- 1Dipartimento di Scienze della Vita e dell’Ambiente, Università Politecnica delle Marche, Ancona, Italy
- 2National Biodiversity Future Centre, Palermo, Italy
Seagrass meadows are regressing due to the cumulative impacts that affect coastal ecosystems worldwide. Seagrass restoration has been repeatedly proposed to reverse this trend, although with contrasting results due to the difficulty in maintaining the transplanted rhizomes. Enhancing the vegetative propagation of the rhizome plantings (e.g., employing growth-promoters) could represent a reliable tool to increase the success of seagrass restoration. Here we tested the effects of physio-activators, as plant growth-promoting bacteria (PGPB), and synthetic hormones, as plant growth regulators (PGRs), on a seagrass species to assess their potential utilization to enhance restoration efficiency. We conducted two separate experiments in aquaria on Cymodocea nodosa fragments: in the first one, the fragments were exposed to PGRs for six weeks, while in the second experiment, the fragments were exposed to PGPB for four weeks. For each experiment (PGRs and PGPB), the formation of new roots and new leaves, the survivorship, and the trend of maximum leaf length were compared between the treated and control (not exposed to PGRs or PGPB) fragments. It was observed that only the PGPB had a significant effect on the fragments’ survivorship (90% in treated fragments vs. 25% in control ones) and contributed significantly to the formation of new leaves and roots of C. nodosa fragments. On the contrary, in the experiments with PGRs, no significant effects were observed between treated and control fragments, and both showed a survivorship of 100% at the end of the experiment. Our study showed that the application of growth-promoters (particularly PGPB) on fragments could increase their survival and the formation of new roots and leaves. Therefore, the use of PGPB on C. nodosa fragments can allow their re-employment in restoration interventions, without damaging the individuals of natural populations.
1 Introduction
Seagrasses are marine angiosperms playing a crucial role in temperate and tropical coastal habitats, as they provide important ecosystem services (Costanza et al., 1997; Boudouresque et al., 2021). They include 67 species worldwide, 7 of which are present in the Mediterranean Sea: Posidonia oceanica is the only endemic species of the Mediterranean Sea, whereas Cymodocea nodosa, Zostera marina and Z. noltei show a broader distribution at temperate latitudes (Boudouresque et al., 2009; Ruiz et al., 2009), Ruppia maritima is almost entirely restricted to brackish lagoons and salt marshes (Shili et al., 2007), and Halophila stipulacea and H. decipiens are non-indigenous species (Winters et al., 2020; Gerakaris et al., 2020).
Over the last decades, seagrasses have severely declined due to anthropogenic activities, and only a few meadows recovered (Waycott et al., 2009; Orth et al., 2006; de los Santos et al., 2019; Sinclair et al., 2021). As a result of direct and indirect anthropogenic pressures, seagrass meadows are shrinking their global distribution at a rate ranging from 1% per year (till 1940) to 7% per year (after 1990, Waycott et al., 2009). In the Mediterranean Sea, the main causes of this decline are coastal development, increased maritime traffic, eutrophication, and chemical contamination (Pergent et al., 2014). Between 1973 and 1989, it was observed that the seagrass meadows of the northern Adriatic Sea were subjected to a decrease in extension due to the explosion of coastal urbanization, the intensification of tourism flow, and a significant increase in eutrophication caused by the Po River flow (Danovaro et al., 2020). Even a long-term analysis conducted from 1869 to 2016 in the European Seas showed that between the 1970s and 1980s, there was a sharp decline in seagrass meadows due to disease, deteriorated water quality, and coastal development (de los Santos et al., 2019).
Climate change and extreme events such as heat waves can exacerbate the rapid loss of shoot density and increase the energy needed to reproduce and produce defense compounds (Pergent et al., 2008). In the Greek Seas, the increase in the thermal regime over two decades (1997-2018) was followed by a decline in P. ocenica production (Litsi-Mizan et al., 2023). Stipcich et al. (2022) tested the effects of current and future Marine Heatwaves (MHWs) through a manipulative experiment in Sardinia (Italy) and observed significant changes in the morphological and biochemical variables of P. oceanica shoots. They found that current and future MHWs could have similar effects, with a difference depending on the intensity of the waves: the number of leaves, the maximum leaf length, and lipid content decreased, while the leaf necrosis and carbohydrate content increased (Stipcich et al., 2022). Beca-Carretero et al. (2024) applied a novel ecological and spatial model, considering two climate scenarios (RCP 2.6 and RCP 8.5) projected from 2020 to 2100 in four different regions within the Mediterranean (West, Central West, Central East, East Mediterranean). They foresee that with rising temperature and salinity, the habitat of P. ocenica will be lost and colonized by more resilient species such as C. nodosa and the invasive species H. stipulacea. Under the worst scenario (RCP 8.5), the most negative effects have been foreseen in warmer regions (Central and East Mediterranean), while the western region will represent a refuge area for P. oceanica (Beca-Carretero et al., 2024).
In recent decades, thanks to the enforcement of conservation measures (e.g., Habitat Directive, Water and Marine Strategy Framework Directives, Marine Protected Areas institution), some meadows displayed encouraging signs of stabilization or recovery (de los Santos et al., 2019). Moreover, in the last two decades, several restoration interventions have been implemented (Orth et al., 2006; Marbà et al., 2014). Although active restoration is considered an increasingly reliable approach to enhancing the recovery of seagrass ecosystems, to date, restoration results have not always been fully successful, due to many factors, such as the seagrass’s low growth rate and complex reproduction cycle (Bekkby et al., 2020), site selection (Paling et al., 2009; Bayraktarov et al., 2016), and used methodology (Da Ros et al., 2020).
Previous studies demonstrated that one of the major causes of restoration failure is the difficulty in maintaining in situ the transplanted rhizomes (Lepoint et al., 2002). During vegetative propagation, the formation of adventitious roots enables the plant to remain firmly attached to the substrate and to absorb the nutrients (Duguma, 1988; Swamy et al., 2002). Therefore, techniques enabling the development of a robust root system could facilitate the vegetative expansion of the transplanted rhizomes (Balestri and Lardicci, 2006). To accelerate vegetation expansion and improve transplant success (Loquès et al., 1990; Balestri et al., 1998; Balestri and Cinelli, 2001), other studies proposed the transplant of entire plants with the surrounding sediments contained in organic and biodegradable containers (Da Ros et al., 2020).
Several studies have shown the role of physio-activators, such as plant growth-promoting bacteria (PGPB), and synthetic hormones, such as plant growth regulators (PGRs), in the increase of the growth, development, and germination abilities over a wide range of terrestrial plants (Russo and Berlyn, 1990; Crunkilton et al., 1994; Swaminathaan and Srinivasan, 1996; Ortíz-Castro et al., 2009; Small and Degenhardt, 2018; Kumari et al., 2023). These molecules promote vegetative propagation, enhancing root and leaf formation and growth (Salisbury and Ross, 1992; Crunkilton et al., 1994; Katiresan and Moorthy, 1994; Munoz, 1995; Swaminathaan and Srinivasan, 1996). Moreover, the PGPB increase plant resilience against abiotic stressors (i.e., salinity, drought) and protect plants from diseases, inducing defense systems (Adhikari et al., 2001; Bloemberg and Lugtenberg, 2001; Weyens et al., 2009; O’Callaghan, 2016; Sánchez-López et al., 2018; Rossi et al., 2021).
Only a few studies investigated the effects of PGRs on Mediterranean seagrasses, with promising results on the plants’ growth (Munoz, 1995; Balestri and Bertini, 2003; Balestri and Lardicci, 2006; Balestri et al., 2011), but the effects of PGRs and PGPBs have never been investigated for enhancing the restoration efficacy on Mediterranean seagrasses (Loquès et al., 1990; Munoz, 1995), particularly for those interventions requiring ex-situ maintenance or growth and reproduction. The use of plant promoters could indeed increase the restoration effectiveness (Cebrian et al., 2021; Smith et al., 2023). The present study aims to test the effects of PGRs and PGPB on the survival and growth of C. nodosa. To avoid any impact on natural populations we explored their potential to produce new shoots and roots from fragmented plants that could represent a potentially important source of plants for restoration interventions (Campbell, 2002).
2 Materials and equipment
2.1 The species
C. nodosa is a pioneer seagrass (Marín-Guirao et al., 2016), forming dense meadows in shallow waters across the Mediterranean Sea and the Northeast Atlantic, including the Canary Islands (Reyes et al., 1995; Pavón-Salas et al., 2000; Alberto et al., 2008; Cunha and Araújo, 2009). This dioecious species is characterized by horizontal rhizomes, which at each node bring a short vertical rhizome ending dorsally with a leaf tuft of 3–4 leaves, and ventrally with irregularly branched roots. The leaves have a ribbon shape and feature 7–9 parallel ribs, and a rounded and obtuse apex (Rodríguez-Prieto et al., 2013). For its role in ecosystem structuring, C. nodosa is considered the second most important seagrass species in the Mediterranean Sea, after P. ocenica. It shows a wide environmental tolerance: along the sandy coasts, it grows in shallow and sheltered areas, in clear waters, also beyond the deep limit of P. oceanica, but also on dead matte of P. oceanica (Rodríguez-Prieto et al., 2013).
