- 1Lab of Ornamental Plants, Shandong Jianzhu University, Jinan, China
- 2Wetland Research Institute, Shandong Academy of Forestry Sciences, Jinan, Shandong, China
- 3College of Forestry, Wildlife and Environment, Auburn University, Auburn, AL, United States
- 4Institute of Botany, Jinan Academy of Landscape and Forestry Sciences, Jinan, Shandong, China
Salinization is a major environmental challenge that jeopardizes productivity and resilience of plants such as the short rotation woody crops (SRWC) and bioenergy crops. Leveraging beneficial microbes will enhance plant resistance to salinity with physiological adjustments. Here we investigated the efficacy of plant growth promoting fungus (Piriformospora indica) on optimizing growth and salt tolerance of SRWCs and bioenergy tree crops, using Paulownia elongata as an example. Following culture in sterile soil, the chlamydospore of P. indica were found in paulownia plants roots. We treated both inoculated and uninoculated plants with four salt concentrations (0.00%, 0.30%,0.50%, 0.70%) by soaking them in varying concentrations of NaCl solution every 7 days. After 30 days of treatment, we investigated various physiological parameters, i.e., biomass, infection rate, growth rate, photosynthetic parameters, antioxidant enzyme activity, and soluble sugar of paulownia plants. Our two-way ANOVA demonstrated that the interaction between salinity stress and P. indica inoculation significantly enhanced plant height growth rate, leaf net photosynthetic rate, superoxide dismutase (SOD) activity, and soluble protein content in Paulownia seedlings. Inoculated plants exhibited improved salt tolerance due to the mitigating effect of symbiosis across a salinity gradient. Mortality in the P. indica-treated group was reduced by approximately 5.55%, 22.22%, and 27.77% under 0.30%, 0.50%, and 0.70% NaCl treatments. Our study is the first application of P. indica to enhance salinity tolerance in Paulownia, a short-rotation woody crop. Inoculating such endophyte significantly improves the resilience and productivity of Paulownia plantations in saline environments, for a sustainable afforestation effort.
1 Introduction
Soil salinization is a critical global ecological challenging, affecting over one billion ha2 of terrestrial land and leading to land degradation, declining agricultural productivity ecosystem functions, as well as long-term threats to food security and socio-economic stability (Negacz et al., 2022). Salt accumulation in soil increases rhizosphere osmotic pressure, disrupts the plant’s water retention, reduces water uptake capacity, and limits nutrient availability. Salinity stress inhibits plant height growth, causes tissue damage in leaves, and alters biomass accumulation and distribution, compromising overall plant health and productivity (Yadav et al., 2019; He et al., 2022; Ma et al., 2023).
Salinity stress can be divided into two parts: in the short term, elevated NaCl concentrations in the root zone impair water uptake by plants, resulting in osmotic stress and growth inhibition (Acosta-Motos et al., 2017). During the increase of salt stress time, excessive salt ions entering the transpiration flow of plants through roots are transported to branches and leaves, and inhibit photosynthesis occurs and ion toxicity, subsequently inhibiting plant growth (Parihar et al., 2015). In addition to osmotic stress and ion toxicity, salt stress also induces oxidative stress, resulting in membrane lipid peroxidation (Acosta-Motos et al., 2017). Plants eliminate oxygen free radicals through the antioxidant enzyme system, preventing oxidative damage to cells for survival in adverse environments. The antioxidant enzymes will be adjusted under the salinity mainly including superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT) (Muchate et al., 2016). The activities of SOD, POD and CAT are higher in the leaves of alfalfa (Medicago sativa) treated with mixed saline-alkali at seedling stage with the increase of mixed saline-alkali concentration and were higher than those of the control (Al-Farsi et al., 2020). Under certain levels of salinity conditions, the plant itself can eliminate the influence of adversity environment by increasing the activities of SOD, POD and CAT to improve the stress tolerance. However, with a gradual increase concentration of salinity, the activities of SOD, CAT and POD decreased, and the oxygen free radicals cannot be eliminated, resulting in the damage of cell membrane and finally the plant death (Zhang et al., 2013). Plants also alleviate the physiological damages due to salinity stress by regulating the content of endogenous osmotic substances (Wang et al., 2022; Feng et al., 2023). Soluble sugar content as an index reflects the tolerance of plants to salt stress (Saied et al., 2005). Previous studies showed that the level of reducing sugar while sucrose in mulberry (Morus alba) significantly increases at a 150 mM salt concentration (Liu et al., 2019).
Enhancing woody plants’ resilience is key for a sustainable development of bioeconomy (Seth, 2003) ecosystem conservation. Mitigating saline wastewater using short rotation woody crops (SRWC) has been tested and applied for phytoremediation (Dimitriou et al., 2006; Zalesny et al., 2007; Mirck and Zalesny, 2015; Pradana et al., 2023). Fast-growing Paulownia Clon in Vitro 112 is a new bio-energy crop in Europe for fuel production (Świechowski et al., 2019). Improving our knowledge of salt tolerance of short rotation woody crops (SRWC) is critical relative to most other economically important species. Such knowledge gap will slow down the broad deployment of SRWC especially at challenging sites.
In the early 19th century, paulownia (Paulownia elongata) was introduced as an ornamental tree species by the United States and some European countries from China (Snow, 2015). Later, some researchers found that its commercial value could be widely used in the production of various wood commodities such as musical instruments, sculptures, and furniture (Özelçam et al., 2021). Paulownia improves environmental quality through significant absorption of carbon dioxide, sulfur dioxide, and airborne particulate matter (Osmanović et al., 2017). Paulownia is also an excellent garden greening tree species with high economic, ecological, and social benefits because of its tall canopy, wide crown, diverse flower color and fragrant blooms. (Woźniak et al., 2022). found that Paulownia has immense potential in the transformation of abandoned land and the improvement of soil quality. It can increase soil enzyme activity, microbial biomass and microbial metabolic diversity, and is a bioenergy crop (Woźniak et al., 2022). Paulownia provides energy regeneration in short-term, which can be harvested and can regenerate every 3 to 5 years in about 25 years rotation (Abreu et al., 2022). Despite its economic promise, Paulownia’s cultivation is limited to non-saline soils, as its salt tolerance thresholds and physiological adaptations remain uncharacterized. The current research on paulownia emphasizes using paulownia wood (Jakubowski, 2022), breeding salt-tolerant varieties of paulownia (Ayala-Astorga and Alcaraz-Melendez, 2010), and improving the wood properties (Kaygin et al., 2009). The mechanisms underlying salt tolerance in Paulownia and strategies for its enhancement remain poorly understood. (Ivanova et al., 2019). investigated the effects of different salinity on the growth, leaf anatomical structure and gas exchange characteristics in two Paulownia hybrid lines (Paulownia tomentosa 9 fortunei-TF and Paulownia elongata 9 elongata-T4) by pot experiments and reported that compared with T4, the total dry biomass, leaf area, total leaf area/leaf number ratio and leaf K+/Na+ ratio of TF01 decreased more significantly under an elevated the soil salinity. Genetic research showed higher salt tolerance in polyploidization too (Deng et al., 2017; Cseri et al., 2020).
Piriformospora indica is an endophytic fungus obtained by Verma et al. in 1998 from the roots of shrubs in the Thar Desert, India (Varma et al., 1999). The endophytic fungus Piriformospora indica was selected for this study due to its proven efficacy in enhancing abiotic stress tolerance across diverse plant species; It can colonize the roots of diverse plant species, enhancing growth and improving stress tolerance in host plants under various abiotic stress conditions (Johnson et al., 2014), including drought (Boorboori and Zhang, 2022), salinity (Sabeem et al., 2022), waterlogging (Li et al., 2023), low temperature (Liang et al., 2022), heavy metal (Singhal et al., 2017) etc. The biomass of maize (Zea mays), tobacco (Nicotiana tabacum), soybean (Glycine max) significantly increased after colonization by P. indica compared to non-colonized control plants (Sharma, 2008). (Liang et al., 2023). demonstrated that the endophytic fungus P. indica enhances the waterlogging tolerance in peach (Prunus persica) seedlings by increasing the activity of key antioxidant enzymes (Liang et al., 2023) to enhance physiological resilience under anaerobic conditions. P. indica can also enhance the cold resistance in banana (Musa nana) by reducing malondialdehyde (MDA) content, promoting antioxidant enzyme activity via accumulating soluble sugar and increased proline content, up-regulating the expression of cold-responsive genes in banana leaves (Li et al., 2021); thus, these physiological adjustments collectively improve the plant’s cold adaptation. (Su et al., 2021). studied the effect of endophytic fungus Piriformospora indica on alleviating cadmium (Cd) stress in tobacco (Nicotiana tabacum) and found that P. indica enhanced the tolerance of tobacco to Cd by increasing Cd accumulation in tobacco roots, while reducing the Cd translocation and accumulation in leaves; thus the above-ground tissue has limited toxicity by root sequestration (Su et al., 2021). (Xu et al., 2017). showed that Piriformospora indica inoculation in maize enhanced drought tolerance by increasing antioxidant activity and up-regulating drought-related gene expression (Xu et al., 2017). Similarly, under salt stress, tomato plants inoculated with P. indica showed a significantly higher growth compared to the inoculated controls (Xu et al., 2022). P. indica has been shown to increase the absorption of K+ in tomatoes, thereby inhibiting Na+ accumulation, and mitigating salinity-damage of plants by improving the photosynthetic parameters (Ghorbani et al., 2018). P. indica could promote the growth of Arabidopsis thaliana under salt stress, potentially through up-regulating the expression of major Na+ and K+ ion channels (Abdelaziz et al., 2017). Although P. indica has a beneficial effect on a variety of herbaceous plants, there is a lack of studies on tree species.
Here we aimed to investigate the salt tolerance of paulownia mediated by P. indica under a gradient of concentrations, i.e., 0.00%, 0.30%, 0.50%, 0.70%. We assessed the growth and physiological performance of Paulownia seedlings and P. indica to analyze the growth-promoting and stress-resistant effects of P. Indica on Paulownia under NaCl stress as follows after establishing the symbiotic system of P. indica and Paulownia seedlings (1), growth of Paulownia seedlings (2), the antioxidant enzyme activity (e.g., SOD, POD, and CAT) of Paulownia seedlings, (3) osmotic adjustment substances (i.e., soluble sugar) in Paulownia seedlings, (4) photosynthetic rate of Paulownia seedling.
The causes of changes in growth and physiological and biochemical indexes of Paulownia seedlings were evaluated. We hypothesized that P. indica inoculation enhances salt tolerance in Paulownia by modulating antioxidant enzyme activity, osmotic adjustment, and photosynthetic efficiency.
2 Materials and methods
2.1 Plant material and growth conditions
The paulownia (Paulownia elongata) seeds were purchased from Shuyang Green Forest Greening Engineering (Jiangsu China). Plump seeds were selected and rinsed with deionized water. The seeds were disinfected with 75% alcohol for 30s, rinsed 3 times with deionized water, followed by 1% sodium hypochlorite for 10 min, finally rinsed with deionized water several times. The sterilized seeds were spread in petri dishes (d=90mm) covered with wet filter papers. The germinated seeds were sown into substrate (121 °C, 0.1 MPa, 2 h) peat soil (Baltic States) and perlite (Xinyang Guotong Electronic Commerce Company, Henan, China) mixture (4:1, by volume) in sterilized containers (70×67×55 mm, Jiangsu, China). Three plants are planted per container. The plants were grown in a constant environment in the greenhouse, with a temperature of 25 ± 5 °C, humidity of 50 ± 10%, and a photoperiod of 14 hours/day. The plants were fertilized weekly with Merlot nutrient solution (N:P:K=30:14:16, Shanghai Yingxi Company, Shanghai, China). Plants with similar size were selected and watered twice a week and one plant was tested per container before treatments.
