- 1College of Biological Sciences and Technology, Taiyuan Normal University, Taiyuan, China
- 2Modern Agricultural Development Center, Jincheng Agricultural and Rural Bureau, Jincheng, China
Foxtail millet (Setaria italica) is a significant cereal crop, but its yield is limited by extreme temperature, particularly cold stress. In this study, we identified a novel plant-specific gene, SiCST1 (Cold Stress Tolerance in Setaria italica 1) in foxtail millet, through transcriptome deep sequencing (RNA-Seq) of cold-stressed seedlings. We generated a CRISPR/Cas9-mediated knockout mutant of rice homolog of SiCST1 (designated oscst1). Compared to wild-type rice, oscst1 mutant seedlings exhibited cold sensitivity with a 46% survival rate reduction under cold stress. This impaired cold stress tolerance was rescued by complementation with SiCST1, indicating the vital role of SiCST1 in cold stress tolerance. SiCST1 consists of a single exon and contains a predicted ribonuclease H-like domain. Further analysis revealed that SiCST1 was significantly up-regulated in response to cold stress and was localized in nucleus. Additionally, our findings suggested that SiCST1 interacted with the OVATE family protein SiOFP1. The lamina joint bending assays were employed to investigate whether mutation of rice homolog of SiCST1 affected the brassinolide (BR) signaling pathways. It was found that oscst1 exhibited insensitivity to exogenous BR treatment. We propose a regulatory mechanism in which SiCST1 interacted with SiOFP1 to release its inhibition of BR signaling transcription complex, thereby activating BR signaling pathways and conferring cold stress tolerance. Our study provides evidence that SiCST1 is a novel plant-specific protein with an essential function involved in cold stress resistance in foxtail millet.
1 Introduction
Foxtail millet (Setaria italica (L.) Beauv.), a cultivated species within the genus Setaria in the Poaceae family, originated in China approximately 8700 years ago, having been domesticated from its wild relative, Setaria viridis (Bettinger et al., 2010). Foxtail millet is a significant crop and a staple component of human diets, ranking second in the global millet production (Anonymous, 2023). The completion of the whole-genome sequencing of foxtail millet revealed a relatively small genome size (approximately 490 Mb) with a low DNA repetition rate (24%) (Bennetzen et al., 2012). Furthermore, compared to its ancestral grass lineages, the genome structure of foxtail millet is highly conserved, making it an ideal model species for genetic and molecular studies (Devos et al., 1998; Jayaraman et al., 2008; Bennetzen et al., 2012). Additionally, due to its close phylogenetic relationship with important biofuel crops such as switchgrass, napiergrass, and pearl millet, foxtail millet is also regarded as a suitable model for research on these crops (Doust et al., 2009). During its growth cycle, foxtail millet is highly sensitive to cold stress, particularly during the seedling and booting stages, where cold stress can significantly hinder growth and reduce final yield (Lyons, 1973; Thakur et al., 2010; Liu et al., 2020; Zhang et al., 2022; Huang et al., 2023b). Cold stress tolerance in foxtail millet is a crucial determinant of its growing season length and geographical distribution. Therefore, enhancing its cold stress tolerance has become a primary objective in breeding programs. To achieve this goal, it is particularly vital to profoundly investigate the regulatory mechanisms of cold signaling pathways in foxtail millet.
An adaptive response called cold acclimation has evolved in temperate plants to enhance their cold stress tolerance (Thomashow, 1999; Ding and Yang, 2022). Cold acclimation in Arabidopsis is primarily mediated by three CBF/DREB1 genes that play central and redundant roles (Novillo et al., 2007; Jia et al., 2016; Zhao et al., 2016; Wang et al., 2023a). Under cold stress, CBF genes are rapidly and highly induced, and the proteins they encode activate the expression of COR (COLD REGULATED) genes. This lead to the accumulation of protective substances such as osmolytes and cold-protective proteins, ultimately facilitating cold acclimation and increasing cold stress tolerance (Thomashow, 1999; Shi et al., 2018; Ding et al., 2020). The regulation of CBF gene expression involves various transcription factors. ICE1 and its homologous protein ICE2 positively regulate the expression of CBFs and cold stress tolerance (Chinnusamy et al., 2003; Fursova et al., 2009; Tang et al., 2020; Wang et al., 2023b). The circadian clock is closely associated with the cold stress response (Lu et al., 2020; Poikela et al., 2021). The rhythmic expression of CBFs is regulated by core components of the circadian clock, including CCA1, LHY, and PRRs (Kidokoro et al., 2021). The cold signaling pathways are also modulated by light and photoperiod (Franklin and Whitelam, 2007; Jiang et al., 2017; Wang et al., 2019). Plant hormones also play roles in plant response to cold stress, including BR (brassinosteroid), ethylene, and JA (jasmonic acid), among others (Hu et al., 2013; Huang et al., 2023a; He et al., 2024; Wang et al., 2024a, b; Zeng et al., 2025).
BR signaling constitutes a pivotal pathway in the acquisition of cold stress tolerance by plants. During cold stress, an elevation in endogenous BR content was observed in plants. When tomato (Solanum lycopersicum L.) leaves were exposed to 8°C for 8 h, three detectable BRs (brassinolide (BL), castorosterone (CS), and 28-norCS) were found to exhibit increased levels (Fang et al., 2019). Investigation into receptor kinases and transcription factors involved in BR signaling have demonstrated that the overexpression of positive components of BR signaling enhances cold stress tolerance, whereas the overexpression of negative components impairs it. In Arabidopsis, the overexpression of BRI1 markedly improves plant survival under cold stress condition, whereas the survival of bri1–1 and bri1–301 mutant plants was significantly compromised (Eremina et al., 2016). BIN2 functions as a negative component within BR signaling pathways. Notably, triple mutant plants of BIN2 exhibit enhanced stress cold resistance, whereas the overexpression of BIN2 in plants leads to decreased cold stress resistance (Li et al., 2017). Studies have further indicated that the exogenous application of BR increases the expression of COR genes and thereby enhances cold stress tolerance (Kagale et al., 2007).
In our study, based on RNA-Seq analysis of cold-stressed foxtail millet seedlings, SiCST1 (LOC101781117) was selected as one of the most significantly up-regulated genes for functional validation. This study demonstrated its role as a key regulator of cold stress tolerance and revealed the underlying molecular mechanism. The discovery of the interaction between SiCST1 and SiOFP1, as well as its implication for BR signaling and cold stress tolerance, offers new avenues for research into genetic and molecular basis of crop stress resilience. The findings have the potential to facilitate the rapid development of new varieties with enhanced cold stress tolerance.
2 Materials and methods
2.1 Plant material
Plants were grown under natural condition in Jinzhong (36°85’ N, 111°77’ E), Shanxi province, China. Yugu1 (Setaria italica cv. Yugu1), a sequenced wild-type foxtail millet variety with a complete reference genome (Bennetzen et al., 2012), was employed in this study.
The pYLCRISPR was used to construct CRISPR vector targeting OsCST1 (rice homolog of SiCST1), which were subsequently employed for genetic transformation of rice variety Zhonghua11 (Oryza sativa L. ssp. japonica Zhonghua11). CRISPR-P 2.0 was utilized to design the base-pairing sequence of the sgRNA (5′-GCATCAGCAGCAGACGCCAC-3′) targeting the single exon of OsCST1.
To complement oscst1 mutant, genomic fragments of SiCST1 (3609 bp) were cloned into pCAMBIA1301 vector. The resulting construct was transformed into Agrobacterium tumefaciens EHA105 and subsequently transformed into rice callus induced from mature seeds of oscst1 mutant.
2.2 Cold stress treatment
Cold stress treatment were implemented as previously described (Ma et al., 2009). The rice seedlings were cultivated in Kimura B nutrient solution under controlled condition (day/night temperature: 30°C/25°C and light/dark photoperiod: 10 h/14 h) until they reached the three-leaf stage. Subsequently, the seedlings were subjected to cold stress (4°C) for 96 h. After a recovery period of 7 days at 30°C/25°C (day/night), seedling survival rate were assessed. Three biological replicates were established, each comprising 32 rice seedlings.
2.3 RNA-seq and gene expression analysis
For RNA-Seq analysis under cold stress, three-leaf-stage foxtail millet seedlings grown at 28°C/24°C (day/night) were transferred to 4°C. After 24 h of treatment, both cold-treated and untreated seedlings were harvested, immediately frozen in liquid nitrogen, and stored at -80°C for subsequent RNA isolation. Total RNA was extracted from three biological replicates (each comprising five pooled seedlings). High-throughput sequencing was performed on HiSeq 2500 (Novogene, Beijing). Clean reads were aligned to Setaria italica reference genome (v2.2, Phytozome).
For SiCST1 expression analysis during cold stress treatment, foxtail millet seedlings cultivated to the three-leaf stage at 28°C/24°C (day/night) were exposed to 4°C. The seedlings were collected at 0, 1, 3, 6, 12, and 24 h post-treatment, immediately frozen in liquid nitrogen, and stored at -80°C for RNA extraction. Total RNA was extracted from three biological replicates, with each consisting of a pooled sample of five seedlings.
Total RNA was extracted from tissues using the RNeasy plant mini kit (QIAGEN). The isolated RNA was reverse transcribed using SuperScript III reverse transcriptase (Invitrogen). Quantitative RT-PCR (RT-qPCR) was performed utilizing SYBR Green Real-Time PCR Master Mixes (Invitrogen). Gene expression level was normalized with foxtail millet actin gene (Seita.7G294000). Primers used for RT-qPCR are listed in Supplementary Table S1.
