- 1School of Life Sciences, Henan University, Kaifeng, Henan, China
- 2College of Environmental Science and Technology, Yangzhou University, Yangzhou, China
- 3Ningxia Technical College of Wine and Desertification Prevention, Yinchuan, Ningxia, China
Alfalfa (Medicago sativa L.), a vital perennial legume forage, has been widely cultivated owing to a variety of favorable characteristics, including comprehensive ecological resilience, superior nutritive value, digestibility, and nitrogen fixation capacity. The productivity traits of alfalfa, particularly its biomass yield and forage quality, are profoundly influenced by a range of abiotic stress conditions. As a common abiotic stress, drought adversely impacts growth and photosynthetic efficiency, accompanied by increased oxidative damage and stomatal closure as a mechanism to minimize water loss; meanwhile, transgenic approaches have been employed to enhance drought resilience by improving antioxidant activity and water-use efficiency. Salinity stress disturbs ionic balance, resulting in sodium (Na+) toxicity and the generation of oxidative damage; however, alfalfa cultivars exhibit salinity tolerance through mechanisms such as Na+ exclusion, K+ retention, activation of antioxidant defenses, hormonal regulation, and the upregulation of stress-responsive genes. In addition, heavy metals pose a significant challenge to alfalfa production, as they impair plant development and disrupt symbiotic nitrogen fixation, but recent studies have highlighted the potential of microbial-assisted phytoremediation in mitigating these detrimental effects. By integrating recent findings, this review highlights the intricate physiological, biochemical, and molecular mechanisms involved in alfalfa’s responses to key abiotic stressors specifically drought, salinity, and heavy metal toxicity. Breakthroughs in genetic modification, notably the development of transgenic lines exhibiting altered expression of stress-responsive genes, offer valuable potential for improving stress resilience. Future research should employ omics approaches, advanced gene-editing and de novo gene synthesis to target key regulatory elements responsible for stress adaptation.
1 Introduction
Alfalfa (Medicago sativa L.) is a perennial legume forage that belongs to the subfamily Papilionoideae (Turki and Hegazy, 2021; Steier et al., 2022). Cultivated alfalfa is a cross-pollinated crop and is tetraploid in nature (Hawkins and Yu, 2018). Southwestern Asia is the origin of alfalfa whereas Iran is regarded as its geographic center for this crop (Wang and Şakiroğlu, 2021). In Europe and some other countries, this crop is also called “Lucerne” (Baxevanos et al., 2022). Due to its nutritional value such as high protein content, minerals, carbohydrates, vitamin A, B, C, D and E, it is well known as a staple crop for both humans and animals (Baker et al., 2019; Mattioli et al., 2019; Michalczyk et al., 2019), as depicted in (Figure 1). It is one of the oldest plants that was cultivated around 3,300 years ago only for forage purposes with livestock (Michaud et al., 2015). It also acts as a source of essential nutrients, including proteins, vitamins, carbohydrates, and minerals (Hao et al., 2008; Gao et al., 2021). On a dry matter basis, it contains nearly 15 to 22 percent crude proteins along with macro- and trace elements with all the fat- and water-soluble vitamins (Scholtz, 2008).
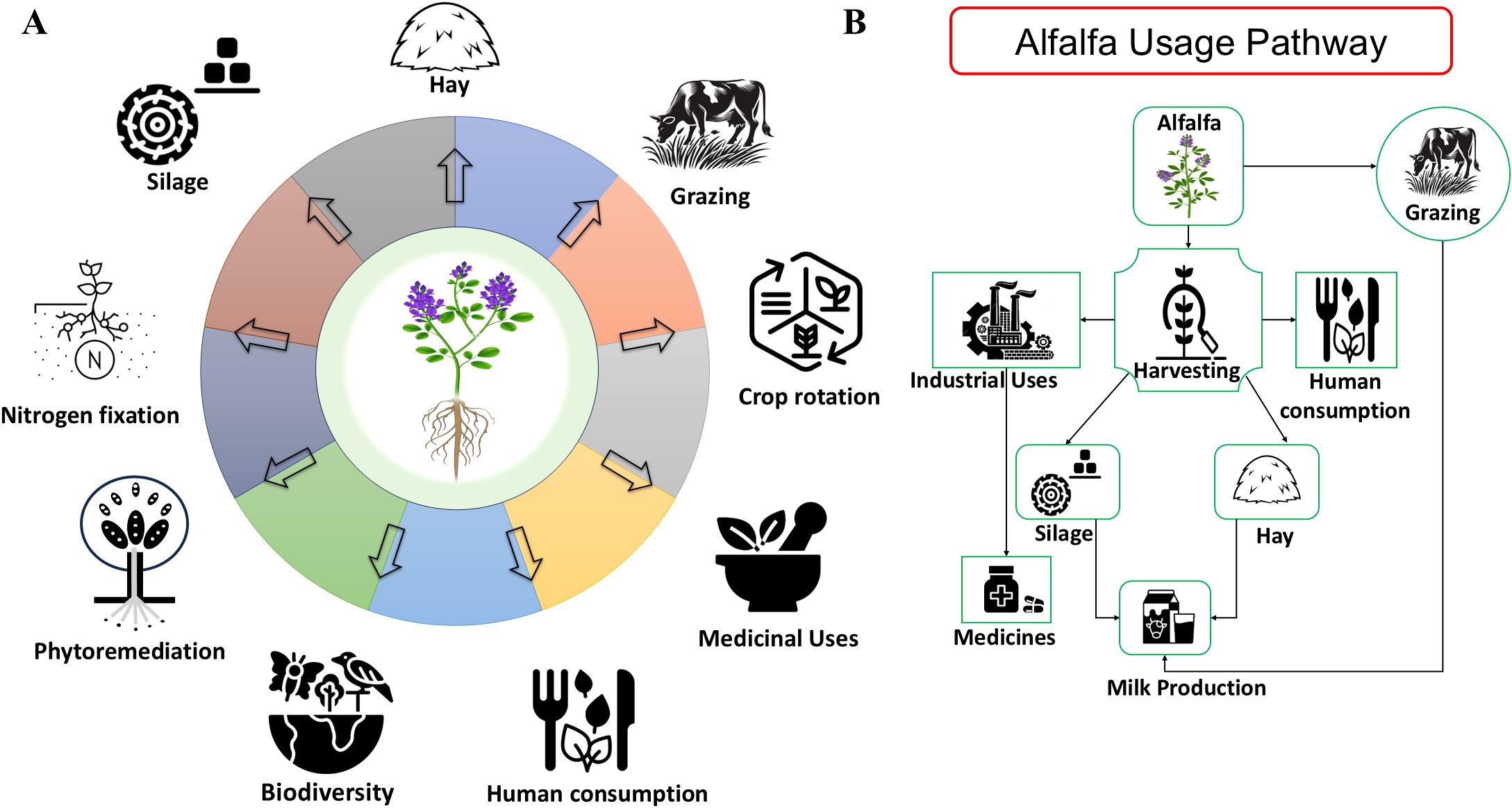
Figure 1. Economic importance of alfalfa. (A) Various uses of alfalfa in agriculture, industry, and animal feed. (B) Pathways of alfalfa utilization from production to end use.
Alfalfa can also be cultivated in a variety of soil types including poor nutrient soils (Lei et al., 2017). There are many advantages of alfalfa in crop rotation, such as the capability to improve the fertility of soil by nitrogen fixation, as depicted in (Figure 1); interestingly, it is found that alfalfa accumulates Nitrogen in large quantities, ranging 300–400 kg/ha/year (Kelner et al., 1997; Angus and Peoples, 2012). Approximately 165 kg/ha of Nitrogen accumulates in the roots and crown, which can be used as a fertilizer for subsequent crops in the same field (Rasse et al., 1999). Generally, this crop is grown for making hay and silage, but because of its high yield and quality of nutrition, it is also used for grazing purposes (Michael and Chandan, 2023), illustrated in (Figure 1). In some Chinese and Hindu societies, doctors recommend young leaves of alfalfa for the cure of some disorders such as water retention, arthritis, and digestive tract (Vaibhavi and Devang, 2024). Proper management of alfalfa fields at both local and landscape levels is crucial to maintain the services of the ecosystem, including those dependent on functional biodiversity, and conservation of threatened species (Julier et al., 2017), as shown in (Figure 1). Alfalfa can also be used in various recipes including: cooked salad, pudding, souffle’, puree saute’, soup, tea, tortilla, and croquettes (Martínez et al., 2016; Apostol et al., 2017). Some farmers in China regard it as a type of vegetable (Zong et al., 2023) (Djordjević et al., 2024), concluded that alfalfa was used to enhance the mineral, protein, vitamin, and dietary fiber content in wheat flour.
Alfalfa cultivation is profoundly influenced by a range of environmental factors, encompassing both biotic and abiotic stressors, which are responsible for reduction in crop productivity (Wang et al., 2023). Considering the importance of alfalfa, agricultural scientists are paying attention to its cultivation under stressful conditions (Stritzler et al., 2018; Annicchiarico et al., 2022). Drought tolerance in alfalfa is relatively high as compared to that of other forage crops, as alfalfa has a deep root system which ranges from 1.5 to 4m (Huang et al., 2018; Han et al., 2020). Due to the robust rooting system of alfalfa, it regrows successfully (Ali et al., 2021). Alfalfa has been noted to be more drought resistant than other grain legumes (Huang et al., 2018; Han et al., 2020). Drought stress remains a major constraint on alfalfa cultivation, as global temperature rise, evapotranspiration is expected to increase, ultimately worsening drought conditions in arid and semi-arid regions worldwide (Huang et al., 2018). Irmak et al. (2007), concluded that rate of evapotranspiration generally ranges from 0.10 to 0.35 inches per day in alfalfa crops, this level of evapotranspiration supports deep root distribution and high yield (Zhu et al., 2016). Various studies explained drought conditions and their responses through morphologically, physiologically, and biochemically as shown in (Table 1).
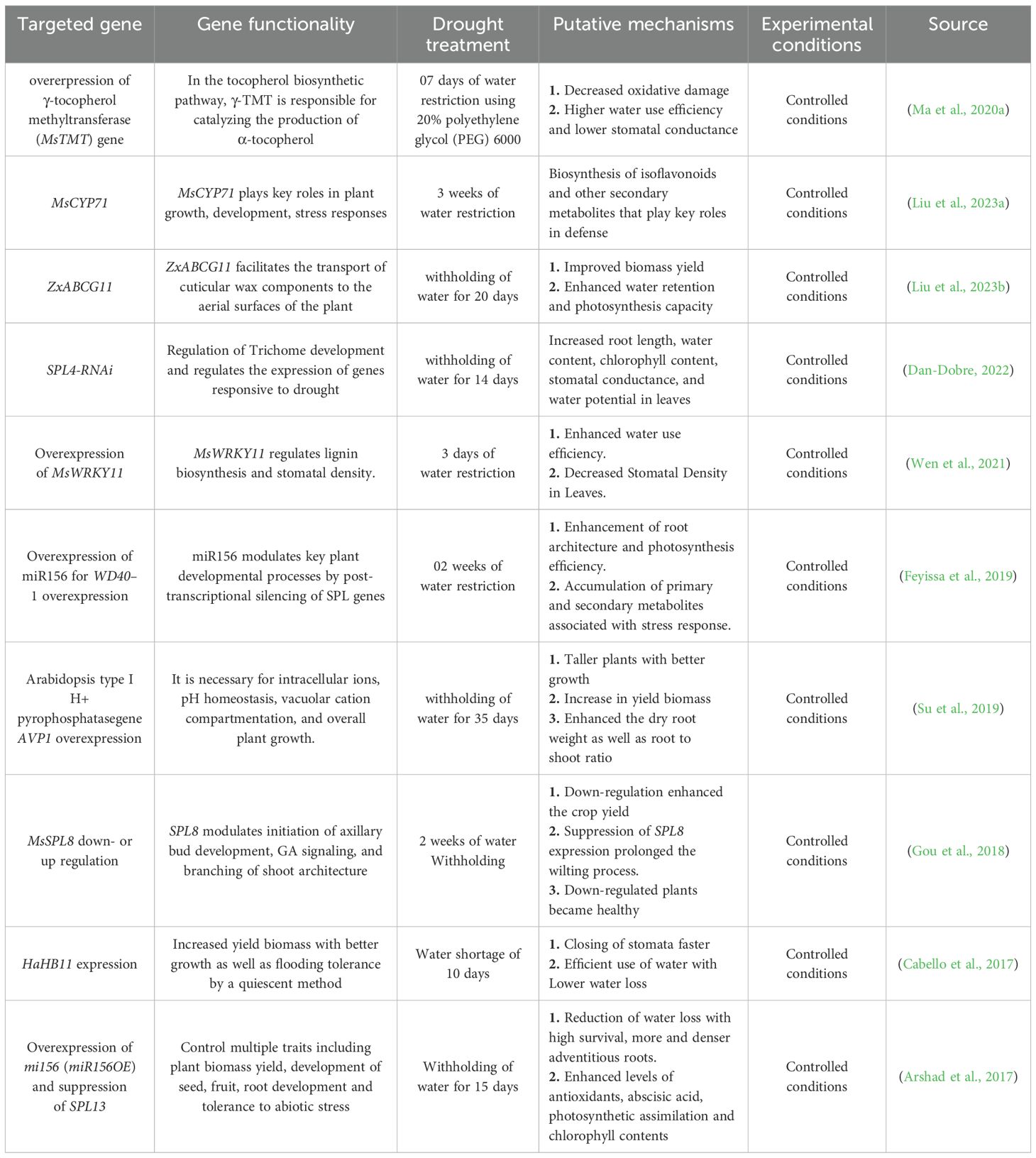
Table 1. Morphological, physiological, and molecular characterization of drought stress tolerance in transgenic and conventional alfalfa.
Several researchers have developed a range of transgenic alfalfa cultivars with enhanced drought tolerance, achieved through the introduction of one or more genes from a single species into another using genetic engineering techniques, including Agrobacterium-mediated transformation or direct gene transfer methods (Gao et al., 2024). Ma et al. (2020a) found that alfalfa resistance to drought stress was improved by the overexpression of the γ-tocopherol methyltransferase gene by alleviated oxidative damage, maintained high water-use efficiency or by lowered the stomatal conductance. Silencing of SPL13 and overexpression of miR156 allowed the alfalfa to become tolerant against drought stress (Arshad et al., 2017), while (Feyissa et al., 2019) successfully used moderate expression of miR156, which improved the ability of alfalfa to withstand drought through WD40–1 overexpression.
