- 1Northeast Institute of Geography and Agroecology, Chinese Academy of Sciences, Changchun, China
- 2College of Advanced Agricultural Sciences, University of Chinese Academy of Sciences, Beijing, China
Introduction: Alkaline stress poses a major challenge to crop productivity, often causing more severe physiological damage than saline stress alone. Maize is particularly sensitive to alkaline conditions, which significantly inhibit germination and early growth. The establishment of accurate evaluation systems for alkali tolerance is therefore crucial for the development of resilient cultivars.
Methods: A total of 42 maize germplasm accessions were evaluated under simulated alkaline stress (100 mM) during germination. A comprehensive analytical framework integrating principal component analysis, membership function analysis, stepwise regression, cluster analysis, and discriminant analysis was used to assess alkalinity tolerance. The Lindeman–Merenda–Gold method was further employed to quantify the relative contribution of each morphological trait to the comprehensive alkali tolerance score.
Results: Alkaline stress significantly inhibited early seedling growth, and several germination-related traits showed strong associations with alkali tolerance. Based on the comprehensive D value, the maize accessions were classified into five tolerance groups. Trait contribution analyses consistently indicated the germination index (GI) and the shoot dry weight (SDW) as the strongest determinants of tolerance, with root length (RL) and root fresh weight (RFW) also playing notable roles. These indicators form a reliable basis for the screening of alkali-tolerant maize germplasm, providing a foundation for future refinement of the evaluation system through physiological or molecular approaches.
Discussion: This integrated evaluation system effectively distinguishes maize germplasm by alkali tolerance level and identifies key morphological determinants. The findings provide a scientific basis for germplasm screening and breeding of alkali-tolerant maize materials, contributing to sustainable agricultural production in saline–alkaline environments.
1 Introduction
Saline–alkali land is a valuable reserve of arable land resources, and its sustainable utilization has become a major research focus in recent years (Baloch et al., 2023). Among these, soda saline–alkali soils are widely recognized as the most challenging type to remediate, which are typically characterized by weak structural stability, high bulk density, surface crusting, and poor permeability, all of which severely inhibit plant growth and development (Nelson et al., 1998). Furthermore, climate change and human activities have exacerbated biodiversity loss and reduced productivity, making the management and restoration of saline–alkali land increasingly challenging (Sahab et al., 2021; Sarath et al., 2021). Against this backdrop, planting salt- and alkali-tolerant species, particularly those adapted to alkaline soils, has been demonstrated to effectively enhance soil productivity, reduce salinity, and improve soil structure (Liang and Shi, 2021). This approach serves as not only a crucial strategy to mitigate the arable land crisis (Rezvi et al., 2022) but also a key priority in saline–alkali land research.
As a major global food and feed crop, maize accounts for approximately one-third of the total global grain production (Ali et al., 2022; McMillen et al., 2022) and is relatively vulnerable to saline–alkali stress. Plant stress tolerance assessments typically focus on the stress responses across developmental stages, with the germination and seedling stages being particularly sensitive to environmental stresses and, thus, critical for the early-stage screening of stress tolerance (Dodd and Donovan, 1999). Research on stress tolerance during these stages not only provides theoretical support for vegetation restoration in saline–alkali soils but also offers crucial evidence for the early identification of stress-tolerant materials in breeding programs (Ren et al., 2025; Hu et al., 2025; Zheng et al., 2023). Therefore, systematically evaluating the salt tolerance of maize seedlings and identifying the key tolerance traits hold significant practical importance for the development of more adaptable salt-tolerant maize germplasm resources.
Robust tolerance evaluation further requires statistical tools that can identify critical traits and reliably classify germplasm. Multivariate analytical methods—including principal component analysis (PCA), cluster analysis (CA), entropy weighting, fuzzy evaluation, grey relational analysis, and TOPSIS (technique for order of preference by similarity to ideal solution)—have been widely applied in crops such as rice (Zhang et al., 2025), wheat (Yang et al., 2025), soybean (Zhao et al., 2022), and potato (Cao et al., 2015) and have also been proven useful in diverse research fields, including food science (Hosseinpour and Martynenko, 2021), ecology (Gonzalez-Martinez et al., 2021), and medicine (Królaczyk et al., 2020), highlighting their versatility in analyzing complex biological systems (Figure 1). However, despite their broad use, these approaches often offer limited interpretability of individual variables and lack explicit validation of their classification performance or quantitative partitioning of trait contributions (Han et al., 2019; Yu et al., 2021; Zhang et al., 2022; Zhao et al., 2023; Song et al., 2024; Baha et al., 2025). Linear discriminant analysis (LDA) can address these gaps by verifying the clustering accuracy (Helm and Eis, 2007; Caturegli et al., 2020; Tang et al., 2024), while the Lindeman–Merenda–Gold (LMG) method decomposes the model R2 to determine the relative importance of each trait (Lindeman et al., 1980). These tools have shown strong utility in plant ecology, grassland productivity assessment, and crop stress physiology (Ding et al., 2025; Liu et al., 2024; Wu et al., 2024; Martini et al., 2019; Kamphorst et al., 2021), providing a robust and interpretable framework for the evaluation of stress tolerance across biological systems.
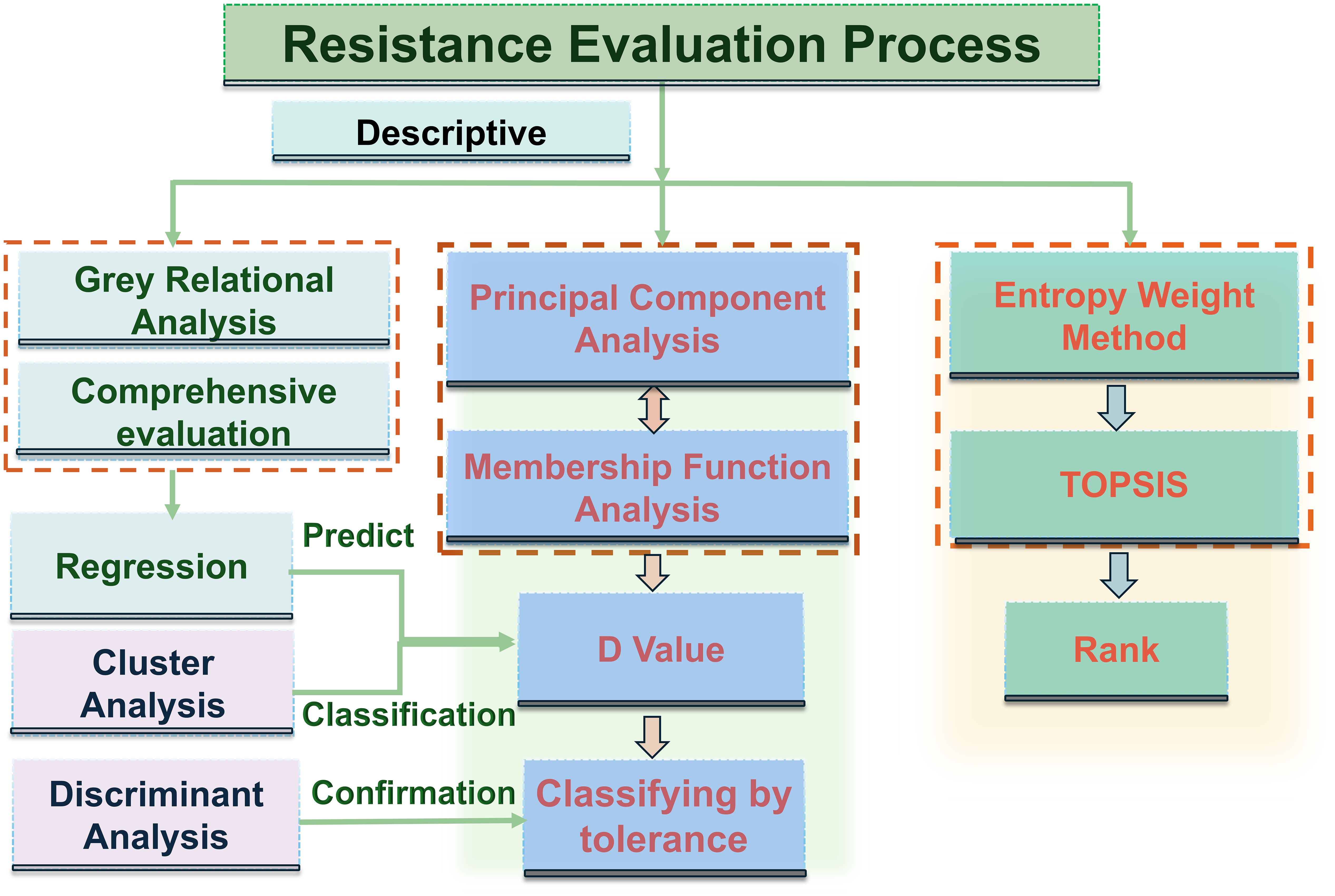
Figure 1. Tolerance evaluation flowchart. Colored background indicates the selection process for tolerant materials. The methods shown in the figure are not limited to those listed. Other analytical methods that perform the same function can be used instead.
Despite these methodological advances, applications in maize remain limited. The majority of the evaluations of alkalinity tolerance employed single or partially integrated analytical strategies, and the contributions of individual early-stage morphological traits to tolerance variations have not been quantitatively resolved. A unified analytical framework that integrates multivariate characterization, classification validation, and trait importance assessment for the alkalinity responses during the germination stage is still lacking.
Building on germplasm studies in rice, wheat, soybean, potato, and bermudagrass, which have demonstrated the analytical value of the integration of multiple statistical perspectives, we extend these approaches to maize alkalinity tolerance. We hypothesize that early morphological traits differ in their contributions to tolerance differentiation, an aspect that has not yet been resolved quantitatively. To address this, we quantified the germination responses under alkaline stress, identified the most informative morphological indicators, and developed an integrated and explicitly data-driven evaluation framework. By combining PCA, membership function analysis (MFA), CA, stepwise regression, LDA, LMG-based trait importance assessment, and t-tests into a coherent analytical pipeline, this study introduces a novel, multi-tiered system that unifies characterization, classification, and trait importance inference. This integrative strategy advances the current screening methodologies and provides an innovative platform for accelerating the development of maize lines with improved alkalinity tolerance.
2 Materials and methods
2.1 Experimental materials
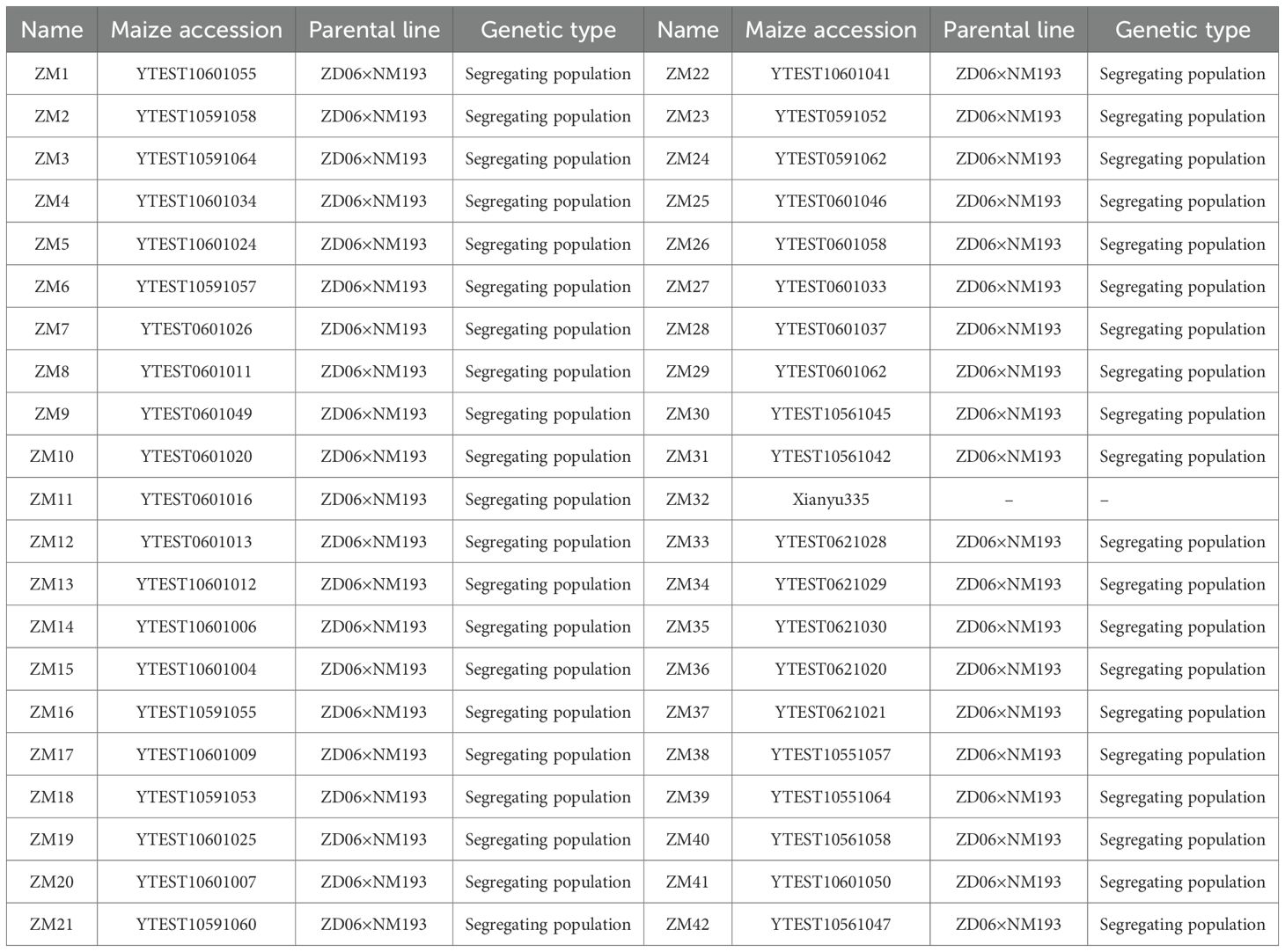
A total of 42 maize accessions were evaluated in this study, all derived from segregating populations of the cross between ZD06, a salt- and alkali-sensitive variety, and NM193, a highly salt- and alkali-tolerant variety. The specific tolerance of each accession was not known prior to the experiment and was assessed through germination and early seedling evaluations under alkaline stress. All accessions, except Xianyu335, were provided by the Maize Genetics and Molecular Breeding Laboratory at the Northeast Institute of Geography and Agroecology. Xianyu335 was purchased online. Full details of all 42 accessions, including the genetic type and parental lines, are provided in Table 1.
