- 1Center for Yunnan Plateau Biological Resources Protection and Utilization, College of Biology and Food Engineering, Qujing Normal University, Qujing, Yunnan, China
- 2School of Medical, Molecular and Forensic Sciences, Murdoch University, Murdoch, WA, Australia
- 3College of Biodiversity Conservation, Southwest Forestry University, Kunming, China
- 4Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai, Thailand
- 5Environmental Science Research Center (ESRC), Chiang Mai University, Chiang Mai, Thailand
- 6Natural Extracts and Innovative Products for Alternative Healthcare Research Group, Faculty of Science, Chiang Mai University, Chiang Mai, Thailand
Ganoderma, a well-known medicinal mushroom, has garnered attention for its broad therapeutic properties, particularly its potent antimicrobial activities. This review focuses on the mechanisms of action and bioactive compounds responsible for the ability of Ganoderma to inhibit various pathogenic microorganisms. The polysaccharides, triterpenoids, proteins, and phenolic compounds in Ganoderma exhibit strong antimicrobial effects by targeting bacterial cell walls, disrupting membrane integrity, and inhibiting key microbial enzymes. These compounds are effective against a wide range of bacteria, including Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and various fungi. Triterpenoids, specifically, have demonstrated efficacy in modulating immune responses, further enhancing the body’s defense mechanisms against infections. Furthermore, the role of Ganoderma in preventing biofilm formation and combating antibiotic-resistant strains highlights its potential as a natural antimicrobial agent. While in vitro and in vivo studies strongly support the antimicrobial properties of Ganoderma, future resety -50arch should focus on large-scale clinical trials to confirm its efficacy and explore its synergistic effects with conventional antibiotics. Establishing standardized dosages and exploring the molecular pathways of its antimicrobial actions will be key to incorporating Ganoderma into clinical practice for infection control.
1 Introduction
Ganoderma is a genus of medicinal mushrooms used for thousands of years in traditional East Asian medicine. Revered for its numerous therapeutic benefits, Ganoderma has gained significant attention in modern scientific research due to its bioactive compounds exhibiting various pharmacological activities (Karunarathna et al., 2024a). Among these activities, its antimicrobial properties stand out as an area of growing interest, particularly in an era where antimicrobial resistance (AMR) poses a significant global health threat (Pandey et al., 2020). Understanding the mechanisms by which Ganoderma exerts its antimicrobial effects is critical for developing novel therapies that harness its bioactive compounds to combat various infectious diseases (Mousavi et al., 2023; Karunarathna et al., 2024b). The antimicrobial properties of Ganoderma are attributed primarily to its rich content of bioactive compounds such as polysaccharides, triterpenoids, phenolic compounds, proteins, and peptides (Ahmad, 2019; Cadar et al., 2023). These compounds have been shown to work synergistically to inhibit the growth of various pathogenic microorganisms, including bacteria, fungi, and viruses. Historically, Ganoderma has been used in traditional medicine to treat infections, improve immune function, and promote overall health. These traditional uses are being validated by scientific research, which has provided evidence for Ganoderma’s effectiveness in inhibiting microbial growth and enhancing immune responses to infections.
One of the most studied bioactive compounds in Ganoderma is polysaccharides, particularly β-glucans, which are known to modulate immune responses and exhibit strong antimicrobial effects. Polysaccharides have been shown to activate macrophages and other immune cells, enhancing the ability of the body to detect and eliminate microbial pathogens. Triterpenoids, another significant class of compounds in Ganoderma, have demonstrated the ability to disrupt microbial cell walls and inhibit the replication of pathogens, particularly bacteria and fungi (Liu et al., 2022). In addition to these, phenolic compounds and polyketides of farnesyl quonines types and peptides isolated from Ganoderma also play crucial roles in its antimicrobial activity by scavenging free radicals, reducing oxidative stress, and enhancing the body’s natural defense mechanisms (Basnet et al., 2017). The antimicrobial properties of Ganoderma have been documented in various in vitro and in vivo studies, which have explored its efficacy against a wide range of pathogens. For instance, Ganoderma has potent inhibitory effects on Gram-positive and Gram-negative bacteria, including Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa. Moreover, it has shown antifungal activity against Candida albicans, a common cause of fungal infections in immunocompromised individuals (Ahmad et al., 2024). Furthermore, emerging studies have investigated its potential antiviral activity, with some evidence suggesting that Ganoderma extracts may inhibit the replication of viruses such as herpes simplex virus (HSV) and influenza virus (Seo and Choi, 2021). These findings suggest that Ganoderma could be a valuable natural alternative or adjunct to conventional antimicrobial therapies, particularly in the context of rising antibiotic resistance. The mechanisms through which Ganoderma exerts its antimicrobial effects are complex and multifaceted. Disruption of microbial cell walls, inhibition of nucleic acid synthesis, and modulation of immune responses are among the primary mechanisms identified in current research. Ganoderma bioactive compounds interact with microbial cells, weakening their structural integrity and preventing proliferation. Moreover, Ganoderma’s ability to modulate the host’s immune system enhances its antimicrobial efficacy, as it not only directly inhibits pathogens but also strengthens the body’s natural defenses against infections (Gao et al., 2005). Despite the promising antimicrobial potential of Ganoderma, several challenges remain. One major limitation is the variability in the composition of bioactive compounds across different Ganoderma species and even within the same species depending on environmental factors and cultivation methods. This variability makes it difficult to standardize extracts for clinical use. In addition, while in vitro and animal studies have provided valuable insights, more human clinical trials are needed to confirm the safety and efficacy of Ganoderma as an antimicrobial agent. Future research should focus on identifying the compounds responsible for antimicrobial effects of Ganoderma and developing standardized formulations for therapeutic use. Ganoderma represents a promising natural source of antimicrobial agents with potential applications in treating various infections. Its ability to modulate the immune system and directly inhibit microbial growth makes it an attractive candidate for developing novel antimicrobial therapies. However, further research is necessary to fully understand its mechanisms of action and overcome the challenges associated with its variability and standardization. As antibiotic resistance continues to rise globally, exploring natural alternatives such as Ganoderma is becoming increasingly important. This review aims to provide a comprehensive overview of the antimicrobial properties of Ganoderma, focusing on recent advances in understanding its bioactive compounds, mechanisms of action, and potential therapeutic applications, particularly in the context of rising AMR. The novelty of this work lies in synthesizing recent findings and highlighting emerging insights into the role of Ganoderma as a promising natural antimicrobial agent.
2 Ganoderma bioactive compounds
Ganoderma species produce a variety of bioactive compounds with significant health benefits, including polysaccharides, triterpenoids, proteins, peptides, and phenolic compounds, each contributing uniquely to their therapeutic potential. This section provides a brief overview of these compounds, highlighting their structures, functions, and mechanisms of action. Detailed phytochemical and bioactivity profiles of Ganoderma have been extensively reviewed (Baby et al., 2015; Blundell et al., 2023).
Among the most studied bioactive compounds are the polysaccharides, particularly β-glucans from G. lucidum. These complex carbohydrates, characterized by β-D-glucose linkages, are categorized by molecular weight and solubility, factors that influence their biological activities (Karunarathna et al., 2024a). β-glucans are known to modulate the immune system by activating macrophages and natural killer cells, enhancing the immune response of the host (Chen et al., 2023). They also impact cellular signaling pathways, regulating cytokine production and inhibiting tumor growth (Zhang et al., 2023). The structural features of Ganoderma polysaccharides, such as branching patterns and molecular configurations, play a critical role in determining their therapeutic efficacy (Wu et al., 2025).
Triterpenoids, another major class of Ganoderma bioactive compounds, include ganoderic and lucidenic acids. These compounds, with their multi-ring structures and diverse functional groups, contribute to a wide range of biological activities (Raza et al., 2024; Pan et al., 2025). Triterpenoids have shown potent immunomodulatory effects by modulating cytokine production and enhancing the activity of immune cells like T cells and macrophages (Jin et al., 2025; Lucius, 2025). They also demonstrate broad-spectrum antimicrobial activity by disrupting microbial cell membranes and interfering with enzymatic processes critical for pathogen survival (Ahmad et al., 2024; Wang et al., 2017; Ewunkem et al., 2024; Liang et al., 2024). Phenolic compounds in Ganoderma, such as flavonoids, phenolic acids, and polyphenols, are well-known for their antioxidant properties. They reduce oxidative stress by neutralizing free radicals and reactive oxygen species (ROS). Their antioxidant effects are largely due to their electron-donating ability, stabilizing free radicals and preventing cellular damage and inflammation (Kebaili et al., 2021; Plosca et al., 2025). In addition to their antioxidative functions, phenolic compounds also exert antimicrobial activity by disrupting microbial cell structures and inhibiting key enzymatic functions necessary for pathogen survival (Rašeta et al., 2023). The multifunctional roles of these compounds underscore their significance in maintaining health and preventing disease.
3 Mechanisms of antimicrobial action
Ganoderma species possess many bioactive compounds that exhibit significant antimicrobial activities. The mechanisms by which these compounds act against pathogens are multifaceted, involving direct effects on microbial structures and functions and modulation of the host immune system (Figure 1).
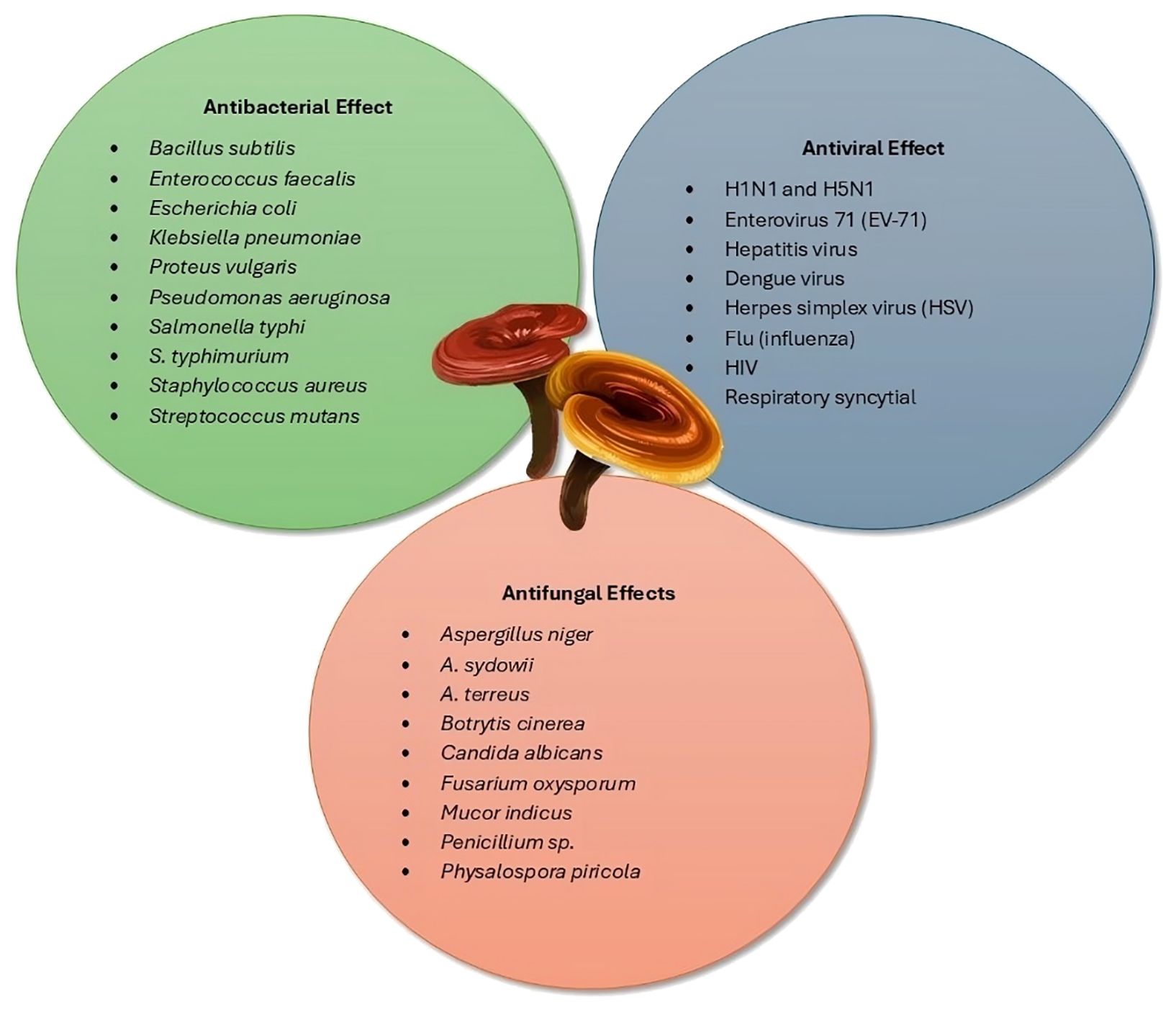
Figure 1. Potential antimicrobial properties of Ganoderma (Ahmad et al., 2024).
3.1 Disruption of microbial cell walls
One of the primary antimicrobial mechanisms of Ganoderma bioactive compounds is the disruption of microbial cell walls. Triterpenoids, such as ganoderic acids found in Ganoderma lucidum, interact with the lipid components of bacterial and fungal cell membranes, leading to increased permeability and cell lysis. This disruption compromises the integrity of the microbial cell wall, causing leakage of cellular contents and, ultimately, cell death (Ewunkem et al., 2024; Ojha, 2025).
3.2 Inhibition of nucleic acid synthesis
Ganoderma bioactive compounds also inhibit microbial proliferation by interfering with nucleic acid synthesis. Polysaccharides extracted from Ganoderma species have been reported to inhibit DNA and RNA synthesis in pathogenic microbes. They achieve this by binding to nucleic acids or key enzymes involved in replication and transcription processes, thereby hindering microbial growth and replication. This inhibition of genetic material synthesis is crucial in preventing the spread and survival of the pathogen (Sułkowska-Ziaja et al., 2022; Liang et al., 2024).
3.3 Immune modulation
Ganoderma compounds enhance the immune response of the body, providing an indirect mechanism to combat infections. Polysaccharides, especially beta-glucans, are known to modulate the immune system by activating macrophages, dendritic cells, and natural killer cells (Zhang et al., 2023; Zhong et al., 2024). This activation increases cytokine and antibody production, bolstering the body’s ability to fight microbial invaders. The immunomodulatory effects of Ganoderma not only enhance the innate immune response but also promote adaptive immunity. By stimulating immune cell proliferation and differentiation, these compounds help establish long-term immunity against specific pathogens (Seweryn et al., 2021; Zhong et al., 2023). This dual action makes Ganoderma an effective agent in preventing and managing infections.
3.4 Oxidative stress regulation
Oxidative stress plays a significant role in the pathogenesis of many microbial infections. Phenolic compounds of Ganoderma exhibit strong antioxidant properties, which help balance ROS within microbial cells (Zahmoul et al., 2024; Plosca et al., 2025). By inducing oxidative stress beyond the tolerance levels of microbes, these compounds can lead to cellular damage and death of the pathogens. Conversely, in host cells, Ganoderma antioxidants protect against oxidative damage caused by infections. They scavenge excess ROS, reducing inflammation and preventing tissue damage (Ahmad et al., 2024; Chen et al., 2024). This protective effect supports the healing process and restores normal cellular functions.
4 Synergistic effects of compounds
The antimicrobial efficacy of Ganoderma species, particularly G. lucidum, is not solely attributed to individual bioactive compounds. Instead, the interactions between various compounds—such as polysaccharides, triterpenoids, proteins, peptides, and phenolic compounds—create synergistic effects that significantly enhance their therapeutic potential. Synergy refers to the increased effectiveness when these compounds work together, often producing results greater than the sum of their actions.
4.1 Interaction between different bioactive compounds
Polysaccharides and triterpenoids are two of the most studied bioactive compounds in Ganoderma. Polysaccharides are known for their immunomodulatory properties, while triterpenoids have potent antimicrobial and anti-inflammatory activities. Combined, these two compounds demonstrate enhanced immunomodulatory effects, stimulating the body’s immune system to fight off infections more effectively (Gao et al., 2005). For example, while triterpenoids may directly disrupt microbial cell membranes, polysaccharides boost the production of immune cells like macrophages and natural killer (NK) cells, leading to a synergistic antimicrobial action (Seweryn et al., 2021; Zhong et al., 2023).
4.1.1 Proteins and peptides with triterpenoids
Proteins and peptides in Ganoderma also exhibit antimicrobial properties, particularly against bacteria and fungi. When these are used with triterpenoids, the compounds together demonstrate enhanced efficacy. The peptides may disrupt microbial membranes, while triterpenoids inhibit nucleic acid synthesis, thereby preventing microbial replication. This dual mechanism increases the effectiveness of the antimicrobial response, especially in pathogens resistant to single-compound treatments (Cör Andrejč et al., 2022; Cadar et al., 2023).
4.1.2 Phenolic compounds and polysaccharides
Phenolic compounds in Ganoderma contribute significantly to its antioxidant activity, reducing oxidative stress within cells. When combined with polysaccharides, these phenolic compounds enhance the immune response and improve the organism’s overall resistance to microbial infections. The phenolic compounds neutralize ROS, while polysaccharides improve immune cell signaling. This combination leads to a more efficient and sustained immune response to pathogens, particularly in cases of chronic infections (Seweryn et al., 2021; Chen et al., 2024).
4.2 Enhanced antimicrobial activity
Research shows that combining polysaccharides and triterpenoids from Ganoderma results in enhanced antibacterial activity. For example, studies on E. coli and S. aureus have shown that combining these two compounds leads to stronger inhibition of bacterial growth compared to their individual effects. The synergy is observed in the disruption of bacterial cell walls by triterpenoids and the enhancement of immune responses by polysaccharides, which work together to eliminate bacterial infections more efficiently (Ahmad et al., 2024).
4.2.1 Synergistic effects against fungal infections
In the case of fungal infections, particularly C. albicans, combining polysaccharides with phenolic compounds has been shown to enhance antifungal activity. This combination disrupts fungal cell walls while simultaneously inducing oxidative stress within the fungal cells. The phenolic compounds reduce ROS accumulation, which damages fungal cells, and the polysaccharides enhance the immune response, creating a powerful antifungal effect. The result is a more effective inhibition of C. albicans growth and biofilm formation, critical for fungal survival and virulence (Roychoudhury et al., 2024).
4.2.2 Viral infections
Emerging research also suggests that the synergistic effects of polysaccharides and triterpenoids in Ganoderma may extend to viral infections. For instance, in studies on the HSV, a combination of these compounds has demonstrated the ability to inhibit viral replication more effectively than when either compound is used alone. Polysaccharides stimulate immune responses, such as activating macrophages and NK cells, while triterpenoids interfere with viral entry into host cells, resulting in enhanced antiviral activity (Eo et al., 2000; Bharadwaj et al., 2019).
5 Research on specific microorganisms
Ganoderma species, particularly G. lucidum, have gained recognition for their potent antimicrobial properties against various pathogens. The bioactive compounds in Ganoderma exhibit broad-spectrum activity against bacteria, fungi, and viruses, making it a promising natural remedy in combating infections. Below is a detailed review of research focusing on the effects of Ganoderma on specific microorganisms. GTs are the most common antimicrobial and antiparasitic compounds reported from Ganoderma sp. Farnesyl quinone, a polyketide type, is the second most common antimicrobial and antiparasitic compound from Ganoderma sp. Quinones are known to be oxidized derivatives of aromatic compounds and are often readily made from reactive aromatic compounds with electron-donating substituents such as catechols and phenols. Besides GTs, polypeptides, small peptides such as ganodermin, polysaccharides such as sacchachitin, and chitosan also possess antimicrobial and antiparasitic properties (Mothana et al., 2000; Wang and Ng, 2006; Sanodiya et al., 2009; Chuang et al., 2013). Extracts from fruiting bodies, both wild and cultivated, and mycelia from fermentation broth are used for the isolation of antimicrobial and antiparasitic bioactive compounds. Literature divulges that, most commonly, ethanol (EtoAc) is used to prepare crude extract; sometimes, some researchers prefer other solvents such as chloroform (CHCl3), EtOH, and acetone (Isaka et al., 2016). In addition, our review reveals that hexane and ether are poorly used for the preparation of extract from Ganoderma sp. Moreover, some techniques such as microwave, ultrasound, and enzyme treatments can facilitate the breakdown of the cell wall (Ferreira et al., 2015). Solvents like MeOH, EtOH, CH2Cl2, CHCl3, and aqueous—both cold and hot—are used for further purification and isolation. Techniques such as thin-layer chromatography (TLC), high-performance liquid chromatography (HPLC), and column chromatography (CC) are used to facilitate the purification and isolation process (Huie and Di, 2004).
5.1 Bacterial infections
Several studies have demonstrated the efficacy of Ganoderma bioactive compounds against pathogenic bacteria, including both Gram-positive and Gram-negative strains. Key compounds, such as triterpenoids, polysaccharides, and peptides, have shown significant antibacterial effects (Figure 2). Ganoderma has been reported as an important source of antimicrobial bioactive compounds. Terpenes, terpenoids, and polyketides of farnesyl quonine types are the major secondary metabolites produced by Ganoderma sp. In Ganoderma species, more than 316 terpenes have been reported, with the majority of compounds from G. lucidum (Xia et al., 2014).
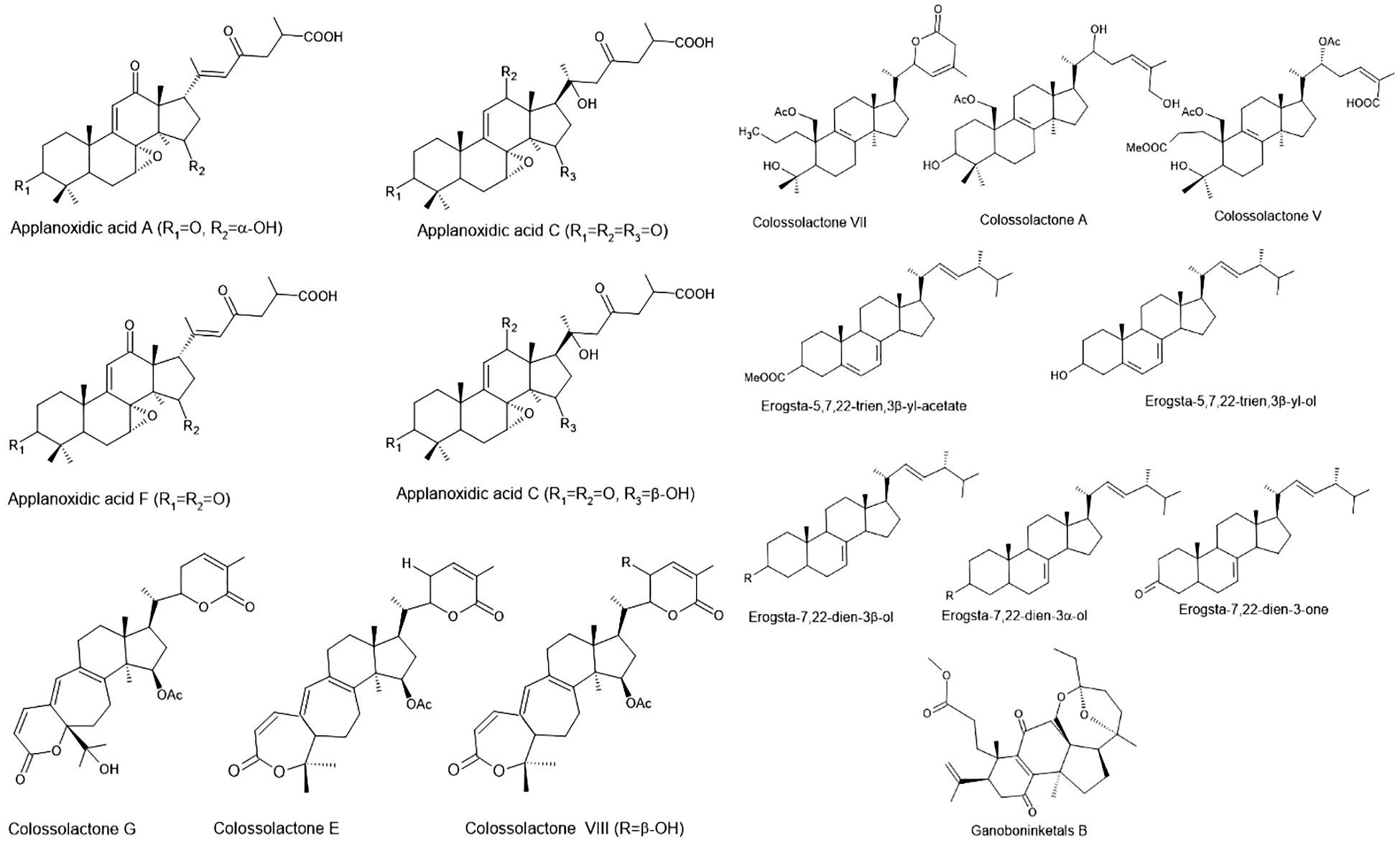
Figure 2. Structures of bioactive compounds from Ganoderma species with antimicrobial and antiparasitic effects (Basnet et al., 2017).