Excessive salt accumulation in the soil is also a major limiting factor for crop productivity (Yadav et al., 2019; Hao et al., 2021). As saline soil contains an excess of soluble salts including calcium, sodium, magnesium, chloride, potassium, and sulfate in their root zones, as a result plants fail to absorb nutrients and water from the soil and causes plant injury (MaChado and Serralheiro, 2017; Behdad et al., 2021). Interestingly, alfalfa is also considered a moderately salt-tolerant legume crop (Bertrand et al., 2015). Usingconventional breeding techniques, different cultivars of alfalfa have been developed with salt tolerance (Sandhu et al., 2017); however, attaining salt tolerance in this crop through genetic engineering is very difficult, moreover the response is genetically and physiologically complex against the salt stress because multiple genes are used in controlling salt tolerance including both physiological and biological mechanisms (Smethurst et al., 2008; Hrbáčková et al., 2020). Comprehending salt resistance pathways and detecting genetic traits suitable for evaluating improved salinity tolerance, play a vital role in alfalfa breeding programs (Kaundal et al., 2021). It is essential to identify the genes responsible for salt tolerance in alfalfa crops for the development of molecular markers, precise screening and advancements in plant breeding and genetics (Bhattarai et al., 2021). Recent technologies used in alfalfa salt stress research include RNA-Seq analysis, salt-resistant breeding, and cutting-edge Synchrotron beamlines (Peng et al., 2025).
Heavy metals stress is also a significant concern to discuss after drought and salt stress (Anwar et al., 2021). Human industrialization and agricultural activities lead to environmental contamination and ultimately affecting plant quality and biomass, so it is very important to study the contaminants that are harmful to plants growth (Raza Altaf et al., 2021; Adil et al., 2024a; Ahamad et al., 2024; Razzaq et al., 2024b, 2024a). All harmful substances released into the biosphere have an impact on different types of living organisms, including plants (Migda et al., 2024). These toxic substances (heavy metals) create problems not only for plant health but also for soil integrity. The use of contaminated crops for food and feed, poses threats to human health globally (Bandyopadhyay et al., 2015; Agnello et al., 2016; Gan et al., 2020). As a leguminous plant, alfalfa forms a symbiotic relationship with Gram-negative soil bacteria of the genus Rhizobium, both experience detrimental effects due to the presence of heavy metals (HMs) because HMs reduce the symbiotic capacity and ultimately the capacity of alfalfa to fix nitrogen (Li et al., 2014). Recent work done on protection of plants and environment, focused on mitigating the detrimental impact of pollution on plants and soil; however, it has led to the emergence of a relatively recent approach known as stress mitigation, which involves applying external phytochemicals and microbial agents to enhance plant homeostasis or make the plant tolerant against different stresses caused by environment (Jócsák et al., 2022). Overall, this review provides an in-depth understanding of how alfalfa responds at physiological, biochemical, and molecular levels to major abiotic stress factors, specifically drought, salinity, and heavy metal exposure, aiming to support the development of stress-resilient cultivars and guide improved cultivation strategies under such stressful conditions.
2 Drought stress
2.1 Effect of drought stress on alfalfa growth
A decline in water supply restricts the plant’s nutrient uptake, leading to slower growth and reductions in various growth parameters such as plant height, biomass accumulation (fresh and dry weight), branching intensity, leaf production per plant, leaf area, cell wall thickness in leaves, stomatal density, cutinization of leaf surface, formation of defective vascular tissue—as well as premature leaf senescence (Singh et al., 2021; Zia et al., 2021; Adil et al., 2022b, 2022c, 2024b, 2024c). Partial closing of stomata has been noted an early response to water scarcity to reduce water loss through transpiration, however, it also limits photosynthesis and carbon assimilation (Devi and Reddy, 2020). The reduction in transpiration rate due to stomatal closure can improve the water-use efficiency but it negatively affects the transport and uptake of nutrients (Ranawana et al., 2021). Many studies on drought stress have demonstrated that stomatal closure can significantly lessen the negative effects of drought stress in alfalfa crops (Arshad et al., 2017; Luo et al., 2022). In response to water scarcity conditions, non-stomatal mechanisms may include decreased carboxylation enzyme activity, a decline in ATP (adenosine triphosphate) production, and structural damage to the photosynthetic system (Zhang et al., 2019).
The results indicated that drought stress negatively affected alfalfa plants by reducing morphological growth (by 12 to 54%), gas exchange efficiency (by 37 to 88%), and chlorophyll content (Chl a and Chl b declined by 29% and 40%, respectively), along with reducing mineral content; furthermore, it increased lipid peroxidation by 69% and increased the accumulation of reactive oxygen species (ROS) (Roy et al., 2021). The findings also revealed that plants experiencing drought stress exhibit decreased plasma membrane permeability and stomatal conductance while limiting malondialdehyde accumulation, and increasing proline levels and related hormones, which ultimately strengthens their drought resistance (Yasmin et al., 2021, 2022). The experiment demonstrated that increasing drought stress in alfalfa plants resulted in a significant rise in H2O2, O2-, and malondialdehyde levels by 323%, 247%, and 235% respectively, while the enzymatic activities of superoxide dismutase (SOD), catalase (CAT), and Ascorbate Peroxidase (APX) also increased by 18.01%, 15.56%, and 587% under 15% PEG (polyethylene glycol-6000) treatment (Chen et al., 2021). Further research concluded that drought stress in alfalfa plants led to variations in hormone levels such as (Gibberellin (GA3), Zeatin (ZA), Abscisic acid (ABA), indole-3-acetic acid (IAA) levels, where GA3, ZA, and the GA3/ABA ratio reached their highest levels under moderate stress, whereas IAA and IAA/ABA dropped significantly under severe stress, accompanied by an increase in ABA (Wang et al., 2024).
2.2 The molecular mechanisms of drought tolerance in alfalfa
Efforts to increase alfalfa stress tolerance under varying growing conditions has recently focused on physiological responses, metabolic activities, morphological adaptions, and genetic modification (Song et al., 2019). Alfalfa demonstrates superior drought resistance over many forages as a result of its deep-penetrating roots (1.5–4.0 m) (Huang et al., 2018; Han et al., 2020). Although chlorophyll content and the rate photosynthesis decline under water scarcity conditions although maintaining chlorophyll under such conditions is associated with better drought resilience (Rokebul Anower et al., 2017). It has been proposed that enhanced stomatal conductance and restricted water loss during drought contribute to the maintenance of higher chlorophyll content, thereby reinforcing drought tolerance in plants (Zheng et al., 2017; Iqbal and Yaning, 2024). Studies suggest that the enhanced drought resilience of alfalfa is closely associated with the accumulation of both organic and inorganic osmolytes (Shanker et al., 2014). Among these, Proline is one of the most well-studied osmolytes in plants like alfalfa, owing to its essential function in preserving leaf relative water content under low water potential, thereby boosting drought resilience (Ni et al., 2012). Legume plants experience a decline in both nodule formation and biological nitrogen fixation under drought conditions (Dollete et al., 2024). Therefore, sustained nitrogen fixation under water-deficit conditions has been linked to increased drought resilience in plants (Xu et al., 2012). Plant breeding approaches, including both traditional methods and genetic engineering, have exhibited considerable potential in strengthening plant tolerance against abiotic challenges (Villalobos-López et al., 2022).
A Numerous genes have the potential to encode transcriptional regulators, including zinc finger proteins (Tang et al., 2013) and NAC transcription factors (Min et al., 2020) associated with stress responses (Figure 2). Certain compounds such as proline, glycinebetaine, LEA proteins, abscisic acid and other anti-oxidants are synthesized and over expressed to maintain osmotic balance and protect the structural integrity of the cell under drought stress (Banerjee and Roychoudhury, 2016). Moreover, certain families like MAPK, CDPK, and antioxidants can be a direct or indirect target to enhance drought tolerance (Puri, 2019), (Figure 2). Overexpression of PEPcase, pyruvate orthophosphate dikinase (PPDK), NADP-malic enzyme (NADP-ME), and NADP-malate dehydrogenase (NADP-MDH) from Medicago sativa L. enhanced alfalfa tolerance by increasing photosynthetic efficiency and promoting nodule formation (Luo et al., 2024). To enhance drought stress tolerance more effectively, numerous transgenic alfalfa plants with enhanced resilience have been developed by various scientists, as shown in (Table 1). Overexpression of the γ-tocopherol methyltransferase gene showed greater alfalfa drought resistance by mitigating oxidative stress, inducing the accumulation of osmoregulatory compounds, modulating stomatal conductance, and optimizing water use efficiency in comparison to untreated plants (Ma et al., 2020a). Studies found that overexpressing miR156 and suppressing SPL13 can effectively enhance drought stress tolerance in alfalfa (Arshad et al., 2017). Research also concluded that a moderate expression of miR156 contributed to alfalfa drought resistance through the upregulating of WD40–1 (Feyissa et al., 2019). The study demonstrated that overexpression of MsNTF2L (M. sativa NUCLEAR TRANSPORT FACTOR 2-LIKE) is a key regulator of drought tolerance in alfalfa; furthermore, scientists determined that it enhanced drought resistance by promoting ROS scavenging, decreasing stomatal density, improving stomatal closure in response to ABA, and increasing the accumulation of epicuticular wax crystals (Luo et al., 2022). Overexpressing MsTHI1 (Medicago sativa Thiamine Thiazole Synthase 1) improved drought resistance through enhanced levels of vitamin B1, chlorophyll a (Chl a), chlorophyll b (Chl b), enhanced antioxidant activity, photosynthetic efficiency, signal transduction, and the activation of stress-related genes (Yin et al., 2022). Additionally, it is found that the bacterial strain DGL1 enhanced alfalfa’s drought resistance through the production of extracellular polysaccharides, deaminase, and solubilizing phosphorus (Yang et al., 2024).
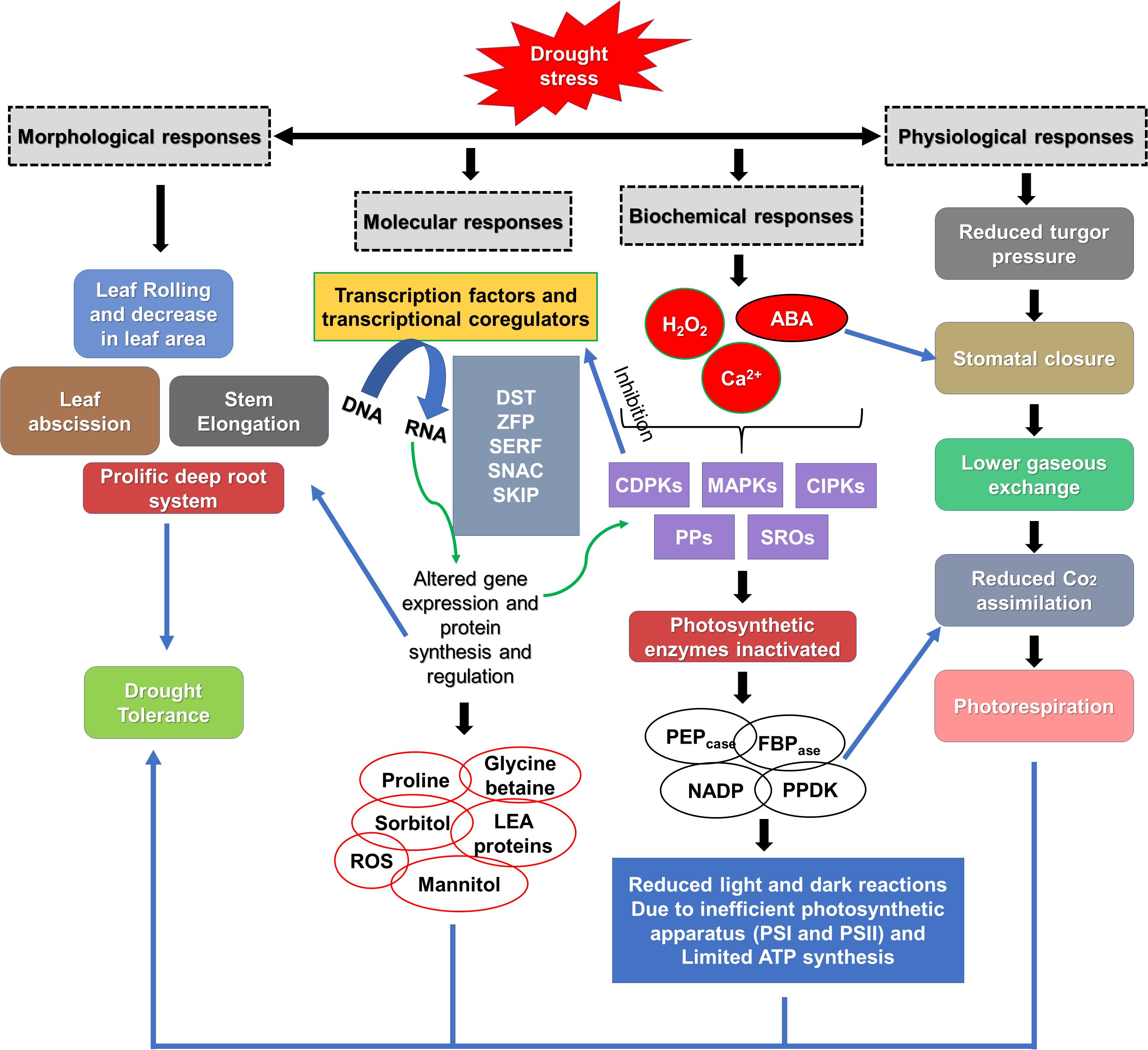
Figure 2. Plant mechanisms to overcome drought conditions. SERF, serum response factor; DST, drought and salt tolerant; SKIP, Ski-interacting protein; ZFP, zinc finger transcription factor; SNAC, stress responsive NAC transcription factor; LEA, late embryogenesis abundant proteins; ROS, reactive oxygen species; SROs, similar to RCD-ONE; CDPKs, calcium dependent protein kinases; CIPKs, CBL interacting protein kinases; PPs, protein phosphatases; MAPKs, mitogen activated protein kinases; ABA, abscisic acid; NADP-ME, NADP malic enzyme; PEPcase, phosphoenol pyruvate carboxylase; PPDKs, pyruvate phosphate dikinases; FBPase, fructose 1,6-bisphosphatase. Preproduced from Zargar et al. (2017) Copyright 2017 Elsevier.
