- 1Faculty of Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran
- 2Faculty of Medicine, Tehran Medical Sciences, Islamic Azad University, Tehran, Iran
- 3Faculty of Medicine, Tehran Medical Science Islamic Azad University, Tehran, Iran
- 4Faculty of Allied Medicine, Tehran University of Medical Sciences, Tehran, Iran
- 5Faculty of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 6Student Research Committee, Qazvin University of Medical Sciences, Qazvin, Iran
- 7Faculty of Medicine, Shahroud University of Medical Sciences, Semnan, Iran
- 8School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
- 9Faculty of Medicine, Guilan University of Medical Sciences, Rasht, Iran
- 10Faculty of Medicine, Ahvaz University of Medical Sciences, Ahvaz, Iran
Introduction: Malignancies of the GI tract account for one-third of cancer-related deaths globally and more than 25% of all cancer diagnoses. The rising prevalence of GI tract malignancies and the shortcomings of existing treatment approaches highlight the need for better predictive prediction models. RF’s machine-learning method can predict cancers by using numerous decision trees to locate, classify, and forecast data. This systematic study aims to assess how well RF models predict the prognosis of GI tract malignancies.
Methods: Following PRISMA criteria, we performed a systematic search in PubMed, Scopus, Google Scholar, and Web of Science until May 28, 2024. Studies used RF models to forecast the prognosis of GI tract malignancies, including esophageal, gastric, and colorectal cancers. The QUIPS approach was used to evaluate the quality of the included studies.
Results: Out of 1846 records, 86 studies met inclusion requirements; eight were disqualified. Numerous studies showed that when combining clinical, genetic, and pathological data, RF models were very accurate and dependable in predicting the prognosis of GI tract malignancies, responses, recurrence, survival rates, and metastatic risks, distinguishing between operable and inoperable tumors, and patient outcomes. RF models outperformed conventional prognostic techniques in terms of accuracy; several research studies reported prediction accuracies of over 80% in survival rate estimates.
Conclusion: RF models, in terms of accuracy, performed better than the conventional approaches and provided better capabilities for clinical decision-making. Such models can increase the life quality and survival of patients by personalizing their treatment regimens for cancers of the GI tract. These models can, in a significant manner, raise patients’ survival and quality of life through hastening clinical decision-making and providing personalized treatment options.
Introduction
Over 25% of all cancer cases worldwide and about one-third of cancer-related deaths are caused by gastrointestinal (GI) tract malignancies, which are a result of major lifestyle changes brought on by socioeconomic growth, increased consumption of processed foods, and higher rates of alcohol and tobacco use (Huang et al., 2023). By 2040, GI cancer mortality is expected to increase by 58 and 73%, to 7.5 million and 5.6 million cases, respectively, due to demographic shifts and global population expansion (Ferlay et al., 2021).
Despite the potential of novel therapeutic techniques like immunotherapy, their effectiveness varies according to the patient’s demographics (Wang et al., 2023). Patients with high PD-L1 expression or microsatellite instability-high (MSI-H) usually experience poor outcomes from immune checkpoint drugs targeting PD-1 and PD-L1 inhibitors (Shen and Zhao, 2018). Especially in the cases of patients receiving immunotherapy, conventional prognostic factors, including clinical staging and tumor markers, sometimes may not be able to precisely predict the treatment outcomes (Zhou et al., 2024). The clinical variability of GI malignancies, which arise from a range of genetic, pathological, and clinical factors, complicates the establishment of conventional therapeutic approaches (Chen et al., 2022).
Esophageal cancer (EC) is the eighth most common and sixth most lethal gastrointestinal disease worldwide, with a mere 20% five-year survival rate (Siegel et al., 2022; Uhlenhopp et al., 2020; Kasai et al., 2024). Despite improvements in early identification and treatment, gastric cancer (GC) continues to rank third in the world for cancer-related fatalities (Yu et al., 2024; Bray et al., 2018). Colorectal cancer (CRC) is the second most common cause of cancer-related death in the United States and one of the most common cancers in Western countries (Du et al., 2022; Van Cutsem et al., 2014).
Most of the models now used for the clinical assessment of GI malignancies are based on conventional statistical methods. However, these techniques frequently fail to analyze the high-dimensional and complicated data inherent in cancer prediction. Because machine learning (ML) techniques, in particular Random Forest (RF), can handle diverse datasets, manage missing values, and capture nonlinear relationships between variables, they provide a tempting alternative (Breiman, 2001; An et al., 2022).
RF is a powerful ensemble learning method that performs better than other machine learning methods such as support vector machines (SVM) and neural networks. It is resistant to missing data, scalable, and interpretable—all of which are typical issues in clinical datasets (Song et al., 2022). Despite their strength, neural networks may need extensive data preparation and are prone to overfitting. However, SVM is less interpretable in a therapeutic setting and has trouble with big datasets (Książek et al., 2019). RF is particularly well-suited for prognostic modeling in GI malignancies because of its ability to integrate several data sources, identify important components, and produce intelligible forecasts (Parmar et al., 2015; Tang and Ishwaran, 2017; Bennett and Campbell, 2000). The concepts of personalized medicine align with RF’s shown capacity to evaluate intricate clinical datasets and its resilience in handling missing data (Kaur et al., 2024).
Although the majority of recent research has focused on machine learning approaches in general, few studies have focused on RF’s unique advantages in addressing the unique challenges of GI malignancies, including clinical heterogeneity and missing data (Abbas et al., 2024; Christou and Tsoulfas, 2021). By filling up these gaps, the current study investigates how well RF models could predict clinical outcomes for patients with gastrointestinal cancer as compared to conventional statistical methods.
To improve the predicted accuracy of RF models, future research should evaluate them using bigger and more varied datasets. There should be efforts to incorporate these models into routine clinical decision-making due to their effectiveness in handling missing data and identifying key factors. RF models can also support personalized treatment by integrating various patient data (Rizinde et al., 2023). Addressing implementation challenges, like data preprocessing and clinician training, is essential for practical use. Bioinformaticians, data scientists, and oncologists must collaborate to standardize these methods (Asif et al., 2023). Finally, research must examine the broader use of RF models in other cancer types. The results of the review study demonstrate the propensity of RF algorithms to guide future machine learning research and enhance clinical decision-making in patient care and treatment planning by accurately predicting outcomes for various GI cancer types.
Methods
This investigation was conducted in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA2020) criteria to enhance the coordination and design of systematic reviews (Page et al., 2021). The registration DOI for our Open Science Framework (OSF) systematic review is DOI 10.17605/OSF. IO/X25ZN (OSF, 2025).
Information sources and search strategy
We developed a thorough search approach to find research using RF-based models for GI cancer prognostication. A comprehensive and detailed search of several databases, from each database’s inception to May 28, 2024, was conducted. The databases included PubMed/MEDLINE, Scopus, Google Scholar, and Web of Science. The results shown in Table 1 show that a controlled vocabulary along with keywords from each database was used to find the use of RF-based models for the prediction of GI tract cancers.
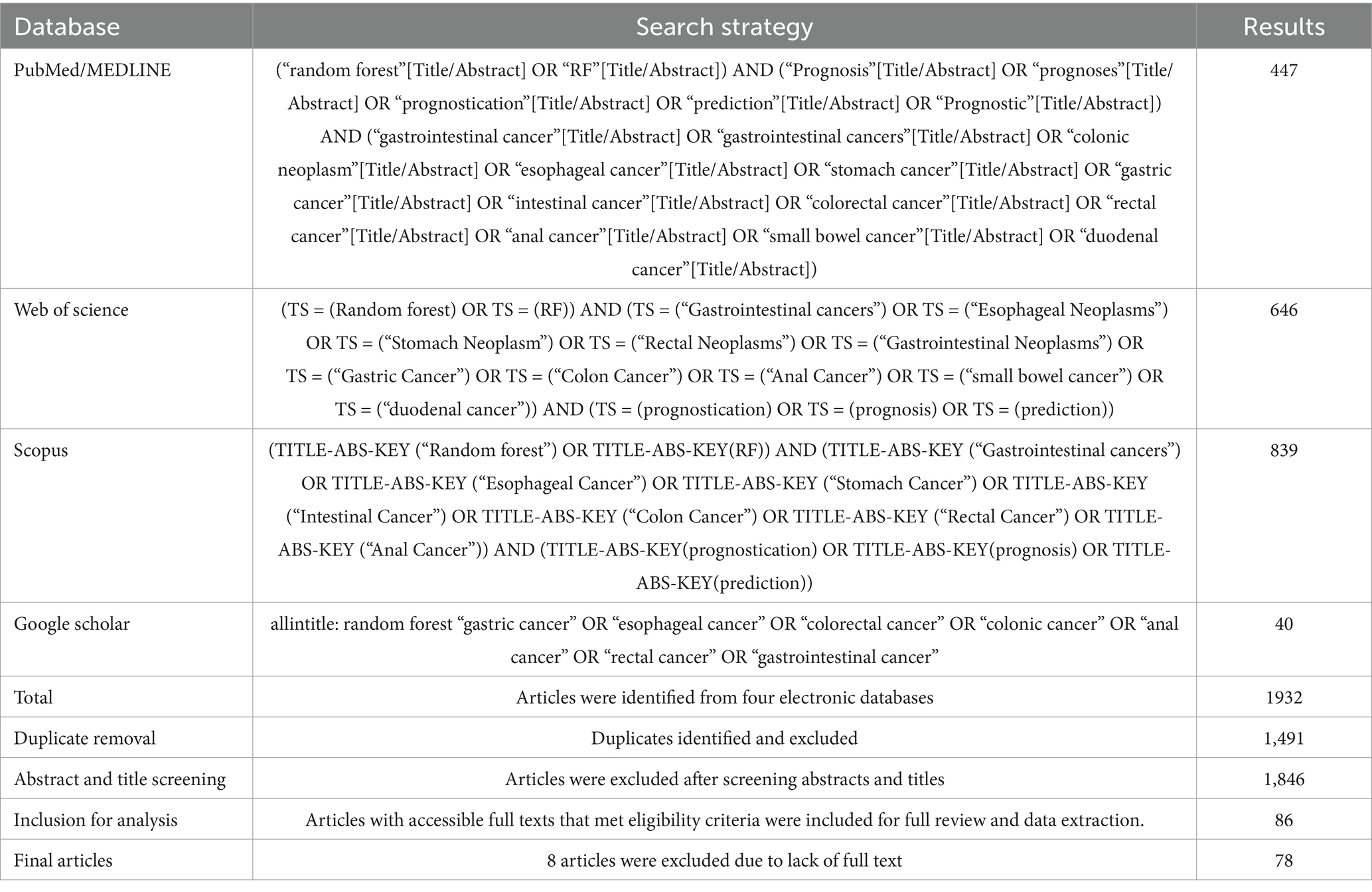
Table 1. Search strategy and article retrieval process for the application of random forest-based (RF) models in prognostication of gastrointestinal tract malignancies.
