- 1Department of Plant Sciences, School of Life Sciences, University of Hyderabad, Hyderabad, Telangana, India
- 2Department of Life Sciences and Biotechnology, Chhatrapati Shahu Ji Maharaj University, Kanpur, Uttar Pradesh, India
- 3Seri-Biotech Research Laboratory, Central Silk Board, Bangalore, India
Reduced crop productivity results from altered plant physiological processes caused by dysfunctional proteins due to environmental stressors. In this study, a novel DnaJ Type-I encoding gene, VaDJI having a zinc finger motif in its C-terminal domain was found to be induced early upon treatment with heat stress (within 5 min) in a heat tolerant genotype of Vigna aconitifolia RMO-40. VaDJI is induced by multiple stresses. In tobacco, ectopic expression of VaDJI reduced ABA sensitivity during seed germination and the early stages of seedling growth of transgenic tobacco plants. Concomitantly, it also improved the ability of transgenic tobacco plants to withstand drought stress by modulating the photosynthetic efficiency, with the transgenic plants having higher Fv/Fm ratios and reduced growth inhibition. Additionally, transgenic plants showed a reduced build-up of H2O2 and lower MDA levels and higher chlorophyll content during drought stress, which attenuated cell damage and reduced oxidative damage. An analysis using the qRT-PCR study demonstrated that VaDJI overexpression is associated with the expression of some ROS-detoxification-related genes and stress-marker genes that are often induced during drought stress responses. These findings suggest a hypothesis whereby VaDJI positively influences drought stress tolerance and ABA signalling in transgenic tobacco, and suggests that it is a potential gene for genetic improvement of drought and heat stress tolerance in crop plants.
1 Introduction
The detrimental environmental factors coming under abiotic stresses include temperature extremes, salinity, drought and nutrient deficiencies, which limit normal growth and development in plants thereby reducing their productivity (He et al., 2018; Ma et al., 2020). Among them, drought and heat stresses are considered the two most important abiotic stresses limiting productivity due to their complex nature (Fahad et al., 2017; Dos Santos et al., 2022). Therefore, understanding the plant response mechanism associated with these stresses is fundamentally needed to protect the plants from climate vulnerability and sustain their productivity. Given their vulnerability to toxicity produced by abiotic stresses in nature, plants heavily rely on the intrinsic signalling network of adaptive mechanisms that operate at molecular and physiological levels, which vary from species to species (Bohnert et al., 1995; Zhu, 2016). Some signalling mechanisms regulate the folding, degradation and trafficking of proteins to ensure that protein homeostasis is maintained in plants under stressful conditions.
Generally, environmental adversity in plants causes denaturation of proteins; thus, the maintenance of proteins in their respective conformation and the prevention of protein aggregation are imperative for plant cell survivability under stress conditions (Nakajima and Suzuki, 2013). Molecular chaperones are proteins that are ubiquitous in nature and are shown to be involved in various stress-related functions and different cell-related processes including cytoprotection and modulate stress-related regulatory networks both at transcriptional and post-transcriptional levels (Al-Whaibi, 2011; Jacob et al., 2017; Zhang et al., 2020; Ling et al., 2021). They are involved in the protein folding and unfolding as well as repair of damaged proteins and their reactivation which constitute the mainstay of stress-related metabolism in plants (Parsell and Lindquist, 1993; Sun et al., 2021). Heat shock proteins (Hsps) or molecular chaperones have been classified into different families according to their sequence homology and molecular weight, including small Hsp, Hsp40, Hsp60, Hsp70, Hsp90 and Hsp100 (Gupta et al., 2010; Fan et al., 2017; Zarouchlioti et al., 2018; Wang et al., 2019a; Wang et al., 2020; Li et al., 2021).
Hsp70 proteins are ubiquitous and versatile proteins that are associated with various activities related to protein function including their folding and refolding, degradation and several other related activities. The J-domain-containing Hsp40 proteins are the main co-chaperones of the Hsp70 proteins and are involved in modulating their functions (Walsh et al., 2004; Kampinga and Craig, 2010). The J-domain of the proteins is involved in the interaction with the Hsp70 proteins and hence, the J-domain containing proteins exhibit different expression patterns and, protein structure and sequence, which control the stress ameliorating activity of the Hsp complexes (Pulido and Leister, 2018). They are generally expressed as families of proteins and the domains other than the J-domain in them drive the “specificity of the system by delivering specific substrate polypeptides or by attracting Hsp70 partners to their site of actions” (Kampinga et al., 2019). These Hsp40 proteins are also expressed in mammalian and avian cell lines under heat-stress conditions (Ohtsuka et al., 1990). The downregulation of a DnaJA1 protein has been linked to pancreatic cancer in humans and its overexpression reduced the detrimental effects of the c-Jun protein and consequently enhanced cell survival (Stark et al., 2014).
Hsp40s, also termed as J-domain proteins or DnaJ proteins constitute one of the important plants Hsps. The J proteins are categorized into Type-I, Type-II, and Type-III according to the presence or absence of the domains as depicted in Supplementry Figure 1 (Liu and Whitham, 2013). Type-I DnaJ proteins usually contain J-domain (approximately 70 amino acid sequences), which interacts with Hsp70/DnaK (its bacterial counterpart), a proximal Gly/Phe-rich region (G/F), a zinc finger domain (CxxCxGxG) and a C terminal domain (less conserved) (Silver and Way, 1993; Craig et al., 2006). The occurrence of the HPD motif (histidine, proline and aspartic acid) is the hallmark of the J-domain and is crucial for the ATPase activity of Hsp70 and protein folding (Kampinga and Craig, 2010). Type-II proteins lack the zinc finger domain and the Type-III proteins exhibit only the J-domain whereas Type-IV (J like proteins) possess significantly similar sequences and structures like the J-domain, which lacks the HPD motif (Liu and Whitham, 2013). Pulido and Leister used the classification of DnaJA, DnaJB, DnaJC and DnaJD representing the four classes listed earlier (Pulido and Leister, 2018). Further, they have identified some novel DnaJ-like proteins classifying them as DnaJE and DnaJF proteins.
Molecular chaperones help avoid inappropriate protein association or aggregation of naked hydrophobic regions of unfolded or partly folded proteins and guide them for productive folding, transport and also promote non-productive interaction and aggregation with other proteins (Miernyk, 1999; Yang et al., 2010). Several studies on the role of Hsps report that some of the members are also induced by soil salinity, water, cold, high temperature and osmotic stresses in plants (Waters et al., 1996; Wang et al., 2004; Yang et al., 2010). Interestingly, plants DnaJ proteins have been reported to play diversified roles in physiological processes such as chloroplast movement (Suetsugu et al., 2005), flowering time (Park et al., 2011; Shen et al., 2011), and male sterility (Tamura et al., 2007; Liu and Whitham, 2013). In biotic stress responses, J proteins have been shown to interact with viral coat and movement proteins, thus facilitating viral assembly and the movement of viral particles during host-pathogen interactions (Shimizu et al., 2009; Liu and Whitham, 2013). A recent study showed that virulence effector HopI (class III J protein) from Pseudomonas syringae suppresses the SA (salicylic acid) accumulation and host defense responses in Arabidopsis plants (Jelenska et al., 2007). Several studies on the role of Hsps reported that some of its members are also induced by soil salinity, water, cold, high temperature and osmotic stresses in plants (Waters et al., 1996; Wang et al., 2004; Yang et al., 2010). In addition, overexpression of DnaJ (Type-III) from Arachis diogoi potentiated tolerance to multiple stresses in tobacco (Rampuria et al., 2018).
Vigna aconitifolia (moth bean) is a heat- and drought-tolerant legume crop grown in tropical, sub-tropical, and warm-temperate regions of various countries (Tiwari et al., 2018). This crop is an important biological resource to study the tolerance mechanisms due to its evolved morpho-physiological and gene-reservoir features for drought amelioration (Iseki et al., 2018). A previous study using the construction of suppression subtractive hybridization (SSH) cDNA library in the heat-tolerant genotype of Vigna aconitifolia RMO-40, (moth bean) identified the DnaJ-like protein to be induced upon treatment at an early stage of heat stress (within 5 min) (Rampuria et al., 2012). In line with this observation, the DnaJ family members appeared to be induced in pepper under heat stress conditions (Fan et al., 2017). A DnaJ protein is also reported to be induced in the thermotolerance of Lentinula edodes in combination with IAA metabolism (Wang et al., 2018b).
In the present study, we cloned this candidate gene from Vigna and analysis of its nucleotide and amino-acid sequences depicted that it is a DnaJ Type-I encoding gene, VaDJI exhibiting the zinc finger motif in its C-terminal domain. Overexpression of this gene in Nicotiana benthamiana suggested a pivotal role of VaDJI in ABA insensitivity and improved drought tolerance by modulating photosystem II efficiency and photosynthesis. The mechanism of drought tolerance due to VaDJI overexpression was outlined.
2 Materials and methods
2.1 Plant material
For cloning VaDJI gene, Vigna aconitifolia RMO-40 was used. The transformation was carried out in the tobacco Nicotiana tabacum (cv. Samsun) and the transgenic tobacco lines were used to perform molecular experiments in this study.
2.2 Hormonal and stress treatments of Vigna aconitifolia
Seeds of Vigna aconitifolia planted in pots filled with sterilized vermiculite were watered with 1x Hoagland’s solution. The seedlings were raised normally for seven days before subjecting to hormonal, salt and heat stress treatments (Harsh et al., 2016). The seedlings were kept in the relevant solutions for various chemical treatments. Treatments included NaCl (100 µM), methyl jasmonate (100 µM), abscisic acid (100 µM), ethephon (250 µM), heat stress at 42°C and polyethylene glycol (PEG-6000). Water was used as the mock for control.
2.3 Construct preparation and sequence analysis of VaDJI from Vigna aconitifolia
The full-length VaDJI CDS sequence was amplified from Vigna aconitifolia (RMO-40) using gene-specific primers listed in the earlier cDNA-AFLP study (Rampuria et al., 2012). The PCR product of VaDJI was cloned into a pRT100 vector expression cassette using ApaI and KpnI restriction sites at the 5′/3′ end of the sequence (Gautam et al., 2019). The VaDJI cassette was excised from pRT100 using PstI restriction digestion and it was then cloned into the appropriate site of the binary vector pCAMBIA2300. The recombinant pCAMBIA2300-VaDJI/35S-nptII construct contains CaMV35S (cauliflower mosaic virus) promoter and nptII as plant selectable marker. The recombinant construct was further mobilized into the Agrobacterium strain, LBA4404 and transformed into tobacco plants using the standard leaf disc method (Gallois and Marinho, 1995). Sequence analysis was done using SMART (http://smart.embl-heidelberg.de/), NCBI-CDD (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) and the Phylogenetic trees were constructed using MEGA 7.0 software and ClustalW (https://www.genome.jp/tools-bin/clustalw), respectively to conduct multiple sequence alignment (Thompson et al., 1994; Kumar et al., 2016).
2.4 Plant transformation
Tobacco leaves from a one-month-old plant were surface sterilized in aqueous 0.1% mercuric chloride for 3 min. After rinsing five times in sterile distilled water, the leaf sections of 1.0 cm length were cut and incubated with Agrobacterium tumefaciens strain LBA4404 harboring the binary vector with the VaDJI cassette for 30 min in LB medium with the combination of antibiotics (25 mg/l each of Rifampicin and Kanamycin) at 28°C. The infected explants were further co-cultivated for 48 h on full-strength Murashige and Skoog (MS) culture medium having 2.0 mg/l 6-benzylaminopurine (BAP), 0.01 mg/l naphthalene acetic acid (NAA). After cocultivation, the explants were washed thrice for 5 min each in sterile water containing 250 mg/l Cefotaxime and then blotted dry on sterile filter paper. The Agrobacterium-treated leaf explants were kept on MS shoot regeneration medium (2 mg/l BAP + 0.1 mg/l NAA + 125 mg/l Kanamycin and 250 mg/l Cefotaxime) for two weeks. The induced shoots were transferred to shoot elongation medium (MS + 1.0 mg/l, BAP + 125 mg/l Kanamycin + 250 mg/l Cefotaxime). The elongated green shoots were excised and placed on rooting media (MS + 0.5 mg/l, NAA + 125 mg/l Kanamycin + 250 mg/l Cefotaxime) for rooting. The rooted plantlets were then transferred to the greenhouse for acclimatization in pots filled with soil and vermiculite mixed in a ratio of 4:1.
2.5 Selection of positive VaDJI tobacco transgenic plants
The three-week-old T2 transgenic tobacco plants were screened by PCR for the presence of various elements in the T-DNA region using nptII and VaDJI gene-specific forward and reverse primers (Supplementary Table 1). Semi-Q PCR was done using tobacco transgenic plants that were one week old (T2 generation) to determine the high and low expression VaDJI transgenic lines. To identify susceptible and resistant seedlings, the seeds from the confirmed T1 plants were germinated on MS medium supplemented with the selective drug Kanamycin (150 mg/l). Transgenic lines that remained green after Kanamycin selection and displayed a 3:1 segregation ratio (as determined by the χ2 test) were continued to T2 generation. Each resistant transgenic plant’s 60–110 seeds were plated on germination media (MS + Kanamycin), and 100% seed germination was utilized to determine homozygosity (Gautam et al., 2020). The seedlings that revealed Kanamycin sensitivity and lacked trans-gene amplification were classified as null segregants (NS) in the first generation. These sensitive segregants segregated from the primary transgenic plants served as negative controls in all experiments. The list of primers used in the VaDJI functional characterization is listed in Supplementary Table 1.