3 Toxicological effects of heavy metals on alfalfa’s growth patterns
3.1 Influence of essential heavy metals on alfalfa growth
Zinc (Zn): High concentration of Zn results in leaf chlorosis, inhibition of growth and reduction in photosynthetic rate due to Zn toxicity (Reddy and Kumari, 2022) (Bandyopadhyay et al., 2015), concluded that an excess level of Zn (750 mg/kg soil) accumulatesin the root zones, approximately 300–400 mg kg-1 DW. Research conducted by (Ibekwe et al., 1996), stated that treatment with 4–7.3 mM Zn after 10 days of exposure resulted in chlorotic symptoms with poor root development and necrotic spots. Yahaghi et al. (2019), showed that Zn treatment with 1.5–24 mM Zn affected the rate of germination.
Manganese (Mn): (Li et al., 2019), investigated the symptoms of Mn toxicity as interveinal chlorosis found in mature leaves, roots browning, nutrient uptake disruption, necrotic spots found in mature leaves. (Sale et al., 1992) summarized the effect of Mn toxicity on alfalfa with approximately (60 mg L-1) resulted 20% less dry weight as compared to plants in control (Gherardi and Rengel, 2003), summarized the Mn symptoms with 500 mg g-1 and noticed a reduction in roots as well as shoots of alfalfa plants.
Nickel (Ni): Nickel is considered an essential heavy metal because of its presence in glyoxalase enzymes, the porphyrin compound F430, peptide deformylases, and because it acts as a central metal atom of some hydrogenases and superoxide dismutases (Dixon et al., 1975). Alfalfa is capable of absorbing nickel, after sixty days of exposure with Ni (0, 50, 150, 250, and 500 mg kg-1) resulted an increase in MDA levels and the activities of glutathione-S-transferase (GST) and peroxidase (POX); whereas GST, phytochelatin synthase (PCs) and Prx1C were also upregulated in roots and shoots of alfalfa (Helaoui et al., 2020) (Table 2).
Copper (Cu): Diazotrophic bacteria are restricted to grow and reproduce due to the presence of copper, which is responsible for the fixation of nitrogen in alfalfa plants (Sharaff and Archana, 2016). when copper is present in high amounts, it accumulates in the stem apoplasts of plant, then it influences the properties of cell wall and ultimately affects the alfalfa quality; furthermore, it leads to a reduction in ion concentration in alfalfa stems and reduces the concentration of ferritins—ubiquitous proteins that regulate the amounts of Fe in the redox state of cells (Strozycki et al., 2010).
3.2 Adverse impacts of non-essential heavy metals on alfalfa
Lead (Pb): Alfalfa plants exposed to pb, showed symptoms of chlorosis, reduced growth and reduction in photosynthetic rate (Yan et al., 2010) (Lopez et al., 2007), performed an experiment in alfalfa plants which were exposed with 40 mg/L of lead and concluded that activity of CAT decreases, but total amylase activity (TAA) increases in alfalfa leaves. (Hattab et al., 2016) conducted research to measure the amount of stress in alfalfa by applying Pb with 0, 10, and 100 mM for 2 and 7 days; furthermore, he observed a reduction in levels of homoglutathione (hGSH) as well as root glutathione (GSH). Research concluded that root growth and development were hindered by lead toxicity, disrupted the early stages of the legume-Rhizobium symbiotic relationship and affected the biochemical signaling involved (Besharati and Memar, 2017). The accumulation of heat shock proteins such as HSP70 and HSP17.7 was found to be higher in plant shoots, reflecting that lead toxicity triggered protective cellular responses against lead stress (Hattab et al., 2016).
Cadmium (Cd): In some crops the toxicity of Cd affects the uptake of water and nutrients (Andresen and Küpper, 2013). In alfalfa, Ca, Fe, Mg, and K contents were decreased by Cd concentrations at 3 and 5 mg/kg soil; furthermore, reduction was also reported in dry matter, root and shoot length (Dražić et al., 2006). It not only affects the process of germination, but also affects the growth of seedling after germination (Yahaghi et al., 2019). When Cd is exposed to alfalfa plant, it also affected the physiological, morphological functions as well as metabolism (Dražić et al., 2006; Haider et al., 2021). Cd also exhibits negative effects on photosynthesis, oxidative stress, root metabolism inhibition, and genotoxicity (Andresen and Küpper, 2013).
Chromium (Cr): Hexavalent chromium [Cr (VI)] exhibits solubility within the pH range of natural water, and can be found in irrigation water, it is considered a toxic metal for aquatic and terrestrial (Salmani Abyaneh and Fazaelipoor, 2016). Study concluded that hexavalent chromium [Cr (VI)] exposed at 5 and 10 mg L-1 K2Cr2O7, reduced the size of leaf, number of photosynthetic pigments, reduction of biomass, but increasing SOD, NO, H2O2, and CAT activities, which were partially maintained through the transcriptional regulation of Cu/ZnSOD, FeSOD, MnSOD and CAT genes (Christou et al., 2020) (Table 2).
Mercury (Hg): Mercury toxicity hinders the growth and development of alfalfa while also disrupting iron and sulfur balance and promoting oxidative stress (El-Shehawi et al., 2022). By applying Hg with a quantity of 4, 5, 10, 20, and 40 M along with an exposure of O2- and H2O2 generation in leaves of alfalfa plant and recorded increase in lipoxygenase (LOX), POD, NADH-oxidase, APX, and CAT activities (Zhou et al., 2008, 2009). Findings showed that mercury exposure in alfalfa plants resulted in increased lipid peroxidation, reduced chlorophyll levels, and impaired glutathione reductase (GR) activity in roots, as well as the production of a new root peroxidase isoform, reflecting redox imbalance (Sobrino-Plata et al., 2009).
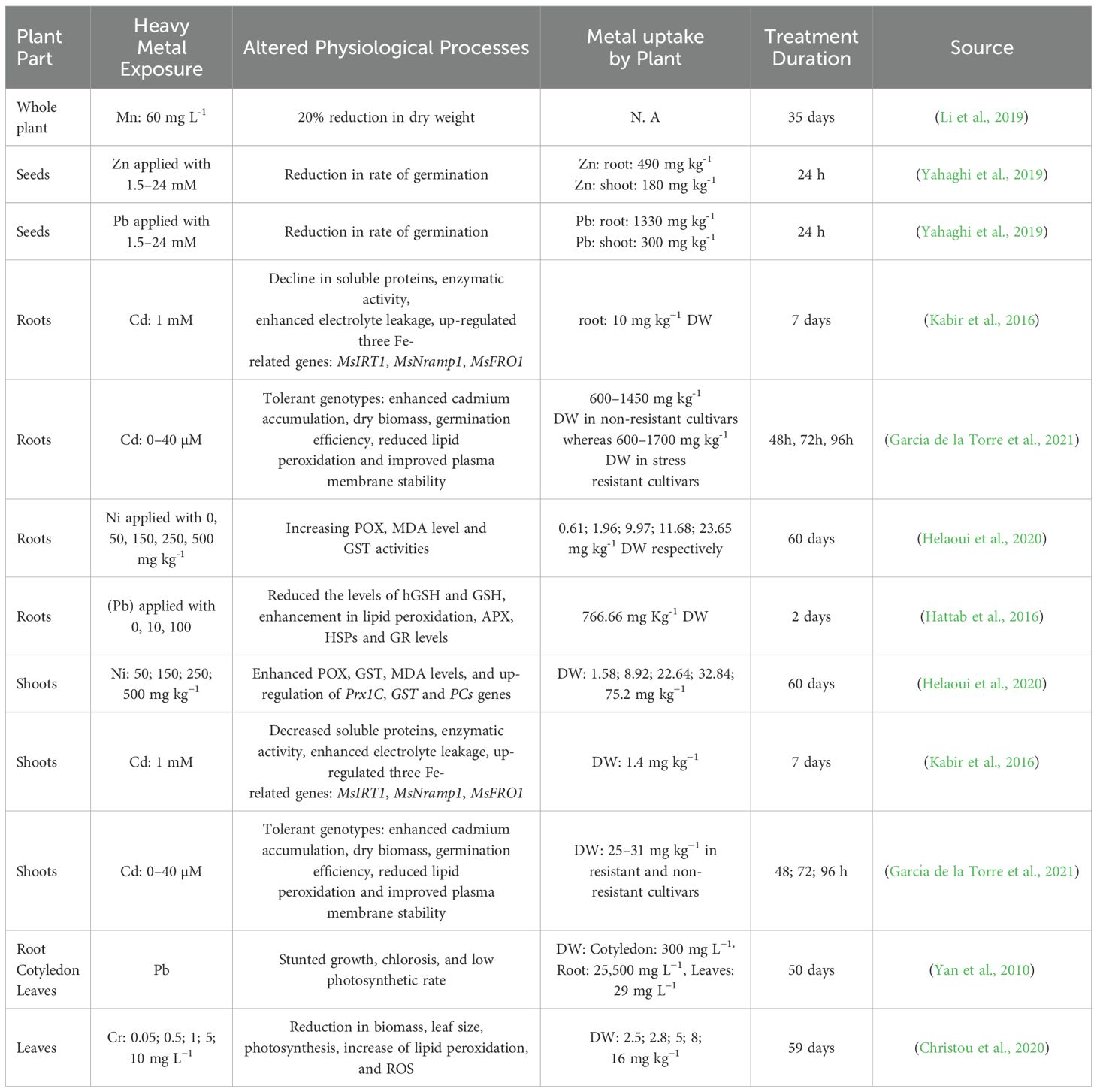
Table 2. Heavy metal exposure in alfalfa: affected parts, concentration levels, and duration of stress.
3.3 Heavy metals tolerance
The cellular redox balance under heavy metal stress is maintained through the prompt quenching of reactive oxygen species by a coordinated action of enzymatic (SOD: superoxide dismutase, CAT: catalase, APX: ascorbate peroxidase, GR: glutathione reductase, MDHAR: monodehydroascorbate reductase, DHAR: dehydroascorbate reductase, GPX: glutathione peroxidase, and glutathione-S transferase) as well as non-enzymatic (ascorbate, glutathione, proline, and α-tocopherol) antioxidant defense systems (Singh et al., 2016), (Figure 3). Glutathione (GSH), as a low-molecular-weight, water-soluble tripeptide (γ-Glu-Cys-Gly), functions as a critical component of the cellular defense system is crucial in mitigating the toxic effects of heavy metal exposure (Flores-Cáceres et al., 2015). Glutathione reductase (GR) efficiently catalyzes the conversion of oxidized glutathione (GSSG) back to its reduced form (GSH), and possesses a conserved disulfide linkage that is susceptible to disruption under metal-induced stress (Hajiboland et al., 2015), and contributes significantly to cellular defense by facilitating the reduction of GSSG, thereby sustaining a high GSH to GSSG ratio essential for redox homeostasis (Figure 3). Surprisingly, the role of arbuscular mycorrhizal (AM) fungi to tolerate and accumulate heavy metals including nickel, lead, cadmium, mercury, chromium, and arsenic has been widely recognized in scientific studies (Helaoui et al., 2020; Boorboori and Zhang, 2022). The study revealed that lead (Pb) stress hindered plant growth and disrupted photosynthesis, but the presence of AM fungi (Glomus intraradices) helped mitigate these harmful effects (Kahromi and Najafi, 2020). Results indicated that inoculating seedlings with the bacterial species (Sinorhizobium meliloti) alleviated growth suppression caused by copper stress and enhanced nitrogen uptake in seedlings, leading to an overall increase in plant nitrogen concentration (Chen et al., 2018; Duan et al., 2022). The application of silicon to plants under Cd stress significantly improved their morpho-physiological characteristics, increased total protein levels, and maintained membrane integrity, highlighting silicon’s crucial role in alleviating Cd-induced stress (Kabir et al., 2016). The NT27 isolate as a strain of Pseudomonas sp. significantly boosted Medicago sativa growth, increasing shoot dry weight (97.6%) and root dry weight (95.4%) under chromium stress; furthermore, it also enhanced chlorophyll content, reduced stress markers, and promoted Phytostabilization in plants (Tirry et al., 2021). Research revealed that plants inoculated with a Rhizobium tibeticum strain at a 0.005 mM Ni concentration led to a notable increase in nodule formation, root length, shoot length, and shoot dry mass compared to non-inoculated alfalfa plants under Nickel stress (Pešić et al., 2025).
