- 1Laboratory of Natural Products Pesticides, College of Plant Protection, Southwest University, Chongqing, China
- 2Yibin Tobacco Company of Sichuan Province, Yibin, China
- 3College of Agronomy and Biotechnology, Southwest University, Chongqing, China
- 4Zunyi Branch Company of Guizhou Tobacco Company, Zunyi, China
Plants will display typical dehydration and wilting symptoms after Ralstonia solanacearum infection. Many studies have shown that abscisic acid (ABA) has been implicated in wilting, but the role of ABA after R. solanacearum infection remains largely unexplored. The plant water potential and endogenous ABA content of tobacco plants were investigated after R. solanacearum infection, and we assessed the preliminary mechanisms and control effect of exogenous ABA on tobacco bacterial wilt. Here we show that R. solanacearum can reduce leaf water content (LWC) and leaf water potential (Ψleaf) and promote the accumulation of ABA on leaves. Notably, foliar spraying 0.78 mg/L ABA could alleviate the wilting by increasing Ψleaf and decreasing the stomatal size, stomatal conductance (Gs), and transpiration rate (Tr). Furthermore, 0.78 mg/L ABA application promoted plant growth, reduced the colonization of R. solanacearum, increased the activities of defense enzymes, upregulated the expression of JA/ET-related and ROS-related genes, and suppressed the expression of SA-related gene. Moreover, 0.78 mg/L ABA could reduce the incidence of tobacco bacterial wilt, with the control efficiency reaching up to 54.94% at 11 dpi, significantly higher than that of benzothiazole (BTH) with 19.33%. Our findings provided a new result for exogenous ABA controlling tobacco bacterial wilt by reducing water loss and enhancing plant resistance.
Highlights
● R. solanacearum promoted plant water loss.
● Plants accumulated ABA to reduce water loss caused by R. solanacearum infection.
● Foliar spraying 0.78 mg/L ABA promoted plant growth and enhanced the activity of defense enzymes.
● Spraying 0.78 mg/L ABA upregulated the expression of JA/ET and ROS-related genes.
● Spraying 0.78 mg/L ABA delayed the progression of tobacco bacterial wilt.
1 Introduction
Plant diseases are important determinants of food yield losses and quality reduction, which threaten food security and social stability (Savary et al., 2019). The second most destructive bacterial plant pathogen R. solanacearum is widespread in tropical, subtropical, and warm temperate areas and infects 450 plant species in over 54 families, such as tobacco, potato, pepper, tomato, and eggplant, with production losses of solanaceae crops of up to 15%–70% or even extinction in severe cases (Aslam and Mukhtar, 2023a; Genin, 2010; Mansfield et al., 2012). At present, the engineered nanoparticles (DA@CMCS-NPs), crop rotation (maize or lily rotation), biological control agents (Trichoderma harzianum), and plant-derived natural products (Hydroxycoumarins) have been studied, but these technologies have been less widely applied on the control of the disease (Niu et al., 2016; Wang et al., 2023; Wu et al., 2017; Yang et al., 2016; Yaseen et al., 2025). It is well known that R. solanacearum, as a species complex, has high variability and strong adaptability, owing to differences in soil types, temperature, moisture, and other edaphic factors of various areas (Shahbaz et al., 2015; Aslam and Mukhtar, 2023b). R. solanacearum invaded plant roots from wounds (or root tips) to rapidly multiply in the xylem (Milling et al., 2011). Subsequently, producing exopolysaccharide (EPS) promoted the colonization of R. solanacearum but also hindered water transport in plants, which ruptured the canal due to excessive hydrostatic pressure, causing wilting and plant death (Aslam and Mukhtar, 2024). Interestingly, we found that the early wilting caused by R. solanacearum infection could be naturally restored under sufficient plant water. Therefore, plant water regulation is particularly important. It is well known that the plant water potential is a physiological indicator of water regulation capacity (Boanares et al., 2020; Spinelli et al., 2017). The more water at high plant water potential will be transported to water-deficient cells, and the ability to absorb water is stronger (Kannenberg et al., 2022). In addition, the higher Ψleaf promoted nutrient uptake, and the lowest Ψleaf was conducive to occurrence and spread of coffee brown spot (Vilela et al., 2022). Pseudomonas syringae could sense water potential (-1.6 to -2.2 MPa), and a low water potential was able to decrease the number of endophytic bacterial population (Wright and Beattie, 2004). It can be seen that water potential is a limiting factor for the growth of pathogens. Water potential can regulate the osmotic pressure of plant tissues to maintain water balance, which is closely linked to ABA.
ABA is a major actor in regulating the water shortage of plants; it also stimulates stomata closure and inhibits stomata opening, mitigating the decline in water potential and preventing plant death due to water deficiency (Mphande et al., 2021; Yari Kamrani et al., 2022). Stomatal conductance controlled by ABA on different time scales suppresses water loss (Franks et al., 2017), and the timing and rate of stomatal closure vary considerably among species before early xylem cavitation, with some species closing their pores rapidly to maintain a high water potential, while others close their pores slowly as water potential decreases (Brodribb and McAdam, 2011; Martin-StPaul et al., 2017). In terms of activating plant resistance, ABA stimulates the expression of defense-related genes and, subsequently, the biosynthesis of protective compounds, e.g., dehydrins (Schwarzenbacher et al., 2020; Sun et al., 2021; You et al., 2010). Simultaneously, in the responses to biotic stresses, ABA was in cooperation with jasmonic acid (JA) for responding to the attack of herbivorous insect. In contrast, JA interacts with ethylene upon necrotroph attack, thereby improving plant disease resistance (Adie et al., 2007; Rekhter et al., 2019). Exogenous ABA application increased the activity of tomato leaf defense enzymes (PAL, PPO, and POD) and defense genes (GLU, PR1, PPO, SOD, and POD genes) expression to resist tomato black spot disease (Song et al., 2011). Moreover, among different resistant varieties, the amount of ABA was higher in KCB-1 resistant variety than in CB-1 susceptible variety 24 h after R. solanacearum infection (Shi et al., 2022). On the contrary, ABA inactivated SOD and CAT activities, inhibited the accumulation of reactive oxygen species (ROS) and the JA pathway, and promoted rice black streak dwarf virus infection (Xie et al., 2018). However, there are not a lot of reports about the changes of ABA and water potential on the development of tobacco bacterial disease and the mechanism of exogenous ABA-induced disease resistance in tobacco after infection.
Therefore, the purposes of this study were to clarify the effect of ABA-mediated Ψleaf and plant immunity against R. solanacearum. Thus, we investigated the variation of plant water potential (Ψroot, Ψstem, and Ψleaf) in tobacco plant after R. solanacearum infection and measured the ABA content and Ψleaf of resistant–susceptible tobacco cultivar. Meanwhile, we analyzed the effect of ABA content in plant on the disease by foliar spraying Na2WO4, investigated the optimal concentration and preliminary mechanism of ABA inducing plant resistance to the disease, and studied the effect of ABA on plant growth, defense enzyme activities, and defense-associated genes. This study will further promote the development and application of ABA and will also provide a new idea for the management of tobacco bacterial wilt.
2 Materials and methods
2.1 Materials
R-solanacearum CQPS-1, the plant pathogen, was stored by Natural Products Pesticide Laboratory (Chongqing, China), and it was grown in B media (nutrient-rich media) at 30°C for 12 h. Abscisic acid (ABA, 99%) was purchased from Shanghai Yuanye Biotechnology Company. Seeds of K326 and Yunyan 87 were provided by Yuxi Zhong Yan Tobacco Seed Co., Ltd.
2.2 Plant cultivation and inducting method
The selected seeds were dipped in 75% ethanol for 30 s and then 30% H2O2 for 10 min. Then, the seeds were placed on a substrate of Danish Pinchotop charcoal soil, in which the culture condition was a relative humidity of 60%, a day/night temperature of 28°C/20°C, and 14/10 h light/dark cycle. The seed cultures of Yunyan 87 and K326 were the same, which could be used after growing into three-leafed tobacco seedlings.
The foliar spraying method was used to induce tobacco resistance. Briefly, the prepared solutions were sprayed evenly on the leaf surface for a total of two times. Afterward, the 6-week-old unwounded tobacco plants were inoculated with 10 mL of fresh R. solanacearum suspension (OD600 = 0.1, ≈108 CFU/mL).
2.3 Water content of different plant parts
To investigate the plant water content with different disease grades, we measured the root, stem, and leaf water content (RWC, SWC, and LWC) of Yunyan 87. Firstly, the method of inoculation with R. solanacearum was referred to in Section 2.2. After 3 days, collected tobacco seedlings with different disease grades (grades 0, 1, 2, 3, and 4, respectively) were weighed to measure the fresh weight. Each disease grade was 10 tobacco seedlings (the stem and root length were approximately 4.4–4.5 and 8.9–9.1 cm, and the number of leaves was three maximum leaves. Each treatment had three biological replicates, each comprising 10 plants. Next, all of the fresh samples were transferred to an oven at 105°C for 30 min and then were placed in 80°C for 48 h, and the dry weight was measured (Zhou et al., 2021). The plant water content was calculated by the following Equation 1:
where FW is fresh weight and DW is dry weight. The different disease grades were defined as follows (Han et al., 2021): 0, no wilt symptom; 1, 1%–25% of leaves wilted; 2, 26%–50% of leaves wilted; 3, 51%–75% of leaves wilted; 4, 76%–100% of leaves wilted.
2.4 Determination of plant water potential
To clarify the relationship between the different disease levels and plant water potential (Ψleaf, Ψstem, and Ψroot), a PSYpro instrument, dew point hydrodynamics, was used to measure the plant water potential (Shelden et al., 2017), with minor modifications. Briefly, after the occurrence of the disease, leaf, stem, and root of tobacco seedlings were harvested. The plant with different disease levels (grades 0, 1, 2, 3, and 4, respectively) had the same size of round leaves (6 mm in diameter) taken with a hole punch. In addition, stem segment with thickness of 0.05 mm and root with 1 cm were cut off separately from tobacco stem and root and then quickly put into the sample chamber to investigate Ψleaf, Ψstem, and Ψroot. Each treatment consisted of three biological replicates, each comprising three plants. The Ψleaf of susceptible tobacco cultivar (Yunyan 87) and resistant tobacco cultivar (K326), respectively, was determined as detailed above.
2.5 Observation of leaf stomata after R. solanacearum infection
The observation of leaf stomata referred to a method reported in previous studies (Wang et al., 2023). Briefly, leaf samples (0.6 cm × 0.6 cm) of different disease grades (grades 0, 1, 2, 3, and 4) were immediately transferred onto a 1.5-mL EP tube with 1.2 mL 2.5% glutaraldehyde solution at 4°C for 24 h. Each treatment consisted of three biological replicates, each comprising three plants. Next, all samples were rinsed with 0.1 M PBS buffer (pH 6.8) four times (10 min each time) and dehydrated in 2 mL ethanol solution with mass concentrations of 10% to 100%. Finally, the leaf samples were freeze-dried, tiled in conductive adhesive, and observed by SEM (ApreoC, ThermoFisher).
2.6 ABA content measurement
To identify the changes of ABA content in plant leaves in different disease levels, firstly, the method of inoculation with R. solanacearum was referred to in Section 2.2. Meanwhile, leaf samples of different disease levels were taken. The ABA content was extracted and determined using the Shanghai KAIB ABA biological kit, which was the method of enzyme-linked immunosorbent Assay (ELISA) (Yang et al., 2001), with minor modifications. Briefly, leaf tissues weighing 0.1 g were transferred to liquid nitrogen for complete grinding. Then, 0.9 mL of PBS buffer (0.01 M, pH = 7.4) was added, followed by homogenization and centrifugation at 5,000 r for 10 min at 4°C. After centrifugation, the supernatant was removed and stored at -20°C. Each treatment consisted of three biological replicates, each comprising five plants. The OD450 was measured by 1500 model full-wavelength enzyme labeling instrument (Thermo, USA), and the regression curve was drafted using the standard concentration and the corresponding OD450. Subsequently, each sample concentration was determined by the curve.
2.7 Assessment of ABA on tobacco bacterial wilt
To obtain the best concentration of foliar spraying ABA application, we used a pot experiment to assess the controlling effect on tobacco bacterial wilt (Wang et al., 2023). Briefly, ABA dissolved in DMSO was diluted with DI water to mass concentrations of 0.38, 0.78, 1.56, 3.13, and 6.26 mg/L. Next, 0.1% DMSO was used for negative control (CK) and BTH for positive control. Each treatment consisted of biological replicates, each comprising 10 plants. Finally, disease index (DI) was scored 3 days after inoculation, and the mean of the DI was calculated relative to defense efficiency with the following Equation 2:
where H represents the disease index for treatment and CK represents the same for the control group.
