- 1Department of Bio-Resources, Government Degree College for Women Pulwama, University of, Kashmir, J&K, India
- 2Department of Botany, School of Biological Sciences, University of Kashmir, Srinagar, J&K, India
- 3Plant Molecular Biology and Biotechnology Lab, Council of Scientific and Industrial Research (CSIR)-Indian Institute of Integrative Medicine, Srinagar, J&K, India
- 4Department of Botany, S. P. College, Cluster University, Srinagar, J&K, India
- 5Department of Biotechnology, Central University of Kashmir, Ganderbal, J&K, India
Soils contaminated with heavy metals (HMs) pose severe consequences to living organisms, primarily affecting human health. During the past two decades, researchers have focused on hyperaccumulator plant species to augment the cleanup efforts of contaminated soils. Plants are continually exposed to HMs in the environment since they are sessile organisms. Plants that do not hyperaccumulate metals are vulnerable to high metal concentrations. Their root vacuoles create complexes with metal ligands as a detoxifying approach. On the other hand, metal-hyperaccumulating plants have evolved internal regulatory systems that allow them to hyperaccumulate excess HMs in their above-ground tissues. Unlike metal non-hyperaccumulators, they have the unusual ability to successfully carry out regular physiological activities without displaying any evident stress signs. The capacity of hyperaccumulators to acquire extra metals is due to the overexpression of constitutive metal transporter and their translocation capacity. To accomplish this, plants respond to HMs stress by inducing specifying key genes and enzymes involved in HMs chelation and compartmentalization in plants, such as phytochelatin synthases (PCS), which synthesize phytochelatins for metal binding, and metallothionein’s (MTs), which also participate in metal detoxification. Additionally, transporters like ATP-binding cassette (ABC) transporters, natural resistance-associated macrophage proteins (NRAMPs), and heavy metal ATPases (HMAs) facilitate metal sequestration into vacuoles or apoplasts. Genes encoding these proteins (e.g., PCS1, MT1/2, HMA3/4, and NRAMP3/4) are often upregulated under heavy metal stress, enabling plants to mitigate toxicity through chelation and compartmentalization. The current review provides an updated overview of major hyperaccumulator plants, explores insights into metal ion transporters and their expression patterns, and discusses the possible molecular mechanisms underlying metal ion hyperaccumulation. In addition, the evolution of various metal ion transporters and their tissue-specific expression patterns have been documented.
1 Introduction
Environmental contamination by HMs and toxic pollutants is a growing global concern, posing severe risks to ecosystems, agriculture, and human health (Briffa et al., 2020; Hembrom et al., 2020). Industrial activities, mining, improper waste disposal, and the excessive use of chemical fertilizers have led to the accumulation of hazardous substances such as cadmium, lead, arsenic, and mercury in soil and water. These contaminants disrupt soil fertility, reduce crop yields, and enter the food chain, leading to chronic diseases in humans and animals (Pirzadah et al., 2019; Dehkordi et al., 2024). Traditional remediation methods, such as chemical treatments and excavation, are often expensive, energy-intensive, and can further degrade the environment. As a result, there is an urgent need for sustainable and cost-effective solutions to detoxify polluted environments (Kuppan et al., 2024; Sangeetha and Jagtap, 2024). HMs are phytotoxic compounds of metals and metalloids, which may be toxic to plants even at low concentrations. While HMs are essential to plants, substantially, there are non-essential HMs like chromium (Cr), cadmium (Cd), lead (Pb), manganese (Mn), etc. induce devastating negative impacts on plant growth, culminating in poor crop production and toxicity to human health (Varma, 2021; Lone and Gaffar, 2021; Varma, 2021; Raza et al., 2022; Bhat et al., 2023). One of the major global concerns regarding animal health and environmental imbalances is the rapid accumulation of HMs to the extent of toxic levels. Substantial loss in crop production is due to the accumulation of heavy metal ions by selective plant hyperaccumulators (Bharwana et al., 2013; Dar et al., 2020; Sharma and Kumar, 2021). Phytotoxic effects of HMs include damage to various physiological and metabolic networks at the cellular and molecular levels (Raza et al., 2022; Bhardwaj et al., 2023). Several metal ions act as potent carcinogens and toxins to animals, usually accumulated in food chains through anthropogenic activities (Arora and Chauhan, 2021; Priyadarshanee et al., 2022; Basu et al., 2023). These HMs in diverse soils may be emitted from coal mines, petrochemical spillages, metal disposals, industrial areas, animal manures, atmospheric depositions, and sewage-sludge treatment plants (Adeyemi, 2021; Oladoye et al., 2021; Tariq et al., 2023). Heavy metal ion toxicity is primarily due to their oxidation ability. In this state, they manifest heavy damage to plants through their negative impacts on physiology, biochemical network, and morpho-anatomy. In addition, HMs inactivate critical enzymes, proteins, and respiratory metabolism and mediate photosynthetic inhibition (Bharwana et al., 2013; Mushtaq et al., 2021b; Thakur et al., 2021). The interaction between hyperaccumulator plants and their environment has far-reaching implications. On one hand, they can improve soil quality by removing toxic elements, making land safer for agriculture (Zhakypbek et al., 2024). On the other hand, their ability to concentrate HMs may affect neighboring plant growth, either by reducing competition (since few plants thrive in metal-rich soils) or by altering microbial communities in the rhizosphere (Barra Caracciolo and Terenzi, 2021; Solomon et al., 2024). For agriculture, hyperaccumulators can be strategically used in phytomining recovering valuable metals like nickel or zinc while also rehabilitating contaminated fields for future crop production. Additionally, their integration into agroecological systems could reduce dependency on chemical remediation, promoting sustainable farming practices (Cao et al., 2025). Understanding these dynamics is essential for optimizing phytoremediation strategies and ensuring their benefits extend to food security, ecosystem restoration, and human well-being.
The hyperaccumulation of metal ions is a highly complex natural phenomenon, mainly due to the expression of unique traits, which are easy to assess. The flexibility of assessing these metal ions largely relies on simple analytical techniques. In addition, hyperaccumulators are of great interest as an alternate strategy to reduce the contamination of soils by toxic metal ions (Pp and Puthur, 2021; Rai et al., 2022; Tariq et al., 2022). Several studies paved the way for opening doors to evolving diverse processes, such as phytomining/bio-fortification, phytoremediation, etc., to improve the efficiency of crops in accumulating nutrients (Clemens et al., 2002; Pirzadah et al., 2015; Yan et al., 2020; Raza et al., 2021). At least 450 species of angiosperms have been identified as potential sinks to hyperaccumulate HMs viz. As, Cu, Cd, Co, Mn, Ni, Pb, Se, Sb, Ti, and Zn. The hyperaccumulation of metal ions is a potential defense against attacks by pathogens or herbivores (Dueli et al., 2021; Mushtaq et al., 2021a; Mir et al., 2022). They employ at least four mechanisms to accumulate metal ions, viz. transport of metals through roots from the soil, radial metal ion transport in roots, root to shoot metal accumulation, and detoxification at storage sites (Clemens, 2001; Clemens et al., 2002; Mari and Lebrun, 2005; Merlot et al., 2018; Corso and de la Torre, 2020). Almost all hyperaccumulators have genetically adapted to accumulate various metal ions and have been used in phytomining technologies for their extraction (van der Ent et al., 2015; Yaqoob et al., 2023; Bhat et al., 2024). Metal homeostasis and stress tolerance are linked to understanding the hyperaccumulation mechanism of hyperaccumulators. In addition, these plant species have evolved to possess adaptations for hypertolerance and detoxification of metal and metalloids (Angulo-Bejarano et al., 2021; Peng et al., 2021; Rai et al., 2021).
Adapting hyperaccumulators to survive under extreme metal ion concentration may further facilitate understanding molecular mechanisms to detoxify the soils. Unraveling the molecular basis of the hyperaccumulation mechanism helps develop proper phytoremediation and phytoextraction techniques. Higher biomass and enhanced growth of roots and shoots may further be augmented by employing ideal hyperaccumulators through phytoremediation (Malik et al., 2015; Quarshie et al., 2021; Ali et al., 2022; Ojuederie et al., 2022). In addition, targeting contaminated soils by specific hyperaccumulators will further enhance crop production and homeostasis of metal ions. The traits possessed by hyperaccumulators serve two essential aspects of the ecosystem: phytoremediation and the other is the biofortification of metal ions (Pirzadah et al., 2019; Jiang et al., 2021; Yang et al., 2021; Pirzadah et al., 2022). In other words, hyperaccumulators accumulate a particular metal ion a hundred or thousand times more than the normal concentration accumulated by common plants. In addition, they also can detoxify these metal ions to maintain their growth and metabolism. Only a few plant species accumulate large amounts of metalloids or transition metal ions like Zn, Cd, Ni, Se, As, Cu, Pb, Mn, Tl, Co, or Sb in their aerial part at higher concentrations as compared to other plant species. Reeves et al. (2018) reported several hyperaccumulators of metal ions, such as Ni (532 species), followed by Cd (07 species) and (05 species).
This review summarizes the molecular mechanism behind the transport and sequestration of metal ions such as Ni, Cu, Zn, Cd, Mn, As, and Se through the intervention of hyperaccumulators. In addition, updated information regarding the expression pattern of transporter genes is provided.
2 Heavy metal ion hyperaccumulators-an update
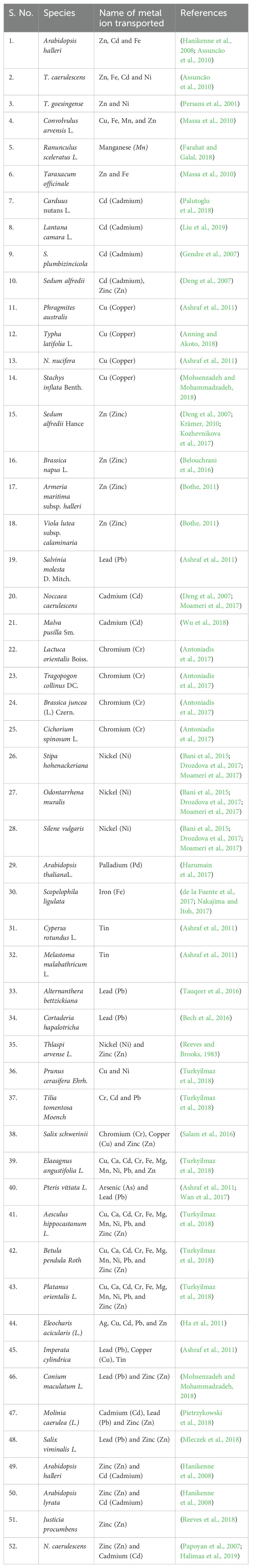
Hyperaccumulators are plant species that accumulate metal ions at high concentrations from contaminated soils in xylem from roots to shoots through bulk flow. The prominent families of plants belonging to the hyperaccumulator category include families such as Asteraceae, Brassicaceae, Buxaceae, Cunoniaceae, Euphorbiaceae, Flacourtiaceae, Phyllanthaceae, Rubiaceae, Salicaceae, and Violaceae (Reeves, 2000; Krämer, 2010; Reeves et al., 2018; Zhang et al., 2021). Numerous metal ion hyperaccumulators have been identified (Table 1), possessing great potential to be employed in phytoextraction and phytoremediation techniques.
In addition, several plants, such as Brassicaceae, Noccaea caerulescens, Arabidopsis thaliana, Chicorium spinosum, and Sedum alfredii. Hance, and Silene vulgaris O. muralis, are studied in detail to have deep insights into understanding the mechanism of hyperaccumulation (Kaushal et al., 2021; Zhang et al., 2021). Several hyperaccumulators, such as Aesculus hippocastanum L., Betula pendula Roth, Elaeagnus angustifolia L., Fraxinus excelsior L., Platanus orientalis L., and Tilia tomentosa Moench, have been employed in biomonitoring of metal ions, such as Cd, Cr, Cu, Ca, Fe, Mg, Mn, Ni, Pb, and Zn (Turkyilmaz et al., 2018). It is reported that various populations of N. caerulescens vary in their hyperaccumulation of metal ions, such as; Zn, Ni, and Cd (Assunção et al., 2003; Manara et al., 2020; Sytar et al., 2020; Tariq et al., 2021). Studies reveal that transporter genes and proteins expressed by hyperaccumulators are highly efficient in contributing to metal tolerance and detoxification of HMs (Sharma et al., 2021b; Bhat et al., 2022b). Since, HMs have the least mobility in soils, plants must adopt diverse mechanisms to transport metal ions efficiently. The underlying molecular mechanisms of heavy metal ion hyperaccumulation are unraveled by employing molecular and genetic systems of hyperaccumulators.
Furthermore, Arabidopsis CPx P1B-type ATPases such as HMA3 (engaged in lead storage) and HMA4 (involved in lead transport) translocate this metal across biological membranes in an energetically-driven process (Gupta et al., 2013). The fact that lead competes with calcium in this transport system explains why lead inhibits voltage-gated Ca-channels (Kumar et al., 2017). As a defensive strategy, phytochelatin complexation sequesters lead into vacuoles via vascular flow, while the remaining lead is transferred through the xylem, and the apoplast is translocated to the leaf.
2.1 Tissue-specific hyperaccumulation
Metal ion hyperaccumulation is tissue/organ-specific depending on the type of species and the transporters (Pasricha et al., 2021; van der Ent et al., 2021). For example, in a comparative study, it was observed that A. maritima subsp. halleri accumulated 88- and 20-times Cu and Pd in roots, respectively, compared to leaves. In addition, experiments also reveal that Pb, Cd, Zn, and Cu were found 3 to 8 times more in brown leaves than green leaves of A. maritima subsp. halleri (Dahmani-Muller et al., 2000). In A. halleri, differential accumulation of Zn (>20,000 mg kg-1) and Cd (>100 mg kg-1) was observed in leaves rather than in other aerial tissues (Dahmani-Muller et al., 2000). Lantana camara L., a native plant of America and Africa, accumulated>100 mg kg-1 of Cd in its shoots (Liu et al., 2019). Moameri et al. (2017) reported that Cd hyperaccumulated up to 68.47 mg kg-1 in Lactuca orientalis and up to 68.1 mg kg-1 in roots of T. collinus and 62 mg kg-1 in both shoots and roots of B. juncea (Moameri et al., 2017). Scanning and transmission electron microscopy coupled with energy-dispersive X-ray (SEM and TEM with EDX), histochemical staining, inductively coupled plasma mass spectrometry (ICP-MS), and optical microscopy (OM) revealed that Imperata cylindrica (L.) P. Beauv hyperaccumulates iron (Fe) in the intercellular spaces of aerial tissues (de la Fuente et al., 2017). Cadmium is accumulated in the edges of leaves, epidermal cells, cell walls, and metabolically less active parts of leaves in Noccaea caerulescens (Cosio et al., 2005). In addition, Cd is also accumulated in mesophyll cells of leaves in A. halleri (Küpper and Kochian, 2010).
2.2 Nickel hyperaccumulators
Nickel (Ni) belongs to the essential category of metal ions, but its serious negative consequences appear when its concentration in plants exceeds 0.85 mM kg−1 plant dry biomass (Rosatto et al., 2021). The Ni toxicity leads to inhibitory effects on the enzymes necessary for operating the Calvin cycle and chlorophyll biosynthesis. It may produce reactive oxygen species (ROS) (Sachan and Lal, 2017). Several plant species have adapted mechanisms for hyperaccumulation and detoxification to circumvent Ni toxicity. Global hyperaccumulator databases have documented 721 metal ion hyperaccumulators, among which Ni is hyperaccumulated by about 532 plant species (Reeves et al., 2018). Most hyperaccumulators (340 plant species) belong to only five families having 180 species to Phyllanthaceae, 87 species to Brassicaceae, 48 species to Cunoniaceae, and 42 species to Euphorbiaceae. Recent Ni hyperaccumulator additions include Senecio conrathii and Phyllanthus rufuschaneyi (Bouman et al., 2018; Siebert et al., 2018). Ni hyperaccumulators are majorly found in serpentine soils, including Alyssum sibiricum and Senecio coronatus (Reeves and Adigüzel, 2004; Boyd et al., 2008) and C. bursa-pastoris (Seregin and Kozhevnikova, 2006). Ni hyperaccumulators outnumber among plant species viz.-a-viz. other metal ions (Krämer, 2010; Reeves et al., 2018). Numerous hyperaccumulators, such as Berkheya coddii Roessler, Echium amoenum Fisch. & C.A. Mey, Stipa hohenackeriana, Lens orientalis, and Taeniatherum crinitum (Schreb.) Nevski have also been identified for Ni accumulation (Robinson et al., 2003; Moameri et al., 2017). Several species of ferns, liverworts, and mosses hyperaccumulate Ni from their habitats (Seregin and Kozhevnikova, 2021). O. muralis has been identified as a hyperaccumulator of Ni using X-ray diffraction (XRD), gravimetric analysis, and inductively coupled plasma atomic emission spectroscopy (ICP-AES) (Zhang et al., 2016). In addition, the Brassicaceae family has been reported to accumulate three times more Zn and six times more Ni than other hyperaccumulators (Krämer, 2010).
Angiosperms predominantly accumulate Ni, as evident in the reports of 140 species grown on the islands of New Caledonia and Cuba (Whiting et al., 2004; Jaffré et al., 2013). Hyperaccumulators like Alyssum bertolonii and Hybanthus floribundus were reported to hyperaccumulate Ni (Minguzzi, 1948; Severne and Brooks, 1972). The Pycnandra (previously Sebertia) acuminate accumulates 2 to 3 orders of Ni in their shoots compared to non-accumulator plant species (Jaffré et al., 1976). Drozdova et al. (2017) also reported that O. muralis hyperaccumulates Ni in their aerial parts. Moreover, it is said that S. hohenackeriana hyperaccumulates Ni in roots up to 195 mg kg-1 and shoots up to 119 mg kg-1 (Moameri et al., 2017). Differential hyperaccumulation rate of Ni was observed in plants; for example, E. amoenum accumulates up to 21 mg kg-1 of Ni in roots and 57 mg kg-1 in shoots, whereas, L. orientalis accumulates up to 32 mg kg-1 Ni in roots and 36 mg kg-1 in shoots (Moameri et al., 2017). The results of almost similar accumulation rates are displayed by T. crinitum, with an accumulation rate of 40 mg kg-1 of Ni in roots and 34 mg kg-1 in shoots (Moameri et al., 2017). Likewise, S. hohenackeriana hyperaccumulates up to 54 mg kg-1 of Ni in shoots and 59 mg kg-1 in roots (Moameri et al., 2017). The available hyperaccumulator database may serve as the baseline for employing the remediation measures to decontaminate Ni toxicity from agricultural soils.