2.6 Seed germination assay
The seed germination analysis of NS and transgenic lines seeds of tobacco was performed according to the protocol as described in earlier reports of Ahmed et al. (2018). The seed germination assay was performed with approximately 100 seeds and the germination percentage was calculated on daily basis while taking the seedlings with green cotyledons into account. The seed germination percentage of VaDJI tobacco seeds was calculated using medium supplemented with half strength MS medium containing ABA (0, 7 µM and 10 µM) and PEG-6000 (0, 5% and 7%) using NS as control. To ensure data repeatability, the experiment was performed with three biological replicates
2.7 Seedling assay
The seedling assay of VaDJI transgenics plants was carried out with 10-days-old T2 seedlings on media containing various concentration of ABA (0, 7 µM and 10 µM) and PEG-6000 (0, 5% and 7%). To ensure data reproducibility, the experiment was performed with three biological replicates. Images of seedling morphology and measurements of root length were taken twelve-days after treatment.
2.8 Relative water content
Three-week-old seedlings grown on half strength MS medium were transferred to liquid half strength MS media enriched with ABA (0, 7 µM and 10 µM) and PEG-6000 (0, 5% and 7%) for 72 hours in order to determine the relative water content. The seedling fresh weight (W) was measured, and the samples were fully hydrated for 12 hours to yield a turgid weight (TW). To calculate the dry weight, samples were oven-dried for 48 hours at 60°C (DW). The following equation was used to calculate the relative water content.
2.9 Thermotolerance assay
The 15-day-old seedlings of NS and transgenic lines #4, #14, and #20 were transplanted and cultivated for three weeks in a growth chamber (Orbitek, Scigenics, Tamil Nadu, India) at 26°C, 70% humidity in plastic cups holding an identical weight of soil mixture (soilrite: soil = 1:4). The temperature of the growth chamber was gradually raised to 45°C for three hours and withering of the plantlets were recorded to evaluate thermotolerance (Dai et al., 2020).
2.10 Biochemical parameters
2.10.1 Total chlorophyll and carotenoid estimation in transgenic plants
Ten seedlings from the control and treated samples in each trial were utilized for the biochemical estimation. The Hiscox and Israelstam technique was used to estimate the total chlorophyll and carotenoid levels using dimethyl sulfoxide (DMSO) (Hiscox and Israelstam, 1979).
2.10.2 Lipid peroxidation and proline estimation in transgenic plants
Levels of lipid peroxidation were measured by determining the malondialdehyde (MDA) concentrations in control and stress-treated tissue samples using the TBARS (thiobarbituric acid reactive substances) method (Heath and Packer, 1968). The absorbance of the various samples was measured at 532 nm using a spectrophotometer (Shimazu spectrophotometer UV-1800, U.S.A.). Proline was extracted using the procedure outlined by Bates et al. (1973), and spectrophotometric measurements were made at 520 nm.
2.11 Tobacco abiotic stress survival assay
Control NS and tobacco plants overexpressing VaDJI were grown under an adequate watering regime for eight weeks in a greenhouse in pots with soil prepared using a mixture of soil: soil rite in a 4:1 ratio before being exposed to drought stress for fourteen days. During the stress treatment period, water to the plants was stopped to put them under drought stress. When dehydration in tobacco plants became fatal, watering in pots was resumed, and the plants were left to recover for three days. After drought treatment and rewatering, the pictures were used for analysis.
2.12 Staining with DAB (3, 3′-diaminobenzidine)
The in-vivo generation of H2O2 in the leaf tissue samples under stress treatments was determined by histochemical staining with diaminobenzidine (DAB) using leaf discs with a diameter of 0.5 cm that was collected from the stressed and control samples grown in pots. DAB was quantified by the procedure described by Wei et al. (2017).
2.13 Photosynthetic efficiency and chlorophyll a fluorescence indices
To monitor the leaf gas exchange in the leaves of control and drought stress-treated plants, a portable LI-6400XT infrared gas exchange equipment was used (Li-COR Inc, Lincoln, NE, USA). Using a broad leaf chamber equipped with PAR (photosynthetically active radiation, LCpro-32070) and a leaf probe, the gas exchange parameters Pn (net photosynthetic rate; mol m-2 s-1), gs (stomatal conductance m-2 s-1), and E (transpiration rate; mmol m-2 s-1) of NS and transgenic plants were measured (ADC, M PLC-011). WUE (water usage efficiency, mol CO2 mol-1 H2O) was computed using the aforementioned information as Pn/E.
A portable plant efficiency analyzer (Handy–PEA; Hansatech Instruments Ltd, Norfolk, UK) was used to record chlorophyll a fluorescence metrics such as PIabs (performance index) and Fv/Fm (maximum quantum yield). The measurements were taken after 30 min of the dark-adapted fully expanded third leaf of control and stressed plants that had been treated with drought stress. The data in the graph are the mean of three separate replications.
2.14 Quantitative RT-PCR of stress marker genes
Following the manufacturer’s instructions, total RNA was extracted from control and treatment samples using Tri-reagent (Takara, Biotech, UK) and further treated with RNase-free DNase1. First-strand cDNA was created using Prime-script First Strand cDNA Synthesis Kit (Takara, Biotech, UK) and 2 µg of total RNA. The cDNA was diluted 2.5 times (1:2.5) and 1 µl of diluted cDNA was used in a total reaction volume of 10 µl containing 10 µM of each primer, 2× Fast start SYBR green, milli-Q water for the analysis using quantitative reverse transcription PCR (Gautam et al., 2019).
The expression patterns of genes like SOS1, SAMDC, ERF5, ERD10, APX, MnSOD, DREB3, ERF5, ERD10, and P5CS1 that have been shown in previous studies to be involved in the instantaneous response to abiotic stress in plants were analyzed to determine whether the ectopic expression of VaDJI is linked to the enhanced expression pattern of various above abiotic stress-specific genes. For the expression analysis by qRT-PCR, samples were collected as three biological replicates, and the Ubiquitin and ADH3 genes were employed as internal controls. Relative expression patterns were determined using the 2-ΔΔCT method (Livak and Schmittgen, 2001).
2.15 Statistical significance
The graphs for each experiment were created using the Graphpad Prism program, and one-way ANOVA with DMRT (Duncan’s multiple range test) was used to statistically analyze each data set. A single asterisk (*) indicates that the statistical difference was significant at the 0.05 level (p ≤ 0.05) and a double asterisk (**) is significant at the 0.01 level (p ≤ 0.01).
3 Results
3.1 Cloning and isolation of cDNA sequence of VaDJI from Vigna aconitifolia
The ORF of VaDJI protein encodes a 418 amino acid polypeptide along with a predicted molecular weight of 46.47 kDa and pI (isoelectric point) of 5.80. Sequence analysis using SMART, NCBI-CDD and phylogenetic study has led to its identification as Type-I DnaJ protein having the N-terminal J-domain consisting of HPD tripeptide, which is highly conserved, followed by G/F- rich region (Gly/Phe domain) and the DIF motif (Supplementary Figure 2). The ZBD (zinc-binding domain) displayed three quintessential CXXCXGXG motifs and a fourth motif ending in N instead of G (Supplementary Figure 2).
The amino acid sequences of DnaJ proteins from various species were used to align the deduced amino acids of VaDJI. VaDJI protein exhibits a maximum similarity of 98.80% to DJI protein from Vigna radiata. It exhibited similarities to the DJI proteins from Glycine max and Phaseolus vulgaris, (97.61 and 96.41% respectively) (Supplementary Figure 3). Interestingly, Nicotiana tabacum genome was found to carry three different isoforms of DnaJI i.e., NtDJI-1, NtDJI-2 and NtDJI-3. These proteins had a sequence similarity of 28%, 29% and 25% with VaDJI. VaDJI phylogenetic relationship revealed that it is closely linked to the Vigna radiata and Phaseolus vulgaris, both having 418-amino acid as opposed to Glycine max DnaJ proteins that carried a 417 amino-acids sequence (Supplementary Figure 4). The fact that all three plants are members of the Fabaceae family may account for their striking similarity in the amino acid sequences.
3.2 VaDJI expression analysis in response to various hormonal and environmental stress treatments
To examine the expression patterns of VaDJI in the Vigna aconitifolia RMO-40 variety in response to abiotic stress (heat stress and PEG) and phytohormonal treatments (ABA, MeJa and ethephon), we carried out qRT-PCR analysis using RNA samples collected at different time intervals (Figure 1). As displayed in Figure 1, VaDJI transcripts rapidly increased by the various treatments. The transcript abundance was strongly elevated with 6 h of ABA and PEG treatment followed by ethephon at 12 h of treatment. VaDJI transcripts recorded significant early upregulation up to 11 and 10 fold for PEG and heat treatment at 3 h and 5 min, which increased at 6 h and 15 min respectively. Interestingly, the observed expression was 4 fold and 5 fold higher than the basal expression level with PEG stress at 12 and 24 h of treatment. A similar pattern of transcript accumulation was evidenced during heat stress where the increment of expression was approximately 5 to 7 fold higher than that of the basal level at 1 and 2 h of treatment.
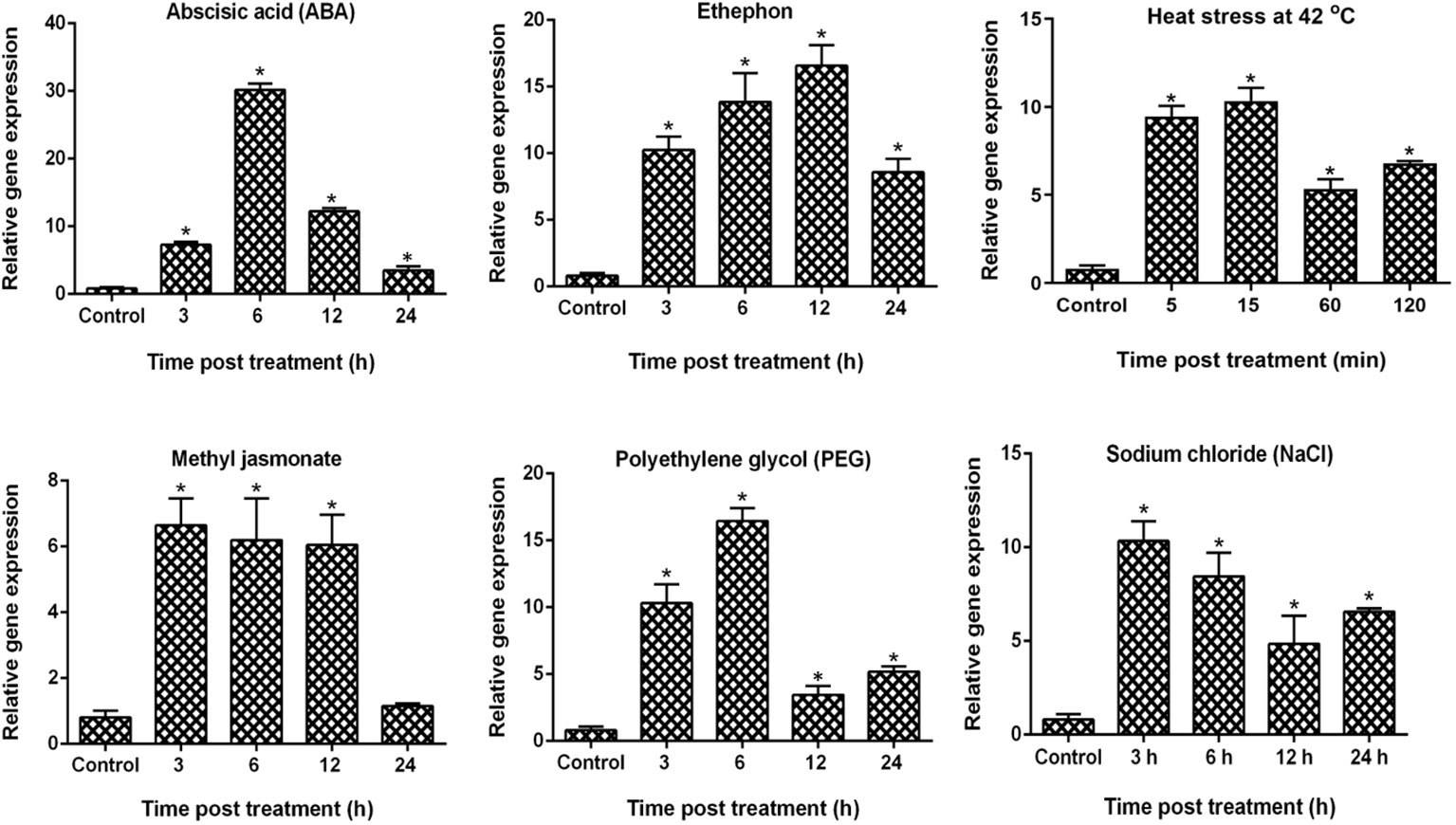
Figure 1 Relative expression analysis of VaDJI gene in V. aconitifolia by using qRT-PCR in response to various hormonal and abiotic stresses. Seedling samples collected at various time points were treated with PEG, heat stress, ABA, NaCl, ethephon and methyl jasmonate. Mean values of the fold change values of three biological and technical replicates for the expression study were used in the qRT-PCR analysis. Livak’s ΔΔCT method was used to calculate the expression level. The experiment here included three biological and three technical repeats. Values are mean ± SE (* at p ≤ 0.05 value).
We observed that MeJa treatment also caused a significant increase in transcript level initially during 3 h, and then further decrease at 6 and 12 h followed by 5 fold decrease at 24 h of treatment. In response to ethephon treatment, VaDJI expression was relatively elevated from 3 to 12 h (10 to 17 fold), with a decline at 24 h up to 8 fold (Figure 1).
ABA is a key signalling molecule in plants in regulating abiotic stress tolerance. Under the ABA treatment, we found that there was an early induction of VaDJI transcripts to nearly 8 fold at 3 h, followed by a 31 fold increment at 6 h, which declined gradually (Figure 1). Strong upregulation was noticed under ABA treatment as compared with the other phytohormones suggests a possibility that ABA serves as an important regulator of VaDJI expression. Overall, upregulation of the VaDJI during the above treatments, indicated it to be involved in various interconnected signalling pathways controlled by these signalling molecules.
