- 1Guizhou Provincial Key Laboratory for Tobacco Quality Improvement and Efficiency Enhancement, Guizhou Academy of Tobacco Science, Guiyang, China
- 2Luohe City Company, Henan Provincial Tobacco Company, Luohe, China
- 3Technology Center, China Tobacco Hunan Industrial Company, Ltd., Changsha, China
- 4Jian City Company, Jiangxi Provincial Tobacco Company, Jian, China
Meloidogyne incognita (M. incognita) is a highly destructive species of Meloidogyne spp., characterized by its ability to cause root-knot nematode (RKN) disease, which is difficult to control and severely inhibits plant growth. Temperature is one of the primary factors affecting M. incognita infection. However, the precise underlying mechanisms have not yet been clarified. The present study aims is to further explore the temperature-influenced resistance mechanisms to M. incognita. Antioxidant enzyme activities, osmotic regulation substance contents, tissue structure changes, and expression of the resistance gene (Rk) in the roots of two tobacco varieties were analyzed under three temperatures (15°C, 25°C, and 35°C) via artificial inoculation. A M. incognita-resistant variety (NC95) and a susceptible variety (CBH) was selected as experimental materials. The results showed that the activities of peroxidase (POD) and catalase (CAT), as well as the contents of soluble sugar, proline, and hydroxyproline-rich glycoprotein (HRGP), increased to varying degrees under M. incognita infection, while superoxide dismutase (SOD) activity decreased. Notably, the activities of POD and CAT, along with the contents of soluble sugar, proline, and HRGP, were all higher in NC95 than in CBH. Meanwhile, antioxidant enzyme activities and osmotic substance contents in both varieties varied most at 25°C and least at 35°C. No giant cells or oocysts were observed in the root tissues of NC95 at any temperature, whereas numerous giant cells and oocysts were present in CBH. The number of giant cells in CBH was highest at 25°C compared to 15°C and 35°C, and the degree of lignification in NC95 was also greater at 25°C. In addition, M. incognita infection induced the expression of Rk gene in NC95, with expression levels at 25°C and 15°C higher than at 35°C. The results indicated that SOD activity and osmotic regulatory substance contents decreased in the roots of the susceptible variety under M. incognita infection, accompanied by the appearance of numerous giant cells in the xylem, contributing to susceptibility. Conversely, the resistant tobacco variety exhibited stronger capabilities in reactive oxygen species (ROS) scavenging and osmotic regulation, no significant changes in root tissue structure, and upregulated expression of the Rk gene, all of which contributed to infection inhibition. Compared with the observations at 25°C, M. incognita infectivity on tobacco roots was effectively reduced by 35°C due to increased antioxidant enzyme activities, enhanced osmotic regulatory substance contents, and well-maintained root tissue structure. Additionally, Rk gene expression was not inactivated but only reduced at 35 °C, and it remained effective in inhibiting M. incognita infection.
1 Introduction
Meloidogyne spp. is one of the most destructive plant-parasites, causing root-knot nematode (RKN) disease and infecting more than 5,500 plant species, including food crops, cash crops, oil crops, vegetables, and fruits (Kaloshian and Teixeira, 2019; Yuan et al., 2023). It also exhibits notable characteristics such as large population variation, strong pathogenicity, a diverse array of hosts, wide distribution range, and concealed damage (Niu et al., 2022; Siddique et al., 2022). Once Meloidogyne spp. infection occurs, giant cells are induced from root tissue cells, leading to the formation of root knots, followed by root atrophy and deformity, and ultimately inhibiting the absorption of water and nutrients by plants (Holbein et al., 2016). In addition, the aboveground parts show slow growth and development, yellowing of leaves, yield reduction by 10%–20%, and, in severe cases, losses exceeding 75% or even complete crop failure, ultimately resulting in significant economic losses (Bird and Kaloshian, 2003; Tapia-Vázquez et al., 2022). With adjustments to the agricultural planting structure in China, the area devoted to high-value-added cash crops has expanded, and the multiple cropping index has also increased, leading to a year-by-year aggravation of Meloidogyne spp. occurrence and damage (Vestergård, 2019). To date, there is no particularly effective method for the prevention and control of Meloidogyne spp.
Chemical methods are widely used in agricultural production due to their fast-acting protective effects (Wang et al., 2024a). However, the use of highly toxic chemical agents not only seriously affects plant quality (Kanwar et al., 2021), but also pollutes the ecological environment (Abd-Elgawad and Askary, 2020; Hu et al., 2023). Therefore, pollution-free control of Meloidogyne spp. has become a current research hotspot (Vashisth et al., 2024).
Temperature is a key factor affecting Meloidogyne spp. infection (Qin, 2022), and the optimal temperature for its growth and development ranges from 15°C to 30°C (Liu, 2000). Devaney (2006) found that warming had a positive enhanced effect on Meloidogyne spp. in a suitable environment but became lethal when the temperature exceeded the optimal range. Matute (2013) indicated that Meloidogyne spp. exhibited pronounced thermophobia, and nearly all Meloidogyne spp. were killed at 49°C within 10 min–15 min (Jiang, 2006). An increase in temperature can enhance plant transpiration, reduce soil moisture content, and ultimately alter the growth microenvironment of Meloidogyne spp (Briones et al., 1999). At the same time, it also clearly promotes root growth and increases the substrate supply for heterotrophic respiration, thereby influencing the Meloidogyne spp. community (Kardol et al., 2011). Bakonyi and Nagy (2000) confirmed that increasing soil temperature reduced the richness and density of the Meloidogyne spp. community and significantly influenced its diversity and trophic structure. Meloidogyne spp. is a type of pest that spreads through soil as its medium. Therefore, controlling soil temperature can effectively alter the living environment, influence infection, reduce plants damage, and ultimately contribute to the prevention and control of Meloidogyne spp. Katan et al. (1976) proposed using the insulating effect of plastic film covering the soil surface to warm the soil and thereby kill Meloidogyne spp. India has made remarkable progress in controlling Meloidogyne spp., benefiting from a climate characterized by high temperature and arid conditions (Ros et al., 2008). In addition, treating soil with high-temperature water can reduce the incidence of Meloidogyne spp. infection, achieving a control rate of over 60% in China (He et al., 2009).
Tobacco (Nicotiana tabacum L.) is an important economic crop worldwide. Since the discovery in 1892, Meloidogyne spp. have been among the main pathogens affecting tobacco production worldwide (Tong et al., 2022). The dominant species, Meloidogyne incognita (M. incognita), has caused significant damage and substantial losses in tobacco due to its widespread occurrence, severe pathogenicity, and difficulty of control (Sang et al., 2024; Shakeel et al., 2020; Sikandar et al., 2020). Currently, several studies have examined the physiological and biochemical responses of tobacco to M. incognita infection (Li et al., 2018a, b; Lu et al., 2023). Each species within Meloidogyne spp. has an optimal growth temperature, but studies on the effects of temperature on the infectivity of M. incognita remain limited.
To date, no resistance genes to Meloidogyne spp. related to tobacco have been cloned, and the resistance mechanism remains unknown. Different resistance genes to Meloidogyne spp. infection exist in resistant varieties, and resistance in tobacco is complex and diverse (Xu et al., 2023). The Rk gene is the only known resistance gene to Meloidogyne spp. in tobacco. It was first discovered in Nicotiana tomentosa (Yi et al., 1998) and confers resistance to Meloidogyne spp., particularly to M. incognita (Adamo et al., 2021). However, to date, this gene has not been cloned, which limits its application potential. In addition, plants can regulate their internal metabolic activity under pathogen infection through a series of physiological and biochemical responses, including antioxidant enzyme activities and osmotic regulation substance content, to adapt and resist infection, minimize damage, and maintain normal physiological functions. Therefore, this study investigated changes in physiological characteristics—including antioxidant enzyme activities, osmotic regulatory substance contents, tissue structure, and expression of the resistance gene Rk—in roots of two tobacco varieties with differing resistance, infected by M. incognita at different temperatures The aim was to further clarify the effects of temperature on M. incognita infectivity and promote a theoretical basis for pollution-free control and the cultivation of resistant varieties.
2 Materials and methods
2.1 Experimental materials
Two tobacco varieties with contrasting responses to M. incognita were selected in the experiments—NC95 (resistant) (Xu et al., 2019) and CBH (susceptible) (Li et al., 2017). Seeds of both varieties were cultivated using the floating seedling method and then transplanted into plastic pots ((27 × 33 × 21 cm, height × caliber × bottom diameter) containing high-temperature-sterilized sand soil (sand-to-soil ratio of 1:3) when five leaves emerged (55 days post-germination), with an average plant height of approximately 12 cm. Each pot contained a single plant.
M. incognita were propagated from roots of greenhouse-grown susceptible tomato plants (Solanum lycopersicum L. ‘Rutgers’) that had been inoculated with the nematode 90 days earlier. After propagation, the infected tomato roots were harvested, and the second-stage juvenile (J2) of M. incognita were extracted using 0.5% NaOCl, following the method described by Tan and Wu (2003), and then hatched at 26 °C in a constant-temperature incubator. Freshly hatched J2 were preserved in deionized water for further inoculation. In addition, Peter’s counting slides were used to quantify the nematodes under a light microscope (XSM-20, China) (Fraher et al., 2024).
2.2 Experimental design
Seedling of two tobacco varieties at the 10-leaf stage were inoculated at the roots with approximately 1,000 J2 in 5 mL of deionized water, while control seedlings were mock-inoculated with the same volume of deionized water.
Temperature treatments were applied after inoculation with M. incognita. Based on studies on temperature during the tobacco growth period in the field (Liu, 2003) and the effects of different temperatures on M. incognita activity (Cao et al., 2012), the temperatures in the present experiment were set to 15°C, 25°C, and 35°C. The experiment was conducted in growth chambers with a photosynthetic photon flux density of 350 μmol m−2 s−1. Relative humidity (RH) was maintained at 70% under a 14-h photoperiod. A randomized complete block design was used with three replicates, and 80 plants per treatment were included for each tobacco variety.
2.3 Test sampling
According to the method described by Wang et al. (2006), samples were collected at 0 d, 14 d, and 28 d after inoculation with M. incognita. For each treatment, three tobacco plants of similar size from each variety were randomly selected. The sampled roots were combined, washed with distilled water, immediately frozen in liquid nitrogen, and stored at −80°C until further analysis.
Three plants from each tobacco variety were randomly selected for each treatment at 28 d after inoculation. The roots were cleaned with distilled water, and small knotted roots were selected and stored in formaldehyde–alcohol–acetic acid (FAA) fixative for paraffin sectioning. Paraffin sections were observed using a Leica 301-185.104–00 microscope (Germany), and images were captured with an Olympus DP70 camera (Japan).
