statistical analysis was subjected to PCA and HCA to visualize the similarities and differences among the A. absinthium samples based on volatile compounds by reducing the number of dimensions without much loss of information. PCA and HCA were performed on the values of the relative volatile concentrations ≥1.0% using the correlation matrix with no rotation and applied to clarify the relationship among the Artemisia samples. PCA resulted in the first two components (PC1 and PC2) with eigenvalues greater than one, with PC1 55.1% and PC2 27.5% of variance, respectively. Bartlett’s sphericity and the Kaiser–Meyer–Olkin (KMO) test were performed to validate the dataset’s suitability and sampling adequacy for a suitable PCA application. The results of Bartlett’s sphericity test (p < 0.05) and KMO test (KMO > 0.5) indicated that the dataset was suitable and adequate, respectively (Table 2).
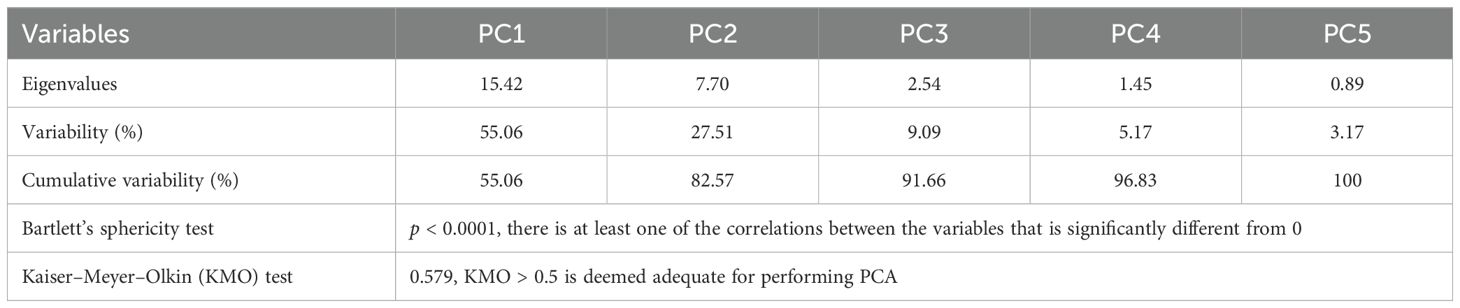
Table 2. Eigenvalues, variability, cumulative variability, Bartlett’s sphericity test, and Kaiser–Meyer–Olkin (KMO) test are associated with each principal component (PC).
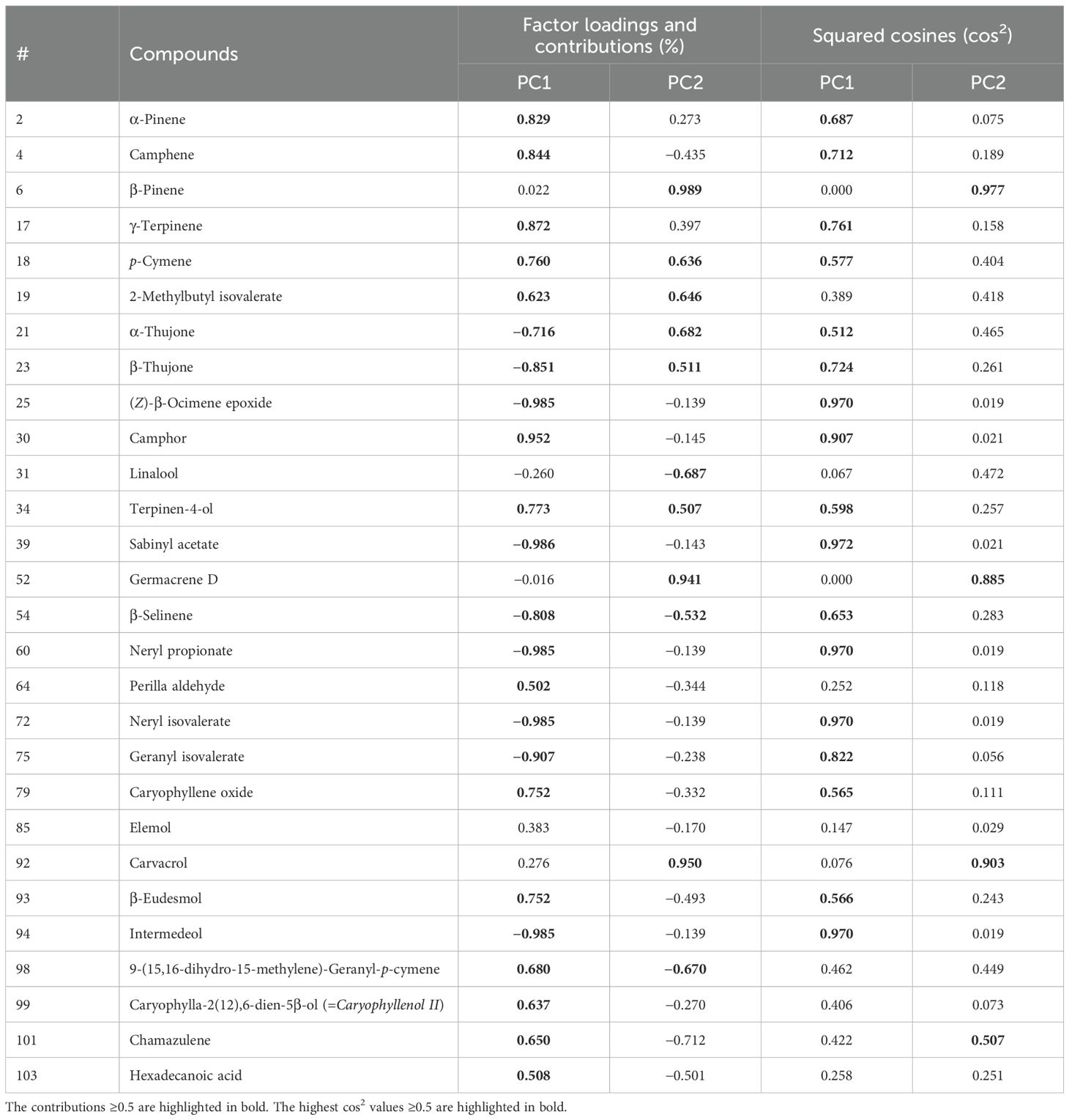
PCA separated the A. absinthium samples into three groups based on their volatile composition. Authenticated sample Asd is located in the lower quadrant at the left-hand side of the plot, displaying negative values for both PC1 and PC2, in contrast to the A1 sample, which is present in the right-hand side of the plot, showing positive values for both PC1 and PC2. Commercial samples A1 to A5 are clustered together on the positive side of PC1 and the negative side of PC2 (Figure 3). Factor loading and high cos2 values determine a strong positive or negative correlation between observations and variables, which is therefore considered an important observation or variable for judgment. Among the 28 Volatile Organic Compounds (VOCs), the highest cos2 values were in PC1: sabinyl acetate (0.972), (Z)-β-ocimene epoxide (0.970), camphor (0.907), neryl propionate (0.970), neryl isovalerate (0.970), intermedeol (0.970), geranyl isovalerate (0.822), γ-terpinene (0.761), β-thujone (0.724), and camphene (0.712). For PC2, the VOCs were β-pinene (0.977), carvacrol (0.903), and germacrene D (0.885) (Table 3).
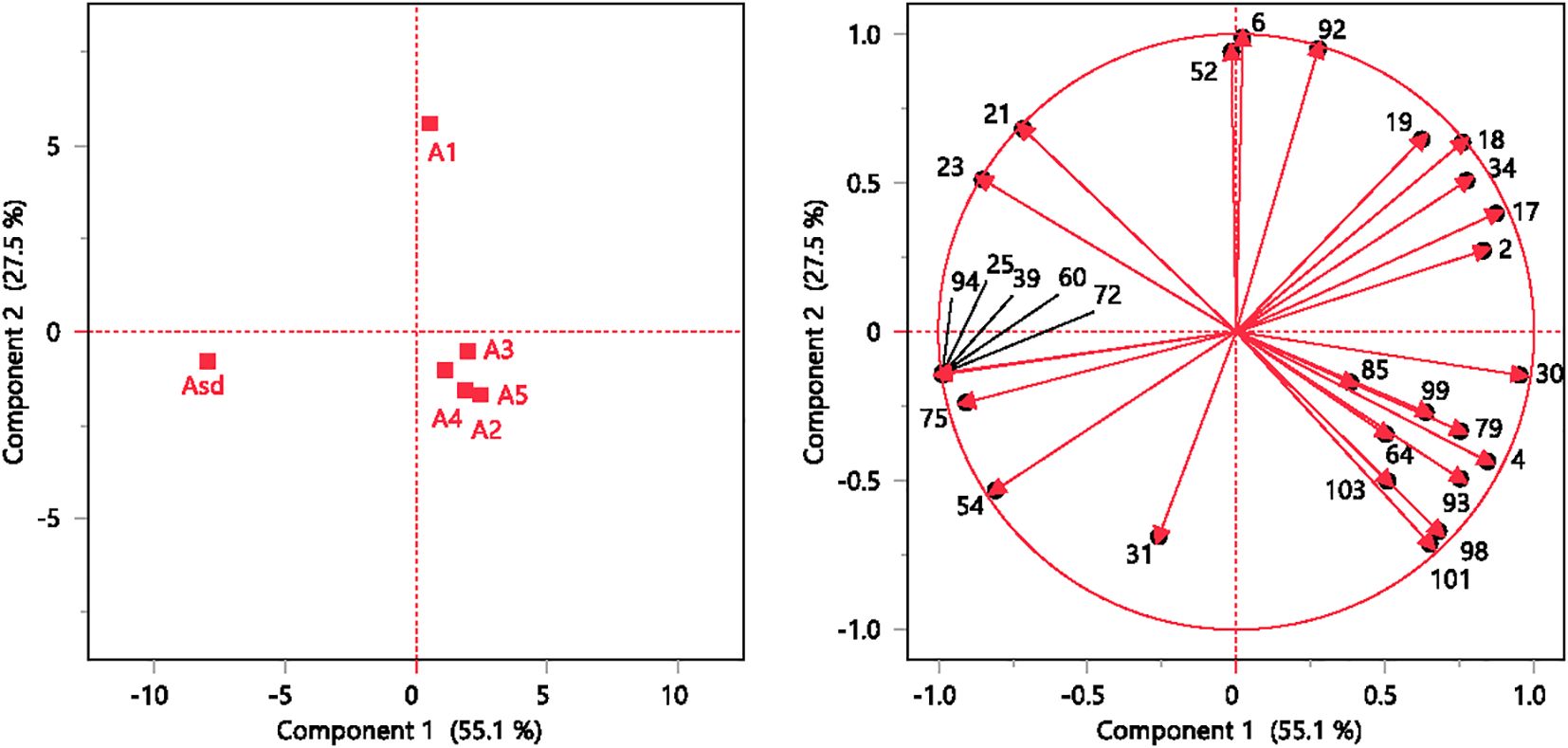
Figure 3. Results of the PCA. PC1 vs. PC2 score plots for the investigated observations (left). PC1 vs. PC2 loading plots for the investigated variables (right). Compound numbers are listed in Table 1. PCA, principal component analysis.
The dendrogram graph generated by HCA based on the differences in EO components of the six A. absinthium samples agreed with the PCA scatter plots of the same samples. A. absinthium samples fell into three clusters. Cluster 1 encompassed Asd with relatively high values of components β-thujone (39.87%), (Z)-β-ocimene epoxide (17.87%), and sabinyl acetate (13.87%). Cluster 2, comprising A1, was characterized by high camphor (30.5%) and β-thujone (28.4%), and samples A2 to A5 were embedded in cluster 3 (Figure 4) with high camphor (40.1%–50.8%), 9-(15,16-dihydro-15-methylene)-geranyl-p-cymene (6.0% to 10.3%), and β-eudesmol (4.3% to 10.2%). PCA and HCA revealed that A. absinthium samples from various sources were effectively identified and classified.
