Abstract
Introduction:
Bolboschoenus planiculmis (F. Schmidt) T. V. Egorova plays an important ecological role in wetland ecosystems by providing essential habitat and food resources for the critically endangered Siberian crane (Grus leucogeranus). It frequently coexists with Phragmites australis (Cav.) Trin. ex Steud. (reed) in natural wetland communities; however, the allelopathic activity of reed on B. planiculmis remains poorly understood.
Methods:
This study investigated the allelopathic effects of reed on B. planiculmis and identified the phenolic allelochemicals involved. Aqueous extracts from individual reed organs (roots, stems, and leaves), as well as from a mixture of these organs in equal mass proportions, were prepared at two concentrations (7% and 14%) using plant materials collected during both the nutrient and reproductive growth stages.
Results and discussion:
Pot experiments revealed that reed aqueous extracts exhibited significant inhibitory activity on the germination and seedling growth of B. planiculmis. The leaf extract showed relatively stronger inhibitory effects compared to the extracts of other organs, especially in the nutrient growth stage. A total of 24 phenolic compounds, including 13 phenolic acids, 9 flavonoids, and 2 coumarins, were identified as potential allelochemicals in reed aqueous extracts. The concentration of phenolic allelochemicals in leaf extract was much higher than that in root and stem extracts. These findings demonstrate the allelopathic inhibitory effect of reed on the germination and seedling growth of B. planiculmis, primarily mediated by active phenolic compounds derived from leaves. Notably, this study is the first to identify flavonoids and coumarins, in addition to phenolic acids, as potential allelochemicals contributing to the allelopathic effects of reed on B. planiculmis in wetland ecosystems. This study enhances our understanding of ecological interactions among wetland plants and provides guidance for the conservation and management of the key functional species B. planiculmis.
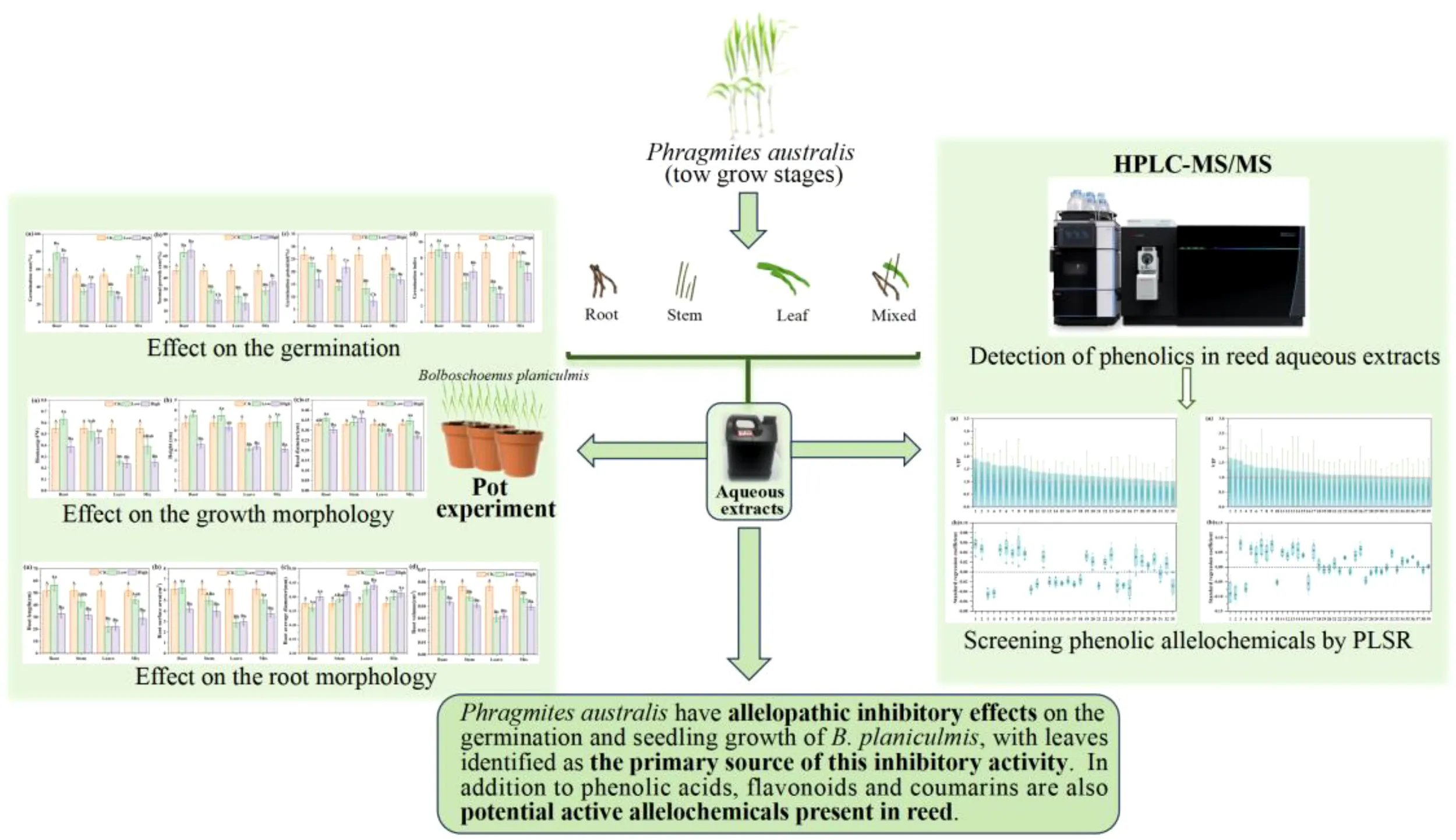
1 Introduction
Phragmites australis (Cav.) Trin. ex Steud. (reed) is one of the most globally widespread wetland plants, found on every continent except Antarctica (Packer et al., 2017). Under favorable conditions, it typically forms dense monospecific stands, and its expansion or invasion can significantly alter ecosystem structure and function (Wails et al., 2021). Allelopathy is a significant mechanism of plant-plant interaction, involving the inhibition of neighboring plant growth through the production and release of secondary metabolites, commonly known as allelochemicals (Hierro and Callaway, 2021). This mechanism is considered an important driver of the reed’s strong interspecific competitive ability and broad distribution (Uddin et al., 2014b, 2017). In southeastern Australia, wetland vegetation dominated by Melaleuca ericifolia is threatened by the expansion of reed, as allelochemicals secreted by its roots can inhibit both germination and growth of M. ericifolia (Uddin et al., 2014b). Similarly, in the coexisting communities of reed and Suaeda salsa in China’s Yellow River Delta, allelopathy serves as a crucial ecological adaptation mechanism that enables reed to maintain a strong interspecific competitive advantage (Gao et al., 2022). Aqueous extracts of reed have been shown to significantly inhibit the germination and seedling growth of S.salsa (Gao et al., 2022).
The Momoge National Nature Reserve serves as a critical stopover site along the migratory route of the critically endangered Siberian Crane (Grus leucogeranus) (Wang Y. et al., 2022). Bolboschoenus planiculmis (F. Schmidt) T. V. Egorova is a key protected plant species within the reserve, with its communities providing primary stopover habitat and its underground tubers serving as an essential food source for Siberian Cranes (An et al., 2021). In recent years, however, B. planiculmis has undergone population decline and a notable reduction in distribution area, posing a serious threat to the cranes’ stopover and survival (An et al., 2018; Zhang et al., 2022). Within the reserve, B. planiculmis frequently co-occurs with reed in the same plant communities, but it exhibits poor growth performance and shows a trend of being gradually displaced by reed (Song et al., 2015; Ding et al., 2021; Tang et al., 2024; Liu et al., 2018). Nevertheless, whether reed influences the growth of B. planiculmis through allelopathic interactions remains uncertain.