2.2 Samples’ collection
During October and November 2023, two samplings were conducted at Torrette site (near Ancona city, North-western Adriatic Sea; 43°36’36”N, 13°27’30”E; Figures 1A, B). In this site, between the coastline and the breakwaters, there is a rock pool formed by artificial reefs, hosting a meadow of C. nodosa (ca. 1 hectare wide) at about 0.5 m depth on muddy substrate.

Figure 1. (A) Location of the study area in the Mediterranean Sea; (B) Detail of the study area (43°36’36”N, 13°27’30”E). Map created using the Free and Open Source QGIS.
During the two samplings, a total of 15 and 40 fragments were collected, respectively, stranded, or manually collected by hand from plants. Since C. nodosa is under conservationist attention, the minimum number of fragments has been collected to run the experiments. For each sampling, the fragments were transported (in transportable aerated tanks, with seawater collected in situ) to the Aquarium Facility of the Department of Life and Environmental Science (Polytechnic University of Marche, Ancona; 43°58’N; 13°51’E), located 8 km from the sampling site, for ca. 14 minutes of transport.
Once arrived, the fragments were acclimatized for one hour through a slow mixing between the seawater used for the transport and the water of the tanks (previously prepared at the same temperature and salinity). The fragments collected during the first and second sampling were used for testing the effect of PGRs and PGPB, respectively, through 2 experiments in mesocosms, running separately.
3 Methods
3.1 Maintenance in the mesocosms
For each experiment, 2 separate aquaria systems were used, each consisting of 2 tanks (volume 50 L, each): one aquarium for the treatment (2 tanks with fragments exposed to PGRs or PGPB, in the first and second experiment, respectively) and 2 used as control (2 tanks with fragments).
The LSS (Life Support System) was used to maintain the plants in the mesocosms. It consists of aquaria, a reserve in which there are three socks of 100 μm for mechanical filtration, and immersed razor clams for biological filtration. Fluorescent lamps produced 260-nm (λ) UV-C rays, sterilizing the water, damaging nucleic acids, and preventing microbes’ proliferation. A Teco TK 500 cooler was used to maintain the temperature. The light intensity was generated by two light-emitting diode lamps (SilverMoon Marine 10,000 K and SilverMoon Universal 6,500 K) 40 cm above the water surface. Irradiance was measured with a Photometer of the Apogee Model MQ-500. The system ensures the maintenance of constant ambient water conditions. The photoperiod was set to a 12:12 h light: dark cycle, with an intensity of 80-100 µmol photons m−2 s−1 to simulate the environmental conditions present during sampling. Temperature, salinity, pH, and light intensity were measured at the sampling site and were set up in aquaria following Marín-Guirao et al. (2011) for the same sampling period. These parameters were maintained throughout the experiments: temperature was 20 ± 1°C, salinity was 37, and pH was 8.2. Furthermore, for routine system maintenance, water loading and unloading, lights, movement pumps, a cooler, and any water leaks at the pipe joints were checked. To ensure the sterilization of the system, the socks were washed, tubs were siphoned to remove organic debris, and 20% of the seawater was exchanged every week. The replacement water was prepared using artificial salts.
3.2 Experimental design
The first experiment consisted of 4 tanks (volume 50 L, each), 2 used for the treatment with PGRs (n = 2) and 2 as control (n = 2). The tanks used as control were labelled as C-PGRs1 and C-PGRs2, containing 2 fragments each. Those used for the treatment with PGRs were labelled as PGRs1 and PGRs2, containing 6 and 5 fragments, respectively (Figure 2). All fragments were fixed to plastic nets with a small weight to maintain them on the bottom of the tanks. In the tanks PGRs1 and PGRs2 it was added one pill of Gibaifar 10 TB, containing gibberellic acid (GA3), and 80 ml of Sprintex New® L, containing alpha-naphtaleneacetic acid (NAA).
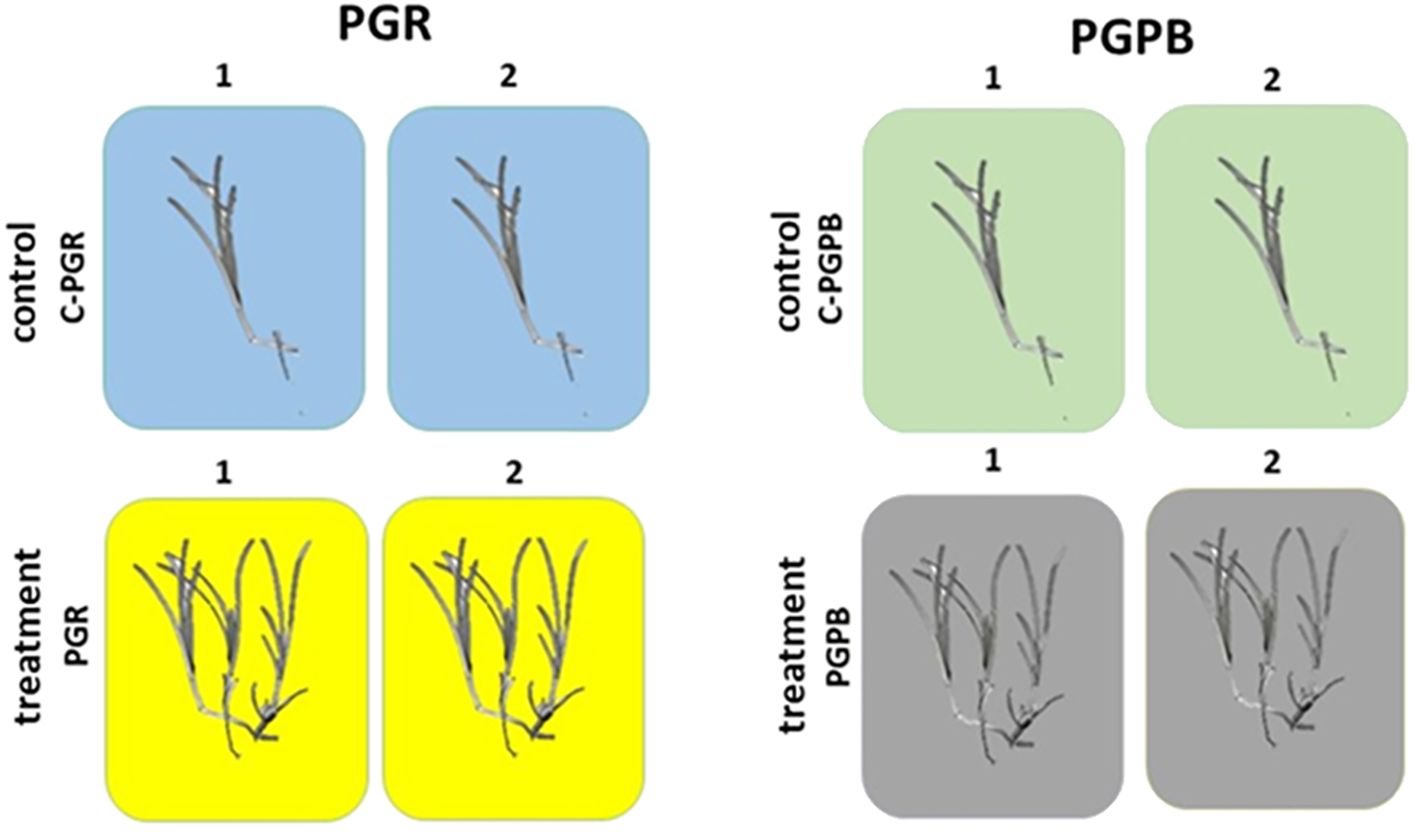
Figure 2. Experimental design for the PGRs and PGPB experiments. In each experiment, C. nodosa fragments were exposed to PGR (left panel) or PGPB (right panel) (n = 2) and compared to fragments not exposed (n = 2). Treatment = treated fragments (with PGR or PGPB, depending on the experiment); Control = fragment not exposed during each experiment (C-PGR and C-PGPB, respectively); 1 and 2 = code of the tank used.
The second experiment consisted of 4 tanks (volume 50 L, each), 2 for the treatment with PGPB (n =2) and 2 as control (n = 2). In this case, tanks used as control were labelled as C-PGPB1 and C-PGPB2. Those used for treatment with PGPB were labelled as PGPB1 and PGPB2 (Figure 2). Each tank contained 10 fragments. In this case, all the fragments were fixed to jute nets with a small weight to maintain them on the bottom of the tanks. In the mesocosms PGPB1 and PGPB2, 100 ml of Microtech Triple eco was added, which contains growth-promoting bacteria and cyanobacteria.
