- Department of Earth Sciences, Faculty of Science, Carleton University, Ottawa, ON, Canada
The origin of terrestrial ecosystems during the Paleozoic is pivotal in the history of life on Earth. This is a fascinating case for testing hypotheses about how ecological novelty arises at the organismal, lineage, and community levels. In this paper, I review research on community assembly and change in deep time and discuss this work in the context of investigating the continentalization of ecosystems. The extensive study of large-scale Phanerozoic trends in taxonomic and autecological diversity, particularly in the marine realm, provides an important theoretical framework. However, the interactions between these trends and community-level properties such as stability and the species carrying capacity are not as well understood. The growing body of paleo-food web literature has returned ambiguous results, and it is not clear whether the bounds of community performance have shifted over time or not. Importantly, these studies are conducted either entirely in the marine realm or in the terrestrial realm, but not yet on communities representing the initial expansion of life into non-marine and, eventually, terrestrial habitats. Modern-day systems such as island colonization might provide some useful insights into continentalization in deep time, but are effectively instances of terrestrial ecosystems being reproduced using extant terrestrial taxa, not terrestrial ecosystems developing de novo. The timeline of Paleozoic continentalization as currently understood is reviewed. Although the process was already underway, the Late Paleozoic (Devonian–Permian) emerged as a key interval for the study of continentalization. Food web modeling methods and hypotheses are discussed. Although challenging, going forward, this area of research has great potential to address questions of relevance to paleontologists, neontologists, and ecologists alike.
1 Introduction
The origin of terrestrial ecosystems during the Paleozoic is pivotal in the development of the global biosphere (Knoll and Bambach, 2000). Research has focused on the chronology of terrestrialization across taxa and its impacts on the Earth system (Knoll and Bambach, 2000; Sahney et al., 2010b; Vecoli et al., 2010; Kenrick et al., 2012; Lenton et al., 2016) and is deeply tied to work on large-scale changes in the taxonomic and autecological diversity of marine organisms (Sepkoski et al., 1981; Jablonski and Sepkoski, 1996; Bambach et al., 2007). Over the last two decades, advances in computing have spurred greater comparative investigations of fossil ecosystems as distinct from the sum of their components. Current issues include: the relationship between taxonomic diversity and ecosystem structure (Dunne et al., 2008; Blanco et al., 2021; Banker et al., 2022); whether ecological communities evolve and are subject to selection (Roopnarine and Angielczyk, 2016; Roopnarine et al., 2019); and the applicability of ecosystem assembly rules (DiMichele et al., 2023b). Most of the effort has gone into the marine or terrestrial realms, while the process of continentalization and the origin of terrestrial ecosystems remain understudied in this regard (Spiridonov and Eldredge, 2024). The aims of this paper are: 1) to review the paleontological and macroecological perspectives on community assembly and 2) to discuss continentalization and the origin of terrestrial communities during the Paleozoic as useful examples for study, with a special focus on the Late Paleozoic and the incorporation of vertebrates into terrestrial ecosystems.
Marine species diversity is commonly portrayed as increasing over the course of the Phanerozoic (Sepkoski et al., 1981; Sepkoski, 1993; Bambach et al., 2004; Bush and Bambach, 2004; Bush et al., 2004), with a sharp increase from the Cretaceous onwards. This biodiversity has also been divided into distinct taxonomic associations in the marine realm—Cambrian, Paleozoic, and Modern—each of the latter two arising after a mass extinction (End-Ordovician and End-Permian, respectively) (Sepkoski et al., 1981). The occupation of marine ecospace, i.e., the number of niches filled, and the breadth of ecospace, i.e., the number of possible niches, have also been described as increasing over the Phanerozoic along similar lines (Vermeij, 1977; Bambach et al., 2007; Patzkowsky and Holland, 2007; Klug et al., 2010, 2010; Villéger et al., 2011; Bush and Novack-Gottshall, 2012; Mondal and Harries, 2016), although there are complexities at smaller spatiotemporal scales. Parts of this interpretation have been challenged as a product of sampling, biotic, and/or tectonic biases in the fossil record (Raup, 1976; Cherns and Wright, 2000; Alroy et al., 2001, 2008; Peters and Foote, 2001; Wall et al., 2009; Zaffos et al., 2017).
Similar correlated increases in species diversity and ecological diversity have been described for tetrapods (Sahney et al., 2010b), suggesting that the pattern of increase might be general for Phanerozoic life. Spatiotemporally distinct taxon associations of terrestrial vertebrates, e.g.,faunachrons or assemblage zones, have been described at various scales, such as the Cenozoic of the Americas (Flynn et al., 1984; Pascual and Jaureguizar, 1990) or the Permo-Triassic of South Africa (Viglietti et al., 2022) and Russia (Olson and Chudinov, 1992; Golubev, 2000; Schneider et al., 2020; Shishkin et al., 2023c). However, thus far, there are no terrestrial analogues for the marine Sepkoski faunas.
If indeed Phanerozoic ecosystems have been assembled from an increasingly large pool of increasingly ecologically diverse species, then understanding the structure of these ecosystems and how they have changed is crucial to understanding the evolution of their component lineages. It is these patterns of species–species ecological interaction that, in conjunction with species–environment interactions, create the evolutionary environment in which species exist at any given time (Valen, 1973; Stenseth and Smith, 1984; Hubbell, 2001; Roopnarine, 2012; Solé, 2022; Spiridonov and Lovejoy, 2022).
2 Ecological assembly rules through time
If we consider the structure of ecosystems as important for understanding the evolution of taxa, the questions then follow of whether and how ecosystems in deep time were different from those in the Modern: were they subject to the same processes of assembly, network interactions, and energy flow (DiMichele et al., 2004)?
Primary productivity has increased over geologic time (Crockford et al., 2023), and it is possible that nutrient availability has increased during the Phanerozoic in particular (Assine et al., 2018; Wang et al., 2020). Increased energy consumption by marine organisms is inferred from the fossil record: the Cambrian rise in bioturbation and the metazoan morphological diversity (Droser et al., 2002; Marshall, 2006; Briggs, 2015; Darroch et al., 2021); the Devonian increases in morphologies for active predation and nektonic swimming (Novack-Gottshall, 2007; Klug et al., 2010); and evidence of increased predation intensity during the Mesozoic (Vermeij, 1977; Kelley and Hansen, 2001; Huntley and Kowalewski, 2007; Finnegan et al., 2011; Buatois et al., 2016). Presumably, this increased energy consumption by marine organisms corresponded with the increased consumption by marine communities (through some combination of bottom-up and top-down effects). Within such a hypothesis of dynamically changing ecological assembly processes over time, organismal identity and traits become more important for understanding past ecosystems (Banker et al., 2022).
On the other hand, some modeling of Cenozoic communities has found that the occupation pattern of niches or guilds can persist despite species extinction and turnover, suggesting that organismal interactions have a stronger control on ecosystem structure than organismal traits (Fraser and Lyons, 2020; Blanco et al., 2021; Whittingham et al., 2024b). These results support the hypothesis that fossil ecosystems are best understood not as groupings of species autecologies but rather as sets of interactions. The taxonomic identity and particular traits of organisms are deemphasized in favor of their niche or functional role within the community (Roopnarine, 2012). The origin of new functional roles, such as herbivory, could have large effects on ecosystem “fitness” (Roopnarine and Angielczyk, 2012; Angielczyk et al., 2020); alternatively, the dynamics inherent to sufficiently-sized networks might have greater importance (Dunne et al., 2002, 2004, 2008). If communities are emergent entities in this way, it is possible that not only do species co-evolve within communities but that the communities themselves undergo selection and evolve (Bambach, 2001; Roopnarine and Angielczyk, 2016; Roopnarine et al., 2019).
Significantly, the ecosystems spanning the continentalization of life have not yet been analyzed in this way. This is quite understandable, given the challenges of the fossil record. However, continentalization in deep time presents a unique opportunity to study ecological assembly rules: the expansion of life into fully novel habitats—beginning with non-marine waters and eventually leading to land—entailed numerous organismal adaptations, the origin of functional roles, and the construction of unprecedented ecosystems in a manner arguably not seen since.
3 Modern models for continentalization?
Ideally, it should be possible to find at least partial Modern analogues for deep-time continentalization. There are three such potential analogues: disturbance (such as that of a volcanic eruption), glacier retreat, and island colonization (Thornton, 2007; Crisafulli and Dale, 2018; Cauvy-Fraunié and Dangles, 2019; Ficetola et al., 2024; Khelidj et al., 2024).
DiMichele and colleagues recently made direct comparisons between recovery following disturbance and the evolution of the first terrestrial floras (DiMichele et al., 2023b). In both cases, the initial plant colonizers were morphologically simple and formed fast-propagating and taxonomically and functionally homogenous floras. Competition was primarily intraspecific. These were then replaced by floras that were progressively more architecturally complex and taxonomically diverse. Within these more complex floras, interspecific interactions (including competition) became more significant, creating a more interconnected and resilient community. Similar successional patterns are seen in the aftermath of the glacier retreat (Cauvy-Fraunié and Dangles, 2019; Ficetola et al., 2024; Khelidj et al., 2024).
Island colonization events differ from both post-disturbance and post-glacier retreat community assembly events in terms of spatial dynamics. Islands are isolated from their mainlands by water bodies that constitute dispersal barriers that colonizers must cross. This requirement of dispersal biases the set of organisms that can colonize. Islands are also limited in area and are generally small, while a glacier retreat can expose large areas on a continental scale relatively quickly (in geological terms). A general pattern of succession and community assembly can be drawn from several extensively researched case studies (Thornton, 2007). Airborne dispersal is primary for both plants and animals. Early colonists depended on airborne or waterborne food sources until a fully terrestrial local community was established. The extinction rates of resident species were initially high, but those species that successfully became established persisted even as the community expanded (Heatwole, 1981).
The order of succession/colonization is, at high taxonomic/ecological levels, the same between the above three scenarios and the fossil record of continentalization, with microorganisms preceding macroorganisms. There were non-marine microfossils ~1,000 Ma (Strother et al., 2011), and geochemical signals have been used to infer the presence of unspecified, presumably microscopic, terrestrial photosynthetic organisms from ~850 Ma (Horodyski and Knauth, 1994; Knauth and Kennedy, 2009). Terrestrial plant fossils have been known from the Late Ordovician onwards (Edwards and Wellman, 2001; Raymond et al., 2006; Steemans et al., 2009; Kenrick et al., 2012; Xue et al., 2018), although, again, earlier origins for land plants have been proposed (Clarke et al., 2011). Body fossils indicate that arthropod terrestrialization was underway by the Middle Silurian (~430 Ma), and fully terrestrial arthropods had evolved by the Late Silurian (Dunlop, 1996; Dunlop and Selden, 2013; Waddington et al., 2015). Hypotheses of a Cambrian radiation of terrestrial arthropods have been proposed on the basis of molecular clock studies (Tihelka et al., 2022). Arthropods appeared to have expanded onto land directly from the ocean without passing through freshwater environments first. During this “intertidal phase,” they likely played an important role in the transfer of marine nutrients into the terrestrial community, as is the case with early arthropod island colonizers in the Modern (Thornton, 2007). The timing and the process of vertebrate terrestrialization are highly contentious and will be discussed later.
4 Limits to modern models
However, there are several major differences between Modern systems of disturbance and colonization and continentalization in the fossil record. Biotic (re)colonization of disturbed areas, previously glaciated areas, and islands can be extremely rapid, on the order of decades for community establishment (Thornton, 2007). In contrast, the invasion of land was protracted across hundreds of millions of years across the Late Precambrian to the Middle Paleozoic. This difference in timing highlights the differences in the biological and ecological problems that need to be solved. In the three Modern scenarios, the foremost problem an organism must solve is that of arriving to the area in question. In cases of disturbance or glacier retreat, this is relatively easy as the distance is small and the climatic/physical barriers minimal. Some heat- or drought-resistant organisms can survive disturbance and persist in place. As discussed, islands are more isolated, but they still draw from a regional pool of colonists at varying scales. In all three cases, the organisms are already adapted to terrestrial life broadly and the new area falls within their habitat parameters (at the very least). Organisms can thus (re)establish and form ecological communities quickly.
In contrast, the greatest problem for organisms during terrestrialization in deep time was adapting to life on land in the first place. This process differed between major clades, although osmotic control and body support were common problems for both plants and animals (Markey and Marshall, 2007b; Clack, 2012; Standen et al., 2016; Lemberg et al., 2021; Tihelka et al., 2022). For vertebrates, terrestriality required extensive modifications to the locomotor and sensory systems, with corresponding neurological and behavioral changes (Clack, 2009; Esteve-Altava et al., 2019; MacIver and Finlay, 2022). In contrast, changes to the feeding system were comparatively modest before, during, and well past the origin of both limbs and terrestriality (Markey and Marshall, 2007b; Anderson et al., 2013; Neenan et al., 2014; Witzmann, 2016; Lemberg et al., 2021; Werneburg, 2024). The components of continental ecosystems had to be created through adaptation and cladogenesis on the scale of millions of years, rather than tens or hundreds, with the correspondingly slow assembly of continental ecosystems.
5 The unrepeatability of the past
In a review of plant and animal transitions between marine, freshwater, and terrestrial habitats, Vermeij and Dudley (2000) found that such transitions were rare overall, but not equally distributed: terrestrial–marine transitions are rarer than the reverse; most marine-terrestrial and terrestrial-marine transitions proceeded via freshwater; and marine–freshwater and terrestrial–freshwater transitions were more common than transitions between marine and freshwater (in either direction) via freshwater (Vermeij and Dudley, 2000). The discrepancy is particularly pronounced in limbed tetrapods, which have repeatedly become secondarily aquatic as far back as the Pennsylvanian (deBraga and Reisz, 1995; Nuñez Demarco et al., 2018) or even earlier (Ahlberg, 2018; Herbst and Hutchinson, 2018).
If, then, terrestrial–aquatic transitions are easier for organismal lineages than the reverse given that terrestriality requires a previous aquatic stage (either marine or non-marine), would we expect there to be an analogous directionality during the initial continentalization of ecosystems? Previous research has indicated that, during biotic invasions, invaders that persist do so via either outcompeting incumbents in the receiving community or filling unoccupied ecospace (Patzkowsky and Holland, 2007; Dudei and Stigall, 2010; Stigall, 2019; Kempf et al., 2020). If the source and receiving habitats are different, invaders might face additional competitive disadvantage due to the lack of suitable adaptations. For the earliest land colonizers, interspecific competition would initially have been low and had a negligible or small role in the taxic (low barrier to invasion) or structural (little or no forced ecospace overlap) composition of ecosystems. These colonizers adapted to increase their fitness in the new environment and became terrestrial residents. As additional organisms made the water–land transition (invaded), competition assumed a greater role that drove further terrestrial adaptation. At some hypothetical point during continentalization, the competitive barrier to invasion became a limiting factor; on the other hand, the cost of returning to the water was lower, although not uniform across lineages (Vermeij and Dudley, 2000).
In summary, just as secondarily aquatic organisms are constrained by a history of terrestrial existence, so might there be forms of continental ecological communities (especially terrestrial ones) that can only have existed in the geologic past. If that is the case, then Modern systems are insufficient as models. Testing this hypothesis, then, requires the extensive study of fossil data.
6 Terrestrialization and continentalization in the Devonian–Carboniferous
Plants and animals were present on land before the Devonian (Kenrick et al., 2012; Kraft et al., 2019; Pšenička et al., 2021; Buatois et al., 2022; Tihelka et al., 2022). However, it is during the Devonian that the terrestrial fossil record improved drastically (Edwards et al., 2018; Xue et al., 2018; Shen et al., 2020; Gess and Prestianni, 2021; Ţabără et al., 2021; Buatois et al., 2022), and these increased data enable us to better understand the emerging terrestrial biosphere. The Rhynie Chert community from the Early Devonian of Scotland preserves a continental community of microorganisms (nematodes), plants (lycopsids and extinct tracheophytes), aquatic invertebrates (e.g., branchiopods and notostracans), and terrestrial invertebrates (e.g., trigonotarbids, opiliones, and myriapods) distributed across multiple trophic levels. Based on the coprolite contents and mouthpart morphology, the terrestrial invertebrates included detritivores, fungivores, and predators (Habgood et al., 2003; Dunlop and Garwood, 2018). This community existed within a volcanic spring environment (Trewin, 1992). If continental communities were restricted to such environments in the Early Devonian, the community from the Gilboa Forest in New York (Shear et al., 1984, 1987; Norton et al., 1988; Shear and Bonamo, 1988; Stein et al., 2012) indicates that such limitations had been overcome by the Middle Devonian time (Givetian).
By the Middle Devonian, progymnosperms and lycopsids had diversified and repeatedly developed shrub and leaf habits (Stein et al., 2007, 2012; Berry and Marshall, 2015; Gensel et al., 2020), leading to the formation of wetland forests and woodlands (Greb et al., 2006; Gensel et al., 2020). Here, terrestrial plants both created new microhabitats and a growing resource pool for terrestrial invertebrates. The advent of root systems in the Devonian (Morris et al., 2015; Xue et al., 2016; Stein et al., 2020, 2020; Wang et al., 2019) facilitated the establishment and the persistence of these communities: roots made plants more physically stable, increased the available nutrients through increased rock weathering, and increased the spread and density of the terrestrial biosphere by extending further underground. Terrestrial trigonotarbid–opilione–myriapod–scorpion assemblages are known from these habitats across the remainder of the Devonian (Parent and Cloutier, 1996; Cressler et al., 2010; Gess, 2013). The evolution of seed reproduction in the Middle Devonian (Gerrienne et al., 2004, 2005) enabled the expansion of terrestrial floras onto less waterlogged sediments during the Late Devonian (Greb et al., 2006; Cressler et al., 2010; Linkies et al., 2010; Gensel et al., 2020).
The increase in Devonian flora also affected aquatic organisms, both continental and marine. Increased organic matter and phosphorous runoff (Wang et al., 2020), as evidenced by the Devonian coastal plankton blooms and corresponding anoxic events (Joachimski and Buggisch, 1993; Algeo and Scheckler, 1998; Boyer et al., 2021). These blooms would have drawn marine organisms (chiefly vertebrates and eurypterids) closer to the shoreline and into non-marine environments. The physical stabilization of sediment by roots increased the persistence of riverbanks and shorelines. These littoral habitats could then be used as sites for reproduction (Gueriau et al., 2016; Olive et al., 2016; Gess and Whitfield, 2020; Piñeiro et al., 2024) and/or feeding on others doing the same before returning to the ocean. Eventually, some of these transients adapted to life in these non-marine habitats as residents. The Devonian–Carboniferous increase in the abundance and diversity of non-marine microinvertebrates such as ostracods was particularly important in providing an expanding prey base for secondary consumers (Bennett et al., 2012). This transition to non-marine environments would be the antecedent for the transition to terrestrial environments in vertebrates (Vermeij and Dudley, 2000; Buatois et al., 2022).