2.2 Culture of fungi
The fungi from the Tree Stress Biology Laboratory of Alberta, Canada, were propagated in the Landscape Architecture Laboratory of Shandong Jianzhu University. Fungi were transferred to the liquid modified melin-norkrans medium (MMN) after cultured on the potato dextrose agar (PDA) medium solidified for 10 d in the dark at room temperature (25 ± 5 °C). After 7 days of shaking culture at a temperature of 26°C and a rotation speed of 150 r·min-1, the liquid fungi filtered and washed with deionized water for 3 times. PDA medium was prepared according to the manufacturer’s instructions (Hope Bio-Technology Co., Ltd, Shandong, China), and 25 g was dissolved in 1 L of distilled water. The medium was sterilized in autoclave for 20 min at 121°C.
2.3 Salinity experimental setup, fungal inoculation and treatment
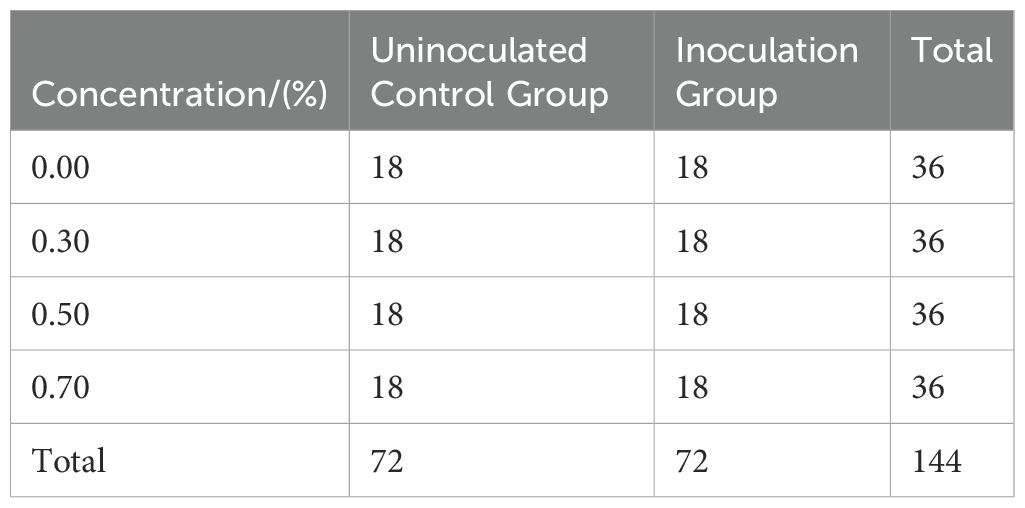
A two-factor (NaCl, P. indica) complete randomized design (CRD) was employed for the study. The first factor, the NaCl treatment group, included a salinity solution gradient of 0.00%, 0.30%, 0.50%, and 0.70% (0.30% represents moderate salinity; 0.50–0.70%, mimics severely saline wastelands targeted for afforestation). The second factor was the treatment group of P. indica, including the control group (CK) without P. indica inoculation and the inoculation group (P) with P. indica inoculation. There were six plants in each treatment group, and each group was repeated 3 times. Each treatment was repeated 18 times in total among 144 individual containers (one plant/container), randomized across treatments (details in Table 1). All containers were clockwise exchanged every 7 days during salinity treatment, systematically changing their position relative to the light source and ventilation to balance the micro-environmental effects.
After two weeks of growth in liquid medium, the fungi were filtered and washed with autoclaved deionized water. The fungi were then homogenized in a blender and suspended in autoclaved water to reach the mycelial concentration of 50 (± 5) g·L-1. Fungal inoculum was injected into the inoculated soil of plant roots by needle tubes (Shuguang Huizhikang Biotechnology, Henan, China) with 40 ml applied per container, repeated twice in plastic containers totally.
After 7-day fungal inoculation, the inoculated and uninoculated paulownia seedlings were immersed in the corresponding NaCl solution for 1h for salinity stress treatment. All paulownia seedlings were subjected to saline immersion (30mm from the top of the pot) at 7-day intervals, and the 0.00% NaCl treatments were immersed in an equal volume of tap water under a 30-day exposure to NaCl stress. The corresponding concentration of salt water was supplemented regularly to keep the soil water content in the range of 50.00%-60.00%.
2.4 P. indica colonization inspection
At the last treatment of salt stress, four plant capillary roots (1 cm) from each treatment (40 in total) were fixed in 70% alcohol FAA (Servicebio, Hubei, China). The roots were rinsed twice with deionized water to remove the FAA from the surface. The roots were cut into about 1 cm segments and fixed on a microscope slide with ten segments randomly as a group for observation under microscope (Olympus Corporation, Tokyo, Japan) (Trouvelot et al., 1986). The colonization degree (D%) of paulownia seedlings was examined by quantifying the chlamydospore of P. indica in the roots as follows
where I denotes the number of infected mycorrhizal root segments; T denotes total number of examined root segments.
2.5 Plant mortality, biomasses, and plant growth rate
Plant mortality in each treatment was recorded following the experiment. At the end of salinity stress, the plants were harvested and weighed for fresh weight for the above- and below-ground parts. After carefully washing the root soil, the above-ground and below-ground parts were separated, and the fresh weight of the above-ground and below-ground parts of each plant was separated and weighed, respectively. Then they were put into paper bags and baked at 105 °C for 30 min, and then baked at 80 °C until constant weight, and then the dry weight of the above-ground and below-ground part was weighed respectively. Due to the inconsistent initial height, we applied the adjusted growth rate to evaluate the growth dynamics. For height growth rate of paulownia plants (R), we measured the plant height at the beginning and end of the salinity stress. The plant height was defined the distance from the soil to the highest branch as follows,
where E is the plant height at the end of the salinity stress; B is plant height at the beginning of the salinity stress.
2.6 Assay of antioxidant enzymes
In each treatment group of the experiment, five strains of Paulownia were randomly selected, and the mature leaves at the same middle canopy region of each plant were sampled to determine the activity of superoxide dismutase (SOD, EC 1.15.1.1), peroxidase (POD, EC 1.11.1.7) and catalase (CAT, EC 1.11.1.6.) enzyme activities.
2.6.1 Assay of SOD activity
The activity of SOD was determined by WST-8 method according to Liu et al (Liu et al., 2010), five strains of paulownia were randomly selected in each treatment, and 0.1 g of mature leaves were weighed. Following homogenization in an ice bath with 0.1 ml of phosphate buffer, the samples were analyzed using assay kits (Keming Biotechnology Co., Ltd, Jiangsu, China). According to the manufacturer’s instructions we measured the absorbance value at 450 nm with a UH5300 spectrophotometer (Hitachi, Japan) determination absorbance values to calculate respective activity values as follows,
S, inhibition percentage÷(1-inhibition percentage); inhibition percentage = (A0-A1)/A0; A0, denotes the reference tube suction light value; A1 is the absorbance value of the measuring tube; V1 is the total volume of the reaction system, ml; W is the sample quality, g; V2 is the sample volume added to the reaction system,ml; V3 is always the volume of added extract, ml.
2.6.2 Assay of POD activity
The activity of POD enzyme in Paulownia leaves was determined by methoxyphenol method (Junfeng, 2006). The POD activity was determined by methoxyphenol method, five strains of paulownia were randomly selected in each treatment, and 0.1 g mature leaves were weighed. After ice bath homogenization with 0.1 ml phosphate buffer, the samples were analyzed with the kits (Keming Biotechnology Co., Ltd, Jiangsu, China). Then according to the manufacturer’s instructions using UH5300 spectrophotometer (Hitachi, Japan) we obtained the 470 nm absorbance values. The POD was calculated as follows
ΔA equals to A2-A1, A1 is the absorbance value at 1 min of reaction; A2 is the absorbance value at 2 min of reaction; V1 is the total volume of the reaction system, ml; W is sample quality, g; V2 is the added sample volume, ml; V3 is always added to the volume of the extract, ml; T is the reaction time, min.
2.6.3 Assay of CAT activity
The activity of CAT was determined by ammonium molybdate colorimetric method (Peng et al., 2009). Five paulownia strains were randomly selected per treatment, and 0.1 g of mature leaves were sampled and weighed. After ice bath homogenization with 0.1 ml phosphate buffer, we analyzed the samples with the kits (Keming Biotechnology Co., Ltd, Jiangsu, China). Then according to the manufacturer’s instructions using UH5300 spectrophotometer (Hitachi, Japan) we determined the 405 nm absorbance values to calculate the activity (CAT) using the following formula,
ΔA denotes A Control-A Determination;V1 is volume of the reaction system, ml; V2 is add sample volume, ml; V3 is add the volume of the extract, ml; T is reaction time, min; W is sample quality, g.
2.7 Soluble sugar content
The soluble sugar content in Paulownia leaves was determined by the anthrone colorimetry method (Maness, 2010). Five paulownia strains were randomly selected for treatment too. Each plant provided 0.15 g of leaf tissue; we added 1 ml of distilled water to grind into a homogenate. According to the manufacturer ‘s instructions, the absorbance at 620 nm was measured using the soluble sugar kit (Keming Biotechnology Co., Ltd, Jiangsu, China) for calculating the soluble sugar content as follows,
ΔA denotes A determination; A is blank; V1 is add sample volume, ml; V2 is add the extract volume, ml; W is sample fresh weight, g.
2.8 Leaf net photosynthetic and transpiration rate
During the determination, nine Paulownia plants were randomly selected from each treatment for analysis. The 2–3 healthy and mature leaves of the plant from top to bottom were selected for determination. The net photosynthetic rate and transpiration rate of Paulownia leaves was measured using LI-6800 portable photosynthesis instrument (LI-COR, USA) from 9:00 am to 11:00 am and from 2:00 pm to 4:00 pm under clear weather (excluding plant photosynthesis noon break period). The environmental factors were set according to the surrounding environment, the humidity was set to 55%, the leaf chamber CO2 was set to 400 umol·mol-1, the leaf chamber temperature was set to 29°C, and the light intensity reference was 1000 umol·m-2·s-1.
2.9 Statistical analysis
One-way ANOVA was used to analyze the above-ground dry and fresh weight, below-ground dry and fresh weight, net photosynthetic rate, transpiration rate, SOD enzyme activity, POD enzyme activity, CAT enzyme activity and soluble sugar content of Paulownia. Levene’s test (p-value >0.05) was performed for testing the equality of variance assumption. Two-way ANOVA was used to compare treatments (including salinity stress and inoculation).The residual distribution and variances were evaluated which met the ANOVA assumptions. As described in each illustration, in a completely randomized design, experiments were performed with at least 5 independent replicates. SPSS 26.0 software was used to calculate the analysis of variance. The significance of differences between data sets was evaluated using paired student’s t-test (p-value<0.05). Statistical charts of data, i.e., bar charts, line graphs and were produced using Sigmaplot 15.0 software.
3 Results
3.1 Colonization efficiency
The colonization of P. indica in the roots of paulownia under different NaCl concentrations for 30 days in the inoculation group and control group (Figure 1). We found the chlamydospores in the roots of paulownia and confirmed the existence of P. indica. The colonization degree (D%) of P. indica ranged from 25.92% to 47.30% under 4 NaCl concentrations (Table 2). The D% value reached a maximum of (42.50% ± 4.79%) in plants with salinity treatment (0.70% NaCl). No chlamydospores were found in the roots of paulownia plants in the control group under all NaCl concentrations.
