- 1Future Energy Center, School of Business, Society and Engineering, Mälardalen University, Västerås, Sweden
- 2Swedish Species Information Centre, Swedish University of Agricultural Sciences, Uppsala, Sweden
- 3Department of Soil and Environment, Swedish University of Agricultural Sciences, Uppsala, Sweden
- 4Department of Green Chemistry and Technology, Ghent University, Ghent, Belgium
With carbon dioxide (CO2) levels continuing to rise in the coming decades and threatening agro-ecosystems worldwide, it is crucial to understand the impact of elevated CO2 on global food production and security. Elevated CO2 levels have been found to reduce micronutrients such as Zinc (Zn) and Iron (Fe) in staple crops, potentially exacerbating the already existing global micronutrient deficiency issue. However, as vegetables serve as another key source of micronutrients, it remains uncertain to what extent this negative effect on micronutrient levels also applies to them. To address this, we investigated the effects of elevated CO2 on Zn and Fe in vegetables using a meta-analysis. As expected, we found a significant increase (27%, 95% CI: 14–41%) in vegetable biomass production under elevated CO2 levels. Elevated CO2 (i) significantly reduced overall Zn concentration in vegetables by 8.9% (95% CI: 4–14%), while this effect was pronounced only in fruit vegetables (11%), but not in leafy and stem vegetables; (ii) consistently exhibited minimal effects on Fe concentration in vegetables. In the context of climate change with rising CO2 levels, these findings suggest that elevated CO2 could potentially exacerbate Zn deficiencies through vegetable consumption, albeit with enhanced vegetable yields. Furthermore, as the global population increasingly adopts vegetarian diets in the future, these results underscore the need for mitigation strategies to address potential future micronutrient deficiencies.
1 Introduction
Atmospheric carbon dioxide (CO2) is projected to increase up to 550 ppm by the middle of the 21st century, nearly doubling the pre-industrial CO2 levels (Friedlingstein et al., 2023; Lan et al., 2024; Smith and Myers, 2018). Such increases in atmospheric CO2 concentrations have been reported to affect human nutrition by influencing global food production and altering nutrient concentrations in staple crops (Beach et al., 2019; Myers et al., 2017; Smith and Myers, 2019). An exemplification of this phenomenon is that several food crops under elevated CO2 levels have shown decreased mineral nutrient concentrations (Myers et al., 2015; Semba et al., 2022; Smith et al., 2017).
Globally, more than two billion people are deficient in micronutrients (Seal and Prudhon, 2007). Among the essential elements, micronutrients such as Zinc (Zn), Iron (Fe) and Selenium (Se) are particularly critical for humans due to their critical roles in numerous biological functions and human physical growth (Belay et al., 2021; Frise et al., 2022; Jones et al., 2017; Phiri et al., 2019). However, the reductions in Zn, Fe, and Se concentrations in plants induced by increased atmospheric CO2 levels may potentially accelerate micronutrient deficiencies for individuals who heavily depend on crops as their primary source of food. A meta-analysis encompassing 143 comparisons of edible portions of crops, including maize, rice, wheat, sorghum and field peas, revealed that elevated CO2 led to significant decreases in Fe and Zn concentrations across all crops except maize (Myers et al., 2014). It was estimated that an additional 175 million people in 2050 will face Zn deficiency and around 1.4 billion individuals are anticipated to experience a reduction of more than 4% in dietary Fe due to elevated CO2 levels (Smith and Myers, 2018). Similarly, Se concentrations also tended to decrease in rice and cucumber under elevated CO2 in research trials (Wang et al., 2023; Wei et al., 2021).
Aside from staple crop consumption, vegetables are highly recommended in daily diets due to their diverse range of beneficial compounds, such as vitamins, antioxidants, minerals, and dietary fiber (Dong et al., 2020). Globally, 1.2 billion tons of vegetables were produced in 2021 and the demand for vegetables is growing (FAO, 2022). Although numerous studies have shown changes in the essential nutrients Zn, Fe and Se in staple crops under elevated CO2, far less attention has been devoted to the effects of elevated CO2 concentration on vegetable growth and quality. Moreover, in climate-controlled vegetable cultivation, elevated CO2 has been widely adopted as an agricultural practice for enhancing plant growth (Dong et al., 2018a, 2020). Thus, understanding vegetable growth and nutrient status under elevated CO2 conditions is crucial for assessing the potential impacts of rising atmospheric CO2 concentrations on food security.
In general, increased CO2 concentrations tend to increase biomass production, but the effects of elevated CO2 on the nutrient status of vegetables are less well recognized due to the predominant focus on biomass enhancement. From experimental observations, the impact of impact of elevated CO2 on nutrients in vegetables varies: some experimental trials suggested that elevated CO2 levels could potentially reduce the Zn, Fe and Se in vegetables including sweet peppers, tomatoes and cucumbers (Dong et al., 2018c; Pinero et al., 2017; Wang et al., 2023), while other experiments showed different outcomes (Baslam et al., 2012; Dong et al., 2018b). This disparity is likely due to the heterogeneity among experimental setups and plant species. For instance, different CO2 enrichment facilities, such as free-air CO2 enrichment systems (FACE), open-top chambers (OTC) and controlled environmental conditions (CEC) have yielded varying results (Long et al., 2006; Taub et al., 2007; Wang et al., 2013). Besides, different plant species have exhibited varying responses to elevated CO2 conditions (Al-Hadeethi et al., 2019; Dong et al., 2020; Taub et al., 2007). As such, a systematic quantification of the effects of elevated CO2 on the Zn, Fe and Se in vegetables is needed. Previous similar research has pointed out a significant reduction in Zn and Fe, but with fewer observations (n=95 versus 51 for Zn; n=97 versus 49 for Fe) (Dong et al., 2018a). In contrast, another meta-analysis focused solely on biomass production without considering nutrient factors (Dong et al., 2020). Our objective was to systematically quantify the impacts of elevated CO2 concentrations on biomass and micronutrients (Fe, Zn and Se) in vegetables using a meta-analysis. We hypothesized that elevated CO2 concentrations would increase vegetable biomass production but decrease Zn, Fe and Se concentrations in vegetables.
2 Materials and methods
2.1 Database compilation
This meta-analysis is based on studies of the effects of elevated CO2 on the essential elements Zn, Fe and Se in common vegetables. An extensive keyword search was performed in the databases Web of Science, and the search engine Google Scholar. The keywords used were “carbon dioxide”, “CO2”, “Zn”, “Zinc”, “Fe”, “Iron”, “Se”, “Selenium”, “vegetable”, “salad” and the name of a specific vegetable was also employed as a keyword (search strings are listed in Supplementary Materials). The vegetables were classified as fruit vegetables, flowery vegetables, leafy vegetables, stem vegetables, and root vegetables. Fruit vegetables include bean, cucumber, eggplant, pea, pepper, squash and tomato. Flowery vegetables include artichoke, broccoli, cauliflower, and kale. Stem vegetables included celery and potato. Leafy vegetables include arugula, basil, cabbage, dill, endive, lettuce, onion, pakchoi, parsley, spinach and Swiss chard. Root vegetables include beet, carrot, radish, sweet potato and turnip. Pea or bean and potato were categorized as fruit vegetables and stem vegetables, respectively, as they are served as vegetables in certain countries (Gopalakrishnan, 2007; Pllana et al., 2018).
Predefined inclusion criteria were applied to determine the eligibility of studies for incorporation into the meta-analysis. First, the study must include experimental treatments (elevated CO2 concentrations at ≥550 and ≤ 1200 µmol mol-1) and controls (ambient CO2 concentrations at ≥200 and ≤ 450 µmol mol-1). When multiple elevated CO2 levels were investigated within the same study, only the outcomes from the elevated CO2 level of approximately double the ambient concentration were incorporated (Lam et al., 2012). Second, the study must present original research on the examination of vegetable biomass production, and Zn and/or Fe and/or Se concentrations in vegetables under elevated CO2 treatments. Third, the mean and sample size for experimental treatments and control groups must be reported.
