- 1Jiangsu Agri-Animal Husbandry Vocational College, Taizhou, Jiangsu, China
- 2Jiangsu Key Laboratory of Crop Genetics and Physiology/Jiangsu Key Laboratory of Crop Cultivation and Physiology/Jiangsu Co-Innovation Center for Modern Production Technology of Grain Crops, Agricultural College of Yangzhou University, Yangzhou, China
Introduction: High surface ozone (O3) concentration presents a significant threat to the growth and development of plants. This research aimed to evaluate the impact of O3 on peony (Paeonia lactiflora), focusing on the physiological mechanisms involved, mainly as peony is widely grown in O3 polluted regions such as Sichuan, Jiangsu, and Shandong.
Methods: Two cultivars of peony Dafugui (DFG) and Heihaibotao (HHBT) were exposed to either non-filtered air (NF, ambient O3 concentration) or elevated O3 (NF60, NF + 60 ppb) for fifty days (April 11 to May 30) in open-top chambers. Key physiological parameters, including gas exchange, pigment levels, leaf mass per area (LMA), stomatal structure, lipid oxidation, and the antioxidant defense system, were measured across three replicate chambers.
Results: The exposure to NF60 resulted in significant reductions in light-saturated photosynthesis rate (Asat), stomatal conductance (gs), and electron transfer rate (ETR), but no changes were observed in intercellular CO2 concentration (Ci). Increased O3 levels accelerated leaf senescence, as indicated by higher malondialdehyde (MDA) and hydrogen peroxide (H2O2) levels, along with a decline in chlorophyll content. Ozone-induced led to a reduction in LMA, but the stomatal area and density were not significantly affected. The total ascorbate (AsA) content was decreased but total antioxidant capacity (TAC), phenolics, and antioxidant enzyme activity showed an increasing trend due to O3. Statistical analysis using ANOVA revealed no significant differences in the responses of leaf indices between the two peony cultivars to O3 stress, as indicated by the absence of significant interaction effects between O3 treatment and cultivar.
Discussion: The above results indicate that under O3 stress, peony leaves exhibit chlorosis and aggravated membrane lipid peroxidation, leading to a decline in Asat and inhibited leaf growth. The findings underscore the necessity of cultivating and promoting ozone-tolerant peony cultivars in heavily O3 polluted areas to improve the production efficiency and quality of the peony.
1 Introduction
Surface ozone (O3) is predominantly produced through photochemical reactions involving primary pollutants, such as nitrogen oxides (NOX) and volatile organic compounds (Monks et al., 2015; Li K, et al., 2018). The increasing levels of NOX, driven by industrial development and the rapid expansion of vehicle ownership, have led to higher concentrations of O3 in the atmosphere (Jiang et al., 2012; Feng et al., 2015). Environmental monitoring data reveal that between 2013 and 2019, the daily maximum 8-hour average O3 concentration in the surface layers of many Chinese cities consistently exceeded 50 ppb, with peak O3 levels increasing at an annual rate of 3 ppb (Lu et al., 2020). In some areas, such as Jiangsu province, the maximum hourly average concentration has exceeded 100 ppb (Hu et al., 2023; Shao et al., 2023). These increased concentrations of surface O3 present significant threats to human health (Sicard et al., 2021; Marco et al., 2022), agricultural production (Feng et al., 2022; Ramya et al., 2023), and biodiversity (Ainsworth, 2017).
The herbaceous peony (Paeonia lactiflora) is a well-known traditional flower in China, valued for its ornamental, medicinal, and health-promoting properties. It has emerged as a commercially significant cut flower in both domestic and international markets due to its substantial economic benefits (Ning et al., 2015; Qi et al., 2020). China has become one of the world’s major producers of cut peonies (Yang et al., 2020), with production concentrated in the Beijing, Sichuan, Jiangsu, and Shandong provinces (Du et al., 2018). Extensive research has been conducted on the effects of various abiotic stressors on peony growth, including heavy metals, saline-alkali conditions, drought, waterlogging, temperature extremes, and insufficient light. Lu et al. (2022) reviewed these stressors and their impacts on peony morphology, internal physiological processes, and secondary metabolites. These findings provided valuable insights into the mechanisms by which peonies adapt to stress and improve their tolerance. However, unlike other environmental stressors, O3 pollution has seasonal variations that make certain crops, including peony, particularly vulnerable during their critical growth phases. Moreover, China’s major peony-producing regions also experience frequent high O3 concentrations (Feng et al., 2022), particularly during the crucial growth and flowering period in May, which coincides with regional O3 pollution peaks (Wang et al., 2001; Li et al., 2007; Xu et al., 2008a; Ding et al., 2013; Wang et al., 2017). Despite numerous studies highlighting the harmful effects of O3 on crops (Feng and Kobayashi, 2009; Peng et al., 2019; Shao et al., 2020; Shang et al., 2024) and woody species (Li et al., 2016; Shang et al., 2018; Dai et al., 2017), minimal research has been conducted on the impacts of O3 on horticultural plants (Mills et al., 2007; Zhang et al., 2015), particularly on peony, to date.
Furthermore, leaves serve as the primary interface for material and energy exchange between the surface atmosphere and the terrestrial biosphere, and they are also the main organs responsible for sensing O3 stress responses (Krupa and Manning, 1988). Ozone predominantly enters plants through the stomata. Prolonged exposure to O3 induces visible leaf injury symptoms in sensitive plants species (Mills et al., 2011; Feng et al., 2014), activates various defense mechanisms, and subsequently leads to progressive chlorophyll degradation, accompanied by a reduction in photosynthetic efficiency (Wittig et al., 2009). These physiological disruptions interfere with carbon assimilation and the distribution of essential mineral nutrients (Shang et al., 2018), ultimately causing growth retardation and premature aging (Matyssek and Sandermann, 2003). Therefore, the plant’s resilience to environmental stress, its ecological fitness, its capacity for carbon sequestration are all compromised (Feng et al., 2022). Significant variability in O3 tolerance has been observed both among crops (Mills et al., 2007; Paoletti et al., 2009) and within species (Biswas et al., 2008; Krupa et al., 1998; Maggs and Ashmore, 1998; Shi et al., 2009).
In crops, susceptibility to O3 is typically measured by reductions in yield and biomass. Whereas, the evaluation of O3 sensitivity in woody and ornamental plants often involves monitoring leaf traits such as specific leaf weight, photosynthetic performance, malondialdehyde (MDA) levels, and antioxidant enzyme activity. Furthermore, high-concentration, short-term O3 exposure often induces oxidative damage in the form of leaf necrosis, accompanied by increased antioxidant metabolism, as a result of programmed cell death. However, the plant’s antioxidant response to chronic exposure to lower O3 concentrations is less well understood and remains a subject of debate. Some studies suggest that chronic O3 stress leads to increased antioxidant activity, as observed in soybeans (Gillespie et al., 2011), while others have observed a reduction in antioxidant pools and key enzymes in wheat leaf tissues under elevated O3 concentrations (Feng et al., 2010; Wang et al., 2014a). No studies have examined how O3 affects peony’s antioxidant system or photosynthetic efficiency. Therefore, a systematic study on the effects of O3 elevation on peony leaf performance is essential to determine its sensitivity to O3 stress. In this study, O3 fumigation experiments were conducted under two distinct concentrations, non-filtered ambient O3 concentration (NF) and NF supplemented with an additional 60 ppb of O3 (NF60), to evaluate the physiological responses of two widely cultivated cultivars of Paeonia lactiflora. The primary objective was to elucidate how O3 exposure influences leaf morphological traits, photosynthetic physiology, and oxidative stress responses in the two peony cultivars and to provide an initial evaluation of cultivar sensitivity to O3 exposure, offering foundational insights for optimizing peony cultivation practices in O3-polluted regions.