2.8 Effect of Na2WO4 on tobacco bacterial wilt
To further identify whether ABA content in plants affects disease occurrence, it was assessed by spraying ABA synthesis inhibitor (Na2WO4). Briefly, 0.5 g/L Na2WO4 was uniformly sprayed on the tobacco leaves. Next, 0.78 mg/L ABA and DI water (CK) were used as the control groups. After the treatment was done twice, R. solanacearum suspension (OD600 = 0.1) was inoculated by root irrigation at 10 mL per plant. Each treatment consisted of three biological replicates, each comprising 10 plants. DI water was used for the control group.
2.9 Transpirational pull and photosynthesis measurement
To further clarify the impact of ABA on transpirational pull and photosynthesis after inoculation with R. solanacearum, we investigated the stomatal conductance (Gs), transpiration rate (Tr), net photosynthetic rate (Pn), and intracellular CO2 concentration (Ci), with minor modifications. Briefly, ABA spray application and inoculation with R. solanacearum were referred to in Section 2.2. Leaf samples were sampled at 1, 3, and 5 days after inoculation with R. solanacearum. Next, Gs, Tr, Pn, and Ci were tested, respectively, by a portable photosynthesizer (USA, LI-6200) from 7:00 to 9:00 p.m. in a greenhouse under LED light conditions. The measurements were repeated three times.
2.10 Effect of ABA application on R. solanacearum infection
To assess whether ABA affects the movement of R. solanacearum, we measured the reproduction of R. solanacearum in tobacco after ABA and Na2WO4 treatments. DI water was used for the control group (CK). Briefly, the method of spraying ABA and inoculation with R. solanacearum were referred to in Section 2.2 and Section 2.7. At 3 days after inoculation, the tobacco stem base and roots were collected. Next, the samples were sterilized with 75% ethanol for 1 min, followed by two washes with sterile water. Afterward, the samples were sufficiently ground with sterile water and collected by centrifugation at 8,000 rpm for 3 min. Then, the obtained bacterial suspensions were gradient-diluted 101, 102, 103, 104, and 105 times, respectively, and the number of R. solanacearum colonized at the stem base and roots, respectively, was determined by plate counting method (Yang et al., 2024). Each concentration was six biological replications, each comprising three plants. Finally, the plate was placed in the incubator at 30°C for 48 h, and the number of single colonies was recorded and calculated.
2.11 Effect of ABA on tobacco growth
ABA is a recognized plant hormone that affects plant growth. To define the effect of different concentrations ABA on tobacco growth, ABA concentrations of 0.38, 0.78, 1.56, 3.13, and 6.26 mg/L were configured with sterile water, respectively, and the method of foliar spraying ABA was performed according to Section 2.2 under non-inoculation. Each concentration has three biological replications, each comprising 10 plants. Then, the agronomic traits of tobacco (dry weight, fresh weight, plant height, and leaf area) were measured on the 10th day after treatment, and leaf area was calculated by the following Equation 3:
where L1 is the maximum leaf length, and L2 is the maximum leaf width. The value 0.6345 is a parameter determined (Xiao et al., 2024).
2.12 Measurement of defense enzyme activities
To clarify the effect of exogenous spraying ABA on defense enzyme activities in tobacco. We measured the enzyme activities as described in the past report (Wang et al., 2024). Firstly, 0.1 g of fresh leaves after foliar spraying 0.78 mg/L ABA was taken at 10 min, 1 h, 6 h, 12 h, and 24 h, respectively, and then rapidly stored in liquid nitrogen for measurement. Furthermore, 0.1% DMSO served as the control group. Each treatment had biological replications, each comprising three plants. The PAL, SOD, POD, and PPO enzyme activities were extracted using a kit (Suzhou Grise Biotechnology Co., Ltd.) and measured by using UV1000 spectrophotometer (Shanghai Tianmei Scientific Instrument Co., Ltd.) at 290, 450, 470, and 420 nm, respectively.
2.13 RT-PCR analysis
The methods of ABA foliar application and in obtaining samples (Yunyan 87) were the same as in Section 2.6. Total RNA in leaves was extracted using an RNA kit (Solarbio, Shanghai), and cDNA was synthesized by a cDNA synthesis kit (Evo M-MLV, Solarbio). The cDNA obtained was amplified with the q-PCR kit, and the primers are shown in Supplementary Table S1. The reaction conditions were as follows: 20 μL reaction system, pre-denatured at 95°C for 30 s, then denatured at 95°C for 5 s, and annealed at 60°C for 30 s, for a total of 40 circulation. The relative gene expression was calculated by using the 2-ΔΔct method (Li et al., 2021; Wang et al., 2019; Xie et al., 2018). Each treatment consisted of three biological replications, each comprising three plants.
2.14 Data analysis
Excel 2016 was used for data organization, GraphPad Prism 8.0.2 for graphing, and SPSS 16.0 for analysis by Tukey’s test or t-tests (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; ns, not significant). All experiments were repeated three times, and representative pictures were selected for display.
3 Results
3.1 Plant water content will decrease after R. solanacearum inoculation in plants
Water is essential for maintaining the health and growth of plants. Water in plants could affect the infection of plant-pathogenic bacteria to the host plant (Wang et al., 2015). As shown in Figures 1a–c, the plant water content (PWC) of the grades 0 to 4 decreased dramatically. The PWC of grade 0 was significantly higher than that of other disease grades, and the PWC of grade 4 was the lowest, and the root water content (RWC), stem water content (SWC), and leaf water content (LWC) decreased with the increase of disease index (DI). However, except for the grade 4, the size of water content was LWC > SWC > RWC (Figure 1d), which decreased by 61.08%, 43.98%, and 24.85%, respectively. In conclusion, PWC decreased with the increase of DI, and the LWC decreased the most.
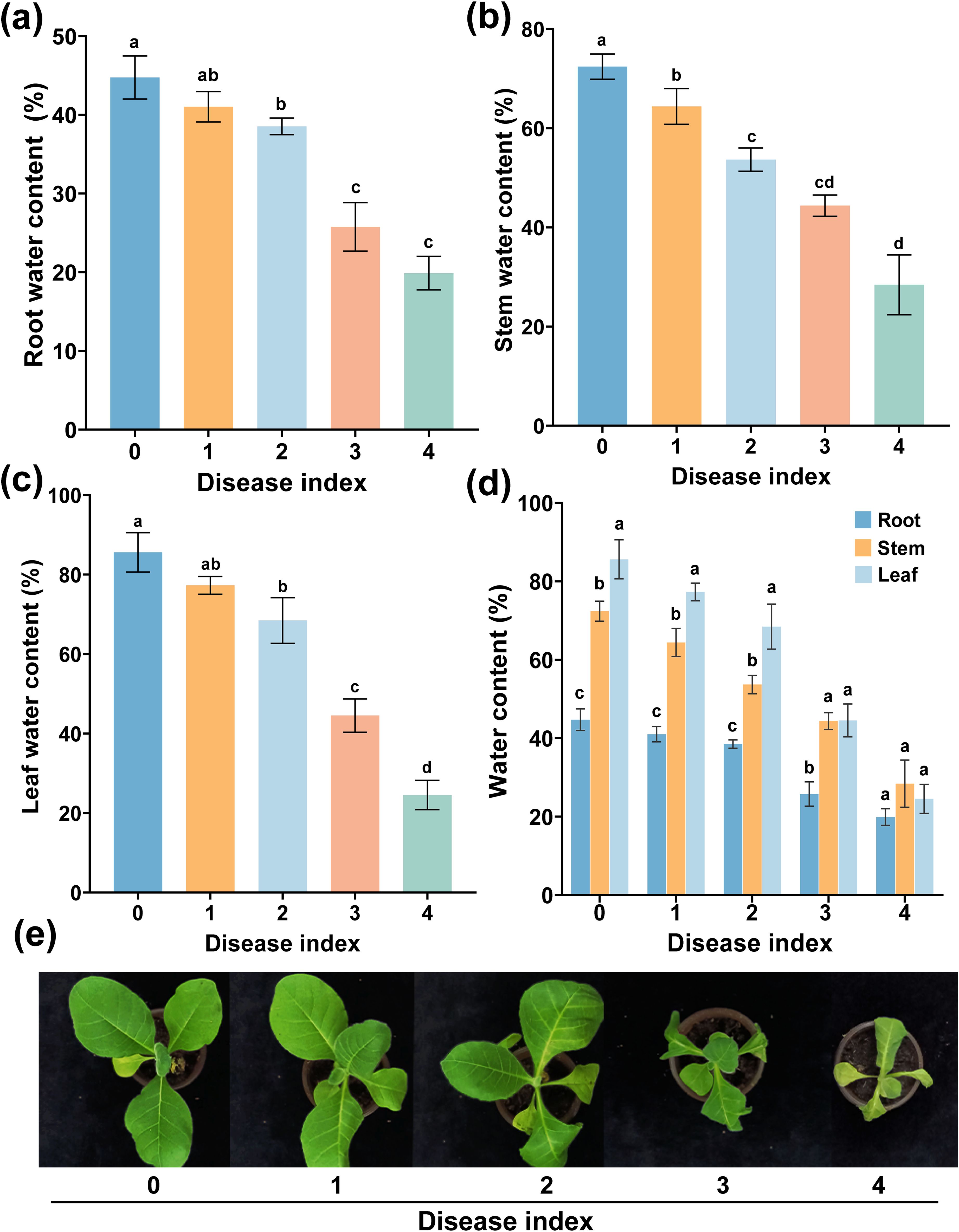
Figure 1. Water content of different plant parts at different disease indexes. Water content of root (a), stem (b), and leaf (c) from grades 0 to 4 and (d) water content of different tissues. (e) The symptoms of different disease index. The data are shown as mean ± standard deviation (SD). The bars with lowercase letters indicate statistically significant differences by Tukey’s test (p < 0.05).
3.2 Plant water potential will decrease after R. solanacearum inoculation in plants
Plant water potential (PWP) is a reflection of PWC, and the low (negative) PWP can cause xylem embolism and the death of a plant (Choat et al., 2012). Based on the variation of PWC, the leaf water potential, stem water potential, and root water potential (Ψleaf, Ψstem, and Ψroot) were measured using a PSYpro dew point water potential (Wescor, USA) after R. solanacearum infection (10 mL/per plant, OD600 nm = 0.1). As shown in Figure 2a, the method of measured PWC at different time points was described. The Ψleaf, Ψstem, and Ψroot all sharply declined after inoculation from 24 to 90 h, and the Ψleaf, Ψstem, and Ψroot of non-inoculated samples showed an upward and downward fluctuation trend (Figures 2b–d). In addition, the Ψleaf, Ψstem, and Ψroot of non-inoculated samples, after 42 h, were higher than that of the inoculated ones. The Ψleaf showed significant changes in PWC after inoculation from 24 to 90 h. Interestingly, in Figure 2b, the Ψleaf of the non-inoculated sample was significantly higher than those inoculated at 90 h (p < 0.001). The results showed that PWC will decrease after R. solanacearum infection, and Ψleaf decreased the most, which was similar to the change of LWC.