Data screening and eligibility criteria
We used RAYYAN.ai to assess the search results (Ouzzani et al., 2016). Using a variety of artificial intelligence tools, this platform facilitates the screening and decision-making procedures in systematic reviews. Five reviewers (Zh. M., Y. A., A. S., F. Sh., and M. A.) evaluated the abstracts and titles of the papers found using our search approach impartially and independently. After the two reviewers had resolved their disagreements through a consensus-building process, a third independent reviewer (A. ZKH) verified the final choices. The RAYYAN platform helped with the process by eliminating unnecessary information from the search results and resolving any overlaps or discrepancies in reviewer scores.
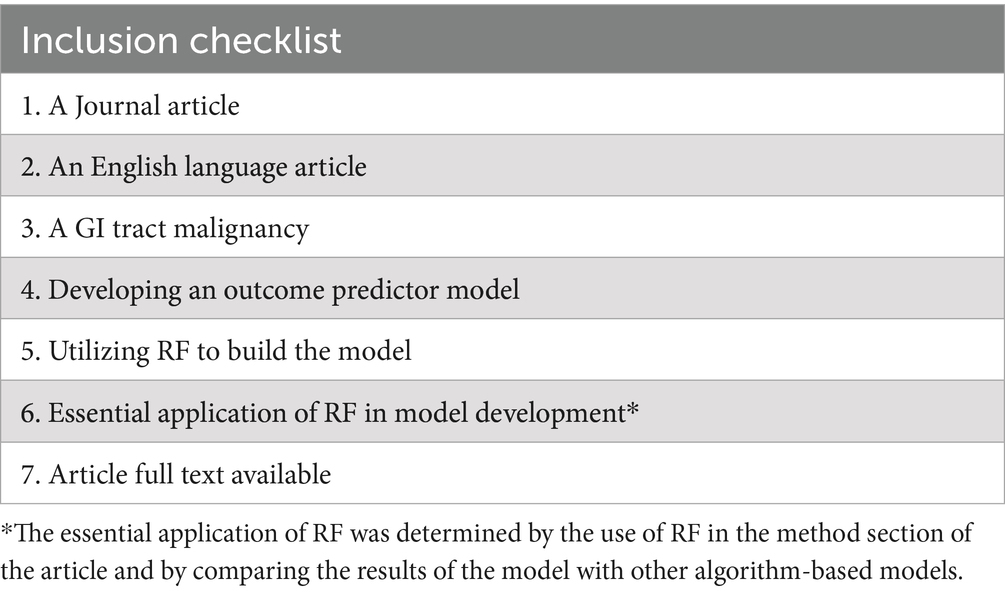
To find research that used RF-based models to create or validate prognostic models for GI malignancies, the inclusion criteria were specially created. This supports our goal of assessing RF’s performance and application in this situation. This investigation includes every original English-language study that used RF to develop a model for predicting the prognosis of a particular kind of GI cancer (Table 2). The inclusion criteria were only looked at the level of the article titles and abstracts during the first screening stage. However, we checked the contained articles’ entire texts in the next step. Articles where the whole text was unavailable or where the usage of RF was not required for model creation were not included in the review.
Data extraction and quality assessment
The retrieved variables were selected to guarantee a thorough assessment of RF’s usefulness in prognostic modeling for GI malignancies. Using the full texts of the included articles, we extracted the following information to align with our research objectives: Title, Author, Year, Nation, Study Objective, Population, pathology kind, RF’s use in the model, development or validation of a model, Validation type, prognostication factor or factors, method, result, conclusion, and evidence quality (Table 3).
The models that were previously used in studies on GI cancer were considered validation models. The process of verifying the applicability of a model using a population different from the sample population described in the data extraction phase was referred to as external validation in this context. While external validation findings are meant to be generalizable to the reference population, internal validation focuses mainly on the results’ robustness when applied to the training dataset (Altman and Royston, 2000). Using the QUIPS tool, four assessors (F. Sh, A. Kh, Y. A., P. G., and M. A.) independently assessed each article to determine the risk of bias and the quality of the included studies (Hayden et al., 2006). The instrument consists of six distinct domains: study participation, study confounding, study attrition, outcome measurement, prognostic factor measurement, statistical analysis, and reporting. For each domain, a set of three to seven prompting items is utilized. Studies evaluated as high quality in these domains were prioritized in the synthesis of data to guarantee strong conclusions.
Due to observation of considerable heterogeneity in RF model implementation across studies, instead of conducting a meta-analysis, a narrative and systematic approach, highlighting trends in predictive performance for each cancer type, was chosen.
Results
Study selection
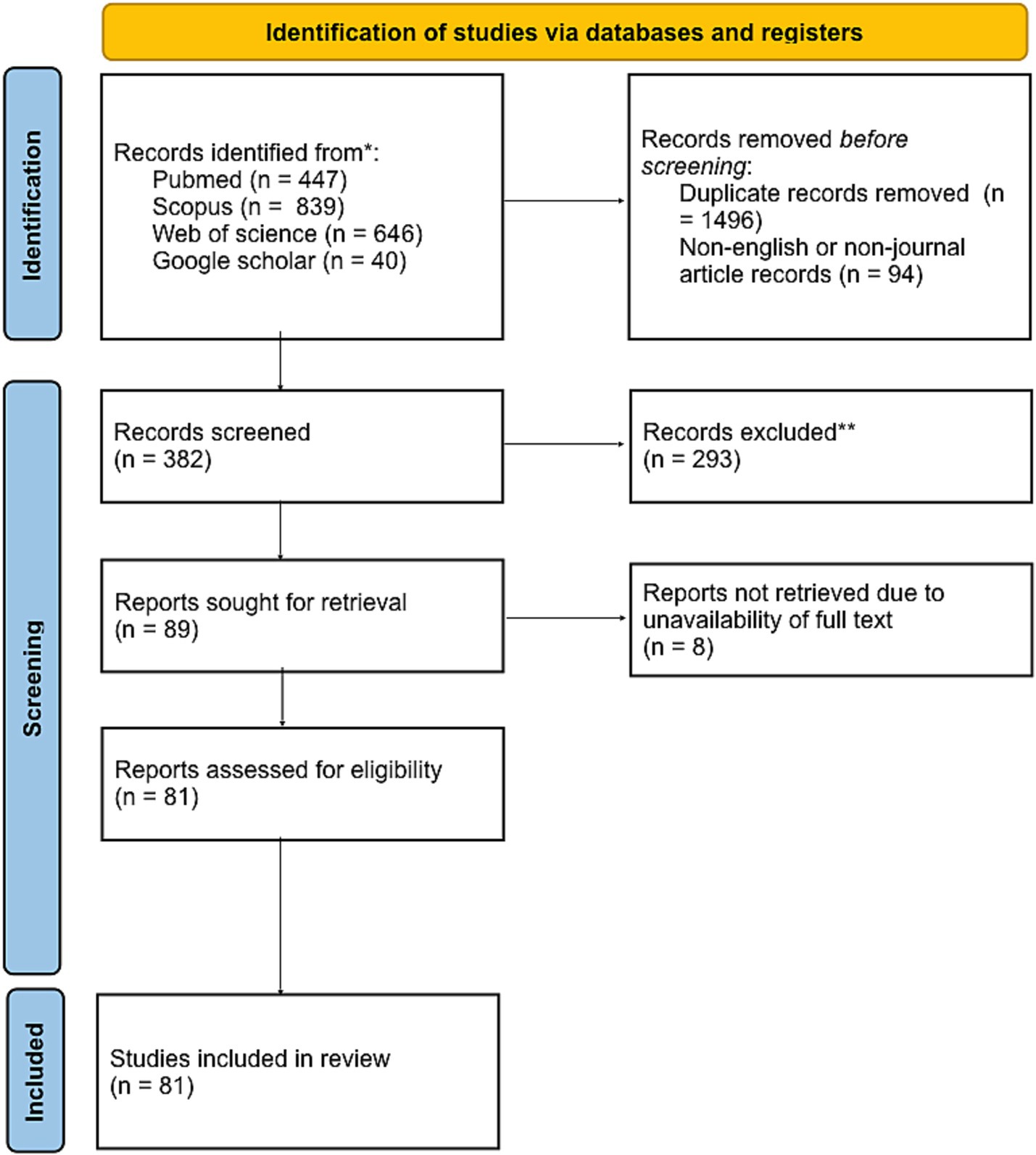
A systematic exploration of four electronic databases identified 1,932 articles. After removing 1,846 items, 1,491 were duplicates. The remaining articles were split into 86 accessible and 8 non-accessible (Figure 1). We extracted data from our 86 full-text articles using 16 factors (author’s name, title, year of publication, country, aim of a study, population studied, type of pathology, use of RF in model, model development or validation, type of validation, model used for validation, factors used for prognostication, method, outcome, conclusion, quality of the evidence).
Study characteristics
The reviewed papers span the years 2011 through 2024. Fewer studies came from North America, Europe, and the Middle East, with the majority of the research being done in Asia, particularly China. Studies that are cross-sectional and retrospective make up the majority of the literature. The majority of the papers (33 and 36, respectively) showed moderate and low risk of bias, according to the risk of bias evaluation, while only a small subgroup (Ferlay et al., 2021) was found to have a high risk of bias. 27 of the 86 included studies briefly noted their approach to handle missing data.