2 Materials and methods
2.1 Plant material and cultivation
Using Dafugui (DFG) and Heihaibotao (HHBT) as experimental materials, both cultivars were transplanted by division in 2023. This experiment used pot cultivation management with a soil mixture comprising a 1:1:1 ratio of soil, peat, and coarse sand. The cultivation pot is a plastic container with a lower base diameter of 18 cm, an upper opening diameter of 25 cm, and a height of 27.5 cm. The soil in the pot with the following properties: soil organic matter 14.1 ± 0.8%, total nitrogen (N) 1.48 ± 0.01 g kg-1, available phosphorus (P) 17.2 ± 4.8 mg kg-1, available potassium (K) 40.4 ± 2.1 mg kg-1, and pH6.6. The fertilizer and water management for the potted plants were consistent with standard field-level practices. In brief, throughout the trial period, water the plants when the top 2–3 cm of soil becomes dry, while avoiding waterlogging. In late April, apply 20 g of monopotassium phosphate per pot and then water thoroughly.
2.2 Fumigation treatment
The O3 fumigation experiment was initiated in April 2024 at the Yangzhou Green Agriculture Research and Demonstration Base. The experimental design utilized open-top chambers (OTCs) of regular octagonal geometry (4.8 m diameter, 2.3 m height, and a 3 m diameter top opening). Two O3 treatments were applied: ambient O3 concentration (NF) and ambient air supplemented with 60 ppb O3 (NF60), with each treatment replicated across three OTCs under comparable environmental conditions (temperature, humidity, illumination). Ozone was produced from high-purity oxygen using an electrical discharge O3 generator (HY003, Jinan Chuangcheng Technology Co., Ltd.) and delivered to the chambers by blowers (CX-125, Shanghai Quanfeng Industrial Co., Ltd.). Canopy-level O3 concentrations was continuously monitored in real-time (at 1-minute intervals) using Thermo Scientific Model 49i analyzers. Ozone delivery was fine-tuned via mass flow controllers to match set-point concentrations. Ozone levels were regulated by adjusting oxygen flow rates through mass flow controllers based on deviations between measured and target concentrations. Three uniformly growing Paeonia lactiflora pots per cultivar were randomly placed in each OTC starting April 11. Fumigation was carried out for 10 hours daily (08:00–18:00) over 50 days ending May 30. The average O3 concentrations during this period were 43.7 ± 1.1 ppb (NF) and 98.2 ± 1.4 ppb (NF60).
2.3 Parameter measurements
Leaf samples were collected from three plants per cultivar in each chamber. The uppermost three leaves from a single stem of each plant were pooled to create a composite sample. Leaves were punched, immediately frozen in liquid nitrogen, and milled to a fine powder using a miller (MM400, Retsch, Arzberg, Germany). These samples were analyzed for chlorophyll content, MDA, total ascorbate (AsA), total antioxidant capacity (TAC), hydrogen peroxide accumulation (H2O2), phenolic, and antioxidant enzyme activities.
Chlorophyll content in leaf samples was determined following the method described by Wang et al. (2014b), with minor modifications. Approximately 30 mg of fresh leaf tissue was extracted using 1 mL of 95% ethanol and incubated for 4 hours at 30°C in a shaker incubator (QYC 2102C, FuMa Experimental Equipment Co., Ltd., Shanghai, China). After centrifugation, the absorbance of the supernatant was measured at 645, 663, and 470 nm using a microplate reader (Lambda 35, PerkinElmer, Norwalk, CT, USA). Based on these values and according to established protocols (Wang et al., 2014b), the concentrations of chlorophyll a, chlorophyll b, total chlorophyll (a + b), and carotenoids were calculated.
Leaf mass per area (LMA): The top three leaves from each of the three single stems of each plant were first collected. The leaf length and surface area were measured using Image J software. The leaves were then combined and placed into paper bags, dried at 105°C for 30 minutes, followed by 72 hours at 80°C, to obtain dry weight. The leaf mass per area of each peony cultivar was estimated using the area and dry weight of the upper three leave.
To evaluate stomatal characteristics, three plants from each cultivar were selected, and the uppermost fully expanded leaves were sampled. After gently removing the dust, a layer of transparent nail polish was applied to a 1 cm × 1 cm area of the abaxial leaf surface. Once dried, the film was lifted with adhesive transparent tape and mounted onto a microscope slide with the impression side facing up. Observations were performed using a Leica DM 2500 microscope, and stomatal density was calculated by counting stomata within a defined area (Laza et al., 2010). The lengths and widths of 10 randomly selected stomata were measured per sample, and the stomatal area was calculated as length × width.
Gas exchange parameters were recorded using a portable photosynthesis system (LI-6800, LI-COR Inc., Lincoln, NE, USA). Measurements included the light-saturated net photosynthetic rate (Asat), stomatal conductance (gs), intercellular CO2 concentration (Ci), and electron transfer rate (ETR) of three fully expanded upper leaves from a single plant, randomly selected in each chamber. All measurements were conducted between 8:30 and 11:30 a.m. on sunny days, with saturation-level photosynthetic active radiation (1200 μmol m-2·s-1), 400 ppm of CO2, leaf temperatures of 28°C, and relative humidity readings of 50-60%.
The concentration of MDA in leaf tissues was measured using microplate-based spectrophotometric analysis as described by Wang et al. (2014b). The TAC was assessed using the ferric reducing antioxidant power (FRAP) assay, which reflects the collective action of non-enzymatic antioxidants and provides an indicative measure of the leaf’s resistance to oxidative stress (Benzie and Strain, 1996). The AsA concentration was determined spectrophotometrically by measuring absorbance at 265 nm after ascorbate oxidase addition, using an extinction coefficient of 14–14 mM-1 cm-1 (Luwe and Heber, 1995). Phenolic content in the leaf supernatant was analyzed using the Folin–Ciocalteu reagent following the protocol of Gillespie and Ainsworth (2007), with results expressed in gallic acid equivalents. Leaf hydrogen peroxide (H2O2) levels were determined using the method described by Mukherjee and Choudhuri (1983).
The activities of the antioxidant enzymes, including superoxide dismutase (SOD), peroxidase (POD), and hydrogen peroxidase(CAT), were analyzed using specific commercial kits from Jiancheng Bioengineering Institute (kit codes A123-1-1, A084-1, A007-1, and A001-4), with spectrophotometric detection. Leaf samples (~20 mg) were homogenized in 2 mL of pH 7.8 extraction buffer and centrifuged at 10,000 × g at 4 °C for 15 minutes. The enzyme activities were determined in the clarified supernatant, following the manufacturer’s protocol.
2.4 Statistical analysis
The statistical analysis was performed using SAS 9.2 software (SAS Institute Inc., Cary, NC). A mixed-effects model was used, following the approach described by Frei et al. (2011), where the fixed effects included O3, cultivar, and their interactions, and the random effect was the chambers. This mixed model did not consider multiple plants within one treatment chamber as replicates on the treatment level. All the data were presented as the mean ± standard error from three chamber replicates.
3 Results
3.1 Leaf gas exchange parameters
As presented in Table 1, exposure to NF60 significantly reduced the Asat and gs of peony leaves by an average of 23.0% and 25.6%, respectively. The reduction in DFG leaves was greater than that in HHBT, although no significant interaction was detected between O3 treatment and cultivar. A 12.7% significant reduction in Ci was observed in DFG leaves under NF60, while no significant changes were observed in HHBT, as indicated by the significant interaction between O3 exposure and cultivar. Moreover, NF60 exposure resulted in a significant 16.1% decrease in ETR, with similar reductions observed in both cultivars.