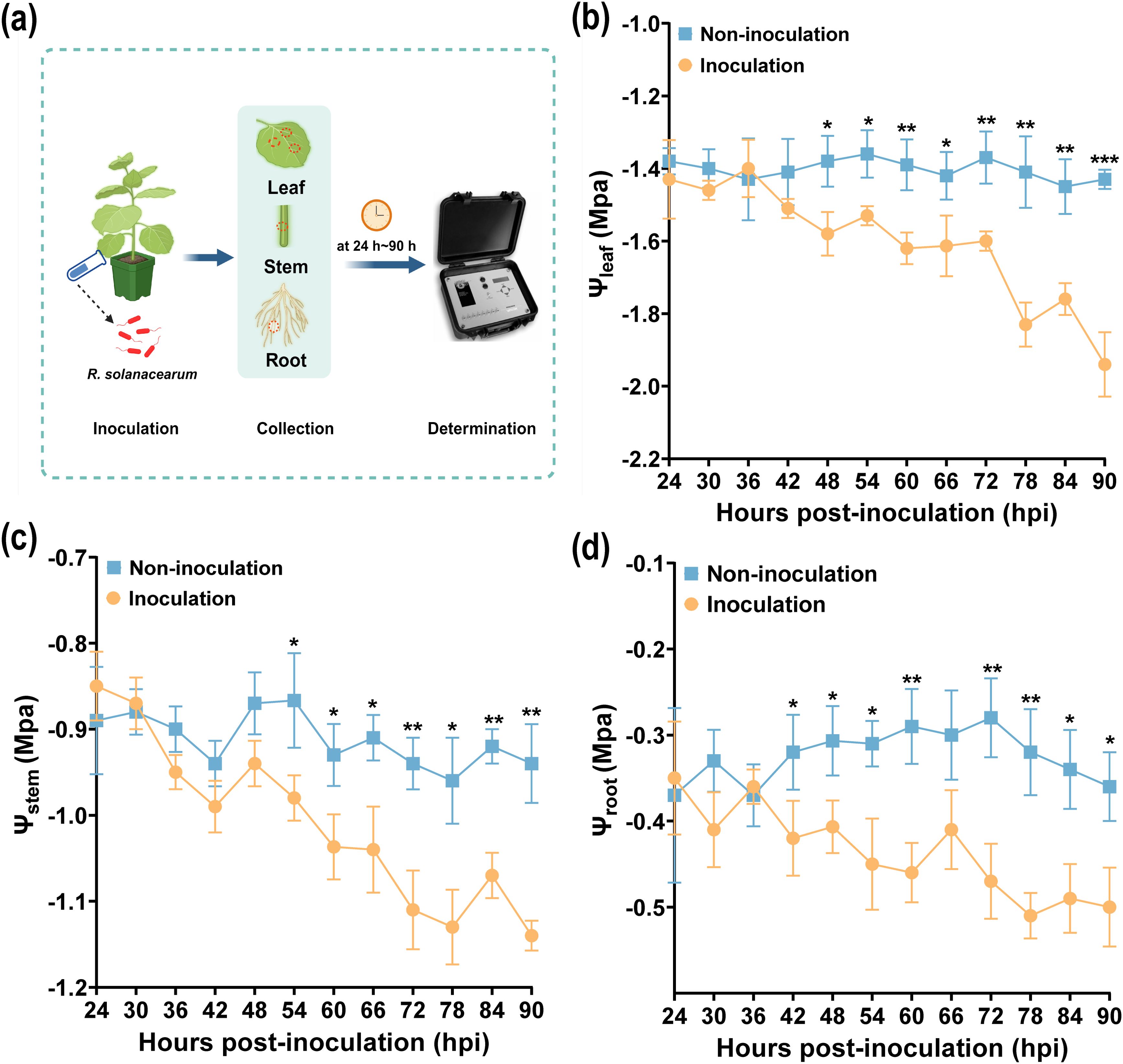
Figure 2. Variations of plant water potential after R. solanacearum inoculation. (a) Schematic drawing of measured Ψstem, Ψroot, and Ψleaf. (b-d) Variations of Ψleaf, Ψstem, and Ψroot, respectively. An asterisk (*) indicates a statistically significant difference between inoculation and non-inoculation by t-tests. The data are shown as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001.
3.3 The stomatal aperture of leaves decreased first and then increased in R. solanacearum-inoculated plants
Shown in Figure 3a are plant leaves in which the wilting symptom appeared (at 3–5 dpi) after R. solanacearum infection. However, this early wilting has similar symptoms to that when there is natural water shortage in plants. To further study the symptom at different disease levels, we measured the Gs, Tr, and stomatal aperture after R. solanacearum infection. As shown in Figures 3b, c, from grades 0 to 4, the values of Gs and Tr showed a trend of first decreasing and then increasing, and the Gs and Tr of grade 2 were significantly lower than those of other disease grades (p < 0.05). Specifically, the Gs and Tr of healthy plants were 16.967 mol m-2 s-1 and 0.286 mmol m-2 s-1, respectively, which were significantly higher than the 13.367 mol m-2 s-1 and 0.223 mmol m-2 s-1 of diseased plants (grade 1). Moreover, the stomatal width across different disease levels was consistent with the trends observed in Gs and Tr, with the stomatal width in grade 2 being the minimum compared to grades 1, 3, and 4 as shown in Figure 3d. This shows that the Gs, Tr, and stomatal width will decrease after R. solanacearum infection, thereby indirectly reducing plant water loss.
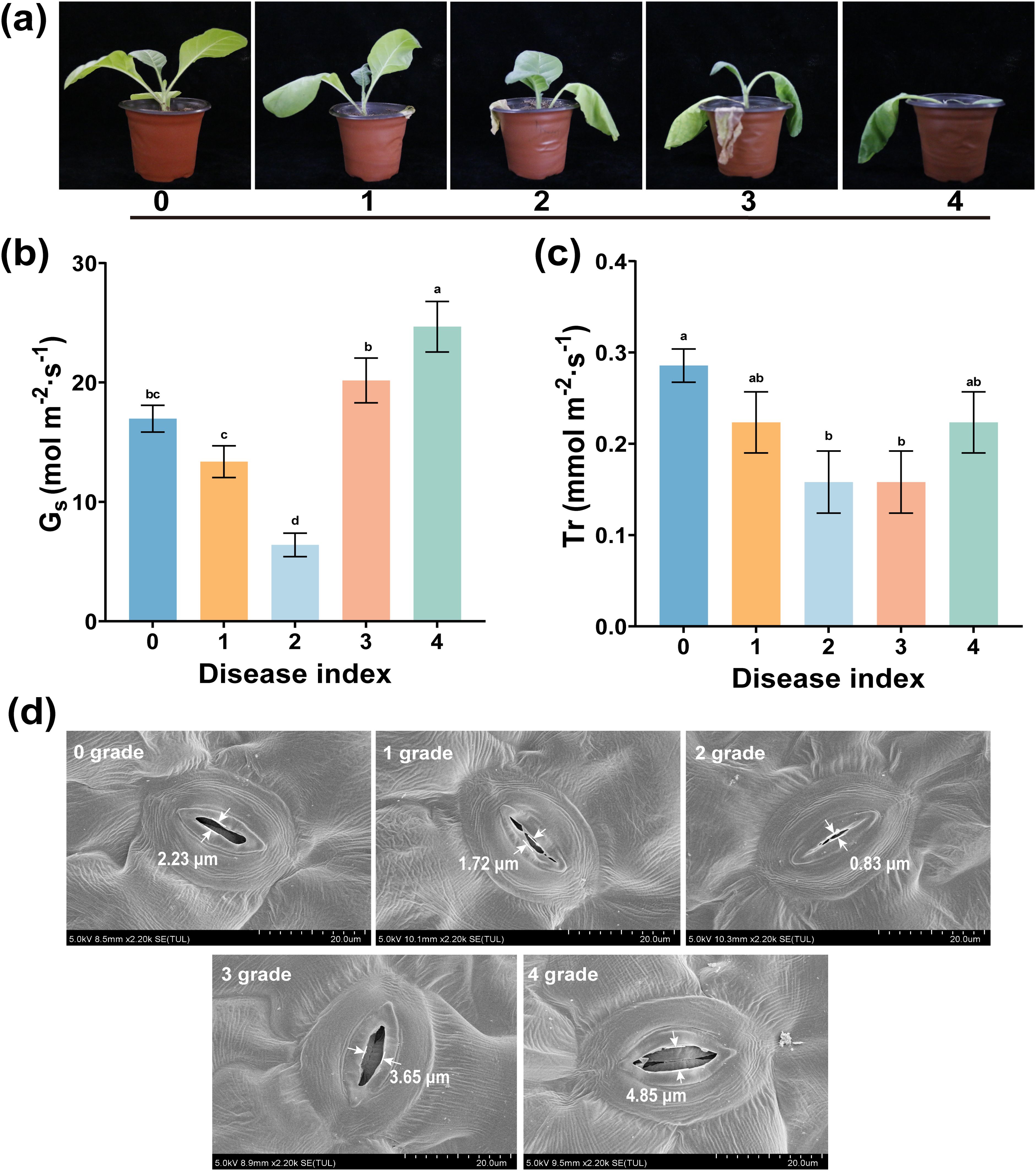
Figure 3. Leaf stomata in the lower epidermis of different disease levels. (a) Wilting symptoms of different disease levels, (b) stomatal conductance, Gs, and (c) transpiration rate, Tr. (d) Stomatal size for different disease levels. The data are shown as mean ± SD. The bars with lowercase letters indicate statistically significant differences by Tukey’s test (p < 0.05).
3.4 Endogenous ABA regulates Ψleaf to counteract water loss caused by R. solanacearum
Compared to Ψroot and Ψstem, the variation of Ψleaf varied greatly. To further analyze whether there were similar changes in Ψleaf among different tobacco varieties after R. solanacearum inoculation, the Ψleaf of susceptible tobacco cultivar (Yunyan 87) and resistant tobacco cultivar (K326), respectively, at different times and disease levels was measured. As shown in Figure 4, the Ψleaf of K326 was higher than that of Yunyan 87. At 1–3 days post-inoculation (dpi), the Ψleaf of K326 did not change much, while that of Yunyan 87 showed an increasing trend from 1 to 2 dpi (Figure 4a). At the same time, both Ψleaf of Yunyan 87 and K326 showed a downward and then upward trend during the process of disease index rising from 0 to 4, and grades 2–4 showed a significant difference (Figure 4b). It is known that ABA phytohormone plays a critical role in plants to respond to drought stress (Jamnická et al., 2019). Based on this, the ABA content of Yunyan 87 and K326, respectively, after R. solanacearum infection were investigated. As shown in Figure 4c, the ABA content in leaves was significantly higher in K326 compared to Yunyan 87 during 1–3 dpi, and the ABA content in the leaves of grades 0 to 4 were also significantly higher in K326 than in Yunyan 87 (Figure 4d). The ABA content of K326 exhibited a pattern of initially increasing and then decreasing. In addition, in Yunyan 87, the ABA content also followed a similar pattern (Figure 4d). The Ψleaf of tobacco plants (K326 and Yunyan 87) was regulated by endogenous ABA after R. solanacearum infection. It could be seen that the increase of Ψleaf and endogenous ABA may be in response to the wilting caused by R. solanacearum.
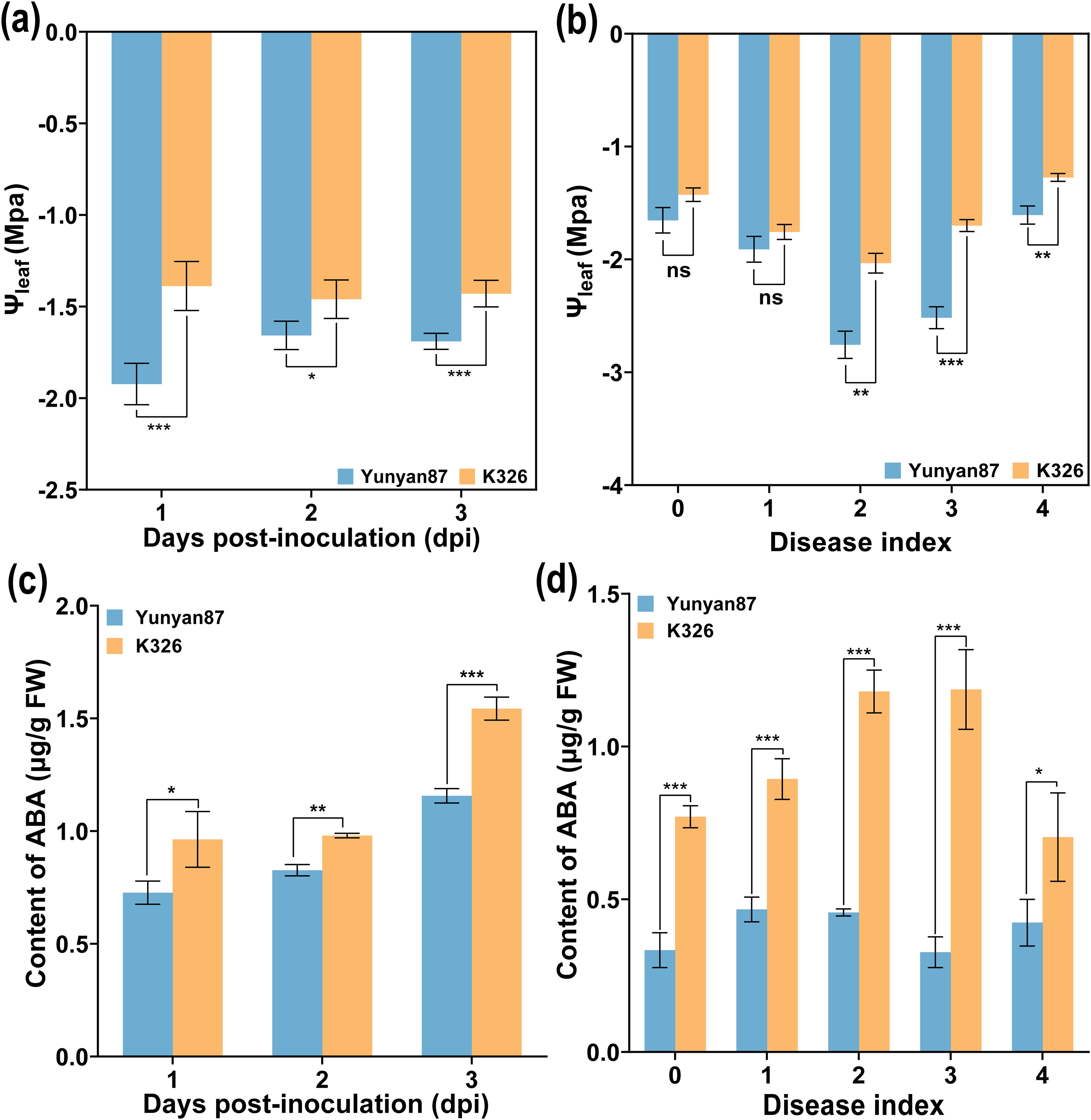
Figure 4. Variations of Ψleaf and ABA content of different varieties after R. solanacearum inoculation. (a) Ψleaf of different time, (b) Ψleaf of different disease levels, (c) content of ABA at different times, and (d) content of ABA at different disease levels. The data are shown as mean ± SD. An asterisk (*) indicates a statistically significant difference between Yunyan87 and K326 by t-tests. *p < 0.05, **p < 0.01, ***p < 0.001. ns, not significant.