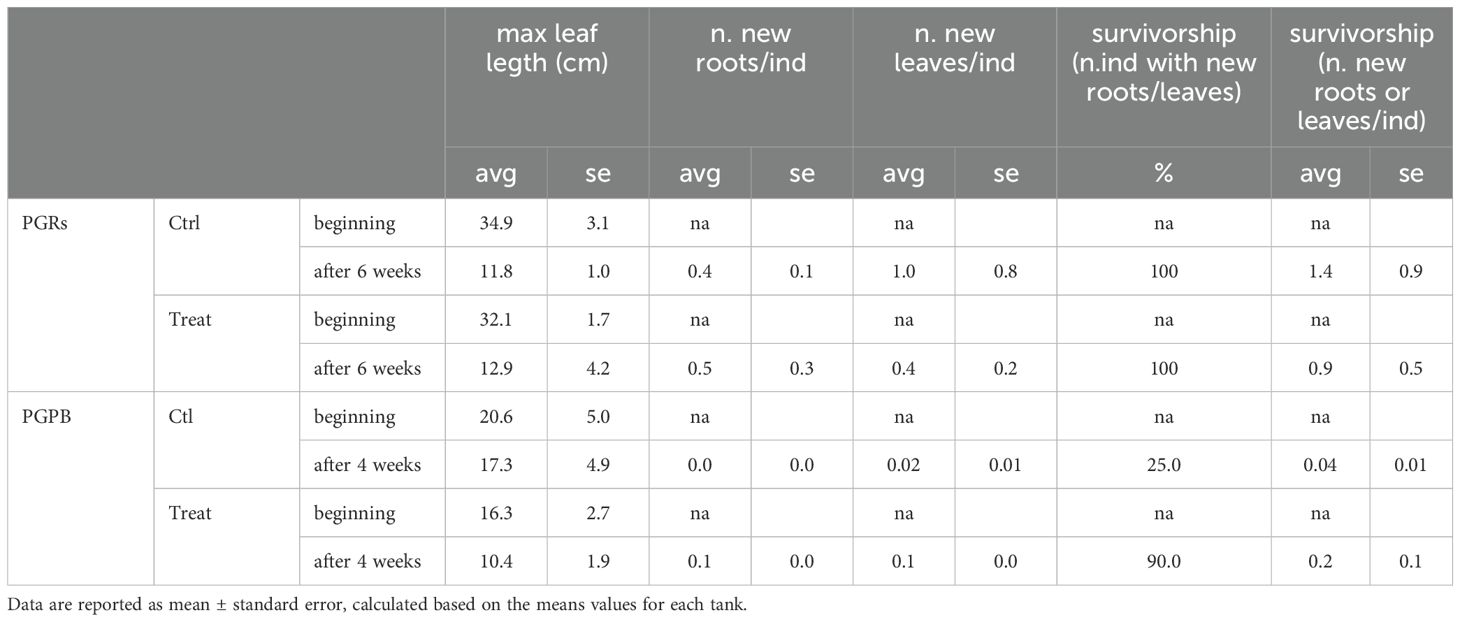
In both experiments, all fragments were tagged, photographed, and their growth measured. The following variables were checked once a week, for 6 and 4 weeks for the first and second experiment, respectively: number of shoots, roots, and leaves, and maximum leaf length. The collection of the abovementioned data allowed us to estimate: the number of new roots, new leaves, survivorship (number of individuals showing new leaves or roots), and trend of the maximum leaf length (following Balestri and Lardicci, 2006; Balestri et al., 2011). All the variables were measured for all the individuals in each tank and reported as tanks’ mean ± standard error.
3.3 Statistical analyses
To test differences in the abovementioned variables (maximum leaf length, number of new roots and leaves, and survivorship), separately for the 2 experiments, one- or two-way permutational analysis of variance (PERMANOVA) was performed, applying two experimental designs.
For the maximum leaf length, two factors of variance were considered: “time” (fixed, 2 levels: beginning and end of the experiments, corresponding to 6 and 4 weeks for the PGRs and PGPB experiment, respectively) and “treatment” (fixed, 2 levels: control and treatment, with the factor “tank” nested in “treatment”).
All other variables (i.e., number of new roots and leaves, and survivorship) were considered as a single factor of variance in the “treatment” (fixed, 2 levels: control and treated, with the factor “tank” nested in “treatment”) since at the beginning there were no new roots and leaves.
Before PERMANOVA, PERMDISP tests were carried out to test the dispersion among groups: “time x treatment(tank)” for max leaf length or “treatment(tank)” for new roots, new leaves, and survivorship. When PERMDISP was significant, the data were fourth root transformed before PERMANOVA.
Statistical analyses were performed by using the software package PRIMER7 (Clarke and Gorley, 2015).
4 Results
All the data are reported in Table 1.
The results of the PERMDISP testing for dispersion among groups for PGRs and PGPB experiments are reported in Tables 2A, B, respectively. The results of the PERMANOVA analyses testing for the effect of treatment and time or only treatment, depending on the variable, on all the considered response variables, for PGRs and PGPB experiments, are reported in Tables 3A, B, respectively.
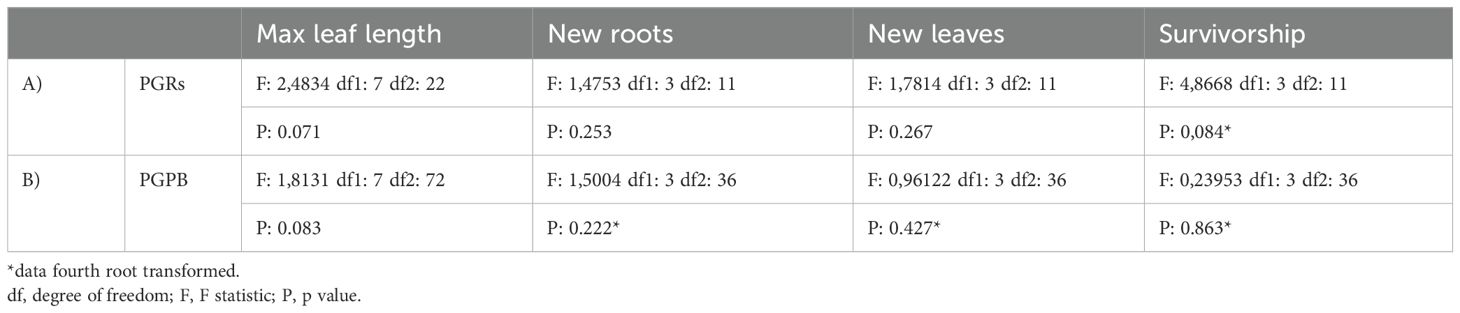
Table 2. Output of the PERDISP conducted after PGRs (A) and PGPB (B) experiments, on all variables, testing for the dispersion among groups: “treatment(tank) x time” for max leaf length or “treatment(tank)” for new roots, new leaves, and survivorship.
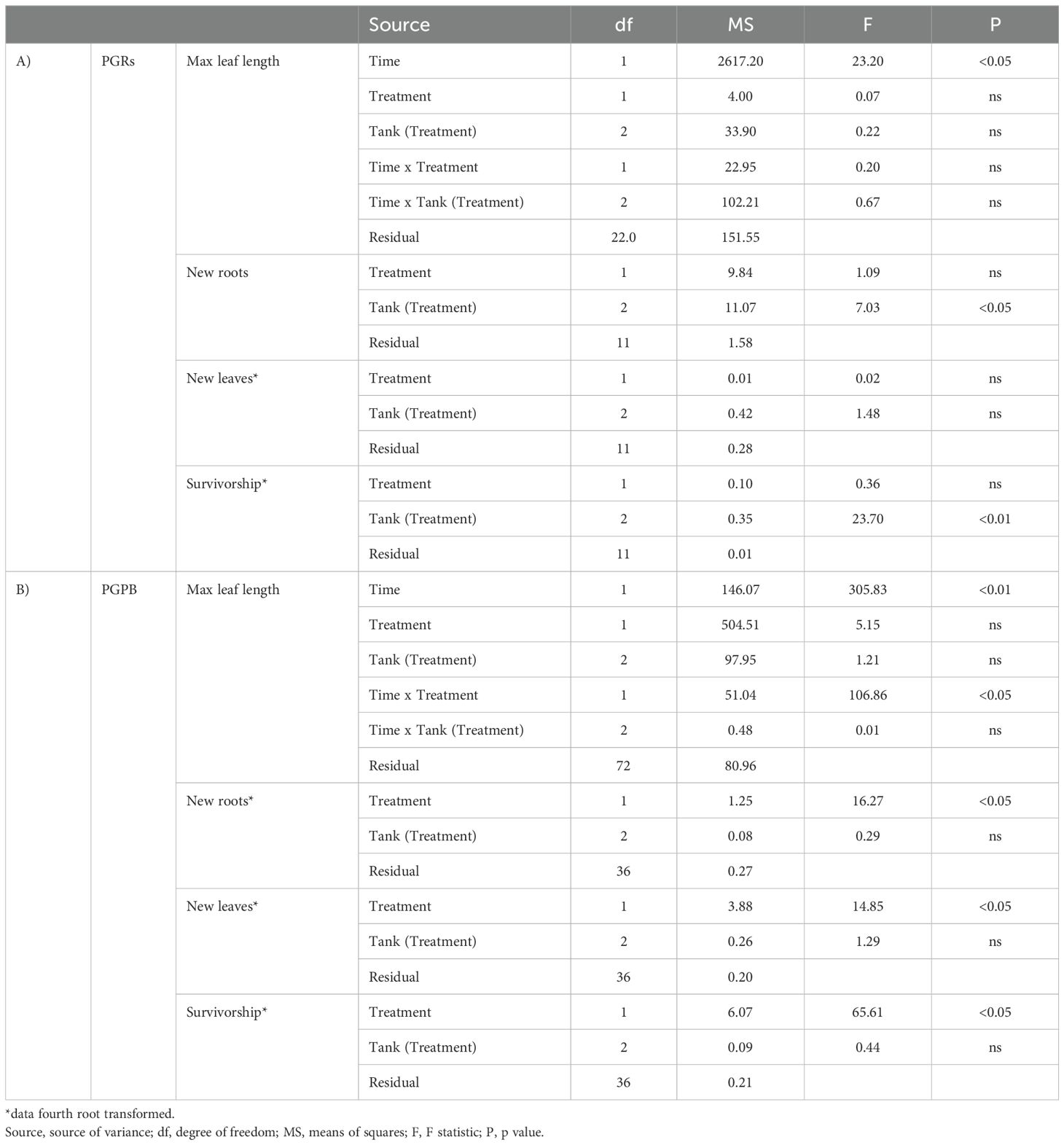
Table 3. Output of PERMANOVA conducted after PGRs (A) and PGPB (B) experiments, testing for differences between treatment (tanks) and times (for the max leaf length) or treatment (tanks) (for new root, new leaves, and survivorship).
