- 1Xinjiang Key Laboratory for Ecological Adaptation and Evolution of Extreme Environment Organisms, College of Life Sciences, Xinjiang Agricultural University, Urumqi, Xinjiang, China
- 2State Key Laboratory of Ecological Safety and Sustainable Development in Arid Lands, Xinjiang Institute of Ecology and Geography, Chinese Academy of Sciences, Urumqi, China
Understanding how environmental heterogeneity shape genetic structure is fundamental to unraveling adaptive evolution and guiding conservation practices. Northern Xinjiang, characterized by its unique geological history and complex ecosystems, provides an ideal system to explore species-environment interactions. The riverine system, topographic relief, arid climate, and other factors have collectively shaped the genetic structure and adaptive trajectories of regional species. Leymus racemosus, a species well adapted to drought conditions, is primarily distributed along the Irtysh River basin. This study aims to examine the relative contributions of geographic distance, environmental factors, and their interactions to the genetic divergence of L. racemosus. Genomic SNP data from 135 individuals across 27 populations were generated through Double-digest restriction site-associated DNA (dd-RAD) sequencing. Population structure analyses employing ADMIXTURE, PCA, and phylogenetics revealed distinct genetic clusters. Gradient forest (GF) and redundancy analysis (RDA) were conducted to assess the environmental effects on genetic variation. Results suggest that low population genetic diversity and population structure analyses identified two evolutionarily distinct lineages (A and U). The divergence time between these two lineages can be traced back to the mid-to-late Last Glacial Period (0.0295 million years ago), a timescale that is highly consistent with the repeated glacial advances and retreats driven by the intense climatic fluctuations of the Quaternary Ice Age. Environmental association analyses (EAA) revealed significant correlations between allelic variation and climatic gradients, particularly with annual precipitation (bio12) and minimum temperature in the coldest month (bio6). Analyses of isolation by distance (IBD) and isolation by environment (IBE) further underscore the role of geographic distance and environment distance in the genetic differentiation of L. racemosus. This study reveals the pattern of genetic structure of L. racemosus populations and its association with environmental factors, contributing to the development of targeted strategies for species conservation and ecological restoration.
1 Introduction
Revealing the driving factors and processes that shape genetic variation in species is of significant interest to molecular ecology, evolutionary biology, and conservation biology (Dorogina and Zhmud, 2020; DeWoody et al., 2021; Kirschner et al., 2023; Walter et al., 2023; Wang and Qiu, 2024). The complex interplay of natural selection, gene flow, and genetic drift results in the formation of distinct spatial genetic structures within species (Eckert et al., 2008). Within this evolutionary framework, geography and environment act as two key determinants that mediate these processes, thereby influencing species-level genetic structure (Buiteveld et al., 2007). The Isolation-by-Distance (IBD) model posits that geographic distance and physical barriers restrict gene flow, leading to the local accumulation of genetic variants, such that genetic divergence increases with geographic separation (Wright, 1943; Orsini et al., 2013; Chang et al., 2022). In contrast, the Isolation-by-Environment (IBE) theory proposes that divergent natural selection driven by environmental heterogeneity constrains gene flow, resulting in positive associations between genetic differentiation and heterogeneous environments (Wang and Summers, 2010; Sexton et al., 2014; Wang and Bradburd, 2014; Jiang et al., 2019). Empirical evidence from multiple studies indicates that both IBD and IBE patterns are often simultaneously present within natural populations of the same species (Chang et al., 2022; Tumpa et al., 2022; Liu et al., 2023). Unraveling the relative importance of geography and environment in shaping patterns of genetic variation can provide deeper insights into how landscape factors influence evolutionary processes at both intra- and interspecific levels (De Kort et al., 2021).
The unique mountain-basin-desert-oasis geomorphology of Xinjiang, China, has been shaped by the combined effects of the uplift of the Qinghai-Tibet Plateau and Quaternary aridification, along with other geological and climatic processes (Liu, 2004; Zhang et al., 2022). In northern Xinjiang, the interplay between hydrologic networks, paleogeological dynamics, and extreme aridity has profoundly influenced the genetic structure and evolutionary trajectories of various species (Ju et al., 2015; Yang et al., 2016; Gao, 2017; Jiang et al., 2019; Xue et al., 2020). This region encompasses the interconnected Irtysh-Ulungur dual-basin system, a biogeographic nexus where drainage patterns and historical climate fluctuations have left measurable genomic imprints. Research on local taxa, including Leuciscus leuciscus baicalensis (Yang et al., 2017) and Esox lucius (Luan et al., 2021), reveals genetic divergence patterns that are associated with Pleistocene glacial cycles in the Altai Mountains. Additionally, Populus alba populations in the Irtysh basin exhibit low-frequency polymorphisms that are consistent with postglacial recolonization following a genetic bottleneck (Liu et al., 2019a). These findings provide critical insights into how climate and environmental change have shaped genetic differentiation in the different taxonomic groups of flora and fauna in this arid land basin system.
The genus Leymus (Poaceae),with its exceptional salt and drought tolerance, plays vital roles in ecological restoration and grassland management across Eurasian steppes (Su et al., 2013). The Altai region of Xinjiang harbors the highest species diversity of Leymus, which were considered as a primary diversification center for the genus (Wu, 1979; Zhi and Teng, 2005; Liu et al., 2017a). Current evidence indicates significant interspecific genetic divergence within Leymus, while intraspecific populations exhibit high genetic homogeneity and close phylogenetic relationships (Yang et al., 2006). Notably, populations of Leymus chinensis across China display east-west genetic clines, with significantly higher diversity observed in northeastern regions compared to the western arid zones (Wang et al., 2005; Yuan et al., 2016). However, habitat patch size and environmental heterogeneity strongly influence the genetic structure of L. chinensis (Liu et al., 2004; Guo et al., 2021). In conclusion, research about the genetic of Leymus is mainly focused on the specific species L. chinensis, while the genetic diversity of other Leymus plants is insufficient, limiting our further development and application of Leymus resources.
Leymus racemosus (Lam.) Tzvelev, a member of Leymus, is a perennial grass endemic to the mobile dunes and sandy steppes of China, Russia and Central Asia. In China, it is exclusively found in northern Xinjiang, where it primarily inhabits stabilized or semi-stabilized dunes along the Irtysh River (Wu, 1996). This species exhibits distinctive morphological adaptations, including large panicles, sturdy stems, and extensive rhizome networks (Figure 1), which enhance dune stabilization by increasing surface roughness and effectively mitigating wind erosion (Dong et al., 1985; Huang et al., 1997). As a wild relative of wheat (Triticeae), L. racemosus is tolerant to salt and drought and resistant to various diseases, such as Fusarium graminearum (Wang and Zhang, 2019), providing excellent genetic resources for wheat improvement (Edet et al., 2018). Therefore, understanding its population genetic structure and genotype-environment interactions is essential for effective utilization of these genetic resources, guiding future breeding efforts to develop varieties that are resilient to climate change and other environmental stressors.
In recent years, research on L. racemosus has predominantly focused on various aspects, including wheat breeding, karyotype studies, mating systems, seed characteristics, fruiting patterns, and genetic diversity (Liu and Zhuo, 2008; Holderegger et al., 2010; Gulnar et al., 2014; Hu et al., 2014; Xue et al., 2015). However, the genetic variation data obtained from previous studies utilizing molecular markers such as ISSR and AFLP are relatively limited. Crucially, no studies to date have integrated environmental data with high-throughput genomic markers to unravel genotype-environment associations in this species. Particularly in the realm of adaptive evolution in response to environmental changes, relevant studies remain sparse. In the context of climate change and ecosystem degradation, research on the genetic structure of L. racemosus populations and their associations with environmental variables is still insufficient. As this species exhibits a fragmented population pattern across its range, investigating the relationship between genetic diversity and landscape features is of considerable theoretical importance for elucidating the species’ adaptive influencing factors and optimizing conservation strategies. The objectives of this study were to: (1) assess the genetic diversity and genetic structure of L. racemosus; (2) estimate the timing and pattern of divergence among populations of L. racemosus; and (3) investigate the roles of geographic distance and environmental factors on genetic variation of L. racemosus.
2 Materials and methods
2.1 Sample collection and DNA extraction
A total of 135 L. racemosus individuals were sampled from 27 geographically distinct populations in northern Xinjiang of China (Figure 2A; Supplementary Table S1). To minimize clonal sampling, individuals within each population were spaced at least 10 m apart. Fresh leaf tissues from healthy plants per individual were collected and immediately desiccated using indicating silica gel, followed by long-term storage at -80°C. Genomic DNA was extracted using a modified CTAB protocol (Porebski et al., 1997) and processed by Shanghai Personal Biotechnology Co., Ltd. The quality of the DNA was verified through 0.8% agarose gel electrophoresis and spectrophotometry.