2.2 Experimental design
2.2.1 Germination assay under alkaline stress
A completely randomized design (CRD) was used to assess the maize germination performance under alkaline stress. Two treatments were established: 1) control, which used distilled water, and 2) alkaline stress, which used a mixed alkaline solution of Na2CO3/NaHCO3 = 1:9 at 100 mM. Each treatment contained three replicates, with 10 seeds per replicate, resulting in 60 experimental units in total (2 treatments × 3 replicates × 10 seeds). Uniform and fully developed seeds were carefully selected for the experiment. The seeds were surface-sterilized by immersion in anhydrous ethanol for 60 s, followed by three to five rinses with tap water and distilled water. The seeds were then immersed in 1% NaClO for 15 min, rinsed thoroughly with distilled water, air-dried, and stored for subsequent use. For the germination assays, the seeds were placed in 9-cm Petri dishes lined with a double-layered filter paper, with each dish corresponding to one experimental unit. The dishes were incubated in a controlled growth chamber under a 14-h/10-h light/dark cycle, with temperatures maintained at 25 °C/20 °C (day/night). Germination was recorded when the radicle protruded ≥2 mm, starting on day 3 and continuing for 7 days. Daily weighing was conducted to maintain constant solution volume, and evaporated water was replenished with the corresponding treatment solution.
2.2.2 Measurement and statistics of the morphological index
After 7 days of incubation, 11 germination and early seedling traits were measured for each treatment: the following formulas were employed to ascertain the germination potential (GP), the germination rate (GR), the germination index (GI), and the vigor index (VI). The amount of seedling growth [root length (RL) + shoot length (SL)] was multiplied by the GI to obtain the VI (Zhou et al., 2023). Calipers were utilized to measure the RL and SL, the samples were weighed on a balance, dried in an oven at 105 °C for 6 h, and then reweighed. The alkali tolerance coefficient (ATC) was determined for each indication (refer to Equation 6).
2.2.2.1 Germination potential
GP refers to the proportion of seeds that have successfully germinated by the third day relative to the total number of seeds. The germination energy of the tested materials was calculated using Equation 1.
2.2.2.2 Germination rate
GR refers to the proportion of seeds that have successfully germinated by the seventh day relative to the total number of seeds. The GR of the tested materials was calculated using Equation 2.
2.2.2.3 Germination index
The GI of the tested materials was calculated using Equation 3, where Gt refers to the number of seeds germinated within t days and Dt represents the corresponding germination days.
2.2.2.4 Vigor index
The VI of the tested materials was calculated using Equation 4, where GI represents the germination index and S represents seedling growth.
2.2.2.5 Root-to-shoot ratio
The root-to-shoot ratio (RSR) of the tested materials was calculated using Equation 5.
2.3 Data analysis
2.3.1 Analysis
In this study, we integrated correlation analysis, PCA, MFA, stepwise regression, CA, LDA, and LMG-based variable importance analysis to construct a multidimensional and systematic evaluation framework, thereby enabling a more comprehensive, robust, and accurate screening of germplasm resources.
2.3.1.1 Alkali tolerance indicator for various indicators of maize
According to Yu et al. (2021), and with suitable amendments, analysis was performed using the relative values of each maize material, and based on this, the ATC for each indicator was calculated.
where ATCij is the alkali tolerance coefficient of the indicator (j) for variety (i). Xij-control and Xij-treat represent the indicator values of each variety assessed under the no-alkali treatment and the alkali treatment, respectively.
2.3.1.2 Membership function values for the comprehensive indicators of maize
The membership function value μ was used to evaluate the alkali tolerance of multiple indicators, which was calculated using Equation 7.
2.3.1.3 Weights of the comprehensive indicators
Equation 8 was used to calculate the weight function Wi, which represents the relative importance of the comprehensive indicators for a variety. Pi denotes the contribution of the comprehensive indicators.
2.3.1.4 Alkali tolerance of maize germplasm resources
The comprehensive evaluation parameter (D) for the alkali tolerance resilience of each variety was calculated using Equation 9 to assess the alkali tolerance of the different maize materials.
2.3.1.5 Relative contribution
Standardized regression coefficients (β) were used to represent the relative effect size of each variable on the dependent variable. The contribution rate of each indicator was calculated as follows:
where Ci represents the relative contribution (in percent) of the i-th indicator and βi is the standardized regression coefficient obtained from the regression model. This method provides an intuitive estimation of the relative importance of predictors and has been widely applied in quantitative studies evaluating complex traits (Nathans et al., 2012).
2.3.1.6 LMG method
To quantify the relative contribution of each indicator to maize alkali tolerance, this study employed the LMG method.
where Y is the response variable, Xi is the explanatory variable, βa is the regression coefficient, and ϵ is the residual. LMG calculates the average contribution of each variable to the total R2 (LMGi) by considering all possible variable entry orders. The relative contribution of each indicator to the model was assessed using the LMG metric implemented in the relaimpo package in R (Groemping, 2006).
Data are presented as the mean ± SE. Data reduction was performed using Excel 24. PCA, regression analysis, correlation analysis, CA, and discriminant analysis were performed using IBM SPSS Statistics version 26. The relative contributions of the variables (LMG) were calculated in R 4.4.3 using the relaimpo package [calc.relimp(), type = “lmg”, rela = TRUE]. Hierarchical clustering with significance annotation was conducted via the online platform OmicStudio (https://www.bioinformatics.com.cn, last accessed August 18, 2025). All other figures were generated using Origin 25.
3 Results
3.1 Genetic analysis of the alkali tolerance coefficients for morphological traits in 42 maize materials
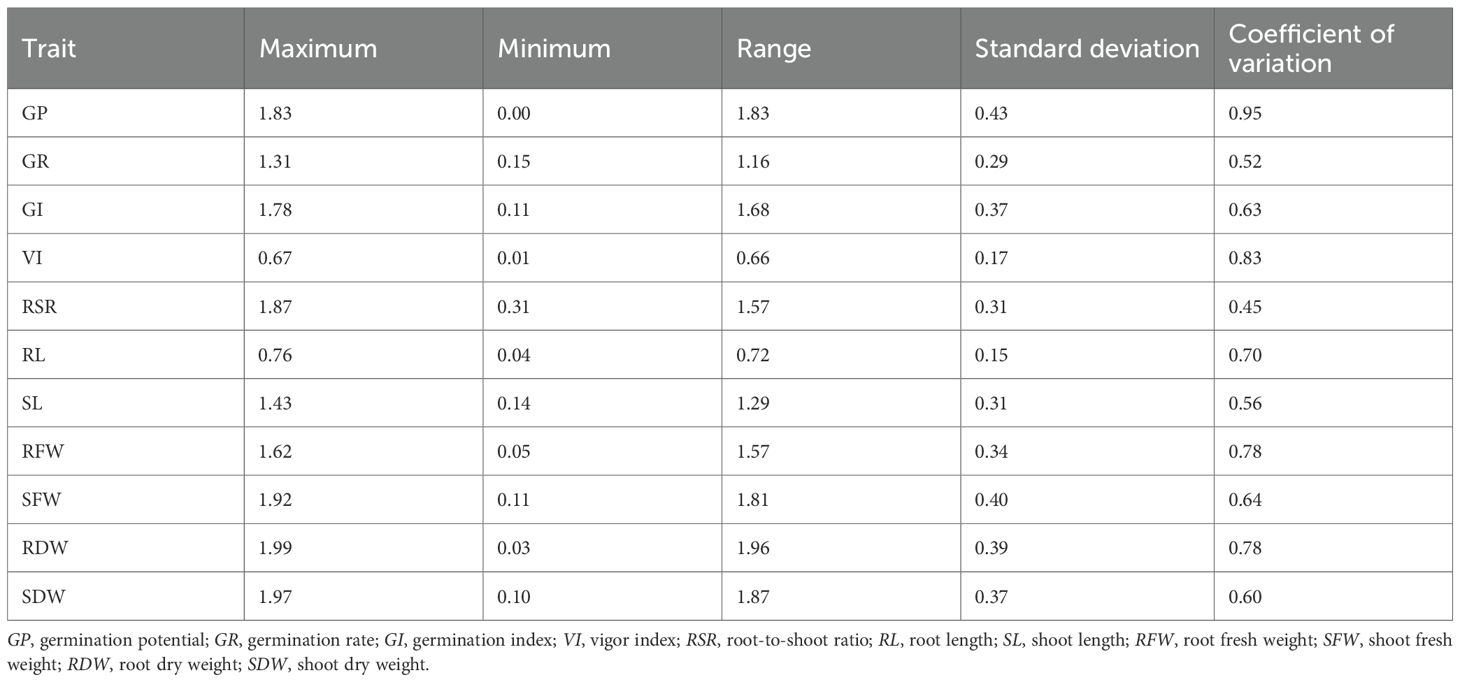
Extensive genetic variation is a fundamental prerequisite for the effective screening of alkaline-tolerant maize materials (Table 2). Alkaline stress exerts a pronounced effect on the germination traits of maize, inducing variations across 11 measured parameters, with coefficients of variation ranging from 45% (RSR) to 95% (GP). These results indicate substantial diversity in the responses of maize genotypes to alkaline stress, thereby providing a robust basis for the identification and selection of salt- and alkali-tolerant germplasm.
3.2 Coefficients of the morphological traits in maize during germination under alkaline stress
A correlation analysis was conducted on the ATCs of all the measured indicators. The results are presented in Figure 2. GP showed highly significant positive correlations (p < 0.01) with GR, GI, VI, shoot fresh weight (SFW), and shoot dry weight (SDW), indicating that a higher GP reflects an enhanced early germination and seedling vigor under alkaline stress. In contrast, GP was not significantly correlated (p > 0.05) with the RSR, RL, SL, root fresh weight (RFW), or root dry weight (RDW), suggesting that GP primarily reflects aboveground early growth rather than root development. GR and GI showed similar correlation patterns to GP, emphasizing their roles in capturing early germination dynamics. VI was significantly correlated (p < 0.01) with the majority of the traits, reflecting its integrative representation of the overall seedling vigor. RSR correlated strongly with RL, RFW, and RDW, highlighting its function in root allocation. RL, along with RFW, RDW, SL, and SDW, showed strong intercorrelations, indicating coordinated development of the root and shoot biomass, which is critical for seedling establishment under alkaline stress. These results demonstrate that the 11 morphological indicators (i.e., GP, GR, GI, VI, RSR, RL, SL, RFW, SFW, RDW, and SDW) capture distinct aspects of the maize seedling responses to alkaline stress, including early germination, root–shoot allocation, and biomass accumulation, providing a detailed understanding of the physiological and morphological effects of alkalinity.