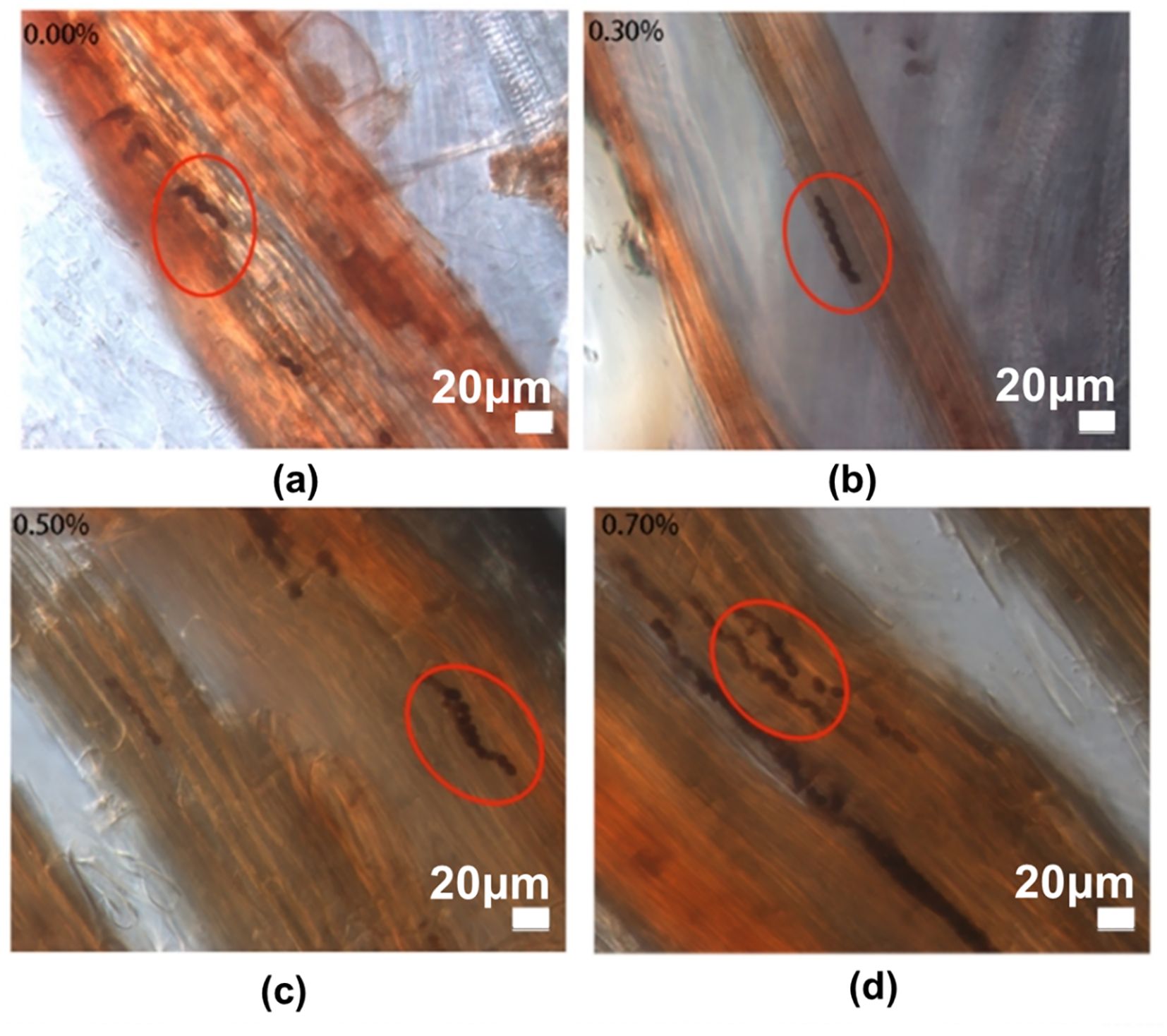
Figure 1. Root colonization of P. indica in Paulownia elongata. (a) Inoculated in 0.00% NaCl, (b) Inoculated in 0.30% NaCl, (c) Inoculated in 0.50% NaCl, and (d) Inoculated in 0.70% NaCl.
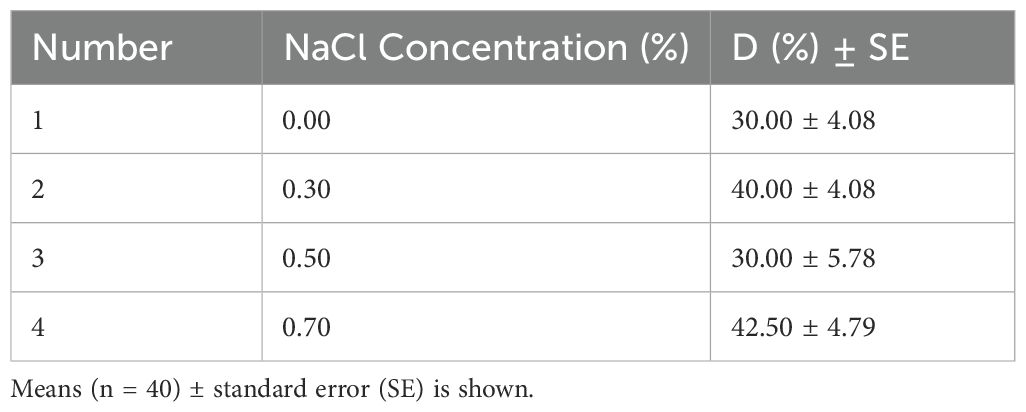
Table 2. The colonization degree (D%) of P. indica in paulownia root under different NaCl concentrations for 30 days.
3.2 Growth and biomass
3.2.1 Mortality
The most striking effects of P. indica inoculation were observed in survival, growth, and stress mitigation. We found no plant mortality in the inoculated and control groups without salinity stress (0.00% NaCl). Among the three salinity stress treatment groups, as the NaCl concentration increased, the mortality increased in both the paulownia plants in the inoculated group and the control group. However, the inoculated group showed significantly lower mortality than that in the control group. Briefly, compared with the control group P. indica-inoculated plants showed the mortality decreased by 5.55%, 22.22% and 27.77% under 0.30%, 0.50% and 0.70% NaCl treatments, respectively (Table 3). The highest mortality of paulownia plants of 50.00% was observed in the uninoculated paulownia under the 0.70% NaCl treatment.
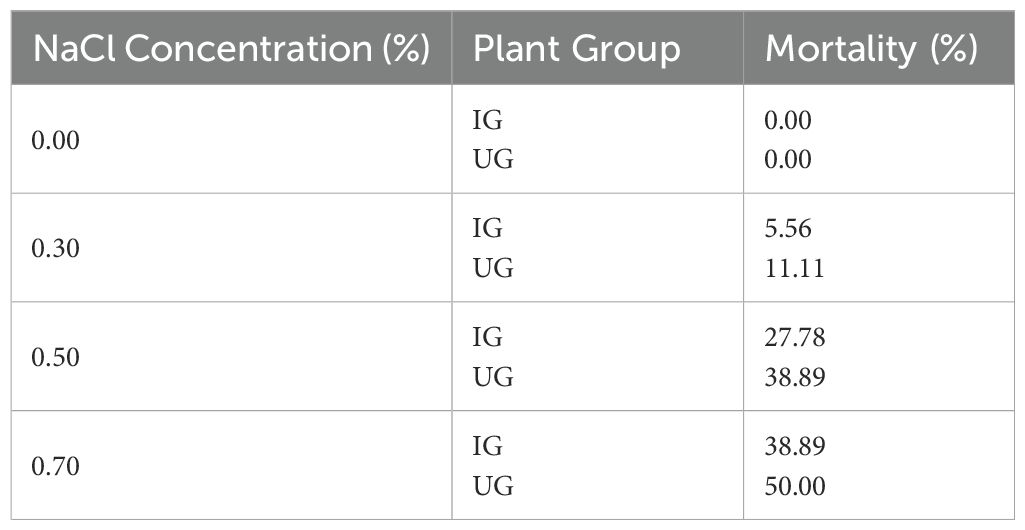
Table 3. Mortality (%) of paulownia plants with 5 salinity concentrations in control group (UG) and inoculated group (IG) for 30 days of salinity stress treatment (n=18).
3.2.2 Height growth
Figure 2 illustrates the effect of P. indica on the growth of paulownia plants under salinity treatment. When the NaCl concentration increased, the growth declined in both the inoculated group and the control group. However, the inoculated paulownia exhibited a higher growth rate compared to that of the control group. The inoculated group showed greater height growth under the 0.30% NaCl treatments. The plant height of paulownia decreased by 53.79%, 37.43%, 26.57% under 0.30%, 0.50% and 0.70% NaCl concentration respectively compared with that under 0.00% NaCl concentration in the inoculated group; however, the uninoculated plant height decreased by 68.88%, 81.99%, 81.00%, respectively. P. indica-inoculated plants showed 21.55%, 24.95%, 37.70%, and 7.41% greater height growth than controls, under the same NaCl concentration treatment (0.00%, 0.30%, 0.50%, and 0.70%).
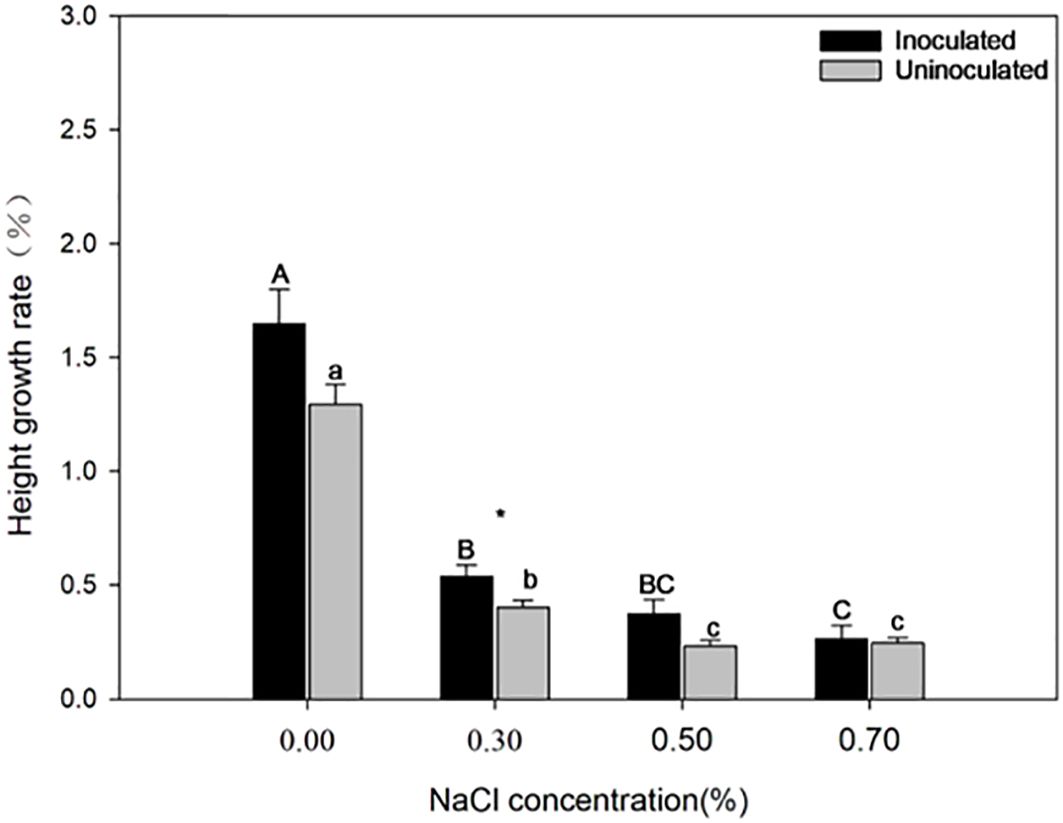
Figure 2. The height growth rate of paulownia plants in the inoculated group and the control group under different NaCl concentrations (0.00%, 0.30%, 0.50%, 0.70%). Note, “*”, p<0.05. Different majuscules indicate that there are significant differences in growth of paulownia plants in different salt concentrations for inoculated group, and different minuscules indicate that there are significant differences in growth of paulownia plants in different salt concentrations for uninoculated group.
