- 1State Key Laboratory of Crop Gene Exploration and Utilization in Southwest China, Sichuan Agricultural University, Chengdu, China
- 2Institute of Rice and Sorghum Sciences, Sichuan Academy of Agricultural Sciences, Deyang, China
- 3Institute of Millet Crops, Hebei Academy of Agricultural and Forestry Sciences, Shijizhuang, China
Sorghum anthracnose, caused by Colletotrichum sublineola, poses a severe threat to global sorghum production. In China, the rising demand for organic sorghum used in Baijiu brewing underscores the urgent need for effective biocontrol agents or microbial formulations, which remain scarce. In this study, we assessed the efficacy of six biofungicides and two microbial agents against sorghum anthracnose. All tested biocontrol agents and microbial inoculants significantly inhibited mycelial growth and spore germination of C. sublineola in vitro. Iron chlorin exhibited the lowest EC50; values for inhibition mycelial growth and spore germination, followed by pterostilbene. In greenhouse trials, Pterostilbene and Iron chlorin significantly reduced disease severity, with control efficacies of 41.3% and 51.7%, respectively, whereas Bacillus subtilis and Trichoderma harzianumachieved higher efficacies of 73.0% and 65.5%, respectively. Field trials conducted at two sites in Southwest China further confirmed pterostilbene as the most effective treatment, followed by B. subtilis. Collectively, our results highlight pterostilbene and B. subtilis as promising biocontrol agents. Their application could reduce reliance on chemical fungicides and mitigate associated environmental risks. These findings provide practical and eco-friendly strategies for anthracnose management, supporting the sustainable cultivation of organic sorghum.
1 Introduction
Sorghum (Sorghum bicolor L. Moench) is a vital global cereal crop for food, feed, and bioenergy. In China, it holds distinctive value as the primary raw material for Baijiu production, a traditional distilled liquor with profound economic and cultural significance (Zhang et al., 2025). To enhance the quality and safety of Baijiu, leading distilleries like Moutai and Luzhou Laojiao are increasingly promoting the use of organically grown sorghum in southwestern China. However, sorghum production in this region is severely threatened by anthracnose, a devastating disease caused by Colletotrichum sublineola that can result in yield losses exceeding 50% (Stutts and Vermerris, 2020; Yu et al., 2025). This disease not only causes severe yield losses but also compromises sorghum grain quality (Acharya et al., 2023; Fu et al., 2020; Abreha et al., 2021).
Currently, chemical fungicides represent the primary approach for managing sorghum anthracnose and other crop diseases. Agrochemicals such as carbendazim, pyraclostrobin, tebuconazole, and phenamacril are generally effective in suppressing fungal pathogens (Zhang et al., 2023). However, their overuse poses substantial risks, such as the emergence of fungicide-resistant pathogen strains, adverse impacts on soil and environmental health, the persistence of chemical residues in agricultural products, and potential threats to human safety. Growing public concern over food safety, combined with the brewing industry’s demand for organic sorghum, has accelerated the pursuit of sustainable alternatives to chemical control. Furthermore, the recurrent anthracnose epidemics in major sorghum-producing regions worldwide highlight the urgent need for the development of eco-friendly and effective management strategies.
In recent years, a diverse range of biofungicides and plant immunity inducers have been developed and applied in crop protection (Abbey et al., 2019; Tian et al., 2024). These products can be broadly classified into three categories: microbial agents, plant-derived vaccines, and natural compounds. Microbial agents such as Bacillus subtilis and Trichoderma harzianum have been widely documented to suppress fungal pathogens and enhance host immunity, with several corresponding formulations successfully commercialized (Zaim et al., 2018; Wang et al., 2019). Plant-derived elicitors include various compounds like plant-activating proteins, oligosaccharides, and salicylic acid. These substances activate plant defense mechanisms through distinct pathways: plant-activating proteins regulate metabolic processes and induce defense-related gene expression; oligosaccharides function as elicitors that trigger systemic acquired resistance; and salicylic acid, a key defense signaling molecule, modulates the expression of defense genes, maintains cell membrane integrity, and enhances antioxidant activity (Trujillo and Angulo, 2025; Mardanova et al., 2024). Abscisic acid is a potent resistance inducer known to activate over 150 defense-related genes (Qiao et al., 2023). A notable commercial application of such immunity-inducing technology is Atailing, a protein–oligosaccharide biopesticide developed by the Institute of Plant Protection at the Chinese Academy of Agricultural Sciences (Dewen et al., 2017). In addition, novel agents such as Iron chlorin, a porphyrin-based plant growth regulator, can enhance photosynthesis, root development, and stress tolerance (Zhou et al., 2024). Another example is the hypersensitive protein preparation HhrpEcc, which combines broad-spectrum disease resistance with growth promotion (Jeblick et al., 2023). Natural phenolics such as Pterostilbene, a sorghum-specific stilbene compound inducible upon anthracnose infection, further illustrate the potential of crop-derived metabolites in disease resistance (Liu et al., 2023). Similarly, erucamide, a newly identified phytoalexin, has demonstrated antifungal activity against bacterial pathogens such as Xanthomonas oryzae and Ralstonia solanacearum (Miao et al., 2025). Together, these agents highlight the diversity of natural and microbial-derived options for eco-friendly anthracnose management.
In this study, we systematically evaluated the antifungal activities of three chemical fungicides (Tebuconazole, Carbendazim, and Pyraclostrobin), six biofungicides (Iron chlorin, HhrpEcc, Alexin, S-abscisic acid, Atailing, and Pterostilbene), and two microbial agents (B. subtilis and T. harzianum) for the control of sorghum anthracnose. Using integrated in vitro and in vivo assays, we evaluated their capacities to inhibit mycelial growth, spore germination, and disease progression. Our results identify highly effective biocontrol alternatives, offering practical solutions to minimize chemical inputs, secure the production of organic brewing sorghum, and advance sustainable disease management in China’s Baijiu industry.
2 Materials and methods
2.1 Pathogen isolation and identification
Typical anthracnose-infected sorghum leaves were collected from Da’an District, Zigong City, Sichuan Province, China, in 2024. The leaf samples were surface-sterilized by sequential immersion in 75% ethanol for 30 s and 1% sodium hypochlorite for 1 min, followed by three rinses with sterile distilled water. Small pieces (approximately 5 mm²) of sterilized tissue from the lesion margins were plated on potato dextrose agar (PDA) medium and incubated at 28°C for 3 days in the dark. Emerging fungal colonies were purified by subculturing on fresh PDA. Conidial morphology was examined under a light microscope (Olympus BX53, Japan) at 400× magnification. For molecular identification, genomic DNA was extracted from 7-day-old mycelial cultures using a commercial fungal DNA extraction kit (Solarbio, Beijing, China) following the manufacturer’s instructions. The internal transcribed spacer (ITS) region of the ribosomal DNA was amplified using the universal primers ITS1 (5’-TCCGTAGGTGAACCTGCGG-3’) and ITS4 (5’-TCCTCCGCTTATTGATATGC-3’) (White et al., 1990). PCR reactions were performed in a 25 μL volume containing 12.5 μL 2× Taq Master Mix (Vazyme, Nanjing, China), 1 μL each primer (10 μmol/L), 1 μL template DNA (50 ng/μL), and 9.5 μL ddH2O. The thermal cycling protocol comprised an initial denaturation at 94°C for 5 min; 35 cycles of denaturation 94°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 1 min; followed by a final extension at 72°C for 10 min. The resulting PCR products were sequenced bidirectionally by Sangon Biotech (Shanghai, China). Sequences were analyzed using BLASTn against the NCBI GenBank database for species identification. The isolated strain, designated ZG-DA-20, was preserved on PDA slants at 4°C for subsequent studies.
2.2 Pathogenicity confirmation
Pathogenicity of the isolated strain ZG-DA-20 was verified in accordance with Koch’s postulates. Healthy sorghum seedlings (cv. ‘GJH’, susceptible to anthracnose) at the 5-leaf stage were inoculated with a conidial suspension (1 × 106 conidia/mL) prepared in sterile distilled water with 0.1% Tween-20. The suspension was sprayed onto leaves until runoff using a handheld atomizer. Control plants were treated similarly with sterile distilled water containing 0.1% Tween-20. All inoculated plants were maintained in a growth chamber at 28°C with 90% relative humidity and a 12-h photoperiod for 7 days. The pathogen was subsequently re-isolated from the developing lesions on the inoculated leaves and confirmed to be identical to the original strain ZG-DA-20 through both morphological and molecular characterization.
2.3 Biocontrol agents
Eleven agents were evaluated for their efficacy in controlling sorghum anthracnose caused by C. sublineola strain ZG-DA-20 (Supplementary Table S1). Three chemical fungicides served as positive controls: Pyraclostrobin (250 g/L EC, BASF Crop Protection, Jiangsu, China), Carbendazim (50% WP, Anhui Guangxin Agrochemical Co., Ltd., China), and Tebuconazole (430 g/L SC, Jiangsu Qizhou Green Chemical Co., Ltd., China). Six biofungicides were tested: S-abscisic acid (5% SL, Sichuan Longmang Fusheng Technology Co., Ltd., China), Alexin (0.3% Matrine AS, Xiangyu Agricultural Technology Co., Ltd., China), Atailing (0.5% Physcion AS, Hebei Zhongbao Lunong Crop Technology Co., Ltd., China), Iron chlorin (also known as dihydroporphyrin iron, 0.5% SL, Anqing Baite Biological Engineering Co., Ltd., China), HhrpEcc (0.5% Osthole AS, Sichuan Haiboshi Biological Technology Co., Ltd., China), and Pterostilbene (99.5% SC, Beijing Solebo Technology Co., Ltd., China). Additionally, two microbial antagonists were incorporated: Bacillus subtilis (1010 CFU/g WP, Hebei Guanlong Agrochemical Co., Ltd., China) and Trichoderma ha0rzianum (2 × 109; CFU/g WP, Hubei Qiming Biological Engineering Co., Ltd., China). All agents were initially prepared as stock solutions according to the field concentrations recommended by the manufacturers, followed by serial dilutions. Based on these tests and preliminary in vitro experiments, we determined the final concentration ranges (Supplementary Table S2) used for EC50; estimation (Abo-Zaid et al., 2025). Sterile distilled water served as the negative control throughout the study.