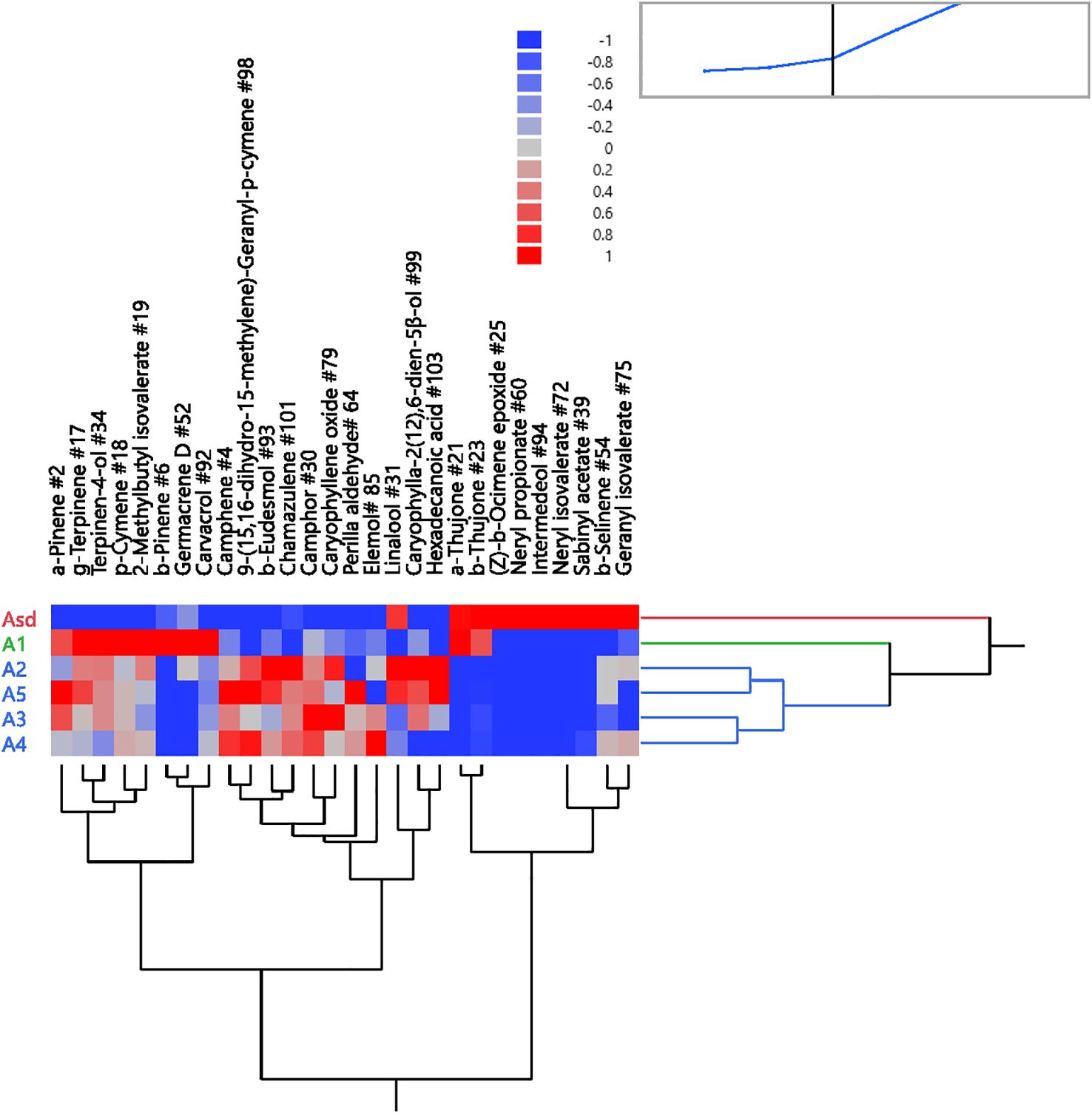
Figure 4. Two-way dendrogram of the hierarchical cluster analysis (HCA) was performed on the chemical compositions of six Artemisia absinthium samples (Asd and A1 to A5). The color box indicates the abundance of each compound from Table 1. Red represents a high density of compounds, and blue represents low density.
3.3 Behavioral responses of the sterile male medflies to A. absinthium oils in short-range assays
3.3.1 Preliminary short-range assays: A. absinthium attraction
In our initial short-range assays, male C. capitata showed varied attraction to A. absinthium EOs. TTO, used as a positive control, was the most attractive after 30 minutes, followed by samples A1, Asd, A5, A2, A4, and A4 in decreasing order of attractiveness (Supplementary Figure S1). The attraction pattern for Asd changed over time and captured more flies after 30 minutes. Based on the two-way hierarchical clustering dendrogram and heat map correlation, it indicates that α,β-thujones are higher than the mean levels (Figure 4). Therefore, we presumed that α,β-thujones may be the main components contributing to the bioactivity mediated by male C. capitata, and further bioassays were carried out to compare thujones. Additionally, unidentified components may also have contributed to this attraction but were not tested in this study.
3.3.2 Bioassays with tea tree oil, α-thujone, and α,β-thujone
We then conducted a second set of bioassays to compare C. capitata response to TTO, α-thujone, and α,β-thujone against an acetone control (Supplementary Table S2).
3.3.2.1 Tea tree oil and acetone
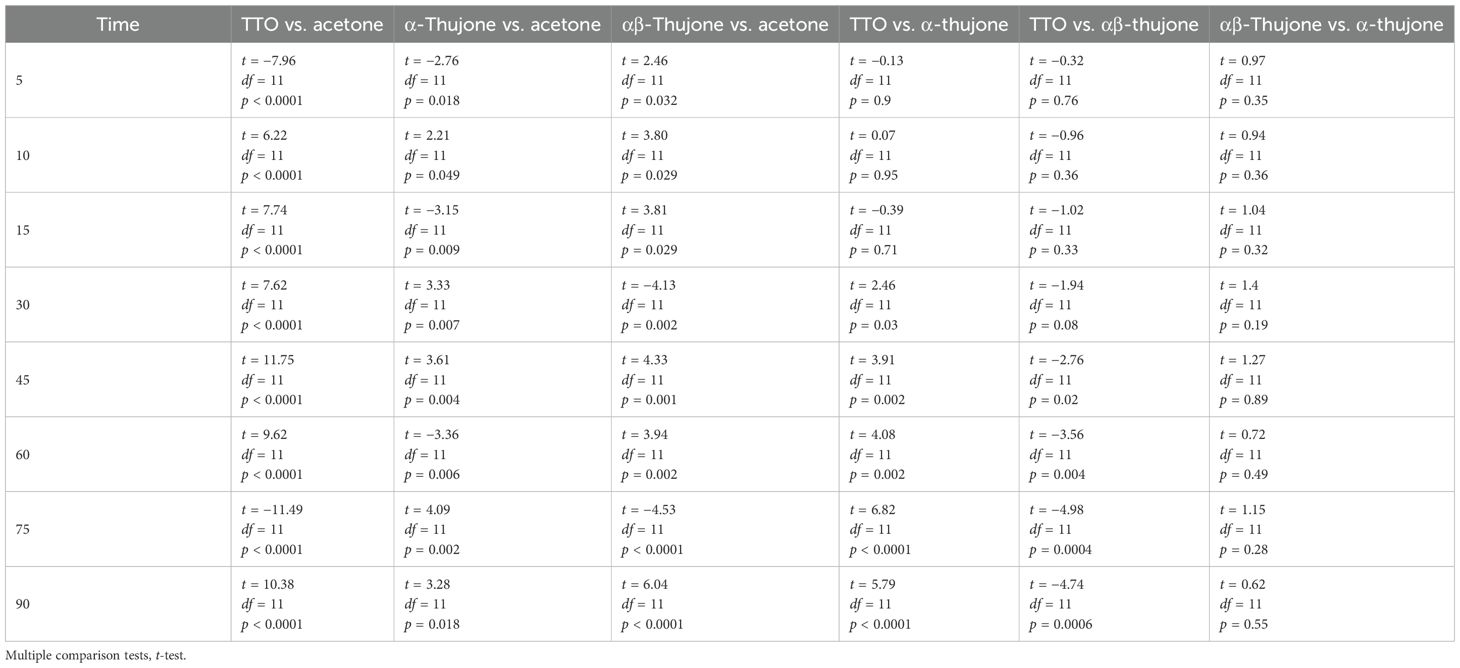
Fly age did not significantly affect responses to TTO (F = 2.59; df = 5; p = 0.14) or acetone (F = 0.64; df = 5; p = 0.67). However, time significantly influenced responses to both tea tree oil (F = 32.15; df = 7; p < 0.0001) and acetone (F = 2.75; df = 7; p = 0.01). Attraction to tea tree oil increased from 5 to 45 minutes and then decreased from 60 to 90 minutes. More flies were attracted to tea tree oil at all time points (Figure 5; Table 4).
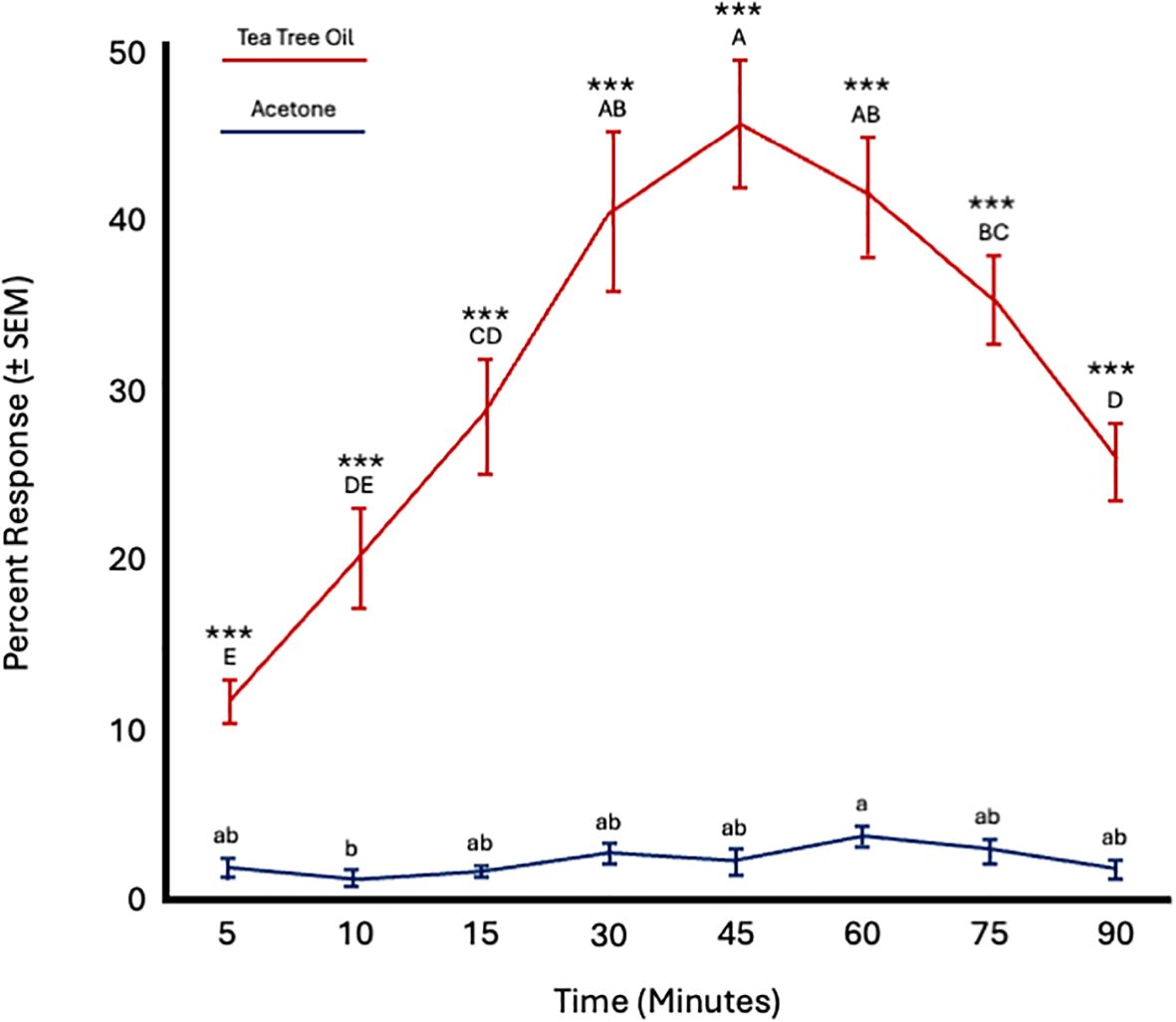
Figure 5. Percent attraction of sterile male Ceratitis capitata to tea tree oil vs. acetone control in small cage bioassays. Fifty flies were tested per cage across 12 replicates (n = 12). An asterisk (*p ≤ 0.05; **p ≤ 0.01) indicates a significant difference between responding flies at a specific time point (matched pairs t-test, p < 0.05). Means sharing the same letter are not significantly different within each test compound (mixed model: fly age and time as fixed effects, replicate as random effect, and time as repeated structure with replicate as subject; compound symmetry structure; Tukey's pairwise comparisons, p < 0.05).
3.3.2.2 α-Thujone and acetone
Similarly, fly age did not significantly impact responses to α-thujone (F = 1.09; df = 5; p = 0.45) or acetone (F = 0.89; df = 5; p = 0.54). There was a significant effect of time on the response to α-thujone (F = 6.53; df = 7; p < 0.0001), but not to acetone (F = 1.07; df = 7; p = 0.39). Attraction to α-thujone increased from 5 to 30 minutes and then decreased from 45 to 90 minutes. More flies were attracted to α-thujone at all time points (Figure 6; Table 4).