3.5 0.78 mg/L ABA was optimal concentration against tobacco bacterial wilt
To obtain the optimal concentration of ABA that induces a tobacco plant’s resistance to R. solanacearum, ABA concentrations at 0.38, 0.78, 1.56, 3.13, and 6.26 mg/L were assessed. As shown in Figure 5, foliar spraying ABA provided a better control effect on tobacco bacterial wilt and alleviated the occurrence of the disease. ABA at concentrations from 0.38 to 6.26 mg/L had a lower disease index than CK. At 11 dpi, the disease index (DI) of ABA treatment at 0.78 and 6.26 mg/L were 2.30 and 2.73, respectively, both lower than BTH being 3.23 and markedly lower than that of CK being 4 (p < 0.05) (Figure 5a). Specifically, there were large differences between different concentrations of ABA in Figure 5b. In 5 days, the control efficacy of ABA (0.78 mg/L) was slightly higher than that of the BTH, the control efficacy of which was 84.80% and 73.35%, respectively. At 11 dpi, the control efficacy of foliar spraying ABA was 54.96%, which was significantly higher than BTH at 19.33%. These results indicated that ABA at 0.78 mg/L was the optimal concentration for induced tobacco resistance to R. solanacearum.
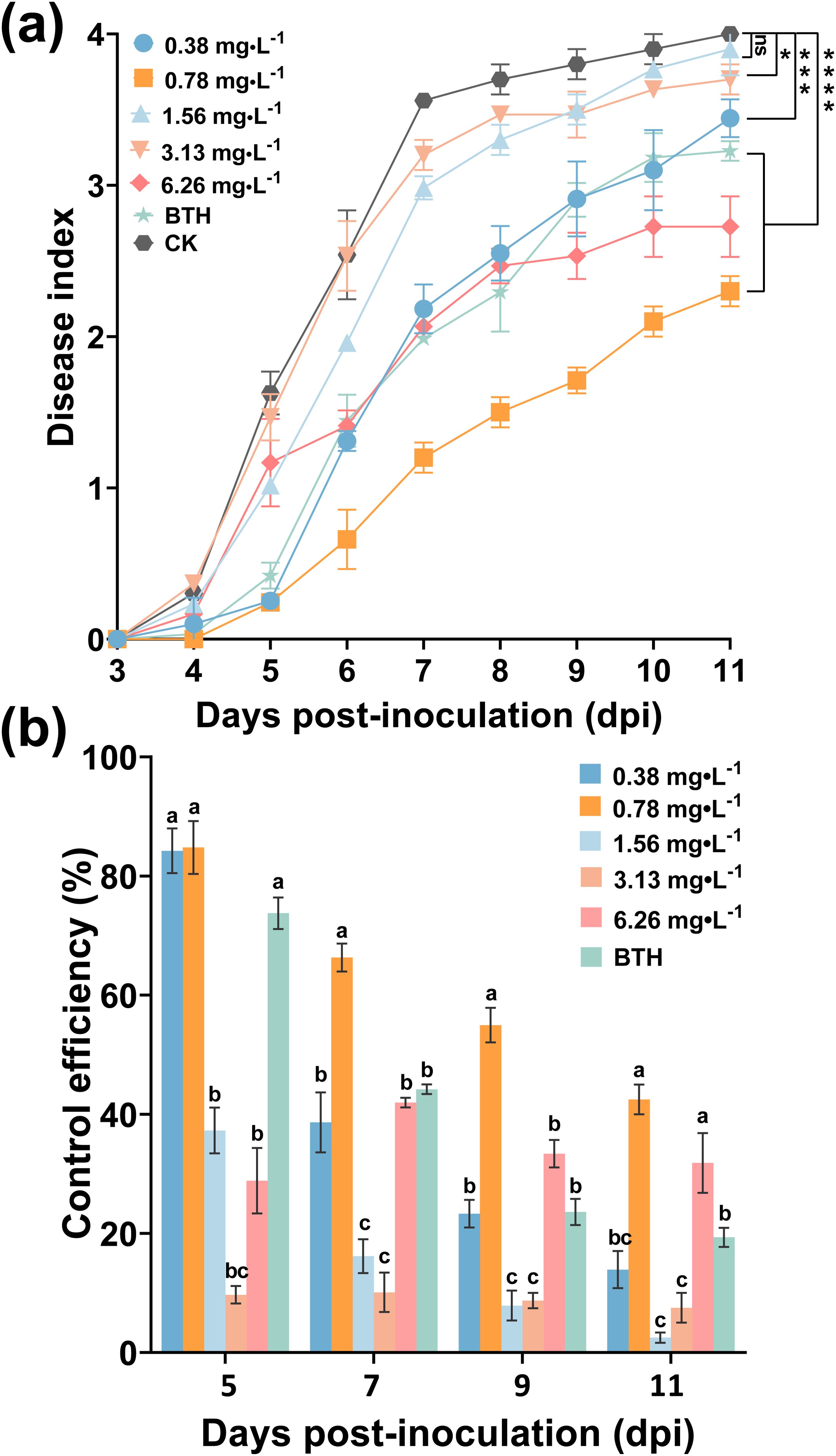
Figure 5. Control efficiency of foliar spraying ABA on tobacco bacterial wilt. (a) Disease index after treatment with ABA, BTH, and CK (DI water). (b) Control efficacy of ABA and BTH. The data are shown as mean ± SD. An asterisk (*) indicates a statistically significant difference between ABA and CK by t-tests (a). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. ns, not significant. The bars with lowercase letters indicate statistically significant differences by Tukey’s test (p < 0.05).
3.6 Foliar spraying Na2WO4 promoted the occurrence of tobacco bacterial wilt
To further investigate whether ABA regulated Ψleaf and how it affected DI, DI and Ψleaf were measured after foliar spraying Na2WO4. As shown in Figure 6a, it was evident that the DI of Na2WO4 treatment was higher than that of CK at 3–13 dpi. In other words, when ABA synthesis in plant leaves was inhibited, the tobacco plant was more susceptible to infection by R. solanacearum, leading to a more serious incidence of the disease (Figure 6b). Interestingly, the Ψleaf of Na2WO4 treatment was also lower than that of CK and ABA treatments (Figure 6c). This result further confirmed that ABA may increase Ψleaf to delay the occurrence of the disease.
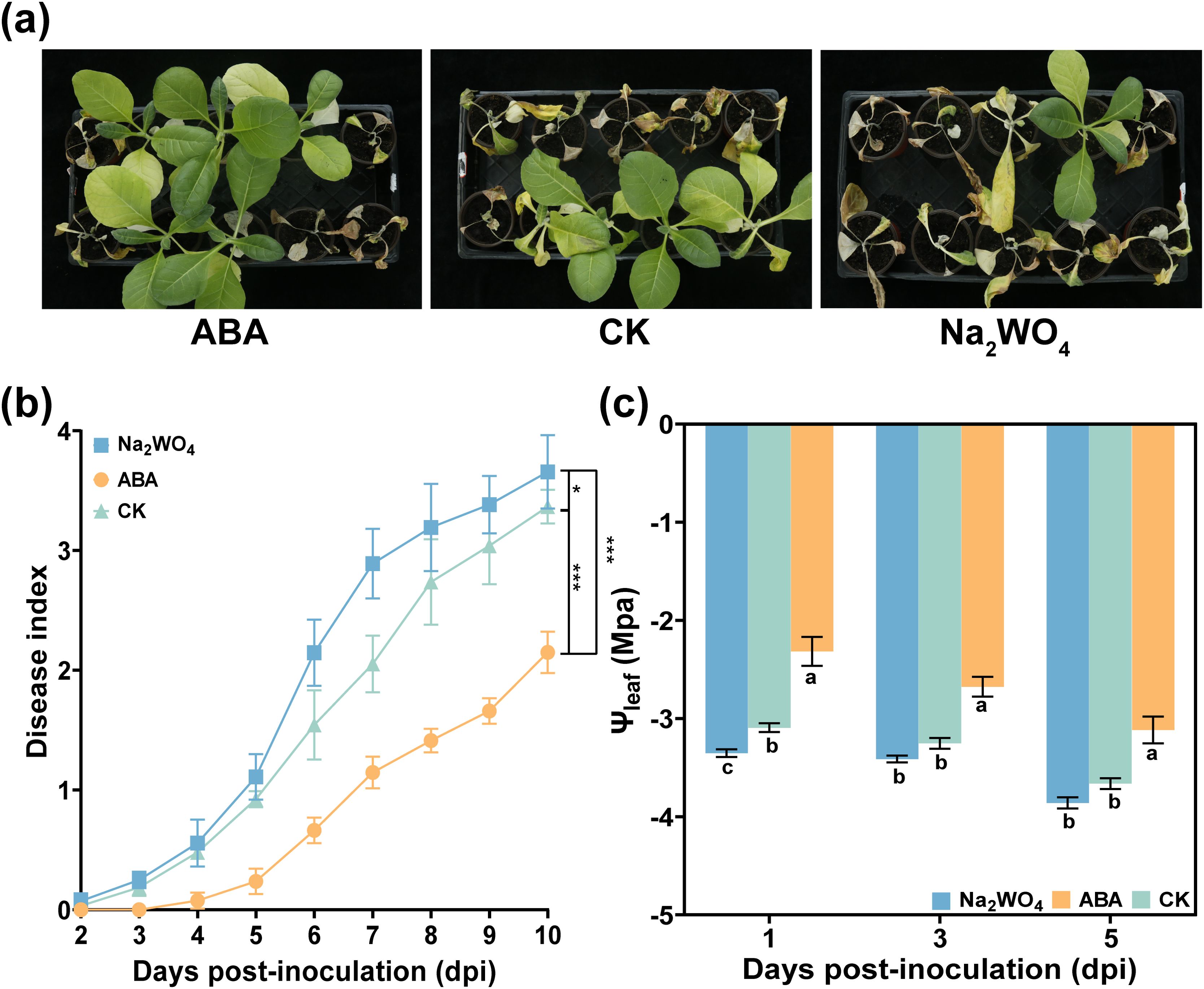
Figure 6. Effect of Na2WO4 on tobacco bacterial wilt and Ψleaf after R. solanacearum inoculation. (a) Occurrence of tobacco bacterial wilt at 9 dpi. (b) Disease index of tobacco bacterial wilt after Na2WO4 and CK (DI water) treatment. (c) Variations of Ψleaf after Na2WO4 treatment. The data are shown as mean ± SD. An asterisk (*) indicates a statistically significant difference between ABA and CK by t-tests. *p < 0.05, **p < 0.01, *** p<0.001. ns, not significant. The bars with lowercase letters indicate statistically significant differences by Tukey’s test (p < 0.05).
3.7 ABA promoted tobacco growth
To further analyze the role of ABA application at varied concentrations (0.38, 0.78, 1.56, 3.13, and 6.26 mg/L) on plant growth, at 15 days after spraying ABA, leaf area, plant height, and fresh and dry weight were investigated. As displayed in Figure 7, ABA did not inhibit tobacco plant growth. On the contrary, spraying 0.78 mg/L ABA could promote plant growth. The fresh weight, plant height, and leaf area with 0.78 mg/L ABA were 4.40 g, 12.50 cm, and 39.83 cm2, respectively, which were significantly higher than those of CK (3.66 g, 10.17 cm, and 29.39 cm2) as shown in Figures 7a, b. In addition, 0.78 mg/L ABA increased the fresh (or dry) weight by 20.21% (or 12.28%) compared with CK as shown in Figures 7c, d. Our study found that 0.78 mg/L ABA had a beneficial effect on promoting plant growth.
