Abstract
Warming oceans are reshaping coastal ecosystems, yet the thermal sensitivity of many foundation macroalgae remains poorly constrained. Under SSP2-4.5, sea surface temperatures are expected to rise by ~2.7 °C by 2100, with unknown consequences for the canopy-forming kelp Lessonia spicata. We exposed thalli to control (15 °C) and warming (18 °C) conditions for 14 days to simulate near-future thermal scenarios, assessing photophysiology, oxidative stress and pigment composition. Warming caused marked declines in Fv/Fm and ETRmax and increased NPQmax, signaling reduced photochemical efficiency and enhanced thermal energy dissipation. Simultaneously, pigment concentrations decreased, ROS and MDA accumulated, and antioxidant capacity dropped, indicating rapid disruption of cellular redox homeostasis. Our results demonstrate that even modest warming consistent with mid-century projections triggers immediate photophysiological impairment and oxidative stress in L. spicata. These mechanistic insights highlight potential vulnerability of kelp forests in central Chile as ocean temperatures continue to rise.
Graphical Abstract

Introduction
Canopy-forming brown macroalgae (kelp), such as L. spicata (Suhr) (resilient Phaeophyceae, Ochrophyta), are foundational species and among the most ecologically important primary producers in temperate coastal habitats worldwide. They play a critical role in structuring intertidal and subtidal habitats, contributing substantially to nutrient cycling, primary production, and providing essential habitat complexity (Buschmann et al., 2014; Gómez et al., 2016; Rosenfeld et al., 2019). Climatic change (CC) is one of the most urgent environmental threats of the 21st century. Its impacts are particularly severe in marine ecosystems, where rising greenhouse gas emissions elevate sea surface temperatures (SST), leading to widespread ecological disruption (Dubash, 2020; IPCC, 2023). In temperate coastal regions, increasing SST imposes multiple stressors on kelp species, affecting key physiological processes such as photosynthesis, respiration, nutrient uptake, and life-cycle dynamics (Harley et al., 2006; Smale, 2020; Gundersen et al., 2021; Tregarot et al., 2024). These physiological disturbances can compromise not only individual performance but also the persistence of entire populations of primary producers over time. Moreover, warming is causing unprecedented shifts in species distributions, triggering biodiversity loss, habitat degradation, and reorganization of biological communities, and the emergence of novel species assemblages, which may alter ecosystem services in ways we are only beginning to understand (Wernberg et al., 2019; Duarte et al., 2022). Such distributional shifts have already been predicted for canopy-forming macroalgae under climate warming scenarios; similar patterns have been predicted for other canopy-forming species using ecological niche models under warming conditions (Carneiro et al., 2023).
Projections under SSP2–4.5 scenarios, estimate a rise of +2.7 °C in SST by 2100 (IPCC, 2023). While moderate, this warming still poses critical risks to macroalgae, particularly foundational species in temperate marine ecosystems, which may exceed their physiological thermal limits. This is consistent with findings in other cold-adapted macroalgae exhibiting reduced thermal tolerance when exposed to increased irradiance and temperature (Marambio and Bischof, 2021). When such thresholds are surpassed, abrupt changes in abundance and geographical range can occur, with cascading consequences for ecosystem structure and function (Smale, 2020). The loss of kelp forests, for example, has already been documented in several regions, including a 43% decline in Western Australia between 2011 and 2015 because of extreme marine heatwaves (Coleman and Wernberg, 2017).
Their productivity and resilience strongly depend on efficient photosynthetic and photoprotective mechanisms, which are sensitive to temperature and irradiance. Photochemical indicators such as the maximum quantum yield of Photosystem II (Fv/Fm), the maximum electron transport rate ETRmax and non-photochemical quenching NPQ are widely used to quantify photosynthetic performance and stress tolerance in kelps. Fv/Fm is the primary measure of photosynthetic efficiency and is highly sensitive to stress; its decline indicates photoinhibition and cellular damage to the photosystem II reaction centers. ETRmax represents the maximum rate of light-driven electron flow through the thylakoid membranes, serving as an indicator of maximum photosynthetic capacity. Finally, NPQ quantifies the dissipation of excess light energy as heat, acting as a crucial photoprotective mechanism under stress conditions (Figueroa et al., 2019). Integrating these metrics provides a robust understanding of the physiological status of macroalgae.
Multiple studies have documented strong physiological and biochemical impacts of elevated temperature on brown macroalgae. The decline in the maximum quantum yield of PSII (Fv/Fm) is a consistent indicator of thermal damage. For instance, Cladosiphon okamuranus, Nemacystus decipiens, and Turbinaria ornata exhibited significant drops in Fv/Fm when exposed to elevated temperatures (Fukumoto et al., 2019; Narrain et al., 2023; Bell et al., 2024). Observed seasonal variation in Fv/Fm values has been documented in L. spicata, highlighting its ecophysiological plasticity and capacity for photoacclimation to predictable environmental shifts (Zúñiga et al., 2021; Celis-Plá et al., 2022). However, this acclimation capacity is finite, and vulnerability increases when physiological thresholds are exceeded by acute events. Reductions in the maximum electron transport rate (ETRmax) have been observed in Fucus serratus under elevated temperature, indicating impaired capacity for electron flow a distribution ranges and energy production (Figueroa et al., 2019). Similarly, elevated temperature exposure in other brown algae led to lower ETR, signaling compromised photochemical efficiency (Fukumoto et al., 2019). Additional impacts on photosynthetic performance have been reported in Lessonia corrugata, which exhibited reduced net productivity and increased erosion near its upper thermal threshold (James et al., 2023). Meanwhile, non-photochemical quenching (NPQ) often increases as a compensatory response, as in Turbinaria ornata, which raised NPQ to dissipate excess excitation energy under stress (Narrain et al., 2023).
