- College of Horticulture, Nanjing Agricultural University, Nanjing, China
5-Aminolevulinic acid (ALA), a newly proved natural plant growth regulator, is well known to improve plant photosynthesis under both normal and stressful conditions. However, its underlying mechanism remains largely unknown. Stomatal closure is one of the major limiting factors for photosynthesis and abscisic acid (ABA) is the most important hormone in provoking stomatal closing. Here, we showed that ALA significantly inhibited ABA-induced stomatal closure using wild-type and ALA-overproducing transgenic Arabidopsis (YHem1). We found that ALA decreased ABA-induced H2O2 and cytosolic Ca2+ accumulation in guard cells with stomatal bioassay, laser-scanning confocal microscopy and pharmacological methods. The inhibitory effect of ALA on ABA-induced stomatal closure was similar to that of AsA (an important reducing substrate for H2O2 removal), CAT (a H2O2-scavenging enzyme), DPI (an inhibitor of the H2O2-generating NADPH oxidase), EGTA (a Ca-chelating agent), and AlCl3 (an inhibitor of calcium channel). Furthermore, ALA inhibited exogenous H2O2- or Ca2+-induced stomatal closure. Taken together, we conclude that ALA inhibits ABA-induced stomatal closure via reducing H2O2, probably by scavenging, and Ca2+ levels in guard cells. Moreover, the inhibitive effect of ALA on ABA-induced stomatal closure was further confirmed in the whole plant. Finally, we demonstrated that ALA inhibits stomatal closing, but significantly improves plant drought tolerance. Our results provide valuable information for the promotion of plant production and development of a sustainable low-carbon society.
Introduction
5-Aminolevulinic acid (ALA) is an essential precursor in tetrapyrrole biosynthesis in organisms, such as such as chlorophyll and heme in plants. Since 1998, hormonal activities of ALA have been found in plant tissue culture (Bindu and Vivekanandan, 1998). In recent 20 years, more research indicates that ALA is not only an important intermediate in biological metabolism, but also a vital plant growth regulator which regulates several key physiological processes such as promoting plant growth and increasing plant stress tolerance (Akram and Ashraf, 2013). One of ALA’s outstanding roles is improving plant photosynthesis and thereby increasing growth. And, it is worth emphasizing that ALA improves plant photosynthesis efficiency not only under normal conditions (Hotta et al., 1997), but also under various stresses, such as cold (Hotta et al., 1998), salt (Nishihara et al., 2003), low light (Wang et al., 2004), water deficit (Liu et al., 2011), heat (Zhang et al., 2012), and heavy metal stresses (Ali et al., 2013; Tian et al., 2014), suggesting its great application potential in agriculture and forestry. However, to date, the proposed mechanisms underlying ALA-promoted photosynthesis include only the following: (1) boosting light-harvesting capability by increasing chlorophyll content (Youssef and Awad, 2008), (2) improving photosynthetic electron transport activity (Wang et al., 2010), (3) promoting antioxidant activity (Nishihara et al., 2003), and (4) increasing rubisco activity by up-regulating transcription of gene encoding Rubisco small unit (Shen et al., 2011). Therefore, the mechanism how ALA regulates plant photosynthesis and growth is still in its infancy.
Except non-stomatal factors, stomatal behavior also plays important roles in plant photosynthesis. In fact, stomatal resistance is thought to be the major limiting factor for CO2 uptake by plants (Wang et al., 2014). And many reports have demonstrated that stomatal aperture is a limiting factor in photosynthesis and plant growth (Lawson and Blatt, 2014; Wang et al., 2014). Wang et al. (2004) firstly showed that exogenous ALA significantly increased stomatal conductance of melon (Cucumis melo) seedlings. Subsequently, several researchers reported that ALA could reduce stomatal limitation in date palm (Phoenix dactylifera) seedlings (Youssef and Awad, 2008), and enhance stomatal conductance in leaves of pepper (Capsicum annuum; Korkmaz et al., 2010), oilseed rape (Brassica napus; Naeem et al., 2010), ‘Summer Black’ grape (Xie et al., 2013a) and apple (Gao et al., 2013) seedlings. Based on the above findings, we assume that the promoting effect of ALA on stomatal aperture might be universal in plant, and ALA-induced stomatal opening should be a critical mechanism for improvement of plant photosynthesis. However, to our knowledge, no specific information is available regarding the regulatory effects of ALA on stomatal movement and its functional mechanisms.
Stomatal movement is a highly complex process and modulated by many stimuli. Abscisic acid (ABA) was considered as the most important regulatory signaling molecule (Dodd, 2003; Tanaka et al., 2005). ABA-induced stomatal movement is one of the best characterized signaling systems in plants. More than 20 components, including secondary metabolites and ion channels, have been shown to participate in ABA-induced stomatal closure (Li et al., 2006). Therefore, our research on ALA-induced stomatal movement is started with a question whether ALA influence ABA-induced stomatal closure. Hydrogen peroxide (H2O2) and Ca2+ are signaling molecules of widespread importance in plant responses to various biotic and abiotic stimuli, including pathogen challenge, drought stress, atmospheric pollutants, extremes of temperatures, gravitropism, hormones, cell development, and senescence (Neill et al., 2002; Tuteja and Mahajan, 2007). It has been demonstrated that increasing H2O2 production and the H2O2-activated elevation of cytosolic Ca2+ concentration ([Ca2+]cyt) in guard cells are important mechanisms for ABA-induced stomatal closing (Pei et al., 2000). Interestingly, exogenous applications of ALA could significantly decrease H2O2 content and increase activities of antioxidant enzymes including catalase (CAT), ascorbate peroxidase (APX), glutathione reductase (GR), and superoxide dismutase (SOD) in leaves of several plant species (Balestrasse et al., 2010; Korkmaz et al., 2010; Liu et al., 2011; Zhen et al., 2012). However, there is no information available on the effect of ALA on H2O2 content in guard cells. Based on the above clues, we hypothesized that ALA might inhibit ABA-induced stomatal closure by decreasing H2O2 accumulation and hence [Ca2+]cyt in guard cells.
5-Aminolevulinic acid-overproducing transgenic Arabidopsis (YHem1) have been obtained by expressing yeast Hem1 gene under the control of Arabidopsis HemA1 promoter (Zhang et al., 2010). To test our hypothesis, here, we first investigated whether ALA inhibited ABA-induced stomatal closure. The results showed that both exogenous and YHem1 expression inhibit ABA-induced stomatal closure. The mechanism behind ALA’s regulation of stomatal movement was then dissected, and the way ALA regulates stomatal aperture, through ALA itself or its metabolites such as chlorophyll, was discussed. Effect of ALA on drought tolerance of Arabidopsis was further evaluated to exclude the possibility of increase in plant sensitivity to drought stress by ALA-inhibited stomatal closure. Our results provide valuable information for understanding the function mechanisms of ALA and the promotion of plant production.
Materials and Methods
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) of wild-type (Col-0) and ALA-over-producing transgenic lines (YHem1; Zhang et al., 2010) that derived from Col-0 background were used in this study. Seeds were surface sterilized with bleaching power (5%, w/v) for 20 min, washed with sterilized water three times, then germinated and grown on vermiculite. Seedlings were irrigated every other day with half-strength Hoagland nutrition, in a growth chamber at 23°C, a relative humidity of 60%, and under a PPFD of 150 μmol⋅m-2⋅s-1 in 8 h light/16 h dark cycles.
Guard Cell Viability Test
Epidermal strips were pretreated for 2 h in opening buffer (50 mM KCl, 10 mM MES, and 0.1 mM CaCl2, pH 6.2) with different treatments. Strips were then incubated with 0.25 μM fluorescein diacetate (FDA) for 5 min. The guard cell viability was detected according to the method of Garcia-Mata and Lamattina (2010). Fluorescence pictures were obtained with a Nikon-TE300 digital camera coupled to a laser scanning confocal microscope (Leica TCS SP8 STED 3X, LSCM). Cell viability was quantified by counting the percentage of fluorescent guard cells relative to total guard cells in the bright field.
Stomatal Bioassay
Stomatal bioassay was performed on abaxial epidermal strips which were peeled from the rosette leaves of 5–6-week-old plants 4 h after the beginning of the light period. Epidermal peels were floated, peeled-side down, on opening buffer and incubated under light conditions (PPFD 240 μmol m-2 s-1) for 2 h to open the stomata. For the application of ALA or various inhibitors, the epidermal peels with pre-opened stomata were transferred to the same buffer supplemented with 10 μM ABA (Sigma–Aldrich, St. Louis, MO, USA), with or without the addition of ALA (0.05–5 mg L-1), 1 mM LA (an inhibitor of ALA metabolism), 100 U mL-1 CAT (a hydrogen peroxide-scavenging enzyme), 100 μM AsA (an important reducing substrate for H2O2 removal), 10 μM DPI (an inhibitor of the H2O2-generating enzyme NADPH oxidase), 5 mM EGTA (a Ca-chelating agent), or 50 μM AlCl3 (an inhibitor of calcium channel) for a further 1 h under light conditions. Stomatal apertures were observed by a light microscope (Nikon TE100, 400×), using a fitted camera (MShot Digital Imaging System), and measured with a digital ruler in Adobe Photoshop 6.0 (Adobe systems, San Jose, CA, USA).
To avoid any potential rhythmic effects on stomatal aperture, experiments were always started at the same time of the day. In each treatment, 30 randomly selected apertures were scored and experiments were repeated three times. The data presented are means of 90 measurements ± SEs.
Scanning Electron Microscopy
The rosette leaves of 5–6-week-old wild-type and ALA-over-producing transgenic plants were immersed in opening buffer (50 mM KCl, 10 mM MES, and 0.1 mM CaCl2, pH 6.2). For wild-type samples, three treatments were designed by applying 0.5 mg L-1 ALA, 10 μM ABA, 10 μM ABA and 0.5 mg L-1 ALA, respectively. And the opening buffer without ABA and ALA were set as control treatment. For transgenic samples (P0 and P3), leaves of each line were treated with or without 10 μM ABA. All samples were incubated under light conditions (PPFD 240 μmol m-2 S-1) for 2 h at 25°C, then rinsed with phosphate buffer (pH 7.2) and fixed in 2% glutaraldehyde for 1 h and 1% glutaraldehyde for another 7 h. Leaves were then rinsed with phosphate buffer (pH 7.2), and dehydrated in an ethanol series (30 to 50 to 60 to 70 to 80 to 90 to 97 to 2 × 100%). These fixed and dehydrated samples were critical point dried with CO2, sputter-coated with a thin layer of gold and photographed under a scanning electron microscopy (PHILIPS-XL30E SEM) at 500× magnification. Stomata were counted and stomatal apertures were measured with a digital ruler in Adobe Photoshop 6.0 (Adobe systems, San Jose, CA, USA).
Determination of Endogenous ALA
Endogenous ALA content in Arabidopsis leaves was measured according to Harel and Klein (1972). Random 0.1 g leaves were homogenized in 200 mM acetic acid buffer (pH 4.6), and centrifuged at 5,000 ×g for 15 min. One milliliter of supernatant were added to 0.5 mL acetylacetone, and boiled for 10 min. After cooling, 0.5 mL Ehrlich’s reagent was added. The absorbance was recorded at 553 nm after static hierarchy for 7 min by spectrophotometer.
Measurement of Endogenous H2O2 Using Confocal Laser-Scanning Microscopy
Endogenous H2O2 were measured with fluorescent indicator dye H2DCF-DA as described by He et al. (2011) with slight modifications. The epidermal strips, previously incubated for 4 h under conditions promoting stomatal opening, were placed into Tris-KCl buffer (10 mM Tris and 50 mM KCl, pH 7.2) containing H2DCF-DA (Sigma–Aldrich, USA) at 50 μM for 30 min, in the dark at 25°C. Excess dye was removed with fresh Tris-KCl buffer in the dark. Peels of wild-type Arabidopsis were then transferred to the opening buffer alone or opening buffer supplemented with 10 μM ABA (Sigma–Aldrich, USA), with or without the addition of ALA. And peels of YHem1 transgenic plants were transferred to the opening buffer alone or opening buffer supplemented with 10 μM ABA. Peel fluorescence were observed 5 min later using a laser scanning confocal microscope (Leica TCS SP8 STED 3X, LSCM), with the following settings: ex = 488 nm, em = 525 ± 15 nm, power 10%, zoom 2, mild scanning, frame 512 × 512, and Time-course and Photoshop software.