There were 5 different cancers mentioned in the 86 articles found in full text, which are categorized by the number of articles they are mentioned in and the type of validation used for them. An abstract of the methods used in the articles with the highest quality of evidence for each of the 5 cancers is gathered and tabled along with the other mentioned information (Table 4).
Findings
86 eligible articles were identified, of which 50 presented model development, 46 presented only internal validation, 27 presented only external validation, and 13 models had both internal and external validation. All of the articles were validated using an RF model, a tool mostly used for assessing the performance and robustness of the prognostic models, minimizing the effect of overfitting (a major cause for diminishing predictive ability for unknown data) or predicting the outcome (in 50% of the cases). The number and type of validation utilized for each cancer is visible in Table 4.
Esophageal cancer (EC)
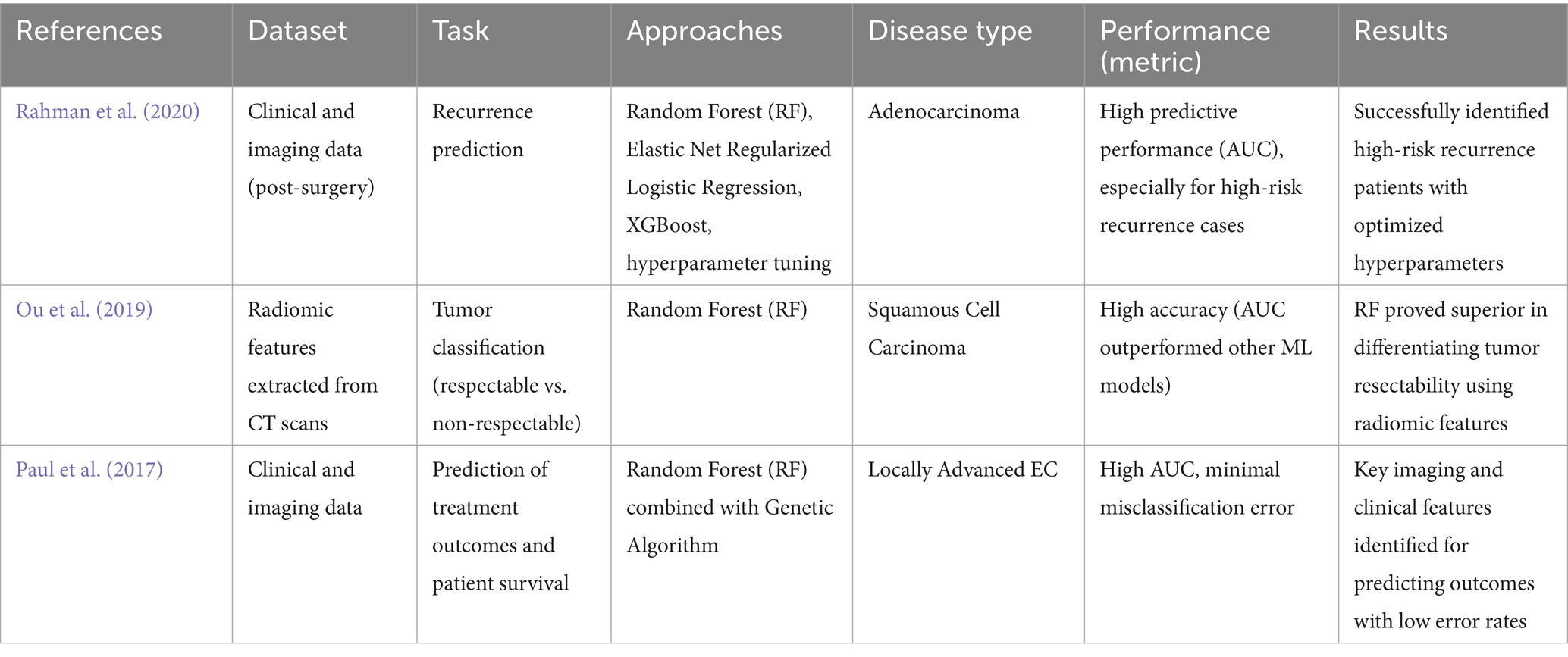
EC is generally divided into squamous cell carcinoma (SCC) and adenocarcinoma and typical treatments for EC are chemotherapy, radiation, and surgery (Acharya et al., 2023). Moreover, tumor stage and respectability typically determine the outcome of this type of cancer (Quail and Joyce, 2013). To conduct an improved clinical decision analysis, it is necessary to predict EC patients’ response to treatment, recurrence, and survival rates. Previous studies have applied machine learning models, especially RF, to predict these outcomes with remarkable success. RF is strong against high-dimensional data, where there are complex relationships among variables. The methodology employed by RF involves the creation of several uncorrelated decision trees, each derived from distinct random samples of the dataset. This will outperform classification and reduce overfitting in the models. In the OCCAMS Consortium study, RF was used in a group model with Elastic Net Regularized Logistic Regression and XGBoost to find out the recurrence rate of esophageal adenocarcinoma after surgery. An optimized version with hyperparameter tuning showed excellent predictive performance, especially in identifying patients at high risk of recurrence (Rahman et al., 2020). Another study on SCC looked at how RF could be used on patients to separate tumors that could be removed from their bodies from those that could not use radiomic features taken from CT scans. The model behaved with high accuracy AUCs and outperformed several other machine learning models (Ou et al., 2019). In a research initiative by GARF focusing on patients with locally advanced EC, RF was combined with a genetic algorithm to identify the most pertinent imaging and clinical characteristics that might predict treatment outcomes and patient lifespan (Paul et al., 2017). The RF classifier provided the minimum misclassification error and gave the maximum AUC score; thus, its strength in handling complex medical images and clinical data has been demonstrated (Chaddad et al., 2018). Generally, RF has constantly shown that it is one of the most powerful tools in providing predictions of the outcomes of EC, and hence, it has allowed relevant and important insight into patient management and treatment (Thavanesan et al., 2024).
RF has been successful in predicting the treatment responses, recurrence, and survival rates of EC patients. With RF, many decision trees are grown on random samples, which decrease the overfitting and increase the accuracy of prediction. Previous studies have shown the role of RF in regulating the outcome of ECs by demonstrating its ability to identify operable tumors and predict cancer recurrence accurately.
Gastric cancer (GC)
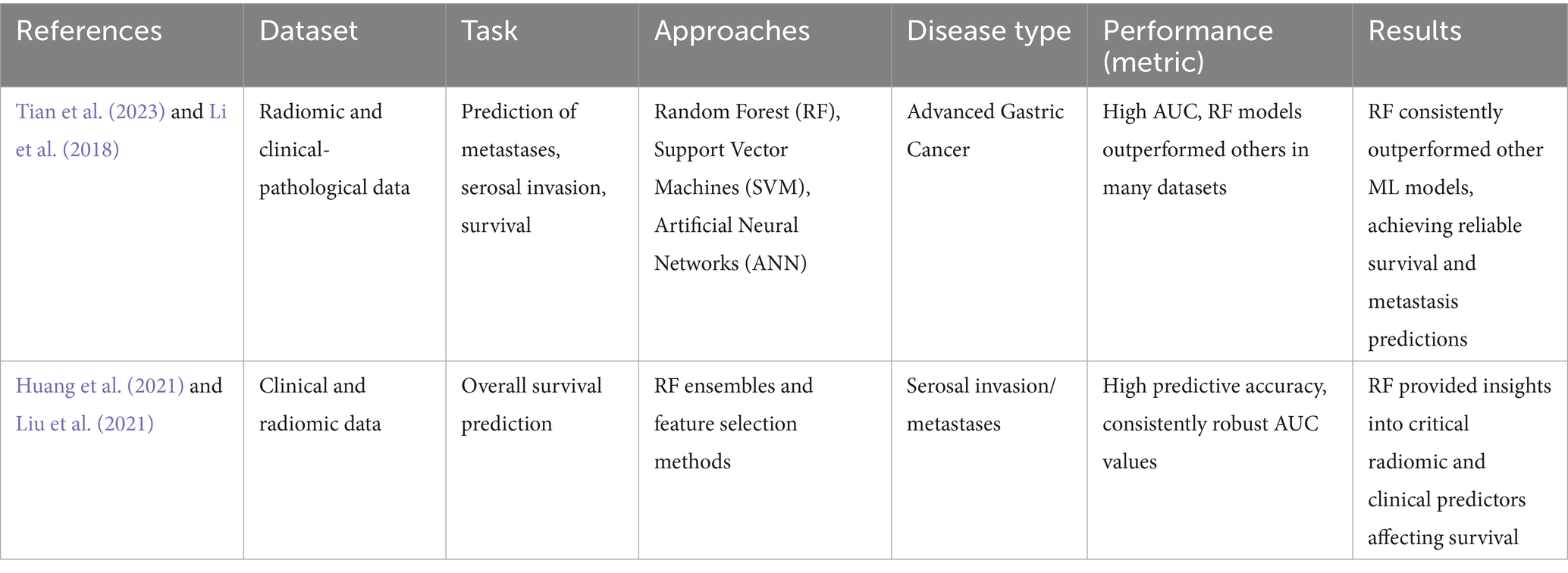
Starting in the gastric mucosa, GC is a cancer that can progress from early-stage T1 GC to advanced metastatic illness (Rawla and Barsouk, 2019). The size and location of the tumor, the level of histological differentiation, and the existence of certain indicators like Helicobacter pylori infection are all factors that increase the chance of having this illness (Hallinan and Venkatesh, 2013). For the illness to be effectively managed, a precise evaluation of the patient’s prognosis—including survival rates, the possibility of distant metastases, and the reaction to chemotherapy—is essential (Stone et al., 2023). To forecast the clinical outcomes of GC patients, including distant metastases, serosal invasion, or survival, this research created machine learning models. These models have been created using tumor markers, radiomic characteristics taken from CT images, and clinical-pathological data. RF, SVM, and ANN are the main machine-learning algorithms examined in this regard. The AUC, sensitivity, and accuracy of these models were compared against one another. Notably, in the majority of the examined datasets, some RF models had competitive or better prediction accuracies, making them extremely predictive of patient outcomes (Tian et al., 2023; Li et al., 2018). To ensure that these models are more resilient across various datasets, external validation is helpful. In general, the ease of handling and processing a dataset with numerous variables has led to the widespread use of RF. The RF models were trained using clinical and radiomic data to predict the overall survival time of patients with GC who had serosal invasion or close metastases (Huang et al., 2021; Liu et al., 2021). RF ensembles various tree-only models, which reduces the risk of single-model overfitting and improves substantial prediction quality (Tian et al., 2023; Liu et al., 2023). RF demonstrated strong predictive performance by consistently achieving high AUC scores in each of these. For instance, in many situations, RF could accurately predict survival and metastasis; in some cases, it could even outperform other models like SVM or neural networks (Li et al., 2018; Liu et al., 2023). Moreover, the feature importance measures calculated by the RF models have pointed out the most critical factors affecting patients’ outcomes and helped clinical decision-making in the treatment of GC (Tian et al., 2023; Liu et al., 2021).