The PRISMA flow chart is given in Supplementary Figure S1 in Supplementary Materials to present the screening and paper selection process. The final dataset contains 433 observations from 27 studies, with 95 observations for Zn, 97 observations for Fe, 3 observations for Se and 238 observations for biomass production. Additionally, to identify influencing factors and assess potential variation of CO2 impacts on biomass and nutrient status in vegetables, we collected and compiled information on the vegetable types, plant tissues, CO2 enrichment facilities and plant growth substrate. Selected studies of the meta-analysis were presented in Supplementary Materials.
2.2 Meta-analysis
Each effect size statistic was calculated as the log-transformed response ratio (LnRR) (Hedges et al., 1999).
Where and represents the mean values in the elevated CO2 treatments and control groups, respectively.
The variance (v) of each LnRR was calculated as:
Where v is the sampling variance, SD and n are the corresponding standard deviation and sampling size, respectively, and CV is the coefficient of variation.
If standard error (SE) instead of standard deviation (SD) were presented in studies, the transformation from SE to SD was performed utilizing the following mathematical equation:
Where n represents the sample size.
The weighting factor (w) was computed as:
The weighted response ratio (LnRR+) for all experiments was calculated as
Where wi and LnRRi are the w and LnRR from the ith study.
The 95% confidence interval (95%CI) for LnRR+ was computed as
The random effect model was employed to obtain the results described above with the “metafor” package in R v.4.3.2. Elevated CO2 effects were considered significant if the 95% confidence interval values did not overlap with zero. The effect sizes were transformed into percentages using the equation below to better illustrate the impacts of elevated CO2 addition:
For empirical papers that did not present standard deviations or statistics that allow the calculation of SD, a method called “All cases” addressing missing standard deviations (SDs) through an improved LnRR2 and a weighted average CV, estimated from studies that do report SDs in the dataset, was adopted as described by Nakagawa et al. (2023). Briefly, a weighted average of CVs within studies was first calculated when multiple effect sizes were reported in one study. The pooled average of CVs between studies was then computed for variance calculations and the variance was used to substitute cases that lack SDs.
The details and equations for the estimators for each effect size and variance can be found in the research of Nakagawa et al. (2023).
2.3 Statistical analysis
The title and abstract screening process was conducted using Covidence. Data from the selected studies were collected and extracted using WebPlotDigitizer software and directly from tables. We applied intercept-only multivariate meta-analysis models, setting ‘1|Observation’ as the random effect, to test whether the lnRRs significantly differed from 0. The data were subsequently categorized into subgroups based on vegetable type, plant tissue, CO2 enrichment facility, and plant growth substrate. For each subgroup, similar intercept-only multivariate meta-analysis models were applied to test whether their lnRRs significantly differed from 0. The meta-analysis and visualization of the results were conducted using the metafor package and ggplot package in R v.4.3.2.
3 Results
3.1 Overall effects of elevated CO2 on biomass and Zn, Fe and Se in vegetables
Overall, elevated CO2 enhanced vegetable biomass production significantly by 27% (95% CI: 14–41%) (Figure 1). However, Zn concentration in vegetables significantly decreased by 8.9% (95% CI: 4–14%; Figure 1) under elevated CO2. A similar trend was also found for Se concentrations in vegetables, with a significant 16% (95% CI: 0.5%–29%) reduction. However, this finding for Se should be interpreted with caution due to the limited number of studies available, which may affect the robustness of the estimated effect size. In contrast, no significant effect from elevated CO2 on Fe concentrations was detected.
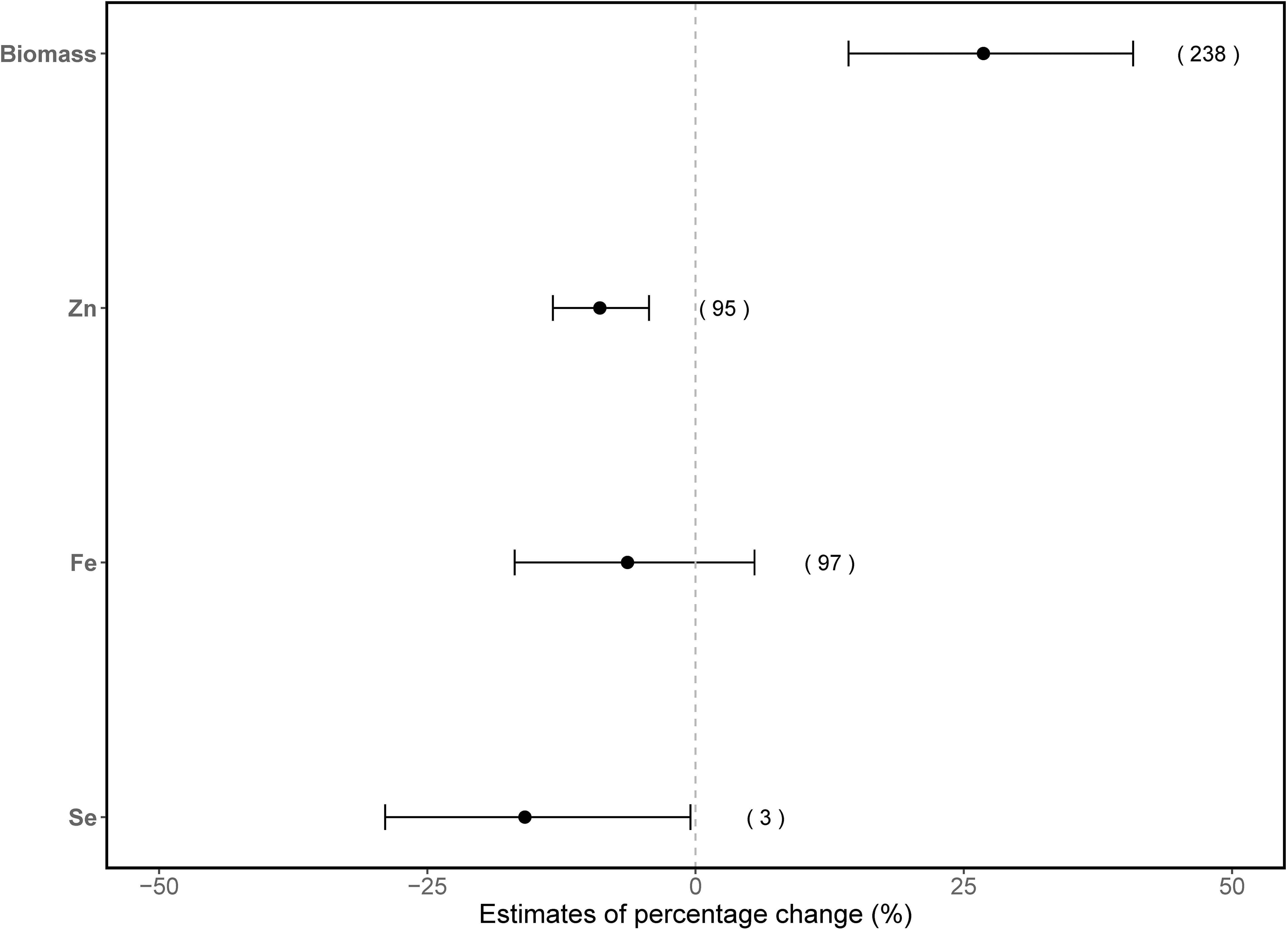
Figure 1. Overall effects of elevated CO2 on biomass production, and Zn, Fe and Se concentrations in vegetables. The x-axis values indicate estimates of percentage change with 95% confidence intervals. The numbers in parentheses represent the experimental observations of each respective indicator. Overlapping with the dashed line indicates no effect of elevated CO2.