4.1 PGRs treatment
Maximum leaf length - A significant effect of time was observed on the maximum leaf length (Table 2A). The maximum leaf length was significantly lower at the end of the experiment only in control fragments. However, the values were similar in the controls and treated fragments in the PGRs experiment, both at the beginning and the end of the experiment (i.e., after 6 weeks, Figure 3).
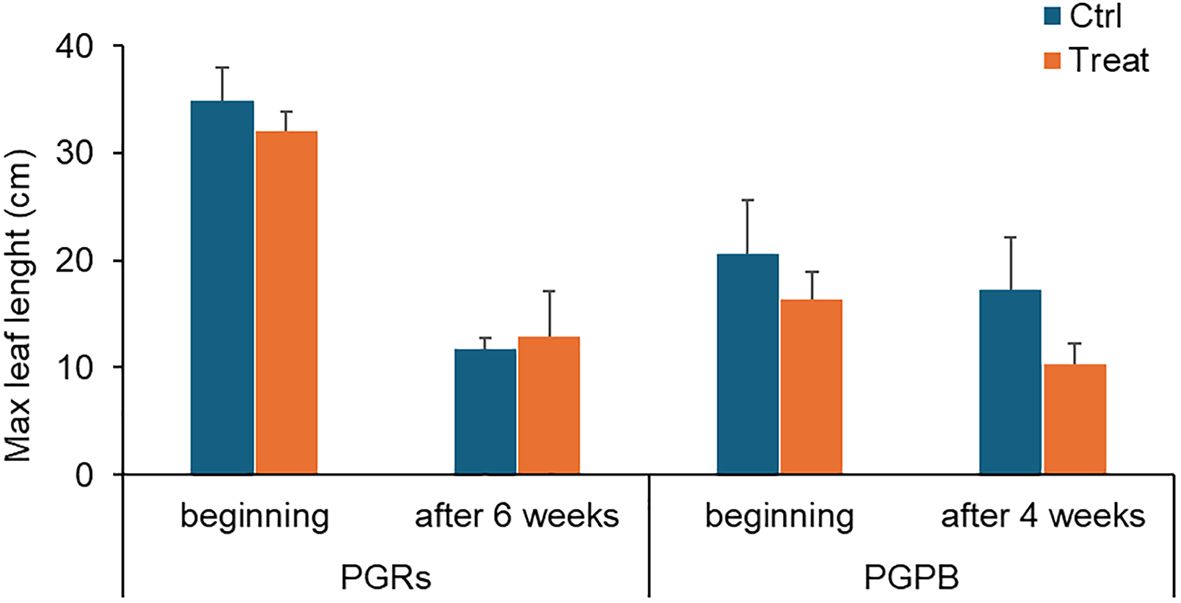
Figure 3. Max leaf length measured in fragments used as control (in light blue) and those exposed to PGRs or PGPB (in orange), at the beginning and after 6 and 4 weeks, respectively. Data (in cm) are reported as the average of values measured in the tanks ± standard error. Ctrl, control; Treat, treatment.
Formation of new roots and new leaves– The formation of new roots and new leaves was observed at the end of the experiment (after 6 weeks), both in control and treated fragments. However, no significant differences were observed between control and treated fragments, for both variables (Table 2A; Figures 4A, B). A significant effect of the factor tank was observed for new roots’ formation.
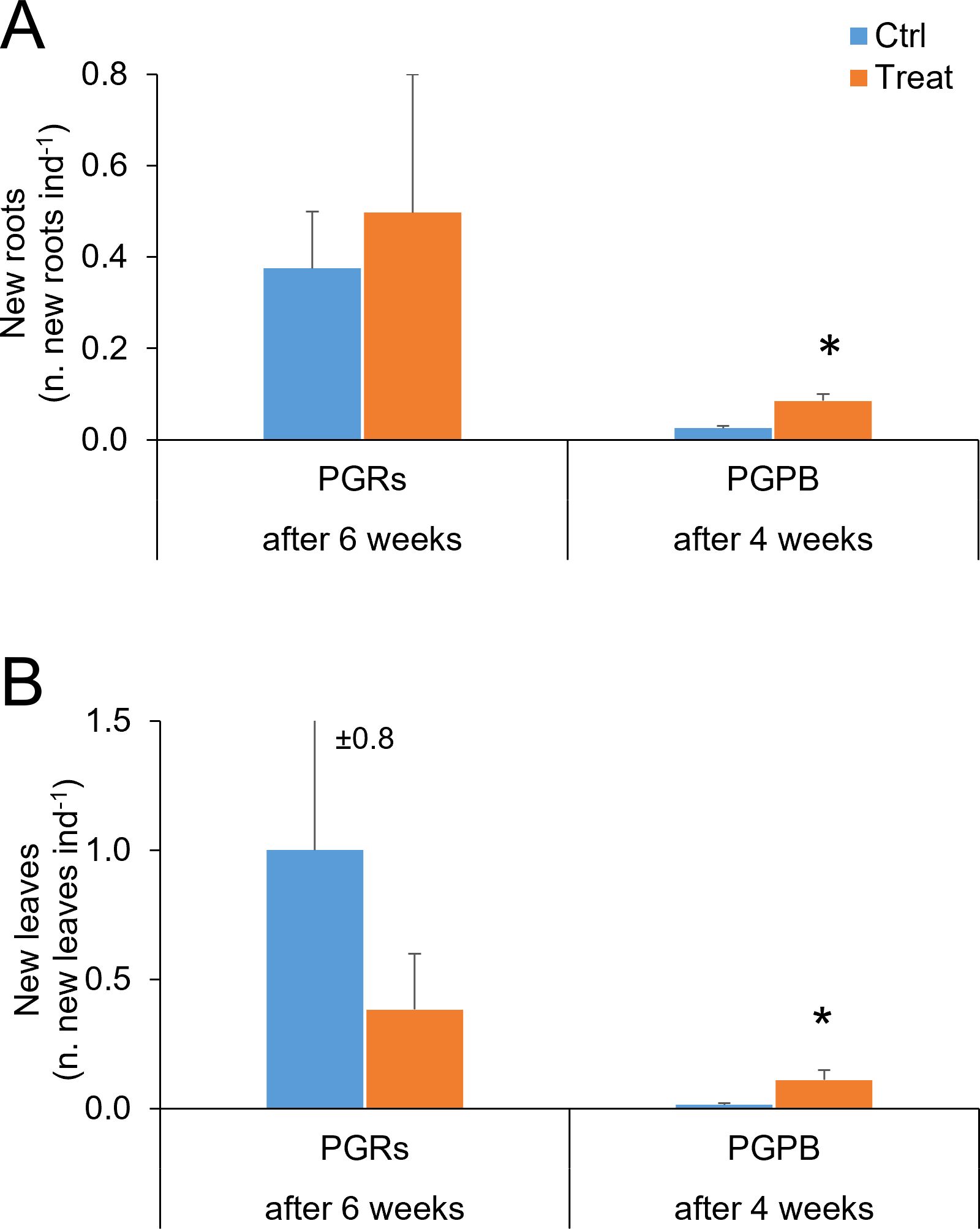
Figure 4. New roots (A) and new leaves (B) observed in fragments used as control (in light blue) and those exposed to PGRs or PGPB (in orange), after 6 and 4 weeks, respectively. Data (as number of new roots and leaves per fragment) are reported as the average of values measured in the tanks ± standard error. Ctrl, control; Treat, treatment; ind., individual (= fragment); *P < 0.05.