The inhibitory allelopathic effects of reed on seed germination and seedling growth have been documented in a variety of plant species, including model species such as Lactuca sativa and Arabidopsis thaliana, economic crops such as Vigna radiata and Nicotiana tabacum (Rudrappa et al., 2007), and common weeds like Poa labillardierei and Avena fatua (Rudrappa et al., 2007; Uddin et al., 2017, 2014a, 2014b). Moreover, the allelopathic activity of reed appears to vary among its different organs. For instance (Gao et al., 2022) found that the underground parts (rhizomes and roots) exhibit stronger phytotoxic effects than the aboveground parts (stems and leaves), whereas (Uddin et al., 2012) reported that the leaves possess greater allelopathic inhibition activity than the rhizomes and roots. However, allelopathic effects are known to exhibit species-specific variability (Zhang et al., 2021). To date, the allelopathic effects of reed on the germination and growth of B. planiculmis, as well as the organ-specific variations in allelopathic activity, remain largely unexplored.
Phenolic compounds represent one of the most common classes of plant allelochemicals, encompassing phenolic acids, flavonoids, and coumarins (Macías et al., 2019). Reed synthesizes and releases phenolic compounds into its surrounding environment (Uddin and Robinson, 2017b), and the concentration of soluble phenolics in soils within reed-colonized areas has been reported to be more than twice that in non-colonized areas (Uddin et al., 2017). Gallic acid is a key allelopathic compound found in the root exudates of reed, capable of inhibiting root growth in plants (Rudrappa et al., 2007). Subsequent studies have also detected gallic acid in other reed tissues, including the leaves, flowers, and stems (Uddin et al., 2014). Moreover, the decomposition of reed litter releases a range of phenolic acids—such as p-coumaric acid, vanillic acid, sinapic acid, syringic acid, caffeic acid, protocatechuic acid, and gallic acid—that have been shown to inhibit algal growth (Nakai et al., 2006). In other Poaceae species, flavonoids also function as important allelochemicals (Anwar et al., 2021; Alghamdi et al., 2022; Vieites-Álvarez et al., 2023). For example, aqueous extracts of the invasive grass Imperata cylindrica contain compounds such as 5-methoxyflavone and 5,2′-dimethoxyflavone, which effectively suppress the growth of various weed species (Suzuki et al., 2018). However, flavonoids and coumarins with allelopathic activity have not yet been identified in reed.
This study aims to investigate the allelopathic effects of reed on B. planiculmis and to identify the active allelochemicals involved. Through pot experiments, we assessed the allelopathic effects of aqueous extracts derived from different reed organs and growth stages on the germination and seedling growth of B. planiculmis. In addition, we screened and identified potential phenolic allelochemicals in the reed extracts. We hypothesize that (1) aqueous extracts of reed inhibit the germination and seedling growth of B. planiculmis, with the inhibitory strength varying depending on the reed organ and growth stage; and (2) phenolic acids, flavonoids, and coumarins present in the extracts collectively contribute to the allelopathic effects of reed on B. planiculmis. The findings of this study will enhance our understanding of the ecological interactions between reed and B. planiculmis.
2 Materials and methods
2.1 Collection of experimental materials
The materials required for the experiment were collected from the buffer zone of the Momoge National Nature Reserve (123°46’98”E, 46°00’73”N). Whole reed plants were collected separately in early June 2023 during the nutrient growth stage and in late August 2023 during the reproductive growth stage. The whole reed plants were separated into roots, stems, and leaves, washed with tap water, then rinsed several times with ultrapure water, air-dried in the shade, and cut into 2 cm segments. Corms and in-situ soil (0–30 cm) of B. planiculmis were collected in early April 2023. Harvested corms were rinsed with tap water, disinfected in 5% sodium hypochlorite for 10 minutes, thoroughly washed with ultrapure water, blotted dry with absorbent paper, and stored at 4°C. Soil was air-dried, sieved through a 2 mm mesh, and homogenized to serve as the growth substrate for B. planiculmis in the pot experiment. Species identification was confirmed by Professor Yong Wang, and voucher specimens were deposited at the Natural History Museum of Northeast Normal University under registration numbers 1-001336-0010 (reed) and 1-001335-0008 (B. planiculmis).
2.2 Preparation of reed aqueous extracts
Based on previous literature (Khan et al., 2016; Wang C. et al., 2022) and preliminary experiments (Supplementary Table S1), concentrations of 7% and 14% were selected for the reed aqueous extracts. Each reed organ (140 g) was placed in a sterilized black bucket with 1 L of ultrapure water and soaked at 25°C for 48 hours, with ultrasonic treatment for 30 minutes every 12 hours to obtain crude extracts from the reed organs. The extract was then filtered and purified using 0.45 μm filter paper, resulting in a 14% (m/v) aqueous extract of reed roots, stems, and leaves. The roots, stems, and leaves were mixed in equal mass proportions and extracted using the aforementioned method to obtain the mixed aqueous extract. This extract simulated the natural coexistence of reed organs and their combined allelopathic potential. A portion of the prepared extract was stored in a 4°C refrigerator for pot experiments and diluted to a 7% concentration when needed. Another portion was stored in a –80°C freezer for the identification of phenolic allelochemicals.
2.3 Pot experiment
Pot experiments were conducted to investigate the allelopathic effects of reed from both the nutrient growth and reproductive growth stages on B. planiculmis. Eight different aqueous extracts (four organs × two concentrations) were prepared from reed for each growth stage. 0.9 kg in-situ soil of B. planiculmis was added to each pot (diameter: 18.5 cm, height: 8.8 cm), and the soil was moistened with 200 mL of ultrapure water. Fifteen healthy, similarly sized corms of B. planiculmis were evenly buried in the soil at the same depth. The pots were placed in an incubator set to 25°C, 60% humidity, and a 12 h light/12 h dark cycle with 250 μmol m−2 s−1 photosynthetic photon flux density during the light period for 10 days, during which the reed aqueous extract treatment was applied (Table 1).
Table 1
| Reed aqueous extracts | Treatment method | ||
|---|---|---|---|
| Growth stage | Organ | Concentration | |
| nutrient growth stage/reproductive growth stage | root | Low: 7% | Root extract (100 mL) and ultrapure water (100 mL) were applied on alternate days. |
| High: 14% | |||
| stem | Low: 7% | Stem extract (100 mL) and ultrapure water (100 mL) were applied on alternate days. | |
| High: 14% | |||
| leaf | Low: 7% | Leave extract (100 mL) and ultrapure water (100 mL) were applied on alternate days. | |
| High: 14% | |||
| mixed | Low: 7% | Mixed extract (100 mL) and ultrapure water (100 mL) were applied on alternate days. | |
| High: 14% | |||
The reed aqueous extract treatments in the pot experiment.
Each treatment consisted of four replicates, with each pot serving as an individual replicate. A daily addition of 100 mL ultrapure water was used as the blank control (CK).
The germination status was recorded daily. The following formulas were used to calculate the germination indicators: germination rate (GR), normal growth rate (NR), germination potential (GP), and germination index (GI):
where n is the number of germinated corms within 10 days, nj is the number of corms that germinated and grew normally (seedlings with true leaves and complete roots) within 10 days, nk is the number of germinated corms within the first 4 days, Gt is the number of germinated corms within t days, Dt is the corresponding number of germination days, and N is the total number of corms tested.
After 10 days, the growth parameters of the seedlings were measured, including biomass (fresh weight), plant height, and basal diameter. Root morphological parameters, including root length, root surface area, average root diameter, and root volume, were scanned using an Epson Perfection V850 Pro and analyzed with WinRHIZO 2017. The values for these indicators were obtained by calculating the average of all seedlings in each pot.
2.4 Detection of phenolic compounds in reed aqueous extracts
Phenolic compounds in the reed aqueous extract (high-concentration: 14%) were detected using high-performance liquid chromatography coupled with tandem mass spectrometry (HPLC-MS/MS). The reed extracts (100 µL) were placed in EP tubes and resuspended in pre-chilled 80% methanol by vortexing. The supernatants were collected following the standard procedure from Novogene (Deng et al., 2023). Then, the supernatant was injected into the HPLC-MS/MS analyzer using a Vanquish HPLC system (ThermoFisher, Germany) coupled with an Orbitrap Q Exactive HF mass spectrometer (Thermo Fisher, Germany) at Novogene Co., Ltd. (Beijing, China). In positive ion mode, mobile phase A was a 0.1% formic acid solution, while in negative ion mode, mobile phase A was a 5 mmol/L ammonium acetate solution. Mobile phase B was methanol, and the flow rate was 0.2 mL/min. The mobile phase gradient was set as follows: 0–2% B, 0–1.5 min; 2–85% B, 1.5–3 min; 85–100% B, 3–10 min;100–2% B, 10–10.1 min; 2% B, 10.1–12 min. The Q Exactive™ HF mass spectrometer was operated in both positive and negative polarity modes.
