- College of Life Science, Shanxi Normal University, Taiyuan, Shanxi, China
Calcium ions (Ca2+) are vital in plants, functioning both as structural cellular components and key secondary messengers that regulate growth, development, and stress responses. Nitric oxide (NO), a ubiquitous gaseous signaling molecule in organisms, also modulates diverse plant physiological processes. These two signaling molecules form a bidirectional interaction network, though the molecular mechanisms underlying their crosstalk remain poorly understood. Previous studies suggest that the calmodulin-like (CML) protein family mediates the interplay between NO and Ca2+ signaling. Our earlier RNA-seq data indicated that CML38 expression is responsive to exogenous NO in Arabidopsis seedlings, prompting the hypothesis that NO and Ca2+ signaling may interact with each other via CML38 regulation. To test this hypothesis, we employed Arabidopsis thaliana as a model plant and integrated genetic, biochemical, and molecular approaches to elucidate CML38’s role in NO-mediated hypocotyl growth inhibition. Our findings demonstrate that NO treatment significantly suppresses hypocotyl elongation in wild-type plants but not in CML38 loss-of-function mutant. CML38 binds Ca2+ and its calcium-binding capacity is unaffected by NO. Transcriptomic analysis revealed that CML38 participates in the crosstalk between NO and Ca2+ signaling, light signaling, as well as phytohormones. This study advances our understanding of the NO-Ca2+ interaction network in plants and provides insights into the molecular mechanisms by which these signals coordinately regulate plant growth and stress adaptation.
1 Introduction
Calcium ions (Ca2+) act as universal secondary messengers in plants, coordinating growth, development, and stress responses through dynamic regulation of cytosolic Ca2+ concentration ([Ca2+]cyt) (Kudla et al., 2010). Under homeostatic conditions, [Ca2+]cyt is maintained at nanomolar levels, but plants rapidly generate stimulus-specific Ca2+ signatures in response to environmental cues (e.g., light, temperature), hormonal pathways, pathogen challenges, and abiotic stresses (e.g., salinity, drought). The [Ca2+]cyt fluctuations trigger downstream signaling cascades that regulate distinct physiological responses (Pirayesh et al., 2021). Importantly, Ca2+ signals extensively interact with other secondary messengers, including nitric oxide (NO). NO modulates Ca2+ dynamics through activation of Ca2+-dependent protein kinases (e.g., CDPKs) and post-translational modification of Ca2+ channels via S-nitrosylation (Courtois et al., 2008; Jeandroz et al., 2013; Hu et al., 2022). This intricate crosstalk between NO and Ca2+ signaling enables precise spatiotemporal regulation of cellular processes, ranging from cell division and elongation to complex physiological adaptations, forming an integrated signaling network that processes multiple environmental and developmental cues.
Ca2+ response proteins decode cytosolic Ca2+ fluctuations into specific physiological outputs, with calmodulin-like proteins (CMLs) representing a large family of Ca2+ sensors with specialized functions beyond the highly conserved calmodulin (CaM). Arabidopsis CMLs possess the characteristic EF-hand Ca2+-binding domains and exhibit remarkable functional diversification in plant growth and stress responses (McCormack and Braam, 2003). During early development, CMLs precisely regulate seed germination through hormone signaling pathways. CML14 and CML24 promote germination, whereas CML39 acts as a negative regulator by enhancing ABA sensitivity and suppressing gibberellin responses, with cml39 showing accelerated germination and reproductive defects including shortened siliques and reduced seed set (Delk et al., 2005; Bender et al., 2013; Symonds et al., 2024). In reproductive processes, multiple CMLs (CML3, 14, 16, 49) show pollen-specific expression patterns, with CML24 and CML25 directly facilitating pollen tube growth via modulation of potassium channel activity, and CML36 participating in floral transition through activation of ACA8 Ca2+-ATPase (Tsai et al., 2007; Wang et al., 2008, 2015; Yang et al., 2014). CMLs also integrate Ca2+ signaling with developmental senescence and environmental responses. MdCML15 accelerates ethylene-mediated leaf and fruit senescence in apple through formation of a transcriptional regulatory complex with MdVQ10 and MdWRKY75, while papaya CpCML15 directly promotes ripening by modulating senescence-related gene networks (Ding et al., 2018). In light signaling, CML39 functions as a photoreceptor-coupled Ca2+ sensor regulating photomorphogenesis, with mutants displaying aberrant hypocotyl elongation (Bender et al., 2013), whereas CML9 mediates root growth responses to microbial elicitors like flagellin (Leba et al., 2012). The functional diversification of CMLs enables plants to generate appropriate responses to myriad internal and external stimuli through Ca2+ signaling networks.
Based on our previous transcriptomic data, which showed that NO treatment markedly alters the expression of several CML family members, we focused on the five most responsive genes: CML23, CML24, CML37, CML38, and CML39 (Ren et al., 2024). Among these, phenotypic analysis of loss-of-function mutants revealed that both cml23 and cml38 mutants were insensitive to NO-mediated inhibition of hypocotyl elongation (data unpublished). Since the interaction between NO and CML23 has been extensively detailed elsewhere (Wang et al., 2025), we focused this study on characterizing the novel role of CML38 in NO signaling pathway. CML38 is a multifunctional Ca2+ response protein, which integrates diverse signaling inputs—including Ca2+ fluctuations, hormonal (e.g., brassinosteroid) cues, nutrient (e.g., nitrate) availability, and environmental (e.g., hypoxia) stimuli—to orchestrate plant development and stress adaptation (Lokdarshi et al., 2016; Field et al., 2021; Song et al., 2021). It exerts its regulatory roles through modulating gene expression, mRNA metabolism, and autophagy, positioning it as a central hub in cellular signaling networks.
Previous studies have shown that the CML protein family plays a role in the crosstalk between NO and Ca2+ signaling, potentially by translating cytoplasmic Ca²+ fluctuations into cellular responses via modulation of NO biosynthesis or turnover (Tsai et al., 2007). Here, we investigate the molecular mechanisms by which CML38 mediates NO-induced suppression of hypocotyl elongation, providing direct evidence that CML38 operates downstream of NO perception to regulate Ca2+-dependent signaling pathways.
2 Materials and methods
2.1 Plant growth condition
Arabidopsis thaliana ecotype Columbia-0 (Col-0) was used as the wild-type. The T-DNA insertion mutant cml38 (SALK_066538C) was obtained from the Arashare Center (Fuzhou, China) and genotypically verified by PCR (Supplementary Figure S1).
Seeds were surface-sterilized sequentially with 70% (v/v) ethanol for 30 s, followed by 10% (v/v) sodium hypochlorite for 15min, and rinsed five times with sterile distilled water. Sterilized seeds were stratified at 4°C for 48h to synchronize germination, then sown vertically on half-strength Murashige and Skoog (1/2 MS) medium in growth chambers maintained at 22°C under a 12-h photoperiod. After 48h of germination, uniform seedlings were transferred to fresh 1/2 MS plates (9cm diameter) containing the following treatments: sodium nitroprusside (SNP; NO donor) fumigation, 1 mM CaCl2, 1 mM EGTA (calcium chelator), or 50 μM ruthenium red (RR; calcium channel inhibitor). For SNP fumigation, sterilized microcentrifuge tubes were placed on the medium surface, and 10 μL of 100 mM SNP solution was added to each tube to allow gradual NO release under light conditions. The Petri dishes were sealed and the SNP solution was applied only once at the beginning of the experiment. Seedlings were grown for 7 additional days under the same conditions. Hypocotyl length was quantified using ImageJ software (NIH, USA) for precise image analysis and morphometric measurements. Graphs were generated using GraphPad Prism 9 (GraphPad Software, USA). Statistical significance between two groups was determined using unpaired two-tailed Student’s t-test.
2.2 Bioinformatics analysis
The Ca2+ binding sites of CML38 were predicted using the MIB2 online server (Lu et al., 2022), and the highest-scoring model was visualized in PyMOL (version 2.4.0).
2.3 Recombinant CML38 protein expression and purification
Arabidopsis thaliana ecotype Columbia-0 (Col-0) was used as the wild-type. The T-DNA inser The Arabidopsis CML38 gene was synthesized and cloned into the EcoRI and SalI restriction sites of the pET28a(+) prokaryotic expression vector (GenScript Biotech Corporation, Nanjing, China), and verified by sequencing. The recombinant pET28a: CML38 plasmid was then transformed into E. coli BL21 (DE3) competent cells. Transformed colonies were cultured in LB medium at 37°C to OD600=0.6, then induced with 0.2 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) at 16°C for 18h. His-tagged recombinant CML38 protein was subsequently purified using BeyoGold™ His-tag Purification Resin (Beyotime, P2218) according to the manufacturer’s protocol.
2.4 [Ca2+]cyt concentration detection
The Fluo-4 AM fluorescence probe (Beyotime) was used to detect [Ca2+]cyt concentration in Arabidopsis hypocotyls, and the detection process was carried out on a BX53 fluorescence microscope (Olympus, Tokyo, Japan). Hypocotyl samples from 9-day-old seedlings were first incubated to load Fluo-4 AM probe, following the manufacture’s protocol. Then the samples were treated with 20 μM GSNO (an alternative NO donor) for 10 minutes, as this donor was chosen to avoid light exposure—which is detrimental to the Fluo-4 AM probe yet essential for NO release from SNP. The relative fluorescence intensity (indicative of [Ca2+]cyt concentration) was quantified using ImageJ. The graphing and statistical analysis methods were the same as those employed in the phenotypic detection assay.
