- 1Department of Biological Sciences, College of Science, King Faisal University, Al Ahsa, Saudi Arabia
- 2Department of Horticulture, Faculty of Agriculture, Suez Canal University, Ismalia, Egypt
Overuse of artificial chemical fertilizers could be detrimental to the environment. Utilizing beneficial microorganisms as biofertilizers is a sustainable technique that promotes soil health, crop yield, and ecosystem preservation. Curcuma longa L. is utilized as a medication since it has its antibacterial, anti-microbial, and anti-tumor characteristics, which reduce inflammation and hasten wound healing. The effect of E. cloacae strain MSR1, which is common in the roots of alfalfa grown in the Al-Ahsaa region, on C. longa plants is being investigated. C. longa rhizomes were planted under greenhouse conditions after being submerged in a solution of E. cloacae strain MSR1 (OD 500) or water treatment as a control for 12 hours. After 240 days of growing, ten randomly selected plants from each treatment were collected, and the vegetative growth and yield metrics were assessed. To investigate how E. cloacae influences C. longa production and chemical composition (photosynthetic pigment, nitrogen, phosphorus, potassium, and curcuminoid), measurements were conducted as well as genes diketide-CoA and curcumin synthases genes. Our research showed that C. longa's growth and yield were favorably impacted by E. cloacae. Significant increases in the related plants' chlorophyll a,b, carotenoid, nitrogen, phosphorus, and potassium levels were likewise a reflection of the enhanced effects shown in the growth and yield parameters. Treatment with E. cloacae raised the curcuminoid's three sub-components' compositions to varying degrees: bisdemethoxycurcumin, demethoxycurcumin, and curcumin. Comparing E. cloacae treated plants to the control, high expression levels of the genes diketide-CoA and curcumin synthase-1, -2, and 3 were also found. The treatment of E. cloacae is a good biostimulant candidate for boosting growth and yield as well as raising the medicinal qualities of C. longa, according to the overall results.
Introduction
The excess use of fertilizers made from synthetic chemicals could be detrimental to the environment. Consequently, suggestions have been made to replace chemical fertilizers entirely or in part with alternative sources, particularly biofertilizers (Bisht and Singh Chauhan, 2021). These biofertilizers are affordable and sustainable. The use of biofertilization techniques to produce organic food has emerged as a key technological advance for safeguarding public health, cropland, and the environment. Food production methods have multiplied, particularly for crops such as turmeric that have high commercial value and promising medical applications (Singh et al., 2016).
Turmeric, scientifically known as Curcuma longa L., is a perennial herbaceous plant belonging to the Zingiberaceae family (Sotiboldieva and Mahkamov, 2020). Rhizomes are commonly consumed plant parts that are rich in volatile oil compounds (e.g., mono- and sesquiterpenoids) and non-volatile curcuminoids, which have bioactive properties (Sharifi-Rad et al., 2020; Klopchevska et al., 2022). The bioactive compound in turmeric responsible for its health benefits is called curcumin. Curcumin has antioxidant, anti-inflammatory, neuroprotective, and anticancer properties (Sharifi-Rad et al., 2020; Aminnezhad et al., 2023). Dipeptide-CoA synthase (DCS) and curcumin synthases 1, 2, and 3 are the genes that mediate curcuminoid metabolism in C. longa (Chakraborty et al., 2021; Katsuyama et al., 2009a; Katsuyama et al., 2009b; Sandeep et al., 2017).
The use of beneficial microorganisms as biofertilizers is a sustainable technique that promotes soil health and crop yield. Beneficial microorganisms employ two distinct ways that either directly or indirectly enhance plant productivity: phytopathogen suppression and plant growth promotion (PGP) (Koskey et al., 2021). Enterobacter cloacae belongs to the Enterobacteriaceae family and is a Gram-negative, short rod bacterium (García-González et al., 2018).
E. cloacae strains that exhibit numerous growth-promoting properties, such as phosphate solubilization, nitrogen fixation, phytohormone production, and exopolysaccharide production, have been found to be plant growth promoters (Gupta et al., 2022). E. cloacae has been essential in establishing and maintaining soil fertility, which increases the development and yield of several agricultural crops. These qualities are due to the various traits of this bacterium that encourage plant growth, including its solubility in phosphate, its ability to produce phytohormones such as acetoin and phosphate, and its ability to produce bioactive compounds (Khalifa et al., 2016; García-González et al., 2018; Al Dayel and El Sherif, 2021; Sritongon et al., 2023).
The objective of this study was to comprehensively examine and assess the effects of treatment with E. cloacae on the plant growth, yield, and bioactive substances of C. longa. The curcuminoid gene expression patterns in the rhizomes were thoroughly investigated to improve our understanding of the underlying molecular mechanisms and obtain a deeper understanding of the molecular mechanisms involved. The effect of the E. cloacae strain MSR1, which is present in the roots of alfalfa growing in the Al-Ahsaa region, on C. longa plants is studied for the first time in this paper.
Materials and methods
Turmeric cultivation and bacterial treatment
The turmeric rhizomes (40 g) (Narendhiran et al., 2024) were submerged in a solution of the E. cloacae strain MSR1 (Khalifa et al., 2016) at 25°C for 12 h, as provided by the Microbiology Laboratory at the Department of Biological Science, Faculty of Science, King Faisal University. The optical density of the solution was measured at OD500)the concentration used in the experiment was 106 microbial cells per 1 ml fluid), while the control method involved soaking in distilled water. On April 1, 2022, the turmeric rhizomes were planted in sand-filled germination trays within the greenhouse of the King Faisal University Agriculture and Veterinary Research and Training Centre, King Faisal University (25.266184323290517 and 49.695981580002844). After 1 month, the seedlings (5 cm long and with three leaf pairs) were cultured into 20-cm-wide and 15-cm-deep plastic pots filled with 4.5 kg of sand soil per pot (one plant per pot).