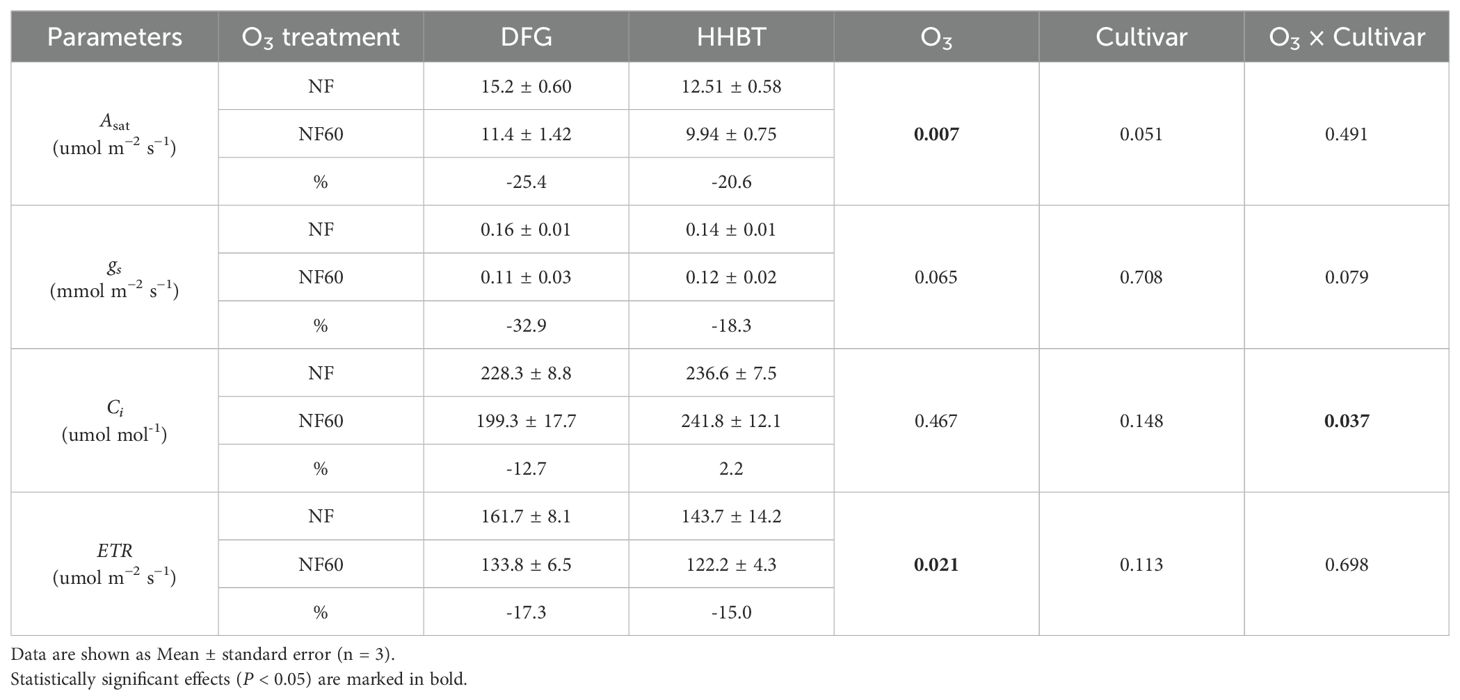
Table 1. Leaf photosynthesis parameters (light-saturated photosynthesis rate (Asat), stomatal conductance (gs), intercellular CO2 concentration (Ci) and electron transfer rate (ETR)) of two cultivars of peony seedlings growing in nonfiltered ambient air (NF) and NF with a targeted O3 addition of 60 ppb (NF60).
3.2 Leaf pigments
As shown in Table 2, the NF60 exposure resulted in an average 12.7% decrease in chlorophyll a+b content in peony leaves compared to NF (P < 0.1), with reductions of 7.1% in DFG and 18.4% in HHBT. The contents of chlorophyll a and b were also significantly decreased by 13.7% and 9.9%, respectively, under NF60. The reduction in chlorophyll content was less marked in DFG than in HHBT. However, no significant interaction was observed between O3 exposure and cultivar (Table 2).
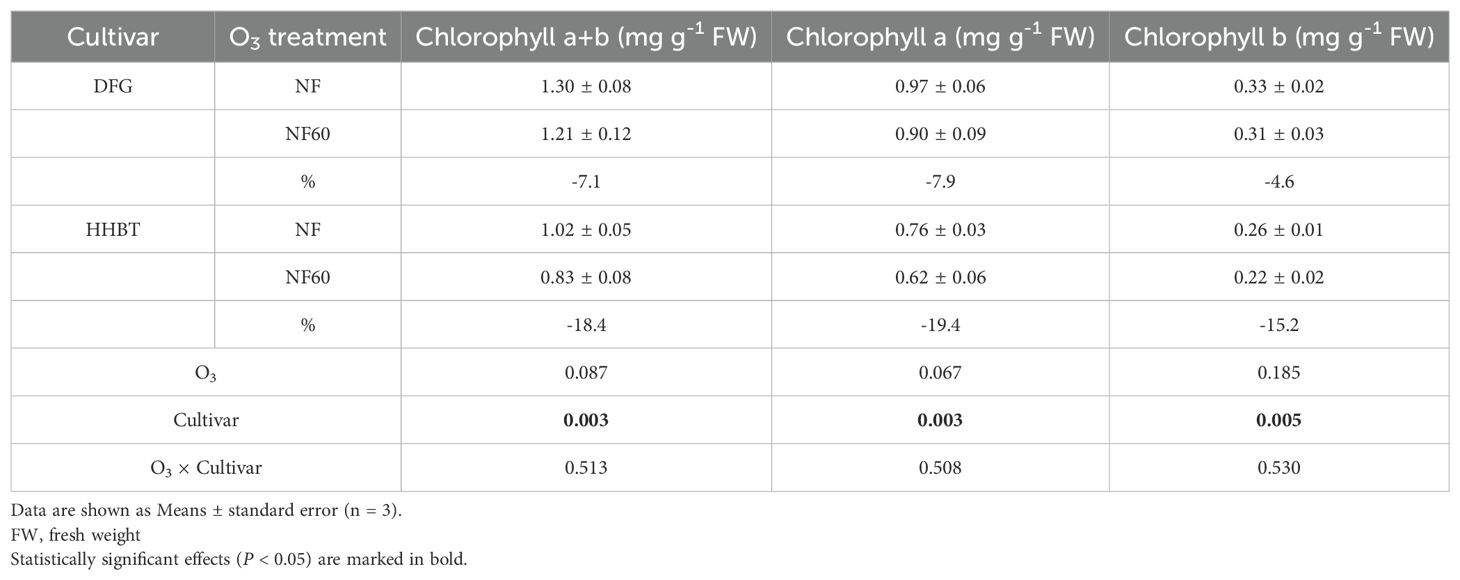
Table 2. Leaf chlorophyll a+b, chlorophyll a and chlorophyll b of two cultivars of peony seedlings growing in nonfiltered ambient air (NF) and NF with a targeted O3 addition of 60 ppb (NF60).
3.3 Leaf mass per area and stomata structure
As presented in Table 3, the dry weight per unit area of herbaceous peony leaves was determined based on leaf area and corresponding dry weight. Compared to NF, NF60 resulted in a 12.8% reduction in dry weight per unit area on average (P = 0.094), with DFG and HHBT showing reductions of 12.1% and 13.4%, respectively. Analysis using ANOVA indicated no significant effects of cultivar or the interaction between cultivar and O3 treatment on this parameter.
