- State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, National Medical Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
Introduction: Metabolic dysfunction-associated steatotic liver disease (MASLD) is a global health burden with an increasing incidence of hepatocellular carcinoma (HCC), yet early risk predictors remain elusive. This study investigated trans-palmitoleic acid (TPA) as a potential biomarker for MASLD-HCC risk.
Methods: Using National Health and Nutrition Examination Survey (NHANES) data (n = 548), propensity score matching (PSM) minimized sociobehavioral confounders. Multivariable logistic regression and restricted cubic spline (RCS) models were employed to assess the association between TPA and HCC risk.
Results: A striking nonlinear association between TPA and HCC risk was observed (P-nonlinear <0.001). Each unit increase in TPA elevated HCC risk by 52.4% (OR = 1.524, 95% CI = 1.397–1.677), with quartile analysis showing exponential risk escalation (Q4 OR = 753.7). Subgroup analyses identified heightened susceptibility in women (Q4 OR = 1148.83) and younger individuals (OR = 612.11). TPA correlated positively with lipid factors (triglycerides β = 1.236, LDL β = 0.557) and hematologic indices, while exhibiting a negative association with BMI (β = −0.037). Mediation analysis implicated triglycerides as a key metabolic intermediary (39.18% effect proportion).
Discussion: These findings establish TPA as an independent MASLD-HCC risk factor with distinct demographic variability, potentially mediated through lipid dysregulation. While limited by observational design, this study highlights TPA’s prognostic value for HCC risk stratification, especially in non-diabetic populations (OR = 257.14). Future mechanistic studies should validate TPA’s oncogenic pathways and explore therapeutic targeting of trans-fatty acid metabolism to mitigate MASLD progression. The robust dose-response relationship and metabolic mediation effects position TPA as a promising candidate for incorporation into existing HCC prediction models.
1 Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD) has emerged as a global health crisis, affecting over 30% of the adult population worldwide (1–3). This condition represents a spectrum of liver disorders ranging from simple steatosis to progressive inflammation and fibrosis, with hepatocellular carcinoma (HCC) being the most devastating endpoint (4). The annual incidence of MASLD-related HCC has shown a concerning upward trajectory, contributing significantly to liver-related mortality and imposing substantial economic burdens on healthcare systems (5). Current epidemiological data reveal that MASLD-associated HCC accounts for 10%–30% of all HCC cases in Western countries (6–8), with projections suggesting this proportion will continue to rise in parallel with the obesity pandemic (9–11). The transition from MASLD to HCC involves complex pathophysiological processes that remain incompletely understood (12), highlighting the urgent need for mechanistic insights and predictive biomarkers.
Current diagnostic and therapeutic approaches for MASLD-HCC face significant limitations (13). Non-invasive diagnostic tools, including ultrasound and transient elastography, often fail to detect early malignant transformation (14, 15), while serum biomarkers such as alpha fetoprotein lack sufficient specificity for clinical implementation (16). Therapeutic strategies primarily focus on lifestyle modifications and metabolic control, with limited efficacy in preventing HCC development (17–20). Pharmacological interventions targeting metabolic pathways show promise but have not demonstrated consistent benefits in HCC prevention (4). The absence of reliable early warning indicators and incomplete understanding of the molecular mechanisms driving MASLD progression to HCC represent critical knowledge gaps in clinical hepatology. This underscores the necessity for identifying novel risk factors and elucidating their pathogenic roles in hepatocarcinogenesis.
A significant research gap exists regarding the role of trans-palmitoleic acid (trans-9-hexadecenoic acid, trans-C16:1 n-7, TPA), an understudied fatty acid metabolite, in MASLD-HCC development. This unusual fatty acid, primarily derived from dairy products, exhibits unique biological properties that distinguish it from other fatty acids (21). Interestingly, TPA levels have been associated with both beneficial and detrimental metabolic effects in epidemiological studies, creating a paradoxical scenario that warrants further investigation. While some reports link TPA to improved glucose metabolism (22), others highlighted a neutral role of TPA on diabetes (23), and others report elevated erythrocyte membrane TPA levels correlate with increased diabetes risk (24). Thus, understanding whether TPA serves as a biomarker of risk or an active participant in hepatocarcinogenesis could provide valuable insights for both risk stratification and therapeutic development.
To address these limitations, we employed a rigorous analytical approach utilizing data from the National Health and Nutrition Examination Survey (NHANES), a nationally representative dataset with extensive biochemical and clinical measurements. Our study design incorporated multiple statistical techniques: propensity score matching (PSM) to balance potential confounders across exposure groups (25); linear and nonlinear models [box plots, linear regression, and restricted cubic splines (RCSs)] to elucidate the dose-response relationship between TPA and HCC (26); subgroup stratification by gender, age and socioeconomic status to assess population differences in risk effects; and mediation effect models to dissect the mediating roles of various metabolic factors in the pathway linking TPA to HCC development (27). Our primary objectives were to establish the strength and nature of the association between TPA and MASLD-HCC, characterize population-specific risk patterns, and identify key metabolic mediators in this relationship. These findings may contribute to the development of novel risk prediction tools and inform targeted prevention strategies for high-risk populations.
2 Materials and methods
2.1 Data and preprocessing
The NHANES represents an ongoing, cross-sectional, and nationally representative investigation conducted in the United States. This significant research initiative is managed by the National Center for Health Statistics (NCHS) and receives authorization and funding from the Centers for Disease Control and Prevention (CDC) with the aim of evaluating the health and nutritional conditions of the civilian population in the United States who are not institutionalized. Data collection occurs biennially through a sophisticated multistage probability sampling methodology, which encompasses in-home, face-to-face interviews followed by comprehensive physical assessments at the Mobile Examination Center (MEC), during which blood and urine specimens are obtained. The NHANES protocol undergoes scrutiny and receives endorsement from the NCHS Research Ethics Review Board, ensuring that informed consent has been acquired from all participants involved. In our study, we utilized data from 10 cycles (1999–2018) of the NHANES database, which contains comprehensive test data on MASLD-HCC for participants. To account for inter-cycle variability, we applied survey weights provided in the NHANES documentation. Missing data were handled using multiple imputation methods. TPA level in plasma were measured by GC/MS as described in NHANES trans fatty acids procedure. The specific procedures included: converting fatty acids into their free forms for extraction, derivatization with pentafluorobenzyl bromide, separation via capillary gas chromatography, and detection by negative chemical ionization mass spectrometry. Fatty acids were identified by comparing retention times with standards and specific mass-to-charge ratios, while quantitative analysis was performed using a stable isotope-labeled internal standard method.
2.2 Diagnostic criteria for MASLD
The initial stage of MASLD is considered to be characterized by abnormal hepatic fat accumulation. Due to the absence of transient elastography data for liver assessment in the 1998–2018 NHANES database cycle, this study employs the Fatty Liver Index (FLI) as a surrogate assessment indicator. The FLI is calculated using the formula:
TG denotes total triglycerides, BMI refers to body mass index, GGT represents gamma-glutamyl transferase, and WC stands for waist circumference (28–32). After excluding other liver diseases associated with the aforementioned etiological factors, participants with a FLI > 60 were diagnosed with MASLD.