2.4 Mycelial growth inhibition assay
Stock solutions of each agent were serially diluted to obtain a graded concentration series, as determined by preliminary range-finding tests. One milliliter of each diluted solution was mixed with 99 mL of molten potato sucrose agar (PSA) medium (cooled to 45-50°C) to prepare agent-amended plates. Control plates received 1 mL of sterile distilled water. Mycelial plugs (5 mm diameter) were excised from the actively growing margins of 7-day-old ZG-DA-20 colonies on PDA and placed centrally on the amended PSA plates. Plates were incubated at 28°C for 6 to 8 days in the dark. Colony diameters were measured using the cross-measurement method (two perpendicular diameters, subtracting the plug diameter). Each treatment consisted of four times. Mycelial growth inhibition rate was according to the following formula (Lü et al., 2023):
where Dc is the average colony diameter on control plates, and Dt is the average colony diameter on treatment plates.
2.5 Conidial germination assay
Stock solutions of each agent were diluted to the desired concentrations and incorporated into water agar (WA) medium at a 1% (v/v) ratio. Control plates were prepared by adding an equivalent volume of sterile distilled water. Conidial suspensions of ZG-DA-20 were prepared by flooding 7-day-old PDA cultures with sterile distilled water containing 0.1% Tween-20, scraping the surface, and filtering through double-layer cheesecloth. The concentration was adjusted to 1 × 106 conidia/mL using a hemocytometer. Fifty microliters of the suspension were spread evenly onto each WA plate. Plates were incubated at 28°C for 9 h in darkness. Germination was assessed microscopically by examining 100 randomly selected conidia per plate; a conidium was considered germinated if the germ tube length exceeded half the conidial length. Each treatment was replicated four times. Conidial germination rate was calculated as follows:
Inhibition rate (%) was then determined as (Hu et al., 2021):
where Gc is the germination rate in the control, and Gt is the germination rate in the treatment.
Virulence regression equations were constructed using the agent concentration as the x-axis and the probit-transformed inhibition rate as the y-axis. Regression parameters, correlation coefficients (r), and median effective concentrations (EC50;) with 95% confidence intervals were estimated using probit analysis in SPSS 26.0 software. EC50; values were used to compare inhibitory potencies and select agents with high efficacy at low doses.
2.6 Greenhouse efficacy trials
Sorghum seeds (cv. ‘GJH1’) were sown in plastic pots (15 cm diameter) filled with sterilized potting mix (peat:vermiculite:perlite = 2:1:1, v/v/v) and grown in a greenhouse at 28 °C with a 12-h photoperiod. At the six-leaf stage, uniform seedlings were selected and transferred to an inoculation chamber maintained at approximately 75% relative humidity. To sustain high humidity, the chamber was covered with white plastic film. Plants were inoculated by spraying their leaves with a conidial suspension of ZG-DA-20 (1 × 106 conidia/mL in 0.1% Tween-20) until runoff occurred. After 36 h, agents were applied at their respective effective concentrations (from in vitro assays) using a handheld sprayer. The experimental design comprised the following treatments: sterile water (blank control), chemical fungicides (positive controls), and pathogen inoculation without agent (negative control). Each treatment had 10 pots with 3 plants per pot, arranged in a completely randomized design. After 7 days, plant growth parameters (fresh weight, root length, and plant height) were measured using a digital balance and ruler. Specifically, root length was measured as the distance from the stem base to the tip of the longest root using a ruler, with each plant measured individually. Mean values for each treatment were used for analysis. Disease severity was rated on a 1–5 scale (Zhang et al., 2025): 1 = no symptoms (healthy); 2 = hypersensitive lesions on lower leaves; 3 = acervuli on bottom leaves; 4 = acervuli on middle to bottom leaves; 5 = acervuli on whole plant including flag leaf. Disease index (DI) was calculated as:
Control efficacy (%) was determined as (Egel et al., 2019):
2.7 Field efficacy trials
Field trials were conducted during May 2025 in experimental sorghum fields in Chouzhou (103.66°E, 30.57°N) and Luhzhou (105.35°E, 28.96°N), Sichuan Province, China. Sorghum plants (cv. ‘GJH1’) were cultivated at a spacing of 40 cm × 20 cm in plots of 20m², arranged in a randomized complete block design with four replicates per treatment. Five selected agents (Atailing, Iron chlorin, T. harzianum, pterostilbene, and B. subtilis) were tested, along with a water control, resulting in 50 plots (2 plants per plot). A conidial suspension of ZG-DA-20 (1 × 106 CFU/mL) was applied to leaves using a backpack sprayer, followed 30 min later by agent application at manufacturer-recommended field concentrations. Standard agronomic practices were followed, without additional fungicide applications. For field evaluations, however, the rating standards were manually adjusted by taking into account local meteorological data (rainfall and average daily temperature) recorded after inoculation, the rate of disease progression, and the proportional distribution of severity levels observed under controlled greenhouse conditions. Fourteen days post-inoculation, disease incidence (percentage of infected plants), severity (1–5 scale as in greenhouse), and disease index were recorded for all plants per plot. Control efficacy was calculated as in the greenhouse trials. Grading criteria for anthracnose severity were adjusted based on natural field conditions, incorporating environmental factors such as rainfall and temperature.
2.8 Statistical analysis
All data were analyzed with SPSS 26.0 software (IBM Corp., Armonk, NY, USA). Means of plant growth parameters, inhibition rates, EC50; values, disease indices, and control efficacies were compared using one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) test at P< 0.05.
3 Results
3.1 Pathogen isolation and identification
A highly virulent fungal strain of C. sublineola, designated ZG-DA-20, was isolated from symptomatic sorghum leaves collected in Da’an District, Zigong City, in southwestern China. The disease symptoms and morphological characteristics of the pathogen confirmed its identity as C. sublineola (Figure 1A). When cultivated on PDA at 28°C for 7 days, the colonies initially exhibited a white coloration that gradually turned gray, forming abundant acervuli with setae (Figure 1B). The conidia were crescent-shaped, hyaline, and aseptate, measuring 15-25 μm in length and 3-5 μm in width, aligning with previously described characteristics for C. sublineola (Figure 1C). Molecular identification based on the ITS region showed that the sequence of ZG-DA-20 (539/539 bp) was 100% identical to the reference strain C. sublineola GD202206 (GenBank: ON680862.1). Pathogenicity was then confirmed via Koch’s postulates. Sorghum seedlings inoculated with a conidial suspension developed characteristic anthracnose symptoms, including red-brown lesions bearing acervuli at 7 dpi. In contrast, mock-inoculated control plants remained healthy and symptom-free (Figure 1D). The same fungus was successfully re-isolated from the diseased tissues, thereby fulfilling Koch’s postulates and confirming C. sublineola as the causal agent of the observed symptoms.
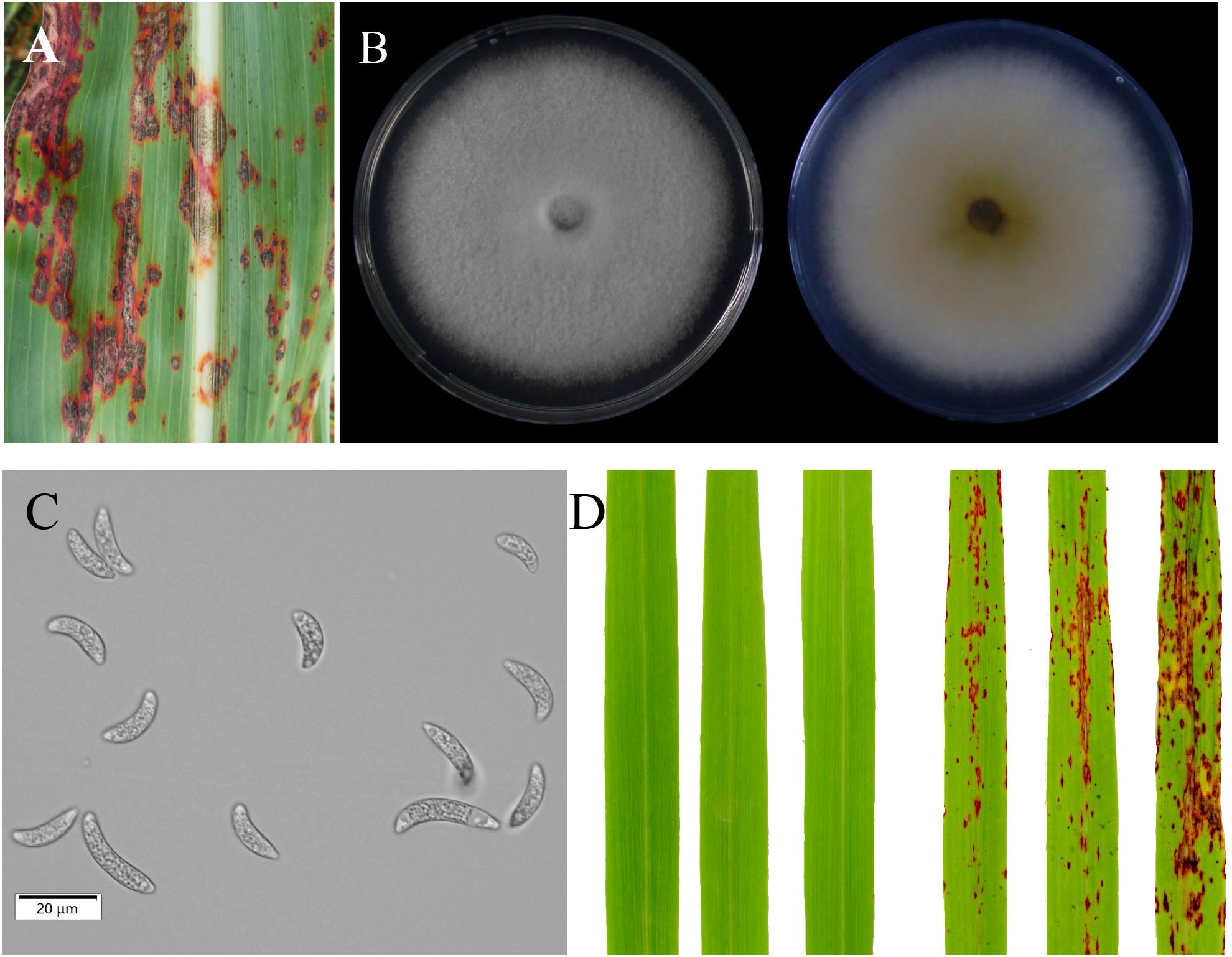
Figure 1. Isolation and identification of C. sublineola. (A) Field symptoms of sorghum anthracnose caused by C. sublineola isolate ZG-DA-20. (B) Colony morphology of ZG-DA-20. (C) Conidial morphology of ZG-DA-20. (D) Pathogenicity assessment of ZG-DA-20 on sorghum, comparing a mock-inoculated control plant (left, sprayed with water) with a pathogen-inoculated plant (right) at 7 days post inoculation.
