- Development Center of Plant Germplasm Resources, College of Life Sciences, Shanghai Normal University, Shanghai, China
CrRLK1L receptor-like kinases have attracted significant interest in plant biology owing to their pivotal roles in development, stress response, and signal transduction. However, our understanding of their importance in the extreme saline-alkali tolerant mechanisms of the halophyte alkaligrass (Puccinellia tenuiflora) remains limited. In this work, we analyzed the homology and evolutionary relationships of the CrRLK1L gene family across 30 species. Through genome-wide analysis we identified 25 PutCrRLK1L genes, which revealed conserved malectin-like and kinase domain, unique intronless structures, and stress-/hormone-responsive promoter elements. Transcriptomic and RT-qPCR analyses revealed salinity response of nine PutCrRLK1L genes’ expression in roots and leaves. Functional characterization of FER and antioxidant enzyme activities demonstrated that PutFER1-overexpressing Arabidopsis lines exhibited enhanced root growth under salt stress and reduced ROS accumulation compared to wild-type plants. These findings may open new avenues for research into halophyte-specific salt tolerance mechanisms and provide potential candidate genes for improving crop resilience to saline-alkaline soils.
Introduction
The CrRLK1L (Catharanthus roseus RECEPTOR-LIKE KINASE 1-LIKEs) family is named after its first member, CrRLK1, isolated from Madagascar periwinkle (Schulze-Muth et al., 1996). These proteins have long been recognized as key regulators of various developmental processes (e.g., cell elongation, polarized growth, and fertilization) and abiotic stresses (e.g., salt stress, extreme temperature, and nutritional deficiency) (Lindner et al., 2012; Nissen et al., 2016; Franck et al., 2018a; Zhu et al., 2021; Zhang et al., 2025). The CrRLK1L family proteins are characterized by two tandemly-linked malectin-like domains (MLDs) required for binding to ligands and cell wall polymers (such as pectin), a transmembrane domain (TMD), and an intracellular kinase domain that relays apoplastic signals to intracellular components via phosphorylation (Schallus et al., 2008; Boisson-Dernier et al., 2011; Kessler et al., 2014). In Arabidopsis, there are 17 CrRLK1Ls, among which the biological functions of FERONIA (FER), THESEUS1 (THE1), HERCULES1 (HERK1), ANXUR1/2 (ANX1/2), ANJEA (ANJ), ERULUS (ERU) and BUDDHA’S PAPER SEAL1/2 (BUPS1/2) have been well addressed (Guo et al., 2009; Boisson-Dernier et al., 2015; Schoenaers et al., 2018; Galindo-Trigo et al., 2019; Ge et al., 2019; Liu et al., 2021a; Cheung, 2024). They regulate diverse processes including cell wall/membrane integrity (e.g., FER, HERK1, and THE1), pollen-ovule interactions (e.g., ANJ and HERK1), pollen tube development (e.g., FER, ANJ, HERK1, ANX1/2, BUPS1/2, and ERU), cell elongation (e.g., THE1 and HERK1), and trichome/tapetum morphogenesis (i.e., CVY1) (Nasrallah et al., 2013; Gachomo et al., 2014; Kessler et al., 2014; Richter et al., 2017; Schoenaers et al., 2018; Zhong et al., 2022). Moreover, some members involved in immune and abiotic stress responses. For instance, FER and THE1 play crucial roles in maintaining cell wall integrity and plasma membrane homeostasis (Richter et al., 2017), while the LETUM1/2 (LET1/2)-LRE-like GPI-AP1 (LLG1) complex participates in disease resistance by activating the SUPPRESSOR of mkk1 mkk2 (SUMM2)-mediated immune pathway (Huang et al., 2020a). Additionally, MEDOS (MDS) 1–4 are involved in metal ion-regulated developmental processes (Richter et al., 2018). However, their functions in salinity tolerance are largely unknown.
FER, originally described as a regulator of female fertility, is a critical modulator of pollen tube-ovule interaction (Huck et al., 2003; Rotman et al., 2003). Arabidopsis FER is universally expressed in both vegetative and reproductive organs and is the most extensively studied and best-characterized CrRLK1L family protein (Escobar-Restrepo et al., 2007; Franck et al., 2018b; Cheung, 2024). In Arabidopsis, FER regulates the initiation and elongation of plant root hairs by regulating the homeostasis of reactive oxygen species (ROS) (Duan et al., 2010; Yu et al., 2012; Huang et al., 2013). In addition, FER also regulates seed germination, plant cell expansion, cell polar growth, pathogen defense, and abiotic stress tolerance by modulating the homeostasis of phytohormone such as jasmonic acid (JA), salicylic acid (SA), abscisic acid (ABA), brassinosteroid (BR), and ethylene (Guo et al., 2009; Deslauriers and Larsen, 2010; Guo et al., 2018; Zhao et al., 2021; Wang et al., 2024b; Chaudhary et al., 2025; Fu et al., 2025).
Importantly, FER regulates several salt-responsive processes to maintain cellular homeostasis such as activation of pectin methyl esterase (PME), mitogen-activated protein kinase (MAPK) cascade, and intracellular Ca2+ signal transduction (Feng et al., 2018; Gigli-Bisceglia et al., 2022). Under salt stress, disassociated RAPID ALKALINIZATION FACTOR (RALF) peptides RALF22/23 bind to FER, inducing its internalization to activate stress responses (Zhao et al., 2018). It has been reported that FER coordinates salt stress responses by modulating stomatal dynamics via G protein β subunit (AGB1) interaction to limit ion influx, phosphorylating serine hydroxymethyltransferase1 (SHM1) to enhance photorespiration for toxicity mitigation, and inactivating phytochrome B (phyB) phosphorylation to inhibit PIF-mediated growth (Yu and Assmann, 2018; Yu et al., 2018; Liu et al., 2023; Jiang et al., 2024a; Liu et al., 2024). Similar to fer-4 mutation, herk1 the1–4 mutation also leads to leaf bleaching phenotype under salt stress suggesting that other CrRLK1L family proteins are required for salt tolerance in plants (Gigli-Bisceglia et al., 2022).
Additionally, genome-wide analyses of different species identified 852 members of the CrRLK1L gene families (Zhang et al., 2016; Yang et al., 2020; Rao et al., 2021; Tang et al., 2022; Ma et al., 2023a; Qiao et al., 2023). Among them, 45 in soybean (Glycine max), 48 in tobacco (Nicotiana tabacum), 24 in maize (Zea mays), 43 in wheat (Triticum aestivum), and 26 in quinoa (Chenopodium quinoa) play important roles under salt stress (Wang et al., 2021; Jiang et al., 2022; Li et al., 2022; Gawande and Sankaranarayanan, 2024; Jiang et al., 2024b; Wang et al., 2024a). Representatively, the highly salinity-induced mRNA levels for soybean GmCrRLK1L2/20, tobacco NtCrRLK1L47, maize ZmCrRLK1L1/2, wheat TaCrRLK1L1, and quinoa CqCrRLK1L5/7/9 indicate that CrRLK1L members in these species could participate in salt response (Wang et al., 2021; Jiang et al., 2022; Li et al., 2022; Gawande and Sankaranarayanan, 2024; Jiang et al., 2024b; Wang et al., 2024a).
Alkaligrass (Puccinellia tenuiflora) is a monocotyledonous halophyte widely distributed in the saline-alkaline land in North-eastern China. This species can survive under extreme saline-alkaline (600 mM NaCl and 150 mM Na2CO3) as well as drought and chilling stresses (Zhang et al., 2013; Meng et al., 2016). Due to its high nutritional value and stress tolerance property, alkaligrass serves as a model organism for studying alkali tolerance mechanisms. Recently, the high-quality chromosome-level whole-genome sequencing has revealed that the genome size of alkaligrass is approximately 1.5 Gb and contains 38,387 protein-encoding genes (Zhang et al., 2020). In addition, stress-treated transcriptome profiling uncovers a series of unique saline- and alkaline-responsive genes and a large number of genes in FER and FLS2 signaling pathways were induced by salinity and alkali in roots of alkaligrass (Zhang et al., 2020). Quantitative proteomic studies have revealed stress-induced changes in protein abundance and post-translational modifications (PTMs) under diverse conditions, such as salt stress (NaCl, Na2CO3, and NaHCO3), low temperature, and oxidative stress (Yu et al., 2011, 2013; Meng et al., 2016; Zhao et al., 2016; Yin et al., 2019; Zhang et al., 2019; Suo et al., 2020). Analyses of 25 glutaredoxins (PutGRXs) and 54 germin-like protein (PutGLPs) genes indicate that PutGRXs and PutGLPs play critical roles in the saline-alkaline stress response of P. tenuiflora (Li et al., 2025; Liu et al., 2025). However, the molecular mechanisms by which the CrRLK1L family members in alkaligrass participates in salt stress responses remain poorly understood. Identification of PutCrRLK1Ls required for salt tolerance would be critical for full understanding of salt-tolerant mechanisms in alkaligrass.
In this study, we identified 25 PutCrRLK1L family genes in P. tenuiflora through genome-wide screening. We analyzed their phylogenetic relationships, chromosomal distribution, gene structures, conserved functional domains, and subcellular localization. Furthermore, we characterized PutCrRLK1L expression patterns under various salinity stress conditions. Phenotypic and physiological analyses of PutFER1-overexpressing lines demonstrated that PutFER1 plays an essential role in root growth and ROS scavenging during salt stress adaptation.
Materials and methods
Identification and cloning of PutCrRLK1L genes in P. tenuiflora
Genome, transcripts, coding sequences (CDS), and peptide sequences data of P. tenuiflora were downloaded from the P. tenuiflora Ensembl database (http://www.xhhuanglab.cn/data/alkaligrass.html). Seventeen CrRLK1Ls protein sequences from Arabidopsis thaliana (including FER and its relatives) were downloaded from TAIR (https://www.arabidopsis.org/). We employed two complementary approaches to identify putative PutCrRLK1L genes in alkaligrass: (I) BLAST Search: 17 Arabidopsis CrRLK1L protein sequences were used as queries to search the alkaligrass protein database using the Basic Local Alignment Search Tool (BLAST). Putative PutCrRLK1L genes were screened based on sequence homology. (II) HMMER Scan: The Malectin-like domain (MLD, PF12819) and Protein kinase domain (Pkinase, PF07714) Hidden Markov Model (HMM) profiles were retrieved from the Pfam database (https://pfam.xfam.org). These profiles were used to scan the alkaligrass protein database using HMMER 3.0 software. Candidate sequences identified through both methods were subsequently validated using the Conserved Domain Database (CDD) tool (https://www.ncbi.nlm.nih.gov/cdd) from the National Center for Biotechnology Information (NCBI). Finally, each putative PutCrRLK1L protein sequence was manually verified using the Simple Modular Architecture Research Tool (SMART, http://smart.embl-heidelberg.de/) to confirm the presence of both the conserved malectin-like domain and protein kinase domains. The protein sequences of 25 PutCrRLK1Ls were displayed in Supplementary Table 2.
Phylogenetic analysis of CrRLK1Ls from different plant species
Multiple sequence alignments were performed using Multiple Sequence Comparison by Log-Expectation (MUSCLE) v5.0 software with 904 CrRLK1L family members from 30 species. A phylogenetic tree was constructed from the CrRLK1L sequence alignment using FastTree v2.1 software. The resulting tree files were then submitted to iTOL (https://itol.embl.de/) for further modification.
Collinearity analysis of CrRLK1Ls between alkaligrass and other plants
Collinearity analysis of the CrRLK1Ls between the alkaligrass and other plants was verified and visualized using One Stem MCScanX and Dual Systeny Plot for MCScanX in TBtools-II software, respectively.
Analyses of gene structure and conserved motifs of PutCrRLK1Ls
The exon-intron structure of each PutCrRLK1L gene was examined using TBtools-II, based on its genomic sequence and CDS. Conserved motifs of 25 PutCrRLK1L proteins (with a maximum of 10 motifs) were identified using (Multiple Em for Motif Elicitation) MEME (https://meme-suite.org/meme/tools/meme) and visualized using TBtools-II.
Identification of cis-regulatory elements in PutCrRLK1Ls promoters
Promoter sequences (2,000 bp upstream of the start codon) of all PutCrRLK1Ls were extracted from the P. tenuiflora Ensembl database (http://www.xhhuanglab.cn/data/alkaligrass.html). Putative cis-regulatory elements were identified using PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) and visualized using TBtools-II.