The percentage of rhizomes that germinated was recorded. The tests utilized a completely randomized block design with 15 replicates (pots), two treatment groups of E. cloacae, and distilled water as the control (Al Dayel and El Sherif, 2021). Groundwater irrigated each plant, as needed (Supplementary Table S1). The soil and irrigation water components were identified using the method of Buurman et al. (1996) (Supplementary Tables S1, S2). After 240 days of cultivation, 10 randomly chosen plants from each treatment were collected. The following measurements were made: plant height (in centimeters); rhizome diameter (in millimeters); dry weight of the roots, leaves, and rhizomes per plant (in grams); and the number of roots, leaves, and rhizomes per plant (n).
Chemical analysis
Photosynthetic pigment measurement
A random sample of four 240-day-old turmeric plants was selected. The photosynthetic pigment content was measured in the third leaf from the apex of each plant. Chlorophylls a and b, as well as the carotenoids, were extracted and computed according to the methods described in House et al. (2019).
Mineral composition
After collection (240 days from planting in the field) from various treatments, the leaves from the turmeric plant were dried at 60°C for 48 h. Subsequently, the leaves were degraded with sulfuric acid following Piper’s description in 1942 (Piper, 2019). The modified micro-Kjeldahl technique introduced by Jackson in 1967 (Nirere et al., 2021) was employed to determine the nitrogen content.
Similarly, to determine the phosphorus level, calorimetry, as suggested by Murphy and Riley in 1962 (Amm et al., 2023), was performed, while the potassium concentration was determined through atomic absorption flame photometry, as proposed by Mazumdar and Majumder in 2003 (Banerjee and Prasad, 2020). Finally, the soil samples were collected and assessed at the conclusion of the study based on the water and soil studies conducted by Page in 1982 (Bottomley et al., 2020).
HPLC analysis of the curcuminoid content in C. longa rhizome
This study used air-dried powdered C. longa rhizome from three plants randomly selected for each treatment of E. cloacae and the control. The concentrations of curcumin, bisdemethoxycurcumin, and demethoxycurcumin were determined with a Waters 2690 Alliance HPLC system. The system was equipped with a C18 Inertsil column (4.6 mm × 250 mm, 5 m) and a Waters 996 photodiode array detector. The analysis was conducted following the guidelines outlined by Field (El Sherif et al., 2022).
Real-time RT-PCR analysis of curcuminoid gene expression
Real-time reverse transcriptase polymerase chain reaction (RT-PCR) was used to measure the transcript concentrations of the curcuminoid genes (CURS1, CURS2, CURS3, and DCS) in C. longa rhizomes. From each experimental group, four 240-day-old plants were chosen at random. The procedures were outlined as shown in Supplementary Table S3 (Katsuyama et al., 2009a; Katsuyama et al., 2009b; El Sherif et al., 2022).
Statistical evaluation
The experiment utilized a completely randomized block design, which was repeated 10 times. Data were compared using an independent-samples t-test in SPSS 21 software package (version 21.0; IBM Corp., Armonk, NY, USA).
Results
Influence of E. cloacae in enhancing plant production
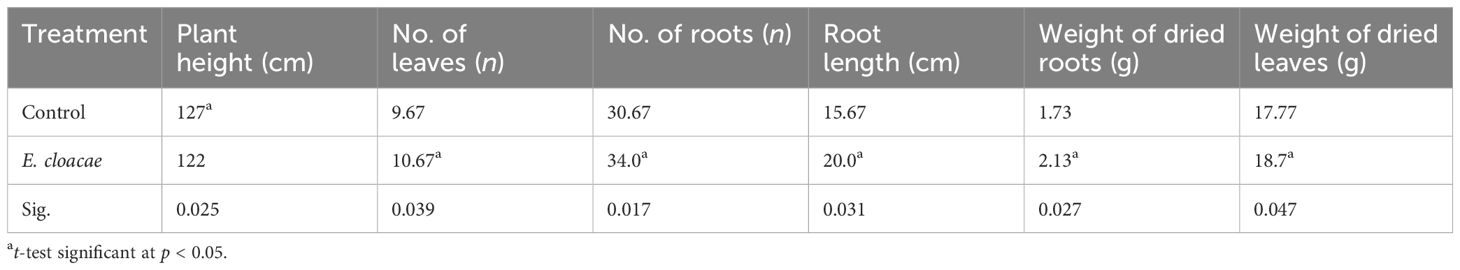
Table 1 displays the findings from the measurements of vegetative development. The results showed that treatment with E. cloacae led to a statistically considerable increase in the amount of leaves and roots, as well as the dry weight and length of C. longa roots, as compared with the control group. In contrast, compared with the control treatment, the administration of E. cloacae led to significant reductions in plant height.