The raw data files were processed using Compound Discoverer 3.1 (ThermoFisher). The peak intensities were normalized to the total spectral intensity and used to predict the molecular formula based on additive ions, molecular ion peaks, and fragment ions. The peaks were matched to the mzCloud, mzVault, and MassList databases to obtain accurate qualitative and relative quantitative results.
2.5 Screening of reed phenolic allelochemicals
Partial Least Squares Regression (PLSR) analysis can address collinearity and noise among variables, and it is suitable for the complex analysis of small sample sizes and multiple variables (Wold et al., 2001). It has also been used in the screening of allelopathic substances (Reiss et al., 2018; Niazipoor et al., 2024). PLSR analysis was performed to investigate the relationship between the independent variable, “relative content of 91 phenolic compounds in reed aqueous extract,” and the dependent variable, “germination and seedling growth characteristics of B. planiculmis,” and screen the potential phenolic allelochemicals (Zhang et al., 2020). The dependent variables were divided into three groups based on their correlations, and each group was modeled separately (Wold et al., 2001). Model 1 focused on four germination indicators (GR, NR, GP, and GI), Model 2 on three growth indicators (biomass, height, and basal diameter), and Model 3 on four root morphology indicators (root length, root surface area, average root diameter, and root volume). PLSR reduces the dimensionality of the original variables, extracting key feature components, which are then used as new variables to build the regression model. The predictive variance of model for the dependent variable (Q2) was calculated using cross-validation. The root mean square error of estimation (RMSEE) was used to evaluate the accuracy of the model predictions. To prevent overfitting of the PLSR model, an appropriate number of PLSR components was determined by an optimal balance between R2 (model correlation coefficient, the explained variance of model for the dependent variables) and Q2. Variable importance for projection (VIP) is used to assess the degree of influence of the independent variables on the dependent variable. Phenolic compounds with a VIP > 1 and a standard regression coefficient< 0 are considered allelochemicals that inhibit the germination and seedling growth of B. planiculmis in reed aqueous extract (Zhang et al., 2020; Niazipoor et al., 2024).
2.6 Data analysis
Analysis of variance (ANOVA) was performed on germination, growth, and root morphology indicators of B. planiculmis using SPSS (version 25.0 (SPSS Inc., Chicago, USA). Before conducting the ANOVA, the normality of the data was assessed using the Shapiro-Wilk test, and the homogeneity of variance was tested using Levene’s test. The least significant difference test was performed to determine significant differences among treatments (P < 0.05). The Response Index (RI) was used to quantify the impact of a specific reed aqueous extract treatment on a particular indicator of B. planiculmis, where a positive value indicates promotion, a negative value indicates inhibition and the absolute value represents the intensity of the effect. The calculation formula is as follows (Bruce Williamson and Richardson, 1988):
Where C represents the control value and T represents the treatment value. The synergistic allelopathic effect index (SE) is the arithmetic mean of the RI values for different measured indicators of B. planiculmis under the same reed aqueous extract treatment, thus quantifying the allelopathic activity of different extracts (Wang et al., 2024). The sensitivity index (SI) is the arithmetic mean of the RI values of the same indicator of B. planiculmis under different reed aqueous extract treatments; thus, it evaluates the sensitivity of the indicators to the treatment (Wang et al., 2024).
Principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) were employed to evaluate the differences in the relative content of phenolic allelochemicals across different reed extracts. Phenolic allelochemicals with VIP > 1, a P-value from the t-test< 0.05, and fold change (FC) > 1.5, or FC< 0.67 were considered differential allelochemicals (Li et al., 2022). PLSR, PCA, and PLS-DA analyses were performed using the SIMCA17.0 software (Umetrics AB, Sweden).
3 Result
3.1 Effect of reed aqueous extracts on Bolboschoenus planiculmis
3.1.1 Effect of reed aqueous extracts on the germination of Bolboschoenus planiculmis
Equations 1-4 were used to calculate the GR, NR, GP, and GI, respectively. For reed in the nutrient growth stage, stem, leaf, and mixed extracts inhibited the B. planiculmis germination, while root extract promoted it (Figure 1). The mixed extract reduced NR and GP by 21%–39%; stem extract reduced NR, GP, and GI by 9%–57%; leaf extract reduced all four germination indicators by 34%-69%; but root extract increased GR and NR by 36%–47% (P< 0.05). Among all treatments, the high-concentration leaf extract caused the greatest reduction in GR, NR, GP, and GI, with decreases of 47%, 64%, 69%, and 60% compared to the control, respectively. For reed in the reproductive growth stage, leaf and root extracts showed inhibitory effects on B. planiculmis germination, while stem and mixed extracts had a low-promoting, high-inhibitory effect (Figure 1). The leaf extract reduced GR, NR, GP, and GI by 11%–71%, and the root extract reduced NR, GP, and GI by 9%–29%. Among all treatments, the high-concentration leaf extract caused the greatest reduction in GR, NR, GP, and GI, with decreases of 18%, 39%, 71%, and 44% compared to the control, respectively. The interaction between reed growth stage and organ significantly affected the GR, NR, and GI of B. planiculmis (Supplementary Table S2, P< 0.001).
Figure 1
Based on Equation (5), RI was computed, and SE was subsequently determined. For reed in the nutrient growth stage, the SE of different extracts on germination was ranked as “LH< LL< SL< SH< MH< ML< RH< RL”. In the reproductive growth stage, it was “LH< LL< SH< RH< MH< RL< ML< SL” (Table 2). The leaf extract had the strongest inhibitory effect in both two growth stages. The root extract exhibited a promoting effect in the nutrient growth stage and an inhibitory effect in the reproductive growth stage.
Table 2
| Reed aqueous extracts | RI | SE | ||||
|---|---|---|---|---|---|---|
| GR | NR | GP | GI | |||
| nutrient growth stage | RL | 0.32 | 0.26 | -0.13 | 0.04 | 0.12 |
| RH | 0.27 | 0.28 | -0.38 | 0.00 | 0.04 | |
| SL | -0.34 | -0.39 | -0.44 | -0.44 | -0.40 | |
| SH | -0.19 | -0.57 | -0.19 | -0.27 | -0.30 | |
| LL | -0.34 | -0.50 | -0.50 | -0.50 | -0.46 | |
| LH | -0.47 | -0.64 | -0.69 | -0.60 | -0.60 | |
| ML | 0.16 | -0.39 | -0.25 | -0.12 | -0.15 | |
| MH | -0.03 | -0.21 | -0.38 | -0.29 | -0.23 | |
| SI | -0.08 | -0.27 | -0.37 | -0.27 | - | |
| reproductive growth stage | RL | 0.10 | -0.09 | -0.14 | -0.13 | -0.07 |
| RH | 0.03 | -0.13 | -0.29 | -0.16 | -0.14 | |
| SL | 0.22 | 0.30 | 0.30 | 0.21 | 0.26 | |
| SH | 0.00 | -0.13 | -0.29 | -0.22 | -0.16 | |
| LL | -0.11 | -0.22 | -0.43 | -0.32 | -0.27 | |
| LH | -0.18 | -0.39 | -0.71 | -0.44 | -0.43 | |
| ML | 0.12 | 0.09 | 0.22 | 0.07 | 0.13 | |
| MH | 0.03 | -0.09 | -0.14 | -0.15 | -0.09 | |
| SI | 0.03 | -0.08 | -0.18 | -0.14 | - | |
Allelopathic effect evaluation of reed aqueous extracts on germination of Bolboschoenus planiculmis.
RL/RH, SL/SH, LL/LH, and ML/MH represent the low (L) and high (H) concentrations of aqueous extracts from the reed root (R), stem (S), leaf (L), and mixture (M), respectively. GR, germination rate; NR, normal growth rate; GP, germination potential; GI, germination index; RI, response index; SI, sensitivity index; SE, synergistic allelopathic effect index.