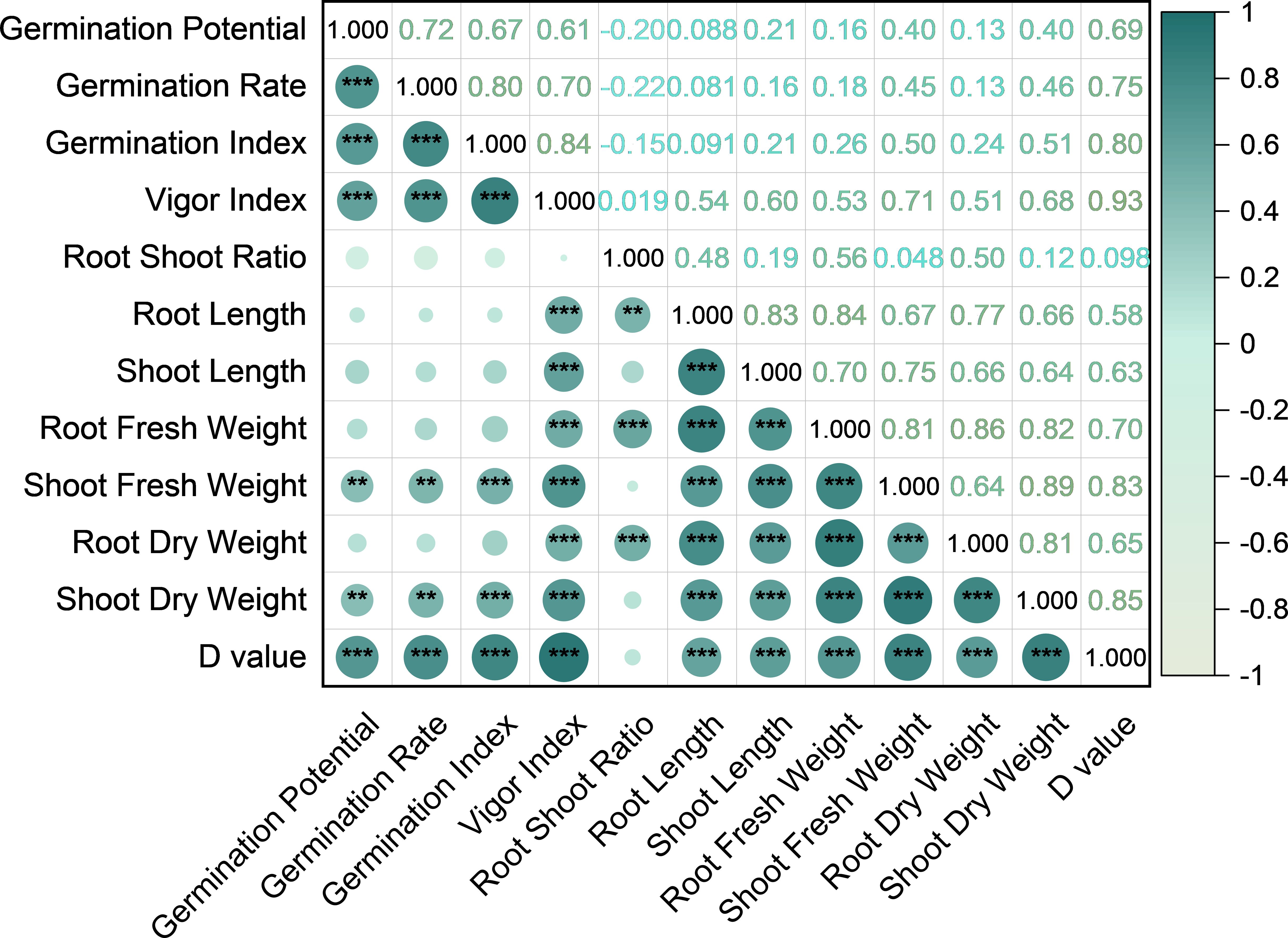
Figure 2. Correlation of the salt tolerance coefficients among traits, correlation of the salt tolerance coefficient of each trait, and comprehensive evaluation of the D value. *p < 0.05, **p < 0.01, ***p < 0.001.
3.3 Principal component analysis and membership function analysis of the alkali tolerance indicators in different maize materials
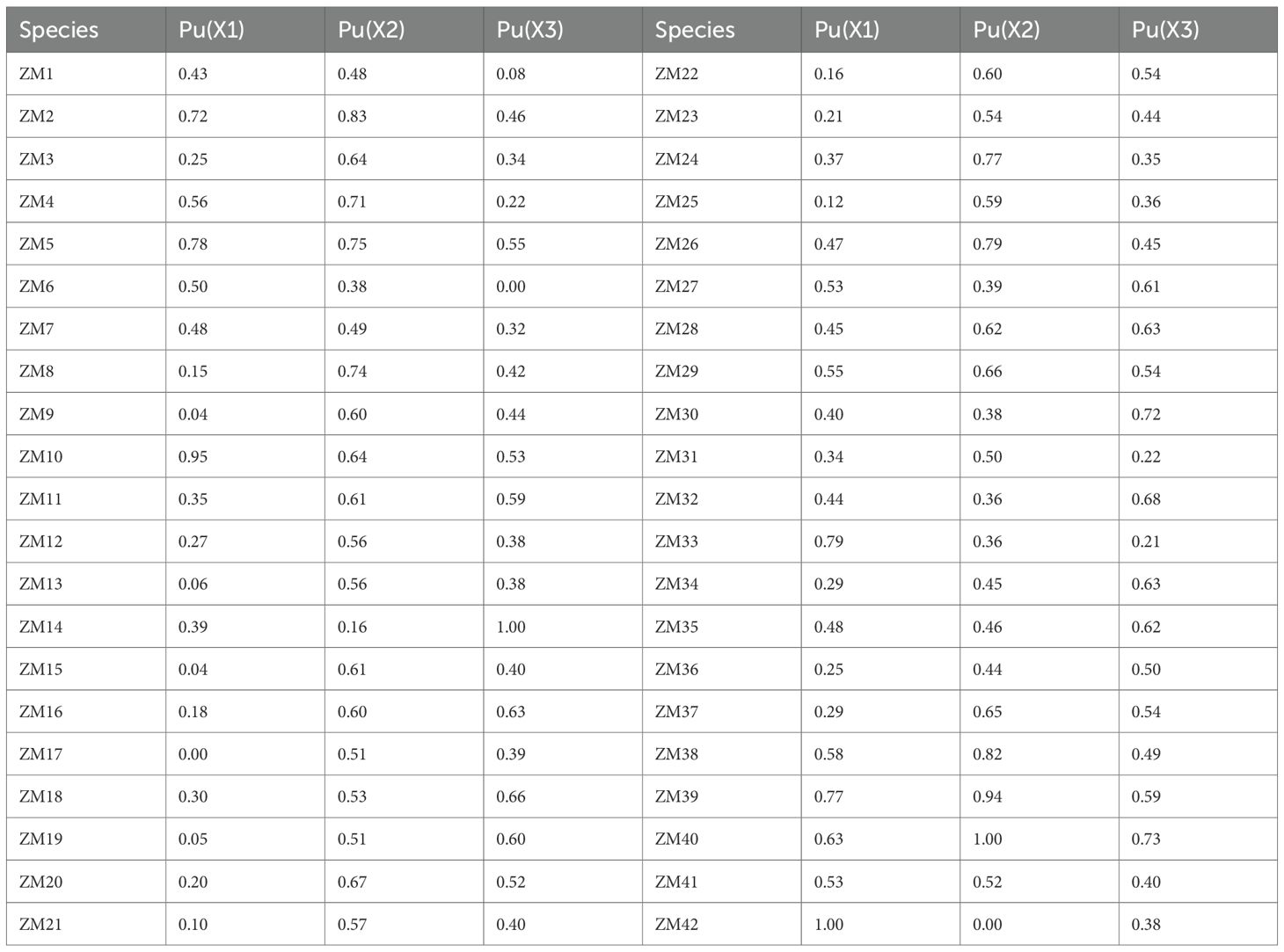
PCA was conducted to reduce the dimensionality of the ATCs under the different salt stress concentrations (Table 3). Factor analysis of the 11 original indicators yielded three principal components (PC1, PC2, and PC3), which accounted for 54.4%, 24.35%, and 7.45% of the total variance, respectively. Collectively, these three components explained 86.2% of the overall variance, effectively capturing the majority of information contained in the original indicators. Subsequently, membership functions were applied to calculate composite scores for the three principal components, which were used for a comprehensive evaluation of alkali tolerance (Table 4). The membership function (μ) values for the composite score of each variety were calculated according to Equation 7. As shown in Table 4, these μ(Xi) values reflect the relative alkali tolerance of each variety, where μ(Xi) = 1 represents the highest tolerance and μ(Xi) = 0 indicates the highest sensitivity. For the Pu1 composite component, ZM42 exhibited the strongest alkali tolerance, whereas ZM17 was the most sensitive. For Pu2, ZM40 showed the highest tolerance, while ZM42 was the most sensitive. For Pu3, ZM14 demonstrated the greatest tolerance, while ZM6 was the most sensitive. Because the rankings based on the three composite indicators occasionally differed, an integrated assessment was performed to provide a more reliable basis for the selection of alkali-tolerant maize materials.
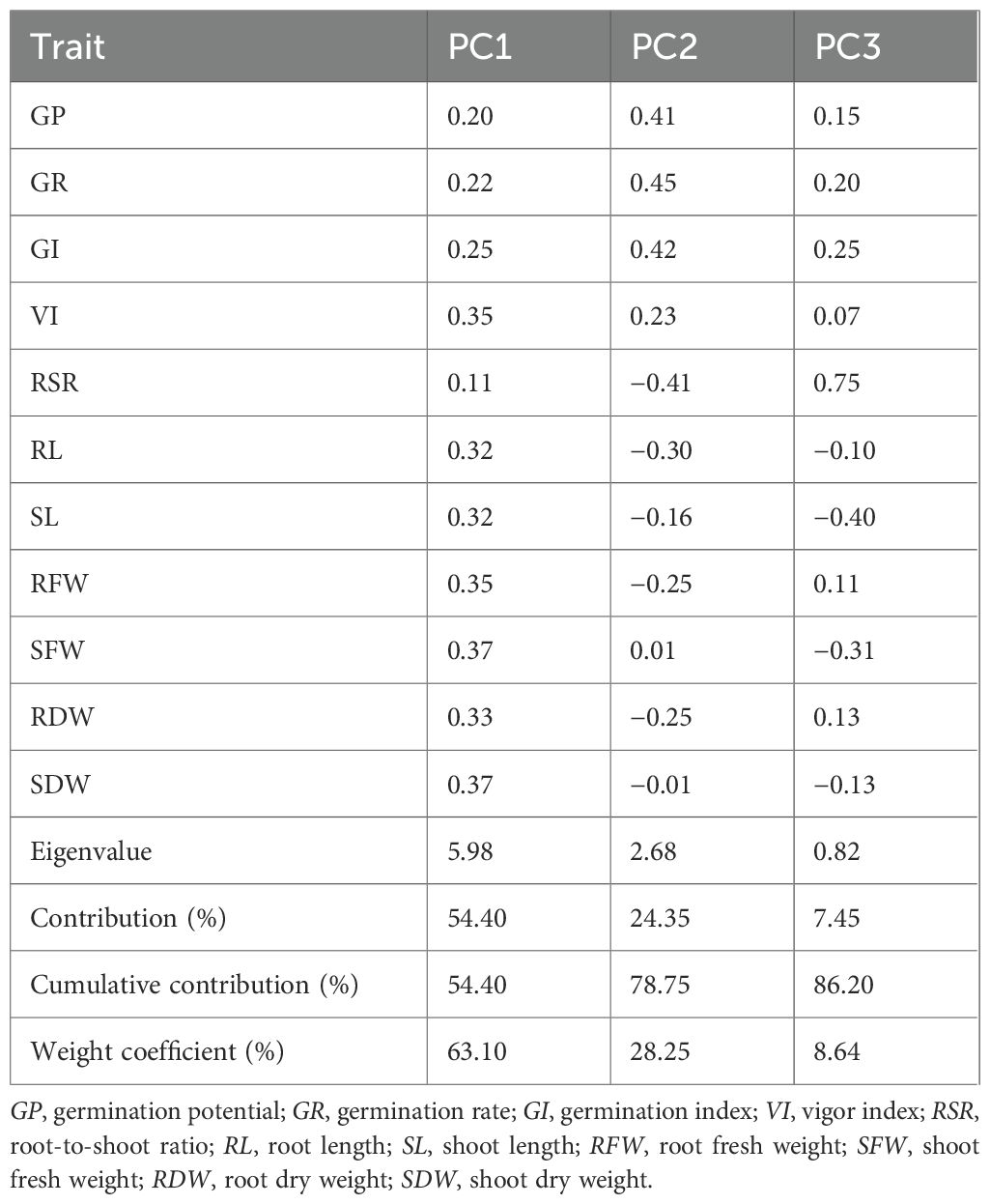
Table 3. Eigenvalues representing the contribution of maize traits extracted using principal component analysis.
3.4 Comprehensive evaluation of maize alkali tolerance
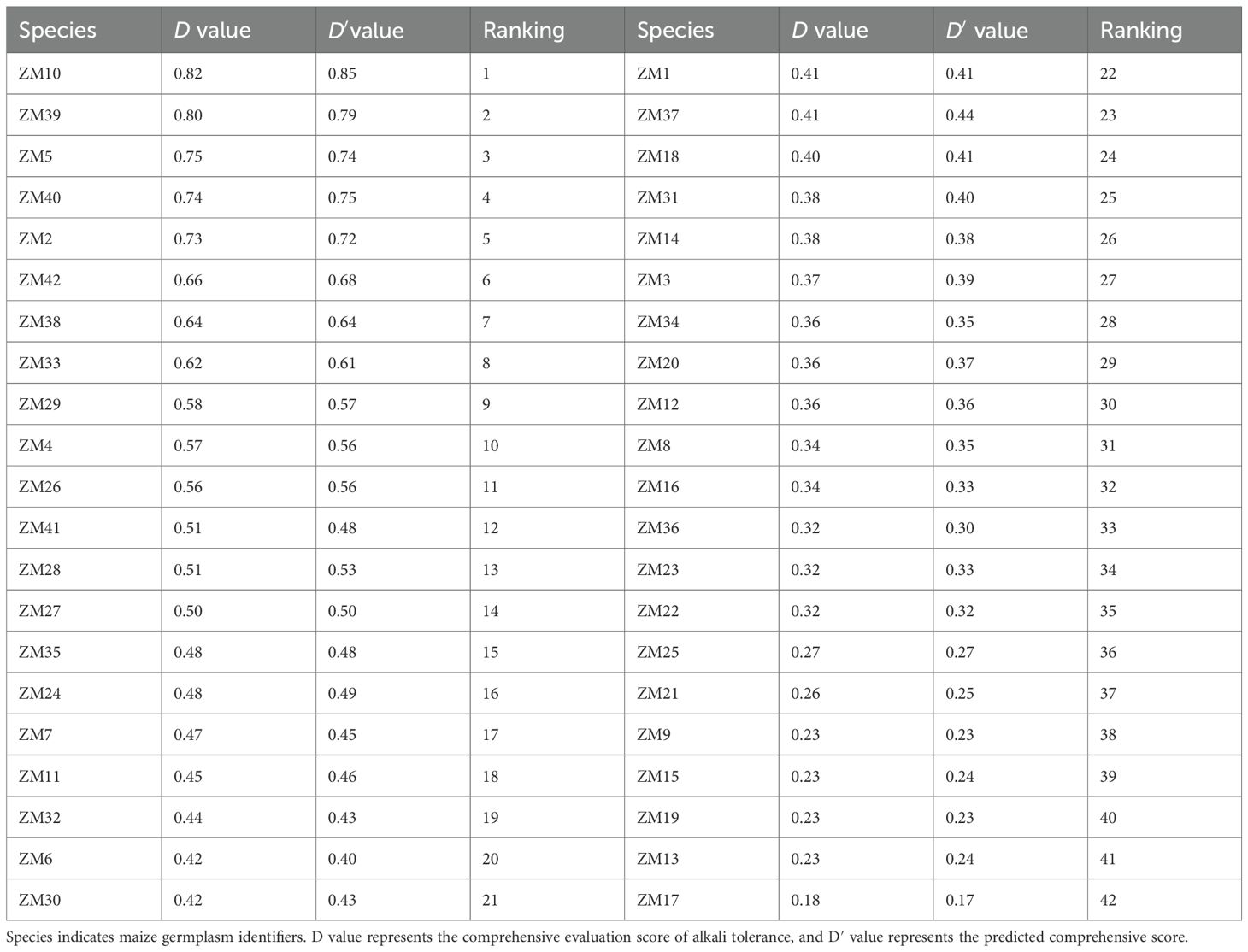
The comprehensive trait (μ value) was calculated using the membership function method, and the D value was determined based on the weights obtained from the PCA (Equations 8, 9). A higher D value indicates stronger alkali tolerance, whereas a lower D value reflects weaker tolerance. The comprehensive scores and the rankings of the germination traits for 42 maize materials were thus determined. Higher comprehensive scores corresponded to greater tolerance to saline–alkaline stress, and all materials were ranked from the highest to the lowest accordingly (Table 5). The D values ranged from 0.82 to 0.18. Material ZM10 ranked first, with a D value of 0.82, indicating superior performance in the alkali tolerance evaluation system. In contrast, material ZM17 had the lowest D value (0.18), ranking last and exhibiting high sensitivity to alkaline stress.