Thermal stress also alters key biochemical traits in brown algae. For instance, Sargassum horneri exhibited significant reductions in chlorophylls above 30°C, indicating pigment degradation (Yong et al., 2021), while Sargassum stenophyllum showed declines in chlorophyll a after 5°C increase in temperature (Urrea-Victoria et al., 2020; Schmid et al., 2021) reported carotenoid adjustments and reductions in total pigment content in temperate kelps Laminaria digitata under heat stress, and Macrocystis pyrifera experienced losses in chlorophyll c and fucoxanthin under elevated temperatures (Mabin et al., 2019). Furthermore, recent work demonstrated that L. spicata exposed to thermal and light stress accumulates hydrogen peroxide (H2O2) and malondialdehyde (MDA), indicating oxidative damage and membrane destabilization (Celis-Plá et al., 2025). L. spicata (Suhr) (Laminariales, Phaeophyceae, Ochrophyta) is distributed along the temperate coast of Chile (29° to 41°S) and is one of the most ecologically important brown macroalgae in the Pacific Ocean (Buschmann et al., 2014; Gómez et al., 2016). It plays a foundational role in structuring intertidal and subtidal habitats, contributing to nutrient cycling, primary production, and habitat complexity.
In central Chile, where L. spicata is ecologically dominant, water temperatures can vary throughout the year, peaking during the summer months (December, January, and February) when temperatures range between 15–22 °C. However, significant increases in maximum temperatures have been recorded in recent years, attributable to global warming (Pujol et al., 2022; IPCC, 2023; Rojo-Garibaldi et al., 2023). These thermal anomalies such as the negative impacts observed on kelp beds in northern Chile (Tarapacá) during the recent El Niño event (Avilés and Parker, 2024), are altering distribution patterns. Notably, while L. spicata has historically dominated central Chile, recent findings document its expansion poleward (southward), reporting the first record of the species in the Sub-Antarctic Magellan Channels (Rosenfeld et al., 2019). This raises concerns about its long-term ecological viability and the potential loss of ecosystem services it supports. In this context, in vivo chlorophyll a fluorescence remains a valuable tool for detecting physiological impairment, as reductions in Fv/Fm and ETRmax are widely used as indicators of photoinhibition and stress in kelps (Abdala-Díaz et al., 2006; Figueroa et al., 2016; Celis-Plá et al., 2016). These measures offer rapid, non-invasive insights into the photosynthetic status of macroalgae exposed to changing environments. Integrating these photophysiological metrics with biochemical traits such as pigment degradation, antioxidant capacity, and oxidative markers provides a comprehensive perspective on algal stress physiology.
Given regional warming trends and the increasing frequency of marine heat anomalies along central Chile, we tested a +3°C increment relative to 15°C to represent a realistic, short-term thermal stressor. This short-term stress is relevant because the degree of cellular damage and redox imbalance measured here is the mechanistic foundation that determines longer-term organismal fitness and demographic performance under chronic or repeated stress, as physiological responses underpin individual life-history traits and, consequently, population dynamics (Coleman, 2024). Our objective was strictly mechanistic: to quantify short-term photophysiological and biochemical responses of L. spicata to moderate warming under controlled conditions.
Materials and methods
Algae collection and experimental design
Thalli of L. spicata were collected in September 2023 from the rocky intertidal zone (upper intertidal) at Cochoa Beach, located north of Valparaíso Bay, Chile (32°57’19” S; 71°32’52” W). To avoid pseudoreplication, each replicate corresponded to a distinct thallus of Lessonia spicata collected from independent holdfasts at least 1 m within the sampling site. From each thallus, only one healthy blade segment was used per experimental unit, ensuring that all replicates represented independent biological individuals rather than subsamples from the same organism. A total of approximately 50 individuals were collected. The approximate sporophyte size ranged from 20 to 50 cm in length. During the sampling the sea surface temperature (SST) was 15 ± 1°C under low tide.
The samples were transported in plastic containers under cooled (13-15°C) conditions to the Laboratory of Environmental Research (LACER) at the HUB Ambiental UPLA research center of Universidad de Playa Ancha, Chile. Upon arrival, thalli were maintained with constant aeration and periodic seawater exchange to stabilize temperature to (15°C) until the experiment began. Fronds from 10 to 11 different individuals were placed in each experimental unit (tank). During the entire experimental period, algae were exposed to a natural daily light regime simulating local conditions (PAR: 953 kJ m-2, UVA: 76.3 kJ m-2, UVB: 4.7 kJ m-2), under a 12:12 h light: dark photoperiod (Figure 1).
Figure 1

Explanatory diagram of the steps to follow during the experiment, from the sampling site to the collection of material for analysis.
Two temperature treatments were applied: a control at (15°C) and a warming treatment of (18°C), as projected by the IPCC, 2023 scenario SSP2-4.5. The experimental unit for the temperature factor was the tank (n=3 per treatment), totaling 6 experimental units (n = 2 × 3). The temperature was regulated using a chiller system (Sunsun Chiller Mod. HYH 05D, Sensen, China). The tanks were assigned randomly to the two temperature treatments, and the temperature regulation was performed via an external water bath, ensuring no water circulation or exchange between the temperature treatments. Fronds were repositioned regularly to prevent self-shading, and fresh filtered seawater was changed every three days to avoid nutrient depletion and microbial overgrowth.
Sampling was performed at 10:00 h (local time) on day-1, day-7, and day-14. The selection of these time points was designed to capture both the immediate, acute physiological response (day-1) and the potential long-term response or compensatory acclimation (day-7; day-14). Photosynthetic parameters were measured in situ, on different adults’ fronds at each time point (days-1-7-14) to avoid time-series pseudo-replication and the influence of the measurement process. Algal tissue for biochemical and molecular analyses was collected from adult fronds using sterile scissors and immediately froze in liquid nitrogen to prevent degradation due to manipulation.
Photosynthetic performance
In vivo chlorophyll a fluorescence was measured using a pulse amplitude modulated fluorometer (Junior-PAM, Walz GmbH, Germany).
The effective quantum yield (ΔF/Fm’) Equation 1 was calculated from initial and maximum fluorescence under illuminated conditions, and maximum quantum yield (Fv/Fm) was determined after 15 minutes of dark adaptation (Figueroa et al., 2019; Celis-Plá et al., 2018, 2022, 2025).
