- Department of Soil Science and Plant Nutrition, Faculty of Agriculture, Selcuk University, Konya, Türkiye
Wheat is an important crop that often suffers from combined drought and salinity stress in agricultural fields, which adversely affects its growth, yield, and nutrient uptake. Understanding the response of genotypes to this combined stress is crucial to developing resilient cultivars. Nutrient uptake patterns in plants under stress not only reveal the physiological effects but also reflect their adaptive strategy and tolerance potential. Neglected and underutilized wheat species with high genetic diversity offer a valuable resource to explore different traits under combined stresses. Notably, no study has thoroughly assessed nutrient uptake in different hexaploid wheat species under combined drought and salinity stress. Thus, this study provides new insights into the individual and combined effects of drought and salinity stresses on nutrient uptake and accumulation of 30 hexaploid wheat genotypes of seven different species grown in a hydroponic system. The combined stress had a synergistic negative effect on nutrient accumulation in wheat genotypes as compared to single stresses. While species-based genetic variability was observed in individual stresses, a greater genotypic diversity was noticed under combined stress. A considerable genotypic variation ranging from 33.6% to 62.6% was observed in traits such as root-shoot phosphorus, manganese, zinc, as well as root copper, iron, and dry weight, while traits like shoot calcium, iron, potassium, and dry weight showed lower genotypic variation (8.3% to 25.8%). Among the studied genotypes, Tc4 (PI 164160, Kanak, India) was the best performing genotype across all three stress conditions, followed by Ta3 (CItr 17028, CAR 1101, Chile) and Tsh2 (PI 42013, India). The patterns of nutrient accumulation proposed that combined stress encompasses a complex interaction of multiple stress pathways. The results yielded valuable insights underscoring the significance of nutrient profiling as a critical component of breeding frameworks for climate-resilient wheat.
1 Introduction
In the face of climate change, multiple abiotic stresses such as drought, heat, and salinity occur simultaneously in agricultural regions that severely damage crop production and yield (Pandey et al., 2023; Jing et al., 2024). Shifting precipitation regimes, degradation of soil, rising water salinity, and elevated temperatures together intensify the co-occurrence of these stresses in major crop-growing areas. While plants behave specifically towards individual stresses, their responses can be more complex under combined stresses (Pandey et al., 2023). Two or more stresses can act synergistically, where the response can be more severe than the sum of the individual stresses, or antagonistically, where one stress can reduce the effect of the other (Suzuki et al., 2014; Rivero et al., 2022; Zandalinas and Mittler, 2022; Pandey et al., 2023). Since individual stresses trigger distinct signaling pathways that can be contradictory sometimes, their combination can develop novel responses that cannot be interpreted through individual stress-based studies (Zhang and Sonnewald, 2017; Zandalinas et al., 2020).
Drought and salinity are the two major individual abiotic stress conditions that often coexist together in arid and semiarid zones as combined drought and salinity stress. Individually, both drought and salinity reduce water uptake, hamper plant water relations and develop osmotic stress that further leads to a decrease in cell turgor and disrupts key physiological processes such as photosynthesis and stomatal conductance (Ors et al., 2021; Angon et al., 2022; Pandey et al., 2023; Khan et al., 2025). A decrease in photosynthetic pigments, gas exchange, and overall plant biomass, along with an increase in the production of reactive oxygen species and oxidative stress, has also been reported in both the stresses (Dugasa et al., 2019; Ibrahim et al., 2019; Begum et al., 2022). Drought stress restricts nutrient uptake by suppressing root development and reducing the efficiency of proteins involved in nutrient absorption (Hu and Schmidhalter, 2005; Bista et al., 2018; Cheraghi et al., 2023). When occur together with drought, salinity often enhances the stress by accumulating more sodium and chloride ions that compete with essential nutrients such as nitrogen, potassium, and calcium at the transport sites, and thus, impairing the nutrient uptake due to ionic imbalance (Hu and Schmidhalter, 2005; Alam et al., 2020; Ors et al., 2021; Pandey et al., 2023).
Under drought and salinity stress, plants stimulate their defense mechanisms such as closure of stomata, accumulation of osmolytes, and production of antioxidants that remain inadequate under combined stress due to disturbance in coordinated root-shoot signaling (Pierik and Testerink, 2014; Hussain et al., 2021; Angon et al., 2022). The combined drought and salinity stress has been reported to be more detrimental with decreased plant growth and physiological efficiency as compared to drought and salinity stress alone (Dugasa et al., 2019, 2021; Argentel-Martínez et al., 2024; Naqi et al., 2024). Despite the well-recognized damage due to combined drought and salinity stress, most studies focused on drought and salinity stress alone. Due to this gap, there is a limited understanding of how plants survive in actual agricultural fields, where they usually face more than one stress at a time. It is not clear that physiological and molecular responses that provide tolerance under individual drought or salinity stress will be enough to confer tolerance under combined stress conditions. Plants tolerant to individual drought and salinity stress may not be resilient to combined drought and salinity stress, emphasizing the need for screening wide germplasm under combined stress (Pandey et al., 2023).
Macronutrients (such as phosphorus, potassium, calcium, and magnesium) and micronutrients (such as iron, zinc, copper, and manganese) play an important role in plant growth and development (Gupta et al., 2024). While macronutrients are needed in larger amounts and are stable, participating in core physiological processes, micronutrients are needed in trace amounts and show greater plasticity under environmental fluctuations (Khan et al., 2022). The nutrient uptake, translocation, and accumulation can drastically change under abiotic stresses such as drought and salinity. These changes in nutrients not only show the effect of stresses in plants but also reflect their capacity to tolerate stress (Hu and Schmidhalter, 2005). Thus, nutrient accumulation profiles under stress conditions can be used as physiological markers for stress tolerance. However, changes in nutrient dynamics under combined drought and salinity stress have not been well explored, especially in wheat.
Wheat is one of the most important cereal crops largely affected by combined drought and salinity stress mostly in arid and semi-arid regions of the world (Dugasa et al., 2019; Fu et al., 2023; Pandey et al., 2023). Hence, there is an urgent need to screen potential wheat germplasm to identify combined drought and salinity-tolerant genotypes that can be grown in such regions and understand the possible mechanisms behind their tolerance. Neglected wheat species can serve as a potential source of tolerance alleles for combined drought and salinity stress (Ahmadi et al., 2020; Ali et al., 2023; Khan et al., 2025). Hence, it is necessary to utilize their inherent genetic diversity and adaptation capacity in marginal environments for crop improvement in breeding programs. However, there are limited studies investigating their performance against modern wheat cultivars under combined stresses. In fact, no study to date has thoroughly examined the effect of combined drought and salinity stress on nutrient accumulation of different hexaploid wheat species.
Thus, filling this research gap, the present study is the first study investigating the changes in nutrient uptake and accumulation of 30 hexaploid wheat genotypes belonging to seven different species under combined drought and salinity stress. Specifically, the study was conducted to answer the following research questions: 1. Does combined stress have a more pronounced effect on nutrient accumulation in wheat genotypes compared with individual drought and salinity stresses? 2. Are neglected/underutilized wheat species more tolerant to combined drought and salinity stress than modern wheat cultivars? 3. Is there any species-specific accumulation of any particular nutrient under combined drought and salinity stress? 4. Are roots more adversely affected than shoots under combined stress in terms of nutrient accumulation? 5. What is the level of genetic variability in nutrient accumulation in root-shoot tissues of hexaploid wheat genotypes under combined drought and salinity stress? The feedback to these questions can offer critical insights into the role of nutrient accumulation in conferring combined drought and salinity tolerance to hexaploid wheat genotypes, which is crucial for the development of resilient cultivars.
2 Materials and methods
2.1 Plant material
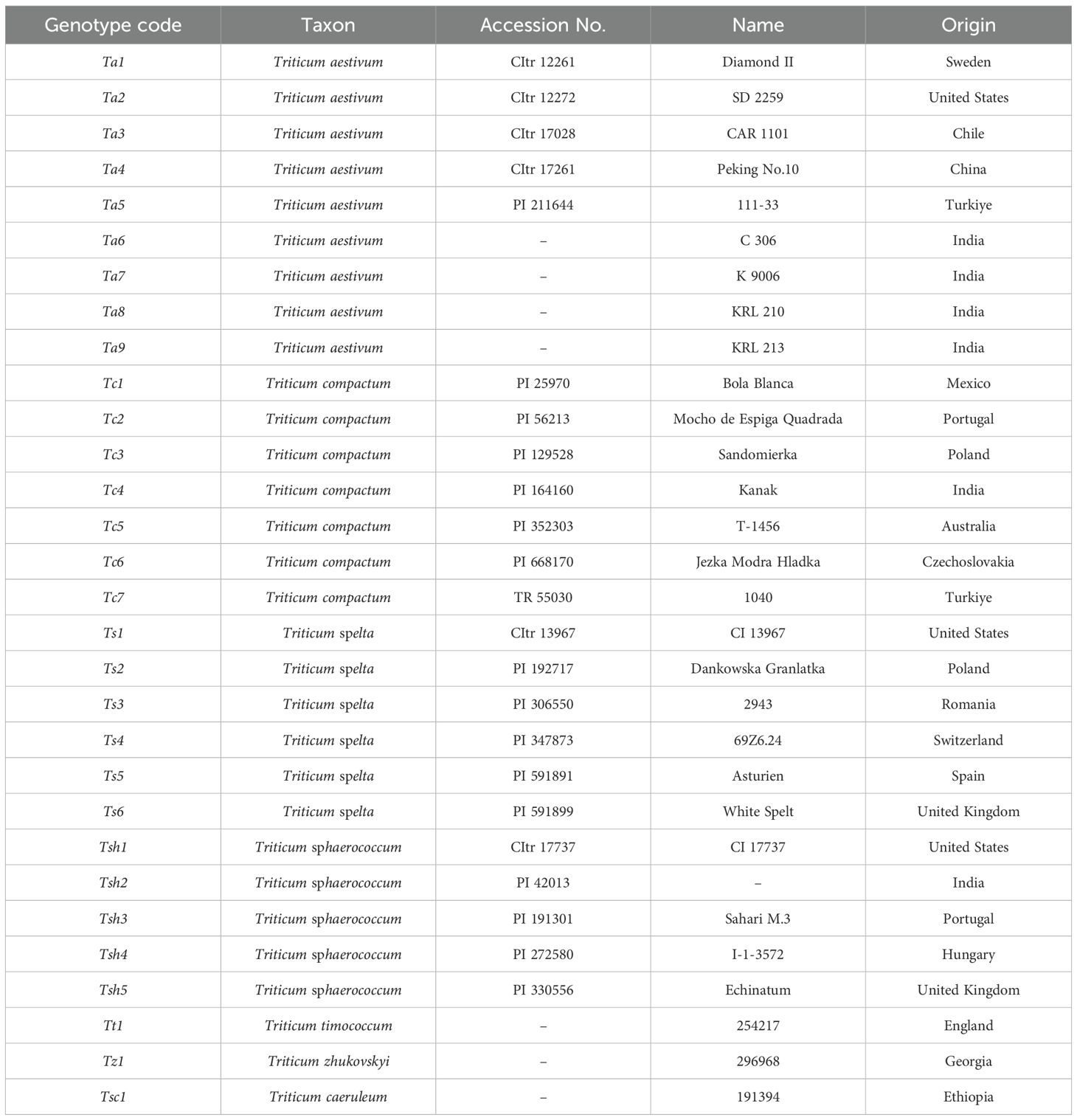
The experimental material consists of 30 hexaploid accessions of seven different species obtained from the National Small Grains Collection (NSGC), USA, and AARI National Gene Bank and Selcuk University, Turkiye. One of the genotypes, C-306, and three of the genotypes, K 9006, KRL-210, and KRL-213, are well-recognized drought and salinity-tolerant bread wheat cultivars, respectively. The details of the genotypes are provided in Table 1.
2.2 Experimental design and plant harvest
The experiment was conducted in a hydroponic chamber at Selcuk University, Turkiye, with 22 ± 1 °C temperature, 16000 Lx/day light intensity, 45-55% humidity, and 16 hours light and 8 hours dark photoperiod. Initially, for germination, seeds of each genotype were surface sterilized with sodium hypochlorite followed by 3–4 rinses with distilled water and kept in plastic trays in the dark for 4 days. Four-day-old healthy seedlings were transferred to sterile hydroponic pots containing half-strength Hoagland’s solution. After 4 days of growth in half-strength Hoagland’s solution, plants were subjected to Control (half strength Hoagland solution); Drought stress (15% (w/v) PEG 6000) (Sharma et al., 2022); Salinity stress (150 mM NaCl) (Uzair et al., 2022; Tang et al., 2024); and Combined Drought and Salinity stress [(15% (w/v) PEG 6000 and 150 mM NaCl)] treatments for 9 days and each treatment was replicated three times with five plants per replicate. The 9-day seedling stage treatment was chosen as this stage is commonly used for initial screening of tolerant germplasm. The results obtained may serve as a baseline for identifying promising genotypes and can be further evaluated at reproductive and grain-filling stages in future greenhouse and field studies. For salinity stress treatment, salt was added with 50 mM NaCl increment twice daily to make up to 150 mM NaCl salinity stress to avoid osmotic shocks. The nutrient solutions were changed every 2 days, and after 9 days of stress treatments, roots and shoots were harvested for biomass measurement and nutrient analysis.