2.4 Subcellular localization
The full-length cDNA of SiCST1 was cloned into pBI221-GFP vector to create fusion protein with GFP at the C-terminus of SiCST1. SiCST1-GFP and the nucleus marker H2B-mCherry were co-transformed into foxtail millet protoplasts via the polyethylene glycol-mediated transformation method, as previously described (Chen et al., 2006). After culturing at 25°C for 16 h in darkness, the transformed protoplasts were observed using a confocal scanning microscope.
2.5 Yeast two-hybrid analysis
For bait construction, the full-length SiCST1 cDNA was cloned into pGBKT7 (Clontech) and transformed into yeast strain Y2HGold. For prey cDNA library construction, total RNA was isolated from 2-week-old foxtail millet seedlings (Yugu1) using the RNeasy Plant Kit (QIAGEN). Synthesized cDNA was co-transformed with linearized pGADT7-Rec vector (Clontech) into Saccharomyces cerevisiae strain Y187 via the Make Your Own Mate & Plate Library System (Clontech).
For Y2H screening, bait strain Y2HGold (harboring pGBKT7-SiCST1) and prey strain Y187 (containing the cDNA library) were mated in YPDA liquid medium at 30°C for 24 h with 40 rpm shaking. Mating diploids were then plated onto SD/-Leu/-Trp/-His/-Ade + X-α-Gal plates. Following 7-day incubation at 30°C, colonies exhibiting blue coloration were restreaked for confirmation. Validated positive clones underwent PCR amplification of insert fragments, followed by DNA sequencing. Sequence alignment against Setaria italica genome using BLASTn identified SiOFP1 (LOC101755245) as a high-confidence interactor detected in 12 out of 20 sequenced clones.
2.6 Bimolecular fluorescence complementation analysis
The full-length cDNA of SiCST1 was cloned into pUC-SPYNE173 vector and the full-length cDNA of SiOFP1 was cloned into pUC-SPYCE (M) vector. To evaluate potential false positives, the pUC-SPYNE173-SiCST1 construct was co-transformed with the empty pUC-SPYCE (M) vector as a negative control.
Protoplasts were isolated from 10-day-old etiolated foxtail millet leaves (Yugu1) by enzymatic digestion. Leaves were sliced into 0.5-mm strips and incubated in digestion solution [1.5% (w/v) Cellulase R10, 0.4% (w/v) Macerozyme R10, 0.5 M D-mannitol, 20 mM KCl, 20 mM MES (pH 5.7), 10 mM CaCl2, 0.1% BSA] at 28°C for 4 h with gentle shaking (40 rpm). Protoplasts were filtered through 75-μm nylon mesh, washed twice with W5 solution [154 mM NaCl, 125 mM CaCl2, 5 mM KCl, 2 mM MES (pH 5.7)], and resuspended in MMg solution [0.5 M mannitol, 15 mM MgCl2, 4 mM MES (pH 5.7)] at a density of 1 - 5 × 106 cells/mL.
For transformation, 10 μg each of pUC-SPYNE173-SiCST1 (N-terminal YFP fragment) and pUC-SPYCE(M)-SiOFP1 (C-terminal YFP fragment) plasmids were added to 100 μL protoplast suspension, followed by 110 μL PEG solution (40% PEG4000, 0.4 M mannitol, 0.1 M CaCl2). After 15-min incubation at 25°C, reactions were quenched with 1 mL W5 solution. Transfected protoplasts were cultured in WI solution [0.5 M D-mannitol, 4 mM MES (pH 5.7), 20 mM KCl] at 25°C for 16 h in darkness (Walter et al., 2004). Following incubation, YFP fluorescence was observed using a confocal scanning microscope.
2.7 Co-immunoprecipitation
The pA7-SiOFP1-GFP and FLAG-SiCST1 plasmids were co-transformed into foxtail millet protoplasts with FLAG-SiCST1 and the empty pA7-GFP vector serving as control to assess false positives. Transfected protoplasts were incubated at 25°C in darkness for 14–16 h. Upon microscopic confirmation of GFP fluorescence expression, the protoplasts were lysed with 400 μL IP buffer (10 mM HEPES, 100 mM NaCl, 1 mM EDTA, 10% glycerol, 0.5% Triton X-100, 1×protease inhibitor cocktail, pH 7.5). The lysate was centrifuged at 5,000 g for 5 min at 4°C. The resultant supernatant was incubated overnight at 4°C with 20 μL of anti-FLAG agarose beads (MBL). The beads were collected and washed five times with IP buffer, followed by boiling in SDS buffer. The samples were examined by western blotting using anti-GFP or anti-FLAG antibody.
2.8 Lamina joint bending assays
Lamina joint bending assays were performed on the second fully expanded leaves of two-week-old WT and oscst1 rice seedlings as previously described (Feng et al., 2016). Excised leaf segments were placed in media supplemented with 0, 0.01, 0.1, or 1 μM 24-epibrassinolide, with three biological replicates per concentration and five seedlings per replicate. After 48 h incubation in darkness at 28°C, the lamina joint angle was quantified using ImageJ software.
2.9 Statistical analysis
Significant differences between control and treatment were analyzed using Student’s t-test within SPSS version 25 software (IBM SPSS Statistics). Differences were considered significant at P < 0.05 and highly significant at P < 0.01.
3 Results
3.1 SiCST1 exhibits the ability to confer cold stress tolerance
To identify genes associated with cold stress tolerance in foxtail millet at the seedling stage, we conducted transcriptome deep sequencing (RNA-Seq) analysis on three-leaf-stage seedlings of Yugu1, which were exposed to 4°C cold stress for 24 h. We thus identified SiCST1 (LOC101781117) was up-regulated 4-fold by cold stress treatment. To explore the possible involvement of SiCST1 in cold stress tolerance, we developed CRISPR/Cas9 knockout mutant line, oscst1, targeting the homolog of SiCST1 in rice (OsCST1, LOC4329262). OsCST1 was identified by conducting a blastp search of SiCST1 protein sequence against rice protein database (Oryza sativa ssp. japonica). Genomic analysis confirmed that OsCST1 is a single-copy gene in rice. Knockout of OsCST1 yielded oscst1 mutant. Sequencing of PCR products from oscst1 T1 generation plants confirmed a 1-bp insertion in the single exon of OsCST1, causing a frameshift and premature termination (Supplementary Figures S1A–C). The truncated protein consisting of 405 amino acids lacks the C-terminal domain (Supplementary Figure S1D).
We then proceeded to evaluate the cold stress tolerance of T1 generation plants of oscst1 by exposing seedlings to 4°C cold stress followed by recovery at 30°C. We defined plants possessing cold stress tolerance as those that displayed continued leaf growth or newly differentiated leaves upon being returned to normal condition following cold stress treatment. A significant difference in survival rate (percentage of seedlings that survived the treatment) and seedling height was observed between wild-type (WT) and oscst1 mutant (Figures 1A–C; Supplementary Figure S2). Notably, oscst1 mutant seedlings demonstrated cold sensitivity compared to WT. Furthermore, we genetically transformed oscst1 with FLAG-tagged genomic fragments of SiCST1. The resultant complementary line (Cp) rescued the impaired cold stress tolerance observed in oscst1 (Figures 1D–F; Supplementary Figure S2). Taken together, these findings imply a pivotal regulatory role for SiCST1 in modulating cold stress tolerance.
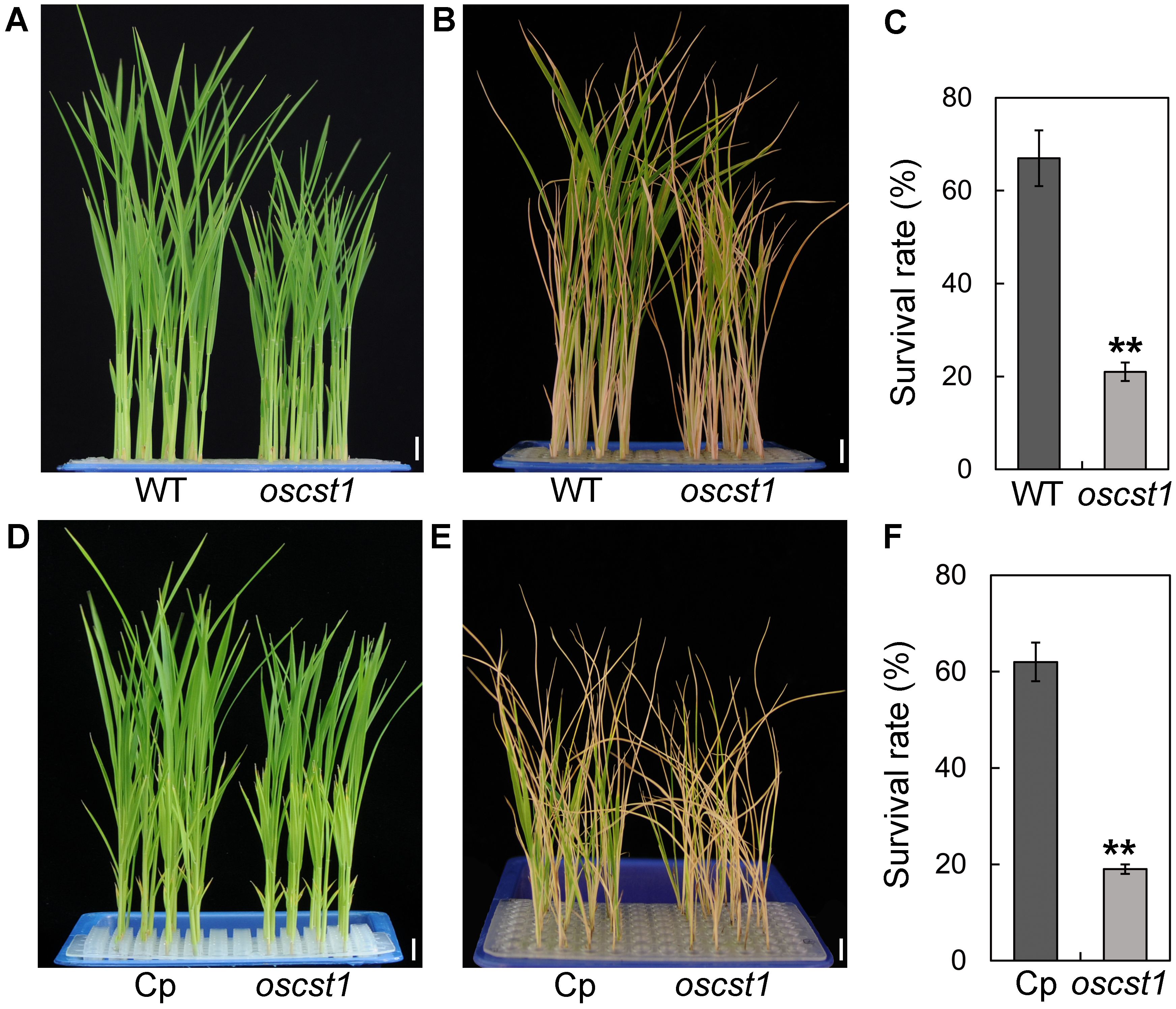
Figure 1. SiCST1 is essential for cold stress tolerance. (A, D) Nontreatment control. (B, E) Cold stress treatment. (C, F) Survival rate was assessed following cold stress treatment at 4°C for 96 h and subsequent recovery at 30°C for 7 days. WT, wild-type; Cp, complementary line. Scale bar, 1 cm. Student’s t-test, **P < 0.01.