Three NC95 plants from each treatment were sampled at 0 d, 2 d, 7 d, 14 d, 21 d, and 28 d after inoculation. The roots were washed with distilled water, immediately frozen in liquid nitrogen, and stored at −80°C to determine the relative expression levels of the resistance gene (Rk).
2.4 Indicators and methods for determination
Superoxide dismutase (SOD) activity was measured as described by Wang et al. (2024b). Peroxidase (POD) activity was assayed according to Yingsanga et al. (2008). Catalase (CAT) activity was determined using the method of Lu et al. (2017). Proline and soluble sugar were measured as described by Zhai et al. (2016) and Li (2000), respectively. Hydroxyproline-rich glycoprotein (HRGP) content was measured according to the method of Kivirikko et al. (1967).
Primer sequence information for the Rk gene is listed in Table 1, and the Actin gene was used as an internal control (Zhang et al., 2023). Total RNA was extracted using RNAiso Reagent (TaKaRa Inc., Japan). After confirming an OD260/OD280 ratio of approximately 2.0, cDNA was synthesized using a TaKaRa reverse transcription kit, and the resulting cDNA was used as a template for qRT-PCR amplification. The qRT-PCR reaction conditions were as follows: one cycle at 94°C for 5 min (initial denaturation), followed by 40 cycles of denaturation at 94°C for 15 s, annealing at 60°C for 15 s, and extension at 72°C for 30 s, with a final extension at 72°C for 10 min (Li et al., 2018b). Each amplification reaction was repeated four times. Data were analyzed using the 2−ΔΔCT method to calculate relative gene expression levels (Yang et al., 2022).
2.5 Statistical analysis
The relative increase of each physiological indicator was calculated according to the method of Xu et al. (2008), using the formula: relative increase = (measured value in treatment group/measured value in control group) × 100%. The relative increase reflects the ability of each physiological indicator in roots under M. incognita inoculation to maintain normal growth. In addition, a greater the deviation from a value from “1” indicates a stronger influence of M. incognita, whereas, a smaller deviation indicates a lesser degree of influence.
All the measurements were conducted with three independent biological replicates per determination, and mean values were presented with standard errors. Data were analyzed using SPSS 21.0 (Statistical Software Package) with one-way ANOVA, and differences between means were separated using the least significant difference (LSD) test at a 0.05 probability level, following the method of Hosseini et al. (2022).
3 Results
3.1 Effects of M. incognita infection on POD activity at different temperatures
The relative increase in POD activity in NC95 roots gradually increased at 25°C under M. incognita infection and showed similar trends at 15°C and 35°C, with peaks appearing at 14 d (Figure 1A). The relative increase in NC95 was highest at 25°C and lowest at 35°C throughout the infection period. Compared with NC95 at the same temperatures, the relative increase in POD activity in CBH roots showed a similar trend but remained significantly lower (p ≤0.05).
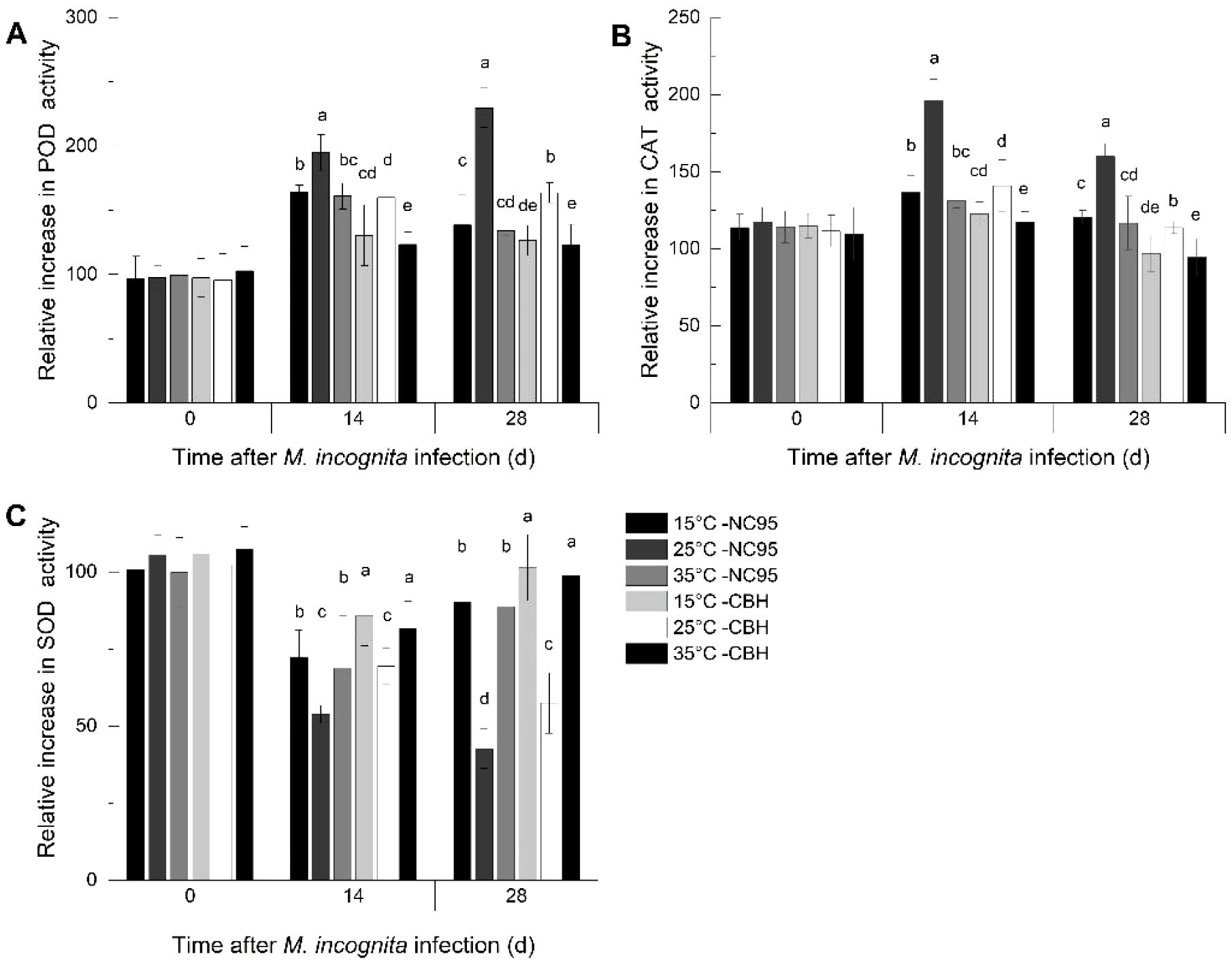
Figure 1. Effects of Meloidogyne incognita infection on antioxidant enzyme activities in the roots of two resistant and susceptible tobacco varieties at different temperatures. (A) Peroxidase (POD); (B) Catalase (CAT); and (C) Superoxide dismutase (SOD). Bars with different letters indicate significant differences at p ≤0.05.
3.2 The effects of M. incognita infection on CAT activity at different temperatures
As shown in Figure 1B, the relative increase in CAT activity in the roots of both varieties peaked at 14 d and declined by 28 d across all temperatures, respectively, with the largest change observed at 25°C. In contrast, no significant difference was observed at 15°C and 35°C. Furthermore, the relative increase in CAT activity in NC95 roots was significantly higher than that in CBH at the same temperature throughout the M. incognita infection period (p ≤0.05).
3.3 Effects of M. incognita infection on SOD activity at different temperatures
As illustrated in Figure 1C, the relative increase in SOD activity in NC95 roots decreased over time at 25°C, while it was the lowest at 14 d and increased again at 28 d at both 15°C and 35°C. During the M. incognita infection period, the relative increase in NC95 was lowest at 25°C, with no significant difference observed between 15°C and 35°C. The relative increase in SOD activity in CBH followed a similar trend to that in NC95 at the same temperatures but showed a greater variation during the same period (p ≤0.05).
3.4 Effects of M. incognita infection on soluble sugar content at different temperatures
As shown in Figure 2A, the relative increase in soluble sugar content in both NC95 and CBH roots rose rapidly during the 0–14 d infection period, then declined, with the greatest change observed at 25°C. At 0 d, 14 d, and 28 d, the differences in relative increase between the two varieties at 15°C and 35°C were not significant. Moreover, throughout the entire M. incognita infection period, the relative increase in soluble sugar content in NC95 roots was significantly higher than that in CBH at the same temperatures (p ≤0.05).
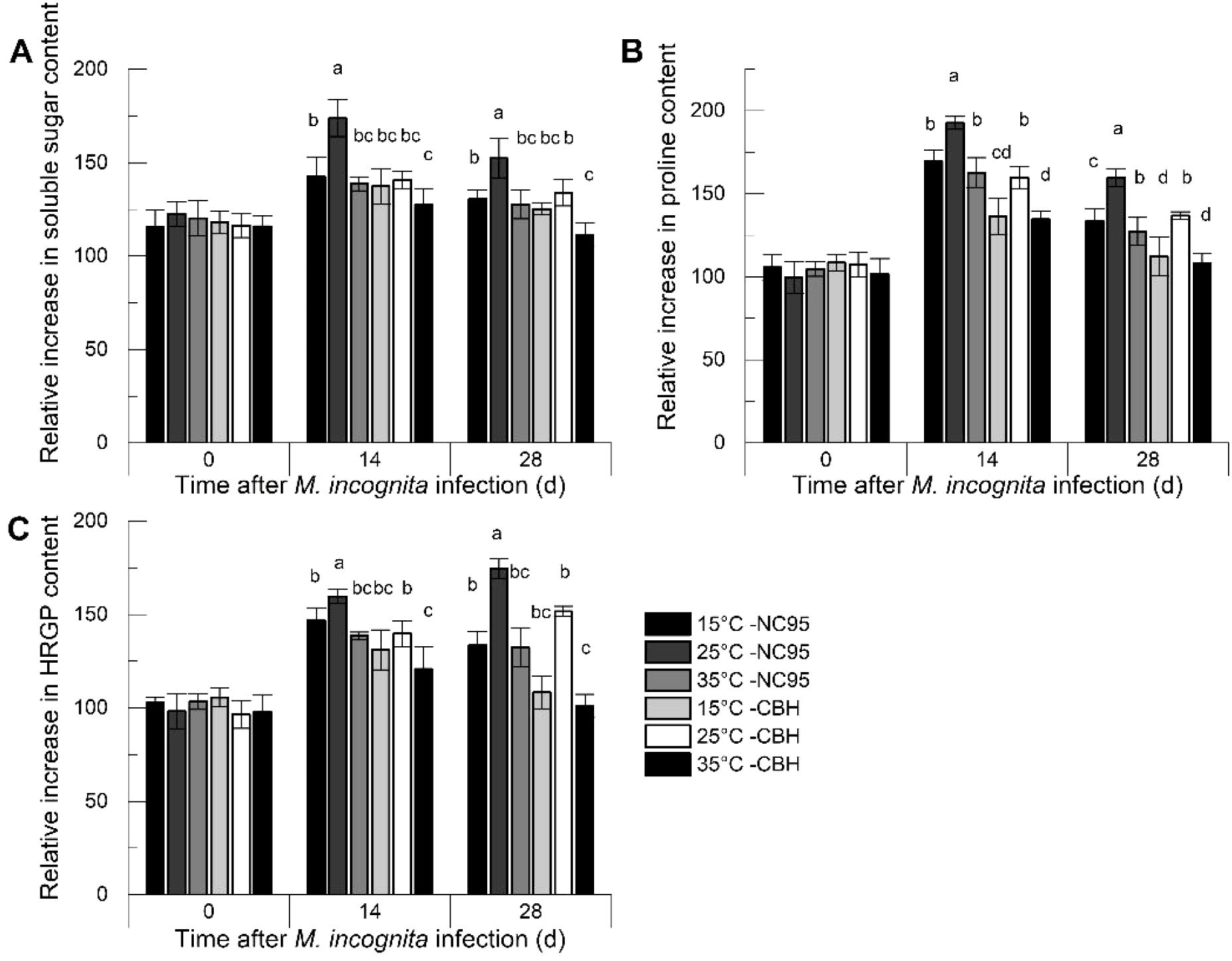
Figure 2. Effects of Meloidogyne incognita infection on osmotic regulation substance contents in the roots of two resistant and susceptible tobacco varieties at different temperatures. (A) Soluble sugar; (B) Proline; (C) Hydroxyproline-rich glycoprotein (HRGP). Bars with different letters indicate significant differences at p ≤0.05.