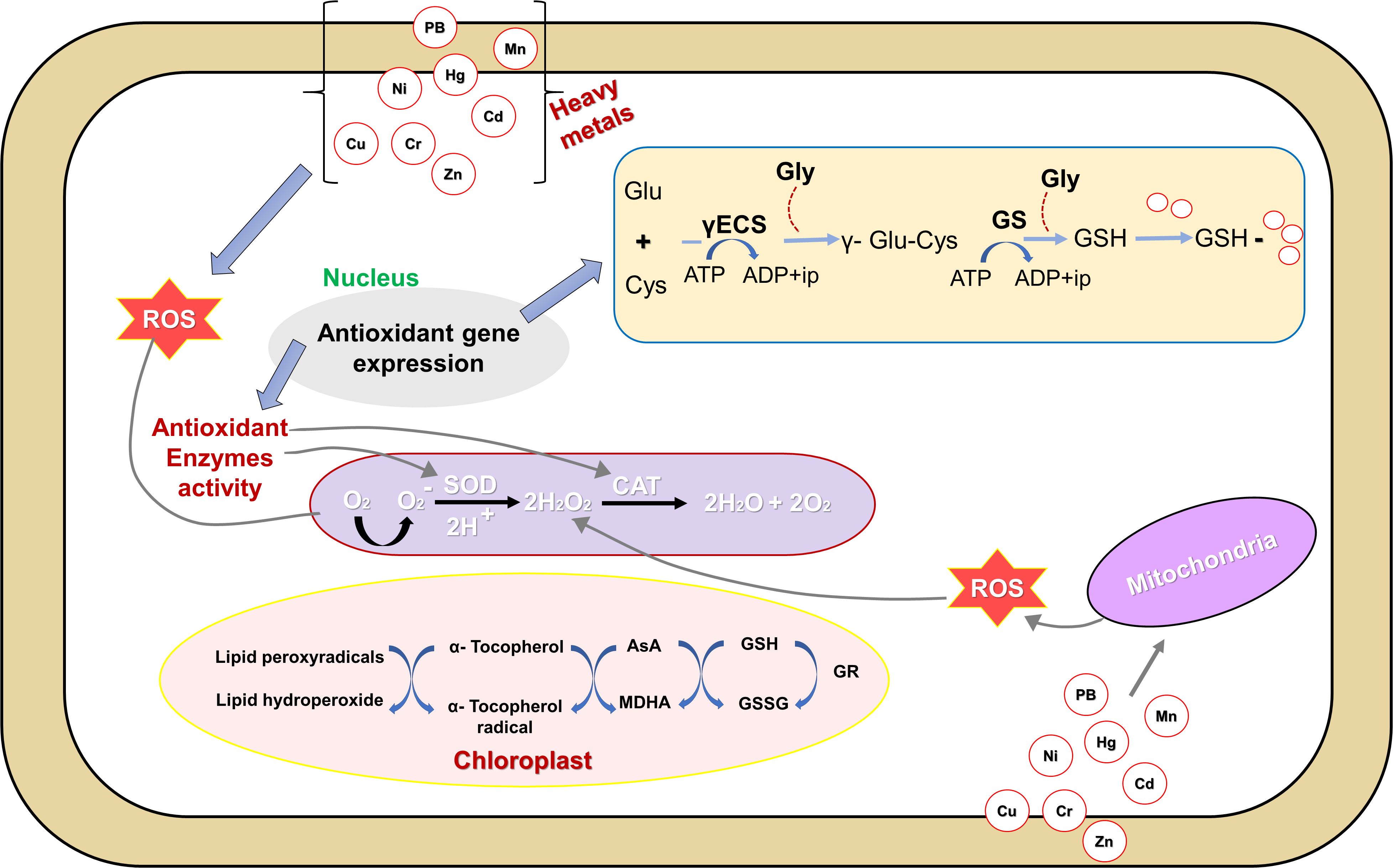
Figure 3. Mechanisms of oxidative stress, tolerance, and detoxification in plant cells under the exposure of heavy metals. ROS, reactive oxygen species; O− 2, superoxide radicals; O2, oxygen molecule; H2O2, hydrogen peroxide; CAT, catalase; SOD, superoxide dismutase; AsA, ascorbic acid; GSH, glutathione (reduced); MDHA, monodehydroascorbate; GSSG, oxidized glutathione; GR, glutathione reductase; Glu, glutamine; Cys, cysteine; GS, glutathione synthetase; Gly, glycine; Pb; Mn; Hg; Ni; Cd; Cu; Cr, Zn: Heavy metals. Reproduced from Singh et al. (2016) Copyright 2016 Frontiers Media SA.
4 Salt stress
4.1 Impact of salt stress on the growth and development of alfalfa
Excessive accumulation of soluble salts, including chloride, sulfate, and carbonate compounds of key cations like sodium, calcium, magnesium, and potassium, significantly disrupts the efficiency of water and nutrient acquisition by plants (Bhattarai et al., 2020; Liu and Wang, 2021). In severe conditions of salt stress, the nature of soil solution becomes hyper-osmotic, which is responsible for leading to water loss, as a result, plants experience wilting and premature senescence (An et al., 2016; Long et al., 2019). In early stages of osmotic stress due to the shortage of water in plant tissues, alfalfa plant reduces the growth of leaves and then decreases the development of shoot and reproductive growth (Farooq et al., 2015, 2017). Salt stress in alfalfa causes a decrease in rate of photosynthesis, as osmotic stress induces partial closure of stomata (Farooq et al., 2017). Absorption of sodium ions in the roots of alfalfa can be dangerous for its growth if present in cytosol at high concentrations (Assaha et al., 2017). High concentrations of sodium and chloride ions in the cytoplasm can disturb the cellular processes, also causes dehydration in cells as well as disturbs the process of photosynthesis (Munns and Tester, 2008; Ashraf and Harris, 2013). Increasing the ratio of cellular potassium to sodium as well as limiting the concentration of sodium in cytosol promotes salt tolerance in alfalfa cultivars (Sandhu et al., 2017). It is found that NaCl stress in alfalfa caused a significant increase in the activity levels of SOD, POD, CAT, and APX by 132.14%, 315.60%, 102.78%, and 27.61%, and a marked upregulation of two genes associated with salt stress (Chen et al., 2021).
Research has shown that stomatal opening can improve photosynthesis and biomass yield, but high concentrations of Na+ may cause stomatal closure, disrupting photosynthesis and causing an overproduction of reactive oxygen species (Kamran et al., 2020; Kimura et al., 2020; Adil et al., 2022a, 2023; Niu et al., 2022). In conditions of high salinity, Na+ in the apoplast surrounding the guard cells leading to stomatal closure (Kerstiens et al., 2002). A key mechanism to limit Na+ accumulation in the shoot is the reduction of transpiration rate by stomatal regulation (Yu and Assmann, 2016). The effect of Na2SO4 solution on alfalfa plants was studied from emergence to maturity, and reduction was recorded in relative emergence (%) at 12.7 dS m-1, with no survival of plants at 30 dSm-1 (Cornacchione and Suarez, 2015). The root growth of alfalfa is adversely less affected by salt stress as compared to that of shoot growth (Bertrand et al., 2020). Research was conducted on 15 populations of alfalfa under salt stress conditions, treated with a mixture of NaCl, Na2SO4, CaCl2 and MgSO4, and KCl, concluded that mass of root per plant at 18.4 dSm-1 and 24.5 ds m-1 electrical conductivity was decreased by 18% and 49% respectively whereas the recorded shoot mass reduction was nearly 50% and 73% (Cornacchione and Suarez, 2017). Alfalfa experienced a decline in biomass by 43%–86% and a 58%–91% decrease in nitrogen content; moreover, it negatively impacted nitrogen fixation and atmospheric nitrogen uptake by hindering nodule formation and decreased nitrogen fixation efficiency when salt levels surpassed 100 mmol Na2SO4 L-¹ (Wan et al., 2023). Salt (NaCl) applied at 9 dSm-1 reduced the size of leaf by 34%, mass of stem by 35% as well as height of plant by 32% respectively (Valizadeh et al., 2013).
4.2 Salt tolerance mechanisms
Salinity resistance in plant involves diverse mechanisms, such as production of osmolytes, stimulation of antioxidant defenses, acidification of the apoplast, ionic stability, and hormonal response regulation (Al-Farsi et al., 2020; Iqbal et al., 2023) (Table 3). Soil salinity interferes with ionic equilibrium in alfalfa, resulting in excessive buildup of Na+ and Cl– in both roots and shoots (Rogers et al., 2003; Li et al., 2010). Thus, maintaining ionic balance under salt stress is crucial for enhancing salinity tolerance in alfalfa, which is crucial for regulating cell volume, sustaining membrane potential, and supporting enzymatic activities (Amin et al., 2021). Salt stress disrupts hormone levels, impacting osmotic regulation and photosynthesis, which ultimately hinders legume growth (Farooq et al., 2017). Primary plant hormones, namely auxins, gibberellins, ethylene, cytokinins, and abscisic acid (ABA), act as crucial regulators engaging various developmental signaling pathways in plants (Agudelo-Morales et al., 2021). Modifications in ABA and ethylene signaling have been observed in response to salinity stress (Farooq et al., 2017). and they are vital for salt tolerance (Sah et al., 2016; Nykiel et al., 2023). Higher ABA levels under salt stress stimulate stress protein production and induce osmotic regulation, hereby enhancing salt tolerance (Chen et al., 2022). The exogenous use of osmolytes and phytohormonal agents may reduce salinity-related losses in alfalfa (Deinlein et al., 2014). The accumulation of compatible solutes under stress conditions contributes to osmotic tolerance by both regulating intercellular osmotic pressure and protecting membranes from ROS damage (Deinlein et al., 2014; Farooq et al., 2015).
The identification of salt-tolerant alfalfa lines has been extensively achieved by screening their resistance to salinity in various studies (Yu et al., 2021). Initiation of plant adaptation to saline conditions involves the early sensing of stress stimuli via molecular detectors like cyclic nucleotide-gated channels (CNGCs), autoinhibited calcium ATPases ACAs), as well as key regulators within the salt overly sensitive (SOS) network (Li et al., 2022), (Figure 4). Whereas the stress perception initiates signal transduction cascades, including salicylic acid and abscisic acid pathways, leading to the induction of multiple downstream genes and regulatory transcription factors (Bose and Howlader, 2020), (Figure 4). Latest findings demonstrated that several differentially expressed genes (DEGs) encode regulatory transcription factors such as DREB, NAC, WRKY, and MYB, that are believed to play a crucial role in transcriptional response to salinity stress in alfalfa (He et al., 2022), (Figure 4). Transcriptomic analysis revealed significant enrichment of heat shock proteins (HSPs), likely functioning within the MAPK signaling cascade, in salt-tolerant alfalfa, while marked upregulation of LEA family genes suggests their role in osmotic adjustment under salt (Hang et al., 2024), (Figure 4). Advances in molecular biology have made transgenic technology a popular and effective method for single trait improvement in plants, as compared to that of traditional breeding practices (Sun et al., 2024). For the development of salt-resistant alfalfa, the introduction of exogenous genes like the receptor kinase gene GsSRK (Sun et al., 2018), ZxNHX and ZxVP1-1 (Kang et al., 2016), thiamine thiazole synthase (THI1) gene MsTHI1 (Yin et al., 2022), Na+/H+ reverse transporter genes AtNHX1 (Stritzler et al., 2018), calcineurin B-like (CBL) gene MsCBL4 (An et al., 2020), and a rare cold-inducible 2/plasma membrane protein 3 (RCI2/PMP3) gene MsRCIs (Li et al., 2021) reported an improvement in salt tolerance in genetically modified alfalfa plants, aided by advancements in high-throughput sequencing and bioinformatics, coupled with transcriptomics, proteomics, and metabolomics has emphasized the vital role of transcription factors (TFs) (Ma et al., 2023; Zhang et al., 2023), metabolite biosynthesis and other abiotic genes related to stress resistance (Bhattarai et al., 2021; Kaundal et al., 2021), and miRNAs (Long et al., 2015; Ma et al., 2020b), are crucial for salt tolerance (Huang et al., 2020; Zhao et al., 2020). Compared to other crops, the mechanisms at the genetic, molecular, and physiological levels that confer salt resistance in alfalfa are still inadequately understood (He et al., 2022). Recent studies have shown that miR156 plays a key role in alfalfa’s response to salt stress by regulating the expression of target genes, including those coding for SPL protein family (Wang et al., 2021; Zhang et al., 2022a, 2022b, 2022c).
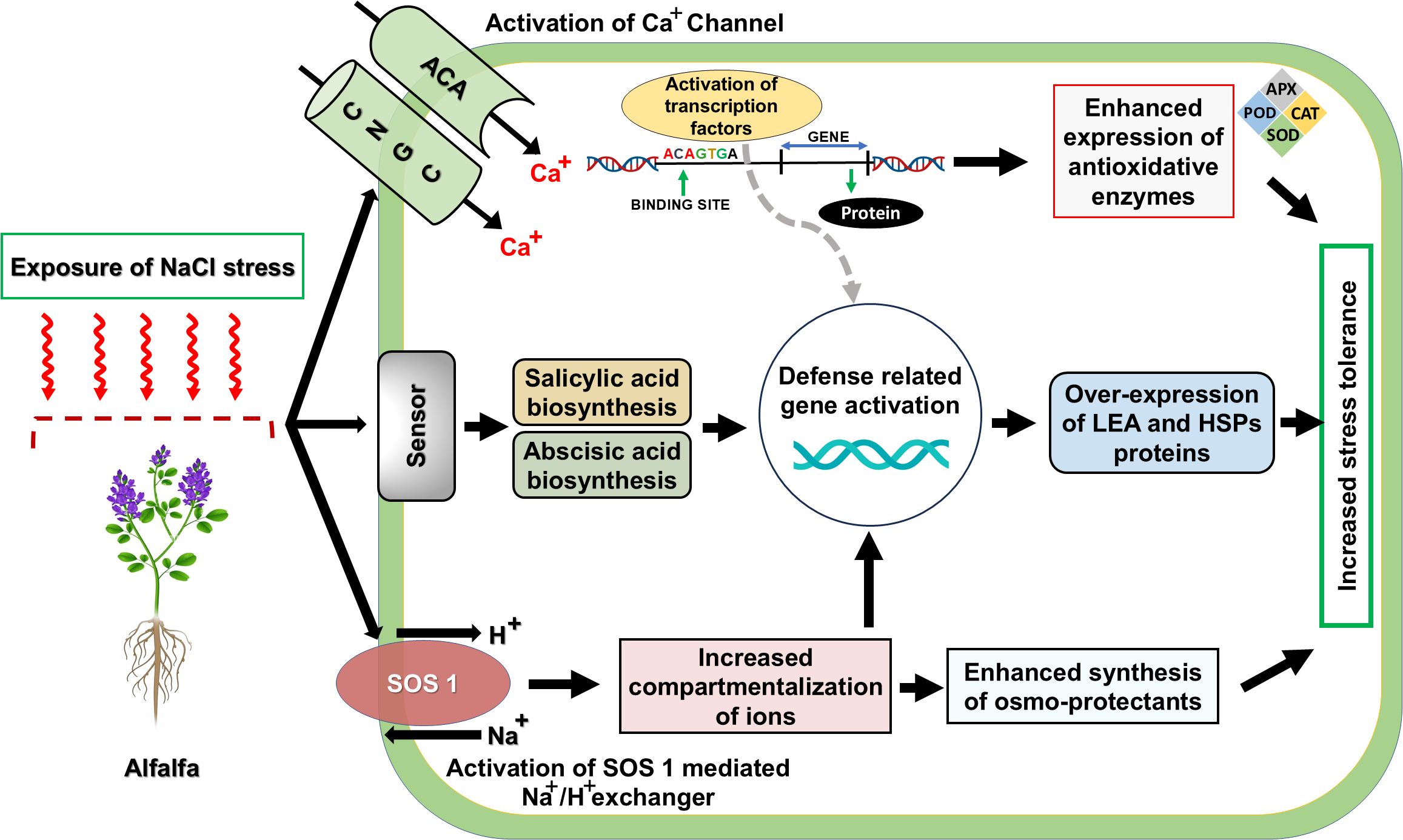
Figure 4. A Graphical Depiction of Plant Adaptations to Salinity Stress. CNGC: Cyclic nucleotide-gated channels, ACA, autoinhibited calcium ATPase; SOS, salt overly sensitive; Transcription factors (DREB; WRKY; NAC; MYB), Proteins (LEA, Late Embryogenesis Abundant proteins; HSPs, Heat Shock Proteins). Reproduced from Kashyap et al. (2021) Copyright 2021 Springer Nature.