3.2 Variation of elevated CO2 effects on vegetables from different subgroups
A consistent positive effect of elevated CO2 on biomass production was observed across different vegetable groups (Figure 2), with significant 19%, 35% and 51% increases in biomass production for fruit vegetables, leafy vegetables, and stem vegetables, respectively. The response of biomass to elevated CO2 exhibited variations based on the plant tissue classification. The increase in biomass production was 54%, 20%, 32% and 66%, respectively, for fruit, leaves, stems and tubers of vegetables. In contrast, vegetable root biomass did not exhibit any changes. Moreover, the impacts of CO2 varied depending on CO2 enrichment technologies applied in agricultural practices. The biomass production of vegetables grown in controlled environmental conditions (CEC) and open-top chambers (OTC) increased by 23% and 37%, respectively, under elevated CO2 conditions. However, this increase was not observed with vegetables grown under free-air CO2 enrichment (FACE) systems, suggesting that results from controlled environments may not fully capture plant responses under field conditions. Furthermore, CO2 had a consistently positive effect on vegetables grown using different substrates, with 44%, 38% and 20% increases when growing in field soils, hydroponic systems and pots, respectively.
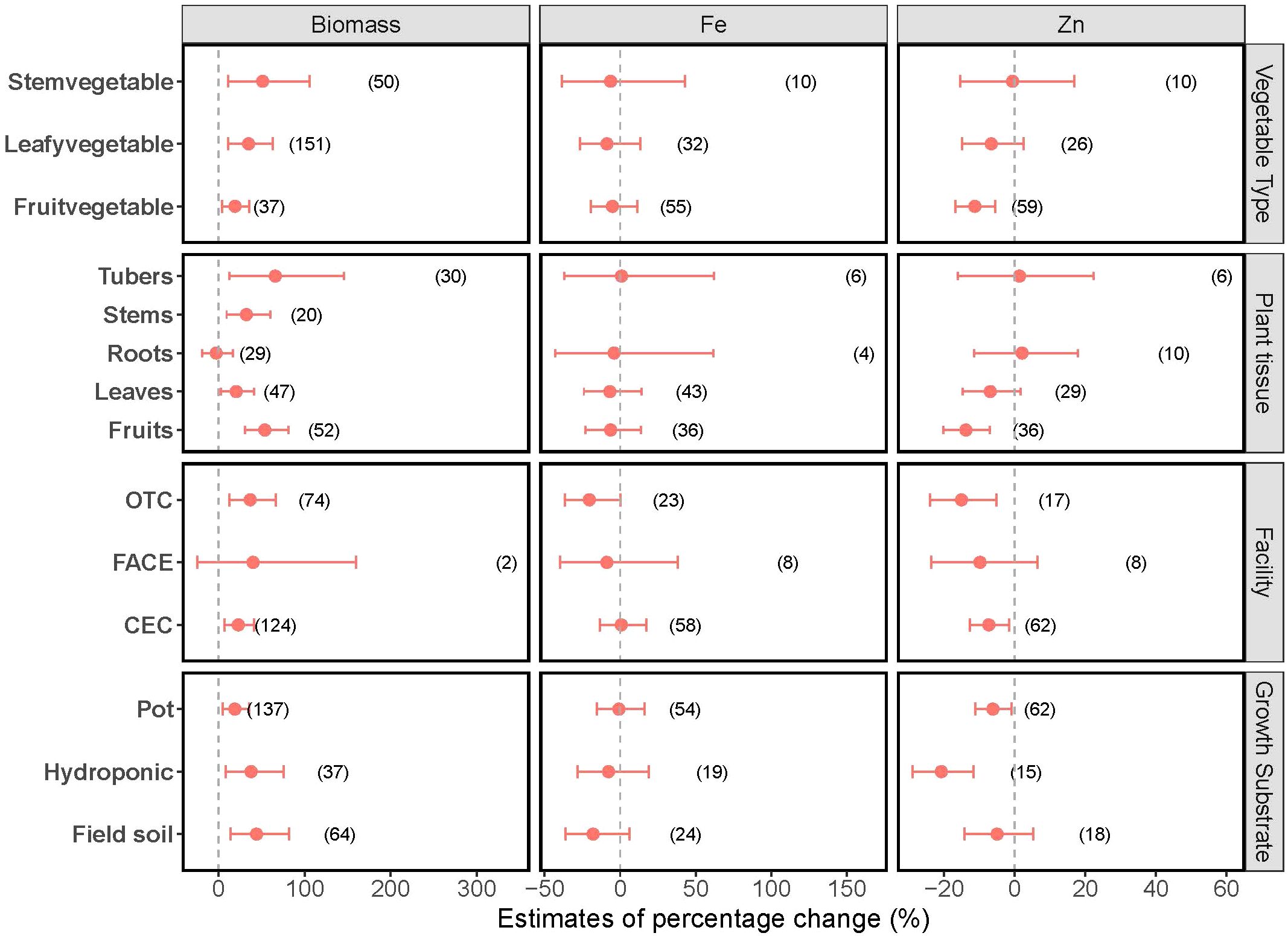
Figure 2. Variation in the effects of elevated CO2 on biomass production and Zn and Fe concentrations in vegetables across different subgroups. The x-axis values indicate estimates of percentage change with 95% confidence intervals. The numbers represent in parentheses the experimental observations of each respective indicator. Overlapping with the dashed line indicates no effect of elevated CO2.
While elevated CO2 generally led to a decrease in Zn concentrations in vegetables, the variations manifest differently within distinct subgroups (Figure 2). Elevated CO2 resulted in a significant decrease (11%) in Zn concentration in fruit vegetables, while Zn concentrations in leafy and stem vegetables appeared unaffected. Likewise, for the fruit of vegetables, increasing CO2 levels led to an evident 14% reduction in Zn concentrations, while no such effect was observed for other plant parts. In studies employing CO2 enrichment technologies including CEC and OTC, the elevated CO2 induced a notable reduction of 7.3% and 15% in Zn concentrations, respectively, while FACE exhibited minimal effects. Vegetables cultivated in both hydroponic systems and pots exhibited a pronounced negative impact from elevated CO2 on Zn, leading to a 21% and 6.2% reduction in Zn levels, respectively. However, these effects were not observed in vegetables grown in the field at elevated CO2 conditions.
No significant changes in Fe concentrations were observed in vegetables under the elevated CO2 condition, neither overall nor within any subgroups (Figure 2).
4 Discussions
4.1 Effects of elevated CO2 on vegetable biomass
Elevated CO2 increases (27%) vegetable biomass significantly (Figure 1), which is comparable with the results from a previous meta-analysis (Dong et al., 2020) as well as the response of staple crops biomass to elevated CO2, with wheat increased by 23% (Ainsworth, 2008), soybean by 37% (Ainsworth et al., 2002), barley by 24% (Gardi et al., 2022) and rice by 24% (Wang et al., 2015). There are two possible explanations for the positive effects of elevated CO2 on plant biomass. First, elevated CO2 increases photosynthetic efficiency by increasing photosynthetic rate while in the meantime reducing stomatal conductance (Seneweera and Norton, 2011). A meta-analysis of 12 large-scale FACE experiments revealed that elevated CO2 resulted in a 31% increase in the light-saturated photosynthetic rate for 40 species (Ainsworth and Long, 2005). More specifically, elevated CO2 can enhance the carboxylation rate of Ribulose-1,5-bisphosphate (RuBP) carboxylase/oxygenase (RuBisCO), which is an important enzyme in plants catalyzing the initial step in the net photosynthetic CO2 assimilation (Spreitzer and Salvucci, 2002). Although RuBisCO exhibits a high affinity for CO2, it is typically not saturated at current atmospheric CO2 levels in C3 plants as it also binds with oxygen to catalyze the oxygenation of RuBP. However, with increased CO2 concentrations, the carboxylation rate of RuBisCO can be augmented, as the competitive inhibition of oxygen on RuBisCO is alleviated (Ainsworth and Long, 2005). This leads to an increase in the rate of CO2 fixation and the decrease in photorespiration, thereby contributing to higher photosynthetic rates and increased biomass production (Allen, 1994; Long et al., 2006, 2004). Second, biomass increase can be associated with the cultivation environment (Dong et al., 2020). Vegetables cultivated in climate-controlled environment chambers or greenhouses usually benefit from optimal growing conditions such as warmer temperature, sufficient water and nutrients. Consequently, plants exhibited greater response in terms of photosynthesis and biomass production under suitable and stable environments when elevated CO2 is supplied (Dong et al., 2020; Long et al., 2004). This is in agreement with our results, where a significant biomass enhancement of 23% and 37% was observed in vegetables grown in well-controlled CEC and OTC systems, respectively, while no changes were found in FACE systems.