5.1.1 Ganoderma extracts and fermentation broths
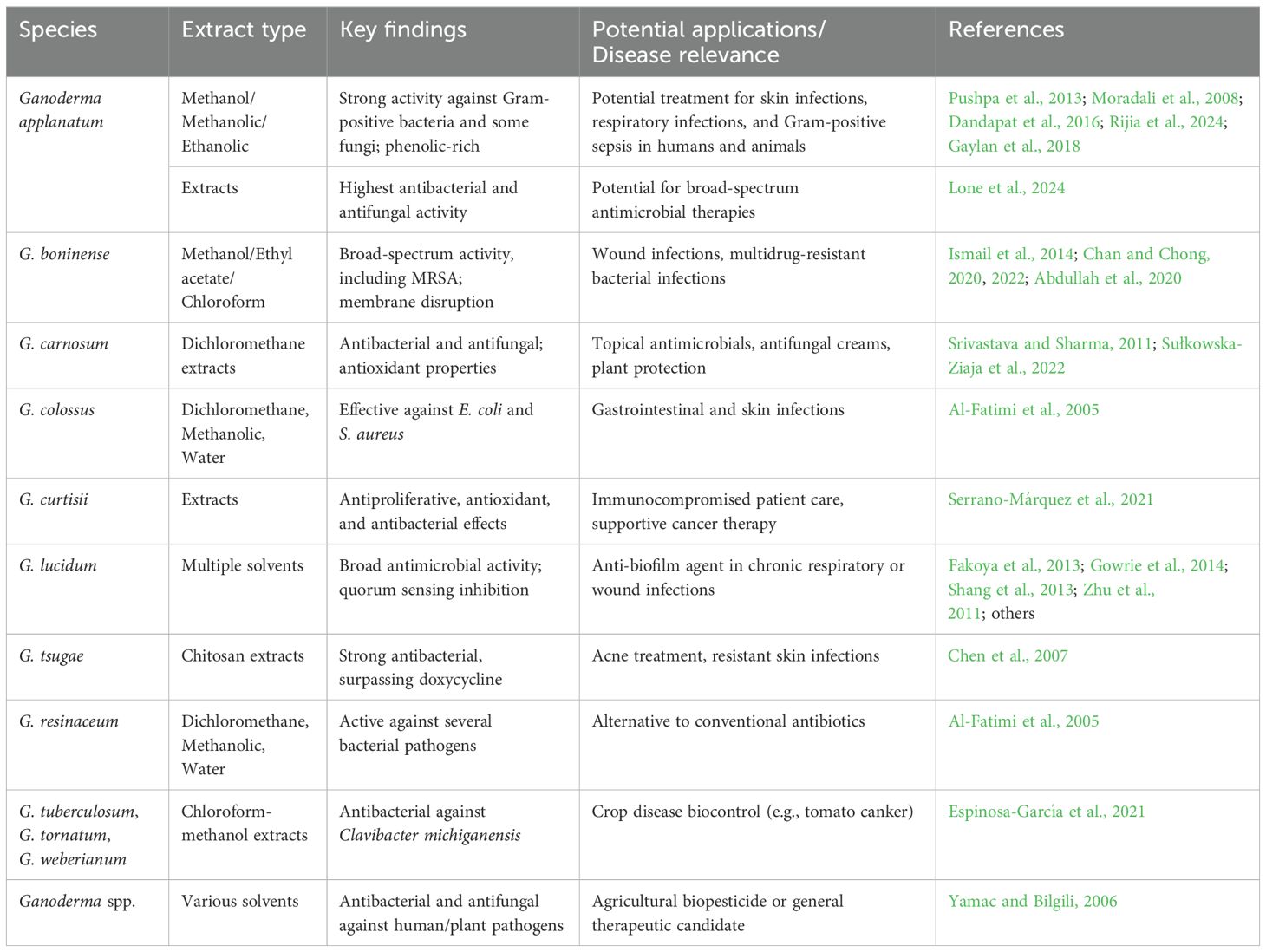
The methanol extract of G. lucidum showed antibacterial activity against E. coli, Salmonella typhimurium, and Bacillus subtilis [minimum inhibitory concentration (MIC): 1 mg/well], with bioactive polyphenols, flavonoids, quinones, and terpenes identified (Sheena et al., 2003). Among 23 Yemeni Basidiomycetes, Agaricus sp., Coriolopsis caperata, Ganoderma colossus, Ganoderma resinaceum, Phellorinia herculea, and Tulostoma obesum exhibited potent antibacterial effects, while G. resinaceum, Inonotus ochroporus, Phellinus rimosus, and P. herculea displayed strong antioxidant activity (Al-Fatimi et al., 2005). G. lucidum butanol extracts inhibited microbial growth and disrupted fungal spore formation, suggesting potential for antimicrobial tea formulations (Rofuli et al., 2005). Ganoderma applanatum, Tricholoma crassum, and Trametes corrugata showed peak antibacterial activity (terpenoids and polysaccharides) after 16 days of fermentation (Bhattacharyya et al., 2006). Ganoderma spp. (e.g., G. carnosum) exhibited static, heat-stable effects against pathogens like E. coli and C. albicans (Yamac and Bilgili, 2006). Furthermore, chitosan from Ganoderma tsugae outperformed doxycycline against Actinobacillus actinomycetemcomitans, retaining 56.58% activity after 18 days, highlighting dental applications (Chen et al., 2007). G. lucidum aqueous extracts (from Persia americana logs) showed stronger antibacterial effects than methanol extracts (Ofodile and Bikomo, 2008), while its chloroform extracts inhibited Gram-positive and Gram-negative bacteria via sterols and triterpenoid acids (Keypour et al., 2008). In addition, G. applanatum methanol extracts (rich in palmitic acid) selectively inhibited Gram-negative bacteria (Moradali et al., 2008).
G. applanatum exhibited antimicrobial activity against E. coli, S. aureus, C. albicans, Mycobacterium smegmatis, and Sporothrix schenckii, highlighting its therapeutic potential (Barranco et al., 2010). G. lucidum methanol, ethanol, and aqueous extracts showed potent activity against pathogens like Listeria monocytogenes and methicillin-resistant S. aureus (MRSA), with methanol being the most effective solvent (Aneeshia and Sornaraj, 2010). G. applanatum methanol extract displayed strong DPPH scavenging (82.80%), while G. lucidum chloroform extract had notable antioxidant and antibacterial effects, linked to high phenol content (Karaman et al., 2010). G. lucidum inhibited spore germination of Alternaria brassicicola, suggesting its potential as a biocontrol agent (Chen and Huang, 2010). Methanol, acetone, chloroform, and aqueous extracts of G. lucidum mycelia inhibited Gram-positive and Gram-negative bacteria (100 mg/mL), with Gram-positive strains more susceptible (Kamble et al., 2011). Furthermore, in Pakistan, Lahore isolates of G. lucidum (G-1, G-3, and G-5) inhibited Xanthomonas spp., while G-2 and G-4 were effective against E. coli and Pseudomonas spp., respectively (Nasim and Ali, 2011). G. lucidum aqueous extract (200 mg) showed a 31-mm inhibition zone against S. typhi and S. aureus, while its methanolic extract was most antifungal (30 mm against Mucor indicus) (Sekaran et al., 2011). Ethyl acetate extracts of Ganoderma praelongum sesquiterpenoids were highly active against MRSA (MIC: 0.390–6.25 mg/mL), unlike ineffective polysaccharides (Ameri et al., 2011). Ganoderma carnosum dichloromethane extracts strongly inhibited S. aureus and Micrococcus luteus (Srivastava and Sharma, 2011). Ganoderma formosanum polysaccharides (d-mannose, d-galactose, and d-glucose) enhanced macrophage activity (TNF-α, nitric oxide, and phagocytosis) and protected mice against L. monocytogenes (Wang et al., 2011).
G. lucidum ethyl acetate extracts showed the strongest antibacterial activity (containing carbohydrates, saponins, and terpenoids), being most effective against Corynebacterium pyogenes, B. subtilis, and Klebsiella pneumoniae though less potent than AmpicloxR (Shamaki et al., 2012). Water extracts inhibited P. aeruginosa, Proteus vulgaris, and Enterococcus faecalis but not L. monocytogenes, while hexane/dichloromethane/ethyl acetate showed limited antimicrobial isolation potential (Kamra and Bhatt, 2012). In Central India, G. lucidum aqueous extracts enhanced synthetic antibiotics against S. aureus, K. pneumoniae, Bacillus cereus, and P. aeruginosa (Karwa and Rai, 2012). Acetone extracts showed the strongest activity against P. aeruginosa (33 mm zone) and the weakest against S. aureus/K. pneumoniae (7 mm), with MICs of 4–35 mg/mL (Mehta and Jandaik, 2012). G. lucidum spore and G. applanatum polysaccharides inhibited S. aureus, B. cereus, and Salmonella enteritidis, suggesting potential as food supplements (Klaus et al., 2012). Comparative studies showed that G. lucidum had the largest inhibition zones against E. coli/Klebsiella sp., though less than standard antibiotics (Krishnaveni and Manikandan, 2014). Solvent choice significantly impacted activity: benzene extracts best inhibited E. coli/Neisseria meningitidis (Shikongo, 2012), while methanol extracts surpassed ampicillin/streptomycin against S. aureus/B. cereus (MIC: 0.0125–0.75 mg/mL) (Heleno et al., 2013). Diethyl ether/chloroform extracts showed strong antagonistic effects (Nithya et al., 2013). Traditional Namibian uses were validated as Ganoderma spp. showed potent Gram-positive/negative activity (Shikongo et al., 2013). The anti-S. aureus activity of G. applanatum was linked to soluble saponins/phenols (Nagaraj et al., 2013). G. lucidum, Pleurotus spp., and Agaricus bisporus demonstrated broad therapeutic potential (Mondal, 2013).
G. lucidum extracts showed significant antimicrobial activity against P. aeruginosa, E. coli, S. aureus, Proteus mirabilis, and K. pneumoniae. Aqueous extracts produced 11.0- to 14.0-mm inhibition zones, with bioactive tannins, phenolics, flavonoids, and saponins identified (Fakoya et al., 2013). HPTLC analysis revealed six flavonoids and four phenolics, with methanol extracts most effective against K. pneumoniae (24 ± 0.666 mm), while Gram-negative bacteria showed greater susceptibility than Gram-positive S. aureus (Sakthivigneswari and Dharmaraj, 2013). G. praelongum (0.3%) combined with Glycyrrhiza glabra (2.5%) in topical gels significantly inhibited MRSA and enhanced wound healing (Ameri et al., 2013). G. tsugae methanol extracts were most active against E. coli (20 ± 0.577 mm), with Gram-negatives more susceptible than Gram-positives (Ganesan and Dharmaraj, 2013). G. applanatum showed particular efficacy against Gram-positive bacteria (Pushpa et al., 2013). G. lucidum ethanol extracts inhibited Helicobacter pylori (MIC < 3 mg/mL) and S. aureus (MIC 10 mg/mL) but not E. coli (Shang et al., 2013). G. lucidum methanol extracts were active against E. coli, S. aureus, B. cereus, Enterobacter aerogenes, and P. aeruginosa (Alves et al., 2013).
Recent studies have demonstrated significant antimicrobial potential in various Ganoderma species. Ganoderma boninense methanol extracts exhibited strong activity against foodborne pathogens E. coli and S. aureus, with GC-MS analysis identifying dodecanoic acid and octadecanoic acid as key bioactive compounds (Ismail et al., 2014). Comparative research on G. lucidum strains revealed distinct bioactive profiles, with Serbian specimens showing higher sugar content and anticancer properties, while Chinese varieties contained more organic acids and demonstrated superior antioxidant capacity—both strains displayed antimicrobial effects that occasionally surpassed standard drugs (Stojković et al., 2014). The extraction method significantly influenced activity, as G. lucidum methanolic extracts (500 µg/disc) produced the largest inhibition zones (13.04 mm) against S. aureus and P. aeruginosa (Djide et al., 2014). Chloroform extracts showed notable efficacy against S. typhi (18 mm) and C. albicans (17 mm), with analytical techniques confirming triterpenoids and polysaccharides as active components (Gowrie et al., 2014). Optimized fermentation protocols yielded extracts with antioxidant activity exceeding ascorbic acid and antimicrobial effects against Shigella dysenteriae, E. faecalis, and K. pneumoniae (Paliya et al., 2014). Additional studies confirmed variable but promising activity of G. lucidum against P. aeruginosa, E. coli, E. faecalis, S. aureus, and C. albicans, with ethanol and chloroform extracts proving most effective (Avcı et al., 2014).
Comparative studies of mushroom species revealed that G. tsugae had the highest dry weight (16.1 g/100 g), while A. bisporus contained superior protein (32.0 mg/g) and glucose (13.2 mg/g) content. A. bisporus acetone extracts showed antimicrobial activity against E. coli (13 mm) and P. aeruginosa (14 mm), whereas G. tsugae displayed stronger antibacterial effects in DMSO extracts (Dharmaraj et al., 2014). Nigerian studies of G. lucidum ethanolic extracts identified steroids, triterpenoids, and glycosides with activity against E. coli (12 mm), K. pneumoniae (12 mm), P. mirabilis (13 mm), and Streptococcus spp. (14 mm) at 1,000 mg/mL (Etim et al., 2014). Ganoderma sp. DKR1 contained saponins, tannins, and terpenoids, with ethyl acetate extracts active against Micrococcus sp., S. aureus, and Salmonella sp., while chloroform extracts inhibited E. faecalis and Candida sp (Rajesh and Dhanasekaran, 2014). G. lucidum acetone extracts (50 µg/mL) showed potent antibacterial activity (31.60 ± 0.10 mm) against six bacterial species and antifungal effects at 1,000 mg/mL (Singh et al., 2014). With rising drug resistance, G. lucidum methanolic extracts containing carbohydrates, triterpenoids, and phenolics demonstrated strong antibacterial effects (Shah et al., 2014). G. lucidum spore powder inhibited Prevotella intermedia (MIC 3.62 mcg/mL) in 65% of periodontal samples (Nayak et al., 2015). Ganoderma australe exhibited antimicrobial and antioxidant activity from alkaloids, while G. applanatum and Flammulina velutipes showed medium-dependent effects enhanced by wine yeast (Liew et al., 2015; Fidler et al., 2015). Ganoderma mycelium extracts outperformed fruiting bodies with lower MIC values against pathogens (Sharma et al., 2015). G. lucidum-enriched soap demonstrated antibacterial activity against S. aureus and antioxidant capacity (IC50 1.53 mg/mL) (Hayati et al., 2020). G. resinaceum methanol extracts showed significant antioxidant and antimicrobial potential (Zengin et al., 2015), corroborated by other studies (Hoque et al., 2015; Kirar et al., 2015). G. applanatum methanolic extracts inhibited S. typhi (3.21 mm ZOI) and P. mirabilis (3.02 mm ZOI), containing phenolics (20.81 mg/100 g) and flavonoids (23.89 mg/100 g), with nutritional analysis revealing 222.08 Kcal/100 g and 42.72% carbohydrates (Dandapat et al., 2016).
Recent studies have demonstrated significant antimicrobial and antioxidant properties in various Ganoderma species. G. lucidum showed strongest inhibition against Candida glabrata (25 ± 1 mm) compared to C. albicans and B. subtilis (10 ± 1 mm), with its methanolic extract exhibiting exceptional DPPH radical scavenging activity (IC50 = 3.82 ± 0.04 μg/mL) attributed to phenolic compounds (Celık et al., 2014). Ethanol mycelial extracts of Ganoderma species, particularly G. lucidum BEOFB 433, displayed both antibacterial effects and antifungal activity against Aspergillus glaucus and Trichoderma viride (Ćilerdžić et al., 2016a). Ganoderma pfeifferi volatile oil, containing 73.6% 1-octen-3-ol, showed strong antimicrobial activity against S. aureus and C. albicans along with significant antioxidant capacity (Al-Fatimi et al., 2016), while G. lucidum fermentation broths demonstrated 39.67% antioxidant activity, with strain BEOFB 432 being particularly effective (Ćilerdžić et al., 2016b). Kenyan G. lucidum extracts exhibited activity against MRSA and common bacteria (up to 10.0 mm inhibition), highlighting its antimicrobial potential (Reid et al., 2016; Sande and Baraza, 2019). Ganoderma tropicum showed promise as a biocide and corrosion inhibitor against sulfate-reducing bacteria in industrial applications (Stanley et al., 2016). Comparative studies of eight mushroom species revealed that G. applanatum, Laetiporus sulphureus, F. velutipes, Trametes versicolor, Hericium coralloides, and Agaricus campestris had significant antimicrobial activity against B. subtilis ATCC 6633, while G. lucidum and Pleurotus eryngii showed no effects (Nicolcioiu et al., 2017). However, G. lucidum culture broth demonstrated antibacterial activity against Staphylococcus epidermidis and P. aeruginosa, suggesting potential for cosmetic and nutraceutical applications (Sarnthima et al., 2017).
GC-MS analysis of G. lucidum mycelia and fruiting bodies revealed that the mycelial aqueous extract possessed the highest anti-Candida activity (against C. albicans and C. glabrata biofilms) and ascorbic acid content, suggesting biofilm prevention potential. Chemometric analysis showed variability in volatile organic compounds between extracts (Bhardwaj et al., 2017). Antimicrobial testing of G. lucidum (GL) showed MICs of 200–400 µg/mL against S. aureus, E. faecalis, L. monocytogenes, K. pneumoniae, P. aeruginosa, E. coli, and Candida spp. While non-cytotoxic to NIH3T3 cells, GL showed genotoxicity (2.71-fold genetic damage at 5 mg/mL) (Ergun, 2017). G. lucidum ethanol extracts showed superior antibacterial activity (lower MICs against S. aureus, M. luteus, B. terom, and B. subtilis), while water extracts had higher DPPH scavenging (56.22% vs. 20.67%) (Wang et al., 2017). Philippine G. applanatum and G. lucidum ethanol extracts inhibited S. aureus (6.55 ± 0.23 mm to 7.43 ± 0.29 mm zones) with MIC50 values of 1,250–10,000 μg/mL (Gaylan et al., 2018). G. lucidum extract inhibited MDR Mycobacterium tuberculosis (complete inhibition at 25%–50% concentration) (Erawati et al., 2018). Bangladeshi G. lucidum exhibited antioxidant activity (IC50 89.05 µg/mL), cytotoxicity (LC50 142.49 µg/mL), and antibacterial effects against antibiotic-resistant strains (Islam et al., 2018). Antimicrobial peptides from G. lucidum fruiting bodies (GLF) and mycelium (GLM) showed activity against E. coli and S. typhi via ROS and protein leakage mechanisms (Mishra et al., 2018a). G. lucidum-based Kombucha beverage achieved 22.8 g/L acidity by day 2, with strong antioxidant/antibacterial activity (especially against S. epidermidis and R. equi), though the vacuum-dried form was less potent (Sknepnek et al., 2018).
Australian G. lucidum extracts demonstrated significant wound-healing properties, with ethanol/methanol-extracted triterpenes and water-extracted polysaccharides (50 mg/mL) showing antimicrobial activity against S. aureus (including MRSA), B. cereus, S. pyogenes, and E. coli. Alkali-extracted compounds were effective against P. aeruginosa (Montalbano, 2018). In food preservation, sausages with 0.5% G. lucidum powder maintained lower lipid oxidation and microbial levels while matching sensory acceptability of conventional preservatives (Ghobadi et al., 2018). Ganoderma lipsiense extract specifically inhibited P. aeruginosa (via phenolic compounds like caffeic acid) but not E. coli or S. aureus (Costa et al., 2019). Turkish G. lucidum exhibited high antioxidant potential (TAS/TOS/OSI assays) and antimicrobial activity against nine pathogens (Celal, 2019). Ethanol extracts (20 g/mL) showed the strongest activity against S. aureus, P. aeruginosa, and Fusarium sp (Tamilselvan and Rajesh, 2019). Serbian Ganoderma species revealed species-specific efficacy: G. resinaceum chloroform extract against P. aeruginosa, G. pfeifferi water extract against E. coli/S. aureus, and G. lucidum showing antiviral potential (Rašeta et al., 2023). South Jakarta G. lucidum ethanol extract only affected S. aureus, with no dose-dependent improvement (Noverita and Ritchie, 2020). GC-MS analysis of Nigerian G. lucidum identified 48 bioactive compounds (including BHA), with methanol extracts showing the strongest antibacterial effects (except against resistant P. aeruginosa) (Anyakorah et al., 2020). Kenyan studies confirmed G. lucidum and Termitomyces letestui activity against MRSA and S. pyogenes (Anyimba, 2020). Methanol extracts from Yeast Wine Media completely inhibited fungal growth (500–1,000 ppm) and showed superior activity against Xanthomonas oryzae/Ralstonia solanacearum, with higher antioxidant capacity (Suansia and John, 2021). Finally, G. lucidum methanol extracts exhibited potent antibacterial effects against MDR E. coli and P. aeruginosa (19.3 ± 0.4 mm zones, MBC 266 ± 23.6) (Radhika, 2021).