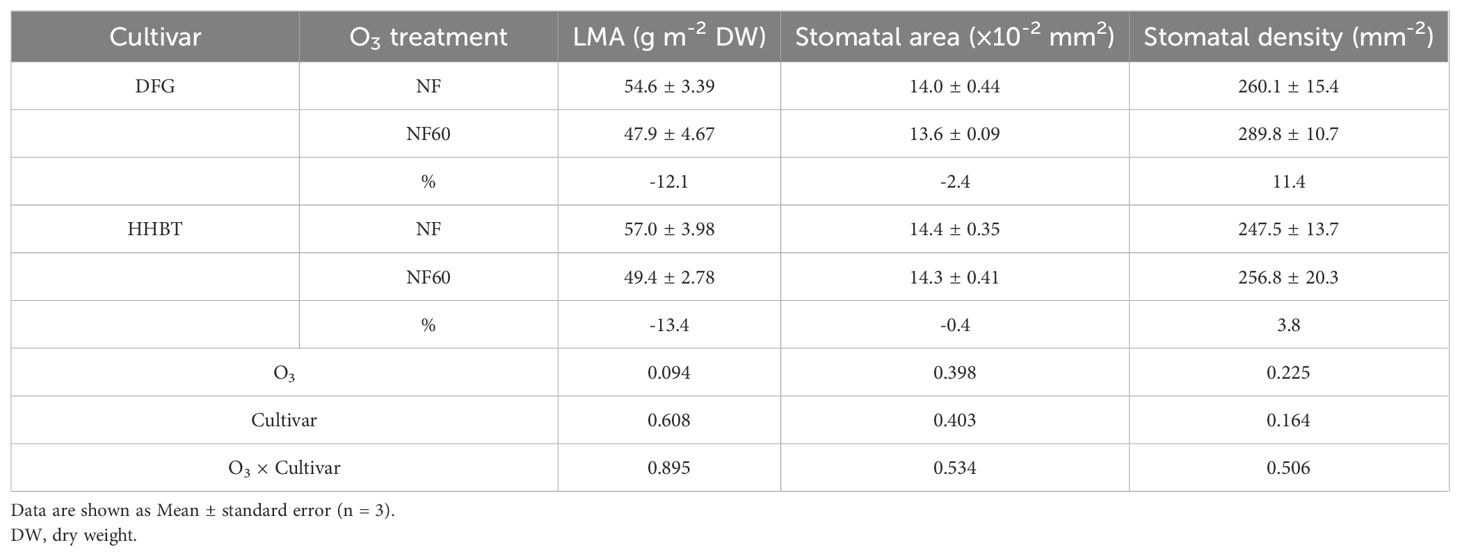
Table 3. Leaf mass per area (LMA), stomatal area and density of two cultivars of peony seedlings growing in nonfiltered ambient air (NF) and NF with a targeted O3 addition of 60 ppb (NF60).
3.4 Leaf stomata structure
The effect of O3 stress on leaf stomatal structure was shown in Table 3. Evaluation of stomatal structure revealed no significant differences in stomatal density between the cultivars; however, HHBT had significantly larger stomata than DFG. Neither O3 treatment nor its interaction with the cultivar significantly affected stomatal size or density (Table 3).
3.5 Leaf lipid peroxidation and antioxidant system
As shown in Table 4, O3 exposure resulted in significant increases in MDA (6.9%) and H2O2 (8.4%) contents in peony leaves, while carotenoid content decreased by 10.0%. No significant effects were observed on AsA, TAC, or phenolic contents. Under NF60 treatment, TAC content in HHBT leaves increased by 13.3%, whereas DFG showed a slight decrease, highlighting a significant interaction between O3 treatment and cultivar.
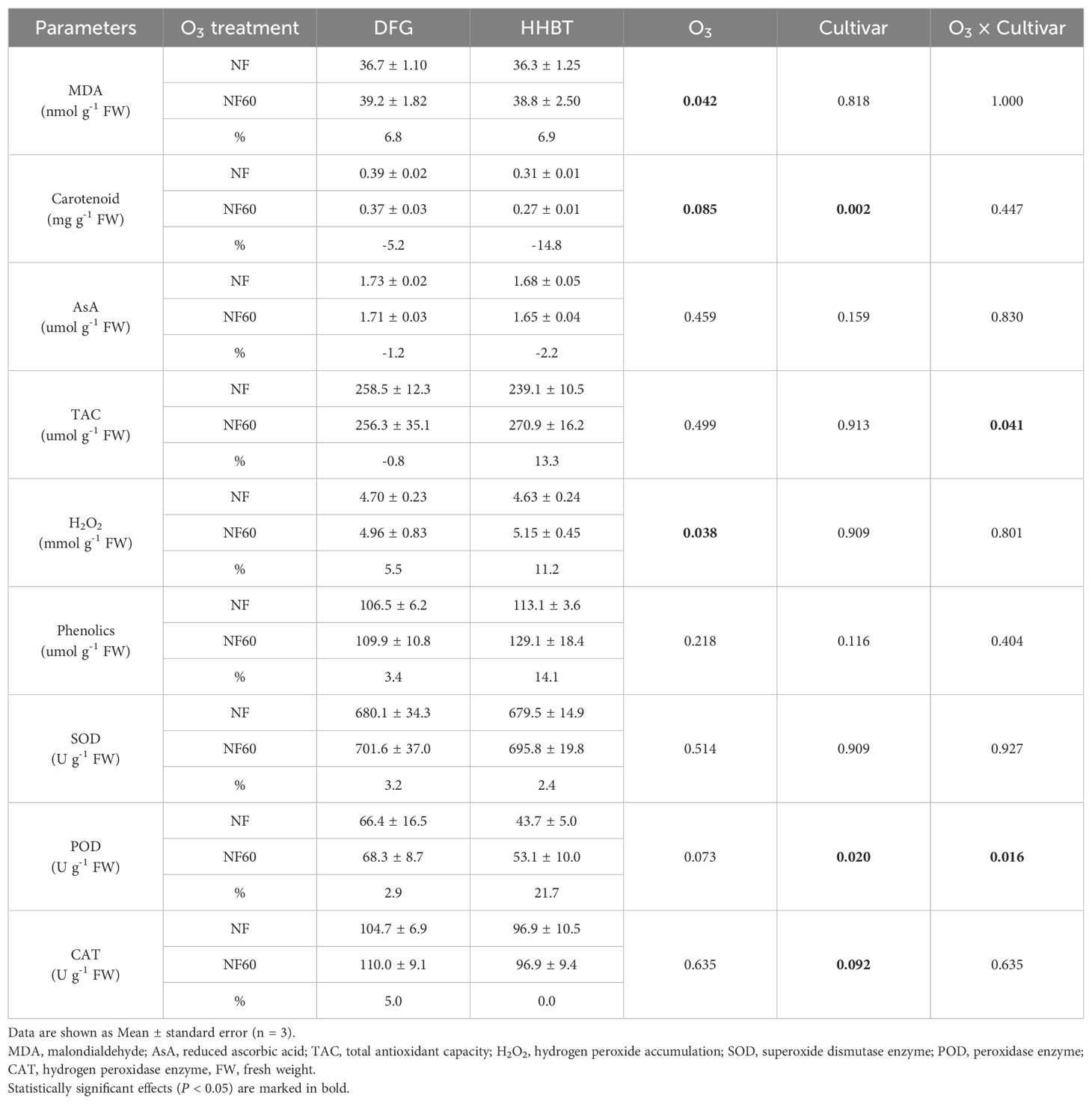
Table 4. Leaf lipid peroxidation and antioxidant system of two cultivars of peony seedlings growing in nonfiltered ambient air (NF) and NF with a targeted O3 addition of 60 ppb (NF60).
Moreover, the activities of SOD and CAT enzymes were not significantly altered by O3 exposure, but POD activity increased by 12.3% (Table 4), this increase was greater in HHBT (21.7%) than in DFG (2.9%). The statistical analysis revealed that the O3-cultivar interaction significantly influenced POD activity alone.