The timing and process of vertebrate terrestrialization is currently contentious (King et al., 2011; Pierce et al., 2012; Mansky and Lucas, 2013; Ahlberg, 2018, 2024). Tetrapodomorph fishes had begun invading non-marine environments early in their evolutionary history and are common components of such brackish and freshwater assemblages from the Middle Devonian onward (Gray, 1988; Cressler et al., 2010; Sallan and Coates, 2010; Swartz, 2012; Schultze, 2013; Zhu et al., 2017; Laurin, 2024). Sedimentological and isotopic evidence suggests that a panderichthyid tolerance for variable salinity was maintained by tetrapods well into the Carboniferous (Laurin and Soler-Gijón, 2001, 2010; Gogáin et al., 2016; Bennett et al., 2017; Goedert et al., 2018; Laurin, 2024). Current phylogenies agree that panderichthyid (=elpistostegalian) tetrapodomorphs comprise the immediate outgroup to limbed tetrapods (Swartz, 2012; Cloutier et al., 2020; Stewart et al., 2022), although it is unclear whether they are a clade or a grade (a series of successive sister groups). The traditional ecological interpretation of panderichthyids has been as shallow-water aquatic predators, their well-developed, jointed fins being used to push against the substrate and navigate around obstacles (Shubin et al., 2006; Clack, 2012; Stewart et al., 2019). The dorsal shift in orbit placement and the increase in orbit size in panderichthyids and Devonian tetrapods relative to more conventionally “fish-shaped” tetrapodomorphs (e.g., Eusthenopteron) suggest an increasing role for vision in these animals, particularly vision through air (MacIver et al., 2017). Predation across the water–land interface as a learned behavior—sans morphological adaptation—has been documented in Modern catfish (Cucherousset et al., 2012). A similar behavior by panderichthyids and the earliest tetrapods might have been part of the changing neuroecology during the origin of tetrapods (MacIver and Finlay, 2022). Nevertheless, there is little “hard” evidence for vertebrates as “resident” components of terrestrial food webs by the end of the Late Devonian. That said, there are hypotheses that posit terrestrial locomotion for both panderichthyids and Devonian tetrapods (Ahlberg, 2018, 2024). The substantial (Warren and Wakefield, 1972; Rogers, 1990; Stössel, 1995; Niedźwiedzki et al., 2010; Stössel et al., 2016; Qvarnström et al., 2018) and growing (Ahlberg, 2024) Devonian trackway record attributed to tetrapod trackmakers remains temporally disjunct from the body fossil record, but represents a tantalizing—if ambiguous—additional source of data.
The End-Devonian mass extinction (EDME), also known as the Hangenberg event, included mass extinctions of marine and non-marine vertebrates (Sallan and Coates, 2010) and terrestrial plants (Myrow et al., 2014; Silvestro et al., 2015; Marshall, 2020; Marshall et al., 2020). There are earliest Mississippian tetrapod trackways (Mansky and Lucas, 2013; Lucas, 2019), but no body fossil evidence for tetrapod terrestriality (Lennie et al., 2021; Long et al., 2025). Terrestrial arthropod assemblages during the Tournaisian were similar to their Late Devonian counterparts in both taxonomic composition and trophic structure (Clarkson, 1985; Mansky and Lucas, 2013; Otoo et al., 2018). The Visean East Kirkton fauna represents a key biota within the context of continentalization. In addition to a more species-rich arthropod fauna (although still only composed of predators and detritivores), the East Kirkton community includes the oldest terrestrial tetrapod body fossils (Milner and Sequeira, 1993; Smithson et al., 1993; Ruta and Clack, 2006; Ruta et al., 2020). This makes East Kirkton the oldest terrestrial vertebrate assemblage at the time of writing (Clarkson et al., 1993). The lack of similar assemblages of terrestrial tetrapods elsewhere in the Mississippian (even from the Scottish Midland Valley succession, despite years of determined search) suggests that terrestrial tetrapods remained relatively rare until at least the Early Pennsylvanian. The Serpukhovian Loanhead community, also from the Scottish Midland Valley succession, contains primarily aquatic vertebrates alongside a much smaller number of likely semi-aquatic tetrapods (Smithson, 1985) and is much more representative of the Mississippian tetrapod communities (Godfrey, 1988; Schultze and Bolt, 1996; Snyder, 2006; Bolt and Lombard, 2010; Clack, 2012; Otoo et al., 2018, 2021).
There is trace fossil evidence of invertebrate herbivory on liverworts by the Middle Devonian (Labandeira et al., 2014), but traces of arthropod herbivory on living vascular plant tissue (folivory) appeared in the Late Mississippian (Iannuzzi and Labandeira, 2008). The latter is closely tied to the Carboniferous radiations of insects, in particular blattodeans, orthopterans, and palaeodictyopterans, that took advantage of expanding terrestrial floras (Grimaldi and Engel, 2005; Donovan et al., 2023). These radiations are primarily recorded in a few exceptionally preserved Pennsylvanian entomofaunas, such as Joggins (Bashkirian), Mazon Creek (Moscovian), and Carrizo Arroyo (Gzhelian) (Rowland, 1997, 1997; Shabica and Hay, 1997; Rasnitsyn et al., 2004, 2015; Grimaldi and Engel, 2005; Falcon-Lang, 2006; Falcon-Lang et al., 2006; Clements et al., 2019; Burke et al., 2024). Fossil coleopterans first appeared in the Early Permian, although they might also be part of these Carboniferous radiations (Kirejtshuk and Nel, 2013; Kirejtshuk et al., 2014; Beutel et al., 2019; Schädel et al., 2022; Boudinot et al., 2023).
Although body sizes generally remained small during the Early Pennsylvanian (Bashkirian–Moscovian), terrestrial tetrapods expanded into both fossoriality (Maddin et al., 2011; Pardo et al., 2015; Mann et al., 2019, 2021a) and scansoriality (Mann et al., 2021b). From the Mississippian through the end of the Early Pennsylvanian, the set of terrestrial tetrapods was taxonomically skewed toward “lepospondyls” (especially “microsaurs”) and total group amniotes (Hook and Baird, 1986, 1993; Clarkson et al., 1993; Milner and Sequeira, 1998, 2011; Falcon-Lang, 2006; Stimson et al., 2013; Mann et al., 2020). The phylogenetic status and affinities of Lepospondyli and its various putative subclades are topics of active debate among early tetrapod specialists (Ruta and Coates, 2007; Pardo et al., 2017; Clack et al., 2019; Marjanović and Laurin, 2019; Mann et al., 2023b; Pardo, 2023; Igielman, 2024; Smithson et al., 2024), but beyond the scope of this paper.
During the Late Pennsylvanian (Kasimovian–Gzhelian), tetrapods began adapting for omnivory and, later, herbivory (Beerbower et al., 1992; Mann et al., 2023a; Ponstein et al., 2024). The advent of tetrapod herbivory greatly increased the influence of tetrapods on plants (Brocklehurst et al., 2020) and compounded the structural changes to food webs induced by insect herbivory. The upper limit of terrestrial tetrapod body size among herbivores and predators increased markedly around this time (Brocklehurst and Brink, 2017). It is during the Late Pennsylvanian that crown amniote lineages began returning to the water (deBraga and Reisz, 1995; Nuñez Demarco et al., 2018), although the specifics are disputed (Romer, 1974; Canoville and Laurin, 2010; Felice and Angielczyk, 2014; Laurin and Piñeiro, 2017; Nuñez Demarco et al., 2018). Thus, by the end of the Pennsylvanian, the fundamental components of terrestrial vertebrate ecosystems appeared to have been in place: multitaxic, architecturally diverse floras; terrestrial invertebrate primary and secondary consumers; and vertebrate primary and secondary consumers.
7 The Late Paleozoic origin of terrestrial vertebrate ecosystems
Olson proposed a scenario for the development of terrestrial vertebrate communities during the Permian (Olson, 1952, 1966, 1971, 1977). He defined a terrestrial (vertebrate) community as one for which the energy base is terrestrial primary productivity. Olson began with (at the time) the oldest known communities with terrestrial vertebrates. These communities were still considered aquatic (but still continental) as aquatic primary activity forms the energy basis of the community, conveyed to terrestrial organisms via predation on semi-aquatic and aquatic organisms. Olson developed this characterization of “type I” communities based primarily on observations of lowland redbed communities from the Late Pennsylvanian/Early Permian of the Southwestern United States (Carpenter, 1970; Olson, 1971, 1977; Fracasso, 1980; Rowland, 1997; Krainer and Lucas, 2004; Schneider et al., 2004; Lucas et al., 2005, 2010; Huttenlocker et al., 2018). Among these, tetrapod herbivores were greatly outnumbered by predators. Terrestrial insects were the primary terrestrial herbivores.
Type III communities overlapped with type I communities in time, but developed in upland, rather than lowland, settings. The communities were based on terrestrial primary productivity, which supported insect herbivores that were, in turn, preyed upon by small tetrapods. Olson considered type III communities to be rare (in large part due to the lack of favorable preservational conditions), but suggested that the Early Permian Fort Sill and the Middle Permian Mezen, Shikhovo-Chirki, and Belebei assemblages from Russia could represent type III communities (Olson, 1962, 1966, 1971). Olson variously hypothesized that the Middle Permian “caseid chronofauna” assemblages—so named for the overwhelming abundance of herbivorous caseids—were type II or type III communities (Olson and Beerbower, 1953; Olson, 1958, 1962, 1966, 1971).
Over the course of the Permian, type I and type III communities were replaced by “fully” terrestrial type II communities. The increased diversity of tetrapod herbivores created the necessary terrestrial food source to effectively reduce the dependence of terrestrial predators on aquatic or semi-aquatic prey, thereby shifting the energy base for the community to terrestrial primary productivity. Olson hypothesized that type II communities developed various combinations of type I and type III communities approximately by the Middle Permian. Olson’s first clear examples of type II communities are pareiasaur–therapsid assemblages from the Late Permian of Russia (Olson and Chudinov, 1992; Golubev, 2000; Benton et al., 2004; Tverdokhlebov et al., 2005; Shishkin, 2022a, b) and South Africa (Olson, 1965, 1966, 1971; Damiani, 2004; Day, 2013; Sidor et al., 2013; Fröbisch, 2014; Brocklehurst et al., 2017; Groenewald et al., 2019; Day and Rubidge, 2021; Prevec et al., 2022; Viglietti et al., 2022; Ronchi et al., 2023). Since the Late Permian, type II of various taxonomic compositions have gone on to dominate the terrestrial portion of the continental biosphere (Olson, 1966).
Substantial data have been added since Olson’s articulation of the above hypothesis. East Kirkton approximates a small type III community, albeit one based mostly on the exploitation of detritus rather than living plant matter (Clarkson et al., 1993). Such “pseudo-type III” communities might have been relatively common during the terrestrialization of arthropods (e.g., Gilboa) and tetrapods at least through the Early Pennsylvanian. New data from Richards Spur (=Fort Sill) (Anderson and Reisz, 2003; Anderson et al., 2009; Modesto et al., 2018b; Brink et al., 2019; deBraga et al., 2019; Gee et al., 2019; Gee, 2020; Hannibal and May, 2020), Bally Mountain (Fox and Bowman, 1966; LeBlanc et al., 2015; Goss, 2023), and Bromacker (Berman et al., 2000, 2014; Eberth et al., 2000) localities indicate the presence of type II communities in the Early Permian. In all three, tetrapod herbivores were a dominant component, chiefly captorhinomorphs (Richards Spur and Bally Mountain) or diadectids (Bromacker). Dental morphologies support high-fiber herbivory in both groups by the Early Permian (Hotton et al., 1997; Ponstein et al., 2024). The composition of these assemblages suggests that type II communities were present by the Early Permian and were less tied to the evolution of mammal-line tetrapods than Olson supposed.
At the same time, data from South America are difficult to interpret. Although records of Early Permian captorhinids (Cisneros et al., 2020), Middle Permian therapsids (Langer, 2000; Cisneros et al., 2012), and Late Permian temnospondyls (Piñero et al., 2007) suggest similar faunal turnover to that observed in Russia and South Africa (Cisneros et al., 2012), it is not clear that the same was the case for changes in community structure. For example, the Early Permian dvinosaur–captorhinid fauna from the Parnaíba Basin of Brazil (Iannuzzi et al., 2018; Cisneros et al., 2020; Marsicano et al., 2021; Richter et al., 2022) could represent a partially preserved type I or type II community, or another form of organization entirely.
Under Olson’s hypothesis, the change in community structure, either through transformation or replacement, was gradual rather than catastrophic. However, he did suggest that older community structures could reemerge:
“Later, during the Triassic, new versions of type I appeared once more. With the development of birds and mammals, the chances of such communities were reduced. Type I, however, is approximated in some of the largely fish-based communities that exist in subtropical swamp regions. The assemblages of the Florida Everglades, in their unmodified form, depart only moderately from this type of community.” (Olson, 1966, p. 296).
The aberrance of the Early Triassic continental biosphere in the aftermath of the End-Permian mass extinction (EPME) is well attested (Benton et al., 2004; Roopnarine et al., 2007, 2018, 2019; Irmis and Whiteside, 2012; Roopnarine and Angielczyk, 2012, 2015; Whiteside et al., 2015; Huang et al., 2021; Viglietti et al., 2022; Hoffman et al., 2023; Rey et al., 2023; Shishkin et al., 2023a, 2023; Pinheiro et al., 2024). In southern Africa, food web modeling found that Early Triassic communities were more unstable and vulnerable to collapse than those prior to the EPME (Roopnarine et al., 2007, 2018; Roopnarine and Angielczyk, 2012). The Early Triassic communities were greatly depleted in terrestrial herbivores and enriched in small terrestrial predators. Taxonomically, these communities were dominated by multiple clades of aquatic and terrestrial temnospondyls (Damiani and Rubidge, 2003; Roopnarine et al., 2007; Mehmood et al., 2025). Intense competition among predators, predation pressure on insect prey, and a lack of herbivores created a boom-and-bust dynamic in the community. These communities might represent type III communities, or a (partial) recapitulation of the type I structure (Huttenlocker, pers. comm.; Irmis, pers. comm.). In either case, it is possible that instability was a feature of these community structures that contributed to their marginalization by type II communities. Alternatively, these could represent dysfunctional, “incomplete” type II communities that have had their terrestrial component temporarily pared back by extinction, or type III communities alongside their aquatic counterparts/components. It is possible, then, that mass extinctions generally might not only degrade the structure of preexisting communities but also temporarily allow otherwise marginal forms of organization to proliferate.
8 Methods and hypothesis testing
Testing hypotheses of community-level selection and competition will be an important step toward reconciling paleontological and ecological modeling perspectives on the uniformity of processes over geologic time. Categorizing the autecology of fossil species—which is necessary to constraining their possible ecological interactions for the purpose of modeling (Bartley et al., 1993)—has been done across numerous studies, most of which focused on marine invertebrates (Bambach et al., 2007; Novack-Gottshall, 2007; Sahney et al., 2010b; Mondal and Harries, 2016; Reeves et al., 2020; Whalen et al., 2020). The categorical approach must necessarily balance precision (the ability to capture the autecology of a taxon) and breadth (the ability to be applied to taxa across space and time) and is best suited for larger datasets and cases in which autecology is difficult to infer from available data.
Once species autecologies have been established, the paleoecological community can be reconstructed from the fossil assemblage (Behrensmeyer, 1982; Bartley et al., 1993; Flessa, 2001). A paleoecological community can be analyzed as either a collection of the autecologies of its component organisms or as a network of interactions such as a food web (Dunne et al., 2008; Mitchell et al., 2012; Roopnarine and Angielczyk, 2015; Chevrinais et al., 2017; Codron et al., 2017; Roopnarine et al., 2018). When modeling the community as a food web, species–species feeding interactions can either be specified by the user (Dunne et al., 2004, 2008; Cortés and Larsson, 2023) or drawn stochastically from a set of possible interactions (Roopnarine, 2009; Mitchell et al., 2012; Roopnarine et al., 2018, 2019; Kempf et al., 2020; Huang et al., 2021, 2023). Both methods allow for model simplification, either through the collapsing of species with identical specified feeding relationships into trophic species or the collapsing of species with identical potential feeding relationships into guilds, respectively. The structure of the community can then be described, usually with ordinations and network statistics (Dunne et al., 2008; Chevrinais et al., 2017; Roopnarine et al., 2018; Banker et al., 2022), and compared to other communities in a dataset. Standard metrics include connectance, link density, and network trophic position (Table 1).
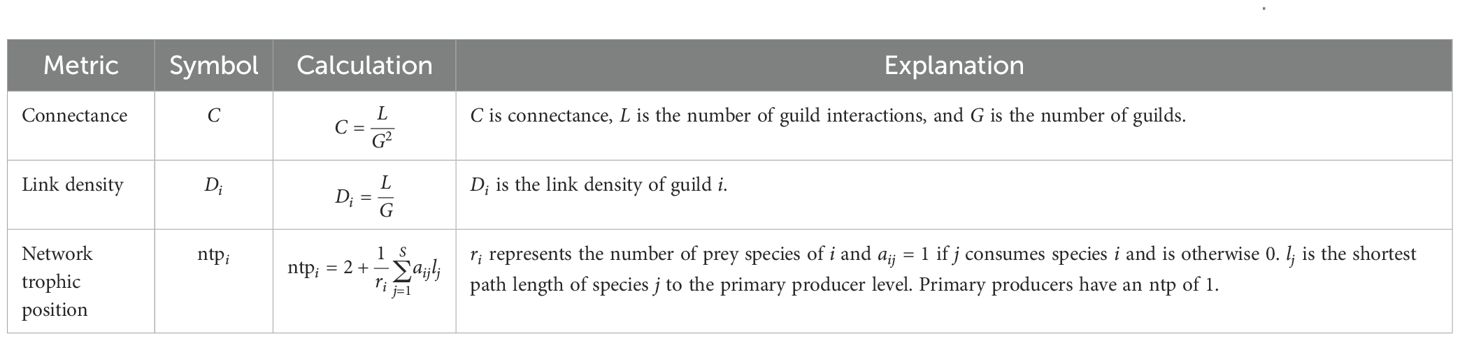
Table 1. Network statistics commonly calculated for food web studies. Formulae and explanations drawn from Banker et al., 2022.
