- 1Shandong Provincial Key Laboratory of Water and Soil Conservation and Environmental Protection, College of Resources and Environment, Linyi University, Linyi, China
- 2Linyi Ecological Environmental Bureau, Linyi, China
Introduction: Soil nitrous oxide (N2O) emissions are a major contributor to global warming and climate change. Microbial N2O reduction via the nosZ gene—classified into clade I and clade II—is the only known biological sink for N2O.
Methods: In this study, we isolated two N2O-reducing bacterial strains: an Enterobacter sp. harboring nosZ clade I (nosZI) and a Pseudomonas sp. harboring nosZ clade II (nosZII). We evaluated their performance under different environmental conditions and their effects on N2O emissions from agricultural soils subjected to varying fertilization strategies.
Results: Pure culture experiments revealed that growth and N2O-reducing activity in both strains were significantly influenced by pH, oxygen concentration, nitrate levels, and carbon source. Notably, the nosZII strain demonstrated broader environmental adaptability and higher N2O-reduction efficiency across a range of conditions. In soil microcosm experiments, N2O emissions were strongly affected by fertilization type, with mixed organic-inorganic treatments producing the highest emissions. Inoculation with the nosZII strain significantly reduced N2O emissions in soils receiving inorganic or combined fertilizers. In contrast, the nosZI strain tended to increase emissions, except under the mixed fertilization regime.
Discussion: These findings highlight the importance of selecting N2O-reducing microbial strains based on their functional capacity and environmental tolerance. This work advances the development of targeted microbial strategies for mitigating N2O emissions, supporting more sustainable and climate-resilient agricultural practices.
1 Introduction
Nitrous oxide (N2O) is a potent greenhouse gas that significantly contributes to global warming and is currently the leading cause of stratospheric ozone depletion (Ravishankara et al., 2009; World Meteorological Organization, 2022). Agricultural soils represent a major source of N2O emissions, with nitrogen fertilizer applications accounting for approximately 53% of global anthropogenic N2O emissions (Tian et al., 2020). Driven by global population growth and intensified land use, the increasing application of nitrogen fertilizers in agriculture has led to a substantial rise in N2O emissions (Bahram et al., 2022). Thus, developing effective strategies to mitigate N2O emissions while sustaining crop productivity and environmental health is crucial, especially under mounting pressure on the global food supply system.
Although soil amendments—such as biochar and biological inhibitors—have been explored for reducing N2O emissions, their effectiveness remains inconsistent and context-dependent. For instance, the efficacy of biochar varies widely depending on factors such as feedstock type, soil properties, climatic conditions, and management practices, with some studies reporting neutral or even adverse effects on greenhouse gas emissions (Sanford et al., 2012; Jones et al., 2013; Zhang et al., 2020a). Likewise, biological inhibitors, including urease inhibitors (UIs) and nitrification inhibitors (NIs) like dicyandiamide (DCD) and 3,4-dimethylpyrazole phosphate (DMPP), have shown variable performance and may negatively affect microbial biomass or plant health when overapplied (Carneiro et al., 2010; Suenaga et al., 2019; Maheshwari et al., 2023). Moreover, recent studies have questioned the effectiveness of DCD in reducing N2O emissions relative to conventional urea-based fertilization (Maheshwari et al., 2023). There is a clear need for alternative mitigation strategies that are both effective and environmentally sustainable.
A promising biological approach lies in harnessing the activity of nitrous oxide reductase (Nos), the only known enzyme capable of reducing N2O to dinitrogen (N2), thereby serving as the sole biological sink for N2O in the nitrogen cycle (Conthe et al., 2018). The nosZ gene, encoding this enzyme, is categorized into two phylogenetically distinct clades—clade I and clade II—which differ in their genetic makeup, physiological traits, and ecological functions (Jones et al., 2013; Domeignoz-Horta et al., 2016; Hallin et al., 2018). Genomic analyses have identified the taxonomic affiliations of these clades: nosZ clade I is primarily found in genera such as Bradyrhizobium, Pseudomonas, Paracoccus, Ralstonia, Thiobacillus, and Rhodopseudomonas, while nosZ clade II is more widely distributed across diverse genera including Anaeromyxobacter, Gemmatimonas, Opitutus, and Hydrogenobacter (Jones et al., 2013; Graf et al., 2014; Orellana et al., 2014; Domeignoz-Horta et al., 2016; Avşar and Aras, 2020). Clade II bacteria, in particular, have been observed to dominate in systems where N2O serves as the primary electron acceptor, whereas clade I bacteria are more abundant under nitrate-rich conditions (Suenaga et al., 2019).
The abundance and functional efficiency of nosZ-carrying microorganisms are influenced by a range of environmental variables, including pH, oxygen availability, carbon sources, and nitrate concentrations (Liu et al., 2010; Lu and Chandran, 2010; Desloover et al., 2014; Park et al., 2017; Suenaga et al., 2018; Qi et al., 2022; Shaaban et al., 2023). Despite advances in understanding the phylogenetic and functional diversity of nosZ communities, there remains a significant knowledge gap regarding how nosZ clade I and II bacteria compare in their N2O reduction capacities under different fertilization regimes and environmental conditions in agricultural soils.
Given their distinct genetic and ecological characteristics, we hypothesize that: (1) nosZ clade II bacteria exhibit broader environmental tolerance—particularly to variations in pH, oxygen, nitrate, and carbon sources—compared to nosZ clade I bacteria; and (2) the N2O-reducing performance of nosZ clade I and II bacteria will differ across soils managed with different fertilization strategies (e.g., inorganic, organic, or mixed). To test these hypotheses, we isolated and characterized nosZ clade I and II bacteria from fertilized agricultural soils using nitrogen-limited, N2O-enriched media under anaerobic conditions. We conducted both pure culture experiments and soil microcosm trials to assess the growth and N2O reduction efficiency of each clade. The objectives of this study are: (1) to compare the growth responses and N2O reduction activity of nosZ clade I and II isolates under a range of environmental conditions relevant to agricultural soils; (2) to evaluate the effects of these isolates on soil N2O emissions under different fertilization strategies.
