- 1Guangdong Key Laboratory for Innovative Development and Utilization of Forest Plant Germplasm, College of Forestry and Landscape Architecture, South China Agricultural University, Guangzhou, China
- 2State Key Laboratory of Conservation and Utilization of Subtropical Agro-Bioresources, Guangdong Laboratory for Lingnan Modern Agriculture, South China Agricultural University, Guangzhou, China
Introduction: Eucalyptus is one of the most productive trees, with short-rotation and high values. Fertilization and inoculating arbuscular mycorrhizal (AM) fungi can increase Eucalyptus productivity. Many studies have concentrated on plant absorption of inorganic nitrogen, but less on organic nitrogen and the effect of AM fungi on organic nitrogen acquisition.
Methods: In this study, we set 28 treatments including with or without Rhizophagus irregularis inoculation, three organic nitrogen sources (glycine, L-glutamine and peptone) and four levels of nitrogen and phosphorus fertilization, with no application of organic nitrogen fertilizer as control.
Results and Discussion: The results indicated that the height, ground diameter and crown width of Eucalyptus seedlings under 2.5 mM glycine and 0.3 mM Pi (G2P2) treatment were 3.23, 0.82 and 3.70 times higher than those without organic nitrogen under 0.03 mM Pi (N0P1) treatment, respectively. Inoculation with AM fungi contributed 10.85%-162.00% increase in dry weight compared to non-mycorrhizal (NM) treatments. Mycorrhizal seedlings also showed significantly higher total phosphorus contents, nitrogen use efficiency and activity of glutamine synthetase, relative to NM treatments. The relative expressions of genes related to organic nitrogen transport (EgAAP3, EgLHT1, EgProt2) and nitrate nitrogen transport (EgNPF4.5, EgNPF6.3, EgNPF8.1) were up-regulated in mycorrhizal Eucalyptus roots, compared to the NM treatment. Non-mycorrhizal Eucalyptus favored peptone, while mycorrhizal Eucalyptus preferred glycine. Eucalyptus growth under 2.5 mM organic nitrogen and 0.3 mM Pi (N2P2) treatments were promoted markedly, regardless of organic nitrogen source. Whereas, the growth of mycorrhizal Eucalyptus under 0.25 mM organic nitrogen and 0.3 mM Pi (N1P2) treatments were increased the most. This study explored the organic nitrogen and AM fungi fertilizer mode for Eucalyptus, this has potential to improve production practice of Eucalyptus under low nitrogen and phosphorus condition.
1 Introduction
Eucalyptus is one of the most widely planted broad-leaf forest species in the world. This tree is characterized by rapid growth, high timber yield, stress resistance, economic value, tolerance to infertile soil, salinity and moisture as well as ideal trunk shape for timber production (Xie et al., 2017; Zhang and Wang, 2021; Shang et al., 2024). However, successive planting of Eucalyptus and its strong abilities to absorb water and nutrients cause the soil fertility decline significantly (Cui et al., 2023; Tang et al., 2023). Therefore, fertilization plays a vital role on sustainable development and enhancing Eucalyptus timber yield (Yu et al., 2019; Wang et al., 2024).
Nitrogen (N) and phosphorus (P) are the most important and common limiting elements that participate in numerous growth and metabolic processes in plant tissues, thereby determine plants productivity (Marro et al., 2022; Rengel et al., 2022; Yahaya et al., 2023). The two fertilizers are coordinated and interact with each other during crop uptake and utilization (Kumar et al., 2021). P and N-related signaling pathways can be categorized into three classes: phosphate starvation response, nitrogen starvation response and primary nitrate response (Bouain et al., 2019; Krouk and Kiba, 2020; Zhang et al., 2024). Under typical conditions, N application enhances P uptake and utilization by increasing soil phosphatase activity, which accelerates P conversion in the soil (Hu et al., 2023). This mutual promotion improves soil fertility and reduces fertilizer waste. Consequently, the combined application of N and P fertilizers is more effective than the single application of either fertilizer (Zheng et al., 2023). However, excessive reliance on inorganic fertilizers can exacerbate soil degradation and environmental pollution. In low-fertility soils, traditional fertilization strategies often fail to address the complex nutrient limitations faced by Eucalyptus, highlighting the need for alternative approaches to improve nutrient use efficiency and sustainability.
Arbuscular mycorrhizal (AM) fungi are soil microorganisms that form symbiotic relationships with most terrestrial plants (Duan et al., 2024). Previous studies showed that mycorrhizal fungi induce plants to produce various phytohormones and growth-regulating substances (Pons et al., 2020; Ahmed et al., 2025). They also stimulate the production of antimicrobial compounds, activate defense-related enzymes, and regulate plant physiological metabolism (Weng et al., 2022; Wahab et al., 2023). These actions promote healthy growth and enhance disease resistance. AM fungi can extract substantial amounts of N from organic matter (Vaishnav et al., 2025). They may play a previously unappreciated role in N cycle by intercepting inorganic N released from decomposing organic matter before it reaches the root system, transferring some to the plant (Hodge and Fitter, 2010). AM fungi influence the soil N cycle (Fall et al., 2022). AM fungi also enhance plant growth and nutrition by acquiring more N, P, and other less mobile nutrients (Saia et al., 2019; Kuila and Ghosh, 2022). At the molecular level, AM fungi regulate the expression of phosphate transporter protein genes (Loth-Pereda et al., 2011; Duan et al., 2024). This enhances effective P uptake and transport processes in response to environmental P fluctuations (Navarro and Morte, 2024). This symbiotic relationship not only enhances nutrient acquisition, but also reduces fertilizer dependency, making it a promising strategy for sustainable forestry (Xie K, et al., 2022; Sun K, et al., 2024). Recently, it was showed the strongest effect of AM fungi inoculation under low fertilization (Sun J, et al., 2024; Mbodj et al., 2025). Under low fertilization, AM fungi inoculation reduced yield losses and resulted in higher yields.
Most N in soil exists mainly as insoluble polymeric N-containing compounds. In natural ecosystems, symbionts predominantly utilize organic N through the uptake of soluble organic N sources. These insoluble macromolecular organic N compounds are absorbed and utilized by host plants, mycorrhizae, and mycorrhizal fungal hyphae when they are enzymatically broken down into soluble small molecule (Jones et al., 2005; Wahab et al., 2024). Previous study has found that organic fertilization can improve soil aggregation by increasing the products of AM fungi (Řezáčová et al., 2021). AM fungi can fix N from organic sources, while organic N is not transferred to the plant in its intact form (Hodge et al., 2001; Hodge and Fitter, 2010; Jansa et al., 2019). AM fungi acquired approximately 1/3 of the N from degraded organic N matter and transferred 3% of this content to plants (Hodge and Fitter, 2010). The quantum dots labeling method was used to demonstrate that AM fungi can directly uptake and transport glycine, and quantum dots-labeled glycine was present in soil extra-radical hyphae, plant roots and plant stems (Whiteside et al., 2009, 2012). Besides, AM fungi assimilate NH4+ mainly through the glutamine synthetase-glutamate synthetase (GS-GOGAT) pathway, with glutamine and glutamate being abundant in AM fungi mycelium (Wu XL, et al., 2024). Root uptake of amino acids involves transporters such as lysine histidine transporter 1 (LHT1), which plays a key role in organic N acquisition in model plants like Arabidopsis thaliana (Hirner et al., 2006; Svennerstam et al., 2011; Ganeteg et al., 2017). These findings suggest that AM fungi may preferentially utilize organic N sources, offering a distinct advantage in low-fertility soils where inorganic N is scarce.
In recent years, N fertilizer mainly focused on the study of plant uptake of inorganic N and metabolic mechanism. However, there is less research on organic N. In addition, the symbiosis of arbuscular mycorrhiza and plants uptake organic N has not been studied much. This study establishes a symbiosis system between AM fungi and Eucalyptus. The purpose is to explore suitable organic N fertilizer and fertilization mode. We hypothesized that (1) mycorrhizal treatments under organic N and P fertilization would markedly promote Eucalyptus growth, and (2) mycorrhization would promote Eucalyptus organic N metabolism and N use efficiency.
2 Materials and methods
2.1 Plant material, AM fungi inoculation and experimental design
The pot sample were three-month-old asexual group-cultivated seedlings of Eucalyptus urophylla × Eucalyptus grandis DH3229 from Gaoyaojiayao forestry development company. The inoculated AM fungi was Rhizophagus irregularis DAOM 197198, from the mycorrhizal biology team, College of Forestry and Landscape Architecture, South China Agricultural University. The conditions for the germination of R. irregularis were as follows: spores were washed with sterile water several times until the inhibitors were cleaned and stored in the refrigerator at 4°C. The spores were stored in a 12-well cell culture plate containing sterile water and placed in a dark incubator at 25°C to induce the spores to produce germination tubes. After 7 days of cultivation, the germinated spores were collected in centrifuge tubes and used for plant inoculation tests. The host plant was Zea mays and the resulting mycorrhizal agent was mixture of AM fungal spores, mycelium, root segments and soil. Spores were collected by wet sieving and sucrose centrifugation (Yamamura et al., 2003). Seedling roots were inoculated with R. irregularis, pipetting 1 mL of an aqueous solution containing approximately 400 spores to the vicinity of the root system. Each non-mycorrhizal seedling received the same amount of sterilized autoclaved inoculum (15 min in an autoclave at 121°C). One month after inoculating AM fungi, the root symbiosis of the seedlings was observed under a microscope to confirm successful inoculation, and then samples were collected.
In this study, there were 28 treatments in total, including with or without AM fungi inoculation, three organic N sources (glycine, L-glutamine and peptone) and four levels of N and P fertilization, with no application of organic N fertilizer as control. Four biological replicates were set up for each treatment. The plant growth conditions were: photoperiod of 16 h at 24°C during the day with a light intensity of 100-200 W m-2 and 8 h at 19°C at night. Watering with Low (30 µM L-1 NaH2PO4) Long Ashton (mLA) nutrient solution (Hewitt, 1966) during the previous month of mycorrhizal colonization. After mycorrhizal symbiosis, the improved LA nutrient solution with different levels of organic N, N and P was used for watering, with 50 mL of water per Eucalyptus seedling every three days. N concentration of 0.25 mM NaNO3 was added to the base of organic N (glycine, L-glutamine, and peptone). Two level of organic N concentrations were set- in the study, 0.25 mM and 2.5 mM. Two level of P concentrations were set, 0.03 mM and 0.3 mM. N concentration of 1 mM NaNO3 was added as control treatments with no organic N fertilization. The specific treatment of applying organic N and P concentration is shown in the table below (Table 1).
The diameter and height of the seedlings were measured monthly from December 2023 to January 2024 using straightedge and vernier calipers. Eucalyptus seedling roots and leaves were harvested and weighed freshly, killed at 105°C for 15 min, then dried at 70°C until constant weight and weighed to calculate the biomass. Root length, diameter, surface area and root volume of seedlings were determined using a root scanner (WinRHIZO, China). The aboveground and underground part of each biological replicate was randomly divided into 5 groups for the determination of chlorophyll concentration, photosynthetic gas exchange parameters, enzyme activity, total nitrogen (TN) contents, total phosphorus (TP) contents and gene levels, respectively.
2.2 Eucalyptus leaf photosynthetic characteristic measurements
Gas exchange parameters of Eucalyptus leaves were determined with Li-6400 portable photosynthesis meter (LI-COR, USA), mainly including net photosynthetic rate, stomatal conductance, transpiration rate and intercellular CO2 concentration. The measurements were made on the top 3 unfolded leaves of Eucalyptus seedlings between 08:00 to 12:00 am in the morning on a sunny day. Three measurement points were randomly selected for each leaf (Zhang et al., 2018).
For the determination of photosynthetic pigments, the ethanol extraction method was used. 0.2 g leaves were taken into a 50 mL centrifuge tube, and 25 mL of 95% ethanol was added sequentially to the tube and sealed, and then extracted for 24-36 h at room temperature in the dark, and then the extracts were diluted twice, and then colorimetrically compared with the UV-visible spectrophotometer at wavelengths of 665 and 649 nm. The concentrations of chlorophyll a, b and the total chlorophyll were calculated according to the OD values at each wavelength (Lichtenthaler and Wellburn, 1983).