3.1.2 Effect of reed aqueous extracts on the growth of Bolboschoenus planiculmis seedlings
3.1.2.1 Effect of reed aqueous extracts on the growth morphology of Bolboschoenus planiculmis seedlings
For reed in the nutrient growth stage, the leaf extract strongly inhibited seedling growth of B. planiculmis, the mixed extract inhibited growth only at high concentrations, and the root extract showed a low-promoting, high-inhibitory effect (Figure 2). The high-concentration root extract reduced biomass, height, and basal diameter by 30%, 32%, and 8%, respectively; mixed extract by 55%, 39%, and 19%. The leaf extract significantly reduced biomass and height by 54% and 38% even at low concentrations (P< 0.05). For reed in the reproductive growth stage, the root extract inhibited the seedling growth of B. planiculmis, the stem and mixed extracts were inhibitory only at high concentrations (Figure 2). The high-concentration stem extract reduced biomass, height, and basal diameter by 18%, 27%, and 19%, respectively; mixed extract by 26%, 31%, and 26%; and root extract by 31%, 37%, and 28% (P< 0.05). The growth morphology of B. planiculmis seedlings was significantly influenced by the interaction among growth stage, organ, and concentration (Supplementary Table S3). The interactions between growth stage and organ, as well as between organ and concentration, significantly affected the biomass, height, and basal diameter of B. planiculmis (P< 0.05). The three-way interaction of these factors had a significant effect on seedling height and basal diameter (P< 0.05).
Figure 2
3.1.2.2 Effect of reed aqueous extracts on the root morphology of Bolboschoenus planiculmis seedlings
For reed in the nutrient growth stage, stem, leaf, and mixed extracts inhibited root growth of B. planiculmis, the root extract was inhibitory only at high concentrations (Figure 3). The high-concentration root extract reduced root length, surface area, and volume by 36%, 31%, and 23%, respectively; stem extract by 39%, 35%, and 28%; and mixed extract by 44%, 39%, and 30%. The leaf extract exhibited strong inhibition even at low concentrations, reducing them by 57%, 53%, and 47% (P< 0.05). For reed in the reproductive growth stage, the leaf extract showed mild inhibition on root growth of B. planiculmis; stem and mixed extracts exhibited a low-promoting, high-inhibitory effect; and the root extract had a strong inhibitory effect even at low concentrations (Figure 3). The high-concentration root extract caused the greatest reduction in root length, root surface area, and root volume among all treatments, with decreases of 38%, 45%, and 28% compared to the control, respectively (P< 0.05). The root morphology of B. planiculmis seedlings was significantly influenced by the interaction among growth stage, organ, and concentration (Supplementary Table S4). The interactions between growth stage and organ, as well as between organ and concentration, significantly affected the root length, surface area, and volume of B. planiculmis (P< 0.05). The three-way interaction of these factors had a significant effect on root length and surface area (P< 0.05).
Figure 3
For reed in the nutrient growth stage, the SE of different extracts on seedling growth was ranked as “ LH = LL< MH< RH< SH< ML< SL< RL”. In the reproductive growth stage, it was “RH< MH< RL< SH< LH< LL< ML< SL” (Table 3). The leaf extract had the strongest inhibitory effect in the nutrient growth stage, while the root extract was most inhibitory in the reproductive growth stage.
Table 3
| Reed aqueous extracts | RI | SE | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Biomass | Height | Basal diameter | Root length | Root surface area | Average root diameter | Root volume | |||
| nutrient growth stage | RL | 0.13 | 0.10 | 0.07 | 0.08 | 0.01 | -0.04 | 0.00 | 0.05 |
| RH | -0.30 | -0.32 | -0.08 | -0.36 | -0.31 | 0.06 | -0.23 | -0.22 | |
| SL | -0.06 | 0.10 | 0.04 | -0.17 | -0.18 | 0.04 | -0.16 | -0.06 | |
| SH | -0.15 | -0.07 | 0.08 | -0.39 | -0.35 | 0.10 | -0.28 | -0.15 | |
| LL | -0.54 | -0.38 | -0.07 | -0.57 | -0.53 | 0.12 | -0.47 | -0.35 | |
| LH | -0.56 | -0.36 | -0.15 | -0.57 | -0.51 | 0.14 | -0.43 | -0.35 | |
| ML | -0.29 | 0.02 | 0.05 | -0.15 | -0.17 | 0.06 | -0.18 | -0.09 | |
| MH | -0.55 | -0.39 | -0.19 | -0.44 | -0.39 | 0.09 | -0.30 | -0.31 | |
| SI | -0.29 | -0.16 | -0.03 | -0.32 | -0.30 | 0.07 | -0.26 | – | |
| reproductive growth stage | RL | -0.18 | -0.23 | -0.16 | -0.37 | -0.41 | 0.03 | -0.27 | -0.23 |
| RH | -0.31 | -0.37 | -0.28 | -0.38 | -0.45 | 0.06 | -0.29 | -0.29 | |
| SL | 0.00 | 0.03 | 0.04 | 0.08 | 0.03 | 0.00 | 0.03 | 0.03 | |
| SH | -0.18 | -0.27 | -0.19 | -0.22 | -0.36 | 0.02 | -0.16 | -0.19 | |
| LL | -0.04 | 0.02 | -0.03 | -0.07 | -0.12 | 0.01 | -0.04 | -0.04 | |
| LH | -0.05 | -0.03 | -0.02 | -0.19 | -0.27 | 0.01 | -0.09 | -0.09 | |
| ML | 0.05 | 0.05 | 0.03 | 0.05 | 0.06 | 0.01 | 0.02 | 0.04 | |
| MH | -0.26 | -0.31 | -0.26 | -0.31 | -0.37 | 0.03 | -0.18 | -0.24 | |
| SI | -0.12 | -0.14 | -0.11 | -0.18 | -0.24 | 0.02 | -0.12 | – | |
Allelopathic effect evaluation of reed aqueous extracts on the growth of Bolboschoenus planiculmis seedlings.
RL/RH, SL/SH, LL/LH, and ML/MH represent the low (L) and high (H) concentrations of aqueous extracts from the reed root (R), stem (S), leaf (L), and mixture (M), respectively. RI, response index; SI, sensitivity index; SE, synergistic allelopathic effect index.
3.2 Phenolic compounds in reed aqueous extracts
A total of 91 phenolic compounds were identified in reed aqueous extracts using untargeted metabolomics with HPLC-MS/MS, including 71 phenolic acids (Supplementary Table S5), 16 flavonoids (Supplementary Table S6), and four coumarins (Supplementary Table S7).
3.3 Phenolic allelochemicals in reed aqueous extracts
3.3.1 Screening of phenolic allelochemicals
The maximum Q2 values for M1 and M2 were obtained with three components, explaining 73.4% and 70.6% of the variance in the dependent variables, respectively (Table 4). M3 was excluded due to its poor explanatory and predictive performance. M1 and M2 exhibited strong predictive capabilities for the germination and seedling growth indicators of B. planiculmis, with minimal differences between predicted and observed values (Figure 4).
Table 4
| Model | Dependent variable | Component | RX2 | RX2(cum) | RY2 | RY2(cum) | Q2 | Q2(cum) |
|---|---|---|---|---|---|---|---|---|
| M1 | GR/NR/GP/GI | 1 | 0.245 | 0.245 | 0.508 | 0.508 | 0.402 | 0.402 |
| 2 | 0.277 | 0.522 | 0.124 | 0.632 | 0.116 | 0.471 | ||
| 3 | 0.127 | 0.649 | 0.101 | 0.734 | 0.084 | 0.516 | ||
| M2 | Biomass/Height/Basal diameter | 1 | 0.304 | 0.304 | 0.303 | 0.303 | 0.192 | 0.192 |
| 2 | 0.159 | 0.463 | 0.253 | 0.556 | 0.187 | 0.343 | ||
| 3 | 0.120 | 0.583 | 0.150 | 0.706 | 0.181 | 0.461 | ||
| M3 | Root length/Root surface area/Average root diameter/Root volume | 1 | 0.177 | 0.177 | 0.359 | 0.359 | 0.033 | 0.033 |
Parameters of the PLSR models.
RX2(cum) represents the cumulative explained variance of the component for the independent variable, RY2(cum) represents the cumulative explained variance of the component for the dependent variable, and Q2(cum) represents the cumulative predictive variance of the component for the dependent variable.