2 Materials and methods
2.1 Study site and soil sample collection
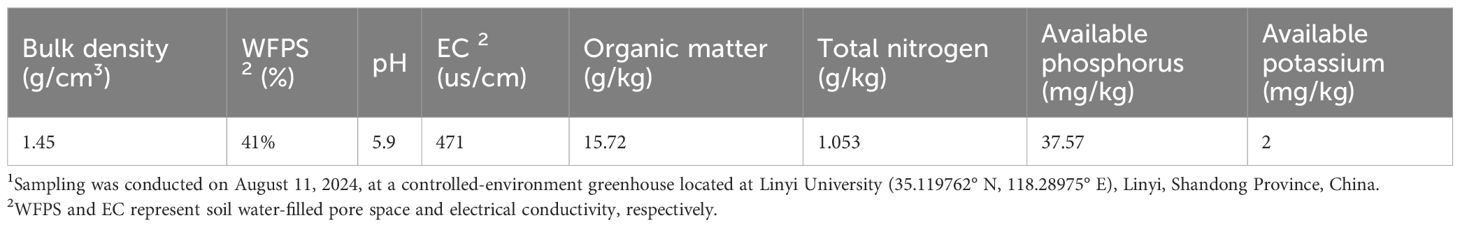
Soil samples were collected from a controlled-environment greenhouse at Linyi University (35.119762° N, 118.28975° E), Linyi, China. The field was cultivated with cucumber (Cucumis sativus L. cv. ‘Zhongnong 62’) and pepper (Capsicum annuum L. cv. ‘Shifeng 802’). The basic physicochemical characteristics of the soil are summarized in Table 1. Topsoil (0–15 cm) was collected during the non-growing season (totaling 10 kg), homogenized, sieved through a 2 mm mesh, and partitioned into 300 g for bacterial screening and 8 kg for soil incubation experiments.
2.2 Isolation, growth, and N2O reduction capacity of isolates
Two isolates were selected for this study: one harboring the nosZ clade I gene (hereafter referred to as the nosZI strain) and the other harboring the nosZ clade II gene (nosZII strain). Morphologically, both isolates formed circular, smooth, mucoid colonies. The nosZI strain produced opaque, white colonies, whereas the nosZII strain formed translucent, creamy-white colonies.
Isolation Procedure: Sterilized Schott bottles were filled with dried soil and sterile water, then pre-incubated anaerobically for 7 days. The soil suspension was homogenized with sterile glass beads, transferred to fresh Schott bottles, amended with sodium acetate anhydrous, flushed with high-purity helium, and spiked with N2O to a final concentration of 8%. Cultures were incubated at 30°C with shaking at 180 rpm. Helium and N2O were replenished every 2 days. After 7 days, samples were serially diluted and plated on nitrogen-free medium, then transferred to a helium-flushed, sealed incubation chamber supplemented with N2O and incubated anaerobically for 48 hours at 30°C. Distinct colonies were subcultured, and genomic DNA was extracted for PCR-based identification of nosZ clade I and II genes using specific primers. Two isolates showing strong N2O reduction capacity were selected for further study (see Supplementary Material for full protocols).
Experimental Conditions: The effects of environmental factors on growth and N2O reduction were assessed under varying conditions of pH (4, 6, 7, 8, 10), oxygen (0%, 0.1%, 1%, 5%, 21% v/v), nitrate (0, 5, 50, 100, 500 mg N L-¹), and carbon source (sodium acetate, glycerol, disodium succinate, glucose, and hydroxyethyl cellulose). Each treatment was replicated three times.
Culture Medium and Setup: Tryptic Soy Broth (TSB; Land Bridge Co., China) was prepared by dissolving 30 g in 1 L of distilled water. The formulation included: tryptic peptone (17.0 g), plant-derived peptone (3.0 g), NaCl (5.0 g), K2HPO4 (2.5 g), and glucose (2.5 g). After sterilization, 20 mL of TSB was added to 500 mL Schott bottles. The pH was adjusted to one of five levels (4–10), and the medium was inoculated with 1% (v/v) bacterial culture and 1.5 mL of 99.9% N2O. Bottles were incubated at 30°C and 180 rpm for 10 hours.
After incubation, 30 mL of headspace gas was withdrawn using a syringe and transferred to pre-evacuated vials for N2O measurement via gas chromatography (Agilent 7890A, USA). Optical density at 600 nm (OD600) was measured immediately afterward.
In O2 and NO3- experiments, pH was held constant at 7, while O2 or nitrate concentrations were varied. For carbon source treatments, an inorganic salt base medium (carbon-free) was used, supplemented with a carbon source providing 50 mg of carbon, and pH was maintained at 7.
2.3 Soil microcosm experiment
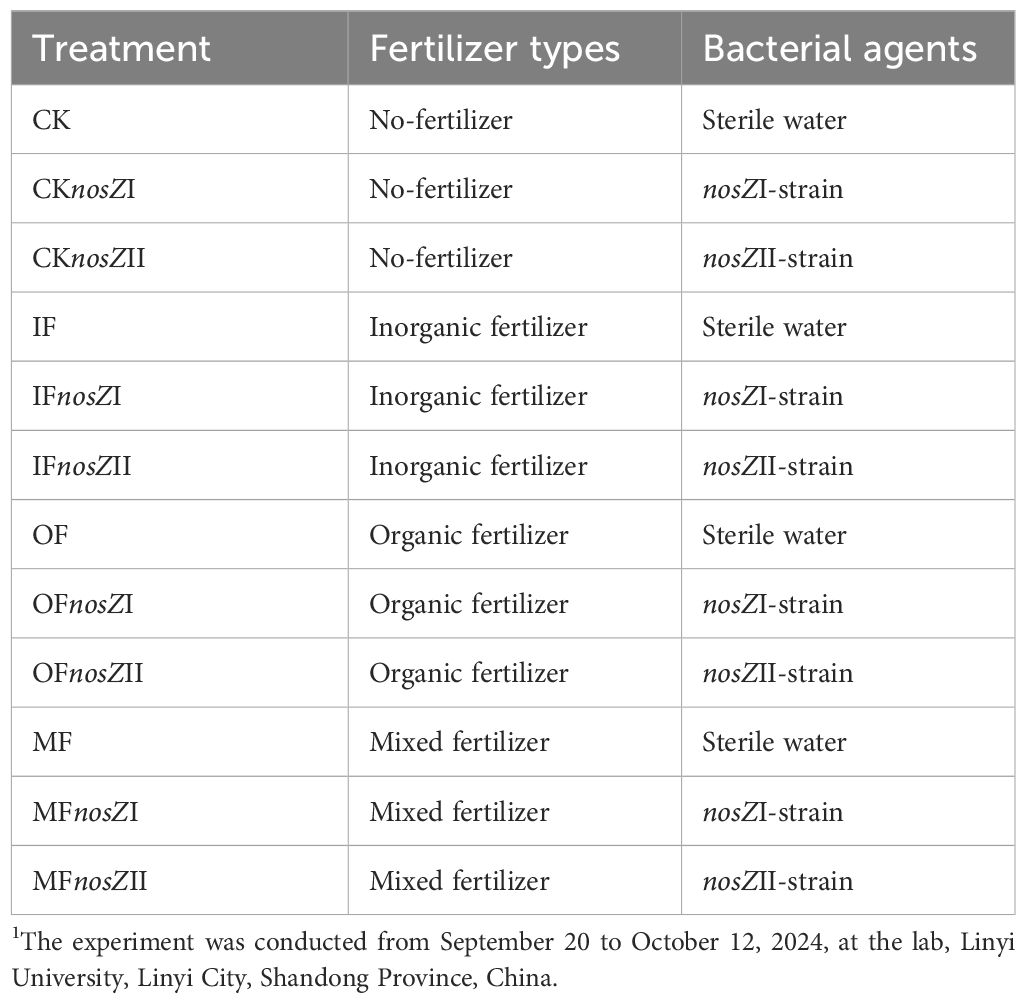
The microcosm experiment consisted of 12 treatments (described in Table 2), each with three replicates and 12 sampling intervals for N2O analysis. Treatments included: CK (no fertilizer or bacteria), IF (inorganic fertilizer), OF (organic fertilizer), and MF (70% organic + 30% inorganic fertilizer). Fertilizer application was standardized at 2.5 mg N g-¹ dry soil. The inorganic fertilizer was a compound formula (15% N–P2O5–K2O; Stanley Co., China), and the organic fertilizer was sheep manure compost (1.72% N, 2.24% P2O5, 2.42% K2O; JiYa Co., China). Treatments suffixed with ‘nosZI’ or ‘nosZII’ indicate inoculation with the respective strain at a concentration of 109 CFU per bottle (based on standard plate count method).