2.3 Total N P contents, nitrogen use efficiency and enzyme activity measurements
Dry roots were fully ground and homogenized for nutrient analysis. The total nitrogen (TN) content was measured from 0.2 g of dried roots powder by the Kjeldahl method (Schuman et al., 1973). Nitrogen use efficiency (NUE) was calculated as the dry weights of the seedlings divided by the TN contents of seedlings (Shi et al., 2017).
To measure the total phosphorus (TP) contents, 0.4 g of dried roots powder was digested with HNO3. The specimens were determined by inductively coupled plasma mass spectrometry (ICP-MS) after digestion and the internal standard method was used for determination of TP contents (Hansen et al., 2013). The glutamate synthase (GOGAT) activity of Eucalyptus in the leaves was determined using the Ionization assay (Singh and Srivastava, 1986). The GOGAT activity was expressed as the reduction of NADH in 0.1 g of sample after ten minutes’ reaction. The nitrate reductase (NR) activity of Eucalyptus in the leaves was determined using the ex vivo assay (Srivastava, 1980). The NR activity was expressed as the amount of nitrite produced by 0.15 g of sample after ten minutes’ reaction. The glutamine synthetase (GS) activity of Eucalyptus in the leaves was determined using the GS activity assay (Magalhaes and Huber, 1991). The GS activity was expressed as the amount of γ-glutamyl isohydroxycyclohexanoic acid produced by 0.05 g of sample.
2.4 Determination of nitrogen relative gene expression levels
Real-time fluorescence quantitative PCR (qRT-PCR) was used to investigate the relative expression of N transport-related genes in the root of Eucalyptus (Zhao et al., 2019). The samples were taken in liquid nitrogen and transferred to -80°C refrigerator for storage. RNA was extracted using E.Z.N.A.™ Plant RNA Protocol II kit (Guangzhou Omega Biotek, Ltd). HiScript® III RT SuperMix for qPCR (+gDNA wiper) kit (Nanjing Vazyme Biotech Co., Ltd) was used to reverse transcription of the first strand cDNA. Furthermore, gene expression levels were determined in a 96-well Real time PCR system instrument (BioRed, Hercules, CA, USA), using the SGExcel FastSYBR Master Mix kit (Shanghai Sangon Biotech Co., Ltd.), with a reaction mixture of 10 µL SYBR qPCR Master Mix, 0.5 µL each of forward and reverse primers, 2 µL cDNA, and 7 µL ddH2O. Nine primers were selected to be used in the qRT-PCR, with EgUBI3 applied to be the endogenous reference (Huang et al., 2014). Each PCR protocol was conducted with 3 biological replicates. Supplementary Table 1 presents all the primers used in the qRT-PCR.
2.5 Statistical analysis
In the current work, SPSS 27.0 (SPSS Inc., Chicago, IL, USA) was adopted for statistical analysis of the result data. The result data were analyzed based on one-way ANOVA (posthoc comparisons using Duncan’s test, P < 0.05) and two-way ANOVA (the variation sources were AM fungi inoculation, N application, P application, organic N and their association). T-test was analyzed between NM and AM treatments. After performing the homogeneity analysis of variance, the data were subjected to ANOVA. Pearson’s correlation coefficient was utilized to evaluate the correlation between different growth and physiological characters. Origin 8.0 software (Systat Software Inc., San Jose, CA, USA) was employed to construct figures.
3 Results
3.1 Growth of mycorrhizal Eucalyptus seedlings under different organic N and P conditions
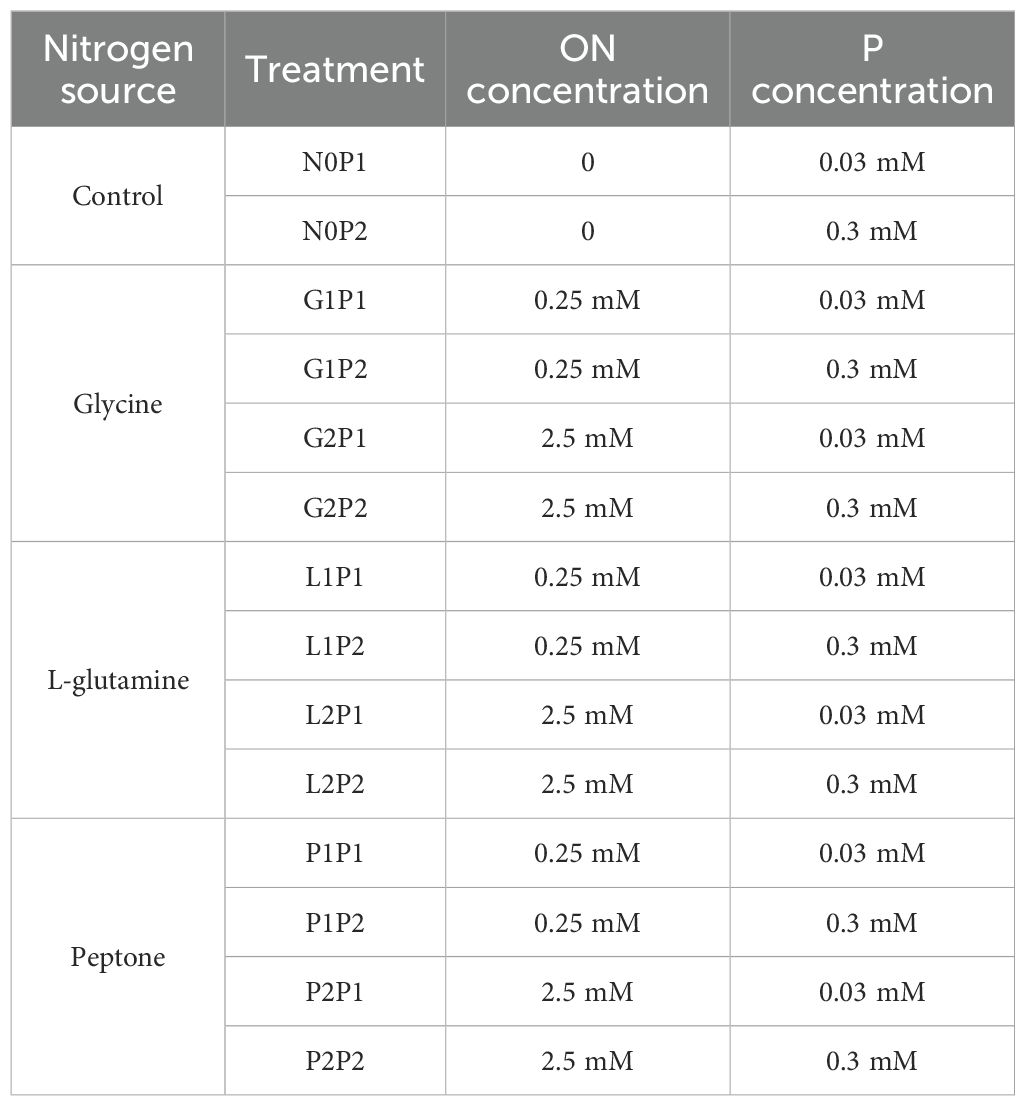
The G2P2 treatment showed the most notable increase in Eucalyptus growth among various treatments. Eucalyptus seedlings treated with G2P2 had heights, ground diameters, and crown widths that were 3.23, 0.82, and 3.70 times higher than N0P1 treatment, respectively. Compared to non-mycorrhizal Eucalyptus seedlings, mycorrhizal Eucalyptus seedlings under both N1P1 and N2P1 treatments showed a substantial increase in plant height and crown width with glycine and peptone treatments (Figures 1B, D). The results indicated that glycine outperformed NM treatments among the three organic N treatments in terms of promoting mycorrhizal Eucalyptus growth (Figure 1). Specifically, the height and crown width of mycorrhizal Eucalyptus seedlings treated with G1P1 were elevated by 60.72% and 41.56%, compared to NM treatments (Figures 1B, C). This implies that at the same N level, mycorrhizal Eucalyptus has a considerable growth advantage in low P conditions. However, the growth of Eucalyptus seedlings did not significantly increase after high N treatments at the same P level, suggesting that P is a more important factor restricting Eucalyptus seedling growth (Figure 1).
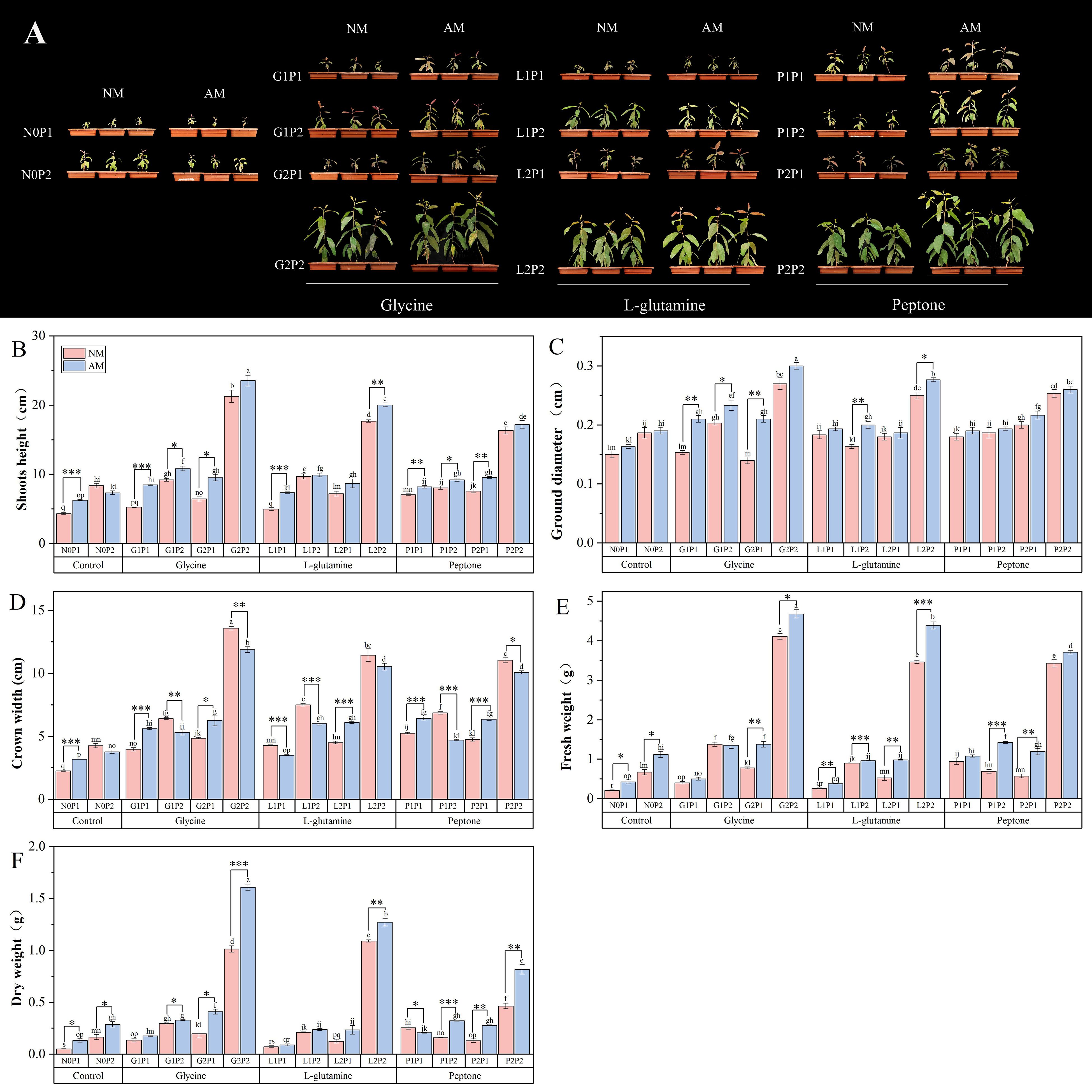
Figure 1. Mycorrhizal Eucalyptus seedlings growth under different organic N and P treatments. Growth phenotypes (A), height (B), ground diameter (C), crown width (D), dry weight (E), fresh weight (F). N0P1: 1 mM N-NO3- and 0.03 mM Pi treatment; N0P2: 1 mM N-NO3- and 0.3 mM Pi treatment; G1P1: 0.25 mM N-NO3-, 0.25 mM glycine and 0.03 mM Pi treatment; G1P2: 0.25 mM N-NO3-, 0.25 mM glycine and 0.3 mM Pi treatment; G2P1: 0.25 mM N-NO3-, 2.5 mM glycine and 0.03 mM Pi treatment; G2P2: 0.25 mM N-NO3-, 2.5 mM glycine and 0.3 mM Pi treatment; L1P1: 0.25 mM N-NO3-, 0.25 mM L-glutamine and 0.03 mM Pi treatment; L1P2: 0.25 mM N-NO3-, 0.25 mM L-glutamine and 0.3 mM Pi treatment; L2P1: 0.25 mM N-NO3-, 2.5 mM L-glutamine and 0.03 mM Pi treatment; L2P2: 0.25 mM N-NO3-, 2.5 mM L-glutamine and 0.3 mM Pi treatment; P1P1: 0.25 mM N-NO3-, 0.25 mM peptone and 0.03 mM Pi treatment; P1P2: 0.25 mM N-NO3-, 0.25 mM peptone and 0.3 mM Pi treatment; P2P1: 0.25 mM N-NO3-, 2.5 mM peptone and 0.03 mM Pi treatment; P2P2: 0.25 mM N-NO3-, 2.5 mM peptone and 0.3 mM Pi treatment. NM, non-mycorrhizal treatment; AM, mycorrhizal treatment. Control, no organic N treatment. Glycine, glycine treatment; L-glutamine, L-glutamine treatment; Peptone, peptone treatment. Using Duncan test for inter group differences, different letters meant that the factors changed significantly among the four strategies (P < 0.05). Using t-test between NM and AM treatments, *P < 0.05 level is significant; **P < 0.01 level is very significant; ***P < 0.001 level is extremely significant.