RNA extraction and RT-qPCR
Total RNA was isolated from two-week-old seedlings using an RNAiso Plus reagent (TaKaRa, China) and was subsequently reverse transcribed using the TransScript® One-Step gDNA Removal and cDNA Synthesis SuperMix Kit (TaKaRa, China). Quantitative PCR analysis was conducted with PerfectStart® Green Quantitative Polymerase Chain Reaction SuperMix (TransGen Biotech, China) on a LC96 Real-Time System (Roche, Germany). RT-qPCR analyses were performed with three independent biological replicates, and the data were normalized using the 2−ΔΔCt method (Livak and Schmittgen, 2001). The primer sequences are provided in Supplementary Table 6.
CrRLK1Ls 3D protein structure prediction
The 3D protein structures of CrRLK1L proteins from different plant species were predicted using the AlphaFold database (https://alphafold.ebi.ac.uk/). Automatic modeling mode was selected within the AlphaFold interface and predicted protein structures were visualized using PyMOL software.
Vector construction and plant transformation
The pCAMBIA1300-GFP plasmid was digested with XbaI and SmaI. The target PutFER1 CDS fragment was amplified using alkaligrass cDNA as template and inserted into the digested pCAMBIA1300-GFP vector via homologous recombination at 37 °C for 30 minutes. The recombinant plasmid was transformed into E. coli DH5α. A sequence-verified positive clone was then transformed into Agrobacterium tumefaciens strain GV3101. GV3101 cells harboring the pCAMBIA1300-PutFER1-GFP plasmid were introduced into Arabidopsis wild-type seedlings via the floral dip method to generate PutFER1-overexpressing lines (Zhang et al., 2006). Transformed seedlings were selected on 1/2 MS agar medium containing 50 μg/mL hygromycin.
Confocal imaging
Confocal microscopy was performed using a Leica STELLARIS 8 confocal laser scanning microscope. Green fluorescent protein (GFP) was excited at wavelengths of 488 nm and detected with a 500–550 nm spectral range.
NBT staining assay
Accumulation of O2•− in plant leaves was detected by nitroblue tetrazolium (NBT) staining as previously described (Zhao et al., 2021). Briefly, Arabidopsis seeds were germinated vertically on 1/2 MS agar plates for five days. Seedlings were then transferred to liquid 1/2 MS medium and incubated overnight with gentle shaking. After treatment with 150 mM NaCl for one hour, seedlings were stained with NBT solution (1 mg/mL NBT dissolved in distilled water) and incubated overnight at room temperature in the dark. Finally, stained seedlings were destained in ethanol overnight and visualized using a Leica EZ4 HD microscope.
Determination of antioxidant enzyme activity
Antioxidant enzyme activities were determined using commercially available kits. In brief, total seedlings (0.1 g FW) were homogenized in the respective extraction buffer provided with the assay kit. The homogenate was centrifuged at 8,000 g for 10 min, and the supernatant was used as the enzyme extract for measurement. The activities of superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and ascorbate peroxidase (APX) in wild-type and two PutFER1-overexpressing Arabidopsis lines were determined using Solarbio Assay Kits (Kit Codes: BC0170, BC0090, BC0200, and BC0220) following the manufacturer’s instructions (Solarbio, China).
Quantification and statistical analysis
All graphs were generated using GraphPad Prism v8.0 for Windows. Statistical analyses were performed using Student’s t-test (two-sided) for two groups or one-way analysis of variance (ANOVA) followed by Duncan analysis for multiple groups in IBM SPSS Statistics 25.0 software (https://www.ibm.com/products/spss-statistics). A P value less than 0.05 was considered significant.
Results
CrRLK1L gene family expanded and FER is conserved during plant evolution
We comprehensively analyzed the genomes and 904 CrRLK1L genes across 30 phylogenetically diverse plant species with sequenced genomes (Figure 1A, Supplementary Table 1). The genome analysis indicated their evolutionary relationships among algae, moss, ferns, early angiosperm Amborella, monocotyledon, and dicotyledon (Figure 1A). Most studies of CrRLK1L gene family focused on Solanaceae (five plant species) and Poaceae (seven plant species) (Figure 1A, Supplementary Table 1). Among these species, unicellular charophycean alga (Closterium peracerosum-strigosum-littorale complex), liverwort (Marchantia polymorpha), moss (Physcomitrella patens), and bog moss (Sphagnum fallax) are haploid, tobacco (N. tabacum), cotton (Gossypium hirsutum), quinoa, and peanut (Arachis hypogaea) are tetraploid, while the remaining 21 plant species are diploid. Overall, the number of CrRLK1Ls in tetraploid and hexaploid plants was larger than those in most diploid and haploid plants (Figure 1A). The number of CrRLK1L copies ranges from one in unicellular charophycean alga to 89 in peanut (Figure 1A, Supplementary Table 1). Importantly, the study of CrRLK1Ls across eight monocotyledonous and 16 dicotyledonous plant species revealed that wheat (T. aestivum, TaCrRLK1Ls) from the Poaceae family and peanut (A. hypogaea, AhCrRLK1Ls) from the Fabaceae family were the most extensively studied, with 43 and 89 CrRLK1Ls investigated, respectively (Figure 1A). These findings indicate that CrRLK1L genes are widely distributed among different plant species and that this gene family has undergone lineage-specific expansion during the evolution of plant species.
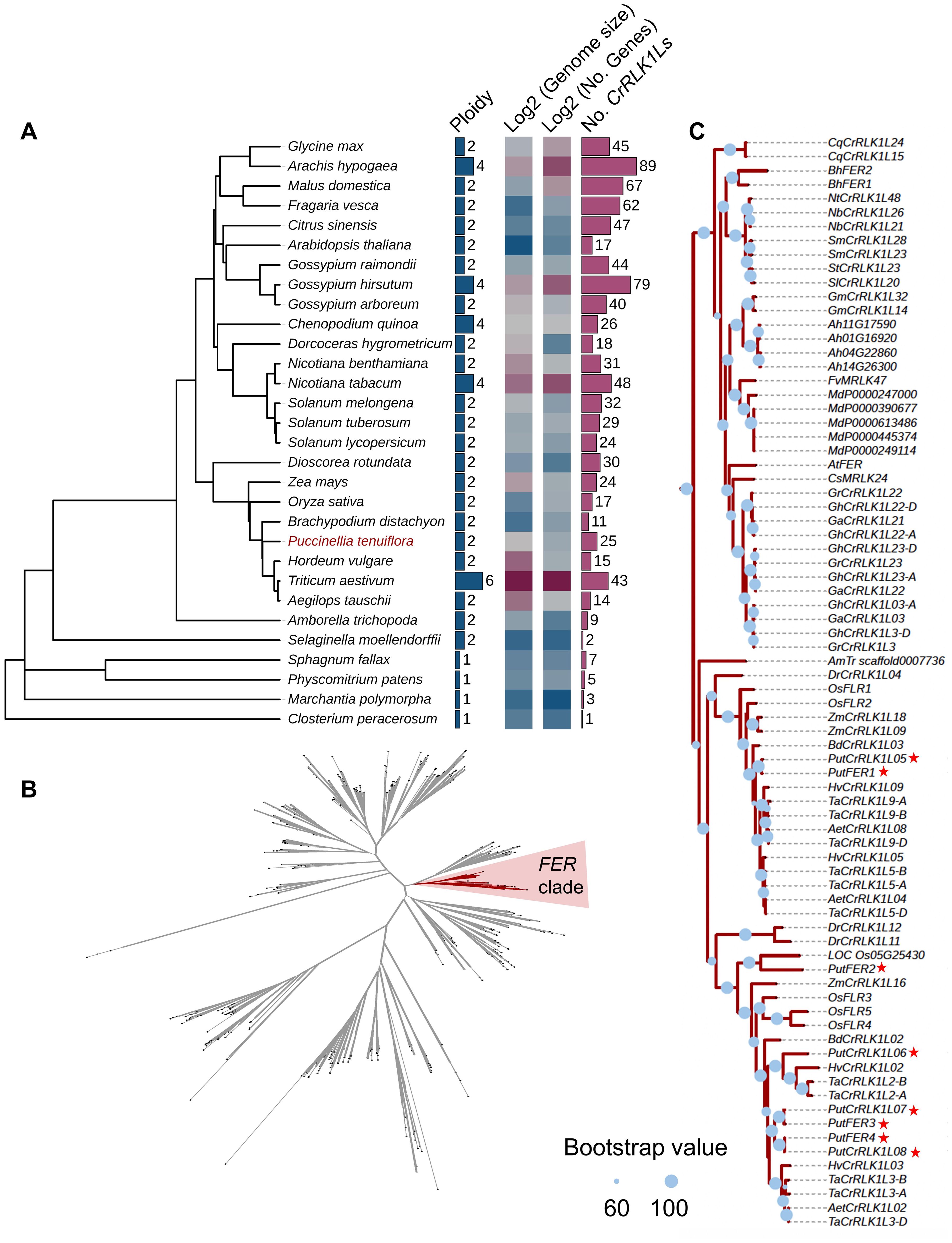
Figure 1. Phylogenetic analysis of Catharanthus roseus RLK1-Like (CrRLK1L) and FERONIA (FER) in different species. (A) Analysis of phylogenetic tree, ploidy, genome size, and gene and CrRLK1L numbers in diverse species. (B) CrRLK1L phylogenetic tree analysis in diverse species. The phylogenetic tree was constructed using the amino acid sequences of the CrRLK1Ls with FastTree, which indicates the phylogenetic relationships among 904 CrRLK1Ls from diverse species. The red colored ranges indicate the FER clade. Branches with a bootstrap value > 0.6 are indicated by thick lines. (C) FER phylogenetic tree. The phylogenetic tree was built based on the amino acid sequences from the FER clade in (B). The red star represents the alkigrass PutCrRLK1L members. Blue circles represent bootstrap values. They are displayed on branches with Bootstrap > 60, where a larger circle indicates higher support.
Given that FER is the most widely studied and best characterized CrRLK1L family protein, we explored its evolution within the CrRLK1L gene family by constructing a phylogenetic tree using the full-length sequences of all 904 CrRLK1L proteins from 30 published plant species (Figures 1B, C, Supplementary Table 1). The phylogenetic tree reveals that FER genes from vast majority plant species are classified into a single clade, indicating that FER has maintained structural homology throughout its evolutionary history (Figure 1B). Notably, we identified 25 CrRLK1L members in P. tenuiflora, including eight FER orthologs of A. thaliana (Supplementary Table 2). Among them, we cloned four PutFER genes (PutFER1 ~ 4) and verified their sequences by DNA sequencing (Supplementary Table 2). Additionally, the copy number of FER genes varies across species, ranging from one in the Amborella trichopoda genome to eight in the P. tenuiflora genome and nine in the T. aestivum genome, indicating that the FER gene family has expanded during species evolution, and that whole-genome duplication events triggered the expansion (Figure 1C; Supplementary Table 1). Eight FER genes from four species (C. psl. complex, M. polymorpha, S. fallax, and P. patens) out of the 30 species do not fall within this clade (Figure 1C). These four species represent basal lineages that diverged prior to Am. trichopoda, one of the earliest diverging angiosperms. This suggests that the FER gene family likely originated after the divergence of angiosperms and subsequently underwent lineage-specific expansion. In conclusion, our results indicate that FER underwent expansion during plant evolution, while it was conserved during this process.
Chromosomal distribution and collinearity analysis of PutCrRLK1Ls
Chromosome distribution analysis revealed that 25 PutCrRLK1Ls exhibited an uneven and non-random distribution across the seven chromosomes in alkaligrass (Figure 2A). Chromosome 1 harbored seven PutCrRLK1Ls, chromosome 3 contained six, chromosome 5 had five, chromosome 4 had three, chromosome 6 had two, and chromosomes 2 and 7 each contained one PutCrRLK1L (Figure 2A). Among them, four PutFERs were evenly distributed on chromosomes 1, 5, and 6. These results indicate that PutCrRLK1Ls may have undergone functional divergence during evolution and developed independent gene expression regulation, thereby enhancing alkaligrass adaptation to the environments (Figure 2A).