3.5 Effects of M. incognita infection on proline content at different temperatures
Under M. incognita infection (Figure 2B), the relative increase in proline content in NC95 roots was highest at 25°C, with no significant difference between 15°C and 35°C. The trend in CBH roots was similar to that in NC95 at the same temperatures; however, the relative increase in proline content was significantly lower during the same period (p ≤0.05).
3.6 Effects of M. incognita infection on HRGP content at different temperatures
Under M. incognita infection (Figure 2C), the relative increase in HRGP content in the roots of both varieties was highest at 25°C and lowest at 35°C Additionally, the relative increase in CBH was significantly lower than that in NC95 (p ≤0.05). Specifically, the relative increase in content in both NC95 and CBH increased gradually at 25°C, whereas at 15°C and 35°C, peaks occurred at 14 d, followed by a decline.
3.7 Effects of M. incognita infection on root tissue structure at different temperatures
As shown in Figures 3A, C, E, G, I, K, there were no differences in the root tissue structure of NC95 and CBH uninoculated with M. incognita at different temperatures at 28 d post-inoculation. Additionally, vascular bundle tissues showed normal growth and development, with closely arranged xylem and phloem parenchyma and intact cell morphology.
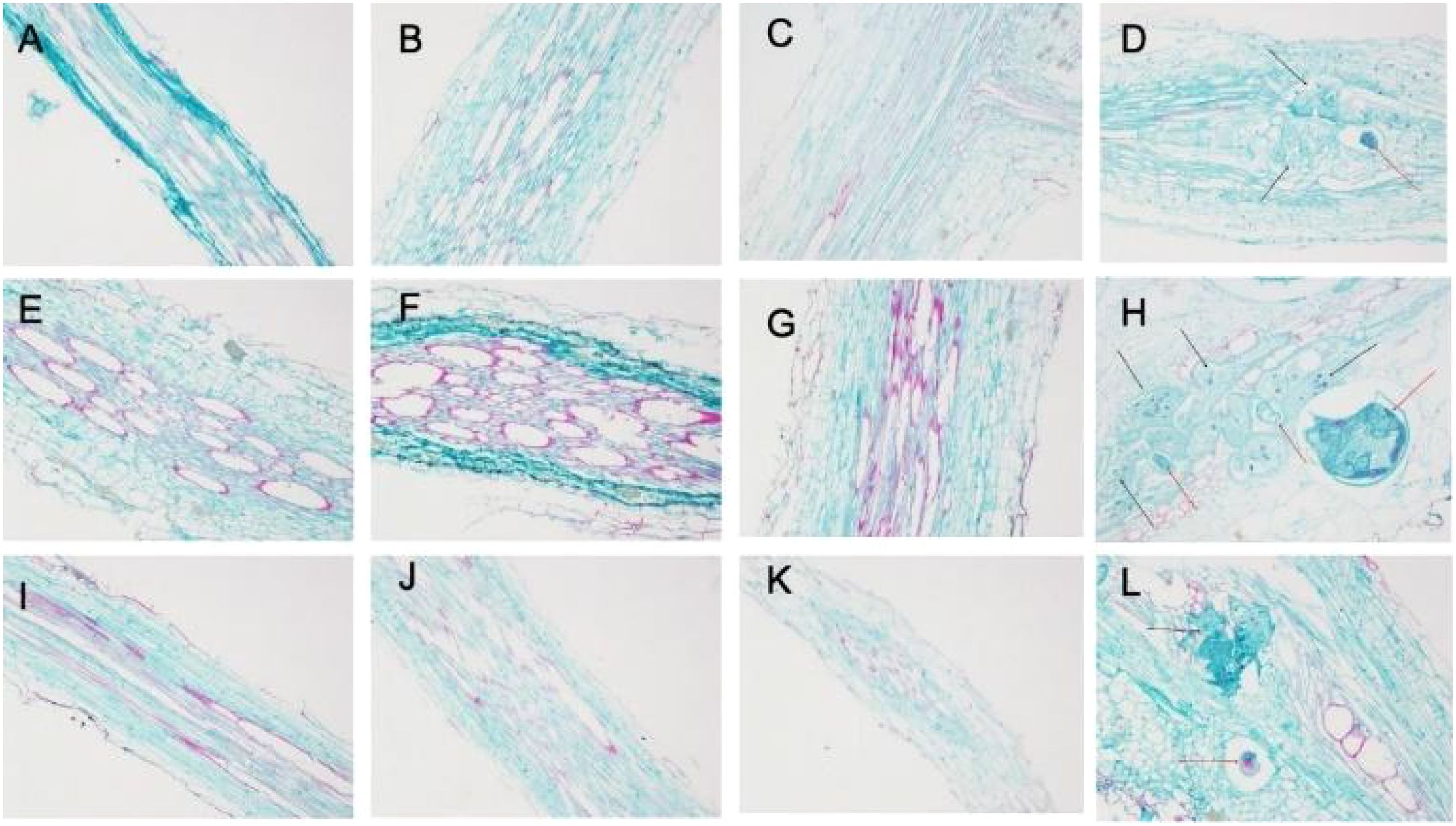
Figure 3. Effects of Meloidogyne incognita infection on the root tissue structure of two resistant and susceptible tobacco varieties at different temperatures. (A) 15°C–NC95–Control. (B) 15°C–NC95–T. (C) 15°C–CBH–Control. (D) 15°C–CBH–T. (E) 25°C–NC95–Control. (F) 25°C–NC95–T. (G) 25°C–CBH–Control. (H) 25°C–CBH–T. (I) 35°C–NC95–Control. (J) 35°C–NC95–T. (K) 35°C–CBH–Control. (L) 35°C–CBH–T.
Under M. incognita infection, the root tissue structure of NC95 showed minimal changes compared with the CK at the same temperature. However, the degree of lignification increased, with the most pronounced lignification observed at 25°C compared to 15°C and 35°C (Figures 3B, F, J).
The tissue structure of CBH was seriously disrupted throughout the M. incognita infection period, exhibiting irregularly arranged xylem and phloem parenchyma and incomplete cell morphology. Meanwhile, oocysts appeared in the xylem (as indicated by the red arrows in Figures 3D, H, L), surrounded by giant cells with multiple nuclei (black arrows in Figures 3D, H, L), thickened cell walls, darker cytoplasm, and highly irregular morphology and chaotic arrangement. These changes led to severe deformation of xylem structure and ultimately to the formation of root knots, as the local root diameter became abnormally large. In addition, the number of giant cells and oocysts in CBH root tissues was greater at 25°C than at 15°C or 35°C. Fewer giant cells and oocysts were observed at 15°C and 35°C, with no significant difference in their numbers.
3.8 Effects of M. incognita infection on Rk gene expression at different temperatures
The results in Figure 4 indicate that at 15°C under M. incognita infection, the expression level of the Rk gene in NC95 roots first increased and then decreased, reaching a peak at 2 d, which was 108.28 times higher than at 0 d. Furthermore, expression gradually declined from 21 d to 28 d and returned to the initial level by the end of the experiment.
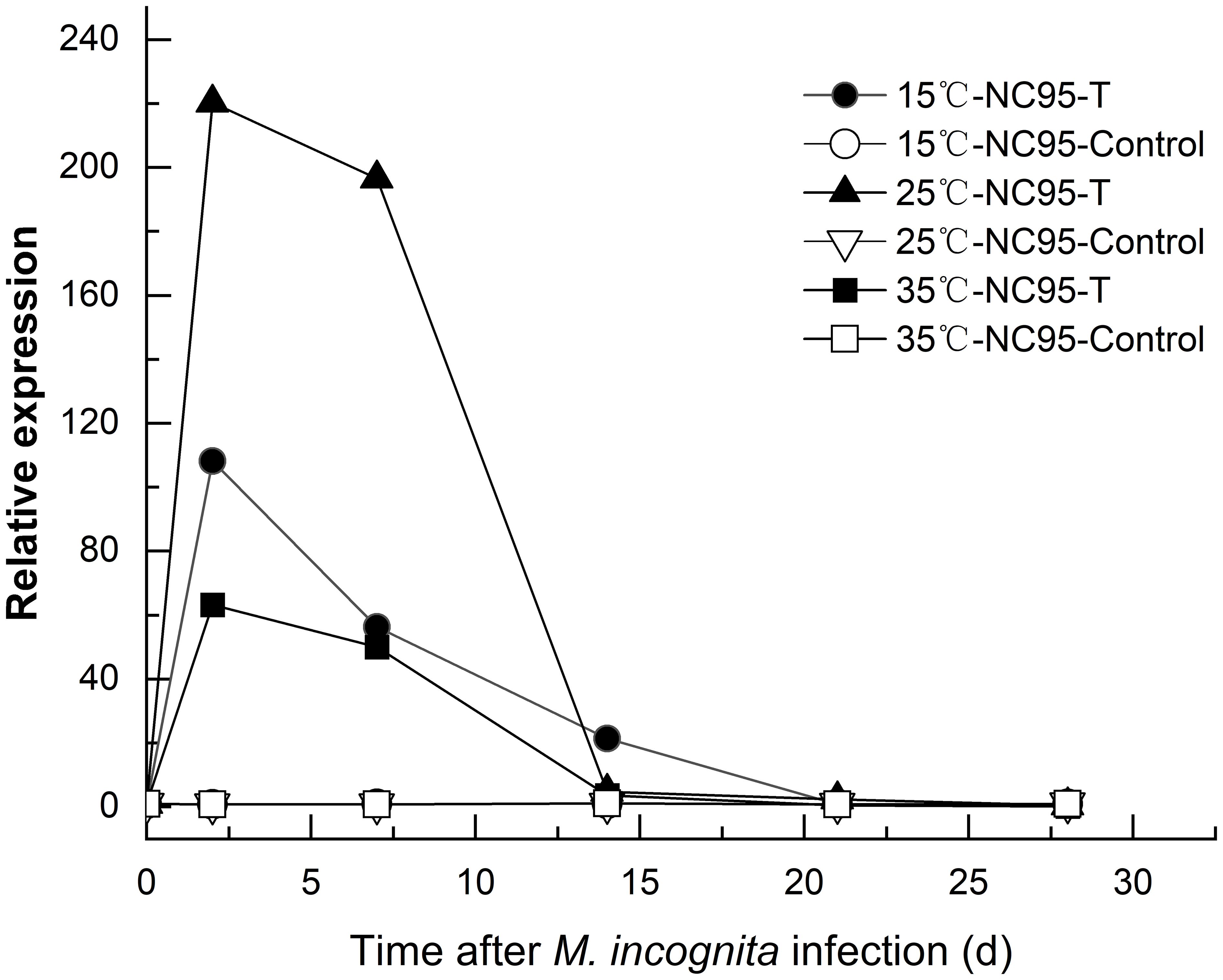
Figure 4. Effects of Meloidogyne incognita infection on Rk gene expression in the roots of the resistant tobacco variety NC95 at different temperatures.