2.5 Ca2+-binding assay
Electrophoresis mobility shift assays were performed as described (Vanderbeld and Snedden, 2007) with modifications. Prior to denaturation, purified recombinant protein was treated with either 200 µM fresh SNP solution, or 48h light-exposed SNP (NO-depleted control). The denatured protein samples were then incubated with 5 mM CaCl2 or 5 mM EGTA for 30min at 4°C, then separated by SDS-PAGE using gels and running buffers containing either 2 mM CaCl2 or 2 mM EGTA to maintain consistent Ca2+ conditions.
2.6 RNA extraction and RT-qPCR analysis
The aboveground parts of 9-day-old seedlings were flash-frozen in liquid nitrogen and homogenized. Total RNA was isolated using CTAB-PBIOZOL reagent (Takara, Beijing, China) following the manufacturer’s protocol, and integrity was assessed with an Agilent 2100 Bioanalyzer.
Quantitative real-time PCR (RT-qPCR) analysis was applied using the QuantStudio™ 5 Real-Time PCR System (Applied Biosystems) in 96-well plates. Each 20 µL reaction contained 10 µL 2× PerfectStart™ Green qPCR SuperMix (Transgene, Beijing, China), 0.5 µL each of forward and reverse primers (listed in Supplementary Table S1) (10 µM), 1 µL cDNA template (~20 ng), and 8 µL nuclease-free water. Thermal cycling conditions: initial denaturation at 94°C for 3min, followed by 40 cycles of 94°C for 30 s, 60°C for 15 s, and 94°C for 10 s. Gene expression levels were normalized to the reference gene β-ACTIN using the ΔΔCt method, with technical triplicates performed for each sample to ensure data reproducibility, and melting curve analysis was conducted to verify amplification specificity.
2.7 Sequencing
Libraries were constructed as follows: (1) genomic DNA elimination and mRNA enrichment using oligo(dT) magnetic beads; (2) RNA fragmentation in NEBNext First Strand Synthesis Buffer (5×) with divalent cations at 94°C for 5–7 minutes; (3) double-stranded cDNA synthesis using M-MuLV Reverse Transcriptase (RNase H-) and DNA Polymerase I; (4) cDNA end repair, 3’ adenylation, and NEBNext adaptor ligation; and (5) size selection (250–300 bp fragments) with AMPure XP beads (Beckman Coulter, Germany).
The libraries underwent: (i) USER Enzyme (NEB) treatment (37°C, 15min; 95°C, 5min); (ii) 12-cycle PCR amplification with Phusion High-Fidelity DNA polymerase; and (iii) final purification with AMPure XP beads. Quality control was performed using the Agilent Bioanalyzer 2100 system prior to 2 × 150 bp paired-end sequencing on the Illumina NovaSeq 6000 platform (Wekemo Tech Group, Shenzhen), generating approximately 40 million reads per sample.
2.8 Analysis of deferentially expressed genes
RNA-seq analysis generated robust datasets with each biological replicate producing >46 million raw paired-end reads. Following sequencing, we implemented a rigorous quality control pipeline: (1) adapter trimming and quality filtering using FastQC (v0.23.1) with custom Perl scripts to remove low-quality bases (Q<20), poly-N sequences (>10% N content), and reads shorter than 50 bp; (2) alignment of processed reads to the Arabidopsis reference genome (GCF_000001735.4_TAIR10.1) using HISAT2 (v2.0.5) with default parameters, achieving >85% mapping efficiency across all samples; and (3) transcript quantification through reference-guided assembly with StringTie (v1.3.3b), expressing gene abundance as FPKM values after normalization.
For differential expression analysis, we employed DESeq2 (v1.4.5) with a stringent threshold of |log2FC| > 1 (equivalent to 2-fold change) and P-value < 0.05. Functional annotation of differentially expressed genes (DEGs) involved: (i) GO term enrichment analysis using GOSeq (v1.34.1) with Wallenius approximation to correct for transcript length bias; and (ii) pathway mapping to KEGG databases through the ClusterProfiler R package (v3.14.3), with significance determined by FDR < 0.05 after Benjamini-Hochberg correction. All analyses were performed with triplicate biological samples.
3 Results
3.1 CML38 is involved in NO inhibition of hypocotyl elongation
CML38 was previously identified as a NO-responsive gene in Arabidopsis seedling (Ren et al., 2024). Here, RT-qPCR showed that 7-day exogenous NO treatment significantly downregulated CML38 expression in Col-0 (p < 0.05; Figures 1A, B). Phenotypic analysis revealed that while NO effectively inhibited hypocotyl growth in Col-0 (p < 0.05), but not in cml38 (Figures 1C, D). These results demonstrate that CML38 functions as a key mediator in NO-induced suppression of hypocotyl elongation.
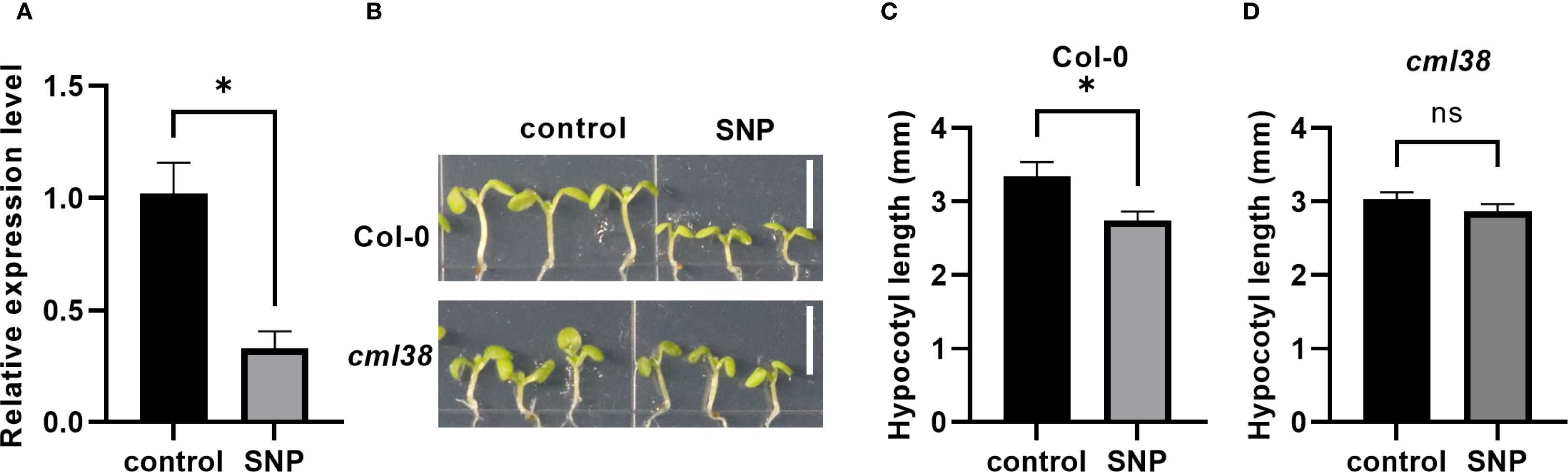
Figure 1. CML38 mediates NO-induced inhibition of hypocotyl elongation. (A) CML38 expression level in Col-0 under NO treatment. (B) Phenotype of Col-0 and cml38. Scale bars represent 3mm. (C) Hypocotyl length of Col-0. (D) Hypocotyl length of cml38. Data are presented as mean ± SEM; *p < 0.05, ns, not significant.
3.2 Ca2+ modulates the regulation of NO on CML38
To investigate whether the regulatory effect of NO on CML38 is Ca2+-dependent, we examined hypocotyl elongation under pharmacological modulation of Ca2+ availability (Figure 2). Exogenous CaCl2 partially alleviated NO-induced hypocotyl growth inhibition in Col-0. Conversely, disrupting Ca2+ signaling with either the extracellular chelator EGTA or the channel blocker RR enhanced NO sensitivity in cml38, suggesting CML38 is involved in NO-Ca2+ crosstalk. However, cml38 mutants exhibited a similar trend to Col-0 in NO-triggered [Ca2+]cyt increases (Figure 3), indicating that CML38 may act downstream of the initial calcium flux to mediate the growth response.