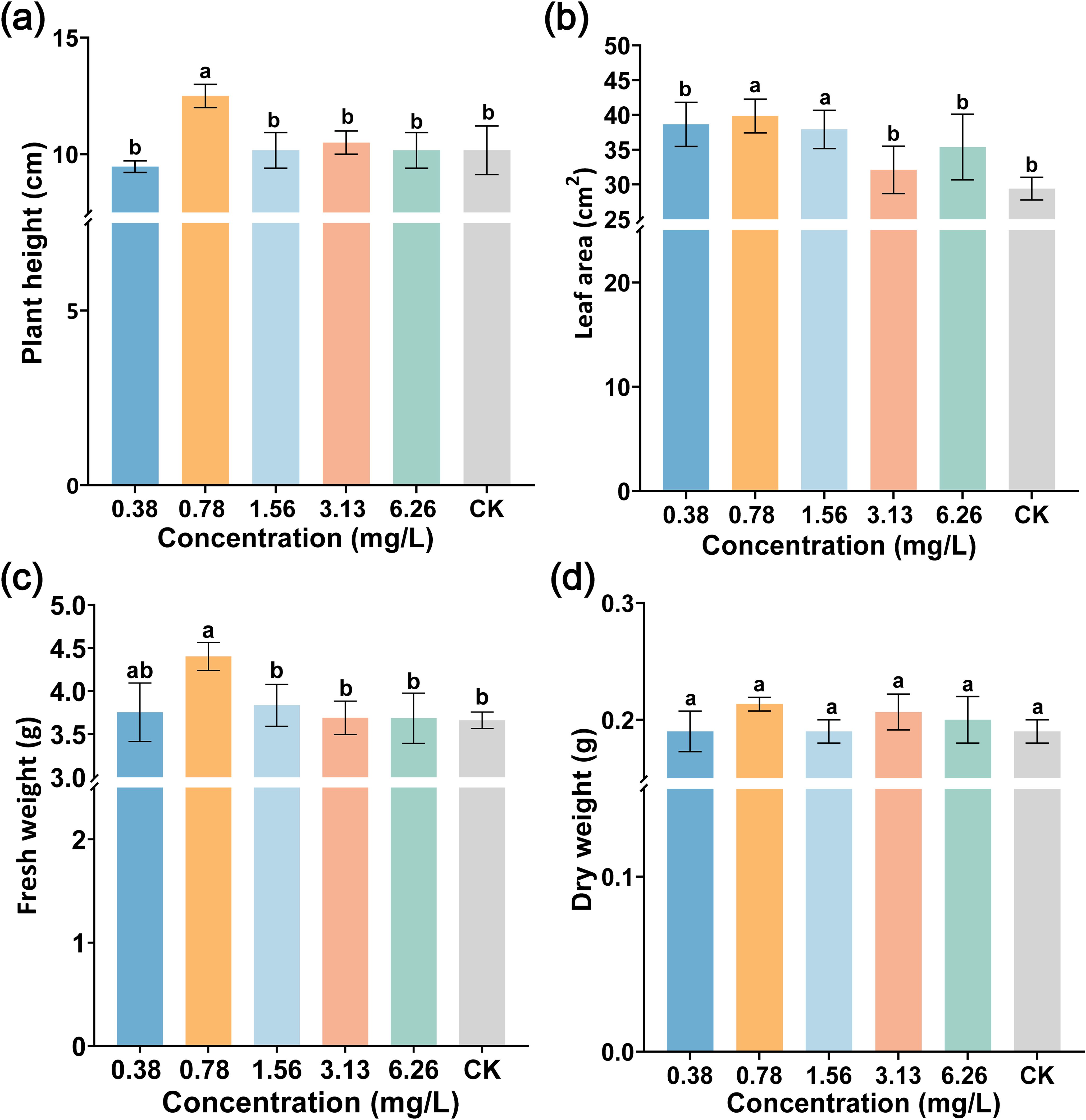
Figure 7. The effect of foliar spraying ABA on tobacco growth. (a) Plant height, (b) leaf area, (c) fresh weight, and (d) dry weight. The data are shown as mean ± SD. The bars with lowercase letters indicate statistically significant differences by Tukey’s test (p < 0.05).
3.8 ABA reduced R. solanacearum infection in plants by reducing its mobility
Pn and Ci are important indicators of plant photosynthesis. To confirm that ABA regulates transpiration pull and plant photosynthesis, we tested the changes in Gs, Tr, Pn, and Ci after ABA and Na2WO4 application. As shown in Figures 8a, b, the Tr and Gs of all treatments decreased from 1 to 5 dpi, and the Tr and Gs in ABA-treated leaves were significantly lower than that with Na2WO4 and CK at 1, 3, and 5 dpi. In the long-term (or multiple) pot experiments, we found that the tobacco seedlings showed obvious wilting symptoms at 3 dpi. In this period, the Tr and Gs of ABA were 16.57 mmol m-2 s-1 and 0.26 mol m-2 s-1, which were significantly lower than those of CK (19.17 mmol m-2 s-1 and 0.31 mol m-2 s-1) and Na2WO4 (24.17 mmol m-2 s-1 and 0.37 mol m-2 s-). In addition, Pn and Ci (except for 5 dpi) also had similar results as shown in Supplementary Figures S1a, b, and Pn and Ci, related to photosynthesis after ABA application, were also significantly lower than those of CK and Na2WO4 at 1 and 3 dpi. At the same time, we further determined the infection of R. solanacearum in different tissues. As anticipated in Figures 8c, d, ABA treatment significantly reduced R. solanacearum infection compared to CK (DI water) and Na2WO4 at 3 dpi. The populations of R. solanacearum in root after ABA, CK, and Na2WO4 treatments were 2.30 × 105 CFU/g, 2.78 × 105 CFU/g, and 2.65 × 105 CFU/g, respectively. Interestingly, compared to CK and Na2WO4 treatments, the populations of R. solanacearum in stem after ABA treatment were the lowest and reduced by 8.15% and 10.33%. The results also proved that ABA reduced the infection of R. solanacearum by decreasing the transpiration pull.
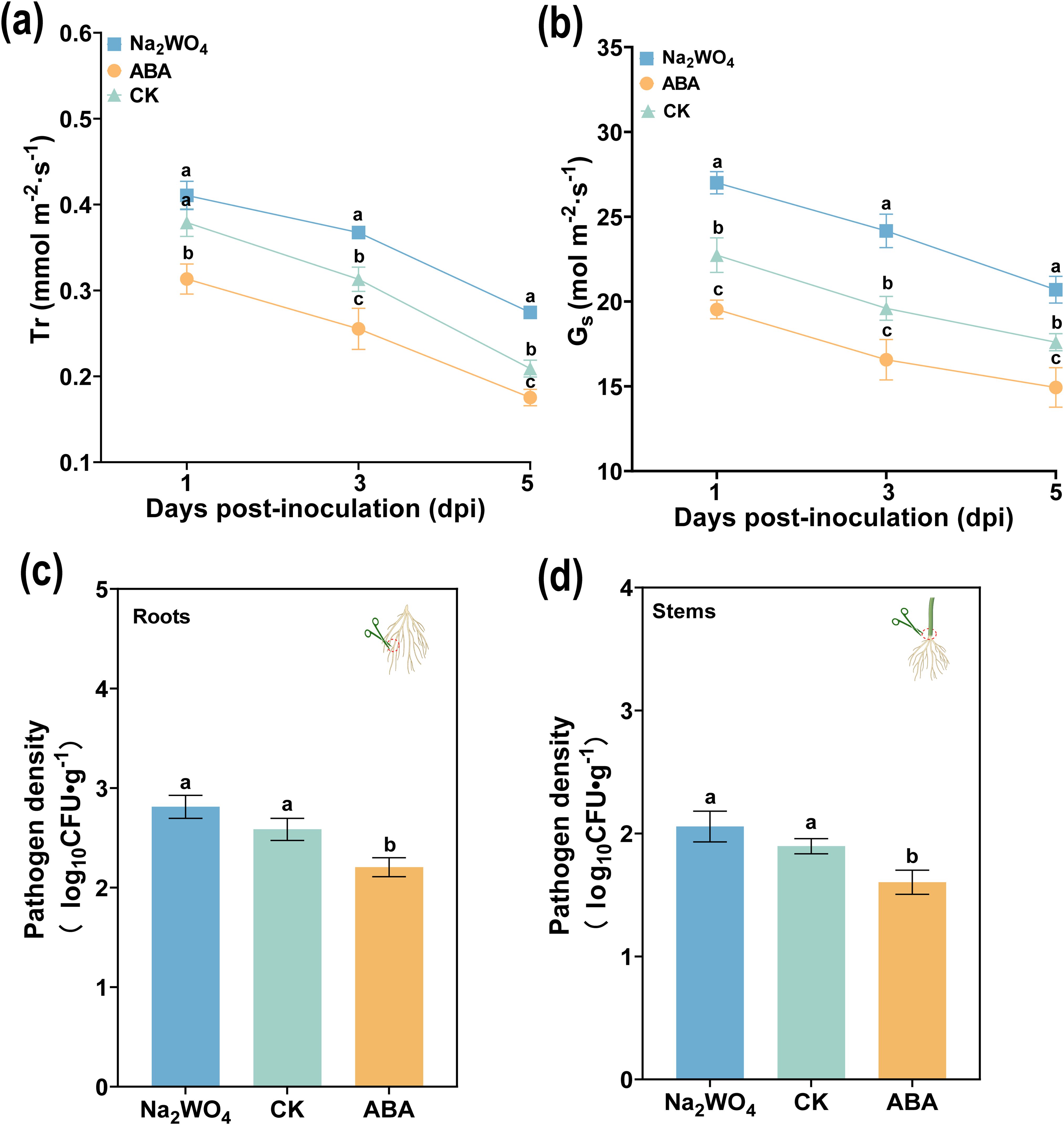
Figure 8. Effects of ABA on stomatal opening degree and transpiration rate of leaf, and bacterial population of R. solanacearum infection in tobacco. (a) Transpiration rate (Tr) and (b) stomatal conductance (Gs) of tobacco at 1, 2, and 3 dpi. (c, d) The pathogen density in the root (c) and stem base (d) after 3 dpi. The data are shown as mean ± SD. The bars with lowercase letters indicate statistically significant differences by Tukey’s test (p < 0.05).
3.9 ABA improved defense enzyme activities
To clarify whether ABA enhances plant resistance to R. solanacearum by activating the plant defense enzyme, PAL, POD, SOD, and PPO activities were measured. As displayed in Figure 9, 0.78 mg/L ABA increased the activities of PAL, POD, SOD, and PPO enzymes. The PPO activity showed a different trend with PAL, SOD, and POD activities (Figure 9a), which tended to increase first and then decrease within 24 h and peaked at 1 h. The PPO activity was at 57.25 U/g, which was significantly higher than CK (40.09 U/g). Notably, the activities of POD, PAL, and SOD showed a rising trend and peaked at 24 h (Figures 9b–d), which were 144.83, 89.99, and 815.43 U/g, respectively, significantly higher than CK (40.09, 36.66, and 354.00 U/g), and were increased by 2.61, 1.45, and 1.30 times, respectively. These results showed that foliar spraying ABA could activate the activities of plant defense enzymes.