The maximal electron transport rate (ETRmax) (Equation 1) was estimated by exposing algal tissue to twelve different intensities of actinic light for 20 seconds during the rapid light curve (Schreiber et al., 2995).
ETR parameters, including ETRmax and the initial slope of the ETR versus irradiance relationship (αETR), were determined using the tangential model (Eilers and Peeters, 1988).
Non-photochemical quenching was calculated as follows (Celis-Plá et al., 2016) according to Equation 2:
The maximum non-photochemical quenching (NPQmax) was obtained by the tangential model contained in (Eilers and Peeters, 1988).
Biochemical variables
All spectrophotometric measurements, including pigments, phenolics, antioxidant capacity, and malondialdehyde (MDA), were conducted using a microplate reader (SPECTROstar Nano, BMG Labtech, Offenburg, Germany), except for ROS fluorescence quantification, which was performed with a Cytation 5 instrument (Agilent BioTek, Santa Clara, USA).
Chlorophyll a and c were extracted from 20 mg of fresh algal tissue using 90% acetone, following (Ritchie, 2008). After 30 minutes of dark incubation at 4°C, samples were centrifuged at 16,200 g for 10 minutes. Absorbance was measured in microplates, and concentrations were calculated using the following Equations 3 and 4:
Fucoxanthin concentration was determined from 25 mg of algal tissue using an extract in dimethyl sulfoxide (DMSO), following the modified protocol of (Seely et al., 1972) according to (Celis-Plá et al., 2918) (Equation 5). Absorbance was read at 480 nm and fucoxanthin concentration calculated using:
Total phenolic compounds were extracted from 250 mg of fresh-frozen algal tissue using 80% methanol and quantified using the Folin–Ciocalteu method with phloroglucinol as a standard, following (Celis-Plá et al., 2022). The antioxidant capacity was assessed via the DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging assay using 150 µL aliquots of the extract prepared from 250 mg of biomass, and results were expressed as μmol TEAC g-1 DW using Trolox as a standard (Álvarez-Gómez et al., 2017). Reactive oxygen species (ROS) production was measured from 30 mg of frozen algal tissue using a commercial detection kit (Sigma-Aldrich), following the manufacturer’s instructions. The tissue was extracted with HCL and reacted with the fluorophore probe. Fluorescence was read at 540/570 nm, and values were expressed as mmol g-1 DW. Lipid peroxidation was estimated from 50 mg of algal biomass via the quantification of malondialdehyde (MDA) using the thiobarbituric acid reactive substances (TBARS) method, following protocols described in Celis-Plá et al., 2025 and (Zúñiga et al., 2021), with absorbance measured at 532 nm. Results were expressed as ng MDA g-1 DW.
Statistical analysis
We analyzed temperature (2 levels) × time (3 levels) with two-way ANOVA. For each response variable we report F, p, and partial η² with 95% CIs. Where interactions were significant, we provide simple effects with Hedges’ g and 95% CIs. Previously, the data were assessed for homogeneity and homoscedasticity of variance using Bartlett’s test, and student-Newman-Keuls post hoc tests were applied. All diagnostics and code are in Supplementary Information. The experimental unit and replication (n = … per treatment × time) are stated in each figure caption. Where significant interactions were observed, the. All statistical analyses were performed using R version 3.3.0 (R Cote Team).
Principal Coordinates Ordination (PCO) was conducted to integrate all photophysiological and biochemical variables and to visualize multivariate similarities among treatments. This complementary analysis allowed the identification of coordinated response patterns of L. spicata under control (15°C) and warming (+3°C) conditions. All variables—maximum quantum yield (Fv/Fm), maximum electron transport rate (ETRmax), photosynthetic efficiency (αETR), photosynthetic saturation irradiance (EkETR), non-photochemical quenching (NPQmax), chlorophyll a (Chl a), chlorophyll c (Chl c), fucoxanthin (FX), total phenolic compounds, antioxidant capacity (DPPH), reactive oxygen species (ROS), and malondialdehyde (MDA), Insoluble Phenolics compounds (PCI) and Soluble Phenolics Compounds (PCS) —were standardized by their maximum values to ensure comparability. The analysis was executed using the PRIMER v7 software package, following standard procedures for ecological datasets.
Results
Multivariate analysis (PCO)
The multivariate Principal Coordinates Ordination (PCO) analysis revealed a clear separation between treatments along the primary axis (PCO1), which explained 25.9% of the total variance (Figure 2). Samples exposed to 18 °C clustered towards the negative side of PCO1, associated with higher levels of non-photochemical quenching (NPQ), reactive oxygen species (ROS), and malondialdehyde (MDA). In contrast, control samples at 15 °C grouped on the positive side, characterized by higher values of maximum quantum yield (Fv/Fm), maximum electron transport rate (ETRmax), photosynthetic efficiency (αETR), and pigment contents (Chl a, Chl c, fucoxanthin), as well as greater antioxidant capacity (DPPH). The second axis (PCO2; 17.9% of total variance) captured minor within-treatment variability. Overall, this ordination underscores the consistent multivariate distinction between control and warming conditions, indicating that short-term exposure to +3 °C induces a coordinated decline in photochemical performance and redox homeostasis in L. spicata.
Figure 2

Principal Coordinates Ordination (PCO) of all photophysiological and biochemical variables. The ordination shows the separation between the 15°C (green triangles) and 18 °C (blue inverted triangles) treatments. Vectors indicate the contribution and direction of each variable. PCO1 and PCO2 explain 25.9% and 17.9% of the total variance, respectively.
Physiological responses
Thermal stress produced consistent and statistically significant changes in the photophysiological performance of L. spicata (Supplementary Table S1). Fv/Fm decreased progressively under 18°C treatment, with significant differences at days-7 and -14 relative to the control (F (2, 30) = 18.9, p < 0.05, η² = 0.46). (Figure 3A). The corresponding Hedges’ g effect size for day-14 (–1.27, 95% CI = –1.98 to –0.56) confirms a strong impairment of PSII efficiency.