3.2 SiCST1 is a plant‐specific protein with a ribonuclease H‐like domain
A full-length SiCST1 cDNA was synthesized using total RNA extracted from two-week-old seedlings of foxtail millet (Yugu1). Sequence analysis revealed that SiCST1 consists of a single exon and has an open reading frame (ORF) encoding 798 amino acid residues, with predicated molecular weight of 87032.1 and isoelectric point of 8.6. Domain analysis revealed that SiCST1 possesses a ribonuclease H‐like (RNase H‐like) domain, which was conserved in SiCST1 and its homologs (Figure 2). Sequence homology searches indicated the presence of SiCST1 homologs in various spermatophytes, but notably absent in animals. Phylogenetic analysis distinctly categorized these sequences into separate clades corresponding to monocots and dicots (Figure 3), suggesting that the emergence of these sequences may be associated with the differentiation of spermatophytes.
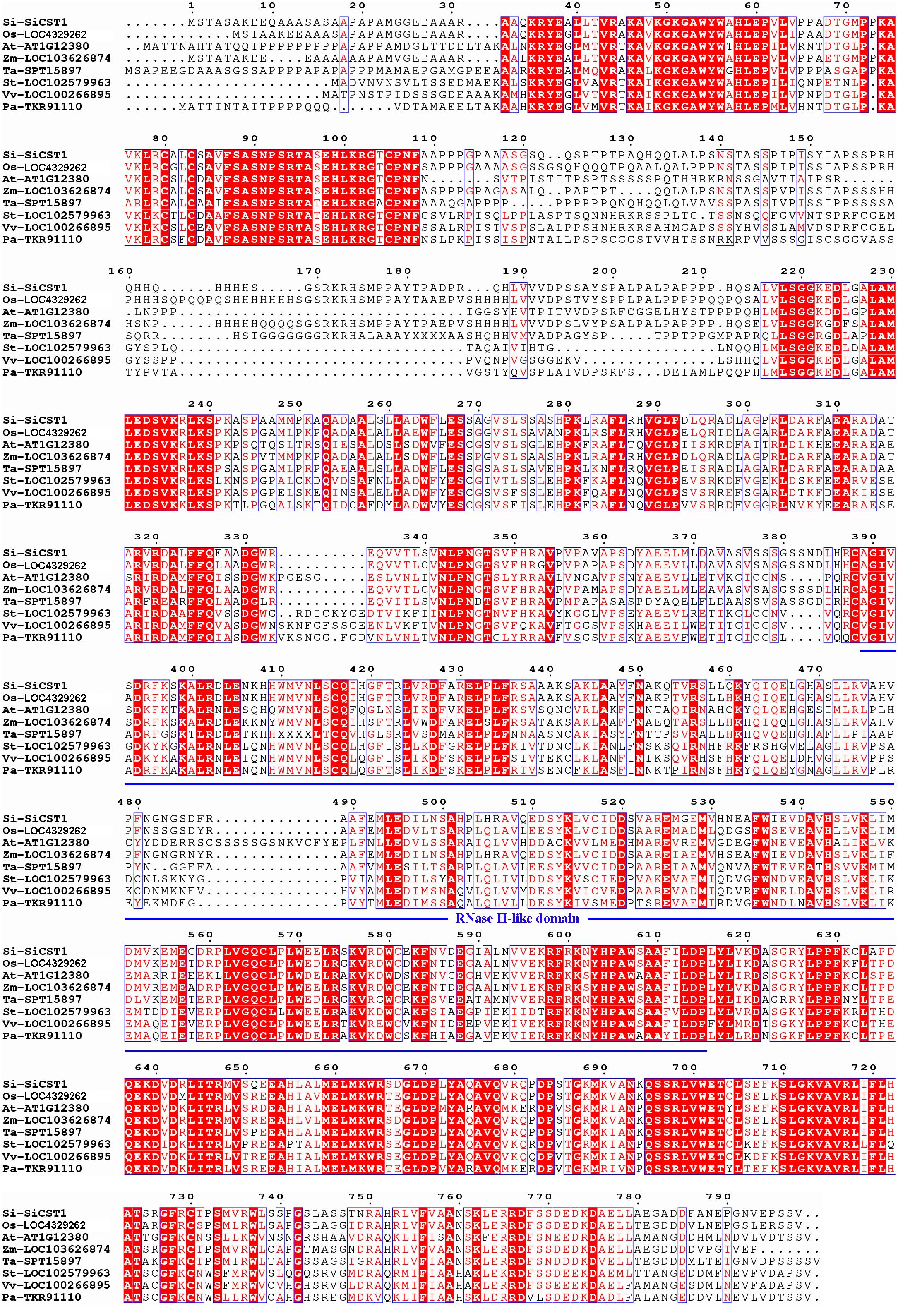
Figure 2. Multiple sequence alignments were performed using MEGA software. These homolog sequences used are LOC4329262 from Oryza sativa (Os), At1g12380 from Arabidopsis thaliana (At), LOC103626874 from Zea mays (Zm), SPT15897 from Triticum aestivum (Ta), LOC102579963 from Solanum tuberosum (St), LOC100266895 from Vitis vinifera (Vv) and TKR91110 from Polulus alba (Pa). The RNase H‐like domain was marked with a blue underline.
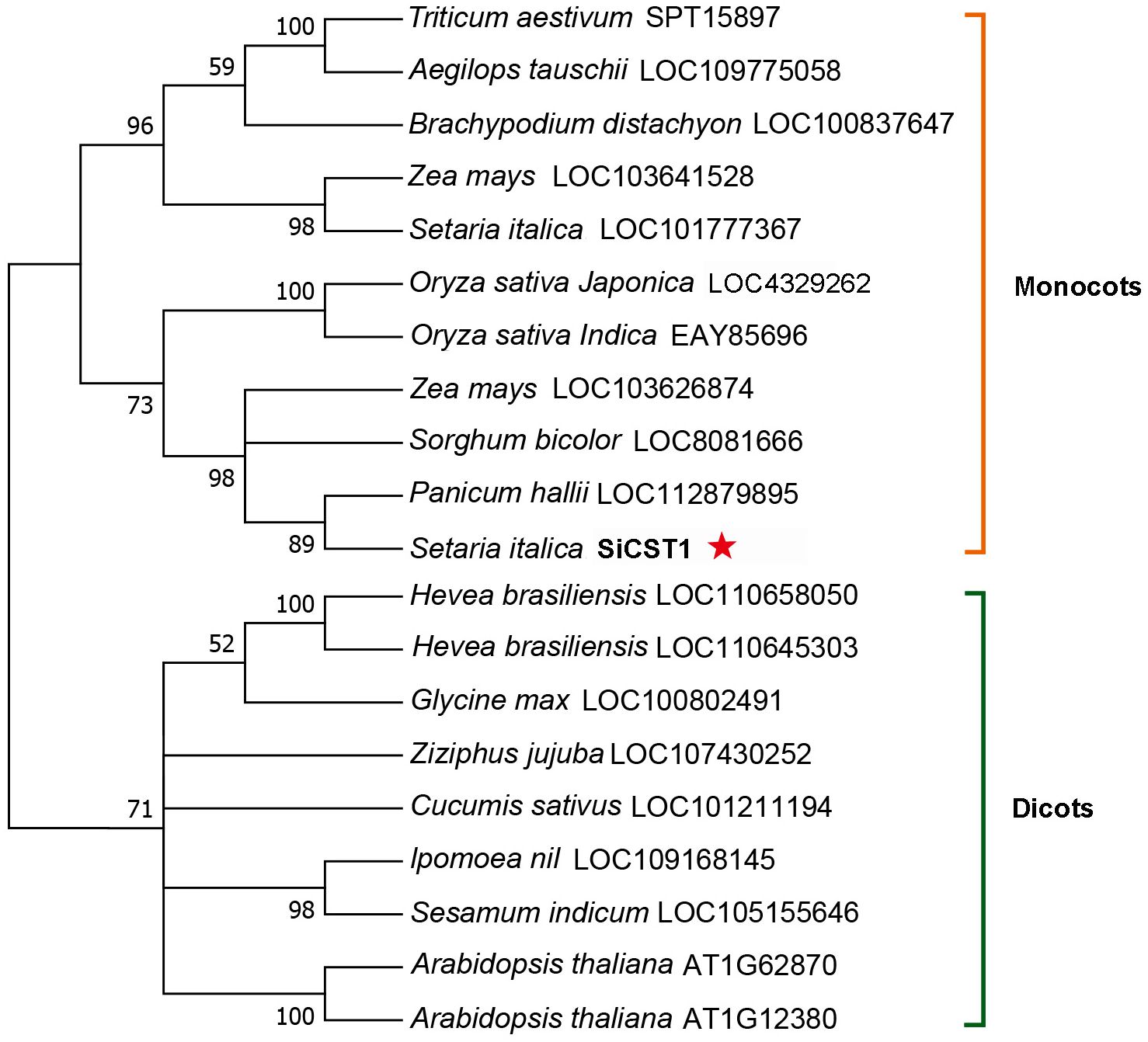
Figure 3. Phylogenetic tree of SiCST1 and its homologs across different species. The construction of the phylogenetic tree was accomplished utilizing MEGA11 software, employing the maximum-likelihood method with 1000 bootstrap replicates.