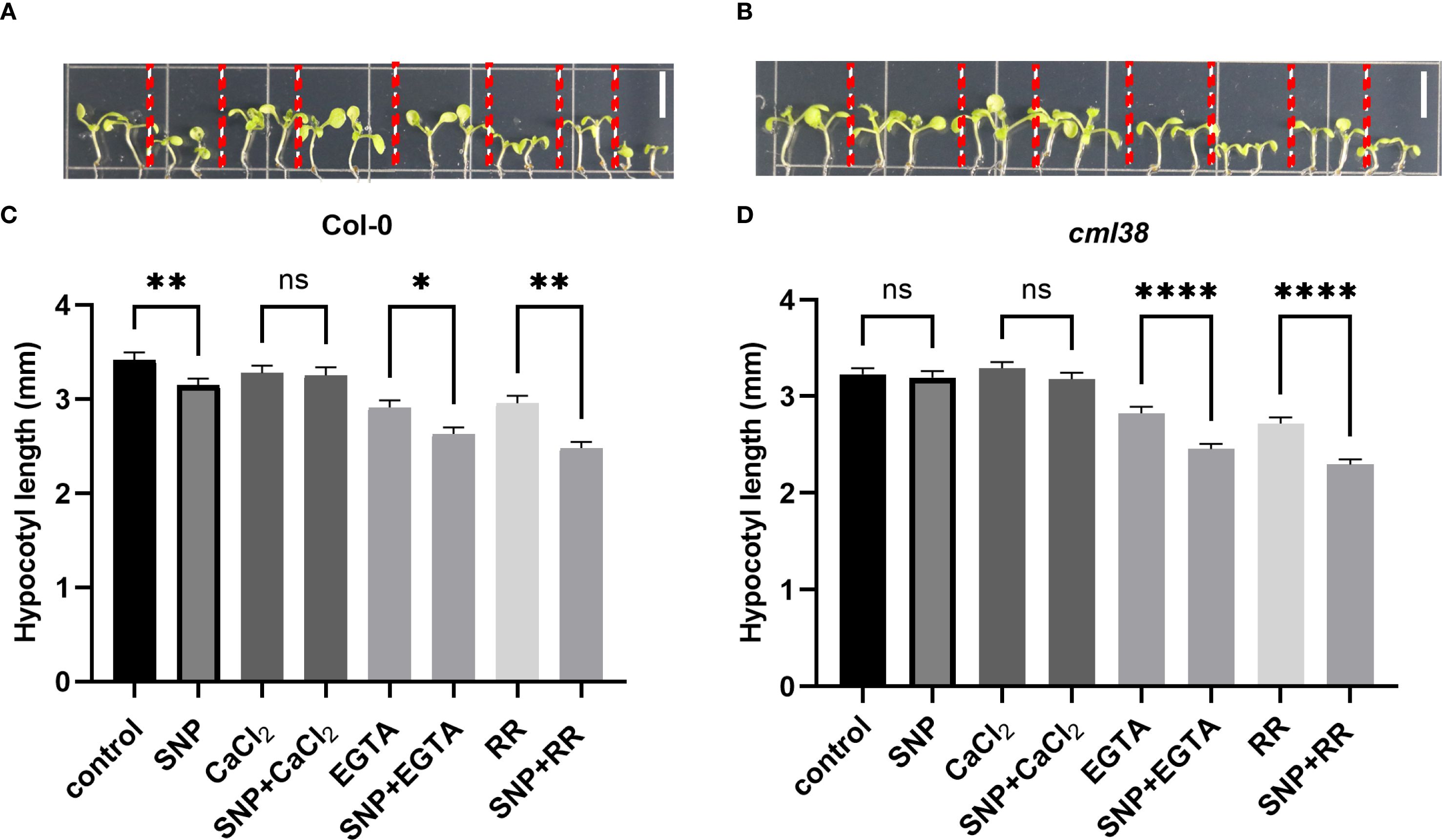
Figure 2. Ca2+ homeostasis modulates NO signaling in hypocotyl elongation. (A) Phenotype of Col-0. (B) Phenotype of cml38. (C) Hypocotyl length of Col-0. (D) Hypocotyl length of cml38. Scale bars in A and B represent 3mm. Data are presented as mean ± SEM; *p < 0.05, **p < 0.01, ****p < 0.0001, ns, not significant.
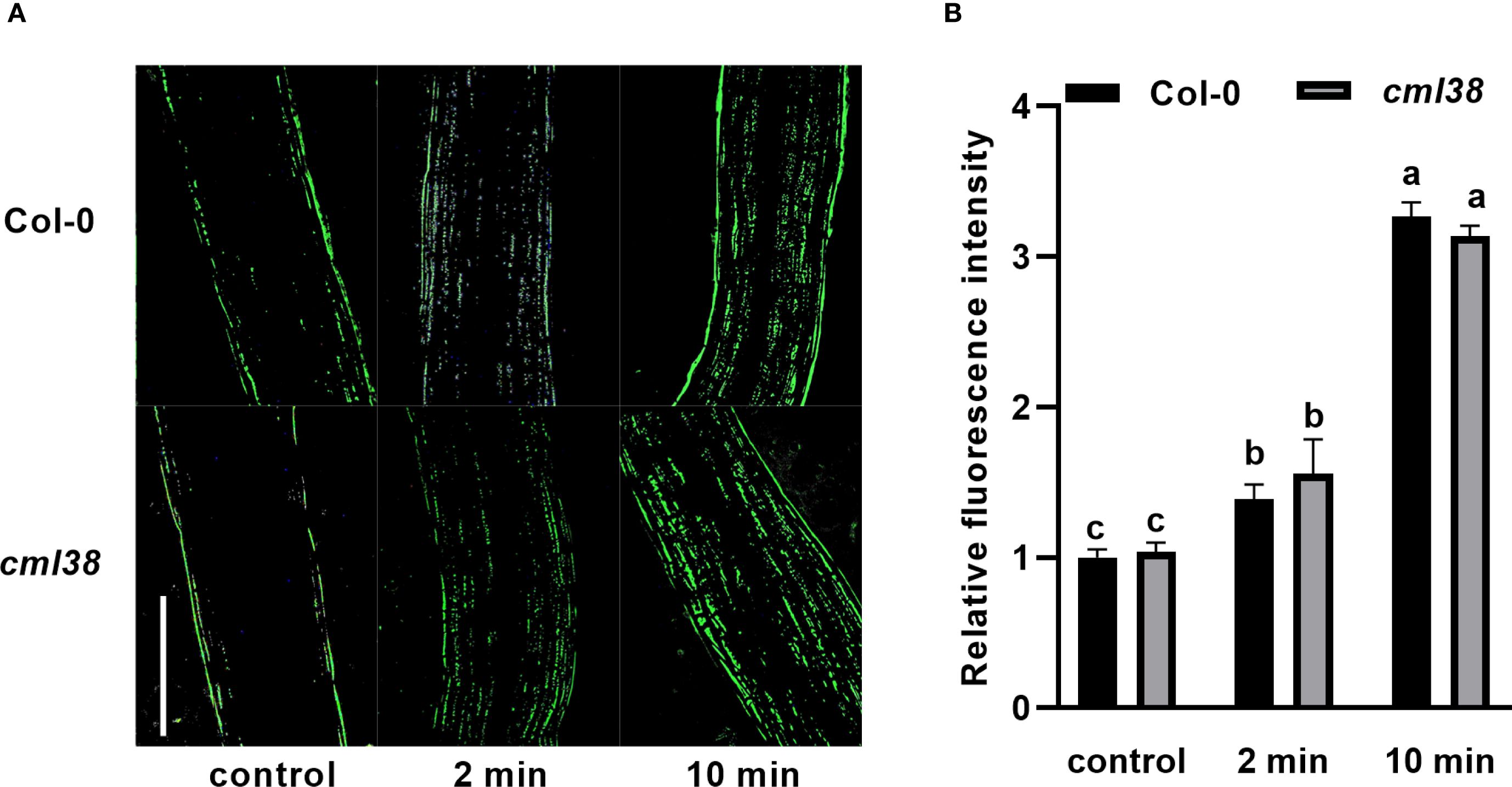
Figure 3. [Ca2+]cyt elevation in response to GSNO treatment is independent of CML38. (A) Representative fluorescent images of hypocotyls from Col-0 and cml38 treated with 20 μM GSNO for the indicated times. Scale bars represent 200 µm. (B) Quantification of relative fluorescence intensity from (A). Different letters indicate significant differences (p < 0.05).
3.3 NO does not affect CML38’s Ca2+-binding capability
The CML protein family represents a group of putative Ca2+ sensors in plants. CML38 is a 177-amino acid protein with a predicted molecular mass of 23 kDa. Notably, CML38 contains three evolutionarily conserved EF-hand motifs, characteristic calcium-binding domains that suggest its functional role in calcium signal perception and transduction. To assess the Ca2+-binding capability of CML38, we performed structural predictions. Computational modeling revealed that CML38 likely coordinates Ca2+ ions through specific residues including Met, Asp and Glu (Figure 4A).