Overall, elevated CO2 has a consistent positive effect on biomass production across various subgroups, with a few exceptions such as the biomass from the FACE system (Figure 2). This result is in line with other studies. For instance, Long et al. (2006) revealed that the enhanced yield of crops was approximately 50% less in FACE studies than in enclosure studies. One possible explanation for this lack of response in biomass in the FACE system is the co-vary factors, such as variations in temperature and precipitation fluctuations and soil heterogeneity under field conditions, influencing photosynthetic rate and biomass (Reich et al., 2014). Thus, future studies should explore the interactive effects of elevated CO2 with other factors on biomass production. Controlled-environment studies can provide valuable mechanistic insights, however, incorporating FACE experiments will enhance the applicability of findings to real-world scenarios.
It is important to note that while elevated CO2 increased biomass production for fruit, leaves, stems, and tubers of vegetables significantly, it had limited impacts on vegetable root biomass (Figure 2). The observed increase in tuber biomass production aligns with the findings of Miglietta et al. (2002) who reported that rising CO2 levels significantly enhanced tuber yield of potatoes. This difference between tubers and roots can be attributed to their distinct functions. Tubers act as storage organs where excess carbon fixed during photosynthesis is deposited as starch (Turesson, 2014). Elevated CO2 enhances photosynthetic activity, leading to increased carbohydrate production, which is preferentially allocated to storage tissues like tubers, thus increasing the biomass production of tubers. The unchanged root biomass aligns with the limited effect of elevated CO2 on root biomass previously observed in grassland ecosystems (Arnone et al., 2000) and barley (Martín-Olmedo et al., 2002). This may be attributed to the increased water use efficiency via reduced stomatal conductance under elevated CO2, decreasing the demand for water supply and thereby mitigating the necessity for a larger root system (Phillips et al., 2005; Reich et al., 2014). However, this result does not align with the findings that elevated CO2 increases root production from grassland, forest and agriculture systems (Nie et al., 2013). This discrepancy might result from the short-term exposure to rising CO2 levels since vegetables have shorter growth cycles compared to other staple crops and tree species. Further research is needed to verify this explanation. Nevertheless, our result suggests that the effects of elevated CO2 primarily manifest in non-root tissues.
4.2 Effects of elevated CO2 on Zn, Fe and Se concentration in vegetables
Aligned with our expectation, elevated CO2 led to an average 8.9% decrease in Zn concentrations in vegetables (Figure 1). Rising atmospheric CO2 levels have been reported to decrease mineral concentrations in staple crops. For example, a meta-analysis of the response of diverse species to elevated CO2 has demonstrated that Zn concentration was decreased by 9.1% in wheat, 3.4% in rice, 5.6% in soybeans and 5.2% in corn, respectively (Al-Hadeethi et al., 2019). Additionally, meta-analyses focusing on individual species further corroborated these findings, with a 3.7% decrease in Zn concentration in rice (Hu et al., 2022) and 12% in wheat (Broberg et al., 2017). Likewise, with increasing CO2 levels, experimental studies have observed a decreased Zn concentration in vegetables, such as tomatoes (Khan et al., 2013), potatoes (Kumari and Agrawal, 2014) and cucumbers (Dong et al., 2018b). The mechanism behind the reduction in mineral contents such as Zn associated with increasing CO2 levels has not been fully elucidated (Myers et al., 2014; Soares et al., 2019). “Dilution effect” has been proposed to account for this phenomenon, wherein the increased carbohydrate production leads to a decrease in mineral concentration (Jarrell and Beverly, 1981; Myers et al., 2014). This appears to explain an overall 27% increase in biomass and an 8.9% decrease in Zn concentration in our study. Besides, resulting from the reduced stomatal conductance in response to the rising CO2 levels, plants tend to exhibit decreased transpiration. This reduction in transpiration could result in diminished mass flow, consequently leading to reduced nutrient uptake such as the uptake of Zn (Ben Mariem et al., 2021; Li et al., 2018; McGrath and Lobell, 2013).
Across all studies in the present analysis, elevated CO2 decreased Zn concentration in fruit vegetables but not in leafy vegetables and stem vegetables (Figure 2). When it comes to different tissues of vegetables, similarly, elevated CO2 decreased Zn concentration in fruit tissues rather than in other parts of vegetables. This may be ascribed to the slower re-translocation of Zn within plants via phloem under elevated CO2, making it less easily redistributed to fruits after root uptake (Olsen and Palmgren, 2014; Page and Feller, 2015; Ujiie et al., 2019). Besides, the biomass of fruits appears to be more sensitive to elevated CO2 concentrations, showing the highest biomass increase (55%, Figure 2) compared to other plant parts, which can be partially explained by the “dilution effects” in fruits.
CO2 enrichment facilities also impacted the response of Zn concentration in vegetables to elevated CO2. Cultivating OTC and CEC systems resulted in a greater decrease in Zn compared to the FACE system. This aligns with the increased biomass production in vegetables cultivated with OTC and CEC systems under elevated CO2, which might be again explained as the “dilution effect”. There are also two other possible explanations. First, the variations in weather conditions such as temperature and water fluctuations in FACE system might account for limited effects of elevated CO2 on Zn. This can be further supported by the observed larger decrease of Zn in vegetables cultivated in pot and hydroponic systems than in field soil conditions (Figure 2). In pot system, the volume of soil substrate available for root exploration is more limited compared to field-grown plants. This constraint can affect plant growth and nutrient uptake, as roots in field conditions can expand freely, accessing a larger volume of nutrients. Second, edge effects in OTC and CEC systems might influence the response due to the warmer conditions induced in these systems compared to the FACE system (Taub et al., 2007). Together with the greater biomass production and Zn decrease, elevated CO2 showed greater effects on vegetables in OTC and CEC systems compared to FACE.
Similar to Zn, Se concentration in vegetables exhibited a decreasing trend under elevated CO2. However, we should note that there was a limited number of comparative observations in this study and the result must be interpreted with care. Current research on the response of Se in plants to CO2 fertilization in different plant species is limited and inconsistent. For instance, elevated CO2 increased Se concentration by 30% in cucumbers when 0.5 mg Se L-1 was applied, while no significant changes were observed at lower Se doses (Wang et al., 2023). Similarly, in staple crops, Se concentration in rice was decreased under elevated CO2 (Wei et al., 2021), while other studies did not observe any changes in Se levels in wheat (Högy et al., 2013) and soybeans (Köhler et al., 2019). These discrepancies, along with the limited studies of elevated CO2 effects on Se in vegetables, underscore the need for further research to validate the observed trend and clarify the underlying mechanisms.