Survivorship – At the end of the experiment, in control and treated experimental units, the 100% of individuals showed survivorship (measured as new leaves or roots). However, no significant difference was observed comparing control and treated fragments (Table 2A; Figure 5). A significant effect of the factor “tank” was observed.
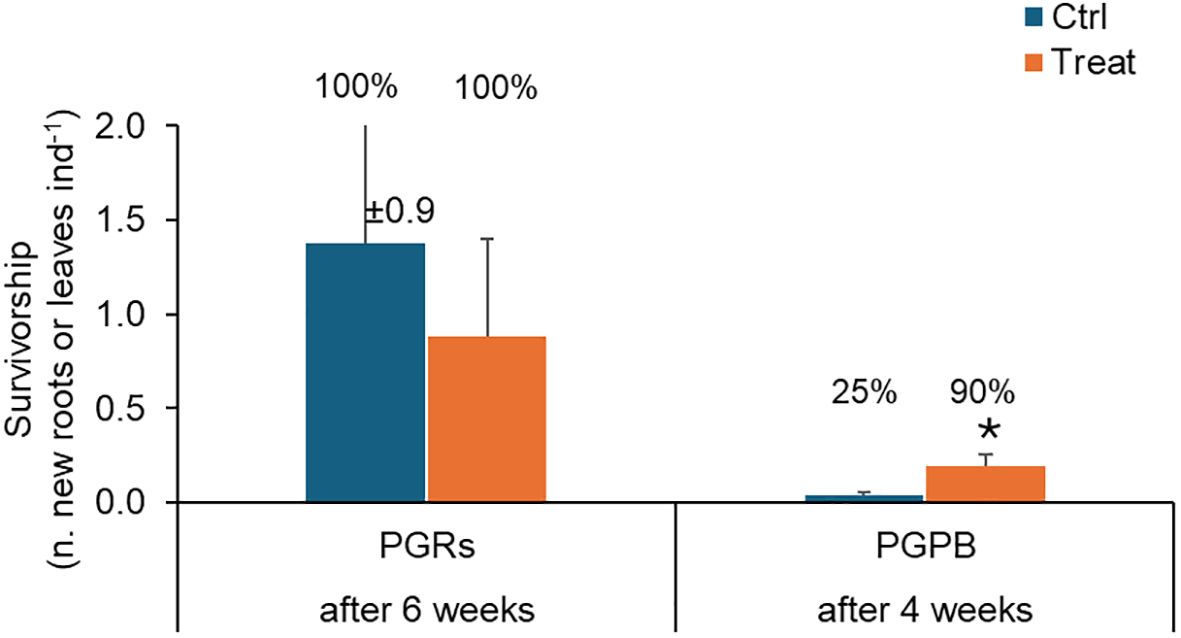
Figure 5. Survivorship observed in fragments used as control (in light blue) and those exposed to PGRs or PGPB (in orange), after 6 and 4 weeks, respectively. Data (as the number of new roots or leaves per fragment) are reported as the average of values measured in the tanks ± standard error. Reported are also the % of individuals developing new roots or leaves. Ctrl, control; Treat, treatment; ind., individual (= fragment); *P < 0.05.
4.2 PGPB treatment
Maximum leaf length – A significant effect of time and time x treatment was observed on the maximum leaf length (Table 2B; Figure 3). No significant differences were observed between the control and treated fragments.
Formation of new roots and new leaves – The formation of new roots and new leaves was observed at the end of the experiment (after 4 weeks). A significant difference was observed between control and treated fragments, with higher values observed in treated fragments, for both variables (Table 2B; Figures 4A, B).
Survivorship – Overall, in control and treatment experimental units, 25 and 90% of individuals showed survivorship (measured as new leaves or roots). A significant effect of the treatment on survivorship was observed (Table 2B), with higher values observed in treated fragments (Figure 5).
5 Discussion
Mediterranean seagrasses are crucially important species, most of which are protected by international conventions such as the Bern Convention, the SPAMI-Barcelona Convention, and the Action Plan for the Conservation of Marine Vegetation in the Mediterranean Sea. They are also part of the Habitat Directive (Curiel et al., 2021). Due to their role in blue carbon sequestration and the coastal ecosystem functioning, marine meadows’ restoration has been proposed as a tool for climate change mitigation (Orth et al., 2006; Marbà et al., 2014; United Nations Environment Agency, 2019; United Nations Environment Programme, 2019). To upscale these interventions, recent studies highlighted the importance of improving transplanting operations by reducing the cost and increasing intervention efficacy (Boudouresque et al., 2021). Moreover, the restoration intervention should be planned to avoid any possible damage to healthy populations. This can be done only by optimizing protocols, also considering phases implemented ex situ, and using laboratory facilities to improve the reproduction or cultivation of individuals used for the outplants or transplants at sea.
Compared to other Mediterranean species, C. nodosa offers the greatest chance for restoration success, due to its high tolerance to varying environmental conditions (Bellato et al., 1994; Rismondo et al., 1997; Sfriso and Ghetti, 1998). The formation of new roots allows the vegetative expansion of the plant, increasing the probability of permanent establishment of the new seagrass beds (Balestri and Lardicci, 2006). However, extreme environmental events such as storm surges, which have increased in intensity and frequency due to climate change, can cause the loss of large portions of natural seagrass meadows (Oprandi et al., 2020).
The implementation of growth-promoters has proven to be successful in stimulating the rooting capacity of seagrasses (Balestri and Bertini, 2003; Balestri and Lardicci, 2006). Our study showed that PGPB have a significantly positive effect on C. nodosa and its stranded fragments. This response allows more efficient use of stranded fragments of seagrasses for habitat restoration, a strategy successfully used for macroalgal forest restoration (Marletta et al., 2024). This would allow to use only some parts of the plants, avoiding using entire portion of the meadow for the restoration interventions.
The effects of these growth-promoters can depend on the molecule (PGPB or PGRs). Our results indicated survivorship (i.e., formation of new roots or leaves) both in control and treated fragments, suggesting that the cultivation conditions were optimal in the aquaria facility. However, only the PGPB growth-promoters had positive effects on the fragments’ survivorship and contributed significantly to the increase in the formation of new leaves and roots in C. nodosa, when compared with control fragments. Therefore, the future use of growth promoters needs to be previously tested, since different promoters (i.e., different molecules) could have a different (or null) effect on the plants’ growth. This is particularly important when planning a cost-effective restoration process, which includes an ex-situ phase. In the experiment with PGPB, the fragments had a higher survivorship when exposed to the growth-promoters (90%) than in the control ones (25%). This could be related to the ability of the plant to incorporate the PGPB through the leaves, with a process possibly catalyzed by nitrogen-fixing cyanobacteria (Kollmen and Strieth, 2022). Moreover, cyanobacteria can optimize the mineralization of organic compounds and nutrient availability (Tarquinio et al., 2019). In this regard, the potential role of microbiota for holobiont health and restoration efficacy has been recently highlighted (Corinaldesi et al., 2023).
Our study also shows that the application of growth-promoters (particularly PGPB) on the fragments increases their survival and the formation of new roots and leaves. The use of PGPB on C. nodosa fragments could allow the re-employment in restoration interventions, also when they are found broken or stranded along the beaches, without damaging the natural populations. This could be particularly important for restoration purposes, as already observed for macroalgae (Marletta et al., 2024). In the Mediterranean Sea, the probability of detached fragments returning back to the sea is very low, also due to the limited tide excursion, and generally, these fragments dry up on the beach (Balestri et al., 2011), but can be salvaged and used directly as a source for restoration/mitigation efforts (Balestri et al. (2011); Marletta et al., 2024) to promote habitat restoration.