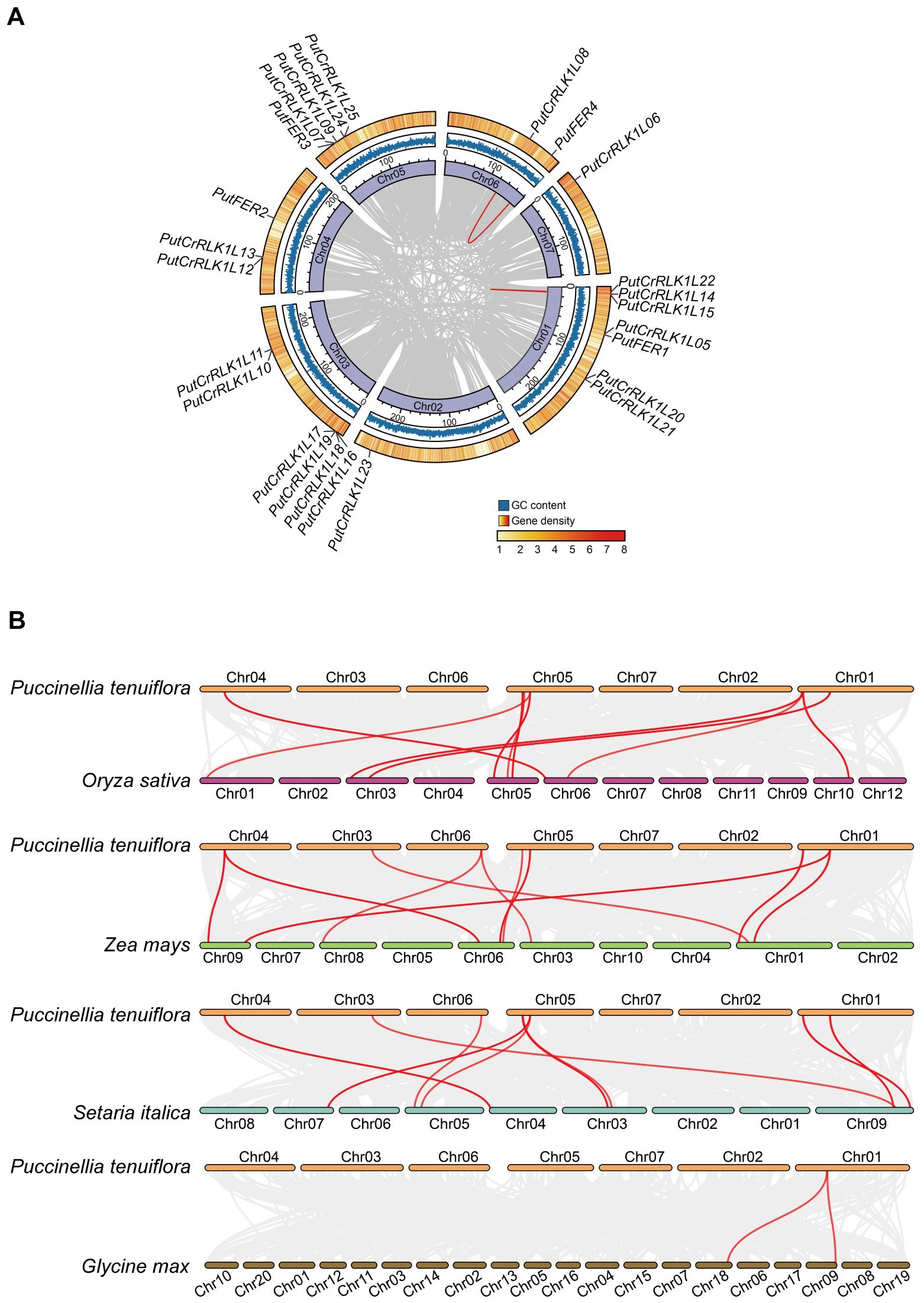
Figure 2. Distribution of PutCrRLK1Ls on chromosomes and collinearity analysis of CrRLK1Ls between Puccinellia tenuiflora and other plant species. (A) Distribution of PutCrRLK1Ls in chromosomes and duplication events analysis of PutCrRLK1Ls. Gray lines in the background indicate the collinear blocks within P. tenuiflora, while the red lines highlight the PutCrRLK1L gene pairs that are syntenic. (B) Collinearity analysis of PutCrRLK1Ls among alkaligrass (P. tenuiflora), rice (Oryza sativa), maize (Zea mays), foxtail millet (Setaria italica), and soybean (Glycine max). Grey lines in the background indicate the collinear blocks within P. tenuiflora and other plant genomes, while the red lines highlight the syntenic CrRLK1L gene pairs.
To gain insight into the expansion pattern of the PutCrRLK1L gene family, we analyzed the duplication events using MCScanX (Figure 2A). This analysis identified two highly homologous gene pairs (PutCrRLK1L08/PutFER4 and PutCrRLK1L14/PutCrRLK1L15) that exhibited high levels of homozygosity, with sequence identity of 97.35% and 99.53%, respectively. Furthermore, the non-synonymous substitution rate (Ka) over the synonymous substitution rate (Ks) (Ka/Ks values) in these gene pairs were less than one (Supplementary Table 3). This demonstrates that CrRLK1L gene family in alkaligrass has undergone purifying selection during its expansion process.
To further understand the evolutionary mechanisms of PutCrRLK1Ls, a collinearity analysis was performed to compare the homologous CrRLK1L genes among alkaligrass, rice (Oryza sativa), maize, foxtail millet (Setaria italica), and soybean (Figure 2B). The results revealed that alkaligrass PutCrRLK1Ls had 15, 16, 14, and two homologous gene pairs in rice, maize, foxtail millet, and soybean, respectively (Figure 2B, Supplementary Table 4). On the other hand, maize, rice, foxtail millet, and soybean CrRLK1Ls have ten, nine, nine, and two genes exhibiting synteny with PutCrRLK1Ls, respectively (Figure 2B, Supplementary Table 4). These findings indicate that the CrRLK1L genes in alkaligrass share stronger phylogenetic relationships with orthologs from monocotyledons such as rice and maize than with those from dicotyledons such as soybean.
Gene structure and protein function domain analysis of PutCrRLK1Ls
To further elucidate the evolutionary relationships of the PutCrRLK1L gene family, we conducted a comprehensive analysis of gene structures, conserved protein domain and motifs. Our results reveal that only three genes (PutCrRLK1L07, PutCrRLK1L22 and PutCrRLK1L25) possess both a 5’ UTR and a 3’ UTR, whereas the remaining PutCrRLK1Ls lack UTRs (Figure 3A). Additionally, five genes (PutCrRLK1L06, PutCrRLK1L07, PutCrRLK1L08, PutCrRLK1L22, and PutCrRLK1L25) have introns, whereas the others do not (Figure 3A). The conserved and simplified gene architecture of PutCrRLK1Ls members suggests that they may enhance gene expression efficiency by reducing alternative splicing processes, thereby enabling a rapid response to fluctuating environments.
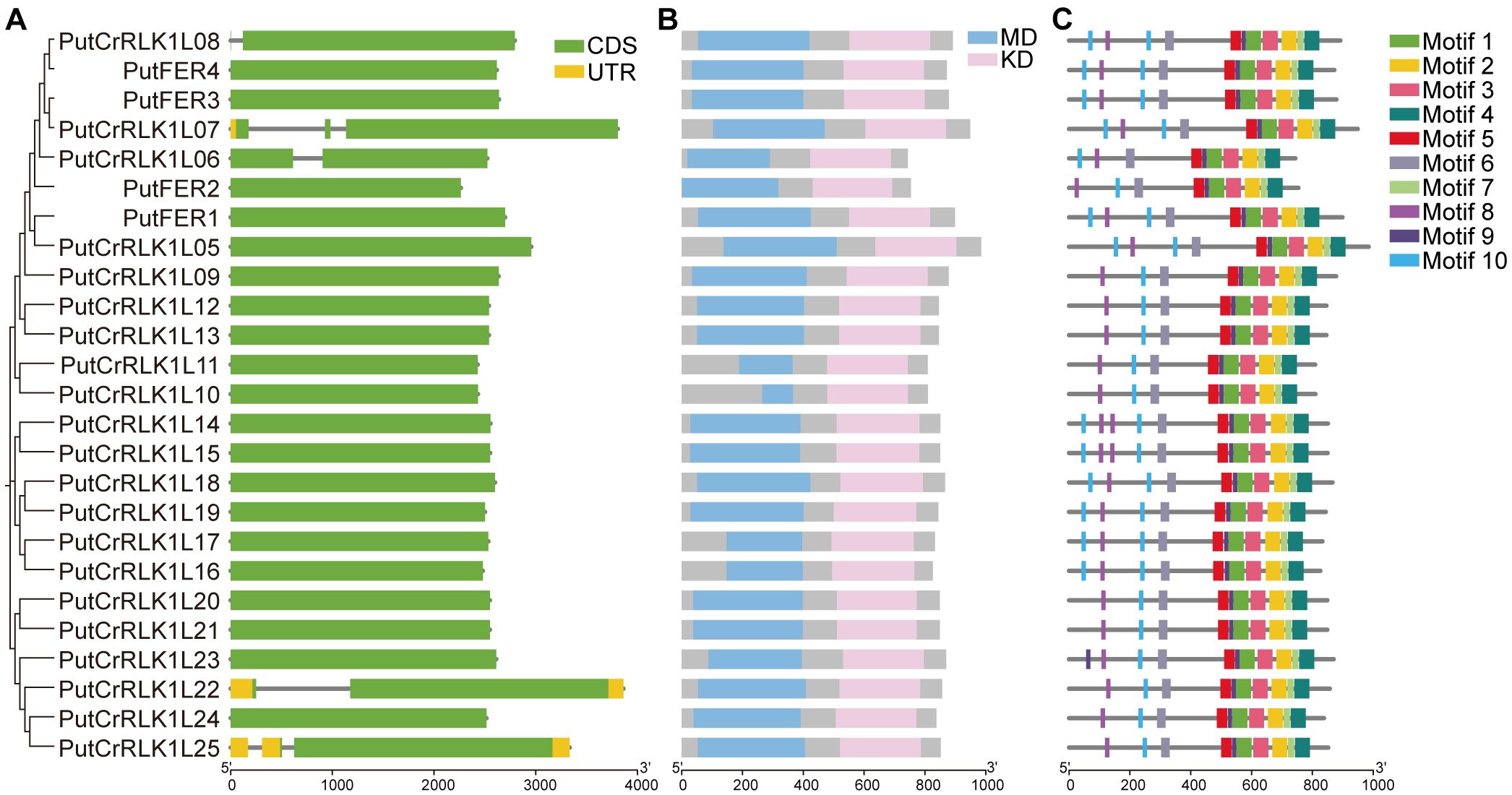
Figure 3. Gene structure, protein domain, and conserved motifs of PutCrRLK1Ls. (A) The structure of PutCrRLK1Ls includes exons, introns, and untranslated regions (UTRs). Yellow boxes indicate UTR, gray lines indicate introns, and green boxes indicate exons. (B) Protein domain of PutCrRLK1Ls. Blue boxes indicate malectin domain (MD) and pink boxes indicate kinase domain (KD). (C) Conserved motifs in PutCrRLK1Ls. Different colors represent different motifs.
Protein domain analysis indicates that PutCrRLK1L family members possess conserved extracellular malectin domains and intracellular kinase domain (Figure 3B). These results provide strong evidence for the functional conservation of PutCrRLK1Ls throughout evolution. Furthermore, we predicted the distribution of conserved motifs in PutCrRLK1L proteins and identified ten conserved motifs (Figure 3C, Supplementary Figure 1). Notably, motifs one to ten are universally present in the majority of PutCrRLK1L family members, suggesting they play a crucial role in maintaining structural or functional integrity (Figure 3C). However, an interesting observation is that the number of motifs eight and ten, which are extracellular MLDs, varies (two to four copies) among PutCrRLK1L family members, suggesting potential functional divergence in signal perception and interaction with other molecules (Figure 3C). Motifs one, two, three, four, five, seven, and nine, which are located in the intracellular domain, are present in all PutCrRLK1L members (Figure 3C). Furthermore, the number and variety of intracellular motifs exceed that of extracellular motifs (Figure 3C). Taken together, our findings suggest that these proteins may play a significant role in maintaining intracellular signaling homeostasis during development and in response to environmental stress in alkaligrass.
Analysis of cis-acting elements in PutCrRLK1Ls promoters
To investigate the potential functions of the 25 PutCrRLK1L genes, cis-acting element prediction was performed on their promoter regions and a schematic map showing the prevalent distribution of stress- and hormone-responsive cis-acting elements was created (Figure 4A; Supplementary Table 5). Overall, the 25 PutCrRLK1L genes in alkaligrass show significant enrichment of phytohormones (nine elements) and light (11 elements) (Figure 4B). Certain elements are responsive to phytohormones (e.g., ABRE, TGACG motif and CGTCA motif), light (e.g., G-box, Sp1 and GT1 motif), anaerobism (e.g., GC motif and ARE) and drought (e.g., MBS). Some of these elements are conserved among the 25 PutCrRLK1Ls (Figure 4B). These findings suggest that PutCrRLK1Ls play a vital role in plants response to drought, light, anaerobism, and hormonal signals. Notably, a total of 234 light-responsive elements were identified. Additionally, 227 hormone-responsive elements were detected, including 70 abscisic acid (ABA) elements (also known as ABREs), 116 jasmonate elements (specifically TGACG and CGTCA motifs), nine salicylic acid elements (identified as the TCA element), 13 gibberellin elements (specifically GARE, P-box, and TATC-box elements), and 19 auxin elements (specifically TGA elements and AuxRR-core elements) (Figure 4B). The conserved elements found in the promoter region suggest that PutCrRLK1Ls play a crucial role in responding to abiotic stress and phytohormone treatment.