The following is the detailed procedure of the plate counting method: 1 mL of bacterial suspension was inoculated into 100 mL of TSB liquid medium and incubated at 30 ± 0.5°C with shaking (180 rpm) for 12 h. Subsequently, serial dilutions were performed to achieve a 10-6 gradient. Aliquots (10 μL) of 10-4, 10-5, and 10-6 dilutions were spread onto nitrogen-free agar plates and incubated at 30 ± 0.5°C for 24 h. Colonies within the valid range (30–300 CFU/plate) were enumerated, and the original cell concentration (N, CFU/mL) was calculated using the formula:
Where: C is the colony count (CFU); D is the dilution factor (10-n); V is the inoculation volume (mL).
For the nosZI strain, the valid dilution (colony count C=203, dilution factor D=10-5, inoculation volume V=0.01 mL) yielded a calculated cell concentration of 2.03×108 CFU/mL. Similarly, for the nosZII strain (C=246, D=10-5, V=0.01 mL), the concentration was determined as 2.46×108 CFU/mL. To achieve the target inoculum density of 1×109 CFU/mL, the required volumes were adjusted to 5 mL for nosZI and 4 mL for nosZII, ensuring standardized inoculation across experimental replicates.
Microcosm Setup: A total of 150 g of soil was placed into 500 mL Schott bottles. Fertilizer was thoroughly mixed into the soil, and 50 g of untreated soil was layered on top. Bacterial cultures and sterile water (total 50 mL) were sprayed onto the surface to adjust moisture to 20%. Control treatments received sterile water only. Bottles were incubated at 26°C in darkness, with soil moisture maintained at 20% via periodic weighing and replenishment with sterile water. The sterile water used was deionized water, with a pH of 7 and a resistivity of 18.2 MΩ·cm.
2.4 Gas sampling and N2O measurement
Gas samples were collected at 6 h, and 1, 2, 3, 4, 5, 7, 9, 12, 15, 18, and 21 days post-treatment. Prior to each sampling, bottles were flushed with ambient air and sealed with gas-tight lids equipped with a three-way valve. At each interval, 30 mL of headspace gas was sampled and stored in pre-evacuated vials. During each sampling, gas was collected at 0, 1, 2, and 3 hours to determine emission rates. N2O concentrations were measured using gas chromatography (Agilent 7890A, USA).
The formula for calculating the soil N2O emission rate (F) is as follows:
Where: represents the density of N2O-N under standard conditions (1.25 g L-¹); represents the rate of change in N2O concentration in the bottle (ppm h-¹); T is the incubation temperature (C); V is the volume of the incubation bottle (m³); m is the dry weight of the incubated soil (kg).
The formula for calculating the cumulative soil N2O emissions (E) is as follows:
Where: is the average emission flux of the gas collected during the i+1 sampling (mg g-¹ h-¹); is the average emission flux of the gas collected during the i sampling (mg g-¹ h-¹); is the time interval between the i+1 and i gas sampling events (days).
2.5 Statistical analysis
In the pure culture experiments, one-way ANOVA followed by Duncan’s multiple range test was used to assess differences in OD600 and N2O reduction efficiency across treatments. Homogeneity of variance was tested using Levene’s test; data were log-transformed if necessary. Statistical significance was set at p < 0.05. Pearson correlation analysis was used to explore relationships between environmental factors, bacterial growth, and N2O reduction, with p < 0.05 considered significant.
In the soil microcosm experiment, two-way ANOVA was performed to assess interactions between fertilizer type and nosZ genotype on N2O emissions. Repeated measures ANOVA, combined with the least significant difference (LSD) test, was used to analyze temporal trends. One-way ANOVA with Duncan’s test was used to compare emissions among treatments at each time point. Homogeneity of variance was again checked with Levene’s test, and log transformations were applied as needed.
All statistical analyses were performed using SPSS v21.0 (SPSS Inc., Chicago, IL, USA). Figures were generated using Origin 2022 (OriginLab Corp., Northampton, MA, USA).
3 Results
3.1 Growth and N2O-reducing capacity of isolates under different environmental conditions
The pure culture experiments demonstrated that environmental factors significantly influenced the growth and N2O-reducing activity of the nosZI and nosZII strains (Figure 1). One-way ANOVA revealed that the nosZI strain exhibited optimal growth under acidic conditions (pH 4 and 6), while its highest N2O reduction efficiency was observed at neutral pH (pH 7), which differed significantly from other pH levels (p < 0.05). In contrast, the nosZII strain thrived under alkaline (pH 8 and 10) and mildly acidic (pH 6) conditions, achieving peak N2O reduction at pH 6 and 8. Bivariate correlation analysis showed a significant negative correlation between pH and the growth of the nosZI strain (r = -0.88, p < 0.01), and a significant positive correlation for the nosZII strain (r = 0.78, p < 0.01).