The implementation of these growth-promoters, particularly PGPB, represents a yet unexplored field for marine plant research and it can offer a new way to improve seagrass health, and resilience, and increase restoration success (Tarquinio et al., 2019), reducing the time and costs of plant maintenance in mesocosms and ensuring long-term transplant success (Pansini et al., 2024) speeding up the process of roots and leaves’ formation (Balestri and Bertini, 2003). Nature-based solutions relying on microbiome analyses (and also through omics approaches) enable health monitoring of transplanted organisms/metacommunities and potential identification/production of probiotics/bio-promoters to stabilize unhealthy conditions of transplants (Corinaldesi et al., 2023). The use of microbes in ecosystem restoration is gaining increasing attention (Farrell et al., 2020; Watson et al., 2022). Microbe-assisted restoration has been implemented for different purposes in both terrestrial and aquatic environments (Robinson et al., 2023): plants’ and animals’ health (Gao et al., 2010; Contos et al., 2021; Birnbaum and Trevathan-Tackett, 2023), nutrient cycling (Garcia and Kao-Kniffin, 2018; Singh Rawat et al., 2023), drought stress tolerance (Ma et al., 2020; Sarabi and Arjmand-Ghajur, 2021), hormone production in plants and animals (Eichmann et al., 2021), climate regulation (Wu et al., 2022), pollination (Martin et al., 2022) and phytoremediation in degraded habitats (Haldar and Ghosh, 2020; Sarker et al., 2023; Agrawal et al., 2024). This study could contribute to the knowledge of new protocols for the conservation and restoration of seagrass meadows to reverse their loss and to optimize both the biodiversity and ecosystem services they provide (Possingham et al., 2015). This is an important target for the “UN Decade on Ecosystem Restoration” (Waltham et al., 2020) and the EU Biodiversity Strategy for 2030, aiming at restoring ecosystems across land and sea, especially those with considerable value in terms of goods and services such as seagrasses (Costanza et al., 2014; Vassallo et al., 2013).
Recent scientific advancements indicate that marine habitat restoration is feasible and should be upscaled, but it is constrained by: 1) the high costs when compared with terrestrial restoration and 2) the potential impact on source populations. This is particularly critical for habitat-forming species, a key targets of marine ecosystem restoration. Due to their successful application on terrestrial plants, growth promoters can be useful to enhance the recovery also of marine plants. In particular, we suggest the use of growth promoters in stranded plants, which would otherwise be lost. This phenomenon is expected to become more frequent as a consequence of climate change, or due to the increasing occurrence of storms. The use of the plant promoters tested here could make stranded organisms an important resource of viable material to be used in transplanting interventions. The set up and implementation of restoration methodologies are particularly important in the framework of the Nature Restoration Regulation recently approved by the European Union, which has binding restoration targets also for marine habitats (at least 30% of the EU’s land and sea areas by 2030, 60% by 2040 and 90% by 2050).
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
GM: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. DS: Data curation, Investigation, Methodology, Writing – review & editing. RD: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review & editing. SB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was conducted in the framework of the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.4-Call for tender No. 3138 of 16 December, 2021, rectified by Decree n.3175 of 18 December, 2021 of Italian Ministry of University and Research funded by the European Union-NextGenerationEU; Award Number: Project code CN_00000033, Concession Decree No. 1034 of 17 June, 2022 adopted by the Italian Ministry of University and Research, Project title “National Biodiversity Future Center-NBFC” and within the frame of the European Biodiversity Partnership: Biodiversa+ funded project entitled: “Innovative approaches FORESCUE and management of algal forests in the Mediterranean Sea FORESCUE”.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adhikari, T. B., Joseph, C. M., Yang, G., Phillips, D. A., and Nelson, L. M. (2001). Evaluation of bacteria isolated from rice for plant growth promotion and biological control of seedling disease of rice. Can. J. Microbiol 47, 916–924. doi: 10.1139/w01-097
Agrawal, K., Ruhil, T., Gupta, V. K., and Verma, P. (2024). Microbial assisted multifaceted amelioration processes of heavy-metal remediation: a clean perspective toward sustainable and greener future. Crit. Rev. Biotechnol. 44, 429–447. doi: 10.1080/07388551.2023.2170862
Alberto, F., Massa, S., Manent, P., Diaz-Almela, E., Arnaud-Haond, S., Duarte, C. M., et al. (2008). Genetic differentiation and secondary contact zone in the seagrass Cymodocea nodosa across the Mediterranean-Atlantic transition zone. J. Biogeography 35, 1270–1294. doi: 10.1111/j.1365-2699.2007.01876.x
Balestri, E. and Bertini, S. (2003). Growth and development of Posidonia oceanica seedlings treated with plant growth regulators: possible implications for meadow restoration. Aquat Bot. 76, 291–297. doi: 10.1016/S0304-3770(03)00074-3
Balestri, E. and Cinelli, F. (2001). Isolation and cell wall regeneration of protoplasts from Posidonia oceanica and Cymodocea nodosa. Aquat Bot. 70, 237–242. doi: 10.1016/S0304-3770(01)00157-7
Balestri, E. and Lardicci, C. (2006). Stimulation of root formation in Posidonia oceanica cuttings by application of auxins (NAA and IBA). Mar Biol. 149, 393–400. doi: 10.1007/s00227-005-0193-0
Balestri, E., Piazzi, L., and Cinelli, F. (1998). ). In vitro germination and seedling development of Posidonia oceanica (L.) Delile. Aquat Bot. 60, 83–93. doi: 10.1016/S0304-3770(97)00017-X
Balestri, E., Vallerini, F., and Lardicci, C. (2011). Storm-generated fragments of the seagrass Posidonia oceanica from beach wrack–A potential source of transplants for restoration. Biol. Conserv 144, 1644–1654. doi: 10.1016/j.biocon.2011.02.020
Bayraktarov, E., Saunders, M. I., Abdullah, S., Mills, M., Beher, J., Possingham, H. P., et al. (2016). The cost and feasibility of marine coastal restoration. Ecol. Appl. 26, 1055–1074. doi: 10.1890/15-1077
Beca-Carretero, P., Winters, G., Teichberg, M., Procaccini, G., Schneekloth, F., Zambrano, R. H., et al. (2024). Climate change and the presence of invasive species will threaten the persistence of the Mediterranean seagrass community. Sci. Total Environ. 910, 168675. doi: 10.1016/j.scitotenv.2023.168675
Bekkby, T., Papadopoulou, N., Fiorentino, D., McOwen, C. J., Rinde, E., Boström, C., et al. (2020). Habitat features and their influence on the restoration potential of marine habitats in Europe. Front. Mar Sci. 7. doi: 10.3389/fmars.2020.00184
Bellato, A., Rismondo, A., Curiel, D., and Marzocchi, M. (1994). Ritrovamento di frutti e semi in germinazione di Cymodocea nodosa (Ucria) Ascherson in Laguna di Venezia. Lav Soc. Ven Sc Nat. 20, 16.
Birnbaum, C. and Trevathan-Tackett, S. M. (2023). Aiding coastal wetland restoration via the belowground soil microbiome: an overview. Restor. Ecol. 31, e13824. doi: 10.1111/rec.13824
Bloemberg, G. V. and Lugtenberg, B. J. J. (2001). Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Curr. Opin. Plant Biol. 4, 343–350. doi: 10.1016/S1369-5266(00)00183-7
Boudouresque, C. F., Bernard, G., Pergent, G., Shili, A., and Verlaque, M. (2009). Regression of Mediterranean seagrasses caused by natural processes and anthropogenic disturbances and stress: A critical review. Bot. Mar 52, 395–418. doi: 10.1515/BOT.2009.057
Boudouresque, C. F., Blanfuné, A., Pergent, G., and Thibaut, T. (2021). ). Restoration of seagrass meadows in the mediterranean sea: A critical review of effectiveness and ethical issues. Water 13, 1034. doi: 10.3390/w13081034
Campbell, M. L. (2002). Getting the foundation right: a scientifically based management framework to aid in the planning and implementation of seagrass transplant efforts. Bull. Mar Sci. 71, 1405–1414.