2.3 Biomass measurement and element quantification
Roots and shoots of all the harvested plants were washed with 0.1 N HCl solution and deionized water, and excess water was collected on the blotting papers (Khan et al., 2022). This was followed by air-drying of root-shoot samples at 70 °C in a hot air oven for 72 hrs, and dry weights were estimated. Dried samples were crushed and 0.20-0.30 g of powdered samples were dissolved in 5 ml of 65% HNO3 and 2 ml of 35% H2O2 and digested in a closed microwave accelerating reaction system (Cem Marsxpress, Matthews, NC, USA) succeeded by the estimation of nutrient amount in the stock solution using ICP-OES (Varian, Vista, Palo Alto, CA, USA). The measurement of the elemental concentration was checked by the certified values of the elements in the reference material provided by the National Institute of Standards and Technology (NIST, Gaithersburg, MD, USA).
2.4 Statistical analysis
From the obtained ICP-OES data, initially accumulated nutrient content was calculated using the following formula, and the variation in the accumulated content of different nutrients along with biomass under different treatments has been depicted as Bar diagrams.
In statistical analysis, the obtained biomass and accumulated nutrient data were subjected to two-way analysis of variance (ANOVA) using the Graphpad Prism 9.0 program, where treatments and genotypes were the two factors and the trait observed was the response. The role of genotypes (G) and treatments (T) in the variability in the expression of traits was considered to be highly significant for the values with P < 0.001. The mean differences among the treatments as compared to the Control was calculated for different traits using Dunnett’s multiple comparisons test, and the comparisons with P < 0.001 were considered to be significantly different. The percentage changes in nutrient accumulation under drought, salinity, and combined drought and salinity stress treatments as compared to the Control treatment were calculated using MS Excel 2010. Minitab (version 16, State College, PA, USA) program-based Pearson correlation analysis was used to determine the association between relative changes in nutrient levels and biomass, considering p < 0.05 to be significant correlation. Heatmaps based on Euclidean distance matrix generated from the average linkage method were drawn to understand the clustering of the genotypes based on the relative changes in their nutrient content and biomass under drought, salinity, and combined drought and salinity stress treatments as compared to the Control.
3 Results
3.1 Analysis of variance
The analysis of variance (ANOVA) revealed a significantly high genotypic variation (ranging from 33.6% to 62.6%) in traits such as root-shoot phosphorus, root-shoot manganese, root copper, root iron, root-shoot zinc, and root dry weight (Table 2). In contrast, shoot calcium, root sodium, shoot iron, root-shoot potassium, and shoot dry weight showed significant but lower genotypic variation (8.3% to 25.8%) (Table 2). These observations indicate considerable genetic diversity among the studied genotypes in their nutrient uptake and accumulation capacity under variable growth conditions. In addition to genotypic effects, treatments significantly affected all the studied traits, with the greatest effect on root-shoot sodium content (Table 2).
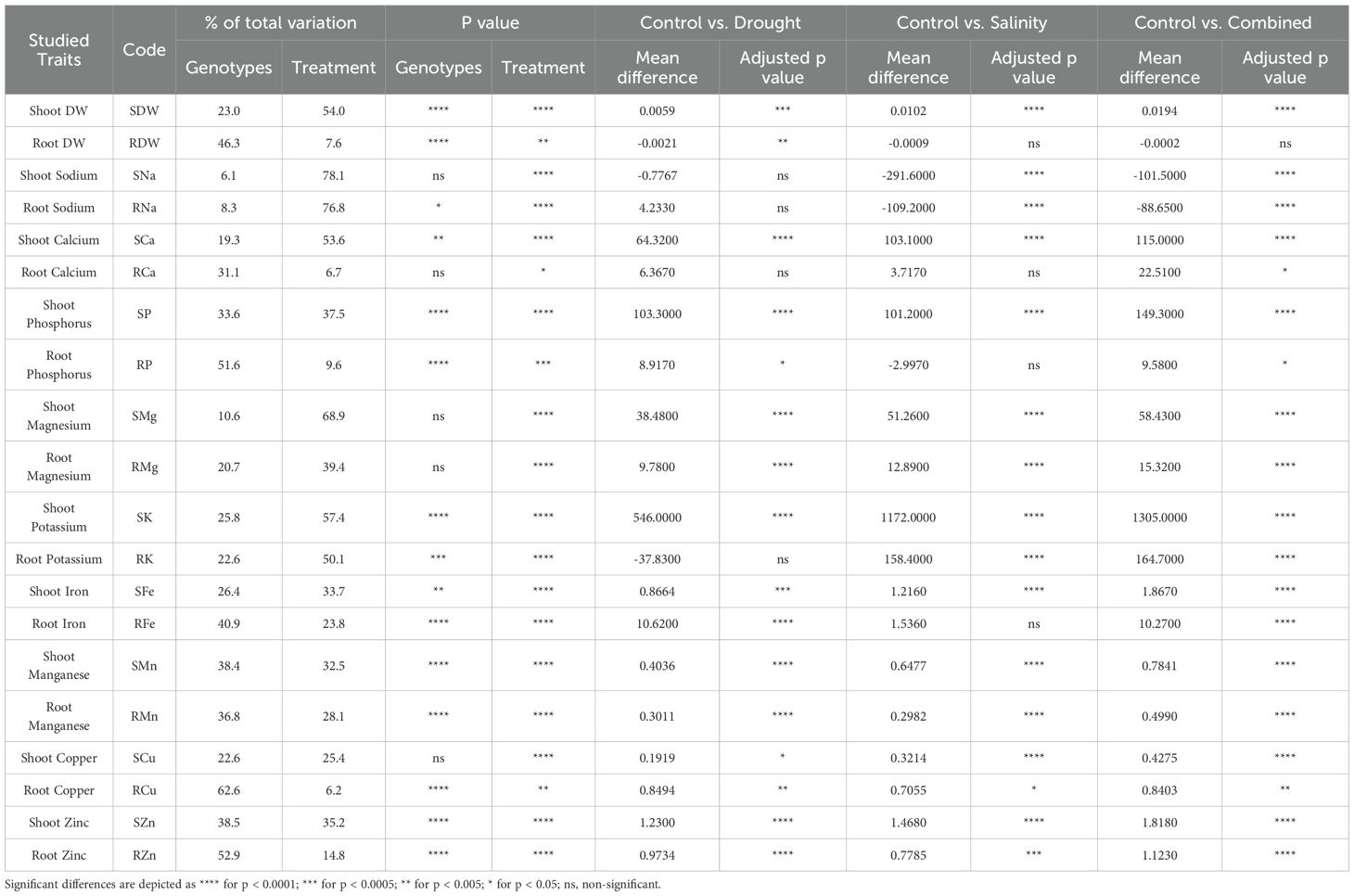
Table 2. Results of the two-way analysis of variance (ANOVA) for all the evaluated parameters in shoots and roots of 30 hexaploid wheat genotypes grown under Control, Drought stress, Salinity stress, and Combined Drought and Salinity stress treatments.
3.2 Growth
For shoot growth, all three stresses, drought, salinity, and combined drought and salinity stress, significantly affected the dry weights as compared to the Control treatment (Table 2). Drought and salinity stress, individually or in combination, had an overall negative effect on shoot dry weights (Table 3, Supplementary Table S1, Supplementary Figure S1). At an individual level, the effects of drought stress were similar to those of salinity stress (Table 3). However, the shoot growth inhibition was more severe in combined drought and salinity stress as compared to drought and salinity stress alone (Table 3, Supplementary Table S1). Though most of the genotypes showed severe reductions under combined drought and salinity stress as compared to the Control, a significant increase was observed in the Tc4 genotype (+6%), and two of the genotypes, Ts3 and Tsh3, showed moderate reduction (< 30%) (Table 3). Under drought stress alone, Tc4 showed the highest increase of 62% in SDW, followed by Ts6 and Tsh5. Similar to the other two stress types, in salinity stress as well, Tc4 was the top-performing genotype with a 13% increase in SDW, succeeded by Tc3 and Tsh2.
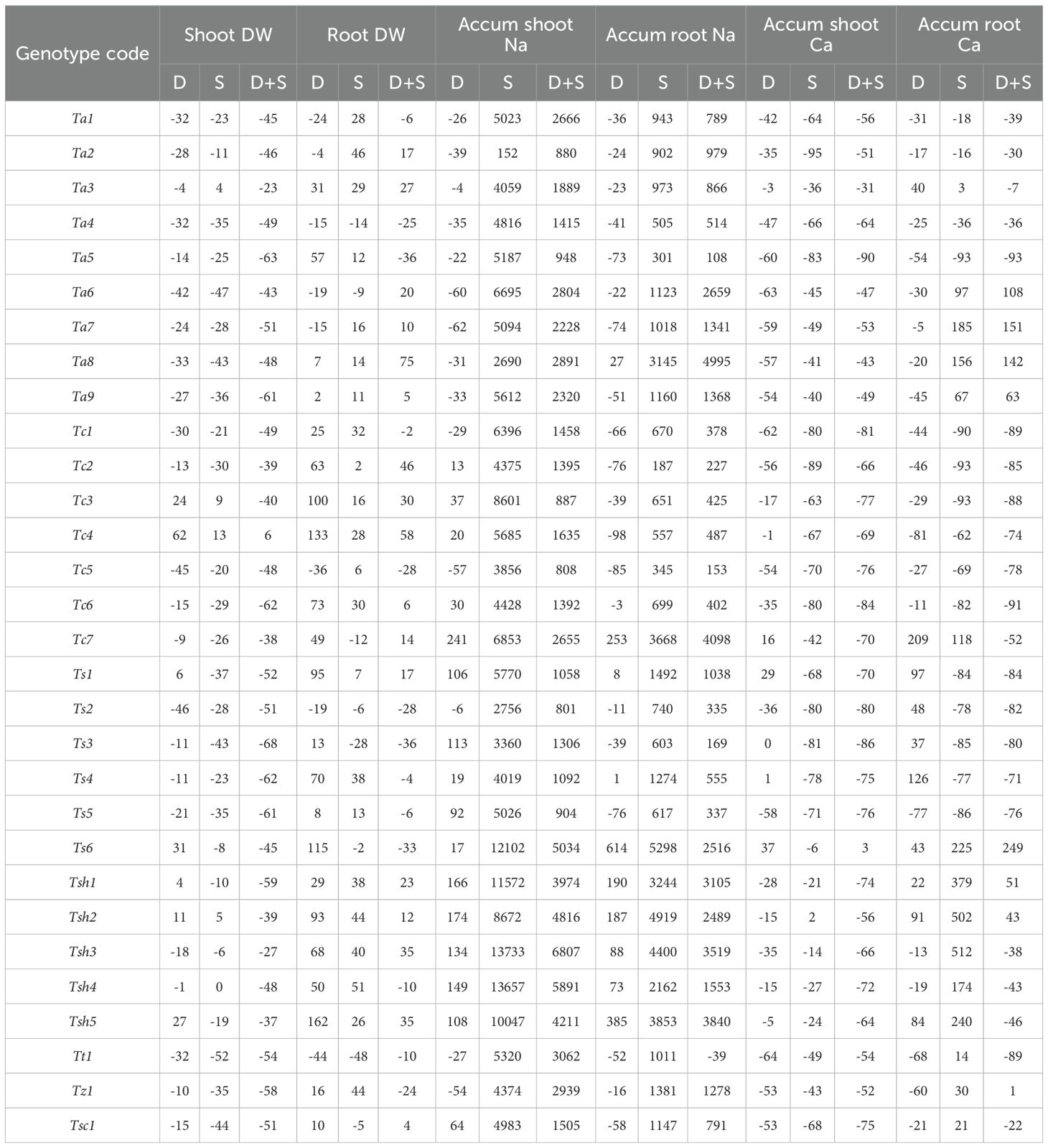
Table 3. The relative changes (in percentage) in the shoot and root biomass and accumulation of sodium (Na), and calcium (Ca) in 30 hexaploid wheat genotypes in drought (D), salinity (S) and combined drought and salinity (D+S) stress treatments as compared to Control conditions.
In case of root growth, only drought stress had a significant effect on dry weights, while salinity and combined stress did not affect them significantly (Table 2). In drought stress, Tsh5 and Tc4 showed the highest increase of 162% and 133% respectively, in root dry weights (RDW) as compared to Control (Table 3, Supplementary Figure S2). Additionally, concerning dry weights, shoots were more affected than roots, either by individual drought and salinity stress or combined drought and salinity stress (Table 3).