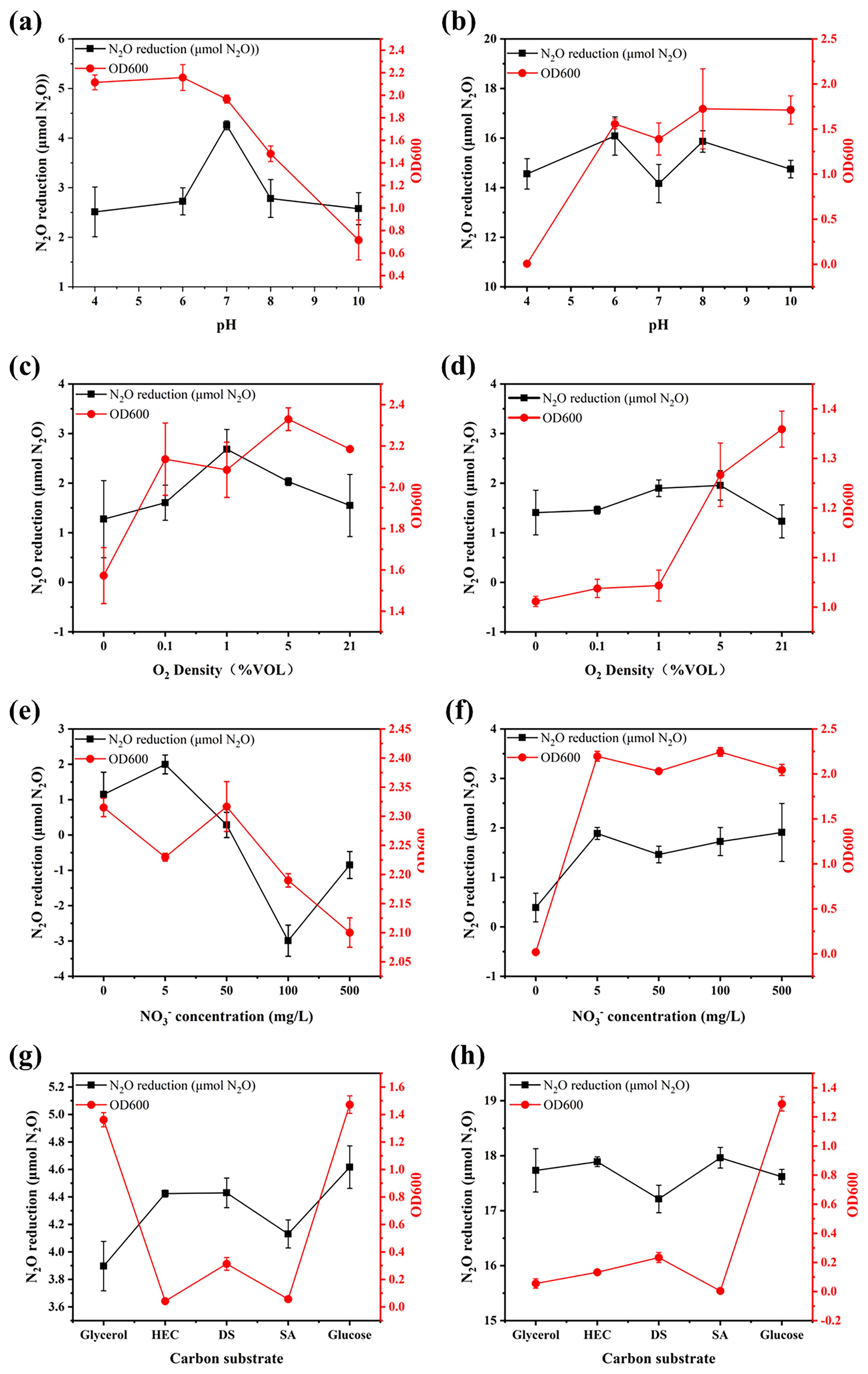
Figure 1. The growth (OD600) and N2O-reducing activity of nosZI (a, c, e, g) and nosZII (b, d, f, h) strains responding to environmental factors through pure culture. Environmental factors were pH (4, 6, 7, 8, 10), O2 concentrations (0, 0.1%, 1%, 5%, 21% v/v), NO3- concentrations (0, 5, 50, 100, 500 mg/L), and carbon sources (Sodium acetate (SA), Glycerol, Disodium succinate (DS), Glucose, hydroxyethyl cellulose (HEC)). The data are expressed as the mean ± SEM for each treatment (n = 3).
Both strains grew well under fully aerobic conditions (21% O2). However, the nosZI strain achieved its highest N2O reduction at 5% O2, while the nosZII strain exhibited optimal N2O reduction under low-oxygen conditions (1% and 5% O2). A significant positive correlation between oxygen concentration and growth was observed only for the nosZII strain (r = 0.84, p < 0.01).
Nitrate concentration had contrasting effects. The nosZI strain grew best under low or absent nitrate, with maximum N2O reduction at 5 mg N L-¹. Higher nitrate levels led to reduced reduction efficiency and even net N2O emissions. Its growth was significantly negatively correlated with nitrate concentration (r = -0.75, p < 0.01), while N2O reduction was positively correlated with growth (r = 0.43, p < 0.05). Conversely, the nosZII strain maintained stable growth across all nitrate levels, except under nitrate deficiency, where growth declined sharply. A strong positive correlation was observed between growth and N2O reduction for the nosZII strain (r = 0.73, p < 0.01).
In terms of carbon sources, both strains exhibited optimal growth with glucose. The nosZI strain also displayed its highest N2O reduction with glucose. However, the nosZII strain achieved greater N2O reduction efficiency when sodium acetate (SA) or hydroxyethyl cellulose (HEC) served as the carbon source.
3.2 Soil N2O emissions with fertilization types and nosZ-clade type strains
N2O emission rates varied across treatments and over time (Figure 2). In soils treated with inorganic fertilizer (IF), emissions remained low for the first 9 days and then increased significantly after day 12, with no peak reached within the 21-day observation period. Soils treated with organic fertilizer (OF) exhibited peak emissions as early as day 4. In contrast, soils treated with a mixed fertilizer (MF) showed steadily increasing emissions, peaking on day 12. Repeated measures ANOVA showed that treatments inoculated with the nosZII strain exhibited significantly lower N2O emission rates than their non-inoculated controls throughout the experiment (p < 0.01). However, this suppression effect was significant only in the IFnosZII and MFnosZII treatments. For nosZI-amended soils, all treatments except IFnosZI showed significantly altered emission rates compared to controls (p < 0.01), with emission suppression observed only in the MFnosZI treatment. One-way ANOVA indicated that IFnosZII (inorganic fertilizer + nosZII) reduced N2O emissions by 55–91% during days 4–8 compared to IF alone (p < 0.05). The MFnosZII treatment also significantly reduced emissions by 1.3–51% between days 3 and 21 (p < 0.05), although a slight increase in emissions was observed during the early incubation phase. In the MFnosZI treatment, N2O emissions were reduced by 27–66% on days 1, 3, 15, 18, and 21 compared to MF (p < 0.05). At other time points, nosZI either increased emissions or had no significant effect.

Figure 2. Soil N2O flux under different fertilization types inoculated with the nosZI (a) or nosZII (b) strain. OF represents organic fertilizer treatment; IF represents inorganic fertilizer treatment; MF represents mixed inorganic-organic fertilizer treatment; The suffix with nosZI or nosZII represent nosZI or nosZII strains adding treatments. CK represents control treatment without fertilization and strain. The data are expressed as the mean ± SEM for each treatment (n = 3).