Comparative analysis of G. lucidum mycelium and spores against P. intermedia from periodontitis patients revealed mean MIC values of 5.64 mcg/mL (mycelium) and 3.62 mcg/mL (spores), demonstrating comparable antimicrobial efficacy for adjunct periodontal therapy (Nayak et al., 2021). Mexican G. curtisii strains exhibited notable biological activities, including tumor cell line inhibition (GI50 ≤50 µg/mL), anti-S. aureus effects, and antioxidant properties, with strain GH-16–023 showing particularly low toxicity (Serrano-Márquez et al., 2021). Kenyan G. lucidum extracts contained terpenoids, phenolics, and glycosides, displaying significant activity against MRSA and Streptococcus pyogenes, with the isolated compound Ergosta-5,7,22-triene-3β,14α-diol showing potent antibacterial effects (Baraza et al., 2021). G. lucidum spore powder aqueous extracts demonstrated remarkable antibacterial activity with MIC values of 125 µg/mL (S. aureus and E. coli), <2 µg/mL (E. faecalis), and 62.5 µg/mL (K. pneumoniae) (Nayak et al., 2010a). Comparative studies of G. boninense extracts revealed that chloroform-extracted mycelium (GBMA) exhibited the strongest antibacterial activity, particularly through chloroform-methanol-water extraction, suggesting novel antimicrobial metabolites (Abdullah et al., 2020). Further analysis of G. boninense fruiting bodies showed that ethyl acetate extracts had broad-spectrum inhibition (especially against P. mirabilis), while methanol extracts showed the lowest MIC (0.625 mg/mL) against Coagulase-Negative Staphylococci, with LC-MS identifying alkaloids, fatty acids, and glycosides as potential bioactive compounds (Chan and Chong, 2020).
Medicinal polypores including G. adspersum, G. applanatum, and G. australe yielded bioactive ergostane compounds (ergosta-7,22-dien-3-one and ergosta-7,22-diene-3β-ol) through methanol/ethyl acetate extractions, showing significant inhibition against S. pyogenes but not Gram-negative bacteria, suggesting potential for novel myco-medicines (Mayaka, 2020). In biofilm-related studies, G. lucidum demonstrated notable anti-biofilm activity against multidrug-resistant (MDR) Enterococcus strains, offering alternatives for challenging infections (Karaca et al., 2020). Phytochemical analysis revealed that wild Ganoderma species contained saponins and flavonoids, with G. lucidum showing the highest cyanide content. Ethanolic extracts inhibited Salmonella spp., E. coli, S. aureus, and Streptococcus spp., with G. applanatum particularly effective against E. coli (19.50 mg/mL) and all species showing similar MBC (~250 mg/mL) (Wood et al., 2021). Optimized cultivation of Philippine G. lucidum on sawdust/PDA yielded ethanol extracts (100–200 mg/mL) that outperformed standard antibiotics in antibacterial tests, with fruiting bodies showing superior antioxidant activity to mycelia (Subedi et al., 2021). Nine Ganoderma species extracts, including G. tuberculosum and G. tornatum, inhibited Clavibacter michiganensis (31.5–1,000 μg/mL), suggesting applications for tomato canker management (Espinosa-García et al., 2021).
Comparative studies of medicinal mushrooms revealed that Taiwanofungus camphoratus methanolic extracts showed strong antimicrobial activity, while G. lucidum extracts displayed no significant effects, with concerns about Penicillium expansum developing tolerance (Kim et al., 2022). G. boninense demonstrated exceptional anti-MRSA activity (41.08 mm zone, MIC 0.078 mg/mL) through membrane disruption, with LC-MS identifying eight bioactive compounds (Chan and Chong, 2022). Iraqi studies showed that G. lucidum methanol extract (200 mg/mL) was most effective against UTI pathogens (K. pneumoniae, S. aureus, and P. mirabilis), containing flavonoids, alkaloids, phenols, and terpenoids (Shawkat and Aedan, 2022). Metabolite profiling of six Ganoderma species identified G. pfeifferi as the richest in phenolic acids (114.07 mg/100 g DW) and G. lucidum as the richest in indole compounds, with all showing antioxidant and enzyme inhibitory potential (Sułkowska-Ziaja et al., 2022). G. lucidum methanol extract demonstrated dual anti-MRSA activity in vitro and in vivo, reducing lung inflammation and LDH levels in infected rats (Soliman et al., 2022). Moroccan studies revealed the potent antimicrobial activity of G. lucidum extract (especially against Epidermophyton floccosum) and high phenolic/flavonoid content (Erbiai et al., 2023). Further studies confirmed antimicrobial (MIC 50 μg/mL against S. aureus/E. coli) and antioxidant (85.9%–90.12% radical scavenging at 400 μg/mL) properties of G. lucidum (Tehranian et al., 2023). Turkish specimens showed 90.81% DPPH scavenging and notable anti-E. faecalis activity (17.67 ± 0.47 mm zone), with GC-MS identifying key fatty acids (Canpolat and Canpolat, 2023). Ganoderma mbrekobenum methanol extracts showed strong anti-Bacillus (15- to 18-mm zones) and anti-Fusarium activity, with 46 bioactive compounds identified (El-Dein et al., 2023). Antifungal studies demonstrated that G. lucidum pure extract achieved 100% inhibition of Colletotrichum gloeosporioides and 94.4% against Alternaria solani (Saludares et al., 2023). Comparative analysis showed that G. lucidum surpassed G. neo-japonicum in protein content (24.3 vs. 15.6 mg/g), phenolics (14.3 vs. 9.8 mg GAE/g), and antioxidant capacity (FRAP 403.9 μmol Fe2+/g) (Ayimbila et al., 2023). The extraction method significantly influenced bioactivity—Soxhlet ethanol extracts showed strongest anticancer effects (MCF-7 IC50 4.797 μg/mL) while UAE water extracts had the best anti-S. aureus activity (20–23 mm) (Azahar et al., 2023).
5.1.2 Triterpenoids
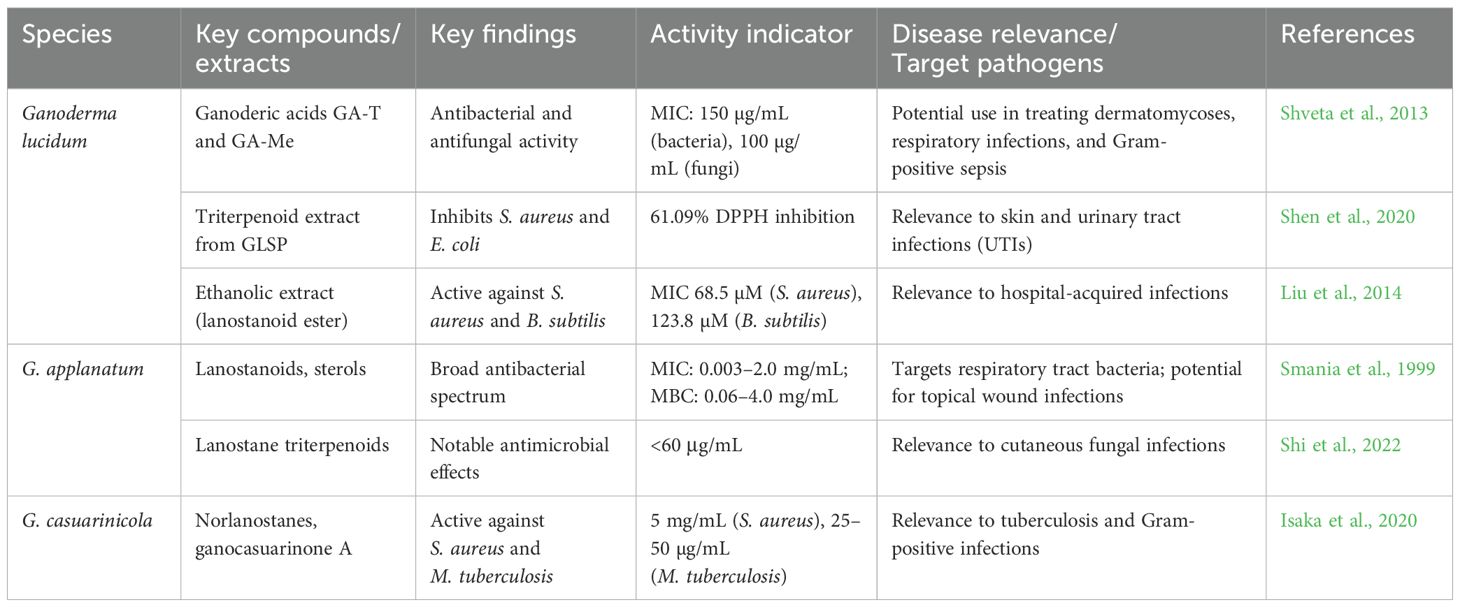
Infectious diseases caused by bacteria, fungi, viruses, and parasites remain a leading cause of global morbidity and mortality, particularly in low- and middle-income countries. The rise of AMR, emerging viral pathogens, and neglected tropical diseases underscores the urgent need for new therapeutic agents. Ganoderma species, especially through their triterpenoid-rich extracts, represent a promising yet underutilized resource in addressing these critical health challenges. Triterpenoids, particularly lanostane-type compounds, are among the most bioactive secondary metabolites in Ganoderma spp., exhibiting broad-spectrum antimicrobial and antiviral activity (Table 1). Their multifaceted mechanisms include membrane disruption, enzyme inhibition, and immunomodulation.
Early studies on G. applanatum identified three sterols and a novel lanostanoid with potent antibacterial activity, showing Gram-positive specificity (MIC: 0.003–2.0 mg/mL; MBC: 0.06–4.0 mg/mL) (Smania et al., 1999). Nigerian G. colossum yielded new colossolactones including 23-hydroxycolossolactone E with antimicrobial potential (Ofodile et al., 2005). Modified applanoxidic acids from Ganoderma spp. maintained activity against E. coli, S. aureus, C. albicans, and T. mentagrophytes (MIC: 1.0 to >2.0 mg/mL) (Smania et al., 2006). Western Ghats Ganoderma sesquiterpenoids surpassed standard antibiotics against bacteria and C. albicans, while triterpenes showed weaker effects (Bhosle et al., 2010). Colossolactones E and 23-hydroxycolossolactone E demonstrated activity against B. subtilis and P. syringae (Ofodile et al., 2011), with G. lucidum and G. mazandaran showing the lowest MICs (128 µl/mL) against B. subtilis and P. mirabilis (Ofodile et al., 2012). Haryana G. lucidum yielded ganoderic acids (GA-T and GA-Me) with MICs of 150 µg/mL (bacteria) and 100 µg/mL (fungi) (Shveta et al., 2013). Ganoderma sp. BCC 16,642 produced ganoderic acids/lanostanoids active against S. aureus and B. subtilis (Liu et al., 2014). Ethyl acetate extracts of G. lucidum contained novel antimicrobial triterpenoids (Liu et al., 2014), while its GA showed cytotoxicity and antibacterial effects (Upadhyay et al., 2014). Two triterpenoids (GLTA and GLTB) exhibited anti-EV71 activity by blocking viral adsorption and RNA replication (Zhang et al., 2014). Ganoderma triterpenoids inhibited S. aureus biofilms and E. coli (Basnet et al., 2017). Recent studies revealed that G. lucidum spore powder triterpenoids had 61.09% DPPH inhibition (600 µg/mL) and anti-S. aureus/E. coli activity (Shen et al., 2020). Ganoderma casuarinicola norlanostanes showed anti-S. aureus (5 mg/mL) and anti-TB (25–50 µg/mL) effects (Isaka et al., 2020). G. applanatum yielded three new antimicrobial lanostane triterpenoids (Shi et al., 2022). Given their demonstrated efficacy against MDR bacteria (e.g., MRSA), biofilm-producing strains, and even viruses, Ganoderma-derived triterpenoids offer a compelling lead for drug discovery targeting difficult-to-treat infections. Their ability to address current gaps in antifungal and antiviral therapeutics, coupled with favorable safety profiles in traditional use, reinforces their potential for clinical translation. Table 2 provides an overview of the antibacterial properties exhibited by various extracts of Ganoderma species.
5.1.3 Polysaccharides
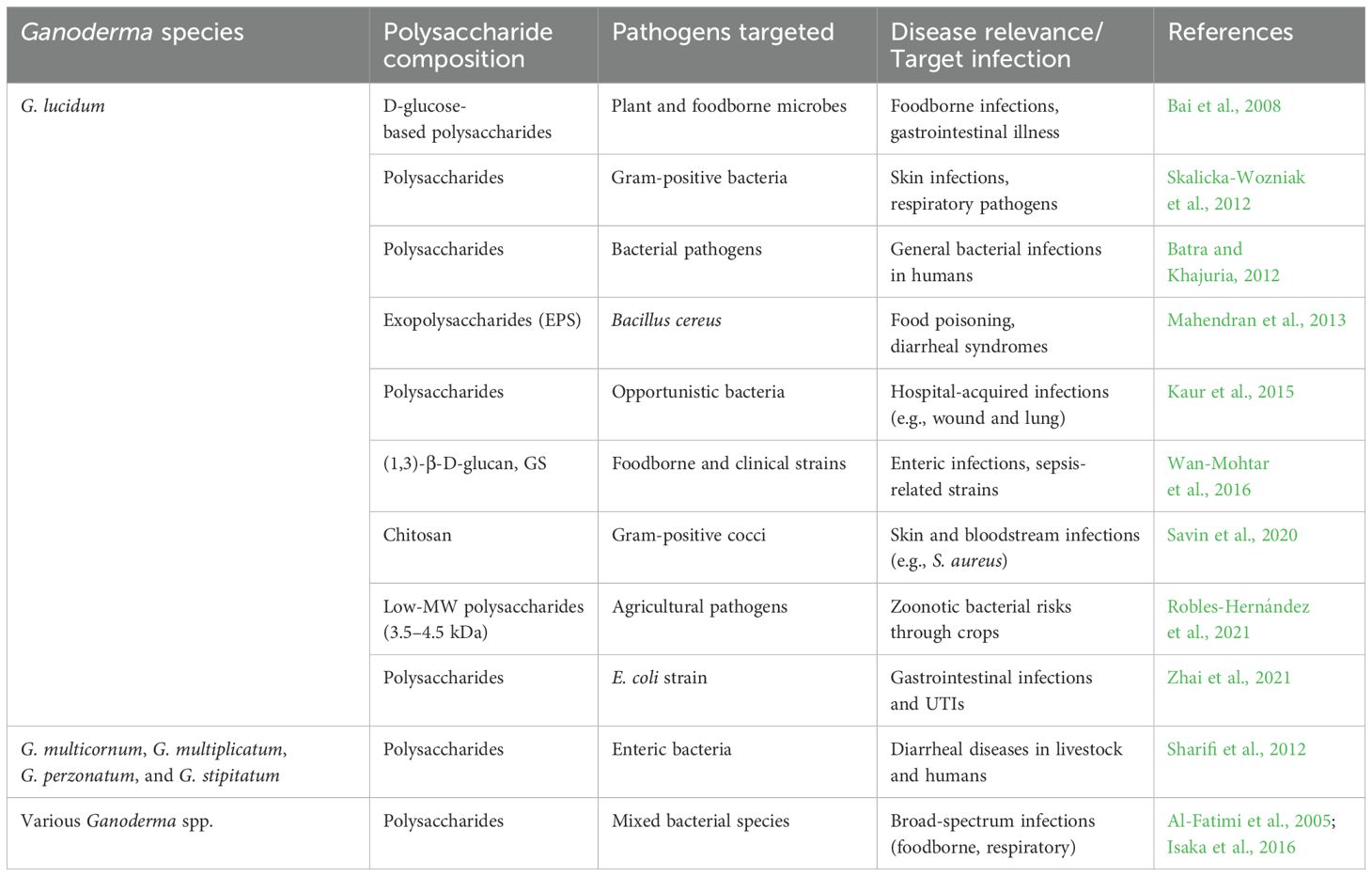
Polysaccharides from Ganoderma species, particularly G. lucidum, offer compelling bioactivity that aligns with global efforts to combat infectious diseases. As AMR and gastrointestinal infections continue to rise globally, especially in immunocompromised populations and developing regions, the need for non-antibiotic, immune-enhancing alternatives becomes critical. Ganoderma-derived polysaccharides, rich in β-glucans and heteropolysaccharides, are emerging as promising immunomodulatory and antimicrobial agents that could complement or replace conventional antimicrobials (Table 3).
Hot water extracts of G. lucidum fruiting bodies, primarily composed of D-glucose, have demonstrated activity against plant pathogens (Erwinia carotovora and Penicillium digitatum) and foodborne microbes (B. cereus, E. coli, and Aspergillus niger) (Bai et al., 2008). G. lucidum polysaccharides also strongly inhibit M. luteus (MIC 0.62–1.25 mg/mL) (Skalicka-Wozniak et al., 2012), and fractions isolated from G. multicornum and related species show activity against E. coli and P. mirabilis (Sharifi et al., 2012). Additional studies revealed inhibition zones up to 19 mm against Staphylococcus sp. (Batra and Khajuria, 2012) and potent activity (18- to 23-mm inhibition zones) from exopolysaccharides (EPS) cultivated on basal and malt media (Mahendran et al., 2013). Strain-specific studies showed that G. lucidum GL-2 and GL-3 produce polysaccharides that inhibit Staphylococcus and Enterobacter spp (Kaur et al., 2015). Mechanistically, these polysaccharides exert their antimicrobial action by disrupting microbial cell walls and modulating oxidative stress. Their synergy with phenolic compounds enhances antimicrobial efficacy, suggesting a multi-targeted mode of action (Al-Fatimi et al., 2005; Isaka et al., 2016).
G. lucidum strain BCCM 31549 produces both (1,3)-β-D-glucan (G) and its sulfated derivative (GS), with GS exhibiting not only superior antimicrobial activity but also selective cytotoxicity against U937 cancer cells (Wan-Mohtar et al., 2016), pointing to potential dual anti-infective and anticancer utility. Enzymatically extracted chitosan from G. lucidum shows superior antibacterial effects against Gram-positive bacteria and improved antioxidant activity compared to chemically extracted counterparts (Savin et al., 2020). Small-molecular-weight polysaccharides (3,500–4,500 Da) isolated from culture fluids have recently demonstrated strong antibacterial effects against plant pathogens (Robles-Hernández et al., 2021), offering a sustainable source for agricultural biocontrol. In a more clinically relevant context, G. lucidum polysaccharides at concentrations of 5–100 μg/mL not only inhibited E. coli proliferation but also modulated immune response pathways in intestinal porcine epithelial cells (IPEC-1), suggesting potential for treating or preventing bacterial gut infections (Zhai et al., 2021).
Collectively, these findings suggest that Ganoderma polysaccharides can address important global health challenges such as antibiotic-resistant bacterial infections, especially gastrointestinal and foodborne diseases. Their natural origin, immunostimulatory properties, and low toxicity support their further development as functional antimicrobial agents or as adjuncts to conventional therapies.
5.1.4 Other compounds
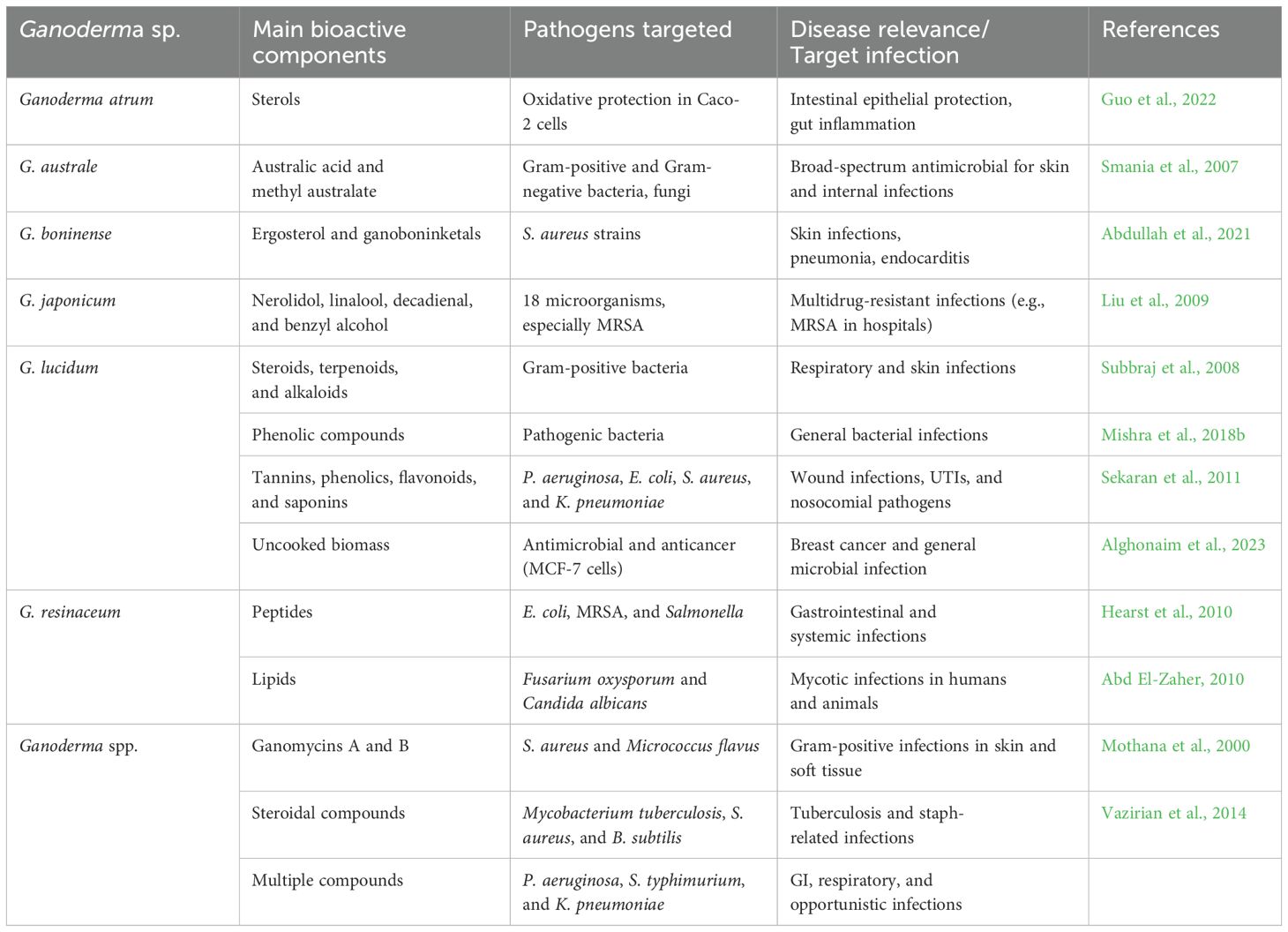
In addition to triterpenoids and polysaccharides, Ganoderma species produce a chemically diverse repertoire of secondary metabolites—including essential oils, steroids, phenolics, alkaloids, and proteins—that contribute to their antimicrobial properties (Table 4). These compounds are increasingly viewed as promising leads in the search for novel anti-infective agents, particularly against MDR pathogens. Given the growing global burden of AMR, notably S. aureus, M. tuberculosis, and nosocomial Gram-negative infections, such natural compounds represent a valuable reservoir for alternative therapies and adjunct treatments. Essential oils derived from G. japonicum mycelia, rich in nerolidol and linalool, exhibited potent activity against MRSA, with a minimum bactericidal concentration (MBC) of 1.03 mg/mL (Liu et al., 2009). G. pfeifferi produced ganomycins A and B, which demonstrated pronounced anti-Gram-positive activity (MIC 2.5–25 µg/mL) (Mothana et al., 2000), suggesting potential as topical agents or adjuvants for skin and wound infections. Novel metabolites from G. australe, including australic acid, showed broad-spectrum antimicrobial effects (Smania et al., 2007), while solvent extracts of G. lucidum yielded terpenoids, alkaloids, and steroids with wide-ranging antimicrobial activity (Subbraj et al., 2008). Proteinaceous extracts from G. resinaceum also demonstrated notable activity against hospital-associated pathogens, including E. coli, S. aureus, and K. pneumoniae (Hearst et al., 2010), while G. lucidum extracts produced inhibition zones up to 16 mm against MDR clinical isolates (Sekaran et al., 2011). Steroidal compounds from several Ganoderma species were shown to inhibit M. tuberculosis (MIC 0.781–50 µg/mL) and Gram-positive cocci (Vazirian et al., 2014), underscoring their relevance for neglected and resurgent infectious diseases such as tuberculosis. Innovative processing and analytical techniques have recently advanced the identification of bioactives from Ganoderma. Gamma irradiation enhanced the antimicrobial potency of G. resinaceum (Abd El-Zaher, 2010), while LC-MS analysis detected bioactive compounds such as hesperetin and ganocin B in G. lucidum (Abdullah et al., 2021). Optimized extraction protocols yielded phenolic-rich fractions (16.01 mg/g total phenolics) from G. lucidum, which showed potent activity against S. aureus (10.6-mm inhibition zone) (Masjedi et al., 2022). These effects are partly attributed to ROS-mediated bacterial protein leakage, as evidenced by phenolic fractions of G. lucidum (Mishra et al., 2018b). Moreover, uncooked Ganoderma biomass has shown dual antimicrobial and anticancer activity, offering potential for functional food or nutraceutical applications (Alghonaim et al., 2023). Altogether, these studies reveal that non-triterpenoid Ganoderma metabolites—especially essential oils, phenolics, and proteins—may offer novel solutions to combat AMR and opportunistic infections. However, their clinical translation remains limited due to a lack of in vivo validation, pharmacokinetic profiling, and toxicity assessments. Future work should prioritize preclinical testing of these compounds in infection models, particularly for high-burden diseases such as tuberculosis, hospital-acquired infections, and drug-resistant enteric pathogens.