This research developed machine-learning models using tumor markers, CT image features, and clinical data to predict clinical outcomes in GC patients. The main algorithms studied were RF, SVM, and ANN, with RF often showing competitive prediction accuracy. RF models were trained to predict survival in patients with serosal invasion or metastases and identified key factors that influence outcomes, aiding treatment decisions.
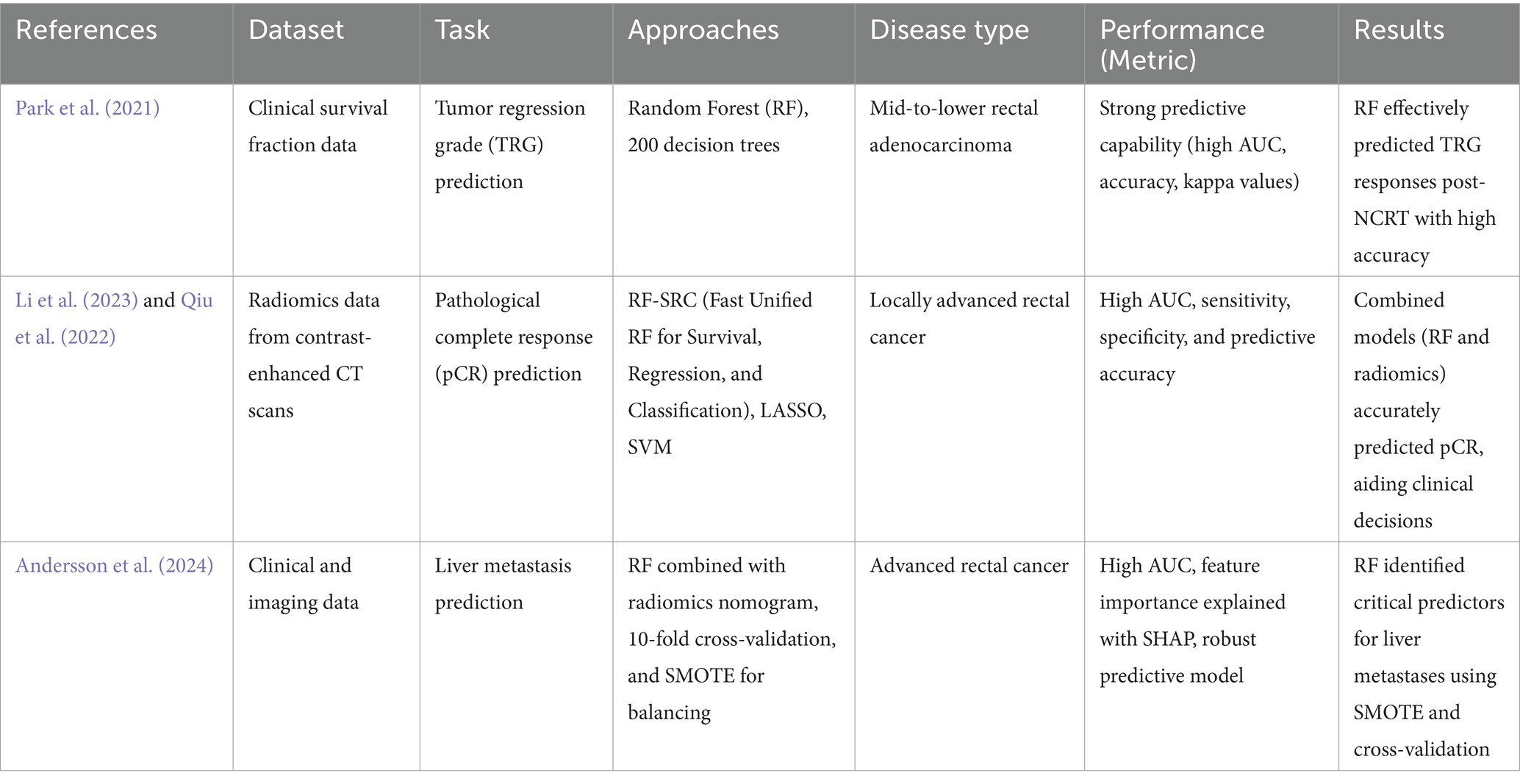
Colon cancer (CC)
CC originates in the colon or rectum and often presents with unnatural growths that can spread and invade adjacent tissues is known as CC (Alzahrani et al., 2021). This presented study aims to enhance the classification of CC by developing predictive models using the RF algorithm together with feature selection techniques. Methodological steps include the four major phases: acquiring gene expression data, model construction without and with feature selection, and comparative analysis. Data consisted of 62 CC samples, where 40 were tumor biopsies and 22 were normal controls. Before using feature selection approaches such as MDA and MDG to modify model performance, RF-based models were developed. The RF classifier is used here because of its excellent performance in managing complex, noisy, and high-dimensional data. RF works particularly effectively in datasets with large feature dimensions because it builds several trees from random picks and utilizes the outputs of those trees to generate predictions. In the present investigation, RF showed excellent prediction ability in distinguishing between normal tissues and CC (Yao et al., 2022). In turn, feature selection with the MDA and MDG methods improved the general performance of the model in terms of much higher accuracy, precision, recall, and F1 score because only the most relevant features were used for classification. The comparative analysis showed that the RF model’s predictive performance was improved by the feature selection procedure. Additionally, the use of RF in several settings in CC research points to its possible value for both predictive and diagnostic evaluations (Li et al., 2021). By adding more information like immune cell fractions, histopathological images, and genomic profiles (Zhou et al., 2019), RF was able to find key markers that set tumor tissue apart from normal samples. When RF was combined with LASSO, it became even easier to find the immune cells and genetic markers that were involved. This improved the accuracy of the prognostic and diagnostic models. This model’s strong performance was validated by tests that used AUC and Harrell’s concordance index (c-index), which are essential for forecasting a patient’s outcome and creating more accurate forecasts (Shafi et al., 2020). The RF consistently outperformed the other approaches in terms of classifying, predicting, and offering useful information about many aspects of CC outcomes.
Shortly, RF showed strong performance in distinguishing between normal and cancerous tissues. Using feature selection methods like MDA and MDG enhanced model accuracy and other performance metrics. RF also provided valuable insights for diagnostic and prognostic evaluations of CC outcomes (Table 5).
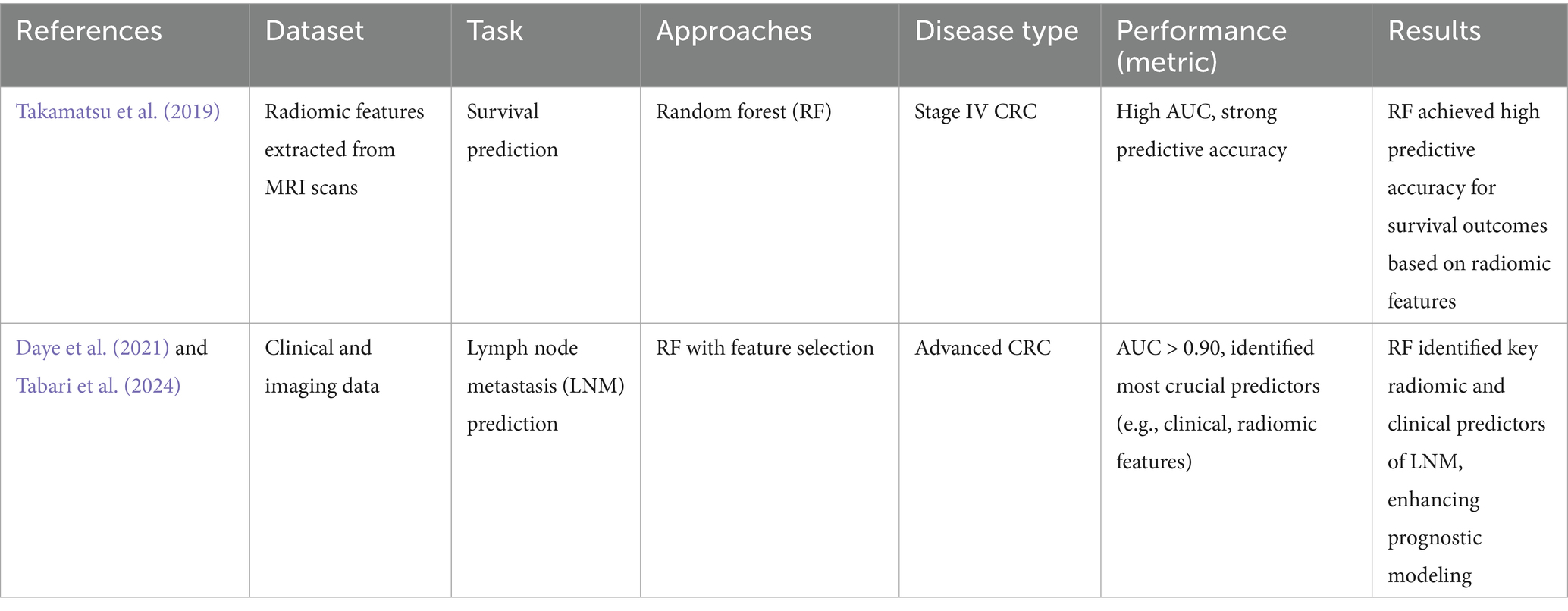
Rectal cancer (RC)
RC remains an important focus for predictive modeling across several studies, predicting improved outcomes and prognosis (Peng et al., 2007). Therefore, in one study, a total of 33 patients diagnosed with mid-to-lower rectal adenocarcinoma underwent NCRT, following which they received radical surgery. RF was applied to develop a predictive model that could provide a prediction about the tumor regression grade, which is a measure of response related to NCRT. The RF model was trained on the survival fraction data with 200 trees and tested by AUC, accuracy, and kappa values. Strong predictive capability and highly effective classification for treatment responses were shown for this RF model (Park et al., 2021). There was another study that involved 211 patients with locally advanced RC, which involved chemoradiotherapy and surgery. Radiomics features were taken from contrast-enhanced CT images taken before treatment, and RF was used to choose the most relevant features that could predict a pathological complete response. Feature ranking regarding its importance and minimizing errors was done by the approach known as the Fast Unified RF for Survival, Regression, and Classification, referred to as RF-SRC (Li et al., 2023). RF was combined with other machine learning methods, namely LASSO and SVM, to develop a radiomics score that predicted response to treatment, which is named Radscore. Based on several performance criteria, including improved AUCs, accuracy, and sensitivity, the suggested model appeared to perform well (Qiu et al., 2022). A radiomics nomogram was developed for clinical decision-making based on predictions by RF and clinical indicators in which, data from the Surveillance, Epidemiology, and End Results database and two hospitals in China supported a different study that developed a predictive model for liver metastasis in RC patients. RF was core in this process among the other machine learning algorithms. The model was trained with a well-established technique called 10-fold cross-validation, whereas SMOTE was used to handle class balancing (Li et al., 2023). Performance was quantified using AUC, accuracy, sensitivity, specificity, and F1-score, and in this regard, it was robust for the RF model. Feature importance was evaluated by permutation importance, while the SHAP method gave further insight into the contribution of each variable. RF has always been good at working with big datasets, picking out the most important features, and then building a good model for predicting what will happen with RC, like TRG response, and likelihood of pCR. In these models, its application underlines effectiveness in improving decision-making and planning treatments for RC patients (Andersson et al., 2024).