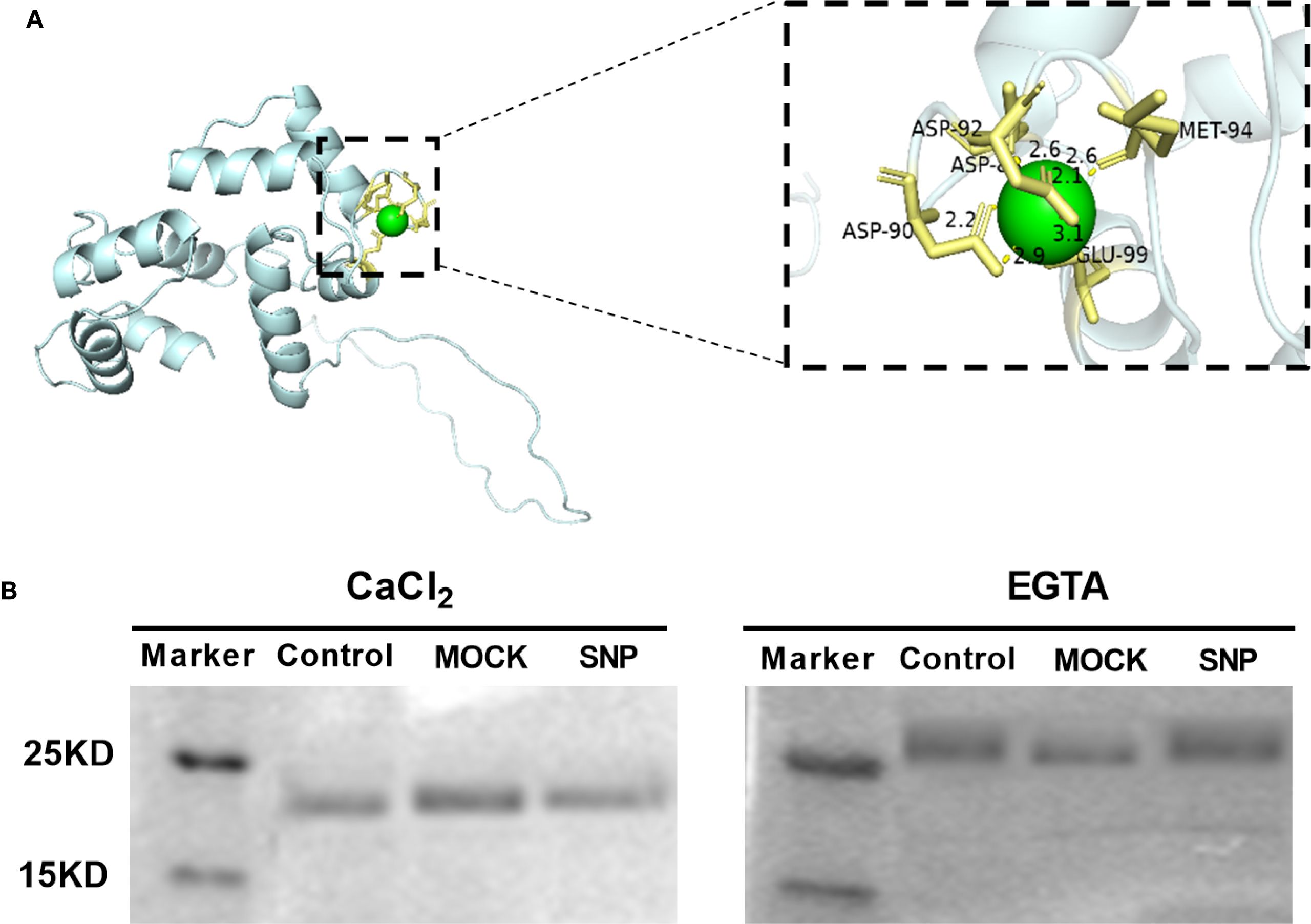
Figure 4. CML38 maintains Ca2+-binding activity independent of NO signaling. (A) Structural prediction of conserved Ca2+-binding site in CML38. (B) Ca2+-dependent electrophoretic mobility shift assay of recombinant CML38 under different treatments: Experimental: 200 µM fresh SNP (NO-producing); Negative control: equal volume of distilled H2O; Mock control: 200 µM light-exposed SNP (48-hour irradiated, NO-depleted).
SDS-PAGE mobility shift assays confirmed Ca2+-binding by CML38: faster migration was observed in the presence of Ca2+ versus EGTA (Figure 3B). To test NO effects, CML38 was treated with fresh SNP (NO-releasing) or light-exposed SNP (NO-depleted control). NO did not alter CML38’s migration pattern under identical Ca2+ conditions, indicating NO does not affect its Ca2+ binding capacity (Figure 4B).
3.4 Genome-wide identification of CML38-target genes in NO signaling
To gain deeper insights into the molecular pathways through which CML38 mediates NO-dependent regulation of hypocotyl elongation, we conducted comparative transcriptomic analysis between Col-0 and cml38 under SNP-treated condition. Using stringent thresholds (|log2FC| > 1, p < 0.05), we found that CML38 deficiency significantly altered the Arabidopsis transcriptome, with 168 genes significantly up-regulated and 351 genes down-regulated (Figure 5).
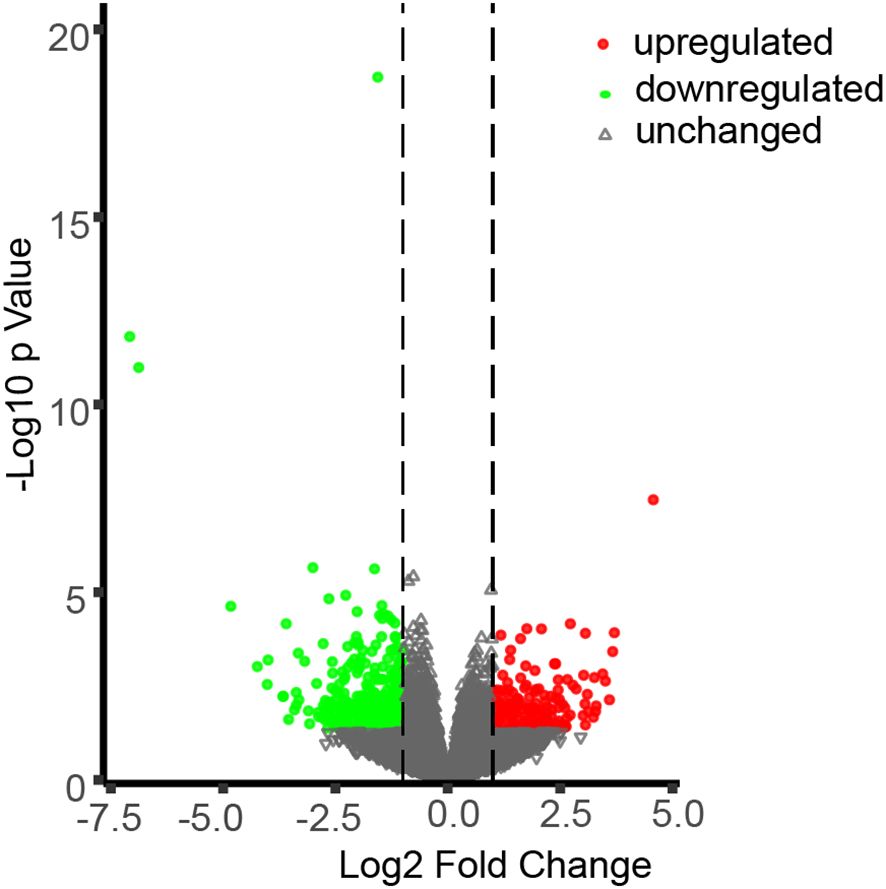
Figure 5. Differentially expressed genes (DEGs) in cml38 under SNP-treated condition. Green, red and grey symbols represent for significantly down-regulated, upregulated and unchanged genes in cml38, compared to Col-0 (|log2FC| > 1, p < 0.05).
To visually summarize the transcriptomic landscape and validate the distinctiveness of the experimental groups, we performed unsupervised hierarchical clustering of the samples based on the expression profiles of the top 30 most DEGs between Col-0 and cml38. As shown in Figure 6, the resulting heatmap revealed a clear and striking separation of the samples into two primary clusters that corresponded perfectly to Col-0 and cml38. This pronounced segregation provides strong evidence that the transcriptional profiles are fundamentally distinct between the two lines.
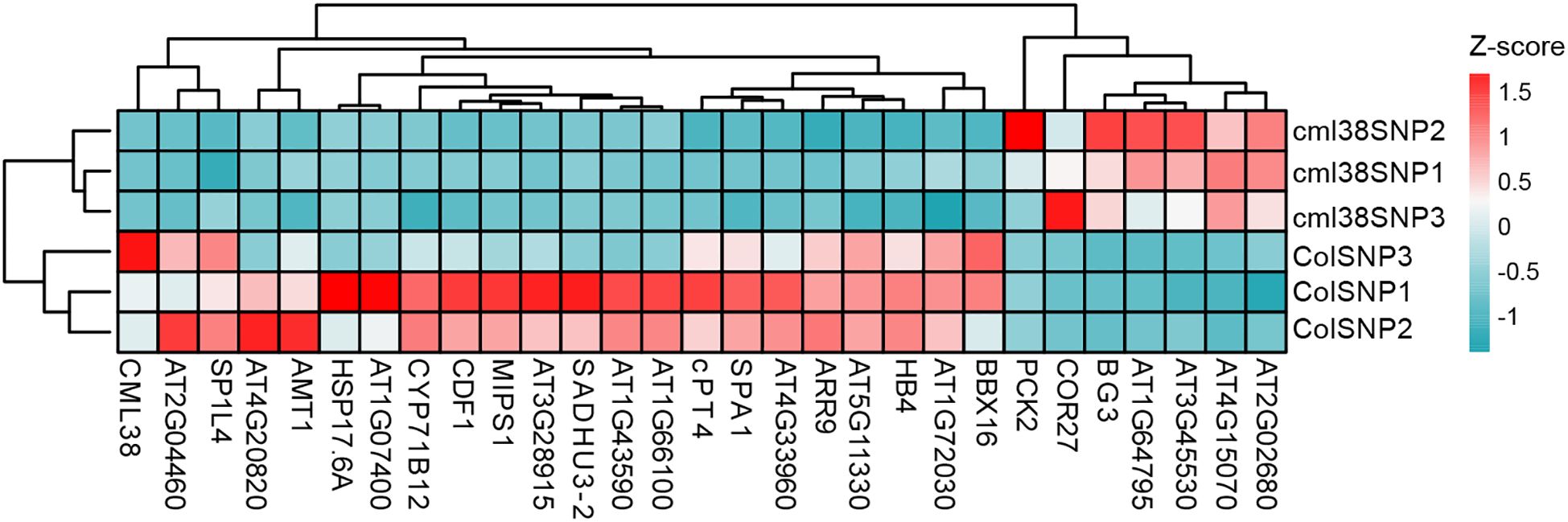
Figure 6. Unsupervised hierarchical clustering of 30 DEGs across all samples. The heatmap displays z-score normalized expression across all samples. The color key indicates relative expression levels: red represents higher expression, and blue represents lower expression.