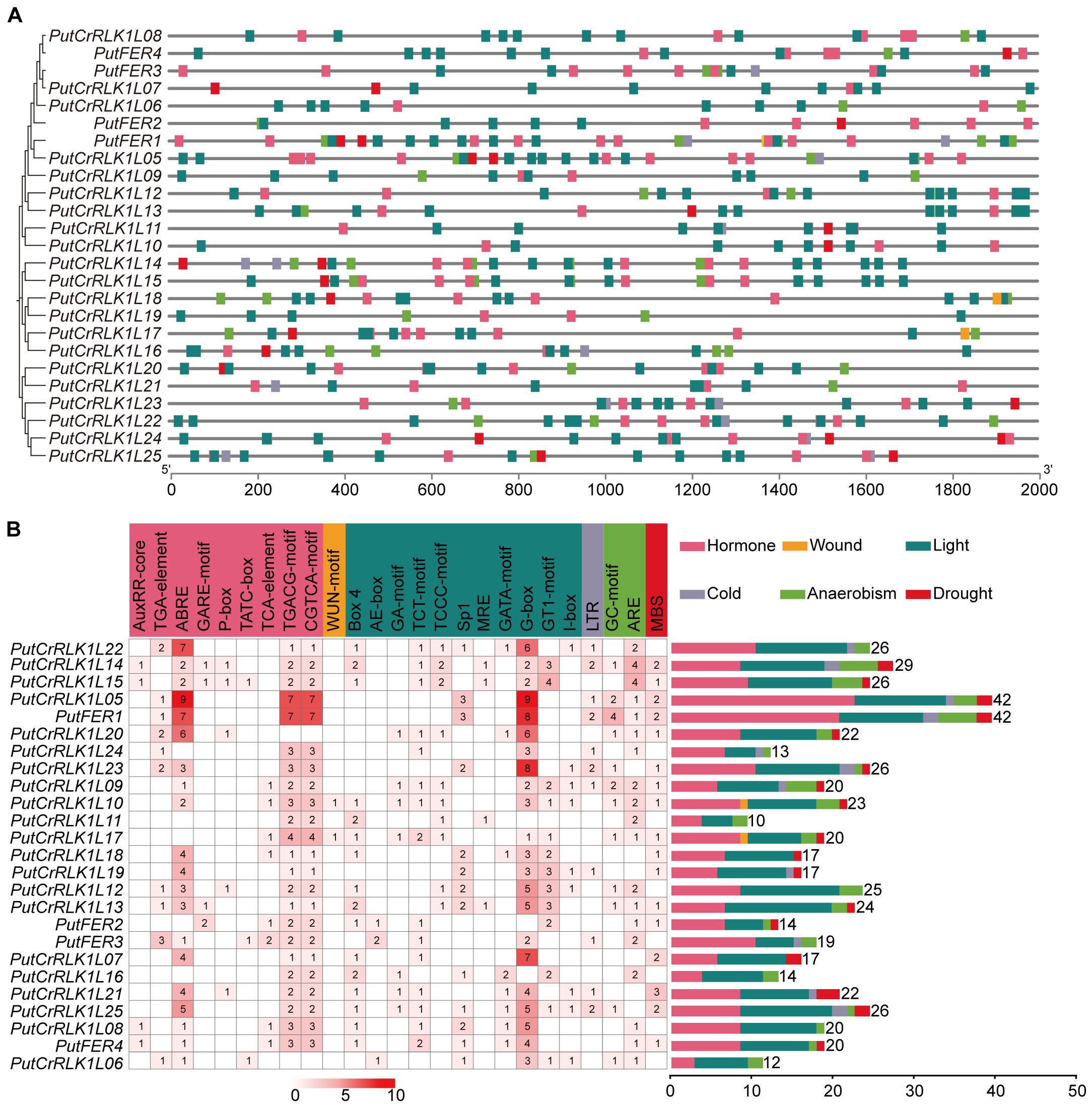
Figure 4. Analysis of cis-acting elements in the promoter region of the PutCrRLK1Ls. (A) The cis-acting elements distribution in PutCrRLK1Ls promoter regions. (B) The names and numbers of cis-acting elements in PutCrRLK1Ls promoters. The heatmap in grid and the color columns indicated the numbers of cis-acting elements. ABRE, ABA responsive element; ARE, Anaerobic-responsive element; GARE, Gibberellin-responsive element; LTR, Low temperature-responsive element; MBS, MYB-binding site; MRE, Metal response element.
Transcriptional analysis of PutFERs in response to salt stress
Given the pivotal role of Arabidopsis FER in regulating plant salt stress responses (Feng et al., 2018), we were interested in elucidating whether PutFERs in alkaligrass are also required for salt tolerance. We have performed a series of deep transcriptome sequencing treated with NaCl, Na2CO3, and NaHCO3 using the Illumina sequencing platform (Zhang et al., 2020). Based on the transcriptome sequencing data, we analyzed the expression profiles of PuCrRLK1L family members in roots and leaves under different salt stress conditions (Figure 5A). Overall, most PutCrRLK1L family genes were significantly more highly expressed in roots than in leaves under all salt stress conditions (Figure 5A).
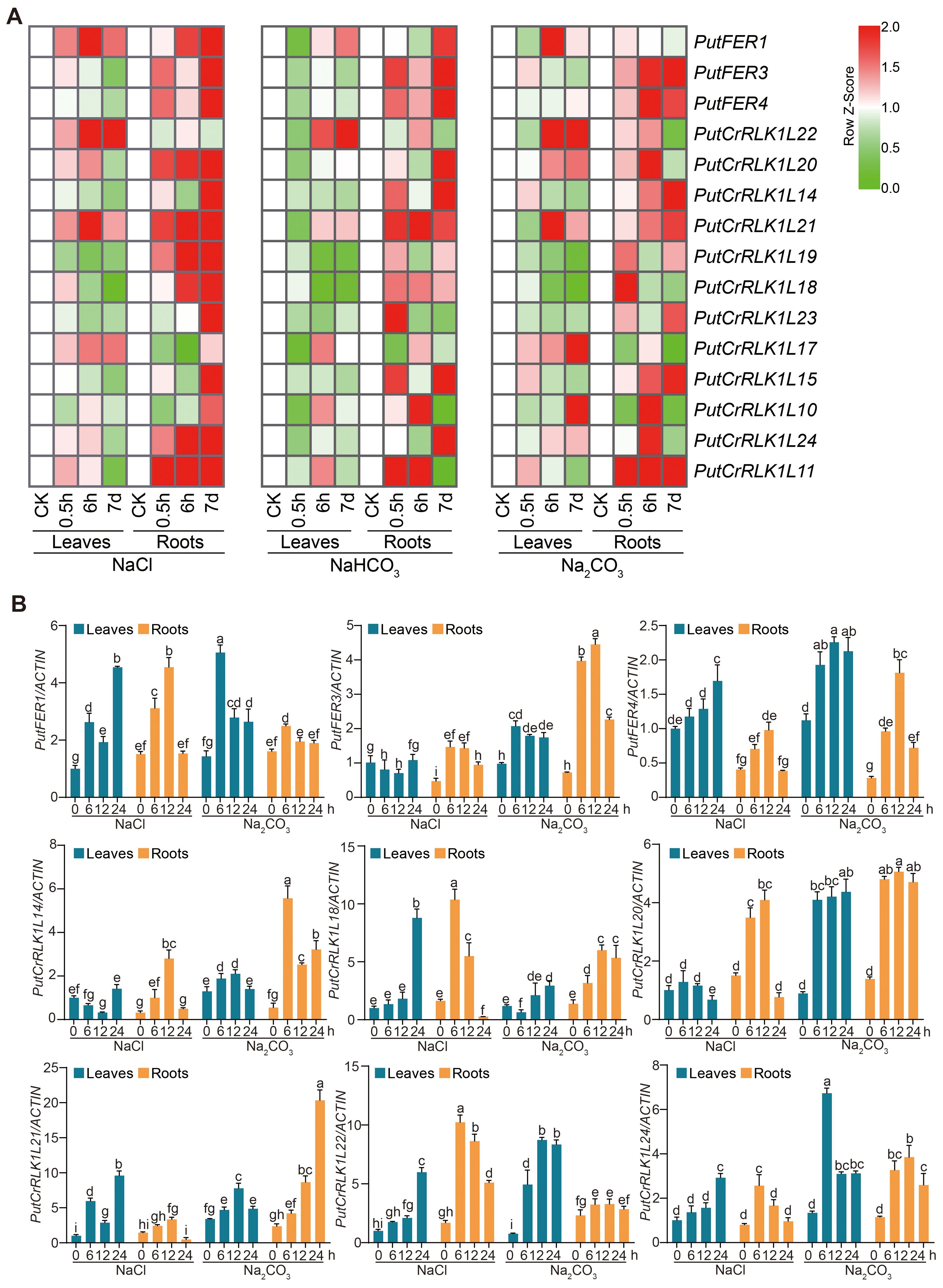
Figure 5. Salt stress induces PutFER1 in alkaligrass roots and leaves. (A) The heat map of PutCrRLK1Ls expression in root and leaf samples treated with NaCl, NaHCO3, and Na2CO3 for 0.5 h, 6 h, and 7 d, based on gene expression profiles. Red and green colors indicated up- and down-regulated genes, respectively. The color scale in the heatmap indicates the relative expression level of each gene across leaves and roots, based on the row Z-score of FPKM values. (B) Expression levels of PutCrRLK1Ls in leaves and roots using RT-qPCR analysis under salt and alkali stress conditions. The RT-qPCR results were calculated using the 2−ΔΔCt method, with PutACTIN serving as reference genes for normalization. The expression levels of PutCrRLK1Ls were analyzed in 14-day-old alkaligrass seedlings treated with 200 mM NaCl or 100 mM NaHCO3 (pH 9.0) for the indicated durations. Different letters indicate statistically significant differences (P < 0.01, one-way ANOVA).
In roots, PutFER3 and PutCrRLK1L11 were significantly induced to express under all salt stress conditions, whereas PutFER4, PutCrRLK1L18, and PutCrRLK1L21 were increased under NaCl and NaHCO3 treatments. Interestingly, PutCrRLK1L17 and PutCrRLK1L22 were initially reduced and subsequently induced under NaCl and NaHCO3 treatments, whereas they induced under Na2CO3 treatment (Figure 5A).
In leaves, the expression of four genes (PutFER1, PutCrRLK1L17, PutCrRLK1L21, and PutCrRLK1L22) were induced under NaCl conditions, while that of five genes (PutFER4, PutCrRLK1L14, PutCrRLK1L15, PutCrRLK1L19, and PutCrRLK1L23) were reduced (Figure 5A). Under Na2CO3 conditions, only PutCrRLK1L24 was induced, while three genes (PutCrRLK1L18, PutCrRLK1L19, and PutCrRLK1L23) were reduced. Interestingly, under NaHCO3 conditions, all screened PutCrRLK1L genes were reduced at 0.5 h (Figure 5A). Additionally, PutFER1 and PutCrRLK1L21 were significantly induced in both leaves and roots under NaCl conditions.
We subsequently selected nine PutCrRLK1L genes that showed significant differential expression under saline-alkali stress and analyzed their transcript levels using RT-qPCR. The results revealed that the majority of these genes were significantly induced by saline-alkali stress in both leaves and roots (Figure 5B; Supplementary Figures 2A, B). In addition, the expression of PutFER3 and PutCrRLK1L14 in leaves was significantly downregulated, which is consistent with the trend observed in the transcriptome data.
Protein structure and subcellular localization of PutFER1
To further investigate the function of PutFER1, we conducted a conservation analysis of kinase active sites within the FER kinase domain, specifically residues K565 and K663 (based on the Arabidopsis sequence), using published data (Minkoff et al., 2017). The sequence alignment among FERs from 11 plant species revealed that the kinase domain and the active sites are highly conserved (Figure 6A). This implied that PutFER1 could play a conserved role in maintaining the intracellular downstream signaling cascade (Figure 6A).