2.3 Patients inclusion criteria
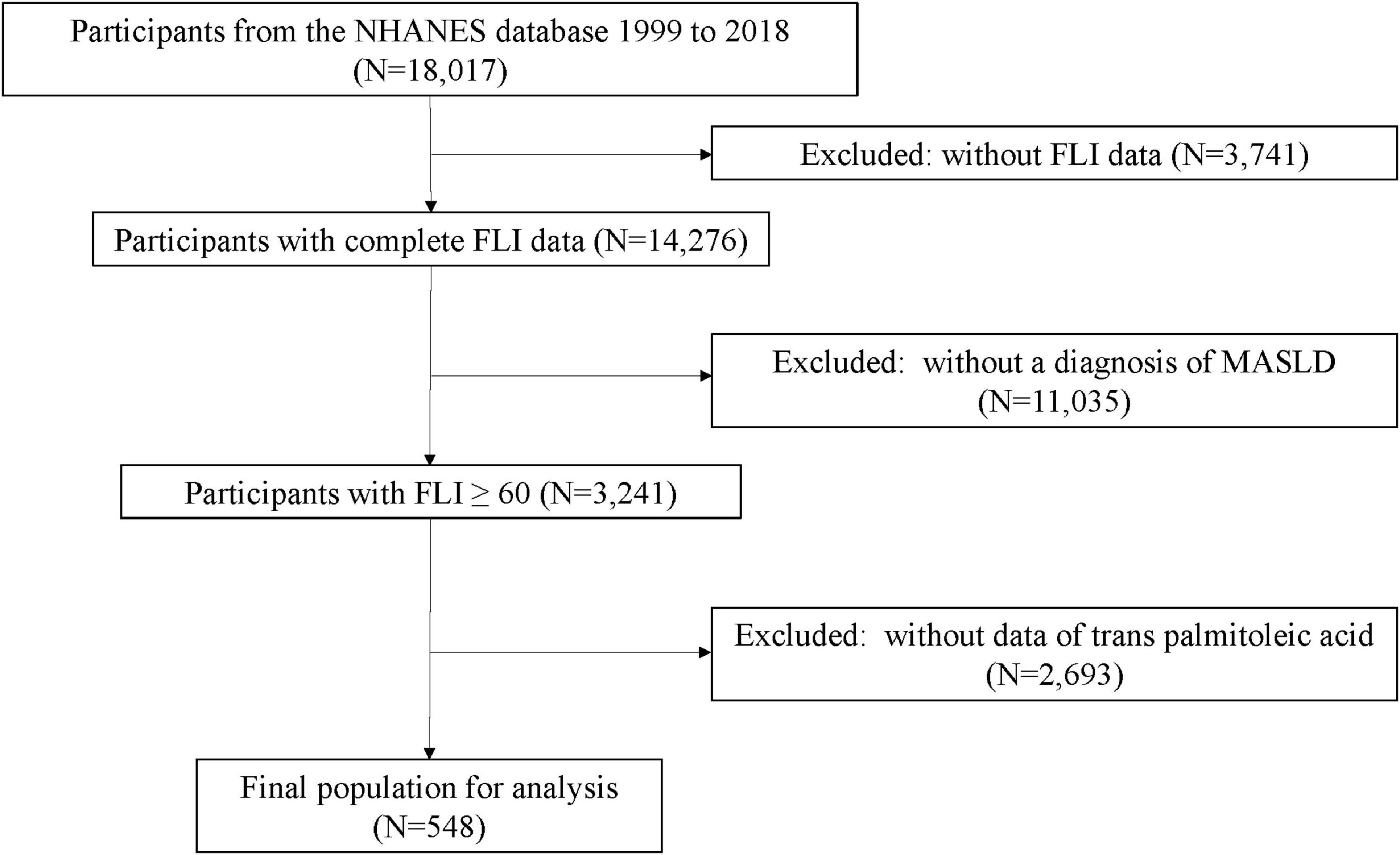
From the initial cohort of 18,017 participants, exclusions were made for the following reasons: (1) removal of samples lacking FLI data (N = 3,741), (2) exclusion of samples without MASLD (N = 11,035), and (3) exclusion of samples lacking TPA (N = 2,693). The final study population comprised 548 participants, including 239 cases of MASLD-HCC and 309 MASLD samples (Figure 1).
2.4 Propensity score matching
Propensity score matching is a statistical technique utilized to address data from observational studies. In observational studies, data bias and the presence of confounding variables are common due to various reasons. The method of PSM is designed to reduce the impact of these biases and confounding factors, thereby enabling a more rational comparison between the experimental group and the control group. This method was first introduced by Rosenbaum and Rubin (33) and is frequently employed in fields such as medicine, public health, and economics to match baseline characteristics (34). In our study, we implemented a 1:1 nearest-neighbor matching algorithm to ensure balanced group comparisons.
2.5 Weighted logistic regression analysis
Weighted binary logistic regression was utilized to investigate explore the possible association between levels of TPA and the occurrence of MASLD-HCC. In the regression model, TPA levels were incorporated as both continuous and categorical variables. This methodological framework enabled the estimation of odds ratios (ORs) along with their respective 95% confidence intervals (95% CIs). The TPA concentrations were first treated as a continuous variable. Subsequently, these concentrations were categorized into quartiles according to the interquartile range, organized from the lowest to the highest levels. These quartiles were designated as the first quartile (Q1), second quartile (Q2), third quartile (Q3), and fourth quartile (Q4), and were also assessed as categorical variables. In cases where TPA levels were analyzed as categorical variables, the lowest quartile (Q1) served as the reference group. The study delineated three distinct statistical analysis groups: model 1 constituted the unadjusted model, whereas model 2 incorporated adjustments for demographic factors including gender, age, race, education, and poverty-income ratio (PIR). Building on the adjustments made in model 2, model 3 additionally accounted for factors such as diabetes, lipid profiles, cholesterol levels, low-density lipoprotein, high-density lipoprotein, albumin, creatinine, blood urea nitrogen, hemoglobin, platelet count, and glycated hemoglobin. All regression analyses were conducted with the inclusion of survey weights, and non-normally distributed continuous covariates underwent transformation utilizing weighted quartiles. Furthermore, the study evaluated potential interactions among factors associated with TPA levels through the application of the variance inflation factor (VIF), where a VIF value of less than 10 indicated no interaction between the examined factor and other variables. The P values were adjusted to account for the false discovery rate (FDR).
2.6 Clinical subgroup analysis
Forest plots are primarily used for visualizing the comparison of effect sizes and CIs across multiple study results. They are commonly employed to summarize and compare outcomes from different studies, providing a more intuitive representation of the effect size (such as RR, OR, HR, or WMD) and their corresponding 95% CIs. This visualization aids in better understanding the consistency and discrepancies between different studies.
2.7 Restricted cubic spline
Restricted cubic spline is a concept in statistics, particularly frequently used in regression analysis and curve fitting. It is a method for fitting and modeling continuous variables by dividing the data range into several intervals and using a cubic polynomial for fitting within each interval to create a smooth curve. These polynomials are smoothly connected across adjacent intervals, often with additional smoothness constraints to avoid sharp fluctuations in the curve. In statistical modeling, RCS is commonly used to model the relationship between continuous variables and the dependent variable. In regression analysis, it allows for capturing nonlinear relationships while maintaining smoothness and avoiding overfitting. In this study, the RCS analysis was performed using the R “rms” package, with three knots automatically selected as the optimal number based on statistical algorithms.
2.8 Multilevel logistic regression model
In the realm of statistics, the multilevel logistic regression model serves as an advanced classification technique that extends traditional logistic regression to accommodate multicategory scenarios. This model is particularly designed to forecast the probabilities associated with various potential outcomes of a dependent variable that has a categorical distribution. Within the framework of a multilevel logistic regression model, the dependent variable’s predictions are made utilizing a series of independent variables, also known as features or observed variables. This model operates by computing the likelihood of achieving a specific outcome in the dependent variable through a linear combination of the independent variables along with their respective parameters, all framed within a probabilistic model. The parameters linked to the independent variables are derived from the training dataset, and they are commonly referred to as regression coefficients. Importantly, the multilevel logistic regression model empowers researchers to account for the inherent clustered structure of the data, explore the origins of variation both within and between clusters, identify which variables account for individual differences, and ascertain which factors influence variations at the cluster level.
2.9 Statistical analysis
All data processing and analytical procedures were performed utilizing R software (version 4.4.0). For the analysis of baseline characteristics, medians and interquartile ranges were utilized to characterize continuous variables that exhibited non-normal distributions. Classification variables were reported as sample counts and weighted percentages. To check for changes in variable characteristics between groups of TPA (quartiles), we used Wilcoxon rank sum tests to test continuous variables and Rao-Scott Chi-square tests to test weighted percentages of categorical variables, providing a comprehensive description of the entire population. The “mediation” package was used for mediation analysis to assess the mediating effects of lipid indicators (including cholesterol, low density lipoprotein, high density lipoprotein, and triglycerides) on the association between TPA and MASLD-HCC. The existence of mediation effects was characterized by the presence of a notable indirect effect, a significant overall effect, and a positive ratio of the mediation effect. All statistical evaluations were conducted as two-tailed tests, with a P-value of less than 0.05 deemed to indicate statistical significance. For all hypothesis tests (including primary, subgroup, and mediation analyses), Benjamini–Hochberg method was applied to adjust P values for FDR.