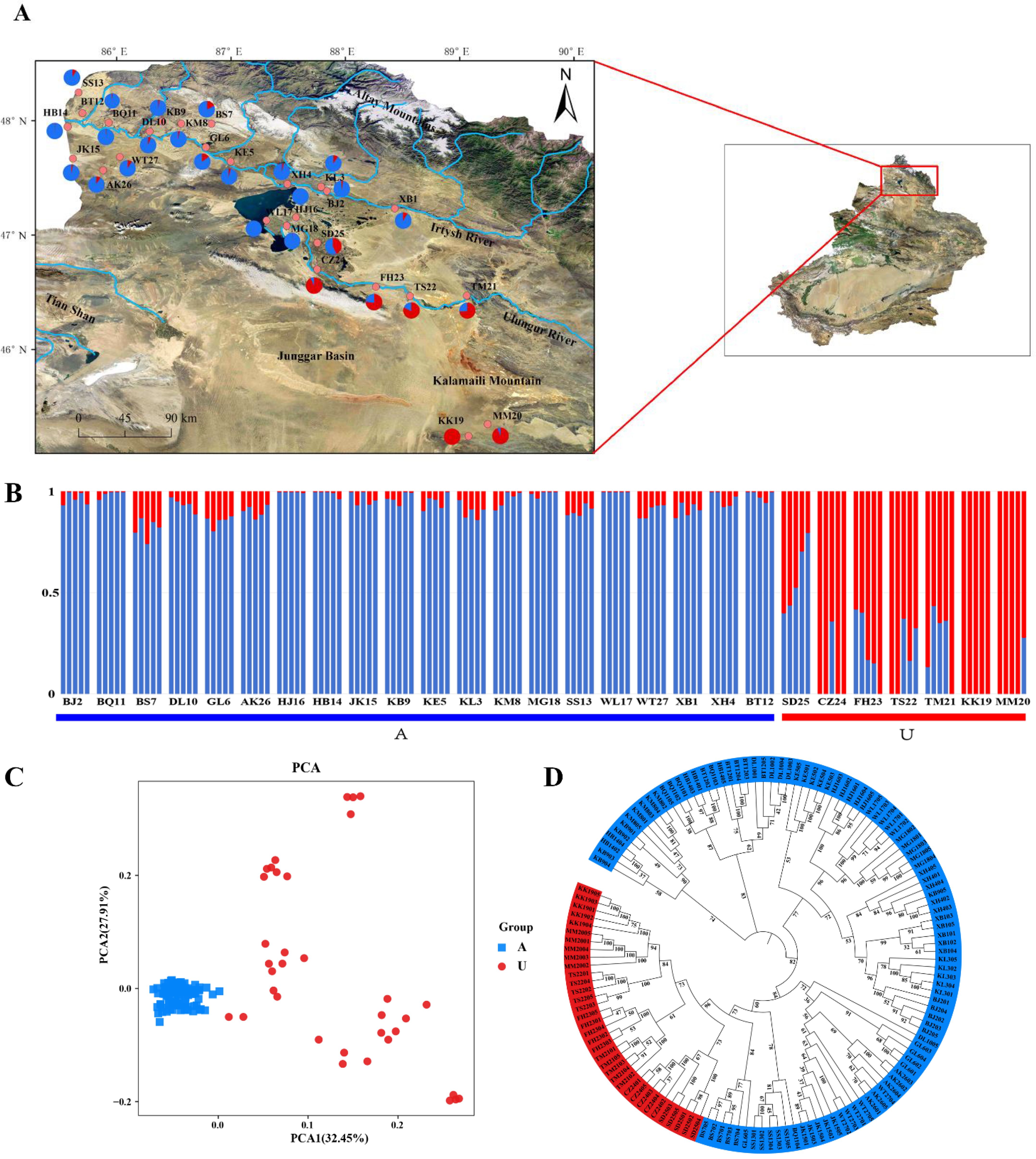
Figure 2. Genetic structure analysis of 27 populations and 135 individuals of Leymus racemosus. (A), The geographical locations of 27 Leymus racemosus populations. The pie chart shows the average proportion of the two genetic lineages identified in each group. Blue represents lineage A and red represents lineage U. (B), ADMIXTURE phylogenetic typing. The blue and red bars represent the likelihood Q values of lineages A and U respectively. (C), Principal component analysis (PCA). (D), Neighbor-Joining (NJ) phylogenetic tree.
2.2 Genome sequencing and SNP filtering
Double-digest restriction site-associated DNA (dd-RAD) libraries were constructed using HindIII and MspI (New England Biolabs), targeting size selection of 300–500 bp fragments. The libraries were quantified with an Agilent High Sensitivity DNA Kit and a Quant-iT PicoGreen dsDNA Assay Kit, followed by paired-end sequencing (2×150 bp) on an Illumina NovaSeq 6000 platform. Approximately 34,971,347 raw reads per sample were generated, with Q30 scores exceeding 95% (Supplementary Table S2). Raw reads were processed using fastp (https://github.com/OpenGene/fastp) for adapter trimming and quality filtering via a sliding window approach (5 bp window), removing reads with more than 10% ambiguous bases or adapter contamination. Clean reads were aligned to the L. chinensis reference genome (https://doi.org/10.6084/m9.figshare.24032238.v1) using BWA-MEM (Li and Durbin, 2009), SNP calling was performed with GATK and filtered using VCFtools (Danecek et al., 2011; Zhu et al., 2014) with the following thresholds: Quality Depth (QD) <2.0; Fisher’s Strand Bias (FS) >60.0; Mapping Quality (MQ) <40.0; minor allele frequency (MAF) >0.05; maximum missing data per site ≤20%; read depth (DP) between 2 and 1,000; and linkage disequilibrium (r²) <0.2 in 50 bp sliding windows with 5 bp steps. This stringent filtering resulted in the retention of 56,655 high-confidence SNPs for downstream analyses.
2.3 Genetic diversity analysis.
Genetic diversity metrics were calculated for the filtered SNP dataset using PLINK (Purcell et al., 2007). Observed heterozygosity (HO), expected heterozygosity (HE), and the inbreeding coefficient (FIS) were estimated to quantify genetic variation at the population level. Values of FIS greater than 0 indicate an excess of homozygosity (inbreeding), while values less than 0 suggest a surplus of heterozygotes relative to Hardy-Weinberg expectations. Population differentiation was assessed through pairwise FST estimates using VCFtools, with interpretations as follows: 0-0.05 (negligible differentiation), 0.05-0.15 (moderate differentiation), and greater than 0.15 (strong differentiation). Neutrality tests were conducted by calculating Tajima’s D across sliding windows (5 kb window, 10 kb step), where significantly positive values indicate balancing selection or population contraction, while negative values suggest directional selection or population expansion. Nucleotide diversity (Pi) was computed as the average pairwise differences per site, with lower values (<0.005) reflecting reduced genetic variation within populations.
2.4 Population genetic structure analysis
Use VCFtools and Plink software successively to convert files into plink format and the “bed” format required by admixture. Subsequently, ADMIXTURE (Patterson et al., 2012) is employed to construct the genetic structure, with the range of genetic clustering values (K) set from 2 to 10, utilizing the software’s default parameters. The optimal K value is defined as the one with the smallest cross-validation (CV) error value. The population structure diagram is generated using the R language (v4.1.0) and the Pophelper package. Additionally, we conducted Principal Component Analysis (PCA) on the samples using PLINK software to further analyze the population’s genetic structure. To elucidate the evolutionary relationships and genetic differences among 135 individuals, we performed Neighbor-Joining (NJ) tree-building analysis using MEGA(v11.0.13) software (Tamura et al., 2021). The phylogenetic tree was constructed using the Kimura 2-parameter model, with bootstrap support assessed from 1,000 replicates. All statistical analyses adhered strictly to the usage specifications and parameter settings of the respective software to ensure the reproducibility and scientific integrity of the results.
2.5 Population evolutionary history
To facilitate the analysis of population dynamic history using DIY ABC software (Cornuet et al., 2010), we further filtered SNPs using VCFtools at intervals of 300 kb, resulting in a total of 12,700 high-quality SNPs. Based on Approximate Bayesian Computation (ABC) at a 95% confidence level, combined with logistic regression, we estimated the posterior probability (PP) of each evolutionary event. The evolutionary event with the highest posterior probability was identified as the optimal evolutionary event. The results of the DIYABC calculations expressed intergroup differentiation in terms of generations. To determine the timing of intergroup differentiation, it is essential to multiply the generation time of the species. For perennial herbaceous plants, accurately determining their generation time is often challenging. Consequently, this study calculated the divergence time based on the generation time of the closely related species L. chinensis, which is 2 years.
2.6 Environmental association analysis
To investigate the impact of climatic and soil factors on the genetic variation of Leymus racemosus populations, 19 bioclimatic variable layers were downloaded from the PaleoClim database (http://www.PaleoClim.org/) and climate data for each population were extracted using the raster package in R. Using ArcGIS v10.8 based on the longitude and latitude of the sampling points, 15 soil factor data were extracted from the Harmonized World Soil Database (HWSD). All climatic and soil data underwent preprocessing. To mitigate multicollinearity issues, Pearson correlation analysis (Cleophas and Zwinderman, 2018) was conducted on the climatic and soil data. Variables with a correlation coefficient (r) greater than 0.8 were removed, and the contribution rate ranking was assessed using the maximum entropy model algorithm. Ultimately, 8 climate factors and 8 soil factors with high contribution rates and no overfitting were selected for subsequent Gradient Forest Analysis (GF) and Redundancy Analysis (RDA) (Supplementary Table S3) (Ellis et al., 2012; Capblancq and Forester, 2021).
The Mantel test was employed to assess potential patterns of Isolation by Distance (IBD) and Isolation by Environment (IBE) in Leymus racemosus populations, evaluating the correlation between genetic distance and both geographical and environmental distances. Genetic distance was calculated using the population differentiation index (FST), with a linearized FST/(1 - FST) transformation applied to quantify genetic distance. The geographical distance between populations was determined using the R package geosphere (Karney, 2013). Eight climate factors and eight soil factors, extracted from the HWSD and PaleoClim databases, were utilized to compute the Euclidean distance matrix between the variables. The R package vegan was employed to conduct 1000 permutation tests (Dixon, 2003) on the genetic distance matrix and the geographical or environmental distance matrices, calculating the correlation coefficient (r) and its significance level (p-value). Scatter plots and trend lines depicting genetic distance versus geographical or environmental distance were generated to illustrate significant correlation patterns.
