- 1Department of Horticulture, School of Life Sciences, Central University of Tamil Nadu, Thiruvarur, Tamil Nadu, India
- 2Department of Microbiology, School of Life Sciences, Central University of Tamil Nadu, Thiruvarur, Tamil Nadu, India
Drought stress is a prime abiotic constraint that reduces microgreen growth and nutritional quality. This research explores a new strategy involving using green-synthesized selenium nanoparticles (SeNPs) to improve drought stress tolerance and biofortification of Amaranthus microgreens (var. Arka Suguna). SeNPs were synthesized from Cassia auriculata leaf extract and characterized via UV-Vis spectroscopy, TEM, XRD, FT-IR, and DLS, establishing their crystalline nature, spherical shape (80.6–135 nm), and phytochemical capping. Toxicity screening indicated 1,000 ppm as growth-inhibitory, whereas 100 ppm was optimal for plant growth. Drought assays employing PEG-induced stress indicated that 100 ppm SeNPs greatly enhanced germination (97.5%), yield (330 mg), plant height (5.6 cm), and biochemical profiles. Treated microgreens exhibited higher total protein (377.2 mg/100 g), carbohydrates (951 mg/100 g), flavonoids (11.4 mg/g), vitamin C (36.67 mg/100 g), and antioxidant enzyme activities (SOD: 0.065 U/mg/min; CAT: 13.5 U/mg/min). SeNPs also promoted selenium accumulation (10.69 mg/g DW) and had no negative impacts on valuable soil microbes, including Pseudomonas aeruginosa, Bacillus subtilis, and Trichoderma viride. This paper is the first comprehensive report on Cassia auriculata-mediated SeNPs administered through seed, soil, and foliar application to enhance drought tolerance and nutrient status in Amaranth microgreens. The findings indicate SeNPs as a green nano-priming approach for promoting crop yield under abiotic stress conditions.
1 Introduction
Selenium (Se), an essential micronutrient, exhibits potent antioxidant and antimicrobial properties, making it a cost-effective and safer option for agricultural and biomedical applications (Abbas et al., 2020). Compared to selenate or selenite, selenium nanoparticles (SeNPs) demonstrate lower toxicity while promoting plant growth, enhancing antioxidant activity, and improving vegetable nutrient accumulation (Hernández-Hernández et al., 2019). The antioxidant and anti-cancer potential of SeNPs is well-documented, but their synthesis method critically influences their safety and efficacy. Chemical synthesis using reducing agents like ascorbic acid often yields hazardous byproducts (Lin et al., 2021). In contrast, green synthesis utilizes plant extracts as reducing, stabilizing, and capping agents, offering an eco-friendly alternative that enhances SeNPs’ biocompatibility and functional properties (Kamal et al., 2019; Alsafran et al., 2025). This biogenic approach reduces toxicity and improves the antioxidant and anti-cancer effects of SeNPs without harming healthy cells (Budhani et al., 2019).
Nanotechnology has transformative applications across diverse sectors, including agriculture, where nanoparticles (1–100 nm) leverage unique physicochemical properties to enhance crop yields and sustainability (Usman et al., 2020). Innovations such as gold nanoparticles for COVID-19 antibody detection and 3D-printed nanomaterials underscore nanotechnology’s versatility in medicine and materials science. In agriculture, nano-agrochemicals enable targeted delivery, controlled release, and improved solubility of nutrients, thereby minimizing environmental harm (Ali et al., 2018; Chhipa, 2019). Despite these advances, challenges like scalability and long-term ecological impacts require resolution (Du et al., 2023). Commonly used silver, titanium dioxide, Zinc oxide and silica nanoparticles have shown promise in boosting germination and stress resilience (Rastogi et al., 2019; Francis et al., 2024a). In addition, these metal nanoparticles enhance crop productivity (20% increase), stress tolerance, and nutrient efficiency while reducing disease (50%) and nutrient leaching (30%). However, risks like toxicity and environmental accumulation must be addressed for sustainable agricultural use (Francis et al., 2024b).
Microgreens, such as amaranth, are nutrient-dense crops valued for their high concentrations of secondary metabolites, minerals (Fe, Mg, K), and digestible proteins (Ayeni, 2021). However, abiotic stresses (e.g., drought, salinity) often limit their productivity, which triggers oxidative damage, impairing growth and metabolism (El-Saadony et al., 2022). Selenium nanoparticles (SeNPs) effectively counteract abiotic stress by modulating key physiological processes, including the upregulation of chlorophyll biosynthesis, enhancement of photosynthetic efficiency, and promotion of osmoprotectant accumulation (Rady et al., 2020). Furthermore, SeNPs stimulate the activity of critical antioxidant enzymes, such as ascorbate peroxidase (APX), superoxide dismutase (SOD), and catalase (CAT), which collectively mitigate oxidative damage through efficient scavenging of reactive oxygen species (ROS). This dual mechanism significantly bolsters plant stress adaptation and tolerance. The SeNPs, particularly biosynthesized ones, improve crops’ resilience to abiotic stresses (e.g., drought, salinity) by increasing germination, antioxidant activity, and stress-responsive genes. Their size and application method influence their efficacy in enhancing growth, photosynthesis, yield, and plant physiology (Fatima et al., 2024). Additionally, SeNPs exhibit antimicrobial activity by catalyzing the oxidation of intracellular thiols (Webster and Ramos, 2012). Earlier studies have shown that green-synthesized selenium nanoparticles (SeNPs) have been reported to act as biostimulants of the antioxidant defense and physiological processes. Recent study shows that the application of green-synthesized SeNPs in the alleviation of drought stress in numerous crops like Soyabean, Purple coneflower, Rice, and Wheat (Zeeshan et al., 2024; Rezagholi et al., 2025; Iqbal et al., 2025; El-Saadony et al., 2021). While numerous studies have investigated the potential of selenium nanoparticles (SeNPs) to alleviate abiotic stress in crops, most of these studies concentrate on conventional vegetables and field crops, frequently involving chemically synthesized particles.
The current study presents novel approaches, such as Cassia auriculata leaf extract, for the green synthesis of SeNPs, a less commonly investigated phytochemical source. Second, the research incorporates a combined application strategy, seed priming, soil drenching, and foliar spraying, which have not been thoroughly tested in microgreens under drought conditions. Third, we employed Amaranthus microgreens, a high-value crop with a short crop cycle characterized by nutritional density but underrepresented in SeNP research. Unlike previous reports, this study includes an in-depth characterization (UV-Vis, DLS, TEM, FT-IR, XRD), toxicity studies, and compatibility studies with beneficial soil microorganisms. Finally, our study provides a comprehensive assessment that includes growth, biochemical, enzymatic, and microbial compatibility parameters. It thus brings new information to the nano-enabled biofortification and stress tolerance area of short-cycle edible crops. By harnessing the synergistic potential of biogenic SeNPs and microgreens, this research aims to develop sustainable strategies for enhancing crop resilience in drought-stressed environments.
2 Materials and methods
2.1 Materials source
Sodium selenite (Na2SeO3) (MW of 172.94) was purchased from Hi Media, India. Fresh leaves of C. auriculata were collected from Tiruvannamalai district, Tamil Nadu, India (latitude 12.3841 and longitude 79.1178). The amaranthus variety used in the study is Arka Suguna, which is resourced from IIHR, Bangalore, India.
2.2 Biosynthesis of SeNPs
Cassia auriculata leaf powder was tested at three concentrations (0.5, 2.5, and 5 g) suspended in 100 mL of double-distilled water (ddH2O) for optimal extract preparation. The suspensions were maintained in a water bath at 60°C with continuous stirring (200 rpm) for 30 min. The resulting mixtures underwent sequential filtration: primary filtration through sterile muslin cloth to remove particulate matter, followed by vacuum filtration using Whatman No. 42 filter paper to eliminate residual macro particles. The clarified, particle-free extracts were aseptically transferred to sterile reagent bottles and stored at 4°C for immediate use in SeNP synthesis. Sodium selenite (Na2SeO3; Molecular Weight: 172.94 g/mol; HiMedia Laboratories) solutions were prepared in five concentrations (5, 8, 10, 12, and 15 mM) using ddH2O as solvent. For SeNP synthesis, each concentration was combined with the standardized leaf extract (5 g/100 mL) in a 1:4 (v/v) ratio. The 1:4 ratio was chosen due to its demonstrated efficacy in prior optimization studies of NP production (Khurana et al., 2019; El-Saadony et al., 2021). The reaction mixtures were incubated at 75°C (±1°C) for 25 min in a hot plate magnetic stirrer (800 rpm) to ensure complete reduction of Se4+ to Se0. Temperature and stirring speed were rigorously controlled to maintain consistent reaction kinetics across all trials.
To achieve optimal nanoparticle stability, the biosynthesized SeNP solution underwent controlled incubation in an orbital shaker (120 rpm) under sequential temperature conditions: initial stabilization at 72°C (±0.5°C) for 3 h, followed by gradual cooling to 37°C (±0.5°C) for extended incubation periods (6, 12, 24, 48, 60, and 72 h). At each time interval, aliquots were aseptically collected and analyzed by UV-Vis spectroscopy to monitor plasmon resonance peak stability at 230 nm. This dual-phase thermal protocol ensured progressive nanoparticle maturation while preventing aggregation.
2.3 Characterization of biosynthesized SeNPs
The synthesized biogenic selenium nanoparticle’s physicochemical properties were characterized using advanced analytical techniques. UV-Vis spectral analysis was carried out on a Genesys 180 spectrophotometer (Thermo Fisher Scientific, United States), with absorbance measurements recorded across 200–800 nm wavelengths at 1 nm intervals. Nanoparticle morphology and elemental composition were evaluated through transmission electron microscopy (Quanta 200 FEG, FEI, Netherlands) coupled with energy-dispersive X-ray spectroscopy (Oxford Instruments X-MaxN EDX system), performed at 200 kV accelerating voltage at Tamil Nadu Agricultural University’s facility. Crystalline structure was determined by X-ray diffraction analysis (Bruker D8 Advance, United States) employing a 2θ range of 10°–80° with 0.02° step resolution. Surface functional group identification was achieved using FT-IR spectroscopy (PerkinElmer Spectrum Two) scanning the 4,000–400 cm−1 spectral range at Central University of Tamil Nadu’s analytical laboratory. For quantitative analysis, TEM micrographs were processed using ImageJ software (v1.45, NIH) with threshold-based particle detection. Size distribution histograms and statistical parameters (mean ± SD) were generated using OriginPro 2022 (OriginLab Corporation) with Gaussian curve fitting. All measurements were performed in triplicates.