The expression of the Rk gene in NC95 was upregulated under infection at 25°C, reaching its highest level at 2 d—220.31 times that at 0 d—before declining under M. incognita infection.
The expression trend of the Rk gene in NC95 at 35°C was similar to that at both 15°C and 25°C under M. incognita infection. The expression level peaked at 2 d—63.30 times that at 0 d—and then continued to decline. However, under normal growth conditions, the expression level in NC95 remained relatively stable around “1” across all temperatures.
These results indicate that M. incognita infection led to the upregulation of Rk gene expression in NC95 roots, with the greatest increase observed at 2 d. Meanwhile, gene expression was highest at 25°C and lowest at 35°C (Figure 5), indicating that 35 °C exerts an inhibitory effect on Rk gene expression.
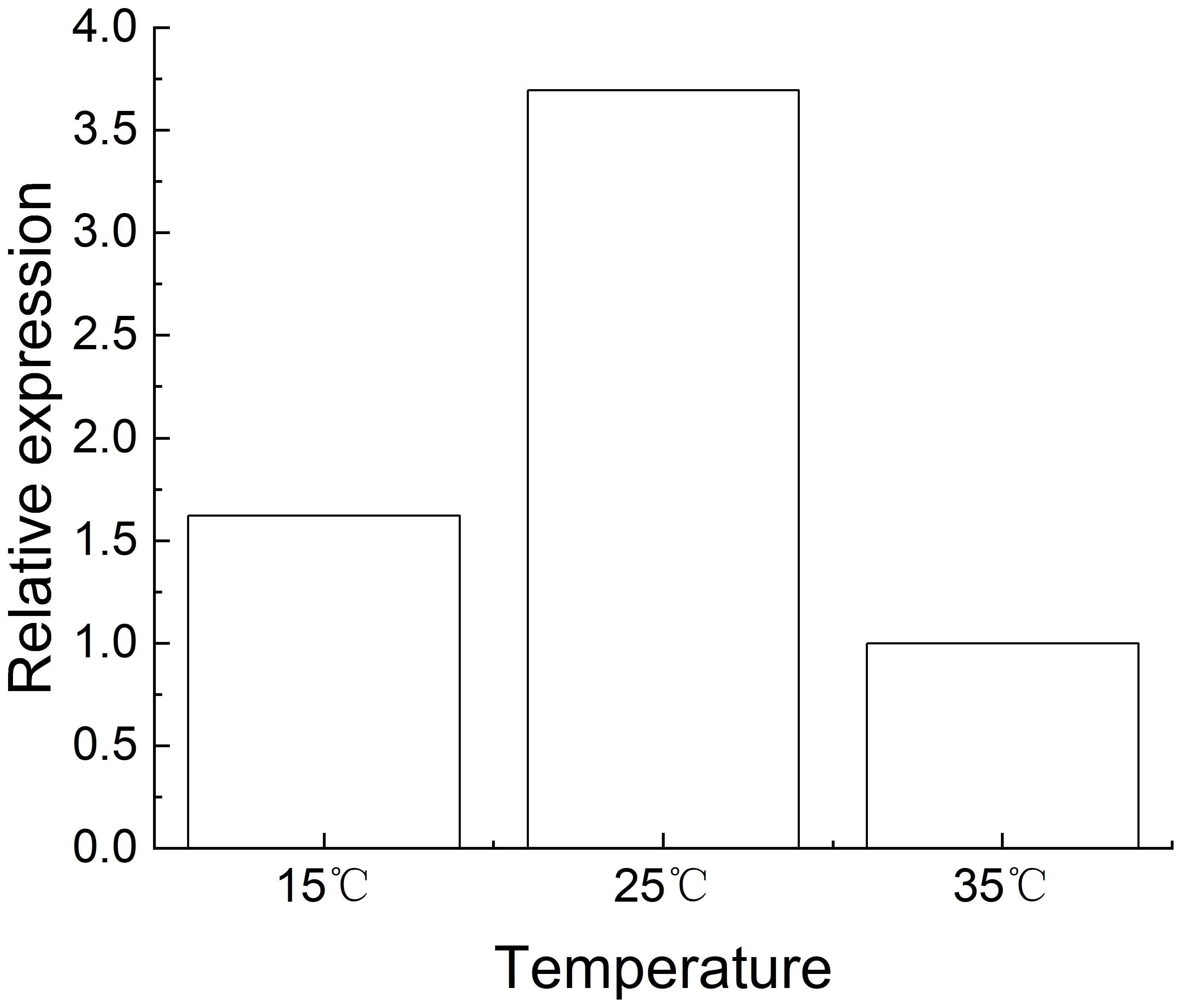
Figure 5. Effects of Meloidogyne incognita infection for 2 d on Rk gene expression in the roots of the resistant tobacco variety NC95 at different temperatures.
4 Discussion
4.1 High temperatures attenuate M. incognita infection by increasing antioxidant enzyme activities and osmotic regulation substance contents
Pathogen infection can increase the levels of reactive oxygen species (ROS) in host cells, making them more vulnerable to oxidative damage. However, the antioxidant enzyme system—of which SOD, POD, and CAT are key components—plays an essential role in scavenging ROS toxicity (Choudhury et al., 2013; Wei et al., 2022a). Normally, these three enzymes function in equilibrium, maintaining ROS production and scavenging at levels that do not harm the plants (Jiang and Huang, 2001; Sugimoto et al., 2014; Zhang et al., 2017). Under pathogen infection, host equilibrium is disrupted, reducing ROS scavenging capacity and resulting in the excessive accumulation of O2•− and H2O2. SOD catalyzes the reaction of O2•− with H+ to generate O2 and H2O2 (Bafana et al., 2011; Nathan and Ding, 2010) The resulting H2O2 is then catalyzed by POD and CAT to form O2 and H2O (Gill and Tuteja, 2010; Khan et al., 2023), thereby reducing the reaction of H2O2 a O2•−, a highly reactive species that can damage all cell membranes (Schmitt et al., 2014; Sewelam et al., 2016). In Dimocarpus longan infected by Lasiodiplodia theobromae (Pat.) Griff. & Maubl. (L. theobromae), SOD and CAT activities initially increased and then decreased, leading to ROS accumulation and the loss of resistance (Sun et al., 2018a). Furthermore, in resistant wheat cultivars, SOD and CAT activities increased under Pyricularia oryzae infection, and symptoms remained mild (Debona et al., 2012). In addition, tobacco strains overexpressing the ScCAT2 gene, which encodes CAT from sugarcane, exhibited enhanced resistance to Ralstonia solanacearum and Fusarium solani var. coeruleum (Sun et al., 2018b). Therefore, the activities of SOD, POD, and CAT is closely related to plants stress resistance (Dumanović et al., 2011; Najafi et al., 2024).
Tomato responded to stress through the production of ROS 12 h after M. incognita infection (Melillo et al., 2006). Zacheo and Bleve-Zacheo (1988) also found that SOD activity was negatively correlated with resistance to Meloidogyne spp. in tomato. Furthermore, resistant varieties showed reduced SOD activity under Meloidogyne spp. infection, whereas susceptible varieties exhiited higher activity with an upward trend. However, POD activity increased in both resistant and susceptible varieties (Rajasekhar et al., 1997). In contrast, Montes et al. (2004) reported that POD activity significantly increased in resistant wheat varieties under cereal cyst nematode (CCN) infection, accompanied by lower SOD and POD activities in susceptible varieties. In the present study, SOD activity decreased in both tobacco varieties with differing resistance under M. incognita infection at all tested temperatures. By contrast, POD and CAT activities showed an upward trend, with greater increases in the resistant variety than in the susceptible one, consistent with the findings of Ye et al. (2009). This may be because the increase in O2•−, influenced by lower SOD activity under M. incognita infection, triggered hypersensitive responses and ultimately produced toxic effects on M. incognita (Kerchev and Breusegem, 2022). Meanwhile, higher POD and CAT activities catalyzed H2O2 into O2 and H2O, which helped reduce ROS accumulation. Furthermore, increased POD activity contributed to cell wall thickening and lignification, thereby enhancing resistance to M. incognita infection in roots. Compared to the susceptible variety, POD and CAT activities were higher in the roots of the resistant variety under M. incognita infection, while SOD activity was lower, indicating stronger ROS scavenging capacity. This contributed to the preservation of root cell structure and function and help maintain physiological balance, ultimately alleviating the damage caused by M. incognita.