3 Results
3.1 SNP screening and population genetic diversity
After stringent quality control, a total of 56,655 SNPs were retained for downstream analyses. The genetic diversity analysis revealed that the inbreeding coefficient (Fis) ranged from 0.5810 to 0.7019, indicating a high degree of genetic similarity and low heterozygosity within the population (Table 1). This pattern is consistent with the species’ known clonal propagation strategy. The expected heterozygosity (HE) ranged from 0.1705 to 0.2171, while the observed heterozygosity (HO) ranged from 0.05901 to 0.0830. The heterozygosity of the L. racemosus population is significantly lower than the expected value, suggesting that factors such as inbreeding, population structure, or genetic drift may be present, resulting in heterozygosity that is lower than theoretical expectations and a reduction in genetic diversity. The Pi value ranged from 0.0353 to 0.0380, reflecting significant DNA sequence variation among individuals and a low level of genetic diversity. The Tajima’s D values (0.518-0.655) suggested potential balancing selection or a recent contraction of the population. Genetic differentiation metrics revealed that populations KK19 and MM20 exhibited pronounced genetic divergence from other populations, whereas populations TM21, TS22, FH23, CZ24 and SD25 displayed relatively low genetic differentiation, and minimal genetic divergence was observed among the remaining populations (Supplementary Figure S1).
3.2 Population genetic structure
STRUCTURE analysis identified K = 2 as the optimal number of clusters using the cross-validation error (CV) method (Figures 2A, B; Supplementary Figures S2, S3). Lineage A consisted of 20 populations from the Irtysh basin, while lineage U included seven populations (KK19, MM20, TM21, TS22, FH23, CZ24, SD25) from the Ulungur basin and Kalamaili Mountains. Five populations (TM21, TS22, FH23, CZ24, SD25) exhibit varying degrees of genetic admixture, suggesting that these populations may occupy a transitional genetic zone between the lineages. Notably, the three populations near Ulungur Lake (HJ16, MG18, WL17) exhibited genetic proximity to those in the Irtysh River Basin. This observation is related to the geographical characteristics of Ulungur Lake, which serves as the connection point between the Irtysh River and the Ulungur River water systems. Principal Component Analysis (PCA) revealed that PC1 and PC2 accounted for 32.45% and 27.91% of the total genetic variation, respectively (Figure 2C). Individuals from distinct lineages exhibited significant genetic divergence, further corroborating the patterns identified in the STRUCTURE analysis. Phylogenetic tree analysis, supported by well-calibrated posterior probabilities, also confirmed this genetic structure (Figure 2D).
3.3 DIY-ABC-based inference of demographic history
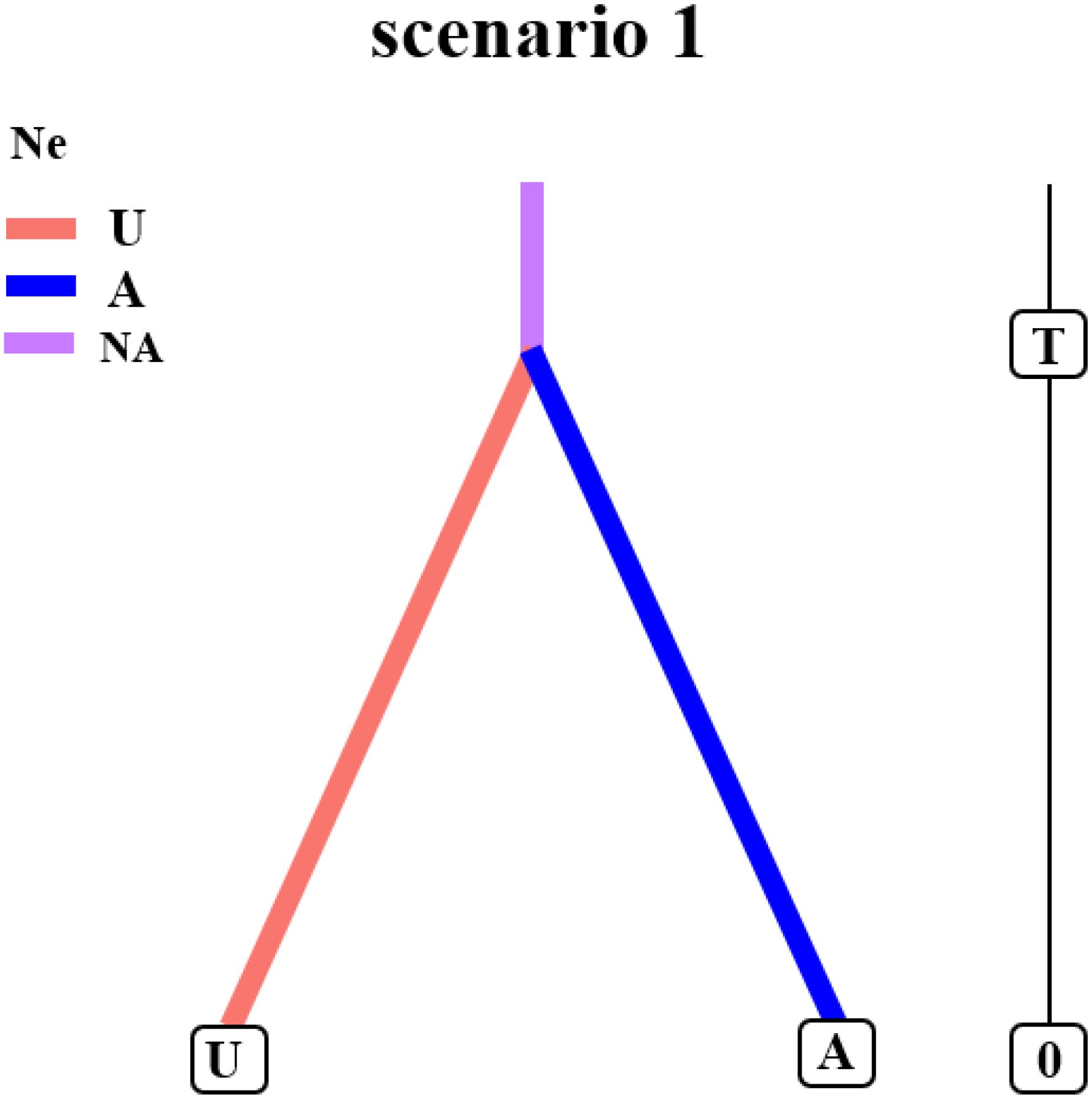
We employed Approximate Bayesian Computation (ABC) as implemented in DIYABC v2.1.0 to evaluate three evolutionary scenarios for L. racemosus. Our analysis provides the strongest support for Model 1, which best explains the divergence of the A and U lineages (Figure 3; Supplementary Figure S4; Supplementary Tables S4, S5). The divergence time was estimated by scaling the expected value of the time parameter (t) by the generation time of L. racemosus. This analysis indicates that the A and U lineages diverged from a common ancestor approximately 0.0295 million years ago (Mya; 95% CI), during the mid-Pleistocene. These historical events have shaped the current phylogeographic structure, wherein the distribution of lineage U is centered in the Ulungur River basin, while lineage A is predominantly found in the Irtysh River basin.
3.4 Genetic and environmental association analysis
Gradient forest analysis of 16 environmental predictors, comprising 8 climatic and 8 edaphic factors (Figure 4), revealed that bio12 (annual precipitation), bio6 (minimum temperature in the coldest month), bio9 (average temperature in the driest quarter), bio8 (average temperature in the wettest quarter), bio7 (annual temperature range), and bio10 (average temperature in the hot quarter) were identified as the six most significant climate factors influencing changes in allele frequencies within the L. racemosus population. The weights of the remaining 10 ecological factors were small, limited impact on genetic variation. Annual precipitation emerged as the most critical climatic factor, with its maximum weight underscoring the decisive role of water conditions in the growth and reproduction. Additionally, bio6, bio9, bio8, bio7, and bio10 suggest that temperature fluctuations significantly affect the physiological adaptability and genetic diversity. In contrast, the impact of soil factors on the genetic variation of L. racemosus appears to be relatively minor, likely due to soil homogeneity across the study area.