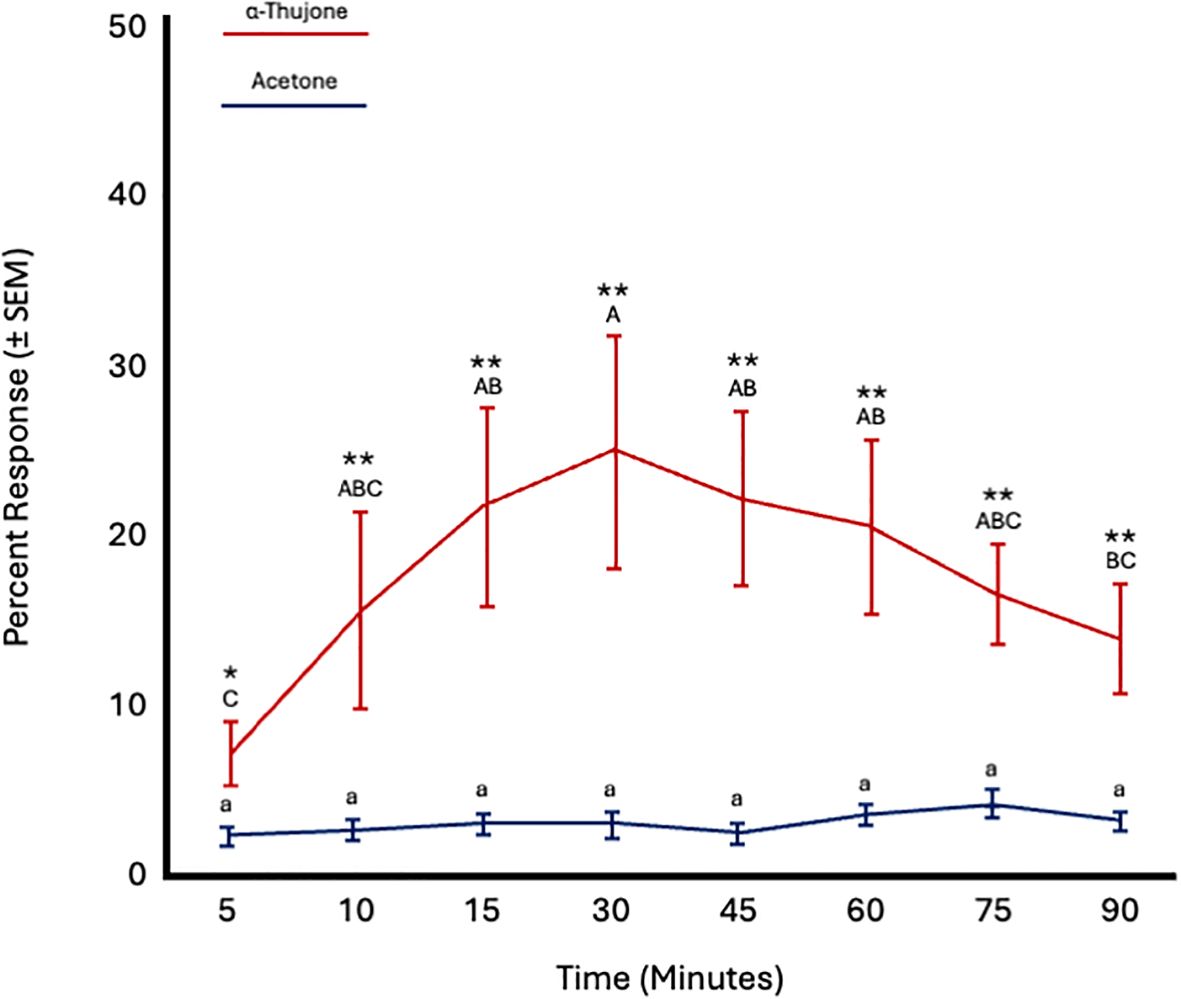
Figure 6. Percent attraction of sterile male Ceratitis capitata to α-thujone vs. acetone control in small cage bioassays. Fifty flies were tested per cage across 12 replicates (n = 12). An asterisk (*p ≤ 0.05; **p ≤ 0.01) indicates a significant difference between responding flies at a specific time point (matched pairs t-test, p < 0.05). Means sharing the same letter are not significantly different within each test compound (mixed model: fly age and time as fixed effects, replicate as random effect, and time as repeated structure with replicate as subject; compound symmetry structure; Tukey's pairwise comparisons, p < 0.05).
3.3.2.3 α,β-Thujone and acetone
Fly age had no significant effect on responses to α,β-thujone (F = 0.55; df = 5; p = 0.73) or acetone (F = 0.43; df = 5; p = 0.81). However, time significantly affected the response to α,β-thujone (F = 4.04; df = 7; p = 0.0008), but not to acetone (F = 1.06; df = 7; p = 0.39). Attraction to α,β-thujone increased from 5 to 10 minutes and then remained constant from 10 to 90 minutes. More flies were attracted to α,β-thujone at all time points (Figure 7; Table 4).
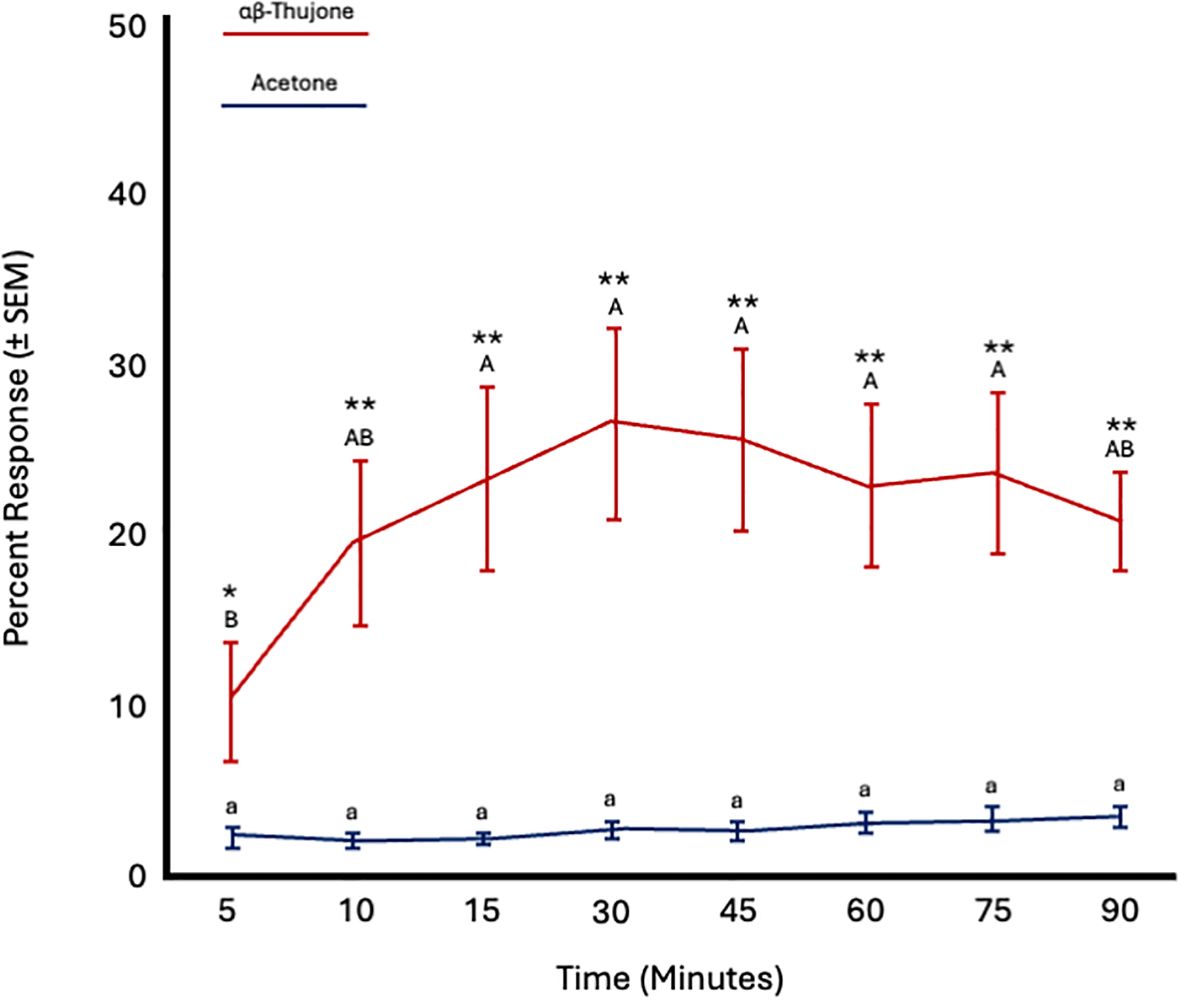
Figure 7. Percent attraction of sterile male Ceratitis capitata to αβ-thujone vs. acetone control in small cage bioassays. Fifty flies were tested per cage across 12 replicates (n = 12). An asterisk (*p ≤ 0.05; **p ≤ 0.01) indicates a significant difference between responding flies at a specific time point (matched pairs t-test, p < 0.05). Means sharing the same letter are not significantly different within each test compound (mixed model: fly age and time as fixed effects, replicate as random effect, and time as repeated structure with replicate as subject; compound symmetry structure; Tukey's pairwise comparisons, p < 0.05).
3.3.2.4 Bioassays with combinations of tea tree oil, α-thujone, and α,β-thujone
Our third set of bioassays examined C. capitata responses to three different combinations of these compounds.
3.3.2.5 Tea tree oil versus α-thujone
Fly age did not significantly affect responses to tea tree oil (F = 0.5; df = 5; p = 0.77) or α-thujone (F = 0.43; df = 5; p = 0.82). However, time significantly influenced responses to both TTO (F = 27.27; df = 7; p < 0.0001) and α-thujone (F = 2.62; df = 7; p = 0.018). Fly attraction to TTO increased from 15 to 30 minutes and remained constant thereafter until 90 minutes. Attraction to α-thujone increased from 5 to 10 minutes and then remained constant until 90 minutes. More flies were attracted to TTO from 30 to 90 minutes (Figure 8; Table 4).
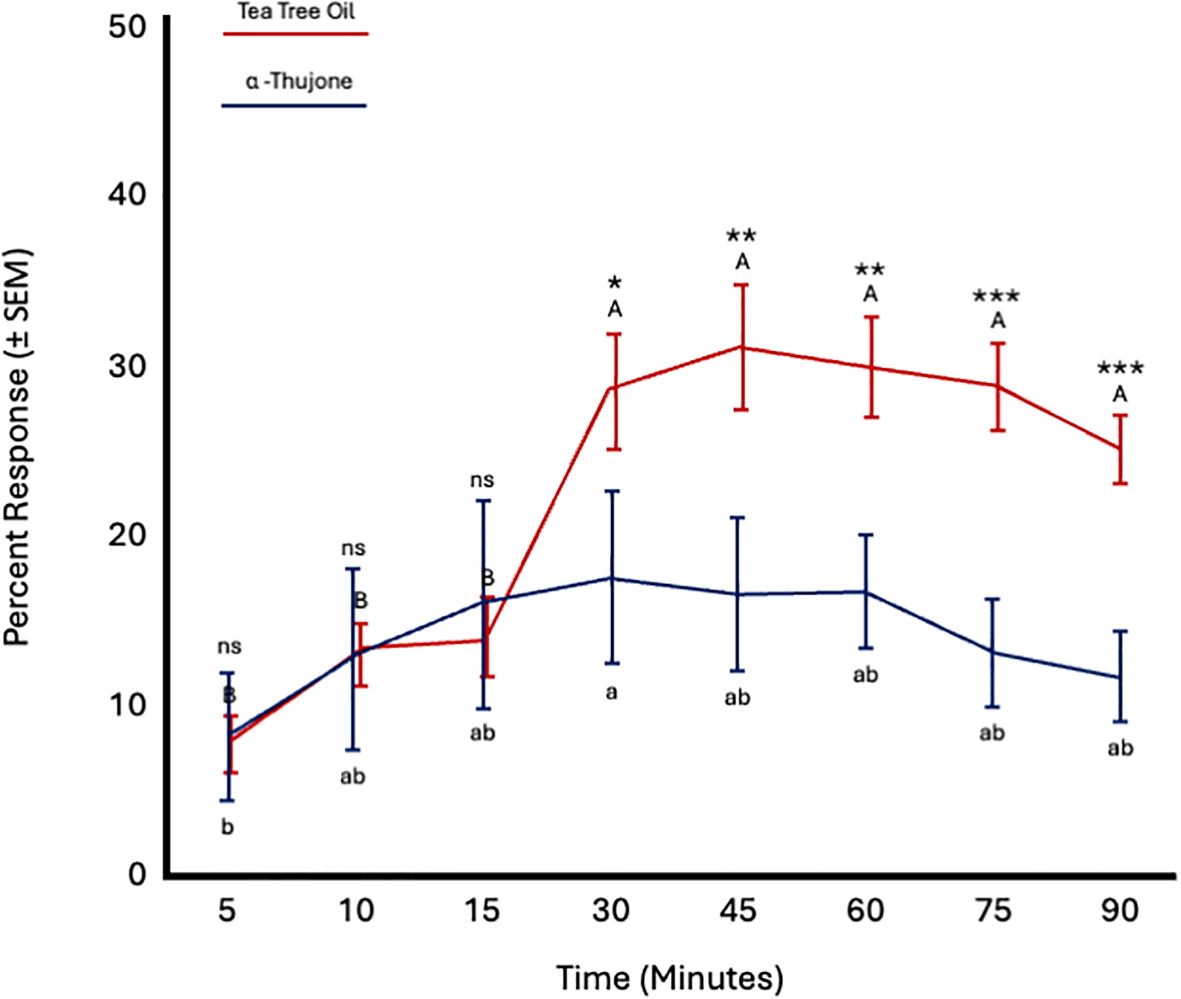
Figure 8. Percent attraction of sterile male Ceratitis capitata to tea tree oil vs. α-thujone in small cage bioassays. Fifty flies were tested per cage across 12 replicates (n = 12). An asterisk (*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001) indicates a significant difference between responding flies at a specific time point (matched pairs t-test, p < 0.05). Means sharing the same letter are not significantly different within each test compound (mixed model: fly age and time as fixed effects, replicate as random effect, and time as repeated structure with replicate as subject; compound symmetry structure; Tukey's pairwise comparisons, p < 0.05). ns: not significant, p-value≥0.05.
3.3.2.6 Tea tree oil versus α,β-thujone
Fly age had no significant effect on responses to tea tree oil (F = 0.61; df = 5; p = 0.69) or α,β-thujone (F = 0.98; df = 5; p = 0.49). Conversely, time significantly affected responses to both TTO (F = 11.07; df = 7; p < 0.0001) and α,β-thujone (F = 3.56; df = 7; p = 0.002). Fly attraction to TTO increased from 15 to 60 minutes and remained constant from 60 to 90 minutes. Attraction to α,β-thujone increased from 5 to 10 minutes and remained constant from 15 to 90 minutes. More flies were attracted to tea tree oil than α,β-thujone from 45 to 90 minutes (Figure 9; Table 4).