Figure 3

Photophysiological responses: (A) Maximal quantum yield (Fv/Fm), (B) maximal electron transport rate (ETRmax). (C) Photosynthetic efficiency (αETR) and (D) Maximal non-photochemical quenching (NPQmax). Lowercase letters denote significance after the SNK test.
Similarly, ETRmax was markedly lower at 18°C by day-14 (F (2, 30) = 0.07, p < 0.05, η² = 0.39), whereas control individuals exhibited a recovery trend (Figure 3B). The photosynthetic efficiency (αETR) also declined at 18 °C, indicating reduced light-use capacity (F (2, 30) = 5.66, p < 0.05) (Figure 3C). Although the photosynthetic saturation irradiance (EkETR) has no significant differences (data not shown). In contrast, maximum non-photochemical quenching (NPQmax) increased significantly at 18 °C from day-14 onward (F (2, 30) = 25.8, p < 0.05, η² = 0.51) (Figure 3D), consistent with enhanced energy dissipation as a protective but transient mechanism. Correlation analysis revealed strong negative relationships between Fv/Fm and NPQmax (r = –0.78, p < 0.01) and between ETRmax and ROS (r = –0.71, p < 0.05), highlighting the functional coupling between photochemical inhibition and oxidative stress (Figure 2).
Biochemical responses
Pigment content declined under elevated temperature (Supplementary Table S2). Chlorophyll a decreased by 32% and chlorophyll c by 27% after 14 days at 18 °C (p < 0.05) (Figures 4A, B), whereas fucoxanthin declined by 23%, confirming pigment degradation (Figure 4C). In the same context, photoprotection as phenolic compounds exhibited a biphasic response: a transient rise at 24 h under 18 °C (early stress activation; F (2, 30) = 13.7, p < 0.05) (Figures 5A, B), followed by a pronounced decrease on day-14. Antioxidant capacity (DPPH assay) dropped by 40% relative to the control (p < 0.05) (Figure 5C), while reactive oxygen species (ROS) (Figure 6A) and malondialdehyde (MDA) (Figure 6B) increased ~60% and 45%, respectively (p < 0.05) (Figure 6).
Figure 4

Photosynthetic pigments: (A) Chlorophyll a and (B) Chlorophyll c (c1+c2) and (C) Fucoxanthin pigment content. Lowercase letters denote significance after the SNK test.
Figure 5
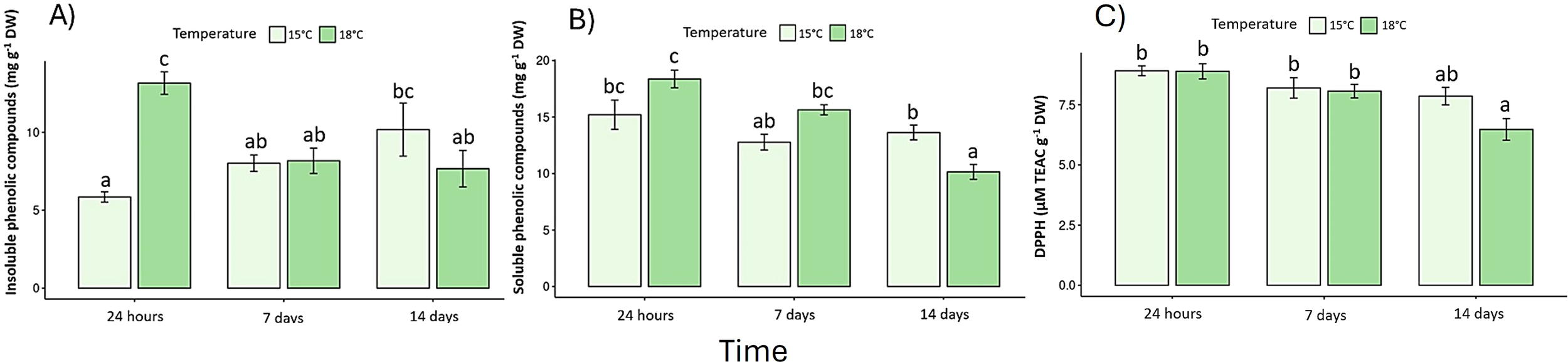
Phenolic compounds: (A) Insoluble phenolic content, (B) Soluble phenolic content, and (C) Total antioxidant capacity, measured by the DPPH assay and expressed as Trolox equivalents. Lowercase letters denote significance after the SNK test.
Figure 6

Oxidative stress markers: (A) Total Reactive Oxygen Species (ROS) and (B) lipid peroxidation (TBARS). Lowercase letters denote significance after the SNK test.
Discussion
This study provides new experimental evidence of how L. spicata responds physiologically and biochemically to short-term thermal stress. Although integrative analyses of photophysiological and biochemical responses remain scarce for kelps from the southeastern Pacific, we simulated a realistic +3 °C increase relative to 15 °C – the mean summer temperature in central Chile – to quantify the immediate physiological and biochemical consequences of moderate warming under controlled laboratory conditions. The objective was explicitly mechanistic and time-bound, providing baseline data to interpret the cellular vulnerability of this species to near-future ocean warming. The multivariate analysis highlights the coordinated impairment of photosynthetic efficiency and redox balance in response to moderate warming. Here, the PCO analysis provides an integrated view of the multivariate stress response in L. spicata under moderate thermal stress. The clear separation of treatments along the first ordination axis demonstrates that even a +3 °C increment triggers a coherent shift in the species’ physiological state. The association of Fv/Fm, ETRmax, αETR, and pigment variables with the control treatment reflects efficient photochemical functioning and stable redox balance under ambient temperature. Conversely, the clustering of NPQ, ROS, and MDA vectors with the 18 °C samples highlight the convergence of photochemical down-regulation and oxidative stress as dominant processes under warming. This multivariate pattern supports the interpretation that short-term exposure to elevated temperature elicits a systemic response, coupling photophysiological impairment with biochemical redox disruption. Such integrative ordination evidence reinforces the notion that L. spicata exhibits limited short-term resilience to moderate thermal anomalies, aligning with experimental findings in other kelps subjected to comparable stress conditions (Li et al., 2020; Graiff and Karsten, 2021; Narrain et al., 2023).