3.3 Screening and molecular characterization of VaDJI expressing tobacco transgenic plants
Several three-week-old T2 transgenic tobacco plants grown on Kanamycin selection overexpressing a cDNA cassette of VaDJI (pCAMBIA2300-VaDJI/35S-nptII) were screened by PCR using nptII and VaDJI gene specific primers. The transgenic plants displayed the presence of 564 bp and 1.27 kb corresponding to nptII and VaDJI respectively, whereas the negative control NS plants did not exhibit the presence of any amplification (Supplementary Figures 5A, B). The transgenic plantlets were screened by semi-quantitative RT-PCR to identify the high and low expression lines among the transgenic plants (Supplementary Figures 6A, B and Figures 2A, B). Based on the band intensity of semi-quantitative RT-PCR analysis, lines L-20, L-25 and L-17 were considered low expression lines whereas Line L-1, L-4, L-14, L-18 and L-22 were considered as high expression lines (Figures 2A, B).
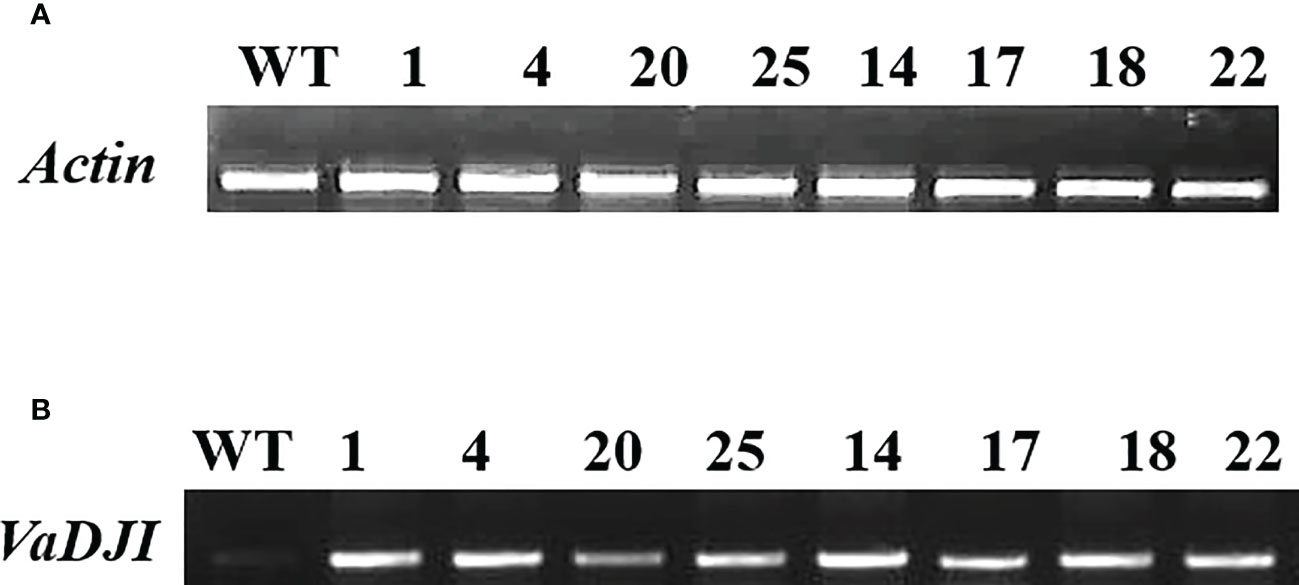
Figure 2 Semi-Quantitative real-time analysis of VaDJI T2 transgenic lines using specific primers for VaDJI gene and actin (as an internal reference). (A, B)- Lane1: represents cDNA from wild type (WT). Lane 2-9: represents cDNA from transformants. Depending on the level of expression, lines L-20, L-25 and L-17 were considered low expression lines. Lines L-1, L-4, L-14 and L-18 were considered high expression lines.
3.4 Overexpression of VaDJI led to insensitivity to exogenously applied ABA during germination and seedling growth stage
The phytohormone ABA is considered to be a major mediator of abiotic stresses and plant development (Vishwakarma et al., 2017; Miao et al., 2018; Chen et al., 2020). Since VaDJI expression was strongly upregulated during ABA treatment, we examined whether VaDJI participates in the ABA-mediated reduction of seed germination and/or seedling growth stage by observing the seed germination percentage in NS and transgenic lines in presence of exogenous ABA at the different concentrations on daily basis (0, 7 µM and 10 µM) (Figures 3A–F). In presence of 7 µM and 10 µM ABA, we observed severe inhibition in the pattern of seed germination of NS lines compared (Figures 3C–F) with the ABA-free medium where a similar pattern of seed germination was clearly visible in all the lines (Figures 3A, B). At the end of the 6th day of 10 µM ABA treatment, only 33.3 ± 6.0 of NS seeds showed germination as compared to 60- 98.6% of seed germination rate in transgenic lines (Figure 3F).
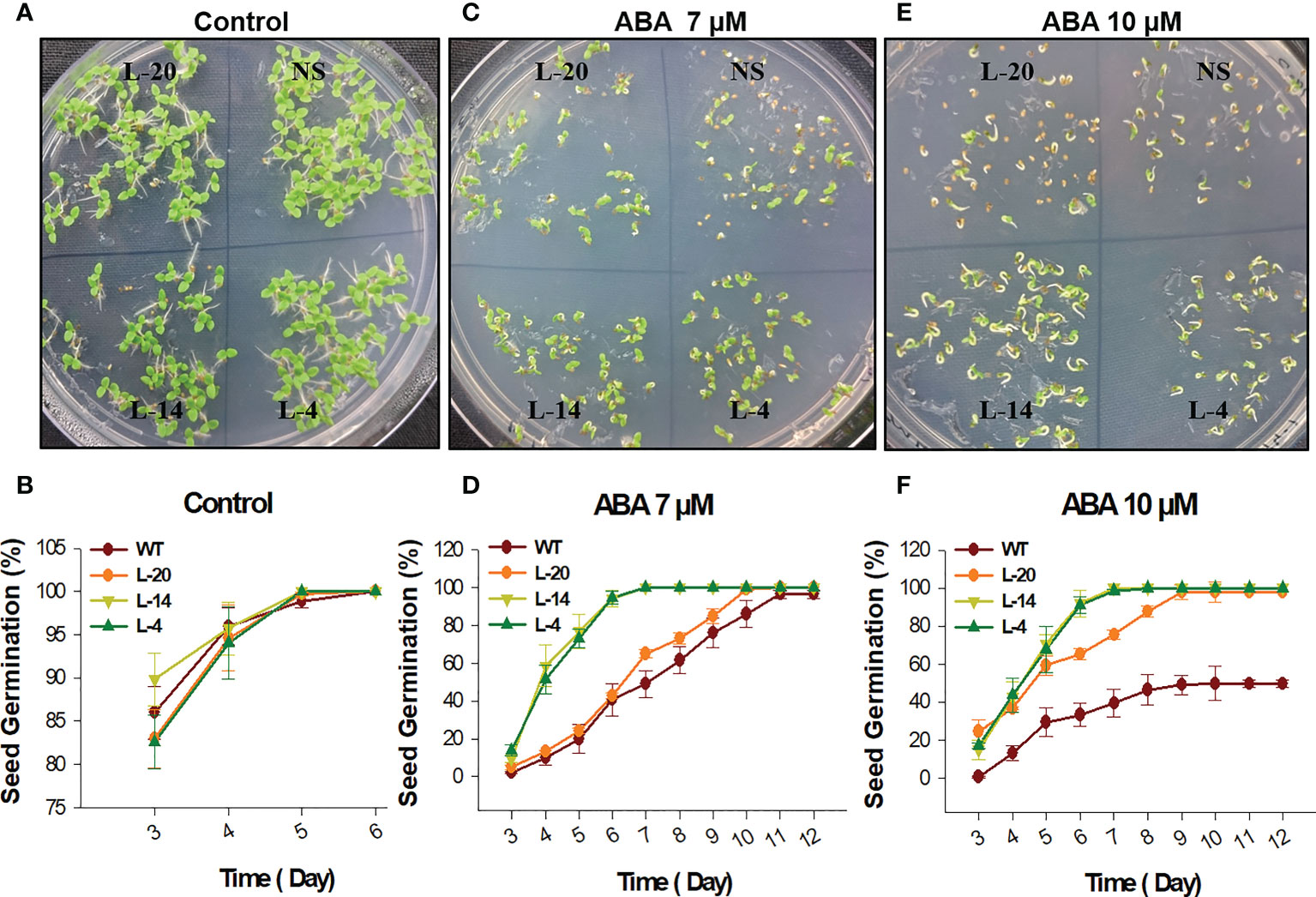
Figure 3 Seed germination analysis of VaDJI transgenic lines for ABA insensitivity; (A, C, E) Represents phenotypic difference during control, 7 µM ABA and 10 µM ABA treatments, (B, D, F) germination percentage of NS and VaDJI transgenic lines under control, 7 µM ABA and 10 µM ABA stresses. Experiments were repeated with three technical and three biological repeats. Error bar depicts mean ± S.E.
We then checked the ABA insensitivity at the seedling stage of these lines. We found that seven-day-old VaDJI expressing T2 tobacco lines L-4, L-14 and L-20 showed an increase in root length when moved to media containing 7 µM ABA whereas the NS lines continued to maintain the length of roots under ABA free medium (control condition) (Figures 4A–D). As the concentration of ABA increased from 7 µM to 10 µM, root length and fresh weight drastically reduced in NS against the transgenic lines which exhibited longer roots, depicting that the seedlings are ABA insensitive (Figures 4C–E).
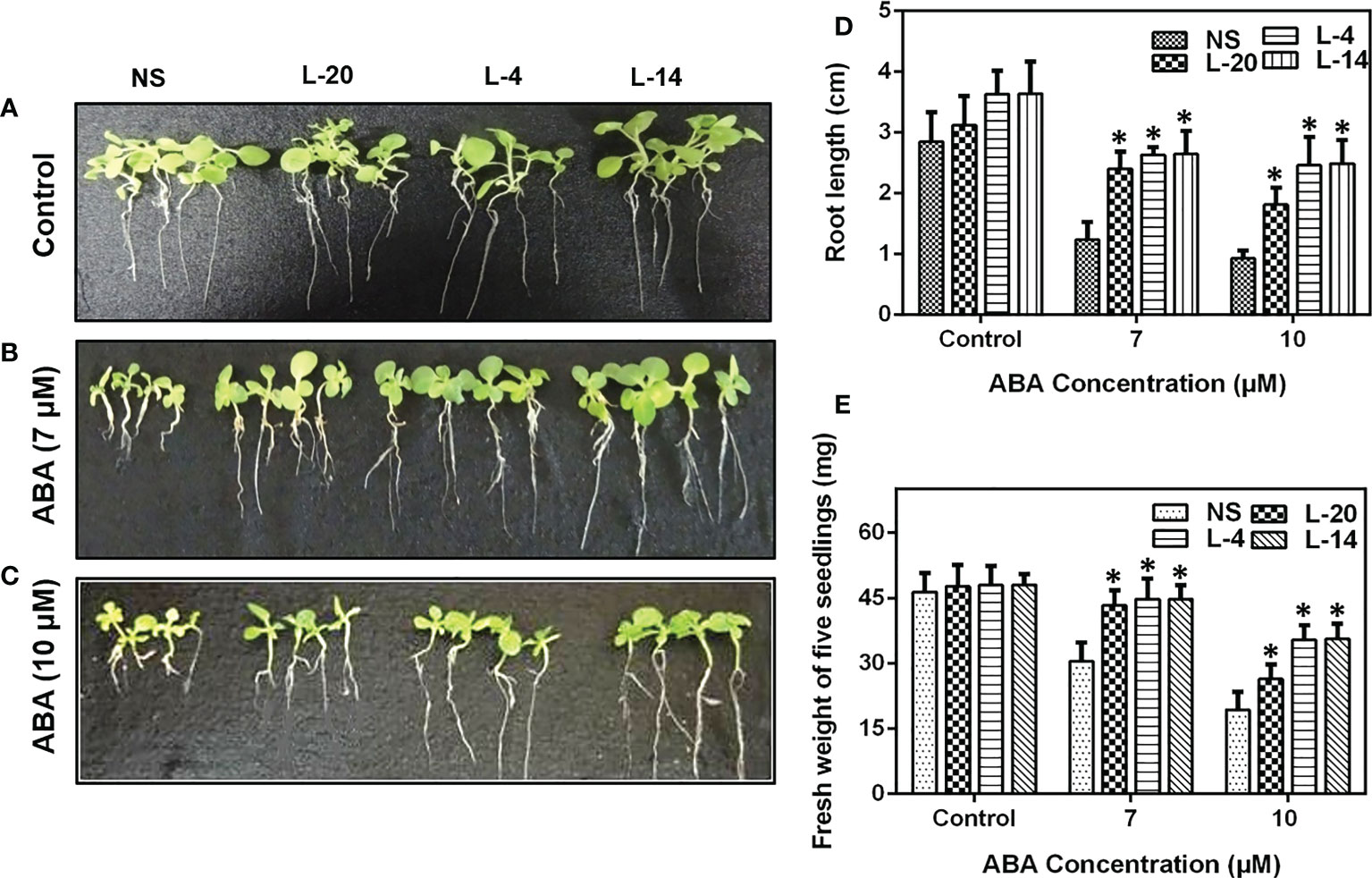
Figure 4 Analysis of enhanced ABA insensitivity in transgenic lines of VaDJI; (A) Under control condition, (B) 7 µM ABA, (C) 10 µM ABA. Photographs of NS and transgenic lines were taken after giving exogenous ABA stress for 12 days of growth. The difference observed in the seedlings (D) root length, (E) fresh weight in the presence of 7 µM ABA and 10 µM ABA. Data were represented as mean ± SE. Statistical significance was tested using one-way analysis of variance (ANOVA) and *(single asterisk) indicates p ≤ 0.05.