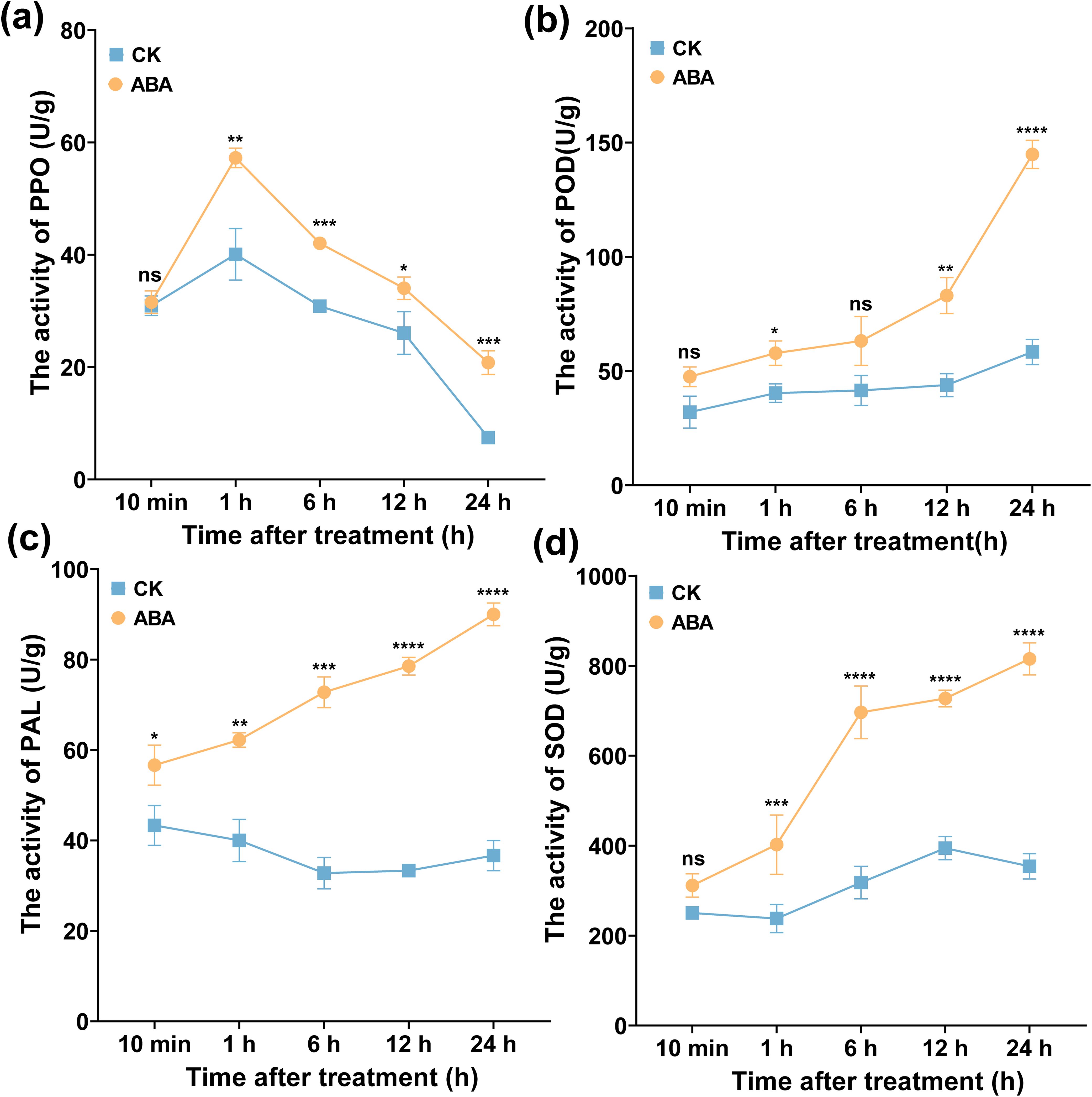
Figure 9. Effect of ABA on leaves’ defense enzymes. (a) PPO, (b) POD, (c) PAL, and (d) SOD, respectively. The data are shown as mean ± SD. An asterisk (*) indicates a statistically significant difference between the ABA and CK by t-tests. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. ns, not significant.
3.10 ABA induced the expression of defense-associated genes
To further analyze the underlying mechanism of ABA in inducing tobacco resistance, expression of defense-associated genes at different time points (6, 12, and 24 h) after ABA application was measured by using qRT-PCR. As shown in Figure 10, compared with CK, the expression levels of JA-related gene NtPDF1.2, ET synthesis gene NtET, and ROS-related gene NtOA increase first and then decrease from 6 to 24 h after spraying ABA, while the expression level of SA-related gene NtPRa1 was significantly downregulated. In addition, the expression levels of NtPDF1.2, NtET, and NtOA genes were significantly upregulated compared with those of CK. Moreover, the expression level of NtPR1a gene after ABA application was significantly inhibited at 12 h (Figure 10a), and the expression level of CK was 1.72 times that of ABA, while the expression levels of NtPDF1.2, NtET, and NtOA genes were significantly upregulated, peaking at 12 h (Figures 10b–d), and were 3.59, 1.63, and 1.10 times, respectively, greater than those of CK. The results showed that ABA enhanced the stress resistance and defense ability of plants mainly by the JA/ET signaling pathway and the expression of ROS-related genes.
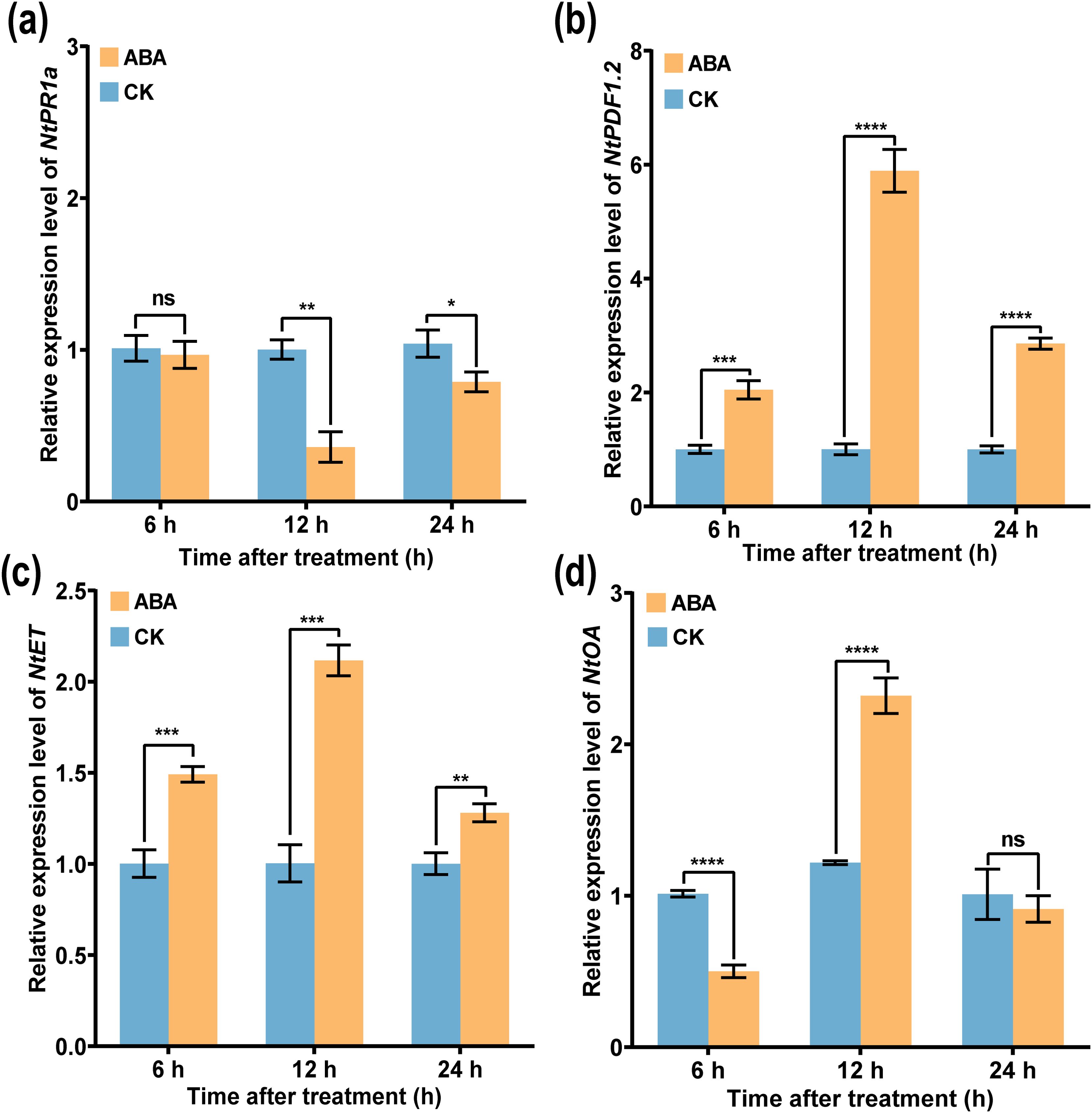
Figure 10. Expression of tobacco resistance genes induced by ABA. Relative expression of (a) NtPR1, (b) NtPDF1.2, (c) NtET, and (d) NtOA, respectively. An asterisk (*) indicates a statistically significant difference between the ABA and CK by t-tests. The data are shown as the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. ns, not significant.
4 Discussion
It is well known that the leaf stomata could alleviate the symptom by reducing plant water loss. ABA widely regulated plants for the uptake and utilization of water in conditions of water deprivation (Du et al., 2018; Kuromori et al., 2018). In particular, ABA was also utilized in crop production and disease management. In addition, it was widely known that ABA played a part in regulating plant stomatal closure (Meigas et al., 2024; Yoshida and Fernie, 2018). Its action pathway (Lim et al., 2015) and signaling mechanism (Hsu et al., 2021) had been previously studied. In this study, we discovered that PWC and plant water potential gradually decreased after R. solanacearum infection, while the stomatal size, Gs, and Tr decreased first and then increased. Notably, ABA content increased first and then decreased, which indicated that ABA was involved in the regulation of Ψleaf, Ψstem, and Ψroot after the process of R. solanacearum infection. In addition, foliar spraying ABA could reduce plant water loss by reducing Gs and Tr, a finding which was similar to previous research (Li et al., 2022; Lim et al., 2015). Interestingly, the Ψleaf, Ψstem, and Ψroot decreased from 24 to 96 h, which may be between grades 1 and 2, and the grade may exhibit greater water mobility and have a lower water potential. Similarly, at higher levels of ABA, the stomatal size, stomatal aperture, and leaf area decreased, which reduced the leaf Tr and improved the water utilization efficiency (Zhang et al., 2018). Water potential was a physiological index to measure water balance in plants (Tao et al., 2016; Wang et al., 2022). A previous study found that change of water potential promoted the accumulation of ABA primarily in the leaves, and ABA primarily enhances drought resistance in tomato by acting on leaf stomata rather than on the xylem (Haverroth et al., 2023). This study found that the Ψleaf and ABA content, respectively, were significantly higher in K326 than in Yunyan 87. The Ψleaf of tobacco plants (K326 and Yunyan 87) was increased and regulated by ABA after R. solanacearum infection. Furthermore, the application of Na2WO4, an inhibitor of ABA synthesis, promoted the occurrence of tobacco bacterial wilt, and spraying 0.78 mg/L ABA could delay the occurrence of the disease. These results indicated that ABA enhanced plant resistance to R. solanacearum, consistent with previous findings reported by Yop et al. (2023). Conversely, Zhou et al. reported a negative regulation of ABA to R. solanacearum in tobacco (Zhou et al., 2008), which may be related to the concentration of ABA. Zhou et al. used 50 μM ABA (about 0.013 mg/L), while we used 0.78 mg/L. In a previous indoor experiment, we observed significant differences in the effect of ABA on tobacco’s resistance to R. solanacearum. Specifically, disease incidence increased with 12.52 mg/L of ABA but decreased with 0.78 mg/L of ABA (Supplementary Figure S2). Furthermore, the ability of ABA to activate plant resistance was related to the concentration of ABA application.
The vital role played by ABA in plant growth and development is increasingly emphasized. Studies have shown that ABA impacted plant growth to a certain extent, and plants lacking ABA exhibit delayed growth, a phenomenon associated with ABA concentration (Brookbank et al., 2021). In addition, the exogenous application of ABA promoted rice root hair elongation by regulating auxin homeostasis (Wang et al., 2021). We also investigated the effect of ABA on tobacco plant growth. The results revealed that 0.78 mg/L ABA had a positive role in increasing plant height, leaf area, and fresh (or dry) weight. In the aspect of plant disease management, in the interaction between host plant and pathogenic bacteria, plant hormones (ABA, SA, and JA) mediated the plant immunity. A past study had demonstrated that ABA could promote JA synthesis and inhibit SA synthesis after pathogenic bacteria invasion (Long et al., 2019). In addition, a study on the involvement of ABA in JA signal transduction in Arabidopsis found that there was a close relationship between ABA and JA, and ABA receptors participated in JA signal transduction through JAZ, MYC2, and COI transcript regulation (Yu et al., 2021). Similarly, in our study, we found that spraying ABA at 0.78 mg/L could upregulate the expression levels of JA-related gene NtPDF1.2 and repress the expression levels of SA-related gene NtPR1a. Interestingly, the expression levels of ET synthesis gene NtET and ROS-related gene NtOA upregulate after ABA treatment, which showed that ABA improved the tobacco plant’s resistance by activating the synergistic regulation of JA, ET, and ROS signaling pathways. However, another study found that JA and ABA were antagonistic after infection with pathogenic bacteria (Du et al., 2024; Han et al., 2023), which was different from our results. The concentration of ABA may have changed after exogenous application, leading to subsequent changes in physiological and biochemical mechanisms.