3.2.3 Biomass
3.2.3.1 Above-ground fresh weight
The fresh weight of plants is a key indicator of growth. As salinity stress intensifies, reduction of fresh weight is greater. Figure 3A reflects the changes of above-ground fresh weight of paulownia plants across rising NaCl concentration in the inoculated group and the control group. The above-ground fresh weight of paulownia plants decreased significantly with the reduction of 54.92%, 88.10% and 96.54% respectively under 0.30%, 0.50% and 0.70% NaCl concentration compared with that under 0.00% NaCl concentration in the inoculated group; in the control group, the above-ground fresh weight decreased by 49.13%, 91.17% and 92.96%, respectively, compared to the 0.00% NaCl control. Under the same NaCl concentration P. indica-inoculated plants showed 30.92%, 22.03%, 48.80% and -40.57% greater above-ground fresh weight than controls respectively, highlighting the mitigating effect of inoculation on salinity stress.

Figure 3. The fresh weight of paulownia plants in the above-ground (A) and below-ground (B) inoculated group and control group under different NaCl concentrations (0.00%, 0.30%, 0.50%, 0.70%). the dry weight of paulownia in the above-ground (C) and below-ground (D) for the inoculated group and control group under different NaCl concentrations (0.00%, 0.30%, 0.50%, 0.70%). Values are means (n=5) ± SE, Different majuscules indicate that there are significant differences in fresh weight of paulownia plants in different salt concentrations for inoculated group, and different minuscules indicate that there are significant differences in fresh weight of paulownia plants in different salt concentrations for control group. One-way ANOVA was performed followed by Duncan’s test (p<0.05); “*” indicates that there is a significant difference between the control group and the inoculated group under the same NaCl concentration stress (p< 0.05).
3.2.3.2 Below-ground fresh weight
The below-ground fresh weight of paulownia plants declined with increasing NaCl concentrations (Figure 3B). The below-ground fresh weight of paulownia plants decreased by 17.27%, 45.39% and 46.61% respectively under 0.30%, 0.50% and 0.70% NaCl concentration compared with that under 0.00% NaCl concentration in the inoculated group. In the control group, the fresh weight decreases were more pronounced, with reductions of 6.25%, 46.64%, and 71.40% at the same NaCl levels compared with that under 0.00% NaCl concentration. Under the same NaCl concentration treatment P. indica-inoculated plants showed 8.49%, -3.70%, 3.89%, and 50.99% greater below-ground fresh weight than controls.
3.2.3.3 Above-ground dry weight
Elevated NaCl concentrations led to a reduction in the above-ground dry weight of Paulownia plants in both inoculated and control groups. In each salinity treatment, except for 0.70% NaCl salinity concentration, the above-ground dry weight of Paulownia plants inoculated with P. indica was higher than that of plants uninoculated with P. indica (Figure 3C). Under the same NaCl concentration treatment P. indica-inoculated plants showed 8.49%, -3.70%, 3.89%, and 50.99% greater above -ground fresh dry weight than controls. Moreover, at 0.30% NaCl concentration, the above-ground dry weight of inoculated Paulownia plants was significantly higher than that of uninoculated Paulownia with P. indica (controls).
3.2.3.4 Below-ground dry weight
The below-ground dry weight of Paulownia plants exhibited an initial increase followed by a decline as NaCl concentrations rose. Across most salinity levels, Paulownia inoculated with P. indica showed higher below-ground dry weight compared to non-inoculated plants, except at 0.30% NaCl. Notably, P. indica significantly boosted below-ground dry weight under 0.7% NaCl concentration (Figure 3D).
3.3 Effects of P. indica on SOD, POD and CAT activities of paulownia plants
The activities of SOD, POD, and CAT are key indicators of a plant’s ability to detoxify reactive oxygen species (ROS). Under increasing NaCl concentrations, SOD activity in both inoculated and uninoculated plants exhibited an initial rise followed by a decline (Figure 4A). The SOD activity of inoculated paulownia plants under 0.30%, 0.50% and 0.70% NaCl concentration increased by 52.23%, -13.89% and 3.57% respectively, compared to the control group (0.00% NaCl). In the uninoculated plants, the SOD activity increased by 16.70%, -12.18% and -40.90% respectively, compared to the control group. Except for the plants treated with 0.50% NaCl, the increase of SOD activity in the inoculated group was higher than that in the control group. Under the same NaCl treatment P. indica-inoculated plants showed -26.73%, 2.84%, -29.25%, and 27.68% greater SOD activity than controls. As the NaCl concentration increased, POD activity initially rose and then declined (Figure 4B). The POD activity of paulownia plants peaked at 0.30% NaCl concentration in the inoculated group and was significantly higher than that in the control group (0.30% NaCl).
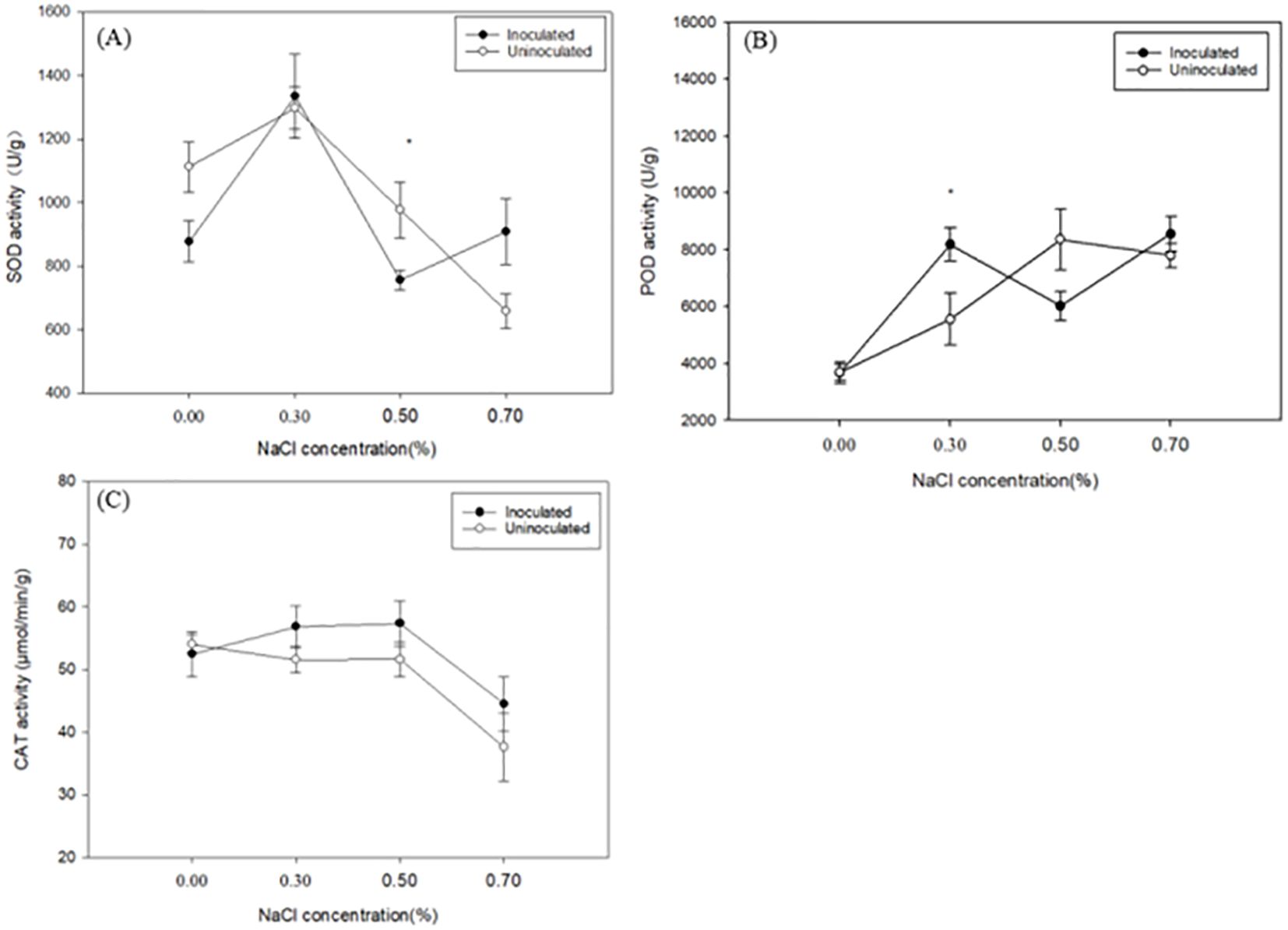
Figure 4. The SOD activity (A) POD activity (B) and CAT activity (C) of paulownia plants in the inoculated group and control group under different NaCl concentrations (0.00%, 0.30%, 0.50%, 0.70%). Values are means (n=5) ± SE. One-way ANOVA was performed followed by Duncan’s test(p<0.05); “*” indicates that there is a significant difference between the control group and the inoculated group under the same NaCl concentration stress (p< 0.05).
POD activity of the inoculated paulownia plants increased by 123.12%, 64.24% and 133.31% at 0.30%, 0.50% and 0.70% (NaCl), respectively, compared with 0.00% NaCl concentration. In the control group, POD activity increased by 50.75%, 126.87% and 111.62% under the same NaCl concentrations, respectively, compared with that under 0.00% NaCl concentration. Consistent with SOD activity, the increase of SOD activity in the inoculated group was higher than that in the control group compared with their respective 0.00% concentration of plants except for the plants treated with 0.50% NaCl. Under identical salt concentrations, POD activity in the non-inoculated group changed by -0.04%, 32.13%, -38.75%, and 8.89%. At 0.30% and 0.70% NaCl levels, SOD and POD activities in inoculated paulownia plants were higher compared to the control group, indicating enhanced antioxidant defense mechanisms in response to salt stress.
With the increase of NaCl concentration, CAT enzyme activity showed a downward trend in uninoculated paulownia, which initially increased and then decreased in uninoculated Paulownia (Figure 4C). CAT activity in the inoculated paulownia plants increased by 8.37%, 9.37% and -15.10% at 0.30%, 0.50% and 0.70% (NaCl), respectively, compared with 0.00% NaCl concentration. In the control group, CAT activity decreased by 4.51%, 4.45% and 30.7%, respectively compared with that under 0.00% NaCl concentration. When assessing CAT activity under identical NaCl concentrations, the uninoculated plants showed reductions of 2.13% to 0.40% at 0.30%, 0.50%, and 0.70% NaCl. Notably, except at 0.00% NaCl, CAT activity in inoculated plants was consistently higher than in uninoculated plants across the remaining salt treatments.
3.4 Effects of P. indica on soluble sugar of paulownia plants
With the increase of NaCl concentration, the soluble sugar content of paulownia plants peaked at 0.3% NaCl (Figure 5). At 0.00%, 0.30%, 0.50%, 0.70% NaCl concentrations, the soluble sugar content of paulownia plants inoculated group was higher than the control group. The soluble sugar content of paulownia plants under 0.30%, 0.50% and 0.70% NaCl concentration increased by 34.01%, -17.89% and -4.70% respectively compared with that under 0.00% NaCl concentration in the inoculated group, and increased by 43.89%, 2.63% and 25.20% respectively compared with that under 0.00% NaCl concentration in the control group. Under the same NaCl concentration treatment P. indica-inoculated plants showed -31.93%, 26.92%,14.92% and 10.58% greater soluble sugar than controls.