Cumulative N2O emissions across treatments are shown in Figure 3. One-way ANOVA revealed that fertilization type significantly influenced cumulative emissions (p < 0.05). Repeated measures ANOVA further confirmed that inoculation with nosZI or nosZII led to significant differences in cumulative emissions compared to uninoculated controls (p < 0.01), although a suppression effect was observed only in the IFnosZII and MFnosZII treatments. Cumulative emissions from IF, OF, and MF treatments were 25.32, 54.96, and 1431 mg kg-¹, respectively—representing 6-, 12-, and 312-fold increases relative to the CK control (4.59 mg kg-¹). In terms of reduction efficiency: IFnosZII reduced cumulative N2O emissions by 72% (p < 0.01), MFnosZII by 10% (p < 0.01), and MFnosZI by 3.4% (p < 0.01), relative to their respective fertilized controls. All other inoculated treatments resulted in increased cumulative emissions. Specifically: OFnosZII and OFnosZI increased emissions by 20% and 10%, respectively, over the OF treatment. IFnosZI increased emissions by 10% compared to IF. Two-way ANOVA revealed that fertilizer type, nosZ clade, and their interaction had significant effects on cumulative N2O emissions (p < 0.01). Notably, in the absence of fertilizer, inoculation with nosZII or nosZI resulted in 80% and 89% higher emissions, respectively, compared to the CK control.
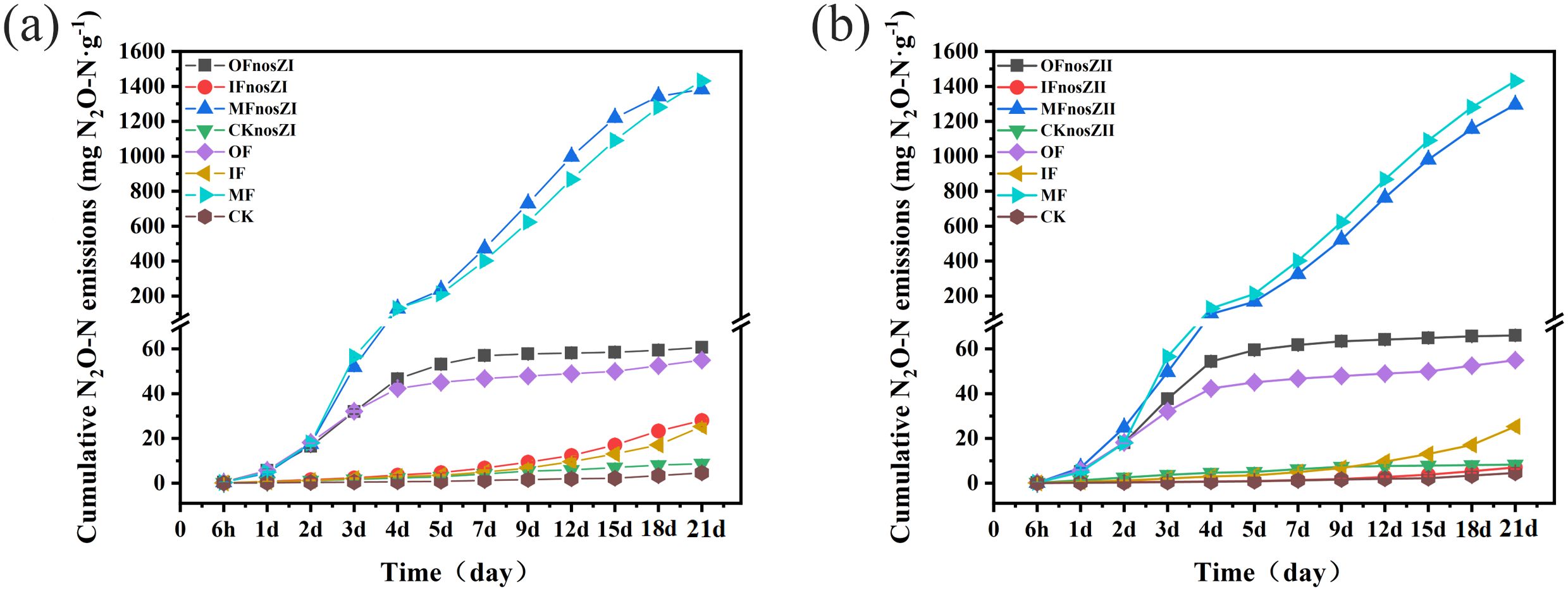
Figure 3. Cumulative N2O emissions under different fertilization types inoculated with the nosZI (a) or nosZII (b) strain. OF represents organic fertilizer treatment; IF represents inorganic fertilizer treatment; MF represents mixed inorganic-organic fertilizer treatment; The suffix with nosZI or nosZII represent nosZI or nosZII strains adding treatments. CK represents control treatment without fertilization and strain. The data are expressed as the mean ± SEM for each treatment (n = 3).
4 Discussion
4.1 Responses of selected isolates to pH, O2, NO3-, and carbon source
Our findings demonstrate that nosZII strains exhibit greater resilience in both growth and N2O-reducing activity under varying environmental conditions compared to nosZI strains. Key environmental factors affecting nosZ-containing bacteria include pH, oxygen concentration, nitrate availability, and carbon source type.
Among these, pH is widely recognized as one of the most critical factors influencing microbial activity. Extreme pH conditions typically inhibit microbial metabolism, with most microbial inoculants favoring neutral to mildly acidic or alkaline environments. In our pure culture experiments, nosZII strain growth was positively correlated with pH across a broad range (pH 4–10), while nosZI strain growth showed a significant negative correlation. This indicates that nosZII strains prefer neutral to alkaline conditions, whereas nosZI strains perform better in neutral to acidic environments. Although nosZ enzyme activity is generally sensitive to both low pH and high salinity, previous studies have shown that certain strains—such as Rhodanobacter and Cloacibacterium—retain N2O reduction capability in low-pH environments (Lycus et al., 2017; Wang et al., 2023). Consistent with these findings, our nosZII strain maintained high N2O-reduction activity across the entire tested pH range, suggesting it could be effective in both acidic and alkaline soils. In contrast, the nosZI strain showed high activity only at neutral pH, with reduced performance under other conditions. The broader pH adaptability of the nosZII strain may be due to enhanced enzyme efficiency and more robust stress response mechanisms.
Although both strains grew optimally under aerobic conditions, they were capable of reducing N2O under all tested O2 concentrations (0–21%), indicating a low dependence on oxygen. This trait suggests their potential effectiveness for field-level N2O mitigation under fluctuating oxygen conditions. Membrane-bound denitrification reductases are typically active under anaerobic conditions, while periplasmic reductases can function under both aerobic and anaerobic environments (Bell et al., 1990; Chen and Strous, 2013; Duan et al., 2015; Yang et al., 2020). Our findings suggest that both isolates may possess periplasmic nitrous oxide reductases, which would explain their flexibility under varying oxygen levels (Liu et al., 2023).