3.2 In vitro inhibition of mycelial growth rate and germination of conidium
The three chemical fungicides and five biofungicides (Iron chlorin, HhrpEcc, Alexin, S-abscisic acid, and Pterostilbene) exhibited varying degrees of inhibitory activity against the mycelial growth and spore germination of C. sublineola in vitro (Figures 1, 2; Tables 1, 2). For mycelial growth inhibition, all agents significantly reduced growth compared with the control, with the three chemical agents showing particularly strong inhibition (Figures 1, 2). Among the five biofungicides, iron chlorin was the most effective, with the lowest median effective concentration (EC50 = 0.0790 µg/mL), followed by Pterostilbene (EC50 = 113.3241 µg/mL). Notably, HhrpEcc displayed a steep regression slope (k = 3.2209), indicating a rapid decline in pathogen viability with increasing concentrations. The correlation coefficients (r) ranged from 0.9113 to 0.9969, implying that all agents exhibited significant dose-response relationships (Table 1).
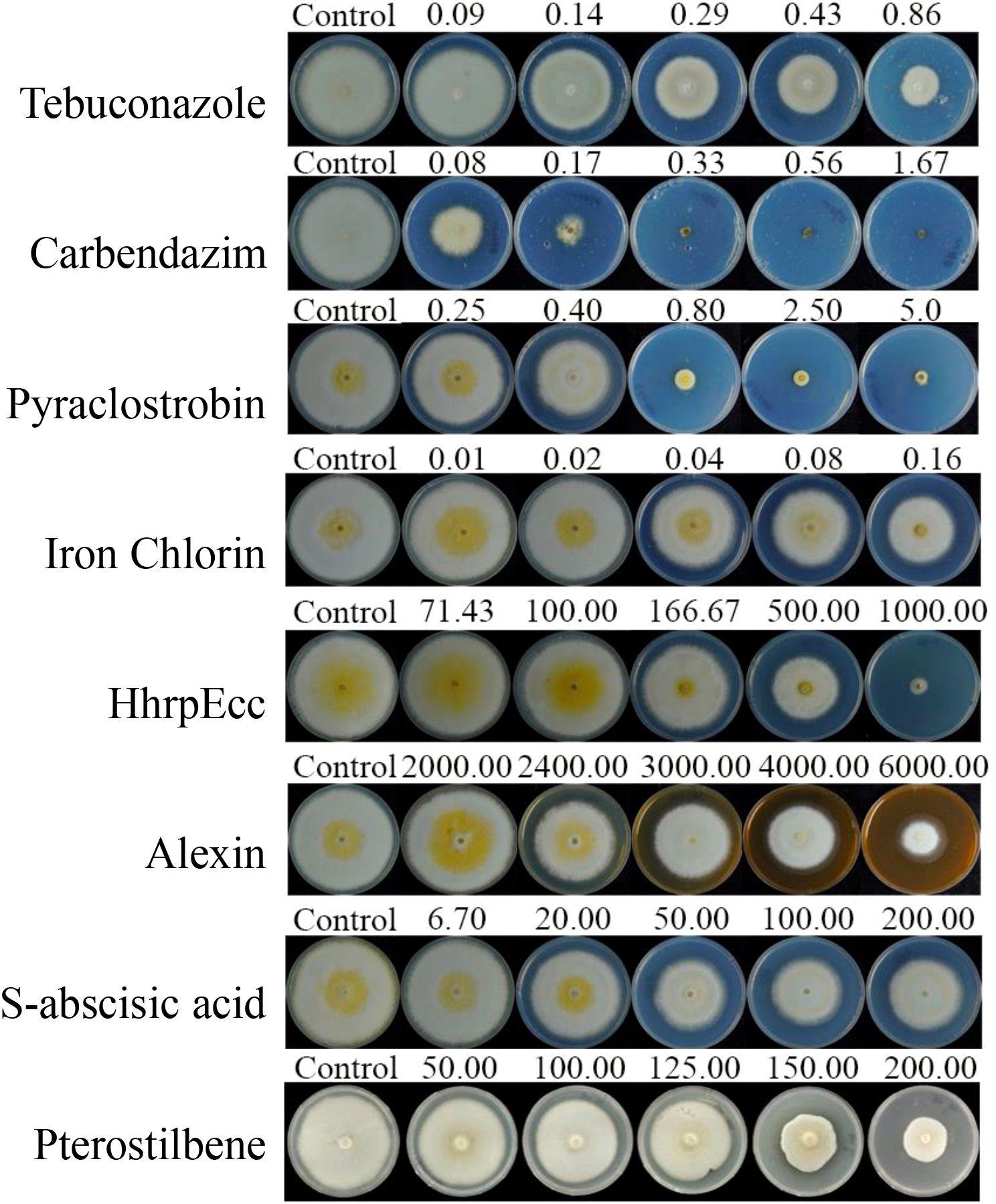
Figure 2. Inhibitory effects of three chemical fungicides and five biofungicides on the mycelial growth of C. sublineola. Fungicides tested: Tebuconazole, Carbendazim, Pyraclostrobin, Iron chlorin, HhrpEcc, Alexin, S-abscisic acid, and Pterostilbene.
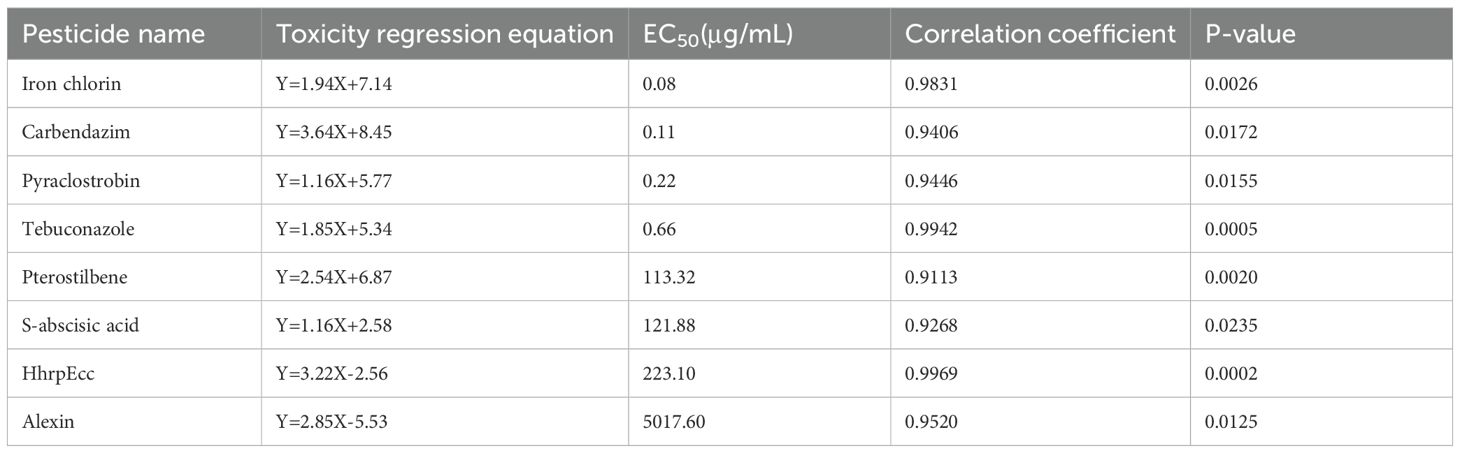
Table 1. Toxicity of three chemical fungicides and five biofungicides on the mycelial growth of C. sublineola.