2.3 Zinc hyperaccumulators
In general, zinc (Zn) is classified as an essential micronutrient due to its direct physiological and metabolic significance in plants. However, when present in concentrations exceeding the threshold level (>10,000 mg/kg), zinc becomes toxic, leading to impaired growth, disrupted physiological functions, and even plant death. At such elevated levels, zinc exerts several inhibitory effects, particularly on the photosynthetic metabolism and overall growth processes of crops (Ali et al., 2020; Bhat et al., 2020; Angulo-Bejarano et al., 2021; Kaur and Garg, 2021). Many plant species accumulate Zn at concentrations of up to 1% of the dry weight of plant biomass (Noulas et al., 2018; Sytar et al., 2021). At least nine species of Zn hyperaccumulators belong to the Brassicaceae family, most of which have been found in contaminated soils. Zn is also hyperaccumulated by L. ruderale, C. bursa-pastoris, and A. halleri (Küpper et al., 2000; Kozhevnikova et al., 2017; Raza et al., 2020). Interestingly, numerous species belonging to the family Brassicaceae accumulate multiple heavy metal ion (Dar et al., 2018). Ni is accumulated at 1000–30000 μg g-1 of dry mass basis, whereas Zn up to 1000 μg g-1 of dry mass basis) Thlaspi species belonging to Cruciferae (mustard family) were collected from Europe (Reeves and Brooks, 1983). Few populations of N. caerulescens show minor symptoms upon accumulating Cd up to 4000 mg kg-1 dry weight and 30,000–40,000 mg kg-1 dry weight of Zn (Shen et al., 1997; Ebbs et al., 2002; Natasha et al., 2022). Zn is hyper-accumulated by A. halleri compared to facultative accumulation of Pb and Cd (Bert et al., 2000; Küpper et al., 2000; Bert et al., 2002; Pauwels et al., 2006; Stein et al., 2017). In addition, A. halleri shows species-wide Zn and Cd hypertolerance, with significant variation among its various populations (Pauwels et al., 2006; Meyer et al., 2010; Corso et al., 2018). It is evident from the above findings that a more significant amount of metal ions is accumulated in aerial parts of the hyperaccumulators. Zn and Cd are hyperaccumulated by almost all the subspecies and populations of Arabidopsis halleri found in the soils of both contaminated and non-contaminated habitats (Bert et al., 2002). Few populations of N. caerulescens found in southern France accumulate up to 2908 μg g−1 of Cd in their leaves (Reeves et al., 2001). In addition, many N. caerulescens populations hyperaccumulate Ni and Zn from ultramafic soils (Reeves et al., 2001). Significant variation in Cd, Ni, and Zn accumulation has been reported in different populations of N. caerulescens (Lloyd-Thomas, 1995; Reeves et al., 2001). Hydroponic experiments reveal that varying concentrations of Cd and Zn are hyperaccumulated by three species of Sedum alfredii (Deng et al., 2007; Li et al., 2007). These reports will pave the way to understanding the physiological process modulated by the Zn accumulation in cellular compartments and tissues in a specific crop. In addition, one can employ newer technology like genome editing to understand the mechanism of heavy metal ion transport and sequestration in plants (Riyazuddin et al., 2022; Venegas-Rioseco et al., 2022).
2.4 Mercury hyperaccumulators
In plants, does not play any physiological role (Rascio and Navari-Izzo, 2011). It is adsorbed from the soil as a soluble complex and precipitated as phosphate, carbonate, sulphide, and hydroxide (Tangahu et al., 2011). Phytotoxic effects of high mercury levels on plants are possible (Azevedo and Rodriguez, 2012; Rocha et al., 2019). It affects oxidative metabolism and photosynthesis by interfering with the electron transport mechanism in mitochondria and chloroplast. This induces cell disruption by causing the creation of ROS. Hg is also responsible for limiting aquaporin activity and lowering plant water absorption. The permeability of cell membranes and the number of palisades may be reduced in the presence of Hg, resulting in the buildup of Fe and the loss of essential elements like Mgand K (Shiyab et al., 2009; Tangahu et al., 2011; Azevedo and Rodriguez, 2012). Hg interaction with thiol (SH) groups in tissues rich in SH ligands, such as seed and embryo, results in the development of an S-Hg-S bridge, which disrupts the group’s stability. Seed germination and embryo development are both affected by this binding. Hg also affects the antioxidant defense system by altering enzymatic and non-enzymatic antioxidants and disrupting cells (Azevedo and Rodriguez, 2012), negatively affecting light and dark photosynthetic responses. Photosynthesis is disrupted when Hg replaces the central Mg atom in chlorophyll (Muddarisna et al., 2013; Zhao et al., 2014). Although it is mostly stored in roots, it can accumulate in tiny amounts in shoots by translocating soluble forms or directly absorbing the vapor form (Ranieri et al., 2021). The plant absorbs ionic, methyl, and phenyl forms of mercury from the soil. The phenyl form is used for absorption, whereas the methyl form is used for sequestration. The change of phenyl mercury to methyl mercury is high in apical regions, whereas the transformation of phenyl mercury to ionic mercury is strong in subtending internode regions (Gay and Butler, 1977).
2.5 Cadmium hyperaccumulators
Cadmium (Cd) is another metal ion imparting high toxicity with high transport mobility to the plant. It is found to cause extensive damage to metabolic networks (Liao et al., 2015; Liu et al., 2022). Cd is hyperaccumulated by A. halleri, A. halleri ssp. Gemmifera, and A. lyrata (Huguet et al., 2012; Isaure et al., 2015; Fukuda et al., 2020). Cd is a non-essential element usually found in hyperaccumulators’ roots and aerial organs (Conn and Gilliham, 2010; Imperiale et al., 2022). Several hyperaccumulator species have accumulated Cd, including A. halleri, B. juncea, Lactuca orientalis Boiss., N. caerulescens, Tragopogon collinus DC., and S. hohenackeriana (Cosio et al., 2005; Küpper and Kochian, 2010; Moameri et al., 2017). Hydroponics-based experiments reveal that Noccaea caerulescens was more tolerant to Cd grown under high zinc concentration and accumulated higher concentrations of Cd/Zn (Papoyan et al., 2007). Through biomonitoring analysis of (HMs), it was observed that Ni and Cu were hyperaccumulated by Prunus cerasifera Ehrh., whereas Pb and Cd were effectively accumulated by T. tomentose (Turkyilmaz et al., 2018). Analysis based on Atomic Absorption Spectroscopy (AAS) showed that T. latifolia hyperaccumulated Cu and Cd, while E. crassipes accumulated Pb when grown in wetlands supplied with effluents (Sukumaran, 2013). It has been reported that Iron (Fe) was hyperaccumulated by Scopelophila ligulata (Spruce) 10 to 61 times more than normal mosses (Nakajima and Itoh, 2017). The hyperaccumulators Lactuca orientalis, T. collinus, and B. juncea accumulates Cd in roots and shoots (Moameri et al., 2017). Cd hyperaccumulation and tolerance are modulated by overexpression of NRAMPs transporters in N. caerulescens (Oomen et al., 2009; Wei et al., 2009; Takahashi et al., 2011). Efficient remediation of Cd from the agricultural and urban soils is critical for sustainable agriculture development in current food insecurity trends. An update on mechanism of hyperaccumulators to initiate phytoremediation of Cd provides efficient ways to restore the polluted habitats to healthy state since it is evident from the existing literature that Cd has negative impacts on the metabolic and physiological networks of plants.
2.6 Manganese hyperaccumulators
Manganese (Mn) is an essential category of micronutrients, although in certain climatic-cum-edaphic conditions, primarily in acidic soils, it is toxic to crops (Rashed et al., 2019). It is believed that Mn adversely affects photosynthetic metabolism and enhances ROS generation (Cui et al., 2021). Numerous Mn hyperaccumulator plant species belonging to various families such as Araliaceae, Apocynaceae, Celastraceae, Clusiaceae, Myrtaceae, Polygonaceae, Proteaceae, and Theaceae have been identified (Fernando et al., 2013). The deposition of higher concentrations of Mn in the vacuoles of photosynthetic cells of the upper epidermis was reported in Maytenus fournieri L (Doncheva et al., 2009; Fernando et al., 2012). In addition, in Gossia. Amplexicaulis L. Mn was deposited in entire leaves, whereas in Trapa natans L and Gossia hillii L., Mn was hyperaccumulated in the floating lamina and photosynthetic tissues, respectively (Fernando et al., 2012, 2013). Moreover, trichomes of Alyssum murale L. and Helianthus annus L. also hyperaccumulate Mn (Blamey et al., 1986; Broadhurst et al., 2004).
2.7 Lead hyperaccumulators
Lead (Pb) is a non-essential heavy metal with little understanding of its biological use in plants due to its high toxicity. Pb poses significant health threats even at low doses, particularly in child brain development and renal failure (Gaur et al., 2014; Kumar et al., 2017). Lead phytotoxicity inhibits metabolic processes by interfering with enzymes, affecting root elongation, seed germination, and plant development. A high quantity of lead affects chlorophyll and ATP synthesis, cell membrane permeability, water, nutrient intake, seedling growth, and biomass output. Lead poisoning causes oxidative stress-mediated by ROS, which causes protein oxidation, lipid, nucleic acid peroxidation, and eventually death (Nagajyoti et al., 2010; Dewanjee et al., 2015; Li et al., 2016). This is supported by the fact that lead poisoning increases the catalytic activity of antioxidant enzymes (Li et al., 2016). Due to its sorption with soil, lead is not readily accessible in biological systems and has a limited solubility at normal pH, making it unavailable for plant absorption even by hyperaccumulators (Chen et al., 2004; Bahraminia et al., 2016). Some recognized lead hyperaccumulators, such as Brassica napus and Euphorbia cheiradenia, have been shown to collect more than 1000 mg kg-1 of lead in dry weight (Major, 2010; Ali et al., 2013). The apoplastic route or Ca2+ channels are both involved in absorbing lead by roots. Alternative mechanisms for lead absorption by roots include cyclic nucleotide-gated ion channels and cation transporters. Lead follows the apoplastic route after absorption by roots, although its transport beyond endodermis is limited by the Casparian strip owing to phytochelatin binding. Sequestration in root vacuoles after complex formation, accumulation in plasma membranes, and complexation with phytochelatins, glutathions, and amino acids like proline all limit lead translocation. By establishing a metal-ligand combination, lead immobilisztion can also occur in the form of phosphates, resulting in reduced negative effects and greater phytoextraction (Kumar et al., 2017). Ethylenediamine tetraacetic acid (EDTA), nitrilotriacetic acid (NTA), and malate are chelating chemicals that can be used to immobilize HM ions (Tangahu et al., 2011; Gaur et al., 2014). Rhizofiltration is when the lead is absorbed and deposited in the roots, with only a small quantity being translocated to the aerial sections of plants like Typha domingensis (Bindu et al., 2010).
2.8 Selenium hyperaccumulators
Selenium (Se) is another potentially toxic heavy metal ion distributed in trace amounts in the earth’s crust in the form of metalloids (Lima et al., 2018; Reynolds et al., 2020). Toxicity mediated by Se above threshold level exhibits several pathological conditions in plants, such as stunted growth, withering, drying of leaves, reduced protein synthesis, and chlorosis (Mengel and Kirkby, 1987; Van Hoewyk, 2013; Gupta et al., 2022). Several plant species such as Xylohiza and Conopis hyperaccumulate Se >1000 mg Se kg-1 dry weight if grown in Se-rich soils. Two important hyperaccumulators of Se identified are Astragalus bisulcatus (Hook.) A. Gray and Stanleya pinnata (Pursh) Britton, accumulating Se in reproductive organs and young growing leaves (Freeman et al., 2006). On a side note, (Antoniadis et al., 2017), identified C. spinosum, a wild edible vegetable, as a hyperaccumulator of chromium (Cr).
2.9 Arsenic hyperaccumulators
Arsenic (As) is a non-essential heavy metal ion highly toxic to crops. It is transported to its roots through specific transporters (Sytar et al., 2021). Hyperaccumulators accumulate about 2% of As in aerial tissues of plants (Chen et al., 2021). More than 21 hyperaccumulators of Arsenic (As) have been identified, and most of them belong to the genus Pteridaceae (Xie et al., 2009). Pteris vittata (Chinese Brake fern) was identified as a potential hyperaccumulator of As grown at four mining sites in Hunan Province of China (Wan et al., 2017). In addition, Pteris vittata (Chinese Brake fern) used phytate, a root exudate, to enhance the uptake of As and increase plant growth and development (Liu et al., 2017). Similarly, two species of Brassicaceae also hyperaccumulate arsenic (As) (Karimi et al., 2009). In submerged plants, Callitriche stagnalis and Myriophyllum propinquum, As is accumulated at 1000 mg kg–1 dry weight (Robinson et al., 2006). Eriophorum angustifolium hyperaccumulates As in root tissues, while in Wolffia globose, up to 400 mg kg–1 of As is accumulated (Stoltz and Greger, 2002; Zhang et al., 2009). Certain gymnosperms, such as Pseudotsuga menziesie, hyperaccumulate As in needles and stems (Haug et al., 2004).
3 Hyperaccumulators as a prelude to solving the heavy metal toxicity: an overview
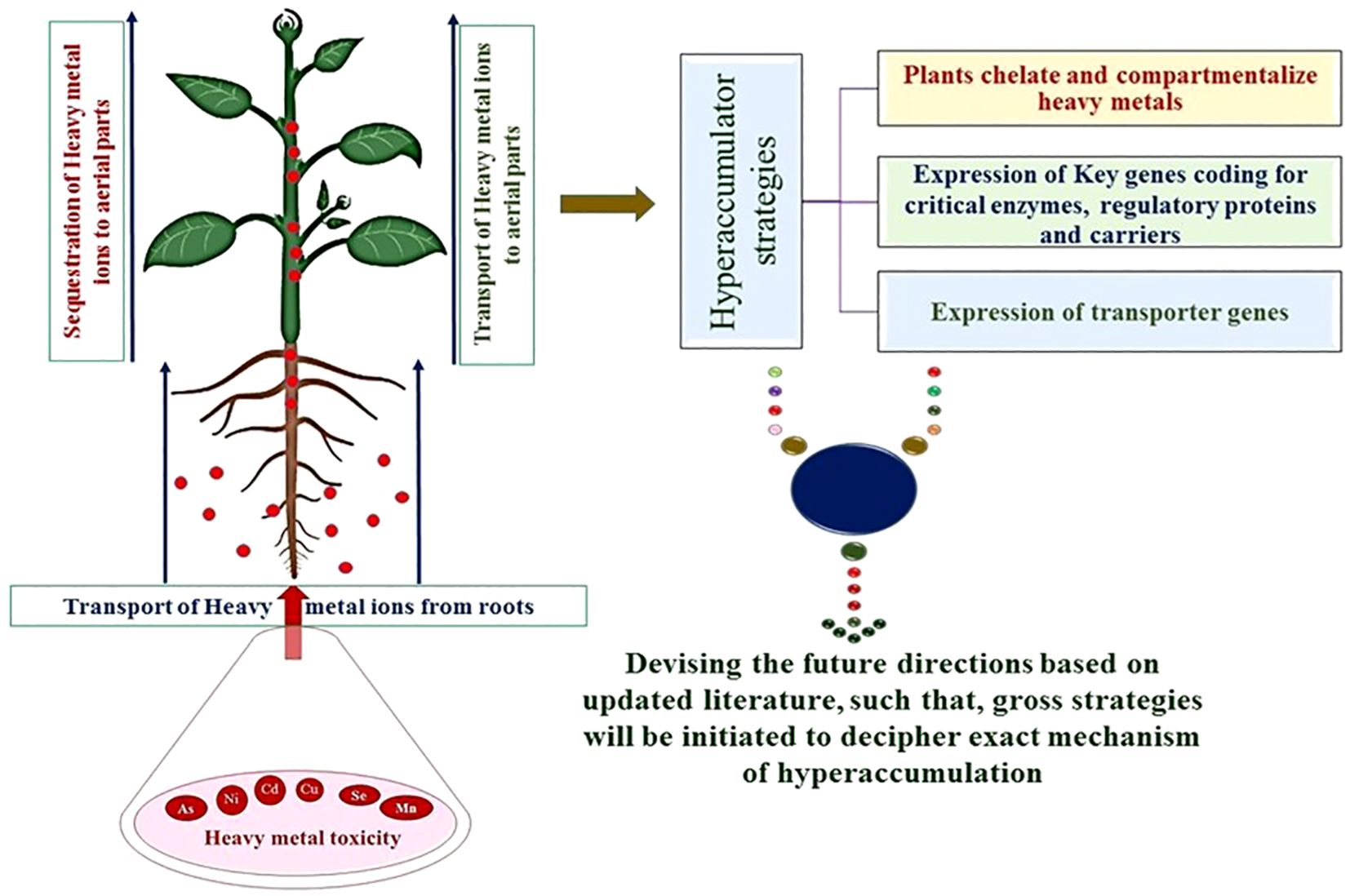
The molecular mechanism of hyperaccumulation has been primarily based on physiological adaptations by hyperaccumulators, such as increased metal ion uptake, loading in the xylem, and detoxification in aerial parts of the plant (Pasricha et al., 2021). Hyperaccumulators display variable mechanisms to accumulate the metal ions from the contaminated and normal soils. The uptake of excess accumulated metal ions is modified or stored to tolerate their ill effects on the growth and metabolism of hyperaccumulators (Pasricha et al., 2021; Sytar et al., 2021). Moreover, bacteria and fungi generally occur in bound form and are converted into a simple form. In addition, several chelating agents secreted in the rhizosphere further help in the absorption of metal ions by several plasma-bound proteins and for specific metal, ion reductases to facilitate their transportation into aerial parts of plants through xylem (Dotaniya et al., 2015). Several plant species are hyperaccumulators of economically essential metal ions and display considerable tolerance to specific classes of metal ions (Pasricha et al., 2021; Sytar et al., 2021; Tariq et al., 2022).
Elevated expression of genes coding for transporters and proteins for chelation plays a critical role in hypertoleranace and hyper-accumulation in several plant hyperaccumulators. Several studies have reported the foliar heavy metal concentration in hyperaccumulators (Krämer, 2010; Goolsby and Mason, 2015; Bhat et al., 2022a). The Alyssum bertolonii/Brassicaceae (Minguzzi, 1948) was first reported to hyperaccumulate Ni, whereas, Noccaea caerulescens (formerly, Thlaspi caerulescens)/Brassicaceae was reported to hyperaccumulate Zn (Sachs, 1865; Baumann, 1885; Reeves, 2000). These reports attracted scientific communities in the early 1990s to employ hyperaccumulators as alternative strategies to circumvent HM toxicity issues. These plant species evolved our mechanistic understanding of molecular mechanisms associated with hyperaccumulation and strategy to detoxify the metal ions. At least tree Quantitative trait locus (QTLs) possibly belonging to hypertolerance of Cd and Zn have been mapped (Courbot et al., 2007; Willems et al., 2007). Moreover, an overlapping QTL identified in the AhHMA4 (Heavy Metal ATPase 4) gene in Arabidopsis halleri, a hyperaccumulator of Cd and Zn, is also screened (Courbot et al., 2007; Willems et al., 2007) (Figure 1).
3.1 Expression pattern of metal ion transporters
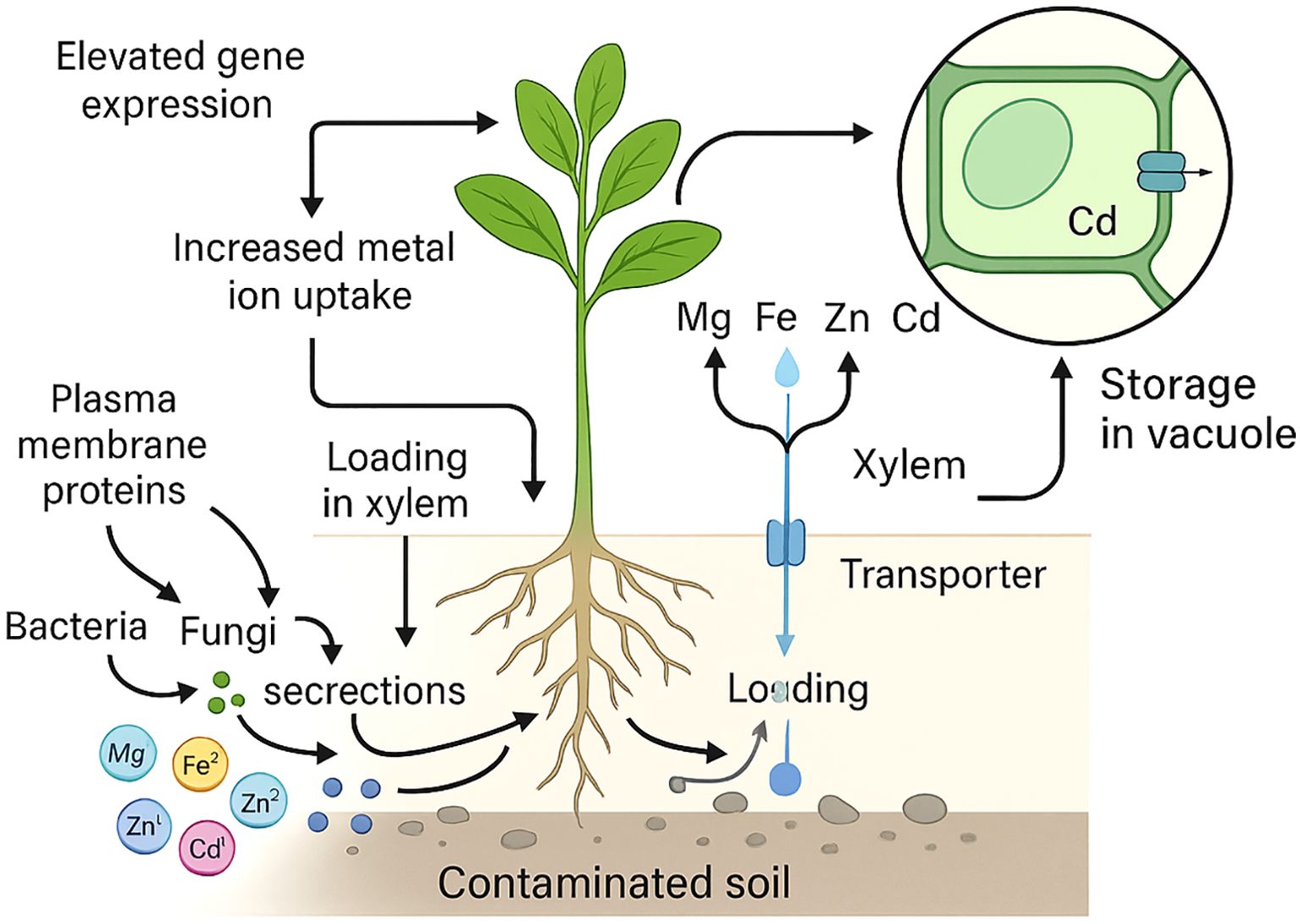
Insights into the metal hyperaccumulation mechanism are deepened by conducting a comparative transcriptome analysis of many genes encoding metal ion transporters and detoxifying proteins (Wu et al., 2021). The hyperaccumulation and hypertoleranace traits are independent of genetic control and are not species-specific. Many reports depict that transporter genes are overexpressed by hyperaccumulators depending on the concentration of metal ions in diverse soil types (Table 2, Figure 2).