5 Conclusion and future perspectives
Environmental fluctuations and abiotic stress factors significantly disrupt agricultural productivity and reduce crop quality worldwide. As a vital forage legume valued for its substantial biomass production and rich nutritional profile, alfalfa remains susceptible to yield declines under abiotic challenges such as salinity, drought, and metal toxicity. Under these stress conditions, alfalfa engages complex regulatory systems at both the physiological and molecular levels, leading to changes in cellular structure, biochemical pathways, and transcriptional regulation. Despite advancements in model plant systems, significant gaps persist in our understanding of alfalfa’s molecular adaptations to stress, primarily due to its complex genome and outcrossing reproductive behavior, which make it a challenging experimental subject. In this review, we consolidate existing insights into the physiological adjustments and molecular adaptations of alfalfa under salinity, drought, and heavy metal stress conditions. These abiotic challenges activate intricate signaling cascades initiated at the cell wall or plasma membrane level through the perception of phytohormones, ions, and gaseous signaling molecules, leading to the regulation of subsequent stress-response pathways.
Deciphering the mechanisms by which alfalfa responds to stress is vital for enhancing breeding strategies focused on generating cultivars with improved tolerance to multiple environmental constraints. In this regard, numerous studies have identified diverse mechanisms of stress tolerance under controlled conditions, although findings from open-field experiments are still insufficient. Hence, to close this knowledge gap, implementing phenotyping at the cellular and tissue scale could offer new insights into how plants adapt to stress conditions, which can further boost our proficiency in designing stress-resistant cultivars of alfalfa. Furthermore, existing studies highlight those advancements in genetic transformation methods have led to the identification of several genes and signaling pathways related to stress resilience and adaptation. Nonetheless, considerable gaps persist regarding the identification of key genes, number of genes to target and understanding their specific involvement in regulating plant responses to stress. There is still an ongoing debate over whether all key genes are linked to particular stress conditions or if targeting a selective set of genes is a more effective strategy. This highlights the need for more comprehensive studies and cutting-edge approaches, such as single-cell omics, to pinpoint precise genetic elements crucial for enhancing stress tolerance in alfalfa.
No single strategy will suffice as plants often face multiple stresses simultaneously in natural environments. Thus, future studies should aim to examine the interactive impacts of multiple tolerance strategies and identify key genetic and biochemical pathways that can be targeted for breeding. Moving beyond conventional studies, we anticipate that a comprehensive approach combining genomics, bioinformatics, and functional genomics, focusing on the study of protein-nucleic acid interactions and gene regulation, is also crucial for exploring alfalfa’s genetic framework. These investigations will reveal new genes that can be linked to targeted traits, providing valuable insights to support the genetic enhancement of alfalfa. In addition, the application of modern molecular tools will play a vital role in advancing genetic manipulation methods including gene overexpression, precise gene editing, and de novo synthesis of genes for specific traits to strengthen stress adaptability and productivity.
Author contributions
MD: Conceptualization, Validation, Visualization, Writing – original draft, Writing – review & editing. SX: Conceptualization, Supervision, Writing – review & editing. HQ: Visualization, Writing – original draft, Writing – review & editing. XH: Visualization, Writing – review & editing. MA: Visualization, Writing – review & editing. YL: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was financially supported by the Ministry of Education's Cultivation Program for Master Teachers (Master Artisans) and Distinguished Principals of Vocational Schools in the New Era (2023–2025) (205), and the Natural Science Foundation of Ningxia (2024AAC03363).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adil, M., Bashir, S., Bashir, S., Aslam, Z., Ahmad, N., Younas, T., et al. (2022a). Zinc oxide nanoparticles improved chlorophyll contents, physical parameters, and wheat yield under salt stress. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.932861
Adil, M., Gul, I., Lv, F., Li, T., Chen, Y., Lu, H., et al. (2024a). Microbial strategies for lead remediation in agricultural soils and wastewater: mechanisms, applications, and future directions. Front. Microbiol. 15. doi: 10.3389/fmicb.2024.1434921
Adil, M., Lu, S., Yao, Z., Zhang, C., Lu, H., Bashir, S., et al. (2024b). No-tillage enhances soil water storage, grain yield and water use efficiency in dryland wheat (Triticum aestivum) and maize (Zea mays) cropping systems: a global meta-analysis. Funct. Plant Biol. 51. doi: 10.1071/FP23267
Adil, M., Lv, F., Cao, L., Lu, H., Lu, S., Gul, I., et al. (2024c). Long-term effects of agronomic practices on winter wheat yield and NUE in dryland regions of USA and China: a long-term meta-analysis. Sci. Rep. 14, 24777. doi: 10.1038/s41598-024-74910-7
Adil, M., Shah, A. N., Khan, A. N., Younas, T., Mehmood, M. S., Mahmood, A., et al. (2023). Amelioration of harmful effects of soil salinity in plants through silicon application: a review. Pakistan J. Bot. 55, 9–18. doi: 10.30848/PJB2023-1(24
Adil, M., Yao, Z., Zhang, C., Lu, S., Fu, S., Mosa, W. F. A., et al. (2022b). Climate change stress alleviation through nature based solutions: A global perspective. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.1007222
Adil, M., Zhang, S., Wang, J., Shah, A. N., Tanveer, M., and Fiaz, S. (2022c). Effects of fallow management practices on soil water, crop yield and water use efficiency in winter wheat monoculture system: A meta-analysis. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.825309
Agnello, A. C., Huguenot, D., van Hullebusch, E. D., and Esposito, G. (2016). Citric acid- and Tween® 80-assisted phytoremediation of a co-contaminated soil: alfalfa (Medicago sativa L.) performance and remediation potential. Environ. Sci. pollut. Res. 23, 9215–9226. doi: 10.1007/s11356-015-5972-7
Agudelo-Morales, C. E., Lerma, T. A., Martínez, J. M., Palencia, M., and Combatt, E. M. (2021). Phytohormones and plant growth regulators - A review. J. Sci. Technological Appl. 10, 27–65. doi: 10.34294/j.jsta.21.10.66
Ahamad, M. I., Yao, Z., Ren, L., Zhang, C., Li, T., Lu, H., et al. (2024). Impact of heavy metals on aquatic life and human health: a case study of River Ravi Pakistan. Front. Mar. Sci. 11. doi: 10.3389/fmars.2024.1374835
Al-Farsi, S. M., Nawaz, A., Anees-Ur-Rehman, Nadaf, S. K., Al-Sadi, A. M., Siddique, K. H. M., et al. (2020). Effects, tolerance mechanisms and management of salt stress in lucerne (Medicago sativa). Crop Pasture Sci. 71, 411–428. doi: 10.1071/CP20033
Ali, G., Wang, Z., Li, X., Jin, N., Chu, H., and Jing, L. (2021). Deep soil water deficit and recovery in alfalfa fields of the Loess Plateau of China. Field Crops Res. 260. doi: 10.1016/j.fcr.2020.107990
Amin, I., Rasool, S., Mir, M. A., Wani, W., Masoodi, K. Z., and Ahmad, P. (2021). Ion homeostasis for salinity tolerance in plants: a molecular approach. Physiologia Plantarum 171, 578–594. doi: 10.1111/ppl.13185
An, Y. M., Song, L. L., Liu, Y. R., Shu, Y. J., and Guo, C. H. (2016). De novo transcriptional analysis of alfalfa in response to saline-alkaline stress. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.00931
An, Y., Yang, X. X., Zhang, L., Zhang, J., Du, B., Yao, L., et al. (2020). Alfalfa MsCBL4 enhances calcium metabolism but not sodium transport in transgenic tobacco under salt and saline–alkali stress. Plant Cell Rep. 39, 997–1011. doi: 10.1007/s00299-020-02543-x
Andresen, E. and Küpper, H. (2013). “Cadmium toxicity in plants,” in Metal Ions in Life Sciences, 395–413. doi: 10.1007/978-94-007-5179-8_13
Angus, J. F. and Peoples, M. B. (2012). Nitrogen from Australian dryland pastures. Crop Pasture Sci. 63, 746–758. doi: 10.1071/CP12161
Annicchiarico, P., Nazzicari, N., Bouizgaren, A., Hayek, T., Laouar, M., Cornacchione, M., et al. (2022). Alfalfa genomic selection for different stress-prone growing regions. Plant Genome 15. doi: 10.1002/tpg2.20264
Anwar, K., Joshi, R., Dhankher, O. P., Singla-Pareek, S. L., and Pareek, A. (2021). Elucidating the response of crop plants towards individual, combined and sequentially occurring abiotic stresses. Int. J. Mol. Sci. 22. doi: 10.3390/ijms22116119
Apostol, L., Iorga, S., Mosoiu, C., Racovita, R. C., Niculae, O. M., and Vlasceanu, G. (2017). Alfalfa concentrate – a rich source of nutrients for use in food products. Int. Sci. Publications 5, 66–73. Available online at: https://www.scientific-publications.net/en/article/1001395/.
Arshad, M., Feyissa, B. A., Amyot, L., Aung, B., and Hannoufa, A. (2017). MicroRNA156 improves drought stress tolerance in alfalfa (Medicago sativa) by silencing SPL13. Plant Sci. 258, 122–136. doi: 10.1016/j.plantsci.2017.01.018
Ashraf, M. and Harris, P. J. C. (2013). Photosynthesis under stressful environments: An overview. Photosynthetica 51, 163–190. doi: 10.1007/s11099-013-0021-6
Assaha, D. V. M., Ueda, A., Saneoka, H., Al-Yahyai, R., and Yaish, M. W. (2017). The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front. Physiol. 8. doi: 10.3389/fphys.2017.00509
Baker, K. A., Beecher, L., and Northcutt, J. K. (2019). Effect of irrigation water source and post-harvest washing treatment on the microflora of alfalfa and mung bean sprouts. Food Control 100, 151–157. doi: 10.1016/j.foodcont.2019.01.015
Bandyopadhyay, S., Plascencia-Villa, G., Mukherjee, A., Rico, C. M., José-Yacamán, M., Peralta-Videa, J. R., et al. (2015). Comparative phytotoxicity of ZnO NPs, bulk ZnO, and ionic zinc onto the alfalfa plants symbiotically associated with Sinorhizobium meliloti in soil. Sci. Total Environ. 515, 60–69. doi: 10.1016/j.scitotenv.2015.02.014
Banerjee, A. and Roychoudhury, A. (2016). Group II late embryogenesis abundant (LEA) proteins: structural and functional aspects in plant abiotic stress. Plant Growth Regul. 79, 1–17. doi: 10.1007/s10725-015-0113-3
Baxevanos, D., Voulgari, O., Pankou, C., Yiakoulaki, M. D., and Tsialtas, I. T. (2022). Comparing adaptive responses of new and old lucerne (Medicago sativa) genotypes under irrigated Mediterranean conditions. Crop Pasture Sci. 73, 679–691. doi: 10.1071/CP21234
Behdad, A., Mohsenzadeh, S., and Azizi, M. (2021). Growth, leaf gas exchange and physiological parameters of two Glycyrrhiza glabra L. populations subjected to salt stress condition. Rhizosphere 17. doi: 10.1016/j.rhisph.2021.100319
Bertrand, A., Dhont, C., Bipfubusa, M., Chalifour, F. P., Drouin, P., and Beauchamp, C. J. (2015). Improving salt stress responses of the symbiosis in alfalfa using salt-tolerant cultivar and rhizobial strain. Appl. Soil Ecol. 87, 108–117. doi: 10.1016/j.apsoil.2014.11.008
Bertrand, A., Gatzke, C., Bipfubusa, M., Lévesque, V., Chalifour, F. P., Claessens, A., et al. (2020). Physiological and biochemical responses to salt stress of alfalfa populations selected for salinity tolerance and grown in symbiosis with salt-tolerant rhizobium. Agronomy 10. doi: 10.3390/agronomy10040569
Besharati, H. and Memar Kouche-Bagh, S. (2017). Effect of lead pollution stress on biological nitrogen fixation of alfalfa plant. Environ. Stresses Crop Sci., 10, 163-171. doi: 10.22077/escs.2017.539
Bhattarai, S., Biswas, D., Fu, Y. B., and Biligetu, B. (2020). Morphological, physiological, and genetic responses to salt stress in alfalfa: A review. Agronomy 10. doi: 10.3390/agronomy10040577
Bhattarai, S., Fu, Y. B., Coulman, B., Tanino, K., Karunakaran, C., and Biligetu, B. (2021). Transcriptomic analysis of differentially expressed genes in leaves and roots of two alfalfa (Medicago sativa L.) cultivars with different salt tolerance. BMC Plant Biol. 21. doi: 10.1186/s12870-021-03201-4
Boorboori, M. R. and Zhang, H. Y. (2022). Arbuscular mycorrhizal fungi are an influential factor in improving the phytoremediation of arsenic, cadmium, lead, and chromium. J. Fungi 8. doi: 10.3390/jof8020176
Bose, S. K. and Howlader, P. (2020). Melatonin plays multifunctional role in horticultural crops against environmental stresses: A review. Environ. Exp. Bot. 176. doi: 10.1016/j.envexpbot.2020.104063
Cabello, J. V., Giacomelli, J. I., Gómez, M. C., and Chan, R. L. (2017). The sunflower transcription factor HaHB11 confers tolerance to water deficit and salinity to transgenic Arabidopsis and alfalfa plants. J. Biotechnol. 257, 35–46. doi: 10.1016/j.jbiotec.2016.11.017
Chen, Z., Guo, H., Sui, C., Gao, Z., Wang, T., Luo, Y., et al. (2021). Effects of drought and salt stress on activities of antioxidant protective enzymes and expression of stress genes in alfalfa (Medicago sativa L.) seedlings. J. Biobased Materials Bioenergy 15, 553–558. doi: 10.1166/jbmb.2021.2083
Chen, J., Liu, Y. Q., Yan, X. W., Wei, G. H., Zhang, J. H., and Fang, L. C. (2018). Rhizobium inoculation enhances copper tolerance by affecting copper uptake and regulating the ascorbate-glutathione cycle and phytochelatin biosynthesis-related gene expression in Medicago sativa seedlings. Ecotoxicology Environ. Saf. 162, 312–323. doi: 10.1016/j.ecoenv.2018.07.001
Chen, G., Zheng, D., Feng, N., Zhou, H., Mu, D., Zhao, L., et al. (2022). Physiological mechanisms of ABA-induced salinity tolerance in leaves and roots of rice. Sci. Rep. 12. doi: 10.1038/s41598-022-11408-0
Christou, A., Georgiadou, E. C., Zissimos, A. M., Christoforou, I. C., Christofi, C., Neocleous, D., et al. (2020). Hexavalent chromium leads to differential hormetic or damaging effects in alfalfa (Medicago sativa L.) plants in a concentration-dependent manner by regulating nitro-oxidative and proline metabolism. Environ. pollut. 267. doi: 10.1016/j.envpol.2020.115379
Cornacchione, M. V. and Suarez, D. L. (2015). Emergence, forage production, and ion relations of alfalfa in response to saline waters. Crop Sci. 55, 444–457. doi: 10.2135/cropsci2014.01.0062
Cornacchione, M. V. and Suarez, D. L. (2017). Evaluation of alfalfa (Medicago sativa L.) populations’ response to salinity stress. Crop Sci. 57, 137–150. doi: 10.2135/cropsci2016.05.0371
Dan-Dobre, M. A. (2022). Characterization of SPL4’s Role in Drought Stress and Trichome Development in Alfalfa (Canada: Master’s Thesis, The University of Western Ontario).