Osmoregulation is an important physiological mechanism by which plants adapt to adverse stress, reducing cellular osmotic potential through the accumulation of osmotic regulatory substances to alleviate dehydration damage to enzymes, organelles, and cell membranes, thereby improving plant resistance (Munns et al., 2022). Soluble sugars and proline are important osmotic regulatory substances in plants (Bai et al., 2019; Su et al., 2021). Furthermore, soluble sugars serve as carbon skeletons and energy sources for the synthesis of organic solutes and play a protective role under high concentrations of inorganic ions in cells (Dai, 2020). In addition, proline is an important component of proteins and often exists in a free state. Under external environmental stress, protein synthesis is inhibited while protein decomposition is promoted, leading to an increase in free proline content to regulate osmotic balance between the cytoplasm and vacuoles (Wei et al., 2022b). Jia (2012) found that proline and soluble sugar contents increased in the roots of different tomato rootstock varieties under M. incognita infection, with a higher growth rate observed in the resistant variety. HRGP is a major structural component of the cell wall and is closely associated with lignin formation. When infected by pathogens, plants often accumulate large amounts of HRGP to repair the cell wall structure and prevent pathogen penetration, thereby enhancing resistance (Xu et al., 2011; Zeng et al., 2003). Lu (2009) reported that HRGP content in resistant banana varieties was higher than in susceptible ones after M. incognita inoculation. The present study showed that the contents of soluble sugars, proline, and HRGP in NC95 roots were all significantly higher than in CBH under M. incognita infection, indicating that root damage induced by M. incognita could be mitigated by increasing osmotic regulatory substances and strengthening cell wall structure in resistant tobacco varieties.
Temperature is an important climatic factor that affects the growth and development, survival, reproduction, and other life activities of pathogens (Velloso et al., 2022). In addition, appropriate temperature support the normal growth of pathogens, while excessively high or low temperatures are unfavorable for their growth and reproduction (Garbelotto et al., 2021). In our present study, comparison of antioxidant enzyme activities and osmotic regulatory substance contents in two tobacco varieties with different resistance levels under M. incognita infection at varying temperatures showed that the relative increase of each physiological indicator was greatest at 25°C, while the smallest change occurred at 35°C, indicating that these physiological responses in tobacco roots were less affected by M. incognita infection at 35°C compared to 25°C. This may be attributed to the increased antioxidant enzyme activities and osmotic regulatory substance contents at higher temperatures, which effectively suppressed M. incognita infection and reduced its pathogenicity. Conversely, the temperature of 25°C was more favorable for M. incognita infection in tobacco plants. M. incognita primarily inhabit the 5 cm–20 cm soil layer. The suitable temperature range for egg hatching and J2 infection is 15°C–30°C, with 27°C being optimal for hatching (Liu, 2000; Wang et al., 2016). Moreover, Chen et al. (2009) reported that temperatures above 35°C inhibited the growth and development of M. incognita (Chen et al., 2009). Within less than 1 h of treatment at 44°C–45°C, all J2 died, indicating that such high-temperature conditions are detrimental to M. incognita survival (Wang and McSorley, 2008). Therefore, using double-layer transparent film combined with the addition of organic matter can help increase soil temperature and inhibit M. incognita in tobacco production (Hou and Liu, 2007). This study offers a new direction for environmentally friendly and sustainable control strategies against M. incognita.
4.2 High temperatures hinder M. incognita infection by maintaining root tissue structure
Host tissue structure changes following pathogen infection. To some extent, this is an active adaptive response by plants to maintain normal physiological activities; however, it is also a passive reaction that inhibits plant growth and development (Kong et al., 2020). Meloidogyne spp. must first penetrate the outer protective layer of roots to infect plants and ultimately cause disease. However, most of J2 are unable to enter the pericycle and remain confined to the root cortical tissue in resistant peanut varieties under Meloidogyne hapla infection (Lu et al., 2000). Bendezu and Starr (2003) found that Meloidogyne arenaria invaded only the epidermal tissue in resistant varieties, while the susceptible varieties were completely invaded. In contrast, Vovlas et al. (2005) reported that giant cells formed in the roots of Cordyceps sinensis across varieties with different resistance levels under Meloidogyne spp. infection. Moreover, there was no difference in the number of giant cells. Therefore, it is suggested that the resistance mechanism in resistant varieties involves inhibiting the growth, development, and reproduction of Meloidogyne spp. in the roots.
Our present study indicated that no hypersensitive necrosis occurred in the root apical area of either the resistant or susceptible tobacco varieties under M. incognita infection, showing that the hypersensitive response is not a unique feature of resistant varieties (Lukan et al., 2023). Additionally, there was no significant change in the root tissue structure of the resistant tobacco variety NC95, and no giant cells or oocysts were observed under infection. In contrast, a large number of giant cells and oocysts were observed around the root xylem of the susceptible variety CBH, clearly indicating that different defense mechanisms were present in the resistant and susceptible tobacco varieties under M. incognita infection. The resistant varieties exhibited a certain degree of immunity to M. incognita infection, manifested as structural resistance that inhibited the formation of giant cells. This may be because substances secreted by the resistant varieties are toxic to M. incognita or repel the pathogen from the roots, thereby protecting the plants from infection. Alternatively, the emergence of giant cells and formation of feeding sites may be inhibited by resistant varieties, leading to the death of M. incognita invading the roots due to nutrients deficiency (Phan et al., 2018), which is consistent with the study by Fan (2020). However, Wang et al. (2006) found that the resistance mechanism in the resistant varieties involved inducing cavitation in giant cells under M. incognita infection. Additionally, paraffin section analysis of root knots formed by inoculation with M. hapla, M. javanica Treub, and M. arenaria Neal in the resistant wild cucumber variety ‘Hardwickii’ and the susceptible cultivar ‘Smuter’ revealed that elongation of giant cells in resistant varieties resulted in abnormal development of Meloidogyne spp (Walters et al., 2006). Collectively, further in-depth research is needed on the root tissue resistance mechanism in the resistant varieties to M. incognita infection. Moreover, more giant cells and oocysts were observed in the root tissue of the susceptible variety CBH at 25°C than at 35°C. Meanwhile, the degree of lignification in the resistant variety NC95 was also greater at 25°C, indicating that roots maintain better tissue structure at higher temperatures, which may hinder M. incognita infection.
4.3 High temperatures inhibit M. incognita infection by promoting upregulated expression of Rk gene
A complex gene regulatory network is involved in plant responses to adverse stress (Awlia et al., 2021). Under pathogen infection, host resistant responses are induced by the upregulated expression of resistance genes, thereby disrupting the living environment of pathogens and ultimately preventing their growth and development. It has been confirmed that the upregulated expression of resistance genes plays an essential role in the Meloidogyne spp.–plant interaction (Gheysen and Fenoll, 2002; Lambert et al., 1999; Vercauteren et al., 2001). To date, several Meloidogyne spp. resistance genes have been cloned from crops such as wheat, potato, and tomato. Lagudah et al. (1997) identified the wheat resistance gene of Cre3 to cereal cyst nematode (CNN). The Gpa2 gene confers resistance to potato cyst nematode (PCN) infection (van der Vossen et al., 2000). Numerous resistance genes to Meloidogyne spp. have been identified in tomato, among which the Mi gene family is the most important, capable of inhibiting nematode development and reproduction (Ernst et al., 2002; Jablonska et al., 2007; Padilla-Hurtado et al., 2022). In addition, the resistance gene Hs1Pro-1 was discovered in sugar beet by Cai et al. (1997). The expression levels of MAPK20, ICS1, NPR1, and PAD4 were upregulated in the rice resistant variety ‘Phule Radha’ upon infection with Meloidogyne spp., whereas no significant expression was observed in the susceptible variety (Hatzade et al., 2020). The combination of Hs1pro-1 and cZR3 in rapeseed enhances resistance to Heterodera schachtii Schm (Zhong et al., 2019). Furthermore, Mex-1 from coffee, CaMi from pepper, and RKN1 from cotton had demonstrated effective resistance to Meloidogyne spp. infection (Chen et al., 2007; Silva et al., 2013; Wang et al., 2008). In our studies, the resistance gene Rk was derived from the M. incognita-resistant tobacco variety RK42, in which the resistance trait was controlled by a single dominant gene (Pollok et al., 2016; Rufty et al., 1983).
Some resistance genes to Meloidogyne spp. exhibit temperature sensitivity, showing complete or partial loss of resistance at elevated temperatures (Hwang et al., 2000; Jablonska et al., 2007). Ammiraju et al. (2003) found that the relative expression of Mi-1, Mi-7, and Mi-8 were significantly reduced when resistant tomato varieties were exposed to high temperatures for 1–2 days after inoculation with Meloidogyne spp., whereas the expression levels of Mi-2, Mi-3, Mi-4, Mi-5, Mi-6, and Mi-9 remain unchanged. The expression profiling of eight hsp genes (Mh-hsp90, Mh-hsp1, Mh-hsp4, Mh-hsp6, Mh-hsp60, Mh-dnj19, Mh-hsp43, and Mh-hsp12.2) in M. hapla at the egg and J2 stages were highly upregulated under heat stress (at 35°C and 40°C) than under cold stress (at 5°C) (Flis et al., 2024). Consistent with the above studies, our results indicated that the expression of the Rk gene in the roots of the resistant tobacco variety roots showed no significant change across temperatures without M. incognita inoculation; however, its relative expression increased markedly at 2–7 d post infection, indicating that M. incognita infection promoted the upregulation of Rk expression. Moreover, under infection, the expression levels of the Rk gene at 25°C and 15°C were 3.36-fold and 1.62-fold higher, respectively than at 35°C. These findings suggest that Rk gene expression is temperature-dependent (Pollok et al., 2023), Although expression levels decreased at 35°C, the Rk gene was not inactivated and still retained the ability to inhibit M. incognita infection. Studies on the Rk gene remain in its early stages, and further studies are needed to explore how to enhance resistance to M. incognita infection in tobacco and how temperature affects Rk expression.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Author contributions
ZY: Writing – original draft. QC: Writing – original draft, Resources. RW: Methodology, Writing – review & editing. YL: Funding acquisition, Writing – original draft. DK: Supervision, Writing – review & editing. ZW: Data curation, Writing – original draft. XH: Conceptualization, Writing – review & editing. ZH: Data curation, Writing – original draft. YG: Writing – review & editing. HX: Project administration, Writing – review & editing. YC: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (32160648); the Major Science and Technology Program of China National Tobacco Corporation [110202101032(JY-09), 110202101055(LS-15)]; the Guizhou Province Science and Technology Project ([2021]5642, Qiankehepingtai ZSYS[2025]028); the Science and Technology Program of Guizhou Provincial Tobacco Company (2022XM05, 2023XM04, 2024XM01); the Science and Technology Program of Zunyi Tobacco Company (2021XM05); and the Science and Technology Program of Hunan Industrial Company, Ltd. (202343000834060).