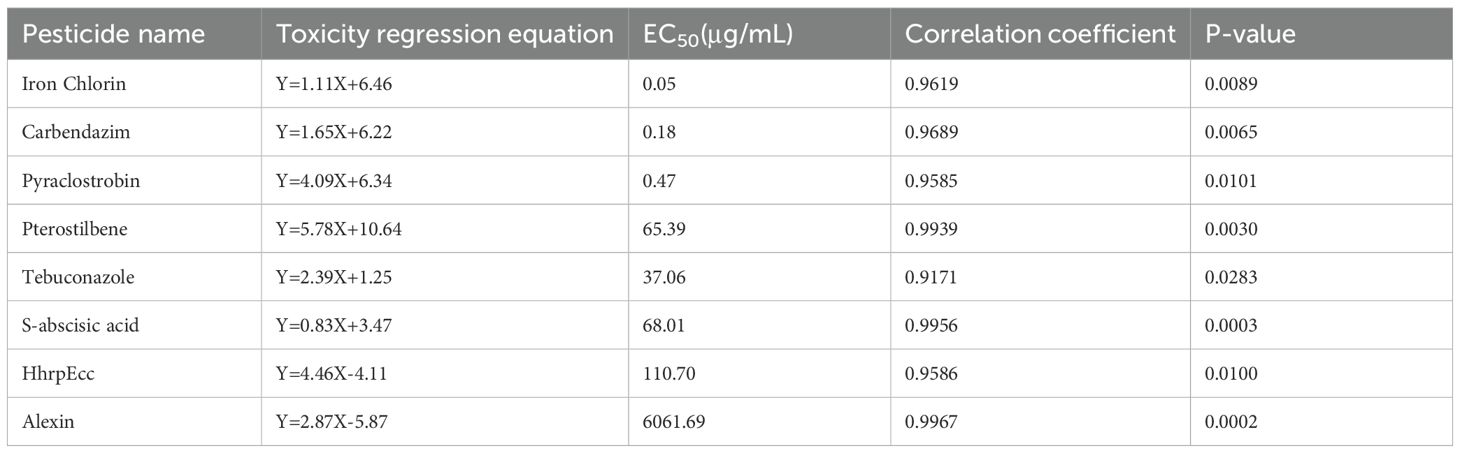
Table 2. Toxicity of three chemical fungicides and five biofungicides on the spore germination of C. sublineola.
Spore germination assays yielded results consistent with those observed for mycelial growth inhibition. Among the five biofungicides tested, iron chlorin again exhibited the strongest inhibitory effect, showing with the lowest EC50; (0.0475 µg/mL), followed by pterostilbene (65.3901 µg/mL). HhrpEcc and S-abscisic acid also displayed moderate effects, with EC50; values of 110.7003 and 68.0103 µg/mL, respectively (Table 2). Notably, the EC50; values for spore germination were consistently lower than those for mycelial growth across all tested agents.
3.3 Greenhouse experiments
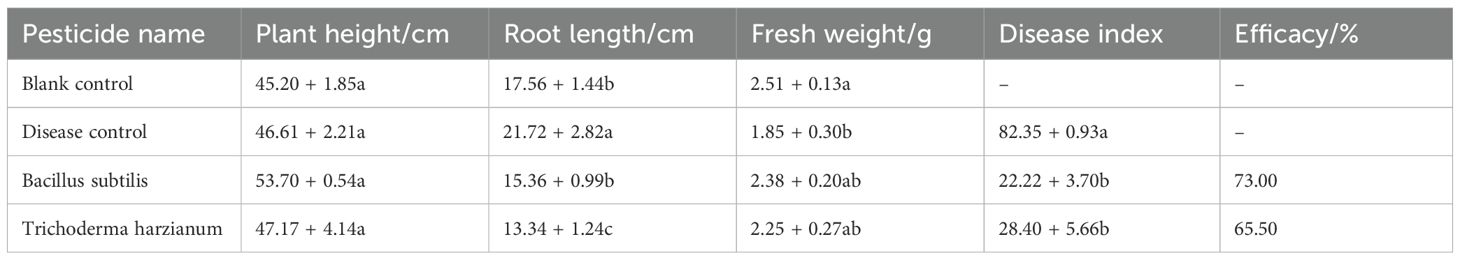
In greenhouse trials, all agents reduced anthracnose severity compared with the inoculated control, which exhibited a disease index of 88.89 and extensive leaf lesions covering over 70% of the leaf area (Figure 3; Table 3). Among the six test biocontrol agents, pterostilbene and iron chlorin provided the most effective protection, achieving disease indices of 51.85 and 42.96, respectively, and preventing the formation of visible red spots on leaves (Figure 3). These results corresponded to control efficacies of 41.3% for pterostilbene and 51.7% for Iron chlorin. Moderate efficacy was observed for HhrpEcc (33.8% efficacy; disease index 58.89), S-abscisic acid (36.7%; 56.30), and Atailing (25.8%; 65.93). Alexin showed the lowest control efficacy (16.7%; 87.40) (Table 3). Regarding plant growth parameters, none of the treatments had a significant impact on plant height or root length. As expected, pathogen inoculation significantly reduced fresh weight, indicating that anthracnose significantly inhibits plant growth. After treatment with biocontrol agents, only pterostilbene restored plant fresh weight to a level statistically comparable to that of the healthy mock-inoculated control, while other treatments still significantly reduced plant fresh weight (Table 3).
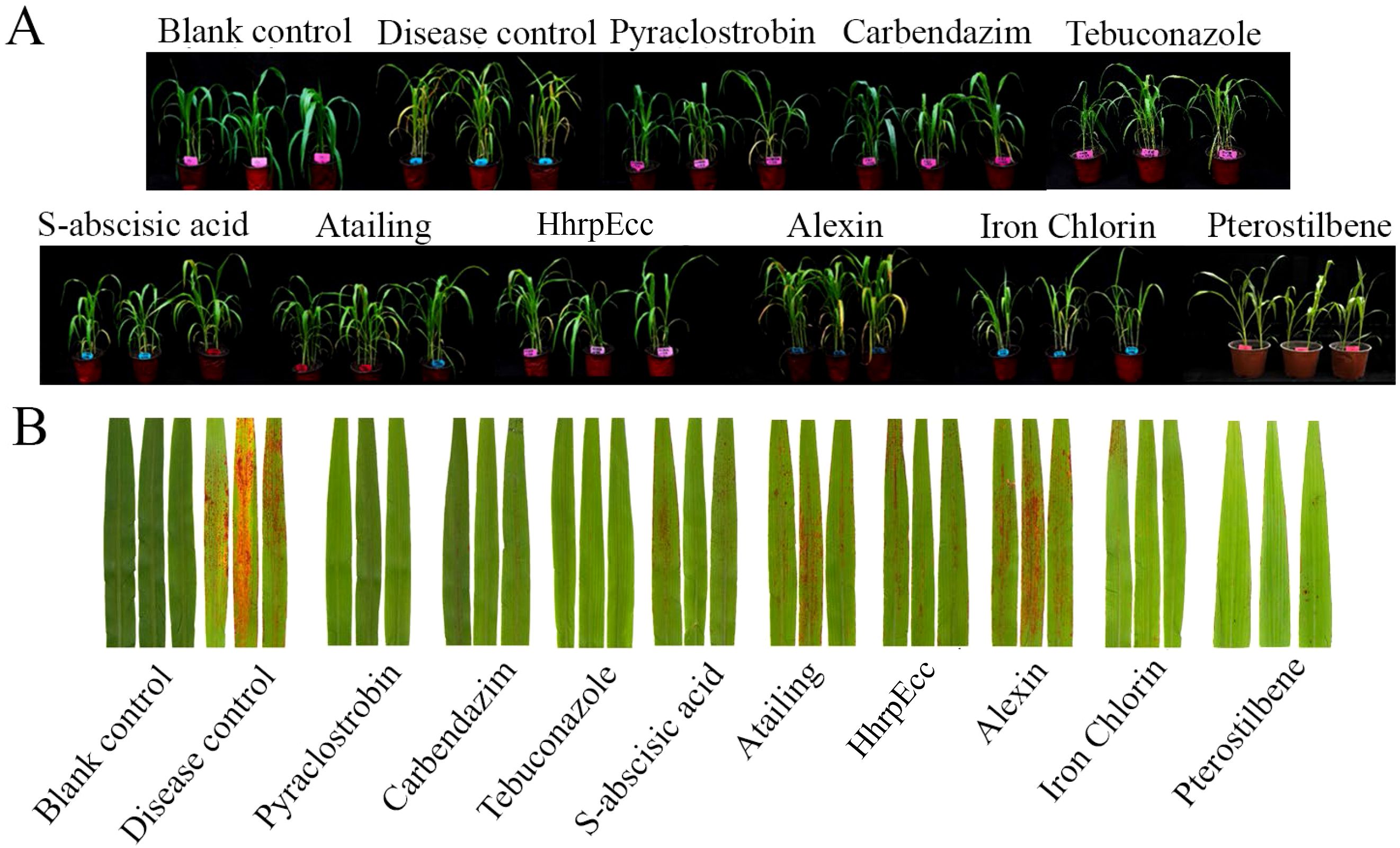
Figure 3. Control efficacy of three chemical fungicides and five biofungicides against sorghum anthracnose in greenhouse experiments. (A) Pot experiment showing effects of different treatments on sorghum plant growth and anthracnose. Blank control: sprayed with sterile water, no C. sublineola inoculation; Disease control: C. sublineola inoculated, no fungicide treatment. (B) Leaf anthracnose symptoms following treatment with different fungicides.