3.5 GO enrichment analysis of CML38-regulated DEGs under NO treatment
GO enrichment analysis identified a total of 769 GO terms, reflecting the broad regulatory scope of CML38 in NO signaling. Considering light and phytohormones also play vital roles during hypocotyl elongation, we focused on three functionally relevant categories of GO terms: (1) calcium ion binding, signaling and homeostasis, (2) photoreceptor pathways (encompassing responses to red, far-red, and blue light responses), and (3) phytohormones biosynthesis and response. This allowed us to identify key regulatory networks potentially involved in CML38-dependent growth regulation.
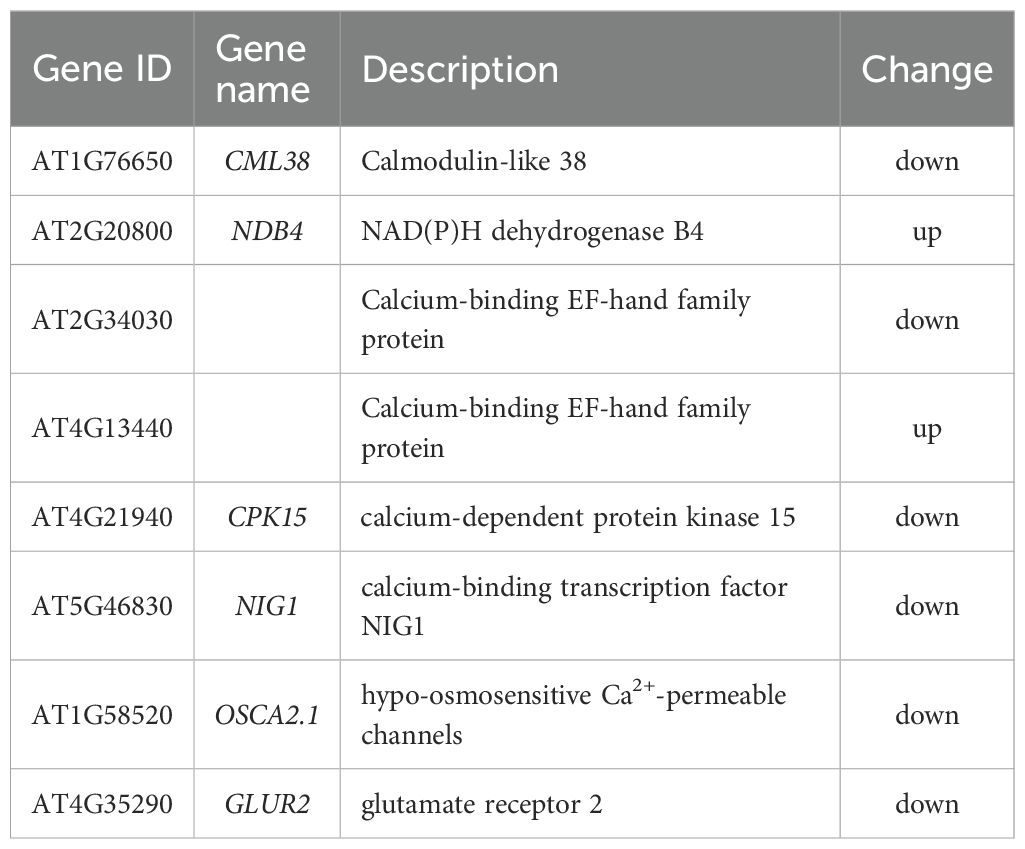
Compared to Col-0, the cml38 mutant exhibited 6 DEGs associated with Ca2+ binding, including 2 up-regulated genes (NDB4 and AT4G13440) and 4 down-regulated genes (CML38, CPK15, NIG1 and AT2G34030) genes in cml38 (Table 1). Furthermore, cml38 showed significant enrichment of 2 DEGs directly involved in Ca2+ transport and signal transduction: OSCA2.1 and GLUR2. These results suggest that CML38 acts as a key regulator of NO-induced Ca2+ signaling, likely by modulating the expression of above-mentioned genes.
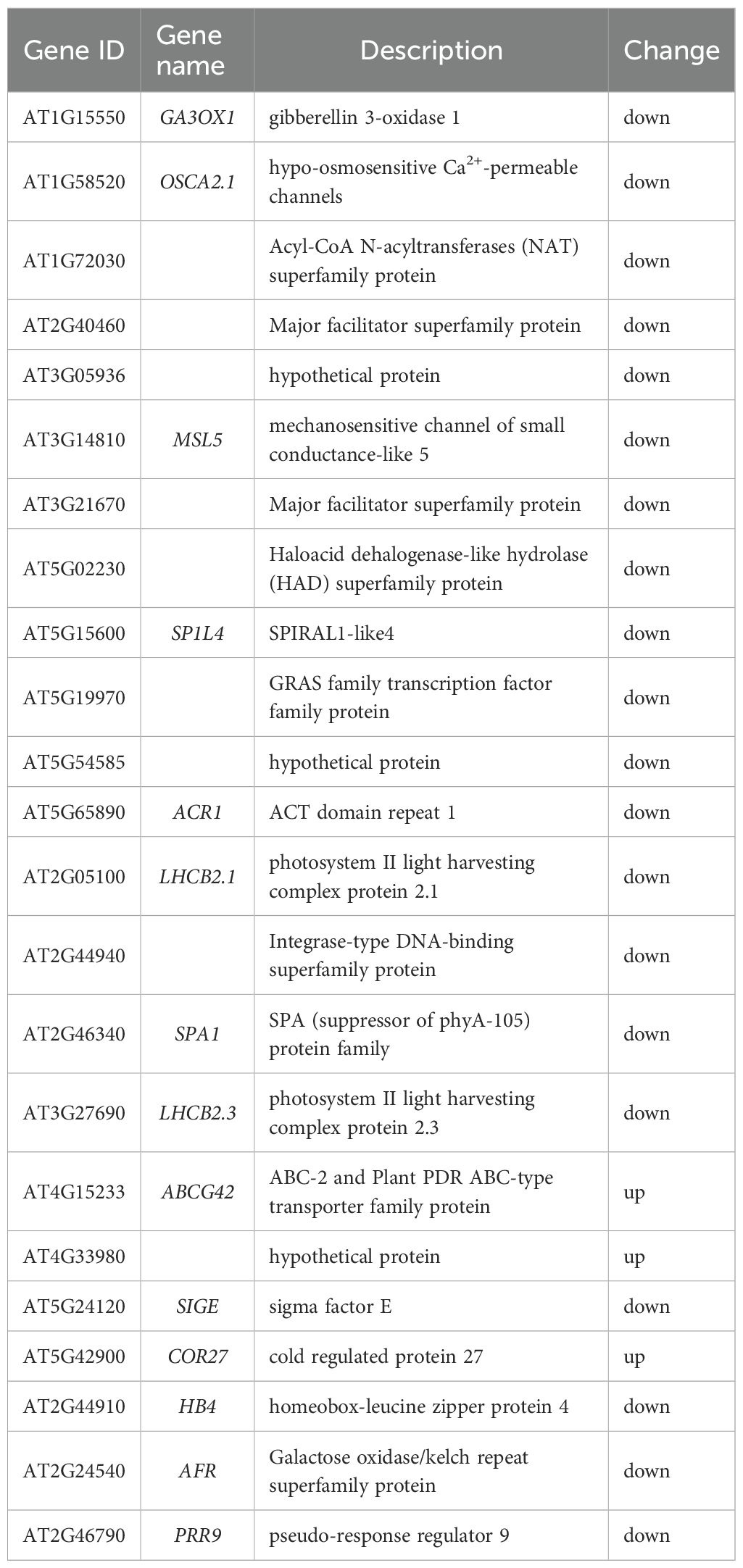
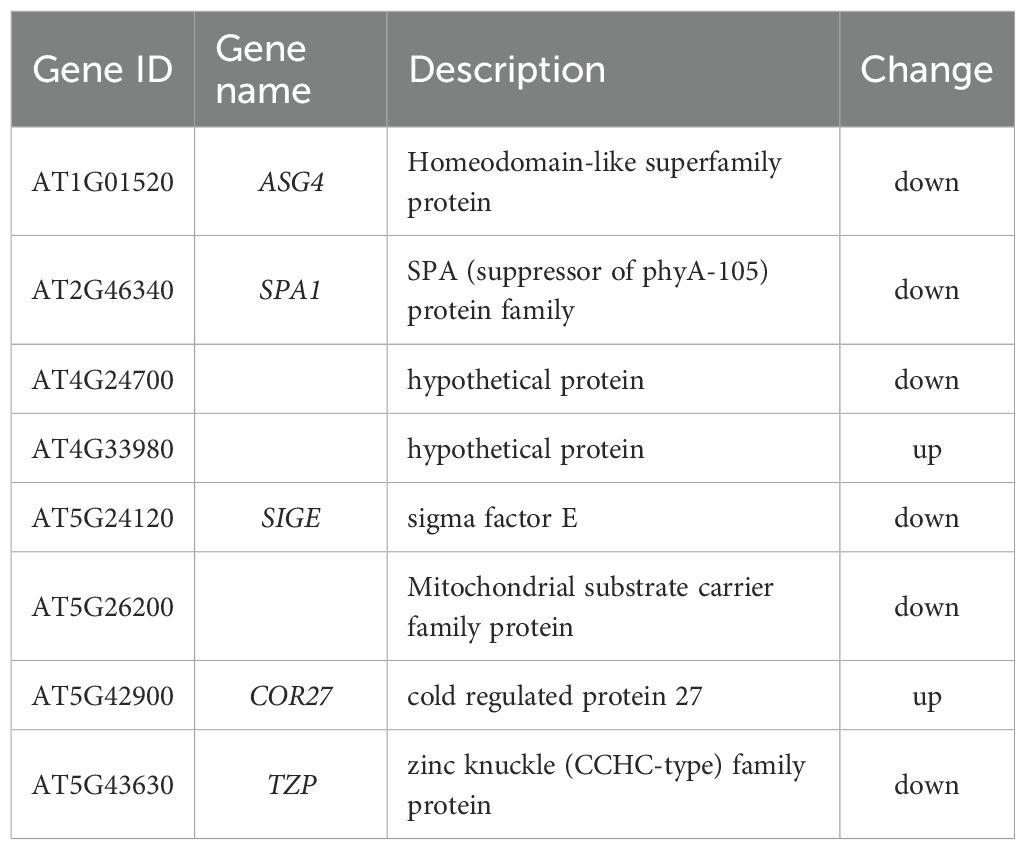
Light is a primary environmental cue that tightly regulates hypocotyl elongation through distinct photoreceptor systems: red light inhibits elongation via phytochrome activation, far-red light reverses this inhibition to promote growth, and blue light independently suppresses elongation through cryptochromes and phototropins (Chen et al., 2023). Emerging evidence indicates that NO serves as a key modulator of these light-mediated growth responses (Sanz et al., 2015), but the molecular links between NO and light signaling remain poorly understood. To investigate whether CML38 contributes to NO-light signal integration, we analyzed the expression of light-responsive genes in Col-0 and cml38 under SNP treatment. Our transcriptomic data revealed striking differences in light-responsive gene expression profiles between the two genotypes. Specifically, CML38 deficiency altered the expression of 23 red/far-red light-responsive genes and 8 blue light-responsive genes in the presence of NO (Tables 2, 3). These findings strongly suggest that CML38 participates in the integration of NO and light signaling pathways during hypocotyl development.