Our results show a significant impairment of photosynthetic performance in L. spicata under moderate thermal stress. The reduction of maximum quantum yield (Fv/Fm) and maximal electron transport rate (ETRmax) at 18°C on days-7 and -14, confirm sustained photoinhibition and damage to photosystem II. Such declines in photochemical efficiency have been widely observed in other brown macroalgae exposed to warming, including Fucus serratus (Figueroa et al., 2019), Saccharina spp. during summer anomalies (Li et al., 2020), and Turbinaria ornata under combined light-temperature stress (Narrain et al., 2023). The parallel reduction in αETR further indicates a decline in light-use efficiency, while the downward trend in EkETR suggests constrained capacity to utilize high irradiance under heat exposure (Zúñiga et al., 2021). The marked increase in non-photochemical quenching (NPQmax) after 14 days reflects an enhanced dissipation of excess excitation energy, which acts as a short-term photoprotective mechanism. Nevertheless, sustained NPQ upregulation typically signals stress compensation rather than successful acclimation (Fukumoto et al., 2019), a pattern consistent with the seasonal NPQ behavior previously described for L. spicata across natural gradients (Zúñiga et al., 2021). Altogether, these physiological responses indicate that L. spicata operates near its upper thermal safety margin under moderate warm conditions. In its natural habitat, recurrent exposure to elevated temperatures and marine heatwaves could therefore push this species beyond its photophysiological compensation range, leading to declines in photosynthetic efficiency, carbon assimilation, and canopy recovery during summer peaks.
At the biochemical level, this study revealed consistent pigment degradation under warming. Chlorophyll a and c concentrations decreased significantly in 18°C treatment, particularly by day-14. These reductions indicate compromised photosynthetic structures, consistent with those observed in S. horneri at >30°C (Yong et al., 2021) and S. stenophyllum following a 5°C increase (Urrea-Victoria et al., 2020). The reduction of fucoxanthin - key for light harvesting and photoprotection - may further weaken the algae’s capacity to manage high light under warming (Mabin et al., 2019; Schmid et al., 2021). In the same context, phenolic compounds, which act as non-enzymatic antioxidants, displayed a biphasic response, a transient early increase at 24 h, indicative of rapid activation of defense pathways, followed by a steep decline over time This depletion coupled with the 40% reduction in overall antioxidant capacity (DPPH), suggests exhaustion of redox buffering mechanisms and the onset of oxidative stress (Cruces et al., 2013; Graiff and Karsten, 2021).
Comparable field patterns have been observed in L. spicata, where phenolic and antioxidant levels rise under moderate stress but collapse under extreme irradiance or warming (Celis-Plá et al., 2022). The increase in oxidative stress was evidenced by significantly elevated ROS and MDA levels in 18 °C treatment after 14 days, indicating lipid peroxidation and progressive membrane destabilization. These biochemical markers are reliable indicators of thermal damage in macroalgae, reflecting the breakdown of antioxidant defenses when stress thresholds are exceeded (Narrain et al., 2023; Celis-Plá et al., 2025). Similar oxidative impairments have been documented in S. latissima under thermal and nutrient stress (Li et al., 2020), and comparable redox imbalances have been reported in S. horneri, S. stenophyllum, and L. digitata under elevated temperatures, where disruptions in redox balance led to protein degradation, metabolic reallocation and compromised cellular integrity (Urrea-Victoria et al., 2020; Yong et al., 2021). In S. mcclurei and F. vesiculosus, even moderate warming has been shown to reduce antioxidant capacity and exacerbate ROS accumulation, particularly during prolonged exposures (Mabin et al., 2019; Graiff and Karsten, 2021). Together, these patterns indicate that the antioxidant system of L. spicata can transiently buffer stress but becomes depleted under sustained warming, reducing its capacity to mitigate oxidative injury. The convergence of pigment loss, oxidative stress, and increased NPQ reveals a shift from transient photoprotection toward persistent oxidative strain, a process likely to define the upper thermal limits of L. spicata under projected warming scenarios (Teagle and Smale, 2018; Schmid et al., 2021; Ji and Gao, 2021). Overall, the convergent photophysiological (lower Fv/Fm, ETRmax, αETR; higher NPQmax) and biochemical signals (lower chlorophylls/fucoxanthin, reduced phenolics/antioxidant capacity, higher ROS and MDA) indicate that L. spicata experienced short-term redox and photochemical stress under a +3 °C increment in controlled treatment. In natural populations, similar physiological disruption could reduce productivity, tissue renewal, and overall canopy persistence during recurrent heat anomalies. These findings align with projections of increased frequency and duration of marine heatwaves in the southeastern Pacific, often exceeding +1 °C for > 40 days (Carrasco et al., 2023; Pujol et al., 2023), which may expose L. spicata to cumulative physiological damage.
The sustained decline in photochemical performance (Fv/Fm, ETRmax) and antioxidant capacity, alongside increased oxidative stress markers (ROS, MDA), suggest that the mechanisms normally used by this species to tolerate environmental variability are insufficient under prolonged moderate thermal stress. Future work should determine whether the short-term photophysiological and biochemical responses documented here persist over longer exposures and whether they co-vary with performance metrics measured independently (e.g., growth, survival, reproduction). Given that L. spicata spans ~29°S–48°S along the Chilean coast (González et al., 2016; Ramírez et al., 2023), future studies comparing northern and southern populations could reveal differential thermal tolerance and local adaptation. Such latitudinal contrasts may also help predict range retractions or shifts under progressive ocean warming. Considering ongoing reports of intensified marine heatwaves and regional warming trends (Carrasco et al., 2023; Pujol et al., 2023), both short- and long-term in situ experiments are warranted to evaluate acclimation and adaptive capacity under natural environmental fluctuations. Related work on seasonal variability in photosynthetic quotients under warming scenarios suggests useful methodological avenues for future assessments of primary production, bridging mechanistic physiology with ecosystem-scale consequences (Piticar, 2018; Demortier et al., 2022; Franke et al., 2024).