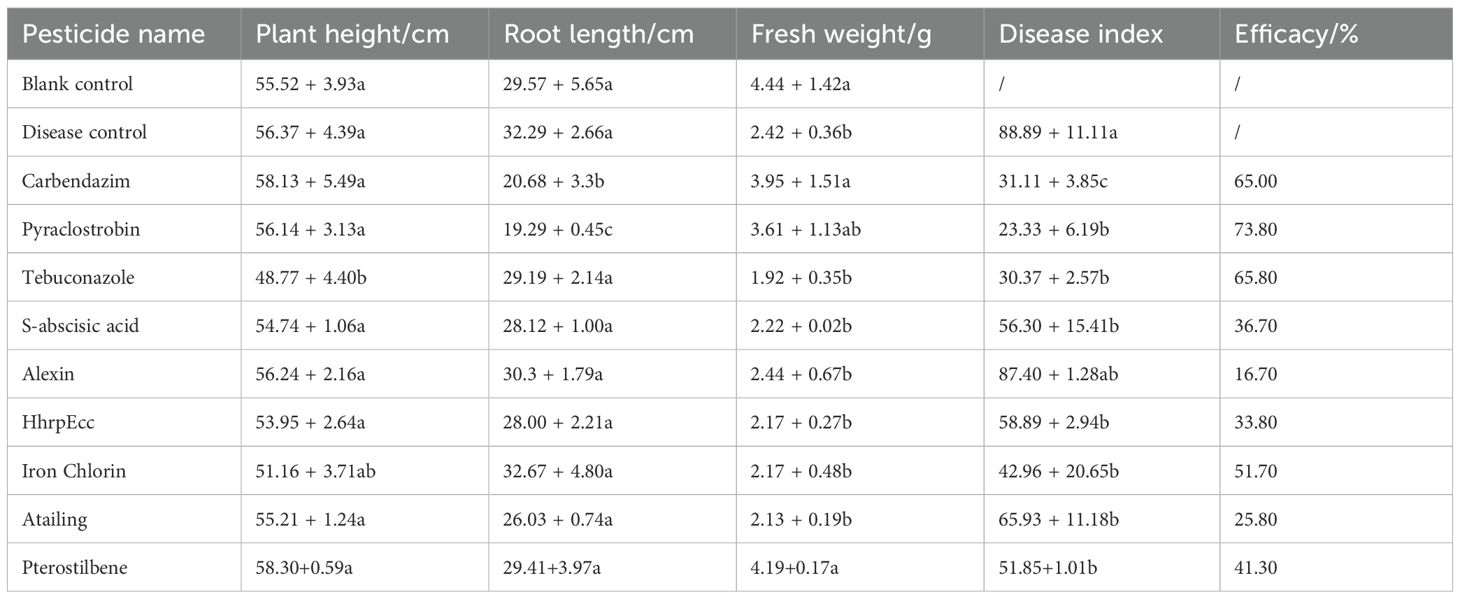
Table 3. Control efficacy of three chemical fungicides and six biofungicides against sorghum anthracnose.
A separate greenhouse pot experiment was also conducted to evaluate the biocontrol efficacy of T. harzianum and B. subtilis against sorghum anthracnose. At 7 dpi after foliar application of spore suspensions at the manufacturer-recommended concentrations, B. subtilis and T. harzianum exhibited high relative control efficacies of 73.0% and 65.5%, respectively (Table 4). To further investigate their modes of action, dual-culture assays were performed, showing that T. harzianum and B. subtilis significantly inhibited the mycelial growth of strain ZG-DA-20, leading to restricted colony expansion (Figure 4). These results demonstrate that T. harzianum and B. subtilis achieve effective control through direct antagonism and possibly by inducing host resistance. Their demonstrated performance underscores the potential of these microbial agents as sustainable alternatives to chemical fungicides, which could help reduce pesticide residues and mitigate the risk of resistance development.
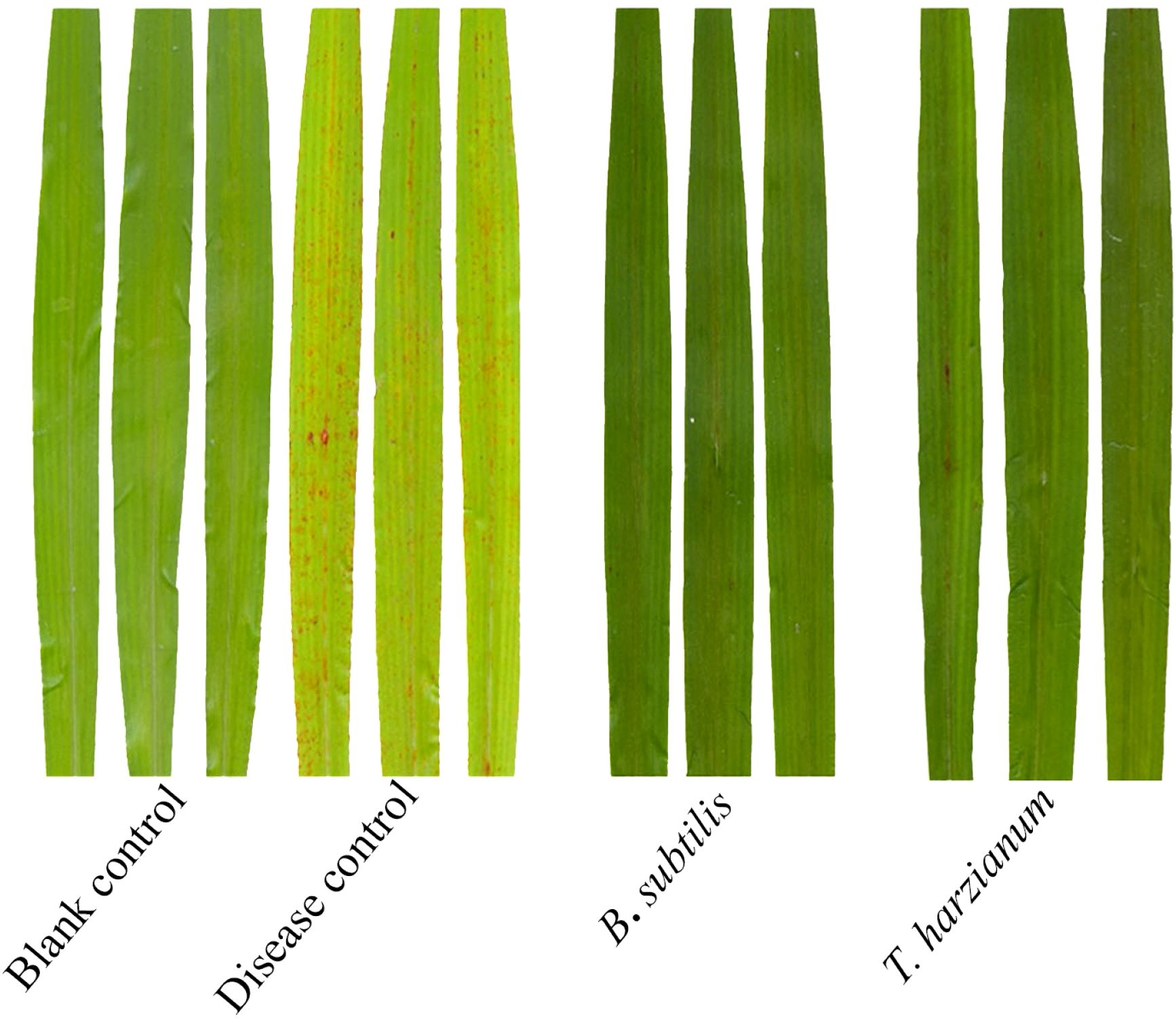
Figure 4. Control effects of B. subtilis and T. harzianum against sorghum anthracnose in greenhouse.
3.4 Field experiments
Field trials conducted in Luzhou and Chongzhou confirmed the consistency of greenhouse results, with pterostilbene and B. subtilis providing consistent control across locations. Disease severity on sorghum leaves was assessed using a 0–4 grading scale, where 0 represented no symptoms and 4 indicated severe lesions covering most of the leaf surface (Figure 5). This grading system highlights progressive deterioration with increasing infection severity. Across the two trial sites, pterostilbene-treated plants exhibited fewer instances of grades 2-4, indicating generally milder disease severity, followed by those treated with B. subtilis, which showed a similar but slightly less pronounced reduction in higher-grade symptoms (Figure 5).