NO coordinates with multiple phytohormone pathways to regulate hypocotyl elongation. CML38 deficiency influenced NO-induced signal transduction of phytohormones. Our GO enrichment analysis revealed that CML38 deficiency significantly impacts NO-induced transcriptional changes in genes associated with 8 major phytohormone pathways: jasmonic acid, auxin, abscisic acid, brassinosteroid, salicylic acid, cytokinin, gibberellin and ethylene, were identified in SNP treated cml38 (Supplementary Table S2).
3.6 KEGG enrichment analysis of DEGs
KEGG pathway enrichment analysis was performed to identify the metabolic pathways regulated by DEGs, elucidating how NO mediated by CML38 influences hypocotyl elongation in Arabidopsis. The results revealed that CML38 deficiency, in the presence of NO, led to the enrichment of 59 pathways, among which plant hormone signal transduction, circadian rhythm, MAPK signaling pathway, ubiquitin mediated proteolysis, zeatin, phenylpropanoid, cutin, suberine and wax biosynthesis, as well as alpha-linolenic acid, inositol phosphate and glycerophospholipid metabolism may involve in hypocotyl elongation (Supplementary Table S3).
3.7 Validation of transcriptome trofiles by RT-qPCR
To independently validate the reliability of the transcriptome profiles obtained by RNA-Seq, we performed reverse RT-qPCR on a subset of 12 DEGs. These genes were randomly selected from those implicated in the Ca2+ signaling, light signaling and phytohormone pathways, which our analysis suggested were interconnected with CML38-mediated signaling (Tables 1–3; Supplementary Table S2). The expression trends of all 12 genes as determined by RT-qPCR were entirely consistent with the patterns observed in the RNA-Seq data (Figure 7). This high degree of concordance demonstrates the high reproducibility and accuracy of our transcriptomic analysis and confirms the differential expression of key genes involved in the interplay between NO/Ca2+, light, and phytohormone signaling.
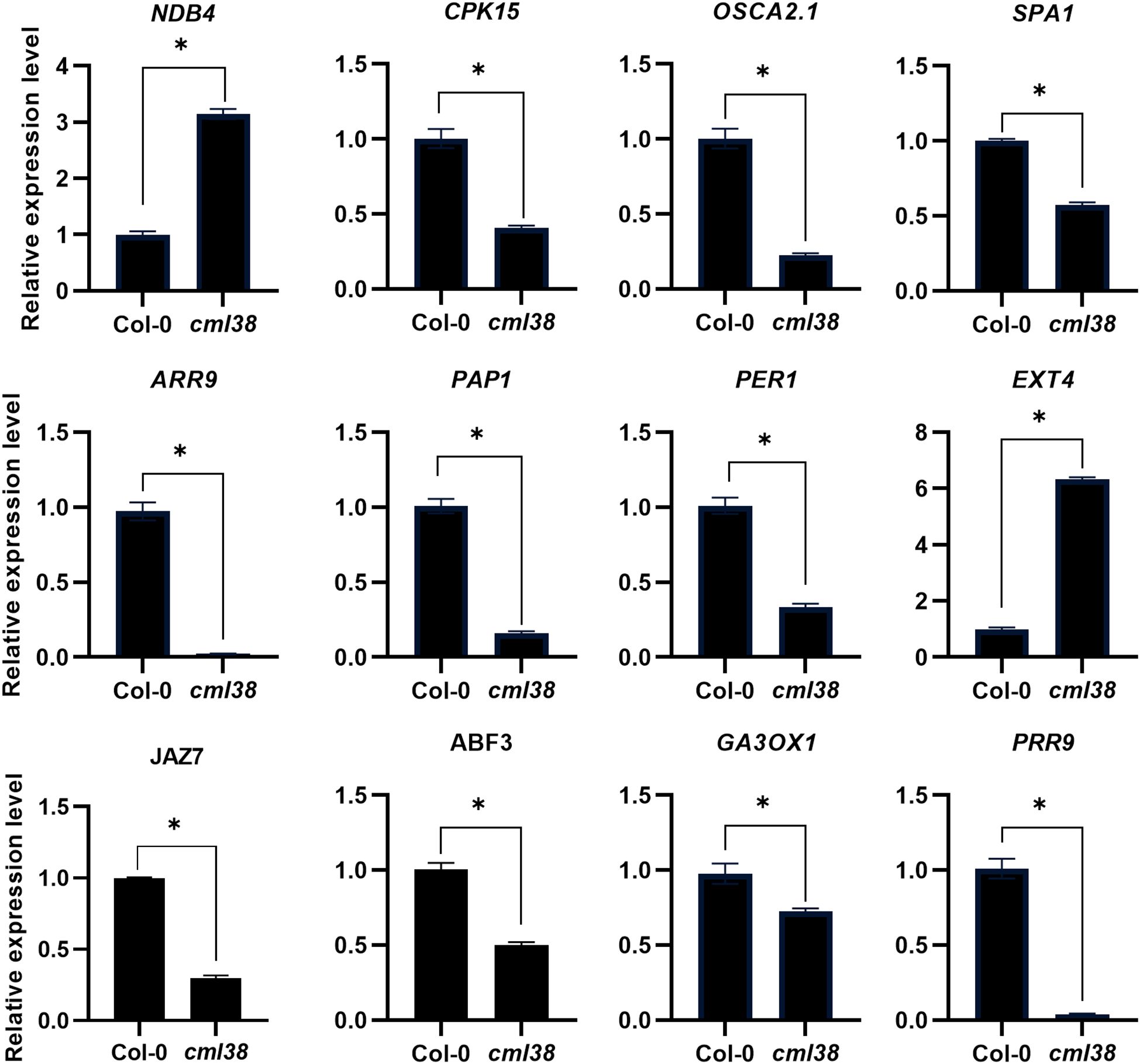
Figure 7. Validation of RNA-seq results and expression analysis of key genes related to Ca2+, light and phytohormone signaling by RT-qPCR. Data are presented as mean ± SEM; *p < 0.05.
4 Discussion
Hypocotyl elongation represents a spatiotemporally regulated developmental process crucial for plant growth and development, playing a pivotal role in ensuring successful life cycle completion. This complex process is precisely controlled by both environmental factors (including light and temperature) and endogenous signaling molecules/growth regulators (Oh et al., 2014). Although exogenous NO treatment has been shown to inhibit hypocotyl elongation in Arabidopsis (Sanz et al., 2015), the precise molecular mechanisms underlying this regulation remain poorly understood. Among CMLs, current research has predominantly investigated their functions in seed germination, flowering, and fruit development (Munir et al., 2016; Wu et al., 2017). To date, two family members, CML23 and CML39, were demonstrated to participate in hypocotyl elongation regulation (Bender et al., 2013; Wang et al., 2025). Similar to CML23, our study identifies CML38 also as a crucial signaling hub integrating NO, Ca2+, light and phytohormones pathways to regulate hypocotyl elongation. However, mechanistic exploration reveals distinct modes of action between these two closely related Ca2+ sensors.