Figure 4
Fourteen potential phenolic allelochemicals that inhibit B. planiculmis germination were screened in reed aqueous extracts through M1 (Figure 5), and twelve allelochemicals that inhibit B. planiculmis seedling growth were screened through M2 (Figure 6). In total, 24 potential phenolic allelochemicals were identified, including 13 phenolic acids, nine flavonoids, and two coumarins (Table 5). 4-Hydroxypropranolol and rutin exhibited inhibitory effects on both germination and seedling growth of B. planiculmis.
Figure 5
Figure 6
Table 5
| Class | Number | Compound name | Inhibited process |
|---|---|---|---|
| Phenolic acid | 1 | 4-Nitrophenol | germination |
| 2 | 3-Coumaric acid | germination | |
| 3 | 6-Gingerol | germination | |
| 4 | Isorhapontigenin | germination | |
| 5 | 4-Hexylresorcinol | germination | |
| 6 | Homogentisate | seedling growth | |
| 7 | Eugenol | seedling growth | |
| 8 | Vanillic acid | seedling growth | |
| 9 | Curcumin | seedling growth | |
| 10 | Ethyl ferulate | seedling growth | |
| 11 | Propylparaben | seedling growth | |
| 12 | Methyl 4-hydroxycinnamate | seedling growth | |
| 13 | 4-Hydroxypropranolol | germination and seedling growth | |
| Flavonoid | 1 | 4’-O-Glucosylvitexin | germination |
| 2 | Isorhamnetin | germination | |
| 3 | Orientin | germination | |
| 4 | Diosmetin | germination | |
| 5 | Quercetin-3β-D-glucoside | germination | |
| 6 | Quercetin | germination | |
| 7 | Quercetin-3-O-beta-glucopyranosyl-6’-acetate | germination | |
| 8 | Naringin | seedling growth | |
| 9 | Rutin | germination and seedling growth | |
| Coumarin | 1 | Esculetin | seedling growth |
| 2 | Isofraxidin | seedling growth |
Potential phenolic allelochemicals in reed aqueous extracts.
3.3.2 Multivariate analysis of phenolic allelochemicals
The PCA score plot revealed differences in the relative content of phenolic allelochemicals across different reed aqueous extracts (Figure 7). The first and second principal components distinguished the extracts based on organ type and growth stage, respectively. The content of phenolic allelochemicals in the leaf extract was significantly different from that in the stem and root extracts, with minimal variation between the two growth stages.
Figure 7
The leaf extract of reed during the nutrient growth stage contained significantly higher levels of 11 differential allelochemicals (VIP > 1, P< 0.05, and FC > 1.5 or FC< 0.67) compared to the stem extract from the same stage, with an average fold change of 46.47. Additionally, the contents of 12 differential allelochemicals in the leaf extract were significantly higher than those in the root extract during the nutrient growth stage, with an average fold change of 24.24 (Table 6).
Table 6
| Class | Compound name | JL vs JR | JL vs JS | ||||
|---|---|---|---|---|---|---|---|
| P | FC | VIP | P | FC | VIP | ||
| Phenolic acid | 4-Nitrophenol | <0.001 | 2.97 | 1.07 | <0.05 | 1.93 | 1.07 |
| 6-Gingerol | <0.001 | 7.75 | 1.07 | <0.001 | 3.76 | 1.07 | |
| 4-Hydroxypropranolol | <0.01 | 81.76 | 1.06 | <0.01 | 14.78 | 1.06 | |
| Isorhapontigenin | <0.001 | 16.16 | 1.07 | <0.001 | 9.44 | 1.07 | |
| 3-Coumaric acid | <0.001 | 2.53 | 1.05 | 0.73 | 0.98 | 1.05 | |
| Vanillic acid | <0.001 | 0.39 | 1.06 | 0.31 | 0.82 | 1.06 | |
| Curcumin | <0.01 | 0.19 | 1.06 | 0.45 | 0.90 | 1.06 | |
| Ethyl ferulate | <0.001 | 0.42 | 1.07 | 0.15 | 1.99 | 1.07 | |
| Propylparaben | <0.01 | 0.52 | 1.00 | 0.87 | 0.91 | 1.00 | |
| Methyl 4-hydroxycinnamate | <0.05 | 0.54 | 0.87 | 0.17 | 2.60 | 0.87 | |
| 4-Hexylresorcinol | 0.11 | 11.12 | 0.87 | 0.11 | 23.84 | 0.87 | |
| Homogentisate | 0.24 | 1.25 | 0.60 | <0.01 | 2.91 | 0.60 | |
| Eugenol | 0.21 | 0.52 | 0.72 | <0.01 | 1.78 | 0.72 | |
| Flavonoid | 4’-O-Glucosylvitexin | <0.001 | 44.04 | 1.07 | <0.001 | 40.13 | 1.07 |
| Orientin | <0.001 | 11.63 | 1.07 | <0.001 | 143.97 | 1.07 | |
| Rutin | <0.01 | 46.17 | 1.06 | <0.01 | 50.42 | 1.06 | |
| Diosmetin | <0.01 | 28.50 | 1.06 | <0.01 | 9.26 | 1.06 | |
| Quercetin-3β-D-glucoside | <0.001 | 15.53 | 1.05 | <0.01 | 106.46 | 1.05 | |
| Quercetin | <0.05 | 22.29 | 1.04 | <0.01 | 78.97 | 1.04 | |
| Quercetin-3-O-beta-glucopyranosyl-6’-acetate | <0.001 | 11.53 | 1.05 | <0.01 | 52.01 | 1.05 | |
| Naringin | 0.06 | 1.69 | 0.85 | <0.001 | 9.76 | 0.85 | |
| Isorhamnetin | <0.01 | 1.35 | 1.00 | 0.13 | 0.61 | 1.00 | |
| Coumarin | Isofraxidin | <0.001 | 0.16 | 1.06 | <0.05 | 1.30 | 1.06 |
| Esculetin | <0.05 | 0.62 | 0.95 | <0.05 | 3.56 | 0.95 | |
Differential allelochemicals among extracts from different organs of reed during the nutrient growth stage.
JR, JS, and JL refer to the high-concentration root (R), stem (S), and leaf (L) extracts of reed during the nutrient growth stage, respectively. P represents the statistical significance from univariate analysis (t-test). FC indicates the fold change in the relative content of phenolic allelochemicals. VIP represents the variable importance for projection based on PLS-DA. Compounds with P< 0.05, VIP > 1, and FC > 1.5 or FC< 0.67 are considered differential allelochemicals between the two aqueous extracts.
4 Discussion
The results confirm the allelopathic activity of reed against B. planiculmis, as reed aqueous extracts significantly inhibited its germination and seedling growth. Previous studies also found such allelopathic effects of reed on other plants (Uddin and Robinson, 2017a; Uddin et al., 2017; Gao et al., 2022; Wang C. H. et al., 2022). For example, reed aqueous extracts have been reported to reduce the germination rate of A. fatua (Ahmad Afridi and Khan, 2015), decrease the root number in Mucuna pruriens (Uddin et al., 2017), shorten the radicle length of S. salsa (Gao et al., 2022), and suppress both plant height and biomass in Rumex crispus (Ahmad Afridi and Khan, 2015). In this study, reed aqueous extracts significantly reduced the germination rate and germination speed of B. planiculmis and strongly inhibited its root growth. Specifically, root length, surface area, and volume were reduced by up to 57%, 53%, and 43%, respectively, compared with the control. Unlike previous studies that used filter paper as the growth substrate in bioassays (Wang C. H. et al., 2022; Gao et al., 2022; Uddin and Robinson, 2017a), this study employed in situ soil collected from natural B. planiculmis populations. Under natural conditions, soil can modulate the allelopathic effects of reed through multiple mechanisms. On one hand, the physical adsorption and microbial degradation of allelochemicals by the soil can reduce their direct inhibitory impact on plants (Revillini et al., 2023; Kong et al., 2024; Yuan et al., 2024). On the other hand, allelopathic interactions may alter the structure and composition of the soil microbial community, thereby indirectly intensifying the inhibitory effects on plant growth (Ge et al., 2018; Zuo et al., 2022). Moreover, studies have shown that soil salinity and water availability can influence the allelopathic potential of plants by regulating the synthesis and release of allelochemicals (Nornasuha and Ismail, 2017; Han et al., 2024; Kostina-Bednarz et al., 2023; Shan et al., 2023). Reed typically inhabits wetland ecosystems characterized by frequent fluctuations in water levels and salinity. Therefore, in natural communities, the strength of reed allelopathic effects against B. planiculmis may fluctuate in response to habitat variations.
