- High Altitude Plant Physiology Research Centre, HemvatiNandan Bahuguna Garhwal University, Srinagar, Uttarakhand, India
Environmental stresses, including temperature extremes (cold and heat), elevated CO2, and ozone, significantly influence the production of plant secondary metabolites (PSMs). These environmental factors can lead to significant changes in the morphology, physiology, and biochemistry of plants. Natural resources, especially medicinal plants, have been used for centuries for their healing properties. PSMs, compounds with unique characteristics, often accumulate in response to stress, playing a crucial role in plant adaptation and stress tolerance. While environmental variables like temperature, light, water availability, humidity, CO2, and mineral nutrition are known to impact plant development and PSM synthesis, research on the effects of climate change on medicinal plants is limited compared to other commercial crops. This review examines the impact of various environmental factors on PSM synthesis in medicinal plants and identifies key knowledge gaps. We highlight the need for further research in this area and suggest potential directions for future studies to better understand and potentially manipulate the relationship between climate change, environmental stress, and the production of therapeutically valuable PSMs.
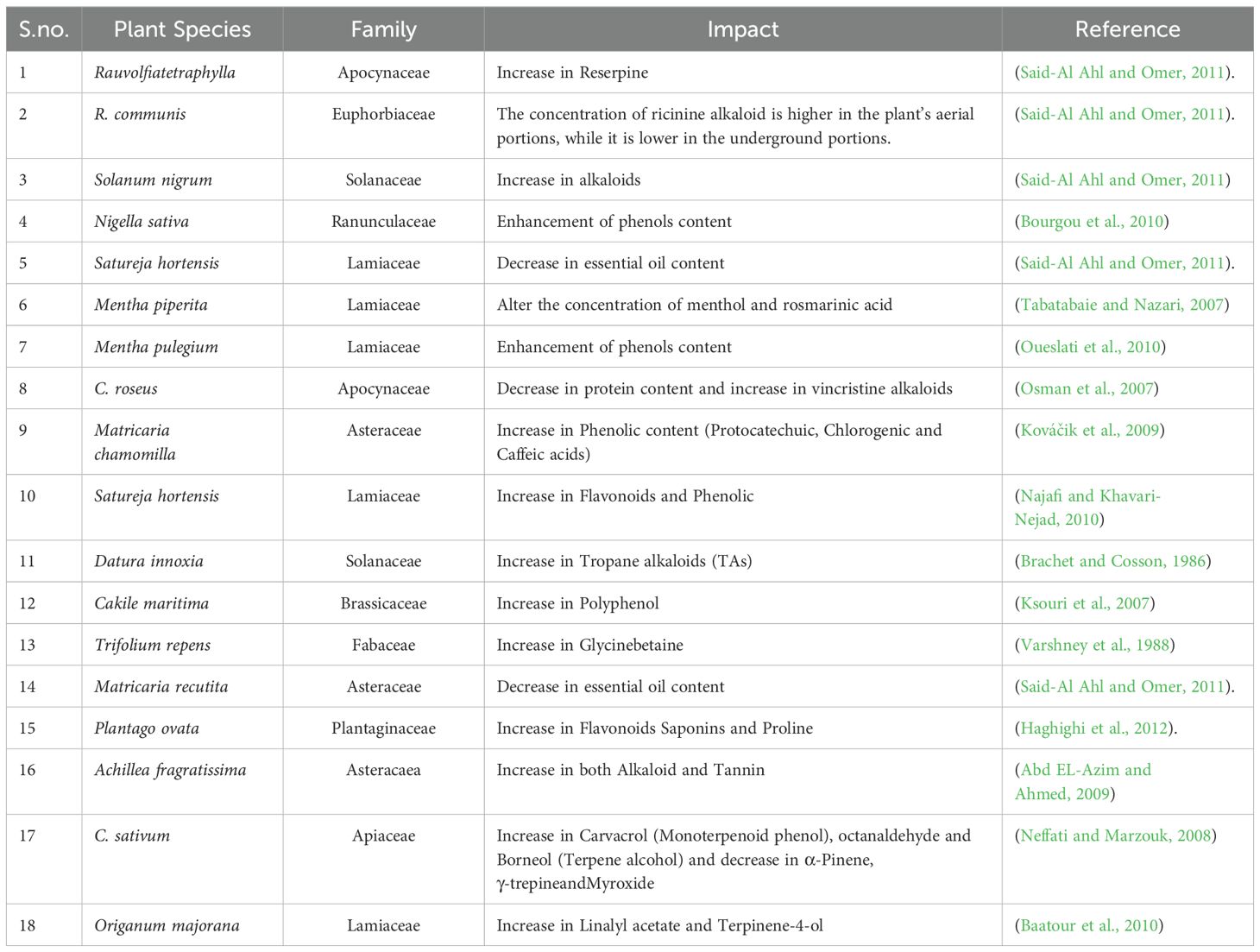
Graphical Abstract. The graphical abstract showing the impact of different abiotic stresses on plant based secondary metabolites. The adaptation strategies to mitigate adverse effects and sustain medicinal plant resources.
1 Introduction
Ecological communities and geographical distributions of plant species worldwide have been suffering the consequences of climate change (Applequist et al., 2020). The Fifth Assessment Report (AR5) of the Intergovernmental Panel on Climate Change (IPCC) projected that the global mean temperature, relative to preindustrial levels, is likely to increase by 1.5°C to 4°C by the end of the twenty-first century. Additionally, the report highlighted the likelihood of experiencing intense and unpredictable weather events in the future (Wu et al., 2018).
Climate change has notably affected medicinal plants, both cultivated and wild, with observable impacts on their growth, distribution, and overall health. A prompt and specific approach to studying these changes is crucial, especially when it comes to the gathering of vital secondary compounds that are essential components and significantly contribute to the preservation of human well-being.
Plant secondary metabolites (PSMs) are produced as byproducts of primary metabolic processes in plants (Khare et al., 2020). These chemical compounds can be volatile or non-volatile and produced through various metabolic pathways in plants, the PSMs have wider applicability including helping plants to adjust to various environmental conditions through affecting the physiological and ecological functioning of plants (Hussein and El-Anssary, 2019). Research studies on medicinal plants in relation to climate change are notably scarce and limited in comparison to other commercial crops.
Medicinal plants serve as promising reservoirs of nutraceuticals and bio-molecules, and it is time that this category of plants cannot be ignored (Harish et al., 2012). This review article thus aims to understand how climate change could affect the production of secondary compounds in medicinal plants. Medicinal plants, known for their diverse array of secondary metabolites with therapeutic properties, are particularly sensitive to a changing climate. The present review explored how changes in temperature, precipitation, elevated CO2 levels, and other climatic variables influence the biosynthesis of secondary metabolites, the gene expression, enzyme activities etc. The findings of this review may help policymakers and researchers to develop strategies for mitigating the impacts of climate change, including conservation efforts, cultivation practices, and biotechnological interventions.
2 Materials and methods
Seven different publication databases including Scopus, PubMed, Web of Science, Research Gate, EBSCO Green FILE, and Google Scholar were searched for the literature using the keywords “Climate change”, “Environmental stress”, “Plant adaptation”, Gene modification”, “Plant ecophysiology”, and “Secondary metabolite synthesis” etc. The articles obtained from these searches were scrutinized in the context of the medicinal plants. Further selected articles were studied and analyzed for a comprehensive review reporting how changing climatic conditions affecting the physiology and biochemistry of medicinal plants.
3 Plant secondary metabolites synthesis
Plant secondary compounds are involved in various defense functions, like protection against UV radiation, inhibiting enzymes, acting as antioxidants, and producing pigments. Secondary metabolites are organic compounds synthesized by plants that do not directly participate in their growth, development, or reproduction; instead, these compounds help plants to sustain in harsh climatic conditions (Hu et al., 2018).
There are about 30,000 compounds that belong to the class of Terpenoids (Mabou et al., 2021), around 8,000 phenolic compounds (Vuolo et al., 2019), and 21,000 compounds are alkaloids. The synthesis of secondary metabolites utilizes various metabolic pathways originating from primary metabolites. These metabolites can be categorized into two types based on their chemical composition:nitrogenous and non-nitrogenous compounds. Nitrogenous compounds are further classified as amines, amino acids, glucosinolates, non-proteinogenic, alkaloids and cyanogenic glycosides. The categorization of alkaloids includes several different types, such as proto alkaloid, cyclopeptide alkaloid, polyamine alkaloid, free alkaloid and pseudo alkaloid. Alkaloids are mainly composed of amino acids, which are considered the fundamental building blocks for their formation (Wink, 2010).
However, non-nitrogenous compounds, such as polyacetylenes and phenolics, are divided into four main categories (like arylpyrones,styrylpyrones, terpenoids and polyketides (Guerriero et al., 2018). Phenolic compounds are formed through two main pathways: the shikimic acid pathway and the malonate acetate pathway. These compounds are broadly classified into two major types: phenolic acids and phenylpropanoids (Naikoo et al., 2019). The phenylpropanoids are of eight types i.e. cinnamic aldehydes, suberin, lignans, flavonoids, stilbenes-coumarins, hydroxyl-cinnamic acid and lignin. The flavonoids compounds are divided into categories such as flavonols, isoflavones, flavones, flavanones, and anthocyanins.
Terpenoids, another category of secondary metabolites, are derived from a compound called isopentenyl diphosphate (IPP) that contains five carbons and serves as a precursor (Thirumurugan et al., 2018). The terpenoids are synthesized from the mevalonate pathway or the methyl erythritol phosphate pathway (Bartram et al., 2006). Terpenoids are classified on the basis of the presence of isoprene units like monoterpenes, triterpenes (which include sterols), diterpenes (such as gibberellins), sesquiterpenes (like ABA), and tetraterpenes (like Carotenoids).
4 Effect of climate change on secondary metabolites production
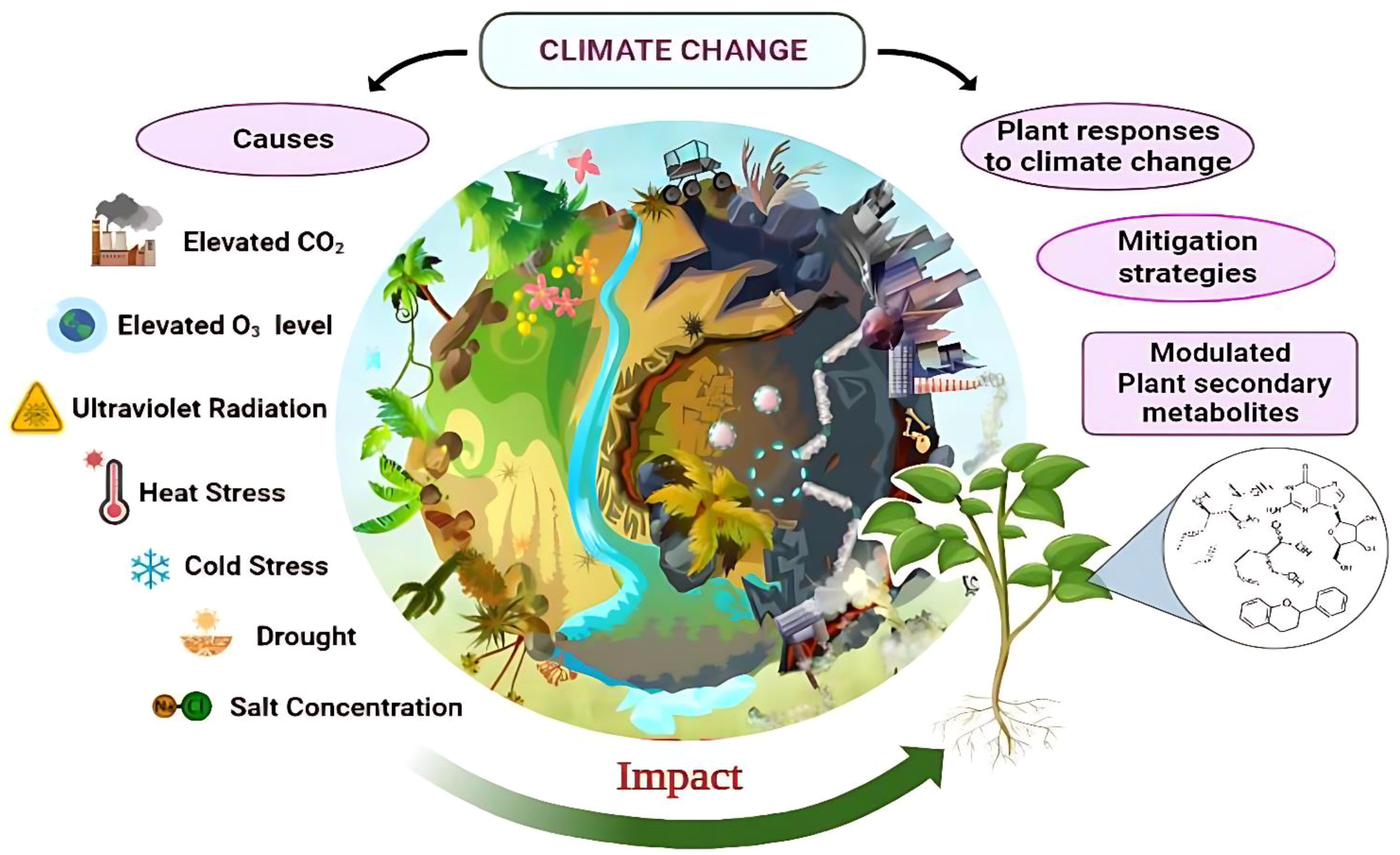
The shifting climate patterns are causing medicinal plants to experience environmental stresses, impacting their growth, development, and production of secondary metabolites (Pant et al., 2021; Figure 1). Increased levels of carbon dioxide (CO2) can impact plant secondary metabolism since CO2 is crucial for photosynthesis and the overall growth of plants (Jamloki et al., 2021). Another factor elevated temperatures also leads to heat stress in plants, affecting their structure, functions, and biochemical processes. The previous studies showed both increased and decreased amounts of plant secondary metabolism under simulated climate change (Pant et al., 2021; Chandra et al., 2022a, 2022). Other abiotic stresses, such as drought, salinity, exposure to heavy metals, and nutrient deficiencies also affect plant secondary metabolism (Punetha et al., 2022). The abiotic stresses induce the activation of genes responsible for the synthesis of secondary metabolites, antioxidants, osmolytes, and phytohormones (Mahajan et al., 2020; Akula and Ravishankar, 2011). Both internal developmental and external environmental factors influence the production of secondary metabolites in medicinal plants (Li et al., 2020). Developmental factors like plant genetics and growth stages interact with environmental factors like light, temperature, water, and soil conditions to determine the characteristics and number of secondary metabolites. An understanding of how these factors affect the plant secondary metabolism may be useful in the development of potent herbal medicine (Li et al., 2020).