Determination of Intracellular Ca2+ Variations Using Confocal Laser-Scanning Microscopy
Intracellular Ca2+ variations were determined with fluorescent dye Fluo-3 AM (Dojindo, Japan). The epidermal strips, previously incubated for 4 h under conditions promoting stomatal opening, were placed into MES-KCl solution containing Fluo-3 AM (dissolved in DMSO, Sigma) at 1 μM for 2 h, in the dark at 4°C. Excess dye was removed with fresh MES-KCl buffer in the dark. Peels of wild-type Arabidopsis were then transferred to the opening buffer alone or opening buffer supplemented with 10 μM ABA (Sigma–Aldrich, St. Louis, MO, USA), with or without the addition of ALA. And peels of YHem1 transgenic plants were transferred to the opening buffer alone or opening buffer supplemented with 10 μM ABA. Peel fluorescence were observed 5 min later using a laser scanning confocal microscope (Leica TCS SP8 STED 3X, LSCM), with the following settings: ex = 488 nm, em = 525 ± 15 nm, power 10%, zoom 2, mild scanning, frame 512 × 512, and Time-course and Photoshop software. In the determination of H2O2 and Ca2+, at least five biological replicates were performed and three images taken for each biological replicate.
Treatment with ABA in Whole Plant
Treatments with ABA in the whole plant, including wild-type and YHem1-transgenic plants, were carried out by irrigation of 10 μM ABA dissolved in distilled water for 30 min in growth chamber. For wild-type plants, four treatments, i.e., control, ALA, ABA, and ABA together with ALA, were set to examine the effects of exogenous ALA on ABA-induced stomatal closure in planta. YHem1-transgenic plants were treated with or without ABA to examine the effects of endogenous ALA. For drought stress, leaves of 5-week-plants were detached from the treated plants and fresh weight (FW) were recorded immediately. Leaves were then placed in the growth chamber for another 1 h, and the reduced weight was measured at 10 min intervals. Ratios of reduced weights to the original FW were calculated to evaluate the rate of FW decrease.
Drought Tolerance Assay
Wild-type and YHem1 transgenic Arabidopsis seeds were surface sterilized with 75% alcohol for 45 s and 10% NaClO for 10 min, wished with sterilized water three times, then placed on MS medium in Petri dishes. Forty-nine seeds were placed in each Petri dish. All materials were incubated in growth chamber at 25°C under PPFD of 150 μmol m-2 S-1 with 12 h light/12 h dark cycle. Ten days later, wild-type plants were randomly divided into four groups and YHem1 transgenic plants were divided into two groups for six treatments. Six milliliter sterilized water or 15% PEG 6 000 were added to each Petri dish with or without 0.5 mg L-1 ALA. Seedlings were allowed to grow in the growth chamber for another 14 days. Then, seedlings were photographed and collected for determination of shoot and root length, stomatal aperture, and leaf chlorophyll contents.
Stomatal apertures were immediately observed by a light microscope (Nikon TE100, 400×), using a fitted camera (MShot Digital Imaging System), and measured with a digital ruler in Adobe Photoshop 6.0 (Adobe systems, San Jose, CA, USA). Leaf chlorophyll were extracted by 95% ethanol and determined according to Lichtenthaler and Wellburn (1982).
Statistical Analysis
All data were taken from at least three independent experiments. Statistical analysis was performed using SPSS statistical computer package (version 16.0 SPSS Inc., Chicago, IL, USA). Data was compared with the control or among treatments by analysis of variance (ANOVA) to discriminate significant differences at P < 0.05 or P < 0.01 followed by least significant difference tests (LSD).
Results
Exogenous ALA Inhibits ABA-induced Stomatal Closure
Abscisic acid is the most well-known elicitor of stomatal closure. To explore the regulatory mechanisms underlying ALA-induced stomatal opening, we examined the effects of ALA on ABA-induced stomatal closure. We employed an in vitro system using isolated epidermal peels in which we could measure stomatal apertures. In our experiments, after 2 h illumination of wild-type plants, the stomata opened and their apertures reached approximately 2.52 μm (Figure 1A). ABA application significantly reduced stomatal aperture to approximately 1.48 μm. When different concentrations of ALA were applied together with ABA to the isolated epidermal peels, ABA-induced stomatal closure was largely suppressed. The inhibitive effect of 0.5 mg⋅L-1 ALA was the most significant, with stomatal apertures increasing to approximately 3.24 μm which was even much higher than control. Therefore, 0.5 mg⋅L-1 was chosen as the final concentration of exogenous ALA for the following experiments. The time course for stomatal movement induced by ABA alone or ABA and ALA together illustrated that the inhibition of ALA on ABA-induced stomatal closure was initiated before 40 min and lasted for at least 2 h (Figure 1B). These observations indicated that exogenous ALA has an inhibitive effect on ABA-induced stomatal closure.
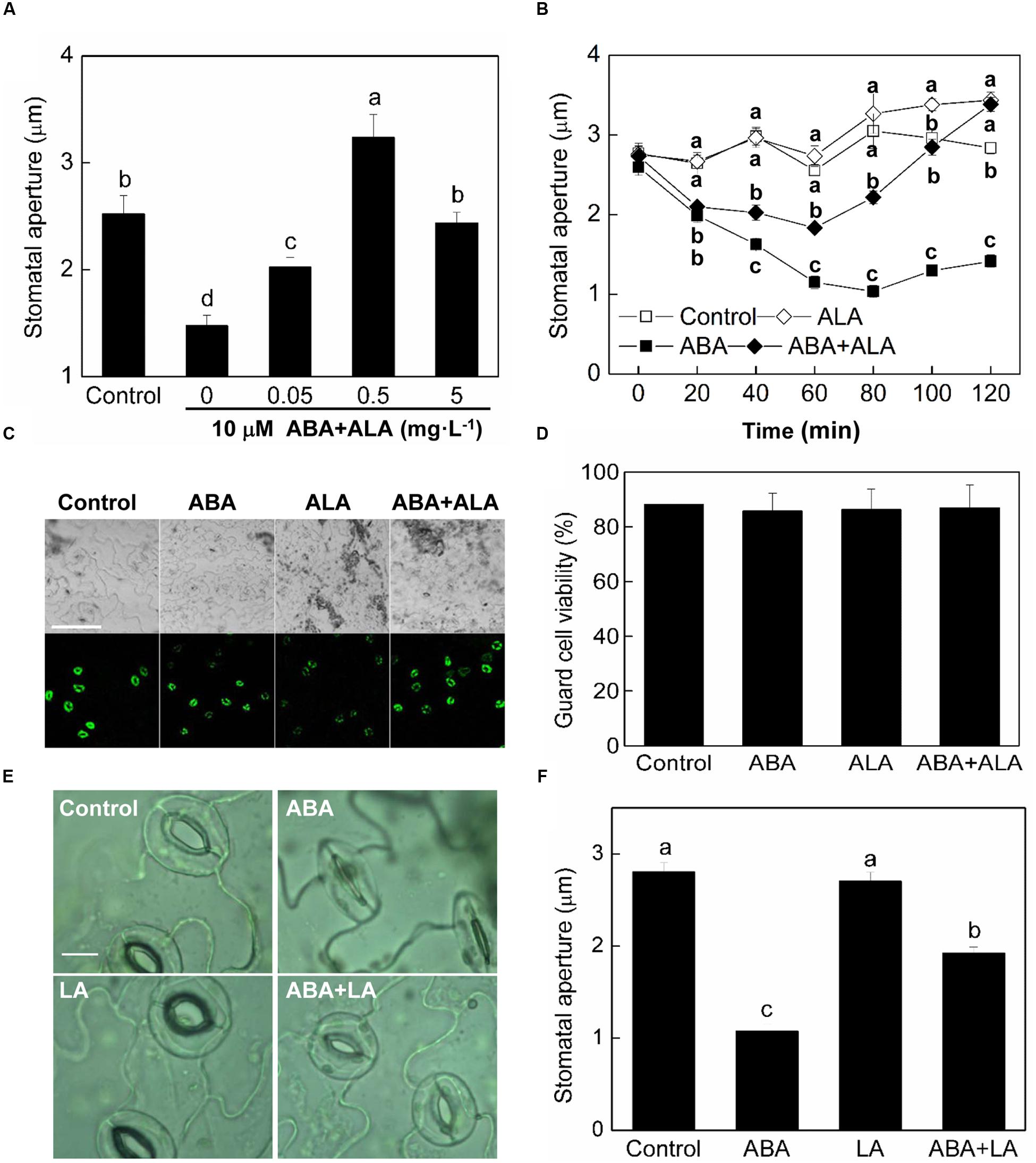
FIGURE 1. ALA inhibits ABA-induced stomatal closure. (A) Effects of different concentrations of ALA on ABA-induced stomatal closure. Isolated epidermal strips of wild-type Arabidopsis were incubated at 25°C in CO2-free MES-KCl buffer without ABA (Control), or containing 10 μM ABA and different concentrations of ALA under light (240 μmol m-2 s-1), and stomatal apertures were determined after 2 h. (B) Time courses of stomatal responses to 0.5 mg L-1 ALA, 10 μM ABA, or 10 μM ABA + 0.5 mg L-1 ALA, respectively. Different letters on the same time point indicate significant differences at P < 0.01. (C,D) Guard cell viability observed by fluorescence microscopy. Isolated epidermal strips of wild-type Arabidopsis were incubated at 25°C in either buffer (Control), or containing 10 μM ABA, 0.5 mg L-1 ALA, 10 μM ABA + 0.5 mg L-1 ALA for 2 h under light (240 μmol m-2 s-1), respectively, and then loaded with 0.25 μM fluorescein diacetate for 5 min. Images (C) obtained with a Nikon-TE300 digital camera depict one representative picture from three independent experiments. The upper image is in bright field and the lower image is in fluorescence. Scale bar: 100 μm. Cell viability (D) was quantified by counting the percentage of fluorescent guard cells relative to total guard cells in the bright field. (E,F) Levulinic acid (LA) inhibits ABA-induced stomatal closure. Isolated epidermal strips of wild-type Arabidopsis were incubated at 25°C in either buffer (Control), or containing 10 μM ABA, 1 mM LA (an analog of ALA which can block ALA metabolism), 10 μM ABA + 1 mM LA for 1 h under light (240 μmol m-2 s-1), respectively, and then images (E) were recorded and stomatal apertures (F) were determined. Scale bar: 10 μm. Values in (A,B,F) are the means of 90 measurements ± SE from three independent experiments. Different small letters represent significant differences among treatments (P < 0.01).
As no data are available in relation to ALA treatments of guard cells, to exclude that ALA could be toxic to guard cells, we detected that guard cell viability under ALA treatment. Epidermal peels were treated with 0.5 mg⋅L-1 ALA solution with or without 10 μM ABA and then loaded with 0.25 μM FDA. Result showed that guard cell viability was not influenced by ALA treatment (Figures 1C,D), indicating that ALA is not toxic for guard cells.
Levulinic acid (LA), an analog of ALA, is a competitive inhibitor of ALA dehydratase (ALAD) and has been used widely to block ALA metabolism, which leads to accumulation of endogenous ALA (Xie et al., 2013b). To confirm that it was ALA per se that was responsible for the inhibitive effect of ALA on ABA-induced stomatal closure, we applied LA instead of ALA. Following treatment with 10 mM LA, ABA-induced stomatal closure was also inhibited (Figures 1E,F). Thus, the inhibitive effect of ALA on ABA-induced stomatal closure probably resulted from ALA itself.
Over-produced Endogenous ALA Inhibits ABA-induced Stomatal Closure
A similar pattern of changes in stomatal aperture was observed in exogenous ALA treated plants by scanning electron microscopy (Figures 2A,B), confirming the inhibitive effect of exogenous ALA on ABA-induced stomatal closure. To further evaluate the effects of ALA on ABA-induced stomatal closure, YHem1-transgenic Arabidopsis lines (P0 and P3) overproducing ALA were used. Stomatal responses of YHem1-transgenic lines and wild-type plants were compared using scanning electron microscopy. The result showed that the stomatal apertures of ALA-overproducing plants were similar to that of wild-type plants under normal condition, but were significantly larger under ABA treatment (Figures 2C,D). This result suggested that the over-production of endogenous ALA also inhibits ABA-induced stomatal closure.