The dry weight and fresh weight of mycorrhizal Eucalyptus seedlings treated with high organic N were found to be considerably higher than NM treatments. In particular, the dry weight of mycorrhizal Eucalyptus seedlings treated with G2P2 was increased by 58.79% compared to NM treatment (Figure 1F). Among the three organic N sources, glycine treatment led to a considerable increase in seedling biomass. There were substantial increases in seedling biomass under N2P2 treatments, the dry weights and fresh weights of Eucalyptus under G2P2 treatment were 13.39 and 12.93 times, respectively, higher than those under N0P1 treatment (Figures 1E, F). This suggests that Eucalyptus seedling biomass was more effectively promoted by higher levels of N and P. In terms of increasing the biomass of Eucalyptus seedlings, the G2P2 treatment performed the best overall.
3.2 Root growth of mycorrhizal Eucalyptus seedlings under different organic N and P conditions
Analysis of Eucalyptus seedlings root growth revealed significant increases in root length under glycine treatments (Figure 2A). Root length under G2P2 treatment was 5.38 times higher than that of N0P1 treatment. The root surface area was increased by 7.60 times under P2P2 treatment compared to N0P1 treatment (Figure 2B). Concurrently, the root average diameter and volume were significantly increased under L-glutamine treatments, compared to control treatment (Figures 2C, D). The average root volume of mycorrhizal Eucalyptus was elevated by 6.07 times compared to non-mycorrhizal Eucalyptus under G1P1 treatment. However, non-mycorrhizal Eucalyptus seedlings exhibited superior root growth, compared to mycorrhizal Eucalyptus seedlings under N2P2 treatment. Furthermore, compared to NM treatments, glycine treatment was more successful in lengthening the mycorrhizal Eucalyptus roots. Under high P treatments, root growth significantly increased in comparison to treatments at the same N level (Figure 2). The root length of G2P2 treatment was increased by 0.84 times compared to G2P1 treatment, indicating that P plays a significant role in the root growth of Eucalyptus seedlings. There was an overall increase in root growth under P2P2 treatment. Compared to N0P1 treatment, the root length, surface area, average diameter and volume of Eucalyptus seedlings were increased by 3.23, 7.60, 3.38 and 5.76 times, accordingly. Overall, P2P2 treatment was the best in enhancing the Eucalyptus root growth.
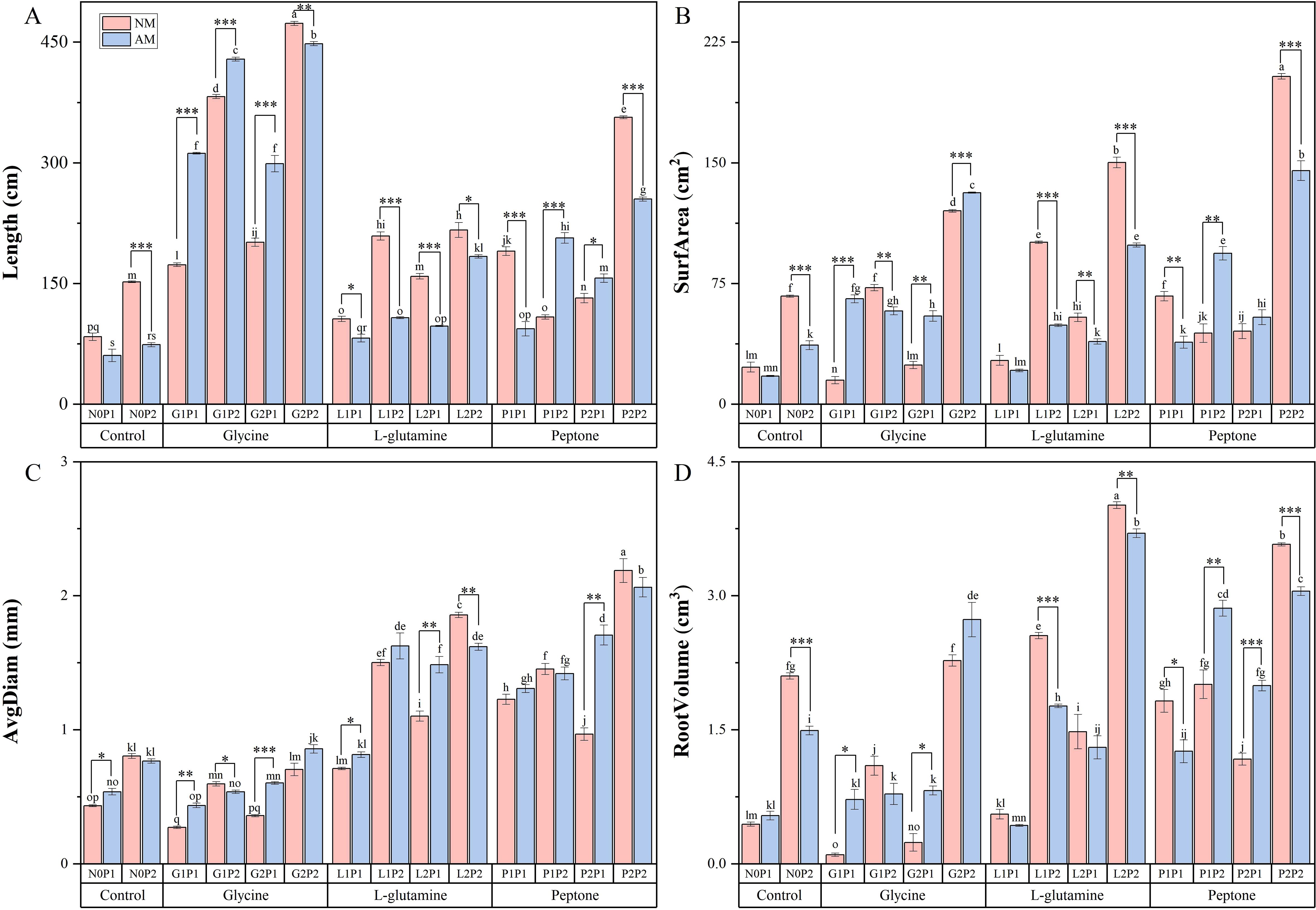
Figure 2. Mycorrhizal Eucalyptus seedlings root growth under different organic N and P treatments. Roots length (A), surfarea (B), avgdiam (C), root volume (D). N0P1: 1 mM N-NO3- and 0.03 mM Pi treatment; N0P2: 1 mM N-NO3- and 0.3 mM Pi treatment; G1P1: 0.25 mM N-NO3-, 0.25 mM glycine and 0.03 mM Pi treatment; G1P2: 0.25 mM N-NO3-, 0.25 mM glycine and 0.3 mM Pi treatment; G2P1: 0.25 mM N-NO3-, 2.5 mM glycine and 0.03 mM Pi treatment; G2P2: 0.25 mM N-NO3-, 2.5 mM glycine and 0.3 mM Pi treatment; L1P1: 0.25 mM N-NO3-, 0.25 mM L-glutamine and 0.03 mM Pi treatment; L1P2: 0.25 mM N-NO3-, 0.25 mM L-glutamine and 0.3 mM Pi treatment; L2P1: 0.25 mM N-NO3-, 2.5 mM L-glutamine and 0.03 mM Pi treatment; L2P2: 0.25 mM N-NO3-, 2.5 mM L-glutamine and 0.3 mM Pi treatment; P1P1: 0.25 mM N-NO3-, 0.25 mM peptone and 0.03 mM Pi treatment; P1P2: 0.25 mM N-NO3-, 0.25 mM peptone and 0.3 mM Pi treatment; P2P1: 0.25 mM N-NO3-, 2.5 mM peptone and 0.03 mM Pi treatment; P2P2: 0.25 mM N-NO3-, 2.5 mM peptone and 0.3 mM Pi treatment. NM, non-mycorrhizal treatment; AM, mycorrhizal treatment. Control, no organic N treatment. Glycine, glycine treatment; L-glutamine, L-glutamine treatment; Peptone, peptone treatment. Using Duncan test for inter group differences, different letters meant that the factors changed significantly among the four strategies (P < 0.05). Using t-test between NM and AM treatments, *P < 0.05 level is significant; **P < 0.01 level is very significant; ***P < 0.001 level is extremely significant.
3.3 Photosynthetic characteristic of mycorrhizal Eucalyptus seedlings under different organic N and P conditions
There was no discernible increase in the net photosynthetic rate with the three organic N treatments. However, the net photosynthetic rate was increased by 0.38 and 0.40 times, respectively, under P1P2 and P2P1 treatment compared to N0P1 treatment (Figure 3A). Under the P1P2 treatment, net photosynthetic rate of mycorrhizal Eucalyptus seedlings was elevated by 113.10%, compared to NM treatment. Both the glycine and peptone treatments resulted in a considerable rise in the intercellular CO2 concentration (Figure 3B). Under glycine treatment, stomatal conductance and transpiration rate increased significantly. Compared to N0P1 treatment, the stomatal conductance and transpiration rate under G2P2 treatment were increased by 1.92 and 3.33 times, respectively (Figures 3C, D). Furthermore, Eucalyptus net photosynthesis rate, intercellular CO2 concentration, stomatal conductance, and transpiration rate were all increased under high P treatments in comparison to treatments at the same N level, suggesting that P is also a crucial component in boosting Eucalyptus seedling photosynthesis. The photosynthesis of Eucalyptus seedlings under the G2P2 treatment were increased notably. Overall, these results showed that glycine was superior in encouraging photosynthesis among the three organic N treatments.