Communities can be compared based not only on their structure and composition but also on their community-level (=emergent) properties. (Global) stability combines persistence (i.e., how long a species continues in a community before going extinct) with stable coexistence (i.e., the ability of species to coexist without external disruption) and can be expressed as the faction of species that can persist different conditions (Chen and Cohen, 2001). Resistance to perturbation is another metric (Roopnarine, 2009), which captures the proportion of species in the community that become extinct (populations decrease to zero) in response to perturbation (usually loss of primary productivity). Roopnarine et al. recently developed a method to compare the stability between both observed communities (those modeled on fossil data) and computer-generated communities of similar species richness but different trophic structure to test for community-level selection for stability (Roopnarine et al., 2019). Community-level properties are key to the question of whether or not (paleo)ecological communities evolve (Eldredge, 1985; Jablonski and Sepkoski, 1996; Bambach, 2001; Spiridonov and Eldredge, 2024).
An attempted synthesis of hypotheses of the changes in community properties and structure during Paleozoic continentalization is presented in Figures 1 and 2 (sources for graphics are presented in Tables 2 and 3). It can be divided into two phases: the first defined by the terrestrialization of plants and arthropods and the second defined by the terrestrialization of vertebrates (tetrapods). Possible community-level properties for selection are conceptually united as “performance.” More precise estimations are made here below.
Table 2. Credits for the silhouettes used in Figure 1.
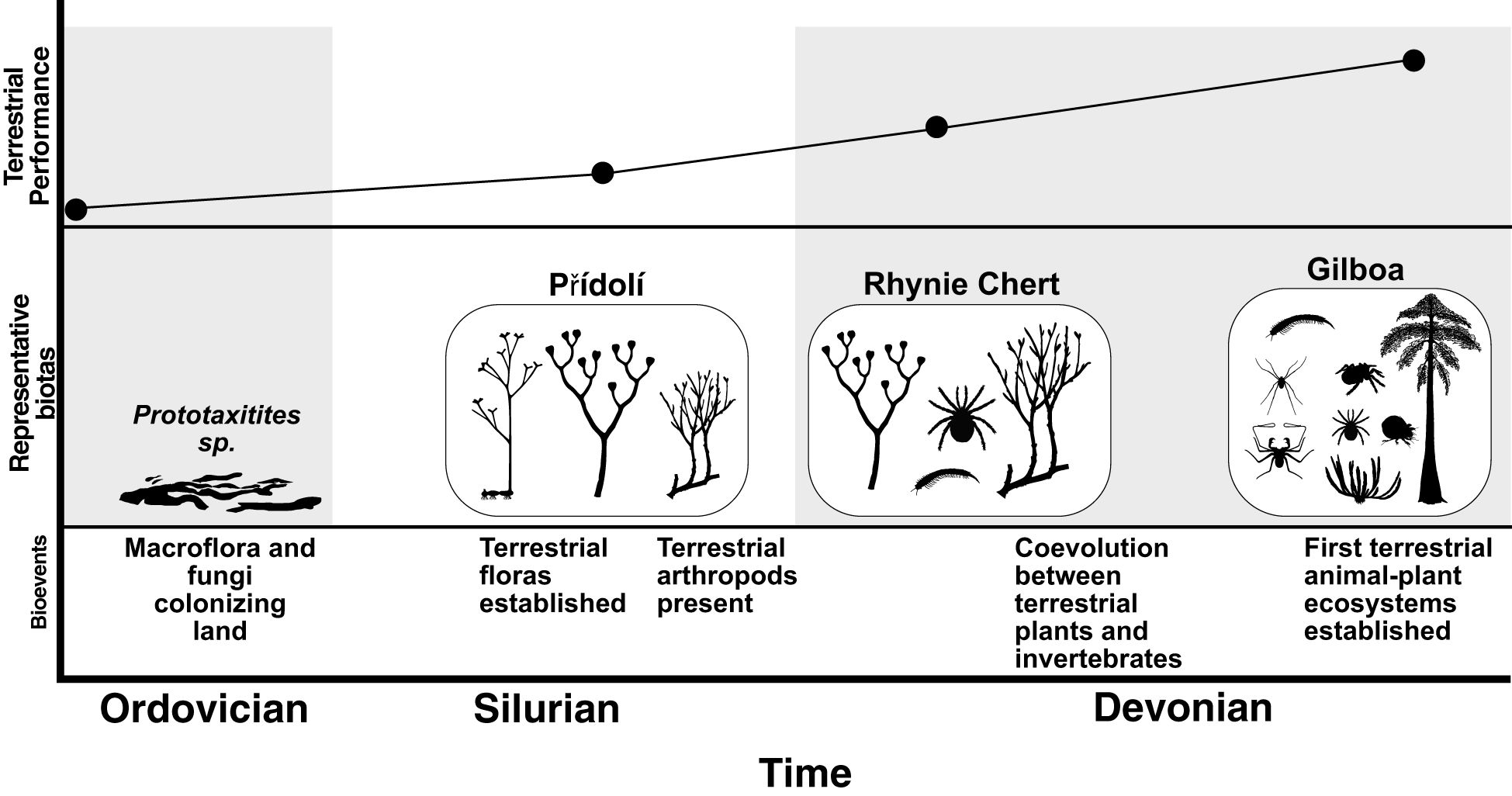
Figure 1. Hypothetical community performance curve during the origin and early development of terrestrial communities during the Ordovician–Devonian. Representative faunas are represented by silhouettes of component organisms (or analogues/equivalents): Přídolí (Libertín et al., 2018; Kraft et al., 2019; Pšenička et al., 2021), Rhynie Chert (Dunlop and Garwood, 2018; Long et al., 2024), and Gilboa (Shear et al., 1984, 1987; Norton et al., 1988; Shear and Bonamo, 1988; Stein et al., 2007, 2012, 2021). Silhouettes from PhyloPic. Individual contributors are listed in Table 2.
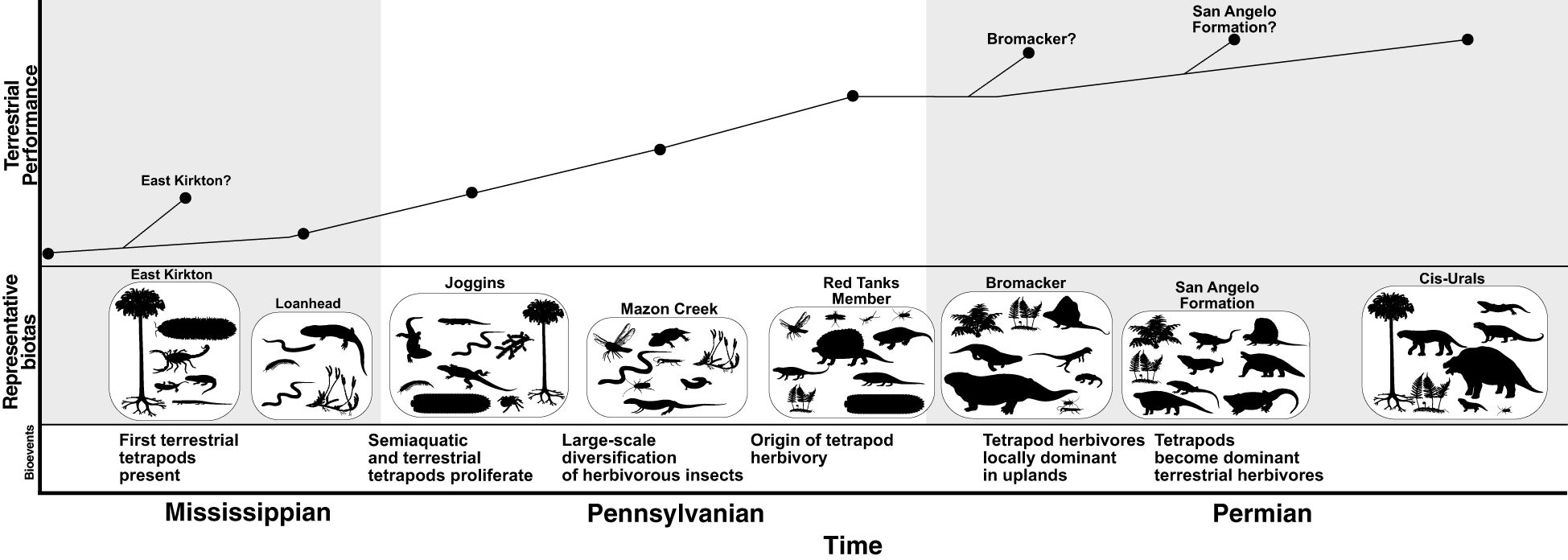
Figure 2. Hypothetical community performance curve during the origin and early development of terrestrial communities during the Mississippian–Permian. Representative faunas are represented by silhouettes of component organisms (or analogues/equivalents): East Kirkton (Clarkson et al., 1993), Joggins (Falcon-Lang, 2006; Falcon-Lang et al., 2006; Mann et al., 2020), Mazon Creek (Shabica and Hay, 1997; Mann and Gee, 2019; Mann et al., 2021a, b), Red Tanks Member (Rowland, 1997; Hannibal et al., 2004; Harris et al., 2004; Rasnitsyn et al., 2004; Schneider et al., 2004), Bromacker (Berman et al., 2000, 2014; Eberth et al., 2000), San Angelo Formation (Olson and Beerbower, 1953; Olson, 1966, 1971; Sidor and Hopson, 1998), and Cis-Urals (Olson, 1962, 1966; Tverdokhlebov et al., 2005; Naugolnykh et al., 2022). Silhouettes from PhyloPic. Individual contributors are listed in Table 3.
Table 3. Credits for the silhouettes used in Figure 2.
The first phase (Figure 2) was underway by the Ordovician and began with the colonization of land by plants, fungi, and arthropods. The terrestrial–terrestrial species feeding interaction strengths were initially outweighed by those of terrestrial–aquatic feeding interactions and, as during island colonization (Heatwole, 1981; Thornton, 2007), turnover was high and the stability of the terrestrial food web was likely low. Increased adaptation to the terrestrial environment continued until stable food webs could persist and replicate across the landscape. Arthropod exploitation of terrestrial detritus, fungi, and plants (Labandeira, 1997; Labandeira et al., 2014) was key in establishing a trophic guild of a fully terrestrial primary consumers. Interspecific competition increased (Vermeij and Dudley, 2000; DiMichele et al., 2023b), as did co-evolution: morphological evidence suggests that, by the Middle Devonian, terrestrial plants had developed chemical defenses against invertebrate herbivory (Labandeira et al., 2014). The Middle Devonian Gilboa assemblage represents the form of the oldest terrestrial community and the culmination of this first phase.
The second phase (Figure 2) began when the effect of vertebrate feeding across the water–land interface started to exert a significant effect on terrestrial food webs, reintensifying the aquatic–terrestrial interactions and effectively “de-terrestrializing” terrestrial communities by more fully integrating them with aquatic communities. This phase was preceded by the invasion of non-marine settings by vertebrates and was arguably a continuation of such. Effects on stability depend on hypotheses of tetrapod terrestrialization (see Section 6), particularly the role of miniaturization. Larger aquatic tetrapods would have been resistant to terrestrial predation and thus represent destabilizing pressure on the terrestrial sub-community. Small aquatic tetrapods, as prey for terrestrial predators (Mansky and Lucas, 2013), would have a stabilizing effect by transferring biomass from the aquatic to the terrestrial sub-community. This phase probably began sometime in the Late Devonian or the earliest Mississippian, but body fossil evidence has yet to be found. Regardless, it was well underway by the middle Mississippian (Clarkson et al., 1993; Garza et al., 2025), from which point on body size diversity was such that tetrapods exerted both effects to varying degrees across faunas. The stability and species carrying capacity of the terrestrial sub-community increased as terrestrial herbivorous insects diversified in the middle–late Pennsylvanian.
By the Early Permian, both of Olson’s type I and type II communities were present. Their relative community-level performance at that time is difficult to assess, in part due to the environmental differences between the two. The diversification of tetrapod herbivores almost certainly had a stabilizing effect, as seen in the natural experiment of their preferential removal in the EPME and scarcity in the protracted post-extinction recovery (Roopnarine et al., 2007, 2018, 2019; Roopnarine and Angielczyk, 2015). What does appear to be the case is that, by the middle Permian, terrestrial communities had (re)emerged as distinct from aquatic communities that contained semi-aquatic and/or terrestrial species. The apparent replacement of type I communities by type II communities in environments occupied by the former in the middle and late Permian, subsequent the global success of type II communities, and the marginalization of type I communities are consistent with type II communities being more stable and/or having a greater carrying capacity.
9 Conclusions
The origin of terrestrial ecosystems during the Paleozoic, part of the broader phenomenon of continentalization during this time, was a crucial episode in the development of the Earth’s biosphere. Since the middle 20th century, the pre-Permian fossil and geobiological records have improved dramatically (Clarkson et al., 1993; Coates et al., 2008; Knauth and Kennedy, 2009; Cressler et al., 2010; Clack, 2012; Kenrick et al., 2012; Gensel et al., 2020; Pšenička et al., 2021). Advances in biomechanics (Markey and Marshall, 2007a; Rayfield, 2007; Brainerd et al., 2010) and ecological modeling (Chen and Cohen, 2001; Dunne et al., 2002; Roopnarine, 2009; Villéger et al., 2011; Slatyer et al., 2013) have greatly expanded our capacity for reconstructing the ecology of fossil organisms and communities, respectively. Much of the incorporation of these techniques into paleontology is the result of increasing interdisciplinary and international collaboration.
Substantial data gaps and analytical challenges remain. Hypotheses of early tetrapod terrestrialization remain complicated by the incongruity between the body fossil and trackway records (Ahlberg, 2018; Long et al., 2025). While much more is now known from previously understudied regions, such as South America (Piñeiro et al., 2003, 2012; Piñero et al., 2007; Cisneros et al., 2012, 2015, 2020; Abrantes et al., 2016, 2019; Araújo et al., 2016; Iannuzzi et al., 2018), Southern Europe (Matamales-Andreu et al., 2022), China (Jun et al., 2014; Chen and Liu, 2020; Huang et al., 2021), and Africa (O’Keefe et al., 2005; Damiani et al., 2006; Steyer et al., 2006; Smiley et al., 2008; Sidor, 2013; Sidor et al., 2013, 2021, 2023; Tsuji et al., 2013; Smith et al., 2015; Turner et al., 2015; Peecook et al., 2017, 2021; Modesto et al., 2018a; Roopnarine et al., 2018), we still know much less about these areas during the Paleozoic than their North American and European counterparts. Reinvestigation of the latter in the context of current dating and stratigraphic schemes (Smith, 1974; Clack et al., 2016; Naugolnykh et al., 2022; Viglietti et al., 2022; Garza et al., 2025) is also warranted. This knowledge deficit hampers attempts to understand the temporal and geographic distributions of not only taxa but also forms of the ecological structure. This paper has focused on animals, especially tetrapods, but there is a pressing need to integrate the fossil records of vertebrates, invertebrates (both micro- and macroinvertebrates), and plants (Bartley et al., 1993; Falcon-Lang, 2006; Falcon-Lang et al., 2006; Iannuzzi and Labandeira, 2008; Sahney et al., 2010a; Stimson et al., 2013; van Hoof et al., 2013; Labandeira et al., 2014; Clack et al., 2016; Kearsey et al., 2016; Dunne et al., 2018; Schachat et al., 2018b, 2018; Bennett et al., 2021; Buatois et al., 2022; Whittingham et al., 2022, 2024; DiMichele et al., 2023a, 2023; Whittingham, 2023; Knecht et al., 2024).
Current community modeling methods have been applied to individual faunas (Dunne et al., 2008; Chevrinais et al., 2017) or biotic crisis intervals (Sidor et al., 2013; Roopnarine and Angielczyk, 2015; Roopnarine et al., 2018, 2019). Recent large-scale biogeographic datasets (Brocklehurst and Fröbisch, 2018; Brocklehurst et al., 2018; Pardo et al., 2019) could represent the foundation of comparative analyses that could test hypotheses such as those of Olson (Olson, 1952, 1966, 1971), Romer (Romer, 1958, 1974), and others (Sahney et al., 2010a; Mansky and Lucas, 2013; Ahlberg, 2018; Lucas, 2019) about the ecological context for evolutionary events and the development of communities themselves. Such investigations are work- and data-intensive, but have proven highly informative and productive (Sidor et al., 2013; Roopnarine and Angielczyk, 2015; Roopnarine et al., 2018, 2019; Angielczyk et al., 2020). Future studies of deep-time continentalization can begin to test hypotheses about the universality of ecological rules, the interactions between ecological and evolutionary processes on multiple scales (Spiridonov and Eldredge, 2024), and more.
Author contributions
BKAO: Conceptualization, Writing – review & editing, Investigation, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by a National Science Foundation Postdoctoral Research Fellowship in Biology (DBI-2209043).
Acknowledgments
I would like to thank the following people for useful discussions and comments on earlier versions of this manuscript and the material therein: Misha A.J.B. Whittingham, Peter D. Roopnarine, Kenneth D. Angielczyk, Jason D. Pardo, Tanner Frank, Sarah Sjoster, Danielle Fraser, Michael I. Coates, Tetsuto Miyashita, Matthew Stimson, Hillary Maddin, Eleanor E. Spence, Caleb P.W. Bohus, Andrew Traynor, Conrad Wilson, Caelan Libke, and Logan Micucci. This manuscript also benefitted from the careful attention and feedback of Antionio Garcia-Alix and three anonymous reviewers.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abrantes F. R., Nogueira A. C. R., Andrade L. S., Bandeira J., Soares J. L., and Medeiros R. S. P. (2019). Register of increasing continentalization and palaeoenvironmental changes in the west-central pangaea during the Permian-Triassic, Parnaíba Basin, Northern Brazil. J. South Am. Earth Sci. 93, 294–312. doi: 10.1016/j.jsames.2019.05.006
Abrantes F. R., Nogueira A. C. R., and Soares J. L. (2016). Permian paleogeography of west-central Pangea: Reconstruction using sabkha-type gypsum-bearing deposits of Parnaíba Basin, Northern Brazil. Sedimentary Geology 341, 175–188. doi: 10.1016/j.sedgeo.2016.06.004
Ahlberg P. E. (2018). Follow the footprints and mind the gaps: a new look at the origin of tetrapods. Earth Environ. Sci. Trans. R. Soc. Edinburgh 109, 115–137. doi: 10.1017/S1755691018000695
Ahlberg P. E. (2024). A new look at the origin of tetrapods (Rimouski, Quebec, Canada: International Symposium on Early and Lower Vertebrates).