3.3 Sodium uptake and accumulation
Compared with the Control treatment, drought stress did not significantly affect the root and shoot sodium content, while salinity stress and combined stress significantly affected them (Table 2). As expected, the sodium content of all the genotypes increased under salinity stress and combined stress as compared to the Control due to the external supply of 150 mM NaCl (Table 3, Supplementary Table S1, Supplementary Figure S3). There was a great variation in sodium accumulation in shoots and roots under both salinity and combined stress.
In shoots, salinity stress led to a maximum and minimum increase of more than 13000% and 152% in the sodium content of Tsh3 and Ta2, respectively. However, combined stress showed a maximum and minimum increase of more than 6000% and 800% in Tsh3 and Ta2, respectively (Table 3). Interestingly, other than Ta2 and Ta8, for all the genotypes combined stress had a diminishing effect on sodium accumulation as compared to salinity stress alone. Similarly, in roots, while salinity stress led to a maximum and minimum increase of more than 5000% in Ts6 and 187% in Tc2, respectively, combined stress showed a maximum increase of more than 4000% in Tc7 and a minimum decrease 39% in Tt1, respectively, in the sodium content (Table 3, Supplementary Figure S4). Similar to shoots, in roots also, combined stress had a diminishing effect on sodium uptake as compared to salinity stress for most of the genotypes (Table 3, Supplementary Table S1).
3.4 Calcium uptake and accumulation
In case of shoots, all three stresses, drought, salinity, and combined drought and salinity stress significantly affected the calcium content as compared to the Control treatment (Table 2). Other than a few genotypes, drought and salinity stress individually or in combination had an overall negative effect on calcium content (Table 3, Supplementary Table S1). Although it was a mixed genotype-dependent response, salinity and combined stress seem to have a more negative effect on calcium accumulation than drought stress (Supplementary Figure S5). Though most of the genotypes showed severe reductions under combined stress as compared to the Control, genotype Ts6 showed an increase of 3% and genotype Ta3 showed a moderate reduction of 30% (Table 3, Supplementary Table S1). Under drought stress alone, Ts6 showed the highest increase of 37% in calcium content, followed by Ts1 and Tc7. In salinity stress, only one of the studied genotypes, Tsh2, showed an increase of 2% in calcium content as compared to Control; all the other genotypes showed a reduction (Table 3, Supplementary Table S1).
In the case of roots, only combined stress had a significant effect on calcium uptake, while drought and salinity stress did not affect them significantly. In combined stress, Ts6 and Ta7 showed the highest increase of 249% and 151% respectively, in calcium uptake as compared to Control (Table 3, Supplementary Table S1, Supplementary Figure S6). Additionally, concerning calcium content, shoots were overall more affected than roots by combined stress.
3.5 Phosphorus uptake and accumulation
In case of shoot, all three stresses, drought, salinity, and combined stress significantly affected the phosphorus content as compared to the Control treatment (Table 2). Drought and salinity stress, individually or in combination, had an overall negative effect on phosphorus accumulation in shoots (Table 4, Supplementary Table S2). Although it was a mixed genotype-dependent response, combined stress seems to have a more negative effect on phosphorus accumulation than drought and salinity stresses (Supplementary Figure S7). Though most of the genotypes showed severe reductions under combined stress as compared to the Control, a significant increase was observed in the Tc4 genotype (+48%), and genotype Tc5 showed a moderate reduction (< 30%) in phosphorus (Table 4). Under drought stress alone, Tc4 and Ts1 showed the greatest increase of 72% and 8% in phosphorus content. Similar to the other two stress types, in salinity stress as well, Tc4 was the top performing genotype with 48% increase in phosphorus accumulation, followed by Ta3 and Tc5 (Table 4).
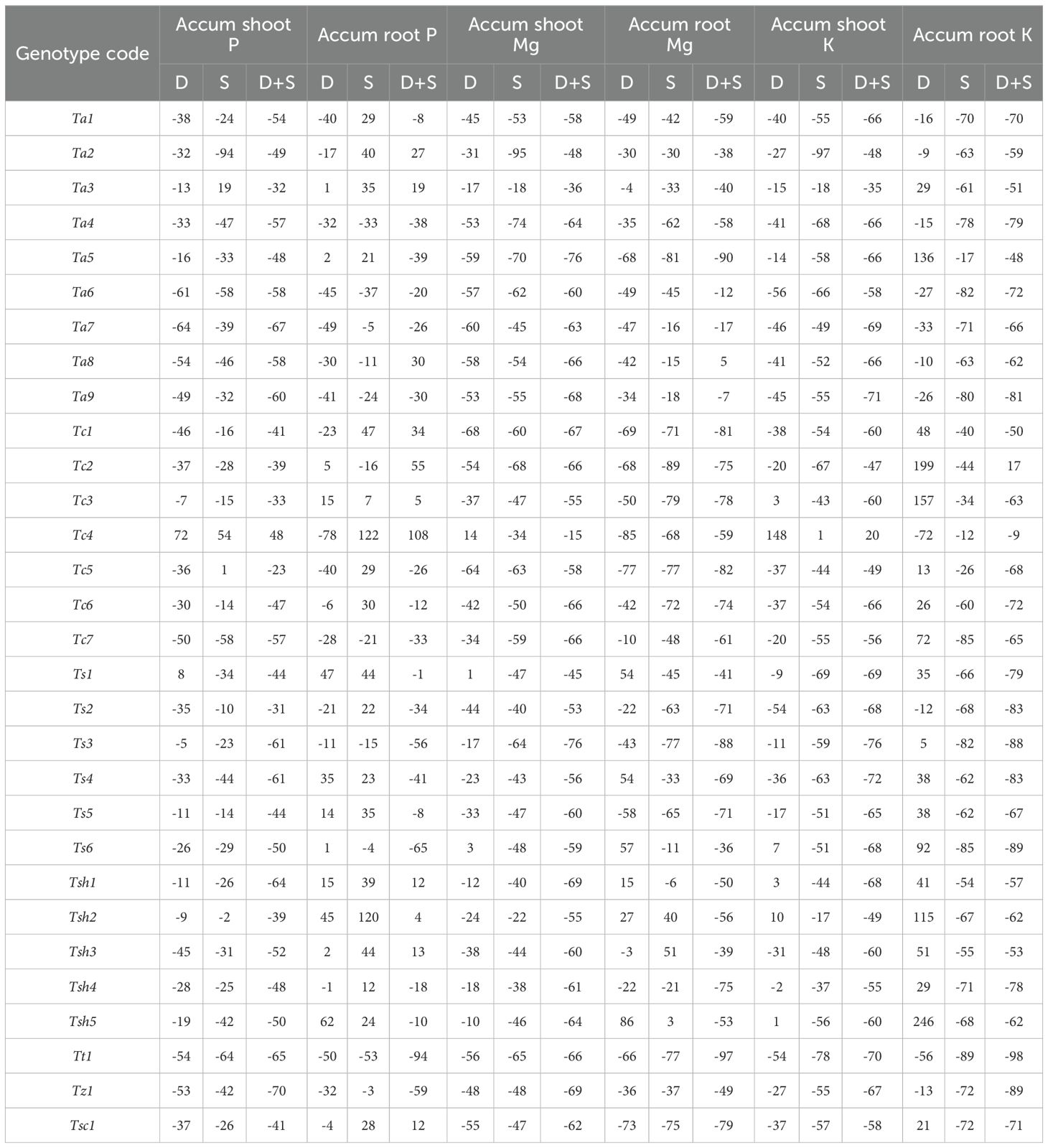
Table 4. The relative changes (in percentage) in the shoot and root accumulation of phosphorus (P), magnesium (Mg), and potassium (K) in 30 hexaploid wheat genotypes in drought (D), salinity (S) and combined drought and salinity (D+S) stress treatments as compared to Control conditions.
In the case of roots, drought and combined stress had a significant effect on phosphorus uptake, while salinity stress did not affect them significantly. The effect was genotype dependent and not overall positive or negative under both drought and combined stress conditions (Table 4, Supplementary Table S2, Supplementary Figure S8). While in drought stress, Tsh5 and Ts1 showed the highest increase of 62% and 47% respectively, as compared to Control, in combined stress, Tc4 and Tc2 were the best performing genotypes with 108% and 55% increases in phosphorus accumulation, respectively (Table 4). Additionally, concerning phosphorus content, shoots were more affected than roots by combined stress (Table 4).
3.6 Magnesium uptake and accumulation
Compared with the Control treatment, all three stresses, drought, salinity, and combined stress, significantly affected the root and shoot magnesium content (Table 2). Drought and salinity stress, individually or in combination, had an overall negative effect on magnesium accumulation in both roots and shoots (Table 4, Supplementary Table S2). Combined stress and salinity stress seem to have a more negative effect on magnesium accumulation than drought stress (Supplementary Figure S9). In shoots, combined stress had a diminishing effect on magnesium accumulation of all the genotypes, with minimum decrease of 15% in Tc4, followed by Ta3. While drought stress led to the greatest increase of 14% in Tc4 and 1% in Ts1, respectively, salinity stress showed a minimum decrease of 18% in Ta3 followed by Tsh2 (Table 4, Supplementary Figure S10). Similarly, in roots, combined stress had diminishing effects on the magnesium content of all the genotypes except Ta8, which showed an increase of 5% (Table 4). While drought stress led to the greatest increase of 86% and 57% in Tsh5 and Ts6, respectively, salinity stress showed an increase of 51% and 40% in genotypes Tsh3 and Tsh2, respectively (Table 4). Additionally, concerning magnesium content, shoots were more affected than roots by combined drought and salinity stress (Table 4).
3.7 Potassium uptake and accumulation
In case of shoot, all three stresses, drought, salinity, and combined stress significantly affected the potassium content as compared to the Control treatment (Table 2). Drought and salinity stress, individually or in combination, had an overall negative effect on potassium accumulation in shoots (Table 4, Supplementary Table S2). Although it was a mixed genotype-dependent response, combined stress seems to have a more negative effect on potassium accumulation than drought and salinity stresses (Supplementary Figure S11). Under salinity and combined stress as compared to Control, all the genotypes showed severe reductions, except one genotype, Tc4, that showed an increase of 1% and 20%, respectively (Table 4). Under drought stress alone, Tc4 and Ts1 showed the greatest increase of 72% and 8% in phosphorus content. Similar to the other two stress types, in drought stress as well, Tc4 showed the greatest increase of 148% followed by Tsh2 and Ts6 (Table 4).
In the case of roots, salinity and combined stress had a significant effect on potassium accumulation, while salinity stress did not affect them significantly. While Tc2 and Tc4 showed the least suppressive effect of combined stress, under salinity stress, the potassium content of Tc4 and Ta5 was the least affected (Table 4, Supplementary Table S2, Supplementary Figure S12). Contrary to other macronutrients, the potassium content of roots was more decreased than in shoots under combined stress as compared to the Control treatment.
3.8 Uptake and accumulation of micronutrients
The iron accumulation in shoots is significantly affected by all three stresses, drought, salinity, and combined stress, while in roots, it is significantly affected by drought and combined stress only (Table 2). Although it was a mixed geno-type-dependent response, combined stress seems to have a more negative effect on iron accumulation than drought and salinity stresses (Supplementary Figure S13). In both roots and shoots, combined stress has overall reduced the iron accumulation (Supplementary Figure S13, Supplementary Figure S14). In shoots, Tsh5 and Ts6 were the genotypes with a minimum decrease in iron accumulation under combined stress as compared to Control (Table 5, Supplementary Table S3). However, in roots, Tsc1 showed an increase of 27% followed by Ta2, which showed a decrease of only 1% under combined drought and salinity stress (Table 5). Under drought stress alone, Ts6 and Ts4 were the genotypes with maximum iron accumulation in shoots and roots, respectively (Table 5).