3 Results
3.1 Baseline characteristics of the participants
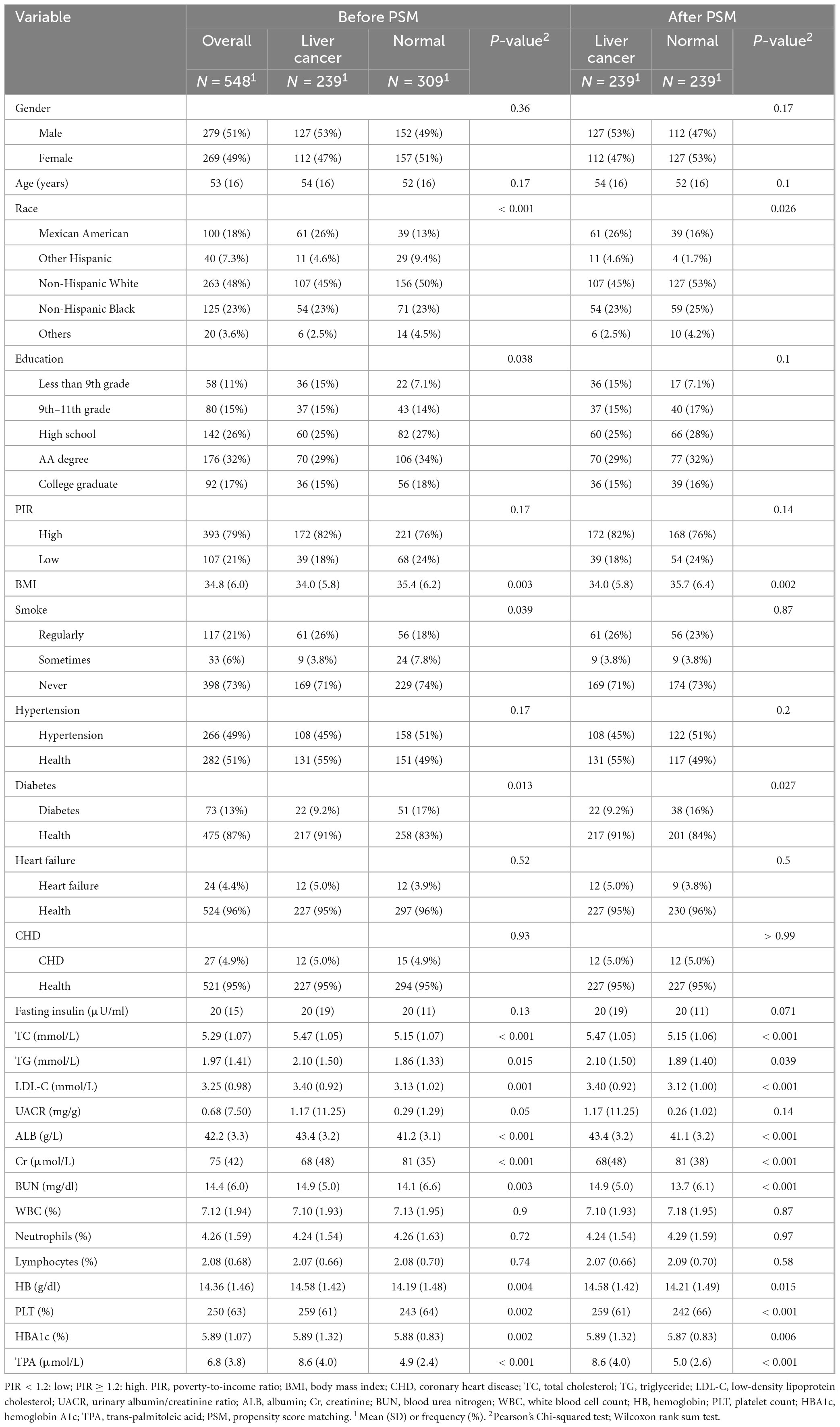
In this study, a final cohort of 548 patients was incorporated to evaluate the baseline characteristics, including factors such as gender, age, and race, in connection with the occurrence of HCC. The baseline characteristics of participants were presented in Table 1. Before PSM, education and smoking showed differences between the HCC and non-HCC groups (P-value = 0.038, P-value = 0.039), whereas no differences were observed after PSM (P-value = 0.1, P-value = 0.87). Some other baseline data also showed differences before and after PSM in both the HCC and non-HCC groups, such as race (P-value < 0.001 before PSM, P-value = 0.026 after PSM), BMI (P-value = 0.003 before PSM, P-value = 0.002 after PSM), diabetes (P-value = 0.013 before PSM, P-value = 0.027 after PSM), total cholesterol (P-value < 0.001 before PSM, P-value < 0.001 after PSM), triglycerides (P-value = 0.015 before PSM, P-value = 0.039 after PSM), and low-density lipoprotein (P-value = 0.001 before PSM, P-value < 0.001 after PSM). These indicators, which showed differences before and after PSM in both the HCC and non-HCC groups, could be considered for mediation effect analysis. Some baseline indicators, however, showed no differences before and after PSM in both the HCC and non-HCC groups, such as gender, age, education, and PIR. This indicates that these indicators were relatively balanced between the HCC and non-HCC groups, and their confounding factors were excluded when comparing the two groups.
3.2 Association of MASLD-HCC with TPA
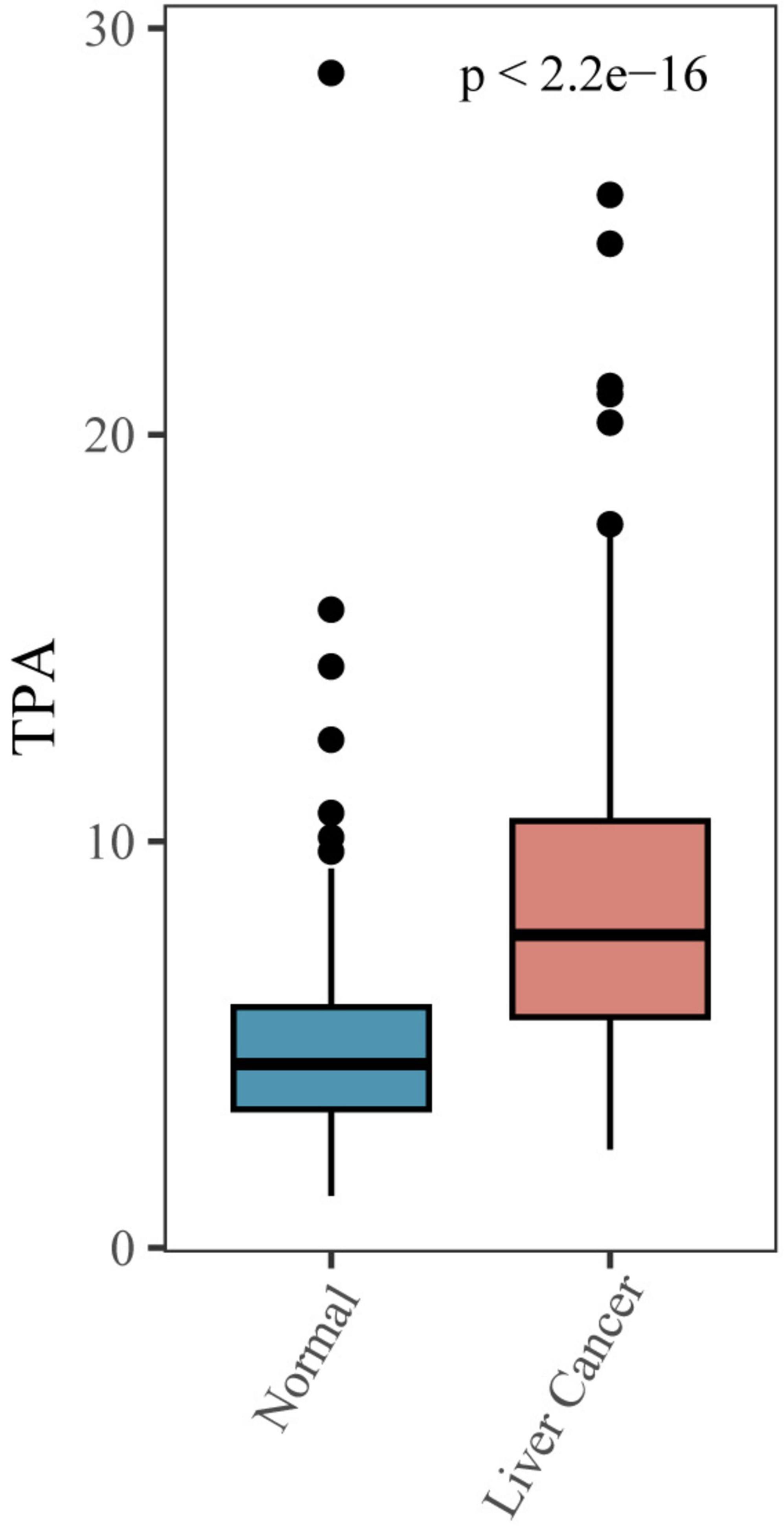
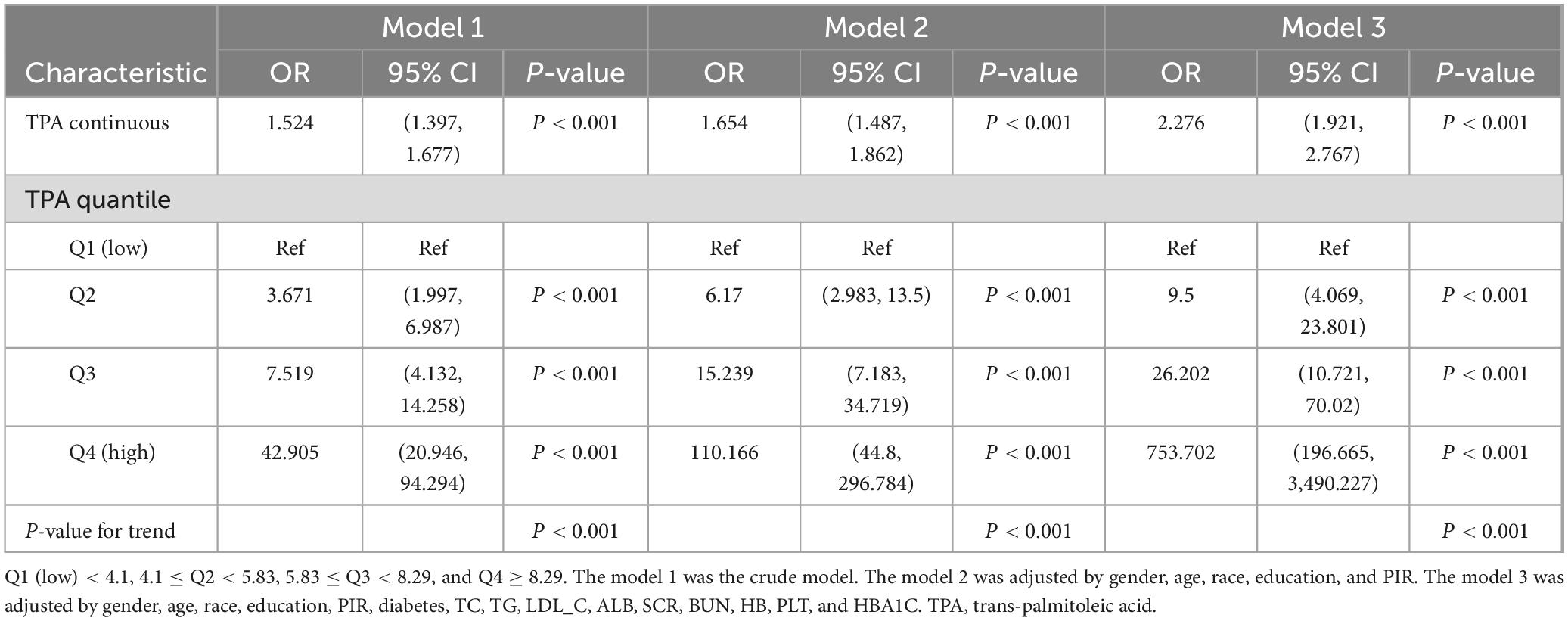
We utilized box plots to illustrate the relationship between MASLD-HCC and TPA, revealing a significant difference in TPA levels between the cancer and non-cancer groups (Figure 2). To further analyze this difference, we utilized linear regression analysis and multilevel logistic regression model to demonstrate the relationship (Table 2). Model 1, without covariates, revealed a positive correlation between TPA levels and MASLD-HCC (OR = 1.524, 95% CI = 1.397–1.677, P-value < 0.001); when compared to Q1, Q2 had an OR of 3.671 (95% CI = 1.997–6.987, P-value < 0.001), Q3 had an OR of 7.519 (95% CI = 4.132–14.258, P-value < 0.001), and Q4 had an OR of 42.905 (95% CI = 20.946–94.294, P-value < 0.001), indicating that with each increase in TPA, the probability of developing MASLD-HCC increased. Model 2, with gender, age, race, education, and PIR as covariates, and model 3, further adding diabetes, blood lipids, cholesterol, low-density lipoprotein, high-density lipoprotein, albumin, creatinine, blood urea nitrogen, hemoglobin, platelet count, and glycated hemoglobin as covariates, both showed a similar positive correlation between TPA and MASLD-HCC (model 2 OR = 1.654, 95% CI = 1.487–1.862, P-value < 0.001; model 3 OR = 2.276, 95% CI = 1.921–2.767, P-value < 0.001). When grouped by quartile intervals, both model 2 and model 3 revealed that, compared to Q1, the OR values for Q2 (model 2 OR = 6.170, 95% CI = 2.983–13.500, P-value < 0.001; model 3 OR = 9.500, 95% CI = 4.069–23.801, P-value < 0.001), Q3 (model 2 OR = 15.239, 95% CI = 7.183–34.719, P-value < 0.001; model 3 OR = 26.202, 95% CI = 10.721–70.020, P-value < 0.001), and Q4 (model 2 OR = 110.166, 95% CI = 44.800–296.784, P-value < 0.001; model 3 OR = 753.702, 95% CI = 196.665–3,490.227, P-value < 0.001) increased progressively, indicating that with the addition of covariates, the probability of developing MASLD-HCC also increased with increasing TPA.
3.3 Restricted cubic spline curves for MASLD-HCC and its subgroups
Restricted cubic spline curves were utilized to explore the association between TPA levels and the incidence of MASLD-HCC, while accounting for all pertinent covariates. A statistically significant nonlinear relationship was observed between TPA and MASLD-HCC (P non-linear < 0.001, cutoff value = 5.85 μmol/L, Figure 3A). Furthermore, a significant nonlinear relationship was found between TPA and the incidence of MASLD-HCC in the male subgroup (P non-linear < 0.001, cutoff value = 5.92 μmol/L, Figure 3B), as well as in the female subgroup (P non-linear < 0.001, cutoff value = 5.83 μmol/L, Figure 3C).
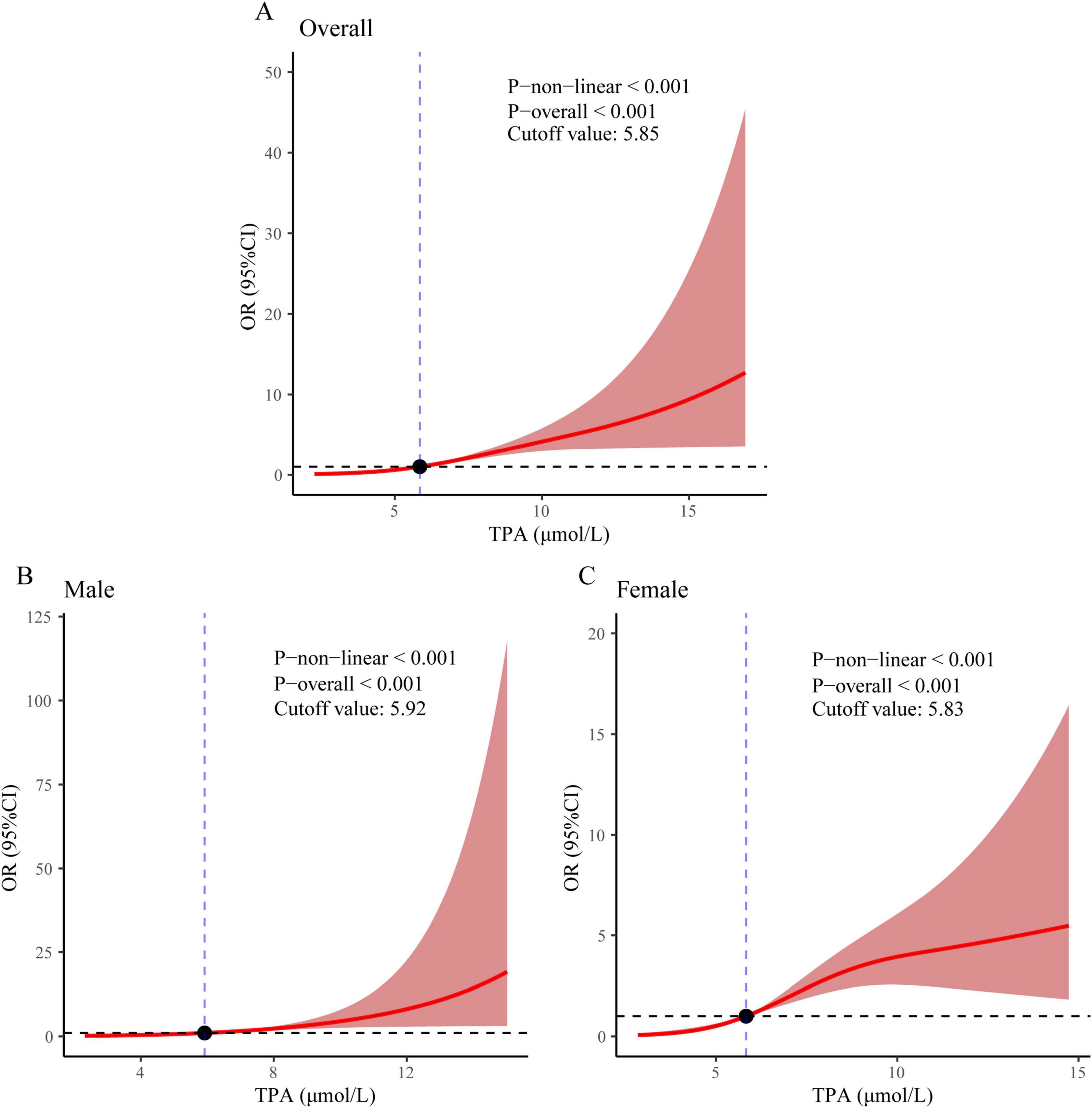
Figure 3. Restricted cubic spline regression analysis revealed an association between MASLD-HCC and TPA levels in (A) the overall population, (B) males, and (C) females.