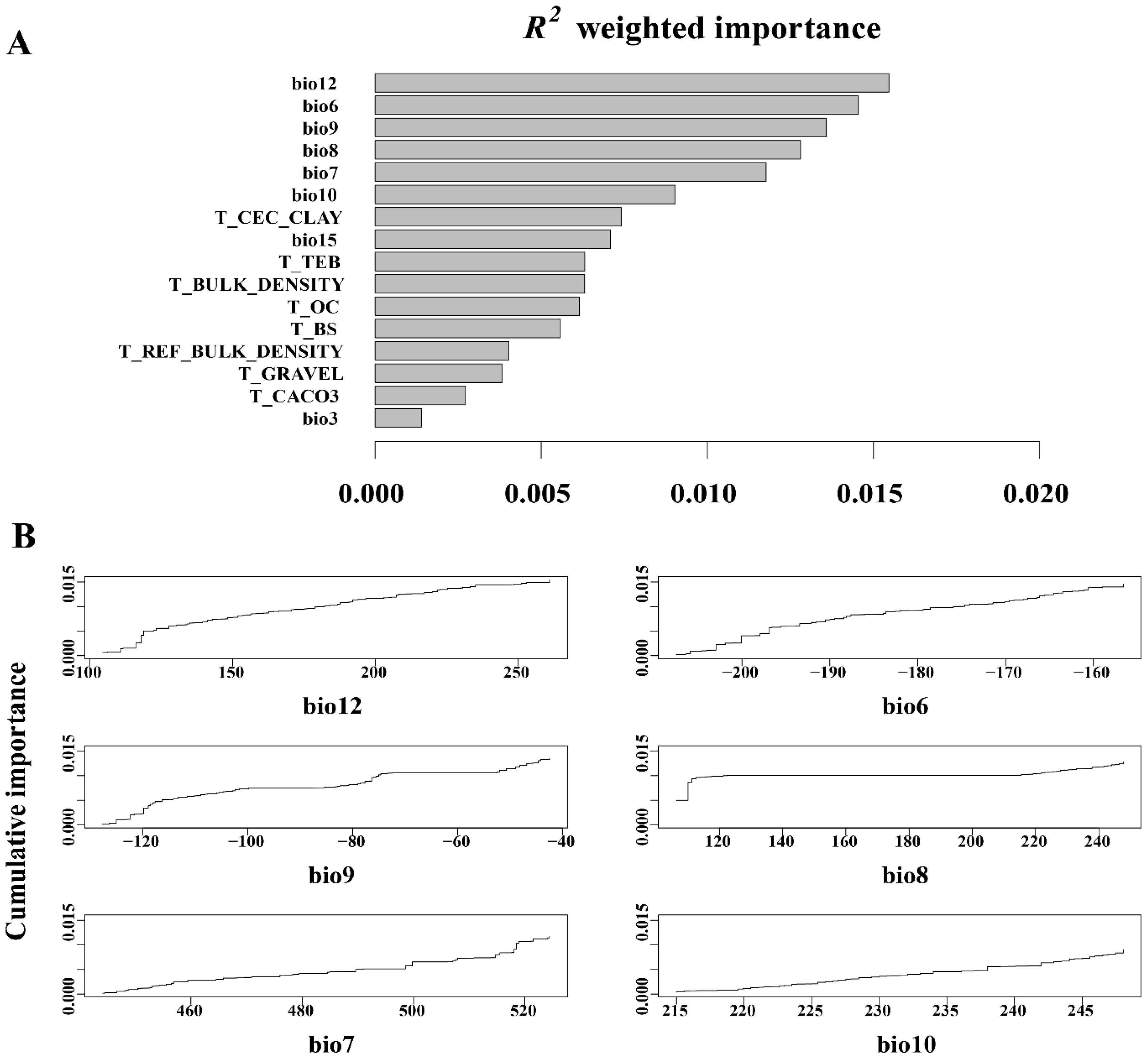
Figure 4. Leymus racemosus gradient forest (GF) analysis. (A), R2-weighted importance of environmental variables that elucidate genetic gradients; (B), The cumulative importance of allele variation in the first six environmental gradients.
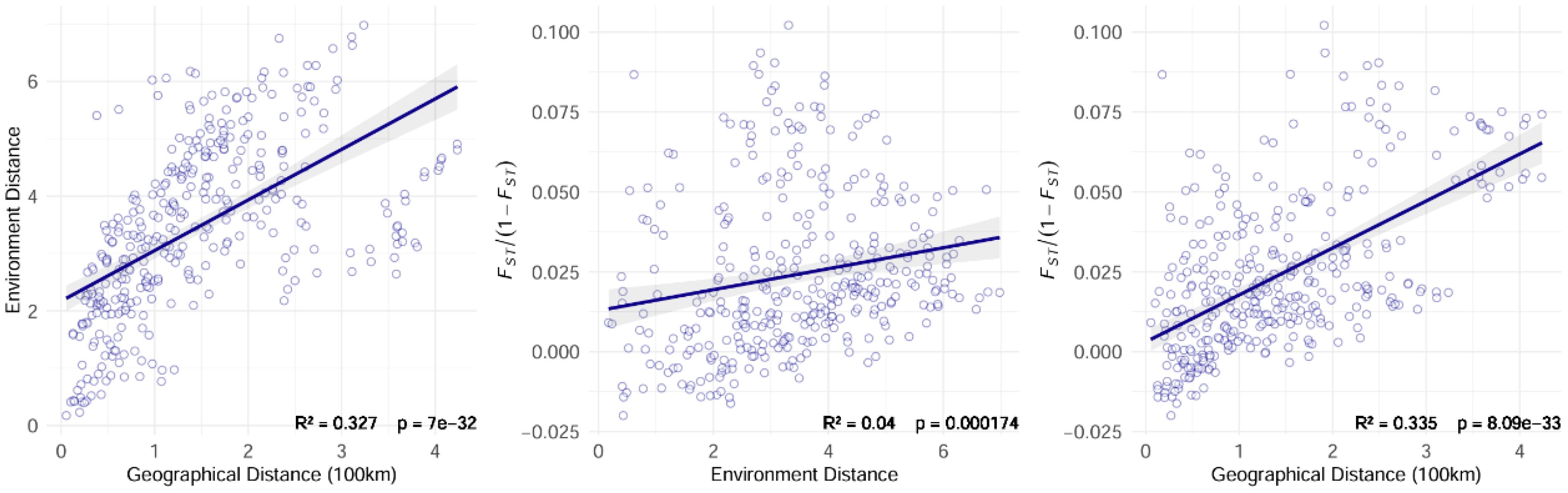
Redundancy analysis (RDA) revealed that climatic variables accounted for 39.6% of the total genetic variation, with RDA1 (21.7%) and RDA2 (17.9%) capturing the primary environmental-genetic covariation (Figure 5). Three thermal parameters exhibited the strongest loadings: the minimum temperature of the coldest month (bio6), the mean temperature of the wettest quarter (bio8), and the mean temperature of the driest quarter (bio9). These temperature extremes also emerged as the top predictors in gradient forest analysis, thereby confirming their dual roles as selective pressures and dispersal filters. Mantel tests quantified isolation patterns, revealing that genetic distance exhibited the strongest correlation with geographic distance (R² = 0.335, p<0.001) (Figure 6), followed by environment-geography covariance (R² = 0.327, p<0.001), while direct genetic-environment relationships were the weakest (R² = 0.04, p<0.001).
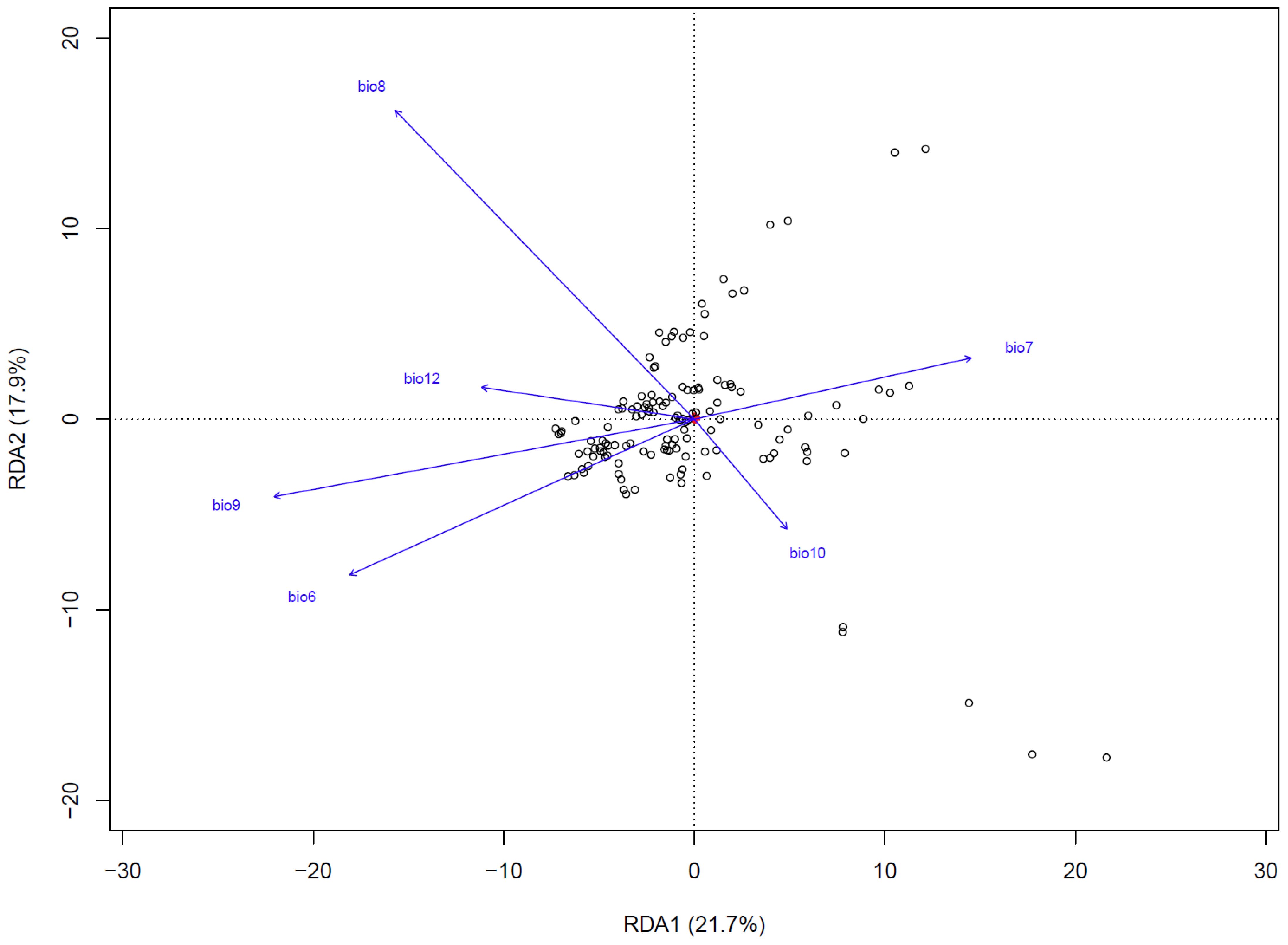
Figure 5. Redundancy analysis (RDA) of the relationship between 6 important environmental factors and genetic variation in Leymus racemosus. White circle, different individuals; small red points (located at the center of the figure), single nucleotide polymorphism (SNP) site.