Here, RF selected relevant features and combined them with other machine learning methods to create a radiomics score, named Radscore, which performed well on various metrics. Additionally, RF was used in creating a predictive model for liver metastasis, demonstrating effectiveness in handling large datasets and improving treatment decisions for RC patients (Table 6).
Colorectal cancer (CRC)
CRC is a malignancy that can develop in the colon or rectum. When it reaches an advanced level, it might cause issues like stage IV liver metastases, which calls for intricate therapeutic measures (Kow, 2019). Chemotherapy, radiation therapy, and surgery are the main forms of treatment. The prognosis of patients is significantly impacted by many variables, such as the kind of cancer, histological aspects, and genetic features, including KRAS mutations (Mishra et al., 2013). Particularly for lymph node metastases, exact survival and metastatic risk prediction are crucial for good treatment planning (Kim and Choi, 2019). To predict patient outcomes, including survival and the chance of metastasis, this research was designed to take advantage of machine learning techniques, namely the RF algorithm. In one study, we used receiver operating characteristic analysis to assess the performance of an RF model we constructed based on radiomic characteristics taken from MRI images, which showed a very high predicted accuracy of patient survival (Takamatsu et al., 2019). Another study of stage IV CRC patients developed an RF model to predict the risk of lymph node metastasis (LNM) based on a wide range of clinical and imaging features (Daye et al., 2021). The AUC values >0.90 (Tabari et al., 2024), reflect these models’ extremely high predictive power. In each of the reviewed investigations, the most commonly used was the RF algorithm. Researchers accepted the use of RF in analyzing variant clinical, radiomic, and imaging data to predict both survival outcomes and LNM risk. As illustrated in Figure 2, RF constructs multiple decision trees based on randomly selected features from the dataset to reduce overfitting and optimize accuracy. The capacity to categorize patient outcomes based on survival or risk of LNM is demonstrated by the RF models’ AUC values. Since they show which elements—such as radiomic or clinical features—are more pertinent to the model’s predictions, these feature importance measurements were quite helpful. Because of this, the outcomes were simpler to comprehend (Pourhoseingholi et al., 2017).
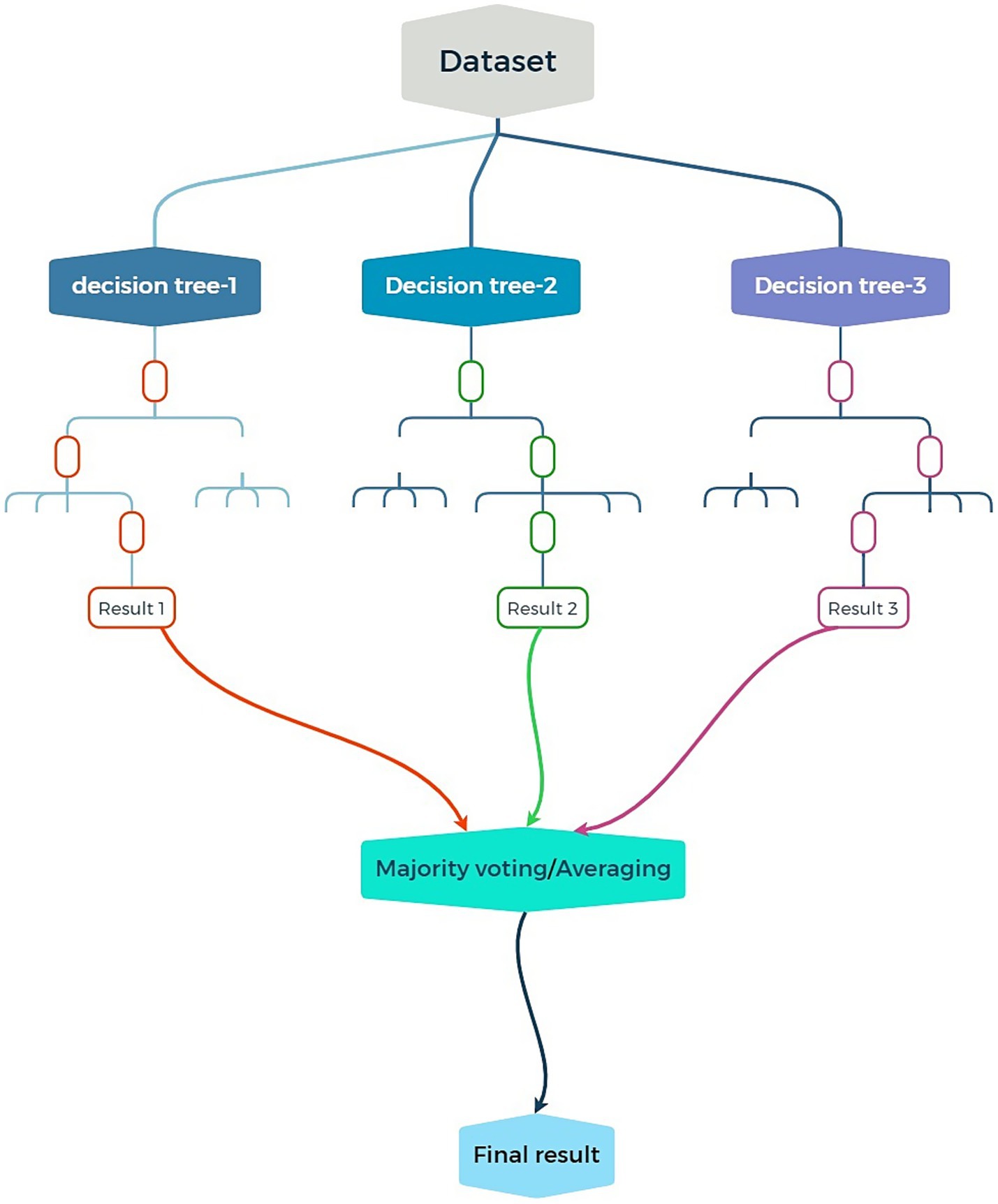
Figure 2. Schematic illustration of an RF model: combining multiple decision trees to improve accuracy and reduce overfitting in predictions.
Overall, predicting patient outcomes using machine learning techniques, particularly the RF algorithm, is key for effective treatment planning. Studies showed high accuracy in predicting survival and lymph node metastasis risk using RF models based on various clinical and imaging data. Feature importance measures helped clarify the most crucial prediction elements (Tables 7, 8).
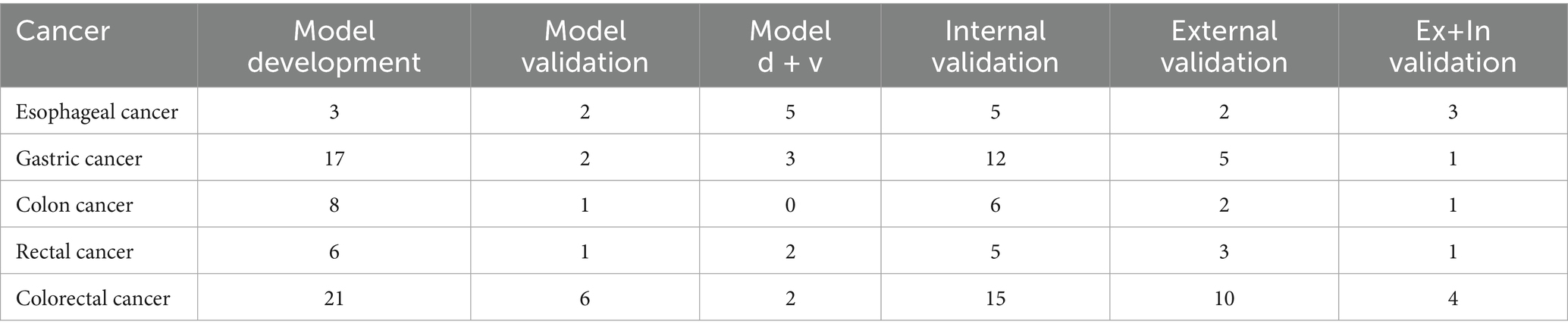
Table 8. Comparison of the included articles discussed 2 cancers, and 13 studies were both internally and externally validated.