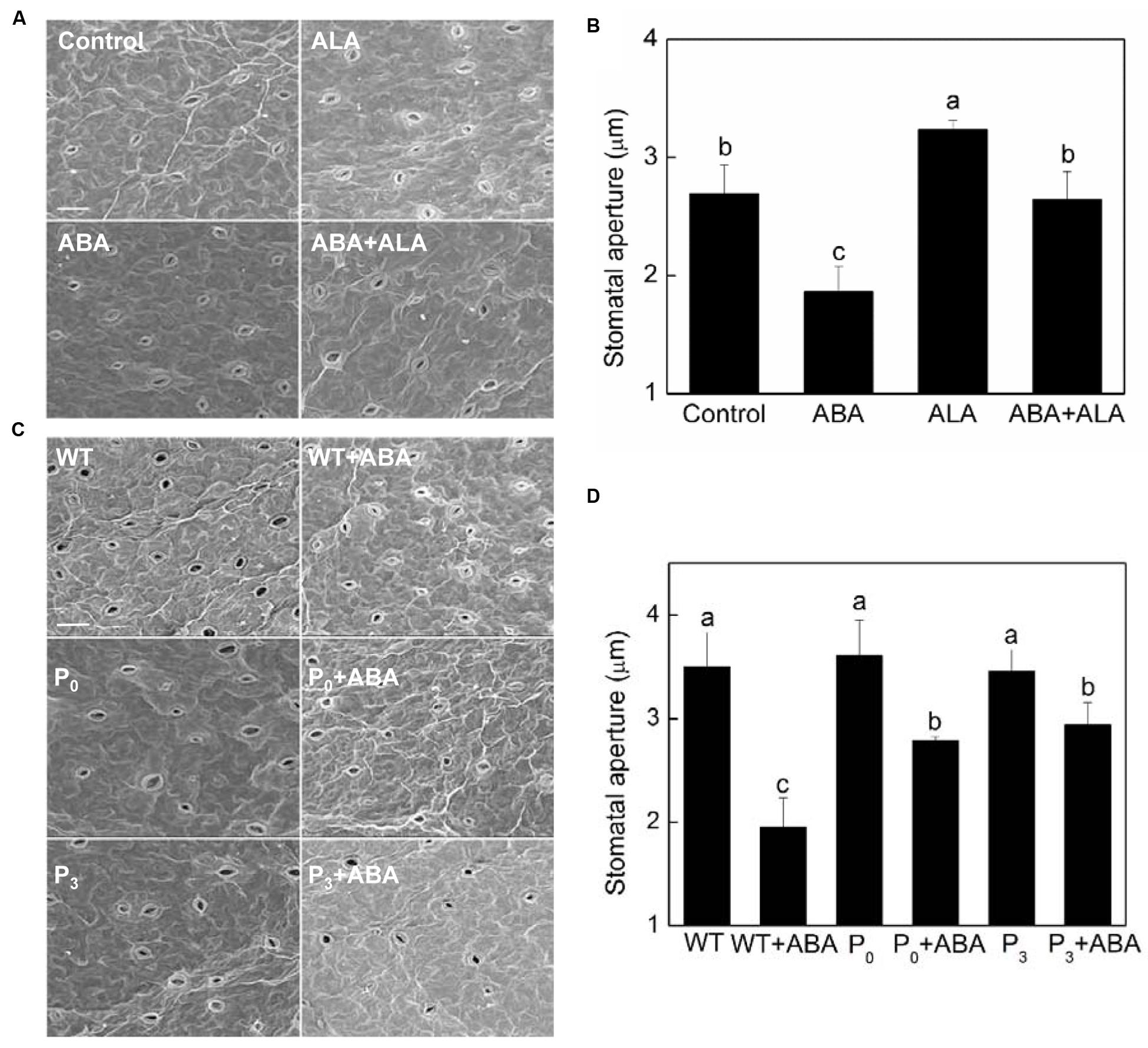
FIGURE 2. Stomatal aperture observed by a scanning electron microscopy. (A,B) Exogenous ALA inhibits ABA-induced stomatal closure. Wild-type Arabidopsis leaves were floated on either MES-KCl buffer (Control), or containing 10 μM ABA, 0.5 mg L-1 ALA, 10 μM ABA, and 0.5 mg L-1 ALA, respectively, at 25°C for 2 h, and then scanning electron microscopy (PHILIPS-XL30E SEM) images (A) of these leaves were recorded, based on which, stomatal apertures (B) were measured. (C,D) Over-produced endogenous ALA inhibits ABA-induced stomatal closure. Leaves of wild-type and YHem1 transgenic (P0 and P3) Arabidopsis were floated on either CO2-free MES-KCl buffer alone or containing 10 μM ABA at 25°C for 2 h. Then scanning electron microscopy (PHILIPS-XL30E SEM) images (C) of these leaves were recorded, based on which, stomatal apertures (D) were measured. Scale bar: 25 μm. Values are the means of 90 measurements ± SE from three independent experiments. Different small letters represent significant difference between treatments (P < 0.01).
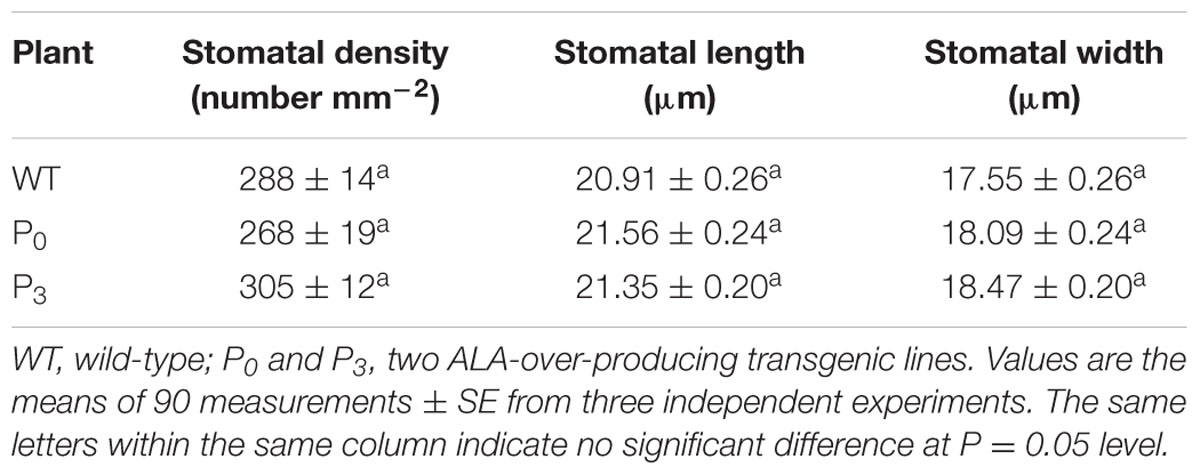
Except stomatal aperture, stomatal development also has critical impact on plant photosynthetic capacity. To determine whether ALA influence plant stomatal development, we compared the stomatal density and size of ALA-overproducing transgenic plants to those of wild-type plants. The result showed that no significant differences were observed between them (Table 1), indicating that ALA does not affect stomatal development. Taken the results of exogenous ALA treatment together, we showed that ALA inhibits ABA-induced stomatal closure.
Levels of Endogenous ALA in WT and YHem1-transgenic Arabidopsis under Different Treatments
To further confirm that it was the endogenous ALA that regulated guard cell ABA signaling, the effect of ABA on endogenous ALA level were determined during ABA-induced stomatal closure in the YHem1 transgenic plants and ALA-, LA-treated wild-type plants. In wide-type plants, endogenous ALA content was significantly reduced by ABA alone treatment, but dramatically increased by exogenous ALA or LA treatment (Figure 3). When ABA was applied together with ALA or LA, endogenous ALA content in wild-type plants also significantly increased. Compared to ABA-treated wild-type plants, ABA-treated YHem1 transgenic plants showed significantly higher level of endogenous ALA. These results indicated that endogenous ALA level increases during the inhibition of ABA-induced stomatal closure, confirming that endogenous ALA regulates guard cell ABA signaling.
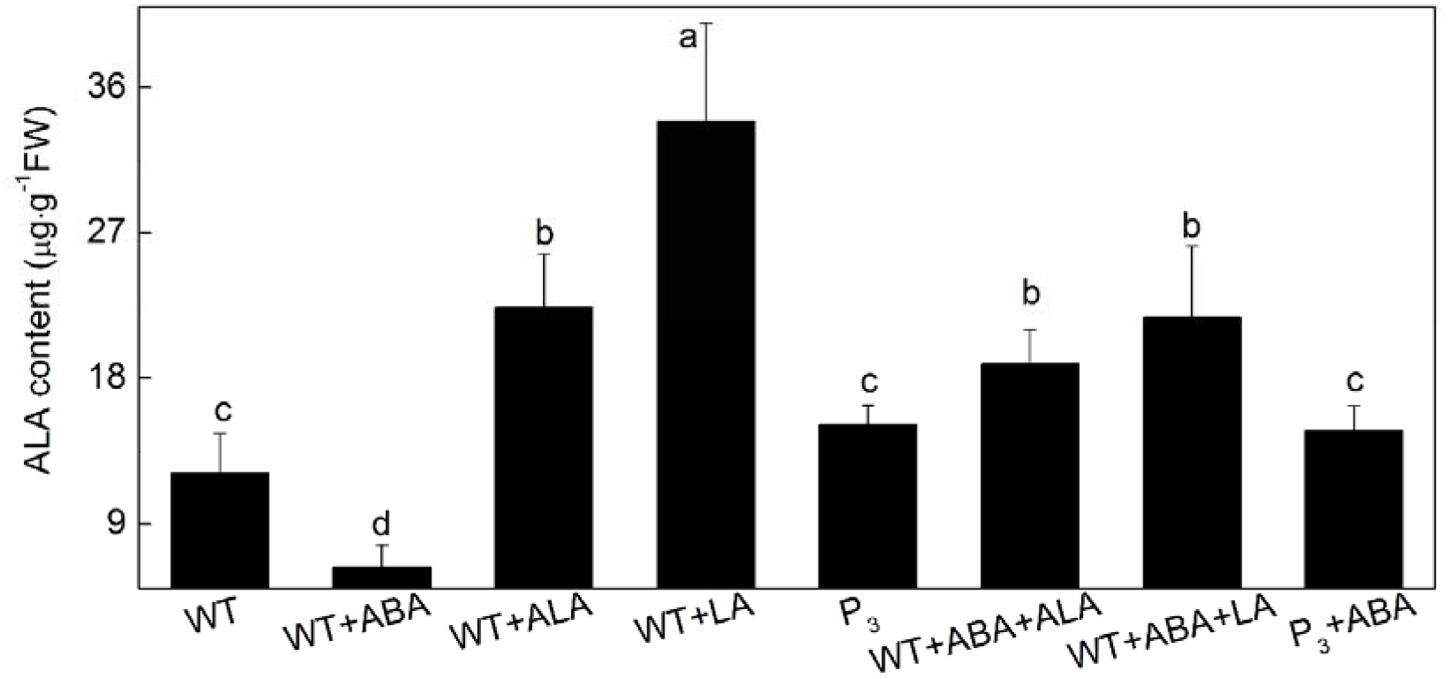
FIGURE 3. Leaf endogenous ALA content. Leaves of wild-type Arabidopsis were floated on either CO2-free MES-KCl buffer alone or containing 10 μM ABA, 0.5 mg L-1 ALA, 1 mM LA, 10 μM ABA + 0.5 mg L-1 ALA, 10 μM ABA + 1 mM LA at 25°C for 1 h under light (240 μmol m-2 s-1), respectively, and YHem1 transgenic (P3) Arabidopsis were floated on either buffer alone or containing 10 μM ABA at 25°C for 1 h under light (240 μmol m-2 s-1). Then, leaves were collected and wished with distilled water for determination of endogenous ALA content. Values are the means of nine measurements ± SE from three independent experiments. Different small letters represent significant difference between treatments (P < 0.05).
ALA Reduces H2O2 Content in Guard Cells
Hydrogen peroxide (H2O2) is an important signaling molecule in guard cells (Neill et al., 2002), and its role in ABA-induced stomatal closure has been well established (Pei et al., 2000; Zhang et al., 2001a). To determine whether ALA inhibits ABA-induced stomatal closure via manipulating H2O2 content in guard cells, we first investigated the effect of ALA on H2O2 content in guard cells using a fluorescent dye, H2DCF-DA. We found that ABA increased H2O2 content in guard cells rapidly indicated by the increase in fluorescence intensity (Figure 4A). Significant H2O2 production was observed within 10 min after the application of ABA, and H2O2 content continuously increased with time. When ALA was applied together with ABA, ABA-induced H2O2 was largely impaired after 18 min and continuously weakened (Figures 4A,B). The transgenic plants were also used here to confirm ALA effect on ABA-induced H2O2. Similarly, the H2DCF-fluorescence in guard cell of transgenic plants was continuously weakened after 18 min as compared with the wild-type (Figures 4A,B) under ABA treatment. These results indicated that exogenous ALA and the over-produced endogenous ALA can both decrease ABA-induced H2O2 accumulation in guard cells.