2.4 Biocompatibility assay and toxicity assay of biosynthesized SeNP
The biocompatibility of biosynthesized SeNPs was evaluated against two beneficial soil microorganisms: Bacillus subtilis (MTCC 121) and Pseudomonas aeruginosa (MTCC 7903). Sterile nutrient agar (20 mL; HiMedia, M001) was supplemented with 5 mL of SeNP solution (100 ppm) in Petri plates (90 mm diameter). Bacterial cultures (100 µL of 108 CFU/mL suspension) were spread-plated and incubated for 48 h at 37°C (±0.2°C). Microbial growth was assessed by measuring colony diameter (mm) and optical density (600 nm) compared to untreated controls.
Seeds of Amaranthus var. Arka Suguna underwent surface sterilization using 0.4% (v/v) sodium hypochlorite solution for 5 min, followed by three thorough rinses with sterile distilled water. After air-drying under aseptic conditions in a laminar flow hood, the sterilized seeds were transferred to Petri plates containing 2% plain agar media (HiMedia, GRM026), which had been pre-treated with either 100 ppm SeNPs solution or control treatments. The plates were maintained in a growth chamber set at 25°C with a 16-h photoperiod/8-h dark cycle. Daily observations were conducted over 7 days to quantify germination percentage and measure radicle elongation (in mm).
2.5 Standardization of PEG concentration for artificial induction of drought
Artificial drought conditions were established using polyethylene glycol 6000 (PEG-6000; HiMedia, PCT1306). Four osmotic stress levels were tested: 16.7 mM (−0.05 MPa), 25 mM (−0.10 MPa), 37.5 mM (−0.20 MPa), and 56.3 mM (−0.40 MPa) PEG solutions. Each concentration was applied at 5 mL per potray cell (4 cm diameter × 5 cm depth) containing the growth medium. The optimal PEG concentration was determined based on germination percentage and Amaranthus var seedling vigor index. A standardized growth medium was prepared by homogenizing farmyard manure (FYM), river sand, and vermicompost in a 1:1:1 (v/v/v) ratio. Physical properties of the substrate were characterized (pH 6.8 ± 0.2, EC 1.2 ± 0.3 dS/m).
2.6 Application methods of biosynthesized SeNPs
Surface-sterilized Amaranthus var. Arka Suguna seeds were nano-primed via incubation in 25 or 100 ppm biosynthesized SeNP solutions (1:2 seed: solution ratio) for 60 min at 25 rpm (25°C ± 1°C) using a benchtop tube rotator before sowing. Soil application was performed on day 3 post-germination (D3) via root zone drenching with 100 ppm SeNP solution (5 mL/portray cell). In comparison, foliar spraying (25 ppm, 1 mL/portray cell) was conducted at the first true leaf stage (D6) using an atomizer (50–100 μm droplets) during peak stomatal activity to optimize uptake. All treatments were employed in controlled environmental conditions (28°C ± 2°C, 70% RH).
2.7 Determination of growth parameters
Amaranthus var. Arka Suguna microgreens were harvested at physiological maturity (9 days post-sowing) for growth and yield evaluation. Germination percentage was calculated as (number of germinated seeds/total seeds sown) × 100, with radicle emergence ≥2 mm as the germination criterion. Plant height was measured from the growth medium surface to the apical meristem using digital calipers (Mitutoyo, ±0.01 mm precision). For yield analysis, fresh weight was recorded immediately post-harvest (Shimadzu balance, ATX224R, Japan), while dry weight measurements followed 48-h dehydration in a forced-air oven at 60°C ± 1°C (P-Lab, Precession lab industries, India) until constant mass was achieved. All measurements were conducted in triplicate across three independent experimental runs.
2.8 Determination of biochemical parameters
2.8.1 Chlorophyll and carotenoid content
Chlorophyll and carotenoid content were analyzed using a modified acetone extraction method. Fresh leaf tissue (0.1 g) was homogenized in 10 mL of 80% acetone (HI-AR, AS025, Himedia laboratories, India) and then centrifuged at 5,000 × g for 10 min at 4°C to separate debris. The absorbance of the supernatant was measured at 643 nm and 660 nm (GENESYS 180, Thermo Fisher Scientific) for chlorophyll quantification using established equations:
where V = extract volume (mL) and W = sample fresh weight (g).
The procedure proposed by Mazumdar and Majumder (2021) was employed to determine the carotenoid content with minor modifications. 10 mL of 80% acetone was used to pulverize 0.1 g of the plant sample. The extract was filtered and the volume was increased to 50 mL using ddH2O. Using a spectrophotometer, the value was determined by measuring the carotenoid content at a specific wavelength (645, 660, and 663). The following formula was used to calculate the value:
All extractions were performed in triplicate under dim light to prevent pigment degradation.
2.8.2 Total soluble protein quantification
The soluble protein content was assessed using the Lowry method (Lowry et al., 1951), with modifications. Fresh plant tissue (0.1 g) was homogenized in 5 mL of ice-cold phosphate buffer (0.1 M, pH 7.0) and subsequently centrifuged at 10,000 × g for 15 min at 4°C. One milliliter of supernatant was mixed with 5 mL of alkaline copper reagent and incubated at 25°C ± 1°C for a duration of 10 min. Following the addition of 0.5 mL of Folin-Ciocalteu reagent (1:1 dilution), absorbance was assessed at 660 nm utilizing a UV-VIS Spectrophotometer (GENESYS 180), with a phosphate buffer blank serving as the reference. Protein concentration was assessed using a bovine serum albumin (BSA) standard curve with a range of 0–100 μg/mL.
2.8.3 Total carbohydrate estimation
Carbohydrate content was analyzed via the anthrone-sulfuric acid method. Lyophilized samples (50 mg) were hydrolyzed with 1.25 mL 2.5 N HCl at 100°C for 3 hours, neutralized with sodium carbonate and diluted to 50 mL with ddH2O. After centrifugation (1,000 × g, 10 min), aliquots (1 mL) were reacted with 4 mL ice-cold anthrone reagent at 100°C for 8 min. The green-to-dark green color transition was quantified at 630 nm using a UV-VIS Spectrophotometer. All extractions and reactions were performed in triplicate.
2.8.4 Total phenolic content
Phenolic compounds in fresh leaf samples were quantified using the Folin-Ciocalteu assay. Methanolic extracts (0.5 mL, 70% v/v) were mixed with 0.2 mL F-C reagent (1N), 3.25 mL ddH2O, and 1 mL 20% (w/v) Na2CO3. After vortexing (30 s), samples were incubated in amber vials (25°C ± 1°C, 30 min) to develop the chromogenic reaction. The absorbance at 700 nm was compared to a methanol blank using a UV-VIS Spectrophotometer. The calibration curve (Y = 0.012X + 0.021; R2 = 0.998) was generated by gallic acid standards (0–100 μg/mL), and the results were expressed as mg gallic acid equivalents (GAE) per g fresh weight.
2.8.5 Total flavonoid content
Flavonoids were analyzed via aluminum chloride complexation (Ordonez et al., 2006). Methanolic extracts (1 mL) were combined with 1.5 mL 80% methanol, 0.1 mL 10% (w/v) AlCl3, 0.1 mL 1M CH3COONa, and 2.8 mL ddH2O. Following incubation (25°C, 30 min, dark), absorbance at 415 nm was compared against quercetin standards (0–50 μg/mL; Y = 0.025X–0.112; R2 = 0.991). Data were expressed as mg quercetin equivalents (QE) per g fresh weight.
2.8.6 FRAP assay
Total antioxidant activity was assessed using the FRAP protocol by Benzie and Strain (1999). The working solution consisted of 10 mL of 300 mM acidified acetate buffer, 1 mL of 10 mM TPTZ in hydrochloric acid, and 1 mL of 20 mM ferric chloride. Plant extracts (0.5 mL) were mixed with 1.8 mL of the FRAP reagent and 1.2 mL of ultrapure water, then incubated at room temperature for 30 min. Absorbance at 593 nm was measured spectrophotometrically and converted to antioxidant equivalents using a calibration curve based on ascorbic acid standards, showing good linearity (r2 = 0.964). Antioxidant capacity was normalized to sample mass and expressed as micromolar ascorbic acid equivalents per gram of fresh tissue weight (μmol AAE/g FW).
2.8.7 Total soluble sugars
Glucose content (% w/w) and total soluble solids (TSS, % Brix) were quantified using digital refractometry. Fresh tissue (1 g) was homogenized in 10 mL ddH2O (1:10 w/v) and filtered through a muslin cloth. Clear extracts were analyzed using calibrated digital meters: glucose (HI96803, HANNA range 0%–85%) and TSS (HI96801, HANNA, range 0–85°Brix). Results represent the mean of three technical replicates per biological sample.
2.8.8 Vitamin C quantification
L-ascorbic acid was measured via redox titration (Contreras-Calderón et al., 2010). Samples (0.05 g) were homogenized in 10 mL 4% (w/v) oxalic acid and centrifuged (8,000 × g, 10 min). The supernatant (5 mL) was titrated against standardized 2,6-dichlorophenolindophenol (DCPI, 0.1 mg/mL) until a persistent pink endpoint (pH 3.0–4.0). Vitamin C content (mg/100 g FW) was calculated.
2.9 Antioxidant enzymatic activities
2.9.1 Superoxide dismutase (SOD) activity assay
The superoxide dismutase (SOD; EC 1.15.1.1) enzymatic assay was performed according to established photochemical methods (Beyer and Fridovich, 1987). Plant tissue samples were homogenized in 50 mM phosphate buffer (pH 7.8) containing 1% (w/v) polyvinylpyrrolidone to minimize phenolic compound interference. The complete reaction system (4 mL final volume) incorporated 50 mM phosphate buffer (pH 7.8), 0.15 mM EDTA, 0.12 mM NBT, 20 mM methionine, and 0.075 mM riboflavin. Following addition of 500 μL enzyme extract, the mixture was exposed to 5,000 lux fluorescent illumination for 30 min to generate superoxide radicals and initiate NBT reduction. Absorbance measurements at 560 nm determined the extent of reaction inhibition, with one SOD unit defined as the enzyme quantity required for 50% suppression of NBT reduction compared to light-exposed controls. Activity calculations employed the formula: % inhibition = [(A560 control - A560 sample)/A560 control] × 100. All experimental measurements included triplicate analyses along with appropriate light-exposed and dark control samples to ensure methodological reliability.