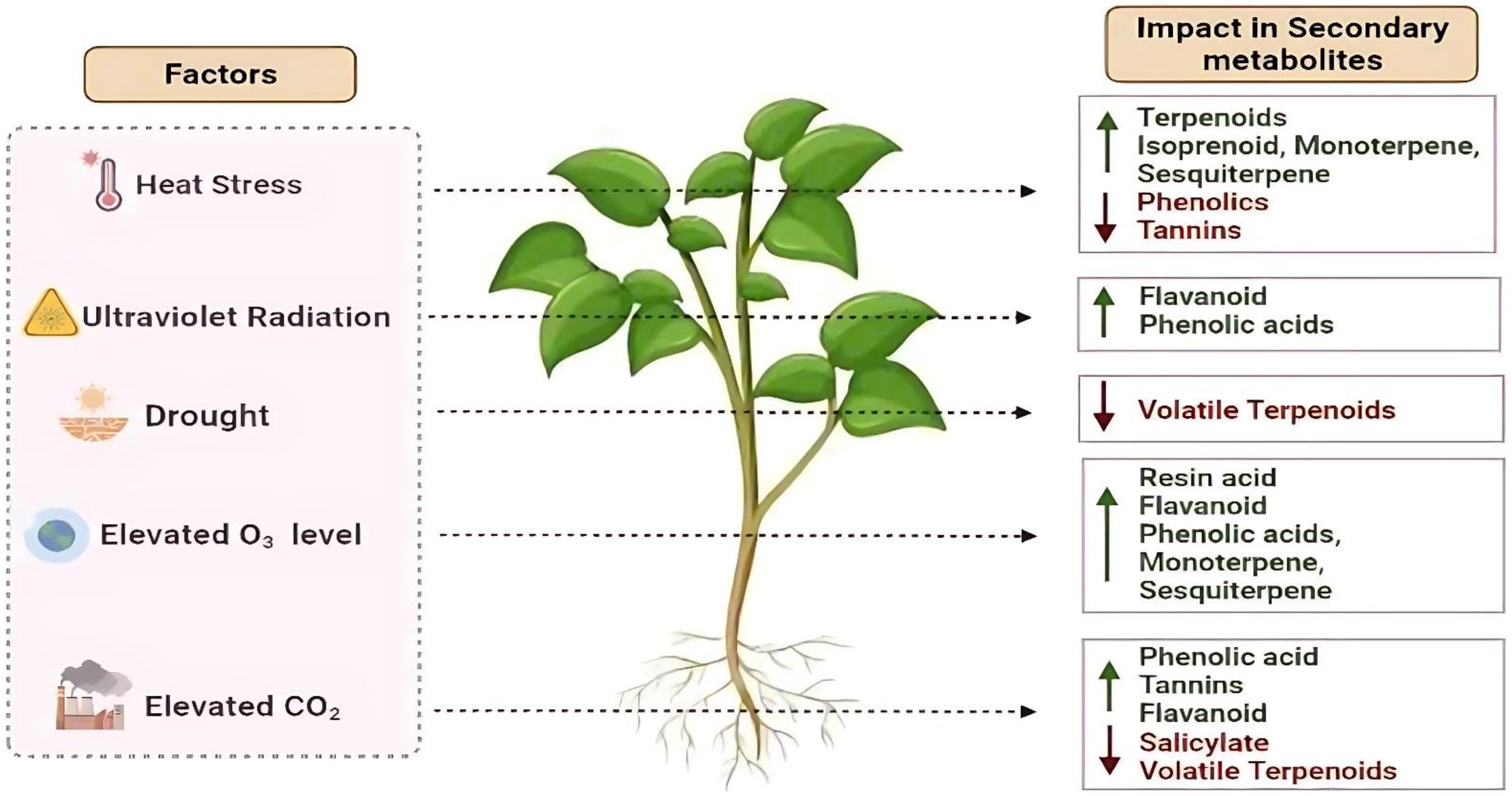
Figure 1. Schematic representation showing the impact of different abiotic stresses (e.g., drought, temperature extremes, salinity, elevated CO2, ozone, UV-B) on the production levels of various plant secondary metabolites. Upward and downward arrows indicate increases or decreases in metabolite levels, respectively.
4.1 Gene regulation during abiotic stress in medicinal plants
Omics technologies play a pivotal role in deciphering plant responses to stress at the gene regulatory level. They emphasize the importance of functional genomics, including metabolomics, transcriptomics, and proteomics, in understanding plant molecular responses to stress (Razzaq et al., 2021). The study of epigenomics is considered a valuable method for examining how plants adapt to environmental stresses at the molecular level and how epigenetic information is passed down from one generation to another (Perrone and Martinelli, 2020). Additionally, the role of bioinformatics in analyzing and identifying genetic elements related to stress tolerance is emphasized (Kumar D. et al., 2021). The complex genetic processes underlying stress tolerance and the promise of “omics” technologies in unravelling these mechanisms (Bagati et al., 2018).
Epigenetic modifications, such as histone methylation and DNA methylation, play a crucial role in regulating gene expression in response to abiotic stresses like drought, high salinity, and extreme temperatures (Santos et al., 2011; Shi et al., 2024). These modifications can lead to chromatin remodeling, altering the accessibility of transcription machinery and ultimately affecting plant homeostasis responses (Cadavid et al., 2023). In medicinal plants, these epigenetic changes can influence the production of secondary metabolites, which are responsible for their medicinal properties. Transcription factors, particularly those belonging to families such as WRKY, MYB, AP2/ERF, and NAC, act as mediators in regulating stress responses in plants. NAC transcription factors, for instance, are involved in regulating different signaling pathways of plant hormones that direct a plant’s immunity against pathogens and affect their responses to abiotic stresses. These transcription factors can also influence the biosynthesis of secondary metabolites in medicinal plants, potentially impacting their medicinal properties (Kumar R. et al., 2021).
Researchers have examined how Polygonatumkingianum, a medicinal plant in Traditional Chinese Medicine, responds to stress at the gene regulatory level (Qian et al., 2021). The research revealed that drought stress caused specific genes related to various pathways like phenylpropanoid biosynthesis, flavonoid biosynthesis, starch and sucrose metabolism, stilbenoid diarylheptanoid and gingerol biosynthesis, and carotenoid biosynthesis to exhibit different levels of activity an increase in starch and sucrose biosynthesis was evident from transcriptomic changes under drought stress. However, when the plants were rewatered following the period of drought stress, the tubers recovered, and there was an enhanced expression observed in certain genes. Phenylpropanoid and flavonoid biosynthesis pathways were commonly affected across multiple plant species under drought stress. In Helianthus tuberosus, genes related to these pathways were differentially expressed (Zhou et al., 2021). Similarly, in Polygonatumkingianum, drought stress reduced the expression of genes involved in lignin, gingerol, and flavonoid biosynthesis (Qian et al., 2021). In Arabidopsis thaliana, drought conditions led to significant changes in enzymes involved in lignin biosynthesis, such as phenylalanine lyase (PAL) and Caffeoyl Coenzyme A 3-O-methyltransferase 1 (COMT) (Lindberg et al., 2014). Ligularia fischeri, drought stress increased the expression of flavone synthase (LfFNS) and anthocyanin 5-O-glucosyltransferase (LfA5GT1), leading to higher levels of flavones and anthocyanins, while decreasing the expression of genes involved in caffeoylquinic acid biosynthesis (Park et al., 2023). Key genes in these pathways, such as phenylalanine ammonia-lyase (PAL), chalcone synthase (CHS), flavonol synthase (FLS), and anthocyanidin synthase (ANS), show increased expression under drought conditions (Ahmed et al., 2021; Park et al., 2023).
5 The effects of climate change on medicinal plants
The life cycles of plants are indeed intricately linked with seasonal changes and are susceptible to alterations due to both natural variations and climate change. The phenomenon of global climate change has brought about disruptions in ecosystems and biodiversity, affecting various species. While many plants are impacted, it is noteworthy that only a subset of medicinal plant species is experiencing adverse effects due to these shifts in plant phenology. These changes primarily involve modifications in crucial aspects such as fruit and flower production (Chandra et al., 2020, 2022a), the growth patterns of leaves and buds, and the timing of leaf shedding, especially in autumn or during dry spells (Bidart-Bouzat and Imeh-Nathaniel, 2008).
The rise in global temperatures is expected to significantly affect the synthesis of secondary metabolites in medicinal plants. Metabolites present in medicinal plants, including immune suppressants, anti-diabetic, and anti-cancer agents, play pivotal roles in the plant’s interactions with its environment and have significant implications for human health. These natural compounds are essential for maintaining the delicate balance between plants and their surroundings, while also providing us with crucial remedies to combat various diseases (Sun et al., 2023). Recent research indicates that plants produce secondary metabolites in response to stressful conditions. Furthermore, it is anticipated that the secondary metabolism of plants will be significantly influenced by the major shifts in global climate that are predicted to occur (Pant et al., 2021). Although there is still insufficient research conducted on the impact of climate change-induced temperature rise on plant secondary metabolism (Loreto et al., 2006). The impact of various abiotic factors on medicinal plants has been discussed under the following subheadings.