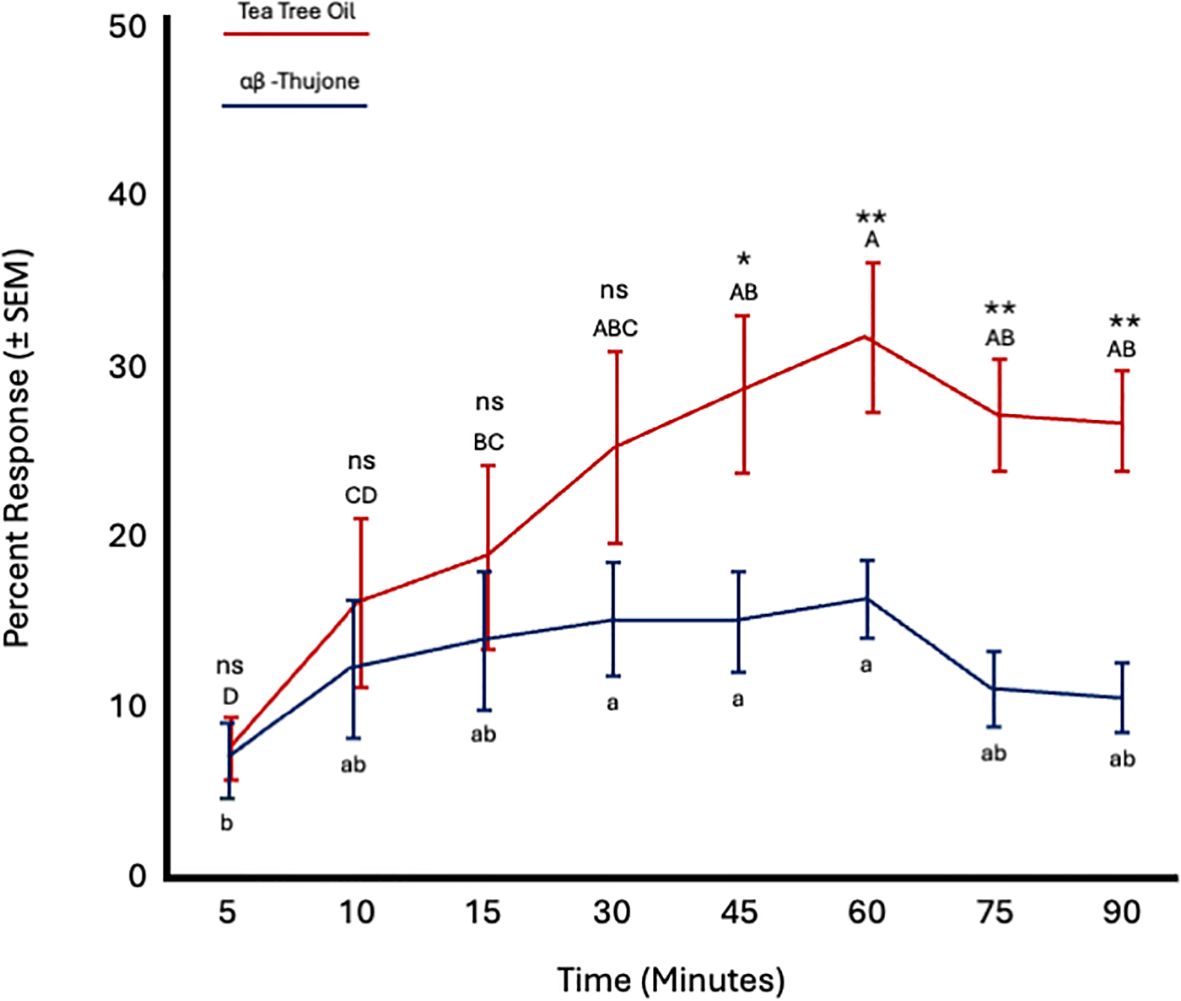
Figure 9. Percent attraction of sterile male Ceratitis capitata to tea tree oil vs. αβ-thujone in small cage bioassays. Fifty flies were tested per cage across 12 replicates (n = 12). An asterisk (*p ≤ 0.05; **p ≤ 0.01) indicates a significant difference between responding flies at a specific time point (matched pairs t-test, p < 0.05). Means sharing the same letter are not significantly different within each test compound (mixed model: fly age and time as fixed effects, replicate as random effect, and time as repeated structure with replicate as subject; compound symmetry structure; Tukey's pairwise comparisons, p < 0.05). ns: not significant, p-value≥0.05.
3.3.2.7 α-Thujone versus α,β-thujone
In the final set of short-range bioassays, fly age did not significantly affect C. capitata response to either α-thujone (F = 0.77; df = 5; p = 0.6) or α,β-thujone (F = 0.75; df = 5; p = 0.61). However, time significantly influenced responses to both compounds (α-thujone: F = 9.7; df = 7; p < 0.0001; α,β-thujone: F = 9.46; df = 7; p < 0.0001). Attraction to both α-thujone and α,β-thujone increased from 5 to 15 minutes and then remained constant from 30 to 90 minutes. Flies were similarly attracted to both α-thujone and α,β-thujone at all time points (Figure 10; Table 4).
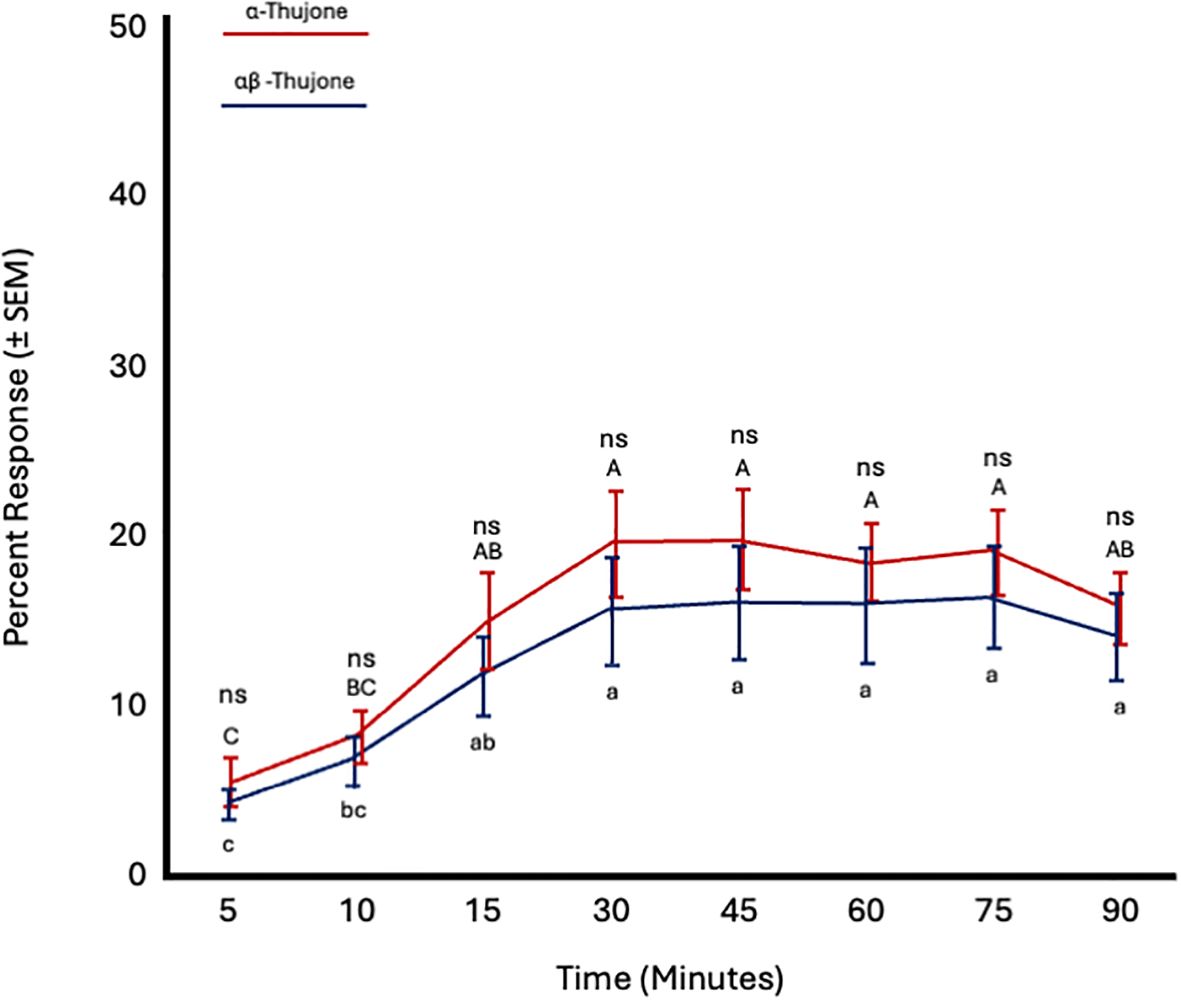
Figure 10. Percent attraction of sterile male Ceratitis capitata to α-thujone vs. αβ-thujone in small cage bioassays. Fifty flies were tested per cage across 12 replicates (n = 12). Means sharing the same letter are not significantly different within each test compound (mixed model: fly age and time as fixed effects, replicate as random effect, and time as repeated structure with replicate as subject; compound symmetry structure; Tukey's pairwise comparisons, p < 0.05). ns: not significant, p-value≥0.05.
3.4 Exploring the effects of α,β-thujones on female caribflies
The present study showed that both α-thujone and αβ-thujone demonstrated strong toxicity to adult female A. suspensa. Our results showed that the median lethal doses (LD50) of α-thujone and α,β-thujone were 0.21 and 0.14 μg/μL, respectively, and the lethal doses responsible for a 99% reduction were 0.66 and 0.71 μg/μL, respectively (Table 5). Our results also showed that both untreated and acetone controls had 0% mortality in female adults. A dose of 0.14 μg of α,β-thujone was required to achieve 50% mortality of female adults, which was lower than the dose required by α-thujone, 0.21 μg/μL. However, a higher dose of α,β-thujone was required than that of α-thujone (Table 5).
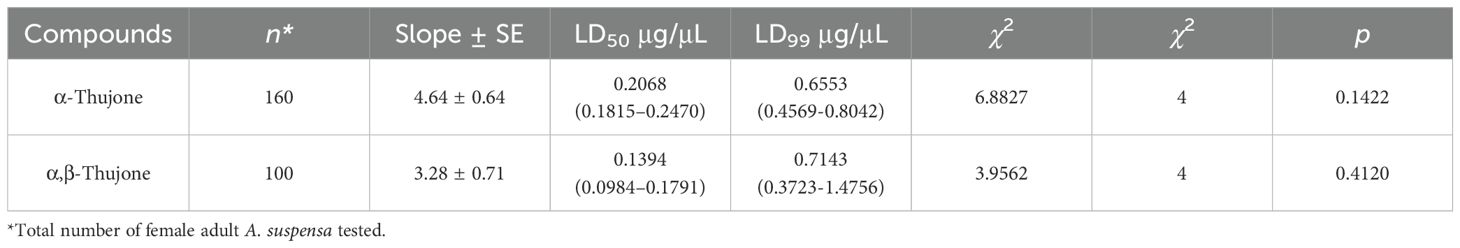
Table 5. Lethal dose (LD50 and LD99) of α,β-thujone and α-thujone for the control of the adult female Caribbean fruit fly, Anastrepha suspensa, under laboratory conditions.
4 Discussion
4.1 Chemical survey
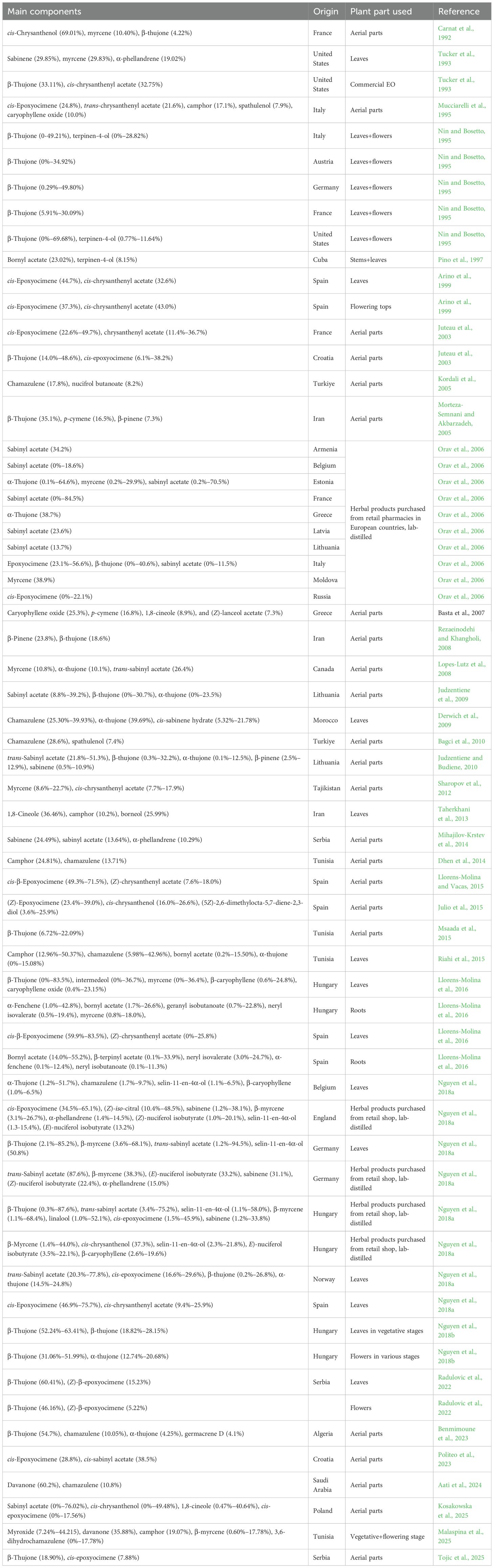
Literature surveys showed that the chemical composition and essential oil profiles of A. absinthium (wormwood) varied significantly among different populations and even within the same species (Table 6). This high level of “intraspecific variability” is a key indicator of a genetic component and ecological factors. Studies comparing A. absinthium from different European countries (e.g., Austria, France, Germany, Hungary, Italy, and Spain) found that their A. absinthium EOs were significantly different. Ecological factors such as altitude, temperature, and precipitation can influence the plant’s metabolism, leading to variations in the production of secondary metabolites. Studies comparing A. absinthium from different European countries (e.g., Austria, Belgium, Croatia, England, Estonia, Germany, Georgia, Hungary, Italy, Latvia, Lithuania, Moldova, Poland, Scotland, Serbia, Spain, and The Netherlands) found that their A. absinthium EOs showed significant qualitative and quantitative differences. Studies have identified several chemotypes based on the dominant compounds in A. absinthium EOs within any plant tissues, including i) thujone-rich chemotypes (Tucker et al., 1993; Nin and Bosetto, 1995; Juteau et al., 2003; Orav et al., 2006; Msaada et al., 2015; Llorens-Molina et al., 2016; Nguyen et al., 2018a, 2018; Radulovic et al., 2022; Raal et al., 2024; Tojic et al., 2025), ii) epoxyocimene+chrysanthenyl acetate or epoxyocimene+chrysanthenol (Carnat et al., 1992; Mucciarelli et al., 1995; Arino et al., 1999; Juteau et al., 2003; Llorens-Molina and Vacas, 2015; Julio et al., 2015; Llorens-Molina et al., 2016; Nguyen et al., 2018a, 2018; Politeo et al., 2023), iii) sabinyl acetate-rich chemotypes (Orav et al., 2006; Judzentiene and Budiene, 2010; Nguyen et al., 2018a; Kosakowska et al., 2025), and iv) mixed terpenoids (Rezaeinodehi and Khangoli, 2008; Taherkhani et al., 2013; Mihajilov-Krstev et al., 2014; Dhen et al., 2014; Riahi et al., 2015; Malaspina et al, 2025). Chrysanthenol chemotype was quite rare, and it was determined in wormwood oils from European countries, such as Hungary, Spain, and Poland (Bailen et al., 2013; Julio et al., 2015; Nguyen et al., 2018a; Kosakowska et al., 2025). Studies have also shown that A. absinthium chemical components are differentiated from different continents. For example, a study on A. absinthium growing in Italy found high concentrations of cis-epoxyocimene (24.8%) and trans-chrysanthenyl acetate (21.6%) (Mucciarelli et al., 1995), while a Cuban sample had bornyl acetate (23.02%) and terpinen-4-ol (8.15%) (Pino et al., 1997). The chamazulene-rich dark blue color of A. absinthium EOs was found in high concentrations in some of the samples, such as the eastern part of Turkey (17.8% and 28.6%) (Kordali et al., 2005; Bagci et al., 2010), Tunisia (5.98%-42.965) (Riahi et al., 2015), Algeria (10.05%) (Benmimoune et al., 2023), and Saudi Arabia (10.8%) (Aati et al., 2024). Another interesting compound, davanone, a sesquiterpene ketone, was found in 60.2% in Saudi Arabian A. absinthium EO (Aati et al., 2024), 35.88% in a Tunisian sample (Malaspina et al., 2025), and 16.4% in an Ethiopian sample (Tariku et al., 2011). This could be related to heat and drought stress. This explains why certain chemotypes are more prevalent in specific regions.