2.9.2 Catalase assay
The enzymatic activity of catalase (EC 1.11.1.6) was quantified using UV-visible spectrophotometry based on hydrogen peroxide decomposition kinetics. Following the established protocol of Aebi (1984), enzyme extraction was performed in ice-cold 50 mM phosphate buffer (pH 7.0), with subsequent clarification through centrifugation (10,000 × g, 15 min, 4°C). The assay system consisted of 3 mL reaction volume containing: (1) 50 mM phosphate buffer (pH 7.0), (2) 10 mM H2O2 substrate solution, and (3) 100 μL of enzyme extract. Catalase-mediated H2O2 breakdown was monitored by measuring absorbance decline at 240 nm (extinction coefficient = 39.4 mM−1cm−1) for 60 s at 25°C. Enzyme activity calculations were normalized to total protein content and reported as micromoles of H2O2 catabolized per minute per milligram of protein.
2.10 Estimation of selenium content
Selenium concentration in Amaranthus microgreens was determined using a modified (Duff and Chessin, 1965) acid digestion method. 0.5 g of tissue was digested in a 10 mL acid mixture (7.5:2.5 v/v nitric: perchloric acids) at 85°C–100°C until white fumes appeared. After cooling, 3 mL 30% hydrogen peroxide (H2O2) was added and reheated (5–10 min) to complete oxidation. The digestate was reacted with 10 mL 3% hydrazine sulfate (reducing agent) and 3 mL 2.5% gum arabic (stabilizer) in a boiling water bath (10 min), producing an orange-red Se-hydrazine complex. The final volume was adjusted to 25 mL using deionized water, and absorbance was recorded at 420 nm relative to sodium selenite standards (0–5 ppm). Spike recovery tests (90%–105%) and reagent blanks validated the protocol, with results expressed as mg Se/g dry weight (triplicate measurements).
2.11 Statistical analysis
Three independent biological replicates were performed for all experimental treatments. Data analysis was conducted using R Statistical Software (version 4.2.3) with specialized packages for agricultural research (dplyr v1.1.0, agricolae v1.3-6, and multicomp v1.4-23). Treatment comparisons were assessed through one-way analysis of variance (ANOVA) followed by post hoc testing using Tukey’s honestly significant difference (HSD) method at α = 0.05. Significant differences between treatment groups were indicated using compact letter display notation following standard agricultural research conventions. For additional verification, Fisher’s least significant difference (LSD) test was performed at the 5% probability level. All graphical representations were generated using GraphPad Prism software (version 8.4.3) to visualize treatment effects and statistical relationships.
3 Result and discussion
3.1 Standardization of leaf extract, sodium selenite concentrations and incubation time for the biosynthesis of SeNP
The biosynthesis of selenium nanoparticles (SeNPs) was optimized using C. auriculata leaf extract with various concentrations of sodium selenite (5 mM, 8 mM, 10 mM, 12 mM, 15 mM) and incubation hours (6, 12, 24, 48, 60, 72 h). Among tested concentrations, 5% leaf extract combined with 12 mM sodium selenite yielded the most effective SeNP synthesis, exhibiting peak absorbance at 230 nm with high intensity (Figure 1A). The reduction process commenced immediately upon mixing. Subsequent stabilization studies revealed that a 72-h incubation period at 120 rpm produced SeNPs with the highest absorbance peak (Figure 1B). These standardized conditions 5% leaf extract, 12 mM sodium selenite, and 72-h incubation were thus established as optimal for efficient and stable SeNP biosynthesis.
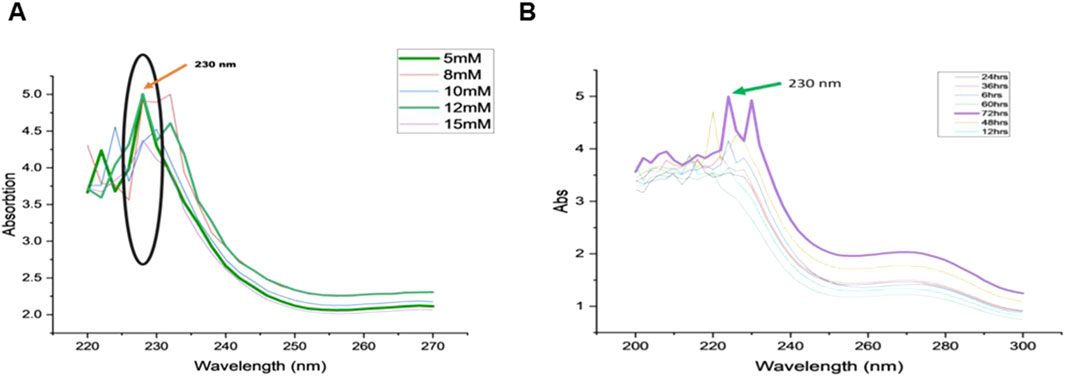
Figure 1. Variations in the absorption spectra of (A) Sodium selenite with increasing concentrations (5 mM, 8 nM, 10 nM, 12 nM, 15 nM), (B) Incubation time for SeNP reduction (6, 12, 24, 36, 48, 60, 72 h).
3.2 Standardization of PEG concentration for artificial induction of drought
To establish an effective in vitro drought induction system, polyethylene glycol (PEG) was tested at varying concentrations (16.7, 25, 37.5, 56.3 mM). Results demonstrated that 37.5 mM and 56.3 mM PEG most effectively simulated drought stress (Figure 2), as evidenced by pronounced physiological responses. Thus, 37.5 mM PEG concentration was selected for subsequent experiments to evaluate drought-induced changes in plant biochemical and physiological parameters.

Figure 2. Standardization of PEG concentrations (16.7, 25, 37.5, 56.3 mM) for artificial drought induction in Amaranth microgreens var. Arka Suguna.
3.3 Biocompatibility of biosynthesized SeNP with beneficial microorganisms and toxicity test
The biocompatibility assessment of biosynthesized SeNPs (100 ppm) demonstrated a stimulatory effect on beneficial soil microorganisms, including P. aeruginosa and B. subtilis. Unlike exhibiting inhibitory effects, the SeNPs enhanced microbial growth compared to untreated controls (Figures 3A,B). These findings suggest that the biosynthesized SeNPs maintain compatibility with essential soil microbiota while potentially promoting their proliferation. A toxicity assessment of biosynthesized SeNPs was conducted on Amaranthus var. Arka Suguna microgreens using four concentrations (100, 250, 500 and 1,000 ppm). While the highest concentration (1,000 ppm) exhibited phytotoxic effects, impairing plant development, lower concentrations (100–500 ppm) showed no adverse effects and instead promoted normal growth (Figure 3C). These results establish a concentration-dependent response to SeNPs, with 1,000 ppm representing the toxicity threshold for this cultivar.
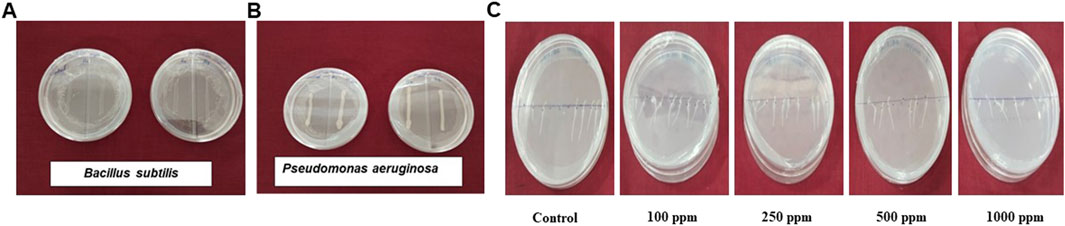
Figure 3. Biocompatibility of biosynthesized SeNPs with beneficial microorganisms (A) Bacillus subtilis, (B) Pseudomonas aeruginosa and (C) Effects of SeNPs on toxicity levels of Invitro germinated Amaranthus seeds.
3.4 Characterization of biosynthesized SeNPs
UV-Vis spectroscopic analysis of biosynthesized SeNPs (prepared with 12 mM sodium selenite and 5% C. auriculata leaf extract) revealed characteristic absorbance peaks at 230 nm for all tested samples, including 100 ppm SeNPs, pure selenium powder, and crude leaf extract (Figure 4A). Dynamic light scattering analysis indicated the synthesized nanoparticles had an average size distribution of 337.3 nm with a polydispersity index (PdI) of 0.321 (Figure 4B), demonstrating moderate size variation among the nanoparticles. The particle size measurements, polydispersity index (PDI), and zeta potential were carried out in Milli-Q water as the dispersion medium. Nevertheless, it is established that such physicochemical features are highly affected by the matrix surrounding it, e.g., ionic strength, pH, and the occurrence of macromolecules like root exudates and soil colloids (El-Saadony et al., 2021; Iqbal et al., 2025). Thus, under real application conditions like seed coating, soil correction, or foliar spraying, nanoparticle behavior can differ significantly from that seen in water. Environmental interactions may influence nanoparticle stability, uptake efficiency, and biological efficacy. Future studies should consider evaluating nanoparticles’ size distribution and surface charge directly in the relevant matrices to better correlate their physicochemical properties with plant responses and agronomic outcomes.