3.4 Relationship of TPA with baseline characteristics in various subgroups
Figure 4 illustrates the association between TPA and MASLD-HCC, with the relationship analyzed across various subgroups stratified by gender, age, PIR, hypertension, and diabetes using fully adjusted multivariate logistic regression. We found that in both male and female gender subgroups, the incidence of HCC increased with increasing levels of TPA (male group: Q2 vs. Q1, OR = 4.10, 95% CI = 1.61–10.43, P-value = 0.003; Q3 vs. Q1, OR = 7.80, 95% CI = 2.95–20.64, P-value < 0.001; Q4 vs. Q1, OR = 89.27, 95% CI = 18.20–437.98, P-value < 0.001; female group: Q2 vs. Q1, OR = 10.92, 95% CI = 2.79–42.63, P-value = 0.001; Q3 vs. Q1, OR = 24.35, 95% CI = 5.99–98.90, P-value < 0.001; Q4 vs. Q1, OR = 1,148.83, 95% CI = 152.86–8,634.14, P-value < 0.001). The age young subgroup appeared to be more sensitive to TPA, with increasing OR values for Q2, Q3, and Q4 compared to Q1 (Q2 vs. Q1, OR = 6.84, 95% CI = 2.91–16.10, P-value < 0.001; Q3 vs. Q1, OR = 13.76, 95% CI = 5.64–33.54, P-value < 0.001; Q4 vs. Q1, OR = 612.11, 95% CI = 130.62–2,868.48, P-value < 0.001), while the tendency was observed in the age old subgroup only when comparing Q3 and Q4 to Q1 (Q3 vs. Q1, OR = 5.96, 95% CI = 1.16–30.71, P-value = 0.033; Q4 vs. Q1, OR = 15.17, 95% CI = 1.63–141.21, P-value = 0.017). A similar trend was observed in the PIR subgroup, with significant differences in Q2, Q3, and Q4 compared to Q1 in the high PIR group (Q2 vs. Q1, OR = 7.99, 95% CI = 3.33–19.16, P-value < 0.001; Q3 vs. Q1, OR = 18.29, 95% CI = 7.28–45.94, P-value < 0.001; Q4 vs. Q1, OR = 437.48, 95% CI = 102.84–1,860.99, P-value < 0.001), and in Q3 and Q4 compared to Q1 in the low PIR group (Q3 vs. Q1, OR = 10.00, 95% CI = 1.13–88.71, P-value = 0.039; Q4 vs. Q1, OR = 913.50, 95% CI = 13.51–61,760.65, P-value = 0.002). In the hypertension subgroup, an increase in the incidence of HCC was observed with increasing levels of TPA, regardless of whether the individual had hypertension. In the diabetes subgroup, patients without diabetes appeared to be more sensitive to TPA levels (Q2 vs. Q1, OR = 6.10, 95% CI = 2.74–13.59, P-value < 0.001; Q3 vs. Q1, OR = 13.63, 95% CI = 5.91–31.43, P-value < 0.001; Q4 vs. Q1, OR = 257.14, 95% CI = 67.62–977.92, P-value < 0.001).
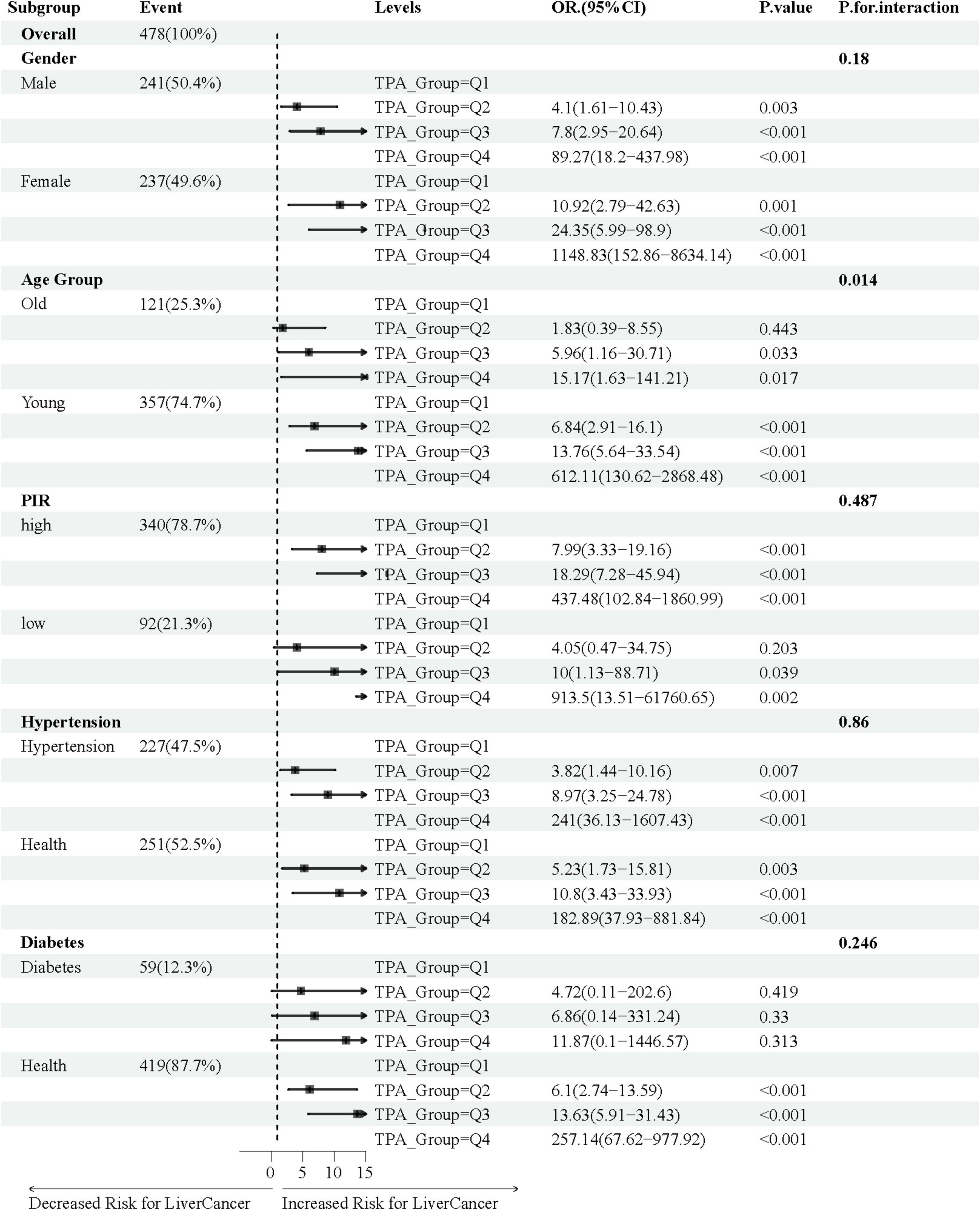
Figure 4. Association of TPA with MASLD-HCC in various subgroups of baseline characteristics. In the age subgroup, “Young” refers to age ≤ 65 years, and “Old” refers to age > 65 years; in the poverty-income ratio (PIR) subgroup, “high” is defined as PIR ≥ 1.2, and “low” is defined as PIR < 1.2.