Uddin et al. (2014a) compared the allelopathic activity of aqueous extracts from different reed organs and found that the leaves exhibited the strongest inhibitory activity, followed by rhizomes, roots, stems, and inflorescences. In contrast, Gao et al. (2022) reported that the underground parts displayed greater inhibition activity than the aboveground parts. This study assessed the allelopathic activity of reed at two growth stages and revealed that its allelopathic activity is influenced by both the developmental stage and the plant organ. This may partly account for the inconsistencies reported in previous research. In this study, reed leaves from both growth stages exhibited notable allelopathic inhibitory activity. In particular, aqueous extracts of leaves collected during the nutrient stage significantly suppressed the seed germination and seedling growth of B. planiculmis, even at low concentrations. Similar findings have been reported in other allelopathic species such as Juglans regia (Đorđević et al., 2022), Asclepias syriaca (Gajić Umiljendić et al., 2025), and Parthenium hysterophorus (Bashar et al., 2022), whose leaf extracts also exhibit strong allelopathic inhibition activity and considerable potential for development as bioherbicides. As a perennial species with high biomass, reed produces large quantities of leaf litter annually, which accumulates in soil or aquatic environments (Packer et al., 2017). Under natural conditions, reed leaves are likely a major source of its allelopathic activity on B. planiculmis.
Previous studies have confirmed that phenolic acids are key active allelochemicals present in reed root exudates and decomposition products (Rudrappa et al., 2007; Nakai et al., 2006). This study demonstrates that phenolic acids are also important active constituents in reed aqueous extracts and reports several phenolic acids that had not been previously identified in this species. Among the 24 potential phenolic allelochemicals identified, 13 were classified as phenolic acids, 12 of which—excluding vanillic acid—are reported for the first time in reed (Table 5). Moreover, this study is the first to reveal that the allelopathic activity of reed originates not only from phenolic acids but also from flavonoids and coumarins. The biological activity of these potential allelochemicals has also been reported in other studies. Phytotoxicity studies demonstrated that rutin can inhibit root growth, chlorophyll content, and PSII photosynthetic efficiency of A. thaliana (Hussain and Reigosa, 2014, 2016). Notably, rutin has been proposed as a promising natural herbicide due to its allelopathic inhibition (Hussain and Reigosa, 2014; Fonseca et al., 2017). Xin et al. (2022) reported that quercetin significantly suppressed the growth of L. sativa seedlings at 50 μg/mL. Facenda et al. (2023) confirmed that esculetin at a concentration of 30 mg/L can significantly inhibit root growth in L. sativa. Hu et al. (2023) reported that isofraxidin exhibited notable activity against planktonic Staphylococcus aureus, with an IC50 value of 13.51 µg/mL. The strong bioherbicidal potential of esculetin and isofraxidin is attributed to the presence of a hydroxyl group at the C7 position in their molecular structures (Pan et al., 2015; Zhao et al., 2021). Therefore, in reed, the aforementioned compounds may individually affect germination and seedling growth of B. planiculmis. Furthermore, synergistic effects have also been observed among phenolic allelochemicals (Huang et al., 2020). α-Amylase plays a crucial role in breaking down starch to supply energy for plant growth. Research has demonstrated that quercetin and rutin can synergistically inhibit α-amylase activity, thereby suppressing seed germination (Oboh et al., 2015). Our study found that the concentrations of rutin and quercetin in reed leaves were significantly higher than those in the roots and stems (Table 6). The stronger allelopathic inhibition observed in the leaves may be attributed to the enhanced synergistic and additive interactions among the allelochemicals they contain.
The concentration of phenolic allelochemicals varies among different reed organs. Leaves are recognized as primary storage sites for phenolic secondary metabolites in plants (Pinhatti et al., 2010). Silva et al. (2007) proposed that photosynthetic activity promotes the accumulation of flavonoids and phenolic acids in leaves. Studies have reported high concentrations of phenolic compounds in the leaves of various species (Sultana and Anwar, 2008; Chen et al., 2019). Consistent with previous findings, this study demonstrated that the concentration of phenolic allelochemicals in reed leaves is much higher than that in roots and stems, which may account for the greater allelopathic inhibition activity of reed leaf extracts. Reed produces abundant biomass, with its leaves typically shedding onto the soil surface at the end of the growing season. In natural wetlands, these reeds often form thick layers of litter accumulating on the ground (Packer et al., 2017). Considering the potential allelopathic inhibitory effects of reed on co-occurring species, regular monitoring of reed biomass, along with periodic removal of litter deposits, are essential.
5 Conclusion
This study confirms that reed exerts significant allelopathic inhibitory effects on the germination and seedling growth of B. planiculmis. In natural wetland ecosystems, such allelopathy may pose a potential threat to the individual performance and population dynamics of B. planiculmis. A total of 24 phenolic compounds were identified in reed extracts, constituting the chemical basis of its allelopathic inhibitory activity. Among different reed organs, leaves were identified as the important source of allelopathic inhibitory activity, with the concentrations of phenolic allelochemicals substantially higher than those in roots and stems. Importantly, this study is the first to demonstrate that, in addition to phenolic acids, flavonoids and coumarins also serve as key allelochemicals in reed.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
LY: Validation, Formal analysis, Data curation, Writing – original draft, Software, Conceptualization, Methodology, Investigation, Visualization. CC: Visualization, Writing – original draft, Conceptualization, Software. YW: Supervision, Conceptualization, Project administration, Resources, Writing – review & editing. JW: Data curation, Software, Investigation, Writing – original draft. FS: Writing – original draft, Investigation, Supervision. KY: Writing – original draft, Investigation. XW: Writing – original draft, Resources. CH: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the General program of National Natural Science Foundation of China (Grant No. 32271624).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1607628/full#supplementary-material
References