4 Discussion
4.1 The genetic structure of L. racemosus population and its geographical distribution pattern
The level of genetic diversity is closely linked to population reproductive strategies, as well as a species’ evolutionary potential and its ability to adapt to environmental changes (Jump and Peñuelas, 2005; Kim et al., 2019). In contrast to the high genetic diversity observed in its congeneric species, Leymus chinensis (HO = 0.780, HE = 0.916), we found that populations of L. racemosus exhibit relatively low genetic diversity (HO = 0.0674, HE = 0.2261). These results are comparable to those reported for other low genetic diversity species, such as Albizia odoratissima (HO = 0.189, HE = 0.189) and Myricaria laxiflora (HO = 0.1394, HE = 0.1468) (Li et al., 2025; Ou et al., 2025; Xiao et al., 2025). We attribute this low genetic diversity to the species’ facultative reproductive strategy. Under multiple environmental pressures, including drought stress, wind erosion, and human disturbance, the species exhibits reduced natural regeneration and lower seed set during the sexual reproduction phase (Wang et al., 2011; Gulnar et al., 2014). This has driven the species to evolve a survival strategy predominantly reliant on asexual reproduction. Although asexual reproduction enables rapid niche occupation, it leads to high genotypic homogeneity within populations, consequently reducing genetic diversity. Low genetic diversity is also observed in other species that have facultative reproduction, for example, Scutellaria floridana (Hanko et al., 2023), Santalum lanceolatum (Brunton et al., 2021), Nypa fruticans (Sugai et al., 2016), and Santalum insulare (Lhuillier et al., 2006). These similar genetic characteristics highlight the critical influence of reproductive modes on the genetic diversity levels of populations. Against the backdrop of low overall genetic diversity in the species, our results revealed that high genetic differentiation exists not only between lineages but also among certain populations (e.g., KK19 and MM20). We hypothesize that this differentiation is associated with the heterogeneity of their habitats: KK19 population is located on a severely wind-eroded, species-poor mountain ridge, while MM20 population is distributed across a relatively species-rich desert steppe. This pronounced difference in microhabitats is likely to be an important contributor to the strong genetic differentiation observed between these two populations (Xia et al., 2019). In addition, field surveys indicate that the L. racemosus population currently exhibits distinct patchy distribution characteristics. This phenomenon may be attributed to population isolation resulting from landscape fragmentation, which subsequently affects the genetic diversity and ecological adaptability of the population. Factors such as habitat loss, invasive species, water diversion, and alterations in river flow may drive this fragmentation (Rood et al., 2005; Thaulow et al., 2013; Mayonde et al., 2019; Pflueger et al., 2019), ultimately result in the loss of genetic connectivity at the landscape level, thereby causing a reduction in gene flow and change in the genetic diversity of species (Cushman et al., 2014). This phenomenon may further diminish genetic variation within populations, thereby compromising their long-term adaptive capacity.
Genome SNP analysis has revealed a significant phylogeographic structure within L. racemosus, which can be divided into two major lineages, designated A and U. The spatial distribution of these two lineages exhibits a clear pattern of river basin differentiation: lineage A is centered on the Irtysh River basin, whereas lineage U primarily occupies the Ulungur River basin, with gradual expansion into the Junggar Basin. This distribution pattern strongly suggests that lineage divergence within this species has been driven by a combination of geographical isolation and differential selection pressures (Liu et al., 2019b; Tóth et al., 2019). By integrating genetic structure analysis with geographical data, we found that the differentiation between lineages U and A may have been profoundly influenced by the regional river network. Within the Irtysh River basin, the genetic structure exhibits higher homogeneity, likely due to more extensive habitats and fewer geographical barriers, thereby promoting gene flow (Ma et al., 2016; Luo et al., 2023). In contrast, populations in the Ulungur River basin show signs of genetic admixture, which may reflect gene introgression during the differentiation process in this region, superimposed with the impacts of human activities (Mairal et al., 2022; Yan et al., 2024). Consequently, the two major basins, the Irtysh and Ulungur Rivers, can each act as corridors for gene exchange, but the area between these distinct basins constitutes a geographical barrier that promotes lineage differentiation. This dual role has been observed in a similar pattern in Populus fremontii, whereby small rivers and highlands restrict gene flow, whereas medium and large rivers facilitate genetic exchange (Cushman et al., 2014). Furthermore, the correlation between hydrological features—such as river gradient, confluence frequency, basin size, seasonal flow, and high-discharge events—and the spatial genetic structure further underscores the critical role of the riverine landscape in intraspecific lineage differentiation (Olsen et al., 2010; Cook et al., 2011; Kanno et al., 2011; Zhang and Sun, 2011). The complex interplay between hydrological connectivity and geographical isolation within these watersheds has fundamentally shaped the contemporary genetic architecture of L. racemosus.
4.2 The adaptation of L. racemosus to the pattern of natural landscape
This study investigates the adaptive evolution of L. racemosus in response to natural landscape patterns, with particular emphasis on how climate fluctuations and geomorphic changes have influenced population differentiation across various temporal and spatial scales (Bay et al., 2018; Du et al., 2020; Tian et al., 2024). Demographic analyses indicate that the initial divergence of L. racemosus populations occurred approximately 0.0295 million years ago. This timeframe corresponds to the mid-to-late phase of the Last Glacial Period (70-11.5 ka before present (BP)), suggesting that their divergence was driven by climatic fluctuations associated with Quaternary glaciation (Li et al., 2004). Since the Pleistocene, the continuous aridification of Northwest China, compounded by periodic cold events during the Last Glacial Period, has collectively reshaped the evolutionary trajectory of L. racemosus (Guo et al.,1999; Xu et al., 2010, Xu et al., 2010). Intense climatic fluctuations led to a sharp reduction in river flow and increasing habitat fragmentation (Huang et al., 2023), imposing strong natural selection pressures on the species while simultaneously restructuring the spatial configuration of its habitat through geomorphological changes (Zhang and Sun, 2011). Previous studies have shown that glacial activity can reshape river networks, thereby triggering genetic differentiation in species (Zhang et al., 2008; Zhang et al., 2011; Zhao et al., 2013; Panin et al., 2020). With the repeated advances and retreats of glaciers during glacial periods, the periodic formation and disappearance of ephemeral river networks may have provided a unique dynamic of dispersal-isolation for L. racemosus. These river networks acted as corridors for gene flow, during humid periods, whereas they transformed into geographical barriers during arid, cold periods, reinforcing local adaptation. The onset of vegetation succession during this interval was closely synchronized with the severe climatic changes, showing no apparent lag effect (Xu et al., 2023). Therefore, the intense climatic fluctuations of the Quaternary glacial periods, coupled with persistent aridification, likely represent key factors driving the population genetic differentiation of L. racemosus.
The interplay between isolation by distance (IBD) and isolation by environment (IBE) highlights the complex nature of genetic divergence in natural environments, where geographic isolation restricts gene flow while environmental variability exacerbates differentiation by creating that favor specific genetic traits (Tian et al., 2024; Ma et al., 2025). Gradient forest (GF) and redundancy analysis (RAD) identified precipitation and temperature as the primary environmental variables influencing genetic variation and structure in L. racemosus, while soil factors played a minimal role. This pattern is likely related to the species’ ecological preference for dune habitats characterized by limited rainfall and pronounced environmental selectivity (Dong et al., 1985). The restricted precipitation and fluctuating temperatures significantly affect both the distribution and genetic characteristics of L. racemosus. Our study is in high agreement with previous studies, for instance, genetic divergence and adaptive divergence in populations of the Caragana genus have been significantly associated with variations in precipitation (Yang et al., 2013). Additionally, changes in temperature and precipitation have been linked to adaptive differentiation in Fagus sylvatica and Microhyla fissipes in Europe (Postolache et al., 2021; Jin et al., 2022). Similarly, the genetic variation and adaptive evolution of Actinidia eriantha are influenced by both precipitation and solar radiation (Jiang et al., 2025). In natural environments, climate variability alters resource distribution and selection pressures, while geomorphic evolution shapes genetic structures by influencing isolation and migration corridors (Liu et al., 2017b; Harris et al., 2018).
4.3 Conservation recommendations
Studies on population genetic diversity and genetic structure in relation to environmental factors are fundamental for developing effective conservation strategies and rationally utilizing genetic resources (Li et al., 2025). L. racemosus, a wild relative of wheat, has been designated as a Category III protected plant in Xinjiang, China (Yin et al., 2006). Given the low genetic diversity of L. racemosus and its significant shelter vulnerability to environmental factors, we propose establishing protected areas within its current distribution range, including the Irtysh-Ulungur dual-basin and Kalamiris Mountain. This conservation measure aims to safeguard both the species and its critical habitats while simultaneously mitigating human-induced disturbances. Additionally, systematically collecting seeds and rhizome materials from geographically distinct populations will facilitate the establishment of cryopreserved seed banks and ex situ living gene banks (via in situ cultivation). These measures will ensure the effective preservation of existing genetic resources, safeguarding the evolutionary potential of L. racemosus in the face of environmental challenges.
5 Conclusion
In summary, this study provides a comprehensive analysis of the interplay between genetic structure and natural landscape patterns in L. racemosus. It demonstrates that geographic distance, environmental factors, and their interactions play critical roles in driving genetic divergence. The findings indicate that the river system is the primary factor influencing genetic divergence among lineages, while climatic variables such as precipitation and temperature also significantly shape genetic variation and structure. These results enhance our understanding of how genetic structure correlates with natural landscape patterns and provide theoretical support for the conservation and genetic resource utilization of L. racemosus.
Data availability statement
The data presented in this study are deposited in the NGDC repository, accession number CRA023768.