Algeo T. J. and Scheckler S. E. (1998). Terrestrial-marine teleconnections in the Devonian: links between the evolution of land plants, weathering processes, and marine anoxic events. Phil. Trans. R. Soc Lond. B 353, 113–130. doi: 10.1098/rstb.1998.0195
Alroy J., Aberhan M., Bottjer D. J., Foote M., Fürsich F. T., Harries P. J., et al. (2008). Phanerozoic trends in the global diversity of marine invertebrates. Science 321, 97–100. doi: 10.1126/science.1156963
Alroy J., Marshall C. R., Bambach R. K., Bezusko K., Foote M., Fürsich F. T., et al. (2001). Effects of sampling standardization on estimates of Phanerozoic marine diversification. Proc. Natl. Acad. Sci. U.S.A. 98, 6261–6266. doi: 10.1073/pnas.111144698
Anderson J. S. and Reisz R. R. (2003). A new microsaur (Tetrapoda: Lepospondyli) from the Lower Permian of Richards Spur (Fort Sill), Oklahoma. Can. J. Earth Sci. 40, 499–505. doi: 10.1139/e02-066
Anderson J. S., Scott D., and Reisz R. R. (2009). Nannaroter mckinziei, a new ostodolepid ‘microsaur’ (Tetrapoda, Lepospondyli, Recumbirostra) from the Early Permian of Richards Spur (Ft. Sill), Oklahoma. J. Vertebrate Paleontology 29, 379–388. doi: 10.1671/039.029.0222
Anderson P. S. L., Friedman M., and Ruta M. (2013). Late to the table: diversification of tetrapod mandibular biomechanics lagged behind the evolution of terrestriality. Integr. Comp. Biol. 53, 197–208. doi: 10.1093/icb/ict006
Angielczyk K. D., Roopnarine P. D., Olroyd S. L., Kammerer C. F., and Pardo J. D. (2020). The changing roles of insect and tetrapod herbivores in promoting terrestrial ecosystem stability from the Pennsylvanian to the Triassic. (Online: Society of Vertebrate Paleontology).
Araújo R. N., Nogueira A. C. R., Bandeira J., and Angélica R. S. (2016). Shallow lacustrine system of the Permian Pedra de Fogo Formation, Western Gondwana, Parnaíba Basin, Brazil. J. South Am. Earth Sci. 67, 57–70. doi: 10.1016/j.jsames.2016.01.009
Assine M. L., de Santa Ana H., Veroslavsky G., and Vesely F. F. (2018). Exhumed subglacial landscape in Uruguay: Erosional landforms, depositional environments, and paleo-ice flow in the context of the late Paleozoic Gondwanan glaciation. Sedimentary Geology 369, 1–12. doi: 10.1016/j.sedgeo.2018.03.011
Bambach R. K. (2001). “Do communities evolve?,” in Palaeobiology II. Eds. Briggs D. E. G. and Crowther P. R. (Oxford, England, UK: Wiley), 437–440. doi: 10.1002/9780470999295.ch106
Bambach R. K., Bush A. M., and Erwin D. H. (2007). Autecology and the filling of ecospace: key metazoan radiations. Palaeontology 50, 1–22. doi: 10.1111/j.1475-4983.2006.00611.x
Bambach R. K., Knoll A. H., and Wang S. C. (2004). Origination, extinction, and mass depletions of marine diversity. Paleobiology 30, 522–542. doi: 10.1666/0094-8373(2004)030<0522:OEAMDO>2.0.CO;2
Banker R. M. W., Dineen A. A., Sorman M. G., Tyler C. L., and Roopnarine P. D. (2022). Beyond functional diversity: The importance of trophic position to understanding functional processes in community evolution. Front. Ecol. Evol. 10. doi: 10.3389/fevo.2022.983374
Bartley D., Behrensmeyer A. K., Damuth J. D., DiMichele W. A., Potts R., Sues H.-D., et al. (1993). Terrestrial ecosystems through time. J. Anim. Ecol. 62, 594. doi: 10.2307/5209
Beerbower R., Olson E. C., and Hotton N. (1992). The early development of tetrapod herbivory. Spec. publ. (Paleontol. Soc.) 6, 21–21. doi: 10.1017/S2475262200005815
Behrensmeyer A. K. (1982). Time resolution in fluvial vertebrate assemblages. Paleobiology 8, 211–227. doi: 10.1017/S0094837300006941
Bennett C. E., Howard A. S., Davies S. J., Kearsey T. I., Millward D., Brand P. J., et al. (2017). Ichnofauna record cryptic marine incursions onto a coastal floodplain at a key Lower Mississippian tetrapod site. Palaeogeography Palaeoclimatology Palaeoecol. 468, 287–300. doi: 10.1016/j.palaeo.2016.12.018
Bennett C. E., Kearsey T. I., Davies S. J., Leng M. J., Millward D., Smithson T. R., et al. (2021). Palaeoecology and palaeoenvironment of Mississippian coastal lakes and marshes during the early terrestrialisation of tetrapods. Palaeogeography Palaeoclimatology Palaeoecol. 564, 110194. doi: 10.1016/j.palaeo.2020.110194
Bennett C. E., Siveter D. J., Davies S. J., Williams M., Wilkinson I. P., Browne M., et al. (2012). Ostracods from freshwater and brackish environments of the Carboniferous of the Midland Valley of Scotland: the early colonization of terrestrial water bodies. Geological Magazine 149, 366–396. doi: 10.1017/S0016756811000719
Benton M. J., Tverdokhlebov V. P., and Surkov M. V. (2004). Ecosystem remodelling among vertebrates at the Permian–Triassic boundary in Russia. Nature 432, 97–100. doi: 10.1038/nature02950
Berman D. S., Henrici A. C., Sumida S. S., and Martens T. (2000). Redescription of Seymouria sanjuanensis (Seymouriamorpha) from the Lower Permian of Germany based on complete, mature specimens with a discussion of paleoecology of the Bromacker locality assemblage. J. Vertebrate Paleontology 20, 253–268. doi: 10.1671/0272-4634(2000)020[0253:ROSSSF]2.0.CO;2
Berman D. S., Henrici A. C., Sumida S. S., Martens T., and Pelletier V. (2014). “First european record of a varanodontine (Synapsida: varanopidae): member of a unique early permian upland paleoecosystem, tambach basin, central Germany,” in Early Evolutionary History of the Synapsida. Eds. Kammerer C. F., Angielczyk K. D., and Fröbisch J. (Springer Netherlands, Dordrecht), 69–86. doi: 10.1007/978-94-007-6841-3_5
Berry C. M. and Marshall J. E. A. (2015). Lycopsid forests in the early Late Devonian paleoequatorial zone of Svalbard. Geology 43, 1043–1046. doi: 10.1130/G37000.1
Beutel R. G., Yan E. V., and Kukalová-Peck J. (2019). Is †Skleroptera (†Stephanastus) an order in the stemgroup of Coleopterida (Insecta)? Insect Syst. Evol. 50, 670–678. doi: 10.1163/1876312X-00002187
Blanco F., Calatayud J., Martín-Perea D. M., Domingo M. S., Menéndez I., Müller J., et al. (2021). Punctuated ecological equilibrium in mammal communities over evolutionary time scales. Science 372, 300–303. doi: 10.1126/science.abd5110
Bolt J. R. and Lombard R. E. (2010). Deltaherpeton hiemstrae, a new colosteid tetrapod from the Mississippian of Iowa. J. Paleontology 84, 1135–1151. doi: 10.1666/10-020.1
Boudinot B. E., Yan E. V., Prokop J., Luo X., and Beutel R. G. (2023). Permian parallelisms: Reanalysis of †Tshekardocoleidae sheds light on the earliest evolution of the Coleoptera. Systematic Entomology 48, 69–96. doi: 10.1111/syen.12562
Boyer D. L., Martinez A. M., Evans S. D., Cohen P. A., Haddad E. E., Pippenger K. H., et al. (2021). Living on the edge: The impact of protracted oxygen stress on life in the Late Devonian. Palaeogeography Palaeoclimatology Palaeoecol. 566, 110226. doi: 10.1016/j.palaeo.2021.110226
Brainerd E. L., Baier D. B., Gatesy S. M., Hedrick T. L., Metzger K. A., Gilbert S. L., et al. (2010). X-ray reconstruction of moving morphology (XROMM): precision, accuracy and applications in comparative biomechanics research. J. Exp. Zoology Part A: Ecol. Genet. Physiol. 313, 262–279. doi: 10.1002/jez.589
Briggs D. E. G. (2015). The cambrian explosion. Curr. Biol. 25, R864–R868. doi: 10.1016/j.cub.2015.04.047
Brink K. S., MacDougall M. J., and Reisz R. R. (2019). Dimetrodon (Synapsida: Sphenacodontidae) from the cave system at Richards Spur, OK, USA, and a comparison of Early Permian–aged vertebrate paleoassemblages. Sci. Nat. 106, 1–10. doi: 10.1007/s00114-018-1598-1
Brocklehurst N. and Brink K. S. (2017). Selection towards larger body size in both herbivorous and carnivorous synapsids during the Carboniferous. FACETS 2, 68–84. doi: 10.1139/facets-2016-0046
Brocklehurst N., Day M. O., Rubidge B. S., and Fröbisch J. (2017). Olson’s Extinction and the latitudinal biodiversity gradient of tetrapods in the Permian. Proc. R. Soc. B: Biol. Sci. 284, 20170231. doi: 10.1098/rspb.2017.0231
Brocklehurst N., Dunne E. M., Cashmore D. D., and Fröbisch J. (2018). Physical and environmental drivers of Paleozoic tetrapod dispersal across Pangaea. Nat. Commun. 9, 1–12. doi: 10.1038/s41467-018-07623-x
Brocklehurst N. and Fröbisch J. (2018). The definition of bioregions in palaeontological studies of diversity and biogeography affects interpretations: palaeozoic tetrapods as a case study. Front. Earth Sci. 6, 200. doi: 10.3389/feart.2018.00200
Brocklehurst N., Kammerer C. F., and Benson R. J. (2020). The origin of tetrapod herbivory: effects on local plant diversity. Proc. R. Soc B 287, 20200124. doi: 10.1098/rspb.2020.0124
Buatois L. A., Carmona N. B., Curran H. A., Netto R. G., Mángano M. G., and Wetzel A. (2016). “The mesozoic marine revolution,” in The Trace-Fossil Record of Major Evolutionary Events. Eds. Mángano M. G. and Buatois L. A. (Springer Netherlands, Dordrecht), 449–473. doi: 10.1007/978-94-017-9597-5_9
Buatois L. A., Davies N. S., Gibling M. R., Krapovickas V., Labandeira C. C., MacNaughton R. B., et al. (2022). The invasion of the land in deep time: integrating paleozoic records of paleobiology, ichnology, sedimentology, and geomorphology. Integr. Comp. Biol. 62, 297–331. doi: 10.1093/icb/icac059
Burke P. J. C., Mayer P. S., and McCoy V. E. (2024). Mazon Creek fossils brought to you by coal, concretions and collectors. SP 543, SP543-2022–317. doi: 10.1144/SP543-2022-317
Bush A. M. and Bambach R. K. (2004). Did alpha diversity increase during the phanerozoic? Lifting the veils of taphonomic, latitudinal, and environmental biases. J. Geology 112, 625–642. doi: 10.1086/424576
Bush A. M., Markey M. J., and Marshall C. R. (2004). Removing bias from diversity curves: the effects of spatially organized biodiversity on sampling-standardization. Paleobiology 30, 666–686. doi: 10.1666/0094-8373(2004)030<0666:RBFDCT>2.0.CO;2
Bush A. M. and Novack-Gottshall P. M. (2012). Modelling the ecological-functional diversification of marine Metazoa on geological time scales. Biol. Lett. 8, 151–155. doi: 10.1098/rsbl.2011.0641
Canoville A. and Laurin M. (2010). Evolution of humeral microanatomy and lifestyle in amniotes, and some comments on palaeobiological inferences. Biol. J. Linn. Soc. 100, 384–406. doi: 10.1111/j.1095-8312.2010.01431.x
Carpenter F. M. (1970). Fossil insects from New Mexico. Psyche: A J. Entomology 77, 400–412. doi: 10.1155/1970/84847
Cauvy-Fraunié S. and Dangles O. (2019). A global synthesis of biodiversity responses to glacier retreat. Nat. Ecol. Evol. 3, 1675–1685. doi: 10.1038/s41559-019-1042-8
Chen X. and Cohen J. E. (2001). Global stability, local stability and permanence in model food webs. J. Theor. Biol. 212, 223–235. doi: 10.1006/jtbi.2001.2370
Chen J. and Liu J. (2020). The youngest occurrence of embolomeres (Tetrapoda: Anthracosauria) from the Sunjiagou Formation (Lopingian, Permian) of North China. Foss. Rec 23, 205–213. doi: 10.5194/fr-23-205-2020
Cherns L. and Wright V. P. (2000). Missing molluscs as evidence of large-scale, early skeletal aragonite dissolution in a Silurian sea. Geology 28, 711–794. doi: 10.1130/0091-7613(2000)28<791:MMAEOL>2.0.CO;2
Chevrinais M., Jacquet C., and Cloutier R. (2017). Early establishment of vertebrate trophic interactions: Food web structure in Middle to Late Devonian fish assemblages with exceptional fossilization. Bull. Geosciences 92, 491–510. doi: 10.3140/bull.geosci.1651
Cisneros J. C., Abdala F., Atayman-Güven S., Rubidge B. S., Şengör A. M. C., and Schultz C. L. (2012). Carnivorous dinocephalian from the Middle Permian of Brazil and tetrapod dispersal in Pangaea. Proc. Natl. Acad. Sci. U.S.A 109, 1584–1588. doi: 10.1073/pnas.1115975109
Cisneros J. C., Angielczyk K., Kammerer C. F., Smith R. M. H., Fröbisch J., Marsicano C. A., et al. (2020). Captorhinid reptiles from the lower Permian Pedra de Fogo Formation, Piauí, Brazil: the earliest herbivorous tetrapods in Gondwana. PeerJ 8, e8719. doi: 10.7717/peerj.8719
Cisneros J. C., Marsicano C., Angielczyk K. D., Smith R. M. H., Richter M., Fröbisch J., et al. (2015). New Permian fauna from tropical Gondwana. Nat. Commun. 6, 1–8. doi: 10.1038/ncomms9676
Clack J. A. (2009). The fin to limb transition: new data, interpretations, and hypotheses from paleontology and developmental biology. Annu. Rev. Earth Planetary Sci. 37, 163–179. doi: 10.1146/annurev.earth.36.031207.124146
Clack J. A. (2012). Gaining Ground: The Origin and Evolution of Tetrapods. 2nd ed (Bloomington: Indiana University Press).
Clack J. A., Bennett C. E., Carpenter D. K., Davies S. J., Fraser N. C., Kearsey T. I., et al. (2016). Phylogenetic and environmental context of a Tournaisian tetrapod fauna. Nat. Ecol. Evol. 1, 2. doi: 10.1038/s41559-016-0002
Clack J. A., Ruta M., Milner A. R., Marshall J. E. A., Smithson T. R., and Smithson K. Z. (2019). Acherontiscus caledoniae: the earliest heterodont and durophagous tetrapod. R. Soc. Open Sci. 6, 182087. doi: 10.1098/rsos.182087
Clarke J. T., Warnock R. C. M., and Donoghue P. C. J. (2011). Establishing a time‐scale for plant evolution. New Phytol. 192, 266–301. doi: 10.1111/j.1469-8137.2011.03794.x
Clarkson E. N. K. (1985). Palaeoecology of the dinantian of foulden, berwickshire, Scotland. Trans. R. Soc. Edinburgh: Earth Sci. 76, 97–100. doi: 10.1017/S0263593300010336
Clarkson E. N. K., Milner A. R., and Coates M. I. (1993). Palaeoecology of the viséan of East Kirkton, West Lothian, Scotland. Trans. R. Soc. Edinburgh: Earth Sci. 84, 417–425. doi: 10.1017/S0263593300006210
Clements T., Purnell M., and Gabbott S. (2019). The Mazon Creek Lagerstätte: a diverse late Paleozoic ecosystem entombed within siderite concretions. J. Geological Soc. 176, 1–11. doi: 10.1144/jgs2018-088
Cloutier R., Clement A. M., Lee M. S. Y., Noël R., Béchard I., Roy V., et al. (2020). Elpistostege and the origin of the vertebrate hand. Nature 579, 549–554. doi: 10.1038/s41586-020-2100-8
Coates M. I., Ruta M., and Friedman M. (2008). Ever since owen: changing perspectives on the early evolution of tetrapods. Annu. Rev. Ecology Evolution Systematics 39, 571–592. doi: 10.1146/annurev.ecolsys.38.091206.095546
Codron J., Botha-Brink J., Codron D., Huttenlocker A. K., and Angielczyk K. D. (2017). Predator-prey interactions amongst Permo-Triassic terrestrial vertebrates as a deterministic factor influencing faunal collapse and turnover. J. Evolutionary Biol. 30, 40–54. doi: 10.1111/jeb.12983
Cortés D. and Larsson H. C. E. (2023). Top of the food chains: an ecological network of the marine Paja Formation biota from the Early Cretaceous of Colombia reveals the highest trophic levels ever estimated. Zoological J. Linn. Soc. 202, zlad092. doi: 10.1093/zoolinnean/zlad092
Cressler W. L., Daeschler E. B., Slingerland R., and Peterson D. A. (2010). Terrestrialization in the Late Devonian: a palaeoecological overview of the Red Hill site, Pennsylvania, USA. Geological Society London Special Publications 339, 111–128. doi: 10.1144/SP339.10
Crisafulli C. M. and Dale V. H. (Eds.) (2018). Ecological Responses at Mount St. Helens: Revisited 35 years after the 1980 Eruption (New York, NY: Springer New York). doi: 10.1007/978-1-4939-7451-1
Crockford P. W., Bar On Y. M., Ward L. M., Milo R., and Halevy I. (2023). The geologic history of primary productivity. Curr. Biol. 33, 4741–4750.e5. doi: 10.1016/j.cub.2023.09.040
Cucherousset J., Boulêtreau S., Azémar F., Compin A., Guillaume M., and Santoul F. (2012). Freshwater killer whales”: beaching behavior of an alien fish to hunt land birds. PloS One 7, e50840. doi: 10.1371/journal.pone.0050840
Damiani R. J. (2004). Temnospondyls from the beaufort group (Karoo basin) of South Africa and their biostratigraphy. Gondwana Res. 7, 165–173. doi: 10.1016/S1342-937X(05)70315-4
Damiani R. J. and Rubidge B. S. (2003). A review of the South African temnospondyl amphibian record. Palaeontologica Africana 39, 21–36.