5.1 Impact of elevated CO2
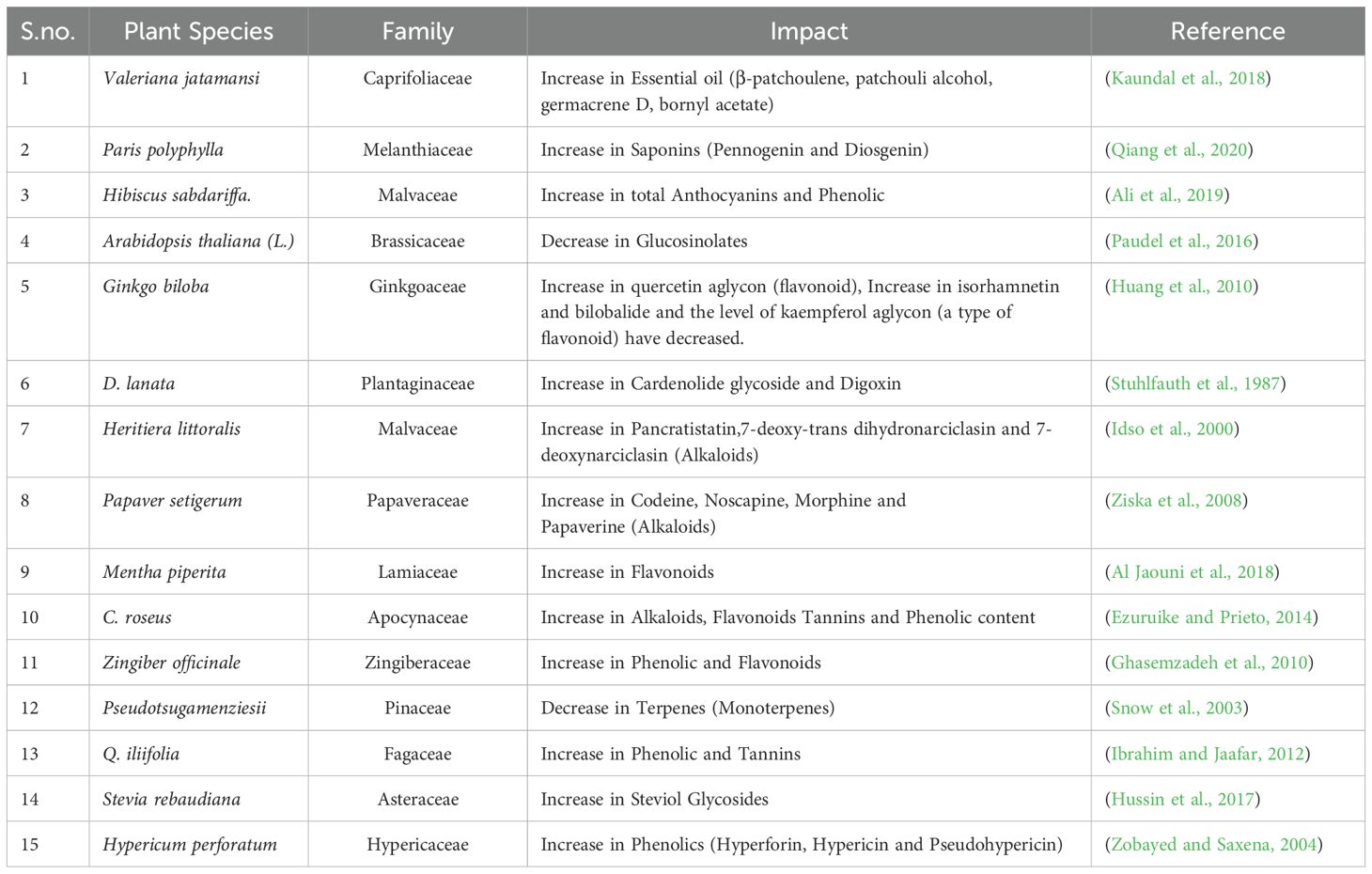
The presence of secondary metabolites in medicinal plant species helps them to sustain in adverse climatic conditions. These compounds in plants are secreted through various metabolic pathways (Figure 2) and the concentration of these secondary metabolites increases or decreases in environmental stresses to prevent cellular damage. This adaptive behavior affects the therapeutic properties of plants which is due to the concentration of secondary metabolites (Mishra, 2016).The results of experiments conducted under normal climate conditions indicate that the increase of carbon dioxide (CO2) has positive effects on various plant parts and products that are utilized in pharmaceuticals. The comparison was made between shoots that were grown in a culture medium with ambient air containing 3000μL CO2/L and those that were grown in an elevated CO2 environment. The plant species, including Mentha spicata, Thymus vulgaris, Ocimumbasilicum, and Origanum vulgare, demonstrated encouraging outcomes by displaying a notable rise in both leaf and root counts, accompanied by an increase in overall fresh weight. In other words, these plants responded positively to the treatment, resulting in a significant boost in their growth and overall health (Tisserat and Vaughn, 2001; Table 1).
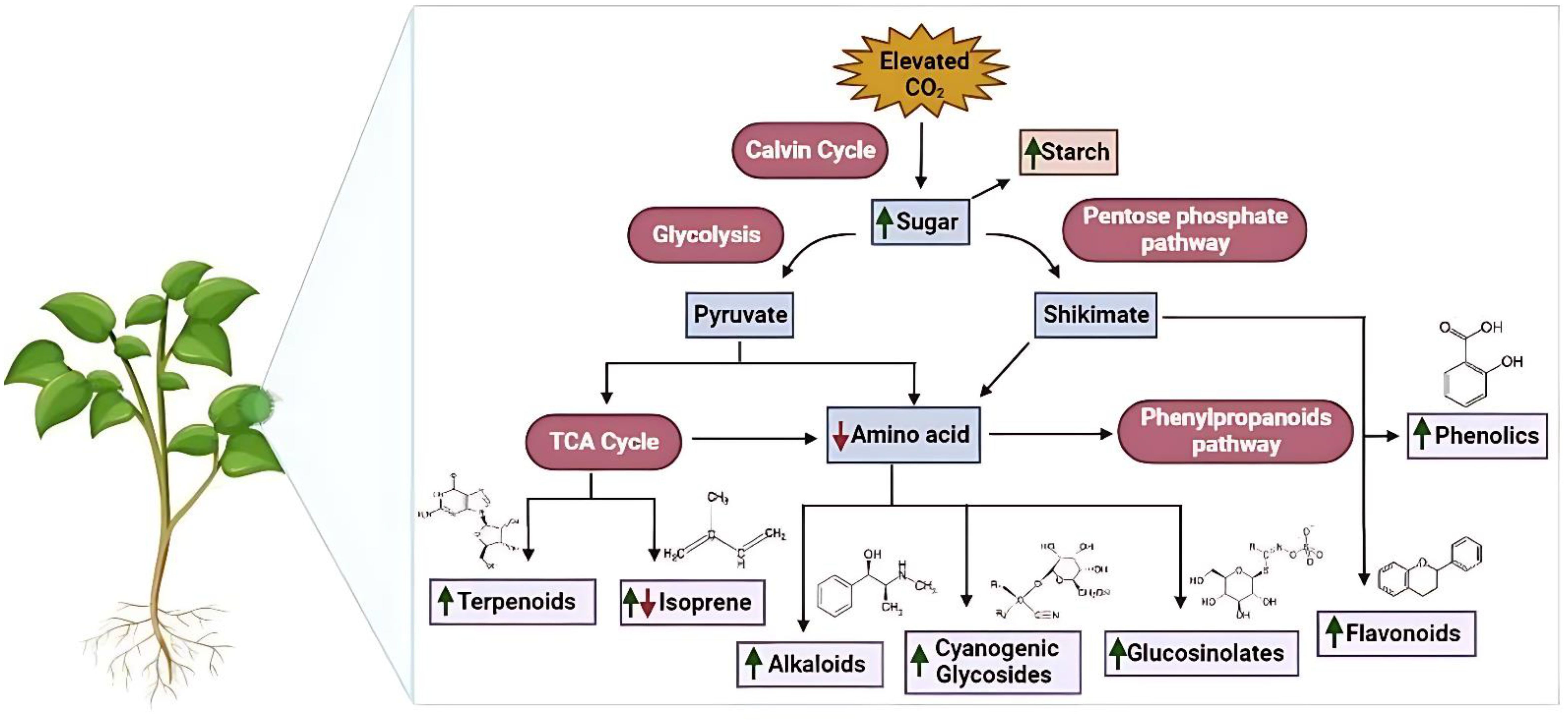
Figure 2. Elevated CO2 effects on secondary metabolite synthesis in medicinal plants. The figure highlights metabolic pathways influenced by elevated CO2 and associated physiological responses such as enhanced biomass and altered phytochemical concentrations.
The concentration of secondary metabolites in plants is influenced not only by the concentration of CO2 but also by the duration of exposure. For instance, the bulbs of the beach spider lily, scientifically known as Hymenocallis littoralis, are famous for their ability to combat cancer and viruses. In a study conducted on these bulbs, the concentrations of three alkaloids, namely pancratistatin, 7-deoxy-trans dihydronarciclasin, and 7-deoxynarciclasine, were found to increase steadily during the first year of the experiment, but after that period, there was a notable reduction in their level of concentration (Idso et al., 2000).
5.2 Effect of elevated ozone
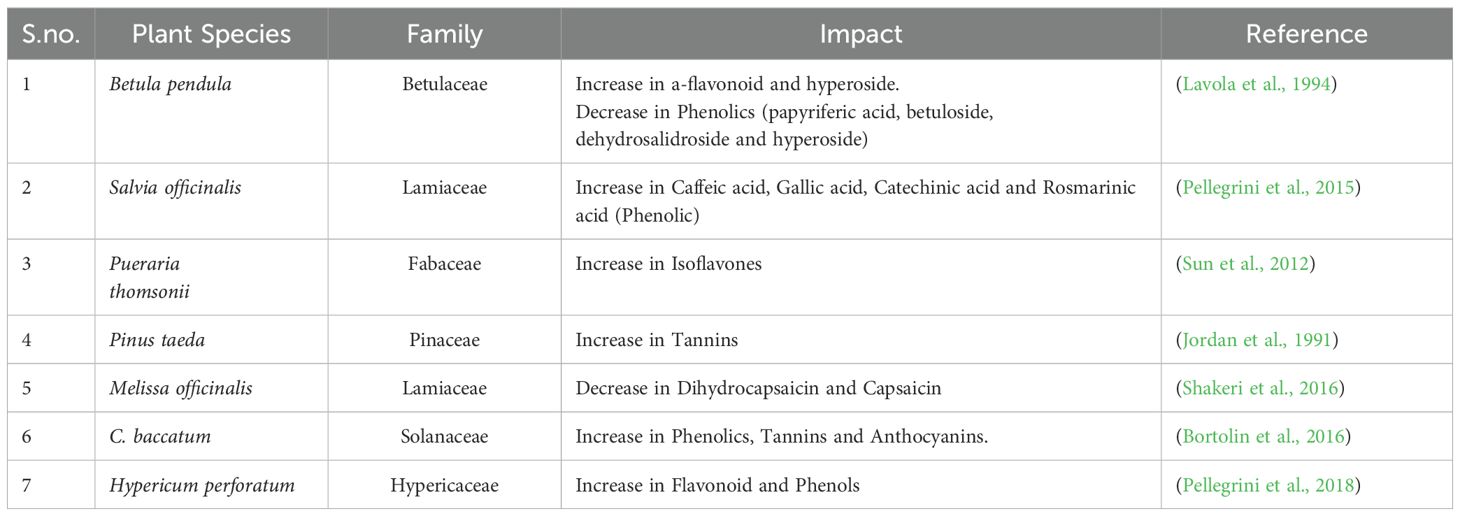
The presence of ozone in the environment can impact the synthesis of secondary compounds in plants (Table 2). Elevated levels of ozone (O3) have been shown to activate important metabolic pathways, including the jasmonic acid and salicylic acid pathways, which are essential for the synthesis of secondary metabolites in plants. These pathways are typically triggered in response to physiological stress. When plants are exposed to increased levels of ozone, these metabolic pathways can be activated as a natural response to the stress induced by elevated ozone levels. This activation stimulates the generation of secondary metabolites, helping plants cope with the environmental challenge without compromising their overall health and survival (Mishra, 2016).
5.3 Impact of ultraviolet-B radiation
UV-B radiation has the potential to inflict harm on a range of biomolecules, including DNA, proteins, and other essential cellular constituents. The exposure to this form of radiation holds the capacity to influence the overall growth and developmental processes of plants, resulting in alterations to their reproductive and vegetative biomass, height, flowering schedules and leaf characteristics (Mishra, 2016; Table 3).