The two strains also exhibited contrasting responses to nitrate availability. The nosZI strain performed best under low or absent NO3-, with high concentrations (100–500 mg/L) inhibiting its activity and even resulting in net N2O emissions. This may indicate an N2O/N2 production ratio greater than 1 due to partial denitrification or enzyme inhibition (Senbayram et al., 2019). In contrast, the nosZII strain maintained stable growth and high N2O reduction efficiency across all NO3- levels, including high concentrations, suggesting it is better adapted for nitrate-rich environments. These differences may be attributed to distinct enzymatic kinetics and substrate affinities between nosZI and nosZII nitrous oxide reductases.
Both strains showed optimal growth with glucose as a carbon source. However, nosZII displayed the highest N2O reduction efficiency with sodium acetate, while nosZI achieved peak efficiency with glucose and moderate efficiency with glycerol. The availability of different electron donors influences the structure of N2O-reducing microbial communities, often driving niche differentiation (Maheshwari et al., 2023). In our study, nosZII demonstrated broader carbon source adaptability, maintaining consistently high N2O reduction across all tested sources, consistent with its widespread occurrence in diverse environments. This may reflect its capacity to metabolize a wider range of organic substrates, enhancing its survival and function under field conditions.
4.2 Soil N2O emissions and functional performance of selected isolates
In the soil microcosm experiment, the MF treatment (mixed fertilizer) produced significantly higher cumulative N2O emissions than the IF (inorganic), OF (organic), or CK (control) treatments (p < 0.01), aligning with previous studies (Feng et al., 2019; Sompouviset et al., 2023). This effect likely stems from the abundant labile carbon in composted sheep manure, which stimulates microbial activity, and the high nitrogen content in chemical fertilizers, both of which enhance denitrification and N2O emissions (Jassal et al., 2011; Senbayram et al., 2012; Montes et al., 2013; Zhou et al., 2017; Shakoor et al., 2021).
While some studies report N2O mitigation effects from organic fertilizers (Xie et al., 2024), our results showed that organic amendments led to higher emissions than inorganic ones, consistent with field trials in Northeast China (Liu et al., 2023). This discrepancy may stem from differences in soil properties, organic matter composition, microbial communities, and experimental conditions. For example, the C/N ratio of organic material is critical; cattle and pig manures often reduce N2O emissions, whereas rapeseed cake increases them (Zou et al., 2003; Zhang et al., 2020b).
Across treatments, the nosZII strain consistently outperformed the nosZI strain in terms of N2O reduction potential. Although both strains contained the nosZ and nirK genes, neither harbored nirS, indicating they can denitrify via the NO2- → N2O → N2 pathway. However, in some inoculated treatments, N2O emissions increased, suggesting that nosZ-containing bacteria—particularly clade I—may contribute more to N2O production than reduction, depending on environmental context.
For example, in the IF and MF treatments inoculated with nosZII, significant reductions in N2O emissions were observed, whereas in OF and CK treatments, emissions increased. These outcomes suggest that nosZII strains are more effective in soils with high inorganic nitrogen, where nitrate levels may enhance their denitrifying capacity. Conversely, in low nitrate or high organic C/N environments, microbial N2O production may exceed reduction, resulting in net emissions. This aligns with our pure culture findings: nosZI strain activity declined under increasing nitrate, while nosZII activity remained high.
4.3 Practical implications, limitations and future perspectives
This study demonstrates the potential of II-containing bacteria for mitigating N2O emissions, especially in soils with high inorganic nitrogen content. The development of bio-organic fertilizers enriched with nosZII strains represents a promising approach to reduce greenhouse gas emissions in agriculture, contributing to both climate mitigation and sustainable crop production.
Our study has several limitations. First, we used only two bacterial strains under a limited range of environmental conditions. Future research should expand the diversity of nosZ-containing isolates, examining their physiological traits and N2O-reducing capacities under field-relevant and extreme conditions. Second, future efforts should prioritize the isolation of strains containing nosZ independent of nir genes, to avoid potential N2O accumulation during partial denitrification. Such strains could serve as ideal candidates for bio-organic fertilizers aimed specifically at mitigating N2O emissions.
Based on our findings, we propose the following future directions: (1) Investigate the genetic and enzymatic differences between nosZI and nosZII strains that explain their divergent environmental responses. (2) Conduct field-scale experiments using diverse nosZ-containing isolates to evaluate their effectiveness in real agricultural systems. (3) Explore the development of bioformulations combining nosZII strains with other beneficial microbes or soil amendments to enhance N2O mitigation while supporting soil health.
5 Conclusions
This study underscores the important role of nosZ-containing microbial isolates in regulating soil N2O emissions under different fertilization regimes. Our results show that the application of mixed organic and inorganic fertilizers significantly increases N2O emissions compared to single-source fertilizers, likely due to enhanced microbial activity driven by combined nutrient availability. Among the tested strains, the nosZII isolate exhibited superior N2O-reducing capacity compared to the nosZI isolate, particularly under conditions with elevated inorganic nitrogen. This highlights its potential as a targeted microbial agent for N2O mitigation in intensively managed agricultural soils. Environmental factors significantly influenced the performance of both strains. However, the nosZII strain demonstrated greater resilience, showing broader pH tolerance, higher nitrate and oxygen tolerance, and greater adaptability to diverse carbon sources. While these findings offer promising insights for the development of microbial biocontrol strategies, the complexity of soil N2O dynamics warrants further investigation. Future research should prioritize the isolation of high-efficiency N2O-reducing strains that possess the nosZ gene independently of other denitrification genes (e.g., nirS, nirK), to minimize potential N2O production and enhance the reliability of microbial inoculants. Integrating these selected microbial strains into bio-organic fertilizers could serve as a practical and scalable solution for reducing N2O emissions from agriculture, contributing to both climate change mitigation and the advancement of sustainable agricultural practices.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
ZX: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Software, Supervision, Writing – review & editing. HL: Investigation, Visualization, Writing – original draft. QL: Visualization, Writing – original draft. LS: Data curation, Writing – review & editing. HX: Data curation, Writing – review & editing. XH: Investigation, Writing – review & editing. KZ: Investigation, Writing – review & editing. ML: Investigation, Writing – review & editing. BL: Formal Analysis, Writing – review & editing. WJ: Formal Analysis, Writing – review & editing. JG: Formal Analysis, Writing – review & editing. ZC: Resources, Writing – review & editing. LH: Funding acquisition, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the National Natural Science Foundation of China (No. 31770531, 31400427).