Damiani Ro., Sidor C. A., Steyer J. S., Smith R. M. H., Larsson H. C. E., Maga A., et al. (2006). The vertebrate fauna of the Upper Permian of Niger—V. The primitive temnospondyl Saharastega moradiensis. J. Vertebrate Paleontology 26, 559–572. doi: 10.1080/02724634.2006.10010015
Darroch S. A. F., Cribb A. T., Buatois L. A., Germs G. J. B., Kenchington C. G., Smith E. F., et al. (2021). The trace fossil record of the Nama Group, Namibia: Exploring the terminal Ediacaran roots of the Cambrian explosion. Earth-Science Rev. 212, 103435. doi: 10.1016/j.earscirev.2020.103435
Day M. (2013). Charting the fossils of the Great Karoo: a history of tetrapod biostratigraphy in the Lower Beaufort Group, South Africa. Palaeontologica Africana 48, 41–47.
Day M. O. and Rubidge B. S. (2021). The late capitanian mass extinction of terrestrial vertebrates in the karoo basin of South Africa. Front. Earth Sci. 9. doi: 10.3389/feart.2021.631198
deBraga M., Bevitt J. J., and Reisz R. R. (2019). A new captorhinid from the permian cave system near richards spur, oklahoma, and the taxic diversity of captorhinus at this locality. Front. Earth Sci. 7. doi: 10.3389/feart.2019.00112
deBraga M. and Reisz R. R. (1995). A new diapsid reptile from the uppermost Carboniferous (Stephanian) of Kansas. Palaeontology 38, 199–212.
DiMichele W. A., Behrensmeyer A. K., Olszewski T. D., Labandeira C. C., Pandolfi J. M., Wing S. L., et al. (2004). Long-term stasis in ecological assemblages: evidence from the fossil record. Annu. Rev. Ecol. Evol. Syst. 35, 285–322. doi: 10.1146/annurev.ecolsys.35.120202.110110
DiMichele W. A., Eble C. F., Pfefferkorn H. W., Elrick S. D., Nelson W. J., and Lucas S. G. (2023a). Kasimovian floristic change in tropical wetlands and the Middle–Late Pennsylvanian Boundary Event. SP 535, 293–335. doi: 10.1144/SP535-2022-228
DiMichele W. A., Hotton C. L., Labandeira C. C., and Sues H.-D. (2023b). A paleontological perspective on ecosystem assembly rules in the Paleozoic terrestrial realm. Evolving Earth 1, 100020. doi: 10.1016/j.eve.2023.100020
Donovan M. P., Schachat S. R., and Monarrez P. M. (2023). Ecological and evolutionary responses of terrestrial arthropods to Middle–Late Pennsylvanian environmental change. SP 535, SP535-2022–209. doi: 10.1144/SP535-2022-209
Droser M. L., Jensen S., and Gehling J. G. (2002). Trace fossils and substrates of the terminal Proterozoic–Cambrian transition: Implications for the record of early bilaterians and sediment mixing. Proc. Natl. Acad. Sci. U.S.A 99, 12572–12576. doi: 10.1073/pnas.202322499
Dudei N. L. and Stigall A. L. (2010). Using ecological niche modeling to assess biogeographic and niche response of brachiopod species to the Richmondian Invasion (Late Ordovician) in the Cincinnati Arch. Palaeogeography Palaeoclimatology Palaeoecol. 296, 28–43. doi: 10.1016/j.palaeo.2010.06.012
Dunlop J. A. (1996). A trigonotarbid arachnid from the Upper Silurian of Shropshire. Palaeontology. 39, 605–614.
Dunlop J. A. and Garwood R. J. (2018). Terrestrial invertebrates in the Rhynie chert ecosystem. Phil. Trans. R. Soc B 373, 20160493. doi: 10.1098/rstb.2016.0493
Dunlop J. A. and Selden P. A. (2013). Scorpion fragments from the silurian of powys, wales. Arachnology 16, 27–32. doi: 10.13156/arac.2013.16.1.27
Dunne E. M., Close R. A., Button D. J., Brocklehurst N., Cashmore D. D., Lloyd G. T., et al. (2018). Diversity change during the rise of tetrapods and the impact of the ‘Carboniferous rainforest collapse’. Proc. R. Soc. B: Biol. Sci. 285, 20172730. doi: 10.1098/rspb.2017.2730
Dunne J. A., Williams R. J., and Martinez N. D. (2002). Network topology and biodiversity loss in food webs: robustness increases with connectance. Ecol. Lett. 5, 558–567. doi: 10.1046/j.1461-0248.2002.00354.x
Dunne J. A., Williams R., and Martinez N. (2004). Network structure and robustness of marine food webs. Mar. Ecol. Prog. Ser. 273, 291–302. doi: 10.3354/meps273291
Dunne J. A., Williams R. J., Martinez N. D., Wood R. A., and Erwin D. H. (2008). Compilation and network analyses of cambrian food webs. PloS Biol. 6, e102. doi: 10.1371/journal.pbio.0060102
Eberth D. A., Berman D. S., Sumida S. S., and Hoff H. (2000). Lower permian terrestrial paleoenvironments and vertebrate paleoecology of the tambach basin (Thuringia, central Germany): the upland holy grail. PALAIOS 15, 293–313. doi: 10.1669/0883-1351(2000)015<0293:LPTPAV>2.0.CO;2
Edwards D., Kenrick P., and Dolan L. (2018). History and contemporary significance of the Rhynie cherts—our earliest preserved terrestrial ecosystem. Phil. Trans. R. Soc B 373, 20160489. doi: 10.1098/rstb.2016.0489
Edwards D. and Wellman C. (2001). “2. Embryophytes on land: the ordovician to lochkovian (Lower devonian) record,” in Plants Invade the Land. Eds. Gensel P. G. and Edwards D. (New York City, New York, USA: Columbia University Press), 3–28. doi: 10.7312/gens11160-003
Eldredge N. (1985). Unfinished synthesis: biological hierarchies and modern evolutionary thought (New York: Oxford University Press).
Esteve-Altava B., Pierce S. E., Molnar J. L., Johnston P., Diogo R., and Hutchinson J. R. (2019). Evolutionary parallelisms of pectoral and pelvic network-anatomy from fins to limbs. Sci. Adv. 5, 1–12. doi: 10.1126/sciadv.aau7459
Falcon-Lang H. J. (2006). A history of research at the Joggins Fossil Cliffs of Nova Scotia, Canada, the world’s finest Pennsylvanian section. Proc. Geologists’ Assoc. 117, 377–392. doi: 10.1016/S0016-7878(06)80044-1
Falcon-Lang H. J., Benton M. J., Braddy S. J., and Davies S. J. (2006). The Pennsylvanian tropical biome reconstructed from the Joggins Formation of Nova Scotia, Canada. J. Geological Soc. 163, 561–576. doi: 10.1144/0016-764905-063
Felice R. N. and Angielczyk K. D. (2014). “Was ophiacodon (Synapsida, eupelycosauria) a swimmer? A test using vertebral dimensions,” in Early Evolutionary History of the Synapsida. Eds. Kammerer C. F., Angielczyk K. D., and Fröbisch J. (Springer Netherlands, Dordrecht), 25–51. doi: 10.1007/978-94-007-6841-3_3
Ficetola G. F., Marta S., Guerrieri A., Cantera I., Bonin A., Cauvy-Fraunié S., et al. (2024). The development of terrestrial ecosystems emerging after glacier retreat. Nature 632, 336–342. doi: 10.1038/s41586-024-07778-2
Finnegan S., McClain C. M., Kosnik M. A., and Payne J. L. (2011). Escargots through time: an energetic comparison of marine gastropod assemblages before and after the Mesozoic Marine Revolution. Paleobiology 37, 252–269. doi: 10.1666/09066.1
Flessa K. W. (2001). “Time‐Averaging,” in Palaeobiology II. Eds. Briggs D. E. G. and Crowther P. R. (Oxford, England, UK: Wiley), 292–296. doi: 10.1002/9780470999295.ch66
Flynn J. J., MacFadden B. J., and McKenna M. C. (1984). Land-mammal ages, faunal heterochrony, and temporal resolution in cenozoic terrestrial sequences. J. Geology 92, 687–705. doi: 10.1086/628906
Fox R. C. and Bowman M. C. (1966). “Osteology and relationships of Captorhinus aguti (Cope) (Reptilia: Captorhinomorpha),” in Vertebrata (Article 11) (University of Kansas Paleontological Contributions) 11, 1–79.
Fracasso M. A. (1980). Age of the permo-carboniferous cutler formation vertebrate fauna from el cobre canyon, New Mexico. J. Paleontology 54, 1237–1244.
Fraser D. and Lyons S. K. (2020). Mammal community structure through the paleocene-eocene thermal maximum. Am. Nat. 196, 271–290. doi: 10.1086/709819
Fröbisch J. (2014). “Synapsid diversity and the rock record in the permian-triassic beaufort group (Karoo supergroup), South Africa,” in Early Evolutionary History of the Synapsida. Eds. Kammerer C. F., Angielczyk K. D., and Fröbisch J. (Springer Netherlands, Dordrecht), 305–319. doi: 10.1007/978-94-007-6841-3_18
Garza H. K., Catlos E. J., Lapen T. J., Clarke J. A., and Brookfield M. E. (2025). New U-Pb constraints and geochemistry of the East Kirkton Quarry, Scotland: Implications for early tetrapod evolution in the Carboniferous. PloS One 20, e0321714. doi: 10.1371/journal.pone.0321714
Gee B. M. (2020). Ecology, ontogeny, and taxonomy of the diverse early Permian dissorophoid assemblage from Richards Spur (Oklahoma. Ontario, Canada: University of Toronto).
Gee B. M., Bevitt J. J., and Reisz R. R. (2019). Dissorophid diversity at the early Permian cave system near Richards Spur, Oklahoma, USA. Palaeontol Electron 22, 1–32. doi: 10.26879/976
Gensel P. G., Glasspool I., Gastaldo R. A., Libertin M., and Kvaček J. (2020). “Back to the beginnings: the silurian-devonian as a time of major innovation in plants and their communities,” in Nature through Time. Eds. Martinetto E., Tschopp E., and Gastaldo R. A. (Springer International Publishing, Cham), 367–398. doi: 10.1007/978-3-030-35058-1_15
Gerrienne P., Meyer-Berthaud B., and Fairon-Demaret M. (2005). The significance of Runcaria (Middle Devonian, Belgium) in the evolution of seed plants. Carnets géologie (Notebooks geology). 2, 15–19. doi: 10.4267/2042/4357
Gerrienne P., Meyer-Berthaud B., Fairon-Demaret M., Streel M., and Steemans P. (2004). Runcaria, a middle devonian seed plant precursor. Science 306, 856–858. doi: 10.1126/science.1102491
Gess R. W. (2013). The earliest record of terrestrial animals in gondwana: A scorpion from the famennian (Late devonian) witpoort formation of South Africa. Afr. Invertebrates 54, 373–379. doi: 10.5733/afin.054.0206
Gess R. W. and Prestianni C. (2021). An early Devonian flora from the Baviaanskloof Formation (Table Mountain Group) of South Africa. Sci. Rep. 11 95, 11859. doi: 10.1038/s41598-021-90180-z
Gess R. W. and Whitfield A. K. (2020). Estuarine fish and tetrapod evolution: insights from a Late Devonian (Famennian) Gondwanan estuarine lake and a southern African Holocene equivalent. Biol. Rev., brv.12590. doi: 10.1111/brv.12590
Godfrey S. J. (1988). Isolated tetrapod remains from the Carboniferous of West Virginia. Kirtlandia, 27–36.
Goedert J., Lécuyer C., Amiot R., Arnaud-Godet F., Wang X., Cui L., et al. (2018). Euryhaline ecology of early tetrapods revealed by stable isotopes. Nature 558, 68–72. doi: 10.1038/s41586-018-0159-2
Gogáin A.Ó., Falcon-Lang H. J., Carpenter D. K., Miller R. F., Benton M. J., Pufahl P. K., et al. (2016). Fish and tetrapod communities across a marine to brackish salinity gradient in the Pennsylvanian (early Moscovian) Minto Formation of New Brunswick, Canada, and their palaeoecological and palaeogeographical implications. Palaeontology 59, 689–724. doi: 10.1111/pala.12249
Golubev V. K. (2000). The faunal assemblages of permian terrestrial vertebrates from Eastern Europe. Palaeontological J. 34, S211–S224.
Goss G. (2023). Analysis of Early Permianfauna of the upland region Bally Mountain from collected blocks (Fort Worth, Texas, USA: Texas Christian University).
Gray J. (1988). Evolution of the freshwater ecosystem: The fossil record. Palaeogeography Palaeoclimatology Palaeoecol. 62, 1–214. doi: 10.1016/0031-0182(88)90054-5
Greb S. F., DiMichele W. A., and Gastaldo R. A. (2006). “Evolution and importance of wetlands in earth history,” in Wetlands through Time (Boulder, Colorado, USA: Geological Society of America). doi: 10.1130/2006.2399(01
Grimaldi D. A. and Engel M. S. (2005). Evolution of the insects (Cambridge [U.K.] ; New York: Cambridge University Press).
Groenewald D. P., Day M. O., and Rubidge B. S. (2019). Vertebrate assemblages from the north-central Main Karoo Basin, South Africa, and their implications for mid-Permian biogeography. LET 52, 486–501. doi: 10.1111/let.12326
Gueriau P., Rabet N., Clément G., Lagebro L., Vannier J., Briggs D. E. G., et al. (2016). A 365-million-year-old freshwater community reveals morphological and ecological stasis in branchiopod crustaceans. Curr. Biol. 26, 383–390. doi: 10.1016/j.cub.2015.12.039
Habgood K. S., Hass H., and Kerp H. (2003). Evidence for an early terrestrial food web: coprolites from the Early Devonian Rhynie chert. Trans. R. Soc. Edinburgh: Earth Sci. 94, 371–389. doi: 10.1017/S0263593300000754
Hannibal J. T., Lerner A. J., Zeigler K. E., and Lucas S. G. (2004). A juliform milliped from the Upper Pennsylvanian (Virgilian) Bursum Formation, Carrizo Arroyo, of central New Mexico. New Mexico Museum Natural History Sci. Bull. 25, 211–214.
Hannibal J. T. and May W. J. (2020). Permian millipedes from the Fort Sill fissures of southwestern Oklahoma, with comments on allied taxa and millipedes preserved in karstic environments. J. Paleontol. 95, 586–600. doi: 10.1017/jpa.2020.100
Harris S. K., Lucas S. G., Berman D. S., and Henrici A. C. (2004). Vertebrate fossil assemblage from the Upper Pennsylvanian Red Tanks Member of the Bursum Formation, Lucero Uplift, central New Mexico. New Mexico Museum Natural History Sci. Bull. 25, 267–284.
Herbst E. C. and Hutchinson J. R. (2018). New insights into the morphology of the Carboniferous tetrapod Crassigyrinus scoticus from computed tomography. Earth Environ. Sci. Trans. R. Soc. Edinburgh 109, 157–175. doi: 10.1017/S1755691018000804
Hoffman D. K., Hancox J. P., and Nesbitt S. J. (2023). A diverse diapsid tooth assemblage from the Early Triassic (Driefontein locality, South Africa) records the recovery of diapsids following the end-Permian mass extinction. PloS One 18, e0285111. doi: 10.1371/journal.pone.0285111
Hook R. W. and Baird D. (1986). The diamond coal mine of linton, ohio, and its pennsylvanian-age vertebrates. J. Vertebrate Paleontology 6, 174–190. doi: 10.1080/02724634.1986.10011609
Hook R. W. and Baird D. (1993). A new fish and tetrapod assemblage from the Allegheny group (late Westphalian, upper Carboniferous) of eastern Ohio, USA. Pollichia-Buch 29, 141–154.
Horodyski R. J. and Knauth L. P. (1994). Life on land in the precambrian. Science 263, 494–498. doi: 10.1126/science.263.5146.494
Hotton N. I., Olson E. C., and Beerbower R. (1997). “Amniote origins and the discovery of herbivory,” in Amniote Origins (San Diego, California, USA: Academic Press), 207–264. doi: 10.1016/B978-012676460-4/50008-1
Huang Y., Chen Z.-Q., Roopnarine P. D., Benton M. J., Yang W., Liu J., et al. (2021). Ecological dynamics of terrestrial and freshwater ecosystems across three mid-Phanerozoic mass extinctions from northwest China. Proc. R. Soc B 288, rspb.2021.0148. doi: 10.1098/rspb.2021.0148
Huang Y., Chen Z.-Q., Roopnarine P. D., Benton M. J., Zhao L., Feng X., et al. (2023). The stability and collapse of marine ecosystems during the Permian-Triassic mass extinction. Curr. Biol. 33, 1059–1070. doi: 10.1016/j.cub.2023.02.007
Hubbell S. P. (Ed.) (2001). The unified neutral theory of biodiversity and biogeography (Princeton: Princeton University Press).
Huntley J. W. and Kowalewski M. (2007). Strong coupling of predation intensity and diversity in the Phanerozoic fossil record. Proc. Natl. Acad. Sci. U.S.A 104, 15006–15010. doi: 10.1073/pnas.0704960104
Huttenlocker A. K., Henrici A., John Nelson W., Elrick S., Berman D. S., Schlotterbeck T., et al. (2018). A multitaxic bonebed near the Carboniferous–Permian boundary (Halgaito Formation, Cutler Group) in Valley of the Gods, Utah, USA: Vertebrate paleontology and taphonomy. Palaeogeography Palaeoclimatology Palaeoecol. 499, 72–92. doi: 10.1016/j.palaeo.2018.03.017
Iannuzzi R. and Labandeira C. C. (2008). The oldest record of external foliage feeding and the expansion of insect folivory on land. Ann. Entomological Soc. America 101, 79–94. doi: 10.1603/0013-8746(2008)101[79:TOROEF]2.0.CO;2
Iannuzzi R., Neregato R., Cisneros J. C., Angielczyk K. D., Rößler R., Rohn R., et al. (2018). Re-evaluation of the Permian macrofossils from the Parnaíba Basin: biostratigraphic, palaeoenvironmental and palaeogeographical implications. Geological Society London Special Publications 472, 223–249. doi: 10.1144/SP472.14
Igielman B. (2024). The impact of taxonomic representation and “representative taxa”; a novel, comprehensive phylogenetic analysis of early amniotes (Minneapolis, Minnesota, USA: Society of Vertebrate Paleontology).