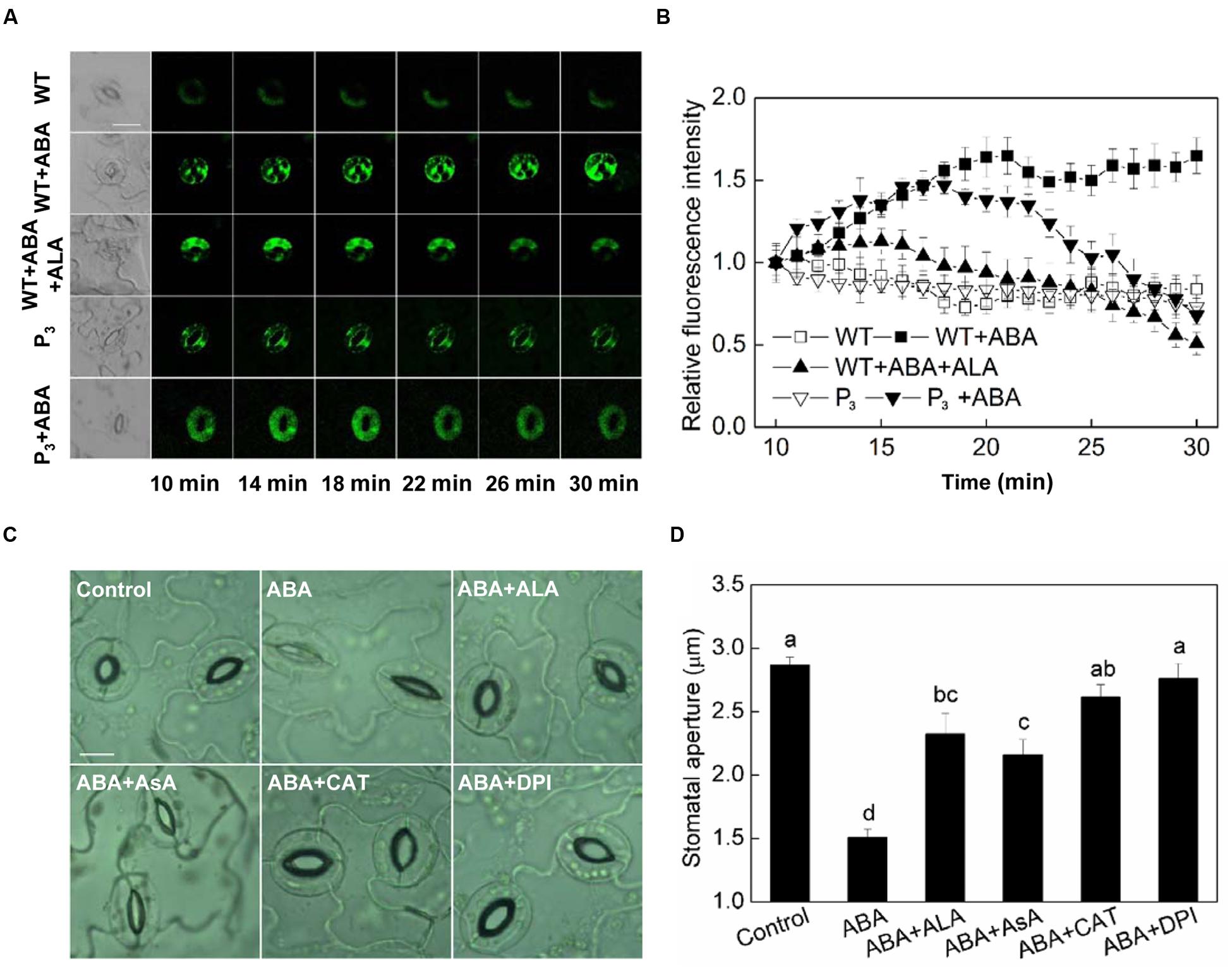
FIGURE 4. ALA reduces ABA-induced H2O2 accumulation in guard cells. (A,B) Changes in H2O2 content in guard cells. Isolated epidermal peels of pre-illuminated wild-type and YHem1 transgenic (P3) Arabidopsis were loaded with H2DCF-DA for 30 min in darkness at 25°C, then excess dye was removed for the following treatments. Wild-type peels were transferred to either the opening buffer alone or supplemented with 10 μM ABA, 10 μM ABA + 0.5 mg L-1 ALA, and P3 peels were transferred to either the opening buffer alone or supplemented with 10 μM ABA. Five minutes later, fluorescence of the above-treated peels (A) was observed using a laser scanning confocal microscope (Leica TCS SP8 STED 3X, LSCM) and Time-course and Photoshop software. For each treatment, the first picture is bright field image and the following are fluorescence images corresponding to the bright field image at 1 min intervals. Scale bar: 20 μm. (B) Time course changes of the relative fluorescence in guard cells of each treatment. Data are normalized by calculating the relative changes in fluorescence over initial values. Values are the means of 15 measurements ± SE from three independent experiments. (C,D) The inhibitive effect of ALA on ABA-induced stomatal closure is similar to AsA, CAT, and DPI, all of which inhibit ABA-induced stomatal closure via decreasing H2O2 in guard cells. Isolated epidermal strips of wild-type Arabidopsis were incubated at 25°C in either buffer (Control), or containing 10 μM ABA, 10 μM ABA + 0.5 mg L-1 ALA, 10 μM ABA + 100 μM AsA, 10 μM ABA + 100 U mL-1 CAT, 10 μM ABA + 10 μM DPI for 1 h under light (240 μmol m-2 s-1), respectively, and then images (C) were recorded and stomatal apertures (D) were determined. Images (C) were recorded by light microscopy (Nikon TE100, 400×), using a fitted camera (MShot Digital Imaging System). Scale bar: 10 μm. Values are the means of 90 measurements ± SE from three independent experiments. The same letters represent no significant differences between treatments (P < 0.01).
AsA, CAT, and DPI are the most important reducing substrate for H2O2 removal, a H2O2-scavenging enzyme and an inhibitor of the ROS-generating enzyme, NADPH oxidase, respectively. To investigate the relationship between ALA-inhibited stomatal closure and the levels of H2O2 in guard cells, the ABA-treated epidermal strips were applied simultaneously with AsA, CAT, and DPI. Similar to ALA, AsA, CAT, and DPI all inhibited ABA-induced stomatal closure (Figures 4C,D). These results suggested that the inhibitive effect of ALA on ABA-induced stomatal closure is associated with a decrease of H2O2 levels in guard cells.
ALA Reduces Cytosolic Ca2+ in Guard Cells
Calcium ion is another important second messenger. It has been reported that ABA-induced H2O2 accumulation and the H2O2-activated cytosolic Ca2+ increase are important mechanisms for ABA-induced stomatal closure (Pei et al., 2000; An et al., 2008). Since we have showed that H2O2 play a crucial role in ALA-inhibited ABA-induced stomatal closure, we assumed that Ca2+ signal may be also involved in the inhibitive process of ALA on ABA-induced stomatal closure. To test this hypothesis, we first investigated the effect of ALA on ABA-induced cytosolic Ca2+ accumulation. The results showed that both exogenous ALA and the over-produced endogenous ALA decreased ABA-induced cytosolic Ca2+ accumulation in guard cells after 14 min (Figures 5A,B). Then, we compared the effect of ALA with EGTA (a Ca2+ chelator) and AlCl3 (a blocker of Ca2+ channel) on ABA-induced stomatal closure. Similar to ALA, both EGTA and AlCl3 suppressed ABA-induced stomatal closure (Figures 5C,D). These results suggested that Ca2+ signal is also involved in ALA-inhibited stomatal closure.
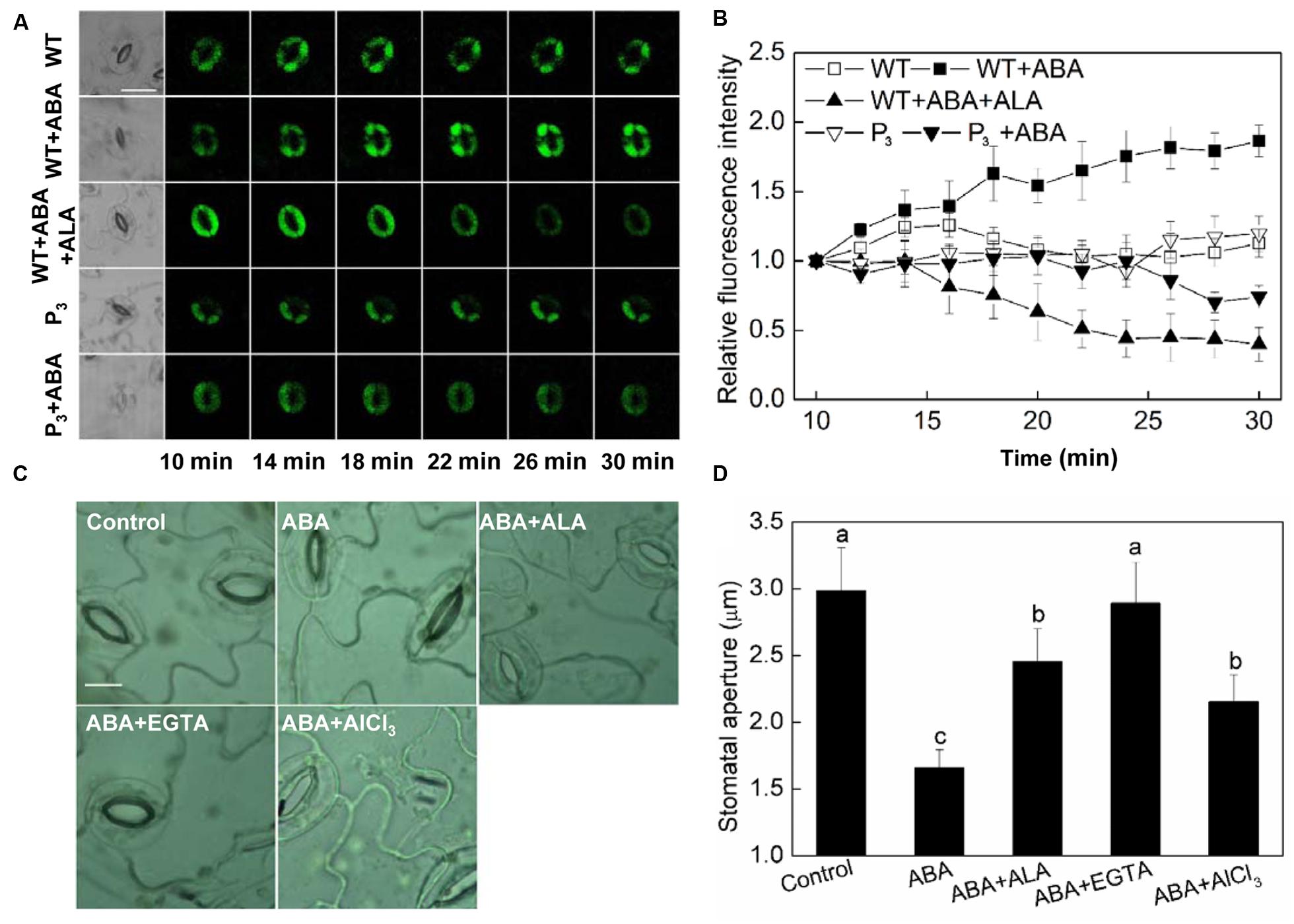
FIGURE 5. ALA reduces ABA-induced cytosolic Ca2+ accumulation in guard cells. (A,B) Changes in Ca2+ content in guard cells. Isolated epidermal peels of pre-illuminated wild-type and YHem1 transgenic (P3) Arabidopsis were loaded with 1 μM Fluo-3 AM (dissolved in DMSO) for 2 h in darkness at 4°C, then excess dye was removed for the following treatments. Wild-type peels were transferred to either the opening buffer alone or supplemented with 10 μM ABA, 10 μM ABA + 0.5 mg L-1 ALA, and P3 peels were transferred to either the opening buffer alone or supplemented with 10 μM ABA. Five minutes later, fluorescence of the above-treated peels (A) was observed using a laser scanning confocal microscope (Leica TCS SP8 STED 3X, LSCM) and Time-course and Photoshop software. For each treatment, the first picture is bright field image and the following are fluorescence images corresponding to the bright field image at 2 min intervals. Scale bar: 20 μm. (B) Time courses changes of the relative fluorescence in guard cells of each treatment. Data are normalized by calculating the relative changes in fluorescence over initial values. Values are the means of 15 measurements ± SE from three independent experiments. (C,D) The inhibitive effect of ALA on ABA-induced stomatal closure is similar to EGTA and AlCl3, both of which inhibit ABA-induced stomatal closure via decreasing cytosolic Ca2+ in guard cells. Isolated epidermal strips of wild-type Arabidopsis were incubated at 25°C in either buffer (Control), or containing 10 μM ABA, 10 μM ABA + 0.5 mg L-1 ALA, 10 μM ABA + 5 mM EGTA, 10 μM ABA + 50 μM AlCl3 for 1 h under light (240 μmol m-2 s-1), respectively, and then images (C) were recorded and stomatal apertures (D) were determined. Images (C) were recorded by light microscopy (Nikon TE100, 400×), using a fitted camera (MShot Digital Imaging System). Scale bar: 10 μm. Values are the means of 90 measurements ± SE from three independent experiments. Different small letters represent significant difference between treatments (P < 0.01).