The genetic factors of the plant populations are characteristic core components. Different accessions of the same species can be grouped into distinct chemical clusters, suggesting that genetic traits control the biosynthetic pathways for chemical diversity. One study on Polish populations of A. absinthium found significant variability in both developmental and chemical traits, distinguishing several distinct chemotypes based on EO profiles. These included a pure sabinyl acetate chemotype, mixed chemotypes, and thujone chemotype (Kosakowska et al., 2025). This suggests that genetic factors are crucial in determining the chemotypes of A. absinthium EOs. This is demonstrated by the high degree of chemical diversity and the existence of chemical types in different populations, even when grown under similar conditions.
The phenological stage of the plant’s growth at the time of harvest is another critical characteristic. The concentration of essential oil and its chemical profile can change as the plant matures from the vegetative stage to the flowering stage. Studies have shown that the chemical composition of A. absinthium EOs can also vary between different parts of the plant, such as the leaves and the roots. Thujones were characterized by high concentrations in mostly leaves and flower oils compared to oils obtained from aerial parts (Table 6). For example, thujone and trans-sabinene acetate chemotypes were investigated at the different stages. The concentration of β-thujone (31.06%–63.41%) was higher than that of α-thujone (12.74%–28.15%) in both leaf and flower samples at the development stages. The concentration of trans-sabinyl acetate reached 70.84% in leaf oils at floral budding (Nguyen et al., 2018a). Judzentiene and Budiene (2010) determined that the concentrations of thujone isomers were 0%–0.8% in leaves, 9.3%–10.9% in flowers, and 4.5%–5.9% in fruit oils. This indicates that the maturation process influences the concentration of specific compounds. The observed chemical complexity and variety within A. absinthium EOs are thus a direct outcome of its geographic origin, developmental stage, harvesting time, and the specific plant organs used.
Thujones are found in various EOs in A. absinthium L., Artemisia arborescens L., Artemisia herba-alba Asso, Artemisia rutifolia Steph. Ex Spreng., Artemisia sieberi Besser, Artemisia verlotiorum Lamotte, Artemisia vulgaris L., Salvia officinalis L., Tanacetum vulgare L., and Thuja occidentalis L (Judzentiene and Buzelyte, 2006; Blagojevic et al., 2006; Mighri et al., 2010; Sharopov and Setzer, 2011; Tabari et al., 2017; Nemeth and Nguyen, 2020; Russo et al., 2020; Politeo et al., 2023). The aerial parts of A. absinthium are largely used in the alcoholic drink absinthe. The chronic abuse of absinthe led to a syndrome called absinthism and stimulated hallucinations after consuming it. Prolonged drinking of absinthe had caused convulsions, blindness, and mental deterioration (Nemeth and Nguyen, 2020). The toxicity of absinthe is attributed to the presence of thujones in wormwood oil. α-Thujone is more potent than β-thujone in the gamma-aminobutyric acid (GABAA) receptor in the central nervous system. The GABAA receptor is the main inhibitory neurotransmitter receptor in the brain, responsible for calming neuronal activity. By blocking this receptor, thujone prevents inhibitory signals, leading to hyperexcitability of neurons. This can cause a range of symptoms from muscle spasm and restlessness to convulsions and seizures. A study in rats and mice showed that the intraperitoneal LD50 of α-thujone in mice is approximately 45 mg/kg. A “no-effect” level in rats was found to be 12.5 mg·kg−1·day−1 (Lachenmeier and Uebelacker, 2010; Pelkonen et al., 2013; Nemeth and Nguyen, 2020). In a clinical study by Dettling et al. (2004), 25 volunteers were exposed to absinthe containing high (100 mg/mL) and low (10 mg/L) concentrations of thujone. A high concentration of thujone had a negative effect on attention performance and some mood dimensions at the 30-minute examination time. Alcohol alone or with a low concentration of thujone did not result in similar effects (Dettling et al., 2004). The use of essential oils containing thujone in foods and beverages is strictly regulated. The content of thujones in wormwood EOs is up to 35% (Orav et al., 2006). In the United States, the Food and Drug Administration (FDA) and Department of the Treasury Alcohol and Tobacco Tax and Trade Bureau (TTB) consider products “thujone-free” if they contain less than 10 ppm of thujone (Department of the Treasury Alcohol and Tobacco Tax and Trade Bureau, 2007).
The market quality of essential oils, particularly those of A. absinthium, can vary significantly. Studies have confirmed that the essential oil composition of A. absinthium can vary dramatically depending on the plant’s geographical origin, genetic chemotype, phenological stage, and the specific plant tissue from which the essential oil is extracted. This supports that a wormwood essential oil from one supplier may have a high concentration of thujone. At the same time, another from a different region may be rich in epoxyocimene, chrysanthenyl acetate, sabinyl acetate, low thujone, or other compounds. Commercial Absinthii herba from various retailers may lead to the use of the wrong herbs, which causes not only serious health issues but also incorrect biological activity. Analytical methods would help to detect intentional and unintentional toxic chemical compounds in A. absinthium botanicals.
4.2 Bioinsecticidal survey
In preliminary short-range bioassays, A. absinthium samples (Asd and A1), which contained α-thujone and α,β-thujone, were the most attractive A. absinthium samples (Supplementary Figure 1). Both α-thujone and α,β-thujone were initially as attractive as the positive control, TTO, for the first 30–45 minutes in competitive short-range bioassays. However, when α-thujone and α,β-thujone were directly compared in a competitive assay, only approximately 20% of male C. capitata were attracted to the treated filter paper during the peak attraction period (30–45 minutes). This limited attraction suggests that α-thujone and α,β-thujone may not be effective short-range attractants at the 10% concentration tested or that this concentration was either too low or too high. In contrast, α-thujone (<96%) as a single compound and α,β-thujone (70% of α-thujone and 10% of β-thujone; Sigma-Aldrich) as a mixture were evaluated in the short-range bioassays, whereas α-thujone was 1.17% and β-thujone was 39.87% in the total of Asd oil. Further studies are needed to determine if different concentrations of these compounds can elicit greater C. capitata attraction in short-range bioassays. Additionally, further studies are needed using long-range attraction studies to investigate the effects of desensitization and reduced compound concentration over time on C. capitata attraction.
While TTO was the most attractive compound in short-range behavioral assays (Blythe et al., 2020; Tabanca et al., 2020), it performed poorly as a long-range attractant, capturing 50% fewer male C. capitata in field tests than trimedlure (Shelly and Epsky, 2015). TTO’s ineffectiveness at long range may be due to its complex composition, being a mixture of various monoterpenes and sesquiterpenes with molecular weights ranging from 134 to 222 g/mol, and varying attractiveness to C. capitata (Tabanca et al., 2020). α- and β-Thujone (152 g/mol of each) fall into this molecular weight range, and they may make them more effective long-range attractants, as their volatility and dispersion characteristics could be more consistent than a complex mixture like TTO.
Previous research by Kurtca et al. (2021) found that EO isolated from J. foetidissima fruit was attractive to C. capitata in short-range bioassays, attracting approximately 35% of male C. capitata. These fruit EOs contained approximately 12% α-thujone and 25% β-thujone (Kurtca et al., 2021). The higher attraction observed with the J. foetidissima fruit EOs compared to pure α-thujone and α,β-thujone suggests that other attractive components, in addition to the thujones, may be present in the J. foetidissima fruit EOs.
α-Thujone and α,β-thujone show promise as novel attractants for C. capitata, warranting further investigation through field tests. These compounds, when used as single components, may prove more effective as long-range attractants compared to complex natural mixtures. Additional field studies are essential to evaluate the long-range attraction of α-thujone and α,β-thujone to wild C. capitata. These studies should compare their attractiveness directly to trimedlure, a known and established C. capitata attractant, to assess their potential as new pest control agents fully.
Furthermore, both α-thujone and α,β-thujone demonstrated contact toxicity attributes when exposed to A. suspensa female adults. A previous study on J. foetidissima fruit EO found that A. suspensa was associated with its higher concentration of oxygenated monoterpenes, specifically identifying α- and β-thujone as major components (Kurtca et al., 2021). The median lethal dose (LD50) for J. foetidissima fruit EO was 10.46 μg/μL, while for J. foetidissima leaf EO, it was 22.07 μg/μL. This difference in toxicity was explicitly attributed to variations in the chemical composition between the two essential oils, including α,β-thujone. This strong correlation suggested thujone’s direct contribution to the observed toxicity. In the current study, α,β-thujone exhibited 1.5 times more toxicity than α-thujone. β-Thujone is quite promising for toxicity testing against A. suspensa female adults. Supporting their insecticidal property, α,β-thujone also induced 75% mortality in adult B. dorsalis via an ingestion toxicity test (Jaffar and Lu, 2022). A study found that thujone was topically toxic against western corn rootworms (Diabrotica virgifera virgifera) (Lee et al., 1997). Both thujones are known as neurotoxins, especially α-thujone, which is considered more potent than β-thujone. Their neurotoxic effect stems from their ability to block GABA receptors, which are crucial for inhibitory neurotransmission in insects. By blocking GABA receptors, thujones disrupt normal nerve function, leading to paralysis, convulsions, and ultimately death (Hold et al., 2000 and 2001). While α-thujone displayed higher toxicity, β-thujone also showed insecticidal effects and had significant behavioral impacts. For example, β-thujone was found to modify the probing behavior of the peach potato aphid (Myzus persicae), acting as a deterrent, limiting aphids’ settling and preventing phloem penetration (Wróblewska-Kurdyk et al., 2019). The current toxicity finding of thujone provides practical implications for fruit fly management in orchards. With bait spray application, although it had limited chances for adult pest flies to be directly sprayed, the sprayed fruits, leaves, and other parts of the tree provide EO-coated surfaces that will increase the chances of landed adult flies of being killed. Also, there was potential for EO to be incorporated into bait as a component and contribute to the attract-and-kill management of fruit flies. However, further studies will be needed to identify the toxicity of thujones against fruit flies via ingestion.
Beyond direct toxicity, the α,β-thujone+camphor mixture has shown antifeedant activity against the Colorado potato beetle larvae and adults (Lazarević et al., 2022). A report on the effectiveness of the alcoholic extract of A. arborescens ‘Powis Castle’ in preventing apple infestation by codling moth (Cydia pomonella) larvae identified α-thujone as a key deterrent (Creed et al., 2015). Thujones and thujone-rich EOs may be a potential feeding deterrence at specific concentrations against certain insect pests.