Discussion
This systematic review shows the effectiveness of randomized RF models in detecting and predicting GI cancers. RF models have consistently shown good accuracy and reliability in diagnosing GI tract malignancy types, including EC, RC, GC, CC, and CRC, in several investigations (Zhang et al., 2023; Kolisnik et al., 2023). They also provided reliable predictions for patient outcomes, including survival rates and the likelihood of relapse (Zhang et al., 2023). RF’s strengths, such as managing complex and heterogeneous datasets and handling missing values, make it an attractive tool for doctors and researchers. In addition, RF models are known for their ability to combine molecular, clinical, and pathological data, thus providing a holistic approach to cancer prediction (Xu et al., 2022).
The findings of the research show that RF-based models are more effective than traditional prediction methods that depend on statistical methods and experimental guidelines in predicting outcomes associated with GI cancer. The reason RF is better than conventional models is because it can capture nonlinear correlations and interactions between variables. Intrinsic algorithms can reduce the nature of excessive risk. Also, these algorithms can increase the generalizability of RF to diverse data sets. In addition, RF’s ability provides a more holistic approach to understanding the progression of cancer to integrate multimodal data, such as genetic, clinical, and demographic factors, potentially leading to more personalized treatment strategies. These results imply that RF models may effectively address the deficiencies in treatment prediction and decision-making associated with GI cancers, which are not adequately managed by conventional markers (Afrash et al., 2023; Liu et al., 2024). RF-based models are valuable tools for early detection and accurate prediction, which is essential to improve survival rates in patients with GI cancer (Alghafees et al., 2024). Application of these models helps healthcare providers to better diagnose high-risk patients and also determine whether immunotherapy or aggressive interventions are beneficial to them (D'Orsi et al., 2024). Furthermore, the adoption of RF models leads to saving costs by optimizing the use of healthcare resources and reducing diagnostic errors (Orji and Ukwandu, 2024). The results suggest the importance of RF models for GI cancers and that these models have a vital role in the future of personalized medicine in oncology (Chen et al., 2024).
RF strengths and applications
The effectiveness of Random Forest (RF) models in identifying and forecasting the course of gastrointestinal (GI) malignancies is highlighted in this comprehensive study. Several studies utilizing RC, GC, and CRC have shown the significant accuracy of RF models (e.g., Rahman et al., 2020; Qiu et al., 2022 RF’s ability to comprehend complex, high-dimensional data and accommodate for missing values makes it a valuable approach in the research of cancer). Additionally, to enhance survival prediction and personalized treatment plans for patients with GI cancer, RF models use multimodal data, such as genetic, clinical, and pathological features (Xu et al., 2022). For example, studies on CC and RC have shown that using RF models in conjunction with feature selection methods such as LASSO greatly improved prediction accuracy (Li et al., 2021; Qiu et al., 2022).
As mentioned earlier, Random Forest models are naturally robust to missing values, and many studies leveraged this strength without extensive preprocessing. Most of the included studies did not mention their approach to handle missing data, but still successfully trained RF models. Given RF’s built-in mechanisms for handling gaps, we consider this acceptable for the scope of our review, though future work could benefit from more detailed reporting of imputation strategies.
Interpretability of RF models
Even though RF models are strong, clinical practitioners nevertheless have a significant difficulty with their interpretability. Effective use of techniques like SHAP values and permutation significance can improve decision-making transparency. While permutation significance analysis aids in determining the most important factors influencing outcomes, SHAP values offer insights into how each feature contributes to model predictions, allowing doctors to have a more nuanced view (Ali et al., 2023). Additionally, by including feature significance charts in clinical reports, doctors may be able to have a better understanding of the key predictors and use the results of the RF model to influence their treatment choices (Sadeghi et al., 2024).
Clinical applicability
To successfully incorporate RF models into clinical procedures, a few obstacles need to be removed. Medical professionals’ lack of technical expertise is one of the main problems; many of them might not understand machine learning principles or particular RF applications well enough (Esmaeilzadeh, 2024). Furthermore, because hospitals can lack the infrastructure required to support more complex modeling techniques, incorporating these sorts of tools into standard clinical practice could be difficult. By developing automated, user-friendly RF-based clinical apps and enhancing training programs to educate medical personnel about machine learning, these problems may be addressed and they will be prepared to deploy machine learning in patient care (Stoumpos et al., 2023).
Limitations
Despite the sector’s extremely positive results, several limitations must be noted. One major problem discouraging physicians from using RF algorithms in their daily practice is their complicated clinical applicability. For instance, in research on EC analysis, different clinical conditions and patient characteristics may yield surprising model performance, and hence physicians cannot consistently apply the results. Moreover, most of the included studies are retrospective, which restricts the generalizability of their findings since they cannot precisely reflect the variety that would have been present in real clinical settings because they usually rely on data that has already been collected. For example, because the majority of research predicting GC outcomes relied on historical data that could be subject to selection bias or lack adequate patient demographics, results often were biased and the model’s dependability was poor in new and diverse patient populations.
Moreover, there are also problems concerning consistency with specific input variables in the RF models identified across the different research studies. Indeed, small changes in the imaging data, biomarkers used, or clinical scenario can greatly influence the performance and consistency of such models. These differences render it challenging to confirm findings across patient groups or various healthcare settings, while limiting comparisons across studies. In this respect, the present work aims to point out that the application of machine learning models, such as RF, in various settings regarding patient demographics and treatment should be demonstrated, as well as the need for carefully designed prospective clinical trials and the need for external validation. To realize the full potential of predictive modeling in enhancing cancer treatment and patient outcomes, several steps should be taken.
Future directions
To increase the efficacy and usefulness of RF models in cancer, future research should focus on developing user-friendly interfaces that facilitate their integration into clinical procedures. Standardizing input variables is necessary to improve repeatability across investigations, and prospective trials and extensive validations across a range of patient groups are needed to evaluate the model’s effectiveness. Furthermore, examining hybrid approaches that combine RF and neural networks may significantly improve predictions for complex outcomes like survival and metastasis, ultimately leading to better patient care and clinical judgment. Further, to ensure that these prognostic tools generalize across diverse patient populations and can be safely deployed in clinics, future efforts should prioritize prospective, multi-center external validation and real-world integration studies.
Conclusion
This extensive review shows that machine learning models are very reliable and accurate in finding out and forecasting GI malignancies like EC, RC, GC, CC, and CRC. Moreover, there exists a large body of literature on how well RF handles such intricate pathological and molecular clinical data for the accurate forecasting of patient outcomes related to survival rates and recurrence risks. On the other hand, traditional prediction algorithms could not identify complex patterns or even recognize and control the diversity of GI malignancies. The results of this study identify the importance of RF models in improving the accuracy of the prediction that will inform treatment options for patients with GI cancers. The ability of these models to handle high volumes of diverse data and identify nonlinear relationships between variables makes them efficient in clinical applications. It aids doctors to better identify high-risk patients and adopt more appropriate treatments for them. RF has been able to act as a powerful tool to reduce the distances in treatment predictions, especially in cases where traditional prediction markers are inadequate.