ALA Inhibits H2O2- and Ca2+-induced Stomatal Closure
To further clarify whether ALA-induced stomatal movement by decreasing H2O2 content in guard cells, we first examined the effect of exogenous ALA on H2O2-induced stomatal closure in wild-type plants. H2O2 significantly reduced stomatal aperture (Figures 6A,B). However, when ALA was applied together with H2O2, H2O2-induced stomatal closure was largely repressed. We next compared the stomatal responses of wild-type plants with those of ALA-overproduced transgenic plants. Contrary to that of wild-type plants, stomatal aperture of transgenic plants was not reduced by H2O2 (Figures 6A,B). These observations indicated that both endogenous and exogenous ALA can scavenge H2O2, and then prevent stomatal closure induced by exogenous H2O2.
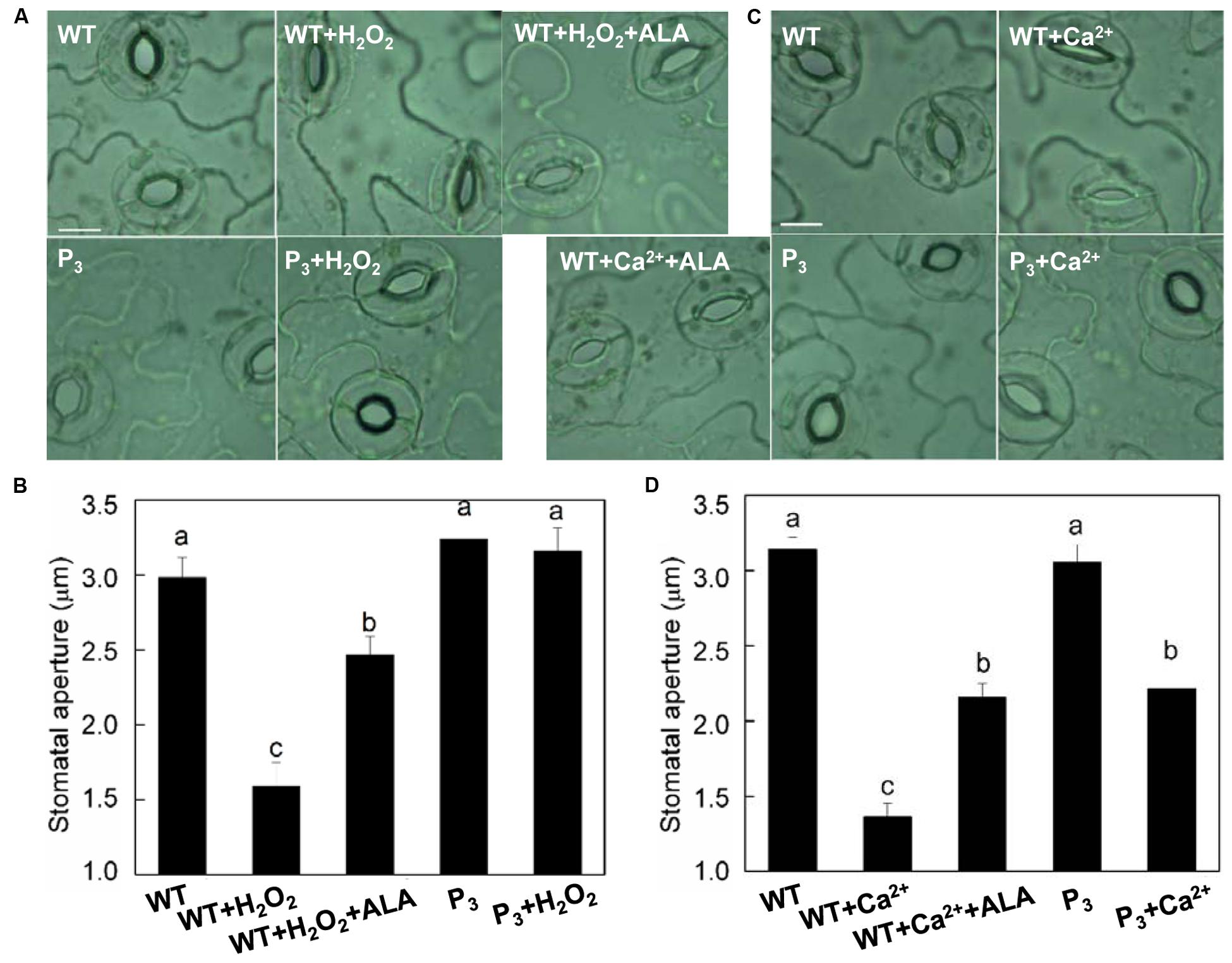
FIGURE 6. ALA inhibits H2O2- and Ca2+-induced stomatal closures. (A,B) ALA inhibits exogenous H2O2-induced stomatal closure. Isolated epidermal strips of wild-type Arabidopsis were incubated in either buffer alone or containing 200 μM H2O2, 200 μM H2O2 + 0.5 mg L-1 ALA, respectively, while P3 peels were incubated in either buffer or containing 200 μM H2O2 at 25°C under light (240 μmol m-2 s-1). One hour later, images (A) were recorded and stomatal apertures (B) were determined. (C,D) ALA inhibits exogenous Ca2+-induced stomatal closure. Isolated epidermal strips of wild-type Arabidopsis were incubated in either buffer alone or containing 2 mM CaCl2, 2 mM CaCl2 + 0.5 mg L-1 ALA, respectively, while P3 peels were incubated in either buffer or containing 2 mM CaCl2 at 25°C under light (240 μmol m-2 s-1). One hour later, images (C) were recorded and stomatal apertures (D) were determined. Images (A,C) were recorded by light microscopy (Nikon TE100, 400×), using a fitted camera (MShot Digital Imaging System). Scale bar: 10 μm. Values are the means of 90 measurements ± SE from three independent experiments. Different small letters represent significant difference between treatments (P < 0.01).
Similarly, both exogenous and endogenous ALA repressed Ca2+-induced stomatal closure significantly (Figures 6C,D), confirming that ALA inhibits ABA-induced stomatal closure by decreasing [Ca2+]cyt.
ALA Inhibits ABA-induced Stomatal Closing in the Whole Plants
To verify the effect of ALA on stomatal movement in the whole plants, we next carried out an examination of whether ALA represses stomatal closure even in planta. Since stomata are known to close in response to drought to limit water loss by transpiration, we monitored time courses of leaf FW decrease after detachment from the whole plant. Results showed that ABA significantly reduced FW decrease rate, while exogenous ALA and expressing YHem1 notably increased it, compared to that in non-treated wild-type plants (Figure 7A). When ABA was applied together with ALA to wild-type plants, the FW decrease rate in ABA-treated plants was obviously accelerated. Similarly, FW decrease rate in ABA-treated transgenic plants was faster than that in ABA-treated wild-type plants. The rate of FW decrease per 20 min also revealed that FW decrease rate in P3 and ALA-treated plants was higher than in non-treated plants within 40 min after detachment (Figure 7B). And FW decrease rate in ABA-treated wild-type plants was also higher in the presence of ALA, regardless of its source, during the whole experiment (Figure 7B). These results indicated that both exogenous and endogenous ALA accelerates plant transpirational water loss, reflecting the stomatal opening induced by ALA.
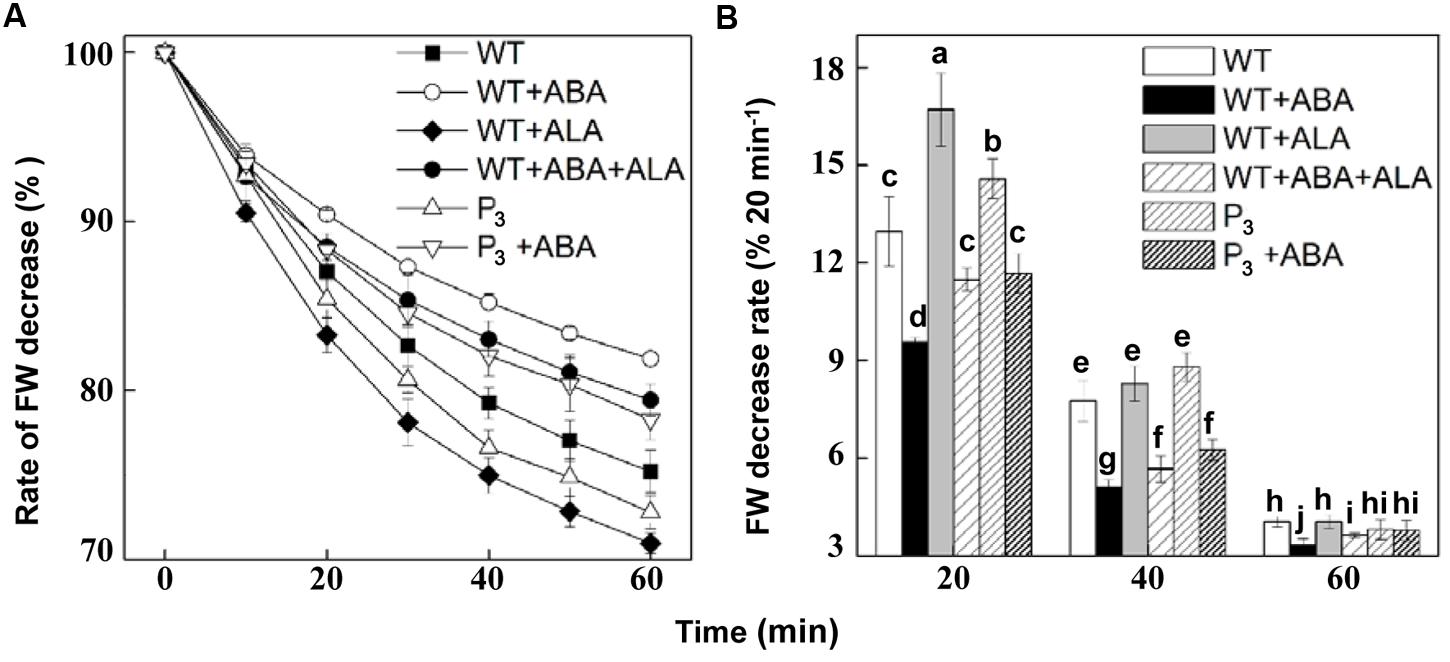
FIGURE 7. ALA accelerates fresh weight (FW) decrease rate of plants. (A) Changes in FW decrease ratio of detached leaves of wild-type plants, wild-type plants treated with 10 μM ABA, 0.5 mg L-1 ALA, or 10 μM ABA + 0.5 mg L-1 ALA, YHem1 transgenic plants (P3) and YHem1 transgenic plants treated with 10 μM ABA. (B) Leaf FW decrease rate calculated every 20 min after detachment from plants. FWs of the detached leaves were measured, and the ratios of reduced weights and original FWs were then calculated. Values are the means of four independent experiments, and five leaves were used for each treatment in one experiment. Error bars represent SEs. The same letters represent no significant differences between treatments (P < 0.05).