4 Discussion
The Herbaceous peony (Paeonia lactiflora Pall.), renowned for its majestic beauty, is often compared to its tree (Paeonia suffruticosa Andr.) and is regarded as the “monarch of herbaceous flowers”. Its large, vibrant, and fragrant flowers have made it a popular choice for urban landscaping, garden cultivation, potted displays, and the premium cut flower market (Holloway and Buchholz, 2013). However, during the introduction and cultivation of the peony, the plant faces numerous abiotic stresses, including fluctuations in temperature, light exposure, water availability, saline-alkali conditions, and heavy metal contamination (Haak et al., 2017). While moderate stress can stimulate growth, excessive stress inhibits development. It causes morphological abnormalities in roots, stems, and leaves, altering internal organic compounds, inorganic ions, and enzymatic activities, which may ultimately lead to plant mortality (Zhang et al., 2018). Among the various abiotic stresses, elevated ground-level O3 concentrations have emerged as a key stress factor for plant health (Ashmore, 2005). Despite this, research on the impact of O3 stress in peony remains limited.
The leaf photosynthesis is an essential physiological process that sustains plant life. There are few reports on the effects of O3 stress on ornamental plants. Qin et al. (2020) conducted a study on O3 stress in Cleome spinosa, but unfortunately, the study did not measure the response of leaf photosynthetic parameters. The impact of O3 stress on leaf photosynthesis has been the subject of substantial research, revealing that O3 exposure significantly reduces photosynthetic rates in various crops and tree species. Studies on wheat (Feng and Kobayashi, 2009; Xu et al., 2025), rice (Ainsworth, 2008; Shang et al., 2024), maize (Yendrek et al., 2017), and trees (Li et al., 2016; Shang et al., 2019) demonstrate that O3 impairs photosynthetic capacity. The present study showed that NF60 treatment resulted in a 23.0% significant decrease in the photosynthetic rate in peony leaves compared to NF (Table 1), with similar reductions in both cultivars, indicating significant O3-induced damage. Moreover, the reduction in photosynthetic rate in peony leaves under NF60 treatment was lower compared to the reductions observed in rice (Ainsworth, 2008; Shao et al., 2023), wheat (Feng et al., 2016), maize (Yendrek et al., 2017), and soybeans (Morgan et al., 2003). The observed decrease in photosynthesis can be attributed to both stomatal and non-stomatal limitations (Dai et al., 2017; Shang et al., 2024). Furthermore, under NF60, stomatal conductance was significantly reduced, with DFG showing a greater reduction than HHBT. However, O3 exposure also reduced intercellular CO2 concentration by 12.7% on average in DFG, but no significant effect or a slight increase was observed in HHBT, as indicated by a significant O3 × cultivar interaction (Table 1). This suggests that stomatal limitations play a key role in the decline of photosynthesis in DFG, while both stomatal and non-stomatal factors contribute to the decrease in HHBT. Moreover, significant O3-induced decreases in leaf ETR were identified, which may represent another non-stomatal mechanism of photosynthetic inhibition (Feng et al., 2016; Schcidegger and Schroeter, 1995).
Chlorophyll, a crucial component in leaf photosynthesis, plays a vital role in absorbing light, transferring energy, and converting it into chemical forms. Fluctuations in chlorophyll levels significantly influence nitrogen fixation and are indicative of leaf senescence (Singh et al., 2015). Consistent with the observed photosynthetic rate patterns, NF60 treatment led to reductions in chlorophyll a+b and its components in peony leaves, with chlorophyll a showing a more considerable decrease than chlorophyll b. While the high O3 concentration affected DFG’s chlorophyll content to a lesser extent than HHBT, no significant interaction was found between cultivar and O3 exposure. The changes in chlorophyll content under O3 stress likely reflect an imbalance in the production and scavenging of reactive oxygen species (ROS). Specifically, O3 exposure intensified lipid peroxidation in membranes, increasing MDA and H2O2 concentrations (Table 4), and allowing excess ROS to infiltrate chloroplasts, where they contributed to the degradation of chlorophyll. This is primarily due to the fact that these excess ROS exacerbate the peroxidation of chloroplast membranes, impairing the synthesis of chlorophyll-protein complexes (Langebartels et al., 2002). This process accelerates chlorophyll depletion and leaf yellowing, as extensively documented in crops such as rice (He et al., 2024) and wheat (Feng et al., 2016).
Leaf morphology and structure, shaped by the evolutionary processes of natural selection, are closely associated with physiological functions and represent key factors in determining a plant’s ability to adapt to its environment (Liu and Liang, 2016). The LMA is recognized as a reflection of plant adaptations and acclimation to prevailing environmental conditions. Species with low LMA display greater responsiveness to environmental stimuli and higher stress sensitivity compared to high-LMA species, which demonstrate enhanced stress tolerance (Bussotti, 2008; Li et al., 2016). The findings of this study indicated that NF60 exposure reduced leaf LMA by 12-13% (P < 0.1) in both peony cultivars, without any significant interaction observed between O3 exposure and cultivar, which aligns with similar reports in peach trees (Dai et al., 2017), maize (Peng et al., 2019), and rice (Fu et al., 2021). Stomatal traits, including density and size, are known to be highly responsive to environmental variations (Zheng and Shangguan, 2005). Previous research has reported differing responses of stomatal density to elevated O3, including increases (Wen et al., 2014), decreases (Li P. et al., 2018), or no significant effects (Xu et al., 2008b), which may be attributed to differences in exposure duration, O3 concentration, plant species, and the developmental stage of leaves (Li P, et al., 2018). Furthermore, no significant effects on stomatal density or size were found in peony leaves under O3 exposure (Table 3). This may be because the leaves were fully expanded before treatment, suggesting that the stomatal structure and number had already been established, thus minimizing the potential impact of the O3 exposure. Therefore, further long-term studies are needed to determine the effects of increased O3 on stomatal development throughout the lifespan of peony leaves.
The leaf antioxidant system plays a crucial role in defending against and repairing the oxidative damage caused by O3 stress. However, when environmental stress exceeds a critical threshold, it disrupts the plant’s protective defense mechanisms (Xu et al., 2020). Several studies have shown that O3-resistant species or cultivars typically display higher levels of antioxidant substances (Dai et al., 2017). In this study, although most leaf antioxidant parameters (such as AsA, TAC, total phenols, and the activities of enzymes like SOD, POD, and CAT) did not show statistically significant responses to O3 stress (Table 4), peony leaves treated with NF60 showed partial positive responses from their antioxidant system. This was accompanied by an increase in H2O2 and MDA content, with all antioxidant enzyme activities showing an upward trend. The results were largely consistent with the findings reported by Qin et al. (2020) regarding 80 ppb O3 stress on Cleome spinose. Antioxidant indicators generally undergo dynamic changes, starting with an initial increase followed by a decrease as stress intensity increases (Wu et al., 2011). Therefore, measurements taken at a single time point may not adequately reflect the complete dynamic response. Future research should conduct multi-year experiments to elucidate the temporal variations of antioxidants in the cellular repair mechanisms of peony leaves.