Acknowledgments
We wish to thank Shuang Kong for her help in the laboratory.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1537010/full#supplementary-material
Supplementary Table 1 | The amplification primers of PCR. Isolation and identification of nosZI or nosZII clade type strains.
References
Avşar, C. and Aras, E. S. (2020). Quantification of denitrifier genes population size and its relationship with environmental factors. Arch. Microbiol. 202, 1181–1192. doi: 10.1007/s00203-020-01826-x
Bahram, M., Espenberg, M., Pärn, J., Lehtovirta-Morley, L., Anslan, S., Kasak, K., et al. (2022). Structure and function of the soil microbiome underlying N2O emissions from global wetlands. Nat. Commun. 13, 1430. doi: 10.1038/s41467-022-29161-3
Bell, L. C., Richardson, D. J., and Ferguson, S. J. (1990). Periplasmic and membrane-bound respiratory nitrate reductases in Thiosphaera pantotropha. The periplasmic enzyme catalyzes the first step in aerobic denitrification. FEBS Lett. 265, 85–87. doi: 10.1016/0014-5793(90)80889-q
Carneiro, J., Cardenas, L., Hatch, D., Trindade, H., Scholefield, D., Clegg, C., et al. (2010). Effect of the nitrification inhibitor dicyandiamide on microbial communities and N2O from an arable soil fertilized with ammonium sulphate. Environ. Chem. Lett. 8, 237–246. doi: 10.1007/s10311-009-0212-3
Chen, J. and Strous, M. (2013). Denitrification and aerobic respiration, hybrid electron transport chains and co-evolution. Biochim. Biophys. Acta 1827, 136–144. doi: 10.1016/j.bbabio.2012.10.002
Conthe, M., Wittorf, L., Kuenen, J. G., Kleerebezem, R., Van Loosdrecht, M. C., and Hallin, S. (2018). Life on N2O: deciphering the ecophysiology of N2O respiring bacterial communities in a continuous culture. ISME J. 12, 1142–1153. doi: 10.1038/s41396-018-0063-7
Desloover, J., Roobroeck, D., Heylen, K., Puig, S., Boeckx, P., Verstraete, W., et al. (2014). Pathway of nitrous oxide consumption in isolated Pseudomonas stutzeri strains under anoxic and oxic conditions. Environ. Microbiol. 16, 3143–3152. doi: 10.1111/1462-2920.12404
Domeignoz-Horta, L. A., Putz, M., Spor, A., Bru, D., Breuil, M.-C., Hallin, S., et al. (2016). Non-denitrifying nitrous oxide-reducing bacteria-an effective N2O sink in soil. Soil Biol. Bioch. 103, 376–379. doi: 10.1016/j.soilbio.2016.09.010
Duan, J., Fang, H., Su, B., Chen, J., and Lin, J. (2015). Characterization of a halophilic heterotrophic nitrification–aerobic denitrification bacterium and its application on treatment of saline wastewater. Bioresour. Technol. 179, 421–428. doi: 10.1016/j.biortech.2014.12.057
Feng, X., Gao, H., Lal, R., Zhu, P., Peng, C., Deng, A., et al. (2019). Nitrous oxide emission, global warming potential, and denitrifier abundances as affected by long-term fertilization on Mollisols of Northeastern China. Arch. Agron. Soil Sci. 65, 1831–1844. doi: 10.1080/03650340.2019.1578959
Graf, D. R., Jones, C. M., and Hallin, S. (2014). Intergenomic comparisons highlight modularity of the denitrification pathway and underpin the importance of community structure for N2O emissions. PloS One 9, e114118. doi: 10.1371/journal.pone.0114118
Hallin, S., Philippot, L., Löffler, F. E., Sanford, R. A., and Jones, C. M. (2018). Genomics and ecology of novel N2O-Reducing Microorganisms. Trends Microbiol. 26, 43–55. doi: 10.1016/j.tim.2017.07.003
Jassal, R. S., Black, T. A., Roy, R., and Ethier, G. (2011). Effect of nitrogen fertilization on soil CH4 and N2O fluxes, and soil and bole respiration. Geoderma 162, 182–186. doi: 10.1016/j.geoderma.2011.02.002
Jones, C. M., Graf, D. R., Bru, D., Philippot, L., and Hallin, S. (2013). The unaccounted yet abundant nitrous oxide-reducing microbial community: a potential nitrous oxide sink. ISME J. 7, 417–426. doi: 10.1038/ismej.2012.125
Liu, B., Mørkved, P. T., Frostegård, Å., and Bakken, L. R. (2010). Denitrification gene pools, transcription and kinetics of NO, N2O and N2 production as affected by soil pH. FEMS Microbiol. Ecol. 72, 407–417. doi: 10.1111/j.1574-6941.2010.00856.x
Liu, C., Sheng, R., Chen, X., Liu, Y., and Wei, W. (2023). Comparison of N2O-reducing abilities and genome features of two nosZ-containing denitrifying bacteria, Pseudomonas veronii DM15 and Pseudomonas frederiksbergensis DM22. J. Appl. Microbiol. 134, lxac011. doi: 10.1093/jambio/lxac011
Lu, H. and Chandran, K. (2010). Factors promoting emissions of nitrous oxide and nitric oxide from denitrifying sequencing batch reactors operated with methanol and ethanol as electron donors. Biotechnol. Bioeng. 106, 390–398. doi: 10.1002/bit.22704
Lycus, P., Lovise Bøthun, K., Bergaust, L., Peele Shapleigh, J., Reier Bakken, L., and Frostegård, Å. (2017). Phenotypic and genotypic richness of denitrifiers revealed by a novel isolation strategy. ISME J. 11, 2219–2232. doi: 10.1038/ismej.2017.82
Maheshwari, A., Jones, C. M., Tiemann, M., and Hallin, S. (2023). Carbon substrate selects for different lineages of N2O reducing communities in soils under anoxic conditions. Soil Biol. Bioch. 177, 108909. doi: 10.1016/j.soilbio.2022.108909
Montes, F., Meinen, R., Dell, C., Rotz, A., Hristov, A. N., Oh, J., et al. (2013). SPECIAL TOPICS—Mitigation of methane and nitrous oxide emissions from animal operations: II. A review of manure management mitigation options. J. Anim. Sci. 91, 5070–5094. doi: 10.2527/jas.2013-6584
Orellana, L., Rodriguez-R, L., Higgins, S., Chee-Sanford, J., Sanford, R., Ritalahti, K., et al. (2014). Detecting nitrous oxide reductase (nosZ) genes in soil metagenomes: method development and implications for the nitrogen cycle. mBio 5, 01193–01114. doi: 10.1128/mBio.01193-14