When reactive oxygen species (ROS) are generated, they initiate a defensive signaling pathway, stimulating the synthesis of secondary metabolites. These metabolites can absorb Ultraviolet-B radiation and include compounds such as anthocyanins, alkaloids, flavonoids, lignin, tannins, phytosterols and saponins (Takshak and Agrawal, 2014; dos Santos Nascimento et al., 2015). Furthermore, changes in the functioning of antioxidant enzymes can be noted, as demonstrated by enzymes originating from the phenylpropanoid pathway (Takshak and Agrawal, 2014). Anthocyanins, a crucial class of pigments discovered in fruits, flowers, and leaves, are predominantly located within the epidermal cells of flowers and other plant components. They play a regulatory role in absorbing ultraviolet-B radiation. Phenolic compounds are abundantly found in plants, and numerous investigations have substantiated their augmentation in enzymatic activities across diverse studies. During exposure to ultraviolet-B stress, flavonoids are produced through enzymatic processes within the phenylpropanoid pathway. Exposure to ultraviolet-B radiation enhances the activation of specific genes responsible for encoding enzymes directly engaged in anthocyanin biosynthesis. Increased enzyme activities, such as Chalcone synthase (CHS), Flavanone 3-hydroxylase (F3H), and Dihydroflavonol reductase (DFR), enhance the anthocyanin content within the plant (Park et al., 2007).
5.4 Impact of temperature on secondary metabolites
A suitable temperature is necessary for plant development. Plants that experience heat or cold stress are negatively impacted by high and low temperatures, respectively (Yadav, 2010).
5.4.1 Impact of heat stress
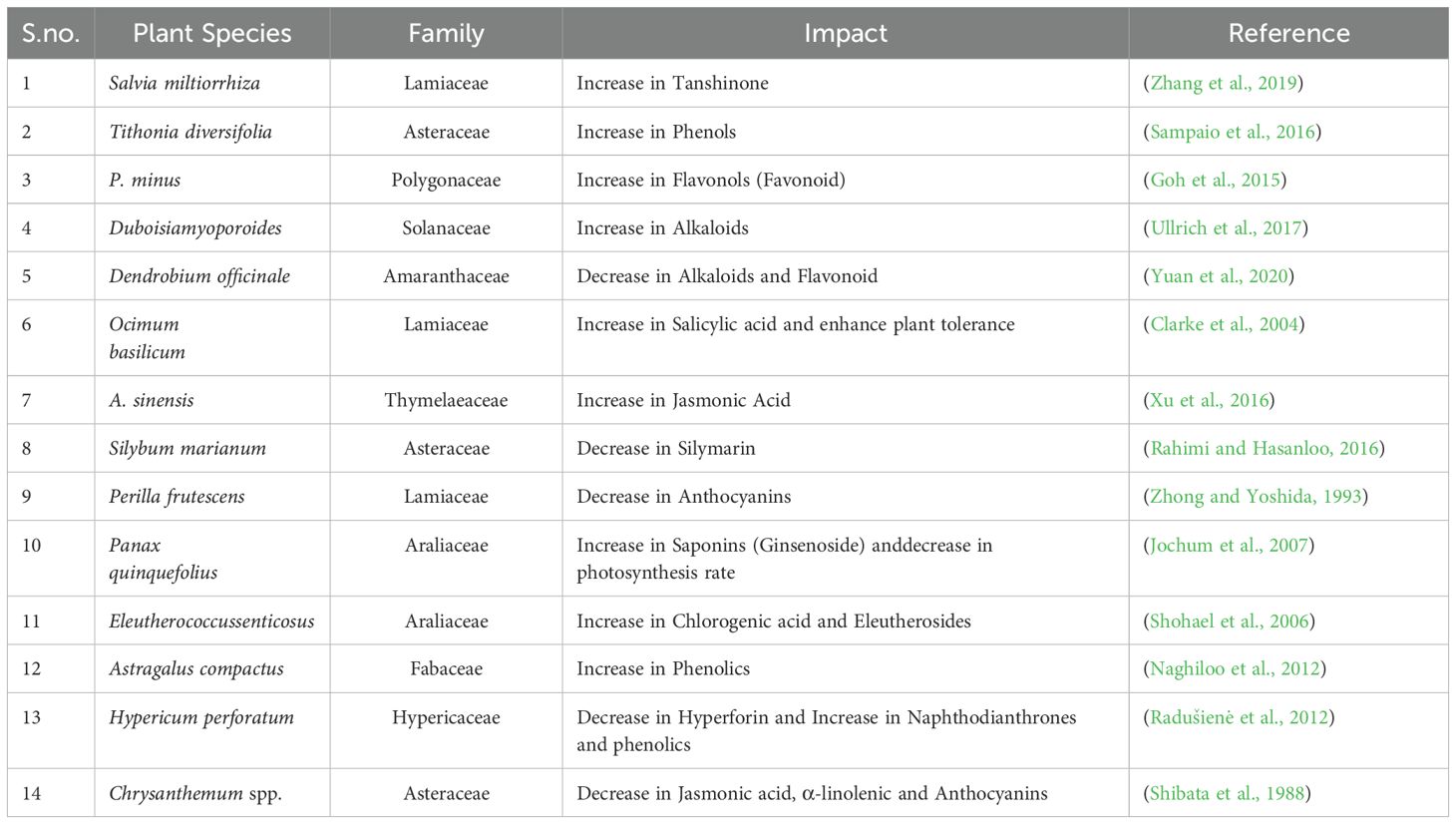
At temperatures higher than optimal, plants experience heat stress. It affects the stomatal conductance, which in turn slows down photosynthesis and plant development. The synthesis of PSMs is also affected by high temperatures. The photosystem II exhibits a decrease in photochemical effectiveness at high temperatures, which increases plant stress. Several research studies have provided evidence indicating that elevated temperatures can trigger the increased production of secondary metabolites (Table 4). However, contrasting findings have also been observed in certain studies, where secondary metabolite levels showed a decline. For instance, in the case of Panax quinquefolius plants, an increase in temperature was found to result in an augmentation of root ginsenoside quantities and in another study on two high-altitude aromatic plants viz., Angelica glauca and Nardostachysjatamansi, monoterpenes were found to be decreased in response to elevated CO2 and temperature gradients (Jochum et al., 2007; Dobhal et al., 2024).
Heat stress induces changes in gene expression that facilitate the activation of molecular mechanisms protecting plants against heat stress (Zhu et al., 2013). A majority of these genes control the production of regulatory, transporter, detoxifying, and Osmo-protective proteins. When a plant is exposed to high temperatures, it can develop an ability to tolerate heat through either adaptation or acclimation. This is achieved by changing its biochemical and physiological processes, which are influenced by modifications in gene expression (Mirza et al., 2010).
5.4.2 Impact of cold stress
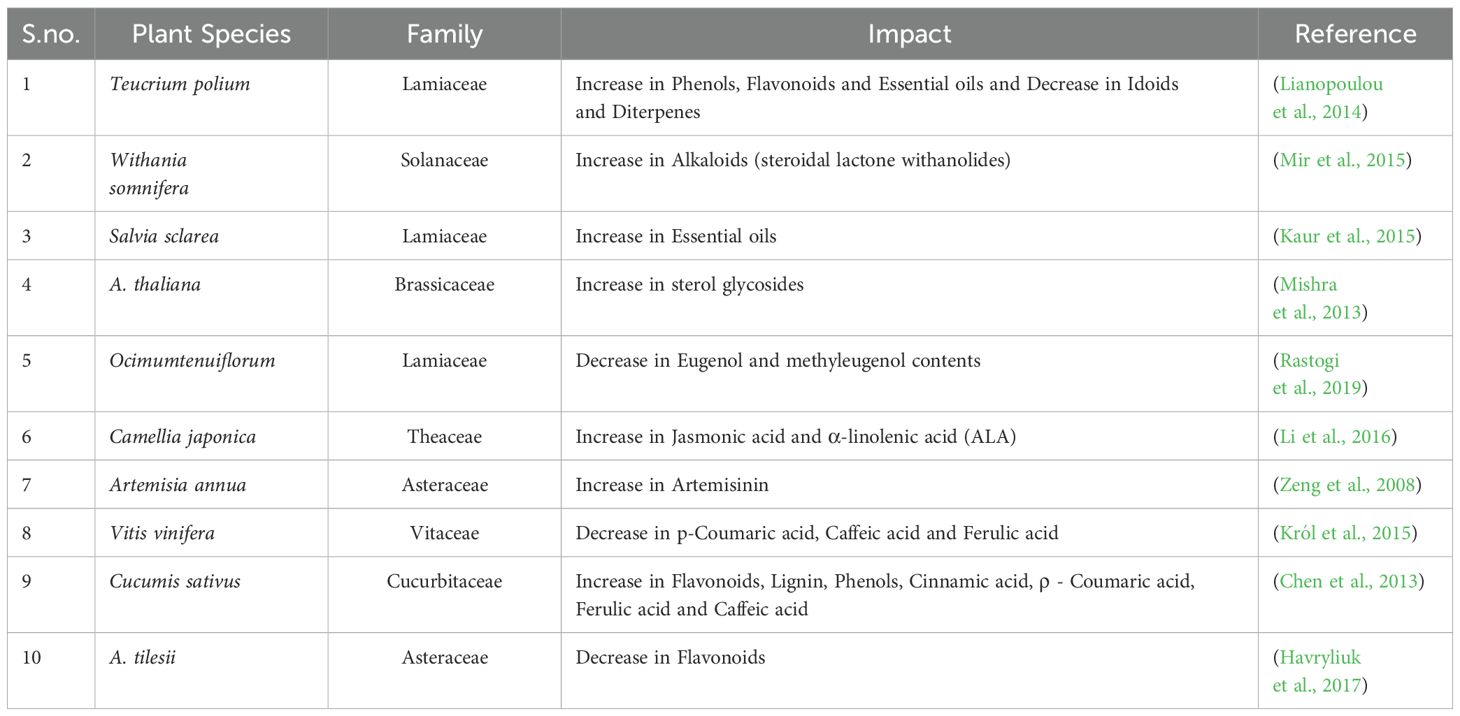
Plants are adversely affected by low temperatures, which can result in stress and various negative consequences such as decreased growth, reduced productivity, loss of diversity, and limited distribution (Chinnusamy et al., 2007; Rahman, 2013) (Table 5). Plants can alter their coping mechanisms to tolerate cold stress by transferring resources and slowing development (Eremina et al., 2016). Plant physiology is directly impacted by low temperatures (Ruelland et al., 2009). The concentration of cellular membrane fluidity is altered by low temperatures (Upchurch, 2008; Sevillano et al., 2009). Plants possess the capacity to undergo physiological, biochemical, and molecular alterations in response to low temperatures, a phenomenon commonly referred to as cold acclimation. This adaptive mechanism enables them to better tolerate and survive in colder environments. When plants are exposed to cold stress, they frequently show a reduction in chlorophyll a levels and overall chlorophyll content, accompanied by an increase in leaf apoplastic and total soluble protein levels (Zhou et al., 2021; Aazami et al., 2021). These adaptations serve as strategies employed by plants to effectively adapt to and endure the adverse effects of low temperatures. By modifying these sentences, the phrasing and structure have been altered to reduce plagiarism and paraphrase the information (Esra et al., 2010). When plants are exposed to low temperatures, the production of free oxygen radicals increases, leading to elevated internal stress levels in plant cells. In response, plants activate antioxidants to counteract and eliminate these radicals (Sevillano et al., 2009 & Ruelland et al., 2009).To endure unfavorable circumstances, plant cells employ strategies to uphold their structural integrity, including augmenting their amino acid content, soluble solids, and cryoprotective proteins. This process involves the activation of different enzymatic and metabolic pathways (Ruelland et al., 2009; Eremina et al., 2016).
Certain medicinal plants, including Teucrium polium, Thymus sibthorpii, Phlomisfruticosa, Saturejathymbra, and Cistus incanus, exhibit a phenomenon known as seasonal dimorphism. This characteristic enables them to employ specific defense strategies that vary depending on the particular season (Lianopoulou et al., 2014). Origanum dictamnus plants exhibit seasonal variation through different defense mechanisms triggered by various hormones. To cope with cold temperatures, these plants undergo several adaptations such as altering leaf size, shape, and arrangement. They also develop a waxy coating, thicker cuticles, and a dense layer of non-glandular hairs on the leaf epidermis. These modifications aid in cold tolerance. Similarly, in high temperatures, the plants’ mesophyll cells possess enlarged intracellular spaces to efficiently store air (Lianopoulou and Bosabalidis, 2014). The essential oils in these plants seem to vary in composition depending on external temperature changes, as evidenced by the fact that during winter, p-Cymene concentrations were at 60%, while during summer, carvacrol concentrations were at 42% (Lianopoulou and Bosabalidis, 2014).