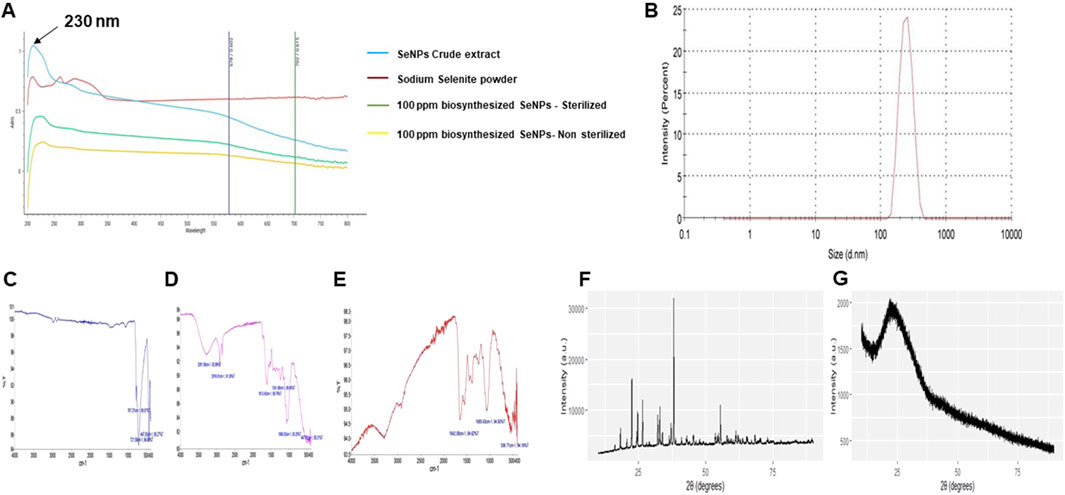
Figure 4. (A) UV-visible spectrums after 72 h of incubation of crude extract of biosynthesized SeNPs, pure sodium selenite powder 99%, biosynthesized SeNPs (100 ppm)- sterilized, biosynthesized SeNPs (100 ppm)- non sterilized; (B) dynamic light scattering pattern of biosynthesized SeNPs; (C) Fourier transform intra-red spectroscopy (FTIR) spectra of - Cassia auriculata leaf extract, (D) sodium selenite, (E) biosynthesized SeNPs; (F) X-ray diffraction pattern of - chemically synthesized SeNPs (ascorbic acid as reducer), (G) biosynthesized SeNPs (C. auriculata extract as reducer).
FT-IR spectroscopy confirmed the involvement of functional groups from C. auriculata leaf extract in the reduction and stabilization of biosynthesized SeNPs. Comparative analysis of the leaf extract (Figure 4C), sodium selenate (Figure 4D), and biosynthesized SeNPs (Figure 4E) identified key vibrational frequencies, including a broad OH stretch at 3291.09 cm−1 (indicative of aromatic rings and ether-methoxy groups) and C–H asymmetric bending at 1066.03 cm−1 and 92.99 cm−1. The observed redshift in the OH band of SeNPs suggested hydroxyl group interactions with nanoparticle surfaces, a critical factor in their stabilization. Spectral changes, including the retention or disappearance of–OH/COO–peaks, further supported the role of these functional groups in capping and stabilizing the SeNPs. These findings align with reported mechanisms of plant-mediated nanoparticle synthesis and stabilization. The X-ray diffraction (XRD) result reveals that the biosynthesized SeNPs are amorphous in nature (Figures 4F, G). The result matches with the previous reported values (Chen et al., 2009).
TEM analysis revealed spherical selenium nanoparticles (SeNPs) with distinct size distributions: chemically synthesized SeNPs ranged from 100 to 200 nm (Figure 5A), while biosynthesized SeNPs using C. auriculata leaf extract exhibited smaller sizes (80.6–135 nm; Figures 5B,C). EDAX confirmed Se presence in all samples, with characteristic absorption peaks at 0.1–0.5 keV and 10–10.5 keV (Figures 5D–F). The 5 mM Na2SeO3-derived SeNPs showed higher peak intensity in the lower energy range than 12 mM Na2SeO3 or ascorbic acid-synthesized particles. Size distribution analysis further demonstrated that ascorbic acid-produced SeNPs had the largest mean area (953.7 nm2) and particle size (163.71 nm). At the same time, biosynthesized SeNPs (5 mM and 12 mM Na2SeO3) displayed smaller, more uniform dimensions (Figures 5G–L). Copper signals in EDAX spectra originated from the TEM grid substrate.
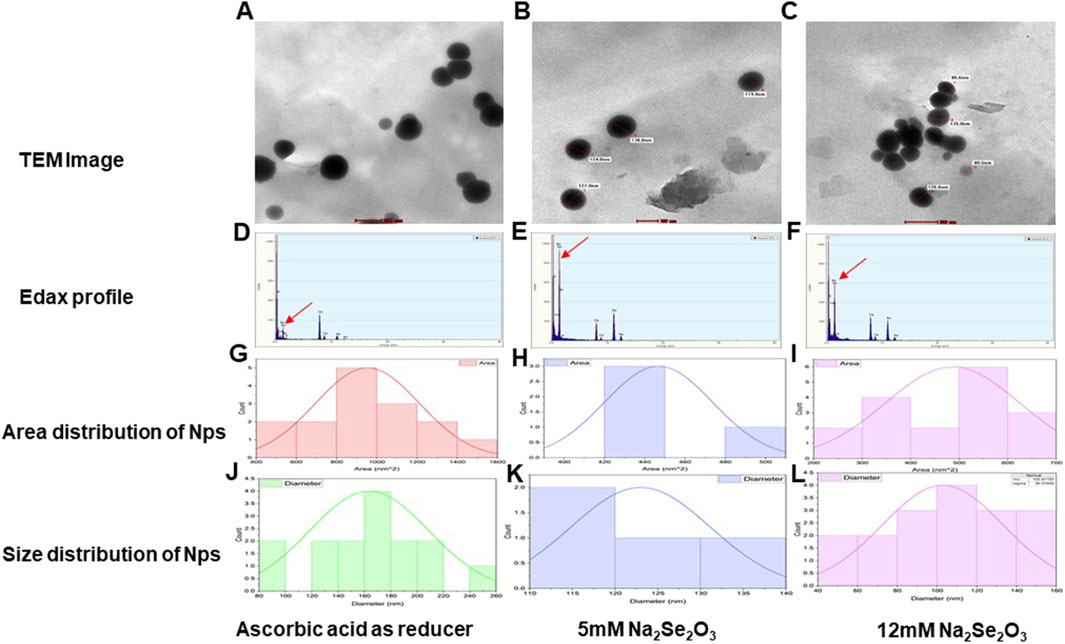
Figure 5. (A) TEM image of synthesized SeNP using Ascorbic acid as a reducer. (B) TEM image of synthesized SeNP using 5 mM Na2Se2O3. (C) TEM image of synthesized SeNP using 12 mM Na2Se2O3. (D) EDAX profile of synthesized SeNP using Ascorbic acid as a reducer. (E) EDAX profile of synthesized SeNP using 5 mM Na2Se2O3. (F) EDAX profile of synthesized SeNP using 12 mM Na2Se2O3. (G) Area distribution (nm2) of synthesized SeNP using Ascorbic acid as a reducer. (H) Area distribution (nm2) of synthesized SeNP using 5 mM Na2Se2O3. (I) Area distribution (nm2) of synthesized SeNP using 12 mM Na2Se2O3. (J) Size distribution (nm) of synthesized SeNP using Ascorbic acid as a reducer. (K) Size distribution(nm) of synthesized SeNP using 5 mM Na2Se2O3. (L) Size distribution(nm) of synthesized SeNP using 12 mM Na2Se2O3.
3.5 Effect of SeNP on amaranthus growth and yield parameters
Se is generally considered a non-essential micronutrient for the plant growth (Zhan et al., 2021). Recent studies highlighted the potential of SeNp to improve germination percentage, growth and stress tolerance in various crops (Abouelhamd et al., 2023; Ghanbari et al., 2023). The study investigated the effects of biogenic synthesized SeNp on the growth attributes of amaranth microgreens (Amaranthus var Arka Suguna) under artificially induced drought conditions with PEG @ 37.5 mM). The combined application method involves seed treatment (ST) and soil application of SeNp at 100 ppm conc. (SA) and foliar application at 25 ppm conc. (FA) had a significant impact on all growth parameters. The combined application (ST+SA+FA) enhanced seed germination to 96.67% under artificial drought stress while also increasing plant height (5.63 cm), fresh weight (332.24 mg) and dry weight (24.14 mg) of harvested amaranth microgreens (Table 1; Figure 6). The seed treatment combined with the soil application (ST+SA) followed the ST+SA+FA treatment in terms of germination percentage, and plant height, showing similar results. However, the ST+SA treatment did not significantly differ in fresh weight (283.87 mg) and dry weight (23.93 mg) compared to the combined application. The lowest germination percentage of 72.50 percent, plant height of 4.43 cm, fresh weight 205.48, and dry weight 14.91 mg was observed in control (Figure 6). The findings of this study align with previous research indicated that biosynthesized SeNPS significantly increases the germination percentage of Amaranth microgreens. Similar growth promotion by SeNp has been reported in carrot plants (Abouelhamd et al., 2023), green beans (Ismail et al., 2023), and potatoes (Perfileva et al., 2023) increased plant height and physiological tolerance (Nagdalian et al., 2023). reported that applying SeNp at 20 mg/L concentration enhances the growth in barley. These results support the role of SeNp in enhancing plant growth under stress conditions (Figures 6A–D).
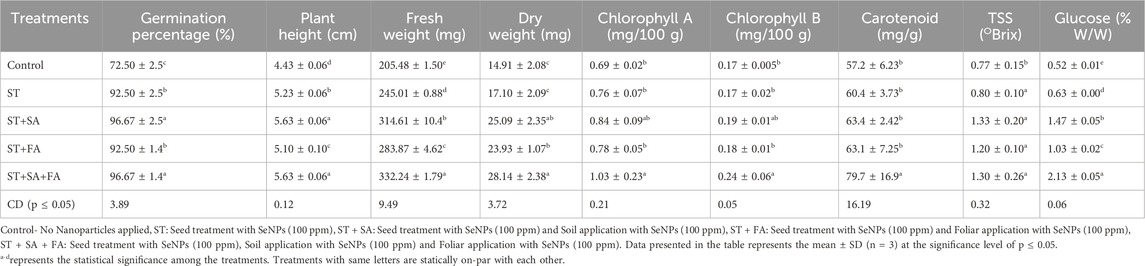
Table 1. Effect of SeNPs on growth and yield parameters of Amaranth microgreens var. Arka Suguna in In-vivo.