Irmis R. B. and Whiteside J. H. (2012). Delayed recovery of non-marine tetrapods after the end-Permian mass extinction tracks global carbon cycle. Proc. R. Soc. B: Biol. Sci. 279, 1310–1318. doi: 10.1098/rspb.2011.1895
Jablonski D. and Sepkoski J. J. (1996). Paleobiology, community ecology, and scales of ecological pattern. Ecology 77, 1367–1378. doi: 10.2307/2265534
Joachimski M. M. and Buggisch W. (1993). Anoxic events in the late Frasnian—Causes of the Frasnian-Famennian faunal crisis? Geol 21, 675. doi: 10.1130/0091-7613(1993)021<0675:AEITLF>2.3.CO;2
Jun L., Li X., Song-Hai J., Han-Yong P., and Xiao-Ling L. (2014). The Jiyuan tetrapod fauna of the Upper Permian of China—2. stratigraphy, taxonomical review, and correlation. Vertebrata PalAsiatica 52, 328–339.
Kearsey T. I., Bennett C. E., Millward D., Davies S. J., Gowing C. J. B., Kemp S. J., et al. (2016). The terrestrial landscapes of tetrapod evolution in earliest Carboniferous seasonal wetlands of SE Scotland. Palaeogeography Palaeoclimatology Palaeoecol. 457, 52–69. doi: 10.1016/j.palaeo.2016.05.033
Kelley P. H. and Hansen T. A. (2001). “Mesozoic marine revolution,” in Palaeobiology II. Eds. Briggs D. E. G. and Crowther P. R. (Oxford, England, UK: Wiley), 94–97. doi: 10.1002/9780470999295.ch19
Kempf H. L., Castro I. O., Dineen A. A., Tyler C. L., and Roopnarine P. D. (2020). Comparisons of Late Ordovician ecosystem dynamics before and after the Richmondian invasion reveal consequences of invasive species in benthic marine paleocommunities. Paleobiology 46, 320–336. doi: 10.1017/pab.2020.26
Kenrick P., Wellman C. H., Schneider H., and Edgecombe G. D. (2012). A timeline for terrestrialization: consequences for the carbon cycle in the Palaeozoic. Phil. Trans. R. Soc B 367, 519–536. doi: 10.1098/rstb.2011.0271
Khelidj N., Caccianiga M., Cerabolini B. E. L., Tampucci D., and Losapio G. (2024). Glacier extinction homogenizes functional diversity via ecological succession. J. Vegetation Sci. 35, 1–12. doi: 10.1111/jvs.13312
King H. M., Shubin N. H., Coates M. I., and Hale M. E. (2011). Behavioral evidence for the evolution of walking and bounding before terrestriality in sarcopterygian fishes. Proc. Natl. Acad. Sci. 108, 21146–21151. doi: 10.1073/pnas.1118669109
Kirejtshuk A. G. and Nel A. (2013). Skleroptera, a new order of holometabolous insects (Insecta) from the Carboniferous. Zoosystematica Rossica 22, 247–257. doi: 10.31610/zsr/2013.22.2.247
Kirejtshuk A. G., Poschmann M., Prokop J., Garrouste R., and Nel A. (2014). Evolution of the elytral venation and structural adaptations in the oldest Palaeozoic beetles (Insecta: Coleoptera: Tshekardocoleidae). J. Systematic Palaeontology 12, 575–600. doi: 10.1080/14772019.2013.821530
Klug C., Kröger B., Kiessling W., Mullins G. L., Servais T., Frýda J., et al. (2010). The Devonian nekton revolution: Devonian nekton revolution. Lethaia 43, 465–477. doi: 10.1111/j.1502-3931.2009.00206.x
Knauth L. P. and Kennedy M. J. (2009). The late Precambrian greening of the Earth. Nature 460, 728–732. doi: 10.1038/nature08213
Knecht R. J., Benner J. S., Swain A., Azevedo-Schmidt L., Cleal C. J., Labandeira C. C., et al. (2024). Early Pennsylvanian Lagerstätte reveals a diverse ecosystem on a subhumid, alluvial fan. Nat. Commun. 15, 7876. doi: 10.1038/s41467-024-52181-0
Knoll A. H. and Bambach R. K. (2000). Directionality in the history of life: diffusion from the left wall or repeated scaling of the right? Paleobiology 26, 1–14. doi: 10.1666/0094-8373(2000)26[1:DITHOL]2.0.CO;2
Kraft P., Pšenička J., Sakala J., and Frýda J. (2019). Initial plant diversification and dispersal event in upper Silurian of the Prague Basin. Palaeogeography Palaeoclimatology Palaeoecol. 514, 144–155. doi: 10.1016/j.palaeo.2018.09.034
Krainer K. and Lucas S. G. (2004). The Upper Pennsylvanian Red Tanks Member of the Bursum Formation at Carrizo Arroyo, central New Mexico: transition from shallow marine to nonmarine facies. New Mexico Museum Natural History Sci. Bull. 25, 53–70.
Labandeira C. C. (1997). Insect mouthparts: ascertaining the paleobiology of insect feeding strategies. Annu. Rev. Ecol. Systematics 28, 153–193. doi: 10.1146/annurev.ecolsys.28.1.153
Labandeira C. C., Tremblay S. L., Bartowski K. E., and VanAller Hernick L. (2014). Middle Devonian liverwort herbivory and antiherbivore defence. New Phytol. 202, 247–258. doi: 10.1111/nph.12643
Langer M. C. (2000). The first record of dinocephalians in South America: Late Permian (Rio do Rasto Formation) of the Paraná Basin, Brazil. Njgpa 215, 69–95. doi: 10.1127/njgpa/215/2000/69
Laurin M. (2024). Habitat of early stegocephalians (Chordata, Vertebrata, Sarcopterygii): a little saltier than most paleontologists like? foss-rec 27, 299–332. doi: 10.3897/fr.27.e123291
Laurin M. and Piñeiro G. H. (2017). A reassessment of the taxonomic position of mesosaurs, and a surprising phylogeny of early amniotes. Front. Earth Sci. 5. doi: 10.3389/feart.2017.00088
Laurin M. and Soler-Gijón R. (2001). The oldest stegocephalian from the Iberian Peninsula: evidence that temnospondyls were euryhaline. Comptes Rendus l’Académie Des. Sci. - Ser. III - Sci. la Vie 324, 495–501. doi: 10.1016/S0764-4469(01)01318-X
Laurin M. and Soler-Gijón R. (2010). Osmotic tolerance and habitat of early stegocephalians: indirect evidence from parsimony, taphonomy, palaeobiogeography, physiology and morphology. Geological Society London Special Publications 339, 151–179. doi: 10.1144/SP339.13
LeBlanc A. R. H., Brar A. K., May W. J., and Reisz R. R. (2015). Multiple tooth-rowed captorhinids from the Early Permian fissure fills of the Bally Mountain Locality of Oklahoma. Vert. Anat. Morph. Palaeo 1, 35–49. doi: 10.18435/B5RP4N
Lemberg J. B., Daeschler E. B., and Shubin N. H. (2021). The feeding system of Tiktaalik roseae: an intermediate between suction feeding and biting. Proc. Natl. Acad. Sci. U.S.A. 118, e2016421118. doi: 10.1073/pnas.2016421118
Lennie K. I., Manske S. L., Mansky C. F., and Anderson J. S. (2021). Locomotory behaviour of early tetrapods from Blue Beach, Nova Scotia, revealed by novel microanatomical analysis. R. Soc Open Sci. 8, 210281. doi: 10.1098/rsos.210281
Lenton T. M., Dahl T. W., Daines S. J., Mills B. J. W., Ozaki K., Saltzman M. R., et al. (2016). Earliest land plants created modern levels of atmospheric oxygen. Proc. Natl. Acad. Sci. U.S.A 113, 9704–9709. doi: 10.1073/pnas.1604787113
Libertín M., Kvaček J., Bek J., and Štorch P. (2018). Plant diversity of the mid silurian (Lower wenlock, sheinwoodian) terrestrial vegetation preserved in marine sediments from the barrandian area, the Czech Republic. FI 74, 327–333. doi: 10.2478/if-2018-0020
Linkies A., Graeber K., Knight C., and Leubner‐Metzger G. (2010). The evolution of seeds. New Phytol. 186, 817–831. doi: 10.1111/j.1469-8137.2010.03249.x
Long E. J., Edgecombe G. D., Clark B., Hatch C., Ball A. D., and Ma X. (2024). Mouthpart morphology and feeding structures in the palaeocharinid trigonotarbids of the Rhynie chert: insights from comparisons to modern arachnids. Palaeontology 67, e12717. doi: 10.1111/pala.12717
Long J. A., Niedźwiedzki G., Garvey J., Clement A. M., Camens A. B., Eury C. A., et al. (2025). Earliest amniote tracks recalibrate the timeline of tetrapod evolution. Nature. 641, 1193–2000. doi: 10.1038/s41586-025-08884-5
Lucas S. G. (2019). An ichnological perspective on some major events of Paleozoic tetrapod evolution. Bollettino della Società Paleontologica Italiana 58, 223–266. doi: 10.4435/BSPI.2019.20
Lucas S. G., Harris S. K., Spielmann J. A., Berman D. S., and Henrici A. C. (2005). Vertebrate biostratigraphy and biochronology of the Pennsylvanian-Permian Cutler Group, El Cobre Canyon, northern New Mexico. New Mexico Museum Natural History Sci. Bull. 31, 128–139.
Lucas S. G., Harris S. K., Spielmann J. A., Rinehart L. F., Berman D. S., Henrici A. C., et al. (2010). Vertebrate paleontology, biostratigraphy and biochronology of the Pennsylvanian-Permian Cutler Group, Cañon del Cobre, northern New Mexico. New Mexico Museum Natural History Sci. Bull. 49, 115–124.
MacIver M. A. and Finlay B. L. (2022). The neuroecology of the water-to-land transition and the evolution of the vertebrate brain. Phil. Trans. R. Soc B 377, 20200523. doi: 10.1098/rstb.2020.0523
MacIver M. A., Schmitz L., Mugan U., Murphey T. D., and Mobley C. D. (2017). Massive increase in visual range preceded the origin of terrestrial vertebrates. Proc. Natl. Acad. Sci. 114, E2375–E2384. doi: 10.1073/pnas.1615563114
Maddin H. C., Olori J. C., and Anderson J. S. (2011). A redescription of Carrolla craddocki (Lepospondyli: Brachystelechidae) based on high‐resolution CT, and the impacts of miniaturization and fossoriality on morphology. J. Morphology 272, 722–743. doi: 10.1002/jmor.10946
Mann A., Calthorpe A. S., and Maddin H. C. (2021a). Joermungandr bolti, an exceptionally preserved ‘microsaur’ from the Mazon Creek Lagerstätte reveals patterns of integumentary evolution in Recumbirostra. R. Soc Open Sci. 8, 210319. doi: 10.1098/rsos.210319
Mann A., Dudgeon T. W., Henrici A. C., Berman D. S., and Pierce S. E. (2021b). Digit and ungual morphology suggest adaptations for scansoriality in the late carboniferous eureptile anthracodromeus longipes. Front. Earth Sci. 9. doi: 10.3389/feart.2021.675337
Mann A. and Gee B. M. (2019). Lissamphibian-like toepads in an exceptionally preserved amphibamiform from Mazon Creek. J. Vertebrate Paleontology 39, e1727490. doi: 10.1080/02724634.2019.1727490
Mann A., Gee B. M., Pardo J. D., Marjanović D., Adams G. R., Calthorpe A. S., et al. (2020). Reassessment of historic ‘microsaurs’ from Joggins, Nova Scotia, reveals hidden diversity in the earliest amniote ecosystem. Pap Palaeontol, spp2.1316. doi: 10.1002/spp2.1316
Mann A., Henrici A. C., Sues H.-D., and Pierce S. E. (2023a). A new Carboniferous edaphosaurid and the origin of herbivory in mammal forerunners. Sci. Rep. 13, 4459. doi: 10.1038/s41598-023-30626-8
Mann A., Pardo J. D., and Maddin H. C. (2019). Infernovenator steenae, a new serpentine recumbirostran from the ‘Mazon Creek’ Lagertätte further clarifies lysorophian origins. Zoological J. Linn. Soc. 187, 506–517. doi: 10.1093/zoolinnean/zlz026
Mann A., Pardo J. D., and Sues H.-D. (2023b). Osteology and phylogenetic position of the diminutive ‘microsaur’ Odonterpeton triangulare from the Pennsylvanian of Linton, Ohio, and major features of recumbirostran phylogeny. Zoological J. Linn. Soc. 197, 641–655. doi: 10.1093/zoolinnean/zlac043
Mansky C. F. and Lucas S. G. (2013). Romer’s Gap revisited: continental assemblages and ichno-assemblages from the basal Carboniferous of Blue Beach, Nova Scotia, Canada. New Mexico Museum Natural History Sci. Bull. 60, 244–273.
Marjanović D. and Laurin M. (2019). Phylogeny of Paleozoic limbed vertebrates reassessed through revision and expansion of the largest published relevant data matrix. PeerJ 6, e5565. doi: 10.7717/peerj.5565
Markey M. J. and Marshall C. R. (2007a). Linking form and function of the fibrous joints in the skull: A new quantification scheme for cranial sutures using the extant fish Polypterus endlicherii. J. Morphology 268, 89–102. doi: 10.1002/jmor.10504
Markey M. J. and Marshall C. R. (2007b). Terrestrial-style feeding in a very early aquatic tetrapod is supported by evidence from experimental analysis of suture morphology. Proc. Natl. Acad. Sci. 104, 7134–7138. doi: 10.1073/pnas.0701706104
Marshall C. R. (2006). Explaining the cambrian “explosion” of animals. Annu. Rev. Earth Planet. Sci. 34, 355–384. doi: 10.1146/annurev.earth.33.031504.103001
Marshall J. E. A. (2020). A terrestrial Devonian-Carboniferous boundary section in East Greenland. Palaeobio Palaeoenv. 101, 541–559. doi: 10.1007/s12549-020-00448-x
Marshall J. E. A., Lakin J., Troth I., and Wallace-Johnson S. M. (2020). UV-B radiation was the Devonian-Carboniferous boundary terrestrial extinction kill mechanism. Sci. Adv. 6, eaba0768. doi: 10.1126/sciadv.aba0768
Marsicano C., Angielczyk K. D., Cisneros J. C., Richter M., Kammerer C. F., Fröbisch J., et al. (2021). Brazilian permian dvinosaurs (Amphibia, temnospondyli): revised description and phylogeny. J. Vertebrate Paleontology, e1893181. doi: 10.1080/02724634.2021.1893181
Matamales-Andreu R., Mujal E., Dinarès-Turell J., Kustatscher E., Roghi G., Oms O., et al. (2022). Early–middle Permian ecosystems of equatorial Pangaea: Integrated multi-stratigraphic and palaeontological review of the Permian of Mallorca (Balearic Islands, western Mediterranean). Earth-Science Rev. 228, 103948. doi: 10.1016/j.earscirev.2022.103948
Mehmood A., Singh S. A., Elsler A., and Benton M. J. (2025). The ecology and geography of temnospondyl recovery after the Permian–Triassic mass extinction. R. Soc Open Sci. 12, 241200. doi: 10.1098/rsos.241200
Milner A. R. and Sequeira S. E. K. (1993). The temnospondyl amphibians from the Viséan of East Kirkton, West Lothian, Scotland. Trans. R. Soc. Edinburgh: Earth Sci. 84, 331–361. doi: 10.1017/S0263593300006155
Milner A. R. and Sequeira S. E. K. (1998). A cochleosaurid temnospondyl amphibian from the Middle Pennsylvanian of Linton, Ohio, U.S.A. Zoological J. Linn. Soc. 122, 261–290. doi: 10.1111/j.1096-3642.1998.tb02532.x
Milner A. R. and Sequeira S. E. K. (2011). The amphibian Erpetosaurus radiatus (Temnospondyli, Dvinosauria) from the Middle Pennsylvanian of Linton, Ohio- morphology and systematic position. Special Papers Palaeontology 86, 57–73.
Mitchell J. S., Roopnarine P. D., and Angielczyk K. D. (2012). Late Cretaceous restructuring of terrestrial communities facilitated the end-Cretaceous mass extinction in North America. Proc. Natl. Acad. Sci. 109, 18857–18861. doi: 10.1073/pnas.1202196109
Modesto S. P., Richards C. D., Ide O., and Sidor C. A. (2018a). The vertebrate fauna of the Upper Permian of Niger—X. The mandible of the captorhinid reptile Moradisaurus grandis. J. Vertebrate Paleontology 38, e1531877. doi: 10.1080/02724634.2018.1531877
Modesto S. P., Scott D., and Reisz R. R. (2018b). A new small captorhinid reptile from the lower Permian of Oklahoma and resource partitioning among small captorhinids in the Richards Spur fauna. Papers Palaeontology 4, 293–307. doi: 10.1002/spp2.1109
Mondal S. and Harries P. J. (2016). Phanerozoic trends in ecospace utilization: The bivalve perspective. Earth-Science Rev. 152, 106–118. doi: 10.1016/j.earscirev.2015.10.005
Morris J. L., Leake J. R., Stein W. E., Berry C. M., Marshall J. E. A., Wellman C. H., et al. (2015). Investigating Devonian trees as geo-engineers of past climates: linking palaeosols to palaeobotany and experimental geobiology. Palaeontology 58, 787–801. doi: 10.1111/pala.12185
Myrow P. M., Ramezani J., Hanson A. E., Bowring S. A., Racki G., and Rakociński M. (2014). High‐precision U–Pb age and duration of the latest Devonian (Famennian) Hangenberg event, and its implications. Terra Nova 26, 222–229. doi: 10.1111/ter.12090
Naugolnykh S. V., Ivanov A. V., Uliakhin A. V., and Novikov I. V. (2022). Paleoecological and depositional environment of permian copper-bearing sandstone fossil plants and tetrapod localities: records from bashkortostan and kargalka river basin, orenburg region, Russia. Paleontological J. 56, 1538–1555. doi: 10.1134/S0031030122110120
Neenan J. M., Ruta M., Clack J. A., and Rayfield E. J. (2014). Feeding biomechanics in Acanthostega and across the fish-tetrapod transition. Proc. R. Soc. B: Biol. Sci. 281, 20132689–20132689. doi: 10.1098/rspb.2013.2689
Niedźwiedzki G., Szrek P., Narkiewicz K., Narkiewicz M., and Ahlberg P. E. (2010). Tetrapod trackways from the early Middle Devonian period of Poland. Nature 463, 43–48. doi: 10.1038/nature08623
Norton R. A., Bonamo P. M., Grierson J. D., and Shear W. A. (1988). Oribatid mite fossils from a terrestrial Devonian deposit near Gilboa, New York. J. Paleontol 62, 259–269. doi: 10.1017/S0022336000029905
Novack-Gottshall P. M. (2007). Using a theoretical ecospace to quantify the ecological diversity of Paleozoic and modern marine biotas. Paleobiology 33, 273–294. doi: 10.1666/06054.1
Nuñez Demarco P., Meneghel M., Laurin M., and Piñeiro G. (2018). Was mesosaurus a fully aquatic reptile? Front. Ecol. Evol. 6. doi: 10.3389/fevo.2018.00109
O’Keefe F. R., Sidor C. A., Larsson H. C. E., Maga A., and Ide O. (2005). The vertebrate fauna of the Upper Permian of Niger—III, morphology and ontogeny of the hindlimb of Moradisaurus grandis (Reptilia, Captorhinidae). J. Vertebrate Paleontology 25, 309–319. doi: 10.1671/0272-4634(2005)025[0309:TVFOTU]2.0.CO;2
Olive S., Clément G., Daeschler E. B., and Dupret V. (2016). Placoderm assemblage from the tetrapod-bearing locality of strud (Belgium, upper famennian) provides evidence for a fish nursery. PloS One 11, e0161540. doi: 10.1371/journal.pone.0161540
Olson E. C. (1952). The evolution of a Permian vertebrate chronofauna. Evolution 6, 181–196. doi: 10.1111/j.1558-5646.1952.tb01413.x
Olson E. C. (1958). Fauna of the Vale and Choza: summary, review, and integration of the geology and the faunas. Fieldiana Geology 10, 397–448. doi: 10.5962/bhl.title.16955
Olson E. C. (1962). Late permian terrestrial vertebrates, U.S.A. and U.S.S.R. Trans. Am. Philos. Soc. 52, 1–224. doi: 10.2307/1005904
Olson E. C. (1965). Relationships of seymouria, diadectes, and chelonia. Am. Zoologist 5, 295–307. doi: 10.1093/icb/5.2.295
Olson E. C. (1966). Community evolution and the origin of mammals. Ecology 47, 291–302. doi: 10.2307/1933776
Olson E. C. (1971). Vertebrate paleozoology (New York City, New York, USA: John Wiley & Sons Ltd). doi: 10.1177/0971102319750120
Olson E. C. (1977). Permian lake faunas: a sutdy in community evolution. J. Palaeontological Soc. India 20, 146–163.