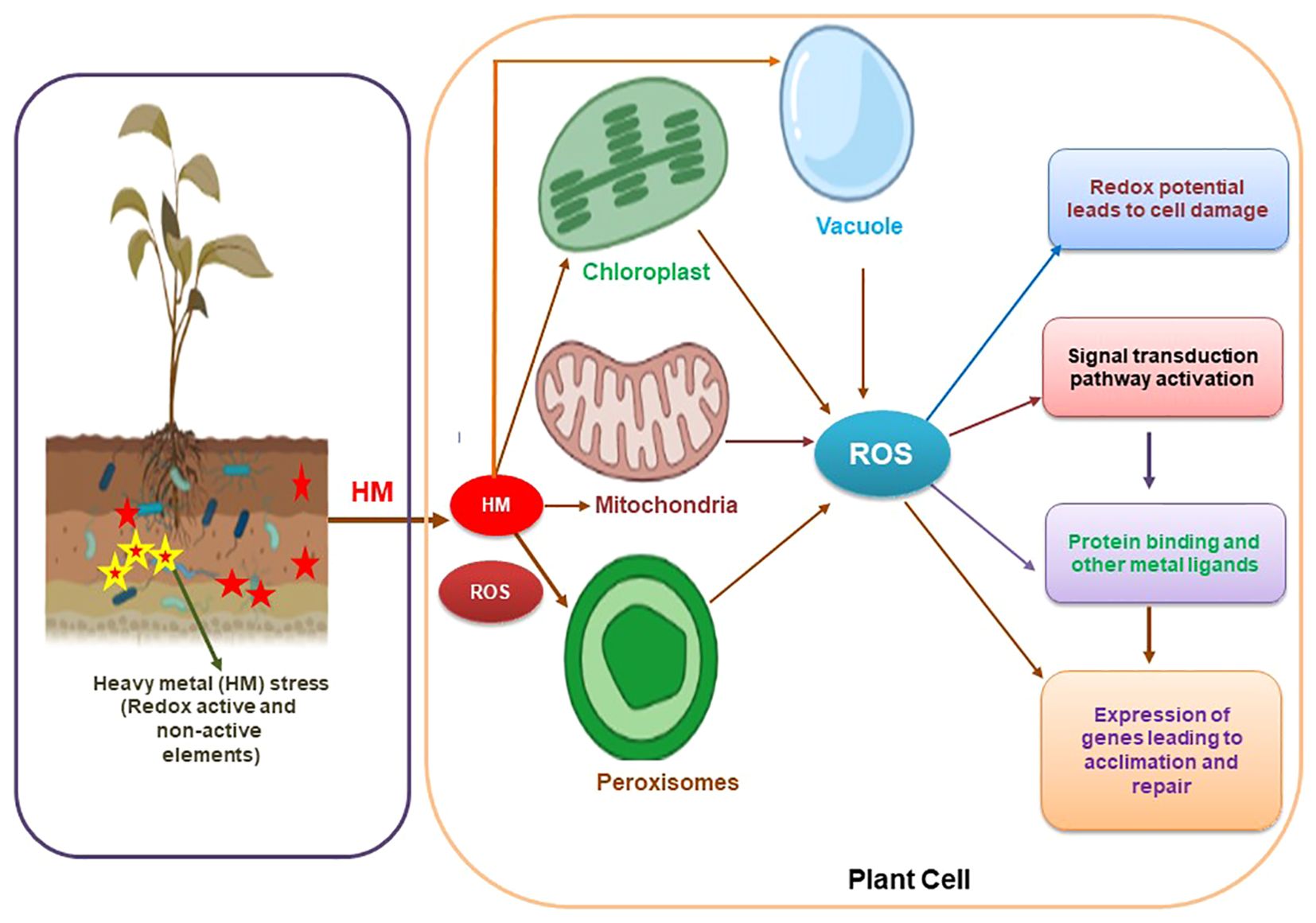
Figure 2. Diagrammatic representation of cellular response to heavy metal stress through the expression of genes coding for transporters and other related proteins.
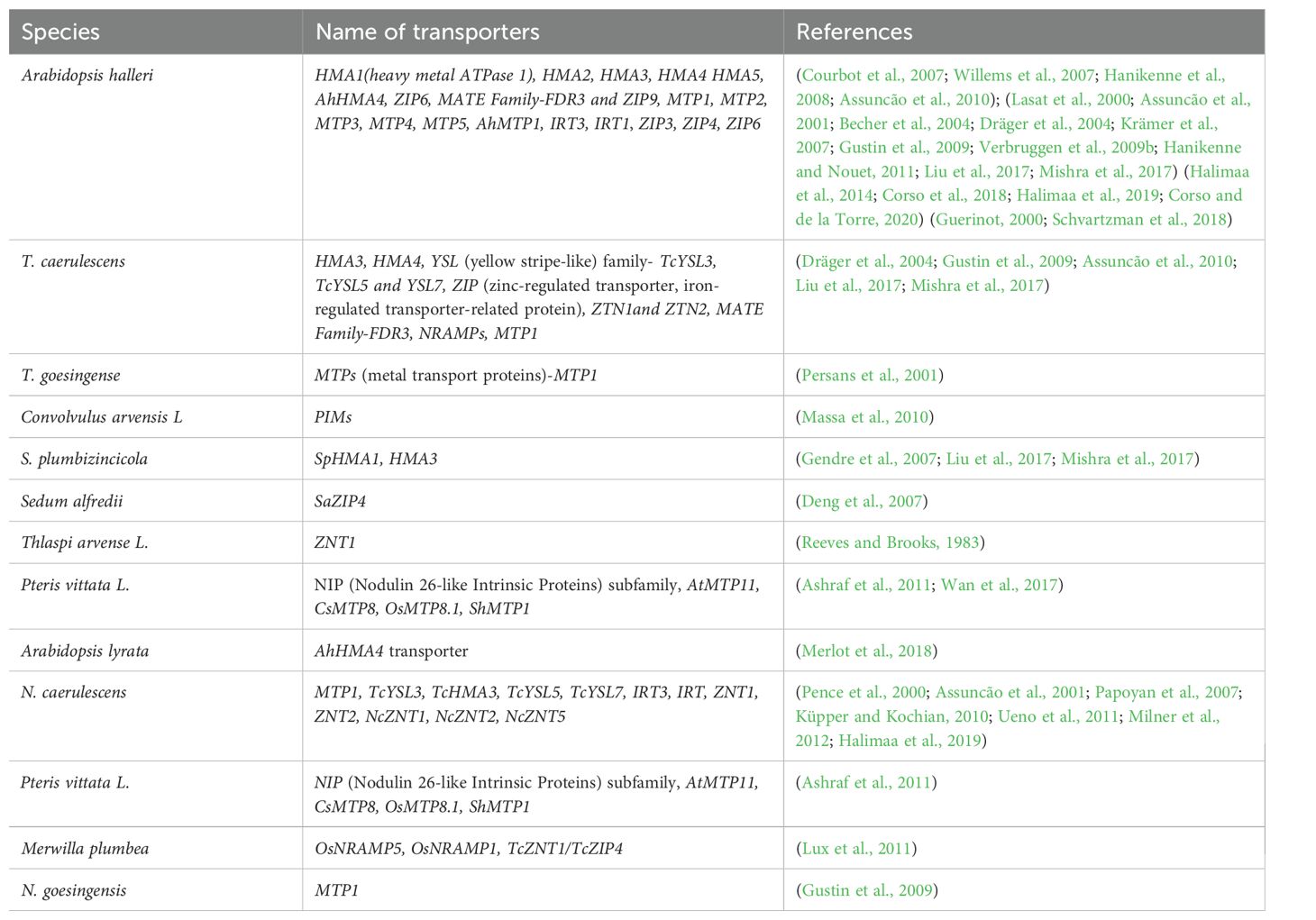
The copy number expansion of transporter genes within the genome and the strong promoter are usually activated by cis-regulatory elements resulting in the hyperexpression of genes. The central mechanism of metal ion hyperaccumulation involves many genes expressed for metal ion transporters. Therefore, it is necessary to understand the mechanism behind the transport of metal ions and trace the expression of genes to devise strategies for developing transgenic plants for heavy metal hyperaccumulation. A comparative expression analysis reported that later genomic evolution was displayed by the enhanced copy number of transporters such as; HMA4 to 3 and MTP1 to 5 in Arabidopsis halleri compared to single-copy found in wild-type A. thaliana (Hanikenne et al., 2008; Shahzad et al., 2010). Similar types of enhancement in expression were due to a five times more copy number of TcHMA3 transporter to accumulate Cd in ecotypes of N. caerulescens (Ueno et al., 2011). Upon expression profiling of two hyperaccumulators, overexpression of genes occurred, which are involved in Zn/Cd uptake, their loading to xylem, transport, and chelation (Balafrej et al., 2020; Sharma et al., 2021a). In addition, it was observed that a higher copy number resulted in over-expression of TcHMA3 gene encoding Cd transporters in Saint- Laurent-le-Minier (Ganges) population compared to the Prayon population of N. caerulescens (Ueno et al., 2011). In A. halleri, the AhMTP1 gene encodes vacuolar membrane Zn/H+ antiporter, expressed 20-folds higher due to high copy number in leaves than A. thaliana (Becher et al., 2004; Dräger et al., 2004). Due to the higher copy number of MTP1, a Zn transporter is highly expressed in A. halleri to mediate hypertolerance (Dräger et al., 2004).
Microarray-based transcriptome analysis has led to the identification of several genes responsible for metal ion transport and their chelation in model organisms like A. halleri or N. caerulescens (Becher et al., 2004; Chiang et al., 2006; Filatov et al., 2006; Hammond et al., 2006; Talke et al., 2006; van de Mortel et al., 2006; Weber et al., 2006). Hyperaccumulators result in overexpression of transporters such as AhHMA4, in xylem parenchyma and pericycle of the root, and in A. halleri shoot tissues viz., cambium and xylem parenchyma (Hanikenne et al., 2008). Moreover, MTP1 was overexpressed in A. halleri and N. caerulescens to transport Zn and Cd (Dräger et al., 2004; Gustin et al., 2009). Transport of Cd was mediated by overexpression of HMA3 in leaf epidermal cells of Sedum plumbizincicola. At the same time, HMA3 was predominantly expressed in bundle sheath and mesophyll cells in A. halleri and N. caerulescens (Liu et al., 2017: Mishra et al., 2017). The transformation of TgMTP1 in A. thaliana resulted in enhanced accumulation of Zn into vacuoles, attributing to Zn tolerance (Gustin et al., 2009). Several transporters were overexpressed, such as SpHMA1 in S. plumbizincicola, SaZIP4 in Sedum alfredii, and TcYSL3, TcHMA3, TcYSL5, and TcYSL7 in N. caerulescens plants for transport of metal ions (Gendre et al., 2007; Ueno et al., 2011; Zhao et al., 2019). It is pertinent to mention that the expression of transporter genes very often varies concerning metal ion supply, tissue, organ, and populations of hyperaccumulators (Krämer et al., 2007; Verbruggen et al., 2009b; Küpper and Kochian, 2010; Visioli et al., 2014). Under sufficient Zn supply, N. caerulescens and A. halleri overexpress several transporter genes, such as IRT3, IRT1, and ZIP genes, for higher accumulation of metal ions (Assunção et al., 2001; Becher et al., 2004; Krämer et al., 2007; Verbruggen et al., 2009b; Hanikenne and Nouet, 2011; Halimaa et al., 2014; Corso et al., 2018; Schvartzman et al., 2018; Halimaa et al., 2019; Corso and de la Torre, 2020). In contrast, during the low availability of Zn, ZNT1 and ZNT2 genes encoding transporters were overexpressed in N. caerulescens (Pence et al., 2000; Assunção et al., 2001). Differential expressions of NcZNT1 and NcZNT2 transporter genes were reported in the roots and shoots of N. caerulescens. Moreover, tissue-specific expression was identified for expression of NcZNT1 in stellar parenchyma cells, pericycle, and very low expression in cortex and rhizodermis in N. caerulescens (Milner et al., 2012).
Upon exposure to Zn deficient soil, a tissue-specific expression pattern of NcZNT1 was observed in N. caerulescens (Milner et al., 2012). In addition, it is reported that NcZNT1 was differentially expressed in shoot apical meristem and mesophyll, bundle sheath, and stomatal guard cells. On the contrary, NcZNT5 expression was limited to young leaves, especially its epidermal cells (Küpper and Kochian, 2010; Milner et al., 2012). Expression of the HMA4 gene occurs in roots as well as shoots of A. halleri and N. caerulescens to load and unload Cd and Zn into the xylem (Bernard et al., 2004; Papoyan and Kochian, 2004; Talke et al., 2006; van de Mortel et al., 2006; Craciun et al., 2012; Visioli et al., 2014; Mishra et al., 2017). Moreover, it is reported that HMA4 encoding Cd and Zn metal ion transporter is overexpressed in the roots and shoots of both A. halleri and T. caerulescens metal ion hyperaccumulators (Bernard et al., 2004; van de Mortel et al., 2006; Courbot et al., 2007). The NcZNT5 overexpression was observed in epidermal cells instead of in guard and subsidiary cells in young leaves of N. caerulescens (Küpper and Kochian, 2010). In contrast, an opposite expression pattern was observed in mature leaves, wherein, NcZNT5 was overexpressed in guard cells rather than epidermal cells (Küpper and Kochian, 2010). Both these findings back up the adaptation of plants to hyperaccumulation of metal ions in correlation with the developmental stage of plants. Further, the RNA interference technique showed that higher expression of AhHMA4 was responsible for hypertolerance to Cd and Zn metal ions in Arabidopsis halleri (Papoyan and Kochian, 2004). In addition, the RNAi technique showed higher transcription of NcZNT1 (Zn Transporter 1) genes encoding a transporter of Cd and Zn in N. caerulescens (Pence et al., 2000). Under higher concentration of Zn, several genes of the ZIP family have been highly expressed in A. halleri and N. caerulescens (Assunção et al., 2001; Becher et al., 2004; Weber et al., 2004; Talke et al., 2006; van de Mortel et al., 2006; Lin et al., 2009). In A. halleri, several transporter genes, such as ZIP family members viz. ZIP3, ZIP4, and ZIP6 are responsible for the influx of metal ions from the rhizosphere to roots and are highly expressed due to high copy numbers (Guerinot, 2000). In addition, overexpression of several ZIP family member transporters has been reported in A. halleri and T. caerulescens to hyperaccumulate Zn metal ions (Becher et al., 2004; Weber et al., 2004; Filatov et al., 2006; Hammond et al., 2006; Talke et al., 2006; Krämer et al., 2007). Expression analysis and RNAi-produced lines identified tissue-specific expression of NgMTP1encoding MTP1 to accumulate Zn in shoots of hyperaccumulators (Desbrosses-Fonrouge et al., 2005).
Comparative analysis showed that ZNT1 was overexpressed in roots of T. caerulescens in comparison to non-accumulator T. arvense (Pence et al., 2000). The overexpression of ZNT1 was further confirmed by microarray analysis (Hammond et al., 2006; van de Mortel et al., 2006). Another transporter, ZTP1, a homolog of AtMTP1, was highly expressed in T. caerulescens to accumulate metal ions in vacuoles (Assunção et al., 2001). Two populations of T. caerulescens were found to differentially express two ABC (ATP-binding cassette) transporters in their shoots to hyperaccumulate Zn (Hassinen et al., 2007). Zn compartmentation and transport is efficiently mediated by overexpression of HMA3 gene coding P1B-ATPase T. caerulescens and A. halleri (Craciun et al., 2006; van de Mortel et al., 2008). In addition, CAX gene encoding cation exchange mediates enhanced Cd sequestration (Craciun et al., 2006; van de Mortel et al., 2008). FDR3 is another transporter belonging to the MATE (Multidrug and Toxin Efflux) gene family of transporters overexpressed in the root pericycle of A. halleri and T. caerulescens to hyperaccumulate Fe (Talke et al., 2006; van de Mortel et al., 2006). In T. caerulescens three genes viz. TcYSL3, TcYSL5, and YSL7 are over-expressed to mediate vascular loading and transport of Ni and Fe in the form of Nicotinamide-Ni complex and Nicotainmide-Fe complex (Gendre et al., 2007). The overexpression of NIP genes might be responsible for transporting As from roots to the xylem vessels in Pteris vitata (Zhao et al., 2009). The comparative RNA-seq analysis reported that Ni transport is highly regulated by overexpression of the ZIP family in S. coronatus. Moreover, the expression pattern of IRT1 and ZIP10 varied between various populations of N. caerulescens (Corso and de la Torre, 2020). The overexpression of Ni transporter genes through high copy number and changing dynamics of promoter activity helps decode Ni’s transport mechanism by hyperaccumulators.
3.2 Heavy metal transporters
The transport of metal ions occurs through the active accumulation of metal ions, usually generated through the air or deposited metalloids on the leaves of plants (Jogawat et al., 2021; Yang et al., 2021). In addition, inactive accumulation of metals occurs from the soil through roots and their transport through the xylem to the aerial parts of plants, such as stems, leaves, and other parts of shoots, by diverse classes of metal ion transporters (Feki et al., 2021; Jamla et al., 2021). Coefficient bioaccumulation of metal ions needs transport through pumps against the concentration gradient. Consequently, the transport of HMs from roots to aerial parts involves active transporters found in the plasma membrane and vacuolar membrane, these transporters require energy, usually in form of ATP to concentrate metal ions into storage organelles, such as vacuoles (Figure 3). It must be noted that vacuoles of epidermal cells store higher concentrations of metal ions as compared to other cellular organelles due presence of enzymes like proteinases, phosphatases and lipases (Feki et al., 2021).