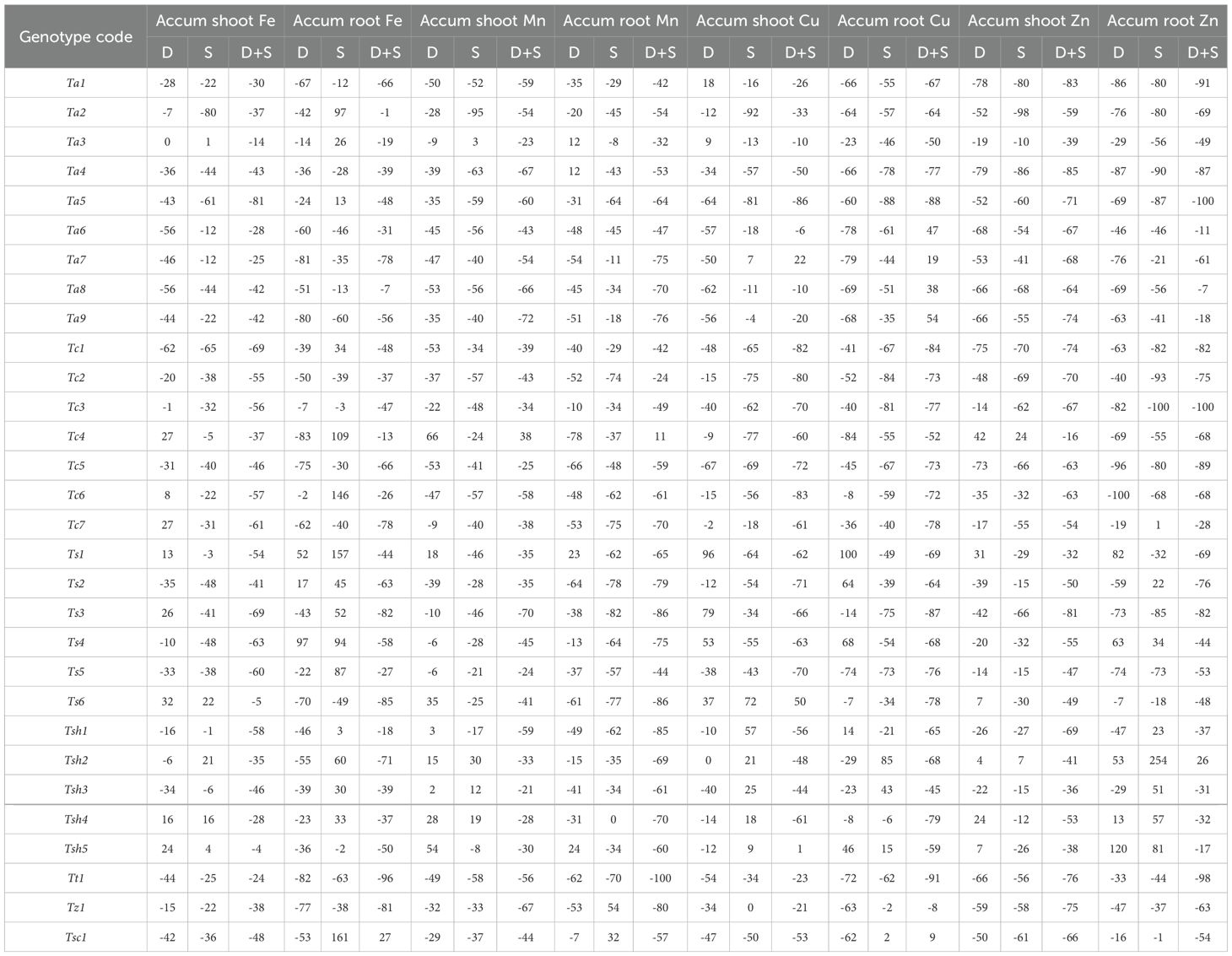
Table 5. The relative changes (in percentage) in the shoot and root accumulation of iron (Fe), manganese (Mn), copper (Cu) and zinc (Zn) in 30 hexaploid wheat genotypes in drought (D), salinity (S) and combined drought and salinity (D+S) stress treatments as compared to Control conditions.
Compared with the Control treatment, all three stresses, drought, salinity, and combined drought and salinity, significantly affected the manganese accumulation in both roots and shoots (Table 2). Under drought and salinity stress, some of the genotypes showed increased manganese accumulation in both roots and shoots (Supplementary Figure S15, Supplementary Figure S16). However, under combined drought and salinity stress, reduced accumulation was observed in all the genotypes but one, Tc4 which showed 38% and 11% increase in shoots and roots, respectively (Table 5, Supplementary Table S3).
The copper accumulation in both roots and shoots is significantly affected by all three stresses, drought, salinity, and combined stress (Table 2). Although it was a mixed genotype-dependent response, combined stress seems to have a more negative effect than drought and salinity stresses on copper accumulation (Supplementary Figures S17, S18). In shoots, Ts6 and Ta7 were the genotypes with a maximum increase of 50% and 22% in copper accumulation under combined stress as compared to the Control (Table 5, Supplementary Table S4). However, in roots, Ta9 and Ta6 showed an increase of 54% and 47% respectively, under combined drought and salinity stress. Under drought stress alone, genotype Ts1 showed the maximum copper accumulation (96% in shoots and 100% in roots), while in salinity stress, genotypes Ts6 and Tsh2 were the ones with maximum shoot and root content (Table 5, Supplementary Table S4).
Compared with the Control treatment, all three stresses, drought, salinity, and combined significantly affected the zinc accumulation in both roots and shoots. Under combined stress as compared to Control, roots and shoots of all the genotypes showed severe reductions except one genotype, Tsh2, which showed an increase of 26% in roots (Table 5, Supplementary Table S4). Interestingly, in shoots, genotype Tc4 had the least effect of drought, salinity, and combined stresses on zinc accumulation. In addition, some of the genotypes, such as Tsh2 and Tsh5, were among the ones with the greatest zinc accumulation in both roots and shoots (Table 5, Supplementary Figures S19, S20).
3.9 Association between accumulated nutrients and dry weights of studied genotypes under combined drought and salinity stress
The association between relative nutrient accumulation under combined drought and salinity stress, as compared to the Control treatment, was estimated using correlation analysis (Table 6). Interestingly, except for magnesium and iron, relative changes of all the other nutrients in shoots were significantly correlated to their relative changes in roots under combined drought and salinity stress (Table 6).
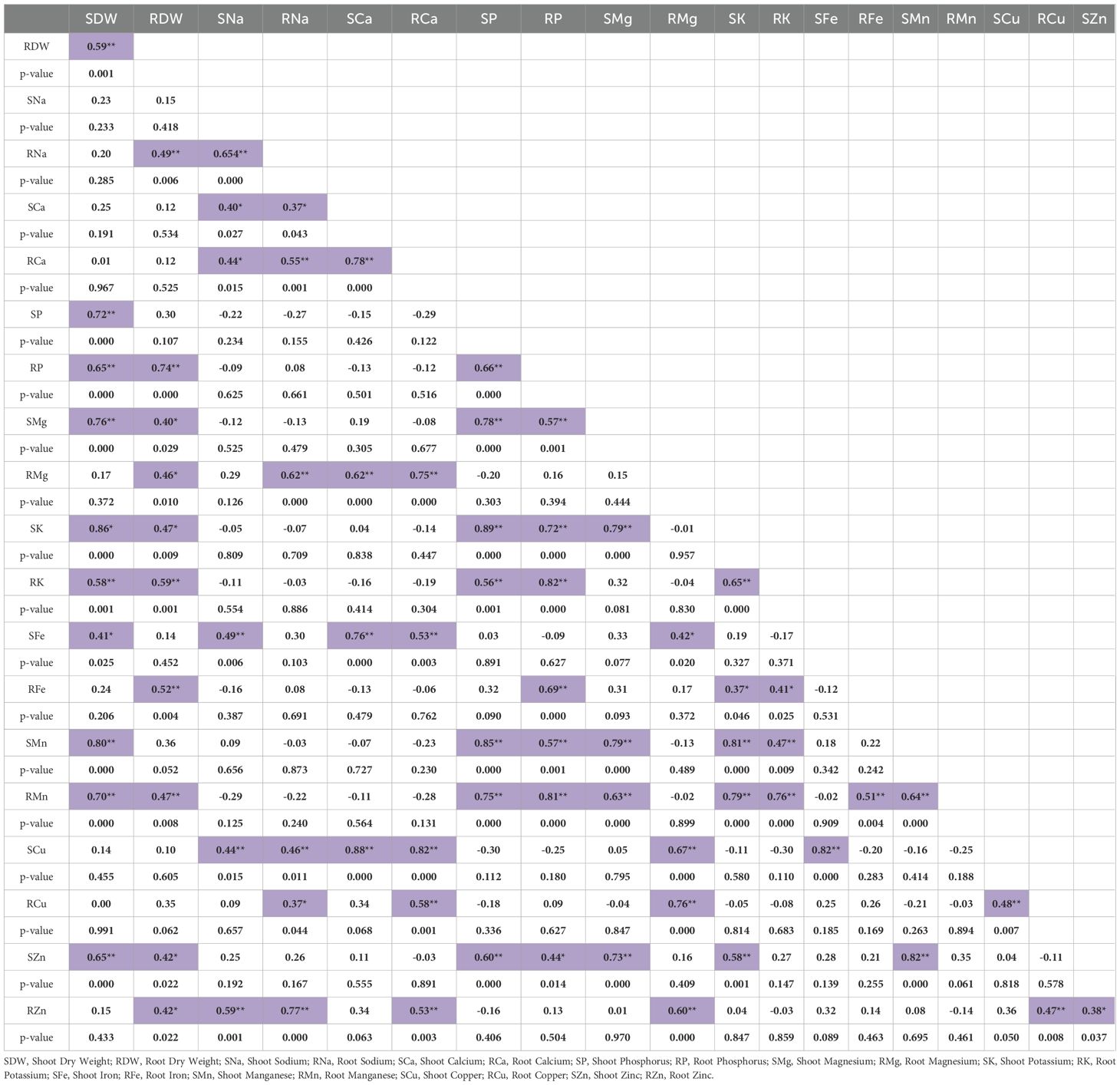
Table 6. Association between relative accumulated nutrients and dry weights of 30 hexaploid wheat genotypes under combined drought and salinity stress (p < 0.05 was considered to be significant correlation; purple marked columns showed the significant correlation between the corresponding traits).
The relative changes in root-shoot phosphorus, root-shoot potassium, root-shoot manganese, shoot magnesium, and shoot zinc content were found to be significantly associated with the relative change in shoot dry weight. Similarly, relative change in root dry weight was significantly correlated to those of root-shoot magnesium, root-shoot potassium, root-shoot zinc, root sodium, root phosphorus, root iron, and root manganese content (Table 6). A strong significant association was found between the shoot sodium content and root-shoot calcium, shoot iron, shoot copper, and root zinc. Similarly, root sodium was significantly related to root-shoot calcium, root magnesium, root-shoot copper, and root zinc (Table 6). While shoot calcium was significantly associated relative change in root magnesium, shoot iron, and shoot copper, root calcium was connected to root magnesium, shoot iron, root-shoot copper, and root zinc. Both root and shoot phosphorus were strongly associated with shoot magnesium, root-shoot potassium, root-shoot manganese, and shoot zinc content under combined drought and salinity stress (Table 6). In shoots, magnesium showed a significant positive correlation with shoot potassium, shoot zinc, and root-shoot manganese. However, in roots, it was associated with root-shoot copper, shoot iron, and root zinc. Similarly, root-shoot potassium was closely related to root-shoot magnesium and root iron content (Table 6).
3.10 Clustering of genotypes based on the relative changes in their nutrient content under drought, salinity, and combined drought and salinity stresses as compared to the control treatment
The generated heatmaps for all the three stress conditions clustered the genotypes on the basis of column Z-scores of the measured traits. While the red color indicates relatively low trait values, green color shows relatively high trait values. It means green patches suggest comparatively higher values for the associated traits (either dry weights or nutrient uptake) reflecting greater tolerance or adaptive responses and red patches suggest comparatively lower values for the associated traits reflecting greater sensitivity towards stress condition. The genotypes with more number of green columns especially for shoots were more tolerant to that particular stress condition.
On comparing heat maps of three stress conditions, it can be observed that in the drought stress heatmap, two clear patches of green and red colors can be seen (Figure 1). The green color patch include genotypes Tc4, Tc7, Ts1, Ts4, Ts6, Tsh1, Tsh2, Tsh3, Tsh4, and Tsh5, while the red color patch includes genotypes Tt1, Tz1, Ta1, Ta2, Ta4, Ta6, Ta7, Ta8, Ta9, Tc1, and Tc5 (Figure 1). Similarly, in the salinity stress heatmap, the green color patch includes genotypes Tsh1, Tsh2, Tsh3, Tsh4, Tsh5, and Ts6, while the red color patch includes all the remaining genotypes except Ta3 and Tc4 (Figure 2). However, in the combined drought and salinity stress heatmap, no clear patch of red or green color revealing any clear grouping based on the studied parameters is present (Figure 3).