ALA Improves Drought Tolerance of Arabidopsis
Abscisic acid-induced stomatal closure is a well-known mechanism behind drought tolerance of plants. Since ALA inhibited ABA-induced stomatal closure, to determine whether ALA reduces plant drought tolerance, 15% PEG 6 000 were used to create drought stress, and ALA’s effect on growth characteristics, leaf chlorophyll content and stomatal aperture of Arabidopsis plants were investigated. Notably, the exogenous ALA-treated wild-type plants and the YHem1 transgenic plants, which exhibited wider stomatal aperture, produced larger rosette leaves and longer roots, indicating they grew better than untreated wild-type plants under drought treatment (Figures 8A–E). Under 15% PEG 6 000, shoot length of the exogenous ALA-treated wild-type plants and the YHem1 transgenic plants were 1.29 and 1.33 times, respectively, higher than that of the untreated wild-type plants, and root length were 0.64 and 0.37 times higher, respectively (Figures 8B–D). Under normal condition, no significant difference were found between chlorophyll content in untreated wild-type plants with YHem1 transgenic plants or ALA-treated wild-type plants (Figure 8F). Drought stress significantly decreased chlorophyll content in untreated wild-type plants, but did not change that in YHem1 transgenic plants or ALA-treated wild-type plants, resulting in significant higher level of chlorophyll in YHem1 transgenic plants or ALA-treated wild-type plants than untreated wild-type plants. These results indicated that ALA-induced increase in stomatal aperture did not engender the sensitivity of plants to drought stress as expected. On the contrary, ALA significantly improved plant drought tolerance while increasing stomatal aperture. The significant positive correlation between chlorophyll content and stomatal aperture (r = 0.900; P = 0.014) in this experiment suggested that ALA-inhibited stomatal closure might be related to the improvement of chlorophyll synthesis.
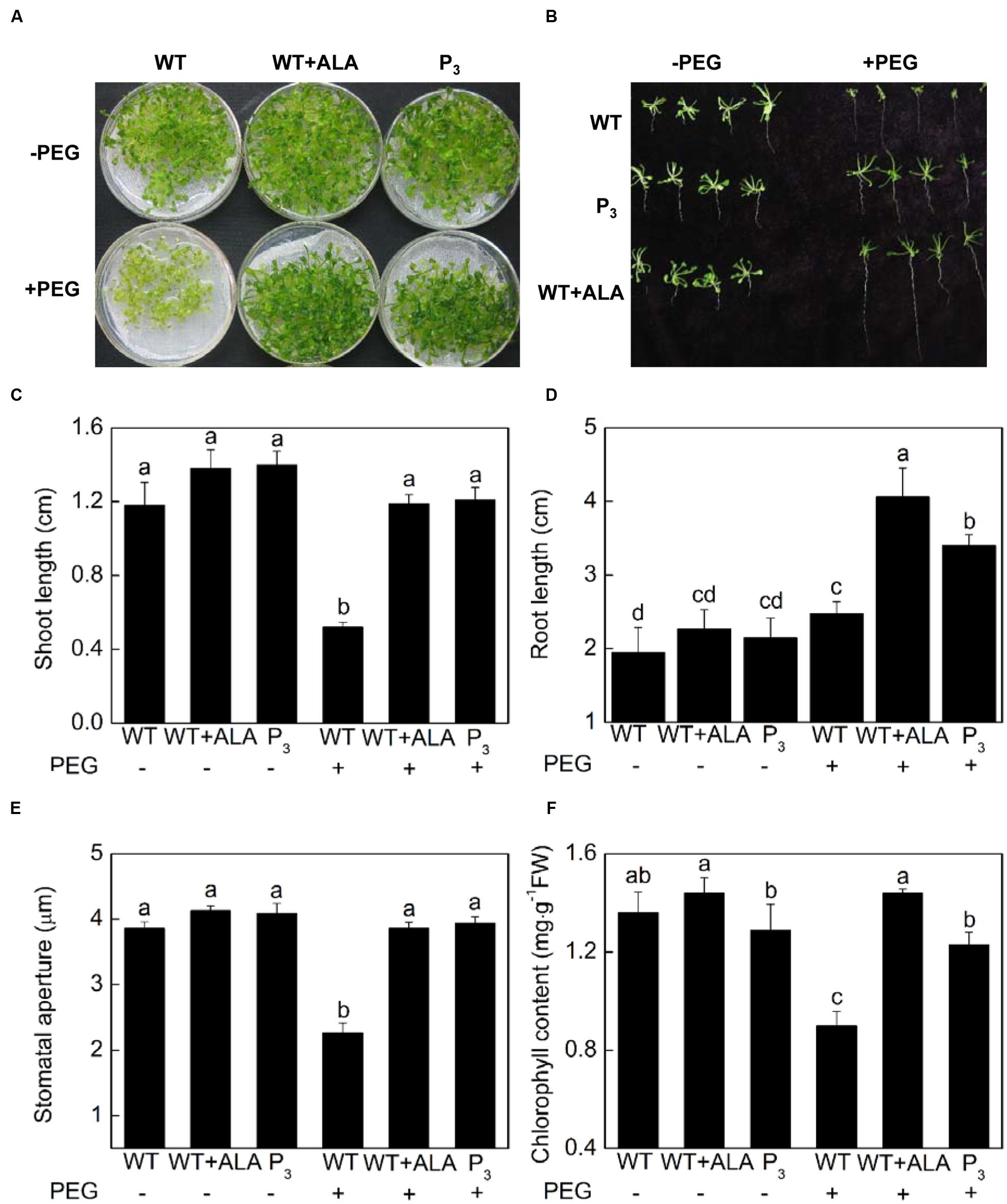
FIGURE 8. ALA increases drought tolerance of Arabidopsis. (A,B) ALA improves seedling growth under drought condition. 18-day-old wild-type Arabidopsis were cultured for another 14 days in either MS medium alone or containing 15% PEG 6 000 with or without 0.5 mg L-1 ALA. And YHem1 transgenic (P3) Arabidopsis were cultured for another 14 days in MS medium alone or with 15% PEG 6 000. Then images were recorded. (C,D) ALA increases shoot length and root length of Arabidopsis seedlings exposed to drought condition. Values are the means of 18 measurements ± SE from three independent experiments. (E) ALA increases stomatal aperture of seedlings exposed to drought condition. Abaxial epidermal strips were peeled from leaves of the above treated plants and then stomatal apertures were determined. Values are the means of 90 measurements ± SE from three independent experiments. (F) ALA increases leaf chlorophyll content in seedlings exposed to drought condition. Values are the means of nine measurements ± SE from three independent experiments. The same letters represent no significant differences between treatments (P < 0.05).
Discussion
ALA Inhibits ABA-induced Stomatal Closure
5-Aminolevulinic acid can significantly improve leaf photosynthesis and widely promote plant growth and yield (Hotta et al., 1997, 1998; Nishihara et al., 2003; Wang et al., 2004; Liu et al., 2011). Stomata controls CO2 uptake for photosynthesis, determining plant productivity (Lawson and Blatt, 2014). Here, we showed that ALA does not affect stomata development, but significantly inhibits stomatal closure.
ABA is well-known in provoking stomatal closing (Aliniaeifard and van Meeteren, 2013; Kollist et al., 2014). In our study, exogenous ALA dramatically increased stomatal aperture under ABA treatment, indicating that ALA inhibits ABA-induced stomatal closure. This partially explains why exogenous ALA significantly improved plant photosynthesis even under stressful conditions (Hotta et al., 1997, 1998; Nishihara et al., 2003; Wang et al., 2004; Liu et al., 2011; Zhang et al., 2012). In addition, the stomatal aperture of P0 and P3, two lines of YHem1-transgenic plants (Zhang et al., 2010), were notably larger than that of wild-type plants under ABA treatment, indicating endogenous ALA also inhibits ABA-induced stomatal closure. This result further interprets why P0 and P3 grew more vigorously than wild-type plants which have been reported before (Zhang et al., 2010).
The effects of ALA on stomatal movement could be due to ALA itself or its metabolites. Wang et al. (2005) demonstrated that ALA-induced improvement of plant salt tolerance was dependent on its conversion into tetrapyrrole compounds. Similarly, Xie et al. (2013b) illustrated that ALA-induced promotion of anthocyanin accumulation in apple skins was not due to itself but its conversion to porphyrins. In the present study, however, LA showed similar inhibitive effect to ALA on ABA-induced stomatal closure. Since LA blocks ALA metabolism and hence leads to ALA accumulation (Xie et al., 2013b), the inhibitive effect of ALA on ABA-induced stomatal closure is probably due to ALA itself. The higher endogenous ALA contents in YHem1-transgenic plants and ALA-, LA-treated wild-type plants under ABA treatment confirmed that ALA itself participates in guard cell signaling. However, the significantly higher endogenous ALA but lower stomatal aperture in plants treated with ABA and LA than that in control plants suggested that ALA’s metabolites might also involve in regulating stomatal movement. ALA is the first key precursor of chlorophyll synthesis, and studies have demonstrated that exogenous ALA and YHem1 expression significantly increase chlorophyll content in plants under stresses (Akram and Ashraf, 2013). Similarly, in this study, ALA increased chlorophyll content in Arabidopsis under osmotic stress (Figure 8E). A correlation has been previously suggested between chlorophyll synthesis and stomatal movement (Shimshi, 1967; Matsumoto et al., 2005). These studies showed that stomatal conductance was dependent on chlorophyll content to some extent. In the present study, stomatal apertures were significantly positively correlated with leaf chlorophyll content. These results indicate that ALA-improved chlorophyll synthesis may be involved in ALA-inhibited stomatal closure. But further studies are needed to elucidate whether and how ALA’s metabolites induce stomatal opening.
ALA Inhibits ABA-induced Stomatal Closure through Reducing H2O2 and Ca2+ Levels in Guard Cells
Inhibition of ABA-induced stomatal closure results from either degradation of ABA or impairment of ABA signal. We did not measure ABA amount in our experiments, but ALA seems not to interfere with the early ABA-signaling pathway since the stomata started to close by ABA application even in the presence of ALA (Figure 1B). The stomata were kept half-opened or reopened by ALA treatment, indicating that ALA probably functions in some later stage of ABA-signaling. The increase in hydrogen peroxide (H2O2) production and the H2O2-activated elevation of [Ca2+]cyt in guard cells are important downstream signaling components for ABA-induced stomatal closure (Pei et al., 2000). Previous studies have showed that ALA could decrease H2O2 levels in leaves or/and roots of several plant species (Balestrasse et al., 2010; Liu et al., 2011). However, little was known about the effect of ALA on H2O2 level in guard cells. In the present study, we showed that both exogenous and endogenous ALA decreased ABA-induced H2O2 accumulation in guard cells. The similarity of ALA’s effect to AsA, CAT, and DPI on ABA-induced stomatal closure further indicate that the inhibitive effect of ALA on ABA-induced stomatal closure results from the decrease of H2O2 levels in guard cells (Zhang et al., 2001a,b; Song et al., 2006; Wei et al., 2014).
Both cytokinins and auxins have been reported to induce stomatal opening by decreasing H2O2 levels in guard cells (Song et al., 2006). But the former probably initiates H2O2-scavenging systems, while the later mainly limits the production of H2O2 (Song et al., 2006). In the present study, both exogenous and endogenous ALA inhibited exogenous H2O2-induced stomatal closure, indicating that, similar to cytokinins, ALA reduced H2O2 levels probably by scavenging H2O2. Furthermore, ABA-induced H2DCF-fluorescence in our experiment first raised and then dropped after 18 min upon ALA application or in YHem1-transgenic plants (Figure 4), suggesting that H2O2 generated first and then was scavenged. This result confirmed that ALA reduced H2O2 levels mainly through accelerating its elimination. Until now, little is known about how ALA scavenge H2O2 in guard cells. However, many reports have revealed that ALA enhances plant antioxidant capacity in leaves or/roots. For example, ALA significantly improved ratio of GSH/GSSG and AsA/DHA and enhanced activities of several antioxidant enzymes including CAT, APX, and GR in oilseed rape (Brassica napus) under water-deficit stress (Liu et al., 2011). Similarly, ALA markedly increased APX, GR, and CAT activity and up-regulated the expressions of CAT, cAPX, and GR gene in NaCl-treated cucumber plants (Zhen et al., 2012). Therefore, in guard cells, ALA may also activate the antioxidant defense system to reduce H2O2 content. But this speculation and the specific antioxidant mechanisms in guard cells needs further testing.