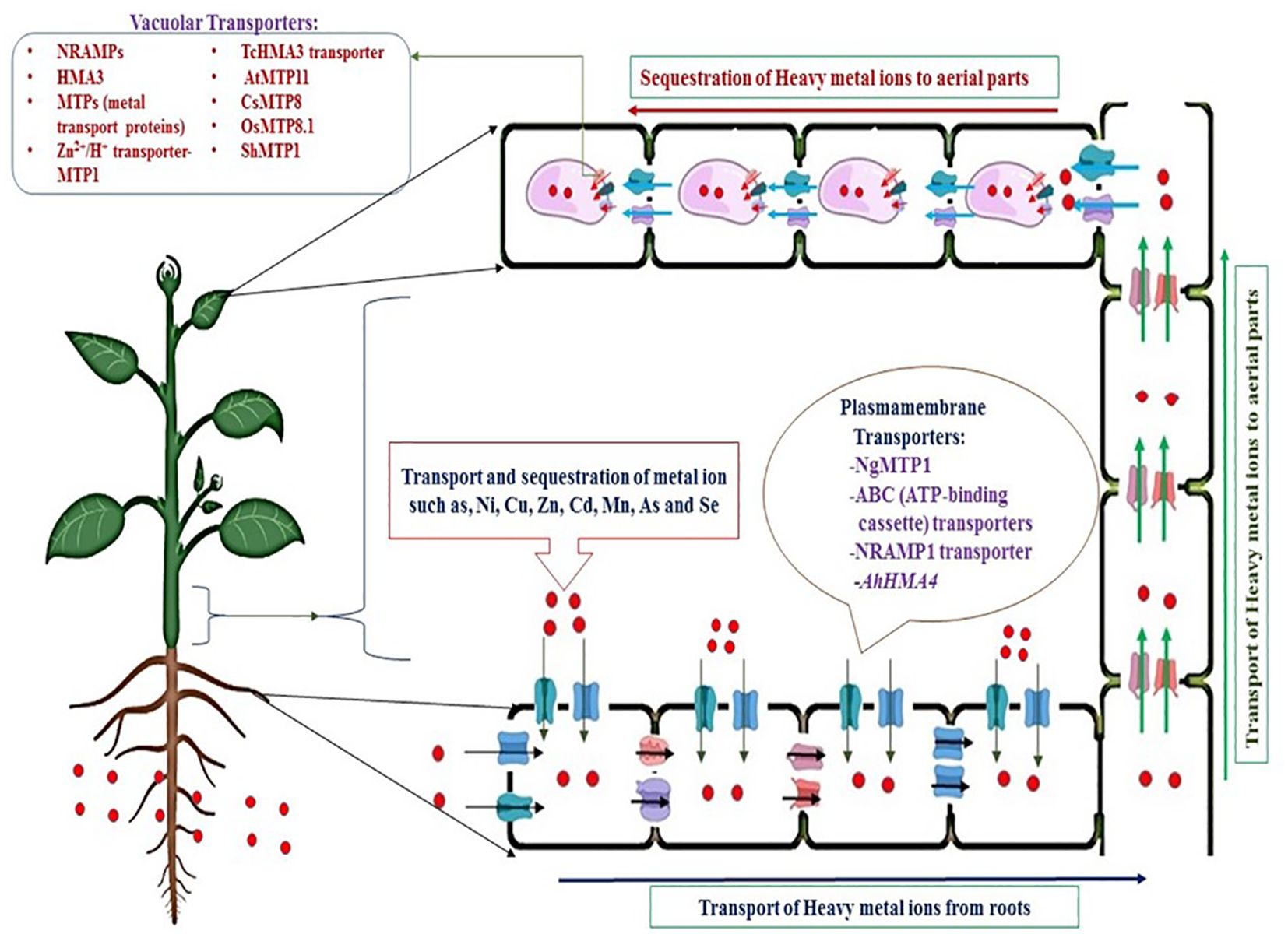
Figure 3. Mechanisms of transportation of heavy metals from contaminated soils through metal ion transporters by active transport.
On average, up to 200 times more metal ion accumulation is mediated by transporter genes overexpressed by hyperaccumulator species compared to non-hyperaccumulators (Pence et al., 2000; Assunção et al., 2001; Becher et al., 2004; Hanikenne et al., 2008; Verbruggen et al., 2009b). Transcriptome analysis identified several metal ion transporter hyperaccumulators in Brassicaceae. A great deal of metal ion transport has been explored through studies on ZIP transporters, as they are involved mainly in the uptake of metal ions (Krämer et al., 2007; Andresen et al., 2018). The hyperaccumulation of metal ions is directly related to a higher number of ZIP transporters in the plasma membrane of leaf epidermal cells and transpiration termination in these cells (Küpper et al., 2009; Schneider et al., 2013). In addition, (Pence et al., 2000) reported that the ZNT1 transporter, a homolog of AtZIP4, transports Zn with higher affinity and Cd uptake with low affinity. The CDF (cation diffusion facilitators) family of the transporter are involved in the transport of metal ions such as Fe2+, Co2+, Zn2+, Mn2+, and Cd2+ from the cytoplasm to organelles like vacuoles, endoplasmic reticulum, etc. (Figure 4) (Peiter et al., 2007). Moreover, metal ion transport into vacuoles occurs through CDF (cation diffusion facilitator), MTP (metal tolerance protein), and P1B type ATPase HMA3 (Krämer et al., 2007; Verbruggen et al., 2009b; Andresen et al., 2018). Many studies have reported on two Zn/Cd hyperaccumulator models, T. caerulescens and A. halleri (Frérot et al., 2010; Krämer, 2010). These findings strongly suggested that the differential expression and regulation of common genes rather than a novel gene set was mainly involved in the metal hyperaccumulation mechanisms (Verbruggen et al., 2009a). The cation transporters belonging to the ZIP family (zinc-regulated and iron-regulated transporter proteins) are primarily located at the plasma membrane of roots in both Cd/Zn hyper and non-hyperaccumulators. The constitutive overexpression of ZIPs genes in T. caerulescens (ZTN1 and ZTN2) and A. halleri (ZIP1 and ZIP6) led to enhanced uptake of Zn irrespective of the exterior Zn concentration (Assunção et al., 2010; Lira-Morales et al., 2019). While in Cd/Zn non-hyperaccumulator plants, the expression of ZIPs was detected only under Zn-deficient conditions, suggesting a Zn-mediated regulation unlike the constitutive expression observed in the hyperaccumulator counterpart (Weber et al., 2004; Yan et al., 2020). Although Zn transporters mediate the uptake of Cd in A. halleri and most ecotypes of T. caerulescens, Zn is preferably being transported over Cd (Zhao et al., 2002). Interestingly, (Lombi et al., 2001) reported that the Ganges ecotype of T. caerulescens could accumulate a very high level of Cd in their aerial parts. Cd uptake is not influenced by Zn concentration, indicating the presence of an efficient cadmium-specific transport system in the roots of this ecotype.
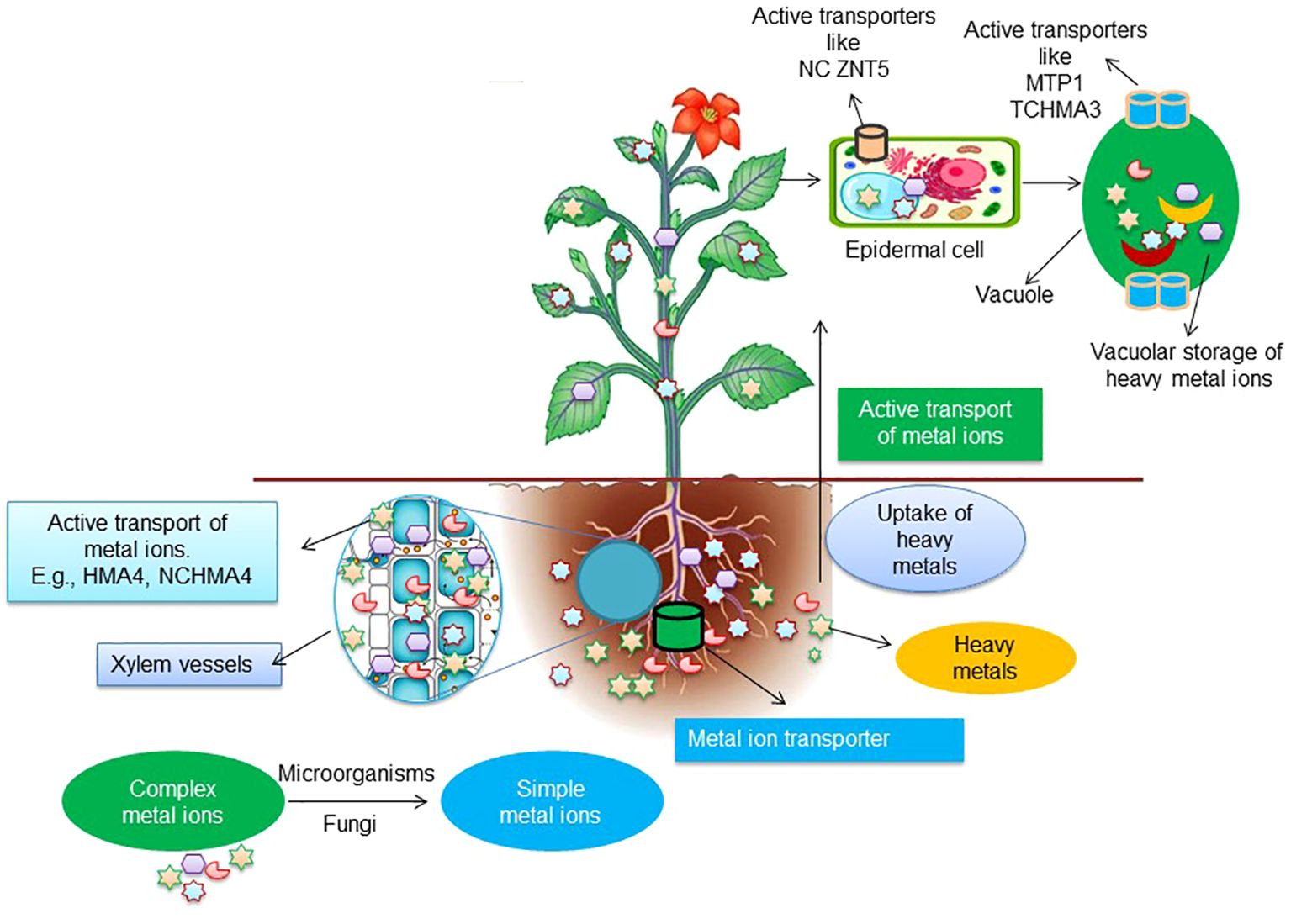
Figure 4. A model demonstrating the mechanism of transportation of heavy metals ions from roots to the aerial parts of plants.
ZIP family of selective cation transporters catalyzes the transport of Ni. This family of transporters encodes ZRT (zinc-regulated transporter)/IRT (iron-regulated transporter) proteins consisting of 8 transmembrane domains and a metal-binding domain in the extracellular loop of the transporter (Guerinot, 2000; Kochian, 2000; Rogers et al., 2000; Potocki et al., 2013). The IRT1 transporter uptakes metal ions via plasma membrane and through the trans-Golgi network, where they are found predominantly (Barberon et al., 2011). Even though the specificity of IRT1 varies among species, it is versatile for the transport of a wide range of metal ions such as Co, Cd, Ni, Fe, Zn, and Mn (Rogers et al., 2000; Schaaf et al., 2006; Halimaa et al., 2014, 2019). Vert et al. (2002) reported that IRT1 depends on the availability of the metal ion; for example, on the scarce availability of Fe, other metal ions are transported to IRT1 found in rhizodermal root cells. The uptake of metal ions occurs through cellular metal ion transporters such as ZIP4 for Cd, Cu, Zn, ZIP6 for transport of Zn and Mn, and only Zn by ZIP10/11 (Krämer et al., 2007; Corso and de la Torre, 2020). Other transporters help accumulate metal ions; for example, Cu influx occurs via ATPase HMA5I, and transport from apoplast to the cytosol occurs via NRAMP1 transporter. The long-distance transport of metal ions is regulated by several transporters, such as HMA4 (heavy metal ATPase 4), the YSL (yellow stripe-like) family, and ZIP (zinc-regulated transporter, iron-regulated transporter-related protein) family members (Verbruggen et al., 2009b). Moreover, other transporters involved in long-distance metal transport include TcYSL3 and TcYSL7 (Gendre et al., 2007). It is also reported that vacuolar sequestration of metal ions occurs through NRAMPs (natural resistance-associated macrophages), HMA3, and MTPs (metal transport proteins) (Verbruggen et al., 2009b). The AhHMA4 encodes P-type ATPases found on the plasma membrane of hyperaccumulators and is responsible for pumping metal ions such as Zn and Cd to confer their tolerance (Talke et al., 2006; Courbot et al., 2007).
Expression analysis identified the AhHMA4 transporter (Hanikenne et al., 2008) to mediate the xylem loading of metal ions in roots and leaves. In addition, the transport of Zn from roots and shoots was reported to be mediated by SaZIP4 transporter S. alfredii (Yang et al., 2018). It was further validated by deploying RNA interference studies that Zn and Cd were highly transported by AhHMA4 transporter (Hanikenne et al., 2008). Accumulation and sequestration of Zn metal ions was subjected to the overexpression of Zn2+/H+ transporter-MTP1 on the vacuolar membranes (Desbrosses-Fonrouge et al., 2005; Gustin et al., 2009) as the MTP1 found in epidermal, and mesophyll cells have been reported to be involved in the influx of Cd, Ni and Zn in the vacuoles (Küpper and Kochian, 2010; Schneider et al., 2013).
Notably, transporters differ in their mechanism of metal ion transport, such as sequestration of Cd by TcHMA3 transporter onto foliar vacuoles and decreasing the effect of Cd on photosynthesis SpHMA1chloroplast Cd exporters (Ueno et al., 2011; Zhao et al., 2019). Puig (2014) reported that copper hyperaccumulation occurs through a COPT1 transporter belonging to the Ctrs family on the plasma membrane. The primary expression sites of this transporter include embryos, cotyledons, root tips, trichomes, pollen grains, and guard cells (Sancenón et al., 2004). CDF transporters predominantly mediate the transport of metal ions into the vacuole and Golgi complex. Metal ions modulated these transporters to induce conformational changes and transport specific metal ions (Andresen et al., 2018). MTP1 is the versatile transporter identified in the vacuolar membranes to transport Zn, in some instances Co, Cd, Fe (II), and Ni (Krämer et al., 2007; Krämer, 2010; Hanikenne and Nouet, 2011; Sharma et al., 2016). Both A. halleri and N. goesingensis possess tolerance to Zn due to the presence of MTP1 transporter on vacuolar membranes of shoot cells (Dräger et al., 2004; Gustin et al., 2009; Meyer et al., 2010; Shahzad et al., 2010). Cd is primarily accumulated through plant roots by transporters such as AtNRAMP6, OsNRAMP5, OsNRAMP1, AtIRT1 and TcZNT1/TcZIP4 (Lux et al., 2011; Sasaki et al., 2012). These transporters are involved in several hyperaccumulators metal ion uptake, transport, and detoxification. The aqua glyceroporins of the NIP (Nodulin 26-like Intrinsic Proteins) are reported as probable transporter proteins of arsenic (As) in plants such as P. vitata (Ma et al., 2008; Kamiya et al., 2009). In addition, transporters, such as AtMTP11, CsMTP8, OsMTP8.1, and ShMTP1, are identified to help transport manganese into vacuoles (Chen et al., 2013; Migocka et al., 2014). Besides, plant-microbe interactions significantly enhance hyperaccumulation and metal tolerance by facilitating metal mobilization, uptake, and detoxification. Beneficial microbes, such as rhizobacteria and mycorrhizal fungi, produce siderophores, organic acids, and phytohormones that solubilize metals, making them more bioavailable for plant uptake, while also improving root growth and nutrient acquisition. Additionally, endophytic and rhizospheric microbes can sequester metals within their cells or bind them extracellularly, reducing toxicity to the plant. These interactions further induce plant stress responses, such as the upregulation of metal transporters (e.g., ZIP, NRAMP) and phytochelatin synthesis, enhancing metal accumulation and tolerance. Thus, symbiotic microbial communities play a crucial role in optimizing hyperaccumulator efficiency for phytoremediation.
3.3 Hyperaccumulators in phytoremediation: promise, pitfalls, and the path forward
Hyperaccumulators have garnered significant attention for their potential in phytoremediation due to their ability to absorb and tolerate high concentrations of HMs and other pollutants (Yang et al., 2022). However, an overly optimistic focus on their capabilities often overlooks critical limitations that hinder their practical application. One major constraint is their typically low biomass production, which limits the total quantity of contaminants that can be extracted from the soil within a given timeframe (Yang et al., 2022; Deng et al., 2024). Additionally, many hyperaccumulator species exhibit slow growth rates, further reducing their efficiency in large-scale remediation projects. These biological constraints are compounded by environmental factors, such as soil composition and climate conditions, which may restrict their adaptability to diverse contaminated sites (Sánchez-Castro et al., 2023; Aryal, 2024). Moreover, the exclusive focus on hyperaccumulation tends to disregard potential trade-offs, such as; reduced competitive ability in natural ecosystems or increased susceptibility to pests and diseases. Without addressing these limitations, the feasibility of deploying hyperaccumulators in real-world remediation scenarios remains uncertain. A more balanced assessment that acknowledges both their potential and their shortcomings is necessary to develop realistic strategies for effective phytoremediation (Skuza et al., 2022). Future research should prioritize overcoming these challenges through genetic engineering, agronomic practices, or complementary technologies to enhance their practicality and scalability.
4 Conclusion and future research gaps in understanding transport regulations and metal specificity
Heavy metal pollution is a global problem worsened by different anthropogenic activities. The latter reason has pushed the scientific communities to intensify research on dealing with the phytotoxicity meditated by heavy metal ions. The metal ion toxicity can be significantly dealt with by the intervention of metal ion hyperaccumulators, which can accumulate metal ions 100-folds more than non-accumulators. Hyperaccumulator plants, the versatile plant species employed for enhancing phytoextraction, phytomining, and metal ion detoxification, are great reservoirs of genes utilized to circumvent the metal ion toxicity and remediation of contaminated soils. Many hyperaccumulators have been documented, which reduce the concentration of metal ions and detoxify them to the optimum level. In conjunction with physiological and adaptive mechanisms, their molecular studies have provided deep insights into understanding the importance of hyperaccumulators as a potential remedy for obtaining contamination-free soils. For example, physiological studies carried out in different populations of T. caerulescens revealed differential affinities to metal ions and the mechanism of hyperaccumulation (Zhao et al., 2002; Assunção et al., 2008). High copy number and gene duplication of transporter genes such as ZIP4/ZNT1-2 and IRT1 in T. caerulescens and HMA4, MTP1, ZIP3, and ZIP9 in A. halleri have primarily contributed to the attribute of hyperaccumulation (Hanikenne et al., 2008). Expression analysis revealed hyperaccumulators differentially express transporter genes against the metal ion concentration in contaminated soils. They respond to the higher concentration of metal ions in elevating the expression of many genes, as mentioned in the above sections of this review. Most of these genes encode transport proteins for long-distance transport and transport with cells via plasma membrane and cellular organelles for accumulation and detoxification. Consequently, it is evident from the reviewed literature that further quest is required to unravel the physiological and molecular mechanism adapted by metal ion hyperaccumulators to withstand and hyperaccumulate toxic metals from diverse soils. In addition, insight into understanding metal ion homeostasis and regulation of the metal ion concentration in cells is of prime interest.