3.5 VaDJI tobacco lines showed enhanced tolerance under heat stress
To study the role of VaDJI under heat stress five-week-old plants of NS and transgenic lines L-4, L-14, and L-20 were exposed to 45°C in a growth chamber. The high expression lines L-4 and L-14 remained healthy while the NS plants displayed signs of severe withering and chlorosis, a sign of heat damage after 2 hours and were fully wilted after 3 hours of treatment (Supplementary Figures 7A, B). The top crown of leaves of low expression line L-20 also appeared robust though it showed some signs of wilting (Supplementary Figures 7A, B).
3.6 Drought stress tolerance of seedlings harboring VaDJI
The T2 generation transgenic lines of VaDJI were subjected to plate-based using PEG that simulates drought stress. The seeds used in the tests were consistently 100% germinated from transgenic lines on a seed germination medium containing Kanamycin. Seed germination rates for the NS and VaDJI lines were nearly identical in the absence of a stress media. We observed that a rise in the PEG concentration from 5% to 7% in the germination medium had a significantly negative impact on the germination percentage of NS seeds (Figures 5A–F). Also, compared to NS plants, transgenic seedlings grew more rapidly when exposed to abiotic stress. Significant variations in root length between VaDJI expressing and NS seedlings were seen as PEG concentration increased from 5% to 7% (Figures 6A, B). During PEG stress, the transgenic lines significantly increased the length of their roots compared to NS plants by 50-80%.
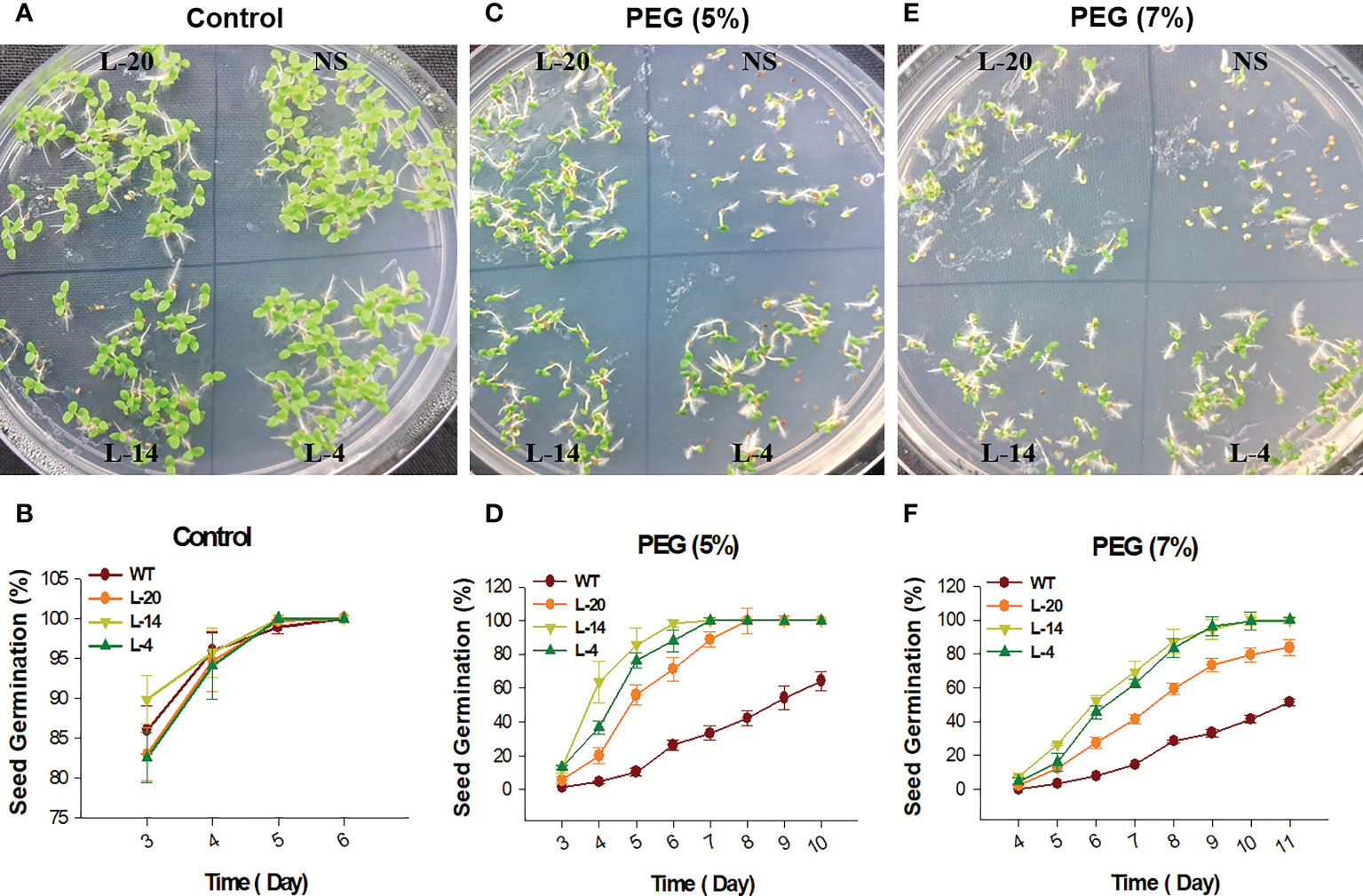
Figure 5 Seed germination analysis for PEG stresses tolerance; (A, C, E) Represents phenotypic difference during control, and PEG treatments, (B, D, F) germination percentage of NS and VaDJI transgenic lines under mock, 5% PEG and 7% PEG stress. Experiments were repeated with three technical and three biological repeats. Error bar depicts mean ± S.E. Statistical significance was tested using one-way analysis of variance (ANOVA) and *(single asterisk) indicates p ≤ 0.05.
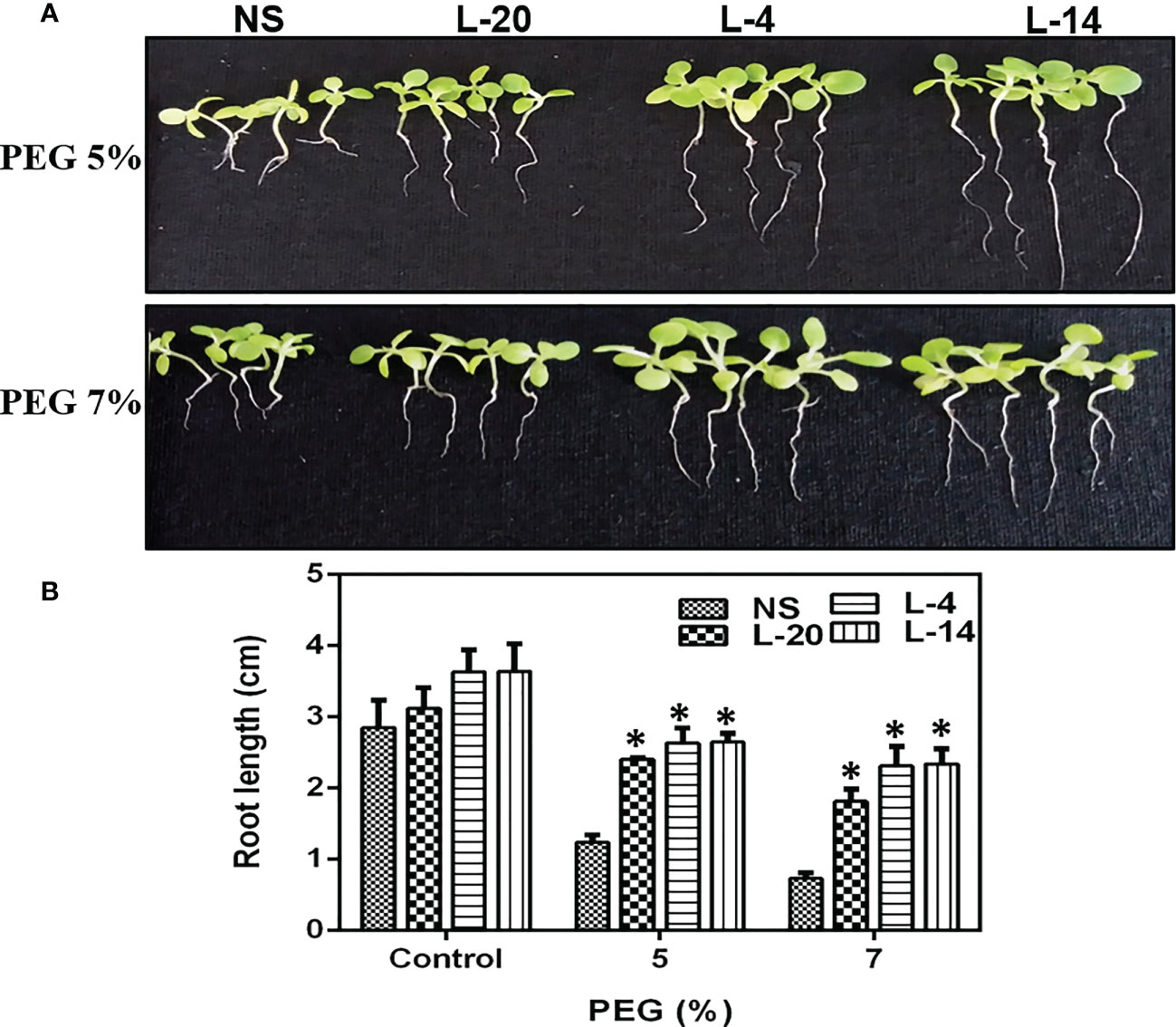
Figure 6 Analysis of enhanced PEG endurance in transgenic lines of VaDJI; (A) under 5% PEG, and 7% PEG. Photographs of NS and transgenic lines were taken after drought stress for 12 days of growth., (B) Difference observed in the seedlings root growth in the presence of 5% and 7% PEG 6000. Data were represented as mean ± SE. Statistical significance was tested using one-way analysis of variance (ANOVA) and *(single asterisk) indicates p ≤ 0.05.
3.7 Overexpression of VaDJI resulted in increased drought tolerance
To explore whether VaDJI overexpression could alter the drought responses in transgenic tobacco plants, we investigated the performance of VaDJI and NS plants under drought stress in pots. The VaDJI transgenic lines after drought stress exhibited reduced wilting, growth inhibition and curling of leaves compared with NS lines. Moreover, NS lines also displayed retarded growth followed by chlorosis and wilting (Figures 7A, B). Interestingly, the tobacco transgenic lines after resuming the supply of water demonstrated complete recovery of the normal phenotype with the presence of healthy leaves, while the NS plant showed wilting and bleached symptoms even after three days of rewatering conditions (Figure 7C).
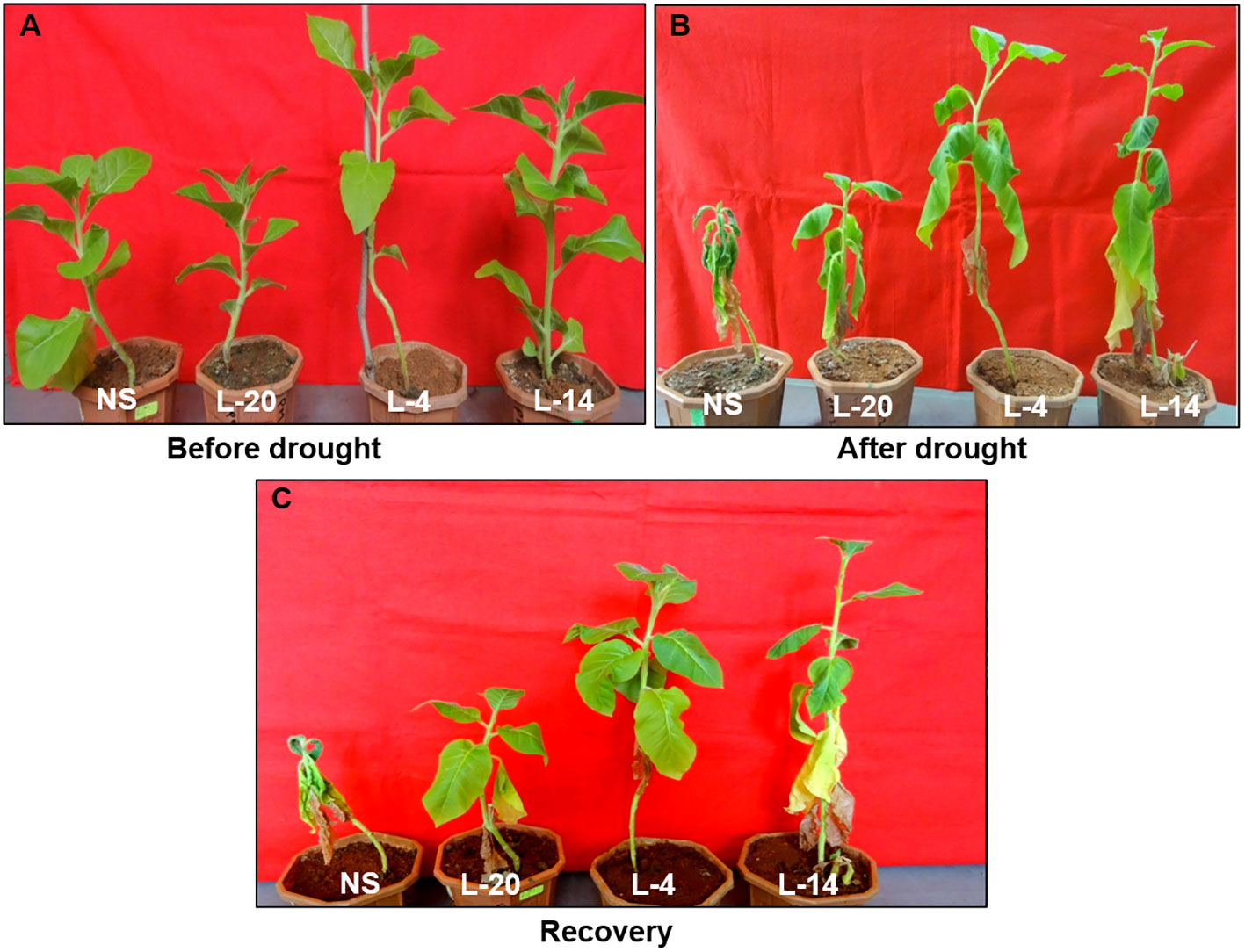
Figure 7 Assessment of drought tolerance observed in eight week NS and VaDJI transgenic tobacco plants subjected to drought stress at pot level; (A) Under control condition, (B) After 14 days of drought stress, (C) recovery phase after drought.