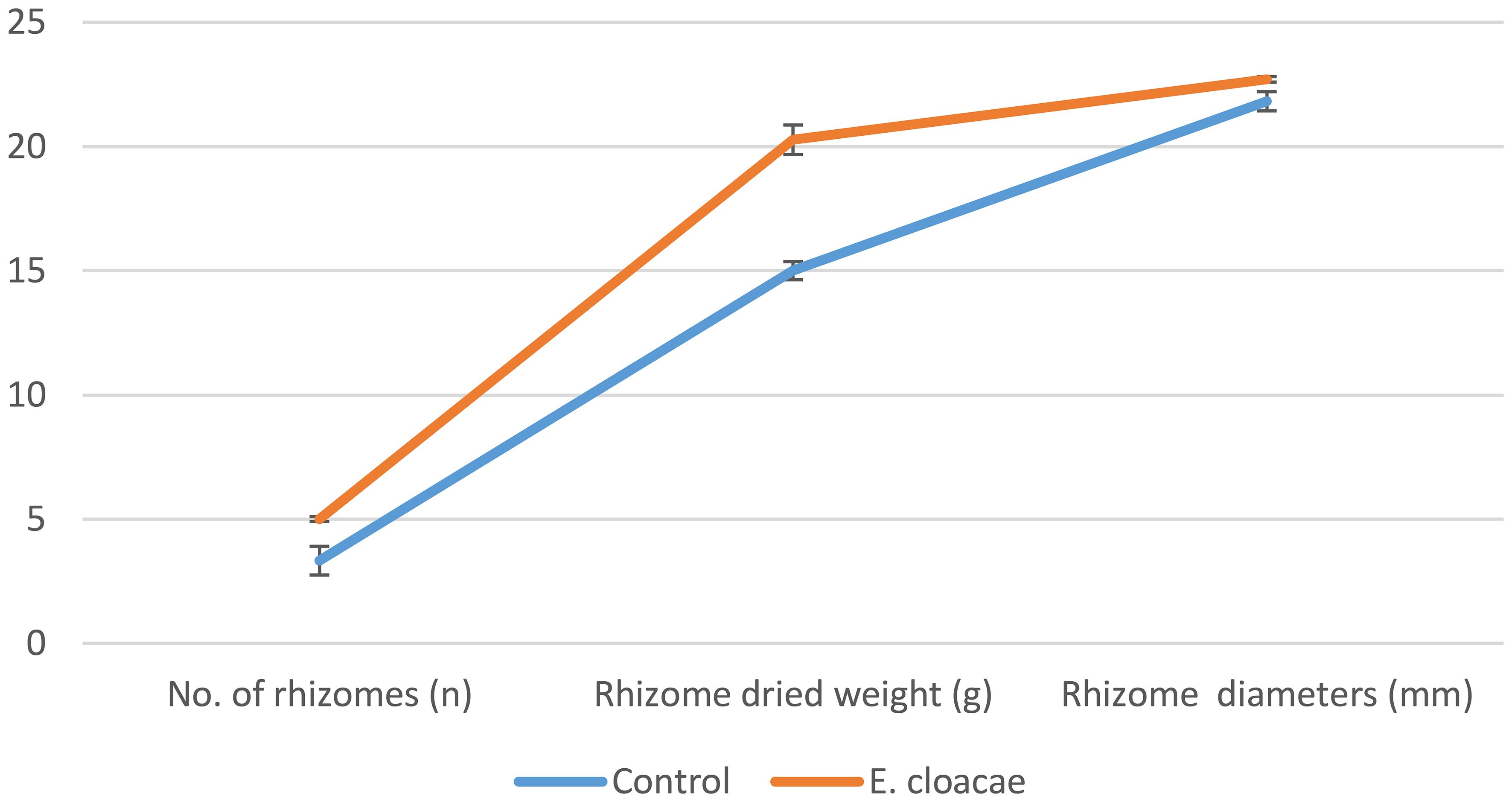
As shown in Figure 1, treatment with E. cloacae significantly increased the amount, dry weight, and the diameter of rhizomes by approximately 1.5, 1.35, and 1.04 times, respectively, in contrast to the control treatment.
Influence of E. cloacae on the photosynthetic pigment contents of C. longa leaves
According to Table 2, it appears that treatment of C. longa with E. cloacae resulted, in comparison to the control, in a notable increase in the quantity of carotenoid and chlorophylls a and b. As shown in the table, the increments were approximately 1.01-, 1.99-, and 1.25-fold, respectively.
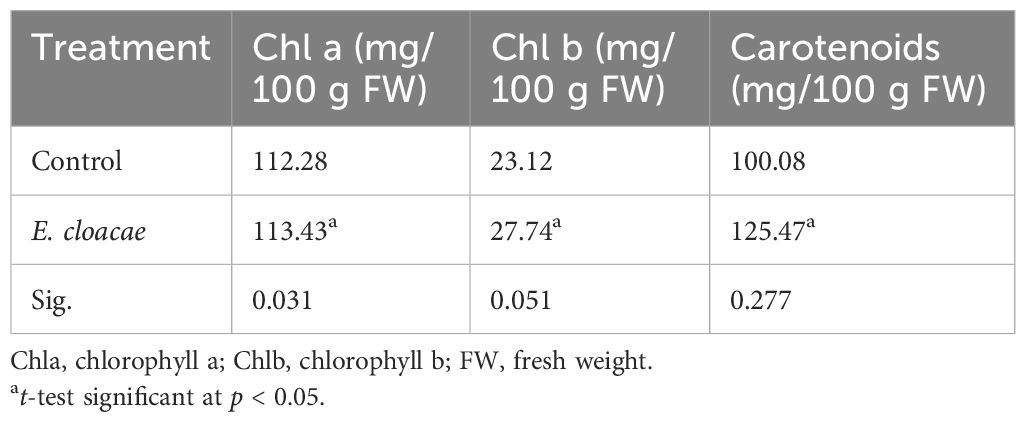
Table 2 Effects of Enterobacter cloacae treatment on the photosynthetic pigments of Curcuma longa leaves.
Influence of E. cloacae on the nitrogen, phosphorus, and potassium contents of C. longa leaves
Table 3 presents the data on the impact of E. cloacae on the potassium, phosphorus, and nitrogen levels in the leaves of C. longa. Treatment with E. cloacae led to 1.09- and 1.14-fold increases in the phosphorus and nitrogen contents, and these increments were statistically significant. On the other hand, no significant increase in potassium content was observed.
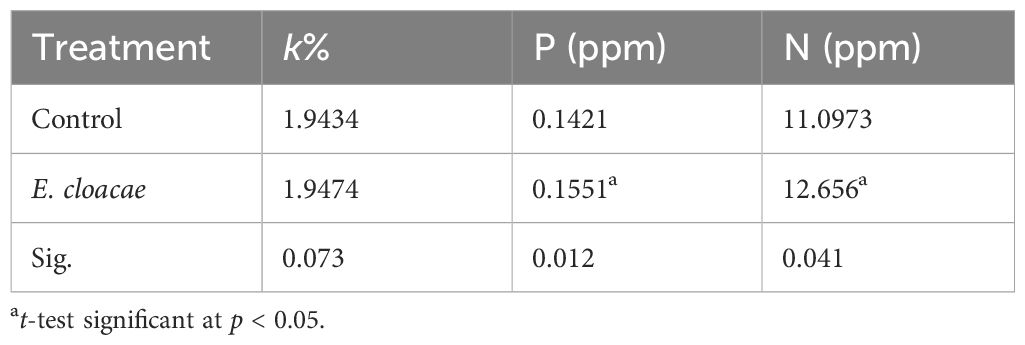
Table 3 Influence of Enterobacter cloacae on the percentage of nitrogen, phosphorus, and potassium in Curcuma longa leaves.