1
Ahmad AfridiR.KhanM. A. (2015). Comparative effect of water extract of parthenium hysterophorus, datura alba, phragmites australis and oryza sativa on weeds and wheat. JSM44, 693–699. doi: 10.17576/jsm-2015-4405-08
2
AlghamdiS. A.Al-NehmiA. A.IbrahimO. H. M. (2022). Potential allelopathic effect of wheat straw aqueous extract on Bermudagrass noxious weed. Sustainability14, 15989. doi: 10.3390/su142315989
3
AnY.GaoY.TongS. (2018). Emergence and growth performance of Bolboschoenus planiculmis varied in response to water level and soil planting depth: Implications for wetland restoration using tuber transplantation. Aquat. Bot.148, 10–14. doi: 10.1016/j.aquabot.2018.04.005
4
AnY.GaoY.TongS.LiuB. (2021). Morphological and physiological traits related to the response and adaption of bolboschoenus planiculmis seedlings grown under salt-alkaline stress conditions. Front. Plant Sci.12. doi: 10.3389/fpls.2021.567782
5
AnwarS.NaseemS.KarimiS.AsiM. R.AkremA.AliZ. (2021). Bioherbicidal activity and metabolic profiling of potent allelopathic plant fractions against major weeds of wheat—Way forward to lower the risk of synthetic herbicides. Front. Plant Sci.12. doi: 10.3389/fpls.2021.632390
6
BasharH. K.JuraimiA. S.Ahmad-HamdaniM. S.UddinM. K.AsibN.AnwarM. P.et al. (2022). Determination and quantification of phytochemicals from the leaf extract of parthenium hysterophorus L. and their physio-biochemical responses to several crop and weed species. Plants11, 3209. doi: 10.3390/plants11233209
7
Bruce WilliamsonG.RichardsonD. (1988). Bioassays for allelopathy: Measuring treatment responses with independent controls. J. Chem. Ecol.14, 181–187. doi: 10.1007/BF01022540
8
ChenY.WangE.WeiZ.ZhengY.YanR.MaX. (2019). Phytochemical analysis, cellular antioxidant and α-glucosidase inhibitory activities of various herb plant organs. Ind. Crops Prod.141, 111771. doi: 10.1016/j.indcrop.2019.111771
9
DengY.YangX.ChenJ.YangS.ChiH.ChenC.et al. (2023). Jute (Corchorus olitorius L.) Nanocrystalline Cellulose Inhibits Insect Virus via Gut Microbiota and Metabolism. ACS Nano. 17, 21662–21677. doi: 10.1021/acsnano.3c06824
10
DingZ.LiuY.LouY.JiangM.LiH.LüX. (2021). How soil ion stress and type influence the flooding adaptive strategies of Phragmites australis and Bolboschoenus planiculmis in temperate saline–alkaline wetlands? Sci. Total Environ.771, 144654. doi: 10.1016/j.scitotenv.2020.144654
11
ĐorđevićT.Đurović-PejčevR.StevanovićM.Sarić-KrsmanovićM.RadivojevićL.ŠantrićL.et al. (2022). Phytotoxicity and allelopathic potential of Juglans regia L. leaf extract. Front. Plant Sci.13. doi: 10.3389/fpls.2022.986740
12
FacendaG.RealM.Galán-PérezJ. A.GámizB.CelisR. (2023). Soil effects on the bioactivity of hydroxycoumarins as plant allelochemicals. Plants12, 1278. doi: 10.3390/plants12061278
13
FonsecaJ. C.BarbosaM. A.SilvaI. C. A.Duarte-AlmeidaJ. M.CastroA. H. F.Dos Santos LimaL. A. R. (2017). Antioxidant and allelopathic activities of Smilax brasiliensis Sprengel (Smilacaceae). South Afr. J. Bot.111, 336–340. doi: 10.1016/j.sajb.2017.04.003
14
Gajić UmiljendićJ.Sarić-KrsmanovićM.RadivojevićL.ŠantrićL.ŠćepanovićM.ŠoštarčićV.et al. (2025). Phytochemical analysis of Asclepias Syriaca L. leaf extract and its potential phytotoxic effect on some invasive weeds. Sci. Rep.15, 1–14. doi: 10.1038/s41598-025-90940-1
15
GaoJ.GuanB.GeM.EllerF.YuJ.WangX.et al. (2022). Can allelopathy of Phragmites australis extracts aggravate the effects of salt stress on the seed germination of Suaeda salsa? Front. Plant Sci.13. doi: 10.3389/fpls.2022.990541
16
GeY.WangQ.WangL.LiuW.LiuX.HuangY.et al. (2018). Response of soil enzymes and microbial communities to root extracts of the alien Alternanthera philoxeroides. Arch. Agron. Soil Sci.64, 708–717. doi: 10.1080/03650340.2017.1373186
17
HanM.YangH.HuangH.DuJ.ZhangS.FuY. (2024). Allelopathy and allelobiosis: efficient and economical alternatives in agroecosystems. Plant Biol.26, 11–27. doi: 10.1111/plb.13582
18
HierroJ. L.CallawayR. M. (2021). The ecological importance of allelopathy. Annu. Rev. Ecol. Evol. Systematics52, 25–45. doi: 10.1146/annurev-ecolsys-051120-030619
19
HuH.TekinV.HuB.YaghoobiM.KhanA.GhoshA. K.et al. (2023). Metabolic profiling of Chimonanthus grammatus via UHPLC-HRMS-MS with computer-assisted structure elucidation and its antimicrobial activity. Front. Plant Sci.14. doi: 10.3389/fpls.2023.1138913
20
HuangS.ZhuJ.ZhangL.PengX.ZhangX.GeF.et al. (2020). Combined effects of allelopathic polyphenols on microcystis aeruginosa and response of different chlorophyll fluorescence parameters. Front. Microbiol.11. doi: 10.3389/fmicb.2020.614570
21
HussainM. I.ReigosaM. J. (2014). Higher peroxidase activity, leaf nutrient contents and carbon isotope composition changes in Arabidopsis thaliana are related to rutin stress. J. Plant Physiol.171, 1325–1333. doi: 10.1016/j.jplph.2014.05.009
22
HussainM. I.ReigosaM. J. (2016). Plant secondary metabolite rutin affects the photosynthesis and excitation energy flux responses in Arabidopsis thaliana. Allelopathy J.38, 215–228.
23
KhanM. A.AfridiR. A.HashimS.KhattakA. M.AhmadZ.WahidF.et al. (2016). Integrated effect of allelochemicals and herbicides on weed suppression and soil microbial activity in wheat (Triticum aestivum L.). Crop Prot.90, 34–39. doi: 10.1016/j.cropro.2016.08.018
24
KongC.-H.LiZ.LiF.-L.XiaX.-X.WangP. (2024). Chemically mediated plant–plant interactions: allelopathy and allelobiosis. Plants13, 626. doi: 10.3390/plants13050626
25
Kostina-BednarzM.PłonkaJ.BarchanskaH. (2023). Allelopathy as a source of bioherbicides: challenges and prospects for sustainable agriculture. Rev. Environ. Sci. Biotechnol.22, 471–504. doi: 10.1007/s11157-023-09656-1
26
LiJ.ZhangD.YinL.LiZ.YuC.DuH.et al. (2022). Integration analysis of metabolome and transcriptome profiles revealed the age-dependent dynamic change in chicken meat. Food Res. Int.156, 111171. doi: 10.1016/j.foodres.2022.111171
27
LiuY.DingZ.BachofenC.LouY.JiangM.TangX.et al. (2018). The effect of saline-alkaline and water stresses on water use efficiency and standing biomass of Phragmites australis and Bolboschoenus planiculmis. Sci. Total Environ.644, 207–216. doi: 10.1016/j.scitotenv.2018.05.321
28
MacíasF. A.MejíasF. J.MolinilloJ. M. (2019). Recent advances in allelopathy for weed control: from knowledge to applications. Pest Manage. Sci.75, 2413–2436. doi: 10.1002/ps.5355
29
NakaiS.ZhouS.HosomiM.TominagaM. (2006). Allelopathic growth inhibition of cyanobacteria by reed. AllelopathyJoural18, 277–286.
30
NiazipoorG.AghaAlikhaniM.Mokhtassi-BidgoliA.IritiM.VitaliniS. (2024). Phytochemical analysis and allelopathic potential of essential oil of yarrow (Achillea spp.) ecotypes against redroot pigweed (Amaranthus retroflexus L.). Heliyon10, e26101. doi: 10.1016/j.heliyon.2024.e26101
31
NornasuhaY.IsmailB. S. (2017). Sustainable weed management using allelopathic approach. Malays Appl. Biol.46, 1–10.