3.5 Association of metabolic indicators with TPA and MASLD-HCC
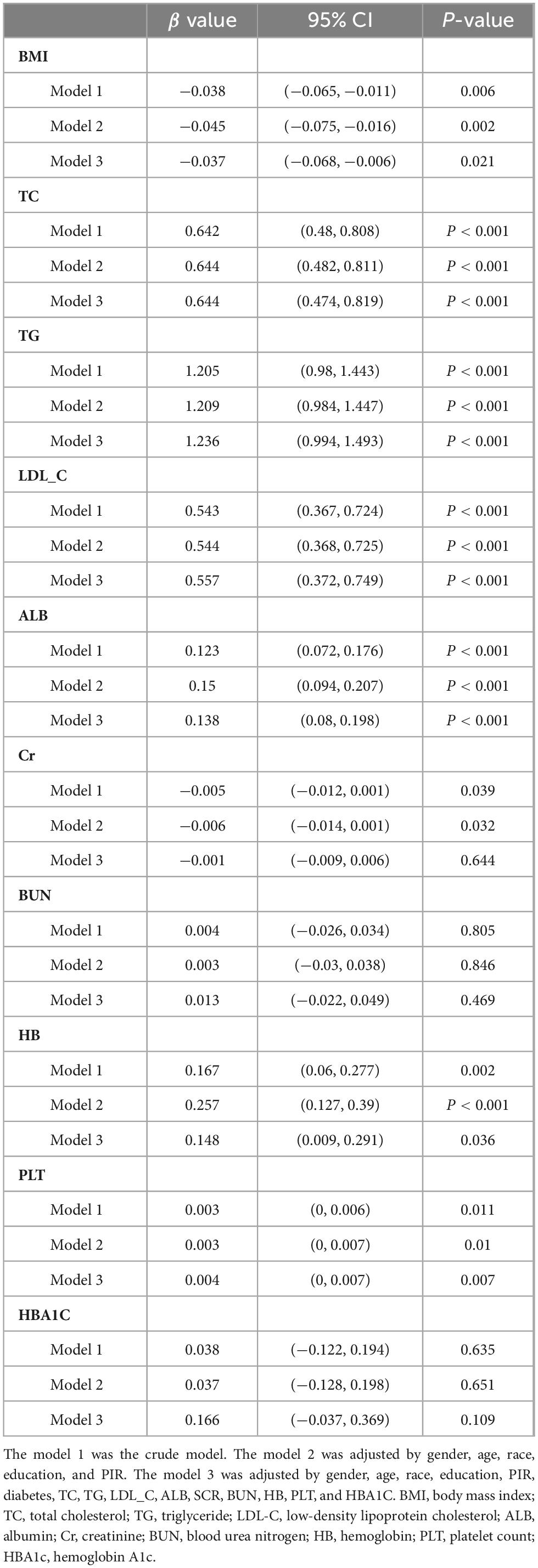
Table 3 presents the associations of TPA with metabolic indicators following multivariate logistic regression. Upon controlling for all potential confounding variables, a positive correlation was observed between TPA and cholesterol levels (β = 0.644, 95% CI = 0.474–0.819, P-value < 0.001), triglycerides (β = 1.236, 95% CI = 0.994–1.493, P-value < 0.001), low-density lipoprotein (β = 0.557, 95% CI = 0.372–0.749, P-value < 0.001), albumin (β = 0.138, 95% CI = 0.08–0.198, P-value < 0.001), hemoglobin (β = 0.148, 95% CI = 0.009–0.291, P-value = 0.036), and platelet count (β = 0.004, 95% CI = 0–0.007, P-value = 0.007). It was negatively correlated with BMI (β = −0.037, 95% CI = −0.068–0.006, P-value = 0.021).
3.6 Analysis of the mediating role of metabolic indicators in the association with TPA
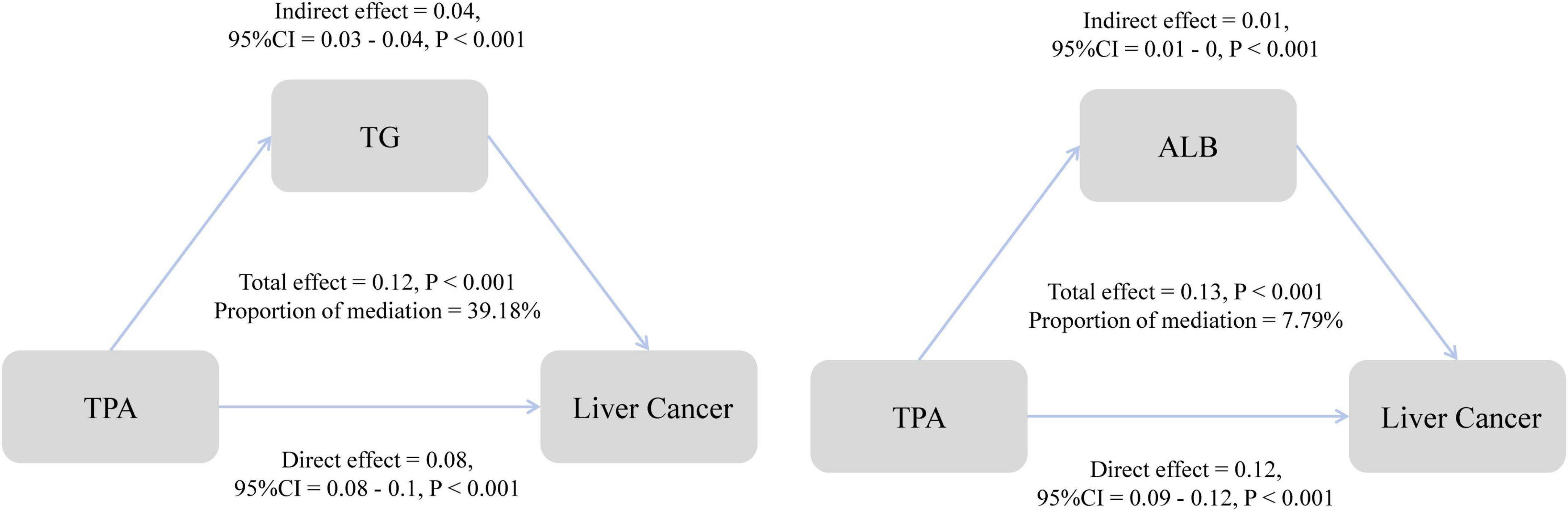
Building upon the results of the analysis of metabolic indicators, we conducted further mediation analysis to investigate the role of these indicators in the association between TPA and MASLD-HCC. The study revealed that in the mediation by triglycerides, the direct effect of TPA on HCC was 0.08, the indirect effect was 0.04, and the total effect was 0.12. Triglycerides mediated 39.18% of the association between TPA and the incidence of HCC (Figure 5). In the case of albumin, the direct effect was 0.12, the indirect effect was 0.01, and the total effect was 0.13, with a mediating proportion of 7.79%. Additionally, we evaluated the mediating roles of various metabolic parameters, including cholesterol and low-density lipoprotein, in the association (Supplementary Table 1).
4 Discussion
The present study provides compelling evidence that TPA serves as an independent risk factor for HCC in patients with MASLD, with a striking nonlinear dose-response relationship. Our findings significantly advance the current understanding of MASLD-HCC pathogenesis by identifying TPA as a novel biomarker and elucidating its metabolic mediation pathways. Unlike previous studies that primarily focused on traditional risk factors such as diabetes (35, 36), our research highlights the unique role of TPA in driving hepatocarcinogenesis, particularly through its association with triglycerides (39.18% mediation effect). Notably, the exponential risk escalation in high-TPA quartiles (Q4 OR = 753.7) and pronounced gender disparity (female Q4 OR = 1,148.83) represent previously unrecognized dimensions of MASLD-HCC susceptibility, challenging the conventional paradigm of metabolic liver disease progression.