Cytosolic Ca2+ in guard cells plays pivotal roles in stomatal function (Harada and Shimazaki, 2009). H2O2 activation of plasma membrane Ca2+ channel in guard cells is known as an important downstream component in ABA signaling (Pei et al., 2000). In the present study, ALA decreased ABA-induced [Ca2+]cyt in guard cells, and ALA’s effect on ABA-induced stomatal closure was similar to EGTA, a Ca2+ chelator (Schwartz, 1985), and AlCl3, a blocker of Ca2+ channel (Zhao et al., 2007). In addition, ALA significantly inhibited Ca2+-induced stomatal closure. These results indicate that ALA inhibits ABA-induced stomatal closure by decreasing [Ca2+]cyt. As ABA-induced H2O2 accumulation was inhibited, the decrease of [Ca2+]cyt might be a result of the suppressed Ca2+ channel activity. Besides, decrease of [Ca2+]cyt start in 14 min after treatment (Figure 5), while reduction of H2O2 content begins after 18 min (Figure 4), suggesting that the time for [Ca2+]cyt began to decrease was ahead of the reduction of H2O2 content. These results indicate that there are some H2O2-independent signal pathways leading to the decrease of [Ca2+]cyt under ALA treatment. P-type Ca2+-ATPase can remove Ca2+ from the cytosol and hence reduce [Ca2+]cyt, and play an important role in Ca2+ signal (Kollist et al., 2014). Recently, We found that ALA increased Ca2+-ATPase activity, decreasing [Ca2+]cyt in pollen tube of pear (Pyrus pyrifolia), and hence reduced the growth of pollen tube (An et al., 2016). Therefore, maybe this mechanism is also employed by ALA to regulate [Ca2+]cyt in guard cells.
The above results demonstrate that ALA inhibits ABA-induced stomatal closure via reducing H2O2 and Ca2+ levels in guard cells, and the mechanisms on how ALA decrease H2O2 and Ca2+ levels in guard cells need to be further studied.
ALA Improves Drought Tolerance while Increasing Stomatal Aperture
In the whole plant, upon drought stress, the rate of transpiration was greater in YHem1 and wild-type plants exposed to ALA than in untreated wild-type control plants, indicating the inhibitive effects of ALA on ABA-induced stomatal closure were also observed in planta. However, inhibition of transpirational water loss by ABA- or stress-induced stomatal closing has been generally accepted as an important mechanism improving stress tolerance, especially the drought tolerance (Li et al., 2006). Whether ALA impairs plant drought tolerance? Here, we showed that 0.5 mg⋅L-1 exogenous ALA and YHem1 expression, both of which inhibit ABA-induced stomatal closure, significantly improved drought tolerance of Arabidopsis (Figure 8). These results indicate that ALA-inhibited stomatal closure does not increase plant sensitivity to drought stress.
Actually, there have been a few studies about the effects of ALA on plant drought tolerance, and they proved that ALA improves drought tolerance of various plants, including wheat (Triticum aestivum vulgare L.; Al-Thabet, 2006; Kosar et al., 2015), barley (Hordeum vulgare L.; Al-Khateeb, 2006), oilseed rape (Brassica napus L.; Liu et al., 2011, 2013), and cucumber (Cucumis sativus L.; Li et al., 2011). Chlorophyll accumulation, improvement of photosynthetic electron transfer ability, photosynthesis, and antioxidant capacity play important roles in ALA-conferred drought tolerance (Li et al., 2011; Liu et al., 2011, 2013). Recently, Kosar et al. (2015) found that ALA significantly improved leaf glycine betaine accumulation and root K+ content under drought stress, indicating osmotic regulation also contributes to the improvement of drought tolerance by ALA. In addition, ALA significantly improved root dry weight under drought stress (Kosar et al., 2015), indicating ALA enhances plant root growth. Consistently, our data showed that both 0.5 mg⋅L-1 exogenous ALA and YHem1 expression significantly improved root length (Figures 8B,D) of Arabidopsis under drought stress. Root system plays a critical role in plant adaptation to drought environments, in terms of signal perception and transmission, water and nutrient uptake during both drought and rewetting conditions (Sharp et al., 2003; Schachtman and Goodger, 2008). Therefore, improved root growth is an important mechanism behind ALA’s improvement of drought tolerance. In addition, aquaporins are known to significantly contribute to water movement and hence play important roles in plant drought tolerance (Zhao et al., 2015). Zhao et al. (2015) found that ALA controls aquaporin expression and consequently regulates plant water homeostasis under salt stress. Therefore, various mechanisms are involved in ALA-conferred drought tolerance, and the stronger root water uptake and aquaporin regulation maybe offset the adverse effect of ALA-induced stomatal opening on water loss. However, how ALA improves plant drought tolerance, especially the molecular mechanisms behind the paradox between enhanced drought tolerance and promoted stomatal opening, needs to be further studied.
Physiological Role of ALA Inhibition of ABA-induced Stomatal Closure
Increasing reports indicate that stomatal resistance is an important limiting factor in photosynthesis and plant growth (Yang et al., 2012; Campos et al., 2014; Wang et al., 2014). However, to date, few studies have been carried out to promote stomatal opening with the goal of improving photosynthesis, perhaps because of the difficulty in balancing the counteracting effects of taking up CO2 while losing water through the stomata. Recently, there were some attempts that trying to improve photosynthesis by manipulating stomatal opening. However, the plant sensitivity to stresses was simultaneously increased in most cases. For example, slac1, an open-stomata mutant of rice, have been shown to increase leaf photosynthesis rate under well-watered conditions (Kusumi et al., 2012). But the slac1 mutation had no effect on plant growth due to an increased sensitivity to drought stress. Therefore, efficient ways balancing plant stomatal opening and stress resistance are of urgent need in the present era of global climate changes and the threat of food insufficiency. In the present study, we demonstrate the inhibitive effect of ALA on stomatal closing and the concurrent improvement of plant drought tolerance, suggesting great application potential of ALA in agriculture and forestry.
Except drought stress, it has been well documented that low concentrations of ALA could markedly improve plant resistance to many other stressful conditions, including cold (Hotta et al., 1998), salt (Nishihara et al., 2003), low light (Wang et al., 2004), heat (Zhang et al., 2012), and heavy metal stress (Ali et al., 2013; Tian et al., 2014). And, in all these studies, improving photosynthesis and growth was proved to play important roles in ALA-increased stress resistance. In addition to the effect of exogenous ALA in these studies, expressing YHem1 in Arabidopsis, which over-produced endogenous ALA, enhanced plant salt tolerance and growth as well (Zhang et al., 2010). Thus, it seems feasible to promote plant photosynthesis and improve plant resistance simultaneously to many abiotic stresses by application of ALA or through genetic modification of ALA biosynthesis.
In summary, the data presented here showed that ALA inhibits ABA-induced stomatal closure via reducing H2O2 and Ca2+ levels in guard cells, and simultaneously improves plant drought tolerance. Since ALA is naturally present in all living cells and has been proved to be of no toxicity and no pollution (Akram and Ashraf, 2013), application of ALA or expressing YHem1 in crops and fuel plants is expected to contribute greatly to the promotion of plant production and a sustainable low-carbon society, especially under stressful conditions. The interaction between ALA and ABA in regulating stomatal movement would no doubt provide clues directing further study about functional mechanisms of ALA.
Author Contributions
YA, LW conceived and designed research. LL and LC carried out all the experiments. YA, LL, and LC analyzed the data. YA and LW wrote the manuscript. All authors read and approved the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (31401820), the Fundamental Research Funds for the Central Universities (KJQN201538), the Natural Science Foundation of Jiangsu Province, China (BK20140702), and Agricultural Independent Innovation Fund of Jiangsu Province, China (CX(11)4004). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Meixiang Zhang from Nanjing Agricultural University for his valuable comments and suggestions that help improve the manuscript.
References
Akram, N. A., and Ashraf, M. (2013). Regulation in plant stress tolerance by a potential plant growth regulator, 5-aminolevulinic acid. J. Plant Growth Regul. 32, 663–679. doi: 10.1007/s00344-013-9325-9
Ali, B., Wang, B., Ali, S., Ghani, M. A., Hayat M. T., Yang, C., et al. (2013). 5-Aminolevulinic acid ameliorates the growth, photosynthetic gas exchange capacity, and ultrastructural changes under cadmium stress in Brassica napus L. J. Plant Growth Regul. 32, 604–614. doi: 10.1007/s00344-013-9328-6
Aliniaeifard, S., and van Meeteren, U. (2013). Can prolonged exposure to low VPD disturb the ABA signalling in stomatal guard cells? J. Exp. Bot. 64, 3551–3566. doi: 10.1093/jxb/ert192
Al-Khateeb, S. A. (2006). Promotive effect of 5-aminolevulinic acid on growth, yield and gas exchange capacity of barley (Hordeum vulgare L.) grown under different irrigation regimes. J. King Saud Univ. Sci. 18, 103–111.