3.5 Screening key indicators for alkalinity tolerance using stepwise regression
Stepwise regression analysis was conducted to examine the relationship between several indicators and the ATC of maize. The candidate variables identified through the initial correlation analysis were used in the stepwise regression, with the D value as the dependent variable and the substantially associated indices as the independent variables for each variety. The resulting optimal regression equation is as follows:
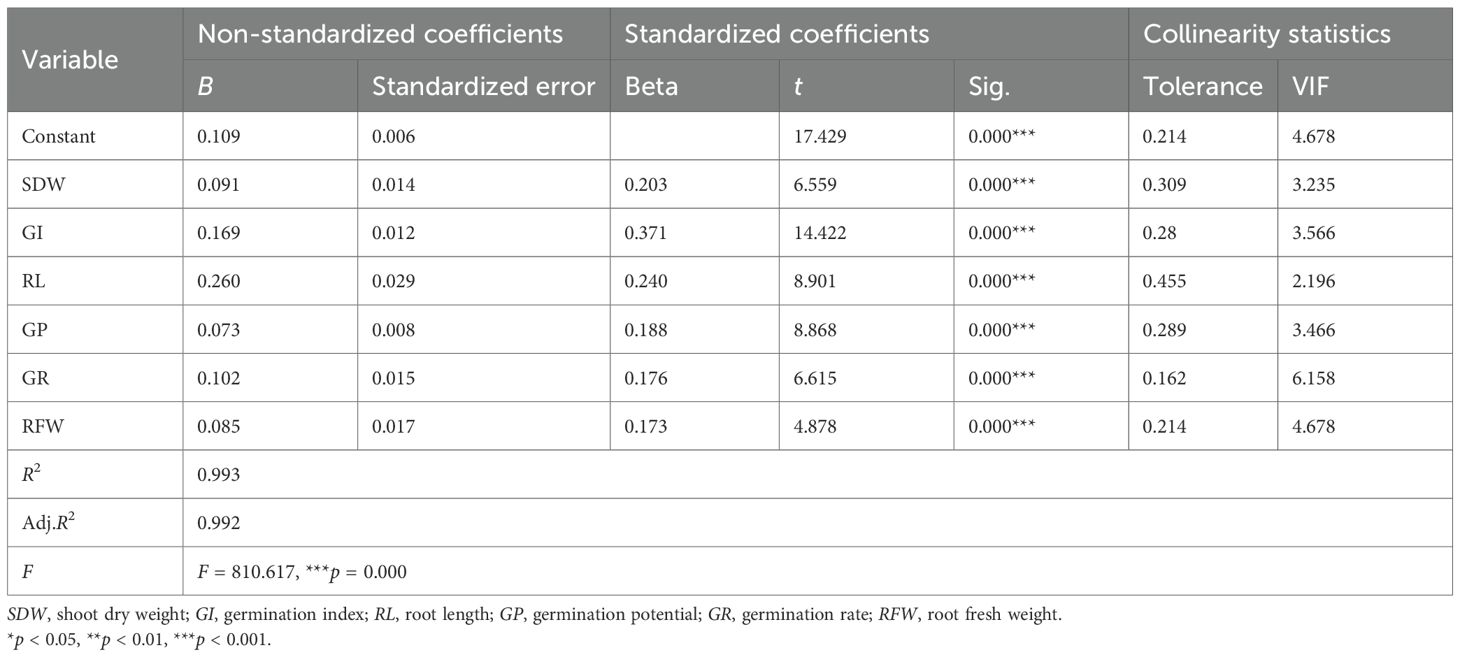
Seven indicators (i.e., GI, SDW, RFW, GR, GP, and RL) were incorporated due to their strong linear correlations with the D value. Model performance was evaluated through residual analysis and comparison of the predicted versus the observed values (Figure 3). The close alignment of the predicted and the observed values along the diagonal line indicates high predictive accuracy. The regression analysis results (Tables 5, 6) further confirmed a good overall model fit and statistical significance (F = 810.617, ***p < 0.001). These findings demonstrate that the stepwise regression model can reliably predict the alkali tolerance of maize based on the D value and that the selected morphological traits can serve as effective indicators for the evaluation and screening of maize germplasm.
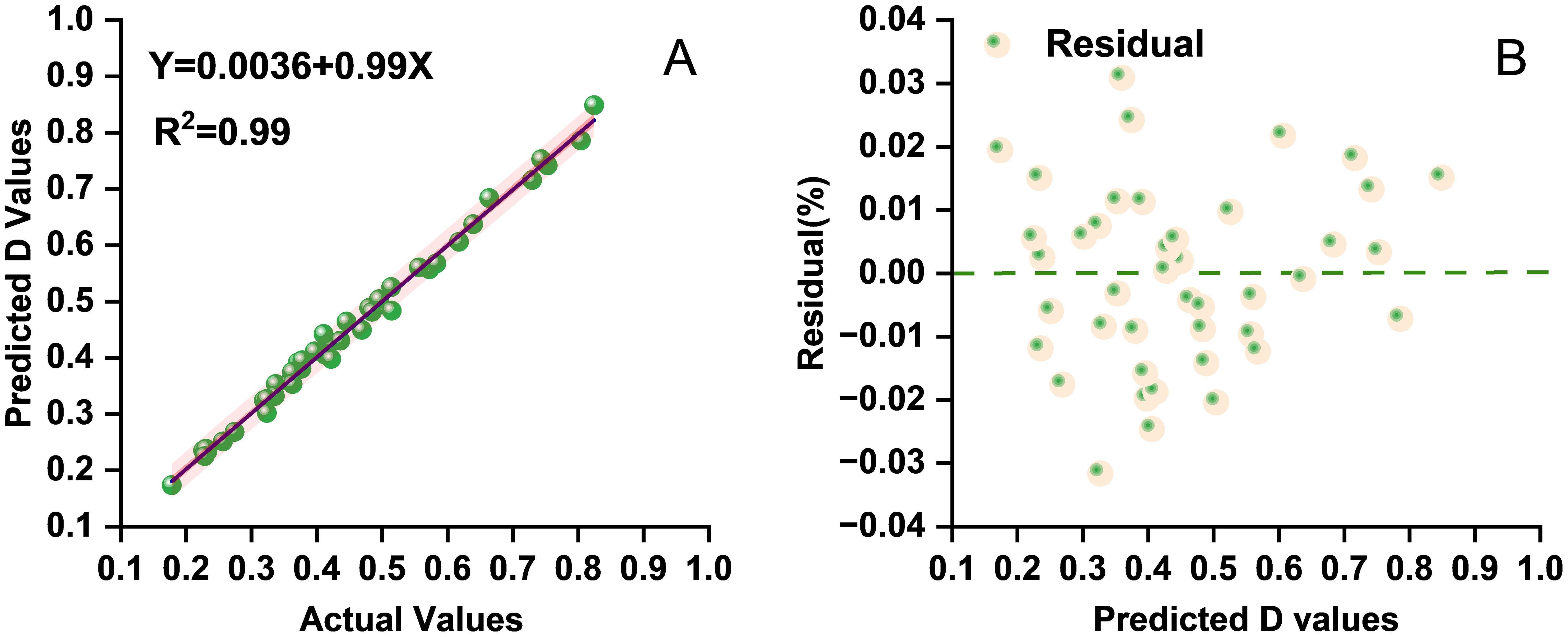
Figure 3. (A) Scatter distribution diagram of the predicted and the actual values on the test set for multiple stepwise regression. (B) Residual scatter plot from the stepwise regression analysis showing the distribution of residuals for the assessment of model fit and variance homogeneity.
3.6 Relative importance analysis based on the LMG method
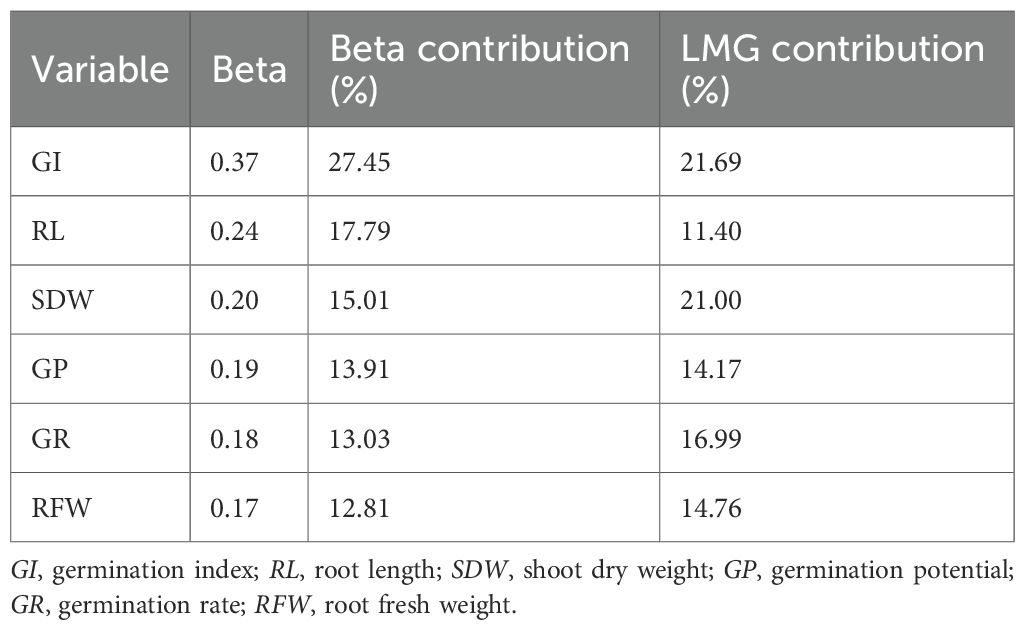
Stepwise regression identified six key traits (i.e., SDW, GI, RL, GP, GR, and RFW) for the construction of the maize alkalinity tolerance model. The standardized beta coefficients indicated (Equation 10) that GI had the strongest direct effect on D (0.37, 27.45%), followed by RL (0.24, 17.79%) and SDW (0.21, 15.01%), while GP, GR, and RFW had smaller direct effects (Table 7). LMG analysis (Equation 11) revealed that GI, SDW, and GR contributed most to the model’s explanatory power (21.69%, 21.00%, and 16.99%), whereas RFW, GP, and RL contributed 14.76%, 14.17%, and 11.40%, respectively (Table 7, Figure 4). The combined contribution of the root traits (RFW + RL, 26.16%) underscores the substantial role of root growth in determining alkalinity tolerance. Moreover, the germination-related traits, particularly GI, along with SDW, showed both strong direct effects and significant overall contributions, highlighting the importance of early seedling vigor and biomass accumulation. The agreement between the beta and LMG results indicates that GI and SDW play dominant roles, whereas the root traits and the other germination indicators provide supportive contributions. These findings provide a quantitative basis for the evaluation of maize seedlings and suggest that GI, SDW, and the root traits should be prioritized when screening germplasm and when selecting materials with enhanced alkalinity tolerance, offering a robust framework to guide breeding programs and improve selection efficiency.