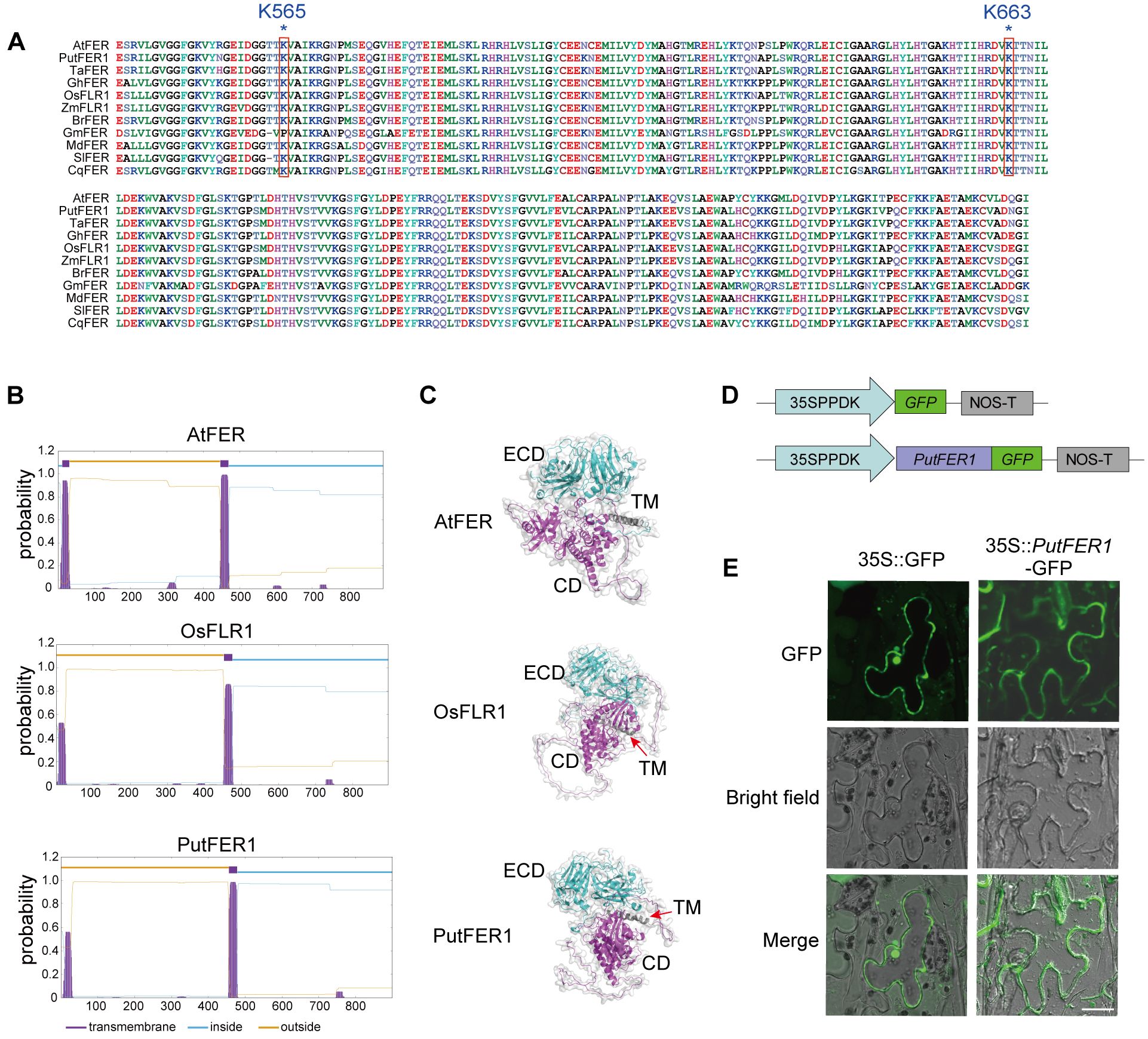
Figure 6. Protein structure and subcellular localization of PutFER1. (A) Amino acid sequence alignment of the kinase domain of FER proteins from 11 plant species. The conserved active sites of Arabidopsis K565 and K663 in 11 plant species are marked with red boxes. Sequence alignment was performed using ClustalW, and results were visualized using Bioedit. (B) Prediction of transmembrane domains in PutFER1 protein. Purple, lightblue, and yellow represent transmembrane domain (TM), intracellular regions, and extracellular regions, respectively. (C) Predicted 3D protein structure of PutFER1. Light blue, gray, and purple represent the extracellular domain (ECD), TM, and cytoplasmic domain (CD), respectively. The structure was predicted using AlphaFold and visualized using PyMOL software. (D) Schematic diagrams of control construct (35S::GFP) and recombinant construct (35S::PutFER1-GFP). 35SPPDK, hybrid promoter consisting of the cauliflower mosaic virus (CMV) 35S enhancer fused to the maize C4PPDK basal promoter. NOS-T, Nopaline synthase terminator. (E) Transient expression of GFP and PutFER1-GFP in tobacco (Nicotiana benthamiana) leaf epidermal cells. Scale bar, 20 µm.
As a receptor-like kinase, Arabidopsis FER possesses a typical single-pass transmembrane domain (Li et al., 2015). We predicted and compared the hydrophobic transmembrane region between PutFER1 and other 10 species (Figure 6B; Supplementary Figure 3). As shown in Figure 6B, PutFER1 has a typical single-pass transmembrane domain, similar to those found in rice and Arabidopsis (Figure 6B). The 3D protein structures of PutFER1 and the other ten plant species indicated that the extracellular domain of PutFER1 contains a high proportion of beta-sheets, while the intracellular domain is rich in alpha-helices (Figure 6C; Supplementary Figure 4). These results suggest that PutFER1 may play a key role in enhancing the efficiency of extracellular ligand recognition and intracellular signal transduction.
To explore the subcellular localization of PutFER1, full-length coding DNA sequence (CDS) of PutFER1 was cloned into the binary vector pCAMBIA1300-GFP and resulting in translational fusion with green fluorescent protein (GFP) (Figure 6D). GFP signal was observed at the plasma membrane using confocal fluorescence imaging in the tobacco (Nicotiana benthamiana) system, suggesting that PutFER1 is located on the plasma membrane (Figure 6E).
PutFER1 is essential for plant salt tolerance and ROS scavenging
In Arabidopsis, FER was shown to be involved in salt tolerance (Zhao et al., 2018), so we were interested in elucidating whether PutFER1 in alkaligrass is also required for salt tolerance. We then generated a PutFER1 construct driven by 35S promoter and transformed it into wild-type Arabidopsis plants. RT-qPCR analysis revealed that the other two transgenic lines exhibited transcript levels >3-fold higher than the line with the lowest expression (Supplementary Figure 5), confirming successful transgene expression. Phenotype analysis revealed that overexpressing PutFER1 in Arabidopsis results in an enhanced root length under salinity conditions (Figures 7A, B), suggesting that PutFER1 in alkaligrass participates in regulating salt tolerance. Additionally, the Arabidopsis fer-4 mutant has been reported to exhibit ROS overaccumulation, which drives salt-induced cell death (Zhao et al., 2021). We assessed ROS homeostasis in the wild-type and two PutFER1-overexpressing Arabidopsis lines. Lower levels of O2•− were observed in the PutFER1-overexpressing lines than in the wild type (Figure 7C), indicating that PutFER1 regulates intracellular ROS homeostasis during salt stress responses. To investigate the physiological impact of PutFER1-overexpression, we assessed the activities of four key antioxidant enzymes including SOD, POD, CAT, and APX in wild-type and PutFER1-overexpressing lines under both normal and salt-stress conditions. The results revealed that salt stress induced a marked increase in the activities of all four enzymes in the two overexpression lines compared to the WT (Figures 7D–G). These findings suggest that PutFER1 may enhance salt stress tolerance by boosting the activity of various antioxidant enzymes to scavenge excess ROS.
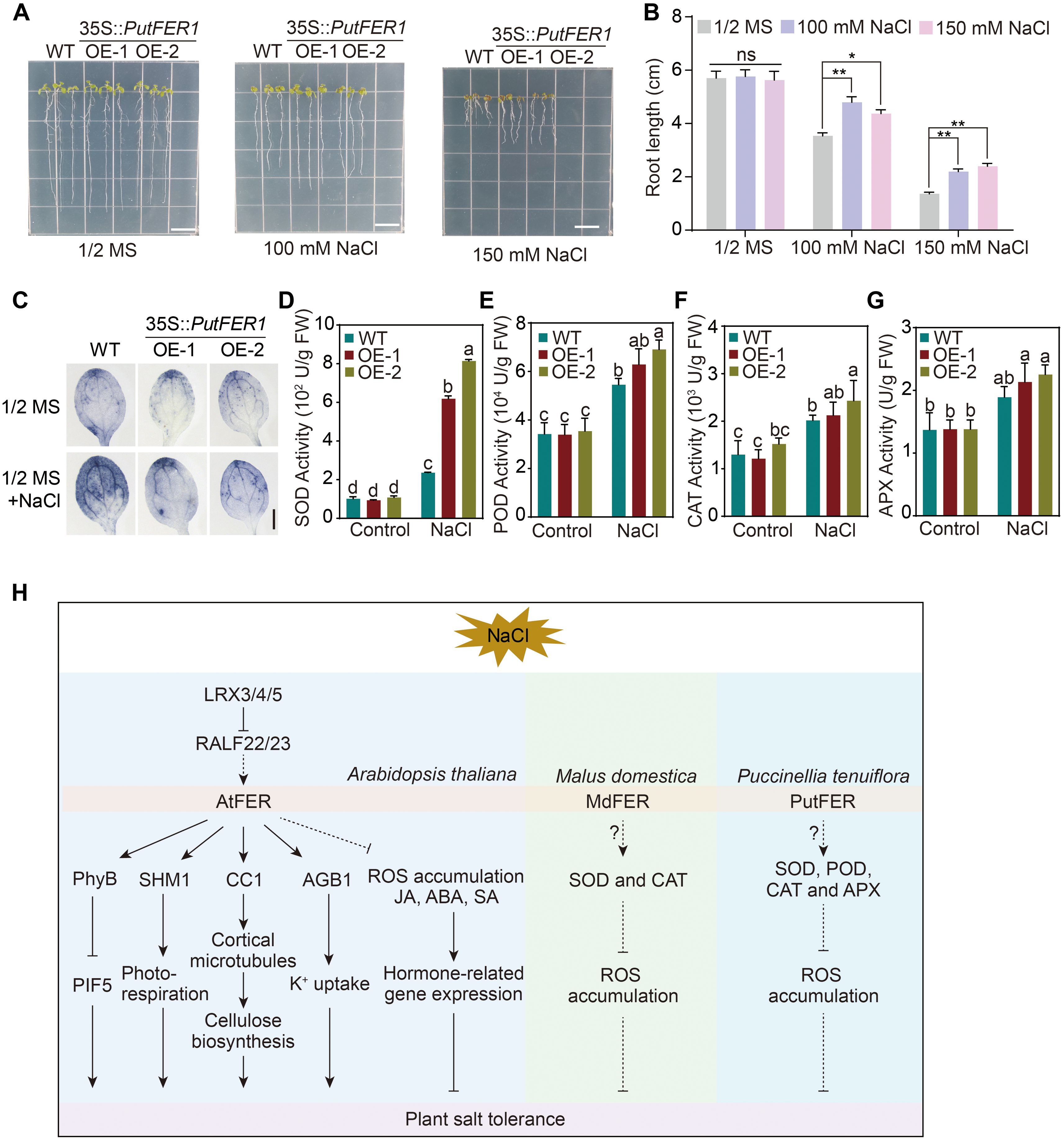
Figure 7. The salt-responsive phenotypes of wild-type, PutFER1-overexpressing Arabidopsis plants. (A) The salt-responsive phenotypes of wild-type and two lines of PutFER1-overexpressing Arabidopsis. Seedlings were grown on 1/2 MS medium for 3 d, and then transferred to 1/2 MS medium with or without NaCl for 5 d. Scale bars, 1 cm. (B) Quantification of root length shown in (A). Data represent means ± S.D. (n=15 in each biological replicates). ** P < 0.01 (two-tailed Student’s t-test). (C) The accumulation of O2•− in the leaves from five-day-old Arabidopsis seedlings. The O2•− levels from wild-type and PutFER1-overexpressing Arabidopsis plants under 0 mM and 150 mM NaCl treatment were detected using NBT staining method. Scale bars, 0.2 cm. (D–G) Activities of superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and ascorbate peroxidase (APX) wild-type and two lines of PutFER1-overexpressing Arabidopsis under control and NaCl conditions. Different letters indicate statistically significant differences (P < 0.01, one-way ANOVA). (H) A proposed model illustrating FER-mediated intracellular signaling pathways in response to salt stress in Arabidopsis thaliana, Puccinellia tenuiflora, and Malus domestica. In Arabidopsis thaliana, salt stress inhibits AtFER kinase activity, leading to the accumulation of phyB in the nucleus, which subsequently suppresses PIF5-mediated plant growth. AtFER enhances photorespiratory flux by phosphorylating SHM1, thereby promoting growth under salt stress. Additionally, AtFER promotes cortical microtubule polymerization and subsequent cellulose synthesis by phosphorylating CC1, further supporting plant growth during salt stress. AtFER, together with AGB1, coordinately regulates K+ uptake under salt stress. The LRX3/4/5-RALF22/23-FER module enhances salt stress tolerance by suppressing intracellular ROS levels and modulating hormone signaling (JA, ABA, and SA). In Puccinellia tenuiflora, PutFER1 contributes to salt stress adaptation by increasing the activity of antioxidant enzymes (SOD, POD, CAT, and APX), which scavenge excess ROS. Similarly, in Malus domestica, MdFER improves salt stress tolerance by enhancing the activities of SOD and CAT to mitigate ROS accumulation. ABA, Abscisic Acid; AGB1, Arabidopsis G protein β subunit; APX, Ascorbate peroxidase; CAT, Catalase; CC1, COMPANION OF CELLULOSE SYNTHASE 1; FER, FERONIA; JA, Jasmonic Acid; LRX3/4/5, LEUCINE-RICH REPEAT EXTENSINS 3/4/5; PhyB, PHYTOCHROME B; PIF5, PHYTOCHROME INTERACTING FACTOR 5; POD, Peroxidase; RALF22/23, RAPID ALKALINIZATION FACTOR 22/23; ROS, Reactive oxygen species; SA, Salicylic Acid; SHM1, Serine Hydroxymethyltransferase 1; SOD, Superoxide dismutase.