5.1.5 Nanoparticles
The global rise of AMR and chronic biofilm-associated infections underscores the urgent need for novel, multi-targeted therapeutics that are both effective and sustainable. Nanotechnology has emerged as a powerful tool in this arena, and Ganoderma-derived nanoparticles—particularly silver nanoparticles (Ag-NPs)—represent a promising frontier in fungal biomedicine. Infections caused by MDR pathogens such as S. aureus, E. coli, and P. aeruginosa remain major contributors to mortality in hospitals worldwide, with the WHO designating these as “priority pathogens.” Numerous studies have demonstrated that Ag-NPs synthesized from G. lucidum, G. resinaceum, and G. sessile exhibit broad-spectrum antibacterial activity, often surpassing the efficacy of conventional antibiotics or potentiating their effects through synergistic mechanisms (Kannan et al., 2014; Ali Syed et al., 2023; Table 5).
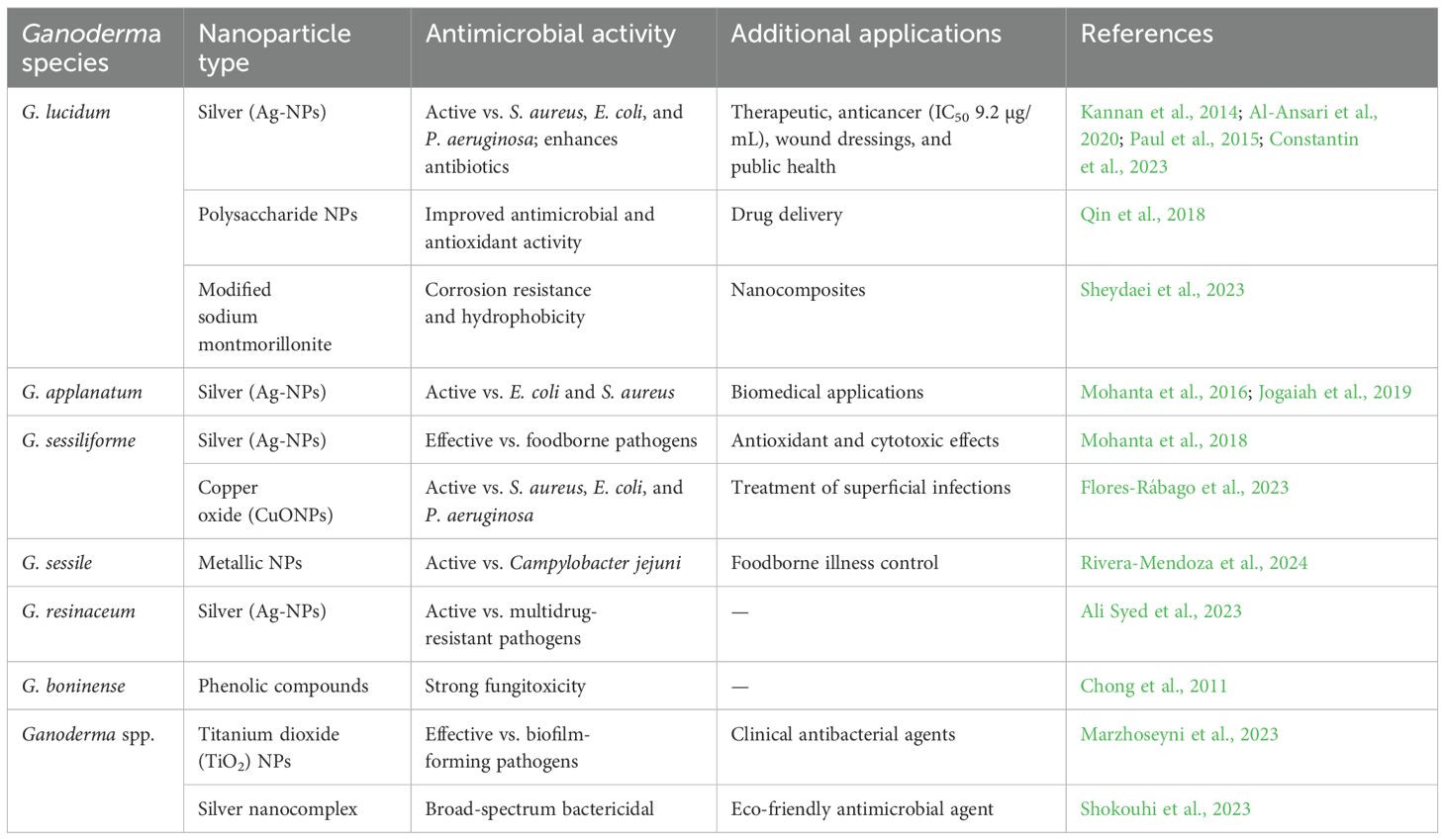
Table 5. Overview of antimicrobial activity and applications of nanoparticles derived from Ganoderma species.
In resource-limited settings where access to antibiotics is restricted, these green-synthesized nanoparticles offer a cost-effective and scalable antimicrobial alternative. Their ability to disrupt bacterial membranes, generate ROS, and inhibit efflux pumps suggests utility in treating persistent infections such as those found in tuberculosis, diabetic wounds, and catheter-associated UTIs (Al-Ansari et al., 2020; Bhardwaj et al., 2016). Moreover, the low toxicity of Ganoderma-synthesized copper oxide nanoparticles (CuONPs) supports their potential use in topical formulations for superficial infections, particularly in low-income regions (Flores-Rábago et al., 2023).
Importantly, Ganoderma-derived nanoparticles also show activity against biofilm-forming pathogens, a major clinical challenge in implant-related infections and chronic wounds. Biofilms protect microbes from host immunity and antibiotics, contributing to prolonged hospital stays and increased mortality. Titanium dioxide nanoparticles combined with Ganoderma extracts have shown antibiofilm efficacy, which could be leveraged in medical device coatings and sterile wound dressings (Marzhoseyni et al., 2023; Paul et al., 2015). The anticancer and antioxidant properties of these nanoparticles add another layer of relevance. Ag-NPs synthesized from G. lucidum and G. sessiliforme have demonstrated cytotoxicity against breast and lung cancer cell lines, potentially addressing cancer-related infections and immune suppression (Mohanta et al., 2018; Bhardwaj et al., 2016). In cancer patients with neutropenia or post-chemotherapy immune suppression, fungal or bacterial co-infections are common. Thus, dual-action nanoparticles offer a novel approach to oncological support therapy.
In food safety and agriculture, Ganoderma-based nanoparticles have been tested against Campylobacter jejuni, a major cause of gastroenteritis and post-infectious sequelae in developing nations (Rivera-Mendoza et al., 2024). This points to a broader public health application, particularly in addressing foodborne diseases and improving sanitation in regions with limited access to refrigeration or clean water. Although current studies are predominantly in vitro, the eco-friendly synthesis, scalability, and multipotent biological activities of Ganoderma-derived nanoparticles position them as strong candidates for next-generation antimicrobials. Future work must address in vivo efficacy, targeted delivery mechanisms, pharmacokinetics, and regulatory considerations to facilitate clinical translation. Hence, Ganoderma-based nanomaterials not only show promise against MDR pathogens and biofilms but also align with global health priorities such as reducing AMR, treating co-infections in cancer or HIV patients, and improving access to antimicrobial materials in underserved regions. These properties highlight their unmet therapeutic potential in both developed and developing healthcare systems.
5.2 Fungal infections
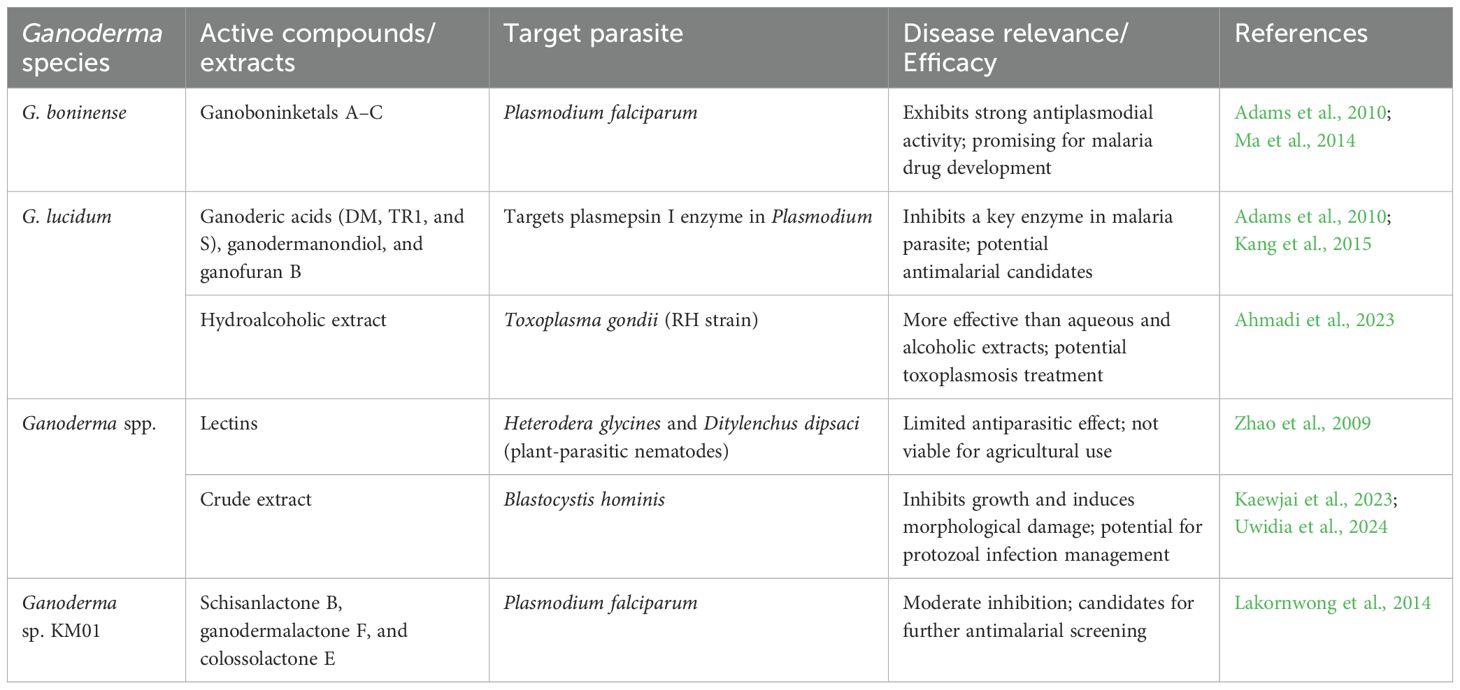
Fungal infections pose a growing threat to global health, particularly among immunocompromised individuals, transplant recipients, and patients undergoing chemotherapy. According to the Global Action Fund for Fungal Infections, over 1.5 million deaths annually are attributed to invasive fungal diseases, and current treatments are limited by toxicity, poor bioavailability, and rising resistance—especially in Candida and Aspergillus species. The pipeline for new antifungal drugs remains dangerously sparse, underlining the urgent need for novel, safer, and more effective agents. Against this backdrop, Ganoderma species, particularly G. lucidum, offer promising antifungal potential with mechanisms distinct from conventional agents (Table 6). G. lucidum has demonstrated broad-spectrum activity against pathogenic fungi, including C. albicans, Aspergillus flavus, and Fusarium oxysporum, with some studies reporting MIC values below 1 µg/mL (Bitew and Abate, 1994). These findings are not merely academic; they suggest that Ganoderma-derived compounds could fill critical therapeutic gaps in treating drug-resistant candidiasis and aspergillosis, which are common and often fatal in ICU patients and those with hematological malignancies.
Among the most notable bioactives is ganodermin, a protein isolated from G. lucidum that inhibits multiple phytopathogens (Wang and Ng, 2006), with potential for further development into topical antifungal formulations. In clinical contexts, G. lucidum has been incorporated into products like antifungal toothpaste and biomaterials such as polymethylmethacrylate (PMMA), where it enhanced mechanical performance while inhibiting C. albicans biofilm formation, a common cause of denture stomatitis (Nayak et al., 2010b; Enaba and El Gendi, 2022).
The unique mode of action of Ganoderma-derived triterpenoids—targeting ergosterol to disrupt fungal membranes—may offer an alternative to existing ergosterol-targeting drugs like amphotericin B but with lower toxicity (Wasser, 2011). In addition, these compounds have demonstrated the ability to interfere with biofilm formation and fungal cell wall synthesis, both of which are key contributors to antifungal resistance and treatment failure (Chan et al., 2013).
Importantly, Ganoderma extracts have shown efficacy against dermatophytes such as Microsporum canis and Trichophyton mentagrophytes, which are prevalent in tropical climates and often undertreated due to limited healthcare access (Smania et al., 2003). In veterinary and agricultural sectors, Ganoderma is also emerging as a natural antifungal for contaminated feed and crops, suggesting a One Health approach to fungal control (El-Fallal et al., 2021). From a pharmaceutical development perspective, molecular docking studies have revealed strong binding affinities of Ganoderma metabolites to key fungal protein targets, offering a rational basis for structure-based drug design (Nguyen et al., 2024). This computational insight strengthens the argument for clinical translation and underscores the need for further in vivo validation and toxicity profiling. Hence, the antifungal properties of Ganoderma are not just promising in vitro but potentially transformative in clinical settings where fungal infections are increasing and treatment options remain inadequate. By targeting resistant strains, disrupting biofilms, and offering low-toxicity alternatives, Ganoderma-derived compounds could represent the next generation of antifungal therapeutics—especially in settings where conventional options fall short.
5.3 Viral infections
Viral infections remain a major global health challenge, with diseases such as HIV/AIDS, hepatitis B (HBV), herpes simplex (HSV), and influenza collectively causing significant morbidity and mortality. According to UNAIDS, approximately 39 million people were living with HIV globally in 2023, while WHO reports over 250 million people chronically infected with HBV. These figures underscore the urgent need for novel antiviral agents, especially in light of emerging drug resistance and the limited efficacy or accessibility of current therapeutics in many regions. Ganoderma species, particularly G. lucidum, have garnered interest for their potential to address these unmet needs through their diverse arsenal of bioactive compounds (Table 7). Isolated triterpenoids, such as ganoderic acid-β, lucidumol B, and ganodermanontriol, have demonstrated significant anti-HIV-1 protease activity, with IC50 values ranging from 20 to 90 μM (Min et al., 1998; El-Mekkawy et al., 1998). Importantly, molecular docking studies suggest that ganoderic acid-B exhibits a binding affinity surpassing that of the standard drug nelfinavir, supporting its potential as a lead compound for drug development (Kang et al., 2015). In addition, enzymatic crude extracts rich in laccase from G. lucidum have shown remarkable in vitro inhibition of HIV-1 replication (Zhang et al., 2011; Flórez-Sampedro et al., 2016), providing an alternative strategy targeting reverse transcription pathways.
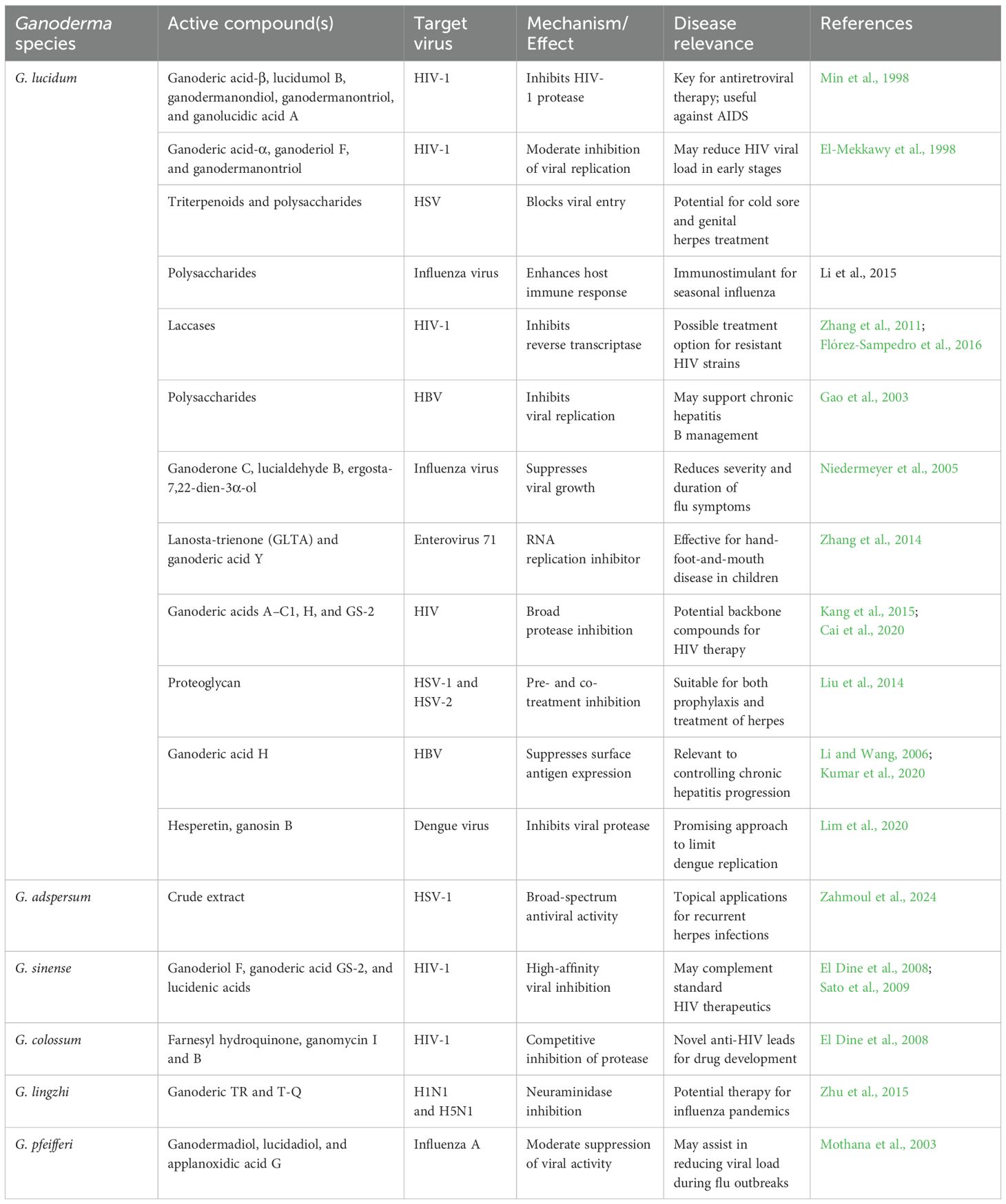
Table 7. Antiviral activity of compounds derived from Ganoderma species against various viral infections.
The antiviral effects of Ganoderma extend beyond HIV. Polysaccharides and triterpenes from G. lucidum have shown inhibitory activity against HSV and influenza virus. These effects are attributed to both direct interference with viral entry and replication, as well as enhancement of host immunity through cytokine stimulation (Basnet et al., 2017). This dual mode of action is particularly relevant in immunocompromised populations where traditional antivirals may fail or cause adverse effects. Notably, Ganoderma adspersum extract demonstrated potent activity against HSV-1 with a high selective index and protective efficacy (Zahmoul et al., 2024), highlighting its therapeutic potential for dermatological or mucosal viral infections. Furthermore, triterpenoids such as ganoderiol F, ganodermadiol, and colossolactones isolated from G. lucidum, G. sinense, and G. colossum have shown broad-spectrum activity against HIV-1, HSV, and influenza viruses with IC50 or ED50 values within pharmacologically relevant ranges (El Dine et al., 2008; Sato et al., 2009; Mothana et al., 2003). These findings suggest that Ganoderma may serve as a platform for developing multitarget antivirals—particularly valuable in resource-limited settings where polyvalent therapies are needed to treat co-infections. Although current evidence is largely preclinical, these studies collectively position Ganoderma-derived compounds as promising candidates for addressing therapeutic gaps in managing persistent and drug-resistant viral infections. Future efforts should focus on validating these compounds in clinical models and elucidating their pharmacokinetics and immunomodulatory effects to advance their development into viable antiviral therapies.