The interplay between NO and Ca2+ constitutes a bidirectional regulatory loop that is central to plant signaling. Ca2+ serve as critical downstream signaling components in NO-mediated plant pathways, with their interplay exhibiting complex, context-dependent regulation. NO influences cytosolic Ca2+ ([Ca2+]cyt) dynamics through multiple mechanisms: it triggers intracellular Ca2+ release and modulates plasma membrane channels while altering membrane potential (Vandelle et al., 2006), yet paradoxically can also reduce [Ca2+]cyt by stimulating Ca2+-ATPases (Courtois et al., 2008). Experimental evidence demonstrates NO-induced Ca2+ elevation in guard cells and intracellular stores (Garcia-Mata et al., 2003), with similar regulation observed during ABA-induced stomatal closure, auxin-mediated root formation (Desikan et al., 2002), and stress responses (Vandelle et al., 2006). However, antagonistic effects exist—NO-generated cGMP activates Ca2+/calmodulin-dependent kinases that inhibit Ca2+ pumps, promoting efflux (Chai et al., 2011). Our data corroborate this duality: CaCl2 rescued NO-induced hypocotyl inhibition in Col-0, whereas application of SNP combined with EGTA or RR suppressed hypocotyl elongation in both Col-0 and cml38. Notably, however, cml38 mutants exhibited a similar trend to Col-0 in NO-triggered [Ca2+]cyt increases, suggesting that CML38 acts independently of initial calcium flux. Electrophoretic mobility assays confirmed that CML38 retains Ca2+-binding activity unaffected by NO, indicating that NO may regulate CML38 through mechanisms beyond calcium binding. This stands in sharp contrast to CML23, whose Ca²+-binding ability is directly modulated by NO via S-nitrosylation and is indispensable for mediating the NO-induced initial cytosolic Ca²+ elevation (Wang et al., 2025), fully revealing the distinct mechanisms by which the two CMLs participate in NO- Ca2+ crosstalk.
Furthermore, compared to CML23, CML38 governs a smaller, more distinct subset of target genes. Within the Ca2+ signaling network, CML38 deficiency impairs NO-mediated regulation of three critical Ca2+-related genes: NDB4, which modulates mitochondrial electron transport in a Ca2+-dependent manner (Geisler et al., 2007); CPK15, as a calcium-dependent protein kinase involved in in plant growth and stress responses (Manimaran et al., 2015); OSCA2.1, a plasma membrane-localized Ca2+-permeable channel that mediates Ca2+ influx in response to osmotic stress (Pei et al., 2024). In light signaling, CML38 deficiency reduces SPA1 and GA3OX1 expression upon NO treatment, implying a potential NO–CML38–COP1/SPA regulatory axis in hypocotyl elongation. Light critically regulates hypocotyl elongation through distinct photoreceptors: phytochrome B (phyB) perceives red light and phytochrome A (phyA) mediates far-red light responses. These photoreceptors converge on the central phy-PIF-COP1/SPA pathway to modulate hypocotyl growth by controlling the stability and activity of PIF transcription factors. PIFs promote cell elongation by activating genes associated with cell expansion and gibberellin biosynthesis. Notably, phyB inhibits while phyA promotes PIF accumulation. In darkness or short days, PIFs accumulate and enhance elongation; under prolonged red light, PIFs are phosphorylated and degraded, inhibiting growth (Chen et al., 2023). The COP1/SPA complex serves as a key regulatory switch: under dark conditions, it targets photomorphogenesis-promoting proteins such as HY5 for degradation, thereby stabilizing PIFs (Ling et al., 2017). In the light, activated phytochromes suppress COP1/SPA activity, leading to HY5 accumulation and PIF degradation, which collectively restrain hypocotyl elongation (Choi and Oh, 2016).
Furthermore, CML38 mediates extensive crosstalk between NO and phytohormone signaling. CML38 deficiency alters zeatin, α-linolenic acid, inositol phosphate, and glycerophospholipid metabolism, suggesting broad effects on phytohormone biosynthesis. Additionally, CML38 deficiency upregulates PRR3 while downregulating PAP1, SPA1, PRR9, and CDF1 in the circadian rhythm pathway, linking photoperiod sensing to hormone-mediated growth regulation. The MAPK signaling pathway, which transduces multiple hormone signals, is also affected—CML38 deficiency downregulates CAT2, VSP2, and HAI1, impacting abscisic acid and jasmonic acid signaling. Meanwhile, ubiquitin-mediated proteolysis promotes auxin and gibberellin signaling by degrading inhibitors (e.g., AUX/IAA and DELLA proteins) (Santner and Estelle, 2010), with UBQ8 upregulated in CML38-deficient plants.
While our transcriptomic and phenotypic data strongly suggest that CML38 operates at the intersection of NO, light, and hormone signaling, a key limitation of this study is that we have not yet definitively established the genetic hierarchy between these pathways. As rightly noted, future research should employ genetic epistasis analysis by characterizing NO sensitivity in higher-order mutants between cml38 and key light signaling mutants (e.g., phyB, pif3, cop1) or hormone biosynthesis/signaling mutants (e.g., ga3ox1, spa1, abf3). Furthermore, utilizing hormone biosynthetic and signaling reporters or ELISA techniques in the cml38 background under NO treatment will be essential to visually quantify and confirm the effect of CML38 on hormonal dynamics (Ondzighi-Assoume et al., 2016). These rigorous genetic and cell biological approaches will be the focus of our future work to precisely map the position of CML38 within this complex regulatory network.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA1255309.
Author contributions
DW: Conceptualization, Funding acquisition, Supervision, Writing – original draft, Writing – review & editing. ZL: Investigation, Writing – original draft, Writing – review & editing. XZ: Investigation, Writing – original draft, Writing – review & editing. YB: Investigation, Writing – original draft, Writing – review & editing. WL: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was supported by Fundamental Research Program of Shanxi Province (202103021224256).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1684245/full#supplementary-material
References
Bender, K. W., Rosenbaum, D. M., Vanderbeld, B., Ubaid, M., and Snedden, W. A. (2013). The Arabidopsis calmodulin-like protein, CML39, functions during early seedling establishment. Plant J. 76, 634–647. doi: 10.1111/tpj.12323
Chai, Y., Zhang, D. M., and Lin, Y. F. (2011). Activation of cGMP-dependent protein kinase stimulates cardiac ATP-sensitive potassium channels via a ROS/calmodulin/CaMKII signaling cascade. PLoS One 6, e18191. doi: 10.1371/journal.pone.0018191
Chen, H., Wang, W., Chen, X., Niu, Y., Qi, Y., Yu, Z., et al. (2023). PIFs interact with SWI2/SNF2-related 1 complex subunit 6 to regulate H2A.Z deposition and photomorphogenesis in Arabidopsis. J. Genet. Genomics 50, 983–992. doi: 10.1016/j.jgg.2023.04.008
Choi, H. and Oh, E. (2016). PIF4 integrates multiple environmental and hormonal signals for plant growth regulation in Arabidopsis. Mol. Cells 39, 587–593. doi: 10.14348/molcells.2016.0126
Courtois, C., Besson, A., Dahan, J., Bourque, S., Dobrowolska, G., Pugin, A., et al. (2008). Nitric oxide signaling in plants: Interplays with Ca2+ and protein kinases. J. Exp. Bot. 59, 155–163. doi: 10.1093/jxb/erm197
Delk, N. A., Johnson, K. A., Chowdhury, N. I., and Braam, J. (2005). CML24, regulated in expression by diverse stimuli, encodes a potential Ca2+ sensor that functions in responses to abscisic acid, daylength, and ion stress. Plant Physiol. 139, 240–253. doi: 10.1104/pp.105.062612
Desikan, R., Griffiths, R., Hancock, J., and Neill, S. (2002). A new role for an old enzyme: Nitrate reductase-mediated nitric oxide generation is required for abscisic acid-induced stomatal closure in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 99, 16314–16318. doi: 10.1073/pnas.252461999
Ding, X., Zhang, L., Hao, Y., Xiao, S., Wu, Z., Chen, W., et al. (2018). Genome-wide identification and expression analyses of the calmodulin and calmodulin-like proteins reveal their involvement in stress response and fruit ripening in papaya. Postharvest Biol. Technol. 143, 13–27. doi: 10.1016/j.postharvbio.2018.04.010