5.5 Impact of drought stress
Drought occurs when plants are unable to obtain an adequate water supply, which leads to a decline in turgor and water potential. This water deficiency hampers their normal physiological processes, resulting in disruptions to their regular functions (Lisar et al., 2012).When stomata close, the photosynthetic rate decreases, the activity of ATP synthesis enzymes diminishes, and cell membranes can suffer damage. Furthermore, osmotic stress caused by drought negatively impacts the production of cereal crops (Valentovic et al., 2006).
Drought stress during the cultivation of spices and medicinal plants can influence the contents of secondary metabolites. The studies found that plants tend to accumulate increased levels of specific natural compounds, including isoprenoids, alkaloids, flavones and phenols under conditions of drought stress (Table 6). This increase in secondary metabolites is attributed to metabolic reactions triggered by a lack of water, which influences the rate of metabolic activities in plants. It is crucial to emphasize that extended periods of drought can result in decreased levels of secondary metabolites in plants. This decline can be attributed to significant reductions in overall plant growth during prolonged drought phases. The application of stress signal transducers, like salicylic acid and methyl jasmonate, can also enhance the concentrations of specific bioactive compounds. Overall, the findings suggest that intentionally causing a significant amount of drought stress while cultivating can enhance the quality and quantity of secondary metabolites in medicinal plants (Gabbish et al., 2015; Shil and Dewanjee, 2022; Kleinwächter and Selmar, 2015).
A number of previous research studies have explored the impact of drought conditions on the synthesis of bioactive compounds in plants. The findings consistently indicate that in response to drought stress, plants augment the production of secondary metabolites, like terpenoids, alkaloids, phenolics, glucosinolates, and cyanogenic glucosides. Concurrently, these plants experience a reduction in their growth rate due to the constraints imposed by limited water availability (Yeshi et al., 2022). When the production of biomass is diminished, it typically leads to an elevation in the levels of secondary metabolites within plants. However, this increase in concentration is not due to a faster rate of metabolite synthesis but rather is dependent on whether the weight of the plant material is measured in terms of fresh or dry weight. In other words, whether the total concentration of secondary metabolites increases or not depends on the method used to measure the weight of the plant material (Kleinwächter and Selmar, 2015).
In a study by (Nowak et al., 2010) on Salvia officinalis, the monoterpene concentration increased dramatically under water stress;this elevation in monoterpene concentration was considerably greater in contrast to the decrease in biomass observed in the control group, which was grown under conditions of adequate water supply. Petroselinum crispum, also known as parsley, was the focus of an experiment that showed increases in monoterpene concentration to be substantially greater than decreases in leaf biomass (Petropoulos et al., 2008). Origanum vulgare had stable essential oil content per plant, but when it was under drought conditions, the amount of metabolites increased (Ninou et al., 2017). According to (Paulsen and Selmar, 2016), the production of monoterpenes remained consistent despite a reduction in the amount of biomass used. They explained the increase in monoterpene content in thyme plants based on this observation. Additionally, the concentration of n-monoterpenes did not change overall.
Dry weight measurements revealed that the rate of synthesis differed between drought-stressed plants and control plants cultivated under optimal watering conditions. Specifically, in the early stages of the experiment, stressed plants showed significantly higher rates of synthesis than the control group, as measured by dry weight. However, this finding changed when the duration of drought was extended (Paulsen and Selmar, 2016). A similar trend was seen in the levels of phenolic compounds in Hypericum brasiliense during periods of drought. Under the specified conditions, there was a noteworthy rise in both the quantity and potency of these compounds. This escalation in the phenolic content resulted in reduced plant sizes in stressed specimens as opposed to those grown under normal conditions (de Abreu and Mazzafera, 2005).
5.6 Impact of salinity
Exposing cells to a high concentration of salt causes them to lose water from their cytoplasm, which creates osmotic pressure. High salt concentrations in plants can induce ionic and osmotic stresses, leading to a decrease in cytosol and vacuole volumes. This exposure can also trigger alterations in different secondary metabolite concentrations (Mahajan and Tuteja, 2005) (Table 7). According to reports, there is evidence to suggest that under conditions of salt stress, there is an observed elevation in the concentrations of anthocyanins (Parida and Das, 2005). Certain species were found to be sensitive to salt stress, and as a result, they showed a reduction in the level of anthocyanin compared to other species that were not affected by salt stress and had no change in the quantity of anthocyanin present (Daneshmand et al., 2010). Plants respond to increased salinity by activating enzymes and regulatory genes that affect the production of secondary metabolites. The quantity of these metabolites that the plant produces changes depending on its particular requirements (Punetha et al., 2022).
6 Adaptation with climate change and global warming
Medicinal plants are facing significant challenges due to climate change and global warming, necessitating adaptation strategies to ensure their survival and continued availability for human use. Climate change is affecting the distribution, growth, and chemical composition of medicinal plants. Rising temperatures and changing precipitation patterns are altering suitable habitats for these species, potentially leading to population declines (Cahyaningsih et al., 2021). Additionally, environmental stresses can impact the production of secondary metabolites, which affect the therapeutic potential of medicinal plants (Harish et al., 2012). The key adaptation strategies may include conserving threatened species (both in-situ and ex-situ), promoting local cultivation, training harvesters in sustainable practices, certifying commercial material, and monitoring raw material quality to ensure efficacy (Applequist et al., 2020; Cahyaningsih et al., 2021).
7 Conclusion and future recommendations
The production and variation of secondary metabolites play a significant role in enabling plants to adapt and thrive in diverse environmental conditions. Indigenous plant species are particularly vulnerable to climate change, which can affect their secondary metabolite production, threatening their survival. The environmental stressors like elevated carbon dioxide levels, temperature stress, drought, and high salinity can enhance the production of secondary metabolites. However, these environmental factors may also negatively affect plant growth and overall productivity. The development of effective and sustainable strategies to enhance secondary metabolite synthesis in plants facing climate change requires future research on the molecular mechanisms controlling biosynthesis and the combined effects of multiple environmental factors on their production.
Author contributions
DJ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Writing – original draft. BP: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – review & editing. VC: Investigation, Writing – review & editing. SC: Investigation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to thank the Director, High Altitude Plant Physiology (HAPPRC), HNB Garhwal University, Srinagar Garhwal, Uttarakhand, India, for their support, cooperation and Assistance throughout this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aazami, M. A., Asghari-Aruq, M., Hassanpouraghdam, M. B., Ercisli, S., Baron, M., and Sochor, J. (2021). Low temperature stress mediates the antioxidants pool and chlorophyll fluorescence in Vitis vinifera L. cultivars. Plants 10, 1877. doi: 10.3390/plants10091877
Abd EL-Azim, W. M. and Ahmed, S. T. (2009). Effect of salinity and cutting date on growth and chemical constituents of Achillea fragratissimaForssk, under Ras Sudr conditions. Res. J. Agr. Biol. Sci. 5, 1121–1129.
Ahmed, U., Rao, M. J., Qi, C., Xie, Q., Noushahi, H. A., Yaseen, M., et al. (2021). Expression profiling of flavonoid biosynthesis genes and secondary metabolites accumulation in populus under drought stress. Molecules 26, 5546. doi: 10.3390/molecules26185546
Akula, R. and Ravishankar, G. A. (2011). Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 6, 1720–1731. doi: 10.4161/psb.6.11.17613
Alavi-Samani, S. M., Kachouei, M. A., and Pirbalouti, A. G. (2015). Growth, yield, chemical composition, and antioxidant activity of essential oils from two thyme species under foliar application of jasmonic acid and water deficit conditions. Hortic. Environ. Biotechnol. 56, 411–420. doi: 10.1007/s13580-015-0117-y
Ali, S. A. M., Latip, J., and Zain, C. R. C. M. (2019). “Growth and phenolic constituents production in roselle (Hibiscus sabdariffa var. UKMR-2) as influenced by irrigation treatment,” in AIP Conference Proceedings, Vol. 2111. (AIP Publishing) (1). doi: 10.1063/1.5111261
Al Jaouni, S., Saleh, A. M., Wadaan, M. A., Hozzein, W. N., Selim, S., and AbdElgawad, H. (2018). Elevated CO2 induces a global metabolic change in basil (Ocimumbasilicum L.) and peppermint (Mentha piperita L.) and improves their biological activity. J. Plant Physiol. 224, 121–131. doi: 10.1016/j.jplph.2018.03.016
Applequist, W. L., Brinckmann, J. A., Cunningham, A. B., Hart, R. E., Heinrich, M., Katerere, D. R., et al. (2020). Scientists’ warning on climate change and medicinal plants. Planta Med. 86, 10–18. doi: 10.1055/a-1113-1659
Azhar, N., Hussain, B., Ashraf, M. Y., and Abbasi, K. Y. (2011). Water stress mediated changes in growth, physiology and secondary metabolites of desi ajwain (Trachyspermumammi L.). Pakistan J. Bot. 43, 15–19.
Baatour, O., Kaddour, R., Aidi Wannes, W., Lachaal, M., and Marzouk, B. (2010). Salt effects on the growth, mineral nutrition, essential oil yield and composition of marjoram (Origanum majorana). Acta Physiol. Plant. 32, 45–51. doi: 10.1007/s11738-009-0374-4
Bagati, S., Nazir, M., Dar, A. A., Zargar, S. M., and Mahajan, R. (2018). “Omics”: a gateway towards abiotic stress tolerance (Springer Singapore), pp. 1–45. doi: 10.1007/978-981-10-7479-0_1
Bartram, S., Jux, A., Gleixner, G., and Boland, W. (2006). Dynamic pathway allocation in early terpenoid biosynthesis of stress-induced lima bean leaves. Phytochemistry 67, 1661–1672. doi: 10.1016/j.phytochem.2006.02.004
Bidart-Bouzat, M. G. and Imeh-Nathaniel, A. (2008). Global change effects on plant chemical defenses against insect herbivores. J. Integr. Plant Biol. 50, 1339–1354. doi: 10.1111/j.1744-7909.2008.00751.x
Bortolin, R. C., Caregnato, F. F., Junior, A. M. D., Zanotto-Filho, A., Moresco, K. S., de Oliveira Rios, A., et al. (2016). Chronic ozone exposure alters the secondary metabolite profile, antioxidant potential, anti-inflammatory property, and quality of red pepper fruit from Capsicum baccatum. Ecotoxicol. Environ. Saf. 129, 16–24. doi: 10.1016/j.ecoenv.2016.03.004
Bourgou, S., Kchouk, M. E., Bellila, A., and Marzouk, B. (2010). Effect of salinity on phenolic composition and biological activity of Nigella sativa. Acta Hortic. 853, 57–60. doi: 10.17660/actahortic.2010.853.5
Brachet, J. and Cosson, L. (1986). Changes in the total alkaloid content of Datura innoxia Mill. subjected to salt stress. J. Exp. Bot. 37, 650–656. doi: 10.1093/jxb/37.5.650
Cadavid, I. C., Balbinott, N., and Margis, R. (2023). Beyond transcription factors: more regulatory layers affecting soybean gene expression under abiotic stress. Genet. Mol. Biol. 46, e20220166. doi: 10.1590/1678-4685-gmb-2022-0166
Cahyaningsih, R., Phillips, J., Brehm, J. M., Gaisberger, H., and Maxted, N. (2021). Climate change impact on medicinal plants in Indonesia. Global Ecol. Conserv. 30, e01752. doi: 10.1016/j.gecco.2021.e01752
Chandra, S., Chandola, V., Nautiyal, M. C., and Purohit, V. K. (2020). Elevated CO 2 causes earlier flowering in an alpine medicinal herb Aconitum heterophyllum Wall. Curr. Sci. 00113891), 118(11).