To identify the subcellular localization of SiCST1, we performed transient protoplast transformation of foxtail millet using constructs of either ubi::SiCST1-GFP (maize ubiquitin promoter-driven fusion) or H2B-mCherry (constituting a nucleus marker). We observed complete overlap between SiCST1-GFP and H2B-mCherry fluorescence signals (Figure 4A), indicating that SiCST1 is localized in nucleus. Constitutive expression of SiCST1 was observed in all examined tissues, with particularly high expression level detected in young tissues (Figure 4B). Additionally, exposure to cold stress (4°C) induced SiCST1 expression, with expression level more than three times higher after 24 h of treatment compared to the untreated control (Figure 4C). This discovery was consistent with the involvement of this gene in seedling cold stress tolerance.
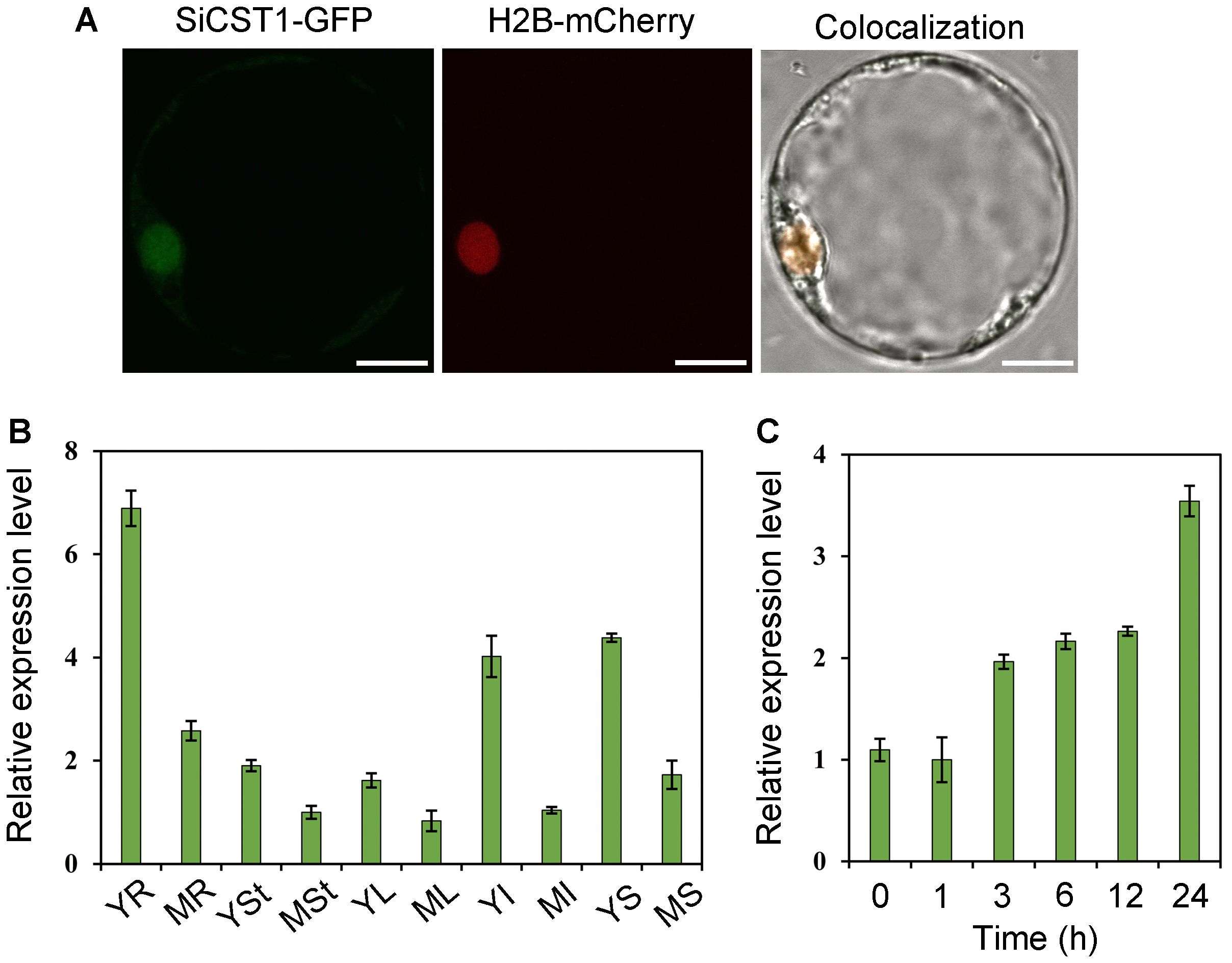
Figure 4. Subcellular localization and expression pattern of SiCST1. (A) Nucleus localization of SiCST1 in foxtail millet protoplasts. Scale bar, 5 μm. (B) Tissue-specific expression of SiCST1. Total RNA was extracted from young roots (YR), mature roots (MR), young stems (YSt), mature stems (MSt), young leaves (YL), mature leaves (ML), young inflorescences (YI), mature inflorescences (MI), young seeds (YS), and mature seeds (MS), respectively. (C) Accumulation of SiCST1 transcripts in response to cold stress treatment. Values represented means ± SE (n = 15).
3.3 SiCST1 interacts with SiOFP1
To elucidate how SiCST1 regulates cold stress tolerance, potential SiCST1-interacting proteins were identified using prey cDNA library generated from foxtail millet seedling RNA and a bait expressing the full-length cDNA of SiCST1. The yeast two-hybrid system (Y2H) revealed a potential interaction between SiCST1 and SiOFP1 (LOC101755245) (Figure 5A). The OFPs are a group of OVATE family transcription factors characterized by a conserved OVATE domain. This interaction was further verified via bimolecular fluorescence complementation (BiFC) and co-immunoprecipitation (CoIP) assays. In BiFC assay, SiCST1 and SiOFP1 were fused to the N- and C-termini of YFP, respectively, generating SiCST1-nYFP and SiOFP1-cYFP constructs. Co-expression of these constructs in foxtail millet protoplasts resulted in fluorescence signals, confirming the interaction between SiCST1 and SiOFP1 through YFP reconstitution. Conversely, protoplasts co-expressing SiCST1-nYFP and the control construct cYFP failed to generate any fluorescence signals (Figure 5B). In CoIP assay, co-expression of FLAG-SiCST1 and GFP-SiOFP1 in foxtail millet protoplasts revealed co-precipitation of FLAG-SiCST1 and GFP-SiOFP1, confirming that SiCST1 directly interacts with SiOFP1 in vivo (Figure 5C).