Author contributions
ZZ: Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. XS: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. HT: Data curation, Methodology, Software, Visualization, Writing – review & editing. DT: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (32460061), the Natural Science Foundation of the Xinjiang Uygur Autonomous Region (2022D01B106), the Tianshan Yingcai Cultivation Program for Leading Talents in Scientific and Technological Innovation (2024TSYCLJ0016), the Natural Science Foundation of the Xinjiang Uygur Autonomous Region(2022D01A195).
Acknowledgments
Thanks to Shanghai Personalbio Technology Co., Ltd. for assisting in sequencing data acquisition.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1675027/full#supplementary-material
References
Bay, R. A., Harrigan, R. J., Underwood, V. L., Gibbs, H. L., Smith, T. B., and Ruegg, K. (2018). Genomic signals of selection predict climate-driven population declines in a migratory bird. Science 359, 83–86. doi: 10.1126/science.aan4380
Brunton, A., Lee, D. J., Conroy, G., and Ogbourne, S. (2021). Clonal reproduction and low genetic diversity in northern Australian santalum lanceolatum (Santalaceae) populations highlights the need for genetic rescue of this commercially significant species. Forests 12, 741. doi: 10.3390/f12060741
Buiteveld, J., Vendramin, G. G., Leonardi, S., Kamer, K., and Geburek, T. (2007). Genetic diversity and differentiation in European beech (Fagus sylvatica L.) stands varying in management history. For. Ecol. Manage. 247, 98–106. doi: 10.1016/j.foreco.2007.04.018
Capblancq, T. and Forester, B. R. (2021). Redundancy analysis: A Swiss Army Knife for landscape genomics. Methods Ecol. Evol. 12, 2298–2309. doi: 10.1111/2041-210X.13722
Chang, C.-W., Fridman, E., Mascher, M., Himmelbach, A., and Schmid, K. (2022). Physical geography, isolation by distance and environmental variables shape genomic variation of wild barley (Hordeum vulgare L. ssp. spontaneum) in the southern levant. Heredity 128, 107–119. doi: 10.1038/s41437-021-00494-x
Cleophas, T. J. and Zwinderman, A. H. (2018). Bayesian pearson correlation analysis. Modern Bayesian Stat Clin. Res. 111–118. doi: 10.1007/978-3-319-92747-3_11
Cook, B. D., Kennard, M. J., Real, K., Pusey, B. J., and Hughes, J. M. (2011). Landscape genetic analysis of the tropical freshwater fish Mogurnda mogurnda (eleotridae) in a monsoonal river basin: importance of hydrographic factors and population history. Freshw. Biol. 56, 812–827. doi: 10.1111/j.1365-2427.2010.02527.x
Cornuet, J.-M., Ravigné, V., and Estoup, A. (2010). Inference on population history and model checking using DNA sequence and microsatellite data with the software DIYABC (v1.0). BMC Bioinf. 11, 401. doi: 10.1186/1471-2105-11-401
Cushman, S. A., Max, T., Meneses, N., Evans, L. M., Ferrier, S., Honchak, B., et al. (2014). Landscape genetic connectivity in a riparian foundation tree is jointly driven by climatic gradients and river networks. Ecol. Appl. 24, 1000–1014. doi: 10.1890/13-1612.1
Danecek, P., Auton, A., Abecasis, G., Albers, C. A., Banks, E., DePristo, M. A., et al. (2011). The variant call format and VCFtools. Bioinform. (Oxf. Engl.) 27, 2156–2158. doi: 10.1093/bioinformatics/btr330
De Kort, H., Prunier, J. G., Ducatez, S., Honnay, O., Baguette, M., Stevens, V. M., et al. (2021). Life history, climate and biogeography interactively affect worldwide genetic diversity of plant and animal populations. Nat. Commun. 12, 516. doi: 10.1038/s41467-021-20958-2
DeWoody, J. A., Harder, A. M., Mathur, S., and Willoughby, J. R. (2021). The long-standing significance of genetic diversity in conservation. Mol. Ecol. 30, 4147–4154. doi: 10.1111/mec.16051
Dixon, P. (2003). VEGAN, a package of R functions for community ecology. J. Vegetation Sci. 14, 927–930. doi: 10.1111/j.1654-1103.2003.tb02228.x
Dong, Y., Sun, Y., Zhong, G., and Cui, N. (1985). Investigation and preliminary research on Leymus racemosus in Altay region of Xinjiang. Chin. Agric. science. 2, 54–56.
Dorogina, O. V. and Zhmud, E. V. (2020). Molecular-genetic methods in plant ecology. Contemp. Probl. Ecol. 13, 333–345. doi: 10.1134/S1995425520040058
Du, F. K., Wang, T., Wang, Y., Ueno, S., and de Lafontaine, G. (2020). Contrasted patterns of local adaptation to climate change across the range of an evergreen oak, Quercus aquifolioides. Evol. Appl. 13, 2377–2391. doi: 10.1111/eva.13030
Eckert, C. G., Samis, K. E., and Lougheed, S. C. (2008). Genetic variation across species’ geographical ranges: the central-marginal hypothesis and beyond. Mol. Ecol. 17, 1170–1188. doi: 10.1111/j.1365-294X.2007.03659.x
Edet, O. U., Kim, J.-S., Okamoto, M., Hanada, K., Takeda, T., Kishii, M., et al. (2018). Efficient anchoring of alien chromosome segments introgressed into bread wheat by new Leymus racemosus genome-based markers. BMC Genet. 19, 18. doi: 10.1186/s12863-018-0603-1
Ellis, N., Smith, S. J., and Pitcher, C. R. (2012). Gradient forests: calculating importance gradients on physical predictors. Ecology 93, 156–168. doi: 10.1890/11-0252.1
Gao, J. (2017). Analysis of the characteristics of ttemperature and pprecipitation cchanges of Irtysh River Basin in Xinjiang. Energ And Energy Conserv., 2, 115–116. doi: 10.3969/j.issn.2095-0802.2017.02.053
Gulnar, A., Zhou, G., and Ayturhan, R. (2014). Fruit-set patterns of Leymus racemosus. J. Desert Res. 34, 1037–1041. doi: 10.7522/j.issn.1000-694X.2013.00405
Guo, Z., Peng, S., Hao, Q., Chen, X., and Liu, T. (1999). Late tertiary development of aridification in northwestern China: line with the arctic ice-sheet formation and tibetan uplifts. Quat. Sci. 19, 556–567.
Guo, J., Richards, C. L., Holsinger, K. E., Fox, G. A., Zhang, Z., and Zhou, C. (2021). Genetic structure in patchy populations of a candidate foundation plant: a case study of Leymus chinensis using genetic and clonal diversity. Am. J. Bot. 108, 2371–2387. doi: 10.1002/ajb2.1771
Hanko, G. R., Vogel, M. T., Negrón-Ortiz, V., and Moore, R. C. (2023). High prevalence of clonal reproduction and low genetic diversity in Scutellaria floridana, a federally threatened florida-endemic mint. Plants (Basel Switz.) 12, 919. doi: 10.3390/plants12040919
Harris, R. M. B., Beaumont, L. J., Vance, T. R., Tozer, C. R., Remenyi, T. A., Perkins-Kirkpatrick, S. E., et al. (2018). Biological responses to the press and pulse of climate trends and extreme events. Nat. Clim Change 8, 579–587. doi: 10.1038/s41558-018-0187-9
Holderegger, R., Buehler, D., Gugerli, F., and Manel, S. (2010). Landscape genetics of plants. Trends Plant Sci. 15, 675–683. doi: 10.1016/j.tplants.2010.09.002
Hu, W., Zhang, Y., and Wang, L. (2014). Effects of leymus racemosus chromosome to the salt tolerance of common wheat seed. Hubei Agric. Sci. 53, 3497–3501. doi: 10.14088/j.cnki.issn0439-8114.2014.15.073
Huang, L., Li, S., Huang, W., Xiang, H., Jin, J., and Oskolski, A. A. (2023). Glacial expansion of cold-tolerant species in low latitudes: megafossil evidence and species distribution modelling. Natl. Sci. Rev. 10, nwad038. doi: 10.1093/nsr/nwad038
Huang, Z., Wu, H., and Hu, Z. (1997). The structures of 30 species of psammophytes and their adaptation to the sandy desert environment in Xinjiang. Acta Phytoecologica Sinica. 21, 521.
Jiang, X., Huang, J., Zhang, Y., and Li, J. (2019). Impact analysis of hydrological regime changes in irtysh river basin valley ecosystem and suggestions for ecological restoration. Rural Water conservancy hydropower China. 10, 12–16.