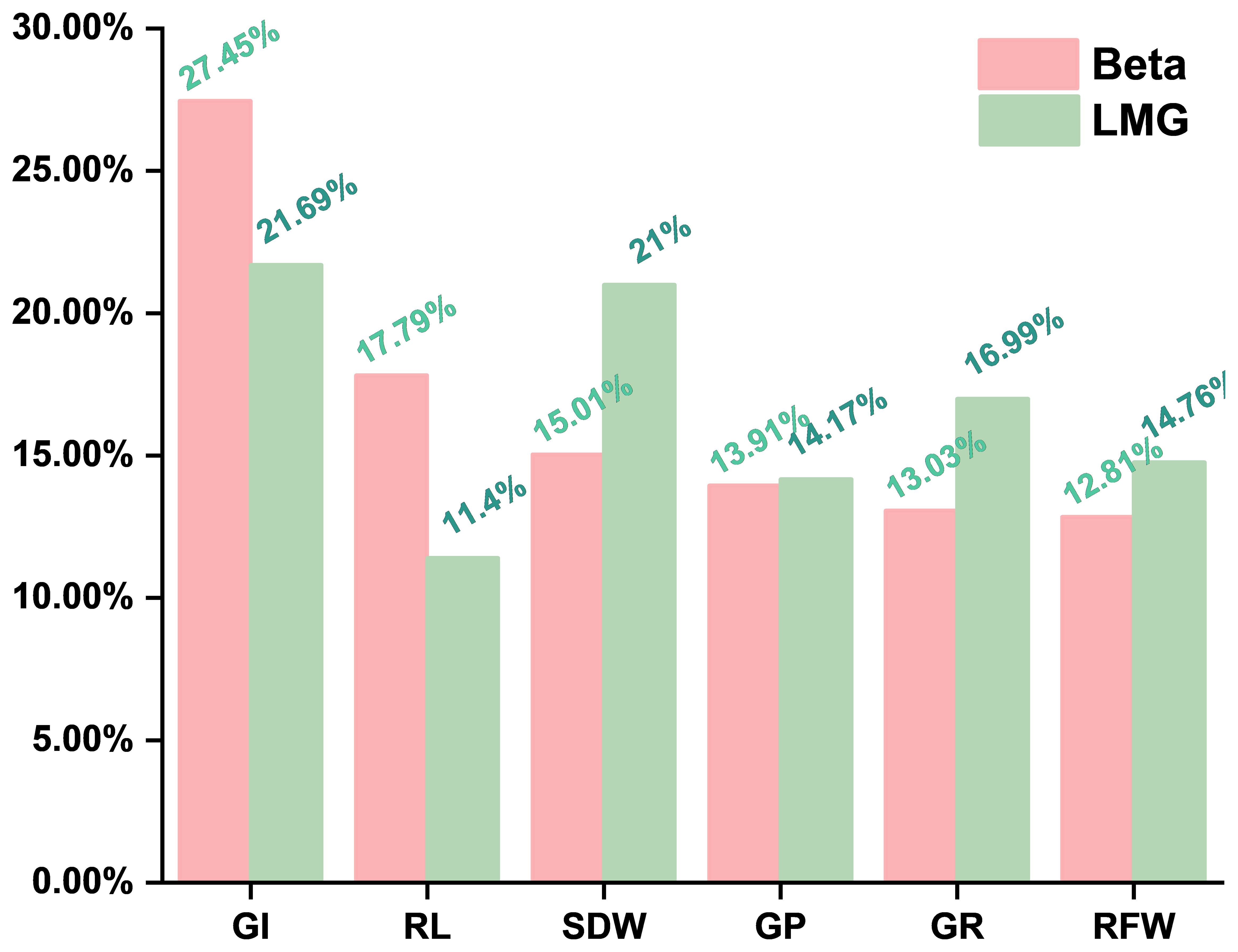
Figure 4. Standardized beta and Lindeman–Merenda–Gold (LMG) contribution, The relative influence of the key traits on alkali tolerance was quantified using standardized beta coefficients and LMG contributions, providing a reliable basis for the screening of alkali-tolerant maize germplasm.
3.7 Cluster analysis of maize materials
Systematic clustering was conducted using intergroup connectivity and squared Euclidean distance, with the composite D value as the primary clustering criterion. The 42 maize accessions were classified into five distinct groups (Figure 5A): highly alkali-resistant (HAR, five accessions), alkali-resistant (AR, six accessions), moderately alkali-resistant (MAR, six accessions), moderately alkali-sensitive (MAS, 18 accessions), and highly alkali-sensitive (HAS, seven accessions). MAS represented the largest proportion. To further illustrate the phenotypic differences among the groups, we plotted the distribution of the 11 morphological indicators across the five clusters (Supplementary Figure S2). The HAR and AR groups exhibited higher values of GP, GR, GI, VI, RL, SL, RFW, SFW, RDW, and SDW, reflecting superior early germination, seedling vigor, and biomass accumulation under alkaline stress. In contrast, the MAS and HAS groups showed lower values in these traits, indicating weaker growth performance and greater sensitivity. Overall, the indicator distribution patterns support the validity of the clustering and demonstrate that these traits can effectively differentiate maize accessions according to their alkali tolerance.
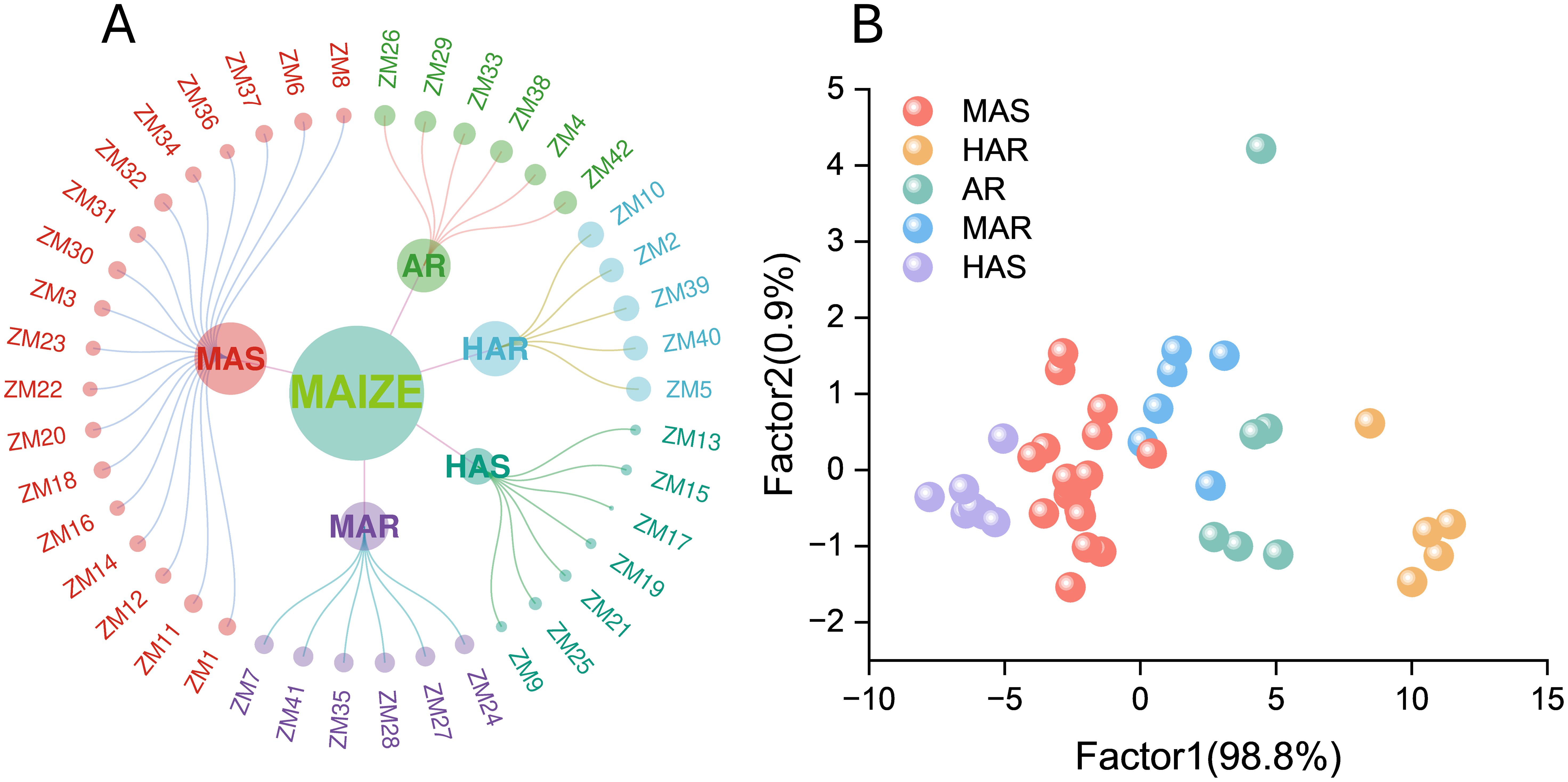
Figure 5. (A) The 42 maize materials classified into five groups via cluster analysis: high alkali tolerance, alkali tolerance, general alkali tolerance, general alkali sensitivity, and high alkali sensitivity. (B) Scatter plot of the discriminant analysis for the maize germplasm categories showing the separation of different maize lines based on the measured traits.
3.8 Discriminant analysis for maize material classification
Fisher’s discriminant analysis based on Bayesian coefficients was used to validate the accuracy of the clustering results and the alkali tolerance groupings (Figure 5B). Under alkaline salt stress, the HAR group exhibited the highest projected scores, followed by the AR and MAR groups, which displayed intermediate scores, while the MAS and HAS groups showed the lowest scores. Comparison of the discriminant function with the squared Euclidean distance method revealed a 92.9% concordance, indicating high reliability. The overall accuracy of the classification reached 95.2%, with a misjudgment rate of 4.8%, further demonstrating the robustness and precision of the evaluation approach.
3.9 Morphological variation analysis among the alkali-tolerant, alkali-sensitive, and overall maize materials
A paired-samples t-test was conducted using the mean values under the control and alkaline conditions for the HAR and HAS groups and the full set of accessions (Table 8). In the HAR group, majority of the traits were not significantly affected by alkaline stress. GP, GR, and GI showed slight increases under stress, reaching values of 0.280, 0.660, and 19.73, respectively, with all p-values greater than 0.05. Similarly, SL, RFW, SFW, RDW, and SDW exhibited minor changes without statistical significance. Only RL and RSR showed notable reductions, decreasing to 1.467 cm and 0.869, respectively (p < 0.05), indicating that the root elongation traits were primarily affected in tolerant lines. In contrast, the HAS group displayed pronounced and highly significant declines across all morphological indicators (p < 0.01). GP, GR, and GI dropped to 0.040, 0.187, and 4.36, respectively, while VI decreased sharply to 3.44. RL and SL were reduced to 0.290 cm and 0.492, respectively, and both the root and shoot fresh and dry biomass declined by over 80%–90%, demonstrating the severe susceptibility of the HAS lines to high-pH stress. Analysis of the full dataset revealed similar trends, with all 11 traits showing highly significant reductions under alkaline conditions (p < 0.01). Table 8 provides the mean values and the t-test results for clear numerical comparisons. The observed differences in the tolerance coefficients between the HAR and HAS groups (Figure 6) further highlighted the contrasting phenotypic responses, supporting the identification of alkali-tolerant accessions. .
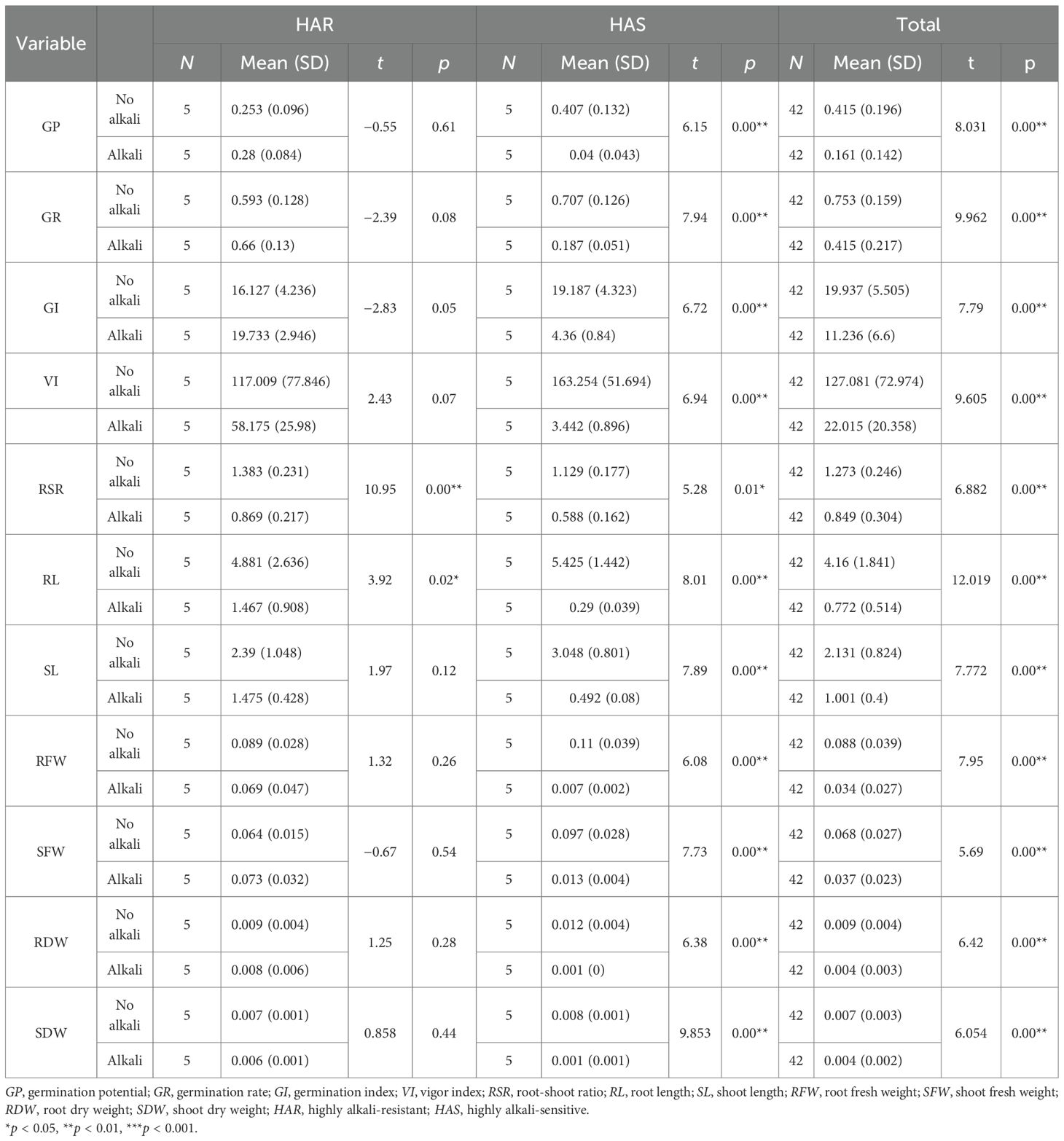
Table 8. Comparison of the various parameters between the alkali-treated and untreated groups using paired-samples t-tests.