Influence of E. cloacae on the curcuminoid content of C. longa rhizome
The bioactive substances bisdemethoxycurcumin, demethoxycurcumin, and curcumin were much more prevalent in the methanolic extracts of C. longa rhizome after the application of E. cloacae. By using HPLC, it was possible to measure these increases, which were found to be 1.86-, 1.35-, and 1.64-fold higher than those of the control. These results are illustrated in Figures 2 and 3.
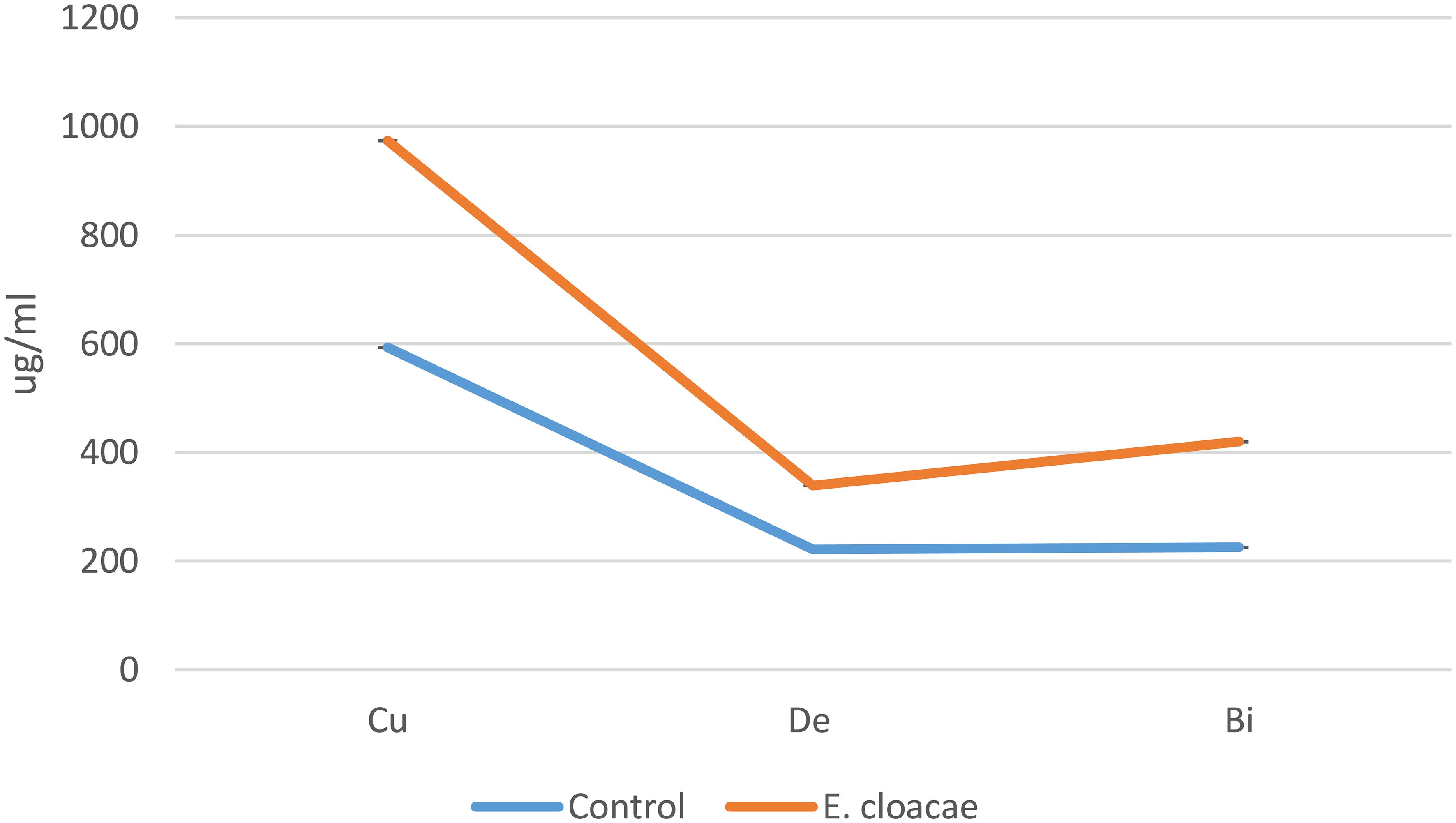
Figure 2 Effects of Enterobacter cloacae treatment on the curcuminoid accumulation (in micrograms per milliliter) of Curcuma longa.