Discussion
Genomic expansion and functional adaptation of the CrRLK1L family in P. tenuiflora
As important members of the plant RLK family, CrRLK1Ls have been identified in 29 species, including angiosperms (e.g., Arabidopsis, rice, and apple), gymnosperms (e.g., Picea abies), and early-diverging lineages (e.g., C. psl. complex, M.polymorpha, and P. patens) (Hirano et al., 2015; Bowman et al., 2017; Westermann et al., 2019; Zhu et al., 2021; Mecchia et al., 2022; Ma et al., 2023b). In this study, we comprehensively analyzed the structural characteristics, expression patterns, and biological functions of CrRLK1Ls in P. tenuiflora. Similar with some plant species, such as apple (Zuo et al., 2018) with 67 genes, tobacco (Li et al., 2022) with 48 genes, cotton (Niu et al., 2016) with 79 genes, and soybean (Jiang et al., 2024b) with 45 genes, the PutCrRLK1L family with 25 members has also expanded significantly. This expansion likely reflects genomic adaptations to the extreme saline-alkaline habitats of P. tenuiflora.
The presence or absence of introns, as well as their abundance, can reflect the evolution of plant genomes and the functional divergence of genes (Liu et al., 2021b). RNA-seq analyses of Arabidopsis and rice revealed that intronless or intron-poor genes, including the AP2, EF-hand, bZIP, FAD-binding and C2 gene families, play a greater role in responding to drought and salt stress than intron-rich genes in the same families (Liu et al., 2021b). Importantly, intronless genes in the S-domain RLK gene family are more likely to be involved in epigenetic processes and plant development (Liu et al., 2021b). We found alkaligrass PutCrRLK1Ls almost universally lack introns and UTRs (Figure 3A), which obviously contrasts with many other CrRLK1L families of maize, tomato (Solanum lycopersicum), yam (Dioscorea rotundata), and citrus (Citrus sinensis) (Luo et al., 2020; Ma et al., 2023b; Wang et al., 2024a; Qiao et al., 2025). For instance, approximately half of the maize ZmCrRLK1L family members have about 1 ~ 7 introns (Wang et al., 2024a). This pattern may imply an adaptive feature in P. tenuiflora, potentially enabling faster transcriptional activation and/or more efficient translation in response to rapidly changing environmental stresses such as salinity.
In addition, similar to those in maize (Wang et al., 2024a), soybean (Jiang et al., 2024b), and potato (Bao et al., 2025), the PutCrRLK1L family members exhibit a comparable pattern of variation in the number of MLD motifs (2 ~ 4). This suggests that PutCrRLK1Ls recognize signals differently in response to various environmental processes.
Comparative cis-regulatory elements of PutCrRLK1Ls
Promoter cis-elements play a crucial role in regulating gene expression patterns, stress responses, and biological functions (Marand et al., 2023). The promoters of CrRLK1L family members in multiple plant species (e.g., wheat, maize, and tomato) are enriched with various cis-acting elements responsive to hormones and abiotic stress (Ma et al., 2023b; Gawande and Sankaranarayanan, 2024; Wang et al., 2024a). For instance, 24 soybean GmCrRLK1L genes possessed the ABA-responsive element ABRE, while 12 and 11 GmCrRLK1L genes contained the drought-responsive element MBS and the low temperature-responsive element LTR, respectively (Wang et al., 2021). In addition, 29 potato StCrRLK1L genes contain at least four cis-elements related to light response, while 10 StCrRLK1L genes contain cis-elements related to phytohormones (Bao et al., 2025). In our studies, 25 PutCrRLK1L genes from alkaligrass exhibit significant enrichment of cis-elements related to phytohormones (nine elements) and light response (11 elements) (Figure 4B). In addition, at least 20 PutCrRLK1Ls contain both ABRE and light-responsive G-box elements, which is consistent with soybean GmCrRLK1Ls and potato StCrRLK1Ls (Jiang et al., 2024b; Bao et al., 2025). This indicates that PutCrRLK1Ls could be involved in hormone and light signaling pathways. Arabidopsis FER confers resistance to photooxidative stress and protects photosystems under moderate light conditions (Shin et al., 2021). Meanwhile, FER fine-tunes the balance between growth and stress by regulating the signaling of hormones such as ABA, JA, and SA (Zhao et al., 2021). Furthermore, the key transcription factor SlBZR1, which is involved in BR signaling, enhances thermotolerance in tomato by directly binding to the E-box (CANNTG) in the SlFERs promoter (Yin et al., 2018). However, no BR relevant regulatory elements were identified in the promoter region of the PutFERs in alkaligrass. Therefore, screening for transcription factors that directly bind to the cis-elements in the FER promoter could deepen our understanding of the FER-mediated signaling pathway.
Functional significance of PutFER1 in salt stress adaptation
As a central member of the CrRLK1L family, FER is widely recognized for its functional versatility in plants, regulating processes span growth, reproduction, immunity, and responses to abiotic stresses such as salinity, drought, and heat (Yin et al., 2018; Huang et al., 2020b, 2023; Jing et al., 2023; Cheung, 2024; Jiang et al., 2024a). In glycophytes like Arabidopsis, FER acts as a polymodal integrator, coordinating salt tolerance through multiple interconnected pathways. FER perceives cell wall integrity via pectin binding, activates Ca²+ and MAPK signaling (Feng et al., 2018; Gigli-Bisceglia et al., 2022), modulates stomatal dynamics through G-protein interactions (Yu and Assmann, 2018), and fine-tunes hormonal and ROS homeostasis via leucine-rich repeat extensins (LRXs)-RALF modules (Zhao et al., 2021). Furthermore, FER regulates metabolic adaptation and growth under stress by targeting SHM1 and phyB-PIF signaling (Liu et al., 2023; Jiang et al., 2024a), thereby mediating a trade-off between stress adaptation and growth suppression. In halophytes such as quinoa (Chenopodium quinoa), FER homologs are rapidly induced under salt stress, indicating a conserved role in stress perception. All these findings suggest that the core FER-dependent pathways appear evolutionarily conserved, while the species-specific functional adaptations have emerged, such as regulating ABA sensitivity in apple (Malus domestica), ion homeostasis in quinoa, and ROS production in Brassica rapa (Zhang et al., 2021a; Bazihizina et al., 2022; Xie et al., 2022). This underscores FER’s versatile role in bridging salt response with developmental plasticity across diverse plant lineages (Figure 7H).
Our study demonstrates that PutFER1, a FER homolog in the halophyte P. tenuiflora, is transcriptionally upregulated under NaCl treatment and localizes to the plasma membrane, consistent with its reported function as a receptor kinase (Boisson-Dernier et al., 2011; Kessler et al., 2014). Functional validation in Arabidopsis revealed that PutFER1 overexpression enhances root growth under salt stress and reduces ROS accumulation, accompanied by elevated activities of key antioxidant enzymes (SOD, POD, CAT, and APX). These results suggest that PutFER1 contributes to salt tolerance by enhancing the ROS-scavenging capacity, thereby mitigating oxidative damage, which could be a critical mechanism that may be particularly critical in halophytes thriving in high-salinity environments.
In summary, our findings establish PutFER1 as a key regulator of salt tolerance through the modulation of ROS homeostasis, offering valuable insights into halophyte adaptation and presenting a promising genetic resource for breeding salt-resilient crops. However, the present study relied on heterologous expression in Arabidopsis, which may not fully recapitulate the native regulatory context of P. tenuiflora. Future work should include functional characterization of PutFER1 in alkaligrass itself, using CRISPR-Cas9-mediated knockout or knockdown lines, to validate its role in a halophyte background (Zhang et al., 2021b). In addition, the precise signaling cascade downstream of PutFER1, including its potential ligands, co-receptors, and phosphorylation targets, remains to be elucidated in alkaligrass. Identifying interaction partners through methods such as co-immunoprecipitation followed by mass spectrometry would provide deeper mechanistic insights. Finally, field trials using PutFER1-overexpressing crop plants could assess its practical potential for improving salinity tolerance in agriculturally important species.
Data availability statement
All relevant data is contained within the article: The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
GL: Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Writing – original draft. YP: Investigation, Validation, Writing – review & editing. YL: Formal analysis, Software, Validation, Writing – review & editing. ZW: Writing – review & editing, Methodology. SD: Funding acquisition, Writing – review & editing. ZQ: Conceptualization, Supervision, Writing – review & editing. MS: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (Grant No. 32441006 and 32070300) to SD and the Fund of Shanghai Engineering Research Center of Plant Germplasm Resources, China (Grant No. 17DZ2252700) to MS.