It must be realized that there is a lack of systematic identification and screening of plants and potential hyperaccumulators (Reeves et al., 2018). The unavailability of novel molecular approaches to decipher the exact mechanism of metal ion hyperaccumulation hinders the fruitful utilization of hyperaccumulators. Unfortunately, significantly little literature has been generated on the importance of microbiomes found in the rhizosphere of hyperaccumulators augmenting the accumulation of metal ions through roots. In addition, it must be of prime interest to the scientific community to further investigate energy allocation to hyperaccumulate metal ions. Understanding genomic evolution in correlation with ecological genomics may further pave the way to broaden our understanding of the mechanism of hyperaccumulation. Strategies including a rise in biomass or the ability to absorb and sequester (HMs) are additional major areas that demand study. However, the introduction of genetically modified plants may threaten the biodiversity of a region through the formation of superweeds, mixing and outcompeting with native species, cross-pollination with other plants, and altering the environment and the sustainable conditions for biological control agents. Before their actual deployment, therefore, substantial research measuring their influence on native biodiversity and the environment should be conducted. The employment of rhizospheric bacteria to drive root proliferation, boost plant development, and increase heavy metal tolerance and plant fitness may also offer feasible alternatives. Knowledge of the exact pathways by which each heavy metal is taken up, translocated, and sequestered in plants, identification and understanding of the role of each component of the pathway, the effect/use of the metal in the metabolic processes, and the long-term effects of large-scale phytoremediation will aid in the design of ideal plant species for hyperaccumulation by genetic engineering and other mentioned methods.
Future research should focus on genetic engineering and breeding strategies to enhance hyper-accumulation traits in high-biomass plants, leveraging CRISPR-Cas9 and omics technologies to optimize metal transporter expression and detoxification pathways (Oubohssaine and Dahmani, 2024). Additionally, integrating phytoremediation with bioenergy production (e.g., using Miscanthus or Helianthus annuus) could improve economic viability (Shackirea et al., 2022). Exploring synthetic biology to design novel chelators or hyper-accumulation pathways may further revolutionize HM remediation (Rafeeq et al., 2023). Field-scale studies, long-term ecological monitoring, and policy frameworks for phytoremediation adoption are essential to translate laboratory successes into real-world applications (Tripathi et al., 2020). Ultimately, interdisciplinary approaches combining plant physiology, microbiology, and biotechnology will be crucial in addressing global HM contamination sustainably. Achieving sustainability in phytoremediation requires a multifaceted approach to minimize phytotoxicity caused by HMs while enhancing plant efficiency (Ashkanani et al., 2024). One promising strategy is the use of soil amendments, such as; biochar, compost, and chelating agents (e.g., EDTA), which can reduce HM bioavailability and mitigate plant stress (Yin et al., 2024). Additionally, microbial-assisted phytoremediation, where plant growth-promoting rhizobacteria (PGPR) and mycorrhizal fungi enhance metal uptake and tolerance, has shown significant potential (Bhat et al., 2022b). Genetic engineering and breeding of hyperaccumulator plants to improve their metal accumulation capacity and stress resilience could further optimize phytoremediation (Nurrahma et al., 2024). The circular economy approach can be integrated by utilizing hyperaccumulator biomass in metal recovery (phytomining) or bioenergy production, reducing waste and creating economic value (Mandal et al., 2024). However, careful management is needed to prevent secondary contamination from harvested biomass. Furthermore, intercropping hyperaccumulators with cash crops could provide dual benefits soil remediation and agricultural productivity while minimizing land-use conflicts (Deng et al., 2024). Besides, understanding epigenetics’s role in adapting hyperaccumulators in extreme environmental conditions needs extensive research.
Despite significant advances, key gaps remain in understanding transporter regulation and metal specificity at the molecular level, particularly in elucidating the dynamic conformational changes that govern metal selectivity and transport efficiency. The precise mechanisms by which post-translational modifications, allosteric effectors, and cellular signaling pathways modulate transporter activity are still unclear, as are the structural determinants that enable certain transporters to discriminate between chemically similar metal ions. Additionally, the interplay between metal availability, transporter expression, and cellular homeostasis in different physiological and pathological contexts requires further exploration. High-resolution structural studies under physiologically relevant conditions, combined with advanced computational and functional assays, are needed to uncover these complexities and provide a comprehensive understanding of transporter regulation and metal specificity.
Author contributions
BB: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. MR: Writing – original draft, Writing – review & editing. TB: Writing – original draft, Writing – review & editing. RN: Writing – original draft, Writing – review & editing. RM: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. RQ: Conceptualization, Software, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adeyemi, D. A. (2021). Evaluation of Selected Heavy Metals in Soil and Well Water Around Industrial Locations in Ilorin Metropolis. (Master's thesis, Nigeria: Kwara State University)
Ali, A., Bhat, B. A., Rather, G. A., Malla, B. A., and Ganie, S. A. (2020). “Proteomic studies of micronutrient deficiency and toxicity,” in Plant micronutrients (Springer), 257–284.
Ali, H., Khan, E., and Sajad, M. A. (2013). Phytoremediation of heavy metals—concepts and applications. Chemosphere 91, 869–881. doi: 10.1016/j.chemosphere.2013.01.075
Ali, S., Tyagi, A., Park, S., Mir, R. A., Mushtaq, M., Bhat, B., et al. (2022). Deciphering the plant microbiome to improve drought tolerance: mechanisms and perspectives. Environ. Exp. Bot. 201, 104933. doi: 10.1016/j.envexpbot.2022.104933
Andresen, E., Peiter, E., and Küpper, H. (2018). Trace metal metabolism in plants. J. Exp. Bot. 69, 909–954. doi: 10.1093/jxb/erx465
Angulo-Bejarano, P. I., Puente-Rivera, J., and Cruz-Ortega, R. (2021). Metal and metalloid toxicity in plants: an overview on molecular aspects. Plants 10, 635. doi: 10.3390/plants10040635
Anning, A. K. and Akoto, R. (2018). Assisted phytoremediation of heavy metal contaminated soil from a mined site with Typha latifolia and Chrysopogon zizanioides. Ecotoxicol. Environ. Saf. 148, 97–104. doi: 10.1016/j.ecoenv.2017.10.014
Antoniadis, V., Polyzois, T., Golia, E. E., and Petropoulos, S. A. (2017). Hexavalent chromium availability and phytoremediation potential of Cichorium spinosum as affect by manure, zeolite and soil ageing. Chemosphere 171, 729–734. doi: 10.1016/j.chemosphere.2016.11.146
Arora, N. K. and Chauhan, R. (2021). Heavy metal toxicity and sustainable interventions for their decontamination. Environ. Sustain. 4, 1–3. doi: 10.1007/s42398-021-00164-y
Aryal, M. (2024). Phytoremediation strategies for mitigating environmental toxicants. Heliyon 10. doi: 10.1016/j.heliyon.2024.e38683
Ashkanani, Z., Mohtar, R., Al-Enezi, S., Smith, P. K., Calabrese, S., Ma, X., et al. (2024). AI-assisted systematic review on remediation of contaminated soils with PAHs and heavy metals. J. Hazardous Materials 468, 133813. doi: 10.1016/j.jhazmat.2024.133813
Ashraf, M. A., Maah, M. J., and Yusoff, I. (2011). Heavy metals accumulation in plants growing in ex tin mining catchment. Int. J. Environ. Sci. Technol. 8, 401–416. doi: 10.1007/BF03326227
Assunção, A. G., Bleeker, P., Wilma, M., Vooijs, R., and Schat, H. (2008). Intraspecific variation of metal preference patterns for hyperaccumulation in Thlaspi caerulescens: evidence from binary metal exposures. Plant Soil 303, 289–299. doi: 10.1007/s11104-007-9508-x
Assunção, A. G. L., Herrero, E., Lin, Y.-F., Huettel, B., Talukdar, S., Smaczniak, C., et al. (2010). Arabidopsis thaliana transcription factors bZIP19 and bZIP23 regulate the adaptation to zinc deficiency. Proc. Natl. Acad. Sci. 107, 10296–10301. doi: 10.1073/pnas.1004788107
Assunção, A., Martins, P. D. C., De Folter, S., Vooijs, R., Schat, H., and Aarts, M. (2001). Elevated expression of metal transporter genes in three accessions of the metal hyperaccumulator Thlaspi caerulescens. Plant Cell Environ. 24, 217–226. doi: 10.1111/j.1365-3040.2001.00666.x
Assunção, A. G. L., Schat, H., and Aarts, M. G. M. (2003). Thlaspi caerulescens, an attractive model species to study heavy metal hyperaccumulation in plants. New Phytol. 159, 351–360.
Azevedo, R. and Rodriguez, E. (2012). Phytotoxicity of mercury in plants: a review. J. Bot., 2012848614.
Bahraminia, M., Zarei, M., Ronaghi, A., and Ghasemi-Fasaei, R. (2016). Effectiveness of arbuscular mycorrhizal fungi in phytoremediation of lead-contaminated soil by vetiver grass. Int. J. phytoremediation 18, 730–737.
Balafrej, H., Bogusz, D., Triqui, Z.-E. A., Guedira, A., Bendaou, N., Smouni, A., et al. (2020). Zinc hyperaccumulation in plants: A review. Plants 9, 562. doi: 10.3390/plants9050562
Bani, A., Echevarria, G., Sulçe, S., and Morel, J. L. (2015). Improving the agronomy of Alyssum murale for extensive phytomining: a five-year field study. Int. J. Phytoremediation 17, 117–127. doi: 10.1080/15226514.2013.862204
Barberon, M., Zelazny, E., Robert, S., Conéjéro, G., Curie, C., Friml, J., et al. (2011). Monoubiquitin-dependent endocytosis of the iron-regulated transporter 1 (IRT1) transporter controls iron uptake in plants. Proc. Natl. Acad. Sci. 108, E450–E458. doi: 10.1073/pnas.1100659108
Barra Caracciolo, A. and Terenzi, V. (2021). Rhizosphere microbial communities and heavy metals. Microorganisms 9, 1462. doi: 10.3390/microorganisms9071462
Basu, U., Riaz Ahmed, S., Bhat, B. A., Anwar, Z., Ali, A., Ijaz, A., et al. (2023). A CRISPR way for accelerating cereal crop improvement: Progress and challenges. Front. Genet. 13, 866976. doi: 10.3389/fgene.2022.866976
Baumann, A. (1885). Das verhalten von zinksalzen gegen pflanzen und im boden. Landwirtschaftliche Versuchsstation 31, 1–53.
Bech, J., Roca, N., Tume, P., Ramos-Miras, J., Gil, C., and Boluda, R. (2016). Screening for new accumulator plants in potential hazards elements polluted soil surrounding Peruvian mine tailings. Catena 136, 66–73. doi: 10.1016/j.catena.2015.07.009
Becher, M., Talke, I. N., Krall, L., and Krämer, U. (2004). Cross-species microarray transcript profiling reveals high constitutive expression of metal homeostasis genes in shoots of the zinc hyperaccumulator Arabidopsis halleri. Plant J. 37, 251–268. doi: 10.1046/j.1365-313X.2003.01959.x
Belouchrani, A. S., Mameri, N., Abdi, N., Grib, H., Lounici, H., and Drouiche, N. (2016). Phytoremediation of soil contaminated with Zn using Canola (Brassica napus L). Ecol. Eng. 95, 43–49. doi: 10.1016/j.ecoleng.2016.06.064
Bernard, C., Roosens, N., Czernic, P., Lebrun, M., and Verbruggen, N. (2004). A novel CPx-ATPase from the cadmium hyperaccumulator Thlaspi caerulescens. FEBS Lett. 569, 140–148. doi: 10.1016/j.febslet.2004.05.036
Bert, V., Bonnin, I., Saumitou-Laprade, P., De Laguérie, P., and Petit, D. (2002). Do Arabidopsis halleri from nonmetallicolous populations accumulate zinc and cadmium more effectively than those from metallicolous populations? New Phytol. 155, 47–57.
Bert, V., Macnair, M. R., De Laguerie, P., Saumitou-Laprade, P., and Petit, D. (2000). Zinc tolerance and accumulation in metallicolous and nonmetallicolous populations of Arabidopsis halleri (Brassicaceae). New Phytol. 146, 225–233. doi: 10.1046/j.1469-8137.2000.00634.x
Bhardwaj, S., Verma, T., Raza, A., and Kapoor, D. (2023). Silicon and Nitric Oxide-Mediated Regulation of Growth Attributes, Metabolites and Antioxidant Defense System of Radish (Raphanus sativus L.) under Arsenic Stress. Phyton-International J. Exp. Bot. 92, 763–782.
Bharwana, S. A., Ali, S., Farooq, M. A., Iqbal, N., Abbas, F., and Ahmad, M. S. A. (2013). Alleviation of lead toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes suppressed lead uptake and oxidative stress in cotton. J. Bioremed. Biodeg. 4, 10–4172.
Bhat, B. A., Islam, S. T., Ali, A., Sheikh, B. A., Tariq, L., Islam, S. U., et al. (2020). “Role of micronutrients in secondary metabolism of plants,” in Plant micronutrients (Springer), 311–329.
Bhat, B. A., Mir, R. A., Mir, W. R., Hamdani, S. S., and Mir, M. A. (2024). Transcription factors-golden keys to modulate the plant metabolism to develop salinity tolerance. Plant Stress, 100409. doi: 10.1016/j.stress.2024.100409
Bhat, B. A., Mir, R. A., Qadri, H., Dhiman, R., Almilaibary, A., Alkhanani, M., et al. (2023). Integrons in the development of antimicrobial resistance: critical review and perspectives. Front. Microbiol. 14, 1231938. doi: 10.3389/fmicb.2023.1231938
Bhat, B. A., Tariq, L., Nissar, S., Hamdani, S. S., Dar, M. A., Mehraj, S., et al. (2022a). “Role of Methyl Jasmonate in Mitigating Plant Stress and Its Interaction with Salicylic Acid,” in Plant Abiotic Stress Physiology (Apple Academic Press), 217–239.
Bhat, B. A., Tariq, L., Nissar, S., Islam, S. T., Islam, S. U., Mangral, Z., et al. (2022b). The role of plant-associated rhizobacteria in plant growth, biocontrol and abiotic stress management. J. Appl. Microbiol. 133, 2717–2741. doi: 10.1111/jam.15796
Bindu, T., Sumi, M. M., and Ramasamy, E. V. (2010). Decontamination of water polluted by heavy metals with Taro (Colocasia esculenta) cultured in a hydroponic NFT system. Environmentalist 30, 35–44. doi: 10.1007/s10669-009-9240-6
Blamey, F. P. C., Joyce, D. C., Edwards, D. G., and Asher, C. J. (1986). Role of trichomes in sunflower tolerance to manganese toxicity. Plant Soil 91, 171–180. doi: 10.1007/BF02181785
Bothe, H. (2011). “Plants in heavy metal soils,” in Detoxification of heavy metals (Springer), 35–57.
Bouman, R., Van Welzen, P., Sumail, S., Echevarria, G., Erskine, P. D., and van der Ent, A. (2018). Phyllanthus rufuschaneyi: a new nickel hyperaccumulator from Sabah (Borneo Island) with potential for tropical agromining. Botanical Stud. 59, 9. doi: 10.1186/s40529-018-0225-y
Boyd, R. S., Davis, M. A., and Balkwill, K. (2008). Elemental patterns in Ni hyperaccumulating and non-hyperaccumulating ultramafic soil populations of Senecio coronatus. South Afr. J. Bot. 74, 158–162. doi: 10.1016/j.sajb.2007.08.013
Briffa, J., Sinagra, E., and Blundell, R. (2020). Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 6. doi: 10.1016/j.heliyon.2020.e04691
Broadhurst, C. L., Chaney, R. L., Angle, J. S., Maugel, T. K., Erbe, E. F., and Murphy, C. A. (2004). Simultaneous hyperaccumulation of nickel, manganese, and calcium in Alyssum leaf trichomes. Environ. Sci. Technol. 38, 5797–5802. doi: 10.1021/es0493796
Cao, X., Dong, Q., Mao, L., Yang, X., Wang, X., and Zou, Q. (2025). Enhanced phytoextraction technologies for the sustainable remediation of cadmium-contaminated soil based on hyperaccumulators—A review. Plants 14, 115. doi: 10.3390/plants14010115
Chen, Z., Fujii, Y., Yamaji, N., Masuda, S., Takemoto, Y., Kamiya, T., et al. (2013). Mn tolerance in rice is mediated by MTP8. 1, a member of the cation diffusion facilitator family. J. Exp. Bot. 64, 4375–4387. doi: 10.1093/jxb/ert243
Chen, T., Lei, M., Wan, X., Zhou, X., Yang, J., Guo, G., et al. (2021). “Element case studies: arsenic,” in Agromining: Farming for Metals (Springer), 443–451.
Chen, Y., Shen, Z., and Li, X. (2004). The use of vetiver grass (Vetiveria zizanioides) in the phytoremediation of soils contaminated with heavy metals. Appl. Geochem. 19, 1553–1565. doi: 10.1016/j.apgeochem.2004.02.003
Chiang, H.-C., Lo, J.-C., and Yeh, K.-C. (2006). Genes associated with heavy metal tolerance and accumulation in Zn/Cd hyperaccumulator Arabidopsis halleri: a genomic survey with cDNA microarray. Environ. Sci. Technol. 40, 6792–6798. doi: 10.1021/es061432y
Clemens, S. (2001). Molecular mechanisms of plant metal tolerance and homeostasis. Planta 212, 475–486. doi: 10.1007/s004250000458
Clemens, S., Palmgren, M. G., and Krämer, U. (2002). A long way ahead: understanding and engineering plant metal accumulation. Trends Plant Sci. 7, 309–315. doi: 10.1016/S1360-1385(02)02295-1
Conn, S. and Gilliham, M. (2010). Comparative physiology of elemental distributions in plants. Ann. Bot. 105, 1081–1102. doi: 10.1093/aob/mcq027
Corso, M. and de la Torre, V. S. G. (2020). Biomolecular approaches to understanding metal tolerance and hyperaccumulation in plants. Metallomics 12, 840–859. doi: 10.1039/d0mt00043d
Corso, M., Schvartzman, M. S., Guzzo, F., Souard, F., Malkowski, E., Hanikenne, M., et al. (2018). Contrasting cadmium resistance strategies in two metallicolous populations of Arabidopsis halleri. New Phytol. 218, 283–297. doi: 10.1111/nph.14948
Cosio, C., Desantis, L., Frey, B., Diallo, S., and Keller, C. (2005). Distribution of cadmium in leaves of Thlaspi caerulescens. J. Exp. Bot. 56, 765–775. doi: 10.1093/jxb/eri062
Courbot, M., Willems, G., Motte, P., Arvidsson, S., Roosens, N., Saumitou-Laprade, P., et al. (2007). A major quantitative trait locus for cadmium tolerance in Arabidopsis halleri colocalizes with HMA4, a gene encoding a heavy metal ATPase. Plant Physiol. 144, 1052–1065. doi: 10.1104/pp.106.095133
Craciun, A. R., Courbot, M., Bourgis, F., Salis, P., Saumitou-Laprade, P., and Verbruggen, N. (2006). Comparative cDNA-AFLP analysis of Cd-tolerant and-sensitive genotypes derived from crosses between the Cd hyperaccumulator Arabidopsis halleri and Arabidopsis lyrata ssp. petraea. J. Exp. Bot. 57, 2967–2983. doi: 10.1093/jxb/erl062
Craciun, A. R., Meyer, C.-L., Chen, J., Roosens, N., De Groodt, R., Hilson, P., et al. (2012). Variation in HMA4 gene copy number and expression among Noccaea caerulescens populations presenting different levels of Cd tolerance and accumulation. J. Exp. Bot. 63, 4179–4189. doi: 10.1093/jxb/ers104
Cui, X., Zhang, J., Wang, X., Pan, M., Lin, Q., Khan, K. Y., et al. (2021). A review on the thermal treatment of heavy metal hyperaccumulator: Fates of heavy metals and generation of products. J. Hazardous Materials 405, 123832. doi: 10.1016/j.jhazmat.2020.123832
Dahmani-Muller, H., Van Oort, F., Gélie, B., and Balabane, M. (2000). Strategies of heavy metal uptake by three plant species growing near a metal smelter. Environ. pollut. 109, 231–238. doi: 10.1016/S0269-7491(99)00262-6
Dar, M. I., Naikoo, M. I., Green, I. D., Sayeed, N., Ali, B., and Khan, F. A. (2018). “Heavy metal hyperaccumulation and hypertolerance in Brassicaceae,” in Plants under Metal and Metalloid Stress (Springer), 263–276.
Dar, F. A., Pirzadah, T. B., and Malik, B. (2020). Accumulation of heavy metals in medicinal and aromatic plants. Plant Micronutrients: Deficiency Toxicity Manage. 113–127.