Conflict of interest
Author QC was employed by the company Luohe City Company, Henan Provincial Tobacco Company. Author ZH was employed by the company Jian City Company, Jiangxi Provincial Tobacco Company. Author XH was employed by the China Tobacco Hunan Industrial Company, Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that this study received funding from China National Tobacco Corporation, Guizhou Provincial Tobacco Company, Zunyi Tobacco Company and Hunan Industrial Company, Ltd. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abd-Elgawad, M. M. M. and Askary, T. H. (2020). Factors affecting success of biological agents used in controlling the plant-parasitic nematodes. Egypt. J. Biol. Pest Co. 30, 17. doi: 10.1186/s41938-020-00215-2
Adamo, N., Johnson, C. S., Reed, T. D., and Eisenback, J. D. (2021). Reproduction of Meloidogyne arenaria race 2 on flue-cured tobacco possessing resistance genes Rk1 and/or Rk2. J. Nematol. 53, e2021–e2042. doi: 10.21307/jofnem-2021-042
Ammiraju, J. S. S., Veremis, J. C., Huang, X., Roberts, P. A., and Kaloshian, I. (2003). The heat-stable root-knot nematode resistance gene Mi-9 from Lycopersicon Peruvianum is localized on the short arm of chromosome 6. Theor. Appl. Genet. 106, 478–484. doi: 10.1007/s00122-002-1106-y
Awlia, M., Alshareef, N., Saber, N., Korte, A., Oakey, H., Panzarov, K., et al. (2021). Genetic mapping of the early responses to salt stress in Arabidopsis thaliana. Plant J. 107, 544–563. doi: 10.1111/tpj.15310
Bafana, A., Dutt, S., Kumar, A., Kumar, S., and Ahuja, P. S. (2011). The basic and applied aspects of superoxide dismutase. J. Mol. Catal B.: Enzym. 68, 129–138. doi: 10.1016/j.molcatb.2010.11.007
Bai, X. F., Dai, L. Q., Sun, H. M., Chen, M. T., and Sun, Y. L. (2019). Effects of moderate soil salinity on osmotic adjustment and energy strategy in soybean under drought stress. Plant Physiol. Bioch. 139, 307–313. doi: 10.1016/j.plaphy.2019.03.029
Bakonyi, G. and Nagy, P. (2000). Temperature- and moisture-induced changes in the structure of the nematode fauna of a semiarid grassland-patterns and mechanisms. Global Change Biol. 6, 697–707. doi: 10.1046/j.1365-2486.2000.00354.x
Bendezu, I. F. and Starr, J. L. (2003). Mechanism of resistance to Meloidogyne arenaria in the peanut cultivar COAN. J. Nematol. 35, 115–118.
Bird, D. M. and Kaloshian, I. (2003). Are roots special? Nematodes have their say. Physiol. Mol. Plant P. 62, 115–123. doi: 10.1016/S0885-5765(03)00045-6
Briones, M. J. I., Ineson, P., and Sleep, D. (1999). Use of δ13 C to determine food selection in collembolan species. Soil Biol. Biochem 31, 937–940. doi: 10.1016/s0038-0717(98)00179-5
Cai, D., Kleine, M., Kifle, S., Harloff, H. J., Sandal, N. N., Marcker, K. A., et al. (1997). Positional cloning of a gene for nematode resistance in sugar beet. Science 275, 832–834. doi: 10.1126/science.275.5301.832
Cao, S. F., Qi, Y. H., Du, H., and Chen, S. L. (2012). Effects of temperature and moisture on the survival of Meloidogyne incognita. Plant Protect. 38, 108–111.
Chen, R. G., Li, H. X., Zhang, L. Y., Zhang, J. H., Xiao, J. H., and Ye, Z. B. (2007). CaMi, a root-knot nematode resistance gene from hot pepper (Capsicum annuum L.) confers nematode resistance in tomato. Plant Cell Rep. 26, 895–905. doi: 10.1007/s00299-007-0304-0
Chen, L. J., Wei, F., Duan, Y. X., Bai, C. M., Huo, J. X., and Zhu, X. F. (2009). Effects of temperature and moisture on egg hatching and the second instars of Meloidogyne incognita. Plant Prot. 35, 48–52.
Choudhury, S., Panda, P., Sahoo, L., and Panda, S. K. (2013). Reactive oxygen species signaling in plants under abiotic stress. Plant Signal. Behav 8, e23681. doi: 10.4161/psb.23681
Dai, H. H. (2020). Effects of exogenous 2,4-epibrassinolide on primary metabolism and secondary metabolism of Atropa belladonna L. seedlings under salt stress (Chongqing: Southwest University).
Debona, D., Rodrigues, F. Á., Rios, J. A., and Nascimento, K. J. T. (2012). Biochemical changes in the leaves of wheat plants infected by Pyricularia oryzae. Phytopathology 102, 1121–1129. doi: 10.1094/PHYTO-06-12-0125-R
Devaney, E. (2006). Thermoregulation in the life cycle of nematodes. Int. J. Parasitol. 36, 641–649. doi: 10.1016/j.ijpara.2006.02.006
Dumanović, J., Nepovimova, E., Natić, M., Kuča, K., and Jaćević, V. (2011). The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.552969
Ernst, K., Kumar, A., Kriseleit, D., Kloos, D.-U., Phillips, M. S., and Ganal, M. W. (2002). The broad-spectrum potato cyst nematode resistance gene (Hero) from tomato is the only member of a large gene family of NBS-LRR genes with an unusual amino acid repeat in the LRR region. Plant J. 31, 127–136. doi: 10.1046/j.1365-313X.2002.0134.x
Fan, Y. L. (2020). Identification of resistance resources of root-knot nematodes Meloidogyne graminicola and study on resistance mechanism (Yangzhou: Yangzhou University).
Flis, Ł., Malewski, T., and Dobosz, R. (2024). Temperature effects on expression levels of hsp genes in eggs and second-stage juveniles of Meloidogyne hapla Chitwoo. Int. J. Mol. Sci. 25, 4867. doi: 10.1046/j.1365-313x.2002.0134.x
Fraher, S. P., Watson, M., Hoang, N., Moore, S., Lewis, R., Kudenov, M., et al. (2024). A comparison of three automated root-knot nematode egg counting approaches using machine learning, image analysis, and a hybrid model. Plant Dis. 108, 2625–2629. doi: 10.1094/PDIS-01-24-0217-SR
Garbelotto, M., Schmidt, D., and Popenuck, T. (2021). Pathogenicity and infectivity of Phytophthora ramorum vary depending on host species, infected plant part, inoculum potential, pathogen genotype, and temperature. Plant Pathol. 70, 287–304. doi: 10.1111/ppa.13297
Gheysen, G. and Fenoll, C. (2002). Gene expression in nematode feeding sites. Annu. Rev. Phytopathol. 40, 191–219. doi: 10.1146/annurev.phyto.40.121201.093719
Gill, S. S. and Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Bioch 48, 909–930. doi: 10.1016/j.plaphy.2010.08.016
Hatzade, B., Singh, D., Phani, V., Kumbhar, S., and Rao, U. (2020). Profiling of defense responsive pathway regulatory genes in Asian rice (Oryza sativa) against infection of Meloidogyne graminicola (Nematoda: Meloidogynidae). 3. Biotech. 10, 60. doi: 10.1007/s13205-020-2055-3
He, C. X., Zhang, Z. B., and Wang, H. S. (2009). Study on effects of hot water injection treatment to soil in preventing tomato root-knot nematodes. Northe. Hortic. 5, 140–142.
Holbein, J., Grundler, F. M. W., and Siddique, S. (2016). Plant basal resistance to nematodes: an update. J. Exp. Bot. 67, 2049–2061. doi: 10.1093/jxb/erw005
Hosseini, A., Hosseini, M., and Schausberger, P. (2022). Plant growth-promoting rhizobacteria enhance defense of strawberry plants against spider mites. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.783578
Hou, M. L. and Liu, X. Y. (2007). Influence of transparent film mulch, soil type and organic soil amendmenton on temperature of soil under solarization for root-knot nematode control. Chin. J. Eco-Agr. 15, 46–50.
Hu, Y., Wu, S., Lyu, W., Ning, J., and She, D. (2023). Risk assessment of human exposure to airborne pesticides in rural greenhouses. Sci. Rep. 13, 5138. doi: 10.1038/s41598-023-32458-y
Hwang, C. F., Bhakta, A. V., Truesdell, G. M., Pudlo, W. M., and Williamson, V. M. (2000). Evidence for a role of the N terminus and leucine-rich repeat region of the Mi gene product in regulation of localized cell death. Plant Cell 12, 1319–1329. doi: 10.1105/tpc.12.8.1319
Jablonska, B., Ammiraju, J. S., Bhattarai, K. K., Mantelin, S., de Ilarduya, O. M., Roberts, P. A., et al. (2007). The Mi-9 gene from Solanum arcanum conferring heat-stable resistance to root-knot nematodes is a homolog of Mi-1. Plant Physiol. 143, 1044–1054. doi: 10.1104/pp.106.089615
Jia, S. S. (2012). Study on evaluation and mechanism of tomato rootstocks for resistance to Meloidogyne Incognita (Tai'an: Shandong Agricultural University).
Jiang, Y. L. (2006). Occurrence and control of root knot nematodes in vegetables. Chin. Seed. Ind. 4, 37–38.
Jiang, Y. W. and Huang, B. R. (2001). Drought and heat stress injury to two cool-season turf grasses in relation to antioxidant metabolism and lipid peroxidation. Crop Sci. 41, 436–442. doi: 10.2135/cropsci2001.412436x
Kaloshian, I. and Teixeira, M. (2019). Advances in plant–nematode interactions with emphasis on the notorious nematode genus Meloidogyne. Phytopathology 109, 1988–1996. doi: 10.1094/PHYTO-05-19-0163-IA
Kanwar, R. S., Patil, J. A., and Yadav, S. (2021). Prospects of using predatory nematodes in biological control for plant parasitic nematodes–A review. Biol. Control. 160, 104668. doi: 10.1016/j.biocontrol.2021.104668
Kardol, P., Nicholas Reynolds, W., Norby, R. J., and Classen, A. T. (2011). Climate change effects on soil microarthropod abundance and community structure. Appl. Soil Ecol. 47, 37–44. doi: 10.1016/j.apsoil.2010.11.001
Katan, J., Greenberger, A., Alon, H., and Grinstein, A. (1976). Solar heating by polyethylene mulching for the control of diseases caused by soil-borne pathogens. Phytopathology 66, 683–688. doi: 10.1094/Phyto-66-683
Kerchev, P. I. and Breusegem, F. V. (2022). Improving oxidative stress resilience in plants. Plant J. 109, 359–372. doi: 10.1111/tpj.15493
Khan, W. U., Khan, L. U., Chen, D., and Chen, F. (2023). Comparative analyses of superoxide dismutase (SOD) gene family and expression profiling under multiple abiotic stresses in water lilies. Horticulturae 9, 781. doi: 10.3390/horticulturae9070781
Kivirikko, K. I., Laitinen, O., and Prockop, D. J. (1967). Modifications of a specific assay for hydroxyproline in urine. Anal. Biochem. 19, 249–253. doi: 10.1016/0003-2697(67)90160-1
Kong, X. P., Zhang, C. L., Zheng, H. H., Sun, M., Zhang, F., Zhang, M. Y., et al. (2020). Antagonistic interaction between auxin and SA signaling pathways regulates bacterial infection through lateral root in Arabidopsis. Cell Rep. 32, 108060. doi: 10.1016/j.celrep.2020.108060
Lagudah, E. S., Moullet, O., and Appels, R. (1997). Map-based cloning of a gene sequence encoding a nucleotide binding domain and leucine rich region at the Cre3 nematode resistance locus of wheat. Genome 40, 659–665. doi: 10.1139/g97-087
Lambert, K. N., Ferrie, B. J., Nombela, G., Brenner, E. D., and Williamson, V. M. (1999). Identification of genes whose transcripts accumulate rapidly in tomato after root-knot nematode infection. Physiol. Mol. P. Pathol. 55, 341–348. doi: 10.1006/pmpp.1999.0239
Li, H. S. (2000). Principle and technology of plant physiology and biochemistry experiment (Beijing: Higher Education Press).