Against our expectation, there was no significant changes in Fe concentrations in vegetables under elevated CO2 conditions (Figures 1, 2). This finding differed from the meta-analysis by Dong et al. (2018a), who found a 16% reduction in Fe in vegetables with rising CO2 levels. This discrepancy can be attributed to the fewer comparison observations (n=49) included in their analysis compared with our work (n=97) since fewer observations might lead to a narrower data cope. The non-significant changes of Fe in vegetables observed in the present study also contrast with the findings reported for staple crops. For instance, in a meta-analysis, elevated CO2 conditions decreased around 4-6% of Fe content in staple crops (Al-Hadeethi et al., 2019). This variation in the Fe response might be attributed to the differences among the various species investigated. For example, Myers et al. (2014) also revealed that elevated CO2 was associated with significant decreases in Fe levels in wheat (5.5%), rice (7.3%), barley (10.5%) and soybean (4.1%), but no significant changes were observed in potato and sorghum. Nevertheless, the unchanged Fe level under increasing CO2 levels cannot be explained by the “dilution effect” theory. One potential explanation is that the change in Fe concentration in rising CO2 is smaller than the change in Zn. This stems from the distinct mass flow mechanisms governing the transport of these elements in plants, influenced by their differing solubilities. Compared to other micronutrients, such as calcium (Ca) and Zn, Fe is typically present at much lower concentrations in soil solution due to its low solubility, particularly under aerobic and alkaline conditions. As a result, Fe exhibits limited mobility via mass flow and its availability to plants is often constrained (McGrath and Lobell, 2013). Therefore, it is less likely influenced by the altered mass flow induced by elevated CO2. In addition, Fe availability in soil is affected by a complex interplay of factors, such as pH, organic matter, microorganisms and interactions with other nutrients (Colombo et al., 2013). For instance, higher pH in soil solutions reduces Fe availability. Elevated CO2 can further alter soil chemistry and microbial dynamics, potentially affecting nutrient cycling and availability (Blagodatskaya et al., 2010; Terrer et al., 2021). However, many studies on plant nutrition under elevated CO2 have not fully accounted for these soil-mediated processes influencing Fe availability. This gap highlights the need for more comprehensive research that integrates soil chemistry and plant physiology to better understand nutrient dynamics under elevated CO2 conditions. However, the above explanations may not apply to the situation with soilless cultivated vegetables, such as those grown in hydroponic systems, where Fe is assumed to be soluble and available in hydroponic solutions. Another possible explanation for the unchanged Fe concentration is the well-fertilized cultivation conditions in vegetables grown in hydroponic systems, where nitrogen and other nutrients are typically well-supplied, reducing nutrient limitations that could constrain Fe acquisition. For example, Fe concentration in wheat was not altered under medium nitrogen levels, while it significantly decreased under low nitrogen levels when exposed to elevated CO2 conditions compared to ambient CO2 levels (Al-Hadeethi et al., 2019). As an enzymatic cofactor of nitrogen metabolism (such as nitrite and nitrate reductase), Fe plays an important role in nitrogen assimilation in plants (Borlotti et al., 2012; Nasar et al., 2022). Thus, the well-supplied nitrogen may enhance plant health and the physiological requirements of Fe as an enzymatic cofactor, possibly altering Fe uptake in plants (McGrath and Lobell, 2013). Moreover, unlike soil-based cultivation, where nitrogen can influence Fe availability by affecting soil pH, hydroponic systems offer a controlled environment where pH is relatively stabilized, minimizing variability in Fe availability. Furthermore, due to its essential role in plant growth, plants tightly regulate Fe homeostasis and respond to both Fe deficiency and Fe overload (Morrissey and Guerinot, 2009). Thus, there may be regulations in vegetables regarding Fe changes induced by elevated CO2 levels to maintain Fe homeostasis, but such speculation requires further investigation.
Although changes in Zn, Fe, and Se concentrations under elevated CO2 are discussed, the studies included in this analysis did not account for the supply of these nutrients. It is important to note that nutrient distribution and transport in plants can be influenced by the levels of Zn and Fe supply, which may affect the observed nutrient concentrations. For instance, under sufficient Zn supply, Zn is primarily absorbed through root uptake. However, under Zn-deficient conditions, root uptake and Zn remobilization from the roots, stems, and leaves to seeds can occur, as observed in rice (Sperotto, 2013). Similarly, the supply range of Se affects its response to elevated CO2. For instance, Se concentration in cucumbers increased by 30% under elevated CO2 when 0.5 mg Se L-1 was applied, but no significant changes were observed at lower Se doses (Wang et al., 2023). The mechanisms underlying the effects of nutrient supply on plant responses to elevated CO2, however, remain to be explored.
4.3 Implications for nutrients deficiency and future work
Dietary deficiency of Zn, Fe and Se poses a significant global public health challenge. By 2050, an additional 175 million people were estimated to become Zn deficient due to the reduced Zn concentrations in staple crops as CO2 levels reach 550 ppm (Smith and Myers, 2018). From our analysis, elevated CO2 levels did not have a substantial impact on Fe concentration in vegetables. This finding suggests that elevated CO2 levels may not further exacerbate Fe deficiency stemming from vegetable consumption. However, a significant reduction (8.9%) in Zn has been observed in vegetables under rising atmospheric CO2 levels. This implies that rising CO2 levels have the potential to further exacerbate Zn deficiency related to vegetable consumption, particularly among the population who consume little animal flesh or animal-based products. Plant-based foods such as vegetables generally contain a lower Zn content compared to meat (Gibson, 2012) and the presence of inhibitors such as phytates will further impede Zn acquisition (Hunt, 2003). Thus, individuals who exclusively rely on plant-based food may face an increased risk of Zn deficiency under rising CO2 levels due to the reduced Zn concentration. For non-vegetarians, meat can be an important source of essential nutrients such as Zn, Fe and Se (Czerwonka and Tokarz, 2017; Gerber et al., 2009). For instance, animal-based food provides more than 50% of the Zn in adult diets in the United States, with beef alone contributing more than 25% of all Zn intake (Subar et al., 1998). Thus, individuals who can benefit from meat consumption may be less affected by the reduced Zn concentration in staple crops and vegetables under rising CO2 levels. However, meat consumption varies significantly from region to region. For instance, the highest levels for unprocessed red meat consumption range from 60 g to 91 g per day in Latin America and Europe, while the lowest levels range from 7 g to 34 g per day in Asia and Africa (Micha et al., 2015). Those with limited access to meat may still experience Zn deficiency due to reduced Zn concentrations in vegetables and staple crops under rising CO2 levels.
Se deficiency and its associated prevalence have been reported in many parts of the world, such as sub-Saharan Africa (Phiri et al., 2019), China (Chen, 2012) and Germany (Moghaddam et al., 2020), and advocating for improving the Se supply by dietary or supplemental measures has been suggested. Albeit limited observations, our results show elevated CO2 decreases Se concentrations significantly in vegetables. Therefore, under future climate change scenarios with rising CO2 levels, further research with a larger sample size is necessary to verify the trends observed.
Elevated CO2 showed greater effects on vegetables (higher biomass production and greater Zn decreases) cultivated in OTC and CEC systems compared to FACE. This disparity likely derives from the variation of other factors under field conditions. Moreover, given limited observations under field conditions, future field research that resembles realistic environmental conditions is needed. Besides, future work should also investigate long-term effects and consider other essential nutrients. Our current understanding is also hampered by the fact that the data available is that the available data is limited to C3 vegetables. Given the physiological differences between C3 and C4 plants in terms of carbon fixation pathways and response mechanisms to environmental conditions, it is essential to assess whether similar changes in nutrients observed in C3 plants also apply to C4 vegetables.
5 Conclusions
Our results show that elevated CO2 significantly enhanced biomass production in vegetables by 27%. However, it also led to an 8.9% reduction in Zn concentrations, while Fe concentrations in vegetables were not impacted. The severity of nutrient reductions in vegetables induced by elevated CO2 varied with vegetable types, micronutrients, and conditions of CO2 enrichment facilities. These findings suggest a risk of exacerbating Zn and Se deficiencies with the consumption of vegetables under elevated CO2 conditions, but it appears that this issue may not be exacerbated for Fe. The findings underscore the importance of considering the nutritional implications of climate change-induced alterations in vegetable composition, and the need to mitigate potential nutrient deficiencies in vegetables, thereby promoting global food security and human health.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. Data is made available in Figshare: https://doi.org/10.6084/m9.figshare.29370743.v1.