5.4 Parasitic infections
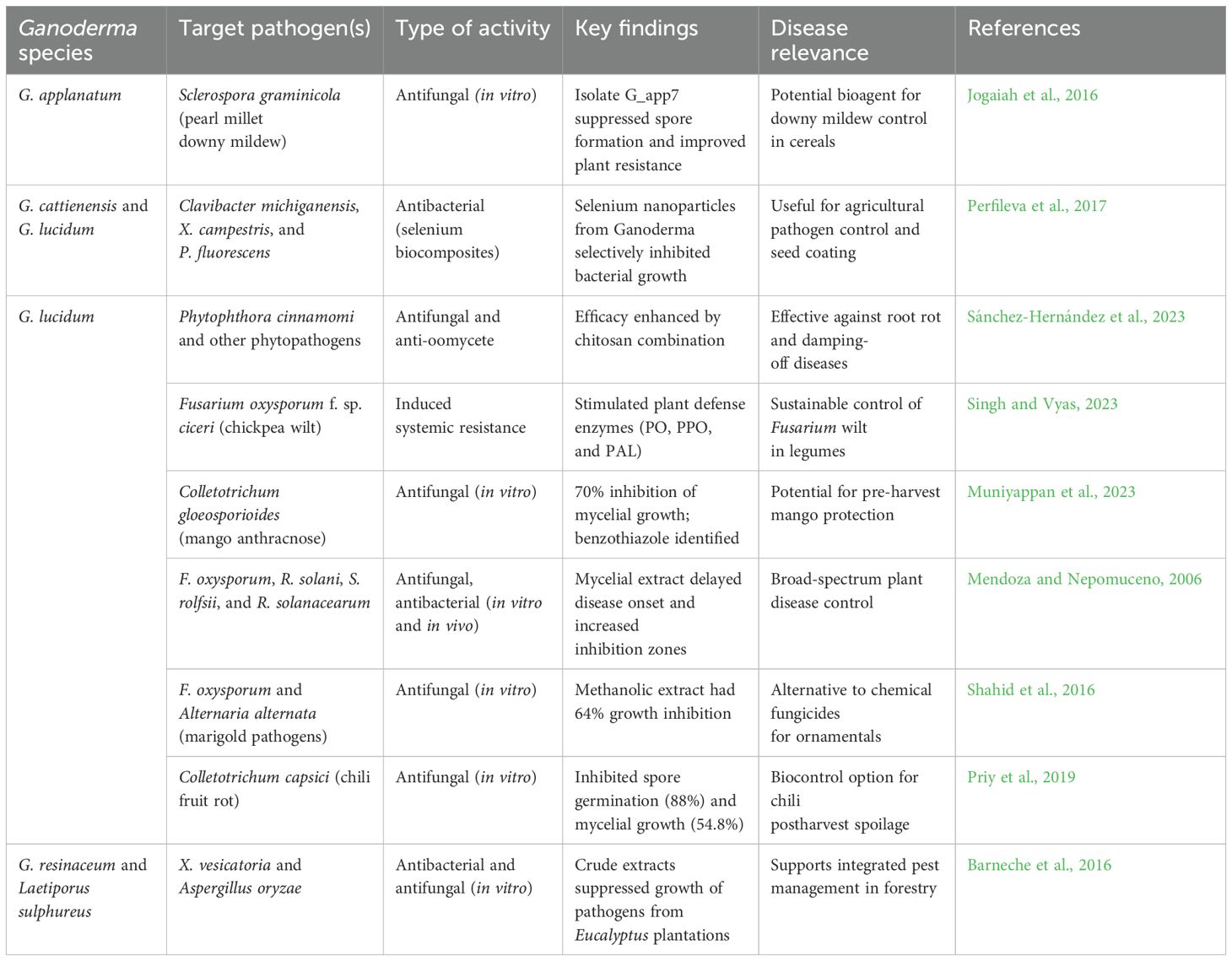
Parasitic diseases continue to exact a significant toll on global health, particularly in tropical and subtropical regions. Malaria alone caused over 600,000 deaths in 2022, predominantly among children under five in sub-Saharan Africa (World Health Organization, 2024). Other parasitic infections, such as toxoplasmosis, giardiasis, leishmaniasis, and blastocystosis, also contribute to considerable morbidity, with limited treatment options, increasing drug resistance, and toxicity issues posing substantial therapeutic challenges. Recent research has highlighted the potential antiparasitic properties of Ganoderma species, revealing promising efficacy against several protozoal and parasitic infections (Table 8). Notably, nortriterpenes ganoboninketals A–C, derived from G. boninense fruiting bodies, demonstrated potent antiplasmodial activity against Plasmodium falciparum with IC50 values of 4.0, 7.9, and 1.7 μM, respectively (Adams et al., 2010; Ma et al., 2014; Figure 2). Additional triterpenes—schisanlactone B, ganodermalactone F, and colossolactone E—isolated from Ganoderma sp. KM01 also showed activity against P. falciparum, with IC50 values ranging from 6.0 to 10.0 μM (Lakornwong et al., 2014). Moreover, G. lucidum-derived compounds such as ganoderic acids (DM, TR1, and S), ganodermanondiol, and ganofuran B, isolated using EtOAc, exhibited inhibitory effects on P. falciparum within a 6.0–20 μM IC50 range (Adams et al., 2010). These activities fall within a biologically relevant range, highlighting their potential as lead compounds for the development of novel antimalarials, especially in the face of rising resistance to artemisinin-based therapies.
In studies on nematode inhibition, Zhao et al. (2009) reported that lectins from Ganoderma exhibited activity against plant nematodes Heterodera glycines and Ditylenchus dipsaci, although their potency was deemed insufficient for practical use. Nonetheless, these findings provide a foundation for future optimization or bioengineering approaches to enhance antihelminthic efficacy. Computational studies further support the antiparasitic potential of Ganoderma compounds. G. lucidum triterpenoids were shown to interact with plasmepsin I, a key enzyme in P. falciparum. Ganodermanondiol demonstrated the highest affinity (binding energy = −7.14 kcal/mol, Ki = 0.005 mM), outperforming the standard inhibitor KNI-10006 (Kang et al., 2015). This suggests a plausible mechanism of action and reinforces the value of Ganoderma constituents in rational drug design against malaria. Ganoderma extracts also displayed antiprotozoal effects against Blastocystis hominis, a parasite increasingly associated with gastrointestinal disorders. Strong inhibitory activity was observed at an MIC of 62.5 μg/mL. At higher concentrations, extracts of Ganoderma and Boesenbergia rotunda reduced B. hominis growth by up to 90% within 12 h and induced notable morphological damage, pointing to their potential in managing treatment-refractory blastocystosis (Kaewjai et al., 2023; Uwidia et al., 2024). In addition, G. lucidum extracts demonstrated anti-Toxoplasma effects, particularly against Toxoplasma gondii RH strain tachyzoites. In vitro studies showed that the hydroalcoholic extract of G. lucidum exhibited the highest toxoplasmacidal activity and selectivity (EC50 = 3.274), outperforming both aqueous (EC50: 76.32) and alcoholic extracts (EC50: 40.18) (Ahmadi et al., 2023). Given the limited efficacy and potential teratogenicity of current anti-toxoplasmosis treatments, such natural alternatives may offer safer and more accessible interventions, especially in immunocompromised populations. Overall, these findings suggest that Ganoderma-derived metabolites hold considerable promise in addressing parasitic diseases where conventional therapies fall short. Future studies should aim to evaluate their efficacy in vivo, explore their mechanisms of action, and assess safety profiles to support clinical translation.
6 Clinical studies on antimicrobial properties of Ganoderma
G. lucidum has been extensively studied for its antimicrobial properties, particularly in laboratory and animal models. In vitro studies have demonstrated that its bioactive compounds—mainly polysaccharides and triterpenoids—possess antiviral, antibacterial, and antifungal activities. Despite these promising findings, human clinical evidence remains limited, with most clinical research to date focusing on immune modulation, cancer therapy, and liver protection rather than direct antimicrobial effects. Some preliminary clinical studies suggest potential antiviral benefits. A pilot clinical trial conducted by Hijikata et al. (2005) evaluated an herbal formula containing G. lucidum in patients with herpes zoster (shingles). Participants who received 750 mg daily experienced rapid symptom relief, with most resolving within 10 days, and no cases of postherpetic neuralgia were reported after 1 year. In a subsequent study by the same group (Hijikata et al., 2007), individuals with recurrent herpes simplex infections who were treated with a hot water extract of G. lucidum at 4 g daily reported faster symptom resolution—genital herpes symptoms improved in 4.9 ± 1.3 days compared to 10.9 ± 6.3 days without treatment. However, both studies involved combination herbal formulas, making it difficult to isolate the specific effects of G. lucidum. To date, there are no human clinical trials specifically evaluating the antibacterial efficacy of G. lucidum, and evidence in this area is limited to in vitro findings. Similarly, while antifungal activity has been reported in laboratory settings—particularly against Candida species and dermatophytes—no human studies have validated these effects clinically. Research on its antiparasitic activity remains scarce, with neither significant preclinical nor clinical data currently available.
7 Ganoderma against plant pathogens
Research on Ganoderma has revealed its potential as a natural biocontrol agent against various plant pathogens. Numerous studies have documented its antimicrobial effects, highlighting its capacity to combat fungal and bacterial infections in plants. G. lucidum mycelia showed moderate antimicrobial activity against soil-borne pathogens, including fungi (F. oxysporum, Rhizoctonia solani, and Sclerotium rolfsii) and bacteria (R. solanacearum and S. aureus). In vitro, mycelial extracts increased inhibition zones, while in vivo tests on tomato seedlings delayed disease symptoms, suggesting G. lucidum as a potential biocontrol agent, particularly against R. solani and S. rolfsii (Mendoza and Nepomuceno, 2006). G. lucidum extracts exhibit antifungal properties effective against plant pathogens F. oxysporum and Alternaria alternata in marigolds. This study compared organic and aqueous extracts of G. lucidum, applying various concentrations (5%, 10%, 15%, and 20%) using Agar absorption, Agar well diffusion, and Vapor assay methods. Methanolic extract showed the highest inhibition (64%) using the Agar absorption method, while aqueous extract showed the lowest inhibition (38%) with Agar well diffusion. These findings highlight the potential of G. lucidum methanolic extract as a biological control agent for marigold plant diseases (Shahid et al., 2016). The antimicrobial activity of extracts from wood-rotting Basidiomycetes mushrooms from Eucalyptus plantations in Uruguay was investigated. Eight extracts, including those from G. resinaceum and L. sulphureus, were active against pathogens such as Xanthomonas vesicatoria and Aspergillus oryzae (Barneche et al., 2016). A compound named G_app7, isolated from G. applanatum, was found to effectively inhibit the growth of Sclerospora graminicola, the pathogen causing downy mildew in pearl millet (Pennisetum glaucum). G_app7 reduced sporangium formation (41.4%), zoospore release (77.5%), and motility (91%), and closely resembles metominostrobin, a fungicide. It remained effective at temperatures between 25 and 80°C and was stable for at least 12 months at 4°C. Seed treatment with G_app7 provided a 63% increase in disease protection compared to controls, highlighting its potential as an environmentally safe agrochemical for pearl millet protection (Jogaiah et al., 2016).
The antibacterial effects of selenium-containing biocomposites from submerged cultures of Ganoderma species were studied against plant pathogenic bacteria. Biocomposites from G. cattienensis and G. lucidum were most effective against C. michiganensis, while those from G. valesiacum and G. lucidum showed strong activity against Xanthomonas campestris. G. colossus exhibited notable activity against Pseudomonas fluorescens. The study highlights the potential of using coumarin-based compounds for producing antimicrobial substances from fungi (Perfileva et al., 2017). Eight mushroom species were screened, including G. lucidum, for their impact on Colletotrichum capsici, the chili fruit rot pathogen. The results revealed that G. lucidum, Auricularia polytricha, and Lentinus edodes demonstrated significant antifungal activity, with G. lucidum achieving the highest mycelial growth inhibition (54.81%). Chloroform extracts from G. lucidum inhibited spore germination (88%) and mycelial growth (60.55%) at 24 h. These findings suggest G. lucidum as a promising source for developing fungicides against C. capsici, warranting further investigation of its active compounds (Priy et al., 2019).
The antimicrobial potential of an aqueous ammonia extract from G. lucidum carpophores, sourced from Quercus ilex trees, was investigated, revealing key chemical constituents such as acetamide and oleic acid. The extract exhibited strong anti-oomycete and antifungal activities, with MIC values of 187.5 μg·mL−1 against Phytophthora cinnamomi and varying MICs against other fungi. When conjugated with chitosan oligomers, the extract’s antimicrobial efficacy significantly increased, showcasing MIC values as low as 78.12 μg·mL−1, demonstrating its potential for protecting holm oak in sustainable agricultural practices (Sánchez-Hernández et al., 2023). The antifungal properties of G. lucidum against the mango anthracnose pathogen C. gloeosporioides were investigated in this study. Ethyl acetate extracts from the fruiting body inhibited mycelial growth by 70.10% at a 1% concentration. Thin-layer chromatography identified two active bands, with the first achieving 53.77% inhibition. Gas chromatography–mass spectrometry detected benzothiazole, which completely inhibited mycelial growth at 50 ppm and caused structural abnormalities in the pathogen. The findings suggest that G. lucidum biomolecules could be effective natural agents against plant pathogens (Muniyappan et al., 2023). The crude extract of G. lucidum was formulated into an emulsion [water in oil (W/O)] to induce systemic resistance in chickpeas against Fusarium wilt caused by F. oxysporum f. sp. ciceri (FOC). Different dilutions of the formulation were applied to chickpeas, which were then challenged with FOC. Enzyme assays showed increased activity of peroxidase (PO), polyphenol oxidase (PPO), and phenylalanine ammonia-lyase (PAL) in treated plants, indicating activation of the plant’s natural defense pathways. GC-MS analysis confirmed bioactive compounds responsible for enhancing enzyme levels. This study suggests the potential for developing bio-formulations to control plant diseases (Singh and Vyas, 2023). Table 9 summarizes the antimicrobial activities of Ganoderma species against plant pathogens.
8 Challenges and limitations of Ganoderma in antimicrobial applications
Although Ganoderma, especially G. lucidum, has demonstrated promising antimicrobial properties, several key challenges limit its broader adoption in medical and agricultural settings. These challenges primarily stem from variability in species, inconsistency in extract composition, and a lack of robust human clinical research specifically targeting antimicrobial use. A major hurdle is the natural variation in bioactive compounds among different Ganoderma species. Each species produces a unique blend of compounds—such as polysaccharides, triterpenoids, and phenolics—which directly influences their antimicrobial efficacy. Even within the same species, factors like geographical origin, climate, substrate, and cultivation conditions can alter the concentration and types of active molecules. This variability makes it difficult to predict or compare the antimicrobial strength of different extracts, reducing their reliability as standardized treatments. Another significant limitation lies in the difficulty of standardizing Ganoderma extracts. Unlike conventional pharmaceuticals that are based on single, well-defined molecules, Ganoderma extracts are complex mixtures. Depending on the extraction method used—whether water-based or alcohol-based—the resulting compounds and their concentrations can vary greatly. This leads to inconsistent therapeutic profiles, making dosage optimization and reproducibility a challenge. Furthermore, there is currently no universally accepted quality control standard for Ganoderma products, which adds another layer of uncertainty for clinical or commercial use. Perhaps the most critical limitation is the lack of extensive human clinical trials specifically designed to assess antimicrobial effects of Ganoderma. While laboratory and animal studies have shown promising results against bacteria, fungi, and viruses, human trials remain scarce. Most clinical research has focused on immune modulation, cancer support, and liver protection, rather than on infectious diseases. Without rigorous clinical testing, questions remain about its safety, appropriate dosing, and real-world efficacy. This lack of data also presents a barrier to regulatory approval and mainstream medical acceptance, hindering the development of Ganoderma-based antimicrobial therapies. Although Ganoderma holds great promise as a natural antimicrobial agent, issues related to species variability, extract standardization, and insufficient clinical evidence must be addressed before it can be reliably integrated into therapeutic or agricultural practices.
9 Future research directions for Ganoderma in antimicrobial applications
The growing recognition of antimicrobial properties of Ganoderma highlights several critical research avenues that could unlock its full therapeutic potential. First and foremost, standardizing Ganoderma extracts is essential to ensure consistency in their bioactive compounds, such as polysaccharides, triterpenoids, and phenolics. Variations in species, cultivation methods, and extraction techniques currently lead to unpredictable antimicrobial effects, limiting reproducibility in both research and clinical applications. Future studies should focus on optimizing extraction protocols and determining minimum effective concentrations to create reliable, high-quality formulations suitable for pharmaceutical use. Another promising direction involves developing Ganoderma-based antimicrobial drugs or supplements. Its bioactive compounds have demonstrated broad-spectrum activity against bacteria, fungi, and viruses, making them strong candidates for novel treatments. Given the escalating threat of AMR, Ganoderma’s multi-target mechanisms—including cell wall disruption, nucleic acid synthesis inhibition, and oxidative stress induction—could provide alternative therapies that pathogens struggle to resist. Perhaps most compelling is the potential for Ganoderma to enhance conventional antibiotics through synergistic combinations. Preliminary evidence suggests that pairing Ganoderma extracts with existing antimicrobials may improve efficacy while reducing required dosages, thereby minimizing side effects and delaying resistance. Future research should systematically investigate these interactions, particularly against drug-resistant strains, as well as explore the role of Ganoderma as an adjunct therapy for fungal and viral infections in immunocompromised patients. By addressing these priorities, Ganoderma could transition from a traditional remedy to a scientifically validated antimicrobial agent, offering new solutions in an era of increasing treatment challenges.
10 Conclusion
Ganoderma exhibits significant promise as a natural source of antimicrobial agents, with its bioactive compounds—polysaccharides, triterpenoids, phenolic compounds, and proteins—demonstrating a variety of mechanisms to combat bacterial, fungal, and viral infections. These compounds function by disrupting microbial cell walls, inhibiting nucleic acid synthesis, modulating the immune system, and regulating oxidative stress, offering a multi-targeted approach to pathogen inhibition. However, it is important to note that there may be potential risks or limitations associated with the use of Ganoderma as an antimicrobial agent, which should be thoroughly investigated in future research. Numerous in vitro and preclinical studies have already illustrated Ganoderma’s potential to be developed into therapeutic agents, especially in light of the growing global concern over AMR. Future research should prioritize clinical trials to validate Ganoderma’s efficacy in human subjects, particularly for its antimicrobial applications. Standardizing Ganoderma extracts is another critical area that would facilitate consistency in research and therapeutic use. In addition, identifying and isolating specific active compounds within Ganoderma may allow for more targeted drug development, potentially leading to the creation of new antimicrobial drugs or supplements. Furthermore, exploring synergistic effects with conventional antibiotics could offer new solutions to enhance treatment efficacy and reduce drug resistance. Continued investigation into these areas will be key to unlocking Ganoderma’s full potential as a vital player in the future of antimicrobial therapies.
Author contributions
SK: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing. NP: Methodology, Software, Writing – original draft, Writing – review & editing. KH: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing. IP: Conceptualization, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Samantha C. Karunarathna thanks the High-Level Talent Recruitment Plan of Yunnan Province (“High-End Foreign Experts” Program), the National Natural Science Foundation of China (Grant No. 32260004), and Key Laboratory of Yunnan Provincial Department of Education of the Deep-Time Evolution on Biodiversity from the Origin of the Pearl River, Qujing Normal University, Qujing, Yunnan 655011, China, for their support. We also extend our gratitude to Chiang Mai University, Thailand, for partial support of this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abd El-Zaher, E. H. F. (2010). Antimicrobial activity of lipid extracted from gamma radiated mycelia of Ganoderma resinaceum. Egypt. J. Exp. Biol. (Bot.). 6, 87–92.
Abdullah, S., Jang, S. E., Kwak, M. K., and Chong, K. (2020). Ganoderma boninense mycelia for phytochemicals and secondary metabolites with antibacterial activity. J. Microbiol. 58, 1054–1064. doi: 10.1007/s12275-020-0208-z
Abdullah, S., Oh, Y. S., Kwak, M. K., and Chong, K. (2021). Biophysical characterization of antibacterial compounds derived from pathogenic fungi Ganoderma boninense. J. Microbiol. 59, 164–174. doi: 10.1007/s12275-021-0551-8
Adams, M., Christen, M., Plitzko, I., Zimmermann, S., Brun, R., Kaiser, M., et al. (2010). Anti plasmodial lanostanes from the G. lucidum mushroom. J. Nat. Prod. 73, 897–900. doi: 10.1021/np100031c
Ahmad, M. F. (2019). “G. lucidum: A macro fungus with phytochemicals and their pharmacological properties,” in Plant and Human Health. Eds. Ozturk, M. and Hakeem, K. (Springer, Cham).
Ahmad, M. F., Alsayegh, A. A., Ahmad, F. A., Akhtar, M. S., Alavudeen, S. S., Bantun, F., et al. (2024). G. lucidum: Insight into antimicrobial and antioxidant properties with development of secondary metabolites. Heliyon 10, e25607. doi: 10.1016/j.heliyon.2024.e25607
Ahmadi, M., Salimi, M., Saraei, M., Nezhad, N. S., Javadi, A., Mohammadi, F., et al. (2023). In vitro anti-Toxoplasma gondii activity of G. lucidum extracts. BMC Res. Notes. 16, 82. doi: 10.1186/s13104-023-06355-6
Al-Ansari, M. M., Dhasarathan, P., Ranjitsingh, A. J., and Al-Humaid, L. A. (2020). G. lucidum inspired silver nanoparticles and its biomedical applications with special reference to drug resistant E. coli isolates from CAUTI. Saudi J. Biol. Sci. 27, 2993–3002. doi: 10.1016/j.sjbs.2020.09.008
Al-Fatimi, M., Wurster, M., Kreisel, H., and Lindequist, U. (2005). Antimicrobial, cytotoxic and antioxidant activity of selected basidiomycetes from Yemen. Pharmazie. 60, 60776–60780. doi: 10.15376/biores.18.4.8037-8061
Al-Fatimi, M., Wurster, M., and Lindequist, U. (2016). Chemical composition, antimicrobial and antioxidant activities of the volatile oil of Ganoderma pfeifferi Bres. Medicines. 3, 10. doi: 10.3390/medicines3020010
Alghonaim, M. I., Alsalamah, S. A., Alsolami, A., and Ghany, T. A. (2023). Characterization and efficiency of G. lucidum biomass as an antimicrobial and anticancer agent. BioResources. 18, 8037.
Ali Syed, I., Alvi, I. A., Fiaz, M., Ahmad, J., Butt, S., Ullah, A., et al. (2023). Synthesis of silver nanoparticles from Ganoderma species and their activity against multi-drug resistant pathogens. Chem. Biodivers. 21, e202301304. doi: 10.1002/cbdv.202301304
Alves, M. J., Ferreira, I. C. F. R., Froufe, H. C., Abreu, R. M. V., Martins, A., and Pintado, M. (2013). Antimicrobial activity of phenolic compounds identified in wild mushrooms, SAR analysis and docking studies. J. Appl. Microbiol. 115, 346–357. doi: 10.1111/jam.12196
Ameri, A., Rajive, B. B., Vaidya, J. G., Apte, K., and Deokule, S. S. (2013). Anti-staphylococcal and wound healing activities of Ganoderma praelongum and Glycyrrhiza glabra formulation in mice. IJARNP. 6, 27–31.
Ameri, A., Vaidya, J. G., and Deokule, S. S. (2011). In vitro evaluation of anti-staphylococcal activity of G. lucidum, Ganoderma praelongum and Ganoderma resinaceum from Pune. India. Afr. J. Microbiol. Res. 5, 328–333.
Aneeshia, C. S. and Sornaraj, R. (2010). Comparativestudy on the antibacterial activity of G. lucidum. Indian J. Environ. Ecoplan. 17, 319–324.
Anyakorah, C. I., Ogunsina, O. A., and Igbo, U. E. (2020). GC/MS analysis and in vitro effect of G. lucidum solvent extracts on microorganisms isolated from the armpit, scalp and urinary tract. JALSI. 25, 37–47. doi: 10.9734/jalsi/2022/v25i130281
Anyimba, E. S. (2020). Extraction, isolation and characterization of compounds From Termitomyces letestui and G. lucidum and their efficacy against some pathogenic micro-organisms. Kakamega, Kenya: Masinde Muliro University of Science and Technology.