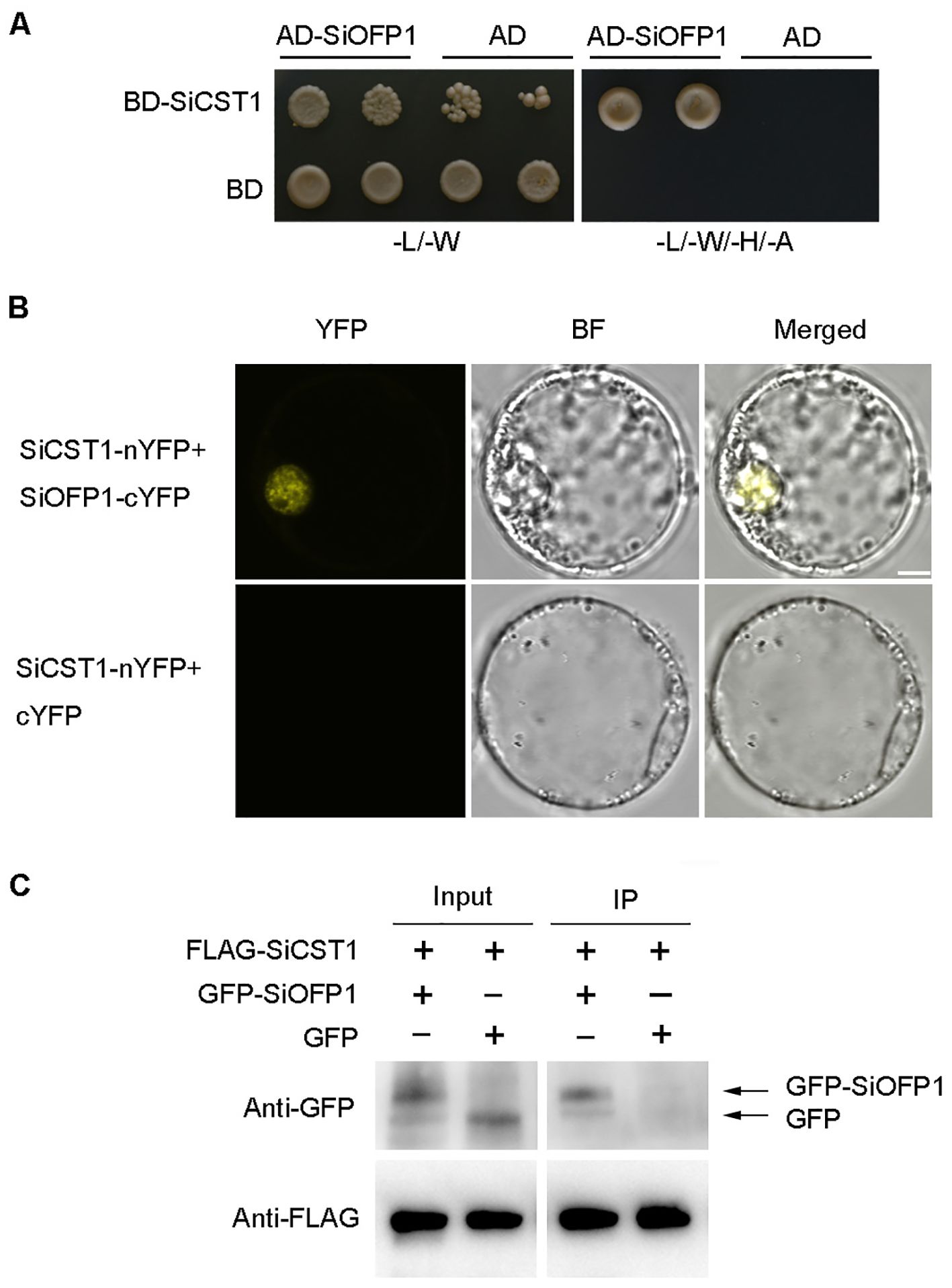
Figure 5. SiCST1 interacts with SiOFP1. (A) Interaction between SiCST1 and SiOFP1 in Y2H assay. (B) BiFC in foxtail millet protoplasts confirmed the interaction between SiCST1 and SiOFP1. Scale bar, 5 μm. (C) CoIP analysis further verified the interaction between SiCST1 and SiOFP1. FLAG-SiCST1 was precipitated from transfected protoplast lysates using anti-FLAG agarose beads, and the interaction was detected by western blotting with anti-GFP or anti-FLAG antibody.
3.4 SiCST1 was involved in BR signaling pathways
Several studies have reported that OFPs play regulatory roles in phytohormone signaling and biosynthesis pathways, particularly in BR signaling (Snouffer et al., 2020), leading us to formulate the speculation that SiCST1 might be implicated in BR signaling pathways. To rigorously test this hypothesis, a series of lamina joint bending assays were conducted on both oscst1 mutant and WT. The results revealed that in WT, the lamina joint angle gradually increased with rising concentrations of 24-epibrassinolide (epiBL). In contrast, oscst1 mutant demonstrated reduced sensitivity to exogenous BR treatment, even at high concentrations of epiBL (Figures 6A, B). These observations provide preliminary evidence supporting the involvement of SiCST1 in BR signaling pathway.
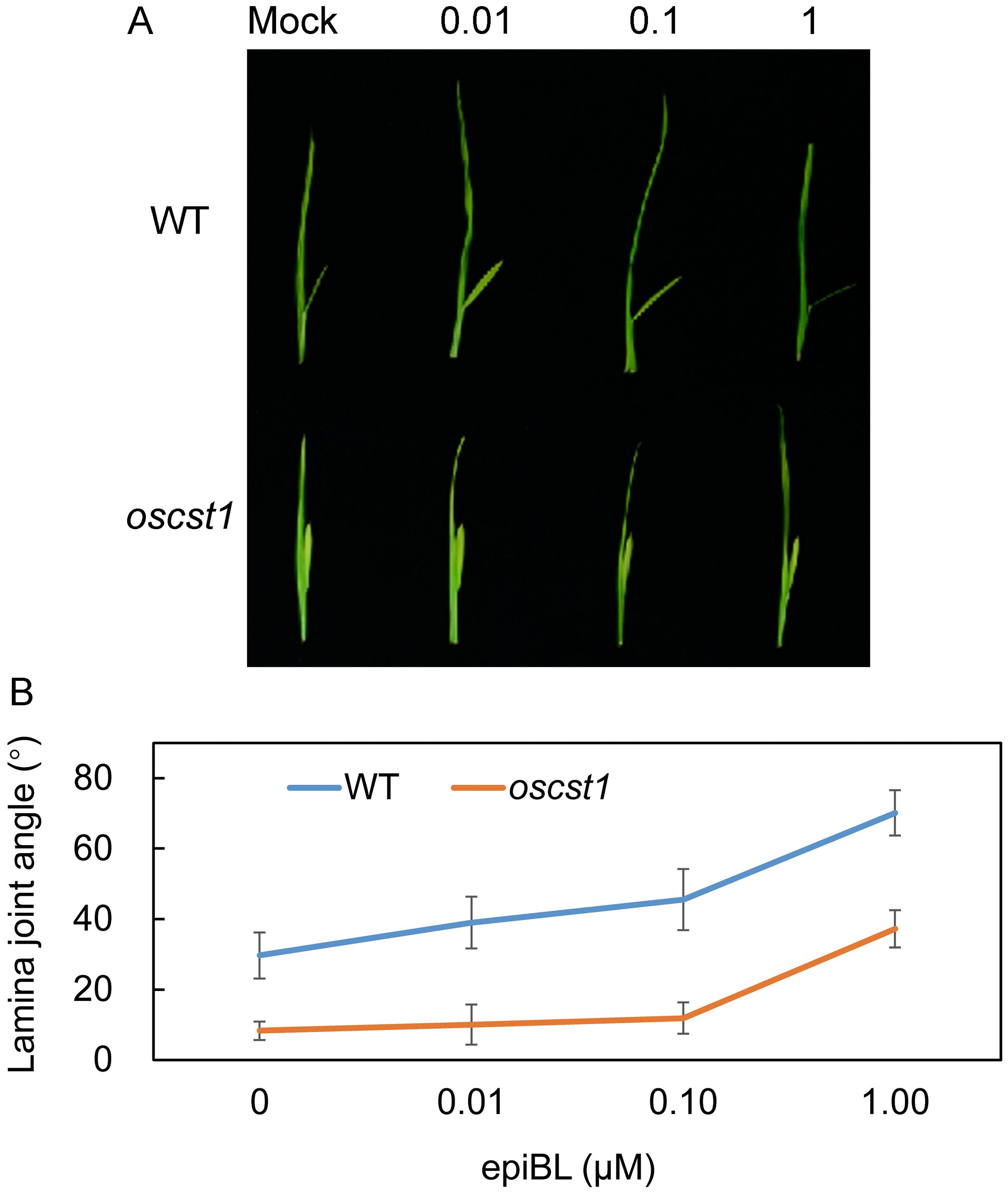
Figure 6. oscst1 exhibited reduced sensitivity to BR. (A) The lamina joint bending response was assessed in WT and oscst1 following application of various concentrations (μM) of epiBL, utilizing the excised leaf segment method. (B) Quantification of lamina joint angles shown in (A). Values represented means ± SD (n = 15).
4 Discussion
4.1 Identification and function of SiCST1 in cold stress tolerance
The exploration of cold stress tolerance in foxtail millet has become a central focus in agricultural research, driven by the urgent need to enhance crop resilience in the face of global climate change. To date, most research efforts concentrated on elucidating the physiological changes, transcriptomic response, and metabolic adaptations that occur in foxtail millet following cold stress exposure (Ananthi et al., 2023; Zhang et al., 2023; Zhao et al., 2023). These studies provided invaluable insights into the multifaceted nature of foxtail millet’s cold tolerance mechanisms. Physiological studies demonstrated that foxtail millet underwent a series of adaptive physiological changes in response to cold stress (Ananthi et al., 2023). Transcriptomic analysis further complemented these findings by revealing a complex network of genes that were either up- or down-regulated in response to cold stress, hinting at the intricate regulatory pathways involved in cold stress tolerance (Zhang et al., 2023). Additionally, metabolic profiling studies identified specific metabolites that accumulated or decreased under cold stress, offering clues into the metabolic reprogramming that occurred in foxtail millet to cope with cold stress (Zhao et al., 2023).