3.8 Drought tolerance of VaDJI overexpression lines was associated with increased water retention, higher proline and reduced MDA levels
Further examination of the relative water content (RWC), proline and MDA levels depicted that these parameters recorded significant differences between NS and high expression lines of VaDJI. RWC is an appropriate tool for measuring drought tolerance and also determining the plant water status whereas total chlorophyll represents the level of chlorosis. RWC, proline and total chlorophyll contents of the transgenic lines were significantly higher than compared to NS lines after they were exposed to fourteen days of drought stress (Figures 8A–C). Under control conditions, the transgenic lines and NS did not show any significant variation in MDA content. Whereas, MDA levels were significantly lower in the transgenic tobacco relative to NS lines under drought stress, suggesting reduced membrane damage (Figure 8D). The leaves of NS and transgenic lines stained by DAB under standard growing conditions displayed a similar pattern of DAB staining. But following drought stress, NS leaves displayed more vivid and profound brown patterns in bleached leaves in comparison to the VaDJI overexpressing lines, and H2O2 buildup was noticeably higher in the NS (Supplementary Figures 8A–C). These physiological parameters established that the VaDJI transgenic plants were more tolerant to drought stress.
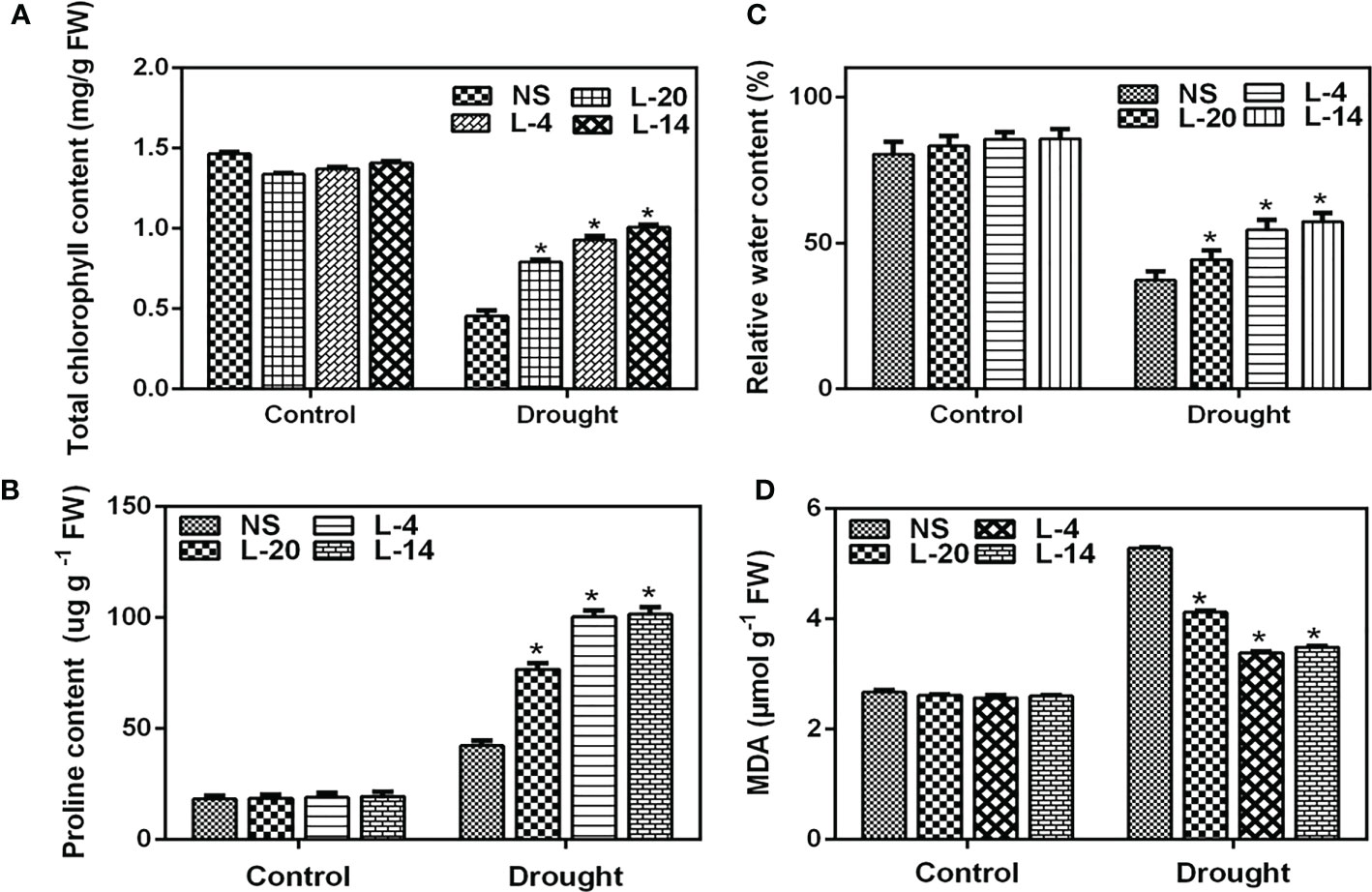
Figure 8 Effect of drought treatment on tobacco transgenics harboring VaDJI after 14 days of drought stress; (A) Estimation of total chlorophyll content, (B) Proline content, (C) RWC relative water content, (D) MDA levels in NS and transgenic tobacco lines. Data were represented as mean ± SE. Statistical significance was tested using one-way analysis of variance (ANOVA) and *(single asterisk) indicates p ≤ 0.05.
3.9 VaDJI alleviates photosynthetic performance and photoinhibition of photosystem II
The reduced photosynthetic efficiency and the production of ROS (reactive oxygen species) are believed to be significant variables that influence plant performance under environmental stress conditions (Huang et al., 2019; Dumanović et al., 2021). Therefore, photosynthetic traits and chlorophyll a fluorescence in null segregants and transgenic plants were evaluated in order to comprehend the effects of drought stress on photosynthesis. We have observed that when tobacco lines were exposed to drought stress, the three transgenic lines viz., L-20, L-4 and L-14 were found to have considerably higher Pn (net photosynthetic rate) and gs (transpiration rate) than those of the NS lines (Figures 9A, B). Similar findings were also made while measuring stomatal conductance E, where the drop in NS was more pronounced than in transgenic lines, especially lines L-4 and L-14 (Figure 9C). The Fv/Fm ratios, which elaborate PSII photochemistry efficiency in all three transgenic lines was similar in well-watered conditions (0.86 to 0.87) and NS (0.85), whereas the high expression lines L-4 and L-14 showed a quantum efficiency of 0.82 to 0.83 under drought stress, which was very similar to the control conditions. Compared to the low expression line L-20, the NS plants displayed a quantum efficiency value of 0.71 (Figure 9D). This demonstrates unequivocally that the photosynthetic efficiency decreased in the drought-stressed NS plants and was much lower than in the VaDJI overexpressing lines. Interestingly, the performance index (PIabs) in the NS lines dropped by 48% whereas the best VaDJI transgenic lines (L-4 and L-14) only showed a decline of 11–15% and the low expression line (L-20) only showed a decline of 34% (Figure 9E).
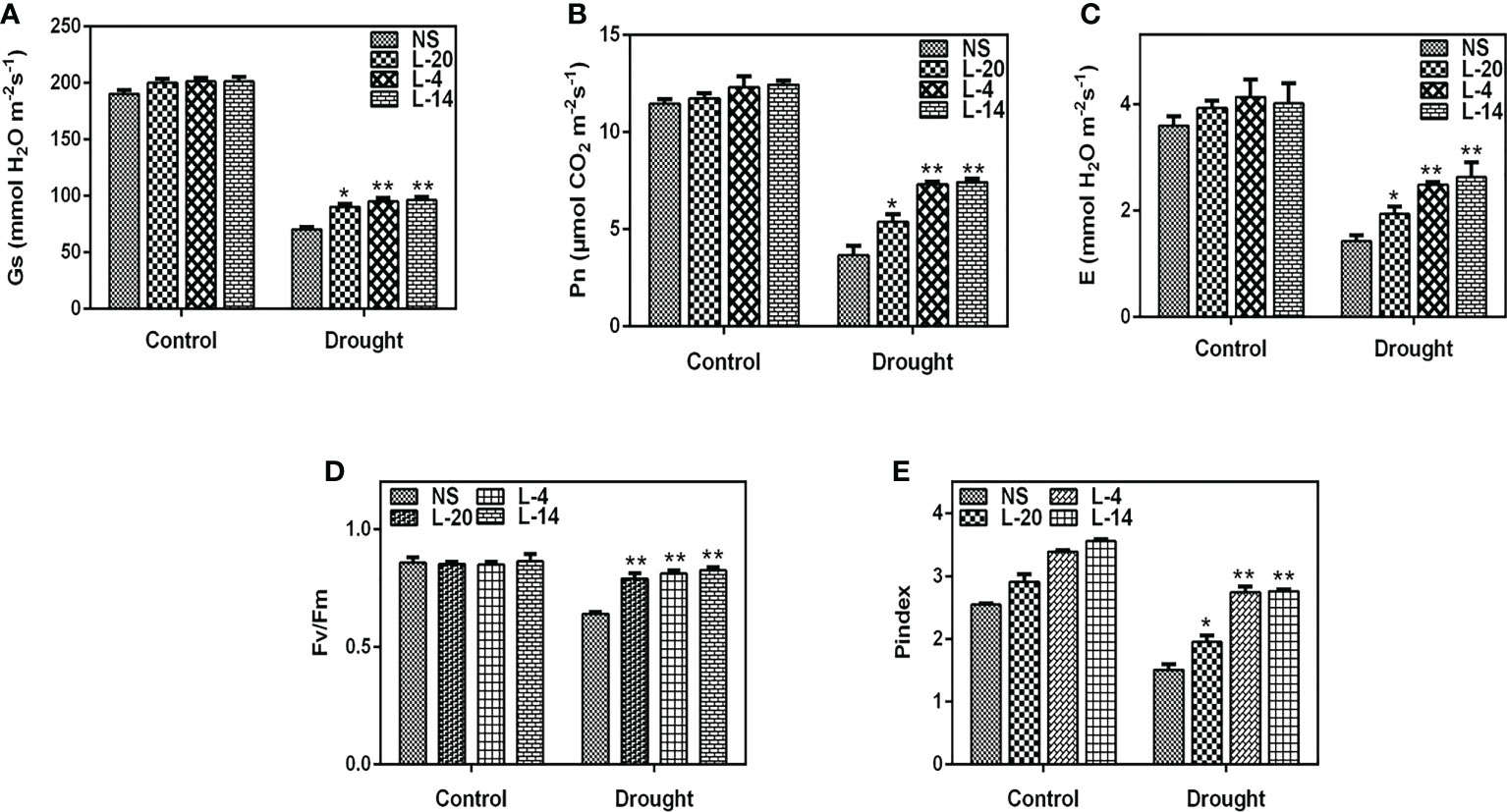
Figure 9 Effect of drought treatment on leaf gas exchange parameter and chlorophyll fluorescence of tobacco transgenics harboring VaDJI after 14 days of drought stress; (A) gs – measurement of stomatal conductance, (B) Pn - measurement of net photosynthetic rate), (C) E- measurement of transpiration rate, (D) Fv/Fm - measurement of PSII photochemistry efficiency, (E) PIabs- measurement of performance index. The values indicate mean ± SE. *statistically significantly different at p ≤.05 and **statistically significantly different at p ≤ 0.01 level as compared to NS.
3.10 VaDJI regulates stress markers and ROS-related genes under drought treatment
To achieve deeper insight into VaDJI role in conferring drought tolerance, expression analysis of stress markers and ROS-related genes under untreated and drought conditions in shoot and root tissues was studied. We found that the transcript levels of DREB3 (Dehydration-responsive element-binding protein), ERF5 (ethylene response factor), ERD10 (early response to dehydration), P5CS1 (Δ1-pyrroline-5-carboxylate synthetase), SOS1 (salt overly sensitive) genes were higher in VaDJI tobacco lines even in control conditions. The transcript levels of the ROS-detoxification-related genes such as APX, SOD and CAT also showed a significant increase in expression in transgenic lines compared with the NS plants under drought stress (Figures 10A, B). The relative expression analysis of stress-marker and ROS-related genes revealed that VaDJI overexpression enhanced drought tolerance in transgenics as compared to NS plants.