Overall, our endpoints reveal rapid and coordinated cellular responses to moderate warming, establishing a mechanistic foundation for future long-term studies that integrate physiological, demographic, and ecological dimensions of thermal tolerance in L. spicata, and by extension, the resilience of kelp forests along the Chilean coast.
Conclusions
This study provides the mechanistic and integrated evidence that a +3 °C thermal increment—consistent with the IPCC SSP2-4.5 scenario—induces rapid declines in photochemical efficiency, pigment content, and antioxidant capacity in L. spicata, leading to oxidative stress within two weeks. By combining effect-size metrics and multivariate analyses, the present revision enhances the mechanistic interpretation of these short-term responses. Although the experiment was limited to a controlled day-14 exposure, the results establish a quantitative physiological baseline from which future long-term and omics-level investigations can be designed. These findings reinforce the ecological concern that projected ocean warming may threaten the persistence and ecosystem functions of this foundational kelp species along the southeastern Pacific coast.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
CN: Data curation, Investigation, Methodology, Software, Supervision, Validation, Writing – original draft, Writing – review & editing, Formal analysis. MT: Formal analysis, Investigation, Methodology, Writing – review & editing. CL: Investigation, Writing – review & editing, Data curation, Supervision. VM: Investigation, Supervision, Writing – review & editing, Validation. PC-P: Investigation, Supervision, Validation, Writing – review & editing, Conceptualization, Data curation, Funding acquisition, Project administration, Resources, Software, Visualization, Writing – original draft.
Funding
The author(s) declared that financial support was received for this work and/or its publication. The financial and logistical support were support by the project FONDECYT 11180197, ANID, Chile.
Acknowledgments
The authors acknowledges to supported by the ANID InES GÉNERO [INGE 220004] – ANID and the “Programa de Apoyo para el pago de Procesamiento de Artículos InES Género 2025, Dirección General de Investigación, Universidad de Playa Ancha, Chile”. CNA acknowledges to national doctoral scholarship 21231539 by ANID, Chile. Also, C.L. was supported by the ANID InES I + D 2021 [INID210013], Marie Curie Postdoctoral Fellowship HORIZON-MSCA-2022-PF-01 project [101106387] and the Severo Ochoa Centre of Excellence accreditation [CEX2019-000928-S] funded by AEI 10.13039/501100011033.
Conflict of interest
The authors declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1727961/full#supplementary-material
References
1
Abdala-Díaz R. T. Cabello-Pasini A. Pérez-Rodríguez E. Conde Álvarez R. M. Figueroa F. (2006). Daily and seasonal variations of optimum quantum yield and phenolic compounds in Cystoseira tamariscifolia (Phaeophyta). Mar. Biol.148, 459–465. doi: 10.1007/s00227-005-0102-6
2
Álvarez-Gómez F. ,. F. Korbee N. Figueroa F. L. Abdala-Díaz R. T. (2017). Combined effects of UVR and nutrients on cell ultrastructure, photosynthesis and biochemistry in Gracilariopsis longissima (Gracilariales, Rhodophyta). Algal Res.26, 190–202. doi: 10.1016/j.algal.2017.07.022
3
Avilés L. Parker U. (2024). Monitoreo de praderas de algas pardas y efectos del evento “El Niño” en la región de Tarapacá (Informe Técnico 3). Centro Investigación Aplicada del Mar. S.A. Available online at: https://www.ciamchile.cl/wp-content/uploads/2024/03/Monitoreo-3-Praderas-Macroalgas_Febrero-2024_El-Nino-2023-24.CIAM_.pdf
4
Bell S. Y. Mabin C. J. T. Britton D. Johnson C. R. (2024). Season influences interspecific responses of canopy-forming kelps to warming and desiccation. Mar. Ecol. Prog. Ser.740, 39–54. doi: 10.3354/meps13524
5
Buschmann A. H. Pereda S. V. Varela D. Rodríguez M. López A. González-Carvajal L. et al . (2014). Ecophysiological plasticity of annual populations of giant kelp (Macrocystis pyrifera) in a seasonally variable coastal environment in the Northern Patagonian Inner Seas of Southern Chile. J. Appl. Phycology26, 837–847. doi: 10.1007/s10811-013-0070-z
6
Carneiro I. M. Paiva P. C. Bertocci I. Lorini M. L. de Széchy M. T. M. (2023). Distribution of a canopy-forming alga along the Western Atlantic Ocean under global warming: The importance of depth range. Mar. Environ. Res.188, 106013. doi: 10.1016/j.marenvres.2023.106013
7
Carrasco J. Montecinos A. Garreaud R. Ramos M. Pizarro C. Piticar A. et al . (2023). Marine heatwaves in the Southeastern Pacific: Intensity, duration, and future projections. Front. Mar. Sci.10. doi: 10.3389/fmars.2023.1129276
8
Celis-Plá P. S. M. Brown M. T. Santillán-Sarmiento A. Korbee N. Sáez C. A. Figueroa F. L. (2018). Eco-physiological and metabolic responses to the interactive exposure to nutrients and excess copper in the brown macroalga Cystoseira tamariscifolia. Mar. pollut. Bull.128, 214–222. doi: 10.1016/j.marpolbul.2018.01.005
9
Celis-Plá P. S. M. Martínez B. Seabra R. Souza-Egipsy V. Quintano E. Lima F. P. (2016). Seasonal biochemical and photophysiological responses in the intertidal macroalga Cystoseira tamariscifolia (Ochrophyta). Mar. Environ. Res.115, 89–97. doi: 10.1016/j.marenvres.2015.11.014
10
Celis-Plá P. S. M. Navarrete C. E. Trabal A. Castro-Varela P. A. Figueroa F. L. Troncoso M. et al . (2025). Ecophysiological and biochemical responses of L. spicata to solar eclipse-induced light deprivation. Plants14, 1810. doi: 10.3390/plants14121810
11
Celis-Plá P. S. M. Trabal A. Navarrete C. Troncoso M. Moenne F. Zúñiga A. et al . (2022). Daily changes on seasonal ecophysiological responses of the intertidal brown macroalga L. spicata: Implications of climate change. Front. Plant Sci.13. doi: 10.3389/fpls.2022.941061
12
Coleman M. A. (2024). Algae in the Anthropocene: Managing, conserving, and utilizing algae in an era of rapid environmental change. J. Phycology60, 1–3. doi: 10.1111/jpy.13409