Author contributions
XW: Conceptualization, Formal analysis, Investigation, Methodology, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing. SZ: Methodology, Software, Formal analysis, Writing – review & editing, Visualization, Supervision. HL: Investigation, Methodology, Supervision, Writing – review & editing. GDL: Writing – review & editing. MO: Funding acquisition, Writing – review & editing. JS: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by The Knowledge Foundation (KK-stiftelsen) under the project Carbon2Food (grant number: 20220040, internal project number at Mälardalen University: 14977). SZ was supported by Formas (ID 2016-20114) and Skogssällskapet (2022-1000-453 Steg2).
Acknowledgments
This study was funded by The Knowledge Foundation (KK-stiftelsen) under the project Carbon2Food (grant number: 20220040, internal project number at Mälardalen University: 14977). SZ was supported by Formas (ID 2016-20114) and Skogssällskapet (2022-1000-Steg2). We also thank Prof. Xun Li for sharing their experimental data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1509102/full#supplementary-material
References
Ainsworth, E. A. (2008). Rice production in a changing climate: a meta-analysis of responses to elevated carbon dioxide and elevated ozone concentration. Global Change Biol. 14, 1642–1650. doi: 10.1111/j.1365-2486.2008.01594.x
Ainsworth, E. A., Davey, P. A., Bernacchi, C. J., Dermody, O. C., Heaton, E. A., Moore, D. J., et al. (2002). A meta-analysis of elevated [CO2] effects on soybean (Glycine max) physiology, growth and yield. Global Change Biol. 8, 695–709. doi: 10.1046/j.1365-2486.2002.00498.x
Ainsworth, E. A. and Long, S. P. (2005). What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 165, 351–371. doi: 10.1111/j.1469-8137.2004.01224.x
Al-Hadeethi, I., Li, Y., Odhafa, A. K. H., Al-Hadeethi, H., Seneweera, S., and Lam, S. K. (2019). Assessment of grain quality in terms of functional group response to elevated [CO2], water, and nitrogen using a meta-analysis: Grain protein, zinc, and iron under future climate. Ecol. Evol. 9, 7425–7437. doi: 10.1002/ece3.5210
Allen, L. H., Jr. (1994). Carbon dioxide increase: Direct impacts on crops and indirect effects mediated through anticipated climatic changes. Physiol. determ. Crop yield, 425–459. doi: 10.2134/1994.physiologyanddetermination.c29
Arnone, J. A., Zaller, J. G., Spehn, E. M., Niklaus, P. A., Wells, C. E., and Körner, C. (2000). Dynamics of root systems in native grasslands: effects of elevated atmospheric CO2. New Phytol. 147, 73–85. doi: 10.1046/j.1469-8137.2000.00685.x
Baslam, M., Garmendia, I., and Goicoechea, N. (2012). Elevated CO2 may impair the beneficial effect of arbuscular mycorrhizal fungi on the mineral and phytochemical quality of lettuce. Ann. Appl. Biol. 161, 180–191. doi: 10.1111/j.1744-7348.2012.00563.x
Beach, R. H., Sulser, T. B., Crimmins, A., Cenacchi, N., Cole, J., Fukagawa, N. K., et al. (2019). Combining the effects of increased atmospheric carbon dioxide on protein, iron, and zinc availability and projected climate change on global diets: a modelling study. Lancet Planet Health 3, e307–e317. doi: 10.1016/S2542-5196(19)30094-4
Belay, A., Gashu, D., Joy, E. J. M., Lark, R. M., Chagumaira, C., Likoswe, B. H., et al. (2021). Zinc deficiency is highly prevalent and spatially dependent over short distances in Ethiopia. Sci. Rep. 11, 6510. doi: 10.1038/s41598-021-85977-x
Ben Mariem, S., Soba, D., Zhou, B., Loladze, I., Morales, F., and Aranjuelo, I. (2021). Climate change, crop yields, and grain quality of C3 cereals: A meta-analysis of [CO2], temperature, and drought effects. Plants (Basel) 10. doi: 10.3390/plants10061052
Blagodatskaya, E., Blagodatsky, S., Dorodnikov, M., and Kuzyakov, Y. (2010). Elevated atmospheric CO2 increases microbial growth rates in soil: results of three CO2 enrichment experiments. Global Change Biol. 16, 836–848. doi: 10.1111/j.1365-2486.2009.02006.x
Borlotti, A., Vigani, G., and Zocchi, G. (2012). Iron deficiency affects nitrogen metabolism in cucumber (Cucumis sativus L.) plants. BMC Plant Biol. 12, 1–15. doi: 10.1186/1471-2229-12-189
Broberg, M., Högy, P., and Pleijel, H. (2017). CO2-induced changes in wheat grain composition: meta-analysis and response functions. Agronomy 7. doi: 10.3390/agronomy7020032
Chen, J. (2012). An original discovery: Selenium deficiency and keshan disease (an endemic heart disease). Asia Pac. J. Clin. Nutr. 21, 320–326. doi: 10.3316/ielapa.557152715796448
Colombo, C., Palumbo, G., He, J.-Z., Pinton, R., and Cesco, S. (2013). Review on iron availability in soil: interaction of Fe minerals, plants, and microbes. J. Soils Sed. 14, 538–548. doi: 10.1007/s11368-013-0814-z
Czerwonka, M. and Tokarz, A. (2017). Iron in red meat-friend or foe. Meat Sci. 123, 157–165. doi: 10.1016/j.meatsci.2016.09.012
Dong, J., Gruda, N., Lam, S. K., Li, X., and Duan, Z. (2018a). Effects of elevated CO2 on nutritional quality of vegetables: A review. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.00924
Dong, J., Gruda, N., Li, X., Tang, Y., Zhang, P., and Duan, Z. (2020). Sustainable vegetable production under changing climate: The impact of elevated CO2 on yield of vegetables and the interactions with environments-A review. J. Clean Prod 253. doi: 10.1016/j.jclepro.2019.119920
Dong, J., Li, X., Nazim, G., and Duan, Z.-Q. (2018b). Interactive effects of elevated carbon dioxide and nitrogen availability on fruit quality of cucumber (Cucumis sativus L.). J. Integr. Agric. 17, 2438–2446. doi: 10.1016/s2095-3119(18)62005-2
Dong, J., Xu, Q., Gruda, N., Chu, W., Li, X., and Duan, Z. (2018c). Elevated and super-elevated CO2 differ in their interactive effects with nitrogen availability on fruit yield and quality of cucumber. J. Sci. Food Agric. 98, 4509–4516. doi: 10.1002/jsfa.8976
FAO (2022). Agricultural production statistics 2000–2021. FAOSTAT Analytical Brief Series No. 60. doi: 10.4060/cc3751en
Friedlingstein, P., O’Sullivan, M., Jones, M. W., Andrew, R. M., Bakker, D. C. E., Hauck, J., et al. (2023). Global carbon budget 2023. Earth Sys. Sci. Data 15, 5301–5369. doi: 10.5194/essd-15-5301-2023
Frise, M. C., Holdsworth, D. A., Johnson, A. W., Chung, Y. J., Curtis, M. K., Cox, P. J., et al. (2022). Abnormal whole-body energy metabolism in iron-deficient humans despite preserved skeletal muscle oxidative phosphorylation. Sci. Rep. 12, 998. doi: 10.1038/s41598-021-03968-4
Gardi, M. W., Haussmann, B. I. G., Malik, W. A., and Högy, P. (2022). Effects of elevated atmospheric CO2 and its interaction with temperature and nitrogen on yield of barley (Hordeum vulgare L.): a meta-analysis. Plant Soil 475, 535–550. doi: 10.1007/s11104-022-05386-5
Gerber, N., Brogioli, R., Hattendorf, B., Scheeder, M. R., Wenk, C., and Gunther, D. (2009). Variability of selected trace elements of different meat cuts determined by ICP-MS and DRC-ICPMS. Animal 3, 166–172. doi: 10.1017/S1751731108003212
Gibson, R. S. (2012). A historical review of progress in the assessment of dietary zinc intake as an indicator of population zinc status. Adv. Nutr. 3, 772–782. doi: 10.3945/an.112.002287
Gopalakrishnan, T. R. (2007). Vegetable crops (No. 4). (New Delhi, India: New India publishing). Available online at: https://books.google.se/books?hl=en&lr=&id=-mTUBjSyo_UC&oi=fnd&pg=PA1&ots=hVDPiu7KZn&sig=xJxYJAbzAwQO867SHmiqxMmkHig&redir_esc=yv=onepage&q&f=false.