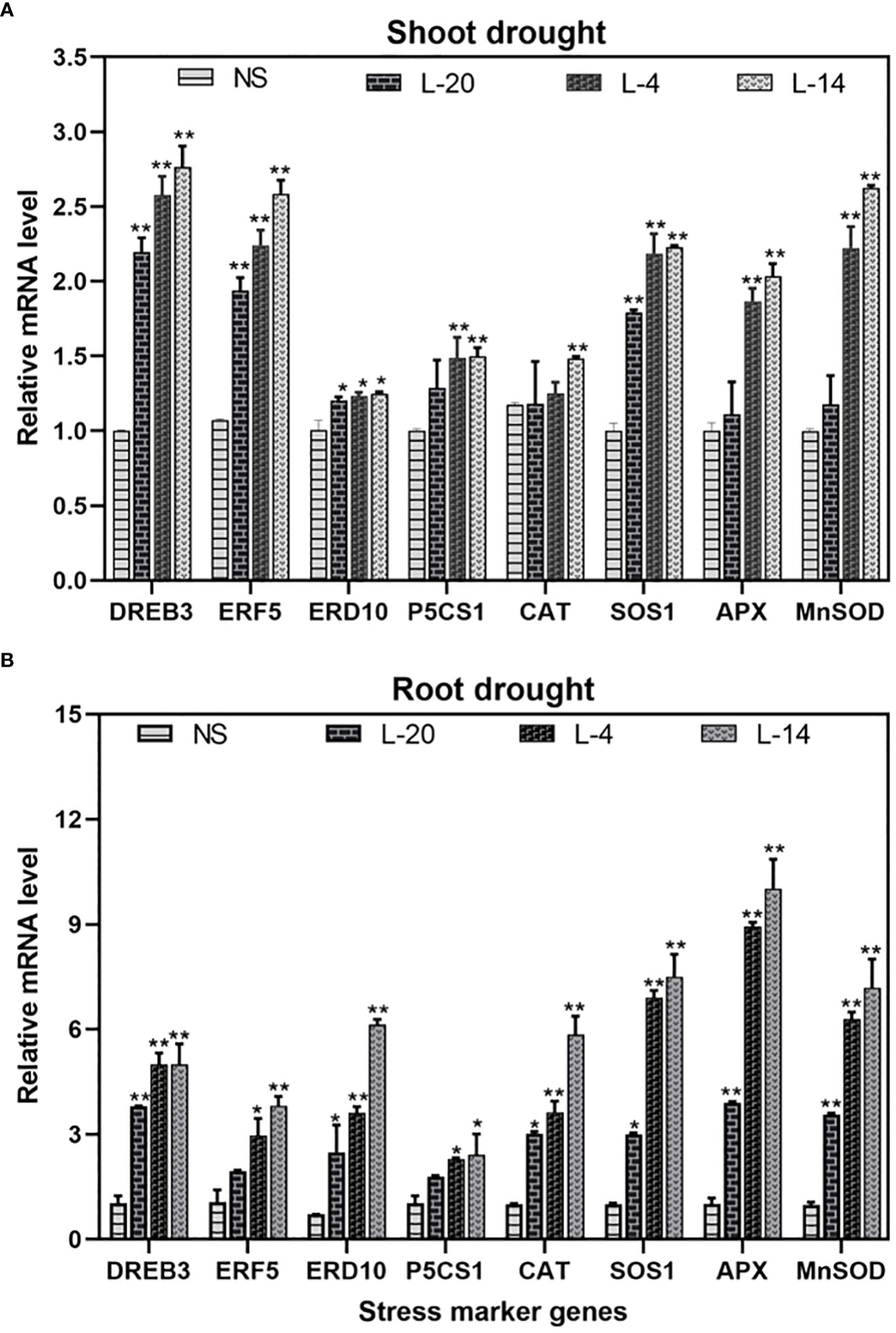
Figure 10 Relative expression analysis of stress- marker genes in (A) Shoot (B) Root of NS and VaDJI transgenic tobacco plants by using qRT-PCR. Plants of tobacco after 14 days of drought stress were used for the expression analysis. Relative expression was normalized with the mean of ADH3 and UBI genes as endogenous control. Livak and Schmittgen’s ΔΔCT method was used to calculate the expression level. The experiment here included three biological and three technical repeats. Values are mean ± SE (*at p ≤ 0.05 value and **at p ≤ 0.01).
4 Discussion
The studied gene VaDJI belongs to Hsp40, a member of type I J-proteins family and these proteins work in association with the Hsp70 proteins function inprotein folding, refolding and transport as well as removal of the damaged cellular proteins. Hsp40 type proteins interact with the Hsp70 proteins through the conserved J-domain present in them. They add specificity to the Hsp70 protein function through their variable domains, which respond to environmental cues. Unfavourable climatic conditions cause an increase in ROS levels above threshold values, which hampers plant physiology and resulting in an increase amount of non-functional proteins in cells compromising the protein cellular homeostasis. All these factors result in restricting plant growth and development. The cellular protein homeostasis is modulated and maintained by the cellular chaperone machinery to alleviate the damage caused to the cellular proteins by abiotic stresses. Therefore, the expression of stress-responsive genes that code for the molecular chaperones like heat shock proteins is one of the tolerance mechanisms to protect plant cellular components and restore cellular homeostasis (Salas-Muñoz et al., 2016).
In plants, limited published information is available on the characterization of the Dna J proteins belonging to Type I class in abiotic stress tolerance. Arabidopsis AtDjB1, a Dna J encoding gene facilitated thermotolerance by shielding the plants against oxidative damage induced by heat stress (Zhou et al., 2012). Similarly, AtDnaJ overexpression also conferred NaCl tolerance in transgenic Arabidopsis lines (Zhichang et al., 2010). However, there is no published evidence on the involvement of Type-I DnaJ proteins of the heat tolerant plant Vigna aconitifolia in response to drought stress so far. Here, we report that ectopic expression of VaDJI conferred ABA insensitivity at the seedling level and drought endurance at vegetative growth stages.
In the current study, VaDJI was isolated from Vigna using an SSR cDNA library and was found to be induced by signalling molecules and abiotic stress treatments (Rampuria et al., 2012). DnaJ proteins are known to be induced by heat, intense light, cold, methyl viologen (MV) and to play roles in signal transduction, development and stress tolerance (Xia et al., 2014; Lee et al., 2018; Rampuria et al., 2018; Wang et al., 2018a; Wang et al., 2019a; Wang et al., 2020; Li et al., 2021). In the present study, VaDJI is rapidly upregulated in response to all the stresses such as MV, ethephon, heat stress and PEG treatments. This early response to a wide range of stimuli suggests that VaDJI plays an important role in arbitrating various stress responses in plants. Being a co-chaperone that works in association with Hsp70, this DnaJ gene may be required for the proper folding of different nascent stress-responsive proteins when they encounter stressful situations.
Emerging evidence indicates that ABA is a crucial plant hormone involved in controlling stress responses and also minimizing water loss. During drought stress conditions, ABA modifies ion transport in guard cells by encouraging stomatal closure and blocking stomatal opening (Sah et al., 2016; Rehman et al., 2021). In our present study, various observations shed light on the function of VaDJI in plants under drought conditions. Our observations suggest that the ABA-driven thermotolerance response in tobacco may be mediated by VaDJI. This interpretation is influenced by following factors: First, qPCR results showed that heat and ABA treatment rapidly elevated the VaDJI transcript levels. Second, the overexpression of VaDJI resulted in drought tolerance and ABA insensitivity at the seed germination and seedling stage. Several studies have previously reported that the constitutive expression of specific genes viz., WRKY20, GmbZIP62 OsPP108, and ABI5 confers ABA insensitivity during the seed germination stage along with abiotic stress tolerance such as drought, salt, and alkalinity (Brocard et al., 2002; Liao et al., 2008; Luo et al., 2013; Singh et al., 2015; Ahmed et al., 2018; Collin et al., 2020). These findings are consistent with present study and suggested that ABA signalling controls seed germination as well as provided resistance to abiotic stimuli through a variety of pathways.
Drought tolerance in plants generally involves drought escape (by short life span or developmental plasticity of the plants), drought avoidance (by reduced water loss and enhanced water uptake in plants) and drought tolerance (by antioxidant capacity, osmotic adjustment and desiccation tolerance) (Yıldırım and Kaya, 2017; Polle et al., 2019). Interestingly, in this study, overexpression of the VaDJI gene in tobacco remarkably improved root growth, plant water status and better solute accumulation under drought stress. Most notably, the transgenic lines showed normal growth, despite the fact that plant height was increased compared to NS lines even under normal conditions and phenotypic differences between them further increased under drought stress. It is assumed that VaDJI gene alters the developmental processes by affecting the phytohormone status i.e., auxin and cytokinin levels in the plant. It has been reported that ARG1 (altered response to gravity) gene encoding a DnaJ like protein in Arabidopsis which has a role in signalling pathways related with gravity and also interact with the cytoskeleton (Sedbrook et al., 1999). It is possible that VaDJI may alter growth in tobacco transgenic plants in a way similar to ARG1 by affecting the cytoskeleton since mutations in the ARG1 locus affected not only root and hypocotyl growth responses to plant hormones but also auxin transport inhibitors and accumulation of starch. ANGULATA7 encodes a DnaJ like protein that is engaged in organization of thylakoidal membrane and leaf development (Muñoz-Nortes et al., 2017). In our study, VaDJI gene promoted the growth of transgenic root system with a in biomass of transgenic lines compared to NS lines. This suggests that tobacco plants can alter organ growth for water uptake to counter the limited water conditions resulting in higher plant water status. Moreover, the promoted vegetative growth is further supported by the comparable chlorophyll levels of the transgenic plants compared with the NS plants.
We have also shown that the better stress endurance of transgenic plants is related with lower MDA content and enhanced accumulation of solutes. MDA represents lipid peroxidation triggered by ROS and is generally used as an indicator of damages mediated by ROS in plants (Moore and Roberts, 1998). Hence, this study signifies that lipid peroxidation mediated by ROS injuries is alleviated in the VaDJI transgenics under drought stress. Proline acts as an osmolyte that assists in protein stability and scavenging of free radicals (Szabados and Savouré, 2010). In comparison with the NS plants, transgenic lines displayed almost similar proline content but the difference in proline accumulation under drought stress increased significantly. Also, the overexpression of P5CS, one of the key enzymes catalyzing the proline biosynthesis pathway has been reported to enhance stress tolerance in plants (Lohani et al., 2022; Ma et al., 2022). For instance, overexpression of P5CS genes in Cajanus cajan, Oryzae sativa and Solanum tuberosum enhanced salt tolerance in the corresponding transgenic plants (Hmida-Sayari et al., 2005; Kumar et al., 2010; Surekha et al., 2014).
The primary component determining the composition of crop productivity is photosynthesis, which serves as the foundation for crop growth and development (Richards, 2000; Fang et al., 2018). Drought stress can negatively affect photosynthetic capacity, denature proteins, increase ROS production, and create a metabolic imbalance (Zhang et al., 2022b). Interestingly, the VaDJI transgenics outperform NS lines in showing strong chlorophyll fluorescence and improved Pn, gs, E, and WUE values under drought stress. The relationship between improved photosynthetic performance and the expression of VaDJI is consistent with earlier reports in which the expression of LeCDJ2 improved the efficiency of photosystem II and net photosynthetic rate (Wang et al., 2014). Similarly, Arabidopsis triple knock-down lines of M-sHSPs (mitochondrial small heat shock proteins) had altered levels of proteins that were mostly involved in photosynthesis and antioxidant defense. Also, in the knock-down plants, heat stress resulted in an unusual pattern of cytosolic response as well as the overexpression of other sHSP members. Overall, the loss of all three M-sHSPs in Arabidopsis had a significant negative impact on core metabolic activities, altering how the plant should grow and develop (Escobar et al., 2021). A pathogen-induced AdDjSKI (DnaJ protein) from a wild peanut Arachis diogoi enhanced the ability of tobacco and E. coli to withstand a variety of stresses (Rampuria et al., 2018). Ectopic expression of the Hsp40 gene (Zjdjb1) of Zostera japonica also enhanced thermotolerance in Arabidopsis (Chen and Qiu, 2022). These results are in line with the present study where transgenic lines exhibited better performance under multiple stress encounters in terms of growth and development as compared to NS.
To further gain an insight into the molecular mechanism of VaDJI in drought stress, the expression of ROS-detoxifying and stress marker genes was explored. The antioxidant system involving SOD provides the first lines of defense in countering the toxic ROS levels by catalyzing the conversion of O2- to oxygen and hydrogen peroxide, of which the latter is further scavenged by combined actions of CAT and POD (Blokhina et al., 2003; Huang et al., 2010; Hu et al., 2013). Present observations demonstrated that the VaDJI transgenic lines recorded significantly higher expression of CAT and SOD genes compared to the NS lines. In the VaDJI transgenic plants, we noticed increased transcript levels of MnSOD and APX which are involved in ROS detoxification. In addition, we observed increased expression of stress marker genes such as ERD10, DREB3, P5CS1, and SOS1 in the transgenic lines. Existing literature suggests that these genes are involved in mitigating plant stress tolerance in plants. ERD10, a member of the dehydrin family acts as a chaperone to shield plants from external stressors and promotes seed development (Kim and Nam, 2010; Gautam et al., 2020). SOS1 encodes a Na+/H+ antiporter that regulates ion transport across the membranes and contributes to salt tolerance (Xie et al., 2022; Zhang et al., 2022a). DREB3, a transcription factor controls the stress responses in plants by interacting with the DRE/CRT cis-elements found in the promoter regions of stress-responsive genes (Shavrukov et al., 2016; Wang et al., 2019b). Based on these results, it can be postulated that the VaDJI overexpression augmented the activation of defense system related with antioxidants and abiotic stress, which further guarded the transgenic lines against injuries caused by ROS in drought stress in transgenic plants.
5 Conclusion
In the current investigation, we identified, cloned, and characterized a heat-induced VaDJI, which has a zinc finger motif in C-terminal domain. Its expression profile reveals that regardless of the type of stress signal, it is universally expressed during various stress treatments. Additionally, the fact that it was upregulated in response to ABA, MeJA, NaCl, PEG and ethephon treatment implies that it is a part of a network related to multiple signalling pathways. In tobacco, ectopic expression of VaDJI conferred ABA insensitivity. Transgenic plants demonstrated improved photosynthetic performance as well as better growth and tolerance to heat and drought stress. We suggest that VaDJI safeguards the photosynthetic apparatus under stress by lowering otherwise excessive ROS levels and ensures retrograde signalling from organelles like chloroplasts, as shown by the transcriptional upregulation of stress-related genes. Further research is needed to determine the upstream and downstream targets of VaDJI to fully clarify its biological function in ABA signalling and drought stress. Also, the results presented in the current investigation provide evidence only for a role of VaDJI in heat shock responses in tobacco, and that more studies will be needed to determine the similar role of the protein in Vigna aconitifolia.This study offers a useful resource for the possible genetic enhancement of drought and heat stress tolerance in agriculturally significant crops to withstand stress.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
PK, RG and RM designed the study. RG, RM and SR performed the experiments. The manuscript was written by PK, RG, RM and PS. All authors contributed to the article and approved the submitted version.