Field, S., Conner, W. C., and Roberts, D. M. (2021). Arabidopsis CALMODULIN-LIKE 38 regulates hypoxia-induced autophagy of SUPPRESSOR OF GENE SILENCING 3 bodies. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.722940
Garcia-Mata, C., Gay, R., Sokolovski, S., Hills, A., Lamattiina, L., and Blatt, M. (2003). Nitric oxide regulates K+ and Cl- channels in guard cells through a subset of abscisic acid-evoked signaling pathways. Proc. Natl. Acad. Sci. U.S.A. 100, 11116–11121. doi: 10.1073/pnas.1434381100
Geisler, D. A., Broselid, C., Hederstedt, L., and Rasmusson, A. G. (2007). Ca2+-binding and Ca2+-independent respiratory NADH and NADPH dehydrogenases of Arabidopsis thaliana. J. Biol. Chem. 282, 28455–28464. doi: 10.1074/jbc.M704674200
Hu, Z., Zhang, B., Lim, L. J. Y., Loh, W. Z. K., Yu, D., Tan, B. W. Q., et al. (2022). S-Nitrosylation-mediated reduction of CaV1.2 surface expression and open probability underlies attenuated vasoconstriction induced by nitric oxide. Hypertension 79, 2854–2866. doi: 10.1161/HYPERTENSIONAHA.122.19103
Jeandroz, S., Lamotte, O., Astier, J., Rasul, S., Trapet, P., Besson-Bard, A., et al. (2013). There’s more to the picture than meets the eye: Nitric oxide cross talk with Ca2+ signaling. Plant Physiol. 163, 459–470. doi: 10.1104/pp.113.220624
Kudla, J., Batistič, O., and Hashimoto, K. (2010). Calcium signals: the lead currency of plant information processing. Plant Cell 22, 541–563. doi: 10.1105/tpc.109.072686
Leba, L. J., Cheval, C., Ortiz-Martín, I., Ranty, B., Beuzón, C. R., Galaud, J., et al. (2012). CML9, an Arabidopsis calmodulin-like protein, contributes to plant innate immunity through a flagellin-dependent signaling pathway. Plant J. 71, 976–989. doi: 10.1111/j.1365-313X.2012.05045.x
Ling, J. J., Li, J., Zhu, D., and Deng, X. W. (2017). Noncanonical role of Arabidopsis COP1/SPA complex in repressing BIN2-mediated PIF3 phosphorylation and degradation in darkness. Proc. Natl. Acad. Sci. U.S.A. 114, 3539–3544. doi: 10.1073/pnas.1700850114
Lokdarshi, A., Craig Conner, W., McClintock, C., Li, T., and Roberts, D. M. (2016). Arabidopsis CML38, a calcium sensor that localizes to ribonucleoprotein complexes under hypoxia stress. Plant Physiol. 170, 1046–1059. doi: 10.1104/pp.15.01407
Lu, C. H., Chen, C. C., Yu, C. S., Liu, C. S., Liu, J. J., Wei, S. T., et al. (2022). MIB2: metal ion-binding site prediction and modeling server. Bioinformatics 38, 4428–4429. doi: 10.1093/bioinformatics/btac534
Manimaran, P., Mangrauthia, S. K., Sundaram, R. M., and Balachandran, S. M. (2015). Constitutive expression and silencing of a novel seed specific calcium dependent protein kinase gene in rice reveals its role in grain filling. J. Plant Physiol. 174, 41–48. doi: 10.1016/j.jplph.2014.09.005
McCormack, E. and Braam, J. (2003). Calmodulins and related potential calcium sensors of Arabidopsis. New Phytol. 159, 585–598. doi: 10.1046/j.1469-8137.2003.00845.x
Munir, S., Khan, M. R. G., Song, J., Munir, S., Zhang, Y., Ye, Z., et al. (2016). Genome-wide identification, characterization and expression analysis of calmodulin-like (CML) proteins in tomato (Solanum lycopersicum). Plant Physiol. Bioch. 102, 167–179. doi: 10.1016/j.plaphy.2016.02.020
Oh, E., Zhu, J. Y., Bai, M. Y., Arenhart, R. A., Sun, Y., and Wang, Z. Y. (2014). Cell elongation is regulated through a central circuit of interacting transcription factors in the Arabidopsis hypocotyl. eLife 2014, e03031. doi: 10.7554/eLife.03031
Ondzighi-Assoume, C. A., Chakrabort, S., and Harri, J. M. (2016). Environmental nitrate stimulates abscisic acid accumulation in arabidopsis root tips by releasing it from inactive stores. Plant Cell 28, 729–745. doi: 10.1105/tpc.15.00946
Pei, S., Tao, Q., Li, W., Qi, G., Wang, B., Wang, Y., et al. (2024). Osmosensor-mediated control of Ca2+ spiking in pollen germination. Nature 629, 1118–1125. doi: 10.1038/s41586-024-07445-6
Pirayesh, N., Giridhar, M., Ben Khedher, A., Vothknecht, U. C., and Chigri, F. (2021). Organellar calcium signaling in plants: An update. Biochim. Biophys. Acta Mol. Cell Res. 1868, 118948. doi: 10.1016/j.bbamcr.2021.118948
Ren, H., Wang, Z., Shang, X., Zhang, X., Ma, L., Bian, Y., et al. (2024). Involvement of GA3-oxidase in inhibitory effect of nitric oxide on primary root growth in Arabidopsis. Plant Biol. 2024, 26. doi: 10.1111/plb.13600
Santner, A. and Estelle, M. (2010). The ubiquitin-proteasome system regulates plant hormone signaling. Plant J. 61, 1029–1040. doi: 10.1111/j.1365-313X.2010.04112.x
Sanz, L., Albertos, P., Mateos, I., Sánchez-Vicente, I., Lechón, T., Fernández-Marcos, M., et al. (2015). Nitric oxide (NO) and phytohormones crosstalk during early plant development. J. Exp. Bot. 66, 2857–2868. doi: 10.1093/jxb/erv213
Song, X., Li, J., Lyu, M., Kong, X., Hu, S., Song, Q., et al. (2021). CALMODULIN-LIKE-38 and PEP1 RECEPTOR 2 integrate nitrate and brassinosteroid signals to regulate root growth. Plant Physiol. 187, 1779–1794. doi: 10.1093/plphys/kiab323
Symonds, K., Teresinski, H., Hau, B., Chiasson, D., Benidickson, K., Plaxton, W., et al. (2024). Arabidopsis CML13 and CML14 have essential and overlapping roles in plant development. Plant Cell Physiol. 65, 228–242. doi: 10.1093/pcp/pcad142
Tsai, Y. C., Delk, N. A., Chowdhury, N. I., and Braam, J. (2007). Arabidopsis potential calcium sensors regulate nitric oxide levels and the transition to flowering. Plant Signal. Behav. 2, 446–454. doi: 10.4161/psb.2.6.4695
Vandelle, E., Poinssot, B., Wendehenne, D., Bentéjac, M., and Pugin, A. (2006). Integrated signaling network involving calcium, nitric oxide, and active oxygen species but not mitogen-activated protein kinases in BcPG1-elicited grapevine defenses. Mol. Plant Microbe In. 19, 429–440. doi: 10.1094/MPMI
Vanderbeld, B. and Snedden, W. A. (2007). Developmental and stimulus-induced expression patterns of Arabidopsis calmodulin-like genes CML37, CML38 and CML39. Plant Mol. Biol. 64, 683–697. doi: 10.1007/s11103-007-9189-0
Wang, D. S., Zhang, X. D., Qiao, Y. Y., and Liu, W. Z. (2025). CML23 mediates NO induced Ca2+ signaling during hypocotyl elongation in Arabidopsis. Plant Sci. 359, 112619. doi: 10.1016/j.plantsci.2025.112619
Wang, S. S., Diao, W. Z., Yang, X., Qiao, Z., Wang, M., Acharya, B. R., et al. (2015). Arabidopsis thaliana CML25 mediates the Ca2+ regulation of K+ transmembrane trafficking during pollen germination and tube elongation. Plant Cell Environ. 38, 2372–2386. doi: 10.1111/pce.12559
Wang, Y., Zhang, W. Z., Song, L. F., Zou, J. J., Su, Z., and Wu, W. H. (2008). Transcriptome analyses show changes in gene expression to accompany pollen germination and tube growth in Arabidopsis. Plant Physiol. 148, 1201–1211. doi: 10.1104/pp.108.126375
Wu, X., Qiao, Z., Liu, H., Acharya, B. R., Li, C., and Zhang, W. (2017). CML20, an Arabidopsis calmodulin-like protein, negatively regulates guard cell ABA signaling and drought stress tolerance. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.00824
Yang, X., Wang, S. S., Wang, M., Qiao, Z., Bao, C., and Zhang, W. (2014). Arabidopsis thaliana calmodulin-like protein CML24 regulates pollen tube growth by modulating the actin cytoskeleton and controlling the cytosolic Ca2+ concentration. Plant Mol. Biol. 86, 225–236. doi: 10.1007/s11103-014-0220-y
Keywords: nitric oxide, calcium, CML38, light signaling, phytohormone signaling
Citation: Wang D, Li Z, Zhang X, Bian Y and Liu W (2025) CML38 is involved in NO-induced inhibition of hypocotyl elongation in Arabidopsis. Front. Plant Sci. 16:1684245. doi: 10.3389/fpls.2025.1684245
Received: 12 August 2025; Accepted: 24 September 2025;
Published: 08 October 2025.
Edited by:
Giampiero Cai, University of Siena, ItalyReviewed by:
Dongchao Ji, Shandong University of Technology, ChinaEhsan Sadeghnezhad, University of Tehran, Iran
Copyright © 2025 Wang, Li, Zhang, Bian and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongsheng Wang, d2FuZ2RzQHN4bnUuZWR1LmNu; Weizhong Liu, bGl1d3poQHN4bnUuZWR1LmNu
†These authors have contributed equally to this work
 Dongsheng Wang
Dongsheng Wang Zhaoyun Li†
Zhaoyun Li†