32
ObohG.AdemosunA. O.AyeniP. O.OmojokunO. S.BelloF. (2015). Comparative effect of quercetin and rutin on α-amylase, α-glucosidase, and some pro-oxidant-induced lipid peroxidation in rat pancreas. Comp. Clin. Pathol.24, 1103–1110. doi: 10.1007/s00580-014-2040-5
33
PackerJ. G.MeyersonL. A.SkálováH.PyšekP.KuefferC. (2017). Biological flora of the british isles: phragmites australis. J. Ecol.105, 1123–1162. doi: 10.1111/1365-2745.12797
34
PanL.LiX.YanZ.GuoH.QinB. (2015). Phytotoxicity of umbelliferone and its analogs: Structure–activity relationships and action mechanisms. Plant Physiol. Biochem.97, 272–277. doi: 10.1016/j.plaphy.2015.10.020
35
PinhattiA. V.de Matos NunesJ.MaurmannN.RosaL. M. G.von PoserG. L.RechS. B. (2010). Phenolic compounds accumulation in Hypericum ternum propagated in vitro and during plant development acclimatization. Acta Physiol. Plant32, 675–681. doi: 10.1007/s11738-009-0446-5
36
ReissA.FomsgaardI. S.MathiassenS. K.StuartR. M.KudskP. (2018). Weed suppression by winter cereals: relative contribution of competition for resources and allelopathy. Chemoecology28, 109–121. doi: 10.1007/s00049-018-0262-8
37
RevilliniD.DavidA. S.ReyesA. L.KnechtL. D.VigoC.AllenP.et al. (2023). Allelopathy-selected microbiomes mitigate chemical inhibition of plant performance. New Phytol.240, 2007–2019. doi: 10.1111/nph.19249
38
RudrappaT.BonsallJ.GallagherJ. L.SeliskarD. M.BaisH. P. (2007). Root-secreted allelochemical in the noxious weed phragmites australis deploys a reactive oxygen species response and microtubule assembly disruption to execute rhizotoxicity. J. Chem. Ecol.33, 1898–1918. doi: 10.1007/s10886-007-9353-7
39
ShanZ.ZhouS.ShahA.ArafatY.Arif Hussain RizviS.ShaoH. (2023). Plant allelopathy in response to biotic and abiotic factors. Agronomy13, 2358. doi: 10.3390/agronomy13092358
40
SilvaE. M.SouzaJ. N. S.RogezH.ReesJ. F.LarondelleY. (2007). Antioxidant activities and polyphenolic contents of fifteen selected plant species from the Amazonian region. Food Chem.101, 1012–1018. doi: 10.1016/j.foodchem.2006.02.055
41
SongK.LeeJ.ChaC.-J.KangH. (2015). Effects of Phragmites invasion on soil microbial activity and structure in a brackish marsh. Plant Soil392, 45–56. doi: 10.1007/s11104-014-2339-7
42
SultanaB.AnwarF. (2008). Flavonols (kaempeferol, quercetin, myricetin) contents of selected fruits, vegetables and medicinal plants. Food Chem.108, 879–884. doi: 10.1016/j.foodchem.2007.11.053
43
SuzukiM.TominagaT.OhnoO.IwasakiA.SuenagaK.Kato-NoguchiH. (2018). Plant growth inhibitory activity and active substances with allelopathic potential of cogongrass (Imperata cylindrica) rhizome. Weed Biol. Manage.18, 92–98. doi: 10.1111/wbm.12144
44
TangH.LiuY.LouY.YuD.ZhouM.LuX.et al. (2024). Nitrogen availability affects the responses of marsh grass and sedge plants (Phragmites australis and Bolboschoenus planiculmis) to flooding time. Sci. Total Environ.908, 168008. doi: 10.1016/j.scitotenv.2023.168008
45
UddinM.RobinsonR. W.CaridiD. (2014a) Phytotoxicity induced by Phragmites australis: an assessment of phenotypic and physiological parameters involved in germination process and growth of receptor plant. J. Plant Interactions. 9, 338–353. doi: 10.1080/17429145.2013.835879
46
UddinM. N.CaridiD.RobinsonR. W. (2012). Phytotoxic evaluation of Phragmites australis: an investigation of aqueous extracts of different organs. Mar. Freshw. Res.63, 777–787. doi: 10.1071/MF12071
47
UddinM. N.RobinsonR. W. (2017a). Allelopathy and resource competition: the effects of Phragmites australis invasion in plant communities. Bot. Stud.58, 29. doi: 10.1186/s40529-017-0183-9
48
UddinM. N.RobinsonR. W. (2017b). Changes associated with Phragmites australis invasion in plant community and soil properties: A study on three invaded communities in a wetland, Victoria, Australia. Limnologica66, 24–30. doi: 10.1016/j.limno.2017.07.006
49
UddinM.RobinsonR. W.BuultjensA.Al Harun Md.A. Y.ShampaS. H. (2017). Role of allelopathy of Phragmites australis in its invasion processes. J. Exp. Mar. Biol. Ecol.486, 237–244. doi: 10.1016/j.jembe.2016.10.016
50
UddinM.RobinsonR. W.CaridiD.Al Harun Md.A. Y. (2014b). Suppression of native Melaleuca ericifolia by the invasive Phragmites australis through allelopathic root exudates. Am. J. Bot.101, 479–487. doi: 10.3732/ajb.1400021
51
Vieites-ÁlvarezY.OteroP.PrietoM. A.Simal-GandaraJ.ReigosaM. J.Sánchez-MoreirasA. M.et al. (2023). Testing the role of allelochemicals in different wheat cultivars to sustainably manage weeds. Pest Manage. Sci.79, 2625–2638. doi: 10.1002/ps.7444
52
WailsC. N.BakerK.BlackburnR.Del ValléA.HeiseJ.HerakovichH.et al. (2021). Assessing changes to ecosystem structure and function following invasion by Spartina alterniflora and Phragmites australis: a meta-analysis. Biol. Invasions23, 2695–2709. doi: 10.1007/s10530-021-02540-5
53
WangY.GongM.ZouC.ZhouT.WenW.LiuG.et al. (2022). Habitat selection by Siberian Cranes at their core stopover area during migration in Northeast China. Global Ecol. Conserv.33, e01993. doi: 10.1016/j.gecco.2021.e01993
54
WangX.WangS.ZhuJ.LiL.MaJ.ZuoL.et al. (2024). Inhibition of co-occurring weeds and young sugarcane seedling growth by perennial sugarcane root extract. Sci. Rep.14, 1–15. doi: 10.1038/s41598-024-58082-y
55
WangC.YuZ.YasenjiangY.TangL.GaoY.ZouC.-J. (2022). Mechanisms of seed-to-seed interactions between dominant species in the yangtze river estuary under saline condition. Diversity14, 1017. doi: 10.3390/d14121017
56
WoldS.SjöströmM.ErikssonL. (2001). PLS-regression: a basic tool of chemometrics. Chemometrics Intelligent Lab. Syst.58, 109–130. doi: 10.1016/S0169-7439(01)00155-1
57
XinA.JinH.YangX.GuanJ.HuiH.LiuH.et al. (2022). Allelochemicals from the rhizosphere soil of potato (Solanum tuberosum L.) and their interactions with the soilborne pathogens. Plants11, 1934. doi: 10.3390/plants11151934
58
YuanL.XieX.ZhangY.LiJ.van KleunenM. (2024). The soil microbial community and nitrogen availability affect the growth, biochemistry and potential allelopathic effects of the invasive plant Solidago canadensis. Plant Soil. 510, 491–505. doi: 10.1007/s11104-024-06934-x
59
ZhangJ.GaoG.LiZ.FuB.GuptaH. V. (2020). Identification of climate variables dominating streamflow generation and quantification of streamflow decline in the Loess Plateau, China. Sci. Total Environ.722, 137935. doi: 10.1016/j.scitotenv.2020.137935
60
ZhangZ.LiuY.YuanL.WeberE.van KleunenM. (2021). Effect of allelopathy on plant performance: a meta-analysis. Ecol. Lett.24, 348–362. doi: 10.1111/ele.13627
61
ZhangM.ZhangD.QiQ.TongS.WangX.AnY.et al. (2022). Flooding effects on population and growth characteristics of Bolboschoenus planiculmis in Momoge wetland, northeast China. Ecol. Indic.137, 108730. doi: 10.1016/j.ecolind.2022.108730
62
ZhaoL.-X.WangZ.-X.ZouY.-L.GaoS.FuY.YeF. (2021). Phenoxypyridine derivatives containing natural product coumarins with allelopathy as novel and promising proporphyrin IX oxidase-inhibiting herbicides: Design, synthesis and biological activity study. Pesticide Biochem. Physiol.177, 104897. doi: 10.1016/j.pestbp.2021.104897
63
ZuoX.WangY.ZhaoH.LiG.WangY.LiG.et al. (2022). Allelopathic effects of amomum villosum lour. Volatiles from different organs on selected plant species and soil microbiota. Plants11, 3550. doi: 10.3390/plants11243550
Summary
Keywords
allelopathy, phenolic allelochemicals, aqueous extract, germination, seedling growth, HPLC-MS/MS
Citation
Yang L, Chen C, Wang Y, Wang J, Shi F, Yue K, Wang X and He C (2025) Allelopathic activity of Phragmites australis against Bolboschoenus planiculmis and the involved active allelochemicals. Front. Plant Sci. 16:1607628. doi: 10.3389/fpls.2025.1607628
Received
07 April 2025
Accepted
30 May 2025
Published
23 June 2025
Volume
16 - 2025
Edited by
Lucian Copolovici, Aurel Vlaicu University of Arad, Romania
Reviewed by
Mirko La Iacona, University of Catania, Italy
Lidia Scrivanti, CCT CONICET Córdoba, Argentina
Updates
Copyright
© 2025 Yang, Chen, Wang, Wang, Shi, Yue, Wang and He.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Wang, wangy833@nenu.edu.cn; Chunguang He, he-cg@nenu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.