Clinically, these findings could revolutionize risk stratification and early intervention strategies for MASLD patients. The robust association between TPA and HCC, coupled with its measurable metabolic correlates (e.g., triglycerides β = 1.236), suggests that routine TPA monitoring could identify high-risk cohorts years before malignant transformation. This is particularly relevant given the limitations of current surveillance tools like ultrasound elastography, which often detect late-stage fibrosis. Notably, our subgroup analyses revealed that females and younger individuals exhibit greater susceptibility to TPA-mediated hepatocarcinogenesis—possibly associated with estrogen-modulated inflammatory pathways and age-related declines in hepatic regenerative capacity. Our RCS analysis further provides actionable thresholds for risk mitigation—the nonlinear inflection points could guide dietary modifications (e.g., reducing dairy/meat-derived TPA) or pharmacologic interventions targeting triglyceride metabolism. TPA also offers several practical advantages, such as low assay cost, rapid turnaround time, and predictable gender- and age-specific risk patterns. For policymakers, these data underscore the need to reevaluate nutritional guidelines in MASLD management, as TPA’s dual role as both a biomarker and modifiable risk factor bridges diagnostic and therapeutic gaps in this burgeoning epidemic.
Current investigations into the relationship between dietary trans fatty acids and the risk of developing cancer are significantly limited, primarily concentrating on trans-11-octadecenoic acid (VA) and trans-9,11,15-octadecatrienoic acid [conjugated linoleic acid (CLA)]. A cohort study conducted in the Netherlands indicated a potential link between the consumption of VA and an elevated risk of breast cancer (37). In addition, another epidemiological investigation established a direct relationship between VA levels in serum or red blood cells and the incidence of both breast and prostate cancers (38, 39). In terms of CLA’s connection to cancer risk, four case-control studies have been performed. One of the investigations revealed a negative correlation between the consumption of CLA and the risk of developing colorectal cancer (40). Another research effort demonstrated that postmenopausal women with breast cancer exhibited significantly lower dietary CLA intake and serum CLA levels than their counterparts without breast cancer (41). It is especially noteworthy that women identified in the uppermost quartile of CLA intake demonstrated a 29% lower risk of developing colorectal cancer in comparison to those in the lowest quartile. Conversely, two other case-control studies did not establish a noteworthy relationship between CLA intake, whether through diet (42) or CLA concentrations in adipose tissue (43), and the risk of breast cancer. Moreover, a prospective cohort analysis indicated a weak association between CLA intake and the incidence of breast cancer, a conclusion derived from a comparison of the highest and lowest quintiles of CLA consumption (37).
Several limitations warrant consideration. First, despite rigorous PSM, residual confounding from unmeasured metabolic variables (e.g., adipose tissue distribution) may persist, though the consistency of effects across multivariable models strengthens causal inference. Second, the observational design precludes mechanistic validation; future studies should employ experimental models to delineate whether TPA directly induces oncogenic mutations or acts via microenvironmental remodeling. Third, while NHANES provides nationally representative data, batch effects from pooled survey cycles may introduce measurement variability. Forth, although FLI enabled MASLD classification in NHANES, its limitations in HCC risk stratification must be noted. Future studies should incorporate imaging and biopsy examinations in MASLD diagnosis. Finally, the modest sample size (n = 548) limited statistical power for subgroup analyses. Addressing these through multicenter cohorts with serial TPA measurements and -omics integration will be critical for translating these findings into precision prevention frameworks. Nevertheless, this study establishes TPA as a pivotal player in MASLD-HCC pathogenesis, opening new avenues for risk prediction and targeted metabolic therapy.
5 Conclusion
This study establishes TPA as a robust, nonlinear risk factor for MASLD-HCC, with effect sizes exceeding conventional metabolic indicators. The pronounced gender/age disparities and the 39.18% mediation by triglycerides underscore TPA’s dual role as a biomarker and potential therapeutic target. Despite methodological constraints, these findings advocate for clinical trials testing TPA-lowering strategies in high-risk subgroups, while highlighting the need for mechanistic studies to decode its oncogenic pathways in hepatic metabolic reprogramming.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://wwwn.cdc.gov/nchs/nhanes/.
Ethics statement
The studies involving humans were approved by Institutional Review Board of the National Center for Health Statistics. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
QrL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review and editing. LL: Funding acquisition, Supervision, Project administration, Writing – review and editing. LZ: Resources, Software, Validation, Writing – original draft. QaL: Visualization, Writing – review and editing. SZ: Investigation, Writing – review and editing. SL: Resources, Writing – review and editing. HJ: Methodology, Writing – review and editing. JC: Funding acquisition, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Fundamental Research Funds for the Central Universities (grant no. 2025ZFJH03) and the Zhejiang Province Public Welfare Technology Application Research Project (grant no. LGF21H030009).
Acknowledgments
We thank Fengfei Zhou for assistance with technical assistance in data processing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1606482/full#supplementary-material
References
1. Eslam M, Sanyal A, George J, International Consensus P. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. (2020) 158:1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312
2. Miao L, Targher G, Byrne C, Cao Y, Zheng M. Current status and future trends of the global burden of MASLD. Trends Endocrinol Metab. (2024) 35:697–707. doi: 10.1016/j.tem.2024.02.007
3. Younossi Z, Kalligeros M, Henry L. Epidemiology of metabolic dysfunction-associated steatotic liver disease. Clin Mol Hepatol. (2025) 31:S32–50. doi: 10.3350/cmh.2024.0431
4. European Association for the Study of the Liver [EASL], European Association for the Study of Diabetes [EASD], European Association for the Study of Obesity [EASO]. EASL-EASD-EASO clinical practice guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J Hepatol. (2024) 81:492–542. doi: 10.1016/j.jhep.2024.04.031
5. Mantovani A, Petracca G, Beatrice G, Csermely A, Tilg H, Byrne C, et al. Non-alcoholic fatty liver disease and increased risk of incident extrahepatic cancers: A meta-analysis of observational cohort studies. Gut. (2022) 71:778–88. doi: 10.1136/gutjnl-2021-324191
6. Giannitrapani L, Zerbo M, Amodeo S, Pipitone E, Galia M, Li Cavoli T, et al. The changing epidemiology of hepatocellular carcinoma: Experience of a single center. Biomed Res Int. (2020) 2020:5309307. doi: 10.1155/2020/5309307
7. Ganslmayer M, Hagel A, Dauth W, Zopf S, Strobel D, Muller V, et al. large cohort of patients with hepatocellular carcinoma in a single European centre: Aetiology and prognosis now and in a historical cohort. Swiss Med Wkly. (2014) 144:w13900. doi: 10.4414/smw.2014.13900
8. Konyn P, Ahmed A, Kim D. Current epidemiology in hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol. (2021) 15:1295–307. doi: 10.1080/17474124.2021.1991792
9. Vilar-Gomez E, Nephew L, Vuppalanchi R, Gawrieh S, Mladenovic A, Pike F, et al. High-quality diet, physical activity, and college education are associated with low risk of NAFLD among the US population. Hepatology. (2022) 75:1491–506. doi: 10.1002/hep.32207