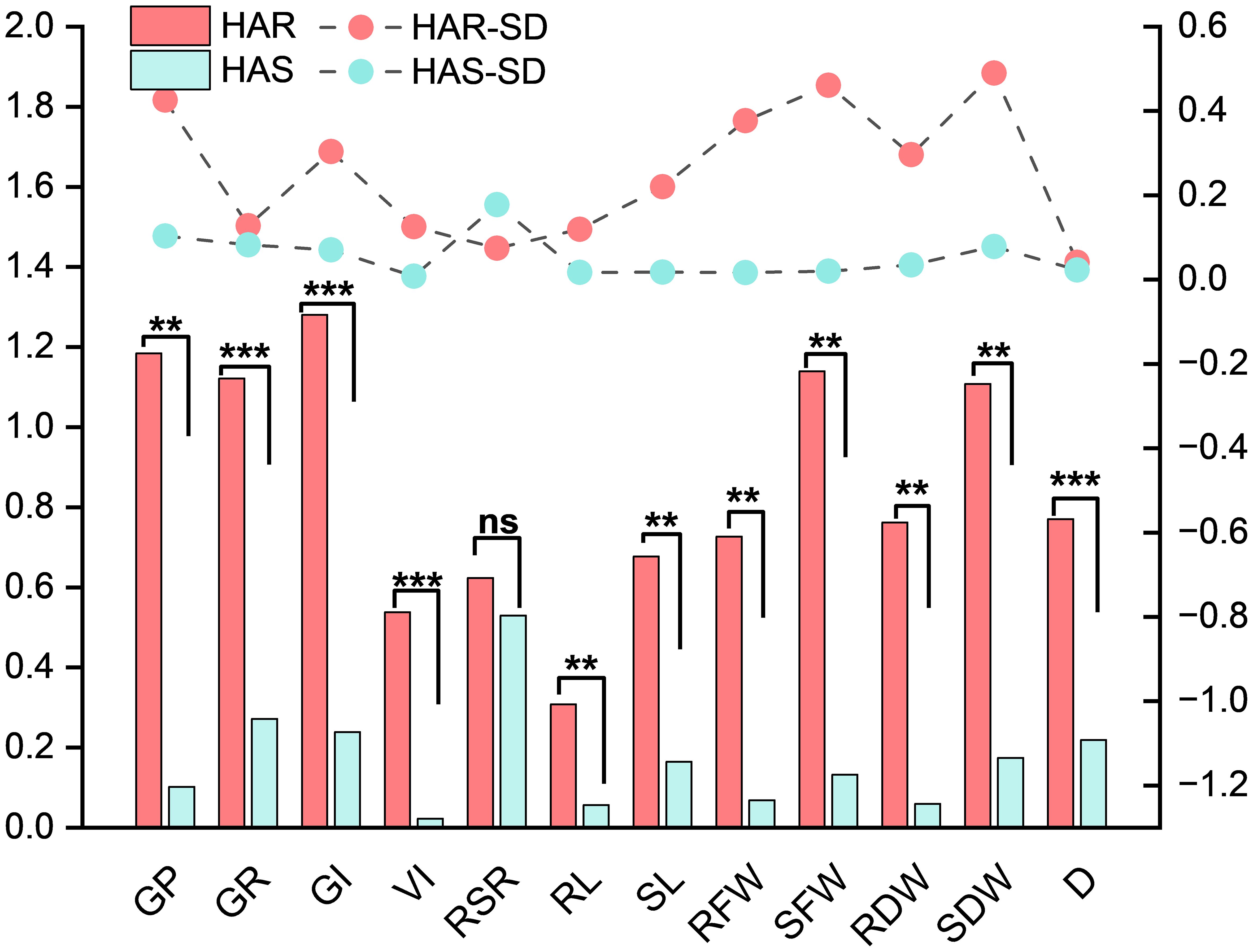
Figure 6. Comparisons of the alkali tolerance coefficients (ATCs) for the germination potential, germination rate, germination index, vigor index, root-to-shoot ratio, root length, shoot length, root fresh weight, shoot fresh weight, root dry weight, shoot dry weight, and D value under stress in five highly alkali-tolerant and five highly alkali-sensitive maize materials using mean values. *p < 0.05, **p < 0.01, ***p < 0.001. ns, not significant.
4 Discussion
4.1 Contribution of the morphological traits under alkaline stress
To identify the key morphological traits that contribute to maize alkalinity tolerance, a correlation analysis was first performed, selecting the traits significantly associated with the D value (i.e., GP, GR, GI, VI, RL, SL, RFW, SFW, RDW, and SDW) while excluding the unrelated trait, i.e., RSR. Stepwise regression was then conducted using these 10 traits as the independent variables and the D value as the dependent variable to determine the most influential indicators. Subsequently, the LMG method was applied to quantify the relative contributions of these key traits. The results revealed GI, SDW, RL, and RFW as the major contributors, jointly explaining over 40% of the total variance. GI and SDW reflect early seedling vigor, while RL and RFW indicate root system development and resource acquisition capacity. Notably, the root-related traits alone contributed approximately 26% of the total R2, highlighting their essential role in sustaining growth under high-pH conditions. These findings are consistent with previous studies reporting that an enhanced root elongation and biomass improve saline–alkali tolerance in maize and other crops (Khan et al., 2024b).
To complement LMG, the standardized regression coefficients (β) were analyzed. While β values quantify direct effects, they can be influenced by multicollinearity, whereas LMG decomposition averages the contribution of each variable across all possible model orderings, providing an unbiased estimate. Both approaches yielded consistent results, with GI and SDW showing the highest values, followed by RL and RFW, confirming the dominant roles of seedling vigor and root performance in the maintenance of growth under alkaline stress.
4.2 Morphological changes under alkaline stress
Building on the identification of the key traits associated with alkalinity tolerance, it is necessary to conduct a comprehensive analysis of the morphological adaptations of maize under alkaline stress. Maize is highly sensitive to salinity and alkalinity, and excessive soil alkalinity can impede water uptake, thereby affecting growth and biomass accumulation. Natural maize populations exhibit considerable genetic diversity, providing a basis for the screening of tolerant lines (Zhang et al., 2019; Zhao et al., 2020; Luo et al., 2021). In this study, alkaline stress markedly affected the germination and early seedling traits, with coefficients of variation ranging from 45% to 95%. The tolerant lines showed minimal reductions, limited to RL and RSR, whereas the sensitive lines exhibited significant decreases across nearly all traits (t-test: p < 0.001). These observations are highly consistent with the contribution rates of the key traits identified by the LMG analysis, further confirming the importance of SDW and RL in the evaluation of alkalinity tolerance and providing a reliable basis for subsequent D-value-based comprehensive assessment.
The root-related traits, particularly RL and RFW, are critical indicators for the screening of stress-tolerant maize (Yu et al., 2021; Sun et al., 2022; Ru et al., 2024), and morphological changes under stress have been extensively characterized (Yu et al., 2021). An elevated soil pH adversely affects root development, reducing the root vitality and limiting the water and nutrient uptake, thereby impairing plant growth (Bibikova et al., 1998; Chen et al., 2021). As the primary organ that perceives stress signals, the maize root system including the primary and lateral roots plays a central role in adaptation. Salt and alkaline stresses inhibit root elongation and biomass accumulation, whereas an enhanced tolerance may involve promoting lateral root proliferation while moderating primary root elongation (Rewald et al., 2012; Bellini et al., 2014; Koevoets et al., 2016). Changes in the RL, biomass, and branching patterns form a crucial physiological basis for the adaptation of maize to alkaline stress (Julkowska et al., 2014; Li et al., 2021).
Furthermore, alkaline soils often cause more severe root damage than saline soils (Pan et al., 2020), and species such as bitter beans, sea buckthorn, and black wheatgrass have demonstrated that root traits are key determinants of tolerance (Matsuoka et al., 2022; Wang et al., 2022). Molecular studies have shown that maize can enhance tolerance by regulating gene expression to control the Na+ efflux from the roots (Cao et al., 2020). Collectively, these findings highlight that root morphological plasticity is a central adaptive mechanism underpinning the resilience of maize under alkaline stress. Building on this understanding of the root and shoot traits under alkaline conditions, we further examined how these morphological variations reflect the underlying physiological and biochemical disruptions during early seedling development. Alkaline stress imposes multiple simultaneous constraints on early seedling growth. High-pH soil conditions may inhibit water imbibition by reducing cell wall acidification and extensibility, impair the root plasma membrane function, and inhibit the enzyme activities necessary for seed germination and growth (Chen et al., 2017; Yang et al., 2024). Collectively, these processes delay radicle emergence and weaken seedling establishment. Moreover, excessive bicarbonate and carbonate ions interfere with nutrient availability and ion uptake, particularly restricting Ca2+, Mg2+, and Fe2+ acquisition, which in turn limits the root elongation and biomass accumulation (Wang et al., 2022; Kumar et al., 2024). The patterns observed in this study reduced the RL, diminished the fresh and dry weights, and inhibited the shoot growth in the sensitive accessions, which align with these physiological disruptions, indicating that the early morphological traits directly reflect the biochemical and cellular consequences of alkaline stress. The strong correspondence between the morphological variations and the physiological expectations reinforces the biological relevance of the 11 measured traits, explaining why RL, RFW, and SDW exhibited high correlations with the comprehensive D value and contributed substantially to the tolerance classification (Lin et al., 2019; Ergun et al., 2024; Guo et al., 2024).
These physiological responses to alkalinity are not unique to maize: they have been widely documented across other species, supporting the generality of the mechanisms observed here. For example, wheat display significant reductions in root biomass and an impaired lateral root development under high-pH stress (Lin et al., 2019), mirroring the patterns observed in maize. Oilseed rape and alfalfa exhibit similar declines in GR and seedling vigor due to bicarbonate-induced osmotic and ionic disturbances. Studies in halophytes such as quinoa and Puccinellia tenuiflora have further demonstrated that tolerance is associated with maintaining root growth, sustaining water absorption (Liu et al., 2018; Ye et al., 2019; Zhang et al., 2023; Bao et al., 2024; Xu et al., 2025), and moderating the ion imbalance physiological strategies, consistent with the superior root performance of the tolerant maize accessions in this study. Similar mechanisms have also been reported in rice and soybean, where tolerant cultivars maintained root growth, regulated the carbon and nitrogen metabolism, and modulated the ion and osmotic balance under alkaline stress (Zhang et al., 2016, 2017; Zhou et al., 2023; Xie et al., 2024; Zhang et al., 2025). Collectively, these cross-species comparisons highlight the importance of root development and early seedling vigor as universal indicators of alkalinity tolerance.
4.3 Comprehensive evaluation of alkali tolerance in 42 maize materials
Comprehensive evaluation of maize alkali tolerance is essential for germplasm screening and breeding program development. The D-value method is a widely used approach for the assessment and differentiation of alkali tolerance among maize genotypes (Khan et al., 2024a; Ren et al., 2025). In this study, 11 morphological traits—GP, GR, GI, VI, RSR, RL, SL, RFW, SFW, RDW, and SDW—were selected for the PCA to reduce dimensionality. PCA was combined with MFA, CA, and LDA to establish a comprehensive evaluation framework for maize alkali tolerance. Using this framework, 42 maize materials were classified into five tolerance levels: highly tolerant (5 accessions), tolerant (6 accessions), moderately tolerant (6 accessions), moderately sensitive (18 accessions), and highly sensitive (7 accessions). The correlation analysis indicated significant associations between the D value and 10 of the 11 traits, except for the RSR. Based on these results, the stepwise regression identified six key indicators—GI, SDW, RFW, GP, GR, and RL—which were used to construct a predictive model (Equation 12) for the evaluation of alkali tolerance during the germination stage.
Several maize materials in this study exhibited enhanced germination under mild alkaline stress, consistent with previous reports (Xu et al., 2020). This suggests that, when the stress intensity is below a harmful threshold, moderately alkaline conditions may stimulate seed germination. The observed pattern of stimulation at low concentrations and inhibition at high concentrations reflects a concentration-dependent response, likely related to cellular physiological regulation. At low concentrations, alkaline solutions may enhance the metabolic activity and water uptake, thereby accelerating germination (Guo et al., 2008). In contrast, high concentrations primarily inhibit germination through osmotic stress and ion toxicity (Zhang et al., 2024). These results indicate that the effects of alkaline stress on early maize growth are not unidirectional, but represent a dynamic balance between stimulation and inhibition, consistent with findings in other plant species (Guo et al., 2008).