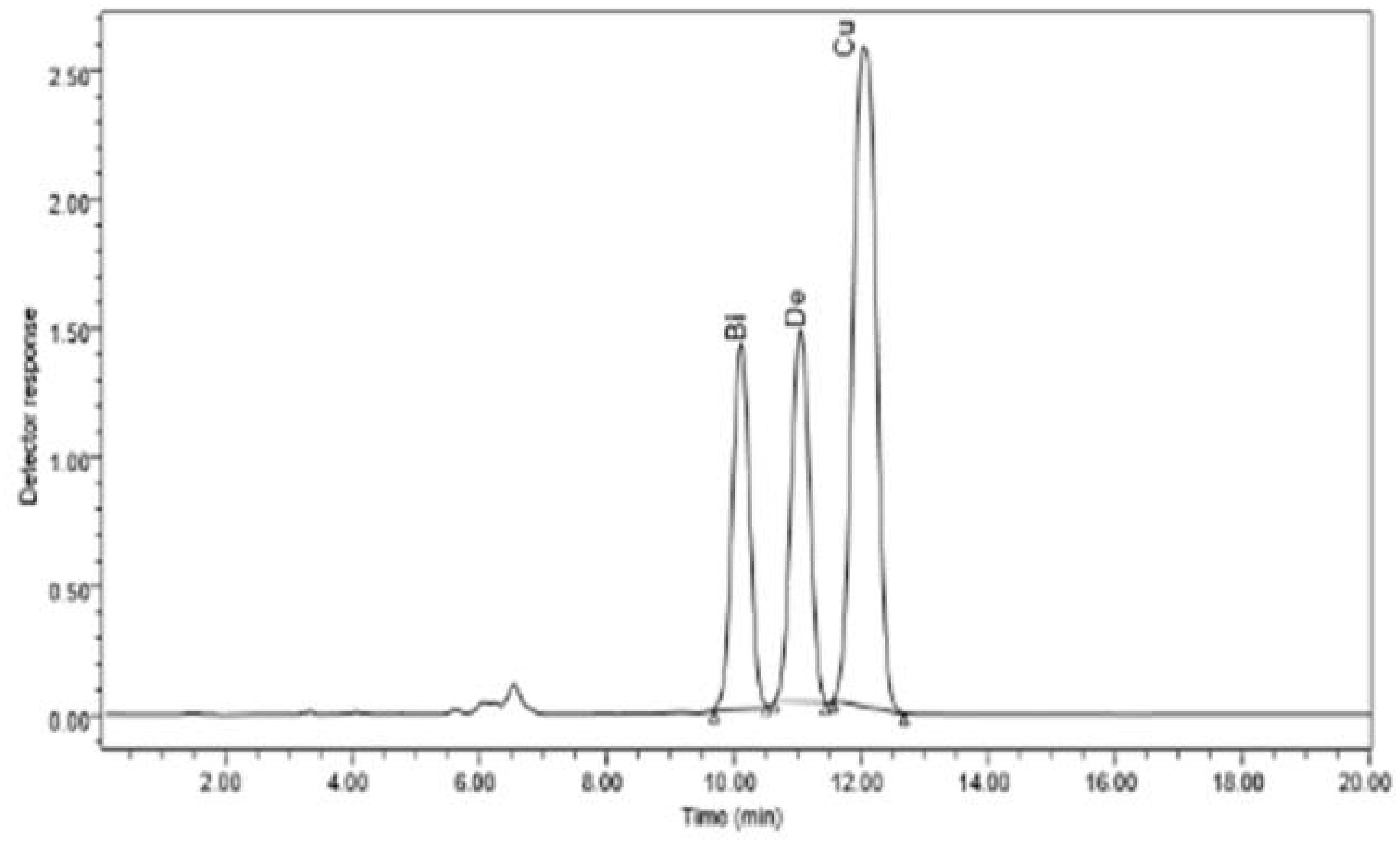
Figure 3 HPLC chromatogram of the organic extract of Curcuma longa after exposure to Enterobacter cloacae, which reveals the presence of curcumin, dimethoxycurcumin, and bisdemethoxycurcumin compounds.
Impact of E. cloacae on the expression of the curcuminoid synthase genes
The results of this study indicated that the genes CURS2, CURS3, and DCS were differentially upregulated by treatments with E. cloacae. The expression levels of DCS, CURS2, and CURS3 were higher following E. cloacae treatment compared with the control (Figure 4). The use of E. cloacae increased the expression of DCS, CURS2, and CURS3 (6.3-, 5.9-, and 5.2-fold respectively) compared with the control (Figure 4). However, a gradual reduction in the expression of the CUR1 gene was observed with E. cloacae application compared with the control treatment.
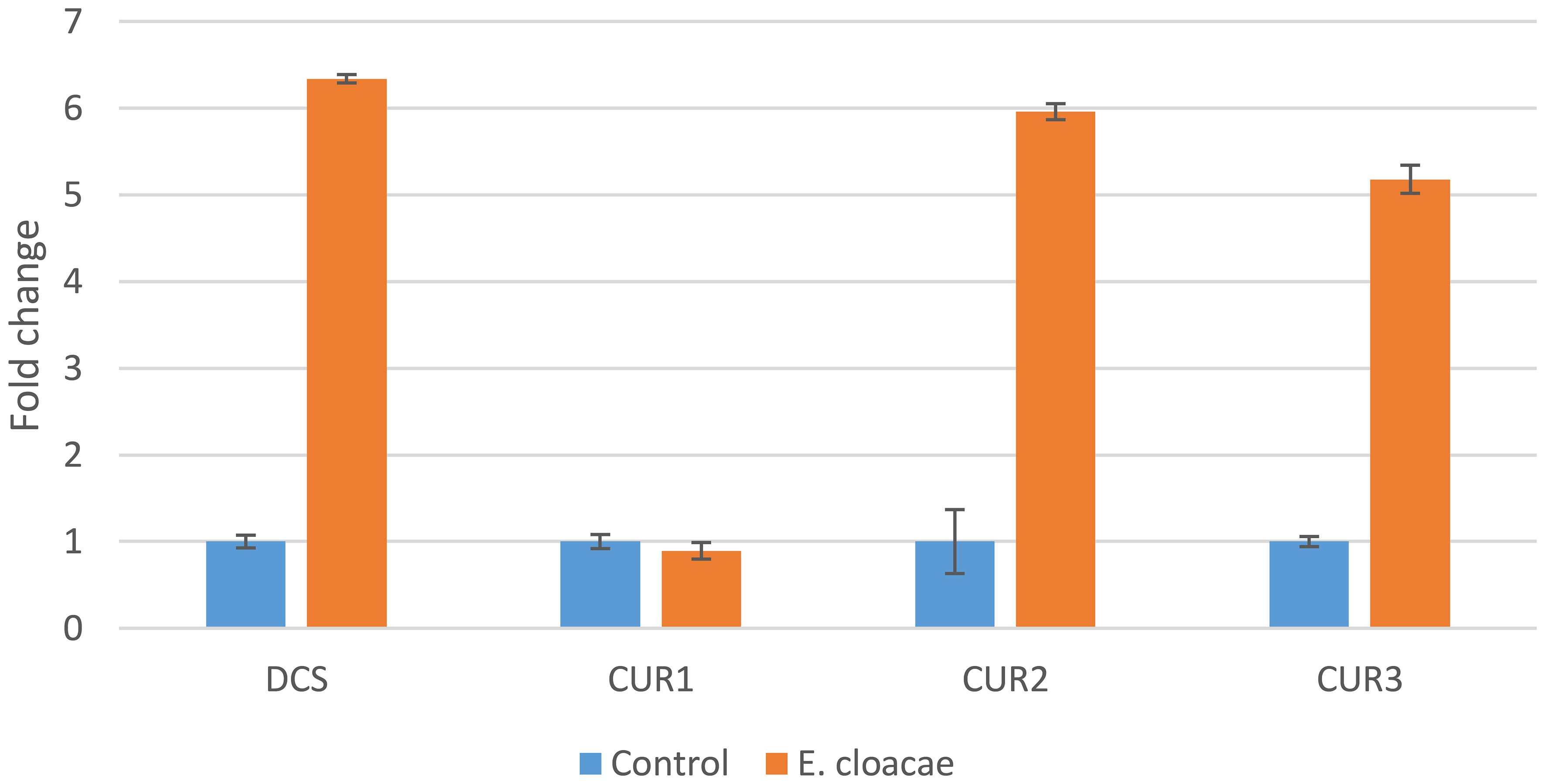
Figure 4 Expression of the curcuminoid synthase genes in Curcuma longa rhizome treated with Enterobacter cloacae. Actin was used as an internal reference gene for standardization.