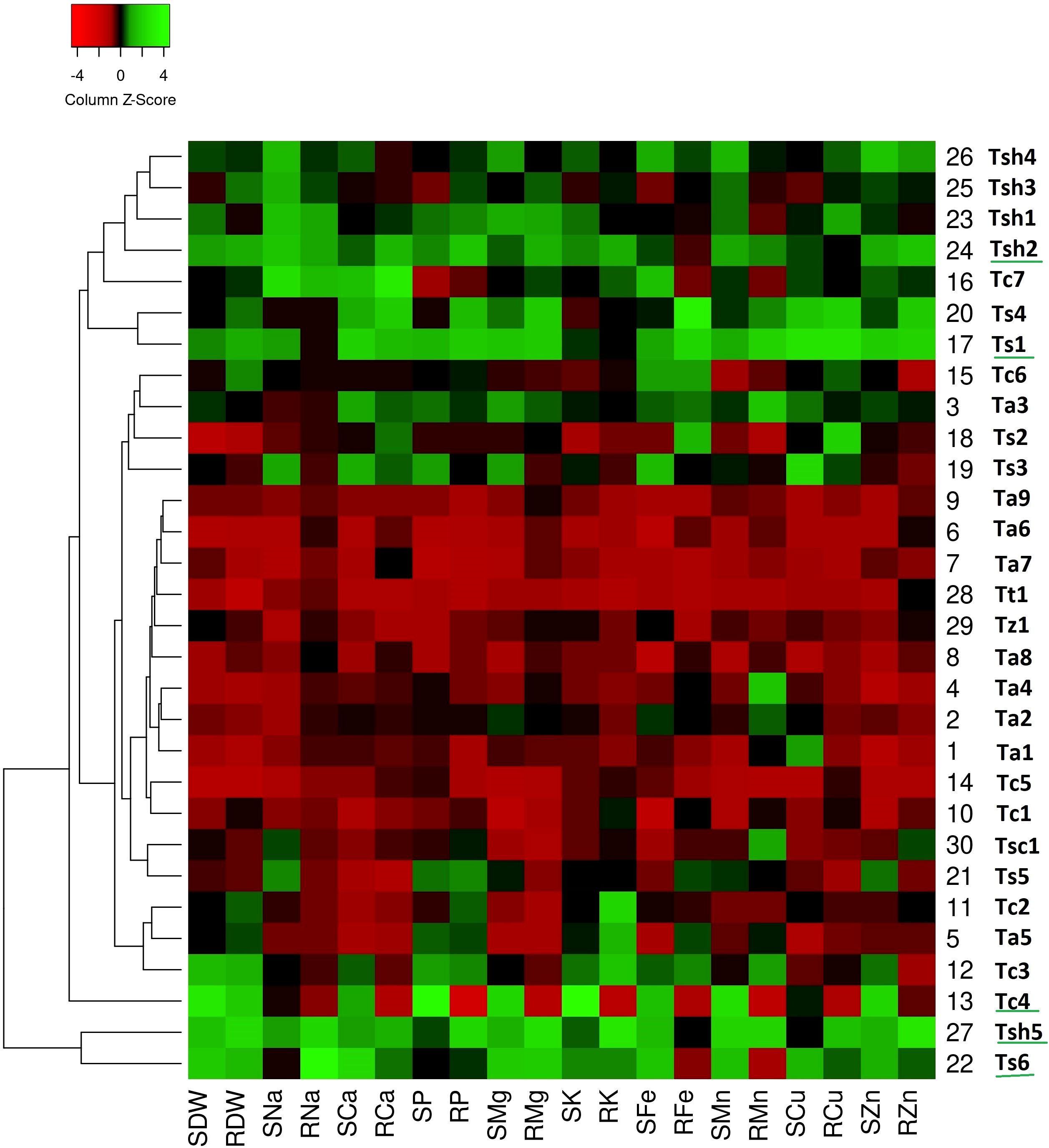
Figure 1. Genotype clustering based on the relative changes in their nutrient content under drought stress as compared to control treatment. [SDW - Shoot Dry Weight; RDW - Root Dry Weight; SNa - Shoot Sodium; RNa - Root Sodium; SCa - Shoot Calcium; RCa - Root Calcium; SP - Shoot Phosphorus; RP - Root Phosphorus; SMg - Shoot Magnesium; RMg - Root Magnesium; SK - Shoot Potassium; RK - Root Potassium; SFe - Shoot Iron; RFe - Root Iron; SMn - Shoot Manganese; RMn - Root Manganese; SCu - Shoot Copper; RCu -Root Copper; SZn - Shoot Zinc; RZn - Root Zinc].
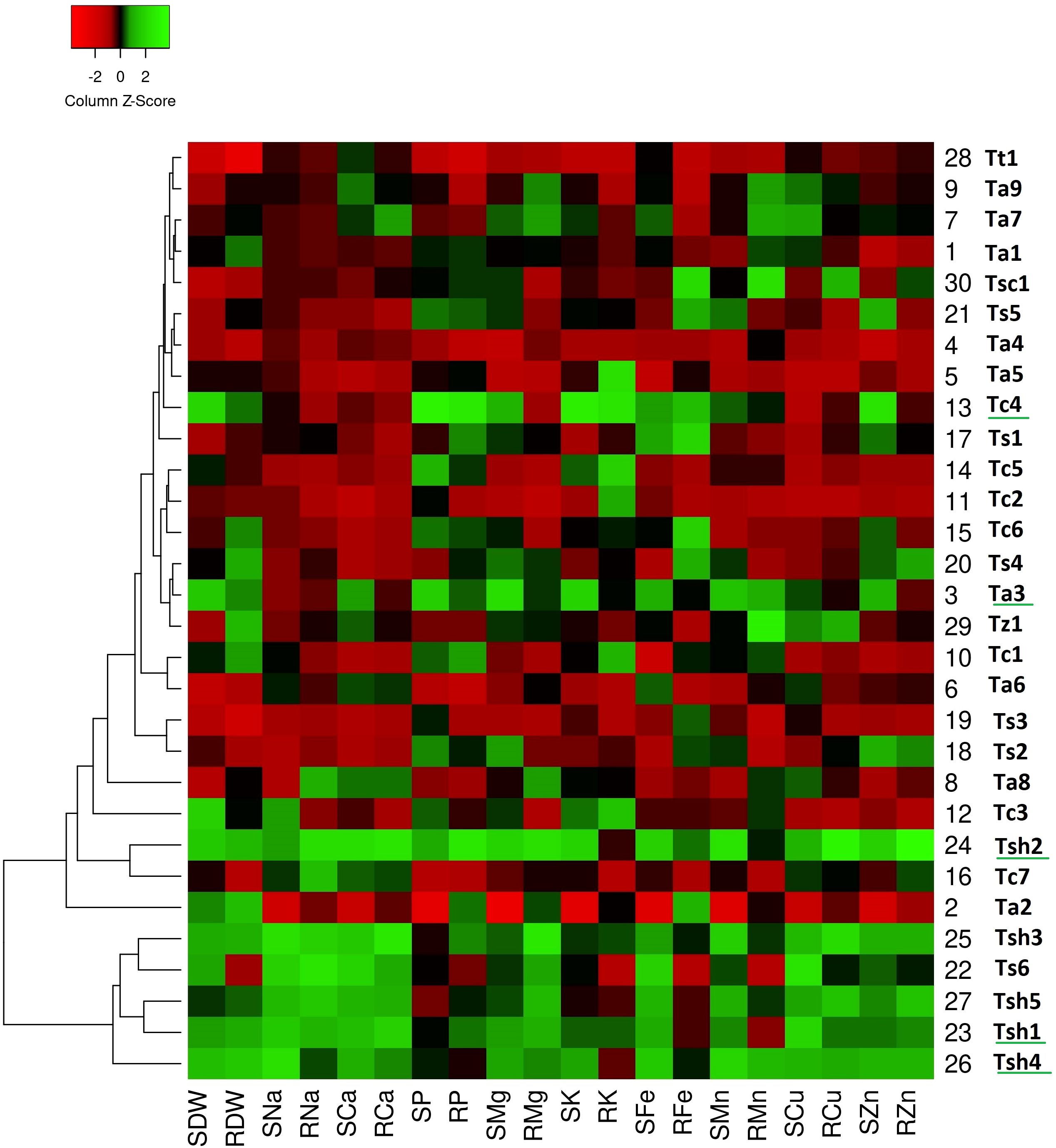
Figure 2. Genotype clustering based on the relative changes in their nutrient content under salinity stress as compared to control treatment. [SDW - Shoot Dry Weight; RDW - Root Dry Weight; SNa - Shoot Sodium; RNa - Root Sodium; SCa - Shoot Calcium; RCa - Root Calcium; SP - Shoot Phosphorus; RP - Root Phosphorus; SMg - Shoot Magnesium; RMg - Root Magnesium; SK - Shoot Potassium; RK - Root Potassium; SFe - Shoot Iron; RFe - Root Iron; SMn - Shoot Manganese; RMn - Root Manganese; SCu - Shoot Copper; RCu -Root Copper; SZn - Shoot Zinc; RZn - Root Zinc].
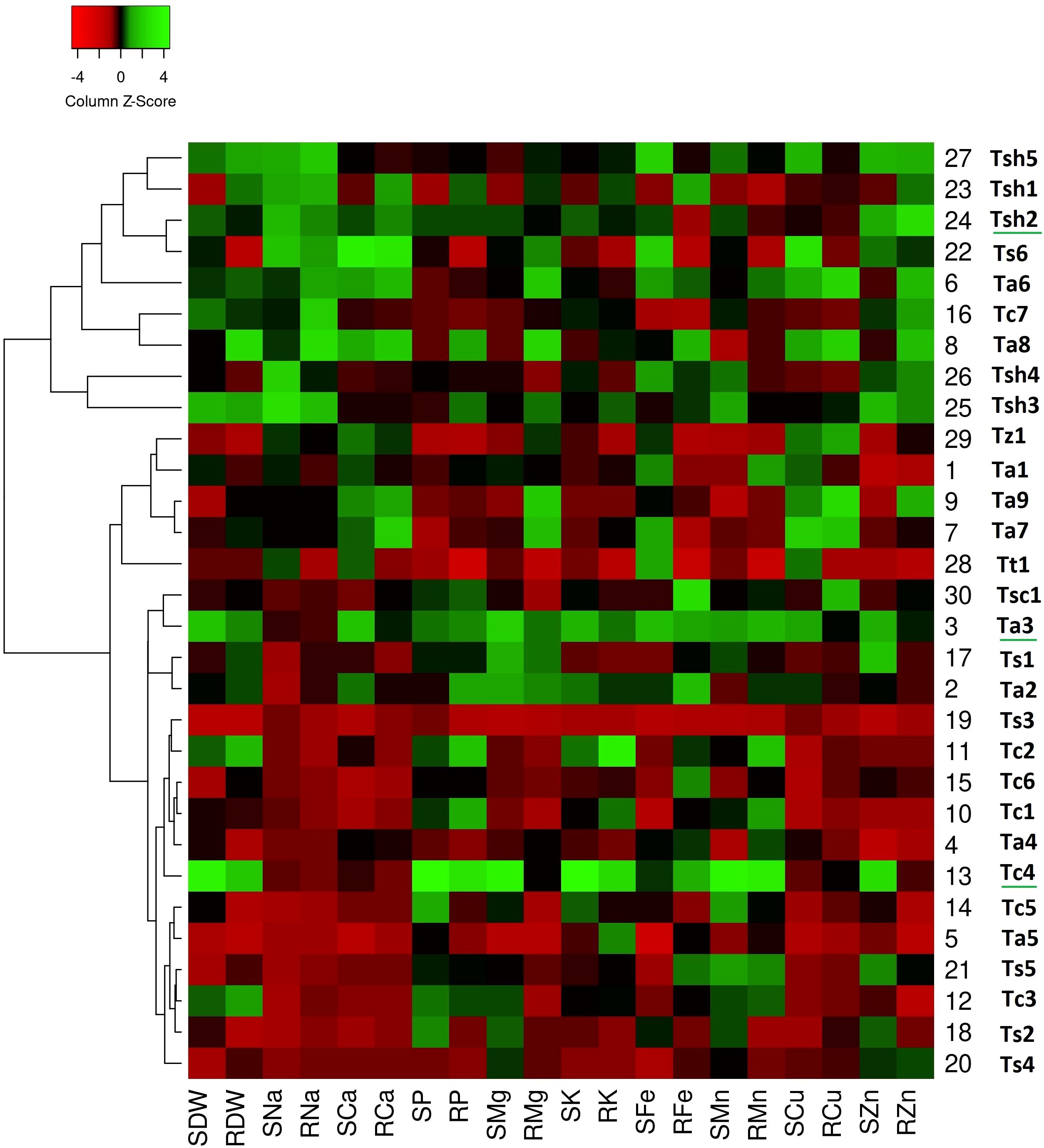
Figure 3. Genotype clustering based on the relative changes in their nutrient content under combined drought and salinity stress as compared to control treatment. [SDW - Shoot Dry Weight; RDW - Root Dry Weight; SNa - Shoot Sodium; RNa - Root Sodium; SCa - Shoot Calcium; RCa - Root Calcium; SP - Shoot Phosphorus; RP - Root Phosphorus; SMg - Shoot Magnesium; RMg - Root Magnesium; SK - Shoot Potassium; RK - Root Potassium; SFe - Shoot Iron; RFe - Root Iron; SMn - Shoot Manganese; RMn - Root Manganese; SCu - Shoot Copper; RCu -Root Copper; SZn - Shoot Zinc; RZn - Root Zinc].