5 Conclusions
Research on the effects of abiotic stress in peony has predominantly focused on factors such as temperature, light, water availability, salinity, and heavy metals. However, there have been no studies on the impact of atmospheric O3 pollution on peony growth. This study used open-top chambers to evaluate the effects of O3 stress on the leaf morphology and physiology of herbaceous peony. Compared with NF, the NF60 treatment led to a significant reduction in leaf photosynthetic capacity, as reflected by decreases in chlorophyll content and increases in MDA content, which contributed to accelerated leaf senescence in both peony cultivars tested. Under the same O3 exposure conditions, the two peony cultivars showed relatively minor changes in leaf morphology and physiology compared to crops such as soybean, rice, maize, and wheat, classifying peony as a species moderately sensitive to O3. Future research should focus on how elevated O3 concentrations affect medicinal compounds and key quality traits in cut flowers, such as stem length, flower color, and size. This knowledge will improve the comprehension of the physiological adaptation of peony to O3 stress, which could inform agronomic practices that optimize peony cultivation and commercialization and improve stress tolerance.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
Author contributions
ZS: Data curation, Funding acquisition, Investigation, Supervision, Writing – original draft, Writing – review & editing. LJ: Data curation, Writing – review & editing. XL: Investigation, Funding acquisition, Writing – original draft. LC: Investigation, Writing – review & editing. WH: Investigation, Writing – original draft. KY: Investigation, Writing – original draft. HZ: Funding acquisition, Investigation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Jiangsu Agri-animal Husbandry Vocational College Project (No. NSF2024CB03), Jiangsu Vocational College of Agriculture and Animal Husbandry Technology Innovation Team Project (NSF2023TC04), and 2024 Taizhou City Science and Technology Support Program -Social Development Project (Applied Research on Key Technologies for Medicinal Peony Production Based on the Substrate Utilization of Agricultural and Forestry Waste). We sincerely appreciate the support and assistance provided by the Environmental Change and Ecological Effects Research Team at Nanjing University of Information Science and Technology for the successful implementation of this experiment.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ainsworth, E. A. (2008). Rice production in a changing climate: A meta-analysis of responses to elevated carbon dioxide and elevated ozone concentration. Glob. Change Biol. 14, 1642–1650. doi: 10.1111/j.1365-2486.2008.01594.x
Ainsworth, E. A. (2017). Understanding and improving global crop response to ozone pollution. Plant J. 90, 886–897. doi: 10.1111/tpj.13298
Ashmore, M. R. (2005). Assessing the future global impacts of ozone on vegetation. Plant Cell Environ. 28, 949–964. doi: 10.1111/j.1365-3040.2005.01341.x
Benzie, I. F. F. and Strain, J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 239, 70–76. doi: 10.1006/abio.1996.0292
Biswas, D. K., Xu, H., Li, Y. G., Sun, J. Z., Wang, X. Z., Han, X. G., et al. (2008). Genotypic differences in leaf biochemical, physiological and growth responses to ozone in 20 winter wheat cultivars released over the past 60 years. Glob. Chang Biol. 14, 46–59. doi: 10.1111/j.1365-2486.2007.01477.x
Bussotti, F. (2008). Functional leaf traits, plant communities and acclimation processes in relation to oxidative stress in trees: a critical overview. Glob. Change Biol. 14, 2727–2739. doi: 10.1111/j.1365-2486.2008.01677.x
Dai, L. L., Li, P., Shang, B., Liu, S., Yang, A. Z., Wang, Y. N., et al. (2017). Differential responses of peach (Prunus persica) seedlings to elevated ozone are related with leaf mass per area, antioxidant enzymes activity rather than stomatal conductance. Environ. pollut. 227, 380–388. doi: 10.1016/j.envpol.2017.04.068
Ding, A. J., Fu, C. B., Yang, X. Q., Sun, J. N., Zheng, L. F., Xie, Y. N., et al. (2013). Ozone and fine particle in the western Yangtze River Delta: an overview of 1 yr data at the SORPES station. Atmos. Chem. Phys. 13, 5813–5830. doi: 10.5194/acp-13-5813-2013
Du, J., Xu, J. G., Lv, M. W., Gao, C. R., Lu, J., Zhang, Q. X., et al. (2018). Study on photosynthetic mechanism of summer shading slowing aging of herbaceous peony (Paeonia lactiflora) leaves. Plant Physiol. J. 54, 773–782. doi: 10.13592/j.cnki.ppj.2017.0579
Feng, Z. Z., Hu, E. Z., Wang, X. K., Jiang, L. J., and Liu, X. J. (2015). Ground-level O3 pollution and its impacts on food crops in China: a review. Environ. pollut. 199, 42–48. doi: 10.1016/j.envpol.2015.01.016
Feng, Z. Z. and Kobayashi, K. (2009). Assessing the impacts of current and future concentrations of surface ozone on crop yield with meta-analysis. Atmos. Environ. 43, 1510–1519. doi: 10.1016/j.atmosenv.2008.11.033
Feng, Z. Z., Pang, J., Nouchi, I., Kobayashi, K., Yamakawa, T., and Zhu, J. G. (2010). Apoplastic ascorbate contributes to the differential ozone sensitivity in two varieties of winter wheat under fully open-air field conditions. Environ. pollut. 158, 3539–3545. doi: 10.1016/j.envpol.2010.08.019
Feng, Z. Z., Sun, J. S., Wan, W. X., Hu, E. Z., and Calatayud, V. (2014). Evidence of widespread ozone-induced visible injury on plants in Beijing, China. Environ. pollut. 193, 296–301. doi: 10.1016/j.envpol.2014.06.004
Feng, Z. Z., Wang, L., Pleijel, H., Zhu, J. G., and Kobayashi, K. (2016). Differential effects of ozone on photosynthesis of winter wheat among cultivars depend on antioxidative enzymes rather than stomatal conductance. Sci. Total Environ. 572, 404–411. doi: 10.1016/j.scitotenv.2016.08.083
Feng, Z. Z., Xu, Y. S., Kobayashi, K., Dai, L. L., Zhang, T. Y., Agathokleous, E., et al. (2022). Ozone pollution threatens the production of major staple crops in East Asia. Nat. Food. 3, 47–56. doi: 10.1038/s43016-021-00422-6
Frei, M., Kohno, Y., Wissuwa, M., Makkar P.S., H., and Becker, K. (2011). Negative effects of tropospheric ozone on the feed value of rice straw are mitigated by an ozone tolerance QTL. Glob. Change Biol. 17, 2319–2329. doi: 10.1111/j.1365-2486.2010.02379.x
Fu, R., Shang, B., Zhang, G. Y., and Feng, Z. Z. (2021). Differential effects of ozone pollution on photosynthesis and growth of rice during two growth stages. J. Agro-Environ. Sci. 40, 2066–2075. doi: 10.11654/jaes.2021-0139
Gillespie, K. M. and Ainsworth, E. A. (2007). Measurement of reduced, oxidized and total ascorbate content in plants. Nat. Protoc. 2, 871–874. doi: 10.1038/nprot.2007.101
Gillespie, K. M., Rogers, A., and Ainsworth, E. A. (2011). Growth at elevated ozone or elevated carbon dioxide concentration alters antioxidant capacity and response to acute oxidative stress in soybean (Glycine max). J. Exp. Bot. 62, 2667–2678. doi: 10.1093/jxb/erq435