Deinlein, U., Stephan, A. B., Horie, T., Luo, W., Xu, G., and Schroeder, J. I. (2014). Plant salt-tolerance mechanisms. Trends Plant Sci. 19, 371–379. doi: 10.1016/j.tplants.2014.02.001
Devi, M. J. and Reddy, V. R. (2020). Stomatal closure response to soil drying at different vapor pressure deficit conditions in maize. Plant Physiol. Biochem. 154, 714–722. doi: 10.1016/j.plaphy.2020.07.023
Dixon, N. E., Carlo, G., Blakeley, R. L., and Zerner, B. (1975). A metalloenzyme. A simple biological role for nickel? J. Am. Chem. Soc. 97. doi: 10.1021/ja00847a045
Djordjević, M., Spychaj, R., Pejcz, E., Djordjević, M., Šereš, Z., Šoronja-Simović, D., et al. (2024). Alfalfa seeds potential in enhancing wheat flour nutritional composition, rheological properties and technological quality of resulting standard and sourdough bread. Eur. Food Res. Technol. 250, 2515–2528. doi: 10.1007/s00217-024-04554-4
Dollete, D., Lumactud, R. A., Carlyle, C. N., Szczyglowski, K., Hill, B., and Thilakarathna, M. S. (2024). Effect of drought stress on symbiotic nitrogen fixation, soil nitrogen availability and soil microbial diversity in forage legumes. Plant Soil 495, 445–467. doi: 10.1007/s11104-023-06348-1
Dražić, G., Mihailović, N., and Lojić, M. (2006). Cadmium accumulation in Medicago sativa seedlings treated with salicylic acid. Biol. Plantarum 50, 239–244. doi: 10.1007/s10535-006-0013-5
Duan, C., Mei, Y., Wang, Q., Wang, Y., Li, Q., Hong, M., et al. (2022). Rhizobium inoculation enhances the resistance of alfalfa and microbial characteristics in copper-contaminated soil. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.781831
El-Shehawi, A. M., Rahman, M. A., Elseehy, M. M., and Kabir, A. H. (2022). Mercury toxicity causes iron and sulfur deficiencies along with oxidative injuries in alfalfa (Medicago sativa). Plant Biosyst. 156, 284–291. doi: 10.1080/11263504.2021.1985005
Farooq, M., Gogoi, N., Hussain, M., Barthakur, S., Paul, S., Bharadwaj, N., et al. (2017). Effects, tolerance mechanisms and management of salt stress in grain legumes. Plant Physiol. Biochem. 118, 199–217. doi: 10.1016/j.plaphy.2017.06.020
Farooq, M., Hussain, M., Wakeel, A., and Siddique, K. H. M. (2015). Salt stress in maize: effects, resistance mechanisms, and management. A review. Agron. Sustain. Dev. 35, 461–481. doi: 10.1007/s13593-015-0287-0
Feyissa, B. A., Arshad, M., Gruber, M. Y., Kohalmi, S. E., and Hannoufa, A. (2019). The interplay between miR156/SPL13 and DFR/WD40–1 regulate drought tolerance in alfalfa. BMC Plant Biol. 19. doi: 10.1186/s12870-019-2059-5
Flores-Cáceres, M. L., Hattab, S., Hattab, S., Boussetta, H., Banni, M., and Hernández, L. E. (2015). Specific mechanisms of tolerance to copper and cadmium are compromised by a limited concentration of glutathione in alfalfa plants. Plant Sci. 233, 165–173. doi: 10.1016/j.plantsci.2015.01.013
Gan, C., Chen, T., and Yang, J.y. (2020). Remediation of vanadium contaminated soil by alfalfa (Medicago sativa L.) combined with vanadium-resistant bacterial strain. Environ. Technol. Innovation 20. doi: 10.1016/j.eti.2020.101090
Gao, R., Wang, B., Jia, T., Luo, Y., and Yu, Z. (2021). Effects of different carbohydrate sources on Alfalfa Silage quality at different ensiling days. Agric. (Switzerland) 11, 1–13. doi: 10.3390/agriculture11010058
Gao, Y., Zhang, Y., Wu, H. Z., Min, X., Zhang, B., Kim, D. S., et al. (2024). Optimization and establishment of Agrobacterium-mediated transformation in alfalfa (Medicago sativa L.) using eGFP as a visual reporter. Plant Cell Tissue Organ Culture 156. doi: 10.1007/s11240-023-02659-4
García de la Torre, V. S., Coba de la Peña, T., Pueyo, J. J., and Lucas, M. M. (2021). Cadmium-tolerant and -sensitive cultivars identified by screening of Medicago truncatula germplasm display contrasting responses to cadmium stress. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.595001
Gherardi, M. J. and Rengel, Z. (2003). Genotypes of lucerne (Medicago sativa L.) show differential tolerance to manganese deficiency and toxicity when grown in bauxite residue sand. Plant Soil 249, 287–296. doi: 10.1023/A:1022872524844
Gou, J., Debnath, S., Sun, L., Flanagan, A., Tang, Y., Jiang, Q., et al. (2018). From model to crop: functional characterization of SPL8 in M. truncatula led to genetic improvement of biomass yield and abiotic stress tolerance in alfalfa. Plant Biotechnol. J. 16, 951–962. doi: 10.1111/pbi.12841
Haider, F. U., Liqun, C., Coulter, J. A., Cheema, S. A., Wu, J., Zhang, R., et al. (2021). Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicology Environ. Saf. 211. doi: 10.1016/j.ecoenv.2020.111887
Hajiboland, R., Rahmat, S., Aliasgharzad, N., and Hartikainen, H. (2015). Selenium-induced enhancement in carbohydrate metabolism in nodulated alfalfa (Medicago sativa L.) as related to the glutathione redox state. Soil Sci. Plant Nutr. 61, 676–687. doi: 10.1080/00380768.2015.1032181
Han, E., Dresbøll, D. B., and Thorup-Kristensen, K. (2020). Core-labelling technique (CLT): A novel combination of the ingrowth-core method and tracer technique for deep root study. Plant Methods 16. doi: 10.1186/s13007-020-00622-4
Hang, J., Song, T., Zhang, L., Hou, W., Liu, X., and Ma, D. (2024). Comparative transcriptomic and proteomic analyses of two salt-tolerant alfalfa (Medicago sativa L.) genotypes: investigation of the mechanisms underlying tolerance to salt. Front. Plant Sci. 15. doi: 10.3389/fpls.2024.1442963
Hao, C.c., Wang, L.j., Li, D., Özkan, N., Wang, D.c., Chen, X. D., et al. (2008). Influence of alfalfa powder concentration and granularity on rheological properties of alfalfa-wheat dough. J. Food Eng. 89, 137–141. doi: 10.1016/j.jfoodeng.2008.04.011
Hao, S., Wang, Y., Yan, Y., Liu, Y., Wang, J., and Chen, S. (2021). A review on plant responses to salt stress and their mechanisms of salt resistance. Horticulturae 7. doi: 10.3390/horticulturae7060132
Hattab, S., Hattab, S., Flores-Casseres, M. L., Boussetta, H., Doumas, P., Hernandez, L. E., et al. (2016). Characterisation of lead-induced stress molecular biomarkers in Medicago sativa plants. Environ. Exp. Bot. 123, 1–12. doi: 10.1016/j.envexpbot.2015.10.005
Hawkins, C. and Yu, L. X. (2018). Recent progress in alfalfa (Medicago sativa L.) genomics and genomic selection. Crop J. 6, 565–575. doi: 10.1016/j.cj.2018.01.006
He, F., Wei, C., Zhang, Y., Long, R., Li, M., Wang, Z., et al. (2022). Genome-wide association analysis coupled with transcriptome analysis reveals candidate genes related to salt stress in alfalfa (Medicago sativa L.). Front. Plant Sci. 12. doi: 10.3389/fpls.2021.826584
Helaoui, S., Boughattas, I., Hattab, S., Mkhinini, M., Alphonse, V., Livet, A., et al. (2020). Physiological, biochemical and transcriptomic responses of Medicago sativa to nickel exposure. Chemosphere 249. doi: 10.1016/j.chemosphere.2020.126121
Hrbáčková, M., Dvořák, P., Takáč, T., Tichá, M., Luptovčiak, I., Šamajová, O., et al. (2020). Biotechnological perspectives of omics and genetic engineering methods in alfalfa. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.00592
Huang, Z., Liu, Y., Cui, Z., Fang, Y., He, H., Liu, B. R., et al. (2018). Soil water storage deficit of alfalfa (Medicago sativa) grasslands along ages in arid area (China). Field Crops Res. 221, 1–6. doi: 10.1016/j.fcr.2018.02.013
Huang, L., Wu, D.z., and Zhang, G.p. (2020). Advances in studies on ion transporters involved in salt tolerance and breeding crop cultivars with high salt tolerance. J. Zhejiang University: Sci. B 21, 426–441. doi: 10.1631/jzus.B1900510
Ibekwe, A. M., Angle, J. S., Chaney, R. L., and van Berkum, P. (1996). Zinc and cadmium toxicity to alfalfa and its microsymbiont. J. Environ. Qual. 25, 1032–1040. doi: 10.2134/jeq1996.00472425002500050015x
Iqbal, H. and Yaning, C. (2024). Redox priming could be an appropriate technique to minimize drought-induced adversities in quinoa. Front. Plant Sci. 15. doi: 10.3389/fpls.2024.1253677
Iqbal, H., Yaning, C., Waqas, M., Raza, S. T., Shareef, M., and Ahmad, Z. (2023). Salinity and exogenous H2O2 improve gas exchange, osmoregulation, and antioxidant metabolism in quinoa under drought stress. Physiologia Plantarum 175. doi: 10.1111/ppl.14057
Irmak, S., Hay, D. R., Anderson, B. E., Kranz, W. L., and Yonts, C. D. (2007). Irrigation Management and Crop Characteristics of Alfalfa, G1778 Vol. 4 (Lincoln, United States: University of Nebraska-Lincoln Extension).