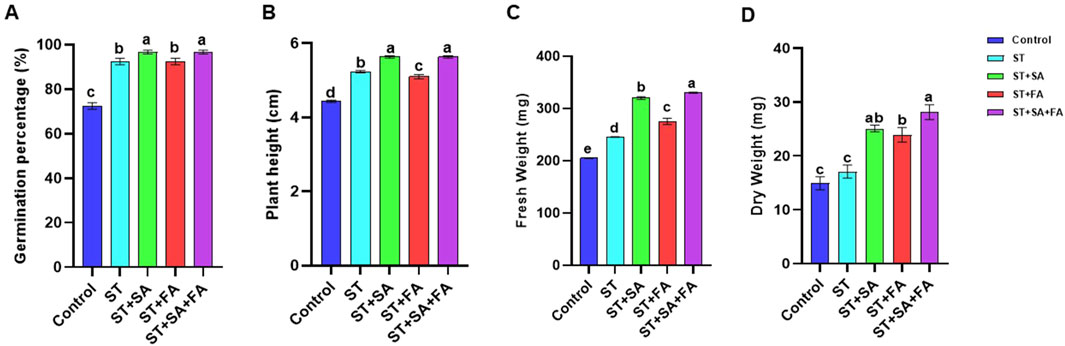
Figure 6. Effects of SeNPs and drought stress on (A) Germination Percentage, (B) Plant height, (C) shoot fresh weight, (D) shoot dry weight in Amaranth microgreens var. Arka Suguna. All the plants were drought-stressed with PEG (37.5 mM). Control- No Nanoparticles applied, ST: Seed treatment with SeNPs (100 ppm), ST + SA: Seed treatment with SeNPs (100 ppm) and Soil application with SeNPs (100 ppm), ST + FA: Seed treatment with SeNPs (100 ppm) and Foliar application with SeNPs (100 ppm), ST + SA + FA: Seed treatment with SeNPs (100 ppm), Soil application with SeNPs (100 ppm) and Foliar application with SeNPs (100 ppm). Different letters demonstrate significant differences among the treatments (p < 0.05).
Application of SeNp significantly increased both fresh and dry weight of amaranth microgreen, which aligns with the findings of de los Santos-Vázquez et al. (2016), who reported that foliar application of Se at 5 mg/L increased the biomass production and vitamin C content in lettuce by overwhelmed stress. Similarly, dry matter production and essential oil content were improved by applying SeNp under drought conditions in Basil (Asghari et al., 2023). The current study findings correlate with these findings and show that the combined application of biosynthesized SeNp (ST+SA+FA) can enhance drought tolerance and biomass production in amaranth microgreens under drought conditions and highlight the potential of SeNp as a sustainable tool for enhancing crop resilience and productivity in stress environments.
3.6 Effect of SeNP on amaranth biochemical parameters of amaranthus microgreens
The biochemical parameters of amaranth microgreens (Amaranthus var. Arka Suguna) viz., chlorophyll content, carotenoids, total phenols, flavonoids, antioxidants, carbohydrates, vitamin c, soluble proteins, total soluble sugars (TSS), enzymatic activity and Se accumulation, was influenced by biogenic synthesized SeNp. The results obtained in the study revealed that SeNp significantly enhances these biochemical parameters, promoting plant growth and stress tolerance under drought conditions. The photosynthetic efficiency of the crop plants was determined by the accumulation of chlorophyll content in the plant systems. In our study, chlorophyll content in the amaranth microgreen was significantly influenced by the combined application of SeNp (ST+SA+FA), recording the highest chlorophyll A and B (1.03 and 0.24 mg/100 g) content, along with higher carotenoid level (79.7 mg/g) followed by ST+SA (Table 1; Figures 7A–C). All other methods of application of SeNp show statistically similar results at the 5 percent significance level. These results were supported by the previous studies conducted by Abbas (2012), who reported that Se influences Chlorophyll and carotenoid levels by controlling the redox status of leaves. Also, Vijayarengan (2013) and Marisamy et al. (2015) identified that Se application has protective effects on chloroplast enzymes and improves photosynthetic pigments. Additionally, applying selenium at 10 and 5 µM enhanced the photosynthetic pigments in cucumber leaves exposed to salt stress (Hawrylak-Nowak, 2009). The result suggested that applying biosynthesized SeNPs increases photosynthetic pigments of amaranth microgreens.
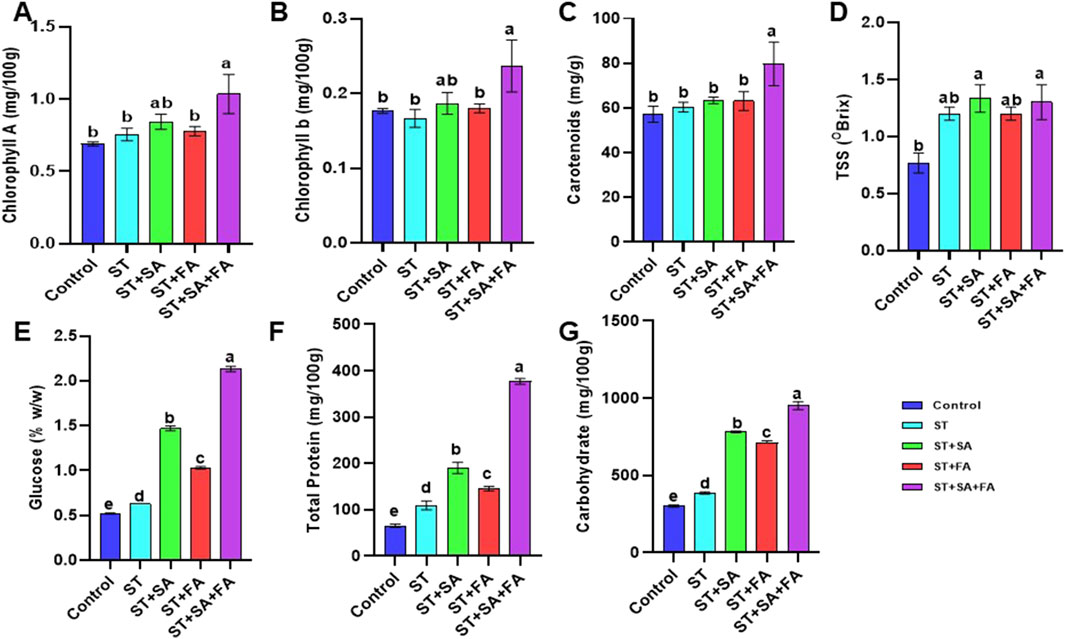
Figure 7. Effects of SeNPs and drought stress on (A) Chlorophyll A, (B) Chlorophyll B, (C) Carotenoid, (D) Total soluble salts, (E) Glucose, (F) Total Proteins, (G) Carbohydrates in Amaranth microgreens var. Arka Suguna. All the plants were drought-stressed with PEG (37.5 mM). Control- No Nanoparticles applied, ST: Seed treatment with SeNPs (100 ppm), ST + SA: Seed treatment with SeNPs (100 ppm) and Soil application with SeNPs (100 ppm), ST + FA: Seed treatment with SeNPs (100 ppm) and Foliar application with SeNPs (100 ppm), ST + SA + FA: Seed treatment with SeNPs (100 ppm), Soil application with SeNPs (100 ppm) and Foliar application with SeNPs (100 ppm). Different letters demonstrate significant differences among the treatments (p < 0.05).
Combined application of SeNp (ST+SA+FA) recorded the highest phenolic content (6.23 mg/g), while ST+SA+FA also showed increased flavonoid levels (11.4 mg/g). The total antioxidant availability was highest in ST+SA (23.6 mg/g), followed by the combination of ST+SA+FA of SeNp (22.3 mg/g) under drought conditions (Table 2; Figures 8A–C). These results are closely related to the findings of Chu et al. (2009), who observed that Se application reduces ROS and malondialdehyde content while increasing phenolic and flavonoid levels in wheat under cold stress. Similarly, antioxidant activity and robust antioxidant potential of SeNps under both saline and non-saline conditions were reported by Shahraki et al. (2022) and Guleria et al. (2020), implying their role in mitigating oxidative stress and enhancing plant growth and development. Earlier studies demonstrated that selenium nanoparticles (SeNPs) synthesized via green methods possess significant antioxidant activity (Kondaparrthi et al., 2019; Mellinas et al., 2019; Boroumand et al., 2019; Dumore and Mukhopadhyay, 2020). These findings align with the observed enhancement in total phenols, flavonoids, and antioxidant levels in Amaranthus microgreens following treatment with biosynthesized SeNPs (Table 2; Figures 8A–C).
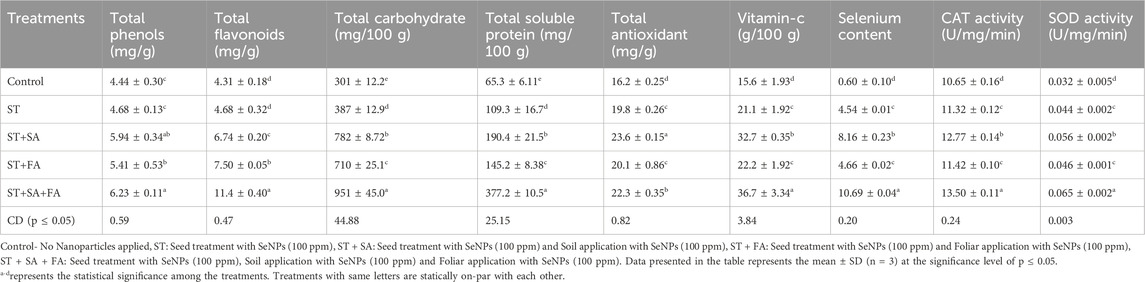
Table 2. Effect of SeNPs on biochemical parameters of Amaranth microgreens var. Arka Suguna in In-vivo.
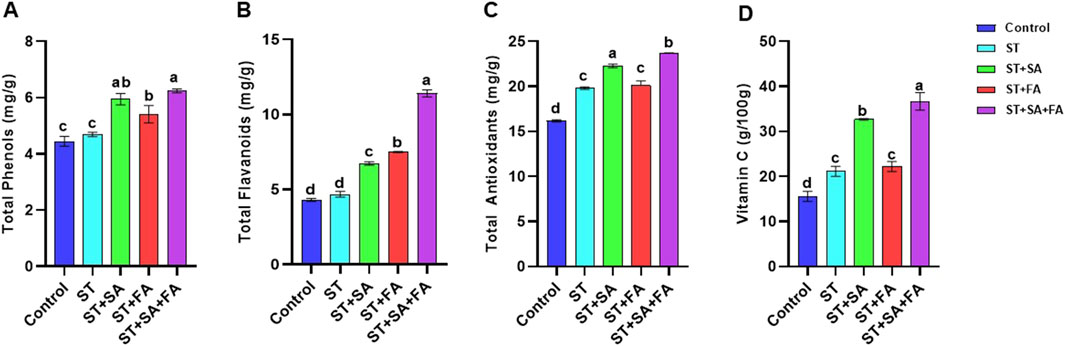
Figure 8. Effects of SeNPs and drought stress on (A) Total Phenol, (B) Total flavonoid, (C) Total Antioxidant, (D) Vit C in Amaranth microgreens var. Arka Suguna. All the plants were drought-stressed with PEG (37.5 mM). Control- No Nanoparticles applied, ST: Seed treatment with SeNPs (100 ppm), ST + SA: Seed treatment with SeNPs (100 ppm) and Soil application with SeNPs (100 ppm), ST + FA: Seed treatment with SeNPs (100 ppm) and Foliar application with SeNPs (100 ppm), ST + SA + FA: Seed treatment with SeNPs (100 ppm), Soil application with SeNPs (100 ppm) and Foliar application with SeNPs (100 ppm). Different letters demonstrate significant differences among the treatments (p < 0.05).