Few studies report that 0.78 mg/L ABA could delay the occurrence of tobacco bacterial wilt. We concluded that foliar spraying ABA alleviated the wilting caused by R. solanacearum by reducing plant water loss and enhancing the plant system’s resistance. To illustrate the role of ABA after R. solanacearum infection, a model of foliar spraying ABA alleviated tobacco bacterial wilt was proposed (Figure 11). ABA maintained the balance of water in the early stage (grades 0 to 1 or grades 0–2) of plants by reducing Gs and Tr, which slowed down R. solanacearum movement from the roots to the stems. Furthermore, ABA improved disease resistance by promoting plant growth, enhanced plant defense enzyme activities, and activated the expression of JA/ET pathway-related genes and ROS-related genes. These findings suggest the possible functions of low-concentration ABA. A follow-up research is required to clarify the specific mechanisms and causal links involved in these processes.
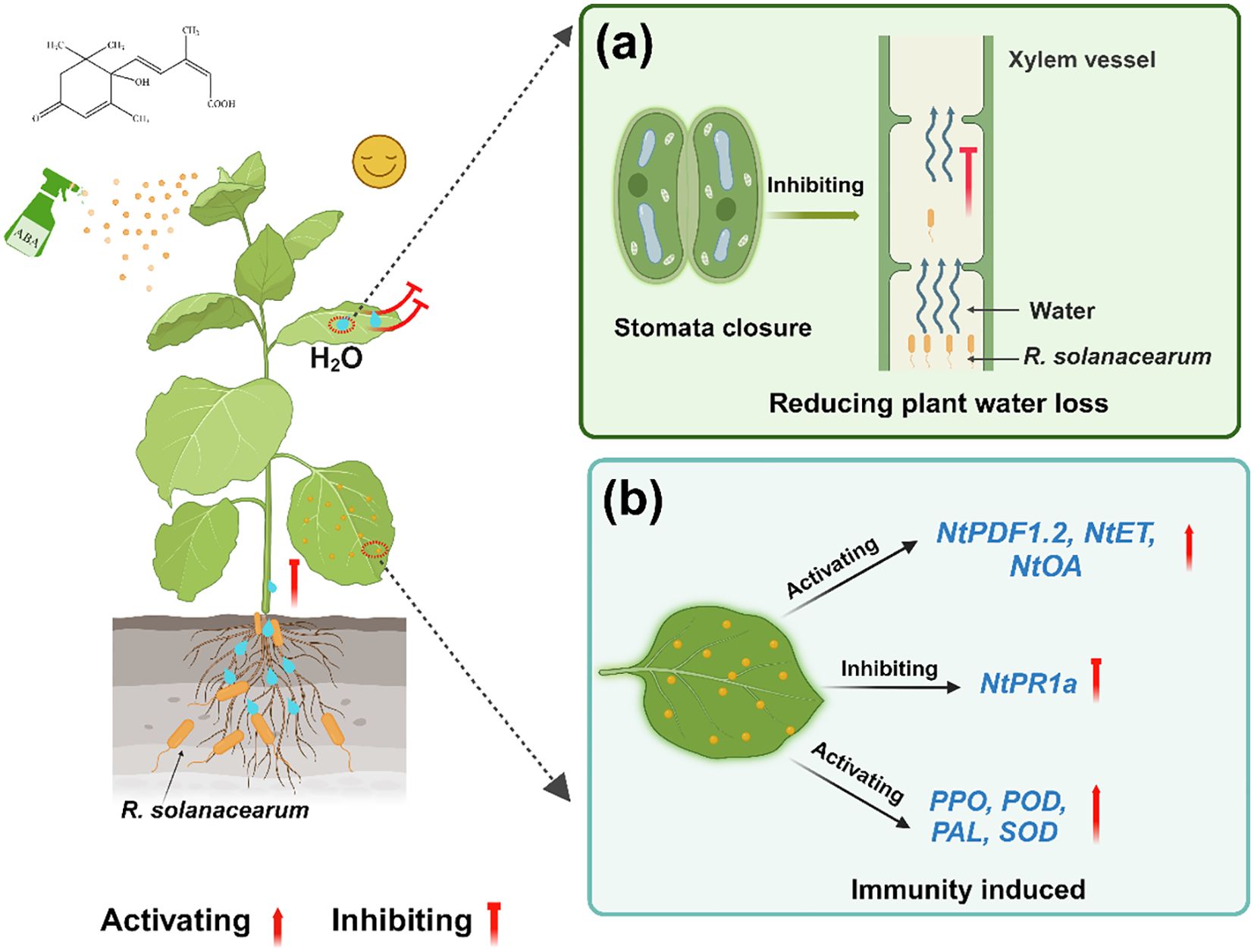
Figure 11. A model of foliar spraying ABA alleviated tobacco bacterial wilt. (a) Reducing plant water loss. (b) Immunity induced.
5 Conclusion
This study analyzed the preliminary mechanism of low-concentration ABA involved in R. solanacearum infection in tobacco plants. The results showed that PWC and Ψplant will decrease after R. solanacearum infection, and LWC and Ψleaf were decreased significantly. In response to biotic stress, plant leaves will accumulate a large amount of ABA to reduce the plant’s water loss. Interestingly, ABA accumulation in the leaves could enhance plant resistance to R. solanacearum, while inhibition of ABA biosynthesis promoted tobacco bacterial wilt. The application of 0.78 mg/L ABA reduced the colonization of R. solanacearum, and stimulated the activity of defense enzymes and resistance-associated genes. Moreover, the application of 0.78 mg/L ABA efficiently delayed the disease. This study clarifies initially the change of ABA-regulated plant water and induced plant resistance to tobacco bacterial wilt.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because in this study, we did not use animal samples.
Author contributions
YW: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. SL: Data curation, Software, Supervision, Validation, Writing – review & editing. AW: Conceptualization, Data curation, Software, Supervision, Writing – review & editing. XM: Data curation, Methodology, Software, Supervision, Writing – review & editing. WT: Funding acquisition, Writing – review & editing. YL: Data curation, Formal Analysis, Supervision, Conceptualization, Writing – review & editing. LZ: Conceptualization, Data curation, Formal Analysis, Supervision, Writing – review & editing. MY: Resources, Software, Supervision, Writing – review & editing. WD: Funding acquisition, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Fundamental Research Funds for the Central Universities (SWU-KR22049), the Science and Technology Project of Yibin Branch, Sichuan Tobacco Company (SCYC202315), the Science and Technology Project of Guizhou Company of China Tobacco Corporation (2022JBXM02), and the National Natural Science Foundation of China (32202321).
Conflict of interest
Author MY was employed by the company Yibin Tobacco Company of Sichuan Province. Author WT was employed by the company Zunyi Branch Company of Guizhou Tobacco Company.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1566215/full#supplementary-material
References
Adie, B. A. T., Pérez-Pérez, J.n., Pérez-Pérez, M. M., Godoy, M., Sánchez-Serrano, J.-J., Schmelz, E. A., et al. (2007). ABA is an essential signal for plant resistance to pathogens affecting JA biosynthesis and the activation of defenses in Arabidopsis. Plant Cell 19, 1665–1681. doi: 10.1105/tpc.106.048041
Aslam, M. N. and Mukhtar, T. (2023a). Distributional spectrum of bacterial wilt of chili incited by Ralstonia solanacearum in Pakistan. Bragantia 82, e20220196. doi: 10.1590/1678-4499-2022-0196
Aslam, M. N. and Mukhtar, T. (2023b). Characterization of Ralstonia solanacearum causing bacterial wilt from chili areas of Pakistan. Bragantia 82, e20230001. doi: 10.1590/1678-4499.20230001
Aslam, M. N. and Mukhtar, T. (2024). Evaluation of virulence among Pakistani isolates of Ralstonia solanacearum inducing bacterial wilt in chilies across different agro-ecological zones. Bragantia 83, e20230181. doi: 10.1590/1678-4499.20230181
Boanares, D., Oliveira, R. S., Isaias, R. M. S., França, M. G. C., and Peñuelas, J. (2020). The neglected reverse water pathway: Atmosphere–plant–soil continuum. Trends Plant Sci. 25, 1073–1075. doi: 10.1016/j.tplants.2020.07.012
Brodribb, T. J. and McAdam, S. A. M. (2011). Passive origins of stomatal control in vascular plants. Science 331, 582–585. doi: 10.1126/science.1197985
Brookbank, B. P., Patel, J., Gazzarrini, S., and Nambara, E. (2021). Role of basal ABA in plant growth and development. Genes 12, 1936. doi: 10.3390/genes12121936
Choat, B., Jansen, S., Brodribb, T. J., Cochard, H., Delzon, S., Bhaskar, R., et al. (2012). Global convergence in the vulnerability of forests to drought. Nature 491, 752–755. doi: 10.1038/nature11688
Du, H., Huang, F., Wu, N., Li, X., Hu, H., and Xiong, L. (2018). Integrative regulation of drought escape through ABA-dependent and -independent pathways in rice. Mol. Plant 11, 584–597. doi: 10.1016/j.molp.2018.01.004
Du, Y., Zhang, H., Jia, K., Chu, Z., Xu, S., Tran, L.-S. P., et al. (2024). Integrative regulation of drought escape through ABA-dependent and -independent pathways in rice. Mol. Plant 176, e14135. doi: 10.1111/ppl.14135
Franks, P. J., Berry, J. A., Lombardozzi, D. L., and Bonan, G. B. (2017). Stomatal function across temporal and spatial scales: Deep-time trends, land-atmosphere coupling and global models. Plant Physiol. 174, 583–602. doi: 10.1104/pp.17.00287
Genin, S. (2010). Stomatal function across temporal and spatial scales: Deep-time trends, land-atmosphere coupling and global models. Plant Physiol. 187, 920–928. doi: 10.1111/j.1469-8137.2010.03397.x
Han, S., Na, L., Rongchao, Z., Xiuqin, H., Wenyu, Z., Bo, Z., et al. (2023). Study on signal transmission mechanism of arbuscular mycorrhizal hyphal network against root rot of Salvia miltiorrhiza. Sci. Rep. 13, 16936. doi: 10.1038/s41598-023-43278-5
Han, S., Yang, L., Wang, Y., Ran, Y., Li, S., and Ding, W. (2021). Preliminary studies on the antibacterial mechanism of a new plant-derived compound, 7-Methoxycoumarin, against Ralstonia solanacearum. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.697911
Haverroth, E. J., Oliveira, L. A., Andrade, M. T., Taggart, M., McAdam, S. A. M., Zsögön, A., et al. (2023). Abscisic acid acts essentially on stomata, not on the xylem, to improve drought resistance in tomato. Plant Cell Environ.46 (11) 3229-3241. doi: 10.1111/pce.14676
Hsu, P.-K., Dubeaux, G., Takahashi, Y., and Schroeder, J. I. (2021). Signaling mechanisms in abscisic acid-mediated stomatal closure. Plant J. 105, 307–321. doi: 10.1111/tpj.15067
Jamnická, G., Fleischer, P., Konôpková, A., Pšidová, E., Kučerová, J., Kurjak, D., et al. (2019). Norway spruce (Picea abies L.) provenances use different physiological strategies to cope with water deficit. Forests 10, 651. doi: 10.3390/f10080651
Kannenberg, S. A., Guo, J. S., Novick, K. A., Anderegg, W. R. L., Feng, X., Kennedy, D., et al. (2022). Opportunities, challenges and pitfalls in characterizing plant water-use strategies. Funct. Ecol. 36, 24–37. doi: 10.1111/1365-2435.13945
Kuromori, T., Seo, M., and Shinozaki, K. (2018). ABA transport and plant water stress responses. Trends Plant Sci. 23, 513–522. doi: 10.1016/j.tplants.2018.04.001
Li, S., Liu, S., Zhang, Q., Cui, M., Zhao, M., Li, N., et al. (2022). The interaction of ABA and ROS in plant growth and stress resistances. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.1050132
Li, L., Zhu, T., Song, Y., Feng, L., Farag, E. A. H., and Ren, M. (2021). ABSCISIC ACID INSENSITIVE5 interacts with RIBOSOMAL S6 KINASE2 to mdiate ABA responses during seedling growth in Arabidopsis. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.598654
Lim, C. W., Baek, W., Jung, J., Kim, J. H., and Lee, S. C. (2015). Function of ABA in stomatal defense against biotic and drought stresses. Int. J. Mol. Sci. 16, 15251–15270. doi: 10.3390/ijms160715251
Long, Q., Xie, Y., He, Y., Li, Q., Zou, X., and Chen, S. (2019). Abscisic acid promotes jasmonic acid accumulation and plays a key role in citrus canker development. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.01634
Mansfield, J., Genin, S., Magori, S., Citovsky, V., Sriariyanum, M., Ronald, P., et al. (2012). Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 13, 614–629. doi: 10.1111/j.1364-3703.2012.00804.x
Martin-StPaul, N., Delzon, S., and Cochard, H. (2017). Plant resistance to drought depends on timely stomatal closure. Ecol. Lett. 20, 1437–1447. doi: 10.1111/ele.12851
Meigas, E., Uusküla, B., and Merilo, E. (2024). Abscisic acid induces stomatal closure in horsetails. New Phytol. 243, 503–505. doi: 10.1111/nph.19542
Milling, A., Babujee, L., and Allen, C. (2011). Ralstonia solanacearum extracellular polysaccharide is a apecific elicitor of defense responses in wilt-resistant tomato plants. PloS One 6, e15853. doi: 10.1371/journal.pone.0015853
Mphande, W., Farrell, A. D., Grove, I. G., Vickers, L. H., and Kettlewell, P. S. (2021). Yield improvement by antitranspirant application in droughted wheat is associated with reduced endogenous abscisic acid concentration. Agric. Water Manag. 244, 106528. doi: 10.1016/j.agwat.2020.106528
Niu, J., Rang, Z., Zhang, C., Chen, W., Tian, F., Yin, H., et al. (2016). The succession pattern of soil microbial communities and its relationship with tobacco bacterial wilt. BMC Microbiol. 16, 233. doi: 10.1186/s12866-016-0845-x
Rekhter, D., Lüdke, D., Ding, Y., Feussner, K., Zienkiewicz, K., Lipka, V., et al. (2019). Isochorismate-derived biosynthesis of the plant stress hormone salicylic acid. Science 365, 498–502. doi: 10.1126/science.aaw1720
Savary, S., Willocquet, L., Pethybridge, S. J., Esker, P., McRoberts, N., and Nelson, A. (2019). The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 3, 430–439. doi: 10.1038/s41559-018-0793-y
Schwarzenbacher, R. E., Wardell, G., Stassen, J., Guest, E., Zhang, P., Luna, E., et al. (2020). The IBI1 receptor of β-Aminobutyric acid interacts with VOZ transcription factors to regulate abscisic acid signaling and callose-associated defense. Mol. Plant 13, 1455–1469. doi: 10.1016/j.molp.2020.07.010
Shahbaz, M. U., Mukhtar, T., Ul-Haque, M. I., and Begum, N. (2015). Biochemical and serological characterization of ralstonia solanacearum associated with chilli seeds from Pakistan. Int. Jagric. Biol. 17, 31–40. Available at: https://www.researchgate.net/publication/269819419.