While previous studies have significantly expanded our understanding of foxtail millet cold stress tolerance, a critical gap persists in our knowledge of the underlying molecular mechanisms. This limitation hinders the development of targeted genetic engineering or breeding strategies to enhance cold tolerance. In this context, the identification of SiCST1 as a novel plant-specific gene with a predicted ribonuclease H-like domain in foxtail millet represents a significant step forward in our understanding of cold stress tolerance mechanisms. Our results demonstrated that SiCST1 played a crucial role in conferring cold stress tolerance, as evidenced by the significant decrease in survival rate observed in oscst1 knockout mutant following cold stress treatment compared to WT and restored cold stress tolerance in complemented plants. Furthermore, we propose a potential molecular mechanism of SiCST1, suggesting its involvement in regulating key molecular processes during cold stress.
To bridge this discovery toward crop improvement, we propose two translational strategies: genetic engineering and marker-assisted selection (MAS) breeding (Xiaodi et al., 2023; Zhang et al., 2024; Wang et al., 2025b). Specifically: (1) We will construct ubiquitin promoter-driven SiCST1 overexpression lines in rice and foxtail millet to enhance cold tolerance; (2) Through haplotype analysis of diverse germplasm, we will develop SiCST1-linked molecular markers for efficient identification of cold-tolerant varieties via MAS. Thus, SiCST1 will serve as both a biotechnological target and a molecular breeding anchor bridging mechanistic insights in cold stress tolerance with practical crop resilience enhancement.
4.2 Interaction between SiCST1 and SiOFP1: implication for BR signaling
SiCST1 encodes a plant-specific protein harboring a conserved RNase-H-like domain. Proteins within the RNase-H-like superfamily (RNHLS), categorized as exonucleases or endonucleases, function in critical nucleic acid metabolism processes including DNA replication or repair, homologous recombination, and RNA interference (Majorek et al., 2014). Intriguingly, recent studies identified Reduced height 8 (Rht8), a key “Green Revolution” gene in wheat, as encoding an RNHLS protein that modulated plant architecture through gibberellin biosynthesis regulation (Lingling et al., 2022) (Hongchun et al., 2022). Notably, orthologs of Rht8 in Arabidopsis and maize exhibited conserved roles in height determination (Lingling et al., 2022). In our study, the identification of SiOFP1 as an interactor of SiCST1 through Y2H screening provided critical insights into the molecular mechanism underlying SiCST1-mediated cold stress tolerance. Recent studies have implicated OFPs in various aspects of plant growth, development, as well as response to biotic and abiotic stresses (Wang et al., 2007, 2011; An et al., 2024). The OFPs were implicated in BR signaling pathways, which are known to play pivotal roles in plant growth, development, and stress response (Xiao et al., 2020; Cheng et al., 2023; Wang et al., 2025a; Zhou et al., 2025). Notably, several OFPs have been demonstrated to interact with components of the BR signaling pathways, such as DLT and BES1/BZR1 transcription factors (Yang et al., 2016, 2018; Xiao et al., 2020). These OFPs act as negative regulators of BR response by inhibiting these BR-signaling transcriptional complexes (Yang et al., 2016, 2018; Xiao et al., 2020). These studies suggest that OFPs may function as regulator of BR signaling, modulating the downstream response to BR. We propose that SiCST1 indirectly participates in BR signaling pathways by interacting with SiOFP1.
4.3 The role of CST1, OFP1 and BR signaling in cold stress tolerance
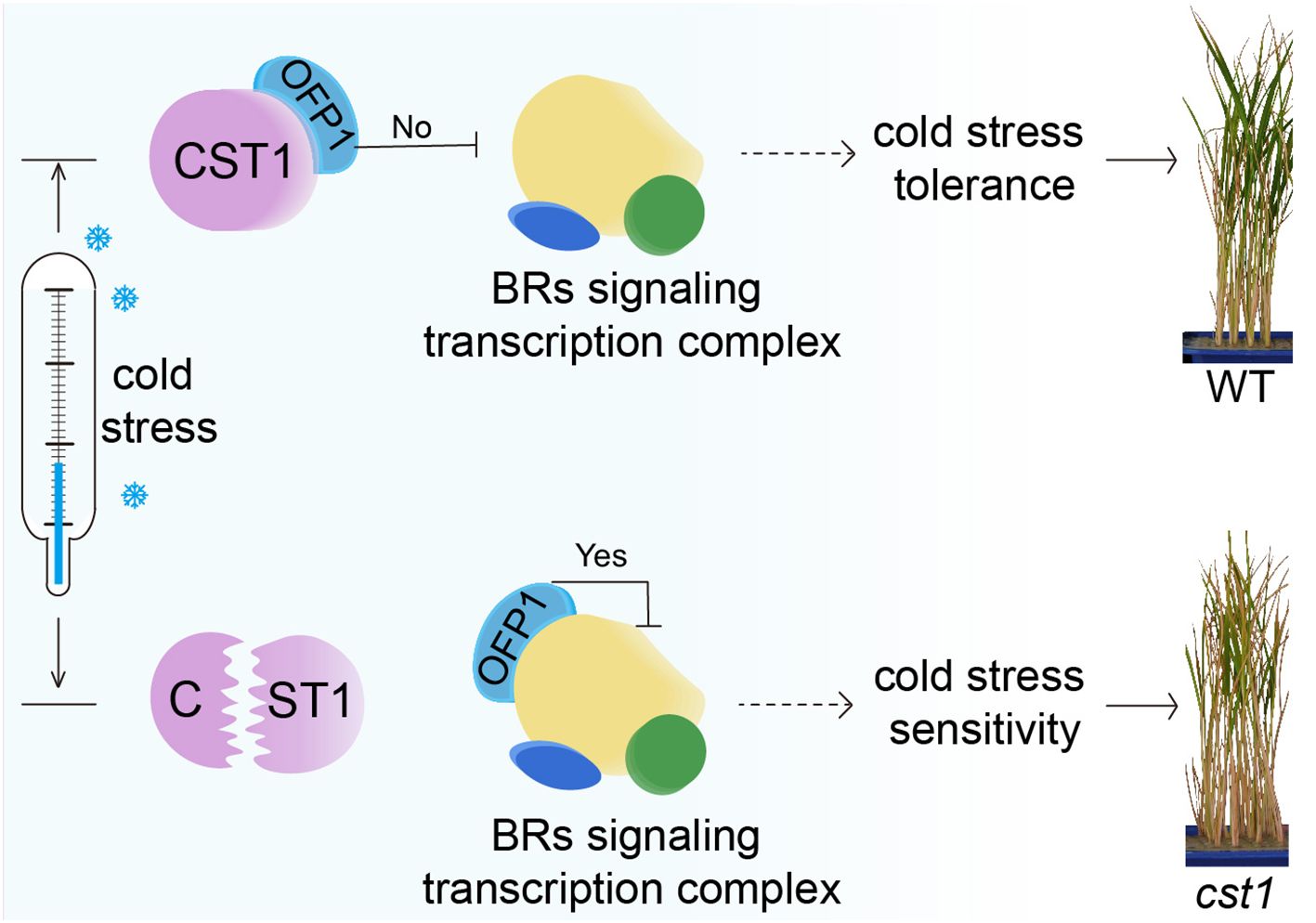
Accumulating evidence suggests that BR signaling pathways were involved in the regulation of cold stress tolerance in plants (He et al., 2024). BR treatment has been shown to enhance the expression of cold-responsive genes and improve plant cold stress tolerance (Kagale et al., 2007; Anwar et al., 2018; Xia et al., 2018). Conversely, mutants defective in BR signaling exhibit reduced cold stress tolerance (Planas-Riverola et al., 2019; Chaudhuri et al., 2022). These findings collectively indicate that BR signaling plays a positive role in conferring cold stress tolerance. In our study, we speculate that SiCST1 may regulate cold stress tolerance by interacting with SiOFP1 and modulating BR signaling. The model is as follows: In WT plants, CST1 interacts with OFP1, to release its inhibition of BR signaling transcription complex, thereby activating BR signaling pathways and conferring cold stress tolerance. On the other hand, in mutant, the mutated CST1 fails to interact with OFP1, allowing OFP1 to maintain its inhibition of BR signaling transcription complex, which renders the mutant plants sensitive to cold stress (Figure 7). Future studies aimed at elucidating the precise molecular mechanisms underlying the interaction between SiCST1 and SiOFP1, as well as their roles in BR signaling and cold stress tolerance, will be crucial for advancing our understanding of this important regulatory pathway.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
FY: Conceptualization, Funding acquisition, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. JQ: Validation, Writing – original draft. XZ: Investigation, Validation, Writing – original draft. ZZ: Validation, Writing – original draft. DH: Conceptualization, Funding acquisition, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Critical Talent Workstation Project (grant numbers TYSGJ202201), the Basic Research Program of Shanxi Province (grant numbers 20210302124365, 202203021222242), and the Higher Education Institutions Science and Technology Innovation Program of Shanxi Province (grant numbers 2021L445).
Acknowledgments
Special thanks to Dr. Ahmad Ali, National Key Laboratory for Tropical Crop Breeding, Institute of Tropical Bioscience and Biotechnology, Chinese Academy ofTropical Agricultural Sciences, for helpful guidance on English writing and Hubei Hygeia Health Technology (Wuhan, China) for providing analytical services.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1618053/full#supplementary-material
References
An, Y., Li, X., Chen, Y., Jiang, S., Jing, T., and Zhang, F. (2024). Genome-wide identification of the OVATE gene family and revelation of its expression profile and functional role in eight tissues of Rosa roxburghii Tratt. BMC Plant Biol. 24, 1068. doi: 10.1186/s12870-024-05775-1
Ananthi, K., Muthukrishnan, N., Sivagamy, K., Kumar, V. A., Jamuna, S., and Yuvaraj, M. (2023). Chilling resistance and physio-chemical changes during cold acclimation in foxtail millet genotypes. Int. J. Plant Soil Sci. 35, 209–215. doi: 10.9734/ijpss/2023/v35i143038
Anonymous (2023). FAO STAT data. Available online at: http://faostat.fao.org/ (Accessed December 19, 2023).