Acknowledgments
We would like to thank Professor Jie Qiu of Shanghai Normal University for his assistance with constructing the phylogenetic tree.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1680452/full#supplementary-material
References
Bao, Y., Zhao, R., Hu, S., Li, X., Wang, L., Wang, J., et al. (2025). Genome-wide identification and expression analysis of CrRLK1-like gene family in potatoes (Solanum tuberosum L.) and its role in PAMP-triggered immunity. Genes 16, 308. doi: 10.3390/genes16030308
Bazihizina, N., Vita, F., Balestrini, R., Kiferle, C., Caparrotta, S., Ghignone, S., et al. (2022). Early signalling processes in roots play a crucial role in the differential salt tolerance in contrasting Chenopodium quinoa accessions. J. Exp. Bot. 73, 292–306. doi: 10.1093/jxb/erab388
Boisson-Dernier, A., Franck, C. M., Lituiev, D. S., and Grossniklaus, U. (2015). Receptor-like cytoplasmic kinase MARIS functions downstream of CrRLK1L-dependent signaling during tip growth. Proc. Natl. Acad. Sci. U.S.A. 112, 12211–12216. doi: 10.1073/pnas.1512375112
Boisson-Dernier, A., Kessler, S. A., and Grossniklaus, U. (2011). The walls have ears: the role of plant CrRLK1Ls in sensing and transducing extracellular signals. J. Exp. Bot. 62, 1581–1591. doi: 10.1093/jxb/erq445
Bowman, J. L., Kohchi, T., Yamato, K. T., Jenkins, J., Shu, S., Ishizaki, K., et al. (2017). Insights into land plant evolution garnered from the marchantia polymorpha genome. Cell 171, 287–304. doi: 10.1016/j.cell.2017.09.030
Chaudhary, A., Hsiao, Y.-C., Jessica Yeh, F.-L., Župunski, M., Zhang, H., Aizezi, Y., et al. (2025). FERONIA signaling maintains cell wall integrity during brassinosteroid-induced cell expansion in Arabidopsis. Mol. Plant 18, 603–618. doi: 10.1016/j.molp.2025.02.001
Cheung, A. Y. (2024). FERONIA: a receptor kinase at the core of a global signaling network. Annu. Rev. Plant Biol. 75, 345–375. doi: 10.1146/annurev-arplant-102820-103424
Deslauriers, S. D. and Larsen, P. B. (2010). FERONIA is a key modulator of brassinosteroid and ethylene responsiveness in Arabidopsis hypocotyls. Mol. Plant 3, 626–640. doi: 10.1093/mp/ssq015
Duan, Q. H., Kita, D., Li, C., Cheung, A. Y., and Wu, H. M. (2010). FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proc. Natl. Acad. Sci. U.S.A. 107, 17821–17826. doi: 10.1073/pnas.1005366107
Escobar-Restrepo, J. M., Huck, N., Kessler, S., Gagliardini, V., Gheyselinck, J., Yang, W. C., et al. (2007). The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception. Science 317, 656–660. doi: 10.1126/science.1143562
Feng, W., Kita, D., Peaucelle, A., Cartwright, H. N., Doan, V., Duan, Q. H., et al. (2018). The FERONIA receptor kinase maintains cell-wall integrity during salt stress through Ca2+ signaling. Curr. Biol. 28, 666–675. doi: 10.1016/j.cub.2018.01.023
Franck, C. M., Westermann, J., and Boisson-Dernier, A. (2018a). Plant malectin-like receptor kinases: from cell wall integrity to immunity and beyond. Annu. Rev. Plant Biol. 69, 301–328. doi: 10.1146/annurev-arplant-042817-040557
Franck, C. M., Westermann, J., Bürssner, S., Lentz, R., Lituiev, D. S., and Boisson-Dernier, A. (2018b). The protein phosphatases ATUNIS1 and ATUNIS2 regulate cell wall integrity in tip-growing cells. Plant Cell 30, 1906–1923. doi: 10.1105/tpc.18.00284
Fu, Q., Li, H., Wang, B., Chen, W., Wu, D., Gao, C., et al. (2025). The RALF1 peptide-FERONIA complex phosphorylates the endosomal sorting protein FREE1 to attenuate abscisic acid signaling. Plant Physiol. 197, kiae625. doi: 10.1093/plphys/kiae625
Gachomo, E. W., Jno Baptiste, L., Kefela, T., Saidel, W. M., and Kotchoni, S. O. (2014). The Arabidopsis CURVY1 (CVY1) gene encoding a novel receptor-like protein kinase regulates cell morphogenesis, flowering time and seed production. BMC Plant Biol. 14, 221. doi: 10.1186/s12870-014-0221-7
Galindo-Trigo, S., Blanco-Touriñán, N., DeFalco, T. A., Wells, E. S., Gray, J. E., Zipfel, C., et al. (2019). CrRLK1L receptor-like kinases HERK1 and ANJEA are female determinants of pollen tube reception. EMBO Rep. 21, e48466. doi: 10.15252/embr.201948466
Gawande, N. D. and Sankaranarayanan, S. (2024). Genome wide characterization and expression analysis of CrRLK1L gene family in wheat unravels their roles in development and stressspecific responses. Front. Plant Sci. 15. doi: 10.3389/fpls.2024.1345774
Ge, Z., Zhao, Y., Liu, M.-C., Zhou, L.-Z., Wang, L., Zhong, S., et al. (2019). LLG2/3 are co-receptors in BUPS/ANX-RALF signaling to regulate Arabidopsis pollen tube integrity. Curr. Biol. 29, 3256–3265. doi: 10.1016/j.cub.2019.08.032
Gigli-Bisceglia, N., van Zelm, E., Huo, W. Y., Lamers, J., and Testerink, C. (2022). Arabidopsis root responses to salinity depend on pectin modification and cell wall sensing. Development 149, dev200363. doi: 10.1242/dev.200363
Guo, H. Q., Li, L., Ye, H. X., Yu, X. F., Algreen, A., and Yin, Y. H. (2009). Three related receptor-like kinases are required for optimal cell elongation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 106, 7648–7653. doi: 10.1073/pnas.0812346106
Guo, H. Q., Nolan, T. M., Song, G. Y., Liu, S. Z., Xie, Z. L., Chen, J. I., et al. (2018). FERONIA receptor kinase contributes to plant immunity by suppressing jasmonic acid signaling in Arabidopsis thaliana. Curr. Biol. 28, 3316–3324. doi: 10.1016/j.cub.2018.07.078
Hirano, N., Marukawa, Y., Abe, J., Hashiba, S., Ichikawa, M., Tanabe, Y., et al. (2015). A receptor-like kinase, related to cell wall sensor of higher plants, is required for sexual reproduction in the unicellular charophycean alga, Closterium peracerosum-strigosum-littorale complex. Plant Cell Physiol. 56, 1456–1462. doi: 10.1093/pcp/pcv065
Huang, G. Q., Li, E., Ge, F. R., Li, S., Wang, Q., Zhang, C. Q., et al. (2013). Arabidopsis RopGEF4 and RopGEF10 are important for FERONIA-mediated developmental but not environmental regulation of root hair growth. New Phytol. 200, 1089–1101. doi: 10.1111/nph.12432
Huang, J., Yang, L., Yang, L., Wu, X., Cui, X., Zhang, L., et al. (2023). Stigma receptors control intraspecies and interspecies barriers in Brassicaceae. Nature 614, 303–308. doi: 10.1038/s41586-022-05640-x
Huang, Y. Y., Liu, X. X., Xie, Y., Lin, X. Y., Hu, Z. J., Wang, H., et al. (2020b). Identification of FERONIA-like receptor genes involved in rice-Magnaporthe oryzae interaction. Phytopathol. Res. 2, 14. doi: 10.1186/s42483-020-00052-z
Huang, Y., Yin, C., Liu, J., Feng, B., Ge, D., Kong, L., et al. (2020a). A trimeric CrRLK1L-LLG1 complex genetically modulates SUMM2-mediated autoimmunity. Nat. Commun. 11, 4859. doi: 10.1038/s41467-020-18600-8
Huck, N., Moore, J. M., Federer, M., and Grossniklaus, U. (2003). The Arabidopsis mutant feronia disrupts the female gametophytic control of pollen tube reception. Development 130, 2149–2159. doi: 10.1242/dev.00458
Jiang, X. M., Cao, J. L., Cao, L. F., Wang, L., and Che, Z. J. (2024b). Genome-wide re-identification of the CrRLK1L family in soybean and functional characterization of GmCrRLK1L2 in salt stress response. Environ. Exp. Bot. 226, 105903. doi: 10.1016/j.envexpbot.2024.105903
Jiang, W., Li, C., Li, L. T., Li, Y. L., Wang, Z. H., Yu, F. Y., et al. (2022). Genome-wide analysis of CqCrRLK1L and CqRALF gene families in Chenopodium quinoa and their roles in salt stress response. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.918594
Jiang, W., Wang, Z. H., Li, Y. L., Liu, X., Ren, Y. Y., Li, C., et al. (2024a). FERONIA regulates salt tolerance in Arabidopsis by controlling photorespiratory flux. Plant Cell 36, 4732–4751. doi: 10.1093/plcell/koae246
Jing, Y., Liu, C., Liu, B., Pei, T., Zhan, M., Li, C., et al. (2023). Overexpression of the FERONIA receptor kinase MdMRLK2 confers apple drought tolerance by regulating energy metabolism and free amino acids production. Tree Physiol. 43, 154–168. doi: 10.1093/treephys/tpac100
Kessler, S. A., Lindner, H., Jones, D. S., and Grossniklaus, U. (2014). Functional analysis of related CrRLK1L receptor-like kinases in pollen tube reception. EMBO Rep. 16, 107–115. doi: 10.15252/embr.201438801
Li, X. X., Guo, C., Wang, Q., Li, Z. Y., Cai, J., Wu, D. S., et al. (2022). Systematic analysis of tobacco CrRLK1L family genes and functional identification of NtCrRLK1L47 in environmental stresses. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.838857
Li, C., Yeh, F.-L., Cheung, A. Y., Duan, Q., Kita, D., Liu, M.-C., et al. (2015). Glycosylphosphatidylinositol-anchored proteins as chaperones and co-receptors for FERONIA receptor kinase signaling in Arabidopsis. eLife 4, e06587. doi: 10.7554/eLife.06587
Li, Y., Zhao, Z., Li, B., Zheng, H., Wu, Z., Li, Y., et al. (2025). Genome-wide identification and salinity response analysis of the germin-like protein (GLP) gene family in Puccinellia tenuiflora. Plants 14, 2259. doi: 10.3390/plants14152259
Lindner, H., Müller, L. M., Boisson-Dernier, A., and Grossniklaus, U. (2012). CrRLK1L receptor-like kinases: not just another brick in the wall. Curr. Opin. Plant Biol. 15, 659–669. doi: 10.1016/j.pbi.2012.07.003
Liu, Y., Han, X., Yu, J., Li, Y., Sun, M., Pang, Q., et al. (2025). Genome-wide identification and expression analysis of glutaredoxin in Puccinellia tenuiflora under salinity stress. BMC Plant Biol. 25, 605. doi: 10.1186/s12870-025-06547-1
Liu, X., Jiang, W., Li, Y. L., Nie, H. Z., Cui, L. N., Li, R. X., et al. (2023). FERONIA coordinates plant growth and salt tolerance via the phosphorylation of phyB. Nat. Plants 9, 645–660. doi: 10.1038/s41477-023-01390-4
Liu, H., Lyu, H. M., Zhu, K. K., Van de Peer, Y., and Cheng, Z. M. (2021b). The emergence and evolution of intron-poor and intronless genes in intron-rich plant gene families. Plant J. 105, 1072–1082. doi: 10.1111/tpj.15088
Liu, C., Shen, L., Xiao, Y., Vyshedsky, D., Peng, C., Sun, X., et al. (2021a). Pollen PCP-B peptides unlock a stigma peptide–receptor kinase gating mechanism for pollination. Science 372, 171–175. doi: 10.1126/science.abc6107
Liu, X., Wang, L., Liu, L. L., Li, Y., Ogden, M., Somssich, M., et al. (2024). FERONIA adjusts CC1 phosphorylation to control microtubule array behavior in response to salt stress. Sci. Adv. 10, eadq8717. doi: 10.1126/sciadv.adq8717
Livak, K. J. and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Luo, C., Sun, Q., Zhang, F., Zhang, D., Liu, C., Wu, Q., et al. (2020). Genome-wide identification and expression analysis of the citrus malectin domain-containing receptor-like kinases in response to arbuscular mycorrhizal fungi colonization and drought. Hortic. Environ. Biotechnol. 61, 891–901. doi: 10.1007/s13580-020-00273-3
Ma, W., Du, J., Yu, X., Chen, K., Ming, Y., Jiang, L., et al. (2023a). Genome-wide identification and analysis of Catharanthus roseus receptor-like kinase 1-like proteins in eggplant. Plants 12, 3379. doi: 10.3390/plants12193379
Ma, W., Liu, X., Chen, K., Yu, X., and Ji, D. (2023b). Genome-wide re-identification and analysis of CrRLK1Ls in tomato. Int. J. Mol. Sci. 24, 3142. doi: 10.3390/ijms24043142
Marand, A. P., Eveland, A. L., Kaufmann, K., and Springer, N. M. (2023). cis-Regulatory elements in plant development, adaptation, and evolution. Annu. Rev. Plant Biol. 74, 111–137. doi: 10.1146/annurev-arplant-070122-030236
Mecchia, M. A., Rövekamp, M., Giraldo-Fonseca, A., Meier, D., Gadient, P., Vogler, H., et al. (2022). The single Marchantia polymorpha FERONIA homolog reveals an ancestral role in regulating cellular expansion and integrity. Development 149, dev200580. doi: 10.1242/dev.200580