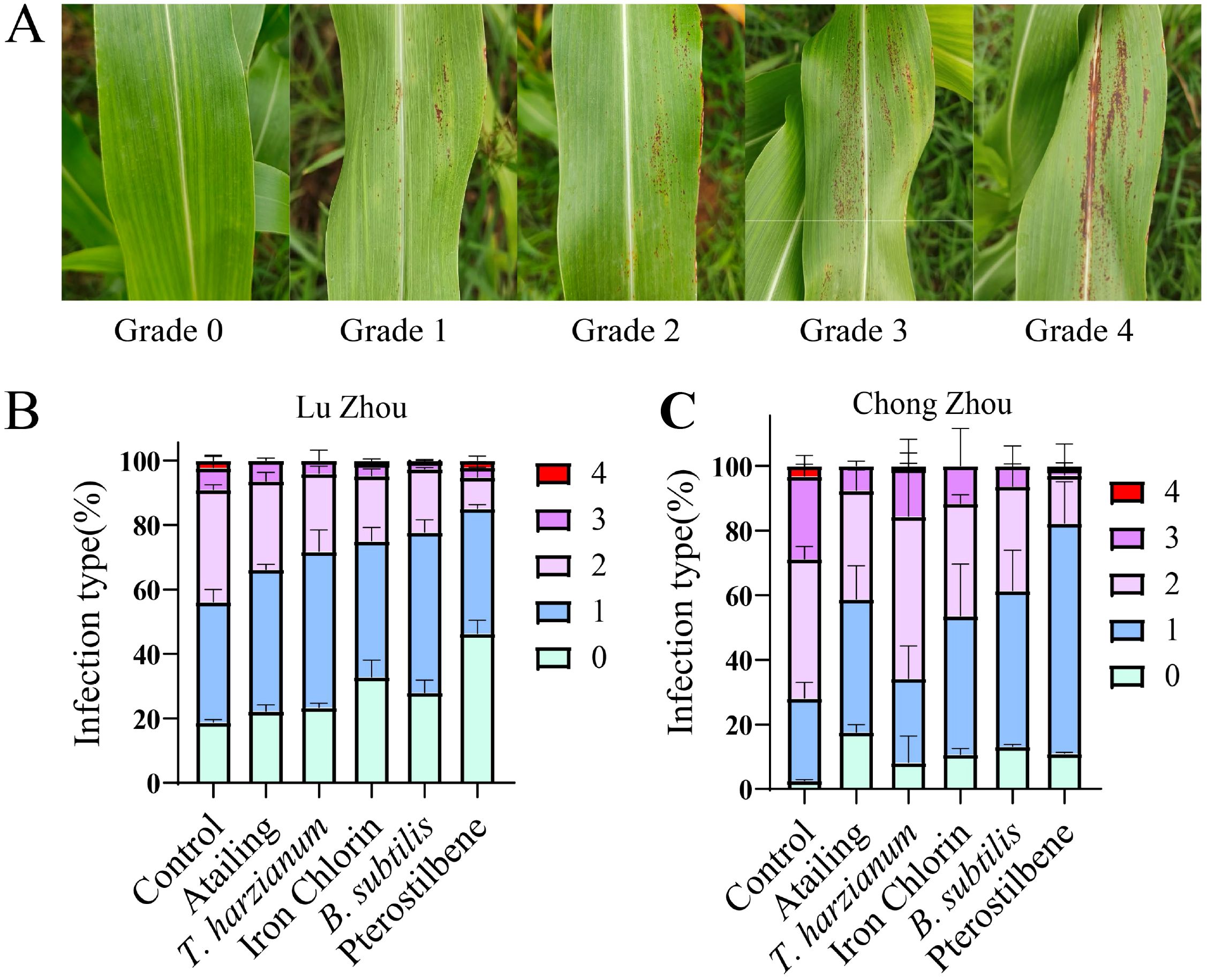
Figure 5. Disease severity grades and incidence of sorghum anthracnose in field trials. (A) Disease severity scoring chart used for filed assessment. Disease severity was scored on a scale of 0 to 4 (0 = no visible infection; 1 = very light infection; 2 = light infection; 3 = moderate infection; 4 = severe infection). (B, C) Distribution of disease-severity levels in Luzhou (B) and Chongzhou (C).
In Luzhou, the inoculated control treatment showed a disease incidence of 81.24% and a disease index of 34.1 (Table 5). Application of pterostilbene reduced the disease incidence to 53.69% and the disease index to 18.98, achieving 44.33% efficacy. B. subtilis achieved 27.43% efficacy, with incidence disease at 71.93% and disease index at 23.89. Iron chlorin and Atailing achieved 26.78% and 19.9% efficacy, respectively. In Chongzhou, where disease pressure was higher, with the inoculated control showing a disease incidence of 97.44% and a disease index of 50.1. However, pterostilbene treatment excelled again at 45.01% efficacy and a disease index of 27.5, followed by B. subtilis at 34.20% efficacy and disease index of 32.7, then Atailing at 34.48% efficacy and disease index of 32.8. T. harzianum demonstrated limited efficacy at 13.28% (Table 5). Statistical analysis confirmed significant differences among treatments, with pterostilbene and B. subtilis outperforming the others in both locations, as evidenced by the skewed distributions toward lower severity grades in the stacked bar charts.
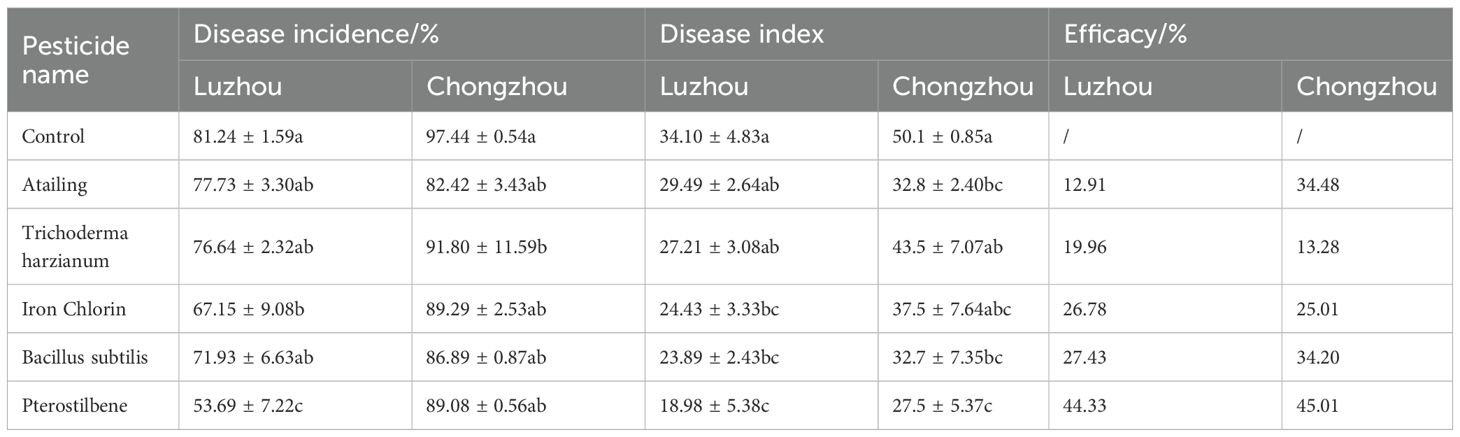
Table 5. Disease incidence, disease index, and control efficacy of five selected biological or microbial agents against sorghum anthracnose.
4 Discussion
This study comprehensively evaluated the potential of various biocontrol agents and microbial formulations as sustainable alternatives to chemical fungicides for managing sorghum anthracnose, a disease caused by C. sublineola that threatens organic sorghum production for China’s Baijiu industry. Integrating in vitro assays, greenhouse trials, and field experiments, we systematically assessed the antifungal activities and practical applications of these agents against the virulent strain ZG-DA-20. We hypothesized that certain bio-based strategies could provide disease control comparable to synthetic fungicides, potentially through combined mechanisms involving direct antagonism and the induction of host resistance.
Our study indicates that all tested agents inhibited the mycelial growth and spore germination of C. sublineola in vitro. Iron chlorin showed the strongest inhibitory effect, with the lowest EC50; values recorded (0.0790 µg/mL for mycelial growth and 0.0475 µg/mL for spore germination), followed closely by pterostilbene (Figure 2; Tables 1, 2). In greenhouse trials, pterostilbene and iron chlorin delivered control efficacies of 41.3% and 51.7%, respectively, while the microbial antagonists B. subtilis and T. harzianum outperformed them with efficacies of 73.0% and 65.5% (Figures 3, 4; Tables 3, 4). Field trials across two sites in Southwest China (Luzhou and Chongzhou) highlighted pterostilbene as the most consistent performer, averaging 44.67% efficacy, with B. subtilis at 30.82% (Figure 5; Table 5). The greenhouse experiments emphasize mechanistic insights and potential resistance, while field trials reflect practical performance and stability. These findings align with previous reports indicating that beneficial microorganisms often perform better under controlled greenhouse conditions than in field environments, likely due to reduced survival and colonization under variable natural conditions (Hu et al., 2021; Luo et al., 2025). While, pterostilbene is more stable under natural environmental conditions. In addition, the induced defense responses of the host may synergize with pterostilbene, thereby enhancing the overall disease control efficacy (Chan et al., 2019). Pterostilbene exhibited potent antifungal activity against multiple pathogens, suggesting its strong potential as an eco-friendly alternative for postharvest disease control (Xu et al., 2018). Notably, plants treated with pterostilbene also exhibited enhanced growth metrics compared to those treated with other biocontrol agents (Table 4), underscoring that this compound not only suppresses disease but also supports plant development without adverse effects.
Following previous studies, we characterized the highly virulent strain ZG-DA-20 using morphological and molecular characterization of the ITS region, further reinforcing its genetic diversity and pathogenicity (Xavier et al., 2018; Chala et al., 2010). In our evaluation, selected biocontrol agents including pterostilbene and iron chlorin demonstrated substantial efficacy in both in vitro and greenhouse trials. The superior in vitro performance of iron chlorin is likely attributable to its porphyrin structure, which can induce oxidative stress in fungal cells, thereby compromising membrane integrity and metabolic pathways (Missall et al., 2004; El-Sayed et al., 2022). Similarly, pterostilbene showed strong inhibitory effects, as reflected by a steep regression slope of 5.7753 (Table 1), indicates potent inhibition via stilbene-mediated disruption of spore germination and mycelial expansion (Xu et al., 2022). Recent metabolomic studies have shown that anthracnose infection in sorghum significantly induces the expression of SbSOMT, a key gene in pterostilbene biosynthesis, leading to its accumulation in the leaves (Lui et al., 2023). Just as licorice extracts modulate defense pathways (Hermann et al., 2022), pterostilbene emerges as a highly effective natural antimicrobial compound. Collectively, these findings advance the current understanding of biocontrol strategies against C. sublineola, particularly in the context of organic sorghum production, where research has historically emphasized genetic resistance over microbial or compound-based interventions (Abreha et al., 2021).