This current study provides a comprehensive analysis of the role of semiochemicals within integrated pest management (IPM) strategies for controlling the Mediterranean fruit fly (C. capitata) and the Caribbean fruit fly (A. suspensa). Identifying novel, cost-effective kairomones is a critical ongoing challenge to make large-scale mass trapping and SIT programs more efficient and sustainable, particularly for small-scale organic farming. When integrated with the SIT, kairomones enhance the efficacy of SIT by reducing wild pest densities and ensuring that the released sterile insects are competitive and successful in the field. In combination with attractive attributes to other tephritid species, managing the dose level in a trap, thujones can potentially be applied in future field studies as an attract-and-kill tool in fruit fly management practices, offering naturally and potentially resistance-mitigating alternatives to conventional synthetic pesticides.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
NT: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing. KC: Formal analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. XY: Investigation, Methodology, Resources, Software, Validation, Writing – original draft, Writing – review & editing. AB: Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing. BD: Investigation, Methodology, Resources, Software, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the United States Department of Agriculture, Agricultural Research Service (USDA-ARS) (Project Number: 6038-22000-007-00D).
Acknowledgments
We are grateful to Micah Gill and Monica Blanco (USDA-ARS, SHRS, Miami, FL, USA) for the bioassays and laboratory experiments. We thank Zeytinburnu Medicinal Plant Garden (Istanbul, Turkiye) for supplying A. absinthium as an authenticated sample and Prof. Dr. Murat Kursat (Bitlis University, Turkiye) for providing A. absinthium photo from the wild habitat. We also acknowledge Dr. Damla Kirci (University of Izmir Katip Celebi, Faculty of Pharmacy, Izmir, Turkiye) and Dr. Nilay Ildiz (Bandirma Onyedi Eylul University, Bandirma, Turkiye) for providing critical reviews of an earlier version of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The mention of firm names or trade products does not imply they are endorsed or recommended by the USDA over other firms or similar products not mentioned. The USDA is an equal-opportunity provider and employer. The findings and conclusions in this publication are those of the authors and should not be construed to represent any official USDA or U.S. Government determination or policy.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1674428/full#supplementary-material
References
Aati, H. Y., Attia, H. A., Alanazi, A. S., AL Tamran, L. K., and Wanner, J. K. (2024). Phytochemical characterization utilizing HS-SPME/GC-MS: Exploration of the antioxidant and enzyme inhibition properties of essential oil from Saudi Artemisia absinthium L. Pharmaceuticals. 17, 1460. doi: 10.3390/ph17111460
Abad, M. J., Bedoya, L. M., Apaza, L., and Bermejo, P. (2012). The Artemisia L. genus: A review of bioactive essential oils. Molecules. 17, 254–2566. doi: 10.3390/molecules17032542
Abbott, A. F. (1925). A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 18, 265–267. doi: 10.1093/jee/18.2.265a
Aluja, M., Arrendondo, J., Fleischer-Diaz, F., Birke, A., Rull, J., Niogret, J., et al. (2014). Susceptibility of 15 mango (Sapindales: Anacardiaceae) cultivars to the attack by Anastrepha ludens and Anastrepha obliqua (Diptera: Tephritidae) and the role of underdeveloped fruit as pest reservoirs: management implications. J. Econ. Entomol. 107, 375–388. doi: 10.1603/EC13045
Antonio, J., Piobesan, B., Bernardi, D., and Nava, D. E. (2023). Search hours for food attractant by Anastrepha fraterculus and Ceratitis capitata (Diptera: Tephritidae) adults in guava orchards. An. Acad. Bras. Cienc. 95, e20201880. doi: 10.1590/0001-3765202320201880
Arino, A., Arberas, I., Renobales, G., Arriaga, S., and Dominguez, J. B. (1999). Seasonal variation in wormwood (Artemisia absinthium L.) essential oil composition. J. Essent. Oil Res. 11, 619–622. doi: 10.1080/10412905.1999.9701105
Bagci, E., Kursat, M., and Civelek, S. (2010). Essential oil composition of the aerial parts of two Artemisia species (A. vulgaris and A. absinthium) from East Anatolian region. J. Essent. Oil Bear. Pl. 13, 66–72. doi: 10.1080/0972060X.2010.10643792
Bailen, M., Julio, L. F., Diaz, C. E., Sanz, J., Martínez-Díaz, R. A., Cabrera, R., et al. (2013). Chemical composition and biological effects of essential oils from Artemisia absinthium L. cultivated under different environmental conditions. Ind. Crop Prod. 49, 102–110. doi: 10.1080/10412905.2007.9699291
Benmimoune, S., Tigrine, C., Mouas, Y., and Kameli, A. (2023). Chemical profiling and assessment of biological activities of wild Artemisia absinthium L. essential oil from Algeria. J. Essent. Oil Bear. Plants 26, 1410–1423. doi: 10.1080/0972060X.2023.2289617
Blagojević, P., Radulović, N., Palić, R., and Stajanović, G. (2006). Chemical composition of the essential oils of Serbian wild-growing Artemisia absinthium and Artemisia vulgaris. J. Agric. Food Chem. 54, 4780–4478. doi: 10.1021/jf060123o
Blythe, E. K., Tabanca, N., Demirci, B., and Kendra, P. E. (2020). Chemical composition of essential oil from Tetradenia riparia and its attractant activity for Mediterranean fruit fly, Ceratitis capitata. Nat. Prod. Commun. 15, 1–6. doi: 10.1177/1934578X2095
Brown, M. T., Cohen, M. J., Bardi, E., and Ingwersen, W. W. (2006). Species diversity in the Florida Everglades, USA: A systems approach to calculating biodiversity. Aquat. Sci. 68, 254–277. doi: 10.1007/s00027-006-0854-1
Carnat, A. P., Madesclaire, M., Chavignon, O., and Lamaison, J. L. (1992). cis-Chrysanthenol, a main component in essential oil of Artemisia absinthium L. growing in Auvergne (Massif Central), France. J. Essent. Oil Res. 4, 487–490. doi: 10.1080/10412905.1992.9698115
Creed, C., Mollhagen, A., Mollhagen, N., and Pszczolkowski, M. A. (2015). Artemisia arborescens “Powis Castle” extracts and α-thujone prevent fruit infestation by codling moth neonates. Pharm. Biol. 53, 1458–1464. doi: 10.3109/13880209.2014.985796
Cunningham, R. T. (1989). ““Parapheromones,”,” in Word crop pests fruit flies: their biology, natural enemies and control, vol. 3A . Eds. Robinson, A. S. and Hooper, G. (Elsevier Science Publishers, Amsterdam, The Netherlands), 221–230.
Davydova, S., Liu, J., and Kandul, N. P. (2023). Next-generation genetic sexing strain establishment in the agricultural pest Ceratitis capitata. Sci. Rep. 13, 19866. doi: 10.1038/s41598-023-47276-5
Department of the Treasury Alcohol and Tobacco Tax and Trade Bureau (2007). Industry circular 07-05. Available online at: https://www.ttb.gov/public-information/industry-circulars/archives/2007/07-05 (Accessed September10, 2025).
Derwich, E., Benziane, Z., and Boukir, A. (2009). Chemical compositions and insecticidal activity of essential oils of three plants Artemisia sp. (A. herba-alba, A. absinthium and A. Pontica (Morocco). Electron. J. Environ. Agric. Food Chem. 8, 1202–1211.
Dettling, A., Grass, H., Schuff, A., Skopp, G., Strohbeck-Kuehner, P., and Haffner, H. T. (2004). Absinthe: attention performance and mood under the influence of thujone. J. Stud. Alcohol 65, 573–581. doi: 10.15288/jsa.2004.65.573.
Dhen, N., Majdoub, O. S., and Souguir, S. (2014). Chemical composition and fumigant toxicity of Artemisia absinthium essential oil against Rhyzopertha Dominica and Spodoptera littoralis. Tunis. J. Plant Prot. 9, 45–53.
Diouf, E. G., Brevault, T., Ndiaye, S., and Piou, C. (2024). Exploration of the potential of a boosted sterile l fruit flies in mango orchards. Pest Manage. Sci. 80, 5212–5223. doi: 10.1002/ps.8248.
Dumont, Y. and Oliva, C. (2024). On the impact of re-mating and residual fertility on the Sterile Insect Technique efficacy: Case study with the medfly, Ceratitis capitata. PloS Comput. Biol. 20, e1012052. doi: 10.1371/journal.pcbi.1012052
Duyck, P. F., Jourdan, H., and Mille, C. (2022). Sequential invasions by fruit flies (Diptera: Tephritidae) in Pacific and Indian Ocean islands: A systematic review. Ecol. Evol. 12, e8880. doi: 10.1002/ece3.8880
Epsky, N. D., Kendra, P. E., and Schnell, E. Q. (2014). ““History and development of food-based attractants,”,” in Trapping and detection, control, and regulation of tephritid fruit flies: lures, area-wide programs, and trade implications. Eds. Shelly, T., Epsky, N. D., Jang, E. B., Reyes-Flores, J., and Vargas, R. (Springer Publishing, New York, NY), 75–118.
Fimiani, P. (1989). ““Mediterranean region,”,” in Word crop pests. Fruit flies: their biology, natural enemies and control, vol. 3A . Eds. Robinson, A. S. and Hooper, G. (Elsevier Science Publishers, Amsterdam), 39–50.
Flath, R. A., Cunningham, R. T., Mon, T. R., and John, J. O. (1994a). Additional male Mediterranean fruit fly (Ceratitis capitata) attractants from angelica seed oil (Angelica archangelica). J. Chem. Ecol. 20, 1969–1984. doi: 10.1007/BF02066237
Flath, R. A., Cunningham, R. T., Mon, T. R., and John, J. O. (1994b). Male lures for Mediterranean fruit fly (Ceratitis capitata Wied.): Structural analogs of α-copaene. J. Chem. Ecol. 20, 2595–2609. doi: 10.1007/BF02036194
Frank, J. H. and McCoy, E. D. (1995). Introduction to insect behavioral ecology: The good, the bad, and the beautiful: Non-indigenous species in Florida. Invasive adventive insects and other organisms in Florida. Fla. Entomol. 78, 1–15. doi: 10.2307/3495661
Froerer, K., Peck, S. L., Mcquate, G. T., Vargas, R. I., Jang, E. B., and Mcinnis, D. O. (2010). Long-distance movement of Bactrocera dorsalis (Diptera: Tephritidae) in Puna, Hawaii: How far can they go? Am. Entomol. 56, 88–94. doi: 10.1093/ae/56.2.88
Gould, W. P. (1998). Cold torpor and flight threshold of Anastrepha suspensa (Diptera: Tephritidae). Fla. Entomol. 81, 211–216. doi: 10.2307/3496088
Heve, W. K., El-Borai, F. E., Carrillo, D., and Dunca, L. W. (2017). Biological control potential of entomopathogenic nematodes for management of Caribbean fruit fly, Anastrepha suspensa Loew (Tephritidae). Pest Manage. Sci. 73, 1120–1228. doi: 10.1002/ps.4447
Hold, K. M., Sirisoma, N. S., and Casida, J. E. (2001). Detoxification of alpha- and beta-thujones (the active ingredients of absinthe): Site specificity and species differences in cytochrome P450 oxidation in vitro and in vivo. Chem. Res. Toxicol. 14, 589–595. doi: 10.1021/tx000242c
Hold, K. M., Sirisoma, N. S., Ikeda, T., Narahashi, T., and Casida, J. E. (2000). Alpha-thujone (the active component of absinthe): Gamma-aminobutyric acid type A receptor modulation and metabolic detoxification. Proc. Natl. Acad. Sci. U.S.A. 97, 3826–3831. doi: 10.1073/pnas.070042397
Hoskins, J. L., Rempoulakis, P., Stevens, M. M., and Dominiak, B. C. (2023). Biosecurity and management strategies for economically important exotic Tephritid fruit fly species in Australia. Insects 14, 801. doi: 10.3390/insects14100801
Hsu, J. C., Chou, M. Y., Mau, R. F., Maeda, C., Shikano, I., Manoukis, N. C., et al. (2011). Spinosad resistance in field populations of melon fly, Zeugodacus cucurbitae (Coquillett), in Hawaii. Pest Manag Sci. 77, 5439–5444. doi: 10.1002/ps.6583
Inserra, R. N., Stanley, J., Steck, G., Anderson, P. J., and Smith, T. R. (2023). Phytosanitary measures and certification programs implemented in Florida. Boll. Accad. Gioenia Nat. Sci. (Catania). 56, 42–69. doi: 10.1002/ps.3448
Jaffar, S. and Lu, Y. (2022). Toxicity of some essential oils constituents against Oriental fruit fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). Insects 13, 954. doi: 10.3390/insects13100954
Juan-Blasco, M., Sabater-Munoz, B., Argiles, R., Jacas, J. A., Castanera, P., and Urbaneja, A. (2013). Molecular tools for sterile sperm detection to monitor Ceratitis capitata populations under SIT programmes. Pest Manag. Sci. 69, 857–864. doi: 10.1002/ps.3448
Judzentiene, A. and Budiene, J. (2006). Chemical composition of essential oils of Artemisia vulgaris L. (mugwort) from North Lithuania. Chemija 17 (1), 12–15.