Carbohydrate content was significantly higher in amaranth microgreens treated with ST+SA+FA of SeNp, recording a maximum of 951 mg/100 g, followed by ST+SA of SeNp-treated microgreens (782 mg/100 g) under drought conditions (Table 2; Figure 7G). Consistent with the reports of Alam et al. (2022) and Zhou et al. (2022), selenium nanoparticles were shown to boost growth performance in stressed plants through enhanced carbohydrate metabolism. Likewise, vitamin C content is also higher in amaranth microgreen treated with ST+SA+FA of SeNp (36.7 g/100 g) under drought conditions (Table 2; Figure 8D), in line with the findings of de los Santos-Vázquez et al. (2016), reported that application of SeNp improves antioxidant and vitamin C content in tomatoes and melons. These results suggest that SeNp enhances nutrient accumulation and drought tolerance in amaranth microgreens.
SeNPs and selenite significantly enhance total selenium accumulation and soluble protein content in crops such as soybean sprouts and potato tubers. However, the effects vary by species, in soybeans (Sarwar et al., 2020) leads to an increase in the accumulation of soluble proteins, whereas in soybean sprouts, soluble proteins get reduced (Rao et al., 2022). In this study, biosynthesized SeNPs elevated total soluble proteins in Amaranthus microgreens, with the highest levels in ST+SA+FA (377.2 mg/100 g) and the lowest in the control (65 mg/100 g) (Table 2; Figure 7F). Glucose content was highest in ST+FA (1.13% w/w), comparable to ST (1.10% w/w), while ST+SA+FA and ST+SA showed slightly lower values (1.03% w/w) (Table 2; Figure 7E). Total soluble solids (TSS) peaked in ST+SA+FA and ST+SA (1.33% Brix), followed by ST and ST+FA (1.20% Brix), with the control exhibiting the lowest (0.76% Brix) (Table 2; Figure 7D). These findings align with reports that SeNPs improve sugar accumulation by enhancing stress resilience and metabolic activity, as seen in tomatoes treated with 10 mg/L SeNPs (Hernández-Hernández et al., 2019). The present study confirms that biosynthesized SeNPs boost total soluble sugars in Amaranthus microgreens (Table 2; Figures 7D,E).
3.7 Effect of SeNP on enzymatic activity
Selenium is an essential component of antioxidant enzymes, critically involved in scavenging reactive oxygen and nitrogen species to mitigate oxidative cellular damage. In the current investigation, catalase (CAT) activity reached its maximum in the ST+SA+FA treatment (13.50 U/mg/min), with ST+SA (12.77 U/mg/min) showing comparable results, whereas the control group displayed the lowest activity (10.65 U/mg/min) (Table 2; Figure 9A). A parallel trend was observed for superoxide dismutase (SOD), where ST+SA+FA exhibited the highest activity (0.065 U/mg/min), significantly surpassing the control (0.032 U/mg/min), with intermediate treatments showing no statistically distinct effects (Table 2; Figure 9B). These results corroborate earlier studies highlighting selenium’s capacity to upregulate antioxidant enzymes, enhancing plant resilience under stress. For instance, Jiang et al. (2017) documented elevated SOD activity in selenium-supplemented maize, and Hussein et al. (2019) reported analogous antioxidant enzyme modulation. Further evidence from Wu et al. (2016) and Marslin et al. (2017) underscores selenium’s ability to augment proline accumulation, peroxidase, and glutathione peroxidase (GPX) activity, collectively attenuating oxidative stress and lipid peroxidation. The present findings validate that biosynthesized selenium nanoparticles (SeNPs) markedly enhance CAT and SOD activity in Amaranthus microgreens (Table 2; Figure 9), solidifying selenium’s pivotal role in fortifying antioxidant defense mechanisms in plants.
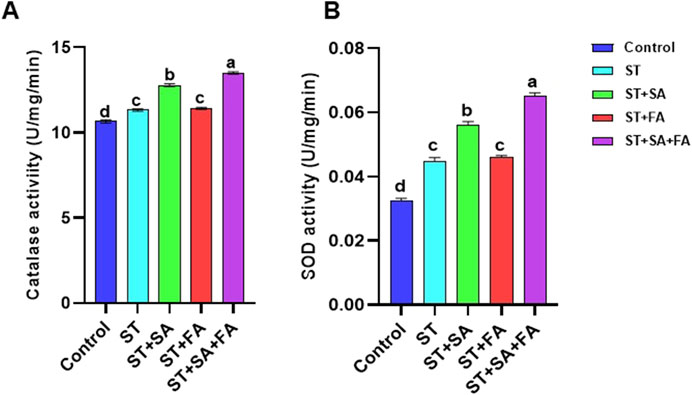
Figure 9. Effects of SeNPs and drought stress on (A) Catalase, (B) SOD in Amaranth microgreens var. Arka Suguna. All the plants were drought stressed with PEG (37.5 mM). Control- No Nanoparticles applied, ST: Seed treatment with SeNPs (100 ppm), ST + SA: Seed treatment with SeNPs (100 ppm) and Soil application with SeNPs (100 ppm), ST + FA: Seed treatment with SeNPs (100 ppm) and Foliar application with SeNPs (100 ppm), ST + SA + FA: Seed treatment with SeNPs (100 ppm), Soil application with SeNPs (100 ppm) and Foliar application with SeNPs (100 ppm). Different letters demonstrate significant differences among the treatments (p < 0.05).
3.8 Estimation of selenium content in amaranth seedling
Selenium is primarily absorbed by plant roots through sulfur transporters and incorporated into organic compounds, with its accumulation in shoots influenced by soil selenium availability and organic matter content (Terry et al., 2000; Arscott and Goldman, 2012). Similar dose-dependent selenium accumulation patterns have been reported in wheat, alfalfa, sunflower (Lintschinger et al., 2000; Lyons et al., 2005), Brassica species (Banuelos et al., 1997), kale (Lefsrud et al., 2006), and onion (Barak and Goldmon, 1997). The selenium content in Amaranthus microgreens was highest in the ST+SA+FA treatment (10.69 mg/g), followed by ST+SA (8.16 mg/g), while the control exhibited minimal accumulation (0.60 mg/g) (Table 2; Figure 10). These findings align with previous studies demonstrating that soil-applied SeNPs significantly enhance selenium uptake in plants, as observed in bok choy, where root concentrations exceeded shoot levels (Ramos et al., 2010).
Figure 10. Effects of SeNPs and drought stress on the Selenium content in Amaranth microgreens var. Arka Suguna. All the plants were drought stressed with PEG (37.5 mM). Control- No Nanoparticles applied, ST: Seed treatment with SeNPs (100 ppm), ST + SA: Seed treatment with SeNPs (100 ppm) and Soil application with SeNPs (100 ppm), ST + FA: Seed treatment with SeNPs (100 ppm) and Foliar application with SeNPs (100 ppm), ST + SA + FA: Seed treatment with SeNPs (100 ppm), Soil application with SeNPs (100 ppm) and Foliar application with SeNPs (100 ppm). Different letters demonstrate significant differences among the treatments (p < 0.05).
The current study confirms that biosynthesized SeNPs effectively promote selenium biofortification in Amaranthus microgreens (Table 2; Figure 10), highlighting their potential for enhancing nutritional quality in leafy vegetables.
4 Conclusion
This study highlights the significant potential of biosynthesized selenium nanoparticles (SeNPs) in enhancing abiotic stress tolerance and improving growth parameters in Amaranthus microgreens (var. Arka Suguna). The most effective treatments are seed treatment, soil application, and foliar spray (ST+SA+FA) of 100 ppm SeNPs, resulting in remarkable improvements across all measured parameters. The treatment achieved a 97.5% germination rate, increased plant height to 5.6 cm, and boosted yield to 330 mg. Nutritionally, it enhanced protein content to 377.20 mg/100 g, carbohydrates to 951 mg/100 g, flavonoids to 11.40 mg/g, and vitamin C to 36.67 mg/100 g. The antioxidant capacity significantly improved with SOD activity reaching 0.07 U/mg/min and CAT activity 13.49 U/mg/min, indicating stronger stress defense mechanisms. Notably, selenium accumulation peaked at 10.68 mg/g, demonstrating effective biofortification. Beyond plant growth, the SeNPs showed compatibility with beneficial soil microorganisms, suggesting broader ecosystem benefits. These comprehensive results position biosynthesized SeNPs as a multifaceted solution for modern agriculture, capable of simultaneously addressing productivity challenges posed by abiotic stresses while improving nutritional quality. The findings strongly support the adoption of SeNPs as sustainable nano-fertilizers, offering an innovative approach to enhance crop performance under stressful environmental conditions. In the future, nano selenium can be mass-produced commercially for agricultural applications. This research provides a practical and environmentally friendly method of enhancing microgreen production and drought resistance, which is particularly beneficial for small-scale and urban agriculture farmers. Improving nutrient content supports food security in areas with limited water availability. The results also complement international sustainability objectives, calling for lesser dependency on chemical inputs in agriculture.