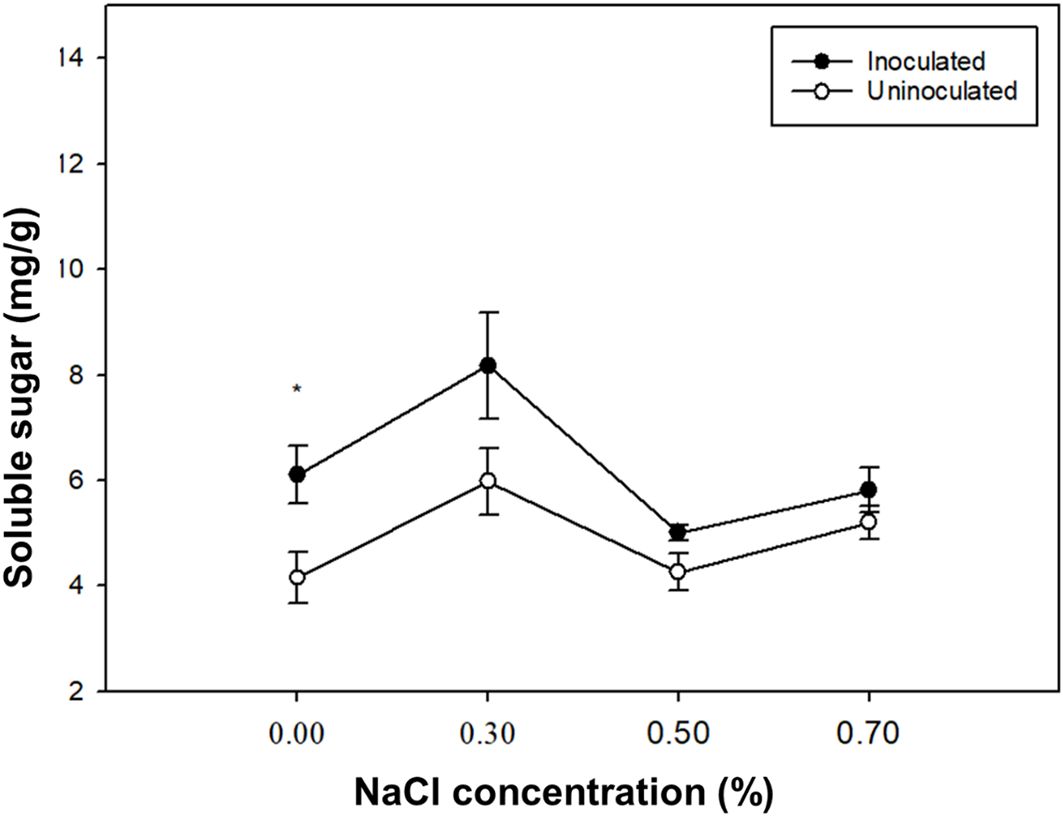
Figure 5. The soluble sugar content of paulownia plants in the inoculated group and control group under different NaCl concentrations (0.00%, 0.30%, 0.50%, 0.70%). Values are means (n=5) ± SE. One-way ANOVA was performed followed by Duncan’s test (p<0.05). * indicates that there is a significant difference between the control group and the inoculated group under the same NaCl concentration stress (p< 0.05).
3.5 Effects of P. indica on leaf net photosynthetic rate and transpiration rate of paulownia plants
The net photosynthetic rate of Paulownia leaves declined as NaCl concentration increased. At all NaCl concentrations, the inoculated group exhibited a higher net photosynthetic rate compared to the control group (Figure 6). The net photosynthetic rate of Paulownia leaves in the inoculated group was significantly higher than that in the control group at NaCl concentrations of 0.50% and 0.70%. In the inoculated group, the net photosynthetic rate decreased by 41.03%, 35.33%, and 44.88% at 0.30%, 0.50%, and 0.70% NaCl, respectively, compared to the rate at 0.00% NaCl. Similarly, the control group showed reductions of 42.15%, 41.60%, and 72.07% at the same NaCl concentrations relative to the 0.00% NaCl level. Under the same NaCl concentrations P. indica-inoculated plants showed 2.13%, 0.40%, 11.61% and 50.41% greater net photosynthesis rate than controls. The transpiration rate of Paulownia decreased with increasing NaCl concentration, with the inoculated group consistently showing lower rates than the control group. Under the same NaCl treatment P. indica-inoculated plants showed 2.95%, 14.93%, 5.36%, and 26.00% lower transpiration rate than controls.
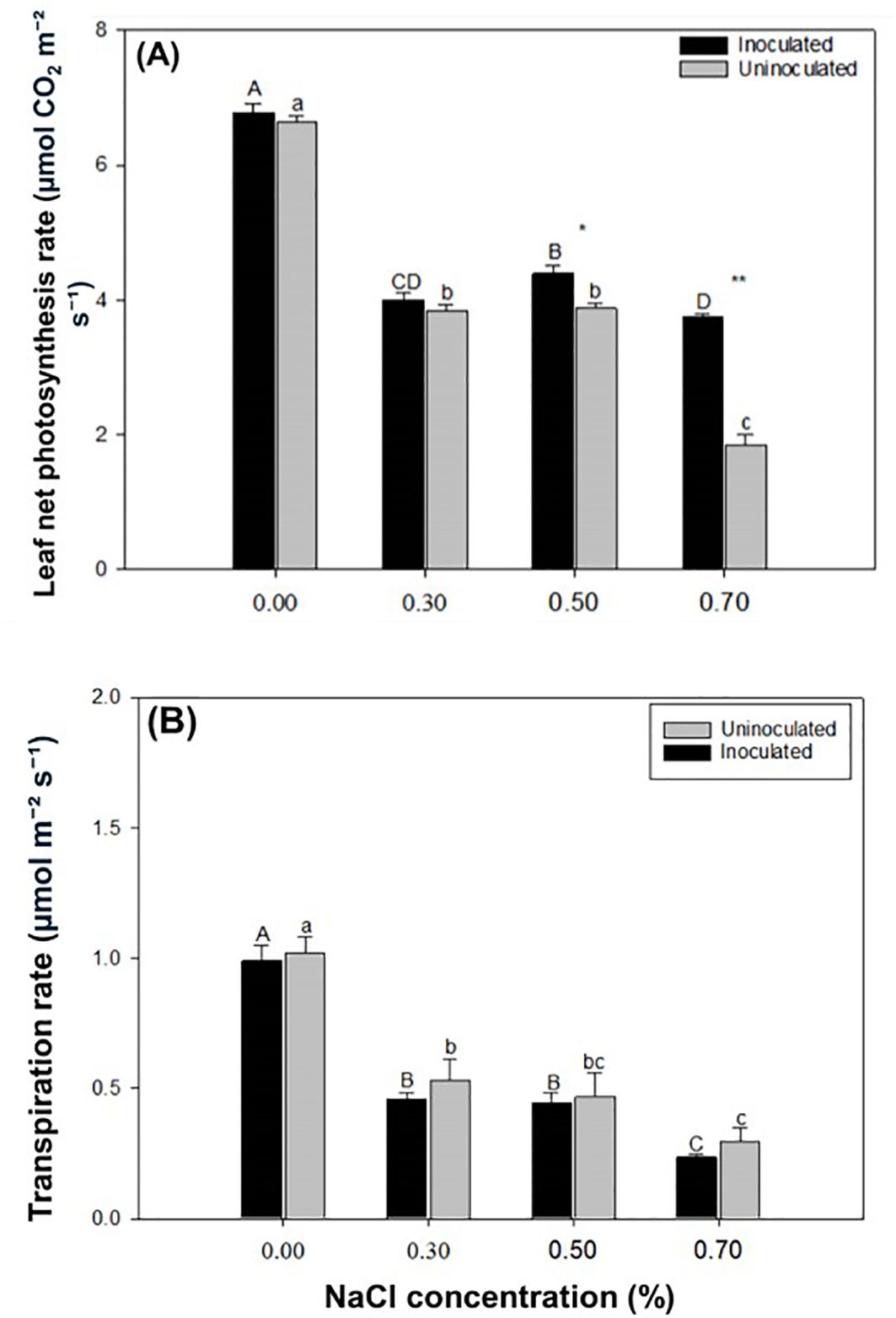
Figure 6. The leaf net photosynthetic rate (A) and transpiration rate of paulownia plants (B) in the inoculated group and control group under different NaCl concentrations (0.00%, 0.30%, 0.50%, 0.70%). Values are means (n=9) ± SE. Different majuscules indicate that there are signifificant differences in soluble sugar content of paulownia plants in different salt concentrations for inoculated group, and different minuscules indicate that there are signifificant differences in soluble sugar content of paulownia plants in different salt concentrations for control group. One-way ANOVA was performed followed by Duncan’s test (p<0.05); “*” indicates that there is a signifificant difference between the control group and the inoculated group under the same NaCl concentration stress (p< 0.05). ** indicates that there is a signifificant difference between the control group and the inoculated group under the same NaCl stress (p< 0.01).
3.6 Comprehensive evaluations of the response of paulownia indicators
The Paulownia indicator values were analyzed and organized, and a two-way ANOVA mean square table was constructed to evaluate the effects of Piriformospora indica inoculation and salt stress on Paulownia under different conditions (Table 4). Results revealed that the interaction between P. indica inoculation and salt stress had a significant effect on net photosynthetic rate and soluble sugar content, as well as a pronounced impact on plant height growth rate and SOD activity.
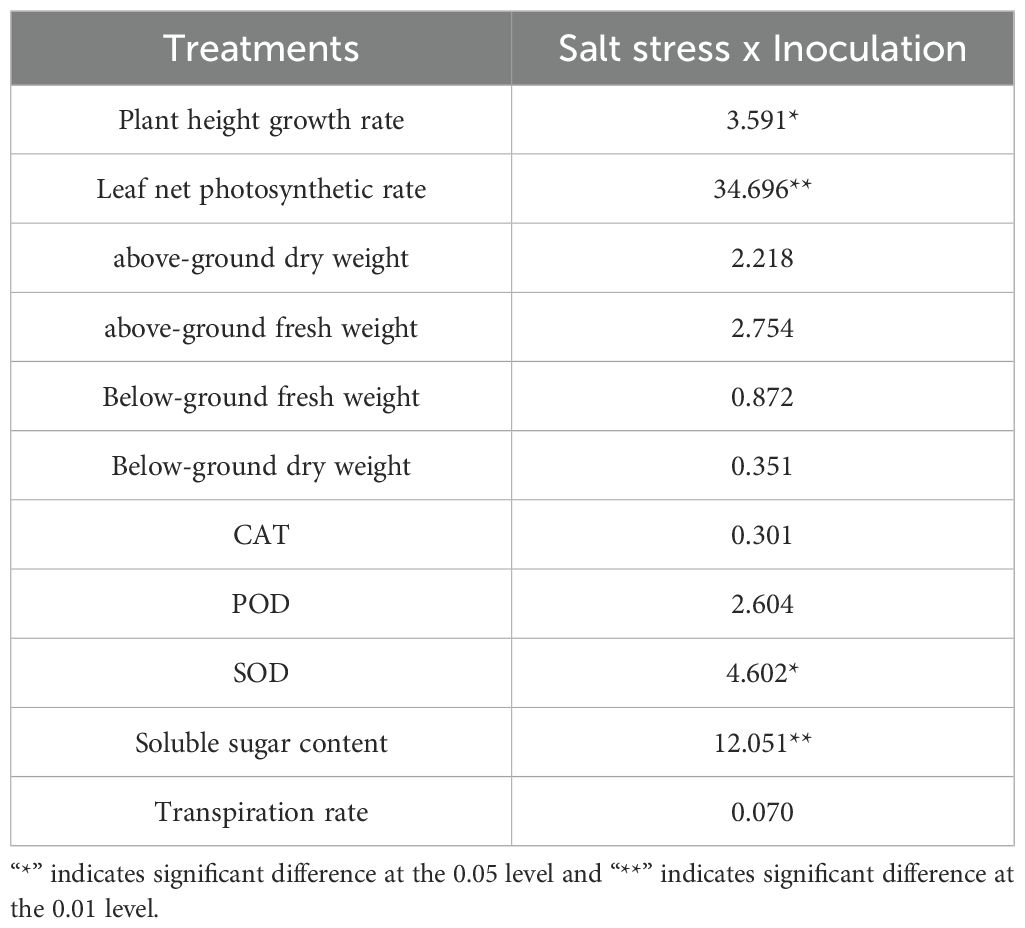
Table 4. Two-way ANOVA analysis of the response of Piriformospora indica to Paulownia elongata under NaCl salt stress.