Figure 3. Mycorrhizal Eucalyptus seedlings photosynthetic characteristic under different organic N and P treatments. Net photosynthetic rate (A), intercellular CO2 concentration (B), stomatal conductance (C), transpiration rate (D), chlorophyll a concentration (E), chlorophyll b concentration (F), total chlorophyll concentration (G). N0P1: 1 mM N-NO3- and 0.03 mM Pi treatment; N0P2: 1 mM N-NO3- and 0.3 mM Pi treatment; G1P1: 0.25 mM N-NO3-, 0.25 mM glycine and 0.03 mM Pi treatment; G1P2: 0.25 mM N-NO3-, 0.25 mM glycine and 0.3 mM Pi treatment; G2P1: 0.25 mM N-NO3-, 2.5 mM glycine and 0.03 mM Pi treatment; G2P2: 0.25 mM N-NO3-, 2.5 mM glycine and 0.3 mM Pi treatment; L1P1: 0.25 mM N-NO3-, 0.25 mM L-glutamine and 0.03 mM Pi treatment; L1P2: 0.25 mM N-NO3-, 0.25 mM L-glutamine and 0.3 mM Pi treatment; L2P1: 0.25 mM N-NO3-, 2.5 mM L-glutamine and 0.03 mM Pi treatment; L2P2: 0.25 mM N-NO3-, 2.5 mM L-glutamine and 0.3 mM Pi treatment; P1P1: 0.25 mM N-NO3-, 0.25 mM peptone and 0.03 mM Pi treatment; P1P2: 0.25 mM N-NO3-, 0.25 mM peptone and 0.3 mM Pi treatment; P2P1: 0.25 mM N-NO3-, 2.5 mM peptone and 0.03 mM Pi treatment; P2P2: 0.25 mM N-NO3-, 2.5 mM peptone and 0.3 mM Pi treatment. NM, non-mycorrhizal treatment; AM, mycorrhizal treatment. Control, no organic N treatment. Glycine, glycine treatment; L-glutamine, L-glutamine treatment; Peptone, peptone treatment. Using Duncan test for inter group differences, different letters meant that the factors changed significantly among the four strategies (P < 0.05). Using t-test between NM and AM treatments, *P < 0.05 level is significant; **P < 0.01 level is very significant; ***P < 0.001 level is extremely significant.
Chlorophyll concentrations in Eucalyptus seedlings were shown to increase following P2P2 treatment over various treatments (Figures 3E–G). The concentrations of chlorophyll a, chlorophyll b and total chlorophyll under P2P2 treatment were 1.12, 0.69 and 1.75 times, accordingly, higher than those of N0P1 treatment. Also, the chlorophyll concentrations under treatments with high N and P increased significantly, revealing that high level of N and P increased the Eucalyptus seedlings’ chlorophyll concentrations. The findings demonstrated that mycorrhizal Eucalyptus seedlings treated with glycine had a higher chlorophyll concentration than non-mycorrhizal Eucalyptus seedlings. However, these results contrasted under G2P2 treatment, implying that higher level of N and P restricted the increase of mycorrhizal Eucalyptus seedlings’ chlorophyll concentrations.
3.4 Enzyme activities related to N metabolism and total N P content under different organic N and P conditions
Under different treatments, the activities of NR and GOGAT were significantly elevated under the P2P2 treatment. Compared to N0P1 treatment, NR and GOGAT activities under P2P2 treatment in Eucalyptus seedlings were increased by 0.81 and 3.82 times, respectively (Figures 4A, B). GS activity in mycorrhizal Eucalyptus was increased significantly, particularly under L1P1 treatment, where it was 1.75 times higher than NM treatment (Figure 4C). The activities of NR, GOGAT and GS in mycorrhizal Eucalyptus seedlings were elevated under G1P1 and G1P2 treatments compared to NM treatment (Figure 4). High P treatments did not effectively promote the GS activity in Eucalyptus. Concurrently, activity of NR and GOGAT under G1P1 treatment was 0.25 and 2.27 times, respectively, higher than NM treatments, indicating that high N and P levels did not promote the activities of NR and GOGAT. The results demonstrated that among the three organic N treatments, glycine treatment was more effective in promoting N metabolism in mycorrhizal Eucalyptus compared to non-mycorrhizal Eucalyptus (Figure 4).
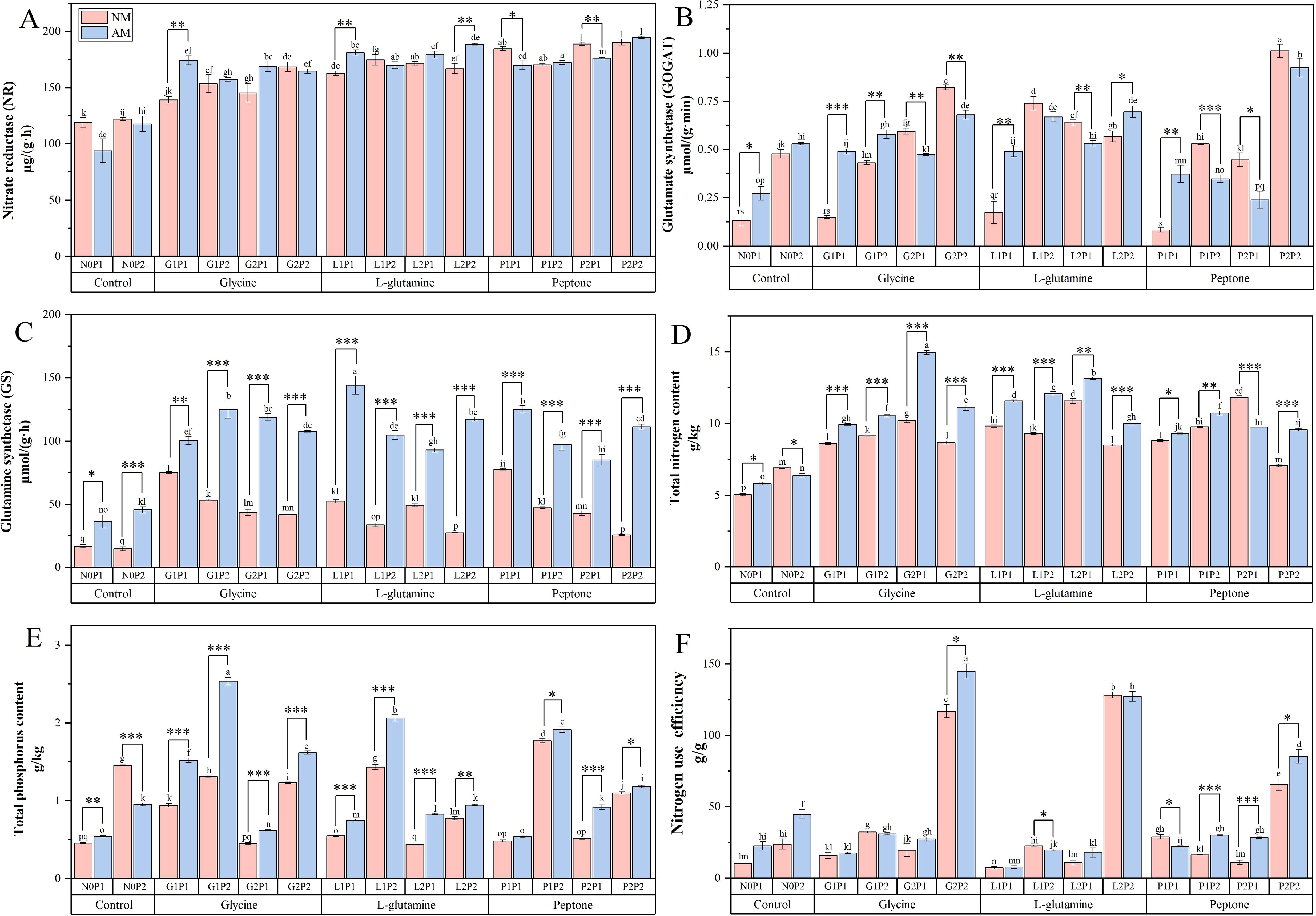
Figure 4. Mycorrhizal Eucalyptus seedlings N metabolism-related enzymes activities, TN TP contents and nitrogen use efficiency under different organic N and P treatments. Activity of nitrate reductase (A), activity of glutamate synthetase (B), activity of glutamine synthetase (C), total nitrogen content (D), total phosphorus content (E), nitrogen use efficiency (F). N0P1: 1 mM N-NO3- and 0.03 mM Pi treatment; N0P2: 1 mM N-NO3- and 0.3 mM Pi treatment; G1P1: 0.25 mM N-NO3-, 0.25 mM glycine and 0.03 mM Pi treatment; G1P2: 0.25 mM N-NO3-, 0.25 mM glycine and 0.3 mM Pi treatment; G2P1: 0.25 mM N-NO3-, 2.5 mM glycine and 0.03 mM Pi treatment; G2P2: 0.25 mM N-NO3-, 2.5 mM glycine and 0.3 mM Pi treatment; L1P1: 0.25 mM N-NO3-, 0.25 mM L-glutamine and 0.03 mM Pi treatment; L1P2: 0.25 mM N-NO3-, 0.25 mM L-glutamine and 0.3 mM Pi treatment; L2P1: 0.25 mM N-NO3-, 2.5 mM L-glutamine and 0.03 mM Pi treatment; L2P2: 0.25 mM N-NO3-, 2.5 mM L-glutamine and 0.3 mM Pi treatment; P1P1: 0.25 mM N-NO3-, 0.25 mM peptone and 0.03 mM Pi treatment; P1P2: 0.25 mM N-NO3-, 0.25 mM peptone and 0.3 mM Pi treatment; P2P1: 0.25 mM N-NO3-, 2.5 mM peptone and 0.03 mM Pi treatment; P2P2: 0.25 mM N-NO3-, 2.5 mM peptone and 0.3 mM Pi treatment. NM, non-mycorrhizal treatment; AM, mycorrhizal treatment. Control, no organic N treatment. Glycine, glycine treatment; L-glutamine, L-glutamine treatment; Peptone, peptone treatment. Using Duncan test for inter group differences, different letters meant that the factors changed significantly among the four strategies (P < 0.05). Using t-test between NM and AM treatments, *P < 0.05 level is significant; **P < 0.01 level is very significant; ***P < 0.001 level is extremely significant.
The TN content in Eucalyptus seedlings was significantly elevated under G2P1 treatment. The TN content in mycorrhizal Eucalyptus seedlings under N2P1 treatment was increased by 0.47 times compared to NM treatment (Figure 4D). Additionally, compared to N0P1 treatment, the TN content in Eucalyptus was 0.67 times higher than that of G2P1 treatment. While high P treatments were less effective, high N fertilization significantly raised the TN contents. Concurrently, the TP content in mycorrhizal Eucalyptus seedlings treated with G1P2 was elevated by 93.13%, compared to non-mycorrhizal Eucalyptus (Figure 4E). Additionally, high N level had no discernible effect on the TP contents. Under various treatments, the N2P2 treatments was the most effective in promoting the NUE of mycorrhizal Eucalyptus. The mycorrhizal Eucalyptus showed notable benefits in boosting NUE when exposed to high levels of organic N fertilization, particularly glycine treatments. NUE of mycorrhizal Eucalyptus under G2P2 treatment was elevated by 24.03%, compared to non-mycorrhizal Eucalyptus. The results indicated that among the three organic N treatments, glycine treatment was more effective in increasing the TN contents, TP contents and NUE of mycorrhizal Eucalyptus compared to non-mycorrhizal Eucalyptus (Figure 4).
3.5 Relative expression of genes related N transport of mycorrhizal Eucalyptus seedlings under different organic N and P conditions
Under different treatments, the relative expression levels of genes associated with N transport in mycorrhizal Eucalyptus significantly increased under G2P2 treatment (Figure 5). The AM treatment markedly elevated the relative expression levels of EgAAP3, EgProt2, EgNPF4.5, EgNPF6.3, EgNPF8.1 and EgAMT2.1, compared to NM treatments under three organic N treatments. The relative expression of genes related to organic N transport (EgAAP3 and EgProt2), nitrate N transport (EgNPF4.5, EgNPF6.3 and EgNPF8.1) in mycorrhizal Eucalyptus were significantly up-regulated under high P treatments compared to treatments at the same organic N level. This suggests that P may be a key factor regulating nitrate N transport and amino acid transport in Eucalyptus (Figure 5). Under G2P2 and L2P2 treatments, mycorrhizal Eucalyptus showed considerably greater relative expression levels of EgAMT2.1 than under NM treatments, whereas under L1P1 and L2P1 treatments, mycorrhizal Eucalyptus showed significantly lower relative expression levels of EgAMT3.1 than under NM treatments. This might indicate that plants use distinct ammonium ion transporter proteins to control ammonium ion absorption under various N supply situations.