Jócsák, I., Knolmajer, B., Szarvas, M., Rabnecz, G., and Pál-Fám, F. (2022). Literature Review on the Effects of Heavy Metal Stress and Alleviating Possibilities through Exogenously Applied Agents in Alfalfa (Medicago sativa L.). Plants 11. doi: 10.3390/plants11162161
Julier, B., Gastal, F., Louarn, G., Badenhausser, I., Annicchiarico, P., Crocq, G., et al. (2017). Lucerne (Alfalfa) in European cropping systems. Legumes Cropping Syst., 168–192. doi: 10.1079/9781780644981.0168
Kabir, A. H., Hossain, M. M., Khatun, M. A., Mandal, A., and Haider, S. A. (2016). Role of silicon counteracting cadmium toxicity in Alfalfa (Medicago sativa L.). Front. Plant Sci. 7. doi: 10.3389/fpls.2016.01117
Kahromi, S. and Najafi, F. (2020). Growth and some physiological characteristics of alfalfa (Medicago sativa L.) in response to lead stress and Glomus intraradices symbiosis. J. Plant Process Funct. 9. Available online at: https://jispp.iut.ac.ir/article-1-1389-en.html
Kamran, M., Parveen, A., Ahmar, S., Malik, Z., Hussain, S., Chattha, M. S., et al. (2020). An overview of hazardous impacts of soil salinity in crops, tolerance mechanisms, and amelioration through selenium supplementation. Int. J. Mol. Sci. 21. doi: 10.3390/ijms21010148
Kang, P., Bao, A. K., Kumar, T., Pan, Y. Q., Bao, Z., Wang, F., et al. (2016). Assessment of stress tolerance, productivity, and forage quality in T1 transgenic Alfalfa co-overexpressing ZxNHX and ZxVP1–1 from Zygophyllum xanthoxylum. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.01598
Kashyap, S. P., Kumari, N., Mishra, P., Moharana, D. P., and Aamir, M. (2021). Tapping the potential of Solanum lycopersicum L. pertaining to salinity tolerance: perspectives and challenges. Genet. Resour. Crop Evol. 68, 2207–2233. doi: 10.1007/s10722-021-01174-9
Kaundal, R., Duhan, N., Acharya, B. R., Pudussery, M. V., Ferreira, J. F. S., Suarez, D. L., et al. (2021). Transcriptional profiling of two contrasting genotypes uncovers molecular mechanisms underlying salt tolerance in alfalfa. Sci. Rep. 11. doi: 10.1038/s41598-021-84461-w
Kelner, D. J., Vessey, J. K., and Entz, M. H. (1997). The nitrogen dynamics of 1-, 2- and 3-year stands of alfalfa in a cropping system. Agriculture Ecosyst. Environ. 64, 1–10. doi: 10.1016/S0167-8809(97)00019-4
Kerstiens, G., Tych, W., Robinson, M. F., and Mansfield, T. A. (2002). Sodium-related partial stomatal closure and salt tolerance of Aster tripolium. New Phytol. 153, 509–515. doi: 10.1046/j.0028-646X.2001.00330.x
Kimura, H., Hashimoto-Sugimoto, M., Iba, K., Terashima, I., and Yamori, W. (2020). Improved stomatal opening enhances photosynthetic rate and biomass production in fluctuating light. J. Exp. Bot. 71, 2339–2350. doi: 10.1093/jxb/eraa090
Lei, Y., Hannoufa, A., and Yu, P. (2017). The use of gene modification and advanced molecular structure analyses towards improving alfalfa forage. Int. J. Mol. Sci. 18. doi: 10.3390/ijms18020298
Li, J., Jia, Y., Dong, R., Huang, R., Liu, P., Li, X., et al. (2019). Advances in the mechanisms of plant tolerance to manganese toxicity. Int. J. Mol. Sci. 20. doi: 10.3390/ijms20205096
Li, Z., Ma, Z., Hao, X., Rensing, C., and Wei, G. (2014). Genes conferring copper resistance in Sinorhizobium meliloti CCNWSX0020 also promote the growth of Medicago lupulina in copper-contaminated soil. Appl. Environ. Microbiol. 80, 1961–1971. doi: 10.1128/AEM.03381-13
Li, R., Shi, F., Fukuda, K., and Yang, Y. (2010). Effects of salt and alkali stresses on germination, growth, photosynthesis and ion accumulation in alfalfa (Medicago sativa L.). Soil Sci. Plant Nutr. 56, 725–733. doi: 10.1111/j.1747-0765.2010.00506.x
Li, C., Song, T., Zhan, L., Cong, C., Xu, H., Dong, L., et al. (2021). Overexpression of MsRCI2A, MsRCI2B, and MsRCI2C in alfalfa (Medicago sativa L.) provides different extents of enhanced alkali and salt tolerance due to functional specialization of MsRCI2s. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.702195
Li, S., Wang, Y., Gao, X., Lan, J., and Fu, B. (2022). Comparative physiological and transcriptome analysis reveal the molecular mechanism of melatonin in regulating salt tolerance in alfalfa (Medicago sativa L.). Front. Plant Sci. 13. doi: 10.3389/fpls.2022.919177
Liu, L., Bao, A., Li, H., Bai, W., Liu, H., Tian, Y., et al. (2023b). Overexpression of ZxABCG11 from Zygophyllum xanthoxylum enhances tolerance to drought and heat in alfalfa by increasing cuticular wax deposition. Crop J. 11, 1140–1151. doi: 10.1016/j.cj.2022.11.007
Liu, J., Shi, K., Wang, S., Zhu, J., Wang, X., Hong, J., et al. (2023a). MsCYP71 is a positive regulator for drought resistance in alfalfa. Plant Physiol. Biochem. 203. doi: 10.1016/j.plaphy.2023.107999
Liu, L. and Wang, B. (2021). Protection of halophytes and their uses for cultivation of saline-alkali soil in China. Biology 10. doi: 10.3390/biology10050353
Long, R. C., Li, M. N., Kang, J. M., Zhang, T. J., Sun, Y., and Yang, Q. C. (2015). Small RNA deep sequencing identifies novel and salt-stress-regulated microRNAs from roots of Medicago sativa and Medicago truncatula. Physiologia Plantarum 154, 13–27. doi: 10.1111/ppl.12266
Long, R., Sun, H., Cao, C., Zhang, T., Kang, J., Wang, Z., et al. (2019). Identification of alkali-responsive proteins from early seedling stage of two contrasting Medicago species by iTRAQ-based quantitative proteomic analysis. Environ. Exp. Bot. 157, 26–34. doi: 10.1016/j.envexpbot.2018.09.021
Lopez, M., Peralta-Videa, J., Castillo-Michel, H., Martinez-Martinez, A., Duarte-Gardea, M., and Gardea-Torresdey, J. (2007). Lead toxicity in alfalfa plants exposed to phytohormones and ethylenediaminetetraacetic acid monitored by peroxidase, catalase, and amylase activities. Environ. Toxicol. Chem. preprint 1. doi: 10.1897/07-302
Luo, D., Liu, J., Wu, Y., Zhang, X., Zhou, Q., Fang, L., et al. (2022). NUCLEAR TRANSPORT FACTOR 2-LIKE improves drought tolerance by modulating leaf water loss in alfalfa (Medicago sativa L.). Plant J. 112, 429–450. doi: 10.1111/tpj.15955
Luo, Y., Wang, X., Zhang, D., Zhan, L., Li, D., Li, C., et al. (2024). Overexpression of phosphoenolpyruvate carboxylase kinase gene MsPPCK1 from Medicago sativa L. increased alkali tolerance of alfalfa by enhancing photosynthetic efficiency and promoting nodule development. Plant Physiol. Biochem. 213. doi: 10.1016/j.plaphy.2024.108764
Ma, L., Li, X., Zhang, J., Yi, D., Li, F., Wen, H., et al. (2023). MsWRKY33 increases alfalfa (Medicago sativa L.) salt stress tolerance through altering the ROS scavenger via activating MsERF5 transcription. Plant Cell Environ. 46, 3887–3901. doi: 10.1111/pce.14703
Ma, J., Qiu, D., Gao, H., Wen, H., Wu, Y., Pang, Y., et al. (2020a). Over-expression of a γ-tocopherol methyltransferase gene in vitamin e pathway confers PEG-simulated drought tolerance in alfalfa. BMC Plant Biol. 20. doi: 10.1186/s12870-020-02424-1
Ma, J., Wang, Y., and Li, J. (2020b). Global identification and analysis of micrornas involved in salt stress responses in two alfalfa (Medicago sativa millennium) Lines. Can. J. Plant Sci. 100, 445–455. doi: 10.1139/cjps-2018-0327
MaChado, R. M. A. and Serralheiro, R. P. (2017). Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae 3, 30. doi: 10.3390/horticulturae3020030
Martínez, R., Kapravelou, G., Porres, J. M., Melesio, A. M., Heras, L., Cantarero, S., et al. (2016). Medicago sativa L., a functional food to relieve hypertension and metabolic disorders in a spontaneously hypertensive rat model. J. Funct. Foods 26, 470–484. doi: 10.1016/j.jff.2016.08.013
Mattioli, S., Dal Bosco, A., Castellini, C., Falcinelli, B., Sileoni, V., Marconi, O., et al. (2019). Effect of heat- and freeze-drying treatments on phytochemical content and fatty acid profile of alfalfa and flax sprouts. J. Sci. Food Agric. 99, 4029–4035. doi: 10.1002/jsfa.9630
Michael, B. and Chandan, S. (2023). Management practices to offset the declining trend of alfalfa hay production. Int. J. Veterinary Sci. Res. 9, 018–026. doi: 10.17352/ijvsr.000133
Michalczyk, M., Fiutak, G., and Tarko, T. (2019). Effect of hot water treatment of seeds on quality indicators of alfalfa sprouts. Lwt 113. doi: 10.1016/j.lwt.2019.108270
Michaud, R., Lehman, W. F., and Rumbaugh, M. D. (2015). “World distribution and historical development,” in Alfalfa and Alfalfa Improvement, 25–91. doi: 10.2134/agronmonogr29.c2
Migda, N. S., Modina, M. A., and Shkoda, V. V. (2024). Effect of atmospheric emissions on ecological and physiological parameters of plants. Bio Web Conferences 103. doi: 10.1051/bioconf/202410300005
Min, X., Jin, X., Zhang, Z., Wei, X., Ndayambaza, B., Wang, Y., et al. (2020). Genome-wide identification of NAC transcription factor family and functional analysis of the abiotic stress-responsive genes in Medicago sativa L. J. Plant Growth Regul. 39, 324–337. doi: 10.1007/s00344-019-09984-z
Munns, R. and Tester, M. (2008). Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59, 651–681. doi: 10.1146/annurev.arplant.59.032607.092911
Ni, Y., Guo, Y. J., Guo, Y. J., Han, L., Tang, H., and Conyers, M. (2012). Leaf cuticular waxes and physiological parameters in alfalfa leaves as influenced by drought. Photosynthetica 50, 458–466. doi: 10.1007/s11099-012-0055-1
Niu, J., Chen, Z., Guo, Z., Xu, N., Sui, X., Roy, M., et al. (2022). Exogenous melatonin promotes the growth of alfalfa (Medicago sativa L.) under NaCl stress through multiple pathways. Ecotoxicology Environ. Saf. 242. doi: 10.1016/j.ecoenv.2022.113938
Nykiel, M., Gietler, M., Fidler, J., Prabucka, B., and Labudda, M. (2023). Abiotic stress signaling and responses in plants. Plants 19, 3405. doi: 10.3390/plants12193405
Peng, W., Cai, W., Pan, J., Su, X., and Dou, L. (2025). Molecular mechanisms of alfalfa response to abiotic stresses. Plants 14. doi: 10.3390/plants14030487
Pešić, M., Tošić Jojević, S., Sikirić, B., Mrvić, V., Jovković, M., Milinković, M., et al. (2025). The plant growth-promoting ability of alfalfa rhizobial strains under nickel stress. Microorganisms 13. doi: 10.3390/microorganisms13020340
Puri, A. (2019). Scholarship @ Western Quantitative proteome analysis of alfalfa in drought stress under the influence of miR156.