Cebrian, E., Tamburello, L., Verdura, J., Guarnieri, G., Medrano, A., Linares, C., et al. (2021). A roadmap for the restoration of Mediterranean macroalgal forests. Front. Mar Sci. 8. doi: 10.3389/fmars.2021.709219
Contos, P., Wood, J. L., Murphy, N. P., and Gibb, H. (2021). Rewilding with invertebrates and microbes to restore ecosystems: Present trends and future directions. Ecol. Evol. 11, 7187–7200. doi: 10.1002/ece3.7597
Corinaldesi, C., Bianchelli, S., Candela, M., Dell’Anno, A., Gambi, C., Rastelli, E., et al. (2023). Microbiome-assisted restoration of degraded marine habitats: a new nature-based solution? Front. Mar Sci. 10. doi: 10.3389/fmars.2023.1227560
Costanza, R., d’Arge, R., De Groot, R., Farber, S., Grasso, M., Hannon, B., et al. (1997). The value of the world’s ecosystem services and natural capital. Nature 387, 253–260. doi: 10.1038/387253a0
Costanza, R., De Groot, R., Sutton, P., van der Ploeg, S., Anderson, J. S., Kubiszewski, I., et al. (2014). Changes in the global value of ecosystem services. Glob Environ. Chang 26, 152–158. doi: 10.1016/j.gloenvcha.2014.04.002
Crunkilton, D. D., Garret, H. E., and Pallardy, S. G. (1994). Growth and ectomycorrhizial development of northern red oak seedlings treated with IBA. Hortic. Sci. 29, 771–773. doi: 10.21273/HORTSCI.29.7.771
Cunha, A. and Araújo, A. (2009). New distribution limits of seagrass beds in West Africa. J. Biogeogr 36, 1621–1622. doi: 10.1111/j.1365-2699.2009.02135.x
Curiel, D., Kraljevíc Pavelíc, S., Kovačev, A., Miotti, C., and Rismondo, A. (2021). Marine seagrasses transplantation in confined and coastal adriatic environments: methods and results. Water 13, 2289. doi: 10.3390/w13162289
Danovaro, R., Nepote, E., Martire, M. L., Carugati, L., Da Ros, Z., Torsani, F., et al. (2020). Multiple declines and recoveries of Adriatic seagrass meadows over forty years of investigation. Mar Pollut. Bull. 161, 111804. doi: 10.1016/j.marpolbul.2020.111804
Da Ros, Z., Corinaldesi, C., Dell’Anno, A., Gambi, C., Torsani, F., and Danovaro, R. (2020). Restoration of Cymodocea nodosa seagrass meadows: Efficiency and ecological implications. Restor. Ecol. 29, e13313. doi: 10.1111/rec.13313
de los Santos, C. B., Krause-Jensen, D., Alcoverro, T., Marbà, N., Duarte, C. M., Van Katwijk, M. M., et al. (2019). Recent trend reversal for declining European seagrass meadows. Nat. Commun. 10, 3356. doi: 10.1038/s41467-019-11340-4
Duguma, B. (1988). Establishment of stakes of Gliricidia sepium (Jacq.) Walp and Leucaena leucocephala (Lam.) De Witt Vol. 6 (Yaounde, Cameroon: NFTA), 6–9.
Eichmann, R., Richards, L., and Schäfer, P. (2021). Hormones as go-betweens in plant microbiome assembly. Plant J. 105, 518–541. doi: 10.1111/tpj.15135
Farrell, H. L., Léger, A., Breed, M. F., and Gornish, E. S. (2020). Restoration, soil organisms, and soil processes: emerging approaches. Restor. Ecol. 28, S307–S310. doi: 10.1111/rec.13237
Gao, F. K., Dai, C. C., and Liu, X. Z. (2010). Mechanisms of fungal endophytes in plant protection against pathogens. Afr J. Microbiol Res. 4, 1346–1351.
Garcia, J. and Kao-Kniffin, J. (2018). Microbial group dynamics in plant rhizospheres and their implications on nutrient cycling. Front. Microbiol 9. doi: 10.3389/fmicb.2018.01516
Gerakaris, V., Lardi, P. I., and Issaris, Y. (2020). First record of the tropical seagrass species Halophila decipiens Ostenfeld in the Mediterranean Sea. Aquat Bot. 160, 103151. doi: 10.1016/j.aquabot.2019.103151
Haldar, S. and Ghosh, A. (2020). Microbial and plant-assisted heavy metal remediation in aquatic ecosystems: a comprehensive review. 3 Biotech. 10, 205. doi: 10.1007/s13205-020-02195-4
Katiresan, K. and Moorthy, P. (1994). Hormone-induced physiological responses of a tropical mangrove species. Bot. Mar 37, 139–141. doi: 10.1515/botm.1994.37.2.139
Kollmen, J. and Strieth, D. (2022). The beneficial effects of cyanobacterial co-culture on plant growth. Life 12, 223. doi: 10.3390/life12020223
Kumari, E., Kumari, S., Das, S. S., Mahapatra, M., and Sahoo, J. P. (2023). Plant growth-promoting bacteria (PGPB) for sustainable agriculture: current prospective and future challenges. AgroEnviron Sustain 1, 274–285. doi: 10.59983/s2023010309
Lepoint, G., Vangeluwe, D., Eisinger, M., Paster, M., van Treeck, P., Bouquegneau, J. M., et al. (2002). Nitrogen dynamics in Posidonia oceanica cuttings: implications for transplantation experiments. Mar Poll Bull. 48, 465–470. doi: 10.1016/j.marpolbul.2003.08.023
Litsi-Mizan, V., Efthymiadis, P. T., Gerakaris, V., Serrano, O., Tsapakis, M., and Apostolaki, E. T. (2023). Decline of seagrass (Posidonia oceanica) production over two decades in the face of warming of the Eastern Mediterranean Sea. New Phytol. 239, 2126–2137. doi: 10.1111/nph.19084
Loquès, F., Caye, G., and Meinesz, A. (1990). Axenic culture of selected tissue of Posidonia oceanica. Aquat Bot. 37, 171–188. doi: 10.1016/0304-3770(90)90090-8
Ma, Y., Dias, M. C., and Freitas, H. (2020). Drought and salinity stress responses and microbe-induced tolerance in plants. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.591911
Marbà, N., Díaz-Almela, E., and Duarte, C. M. (2014). Mediterranean seagrass (Posidonia oceanica) loss between 1842 and 2009. Biol. Conserv 176, 183–190. doi: 10.1016/j.biocon.2014.05.024
Marín-Guirao, L., Ruiz, J. M., Dattolo, E., Garcia-Munoz, R., and Procaccini, G. (2016). Physiological and molecular evidence of differential short-term heat tolerance in Mediterranean seagrasses. Sci. Rep. 6, 28615. doi: 10.1038/srep28615
Marín-Guirao, L., Sandoval-Gil, J. M., Ruíz, J. M., and Sánchez-Lizaso, J. L. (2011). Photosynthesis, growth and survival of the Mediterranean seagrass Posidonia oceanica in response to simulated salinity increases in a laboratory mesocosm system. Estuar Coast Shelf Sci. 92, 286–296. doi: 10.1016/j.ecss.2011.01.003
Marletta, G., Sacco, D., Danovaro, R., and Bianchelli, S. (2024). Stranded seaweeds (Gongolaria barbata): an opportunity for macroalgal forest restoration. Restor. Ecol. 32, e14134. doi: 10.1111/rec.14134
Martin, V. N., Schaeffer, R. N., and Fukami, T. (2022). Potential effects of nectar microbes on pollinator health. Philos. Trans. R Soc. B 377, 20210155. doi: 10.1098/rstb.2021.0155
Munoz, J. T. (1995). Effects of some plant growth regulators on the growth of the seagrass Cymodocea nodosa (Ucria) Ascherson. Aquat Bot. 51, 311–318. doi: 10.1016/0304-3770(95)00481-E
O’Callaghan, M. (2016). Microbial inoculation of seed for improved crop performance: issues and opportunities. Appl. Microbiol Biotechnol. 100, 5729–5746. doi: 10.1007/s00253-016-7590-9
Oprandi, A., Mucerino, L., de Leo, F., Bianchi, C. N., Morri, C., Azzola, A., et al. (2020). Effects of a severe storm on seagrass meadows. Sci. Total Environ. 748, 1–13. doi: 10.1016/j.scitotenv.2020.141373
Orth, R. J., Carruthers, T. J. B., Dennison, W. C., Duarte, C. M., Fourqurean, J. W., Heck, K. L., et al. (2006). A global crisis for seagrass ecosystems. Biosci 56, 987–996. doi: 10.1641/0006-3568(2006)56[987:AGCFSE]2.0.CO;2
Ortíz-Castro, R., Contreras-Cornejo, H. A., Macías-Rodríguez, L., and López-Bucio, J. (2009). The role of microbial signals in plant growth and development. Plant Signal Behav. 4, 701–712. doi: 10.4161/psb.4.8.9047
Paling, E. I., Fonseca, M., Van Katwijk, M. M., and Van Keulen, M. (2009). “Seagrass restoration,” in Coastal wetlands: an integrated ecosystem approach. Eds. Perillo, G., Wolanski, E., Cahoon, D., and Brinson, M. (Elsevier, Amsterdam, The Netherlands).
Pansini, A., Deroma, M., Guala, I., Monnier, B., Pergent-Martini, C., Piazzi, L., et al. (2024). The resilience of transplanted seagrass traits encourages detection of restoration success. J. Environ. Manage 357, 120744. doi: 10.1016/j.jenvman.2024.120744
Pavón-Salas, N., Herrera, R., Hernández-Guerra, A., and Haroun, R. (2000). Distribution pattern of seagrasses in the Canary Islands (Central-East Atlantic Ocean). J. Coast Res. 16, 329–335.