Olson E. C. and Beerbower J. R. (1953). The san angelo formation, permian of texas, and its vertebrates. J. Geology 61, 389–423. doi: 10.1086/626109
Olson E. C. and Chudinov P. (1992). Upper Permian terrestrial vertebrates of the USA and Russia: 1991. Int. Geology Rev. 34, 1143–1160. doi: 10.1080/00206819209465658
Otoo B. K. A., Bolt J. R., Lombard R. E., Angielczyk K. D., and Coates M. I. (2021). The postcranial anatomy of Whatcheeria deltae and its implications for the family Whatcheeriidae. Zoological J. Linn. Soc. 193, 700–745. doi: 10.1093/zoolinnean/zlaa182
Otoo B. K. A., Clack J. A., Smithson T. R., Bennett C. E., Kearsey T. I., and Coates M. I. (2018). A fish and tetrapod fauna from Romer’s Gap preserved in Scottish Tournaisian floodplain deposits. Palaeontology 62, 225–253. doi: 10.1111/pala.12395
Pardo J. D. (2023). New information on the neurocranium of Archeria crassidisca and the relationships of the Embolomeri. Zoological J. Linn. Soc. 201, zlad156. doi: 10.1093/zoolinnean/zlad156
Pardo J. D., Small B. J., Milner A. R., and Huttenlocker A. K. (2019). Carboniferous–Permian climate change constrained early land vertebrate radiations. Nat. Ecol. Evol. 3, 200–206. doi: 10.1038/s41559-018-0776-z
Pardo J. D., Szostakiwskyj M., Ahlberg P. E., and Anderson J. S. (2017). Hidden morphological diversity among early tetrapods. Nature 546, 642–645. doi: 10.1038/nature22966
Pardo J. D., Szostakiwskyj M., and Anderson J. S. (2015). Cranial Morphology of the Brachystelechid ‘Microsaur’ Quasicaecilia texana Carroll Provides New Insights into the Diversity and Evolution of Braincase Morphology in Recumbirostran ‘Microsaurs’. PloS One 10, e0130359. doi: 10.1371/journal.pone.0130359
Parent N. and Cloutier R. (1996). “Distribution and preservation of fossils in the Escuminac Formation,” in Devonian Fishes and Plants of miguasha, Quebec, Canada (Verlag Dr. Friedrich Pfeil, Munich, Germany), 54–78.
Pascual R. and Jaureguizar E. O. (1990). Evolving climates and mammal faunas in Cenozoic South America. J. Hum. Evol. 19, 23–60. doi: 10.1016/0047-2484(90)90011-Y
Patzkowsky M. E. and Holland S. M. (2007). Diversity partitioning of a Late Ordovician marine biotic invasion: controls on diversity in regional ecosystems. Paleobiology 33, 295–309. doi: 10.1666/06078.1
Peecook B. R., Bronson A. W., Otoo B. K. A., and Sidor C. A. (2021). Freshwater fish faunas from two Permian rift valleys of Zambia, novel additions to the ichthyofauna of southern Pangea. J. Afr. Earth Sci. 183, 104325. doi: 10.1016/j.jafrearsci.2021.104325
Peecook B. R., Steyer J. S., Tabor N. J., and Smith R. M. H. (2017). Updated geology and vertebrate paleontology of the Triassic Ntawere Formation of northeastern Zambia, with special emphasis on the archosauromorphs. J. Vertebrate Paleontology 37, 8–38. doi: 10.1080/02724634.2017.1410484
Peters S. E. and Foote M. (2001). Biodiversity in the phanerozoic: a reinterpretation. Paleobiology 27, 583–601. doi: 10.1666/0094-8373(2001)027<0583:BITPAR>2.0.CO;2
Pierce S. E., Clack J. A., and Hutchinson J. R. (2012). Three-dimensional limb joint mobility in the early tetrapod Ichthyostega. Nature 486, 523–526. doi: 10.1038/nature11124
Piñeiro G., Ramos A., Goso C., Scarabino F., and Laurin M. (2012). Unusual environmental conditions preserve a permian mesosaur-bearing konservat-lagerstätte from Uruguay. Acta Palaeontologica Polonica 57, 299–318. doi: 10.4202/app.2010.0113
Piñeiro G., Rodao M., and Núñez Demarco P. (2024). First evidence of reproductive strategies in cephalopods preserved in phosphate and siderite nodules from the devonian of Uruguay. Fossil Stud. 2, 223–244. doi: 10.3390/fossils2030011
Piñeiro G., Verde M., Ubilla M., and Ferigolo J. (2003). First basal synapsids (“pelycosaurs”) from the Upper Permian-?Lower Triassic of Uruguay, South America. J. Paleontology 77, 389–392. doi: 10.1666/0022-3360(2003)077<0389:FBSPFT>2.0.CO;2
Piñero G., Marsicano C. A., Goso C., and Morosi E. (2007). Temnospondyl diversity of the permian-triassic colonia orozco local fauna (Buena vista formation) of Uruguay. RBP 10, 169–180. doi: 10.4072/rbp.2007.3.04
Pinheiro F. L., Eltink E., Paes‐Neto V. D., MaChado A. F., Simões T. R., and Pierce S. E. (2024). Interrelationships among Early Triassic faunas of Western Gondwana and Laurasia as illuminated by a new South American benthosuchid temnospondyl. Anatomical Rec. 307, 726–743. doi: 10.1002/ar.25384
Ponstein J., MacDougall M. J., and Fröbisch J. (2024). A comprehensive phylogeny and revised taxonomy of Diadectomorpha with a discussion on the origin of tetrapod herbivory. R. Soc Open Sci. 11, 231566. doi: 10.1098/rsos.231566
Prevec R., Nel A., Day M. O., Muir R. A., Matiwane A., Kirkaldy A. P., et al. (2022). South African Lagerstätte reveals middle Permian Gondwanan lakeshore ecosystem in exquisite detail. Commun. Biol. 5, 1154. doi: 10.1038/s42003-022-04132-y
Pšenička J., Bek J., Frýda J., Žárský V., Uhlířová M., and Štorch P. (2021). Dynamics of silurian plants as response to climate changes. Life 11, 906. doi: 10.3390/life11090906
Qvarnström M., Szrek P., Ahlberg P. E., and Niedźwiedzki G. (2018). Non-marine palaeoenvironment associated to the earliest tetrapod tracks. Sci. Rep. 8, 1074. doi: 10.1038/s41598-018-19220-5
Rasnitsyn A. P., Aristov D. S., Gorochov A. V., Rowland J. M., and Sinitshenkova N. D. (2004). Important new insect fossils from Carrizo Arroyo and the Permo-Carboniferous faunal boundary. New Mexico Museum Natural History Sci. Bull. 25, 215–246.
Rasnitsyn A. P., Aristov D. S., and Rasnitsyn D. A. (2015). Dynamics of insect diversity during the Early and Middle Permian. Paleontol. J. 49, 1282–1309. doi: 10.1134/S0031030115120102
Raup D. M. (1976). Species diversity in the Phanerozoic: an interpretation. Paleobiology 2, 289–297. doi: 10.1017/S0094837300004929
Rayfield E. J. (2007). Finite element analysis and understanding the biomechanics and evolution of living and fossil organisms. Annu. Rev. Earth Planetary Sci. 35, 541–576. doi: 10.1146/annurev.earth.35.031306.140104
Raymond A., Gensel P., and Stein W. E. (2006). Phytogeography of late silurian macrofloras. Rev. Palaeobotany Palynology 142, 165–192. doi: 10.1016/j.revpalbo.2006.02.005
Reeves J. C., Moon B. C., Benton M. J., and Stubbs T. L. (2020). Evolution of ecospace occupancy by Mesozoic marine tetrapods. Palaeontology 64, 31–49. doi: 10.1111/pala.12508
Rey K., Amiot R., Fourel F., Luyt J., Fluteau F., and Lécuyer C. (2023). Paleoenvironmental implications of Permo-Triassic geographic shift in oxygen stable isotope (δ18Op) from tetrapod bone in the South African Karoo Basin. J. Afr. Earth Sci. 205, 104999. doi: 10.1016/j.jafrearsci.2023.104999
Richter M., Cisneros J. C., Kammerer C. F., Pardo J., Marsicano C. A., Fröbisch J., et al. (2022). Deep-scaled fish (Osteichthyes: Actinopterygii) from the lower Permian (Cisuralian) lacustrine deposits of the Parnaíba Basin, NE Brazil. J. Afr. Earth Sci. 194, 104639. doi: 10.1016/j.jafrearsci.2022.104639
Rogers D. A. (1990). Probable tetrapod tracks rediscovered in the Devonian of N Scotland. JGS 147, 746–748. doi: 10.1144/gsjgs.147.5.0746
Romer A. S. (1958). Tetrapod limbs and early tetrapod life. Evolution 12, 365–369. doi: 10.1111/j.1558-5646.1958.tb02966.x
Romer A. S. (1974). Aquatic adaptation in reptiles- primary or secondary? Ann. South Afr. Museum 64, 221–230.
Ronchi A., Marchetti L., Klein H., and Groenewald G. H. (2023). A middle permian oasis for vertebrate and invertebrate life in a high-energy fluvial palaeoecosystem of southern gondwana (Karoo, Republic of South Africa). Geosciences 13, 325. doi: 10.3390/geosciences13110325
Roopnarine P. D. (2009). “Ecological modeling of paleocommunity food webs,” in Conservation Paleobiology: Using the Past to Manage for the Future. Eds. Dietl G. P. and Flessa K. W. (Westminster, Colorado, USA: The Paleontological Society), 195–220.
Roopnarine P. (2012). Red queen for a day: models of symmetry and selection in paleoecology. Evolutionary Ecol. 26, 1–10. doi: 10.1007/s10682-011-9494-6
Roopnarine P. D. and Angielczyk K. D. (2012). The evolutionary palaeoecology of species and the tragedy of the commons. Biol. Lett. 8, 147–150. doi: 10.1098/rsbl.2011.0662
Roopnarine P. D. and Angielczyk K. D. (2015). Community stability and selective extinction during the Permian-Triassic mass extinction. Science 350, 90–93. doi: 10.1126/science.aab1371
Roopnarine P. D. and Angielczyk K. D. (2016). “The stability of ecological communities as an agent of evolutionary selection,” in Evolutionary Theory: A Hierarchical Perspective, vol. 28 . Eds. Eldredge N., Pievani T., Serrilli E., and Temkin I. (Chicago, Illinois, USA: University of Chicago Press), 307–333.
Roopnarine P. D., Angielczyk K. D., Olroyd S. L., Nesbitt S. J., Botha-brink J., Peecook B. R., et al. (2018). Comparative ecological dynamics of Permian-Triassic communities from the Karoo, Luangwa, and Ruhuhu Basins of southern Africa. J. Vertebrate Paleontology 37, 254–272. doi: 10.1080/02724634.2018.1424714
Roopnarine P. D., Angielczyk K. D., Wang S. C., and Hertog R. (2007). Trophic network models explain instability of Early Triassic terrestrial communities. Proc. R. Soc. B: Biol. Sci. 274, 2077–2086. doi: 10.1098/rspb.2007.0515
Roopnarine P. D., Angielczyk K. D., Weik A., and Dineen A. (2019). Ecological persistence, incumbency and reorganization in the Karoo Basin during the Permian-Triassic transition. Earth-Science Rev. 189, 244–263. doi: 10.1016/j.earscirev.2018.10.014
Rowland J. M. (1997). The Late Paleozoic insect assemblage at Carrizo Arroyo, New Mexico. New Mexico Museum Natural History Sci. Bull. 11, 1–8.
Ruta M. and Clack J. A. (2006). A review of Silvanerpeton miripedes, a stem amniote from the Lower Carboniferous of East Kirkton, West Lothian, Scotland. Trans. R. Soc. Edinburgh: Earth Sci. 46, 115. doi: 10.1016/0016-7037(82)90301-5
Ruta M., Clack J. A., and Smithson T. R. (2020). A review of the stem amniote Eldeceeon rolfei from the Viséan of East Kirkton, Scotland. Earth Environ. Sci. Trans. R. Soc. Edinburgh 111, 173–192. doi: 10.1017/S1755691020000079
Ruta M. and Coates M. I. (2007). Dates, nodes and character conflict: Addressing the Lissamphibian origin problem. J. Systematic Palaeontology 5, 69–122. doi: 10.1017/S1477201906002008
Sahney S., Benton M. J., and Falcon-Lang H. J. (2010a). Rainforest collapse triggered Carboniferous tetrapod diversification in Euramerica. Geology 38, 1079–1082. doi: 10.1130/G31182.1
Sahney S., Benton M. J., and Ferry P. A. (2010b). Links between global taxonomic diversity, ecological diversity and the expansion of vertebrates on land. Biol. Lett. 6, 544–547. doi: 10.1098/rsbl.2009.1024
Sallan L. C. and Coates M. I. (2010). End-Devonian extinction and a bottleneck in the early evolution of modern jawed vertebrates. Proc. Natl. Acad. Sci. 107, 10131–10135. doi: 10.1073/pnas.0914000107
Schachat S. R., Labandeira C. C., and Maccracken S. A. (2018a). The importance of sampling standardization for comparisons of insect herbivory in deep time: a case study from the late Palaeozoic. R. Soc. Open Sci. 5, 1–15. doi: 10.1098/rsos.171991
Schachat S. R., Labandeira C. C., Saltzman M. R., Cramer B. D., Payne J. L., and Boyce C. K. (2018b). Phanerozoic p O 2 and the early evolution of terrestrial animals. Proc. R. Soc. B: Biol. Sci. 285, 20172631. doi: 10.1098/rspb.2017.2631
Schädel M., Yavorskaya M., and Beutel R. (2022). The earliest beetle †Coleopsis archaica (Insecta: Coleoptera) – morphological re-evaluation using Reflectance Transformation Imaging (RTI) and phylogenetic assessment. ASP 80, 495–510. doi: 10.3897/asp.80.e86582
Schneider J. W., Lucas S. G., and Rowland J. M. (2004). The Blattida (Insecta) fauna of Carrizo Arroyo, New Mexico- biostratigraphic link between marine and nonmarine Pennsylvanian/Permian boundary profiles. New Mexico Museum Natural History Sci. Bull. 25, 247–262.
Schneider J. W., Lucas S. G., Scholze F., Voigt S., Marchetti L., Klein H., et al. (2020). Late Paleozoic–early Mesozoic continental biostratigraphy — Links to the Standard Global Chronostratigraphic Scale. Palaeoworld 29, 186–238. doi: 10.1016/j.palwor.2019.09.001
Schultze H.-P. (2013). The paleoenvironment at the transition from piscine to tetrapod sarcopterygians. New Mexico Museum Natural History Sci. Bull. 60, 373–397.
Schultze H.-P. and Bolt J. R. (1996). The lungfish Tranodis and the tetrapod fauna from the Upper Mississippian of North America. Special Papers Palaeontology 52, 31–54.
Sepkoski J. J. (1993). Ten years in the library: new data confirm paleontological patterns. Paleobiology 19, 43–51. doi: 10.1017/S0094837300012306
Sepkoski J. J., Bambach R. K., Raup D. M., and Valentine J. W. (1981). Phanerozoic marine diversity and the fossil record. Nature 293, 435–437. doi: 10.1038/293435a0
Shabica C. W. and Hay A. A. (Eds.) (1997). Richardson’s Guide to the Fossil Fauna of Mazon Creek (Chicago, IL, USA: Northeastern Illinois University).
Shear W. A. and Bonamo P. M. (1988). Devonobiomorpha, A new order of centipeds (Chilopoda) from the middle devonian of gilboa, New York State, USA, and the phylogeny of centiped orders. Am. Museum Novitates 32.
Shear W. A., Bonamo P. M., Grierson J. D., Rolfe W. D. I., Smith E. L., and Norton R. A. (1984). Early land animals in North America: evidence from devonian age arthropods from gilboa, New York. Science 224, 492–494. doi: 10.1126/science.224.4648.492
Shear W. A., Selden P. A., Rolfe W. D. I., Bonamo P. M., and Grierson J. D. (1987). New terrestrial arachnids from the devonian of Gilboa, New York (Arachnida, trigonotarbida). Am. Museum Novitates, 1–74.