4 Discussion
Previous studies have shown that P. indica exhibits the ability to colonize a wide range of plant species. It is not only able to symbiosis with herbaceous plants such as maize (Zea mays) (Xu et al., 2017), barley (Hordeum vulgare), wheat (Triticum aestivum) (Ghabooli and Hosseini, 2021), rice (Oryza sativa) (Tsai et al., 2020), but also to symbiose with woody plants such as peach (Prunus persica) (Liang et al., 2023), longan (Dimocarpus longan) (Cheng et al., 2022), promoting the growth of host plants, and improving their ability to resist abiotic stresses such as salt and drought tolerance. However, P. indica had different effects on the colonization degree of different plants and plants with different initial nutritional status. The results of this experiment showed that P. indica could successfully colonize the roots of Paulownia and form a strong symbiotic relationship. Notably, the degree of root colonization remained relatively stable despite increasing NaCl concentrations. The colonization rates were recorded as 30.00% at 0.00% NaCl, 40.00% at 0.30% NaCl, 30.00% at 0.50% NaCl, and 42.50% at 0.70% NaCl (Table 2). It is well-reported that salt stress can hinder plant growth, accelerate senescence, and ultimately result in plant mortality (Jouyban, 2012). Under all salt stress conditions, the mortality rate of the inoculated group was consistently lower than that of the control group. Thus, P. indica inoculation improves paulownia plants to tolerate salt stress, reducing the salinity-induced damage to a certain extent.
Our results showed that paulownia plants inoculated with P. indica had an elevated growth rate compared to uninoculated plants at all salt concentrations. This is similar to the results for rice (Jogawat et al., 2013). Salt stress reduces plant water use and disrupts plant photosynthesis, which consequently suppresses plant growth and yield (Munns, 2002). Salt stress will also significantly reduce the biomass accumulation of plants (Pour-Aboughadareh et al., 2021). Salt stress inhibits the growth and development of plant roots, which directly limits the ability of plants to absorb water and nutrients from the soil (Nawaz et al., 2010).
We found that for the below-ground biomass, inoculated with P. indica significantly increased the root biomass of Paulownia under the treatment of 0.70% NaCl concentration (Figure 3). It indicated that P. indica significantly promoted the growth of the below-ground part of Paulownia under the treatment of 0.70% NaCl concentration. A similar trend was reported on gerbera (Gerbera jamesonii) (Chen et al., 2022) and tomato (Solanum lycopersicum) (Ghorbani et al., 2018). The observed growth enhancement of Paulownia following inoculation with P. indica is likely attributable to the ability of P. indica to promote plant growth by facilitating the uptake of essential mineral nutrients, particularly phosphate (Kumar et al., 2011). The unexpected biomass reduction in inoculated plants at 0.70% NaCl (Figure 3A) likely reflects a trade-off between survival and growth under extreme stress: Energy reallocation P. indica may prioritize root maintenance overshoot growth at lethal salinity (Pérez-Alonso et al., 2020), as evidenced by the 51% higher root biomass in inoculated plants (Figure 3D).
Plants capture light energy through leaves and convert water and carbon dioxide into glucose and oxygen. Photosynthesis process provides energy and basic substances to support various activities throughout plants’ life cycle (Pessarakli, 2024). Our results showed that the above-ground biomass of Paulownia inoculated group was higher than that of control group under all NaCl concentration treatments except 0.70% NaCl concentration treatment. After inoculation with P. indica, the growth of the above-ground tissues increased to a certain extent, which is consistent with previous findings in Arabidopsis thaliana (Abdelaziz et al., 2017). Although rhizosphere growth-promoting bacteria mainly act on roots, they can also indirectly enhance photosynthesis and improve the photosynthetic efficiency, thus fostering above-ground growth (Sun et al., 2014). Plants have developed many mechanisms to adapt to stress, such as increasing root length to promote water uptake and reducing transpiration rate (Kooyers, 2015). Our results demonstrated that the transpiration rate in the inoculated group was significantly lower than that in the control group under salt stress. This reduction in transpiration played a critical role in conserving water and improving survival chances. These findings highlight that inoculation with P. indica can effectively enhance water retention and plant tolerance to salt stress (Mohsenifard et al., 2017; Atia et al., 2020).
The root-to-shoot ratio of Paulownia increased under high salt stress, likely reflecting an adaptive strategy to optimize water uptake. This adjustment in biomass allocation may help to adapt to reduced water availability and decreased water use efficiency caused by salt stress (Munns, 2002). With NaCl concentration increases, the dry-fresh ratio of Paulownia plants increased, but the dry-fresh ratio of the inoculated group was always lower than that of the control group. High salt concentration will reduce the water absorption capacity of plants. This may be because under salt stress conditions, high salt concentration in the soil will reduce the water absorption capacity of plants, resulting in a decrease in water content in plants, so that the dry-fresh ratio increased (Hailu and Mehari, 2021). The inoculation of P. indica alleviated this phenomenon well because the epitaxial mycelium can help the plant roots to improve the water use efficiency of Paulownia by more effectively absorbing water from the soil (Augé, 2001; Asrar et al., 2012), thereby enhancing the salt tolerance of Paulownia (Boorboori and Zhang, 2022).
Under salt stress, the production of reactive oxygen species (ROS) increased, and the increase of ROS content promoted membrane lipid peroxidation, resulting in the destruction of membrane integrity, the leakage of electrolytes and small molecular organic matter, resulting in a series of metabolic disorders and damage to plants (de Azevedo Neto et al., 2006; Esfandiari et al., 2007; Lum et al., 2014). To alleviate the effect of oxidative stress caused by salt stress on plants, ROS is removed in the body through the antioxidant enzyme system such as superoxide dismutase (SOD), catalase (CAT), peroxide (POD) (Gharsallah et al., 2016). SOD is the main O2-free radical scavenging, and its enzymatic action leads to the formation of H2O2 and O2. The produced H2O2 was then removed by CAT and POD enzyme (Sachdev et al., 2021).
While P. indica universally upregulates antioxidant enzymes across hosts, the magnitude and stress threshold of this response vary: (Abdelaziz et al., 2019). studied the greenhouse tomato, under salt stress, and found the activities of SOD and CAT in colonized plants were significantly higher than those in non-colonized plants (Abdelaziz et al., 2019). (Arora et al., 2020). found that During salt stress, the activities of antioxidant enzymes SOD and CAT were enhanced after Artemisia annua was inoculated with P. indica (Arora et al., 2020). Our results showed that the activities of SOD, POD and CAT in paulownia leaves under salt stress increased first and then decreased, which is consistent with the findings in poplar (Rui et al., 2012). The activities of SOD and POD in the leaves of paulownia inoculated with P. indica increased rapidly and the accumulation was higher than that of paulownia uninoculated with P. indica at a certain concentration of salt treatment, which is similar to the research results of greenhouse tomato and Brassica napus (You, 2013; Abdelaziz et al., 2019). Paulownia seedlings inoculated with P. indica under salt stress had better H2O2 scavenging ability, reduced active oxygen content and membrane lipid peroxidation (Xu et al., 2017). With the increase of salt concentration, the activities of POD and SOD enzymes in paulownia leaves decreased, the membrane lipid peroxidation of plants increased, the activities of various protective enzymes decreased, and the growth of plants was inhibited. Low salt can induce increases of SOD and POD activity, antioxidant enzyme activity, and scavenging excessive reactive oxygen species (Liu et al., 2021). However, the activities of SOD and POD in the leaves of inoculated paulownia seedlings were still higher than those of paulownia seedlings not inoculated with P. indica when the NaCl concentration was 0.70%.
The activity of CAT did not change significantly. In the early stage of salt stress, plants may produce abundant superoxide anion free radicals, as the substrate of SOD, so that it is quickly activated. The hydrogen peroxide produced by SOD can become the substrate of POD, which promotes POD to respond quickly. If the hydrogen peroxide produced in the early stage of salt stress is not the main form of reactive oxygen species, then CAT cannot play a role as quickly as SOD and POD, resulting in a weak response to its activity (Joseph and Jini, 2010). Therefore, it is speculated that the enzymatic mechanism of inoculation of P. indica to improve the salt stress resistance in paulownia seedlings may be that SOD and POD play a key role in alleviating a series of reactive oxygen species damage which aligns with the findings in dandelion (Taraxacum mongolicum) (Yahui et al., 2017).
Osmotic adjustment ability plays a key role in plant salt tolerance (Munns et al., 2020). Soluble sugar is considered to be an osmotic adjustment substance that is significantly accumulated in many plants under salt stress or drought stress (Farhangi-Abriz and Torabian, 2017). Our results showed that the soluble sugar content in paulownia leaves increased first and then decreased under salt stress. The soluble sugar content of Paulownia leaves at each NaCl concentration was higher than that of the control group, indicating that P. indica increases the content of soluble sugar in the cell osmotic substance, reducing the cell osmotic potential, and delaying the loss of intra-cellular water (Redillas et al., 2012).
The results of two-way ANOVA also showed that under the salt-stress treatment, compared with the un-inoculated treatment, the inoculated with P. indica mycorrhizal fungi could significantly improve the plant height growth rate, net photosynthetic rate, and SOD activity of paulownia seedlings, thereby improving the salt-tolerance of paulownia seedlings. More in-depth studies on saline-alkaline stress are promising in the light of ROS scavenging capacity, osmotic adjustment substance content, and photosynthetic capacity, and how these regulations promote plant growth with P. indica inoculation for more SRWCs and bio-energy tree crops (Yahui et al., 2017). Besides, more detailed studies on the light energy utilization efficiency can benefit the understanding of the plant adaptation to the salinity conditions in terms of growth in both shoot and roots, though we provided evidence in the harvest evaluation of the productivity.
Our findings have direct implications for saline land afforestation and sustainable agroforestry: 1) Field-ready inoculant, i.e., P. indica’s axenic cultivability (Varma et al., 1999), enables large-scale production of low-cost inoculants; and combined with Paulownia’s rapid growth, our application could transform marginal saline soils (ECe 4–8 dS/m) into productive plantations within 3–5 years; 2) Water conservation due to reduced transpiration rates of inoculated Paulownia (Figure 6) can be achieved by 30–40% lower irrigation demand compared to uninoculated plants—critical for arid saline regions; 3) Regarding policy integration, promoting afforestation (e.g., China’s ‘Green Belt’ initiative) could prioritize P. indica-Paulownia systems in saline-alkali land reclamation programs nation-wide.
5 Conclusion
P. indica alleviates salt stress-induced mortality in paulownia plants, significantly enhancing plant height and biomass. Inoculation with P. indica also increases the activity of antioxidant enzymes such as POD and SOD under specific salt concentrations and boosts soluble sugar content in the leaves. These results suggest that P. indica mitigates oxidative damage from reactive oxygen species by enhancing antioxidant enzyme activity and protects paulownia from osmotic stress by elevating soluble sugar levels. However, further research is still necessary to verify these effects and explore the potential application of P. indica as a biofertilizer to improve paulownia yield under salt stress.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: Please contact the author for the data access.