13
Coleman M. A. Wernberg T. (2017). Forgotten underwater forests: The key role of fucoid on Australian temperate reefs. Ecol. Evol.7, 8406–8418. doi: 10.1002/ece3.3279r1
14
Cruces E. Huovinen P. Gómez I. (2013). Interactive effects of UV radiation and enhanced temperature on photosynthesis, phlorotannin induction and antioxidant activities of two sub-Antarctic brown algae. Mar. Biol.160, 1–13. doi: 10.1007/s00227-012-2049-8
15
Demortier L. Garreaud R. Montecinos A. Pizarro O. Ramos M. Piticar A. (2022). The coastal ocean response to climate variability and change in the Eastern South Pacific: A synthesis. Front. Mar. Sci.9. doi: 10.3389/fmars.2022.800325
16
Duarte C. M. Gattuso J. Hancke K. Gundersen H. Filbee-Dexter K. Pedersen M. et al . (2022). Global estimates of the extent and production of macroalgal forests’. Global Ecol. Biogeography31, 1422–1439. doi: 10.1111/geb.13515or
17
Dubash N. K. (2020). Climate laws help reduce emissions. Nat. Climate Change10, 709–710. doi: 10.1038/s41558-020-0853-6
18
Eilers P. H. C. Peeters J. C. H. (1988). A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecol. Model.42, 199–215. doi: 10.1016/0304-3800(88)90057-9
19
Figueroa F. L. Celis-Plá P. S. M. Martínez B. Korbee N. Trilla A. Arenas F. (2019). Yield losses and electron transport rate as indicators of thermal stress in Fucus serratus (Ochrophyta). Algal Res.41, 101560. doi: 10.1016/j.algal.2019.101560
20
Figueroa F. L. Hermoso-Bentrán M. Celis-Pl. P. S. M. Bonomi-Barufi J. Álvarez-Gómez F. Korbee N. et al . (2016). Photosynthetic activity estimated as in vivo chlorophyll a fluorescence in calcareous red macroalgae. Cienc. Marinas42, 139–155. doi: 10.7773/cm.v42i2.2587
21
Franke A. Bartsch I. Wiencke C. Graiff A. (2024). Varying photosynthetic quotients strongly influence net kelp primary production and seasonal differences increase under warming. Mar. Environ. Res.195, 106157. doi: 10.1016/j.marenvres.2023.106157
22
Fukumoto R. Yotsukura N. Endo H. Mikami K. (2019). Effect of photosynthetically active radiation and temperature on photosynthesis and growth in two brown algae, Cladosiphon okamuranus and Nemacystus decipiens. J. Appl. Phycology31, 3687–3698. doi: 10.1007/s10811-018-1675-z
23
Gómez I. Español S. Velis K. Houvien P. (2016). Spatial distribution of phlorotannins and its relationship with photosynthetic UV tolerance and allocation of storage carbohydrates in blades of the kelp Lessonia spicata. Mar. Biol.163, 110. doi: 10.1007/s00227-016-2891-1
24
González A. Rojas-Herrera R. Sáez C. A. Riosmena-Rodríguez R. Buschmann A. H. (2016). Frequency of chimerism in populations of the brown alga Lessonia spicata (Phaeophyceae, Laminariales) in Chile. J. Appl. Phycol.28, 103–113. doi: 10.1007/s10811-015-0554-x
25
Graiff A. Karsten U. (2021). Antioxidative Properties of Baltic Sea Keystone Macroalgae (Fucus vesiculosus, Phaeophyceae) under Ocean Warming and Acidification in a Seasonally Varying Environment. Biology10, 1330. doi: 10.3390/biology10121330
26
Gundersen H. Rinde E. Bekkby T. Hancke K. Gitmark JK and Christie H. (2021). Structure and standing stocks of kelp along multiple environmenta gradients and implications for ecosystem services. Front. Mar. Sci.8. doi: 10.3389/fmars.2021.578629
27
Harley C. Randall Hughes A. Hultgren K. Miner B. Sorte C. Thornber C. et al . (2006). The impacts of climate change in coastal marine systems. Ecol. Lett.9, 228–241. doi: 10.1111/j.1461-0248.2005.00871.x
28
IPCC (2023). Summary for Policymakers. In: Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate ChangeCore Writing TeamLeeH.RomeroJ. (eds.) (Geneva, Switzerland: IPCC), pp. 1–34. doi: 10.59327/IPCC/AR6-9789291691647.001
29
James D. A. Johnson C. R. Mabin C. J. T. (2023). The endemic kelp Lessonia corrugata is being pushed to its thermal limits in a warming ocean. Proc. R. Soc. B: Biol. Sci.290, 20232253. doi: 10.1098/rspb.2023.2253
30
Ji Y. Gao K. (2021). Effects of climate change factors on marine macroalgae: A reviewAdvances in Marine Biology88, 91–136. doi: 10.1016/bs.amb.2020.11.001
31
Li H. Monteiro C. Heinrich S. Bartsch I. Valentin K. Harms L. et al . (2020). Responses of the kelp Saccharina latissima (Phaeophyceae) to the warming Arctic: from physiology to transcriptomics. Physiol. Plant168, 5–26. doi: 10.1111/ppl.13009
32
Mabin C. Johnson C. Wright J. (2019). Physiological response to temperature, light, and nitrates in the giant kelp Macrocystis pyrifera, from Tasmania, Australia. Mar. Ecol. Prog. Ser.614, 1–19. doi: 10.3354/meps12900
33
Marambio J. Bischof K. (2021). Differential acclimation responses to irradiance and temperature in two co-occurring seaweed species in Arctic fjords. Polar Res.40, 5702. doi: 10.33265/polar.v40.5702
34
Narrain K. Vairappan C. S. Sumiati S. (2023). Photosynthetic, phytochemical and antioxidant responses of three tropical brown algae to thermal and irradiance stress. Front. Mar. Sci.10. doi: 10.3389/fmars.2021.578629
35
Piticar A. (2018). Changes in heatwaves in Chile. Glob. Planet. Change169, 234–246. doi: 10.1016/j.gloplacha.2018.08.007
36
Pujol C. Montecinos A. Piticar A. Ramos M. Garreaud R. Pizarro C. (2023). Main drivers of marine heatwaves in the eastern South Pacific: Insights from 35 years of satellite data. Front. Mar. Sci.10, 1129276. doi: 10.3389/fmars.2022.800325
37
Pujol C. Pérez-Santos I. Barth A. Alvera-Azcárate (2022). Marine heatwaves offshore central and South Chile: understanding forcing mechanisms during the years 2016-2017. Front. Mar. Sci.9, 2022. doi: 10.3389/fmars.2022.800325
38
Ramírez C. Ávila A. Zamorano J. Troncoso M. (2023). Identificando oportunidades en el manejo del huiro negro (Lessonia spicata) para la adaptación al cambio climático (Santiago, Chile: Fundación Terram). Available online at: https://terram.cl/wp-content/uploads/2023/11/Informe-Huiro-Negro-2023.pdf (Accessed September 4, 2023).