Funding
This work was conducted without any grant. The work was carried out by the candidates UGC-RGNF fellowship contingency recieved for doing Ph.D. work which was around  12000/- per annum for initial two years and
12000/- per annum for initial two years and  25000/- per annum for next three years.
25000/- per annum for next three years.
Acknowledgments
The authors are thankful to Head, Department of Plant Sciences, University of Hyderabad, Hyderabad, Telangana for providing the facilities available under UGC-SAP-DRS and DST-FIST to carry out the experiments. RG gratefully acknowledges the University Grants Commission (UGC), New Delhi for providing RGNF-JRF fellowship to carry out this work. PK is supported by the NASI-Platinum Jubilee Senior Scientist award of the National Academy of Sciences, India.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2023.1135552/full#supplementary-material
References
Ahmed, I., Yadav, D., Shukla, P., Kirti, P. B. (2018). Heterologous expression of Brassica juncea annexin, AnnBj2 confers salt tolerance and ABA insensitivity in transgenic tobacco seedlings. Funct. Integr. Genomic. 18, 569–579. doi: 10.1007/s10142-018-0614-z
Al-Whaibi, M. H. (2011). Plant heat-shock proteins: a mini review. J. King Saud Univ. – Sci. 23, 139–150. doi: 10.1016/j.jksus.2010.06.022
Bates, L. S., Waldren, R. P., Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant Soil 39, 205–207. doi: 10.1007/BF00018060
Blokhina, O., Virolainen, E., Fagerstedt, K. V. (2003). Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann. Bot. 91, 179–194. doi: 10.1093/aob/mcf118
Bohnert, H. J., Nelson, D. E., Jensen, R. G. (1995). Adaptations to environmental stresses. Plant Cell 7, 1099–1111. doi: 10.2307/3870060
Brocard, I. M., Lynch, T. J., Finkelstein, R. R. (2002). Regulation and role of the Arabidopsis abscisic acid-insensitive 5 gene in abscisic acid, sugar, and stress response. Plant Physiol. 129, 1533–1543. doi: 10.1104/pp.005793
Chen, K., Li, G.-J., Bressan, R. A., Song, C.-P., Zhu, J.-K., Zhao, Y. (2020). Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 62, 25–54. doi: 10.1111/jipb.12899
Chen, S., Qiu, G. (2022). Overexpression of Zostera japonica heat shock protein gene ZjHsp70 enhances the thermotolerance of transgenic Arabidopsis. Mol. Biol. Rep. 49, 6189–6197. doi: 10.1007/s11033-022-07411-3
Collin, A., Daszkowska-Golec, A., Kurowska, M., Szarejko, I. (2020). Barley ABI5 (Abscisic acid INSENSITIVE 5) is involved in abscisic acid-dependent drought response. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.01138
Craig, E. A., Huang, P., Aron, R., Andrew, A. (2006). The diverse roles of J-proteins, the obligate Hsp70 co-chaperone. Rev. Physiol. Biochem. Pharmacol. 156, 1–21. doi: 10.1007/s10254-005-0001-0
Dai, X., Wang, Y., Chen, Y., Li, H., Xu, S., Yang, T., et al. (2020). Overexpression of NtDOG1L-t improves heat stress tolerance by modulation of antioxidant capability and defense-, heat-, and ABA-related gene expression in tobacco. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.568489
Dos Santos, T. B., Ribas, A. F., De Souza, S. G. H., Budzinski, I. G. F., Domingues, D. S. (2022). Physiological responses to drought, salinity, and heat stress in plants: a review. Stresses 2, 113–135. doi: 10.3390/stresses2010009
Dumanović, J., Nepovimova, E., Natić, M., Kuča, K., Jaćević, V. (2021). The significance of reactive oxygen species and antioxidant defense system in plants: a concise overview. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.552969
Escobar, M. R., Feussner, I., Valle, E. M. (2021). Mitochondrial small heat shock proteins are essential for normal growth of Arabidopsis thaliana. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.600426
Fahad, S., Bajwa, A. A., Nazir, U., Anjum, S. A., Farooq, A., Zohaib, A., et al. (2017). Crop production under drought and heat stress: plant responses and management options. Front. Plant Sci. 8, 1147. doi: 10.3389/fpls.2017.01147
Fan, F., Yang, X., Cheng, Y., Kang, Y., Chai, X. (2017). The DnaJ gene family in pepper (Capsicum annuum l.): comprehensive identification, characterization and expression profiles. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.00689
Fang, X., Li, Y., Nie, J., Wang, C., Huang, K., Zhang, Y., et al. (2018). Effects of nitrogen fertilizer and planting density on the leaf photosynthetic characteristics, agronomic traits and grain yield in common buckwheat (Fagopyrum esculentum m.). Field Crops Res. 219, 160–168. doi: 10.1016/j.fcr.2018.02.001
Gallois, P., Marinho, P. (1995). Leaf disk transformation using Agrobacterium tumefaciens expression of heterologous genes in tobacco. Method Mol. Biol. (Clifton N.J.) 49, 39–48. doi: 10.1385/0-89603-321-X:39
Gautam, R., Ahmed, I., Shukla, P., Meena, R. K., Kirti, P. B. (2019). Genome-wide characterization of ALDH superfamily in Brassica rapa and enhancement of stress tolerance in heterologous hosts by BrALDH7B2 expression. Sci. Rep. 9, 7012. doi: 10.1038/s41598-019-43332-1
Gautam, R., Meena, R. K., Woch, N., Kirti, P. B. (2020). Ectopic expression of BrALDH7B2 gene encoding an antiquitin from Brassica rapa confers tolerance to abiotic stresses and improves photosynthetic performance under salt stress in tobacco. Environ. Exp. Bot. 180, 104223. doi: 10.1016/j.envexpbot.2020.104223
Gupta, S. C., Sharma, A., Mishra, M., Mishra, R. K., Chowdhuri, D. K. (2010). Heat shock proteins in toxicology: how close and how far? Life Sci. 86, 377–384. doi: 10.1016/j.lfs.2009.12.015
Harsh, A., Sharma, Y. K., Joshi, U., Rampuria, S., Singh, G., Kumar, S., et al. (2016). Effect of short-term heat stress on total sugars, proline and some antioxidant enzymes in moth bean (Vigna aconitifolia). Ann. Agric. Sci. 61, 57–64. doi: 10.1016/j.aoas.2016.02.001
He, M., He, C.-Q., Ding, N.-Z. (2018). Abiotic stresses: general defenses of land plants and chances for engineering multistress tolerance. Front. Plant Sci. 9, 1771. doi: 10.3389/fpls.2018.01771
Heath, R. L., Packer, L. (1968). Photoperoxidation in isolated chloroplasts. i. kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 125, 189–198. doi: 10.1016/0003-9861(68)90654-1
Hiscox, J., Israelstam, G. F. (1979). A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 57, 1332–1334. doi: 10.1139/b79-163
Hmida-Sayari, A., Gargouri-Bouzid, R., Bidani, A., Jaoua, L., Savouré, A., Jaoua, S. (2005). Overexpression of Δ1-pyrroline-5-carboxylate synthetase increases proline production and confers salt tolerance in transgenic potato plants. Plant Sci. 169, 746–752. doi: 10.1016/j.plantsci.2005.05.025
Hu, W., Huang, C., Deng, X., Zhou, S., Chen, L., Li, Y., et al. (2013). TaASR1, a transcription factor gene in wheat, confers drought stress tolerance in transgenic tobacco. Plant Cell Environ. 36, 1449–1464. doi: 10.1111/pce.12074
Huang, X.-S., Liu, J.-H., Chen, X.-J. (2010). Overexpression of PtrABF gene, a bZIP transcription factor isolated from Poncirus trifoliata, enhances dehydration and drought tolerance in tobacco via scavenging ROS and modulating expression of stress-responsive genes. BMC Plant Biol. 10, 230. doi: 10.1186/1471-2229-10-230
Huang, H., Ullah, F., Zhou, D.-X., Yi, M., Zhao, Y. (2019). Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.00800
Iseki, K., Takahashi, Y., Muto, C., Naito, K., Tomooka, N. (2018). Diversity of drought tolerance in the genus Vigna. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.00729
Jacob, P., Hirt, H., Bendahmane, A. (2017). The heat-shock protein/chaperone network and multiple stress resistance. Plant Biotechnol. J. 15, 405–414. doi: 10.1111/pbi.12659
Jelenska, J., Yao, N., Vinatzer, B. A., Wright, C. M., Brodsky, J. L., Greenberg, J. T. (2007). A J domain virulence effector of Pseudomonas syringae remodels host chloroplasts and suppresses defenses. Curr. Biol. 17, 499–508. doi: 10.1016/j.cub.2007.02.028
Kampinga, H. H., Andreasson, C., Barducci, A., Cheetham, M. E., Cyr, D., Emanuelsson, C., et al. (2019). Function, evolution, and structure of J-domain proteins. Cell Stress Chaperones. 24, 7–15. doi: 10.1007/s12192-018-0948-4
Kampinga, H. H., Craig, E. A. (2010). The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat. Rev. Mol. Cell Biol. 11, 579–592. doi: 10.1038/nrm2941
Kim, S. Y., Nam, K. H. (2010). Physiological roles of ERD10 in abiotic stresses and seed germination of Arabidopsis. Plant Cell Rep. 29, 203–209. doi: 10.1007/s00299-009-0813-0
Kumar, V., Shriram, V., Kavi Kishor, P. B., Jawali, N., Shitole, M. G. (2010). Enhanced proline accumulation and salt stress tolerance of transgenic indica rice by over-expressing P5CSF129A gene. Plant Biotechnol. Rep. 4, 37–48. doi: 10.1007/s11816-009-0118-3
Kumar, S., Stecher, G., Tamura, K. (2016). MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. doi: 10.1093/molbev/msw054
Lee, K.-W., Rahman, M. A., Kim, K.-Y., Choi, G. J., Cha, J.-Y., Cheong, M. S., et al. (2018). Overexpression of the alfalfa DnaJ-like protein (MsDJLP) gene enhances tolerance to chilling and heat stresses in transgenic tobacco plants. Turk. J. Biol. 42, 12–22. doi: 10.3906/biy-1705-30
Li, K. P., Wong, C. H., Cheng, C. C., Cheng, S. S., Li, M. W., Mansveld, S., et al. (2021). GmDNJ1, a type-I heat shock protein 40 (HSP40), is responsible for both growth and heat tolerance in soybean. Plant Direct. 5, e00298. doi: 10.1002/pld3.298
Liao, Y., Zou, H.-F., Wei, W., Hao, Y.-J., Tian, A.-G., Huang, J., et al. (2008). Soybean GmbZIP44, GmbZIP62 and GmbZIP78 genes function as negative regulator of ABA signaling and confer salt and freezing tolerance in transgenic Arabidopsis. Planta 228, 225–240. doi: 10.1007/s00425-008-0731-3
Ling, Y., Mahfouz, M. M., Zhou, S. (2021). Pre-mRNA alternative splicing as a modulator for heat stress response in plants. Trends Plant Sci. 26, 1153–1170. doi: 10.1016/j.tplants.2021.07.008
Liu, J. Z., Whitham, S. A. (2013). Overexpression of a soybean nuclear localized type-III DnaJ domain-containing HSP40 reveals its roles in cell death and disease resistance. Plant J. 74, 110–121. doi: 10.1111/tpj.12108
Livak, K. J., Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Lohani, N., Singh, M. B., Bhalla, P. L. (2022). Biological parts for engineering abiotic stress tolerance in plants. Biodes. Res. 2022, 9819314. doi: 10.34133/2022/9819314
Luo, X., Bai, X., Sun, X., Zhu, D., Liu, B., Ji, W., et al. (2013). Expression of wild soybean WRKY20 in Arabidopsis enhances drought tolerance and regulates ABA signalling. J. Exp. Bot. 64, 2155–2169. doi: 10.1093/jxb/ert073
Ma, Y., Dias, M. C., Freitas, H. (2020). Drought and salinity stress responses and microbe-induced tolerance in plants. Front. Plant Sci. 11, 591911. doi: 10.3389/fpls.2020.591911
Ma, C., Wang, M., Zhao, M., Yu, M., Zheng, X., Tian, Y., et al. (2022). The Δ1-pyrroline-5-carboxylate synthetase family performs diverse physiological functions in stress responses in pear (Pyrus betulifolia). Front. Plant Sci. 13. doi: 10.3389/fpls.2022.1066765
Miao, C., Xiao, L., Hua, K., Zou, C., Zhao, Y., Bressan, R. A., et al. (2018). Mutations in a subfamily of abscisic acid receptor genes promote rice growth and productivity. Proc. Natl. Acad. Sci. U. S. A. 115, 6058–6063. doi: 10.1073/pnas.1804774115
Miernyk, J. A. (1999). Protein folding in the plant cell. Plant Physiol. 121, 695–703. doi: 10.1104/pp.121.3.695
Moore, K., Roberts, L. J., 2nd (1998). Measurement of lipid peroxidation. Free Radic. Res. 28, 659–671. doi: 10.3109/10715769809065821
Muñoz-Nortes, T., Pérez-Pérez, J. M., Ponce, M. R., Candela, H., Micol, J. L. (2017). The ANGULATA7 gene encodes a DnaJ-like zinc finger-domain protein involved in chloroplast function and leaf development in Arabidopsis. Plant J. 89, 870–884. doi: 10.1111/tpj.13466