Seed germination and seedling establishment require an appropriate pH, as both excessively high and low pH values are detrimental to growth (Jefferson et al., 1989; Rivard and Woodard, 1989). A low pH can inhibit germination and early seedling development (Shelley et al., 2018), whereas a high pH disrupts cellular homeostasis, ion balance, and enzyme activity; damages the cellular structures; and interferes with metabolism, ultimately leading to tissue degradation or seed death (Guo et al., 2010). Therefore, maintaining an optimal pH is crucial for maize growth under saline–alkali stress and provides insights into the physiological mechanisms underlying alkali tolerance, guiding future breeding efforts.
Notably, Xianyu335 was classified as alkali-sensitive in this study, in agreement with earlier observations (Zhang, 2016). Nevertheless, contrasting reports have described this variety as tolerant to salt–alkali during the germination and seedling stages (Liu et al., 2023), indicating possible differences in the stress responses across developmental stages or testing environments. This discrepancy may reflect differences in the developmental stage responses (Morton et al., 2019) or the specific stress regime applied here (Na2CO3/NaHCO3 = 1:9, pH 9.21 ± 0.1), which may have exerted stronger effects. These observations further support that plants often experience more severe damage from alkaline stress than from salt stress (Li and Yang, 2023; Qi et al., 2024).
The analytical framework used in this study integrated complementary statistical approaches to capture different aspects of the data structure. PCA reduces the trait dimensionality and extracts principal variation axes, while MFA balances the trait groups to avoid bias from the traits with larger variance. The D value synthesizes the PCA–MFA outputs into a single tolerance index. CA subsequently identifies natural grouping patterns among accessions, while LDA validates the classification and improves the discrimination accuracy. The stepwise regression and LMG analyses further quantified the relative contributions of the individual traits. These interconnected methods formed a coherent evaluation system in which dimensionality reduction, classification, and trait attribution mutually support the robustness of the final tolerance assessment. This integrated design strengthens the biological interpretability and reproducibility of the classification outcomes.
4.4 Limitations and future perspectives
Although this study established a comprehensive morphological framework for maize alkali tolerance at the germination stage, only the early-stage traits were assessed and physiological or ionomic measurements were not included, limiting mechanistic insights. The evaluation was conducted under controlled laboratory conditions with a single alkaline concentration, which may not fully capture field variability. Future studies should integrate multi-omics approaches and high-throughput phenotyping, as well as validate candidate tolerant accessions under diverse field conditions. Combining morphological, physiological, and molecular data will enhance the robustness and applicability of the tolerance evaluation system for breeding programs.
5 Conclusion
This study established a systematic framework for the evaluation of alkali tolerance in maize during germination. Using this framework, eight highly tolerant and seven highly sensitive accessions were identified. By quantifying the contribution of key indicators through the LMG method, GI, SDW, RL, and RFW were confirmed as reliable morphological traits for distinguishing tolerant and sensitive accessions. The results demonstrate that the proposed evaluation system effectively achieves the primary objectives of the study: to identify alkali-tolerant genotypes and determine the key traits associated with tolerance. Overall, this framework provides a practical and robust tool for the rapid screening of maize germplasm and lays a solid foundation for subsequent physiological and breeding studies.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
ZP: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing, Visualization. ZG: Data curation, Investigation, Writing – review & editing. FG: Data curation, Investigation, Writing – review & editing. MA: Funding acquisition, Resources, Writing – review & editing. YG: Resources, Writing – review & editing.
Funding
The author(s) declared financial support was received for this work and/or its publication This work was supported by the Chinese Academy of Sciences.
Acknowledgments
We sincerely thank the Chinese Academy of Sciences for financial support. We are grateful to Dr. Xin Shi from the Changchun Branch of the Chinese Academy of Sciences for his thorough proofreading and valuable suggestions on this manuscript.
Conflict of interest
The authors declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1728607/full#supplementary-material
References
Ali, M., Zafar, S., and Ashraf, M. Y. (2022). Assessment of maize genotypes for salt tolerance based on physiological indices. Pakistan J. Bot. 54, 1613–1618. doi: 10.30848/pjb2022-5(34
Baha, J., Liu, W., Ma, X., Li, Y., Zhao, X., Zhai, X., et al. (2025). Comprehensive evaluation of 202 Cotton Varieties (Lines) and their physiological drought resistance response during seedling stage. Plants 14, 1770. doi: 10.3390/plants14121770
Baloch, M. Y. J., Zhang, W., Sultana, T., Akram, M., Al Shoumik, B. A., Khan, M. Z., et al. (2023). Utilization of sewage sludge to manage saline–alkali soil and increase crop production: Is it safe or not? Environ. Technol. Innovation. 32, 103266. doi: 10.1016/j.eti.2023.103266
Bao, Q., Wu, Y., Wang, Y., and Zhang, Y. (2024). Comparative transcriptomic analysis reveals transcriptional differences in the response of quinoa to salt and alkali stress responses. Agronomy. 14, 1596. doi: 10.3390/agronomy14071596
Bellini, C., Pacurar, D. I., and Perrone, I. (2014). Adventitious roots and lateral roots: similarities and differences. Annu. Rev. Plant Biol. 65, 639–666. doi: 10.1146/annurev-arplant-050213-035645
Bibikova, T. N., Jacob, T., Dahse, I., and Gilroy, S. (1998). Localized changes in apoplastic and cytoplasmic pH are associated with root hair development in Arabidopsis thaliana. Development. 125, 2925–2934. doi: 10.1242/DEV.125.15.2925
Cao, X., Jiang, F., Wang, X., Zang, Y., and Wu, Z. (2015). Comprehensive evaluation and screening for chilling-tolerance in tomato lines at the seedling stage. Euphytica 205, 569–584. doi: 10.1007/s10681-015-1433-0
Cao, Y., Zhang, M., Liang, X., Li, F., Shi, Y., Yang, X., et al. (2020). Natural variation of an EF-hand Ca(2+)-binding-protein coding gene confers saline-alkaline tolerance in maize. Nat. Commun. 11, 186. doi: 10.1038/s41467-019-14027-y
Caturegli, L., Matteoli, S., Gaetani, M., Grossi, N., Magni, S., Minelli, A., et al. (2020). Effects of water stress on spectral reflectance of Bermudagrass. Sci. Rep. 10, 15055. doi: 10.1038/s41598-020-72006-6
Chen, H., Zhang, Q., Cai, H., and Xu, F. (2017). Ethylene mediates alkaline-induced rice growth inhibition by negatively regulating plasma membrane H(+)-ATPase activity in roots. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.01839
Chen, J., Li, X., Ye, X., Guo, P., Hu, Z., Qi, G., et al. (2021). An S-ribonuclease binding protein EBS1 and brassinolide signaling are specifically required for Arabidopsis tolerance to bicarbonate. J. Exp. Bot. 72, 1449–1459. doi: 10.1093/jxb/eraa524
Ding, R., Nobrega, R. L. B., and Prentice, I. C. (2025). Global assessment of environmental and plant-trait influences on root: shoot biomass ratios. Global Change Biol. 31, e70543. doi: 10.1111/gcb.70543
Dodd, G. L. and Donovan, L. A. (1999). Water potential and ionic effects on germination and seedling growth of two cold desert shrubs. Am. J. Bot. 86, 1146–1153. doi: 10.2307/2656978
Ergun, N., Akdogan, G., Aydogan, S., Bilir, M., and Kılıc, G. (2024). Determination of early-stage shoot and root traits of cultivated and wild barley genotypes. J. Cent. Eur. Agric. 25, 661–674. doi: 10.5513/jcea01/25.3.4220
Gonzalez-Martinez, A., De-Pablos-Heredero, C., González, M., Rodriguez, J., Barba, C., and García, A. (2021). Usefulness of discriminant analysis in the morphometric differentiation of six native freshwater species from Ecuador. Animals. 11, 111. doi: 10.3390/ani11010111
Groemping, U. (2006). Relative Importance for Linear Regression in R: The Package relaimpo. Journal of Statistical Software. 17, 1–27. doi: 10.18637/jss.v017.i01
Guo, R., Shi, L., Ding, X., Hu, Y., Tian, S., Yan, D., et al. (2010). Effects of saline and alkaline stress on germination, seedling growth, and ion balance in wheat. Agron. Journa. 102, 1252–1260. doi: 10.2134/agronj2010.0022
Guo, Y., Yang, H., and Zhao, J. (2008). Effects of complex saline-alkaloid stress on the seed germination of soybean. Seed. 27, 92–94. doi: 10.16590/j.cnki.1001-4705.2008.12.059
Guo, C., Zhu, L., Sun, H., Han, Q., Wang, S., Zhu, J., et al. (2024). Evaluation of drought-tolerant varieties based on root system architecture in cotton (Gossypium hirsutum L.). BMC Plant Biol. 24, 127. doi: 10.1186/s12870-024-04799-x
Han, W., Yang, Z., Huang, L., Sun, C., Yu, X., and Zhao, M. (2019). Fuzzy comprehensive evaluation of the effects of relative air humidity on the morpho-physiological traits of Pakchoi (Brassica chinensis L.) under high temperature. Scientia Hortic. 246, 971–978. doi: 10.1016/j.scienta.2018.11.079
Helm, D. and Eis, D. (2007). Subgrouping outpatients of an environmental medicine unit using SCL-90-R and cluster analysis. Int. J. Hygiene Environ. Health 210, 701–713. doi: 10.1016/j.ijheh.2006.11.006
Hosseinpour, S. and Martynenko, A. (2021). Food quality evaluation in drying: Structuring of measurable food attributes into multi-dimensional fuzzy sets. Drying Technol. 40, 2293–2307. doi: 10.1080/07373937.2021.1933514
Hu, M., Zhang, D., Du, W., Tian, H., Hao, Y., Ding, S., et al. (2025). Screening for cold tolerance resources in maize seedlings and analysis of leaf cell responses. Front. Plant Sci. 16. doi: 10.3389/fpls.2025.1565831
Jefferson, P., Johnson, D., Rumbaugh, M., and Asay, K. (1989). Water stress and genotypic effects on epicuticular wax production of alfalfa and crested wheatgrass in relation to yield and excised leaf water loss rate. Can. J. Plant Sci. 69, 481–490. doi: 10.4141/cjps89-059
Julkowska, M. M., Hoefsloot, H. C., Mol, S., Feron, R., de Boer, G. J., Haring, M. A., et al. (2014). Capturing Arabidopsis root architecture dynamics with ROOT-FIT reveals diversity in responses to salinity. Plant Physiol. 166, 1387–1402. doi: 10.1104/pp.114.248963
Kamphorst, S. H., Gonçalves, G. M. B., Amaral Júnior, A. T. D., Lima, V. J. D., Schmitt, K. F. M., Leite, J. T., et al. (2021). Supporting physiological trait for indirect selection for grain yield in drought-stressed popcorn. Plants. 10, 1510. doi: 10.3390/plants10081510
Khan, R., Gao, F., Khan, K., Shah, M. A., Ahmad, H., Fan, Z. P., et al. (2024a). Evaluation of maize varieties via multivariate analysis: Roles of ionome, antioxidants, and autophagy in salt tolerance. Plant Physiol. 196, 195–209. doi: 10.1093/plphys/kiae335
Khan, W., Zhu, Y., Khan, A., Zhao, L., Yang, Y.-M., Wang, N., et al. (2024b). Above-and below-ground feedback loop of maize is jointly enhanced by plant growth-promoting rhizobacteria and arbuscular mycorrhizal fungi in drier soil. Sci. Total Environ. 917, 170417. doi: 10.1016/j.scitotenv.2024.170417
Koevoets, I. T., Venema, J. H., Elzenga, J. T., and Testerink, C. (2016). Roots withstanding their environment: exploiting root system architecture responses to abiotic stress to improve crop tolerance. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.01335
Królaczyk, K., Zaborski, D., Dzierzba, E., and Kavetska, K. M. (2020). Redescription of quasiamidostomum fulicae (Rudolphi 1819) lomakin 1991 (Nematoda: Amidostomatidae), a parasite of fulica atra (Gruiformes). J. Veterinary Res. 64, 95. doi: 10.2478/jvetres-2020-0014
Kumar, K., Jaiswal, A., Koppolu, U. M. K., and Kumar, K. R. R. (2024). Alkaline stress disrupts growth, biochemistry, and ion homeostasis of chickpea (Cicer arietinum L.) roots. Front. Agron. 6. doi: 10.3389/fagro.2024.1497054
Li, J. and Yang, Y. (2023). How do plants maintain pH and ion homeostasis under saline-alkali stress? Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1217193
Li, P., Yang, X., Wang, H., Pan, T., Wang, Y., Xu, Y., et al. (2021). Genetic control of root plasticity in response to salt stress in maize. Theor. Appl. Genet. 134, 1475–1492. doi: 10.1007/s00122-021-03784-4
Liang, J. and Shi, W. (2021). Cotton/halophytes intercropping decreases salt accumulation and improves soil physicochemical properties and crop productivity in saline-alkali soils under mulched drip irrigation: A three-year field experiment. Field Crops Res. 262, 8909. doi: 10.1016/j.fcr.2020.108027
Lin, Y., Yi, X., Tang, S., Chen, W., Wu, F., Yang, X., et al. (2019). Dissection of phenotypic and genetic variation of drought-related traits in diverse Chinese Wheat Landraces. Plant Genome. 12, 1–14. doi: 10.3835/plantgenome2019.03.0025
Lindeman, R. H., Merenda, P. F., and Gold, R. Z. (1980). Introduction to bivariate and multivariate analysis (Glenview, IL: Scott, Foresman).