The strong field performance of B. subtili observed in this study aligns with its documented efficacy in other pathosystems. For instance, in pepper anthracnose caused by C. gloeosporioides, lipopeptides produced by B. subtilis suppressed fungal growth by 70-80% (Ashwini and Srividya, 2014). Similarly, the greenhouse efficacy of T. harzianum corresponds with Indian native Trichoderma isolates that effectively control sorghum anthracnose through mycoparasitism, achieving up to 65% inhibition (Yadav et al., 2020). Although T. harzianum displayed strong antagonism in dual-culture assays, its inconsistent field performance suggests sensitivity to environmental factors, such as humidity and soil conditions, that may influence hyperparasitism and mycoparasitic enzyme activity (Pandey et al., 2024). Both greenhouse and field trials have demonstrated that B. subtilis exhibits strong biocontrol effects against the sorghum anthracnose (Figures 4, 5). As supported by previous research, its biocontrol capacity involves multiple mechanisms, including lipopeptide-mediated antibiotic effects and induced systemic resistance (ISR) triggered via salicylic acid (SA) and jasmonic acid (JA) signaling pathways (Yu et al., 2022; Hansel et al., 2024). Overall, our findings support synergistic, system-level effects among microbial agents, where field efficacy depends less on in vitro potency and more on complex host-microbe-environment interactions that enhance biocontrol performance under natural conditions. These findings have broader relevance for managing Colletotrichum diseases across cropping systems, supporting the development of climate-resilient strategies that integrate biofungicides with host resistance, as recently demonstrated in Ethiopian field trials (Tadesse et al., 2024).
Several limitations should be considered when interpreting the findings of this study. First, the evaluation focused on a single virulent strain (ZG-DA-20), which may not fully represent the pathotype diversity reported in C. sublineola populations from the United States and Africa (Prom et al., 2014; Zeleke et al., 2024). Second, methodological constraints hindered the exploration of long-term field persistence, with trials limited to one season, and the absence of data on microbial colonization dynamics prevents a conclusive understanding of ISR mechanisms. Third, disease assessment relied primarily on visual severity grading, which is susceptible to observer bias, while environmental variability between the two field sites (e.g., in rainfall and temperature) may have contributed to the differences in control efficacy observed in Luzhou and Chongzhou (Figure 5). Finally, this study is constrained from addressing interactions with co-occurring sorghum diseases or diverse cultivars, thus limiting broader generalizability.
To address the gap identified in this study, researchers may consider longitudinal field trials across diverse agroecological zones to evaluate agent persistence and environmental resilience. Exploring synergistic formulations that combine pterostilbene with microbial agents such as B. subtilis could enhance control efficacy, potentially exceeding the 70% threshold achieved in comparable pathosystems. In order to overcome the challenges outlined, researchers could adopt molecular techniques, such as qPCR for pathogen quantification and metabolomics for phytoalexin tracking, would provide more accurate and mechanistic insights into agent-pathogen interactions. To deepen our understanding of biocontrol mechanisms, future investigations might focus on greenhouse simulations of abiotic stressors, paving the way for tailored integrated pathogen management strategies in organic sorghum production and contributing to sustainable global food security. Collectively, these approaches would not only address the current limitations but also contribute to the development of sustainable crop protection practices in the face of evolving climate and disease pressures.
5 Conclusion
This study provides a comprehensive evaluation of several biofungicides and microbial agents against C. sublineola. Integrating in vitro, greenhouse, and field data, we found that all tested biological treatments inhibited pathogen growth and spore germination to varying extents. Iron chlorin exhibited strong antifungal activity in vitro but showed reduced efficacy in the field, underscoring the impact of environmental factors and formulation stability. In contrast, pterostilbene and B. subtilis consistently achieved superior control across environments, highlighting their potential as effective and eco-friendly alternatives to synthetic fungicides. Overall, pterostilbene and B. subtilis represent sustainable options for reducing chemical dependence and advancing environmentally responsible anthracnose management in sorghum production.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: https://ngdc.cncb.ac.cn/biosample/browse/SAMC3768278.
Author contributions
J-WZ: Funding acquisition, Writing – review & editing, Supervision, Conceptualization. J-RH: Visualization, Investigation, Formal analysis, Methodology, Writing – original draft, Data curation. F-FZ: Data curation, Methodology, Investigation, Formal analysis, Writing – original draft. X-RH: Validation, Investigation, Writing – original draft, Methodology. J-LH: Methodology, Validation, Formal analysis, Software, Writing – original draft. J-YL: Writing – original draft, Data curation, Validation, Supervision. KZ: Validation, Writing – original draft, Investigation, Formal analysis. LM: Writing – original draft, Methodology, Validation, Supervision. Z-HL: Validation, Formal analysis, Writing – original draft. J-YX: Validation, Supervision, Writing – original draft. J-TS: Validation, Writing – original draft, Formal analysis. Z-HL: Validation, Writing – original draft, Formal analysis. X-GW: Validation, Formal analysis, Writing – original draft. X-LN: Resources, Funding acquisition, Validation, Writing – review & editing. Y-NS: Writing – original draft, Validation, Methodology, Supervision. Z-FW: Writing – original draft, Formal analysis, Validation. Z-YJ: Validation, Writing – original draft, Formal analysis. J-PW: Validation, Writing – original draft, Formal analysis. PL: Methodology, Conceptualization, Writing – review & editing, Validation.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was financially supported the Sichuan Science and Technology Program (2022ZDZX0016, 2025YFHZ0311), the Key Technology Research and Development Program of Sichuan Province (2021YFYZ0017), the China Agriculture Research System (CARS-06-A14.5), Sichuan Province Engineering Technology Research Center of Liquor-Making Grains (2023-03).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2025.1728722/full#supplementary-material
References
Abbey, J. A., Percival, D., Abbey, L., Asiedu, S. K., Prithiviraj, B., and Schilder, A. (2019). Biofungicides as alternative to synthetic fungicide control of grey mould (Botrytis cinerea)–prospects and challenges. Biocontrol Sci. Technol. 29, 207–228. doi: 10.1080/09583157.2018.1548574
Abo-Zaid, A. H., Abdel-Wahab, H., EL-Samman, M. G. E. R., and Helmy, K. G. (2025). Evaluation of botanical and biological control strategies against onion purple blotch disease in Egypt. Egyptian J. Phytopathol. 53, 73–89. doi: 10.21608/ejp.2025.406926.1151
Abreha, K. B., Ortiz, R., Carlsson, A. S., and Geleta, M. (2021). Understanding the sorghum–Colletotrichum sublineola interactions for enhanced host resistance. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.641969
Acharya, P., Ghimire, R., Lehnhoff, E. A., and Marsalis, M. A. (2023). Cover crop forage potential and subsequent sorghum silage yield and nutritive value. Agron. J. 115, 1723–1734. doi: 10.1002/agj2.21334
Ashwini, N. and Srividya, S. (2014). Potentiality of Bacillus subtilis as biocontrol agent for management of anthracnose disease of chilli caused by Colletotrichum gloeosporioides OGC1. 3 Biotech. 4, 127–136. doi: 10.1007/s13205-013-0134-4
Chala, A., Alemu, T., Prom, L. K., and Tronsmo, A. M. (2010). Effect of host genotypes and weather variables on the severity and temporal dynamics of sorghum anthracnose in Ethiopia. Plant Pathol. J. 9, 39–46. doi: 10.3923/ppj.2010.39.46
Chan, E., Wong, C., Tan, Y., Foo, J., Wong, S., and Chan, H. (2019). Resveratrol and pterostilbene: A comparative overview of their chemistry, biosynthesis, plant sources and pharmacological properties. J. Appl. Pharm. Sci. 9, 124–129. doi: 10.7324/JAPS.2019.90717
Dewen, Q., Yijie, D., Yi, Z., Shupeng, L., and Fachao, S. (2017). Plant immunity inducer development and application. Mol. Plant Microbe Interact. 30, 355–360. doi: 10.1094/MPMI-11-16-0231-CR
Egel, D. S., Hoagland, L., Davis, J., Marchino, C., and Bloomquist, M. (2019). Efficacy of organic disease control products on common foliar diseases of tomato in field and greenhouse trials. Crop Protection 122, 90–97. doi: 10.1016/j.cropro.2019.04.022