Dehkordi, M. M., Nodeh, Z. P., Dehkordi, K. S., Khorjestan, R. R., and Ghaffarzadeh, M. (2024). Soil, air, and water pollution from mining and industrial activities: Sources of pollution, environmental impacts, and prevention and control methods. Results Eng., 102729.
De La Fuente, V., Rufo, L., Rodríguez, N., Franco, A., and Amils, R. (2017). Comparison of iron localization in wild plants and hydroponic cultures of Imperata cylindrica (L.) P. Beauv. Plant Soil 418, 25–35. doi: 10.1007/s11104-017-3251-8
Deng, D. M., Shu, W. S., Zhang, J., Zou, H. L., Lin, Z., Ye, Z. H., et al. (2007). Zinc and cadmium accumulation and tolerance in populations of Sedum alfredii. Environ. pollut. 147, 381–386. doi: 10.1016/j.envpol.2006.05.024
Deng, S., Zhang, X., Zhu, Y., and Zhuo, R. (2024). Recent advances in phyto-combined remediation of heavy metal pollution in soil. Biotechnol. Adv., 108337. doi: 10.1016/j.biotechadv.2024.108337
Desbrosses-Fonrouge, A.-G., Voigt, K., Schröder, A., Arrivault, S., Thomine, S., and Krämer, U. (2005). Arabidopsis thaliana MTP1 is a Zn transporter in the vacuolar membrane which mediates Zn detoxification and drives leaf Zn accumulation. FEBS Lett. 579, 4165–4174. doi: 10.1016/j.febslet.2005.06.046
Dewanjee, S., Dua, T. K., Khanra, R., Das, S., Barma, S., Joardar, S., et al. (2015). Water spinach, Ipomoea aquatica (Convolvulaceae), ameliorates lead toxicity by inhibiting oxidative stress and apoptosis. PloS One 10, e0139831. doi: 10.1371/journal.pone.0139831
Doncheva, S. N., Poschenrieder, C., Stoyanova, Z., Georgieva, K., Velichkova, M., and Barceló, J. (2009). Silicon amelioration of manganese toxicity in Mn-sensitive and Mn-tolerant maize varieties. Environ. Exp. Bot. 65, 189–197. doi: 10.1016/j.envexpbot.2008.11.006
Dotaniya, M. L. and Meena, V. D. (2015). Rhizosphere effect on nutrient availability in soil and its uptake by plants: a review. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 85, 1–12.
Dräger, D. B., Desbrosses-Fonrouge, A. G., Krach, C., Chardonnens, A. N., Meyer, R. C., Saumitou-Laprade, P., et al. (2004). Two genes encoding Arabidopsis halleri MTP1 metal transport proteins co-segregate with zinc tolerance and account for high MTP1 transcript levels. Plant J. 39, 425–439.
Drozdova, I. V., Alekseeva-Popova, N. V., Kalimova, I. B., Belyaeva, A. I., and Smirnova, N. A. (2017). The accumulating ability and nickel tolerance of Brassicaceae species of the North Caucasus in connection with the problem of phytoremediation. J. Geochemical Explor. 182, 235–241. doi: 10.1016/j.gexplo.2017.03.001
Dueli, G. F., Desouza, O., and Ribeiro, S. P. (2021). Metal bioaccumulation alleviates the negative effects of herbivory on plant growth. Sci. Rep. 11, 1–11. doi: 10.1038/s41598-021-98483-x
Ebbs, S., Lau, I., Ahner, B., and Kochian, L. (2002). Phytochelatin synthesis is not responsible for Cd tolerance in the Zn/Cd hyperaccumulator Thlaspi caerulescens (J. & C. Presl). Planta 214, 635–640. doi: 10.1007/s004250100650
Farahat, E. A. and Galal, T. M. (2018). Trace metal accumulation by Ranunculus sceleratus: implications for phytostabilization. Environ. Sci. pollut. Res. 25, 4214–4222. doi: 10.1007/s11356-017-0808-2
Feki, K., Tounsi, S., Mrabet, M., Mhadhbi, H., and Brini, F. (2021). Recent advances in physiological and molecular mechanisms of heavy metal accumulation in plants. Environ. Sci. pollut. Res. 28, 64967–64986. doi: 10.1007/s11356-021-16805-y
Fernando, D. R., Marshall, A. T., Forster, P. I., Hoebee, S. E., and Siegele, R. (2013). Multiple metal accumulation within a manganese-specific genus. Am. J. Bot. 100, 690–700. doi: 10.3732/ajb.1200545
Fernando, D. R., Woodrow, I. E., Baker, A. J. M., and Marshall, A. T. (2012). Plant homeostasis of foliar manganese sinks: specific variation in hyperaccumulators. Planta 236, 1459–1470. doi: 10.1007/s00425-012-1699-6
Filatov, V., Dowdle, J., Smirnoff, N., Ford-Lloyd, B., Newbury, H. J., and Macnair, M. R. (2006). Comparison of gene expression in segregating families identifies genes and genomic regions involved in a novel adaptation, zinc hyperaccumulation. Mol. Ecol. 15, 3045–3059. doi: 10.1111/j.1365-294X.2006.02981.x
Freeman, J. L., Quinn, C. F., Marcus, M. A., Fakra, S., and Pilon-Smits, E. A. (2006). Selenium-tolerant diamondback moth disarms hyperaccumulator plant defense. Curr. Biol. 16, 2181–2192. doi: 10.1016/j.cub.2006.09.015
Frérot, H., Faucon, M. P., Willems, G., Godé, C., Courseaux, A., Darracq, A., et al. (2010). Genetic architecture of zinc hyperaccumulation in Arabidopsis halleri: the essential role of QTL× environment interactions. New Phytol. 187, 355–367.
Fukuda, N., Kitajima, N., Terada, Y., Abe, T., Nakai, I., and Hokura, A. (2020). Visible cellular distribution of cadmium and zinc in the hyperaccumulator Arabidopsis halleri ssp. gemmifera determined by 2-D X-ray fluorescence imaging using high-energy synchrotron radiation. Metallomics 12, 193–203. doi: 10.1039/c9mt00243j
Gaur, N., Flora, G., Yadav, M., and Tiwari, A. (2014). A review with recent advancements on bioremediation-based abolition of heavy metals. Environ. Sci.: Processes Impacts 16, 180–193. doi: 10.1039/C3EM00491K
Gay, D. D. and Butler, G. P. (1977). Movement of mercury-203 in plants (Environmental Protection Agency, Office of Research and Development).
Gendre, D., Czernic, P., Conéjéro, G., Pianelli, K., Briat, J. F., Lebrun, M., et al. (2007). TcYSL3, a member of the YSL gene family from the hyper-accumulator Thlaspi caerulescens, encodes a nicotianamine-Ni/Fe transporter. Plant J. 49, 1–15. doi: 10.1111/j.1365-313X.2006.02937.x
Goolsby, E. W. and Mason, C. M. (2015). Toward a more physiologically and evolutionarily relevant definition of metal hyperaccumulation in plants. Front. Plant Sci. 6, 33. doi: 10.3389/fpls.2015.00033
Guerinot, M. L. (2000). The ZIP family of metal transporters. Biochim. Biophys. Acta (BBA)-Biomembranes 1465, 190–198. doi: 10.1016/S0005-2736(00)00138-3
Gupta, D. K., Huang, H. G., and Corpas, F. J. (2013). Lead tolerance in plants: strategies for phytoremediation. Environ. Sci. pollut. Res. 20, 2150–2161. doi: 10.1007/s11356-013-1485-4
Gupta, P. K., Saxena, G., and Yadav, B. (2022). “Selenium and naturally occurring radioactive contaminants in soil–water systems,” in Advances in Remediation Techniques for Polluted Soils and Groundwater (Elsevier), 259–267.
Gustin, J. L., Loureiro, M. E., Kim, D., Na, G., Tikhonova, M., and Salt, D. E. (2009). MTP1-dependent Zn sequestration into shoot vacuoles suggests dual roles in Zn tolerance and accumulation in Zn-hyperaccumulating plants. Plant J. 57, 1116–1127. doi: 10.1111/j.1365-313X.2008.03754.x
Ha, N. T. H., Sakakibara, M., and Sano, S. (2011). Accumulation of Indium and other heavy metals by Eleocharis acicularis: an option for phytoremediation and phytomining. Bioresource Technol. 102, 2228–2234. doi: 10.1016/j.biortech.2010.10.014
Halimaa, P., Blande, D., Baltzi, E., Aarts, M. G., Granlund, L., Keinänen, M., et al. (2019). Transcriptional effects of cadmium on iron homeostasis differ in calamine accessions of Noccaea caerulescens. Plant J. 97, 306–320. doi: 10.1111/tpj.14121
Halimaa, P., Lin, Y.-F., Ahonen, V. H., Blande, D., Clemens, S., Gyenesei, A., et al. (2014). Gene expression differences between Noccaea caerulescens ecotypes help to identify candidate genes for metal phytoremediation. Environ. Sci. Technol. 48, 3344–3353. doi: 10.1021/es4042995
Hammond, J. P., Bowen, H. C., White, P. J., Mills, V., Pyke, K. A., Baker, A. J. M., et al. (2006). A comparison of the Thlaspi caerulescens and Thlaspi arvense shoot transcriptomes. New Phytol. 170, 239–260. doi: 10.1111/j.1469-8137.2006.01662.x
Hanikenne, M. and Nouet, C. (2011). Metal hyperaccumulation and hypertolerance: a model for plant evolutionary genomics. Curr. Opin. Plant Biol. 14, 252–259. doi: 10.1016/j.pbi.2011.04.003
Hanikenne, M., Talke, I. N., Haydon, M. J., Lanz, C., Nolte, A., Motte, P., et al. (2008). Evolution of metal hyperaccumulation required cis-regulatory changes and triplication of HMA4. Nature 453, 391–395. doi: 10.1038/nature06877
Harumain, Z., Parker, H. L., MuñOz GarcíA, A., Austin, M. J., Mcelroy, C. R., Hunt, A. J., et al. (2017). Toward financially viable phytoextraction and production of plant-based palladium catalysts. Environ. Sci. Technol. 51, 2992–3000.
Hassinen, V. H., Tervahauta, A. I., Halimaa, P., Plessl, M., Peräniemi, S., Schat, H., et al. (2007). Isolation of Zn-responsive genes from two accessions of the hyperaccumulator plant Thlaspi caerulescens. Planta 225, 977–989. doi: 10.1007/s00425-006-0403-0
Haug, C. M., Reimer, K. J., and Cullen, W. R. (2004). Arsenic uptake by the Douglas-fir (Pseudotsuga menziesie). Appl. organometallic Chem. 18, 626–630. doi: 10.1002/aoc.656
Hembrom, S., Singh, B., Gupta, S. K., and Nema, A. K. (2020). A comprehensive evaluation of heavy metal contamination in foodstuff and associated human health risk: a global perspective. Contemporary Environmental Issues and Challenges in Era of Climate Change. 33–63.
Huguet, S., Bert, V., Laboudigue, A., Barthès, V., Isaure, M.-P., Llorens, I., et al. (2012). Cd speciation and localization in the hyperaccumulator Arabidopsis halleri. Environ. Exp. Bot. 82, 54–65. doi: 10.1016/j.envexpbot.2012.03.011
Imperiale, D., Lencioni, G., Marmiroli, M., Zappettini, A., White, J. C., and Marmiroli, N. (2022). Interaction of hyperaccumulating plants with Zn and Cd nanoparticles. Sci. Total Environ., 152741.
Isaure, M.-P., Huguet, S., Meyer, C.-L., Castillo-Michel, H., Testemale, D., Vantelon, D., et al. (2015). Evidence of various mechanisms of Cd sequestration in the hyperaccumulator Arabidopsis halleri, the non-accumulator Arabidopsis lyrata, and their progenies by combined synchrotron-based techniques. J. Exp. Bot. 66, 3201–3214. doi: 10.1093/jxb/erv131
Jaffré, T., Brooks, R. R., Lee, J., and Reeves, R. D. (1976). Sebertia acuminata: a hyperaccumulator of nickel from New Caledonia. Science 193, 579–580.
Jaffré, T., Pillon, Y., Thomine, S., and Merlot, S. (2013). The metal hyperaccumulators from New Caledonia can broaden our understanding of nickel accumulation in plants. Front. Plant Sci. 4, 279. doi: 10.3389/fpls.2013.00279
Jamla, M., Khare, T., Joshi, S., Patil, S., Penna, S., and Kumar, V. (2021). Omics approaches for understanding heavy metal responses and tolerance in plants. Curr. Plant Biol. 27, 100213. doi: 10.1016/j.cpb.2021.100213
Jiang, H., Lin, W., Jiao, H., Liu, J., Chan, L., Liu, X., et al. (2021). Uptake, transport, and metabolism of selenium and its protective effects against toxic metals in plants: a review. Metallomics 13, mfab040. doi: 10.1093/mtomcs/mfab040
Jogawat, A., Yadav, B., and Narayan, O. P. (2021). Metal transporters in organelles and their roles in heavy metal transportation and sequestration mechanisms in plants. Physiologia Plantarum. doi: 10.1111/ppl.13370
Kamiya, T., Tanaka, M., Mitani, N., Ma, J. F., Maeshima, M., and Fujiwara, T. (2009). NIP1; 1, an aquaporin homolog, determines the arsenite sensitivity of Arabidopsis thaliana. J. Biol. Chem. 284, 2114–2120. doi: 10.1074/jbc.M806881200
Karimi, N., Ghaderian, S. M., Raab, A., Feldmann, J., and Meharg, A. A. (2009). An arsenic-accumulating, hypertolerant brassica, Isatis capadocica. New Phytol., 41–47. doi: 10.1111/j.1469-8137.2009.02982.x
Kaur, H. and Garg, N. (2021). Zinc toxicity in plants: a review. Planta 253, 1–28. doi: 10.1007/s00425-021-03642-z
Kaushal, J., Mahajan, P., and Kaur, N. (2021). A review on application of phytoremediation technique for eradication of synthetic dyes by using ornamental plants. Environ. Sci. pollut. Res., 1–20. doi: 10.1007/s11356-021-16672-7
Kochian, L. V. (2000). Molecular physiology of mineral nutrient acquisition, transport, and utilization. Biochem. Mol. Biol. Plants, 1204–1249.
Kozhevnikova, A. D., Seregin, I., Gosti, F., and Schat, H. (2017). Zinc accumulation and distribution over tissues in Noccaea сaerulescens in nature and in hydroponics: a comparison. Plant Soil 411, 5–16. doi: 10.1007/s11104-016-3116-6
Krämer, U. (2010). Metal hyperaccumulation in plants. Annu. Rev. Plant Biol. 61, 517–534. doi: 10.1146/annurev-arplant-042809-112156
Krämer, U., Talke, I. N., and Hanikenne, M. (2007). Transition metal transport. FEBS Lett. 581, 2263–2272. doi: 10.1016/j.febslet.2007.04.010
Kumar, B., Smita, K., and Flores, L. C. (2017). Plant mediated detoxification of mercury and lead. Arabian J. Chem. 10, S2335–S2342. doi: 10.1016/j.arabjc.2013.08.010
Kuppan, N., Padman, M., Mahadeva, M., Srinivasan, S., and Devarajan, R. (2024). A comprehensive review of sustainable bioremediation techniques: Eco friendly solutions for waste and pollution management. Waste Manage. Bulletin. doi: 10.1016/j.wmb.2024.07.005
Küpper, H., Götz, B., Mijovilovich, A., Küpper, F. C., and Meyer-Klaucke, W. (2009). Complexation and toxicity of copper in higher plants. I. Characterization of copper accumulation, speciation, and toxicity in Crassula helmsii as a new copper accumulator. Plant Physiol. 151, 702–714. doi: 10.1104/pp.109.139717
Küpper, H. and Kochian, L. V. (2010). Transcriptional regulation of metal transport genes and mineral nutrition during acclimatization to cadmium and zinc in the Cd/Zn hyperaccumulator, Thlaspi caerulescens (Ganges population). New Phytol. 185, 114–129. doi: 10.1111/j.1469-8137.2009.03051.x
Küpper, H., Lombi, E., Zhao, F.-J., and Mcgrath, S. P. (2000). Cellular compartmentation of cadmium and zinc in relation to other elements in the hyperaccumulator Arabidopsis halleri. Planta 212, 75–84. doi: 10.1007/s004250000366
Lasat, M. M., Pence, N. S., Garvin, D. F., Ebbs, S. D., and Kochian, L. V. (2000). Molecular physiology of zinc transport in the Zn hyperaccumulator Thlaspi caerulescens. J. Exp. Bot. 51, 71–79. doi: 10.1093/jxb/51.342.71
Li, X., Cen, H., Chen, Y., Xu, S., Peng, L., Zhu, H., et al. (2016). Physiological analyses indicate superoxide dismutase, catalase, and phytochelatins play important roles in Pb tolerance in Eremochloa ophiuroides. Int. J. phytoremediation 18, 251–260. doi: 10.1080/15226514.2015.1084994
Li, W. C., Ye, Z. H., and Wong, M. H. (2007). Effects of bacteria on enhanced metal uptake of the Cd/Zn-hyperaccumulating plant, Sedum alfredii. J. Exp. Bot. 58, 4173–4182. doi: 10.1093/jxb/erm274
Liao, Q. L., Liu, C., Wu, H. Y., Jin, Y., Hua, M., Zhu, B. W., et al. (2015). Association of soil cadmium contamination with ceramic industry: A case study in a Chinese town. Sci. Total Environ. 514, 26–32. doi: 10.1016/j.scitotenv.2015.01.084
Lima, L. W., Pilon-Smits, E., and Schiavon, M. (2018). Mechanisms of selenium hyperaccumulation in plants: A survey of molecular, biochemical and ecological cues. Biochim. Biophys. Acta (BBA)-General Subj. 1862, 2343–2353. doi: 10.1016/j.bbagen.2018.03.028
Lin, Y. F., Liang, H. M., Yang, S. Y., Boch, A., Clemens, S., Chen, C. C., et al. (2009). Arabidopsis IRT3 is a zinc-regulated and plasma membrane localized zinc/iron transporter. New Phytol. 182, 392–404. doi: 10.1111/j.1469-8137.2009.02766.x
Lira-Morales, J. D., Varela-Bojórquez, N., Montoya-Rojo, M. B., and Sañudo-Barajas, J. A. (2019). The role of ZIP proteins in zinc assimilation and distribution in plants: current challenges. Czech J. Genet. Plant Breed. 55, 45–54. doi: 10.17221/54/2018-CJGPB
Liu, S., Ali, S., Yang, R., Tao, J., and Ren, B. (2019). A newly discovered Cd-hyperaccumulator Lantana camara L. J. hazardous materials 371, 233–242. doi: 10.1016/j.jhazmat.2019.03.016
Liu, Z., Chen, M., Lin, M., Chen, Q., Lu, Q., Yao, J., et al. (2022). Cadmium uptake and growth responses of seven urban flowering plants: hyperaccumulator or bioindicator? Sustainability 14, 619. doi: 10.3390/su14020619
Liu, X., Fu, J. W., Da Silva, E., Shi, X. X., Cao, Y., Rathinasabapathi, B., et al. (2017). Microbial siderophores and root exudates enhanced goethite dissolution and Fe/As uptake by As-hyperaccumulator Pteris vittata. Environ. pollut. 223, 230–237. doi: 10.1016/j.envpol.2017.01.016
Lloyd-Thomas, D. H. (1995). Heavy metal hyperaccumulation by Thlaspi caerulescens J. & C. Presl. PhD disss., University of Sheffield.
Lombi, E., Zhao, F. J., Mcgrath, S. P., Young, S. D., and Sacchi, G. A. (2001). Physiological evidence for a high-affinity cadmium transporter highly expressed in a Thlaspi caerulescens ecotype. New Phytol. 149, 53–60. doi: 10.1046/j.1469-8137.2001.00003.x
Lone, I. A. and Gaffar, M. (2021). “Phytoremediation Potential of Medicinal and Aromatic Plants,” in Medicinal and Aromatic Plants (Springer), 741–760.