Anwar, A., Liu, Y., Dong, R., Bai, L., Yu, X., and Li, Y. (2018). The physiological and molecular mechanism of brassinosteroid in response to stress: a review. Biol. Res. 51, 46. doi: 10.1186/s40659-018-0195-2
Bennetzen, J. L., Schmutz, J., Wang, H., Percifield, R., Hawkins, J., Pontaroli, A. C., et al. (2012). Reference genome sequence of the model plant Setaria. Nat. Biotechnol. 30, 555–561. doi: 10.1038/nbt.2196
Bettinger, R. L., Barton, L., and Morgan, C. (2010). The origins of food production in north China: A different kind of agricultural revolution. Evolutionary Anthropol. 19, 9–21. doi: 10.1002/evan.20236
Chaudhuri, A., Halder, K., Abdin, M. Z., Majee, M., and Datta, A. (2022). Abiotic stress tolerance in plants: brassinosteroids navigate competently. Int. J. Mol. Sci. 23, 14577. doi: 10.3390/ijms232314577
Chen, S. B., Tao, L. Z., Zeng, L. R., Vega-Sanchez, M. E., Umemura, K., and Wang, G. L. (2006). A highly efficient transient protoplast system for analyzing defence gene expression and protein-protein interactions in rice. Mol. Plant Pathol. 7, 417–427. doi: 10.1111/j.1364-3703.2006.00346.x
Cheng, M., Yuan, H., Wang, R., Wang, W., Zhang, L., Fan, F., et al. (2023). Identification and characterization of BES1 genes involved in grain size development of Oryza sativa L. Int. J. Biol. macromolecules 253, 127327. doi: 10.1016/j.ijbiomac.2023.127327
Chinnusamy, V., Ohta, M., Kanrar, S., Lee, B. H., Hong, X., Agarwal, M., et al. (2003). ICE1: a regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev. 17, 1043–1054. doi: 10.1101/gad.1077503
Devos, K. M., Wang, Z., Beales, J., Sasaki, T., and Gale, M. (1998). Comparative genetic maps of foxtail millet (Setaria italica) and rice (Oryza sativa). Theor. Appl. Genet. 96, 63–68. doi: 10.1007/s001220050709
Ding, Y., Shi, Y., and Yang, S. (2020). Molecular regulation of plant responses to environmental temperatures. Mol. Plant 13, 544–564. doi: 10.1016/j.molp.2020.02.004
Ding, Y. and Yang, S. (2022). Surviving and thriving: How plants perceive and respond to temperature stress. Dev. Cell 57, 947–958. doi: 10.1016/j.devcel.2022.03.010
Doust, A. N., Kellogg, E. A., Devos, K. M., and Bennetzen, J. L. (2009). Foxtail millet: a sequence-driven grass model system. Plant Physiol. 149, 137–141. doi: 10.1104/pp.108.129627
Eremina, M., Unterholzner, S. J., Rathnayake, A. I., Castellanos, M., Khan, M., Kugler, K. G., et al. (2016). Brassinosteroids participate in the control of basal and acquired freezing tolerance of plants. Proc. Natl. Acad. Sci. U.S.A. 113, E5982–e5991. doi: 10.1073/pnas.1611477113
Fang, P., Yan, M., Chi, C., Wang, M., Zhou, Y., Zhou, J., et al. (2019). Brassinosteroids act as a positive regulator of photoprotection in response to chilling stress. Plant Physiol. 180, 2061–2076. doi: 10.1104/pp.19.00088
Feng, Z., Wu, C., Wang, C., Roh, J., Zhang, L., Chen, J., et al. (2016). SLG controls grain size and leaf angle by modulating brassinosteroid homeostasis in rice. J. Exp. Bot. 67, 4241–4253. doi: 10.1093/jxb/erw204
Franklin, K. A. and Whitelam, G. C. (2007). Light-quality regulation of freezing tolerance in Arabidopsis thaliana. Nat. Genet. 39, 1410–1413. doi: 10.1038/ng.2007.3
Fursova, O. V., Pogorelko, G. V., and Tarasov, V. A. (2009). Identification of ICE2, a gene involved in cold acclimation which determines freezing tolerance in Arabidopsis thaliana. Gene 429, 98–103. doi: 10.1016/j.gene.2008.10.016
He, Z., Zhou, M., Feng, X., Di, Q., Meng, D., Yu, X., et al. (2024). The role of brassinosteroids in plant cold stress response. Life 14, 1015. doi: 10.3390/life14081015
Hongchun, X., Chunyun, Z., Meiyu, F., Huijun, G., Yongdun, X., Linshu, Z., et al. (2022). Cloning and functional characterization of rht8, a “Green revolution” Replacement gene in wheat. Mol. Plant 15, 373–376. doi: 10.1016/j.molp.2022.01.014
Hu, Y., Jiang, L., Wang, F., and Yu, D. (2013). Jasmonate regulates the inducer of cbf expression-C-repeat binding factor/DRE binding factor1 cascade and freezing tolerance in Arabidopsis. Plant Cell 25, 2907–2924. doi: 10.1105/tpc.113.112631
Huang, Y.-C., Wang, Y.-T., Choong, Y.-C., Huang, H.-Y., Chen, Y.-R., Hsieh, T.-F., et al. (2023b). How ambient temperature affects the heading date of foxtail millet (Setaria italica). Front. Plant Sci. 14, 1147756. doi: 10.3389/fpls.2023.1147756
Huang, J., Zhao, X., Bürger, M., Chory, J., and Wang, X. (2023a). The role of ethylene in plant temperature stress response. Trends Plant Sci. 28, 808–824. doi: 10.1016/j.tplants.2023.03.001
Jayaraman, A., Puranik, S., Rai, N. K., Vidapu, S., Sahu, P. P., Lata, C., et al. (2008). cDNA-AFLP analysis reveals differential gene expression in response to salt stress in foxtail millet (Setaria italica L.). Mol. Biotechnol. 40, 241–251. doi: 10.1007/s12033-008-9081-4
Jia, Y., Ding, Y., Shi, Y., Zhang, X., Gong, Z., and Yang, S. (2016). The cbfs triple mutants reveal the essential functions of CBFs in cold acclimation and allow the definition of CBF regulons in Arabidopsis. New Phytol. 212, 345–353. doi: 10.1111/nph.14088
Jiang, B., Shi, Y., Zhang, X., Xin, X., Qi, L., Guo, H., et al. (2017). PIF3 is a negative regulator of the CBF pathway and freezing tolerance in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 114, E6695–e6702. doi: 10.1073/pnas.1706226114
Kagale, S., Divi, U. K., Krochko, J. E., Keller, W. A., and Krishna, P. (2007). Brassinosteroid confers tolerance in Arabidopsis thaliana and Brassica napus to a range of abiotic stresses. Planta 225, 353–364. doi: 10.1007/s00425-006-0361-6
Kidokoro, S., Hayashi, K., Haraguchi, H., Ishikawa, T., Soma, F., Konoura, I., et al. (2021). Posttranslational regulation of multiple clock-related transcription factors triggers cold-inducible gene expression in Arabidopsis. Proc. Natl. Acad. Sci. 118, e2021048118. doi: 10.1073/pnas.2021048118
Li, H., Ye, K., Shi, Y., Cheng, J., Zhang, X., and Yang, S. (2017). BZR1 positively regulates freezing tolerance via CBF-dependent and CBF-independent pathways in arabidopsis. Mol. Plant 10, 545–559. doi: 10.1016/j.molp.2017.01.004
Lingling, C., Mingming, X., Chaoqun, D., Zhaoyan, C., Huijie, Z., Junhong, Z., et al. (2022). A natural variation in Ribonuclease H-like gene underlies Rht8 to confer “Green Revolution” trait in wheat. Mol. Plant 15, 377–380. doi: 10.1016/j.molp.2022.01.013
Liu, T., Yang, X., Batchelor, W. D., Liu, Z., Zhang, Z., Wan, N., et al. (2020). A case study of climate-smart management in foxtail millet (Setaria italica) production under future climate change in Lishu county of Jilin, China. Agric. For. Meteorol. 292, 108131. doi: 10.1016/j.agrformet.2020.108131
Lu, X., Zhou, Y., Fan, F., Peng, J., and Zhang, J. (2020). Coordination of light, circadian clock with temperature: The potential mechanisms regulating chilling tolerance in rice. J. Integr. Plant Biol. 62, 737–760. doi: 10.1111/jipb.12852
Lyons, J. M. (1973). Chilling injury in plants. Annu. Rev. Plant Biol. 24, 445–466. doi: 10.1146/annurev.pp.24.060173.002305
Ma, Q., Dai, X., Xu, Y., Guo, J., Liu, Y., Chen, N., et al. (2009). Enhanced tolerance to chilling stress in OsMYB3R-2 transgenic rice is mediated by alteration in cell cycle and ectopic expression of stress genes. Plant Physiol. 150, 244–256. doi: 10.1104/pp.108.133454
Majorek, K. A., Stanislaw, D.-H., Kamil, S., Anna, M., Marcin, N., Krzysztof, G., et al. (2014). The RNase H-like superfamily: new members, comparative structural analysis and evolutionary classification. Nucleic Acids Res. 42, 4160–4179. doi: 10.1093/nar/gkt1414