39
R Core Team (2023). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing). Available online at: https://www.R-project.org/ (Accessed November 28, 2023).
40
Ritchie R. J. (2008). Universal chlorophyll equations for estimating chlorophylls a, b, c1 and c2 and total chlorophylls in natural assemblages of photosynthetic organisms using acetone, methanol, or ethanol solvents. Photosynthetica46, 115–126. doi: 10.1007/s11099-008-0019-7
41
Rojo-Garibaldi B. Contreras-López M. Giannerini S. Salas-de-León D. A. Vázquez-Guerra V. H. E. Cartwright J. (2023). Nonlinear time series analysis of coastal temperatures and El Niño–Southern Oscillation events in the eastern South Pacific, Earth Syst. Dynam.14, 1125–1164. doi: 10.5194/esd-14-1125-2023
42
Rosenfeld S. Lagos N. A. Cerda M. (2019). Lessonia spicata as a foundation species: Implications for conservation and restoration. Rev. Biología Marina y Oceanografía.54, 241–254. doi: 10.22370/rbmo.2019.54.2.1601
43
Schmid M. Gonçalves A. G. Seabra R. Faria J. Lima F. P. Nunes C. (2021). Acclimation potential and biochemical responses of four temperate macroalgae to light and future seasonal temperature scenarios. Mar. Environ. Res.168, 105310. doi: 10.1016/j.marenvres.2021.105310
44
Schreiber U. Bilger W. Neubauer C. (1995). Chlorophyll Fluorescence as a Nonintrusive Indicator for Rapid Assessment of In Vivo Photosynthesis in Ecophysiology of Photosynthesis (Berlin, Heidelberg: Springer Berlin Heidelberg), 49–70. doi: 10.1007/978-3-642-79354-7_3
45
Seely G. R. Duccan M. J. Vidiver W. E. (1972). Preparative and analytical extraction of pigments from brown algae with dimethyl sulfoxide. Mar. Biol. doi: 10.1007/BF00350754
46
Smale D. A. (2020). Impacts of ocean warming on kelp forest ecosystems. New Phytol.225, 1447–1454. doi: 10.1111/nph.16107
47
Teagle H. Smale D. A. (2018). Climate-driven substitution of habitat-forming species leads to reduced biodiversity within a temperate marine community. Diversity Distributions24, 1367–1380. doi: 10.1111/ddi.12775
48
Trégarot E. D’Olivo J. Botelho A. Cabrito A. Cardoso G. Casal G. et al . (2024). Effects of climate change on marine coastal ecosystems -A review to guide research and management. Biol. Conserv.289, 110394. doi: 10.1016/j.biocon.2023.110394
49
Urrea-Victoria V. Nardelli A. Floh E. Chow F. (2020). Sargassum stenophyllum (Fucale, Ochrophyta) responses to temperature short-term exposure: photosynthesis and chemical composition. Braz. J. Bot.43, 733–745. doi: 10.1007/s40415-020-00639-y
50
Wernberg T. Krumhansl K. Filbee-Dexter K. Pedersen M. (2019). “ Status and trends for the world’s kelp forests’,” in World Seas: An Environmental Evaluation ( Elsevier), 57–78. doi: 10.1016/B978-0-12-805052-1.00003-6
51
Yong Y. S. Yusoff F. M. Shariff M. Fadhlullah Z. M. (2021). Effects of temperature and light on growth rate and photosynthetic characteristics of Sargassum horneri. J. Appl. Phycology33, 1817–1828. doi: 10.1007/s10811-020-02174-5
52
Zúñiga A. Sáez C. A. Trabal A. Figueroa F. L. Pardo D. Navarrete C. et al . (2021). Seasonal photoacclimation and vulnerability patterns in the brown macroalga Lessonia spicata (Ochrophyta). Water13, 6. doi: 10.3390/w13010006
Summary
Keywords
kelp physiology, photoprotection, reactive oxygen species, antioxidant capacity, phenolics compounds
Citation
Navarrete C, Troncoso M, Lavergne C, Molina V and Celis-Plá PSM (2025) Thermal stress impairs photophysiology and redox balance in the kelp Lessonia spicata. Front. Plant Sci. 16:1727961. doi: 10.3389/fpls.2025.1727961
Received
20 October 2025
Revised
08 November 2025
Accepted
28 November 2025
Published
15 December 2025
Volume
16 - 2025
Edited by
Mizanur Rahman, The University of Texas Rio Grande Valley, United States
Reviewed by
Aikaterini Koletti, Agricultural University of Athens, Greece
Rosalie Harris, University of California, San Diego, United States
Updates
Copyright
© 2025 Navarrete, Troncoso, Lavergne, Molina and Celis-Plá.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paula S. M. Celis-Plá, paulacelispla@upla.cl
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.