Li, M. Y., Jiao, F. C., Zeng, J. M., Wang, B. W., Song, Z. B., Wu, X. F., et al. (2017). Characterization of Meloidogyne incognita resistance in new tobacco varieties (lines). Tob. Sci. Technol. 50, 22–26.
Li, X., Xing, X., Tian, P., Zhang, M., Huo, Z., Zhao, K., et al. (2018a). Comparative transcriptome profiling reveals defense-related genes against Meloidogyne incognita invasion in tobacco. Molecules 23, 2081. doi: 10.3390/molecules23082081
Li, X., Xing, X., Xu, S., Zhang, M., Wang, Y., Wu, H., et al. (2018b). Genome-wide identification and functional prediction of tobacco lncRNAs responsive to root-knot nematode stress. PloS One 13, e0204506. doi: 10.1371/journal.pone.0204506
Lu, G. Y. (2009). Studies on the resistant mechanism of banana to Meloidogyne incognita (Nanning: Guangxi University).
Lu, F. P., Liang, X., Lu, H., Li, Q., Chen, Q., Zhang, P., et al. (2017). Overproduction of superoxide dismutase and catalase confers cassava resistance to Tetranychus cinnabarinus. Sci. Rep. 7, 40179. doi: 10.1038/srep40179
Lu, Z. X., Reighard, G. L., Nyczepir, A. P., Beckman, T. G., and Ramming, D. W. (2000). Inheritance of resistance to root-knot nematodes (Meloidogyne sp.) in Prunus rootstocks. HortScience 35, 1344–1346. doi: 10.21273/HORTSCI.35.7.1344
Lu, P., Shi, H., Tao, J., Jin, J., Wang, S., Zheng, Q., et al. (2023). Metagenomic insights into the changes in the rhizosphere microbial community caused by the root-knot nematode Meloidogyne incognita in tobacco. Environ. Res. 216, 114848. doi: 10.1016/j.envres.2022.114848
Lukan, T., Županič, A., Povalej, T. M., Brunkard, J. O., Kmetič, M., Juteršek, M., et al. (2023). Chloroplast redox state changes mark cell-to-cell signaling in the hypersensitive response. New Phytol. 237, 548–562. doi: 10.1111/nph.18425
Matute, M. M. (2013). Soil nematodes of Brassica rapa: influence of temperature and pH. Adv. Nat. Sci. 6, 20–26. doi: 10.3968/j.ans.1715787020130604.2858
Melillo, M. T., Leonetti, P., Bongiovanni, M., Castagnone-Sereno, P., and Bleve-Zacheo, T. (2006). Modulation of reactive oxygen species activities and H2O2 accumulation during compatible and incompatible tomato-root-knot nematode interactions. New Phytol. 170, 501–512. doi: 10.1111/j.1469-8137.2006.01724.x
Montes, M. J., López-Braña, I., and Delibes, A. (2004). Root enzyme activities associated with resistance to Heterodera avenae conferred by gene Cre7 in a wheat/Aegilops triuncialis introgression line. J. Plant Physiol. 161, 493–495. doi: 10.1078/0176-1617-01165
Munns, R., Passioura, J. B., Colmer, T. D., and Byrt, C. S. (2022). Osmotic adjustment and energy limitations to plant growth in saline soil. New Phytol. 225, 1091–1096. doi: 10.1111/nph.15862
Najafi, M., Esfahani, M. N., Vatandoost, J., Hassanzadeh-Khankahdani, H., and Moeini, M. J. (2024). Antioxidant enzymes activity associated with resistance to Phytophthora melonis-pumpkin blight. Physiol. Mol. Plant P. 129, 102192. doi: 10.1016/j.pmpp.2023.102192
Nathan, C. and Ding, A. H. (2010). Snapshot: reactive oxygen intermediates (ROI). Cell 140, 952 (e1–e2). doi: 10.1016/j.cell.2010.03.008
Niu, Y. R., Xiao, L. Y., de Almeida-Engler, J., Gheysen, G., Peng, D. L., Xiao, X. Q., et al. (2022). Morphological characterization reveals new insights into giant cell development of Meloidogyne graminicola on rice. Planta 255, 70. doi: 10.1007/s00425-022-03852-z
Padilla-Hurtado, B., Morillo-Coronado, Y., Tarapues, S., Burbano, S., Soto-Suárez, M., Urrea, R., et al. (2022). Evaluation of root-knot nematodes (Meloidogyne spp.) population density for disease resistance screening of tomato germplasm carrying the gene Mi-1. Chil. J. Agric. Res. 82, 157–166. doi: 10.4067/S0718-58392022000100157
Phan, N. T., Waele, D. D., Lorieux, M., Xiong, L. Z., and Bellafiore, S. (2018). A Hypersensitivity-Like Response to Meloidogyne graminicola in Rice (Oryza sativa). Phytopathology 108, 521–528. doi: 10.1094/PHYTO-07-17-0235-R
Pollok, J. R., Johnson, C. S., Eisenback, J. D., and Reed, T. D. (2016). Reproduction of Meloidogyne incognita race 3 on flue-cured tobacco homozygous for Rk1 and/or Rk2 resistance genes. J. Nematol. 48, 79–86. doi: 10.21307/jofnem-2017-012
Pollok, J. R., Johnson, C. S., Eisenback, J. D., Reed, T. D., and Adamo, N. (2023). Effect of soil temperature on reproduction of root-knot nematodes in flue-cured tobacco with homozygous Rk1 and/or Rk2 resistance genes. J. Nematol. 55, 20230032. doi: 10.2478/jofnem-2023-0032
Qin, H. X. (2022). Differential analysis of microRNA of Bursaphelenchus xylophilus cultured at two temperatures and study on the function of mog-2 gene (Tai'an: Shandong Agricultural University).
Rajasekhar, S. P., Ganguly, A. K., and Swain, S. C. (1997). Quantitative changes in superoxide dismutase, catalase and peroxidase with reference to resistance in tomato to Meloidogyne incognita. Indian J. Nematol. 27, 79–85. doi: 10.1016/j.mseb.2009.12.053
Ros, M., Garcia, C., Hernandez, M. T., Lacasa, A., Fernandez, P., and Pascual, J. A. (2008). Effects of biosolarization as methyl bromide alternative for Meloidogyne incognita control on quality of soil under pepper. Biol. Fertil. Soils. 45, 37–44. doi: 10.1007/s00374-008-0307-1
Rufty, R. C., Powell, N. T., and Gooding, G. V. (1983). Relationship between resistance to Meloidogyne incognita and a necrotic response to infection by a strain of potato virus Y in tobacco. Phytopathology 73, 1418–1483. doi: 10.1007/BF00224098
Sang, Y. H., Ren, K., Chen, Y., Wang, B., Meng, Y. F., Zhou, W. B., et al. (2024). Integration of soil microbiology and metabolomics to elucidate the mechanism of the accelerated infestation of tobacco by the root-knot nematode. Front. Microbiol. 15. doi: 10.3389/fmicb.2024.1455880
Schmitt, F. J., Renger, G., Friedrich, T., Kreslavski, V. D., Zharmukhamedov, S. K., Los, D. A., et al. (2014). Reactive oxygen species: Re-evaluation of generation, monitoring and role in stress-signaling in phototrophic organisms. BBA-Bioenergetics 1837, 835–848. doi: 10.1016/j.bbabio.2014.02.005
Sewelam, N., Kazan, K., and Schenk, P. M. (2016). Global plant stress signaling: reactive oxygen species at the cross-road. Front. Plant Sci. 23. doi: 10.3389/fpls.2016.00187
Shakeel, A., Khan, A. A., and Haris, M. (2020). Multifaceted strategies used by root-knot nematodes to parasitize plants-A review. Phyton-Int. J. Exp. Bot. 89, 205–215. doi: 10.32604/phyton.2020.08922
Siddique, S., Coomer, A., Baum, T., and Williamson, V. M. (2022). Recognition and response in plant–nematode interactions. Annu. Rev. Phytopathol. 60, 143–162. doi: 10.1146/annurev-phyto-020620-102355
Sikandar, A., Zhang, M. Y., Wang, Y. Y., Zhu, X. F., Liu, X. Y., Fan, H. Y., et al. (2020). Review article: Meloidogyne incognita (root-knot nematodes) a risk to agriculture. Appl. Ecol. Env. Res. 18, 1679–1690. doi: 10.15666/aeer/1801_16791690
Silva, R. V., Oliveira, R. D. L., Ferreira, P. S., Ferreira, A. O., and Rodrigues, F. A. (2013). Defense responses to Meloidogyne exigua in resistant coffee cultivar and non-host plant. Trop. Plant Pathol. 38, 114–121. doi: 10.1590/S1982-56762013000200004
Su, Y. N., Huang, Y. Z., Dong, X. T., Wang, R. J., Tang, M. Y., Cai, J. B., et al. (2021). Exogenous methyl jasmonate improves heat tolerance of perennial ryegrass through alteration of osmotic adjustment, antioxidant defense, and expression of jasmonic acid-responsive genes. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.664519
Sugimoto, M., Oono, Y., Gusev, O., Matsumoto, T., Yazawa, T., Levinskikh, M. A., et al. (2014). Genome-wide expression analysis of reactive oxygen species gene network in Mizuna plants grown in long-term spaceflight. BMC Plant Biol. 14, 4. doi: 10.1186/1471-2229-14-4
Sun, J. Z., Lin, H. T., Zhang, S., Lin, Y. F., Wang, H., Lin, M. S., et al. (2018a). The roles of ROS production-scavenging system in Lasiodiplodia theobromae (Pat.) Griff. & Maubl.-induced pericarp browning and disease development of harvested longan fruit. Food Chem. 247, 16–22. doi: 10.1016/j.foodchem.2017.12.017
Sun, T. T., Liu, F., Wang, W. J., Wang, L., Wang, Z. Q., Li, J., et al. (2018b). The role of sugarcane catalase gene ScCAT2 in the defense response to pathogen challenge and adversity stress. Int. J. Mol. Sci. 19, 2686. doi: 10.3390/ijms19092686
Tapia-Vázquez, I., Montoya-Martínez, A. C., Santos-Villalobos, S. D., Ek-Ramos, M. J., Montesinos-Matías, R., and Martínez-Anaya, C. (2022). Root-knot nematodes (Meloidogyne spp.) a threat to agriculture in Mexico: biology, current control strategies, and perspectives. World J. Microb. Biot. 38, 26. doi: 10.1007/s11274-021-03211-2
Tong, W., Li, J., Cong, W., Zhang, C., Xu, Z., Chen, X., et al. (2022). Bacterial community structure and function shift in rhizosphere soil of tobacco plants infected by Meloidogyne incognita. Plant Pathol. J. 38, 583–592. doi: 10.5423/PPJ.OA.08.2022.0105
van der Vossen, E. A. G., van der Voort, J. R., Kanyuka, K., Bendahmane, A., Sandbrink, H., Baulcombe, D. C., et al. (2000). Homologues of a single resistance-gene cluster in potato confer resistance to distinct pathogens: a virus and a nematode. Plant J. 23, 567–557. doi: 10.1046/j.1365-313x.2000.00814.x
Vashisth, S., Kumar, P., Chandel, V. G. S., Kumar, R., Verma, S. C., and Chandel, R. S. (2024). Unraveling the enigma of root-knot nematodes: from origins to advanced management strategies in agriculture. Planta 260, 36. doi: 10.1007/s00425-024-04464-5
Velloso, J. A., Maquilan, M. A. D., Campos, V. P., Brito, J. A., and Dicks, D. W. (2022). Temperature effects on development of meloidogyne enterolobii and M. Floridensis. J. Nematol. 54, 20220013. doi: 10.2478/jofnem-2022-0013
Vercauteren, I., van der Schueren, E., van Montagu, M., and Gheysen, G. (2001). Arabidopsis thaliana genes expressed in the early compatible interaction with root-knot nematodes. Mol. Plant Microbe In. 14, 288–299. doi: 10.1094/MPMI.2001.14.3.288
Vestergård, M. (2019). Trap crops for Meloidogyne hapla management and its integration with supplementary strategies. Appl. Soil Ecol. 134, 105–110. doi: 10.1016/j.apsoil.2018.10.012
Vovlas, N., Rapoport, H. F., Jiménez Díaz, R. M., and Castillo, P. (2005). Differences in feeding sites induced by root-knot nematodes, Meloidogyne spp., in chickpea. Phytopathology 95, 368–375. doi: 10.1094/PHYTO-95-0368
Walters, S. A., Wehner, T. C., Daykin, M. E., and Barker, K. R. (2006). Penetration rates of root-knot nematodes into Cucumis sativus and C. metuliferus roots and subsequent histological changes. Nematropica 36, 231–242.