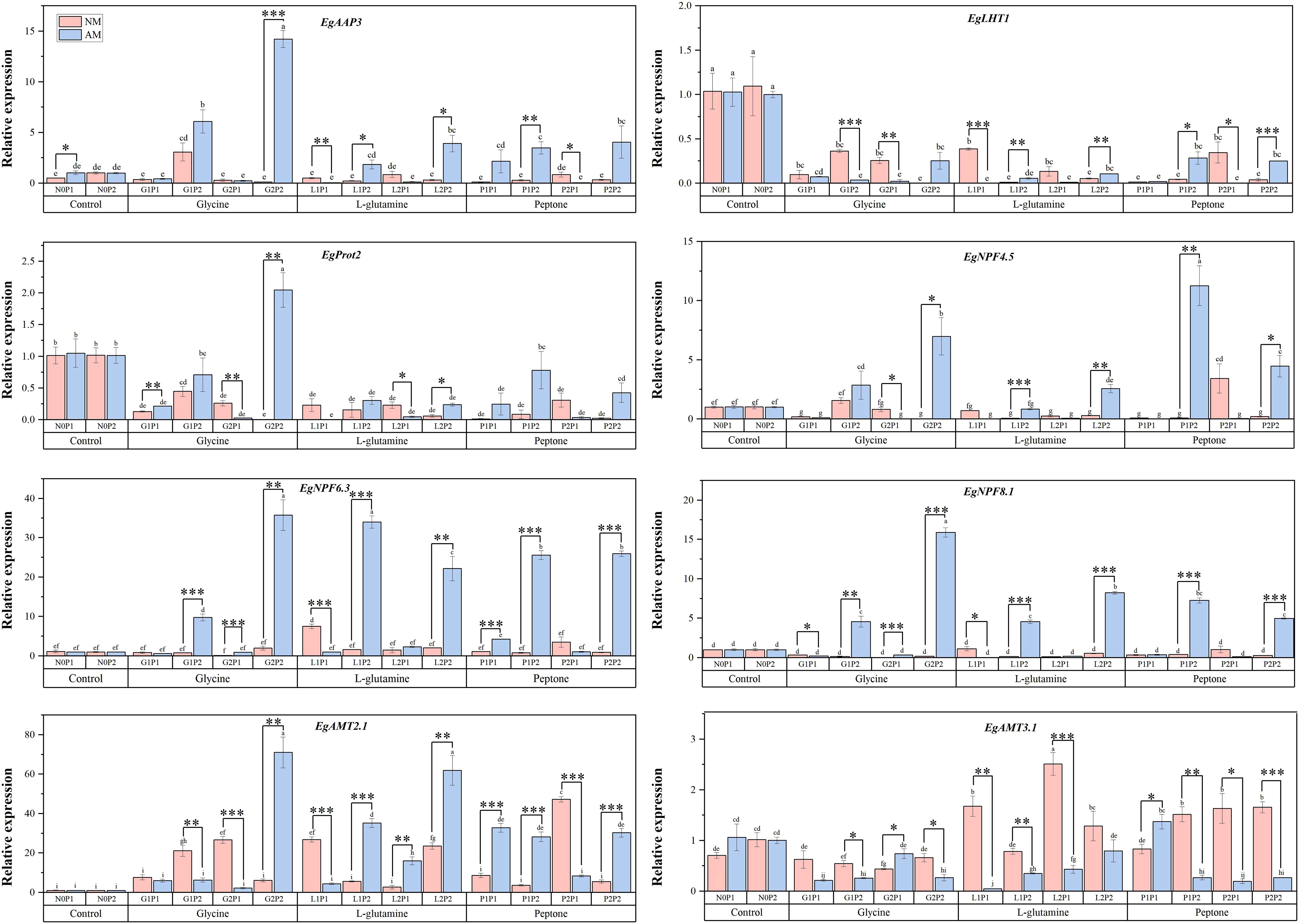
Figure 5. Mycorrhizal Eucalyptus seedlings relative expressions of genes related to N transport under different organic N and P treatments. N0P1: 1 mM N-NO3- and 0.03 mM Pi treatment; N0P2: 1 mM N-NO3- and 0.3 mM Pi treatment; G1P1: 0.25 mM N-NO3-, 0.25 mM glycine and 0.03 mM Pi treatment; G1P2: 0.25 mM N-NO3-, 0.25 mM glycine and 0.3 mM Pi treatment; G2P1: 0.25 mM N-NO3-, 2.5 mM glycine and 0.03 mM Pi treatment; G2P2: 0.25 mM N-NO3-, 2.5 mM glycine and 0.3 mM Pi treatment; L1P1: 0.25 mM N-NO3-, 0.25 mM L-glutamine and 0.03 mM Pi treatment; L1P2: 0.25 mM N-NO3-, 0.25 mM L-glutamine and 0.3 mM Pi treatment; L2P1: 0.25 mM N-NO3-, 2.5 mM L-glutamine and 0.03 mM Pi treatment; L2P2: 0.25 mM N-NO3-, 2.5 mM L-glutamine and 0.3 mM Pi treatment; P1P1: 0.25 mM N-NO3-, 0.25 mM peptone and 0.03 mM Pi treatment; P1P2: 0.25 mM N-NO3-, 0.25 mM peptone and 0.3 mM Pi treatment; P2P1: 0.25 mM N-NO3-, 2.5 mM peptone and 0.03 mM Pi treatment; P2P2: 0.25 mM N-NO3-, 2.5 mM peptone and 0.3 mM Pi treatment. NM, non-mycorrhizal treatment; AM, mycorrhizal treatment. Control, no organic N treatment. Glycine, glycine treatment; L-glutamine, L-glutamine treatment; Peptone, peptone treatment. Using Duncan test for inter group differences, different letters meant that the factors changed significantly among the four strategies (P < 0.05). Using t-test between NM and AM treatments, *P < 0.05 level is significant; **P < 0.01 level is very significant; ***P < 0.001 level is extremely significant.
3.6 Correlation analysis between different growth and physiological characters of Eucalyptus seedlings
Significant positive relationships between seedling growth features and photosynthetic characteristics were found based on the correlation plot among several Eucalyptus growth and physiological characters (Figure 6). This suggests that photosynthesis is a major factor influencing seedling growth. Chlorophyll concentration and seedling growth characteristics showed highly significant positive associations with NUE (P < 0.001). Additionally, TC was shown to have highly significant positive associations (P < 0.001) with both NR and GOGAT activities. In summary, GOGAT activity, photosynthesis, and NUE are important variables affecting Eucalyptus growth. As presented in Supplementary Table 2, the impacts of AM fungi, N application and P application, organic N and the interactions of the previous four significantly (P < 0.01) elevated the levels of evaluated growth and photosynthesis indexes.
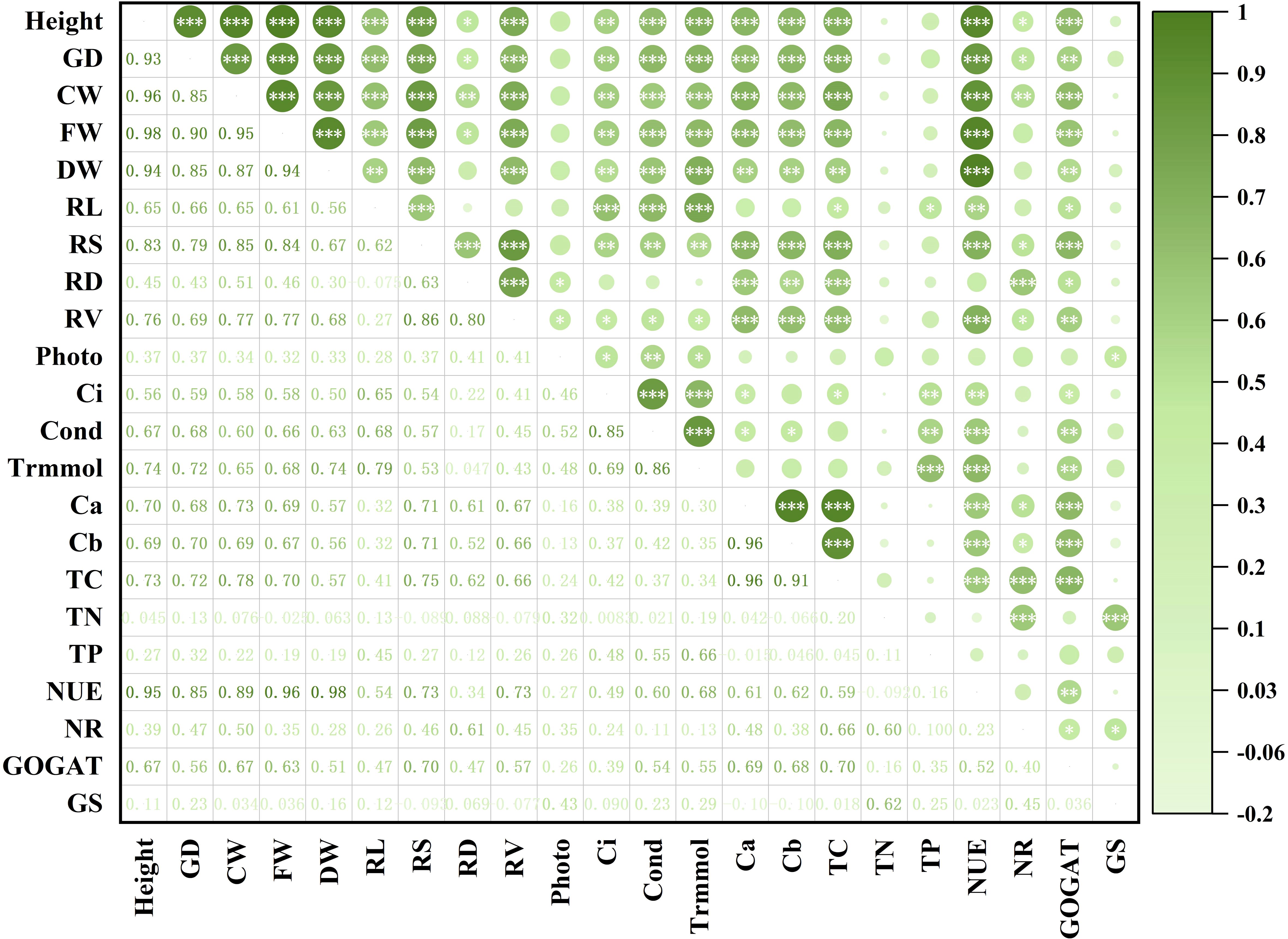
Figure 6. Correlation analysis between different growth and physiological characters of Eucalyptus. Height: seedling height; GD: ground diameter; CW: crown width; FW: fresh weight; DW: dry weight; RL: root length; RS: root average surface area; RD: root average diameter; RV: root average volume; Photo: net photosynthetic rate; Ci: intercellular CO2 concentration; Cond: stomatal conductance; Trmmol: transpiration rate; Ca: chlorophyll a concentration; Cb: chlorophyll b concentration; TC: total chlorophyll concentration; TN: total nitrogen content; TP: total phosphorus content; NUE: nitrogen use efficiency; NR: activity of nitrate reductase; GOGAT: activity of glutamate synthetase; GS: activity of glutamine synthetase. The dark green color represents a positive correlation between the two indicators, and light green represents a negative correlation between the two indicators, and the depth of the color represents the level of correlation. An asterisk (*) represents that the correlation between the two indicators reaches a significant difference level (P < 0.05), two asterisks (**) represent that the correlation between the two indicators reaches a very significant difference level (P < 0.01), three asterisks (***) represent that the correlation between the two indicators reaches an extremely significant difference level (P < 0.001).
4 Discussion
4.1 Effects of AM fungi on plant growth under different N and P conditions
AM fungi play a vital role in enhancing plant growth under nutrient-limited conditions. Our study revealed that AM treatments exhibited significantly greater biomass accumulation, compared to NM treatments, with low P fertilization under the same N level (Figure 1). This aligns with previous observations that AM fungi symbiosis improves P acquisition efficiency in P deficient soil by extending the root absorption zone, thereby compensating for reduced root hair development (Qian et al., 2024). Notably, this growth advantage diminished under N2P2 treatments, where NM treatments outperformed their AM treatments, likely due to suppressed AM fungi colonization when P availability exceeds plant requirements (Figure 2).