Shen Z., Monnet C., Cascales-Miñana B., Gong Y., Dong X., Kroeck D. M., et al. (2020). Diversity dynamics of Devonian terrestrial palynofloras from China: Regional and global significance. Earth-Science Rev. 200, 102967. doi: 10.1016/j.earscirev.2019.102967
Shishkin M. A. (2022a). Disturbance of Organizational Equilibrium during the Change of Ancient Tetrapod Communities: Its Manifestations at the Middle–Late Permian Transition. Palaeontological J. 56, 237–246. doi: 10.1134/S0031030122030170
Shishkin M. A. (2022b). On traces of non-equilibrium states in the evolution of terrestrial vertebrate communities across the paleozoic–mesozoic boundary. Paleontol. J. 56, 1–16. doi: 10.1134/S0031030122010117
Shishkin M. A., Novikov I. V., Sennikov A. G., Golubev V. K., and Morkovin B. I. (2023a). Chapter II. Stages in evolution of Triassic tetrapod assemblages of European Russia. Paleontol. J. 57, 1469–1484. doi: 10.1134/S0031030123120031
Shishkin M. A., Novikov I. V., Sennikov A. G., Golubev V. K., and Morkovin B. I. (2023b). Foreword. Paleontol. J. 57, 1353–1362. doi: 10.1134/S0031030123120067
Shishkin M. A., Novikov I. V., Sennikov A. G., Golubev V. K., and Morkovin B. I. (2023c). Triassic tetrapods of Russia. Paleontol. J. 57, 1353–1539. doi: 10.1134/S0031030123120067
Shubin N. H., Daeschler E. B., and Jenkins F. A. (2006). The pectoral fin of Tiktaalik roseae and the origin of the tetrapod limb. Nature 440, 764–771. doi: 10.1038/nature04637
Sidor C. A. (2013). The vertebrate fauna of the Upper Permian of Niger—VIII. Nigerpeton ricqlesi (Temnospondyli: Cochleosauridae) and tetrapod biogeographic provinces. Comptes Rendus Palevol 12, 463–472. doi: 10.1016/j.crpv.2013.05.005
Sidor C. A. and Hopson J. A. (1998). Ghost lineages and “Mammalness”: assessing the temporal pattern of character acquisition in the synapsida. Paleobiology 24, 254–273. doi: 10.1666/0094-8373(1998)024[0254:GLAATT]2.3.CO;2
Sidor C. A., Ide O., Larsson H. C. E., O’Keefe F. R., Smith R. M. H., Steyer J.-S., et al. (2021). The vertebrate fauna of the upper Permian of Niger—XI. Cranial material of a juvenile Moradisaurus grandis (Reptilia: Captorhinidae). J. Vertebrate Paleontology 41, e2030345. doi: 10.1080/02724634.2021.2030345
Sidor C. A., Mann A., and Angielczyk K. D. (2023). Gorgonops and Endothiodon (Synapsida: Therapsida) from the Madumabisa Mudstone Formation: evidence of a previously unreported tetrapod biozone in the Mid-Zambezi Basin of southern Zambia. J. Vertebrate Paleontology 43, 1–13. doi: 10.1080/02724634.2023.2256812
Sidor C. A., Vilhena D. A., Angielczyk D., Huttenlocker A. K., Nesbitt S. J., Peecook B. R., et al. (2013). Provincialization of terrestrial faunas following the end-Permian mass extinction. Proc. Natl. Acad. Sci. 110, 8129–8133. doi: 10.1073/pnas.1302323110
Silvestro D., Cascales‐Miñana B., Bacon C. D., and Antonelli A. (2015). Revisiting the origin and diversification of vascular plants through a comprehensive Bayesian analysis of the fossil record. New Phytol. 207, 425–436. doi: 10.1111/nph.13247
Slatyer R. A., Hirst M., and Sexton J. P. (2013). Niche breadth predicts geographical range size: a general ecological pattern. Ecol. Lett. 16, 1104–1114. doi: 10.1111/ele.12140
Smiley T. M., Sidor C. A., Maga A., and Ide O. (2008). The vertebrate fauna of the Upper Permian of Niger—VI. First evidence of a gorgonopsian therapsid. J. Vertebrate Paleontology 28, 543–547. doi: 10.1671/0272-4634(2008)28[543:TVFOTU]2.0.CO;2
Smith G. E. (1974). Depositional systems, San Angelo Formation (Permian), north Texas- facies contrl of red-bed copper mineralization (Austin, Texas, USA: Texas Bureau of Economic Geology).
Smith R. M. H., Sidor C. A., Tabor N. J., and Steyer J. S. (2015). Sedimentology and vertebrate taphonomy of the Moradi Formation of northern Niger: A Permian wet desert in the tropics of Pangaea. Palaeogeography Palaeoclimatology Palaeoecol. 440, 128–141. doi: 10.1016/j.palaeo.2015.08.032
Smithson T. R. (1985). Scottish Carboniferous amphibian localities. Scottish J. Geology 21, 123–142. doi: 10.1144/sjg21020123
Smithson T. R., Carroll R. L., Panchen A. L., and Andrews S. M. (1993). Westlothiana lizziae from the Viséan of East Kirkton, West Lothian, Scotland, and the amniote stem. Trans. R. Soc. Edinburgh: Earth Sci. 84, 383–412. doi: 10.1017/S0263593300006192
Smithson T. R., Ruta M., and Clack J. A. (2024). On Ossirarus kierani, a stem tetrapod from the Tournaisian of Burnmouth, Berwickshire, Scotland, and the phylogeny of early tetrapods. foss-rec 27, 333–352. doi: 10.3897/fr.27.e126410
Snyder D. (2006). A study of the fossil vertebrate fauna from the Jasper Hiemstra Quarry, Delta, Iowa and its environment (Iowa City, Iowa, USA: University of Iowa).
Solé R. (2022). Revisiting leigh van valen’s “A new evolutionary law” (1973). Biol. Theory 17, 120–125. doi: 10.1007/s13752-021-00391-w
Spiridonov A. and Eldredge N. (2024). The Bretskyan hierarchy, multiscale allopatry, and geobiomes—on the nature of evolutionary things. Paleobiology 50, 194–213. doi: 10.1017/pab.2023.37
Spiridonov A. and Lovejoy S. (2022). Life rather than climate influences diversity at scales greater than 40 million years. Nature 607, 307–312. doi: 10.1038/s41586-022-04867-y
Standen E. M., Du T. Y., Laroche P., and Larsson H. C. E. (2016). Locomotor flexibility of Polypterus Senegalus across various aquatic and terrestrial substrates. Zoology 119, 447–454. doi: 10.1016/j.zool.2016.05.001
Steemans P., Hérissé A. L., Melvin J., Miller M. A., Paris F., Verniers J., et al. (2009). Origin and radiation of the earliest vascular land plants. Science 324, 353–353. doi: 10.1126/science.1169659
Stein W. E., Berry C. M., Hernick L. V., and Mannolini F. (2012). Surprisingly complex community discovered in the mid-Devonian fossil forest at Gilboa. Nature 483, 78–81. doi: 10.1038/nature10819
Stein W. E., Berry C. M., Hernick L. V., and Mannolini F. (2021). The classic mid-Devonian Eospermatopteris localities, Gilboa NY, USA. Rev. Palaeobotany Palynology 295, 104520. doi: 10.1016/j.revpalbo.2021.104520
Stein W. E., Berry C. M., Morris J. L., Hernick L. V., Mannolini F., Ver Straeten C., et al. (2020). Mid-devonian archaeopteris roots signal revolutionary change in earliest fossil forests. Curr. Biol. 30, 421–431.e2. doi: 10.1016/j.cub.2019.11.067
Stein W. E., Mannolini F., Hernick L. V., Landing E., and Berry C. M. (2007). Giant cladoxylopsid trees resolve the enigma of the Earth’s earliest forest stumps at Gilboa. Nature 446, 904–907. doi: 10.1038/nature05705
Stenseth N.C. and Smith J. M. (1984). Coevolution in ecosystems: red queen evolution or stasis? Evolution 38, 870–880. doi: 10.1111/j.1558-5646.1984.tb00358.x
Stewart T. A., Lemberg J. B., Daly A., Daeschler E. B., and Shubin N. H. (2022). A new elpistostegalian from the Late Devonian of the Canadian Arctic. Nature 608, 563–568. doi: 10.1038/s41586-022-04990-w
Stewart T. A., Lemberg J. B., Taft N. K., Yoo I., Daeschler E. B., and Shubin N. H. (2019). Fin ray patterns at the fin-to-limb transition. Proc. Natl. Acad. Sci. U.S.A. 117, 1612–1620. doi: 10.1073/pnas.1915983117
Steyer J. S., Damiani R., Sidor C. A., O’Keefe F. R., Larsson H. C. E., Maga A., et al. (2006). The vertebrate fauna of the Upper Permian of Niger—IV. Nigerpeton ricqlesi (Temnospondyli: Cochleosauridae), and the Edopoid Colonization of Gondwana. J. Vertebrate Paleontology 26, 18–28. doi: 10.1671/0272-4634(2006)26[18:TVFOTU]2.0.CO;2
Stigall A. L. (2019). The invasion hierarchy: ecological and evolutionary consequences of invasions in the fossil record. Annu. Rev. Ecol. Evol. Syst. 50, annurev–ecolsys-110617-062638. doi: 10.1146/annurev-ecolsys-110617-062638
Stimson M., Calder J., Macrae A., Hebert B., Hanley J., Owen V., et al. (2013). New discoveries of tetrapod-bearing fossil forests at Joggins, Nova Scotia: implications for tetrapod entombment and ecological persistence. New Mexico Museum Natural History Sci. Bull. 60, 425.
Stössel I. (1995). The discovery of a new Devonian tetrapod trackway in SW Ireland. J. Geological Soc. 152, 407–413. doi: 10.1144/gsjgs.152.2.0407
Stössel I., Williams E. A., and Higgs K. T. (2016). Ichnology and depositional environment of the Middle Devonian Valentia Island tetrapod trackways, south-west Ireland. Palaeogeography Palaeoclimatology Palaeoecol. 462, 16–40. doi: 10.1016/j.palaeo.2016.08.033
Strother P. K., Battison L., Brasier M. D., and Wellman C. H. (2011). Earth’s earliest non-marine eukaryotes. Nature 473, 505–509. doi: 10.1038/nature09943
Swartz B. (2012). A marine stem-tetrapod from the devonian of Western North America. PloS One 7, e33683. doi: 10.1371/journal.pone.0033683
Ţabără D., Tudor E., Chelariu C., and Olaru-Florea R. F. (2021). Paleoenvironmental interpretation and palynoflora of Devonian–Carboniferous subsurface sections from the eastern part of the Moesian Platform (Romania). Rev. Palaeobotany Palynology 284, 104338. doi: 10.1016/j.revpalbo.2020.104338
Thornton I. (2007). Island Colonization: The Origin and Development of Island Communities. Ed. New. T. (New York, NY, USA: Cambridge University Press).
Tihelka E., Howard R. J., Cai C., and Lozano-Fernandez J. (2022). Was there a cambrian explosion on land? The case of arthropod terrestrialization. Biology 11, 1516. doi: 10.3390/biology11101516
Trewin N. H. (1992). Depositional environment and preservation of biota in the Lower Devonian hot-springs of Rhynie, Aberdeenshire, Scotland. Trans. R. Soc. Edinburgh: Earth Sci. 84, 433–442.
Tsuji L. A., Sidor C. A., Steyer J.-S., Smith R. M. H., Tabor N. J., and Ide O. (2013). The vertebrate fauna of the Upper Permian of Niger—VII. Cranial anatomy and relationships of Bunostegos akokanensis (Pareiasauria). J. Vertebrate Paleontology 33, 747–763. doi: 10.1080/02724634.2013.739537
Turner M. L., Tsuji L. A., Ide O., and Sidor C. A. (2015). The vertebrate fauna of the upper Permian of Niger—IX. The appendicular skeleton of Bunostegos akokanensis (Parareptilia: Pareiasauria). J. Vertebrate Paleontology 35, e994746. doi: 10.1080/02724634.2014.994746
Tverdokhlebov V. P., Tverdokhlebova G., Minikh A. V., Surkov M. V., and Benton M. J. (2005). Upper Permian vertebrates and their sedimentological context in the South Urals, Russia. Earth-Science Rev. 69, 27–77. doi: 10.1016/j.earscirev.2004.07.003
van Hoof T. B., Falcon-Lang H. J., Hartkopf-Fröder C., and Kerp H. (2013). Conifer-dominated palynofloras in the Middle Pennsylvanian strata of the De Lutte-6 borehole, The Netherlands: Implications for evolution, palaeoecology and biostratigraphy. Rev. Palaeobotany Palynology 188, 18–37. doi: 10.1016/j.revpalbo.2012.09.003
Vecoli M., Meyer-Berthaud B., and Clément G. (2010). The terrestrialization process: modelling complex interactions at the biosphere-geosphere interface-Introduction. SP 339, 1–3. doi: 10.1144/SP339.1
Vermeij G. J. (1977). The Mesozoic marine revolution: evidence from snails, predators and grazers. Paleobiology 3, 245–258. doi: 10.1017/S0094837300005352
Vermeij G. J. and Dudley R. (2000). Why are there so few evolutionary transitions between aquatic and terrestrial ecosystems? Biol. J. Linn. Soc. 70, 541–554. doi: 10.1111/j.1095-8312.2000.tb00216.x
Viglietti P. A., Rojas A., Rosvall M., Klimes B., and Angielczyk K. D. (2022). Network‐based biostratigraphy for the late Permian to mid‐ Triassic Beaufort Group (Karoo Supergroup) in South Africa enhances biozone applicability and stratigraphic correlation. Palaeontology 65, 1–22. doi: 10.1111/pala.12622
Villéger S., Novack-Gottshall P. M., and Mouillot D. (2011). The multidimensionality of the niche reveals functional diversity changes in benthic marine biotas across geological time. Ecol. Lett. 14, 561–568. doi: 10.1111/j.1461-0248.2011.01618.x
Waddington J., Rudkin D. M., and Dunlop J. A. (2015). A new mid-Silurian aquatic scorpion—one step closer to land? Biol. Lett. 11, 20140815. doi: 10.1098/rsbl.2014.0815
Wall P. D., Ivany L. C., and Wilkinson B. H. (2009). Revisiting Raup: exploring the influence of outcrop area on diversity in light of modern sample-standardization techniques. Paleobiology 35, 146–167. doi: 10.1666/07069.1
Wang R., Lang X., Ding W., Liu Y., Huang T., Tang W., et al. (2020). The coupling of Phanerozoic continental weathering and marine phosphorus cycle. Sci. Rep. 10, 5794. doi: 10.1038/s41598-020-62816-z
Wang D., Qin M., Liu L., Liu L., Zhou Y., Zhang Y., et al. (2019). The most extensive devonian fossil forest with small lycopsid trees bearing the earliest stigmarian roots. Curr. Biol. 29, 2604–2615. doi: 10.1016/j.cub.2019.06.053
Warren J. W. and Wakefield N. A. (1972). Trackways of tetrapod vertebrates from the upper devonian of victoria, Australia. Nature 238, 469–470. doi: 10.1038/238469a0
Werneburg I. (2024). Terrestrialisation and the cranial architecture of tetrapods. Foss-Rec 27, 473–497. doi: 10.3897/fr.27.137860
Whalen C. D., Hull P. M., and Briggs D. E. G. (2020). Paleozoic ammonoid ecomorphometrics test ecospace availability as a driver of morphological diversification. Sci. Adv. 6, eabc2365. doi: 10.1126/sciadv.abc2365
Whiteside J. H., Lindström S., Irmis R. B., Glasspool I. J., Schaller M. F., Dunlavey M., et al. (2015). Extreme ecosystem instability suppressed tropical dinosaur dominance for 30 million years. Proc. Natl. Acad. Sci. 112, 7909–7913. doi: 10.1073/pnas.1505252112
Whittingham M. A. J. B. (2023). “Regional-scale trends in the composition of late Pennsylvanian palaeobotanical communities from Atlantic Canada,” in Atlantic Geoscience (Atlantic Geological Society, Truro, Nova Scotia, Canada).
Whittingham M. A. J. B., Fraser D., and Maddin H. C. (2024a). “What is the tempo of turnover? A comparison of observed and neutrally simulated changes in the late carboniferous wetland forests of atlantic Canada,” in Geological Society of America Abstracts (Geological Society of America, Anaheim, California, USA).
Whittingham M. A. J. B., Herx E., Gaidies F., Fraser D., and Maddin H. C. (2022). “Dietary ecology of early amniotes and their relatives as explored through 3d surace modeling,” in Geological Society of America Abstracts (Geological Society of America, Denver, Colorado, USA).
Whittingham M. A. J. B., Korasidis V. A., and Fraser D. (2024b). Functional stasis and changing habitat preferences among mammalian communities from the PETM of the Bighorn Basin, Wyoming. Camb. prisms Extinction 2, e20. doi: 10.1017/ext.2024.25
Witzmann F. (2016). CO 2 -metabolism in early tetrapods revisited: inferences from osteological correlates of gills, skin and lung ventilation in the fossil record. Lethaia 49, 492–506. doi: 10.1111/let.12161
Xue J., Deng Z., Huang P., Huang K., Benton M. J., Cui Y., et al. (2016). Belowground rhizomes in paleosols: The hidden half of an Early Devonian vascular plant. Proc. Natl. Acad. Sci. U.S.A 113, 9451–9456. doi: 10.1073/pnas.1605051113
Xue J., Huang P., Wang D., Xiong C., Liu L., and Basinger J. F. (2018). Silurian-Devonian terrestrial revolution in South China: Taxonomy, diversity, and character evolution of vascular plants in a paleogeographically isolated, low-latitude region. Earth-Science Rev. 180, 92–125. doi: 10.1016/j.earscirev.2018.03.004
Zaffos A., Finnegan S., and Peters S. E. (2017). Plate tectonic regulation of global marine animal diversity. Proc. Natl. Acad. Sci. U.S.A 114, 5653–5658. doi: 10.1073/pnas.1702297114
Keywords: early tetrapods, paleoecology, food web, terrestrialization, continentalization, Paleozoic, water land transition, community ecology
Citation: Otoo BKA (2025) Prospects for studying continentalization and the origin of terrestrial ecosystems during the late Paleozoic. Front. Ecol. Evol. 13:1606225. doi: 10.3389/fevo.2025.1606225
Received: 04 April 2025; Accepted: 09 June 2025;
Published: 18 July 2025.
Edited by:
Antonio Garcia-Alix, University of Granada, SpainReviewed by:
Graciela Helena Piñeiro, Universidad de la República, UruguayLi Tian, China University of Geosciences Wuhan, China
Marcello Ruta, University of Lincoln, United Kingdom
Copyright © 2025 Otoo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benjamin K. A. Otoo, Ym90b28ucGFsZW9AZ21haWwuY29t
 Benjamin K. A. Otoo
Benjamin K. A. Otoo