Chandra, S., Chandola, V., Sultan, Z., Singh, C. P., Purohit, V. K., Nautiyal, B. P., et al. (2022b). Climate change adversely affects the medicinal value of Aconitum species in Alpine region of Indian Himalaya. Ind. Crops Prod. 186, 115277. doi: 10.1016/j.indcrop.2022.115277
Chandra, S., Singh, A., Mathew, J. R., Singh, C. P., Pandya, M. R., Bhattacharya, B. K., et al. (2022a). Phenocam observed flowering anomaly of Rhododendron arboreum Sm. in Himalaya: a climate change impact perspective. Environ. Monit. Assess. 194, 877. doi: 10.1007/s10661-022-10466-1
Chen, S., Jin, W., Liu, A., Zhang, S., Liu, D., Wang, F., et al. (2013). Arbuscular mycorrhizal fungi (AMF) increase growth and secondary metabolism in cucumber subjected to low temperature stress. Scientia Hortic. 160, 222–229. doi: 10.1016/j.scienta.2013.05.039
Chen, Y., Zhang, X., Guo, Q., Liu, L., Li, C., Cao, L., et al. (2018). Effects of UV-B radiation on the content of bioactive components and the antioxidant activity of Prunella vulgaris L. Spica during development. Molecules 23, 989. doi: 10.3390/molecules23050989
Cheng, L., Han, M., Yang, L. M., Li, Y., Sun, Z., and Zhang, T. (2018). Changes in the physiological characteristics and baicalin biosynthesis metabolism of Scutellariabaicalensis Georgi under drought stress. Ind. Crops Prod. 122, 473–482. doi: 10.1016/j.indcrop.2018.06.030
Chinnusamy, V., Zhu, J., and Zhu, J. K. (2007). Cold stress regulation of gene expression in plants. Trends Plant Sci. 12, 444–451. doi: 10.1016/j.tplants.2007.07.002
Clarke, S. M., Mur, L. A., Wood, J. E., and Scott, I. M. (2004). Salicylic acid dependent signalling promotes basal thermotolerance but is not essential for acquired thermotolerance in Arabidopsis thaliana. Plant J. 38, 432–447. doi: 10.1111/j.1365-313X.2004.02054.x
Daneshmand, F., Arvin, M. J., and Kalantari, K. M. (2010). Physiological responses to NaCl stress in three wild species of potato in vitro. Acta Physiol. Plant. 32, 91–101. doi: 10.1007/s11738-009-0384-2
de Abreu, I. N. and Mazzafera, P. (2005). Effect of water and temperature stress on the content of active constituents of Hypericum brasiliense Choisy. Plant Physiol. Biochem. 43, 241–248. doi: 10.1016/j.plaphy.2005.01.020
Dobhal, P., Purohit, V. K., Chandra, S., Rawat, S., Prasad, P., Bhandari, U., et al. (2024). Climate-induced changes in essential oil production and terpene composition in Alpine aromatic plants. Plant Stress 12, 100445. doi: 10.1016/j.stress.2024.100445
dos Santos Nascimento, L. B., Leal-Costa, M. V., Menezes, E. A., Lopes, V. R., Muzitano, M. F., Costa, S. S., et al. (2015). Ultraviolet-B radiation effects on phenolic profile and flavonoid content of Kalanchoe pinnata. J. Photochem. Photobiol. B: Biol. 148, 73–81. doi: 10.1016/j.jphotobiol.2015.03.011
Eichholz, I., Rohn, S., Gamm, A., Beesk, N., Herppich, W. B., Kroh, L. W., et al. (2012). UV-B-mediated flavonoid synthesis in white asparagus (Asparagus officinalis L.). Food Res. Int. 48, 196–201. doi: 10.1016/j.foodres.2012.03.008
Eremina, M., Rozhon, W., and Poppenberger, B. (2016). Hormonal control of cold stress responses in plants. Cell. Mol. Life Sci. 73, 797–810. doi: 10.1007/s00018-015-2089-6
Esra, K.O. Ç., İŞLEK, C., and Üstün, A. S. (2010). Effect of cold on protein, proline, phenolic compounds and chlorophyll content of two pepper (Capsicum annuum L.) varieties. Gazi Univ. J. Sci. 23, 1–6.
Ezuruike, U. F. and Prieto, J. M. (2014). The use of plants in the traditional management of diabetes in Nigeria: pharmacological and toxicological considerations. J. Ethnopharmacol. 155, 857–924. doi: 10.1016/j.jep.2014.05.055
Ferreira, M. I., Uliana, M. R., Costa, S. M., Magro, M., Vianello, F., Ming, L. C., et al. (2016). Exclusion of solar UV radiation increases the yield of curcuminoid in Curcuma longa L. Ind. Crops Prod. 89, 188–194. doi: 10.1016/j.indcrop.2016.05.009
Gabbish, A. A., Klenwachter, M., and Selmar, D. (2015). Influencing the content of secondary metabolites in spice and medicinal plants by deliberately applying drought stress during their cultivation. Jordan J. Biol. Sci. 8, 1–10.
Ghasemzadeh, A., Jaafar, H. Z., and Rahmat, A. (2010). Elevated carbon dioxide increases contents of flavonoids and phenolic compounds, and antioxidant activities in Malaysian young ginger (Zingiber officinale Roscoe.) varieties. Molecules 15, 7907–7922. doi: 10.3390/molecules15117907
Goh, H. ‐H, Sukiran, N. A., Baharum, S. N., Khairudin, K., and Normah, M. N. (2015). Metabolite profiling reveals temperature effects on the VOCs and flavonoids of different plant populations. Plant Biology, 18(S1), 130–139. doi: 10.1111/plb.12403
Guerriero, G., Berni, R., Muñoz-Sanchez, J. A., Apone, F., Abdel-Salam, E. M., Qahtan, A. A., et al. (2018). Production of plant secondary metabolites: Examples, tips and suggestions for biotechnologists. Genes 9, 309. doi: 10.3390/genes9060309
Haghighi, Z., Karimi, N., Modarresi, M., and Mollayi, S. (2012). Enhancement of compatible solute and secondary metabolites production in Plantago ovata Forsk. by salinity stress. J. Medicinal Plants Res. 6, 3495–3500.
Harish, B. S., Dandin, S. B., Umesha, K., and Sasanur, A. (2012). Impact of climate change on medicinal plants-A review. Anc Sci. Life 32, S23.
Havryliuk, O., Мatvieieva, N., Tashyrev, O., and Yastremskaya, L. (2017). Influence of cold stress on growth and flavonoids accumulation in Artemisia tilesii “hairy” root culture. Agrobiodiversity Improv. Nutr. Health Life Qual. (1), 163–167.
Hu, L., Robert, C. A., Cadot, S., Zhang, X. I., Ye, M., Li, B., et al. (2018). Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat. Commun. 9, 2738. doi: 10.1038/s41467-018-05122-7
Huang, W., He, X. Y., Liu, C. B., and Li, D. W. (2010). Effects of elevated carbon dioxide and ozone on foliar flavonoids of Ginkgo biloba. Adv. Mater Res. 113, 165–169. doi: 10.4028/www.scientific.net/AMR.113-116
Hussein, R. A. and El-Anssary, A. A. (2019). Plants secondary metabolites: the key drivers of the pharmacological actions of medicinal plants. In Hebal Medicine. IntechOpen 13–30. doi: 10.5772/intechopen.76139
Hussin, S., Geissler, N., El-Far, M. M., and Koyro, H. W. (2017). Effects of salinity and short-term elevated atmospheric CO2 on the chemical equilibrium between CO2 fixation and photosynthetic electron transport of Stevia rebaudiana Bertoni. Plant Physiol. Biochem. 118, 178–186. doi: 10.1016/j.plaphy.2017.06.017
Ibrahim, M. H. and Jaafar, H. Z. (2012). Impact of elevated carbon dioxide on primary, secondary metabolites and antioxidant responses of Eleais guineensis Jacq.(Oil Palm) seedlings. Molecules 17, 5195–5211. doi: 10.3390/molecules17055195
Idso, S. B., Kimball, B. A., Pettit, G. R., III, Garner, L. C., Pettit, G. R., and Backhaus, R. A. (2000). Effects of atmospheric CO2 enrichment on the growth and development of Hymenocallis littoralis (Amaryllidaceae) and the concentrations of several antineoplastic and antiviral constituents of its bulbs. Am. J. Bot. 87, 769–773. doi: 10.2307/2656884
Jaafar, H. Z., Ibrahim, M. H., and Fakri, N. F. M. (2012). Impact of soil field water capacity on secondary metabolites, phenylalanine ammonia-lyase (PAL), Maliondialdehyde (MDA) and photosynthetic responses of Malaysian Kacip Fatimah (Labisia pumila Benth). Molecules 17, 7305–7322. doi: 10.3390/molecules17067305
Jamloki, A., Bhattacharyya, M., Nautiyal, M. C., and Patni, B. (2021). Elucidating the relevance of high temperature and elevated CO2 in plant secondary metabolites (PSMs) production. Heliyon 7 (8), e07709. doi: 10.1016/j.heliyon.2021.e07709
Jensen, C. R., Mogensen, V. O., Mortensen, G., Fieldsend, J. K., Milford, G. F. J., Andersen, M. N., et al. (1996). Seed glucosinolate, oil and protein contents of field-grown rape (Brassica napus L.) affected by soil drying and evaporative demand. Field Crops Res. 47, 93–105. doi: 10.1016/0378-4290(96)00026-3
Jochum, G. M., Mudge, K. W., and Thomas, R. B. (2007). Elevated temperatures increase leaf senescence and root secondary metabolite concentrations in the understory herb Panax quinquefolius (Araliaceae). Am. J. Bot. 94, 819–826. doi: 10.3732/ajb.94.5.819
Jordan, D. N., Green, T. H., Chappelka, A. H., Lockaby, B. G., Meldahl, R. S., and Gjerstad, D. H. (1991). Response of total tannins and phenolics in loblolly pine foliage exposed to ozone and acid rain. J. Chem. Ecol. 17, 505–513. doi: 10.1007/BF00982121
Kaundal, M., Bhatt, V., and Kumar, R. (2018). Elevated CO2 and temperature effect on essential oil content and composition of Valeriana jatamansi Jones. with organic manure application in a Western Himalayan region. J. Essential Oil-Bearing Plants 21, 1041–1050. doi: 10.1080/0972060X.2018.1497547
Kaur, T., Kumar, A., Koul, S., Bhat, R., Bindu, K., Bhat, H. A., et al. (2015). Physiochemical and antioxidant profiling of Salvia sclarea L. at different climates in northwestern Himalayas. Acta Physiol. Plant 37, 132. doi: 10.1007/s11738-015-1879-7
Khalid, K. A. (2006). Influence of water stress on growth, essential oil, and chemical composition of herbs (Ocimum sp). Int. Agrophys. 20 (40), 289–296.