Meng, X., Zhao, Q., Jin, Y., Yu, J., Yin, Z., Chen, S., et al. (2016). Chilling-responsive mechanisms in halophyte Puccinellia tenuiflora seedlings revealed from proteomics analysis. J. Proteomics 143, 365–381. doi: 10.1016/j.jprot.2016.04.038
Minkoff, B. B., Makino, S. I., Haruta, M., Beebe, E. T., Wrobel, R. L., Fox, B. G., et al. (2017). A cell-free method for expressing and reconstituting membrane proteins enables functional characterization of the plant receptor-like protein kinase FERONIA. J. Biol. Chem. 292, 5932–5942. doi: 10.1074/jbc.M116.761981
Nasrallah, J. B., Boisson-Dernier, A., Lituiev, D. S., Nestorova, A., Franck, C. M., Thirugnanarajah, S., et al. (2013). ANXUR receptor-like kinases coordinate cell wall integrity with growth at the pollen tube tip via NADPH oxidases. PLoS Biol. 11, e1001719. doi: 10.1371/journal.pbio.1001719
Nissen, K. S., Willats, W. G. T., and Malinovsky, F. G. (2016). Understanding CrRLK1L function: cell walls and growth control. Trends Plant Sci. 21, 516–527. doi: 10.1016/j.tplants.2015.12.004
Niu, E., Cai, C., Zheng, Y., Shang, X., Fang, L., and Guo, W. (2016). Genome−wide analysis of CrRLK1L gene family in Gossypium and identification of candidate CrRLK1L genes related to fiber development. Mol. Genet. Genomics 291, 1137–1154. doi: 10.1007/s00438-016-1169-0
Qiao, Q., Fu, X., Ren, Z., Qiao, W., Xiao, D., and He, L. (2023). Genome−wide Identification, evolutionary and expression analyses of CrRLK1L gene family in peanut (Arachis hypogaea L.). Trop. Plant Biol. 17, 24–41. doi: 10.1007/s12042-023-09350-0
Qiao, Q., Li, W., Li, C., Zhou, Y., Qiao, W., Sheng, F., et al. (2025). Genome-wide identification and expression analysis of the CrRLK1L gene family in yam (Dioscorea rotundata): potential roles in growth and tuber development. Trop. Plant Biol. 18, 41. doi: 10.1007/s12042-025-09409-0
Rao, S., Wu, X., Zheng, H., Lu, Y., Peng, J., Wu, G., et al. (2021). Genome-wide identification and analysis of Catharanthus roseus RLK1-like kinases in Nicotiana benthamiana. BMC Plant Biol. 21, 425. doi: 10.1186/s12870-021-03208-x
Richter, J., Ploderer, M., Mongelard, G., Gutierrez, L., and Hauser, M.-T. (2017). Role of CrRLK1L cell wall sensors HERCULES1 and 2, THESEUS1, and FERONIA in growth adaptation triggered by heavy metals and trace elements. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.01554
Richter, J., Watson, J. M., Stasnik, P., Borowska, M., Neuhold, J., Berger, M., et al. (2018). Multiplex mutagenesis of four clustered CrRLK1L with CRISPR/ Cas9 exposes their growth regulatory roles in response to metal ions. Sci. Rep. 8, 12182. doi: 10.1038/s41598-018-30711-3
Rotman, N., Rozier, F., Boavida, L., Dumas, C., Berger, F., and Faure, J.-E. (2003). Female control of male gamete delivery during fertilization in Arabidopsis thaliana. Curr. Biol. 13, 432–436. doi: 10.1016/s0960-9822(03)00093-9
Schallus, T., Jaeckh, C., Fehér, K., Palma, A. S., Liu, Y., Simpson, J. C., et al. (2008). Malectin: a novel carbohydrate-binding protein of the endoplasmic reticulum and a candidate player in the early steps of protein N-glycosylation. Mol. Biol. Cell 19, 3404–3414. doi: 10.1091/mbc.e08-04-0354
Schoenaers, S., Balcerowicz, D., Breen, G., Hill, K., Zdanio, M., Mouille, G., et al. (2018). The auxin-regulated CrRLK1L Kinase ERULUS controls cell wall composition during root hair tip growth. Curr. Biol. 28, 722–732.e726. doi: 10.1016/j.cub.2018.01.050
Schulze-Muth, P., Irmler, S., Schröder, G., and Schröder, J. (1996). Novel type of receptor-like protein kinase from a higher plant (Catharanthus roseus). J. Biol. Chem. 271, 26684–26689. doi: 10.1074/jbc.271.43.26684
Shin, S. Y., Park, J. S., Park, H. B., Moon, K. B., Kim, H. S., Jeon, J. H., et al. (2021). FERONIA confers resistance to photooxidative stress in Arabidopsis. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.714938
Suo, J., Zhang, H., Zhao, Q., Zhang, N., Zhang, Y., Li, Y., et al. (2020). Na2CO3-responsive photosynthetic and ROS scavenging mechanisms in chloroplasts of alkaligrass revealed by phosphoproteomics. Genomics Proteomics Bioinform. 18, 271–288. doi: 10.1016/j.gpb.2018.10.011
Tang, L., Wang, Y., Wang, W., Deng, X., and Wang, X. (2022). Genome-wide identification of CrRLK1L gene family and desiccation-induced expression profiles in Boea hygrometrica. Curr. Plant Biol. 31, 100256. doi: 10.1016/j.cpb.2022.100256
Wang, X. X., Liu, J. W., Wang, M. T., Liu, L. L., Liu, X., and Zhao, C. Z. (2024b). FERONIA controls ABA-mediated seed germination via the regulation of CARK1 kinase activity. Cell Rep. 43, 114843. doi: 10.1016/j.celrep.2024.114843
Wang, K., Xue, B., He, Y., Zhao, H., Liu, B., Jiang, W., et al. (2024a). Evolution, gene duplication, and expression pattern analysis of CrRLK1L gene family in Zea mays (L.). Int. J. Mol. Sci. 25, 10487. doi: 10.3390/ijms251910487
Wang, Z. Q., Yu, T. F., Sun, G. Z., Zheng, J. C., Chen, J., Zhou, Y. B., et al. (2021). Genome-wide analysis of the Catharanthus roseus RLK1-like in soybean and GmCrRLK1L20 responds to drought and salt stresses. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.614909
Westermann, J., Streubel, S., Franck, C. M., Lentz, R., Dolan, L., and Boisson-Dernier, A. (2019). An evolutionarily conserved receptor-like kinases signaling module controls cell wall integrity during tip growth. Curr. Biol. 29, 3899–3908.e3893. doi: 10.1016/j.cub.2019.09.069
Xie, Y. H., Zhang, F. J., Sun, P., Li, Z. Y., Zheng, P. F., Gu, K. D., et al. (2022). Apple receptor-like kinase FERONIA regulates salt tolerance and ABA sensitivity in Malus domestica. J. Plant Physiol. 270, 153616. doi: 10.1016/j.jplph.2022.153616
Yang, Z., Xing, J., Wang, L., Liu, Y., Qu, J., Tan, Y., et al. (2020). Mutations of two FERONIA-like receptor genes enhance rice blast resistance without growth penalty. J. Exp. Bot. 71, 2112–2126. doi: 10.1093/jxb/erz541
Yin, Y. L., Qin, K. Z., Song, X. W., Zhang, Q. H., Zhou, Y. H., Xia, X. J., et al. (2018). BZR1 transcription factor regulates heat stress tolerance through FERONIA receptor-like kinase-mediated reactive oxygen species signaling in tomato. Plant Cell Physiol. 59, 2239–2254. doi: 10.1093/pcp/pcy146
Yin, Z. P., Zhang, H., Zhao, Q., Yoo, M. J., Zhu, N., Yu, J. L., et al. (2019). Physiological and comparative proteomic analyses of saline-alkali NaHCO3-responses in leaves of halophyte Puccinellia tenuiflora. Plant Soil 437, 137–158. doi: 10.1007/s11104-019-03955-9
Yu, Y. and Assmann, S. (2018). Inter-relationships between the heterotrimeric Gβ subunit AGB1, the receptor-like kinase FERONIA, and RALF1 in salinity response. Plant Cell Environ. 41, 2475–2489. doi: 10.1111/pce.13370
Yu, Y., Chakravorty, D., and Assmann, S. (2018). The G protein β-subunit, AGB1, interacts with FERONIA in RALF1-regulated stomatal movement. Plant Physiol. 176, 2426–2440. doi: 10.1104/pp.17.01277
Yu, J., Chen, S., Wang, T., Sun, G., and Dai, S. (2013). Comparative proteomic analysis of Puccinellia tenuiflora leaves under Na2CO3 stress. Int. J. Mol. Sci. 14, 1740–1762. doi: 10.3390/ijms14011740
Yu, J., Chen, S., Zhao, Q., Wang, T., Yang, C., Diaz, C., et al. (2011). Physiological and proteomic analysis of salinity tolerance in Puccinellia tenuiflora. J. Proteome Res. 10, 3852–3870. doi: 10.1021/pr101102p
Yu, F., Qian, L. C., Nibau, C., Duan, Q. H., Kita, D., Levasseur, K., et al. (2012). FERONIA receptor kinase pathway suppresses abscisic acid signaling in Arabidopsis by activating ABI2 phosphatase. Proc. Natl. Acad. Sci. U.S.A. 109, 14693–14698. doi: 10.1073/pnas.1212547109
Zhang, L., Deng, X., Xiao, J., Zhao, W., Zou, P., Liao, R., et al. (2025). Root metabolites regulated by FERONIA promote phosphorus-solubilizing rhizobacteria enrichment induced by Arabidopsis thaliana coping with phosphorus deficiency. Microbiol. Res. 292, 128030. doi: 10.1016/j.micres.2024.128030
Zhang, X., Henriques, R., Lin, S. S., Niu, Q. W., and Chua, N. H. (2006). Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protoc. 1, 641–646. doi: 10.1038/nprot.2006.97
Zhang, L. L., Huang, J. B., Su, S. Q., Wei, X. C., Yang, L., Zhao, H. H., et al. (2021a). FERONIA receptor kinase-regulated reactive oxygen species mediate self-incompatibility in Brassica rapa. Curr. Biol. 31, 3004–3016. doi: 10.1016/j.cub.2021.04.060
Zhang, W., Liu, J., Zhang, Y., Qiu, J., Li, Y., Zheng, B., et al. (2020). A high-quality genome sequence of alkaligrass provides insights into halophyte stress tolerance. Sci. China Life Sci. 63, 1269–1282. doi: 10.1007/s11427-020-1662-x
Zhang, Y., Qin, C., Liu, S., Xu, Y., Li, Y., Zhang, Y., et al. (2021b). Establishment of an efficient Agrobacterium-mediated genetic transformation system in halophyte Puccinellia tenuiflora. Mol. Breed. 41, 55. doi: 10.1007/s11032-021-01247-8
Zhang, X., Wei, L., Wang, Z., and Wang, T. (2013). Physiological and molecular features of Puccinellia tenuiflora tolerating salt and alkaline-salt stress. J. Integr. Plant Biol. 55, 262–276. doi: 10.1111/jipb.12013
Zhang, J.-S., Zhang, Q., Jia, M., Xing, Y., Qin, L., Li, B., et al. (2016). Genome-wide identificationand expression analysis of MRLK family genes associated with strawberry (Fragaria vesca) fruit ripening and abiotic stress responses. PLoS One 11, 163647. doi: 10.1371/journal.pone.0163647
Zhang, Y., Zhang, Y., Yu, J., Zhang, H., Wang, L., Wang, S., et al. (2019). NaCl-responsive ROS scavenging and energy supply in alkaligrass callus revealed from proteomic analysis. BMC Genomics 20, 990. doi: 10.1186/s12864-019-6325-6
Zhao, C., Jiang, W., Zayed, O., Liu, X., Tang, K., Nie, W., et al. (2021). The LRXs-RALFs-FER module controls plant growth and salt stress responses by modulating multiple plant hormones. Natl. Sci. Rev. 8, nwaa149. doi: 10.1093/nsr/nwaa149
Zhao, Q., Suo, J., Chen, S., Jin, Y., Ma, X., Yin, Z., et al. (2016). Na2CO3-responsive mechanisms in halophyte Puccinellia tenuiflora roots revealed by physiological and proteomic analyses. Sci. Rep. 6, 32717. doi: 10.1038/srep32717
Zhao, C., Zayed, O., Yu, Z., Jiang, W., Zhu, P., Hsu, C., et al. (2018). Leucine-rich repeat extensin proteins regulate plant salt tolerance in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 115, 13123–13128. doi: 10.1073/pnas.1816991115
Zhong, S., Li, L., Wang, Z. J., Ge, Z. X., Li, Q. Y., Bleckmann, A., et al. (2022). RALF peptide signaling controls the polytubey block in Arabidopsis. Science 375, 290–296. doi: 10.1126/science.abl4683
Zhu, S. R., Fu, Q., Xu, F., Zheng, H. P., and Yu, F. (2021). New paradigms in cell adaptation: decades of discoveries on the CrRLK1L receptor kinase signalling network. New Phytol. 232, 1168–1183. doi: 10.1111/nph.17683
Keywords: CrRLK1L gene family, expression profiling, PutFER1, Puccinellia tenuiflora, salt stress
Citation: Li G, Peng Y, Liu Y, Wu Z, Dai S, Qin Z and Sun M (2025) Genome-wide analysis of the CrRLK1L gene family in Puccinellia tenuiflora and functional study of PutFER1 in Arabidopsis underpinning salt tolerance. Front. Plant Sci. 16:1680452. doi: 10.3389/fpls.2025.1680452
Received: 06 August 2025; Accepted: 06 November 2025; Revised: 04 November 2025;
Published: 26 November 2025.
Edited by:
Yi-Hong Wang, University of Louisiana at Lafayette, United StatesReviewed by:
Tejas Chimanlal Bosamia, Central Salt & Marine Chemicals Research Institute (CSIR), IndiaGuang Wu, Shaanxi Normal University, China
Copyright © 2025 Li, Peng, Liu, Wu, Dai, Qin and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi Qin, cWluemhpQHNobnUuZWR1LmNu; Meihong Sun, c3VubWVpaG9uZ0BzaG51LmVkdS5jbg==
 Gaojian Li
Gaojian Li Yulin Peng
Yulin Peng Shaojun Dai
Shaojun Dai Zhi Qin
Zhi Qin Meihong Sun
Meihong Sun