Author contributions
DM: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MZ: Conceptualization, Data curation, Formal Analysis, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. YL: Data curation, Methodology, Resources, Validation, Writing – original draft. CD: Visualization, Writing – review & editing. QC: Data curation, Methodology, Resources, Validation, Writing – original draft. XF: Data curation, Methodology, Resources, Validation, Writing – original draft. XM: Data curation, Methodology, Resources, Validation, Writing – original draft. XZ: Data curation, Methodology, Resources, Validation, Writing – original draft. SG: Data curation, Methodology, Resources, Validation, Writing – original draft. DZ: Data curation, Methodology, Resources, Validation, Writing – original draft. YG: Data curation, Methodology, Resources, Validation, Writing – original draft. YW: Investigation, Project administration, Resources, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research was funded by the Forestry Innovation Project of Agricultural Innovation Program in Shandong Province (funding No. 2019LY005, 2019LY009); Study of the capacity of mycorrhizal in promoting saline-alkaline tolerance of main afforestation tree species. (funding No. SCSFP-KY-1).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Świechowski, K., Liszewski, M., Bąbelewski, P., Koziel, J. A., and Białowiec, A. (2019). Fuel properties of torrefied biomass from pruning of oxytree. Data. 4, 55. doi: 10.3390/data4020055
Abdelaziz, M. E., Abdelsattar, M., Abdeldaym, E. A., Atia, M. A., Mahmoud, A. W. M., Saad, M. M., et al. (2019). Piriformospora indica alters na+/K+ Homeostasis, antioxidant enzymes and lenhx1 expression of greenhouse tomato grown under salt stress. Scientia horticulturae 256, 108532. doi: 10.1016/j.scienta.2019.05.059
Abdelaziz, M. E., Kim, D., Ali, S., Fedoroff, N. V., and Al-Babili, S. (2017). The endophytic fungus piriformospora indica enhances arabidopsis thaliana growth and modulates na+/K+ Homeostasis under salt stress conditions. Plant Science 263, 107–115. doi: 10.1016/j.plantsci.2017.07.006
Abreu, M., Silva, L., Ribeiro, B., Ferreira, A., Alves, L., Paixão, S. M., et al. (2022). Low indirect land use change (Iluc) energy crops to bioenergy and biofuels—a review. Energies. 15, 4348. doi: 10.3390/en15124348
Acosta-Motos, J. R., Ortuño, M. F., Bernal-Vicente, A., Diaz-Vivancos, P., Sanchez-Blanco, M. J., and Hernandez, J. A. (2017). Plant responses to salt stress: adaptive mechanisms. Agronomy. 7, 18. doi: 10.3390/agronomy7010018
Al-Farsi, S. M., Nawaz, A., Nadaf, S. K., Al-Sadi, A. M., Siddique, K. H., and Farooq, M. (2020). Effects, tolerance mechanisms and management of salt stress in lucerne (Medicago sativa). Crop Pasture Science 71, 411–428. doi: 10.1071/CP20033
Arora, M., Saxena, P., Abdin, M., and Varma, A. (2020). Interaction between piriformospora indica and azotobacter chroococcum diminish the effect of salt stress in artemisia annua L. By enhancing enzymatic and non-enzymatic antioxidants. Symbiosis. 80, 61–73. doi: 10.1007/s13199-019-00656-w
Asrar, A., Abdel-Fattah, G., and Elhindi, K. (2012). Improving growth, flower yield, and water relations of snapdragon (Antirhinum majus L.) plants grown under well-watered and water-stress conditions using arbuscular mycorrhizal fungi. Photosynthetica 50, 305–316. doi: 10.1007/s11099-012-0024-8
Atia, M. A., Abdeldaym, E. A., Abdelsattar, M., Ibrahim, D. S., Saleh, I., Abd Elwahab, M., et al. (2020). Piriformospora indica promotes cucumber tolerance against root-knot nematode by modulating photosynthesis and innate responsive genes. Saudi J. Biol. Sci. 27, 279–287. doi: 10.1016/j.sjbs.2019.09.007
Augé, R. M. (2001). Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza. 11, 3–42. doi: 10.1007/s005720100097
Ayala-Astorga, G. I. and Alcaraz-Meléndez, L. (2010). Salinity effects on protein content, lipid peroxidation, pigments, and proline in paulownia imperialis (Siebold & Zuccarini) and paulownia fortunei (Seemann & Hemsley) grown in vitro. Electronic J. Biotechnol. 13, 13–14. doi: 10.2225/vol13-issue5-fulltext-13
Boorboori, M. R. and Zhang, H.-Y. (2022). The role of serendipita indica (Piriformospora indica) in improving plant resistance to drought and salinity stresses. Biology. 11, 952. doi: 10.3390/biology11070952
Chen, W., Lin, F., Lin, K.-H., Chen, C., Xia, C., Liao, Q., et al. (2022). Growth promotion and salt-tolerance improvement of gerbera jamesonii by root colonization of piriformospora indica. J. Plant Growth Regul. 41, 1219–1228. doi: 10.1007/s00344-021-10385-4
Cheng, C., Li, D., Wang, B., Liao, B., Qu, P., Liu, W., et al. (2022). Piriformospora indica colonization promotes the root growth of dimocarpus longan seedlings. Scientia horticulturae 301, 111137. doi: 10.1016/j.scienta.2022.111137
Cseri, A., Borbély, P., Poór, P., Fehér, A., Sass, L., Jancsó, M., et al. (2020). Increased adaptation of an energy willow cultivar to soil salinity by duplication of its genome size. Biomass Bioenergy 140, 105655. doi: 10.1016/j.biombioe.2020.105655
de Azevedo Neto, A. D., Prisco, J. T., Enéas-Filho, J., de Abreu, C. E. B., and Gomes-Filho, E. (2006). Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ. Exp. botany 56, 87–94. doi: 10.1016/j.envexpbot.2005.01.008
Deng, M., Dong, Y., Zhao, Z., Li, Y., and Fan, G. (2017). Dissecting the proteome dynamics of the salt stress induced changes in the leaf of diploid and autotetraploid paulownia fortunei. PloS One 12, e0181937. doi: 10.1371/journal.pone.0181937
Dimitriou, I., Aronsson, P., and Weih, M. (2006). Stress tolerance of five willow clones after irrigation with different amounts of landfill leachate. Bioresour Technol. 97, 150–157. doi: 10.1016/j.biortech.2005.02.004
Esfandiari, E., Shekari, F., Shekari, F., and Esfandiari, M. (2007). The effect of salt stress on antioxidant enzymes'activity and lipid peroxidation on the wheat seedling. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 35, 48. doi: 10.1016/j.envexpbot.2005.01.008
Farhangi-Abriz, S. and Torabian, S. (2017). Antioxidant enzyme and osmotic adjustment changes in bean seedlings as affected by biochar under salt stress. Ecotoxicology Environ. Safety 137, 64–70. doi: 10.1016/j.ecoenv.2016.11.029
Feng, D., Gao, Q., Liu, J., Tang, J., Hua, Z., and Sun, X. (2023). Categories of exogenous substances and their effect on alleviation of plant salt stress. Eur. J. Agronomy 142, 126656. doi: 10.1016/j.eja.2022.126656
Ghabooli, M. and Hosseini, A. (2021). Piriformospora indica promotes some morphophysiological traits, yield and ion homeostasis of barley (Hordeum vulgare L.) under drought stress. J. Plant Biol. Sci. 13, 1–18. doi: 10.22108/ijpb.2021.123339.1219
Gharsallah, C., Fakhfakh, H., Grubb, D., and Gorsane, F. (2016). Effect of salt stress on ion concentration, proline content, antioxidant enzyme activities and gene expression in tomato cultivars. AoB Plants 8, plw055. doi: 10.1093/aobpla/plw055
Ghorbani, A., Razavi, S., Ghasemi Omran, V., and Pirdashti, H. (2018). Piriformospora indica inoculation alleviates the adverse effect of nacl stress on growth, gas exchange and chlorophyll fluorescence in tomato (Solanum lycopersicum L.). Plant Biol. 20, 729–736. doi: 10.1111/plb.2018.20.issue-4
Hailu, B. and Mehari, H. (2021). Impacts of soil salinity/sodicity on soil-water relations and plant growth in dry land areas: A review. J. Nat. Sci. Res. 12, 1–10. doi: 10.7176/JNSR/12-3-01
He, H., Zhou, W., Lü, H., and Liang, B. (2022). Growth, leaf morphological and physiological adaptability of leaf beet (Beta vulgaris var. Cicla) to salt stress: A soil culture experiment. Agronomy. 12, 1393. doi: 10.3390/agronomy12061393
Ivanova, K., Geneva, M., Anev, S., Georgieva, T., Tzvetkova, N., Stancheva, I., et al. (2019). Effect of soil salinity on morphology and gas exchange of two paulownia hybrids. Agroforestry Systems 93, 929–935. doi: 10.1007/s10457-018-0186-x
Jakubowski, M. (2022). Cultivation potential and uses of paulownia wood: A review. Forests. 13, 668. doi: 10.3390/f13050668
Jogawat, A., Saha, S., Bakshi, M., Dayaman, V., Kumar, M., Dua, M., et al. (2013). Piriformospora indica rescues growth diminution of rice seedlings during high salt stress. Plant Signaling behavior 8, e26891. doi: 10.4161/psb.26891
Johnson, J. M., Alex, T., and Oelmüller, R. (2014). Piriformospora indica: the versatile and multifunctional root endophytic fungus for enhanced yield and tolerance to biotic and abiotic stress in crop plants. J. Trop. Agriculture 52, 103–122.
Joseph, B. and Jini, D. (2010). Insight into the role of antioxidant enzymes for salt tolerance in plants. Int. J. Bot. 6, 456–464. doi: 10.3923/ijb.2010.456.464
Kaygin, B., Gunduz, G., and Aydemir, D. (2009). Some physical properties of heat-treated paulownia (Paulownia elongata) wood. Drying Technology 27, 89–93. doi: 10.1080/07373930802565921
Kooyers, N. J. (2015). The evolution of drought escape and avoidance in natural herbaceous populations. Plant Science 234, 155–162. doi: 10.1016/j.plantsci.2015.02.012
Kumar, M., Yadav, V., Singh, A., Tuteja, N., and Johri, A. (2011). Piriformospora indica enhances plant growth by transferring phosphate. Plant Signal Behav. 6, 723–725. doi: 10.4161/psb.6.5.15106
Li, D., Bodjrenou, D. M., Zhang, S., Wang, B., Pan, H., Yeh, K.-W., et al. (2021). The endophytic fungus piriformospora indica reprograms banana to cold resistance. Int. J. Mol. Sci. 22, 4973. doi: 10.3390/ijms22094973
Li, L., Feng, Y., Qi, F., and Hao, R. (2023). Research progress of piriformospora indica in improving plant growth and stress resistance to plant. J. Fungi 9, 965. doi: 10.3390/jof9100965
Liang, S.-M., Hashem, A., Abd-Allah, E. F., and Wu, Q.-S. (2023). Root-associated symbiotic fungi enhance waterlogging tolerance of peach seedlings by increasing flavonoids and activities and gene expression of antioxidant enzymes. Chem. Biol. Technol. Agriculture 10, 124. doi: 10.1186/s40538-023-00500-w
Liang, D., Huang, X., Tang, Q., Wei, X., Li, B., Oelmüller, R., et al. (2022). Effect of piriformospora indica on the cold resistance of passion fruit. J. Fujian Agric. For Univ(Nat Sci. Ed) 51, 748–753. doi: 10.3323/j.cnki.j.fafu(nat.sci.)