Discussion
This study examined the impact of E. cloacae (as a plant growth-promoting bacterium, PGPB) on the growth and yield of C. longa. The results of this study contribute to the knowledge needed for the implementation of microorganisms that promote plant growth in an agricultural setting, such as curcuma plants.
It is worth noting that sand soil is considered deficient in mineral nutrients. The results presented in Supplementary Table S1 validate this claim. According to Turan et al. (2017), E. cloacae stimulation of root proliferation could improve the capacity of the seedlings to sequester the limited mineral nutrients found in vermiculite, which may inadvertently promote shoot growth. Seedlings may also be more adept at sequestering minerals from vermiculite, which could sustain the growth observed in plants treated with PGPB. E. cloacae has high levels of nitrogen-fixing bacteria from the rhizosphere, which increases the N content in the soil (Chao et al., 2020). E. cloacae had the most favorable effect on the plant height, number of roots, dry weight of the leaves, and the contents of chlorophylls a and b, carotenoid, and N, P, and K in the leaves, which correlated with the highest rhizome number, rhizome dry weight, and rhizome diameter.The bacterium-associated roots of a plant are essential to its growth and development due to a variety of processes, including the availability of nutrients and the effect of the produced indoleacetic acid (IAA) on the root shape (Saikia et al., 2018; Chouhan et al., 2021; Shankar and Prasad, 2023). According to Overvoorde et al. (2010), with regard to root formation, this hormone primarily influences the length of the main root, the number of lateral roots, and the amount of root hairs. The effects of E. cloacae on plant development and productivity have been thoroughly documented (Ramakrishnan et al., 2023; Wang et al., 2023; Yue et al., 2023; Fan et al., 2024). E. cloacae caused an increase in the amount of photosynthetic pigments, increased the nutrient absorption, and increased the vegetative plant growth, and this enhanced rhizome output was sustained by the turmeric plant (Gupta et al., 2022; Chandarana and Amaresan, 2023; Shankar and Prasad, 2023; Sun et al., 2023). An increase in bioactive compounds resulting from the application of plant growth-promoting rhizome (PGPR) has been previously reported, including rutin and gallic acid in Moringa oleifera (Al Dayel and El Sherif, 2021), glucosinolate in Brassica oleracea (AbdElgawad et al., 2023), and acemannan in Aloe vera (Nikolaou et al., 2023).
The curcuminoid genes have been discovered as being involved in the production of curcuminoid in previous investigations (Katsuyama et al., 2009a; Katsuyama et al., 2009b). In this study, we found a simultaneous increase in the curcuminoid biosynthesis genes (DCS, CURS2, and CURS3) as demonstrated by RT-PCR and in the curcuminoids (curcumin, dimethoxycurcumin, and bisdemethoxycurcumin) as evaluated by HPLC. Similar results have been reported by El Sherif et al. (2022) and Khattab et al. (2023), suggesting that these genes play a role in modifying the levels of curcuminoids in C. longa.
Conclusions
The utilization of E. cloacae as a biofertilizer has opened up numerous opportunities for agriculture. The findings of this study conclusively demonstrated that E. cloacae significantly exceeded the control treatments in terms of growth (root and shoot quantity, root length, and dry weight of leaves and roots) and yield (rhizome number, rhizome diameter, and root dry weight), as well as in the levels of photosynthetic pigments (chlorophylls a and b and carotenoids) and the contents of nitrogen, phosphorus, potassium, and curcuminoids (bisdemethoxycurcumin, demethoxycurcumin, and curcumin), all without the addition of external mineral nutrients, over the 240-day cultivation period. The unique influence of E. cloacae treatment on the expression of the genes CURS2, CURS3, and DCS also uncovered its potential application in plants. By utilizing E. cloacae, the complete potential of C. longa rhizome and other plants.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed.
Author contributions
FE: Conceptualization, Data curation, Formal analysis, Methodology, Resources, Software, Writing – original draft, Writing – review & editing. HA: Funding acquisition, Investigation, Methodology, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The Deanship of Scientific Research at King Faisal University is acknowledged by the authors for sponsoring this research project with grant number 5161.