Khare, S., Singh, N. B., Singh, A., Hussain, I., Niharika, K. M., Yadav, V., et al. (2020). Plant secondary metabolites synthesis and their regulations under biotic and abiotic constraints. J. Plant Biol. 63, 203–216. doi: 10.1007/s12374-020-09245-7
Kleinwächter, M. and Selmar, D. (2015). New insights explain that drought stress enhances the quality of spice and medicinal plants: potential applications. Agron. Sustain. Dev. 35, 121–131. doi: 10.1007/s13593-014-0260-3
Kováčik, J., Klejdus, B., Hedbavny, J., and Bačkor, M. (2009). Salicylic acid alleviates NaCl-induced changes in the metabolism of Matricaria chamomilla plants. Ecotoxicology 18, 544–554. doi: 10.1007/s10646-009-0312-7
Król, A., Amarowicz, R., and Weidner, S. (2015). The effects of cold stress on the phenolic compounds and antioxidant capacity of grapevine (Vitis vinifera L.) leaves. J. Plant Physiol. 189, 97–104. doi: 10.1016/j.jplph.2015.10.002
Ksouri, R., Megdiche, W., Debez, A., Falleh, H., Grignon, C., and Abdelly, C. (2007). Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol. Biochem. 45, 244–249. doi: 10.1016/j.plaphy.2007.02.001
Kumar, R., Das, S., Mishra, M., Choudhury, D. R., Sharma, K., Kumari, A., et al. (2021). Emerging roles of NAC transcription factor in medicinal plants: progress and prospects. 3 Biotech. 11, 1–14. doi: 10.1007/s13205-021-02970-x
Kumar, D., Jha, S. S., Kumar, A., and Singh, S. K. (2021). “Omics and approaches in plant stress management,” In Kumar, A. and Droby, S. (Eds.), Microbial Management of Plant Stresses (Cambridge, United Kingdom: Woodhead Publishing), pp. 107–117. doi: 10.1016/B978-0-323-85193-0.00003-6
Lavola, A., Julkunen-Tiitto, R., and Pääkkönen, E. (1994). Does ozone stress change the primary or secondary metabolites of birch (Betula pendula Roth.)? New Phytol. 126, 637–642. doi: 10.1111/j.1469-8137.1994.tb02959.x
Li, Y., Kong, D., Fu, Y., Sussman, M. R., and Wu, H. (2020). The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 148, 80–89. doi: 10.1016/j.plaphy.2020.01.006
Li, Q., Lei, S., Du, K., Li, L., Pang, X., Wang, Z., et al. (2016). RNA-seq based transcriptomic analysis uncovers α-linolenic acid and jasmonic acid biosynthesis pathways respond to cold acclimation in Camellia japonica. Sci. Rep. 6, 36463. doi: 10.1038/srep36463
Lianopoulou, V. and Bosabalidis, A. M. (2014). Traits of seasonal dimorphism associated with adaptation to cold stress in Origanum dictamnusL. (Lamiaceae). J. Biol. Res. Thessaloniki 21, 1–9. doi: 10.1186/2241-5793-21-17
Lianopoulou, V., Bosabalidis, A. M., Patakas, A., Lazari, D., and Panteris, E. (2014). Effects of chilling stress on leaf morphology, anatomy, ultrastructure, gas exchange, and essential oils in the seasonally dimorphic plant Teucrium polium(Lamiaceae). Acta Physiol. Plant. 36, 2271–2281. doi: 10.1007/s11738-014-1605-x
Lisar, S. Y. S., Motafakkerazad, R., Hossain, M. M., and Rahman, I. M. M. (2012). “Water stress in plants: Causes, effects and responses”, In Rahman, I. M. M. and Hasegawa, H. (Eds.), Water Stress pp. 1–14. In Tech. doi: 10.5772/39363
Liu, H., Wang, X., Wang, D., Zou, Z., and Liang, Z. (2011). Effect of drought stress on growth and accumulation of active constituents in Salvia miltiorrhiza Bunge. Ind. Crops Prod. 33, 84–88. doi: 10.1016/j.indcrop.2010.09.006
Lindberg, J., Milliot, J., Peethambaran, B., Smith, R., Nabbie, F., and Tettamanzi, M. C. (2014). 14-3-3λ Affects Production of a Sinapoyl Derivative in Lignin Biosynthesis during Drought Stress in Arabidopsis Thaliana. Univ. J. Plant Sci. 2(4), 77–85. doi: 10.13189/ujps.2014.020401
Loreto, F., Barta, C., Brilli, F., and Nogues, I. (2006). On the induction of volatile organic compound emissions by plants as consequence of wounding or fluctuations of light and temperature. Plant Cell Environ. 29, 1820–1828. doi: 10.1111/j.1365-3040.2006.01561.x
Ma, C. H., Chu, J. Z., Shi, X. F., Liu, C. Q., and Yao, X. Q. (2016). Effects of enhanced UV-B radiation on the nutritional and active ingredient contents during the floral development of medicinal chrysanthemum. J. Photochem. Photobiol. B: Biol. 158, 228–234. doi: 10.1016/j.jphotobiol.2016.02.019
Mabou, F. D., Belinda, I., and Yossa, N. (2021). TERPENES: Structural classification and biological activities. IOSR J. Pharm. Biol. Sci. 16 (3), 25–40. doi: 10.9790/3008-1603012540
Mahajan, M., Kuiry, R., and Pal, P. K. (2020). Understanding the consequence of environmental stress for accumulation of secondary metabolites in medicinal and aromatic plants. J. Appl. Res. Medicinal Aromatic Plants 18, 100255. doi: 10.1016/j.jarmap.2020.100255
Mahajan, S. and Tuteja, N. (2005). Cold, salinity and drought stresses: an overview. Arch. Biochem. Biophys. 444, 139–158. doi: 10.1016/j.abb.2005.10.018
Mir, B. A., Mir, S. A., Khazir, J., Tonfack, L. B., Cowan, D. A., Vyas, D., et al. (2015). Cold stress affects antioxidative response and accumulation of medicinally important withanolides in Withaniasomnifera (L.) Dunal. Ind. Crops Prod. 74, 1008–1016. doi: 10.1016/j.indcrop.2015.06.012
Mirza, H., Hossain, M. A., and Fujita, M. (2010). Physiological and biochemical mechanisms of nitric oxide induced abiotic stress tolerance in plants. Am. J. Plant Physiol. 5, 295–324. doi: 10.3923/ajpp.2010.295.324
Mishra, M. K., Chaturvedi, P., Singh, R., Singh, G., Sharma, L. K., Pandey, V., et al. (2013). Overexpression of WsSGTL1 gene of Withaniasomniferaenhances salt tolerance, heat tolerance and cold acclimation ability in transgenic Arabidopsis plants. PloS One 8, e63064. doi: 10.1371/journal.pone.0063064
Mishra, T. (2016). Climate change and production of secondary metabolites in medicinal plants: a review. Int. J. Herb. Med. 4 (4), 27–30.
Mohammadi, H., Ghorbanpour, M., and Brestic, M. (2018). Exogenous putrescine changes redox regulations and essential oil constituents in field-grown Thymus vulgaris L. under well-watered and drought stress conditions. Ind. Crops Prod. 122, 119–132. doi: 10.1016/j.indcrop.2018.05.064
Naghiloo, S., Movafeghi, A., Delazar, A., Nazemiyeh, H., Asnaashari, S., and Dadpour, M. R. (2012). Ontogenetic variation of total phenolics and antioxidant activity in roots, leaves and flowers of Astragalus compactus Lam. (Fabaceae). BioImpacts: BI 2 (2), 105–109. doi: 10.5681/bi.2012.015
Naikoo, M. I., Dar, M. I., Raghib, F., Jaleel, H., Ahmad, B., Raina, A., et al. (2019). Role and regulation of plants phenolics in abiotic stress tolerance: An overview. Plant Signaling Mol. 5, 157–168. doi: 10.1016/B978-0-12-816451-8.00009-5
Najafi, F. and Khavari-Nejad, R. A. (2010). The effects of salt stress on certain physiological parameters in summer savory (Satureja hortensis L.) plants. J. Stress Physiol. Biochem. 6, 13–21.
Neffati, M. and Marzouk, B. (2008). Changes in essential oil and fatty acid composition in coriander (Coriandrum sativum L.) leaves under saline conditions. Ind. Crops Prod. 28, 137–142. doi: 10.1016/j.indcrop.2008.02.005
Ninou, E., Paschalidis, K., and Mylonas, I. (2017). Essential oil responses to water stress in greek oregano populations. J. Essential Oil Bear. Plants 20, 12–23. doi: 10.1080/0972060X.2016.1264278
Nowak, M., Kleinwächter, M., Manderscheid, R., Weigel, H.-J., and Selmar, D. (2010). Drought stress increases the accumulation of monoterpenes in sage (Salvia officinalis), an effect that is compensated by elevated carbon dioxide concentration. J. Appl. Bot. Food Qual. 83, 133–136.
Osman, M. E., Elfeky, S. S., El-Soud, K. A., and Hasan, A. M. (2007). Response of Catharanthus roseus shoots to salinity and drought in relation to vincristine alkaloid content. Asian J. Plant Sci. 6 (8), 1223–1228. doi: 10.3923/ajps.2007.1223.1228
Oueslati, S., Karray-Bouraoui, N., Attia, H., Rabhi, M., Ksouri, R., and Lachaal, M. (2010). Physiological and antioxidant responses of Mentha pulegium (Pennyroyal) to salt stress. Acta Physiol. Plant. 32, 289–296. doi: 10.1007/s11738-009-0406-0
Pant, P., Pandey, S., and Dall’Acqua, S. (2021). The influence of environmental conditions on secondary metabolites in medicinal plants: A literature review. Chem. Biodivers. 18, e2100345. doi: 10.1002/cbdv.202100345
Parida, A. K. and Das, A. B. (2005). Salt tolerance and salinity effects on plants: a review. Ecotoxicol. Environ. Saf. 60, 324–349. doi: 10.1016/j.ecoenv.2004.06.010
Park, J. S., Choung, M. G., Kim, J. B., Hahn, B. S., Kim, J. B., Bae, S. C., et al. (2007). Genes up-regulated during red coloration in UV-B irradiated lettuce leaves. Plant Cell Rep. 26, 507–516. doi: 10.1007/s00299-006-0255-x
Park, Y. J., Kwon, D. Y., Koo, S. Y., Truong, T. Q., Hong, S. C., Choi, J., et al. (2023). Identification of drought-responsive phenolic compounds and their biosynthetic regulation under drought stress in Ligularia fischeri. Front. Plant Sci. 14, 1140509. doi: 10.3389/fpls.2023.1140509
Paudel, J. R., Amirizian, A., Krosse, S., Giddings, J., Ismail, S. A. A., Xia, J., et al. (2016). Effect of atmospheric carbon dioxide levels and nitrate fertilization on glucosinolate biosynthesis in mechanically damaged Arabidopsis plants. BMC Plant Biol. 16, 1–12. doi: 10.1186/s12870-016-0752-1
Paulsen, J. and Selmar, D. (2016). Case study: the difficulty of correct reference values when evaluating the effects of drought stress: a case study with Thymus vulgaris. J. Appl. Bot. Food Qual. 89, 191–196. doi: 10.5073/JABFQ.2016.089.037.
Pellegrini, E., Campanella, A., Cotrozzi, L., Tonelli, M., Nali, C., and Lorenzini, G. (2018). Ozone primes changes in phytochemical parameters in the medicinal herb Hypericum perforatum (St. John’s wort). Ind. Crops Prod. 126, 119–128. doi: 10.1016/j.indcrop.2018.10.002
Pellegrini, E., Francini, A., Lorenzini, G., and Nali, C. (2015). Ecophysiological and antioxidant traits of Salvia officinalis under ozone stress. Environ. Sci. pollut. Res. 22, 13083–13093. doi: 10.1007/s11356-015-4569-5
Perrone, A. and Martinelli, F. (2020). Plant stress biology in epigenomic era. Plant Sci. 294, 110376. doi: 10.1016/j.plantsci.2019.110376
Petropoulos, S. A., Daferera, D., Polissiou, M. G., and Passam, H. C. (2008). The effect of water deficit stress on the growth, yield and composition of essential oils of parsley. Scientia Hortic. 115, 393–397. doi: 10.1016/j.scienta.2007.10.008
Punetha, A., Kumar, D., Suryavanshi, P., Padalia, R. C., and Venkatesha, K. T. (2022). Environmental abiotic stress and secondary metabolites production in medicinal plants: a review. J. Agric. Sci. 28, 351–362. doi: 10.15832/ankutbd.999117
Qian, H., Xu, Z., Cong, K., Zhu, X., Zhang, L., Wang, J., et al. (2021). Transcriptomic responses to drought stress in Polygonatumkingianum tuber. BMC Plant Biol. 21, 1–20. doi: 10.1186/s12870-021-03297-8
Qiang, Q., Gao, Y., Yu, B., Wang, M., Ni, W., Li, S., et al. (2020). Elevated CO2 enhances growth and differentially affects saponin content in Paris polyphylla var. yunnanensis. Ind. Crops Prod. 147, 112124. doi: 10.1016/j.indcrop.2020.112124
Radušienė, J., Karpavičienė, B., and Stanius, Ž. (2012). Effect of external and internal factors on secondary metabolites accumulation in St. John’s worth. Botanica Lithuanica 18, 101–108. doi: 10.2478/v10279-012-0012-8
Rahimi, S. and Hasanloo, T. (2016). The effect of temperature and pH on biomass and bioactive compound production in Silybum marianum hairy root cultures. Res. J. Pharmacogn. 3, 53–59.
Rahman, A. (2013). Auxin: a regulator of cold stress response. Physiol. Plant. 147, 28–35. doi: 10.1111/j.1399-3054.2012.01617.x
Rastogi, S., Shah, S., Kumar, R., Vashisth, D., Akhtar, M. Q., Kumar, A., et al. (2019). Ocimum metabolomics in response to abiotic stresses: Cold, flood, drought and salinity. PloS One 14, e0210903. doi: 10.1371/journal.pone.0210903
Razmjoo, K. H. O. R. S. H. I. D., Heydarizadeh, P. A. R. I. S. A., and Sabzalian, M. R. (2008). Effect of salinity and drought stresses on growth parameters and essential oil content of Matricaria chamomile. Int. J. Agric. Biol. 10, 451–454.
Razzaq, M. K., Aleem, M., Mansoor, S., Khan, M. A., Rauf, S., Iqbal, S., et al. (2021). Omics and CRISPR-Cas9 approaches for molecular insight, functional gene analysis, and stress tolerance development in crops. Int. J. Mol. Sci. 22, 1292. doi: 10.3390/ijms22031292
Reifenrath, K. and Müller, C. (2007). Species-specific and leaf-age dependent effects of ultraviolet radiation on two Brassicaceae. Phytochemistry 68, 875–885. doi: 10.1016/j.phytochem.2006.12.008
Ruelland, E., Vaultier, M. N., Zachowski, A., and Hurry, V. (2009). Cold signalling and cold acclimation in plants. Adv. Bot. Res. 49, 35–150. doi: 10.1016/S0065-2296(08)00602-2
Said-Al Ahl, H. A. H. and Omer, E. A. (2011). Medicinal and aromatic plants production under salt stress. A review. Herba olonica 57 (1), 72–87.