Hedges, L. V., Gurevitch, J., and Curtis, P. S. (1999). The meta-analysis of response ratios in experimental ecology. Ecology 80, 1150–1156. doi: 10.1890/0012-9658(1999)080[1150:Tmaorr]2.0.Co;2
Högy, P., Brunnbauer, M., Koehler, P., Schwadorf, K., Breuer, J., Franzaring, J., et al. (2013). Grain quality characteristics of spring wheat (Triticum aestivum) as affected by free-air CO2 enrichment. Environ. Exp. Bot. 88, 11–18. doi: 10.1016/j.envexpbot.2011.12.007
Hu, S., Tong, K., Chen, W., Wang, Y., Wang, Y., and Yang, L. (2022). Response of rice grain quality to elevated atmospheric CO2 concentration: A meta-analysis of 20-year FACE studies. Field Crops Res. 284. doi: 10.1016/j.fcr.2022.108562
Hunt, J. R. (2003). Bioavailability of iron, zinc, and other trace minerals from vegetarian diets. Am. J. Clin. Nutr. 78, 633S–639S. doi: 10.1093/ajcn/78.3.633S
Jarrell, W. M. and Beverly, R. B. (1981). The dilution effect in plant nutrition studies. Adv. Agron. 34, 197–224. doi: 10.1016/s0065-2113(08)60887-1
Jones, G. D., Droz, B., Greve, P., Gottschalk, P., Poffet, D., McGrath, S. P., et al. (2017). Selenium deficiency risk predicted to increase under future climate change. Proc. Natl. Acad. Sci. 114, 2848–2853. doi: 10.1073/pnas.1611576114
Khan, I., Azam, A., and Mahmood, A. (2013). The impact of enhanced atmospheric carbon dioxide on yield, proximate composition, elemental concentration, fatty acid and vitamin C contents of tomato (Lycopersicon esculentum). Environ. Monit. Assess. 185, 205–214. doi: 10.1007/s10661-012-2544-x.pdf
Köhler, I. H., Huber, S. C., Bernacchi, C. J., and Baxter, I. R. (2019). Increased temperatures may safeguard the nutritional quality of crops under future elevated CO2 concentrations. Plant J. 97, 872–886. doi: 10.1111/tpj.14166
Kumari, S. and Agrawal, M. (2014). Growth, yield and quality attributes of a tropical potato variety (Solanum tuberosum L. cv Kufri chandramukhi) under ambient and elevated carbon dioxide and ozone and their interactions. Ecotoxicol. Environ. Saf. 101, 146–156. doi: 10.1016/j.ecoenv.2013.12.021
Lam, S. K., Chen, D., Norton, R., Armstrong, R., and Mosier, A. R. (2012). Nitrogen dynamics in grain crop and legume pasture systems under elevated atmospheric carbon dioxide concentration: A meta-analysis. Global Change Biol. 18, 2853–2859. doi: 10.1111/j.1365-2486.2012.02758.x
Lan, X., Tans, P., and Thoning, K. W. (2024). Trends in globally-averaged CO2 determined from NOAA Global Monitoring Laboratory measurements. doi: 10.15138/9N0H-ZH07
Li, Y., Yu, Z., Jin, J., Zhang, Q., Wang, G., Liu, C., et al. (2018). Impact of elevated CO2 on seed quality of soybean at the fresh edible and mature stages. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.01413
Long, S. P., Ainsworth, E. A., Leakey, A. D., Nosberger, J., and Ort, D. R. (2006). Food for thought: lower-than-expected crop yield stimulation with rising CO2 concentrations. Science 312, 1918–1921. doi: 10.1126/science.1114722
Long, S. P., Ainsworth, E. A., Rogers, A., and Ort, D. R. (2004). Rising atmospheric carbon dioxide: plants FACE the future. Annu. Rev. Plant Biol. 55, 591–628. doi: 10.1146/annurev.arplant.55.031903.141610
Martín-Olmedo, P., Rees, R. M., and Grace, J. (2002). The influence of plants grown under elevated CO2 and N fertilization on soil nitrogen dynamics. Global Change Biol. 8, 643–657. doi: 10.1046/j.1365-2486.2002.00499.x
McGrath, J. M. and Lobell, D. B. (2013). Reduction of transpiration and altered nutrient allocation contribute to nutrient decline of crops grown in elevated CO2 concentrations. Plant Cell Environ. 36, 697–705. doi: 10.1111/pce.12007
Micha, R., Khatibzadeh, S., Shi, P., Andrews, K. G., Engell, R. E., Mozaffarian, D., et al. (2015). Global, regional and national consumption of major food groups in 1990 and 2010: a systematic analysis including 266 country-specific nutrition surveys worldwide. BMJ Open 5, e008705. doi: 10.1136/bmjopen-2015-008705
Miglietta, F., Magliulo, V., Bindi, M., Cerio, L., Vaccari, F. P., Loduca, V., et al. (2002). Free Air CO2 Enrichment of potato (Solanum tuberosum L.): development, growth and yield. Global Change Biol. 4, 163–172. doi: 10.1046/j.1365-2486.1998.00120.x
Moghaddam, A., Heller, R. A., Sun, Q., Seelig, J., Cherkezov, A., Seibert, L., et al. (2020). Selenium deficiency is associated with mortality risk from COVID-19. Nutrients 12. doi: 10.3390/nu12072098
Morrissey, J. and Guerinot, M. L. (2009). Iron uptake and transport in plants: the good, the bad, and the ionome. Chem. Rev. 109, 4553–4567. doi: 10.1021/cr900112r
Myers, S. S., Smith, M. R., Guth, S., Golden, C. D., Vaitla, B., Mueller, N. D., et al. (2017). Climate change and global food systems: potential impacts on food security and undernutrition. Annu. Rev. Public Health 38, 259–277. doi: 10.1146/annurev-publhealth-031816-044356
Myers, S. S., Wessells, K. R., Kloog, I., Zanobetti, A., and Schwartz, J. (2015). Effect of increased concentrations of atmospheric carbon dioxide on the global threat of zinc deficiency: a modelling study. Lancet Global Health 3, e639–e645. doi: 10.1016/S2214-109X(15)00093-5
Myers, S. S., Zanobetti, A., Kloog, I., Huybers, P., Leakey, A. D., Bloom, A. J., et al. (2014). Increasing CO2 threatens human nutrition. Nature 510, 139–142. doi: 10.1038/nature13179
Nakagawa, S., Noble, D. W. A., Lagisz, M., Spake, R., Viechtbauer, W., and Senior, A. M. (2023). A robust and readily implementable method for the meta-analysis of response ratios with and without missing standard deviations. Ecol. Lett. 26, 232–244. doi: 10.1111/ele.14144
Nasar, J., Wang, G. Y., Ahmad, S., Muhammad, I., Zeeshan, M., Gitari, H., et al. (2022). Nitrogen fertilization coupled with iron foliar application improves the photosynthetic characteristics, photosynthetic nitrogen use efficiency, and the related enzymes of maize crops under different planting patterns. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.988055
Nie, M., Lu, M., Bell, J., Raut, S., and Pendall, E. (2013). Altered root traits due to elevated CO2: a meta-analysis. Global Ecol. Biogeogr. 22, 1095–1105. doi: 10.1111/geb.12062
Olsen, L. I. and Palmgren, M. G. (2014). Many rivers to cross: the journey of zinc from soil to seed. Front. Plant Sci. 5. doi: 10.3389/fpls.2014.00030
Page, V. and Feller, U. (2015). Heavy metals in crop plants: transport and redistribution processes on the whole plant level. Agronomy 5, 447–463. doi: 10.3390/agronomy5030447
Phillips, D. L., Johnson, M. G., Tingey, D. T., Catricala, C. E., Hoyman, T. L., and Nowak, R. S. (2005). Effects of elevated CO2 on fine root dynamics in a Mojave Desert community: a FACE study. Global Change Biol. 12, 61–73. doi: 10.1111/j.1365-2486.2005.01085.x
Phiri, F. P., Ander, E. L., Bailey, E. H., Chilima, B., Chilimba, A. D. C., Gondwe, J., et al. (2019). The risk of selenium deficiency in Malawi is large and varies over multiple spatial scales. Sci. Rep. 9, 6566. doi: 10.1038/s41598-019-43013-z
Pinero, M. C., Otalora, G., Porras, M. E., Sanchez-Guerrero, M. C., Lorenzo, P., Medrano, E., et al. (2017). The form in which nitrogen is supplied affects the polyamines, amino acids, and mineral composition of sweet pepper fruit under an elevated CO2 concentration. J. Agric. Food Chem. 65, 711–717. doi: 10.1021/acs.jafc.6b04118
Pllana, M., Merovci, N., Jashari, M., Tmava, A., and Shaqiri, F. (2018). Potato market and consumption. Int. J. Sustain. Econ. Manage. 7, 19–29. doi: 10.4018/ijsem.2018070102
Reich, P. B., Hobbie, S. E., and Lee, T. D. (2014). Plant growth enhancement by elevated CO2 eliminated by joint water and nitrogen limitation. Nat. Geosci. 7, 920–924. doi: 10.1038/ngeo2284
Seal, A. J. and Prudhon, C. (2007). Assessing micronutrient deficiencies in emergencies: current practice and future directions (Geneva, Switzerland, WHO: UNS/Standing Committee on Nutrition).