Ranawana, S. R. W. M. C. J. K., Siddique, K. H. M., Palta, J. A., Stefanova, K., and Bramley, H. (2021). Stomata coordinate with plant hydraulics to regulate transpiration response to vapour pressure deficit in wheat. Funct. Plant Biol. 48, 839–850. doi: 10.1071/FP20392
Rasse, D. P., Smucker, A. J. M., and Schabenberger, O. (1999). Modifications of soil nitrogen pools in response to alfalfa root systems and shoot mulch. Agron. J. 91, 471–477. doi: 10.2134/agronj1999.00021962009100030019x
Raza Altaf, A., Teng, H., Saleem, M., Raza Ahmad, H., Adil, M., and Shahzad, K. (2021). Associative interplay of Pseudomonas gessardii BLP141 and pressmud ameliorated growth, physiology, yield, and Pb-toxicity in sunflower. Bioremediation J. 25, 178–188. doi: 10.1080/10889868.2020.1853028
Razzaq, S., Zhou, B., Adil, M., Ullah, Z., Guo, H., Zia-Ur-Rehman, M., et al. (2024a). Cadmium stress alleviation: interplay of micronutrients and enzymatic/non-enzymatic species in maize by organic and inorganic amendments. Water Air Soil pollut. 235. doi: 10.1007/s11270-024-07086-5
Razzaq, S., Zhou, B., Ullah, Z., Zia-ur-Rehman, M., Guo, H., Adil, M., et al. (2024b). Exploring the impact of organic and inorganic amendments, with foliar application of iron nanoparticles, on cadmium stabilization and growth of maize in wastewater irrigated-soil. J. Hazardous Materials Lett. 5. doi: 10.1016/j.hazl.2024.100111
Reddy, N. S. and Kumari, K. (2022). Importance of zinc in plant nutrition: a review. Asian Jr. of Microbiol. Biotech. Env. Sc. 24, 490–493. doi: 10.53550/ajmbes.2022.v24i02.008
Rogers, M. E., Grieve, C. M., and Shannon, M. C. (2003). Plant growth and ion relations in lucerne (Medicago sativa L.) in response to the combined effects of NaCl and P. Plant Soil 253, 187–194. doi: 10.1023/A:1024543215015
Rokebul Anower, M., Peel, M. D., Mott, I. W., and Wu, Y. (2017). Physiological processes associated with salinity tolerance in an alfalfa half-sib family. J. Agron. Crop Sci. 203, 506–518. doi: 10.1111/jac.12221
Roy, M., Niu, J., Irshad, A., Kareem, H. A., Hassan, M. U., Xu, N., et al. (2021). Exogenous melatonin protects alfalfa (Medicago sativa L.) seedlings from drought-induced damage by modulating reactive oxygen species metabolism, mineral balance and photosynthetic efficiency. Plant Stress 2. doi: 10.1016/j.stress.2021.100044
Sah, S. K., Reddy, K. R., and Li, J. (2016). Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.00571
Sale, P. W. G., Couper, D. I., Cachia, P. L., and Larkin, P. J. (1992). Tolerance to manganese toxicity among cultivars of lucerne (Medicago sativa L.). Plant and soil 146, 45–52. doi: 10.1007/978-94-011-1650-3_6
Salmani Abyaneh, A. and Fazaelipoor, M. H. (2016). Evaluation of rhamnolipid (RL) as a biosurfactant for the removal of chromium from aqueous solutions by precipitate flotation. J. Environ. Manage. 165, 184–187. doi: 10.1016/j.jenvman.2015.09.034
Sandhu, D., Cornacchione, M. V., Ferreira, J. F. S., and Suarez, D. L. (2017). Variable salinity responses of 12 alfalfa genotypes and comparative expression analyses of salt-response genes. Sci. Rep. 7. doi: 10.1038/srep42958
Shanker, A. K., Maheswari, M., Yadav, S. K., Desai, S., Bhanu, D., Attal, N. B., et al. (2014). Drought stress responses in crops. Funct. Integr. Genomics 14, 11–22. doi: 10.1007/s10142-013-0356-x
Sharaff, M. and Archana, G. (2016). Copper-induced modifications in early symbiotic signaling factors of Ensifer (Sinorhizobium)–Medicago interactions. Arch. Microbiol. 198, 701–709. doi: 10.1007/s00203-016-1242-4
Singh, A. D., Kour, J., Dhiman, S., Khanna, K., Kumar, P., Kaur, R., et al. (2021). Prospects of PGPR-mediated antioxidants and S and P metabolism in plants under drought stress. Antioxidants Plant-Microbe Interaction, 499–549. doi: 10.1007/978-981-16-1350-0_24
Singh, S., Parihar, P., Singh, R., Singh, V. P., and Prasad, S. M. (2016). Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics, and ionomics. Front. Plant Sci. 6. doi: 10.3389/fpls.2015.01143
Smethurst, C. F., Rix, K., Garnett, T., Auricht, G., Bayart, A., Lane, P., et al. (2008). Multiple traits associated with salt tolerance in lucerne: Revealing the underlying cellular mechanisms. Funct. Plant Biol. 35, 640–650. doi: 10.1071/FP08030
Sobrino-Plata, J., Ortega-Villasante, C., Laura Flores-Cáceres, M., Escobar, C., Del Campo, F. F., and Hernández, L. E. (2009). Differential alterations of antioxidant defenses as bioindicators of mercury and cadmium toxicity in alfalfa. Chemosphere 77, 946–954. doi: 10.1016/j.chemosphere.2009.08.007
Song, Y., Lv, J., Ma, Z., and Dong, W. (2019). The mechanism of alfalfa (Medicago sativa L.) response to abiotic stress. Plant Growth Regul. 89, 239–249. doi: 10.1007/s10725-019-00530-1
Steier, J. E., Mandáková, T., Wojciechowski, M. F., and Steele, K. P. (2022). Insights into species delimitation of selected species in the flowering plant genus Medicago section buceras (Leguminosae). Systematic Bot. 47, 431–440. doi: 10.1600/036364422X16512564801533
Stritzler, M., Elba, P., Berini, C., Gomez, C., Ayub, N., and Soto, G. (2018). High-quality forage production under salinity by using a salt-tolerant AtNXH1-expressing transgenic alfalfa combined with a natural stress-resistant nitrogen-fixing bacterium. J. Biotechnol. 276–277, 42–45. doi: 10.1016/j.jbiotec.2018.04.013
Strozycki, P. M., Szymanski, M., Szczurek, A., Barciszewski, J., and Figlerowicz, M. (2010). A new family of ferritin genes from lupinus luteus-comparative analysis of plant ferritins, their gene structure, and evolution. Mol. Biol. Evol. 27, 91–101. doi: 10.1093/molbev/msp196
Su, J. H., Bai, T. H., Wang, F., and Bao, A. K. (2019). Overexpression of Arabidopsis H+-pyrophosphatase improves the growth of alfalfa under long-term salinity, drought conditions and phosphate deficiency. Czech J. Genet. Plant Breed. 55, 156–161. doi: 10.17221/134/2018-CJGPB
Sun, L., Lai, M., Ghouri, F., Nawaz, M. A., Ali, F., Baloch, F. S., et al. (2024). Modern plant breeding techniques in crop improvement and genetic diversity: from molecular markers and gene editing to artificial intelligence—A critical review. Plants 13. doi: 10.3390/plants13192676
Sun, M., Qian, X., Chen, C., Cheng, S., Jia, B., Zhu, Y., et al. (2018). Ectopic expression of GsSRK in Medicago sativa reveals its involvement in plant architecture and salt stress responses. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.00226
Tang, L., Cai, H., Ji, W., Luo, X., Wang, Z., Wu, J., et al. (2013). Overexpression of GsZFP1 enhances salt and drought tolerance in transgenic alfalfa (Medicago sativa L.). Plant Physiol. Biochem. 71, 22–30. doi: 10.1016/j.plaphy.2013.06.024
Tirry, N., Kouchou, A., El Omari, B., Ferioun, M., and El Ghachtouli, N. (2021). Improved chromium tolerance of Medicago sativa by plant growth-promoting rhizobacteria (PGPR). J. Genet. Eng. Biotechnol. 19. doi: 10.1186/s43141-021-00254-8
Turki, Z. A. and Hegazy, M. M. (2021). Taxonomic relevance of seed and seedling morphology in some medicago (Leguminosae) species. Egyptian J. Bot. 61, 731–746. doi: 10.21608/EJBO.2021.43277.1558
Vaibhavi, S. and Devang, P. (2024). Traditional, ethnobotanical, phytopharmacological and nutraceutical potential of Medicago sativa Linn. Afr. J. Biol. Sci. 6, 602–611. doi: 10.33472/AFJBS.6.11.2024.602-611
Valizadeh, M., Moharamnejad, S., Ahmadi, M., and Mohammadzadeh jalaly, H. (2013). Changes in activity profile of some antioxidant enzymes in alfalfa half-sib families under salt stress. J. Agric. Sci. Technol. 15, 801–809.
Villalobos-López, M. A., Arroyo-Becerra, A., Quintero-Jiménez, A., and Iturriaga, G. (2022). Biotechnological advances to improve abiotic stress tolerance in crops. Int. J. Mol. Sci. 23. doi: 10.3390/ijms231912053
Wan, W., Liu, Q., Zhang, C., Li, K., Sun, Z., Li, Y., et al. (2023). Alfalfa growth and nitrogen fixation constraints in salt-affected soils are in part offset by increased nitrogen supply. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1126017
Wang, K., Liu, Y., Teng, F., Cen, H., Yan, J., Lin, S., et al. (2021). Heterogeneous expression of Osa-MIR156bc increases abiotic stress resistance and forage quality of alfalfa. Crop J. 9, 1135–1144. doi: 10.1016/j.cj.2020.11.009
Wang, K., Nan, L., and Guo, Q. (2024). Changes in root endogenous hormone levels and rhizosphere fungi diversity in alfalfa under drought stress. Plant Growth Regul. 102, 313–324. doi: 10.1007/s10725-023-01062-5
Wang, Y., Ruan, Q., Zhu, X., Wang, B., Wei, B., and Wei, X. (2023). Identification of Alfalfa SPL gene family and expression analysis under biotic and abiotic stresses. Sci. Rep. 13. doi: 10.1038/s41598-022-26911-7
Wang, Z. and Şakiroğlu, M. (2021). The Origin, Evolution, and Genetic Diversity of Alfalfa. 29–42. doi: 10.1007/978-3-030-74466-3_3
Wen, W., Wang, R., Su, L., Lv, A., Zhou, P., and An, Y. (2021). MsWRKY11, activated by MsWRKY22, functions in drought tolerance and modulates lignin biosynthesis in alfalfa (Medicago sativa L.). Environ. Exp. Bot. 184. doi: 10.1016/j.envexpbot.2021.104373
Xu, J., Li, X. L., and Luo, L. (2012). Effects of engineered Sinorhizobium meliloti on cytokinin synthesis and tolerance of alfalfa to extreme drought stress. Appl. Environ. Microbiol. 78, 8056–8061. doi: 10.1128/AEM.01276-12
Yadav, S. P., Bharadwaj, R., Nayak, H., Mahto, R., Singh, R. K., and Prasad, S. K. (2019). Impact of salt stress on growth, productivity and physicochemical properties of plants: A Review. Int. J. Chem. Stud. 7, 1793–1798.
Yahaghi, Z., Shirvani, M., Nourbakhsh, F., and Pueyo, J. J. (2019). Uptake and effects of lead and zinc on alfalfa (Medicago sativa L.) seed germination and seedling growth: Role of plant growth promoting bacteria. South Afr. J. Bot. 124, 573–582. doi: 10.1016/j.sajb.2019.01.006
Yan, Z. Z., Ke, L., and Tam, N. F. Y. (2010). Lead stress in seedlings of Avicennia marina, a common mangrove species in South China, with and without cotyledons. Aquat. Bot. 92, 112–118. doi: 10.1016/j.aquabot.2009.10.014
Yang, X., Xie, Y., Qiao, Y., Chang, F., Wang, T., Li, J., et al. (2024). Drought stress tolerance and metabolomics of Medicago sativa induced by Bacillus amyloliquefaciens DGL1. Front. Plant Sci. 15. doi: 10.3389/fpls.2024.1378707
Yasmin, H., Bano, A., Wilson, N. L., Nosheen, A., Naz, R., Hassan, M. N., et al. (2022). Drought-tolerant Pseudomonas sp. showed differential expression of stress-responsive genes and induced drought tolerance in Arabidopsis thaliana. Physiologia Plantarum 174. doi: 10.1111/ppl.13497
Yasmin, H., Rashid, U., Hassan, M. N., Nosheen, A., Naz, R., Ilyas, N., et al. (2021). Volatile organic compounds produced by Pseudomonas pseudoalcaligenes alleviated drought stress by modulating defense system in maize (Zea mays L.). Physiologia Plantarum 172, 896–911. doi: 10.1111/ppl.13304
Yin, H., Wang, Z., Li, H., Zhang, Y., Yang, M., Cui, G., et al. (2022). MsTHI1 overexpression improves drought tolerance in transgenic alfalfa (Medicago sativa L.). Front. Plant Sci. 13. doi: 10.3389/fpls.2022.992024
Yu, Y. and Assmann, S. M. (2016). The effect of NaCl on stomatal opening in Arabidopsis wild type and agb1 heterotrimeric G-protein mutant plants. Plant Signaling Behav. 11. doi: 10.1080/15592324.2015.1085275
Yu, R., Wang, G., Yu, X., Li, L., Li, C., Song, Y., et al. (2021). Assessing alfalfa (Medicago sativa L.) tolerance to salinity at seedling stage and screening of the salinity tolerance traits. Plant Biol. 23, 664–674. doi: 10.1111/plb.13271
Zargar, S. M., Gupta, N., Nazir, M., Mahajan, R., Malik, F. A., Sofi, N. R., et al. (2017). Impact of drought on photosynthesis: Molecular perspective. Plant Gene 11, 154–159. doi: 10.1016/j.plgene.2017.04.003
Zhang, H., Jia, S., Zhang, M., Wang, K., Teng, F., Liu, Y., et al. (2022b). Deciphering the regulatory network of miR156 in plant architecture and abiotic stress resistance of alfalfa (Medicago sativa) by transcriptome sequencing. Ind. Crops Products 189. doi: 10.1016/j.indcrop.2022.115828
Zhang, M., Qin, S., Yan, J., Li, L., Xu, M., Liu, Y., et al. (2023). Genome-wide identification and analysis of TCP family genes in Medicago sativa reveal their critical roles in Na+/K+ homeostasis. BMC Plant Biol. 23. doi: 10.1186/s12870-023-04318-4
Zhang, C., Shi, S., Liu, Z., Yang, F., and Yin, G. (2019). Drought tolerance in alfalfa (Medicago sativa L.) varieties is associated with enhanced antioxidative protection and declined lipid peroxidation. J. Plant Physiol. 232, 226–240. doi: 10.1016/j.jplph.2018.10.023
Zhang, X., Yang, H., Li, M., Bai, Y., Chen, C., Guo, D., et al. (2022c). A pan-transcriptome analysis indicates efficient downregulation of the FIB genes plays a critical role in the response of alfalfa to cold stress. Plants 11. doi: 10.3390/plants11223148
Zhang, D., Zhang, Z., Li, C., Xing, Y., Luo, Y., Wang, X., et al. (2022a). Overexpression of MsRCI2D and MsRCI2E enhances salt tolerance in alfalfa (Medicago sativa L.) by stabilizing antioxidant activity and regulating ion homeostasis. Int. J. Mol. Sci. 23. doi: 10.3390/ijms23179810
Zhao, C., Zhang, H., Song, C., Zhu, J. K., and Shabala, S. (2020). Mechanisms of plant responses and adaptation to soil salinity. Innovation 1. doi: 10.1016/j.xinn.2020.100017
Zheng, G., Fan, C., Di, S., Wang, X., Xiang, C., and Pang, Y. (2017). Over-expression of arabidopsis EDT1 gene confers drought tolerance in alfalfa (Medicago sativa L.). Front. Plant Sci. 8. doi: 10.3389/fpls.2017.0
Zhou, Z. S., Guo, K., Elbaz, A. A., and Yang, Z. M. (2009). Salicylic acid alleviates mercury toxicity by preventing oxidative stress in roots of Medicago sativa. Environ. Exp. Bot. 65, 27–34. doi: 10.1016/j.envexpbot.2008.06.001
Zhou, Z. S., Wang, S. J., and Yang, Z. M. (2008). Biological detection and analysis of mercury toxicity to alfalfa (Medicago sativa) plants. Chemosphere 70, 1500–1509. doi: 10.1016/j.chemosphere.2007.08.028
Zhu, L., Zhang, H., Gao, X., Qi, Y., and Xu, X. (2016). Seasonal patterns in water uptake for Medicago sativa grown along an elevation gradient with shallow groundwater table in Yanchi county of Ningxia, Northwest China. J. Arid Land 8, 921–934. doi: 10.1007/s40333-016-0017-8
Zia, R., Nawaz, M. S., Siddique, M. J., Hakim, S., and Imran, A. (2021). Plant survival under drought stress: Implications, adaptive responses, and integrated rhizosphere management strategy for stress mitigation. Microbiological Res. 242. doi: 10.1016/j.micres.2020.126626
Keywords: alfalfa, drought tolerance, salinity stress, heavy metal toxicity, transgenic approaches
Citation: Daud M, Qiao H, Xu S, Hui X, Adil M and Lu Y (2025) Understanding abiotic stress in alfalfa: physiological and molecular perspectives on salinity, drought, and heavy metal toxicity. Front. Plant Sci. 16:1627599. doi: 10.3389/fpls.2025.1627599
Received: 13 May 2025; Accepted: 14 July 2025;
Published: 31 July 2025.
Edited by:
Hassan Iqbal, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Masood Jan, University of Florida, United StatesHao Sun, Henan Agricultural University, China
Copyright © 2025 Daud, Qiao, Xu, Hui, Adil and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shouming Xu, eHVzaG91bWluZ0BoZW51LmVkdS5jbg==; Yan Lu, eXoyMDA0YWFhQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
‡ORCID: Muhammad Daud, orcid.org/0009-0002-4054-7265
Muhammad Adil, orcid.org/0000-0003-2915-8461
 Muhammad Daud
Muhammad Daud Haixia Qiao1†
Haixia Qiao1† Shouming Xu
Shouming Xu Muhammad Adil
Muhammad Adil