Acknowledgments
The authors are grateful for support from Salah Khattab and Yun-Kiam Yap of the Department of Biological Sciences, College of Science, King Faisal University, as well as the greenhouse personnel at King Faisal University’s Agriculture and Veterinary Research and Training Centre, is greatly appreciated.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2024.1393198/full#supplementary-material
References
AbdElgawad, H., Magdy Korany, S., Reyad, A. M., Zahid, I., Akhter, N., Alsherif, E., et al. (2023). Synergistic impacts of plant-growth-promoting bacteria and selenium nanoparticles on improving the nutritional value and biological activities of three cultivars of Brassica sprouts. ACS omega 8, 26414–26424. doi: 10.1021/acsomega.3c02957
Al Dayel, M. F., El Sherif, F. (2021). Evaluation of the effects of Chlorella vulgaris, Nannochloropsis salina, and Enterobacter cloacae on growth, yield and active compound compositions of Moringa oleifera under salinity stress. Saudi J. Biol. Sci. 28, 1687–1696. doi: 10.1016/j.sjbs.2020.12.007
Aminnezhad, S., Zonobian, M. A., Moradi Douki, M., Mohammadi, M. R., Azarakhsh, Y. (2023). Curcumin and their derivatives with anti-inflammatory, neuroprotective, anticancer, and antimicrobial activities: A review. Micro Nano Bio Aspects 2, 25–34. doi: 10.22034/mnba.2023.417415.1047
Amm, M., Dmsh, D., Mim, M. (2023). Enhancement of phoosphorous removal from wastewater using murunnkan clay mixed media. Larhyss J. 53, 145–163. doi: 10.1016/j.jwpe.2024.105282
Banerjee, P., Prasad, B. (2020). Determination of concentration of total sodium and potassium in surface and ground water using a flame photometer. Appl. Water Sci. 10, 1–7. doi: 10.1007/s13201-020-01188-1
Bisht, N., Singh Chauhan, P. (2021). Excessive and disproportionate use of chemicals cause soil contamination and nutritional stress. IntechOpen. doi: 10.5772/intechopen.94593
Bottomley, P. J., Angle, J. S., Weaver, R. (2020). Methods of soil analysis, Part 2: Microbiological and biochemical properties Weaver, R. W. (Richard W. ) (Ed.) (1994). Methods of soil analysis. Part 2, Microbiological and biochemical properties. Soil Science Society of America.
Buurman, P., Van Lagen, B., Velthorst, E. (1996). Manual for soil and water analysis (The Netherlands: Backhuys Publishers, Leiden).
Chakraborty, A., Mahajan, S., Jaiswal, S. K., Sharma, V. K. (2021). Genome sequencing of turmeric provides evolutionary insights into its medicinal properties. Commun. Biol. 4, 1193. doi: 10.1038/s42003-021-02720-y
Chandarana, K. A., Amaresan, N. (2023). Predation pressure regulates plant growth promoting (PGP) attributes of bacterial species. J. Appl. Microbiol. 134, lxad083. doi: 10.1093/jambio/lxad083
Chao, J., Zhaoyang, L., Liping, H., Xin, S., Changdong, W., Yue, L., et al. (2020). Effects of enterobacter cloacae HG-1 on the nitrogen-fixing community structure of wheat rhizosphere soil and on salt tolerance. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.01094
Chouhan, G. K., Verma, J. P., Jaiswal, D. K., Mukherjee, A., Singh, S., de Araujo Pereira, A. P., et al. (2021). Phytomicrobiome for promoting sustainable agriculture and food security: Opportunities, challenges, and solutions. Microbiological Res. 248, 126763. doi: 10.1016/j.micres.2021.126763
El Sherif, F., Alkuwayti, M. A., Khattab, S. (2022). Foliar spraying of salicylic acid enhances growth, yield, and curcuminoid biosynthesis gene expression as well as curcuminoid accumulation in curcuma longa. Horticulturae 8, 417. doi: 10.3390/horticulturae8050417
Fan, Y., Wang, H., Zhang, Z., Li, Y., Zhao, Z., Ni, X. (2024). Mechanisms involved in plant growth promotion by Enterobacter cloacae DJ under salinity-alkalinity stress. Chem. Biol. Technol. Agric. 11, 1–12. doi: 10.1186/s40538-024-00537-5
García-González, T., Sáenz-Hidalgo, H. K., Silva-Rojas, H. V., Morales-Nieto, C., Vancheva, T., Koebnik, R., et al. (2018). Enterobacter cloacae, an emerging plant-pathogenic bacterium affecting chili pepper seedlings. Plant Pathol. J. 34, 1. doi: 10.5423/PPJ.OA.06.2017.0128
Gupta, P. J., Trivedi, M. J., Soni, H. P. (2022). Enterobacter cloacae PNE2 as promising plant growth promoting bacterium, isolated from the kadi vegetable market waste, gujarat. Biosci. Biotechnol. Res. Asia 19, 773–786. doi: 10.13005/bbra/
House, J. D., Hill, K., Neufeld, J., Franczyk, A., Nosworthy, M. G. (2019). Determination of the protein quality of almonds (Prunus dulcis L.) as assessed by in vitro and in vivo methodologies. Food Sci. Nutr. 7, 2932–2938. doi: 10.1002/fsn3.1146
Katsuyama, Y., Kita, T., Funa, N., Horinouchi, S. (2009a). Curcuminoid biosynthesis by two type III polyketide synthases in the herb Curcuma longa. J. Biol. Chem. 284(17), 11160–11170. doi: 10.1074/jbc.M900070200
Katsuyama, Y., Kita, T., Horinouchi, S. (2009b). Identification and characterization of multiple curcumin synthases from the herb Curcuma longa. FEBS Lett. 583(17), 2799–2803. doi: 10.1016/j.febslet.2009.07.029
Khalifa, A. Y., Alsyeeh, A.-M., Almalki, M. A., Saleh, F. A. (2016). Characterization of the plant growth promoting bacterium, Enterobacter cloacae MSR1, isolated from roots of non-nodulating Medicago sativa. Saudi J. Biol. Sci. 23, 79–86. doi: 10.1016/j.sjbs.2015.06.008
Khattab, S., Alkuwayti, M. A., Yap, Y.-K., Meligy, A. M., Bani Ismail, M., El Sherif, F. (2023). Foliar spraying of ZnO nanoparticals on curcuma longa had increased growth, yield, expression of curcuminoid synthesis genes, and curcuminoid accumulation. Horticulturae 9, 355. doi: 10.3390/horticulturae9030355