Lux, A., Vaculík, M., Martinka, M., Lišková, D., Kulkarni, M. G., Stirk, W. A., et al. (2011). Cadmium induces hypodermal periderm formation in the roots of the monocotyledonous medicinal plant Merwilla plumbea. Ann. Bot. 107, 285–292. doi: 10.1093/aob/mcq240
Ma, J. F., Yamaji, N., Mitani, N., Xu, X.-Y., Su, Y.-H., Mcgrath, S. P., et al. (2008). Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc. Natl. Acad. Sci. 105, 9931–9935. doi: 10.1073/pnas.0802361105
Major, J. (2010). Guidelines on practical aspects of biochar application to field soil in various soil management systems. Int. Biochar. Initiative 8, 5–7.
Malik, B., Pirzadah, T. B., Tahir, I., Dar, T. U. H., and Rehman, R. U. (2015). Recent trends and approaches in phytoremediation. Soil remediation Plants 75, 131–146. doi: 10.1016/B978-0-12-799937-1.00006-1
Manara, A., Fasani, E., Furini, A., and Dalcorso, G. (2020). Evolution of the metal hyperaccumulation and hypertolerance traits. Plant Cell Environment. doi: 10.1111/pce.13821
Mandal, R. R., Bashir, Z., Mandal, J. R., and Raj, D. (2024). Potential strategies for phytoremediation of heavy metals from wastewater with circular bioeconomy approach. Environ. Monit. Assess. 196, 502. doi: 10.1007/s10661-024-12680-5
Mari, S. and Lebrun, M. (2005). “Metal immobilization: where and how?,” in Molecular biology of metal homeostasis and detoxification (Springer), 273–298.
Massa, N., Andreucci, F., Poli, M., Aceto, M., Barbato, R., and Berta, G. (2010). Screening for heavy metal accumulators amongst autochtonous plants in a polluted site in Italy. Ecotoxicol. Environ. Saf. 73, 1988–1997. doi: 10.1016/j.ecoenv.2010.08.032
Mengel, K. and Kirkby, E. A. (1987). Principles of plant nutrition (Bern: International Potash Institute), 687–695.
Merlot, S., de la Torre, V. S. G., and Hanikenne, M. (2018). “Physiology and molecular biology of trace element hyperaccumulation,” in Agromining: farming for metals (Springer), 93–116.
Meyer, C. L., Kostecka, A. A., Saumitou-Laprade, P., Créach, A., Castric, V., Pauwels, M., et al. (2010). Variability of zinc tolerance among and within populations of the pseudometallophyte species Arabidopsis halleri and possible role of directional selection. New Phytol. 185, 130–142. doi: 10.1111/j.1469-8137.2009.03062.x
Migocka, M., Papierniak, A., Maciaszczyk-Dziubińska, E., Poździk, P., Posyniak, E., Garbiec, A., et al. (2014). Retracted: Cucumber metal transport protein MTP8 confers increased tolerance to manganese when expressed in yeast and Arabidopsis thaliana (UK: Oxford University Press).
Milner, M. J., Craft, E., Yamaji, N., Koyama, E., Ma, J. F., and Kochian, L. V. (2012). Characterization of the high affinity Zn transporter from Noccaea caerulescens, NcZNT1, and dissection of its promoter for its role in Zn uptake and hyperaccumulation. New Phytol. 195, 113–123. doi: 10.1111/j.1469-8137.2012.04144.x
Minguzzi, C. (1948). Il contenuto di nichel nelle ceneri di Alyssum bertolonii Desv. Mem. Soc Tosc. Sci. Nat. Ser. A 55, 49–74.
Mir, R. A., Bhat, B. A., Yousuf, H., Islam, S. T., Raza, A., Rizvi, M. A., et al. (2022). Multidimensional role of silicon to activate resilient plant growth and to mitigate abiotic stress. Front. Plant Sci. 13, 819658. doi: 10.3389/fpls.2022.819658
Mishra, S., Mishra, A., and Küpper, H. (2017). Protein biochemistry and expression regulation of cadmium/zinc pumping ATPases in the hyperaccumulator plants Arabidopsis halleri and Noccaea caerulescens. Front. Plant Sci. 8, 835. doi: 10.3389/fpls.2017.00835
Mleczek, M., Gąsecka, M., Waliszewska, B., Magdziak, Z., Szostek, M., Rutkowski, P., et al. (2018). Salix viminalis L.-A highly effective plant in phytoextraction of elements. Chemosphere 212, 67–78. doi: 10.1016/j.chemosphere.2018.08.055
Moameri, M., Jafri, M., Tavili, A., Motasharezadeh, B., and Zare Chahouki, M. (2017). Rangeland plants potential for phytoremediation of contaminated soils with lead, zinc, cadmium and nickel (case study: Rangelands around National Lead & Zinc Factory, Zanjan, Iran). J. Rangeland Sci. 7, 160–171.
Mohsenzadeh, F. and Mohammadzadeh, R. (2018). Phytoremediation ability of the new heavy metal accumulator plants. Environ. Eng. Geosci. 24, 441–450. doi: 10.2113/EEG-2123
Muddarisna, N., Krisnayanti, B. D., Utami, S. R., and Handayanto, E. (2013). Phytoremediation of mercury-contaminated soil using three wild plant species and its effect on maize growth. Appl. Ecol. Environ. Sci. 1, 27–32. doi: 10.12691/aees-1-3-1
Mushtaq, M., Ahmad Dar, A., Skalicky, M., Tyagi, A., Bhagat, N., Basu, U., et al. (2021a). CRISPR-based genome editing tools: Insights into technological breakthroughs and future challenges. Genes 12, 797. doi: 10.3390/genes12060797
Mushtaq, M., Dar, A. A., Basu, U., Bhat, B. A., Mir, R. A., Vats, S., et al. (2021b). Integrating CRISPR-Cas and next generation sequencing in plant virology. Front. Genet. 12, 735489. doi: 10.3389/fgene.2021.735489
Nagajyoti, P. C., Lee, K. D., and Sreekanth, T. V. M. (2010). Heavy metals, occurrence and toxicity for plants: a review. Environ. Chem. Lett. 8, 199–216. doi: 10.1007/s10311-010-0297-8
Nakajima, H. and Itoh, K. (2017). Relationship between metal and pigment concentrations in the Fe-hyperaccumulator moss Scopelophila ligulata. J. Plant Res. 130, 135–141. doi: 10.1007/s10265-016-0867-3
Natasha, N., Shahid, M., Bibi, I., Iqbal, J., Khalid, S., Murtaza, B., et al. (2022). Zinc in soil-plant-human system: A data-analysis review. Sci. Total Environ. 808, 152024. doi: 10.1016/j.scitotenv.2021.152024
Noulas, C., Tziouvalekas, M., and Karyotis, T. (2018). Zinc in soils, water and food crops. J. Trace Elements Med. Biol. 49, 252–260. doi: 10.1016/j.jtemb.2018.02.009
Nurrahma, A. H. I., Nuraini, L., Harsonowati, W., and El-Beltagi, H. S. (2024). “Improving hyperaccumulator plant traits for the optimized remediation of heavy metals contaminated soils,” in Bio-organic Amendments for Heavy Metal Remediation (Elsevier), 549–560.
Ojuederie, O. B., Igwe, D. O., and Popoola, J. O. (2022). “Transgenic plant-mediated phytoremediation: Applications, challenges, and prospects,” in Assisted Phytoremediation (Elsevier), 179–202.
Oladoye, P. O., Olowe, O. M., and Asemoloye, M. D. (2021). Phytoremediation technology and food security impacts of heavy metal contaminated soils: A review of literatures. Chemosphere 288, 132555.
Oomen, R. J. F. J., Wu, J., Lelièvre, F., Blanchet, S., Richaud, P., Barbier-Brygoo, H., et al. (2009). Functional characterization of NRAMP3 and NRAMP4 from the metal hyperaccumulator Thlaspi caerulescens. New Phytol. 181, 637–650. doi: 10.1111/j.1469-8137.2008.02694.x
Oubohssaine, M. and Dahmani, I. (2024). Phytoremediation: Harnessing plant power and innovative technologies for effective soil remediation. Plant Stress, 100578. doi: 10.1016/j.stress.2024.100578
Palutoglu, M., Akgul, B., Suyarko, V., Yakovenko, M., Kryuchenko, N., and Sasmaz, A. (2018). Phytoremediation of cadmium by native plants grown on mining soil. Bull. Environ. contamination Toxicol. 100, 293–297. doi: 10.1007/s00128-017-2220-5
Papoyan, A. and Kochian, L. V. (2004). Identification of Thlaspi caerulescens genes that may be involved in heavy metal hyperaccumulation and tolerance. Characterization of a novel heavy metal transporting ATPase. Plant Physiol. 136, 3814–3823. doi: 10.1104/pp.104.044503
Papoyan, A., Piñeros, M., and Kochian, L. V. (2007). Plant Cd2+ and Zn2+ status effects on root and shoot heavy metal accumulation in Thlaspi caerulescens. New Phytol. 175, 51–58. doi: 10.1111/j.1469-8137.2007.02073.x
Pasricha, S., Mathur, V., Garg, A., Lenka, S., Verma, K., and Agarwal, S. (2021). Molecular mechanisms underlying heavy metal uptake, translocation and tolerance in hyperaccumulators-an analysis: Heavy metal tolerance in hyperaccumulators. Environ. Challenges 4, 100197.
Pauwels, M., Frérot, H., Bonnin, I., and Saumitou-Laprade, P. (2006). A broad-scale analysis of population differentiation for Zn tolerance in an emerging model species for tolerance study: Arabidopsis halleri (Brassicaceae). J. evolutionary Biol. 19, 1838–1850. doi: 10.1111/j.1420-9101.2006.01178.x
Peiter, E., Montanini, B., Gobert, A., Pedas, P., Husted, S., Maathuis, F. J., et al. (2007). A secretory pathway-localized cation diffusion facilitator confers plant manganese tolerance. Proc. Natl. Acad. Sci. 104, 8532–8537. doi: 10.1073/pnas.0609507104
Pence, N. S., Larsen, P. B., Ebbs, S. D., Letham, D. L., Lasat, M. M., Garvin, D. F., et al. (2000). The molecular physiology of heavy metal transport in the Zn/Cd hyperaccumulator Thlaspi caerulescens. Proc. Natl. Acad. Sci. 97, 4956–4960. doi: 10.1073/pnas.97.9.4956
Peng, J.-S., Guan, Y.-H., Lin, X.-J., Xu, X.-J., Xiao, L., Wang, H.-H., et al. (2021). Comparative understanding of metal hyperaccumulation in plants: a mini-review. Environ. geochem. Health 43, 1599–1607. doi: 10.1007/s10653-020-00533-2
Persans, M. W., Nieman, K., and Salt, D. E. (2001). Functional activity and role of cation-efflux family members in Ni hyperaccumulation in Thlaspi goesingense. Proc. Natl. Acad. Sci. 98, 9995–10000. doi: 10.1073/pnas.171039798
Pietrzykowski, M., Antonkiewicz, J., Gruba, P., and Pająk, M. (2018). Content of Zn, Cd and Pb in purple moor-grass in soils heavily contaminated with heavy metals around a zinc and lead ore tailing landfill. Open Chem. 16, 1143–1152. doi: 10.1515/chem-2018-0129
Pirzadah, T. B., Malik, B., and Dar, F. A. (2019). Phytoremediation potential of aromatic and medicinal plants: A way forward for green economy. J. Stress Physiol. Biochem. 15, 62–75.
Pirzadah, T. B., Malik, B., Hakeem, K. R., and Rashid, S. (2022). “Nature sucks up explosives,” in Phytoremediation (Elsevier), 351–368.
Pirzadah, T. B., Malik, B., Tahir, I., Kumar, M., Varma, A., and Rehman, R. U. (2015). Phytoremediation: an eco-friendly green technology for pollution prevention, control and remediation. Soil Rem. Plants Prospects Chall. 5, 107–126.
Potocki, S., Valensin, D., Camponeschi, F., and Kozlowski, H. (2013). The extracellular loop of IRT1 ZIP protein—the chosen one for zinc? J. Inorganic Biochem. 127, 246–252. doi: 10.1016/j.jinorgbio.2013.05.003
Pp, S. and Puthur, J. T. (2021). Heavy metal phytoremediation by bioenergy plants and associated tolerance mechanisms. Soil Sediment Contamination: Int. J. 30, 253–274.
Priyadarshanee, M., Mahto, U., and Das, S. (2022). “Mechanism of toxicity and adverse health effects of environmental pollutants,” in Microbial Biodegradation and Bioremediation (Elsevier), 33–53.
Puig, S. (2014). Function and regulation of the plant COPT family of high-affinity copper transport proteins. Adv. Bot. 2014, 476917. doi: 10.1155/2014/476917
Quarshie, S. D.-G., Xiao, X., and Zhang, L. (2021). Enhanced phytoremediation of soil heavy metal pollution and commercial utilization of harvested plant biomass: a review. Water Air Soil pollut. 232, 1–28. doi: 10.1007/s11270-021-05430-7
Rafeeq, H., Afsheen, N., Rafique, S., Arshad, A., Intisar, M., Hussain, A., et al. (2023). Genetically engineered microorganisms for environmental remediation. Chemosphere 310, 136751. doi: 10.1016/j.chemosphere.2022.136751
Rai, G. K., Bhat, B. A., Mushtaq, M., Tariq, L., Rai, P. K., Basu, U., et al. (2021). Insights into decontamination of soils by phytoremediation: A detailed account on heavy metal toxicity and mitigation strategies. Physiologia Plantarum 173, 287–304. doi: 10.1111/ppl.13433
Rai, G. K., Mushtaq, M., Bhat, B. A., Kumar, R. R., Singh, M., and Rai, P. K. (2022). “Reactive oxygen species: friend or foe,” in Thermotolerance in Crop Plants (Springer), 129–162.
Ranieri, E., D’onghia, G., Ranieri, F., Petrella, A., Spagnolo, V., and Ranieri, A. C. (2021). Phytoextraction of cr (VI)-contaminated soil by phyllostachys pubescens: A case study. Toxics 9, 312. doi: 10.3390/toxics9110312
Rascio, N. and Navari-Izzo, F. (2011). Heavy metal hyperaccumulating plants: how and why do they do it? And what makes them so interesting? Plant Sci. 180, 169–181. doi: 10.1016/j.plantsci.2010.08.016
Rashed, M. H., Hoque, T. S., Jahangir, M. M. R., and Hashem, M. A. (2019). Manganese as a micronutrient in agriculture: crop requirement and management. J. Environ. Sci. Nat. Resour. 12, 225–242.
Raza, A., Habib, M., Charagh, S., and Kakavand, S. N. (2021). “Genetic engineering of plants to tolerate toxic metals and metalloids,” in Handbook of Bioremediation (Elsevier), 411–436.
Raza, A., Hafeez, M. B., Zahra, N., Shaukat, K., Umbreen, S., Tabassum, J., et al. (2020). “The plant family brassicaceae: introduction, biology, and importance,” in The plant family brassicaceae. Ed. Hasanuzzaman, M. (Springer), 1–43.
Raza, A., Tabassum, J., Zahid, Z., Charagh, S., Bashir, S., Barmukh, R., et al. (2022). Advances in “Omics” Approaches for improving toxic metals/metalloids tolerance in plants. Front. Plant Sci. 12, 794373. doi: 10.3389/fpls.2021.794373
Reeves, R. D. (2000). Metal-accumulating plants. Phytoremediation of toxic metals: using plants to clean up the environment.
Reeves, R. D. and Adigüzel, N. (2004). Rare plants and nickel accumulators from Turkish serpentine soils, with special reference to Centaurea species. Turkish J. Bot. 28, 147–153.
Reeves, R. D., Baker, A. J., Jaffré, T., Erskine, P. D., Echevarria, G., and van der Ent, A. (2018). A global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytol. 218, 407–411. doi: 10.1111/nph.14907
Reeves, R. and Brooks, R. (1983). European species of Thlaspi L.(Cruciferae) as indicators of nickel and zinc. J. Geochemical Explor. 18, 275–283. doi: 10.1016/0375-6742(83)90073-0
Reeves, R. D., Schwartz, C., Morel, J. L., and Edmondson, J. (2001). Distribution and metal-accumulating behavior of Thlaspi caerulescens and associated metallophytes in France. Int. J. Phytoremediation 3, 145–172. doi: 10.1080/15226510108500054
Reynolds, R. J. B., Jones, R. R., Heiner, J., Crane, K. M., and Pilon-Smits, E. (2020). Effects of selenium hyperaccumulators on soil selenium distribution and vegetation properties. Am. J. Bot. 107, 970–982. doi: 10.1002/ajb2.1500
Riyazuddin, R., Nisha, N., Ejaz, B., Khan, M. I. R., Kumar, M., Ramteke, P. W., et al. (2022). A comprehensive review on the heavy metal toxicity and sequestration in plants. Biomolecules 12, 43.
Robinson, B., Kim, N., Marchetti, M., Moni, C., Schroeter, L., Van Den Dijssel, C., et al. (2006). Arsenic hyperaccumulation by aquatic macrophytes in the Taupo Volcanic Zone, New Zealand. Environ. Exp. Bot. 58, 206–215. doi: 10.1016/j.envexpbot.2005.08.004
Robinson, B. H., Lombi, E., Zhao, F. J., and Mcgrath, S. P. (2003). Uptake and distribution of nickel and other metals in the hyperaccumulator Berkheya coddii. New Phytol. 158, 279–285. doi: 10.1046/j.1469-8137.2003.00743.x
Rocha, J. E., Guedes, T., Bezerra, C. F., Costa, M. D. S., Campina, F. F., De Freitas, T. S., et al. (2019). Mercury chloride phytotoxicity reduction using antioxidative mechanisms evidenced by caffeic acid FTIR. Appl. Geochem. 104, 109–115. doi: 10.1016/j.apgeochem.2019.03.015
Rogers, E. E., Eide, D. J., and Guerinot, M. L. (2000). Altered selectivity in an Arabidopsis metal transporter. Proc. Natl. Acad. Sci. 97, 12356–12360. doi: 10.1073/pnas.210214197
Rosatto, S., Mariotti, M., Romeo, S., and Roccotiello, E. (2021). Root and shoot response to nickel in hyperaccumulator and non-hyperaccumulator species. Plants 10, 508. doi: 10.3390/plants10030508
Sachan, P. and Lal, N. (2017). An overview of nickel (Ni2+) essentiality, toxicity and tolerance strategies in plants. Asian J. Biol., 1–15. doi: 10.9734/AJOB/2017/33931
Sachs, J. (2022). Handbuch der Experimental-Physiologie der Pflanzen: Untersuchungen über die allgemeinen Lebensbedingungen der Pflanzen. BoD–Books on Demand.
Salam, M. M. A., Kaipiainen, E., Mohsin, M., Villa, A., Kuittinen, S., Pulkkinen, P., et al. (2016). Effects of contaminated soil on the growth performance of young Salix (Salix schwerinii EL Wolf) and the potential for phytoremediation of heavy metals. J. Environ. Manage. 183, 467–477. doi: 10.1016/j.jenvman.2016.08.082
Sancenón, V., Puig, S., Mateu-Andrés, I., Dorcey, E., Thiele, D. J., and Peñarrubia, L. (2004). The Arabidopsis copper transporter COPT1 functions in root elongation and pollen development. J. Biol. Chem. 279, 15348–15355. doi: 10.1074/jbc.M313321200
Sánchez-Castro, I., Molina, L., Prieto-Fernández, M.-Á., and Segura, A. (2023). Past, present and future trends in the remediation of heavy-metal contaminated soil-Remediation techniques applied in real soil-contamination events. Heliyon 9.
Sangeetha, P. and Jagtap, S. (2024). “Impact of Novel Remediation Technology: Significant Role in the Removal of Toxic Pollutants via Sustainable Approaches,” in Industrial Microbiology and Biotechnology: An Insight into Current Trends (Springer), 679–701.