Liu, J., Fu, C., Li, G., Khan, M. N., and Wu, H. (2021). Ros homeostasis and plant salt tolerance: plant nanobiotechnology updates. Sustainability. 13, 3552. doi: 10.3390/su13063552
Liu, Y., Ji, D., Turgeon, R., Chen, J., Lin, T., Huang, J., et al. (2019). Physiological and proteomic responses of mulberry trees (Morus alba. L.) to combined salt and drought stress. Int. J. Mol. Sci. 20, 2486. doi: 10.3390/ijms20102486
Liu, J., Zhang, Z., Xing, G., Hu, H., Sugiura, N., and Keo, I. (2010). Potential antioxidant and antiproliferative activities of a hot-water extract from the root of tonh khidum. Oncol. letters 1, 383–387. doi: 10.3892/ol_00000068
Lum, M., Hanafi, M., Rafii, Y., and Akmar, A. (2014). Effect of drought stress on growth, proline and antioxidant enzyme activities of upland rice. JAPS: J. Anim. Plant Sci. 24, 1487–1493. doi: 10.5555/20143378334
Ma, Y., Wang, J., Sun, Y., Dong, Y., Cai, H., Raja, I. H., et al. (2023). Effects of compound salt concentration on growth, physiological and nutritional value of hydroponic forage wheat. Agriculture. 13, 1833. doi: 10.3390/agriculture13091833
Maness, N. (2010). Extraction and analysis of soluble carbohydrates. Plant Stress tolerance: Methods Protoc. 639, 341–370. doi: 10.1007/978-1-60761-702-0_22
Mirck, J. and Zalesny, R. S. (2015). Mini-review of knowledge gaps in salt tolerance of plants applied to willows and poplars. Int. J. Phytoremediation 17, 640–650. doi: 10.1080/15226514.2014.950414
Mohsenifard, E., Ghabooli, M., Mehri, N., and Bakhshi, B. (2017). Regulation of mir159 and mir396 mediated by piriformospora indica confer drought tolerance in rice. J. Plant Mol. Breeding 5, 10–18. doi: 10.22058/jpmb.2017.60864.1129
Muchate, N. S., Nikalje, G. C., Rajurkar, N. S., Suprasanna, P., and Nikam, T. D. (2016). Plant salt stress: adaptive responses, tolerance mechanism and bioengineering for salt tolerance. Botanical Review 82, 371–406. doi: 10.1007/s12229-016-9173-y
Munns, R. (2002). Comparative physiology of salt and water stress. Plant Cell Environment 25, 239–250. doi: 10.1046/j.0016-8025.2001.00808.x
Munns, R., Passioura, J. B., Colmer, T. D., and Byrt, C. S. (2020). Osmotic adjustment and energy limitations to plant growth in saline soil. New Phytologist 225, 1091–1096. doi: 10.1111/nph.v225.3
Nawaz, K., Hussain, K., Majeed, A., Khan, F., Afghan, S., and Ali, K. (2010). Fatality of salt stress to plants: morphological, physiological and biochemical aspects. Afr. J. Biotechnol. 9, 5475–5480. doi: 10.1186/1472-6750-10-61
Negacz, K., Malek, Ž., de Vos, A., and Vellinga, P. (2022). Saline soils worldwide: identifying the most promising areas for saline agriculture. J. Arid Environments 203, 104775. doi: 10.1016/j.jaridenv.2022.104775
Osmanović, Z., Huseinović, S., Bektić, S., and Ahmetbegović, S. (2017). Elongata sy hu in function of improving the quality of the environment. Period. Engineering Nat.Sci. 2 (5), 117–123. doi: 10.21533/pen.v5i2.83
Özelçam, H., İpçak, H. H., Özüretmen, S., and Canbolat, Ö. (2021). Feed value of dried and ensiled paulownia (Paulownia spp.) leaves and their relationship to rumen fermentation, in vitro digestibility, and gas production characteristics. Rev. Bras. Zootecnia 50, e20210057. doi: 10.37496/rbz5020210057
Parihar, P., Singh, S., Singh, R., Singh, V. P., and Prasad, S. M. (2015). Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. pollut. Res. 22, 4056–4075. doi: 10.1007/s11356-014-3739-1
Peng, J., Wang, D., Xu, C., Chen, L., Deng, F., and Zhang, X. (2009). Ammonium molybdate method for detecting the activities of rice catalase. Chin. Agric. Sci. Bulletin 25, 61–64. doi: 10.11924/j.issn.1000-6850.2009-0352
Pérez-Alonso, M.-M., Guerrero-Galán, C., Scholz, S. S., Kiba, T., Sakakibara, H., Ludwig-Müller, J., et al. (2020). Harnessing symbiotic plant–fungus interactions to unleash hidden forces from extreme plant ecosystems. J. Exp. Botany 71, 3865–3877.
Pour-Aboughadareh, A., Mehrvar, M. R., Sanjani, S., Amini, A., Nikkhah-Chamanabad, H., and Asadi, A. (2021). Effects of salinity stress on seedling biomass, physiochemical properties, and grain yield in different breeding wheat genotypes. Acta Physiologiae Plantarum 43, 98. doi: 10.1007/s11738-021-03265-7
Pradana, R., González, I., Oliveira, N., González-González, B. D., de Bustamante, I., and Sixto, H. (2023). Suitability of salicaceae genotypes to produce biomass using industrial wastewater. Biomass Bioenergy 175, 106874. doi: 10.1016/j.biombioe.2023.106874
Redillas, M. C., Park, S.-H., Lee, J. W., Kim, Y. S., Jeong, J. S., Jung, H., et al. (2012). Accumulation of trehalose increases soluble sugar contents in rice plants conferring tolerance to drought and salt stress. Plant Biotechnol. Rep. 6, 89–96. doi: 10.1007/s11816-011-0210-3
Rui, G., Jiandao, Y., Meifeng, J., Limin, L., Cong, J., and Lin, X. (2012). Comparative analysis of salt tolerance of five poplar seedlings. J. Northeast Forestry Univ. 40, 28–33. doi: 10.13759/j.cnki.dlxb.2012.10.026
Sabeem, M., Abdul Aziz, M., Mullath, S. K., Brini, F., Rouached, H., and Masmoudi, K. (2022). Enhancing growth and salinity stress tolerance of date palm using piriformospora indica. Front. Plant Sci. 13, 1037273. doi: 10.3389/fpls.2022.1037273
Sachdev, S., Ansari, S., Ansari, M., Fujita, M., and Hasanuzzaman, M. (2021). Abiotic stress and reactive oxygen species: generation, signaling, and defense mechanisms. Antioxidants 10, 277. doi: 10.3390/antiox10020277
Saied, A. S., Keutgen, A. J., and Noga, G. (2005). The influence of nacl salinity on growth, yield and fruit quality of strawberry cvs.‘Elsanta’and ‘Korona’. Scientia Hortic. 103, 289–303. doi: 10.1016/j.scienta.2004.06.015
Seth, M. (2003). Trees and their economic importance. Botanical Review 69, 321–376. doi: 10.1663/0006-8101(2004)069[0321:TATEI]2.0.CO;2
Sharma, M. (2008). A functional study on the multilateral symbiosis of the fungal order sebacinales with plant hosts and bacteria. Dissertation zur Erlangung des Doktorgrades, der Justus-Liebig-University Gießen. Giessen, Germany. doi: 10.22029/jlupub-10100
Singhal, U., Khanuja, M., Prasad, R., and Varma, A. (2017). Impact of synergistic association of zno-nanorods and symbiotic fungus piriformospora indica dsm 11827 on brassica oleracea var. Botrytis (Broccoli). Front. Microbiol. 8, 1909. doi: 10.3389/fmicb.2017.01909
Snow, W. A. (2015). Ornamental, crop, or invasive? The history of the empress tree (Paulownia) in the USA. Forests Trees Livelihoods 24, 85–96. doi: 10.1080/14728028.2014.952353
Su, Z., Zeng, Y., Li, X., Perumal, A. B., Zhu, J., Lu, X., et al. (2021). The endophytic fungus piriformospora indica-assisted alleviation of cadmium in tobacco. J. Fungi 7, 675. doi: 10.3390/jof7080675
Sun, C., Shao, Y., Vahabi, K., Lu, J., Bhattacharya, S., Dong, S., et al. (2014). The beneficial fungus piriformospora indica protects arabidopsis from verticillium dahliae infection by downregulation plant defense responses. BMC Plant Biol. 14, 1–16. doi: 10.1186/s12870-014-0268-5
Trouvelot, A., Kough, J., and Gianinazzi-Pearson, V. (1986). Evaluation of va infection levels in root systems. Research for estimation methods having a functional significance. Physiol. genetical aspects mycorrhizae 1986, 217–221.
Tsai, H.-J., Shao, K.-H., Chan, M.-T., Cheng, C.-P., Yeh, K.-W., Oelmüller, R., et al. (2020). Piriformospora indica symbiosis improves water stress tolerance of rice through regulating stomata behavior and ros scavenging systems. Plant Signaling behavior 15, 1722447. doi: 10.1080/15592324.2020.1722447
Varma, A., Verma, S., Sudha., Sahay, N., Bütehorn, B., and Franken, P. (1999). Piriformospora indica, a cultivable plant-growth-promoting root endophyte. Appl. Environ. Microbiol. 65 (6), 2741–2744. doi: 10.1128/AEM.65.6.2741-2744.1999
Wang, M., Gong, S., Fu, L., Hu, G., Li, G., Hu, S., et al. (2022). The involvement of antioxidant enzyme system, nitrogen metabolism and osmoregulatory substances in alleviating salt stress in inbred maize lines and hormone regulation mechanisms. Plants. 11, 1547. doi: 10.3390/plants11121547
Woźniak, M., Gałązka, A., Siebielec, G., and Frąc, M. (2022). Can the biological activity of abandoned soils be changed by the growth of paulownia elongata× Paulownia fortunei?—Preliminary study on a young tree plantation. Agriculture. 12, 128. doi: 10.3390/agriculture12020128
Xu, Z., Pehlivan, N., Ghorbani, A., and Wu, C. (2022). Effects of azorhizobium caulinodans and piriformospora indica co-inoculation on growth and fruit quality of tomato (Solanum lycopersicum L.) under salt stress. Horticulturae 8, 302. doi: 10.3390/horticulturae8040302
Xu, L., Wang, A., Wang, J., Wei, Q., and Zhang, W. (2017). Piriformospora indica confers drought tolerance on zea mays L. Through enhancement of antioxidant activity and expression of drought-related genes. Crop J. 5, 251–258. doi: 10.1016/j.cj.2016.10.002
Yadav, S. P., Bharadwaj, R., Nayak, H., Mahto, R., Singh, R. K., and Prasad, S. K. (2019). Impact of salt stress on growth, productivity and physicochemical properties of plants: A review. Int. J. Chem. Stud. 7, 1793–1798.
Yahui, L., Xiuping, W., Yongmei, Z., Guoxin, Z., and Xuelin, L (2017). Determination of salt stress response and salt tolerance threshold of dandelion at seedling stage. Northwest Agric. J. 26, 1223–1229. doi: 10.21608/ejbo.2014.488
You, C. (2013). Preliminary study on mechanisms of drought resistance in brassica napus L. Conferred by piriformospora indica. J. Agric. Biotechnol. doi: 10.3969/j.issn.1674-7968.2013.03.003
Zalesny, J. A., Zalesny, R. S., Coyle, D. R., and Hall, R. B. (2007). Growth and biomass of populus irrigated with landfill leachate. For. Ecol. Management 248, 143–152. doi: 10.1016/j.foreco.2007.04.045
Keywords: Paulownia elongata, Piriformospora indica, antioxidant oxidase, salt tolerance, osmotic adjustment substance
Citation: Mu D, Zhang M, Liang Y, Ding C, Chen Q, Fan X, Meng X, Zhang X, Gao S, Zhai D, Gao Y and Wu Y (2025) Piriformospora indica enhances growth and salt tolerance in a short rotation woody crop, Paulownia elongata, under NaCl stress. Front. Plant Sci. 16:1566470. doi: 10.3389/fpls.2025.1566470
Received: 24 January 2025; Accepted: 26 May 2025;
Published: 23 June 2025.
Edited by:
Kai-Hua Jia, Shandong Academy of Agricultural Sciences, ChinaReviewed by:
Katarzyna Otulak-Kozieł, Warsaw University of Life Sciences, PolandAhsan Ayyaz, Zhejiang University, China
Copyright © 2025 Mu, Zhang, Liang, Ding, Chen, Fan, Meng, Zhang, Gao, Zhai, Gao and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chen Ding, Y3pkMDA4NEBhdWJ1cm4uZWR1; Yawei Wu, NTA5ODI0MjU5QHFxLmNvbQ==
 Deyu Mu
Deyu Mu Meng Zhang1
Meng Zhang1 Chen Ding
Chen Ding Xinpeng Zhang
Xinpeng Zhang Datong Zhai
Datong Zhai