Wang, K. H. and McSorley, R. (2008). Exposure time to lethal temperatures for Meloidogyne incognita suppression and its implication for soil solarization. J. Nematol. 40, 7–12.
Wang, Y. H., Qian, X. J., Ge, J. J., and Liu, C. Z. (2016). Effects of temperature on the survival of Meloidogyne inicognita. Gansu. Agric. Sci. Technol. 7, 43–47.
Wang, G., Shen, Y. H., Wang, J. F., and Wang, R. X. (2006). Histopathological changes of different tobacco varieties infected by Meloidogyne incognita. J. Henan. Agr. Sci. 27, 20–22.
Wang, C. L., Ulloa, M., and Roberts, P. A. (2008). A transgressive segregation factor (RKN2) in Gossypium barbadense for nematode resistance clusters with gene rkn1 in G. hirsutum. Mol. Genet. Genomics 279, 41–52. doi: 10.1007/s00438-007-0292-3
Wang, Y., Wang, M. N., Zhang, Y., Chen, F., Sun, M., Li, S., et al. (2024a). Resistance to both aphids and nematodes in tobacco plants expressing a Bacillus thuringiensis crystal protein. Pest Manage. Sci. 80, 3098–3106. doi: 10.1002/ps.8013
Wang, X., Zhang, J., Shen, J., Zhang, L., Wei, P., Liu, A., et al. (2024b). The alleviating effect on the growth, chlorophyll synthesis, and biochemical defense system in sunflowers under cadmium stress achieved through foliar application of humic acid. BMC Plant Biol. 24, 792. doi: 10.1186/s12870-024-05516-4
Wei, C., Jiao, Q. J., Agathokleous, E., Liu, H. T., Li, G. Z., Zhang, J. J., et al. (2022a). Hormetic effects of zinc on growth and antioxidant defense system of wheat plants. Sci. Total. Environ. 807, 150992. doi: 10.1016/j.scitotenv.2021.150992
Wei, T. L., Wang, Z. X., He, Y. F., Xue, S., Zhang, S. Q., Pei, M. S., et al. (2022b). Proline synthesis and catabolism-related genes synergistically regulate proline accumulation in response to abiotic stresses in grapevines. Sci. Hortic-Amsterdam. 305, 111373. doi: 10.1016/j.scienta.2022.111373
Xu, C. X., Takáč, T., Burbach, C., Menzel, D., and Šamaj, J. (2011). Developmental localization and the role of hydroxyproline rich glycoproteins during somatic embryogenesis of banana (Musa spp. AAA). BMC Plant Biol. 11, 38. doi: 10.1186/1471-2229-11-38
Xu, S., Tian, P., Jiang, Z., Chen, X., Li, B., Sun, J., et al. (2023). Transcriptome analysis of two tobacco varieties with contrast resistance to Meloidogyne incognita in response to PVY MSNR infection. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1213494
Xu, X. M., Xu, K., Yu, Q., and Zhang, X. Y. (2008). Screening and evaluation of eggplant rootstock for resistance to Meloidogyne incognita. Acta Hortic. Sin. 35, 1461–1466.
Xu, X. Y., Zhao, H. L., Li, F. L., Li, M. Y., Wang, Y. X., Zhu, J. H., et al. (2019). Selection of disease resistant to Meloidogyne incognita from cultivated Nicotian tabacum and evaluation on resistance degradation of main varieties. J. Kunming. Univ. 41, 1–6.
Yang, Z. X., Lin, Y. C., Cao, Y., Wang, R. G., Kong, D. J., Hou, Q., et al. (2022). Potassium accumulation characteristics and expression of related genes involved in potassium metabolism in a high-potassium variety: tobacco (Nicotiana tabacum) as a model. Funct. Plant Biol. 49, 887–897. doi: 10.1071/FP22011
Ye, D. Y., Qian, C. T., Jia, Y. Y., Zhang, Y. X., and Chen, J. F. (2009). Cucumber and its related species for resistance to the sourthern root-knot nematode Meloidogyne incognita and respond to changes of enzyme. Acta Hortic. Sin. 36, 1755–1760.
Yi, H. Y., Rufty, R., Wernsman, E., and Conkling, M. (1998). Mapping the root-knot nematode resistance gene (Rk) in tobacco with RAPD markers. Plant Dis. 82, 1319–1322. doi: 10.1094/PDIS.1998.82.12.1319
Yingsanga, P., Srilaong, V., Kanlayanarat, S., Noichinda, S., and McGlasson, W. B. (2008). Relationship between browning and related enzymes (PAL, PPO and POD) in rambutan fruit (Nepheliumlappaceum Linn.) cvs. Rongrien and See-Chompoo. Postharvest. Biol. Tec. 50, 164–168. doi: 10.1016/j.postharvbio.2008.05.004
Yuan, Z., Zhao, D., Duan, Y. X., Chen, L. J., Fan, H. Y., Wang, Y. Y., et al. (2023). AtSWEET1 negatively regulates plant susceptibility to root-knot nematode disease. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1010348
Zacheo, G. and Bleve-Zacheo, T. (1988). Involvement of superoxide dismutases and superoxide radicals in the susceptibility and resistance of tomato plants to Meloidogyne incognita attack. Physiol. Mol. Plant P. 32, 313–322. doi: 10.1016/S0885-5765(88)80026-2
Zeng, Y. W., Shen, S. Q., Li, Z. C., Yang, Z. Y., Wang, X. K., Zhang, et al. (2003). Ecogeographic and genetic diversity based on morphological characters of indigenous rice (Oryza sativa L.) in Yunnan, China. Genet. Resour. Crop Ev. 50, 567–577. doi: 10.1023/A:1024436501289
Zhai, H., Wang, F. B., Si, Z. Z., Huo, J. X., Xing., L., An, Y. Y., et al. (2016). A myo-inositol-1-phosphate synthase gene, IbMIPS1, enhances salt and drought tolerance and stem nematode resistance in transgenic sweet potato. Plant Biotechnol. J. 14, 592–602. doi: 10.1111/pbi.12402
Zhang, X. S., Wang, T., Lin, X. W., Denlinger, D. L., and Xu, W. H. (2017). Reactive oxygen species extend insect life span using components of the insulin-signaling pathway. P. Natl. Acad. Sci. U.S.A. 114, E7832–E7840. doi: 10.1073/pnas.1711042114
Zhang, L., Xu, Z., Jiang, Z., Chen, X., Li, B., Xu, L., et al. (2023). Cloning and functional analysis of the root-knot nematode resistance gene NtRk1 in tobacco. Physiol. Plantarum. 175, e13894. doi: 10.1111/ppl.13894
Keywords: Meloidogyne incognita, temperature, tobacco, root, physiological characteristics, resistance gene
Citation: Yang Z, Chen Q, Wang R, Lin Y, Kong D, Wang Z, He X, Han Z, Guo Y, Xia H and Cao Y (2025) Responses of root physiological characteristics and resistance gene expression to infection by Meloidogyne incognita at different temperatures in tobacco. Front. Plant Sci. 16:1592335. doi: 10.3389/fpls.2025.1592335
Received: 18 March 2025; Accepted: 19 June 2025;
Published: 21 July 2025.
Edited by:
Francesca De Luca, National Research Council (CNR), ItalyReviewed by:
Juan Emilio Palomares-Rius, Spanish National Research Council (CSIC), SpainLintle Mohase, University of the Free State, South Africa
Copyright © 2025 Yang, Chen, Wang, Lin, Kong, Wang, He, Han, Guo, Xia and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yushuang Guo, eXNoZ3VvQDEyNi5jb20=; Haiqian Xia, eGlhaGFpcWFpQDEyNi5jb20=; Yi Cao, Y2FveWkxMDAxQDE2My5jb20=
 Zhixiao Yang
Zhixiao Yang Qilong Chen2
Qilong Chen2 Yingchao Lin
Yingchao Lin Yushuang Guo
Yushuang Guo