Avcı, E., Avcı, G. A., and Kose, D. A. (2014). Determination of antioxidant and antimicrobial activities of medically important mushrooms using different solvents and chemical composition via GC/MS analyses. J. Food Nutr. Res. 2, 429–434. doi: 10.12691/jfnr-2-8-1
Ayimbila, F., Siriwong, S., Chaiyama, V., Srihanant, N., and Keawsompong, S. (2023). Comparative study of bio-functional profile and bioactivities of polysaccharides from G. lucidum and Ganoderma neo-japonicum. Biocatal. Agric. Biotechnol. 53, 102875. doi: 10.1016/j.bcab.2023.102875
Azahar, N. I., Swan, S. Y., Mokhtar, N. M., Abd Aziz, M. A., and Arifin, M. A. (2023). Evaluation of antioxidant, antibacterial and anticancer activities of G. lucidum extracts. Mater. Today: Proc. 107, 82–91. doi: 10.1016/j.matpr.2023.08.030
Baby, S., Johnson, A. J., and Govindan, B. (2015). Secondary metabolites from ganoderma. Phytochemistry 114, 66–101. doi: 10.1016/j.phytochem.2015.03.010
Bai, D., Chang, N.-T., Li, D.-H., Liu, J.-X., and You, X.-Y. (2008). Antiblastic activitiy of G. lucidum. Acta Agric. Boreali-Sin. 23, 282–285. doi: 10.7668/hbnxb.2008.S1.065
Baig, M. N., Shahid, A. A., and Ali, M. (2015). In vitro assessment of extracts of the Lingzhi or Reishi medicinal mushroom, G. lucidum (higher basidiomycetes) against different plant pathogenic fungi. Int. J. Med. Mushrooms. 17, 407–411. doi: 10.1615/IntJMedMushrooms.v17.i4.90
Baraza, D. L., Ooko, S., Nyongesa, P. K., and Sande, E. (2021). Phytochemical analysis, isolated ergosta-5, 7, 22-triene-3?, 14?-diol (22Z) and antimicrobial activity of Kenyan G. lucidum. Curr. Perspect. Chem. Sciences Vol. 7, 65–78. doi: 10.9734/bpi/cpcs/v7/2178E
Barneche, S., Jorcin, G., Cecchetto, G., Cerdeiras, M. P., Vázquez, A., and Alborés, S. (2016). Screening for antimicrobial activity of wood rotting higher basidiomycetes mushrooms from Uruguay against phytopathogens. Int. J. Med. Mushrooms. 18, 261–267. doi: 10.1615/IntJMedMushrooms.v18.i3.90
Barranco, P. G., Ocanas, L. G., Cabrera, L. V., Carmona, M. C., Ocanas, F. G., Gomez, X. S., et al. (2010). Evaluation of antioxidant, immunomodulating, cytotoxic and antimicrobial properties of different strains of Basidiomycetes from Northeastern Mexico. J. Med. Plant Res. 4, 1762–1769.
Basnet, B. B., Liu, L., Bao, L., and Liu, H. (2017). Current and future perspective on antimicrobial and anti-parasitic activities of Ganoderma sp.: An update. Mycology. 8, 111–124. doi: 10.1080/21501203.2017.1324529
Batra, P. and Khajuria, R. (2012). Evaluation of antibacterial activity of polysaccharide extract of G. lucidum. CTBCR.
Bharadwaj, S., Lee, K. E., Dwivedi, V. D., Yadava, U., Panwar, A., Lucas, S. J., et al. (2019). Discovery of Ganoderma lucidum triterpenoids as potential inhibitors against Dengue virus NS2B-NS3 protease. Sci. Rep. 9, 19059. doi: 10.1038/s41598-019-55723-5
Bhardwaj, N., Batra, P., and Beniwal, V. (2016). Biosynthesis, cytotoxicity and antimicrobial effect of silver ganoparticle from polysaccharide extract of G. lucidum. J. Pure Appl. Microbiol. 10, 1427.
Bhardwaj, A., Gupta, P., Kumar, N., Mishra, J., Kumar, A., and Misra, K. (2017). Lingzhi or reishi medicinal mushroom, G. lucidum (Agaricomycetes), inhibits Candida biofilms: A metabolomic approach. Int. J. Med. Mushrooms. 19, 685–696. doi: 10.1615/IntJMedMushrooms.2017021225
Bhattacharyya, C. B., De, S. A., Basak, A. N., Banerjee, M. A., Maitra, S., and Samajpati, N. (2006). Antimicrobial activities of some Basidiomycetous fungi. J. Mycopathol Res. 44, 129–135.
Bhosle, S. R., Bapat, G., Vaidya, J. G., Garad, S. A., and Sonawane, H. B. (2010). Antimicrobial activity of terpenoid extracts from Ganoderma samples. IJPLS. 1, 234–240. doi: 10.5555/20113195350
Bitew, A. and Abate, D. (1994). Antifungal metabolites from submerged culture of G. lucidum (Polypore). Ethiop. J. Health Dev. 8, 63–70.
Blundell, R., Camilleri, E., Baral, B., Karpiński, T. M., Neza, E., and Atrooz, O. M. (2023). The phytochemistry of Ganoderma species and their medicinal potentials. Am. J. Chin. Med. 51, 859–882. doi: 10.1142/S0192415X23500404
Cadar, E., Negreanu-Pirjol, T., Pascale, C., Sirbu, R., Prasacu, I., Negreanu-Pirjol, B. S., et al. (2023). Natural bio-compounds from G. lucidum and their beneficial biological actions for anticancer application: a review. Antioxidants 12, 1907. doi: 10.3390/antiox12111907
Cai, S., Xiao, H., Wang, X., Lin, S., and Zhong, J. J. (2020). Bioconversion of a ganoderic acid 3-hydroxy-lanosta-8, 24-dien-26-oic acid by a crude enzyme from G. lucidum. Process Biochem. 95, 12–16. doi: 10.1016/j.procbio.2020.05.002
Canpolat, Ş. and Canpolat, E. Y. (2023). Antioxidant and antimicrobial activity of a medicinal mushroom, G. lucidum. J. Adv. Biol. Biotechnol. 26, 60–67. doi: 10.9734/jabb/2023/v26i11667
Celal, B. (2019). Antioxidant and antimicrobial capacities of G. lucidum. MedCrave. 7, 5–7. doi: 10.15406/jbmoa.2019.07.00232
Celık, G. Y., Onbaslı, D., Altınsoy, B., and Allı, H. (2014). In vitro antimicrobial and antioxidant properties of G. lucidum extracts grown in Turkey. Eur. J. Medicinal Plants. 4, 709–722.
Chan, Y. S. and Chong, K. P. (2020). Antimicrobial activity and metabolite analysis of Ganoderma boninense fruiting body. J. Pure Appl. Microbiol. 14, 1213–1226. doi: 10.22207/JPAM.14.2.16
Chan, Y. S. and Chong, K. P. (2022). Bioactive compounds of Ganoderma boninense inhibited methicillin-resistant Staphylococcus aureus growth by affecting their cell membrane permeability and integrity. Molecules. 27, 838. doi: 10.3390/molecules27030838
Chan, P.-M., Kanagasabapathy, G., Tan, Y.-S., Sabaratnam, V., and Kuppusamy, U. R. (2013). Amauroderma rugosum (Blume & T. Nees) Torrend: Nutritional composition and antioxidant and potential anti-inflammatory properties. Evid. Based Complementary Altern. Med. 2013, 304713. doi: 10.1155/2013/304713
Chandrawanshi, N. K. and Shukla, S. (2019). Rapid in vitro antifungal property assessment of organic and aqueous extracts of G. lucidum against human pathogenic fungus of. Aspergillus Niger. JETIR. 6, 473–477. doi: 10.5281/zenodo.7080772
Chen, C. C., Cheh, L. W., Yang, J. C., Tsai, C. M., Keh, E. S., Sheu, M. T., et al. (2007). Non-shellfish chitosan from the fruiting body residue of Ganoderma tsugae for long-lasting antibacterial guided-tissue regeneration barriers. J. Dental Sci. 2, 19–29.
Chen, X. J., Deng, Z., Zhang, L. L., Pan, Y., Fu, J., Zou, L., et al. (2024). Therapeutic potential of the medicinal mushroom Ganoderma lucidum against Alzheimer’s disease. Biomedicine Pharmacotherapy. 172, 116222. doi: 10.1016/j.biopha.2024.116222
Chen, J. T. and Huang, J. W. (2010). Antimicrobial activity of edible mushroom culture filtrates on plant pathogens. Plant Pathol. Bull. 19, 261–270.
Chen, S. N., Nan, F. H., Liu, M. W., Yang, M. F., Chang, Y. C., and Chen, S. (2023). Evaluation of immune modulation by β-1, 3; 1, 6 D-glucan derived from G. lucidum healthy adult volunteers randomized Controlled trial. Foods. 12, 659. doi: 10.3390/foods12030659
Chong, K. P., Rossall, S., and Atong, M. (2011). HPC fingerprints and in vitro antimicrobial activity of syringic acid, caffeic acid and 4-hydroxybenzoic acid against. Ganoderma boninense. J. Appl. Sci. 11, 2284–2291. doi: 10.3923/jas.2011.2284.2291
Chuang, C. M., Wang, H. E., Chang, C. H., Peng, C. C., Ker, Y. B., Lai, J. E., et al. (2013). Sacchachitin, a novel chitin-poly- saccharide conjugate macromolecule present in G. lucidum: Purification, composition, and proper- ties. Pharm. Biol. 51, 84–95. doi: 10.3109/13880209.2012.711840
Ćilerdžić, J., Kosanic, M., Stajić, M., Vukojević, J., and Ranković, B. (2016a). Species of genus Ganoderma (Agaricomycetes) fermentation broth: A novel antioxidant and antimicrobial agent. Int. J. Med. Mushrooms. 18, 397–404. doi: 10.1615/IntJMedMushrooms.v18.i5.30
Ćilerdžić, J., Stajic, M., and Vukojevic, J. (2016b). Potential of submergedly cultivated mycelia of Ganoderma spp. as antioxidant and antimicrobial agents. Curr. Pharm. Biotechnol. 17, 17. doi: 10.2174/1389201016666150930115944
Constantin, M., Răut, I., Suica-Bunghez, R., Firinca, C., Radu, N., Gurban, A.-M., et al. (2023). G. lucidum-mediated green synthesis of silver nanoparticles with antimicrobial activity. Materials. 16, 4261. doi: 10.3390/ma16124261
Cör Andrejč, D., Knez, Ž., and Knez Marevci, M. (2022). Antioxidant, antibacterial, antitumor, antifungal, antiviral, anti-inflammatory, and neuro-protective activity of Ganoderma lucidum: An overview. Front Pharmacol. 13, 934982. doi: 10.3389/fphar.2022.934982
Costa, T. M., Kaufmann, V., Paganelli, C. J., Siebert, D. A., Micke, G. A., Alberton, M. D., et al. (2019). Kinetic identification of phenolic compounds and potential production of caffeic acid by Ganoderma lipsiense in solid-state fermentation. Bioprocess Biosyst. Eng. 42, 1325–1332. doi: 10.1007/s00449-019-02131-8
Dandapat, S., Sinha, M. P., and Sarma, T. C. (2016). Antibacterial activity of wild medicinal mushroom: Ganoderma applanatum (Pers.) Pat. Ecoscan. 9, 837–843.
Daruliza, K. M., Fernandez, L., Jegathambigai, R., and Sasidharan, S. (2012). Anti-candida activity and brine shrimp toxicity assay of Ganoderma boninense. Eur. Rev. Med. Pharmacol. Sci. 16, 43–48.
Demir, M. S. and Yamaç, M. (2008). Antimicrobial activities of basidiocarp, submerged mycelium and exopolysaccharide of some native basidiomycetes strains. JABS. 2, 89–93.
Dharmaraj, K., Kuberan, T., and Mahalakshmi, R. (2014). Comparison of nutrient contents and antimicrobial properties of Pleurotus djamor, Agaricus bisporus and Ganoderma tsugae.
Djide, M. N., Sartini Rahman, L., and Hasyim, N. (2014). Antibacterial activity of various extracts from the fruiting bodies of G. lucidum growing at Samanea saman (Jacq.) Merr. trunk. Int. J. Sci. Technol. Res. 3, 15–16.
Dzubak, P., Hajduch, M., Vydra, D., Hustova, A., Kvasnica, M., Biedermann, D., et al. (2006). Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat. Prod Rep; 23, 394–411. doi: 10.1039/b515312n
El-Dein, M. N., El-Fallal, A., El-Sayed, A., and El-Esseily, S. (2023). Antimicrobial activities of Ganoderma mbrekobenum strain EGDA (Agaricomycetes) from Egypt. Int. J. Med. Mushrooms. 25, 31–41. doi: 10.1615/IntJMedMushrooms.2023049502
El Dine, R. S., El Halawany, A. M., Ma, C.-M., and Hattori, M. (2008). Anti- HIV1 protease activity of lanostane triterpenes from the Vietnamese mushroom Ganoderma colossum. J. Nat. Prod. 71, 1022–1026. doi: 10.1021/np8001139
El-Fallal, A. A., El-Sayed, A. K., El-Fawal, M. F., and El-Gharabawy, H. M. (2021). Biocontrol of mycotoxigenic fungi in feedstuff using spices and Ganoderma mushroom. Catrina. 24, 65–73. doi: 10.21608/CAT.2022.107327.1112
El-Mekkawy, S., Meselhy, M. R., Nakamura, N., Tezuka, Y., Hattori, M., Kakiuchi, N., et al. (1998). Anti-HIV-1 and anti-HIV-1-protease substances from G. lucidum. Phytochem. 49, 1651–1657. doi: 10.1016/S0031-9422(98)00254-4
Enaba, L. and El Gendi, H. (2022). Mechanical properties and antifungal activity of G. lucidum modified polymethylmethacrylate: An in-vitro study. Egyptian Dental J. 68, 2651–2658. doi: 10.21608/edj.2022.111817.1923
Eo, S. K., Kim, Y. S., Lee, C. K., and Han, S. S. (2000). Possible mode of antiviral activity of acidic protein bound polysaccharide isolated from Ganoderma lucidum on herpes simplex viruses. J. ethnopharmacology. 72, 475–481. doi: 10.1016/S0378-8741(00)00266-X
Erawati, M., Andriany, M., and Kusumaningrum, N. S. (2018). The potential of G. lucidum as antimicrobial agent for multidrug-resistant Mycobacterium tuberculosis. Anti-Infective Agents. 16, 11–14. doi: 10.2174/2211352516666180227135043
Erbiai, E. H., Amina, B., Kaoutar, A., Saidi, R., Lamrani, Z., Pinto, E., et al. (2023). Chemical characterization and evaluation of antimicrobial properties of the wild medicinal mushroom G. lucidum growing in northern Moroccan forests. Life. 13, 1217. doi: 10.3390/life13051217
Ergun, B. (2017). Evaluation of antimicrobial, cytotoxic and genotoxic activities of G. lucidum (Reishi mushroom). Pak. J. Pharm. Sci. 30, 1991–1995.
Espinosa-García, V., Mendoza, G., Shnyreva, A. V., Padrón, J. M., and Trigos, Á. (2021). Biological activities of different strains of the genus Ganoderma spp.(Agaricomycetes) from Mexico. Int. J. Med. Mushrooms. 23, 67–77. doi: 10.1615/IntJMedMushrooms.2021037451
Etim, V. A., Abubakar, S., Asemota, U. K., Okereke, O. E., and Ogbadu, G. H. (2014). Evaluation of pharmacological potentials of the ethanolic extract of a mushroom (G. lucidum) grown in FCT. Indian J. Pharm. Biol. Res. 2, 1. doi: 10.30750/ijpbr.2.1.1
Ewunkem, A. J., Tshimanga, I., Samson, B., Justice, B., and Singh, D. K. (2024). In-vitro evaluation of antimicrobial activity of aqueous extracts of reishi mushroom (G. lucidum) against a select gram positive and negative bacteria. SJBLS. 3, 1–11. doi: 10.33552/SJBLS.2024.03.000565
Fakoya, S., Adegbehingbe, K. T., and Ogundiimu, A. A. (2013). Biopharmaceutical assessment of active components of Deadaleopsis confragosa and G. lucidum. OJMM. 3, 135–138. doi: 10.4236/ojmm.2013.32020
Ferreira, I. C. F. R., Heleno, S. A., Reis, F. S., Stojkovic, D., Queiroz., M. J. R. P., Vasconcelos, M. H., et al. (2015). Chemical features of Ganoderma polysaccharides with antioxidant, antitumor and antimicrobial activities. Phytochem. 114, 38–55. doi: 10.1016/j.phytochem.2014.10.011
Fidler, G., Butu, A., Rodino, S., Butu, M., and Cornea, P. C. (2015). Antioxidant activity, bioactive compounds and antimicrobial effect of mushrooms extracts. Bull. UASVM Anim. Sci. Biotechnologies. 72, 32–35. doi: 10.15835/buasvmcn-asb:10922
Flores-Rábago, K. M., Rivera-Mendoza, D., Vilchis-Nestor, A. R., Juarez-Moreno, K., and Castro-Longoria, E. (2023). Antibacterial activity of biosynthesized copper oxide nanoparticles (CuONPs) using Ganoderma sessile. Antibiotics 12, 1251. doi: 10.3390/antibiotics12081251
Flórez-Sampedro, L., Zapata, W., Orozco, L. P., Mejía, A. I., Arboleda, C., and Rugeles, M. T. (2016). In vitro anti-HIV-1 activity of the enzymatic extract enriched with laccase produced by the fungi Ganoderma sp. and Lentinus sp. Vitae 23, 109–118. doi: 10.17533/udea.vitae.v23n2a03
Ganesan, S. and Dharmaraj, K. (2013). Screening of Ganoderma tsugae for antimicrobial activities. World J. Pharm. Sci. 2, 5067–5077.
Gao, Y., Tang, W., Gao, H. E., Chan, E., Lan, J., Li, X., et al. (2005). Antimicrobial activity of the medicinal mushroom Ganoderma. Food Rev. Int. 21, 211–229. doi: 10.1081/FRI-200051893
Gao, Y., Zhou, S., Huang, M., and Xu, A. (2003). Antibacterial and antiviral value of the genus Ganoderma P. Karst. species (Aphyllophoromycetideae): A review. Int. J. Med. Mushrooms. 5, 235–246. doi: 10.1615/InterJMedicMush.v5.i3.20
Gaylan, C. M., Estebal, J. C., Tantengco, O. A. G., and Ragragio, E. M. (2018). Anti-staphylococcal and antioxidant properties of crude ethanolic extracts of macrofungi collected from the Philippines. Pharmacognosy J. 10, 106–109. doi: 10.5530/pj.2018.1.19
Ghobadi, R., Mohammadi, R., Chabavizade, J., and Sami, M. (2018). Effect of G. lucidum powder on oxidative stability, microbial and sensory properties of emulsion type sausage. Adv. Biomed. Res. 7, 24. doi: 10.4103/2277-9175.225595
Gowrie, U. S., Chathurdevi, G., and Rani, K. (2014). Evaluation of bioactive potential of basidiocarp extracts of G. lucidum. Int. J. Pharm. Res. Allied. 3, 36–46.
Guo, X., Jia, S., Zeng, F., Yu, Q., Chen, Y., and Xie, J. (2022). Sterol compounds from Ganoderma atrum: Isolation, antimicrobial activity and protective effect on Caco-2 cell oxidation damage. Food Chem. Adv. 1, 100117. doi: 10.1016/j.focha.2022.100117
Hayati, S. N., Rosyida, V. T., Darsih, C., Nisa, K., Indrianingsih, A. W., Apriyana, W., et al. (2020). “Physicochemical properties, antimicrobial and antioxidant activity of Ganoderma transparent soap,” in IOP Conference Series: Earth and Environmental Science, vol. 462. (Bristol, United Kingdom: IOP Publishing), 012047.
Hearst, M., Nelson, D., McCollum, G., Ballard, L. M., Millar, B. C., Moore, S., et al. (2010). Antimicrobial properties of protein extracts from wild mushroom fungi and native plant species against hospital pathogens. J. Pharmacognosy Phytother. 2, 103–107.
Heleno, S. A., Ferreira, I. C., Esteves, A. P., Ćirić, A., Glamočlija, J., Martins, A., et al. (2013). Antimicrobial and demelanizing activity of G. lucidum extract, p-hydroxybenzoic and cinnamic acids and their synthetic acetylated glucuronide methyl esters. Food Chemtoxicol. 58, 95–100. doi: 10.1016/j.fct.2013.04.025
Hijikata, Y., Yamada, S., and Yasuhara, A. (2007). Herbal mixtures containing the mushroom Ganoderma lucidum improve recovery time in patients with herpes genitalis and labialis. J. Altern. Complement Med. 13, 985–987. doi: 10.1089/acm.2006.6297
Hijikata, Y., Yasuhara, A., and Sahashi, Y. (2005). Effect of an herbal formula containing Ganoderma lucidum on reduction of herpes zoster pain: a pilot clinical trial. Am. J. Chin. Med. 33, 517–523. doi: 10.1142/S0192415X05003120
Hoque, N., Ahmed, I., Akanda, M. R., and Chowdhury, N. S. (2015). In vitro antioxidant, antimicrobial and cytotoxic activities of the various extracts of G. lucidum available in Bangladesh. J. Pharmacogn. Phytochem. 4, 42–46.
Huie, C. W. and Di, X. (2004). Chromatographic and electrophoretic methods for Lingzhi pharmacologically active components. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 812, 241–257. doi: 10.1016/S1570-0232(04)00678-6
Isaka, M., Chinthanom, P., Sappan, M., Danwisetkanjana, K., Boonpratuang, T., and Choeyklin, R. (2016). Antitubercular lanostane triterpenes from cultures of the basidiomycete Ganoderma sp. BCC 16642. J. Nat. Prod. 79, 161–169. doi: 10.1021/acs.jnatprod.5b00826
Isaka, M., Chinthanom, P., Rachtawee, P., Choowong, W., Choeyklin, R., and Thummarukcharoen, T. (2020). Lanostane triterpenoids from cultivated fruiting bodies of the wood-rot basidiomycete Ganoderma casuarinicola. Phytochemistry 170, 112225. doi: 10.1016/j.phytochem.2019.112225
Islam, M. S., Rahi, M. S., Koli, H. K., Jerin, I., Sajib, S. A., Hoque, K. M., et al. (2018). Evaluation of phytochemical, antioxidant, cytotoxicity and in vitro antibacterial activity of aqueous extract of G. lucidum cultivated in Bangladeshi habitat. Malaya J. Biosci. 5, 1–3.
Ismail, K. H. A. T. I. J. A. H., Abdullah, S. Y. A. H. R. I. E. L., and Chong, K. P. (2014). Screening for potential antimicrobial compounds from Ganoderma boninense against selected food borne and skin disease pathogens. Int. J. Pharm. Pharm. Sci. 6, 771–774.
Jin, H., Aquili, L., Heng, B. C., Strekalova, T., Fung, M. L., Tipoe, G. L., et al. (2025). lucidum: an emerging nutritional approach to manage depression. Food Rev. Int. 26, 1–22. doi: 10.1080/87559129.2025.2466456
Jogaiah, S., Kurjogi, M., Abdelrahman, M., Hanumanthappa, N., and Tran, L. S. (2019). Ganoderma applanatum-mediated green synthesis of silver nanoparticles: Structural characterization, and in vitro and in vivo biomedical and agrochemical properties. Arab. J. Chem. 12, 1108–1120. doi: 10.1016/j.arabjc.2017.12.002
Jogaiah, S., Shetty, H. S., Ito, S. I., and Tran, L. S. (2016). Enhancement of downy mildew disease resistance in pearl millet by the G_app7 bioactive compound produced by Ganoderma applanatum. Plant Physiol. Biochem. 105, 109–117. doi: 10.1016/j.plaphy.2016.04.006
Kaewjai, C., Tonsomboon, A., Pawiwongchai, J., and Prommano, O. (2023). Antiprotozoal activity of Boesenbergia rotunda (L.) Mansf and G. lucidum (Fr.) Kart extracts against Blastocystis hominis. Vet. World. 16, 187. doi: 10.14202/vetworld.2023.187-193
Kamble, R., Venkata, S., and Gupte, A. M. (2011). Antimicrobial activity of G. lucidum mycelia. J. Pure Appl. Microbiol. 5, 983–986.