Nakajima, Y., Suzuki, S. (2013). Environmental stresses induce misfolded protein aggregation in plant cells in a microtubule-dependent manner. Int. J. Mol. Sci. 14, 7771–7783. doi: 10.3390/ijms14047771
Ohtsuka, K., Masuda, A., Nakai, A., Nagata, K. (1990). A novel 40-kDa protein induced by heat shock and other stresses in mammalian and avian cells. Biochem. Biophys. Res. Commun. 166, 642–647. doi: 10.1016/0006-291X(90)90857-J
Park, H.-Y., Lee, S.-Y., Seok, H.-Y., Kim, S.-H., Sung, Z. R., Moon, Y.-H. (2011). EMF1 interacts with EIP1, EIP6 or EIP9 involved in the regulation of flowering time in Arabidopsis. Plant Cell Physiol. 52, 1376–1388. doi: 10.1093/pcp/pcr084
Parsell, D. A., Lindquist, S. (1993). The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu. Rev. Genet. 27, 437–496. doi: 10.1146/annurev.ge.27.120193.002253
Polle, A., Chen, S. L., Eckert, C., Harfouche, A. (2019). Engineering drought resistance in forest trees. Front. Plant Sci. 9, 1875. doi: 10.3389/fpls.2018.01875
Pulido, P., Leister, D. (2018). Novel DNAJ-related proteins in Arabidopsis thaliana. New Phytol. 217, 480–490. doi: 10.1111/nph.14827
Rampuria, S., Bag, P., Rogan, C. J., Sharma, A., Gassmann, W., Kirti, P. B. (2018). Pathogen-induced AdDjSKI of the wild peanut, Arachis diogoi, potentiates tolerance of multiple stresses in E. coli and tobacco. Plant Sci. 272, 62–74. doi: 10.1016/j.plantsci.2018.03.033
Rampuria, S., Joshi, U., Palit, P., Deokar, A. A., Meghwal, R. R., Mohapatra, T., et al. (2012). Construction and analysis of an SSH cDNA library of early heat-induced genes of Vigna aconitifolia variety RMO-40. Genome 55, 783–796. doi: 10.1139/g2012-064
Rehman, A., Azhar, M. T., Hinze, L., Qayyum, A., Li, H., Peng, Z., et al. (2021). Insight into abscisic acid perception and signaling to increase plant tolerance to abiotic stress. J. Plant Interact. 16, 222–237. doi: 10.1080/17429145.2021.1925759
Richards, R. A. (2000). Selectable traits to increase crop photosynthesis and yield of grain crops. J. Exp. Bot. 51, 447–458. doi: 10.1093/jexbot/51.suppl_1.447
Sah, S. K., Reddy, K. R., Li, J. (2016). Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant Sci. 7, 571. doi: 10.3389/fpls.2016.00571
Salas-Muñoz, S., Rodríguez-Hernández, A. A., Ortega-Amaro, M. A., Salazar-Badillo, F. B., Jiménez-Bremont, J. F. (2016). Arabidopsis AtDjA3 null mutant shows increased sensitivity to abscisic acid, salt, and osmotic stress in germination and post-germination stages. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.00220
Sedbrook, J. C., Chen, R., Masson, P. H. (1999). ARG1 (Altered response to gravity) encodes a DnaJ-like protein that potentially interacts with the cytoskeleton. Proc. Natl. Acad. Sci. U. S. A. 96, 1140–1145. doi: 10.1073/pnas.96.3.1140
Shavrukov, Y., Baho, M., Lopato, S., Langridge, P. (2016). The TaDREB3 transgene transferred by conventional crossings to different genetic backgrounds of bread wheat improves drought tolerance. Plant Biotechnol. J. 14, 313–322. doi: 10.1111/pbi.12385
Shen, L., Kang, Y. G. G., Liu, L., Yu, H. (2011). The J-domain protein J3 mediates the integration of flowering signals in Arabidopsis. Plant Cell 23, 499–514. doi: 10.1105/tpc.111.083048
Shimizu, T., Yoshii, A., Sakurai, K., Hamada, K., Yamaji, Y., Suzuki, M., et al. (2009). Identification of a novel tobacco DnaJ-like protein that interacts with the movement protein of tobacco mosaic virus. Arch. Virol. 154, 959–967. doi: 10.1007/s00705-009-0397-6
Silver, P. A., Way, J. C. (1993). Eukaryotic DnaJ homologs and the specificity of Hsp70 activity. Cell 74, 5–6. doi: 10.1016/0092-8674(93)90287-Z
Singh, A., Jha, S. K., Bagri, J., Pandey, G. K. (2015). ABA inducible rice protein phosphatase 2c confers aba insensitivity and abiotic stress tolerance in Arabidopsis. PloS One 10, e0125168. doi: 10.1371/journal.pone.0125168
Stark, J. L., Mehla, K., Chaika, N., Acton, T. B., Xiao, R., Singh, P. K., et al. (2014). Structure and function of human DnaJ homologue subfamily a member 1 (DNAJA1) and its relationship to pancreatic cancer. Biochem. 53, 1360–1372. doi: 10.1021/bi401329a
Suetsugu, N., Kagawa, T., Wada, M. (2005). An auxilin-like J-domain protein, JAC1, regulates phototropin-mediated chloroplast movement in Arabidopsis. Plant Physiol. 139, 151–162. doi: 10.1104/pp.105.067371
Sun, X., Huang, N., Li, X., Zhu, J., Bian, X., Li, H., et al. (2021). A chloroplast heat shock protein modulates growth and abiotic stress response in creeping bentgrass. Plant Cell Environ. 44, 1769–1787. doi: 10.1111/pce.14031
Surekha, C., Kumari, K. N., Aruna, L. V., Suneetha, G., Arundhati, A., Kavi Kishor, P. B. (2014). Expression of the Vigna aconitifolia P5CSF129A gene in transgenic pigeonpea enhances proline accumulation and salt tolerance. Plant Cell Tissue Organ Cult 116, 27–36. doi: 10.1007/s11240-013-0378-z
Szabados, L., Savouré, A. (2010). Proline: a multifunctional amino acid. Trends Plant Sci. 15, 89–97. doi: 10.1016/j.tplants.2009.11.009
Tamura, K., Takahashi, H., Kunieda, T., Fuji, K., Shimada, T., Hara-Nishimura, I. (2007). Arabidopsis KAM2/GRV2 is required for proper endosome formation and functions in vacuolar sorting and determination of the embryo growth axis. Plant Cell 19, 320–332. doi: 10.1105/tpc.106.046631
Thompson, J. D., Higgins, D. G., Gibson, T. J. (1994). CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680. doi: 10.1093/nar/22.22.4673
Tiwari, B., Kalim, S., Tyagi, N., Kumari, R., Bangar, P., Barman, P., et al. (2018). Identification of genes associated with stress tolerance in moth bean [Vigna aconitifolia (Jacq.) marechal], a stress hardy crop. Physiol. Mol. Biol. Plants 24, 551–561. doi: 10.1007/s12298-018-0525-4
Vishwakarma, K., Upadhyay, N., Kumar, N., Yadav, G., Singh, J., Mishra, R. K., et al. (2017). Abscisic acid signaling and abiotic stress tolerance in plants: a review on current knowledge and future prospects. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.00161
Walsh, P., Bursać, D., Law, Y. C., Cyr, D., Lithgow, T. (2004). The J-protein family: modulating protein assembly, disassembly and translocation. EMBO Rep. 5, 567–571. doi: 10.1038/sj.embor.7400172
Wang, G., Cai, G., Kong, F., Deng, Y., Ma, N., Meng, Q. (2014). Overexpression of tomato chloroplast-targeted DnaJ protein enhances tolerance to drought stress and resistance to Pseudomonas solanacearum in transgenic tobacco. Plant Physiol. Biochem. 82, 95–104. doi: 10.1016/j.plaphy.2014.05.011
Wang, G., Cai, G., Xu, N., Zhang, L., Sun, X., Guan, J., et al. (2019a). Novel DnaJ protein facilitates thermotolerance of transgenic tomatoes. Int. J. Mol. Sci. 20, 367. doi: 10.3390/ijms20020367
Wang, G., Luo, Y., Wang, C., Zhou, Y., Mou, C., Kang, H., et al. (2020). Hsp40 protein LeDnaJ07 enhances the thermotolerance of Lentinula edodes and regulates IAA biosynthesis by interacting LetrpE. Front. Microbiol. 11. doi: 10.3389/fmicb.2020.00707
Wang, G. Z., Ma, C. J., Luo, Y., Zhou, S. S., Zhou, Y., Ma, X. L., et al. (2018b). Proteome and transcriptome reveal involvement of heat shock proteins and indoleacetic acid metabolism process in Lentinula edodes thermotolerance. Cell Physiol. Biochem. 50, 1617–1637. doi: 10.1159/000494784
Wang, W., Vinocur, B., Shoseyov, O., Altman, A. (2004). Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 9, 244–252. doi: 10.1016/j.tplants.2004.03.006
Wang, G., Xu, X., Wang, H., Liu, Q., Yang, X., Liao, L., et al. (2019b). A tomato transcription factor, SlDREB3 enhances the tolerance to chilling in transgenic tomato. Plant Physiol. Biochem. 142, 254–262. doi: 10.1016/j.plaphy.2019.07.017
Wang, G., Zhou, S., Luo, Y., Ma, C., Gong, Y., Zhou, Y., et al. (2018a). The heat shock protein 40 LeDnaJ regulates stress resistance and indole-3-acetic acid biosynthesis in Lentinula edodes. Fungal Genet. Biol. 118, 37–44. doi: 10.1016/j.fgb.2018.07.002
Waters, E. R., Lee, G. J., Vierling, E. (1996). Evolution, structure and function of the small heat shock proteins in plants. J. Exp. Bot. 47, 325–338. doi: 10.1093/jxb/47.3.325
Wei, Q., Luo, Q., Wang, R., Zhang, F., He, Y., Zhang, Y., et al. (2017). A wheat R2R3-type MYB transcription factor TaODORANT1 positively regulates drought and salt stress responses in transgenic tobacco plants. Front. Plant Sci. 8, 1374. doi: 10.3389/fpls.2017.01374
Xia, Z., Zhang, X., Li, J., Su, X., Liu, J. (2014). Overexpression of a tobacco J-domain protein enhances drought tolerance in transgenic Arabidopsis. Plant Physiol. Biochem. 83, 100–106. doi: 10.1016/j.plaphy.2014.07.023
Xie, Q., Zhou, Y., Jiang, X. (2022). Structure, function, and regulation of the plasma membrane Na+/H+ antiporter salt overly sensitive 1 in plants. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.866265
Yang, Y., Qin, Y., Xie, C., Zhao, F., Zhao, J., Liu, D., et al. (2010). The Arabidopsis chaperone J3 regulates the plasma membrane h+-ATPase through interaction with the PKS5 kinase. Plant Cell 22, 1313–1332. doi: 10.1105/tpc.109.069609
Yıldırım, K., Kaya, Z. (2017). Gene regulation network behind drought escape, avoidance and tolerance strategies in black poplar (Populus nigra l.). Plant Physiol. Biochem. 115, 183–199. doi: 10.1016/j.plaphy.2017.03.020
Zarouchlioti, C., Parfitt, D. A., Li, W., Gittings, L. M., Cheetham, M. E. (2018). DNAJ proteins in neurodegeneration: essential and protective factors. Philos. Trans. R. Soc Lond. B Biol. Sci. 373, 20160534. doi: 10.1098/rstb.2016.0534
Zhang, M., Cao, J., Zhang, T., Xu, T., Yang, L., Li, X., et al. (2022a). A putative plasma membrane Na+/H+ antiporter GmSOS1 is critical for salt stress tolerance in Glycine max. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.870695
Zhang, X., Liu, W., Lv, Y., Li, T., Tang, J., Yang, X., et al. (2022b). Effects of drought stress during critical periods on the photosynthetic characteristics and production performance of naked oat (Avena nuda l.). Sci. Rep. 12, 11199. doi: 10.1038/s41598-022-15322-3
Zhang, X., Zhuang, L., Liu, Y., Yang, Z., Huang, B. (2020). Protein phosphorylation associated with drought priming-enhanced heat tolerance in a temperate grass species. Hortic. Res. 7, 207. doi: 10.1038/s41438-020-00440-8
Zhichang, Z., Wanrong, Z., Jinping, Y., Jianjun, Z., Zhen, L., Xufeng, L., et al. (2010). Over-expression of Arabidopsis DnaJ (Hsp40) contributes to NaCl-stress tolerance. Afr. J. Biotechnol. 9, 972–978. doi: 10.5897/AJB09.1450
Zhou, W., Zhou, T., Li, M. X., Zhao, C. L., Jia, N., Wang, X. X., et al. (2012). The Arabidopsis J-protein AtDjB1 facilitates thermotolerance by protecting cells against heat-induced oxidative damage. New Phytol. 194, 364–378. doi: 10.1111/j.1469-8137.2012.04070.x
Keywords: drought stress, heat stress, DnaJ, photosynthetic efficiency, tobacco, gene expression, VaDJI
Citation: Gautam R, Meena RK, Rampuria S, Shukla P and Kirti PB (2023) Ectopic expression of DnaJ type-I protein homolog of Vigna aconitifolia (VaDJI) confers ABA insensitivity and multiple stress tolerance in transgenic tobacco plants. Front. Plant Sci. 14:1135552. doi: 10.3389/fpls.2023.1135552
Received: 01 January 2023; Accepted: 31 March 2023;
Published: 19 April 2023.
Edited by:
David Horvath, Edward T. Schafer Agricultural Research Center (USDA), United StatesReviewed by:
Tahmina Islam, University of Dhaka, BangladeshVivek Ambastha, Washington University in St. Louis, United States
Copyright © 2023 Gautam, Meena, Rampuria, Shukla and Kirti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ranjana Gautam, cmFuamFuYS5nYXV0YW05QGdtYWlsLmNvbQ==; P. B. Kirti, cGJraXJ0aUB1b2h5ZC5hYy5pbg==
†Present address: P. B. Kirti, Agri Biotech Foundation, Professor Jayashankar Telangana State Agricultural University Campus, Hyderabad, Telangana, India
 Ranjana Gautam
Ranjana Gautam Rajesh Kumar Meena
Rajesh Kumar Meena Sakshi Rampuria
Sakshi Rampuria Pawan Shukla
Pawan Shukla P. B. Kirti
P. B. Kirti