Pergent, G., Bazairi, H., Bianchi, C. N., Boudouresque, C. F., Buia, M. C., Calvo, S., et al. (2014). Climate change and Mediterranean seagrass meadows: a synopsis for environmental managers. Mediterr Mar Sci. 15, 462–473. doi: 10.12681/mms.621
Pergent, G., Boudouresque, C. F., Dumay, O., Pergent-Martini, C., and Wyllie-Echeverria, S. (2008). Competition between the invasive macrophyte Caulerpa taxifolia and the seagrass Posidonia oceanica: contrasting strategies. BMC Ecol. 8, 1–13. doi: 10.1186/1472-6785-8-20
Possingham, H. P., Bode, M., and Klein, C. J. (2015). Optimal conservation outcomes require both restoration and protection. PloS Biol. 13, 1–16. doi: 10.1371/journal.pbio.1002052
Reyes, J., Sansón, M., and Afonso-Carrillo, J. (1995). Distribution and reproductive phenology of the seagrass Cymodocea nodosa (Ucria) Ascherson in the Canary Islands. Aquat Bot. 50, 171–180. doi: 10.1016/0304-3770(95)00451-5
Rismondo, A., Curiel, D., Marzocchi, M., and Scattolin, M. (1997). Seasonal pattern of Cymodocea nodosa biomass and production in the lagoon of Venice. Aquat Bot. 58, 55–64. doi: 10.1016/S0304-3770(96)01116-3
Robinson, J. M., Hodgson, R., Krauss, S. L., Liddicoat, C., Malik, A. A., Martin, B. C., et al. (2023). Opportunities and challenges for microbiomics in ecosystem restoration. Trends Ecol. Evol. 38, 1189–1202. doi: 10.1016/j.tree.2023.07.009
Rodríguez-Prieto, C., Ballesteros, E., Boisset, F., and Afonso Carrilo, J. (2013). Guía de las macroalgas y fanerógamas marinas del Mediterráneo Occidental (Barcelona, Spain: Ediciones Omega).
Rossi, M., Borromeo, I., Capo, C., Glick, B. R., Del Gallo, M., Pietrini, F., et al. (2021). PGPB improve photosynthetic activity and tolerance to oxidative stress in brassica napus grown on salinized soils. Appl. Sci. 11, 11442. doi: 10.3390/app112311442
Ruiz, J. M., Boudouresque, C. F., and Enríquez, S. (2009). Mediterranean seagrasses. Bot. Mar 52, 369–382. doi: 10.1515/BOT.2009.058
Russo, R. O. and Berlyn, G. P. (1990). The use of organic biostimulants to help low input sustainable agriculture. J. Sustain. Agric. 1, 19–42. doi: 10.1300/J064v01n02_04
Sánchez-López, A. S., Pintelon, I., Stevens, V., Imperato, V., Timmermans, J. P., González-Chávez, C., et al. (2018). Seed endophyte microbiome of Crotalaria pumila unpeeled: identification of plant-beneficial methylobacteria. Int. J. Mol. Sci. 19, 291. doi: 10.3390/ijms19010291
Sarabi, V. and Arjmand-Ghajur, E. (2021). Exogenous plant growth regulators/plant growth promoting bacteria roles in mitigating water-deficit stress on chicory (Cichorium pumilum Jacq.) at a physiological level. Agric. Water Manag 245, 106439. doi: 10.1016/j.agwat.2020.106439
Sarker, A., Al Masud, M. A., Deepo, D. M., Das, K., Nandi, R., Ansary, M. W. R., et al. (2023). Biological and green remediation of heavy metal contaminated water and soils: A state-of-the-art review. Chemosphere 332, 138861. doi: 10.1016/j.chemosphere.2023.138861
Sfriso, A. and Ghetti, P. F. (1998). Seasonal variation in the biomass, morphometric parameters and production of rhizophytes in the lagoon of Venice. Aquat Bot. 61, 207–223. doi: 10.1016/S0304-3770(98)00064-3
Shili, A., Ben Maiz, N., Boudouresque, C. F., and Trabelsi, E. B. (2007). Abrupt changes in Potamogeton and Ruppia beds in a Mediterranean lagoon. Aquat Bot. 87, 181–188. doi: 10.1016/j.aquabot.2007.03.010
Sinclair, E. A., Sherman, C. D. H., Statton, J., Copeland, C., Matthews, A., Waycott, M., et al. (2021). Advances in approaches to seagrass restoration in Australia. Ecol. Manag Restor. 22, 10–21. doi: 10.1111/emr.12452
Singh Rawat, V., Kaur, J., Bhagwat, S., Arora Pandit, M., and Dogra Rawat, C. (2023). Deploying microbes as drivers and indicators in ecological restoration. Restor. Ecol. 31, e13688. doi: 10.1111/rec.13688
Small, C. C. and Degenhardt, D. (2018). Plant growth regulators for enhancing revegetation success in reclamation: A review. Ecol. Eng 118, 43–51. doi: 10.1016/j.ecoleng.2018.04.010
Smith, C. J., Verdura, J., Papadopoulou, N., Fraschetti, S., Cebrian, E., Fabbrizzi, E., et al. (2023). A decision-support framework for the restoration of Cystoseira sensu lato forests. Front. Mar Sci. 10. doi: 10.3389/fmars.2023.1159262
Stipcich, P., Marín-Guirao, L., Pansini, A., Pinna, F., Procaccini, G., Pusceddu, A., et al. (2022). Effects of current and future summer marine heat waves on Posidonia oceanica: plant origin matters? Front. Clim 4. doi: 10.3389/fclim.2022.844831
Swaminathaan, C. and Srinivasan, V. M. (1996). Seedling invigoration through plant growth substances in teak (Tectona grandis). J. Trop. For Sci. 8, 310–316.
Swamy, S. L., Puri, S., and Singh, A. K. (2002). Effect of auxins (IBA and NAA) and season on rooting of juvenile and mature hardwood cuttings of Robinia pseudoacacia and Grewia optiva. New For 23, 143–157. doi: 10.1023/A:1015653131706
Tarquinio, F., Hyndes, G. A., Laverock, B., Koenders, A., and Säwström, C. (2019). The seagrass holobiont: understanding seagrass-bacteria interactions and their role in seagrass ecosystem functioning. FEMS Microbiol Lett. 366, fnz057. doi: 10.1093/femsle/fnz057
United Nations Environment Agency (2019). “Resolution adopted by the general Assembly on 1 March 2019 73/284,” in United Nations Decade on Ecosystem Restoration, (2021-2030). New York, NY, USA.
United Nations Environment Programme (2019). A new deal for nature – restore the degraded planet. Available online at: https://www.unep.org/resources/policy-and-strategy/new-deal-nature (Accessed 18 June 2024).
Vassallo, P., Paoli, C., Rovere, A., Montefalcone, M., Morri, C., and Bianchi, C. N. (2013). The value of the seagrass Posidonia oceanica: a natural capital assessment. Mar Pollut. Bull. 75, 157–167. doi: 10.1016/j.marpolbul.2013.07.044
Waltham, N. J., Elliott, M., Lee, S. Y., Lovelock, C., Duarte, C. M., Buelow, C., et al. (2020). UN decade on ecosystem restoration 2021–2030—what chance for success in restoring coastal ecosystems? Front. Mar Sci. 7. doi: 10.3389/fmars.2020.00071
Watson, C. D., Gardner, M. G., Hodgson, R. J., Liddicoat, C., Peddle, S. D., and Breed, M. F. (2022). Global meta-analysis shows progress towards recovery of soil microbiota following revegetation. Biol. Conserv 272, 109592. doi: 10.1016/j.biocon.2022.109592
Waycott, M., Duarte, C. M., Carruthers, T. J., Orth, R. J., Dennison, W. C., Olyarnik, S., et al. (2009). Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. U.S.A. 106, 12377–12381. doi: 10.1073/pnas.090562010
Weyens, N., van der Lelie, D., Taghavi, S., Newman, L., and Vangronsveld, J. (2009). Exploiting plant–microbe partnerships to improve biomass production and remediation. Trends Biotechnol. 27, 591–598. doi: 10.1016/j.tibtech.2009.07.006
Winters, G., Beer, S., Willette, D. A., Viana, I. G., Chiquillo, K. L., Beca-Carretero, P., et al. (2020). The tropical seagrass Halophila stipulacea: Reviewing what we know from its native and invasive habitats, alongside identifying knowledge gaps. Front. Mar Sci. 7. doi: 10.3389/fmars.2020.00300
Keywords: habitat-forming species, seagrass, Mediterranean Sea, complementary actions, active restoration
Citation: Marletta G, Sacco D, Danovaro R and Bianchelli S (2025) Effectiveness of growth promoters for the seagrass (Cymodocea nodosa) restoration. Front. Plant Sci. 16:1507804. doi: 10.3389/fpls.2025.1507804
Received: 08 October 2024; Accepted: 12 May 2025;
Published: 03 June 2025.
Edited by:
Amrit Mishra, James Cook University, AustraliaReviewed by:
Sebastian Leuzinger, Auckland University of Technology, New ZealandIoannis-Dimosthenis S. Adamakis, National and Kapodistrian University of Athens, Greece
Copyright © 2025 Marletta, Sacco, Danovaro and Bianchelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Silvia Bianchelli, c2lsdmlhLmJpYW5jaGVsbGlAdW5pdnBtLml0
†These authors have contributed equally to this work
 Giuliana Marletta
Giuliana Marletta Domenico Sacco
Domenico Sacco Roberto Danovaro
Roberto Danovaro Silvia Bianchelli
Silvia Bianchelli