Jiang, S., Luo, M.-X., Gao, R.-H., Zhang, W., Yang, Y.-Z., Li, Y.-J., et al. (2019). Isolation-by-environment as a driver of genetic differentiation among populations of the only broad-leaved evergreen shrub Ammopiptanthus mongolicus in Asian temperate deserts. Sci. Rep. 9, 12008. doi: 10.1038/s41598-019-48472-y
Jiang, Q., Shen, Y., Wu, L., Jiang, Z., and Yao, X. (2025). Genomic signatures of local adaptation to precipitation and solar radiation in kiwifruit. Plant Divers. 47, 733–745. doi: 10.1016/j.pld.2025.02.003
Jin, L., Liao, W. B., and Merilä, J. (2022). Genomic evidence for adaptive differentiation among Microhyla fissipes populations: implications for conservation. Divers. Distrib. 28, 2665–2680. doi: 10.1111/ddi.13433
Ju, B., Zhang, S., and Hu, D. (2015). Characteristics and trend of precipitation changes over irtysh river basin. J. Yangtze River Sci. Res. Institute. 32, 21. doi: 10.11988/ckyyb.20150019
Jump, A. S. and Peñuelas, J. (2005). Running to stand still: adaptation and the response of plants to rapid climate change. Ecol. Lett. 8, 1010–1020. doi: 10.1111/j.1461-0248.2005.00796.x
Kanno, Y., Vokoun, J. C., and Letcher, B. H. (2011). Fine-scale population structure and riverscape genetics of brook trout (Salvelinus fontinalis) distributed continuously along headwater channel networks. Mol. Ecol. 20, 3711–3729. doi: 10.1111/j.1365-294X.2011.05210.x
Karney, C. F. F. (2013). Algorithms for geodesics. J. Geod. 87, 43–55. doi: 10.1007/s00190-012-0578-z
Kim, Y. K., Kim, S. H., Yi, J. M., Park, S. R., and Lee, K. S. (2019). Influence of environmental disturbances and reproductive strategy on genetic diversity and differentiation of Zostera marina populations on the southern coast of Korea. Mar. Ecology. 40, 1–9. doi: 10.1111/maec.12537
Kirschner, P., Zaveska, E., Huelber, K., Wessely, J., Willner, W., Schoenswetter, P., et al. (2023). Evolutionary dynamics of Euphorbia carniolica suggest a complex Plio-Pleistocene history of understorey species of deciduous forest in southeastern Europe. Mol. Ecol. 32, 5350–5368. doi: 10.1111/mec.17102
Lhuillier, E., Butaud, J.-F., and Bouvet, J.-M. (2006). Extensive clonality and strong differentiation in the insular pacific tree Santalum insulare: implications for its conservation. Ann. Bot. 98, 1061–1072. doi: 10.1093/aob/mcl190
Li, H. and Durbin, R. (2009). Fast and accurate short read alignment with burrows-wheeler transform. Bioinform. (Oxf. Engl.) 25, 1754–1760. doi: 10.1093/bioinformatics/btp324
Li, Z., Ji, Q., Yang, Y., Xu, M., and Guan, Y. (2025). Low genetic diversity and weak population structure of Albizia odoratissimaon Hainan Island. BMC Plant Biol. 25, 395. doi: 10.1186/s12870-025-06373-5
Li, J., Shu, Q., Zhou, Z., Zhao, Z., and Zhang, J. (2004). Review and prospects of quaternary glaciation research in China. J. Glaciology Geocryology 03), 235–243. cnki:sun:bcdt.0.2004-03-001
Liu, D. (2004). The evolution of the natural environment in Northwest China and its development trend. Sci. Press. doi: 10.3969/j.issn.1000-1123.2003.09.011
Liu, Y., Dietrich, C. H., and Wei, C. (2019a). Genetic divergence, population differentiation and phylogeography of the cicada Subpsaltria yangi based on molecular and acoustic data: an example of the early stage of speciation? BMC Evol. Biol. 19, 5. doi: 10.1186/s12862-018-1317-8
Liu, H., Gao, Y.-B., Ruan, W., Chen, L., Li, C., Zhao, N., et al. (2004). Genetic differentiation within and between Leymus chinensis populations from different zones of Mid-Eastern Inner Mongolia steppe. Acta Ecologica Sin., 24, 2157–2164.. doi: 10.1007/BF02873086
Liu, Y., Liu, T., Lu, T., Zhou, Y., Zhang, X., and Su, X. (2017a). Geographical distribution, origin, and dispersal of Leymus Hochst. (Poaceae). Plant Sci. J. 35, 305–317. doi: 10.11913/PSJ.2095-0837.2017.30305
Liu, J., Luo, Y., Li, D., and Gao, L. (2017b). Evolution and maintenance mechanisms of plant diversity in the Qinghai-Tibet Plateau and its adjacent areas: Progress and prospects. Biodiversity. 25, 163–174. doi: 10.17520/biods.2016293
Liu, Y., Wang, X., and Zeng, Q. (2019b). De novo assembly of white poplar genome and genetic diversity of white poplar population in Irtysh River Basin in China. Sci. China Life Sci. 62, 609–618. doi: 10.1007/s11427-018-9455-2
Liu, H., Wang, Z., Zhang, Y., Li, M., Wang, T., and Su, Y. (2023). Geographic isolation and environmental heterogeneity contribute to genetic differentiation in Cephalotaxus oliveri. Ecol. Evol. 13, e9869. doi: 10.1002/ece3.9869
Liu, Y. and Zhuo, G. (2008). Seed dormancy and longevity in leymus racemosus (Lam.) tzvel. Xinjiang Agric. Sci. 45, 356–361. doi: CNKI:SUN:XJNX.0.2008-02-035
Luan, P., Huo, T., Ma, B., Song, D., Zhang, X., and Hu, G. (2021). Genomic inbreeding and population structure of northern pike (Esox lucius) in XinJiang, China. Ecol. Evol. 11, 5657–5668. doi: 10.1002/ece3.7469
Luo, H., Fang, D., He, M., Mao, C., Kuang, Z., Qi, H., et al. (2023). Genetic diversity and population structure of Gymnocypris przewalskii based on SNP markers. South China Fisheries Sci. 19, 86–96. doi: 10.12131/20220091
Ma, X., Wang, W., Pang, Y., Chen, W., and Yang, Y. (2016). Microsatellite marker analysis of genetic diversity in the two populations of Yellow River carps. Heilongjiang Anim. Husbandry Veterinary Med. 12, 22–26. doi: 10.13881/j.cnki.hljxmsy.2016.2273
Ma, W., Zhang, J., Tian, H., Li, Y., and Zhang, H. (2025). Genetic structure and local adaptation of Nitraria sphaerocarpa populations from arid northwestern China. Front. Plant Sci. 16. doi: 10.3389/fpls.2025.1623235
Mairal, M., Chown, S. L., Shaw, J., Chala, D., Chau, J. H., Hui, C., et al. (2022). Human activity strongly influences genetic dynamics of the most widespread invasive plant in the sub-Antarctic. Mol. Ecol. 31, 1649–1665. doi: 10.1111/mec.16045
Mayonde, S., Cron, G., Glennon, K. L., and Byrne, M. J. (2019). Genetic diversity assessment of Tamarix in South Africa - biocontrol and conservation implications. S. Afr. J. Bot. 121, 54–62. doi: 10.1016/j.sajb.2018.10.030
Olsen, J. B., Beacham, T. D., Wetklo, M., Seeb, L. W., Smith, C. T., Flannery, B. G., et al. (2010). The influence of hydrology and waterway distance on population structure of chinook salmon Oncorhynchus tshawytscha in a large river. J. Fish Biol. 76, 1128–1148. doi: 10.1111/j.1095-8649.2010.02554.x
Orsini, L., Vanoverbeke, J., Swillen, I., Mergeay, J., and De Meester, L. (2013). Drivers of population genetic differentiation in the wild: isolation by dispersal limitation, isolation by adaptation and isolation by colonization. Mol. Ecol. 22, 5983–5999. doi: 10.1111/mec.12561
Ou, T., Wu, Z., Tian, C., Yang, Y., Gong, W., Niu, J., et al. (2025). Development of genome-wide SSR markers in Leymus chinensis with genetic diversity analysis and DNA fingerprints. Int. J. Mol. Sci. 26, 918. doi: 10.3390/ijms26030918
Panin, A. V., Astakhov, V. I., Lotsari, E., Komatsu, G., Lang, J., and Winsemann, J. (2020). Middle and Late Quaternary glacial lake-outburst floods, drainage diversions and reorganization of fluvial systems in northwestern Eurasia. Earth-Science Rev. 201, 103069. doi: 10.1016/j.earscirev.2019.103069
Patterson, N., Moorjani, P., Luo, Y., Mallick, S., Rohland, N., Zhan, Y., et al. (2012). Ancient admixture in human history. Genetics 192, 1065–1093. doi: 10.1534/genetics.112.145037
Pflueger, F. J., Signer, J., and Balkenhol, N. (2019). Habitat loss causes non-linear genetic erosion in specialist species. Glob.Ecol. Conserv. 17, e00507. doi: 10.1016/j.gecco.2018.e00507
Porebski, S., Bailey, L. G., and Baum, B. R. (1997). Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol. Biol. Rep. 15, 8–15. doi: 10.1007/BF02772108
Postolache, D., Oddou-Muratorio, S., Vajana, E., Bagnoli, F., Guichoux, E., Hampe, A., et al. (2021). Genetic signatures of divergent selection in European beech (Fagus sylvatica L.) are associated with the variation in temperature and precipitation across its distribution range. Mol. Ecol. 30, 5029–5047. doi: 10.1111/mec.16115
Purcell, S., Neale, B., Todd-Brown, K., Thomas, L., Ferreira, M. A. R., Bender, D., et al. (2007). PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575. doi: 10.1086/519795
Rood, S. B., Samuelson, G. M., Braatne, J. H., Gourley, C. R., Hughes, F. M., and Mahoney, J. M. (2005). Managing river flows to restore floodplain forests. Front. Ecol. Environ. 3, 193–201. doi: 10.1890/1540-9295(2005)003[0193:MRFTRF]2.0.CO;2
Sexton, J. P., Hangartner, S. B., and Hoffmann, A. A. (2014). Genetic isolation by environment or distance: which pattern of gene flow is most common? Evol.; Int. J. Org. Evol. 68, 1–15. doi: 10.1111/evo.12258