Recent studies have shown that selenium nanoparticles can induce epigenetic modifications by altering stress-related gene expression, such as DNA methylation in plants like chicory and pepper. These changes may contribute to enhanced abiotic stress tolerance. While transgenerational inheritance has not yet been confirmed, the potential for epigenetic memory warrants investigation. Long-term studies are necessary to assess whether such traits are retained and if they provide adaptive advantages in offspring under abiotic stress. A potential limitation of this study is the use of short-term microgreen trials under controlled conditions, which may not fully reflect the field-level responses. Future work should evaluate SeNPs effects across developmental stages and at different environmental conditions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
AG: Writing – original draft, Writing – review and editing, Conceptualization, Investigation, Data curation, Formal Analysis, Validation. RS: Formal Analysis, Investigation, Software, Validation, Visualization, Writing – review and editing. IA: Conceptualization, Formal Analysis, Methodology, Software, Validation, Writing – review and editing. RK: Data curation, Software, Validation, Visualization, Writing – review and editing. AR: Formal Analysis, Investigation, Software, Validation, Visualization, Writing – review and editing. AA: Data curation, Formal Analysis, Methodology, Software, Validation, Writing – review and editing. SS: Conceptualization, Data curation, Investigation, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors acknowledge the Department of Physics, Central University of Tamil Nadu, Thiruvarur, for sharing their instrumentation facility to complete the work successfully.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnano.2025.1621024/full#supplementary-material
References
Abbas, H. S., Baker, D. H. A., and Ahmed, E. A. (2020). Cytotoxicity and antimicrobial efficiency of selenium nanoparticles biosynthesized by Spirulina platensis. Arch. Microbiol. 203 (2), 523–532. doi:10.1007/s00203-020-02042-3
Abbas, S. M. (2012). Effects of low temperature and selenium application on growth and the physiological changes in sorghum seedlings. J. Stress Physiol. Biochem. 8 (1), 268–286.
Abouelhamd, N., Gharib, F. A. E. L., Amin, A. A., and Ahmed, E. Z. (2023). Impact of foliar spray with Se, nano-Se and sodium sulfate on growth, yield and metabolic activities of red kidney bean. Sci. Rep. 13, 17102. doi:10.1038/s41598-023-43677-8
Aebi, H. (1984). Catalase in vitro. Methods Enzym. CD-ROM/Methods Enzym. 105, 121–126. doi:10.1016/s0076-6879(84)05016-3
Alam, P., Arshad, M., Al-Kheraif, A. A., Azzam, M. A., and Balawi, T. A. (2022). Silicon nanoparticle-induced regulation of carbohydrate metabolism, photosynthesis, and ROS homeostasis in Solanum lycopersicum subjected to salinity stress. ACS Omega 7 (36), 31834–31844. doi:10.1021/acsomega.2c02586
Ali, S., Shafique, O., Mahmood, T., Hanif, M. A., Ahmed, I., and Khan, B. A. (2018). A review about perspectives of nanotechnology in agriculture. Pak. J. Agri. Res. 30, 116–121. doi:10.17582/journal.pjar/2018/31.2.116.121
Alsafran, M., Razavi, M. M., Rizwan, M., and Usman, K. (2025). A review on synthesis and characterization of selenium nanoparticles from plant extracts for applications in agriculture, biomedicine, and environment. Green Chem. Lett. Rev. 18 (1), 2488237. doi:10.1080/17518253.2025.2488237
Arscott, S. A., and Goldman, I. L. (2012). Biomass effects and selenium accumulation in sprouts of three vegetable species grown in selenium-enriched conditions. Hort. Sci. 47, 497–502. doi:10.21273/HORTSCI.47.4.497
Asghari, J., Mahdavikia, H., Rezaei-Chiyaneh, E., Banaei-Asl, F., Machiani, M. A., and Harrison, M. T. (2023). Selenium nanoparticles improve physiological and phytochemical properties of Basil (Ocimum basilicum L.) under drought stress conditions. Land 12 (1), 164. doi:10.3390/land12010164
Ayeni, A. (2021). Nutrient content of micro/baby-green and field-grown mature foliage of tropical spinach (Amaranthus sp.) and roselle (Hibiscus sabdariffa L.). Foods 10 (11), 2546. doi:10.3390/foods10112546
Banuelos, G. S., Ajwa, H. A., Wu, L., Guo, X., Akohoue, S., and Zambrzuski, S. (1997). Selenium-induced growth reduction in Brassica land races considered for phytoremediation. Ecotoxicol. Environ. Saf. 36, 282–287. doi:10.1006/eesa.1996.1517
Barak, P., and Goldman, I. L. (1997). Antagonistic relationship between selenate and sulfate uptake in onion (Allium cepa): implications for the production of organosulfur and organoselenium compounds in plants. J. Agri. Food Chem. 45, 1290–1294. doi:10.1021/jf960729k
Benzie, I. F., and Strain, J. (1999). Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzym. CD-ROM/Methods Enzym. 299, 15–27. doi:10.1016/s0076-6879(99)99005-5
Beyer, W. F., and Fridovich, I. (1987). Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal. Biochem. 161, 559–566. doi:10.1016/0003-2697(87)90489-1
Boroumand, S., Safari, M., Shaabani, E., Shirzad, M., and Faridi-Majidi, R. (2019). Selenium nanoparticles: synthesis, characterization and study of their cytotoxicity, antioxidant and antibacterial activity. Mat. R. Exp. 6, 0850d8. doi:10.1088/2053-1591/ab2558
Budhani, S., Egboluche, N. P., Arslan, Z., Yu, H., and Deng, H. (2019). Phytotoxic effect of silver nanoparticles on seed germination and growth of terrestrial plants. J. Environ. Sci. Health., Part C 37, 330–355. doi:10.1080/10590501.2019.1676600
Chen, Z., Shen, Y., Xie, A., Zhu, J., Wu, Z., and Huang, F. (2009). L-Cysteine-Assisted controlled synthesis of selenium nanospheres and nanorods. Cryst. Growth Des. 9 (3), 1327–1333. doi:10.1021/cg800398b
Chhipa, H. (2019). “Applications of nanotechnology in agriculture,” in Methods in microbiol, 115–142. doi:10.1016/bs.mim.2019.01.002
Chu, J., Yao, X., and Zhang, Z. (2009). Responses of wheat seedlings to exogenous selenium supply under cold stress. Biol. Trace Elem. Res. 136 (3), 355–363. doi:10.1007/s12011-009-8542-3
Contreras-Calderón, J., Calderón-Jaimes, L., Guerra-Hernández, E., and García-Villanova, B. (2010). Antioxidant capacity, phenolic content and vitamin C in pulp, peel and seed from 24 exotic fruits from Colombia. Food R. Intern. 44 (7), 2047–2053. doi:10.1016/j.foodres.2010.11.003
de los Santos-Vázquez, M. E., Benavides-Mendoza, A., Ruiz-Torres, N. A., Cabrera-de la Fuente, M., and Morelos-Moreno, Á. (2016). Sodium selenite treatment of vegetable seeds and seedlings and the effect on antioxidant status. Emi. J. Food Agri. 28, 589–593. doi:10.9755/ejfa.2016-03-270
Du, Y., Zhou, J., He, F., Zang, P., Gong, H., Liu, C., et al. (2023). A bright future: advanced nanotechnology-assisted microwave therapy. Nano Today 52, 101963. doi:10.1016/j.nantod.2023.101963
Duff, R., and Chessin, M. (1965). Rapid method for selenium assay of plant material. Nature 208 (5014), 1001–1001.
Dumore, N. D., and Mukhopadhyay, M. (2020). Antioxidant properties of aqueous selenium nanoparticles (ASeNPs) and its catalysts activity for 1, 1-diphenyl-2-picrylhydrazyl (DPPH) reduction. J. Mol. Struc. 1205, 127637. doi:10.1016/j.molstruc.2019.127637
El-Saadony, M. T., Saad, A. M., Najjar, A. A., Alzahrani, S. O., Alkhatib, F. M., Shafi, M. E., et al. (2021). The use of biological selenium nanoparticles to suppress Triticum aestivum L. crown and root rot diseases induced by Fusarium species and improve yield under drought and heat stress. Saudi J. Biol. Sci. 28 (8), 4461–4471. doi:10.1016/j.sjbs.2021.04.043
El-Saadony, M. T., Saad, A. M., Soliman, S. M., Salem, H. M., Desoky, E. M., Babalghith, A. O., et al. (2022). Role of nanoparticles in enhancing crop tolerance to abiotic stress: a Comprehensive review. Front. Pl. Sci. 13, 946717. doi:10.3389/fpls.2022.946717
Fatima, M., Maqbool, A., Sardar, R., Maqsood, M. F., and Zulfiqar, U. (2024). Nano-Selenium: a green promising approach against abiotic stresses in plants. J. Soil Sci. Pl. Nutrit. 24 (3), 6000–6023. doi:10.1007/s42729-024-01956-x
Francis, D. V., Abdalla, A. K., Mahakham, W., Sarmah, A. K., and Ahmed, Z. F. (2024b). Interaction of plants and metal nanoparticles: exploring its molecular mechanisms for sustainable agriculture and crop improvement. Environ. Intern. 190, 108859. doi:10.1016/j.envint.2024.108859
Francis, D. V., Subhan, A., Mourad, A. H. I., Abdalla, A. K., and Ahmed, Z. F. R. (2024a). Optimizing germination conditions of Ghaf seed using ZnO nanoparticle priming through Taguchi method analysis. Sci. Rep. 14, 15946. doi:10.1038/s41598-024-67025-6
Ghanbari, F., Bag-Nazari, M., and Azizi, A. (2023). Exogenous application of selenium and nano-selenium alleviates salt stress and improves secondary metabolites in lemon verbena under salinity stress. Sci. Rep. 13, 5352. doi:10.1038/s41598-023-32436-4
Guleria, A., Neogy, S., Raorane, B. S., and Adhikari, S. (2020). Room temperature ionic liquid assisted rapid synthesis of amorphous Se nanoparticles: their prolonged stabilization and antioxidant studies. Mat. Chem. Phy. 253, 123369. doi:10.1016/j.matchemphys.2020.123369
Hawrylak-Nowak, B. (2009). Beneficial effects of exogenous selenium in cucumber seedlings subjected to salt stress. Biol. Trace Elem. Res. 132, 259–269. doi:10.1007/s12011-009-8402-1
Hernández-Hernández, H., Quiterio-Gutiérrez, T., Cadenas-Pliego, G., Ortega-Ortiz, H., Hernández-Fuentes, A. D., Cabrera de la Fuente, M., et al. (2019). Impact of selenium and copper nanoparticles on yield, antioxidant system, and fruit quality of tomato plants. Plants 8, 355. doi:10.3390/plants8100355
Hussein, H. A., Darwesh, O. M., and Mekki, B. B. (2019). Environmentally friendly nano-selenium to improve antioxidant system and growth of groundnut cultivars under sandy soil conditions. Biocat. Agric. Biotechnol. 18, 101080. doi:10.1016/j.bcab.2019.101080
Iqbal, M. F., Irshad, I., Ahmed, I., Ahmad, S., Uzair, M., Kausar, R., et al. (2025). Comparative study of the ability of green synthesized Se-NPs and CTS-NPs to overcome drought stress in Oryza sativa L. for regenerative nanoengineering in agriculture. New J. Chem. 49 (18), 7358–7375. doi:10.1039/D4NJ04880F
Ismail, L., Metwally, H., Alshirbiny, A., and El-Behairy, U. (2023). Exogenous nano-selenium application mitigates low-temperature stress in green bean plants. J. Environ. Sci. 51 (12), 0–55. doi:10.21608/jes.2023.174548.1397
Jiang, C., Zu, C., Lu, D., Zheng, Q., Shen, J., Wang, H., et al. (2017). Effect of exogenous selenium supply on photosynthesis, Na+ accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress. Sci. Rep. 7, 42039. doi:10.1038/srep42039
Kamal, A., Nazari, M. V., Yaseen, M., Iqbal, M. A., Ahamed, M. B. K., Majid, A. S. A., et al. (2019). Green synthesis of selenium-N-heterocyclic carbene compounds: evaluation of antimicrobial and anticancer potential. Bioorg. Chem. 90, 103042. doi:10.1016/j.bioorg.2019.103042
Khurana, A., Tekula, S., Saifi, M. A., Venkatesh, P., and Godugu, C. (2019). Therapeutic applications of selenium nanoparticles. Biomed. and Pharmacother. 111, 802–812. doi:10.1016/j.biopha.2018.12.146
Kondaparrthi, P., Flora, S. J. S., and Naqvi, S. (2019). Selenium nanoparticles: an insight on its pro-oxidant and antioxidant properties. Front. Nanosci. Nanotech. 6, 1–5. doi:10.15761/FNN.1000189
Lefsrud, M. G., Kopsell, D. A., Kopsell, D. E., and Curran-Celentano, J. (2006). Irradiance levels affect growth parameters and carotenoid pigments in kale and spinach grown in a controlled environment. Physiol. Plant. 127, 624–631. doi:10.1111/j.1399-3054.2006.00692.x
Lin, M., Shen, M., Wu, J., Chen, H., Xu, Y., and Liu, W. (2021). Effects of zinc oxide nanoparticles on germination and seedling growth of two vegetables. J. Agricul. Res. Environ. 38, 72–78. doi:10.1007/s00128-023-03752-2
Lintschinger, J., Fuchs, N., Moser, J., Kuehnelt, D., and Goessler, W. (2000). Selenium-enriched sprouts. A raw material for fortified cereal-based diets. J. Agric. Food Chem. 48, 5362–5368. doi:10.1021/jf000509d
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. J Biol. Chem. 193, 265–275. doi:10.1016/s0021-9258(19)52451-6
Lyons, G. H., Stangoulis, J. C., and Graham, R. D. (2005). Tolerance of wheat (Triticum aestivum L.) to high soil and solution selenium levels. Plant Soil 270, 179–188. doi:10.1007/s11104-004-1390-1
Marisamy, K., Duraipandian, M., Sevugaperumal, R., and Ramasubramanian, V. (2015). Estimation of barium toxicity mitigating efficacy of Amaranthus caudatus L. Uni. J. Environ. Res. Tech. 5, 295–305.