Haak, D. C., Fukao, T., Grene, R., Hua, Z. H., Ivanov, R., Perrella, G., et al. (2017). Multilevel regulation of abiotic stress responses in plants. Front. Plant Sci. 8, 1–24. doi: 10.3389/fpls.2017.01564
He, L. X., Bao, M. X., Li, Y., Xu, Y. S., Shao, Z. S., Ma, Y. Z., et al. (2024). Leaf biochemical and physiological responses to elevated atmospheric ozone concentration in eight modern rice cultivars. Ecosyst. Health Sustain. 10, 0269. doi: 10.34133/ehs.0269
Holloway, P. S. and Buchholz, K. (2013). The state of the Alaska peony industry 2012. AFES Misc. Pub. 3, 1–8. doi: 10.1093/acprof:oso/9780199744824.003.0006
Hu, S. W., Yang, Y., Tian, C., He, F. H., Wang, Y. X., Wang, Y. L., et al. (2023). Physicochemical characteristics of lodging susceptibility of rice cultivars in response to ozone exposure. Agric. Ecosyst. Environ. 344, 108313. doi: 10.1016/j.agee.2022.108313
Jiang, F., Zhou, P., Liu, Q., Wang, T. J., Zhuang, B. L., and Wang, X. Y. (2012). Modeling tropospheric ozone formation over East China in springtime. J. Atmos. Chem. 66, 303–319. doi: 10.1007/s10874-012-9244-3
Krupa, S. V. and Manning, W. J. (1988). Atmospheric ozone: formation and effects on vegetation. Environ. pollut. 50, 101–137. doi: 10.1016/0269-7491(88)90187-X
Krupa, S., Nosal, M., and Legge, A. (1998). A numerical analysis of the combined open-top chamber data from the USA and Europe on ambient ozone and negative crop responses. Environ. pollut. 101, 157–160. doi: 10.1016/S0269-7491(98)00019-0
Langebartels, C., Wohlgemuth, H., Kschieschan, S., Sebastian, G., and Sandermann, H. (2002). Oxidative burst and cell death in ozone-exposed plants. Plant Physiol. Biochem. 40, 567–575. doi: 10.1016/S0981-9428(02)01416-X
Laza, M. R. C., Kondo, M., Ideta, O., Barlaan, E., and Imbe, T. (2010). Quantitative trait loci for stomatal density and size in lowland rice. Euphytica. 172, 149–158. doi: 10.1007/s10681-009-0011-8
Li, P., Calatayud, V., Gao, F., Uddling, J., and Feng, Z. Z. (2016). Differences in ozone sensitivity among woody species are related to leaf morphology and antioxidant levels. Tree Physiol. 36, 1105–1116. doi: 10.1093/treephys/tpw042
Li, P., Feng, Z. Z., Shang, B., Yuan, X. Y., Dai, L. L., and Xu, Y. S. (2018). Stomatal characteristics and ozone dose- response relationships for six greening tree species. Acta Ecol. Sin. 38, 2710–2721. doi: 10.5846/stxb201705220945
Li, K., Jacob, D. J., Liao, H., Shen, L., Zhang, Q., and Bates, K. H. (2018). Anthropogenic drivers of 2013–2017 trends in summer surface ozone in China. P. Natl. Acad. Sci. U.S.A. 116, 422–427. doi: 10.1073/pnas.1812168116
Li, J., Wang, Z., Akimoto, H., Gao, C., Pochanart, P., and Wang, X. (2007). Modeling study of ozone seasonal cycle in lower troposphere over east Asia. J. Geophys. Res.: Atmos 112, D22S25. doi: 10.1029/2006JD008209
Liu, M. X. and Liang, G. L. (2016). Research progress on leaf mass per area. Chin. J. Plant Ecol. 40, 847–860. doi: 10.17521/cjpe.2015.0428
Lu, J. X., Wang, H. G., Wang, Z., Xu, J., Liu, X. B., and Ma, W. (2022). Research progress in China on the effects of various abiotic stresses on the medicinal plant (Paeonia lactiflora). J. Chin. Med. Mater. 45, 248–254. doi: 10.13863/j.issn1001-4454
Lu, X., Zhang, L., Wang, X., Gao, M., Li, K., Zhang, Y., et al. (2020). Rapid increases in warm-season surface ozone and resulting health impact in China since 2013. Environ. Sci. Technol. Lett. 7, 240–247. doi: 10.1021/acs.estlett.0c00171
Luwe, M. and Heber, U. (1995). Ozone detoxification in the apoplasm and symplasm of spinach, broad bean and beech leaves at ambient and elevated concentrations of ozone in air. Planta. 197, 448–455. doi: 10.1007/BF00196666
Maggs, R. and Ashmore, M. (1998). Growth and yield responses of Pakistan rice (Oryza sativa L.) cultivars to O3 and NO2. Environ. pollut. 103, 159–170. doi: 10.1016/S0269-7491(98)00129-8
Marco, A. D., Garcia-Gomez, H., Collalti, A., Khaniabadi, Y. O., Feng, Z. Z., Proietti, C., et al. (2022). Ozone modelling and mapping for risk assessment: an overview of different approaches for human and ecosystems health. Environ. Res. 211, 113048. doi: 10.1016/j.envres.2022.113048
Matyssek, R. and Sandermann, H. (2003). Impact of ozone on trees: An ecophysiological perspective. Prog. Bot. 64, 349–404. doi: 10.1007/978-3-642-55819-1_15
Mills, G., Buse, A., Gimeno, B., Bermejo, V., Holland, M., Emberson, L., et al. (2007). A synthesis of AOT40-based response functions and critical levels of ozone for agricultural and horticultural crops. Atmos. Environ. 41, 2630–2643. doi: 10.1016/j.atmosenv.2006.11.016
Mills, G., Hayes, F., Simpson, D., Emberson, L., Norris, D., Harmens, H., et al. (2011). Evidence of widespread effects of ozone on crops and (semi-) natural vegetation in Europe, (1990-2006) in relation to AOT40- and flux-based risk maps. Glob. Change Biol. 17, 592–613. doi: 10.1111/j.1365-2486.2010.02217.x
Monks, P. S., Archibald, A. T., Colette, A., Cooper, O., Coyle, M., Derwent, R., et al. (2015). Tropospheric ozone and its precursors from the urban to the global scale from air quality to shortlived climate forcer. Atmos. Chem. Phys. 15, 8889–8973. doi: 10.5194/acp-15-8889-2015
Morgan, P. B., Ainsworth, E. A., and Long, S. (2003). How does elevated ozone impact soybean? A meta-analysis of photosynthesis, growth and yield. Plant Cell Environ. 26, 1317–1328. doi: 10.1046/j.0016-8025.2003.01056.x
Mukherjee, S. P. and Choudhuri, M. A. (1983). Implications of water stress induced changes in the levels of endogenous as corbic adid and hydrogen peroxide in vigna seedlings. Physiol. Plant 58, 166–170. doi: 10.1111/j.1399-3054.1983.tb04162.x
Ning, C. L., Jiang, Y., Meng, J. S., Zhou, C. H., and Tao, J. (2015). Herbaceous peony seed oil: A rich source of unsaturated fatty acids and γ- tocopherol. Eur. J. Lipid Sci. Technol. 117, 532–542. doi: 10.1002/ejlt.201400212
Paoletti, E., Contran, N., Manning, W. J., and Ferrara, A. M. (2009). Use of the antiozonant ethylenediurea (EDU) in Italy: verification of the effects of ambient ozone on crop plants and trees and investigation of EDU’s mode of action. Environ. pollut. 157, 1453–1460. doi: 10.1016/j.envpol.2008.09.021
Peng, J. D., Shang, B., Xu, Y. S., Feng, Z. Z., and Pleijel, H. (2019). Ozone exposure- and flux-yield response relationships for maize. Environ. pollut. 252, 1–7. doi: 10.1016/j.envpol.2019.05.088
Qi, Q. M., Li, Y., Xing, G. P., Guo, J., and Guo, X. F. (2020). Fertility variation among Paeonia lactiflora genotypes and fatty acid composition of seed oil. Ind. Crops Prod. 152, 112540. doi: 10.1016/j.indcrop.2020.112540
Qin, Z. Q., Xu, S., Qi, S. Y., Chen, W., He, X. Y., and Wang, Y. J. (2020). Effects of elevated O3 concentration and warming on oxidative jury and antioxidant enzyme activities in leaves of Cleome spinose. Chin. J. Ecol. 39, 830–837. doi: 10.13292/j.1000-4890.202003.002
Ramya, A., Dhevagi, P., Poornima, R., Avudainayagam, S., Watanabe, M., and Agathokleous, E. (2023). Effect of ozone stress on crop productivity: A threat to food security. Environ. Res. 236, 116816. doi: 10.1016/j.envres.2023.116816