The AM fungi symbiosis further modulated seedlings’ organic N preference by enhancing NUE. Among three organic N sources, glycine emerged as the most effective in promoting mycorrhizal seedlings growth, contrasting with limited benefits observed in NM treatments. This finding corroborates evidence that forest-dominant plants, regardless of mycorrhizal type, preferentially acquire low-molecular-weight organic N compounds like glycine (Näsholm et al., 1998; Naz et al., 2024). Glycine’s efficiency in promoting Eucalyptus growth may be due to its small molecular weight and simple structure, making it easily absorbed by plants directly. The molecular simplicity of glycine likely facilitates direct uptake through AM-mediated pathways, bypassing energy-intensive mineralization processes required for complex N forms.
A synergistic mechanism between AM fungi and organic N was evident through coordinated nutrient interactions. While N fertilization independently promoted root proliferation, AM fungi inoculation amplified this effect by improving P co-utilization efficiency (Chen et al., 2020). The symbiosis induced physiological modifications in root architecture and exudation patterns, creating feedback loops that enhanced both N and P acquisition (Mazumder et al., 2025). This synergy was particularly pronounced under moderate fertilization regimes, where AM fungi optimized the balance between N assimilation and P mobilization. Our results demonstrate that AM-mediated nutrient coordination, rather than isolated nutrient applications, drives sustainable Eucalyptus growth, which is a critical insight for developing precision fertilization strategies in forest management.
4.2 Effects of AM fungi on plant photosynthetic characteristic and total N P contents under different N and P conditions
In our study, treatments with high N and P significantly increased the seedlings chlorophyll concentration (Figure 3), which corresponds with the previous finding that leaf chlorophyll content is positively correlated with N supply level. Increasing N supply typically elevates leaf chlorophyll content (Wu et al., 2017b; Chen et al., 2024). At the same time, AM fungi significantly improved photosynthetic efficiency of Eucalyptus seedlings under P-limited conditions. While high P treatments universally boosted net photosynthesis, stomatal conductance, and transpiration rate (Figure 3), AM treatments exhibited superior photosynthetic performance compared to NM treatments under low P availability. This aligns with P’s critical role as a substrate, and deficiency or low level of P inhibit leaf photosynthetic rates (Yu et al., 2022). Notably, AM fungi symbiosis optimized N partitioning under low N conditions, redirecting N resources from structural components to photosynthetic machinery, thereby amplifying light energy capture and carbon assimilation (Wu et al., 2017a). This reallocation mechanism underscores AM fungi’s ability to mitigate nutrient limitations by prioritizing metabolic processes essential for growth under low fertilization (Sun J, et al., 2024).
The symbiosis between AM fungi and Eucalyptus selectively enhanced organic N utilization, with glycine emerging as the most effective N source for mycorrhizal seedlings. Under glycine treatments, AM treatments showed markedly elevated chlorophyll concentrations compared to NM treatments (Figure 3), a response linked to enhanced NUE and upregulated activities of N metabolism enzymes (NR, GOGAT) (Figure 6). These findings corroborate that AM fungi facilitate direct assimilation of low-molecular-weight organic N compounds, bypassing energy-intensive mineralization pathways (Näsholm et al., 1998; Naz et al., 2024). By increasing chlorophyll biosynthesis, AM fungi amplified capacity on harvesting light, creating a positive feedback loop where improved N metabolism reinforced photosynthetic output. This preferential utilization of glycine highlights AM fungi’s role in fine-tuning N source selection to maximize host plant productivity.
Several studies have demonstrated that AM fungi can facilitate plant uptake of soil nutrients such as N and P, increasing plant biomass, N concentration, P concentration, N uptake of whole plant (Che et al., 2022; Wu Y, et al., 2024). Our findings observed that high P treatments behaved better in increasing mycorrhizal Eucalyptus’s TP contents. This effect is most likely mediated by improved P acquisition in mycorrhizal plants compared to non-mycorrhizal plants under low P conditions. Outsourcing P acquisition to mycorrhiza may limit the value of P loss reduction by plants (Wang D, et al., 2025). AM fungi-organic N synergy drove coordinated improvements in N and P homeostasis. AM treatments exhibited simultaneous increases in TN and TP contents (Figure 4), reflecting synergistic interactions exist between N and P uptake. While AM fungi primarily enhanced P uptake via extraradical hyphae that bypass rhizosphere depletion zones (Smith et al., 2011; Bhupenchandra et al., 2024), organic N fertilization indirectly boosted P availability by stimulating soil phosphatase activity, accelerating organic P mineralization (Gou et al., 2024). This dual mechanism direct AM-mediated P scavenging and N-driven P mobilization, creating a synergistic nutrient loop. High P treatments amplified this interaction, as AM fungi colonization under sufficient P conditions optimized N-P stoichiometry, further elevating chlorophyll synthesis and photosynthetic rates. Such interdependence underscores that AM fungi do not merely supplement nutrient uptake but actively rewire host plant metabolism to exploit nutrient co-limitation, transforming discrete nutrient inputs into multiplicative growth benefits.
4.3 Effects of AM fungi on plant activity of enzymes related to N metabolism under different N and P conditions
AM fungi significantly upregulated key N metabolic enzymes in Eucalyptus leaves, even under N-deficient conditions. Mycorrhizal seedlings exhibited elevated activities of NR, GOGAT, and GS, compared to NM treatments (Figure 4), consistent with AM fungi’s role in enhancing N uptake and assimilation. The GS-GOGAT cycle, which is critical for converting ammonium into glutamine and glutamate, was particularly amplified, thus providing precursors for amino acids, nucleic acids, and chlorophyll synthesis. This aligns with studies showing AM fungi colonization upregulates genes encoding nitrate transporters and N metabolism enzymes, enabling efficient N recycling under low nutrient conditions (Wang et al., 2020; Chen et al., 2023; Wu XL, et al., 2024). Notably, this enzymatic enhancement occurred independently of fertilization levels, suggesting AM fungi constitutively prime N assimilation pathways to mitigate nutrient scarcity.
The symbiosis selectively optimized organic N utilization by prioritizing ammonium assimilation via the GS-GOGAT pathway (Fortunato et al., 2023; Vaishnav et al., 2025). While NM treatments relied heavily on soil nitrate, mycorrhizal Eucalyptus exhibited a metabolic shift toward glutamine synthesis, a hallmark of organic N preference. This reprogramming explains why mycorrhizal seedlings maintained higher GS activity across all treatments (Figure 4), as GS catalyzes the ATP-dependent fixation of ammonium, which is the key step in assimilating organic N breakdown products (Kojima et al., 2021). By bypassing energetically costly nitrate reduction, AM fungi-enabled ammonium assimilation conserved cellular resources, redirecting energy toward chlorophyll biosynthesis and photosynthetic output. Such metabolic flexibility underscores AM fungi’s ability to tailor N source utilization to environmental availability, favoring organic N forms like glycine under nutrient-limited condition.
The symbiosis between AM fungi and Eucalyptus created a nutrient acquisition feedback loop which N assimilation efficiency reinforced P utilization. Elevated GS activity in mycorrhizal roots increased demand for P-rich ATP, driving hyphal P scavenging via the AM pathway. Paradoxically, high N-P co-fertilization disrupted this synergy, excessive P suppressed AM fungi colonization, reducing NR and GOGAT activities despite nutrient abundance (Figure 4). This aligns with the trade-off balance model, where AM fungi benefits diminish when soil P exceeds host demand, destabilizing the symbiosis (Smith et al., 2011; Bennett and Groten, 2022). Crucially, organic N inputs sustained the synergy by stimulating phosphatase-mediated P mineralization, whereas synthetic fertilization disrupted it (Manzoor et al., 2022). These findings reveal that AM fungi-mediated nutrient synergy thrives under moderate, organically balanced fertilization but collapses under inorganic nutrient overload, highlighting the importance of AM fungi-driven stoichiometric regulation in sustainable nutrient management.
4.4 Effects of AM fungi on plant genes expression related to N transport under different N and P conditions
AM fungi significantly upregulated key N transporter genes in Eucalyptus roots under nutrient-limited conditions. Mycorrhizal seedlings exhibited elevated expression of nitrate transporters (EgNPF4.5, EgNPF6.3, EgNPF8.1) and organic N transporters (EgAAP3, EgProt2), compared to NM treatments (Figure 5), mirroring results observed in AM fungi-colonized maize and sorghum (Xie et al., 2022). This transcriptional reprogramming enables efficient N scavenging in low-fertility soils by activating dual acquisition pathways: direct nitrate uptake via NPF transporters and organic N assimilation through transport mediated by AAP3 and Prot2. Notably, P limitation amplified this response, as P starvation signals cross-regulate N transporter expression, positioning AM fungi as critical enhancers of N capture under co-limiting conditions (Zhao et al., 2021).
The symbiosis shifted Eucalyptus N acquisition strategy toward organic sources, evidenced by upregulation of EgAAP3 and EgProt2. While nitrate transporters (EgNPF4.5, EgNPF6.3, EgNPF8.1) were also induced, their activity likely supports secondary nitrate uptake from AM-released N pools in the peri-arbuscular space (Wang et al., 2020). Contrastingly, the downregulation of ammonium transporter EgAMT3.1 in mycorrhizal roots suggests a metabolic trade-off, AM fungi suppress energetically costly ammonium uptake pathways while prioritizing organic N and nitrate assimilation. This aligns with findings in Lycium barbarum, where AM fungi colonization favors nitrate over ammonium uptake, highlighting a conserved mycorrhizal strategy to optimize N source utilization based on symbiotic efficiency (Gong et al., 2023). The coordinated and specific expression of ammonium and nitrate transporters in mycorrhizae-colonized cortical cells suggests the crucial importance of fungal N transfer in plants (Rui et al., 2022).
AM fungi orchestrated a nutrient feedback loop which P availability fine-tuned N transporter expression. High P treatments amplified EgNPFs and EgAAP3 expression (Figure 5), indicating that P sufficiency liberates carbon resources for N transporter synthesis, while P scarcity prioritizes organic N uptake to balance stoichiometric demands. This synergy mirrors the dual role of AM fungi hyphae: (1) delivering P via the AM pathway, which reduces plant investment in root P transporters, and (2) priming N transporters to exploit organic N mineralization driven by phosphatase activity (Wang S, et al., 2025). However, excessive P disrupted the balance, downregulating AM fungi-specific ammonium transporters and demonstrating that optimal AM fungi-driven nutrient synergy occurs under moderate P levels where N-P co-regulation remains intact (Calabrese et al., 2016).