Semba, R. D., Askari, S., Gibson, S., Bloem, M. W., and Kraemer, K. (2022). The potential impact of climate change on the micronutrient-rich food supply. Adv. Nutr. 13, 80–100. doi: 10.1093/advances/nmab104
Seneweera, S. and Norton, R. M. (2011). Plant responses to increased carbon dioxide. Crop adapt to Climate Change 7, 198–121.
Smith, M. R., Golden, C. D., and Myers, S. S. (2017). Potential rise in iron deficiency due to future anthropogenic carbon dioxide emissions. Geohealth 1, 248–257. doi: 10.1002/2016GH000018
Smith, M. R. and Myers, S. S. (2018). Impact of anthropogenic CO2 emissions on global human nutrition. Nat. Climate Change 8, 834–839. doi: 10.1038/s41558-018-0253-3
Smith, M. R. and Myers, S. S. (2019). Global health implications of nutrient changes in rice under high atmospheric carbon dioxide. Geohealth 3, 190–200. doi: 10.1029/2019GH000188
Soares, J., Deuchande, T., Valente, L. M. P., Pintado, M., and Vasconcelos, M. W. (2019). Growth and nutritional responses of bean and soybean genotypes to elevated CO2 in a controlled environment. Plants (Basel) 8. doi: 10.3390/plants8110465
Sperotto, R. A. (2013). Zn/Fe remobilization from vegetative tissues to rice seeds: should I stay or should I go? Ask Zn/Fe supply! Front. Plant Sci. 4. doi: 10.3389/fpls.2013.00464
Spreitzer, R. J. and Salvucci, M. E. (2002). Rubisco: structure, regulatory interactions, and possibilities for a better enzyme. Annu. Rev. Plant Biol. 53, 449–475. doi: 10.1146/annurev.arplant.53.100301.135233
Subar, A. F., Krebs-Smith, S. M., Cook, A., and Kahle, L. L. (1998). Dietary sources of nutrients among US adults 1989 to 1991. J. Am. Diet. Assoc. 98, 537–547. doi: 10.1016/S0002-8223(98)00122-9
Taub, D. R., Miller, B., and Allen, H. (2007). Effects of elevated CO2 on the protein concentration of food crops: a meta-analysis. Global Change Biol. 14, 565–575. doi: 10.1111/j.1365-2486.2007.01511.x
Terrer, C., Phillips, R. P., Hungate, B. A., Rosende, J., Pett-Ridge, J., Craig, M. E., et al. (2021). A trade-off between plant and soil carbon storage under elevated CO(2). Nature 591, 599–603. doi: 10.1038/s41586-021-03306-8
Turesson, H. (2014). Carbon Allocation in Underground Storage Organs (Uppsala, Sweden: Swedish University of Agricultural Sciences]).
Ujiie, K., Ishimaru, K., Hirotsu, N., Nagasaka, S., Miyakoshi, Y., Ota, M., et al. (2019). How elevated CO2 affects our nutrition in rice, and how we can deal with it. PloS One 14, e0212840. doi: 10.1371/journal.pone.0212840
Wang, H., Fan, H., Li, Y., Ge, C., and Yao, H. (2023). Elevated CO2 altered the nano-ZnO-induced influence on bacterial and fungal composition in tomato (Solanum lycopersicum L.) rhizosphere soils. Environ. Sci. pollut. Res. 30, 75894–75907. doi: 10.1007/s11356-023-27744-1
Wang, L., Feng, Z., and Schjoerring, J. K. (2013). Effects of elevated atmospheric CO2 on physiology and yield of wheat (Triticum aestivum L.): A meta-analytic test of current hypotheses. Agricult. Ecosyst. Environ. 178, 57–63. doi: 10.1016/j.agee.2013.06.013
Wang, Z., Li, D., Gruda, N. S., Zhu, C., Duan, Z., and Li, X. (2023). How to efficiently produce the selenium-enriched cucumber fruit with high yield and qualities via hydroponic cultivation? The balance between selenium supply and CO2 fertilization. Agronomy 13. doi: 10.3390/agronomy13030922
Wang, J., Wang, C., Chen, N., Xiong, Z., Wolfe, D., and Zou, J. (2015). Response of rice production to elevated CO2 and its interaction with rising temperature or nitrogen supply: a meta-analysis. Clim. Change 130, 529–543. doi: 10.1007/s10584-015-1374-6
Keywords: elevated CO2, Zn, Fe, Selenium (Se), vegetable, micronutrient deficiency, food security
Citation: Wang X, Zhang S, Li H, Du Laing G, Odlare M and Skvaril J (2025) Elevated CO2 decreases micronutrient Zn but not Fe in vegetables – evidence from a meta-analysis. Front. Plant Sci. 16:1509102. doi: 10.3389/fpls.2025.1509102
Received: 10 October 2024; Accepted: 10 June 2025;
Published: 04 July 2025.
Edited by:
Anoop Kumar Srivastava, Central Citrus Research Institute (ICAR), IndiaReviewed by:
Cristina Sgherri, University of Pisa, ItalyMarkus Weinmann, University of Hohenheim, Germany
Heiplanmi Rymbai, ICAR Research Complex for NEH Region, India
Copyright © 2025 Wang, Zhang, Li, Du Laing, Odlare and Skvaril. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shengmin Zhang, c2hlbmdtaW4uemhhbmdAc2x1LnNl
 Xiaolin Wang
Xiaolin Wang Shengmin Zhang
Shengmin Zhang Haichao Li3
Haichao Li3 Gijs Du Laing
Gijs Du Laing Jan Skvaril
Jan Skvaril