Judzentiene, A. and Budiene, J. (2010). Compositional variation in essential oils of wild Artemisia absinthium from Lithuania. J. Essent. Oil-Bear. Pl. 13, 275–285. doi: 10.1080/0972060X.2010.10643822
Judzentiene, A., Tomi, F., and Casanova, J. (2009). Analysis of essential oils of Artemisia absinthium L. from Lithuania by CC, GC(RI), GC-MS and 13C NMR. Nat. Prod. Commun. 4, 1113–1118. doi: 10.1177/1934578X09004008
Julio, L. F., Burillo, J., Giménez, C., Cabrera, R., Díaz, C. E., Sanz, J., et al. (2015). Chemical and biocidal characterization of two cultivated Artemisia absinthium populations with different domestication levels. Ind. Crops Prod. 76, 787–792. doi: 10.1016/j.indcrop.2015.07.041
Juteau, F., Jerkovic, I., Masotti, V., Milos, M., Mastelic, J., Bessière, J. M., et al. (2003). Composition and antimicrobial activity of the essential oil of Artemisia absinthium from Croatia and France. Planta Med. 69, 158–161. doi: 10.1055/s-2003-37714
Koening, W. A., Rieck, A., Hardt, I., Gehrcke, B., Kubeczka, K. H., and Muhle, H. (1994). Enantiomeric composition of the chiral constituents of essential oils. Part 2: Sesquiterpene hydrocarbons. J. High Resolut. Chromatogr. 17, 315–320. doi: 10.1002/jhrc.1240170507
Kordali, S., Cakir, A., Mavi, A., Kilic, H., and Yildirim, A. (2005). Screening of chemical composition and antifungal and antioxidant activities of the essential oils from three Turkish Artemisia species. J. Agric. Food Chem. 53, 1408–1416. doi: 10.1021/jf048429n
Kosakowska, O., Weglarz, Z., Zuchowska, A., Styczynska, S., Zaras, E., and Baczek, K. (2025). Intraspecific variability of wormwood (Artemisia absinthium L.) occurring in Poland in respect of developmental and chemical traits. Molecules. 30, 2915. doi: 10.3390/molecules30142915
Kurtca, M., Tumen., I., Keskin., H., Tabanca., N., Yang., X., Demirci, B., et al. (2021). Chemical composition of essential oils from leaves and fruits of Juniperus foetidissima and their attractancy and toxicity to two economically important tephritid fruit fly species, Ceratitis capitata and Anastrepha suspensa. Molecules 26, 7504. doi: 10.3390/molecules26247504
Lachenmeier, D. W. and Uebelacker, M. (2010). Risk assessment of thujone in foods and medicines containing sage and wormwood-evidence for a need of regulatory changes? Regul. Toxicol. Pharmacol. 58, 437–443. doi: 10.1016/j.yrtph.2010.08.012
Lazarević, J., Kostić, I., Šešlija Jovanović, D., Ćalić, D., Milanović, S., and Kostić, M. (2022). Pure camphor and a thujone-camphor mixture as eco-friendly antifeedants against larvae and adults of the Colorado potato beetle. Plants 11, 3587. doi: 10.3390/plants11243587
Lee, S., Tsao, R., Peterson, C., and Coats, J. R. (1997). Insecticidal activity of monoterpenoids to western corn rootworm (Coleoptera: Chrysomelidae), twospotted spider mite (Acari: Tetranychidae), and house fly (Diptera: Muscidae). J. Econ. Entomol. 90, 883–892. doi: 10.1093/jee/90.4.883
Li, X. L., Li, D. D., Cai, X. Y., Chen, D. F., and Lu, Y. Y. (2024). Reproductive behavior of fruit flies: courtship, mating, and oviposition. Pest Manage. Sci. 80, 935–952. doi: 10.1002/ps.7816
Llorens-Molina, J. A. and Vacas, S. (2015). Seasonal variations in essential oil of aerial parts and roots of an Artemisia absinthium L. population from a Spanish area with supramediterranean climate (Teruel, Spain). J. Essent. Oil Res. 27, 395–405. doi: 10.1080/10412905.2015.1043400
Llorens-Molina, J. A., Vacas, S., Castell, V., and Németh-Zámboriné, É. (2016). Variability of essential oil composition of wormwood (Artemisia absinthium L.) affected by plant organ. J. Essent. Oil Res. 29, 11–21. doi: 10.1080/10412905.2016.1202152
Lopes-Lutz, D., Alviano, D. S., Alviano, C. S., and Kolodziejczyk, P. P. (2008). Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochem. 69, 1732–1738. doi: 10.1016/j.phytochem.2008.02.014
Lull, C., Gil-Ortiz, R., and Cantín, A. (2023). A chemical approach to obtaining α-copaene from clove oil and its application in the control of the medfly. Appl. Sci. 13, 5622. doi: 10.3390/app13095622
Malaspina, P., Polito, F., Mainetti, A., Khedhri, S., Feo, De, and Cornara, V. (2025). Exploring chemical variability in the essential oil of Artemisia absinthium L. @ in relation to different phenological stages and geographical location. Chem. Biodivers. 19, e00743. doi: 10.1002/cbdv.202500743
Mangan, R. L., Thomas, D. B., Moreno, A. T., and Robacker, D. (2011). Grapefruit as a host for the west Indian fruit fly (Diptera: tephritidae). J. Econ. Entomol. 104, 54–62. doi: 10.1603/ec09220
Manrakhan, A. and Addison, P. (2014). Assessment of fruit fly (Diptera: Tephritidae) management practices in deciduous fruit growing areas in South Africa. Pest Manage. Sci. 70, 651–660. doi: 10.1002/ps.3604
Mason, C. J. (2024). Evaluating impacts of radiation-induced sterilization on the performance and gut microbiome of mass-reared Mediterranean fruit fly (Ceratitis capitata) in Hawai’i. J. Econ. Entomol. 117, 1867–1875. doi: 10.1093/jee/toae173
McLafferty, F. W. and Stauffer, D. B. (1989). The wiley/NBS registry of mass spectral data. (New York, USA: J Wiley and Sons).
Mighri, H., Hajlaoui, H., Akrout, A., Najjaa, H., and Neffati, M. (2010). Antimicrobial and antioxidant activities of Artemisia herba-alba essential oil cultivated in Tunisian arid zone. C. R. Chimie. 13, 380–386. doi: 10.1055/s-0034-1383182
Mihajilov-Krstev, T., Jovanović, B., Jović, J., Ilić, B., Miladinović, D., Matejić, J., et al (2014). Antimicrobial, antioxidative, and insect repellent effects of Artemisia absinthium essential oil. Planta Med. 80, 1698–1705. doi: 10.1055/s-0034-1383182
Morteza-Semnani, K. and Akbarzadeh, M. (2005). Essential Oils Composition of Iranian Artemisia absinthium L. and Artemisia scoparia Waldst. et Kit. J. Essent. Oil Res. 17, 321–322. doi: 10.1080/10412905.2005.9698918
Msaada, K., Salem, N., Bachrouch, O., Bousselmi, S., Tammar, S., and Alfaify. and Marzouk, A. (2015). Chemical composition and antioxidant and antimicrobial activities of wormwood (Artemisia absinthium L.) essential oils and phenolics. J. Chem. 804658, 1–12. doi: 10.1155/2015/804658
Mucciarelli, M., Caramiello, R., Maffei, M., and Chialva, F. (1995). Essential oils from some Artemisia species growing spontaneously in North-West Italy. Flavour Frag. J. 10, 25–32. doi: 10.1603/EN10302
Navarro-Campos, C., Martines-Ferrer, M. T., Campos, J. M., Fibla, J. M., Alcaide, J., Bargues, L., et al (2011). The influence of host fruit and temperature on the body size of adult Ceratitis capitata (Diptera: Tephritidae) under laboratory and field conditions. Environ. Entomol. 40, 931–938. https://doi.org/10.1603/EN10302
Nemeth, E. Z. (2016). ““Natural variability of essential oil components,”,” in Handbook of essential oils science, technology, and applications. Eds. Baser, K. H. C. and Buchbauer, G. (CRC Press, Boca Raton, FL), 87–125.
Nemeth, E. Z. and Nguyen, H. T. (2020). Thujone, a widely debated volatile compound: What do we know about it? Phytochem. Rev. 19, 405–423. doi: 10.1007/s11101-020-09671-
Nguyen, H. T. and Nemeth, E. Z. (2016). Sources of variability of wormwood (Artemisia absinthium L.) essential oil. J. Appl. Res. Med. Aromat. Plants 3, 143–150. doi: 10.1016/j.jarmap.2016.07.005
Nguyen, R. U., Poucher, C., and Brazzel, J. R. (1992). Seasonal occurrence of Anastrepha suspensa (Diptera: Tephritidae) in Indian River County, Florida 1984-1987. J. Econ. Entomol. 85, 813–820. doi: 10.1093/jee/85.3.813
Nguyen, H. T., Radácsi, P., Gosztola, B., Rajhárt, P., and Zámboriné Németh, É. (2018b). Accumulation and composition of essential oil due to plant development and organs in wormwood (Artemisia absinthium L.). Ind. Crops Prod. 123, 232–237. doi: 10.1016/j.indcrop.2018.06.076
Nguyen, H. T., Tavaszi-Sárosi, S. Z., Llorens-Molina, A., and Zámborine-Németh, E. (2018a). Compositional variability in essential oils of twelve wormwood (Artemisia absinthium L.) accessions. J. Essent. Oil Res. 30, 421–430. doi: 10.1080/10412905.2018.1496856
Nin, S. P. and Bosetto, M. (1995). Quantitative determination of some essential oil components of selected Artemisia absinthium plants. J. Essent. Oil Res. 7, 271–277. doi: 10.1080/10412905.1995.9698518
Niogret, J., Gill, M. A., Espinoza, H. R., Kendra, P. E., and Epsky, N. D. (2017). Attraction and electroantennogram responses of male Mediterranean fruit fly (Diptera: Tephritidae) to six plant essential oils. J. Entomol. Zool. Stud. 5, 958–964.
Niogret, J., Montgomery, W. S., Kendra, P. E., Heath, R. R., and Epsky, N. D. (2011). Attraction and electroantennogram responses of male Mediterranean fruit fly (Diptera: Tephritidae) to volatile chemicals from Persea, Litchi and Ficus wood. J. Chem. Ecol. 37, 483–491. doi: 10.1007/s10886-011-9953-0
Orav, A., Raal, A., Arak, E., Muurisepp, M., and Kailas, T. (2006). Composition of the essential oil of Artemisia absinthium L. @ of different geographical origin. Proc. Estonian Acad. Sci. Chem. 55, 155. doi: 10.3176/chem.2006.3.04
Papadopoulos, N. T. (2014). ““Fruit fly invasion: historical, biological, economical aspects and management,”,” in Trapping and the detection, control, and regulation of tephritid fruit flies. Eds. T. Shelly, N. D., Jang, E. B., Reyes-Flores, J., and Vargas, R. (Springer Publishing, New York, NY), 219–252.
Pelkonen, O., Abass, K., and Wiesner, J. (2013). Thujone and thujone-containing herbal medicinal and botanical products: toxicological assessment. Regul. Toxicol. Pharmacol. 65, 100–107. doi: 10.1016/j.yrtph.2012.11.002
Pimentel, R., Lopes, D. J. H., Mexia, A. M. M., and Mumford, J. D. (2017). Seasonality of the mediterranean fruit fly (Diptera: tephritidae) on terceira and sao jorge islands, azores, Portugal. J. Insect Sci. 17, 1–35. doi: 10.1093/jisesa/iew097
Pinero, J. C., Enkerlin, W., and Epsky, N. C. (2014). ““Recent developments and applications of bait stations for integrated pest management of tephritid fruit flies”,” in Trapping and detection, control, and regulation of tephritid fruit flies: lures, area-wide programs, and trade implications. Eds. Shelly, T., Epsky, N. D., Jang, E. B., Reyes-Flores, J., and Vargas, R. (Springer Publishing, New York, NY), 457–492.
Pinkerton, M. G., Thompson, S. M., Casuso, N. A., Hodges, A. C., and Leppla, N. C. (2019). Engaging Florida’s youth to increase their knowledge of invasive species and plant biosecurity. J. Integr. Pest Manage. 10, 1–7. doi: 10.1093/jipm/pmy019
Pino, J. A., Rosado, A., and Fuentes, V. (1997). Chemical composition of the essential oil of Artemisia absinthium L. from Cuba. J. Essent. Oil Res. 9, 87–89. doi: 10.1080/10412905.1997.9700720
Politeo, O., Cajic, I., Simic, A., Ruscic, M., and Bektasevic, M. (2023). Comparative study of chemical composition and cholinesterase inhibition potential of essential oils isolated from Artemisia plants from Croatia. Separations. 10, 546. doi: 10.3390/separations10100546
Puche, H., Midgarden, D. G., Ovalle, O., Kendra, P. E., Epsky, N. D., Rendon, P., et al. (2005). Effect of elevation and host availability on sterile and wild Mediterranean fruit flies (Diptera: Tephritidae) distribution. Fla. Entomol. 88, 83–90. doi: 10.1653/0015-4040(2005)088[0083:EOEAHA]2.0.CO;2
Qin, Y., Paini, D., Wang, C., Fang, Y., and Li, Z. (2015). Global establishment risk of economically important fruit fly species (Tephritidae). PloS One 10, e0116424. doi: 10.1371/journal.pone.0116424
Raal, A., Illina, T., Kovalyona, A., Orav, A., Karileet, M., Dzaniasvili, M., et al. (2024). Variation in the composition of the essential oil of commercial Artemisia absinthium L. herb samples from different countries. ScienceRise Pharm. Sci. 2, 2024. doi: 10.15587/2519-4852.2024.302232
Radulovic, M., Gavrilovic, M., Rajcevic, N., Janakiev, T., Dimkic, I., and Janackovic, P. (2022). The essential oil composition of different parts of Artemisia absinthium and its antibacterial activity against phytopathogenic bacteria. Biol. Nyssana. 13, 179. doi: 10.5281/zenodo.7437345
Raparelli, E., Cesare, D., Scaglione, M., and Lolletti, D. (2025). Exploring four decades of Ceratitis capitata research: Uncovering trends, key players and breakthroughs in pest management. J. Appl. Entomol. 0, 1–11. doi: 10.1111/jen.13476
Rezaeinodehi, A. and Khangholi, S. (2008). Chemical composition of the essential oil of Artemisia absinthium growing wild in Iran. Pak. J. Biol. Sci. 11, 946–949. doi: 10.3923/pjbs.2008.946.949
Riahi, E., Ghazghazi, H., Ayari, B., Aouadhi, C., Klay, I., Chograni, H., et al. (2015). Effect of environmental conditions on chemical polymorphism and biological activities among Artemisia absinthium L. essential oil provenances grown in Tunisia. Ind. Crops Prod. 66, 96–102. doi: 10.1016/j.indcrop.2014.12.036
Roh, G., Kendra, P. E., Zhu, J. J., Roda, A., Loeb, G. M., Tay, J., et al. (2023). Coconut oil derived five-component synthetic oviposition deterrent for oriental fruit fly, Bactrocera dorsalis. Pest Manage. Sci. 79, 3852–3859. doi: 10.1002/ps.7584
Russo, A., Bruno, M., Avola, R., Cardile, V., and Rigano, D. (2020). Chamazulene-rich Artemisia arborescens essential oils affect the cell growth of human melanoma cells. Plants. 9, 1000. doi: 10.3390/plants9081000
Salas, B., Conway, H. E., Vacek, D. C., Vitek, C., and Schuenzel, E. L. (2023). Pathogenicity of multiple Providencia species (Enterobacteriales: Morganellaceae) to the mass-reared Mexican fruit fly (Diptera: Tephritidae). J. Insect Sci. 23, 1–11. doi: 10.1093/jisesa/iead024
Segura, D. F., Belliard, S. A., Vera, M. T., Bachmann, G. E., Ruiz, M. J., Jofre-Barud, F., et al. (2018). Plant chemicals and the sexual behavior of male tephritid fruit flies. Ann. Entomol. Soc Am. 111, 239–264. doi: 10.1093/aesa/say024
Sharopov, F. S. and Setzer, W. N. (2011). Thujone-rich essential oils of Artemisia rutifolia Stephan ex Spreng. growing wild in Tajikistan. J. Essent. Oil-Bear. Plants. 14, 136–139. doi: 10.1080/0972060X.2011.10643913
Sharopov, F. S., Sulaimonova, V. A., and Setzer, W. N. (2012). Composition of the Essential oil of Artemisia absinthium from Tajikistan. Rec. Nat. Prod. 6, 127–134.