Su, X., Liu, Y., and Chen, W. (2013). Taxonomic review of leymus (Poaceae). J. Trop. Subtropical Bot. 21, 471–478. doi: 10.3969/j.issn.1005–3395.2013.05.015
Sugai, K., Watanabe, S., Kuishi, T., Imura, S., Ishigaki, K., Yokota, M., et al. (2016). Extremely low genetic diversity of the northern limit populations of Nypa fruticans (Arecaceae) on Iriomote Island, Japan. Conserv. Genet. 17, 221–228. doi: 10.1007/s10592-015-0773-6
Tamura, K., Stecher, G., and Kumar, S. (2021). MEGA11: molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 38, 3022–3027. doi: 10.1093/molbev/msab120
Thaulow, J., Borgstrom, R., and Heun, M. (2013). Brown trout population structure highly affected by multiple stocking and river diversion in a high mountain national park. Conserv. Genet. 14, 145–158. doi: 10.1007/s10592-012-0438-7
Tian, H., Zhang, H., Shi, X., Ma, W., and Zhang, J. (2024). Population genetic diversity and environmental adaptation of Tamarix hispida in the tarim basin, arid northwestern China. Heredity 133, 298–307. doi: 10.1038/s41437-024-00714-0
Tóth, E. G., Tremblay, F., Housset, J. M., Bergeron, Y., and Carcaillet, C. (2019). Geographic isolation and climatic variability contribute to genetic differentiation in fragmented populations of the long-lived subalpine conifer Pinus cembra L. in the western alps. BMC Evol. Biol. 19, 190. doi: 10.1186/s12862-019-1510-4
Tumpa, K., Šatović, Z., Vidaković, A., Idžojtić, M., Stipetić, R., and Poljak, I. (2022). Population variability of almond-leaved willow (Salix triandra L.) based on the leaf morphometry: isolation by distance and environment explain phenotypic diversity. Forests 13, 420. doi: 10.3390/f13030420
Walter, G. M., Clark, J., Terranova, D., Cozzolino, S., Cristaudo, A., Hiscock, S. J., et al. (2023). Hidden genetic variation in plasticity provides the potential for rapid adaptation to novel environments. New Phytol. 239, 374–387. doi: 10.1111/nph.18744
Wang, I. J. and Bradburd, G. S. (2014). Isolation by environment. Mol. Ecol. 23, 5649–5662. doi: 10.1111/mec.12938
Wang, C., Hasimu, D., and Zhou, G. (2011). Effects of different environmental factors on seed germination of Leymus racemosus. J. Xinjiang Agric. University. 34, 469–473. doi: CNKI:SUN:XJNY.0.2011-06-004
Wang, T. and Qiu, Y. (2024). Application and prospects of landscape genomics in conservation biology. Plant Sci. J. 41, 741–750. doi: 10.11913/PSJ.2095-0837.23166
Wang, I. J. and Summers, K. (2010). Genetic structure is correlated with phenotypic divergence rather than geographic isolation in the highly polymorphic strawberry poison-dart frog. Mol. Ecol. 19, 447–458. doi: 10.1111/j.1365-294X.2009.04465.x
Wang, L. and Zhang, Y. (2019). Molecular and cytogenetic identification of Triticum aestivum -Leym us racem osus translocation line T5AS/5LrL conferring resistance to Fusarium head blight. Acta Phytopathologica Sin. 49, 83–89. doi: 10.13926/j.cnki.apps.000194
Wang, Y., Zhao, L., Wang, H., Wang, J., Huang, D.-M., Hong, R.-M., et al. (2005). Molecular genetic variation in a clonal plant population of Leymus chinensis (Trin.) tzvel. J. Integr. Plant Biol. 47, 1055–1064. doi: 10.1111/j.1744-7909.2005.00091.x
Wu, Z. (1979). On the problem of regionalization of Chinese flora. Acta Botanica Yunnanica. 1, 1–20.
Xia, S., Hu, D., Deng, Y., Zhong, X., Bai, W., Zhang, J., et al. (2019). Habitat partitioning between sympatric golden pheasant and temminck’s tragopan at different spatial scales. Acta Ecol. Sin. 39, 1627–1638. doi: 10.5846/stxb201809051894
Xiao, Z., Liu, H., Huang, G., Wu, D., Qiu, L., Wu, J., et al. (2025). Spatially biased collections and the failure to cover all wild genetic clusters in plant populations under ex situ conservation. Plant Divers. doi: 10.1016/j.pld.2025.09.001
Xu, X., Kleidon, A., Miller, L., Wang, S., Wang, L., and Dong, G. (2010). Late quaternary glaciation in the tianshan and implications for palaeoclimatic change: a review. Boreas 39, 215–232. doi: 10.1111/j.1502-3885.2009.00118.x
Xu, D., Lu, H., Chu, G., Shen, C., Sun, Q., Wu, J., et al. (2023). Fast response of vegetation in east asia to abrupt climatic events during the last deglaciation. PNAS nexus 2, pgad061. doi: 10.1093/pnasnexus/pgad061
Xue, X., Dai, P., and Zhuo, G. (2015). Studies on karyotype of leymus racemosus (Lam.) tzvel of different populations. J. Xinjiang Agric. Univ. 38, 205–211. doi: 10.3969/j.issn.1007-8614.2015.03.006
Xue, J., Pei, Y., Cheng, Y., Ying, C., Hao, L., Hai, B., et al. (2020). Species diversity of freshwater fish and assessment on watershed health in the Irtysh River and Ulungur River basins in Xinjiang, China. Biodiversity Sci. 28, 422. doi: 10.17520/biods.2019071
Yan, L.-J., Fan, P.-Z., Wambulwa, M. C., Qi, H.-L., Chen, Y., Wu, Z.-Y., et al. (2024). Human-associated genetic landscape of walnuts in the Himalaya: implications for conservation and utilization. Diversity Distributions 30, e13809. doi: 10.1111/ddi.13809
Yang, J., Cushman, S. A., Yang, J., Yang, M., and Bao, T. (2013). Effects of climatic gradients on genetic differentiation of Caragana on the Ordos Plateau, China. Landscape Ecol. 28, 1729–1741. doi: 10.1007/s10980-013-9913-x
Yang, T., Meng, W., Hai, S., Niu, J., and Zhuo, Q. (2017). Population Genetic Structure of Siberian Dace (Leuciscus leuciscus baicalensis) in Irtysh River China Based on the Cyt b Gene Sequences. Chin. J. Zoology 52, 304–313. doi: 10.13859/j.cjz.201702014
Yang, H., Xu, F., Yelebolati, T., Cheng, C., Li, Q., Jin, B., et al. (2016). Constructing the river basin scale ecological protection system of ertix - ulungur river basin in xinjiang, China: importance, specificity and the spatial pattern of protected areas. China Population Resour. Envi Ronment. 26, 260–265. doi: CNKI:SUN:ZGRZ.0.2016-S1-060
Yang, R.-W., Zhou, Y.-H., Zhang, Y., Zheng, Y.-L., and Ding, C.-B. (2006). The genetic diversity among Leymus species based on random amplified microsatellite polymorphism (RAMP). Genet. Resour. Crop Evol. 53, 139–144. doi: 10.1007/s10722-004-1933-6
Yin, L., Tan, L., and Wang, B. (2006). Rare endangered endemic higger plants in xinjiang of China (Urumqi: Xinjiang Science and Technology Pres), 134–135.
Yuan, S., Ma, L., Guo, C., and Wang, R. (2016). What drives phenotypic divergence in Leymus chinensis (Poaceae) on large-scale gradient, climate or genetic differentiation? Sci. Rep. 6, 26288. doi: 10.1038/srep26288
Zhang, T.-C., Comes, H. P., and Sun, H. (2011). Chloroplast phylogeography of Terminalia franchetii (Combretaceae) from the eastern Sino-Himalayan region and its correlation with historical river capture events. Mol. Phylogenet Evol. 60, 1–12. doi: 10.1016/j.ympev.2011.04.009
Zhang, T. and Sun, H. (2011). Phylogeographic structure of Terminalia franchetii (Combretaceae) in southwest China and its implications for drainage geological history. J. Plant Res. 124, 63–73. doi: 10.1007/s10265-010-0360-3
Zhang, L., Zhang, Y., Ma, S., Zhang, D., and He, L. (2022). Distribution pattern and driving mechanisms of the sand plant Leymus racemosus in the Junggar Basin. Arid Zone Res. 39, 863–871. doi: 10.13866/j.azr.2022.03.19
Zhang, Z., Fu, B., and Yasuo, A. (2008). Late quaternary systematic stream offsets along the FuYun right-lateral strike-slip fault, Altay Mountains, China. Quat. Sci. 28, 273–279. doi: 10.3321/j.issn:1001-7410.2008.02.009
Zhao, J., Yin, X., Harbor, J. M., Lai, Z., Liu, S., and Li, Z. (2013). Quaternary glacial chronology of the Kanas River Valley, Altai Mountains, China. Quat. Int. 311, 44–53. doi: 10.1016/j.quaint.2013.07.047
Zhi, L. and Teng, Z. (2005). Classification and geographical distribution of Leymus in China. Bull. Botanical Res. 25, 22. doi: 10.3969/j.issn.1673-5102.2005.01.011
Keywords: Leymus racemosus, dd-RAD, genetic diversity, demographic history, environmental factors
Citation: Zhang Z, Shi X, Tian H and Tan D (2025) Drivers of intraspecific genetic differentiation of a wheat’s wild relative Leymus racemosus: roles of isolation by distance and environmental factors. Front. Plant Sci. 16:1675027. doi: 10.3389/fpls.2025.1675027
Received: 28 July 2025; Accepted: 23 October 2025;
Published: 18 November 2025.
Edited by:
Shuai Yuan, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Yiye Liang, Chinese Academy of Sciences (CAS), ChinaQiang Lai, Sichuan University, China
Copyright © 2025 Zhang, Shi, Tian and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaojun Shi, c3hqNjE1MDZAMTYzLmNvbQ==; Dunyan Tan, dGFuZHVueWFuQDE2My5jb20=
 Zhuo Zhang
Zhuo Zhang Xiaojun Shi
Xiaojun Shi Haowen Tian
Haowen Tian Dunyan Tan
Dunyan Tan