In the combined drought and salinity stress heatmap (Figure 3), all the genotypes were grouped into two main clusters, Cluster A and Cluster B, based on the relative changes in their nutrient content under combined stress as compared to Control. While Cluster A consists of nine genotypes, twenty-one genotypes are present in Cluster B. All five T. sphaerococcum genotypes are closely grouped in Cluster A, showing a unique high nutrient and medium biomass pattern. T. sphaerococcum genotypes are the ones with consistently higher micronutrient bioaccumulation. Similarly, except Tc7, all six T. compactum genotypes were clustered in a close subgroup of Cluster B. Among these six genotypes, except Tc4, all the other showed low nutrient and medium biomass.
In a similar way, except for Ts6, all the T. spelta genotypes are present in Cluster B. The T. aestivum genotypes are present in both clusters, with the two T. aestivum genotypes of Cluster A showing high-low nutrient and low biomass. While all the aestivum genotypes of Cluster B showed low nutrient and low biomass, Ta3 showed high nutrient and high biomass.
According to the drought stress heatmap, genotypes Tc4, Ts1, Ts6, Tsh2, and Tsh5 seem to be the top performing genotypes with high biomass and high nutrient accumulation. In the salinity stress heatmap, genotypes Ta3, Tc4, Tsh1, Tsh2, and Tsh4 seem to be the top performing genotypes with high biomass and high nutrient accumulation. In the combined drought and salinity stress heatmap, genotypes Tc4, Ta3, and Tsh2 seem to be the top-performing genotypes with high biomass and high nutrient accumulation.
4 Discussion
Drought and salinity are major abiotic stresses restricting plant growth, particularly in arid and semi-arid regions (Khan et al., 2024). In these regions, they often co-occur as combined drought and salinity stress (Kumar et al., 2019; Romdhane et al., 2020; Ben Gaied et al., 2024). Through the long-term evolutionary processes, plants have developed intricate mechanisms to respond to various environmental stresses. Changes in nutrient uptake, accumulation, and distribution are one of those adaptive responses that help plants to regulate cellular homeostasis and facilitate survival under adverse conditions (Khan et al., 2023; Wang et al., 2025). Distinct germplasms of the same or different species can exhibit variable nutrient response to a particular stress depending on their growth and breeding background (Maharajan et al., 2021; Khan et al., 2022). Hence, in this study, changes in root-shoot nutrient accumulation along with the biomass were observed to understand how differently combined drought and salinity stress affect different Triticum genotypes as compared to drought and salinity stresses alone.
4.1 Significant genotypic variability in root and shoot nutrient accumulation under drought, salinity, and combined drought and salinity stresses
Previously, most of the studies have focused on drought and salinity stress alone as a single stress factor. However, since the last few years, there is a growing body of research on the combined effects of drought and salinity stress in wheat (Ahmed et al., 2013; Dugasa et al., 2019; Fu et al., 2024b; Shamloo-Dashtpagerdi et al., 2024). Despite this, no research has focused on the accumulation of macro- and micronutrients in wheat genotypes under combined drought and salinity stress conditions. The frequent co-existence of drought and salinity stress in field conditions may affect nutrient content in plants positively or negatively (Vasantha et al., 2017; Akhzari et al., 2022; Kucukyumuk et al., 2025). This positive or negative response of plants can direct towards their genotypic variation and hence, nutrient uptake and accumulation patterns under stress are widely used to distinguish between tolerant and susceptible genotypes in crops. In this study, accumulation of nutrients highlighted noticeable variation both at the level of genotypes and species under drought, salinity, and combined stresses.
At the genotypic level, biomass and accumulation of phosphorus, manganese, potassium, zinc, and iron in both roots and shoots, along with copper and sodium in roots and calcium in shoots, significantly contributed to the variability of the studied wheat genotypes. For instance, certain genotypes, such as Tc4 and Ts1, maintained high shoot phosphorus content under drought stress compared to the Control, while Tc4, Tc5, and Ta3 did so under salinity stress. Under combined drought and salinity stress, only Tc4 maintained high phosphorus levels. This ability can be a reason for the high relative shoot dry weight of Tc4 in both drought and combined stress, and of Tc4 and Ta3 under salinity stress. Similarly, the high relative root phosphorus observed in Tsh5 and Ts1 under drought and Tc4 and Tc2 under combined drought and salinity stress can be linked to their correspondingly high relative root dry weights in the same conditions. Under drought stress, three T. compactum genotypes, Tc3, Tc4, and Tc7; two T. spelta, Ts1, and Ts6; one T. aestivum, Ta3, and four T. sphaerococcum, Tsh1, Tsh2, Tsh4, and Tsh5 seem to be more tolerant than other genotypes of the same species, with greatest increase in biomass and nutrient accumulation when compared to Control. Under salinity stress, four T. sphaerococcum, Tsh1, Tsh2, Tsh4, and Tsh5; one T. aestivum, Ta3, and one T. compactum, Tc4, seem to be more tolerant than other genotypes of the same species, with the greatest increase in biomass and nutrient accumulation when compared to the Control. Under combined drought and salinity stress, two T. aestivum, Ta3 and Ta6; three T. compactum genotypes, Tc2, Tc3, and Tc4; and three T. sphaerococcum, Tsh2, Tsh3, and Tsh5, seem to be more tolerant than other genotypes of the same species, with the greatest increase in biomass and nutrient accumulation when compared to the Control. The consistent response of Tc4, Ta3, and Tsh5 across all three stress conditions makes them a potential candidate for breeding programs targeting multi-stressed environments.
If observed at the species level, under drought stress, T. sphaerococcum seems to be the most tolerant species with the highest increase in biomass and accumulation of most of the nutrients, especially macronutrients, when compared to the Control. Among six T. sphaerococcum genotypes, Tsh5 was the best performing one. In contrast, T. aestivum turned out to be the most sensitive species, where all the genotypes, except Ta3, showed maximum decrease in biomass and nutrient accumulation. Surprisingly, the response of T. sphaerococcum genotypes was better than the well-known drought-tolerant bread wheat check cultivar, Ta6 (C 306) used in this study. These findings were in accordance with the outcomes reported by Gaikwad et al. (2024) where average grain yields of the T. sphaerococcum accessions outperform the drought-tolerant cultivar C 306 under restricted irrigation, confirming its potential as a genetic resource for drought stress tolerance.
Similarly, under salinity stress as well, the genotypes of T. sphaerococcum surpassed the performance of T. aestivum genotypes, which also include the three well-recognized salt-tolerant cultivars, Ta7 (K 9006), Ta8 (KRL 210), and Ta9 (KRL 213). Along with increased biomass, T. sphaerococcum genotypes showed better accumulation of nutrients, especially in shoots. Among the six Triticum sphaerococcum genotypes, Tsh2 was the best performing one under salinity stress. The better performance of Tsh5 and Tsh2 than drought- and salt-tolerant check cultivars used in this study emphasizes that T. sphaerococcum possesses untapped allelic diversity that can be used in wheat breeding programs for improving salinity tolerance of modern cultivars (Adhikari et al., 2023; Mazumder et al., 2024). This superior performance of tolerant genotypes can be associated to nutrient translocation mechanisms, possibly involving active transport and compartmentalization of toxic ions like sodium ions and ion homeostasis. Moreover, the increase in the accumulation of nutrients such as phosphorus, potassium, and magnesium in root and shoot tissues may have contributed to osmotic adjustment and membrane stabilization of these genotypes under drought and salinity stress (Hu and Schmidhalter, 2005; Dugasa et al., 2019, 2021).
In contrast to individual drought and salinity stress, under combined drought and salinity stress, no species gave a specific pattern of higher biomass or accumulation of all the nutrients or a particular nutrient in the entire set of genotypes. Instead, some genotypes belonging to different species showed unique patterns in the accumulation of particular nutrients. For instance, while all five T. sphaerococcum genotypes showed high relative shoot sodium and root zinc content under combined drought and salinity stress as compared to the Control, only three of them showed higher shoot biomass, root phosphorus, and shoot manganese. Likewise, only six of the nine T. aestivum genotypes showed high shoot calcium and root magnesium accumulation under combined drought and salinity stress as compared to the Control. This highlighted a genotype-dependent response rather than a species-based response of the studied genotypes under combined stress. In line with our study, several previous studies reported that responses were significant at genotypic level and not the species level (Stavridou et al., 2019; Yadav et al., 2022). Specific combinations of physiological, biochemical, and molecular adaptations based on stress-mitigation mechanisms, such as variations in antioxidant enzyme activity, osmolyte accumulation, ion homeostasis, and stress-responsive gene expression, can be involved in maintaining the growth of some genotypes under combined drought and salinity stress (Ibrahim et al., 2019; Kayacetin, 2022; Yadav et al., 2022; Alsamadany et al., 2024; Alzahrani et al., 2025).
4.2 Comparing the effects of combined drought and salinity stress with drought and salinity stress on the accumulation of different nutrients
Occurrence of multiple stresses can have additive, complementary, or counteractive effects on plants depending on stress type, species, genotypes, and traits (Zandalinas and Mittler, 2022). In this study, while 2 and 3 genotypes showed an increase in shoot phosphorus content under individual drought and salinity stress, as compared to the Control, respectively, under combined drought and salinity stress, only one genotype showed an increase. Similarly, in roots, while drought and salinity stress showed an increase in 12 and 19 genotypes, respectively, only 11 genotypes showed an increase in phosphorus content under combined drought and salinity stress. Similar to phosphorus, other macronutrients, including calcium, potassium, and magnesium, along with all the micronutrients (except root copper), including iron, manganese, and zinc, showed increased accumulation in a greater number of genotypes under drought and salinity stress compared to combined drought and salinity stress. These findings highlighted that overall combined drought and salinity stress was more damaging for the studied wheat genotypes as compared to individual drought and salinity stress. A several impairment or differential regulation of physiological and biochemical pathways responsible for nutrient uptake, transport, and homeostasis under combined drought and salinity stress is suggested. The decrease in the number of genotypes capable of maintaining nutrient content under combined stress directs towards a synergistic negative effect rather than an additive one (Paul et al., 2019; Argentel-Martínez et al., 2024; Naqi et al., 2024).
Both drought and salinity exert osmotic pressure in plants by limiting water availability. However, salinity further develops ionic stress with an accumulation of toxic ions such as sodium (Na+) and chloride (Cl-). These effects are compounded under combined drought and salinity stress, decreasing the water uptake and cellular turgor as well as increasing accumulation of reactive oxygen species that consequently limit cell expansion and biomass development (Neumann, 1995; Angon et al., 2022). This was evidenced in our study by a significant reduction in SDW under combined drought and salinity stress as compared to either stress alone. The reduced sodium accumulation under combined drought and salinity stress, as compared to individual salinity stress, suggests that drought stress stimulates some other changes in the uptake and transport of sodium ions. Despite the reduced sodium levels in combined drought and salinity stress as compared to salinity stress, there are possibly some other factors, such as disturbed ionic balance and increased oxidative stress that reduced the growth under the combined stress (Zhang et al., 2023b).
As wheat plants experience both ionic and osmotic stress under combined drought and salinity stress, plants would have spent more energy on the maintenance of cellular water balance, and ion homeostasis rather than biomass accumulation (Karnik et al., 2017; Ghosh et al., 2021; Mangal et al., 2023).
Moreover, compounded energy is required due to detoxification of reactive oxygen species (ROS) and simultaneous activation of multiple defense pathways such as abscisic acid (ABA), mitogen-activated protein kinases (MAPKs), and calcium signaling and signal transduction (Choudhury et al., 2017; Ravi et al., 2023).
Salinity stress disturbs the uptake and distribution of nutrients such as potassium, calcium, magnesium, and phosphorus due to competition with sodium ions at transport sites. It also disturbs the membrane permeability in roots and interferes with xylem loading and long-distance translocation (Isayenkov, 2019; Khare et al., 2024). These disturbances are further worsened by drought due to a reduction in transpiration rates which is important for nutrient transport from roots to shoots (Hu and Schmidhalter, 2005). Accordingly, in this study, greater reductions of Mg, P, and Ca were observed in combined stress compared to individual drought and salinity stress, especially in shoots. Additionally, shoots were more affected than roots under combined stress in most of the nutrients. Although some genotypes such as Tc4, Ta3, and Tsh2 showed better nutrient accumulation, and biomass under combined drought and salinity stress, most of them showed significant reductions, highlighting the greater negative effect of combined drought and salinity stress.