Shelly, T. E. (2018). Sexual selection on leks: A fruit fly primer. J. Insect Sci. 18, 1–16. doi: 10.1093/jisesa/iey048
Shelly, T. E. and Cloonan, K. R. (2023). Male lures and the detection of tephritid fruit flies: Assessing the relationships between lure amount and release rate and trap captures of invasive pest species. Crop Prot. 176, 106504. doi: 10.1016/j.cropro.2023.106504
Shelly, T. E. and Epsky, N. D. (2015). Exposure to tea tree oil enhances the mating success of male Mediterranean fruit flies (Diptera: Tephritidae). Fla. Entomol. 98, 1127–1133. doi: 10.1653/024.098.0417
Shelly, T. E. and McInnis., D. O. (2001). Exposure to ginger root oil enhances mating success of irradiated, mass-reared males of Mediterranean fruit fly (Diptera: Tephritidae). J. Econ Entomol. 94, 1413–1418. doi: 10.1603/0022-0493-94.6.1413
Shelly, T. E., Nishimoto, J., and Kurashima, R. (2012). Trap capture of three economically important fruit fly species (Diptera: Tephritidae): Evaluation of a solid formulation containing multiple male lures in a Hawaiian coffee field. J. Econ. Entomol. 105, 1186–1193. doi: 10.1603/ec11371
Shelly, T., Oehlschlager, C., and Kurashima, R. (2023). Natural oil lure outperforms trimedlure in capturing males of the Mediterranean fruit fly, Ceratitis capitata (Diptera: Tephritidae). Neotrop. Entomol. 52, 1138–1143. doi: 10.1007/s13744-023-01079-5
Shelly, T. E., Whittier, T. S., and Villalobos, E. M. (1996). Trimedlure affects mating success and mate attraction in male Mediterranean fruit flies. Entomol. Exp. Appl. 78, 181–185. doi: 10.1111/j.1570-7458.1996.tb00780.x
Shikano, I., Gutierrez-Coarite, R., Streit, C., Perez, E., Fujitani, E., and Mau, R. F. L. (2022). Field tests of three alternative insecticides with protein bait for the development of an insecticide rotation program to control melon flies, zeugodacus cucurbitae (Coquillett) (Diptera: tephritidae). Insects 13, 629. doi: 10.3390/insects13070629
Simanton, W. A. (1958). Studies of Mediterranean fruit fly lures in Florida. J. Econ. Entomol. 51, 679–882. doi: 10.1093/jee/51.5.679
Simpson, S. E. (1993). Caribbean fruit fly-free zone certification protocol in Florida (Diptera: Tephritidae). Fla. Entomol. 76, 228–233. doi: 10.2307/3495719
Skelley, P. E., Buss, E. A., Allen, J. S., Bolton, S. J., Deeter, L. A., Fulton, J. C., et al. (2025). Adventive arthropods in Florida as reported by the Florida Department of Agriculture and Consumer Services, Division of Plant Industry 1990-2023. Fla. Entomol. 108, 20240027. doi: 10.1515/flaent-2024-0027
Steck, G., Fox, A., Carrillo, D., Dean, D., Roda, A., and Epsky, N. D. (2019). Oriental fruit fly eradication in Florida 2015-2016: Program implementation, unique aspects and lessons learned. Am. Entomol. 65, 108–121. doi: 10.1093/ae/tmz023
Steiner, L. F., Miyashita, D. H., and Christenson, L. D. (1957). Angelica oils as Mediterranean fruit fly lures. J. Econ. Entomol. 50, 505. doi: 10.1093/jee/50.4.505
Suckling, D. M., Jang, E. B., Holder, P., Carvalho, and Stephens, A. E. A. (2008). Evaluation of lure dispensers for fruit fly surveillance in New Zealand. Pest Manage. Sci. 64, 848–865. doi: 10.1002/ps.1578
Suckling, D. M., Kean, J., Stringer, L. D., Caceres-Barrios, C., Hendrichs, J., Reyes-Flores, J., et al. (2016). Eradication of tephritid fruit fly pest populations: outcomes and prospects. Pest Manage. Sci. 72, 456–465. doi: 10.1002/ps.3905
Suckling, D. M., Woods, B., Mitchell, V. J., Twidle, A., Lacey, I., Jang, E. B., et al. (2011). Mobile mating disruption of light-brown apple moths using pheromone-treated sterile Mediterranean fruit flies. Pest Manage. Sci. 67, 1004–1014. doi: 10.1002/ps.2150
Tabanca, N., Masi, M., Epsky, N. D., Nocera, P., Cimmino, A., Kendra, P. E., et al. (2019). Laboratory evaluation of natural and synthetic aromatic compounds as potential attractants for male Mediterranean fruit fly, Ceratitis capitata. Molecule. 24, 2409. doi: 10.3390/molecules24132409
Tabanca, N., Niogret., J., Kendra., P. E., and Epsky, N. D. (2020). TLC-based bioassay to isolate kairomones from tea tree essential oil that attract male Mediterranean fruit flies, Ceratitis capitata (Wiedemann). Biomolecules 10, 683. doi: 10.3390/biom10050683
Tabari, M. A., Youssefi, M. R., and Benelli, G. (2017). Eco-friendly control of the poultry red mite, Dermanyssus gallinae (Dermanyssidae), using the α-thujone-rich essential oil of Artemisia sieberi (Asteraceae): toxic and repellent potential. Parasitol. Res. 116, 1545–1551. doi: 10.1007/s00436-017-5431-0
Taherkhani, M., Rustaiyan, A., Iraj Rasooli, I., and Taherkhani, T. (2013). Chemical composition, antimicrobial activity, antioxidant and total phenolic content within the leaves essential oil of Artemisia absinthium L. growing wild in Iran. Afr. J. Pharm. Pharmacol. 7, 30–36. doi: 10.5897/AJPP12.945
Tan, K. H., Nishida, R., Jang, E. B., and Shelly, T. E. (2014). ““Pheromones, male lures, and trapping of Tephritid fruit flies,”,” in Trapping and the detection, control, and regulation of tephritid fruit flies. Eds. T. Shelly, N. D., Epsky, E. B., Jang Reyes-Flores, J.J., and Vargas, R. (Springer Publishing, New York, NY), 15–74.
Tariku, Y., Hymete, A., Hailu, A., and Rohloff, J. (2011). In vitro evaluation of antileishmanial activity and toxicity of essential oils of Artemisia absinthium and Echinops kebericho. Chem. Biodiv. 8, 614–662. doi: 10.1002/cbdv.201000331
Tejada, M. T., Arredondo, J., Diaz-Fleischer, F., and Perez-Staples, D. (2020). Does size matter? Mate choice in two lekking flies. J. Insect Sci. 20, 1–6. doi: 10.1093/jisesa/ieaa019
Tojic, T., Dordevic, T., Durovic-Pejcev, R., Acimovic, M., Bozic, D., Radivojevic, L., et al. (2025). Allelopathic potential of Artemisia absinthium and Artemisia vulgaris from Serbia: Chemical composition and bioactivity on weeds. Plants. 14, 1663. doi: 10.3390/plants14111663
Tok, F., Yang, X., Tabanca, N., and Kocyigit-Kaymakcioglu, B. (2023). Synthesis of phthalimide derivatives and their insecticidal activity against Caribbean Fruit Fly, Anastrepha suspensa (Loew). Biomolecule 13, 361. doi: 10.3390/biom13020361
Troetschler, R. G. (1983). Effects on nontarget arthropods of malathion bait sprays used in California to eradicate the Mediterranean fruit fly, Ceratitis capitata (Wiedemann) (Diptera: Tephritidae). Environ. Entomol. 12, 1816–1822. doi: 10.1093/ee/12.6.1816
Tucker, A. O., Maciarello, M. J., and Sturtz., G. (1993). The essential oils of Artemisia “Powis Castle” and its putative parents, A. absinthium and A. arborescens. J. Essent. Oil Res. 5, 239–242. doi: 10.1080/10412905.1993.9698215
Umpiérrez, M. L., Lagreca, M. E., Cabrera, R., Grille, G., and Rossini, C. (2012). Essential oils from Asteraceae as potential biocontrol tools for tomato pests and diseases. Phytochem. Rev. 11, 339–335. doi: 10.1007/s11101-012-9253-5
Vanickova, L., do Nascimento, R. R., Hoskovec, M., Jezkova, Z., Brizove, R., Tomcala, A., et al. (2012). Are the wild and laboratory insect populations different in semiochemical emission? The case of the medfly sex pheromone. J. Agric. Food Chem. 60, 7168–7176. doi: 10.1021/jf301474d
Vargas, R. I., Piñero, J. C., Ronald, F. L., Mau, R. F. L., Jang, E. B., Klungness, L. M., et al. (2010). Area-wide suppression of the Mediterranean fruit fly, Ceratitis capitata, and the Oriental fruit fly, Bactrocera dorsalis, in Kamuela, Hawaii. J. Insect Sci. 10, 135. doi: 10.1673/031.010.13501
Vera, M. T., Rodriguez, R., Segura, D. F., Cladera, J. L., and Sutherst, R. W. (2002). Potential geographical distribution of the Mediterranean fruit fly, Ceratitis capitata (Diptera: Tephritidae), with emphasis on Argentina and Australia. Environ. Entomol. 31, 1009–1021. doi: 10.1603/0046-225X-31.6.1009
Wagner, D. L. and Van Driesche, R. G. (2010). Threats posed to rare or endangered insects by invasions of nonnative species. Annu. Rev. Entomol. 55, 547–568. doi: 10.1146/annurev-ento-112408-085516
Wang, X. G. and Messing, R. H. (2006). Feeding and attraction of non-target flies to spinosad-based fruit fly bait. Pest Manage. Sci. 6, 933–939. doi: 10.1002/ps.1259
Weems, H. V., Heppner, J. B., Fasula, T. R., and Nation, J. L. (2001). Caribbean fruit fly, Anastrepha suspensa (Loew) (Insecta: Diptera: Tephritidae) University of florida, entomology and nematology department, UF/IFAS extension, EDIS EENY-196. Available online at: https://edis.ifas.ufl.edu/publication/IN353 (Accessed September 12, 2025).
Weldon, C. W., Nyamukondiwa, C., Karsten, M., Chown, S. L., and Terblanche, J. S. (2018). Geographic variation and plasticity in climate stress resistance among southern African populations of Ceratitis capitata (Wiedemann) (Diptera: Tephritidae). Sci. Rep. 8, 9849. doi: 10.1038/s41598-018-28259-3
Wróblewska-Kurdyk, A., Gniłka, R., Dancewicz, K., Grudniewska, A., Wawrzeńczyk, C., and Gabryś, B. (2019). β-Thujone and its derivatives modify the probing behavior of the peach potato aphid. Molecules 24, 1847. doi: 10.3390/molecules24101847
Keywords: semiochemical, Artemisia absinthium, wormwood, α-thujone, β-thujone, medfly, caribfly, integrated pest management
Citation: Tabanca N, Cloonan KR, Yang X, Baldemir Kılıc A and Demirci B (2025) Chemical variability for authenticated and commercial Artemisia absinthium L. essential oils with thujones on tephritid fruit flies: Mediterranean fruit fly Ceratitis capitata (Wiedemann) and Caribbean fruit fly Anastrepha suspensa (Loew). Front. Plant Sci. 16:1674428. doi: 10.3389/fpls.2025.1674428
Received: 28 July 2025; Accepted: 16 September 2025;
Published: 20 November 2025.
Edited by:
Islam S. Sobhy, Cardiff University, United KingdomReviewed by:
Weria Weisany, Islamic Azad University, IranJonathan Osei-Owusu, University of Environment and Sustainable Development, Ghana
Sabrine Chouikhi, Institut des Régions Arides, Tunisia
Copyright © 2025 Tabanca, Cloonan, Yang, Baldemir Kılıc and Demirci. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nurhayat Tabanca, bnVyaGF5YXQudGFiYW5jYUB1c2RhLmdvdg==
 Nurhayat Tabanca
Nurhayat Tabanca Kevin R. Cloonan
Kevin R. Cloonan Xiangbing Yang
Xiangbing Yang Ayse Baldemir Kılıc2
Ayse Baldemir Kılıc2