RF-based models are promising to have a huge say in future developments related to customized medicine. The integration of the models into current treatment strategies will likely improve survival rates and the general quality of life in patients with GI cancers. Cost savings can be realized through the use of the models by reducing diagnostic mistakes and improving healthcare resource distribution. Further studies are needed to be carried out to encourage wide application of the models in real-world scenarios.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
ZM: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AS: Data curation, Investigation, Writing – original draft, Writing – review & editing. YA: Data curation, Investigation, Writing – original draft, Writing – review & editing. SS: Data curation, Investigation, Writing – original draft, Writing – review & editing. PG: Data curation, Investigation, Writing – original draft, Writing – review & editing. AK: Data curation, Investigation, Writing – original draft, Writing – review & editing. AV: Data curation, Investigation, Writing – original draft, Writing – review & editing. HA: Data curation, Investigation, Writing – original draft, Writing – review & editing. OB: Writing – review & editing. AZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. YK: Data curation, Investigation, Writing – original draft, Writing – review & editing. KP: Data curation, Investigation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We used Xmind to draw mind maps.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frai.2025.1517670/full#supplementary-material
References
Abbas, S., Asif, M., Rehman, A., Alharbi, M., Khan, M. A., and Elmitwally, N. (2024). Emerging research trends in artificial intelligence for cancer diagnostic systems: a comprehensive review. Heliyon. 10:e36743. doi: 10.1016/j.heliyon.2024.e36743
Acharya, R., Mahapatra, A., Verma, H. K., and Bhaskar, L. (2023). Unveiling therapeutic targets for esophageal cancer: a comprehensive review. Curr. Oncol. 30, 9542–9568. doi: 10.3390/curroncol30110691
Afrash, M. R., Shafiee, M., and Kazemi-Arpanahi, H. (2023). Establishing machine learning models to predict the early risk of gastric cancer based on lifestyle factors. BMC Gastroenterol. 23:6. doi: 10.1186/s12876-022-02626-x
Alghafees, M., Seyam, R. M., Al-Hussain, T., Amin, T. M., Altaweel, W., Sabbah, B. N., et al. (2024). Using machine learning models to predict synchronous genitourinary cancers among gastrointestinal stromal tumor patients. Urol Ann. 16, 94–97. doi: 10.4103/ua.ua_32_23
Ali, S., Akhlaq, F., Imran, A. S., Kastrati, Z., Daudpota, S. M., and Moosa, M. (2023). The enlightening role of explainable artificial intelligence in medical & healthcare domains: a systematic literature review. Comput. Biol. Med. 166:107555. doi: 10.1016/j.compbiomed.2023.107555
Altman, D. G., and Royston, P. (2000). What do we mean by validating a prognostic model? Stat. Med. 19, 453–473. doi: 10.1002/(sici)1097-0258(20000229)19:4<>3.0.co;2-5
Alzahrani, S. M., Al Doghaither, H. A., and Al-Ghafari, A. B. (2021). General insight into cancer: an overview of colorectal cancer (review). Mol. Clin. Oncol. 15:271. doi: 10.3892/mco.2021.2433
An, C., Yang, H., Yu, X., Han, Z. Y., Cheng, Z., Liu, F., et al. (2022). A machine learning model based on health Records for Predicting Recurrence after Microwave Ablation of hepatocellular carcinoma. J. Hepatocell Carcinoma. 9, 671–684. doi: 10.2147/JHC.S358197
Andersson, J., Angenete, E., Gellerstedt, M., and Haglind, E. (2024). Developing a multivariable prediction model of global health-related quality of life in patients treated for rectal cancer: a prospective study in five countries. Int. J. Color. Dis. 39:35. doi: 10.1007/s00384-024-04605-y
Asif, A., Rajpoot, K., Graham, S., Snead, D., Minhas, F., and Rajpoot, N. (2023). Unleashing the potential of AI for pathology: challenges and recommendations. J. Pathol. 260, 564–577. doi: 10.1002/path.6168
Bennett, K. P., and Campbell, C. (2000). Support vector machines: hype or hallelujah? SIGKDD Explor. Newsl. 2, 1–13. doi: 10.1145/380995.380999
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424. doi: 10.3322/caac.21492
Chaddad, A., Niazi, T., Probst, S., Bladou, F., Anidjar, M., and Bahoric, B. (2018). Predicting Gleason score of prostate cancer patients using radiomic analysis. Front. Oncol. 8:630. doi: 10.3389/fonc.2018.00630
Chen, Y., Sun, Z., Wan, L., Chen, H., Xi, T., and Jiang, Y. (2022). Tumor microenvironment characterization for assessment of recurrence and survival outcome in gastric Cancer to predict chemotherapy and immunotherapy response. Front. Immunol. 13:890922. doi: 10.3389/fimmu.2022.890922
Chen, Y., Wang, B., Zhao, Y., Shao, X., Wang, M., Ma, F., et al. (2024). Metabolomic machine learning predictor for diagnosis and prognosis of gastric cancer. Nat. Commun. 15:1657. doi: 10.1038/s41467-024-46043-y
Christou, C. D., and Tsoulfas, G. (2021). Challenges and opportunities in the application of artificial intelligence in gastroenterology and hepatology. World J. Gastroenterol. 27, 6191–6223. doi: 10.3748/wjg.v27.i37.6191
Daye, D., Tabari, A., Kim, H., Chang, K., Kamran, S. C., Hong, T. S., et al. (2021). Quantitative tumor heterogeneity MRI profiling improves machine learning-based prognostication in patients with metastatic colon cancer. Eur. Radiol. 31, 5759–5767. doi: 10.1007/s00330-020-07673-0
D'Orsi, L., Capasso, B., Lamacchia, G., Pizzichini, P., Ferranti, S., Liverani, A., et al. (2024). Recent advances in artificial intelligence to improve immunotherapy and the use of digital twins to identify prognosis of patients with solid tumors. Int. J. Mol. Sci. 25:588. doi: 10.3390/ijms252111588
Du, H., Wang, H., Kong, F., Wu, M., Chen, W., Lyu, J., et al. (2022). Identification and comprehensive analysis of FREM2 mutation as a potential prognostic biomarker in colorectal Cancer. Front. Mol. Biosci. 9:839617. doi: 10.3389/fmolb.2022.839617
Esmaeilzadeh, P. (2024). Challenges and strategies for wide-scale artificial intelligence (AI) deployment in healthcare practices: a perspective for healthcare organizations. Artif. Intell. Med. 151:102861. doi: 10.1016/j.artmed.2024.102861
Ferlay, J., Colombet, M., Soerjomataram, I., Parkin, D. M., Piñeros, M., Znaor, A., et al. (2021). Cancer statistics for the year 2020: An overview. Int. J. Cancer 149, 778–789. doi: 10.1002/ijc.33588
Hallinan, J. T., and Venkatesh, S. K. (2013). Gastric carcinoma: imaging diagnosis, staging and assessment of treatment response. Cancer Imaging 13, 212–227. doi: 10.1102/1470-7330.2013.0023
Hayden, J. A., Côté, P., and Bombardier, C. (2006). Evaluation of the quality of prognosis studies in systematic reviews. Ann. Intern. Med. 144, 427–437. doi: 10.7326/0003-4819-144-6-200603210-00010
Huang, J., Lucero-Prisno, D. E. 3rd, Zhang, L., Xu, W., Wong, S. H., Ng, S. C., et al. (2023). Updated epidemiology of gastrointestinal cancers in East Asia. Nat. Rev. Gastroenterol. Hepatol. 20, 271–287. doi: 10.1038/s41575-022-00726-3
Huang, B., Tian, S., Zhan, N., Ma, J., Huang, Z., Zhang, C., et al. (2021). Accurate diagnosis and prognosis prediction of gastric cancer using deep learning on digital pathological images: a retrospective multicentre study. EBioMedicine 73:103631. doi: 10.1016/j.ebiom.2021.103631
Kasai, A., Miyoshi, J., Sato, Y., Okamoto, K., Miyamoto, H., Kawanaka, T., et al. (2024). A novel CT-based radiomics model for predicting response and prognosis of chemoradiotherapy in esophageal squamous cell carcinoma. Sci. Rep. 14:2039. doi: 10.1038/s41598-024-52418-4
Kaur, S., Kim, R., Javagal, N., Calderon, J., Rodriguez, S., and Murugan, N. (2024). Precision medicine with data-driven approaches: a framework for clinical translation. AIJMR 2:1077. doi: 10.62127/aijmr.2024.v02i05.1077
Kim, H. J., and Choi, G. S. (2019). Clinical implications of lymph node metastasis in colorectal cancer: current status and future perspectives. Ann. Coloproctol. 35, 109–117. doi: 10.3393/ac.2019.06.12
Kolisnik, T., Sulit, A. K., Schmeier, S., Frizelle, F., Purcell, R., Smith, A., et al. (2023). Identifying important microbial and genomic biomarkers for differentiating right- versus left-sided colorectal cancer using random forest models. BMC Cancer 23:647. doi: 10.1186/s12885-023-10848-9
Kow, A. W. C. (2019). Hepatic metastasis from colorectal cancer. J. Gastrointest. Oncol. 10, 1274–1298. doi: 10.21037/jgo.2019.08.06
Książek, W., Abdar, M., Acharya, U., and Pławiak, P. (2019). A novel machine learning approach for early detection of hepatocellular carcinoma patients. Cogn. Syst. Res. 54, 116–127. doi: 10.1016/j.cogsys.2018.12.001
Li, H., Chen, L., Zeng, H., Liao, Q., Ji, J., and Ma, X. (2021). Integrative analysis of histopathological images and genomic data in Colon adenocarcinoma. Front. Oncol. 11:636451. doi: 10.3389/fonc.2021.636451
Li, C., Chen, H., Zhang, B., Fang, Y., Sun, W., Wu, D., et al. (2023). Radiomics signature based on support vector Machines for the Prediction of pathological complete response to Neoadjuvant Chemoradiotherapy in locally advanced rectal Cancer. Cancers (Basel) 15:134. doi: 10.3390/cancers15215134
Li, Z., Zhang, D., Dai, Y., Dong, J., Wu, L., Li, Y., et al. (2018). Computed tomography-based radiomics for prediction of neoadjuvant chemotherapy outcomes in locally advanced gastric cancer: a pilot study. Chin. J. Cancer Res. 30, 406–414. doi: 10.21147/j.issn.1000-9604.2018.04.03
Li, H. Y., Zhou, J. T., Wang, Y. N., Zhang, N., and Wu, S. F. (2023). Establishment and application of three predictive models of anastomotic leakage after rectal cancer sphincter-preserving surgery. World J. Gastrointest. Surg. 15:2201, –2210. doi: 10.4240/wjgs.v15.i10.2201
Liu, Y., Chen, S., Shen, W., Qu, X., Li, S., and Shi, Y. (2024). Construction and validation of a gastric Cancer diagnostic model based on blood groups and tumor markers. J. Cancer 15, 729–736. doi: 10.7150/jca.88190
Liu, Y., Wang, L., Du, W., Huang, Y., Guo, Y., Song, C., et al. (2023). Identification of high-risk factors associated with mortality at 1-, 3-, and 5-year intervals in gastric cancer patients undergoing radical surgery and immunotherapy: an 8-year multicenter retrospective analysis. Front. Cell. Infect. Microbiol. 13:1207235. doi: 10.3389/fcimb.2023.1207235
Liu, S., Xu, M., Qiao, X., Ji, C., Li, L., and Zhou, Z. (2021). Prediction of serosal invasion in gastric cancer: development and validation of multivariate models integrating preoperative clinicopathological features and radiographic findings based on late arterial phase CT images. BMC Cancer 21:1038. doi: 10.1186/s12885-021-08672-0
Mishra, J., Drummond, J., Quazi, S. H., Karanki, S. S., Shaw, J. J., Chen, B., et al. (2013). Prospective of colon cancer treatments and scope for combinatorial approach to enhanced cancer cell apoptosis. Crit. Rev. Oncol. Hematol. 86, 232–250. doi: 10.1016/j.critrevonc.2012.09.014
Orji, U., and Ukwandu, E. (2024). Machine learning for an explainable cost prediction of medical insurance. Mach. Learn. Appl. 15:100516. doi: 10.1016/j.mlwa.2023.100516
OSF (2025). Available online at: https://osf.io/ (Accessed May 28, 2024).
Ou, J., Li, R., Zeng, R., Wu, C. Q., Chen, Y., Chen, T. W., et al. (2019). CT radiomic features for predicting resectability of oesophageal squamous cell carcinoma as given by feature analysis: a case control study. Cancer Imaging 19:66. doi: 10.1186/s40644-019-0254-0
Ouzzani, M., Hammady, H., Fedorowicz, Z., and Elmagarmid, A. (2016). Rayyan—a web and mobile app for systematic reviews. Syst. Rev. 5:210. doi: 10.1186/s13643-016-0384-4
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. doi: 10.1136/bmj.n71
Park, M., Kwon, J., Kong, J., Moon, S. M., Cho, S., Yang, K. Y., et al. (2021). A patient-derived organoid-based Radiosensitivity model for the prediction of radiation responses in patients with rectal Cancer. Cancers (Basel) 13:760. doi: 10.3390/cancers13153760
Parmar, C., Grossmann, P., Rietveld, D., Rietbergen, M. M., Lambin, P., and Aerts, H. J. (2015). Radiomic machine-learning classifiers for prognostic biomarkers of head and neck cancer. Front. Oncol. 5:272.