Park, D., Kim, H., and Yoon, S. (2017). Nitrous oxide reduction by an obligate aerobic bacterium, Gemmatimonas aurantiaca strain T-27. Appl. Environ. Microbiol. 83, e00502–e00517. doi: 10.1128/AEM.00502-17
Qi, C., Zhou, Y., Suenaga, T., Oba, K., Lu, J., Wang, G., et al. (2022). Organic carbon determines nitrous oxide consumption activity of clade I and II nosZ bacteria: Genomic and biokinetic insights. Water Res. 209, 117910. doi: 10.1016/j.watres.2021.117910
Ravishankara, A., Daniel, J. S., and Portmann, R. W. (2009). Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Sci 326, 123–125. doi: 10.1126/science.1176985
Sanford, R. A., Wagner, D. D., Wu, Q., Chee-Sanford, J. C., Thomas, S. H., Cruz-García, C., et al. (2012). Unexpected nondenitrifier nitrous oxide reductase gene diversity and abundance in soils. Proc. Natl. Acad. Sci. U.S.A. 109, 19709–19714. doi: 10.1073/pnas.1211238109
Senbayram, M., Budai, A., Bol, R., Chadwick, D., Marton, L., Gündogan, R., et al. (2019). Soil NO3– level and O2 availability are key factors in controlling N2O reduction to N2 following long-term liming of an acidic sandy soil. Soil Biol. Bioch. 132, 165–173. doi: 10.1016/j.soilbio.2019.02.009
Senbayram, M., Chen, R., Budai, A., Bakken, L., and Dittert, K. (2012). N2O emission and the N2O/(N2O+ N2) product ratio of denitrification as controlled by available carbon substrates and nitrate concentrations. Agr. Ecosyst. Environ. 147, 4–12. doi: 10.1016/j.agee.2011.06.022
Shaaban, M., Wang, X.-L., Song, P., Hou, X., Wu, Y., and Hu, R. (2023). Ascription of nosZ gene, pH and copper for mitigating N2O emissions in acidic soils. Environ. Res. 237, 117059. doi: 10.1016/j.envres.2023.117059
Shakoor, A., Shakoor, S., Rehman, A., Ashraf, F., Abdullah, M., Shahzad, S. M., et al. (2021). Effect of animal manure, crop type, climate zone, and soil attributes on greenhouse gas emissions from agricultural soils—A global meta-analysis. J. Clean. Prod. 278, 124019. doi: 10.1016/j.jclepro.2020.124019
Sompouviset, T., Ma, Y., Sukkaew, E., Zheng, Z., Zhang, A., Zheng, W., et al. (2023). The effects of plastic mulching combined with different fertilizer applications on greenhouse gas emissions and intensity, and apple yield in Northwestern China. Agric 13, 1211. doi: 10.3390/agriculture13061211
Suenaga, T., Hori, T., Riya, S., Hosomi, M., Smets, B. F., and Terada, A. (2019). Enrichment, isolation, and characterization of high-affinity N2O-reducing bacteria in a gas-permeable membrane reactor. Environ. Sci. Technol. 53, 12101–12112. doi: 10.1021/acs.est.9b02237
Suenaga, T., Riya, S., Hosomi, M., and Terada, A. (2018). Biokinetic characterization and activities of N2O-reducing bacteria in response to various oxygen levels. Front. Microbiol. 9. doi: 10.3389/fmicb.2018.00697
Tian, H., Xu, R., Canadell, J. G., Thompson, R. L., Winiwarter, W., Suntharalingam, P., et al. (2020). A comprehensive quantification of global nitrous oxide sources and sinks. Nature 586, 248–256. doi: 10.1038/s41586-020-2780-0
Wang, Y., Xu, W., Cong, Q., Wang, Y., Wang, W., Zhang, W., et al. (2023). Responses of CH4, N2O, and NH3 emissions to different slurry pH values of 5.5–10.0: Characteristics and mechanisms. Environ. Res. 234, 116613. doi: 10.1016/j.envres.2023.116613
World Meteorological Organization (2022). SAOD. Available online at: https://www.csl.noaa.gov/assessments/ozone/2022 (Accessed March 15, 2025).
Xie, L., Li, L., Xie, J., Wang, J., Mumtaz, M. Z., Effah, Z., et al. (2024). Optimal substitution of inorganic fertilizer with organic amendment sustains rainfed maize production and decreases soil N2O emissions by modifying denitrifying bacterial communities in Northern China. Eur. J. Agron. 160, 127287. doi: 10.1016/j.eja.2024.127287
Yang, J., Feng, L., Pi, S., Cui, D., Ma, F., Zhao, H.-P., et al. (2020). A critical review of aerobic denitrification: insights into the intracellular electron transfer. Sci. Total Environ. 731, 139080. doi: 10.1016/j.scitotenv.2020.139080
Zhang, X., Fang, Q., Zhang, T., Ma, W., Velthof, G. L., Hou, Y., et al. (2020b). Benefits and trade-offs of replacing synthetic fertilizers by animal manures in crop production in China: A meta-analysis. Global Change Biol. 26, 888–900. doi: 10.1111/gcb.14826
Zhang, Q., Xiao, J., Xue, J., and Zhang, L. (2020a). Quantifying the effects of biochar application on greenhouse gas emissions from agricultural soils: a global meta-analysis. Sustainability 12, 3436. doi: 10.3390/su12083436
Zhou, M., Zhu, B., Wang, S., Zhu, X., Vereecken, H., and Brüggemann, N. (2017). Stimulation of N2O emission by manure application to agricultural soils may largely offset carbon benefits: a global meta-analysis. Glob. Chang Biol. 23, 4068–4083. doi: 10.1111/gcb.13648
Keywords: nitrous oxide emissions, nosZ clade I, nosZ clade II, nitrous oxide reduction, environmental factors, fertilizer types
Citation: Li H, Luo Q, Sun L, Xu H, Hao X, Zhu K, Li M, Li B, Jiao W, Geng J, Chen Z, Huang L and Xia Z (2025) Comparative study of nosZI and nosZII clade isolates: insights into their responses to environmental variables and soil fertilization types. Front. Plant Sci. 16:1537010. doi: 10.3389/fpls.2025.1537010
Received: 29 November 2024; Accepted: 20 May 2025;
Published: 06 June 2025.
Edited by:
Virgilio Gavicho Uarrota, Universidad de O’Higgins, ChileReviewed by:
Set Pérez Fuentealba, Universidad de O’Higgins, ChileLuiz Peruch, Empresa de Pesquisa Agropecuária e Extensão Rural de Santa Catarina, Brazil
Copyright © 2025 Li, Luo, Sun, Xu, Hao, Zhu, Li, Li, Jiao, Geng, Chen, Huang and Xia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zongwei Xia, eGlhem9uZ3dlaUBseXUuZWR1LmNu; Lihua Huang, aHVhbmdsaWh1YUBseXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Haoran Li1†
Haoran Li1† Kai Zhu
Kai Zhu Zongwei Xia
Zongwei Xia