Novillo, F., Medina, J., and Salinas, J. (2007). Arabidopsis CBF1 and CBF3 have a different function than CBF2 in cold acclimation and define different gene classes in the CBF regulon. Proc. Natl. Acad. Sci. U.S.A. 104, 21002–21007. doi: 10.1073/pnas.0705639105
Planas-Riverola, A., Gupta, A., Betegón-Putze, I., Bosch, N., Ibañes, M., and Caño-Delgado, A. I. (2019). Brassinosteroid signaling in plant development and adaptation to stress. Development 146, 151894. doi: 10.1242/dev.151894
Poikela, N., Tyukmaeva, V., Hoikkala, A., and Kankare, M. (2021). Multiple paths to cold tolerance: the role of environmental cues, morphological traits and the circadian clock gene vrille. BMC Ecol. Evol. 21, 117. doi: 10.1186/s12862-021-01849-y
Shi, Y., Ding, Y., and Yang, S. (2018). Molecular regulation of CBF signaling in cold acclimation. Trends Plant Sci. 23, 623–637. doi: 10.1016/j.tplants.2018.04.002
Snouffer, A., Kraus, C., and van der Knaap, E. (2020). The shape of things to come: ovate family proteins regulate plant organ shape. Curr. Opin. Plant Biol. 53, 98–105. doi: 10.1016/j.pbi.2019.10.005
Tang, K., Zhao, L., Ren, Y., Yang, S., Zhu, J. K., and Zhao, C. (2020). The transcription factor ICE1 functions in cold stress response by binding to the promoters of CBF and COR genes. J. Integr. Plant Biol. 62, 258–263. doi: 10.1111/jipb.12918
Thakur, P., Kumar, S., Malik, J. A., Berger, J. D., and Nayyar, H. (2010). Cold stress effects on reproductive development in grain crops: An overview. Environ. Exp. Bot. 67, 429–443. doi: 10.1016/j.envexpbot.2009.09.004
Thomashow, M. F. (1999). PLANT COLD ACCLIMATION: freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 571–599. doi: 10.1146/annurev.arplant.50.1.571
Walter, M., Chaban, C., Schutze, K., Batistic, O., Weckermann, K., Nake, C., et al. (2004). Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J. 40, 428–438. doi: 10.1111/j.1365-313X.2004.02219.x
Wang, S., Chang, Y., Guo, J., and Chen, J. G. (2007). Arabidopsis Ovate Family Protein 1 is a transcriptional repressor that suppresses cell elongation. Plant J. 50, 858–872. doi: 10.1111/j.1365-313X.2007.03096.x
Wang, S., Chang, Y., Guo, J., Zeng, Q., Ellis, B. E., and Chen, J.-G. (2011). Arabidopsis ovate family proteins, a novel transcriptional repressor family, control multiple aspects of plant growth and development. PloS One 6, e23896. doi: 10.1371/journal.pone.0023896
Wang, L., Chen, H., Chen, G., Luo, G., Shen, X., Ouyang, B., et al. (2024a). Transcription factor SlWRKY50 enhances cold tolerance in tomato by activating the jasmonic acid signaling. Plant Physiol. 194, 1075–1090. doi: 10.1093/plphys/kiad578
Wang, X., Li, Z., Shi, Y., Liu, Z., Zhang, X., Gong, Z., et al. (2023a). Strigolactones promote plant freezing tolerance by releasing the WRKY41-mediated inhibition of CBF/DREB1 expression. EMBO J. 42, e112999. doi: 10.15252/embj.2022112999
Wang, S., Lou, Y., Qi, H., Li, H., Di, X., Sun, H., et al. (2025a). BZR1 targets steroid 22-alpha hydroxylase 4 to negatively regulates cell elongation in bamboo. Int. J. Biol. macromolecules 289, 138832. doi: 10.1016/j.ijbiomac.2024.138832
Wang, Y., Tong, W., Li, F., Samarina, L., Li, P., Yang, T., et al. (2024b). LUX ARRHYTHMO links CBF pathway and jasmonic acid metabolism to regulate cold tolerance of tea plants. Plant Physiol. 196, 961–978. doi: 10.1093/plphys/kiae337
Wang, Z., Wang, Y., and Wong, D. C. J. (2025b). Editorial: Surviving and thriving: how crops perceive and respond to temperature stress. Front. Plant Sci. 15. doi: 10.3389/fpls.2024.1550257
Wang, F., Zhang, L., Chen, X., Wu, X., Xiang, X., Zhou, J., et al. (2019). SlHY5 integrates temperature, light, and hormone signaling to balance plant growth and cold tolerance. Plant Physiol. 179, 749–760. doi: 10.1104/pp.18.01140
Wang, X., Zhang, X., Song, C.-P., Gong, Z., Yang, S., and Ding, Y. (2023b). PUB25 and PUB26 dynamically modulate ICE1 stability via differential ubiquitination during cold stress in Arabidopsis. Plant Cell 35, 3585–3603. doi: 10.1093/plcell/koad159
Xia, X.-J., Fang, P.-P., Guo, X., Qian, X.-J., Zhou, J., Shi, K., et al. (2018). Brassinosteroid-mediated apoplastic HO-glutaredoxin 12/14 cascade regulates antioxidant capacity in response to chilling in tomato. Plant Cell Environ. 41, 1052–1064. doi: 10.1111/pce.13052
Xiao, Y., Zhang, G., Liu, D., Niu, M., Tong, H., and Chu, C. (2020). GSK2 stabilizes OFP3 to suppress brassinosteroid responses in rice. Plant J. 102, 1187–1201. doi: 10.1111/tpj.14692
Xiaodi, W., Ning, L., Tianxiang, Z., Kai, X., Shenghua, G., Yanxu, Y., et al. (2023). Genome-wide analysis of the TIFY family and function of CaTIFY7 and CaTIFY10b under cold stress in pepper (Capsicum annuum L.). Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1308721
Yang, C., Ma, Y., He, Y., Tian, Z., and Li, J. (2018). OsOFP19 modulates plant architecture by integrating the cell division pattern and brassinosteroid signaling. Plant J. Cell Mol. Biol. 93, 489. doi: 10.1111/tpj.13793
Yang, C., Shen, W., He, Y., Tian, Z., and Li, J. (2016). OVATE family protein 8 positively mediates brassinosteroid signaling through interacting with the GSK3-like kinase in rice. PloS Genet. 12, e1006118. doi: 10.1371/journal.pgen.1006118
Zeng, X.-W., Jiang, W.-Z., Zhang, J.-L., Ding, J.-H., Qiu, Y.-M., Wen, W., et al. (2025). Ethylene negatively regulates cold tolerance through HbEIN3-HbICE2 regulatory module in Hevea brasiliensis. Plant Physiol. Biochem. 219, 109397. doi: 10.1016/j.plaphy.2024.109397
Zhang, W., Wang, B., Liu, B., Chen, Z., Lu, G., Ge, Y., et al. (2022). Trait selection for yield improvement in foxtail millet (Setaria italica Beauv.) under climate change in the North China plain. Agronomy 12, 1500. doi: 10.3390/agronomy12071500
Zhang, H., Wang, Y.-Y., Zhao, B., Zhang, L.-L., Qie, Q.-R., Han, Y.-H., et al. (2023). Identification of co-expression genes related to cold stress in foxtail millet by WGCNA. Journal of Agricultural Science & Technology. 25, (10), 22–34. doi: 10.13304/j.nykjdb.2023.0108
Zhang, R., Xi, X., Chen, X., Wang, Y., and Zhou, M. (2024). Comparing time-series transcriptomes between chilling-resistant and -susceptible rice reveals potential transcription factors responding to chilling stress. Front. Plant Sci. 15, 1451403. doi: 10.3389/fpls.2024.1451403
Zhao, X., Ma, K., Li, Z., Li, W., Zhang, X., Liu, S., et al. (2023). Transcriptome Analysis Reveals Brassinolide Signaling Pathway Control of Foxtail Millet Seedling Starch and sucrose metabolism under freezing stress, with implications for growth and development. Int. J. Mol. Sci. 24, 11590. doi: 10.3390/ijms241411590
Zhao, C., Zhang, Z., Xie, S., Si, T., Li, Y., and Zhu, J. K. (2016). Mutational evidence for the critical role of CBF transcription factors in cold acclimation in arabidopsis. Plant Physiol. 171, 2744–2759. doi: 10.1104/pp.16.00533
Keywords: foxtail millet, cold stress, SiCST1, SiOFP1, BR
Citation: Yang F, Qiao J, Zhang X, Zhang Z and Huo D (2025) SiCST1, a novel plant-specific protein of foxtail millet, confers cold stress tolerance in plants. Front. Plant Sci. 16:1618053. doi: 10.3389/fpls.2025.1618053
Received: 25 April 2025; Accepted: 21 July 2025;
Published: 05 August 2025.
Edited by:
Darren Wong, Australian National University, AustraliaReviewed by:
Maria Helena S. Goldman, University of São Paulo, BrazilSaurabh Pandey, Indira Gandhi Krishi Vishwavidyalaya, India
Copyright © 2025 Yang, Qiao, Zhang, Zhang and Huo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongao Huo, ZG9uZ2FvaHVvQDE2My5jb20=
 Fan Yang
Fan Yang Jiaqi Qiao1
Jiaqi Qiao1 Dongao Huo
Dongao Huo