Kamra, A. and Bhatt, A. B. (2012). Evaluation of antimicrobial and antioxidant activity of G. lucidum extracts against human pathogenic bacteria. Int. J. Pharm. Pharm. Sci. 2, 359–362.
Kang, D., Mutakin, M., and Levita, J. (2015). Computational study of triterpenoids of G. lucidum with aspartic protease enzymes for discovering HIV-1 and plasmepsin inhibitors. Int. J. Chem. 7, 62. doi: 10.5539/ijc.v7n1p62
Kannan, M., Muthusamy, P., Venkatachalam, U., and Rajarajeswaran, J. (2014). Mycosynthesis, characterization and antibacterial activity of silver nanoparticles (Ag-NPs) from fungus. G. lucidum. Malaya J. Biosci. 1, 134–142.
Karaca, B., Cihan, A. Ç., Akata, I., and Altuner, E. M. (2020). Anti-biofilm and antimicrobial activities of five edible and medicinal macrofungi samples on some biofilm producing multi drug resistant Enterococcus strains. TURJAF. 8, 69–80. doi: 10.24925/turjaf.v8i1.69-80.2723
Karaman, M., Jovin, E., Malbaša, R., Matavuly, M., and Popović, M. (2010). Medicinal and edible lignicolous fungi as natural sources of antioxidative and antibacterial agents. Phytother. Res. 24, 1473–1481. doi: 10.1002/ptr.v24:10
Karunarathna, S. C., Ediriweera, A., Prasannath, K., Mingfei, Y., and Hapuarachchi, K. K. (2024a). Exploring the health benefits of Ganoderma: bioactive compounds and mechanisms of action; immunomodulatory, and anti-tumour activities. N. Z. J. Bot. 62, 1–85. doi: 10.1080/0028825X.2024.2375996
Karunarathna, S. C., Lu, W., Patabedige, N., Zhao, C. L., and Hapuarachchi, K. K. (2024b). Unlocking the therapeutic potential of edible mushrooms: Ganoderma and their secondary metabolites as novel antiviral agents for combating COVID-19. N. Z. J. Bot. 62, 1–59. doi: 10.1080/0028825X.2024.2384453
Karwa, A. and Rai, M. (2012). Naturally occurring medicinal mushroom-derived antimicrobials: A case-study using lingzhi or reishi G. lucidum (W. Curt.: fr.) P. Karst. (Higher Basidiomycetes). Int. J. Med. Mushrooms 14, 481. doi: 10.1615/intjmedmushr.v13.i5.80
Kaur, H., Sharma, S., Khanna, P. K., and Kapoor, S. (2015). Evaluation of G. lucidum strains for the production of bioactive components and their potential use as antimicrobial agents. J. Appl. Nat. Sci. 7, 298–303. doi: 10.31018/jans.v7i1.605
Kebaili, F. F., Tahar, N., Toumi, M. E., Redouane, R., Chawki, B., Pablo, A., et al. (2021). Antioxidant activity and phenolic content of extracts of wild Algerian Lingzhi or Reishi Medicinal Mushroom, G. lucidum (Agaricomycetes). Int. J. Medicinal Mushrooms. 23. doi: 10.1615/IntJMedMushrooms.2021038424
Keypour, S., Riahi, H., Moradali, M. F., and Rafati, H. (2008). Investigation of the antibacterial activity of a chloroform extract of Ling Zhi or Reishi medicinal mushroom, G. lucidum (W. Curt.: Fr.) P. Karst.(Aphyllophoromycetideae), from Iran. Int. J. Med. Mushrooms. 10, 345–349. doi: 10.1615/IntJMedMushr.v10.i4.70
Kim, J. H., Tam, C. C., Chan, K. L., Mahoney, N., Cheng, L. W., Friedman, M., et al. (2022). Antimicrobial efficacy of edible mushroom extracts: Assessment of fungal resistance. Appl. Sci. 12, 4591. doi: 10.3390/app12094591
Kirar, V., Mehrotra, S., Negi, P. S., Nandi, S. P., and Misra, K. (2015). HPTLC fingerprinting, antioxidant potential and antimicrobial efficacy of Indian Himalayan lingzhi: G. lucidum. Indian. J. Pharm. Sci. 6, 4259–4268. doi: 10.13040/IJPSR.0975-8232.6(10).4259-68
Klaus, A., Kozarski, M., Vunduk, J., and Nikšić, M. (2012). Antimicrobial potential of Ganoderma spp. polysaccharide extracts. CABI Digit Libr., 84–86. doi: 10.5555/20133109956
Krishnaveni, M. and Manikandan, M. (2014). Antimicrobial activity of mushrooms. Res. J. Pharm. Tech. 7, 399–400.
Kumar, S. P., Girija, A. S., and Priyadharsini, J. V. (2020). Targeting NM23-H1-mediated inhibition of tumour metastasis in viral hepatitis with bioactive compounds from G. lucidum: A computational study. Indian J. Pharm. Sci. 82, 300–305. doi: 10.36468/pharmaceutical-sciences.650
Lakornwong, W., Kanokmedhakul, K., Kanokmedhakul, S., Kongsaeree, P., Prabpai, S., Sibounnavong, P., et al. (2014). Triterpene lactones from cultures of Ganoderma sp. KM01. J. Nat. Prod. 77, 1545–1553. doi: 10.1021/np400846k
Li, Y. Q. and Wang, S. F. (2006). Anti-hepatitis B activities of ganoderic acid from G. lucidum. Biotechnol. Lett. 28, 837–841. doi: 10.1007/s10529-006-9007-9
Liang, L., Su, Q., Ma, Y., Zhao, S., Zhang, H., and Gao, X. (2024). Research progress on the polysaccharide extraction and antibacterial activity. Ann. Microbiol. 74, 17. doi: 10.1186/s13213-024-01762-x
Liew, G. M., Khong, H. Y., Kutoi, C. J., and Sayok, A. K. (2015). Phytochemical screening, antimicrobial and antioxidant activities of selected fungi from Mount Singai, Sarawak, Malaysia. Int. J. Res. Stud. Biosci. 3, 191–197.
Lim, W. Z., Cheng, P. G., Abdulrahman, A. Y., and Teoh, T. C. (2020). The identification of active compounds in G. lucidum var. antler extract inhibiting dengue virus serine protease and its computational studies. J. Biomol. Struct. Dyn. 38, 4273–4288. doi: 10.1080/07391102.2019.1678523
Liu, D., Hu, Z., Liu, Z., Yang, B., Tu, W., and Li, L. (2009). Chemical composition and antimicrobial activity of essential oil isolated from the cultured mycelia of Ganoderma japonicum. J. Nanjing Med. Univ. 23, 168–172. doi: 10.1016/S1007-4376(09)60049-6
Liu, G., Zhang, J., Kan, Q., Song, M., Hou, T., An, S., et al. (2022). Extraction, structural characterization, and immunomodulatory activity of a high molecular weight polysaccharide from G. lucidum. Front. Nutr. 9, 846080. doi: 10.3389/fnut.2022.846080
Liu, D. Z., Zhu, Y. Q., Li, X. F., Shan, W. G., and Gao, P. F. (2014). New triterpenoids from the fruiting bodies of G. lucidum and their bioactivities. Chem. Biodivers. 11, 982–986. doi: 10.1002/cbdv.201400004
Lone, S. A., Bhat, Y. M., Wani, A. H., and Bhat, M. Y. (2024). Molecular circumscription, myco-chemical evaluation, antioxidant and antimicrobial potential of some selected mushrooms from Kashmir Himalaya. Vegetos. 12, 1–9. doi: 10.1007/s42535-024-00955-8
Lucius, K. (2025). Clinical evidence for the use of G. lucidum medicinal mushroom. Integr. Complementary Therapies. 31, 17–24. doi: 10.1089/ict.2024.56835.luc
Ma, K., Ren, J. W., Han, J. J., Bao, L., Li, L., Yao, Y. J., et al. (2014). Ganoboninketals A–C, antiplasmodial 3,4-seco-27-norlanostane triterpenes from Ganoderma boninense Pat. J. Nat. Prod. 77, 1847–1852. doi: 10.1021/np5002863
Mahendran, S., Saravana, S., Vijayabaskar, P., Anandapandian, K. T., and Shankar, T. (2013). Antibacterial potential of microbial exopolysaccharide from G. lucidum and Lysinibacillus fusiformis. Int. J. Recent Sci. Res. 4, 501–505.
Marzhoseyni, Z., Rashki, S., and Nazari-Alam, A. (2023). Evaluation of the inhibitory effects of TiO2 nanoparticle and G. lucidum extract against biofilm-producing bacteria isolated from clinical samples. Arch. Microbiol. 205, 59. doi: 10.1007/s00203-023-03403-4
Masjedi, M., Nateghi, L., Berenjy, S., and Eshaghi, M. R. (2022). Optimization of extraction of flavonoid, total phenolic, antioxidant, and antimicrobial compounds from G. lucidum by maceration method. Iran. J. Chem. Chem. Eng. Res. 41, 3127–3140. doi: 10.30492/IJCCE.2021.532590.4801
Mayaka, R. K. (2020). Chemical characterization and antimicrobial activity of compounds from some selected medicinal Kenyan Ganoderma and Trametes species. Njoro, Kenya: Egerton University.
Mehta, S. and Jandaik, S. (2012). In vitro comparative evaluation of antibacterial activity of fruiting body and mycelial extracts of G. lucidum against pathogenic bacteria. J. Pure Appl. Microbiol. 6, 1997–2001.
Mendoza, M. J. L. and Nepomuceno, A. R. T. (2006). G. lucidum mycelia as potential antimicrobial and biological control agents of selected soil-borne plant pathogens. Ermita, Manila, Philippines: University of the Philippines Manila.
Migahed, F. F., El-Fallal, A. A., and Elshobaky, S. S. (2018). Evaluation of antimicrobial activities of mycelia and crude extracts of some Egyptian wild mushrooms, Agaricus and Ganoderma Species. JPP. 9, 387–395. doi: 10.21608/jpp.2018.35750
Min, B. S., Nakamura, N., Miyashiro, H., Bae, K. W., and Hattori, M. (1998). Triterpenes from the spores of G. lucidum and their inhibitory activity against HIV-1 protease. Chem. Pharm. Bull. 46, 1607–1612. doi: 10.1248/cpb.46.1607
Mishra, J., Rajput, R., Singh, K., Puri, S., Goyal, M., Bansal, A., et al. (2018a). Antibacterial natural peptide fractions from Indian Ganoderma lucidum. Int J Pept Res Ther 24, 543–554. doi: 10.1007/s10989-017-9581-6
Mishra, J., Joshi, A., Rajput, R., Singh, K., Bansal, A., and Misra, K.. (2018b). Phenolic rich fractions from mycelium and fruiting body of G. lucidum inhibit bacterial pathogens mediated by generation of reactive oxygen species and protein leakage and modulate hypoxic stress in HEK 293 cell line. Adv Pharmacol Pharm Sci 2018, 6285615. doi: 10.1155/2018/6285615
Mohanta, Y. K., Nayak, D., Biswas, K., Singdevsachan, S. K., Abd_Allah, E. F., Hashem, A., et al. (2018). Silver nanoparticles synthesized using wild mushroom show potential antimicrobial activities against food borne pathogens. Molecules. 23, 655. doi: 10.3390/molecules23030655
Mohanta, Y. K., Singdevsachan, S. K., Parida, U. K., Panda, S. K., Mohanta, T. K., and Bae, H. (2016). Green synthesis and antimicrobial activity of silver nanoparticles using wild medicinal mushroom Ganoderma applanatum (Pers.) Pat. from Similipal Biosphere Reserve, Odisha, India. IET Nanobiotechnol 10, 184–189. doi: 10.1049/iet-nbt.2015.0059
Mondal, T. (2013). Studies on antioxidant and antimicrobial properties of some common mushrooms. J. Today’s Biol. Sci.: Res. Rev. 2, 60–67.
Montalbano, G. (2018). Evaluation of the antimicrobial, anti-inflammatory, regenerative and wound healing properties of the bracket fungus G. lucidum. Brisbane, QLD, Australia: Queensland University of Technology.
Moradali, M. F., Mostafavi, H., Ghods, S., and Hejaroude, G. A. (2008). Investigation of antimicrobial fatty acids from medicinal artist conk mushroom Ganoderma applanatum (Pers.) Pat.(Aphyllophoromycetideae) by TLC and spectroscopic detection. Int. J. Med. Mushrooms. 10, 149–154. doi: 10.1615/IntJMedMushr.v10.i2.50
Mothana, R. A. A., Awadh Ali, N. A., Jansen, R., Wegner, U., Mentel, R., and Lindequist, U. (2003). Antiviral lanostanoid triterpenes from the fungus Ganoderma pfeifferi. Fitoterapia. 74, 177–180. doi: 10.1016/S0367-326X(02)00305-2
Mothana, R. A. A., Jansen, R., Jülich, W. D., and Lindequist, U. (2000). Ganomycins A and B, New antimicrobial farnesyl hydroquinones from the basidiomycete Ganoderma pfeifferi. J. Nat. Prod. 63, 416–418. doi: 10.1021/np990381y
Mousavi, S. M., Hashemi, S. A., Gholami, A., Omidifar, N., Chiang, W. H., Neralla, V. R., et al. (2023). G. lucidum methanolic extract as a potent phytoconstituent: characterization, in-vitro antimicrobial and cytotoxic activity. Sci. Rep. 13, 17326. doi: 10.1038/s41598-023-44135-1
Muniyappan, G., Gurudevan, T., Thangaraj, P., Balamurali, A. S., Iyadurai, A. P., Suppaiah, R., et al. (2023). Benzothiazole—An Antifungal compound derived from medicinal mushroom G. lucidum against mango anthracnose pathogen Colletotrichum gloeosporioides (Penz and (Sacc.)). Molecules. 28, 2476. doi: 10.3390/molecules28062476
Nagaraj, K., Mallikarjun, N., Naika, R., and Venugopal, T. M. (2013). Phytochemical analysis and in vitro antimicrobial potential of Ganoderma applanatum (Pers.) Pat. of Shivamogga district-Karnataka, India. Int. J. Pharm. Sci. Rev. Res. 23, 36–41.
Nasim, G. and Ali, M. (2011). Estimation of antimicrobial potential of G. lucidum (Leyss. ex Fr.) Karst. extracts. Pak. J. Bot. 43, 183–189.
Naveenkumar, C., Swathi, S., Jayalakshmi, G., Chidambaram, R., and Srikumar, R. (2018). Screening of antifungal activity of G. lucidum extract against medically important fungi. Indian J. Public Health Dev. 9, 269–272. doi: 10.5958/0976-5506.2018.00050.5
Nayak, R. N., Dixitraj, P. T., Nayak, A., and Bhat, K. (2015). Evaluation of anti-microbial activity of spore powder of G. lucidum on clinical isolates of Prevotella intermedia: A pilot study. Contemp. Clin. Dent. 6, S248–S252. doi: 10.4103/0976-237X.166834
Nayak, A., Dixitraj, P. T., Nayak, R. N., and Bhat, K. G. (2021). Comparison of antimicrobial activity of mycelium and spore of G. lucidum on Prevotella intermedia isolated from chronic periodontitis patients. CHRISMED J. Health Res. 8, 35–39. doi: 10.4103/cjhr.cjhr_61_20
Nayak, R. N., Nayak, A., and Bhat, K. (2010a). Antimicrobial activity of aqueous extract of spore powder of G. lucidum–An in vitro study. J. Int. Oral. Health 2, 68–74.
Nayak, A., Nayak, R. N., and Bhat, K. (2010b). Antifungal activity of a toothpaste containing G. lucidum against Candida albicans-An in vitro study. J. Int. Oral. Health 2, 51–57.
Nguyen, T. T. T., Nguyen, T. T. T., Nguyen, H. D., Nguyen, T. K., Pham, P. T. V., Tran, L. T. T., et al. (2024). Anti-Staphylococcus aureus potential of compounds from Ganoderma sp.: A comprehensive molecular docking and simulation approaches. Heliyon. 10, e28118. doi: 10.1016/j.heliyon.2024.e28118
Nicolcioiu, M. B., Popa, G., and Matei, F. (2017). Antimicrobial activity of ethanolic extracts made of mushroom mycelia developed in submerged culture. Sci. Bull. Ser. F. Biotechnologies. 21.
Niedermeyer, T. H. J., Lindequist, U., Mentel, R., Gördes, D., Schmidt, E., Thurow, K., et al. (2005). Antiviral terpenoid constituents of Ganoderma pfeifferi. J. Nat. Prod. 68, 1728–1731. doi: 10.1021/np0501886
Nithya, M., Ambikapathy, V., and Panneerselvam, A. (2013). Studies on antimicrobial potential of different strains of G. lucidum (Curt.: Fr.) P. Karst. Int. J. Pharm. Sci. Rev. Res. 21, 56.
Noverita, N. and Ritchie, Y. H. (2020). Antibacterial activities of ethanol extracts fruit bodies of G. lucidum and Rigidoporus microphorus against E. coli and Staphlyococcus aureus. J. Trop. Biodivers. Biotechnol. 1, 35–46. doi: 10.59689/bio.v1i1.25
Ofodile, L. N. and Bikomo, E. O. (2008). Antibacterial activity of G. lucidum from Nigeria. Hamdard Med. 51, 14–17.
Ofodile, L. N., Ogbe, A. O., and Oladipupo, O. (2011). Effect of the mycelial culture of G. lucidum on human pathogenic bacteria. Int. J. Biol. 3, 111–114. doi: 10.5539/ijb.v3n2p111
Ofodile, L. N., Uma, N., Grayer, R. J., Ogundipe, O. T., and Simmonds, M. S. (2012). Antibacterial compounds from the mushroom Ganoderma colossum from Nigeria. Phytother. Res. 26, 748–751. doi: 10.1002/ptr.v26.5
Ofodile, L. N., Uma, N. U., Kokubun, T., Grayer, R. J., Ogundipe, O. T., and Simmons, M. S. (2005). Antimicrobial colossolactones from a Nigerian polypore Ganoderma colossum (Fr.) CF Baker. Int. J. Med. Mushrooms. 7, 437–438. doi: 10.1615/IntJMedMushr.v7.i3.770
Ojha, A. (2025). Comparative phytochemical profiling and antimicrobial efficacy of ganoderma and shiitake mushrooms: A microbiological perspective. Microbiol. Res. J. Int. 35, 1–0. doi: 10.9734/mrji/2025/v35i51569
Paliya, B. S., Chaudhary, H. S., Verma, S., and Prasad, S. (2014). Optimization of fermentation media composition of G. lucidum for improved production of antioxidant and antimicrobial compounds. Phcog. Commn. 4, 35–41.
Pan, Z., Lin, J., Luo, G., Cheng, W., Li, Y., and Wu, C. (2025). Unveiling triterpenoid superiority in a newly developed G. lucidum variety through untargeted metabolomics approach. Front. Nutr. 12. doi: 10.3389/fnut.2025.1541162
Pandey, A. T., Pandey, I., Hachenberger, Y., Krause, B. C., Haidar, R., Laux, P., et al. (2020). Emerging paradigm against global antimicrobial resistance via bioprospecting of mushroom into novel nanotherapeutics development. Trends Food Sci. Technol. 106, 333–344. doi: 10.1016/j.tifs.2020.10.025
Parkash, V. and Sharma, A. (2016). In vitro efficacy of bracket fungi for their potential antimicrobial activity. J. Microbiol. Biotechnol. Food Sci. 6, 818. doi: 10.15414/jmbfs.2016.6.2.818-822
Paul, S. N., Sasikumar, C. S., and Singh, A. R. (2015). Fabrication of silver nanoparticles synthesized from G. lucidum into the cotton fabric and its antimicrobial property. Int. J. Pharm. Pharm. Sci. 7, 53–56.
Perfileva, A. I., Tsivileva, O. M., Ibragimova, D. N., Koftin, O. V., and Fedotova, O. V. (2017). Effect of selenium-containing biocomposites based on Ganoderma mushroom isolates grown in the presence of oxopropyl-4-hydroxycoumarins, on bacterial phytopathogens. Microbiology. 86, 183–191. doi: 10.1134/S0026261717020163
Plosca, M. P., Chiș, M. S., Fărcaș, A. C., and Păucean, A. G. (2025). lucidum—From ancient remedies to modern applications: chemistry, benefits, and safety. Antioxidants. 14, 513. doi: 10.3390/antiox14050513
Priy, K., Thiribhuvanamala, G., Kamalakannan, A., and Krishnamoorthy, A. S. (2019). Antimicrobial activity of biomolecules from mushroom fungi against Colletotrichum capsici (Syd) Butler and bisby, the fruit rot pathogen of Chilli. Inter. J. Curr. Microbiol. Appl. Sci. 8, 1172–1186. doi: 10.20546/ijcmas.2019.806.145
Pushpa, H., Anand, M., Kasimaiah, P., and Penugonda, J. V. S. P. (2013). Evaluation of antimicrobial activity of some of the selected basidiomycetous fungi. Int. J. Pharma Bio Sci. 4, B964–B971.
Qin, Y., Xiong, L., Li, M., Liu, J., Wu, H., Qiu, H., et al. (2018). Preparation of bioactive polysaccharide nanoparticles with enhanced radical scavenging activity and antimicrobial activity. J. Agric. Food Chem. 66, 4373–4383. doi: 10.1021/acs.jafc.8b00388
Radhika, R. (2021). Antibacterial activity of G. lucidum extracts against MDR pathogens. Int. J. Mod. Agric. 10, 3488–3493. doi: 10.33545/26649926.2021.v3.i1a.24
Radhika, R. and Rajan, S. (2021). Antifungal potentials of G. lucidum extracts. Plant Cell Biotechnol. Mol. Biol. 22, 22–27.
Rajesh, K. and Dhanasekaran, D. (2014). Phytochemical screening and biological activity of medicinal mushroom Ganoderma species. Malaya J. Biosci. 1, 67–75.
Rašeta, M., Mišković, J., Čapelja, E., Zapora, E., Petrović Fabijan, A., Knežević, P., et al. (2023). Do Ganoderma species represent novel sources of phenolic based antimicrobial agents? Molecules. 28, 3264. doi: 10.3390/molecules28073264
Raza, S. H., Zhong, R., Li, X., Pant, S. D., Shen, X., BinMowyna, M. N., et al. (2024). lucidum triterpenoids investigating their role in medicinal applications and genomic protection. J. Pharm. Pharmacol. 76, 1535–1551. doi: 10.1093/jpp/rgae133
Reid, T., Kashangura, C., Chidewe, C., Benhura, M. A., and Mduluza, T. (2016). Antibacterial properties of wild edible and non-edible mushrooms found in Zimbabwe. Afr. J. Microbiol. Res. 10, 977–984. doi: 10.5897/AJMR2016.8052
Rijia, A., Krishnamoorthi, R., Rasmi, M., Mahalingam, P. U., and Kim, K. S. (2024). Comprehensive analysis of bioactive compounds in wild Ganoderma applanatum mushroom from Kerala, South India: Insights into dietary nutritional, mineral, antimicrobial, and antioxidant activities. Pharmaceuticals. 17, 509. doi: 10.3390/ph17040509
Rivera-Mendoza, D., Quiñones, B., Huerta-Saquero, A., and Castro-Longoria, E. (2024). Antimicrobial activity of green synthesized silver and copper oxide nanoparticles against the foodborne pathogen Campylobacter jejuni. Antibiotics. 13, 650. doi: 10.3390/antibiotics13070650
Robles-Hernández, L., Salas-Salazar, N. A., and Gonzalez-Franco, A. C. (2021). Purification and characterization of antibacterial activity against phytopathogenic bacteria in culture fluids from G. lucidum. Molecules. 26, 5553. doi: 10.3390/molecules26185553
Rofuli, N. B., III, Cruz, A. G., Medalla, A. P., and Buenavista, M. T. (2005). Antimicrobial and antagonistic properties of G. lucidum (W. Curt.: Fr.) Lloyd. Int. J. Med. Mushrooms. 7, 460. doi: 10.1615/INTJMEDMUSHROOMS.V7.I3.930
Roychoudhury, A., Sarkar, R., and Sarkar, R. (2024). Unlocking the potential of fungal extracts as inhibitors of biofilm formation and improving human health. J. Stress. Physiol. Biochem. 20 (3), 195–217. Available online at: https://cyberleninka.ru/article/n/unlocking-the-potential-of-fungal-extracts-as-inhibitors-of-biofilm-formation-and-improving-human-health (Accessed July 10, 2025).