Paul, D., Su, R., Romain, M., Sébastien, V., Pierre, V., and Isabelle, G. (2017). Feature selection for outcome prediction in oesophageal cancer using genetic algorithm and random forest classifier. Comput. Med. Imaging Graph. 60, 42–49. doi: 10.1016/j.compmedimag.2016.12.002
Peng, J. J., Cai, S. J., Lu, H. F., Cai, G. X., Lian, P., Guan, Z. Q., et al. (2007). Predicting prognosis of rectal cancer patients with total mesorectal excision using molecular markers. World J. Gastroenterol. 13, 3009–3015. doi: 10.3748/wjg.v13.i21.3009
Pourhoseingholi, M. A., Kheirian, S., and Zali, M. R. (2017). Comparison of basic and ensemble data mining methods in predicting 5-year survival of colorectal Cancer patients. Acta Inform Med. 25, 254–258. doi: 10.5455/aim.2017.25.254-258
Qiu, B., Su, X. H., Qin, X., and Wang, Q. (2022). Application of machine learning techniques in real-world research to predict the risk of liver metastasis in rectal cancer. Front. Oncol. 12:1065468. doi: 10.3389/fonc.2022.1065468
Quail, D. F., and Joyce, J. A. (2013). Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 19, 1423–1437. doi: 10.1038/nm.3394
Rahman, S. A., Walker, R. C., Lloyd, M. A., Grace, B. L., van Boxel, G. I., Kingma, B. F., et al. (2020). Machine learning to predict early recurrence after oesophageal cancer surgery. Br. J. Surg. 107, 1042–1052. doi: 10.1002/bjs.11461
Rawla, P., and Barsouk, A. (2019). Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 14, 26–38. doi: 10.5114/pg.2018.80001
Rizinde, T., Ngaruye, I., and Cahill, N. D. (2023). Comparing machine learning classifiers for predicting hospital readmission of heart failure patients in Rwanda. J. Pers. Med. 13:393. doi: 10.3390/jpm13091393
Sadeghi, Z., Alizadehsani, R., Cifci, M. A., Kausar, S., Rehman, R., Mahanta, P., et al. (2024). A review of explainable artificial intelligence in healthcare. Comput. Electr. Eng. 118:109370. doi: 10.1016/j.compeleceng.2024.109370
Shafi, A., Molla, M. M. I., Julakha, J. J., and Mohammad, R. M. M. (2020). Detection of colon cancer based on microarray dataset using machine learning as a feature selection and classification techniques. SN Appl. Sci. 2, 1–8. doi: 10.1007/s42452-020-3051-2
Shen, X., and Zhao, B. (2018). Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: meta-analysis. BMJ 362:k3529. doi: 10.1136/bmj.k3529
Siegel, R. L., Miller, K. D., Fuchs, H. E., and Jemal, A. (2022). Cancer statistics, 2022. CA Cancer J. Clin. 72, 7–33. doi: 10.3322/caac.21708
Song, Y., Yin, Z., Zhang, C., Hao, S., Li, H., Wang, S., et al. (2022). Random forest classifier improving phenylketonuria screening performance in two Chinese populations. Front. Mol. Biosci. 9:986556. doi: 10.3389/fmolb.2022.986556
Stone, P., Buckle, P., Dolan, R., Feliu, J., Hui, D., Laird, B. J. A., et al. (2023). Prognostic evaluation in patients with advanced cancer in the last months of life: ESMO clinical practice guideline. ESMO Open. 8:101195. doi: 10.1016/j.esmoop.2023.101195
Stoumpos, A. I., Kitsios, F., and Talias, M. A. (2023). Digital transformation in healthcare: technology acceptance and its applications. Int. J. Environ. Res. Public Health 20:3407. doi: 10.3390/ijerph20043407
Tabari, A., D'Amore, B., Noh, J., Gee, M. S., and Daye, D. (2024). Quantitative peritumoral magnetic resonance imaging fingerprinting improves machine learning-based prediction of overall survival in colorectal cancer. Explor. Target Antitumor Ther. 5, 74–84. doi: 10.37349/etat.2024.00205
Takamatsu, M., Yamamoto, N., Kawachi, H., Chino, A., Saito, S., Ueno, M., et al. (2019). Prediction of early colorectal cancer metastasis by machine learning using digital slide images. Comput. Methods Prog. Biomed. 178, 155–161. doi: 10.1016/j.cmpb.2019.06.022
Tang, F., and Ishwaran, H. (2017). Random Forest missing data algorithms. Stat. Anal. Data Min. 10, 363–377. doi: 10.1002/sam.11348
Thavanesan, N., Farahi, A., Parfitt, C., Belkhatir, Z., Azim, T., Vallejos, E. P., et al. (2024). Insights from explainable AI in oesophageal cancer team decisions. Comput. Biol. Med. 180:108978. doi: 10.1016/j.compbiomed.2024.108978
Tian, H., Liu, Z., Liu, J., Zong, Z., Chen, Y., Zhang, Z., et al. (2023). Application of machine learning algorithm in predicting distant metastasis of T1 gastric cancer. Sci. Rep. 13:5741. doi: 10.1038/s41598-023-31880-6
Uhlenhopp, D. J., Then, E. O., Sunkara, T., and Gaduputi, V. (2020). Epidemiology of esophageal cancer: update in global trends, etiology and risk factors. Clin. J. Gastroenterol. 13, 1010–1021. doi: 10.1007/s12328-020-01237-x
Van Cutsem, E., Cervantes, A., Nordlinger, B., and Arnold, D. (2014). Metastatic colorectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 25, iii1–iii9. doi: 10.1093/annonc/mdu260
Wang, Z. X., Pan, Y. Q., Li, X., Tsubata, T., and Xu, R. H. (2023). Immunotherapy in gastrointestinal cancers: advances, challenges, and countermeasures. Sci Bull 68, 763–766. doi: 10.1016/j.scib.2023.03.036
Xu, C., Wang, J., Zheng, T., Cao, Y., and Ye, F. (2022). Prediction of prognosis and survival of patients with gastric cancer by a weighted improved random forest model: an application of machine learning in medicine. Arch. Med. Sci. 18, 1208–1220. doi: 10.5114/aoms/135594
Yao, D., Zhang, T., Zhan, X., Zhang, S., Zhan, X., and Zhang, C. (2022). Geometric complement heterogeneous information and random forest for predicting lncRNA-disease associations. Front. Genet. 13:995532. doi: 10.3389/fgene.2022.995532
Yu, D., Yang, J., Wang, B., Li, Z., Wang, K., Li, J., et al. (2024). New genetic insights into immunotherapy outcomes in gastric cancer via single-cell RNA sequencing and random forest model. Cancer Immunol. Immunother. 73:112. doi: 10.1007/s00262-024-03684-8
Zhang, X., Liu, G., and Peng, X. (2023). A random Forest model for post-treatment survival prediction in patients with non-squamous cell carcinoma of the head and neck. J. Clin. Med. 12:15. doi: 10.3390/jcm12155015
Zhang, C., Xu, J., Tang, R., Yang, J., Wang, W., Yu, X., et al. (2023). Novel research and future prospects of artificial intelligence in cancer diagnosis and treatment. J. Hematol. Oncol. 16:114. doi: 10.1186/s13045-023-01514-5
Zhou, Y., Tao, L., Qiu, J., Xu, J., Yang, X., Zhang, Y., et al. (2024). Tumor biomarkers for diagnosis, prognosis and targeted therapy. Signal Transduct. Target. Ther. 9:132. doi: 10.1038/s41392-024-01823-2
Keywords: random forest, prognostication, GI tract cancers, malignancy, prognose
Citation: Mohamadi Z, Shafizadeh A, Aliyan Y, Shayesteh SF, Goudarzi P, Khodabandeh A, Vaghari A, Ashrafi H, Bahrami O, ZarinKhat A, Khodabandeh Y and Pouyan K (2025) The application of random forest-based models in prognostication of gastrointestinal tract malignancies: a systematic review. Front. Artif. Intell. 8:1517670. doi: 10.3389/frai.2025.1517670
Edited by:
Petar Ozretić, Rudjer Boskovic Institute, CroatiaReviewed by:
Muhammad Shahid Iqbal, Anhui University, ChinaTaChen Chen, Nihon Pharmaceutical University, Japan
Copyright © 2025 Mohamadi, Shafizadeh, Aliyan, Shayesteh, Goudarzi, Khodabandeh, Vaghari, Ashrafi, Bahrami, ZarinKhat, Khodabandeh and Pouyan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Armin ZarinKhat, emtoYXJtaW5AZ21haWwuY29t
•ORCID: Zhina Mohamadi, https://orcid.org/0009-0007-6862-0379
Ali Shafizadeh, https://orcid.org/0009-0002-0805-185X
Yasamin Aliyan, https://orcid.org/0009-0006-3208-7181
Seyedeh Fatemeh Shayesteh, https://orcid.org/0000-0001-8421-0205
Parsa Goudarzi, https://orcid.org/0009-0002-0727-0559
Alireza Khodabandeh, https://orcid.org/0009-0008-9180-0279
Amirali Vaghari, https://orcid.org/0009-0003-2391-493X
Helma Ashrafi, https://orcid.org/0009-0008-9562-6562
Omid Bahrami, https://orcid.org/0009-0005-4310-711X
Yalda Khodabandeh, https://orcid.org/0009-0001-4621-8860
Kimia Pouyan, https://orcid.org/0009-0001-1147-6827
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share second authorship
§These authors have contributed equally to this work and share fifth authorship
 Zhina Mohamadi
Zhina Mohamadi Ahmad Shafizadeh2•†
Ahmad Shafizadeh2•† Parsa Goudarzi
Parsa Goudarzi Armin ZarinKhat
Armin ZarinKhat