10. Targher G, Byrne C, Tilg H. MASLD: A systemic metabolic disorder with cardiovascular and malignant complications. Gut. (2024) 73:691–702. doi: 10.1136/gutjnl-2023-330595
11. Singal A, Kanwal F, Llovet J. Global trends in hepatocellular carcinoma epidemiology: Implications for screening, prevention and therapy. Nat Rev Clin Oncol. (2023) 20:864–84. doi: 10.1038/s41571-023-00825-3
12. Phoolchund AGS, Khakoo SI. MASLD and the development of HCC: Pathogenesis and therapeutic challenges. Cancers. (2024) 16:259. doi: 10.3390/cancers16020259
13. Vogel A, Chan S, Dawson L, Kelley R, Llovet J, Meyer T, et al. Hepatocellular carcinoma: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. (2025) 29:iv238–55. doi: 10.1016/j.annonc.2025.02.006
14. Samoylova M, Mehta N, Roberts J, Yao F. Predictors of ultrasound failure to detect hepatocellular carcinoma. Liver Transpl. (2018) 24:1171–7. doi: 10.1002/lt.25202
15. Lee H, Kim K, Ahn S, Lee H, Choi J. The associations between fibrosis changes and liver-related events in patients with metabolic dysfunction-associated steatotic liver disease. Liver Int. (2024) 44:1448–55. doi: 10.1111/liv.15897
16. Tzartzeva K, Obi J, Rich N, Parikh N, Marrero J, Yopp A, et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: A meta-analysis. Gastroenterology. (2018) 154:1706–18e1. doi: 10.1053/j.gastro.2018.01.064
17. Ma Y, Yang W, Simon T, Smith-Warner S, Fung T, Sui J, et al. Dietary patterns and risk of hepatocellular carcinoma among U.S. men and women. Hepatology. (2019) 70:577–86. doi: 10.1002/hep.30362
18. Bogumil D, Park S, Le Marchand L, Haiman C, Wilkens L, Boushey C, et al. High-quality diets are associated with reduced risk of hepatocellular carcinoma and chronic liver disease: The multiethnic cohort. Hepatol Commun. (2019) 3:437–47. doi: 10.1002/hep4.1313
19. Liu Y, Yang W, VoPham T, Ma Y, Simon T, Gao X, et al. Plant-based and animal-based low-carbohydrate diets and risk of hepatocellular carcinoma among US men and women. Hepatology. (2021) 73:175–85. doi: 10.1002/hep.31251
20. Luu H, Neelakantan N, Geng T, Wang R, Goh G, Clemente J, et al. Quality diet indexes and risk of hepatocellular carcinoma: Findings from the Singapore Chinese health study. Int J Cancer. (2021) 148:2102–14. doi: 10.1002/ijc.33367
21. Guillocheau E, Legrand P, Rioux V. Trans-palmitoleic acid (trans-9-C16:1, or trans-C16:1 n-7): Nutritional impacts, metabolism, origin, compositional data, analytical methods and chemical synthesis. A review. Biochimie. (2020) 169:144–60. doi: 10.1016/j.biochi.2019.12.004
22. de Souza R, Mente A, Maroleanu A, Cozma A, Ha V, Kishibe T, et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ. (2015) 351:h3978. doi: 10.1136/bmj.h3978
23. Patel P, Sharp S, Jansen E, Luben R, Khaw K, Wareham N, et al. Fatty acids measured in plasma and erythrocyte-membrane phospholipids and derived by food-frequency questionnaire and the risk of new-onset type 2 diabetes: A pilot study in the European prospective investigation into cancer and nutrition (EPIC)-Norfolk cohort. Am J Clin Nutr. (2010) 92:1214–22. doi: 10.3945/ajcn.2010.29182
24. Kroger J, Zietemann V, Enzenbach C, Weikert C, Jansen E, Doring F, et al. Erythrocyte membrane phospholipid fatty acids, desaturase activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European prospective investigation into cancer and nutrition (EPIC)-Potsdam study. Am J Clin Nutr. (2011) 93:127–42. doi: 10.3945/ajcn.110.005447
25. Benedetto U, Head S, Angelini G, Blackstone E. Statistical primer: Propensity score matching and its alternatives. Eur J Cardiothorac Surg. (2018) 53:1112–7. doi: 10.1093/ejcts/ezy167
26. Greenland S. Dose-response and trend analysis in epidemiology: Alternatives to categorical analysis. Epidemiology. (1995) 6:356–65. doi: 10.1097/00001648-199507000-00005
27. VanderWeele T. Mediation analysis: A practitioner’s guide. Annu Rev Public Health. (2016) 37:17–32. doi: 10.1146/annurev-publhealth-032315-021402
28. Chalasani N, Younossi Z, Lavine J, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American association for the study of liver diseases. Hepatology. (2018) 67:328–57. doi: 10.1002/hep.29367
29. Ruhl C, Everhart J. Fatty liver indices in the multiethnic United States national health and nutrition examination survey. Aliment Pharmacol Ther. (2015) 41:65–76. doi: 10.1111/apt.13012
30. Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The fatty liver index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. (2006) 6:33. doi: 10.1186/1471-230X-6-33
31. Yang Y, Liu J, Sun C, Shi Y, Hsing J, Kamya A, et al. Nonalcoholic fatty liver disease (NAFLD) detection and deep learning in a Chinese community-based population. Eur Radiol. (2023) 33:5894–906. doi: 10.1007/s00330-023-09515-1
32. Sumida Y, Yoneda M, Hyogo H, Itoh Y, Ono M, Fujii H, et al. Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol. (2012) 12:2. doi: 10.1186/1471-230X-12-2
33. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrica. (1983) 70:41–55. doi: 10.1093/biomet/70.1.41
34. Liang J, Hu Z, Zhan C, Wang Q. Using propensity score matching to balance the baseline characteristics. J Thorac Oncol. (2021) 16:e45–6. doi: 10.1016/j.jtho.2020.11.030
35. Mozaffarian D, de Oliveira Otto M, Lemaitre R, Fretts A, Hotamisligil G, Tsai M, et al. trans-Palmitoleic acid, other dairy fat biomarkers, and incident diabetes: The multi-ethnic study of atherosclerosis (MESA). Am J Clin Nutr. (2013) 97:854–61. doi: 10.3945/ajcn.112.045468
36. Korkus E, Szustak M, Madaj R, Chworos A, Drzazga A, Koziolkiewicz M, et al. Trans-palmitoleic acid, a dairy fat biomarker, stimulates insulin secretion and activates G protein-coupled receptors with a different mechanism from the cis isomer. Food Funct. (2023) 14:6496–512. doi: 10.1039/d2fo03412c
37. Voorrips L, Brants H, Kardinaal A, Hiddink G, van den Brandt P, Goldbohm R. Intake of conjugated linoleic acid, fat, and other fatty acids in relation to postmenopausal breast cancer: The Netherlands cohort study on diet and cancer. Am J Clin Nutr. (2002) 76:873–82. doi: 10.1093/ajcn/76.4.873
38. Chajes V, Thiebaut A, Rotival M, Gauthier E, Maillard V, Boutron-Ruault M, et al. Association between serum trans-monounsaturated fatty acids and breast cancer risk in the E3N-EPIC study. Am J Epidemiol. (2008) 167:1312–20. doi: 10.1093/aje/kwn069
39. King I, Kristal A, Schaffer S, Thornquist M, Goodman G. Serum trans-fatty acids are associated with risk of prostate cancer in beta-Carotene and retinol efficacy trial. Cancer Epidemiol Biomark Prev. (2005) 14:988–92. doi: 10.1158/1055-9965.EPI-04-0517
40. Larsson S, Bergkvist L, Wolk A. High-fat dairy food and conjugated linoleic acid intakes in relation to colorectal cancer incidence in the Swedish Mammography Cohort. Am J Clin Nutr. (2005) 82:894–900. doi: 10.1093/ajcn/82.4.894
41. Aro A, Mannisto S, Salminen I, Ovaskainen M, Kataja V, Uusitupa M. Inverse association between dietary and serum conjugated linoleic acid and risk of breast cancer in postmenopausal women. Nutr Cancer. (2000) 38:151–7. doi: 10.1207/S15327914NC382_2
42. McCann S, Ip C, Ip M, McGuire M, Muti P, Edge S, et al. Dietary intake of conjugated linoleic acids and risk of premenopausal and postmenopausal breast cancer, western New York exposures and breast cancer study (WEB Study). Cancer Epidemiol Biomark Prev. (2004) 13:1480–4. doi: 10.1158/1055-9965.1480.13.9
Keywords: MASLD, HCC, trans-palmitoleic acid, NHANES, MASLD-HCC
Citation: Li Q, Zhan L, Li Q, Zhu S, Liu S, Jiang H, Cheng J and Li L (2025) Association between trans-palmitoleic acid and metabolic dysfunction-associated steatotic liver disease-related hepatocellular carcinoma: NHANES 1999–2018. Front. Nutr. 12:1606482. doi: 10.3389/fnut.2025.1606482
Received: 05 April 2025; Accepted: 21 May 2025;
Published: 19 June 2025.
Edited by:
Eric Gumpricht, Independent Researcher, Gilbert, AZ, United StatesReviewed by:
Liu Hailing, Chongqing Medical University, ChinaJingjing Song, Capital Medical University, China
Copyright © 2025 Li, Zhan, Li, Zhu, Liu, Jiang, Cheng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lanjuan Li, bGpsaUB6anUuZWR1LmNu
 Qirui Li
Qirui Li Luqi Zhan
Luqi Zhan Qian Li
Qian Li Shuai Zhu
Shuai Zhu Lanjuan Li
Lanjuan Li