Roots are the main organs that sense water availability in soil which transmit the required hormonal and electrical signals to the shoots for coordinating a proper response towards stress. The abscisic acid and its receptor-mediated pathways that are involved in wheat signaling under drought regulate closure of stomata, osmolyte accumulation and antioxidant responses to prevent water loss (Guizani et al., 2023; Zhang et al., 2023a, 2025). Different macroelements such as potassium and calcium, and microelements participate in the stabilization of ion homeostasis and osmotic adjustment via these pathways (Vuković et al., 2022; Guizani et al., 2023; Fu et al., 2024a). Under salinity stress, several macroelements such as calcium, potassium, and sodium, and micronutrients such as Fe, Zn, Cu, and Mn are involved in signaling. Ca2+ ions act as ubiquitous secondary messenger and their levels rapidly increase under high salt to transfer stress signals and stimulate adaptive responses (Dugasa et al., 2021; Lindberg and Premkumar, 2023). Potassium and sodium ions compete during uptake and signaling pathways such as SOS pathway and Na+/H+ antiporters act to exclude or compartmentalize sodium ions in different wheat tissues (Wu et al., 2015; Lindberg and Premkumar, 2023). While drought induces the abscisic acid accumulation, facilitating the closure of stomata to prevent water loss, salinity induces ion toxicity (Angon et al., 2022). This signaling can become more complex under combined drought and salinity stress. The simultaneous presence of both stresses disturbs the coordinated root-shoot signaling, leading to abrupt physiological responses such as closing of stomata despite continuous influx of sodium ions (Pierik and Testerink, 2014; Hussain et al., 2021; Angon et al., 2022; Pandey et al., 2023). Accordingly, in this study, accumulation of nutrients such as potassium, calcium, and magnesium was much reduced in shoots as compared to roots, highlighting the possible breakdown in coordinated interaction between roots and shoots, and interference in signaling between them (Hussain et al., 2021). A deeper research is required related to molecular and physiological mechanisms underlying root-to-shoot signaling under combined drought and salinity stress.
5 Conclusion
This study explores genotypic variation in nutrient uptake and biomass accumulation among 30 hexaploid wheat genotypes from seven species, evaluated under drought, salinity, and combined drought and salinity stress. Moreover, the study also attempted to understand the mechanisms underlying the variation in nutrient accumulation. Significant species and genotypic level differences were observed in the nutrient accumulation of these genotypes under these stresses, with a more complex and genotype-dependent response noticed under combined drought and salinity stress. Though individual drought and salinity stress resulted in considerable reductions in both dry weights and nutrient accumulation, their simultaneous existence had a more damaging and synergistic negative effect, specifically on dry weights and macronutrients such as phosphorus, potassium, magnesium, and calcium. Among the studied genotypes, Tc4 (PI 164160, Kanak, India) was the best-performing genotype across all three stress conditions, followed by Ta3 (CItr 17028, CAR 1101, Chile) and Tsh2 (PI 42013, India) in terms of improved nutrient accumulation and biomass, and can be used for future genetic improvement.
Superior adaptability of T. sphaerococcum genotypes under both drought and salinity stress suggested that this species is a reservoir of valuable alleles that can be used to improve wheat resilience towards abiotic stresses. The underperformance of T. aestivum genotypes in the study, including the well-established drought and salt-tolerant check cultivars, emphasizes the necessity of exploring a greater number of underutilized wheat species in breeding programs. The contrasting genotype-dependent responses under combined stress, contrary to the uniform species level responses under individual drought and salinity stress, indicated that adaptation under combined stress involves complex and genotype-dependent mechanisms. Hence, a greater number of wheat genotypes must be evaluated under realistic multi-stress environments rather than individual stresses. The greater decreases in nutrient content under combined drought and salinity stress, particularly in shoots may indicate a disruption in root-shoot communication. This also emphasized the need for further transcriptomic or proteomic studies to understand the underlying molecular mechanisms for differential nutrient uptake under nutrient stress. Moreover, root morphology and architecture should be studied in detail to identify physical barriers to nutrient absorption under combined stress.
The results obtained in this study identified potential genotypes and species that can be used for breeding programs targeting individual drought, salinity, and combined drought and salinity stress. In addition, the study emphasized that combined drought and salinity stress is not just a sum of drought and salinity stress, but it involves a complex interaction of multiple stress pathways, leading to a greater physiological and biochemical disruption. Hence, it should be thoroughly explored as a separate stress condition to identify a greater number of genetic resources that are tolerant to this deadly stress combination. The results provided valuable insights emphasizing the relevance of nutrient profiling as a critical component of breeding frameworks for climate-resilient wheat.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
MK: Conceptualization, Methodology, Data curation, Writing – review & editing, Validation, Investigation, Writing – original draft, Formal Analysis.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. I would like to thank TUBITAK, as a part of this study (sodium accumulation and root-shoot dry weight measurement) was conducted by me under TUBITAK-funded 1001 project (No. 123R072). Moreover, a part of APC is funded by BAP project (No. 25601070) of Selcuk University, Turkiye.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author declared that he was an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1682258/full#supplementary-material
References
Adhikari, S., Kumari, J., Bhardwaj, R., Jacob, S., Langyan, S., Sharma, S., et al. (2023). Unlocking the potential of ancient hexaploid Indian dwarf wheat, Triticum sphaerococcum for grain quality improvement. PeerJ 11, e15334. doi: 10.7717/peerj.15334, PMID: 37525662
Ahmadi, J., Pour-Aboughadareh, A., Fabriki Ourang, S., Khalili, P., and Poczai, P. (2020). Unraveling salinity stress responses in ancestral and neglected wheat species at early growth stage: A baseline for utilization in future wheat improvement programs. Physiol. Mol. Biol. Plants. 26, 537–549. doi: 10.1007/s12298-020-00768-4, PMID: 32205929
Ahmed, I. M., Cao, F., Zhang, M., Chen, X., Zhang, G., and Wu, F. (2013). Difference in yield and physiological features in response to drought and salinity combined stress during anthesis in Tibetan wild and cultivated barleys. PloS One 8, e77869. doi: 10.1371/journal.pone.0077869, PMID: 24205003
Akhzari, D., Pessarakli, M., Mahdavi, S., and Ariapour, A. (2022). Impact of drought, salinity, and heavy metal stress on growth, nutrient uptake, and physiological traits of vetiver grass (Chrysopogon zizanioides L.). Commun. Soil Sci. Plant Anal. 53, 1841–1847. doi: 10.1080/00103624.2022.2063327
Alam, A., Ullah, H., Attia, A., and Datta, A. (2020). Effects of salinity stress on growth, mineral nutrient accumulation and biochemical parameters of seedlings of three citrus rootstocks. Int. J. Fruit Sci. 20, 786–804. doi: 10.1080/15538362.2019.1674762
Ali, Z., Hakeem, S., Wiehle, M., Saddique, M., and Habib-Ur-Rahman, M. (2023). Prioritizing strategies for wheat biofortification: Inspiration from underutilized species. Heliyon 9, e20208. doi: 10.1016/j.heliyon.2023.e20208, PMID: 37818015
Alsamadany, H., Abdulbaki, A. S., and Alzahrani, Y. (2024). Unravelling drought and salinity stress responses in barley genotypes: physiological, biochemical, and molecular insights. Front. Plant Sci. 15, 1417021. doi: 10.3389/fpls.2024.1417021, PMID: 39049857
Alzahrani, Y., Abdulbaki, A. S., and Alsamadany, H. (2025). Genotypic variability in stress responses of Sorghum bicolor under drought and salinity conditions. Front. Genet. 15, 1502900. doi: 10.3389/fgene.2024.1502900, PMID: 39845188
Angon, P. B., Tahjib-Ul-Arif, M., Samin, S. I., Habiba, U., Hossain, M. A., and Brestic, M. (2022). How do plants respond to combined drought and salinity stress?-A systematic review. Plants (Basel). 11, 2884. doi: 10.3390/plants11212884, PMID: 36365335
Argentel-Martínez, L., Peñuelas-Rubio, O., Pérez-López, L., Aguilera, J. G., Steiner, F., Zuffo, A. M., et al. (2024). Assessing salinity, drought and high temperature stress in maize (Zea mays L.) and wheat (Triticum aestivum L.) varieties: Theoretical combination as multifactorial stress. J. Agron. Crop Sci. 210, e70001. doi: 10.1111/jac.70001
Begum, N., Hasanuzzaman, M., Li, Y., Akhtar, K., Zhang, C., and Zhao, T. (2022). Seed germination behavior, growth, physiology and antioxidant metabolism of four contrasting cultivars under combined drought and salinity in soybean. Antioxidants 11, 498. doi: 10.3390/antiox11030498, PMID: 35326148
Ben Gaied, R., Brígido, C., Sbissi, I., and Tarhouni, M. (2024). Sustainable strategy to boost legumes growth under salinity and drought stress in semi-arid and arid regions. Soil Syst. 8, 84. doi: 10.3390/soilsystems8030084
Bista, D. R., Heckathorn, S. A., Jayawardena, D. M., Mishra, S., and Boldt, J. K. (2018). Effects of drought on nutrient uptake and the levels of nutrient-uptake proteins in roots of drought-sensitive and-tolerant grasses. Plants 7, 28. doi: 10.3390/plants7020028, PMID: 29601475
Cheraghi, M., Mousavi, S. M., and Zarebanadkouki, M. (2023). Functions of rhizosheath on facilitating the uptake of water and nutrients under drought stress: A review. Plant Soil 491, 239–263. doi: 10.1007/s11104-023-06126-z
Choudhury, F. K., Rivero, R. M., Blumwald, E., and Mittler, R. (2017). Reactive oxygen species, abiotic stress and stress combination. Plant J. 90, 856–867. doi: 10.1111/tpj.13299, PMID: 27801967
Dugasa, M. T., Cao, F., Ibrahim, W., and Wu, F. (2019). Differences in physiological and biochemical characteristics in response to single and combined drought and salinity stresses between wheat genotypes differing in salt tolerance. Physiol. Plant 165, 134–143. doi: 10.1111/ppl.12743, PMID: 29635753
Dugasa, M. T., Feng, X., Wang, N.-H., Wang, J., and Wu, F. (2021). Comparative transcriptome and tolerance mechanism analysis in the two contrasting wheat (Triticum aestivum L.) cultivars in response to drought and salinity stresses. Plant Growth Regul. 94, 101–114. doi: 10.1007/s10725-021-00699-4
Fu, Y., Li, P., Mounkaila Hamani, A. K., Wan, S., Gao, Y., and Wang, X. (2023). Effects of single and combined drought and salinity stress on the root morphological characteristics and root hydraulic conductivity of different winter wheat varieties. Plants 12, 2694. doi: 10.3390/plants12142694, PMID: 37514308
Fu, X., Liu, Z., Du, X., Duan, H., Zhen, W., Zhang, Y., et al. (2024a). Transcriptomic and metabolomic analyses reveal the response to short-term drought stress in bread wheat (Triticum aestivum L.). Agronomy 14, 704. doi: 10.3390/agronomy14040704
Fu, Y., Li, P., Si, Z., Ma, S., and Gao, Y. (2024b). Seeds priming with melatonin improves root hydraulic conductivity of wheat varieties under drought, salinity, and combined stress. Int. J. Mol. Sci. 25, 5055. doi: 10.3390/ijms25095055, PMID: 38732273
Gaikwad, K. B., Dawar, A., Singh, A., Babu, P., Kumar, M., Kumar, N., et al. (2024). Trait phenotyping in an ancient Indian landrace of wheat Triticum sphaerococcum under optimum, terminal heat stress and deficit irrigation conditions. Genet. Resour. Crop Evol. 71, 2779–2795. doi: 10.1007/s10722-023-01817-z
Ghosh, U. K., Islam, M. N., Siddiqui, M. N., and Khan, M. (2021). Understanding the roles of osmolytes for acclimatizing plants to changing environment: a review of potential mechanism. Plant Signal. Behav. 16, 1913306. doi: 10.1080/15592324.2021.1913306, PMID: 34134596
Guizani, A., Askri, H., Amenta, M. L., Defez, R., Babay, E., Bianco, C., et al. (2023). Drought responsiveness in six wheat genotypes: identification of stress resistance indicators. Front. Plant Sci. 14, 1232583. doi: 10.3389/fpls.2023.1232583, PMID: 37780517
Gupta, O. P., Singh, A., Pandey, V., Sendhil, R., Khan, M. K., Pandey, A., et al. (2024). Critical assessment of wheat biofortification for iron and zinc: a comprehensive review of conceptualization, trends, approaches, bioavailability, health impact, and policy framework. Front. Nutr. 10, 1310020. doi: 10.3389/fnut.2023.1310020, PMID: 38239835
Hu, Y. and Schmidhalter, U. (2005). Drought and salinity: a comparison of their effects on mineral nutrition of plants. J. Plant Nutr. Soil Sci. 168, 541–549. doi: 10.1002/jpln.200420516
Hussain, Q., Asim, M., Zhang, R., Khan, R., Farooq, S., and Wu, J. (2021). Transcription factors interact with ABA through gene expression and signaling pathways to mitigate drought and salinity stress. Biomolecules 11, 1159. doi: 10.3390/biom11081159, PMID: 34439825
Ibrahim, W., Qiu, C. W., Zhang, C., Cao, F., Shuijin, Z., and Wu, F. (2019). Comparative physiological analysis in the tolerance to salinity and drought individual and combination in two cotton genotypes with contrasting salt tolerance. Physiol. Plant. 165, 155–168. doi: 10.1111/ppl.12791, PMID: 30006979
Isayenkov, S. V. (2019). Genetic sources for the development of salt tolerance in crops. Plant Growth Regul. 89, 1–17. doi: 10.1007/s10725-019-00519-w
Jing, Z., Liu, N., Zhang, Z., and Hou, X. (2024). Research progress on plant responses to stress combinations in the context of climate change. Plants 13, 469. doi: 10.3390/plants13040469, PMID: 38498439
Karnik, R., Waghmare, S., Zhang, B., Larson, E., Lefoulon, C., Gonzalez, W., et al. (2017). Commandeering channel voltage sensors for secretion, cell turgor, and volume control. Trends Plant Sci. 22, 81–95. doi: 10.1016/j.tplants.2016.10.006, PMID: 27818003
Kayacetin, F. (2022). Assessment of safflower genotypes for individual and combined effects of drought and salinity stress at early seedling growth stages. Turk. J. Agric. For. 46, 601–612. doi: 10.55730/1300-011X.3029
Khan, M. K., Pandey, A., Hamurcu, M., Germ, M., Yilmaz, F. G., Ozbek, M., et al. (2022). Nutrient homeostasis of aegilops accessions differing in B tolerance level under boron toxic growth conditions. Biol. (Basel). 11, 1094. doi: 10.3390/biology11081094, PMID: 35892950
Khan, M. K., Pandey, A., Hamurcu, M., Rajpal, V. R., Vyhnanek, T., Topal, A., et al. (2023). Insight into the Boron Toxicity Stress-Responsive Genes in Boron-Tolerant Triticum dicoccum Shoots Using RNA Sequencing. Agronomy 13, 631. doi: 10.3390/agronomy13030631
Khan, M. K., Pandey, A., Hamurcu, M., Vyhnánek, T., Zargar, M. S., Kahraman, A., et al. (2024). Exploring strigolactones for inducing abiotic stress tolerance in plants. Czech. J. Genet. Plant Breed. 60, 55–69. doi: 10.17221/88/2023-CJGPB
Khan, M. K., Pandey, A., Hamurcu, M., Zargar, S. M., Chaudhry, B., Topal, A., et al. (2025). “Chapter seven - Potential of wheat wild species for salinity tolerance improvement in modern wheat,” in Wheat Wild Relatives. Eds. Khan, M. K., Pandey, A., Hamurcu, M., and Gezgin, S. (United States: Academic Press), 205–221.