Sasaki, A., Yamaji, N., Yokosho, K., and Ma, J. F. (2012). Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell 24, 2155–2167. doi: 10.1105/tpc.112.096925
Schaaf, G., Honsbein, A., Meda, A. R., Kirchner, S., Wipf, D., and Von Wirén, N. (2006). AtIREG2 encodes a tonoplast transport protein involved in iron-dependent nickel detoxification in Arabidopsis thaliana roots. J. Biol. Chem. 281, 25532–25540. doi: 10.1074/jbc.M601062200
Schneider, T., Persson, D. P., Husted, S., Schellenberg, M., Gehrig, P., Lee, Y., et al. (2013). A proteomics approach to investigate the process of Z n hyperaccumulation in N occaea caerulescens (J & C. P resl) FK M eyer. Plant J. 73, 131–142.
Schvartzman, M. S., Corso, M., Fataftah, N., Scheepers, M., Nouet, C., Bosman, B., et al. (2018). Adaptation to high zinc depends on distinct mechanisms in metallicolous populations of Arabidopsis halleri. New Phytol. 218, 269–282. doi: 10.1111/nph.14949
Seregin, I. and Kozhevnikova, A. D. (2006). Physiological role of nickel and its toxic effects on higher plants. Russian J. Plant Physiol. 53, 257–277. doi: 10.1134/S1021443706020178
Seregin, I. V. and Kozhevnikova, A. D. (2021). Low-molecular-weight ligands in plants: role in metal homeostasis and hyperaccumulation. Photosynthesis Res. 150, 51–96. doi: 10.1007/s11120-020-00768-1
Severne, B. C. and Brooks, R. R. (1972). A nickel-accumulating plant from Western Australia. Planta 103, 91–94. doi: 10.1007/BF00394610
Shackirea, A. M., Mirshad, P. P., and Naz, M. (2022). “Global Perspective and Challenges in Utilization of Bioenergy Crops for Phytoremediation,” in Bioenergy Crops (CRC Press), 204–214.
Shahzad, Z., Gosti, F., Frérot, H., Lacombe, É., Roosens, N., Saumitou-Laprade, P., et al. (2010). The five AhMTP1 zinc transporters undergo different evolutionary fates towards adaptive evolution to zinc tolerance in Arabidopsis halleri. PloS Genet. 6, e1000911. doi: 10.1371/journal.pgen.1000911
Sharma, S. S., Dietz, K. J., and Mimura, T. (2016). Vacuolar compartmentalization as indispensable component of heavy metal detoxification in plants. Plant Cell Environ. 39, 1112–1126. doi: 10.1111/pce.12706
Sharma, P. and Kumar, S. (2021). Bioremediation of heavy metals from industrial effluents by endophytes and their metabolic activity: Recent advances. Bioresource Technol., 125589. doi: 10.1016/j.biortech.2021.125589
Sharma, P., Ngo, H. H., Khanal, S., Larroche, C., Kim, S.-H., and Pandey, A. (2021a). Efficiency of transporter genes and proteins in hyperaccumulator plants for metals tolerance in wastewater treatment: Sustainable technique for metal detoxification. Environ. Technol. Innovation 23, 101725. doi: 10.1016/j.eti.2021.101725
Sharma, P., Pandey, A. K., Udayan, A., and Kumar, S. (2021b). Role of microbial community and metal-binding proteins in phytoremediation of heavy metals from industrial wastewater. Bioresource Technol. 326, 124750. doi: 10.1016/j.biortech.2021.124750
Shen, Z., Zhao, F., and Mcgrath, S. (1997). Uptake and transport of zinc in the hyperaccumulator Thlaspi caerulescens and the non-hyperaccumulator Thlaspi ochroleucum. Plant Cell Environ. 20, 898–906. doi: 10.1046/j.1365-3040.1997.d01-134.x
Shiyab, S., Chen, J., Han, F. X., Monts, D. L., Matta, F. B., Gu, M., et al. (2009). Phytotoxicity of mercury in Indian mustard (Brassica juncea L.). Ecotoxicol. Environ. Saf. 72, 619–625. doi: 10.1016/j.ecoenv.2008.06.002
Siebert, S. J., Schutte, N. C., Bester, S. P., Komape, D. M., and Rajakaruna, N. (2018). Senecio conrathii NE Br.(Asteraceae), a new hyperaccumulator of nickel from serpentinite outcrops of the Barberton Greenstone Belt, South Africa. Ecol. Res. 33, 651–658. doi: 10.1007/s11284-017-1541-5
Skuza, L., Szućko-Kociuba, I., Filip, E., and Bożek, I. (2022). Natural molecular mechanisms of plant hyperaccumulation and hypertolerance towards heavy metals. Int. J. Mol. Sci. 23, 9335. doi: 10.3390/ijms23169335
Solomon, W., Janda, T., and Molnár, Z. (2024). Unveiling the significance of rhizosphere: Implications for plant growth, stress response, and sustainable agriculture. Plant Physiol. Biochem. 206, 108290. doi: 10.1016/j.plaphy.2023.108290
Stein, R. J., Höreth, S., De Melo, J. R. F., Syllwasschy, L., Lee, G., Garbin, M. L., et al. (2017). Relationships between soil and leaf mineral composition are element-specific, environment-dependent and geographically structured in the emerging model Arabidopsis halleri. New Phytol. 213, 1274–1286. doi: 10.1111/nph.14219
Stoltz, E. and Greger, M. (2002). Accumulation properties of As, Cd, Cu, Pb and Zn by four wetland plant species growing on submerged mine tailings. Environ. Exp. Bot. 47, 271–280. doi: 10.1016/S0098-8472(02)00002-3
Sukumaran, D. (2013). Phytoremediation of heavy metals from industrial effluent using constructed wetland technology. Appl. Ecol. Environ. Sci. 1, 92–97. doi: 10.12691/aees-1-5-4
Sytar, O., Ghosh, S., Malinska, H., Zivcak, M., and Brestic, M. (2020). Physiological and molecular mechanisms of metal accumulation in hyperaccumulator plants. Physiologia Plantarum. doi: 10.1111/ppl.13285
Sytar, O., Ghosh, S., Malinska, H., Zivcak, M., and Brestic, M. (2021). Physiological and molecular mechanisms of metal accumulation in hyperaccumulator plants. Physiologia Plantarum 173, 148–166.
Takahashi, R., Ishimaru, Y., Senoura, T., Shimo, H., Ishikawa, S., Arao Nakanishi, H., et al. (2011). The OsNRAMP1 iron trans—porter is involved in Cd accumulation in rice. JExp Bot. 62, 4843–4850. doi: 10.1093/jxb/err136
Talke, I. N., Hanikenne, M., and Krämer, U. (2006). Zinc-dependent global transcriptional control, transcriptional deregulation, and higher gene copy number for genes in metal homeostasis of the hyperaccumulator Arabidopsis halleri. Plant Physiol. 142, 148–167. doi: 10.1104/pp.105.076232
Tangahu, B. V., Sheikh Abdullah, S. R., Basri, H., Idris, M., Anuar, N., and Mukhlisin, M. (2011). A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int. J. Chem. Eng. 2011, 939161. doi: 10.1155/2011/939161
Tariq, L., Bhat, B. A., Hamdani, S. S., and Mir, R. A. (2021). “Phytochemistry, Pharmacology and Toxicity of Medicinal Plants,” in Medicinal and Aromatic Plants (Springer), 217–240.
Tariq, L., Bhat, B. A., Hamdani, S. S., Nissar, S., Sheikh, B. A., Dar, M. A., et al. (2022). “Plant growth regulators and their interaction with abiotic stress factors,” in Plant Abiotic Stress Physiology (Apple Academic Press), 115–135.
Tariq, A., Mushtaq, M., Yaqoob, H., Bhat, B. A., Zargar, S. M., Raza, A., et al. (2023). Putting CRISPR-Cas system in action: a golden window for efficient and precise genome editing for crop improvement. GM Crops Food 14, 1–27. doi: 10.1080/21645698.2023.2219111
Tauqeer, H. M., Ali, S., Rizwan, M., Ali, Q., Saeed, R., Iftikhar, U., et al. (2016). Phytoremediation of heavy metals by Alternanthera bettzickiana: growth and physiological response. Ecotoxicol. Environ. Saf. 126, 138–146. doi: 10.1016/j.ecoenv.2015.12.031
Thakur, M., Praveen, S., Divte, P. R., Mitra, R., Kumar, M., Gupta, C. K., et al. (2021). Metal tolerance in plants: Molecular and physicochemical interface determines the “not so heavy effect” of heavy metals. Chemosphere, 131957.
Tripathi, S., Singh, V. K., Srivastava, P., Singh, R., Devi, R. S., Kumar, A., et al. (2020). Phytoremediation of organic pollutants: current status and future directions. Abatement Environ. pollutants, 81–105. doi: 10.1016/B978-0-12-818095-2.00004-7
Turkyilmaz, A., Sevik, H., Cetin, M., and Ahmaida Saleh, E. A. (2018). Changes in heavy metal accumulation depending on traffic density in some landscape plants. Polish J. Environ. Stud. 27. doi: 10.15244/pjoes/78620
Ueno, D., Milner, M. J., Yamaji, N., Yokosho, K., Koyama, E., Clemencia Zambrano, M., et al. (2011). Elevated expression of TcHMA3 plays a key role in the extreme Cd tolerance in a Cd-hyperaccumulating ecotype of Thlaspi caerulescens. Plant J. 66, 852–862. doi: 10.1111/j.1365-313X.2011.04548.x
Van De Mortel, J. E., Schat, H., Moerland, P. D., Van Themaat, E. V. L., van der Ent, S., Blankestijn, H., et al. (2008). Expression differences for genes involved in lignin, glutathione and sulphate metabolism in response to cadmium in Arabidopsis thaliana and the related Zn/Cd-hyperaccumulator Thlaspi caerulescens. Plant Cell Environ. 31, 301–324. doi: 10.1111/j.1365-3040.2007.01764.x
Van De Mortel, J. E., Villanueva, L. A., Schat, H., Kwekkeboom, J., Coughlan, S., Moerland, P. D., et al. (2006). Large expression differences in genes for iron and zinc homeostasis, stress response, and lignin biosynthesis distinguish roots of Arabidopsis thaliana and the related metal hyperaccumulator Thlaspi caerulescens. Plant Physiol. 142, 1127–1147. doi: 10.1104/pp.106.082073
Van Der Ent, A., Baker, A. J., Reeves, R. D., Chaney, R. L., Anderson, C. W., Meech, J. A., et al. (2015). Agromining: farming for metals in the future? (ACS Publications).
Van Der Ent, A., Purwadi, I., Harris, H. H., Kopittke, P. M., Przybyłowicz, W. J., and Mesjasz-Przybyłowicz, J. (2021). “Methods for Visualizing Elemental Distribution in Hyperaccumulator Plants,” in Agromining: Farming for Metals (Springer), 197–214.
Van Hoewyk, D. (2013). A tale of two toxicities: malformed selenoproteins and oxidative stress both contribute to selenium stress in plants. Ann. Bot. 112, 965–972. doi: 10.1093/aob/mct163
Varma, S. (2021). Heavy metals stress and defense strategies in plants: An overview. J. Pharmacognosy Phytochem. 10, 608–614.
Venegas-Rioseco, J., Ginocchio, R., and Ortiz-Calderón, C. (2022). Increase in phytoextraction potential by genome editing and transformation: A review. Plants 11, 86.
Verbruggen, N., Hermans, C., and Schat, H. (2009a). Mechanisms to cope with arsenic or cadmium excess in plants. Curr. Opin. Plant Biol. 12, 364–372. doi: 10.1016/j.pbi.2009.05.001
Verbruggen, N., Hermans, C., and Schat, H. (2009b). Molecular mechanisms of metal hyperaccumulation in plants. New Phytol. 181, 759–776. doi: 10.1111/j.1469-8137.2008.02748.x
Vert, G., Grotz, N., Dédaldéchamp, F., Gaymard, F., Guerinot, M. L., Briat, J. F., et al. (2002). IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 14, 1223–1233.
Visioli, G., Gullì, M., and Marmiroli, N. (2014). Noccaea caerulescens populations adapted to grow in metalliferous and non-metalliferous soils: Ni tolerance, accumulation and expression analysis of genes involved in metal homeostasis. Environ. Exp. Bot. 105, 10–17. doi: 10.1016/j.envexpbot.2014.04.001
Wan, X., Lei, M., and Yang, J. (2017). Two potential multi-metal hyperaccumulators found in four mining sites in Hunan Province, China. Catena 148, 67–73. doi: 10.1016/j.catena.2016.02.005
Weber, M., Harada, E., Vess, C., Roepenack-Lahaye, E. V., and Clemens, S. (2004). Comparative microarray analysis of Arabidopsis thaliana and Arabidopsis halleri roots identifies nicotianamine synthase, a ZIP transporter and other genes as potential metal hyperaccumulation factors. Plant J. 37, 269–281. doi: 10.1046/j.1365-313X.2003.01960.x
Weber, M., Trampczynska, A., and Clemens, S. (2006). Comparative transcriptome analysis of toxic metal responses in Arabidopsis thaliana and the Cd2+-hypertolerant facultative metallophyte Arabidopsis halleri. Plant Cell Environ. 29, 950–963. doi: 10.1111/j.1365-3040.2005.01479.x
Wei, W., Chai, T., Zhang, Y., Han, L., Xu, J., and Guan, Z. (2009). The Thlaspi caerulescens NRAMP homologue TcNRAMP3 is capable of divalent cation transport. Mol. Biotechnol. 41, 15–21. doi: 10.1007/s12033-008-9088-x
Whiting, S. N., Reeves, R. D., Richards, D., Johnson, M. S., Cooke, J. A., Malaisse, F., et al. (2004). Research priorities for conservation of metallophyte biodiversity and their potential for restoration and site remediation. Restor. Ecol. 12, 106–116. doi: 10.1111/j.1061-2971.2004.00367.x
Willems, G., Dräger, D. B., Courbot, M., Godé, C., Verbruggen, N., and Saumitou-Laprade, P. (2007). The genetic basis of zinc tolerance in the metallophyte Arabidopsis halleri ssp. halleri (Brassicaceae): an analysis of quantitative trait loci. Genetics 176, 659–674. doi: 10.1534/genetics.106.064485
Wu, M., Luo, Q., Liu, S., Zhao, Y., Long, Y., and Pan, Y. (2018). Screening ornamental plants to identify potential Cd hyperaccumulators for bioremediation. Ecotoxicol. Environ. Saf. 162, 35–41. doi: 10.1016/j.ecoenv.2018.06.049
Wu, D., Saleem, M., He, T., and He, G. (2021). The mechanism of metal homeostasis in plants: A new view on the synergistic regulation pathway of membrane proteins, lipids and metal ions. Membranes 11, 984. doi: 10.3390/membranes11120984
Xie, Q.-E., Yan, X.-L., Liao, X.-Y., and Li, X. (2009). The arsenic hyperaccumulator fern Pteris vittata L. Environ. Sci. Technol. 43, 8488–8495. doi: 10.1021/es9014647
Yan, A., Wang, Y., Tan, S. N., Mohd Yusof, M. L., Ghosh, S., and Chen, Z. (2020). Phytoremediation: a promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 11, 359. doi: 10.3389/fpls.2020.00359
Yang, Q., Ma, X., Luo, S., Gao, J., Yang, X., and Feng, Y. (2018). SaZIP4, an uptake transporter of Zn/Cd hyperaccumulator Sedum alfredii Hance. Environ. Exp. Bot. 155, 107–117. doi: 10.1016/j.envexpbot.2018.06.021
Yang, Z., Yang, F., Liu, J.-L., Wu, H.-T., Yang, H., Shi, Y., et al. (2021). Heavy metal transporters: Functional mechanisms, regulation, and application in phytoremediation. Sci. Total Environ., 151099.
Yang, Z., Yang, F., Liu, J.-L., Wu, H.-T., Yang, H., Shi, Y., et al. (2022). Heavy metal transporters: Functional mechanisms, regulation, and application in phytoremediation. Sci. Total Environ. 809, 151099. doi: 10.1016/j.scitotenv.2021.151099
Yaqoob, H., Tariq, A., Bhat, B. A., Bhat, K. A., Nehvi, I. B., Raza, A., et al. (2023). Integrating genomics and genome editing for orphan crop improvement: a bridge between orphan crops and modern agriculture system. GM Crops Food 14, 1–20. doi: 10.1080/21645698.2022.2146952
Yin, F., Li, J., Wang, Y., and Yang, Z. (2024). Biodegradable chelating agents for enhancing phytoremediation: mechanisms, market feasibility, and future studies. Ecotoxicol. Environ. Saf. 272, 116113. doi: 10.1016/j.ecoenv.2024.116113
Zhakypbek, Y., Kossalbayev, B. D., Belkozhayev, A. M., Murat, T., Tursbekov, S., Abdalimov, E., et al. (2024). Reducing heavy metal contamination in soil and water using phytoremediation. Plants 13, 1534. doi: 10.3390/plants13111534
Zhang, D., Dyck, M., Filipović, L., Filipović, V., Lv, J., and He, H. (2021). Hyperaccumulators for potentially toxic elements: A scientometric analysis. Agronomy 11, 1729. doi: 10.3390/agronomy11091729
Zhang, X., Laubie, B., Houzelot, V., Plasari, E., Echevarria, G., and Simonnot, M.-O. (2016). Increasing purity of ammonium nickel sulfate hexahydrate and production sustainability in a nickel phytomining process. Chem. Eng. Res. design 106, 26–32. doi: 10.1016/j.cherd.2015.12.009
Zhang, X., Zhao, F. J., Huang, Q., Williams, P. N., Sun, G. X., and Zhu, Y. G. (2009). Arsenic uptake and speciation in the rootless duckweed Wolffia globosa. New Phytol. 182, 421–428. doi: 10.1111/j.1469-8137.2008.02758.x
Zhao, F. J., Hamon, R. E., Lombi, E., Mclaughlin, M. J., and Mcgrath, S. P. (2002). Characteristics of cadmium uptake in two contrasting ecotypes of the hyperaccumulator Thlaspi caerulescens. J. Exp. Bot. 53, 535–543. doi: 10.1093/jexbot/53.368.535
Zhao, F. J., Ma, J. F., Meharg, A. A., and Mcgrath, S. P. (2009). Arsenic uptake and metabolism in plants. New Phytol. 181, 777–794. doi: 10.1111/j.1469-8137.2008.02716.x
Zhao, H.-B., Song, W.-Y., Han, G.-D., Shao, H.-B., and Zhang, S.-W. (2014). Dynamic change of wheat eco-physiology and implications for establishing high-efficient stable agro-ecosystems under Hg stress. Ecol. Eng. 70, 50–55. doi: 10.1016/j.ecoleng.2014.04.022
Keywords: hyperaccumulators, homeostasis, phytoremediation, transporter proteins, metal ion tolerance, microbes
Citation: Bhat BA, Rather MA, Bilal T, Nazir R, Qadir RU and Mir RA (2025) Plant hyperaccumulators: a state-of-the-art review on mechanism of heavy metal transport and sequestration. Front. Plant Sci. 16:1631378. doi: 10.3389/fpls.2025.1631378
Received: 19 May 2025; Accepted: 23 June 2025;
Published: 23 July 2025.
Edited by:
Mohd Irfan Naikoo, King Fahd University of Petroleum and Minerals, Saudi ArabiaReviewed by:
Masood Jan, University of Florida, United StatesMudassara Hasan, Aligarh Muslim University, India
Copyright © 2025 Bhat, Rather, Bilal, Nazir, Qadir and Mir. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rakeeb Ahmad Mir, cmFrZWViYWhtYWRAZ21haWwuY29t; Roof Ul Qadir, cm91ZjA2MjlAZ21haWwuY29t
 Basharat Ahmad Bhat
Basharat Ahmad Bhat Muneeb Ahmad Rather2
Muneeb Ahmad Rather2 Tanveer Bilal
Tanveer Bilal Romaan Nazir
Romaan Nazir Roof Ul Qadir
Roof Ul Qadir