Shelden, M. C., Vandeleur, R., Kaiser, B. N., and Tyerman, S. D. (2017). A comparison of petiole hydraulics and aquaporin expression in an anisohydric and isohydric cultivar of grapevine in response to water-stress induced cavitation. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.01893
Shi, H., Xu, P., Yu, W., Cheng, Y., Ding, A., Wang, W., et al. (2022). Metabolomic and transcriptomic analysis of roots of tobacco varieties resistant and susceptible to bacterial wilt. Genomics 114, 110471. doi: 10.1016/j.ygeno.2022.110471
Song, W., Ma, X., Tan, H., and Zhou, J. (2011). Abscisic acid enhances resistance to Alternaria solani in tomato seedlings. Plant Physiol. Biochem. 49, 693–700. doi: 10.1016/j.plaphy.2011.03.018
Spinelli, G. M., Shackel, K. A., and Gilbert, M. E. (2017). A model exploring whether the coupled effects of plant water supply and demand affect the interpretation of water potentials and irrigation management. Agr. Water Manage. 192, 271–280. doi: 10.1016/j.agwat.2017.07.019
Sun, Z., Li, S., Chen, W., Zhang, J., Zhang, L., Sun, W., et al. (2021). Plant dehydrins: expression, regulatory networks, and protective roles in plants challenged by abiotic stress. Int. J. Mol. Sci. 22, 12619. doi: 10.3390/ijms222312619
Tao, Z.-q., Chen, Y.-q., Li, C., Zou, J.-x., Yan, P., Yuan, S.-f., et al. (2016). The causes and impacts for heat stress in spring maize during grain filling in the North China Plain — A review. J. Integr. Agric. 15, 2677–2687. doi: 10.1016/S2095-3119(16)61409-0
Vilela, M. S., Resende, L. S., Pozza, E. A., Netto, P. M., de Cassia Roteli, K., and Guimarães, R. J. (2022). Nitrogen, phosphorus, and potassium fertilization on the incidence of brown eye spot in coffee crop in vegetative stage. Trop. Plant Pathol. 47, 672–684. doi: 10.1007/s40858-022-00523-y
Wang, X.-p., Berndtsson, R., Pan, Y.-x., Hu, R., Zhang, Y.-f., and Li, Y. (2022). Spatiotemporal variation of soil water potential and its significance to water balance for a desert shrub area. Soil Till. Res. 224, 105506. doi: 10.1016/j.still.2022.105506
Wang, X., Guo, C., Peng, J., Li, C., Wan, F., Zhang, S., et al. (2019). ABRE-BINDING FACTORS play a role in the feedback regulation of ABA signaling by mediating rapid ABA induction of ABA co-receptor genes. New Phytol. 221, 341–355. doi: 10.1111/nph.15345
Wang, H., Li, N., Li, H., Zhang, S., Zhang, X., Yan, X., et al. (2023). Overexpression of NtGCN2 improves drought tolerance in tobacco by regulating proline accumulation, ROS scavenging ability, and stomatal closure. Plant Physiol. Bioch. 198, 107665. doi: 10.1016/j.plaphy.2023.107665
Wang, Y., Liang, Y., Ma, X., Dong, Y., Klakong, M., Wang, A., et al. (2024). An environmental-friendly resistance inducer in crop protection: V2C MXene nanosheets induce plant resistance to Ralstonia solanacearum via the ET/JA signaling pathway. Ind. Crop Prod. 220, 119269. doi: 10.1016/j.indcrop.2024.119269
Wang, T., Li, C., Wu, Z., Jia, Y., Wang, H., Sun, S., et al. (2017). Abscisic acid regulates auxin homeostasis in rice root tips to promote root hair elongation. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.01121
Wang, M., Sun, Y., Sun, G., Liu, X., Zhai, L., Shen, Q., et al. (2015). Water balance altered in cucumber plants infected with Fusarium oxysporum f. sp. cucumerinum. Sci. Rep. 5, 7722. doi: 10.1038/srep07722
Wang, Y., Yang, L., Zhou, X., Wang, Y., Liang, Y., Luo, B., et al. (2023). Molecular mechanism of plant elicitor daphnetin-carboxymethyl chitosan nanoparticles against Ralstonia solanacearum by activating plant system resistance. Int. J. Biol. Macromol. 241, 124580. doi: 10.1016/j.ijbiomac.2023.124580
Wright, C. A. and Beattie, G. A. (2004). Pseudomonas syringae pv. tomato cells encounter inhibitory levels of water stress during the hypersensitive response of Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 101, 3269–3274. doi: 10.1073/pnas.0400461101
Wu, K., Su, L., Fang, Z., Yuan, S., Wang, L., Shen, B., et al. (2017). Competitive use of root exudates by Bacillus amyloliquefaciens with Ralstonia solanacearum decreases the pathogenic population density and effectively controls tomato bacterial wilt. Sci. Hortic. 218, 132–138. doi: 10.1016/j.scienta.2017.01.047
Xiao, Q., Zhao, W., Ju, C., Peng, K., Yuan, M., Tan, Q., et al. (2024). Effects of different tillage depths on soil physical properties and the growth and yield of tobacco in the mountainous Chongqing region of China. Agriculture 14, 276. doi: 10.3390/agriculture14020276
Xie, K., Li, L., Zhang, H., Wang, R., Tan, X., He, Y., et al. (2018). Abscisic acid negatively modulates plant defence against rice black-streaked dwarf virus infection by suppressing the jasmonate pathway and regulating reactive oxygen species levels in rice. Plant Cell Environ. 41, 2504–2514. doi: 10.1111/pce.13372
Yang, L., Ding, W., Xu, Y., Wu, D., Li, S., Chen, J., et al. (2016). New Insights into the Antibacterial Activity of Hydroxycoumarins against Ralstonia solanacearum. Molecules 21, 468. doi: 10.3390/molecules21040468
Yang, L., Wang, Y., Liang, Y., Deng, H., Wang, J., Dai, Y., et al. (2024). pH-responsive bentonite nanoclay carriers control the release of benzothiazolinone to restrain bacterial wilt disease. Pestic. Biochem. Physiol. 198, 105754. doi: 10.1016/j.pestbp.2023.105754
Yang, J., Zhang, J., Wang, Z., Zhu, Q., and Wang, W. (2001). Hormonal changes in the grains of rice subjected to water stress during grain filling. Plant Physiol. 127, 315–323. doi: 10.1104/pp.127.1.315
Yari Kamrani, Y., Shomali, A., Aliniaeifard, S., Lastochkina, O., Moosavi-Nezhad, M., Hajinajaf, N., et al. (2022). Regulatory role of circadian clocks on ABA production and signaling, stomatal responses, and water-use efficiency under water-deficit conditions. Cells 11, 1154. doi: 10.3390/cells11071154
Yaseen, I., Mukhtar, T., Mubarik, A., Arshad, B., Sahu, N., and Somaddar, U. (2025). Management of Ralstonia solanacearum-Meloidogyne incognita complex with Trichoderma harzianum in tomato. Bragantia 84, e20240060. doi: 10.1590/1678-4499.20240060
Yop, G., Gair, L. H. V., da Silva, V. S., MaChado, A. C. Z., Santiago, D. C., and Tomaz, J. P. (2023). Abscisic Acid is involved in the resistance response of Arabidopsis thaliana against meloidogyne paranaensis. Plant Dis. 107, 2778–2783. doi: 10.1094/PDIS-07-22-1726-RE
Yoshida, T. and Fernie, A. R. (2018). Remote control of transpiration via ABA. Trends Plant Sci. 23, 755–758. doi: 10.1016/j.tplants.2018.07.001
You, M. K., Shin, H. Y., Kim, Y. J., Ok, S. H., Cho, S. K., Jeung, J. U., et al. (2010). Novel bifunctional nucleases, OmBBD and AtBBD1, are involved in abscisic acid-mediated callose deposition in Arabidopsis. Plant Physiol. 152, 1015–1029. doi: 10.1104/pp.109.147645
Yu, Q., Hua, X., Yao, H., Zhang, Q., He, J., Peng, L., et al. (2021). Abscisic acid receptors are involves in the Jasmonate signaling in Arabidopsis. Plant Signal. Behav. 16, 1948243. doi: 10.1080/15592324.2021.1948243
Zhang, S.-h., Xu, X.-f., Sun, Y.-m., Zhang, J.-l., and Li, C.-z. (2018). Influence of drought hardening on the resistance physiology of potato seedlings under drought stress. J. Integr. Agric. 17, 336–347. doi: 10.1016/S2095-3119(17)61758-1
Zhou, J., Zhang, H., Yang, Y., Zhang, Z., Zhang, H., Hu, X., et al. (2008). Abscisic acid regulates TSRF1-mediated resistance to Ralstonia solanacearum by modifying the expression of GCC box-containing genes in tobacco. J. Exp. Bot. 59, 645–652. doi: 10.1093/jxb/erm353
Keywords: abscisic acid, plant water content, induced resistance, tobacco bacterial wilt, R. solanacearum
Citation: Wang Y, Yan M, Wang A, Ma X, Tian W, Liu Y, Zhu L, Ding W and Li S (2025) Plants accumulate abscisic acid after Ralstonia solanacearum infection for enhanced dehydration tolerance and plant resistance. Front. Plant Sci. 16:1566215. doi: 10.3389/fpls.2025.1566215
Received: 24 January 2025; Accepted: 07 April 2025;
Published: 05 June 2025.
Edited by:
Youxiong Que, Chinese Academy of Tropical Agricultural Sciences, ChinaReviewed by:
Tariq Mukhtar, Pir Mehr Ali Shah Arid Agriculture University, PakistanKatarzyna Otulak-Kozieł, Warsaw University of Life Sciences, Poland
Copyright © 2025 Wang, Yan, Wang, Ma, Tian, Liu, Zhu, Ding and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shili Li, eXVhbmxzMjAxNkBzd3UuZWR1LmNu; Wei Ding, ZGluZ3dAc3d1LmVkdS5jbg==
†These authors have contributed equally to this work
 Yao Wang1†
Yao Wang1† Liquan Zhu
Liquan Zhu Wei Ding
Wei Ding