Marslin, G., Sheeba, C. J., and Franklin, G. (2017). Nanoparticles alter secondary metabolism in plants via ROS burst. Fron. Pl. Sci. 8, 832. doi:10.3389/fpls.2017.00832
Mazumdar, B. C., and Majumder, K. (2021). Methods on physico-chemical analysis of fruits. Daya Publishing House, a division of Astral International Pvt Limited.
Mellinas, C., Jimenez, A., and Garrigos, M. C. (2019). Microwave-assisted green synthesis and antioxidant activity of selenium nanoparticles using Theobroma cacao L. bean shell extract. Molecules 24, 4048–48. doi:10.3390/molecules24224048
Nagdalian, A. A., Blinov, A. V., Siddiqui, S. A., Gvozdenko, A. A., Golik, A. B., Maglakelidze, D. G., et al. (2023). Effect of selenium nanoparticles on biological and morphofunctional parameters of barley seeds (Hordéum vulgáre L.). Sci. Rep. 13 (1), 6453.
Ordonez, F., Rosety-Plaza, M., and Rosety-Rodriguez, M. (2006). Glucose-6-phosphate-dehydrogenase is also increased in erythrocytes from adolescents with Down syndrome. Down Syndrome Research and Practice 11 (2), 84–87.
Perfileva, A. I., Kharasova, A. R., Nozhkina, O. A., Sidorov, A. V., Graskova, I. A., and Krutovsky, K. V. (2023). Effect of nanopriming with selenium nanocomposites on potato productivity in a field experiment, soybean germination and viability of pectobacterium carotovorum. Horticulturae 9 (4), 458. doi:10.3390/horticulturae9040458
Rady, M. M., Belal, H. E., Gadallah, F. M., and Semida, W. M. (2020). Selenium application in two methods promotes drought tolerance in Solanum lycopersicum plant by inducing the antioxidant defense system. Sci. Horticul. 266, 109290. doi:10.1016/j.scienta.2020.109290
Ramos, S. J., Faquin, V., Guilherme, L. R. G., Castro, E. M., Ávila, F. W., Carvalho, G. S., et al. (2010). Selenium biofortification and antioxidant activity in lettuce plants fed with selenate and selenite. Pl. Soil Environ. 56, 584–588. doi:10.17221/113/2010-PSE
Rao, S., Xiao, X., Wang, Y., Xiong, Y., Cheng, H., Li, L., et al. (2022). Comparative study of the effects of selenium nanoparticles and selenite on selenium content and nutrient quality in soybean sprouts. Folia Horticul 34 (2), 223–234. doi:10.2478/fhort-2022-0017
Rastogi, A., Tripathi, D. K., Yadav, S., Chauhan, D. K., Živčák, M., Ghorbanpour, M., et al. (2019). Application of silicon nanoparticles in agriculture. 3 Biotech. 9, 90–11. doi:10.1007/s13205-019-1626-7
Rezagholi, M., Fard, J. R., and Darvishzadeh, R. (2025). Selenium nanoparticles mitigates drought stress in E. purpurea by enhancing morpho-physiological characteristics and gene expression related to the phenylpropanoid pathway. Industrial Crops Prod. 227, 120833. doi:10.1016/j.indcrop.2025.120833
Sarwar, N., Akhtar, M., Kamran, M. A., Imran, M., Riaz, M. A., Kamran, K., et al. (2020). Selenium biofortification in food crops: key mechanisms and future perspectives. J. Food Comp. Anal. 93, 103615. doi:10.1016/j.jfca.2020.103615
Shahraki, B., Bayat, H., Aminifard, M. H., and Atajan, F. A. (2022). Effects of foliar application of selenium and nano-selenium on growth, flowering, and antioxidant activity of pot marigold (Calendula officinalis L.) under salinity stress conditions. Commun. Soil Sci. Pl. Anal. 53, 2749–2765. doi:10.1080/00103624.2022.2089679
Terry, N., Zayed, A. M., De Souza, M. P., and Tarun, A. S. (2000). Selenium in higher plants. Ann. Rev. Plant Biol. 51 (1), 401–432.
Usman, M., Farooq, M., Wakeel, A., Nawaz, A., Cheema, S. A., ur Rehman, H., et al. (2020). Nanotechnology in agriculture: current status, challenges and future opportunities. Sci. Total Environ. 721, 137778. doi:10.1016/j.scitotenv.2020.137778
Vijayarengan, P. (2013). Changes in growth, biochemical constituents and antioxidant potentials in cluster bean Cyamopsis tetragonoloba L. Taub under zinc stress. Internat. J. Curr. Sci. 5, 37–49.
Webster, T. J., and Ramos, N. (2012). Cytotoxicity of selenium nanoparticles in rat dermal fibroblasts. Int. J. Nanomed 3907, 3907–3914. doi:10.2147/ijn.s33767
Wu, Z., Yin, X., Banuelos, G. S., Lin, Z. Q., Liu, Y., Li, M., et al. (2016). Indications of selenium protection against cadmium and lead toxicity in oilseed rape (Brassica napus L.). Front. Plant Sci. 7, 1875. doi:10.3389/fpls.2016.01875
Zeeshan, M., Wang, X., Salam, A., Wu, H., Li, S., Zhu, S., et al. (2024). Selenium nanoparticles boost the drought stress response of soybean by enhancing pigment accumulation, oxidative stress management and ultrastructural integrity. Agronomy 14 (7), 1372. doi:10.3390/agronomy14071372
Zhan, T., Zhang, Z., Sun, D., Wang, Y., Zhang, L., Li, W., et al. (2021). Chitin combined with selenium reduced nitrogen loss in soil and improved nitrogen uptake efficiency in Guanxi pomelo orchard. Sci. Total Environ. 799, 149414. doi:10.1016/j.scitotenv.2021.149414
Keywords: selenium nanoparticles, amaranthus, microgreens, green synthesis, biofortification, sustainable agriculture, drought stress
Citation: Gomathi A, Sriharini R, Arumuka Pravin I, Kaushik R, Ramesh Kumar A, Anbu Sezhian A and Srivignesh S (2025) Impact of green synthesized selenium nanoparticles on the growth and development of amaranth microgreens. Front. Nanotechnol. 7:1621024. doi: 10.3389/fnano.2025.1621024
Received: 30 April 2025; Accepted: 25 June 2025;
Published: 24 July 2025.
Edited by:
Jayanta Kumar Patra, Dongguk University Seoul, Republic of KoreaReviewed by:
Ilika Ghosh, Max Planck Florida Institute for Neuroscience (MPFI), United StatesZienab F. R. Ahmed, United Arab Emirates University, United Arab Emirates
Rupesh Kumar, O. P. Jindal Global University, India
Copyright © 2025 Gomathi, Sriharini, Arumuka Pravin, Kaushik, Ramesh Kumar, Anbu Sezhian and Srivignesh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sundaresan Srivignesh, c3JpdmlnbmVzaEBjdXRuLmFjLmlu
 Arivalagan Gomathi
Arivalagan Gomathi Ramalingam Sriharini
Ramalingam Sriharini Iyadurai Arumuka Pravin
Iyadurai Arumuka Pravin Rajaram Kaushik
Rajaram Kaushik Alagarsamy Ramesh Kumar
Alagarsamy Ramesh Kumar Ambethgar Anbu Sezhian
Ambethgar Anbu Sezhian Sundaresan Srivignesh
Sundaresan Srivignesh