Al-Thabet, S. S. (2006). Promotive effect of 5-aminolevulinic acid on growth and yield of wheat grown under dry conditions. J. Agron. 5, 45–49. doi: 10.3923/ja.2006.45.49
An, Y. Y., Li, J., Duan, C. H., Liu, L. B., Sun, Y. P., Cao, R. X., et al. (2016). 5-Aminolevulinic acid thins pear fruits by inhibiting pollen tube growth via Ca2+-ATPase-mediated Ca2+ efflux. Front. Plant Sci. 7:121. doi: 10.3389/fpls.2016.00121
An, Z. F., Jing, W., Liu, Y. L., and Zhang, W. H. (2008). Hydrogen peroxide generated by copper amine oxidase is involved in abscisic acid-induced stomatal closure in Vicia faba. J. Exp. Bot. 59, 815–825. doi: 10.1093/jxb/erm370
Balestrasse, K. B., Tomaro, M. L., Batlle, A., and Noriega, G. O. (2010). The role of 5-aminolevulinic acid in the response to cold stress in soybean plants. Phytochemistry 71, 2038–2045. doi: 10.1016/j.phytochem.2010.07.012
Bindu, R. C., and Vivekanandan, M. (1998). Hormonal activities of 5-aminolevulinic acid in callus induction and micropropagation. Plant Growth Regul. 26, 15–18. doi: 10.1023/A:1006098005335
Campos, H., Trejo, C., Pena-Valdivia, C. B., Garcia-Nava, R., Conde-Martinez, F. V., and Cruz-Ortega, M. R. (2014). Stomatal and non-stomatal limitations of bell pepper (Capsicum annuum L.) plants under water stress and re-watering: delayed restoration of photosynthesis during recovery. Environ. Exp. Bot. 98, 56–64. doi: 10.1016/j.envexpbot.2013.10.015
Dodd, I. C. (2003). Hormonal interactions and stomatal responses. J. Plant Growth Regul. 22, 32–46. doi: 10.1007/s00344-003-0023-x
Gao, J. J., Feng, X. X., Duan, C. H., Li, J. H., Shi, Z. X., Gao, F. Y., et al. (2013). Effects of 5-aminolevulinic acid (ALA) on leaf photosynthesis and fruit quality of apples. J. Fruit Sci. 30, 944–951. doi: 10.13925/j.cnki.gsxb.2013.06.015
Garcia-Mata, C., and Lamattina, L. (2010). Hydrogen sulphide, a novel gasotransmitter involved in guard cell signalling. New Phytol. 188, 977–984. doi: 10.1111/j.1469-8137.2010.03465.x
Harada, A., and Shimazaki, K. (2009). Measurement of changes in cytosolic Ca2+ in Arabidopsis guard cells and mesophyll cells in response to blue light. Plant Cell Physiol. 50, 360–373. doi: 10.1093/pcp/pcn203
Harel, E., and Klein, S. (1972). Light dependent formation of δ-aminolevulinic acid in etiolated leaves of higher plants. Biochem. Biophy. Res. Commun. 49, 364–370. doi: 10.1016/0006-291X(72)90419-6
He, J. M., Yue, X. Z., Wang, R. B., and Zhang, Y. (2011). Ethylene mediates UV-B-induced stomatal closure via peroxidase-dependent hydrogen peroxide synthesis in Vicia faba L. J. Exp. Bot. 62, 2657–2666. doi: 10.1093/jxb/erq431
Hotta, Y., Tanaka, T., Bingshan, L., Takeuchi, Y., and Konnai, M. (1998). Improvement of cold resistance in rice seedlings by 5-aminolevulinic acid. J. Pestic. Sci. 23, 29–33. doi: 10.1584/jpestics.23.29
Hotta, Y., Tanaka, T., Takaoka, H., Takeuchi, Y., and Konnai, M. (1997). Promotive effects of 5-aminolevulinic acid on the yield of several crops. Plant Growth Regul. 22, 109–114. doi: 10.1023/A:1005883930727
Kollist, H., Nuhkat, M., and Roelfsema, M. R. G. (2014). Closing gaps: linking elements that control stomatal movement. New Phytol. 203, 44–62. doi: 10.1111/nph.12832
Korkmaz, A., Korkmaz, Y., and Demirkıran, A. R. (2010). Enhancing chilling stress tolerance of pepper seedlings by exogenous application of 5-aminolevulinic acid. Environ. Exp. Bot. 67, 495–501. doi: 10.1016/j.envexpbot.2009.07.009
Kosar, F., Akram, N. A., and Ashraf, M. (2015). Exogenously-applied 5-aminolevulinic acid modulates some key physiological characteristics and antioxidative defense system in spring wheat (Triticum aestivum L.) seedlings under water stress. S. Afr. J. Bot. 96, 71–77. doi: 10.1016/j.sajb.2014.10.015
Kusumi, K., Hirotsuka, S., Kumamaru, T., and Iba, K. (2012). Increased leaf photosynthesis caused by elevated stomatal conductance in a rice mutant deficient in SLAC1, a guard cell anion channel protein. J. Exp. Bot. 63, 5635–5644. doi: 10.1093/jxb/ers216
Lawson, T., and Blatt, M. R. (2014). Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 164, 1556–1570. doi: 10.1104/pp.114.237107
Li, D. M., Zhang, J., Sun, W. J., Li, Q., Dai, A. H., and Bai, J. G. (2011). 5-Aminolevulinic acid pretreatment mitigates drought stress of cucumber leaves through altering antioxidant enzyme activity. Sci. Hort. 130, 820–828. doi: 10.1016/j.scienta.2011.09.010
Li, S., Assmann, S. M., and Albert, R. (2006). Predicting essential components of signal transduction networks: a dynamic model of guard cell abscisic acid signaling. PLoS Biol. 4:e312. doi: 10.1371/journal.pbio.0040312
Lichtenthaler, H. K., and Wellburn, A. R. (1982). Determination of tatal carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 603, 591–592. doi: 10.1042/bst0110591
Liu, D., Pei, Z., Naeem, M., Ming, D., Liu, H., Khan, F., et al. (2011). 5-Aminolevulinic acid activates antioxidative defence system and seedling growth in Brassica napus L. under water-deficit stress. J. Agron. Crop Sci. 197, 284–295. doi: 10.1111/j.1439-037X.2011.00465.x
Liu, D., Wu, L. T., Naeem, M. S., Liu, H. B., Deng, X. Q., Xu, L., et al. (2013). 5-Aminolevulinic acid enhances photosynthetic gas exchange, chlorophyll fluorescence and antioxidant system in oilseed rape under drought stress. Acta Physiol. Plant. 35, 2747–2759. doi: 10.1007/s11738-013-1307-9
Matsumoto, K., Ohta, T., and Tanaka, T. (2005). Dependence of stomatal conductance on leaf chlorophyll concentration and meteorological variables. Agric. Forest Meteorol. 132, 44–57. doi: 10.1016/j.agrformet.2005.07.001
Naeem, M., Jin, Z., Wan, G., Liu, D., Liu, H., Yoneyama, K., et al. (2010). 5-Aminolevulinic acid improves photosynthetic gas exchange capacity and ion uptake under salinity stress in oilseed rape (Brassica napus L.). Plant Soil. 332, 405–415. doi: 10.1007/s11104-010-0306-5
Neill, S., Desikan, R., and Hancock, J. (2002). Hydrogen peroxide signalling. Curr. Opin. Plant Biol. 5, 388–395. doi: 10.1016/S1369-5266(02)00282-0
Nishihara, E., Kondo, K., Parvez, M. M., Takahashi, K., Watanabe, K., and Tanaka, K. (2003). Role of 5-aminolevulinic acid (ALA) on active oxygen-scavenging system in NaCl-treated spinach (Spinacia oleracea). J. Plant Physiol. 160, 1085–1091. doi: 10.1078/0176-1617-00991
Pei, Z. M., Murata, Y., Benning, G., Thomine, S., Klusener, B., Allen, G. J., et al. (2000). Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406, 731–734. doi: 10.1038/35021067
Schachtman, D. P., and Goodger, J. Q. D. (2008). Chemical root to shoot signaling under drought. Trends Plant Sci. 13, 281–287. doi: 10.1016/j.tplants.2008.04.003
Schwartz, A. (1985). Role of Ca2+ and EGTA on stomatal movements in Commelina communis L. Plant Physiol. 79, 1003–1005. doi: 10.1104/pp.79.4.1003
Sharp, R. E., Bohnert, H. J., Springer, G. K., Davis, G. L., Schachtman, D. P., Wu, Y. J., et al. (2003). Root growth maintenance during water deficits: physiology to functional genomics. J. Exp. Bot. 54, 20–20. doi: 10.1093/jxb/erh276
Shen, M., Zhang, Z. P., and Wang, L. J. (2011). Effect of 5-aminolevulinic Acid (ALA) on Leaf Diurnal Photosynthetic Characteristics and Antioxidant Activity in Pear (Pyrus pyrifolia Nakai). Croatia: InTech, 298.
Shimshi, D. (1967). Leaf chlorosis and stomatal aperture. New Phytol. 66, 455–461. doi: 10.1111/j.1469-8137.1967.tb06024.x
Song, X. G., She, X. P., He, J. M., Huang, C., and Song, T. S. (2006). Cytokinin- and auxin-induced stomatal opening involves a decrease in levels of hydrogen peroxide in guard cells of Vicia faba. Funct. Plant Biol. 33, 573–583. doi: 10.1071/FP05232
Tanaka, Y., Sano, T., Tamaoki, M., Nakajima, N., Kondo, N., and Hasezawa, S. (2005). Ethylene inhibits abscisic acid-induced stomatal closure in Arabidopsis. Plant Physiol. 138, 2337–2343. doi: 10.1104/pp.105.063503
Tian, T., Ali, B., Qin, Y. B., Malik, Z., Gill, R. A., and Ali, S. et al. (2014). Alleviation of lead toxicity by 5-aminolevulinic acid is related to elevated growth, photosynthesis, and suppressed ultrastructural damages in oilseed rape. Biomed. Res. Int. 2014, 1–11. doi: 10.1155/2014/530642
Tuteja, N., and Mahajan, S. (2007). Calcium signaling network in plants. Plant Signal. Behav. 2, 79–85. doi: 10.4161/psb.2.2.4176
Wang, L. J., Jiang, W. B., and Huang, B. J. (2004). Promotion of 5-aminolevulinic acid on photosynthesis of melon (Cucumis melo) seedlings under low light and chilling stress conditions. Physiol. Plant. 121, 258–264. doi: 10.1111/j.0031-9317.2004.00319.x
Wang, L. J., Jiang, W. B., Liu, H., Liu, W. Q., Kang, L., and Hou, X. L. (2005). Promotion by 5-aminolevulinic acid of germination of pakchoi (Brassica campestris ssp. chinensis var. communis Tsen et Lee) seeds under salt stress. J. Integr. Plant Biol. 47, 1084–1091. doi: 10.1111/j.1744-7909.2005.00150.x
Wang, L. J., Sun, Y. P., Zhang, Z. P., and Kang, L. (2010). Effects of 5-aminolevulinic acid (ALA) on photosynthesis and chlorophyll fluorescence of watermelon seedlings grown under low light and low temperature conditions. Acta Hortic. 856, 159–166. doi: 10.17660/ActaHortic.2010.856.21
Wang, Y., Noguchi, K., Ono, N., Inoue, S., Terashima, I., and Kinoshita, T. (2014). Overexpression of plasma membrane H+-ATPase in guard cells promotes light-induced stomatal opening and enhances plant growth. Proc. Nat. Acad. Sci. U.S.A. 111, 533–538. doi: 10.1073/pnas.1305438111
Wei, L. L., Xin, X. J., Wang, Y. S., Zhang, C., and Cao, D. M. (2014). SO2-induced guard cells apoptosis and its signal regulation in Hemerocallis fulva. Acta Sci. Circumst. 34, 801–806. doi: 10.13671/j.hjkxxb.2014.0135
Xie, L., Cheng, X. H., Feng, X. X., Yang, T., Zhang, Z. P., and Wang, L. J. (2013a). Effects of an amino acid fertilizer on the leaf photosynthesis and fruit quality of ‘Summer Black’ grape. J. Nanjing Agric. Uni. 36, 31–37. doi: 10.7685/j.issn.1000-2030.2013.02.006
Xie, L., Wang, Z. H., Cheng, X. H., Gao, J. J., Zhang, Z. P., and Wang, L. J. (2013b). 5-Aminolevulinic acid promotes anthocyanin accumulation in Fuji apples. Plant Growth Regul. 69, 295–303. doi: 10.1007/s10725-012-9772-5
Yang, Y., Zheng, Q. S., Liu, M., Long, X. H., Liu, Z. P., Shen, Q. R., et al. (2012). Difference in sodium spatial distribution in the shoot of two canola cultivars under saline stress. Plant Cell Physiol. 53, 1083–1092. doi: 10.1093/pcp/pcs055
Youssef, T., and Awad, M. A. (2008). Mechanisms of enhancing photosynthetic gas exchange in date palm seedlings (Phoenix dactylifera L.) under salinity stress by a 5-aminolevulinic acid-based fertilizer. J. Plant Growth Regul. 27, 1–9. doi: 10.1007/s00344-007-9025-4
Zhang, J., Li, D. M., Gao, Y., Yu, B., Xia, C. X., and Bai, J. G. (2012). Pretreatment with 5-aminolevulinic acid mitigates heat stress of cucumber leaves. Biol. Plant. 56, 780–784. doi: 10.1007/s10535-012-0136-9
Zhang, X., Miao, Y. C., An, G. Y., Zhou, Y., Shangguan, Z. P., Gao, J. F., et al. (2001a). K+ channels inhibited by hydrogen peroxide mediate abscisic acid signaling in Vicia guard cells. Cell Res 11, 195–202. doi: 10.1038/sj.cr.7290086
Zhang, X., Zhang, L., Dong, F. C., Gao, J. F., Galbraith, D. W., and Song, C. P. (2001b). Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol. 126, 1438–1448. doi: 10.1104/pp.126.4.1438
Zhang, Z. P., Yao, Q. H., and Wang, L. J. (2010). Expression of yeast Hem1 gene controlled by Arabidopsis HemA1 promoter improves salt tolerance in Arabidopsis plants. BMB Rep. 43, 330–336. doi: 10.5483/BMBRep.2010.43.5.330
Zhao, M. G., Tian, Q. Y., and Zhang, W. H. (2007). Ethylene activates a plasma membrane Ca2+-permeable channel in tobacco suspension cells. New Phytol. 174, 507–515. doi: 10.1111/j.1469-8137.2007.02037.x
Zhao, Y. Y., Yan, F., Hu, L. P., Zhou, X. T., Zou, Z. R., and Cui, L. R. (2015). Effects of exogenous 5-aminolevulinic acid on photosynthesis, stomatal conductance, transpiration rate, and PIP gene expression of tomato seedlings subject to salinity stress. Genet. Mol. Res. 14, 6401–6412. doi: 10.4238/2015.June.11.16
Keywords: abscisic acid (ABA), 5-aminolevulinic acid (ALA), calcium, hydrogen peroxide, stomatal opening, drought tolerance
Citation: An Y, Liu L, Chen L and Wang L (2016) ALA Inhibits ABA-induced Stomatal Closure via Reducing H2O2 and Ca2+ Levels in Guard Cells. Front. Plant Sci. 7:482. doi: 10.3389/fpls.2016.00482
Received: 26 December 2015; Accepted: 24 March 2016;
Published: 11 April 2016.
Edited by:
Richard Sayre, New Mexico Consortium at Los Alamos National Labs, USAReviewed by:
Nabil I. Elsheery, Tanta University, EgyptMaria Concetta De Pinto, University of Bari, Italy
Copyright © 2016 An, Liu, Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liangju Wang, d2xqQG5qYXUuZWR1LmNu
†These authors have contributed equally to this work.
 Yuyan An
Yuyan An Longbo Liu†
Longbo Liu† Liangju Wang
Liangju Wang