Sampaio, B. L., Edrada-Ebel, R., and Da Costa, F. B. (2016). Effect of the environment on the secondary metabolic profile of Tithonia diversifolia: a model for environmental metabolomics of plants. Sci. Rep. 6, 29265. doi: 10.1038/srep29265
Santos, A. P., Serra, T., Figueiredo, D. D., Barros, P., Lourenço, T., Chander, S., et al. (2011). Transcription regulation of abiotic stress responses in rice: a combined action of transcription factors and epigenetic mechanisms. Omics: J. Integr. Biol. 15, 839–857. doi: 10.1089/omi.2011.0095
Sevillano, L., Sanchez-Ballesta, M. T., Romojaro, F., and Flores, F. B. (2009). Physiological, hormonal and molecular mechanisms regulating chilling injury in horticultural species. Postharvest technologies applied to reduce its impact. J. Sci. Food Agric. 89, 555–573. doi: 10.1002/jsfa.v89:4
Shakeri, A., Sahebkar, A., and Javadi, B. (2016). Melissa officinalis L.–A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 188, 204–228. doi: 10.1016/j.jep.2016.05.010
Shi, L., Cui, X., and Shen, Y. (2024). The roles of histone methylation in the regulation of abiotic stress responses in plants. Plant Stress 11, 100303. doi: 10.1016/j.stress.2023.100303
Shibata, M., Amano, M., Kawata, J., and Uda, M. (1988). “Breeding process and characteristics of” summer queen”, a spray-type chrysanthemum cultivar for summer production,” in Bulletin of the National Research Institute of Vegetables, Ornamental Plants and Tea. Series A. 2 (A), 245–255.
Shil, S. and Dewanjee, S. (2022). Impact of drought stress signals on growth and secondary metabolites (SMs) in medicinal plants. J. Phytopharmacol. 11 (5), 371–376. doi: 10.31254/phyto.2022.11511
Shohael, A. M., Ali, M. B., Yu, K. W., Hahn, E. J., and Paek, K. Y. (2006). Effect of temperature on secondary metabolites production and antioxidant enzyme activities in Eleutherococcussenticosus somatic embryos. Plant Cell Tissue Organ Cult. 85, 219–228. doi: 10.1007/s11240-005-9075-x
Snow, M. D., Bard, R. R., Olszyk, D. M., Minster, L. M., Hager, A. N., and Tingey, D. T. (2003). Monoterpene levels in needles of Douglas fir exposed to elevated CO2 and temperature. Physiol. Plant. 117, 352–358. doi: 10.1034/j.1399-3054.2003.00035.x
Spitaler, R., Schlorhaufer, P. D., Ellmerer, E. P., Merfort, I., Bortenschlager, S., and Stuppner, H. (2006). Altitudinal variation of secondary metabolite profiles in flowering heads of Arnica montana cv. ARBO Phytochem. 67, 409–417. doi: 10.1016/j.phytochem.2005.11.018
Stuhlfauth, T., Klug, K., and Fock, H. P. (1987). The production of secondary metabolites by Digitalis lanata during CO2 enrichment and water stress. Phytochemistry 26, 2735–2739. doi: 10.1016/S0031-9422(00)83581-5
Sun, Y., Alseekh, S., and Fernie, A. R. (2023). Plant secondary metabolic responses to global climate change: a meta-analysis in medicinal and aromatic plants. Global Change Biol. 29, 477–504. doi: 10.1111/gcb.16484
Sun, L., Su, H., Zhu, Y., and Xu, M. (2012). Involvement of abscisic acid in ozone-induced puerarin production of Pueraria thomsnii Benth. suspension cell cultures. Plant Cell Rep. 31, 179–185. doi: 10.1007/s00299-011-1153-4
Szabó, B., Tyihák, E., Szabó, G., and Botz, L. (2003). Mycotoxin and drought stress induced change of alkaloid content of Papaver somniferum plantlets. Acta Botanica Hungarica 45, 409–417. doi: 10.1556/ABot.45.2003.3-4.15
Tabatabaie, S. J. and Nazari, J. (2007). Influence of nutrient concentrations and NaCl salinity on the growth, photosynthesis, and essential oil content of peppermint and lemon verbena. Turkish J. Agric. Forest. 31, 245–253.
Takshak, S. and Agrawal, S. Á. (2014). Secondary metabolites and phenylpropanoid pathway enzymes as influenced under supplemental ultraviolet-B radiation in WithaniasomniferaDunal, an indigenous medicinal plant. J. Photochem. Photobiol. B: Biol. 140, 332–343. doi: 10.1016/j.jphotobiol.2014.08.011
Takshak, S. and Agrawal, S. B. (2015). Defence strategies adopted by the medicinal plant Coleus forskohlii against supplemental ultraviolet-B radiation: Augmentation of secondary metabolites and antioxidants. Plant Physiol. Biochem. 97, 124–138. doi: 10.1016/j.plaphy.2015.09.018
Thirumurugan, D., Cholarajan, A., Raja, S. S., and Vijayakumar, R. (2018). An introductory chapter: secondary metabolites. Secondary metabolites-sources Appl. 1, 1–13. doi: 10.5772/intechopen.79766
Tisserat, B. R. E. N. T. and Vaughn, S. F. (2001). Essential oils enhanced by ultra-high carbon dioxide levels from Lamiaceae species grown in vitro and in vivo. Plant Cell Rep. 20, 361–368. doi: 10.1007/s002990100327
Ullrich, S. F., Rothauer, A., Hagels, H., and Kayser, O. (2017). Influence of light, temperature, and macronutrients on growth and scopolamine biosynthesis in Duboisia species. Planta Med. 83, 937–945. doi: 10.1055/s-0043-106435
Upchurch, R. G. (2008). Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol. Lett. 30, 967–977. doi: 10.1007/s10529-008-9639-z
Valentovic, P., Luxova, M., Kolarovic, L., and Gasparikova, O. (2006). Effect of osmotic stresson compatible solutes content, membrane stability and water relations in twomaize cultivars. Plant Soil Environ. 52, 186–191. doi: 10.17221/3364-PSE
Varshney, K. A., Gangwar, L. P., and Goel, N. (1988). Choline and betaine accumulation in Trifolium alexandrinum L. during salt stress. Egypt J. Bot. 31, 81–86.
Verma, N. and Shukla, S. (2015). Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Medicinal Aromatic Plants 2, 105–113. doi: 10.1016/j.jarmap.2015.09.002
Vuolo, M. M., Lima, V. S., and Junior, M. R. M. (2019). “Phenolic compounds: Structure, classification, and antioxidant power,” in Segura Campos, M. R. (Ed.), Bioactive compounds (Cambridge, United Kingdom: Woodhead Publishing), pp. 33–50. doi: 10.1016/B978-0-12-814774-0.00002-5
Wang, D. H., Du, F., Liu, H. Y., and Liang, Z. S. (2010). Drought stress increases iridoid glycosides biosynthesis in the roots of Scrophularianingpoensis seedlings. J. Med. Plants Res. 4, 2691–2699. doi: 10.5897/JMPR09.338
Wink, M. (2010). “Introduction: Biochemistry, physiology and ecological functions of secondary metabolites,” In Wink, M. (Ed.) Annual Plant Reviews, Volume 40: Biochemistry of plant secondary metabolism, pp. 1–19. doi: 10.1002/9781444320503.ch1
Wu, W., Xu, C., and Liu, X. (2018). “Climate change projections in the twenty-first century,” in Atlas of Environmental Risks Facing China Under Climate Change, pp. 15–30. IHDP/Future Earth–Integrated Risk Governance Project Series. Springer, Singapore. doi: 10.1007/978-981-10-4199-0_2
Xu, Y. H., Liao, Y. C., Zhang, Z., Liu, J., Sun, P. W., Gao, Z. H., et al. (2016). Jasmonic acid is a crucial signal transducer in heat shock induced sesquiterpene formation in Aquilaria sinensis. Sci. Rep. 6, 21843. doi: 10.1038/srep21843
Yadav, S. K. (2010). Cold stress tolerance mechanisms in plants. A review. Agron. Sustain. Dev. 30, 515–527. doi: 10.1051/agro/2009050
Yeshi, K., Crayn, D., Ritmejerytė, E., and Wangchuk, P. (2022). Plant secondary metabolites produced in response to abiotic stresses has potential application in pharmaceutical product development. Molecules 27, 313. doi: 10.3390/molecules27010313
Yuan, Y., Tang, X., Jia, Z., Li, C., Ma, J., and Zhang, J. (2020). The effects of ecological factors on the main medicinal components of Dendrobium officinale under different cultivation modes. Forests 11, 94. doi: 10.3390/f11010094
Zeng, Q., Feng, L., Zhao, C., Yang, X., Yang, R., Yin, L., et al. (2008). Cloning of artemisinin biosynthetic cDNAs and novel ESTs and quantification of low temperature-induced gene overexpression. Sci. China C: Life Sci. 51 (3), 232–244. doi: 10.1007/s11427-008-0032-x
Zhang, L., Wang, Q., Guo, Q., Chang, Q., Zhu, Z., Liu, L., et al. (2012). Growth, physiological characteristics and total flavonoid content of Glechoma longituba in response to water stress. J. Medicinal Plants Res. 6, 1015–1024.
Zhang, C., Yang, D., Liang, Z., Liu, J., Yan, K., Zhu, Y., et al. (2019). Climatic factors control the geospatial distribution of active ingredients in Salvia miltiorrhiza Bunge in China. Sci. Rep. 9, 904. doi: 10.1038/s41598-018-36729-x
Zhong, J. J. and Yoshida, T. (1993). Effects of temperature on cell growth and anthocyanin production in suspension cultures of Perilla frutescens. J. Ferment. Bioeng. 76, 530–531. doi: 10.1016/0922-338X(93)90255-7
Zhou, L., Li, C., White, J. F., and Johnson, R. D. (2021). Synergism between calcium nitrate applications and fungal endophytes to increase sugar concentration in Festuca sinensis under cold stress. PeerJ 9, e10568. doi: 10.7717/peerj.10568
Zhu, Y., Zhu, G., Guo, Q., Zhu, Z., Wang, C., and Liu, Z. (2013). A comparative proteomic analysis of Pinelliaternata leaves exposed to heat stress. Int. J. Mol. Sci. 14, 20614–20634. doi: 10.3390/ijms141020614
Ziska, L. H., Panicker, S., and Wojno, H. L. (2008). Recent and projected increases in atmospheric carbon dioxide and the potential impacts on growth and alkaloid production in wild poppy (Papaver setigerum DC.). Clima. Change 91, 395–403. doi: 10.1007/s10584-008-9418-9
Zobayed, S. M. A., Afreen, F., and Kozai, T. (2007). Phytochemical and physiological changes in the leaves of St. John’s wort plants under a water stress condition. Environ. Exp. Bot. 59, 109–116. doi: 10.1016/j.envexpbot.2005.10.002
Keywords: abiotic stress, climate change, medicinal plants, secondary metabolites, phytochemical biosynthesis, omics technologies, plant adaptation, epigenetic regulation
Citation: Jangpangi D, Patni B, Chandola V and Chandra S (2025) Medicinal plants in a changing climate: understanding the links between environmental stress and secondary metabolite synthesis. Front. Plant Sci. 16:1587337. doi: 10.3389/fpls.2025.1587337
Received: 04 March 2025; Accepted: 16 May 2025;
Published: 13 June 2025.
Edited by:
Kai-Hua Jia, Shandong Academy of Agricultural Sciences, ChinaReviewed by:
Siniša Srečec, Križevci University of Applied Sciences, CroatiaVishnu Mishra, University of Delaware, United States
Copyright © 2025 Jangpangi, Patni, Chandola and Chandra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Babita Patni, YmFiaXRhMjhwYWF0bmlAZ21haWwuY29t
 Devesh Jangpangi
Devesh Jangpangi Babita Patni
Babita Patni Vaishali Chandola
Vaishali Chandola Sudeep Chandra
Sudeep Chandra