5 Conclusion
In conclusion, mycorrhizal Eucalyptus seedlings’ growth was significantly enhanced under organic N and P fertilization, compared to NM treatments. AM fungi contributed to increasing the NUE and N absorption, thus improving N contents and photosynthesis of seedlings, which in turn enhanced seedling growth. Relative expressions of genes related to organic and nitrate N transport in root of mycorrhizal seedlings were significantly higher than NM treatments, thus improving seedlings’ enzymes related to N metabolism. Eucalyptus showed varying preferences for absorption of various organic N sources. Under glycine treatments, mycorrhizal Eucalyptus seedlings’ growth was the highest promoted, whereas non-mycorrhizal Eucalyptus favored peptone. Eucalyptus growth under high level of N and P treatments were promoted markedly, regardless of organic N source, which showed N-P synergistic interaction in Eucalyptus seedlings. In addition, compared to NM treatments, the growth of mycorrhizal Eucalyptus was the most enhanced under low N and high P treatment, which has potential to reduce fertilization and improve biomass.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Author contributions
XL: Investigation, Data curation, Writing – original draft, Formal analysis. CH: Formal analysis, Methodology, Writing – original draft, Data curation, Investigation, Conceptualization. XY: Methodology, Data curation, Writing – original draft, Investigation. QL: Investigation, Writing – original draft, Methodology, Data curation. ZS: Investigation, Writing – original draft, Methodology. XF: Methodology, Investigation, Writing – original draft. ZL: Writing – original draft, Methodology, Investigation. ZC: Conceptualization, Writing – review & editing, Funding acquisition, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Guangdong Basic and Applied Basic Research Foundation, China (2025A1515010996), the Innovation and Entrepreneurship Program for University Students (2024105641209; 2024105641211).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1597451/full#supplementary-material
References
Ahmed, N., Li, J., Li, Y., Deng, L., Deng, L., Chachar, M., et al. (2025). Symbiotic synergy: How arbuscular mycorrhizal fungi enhance nutrient uptake, stress tolerance, and soil health through molecular mechanisms and hormonal regulation. IMA fungus 16, e144989. doi: 10.3897/imafungus.16.144989
Bennett, A. E. and Groten, K. (2022). The costs and benefits of plant-arbuscular mycorrhizal fungal interactions. Annu. Rev. Plant Biol. 73, 649–672. doi: 10.1146/annurev-arplant-102820-124504
Bhupenchandra, I., Chongtham, S. K., Devi, A. G., Dutta, P., Sahoo, M. R., Mohanty, S., et al. (2024). Unlocking the potential of arbuscular mycorrhizal fungi: exploring role in plant growth promotion, nutrient uptake mechanisms, biotic stress alleviation, and sustaining agricultural production systems. J. Plant Growth Regul. 43 (9), 1–39. doi: 10.1007/s00344-024-11467-9
Bouain, N., Krouk, G., Lacombe, B., and Rouached, H. (2019). Getting to the root of plant mineral nutrition: combinatorial nutrient stresses reveal emergent properties. Trends Plant Sci. 24, 542–552. doi: 10.1016/j.tplants.2019.03.008
Calabrese, S., Pérez-Tienda, J., Ellerbeck, M., Arnould, C., Chatagnier, O., Boller, T., et al. (2016). GintAMT3 - a low-affinity ammonium transporter of the arbuscular mycorrhizal Rhizophagus irregularis. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.00679
Che, X., Lai, W., Wang, S., Wang, X., Hu, W., Chen, H., et al. (2022). Multiple PHT1 family phosphate transporters are recruited for mycorrhizal symbiosis in Eucalyptus grandis and conserved PHT1;4 is a requirement for the arbuscular mycorrhizal symbiosis. Tree Physiol. 42, 2020–2039. doi: 10.1093/treephys/tpac050
Chen, J., Liu, L., Wang, Z., Zhang, Y., Sun, H., Song, S., et al. (2020). Nitrogen fertilization increases root growth and coordinates the root-shoot relationship in cotton. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.00880
Chen, L. H., Xu, M., Cheng, Z., and Yang, L. T. (2024). Effects of nitrogen deficiency on the photosynthesis, chlorophyll a fluorescence, antioxidant system, and sulfur compounds in Oryza sativa. Int. J. Mol. Sci. 25, 10409. doi: 10.3390/ijms251910409
Chen, W., Mou, X., Meng, P., Chen, J., Tang, X., Meng, G., et al. (2023). Effects of arbuscular mycorrhizal fungus inoculation on the growth and nitrogen metabolism of Catalpa bungei CA Mey. under different nitrogen levels. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1138184
Cui, R., Wang, C., Cheng, F., Ma, X., Cheng, X., He, B., et al. (2023). Effects of successive planting of Eucalyptus on soil physicochemical properties 1-3 generations after converting Masson Pine forests into Eucalyptus plantations. Pol. J. Environ. Stud. 32 (5), 4503–4514. doi: 10.15244/pjoes/169015
Duan, S., Feng, G., Limpens, E., Bonfante, P., Xie, X. A., and Zhang, L. (2024). Cross-kingdom nutrient exchange in the plant-arbuscular mycorrhizal fungus-bacterium continuum. Nat. Rev. Microbiol. 22, 773–790. doi: 10.1038/s41579-024-01073-7
Fall, A. F., Nakabonge, G., Ssekandi, J., Founoune-Mboup, H., Apori, S. O., Ndiaye, A., et al. (2022). Roles of arbuscular mycorrhizal fungi on soil fertility: contribution in the improvement of physical, chemical, and biological properties of the soil. Front. Fungal Biol. 3. doi: 10.3389/ffunb.2022.723892
Fortunato, S., Nigro, D., Lasorella, C., Marcotuli, I., Gadaleta, A., and de Pinto, M. C. (2023). The role of glutamine synthetase (GS) and glutamate synthase (GOGAT) in the improvement of nitrogen use efficiency in cereals. Biomolecules 13, 1771. doi: 10.3390/biom13121771
Ganeteg, U., Ahmad, I., Jämtgard, S., Aguetoni-Cambui, C., Inselsbacher, E., Svennerstam, H., et al. (2017). Amino acid transporter mutants of Arabidopsis provides evidence that a non-mycorrhizal plant acquires organic nitrogen from agricultural soil. Plant Cell Environ. 40, 413–423. doi: 10.1111/pce.12881
Gong, M., Zhang, Q., Cheng, K., and Zhang, H. (2023). Symbiosis of arbuscular mycorrhizal fungi and Lycium barbarum L. prefers NO3- over NH4+. Hortic 9, 637. doi: 10.3390/horticulturae9060637
Gou, X. M., Ren, Y. Q., Qin, X., Wei, X. R., and Wang, J. J. (2024). Global patterns of soil phosphatase responses to nitrogen and phosphorus fertilization. Pedosphere 34, 200–210. doi: 10.1016/j.pedsph.2023.06.011
Hansen, T. H., De Bang, T. C., Laursen, K. H., Pedas, P., Husted, S., and Schjoerring, J. K. (2013). Multielement plant tissue analysis using ICP spectrometry. Plant Mineral Nutrients: Methods Protoc. 953, 121–141. doi: 10.1007/978-1-62703-152-3_8
Hewitt, E. J. (1966). Sand and water culture methods used in the study of plant nutrition Vol. 22 (England UK: Farnham Royal), 315–709. doi: 10.2134/agronj1952.00021962004400120018x
Hirner, A., Ladwig, F., Stransky, H., Okumoto, S., Keinath, M., Harms, A., et al. (2006). Arabidopsis LHT1 is a high-affinity transporter for cellular amino acid uptake in both root epidermis and leaf mesophyll. Plant Cell 18, 1931–1946. doi: 10.1105/tpc.106.041012
Hodge, A., Campbell, C. D., and Fitter, A. H. (2001). An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 413, 297–299. doi: 10.1038/35095041
Hodge, A. and Fitter, A. H. (2010). Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proc. Natl. Acad. Sci. 107, 13754–13759. doi: 10.1073/pnas.1005874107
Hu, W., Zhang, Y., Rong, X., Fei, J., Peng, J., and Luo, G. (2023). Coupling amendment of biochar and organic fertilizers increases maize yield and phosphorus uptake by regulating soil phosphatase activity and phosphorus-acquiring microbiota. Agric. Ecosyst. Environ. 355, 108582. doi: 10.1016/j.agee.2023.108582
Huang, L., Yan, H., Jiang, X., Yin, G., Zhang, X., Qi, X., et al. (2014). Identification of candidate reference genes in perennial ryegrass for quantitative RT-PCR under various abiotic stress conditions. PloS One 9, e93724. doi: 10.1371/journal.pone.0093724
Jansa, J., Forczek, S. T., Rozmoš, M., Püschel, D., Bukovská, P., and Hršelová, H. (2019). Arbuscular mycorrhiza and soil organic nitrogen: network of players and interactions. Chem. Biol. Technol. Agric. 6, 1–10. doi: 10.1186/s40538-019-0147-2
Jones, D. L., Healey, J. R., Willett, V. B., Farrar, J. F., and Hodge, A. (2005). Dissolved organic nitrogen uptake by plants-an important N uptake pathway? Soil Biol. Biochem. 37, 413–423. doi: 10.1016/j.soilbio.2004.08.008
Kojima, S., Ishiyama, K., Beier, M. P., and Hayakawa, T. (2021). Ammonium assimilation and metabolism in rice. Prog. Bot. Vol. 82, 211–231. doi: 10.1007/978-3-030-68620-8
Krouk, G. and Kiba, T. (2020). Nitrogen and phosphorus interactions in plants: from agronomic to physiological and molecular insights. Curr. Opin. Plant Biol. 57, 104–109. doi: 10.1016/j.pbi.2020.07.002
Kuila, D. and Ghosh, S. (2022). Aspects, problems and utilization of Arbuscular Mycorrhizal (AM) application as bio-fertilizer in sustainable agriculture. Curr. Res. Microb. Sci. 3, 100107. doi: 10.1016/j.crmicr.2022.100107
Kumar, S., Kumar, S., and Mohapatra, T. (2021). Interaction between macro-and micro-nutrients in plants. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.665583
Lichtenthaler, H. K. and Wellburn, A. R. (1983). Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc Transmycol. 11, 591–593. doi: 10.1042/BST0110591
Loth-Pereda, V., Orsini, E., Courty, P. E., Lota, F., Kohler, A., Diss, L., et al. (2011). Structure and expression profile of the phosphate Pht1 transporter gene family in mycorrhizal. Populus trichocarpa. Plant Physiol. 156, 2141–2154. doi: 10.1104/pp.111.180646
Magalhaes, J. R. and Huber, D. M. (1991). Response of ammonium assimilation enzymes to nitrogen form treatments in different plant species. J. Plant Nutr. 14, 175–185. doi: 10.1080/01904169109364193
Manzoor, A., Dippold, M. A., Loeppmann, S., and Blagodatskaya, E. (2022). Two-phase conceptual framework of phosphatase activity and phosphorus bioavailability. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.935829
Marro, N., Lidoy, J., Chico, M.Á., Rial, C., García, J., Varela, R. M., et al. (2022). Strigolactones: New players in the nitrogen-phosphorus signalling interplay. Plant Cell Environ. 45, 512–527. doi: 10.1111/pce.14212
Mazumder, S., Bhattacharya, D., Lahiri, D., and Nag, M. (2025). Rhizobacteria and arbuscular mycorrhizal fungi (AMF) community in growth management and mitigating stress in millets: A plant-soil microbe symbiotic relationship. Curr. Microb. 82, 242. doi: 10.1007/s00284-025-04230-0
Mbodj, D., Diedhiou, A. G., Manneh, B., Ndiaye, C., Laplaze, L., and Kane, A. (2025). AMF inoculation reduces yield losses in rice exposed to alternate wetting and drying and low fertilization. Sci. Rep. 15, 12281. doi: 10.1038/s41598-025-95528-3
Näsholm, T., Ekblad, A., Nordin, A., Giesler, R., Högberg, M., and Högberg, P. (1998). Boreal forest plants take up organic nitrogen. Nature 392, 914–916. doi: 10.1038/31921
Navarro, J. M. and Morte, A. (2024). Arbuscular mycorrhizal fungi as biofertilizers to increase the plant quality of sour-orange seedlings. Agronomy 14, 230. doi: 10.3390/agronomy14010230
Naz, S., Khan, S. U., Kanwal, F., Khan, A., and Zhang, G. (2024). The difference in shoot metabolite profiles of a wild and a cultivated barley genotype in response to four nitrogen forms. Agronomy 14, 621. doi: 10.3390/agronomy14030621
Pons, S., Fournier, S., Chervin, C., Bécard, G., Rochange, S., Frei Dit Frey, N., et al. (2020). Phytohormone production by the arbuscular mycorrhizal fungus Rhizophagus irregularis. PloS One 15, e0240886. doi: 10.1371/journal.pone.0240886
Qian, S., Xu, Y., Zhang, Y., Wang, X., Niu, X., and Wang, P. (2024). Effect of AMF inoculation on reducing excessive fertilizer use. Microorg 12, 1550. doi: 10.3390/microorganisms12081550
Rengel, Z., Cakmak, I., and White, P. J. (2022). Marschner’s mineral nutrition of plants (New York: Academic press).