Klopchevska, J., Chadikovski, A., Kavrakovski, Z., Srbinoska, M., Rafajlovska, V. (2022). Evaluation of solvent efficiency for extraction of bioactive curcuminoids from turmeric (Curcuma longa L.). Macedonian pharmaceutical bulletin 68(Suppl 2), 75–76. doi: 10.33320/MacedPharmBull
Koskey, G., Mburu, S. W., Awino, R., Njeru, E. M., Maingi, J. M. (2021). Potential use of beneficial microorganisms for soil amelioration, phytopathogen biocontrol, and sustainable crop production in smallholder agroecosystems. Front. Sustain. Food Syst. doi: 10.3389/fsufs.2021.606308
Narendhiran, V., Gangadharan, S., Muthulakshmi, K. (2024). Impact of seed rhizome size on growth and yield of turmeric. Asian J. Soil Sci. Plant Nutr. 10, 60–63. doi: 10.9734/ajsspn/2024/v10i1210
Nikolaou, C. N., Chatziartemiou, A., Tsiknia, M., Karyda, A. G., Ehaliotis, C., Gasparatos, D. (2023). Calcium-and Magnesium-Enriched Organic Fertilizer and Plant Growth-Promoting Rhizobacteria Affect Soil Nutrient Availability, Plant Nutrient Uptake, and Secondary Metabolite Production in Aloe vera (Aloe barbadensis Miller) Grown under Field Conditions. Agronomy 13, 482. doi: 10.3390/agronomy13020482
Nirere, D., SR, M., FX, R., FR, M. (2021). Effect of foliar application of nitrogen-phosphorus-potassium fertilizers on nutrient uptake and protein content of maize. African J. Biotech. 20(12), 465–469. doi: 10.5897/AJB2021.17383
Overvoorde, P., Fukaki, H., Beeckman, T. (2010). “Auxin control of root development. Cold Spring Harbor Perspect. Biol. 2, a001537. doi: 10.1101/cshperspect.a001537
Ramakrishnan, P., Ariyan, M., Rangasamy, A., Rajasekaran, R., Ramasamy, K., Murugaiyan, S., et al. (2023). Draft genome sequence of enterobacter cloacae S23 a plant growth-promoting passenger endophytic bacterium isolated from groundnut nodule possesses stress tolerance traits. Curr. Genomics 24, 36. doi: 10.2174/1389202924666230403123208
Saikia, J., Sarma, R. K., Dhandia, R., Yadav, A., Bharali, R., Gupta, V. K., et al. (2018). Alleviation of drought stress in pulse crops with ACC deaminase producing rhizobacteria isolated from acidic soil of Northeast India. Sci. Rep. 8, 3560. doi: 10.1038/s41598-018-21921-w
Sandeep, I. S., Das, S., Nasim, N., Mishra, A., Acharya, L., Joshi, R. K., et al. (2017). Differential expression of CURS gene during various growth stages, climatic condition and soil nutrients in turmeric (Curcuma longa): Towards site specific cultivation for high curcumin yield. Plant Physiol. Biochem. 118, 348–355. doi: 10.1016/j.plaphy.2017.07.001
Shankar, A., Prasad, V. (2023). Potential of desiccation-tolerant plant growth-promoting rhizobacteria in growth augmentation of wheat (Triticum aestivum L.) under drought stress. Front. Microbiol. 14, 1017167. doi: 10.3389/fmicb.2023.1017167
Sharifi-Rad, J., Rayess, Y. E., Rizk, A. A., Sadaka, C., Zgheib, R., Zam, W., et al. (2020). Turmeric and its major compound curcumin on health: bioactive effects and safety profiles for food, pharmaceutical, biotechnological and medicinal applications. Front. Pharmacol. 11, 1021. doi: 10.3389/fphar.2020.01021
Singh, M., Dotaniya, M. L., Mishra, A., Dotaniya, C. K., Regar, K. L., Lata, M. (2016). “Role of biofertilizers in conservation agriculture,” in Conservation agriculture. Eds. Bisht, J., Meena, V., Mishra, P., Pattanayak, A. (Springer, Singapore). doi: 10.1007/978–981-10–2558-7_4
Sotiboldieva, D. I., Mahkamov, T. X. (2020). Component composition of essential oils Curcuma longa L.(Zingiberaceae) introduced in Uzbekistan. Am. J. Plant Sci. 11, 1247–1253. doi: 10.4236/ajps.2020.118088
Sritongon, N., Boonlue, S., Mongkolthanaruk, W., Jogloy, S., Riddech, N. (2023). The combination of multiple plant growth promotion and hydrolytic enzyme producing rhizobacteria and their effect on Jerusalem artichoke growth improvement. Sci. Rep. 13, 5917. doi: 10.1038/s41598-023-33099-x
Sun, T., Yang, Y., Duan, K., Liao, Y., Zhang, Z., Guan, Z., et al. (2023). Biodiversity of endophytic microbes in diverse tea chrysanthemum cultivars and their potential promoting effects on plant growth and quality. Biology 12, 986. doi: 10.3390/biology12070986
Turan, M., Yildirim, E., Kitir, N., Unek, C., Nikerel, E., Ozdemir, B. S., G nes, A., NEP, M.. (2017). Beneficial role of plant growth-promoting bacteria in vegetable production under abiotic stress. Microbial strategies vegetable production, 151–166. doi: 10.1007/978-3-319-54401-4_7
Wang, X., Wu, Z., Xiang, H., He, Y., Zhu, S., Zhang, Z., et al. (2023). Whole genome analysis of Enterobacter cloacae Rs-2 and screening of genes related to plant-growth promotion. Environ. Sci. pollut. Res. 30, 21548–21564. doi: 10.1007/s11356-022-23564-x
Keywords: Turmeric, PGPB, RT-PCR, rhizome, NPK, HPLC
Citation: Alrajeh HS and Sherif FE (2024) Study the effect of Enterobacter cloacae on the gene expression, productivity, and quality traits of Curcuma longa L. Plant. Front. Plant Sci. 15:1393198. doi: 10.3389/fpls.2024.1393198
Received: 28 February 2024; Accepted: 07 May 2024;
Published: 02 August 2024.
Edited by:
Marta Wilton Vasconcelos, Catholic University of Portugal, PortugalReviewed by:
Ertan Yildirim, Atatürk University, TürkiyeRoxana Vidican, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, Romania
Copyright © 2024 Alrajeh and Sherif. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fadia El Sherif, RmVsc2hlcmlmQGtmdS5lZHUuc2E=
 Hind Salih Alrajeh1
Hind Salih Alrajeh1 Fadia El Sherif
Fadia El Sherif