El-Sayed, M. T., Ezzat, S. M., Taha, A. S., and Ismaiel, A. A. (2022). Iron stress response and bioaccumulation potential of three fungal strains isolated from sewage-irrigated soil. J. Appl. Microbiol. 132, 1936–1953. doi: 10.1111/jam.15372
Fu, F., Girma, G., and Mengiste, T. (2020). Global mRNA and microRNA expression dynamics in response to anthracnose infection in sorghum. BMC Genomics 21, 760. doi: 10.1186/s12864-020-07138-0
Hansel, J., Saville, A. C., and Ristaino, J. B. (2024). Evaluation of a formulation of Bacillus subtilis for control of Phytophthora blight of bell pepper. Plant Dis. 108, 1014–1024. doi: 10.1094/pdis-04-23-0807-re
Hermann, S., Orlik, M., Boevink, P., Stein, E., Scherf, A., Kleeberg, I., et al. (2022). Biocontrol of plant diseases using Glycyrrhiza glabra leaf extract. Plant Dis. 106, 3133–3144. doi: 10.1094/PDIS-12-21-2813-RE
Hu, J., Zheng, M., Dang, S., Shi, M., Zhang, J., and Li, Y. (2021). Biocontrol potential of Bacillus amyloliquefaciens LYZ69 against anthracnose of alfalfa (Medicago sativa). Phytopathology 111, 1338–1348. doi: 10.1094/PHYTO-09-20-0385-R
Jeblick, T., Leisen, T., Steidele, C. E., Albert, I., Müller, J., Kaiser, S., et al. (2023). Botrytis hypersensitive response inducing protein 1 triggers noncanonical PTI to induce plant cell death. Plant Physiol. 191, 125–141. doi: 10.1093/plphys/kiac476
Liu, C., Yao, Z., Jiang, B., Yu, W., Wang, Y., Dong, W., et al. (2023). Effects of exogenous auxin on mesocotyl elongation of sorghum. Plants. 12, 944. doi: 10.3390/plants12040944
Lü, Z. W., Liu, H. Y., Wang, C. L., Chen, X., Huang, Y. X., Zhang, M. M., et al. (2023). Isolation of endophytic fungi from Cotoneaster multiflorus and screening of drought-tolerant fungi and evaluation of their growth-promoting effects. Front. Microbiol. 14. doi: 10.3389/fmicb.2023.1267404
Lui, A. C. W., Pow, K. C., Lin, N., Lam, L. P. Y., Liu, G., Godwin, I. D., et al. (2023). Regioselective stilbene O-methylations in Saccharinae grasses. Nat. Commun. 14 (1), 3462. doi: 10.1038/s41467-023-38908-5
Luo, W., Ping, X., Zhou, J., Gao, S., Huang, X., Song, S., et al. (2025). Alternaria alternata JTF001 Metabolites Recruit Beneficial Microorganisms to Reduce the Parasitism of Orobanche aEgyptiaca in Tomato. Biology 14, 116. doi: 10.3390/biology14020116
Mardanova, E. S., Vasyagin, E. A., and Ravin, N. V. (2024). Virus-like particles produced in plants: A promising platform for recombinant vaccine development. Plants 13, 3564. doi: 10.3390/plants13243564
Miao, P., Wang, H., Wang, W., Wang, Z., Ke, H., Cheng, H., et al. (2025). A widespread plant defense compound disarms bacterial type III injectisome assembly. Science 387, eads0377. doi: 10.1126/science.ads0377
Missall, T. A., Lodge, J. K., and McEwen, J. E. (2004). Mechanisms of resistance to oxidative and nitrosative stress: implications for fungal survival in mammalian hosts. Eukaryotic Cell 3, 835–846. doi: 10.1128/EC.3.4.835-846.2004
Pandey, A. K., Yadav, S., Samota, M. K., Sharma, H. K., and Roy, S. (2024). Trichoderma harzianum TIND02 upregulates the expression of pathogenesis-related genes and enzymes and enhances gray blight resistance in tea. Pestic. Biochem. Physiol. 205, 106115. doi: 10.1016/j.pestbp.2024.106115
Prom, L. K., Perumal, R., Cissé, N., and Little, C. R. (2014). Evaluation of selected sorghum lines and hybrids for resistance to grain mold and long smut fungi in Senegal, West Africa. Plant Health Prog. 15, 74–77. doi: 10.1094/php-rs-13-0128
Qiao, Z., Yao, C., Sun, S., Zhang, F., Yao, X., Li, X., et al. (2023). Spraying S-ABA can alleviate the growth Inhibition of corn (Zea mays L.) under water-deficit stress. J. Soil Sci. Plant Nutr. 23, 1222–1234. doi: 10.1007/s42729-022-01116-z
Stutts, L. R. and Vermerris, W. (2020). Elucidating anthracnose resistance mechanisms in sorghum—a review. Phytopathology 110, 1863–1876. doi: 10.1094/PHYTO-04-20-0132-RVW
Tadesse, T., Zenebe, T., Kebede, T., Gizaw, O., and Eguale, T. (2024). Prevalence and antimicrobial susceptibility profile of Listeria monocytogenes in humans, animals, and foods of animal origin in Ethiopia: a systematic review and meta-analysis. Cogent Food Agric. 10, 2306018. doi: 10.1080/23311932.2024.2306018
Tian, Y., Wang, J., Lan, Q., Liu, Y., Zhang, J., Liu, L., et al. (2024). Biocontrol mechanisms of three plant essential oils against Phytophthora infestans causing potato late blight. Phytopathology 114, 1502–1514. doi: 10.1094/PHYTO-06-23-0216-R
Trujillo, E. and Angulo, C. (2025). Plant-made vaccines targeting enteric pathogens—Safe alternatives for vaccination in developing countries. Biotechnol. Bioeng. 122, 457–480. doi: 10.1002/bit.28876
Wang, Z., Li, Y., Zhuang, L., Yu, Y., Liu, J., Zhang, L., et al. (2019). A rhizosphere-derived consortium of Bacillus subtilis and Trichoderma harzianum suppresses common scab of potato and increases yield. Comput. Struct. Biotechnol. J. 17, 645–653. doi: 10.1016/j.csbj.2019.05.003
Xavier, K. V., Mizubuti, E. S. G., Queiroz, M. V., Chopra, S., and Vaillancourt, L. (2018). Genotypic and pathogenic diversity of Colletotrichum sublineola isolates from sorghum (Sorghum bicolor) and johnsongrass (S. halepense) in the southeastern United States. Plant Dis. 102, 2341–2351. doi: 10.1094/PDIS-04-18-0562-RE
Xu, D., Deng, Y., Xi, P., Zhu, Z., Kong, X., Wan, L., et al. (2018). Biological activity of pterostilbene against Peronophythora litchii, the litchi downy blight pathogen. Postharvest Biol. Technol. 144, 29–35. doi: 10.1016/j.postharvbio.2018.05.011
Xu, D., Qiao, F., Xi, P., Lin, Z., Jiang, Z., Romanazzi, G., et al. (2022). Efficacy of pterostilbene suppression of postharvest gray mold in table grapes and potential mechanisms. Postharvest Biol. Technol. 183, 111745. doi: 10.1016/j.postharvbio.2021.111745
Yadav, A. N., Kour, D., Kaur, T., Devi, R., and Yadav, N. (2020). Functional annotation of agriculturally important fungi for crop protection: current research and future challenges. Fungal Biol., 347–356. doi: 10.1007/978-3-030-48474-3_12
Yu, Y., Gui, Y., Li, Z., Jiang, C., Guo, J., and Niu, D. (2022). Induced systemic resistance for improving plant immunity by beneficial microbes. Plants 11, 386. doi: 10.3390/plants11030386
Yu, Z. F., Kami, Y. B., Xia, J. Y., Chang, X. Y., Li, J. Y., Han, J. R., et al. (2025). Identification of major fungal diseases in sorghum and screening of broad-spectrum resistance germplasms in Southwest China. Physiol. Mol. Plant Pathol. 122, 90–97. doi: 10.1016/j.pmpp.2025.102706
Zaim, S., Bekkar, A. A., and Belabid, L. (2018). Efficacy of Bacillus subtilis and Trichoderma harzianum combination on chickpea Fusarium wilt caused by F. oxysporum f. sp. ciceris. Arch. Phytopathol. Plant Prot. 51, 217–226. doi: 10.1080/03235408.2018.1447896
Zeleke, A., Ofga, B., and Megersa, M. (2024). Diversity and ethnobotanical study of homegarden plants in Goba district, Oromia region, Ethiopia. Discov. Plants. 1, 30. doi: 10.1007/s44372-024-00029-8
Zhang, Y., He, K., Guo, X., Jiang, J., Qian, L., Xu, J., et al. (2023). Transcriptomic profiling of fusarium pseudograminearum in response to carbendazim, pyraclostrobin, tebuconazole, and phenamacril. J. Fungi (Basel) 9 (3), 334. doi: 10.3390/jof9030334
Zhang, J. W., Li, J. Y., Yu, Z. F., Chang, X. Y., Han, J. R., Xia, J. Y., et al. (2025). Comparative genomic analysis reveals the difference of NLR immune receptors between anthracnose-resistant and susceptible sorghum cultivars. Phytopathol. Res. 7, 29. doi: 10.1186/s42483-025-00318-4
Zhou, W., Chen, J., Zhou, R., Xiao, J., Li, Y., Ren, Y., et al. (2024). Evaluation of Iron Chlorin e6 disappearance and hydrolysis in soil and garlic using salting-out assisted liquid-liquid extraction coupled with high-performance liquid chromatography and ultraviolet-visible detection. Food Chem. 447, 138960. doi: 10.1016/j.foodchem.2024.138960
Keywords: Sorghum bicolor, biocontrol agents, pterostilbene, Bacillus subtilis, Colletotrichum sublineola
Citation: Zhang J-W, Han J-R, Zhao F-F, Huang X-R, Hu J-L, Li J-Y, Zhu K, Mao L, Li Z-H, Xia J-Y, Su J-T, Liang Z-H, Wang X-G, Ni X-L, Shi Y-N, Wang Z-F, Jiao Z-Y, Wang J-P and Lv P (2025) Evaluation of biocontrol agents for the management of sorghum anthracnose caused by Colletotrichum sublineola. Front. Plant Sci. 16:1728722. doi: 10.3389/fpls.2025.1728722
Received: 20 October 2025; Accepted: 19 November 2025; Revised: 13 November 2025;
Published: 15 December 2025.
Edited by:
Mahalingam Govindaraj, HarvestPlus - Alliance Bioversity- CIAT., ColombiaReviewed by:
Haiming Duan, Anhui University of Science and Technology, ChinaYu Jiang, Liaoning Academy of Agricultural Sciences, China
Copyright © 2025 Zhang, Han, Zhao, Huang, Hu, Li, Zhu, Mao, Li, Xia, Su, Liang, Wang, Ni, Shi, Wang, Jiao, Wang and Lv. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ji-Wei Zhang, emhhbmdqaXdlaUBzaWNhdS5lZHUuY24=; Peng Lv, cGVuZ2wwMDFAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Ji-Wei Zhang
Ji-Wei Zhang Jun-Ru Han1†
Jun-Ru Han1† Yan-Nan Shi
Yan-Nan Shi Zhi-Fang Wang
Zhi-Fang Wang Zhi-Yin Jiao
Zhi-Yin Jiao