Schcidegger, C. and Schroeter, B. (1995). Effects of ozone fumigation on Epiphytic Macrolichens Ultrastucture CO2 gas exchange and chlorophyll fluorescence. Environ. pollut. 88, 345–354. doi: 10.1016/0269-7491(95)93449-A
Shang, B., Deng, T. T., Chen, H., Xu, Y. S., and Feng, Z. Z. (2024). Effects of elevated ozone on physiology, growth, yield and grain quality of rice (Oryza sativa L.): An ozone gradient experiment. Agric. Ecosyst. Environ. 363, 108858. doi: 10.1016/j.agee.2023.108858
Shang, B., Feng, Z. Z., Li, P., and Calatayud, V. (2018). Elevated ozone affects C, N and P ecological stoichiometry and nutrient resorption of two poplar clones. Environ. pollut. 234, 136–144. doi: 10.1016/j.envpol.2017.11.056
Shang, B., Xu, Y. S., Dai, L. L., Yuan, X. Y., and Feng, Z. Z. (2019). Elevated ozone reduced leaf nitrogen allocation to photosynthesis in poplar. Sci. Total Environ. 657, 169–178. doi: 10.1016/j.scitotenv.2018.11.471
Shao, Z. S., Zhang, Y. L., Hu, S. W., Gao, B., Jing, L. Q., Wang, Y. X., et al. (2023). Impacts of ozone stress on stem lodging characteristics of different indica and japonica rice cultivars. Crop Sci. 63, 1–17. doi: 10.1002/csc2.20913
Shao, Z. S., Zhang, Y. L., Mu, H. R., Wang, Y. L., Wang, Y. X., and Yang, L. X. (2020). Ozone-induced reduction in rice yield is closely related to the response of spikelet density under ozone stress. Sci. Total Environ. 712, 136560. doi: 10.1016/j.scitotenv.2020.136560
Shi, G. Y., Yang, L. X., Wang, Y. X., Kobayashi, K., Zhu, J. G., Tang, H. Y., et al. (2009). Impact of elevated ozone concentration on yield of four Chinese rice cultivars under fully open-air field conditions. Agric. Ecosyst. Environ. 131, 178–184. doi: 10.1016/j.agee.2009.01.009
Sicard, P., Agathokleous, E., Marco, A. D., Paoletti, E., and Calatayud, V. (2021). Urban population exposure to air pollution in Europe over the last decades. Environ. Sci. Eur. 33, 1–12. doi: 10.1186/s12302-020-00450-2
Singh, P., Agrawal, M., Agrawal, S. B., Singh, S., and Singh, A. (2015). Genotypic differences in utilization of nutrients in wheat under ambient ozone concentrations: Growth, biomass and yield. Agric. Ecosyst. Environ. 199, 26–33. doi: 10.1016/j.agee.2014.07.021
Wang, T., Cheung, V. T. F., Anson, M., and Li, Y. S. (2001). Ozone and related gaseous pollutants in the boundary layer of eastern China: Overview of the recent measurements at a rural site. Geophys. Res. Lett. 28, 2373–2376. doi: 10.1029/2000GL012378
Wang, T., Xue, L., Brimblecombe, P., Lam, Y. F., Li, L., and Zhang, L. (2017). Ozone pollution in China: A review of concentrations, meteorological influences, chemical precursors, and effects. Sci. Total Environ. 575, 1582–1596. doi: 10.1016/j.scitotenv.2016.10.081
Wang, Y. X., Yang, L. X., Höller, M., Shao, Z. S., Pariasca-Tanaka, J., Wissuwa, M., et al. (2014b). Pyramiding of ozone tolerance QTLs OzT8 and OzT9 confers improved tolerance to season-long ozone exposure in rice. Environ. Exp. Bot. 104, 26–33. doi: 10.1016/j.envexpbot.2014.03.005
Wang, J., Zeng, Q., Zhu, J., Chen, C., Liu, G., and Tang, H. (2014a). Apoplastic antioxidant enzyme responses to chronic free-air ozone exposure in two different ozone-sensitive wheat cultivars. Plant Physiol. Biochem. 82, 183–193. doi: 10.1016/j.plaphy.2014.06.004
Wen, Z., Wang, L., Wang, X. K., Li, L., and Cui, J. (2014). Combined effects of ozone and drought on leaf stomata of Acer truncatum. Chin. J. Ecol. 33, 560–566. doi: 10.13292/j.1000-4890.2014.0042
Wittig, V. E., Ainsworth, E. A., Naidu, S. L., Karnosky, D. F., and Long, S. P. (2009). Quantifying the impact of current and future tropospheric ozone on tree biomass, growth, physiology and biochemistry: a quantitative meta-analysis. Glob. Change Biol. 15, 396–424. doi: 10.1111/j.1365-2486.2008.01774.x
Wu, F. F., Zheng, Y. F., Wu, R. J., and Wang, J. Q. (2011). Concentration of O3 at the atmospheric surface affects the changes characters of antioxidant enzyme activities in Triticum aestivum. Acta Ecol. Sin. 31, 4019–4026. doi: 10.20103/j.stxb.2011.14.018
Xu, Y. S., Feng, Z. Z., Bao, M. X., Li, Y., Xia, J. X., Xu, S. Y., et al. (2025). Warming mitigates ozone damage to wheat photosynthesis in a FACE Experiment. Plant Cell Environ. 48, 2312–2328. doi: 10.1111/pce.15304
Xu, S., He, X. Y., Du, Z., Chen, W., Li, B., Li, Y., et al. (2020). Tropospheric ozone and cadmium do not have interactive effects on growth, photosynthesis and mineral nutrients of Catalpa ovata seedlings in the urban areas of Northeast China. Sci. Total Environ. 704, 135307. doi: 10.1016/j.scitotenv.2019.135307
Xu, X., Lin, W., Wang, T., Yan, P., Tang, J., Meng, Z., et al. (2008a). Long-term trend of surface ozone at a regional background station in eastern China 1991-2006:enhanced variability. Atmos. Chem. Phys. 8, 2595–2607. doi: 10.5194/acp-8-2595-2008
Xu, W. D., Qi, S. Y., He, X. Y., Chen, W., Zhao, G. L., and Zhou, Y. (2008b). Effects of elevated CO2 and O3 concentrations on quantitative characteristics of mature leaf stomata in Ginkgo biloba. Chin. J. Ecol. 27, 1059–1063. doi: 10.13292/j.1000-4890.2008.0215
Yang, Y., Sun, M., Li, S. S., Chen, Q. H., Silva, T. D., Wang, A. J., et al. (2020). Germplasm resources and genetic breeding of Paeonia: A systematic review. Hortic. Res. 7, 107. doi: 10.1038/s41438-020-0332-2
Yendrek, C. R., Erice, G., Montes, C. M., Tomaz, T., Sorginj, C. A., Brown, P. J., et al. (2017). Elevated ozone reduces photosynthetic carbon gain by accelerating leaf senescence of inbred and hybrid maize in a genotype-specific manner. Plant Cell Environ. 40, 3088–3100. doi: 10.1111/pce.13075
Zhang, L., Jia, L. L., Sui, J. X., Zhang, Y., and Chen, Y. J. (2015). Research on the effect of ozone pollution on horticultural crops. North. Hortic. 16, 188–195. doi: 10.11937/bfyy.201516045
Zhang, X. Y., Liu, H. N., Su, J. H., Tao, J., and Zhao, D. Q. (2018). Research progress on the effect of abiotic stress on the growth and development of Paeonia plant. Mol. Plant Breed. 16, 5072–5079. doi: 10.13271/j.mpb.016.005072
Keywords: peony (Paeonia lactiflora), air pollution, abiotic stress, leaf photosynthetic capacity, antioxidant system
Citation: Shao Z, Jing L, Zha L, Lu X, Huo W, Yang K and Zhang H (2025) Peony (Paeonia lactiflora) leaf performance of two cultivars in response to ozone exposure. Front. Plant Sci. 16:1607998. doi: 10.3389/fpls.2025.1607998
Received: 08 April 2025; Accepted: 13 June 2025;
Published: 11 July 2025.
Edited by:
Yongfeng Guo, Chinese Academy of Agricultural Sciences, ChinaReviewed by:
Lia Dinis, University of Trás-os-Montes and Alto Douro, PortugalMohamed T. El-Saadony, Zagazig University, Egypt
Copyright © 2025 Shao, Jing, Zha, Lu, Huo, Yang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zaisheng Shao, c2hhb3phaXNoZW5nQGpzYWh2Yy5lZHUuY24=
 Zaisheng Shao
Zaisheng Shao Liquan Jing
Liquan Jing Li Zha1
Li Zha1