Khare, T., Jamla, M., Mathur, V., and Kumar, V. (2024). Exploring halobiome resources for developing salt-tolerant crops: A perspective review. J. Plant Growth Regul. 43, 2137–2164. doi: 10.1007/s00344-024-11266-2
Kucukyumuk, Z., Suarez, D. L., and Kucukyumuk, C. (2025). Influence of drought and salt stress on almond nutrition. J. Plant Nutr. 48, 727–736. doi: 10.1080/01904167.2024.2414745
Kumar, V., Joshi, S., Pant, N. C., Sangwan, P., Yadav, A. N., Saxena, A., et al. (2019). “Molecular approaches for combating multiple abiotic stresses in crops of arid and semi-arid region,” in Molecular approaches in plant biology and environmental challenges (Springer Singapore: Springer), 149–170.
Lindberg, S. and Premkumar, A. (2023). Ion changes and signaling under salt stress in wheat and other important crops. Plants 13, 46. doi: 10.3390/plants13010046, PMID: 38202354
Maharajan, T., Krishna, T. A., Kiriyanthan, R. M., Ignacimuthu, S., and Ceasar, S. A. (2021). Improving abiotic stress tolerance in sorghum: focus on the nutrient transporters and marker-assisted breeding. Planta 254, 90. doi: 10.1007/s00425-021-03739-5, PMID: 34609619
Mangal, V., Lal, M. K., Tiwari, R. K., Altaf, M. A., Sood, S., Kumar, D., et al. (2023). Molecular insights into the role of reactive oxygen, nitrogen and sulphur species in conferring salinity stress tolerance in plants. J. Plant Growth Regul. 42, 554–574. doi: 10.1007/s00344-022-10591-8
Mazumder, A. K., Budhlakoti, N., Kumar, M., Pradhan, A. K., Kumar, S., Babu, P., et al. (2024). Exploring the genetic diversity and population structure of an ancient hexaploid wheat species Triticum sphaerococcum using SNP markers. BMC Plant Biol. 24, 1188. doi: 10.1186/s12870-024-05968-8, PMID: 39695987
Naqi, S., Khan, A. H., Rana, R. M., Hamza, M. I., Kiedrzyński, M., Tahir, M. N., et al. (2024). Inheritance of cell membrane stability and yield components under drought and salinity stress in bread wheat (Triticum aestivum L.). J. Plant Growth Regul. 44, 1–16. doi: 10.1007/s00344-024-11580-9
Neumann, P. M. (1995). The role of cell wall adjustments in plant resistance to water deficits. Crop Sci. 35, 1258–1266. doi: 10.2135/cropsci1995.0011183X003500050002x
Ors, S., Ekinci, M., Yildirim, E., Sahin, U., Turan, M., and Dursun, A. (2021). Interactive effects of salinity and drought stress on photosynthetic characteristics and physiology of tomato (Lycopersicon esculentum L.) seedlings. S. Afr. J. Bot. 137, 335–339. doi: 10.1016/j.sajb.2020.10.031
Pandey, A., Khan, M. K., Athar, T., Hamurcu, M., Germ, M., and Gezgin, S. (2023). “Chapter 17 - Combined abiotic stresses in wheat species,” in Abiotic stresses in wheat. Eds. Khan, M. K., Pandey, A., Hamurcu, M., Gupta, O. P., and Gezgin, S. (United States: Academic Press), 273–282.
Paul, K., Pauk, J., Kondic-Spika, A., Grausgruber, H., Allahverdiyev, T., Sass, L., et al. (2019). Co-occurrence of mild salinity and drought synergistically enhances biomass and grain retardation in wheat. Front. Plant Sci. 10, 501. doi: 10.3389/fpls.2019.00501, PMID: 31114595
Pierik, R. and Testerink, C. (2014). The art of being flexible: how to escape from shade, salt, and drought. Plant Physiol. 166, 5–22. doi: 10.1104/pp.114.239160, PMID: 24972713
Ravi, B., Foyer, C. H., and Pandey, G. K. (2023). The integration of reactive oxygen species (ROS) and calcium signalling in abiotic stress responses. Plant Cell Environ. 46, 1985–2006. doi: 10.1111/pce.14596, PMID: 37132157
Rivero, R. M., Mittler, R., Blumwald, E., and Zandalinas, S. I. (2022). Developing climate-resilient crops: improving plant tolerance to stress combination. Plant J. 109, 373–389. doi: 10.1111/tpj.15483, PMID: 34482588
Romdhane, L., Radhouane, L., Farooq, M., Dal Cortivo, C., Panozzo, A., and Vamerali, T. (2020). Morphological and biochemical changes in maize under drought and salinity stresses in a semi-arid environment. Plant Biosyst. 154, 396–404. doi: 10.1080/11263504.2019.1635221
Shamloo-Dashtpagerdi, R., Tanin, M. J., Aliakbari, M., and Saini, D. K. (2024). Unveiling the role of the ERD15 gene in wheat’s tolerance to combined drought and salinity stress: a meta-analysis of QTL and RNA-Seq data. Physiol. Plant 176, e14570. doi: 10.1111/ppl.14570, PMID: 39382027
Sharma, V., Kumar, A., Chaudhary, A., Mishra, A., Rawat, S., B., B. Y., et al. (2022). Response of wheat genotypes to drought stress stimulated by PEG. Stresses 2, 26–51. doi: 10.3390/stresses2010003
Stavridou, E., Webster, R. J., and Robson, P. R. (2019). Novel Miscanthus genotypes selected for different drought tolerance phenotypes show enhanced tolerance across combinations of salinity and drought treatments. Ann. Bot. 124, 653–674. doi: 10.1093/aob/mcz009, PMID: 31665760
Suzuki, N., Rivero, R. M., Shulaev, V., Blumwald, E., and Mittler, R. (2014). Abiotic and biotic stress combinations. New Phytol. 203, 32–43. doi: 10.1111/nph.12797, PMID: 24720847
Tang, K., An, C., Li, L., Sun, T., Song, J., and Zhao, J. (2024). Effects of drought and salt stress on the root phenotype of wheat seedlings and underlying gene expression analysis. Front. Plant Sci. 15, 1475500. doi: 10.3389/fpls.2024.1475500, PMID: 39737378
Uzair, M., Ali, M., Fiaz, S., Attia, K., Khan, N., Al-Doss, A. A., et al. (2022). The characterization of wheat genotypes for salinity tolerance using morpho-physiological indices under hydroponic conditions. Saudi. J. Biol. Sci. 29, 103299. doi: 10.1016/j.sjbs.2022.103299, PMID: 35574282
Vasantha, S., Gomathi, R., and Brindha, C. (2017). Growth and nutrient composition of sugarcane genotypes subjected to salinity and drought stresses. Commun. Soil Sci. Plant Anal. 48, 989–998. doi: 10.1080/00103624.2017.1322604
Vuković, R., Čamagajevac, I.Š., Vuković, A., Šunić, K., Begović, L., Mlinarić, S., et al. (2022). Physiological, biochemical and molecular response of different winter wheat varieties under drought stress at germination and seedling growth stage. Antioxidants 11, 693. doi: 10.3390/antiox11040693, PMID: 35453378
Wang, L., Ju, C., Han, C., Yu, Z., Bai, M. Y., and Wang, C. (2025). The interaction of nutrient uptake with biotic and abiotic stresses in plants. J. Integr. Plant Biol. 67, 455–487. doi: 10.1111/jipb.13827, PMID: 39783785
Wu, H., Shabala, L., Liu, X., Azzarello, E., Zhou, M., Pandolfi, C., et al. (2015). Linking salinity stress tolerance with tissue-specific Na+ sequestration in wheat roots. Front. Plant Sci. 6, 71. doi: 10.3389/fpls.2015.00071, PMID: 25750644
Yadav, C., Bahuguna, R. N., Dhankher, O. P., Singla-Pareek, S. L., and Pareek, A. (2022). Physiological and molecular signatures reveal differential response of rice genotypes to drought and drought combination with heat and salinity stress. Physiol. Mol. Biol. Plants. 28, 899–910. doi: 10.1007/s12298-022-01162-y, PMID: 35592483
Zandalinas, S. I., Fichman, Y., Devireddy, A. R., Sengupta, S., Azad, R. K., and Mittler, R. (2020). Systemic signaling during abiotic stress combination in plants. Proc. Natl. Acad. Sci. U.S.A. 117, 13810–13820. doi: 10.1073/pnas.2005077117, PMID: 32471943
Zandalinas, S. I. and Mittler, R. (2022). Plant responses to multifactorial stress combination. New Phytol. 234, 1161–1167. doi: 10.1111/nph.18087, PMID: 35278228
Zhang, H. and Sonnewald, U. (2017). Differences and commonalities of plant responses to single and combined stresses. Plant J. 90, 839–855. doi: 10.1111/tpj.13557, PMID: 28370754
Zhang, Z., Zhang, T., Yin, B., Wang, Z., Li, R., and Li, S. (2023b). The influence of sodium salt on growth, photosynthesis, Na+/K+ homeostasis and osmotic adjustment of Atriplex canescens under drought stress. Agronomy 13, 2434. doi: 10.3390/agronomy13092434
Zhang, Y., Zhao, Y., Hou, X., Ni, C., Han, L., Du, P., et al. (2023a). Wheat ABA receptor TaPYL5 constitutes a signaling module with its downstream partners TaPP2C53/TaSnRK2. 1/TaABI1 to modulate plant drought response. Int. J. Mol. Sci. 24, 7969. doi: 10.3390/ijms24097969, PMID: 37175676
Keywords: abiotic stress, climate change, genetic resources, genetic variation, nutrient profiling, neglected species, salt stress, water stress
Citation: Khan MK (2025) Nutrient uptake under combined drought and salinity stress in hexaploid wheat species. Front. Plant Sci. 16:1682258. doi: 10.3389/fpls.2025.1682258
Received: 08 August 2025; Accepted: 10 October 2025;
Published: 10 November 2025.
Edited by:
Arif Tasleem Jan, Baba Ghulam Shah Badshah University, IndiaReviewed by:
Mohd Asgher, Baba Ghulam Shah Badshah University, IndiaAnna Dimitrova, Bulgarian Academy of Sciences (BAS), Bulgaria
Elias Arazmjoo, Agricultural Research, Education and Extension Organization (AREEO), Iran
Copyright © 2025 Khan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohd. Kamran Khan, bW9oZGthbXJhbi5iaW90ZWNoQGdtYWlsLmNvbQ==; bWtraGFuQHNlbGN1ay5lZHUudHI=
 Mohd. Kamran Khan
Mohd. Kamran Khan