Liu, D., Esquivel-Muelbert, A., Acil, N., Astigarraga, J., Cienciala, E., Fridman, J., et al. (2024). Mapping multi-dimensional variability in water stress strategies across temperate forests. Nat. Commun. 15. doi: 10.1038/s41467-024-53160-1
Liu, D., Liu, M., Liu, X. L., Cheng, X. G., and Liang, Z. W. (2018). Silicon priming created an enhanced tolerance in Alfalfa (Medicago sativa L.) seedlings in response to high alkaline stress. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.00716
Liu, H., Zhang, F., Tian, H., Hu, M., Li, R., Yang, X., et al. (2023). Study on salt tolerance of different maize varieties at germination and seedling stages. Seed. 42, 56–62. doi: 10.16590/j.cnki.1001-4705.2023.03.056
Luo, M., Zhang, Y., Li, J., Zhang, P., Chen, K., Song, W., et al. (2021). Molecular dissection of maize seedling salt tolerance using a genome-wide association analysis method. Plant Biotechnol. J. 19, 1937–1951. doi: 10.1111/pbi.13607
Martini, D., Pacheco-Labrador, J., Perez-Priego, O., van der Tol, C., El-Madany, T. S., Julitta, T., et al. (2019). Nitrogen and phosphorus effect on sun-induced fluorescence and gross primary productivity in mediterranean grassland. Remote Sens-Basel 11, 2562. doi: 10.3390/rs11112562
Matsuoka, T., Asagi, N., and Komatsuzaki, M. (2022). Response of weeds and rice yield to Italian ryegrass as a cover crop and planting density in organic farming. Agron. J. 114, 689–699. doi: 10.1002/agj2.20939
McMillen, M. S., Mahama, A. A., Sibiya, J., Lübberstedt, T., and Suza, W. P. (2022). Improving drought tolerance in maize: Tools and techniques. Front. Genet. 13. doi: 10.3389/fgene.2022.1001001
Morton, M. J., Awlia, M., Al-Tamimi, N., Saade, S., Pailles, Y., Negrão, S., et al. (2019). Salt stress under the scalpel–dissecting the genetics of salt tolerance. Plant J. 97, 148–163. doi: 10.1111/tpj.14189
Nathans, L. L., Oswald, F. L., and Nimon, K. (2012). Interpreting multiple linear regression: a guidebook of variable importance. Practical assessment, research & evaluation. 17, n9. doi: 10.7275/5fex-b874
Nelson, P., Baldock, J., and Oades, J. (1998). Changes in dispersible clay content, organic carbon content, and electrolyte composition following incubation of sodic soil. Soil Res. 36, 883–898. doi: 10.1071/S98024
Pan, T., Liu, M., Kreslavski, V. D., Zharmukhamedov, S. K., Nie, C., Yu, M., et al. (2020). Non-stomatal limitation of photosynthesis by soil salinity. Critical reviews in environmental science and technology. 51, 791–825. doi: 10.1080/10643389.2020.1735231
Qi, Y., Xie, Y., Ge, M., Shen, W., He, Y., Zhang, X., et al. (2024). Alkaline tolerance in plants: the AT1 gene and beyond. J. Plant Physiol. 303, 154373. doi: 10.1016/j.jplph.2024.154373
Ren, S., Tan, J., Zhou, S., Sun, H., Li, H., Li, W., et al. (2025). Germplasm selection and comprehensive evaluation of maize inbred lines at germination and seedling stage for saline–alkali tolerance. Agronomy. 15, 626. doi: 10.3390/agronomy15030626
Rewald, B., Raveh, E., Gendler, T., Ephrath, J. E., and Rachmilevitch, S. (2012). Phenotypic plasticity and water flux rates of Citrus root orders under salinity. J. Exp. Bot. 63, 2717–2727. doi: 10.1093/jxb/err457
Rezvi, H. U. A., Tahjib-Ul-Arif, M., Azim, M. A., Tumpa, T. A., Tipu, M. M. H., Najnine, F., et al. (2022). Rice and food security: Climate change implications and the future prospects for nutritional security. Food Energy Secur. 12, e430. doi: 10.1002/fes3.430
Rivard, P. G. and Woodard, P. M. (1989). Light, ash, and pH effects on the germination and seedling growth of Typha latifolia (cattail). Can. J. Bot. 67, 2783–2787. doi: 10.1139/b89-358
Ru, S., Sanz-Saez, A., Leisner, C. P., Rehman, T., and Busby, S. (2024). Review on blueberry drought tolerance from the perspective of cultivar improvement. Front. Plant Sci. 15. doi: 10.3389/fpls.2024.1352768
Sahab, S., Suhani, I., Srivastava, V., Chauhan, P. S., Singh, R. P., and Prasad, V. (2021). Potential risk assessment of soil salinity to agroecosystem sustainability: Current status and management strategies. Sci. Total Environ. 764, 144164. doi: 10.1016/j.scitotenv.2020.144164
Sarath, N., Nair, G. S., Sruthi, P., Shackira, A. M., and Puthur, J. T. (2021). “Halophytes as effective tool for phytodesalination and land reclamation,” in Frontiers in plant-soil interaction (San Diego, CA: Academic Press), 459–494. doi: 10.1016/B978-0-323-90943-3.00020-1
Shelley, B., Luster, D., Garrett, W., McMahon, M., and Widmer, T. (2018). Effects of temperature on germination of sporangia, infection and protein secretion by Phytophthora kernoviae. Plant Pathol. 67, 719–728. doi: 10.1111/ppa.12782
Song, R., Shi, P., Xiang, L., He, Y., Dong, Y., Miao, Y., et al. (2024). Evaluation of barley genotypes for drought adaptability: based on stress indices and comprehensive evaluation as criteria. Front. Plant Sci. 15. doi: 10.3389/fpls.2024.1436872
Sun, Z., Zou, Y., Xie, C., Han, L., Zheng, X., Tian, Y., et al. (2022). Brassinolide improves the tolerance of Malus hupehensis to alkaline stress. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.1032646
Tang, L., Li, W., Yu, Q., Sun, Z., and Li, P. (2024). Assessing germplasm variation and tolerance thresholds of Bermudagrass (Cynodon dactylon) to neutral and alkaline salt stress in ecological restoration. Agronomy. 14, 2023. doi: 10.3390/agronomy14092023
Wang, W., Zhang, F., Sun, L., Yang, L., Yang, Y., Wang, Y., et al. (2022). Alkaline salt inhibits seed germination and seedling growth of canola more than neutral salt. Front. Plant Sci. 13. doi: 10.3389/fpls.2022.814755
Wu, Y., Qiu, X., Liang, D., Zeng, X., and Liu, Q. (2024). How the characteristics of land cover changes affect vegetation greenness in Guangdong, a rapid urbanization region of China during 2001–2022. Environ. Monit. Assess. 196, 1020. doi: 10.1007/s10661-024-13219-4
Xie, X., Ji, R., Xu, Z., Shao, Q., Pu, L., Jia, Z., et al. (2024). Effect of salt-tolerant rice (Oryza sativa L.) cultivation on soil bacterial community and ecological function groups in coastal saline land. Appl. Soil Ecol. 201, 105511. doi: 10.1016/j.apsoil.2024.105511
Xu, H., Feng, L., Hao, J., Zhang, Y., and Li, R. (2025). Water use enhancement and root function compensatory regulation of biomass accumulation in Quinoa under salt stress by photosynthetic drive advantage. Plants (Basel) 14, 1615. doi: 10.3390/plants14111615
Xu, H. S., Guo, S. M., Zhu, L., and Xing, J. C. (2020). Growth, physiological and transcriptomic analysis of the perennial ryegrass Lolium perenne in response to saline stress. R. Soc. Open Sci. 7, 200637. doi: 10.1098/rsos.200637
Yang, Y., Liu, X., Zhao, Y., Tang, G., Nan, R., Zhang, Y., et al. (2025). Evaluation of wheat drought resistance using hyperspectral and chlorophyll fluorescence imaging. Plant Physiol. Biochem. 219, 109415. doi: 10.1016/j.plaphy.2024.109415
Yang, S., Xu, Y., Tang, Z., Jin, S., and Yang, S. (2024). The Impact of alkaline stress on plant growth and its alkaline resistance mechanisms. Int. J. Mol. Sci. 25, 13719. doi: 10.3390/ijms252413719
Ye, X., Wang, H., Cao, X., Jin, X., Cui, F., Bu, Y., et al. (2019). Transcriptome profiling of Puccinellia tenuiflora during seed germination under a long-term saline-alkali stress. BMC Genomics 20, 589. doi: 10.1186/s12864-019-5860-5
Yu, R., Wang, G., Yu, X., Li, L., Li, C., Song, Y., et al. (2021). Assessing alfalfa (Medicago sativa L.) tolerance to salinity at seedling stage and screening of the salinity tolerance traits. Plant Biol. 23, 664–674. doi: 10.1111/plb.13271
Zhang, J. (2016). Study on the physiological responses of Maize seedlings to combined salt-alkali stress and the regulatory effects of exogenous spermine (China: Heilongjiang Bayi Agricultural University).
Zhang, M.-X., Bai, R., Nan, M., Ren, W., Wang, C.-M., Shabala, S., et al. (2022). Evaluation of salt tolerance of oat cultivars and the mechanism of adaptation to salinity. J. Plant Physiol. 273, 153708. doi: 10.1016/j.jplph.2022.15370
Zhang, X., Cai, H., Lu, M., Wei, Q., Xu, L., Bo, C., et al. (2019). A maize stress-responsive Di19 transcription factor, ZmDi19-1, confers enhanced tolerance to salt in transgenic Arabidopsis. Plant Cell Rep. 38, 1563–1578. doi: 10.1007/s00299-019-02467-1
Zhang, H., Liu, X.-L., Zhang, R.-X., Yuan, H.-Y., Wang, M.-M., Yang, H.-Y., et al. (2017). Root damage under alkaline stress is associated with reactive oxygen species accumulation in Rice (Oryza sativa L.). Front. Plant Sci. 8. doi: 10.3389/fpls.2017.01580
Zhang, J., Wang, J., Jiang, W., Liu, J., Yang, S., Gai, J., et al. (2016). Identification and analysis of NaHCO(3) stress responsive genes in wild Soybean (Glycine soja) Roots by RNA-seq. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.01842
Zhang, M., Wang, Y., Qi, Z., Zhang, Q., Wang, H., Guan, C., et al. (2025). Screening of germplasm resources with low-phosphorus tolerance during the seedling stage of Rice. Plants (Basel) 14, 1543. doi: 10.3390/plants14101543
Zhang, J., Zhang, A., Liu, Z., He, W., and Yang, S. (2023). Multi-index fuzzy comprehensive evaluation model with information entropy of alfalfa salt tolerance based on LiDAR data and hyperspectral image data. Front. Plant Sci. 14. doi: 10.3389/fpls.2023.1200501
Zhang, R., Zhang, H., Wang, L., and Zeng, Y. (2024). Effect of salt-alkali stress on seed germination of the halophyte Halostachys caspica. Sci. Rep. 14, 13199. doi: 10.1038/s41598-024-61737-5
Zhao, X., Huang, M., Huang, X., and Liu, E. (2023). Evaluation of drought resistance and index screening of foxtail millet cultivars. J. Water Climate Change 14, 2384–2396. doi: 10.2166/wcc.2023.086
Zhao, X., Liu, Z., Li, H., Zhang, Y., Yu, L., Qi, X., et al. (2022). Identification of drought-tolerance genes in the germination stage of Soybean. Biology (Basel) 11, 1812. doi: 10.3390/biology11121812
Zhao, C., Zhang, H., Song, C., Zhu, J. K., and Shabala, S. (2020). Mechanisms of plant responses and adaptation to soil salinity. Innovation (camb) 1, 100017. doi: 10.1016/j.xinn.2020.100017
Zheng, Y., Hou, P., Jia, X., Zhu, L., Zhao, Y., Song, W., et al. (2023). Evaluation of the lodging resistance and the selection of identification indexes of maize inbred lines. Food Energy Secur. 12, e499. doi: 10.1002/fes3.499
Keywords: alkali tolerance, germplasm evaluation, LMG analysis, maize, seed germination
Citation: Pan Z, Ge Z, Gao F, Ao M and Guan Y (2026) Comprehensive evaluation of maize germplasm for alkali tolerance during germination. Front. Plant Sci. 16:1728607. doi: 10.3389/fpls.2025.1728607
Received: 20 October 2025; Accepted: 08 December 2025; Revised: 03 December 2025;
Published: 12 January 2026.
Edited by:
Alberto Soares de Melo, State University of Paraíba, BrazilReviewed by:
Faujiah Nurhasanah Ritonga, Faculty of Forestry Universitas Sumatera Utara, IndonesiaAgda Malany Forte De Oliveira, State University of Paraíba, Brazil
Copyright © 2026 Pan, Ge, Gao, Ao and Guan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Man Ao, YW9tYW5AaWdhLmFjLmNu; Yixin Guan, Z3VhbnlpeGluQGlnYS5hYy5jbg==
 Zhuyun Pan1,2
Zhuyun Pan1,2 Zhiqiang Ge
Zhiqiang Ge Man Ao
Man Ao