Řezáčová, V., Czakó, A., Stehlík, M., Mayerová, M., Šimon, T., Smatanová, M., et al. (2021). Organic fertilization improves soil aggregation through increases in abundance of eubacteria and products of arbuscular mycorrhizal fungi. Sci. Rep. 11, 12548. doi: 10.1038/s41598-021-91653-x
Rui, W., Mao, Z., and Li, Z. (2022). The roles of phosphorus and nitrogen nutrient transporters in the arbuscular mycorrhizal symbiosis. Int. J. Mol. Sci. 23, 11027. doi: 10.3390/ijms231911027
Saia, S., Tamayo, E., Schillaci, C., and De Vita, P. (2019). Arbuscular mycorrhizal fungi and nutrient cycling in cropping systems. C.N. Cycl. Soil, 87–115. Singapore: Springer Singapore. doi: 10.1007/978-981-13-7264-3
Schuman, G. E., Stanley, M. A., and Knudsen, D. (1973). Automated total nitrogen analysis of soil and plant samples. Soil Sci. Soc Am. J. 37, 480–481. doi: 10.2136/sssaj1973.03615995003700030045x
Shang, X., Zhang, P., Li, X., Wang, Y., and Wu, Z. (2024). Key traits influencing the resistance of Eucalyptus camaldulensis to wind damage in coastal areas of South China. Front. Plant Sci. 15. doi: 10.3389/fpls.2024.1433670
Shi, H., Ma, W., Song, J., Lu, M., Rahman, S. U., Bui, T. T. X., et al. (2017). Physiological and transcriptional responses of Catalpa bungei to drought stress under sufficient-and deficient-nitrogen conditions. Tree Physiol. 37, 1457–1468. doi: 10.1093/treephys/tpx090
Singh, R. P. and Srivastava, H. S. (1986). Increase in glutamate synthase (NADH) activity in maize seedlings in response to nitrate and ammonium nitrogen. Physiologia Plantarum 66, 413–416. doi: 10.1111/j.1399-3054.1986.tb05944.x
Smith, S. E., Jakobsen, I., Grønlund, M., and Smith, F. A. (2011). Roles of arbuscular mycorrhizas in plant phosphorus nutrition: interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol. 156, 1050–1057. doi: 10.1104/pp.111.174581
Srivastava, H. S. (1980). Regulation of nitrate reductase activity in higher plants. Phytochemistry 19, 725–733. doi: 10.1016/0031-9422(80)85100-4
Sun, J., Tang, Y., Chen, K., Ren, S., Shi, H., Dong, Q., et al. (2024). Nitrogen level determines arbuscular mycorrhizal fungi nitrogen uptake rate of Stipa purpurea in alpine steppe. Plant Soil 504, 1–17. doi: 10.1007/s11104-024-07106-7
Sun, K., Zhang, W., Wang, X., and Dai, C. C. (2024). Decoding the microbiome for sustainable agriculture. Abiotech 5, 408–412. doi: 10.1007/s42994-024-00162-8
Svennerstam, H., Jämtgard, S., Ahmad, I., Huss-Danell, K., Näsholm, T., and Ganeteg, U. (2011). Transporters in Arabidopsis roots mediating uptake of amino acids at naturally occurring concentrations. New Phytol. 191, 459–467. doi: 10.1111/j.1469-8137.2011.03699.x
Tang, J., Zhao, J., Qin, Z., Chen, L., Song, X., Ke, Q., et al. (2023). Structural equation model was used to evaluate the effects of soil chemical environment, fertility and enzyme activity on eucalyptus biomass. R. Soc Open Sci. 10, 221570. doi: 10.1098/rsos.221570
Vaishnav, A., Rozmoš, M., Kotianová, M., Hršelová, H., Bukovská, P., and Jansa, J. (2025). Protists are key players in the utilization of protein nitrogen in the arbuscular mycorrhizal hyphosphere. New Phytol. 246 (6), 2753–2764. doi: 10.1111/nph.70153
Wahab, A., Batool, F., Muhammad, M., Zaman, W., Mikhlef, R. M., Qaddoori, S. M., et al. (2024). Unveiling the complex molecular dynamics of arbuscular mycorrhizae: a comprehensive exploration and future perspectives in harnessing phosphate-solubilizing microorganisms for sustainable progress. Environ. Exp. Bot. 219, 105633. doi: 10.1016/j.envexpbot.2023.105633
Wahab, A., Muhammad, M., Munir, A., Abdi, G., Zaman, W., Ayaz, A., et al. (2023). Role of arbuscular mycorrhizal fungi in regulating growth, enhancing productivity, and potentially influencing ecosystems under abiotic and biotic stresses. Plants 12, 3102. doi: 10.3390/plants12173102
Wang, S., Chen, A., Xie, K., Yang, X., Luo, Z., Chen, J., et al. (2020). Functional analysis of the OsNPF4.5 nitrate transporter reveals a conserved mycorrhizal pathway of nitrogen acquisition in plants. Proc. Natl. Acad. Sci. 117, 16649–16659. doi: 10.1073/pnas.2000926117
Wang, D., Freschet, G. T., McCormack, M. L., Lambers, H., and Gu, J. (2025). Nutrient resorption of leaves and roots coordinates with root nutrient-acquisition strategies in a temperate forest. New Phytol. 246 (2), 515–527. doi: 10.1111/nph.70001
Wang, H., Tian, D., Cao, J., Ren, S., Zhu, Y., Wang, H., et al. (2024). Eucalyptus and native broadleaf mixed cultures boost soil multifunctionality by regulating soil fertility and fungal community dynamics. J. Fungi 10, 709. doi: 10.3390/jof10100709
Wang, S., Ye, H., Yang, C., Zhang, Y., Pu, J., Ren, Y., et al. (2025). OsNLP3 and OsPHR2 orchestrate direct and mycorrhizal pathways for nitrate uptake by regulating NAR2.1-NRT2s complexes in rice. Proc. Natl. Acad. Sci. 122, e2416345122. doi: 10.1073/pnas.2416345122
Weng, W., Yan, J., Zhou, M., Yao, X., Gao, A., Ma, C., et al. (2022). Roles of arbuscular mycorrhizal fungi as a biocontrol agent in the control of plant diseases. Microorganisms 10, 1266. doi: 10.3390/microorganisms10071266
Whiteside, M. D., Digman, M. A., Gratton, E., and Treseder, K. K. (2012). Organic nitrogen uptake by arbuscular mycorrhizal fungi in a boreal forest. Soil Biol. Biochem. 55, 7–13. doi: 10.1016/j.soilbio.2012.06.001
Whiteside, M. D., Treseder, K. K., and Atsatt, P. R. (2009). The brighter side of soils: quantum dots track organic nitrogen through fungi and plants. Ecology 90, 100–108. doi: 10.1890/07-2115.1
Wu, Y., Chen, C., and Wang, G. (2024). Inoculation with arbuscular mycorrhizal fungi improves plant biomass and nitrogen and phosphorus nutrients: A meta-analysis. BMC Plant Biol. 24, 960. doi: 10.2139/ssrn.4498871
Wu, X. L., Hao, Y., Lu, W., Liu, C. Y., and He, J. D. (2024). Arbuscular mycorrhizal fungi enhance nitrogen assimilation and drought adaptability in tea plants by promoting amino acid accumulation. Front. Plant Sci. 15. doi: 10.3389/fpls.2024.1450999
Wu, F., Zhang, H., Fang, F., Liu, H., and Tang, M. (2017a). Arbuscular mycorrhizal fungi alter nitrogen allocation in the leaves of Populus×canadensis ‘Neva’. Plant Soil 421, 477–491. doi: 10.1007/s11104-017-3461-0
Wu, F., Zhang, H., Fang, F., Wu, N., Zhang, Y., and Tang, M. (2017b). Effects of nitrogen and exogenous Rhizophagus irregularis on the nutrient status, photosynthesis and leaf anatomy of Populus×canadensis ‘Neva’. J. Plant Growth Regul. 36, 824–835. doi: 10.1007/s00344-017-9686-6
Xie, Y., Arnold, R. J., Wu, Z., Chen, S., Du, A., and Luo, J. (2017). Advances in eucalypt research in China. Front. Agric. Sci. Eng. 4, 380–390. doi: 10.15302/J-FASE-2017171
Xie, X., Lai, W., Che, X., Wang, S., Ren, Y., Hu, W., et al. (2022). A SPX domain-containing phosphate transporter from Rhizophagus irregularis handles phosphate homeostasis at symbiotic interface of arbuscular mycorrhizas. New Phytol. 234, 650–671. doi: 10.1111/nph.17973
Xie, K., Ren, Y., Chen, A., Yang, C., Zheng, Q., Chen, J., et al. (2022). Plant nitrogen nutrition: The roles of arbuscular mycorrhizal fungi. J. Plant Physiol. 269, 153591. doi: 10.1016/j.jplph.2021.153591
Yahaya, S. M., Mahmud, A. A., Abdullahi, M., and Haruna, A. (2023). Recent advances in the chemistry of nitrogen, phosphorus and potassium as fertilizers in soil: A review. Pedosphere 33, 385–406. doi: 10.1016/j.pedsph.2022.07.012
Yamamura, H., Hayakawa, M., and Iimura, Y. (2003). Application of sucrose-gradient centrifugation for selective isolation of Nocardia spp. from soil. J. Appl. Microbiol. 95, 677–685. doi: 10.1046/j.1365-2672.2003.02025.x
Yu, Q., Ni, X., Cheng, X., Ma, S., Tian, D., Zhu, B., et al. (2022). Foliar phosphorus allocation and photosynthesis reveal plants’ adaptative strategies to phosphorus limitation in tropical forests at different successional stages. Sci. Total Environ. 846, 157456. doi: 10.1016/j.scitotenv.2022.157456
Yu, F., Truong, T. V., He, Q., Hua, L., Su, Y., and Li, J. (2019). Dry season irrigation promotes leaf growth in Eucalyptus urophylla×E. grandis under fertilization. Forests 10, 67. doi: 10.3390/f10010067
Zhang, Y., Feng, H., Druzhinina, I. S., Wang, E. T., Martin, F., and Yuan, Z. L. (2024). Phosphorus/nitrogen sensing and signaling in diverse root-fungus symbioses. Trends Microbiol. 32, 200–215. doi: 10.1016/j.tim.2023.08.005
Zhang, T., Hu, Y., Zhang, K., Tian, C., and Guo, J. (2018). Arbuscular mycorrhizal fungi improve plant growth of Ricinus communis by altering photosynthetic properties and increasing pigments under drought and salt stress. Ind. Crops Prod 117, 13–19. doi: 10.1016/j.indcrop.2018.02.087
Zhang, Y. and Wang, X. (2021). Geographical spatial distribution and productivity dynamic change of eucalyptus plantations in China. Sci. Rep. 11, 19764. doi: 10.1038/s41598-021-97089-7
Zhao, C., Wang, H., Lu, Y., Hu, J., Qu, L., Li, Z., et al. (2019). Deep sequencing reveals early reprogramming of Arabidopsis root transcriptomes upon Ralstonia solanacearum infection. Mol. Plant-Microbe Interact. 32, 813–827. doi: 10.1094/MPMI-10-18-0268-R
Zhao, X., Zhang, X., Liu, Z., Lv, Y., Song, T., Cui, J., et al. (2021). Comparing the effects of N and P deficiency on physiology and growth for fast-and slow-growing provenances of Fraxinus mandshurica. Forests 12, 1760. doi: 10.3390/f12121760
Keywords: arbuscular mycorrhiza, organic nitrogen, nitrogen metabolism, phosphorus, fertilization, Eucalyptus
Citation: Li X, Huo C, Yang X, Liu Q, Su Z, Fan X, Liu Z and Chen Z (2025) AM fungi modulate organic nitrogen preference of Eucalyptus to enhance seedling growth under low fertilization. Front. Plant Sci. 16:1597451. doi: 10.3389/fpls.2025.1597451
Received: 21 March 2025; Accepted: 04 June 2025;
Published: 20 June 2025.
Edited by:
Kai Sun, Nanjing Normal University, ChinaReviewed by:
Song Baiquan, Heilongjiang University, ChinaJie Yuan, Jiangsu Academy of Agricultural Sciences (JAAS), China
Copyright © 2025 Li, Huo, Yang, Liu, Su, Fan, Liu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zujing Chen, enVqaW5nY2hlbkBzY2F1LmVkdS5jbg==
†These authors have contributed equally to this work
 Xinyue Li
Xinyue Li Chunyu Huo1†
Chunyu Huo1† Zujing Chen
Zujing Chen