Sakthivigneswari, G. and Dharmaraj, K. (2013). Studies on analysis of few secondary metabolites and antimicrobial activity of G. lucidum. J. Pharm. Res. 1, 781–786.
Saludares, G. G., Amper, C. D., and Lituanas, I. M. (2023). “Antimicrobial performances of G. lucidum extract against fruits and leaves pathogens,” in IOP Conference Series: Earth and Environmental Science, vol. 1145. (Bristol, United Kingdom: IOP Publishing), 012019.
Sánchez-Hernández, E., Teixeira, A., Pereira, C., Cruz, A., Martín-Gil, J., Oliveira, R., et al. (2023). Chemical constituents and antimicrobial activity of a G. lucidum (Curtis.) P. Karst. aqueous ammonia extract. Plants 12, 2271.
Sande, E. and Baraza, D. L. (2019). Phytochemical screening and antimicrobial activity of Kenyan mushroom G. lucidum. Asian J. Chem. Sci. 6, 1–6. doi: 10.9734/AJOCS/2019/v6i218994
Sanodiya, B. S., Thakur, G. S., Baghel, R. K., Prasad, G., and Bisen, P. S. (2009). G. lucidum: a potent pharmacological macrofungus. Curr. Pharm. Biotechnol. 10, 717–742. doi: 10.2174/138920109789978757
Sarnthima, R., Khammaung, S., and Sa-Ard, P. (2017). Culture broth of G. lucidum exhibited antioxidant, antibacterial and α-amylase inhibitory activities. J. Food Sci. Technol. 54, 3724–3730. doi: 10.1007/s13197-017-2839-6
Sato, N., Zhang, Q., Ma, C.-M., and Hattori, M. (2009). Anti-human immunodeficiency virus-1 protease activity of new lanostane type triterpenoids from Ganoderma sinense. Chem. Pharm. Bull. 57, 1076–1080. doi: 10.1248/cpb.57.1076
Savin, S., Craciunescu, O., Oancea, A., Ilie, D., Ciucan, T., Antohi, L. S., et al. (2020). Antioxidant, cytotoxic and antimicrobial activity of chitosan preparations extracted from G. lucidum mushroom. Chem. Biodivers. 17, e2000175. doi: 10.1002/cbdv.202000175
Sekaran, S., Elumalai, S., Ramalingam, B., and Devendiran, K. (2011). Evaluation of antibacterial and antifungal activity of G. lucidum (Curtis) P. Karst. fruit bodies extracts. World J. Sci. Technol. 1, 8–11.
Seo, D. J. and Choi, C. (2021). Antiviral bioactive compounds of mushrooms and their antiviral mechanisms: A review. Viruses 13, 350. doi: 10.3390/v13020350
Serrano-Márquez, L., Trigos, A., Couttolenc, A., Padrón, J. M., Shnyreva, A. V., and Mendoza, G. (2021). Antiproliferative and antibacterial activity of extracts of Ganoderma strains grown in vitro. Food Sci. Biotechnol. 30, 711–721. doi: 10.1007/s10068-021-00903-1
Seweryn, E., Ziała, A., and Gamian, A. (2021). Health-promoting of polysaccharides extracted from. Ganoderma lucidum. Nutrients. 13, 2725. doi: 10.3390/nu13082725
Shah, P., Modi, H. A., Shukla, M. D., and Lahiri, S. K. (2014). Preliminary phytochemical analysis and antibacterial activity of G. lucidum collected from Dang District of Gujarat, India. Int. J. Curr. Microbiol. App. Sci. 3, 246–255.
Shahid, A. A., Asif, M., Shahbaz, M., and Ali, M. (2016). “Antifungal potential of G. lucidum extract against plant pathogenic fungi of Calendula officinalis L,” in Proceedings of the 5th International Conference on Biological, Chemical and Environmental Sciences (BCES-2016)*. International Institute of Chemical, Biological & Environmental Engineering (IICBE), Mar 24 2016, London, United Kingdom. 24–25.
Shamaki, B. U., Geidam, Y. A., Abdulrahma, F., Ogbe, A. O., and Sandabe, U. K. (2012). Evaluation of phytochemical constituents and in vitro antibacterial activity of organic solvent fractions of G. lucidum methanolic extract. Int. J. Med. Plants. Res. 1, 026–031.
Shang, X., Tan, Q., Liu, R., Yu, K., Li, P., and Zhao, G.-P. (2013). In vitro anti-Helicobacter pylori effects of medicinal mushroom extracts, with special emphasis on the Lion’s Mane mushroom, Hericium erinaceus (higher Basidiomycetes). Int. J. Med. Mushrooms. 15, 165–174. doi: 10.1615/IntJMedMushr.v15.i2.50
Sharifi, A., Khoramrooz, S. S., Jahedi, S., and Khosravani, S. A. (2012). Screening of antimicrobial activity of sesquiterpenoid crude extract of Ganoderma. Life Sci. J. 9, 2516–2519.
Sharma, P. K., Shahi, S. K., Sharma, P. K., Mahesh Kumar, M. K., Ramakant Lawaniya, R. L., and Mandeep Balhara, M. B. (2015). Antimicrobial potential of Ganoderma spp. fruiting bodies and cultured mycelium. Afr. J. Microbiol. Res. 9, 265–274. doi: 10.5897/AJMR2014.7045
Shawkat, M. S. and Aedan, S. A. (2022). Antibacterial activity of G. lucidum (reishi mushroom) alcoholic extract. Biochem. Cell. Arch. 22, 983–988.
Sheena, N., Ajith, T., Mathew, A., and Janardhanan, K. (2003). Antibacterial activity of three macrofungi, G. lucidum, Navesporus floccosa and Phellinus rimosus occurring in South India. Pharmaceut. Biol. 41, 564–567.
Shen, S. F., Zhu, L. F., Wu, Z., Wang, G., Ahmad, Z., and Chang, M. W. (2020). Extraction of triterpenoid compounds from G. lucidum spore powder through a dual-mode sonication process. Drug Dev. Ind. Pharm. 46, 963–974. doi: 10.1080/03639045.2020.1764022
Sheydaei, M., Edraki, M., and Javanbakht, S. (2023). G. lucidum-modified clay epoxy coating: Investigation of thermal, mechanical, anticorrosion, and antimicrobial properties. Polym. Sci. Ser. B. 65, 991–1000. doi: 10.1134/S1560090424600153
Shi, J. X., Chen, G. Y., Sun, Q., Meng, S. Y., and Chi, W. Q. (2022). Antimicrobial lanostane triterpenoids from the fruiting bodies of Ganoderma applanatum. J. Asian Nat. Prod. Res. 24, 1001–1007. doi: 10.1080/10286020.2021.2017899
Shikongo, L. T. (2012). Analysis of the mycochemicals components of the indigenous Namibian Ganoderma Mushrooms. Windhoek, Namibia: University Of Namibia.
Shikongo, L. T., Chimwamurombe, P. M., Lotfy, H. R., and Kandawa-Schulz, M. (2013). Antimicrobial screening of crude extracts from the indigenous G. lucidum mushrooms in Namibia. Afr. J. Microbiol. Res. 7, 4812–4816. doi: 10.5897/AJMR2013.5841
Shokouhi, F., Jamshidian-Mojaver, M., Farzin, H., Tabatabaeizadeh, S. E., Kadoughani Sani, S., and Amiri, M. (2023). Antibacterial effect of Ganoderma+ silver nanocomplex extract on Streptococcus pyogenes, Staphylococcus aureus, Pseudomonas aeruginosa and Acinetobacter baumannii and evaluation of nanocomplex toxicity on vero cell line. NFVM. 5, 79–87. doi: 10.22034/NFVM.2022.339684.1140
Shveta, Sindhu, A., Rohilla, A. K., Ritika, and Singh, A. (2013). Separation and determination of antimicrobial activities of targeted ganoderic acids from G. lucidum (Lingzhi). Mushroom Res. 22, 63–69.
Singh, J., Gupta, S., Malviya, S., and Ahrwar, B. (2014). In-vitro evaluation of antimicrobial activity of G. lucidum. Int. J. Adv. Res. 2, 460–466.
Singh, C. and Vyas, D. (2023). Use of G. lucidum extract to elevate the resistance in chickpea against the Fusarium oxysporum f. sp. ciceris. Arch. Phytopathol. Pflanzenschutz. 56, 605–624. doi: 10.1080/03235408.2023.2207955
Skalicka-Wozniak, K., Szypowski, J., Los, R., Siwulski, M., Sobieralski, K., Glowniak, K., et al. (2012). Evaluation of polysaccharides content in fruit bodies and their antimicrobial activity of four G. lucidum (W Curt.: Fr.) P. Karst. strains cultivated on different wood type substrates. Acta Soc Bot. Pol. 81, 17–21. doi: 10.5586/asbp.2012.001
Sknepnek, A., Pantić, M., Matijašević, D., Miletić, D., Lević, S., Nedović, V., et al. (2018). Novel kombucha beverage from lingzhi or reishi medicinal mushroom, G. lucidum, with antibacterial and antioxidant effects. Int. J. Med. Mushrooms. 20, 243–258. doi: 10.1615/IntJMedMushrooms.2018025833
Smania, E. F. A., DelleMonache, F., Smania, A., Yunes, R. A., and Cuneo, R. S. (2003). Antifungal activity of sterols and triterpenes isolated from Ganoderma annulare. Fitoterapia. 74, 375–377. doi: 10.1016/S0367-326X(03)00064-9
Smania, E. D., Delle Monache, F., Yunes, R. A., Paulert, R., and Smania Junior, A. (2007). Antimicrobial activity of methyl australate from Ganoderma australe. Rev. Bras. Farmacogn. 17, 14–16. doi: 10.1590/S0102-695X2007000100004
Smania, J. A., Monache, F. D., Smania, E. D., and Cuneo, R. S. (1999). Antibacterial activity of steroidal compounds isolated from Ganoderma applanatum (Pers.) Pat. (Aphyllophoromycetideae) fruit body. Int. J. Med. Mushrooms. 1, 325–330. doi: 10.1615/IntJMedMushr.v1.i4.40
Smania, J. A., Smania, E. F., Monache, F. D., Pizzolatti, M. G., and Monache, G. D. (2006). Derivatization does not influence antimicrobial and antifungal activities of applanoxidic acids and sterols from Ganoderma spp. Z. Naturforsch. C. 61, 31–34. doi: 10.1515/znc-2006-1-206
Soliman, A., Abdelbary, S., Yonus, A., and Abdelghany, T. (2022). Trends in assessment of G. lucidum methanol extract against MRSA infection in vitro and in vivo with nutrition support. J. Adv. Pharm. Res. 6, 46–57. doi: 10.21608/aprh.2022.111305.1147
Srivastava, M. P. and Sharma, N. (2011). Antimicrobial activities of basidiocarp of some basidiomycetes strains against bacteria and fungi. J. Mycol. Pl. Pathol. 41, 332.
Stanley, H. O., Abu, G. O., and Immanuel, O. M. (2016). Evaluation of the biocorrosion inhibition potential of ethanol extract of Ganoderma tropicum. Int. J. Curr. Microbiol. App. Sci. 5, 609–618. doi: 10.20546/ijcmas.2016.507.068
Stojković, D. S., Barros, L., Calhelha, R. C., Glamočlija, J., Ćirić, A., Van Griensven, L. J., et al. (2014). A detailed comparative study between chemical and bioactive properties of G. lucidum from different origins. Int. J. Food Sci. Nutr. 65, 42–47. doi: 10.3109/09637486.2013.832173
Suansia, A. and John, P. (2021). Antimicrobial and antioxidant properties of medicinal mushroom Ganoderma P. Karst. GSCBPS. 17, 106–112. doi: 10.30574/gscbps.2021.17.2.0326
Subbraj, T., Michael, A., Rajeshwari, S., and Mani, K. (2008). Presence of antimicrobial substance in G. lucidum, a wild type mushroom. Plant Arch. 8, 261–263.
Subedi, K., Basnet, B. B., Panday, R., Neupane, M., and Tripathi, G. R. (2021). Optimization of growth conditions and biological activities of Nepalese G. lucidum strain Philippine. Adv. Pharmacol. Pharm. Sci. 2021, 4888979. doi: 10.1155/2021/4888979
Sułkowska-Ziaja, K., Zengin, G., Gunia-Krzyżak, A., Popiół, J., Szewczyk, A., Jaszek, M., et al. (2022). Bioactivity and mycochemical profile of extracts from mycelial cultures of Ganoderma spp. Molecules. 27, 275. doi: 10.3390/molecules27010275
Tamilselvan, N. and Rajesh, K. (2019). Antimicrobial efficacy of medicinal mushroom G. lucidum. IJTSRD 3, 1798–1800. doi: 10.31142/ijtsrd23522
Tehranian, M. J., Jouki, M., Shakouri, M. J., and Jafari, S. (2023). Functional properties of G. lucidum extract: Antimicrobial and antioxidant activities. Food Sci. Technol. 43, e21423. doi: 10.5327/fst.21423
Upadhyay, M., Shrivastava, B., Jain, A., Kidwai, M., Kumar, S., Gomes, J., et al. (2014). Production of ganoderic acid by G. lucidum RCKB-2010 and its therapeutic potential. Ann. Microbiol. 64, 839–846. doi: 10.1007/s13213-013-0723-9
Uwidia, I. E., Ikhuoria, E. U., Okojie, R. O., Ifijen, I. H., and Chikaodili, I. D. (2024). Antibacterial properties of rod-like vanadium oxide nanostructures via G. lucidum plant extract approach. Chem. Afr. 7, 1951–1961. doi: 10.1007/s42250-023-00854-6
Vahdani, M., Shoeibi, S., and Sharifan, A. (2022). Antifungal susceptibility of Aspergillus flavus, Aspergillus ochraceus, and Fusarium graminearum to G. lucidum extract. Jundishapur J. Nat. Pharm. Prod. 17, e115715. doi: 10.5812/jjnpp.115715
Vazirian, M., Faramarzi, M. A., Ebrahimi, S. E., Esfahani, H. R., Samadi, N., Hosseini, S. A., et al. (2014). Antimicrobial effect of the Lingzhi or Reishi medicinal mushroom, G. lucidum (higher Basidiomycetes) and its main compounds. Int. J. Med. Mushrooms. 16, 77–84. doi: 10.1615/IntJMedMushr.v16.i1.70
Wang, H. and Ng, T. B. (2006). Ganodermin, an antifungal protein from fruiting bodies of the medicinal mushroom G. lucidum. Peptides. 27, 27–30. doi: 10.1016/j.peptides.2005.06.009
Wang, C. L., Pi, C. C., Kuo, C. W., Zhuang, Y. J., Khoo, K. H., Liu, W. H., et al. (2011). Polysaccharides purified from the submerged culture of Ganoderma formosanum stimulate macrophage activation and protect mice against Listeria monocytogenes infection. Biotechnol. Lett. 33, 2271–2278. doi: 10.1007/s10529-011-0697-2
Wang, H., Wang, L., Xie, K., and Xie, M. (2017). The antimicrobial and antioxidant activity of the powder of G. lucidum. Chin. J. Biochem. Pharmaceutics. 2017, 52–54.
Wan-Mohtar, W. A., Viegelmann, C., Klaus, A., and Lim, S. A. (2017). Antifungal-demelanizing properties and RAW264. 7 macrophages stimulation of glucan sulfate from the mycelium of the mushroom G. lucidum. Food Sci. Biotechnol. 26, 159–165. doi: 10.1007/s10068-017-0021-6
Wan-Mohtar, W.A.A.Q.I., Young, L., Abbott, G. M., Clements, C., Harvey, L. M., and McNeil, B. (2016). Antimicrobial properties and cytotoxicity of sulfated (1,3)-β-D-glucan from the mycelium of the mushroom G. lucidum. J. Microbiol. Biotechnol. 26, 999–1010. doi: 10.4014/jmb.1510.10018
Wasser, S. P. (2011). Current findings, future trends, and unsolved problems in studies of medicinal mushrooms. Appl. Microbiol. Biotechnol. 89, 1323–1332. doi: 10.1007/s00253-010-3067-4
Wood, T. T., Rowaiye, A. B., Okwu, C. O., Popoola, O. A., Unaeze, C. H., AkienAlli, I. J., et al. (2021). Phytochemical screening and antibacterial effects of wild Ganoderma species on selected foodborne bacteria. Int. J. Adv. Res. Biol. Sci. 8, 128–137. doi: 10.22192/ijarbs.2021.08.01.016
World Health Organization. (2024). World Malaria Report 2024. Geneva: World Health Organization. Available online at: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2024 (Accessed July 10, 2025).
Wu, Z. W., Zhao, X. F., Quan, C. X., Liu, X. C., Tao, X. Y., Li, Y. J., et al. (2025). Structure–function insights of natural Ganoderma polysaccharides: advances in biosynthesis and functional food applications. Natural Products Bioprospecting. 15, 1–33. doi: 10.1007/s13659-025-00496-w
Xia, Q., Zhang, H., Sun, X., Zhao, H., Wu, L., Zhu, D., et al. (2014). A comprehensive review of the structure elucidation and biological activity of triterpenoids from Ganoderma spp. Molecules. 19, 17478–17535. doi: 10.3390/molecules191117478
Yamac, M. and Bilgili, F. (2006). Antimicrobial activities of fruit bodies and/or mycelial cultures of some mushroom isolates. Pharm. Biol. 44, 660–667. doi: 10.1080/13880200601006897
Zahmoul, S. H., Chaabouni, R. L., Srih, A., Dogan, H. H., Varıcıoğlu, E., Sbissi, I., et al. (2024). Nutritional and pharmacological potentials of the medicinal mushroom Ganoderma adspersum (Schulz.) Donk. S. Afr. J. Bot. 166, 360–374. doi: 10.1016/j.sajb.2024.01.049
Zengin, G., Sarikurkcu, C., Gunes, E., Uysal, A., Ceylan, R., Uysal, S., et al. (2015). Two Ganoderma species: profiling of phenolic compounds by HPLC–DAD, antioxidant, antimicrobial and inhibitory activities on key enzymes linked to diabetes mellitus, Alzheimer’s disease and skin disorders. Food Funct. 6, 2794–2802. doi: 10.1039/C5FO00665A
Zhai, J. L., Lin, Y., Wang, M. Y., Gao, Y. Y., Wang, C. K., and Jin, L. (2021). Effects of G. lucidum polysaccharides on immune function of IPEC-1 cells infected with. E. coli. CJAN. 33, 7118–7130. doi: 10.3969/j.issn.1006-267x.2021.12.051
Zhang, X.-Q., Ip, F. C. F., Zhang, D.-M., Chen, L.-X., Zhang, W., Li, Y.-L., et al. (2011). Triterpenoids with neurotrophic activity from G. lucidum. Nat. Prod. Res. 25, 1607–1613. doi: 10.1080/14786419.2010.496367
Zhang, W., Tao, J., Yang, X., Yang, Z., Zhang, L., Liu, H., et al. (2014). Antiviral effects of two G. lucidum triterpenoids against enterovirus 71 infection. Biochem. Biophys. Res. Commun. 449, 307–312. doi: 10.1016/j.bbrc.2014.05.019
Zhang, H., Zhang, J., Liu, Y., and Tang, C. (2023). Recent advances in the preparation, structure, and biological activities of β-glucan from Ganoderma species: a review. Foods. 12, 2975. doi: 10.3390/foods12152975
Zhao, S., Guo, Y. X., Liu, Q. H., Wang, H. X., and Ng, T. B. (2009). Lectins but not antifungal proteins exhibit anti-nematode activity. Environ. Toxicol. Pharmacol. 28, 265–268. doi: 10.1016/j.etap.2009.05.003
Zhong, Y., Tan, P., Lin, H., Zhang, D., Chen, X., Pang, J., et al. (2024). A review of ganoderma lucidum polysaccharide: preparations, structures, physicochemical properties and application. Foods. 13, 2665. doi: 10.3390/foods13172665
Zhong, X., Wang, G., Li, F., Fang, S., Zhou, S., Ishiwata, A., et al. (2023). Immunomodulatory effect and biological significance of β-glucans. Pharmaceutics. 15, 1615. doi: 10.3390/pharmaceutics15061615
Zhu, Q., Bang, T. H., Ohnuki, K., Sawai, T., Sawai, K., and Shimizu, K. (2015). Inhibition of neuraminidase by Ganoderma triterpenoids and implications for neuraminidase inhibitor design. Sci. Rep. 5, 13194. doi: 10.1038/srep13194
Zhu, H., Liu, W., Tian, B., Liu, H., and Ning, S. (2011). Inhibition of quorum sensing in the opportunistic pathogenic bacterium Chromobacterium violaceum by an extract from fruiting bodies of Lingzhi or Reishi medicinal mushroom, G. lucidum (W. Curt.: Fr.) P. Karst. (higher Basidiomycetes). Int. J. Med. Mushrooms 13. doi: 10.1615/intjmedmushr.v13.i6.80
Keywords: antimicrobial activity, biofilm inhibition, pathogenic bacteria, polysaccharides, synergistic effects, triterpenoids
Citation: Karunarathna SC, Patabendige NM, Hapuarachchi KK and Promputtha I (2025) Exploring the health benefits of Ganoderma: antimicrobial properties and mechanisms of action. Front. Cell. Infect. Microbiol. 15:1535246. doi: 10.3389/fcimb.2025.1535246
Received: 27 November 2024; Accepted: 28 May 2025;
Published: 18 July 2025.
Edited by:
Blake Billmyre, University of Georgia, United StatesReviewed by:
Sabulal Baby, Jawaharlal Nehru Tropical Botanic Garden and Research Institute, IndiaPrashant R. Desai, University of Wisconsin-Madison, United States
Copyright © 2025 Karunarathna, Patabendige, Hapuarachchi and Promputtha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Itthayakorn Promputtha, aXR0aGF5YWtvcm4ucEBjbXUuYWMudGg=; Kalani K. Hapuarachchi, a2FsYW5pZmlyc3RAeWFob28uY29t
 Samantha C. Karunarathna
Samantha C. Karunarathna Nimesha M. Patabendige2
Nimesha M. Patabendige2 Kalani K. Hapuarachchi
Kalani K. Hapuarachchi Itthayakorn Promputtha
Itthayakorn Promputtha